Note: Descriptions are shown in the official language in which they were submitted.
A8141809CA
METHOD OF INHIBITING DEPOSITION OF SILICON-BASED INORGANIC DEPOSITS
DURING IN-SITU HYDROCARBON PRODUCTION
TECHNICAL FIELD
[0001] The present disclosure generally relates to inhibiting
deposition of
inorganic deposits during in-situ petroleum production. In particular, the
present
disclosure relates to inhibiting deposition of silicon-based inorganic
deposits on surfaces
within the intermediate casing of a production well under high-pressure and/or
high-
temperature conditions.
BACKGROUND
100021 Thermal recovery processes use heat to mobilize viscous hydrocarbons
so
that they can be recovered from a subterranean reservoir. Such processes
typically rely
on steam injection to reduce hydrocarbon viscosity in situ. Steam is a useful
means for
introducing heat energy into a subterranean reservoir, because steam has a
high specific
heat capacity. Steam assisted gravity drainage (SAGD) is one example of a
thermal in-situ
hydrocarbon recovery process that uses steam as the primary means for
transferring heat
energy to the reservoir. In a typical SAGD well configuration, a hydrocarbon-
containing
reservoir is penetrated by an injection well and a production well. The
production well is
horizontally aligned with, and vertically displaced from, the injection well.
In a typical
SAGD process, steam is injected into the hydrocarbon-containing reservoir
where the
steam condenses and transfers latent heat energy to the reservoir. The heat
transfer
mobilizes hydrocarbons within the reservoir by reducing their viscosity. Over
time,
condensed steam and mobilized hydrocarbons drain to the production well
through
which they are produced to the surface.
[0003] A typical production well for a SAGD process comprises a
liner, an
intermediate casing, an electric submersible pump (ESP), and production
tubing. The liner
is configured to allow passage of production fluids (i.e. condensed steam,
mobilized
hydrocarbons, and/or other fluids derived from the reservoir) into the
production well.
The intermediate casing engages with the liner and runs to the surface. The
intermediate
1
CA 3063448 2019-12-02
A8141809CA
casing is configured to provide structural support to the production well and
to house
the ESP, the production tubing, and other primary completion components. The
ESP is
configured to pump production fluids from the area proximate to the liner to
the surface
via the production tubing. Because production fluids are free to flow within
the liner and
the intermediate casing, fluid levels in the intermediate casing rise and fall
as operational
protocols and reservoir behavior vary. As a result, surfaces and fluids within
the
intermediate casing are subjected to a wide range of temperature/pressure
conditions
which may promote the deposition of problematic inorganic materials during
production.
[0004] Scale is a mineral salt deposit that occurs on well
completion components,
and it causes a variety of problems during hydrocarbon recovery processes. A
range of
chemical treatment options have been proposed for scale removal/inhibition.
With
respect to scale removal, chemical treatment protocols are generally selected
based on
the composition of the scale. For example, carbonate scales (such as calcium
carbonate)
are typically treated with aqueous hydrochloric acid, and sulfate scales (such
as calcium
sulfate) are typically treated with chelating agents such as ethylenediamine
teraacetic
acid (EDTA). With respect to scale inhibition, chemical treatments typically
involve
"squeezing" a chemical inhibitor, such as a chelating agent or a polymer, into
the
reservoir for subsequent commingling with produced fluids in an attempt to
mitigate
against further scale precipitation.
[0005] Unlike common scales which comprise primarily mono-valent alkali
metals
(e.g. Na, Li, K+) and/or di-valent alkaline earth metals (e.g. Ca2+, Sr2+,
Ba2+), deposits
formed during SAGD processes tend to be primarily comprised of silicon.
Silicon is a
metalloid element, and it tends to form stable compounds/complexes in a tetra-
valent
state. Silicon-based inorganic deposits typically comprise silica (i.e.
silicon dioxide) and/or
silicates (i.e. salts derived from silica or silicic acids), and they tend not
dissolve in
hydrochloric acid or form stable chelate complexes. Accordingly, typical scale-
treatment
protocols are of limited use for thermal recovery processes like SAGD. The
impact of the
limited utility of typical treatment protocols and the recalcitrant nature of
silicon-based
inorganic deposits is amplified by the dynamic high-temperature and high-
pressure
conditions associated with SAGD. Because, operating conditions and reservoir
character
2
CA 3063448 2019-12-02
A8141809CA
vary widely during recovery, SAGD production wells are routinely exposed to
wide
temperature/pressure swings and varying compositions and quantities of
production
fluids. Temperature and pressure variations can shift solubility equilibria
towards
precipitation, increase flocculation rates, and reduce emulsion stability, all
of which may
contribute to accelerated deposition of silicon-based inorganic deposits.
Deposition of
silicon-based inorganic deposits on surfaces within the intermediate casing is
undesirable, because it can lead to sub-optimal production efficiency,
increased
maintenance requirements, and/or premature failure for casing and primary
completion
components. Accordingly, there is a need for methods of inhibiting deposition
of silicon-
based inorganic deposits on surfaces within the intermediate casing of SAGD
production
wells.
SUMMARY
[0006] In the context of the present disclosure, it has been
determined that
deposition of silicon-based inorganic deposits on surfaces within the
intermediate casing
can be inhibited by providing a deposition inhibitor to the interior volume of
the
intermediate casing by way of a capillary tube. In the context of the present
disclosure, a
capillary tube is a conduit that has a first opening at the surface and a
second opening in
fluid communication with the interior volume of the intermediate casing. In
SAGD
production operations, capillary tubes are typically used to evaluate in-situ
pressure
conditions at the heel of the production tube by counter-pressurizing the
capillary tube
from the surface. In the context of the present disclosure, the deposition
inhibitor is
pushed (under pressure from the surface) through the capillary tube into the
interior
volume of the intermediate casing, such that the deposition inhibitor is
introduced in
proximity to the surfaces that may be at risk of being compromised by silicon-
based
inorganic deposits. Specific parameters with respect to the composition,
density,
pressure, timing, and concentration of the injection of the deposition
inhibitor into the
interior volume of the intermediate casing are provided in the present
disclosure.
Protocols for monitoring the impact of the presence of the deposition
inhibitor within
the interior volume of the intermediate casing are provided in the present
disclosure (e.g.
flow-back testing) as are strategies for modifying related methods based on
the results
3
CA 3063448 2019-12-02
A8141809CA
obtained from such monitoring. The monitoring methods involve analyzing
produced
fluids for quantities of silicon-based inorganic compounds and/or quantities
of the
deposition inhibitor (each in various forms). The monitoring methods allow for
feedback-
based updating of the protocol for providing the deposition inhibitor to the
interior
volume of the intermediate casing. Methods for producing hydrocarbons while
inhibiting
deposition of silicon-based inorganic deposits on surfaces within the interior
volume of
the intermediate casing are also provided.
100071 In select embodiments, the present disclosure relates to a
method of
inhibiting deposition of a silicon-based inorganic deposit within a production
well that is
in fluid communication with a subterranean reservoir. The method comprises
providing
a deposition inhibitor into an internal volume defined by an intermediate
casing of the
production well. The deposition inhibitor is provided by way of a conduit
having an
opening in fluid communication with the internal volume of the intermediate
casing. The
deposition inhibitor is provided into the internal volume of the intermediate
casing
before, during, and/or after the internal volume of the intermediate casing is
occupied
by a fluid having, at one or more times during a hydrocarbon production phase,
a
temperature of between about 80 C and about 285 C, and a pressure of between
about
800 kPag and about 7,000 kPag.
100081 In select embodiments, the present disclosure relates to a
method of
producing hydrocarbons from a subterranean reservoir that is in fluid
communication
with an injection well and a production well. The method comprises injecting
steam,
solvent, or a combination thereof into the subterranean reservoir by way of
the injection
well. The method further comprises providing a deposition inhibitor to an
internal
volume of an intermediate casing within the production well. The deposition
inhibitor is
provided by way of a conduit having an opening in fluid communication with the
internal
volume of the intermediate casing. The providing of the deposition inhibitor
to the
internal volume of the intermediate casing occurs before, during, and/or after
the
internal volume of the intermediate casing is occupied by a fluid having, at
one or more
times during a hydrocarbon production phase, a temperature of between about 80
C
and about 285 C, and a pressure of between about 800 kPag and about 7,000
kPag. The
4
CA 3063448 2019-12-02
A8141809CA
method further comprises producing at least a portion of the fluid by way of
the
production well.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] These and other features of the present disclosure will
become more
apparent in the following detailed description in which reference is made to
the
appended drawings. The appended drawings illustrate one or more embodiments of
the
present disclosure by way of example only and are not to be construed as
limiting the
scope of the present disclosure.
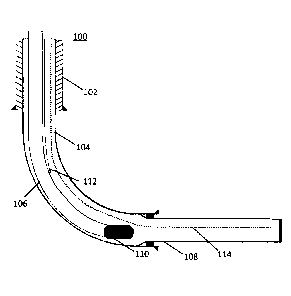
[0010] FIG. 1 provides a schematic illustration of a SAGD
production well that is
configured for use in accordance with one or more methods of the present
disclosure.
[0011] FIG. 2 provides a plot of the saturation concentration of
silica in an
aqueous steam condensate fluid as a function of temperature.
[0012] FIG. 3 provides a plot of the saturation concentration of
silica in an
aqueous steam condensate fluid as a function of pH.
[0013] FIG. 4 provides a plot of pH as a function of time for a produced
fluid from
a SAGD facility located in Foster Creek, Alberta.
[0014] FIG. S provides IR spectra of a deposition inhibitor taken
before (A) and
after (B) the deposition inhibitor was heated to a temperature of 235 C for
22 hours.
[0015] FIG. 6 provides results from a differential scanning
calorimetry
experiment. The results were used to determine the decomposition temperature
of a
deposition inhibitor.
[0016] FIG. 7A provides a photograph of a silicon-based deposit
obtained from
within the interior volume of the intermediate casing, and FIG. 7B provides a
high-
resolution microscopic image of the same.
5
CA 3063448 2019-12-02
A8141809CA
[0017] FIG. 8 provides forecasted results for emulsion rates (A and
B) and
concentration of a deposition inhibitor (C) as a function of time for a well-
pad wide
experiment relating to methods that are in accordance with the present
disclosure.
DETAILED DESCRIPTION
[0018] Embodiments of the present disclosure will now be described with
reference to FIG. 1 to FIG. 8.
[0019] The present disclosure sets out an important finding
regarding the
chemical composition of silicon-based inorganic deposits within the interior
volume of
the intermediate casing of a SAGD production well. Briefly stated,
experimental evidence
suggests that under at least some SAGD recovery conditions a large portion
(e.g. greater
than about 85 %) of surface deposits may be crystalline in form and comprised
of silicon-
containing compounds. Without being bound to any particular theory, the
present
disclosure asserts that because: (i) the solubility (and by proxy the
deposition
characteristics) of crystalline and amorphous forms of silicon-containing
compounds are
often substantially different, and (ii) the majority of the silicon-based
inorganic deposits
formed under SAGD recovery conditions are crystalline in form, methods for
inhibiting
deposition of silicon-containing deposits under SAGD recovery conditions are
best
targeted towards crystalline forms of silicon-containing compounds. Again
without being
bound to any particular theory, the present disclosure asserts that
crystalline forms of
silicon-containing compounds may be inhibited from depositing by reducing the
extent
crystal nucleation and/or crystal growth in situ such that silicon-containing
precipitates
stay suspended and/or dispersed so as to be produced to the surface instead of
deposited
within the interior volume of the intermediate casing. To this end, the
methods of the
present disclosure utilize a deposition inhibitor that is provided to the
interior volume of
the intermediate casing by way of a capillary tube. The deposition inhibitor
is selected
based on its capacity to supress deposition of silica-based inorganic deposits
and based
on its ability to resist degradation under the temperature, pressure, and/or
pH conditions
associated with SAGD production. The capillary tube provides a means to
introduce the
deposition inhibitor in proximity to the surfaces that are most at risk
receiving silicon-
6
CA 3063448 2019-12-02
A8141809CA
based inorganic deposits. Moreover, the capillary tube provides a means to
provide the
deposition inhibitor under targeted, time, concentration, density, rate,
and/or pressure
protocols. Taken together, the desirable attributes of targeted capillary tube
injection
protocols and condition specific deposition inhibitors provide for the
inhibition of
deposition of silicon-containing deposits during thermal recovery of in situ
hydrocarbons.
100201 In the context of the present disclosure, various terms are
used in
accordance with what is understood to be the ordinary meaning of those terms.
For
example, "petroleum" is a naturally occurring mixture consisting predominantly
of
hydrocarbons in the gaseous, liquid, or solid phase. In the context of the
present
disclosure, the words "petroleum" and "hydrocarbon(s)" are used to refer to
mixtures of
widely varying composition. The production of petroleum from a reservoir
necessarily
involves the production of hydrocarbons, but is not limited to hydrocarbon
production
and may include, for example, trace quantities of metals (e.g. Fe, Ni, Cu, V).
Similarly,
processes that produce hydrocarbons from a well will generally also produce
petroleum
fluids that are not hydrocarbons. In accordance with this usage, a process for
producing
petroleum or hydrocarbons is not necessarily a process that produces
exclusively
petroleum or hydrocarbons, respectively. "Fluids", such as petroleum fluids,
include both
liquids and gases.
100211 It is common practice to categorize petroleum substances of
high viscosity
and density into two categories, "heavy oil" and "bitumen". For example, some
sources
define "heavy oil" as a petroleum that has a mass density of greater than
about 900
kg/m3. Bitumen is sometimes described as that portion of petroleum that exists
in the
semi-solid or solid phase in natural deposits, with a mass density greater
than about
1,000 kg/m3 and a viscosity greater than 10,000 centipoise (cP; or 10 Pa.$)
measured at
original temperature in the deposit and atmospheric pressure, on a gas-free
basis.
Although these terms are in common use, references to heavy oil and bitumen
represent
categories of convenience, and there is a continuum of properties between
heavy oil and
bitumen. Accordingly, references to heavy oil and/or bitumen herein include
the
continuum of such substances, and do not imply the existence of some fixed and
universally recognized boundary between the two substances. In particular, the
term
7
CA 3063448 2019-12-02
A8141809CA
"heavy oil" includes within its scope all "bitumen" including hydrocarbons
that are
present in semi-solid or solid form.
[0022] In the context of the present disclosure, a "reservoir" is a
subsurface
formation containing one or more natural accumulations of moveable petroleum,
which
are generally confined by relatively impermeable rock. An "oil sand" or "oil
sands"
reservoir is generally comprised of strata of sand or sandstone containing
petroleum.
"Thermal recovery" or "thermal stimulation" refers to enhanced oil recovery
techniques
that involve delivering thermal energy to a petroleum resource, for example to
a heavy
oil reservoir. There are a significant number of thermal recovery techniques
other than
SAGD, such as cyclic steam stimulation (CSS), in-situ combustion, hot water
flooding,
steam flooding, and electrical heating. In general, thermal energy is provided
to reduce
the viscosity of the petroleum to facilitate production. This thermal energy
may be
provided by a "thermal recovery fluid", which is a fluid that carries thermal
energy, for
example in the form of steam, solvents, or mixtures thereof (with or without
additives
such as surfactants).
[0023] Select embodiments of the present disclosure relate to
methods of
inhibiting deposition of a silicon-based inorganic deposit within a production
well that is
in fluid communication with a subterranean reservoir. The method comprises
providing
a deposition inhibitor into an internal volume defined by an intermediate
casing of the
production well. The deposition inhibitor is provided by way of a conduit
having an
opening in fluid communication with the internal volume of the intermediate
casing. The
deposition inhibitor is provided into the internal volume of the intermediate
casing
before, during, and/or after the internal volume of the intermediate casing is
occupied
by a fluid having, at one or more times during a hydrocarbon production phase,
a
temperature of between about 80 C and about 285 C and .a pressure of between
about
800 kPag and about 7,000 kPag.
[0024] In select embodiments, the present disclosure relates to a
method of
producing hydrocarbons from a subterranean reservoir that is in fluid
communication
with an injection well and a production well. The method comprises injecting
steam,
8
CA 3063448 2019-12-02
A8141809CA
solvent, or a combination thereof into the subterranean reservoir by way of
the injection
well. The method further comprises providing a deposition inhibitor to an
internal
volume of an intermediate casing within the production well. The deposition
inhibitor is
provided by way of a conduit having an opening in fluid communication with the
internal
volume of the intermediate casing. The providing of the deposition inhibitor
to the
internal volume of the intermediate casing occurs before, during, and/or after
the
internal volume of the intermediate casing is occupied by a fluid having, at
one or more
times during a hydrocarbon production phase, a temperature of between about 80
C
and about 285 C and a pressure of between about 800 kPag and about 7,000
kPag. The
method further comprises producing at least a portion of the fluid by way of
the
production well.
[0025] In select embodiments of the present disclosure, the
production well may
be configured for a SAGD process, a cyclic steam stimulation process (CSS), a
solvent
process such as a solvent assisted process (SAP), or a combination thereof.
For example
the production well may comprise a surface casing, an intermediate casing, and
a liner.
In the context of the present disclosure, an intermediate casing may engage a
liner at one
end and may house a production tubing, a pump (such as an electric submersible
pump
(ESP)), and/or other primary completion components. Accordingly, in the
context of the
present disclosure, the interior volume of an intermediate casing may comprise
any
interior surfaces of the intermediate casing, any surfaces of the production
tubing housed
within the intermediate casing, any surfaces of the ESP housed within the
intermediate
casing, and/or any surfaces of any other primary completion components housed
within
the intermediate casing. FIG. 1 provides a schematic of one such production
well 100. In
FIG.1, the production well 100 comprises a surface casing 102, an intermediate
casing
104, a production tubing 106, a liner 108, an ESP 110, a capillary tube 112,
and an
instrumentation coil 114. The surface casing 102 is a J-55 ST&C surface casing
(339.7 mm,
81.105 kg/m). The intermediate casing 104 is an L80 QB2 intermediate casing
(244.5 mm,
59.527 kg/m). The production tubing 106 is a 1-55 EUE production tubing (88.9
mm, 13.84
kg/m), the liner 108 is a 177.8 m liner. The capillary tube 112 is a 316L SS
capillary tube
(12.7 mm, 0.354 kg/m). The instrumentation coil 114 is a 31.75 mm coil.
9
CA 3063448 2019-12-02
A8141809CA
[0026] In the context of the present disclosure, the fluid within
the internal
volume of the intermediate casing may comprise a hydrocarbon fluid, an aqueous
fluid
(e.g. steam condensate), and/or another fluid which may originate from the
reservoir. In
select embodiments of the present disclosure, the temperature of the fluid
within the
intermediate casing may be, at one or more times during the production
process,
between about 100 C and about 230 C (in particular between about 180 C and
about
220 C). Likewise, in select embodiments of the present disclosure, the
pressure of the
fluid within the intermediate casing may be, at one or more times during the
production,
between about 2,500 kPag and about 5,500 kPag (in particular between about
36,000
kPag and 5,000 kPag). Further, in select embodiments of the present
disclosure, the pH
of the fluid within the intermediate casing may be, at one or more times
during the
production, between about 5.0 and about 10.0 (in particular between about 6.0
and 9.0).
Because, operating conditions and reservoir characteristics vary widely during
recovery,
SAGD production wells may be exposed to wide temperature variations, pressure
variations, and/or pH variations. Some such variations may drive solubility
equilibria
towards precipitation, increase flocculation rates, and/or reduce emulsion
stability. The
equilibrium saturation concentration (mg/L) of silica in a steam condensate as
a function
of temperature is shown in FIG. 2. The trend in FIG. 2 indicates a decrease of
greater than
about 25 % of the saturation concentration of silica between 180 C and 220
'C. Without
being bound to any particular theory, the present disclosure contemplates that
reductions in produced-fluid temperature within such a range may be associated
with
increased rates of deposition of silicon-based deposits. Similar correlations
may arise
with respect to pressure and/or pH. The equilibrium saturation concentration
(mg/L) of
silica in a steam condensate as a function of pH is shown in FIG. 3, and the
trend indicates
a sharp increase in silica solubility between about 9.0 and about 10Ø pH
values within
this range may occur within a SAGD reservoir, especially at or near peak
temperature and
pressure conditions where CO2 solubility (in the form of carboxylate anion)
may be
elevated. Without being bound to any particular theory, the present disclosure
contemplates that silicon-containing compounds may be leached from the
reservoir
under such conditions and deposition may be induced in part by pH reduction as
reservoir
fluids cool and drop in pressure within the interior volume of the
intermediate casing.
CA 3063448 2019-12-02
A8141809CA
FIG. 4 which provides a plot of pH as a function of time for a produced fluid
stream from
a SAGD facility located in Foster Creek, Alberta. Large variations in pH over
relatively short
periods of time are readily apparent in FIG. 4 with pH values ranging from
about 5.1 to
about 9.2.
[0027] In the context of the present disclosure, a silicon-based inorganic
deposit
is one that primarily comprises silicon-containing compounds (by mass, volume,
or
elemental analysis). In the context of the present disclosure, silicon-based
inorganic
deposits may comprise primarily crystalline silica, amorphous silica, and/or
another
silicon-containing compound. Examples of silicon-containing compounds that
often occur
in silicon-based inorganic deposits include, but are not limited to,
Quartz/Cristobalite
(SiO2 ¨ various crystal-lattice-structure analogs), Anorthite (CaAl2S1208),
Microcline
(KAISi308), Illite ((K,H30)Al2Si3A1010(OH)2), Albite (NaAlSi308), Magnesium
Aluminum
Silicate (Mg2A1415018), Faujasite ((Ca, Na).Al2S12.509.6.4H20), and Analcime
(Na(Si2A1)06=H20). As will be appreciated by those skilled in the art, the
composition of a
silicon-based inorganic deposit may be determined by a variety of
characterization
techniques including, but not limited to, X-ray diffraction (XRD),
transmission electron
microscopy (TEM), scanning electron microscopy (SEM), energy dispersive X-ray
spectroscopy (EDS), and X-ray absorption fine structure (EXAFS).
[0028] In the context of the present disclosure, a deposition
inhibitor is any
compound or composition that is capable of suppressing deposition of silicon-
based
inorganic deposits under the temperature, pressure, and/or pH conditions set
out herein.
In the context of the present disclosure, deposition inhibitors may: increase
the solubility
of silicon-containing compounds in solution; inhibit chain propagation of
silicon-
containing compounds; decrease the size and/or quantity of silicon-containing
compounds; and/or disperse silicon-containing compounds in a fluid. As will be
appreciated by those skilled in the art, the stability of a deposition
inhibitor under a
particular set of pressure, temperature, and/or pH conditions may be evaluated
by a
variety of methods such as infrared (IR) spectroscopy, nuclear magnetic
resonance (NMR)
spectroscopy, and/or differential scanning calorimetry (DSC). For example,
FIG. 5 shows
IR spectra of a deposition inhibitor taken before (A) and after (B) the
deposition inhibitor
11
CA 3063448 2019-12-02
A8141809CA
was heated to a temperature of 235 C for 22 hours, and FIG. 6 provides
results from a
DSC experiment on a deposition inhibitor. The DSC results indicate a that
first
decomposition event occurred at 118 C (244.2 F), and a second decomposition
event
occurred at 135 C (275 F). On this basis, the deposition inhibitor was
discarded as a
candidate for the high temperature and high pressure conditions set out
herein. Those
skilled in the art, having benefited from the teachings of the present
disclosure, may
select an appropriate deposition inhibitor by evaluating a
compound/composition's
ability to: (i) resist degradation under a particular set of
temperature/pressure/pH
conditions (for example by IR spectroscopy and/or DSC); and (ii) inhibit
deposition of
silica-based inorganic deposits (for example by analysing the form, severity,
and/or
composition of the deposit by XRD) under such conditions.
[0029] In select embodiments of the present disclosure, the
deposition inhibitor
is a composition that comprises an alcohol, a bisulfate salt, and an organo-
phosphorous
salt. The alcohol may be ethylene glycol, the bisulfate sulfate salt may be
sodium
bisulfate, and the organo-phosphorous salt may be an organo-phosphate. In
particular,
the deposition inhibitor may comprise a composition identified by product
number
5CW4481 (as provided by Baker Hughes) which comprises between about 30 % and
about 40 % ethylene glycol (by weight), between about 0.1 % and about 1 %
sodium
bisulfate (by weight), and an undisclosed quantity of an undisclosed organo-
phosphorous
salt (trade secret).
[0030] In the context of the present disclosure, a capillary tube
is an example of
a conduit having an opening in fluid communication with the internal volume of
an
intermediate casing. Capillary tubes are commonly referred to as "bubble
tubes". In the
context of the present disclosure, the deposition inhibitor may be provided to
the
internal volume of the intermediate casing by injecting the deposition
inhibitor through
the capillary tube under a variety of protocols. Such protocols may include a
plurality of
parameters as exemplified by the following:
[0031] As a first example, the trigger for the initial injection
via the capillary tube
may vary. The initial injection may be triggered by: a production-event-based
trigger (e.g.
12
CA 3063448 2019-12-02
A8141809CA
at the onset of fluid communication between the injection well and the
production well,
or at a specific production rate or ratio); an operation-parameter-based
trigger (e.g. at a
specific steam injection pressure and/or temperature, or at a specific
temperature,
pressure, and/or composition of a produced fluid); a reservoir-based-trigger
(e.g. at a
specific reservoir temperature and/or pressure, or once a steam chamber
achieves a
particular size and/or growth rate); a time-based trigger (such as a specific
time period
from the onsite of production or the onsite of injection). In select
embodiments of the
present disclosure, the initial injection via the capillary tube may be
triggered by a
reservoir pressure of between about 3,600 kPag and about 5,000 kPag. In select
embodiments of the present disclosure, the initial injection via the capillary
tube may be
triggered by a drop in reservoir pressure from between about 3,600 kPag and
about 5,000
kPag to between about 1,500 kPag and about 3,600 kPag.
[0032] As a second example, the schedule of sequential injections
via the capillary
tube may vary. For example, the injection of the deposition inhibitor via the
capillary tube
may be continuous, or the deposition inhibitor may be injected via the
capillary tube on
a cyclic basis (e.g. once every two weeks, once every month, or once every six
months)
or an iterative basis. Determinations regarding scheduling sequential
injections via the
capillary tube may be made having regard to: production-event-related
indicators (e.g.
time to peak production); operation-parameter-related indicators (e.g. at a
specific
steam injection pressure and/or temperature, or at a specific temperature,
pressure,
and/or compositions of the produced fluid); and/or reservoir-related
indicators (e.g. at a
specific reservoir temperature and/or pressure, or at a specific steam chamber
size
and/or growth rate).
[0033] As a third example, the trigger for ceasing the injection
via the capillary
tube may vary. For example, the ceasing the injection via the capillary tube
may be
triggered by: a production-event-based trigger (e.g. at a specific production
rate or ratio);
an operation-parameter-based trigger (e.g. at a specific steam injection
pressure and/or
temperature, or at a specific temperature, pressure, and/or composition of a
produced
fluid); a reservoir-based-trigger (e.g. at a specific reservoir temperature
and/or pressure,
or once a steam chamber achieves a particular size or growth rate); and/or a
time-based
13
CA 3063448 2019-12-02
A8141809CA
trigger (e.g. such as a specific time period from the onsite of production or
the onsite of
injection). In select embodiments of the present disclosure, ceasing the
injection via the
capillary tube may be triggered after a drop in reservoir pressure from
between about
3,600 kPag and about 5,000 kPag to between about 1,500 kPag and about 3,600
kPag,
and/or once flow-back testing indicates that dissolved silica concentrations
are at a
relatively steady state (e.g. within +/- 10 % of a median value).
[0034] As a fourth example, the concentration (rate) of the
deposition inhibitor
injected via the capillary tube may be varied. For example, the deposition
inhibitor may
be injected at a first concentration (rate) (e.g. between about 40 ppm and
about 60 ppm
based on emulsion rates) for one or more injections (or for a specific time
period), and
then the inhibitor may be injected at a second concentration (rate) (e.g.
between about
ppm and about 30 ppm based on emulsion rates) for one or more further
injection (or
for a specific time period). Of course, further injections based on further
alternate
concentrations (rates) are also possible via the capillary tube.
Determinations regarding
15 modulating the concentration (rate) of the composition injected via the
capillary tube
may be made having regard to: production-event-related indicators (e.g. time
to peak
production); operation-parameter-related indicators (e.g. at a specific steam
injection
pressure and/or temperature, or at a specific temperature, pressure, and/or
compositions of the produced fluid); and/or reservoir-related indicators (e.g.
at a specific
20 reservoir temperature and/or pressure, or at a specific steam chamber
size and/or
growth rate).
[0035] As a fifth example, the composition of the deposition
inhibitor injected via
the capillary tube may vary. For example, a first composition may be injected
for a first
time period (e.g. between about 1 month and about 4 months) and then a second
composition may be injected for a second period (e.g. between 6 months and 12
months). Of course, further injections based on further alternate compositions
are also
possible via the capillary tube. Determinations regarding modulating the
components of
the composition injected via the capillary tube may be made having regard to:
production-event-related indicators (e.g. time to peak production); operation-
parameter-related indicators (e.g. at a specific steam injection pressure
and/or
14
CA 3063448 2019-12-02
A8141809CA
temperature, or at a specific temperature, pressure, and/or compositions of
the
produced fluid); and/or reservoir-related indicators (e.g. at a specific
reservoir
temperature and/or pressure, or at a specific steam chamber growth rate).
[0036] In the context of the present disclosure, flow-back testing
may be utilized
to inform decisions relating to, for example, protocols based on the foregoing
triggers
and parameters. Generally speaking, flow-back testing comprises analyzing
produced
fluids (and any materials entrained therein) to determine the content of
particular
components such as silicon-containing compounds, deposition inhibitors, and/or
decomposition products thereof. In this way, flow-back testing may provide
indirect
feedback relating to, for example: the extent of deposition of silicon-based
inorganic
deposits within the interior volume of an intermediate casing; the extent to
which a
deposition inhibitor is being degraded within the interior volume of an
intermediate
casing; and/or the effectiveness of the deposition inhibitor. Such information
may be
utilized to tailor a particular deposition-inhibitor-injection protocol to
provide a sufficient
quantity of deposition inhibition to achieve a particular result without using
an undue
quantity of the deposition inhibitor.
[0037] Accordingly, methods of inhibiting deposition of silicon-
based inorganic
deposits in accordance with the present disclosure may further comprise an
additional
step of analyzing produced fluids (and/or any materials entrained therein) and
adjusting
a production parameter in response to the analysis. The production parameter
may be,
for example, steam-injection temperature, steam-injection pressure, steam
quality, ESP
pump rate, the presence/absence of an injection additive, the composition of
the
deposition inhibitor, the concentration of the deposition inhibitor, the
density of the
deposition inhibitor, the injection pressure of the deposition inhibitor, the
initiation time
for injection of the deposition inhibitor, the stop time for injection of the
deposition
inhibitor, and/or the schedule for injection of the deposition inhibitor.
Likewise flow-back
testing may be used to inform decisions regarding trigger points for such
parameters/protocols.
CA 3063448 2019-12-02
A8141809CA
[0038] In the context of the present disclosure, flow-back testing
may utilize
pressurized emulsion sampling procedures and/or non-pressurized emulsion
sampling
procedures. Pressurized emulsion sampling may be used for high pressure sample
points.
The collection of samples from high pressure sampling points at a wellhead may
involve
the use of specialized equipment to mitigate against safety risks associated
with high
pressure and/or high temperature operations (e.g. sampling temperatures
between
about 150 C and about 220 C and/or sampling pressures between about 10,000
kPag
and about 25,000 kPag). Applications of known high-temperature and/or high-
pressure
sampling procedures in the context of the present disclosure are within the
purview of
those skilled in the art having regard to the teachings of the present
disclosure. Likewise,
applications of known non-pressurized sampling procedures in the context of
the present
disclosure are within the purview of those skilled in the art having regard to
the teachings
of the present disclosure.
[0039] In the context of the present disclosure, a variety of
emulsion separation
procedures may be utilized during flow back testing. Centrifugation and Dean-
Stark
extraction are examples of such procedures, and they may allows for oil-water
ratio
quantification.
[0040] In the context of the present disclosure, following
separation, aqueous
phase samples may be analyzed for/to determine a variety of metrics during
flow-back
testing. For example, aqueous phase samples may be analyzed for/to determine:
temperature; pH; oxidation reduction potential (ORP); anion content (e.g.
alkalinity,
chloride, sulfate, and/or sulfide); cation content (Nat, K+, Fe2+/3+, and/or
Ca2 ); total
dissolved solids (TDS); total suspended solids (TSS); total silica; reactive
silica; suspended
silica; trace metals; and/or trace elements associated with the deposition
inhibitor. A
variety of analytical instruments may be used for the foregoing analyses. For
example,
inductively coupled plasma emission spectroscopy (ICP-ES) and ion
chromatography (IC)
may be used to identify solution phase components.
[0041] In the context of the present disclosure, solids entrained
in the oil phase
of flow-back testing samples may be analyzed for/to determine a variety of
metrics. For
16
CA 3063448 2019-12-02
A8141809CA
example, solids entrained in the oil phase may be isolated by centrifugation
and/or
filtration and identified by for example X-ray powder diffraction (XRD), X-ray
fluorescence
(XRF), and/or scanning electron microscopy (SEM).
[0042] In select embodiments of the present disclosure, methods of
inhibiting
deposition of silicon-based inorganic deposits may further comprise collecting
baseline
flow-back data in advance of the providing of the deposition inhibitor to the
intermediate-casing channel. Baseline flow-back data may provide context for
additional
flow-back data obtained during hydrocarbon production in the presence of a
deposition
inhibitor. For example, total silica, reactive silica, and/or suspended silica
may be
analyzed both before and after providing a deposition inhibitor to the
interior volume of
an intermediate casing, and results from such analyses may be utilized to
trigger a
modification to the composition, concentration, pressure, and/or density of
the
deposition inhibitor. Likewise, such analyses may be utilized to trigger a
change to the
schedule for injecting the deposition inhibitor.
Example 1
[0043] At a SAGD production facility in Foster Creek, Alberta, a
SAGD production
well completed as set out above with respect to FIG. 1 was operated through a
typical
SAGD start-up phase, ramp-up phase, and production phase. During the
production
phase, steam injection parameters were modulated to provide a peak reservoir
pressure
between about 3,600 kPag and 5,000 kPag, and then the injection conditions
were
throttled to allow the reservoir pressure to settle. After a period of about
10 months
production tubing was retrieved from within the interior volume of the
intermediate
casing and analyzed. FIG. 7A provides a photograph of a silicon-based deposit
that was
mechanically removed from the production tubing, and FIG. 7B provides a high-
resolution microscopic image of the same. A high degree of crystallinity is
apparent in the
microscopic image. A plurality of samples were obtained from various surfaces
with the
interior volume of the intermediate casing. The samples were mechanically
isolated and
prepared for XRD analysis. Results from a typical XRD analysis are provided in
Table 1.
17
CA 3063448 2019-12-02
A8141809CA
The results of Table 1 indicate that a high percentage (greater than 85%) of
the analysed
deposit is in a crystalline form.
Table 1: Results from XRD analysis of casing deposit from surface exposed to
SAGD
production conditions within the interior volume of the intermediate casing.
Formula Name Percentage
S102 Quartz 85.2%
SiO2 Cristoba lite 4.6%
CaAl2S1208 Anorthite 4.3%
KAIS1308 Microcline 1.3%
(K,H30)Al2Si3A1010(OH)2 Illite 0.4%
SiO2 Silicon Oxide 0.3%
NaAlSi308 Albite 0.1%
Magnesium Aluminum
Mg2A14Si5018 1.2%
Silicate
CaSO4 Anhydrite 1.0%
Ca(Fe,Mg)(CO3)2 Ankerite 0.4%
HFe(SO4)2.4H20 Rhomboclase 0.3%
(Ca,Na).Al2Si2509.6.4H20 Fa ujasite 0.3%
Na2(5406)(H20)2 Sodium Sulfite Hydrate 0.3%
Na(Si2A1)06.1-120 Analcime 0.3%
Example 2:
[0044] A well-pad wide experiment is underway at a SAGD production
facility in
Foster Creek, Alberta. Generally speaking, the reservoir strategy for the
experiment is to
operate at high pressure until peak oil production rate is achieved. Once peak
production
is achieved, steam will be redirected to other areas to provide a decline in
reservoir
pressure. Then, surfaces from within the interior volume of the intermediate
casing will
be retrieved and evaluated to determine the extent of deposition (both
qualitatively and
quantitatively) using the protocols set out in the present disclosure. The
production wells
are generally completed as set out above with respect to FIG. 1. Half of the
wells on the
pad are being operated in the absence of a deposition inhibitor. For the other
half of the
wells, capillary tubes are being used to provide a deposition inhibitor to the
interior
volume of the intermediate casing. The deposition inhibitor is 5CW4481 as
provided by
Baker Hughes . The concentration of the deposition inhibitor is 40 ppm with
respect to
18
CA 3063448 2019-12-02
A8141809CA
emulsion rates, and the deposition is being provided as a constant trickle
until definitive
results are obtained or the trial ends.
[0045] The wells of the pad are configured for flow-back testing.
During
production, samples of the liquid phase (aqueous and oil phases) of the
production fluid
are being taken at regular volume intervals. For each sample, pH, temperature,
and
oxidation/reduction potential are being analyzed on site. Following on-site
analysis, the
samples are being analyzed by ICP-ES and IC to determine the concentrations of
key
components. In particular one or more of the following may be analysed: anion
content;
cation content; TDS; TSS; total silica; reactive silica; suspended silica;
trace metals; and/or
trace elements associated with the deposition inhibitor.
[0046] Forecasts for emulsion production rates and deposition
inhibitor
concentration (rate) for the well-pad wide experiment are set out FIG. 8. In
FIG. 8, total
emulsion rates for the field (A) and emulsion rates only for the test pad (B)
are shown as
a function of time, and the concentration of the deposition inhibitor (with
respect to
emulsion rates) as a function of time is overlaid as plot C. As indicated in
plot C, the
concentration (rate) of deposition inhibitor is forecasted to peak at about
6.2 ppm based
on total emulsion rate and then drop off quickly after injection is ceased.
In the context of the present disclosure, all terms referred to in singular
form are meant
to encompass plural forms of the same. Likewise, all terms referred to in
plural form are
meant to encompass singular forms of the same. Unless defined otherwise, all
technical
and scientific terms used herein have the same meaning as commonly understood
by one
of ordinary skill in the art to which this disclosure pertains.
As used herein, the term "about" refers to an approximately +/-10 % variation
from a
given value. It is to be understood that such a variation is always included
in any given
value provided herein, whether or not it is specifically referred to.
It should be understood that the compositions and methods are described in
terms of
"comprising," "containing," or "including" various components or steps, the
compositions and methods can also "consist essentially of" or "consist of" the
various
19
CA 3063448 2019-12-02
A8141809CA
components and steps. Moreover, the indefinite articles "a" or "an", as used
in the
description and the claims, are defined herein to mean "one or more than one"
of the
element that it introduces.
For the sake of brevity, only certain ranges are explicitly disclosed herein.
However,
ranges from any lower limit may be combined with any upper limit to recite a
range not
explicitly recited, as well as, ranges from any lower limit may be combined
with any other
lower limit to recite a range not explicitly recited, in the same way, ranges
from any upper
limit may be combined with any other upper limit to recite a range not
explicitly recited.
Additionally, whenever a numerical range with a lower limit and an upper limit
is
disclosed, any number and any included range falling within the range are
specifically
disclosed. In particular, every range of values (of the form, "from about a to
about b," or,
equivalently, "from approximately a to b," or, equivalently, "from
approximately a-b")
disclosed herein is to be understood to set forth every number and range
encompassed
within the broader range of values even if not explicitly recited. Thus, every
point or
individual value may serve as its own lower or upper limit combined with any
other point
or individual value or any other lower or upper limit, to recite a range not
explicitly
recited.
Therefore, the present disclosure is well adapted to attain the ends and
advantages
mentioned as well as those that are inherent therein. The particular
embodiments
disclosed above are illustrative only, as the present disclosure may be
modified and
practiced in different but equivalent manners apparent to those skilled in the
art having
the benefit of the teachings herein. Although individual embodiments are
discussed, the
disclosure covers all combinations of all those embodiments. Furthermore, no
limitations
are intended to the details of construction or design herein shown, other than
as
described in the claims below. Also, the terms in the claims have their plain,
ordinary
meaning unless otherwise explicitly and clearly defined by the patentee. It is
therefore
evident that the particular illustrative embodiments disclosed above may be
altered or
modified and all such variations are considered within the scope of the
present
disclosure. Moreover, many obvious variations of the embodiments set out
herein will
CA 3063448 2019-12-02
A8141809CA
suggest themselves to those skilled in the art in light of the present
disclosure. Such
obvious variations are within the full intended scope of the appended claims.
21
CA 3063448 2019-12-02