Note: Descriptions are shown in the official language in which they were submitted.
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
TREATMENT OF INFLAMMATORY DISEASES WITH INHIBITORS OF C5A ACTIVITY
FIELD OF THE INVENTION
The present invention relates to inhibitors of C5a activity and their use in
the treatment
of cutaneous, neutrophilic, inflammatory diseases in a subject.
BACKGROUND OF THE INVENTION
Target C5a in Inflammation
C5a is a 74 amino acid spanning split product of its "mother molecule" C5 and
represents one endpoint of the complement activation cascade. It can be
generated through
activation of at least three well-described pathways (the alternative, the
classical and the MBL
pathway). All pathways merge at the level of C3, forming the C5- or
alternative C5 convertase
leading to cleavage of C5 into C5a and C5b. The latter binds with C6, C7, C8
and multiple C9
molecules ultimately leading to formation of pores in e.g. bacterial membranes
(terminal
Membrane Attack Complex = MAC). C5a is generated when the complement system is
activated in settings of inflammation and other immunological and inflammatory
disorders /
diseases.
Among the complement activation products, C5a is one of the most potent
inflammatory
peptides, with a broad spectrum of functions (Guo and Ward, 2005). C5a exerts
its effects
through the high-affinity C5a receptors (C5aR and C5L2) (Ward, 2009). C5aR
belongs to the
rhodopsin family of G-protein-coupled receptors with seven transmembrane
segments; C5L2
has a similar structure but appears not to be G-protein-coupled. It is
currently believed that C5a
exerts its biological functions primarily through C5a-05aR interaction, as few
biological
responses have been found for C5a-05L2 interaction. However, latest reports
demonstrate
.. signaling also through C5L2 activation (Rittirsch and others, 2008).
C5aR is widely expressed on myeloid cells including neutrophils, eosinophils,
basophils, and monocytes, and non-myeloid cells in many organs, especially in
the lung and
liver, indicative of the importance of C5a/C5aR signaling. Widespread up-
regulation of C5aR
expression occurs during the onset of sepsis, and blockade of C5a/C5aR
interaction by anti-
C5a, or anti-05aR antibodies, or C5aR antagonists renders highly protective
effects in rodent
models of sepsis (Czermak and others, 1999; Huber-Lang and others, 2001;
Riedemann and
others, 2002).
CA 03066689 2019-12-09
WO 2018/234118 2
PCT/EP2018/065676
C5a has a variety of biological functions (Guo and Ward, 2005). C5a is a
strong
chemoattractant for neutrophils and also has chemotactic activity for
monocytes and
macrophages. C5a causes an oxidative burst (02 consumption) in neutrophils and
enhances
phagocytosis and release of granular enzymes. C5a has also been found to be a
vasodilator. C5a
has been shown to be involved in modulation of cytokine expression from
various cell types
and to enhance expression of adhesion molecule expression on neutrophils. High
doses of C5a
can lead to nonspecific chemotactic "desensitization" of neutrophils, thereby
causing broad
dysfunction. Many inflammatory diseases are attributable to the effects of
C5a, including
sepsis, acute lung injury, inflammatory bowel disease, rheumatoid arthritis
and others. In the
experimental setting of sepsis, exposure of neutrophils to C5a can lead to
neutrophil dysfunction
and paralysis of signaling pathways, leading to defective assembly of NADPH
oxidase,
paralysis of MAPK signaling cascades, a great depression of oxidative burst,
phagocytosis and
chemotaxis (Guo and others, 2006; Huber-Lang and others, 2002). Thymocytes
apoptosis and
delayed neutrophil apoptosis are two important pathogenic events for sepsis
development,
which are dependent on the presence of C5a. During experimental sepsis, C5a up-
regulates in-
integrin expression on neutrophils to promote cell migration into organs, one
of the major
causes for multi-organ failure (MOF). It is also found that C5a is
attributable to the activation
of the coagulation pathway that occurs in experimental sepsis. C5a stimulates
the synthesis and
release from human leukocytes of pro-inflammatory cytokines such as TNF-a, IL-
10, IL-6, IL-
8, and macrophage migration inhibitory factor (MIF). Given that complement
activation is an
event occurring during the onset of acute inflammation, C5a may come into play
before
emergence of most of the inflammatory "cytokine storm". It appears that C5a
plays a key role
in orchestrating and amplifying the performance of the cytokine network and
the formation of
systemic inflammatory response syndrome (SIRS).
In the immunological regulatory network tailing to the adaptive immunity, C5a
affects
the crosstalk between dendritic cells (DC) and SET cells, and this may result
in a large
production of inflammatory mediators such as IL-17 (Xu and others, 2010). An
essential role
for C5a has been established and defined in the generation of pathogenic Th17
responses in
systemic lupus erythematosus (SLE) (Pawaria and others, 2014). In addition, it
has been
reported that C5a is a key regulator for Treg cells offering a powerful
suppressive effect for
Treg propagation and induction (Strainic and others, 2013). Given the fact
that Treg and TH17
are the essential players in the autoimmune disease setting, inhibition of C5a
signaling would
be expected to significantly reduce overactive immune status in the autoimmune
diseases.
CA 03066689 2019-12-09
WO 2018/234118 3
PCT/EP2018/065676
IFX-1
IFX-1 is a chimaeric monoclonal IgG4 antibody which specifically binds to the
soluble
human complement split product C5a. IFX-1 is composed of 1328 amino acids and
has an
approximate molecular weight of 148,472 Daltons. The CDR and FR sequences of
IFX-1 are
disclosed in in Table 3 below.
IFX-1 is expressed in a mammalian CHO cell line as recombinant protein and
finally
formulated in a phosphate buffered saline solution for intravenous
administration. The binding
of this antibody to human C5a facilitates a highly effective blockade of C5a-
induced biological
effects by disabling C5a binding to and reacting with its corresponding cell-
bound receptors.
Various nonclinical studies were conducted to assess pharmacological and
toxicological
aspects of IFX-1, which can be divided into in vitro I ex vivo tests and in
vivo studies including
GLP toxicology studies in cynomolgus monkey (using IFX-1). None of the
conducted
nonclinical tests and studies revealed any toxicological or safety concerns
for IFX-1. Human
Phase I trial indicated that safety laboratory parameters, vital signs and ECG
parameters showed
no clinically relevant time or dose-related changes.
In vitro analysis of IFX-1 demonstrates a strong binding capacity to soluble
human C5a
as well as a high blocking activity of C5a-induced biological effects such as
lysozyme release
from human neutrophils or CD1 lb up-regulation in neutrophils in human whole
blood. One
IFX-1 antibody reaches the capability of neutralizing the effects of 2
molecules C5a with close
.. to 100% efficiency in experimental in vitro settings. Clinical trials with
IFX-1 have been
ongoing to test its clinical efficacy in several inflammatory diseases
including septic organ
dysfunction and complex cardiac surgery.
Neutrophils
Neutrophils, terminally differentiated cells with a short lifespan in
circulation, are the
most abundant leukocytes in the human body. As a first line of defense against
invading
microorganisms, neutrophils are characterized by their ability to act as
phagocytic cells, release
lytic enzymes from their granules and produce reactive oxygen species upon
stimulation. In
addition to microbial products, other stimuli such as immune complex can also
induce the
respiratory burst in neutrophils, leading to enhanced inflammation and the
recruitment of
inflammatory cells (Kaplan, 2013).
After infiltrating into inflamed tissues, neutrophils engage in many other
cell types, such
as macrophages, dendritic cells (DCs), natural killer cells, lymphocytes and
mesenchymal stem
cells, regulate innate and adaptive immune responses. For instance,
neutrophils can modulate
CA 03066689 2019-12-09
WO 2018/234118 4
PCT/EP2018/065676
DC maturation and the proliferation and polarization of T cells, and they can
also directly prime
antigen-specific T-helper type 1 and T-helper type 17 cells (Abi Abdallah and
others, 2011). A
variety of stimuli induce neutrophil degranulation, including C5a, formyl-
methionyl-leucyl-
phenylalanine (FMLP), lipopolysaccharide, platelet activating factor, and
Tumor necrosis
factor (TNF) (Kaplan, 2013). The contents released from degranulation and
oxidative species
together with cytokines and chemokines resulted from neutrophil activation are
the primary
inflammatory mediators that cause tissue damage, and this mechanism is
believed to be
attributable to many types of inflammatory tissue injury.
Hidradenitis Suppurativa (HS)
HS is a chronic devastating skin disorder affecting areas rich in apocrine
glands, and it
is considered as one of neutrophil-associated cutaneous inflammatory diseases.
Nodules appear
in the affected areas, and they progressively become swollen and rupture with
the release of
pus. This process occurs repeatedly leading to sinus tract formation and scars
(Jemec, 2004).
This disease course creates a frustrating situation for the patients but also
for physicians. The
point prevalence is reported to range between 1% and 4% (Jemec and others,
1996).
The exact pathophysiology of HS is not well defined. Smoking, dietary habits
and
genetic predisposition have all been linked with HS (Kurzen and others, 2008;
Slade and others,
2003). The percentage of NK cells was increased and that of CD4-lymphocytes
decreased
compared to healthy controls probably implying the existence of an autoimmune
predilection
for the disorder. IL-10 and 1L-17 have been found to be upregulated in the
lesion of HS, being
associated with the activation of inflammasome (Lima and others, 2016).
Hidradenitis
suppurativa (HS) is presented with the high number of neutrophil infiltrates
in the inflamed
skin, especially in the late stage of disease (Lima and others, 2016; Marzano,
2016). Activated
neutrophils could be an important effector cell type causing tissue damage
through direct
harmful effect or indirect regulatory effect toward other effect cells such as
active T cells and
TH17 in this disease setting.
A hypothesis for the implication of some autoimmune or autoinflammatory
mechanism
in the pathogenesis of HS has been created over the last years. The hypothesis
is further
reinforced by positive results from the administration of TNF antagonists in
prospective,
placebo-controlled studies, which result in the approval of Adalimumab (an
antibody directed
against tumor necrosis factor a) in patients with moderate to severe HS. One
major, yet
unanswered question is how neutrophils are recruited to the affected skin
lesion and to what
extent activated neutrophils would contribute to the disease development.
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
The wide range of possible pathogenic mechanisms suggested by different
studies may
imply that HS is associated with host mechanisms rather than exogenous
factors. Taking into
account of the paradox that both anti-infectious (antibiotics) and pro-
infectious (anti-TNF,
corticosteroids, immunosuppressive drugs) therapies may be helpful, HS may
appear as an
5 auto-inflammatory disease based on a defect in the hair follicle innate
immunity (Revuz, 2009),
which is supported by the fact that pro-inflammatory cytokines such as
interleukin (IL)-1 p, and
tumor necrosis factor-a (TNF-a) are markedly increased in lesional and
perilesional skin
(Wollina and others, 2013).
Neutrophilic Dermatoses
The neutrophilic dermatoses (ND) are a group of disorders characterized by
skin lesions
for which histologic examination reveals intense inflammatory infiltrates
composed primarily
of neutrophils with no evidence of infection. ND mainly include Sweet syndrome
(SS),
pyodeima gangrenosum (PG), subcomeal pustular dermatosis (SPD), other well-
defined
entities, and their atypical or transitional forms (Prat and others, 2014).
Hidradenitis suppurativa
(HS) has recently been assigned to the family of ND based on the high number
of neutrophil
infiltrates observed in the inflamed skin (Lima and others, 2016; Marzano,
2016).
Pyoderma gangrenosum (PG) and hidradenitis suppurativa (HS) are prototypic
neutrophilic dermatoses that are regarded as autoinflammatory disease in
origin with the
hallmark of the accumulation of neutrophils in the skin (Braun-Falco and
others, 2012; Marzano
and others, 2014). Autoinflammatory Syndrome represents an emerging group of
inflammatory
conditions that are distinct from autoimmune, allergic, and infectious
disorders. From a
pathophysiological perspective, all the autoinflammatory syndromes such as
PAPA (pyogenic
arthritis, PG and acne), PASH (PG, acne and hidradenitis suppurativa) or
PAPASH (pyogenic
arthritis, acne, PG and hidradenitis suppurativa) share common mechanisms
consisting of over-
activation of the innate immune system and 'sterile' neutrophil-rich cutaneous
inflammation
(Cugno and others, 2017).
BADAS (bowel-associated dermatosis-arthritis syndrome) is characterized by
fever,
flu-like symptoms, arthritis and inflammatory skin involvement. The latter is
characterized by
lesions recalling different neutrophilic dermatoses such as papules and
plaques (Sweet's
syndrome), pustules and ulcers (pyoderma gangrenosum) or nodules, abscesses or
fistulae
(hidradenitis suppurativa). In addition, acne and neutrophilic panniculitis
can be associated.
Patients usually experience a symmetrical, non-erosive polyarthritis that
predominantly
involves small joints (Cugno and others, 2018).
CA 03066689 2019-12-09
WO 2018/234118 6
PCT/EP2018/065676
Synovitis, acne, pustulosis, hyperostosis and osteitis (SAPHO) syndrome was
initially
described in 1987. SAPHO syndrome is a rare condition, possibly to
misdiagnosis. Although
its pathogenesis is still elusive, there is increasing understanding that
SAPHO shares similarities
with other autoinflammatory diseases (Cugno and others, 2018).
Neutrophils and Autoimmune Diseases
Autoimmune diseases are defined by defective differentiation of self and non-
self
molecules, leading to inappropriate recognition of self molecules and tissues
as foreign
structures, and concomitant immune attack against host organs. The
pathogenesis of
autoimmune diseases can generally be divided into two phases, immunization
phase and
effector phase. Immunization phase is characterized by the emergence of
autoreactive T-
lymphocytes. Those T-cells then trigger a secondary response leading to tissue
damaging phase
by activating various other cell types (B-cells, cytotoxic T-cells, NK-cells,
neutrophils,
macrophages, osteoclasts, fibroblasts, etc.). The activation of those effector
cells by the
autoreactive T cells can be considered as the effector phase which can be
mediated by multiple
levels including autoantibody production, cytokine networks or direct
cell¨cell contacts
(Nemeth and Mocsai, 2012).
The role of neutrophils in the pathophysiological development of autoimmune
diseases
has been limitedly defined, but increasingly appreciated. Neutrophils could
participate in the
multiple steps of the autoimmune disease process, including antigen
presentation, regulation of
the activity of other immune cell types, and direct tissue damage. Neutrophils
can
expose/release autoantigens when activated, or when dying by apoptosis, or
during formation
of neutrophil extracellular traps (NETs). They can also contribute to tissue
deposition of
autoantibodies or, as an effector cell type, they can induce tissue damage
themselves.
Accumulative studies have demonstrated that neutrophils play an active role in
the development
of autoimmune diseases, such as, rheumatoid arthritis (RA), systemic lupus
erythematosus
(SLE), bullous pemphigoid, epidermolysis bullosa acquisita, ANCA-associated
vasculitis,
familial Mediterranean fever, cryopyrin-associated periodic disorders (CAPS)
and gout, etc.
(Nemeth and Mocsai, 2012; Nemeth and others, 2016). As the skin being an easy
target for
immune responses, cutaneous inflammation is one of most frequent syndromes
presented by
these autoimmune diseases. However, rheumatoid neutrophilic dermatosis is a
rare cutaneous
manifestation in patients with severe rheumatoid arthritis. It mainly affects
patients with severe
seropositive rheumatoid arthritis, predominantly women (ration 2:1), but it
has been observed
also in seronegative rheumatoid arthritis (Cugno and others, 2018).
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
7
TECHNICAL PROBI FMS UNDERLYING --am PRESENT INVENTION
As explained above, there existed a need in the prior art for effective
therapies for the
treatment of neutrophilic dermatoses, such as Hidradenitis suppurativa (HS),
and cutaneous
neutrophilic autoimmune diseases.
The present inventors have now surprisingly found that molecules inhibiting
C5a
signaling, e.g. an anti-05a antibody, are exceptionally well-suited for the
treatment of
Hidradenitis suppurativa. The present inventors have additionally studied the
physiological
mechanism leading to neutrophil activation and found out that C5a is the key
driver of
neutrophil activation.
Thus, the present inventors expect that inhibiting C5a activity will be a
suitable therapy
approach for the treatment of various neutrophilic disorders, especially
cutaneous, neutrophilic,
inflammatory diseases.
SUMMARY OF THE INVENTION
In a first aspect the present invention relates to a compound for use in the
treatment of
a cutaneous, neutrophilic, inflammatory disease in a subject, wherein the
compound is an
inhibitor of C5a activity, and wherein the cutaneous, neutrophilic,
inflammatory disease is
selected from the group consisting of hidradenitis suppurativa (HS); Pyoderma
gangrenosum
(PG); PAPA (pyogenic arthritis, PG and acne); PASH (PG, acne and hidradenitis
suppurativa);
PAPASH (pyogenic arthritis, acne, PG and hidradenitis suppurativa); Sweet
syndrome (SS);
subcomeal pustular dermatosis (SPD); epiderrnolysis bullosa acquisita,
erythema elevatum
diutinum (EED); neutrophilic panniculitis; bowel-associated dermatosis-
arthritis syndrome
(BADAS); SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis)
syndrome;
rheumatoid neutrophilic dermatosis; familial Mediterranean fever, cryopyrin-
associated
disorders, gout, and Schnitzler syndrome.
In a second aspect, the present invention relates to a method for the
treatment of a
cutaneous, neutrophilic, inflammatory disease in a subject, comprising the
step of:
administering to a subject in need thereof a therapeutic amount of a compound,
wherein
the compound is an inhibitor of C5a activity, and wherein the cutaneous,
neutrophilic,
inflammatory disease is selected from the group consisting of hidradenitis
suppurativa (HS);
Pyoderma gangrenosum (PG); PAPA (pyogenic arthritis, PG and acne); PASH (PG,
acne and
hidradenitis suppurativa); PAPASH (pyogenic arthritis, acne, PG and
hidradenitis suppurativa);
Sweet syndrome (SS); subcomeal pustular dermatosis (SPD); epidermolysis
bullosa acquisita,
CA 03066689 2019-12-09
WO 2018/234118 8
PCT/EP2018/065676
erythema elevatum diutinum (EED); neutrophilic panniculitis; bowel-associated
dermatosis-
arthritis syndrome (BADAS); SAPHO (synovitis, acne, pustulosis, hyperostosis,
and osteitis)
syndrome; rheumatoid neutrophilic dermatosis; familial Mediterranean fever,
cryopyrin-
associated disorders, gout, and Schnitzler syndrome.
In a third aspect, the present invention relates to a use of a compound for
the preparation
of a pharmaceutical composition for the treatment of a cutaneous,
neutrophilic, inflammatory
disease, wherein the compound is an inhibitor of C5a activity, and wherein the
cutaneous,
neutrophilic, inflammatory disease is selected from the group consisting of
hidradenitis
suppurativa (HS); Pyoderma gangrenosum (PG); PAPA (pyogenic arthritis, PG and
acne);
PASH (PG, acne and hidradenitis suppurativa); PAPASH (pyogenic arthritis,
acne, PG and
hidradenitis suppurativa); Sweet syndrome (SS); subcorneal pustular dermatosis
(SPD);
epidermolysis bullosa acquisita, erythema elevatum diutinum (EED);
neutrophilic panniculitis;
bowel-associated dermatosis-arthritis syndrome (BADAS); SAPHO (synovitis,
acne,
pustulosis, hyperostosis, and osteitis) syndrome; rheumatoid neutrophilic
dermatosis; familial
Mediterranean fever, cryopyrin-associated disorders, gout, and Schnitzler
syndrome.
This summary of the invention does not necessarily describe all features of
the present
invention. Other embodiments will become apparent from a review of the ensuing
detailed
description.
BRIEF DESCRIPTION OF THE FIGURES
Figure 1. Blocking activity of IFX-1 to recombinant human C5a (rhC5a)-induced
CD11b upregulation on blood neutrophils. IFX-1-004 and IFX-1-012 represent two
different
production batches. Human whole blood was incubated with buffer, antibody
alone, rhC5a
alone, or combinations of different antibody concentration and rhC5a. After
incubation, cells
were stained with anti-mouse CD11b:FITC and CD1 lb MFI was analysed by flow
cytometry.
Results are presented as mean SD. The percentage of IFX-1 blocking activity
of C5a-induced
CD 1 lb expression is marked (arrow). Statistical differences were calculated
by One-Way-
ANOVA, p values of p<0.05 were statistically significant.
Figure 2. Blocking activity of IFX-1 on endogenous C5a (eC5a)-driven CD11b
upregulation on neutrophils. Zymosan-activated human plasma (ZAP) was used as
the source
of eC5a. Whole blood was incubated with buffer, IFX-1 alone, ZAP alone, or
combinations of
11-X-1 and ZAP. After incubation, cells were stained with anti-mouse
CD11b:FITC and
analysed by flow cytometry. Results are presented as mean SD. The percentage
of IFX-1
CA 03066689 2019-12-09
WO 2018/234118 9
PCT/EP2018/065676
blocking activity of eC5a-induced CD1lb expression is marked (arrow).
Statistical differences
were calculated by One-Way-ANOVA, p values of p<0.05 were statistically
significant.
Figure 3. Activation of blood neutrophils by zymosan and IFX-1 blocking
activity.
Whole blood was incubated with HBSS, rhC5a and zymosan A alone, or
combinations of
different IFX-1 concentrations and rhC5a or zymosan A. After incubation, cells
were stained
with anti-mouse CD11b:FITC, and CD1 lb MFI was analysed by flow cytometry.
Results are
presented as mean SD. The percentage of IFX-1 blocking activity of C5a-
induced CD1 lb
expression is marked (arrow). Statistical differences were calculated by One-
Way-ANOVA, p
values of p<0.05 were statistically significant.
Figure 4. IFX-1 inhibits zymosan-induced generation of IL-8 in human whole
blood. IL-8 concentrations were obtained by ELISA after incubation of human
whole blood
with different concentrations of zymosan A (as indicated on the x-axis) in the
presence (empty
circles) or absence (filled circles) of IFX-1. Results were presented as mean
SD.
Figure 5. Concentrations of C3a (A), C5a (B) and C5b-9 (C) in the plasma of 14
healthy controls and of 54 patients with hidradenitis suppurativa (HS).
Circles denote
outliers and asterisks denote extremes. P values symbolize significant
differences between
patients and controls.
Figure 6. Effect of HS plasma on blood neutrophil activation and the potential
role
of C5a. HS plasma samples were incubated with human whole blood in the
presence and
absence of IFX-1, and CD1 lb expression on blood neutrophils was determined by
flow
cytometric analysis. C5a levels in the control and HS samples were labeled in
the embedded
table.
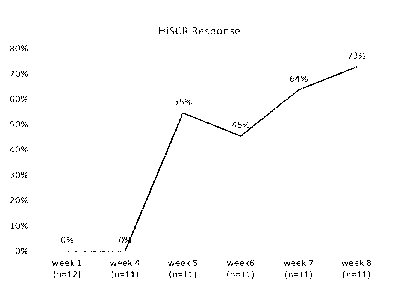
Figure 7. HiSCR response post-IFX-1 treatment in HS patients. HiSCR responder
is defined as a > 50% reduction in inflammatory lesion count (abscesses +
inflammatory
nodules), and no increase in abscesses or draining fistulas when compared with
baseline.
Figure 8. Blockade of C5a-induced CD11b upregulation via different anti-05aR
antibodies. Whole blood as source of neutrophils was incubated with (spiked-)
plasma samples
in the absence or presence of respective anti-05aR antibodies. The blocking
activity of each
inhibitor was indicated as percentage on the corresponding sample.
Figure 9. Blockade of C5a-induced CD11b upregulation via C5aR antagonist
PMX-53. Whole blood as source of neutrophils was incubated with (spiked-)
plasma samples
in the absence or presence of low-concentrated (A) or over-concentrated (B)
C5aR antagonist
PMX-53. The blocking activities of PMX-53 were indicated as percentage on the
corresponding
samples.
10
Figure 10. Blockade of C5a-induced CD11b upregulalion via C5a blocking
antibody IFX-1. Whole blood as source of neutrophils was incubated with
(spiked-) plasma
samples in the absence or presence of IFX-1. The blocking activities of IFX-1
were indicated
as percentage on the corresponding samples.
Figure 11. Blockade of C5a-induced CD11b upregulation via C5aR inhibitor
Avacopan. Whole blood as source of neutrophils was incubated with (spiked-)
plasma samples
in the absence or presence of Avacopan. The blocking activities of Avacopan
were indicated as
percentage on the corresponding samples,
DETAILED DESCRIPTION OF THE INVENTION
Definitions
Before the present invention is described in detail below, it is to be
understood that this
invention is not limited to the particular methodology, protocols and reagents
described herein
as these may vary. It is also to be understood that the terminology used
herein is for the purpose
of describing particular embodiments only, and is not intended to limit the
scope of the present
invention which will be limited only by the appended claims. Unless defined
otherwise, all
technical and scientific terms used herein have the same meanings as commonly
understood by
one of ordinary skill in the art to which this invention belongs.
Preferably, the terms used herein are defined as described in "A multilingual
glossary
of biotechnological terms: (IUPAC Recommendations)", Leuenberger, H.G.W,
Nagel, B. and
Kolbl, H. eds. (1995), Helvetica Chitnica Acta, CH-4010 Basel, Switzerland),
Throughout this specification and the claims which follow, unless the context
requires
otherwise, the won! "comprise", and variations such as "comprises" and
"comprising", will be
understood to imply the inclusion of a stated integer or step or group of
integers or steps but
not the exclusion of any other integer or step or group of integers or steps.
Several documents (for example: patents, patent applications, scientific
publications,
manufacturer's specifications, instructions, GenBank Accession Number sequence
submissions
etc.) are cited throughout the text of this specification. Nothing herein is
to be construed as an
admission that the invention is not entitled to antedate such disclosure by
virtue of prior
invention.
In the event of a conflict between the definitions or teachings of such cited
references and definitions or teachings recited in the present specification,
the text of the present
specification takes precedence.
Da
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
11
Sequences: All sequences referred to herein are disclosed in the attached
sequence
listing that, with its whole content and disclosure, is a part of this
specification.
In the context of the present invention, C5a particularly refers to human C5a.
Human
C5a is a 74 amino acid peptide with the following amino acid sequence:
TLQKKIEEIA AKYKHSVVKK CCYDGACVNN DETCEQRAAR ISLGPRCIKA
FTECCVVASQ LRANISHKDM QLGR (SEQ ID NO: 1).
The amino acid sequence of human C5 can be found under the accession number
UniProtKB P01031 (C05_HUMAN).
As used herein, the term "inhibitor of C5a activity" refers to any compound
that in any
way reduces the activity of C5a. This activity reduction can be achieved by
directly or indirectly
lowering the concentration of C5a, or by reducing the activity of C5a, or by
preventing C5a
from exerting its effects on one or more of its receptors (e.g. on C5aR or
C5L2), or by reducing
the concentration or activity of one or more receptors of C5a.
In the context of the present invention, the expression "C5a receptor" refers
to any
potential C5a binding ligand on the cell surface, especially to any receptor
protein to which C5a
may bind and elicit a reaction on said receptor (e.g. activation or inhibition
of the receptor). The
term "C5a receptor" particularly encompasses the two receptors C5aR and C5L2.
Alternative
names for C5aR are C5aR1 and CD88. An alternative name for C5L2 is C5aR2.
Certain embodiments of the present invention refer to an inhibitor of C5a that
interferes
with a C5a receptor (e.g. by binding to a C5a receptor, or by blocking
expression of a C5a
receptor). In these contexts, the term "a C5a receptor" can refer to (i) C5aR
or to (ii) C5L2 or
to (iii) both C5aR and C5L2. This means that some inhibitors of C5a interfere
with only one of
the C5a receptors (i.e. either C5aR or C5L2), while other inhibitors of C5a
interfere with both
C5a receptors (i.e. both C5aR and C5L2).
In the context of the present invention, the expression "protein ligand"
refers to any
molecule composed of amino acids linked by peptide bonds, irrespective of the
total size of the
molecule, and that is capable of specifically binding to another molecule.
Accordingly, the
expression "protein ligand" comprises oligopeptides (< 100 amino acids) and
polypeptides
(>100 amino acids). The expression "protein ligand" also comprises cyclic
peptides,
irrespective of their size. The expression "protein ligand" particularly
encompasses antibodies,
antigen-binding fragments of antibodies, antibody-like proteins, and
peptidomimetics.
As used herein, a first compound (e.g. a protein ligand or nucleic acid
aptamer) is
considered to "bind" to a second compound (e.g. a target protein), if it has a
dissociation
constant Ka to said second compound of 1 mM or less, preferably 100 tM or
less, preferably
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
12
50 M or less, preferably 30 M or less, preferably 20 M or less, preferably
10 M or less,
preferably 51.iM or less, more preferably 1 M or less, more preferably 900 nM
or less, more
preferably 800 nM or less, more preferably 700 nM or less, more preferably 600
nM or less,
more preferably 500 nM or less, more preferably 400 nM or less, more
preferably 300 nM or
less, more preferably 200 nM or less, even more preferably 100 nM or less,
even more
preferably 90 nM or less, even more preferably 80 nM or less, even more
preferably 70 nM or
less, even more preferably 60 nM or less, even more preferably 50 nM or less,
even more
preferably 40 nM or less, even more preferably 30 nM or less, even more
preferably 20 nM or
less, and even more preferably 10 nM or less.
The term "binding" according to the invention preferably relates to a specific
binding.
"Specific binding" means that a compound (e.g. a protein ligand or nucleic
acid aptamer) binds
stronger to a target such as an epitope for which it is specific compared to
the binding to another
target. A compound binds stronger to a first target compared to a second
target, if it binds to
the first target with a dissociation constant (Kd) which is lower than the
dissociation constant
for the second target. Preferably the dissociation constant (IQ) for the
target to which the
compound binds specifically is more than 10-fold, preferably more than 20-
fold, more
preferably more than 50-fold, even more preferably more than 100-fold, 200-
fold, 500-fold or
1000-fold lower than the dissociation constant (Kd) for the target to which
the compound does
not bind specifically.
As used herein, the term "Kd" (usually measured in "mol/L", sometimes
abbreviated as
"M") is intended to refer to the dissociation equilibrium constant of the
particular interaction
between a compound (e.g. a protein ligand) and a target molecule.
Methods for determining binding affinities of compounds, i.e. for determining
the
dissociation constant Kd, are known to a person of ordinary skill in the art
and can be selected
for instance from the following methods known in the art: Surface Plasmon
Resonance (SPR)
based technology, Bio-layer interferometry (BLI), enzyme-linked immunosorbent
assay
(ELISA), flow cytometry, isothermal titration calorimetry (ITC), analytical
ultracentrifugation,
radioimmunoassay (RIA or IRMA) and enhanced chemiluminescence (ECL).
Typically, the
dissociation constant Kd is determined at 20 C, 25 C, 30 C, or 37 C. If not
specifically
.. indicated otherwise, the Kd values recited herein are determined at 20 C
by ELISA.
An "epitope", also known as antigenic determinant, is the part of a
macromolecule that
is recognized by the immune system, specifically by antibodies, B cells, or T
cells. As used
herein, an "epitope" is the part of a macromolecule capable of binding to a
compound (e.g. an
antibody or antigen-binding fragment thereof) as described herein. In this
context, the term
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
13
"binding" preferably relates to a specific binding. Epitopes usually consist
of chemically active
surface groupings of molecules such as amino acids or sugar side chains and
usually have
specific three-dimensional structural characteristics, as well as specific
charge characteristics.
Conformational and non-conformational epitopes can be distinguished in that
the binding to the
former but not the latter is lost in the presence of denaturing solvents.
A "paratope" is the part of an antibody that binds to the epitope. In the
context of the
present invention, a "paratope" is the part of a compound (e.g. a protein
ligand) as described
herein that binds to the epitope.
The term "antibody" typically refers to a glycoprotein comprising at least two
heavy (H)
chains and two light (L) chains inter-connected by disulfide bonds, or an
antigen-binding
portion thereof. The term "antibody" also includes all recombinant forms of
antibodies, in
particular of the antibodies described herein, e.g. antibodies expressed in
prokaryotes,
unglycosylated antibodies, antibodies expressed in eukaryotes (e.g. CHO
cells), glycosylated
antibodies, and any antigen-binding antibody fragments and derivatives as
described below.
.. Each heavy chain is comprised of a heavy chain variable region (abbreviated
herein as VH or
VII) and a heavy chain constant region. Each light chain is comprised of a
light chain variable
region (abbreviated herein as VL or VI) and a light chain constant region. The
VH and VL
regions can be further subdivided into regions of hypervariability, termed
complementarity
determining regions (CDR), interspersed with regions that are more conserved,
termed
framework regions (FR). Each VH and VL is composed of three CDRs and four FRs,
arranged
from amino-terminus to carboxy-tenninus in the following order: FR1, CDR1,
FR2, CDR2,
FR3, CDR3, FR4. The variable regions of the heavy and light chains contain a
binding domain
that interacts with an antigen. The constant regions of the antibodies may
mediate the binding
of the immunoglobulin to host tissues or factors, including various cells of
the immune system
(e.g., effector cells) and the first component (Clq) of the classical
complement system.
The term "antigen-binding fragment" of an antibody (or simply "binding
portion"), as
used herein, refers to one or more fragments of an antibody that retain the
ability to specifically
bind to an antigen. It has been shown that the antigen-binding function of an
antibody can be
performed by fragments of a full-length antibody. Examples of binding
fragments encompassed
within the term "antigen-binding portion" of an antibody include (i) Fab
fragments, monovalent
fragments consisting of the VL, VH, CL and CH domains; (ii) F(a1302 fragments,
bivalent
fragments comprising two Fab fragments linked by a disulfide bridge at the
hinge region; (iii)
Fd fragments consisting of the VH and CH domains; (iv) Fv fragments consisting
of the VL
and VH domains of a single arm of an antibody, (v) dAb fragments (Ward et al.,
(1989) Nature
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
14
341: 544-546), which consist of a VH domain; (vi) isolated complementarity
determining
regions (CDR), and (vii) combinations of two or more isolated CDRs which may
optionally be
joined by a synthetic linker. Furthermore, although the two domains of the Fv
fragment, VL
and VH, are coded for by separate genes, they can be joined, using recombinant
methods, by a
synthetic linker that enables them to be made as a single protein chain in
which the VL and VI-1
regions pair to form monovalent molecules (known as single chain Fv (scFv);
see e.g., Bird et
al. (1988) Science 242: 423-426; and Huston et al. (1988) Proc. Natl. Acad.
Sci. USA 85: 5879-
5883). Such single chain antibodies are also intended to be encompassed within
the term
"antigen-binding fragment" of an antibody. A further example is a binding-
domain
immunoglobulin fusion protein comprising (i) a binding domain polypeptide that
is fused to an
immunoglobulin hinge region polypeptide, (ii) an immunoglobulin heavy chain
CH2 constant
region fused to the hinge region, and (iii) an immunoglobulin heavy chain CH3
constant region
fused to the CH2 constant region. The binding domain polypeptide can be a
heavy chain
variable region or a light chain variable region. The binding-domain
immunoglobulin fusion
proteins are further disclosed in US 2003/0118592 and US 2003/0133939. These
antibody
fragments are obtained using conventional techniques known to those skilled in
the art, and the
fragments are screened for utility in the same manner as are intact
antibodies. Further examples
of "antigen-binding fragments" are so-called microantibodies, which are
derived from single
CDRs. For example, Heap et al., 2005, describe a 17 amino acid residue
microantibody derived
from the heavy chain CDR3 of an antibody directed against the gp120 envelope
glycoprotein
of HIV-1 (Heap C.J. et al. (2005) Analysis of a 17-amino acid residue, virus-
neutralizing
microantibody. J. Gen. Virol. 86:1791-1800). Other examples include small
antibody mimetics
comprising two or more CDR regions that are fused to each other, preferably by
cognate
framework regions. Such a small antibody mimetic comprising VH CDR1 and VL
CDR3 linked
by the cognate VH FR2 has been described by Qiu et al., 2007 (Qiu X.-Q. et al.
(2007) Small
antibody mimetics comprising two complementary-determining regions and a
framework
region for tumor targeting. Nature biotechnology 25(8):921-929).
Thus, the term "antibody or antigen-binding fragment thereof', as used herein,
refers to
immunoglobulin molecules and immunologically active portions of immunoglobulin
molecules, i.e. molecules that contain an antigen-binding site that
immunospecifically binds an
antigen. Also comprised are immunoglobulin-like proteins that are selected
through techniques
including, for example, phage display to specifically bind to a target
molecule or target epitope.
The immunoglobulin molecules of the invention can be of any type (e.g., IgG,
IgE, IgM, IgD,
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
IgA and IgY), class (e.g., IgG 1 , IgG2, preferably IgG2a and IgG2b, IgG3,
IgG4, IgAl and
IgA2) or subclass of immunoglobulin molecule.
Antibodies and antigen-binding fragments thereof usable in the invention may
be from
any animal origin including birds and mammals. Preferably, the antibodies or
fragments are
5 .. from human, chimpanzee, rodent (e.g. mouse, rat, guinea pig, or rabbit),
chicken, turkey, pig,
sheep, goat, camel, cow, horse, donkey, cat, or dog origin. It is particularly
preferred that the
antibodies are of human or murine origin. Antibodies of the invention also
include chimeric
molecules in which an antibody constant region derived from one species,
preferably human,
is combined with the antigen-binding site derived from another species, e.g.
mouse. Moreover,
10 antibodies of the invention include humanized molecules in which the
antigen-binding sites of
an antibody derived from a non-human species (e.g. from mouse) are combined
with constant
and framework regions of human origin.
As exemplified herein, antibodies of the invention can be obtained directly
from
hybridomas which express the antibody, or can be cloned and recombinantly
expressed in a
15 host cell (e.g., a CHO cell, or a lymphocytic cell). Further examples of
host cells are
microorganisms, such as E. coli, and fungi, such as yeast. Alternatively, they
can be produced
recombinantly in a transgenic non-human animal or plant.
The term "chimeric antibody" refers to those antibodies wherein one portion of
each of
the amino acid sequences of heavy and light chains is homologous to
corresponding sequences
in antibodies derived from a particular species or belonging to a particular
class, while the
remaining segment of the chain is homologous to corresponding sequences in
another species
or class. Typically the variable region of both light and heavy chains mimics
the variable
regions of antibodies derived from one species of mammals, while the constant
portions are
homologous to sequences of antibodies derived from another. One clear
advantage to such
chimeric forms is that the variable region can conveniently be derived from
presently known
sources using readily available B-cells or hybridomas from non-human host
organisms in
combination with constant regions derived from, for example, human cell
preparations. While
the variable region has the advantage of ease of preparation and the
specificity is not affected
by the source, the constant region being human is less likely to elicit an
immune response from
a human subject when the antibodies are injected than would the constant
region from a non-
human source. However, the definition is not limited to this particular
example.
The term "humanized antibody" refers to a molecule having an antigen-binding
site that
is substantially derived from an immunoglobulin from a non-human species,
wherein the
remaining immunoglobulin structure of the molecule is based upon the structure
and/or
16
sequence of a human immunoglobulin. The antigen-binding site may either
comprise complete
variable domains fused onto constant domains or only the complementarity
determining regions
(CDR) grafted onto appropriate framework regions in the variable domains.
Antigen-binding
sites may be wild-type or modified by one or more amino acid substitutions,
e.g. modified to
resemble human immunoglobulins more closely. Some forms of humanized
antibodies preserve
all CDR sequences (for example a humanized mouse antibody which contains all
six CDRs
from the mouse antibody). Other forms have one or more CDRs which are altered
with respect
to the original antibody.
Different methods for humanizing antibodies are known to the skilled person,
as
reviewed by Almagro & Fransson, 2008, Frontiers in Bioscience, 13:1619-1633 .
The review article by Almagro &
Fransson is briefly summarized in US 2012/0231008 Al which is the national
stage entry of
international patent application WO 2011/063980 Al.
As used herein, "human antibodies" include antibodies having variable and
constant
regions derived from human germline immunoglobulin sequences. The human
antibodies of
the invention may include amino acid residues not encoded by human germline
immunoglobulin sequences (e.g., mutations introduced by random or site-
specific mutagenesis
in vitro or by somatic mutation in vivo). Human antibodies of the invention
include antibodies
isolated from human immunoglobulin libraries or from animals transgenic for
one or more
human immunoglobulin and that do not express endogenous immunoglobulins, as
described for
example in U.S. Patent No. 5,939,598 by Kucherlapati & Jakobovits.
The term "monoclonal antibody" as used herein refers to a preparation of
antibody
molecules of single molecular composition. A monoclonal antibody displays a
single binding
specificity and affinity for a particular epitope. In one embodiment, the
monoclonal antibodies
are produced by a hybridoma which includes a B cell obtained from a non-human
animal, e.g.
mouse, fused to an immortalized cell.
The term "recombinant antibody", as used herein, includes all antibodies that
are
prepared, expressed, created or isolated by recombinant means, such as (a)
antibodies isolated
from an animal (e.g., a mouse) that is transgenic or transchromosomal with
respect to the
immunoglobulin genes or a hybridoma prepared therefrom, (b) antibodies
isolated from a host
cell transformed to express the antibody, e.g. from a transfectoma, (c)
antibodies isolated from
a recombinant, combinatorial antibody library, and (d) antibodies prepared,
expressed, created
Date Recue/Date Received 2023-01-26
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
17
or isolated by any other means that involve splicing of immunoglobulin gene
sequences to other
DNA sequences.
The term "transfectoma", as used herein, includes recombinant eukaryotic host
cells
expressing an antibody, such as CHO cells, NS/0 cells, HEK293 cells, HEK293T
cells, plant
cells, or fungi, including yeast cells.
As used herein, a "heterologous antibody" is defined in relation to a
transgenic organism
producing such an antibody. This term refers to an antibody having an amino
acid sequence or
an encoding nucleic acid sequence corresponding to that found in an organism
not consisting
of the transgenic organism, and being generally derived from a species other
than the transgenic
organism.
As used herein, a "heterohybrid antibody" refers to an antibody having light
and heavy
chains of different organismal origins. For example, an antibody having a
human heavy chain
associated with a murine light chain is a heterohybrid antibody.
Thus, "antibodies and antigen-binding fragments thereof" suitable for use in
the present
invention include, but are not limited to, polyclonal, monoclonal, monovalent,
bispecific,
heteroconjugate, multispecific, recombinant, heterologous, heterohybrid,
chimeric, humanized
(in particular CDR-grafted), deimmunized, or human antibodies, Fab fragments,
Fab'
fragments, F(a13')2 fragments, fragments produced by a Fab expression library,
Fd, Fv, disulfide-
linked Fvs (dsFv), single chain antibodies (e.g. scFv), diabodies or
tetrabodies (Holliger P. et
al. (1993) Proc. Natl. Acad. Sci. U.S.A. 90(14), 6444-6448), nanobodies (also
known as single
domain antibodies), anti-idiotypic (anti-Id) antibodies (including, e.g., anti-
Id antibodies to
antibodies described herein), and epitope-binding fragments of any of the
above.
The antibodies described herein are preferably isolated. An "isolated
antibody" as used
herein, is intended to refer to an antibody which is substantially free of
other antibodies having
different antigenic specificities (e.g., an isolated antibody that
specifically binds to C5a is
substantially free of antibodies that specifically bind antigens other than
C5a). An isolated
antibody that specifically binds to an epitope, isoform or variant of human
C5a may, however,
have cross-reactivity to other related antigens, e.g. from other species (e.g.
C5a species
homologs, such as rat C5a). Moreover, an isolated antibody may be
substantially free of other
cellular material and/or chemicals. In one embodiment of the invention, a
combination of
"isolated" monoclonal antibodies relates to antibodies having different
specificities and being
combined in a well-defined composition.
The term "naturally occurring", as used herein, as applied to an object refers
to the fact
that an object can be found in nature. For example, a polypeptide or
polynucleotide sequence
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
18
that is present in an organism (including viruses) that can be isolated from a
source in nature
and which has not been intentionally modified by man in the laboratory is
naturally occurring.
As used herein, the term "nucleic acid aptamer" refers to a nucleic acid
molecule that
has been engineered through repeated rounds of in vitro selection or SEI EX
(systematic
evolution of ligands by exponential enrichment) to bind to a target molecule
(for a review see:
Brody E.N. and Gold L. (2000), Aptamers as therapeutic and diagnostic agents.
J. Biotechnol.
74(1):5-13). The nucleic acid aptamer may be a DNA or RNA molecule. The
aptarners may
contain modifications, e.g. modified nucleotides such as 2'-fluorine-
substituted pyrimidines,
and/or may comprise one or more nucleotides with L-ribose units (or L-
deoxyribose) instead of
the standard D-ribose units (or D-deoxyribose units).
As used herein, the term "antibody-like protein" refers to a protein that has
been
engineered (e.g. by mutagenesis of loops) to specifically bind to a target
molecule. Typically,
such an antibody-like protein comprises at least one variable peptide loop
attached at both ends
to a protein scaffold. This double structural constraint greatly increases the
binding affinity of
the antibody-like protein to levels comparable to that of an antibody. The
length of the variable
peptide loop typically consists of 10 to 20 amino acids. The scaffold protein
may be any protein
having good solubility properties. Preferably, the scaffold protein is a small
globular protein.
Antibody-like proteins include without limitation affibodies, affilins,
affimers, affitins,
alphabodies, anticalins, avimers, DARPins (designed ankyrin repeat proteins),
fynomers,
.. Kunitz domain peptides, and monobodies (for review see: Binz H.K. et al.
(2005) Engineering
novel binding proteins from nonimmunoglobulin domains. Nat. Biotechnol.
23(10):1257-1268).
Antibody-like proteins can be derived from large libraries of mutants, e.g. be
panned from large
phage display libraries and can be isolated in analogy to regular antibodies.
Also, antibody-like
binding proteins can be obtained by combinatorial mutagenesis of surface-
exposed residues in
globular proteins. Antibody-like proteins are sometimes referred to as
"peptide aptamers" or as
"antibody mimetics".
As used herein, a "peptidomimetic" is a small protein-like chain designed to
mimic a
peptide. Peptidomimetics typically arise from modification of an existing
peptide in order to
alter the molecule's properties. For example, they may arise from
modifications to change the
molecule's stability or biological activity. This can have a role in the
development of drug-like
compounds from existing peptides. These modifications involve changes to the
peptide that will
not occur naturally (such as altered backbones and the incorporation of non-
natural amino
acids).
19
In. the context of the present invention, the term "small molecule" refers to
a molecule
with a molecular weight of 2 kDa or less, preferably with a molecular weight
of 1 kDa or less.
The term "small molecule" particularly refers to molecules that are neither
oligopeptides nor
oligonucleotides.
In the context of the present invention, the general expression "wherein A
competes
with B for binding to C", (e.g. in the expression "wherein said antibody or
antigen-binding
fragment thereof competes with one of the antibodies indicated under (a) for
binding to C5a")
is used to define the binding properties of the compound listed in position A.
Said compound
A binds to C and compound B also binds to C but compound A and compound B
cannot bind
to C at the same time; i.e. A and B bind to the same epitope (or at least to
overlapping epitopes)
on C. Such competition in binding can be determined by competitive ELISA or by
Surface
Plasmon Resonance (SPR) based technology or by any of the other techniques
listed above in
the context of the determination of binding affmities. If not explicitly
stated otherwise, the
competing binding properties of a compound are determined by ELISA at 20 C
using equimolar
concentrations of the two competing compounds.
As used herein, a "cutaneous, neutrophilic, inflammatory disease" refers to
any disease
that is associated with an inflammation of the skin and with a neutrophilic
infiltrate into the
skin (e.g. into the epidermis) of an individual afflicted by said disease. The
term "cutaneous,
neutrophilic, inflammatory disease" particularly refers to hidradenitis
suppurativa (HS);
Pyoderrna gangrenosum (PG); PAPA (pyogenic arthritis, PG and acne); PASH (PG,
acne and
hidradenitis suppurativa); PAPASH (pyogenic arthritis, acne, PG and
hidradenitis suppurativa);
Sweet syndrome (SS); subcorneal pustular dermatosis (SPD); epidermolysis
bullosa acquisita,
erythema elevatum diutinum (EED); neutrophilic panniculitis; bowel-associated
dermatosis-
arthritis syndrome (BADAS); SAPHO (synovitis, acne, pustulosis, hyperostosis,
and osteitis)
syndrome; rheumatoid neutrophilic dermatosis; familial Mediterranean fever,
cryopyrin-
associated disorders, gout, and Schnitzler syndrome.
As used herein, the expression "HS-related disease" comprises without
limitation
Pyoderma gangrenosum (PG); PAPA (pyogenic arthritis, PG and acne); PASH (PG,
acne and
hidradenitis suppurativa); PAPASH (pyogenic arthritis, acne, PG and
hidradenitis suppurativa);
Sweet syndrome (SS); and subcomeal pustular dermatosis (SPD).
IFX-1 (alternative name: CaCP29; InflaRx GmbH, Germany) is an antibody
specifically
binding to C5a. The CDR sequences and FR sequences of IFX-1 are disclosed in
WO
2015/140304 Al (Table 3).
Date Recue/Date Received 2023-01-26
20
INab708 (InflaRx GmbH, Germany) is another antibody specifically binding to
C5a.
The CDR sequences and FR sequences of INab708 are also disclosed in WO
2015/140304 Al
(Table 3) .
MEDI-7814 (MedImmune) is a recombinant humanized anti-05a antibody. The
crystal
structure of the human C5a in complex with MEDI7814 is available in the RCSB
Protein Data
Bank under 4UU9 (DOI: 10.2210/pdb4uu9/pdb).
ALXN-1007 (Alexion) is a humanized anti-05a antibody.
NOX-D21 (Noxxon) is a PEGylated mixed L-RNA/DNA-aptamer (Spiegelmer Tm) with
the sequence 40 kDaPEG-aminohexyl-GCG AUG (dU)GG UGG UGA AGG GUU GUU GGG
(dU)GU CGA CGC A(dC)G C (SEQ ID NO: 34). NOX-D21 targets C5a (Hyzewicz 1,
Tanihata
J, Kuraoka M, Nitahara-Kasahara Y, Beylier T, Ruegg UT, Vater A, and Takeda S.
2017. Low-
Intensity Training and the C5a Complement Antagonist NOX-D21 Rescue the mdx
Phenotype
through Modulation of Inflammation. Am. J. Pathol., 187(5):1147-1161;
electronically
published ahead of print: March 18, 2017).
Eculizumab (Alternative names: SolirisTM, 5G1-1; h5G1.1; Alexion
Pharmaceuticals) is
a recombinant humanized monoclonal IgG2/4ic antibody produced by murine
myeloma cell
culture and purified by standard bioprocess technology. Eculizumab
specifically binds to
human C5. Eculizumab contains human constant regions from human IgG2 sequences
and
human IgG4 sequences and murine complementarity-determining regions grafted
onto the
.. human framework light- and heavy-chain variable regions. Eculizumab is
composed of two 448
amino acid heavy chains and two 214 amino acid light chains and has a
molecular weight of
approximately 148 kDa. The heavy chain and light chain of eculizumab are
disclosed, for
example, in WO 2016/061066 Al as SEQ ID NO: 1 and SEQ ID NO: 34, respectively.
Nucleic
acids that encode the heavy and light chains of eculizumab are disclosed, for
example, in U.S.
Patent No. 6,355,245.
TM
ALXN1210 (Alternative name: BNJ441; Alexion Pharmaceuticals) is an anti-05
antibody. The heavy and light chains of ALXN1210 are disclosed in WO
2016/209956 Al as
SEQ ID NOs: 14 and 11, respectively.
ALXN5500 (Alexion) is a humanized anti-05 antibody. It is a next-generation
eculizumab candidate.
TM
LFG316 (Alternative name: Tesidolumab, NOV-4; Morphosys, Novartis) is an anti-
05
antibody.
CoversinTM (alternative names: EV 576; PAS-coversin; rEV 576; Tissue targeted
CoversinTM - Akari; Akari Therapeutics, Evolutec) is a recombinant protein
molecule
Date Recue/Date Received 2023-01-26
21
(16.7 kDa) derived from a salivary molecule from the Ornithodros moubata tick
where it assists
the parasite to feed without provoking a host immunological response. The
amino acid sequence
of the EV576 protein (i.e. Coversin) as well as its coding nucleotide sequence
are shown in Fig.
2 of WO 2008/029167. CoversinTM binds to C5,
RA101495 (Ra Pharma) is a macrocyclic synthetic peptide inhibitor of C5
(Ricardo A,
Arata M, DeMarco S, Dhamnaskar K, Hammer R, Fridkis-Hareli M, Rajagopal V,
Seyb K,
Tang G-Q, lobe S and Treco D. 2015. Preclinical Evaluation of RA101495, a
Potent Cyclic
Peptide Inhibitor of C5 for the Treatment of Paroxysmal Nocturnal
Hemoglobinuria. Blood
126:939).
Zimura (Alternative names: Anti-05 aptamer; ARC-187; ARC-1905; Avacincaptad
pegol sodium; OphthoTech Corporation, Archemix Corporation) is a pegylated RNA
aptamer
that inhibits complement factor C5. The nucleotide sequence of ARC1905 (i.e.
Zimura) is
shown, for example, in WO 2005/079363 A2 as SEQ ID NO: 67, and its structure
is shown in
Fig. 22 of WO 2005/079363 A2.
AMY-201 (Amyndas Pharmaceuticals) is an engineered form of Factor H that
directly
links the regulatory and surface-recognition domains; thus, it is a sort of
mini-FH molecule.
Mirococept (alternative names: APT070 and APT 070C; originator: Adprotech;
developer: Inflazyme Pharmaceuticals) consists of the first three short
consensus domains of
human complement receptor 1, manufactured in recombinant bacteria and modified
with a
membrane-targeting amphiphilic peptide based on the naturally occurring
membrane-bound
myristoyl-electrostatic switch peptide (Souza DG, Esser D, Bradford R, Vieira
AT, and
Teixeira MM. 2005. APT070 (Mirococept), a membrane-localised complement
inhibitor,
inhibits inflammatory responses that follow intestinal ischaemia and
repofusion injury. Br J
Pharmacol 145(8):1027-1034).
BikacioMab (Novelmed) is an F(ab)2 fragment of an anti-factor Bb antibody
termed
NM001. Antibody NM001 is produced by hybridoma cell line 1D3 deposited under
ATCC
accession number PTA-8543.
Lampalizumab (alternative names: Anti-factor D Fab; FCFD4514S; RG7417; TNX-
234; originator: Tanox, Developer: Genentech) is a humanized anti-Factor D Fab
fragment that
inhibits Factor D and the alternative complement pathway, through binding to
an exosite on
factor D.
TM
ALN-CC5 (Alnylam) is an RNAi therapeutic targeting human, primate and rodent
C5.
Exemplary iRNA compositions targeting the C5 gene are described in WO
2016/044419.
Date Recue/Date Received 2023-01-26
22
Avacopan (also known by the name CCX168; Chemocentryx) is a small molecule (MW
= 581.66 g/mol) that has a structure according to foimula I:
HN):3)
1
' 1 = F
N N
H -F
I
5 The
IUPAC/Chemical name of avacopan is (2R,3S)-244-(cyclopentylamino)pheny1]-
1-(2-fluoro-6-methylbenzoy1)-N14-methyl-3-(tHfluoromethyl)phenyl]piperidine-3-
carboxamide. Avacopan is a selective inhibitor of C5aR. In the context of the
present invention,
the term "avacopan" refers to the compound according to formula I as well as
to physiologically
tolerable salts thereof.
10
Compounds similar to Avacopan that are also suitable for practicing the
present
invention are disclosed in international patent applications WO 2010/075257 Al
and WO
2011/163640 Al.
Thus, in some embodiments the inhibitor of C5a activity is a compound having
the formula II
&N'Cl
H
N C3
"k=
C2 0
II
and pharmaceutically acceptable salts, hydrates and rotomers thereof; wherein
C1 is selected from the group consisting of aryl and heteroaryl, wherein the
heteroaryl group
has from 1-3 heteroatoms as ring members selected from N, 0 and S; and wherein
said
aryl and heteroaryl groups are optionally substituted with from 1 to 3 Rl
substituents;
C2 is selected from the group consisting of aryl and heteroaryl, wherein the
heteroaryl group
has from 1-3 heteroatoms as ring members selected from N, 0 and S; and wherein
said
aryl and heteroaryl groups are optionally substituted with from 1 to 3 R2
substituents;
C3 is selected from the group consisting of C1-8 alkyl or heteroalkyl, C3-8
cycloalkyl, C3-8
cycloalkyl-Ci4 alkyl, aryl, aryl-Ci4 alkyl, heteroaryl, heteroaryl-C14 alkyl,
heterocycloalkyl or heterocycloalkyl-C14 alkyl, wherein the heterocycloalkyl
group or
portion has from 1-3 heteroatoms selected from N, 0 and S, and wherein the
heteroaryl
Date Recue/Date Received 2023-01-26
CA 03066689 2019-12-09
WO 2018/234118 23
PCT/EP2018/065676
group has from 1-3 heteroatoms as ring members selected from N, 0 and S, and
each
C3 is optionally substituted with from 1-3 R3 substituents;
each RI is independently selected from the group consisting of halogen, -CN, -
W, -0O212", -
CONWW, -C(0)W, -0C(0)NRaRh, -NleC(0)Ra, -NWC(0)2W, -NRa-C(0)NRaRh, -
NRaC(0)NRaRh, -NR"Rh, -OR', and -S(0)2NRaRh; wherein each Ra and Rh is
independently selected from hydrogen, C1-8 alkyl, and C1-8 haloalkyl, or when
attached
to the same nitrogen atom can be combined with the nitrogen atom to form a
five or six-
membered ring having from 0 to 2 additional heteroatoms as ring members
selected
from N, 0 or S, and is optionally substituted with one or two oxo; each RC is
independently selected from the group consisting of C1-8 alkyl or heteroalkyl,
C1-8
haloalkyl, C3-6 cycloalkyl, heterocycloalkyl, aryl and heteroaryl, and wherein
the
aliphatic and cyclic portions of It', Rh and R' are optionally further
substituted with from
one to three halogen, hydroxy, methyl, amino, alkylamino and dialkylamino
groups; and
optionally when two le sub stituents are on adjacent atoms, are combined to
form a fused
five or six- membered carbocyclic or heterocyclic ring;
each le is independently selected from the group consisting of halogen, -CN, -
NO2, -Re, -
CO2Rd, _coNRdRe, -C(0)Rd, -0C(0)NRdRe, -NReC(0)Rd, -NReC(0)2W, -
NRdC(0)NR1IW, -NRdC(0)NRdRe, -NRdRe, -OW, and -S(0)2NRdW; wherein each Rd
and Re is independently selected from hydrogen, C1-8 alkyl, and CI_Ei
haloalkyl, or when
attached to the same nitrogen atom can be combined with the nitrogen atom to
form a
five or six-membered ring having from 0 to 2 additional heteroatoms as ring
members
selected from N, 0 or S, and is optionally substituted with one or two oxo;
each R is
independently selected from the group consisting of C1_8 alkyl or heteroalkyl,
C1_8
haloalkyl, C3-6 cycloalkyl, heterocycloalkyl, aryl and heteroaryl, and wherein
the
aliphatic and cyclic portions of Rd, W and Re are optionally further
substituted with from
one to three halogen, hydroxy, methyl, amino, alkylamino and dialkylamino
groups, and
optionally when two R2 groups are on adjacent atoms, they are combined to form
a five-
or six-membered ring;
each R3 is independently selected from the group consisting of halogen, -CN, -
Ri, -CO2Rg, -
CONRgRh, -C(0)Rg, -C(0)12`, -0C(0)NRgRh, -NRhC(0)Rg, -NRhCO2Ri, -
NRgC(0)NR8Rh, -NRgRh, -ORg, -S(0)2NR8R
h, K _
-O-X4-1 , -X4-
NRgRh, -X4-NHRi, -X4-CONRgRh, -X4-NWC(0)Rg, -X4-CO2Rg, -0-X4-CO2Rg, -NH-
X4-CO2Rg, -X4-NRhCO2Ri, -0-X4-NRhCO2Ri, -NHRj and -NHCH2Rj, wherein X4 is a
C1-4 alkylene; each Rg and Rh is independently selected from hydrogen, C1-8
alkyl or
24
heteroalkyl, C3-6 cycloalkyl and Cis haloalkyl, or when attached to the same
nitrogen
atom can be combined with the nitrogen atom to form a four-, five- or six-
membered
ring having from 0 to 2 additional heteroatoms as ring members selected from
N, 0 or
S and is optionally substituted with one or two oxo; each Ri is independently
selected
from the group consisting of C1-8 alkyl or heteroalkyl, C1-8 haloalkyl, C3-6
cycloalkyl,
heterocycloalkyl, aryl and heteroaryl; and each R.) is selected from the group
consisting
of C3-6 cycloalkyl, imidazolyl, pyrimidinyl, pyrrolinyl, piperidinyl,
morpholinyl,
tetrahydrofuranyl, tetrahydropyranyl, and S,S-dioxo-tetrahydrothiopyranyl, and
wherein the aliphatic and cyclic portions of Rig, Rh, Ri and Rj are optionally
further
substituted with from one to three halogen, methyl, CF3, hydroxy, C14 alkoxy,
C14
alkoxy-C14 alkyl, -C(0)0-Cmi alkyl, amino, alkylamino and dialkylamino groups,
and
optionally when two R3 groups are on adjacent atoms, they are combined to form
a five-
or six-membered ring; and
X is hydrogen or CH3.
Compounds that are similar to Avacopan but have an improved solubility profile
are
disclosed in WO 2017/176620 A2 .
Thus, in some other embodiments the inhibitor of C5a activity is a compound of
the following formula III:
0 40 Ri
,
CF3
110) N
10 0 f;1
F R2
III
or a pharmaceutically acceptable salt thereof, wherein:
RI is selected from the group consisting of H, -0-CH2-0-P(0)012a0Rh, -0-C(0)-
C1-6 alkylene-
L2-XI, 0-P(0)012'01th , and -0-C(0)-A1-(C1.3 alkylene),-C4-7heterocycly1
wherein the
C4-7 heterocyclyl is optionally substituted with 1 to 6 RC groups;
Al is selected from the group consisting of C6-10 aryl, C3-10 cycloalkyl, C5-
10 heteroaryl and C5-
10 heterocyclyl, each of which is optionally substituted with 1 to 5 R.' which
can be the
same or different;
n=0 or 1;
Date Recue/Date Received 2023-01-26
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
L2 is independently selected from the group consisting of a bond, -0-C(0)-C1_6
alkylene-, and
-NRd-C(0)-C1-6 alkylene-;
X' is independently selected from the group consisting of -NReRf , -P(0)0Ra0Rh
, -0-
P(0)0RaORb, and -CO2H;
5
R2 is selected from the group consisting of H, -L3-C1_6 alkylene-L4-X2 , -L3-
(C1-6 alkylene)m-
A2-X2 , -P(0)0Ra0C(0)-Ci_6 alkyl, -P(0)0RaNRgRh and -P(0)0RaOle;
L3 is independently selected from the group consisting of -C(0)-0-, and -C(0)-
;
L4 is independently selected from the group consisting of a bond, -0-C(0)-C2-6
allcenylene-, -
0-C(0)-C1-6 alkylene-, and -NRd-C(0)-C1_6 alkylene- wherein the C1-6 alkylene
in -
10
NRd-C(0)-Ci_6 alkylene- and -0-C(0)-C1-6 alkylene- is optionally substituted
with
NReRf;
X2 is independently selected from the group consisting of -NRkle, -P(0)0WORb ,
-0-
P(0)0RaORb, and -CO2H;
m=0 or 1;
15
A2 is selected from the group consisting of C6-10 aryl, C3_10 cycloallcyl,
C5_10 heteroaryl and Cs_
10 heterocyclyl, each of which is optionally substituted with 1 to 5 IV which
can be the
same or different;
R3 is H or -L5-P(0)0WORb wherein L5 is independently selected from the group
consisting of
a bond and -CH2-0-;
20
each R.' is independently selected from the group consisting of halogen, C1-6
alkyl, C1-6
haloalkyl, Ci_6heteroalkyl, CN, NRYRz, SR Y and ORY;
each RC is independently selected from the group consisting of halogen, C1_6
alkyl, C1_6
haloalkyl, C1-6heteroalkyl, CN, NRYW, SR Y and ORY;
each Ra, R", Rd, W, Rf, Rg, Rk, RI, RY and W is independently selected from
the group consisting
25 of H and C1_6 alkyl;
each Rh is independently selected from the group consisting of H and C1-6
alkyl wherein the CI-
6 alkyl is optionally substituted with 1 to 5 substituents independently
selected from
CO2H,
C6-io aryl, C3-10 cycloalkyl, C5_10 heteroaryl and C5-10 heterocyclyl,
wherein each Ri and Ri is independently H or C1_6 alkyl;
wherein two of R", R2 and R3 are H, and one of R1, R2 and R3 is other than H.
PMX-53 is a potent antagonist of C5aR (CD88). It is a circular peptide
composed of six
amino acids, with the following sequence: Ac-Phe-cyclo(Orn-Pro-D-Cha-Trp-Arg)
with a
lactam bridge between Om-2 and Arg-6. Since PMX-53 contains at least one D-
amino acid (i.e.
D-Cha), it is not included the enclosed sequence listing of this application.
PMX-53 is
26
commercially available by bio-techne GmbH (Wiesbaden-Nordenstadt, Germany),
Cat. No.
5473.
Compounds similar to PMX-53 that are also suitable for practicing the present
invention
are disclosed in international patent applications WO 99/00406 Al, WO
03/033528 Al, and
WO 2008/009062 Al . Thus, in
some embodiments the inhibitor of C5a activity is a cyclic peptide or
peptidomimetic
compound of the formula IV
0
A
0 NH
0.4044(,)"vi E
(NH
. -
where A is H, alkyl, aryl, NH2, NH-alkyl, N(alkyl)2, NH-aryl, NH-acyl, NH-
benzoyl, NHS03,
NHS02-alkyl, NHS02-aryl, OH, 0-alkyl, or 0-aryl;
B is an alkyl, aryl, phenyl, benzyl, naphthyl or indole group, or the side
chain of a D- or L-
amino acid, but is not the side chain of glycine, D-phenylalanine, L-
homophenylalanine, L-tryptophan, L-homotryptophan, L- tyrosine, or L-
homotyrosine;
C is the side chain of a D-, L- or homo-amino acid, but is not the side chain
of isoleucine,
phenylalanine, or cyclohexylalanine;
D is the side chain of a neutral D-amino acid, but is not the side chain of
glycine or D-alanine,
a bulky planar side chain, or a bulky charged side chain;
E is a bulky substituent, but is not the side chain of D-tryptophan, L-N-
methyltryptophan, L-
homophenylalanine, L-2-naphthyl L-tetrahydroisoquinoline, L-cyclohexylalanine,
D-
leucinc, L-fluorenylalanine, or L-histidine;
F is the side chain of L-arginine, L-homoarginine, L-citrulline, or L-
canavanine, or a bioisostere
thereof; and
Date Recue/Date Received 2023-01-26
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
27
X1 is -(CH2)0NH- or (CH2)nS-, where n is an integer of from 1 to 4; -(CH2)20-;
-(CH2)30; -
(CH2)3-; -(CH2)4-, -CH2-COCHRNH-: or -CH2-CHCOCHRNH-, where R is the side
chain of any common or uncommon amino acid.
In this context, the term "common amino acid" refers to the twenty
proteinogenic amino
acids that are defined by the standard genetic code. The term "uncommon amino
acid" includes,
but is not restricted to, D-arnino acids, homo-amino acids, N-alkyl amino
acids, dehydroamino
acids, aromatic amino acids other than phenylalanine, tyrosine and tryptophan,
ortho-, meta-
or para-aminobenzoic acid, ornithine, citrulline, canavanine, norleucinc, 6-
glutamic acid,
aminobutyric acid, L-fluorenylalanine, L-3-benzothienylalanine, and ct,a-
disubstituted amino
acids.
Specific antagonists of C5aR (CD88) suitable for practicing the present
invention
include PMX95, PMX218, PMX200, PMX273, PMX205, and PMX201, as disclosed in WO
2008/009062 Al.
Clone S5/1 is a monoclonal antibody recognizing the human receptor for C5a
(CD88).
Clone S5/1 was raised against a synthetic peptide comprising the N-terminal
domain of the
C5aR (Metl-Asn31). The antibody has been shown to inhibit the binding of C5a
to its receptor.
It is commercially available via Hycult Biotech (Uden, The Netherlands), Cat.
No. HM2094.
Clone 7H110 is a monoclonal mouse antibody recognizing the human receptor for
C5a
(CD88). It is commercially available via Biomol GmbH (Hamburg, Germany); Cat.
No. C2439-
60N.
As used herein, a "patient" means any mammal or bird who may benefit from a
treatment with the compound described herein (i.e. with an inhibitor of C5a
activity described
herein). Preferably, a "patient" is selected from the group consisting of
laboratory animals (e.g.
mouse or rat), domestic animals (including e.g. guinea pig, rabbit, chicken,
turkey, pig, sheep,
goat, camel, cow, horse, donkey, cat, or dog), or primates including
chimpanzees and human
beings. It is particularly preferred that the "patient" is a human being.
As used herein, "treat", "treating" or "treatment" of a disease or disorder
means
accomplishing one or more of the following: (a) reducing the severity and/or
duration of the
disorder; (b) limiting or preventing development of symptoms characteristic of
the disorder(s)
being treated; (c) inhibiting worsening of symptoms characteristic of the
disorder(s) being
treated; (d) limiting or preventing recurrence of the disorder(s) in patients
that have previously
had the disorder(s); and (e) limiting or preventing recurrence of symptoms in
patients that were
previously symptomatic for the disorder(s).
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
28
As used herein, "prevent", "preventing", "prevention", or "prophylaxis" of a
disease or
disorder means preventing that a disorder occurs in a subject.
An "effective amount" is an amount of a therapeutic agent sufficient to
achieve the
intended purpose. The effective amount of a given therapeutic agent will vary
with factors such
as the nature of the agent, the route of administration, the size and species
of the animal to
receive the therapeutic agent, and the purpose of the administration. The
effective amount in
each individual case may be determined empirically by a skilled artisan
according to established
methods in the art.
"Pharmaceutically acceptable" means approved by a regulatory agency of the
Federal
or a state government or listed in the U.S. Pharmacopeia or other generally
recognized
pharmacopeia for use in animals, and more particularly in humans.
Embodiments of the Invention
The present invention will now be further described. In the following passages
different
aspects of the invention are defined in more detail. Each aspect defined below
may be combined
with any other aspect or aspects unless clearly indicated to the contrary. In
particular, any
feature indicated as being preferred or advantageous may be combined with any
other feature
or features indicated as being preferred or advantageous.
In a first aspect the present invention is directed to a compound for use in
the treatment
of a cutaneous, neutrophilic, inflammatory disease in a subject, wherein the
compound is an
inhibitor of C5a activity, and wherein the cutaneous, neutrophilic,
inflammatory disease is
selected from the group consisting of hidradenitis suppurativa (HS); Pyoderma
gangrenosum
(PG); PAPA (pyogenic arthritis, PG and acne); PASH (PG, acne and hidradenitis
suppurativa);
PAPASH (pyogenic arthritis, acne, PG and hidradenitis suppurativa); Sweet
syndrome (SS);
subcomeal pustular dermatosis (SPD); epidermolysis bullosa acquisita, erythema
elevatum
diutinum (EED); neutrophilic panniculitis; bowel-associated dermatosis-
arthritis syndrome
(BADAS); SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis)
syndrome;
rheumatoid neutrophilic dermatosis; familial Mediterranean fever, cryopyrin-
associated
disorders, gout, and Schnitzler syndrome.
In a second aspect, the present invention is directed to a method for the
treatment of a
cutaneous, neutrophilic, inflammatory disease in a subject, comprising the
step of:
administering to a subject in need thereof a therapeutic amount of a compound,
wherein
the compound is an inhibitor of C5a activity, and wherein the cutaneous,
neutrophilic,
inflammatory disease is selected from the group consisting of hidradenitis
suppurativa (HS);
CA 03066689 2019-12-09
WO 2018/234118 29
PCT/EP2018/065676
Pyoderrna gangrenosum (PG); PAPA (pyogenic arthritis, PG and acne); PASH (PG,
acne and
hidradenitis suppurativa); PAPASH (pyogenic arthritis, acne, PG and
hidradenitis suppurativa);
Sweet syndrome (SS); subcorneal pustular dermatosis (SPD); epidelinolysis
bullosa acquisita,
erythema elevatum diutinum (EED); neutrophilic panniculitis; bowel-associated
dermatosis-
arthritis syndrome (BADAS); SAPHO (synovitis, acne, pustulosis, hyperostosis,
and osteitis)
syndrome; rheumatoid neutrophilic dermatosis; familial Mediterranean fever,
cryopyrin-
associated disorders, gout, and Schnitzler syndrome.
In a third aspect, the present invention is directed to a use of a compound
for the
preparation of a pharmaceutical composition for the treatment of a cutaneous,
neutrophilic,
.. inflammatory disease, wherein the compound is an inhibitor of C5a activity,
and wherein the
cutaneous, neutrophilic, inflammatory disease is selected from the group
consisting of
hidradenitis suppurativa (HS); Pyoderma gangrenosum (PG); PAPA (pyogenic
arthritis, PG
and acne); PASH (PG, acne and hidradenitis suppurativa); PAPASH (pyogenic
arthritis, acne,
PG and hidradenitis suppurativa); Sweet syndrome (SS); subcorneal pustular de
__ inatosis (SPD);
epidermolysis bullosa acquisita, erythema elevatum diutinum (EED);
neutrophilic panniculitis;
bowel-associated dermatosis-arthritis syndrome (BADAS); SAPHO (synovitis,
acne,
pustulosis, hyperostosis, and osteitis) syndrome; rheumatoid neutrophilic
dermatosis; familial
Mediterranean fever, cryopyrin-associated disorders, gout, and Schnitzler
syndrome.
In some embodiments of any aspect of the present invention, the inhibitor of
C5a
activity:
- lowers the concentration of C5 (for example, by inhibiting formation
and/or activity of C3
convertase; by inhibiting formation and/or activity of C5 convertase; by
inhibiting the
transcription of the C5 gene; by blocking translation of the C5 mRNA; by
increasing
degradation of the C5 mRNA; by increasing degradation of the C5 protein; or by
prevention
secretion of C5 from the liver);
- inhibits the cleavage of C5 into C5a and C5b (for example, by
inhibiting the C5 convertase
or by binding to a cleavage site on C5 thereby blocking cleavage);
- lowers the concentration of C5a (for example, by increasing degradation
of the C5a protein);
- inhibits the binding between C5a and a C5a receptor (for example by
binding to C5a or by
binding to a C5a receptor);
- lowers the concentration of a C5a receptor (for example, by inhibiting
transcription of a
C5a receptor gene; by blocking translation of a C5a receptor mRNA; by
increasing
degradation of a C5a receptor mRNA; by increasing degradation of a C5a
receptor protein);
and/or
CA 03066689 2019-12-09
WO 2018/234118 30
PCT/EP2018/065676
- inhibits the activity of a C5a receptor.
In some embodiments of any aspect of the present invention, the inhibitor of
C5a activity
is selected from the group consisting of a protein ligand (as defined above);
an oligonucleotide;
and a small molecule (as defined above). Oligonucleotides acting as inhibitors
of C5a activity
can achieve their inhibitory effect for example by binding to nucleic acid
molecules (thereby
inhibiting transcription and/or translation) or by binding to proteins (e.g.
when the
oligonucleotides are nucleic acid aptamers).
In some embodiments of any aspect of the present invention, the inhibitor of
C5a activity
is a protein ligand that specifically binds to C5 protein, or to C5a protein,
or to a C5a receptor
protein. In further embodiments, the protein ligand is selected from the group
consisting of
(i) antibodies (e.g. anti-05 antibodies, anti-05a antibodies, anti-05aR
antibodies, or anti-
05L2 antibodies),
(ii) antigen-binding fragments of antibodies,
(iii) antibody-like proteins,
(iv) inhibitory variants of C5a,
(v) inhibitory variants of a C5a receptor (e.g. decoy receptors),
(vi) proteins acting on the complement pathway (e.g. Coversin); and
(vii) peptides (e.g. RA101495 (Ra Pharma, Cambridge, MA); PMX-53 (bio-techne
GmbH
(Wiesbaden-Nordenstadt, Germany)).
In some embodiments of any aspect of the present invention, the inhibitor of
C5a activity
is a protein ligand or an oligonucleotide, preferably a protein ligand, that
specifically binds to
a conformational epitope formed by amino acid sequences NDETCEQRA (SEQ ID NO:
2) and
SHKDMQL (SEQ ID NO: 3) of human C5a. Binding to the conformational formed by
the
amino acid sequences according to SEQ ID NOs: 2 and 3 means that the protein
ligand or
oligonucleotide binds to at least one amino acid within the amino acid
sequence according to
SEQ ID NO: 2 and to at least one amino acid within the amino acid sequence
according to SEQ
ID NO: 3. SEQ ID NO: 2 corresponds to amino acids 30-38 of human C5a. SEQ ID
NO: 3
corresponds to amino acids 66-72 of human C5a.
In some embodiments of any aspect of the present invention the protein ligand
or
oligonucleotide, preferably the protein ligand, binds to at least one amino
acid within the amino
acid sequence according to DETCEQR (SEQ ID NO: 4). SEQ ID NO: 4 corresponds to
amino
acids 31-37 of human C5a.
In some embodiments of any aspect of the present invention the protein ligand
or
oligonucleotide, preferably the protein ligand, binds to at least one amino
acid within the amino
CA 03066689 2019-12-09
WO 2018/234118 31
PCT/EP2018/065676
acid sequence according to HKDMQ (SEQ ID NO: 5), more preferably to at least
one amino
acid within the amino acid sequence KDM. SEQ ID NO: 5 corresponds to amino
acids 67-71
of human C5a; the sequence KDM corresponds to amino acids 68-70 of human C5a.
In some embodiments of any aspect of the present invention the protein ligand
or
oligonucleotide, preferably the protein ligand, binds to at least one amino
acid within the amino
acid sequence DETCEQR (SEQ ID NO: 4) and to at least one amino acid within the
amino acid
sequence HKDMQ (SEQ ID NO: 5).
In some embodiments of any aspect of the present invention the protein ligand
or
oligonucleotide, preferably the protein ligand, binds to at least one amino
acid within the amino
acid sequence DETCEQR (SEQ ID NO: 4) and to at least one amino acid within the
amino acid
sequence KDM.
In some embodiments of any aspect of the present invention the two sequences
forming
the conformational epitope of C5a (e.g. sequence pairs according to SEQ IL)
NO: 2 and 3; SEQ
ID NO: 4 and 5; or SEQ ID NO: 4 and sequence KDM) are separated by 1-50
contiguous amino
acids that do not participate in binding to the binding moiety of the
invention. In the following,
such amino acids that do not participate in binding to the binding moiety of
the invention will
be referred to as "non-binding amino acids". The two sequences forming the
conformational
epitope are preferably separated by 6-45 contiguous non-binding amino acids,
more preferably
by 12-40 contiguous non-binding amino acids, more preferably by 18-35
contiguous non-
binding amino acids, more preferably by 24-30 contiguous non-binding amino
acids, more
preferably by 25-29 contiguous non-binding amino acids, even more preferably
by 26-28
contiguous non-binding amino acids, and most preferably by 27 contiguous non-
binding amino
acids.
In some embodiments of any aspect of the present invention the protein ligand
or
oligonucleotide, preferably the protein ligand, specifically binding to a
conformational epitope
of C5a has a binding constant to human C5a with a Kd value of 10 nM or less,
preferably 9 nM
or less, more preferably 8 nM or less, more preferably 7 nM or less, more
preferably 6 nM or
less, more preferably 5 nM or less, more preferably 4 nM or less, more
preferably 3 nM or less,
more preferably 2 nM or less, and even more preferably 1 nM or less. In some
embodiments of
any aspect of the present invention the dissociation constant Ki between the
binding moiety and
human C5a is between 1 pM (picomolar) and 5 nM (nanomolar), more preferably
between 2
pM and 4 nM, more preferably between 5 pM and 3 nM, more preferably between 10
pM and
2 nM, more preferably between 50 pM and 1 nM, more preferably between 100 pM
and 900
CA 03066689 2019-12-09
WO 2018/234118 32
PCT/EP2018/065676
pM, more preferably between 200 pM and 800 pM, more preferably between 300 pM
and 700
pM, and even more preferably between 400 pM and 600 pM.
In some embodiments of any aspect of the present invention the protein ligand
or
oligonucleotide, preferably the protein ligand, specifically binding to C5a
exhibits at least 75%
blocking activity, preferably at least 80% blocking activity, more preferably
at least 85%
blocking activity, more preferably at least 90% blocking activity, more
preferably at least 95%
blocking activity for biological effects induced by one molecule C5a,
particularly human C5a.
These particular blocking activities refer to those embodiments, wherein the
binding moiety
comprises a single paratope binding to C5a, preferably human C5a. In
embodiments, wherein
the binding moiety comprises two or more C5a-specific paratopes, said blocking
activities of
at least 75%, preferably at least 80%, more preferably at least 85%, etc. are
achieved when one
binding-moiety molecule is contacted with a number of C5a molecules equal to
the number of
C5a-specific paratopes present in the binding moiety. In other words, when the
paratopes of a
binding moiety described herein and C5a are present in equimolar
concentrations, the binding
moiety exhibits at least 75% blocking activity, preferably at least 80%
blocking activity, more
preferably at least 85% blocking activity, more preferably at least 90%
blocking activity, and
more preferably at least 95% blocking activity for biological effects induced
by C5a. A
preferred biological effect to be blocked is C5a-induced lysozyme release from
human whole
blood cells. Assays for determining this C5a-induced lysozyme release and its
blocking are
described, for example, in WO 2011/063980 Al and in the corresponding US
national stage
application US 2012/0231008 Al.
In some embodiments of any aspect of the present invention the protein ligand
is an
antibody or an antigen-binding fragment thereof, wherein said antibody or
antigen-binding
fragment thereof comprises
(i) a heavy chain CDR3 sequence as set forth in SEQ ID NO: 6; or
(ii) a heavy chain CDR3 sequence as set forth in SEQ ID NO: 7;
wherein the heavy chain CDR3 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions.
In some embodiments of any aspect of the present invention the protein ligand
is an
antibody or an antigen-binding fragment thereof, wherein said antibody or
antigen-binding
fragment thereof comprises
(iii) a light chain CDR3 sequence as set forth in SEQ ID NO: 8; or
(iv) a light chain CDR3 sequence as set forth in SEQ ID NO: 9;
CA 03066689 2019-12-09
WO 2018/234118 33
PCT/EP2018/065676
wherein the light chain CDR3 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions.
In some embodiments of any aspect of the present invention the protein ligand
is an
antibody or an antigen-binding fragment thereof, wherein said antibody or
antigen-binding
fragment thereof comprises
(i) a heavy chain CDR3 sequence as set forth in SEQ ID NO: 6 and a light
chain CDR3
sequence as set forth in SEQ ID NO: 8; or
(ii) a heavy chain CDR3 sequence as set forth in SEQ ID NO: 7 and a light
chain CDR3
sequence as set forth in SEQ ID NO: 9;
wherein the heavy chain CDR3 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions; and
wherein the light chain CDR3 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions.
In some embodiments of any aspect of the present invention the protein ligand
is an
antibody or an antigen-binding fragment thereof, wherein said antibody or
antigen-binding
fragment thereof comprises at least one of the following sequences:
(v) a heavy chain CDR2 sequence according to SEQ ID NO: 10;
(vi) a heavy chain CDR2 sequence according to SEQ ID NO: 11;
(vii) a light chain CDR2 sequence according to SEQ ID NO: 12;
(viii) a light chain CDR2 sequence according to SEQ ID NO: 13;
(ix) a heavy chain CDR1 sequence according to SEQ ID NO: 14;
(x) a heavy chain CDR1 sequence according to SEQ ID NO: 15;
(xi) a light chain CDR1 sequence according to SEQ ID NO: 16; or
(xii) a light chain CDR1 sequence according to SEQ ID NO: 17;
wherein the heavy chain CDR2 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions;
wherein the light chain CDR2 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions;
CA 03066689 2019-12-09
WO 2018/234118 34
PCT/EP2018/065676
wherein the heavy chain CDR1 sequence optionally comprises 1, 2 or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions; and
wherein the light chain CDR1 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions.
In particular embodiments, the total number of these optional changes recited
above in
each one of the amino acid sequences according to SEQ ID NO: 6, SEQ ID NO: 7,
SEQ ID
NO: 8, SEQ ID NO: 9, SEQ ID NO: 10, SEQ ID NO: 11, SEQ ID NO: 13, SEQ ID NO:
14,
SEQ ID NO: 15, SEQ ID NO: 16, and SEQ ID NO: 17, i.e. the total number of
exchanges,
deletions and additions in each sequence, is 1 or 2.
In particular embodiments the total number of exchanges, deletions, and
additions added
up for all CDRs present in an antibody or antigen-binding fragment thereof is
between 1 and 5
(e.g. 1, 2, 3, 4, or 5).
In some embodiments of any aspect of the present invention the protein ligand
is an
antibody or an antigen-binding fragment thereof, comprises one of the sets A
to H of heavy
chain CDR3, heavy chain CDR2, and heavy chain CDR1 sequences as listed below
in Table 1,
wherein each heavy chain CDR3 sequence optionally comprises 1, 2, or 3 amino
acid
exchanges, preferably conservative amino acid exchanges, 1, 2, or 3 amino acid
deletions,
and/or 1, 2, or 3 amino acid additions;
wherein each heavy chain CDR2 sequence optionally comprises 1, 2, or 3 amino
acid
exchanges, preferably conservative amino acid exchanges, 1, 2, or 3 amino acid
deletions,
and/or 1, 2, or 3 amino acid additions; and
wherein each heavy chain CDR1 sequence optionally comprises 1, 2, or 3 amino
acid
exchanges, preferably conservative amino acid exchanges, 1, 2, or 3 amino acid
deletions,
and/or 1, 2, or 3 amino acid additions:
Table 1: Sets of heavy chain CDR sequences suitable for use in the antibodies
or fragments
thereof of the present invention
Symbol of heavy
CDR3 sequence CDR2 sequence CDR1 sequence
chain set
A SEQ ID NO: 6
SEQ ID NO: 10 SEQ ID NO: 14
SEQ ID NO: 6 SEQ ID NO: 10 SEQ ID NO: 15
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
SEQ ID NO: 6 SEQ TD NO: 11
SEQ ID NO: 14
SEQ ID NO: 6 SEQ ID NO: 11
SEQ ID NO: 15
SEQ ID NO: 7 SEQ ID NO: 10
SEQ ID NO: 14
SEQ ID NO: 7 SEQ ID NO: 10
SEQ ID NO: 15
SEQ ID NO: 7 SEQ ID NO: 11
SEQ ID NO: 14
SEQ ID NO: 7 SEQ ID NO: 11
SEQ ID NO: 15
In some embodiments of any aspect of the present invention the protein ligand
is an
antibody or an antigen-binding fragment thereof, comprises one of the
following sets Ito IV of
light chain CDR3, light chain CDR2, and light chain CDR1 sequences as listed
in Table 2,
5 wherein each light chain CDR3 sequence optionally comprises 1, 2, or 3
amino acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions;
wherein each light chain CDR2 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
10 amino acid additions; and
wherein each light chain CDR1 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions.
15 Table 2: Sets of light chain CDR sequences suitable for use in the
antibodies or fragments
thereof of the present invention
Since the CDR2 light chain sequence of antibody IFX-1 (SEQ ID NO: 12) is
identical to the
CDR2 light chain sequence of antibody INab708 (SEQ ID NO: 13), sets including
SEQ ID NO:
13 would be redundant to sets including SEQ ID NO: 12. Therefore, the table
only lists four
20 sets of light chain CDR sequences.
Number of light
CDR3 sequence CDR2 sequence
CDR1 sequence
chain set
SEQ ID NO: 8 SEQ ID NO: 12
SEQ ID NO: 16
II SEQ ID NO: 8 SEQ ID NO: 12
SEQ ID NO: 17
rn SEQ ID NO: 9 SEQ ID NO: 12
SEQ ID NO: 16
IV SEQ ID NO: 9 SEQ ID NO: 12
SEQ ID NO: 17
CA 03066689 2019-12-09
WO 2018/234118 36
PCT/EP2018/065676
In some embodiments of any aspect of the present invention the protein ligand
is an
antibody or an antigen-binding fragment thereof, comprises one of the heavy
CDR sets A-H
listed above in Table 1 and one of the light chain CDR sets I-IV listed above
in Table 2, i.e. one
of the following combinations of sets: A-I, A-II, A-III, A-IV, B-I, B-IT, B-
IV, C-I, C-II,
C-IV, D-I, D-II, D-IV, E-I, E-TV, F-I, F-II, F-IV, G-I, G-
II,
G-IV, H-I, H-I!, H-DI, or H-IV (wherein the combinations A-I and H-IV are
especially
preferred),
wherein each heavy chain CDR3 sequence optionally comprises 1, 2, or 3 amino
acid
exchanges, preferably conservative amino acid exchanges, 1, 2, or 3 amino acid
deletions,
and/or 1, 2, or 3 amino acid additions;
wherein each heavy chain CDR2 sequence optionally comprises 1, 2, or 3 amino
acid
exchanges, preferably conservative amino acid exchanges, 1, 2, or 3 amino acid
deletions,
and/or 1, 2, or 3 amino acid additions;
wherein each heavy chain CDR1 sequence optionally comprises 1, 2, or 3 amino
acid
exchanges, preferably conservative amino acid exchanges, 1, 2, or 3 amino acid
deletions and/or
1, 2, or 3 amino acid additions;
wherein each light chain CDR3 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
in particular conservative amino acid exchanges, 1, 2, or 3 amino acid
deletions, and/or 1, 2, or
3 amino acid additions;
wherein each light chain CDR2 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions,
and/or 1, 2, or 3
amino acid additions; and
wherein each light chain CDR1 sequence optionally comprises 1, 2, or 3 amino
acid exchanges,
preferably conservative amino acid exchanges, 1, 2, or 3 amino acid deletions
and/or 1, 2, or 3
amino acid additions.
In some embodiments of any aspect of the present invention the protein ligand
is an
antibody or an antigen-binding fragment thereof, comprises a VH domain that
comprises,
essentially consists of or consists of (i) the VH domain of IFX-1 or (ii) the
VH domain of
INab708.
The FR1, CDR1, FR2, CDR2, FR3, CDR3, and FR4 sequences defining the VH
domains of IFX-1 and INab708 are shown below in Table 3.
In some embodiments of any aspect of the present invention the protein ligand
is an
antibody or an antigen-binding fragment thereof, comprises a VL domain that
comprises,
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
37
essentially consists of or consists of (i) the VL domain of IFX-1 or (ii) the
VL domain of
INab708.
The FR1, CDR1, FR2, CDR2, FR3, CDR3, and FR4 sequences defining the VL domains
of IFX-1 and INab708 are shown below in Table 3.
Table 3: CDR and FR sequences of antibodies IFX-1 and INab708 (Chothia
classification
mode)
IFX-1: INab708:
Heavy Chain: Heavy Chain:
FR1: QVQLQQSGPQLVRPGTSVKIS FR1: VQLLESGAELMKPGASVKIS
(= SEQ ID NO: 18) (SEQ ID NO: 26)
CDR1: CKASGYSFTTFWMD CDR1: CKATGNTFSGYWIF.
(= SEQ ID NO: 14) (= SEQ ID NO: 15)
FR2: WVKQRPGQGLEWIGR FR2: WVKQRPGHGLEWIGE
(SEQ ID NO: 19) (SEQ ID NO: 27)
CDR2: IDPSDSESRLDQ CDR2: ILPGSGSTNYNE
(= SEQ ID NO: 10) (= SEQ ID NO: 11)
FR3: FR3:
RFKDRATLTVDKSSSTVYMQLSSPTSE KFKGKATLTADTSSNTAYMQLSSLTSE
DSAVYY DSAVYY
(SEQ ID NO: 20) (SEQ ID NO: 28)
CDR3: CARGNDGYYGFAY CDR3: CTRRGLYDGSSYFAY
(= SEQ ID NO: 6) (= SEQ ID NO: 7)
FR4: WGQGTLVTVSS FR4: WGQGTLVTVSA
(SEQ ID NO: 21) (SEQ ID NO: 29)
CA 03066689 2019-12-09
WO 2018/234118 38
PCT/EP2018/065676
Light Chain: Light Chain:
FR1: DIVLTQSPASLAVSLGQRATIS FR1: DIVLTQSPASLAVSLGQRAT1S
(SEQ ID NO: 22) (SEQ ID NO: 30)
CDR1: CKASQSVDYDGDSYMK CDR1: CKASQSVDYDGDSYMN
(= SEQ TD NO: 16) (= SEQ ID NO: 17)
FR2: WYQQICPGQPPKLL 1-R2: WYQQKPGQPPKLL
(SEQ ID NO: 23) (SEQ ID NO: 31)
CDR2: IYAASNL CDR2: IYAASNL
(= SEQ ID NO: 12) (= SEQ ID NO: 13)
FR3: FR3:
QSGIPARFSGSGSGTDFTLNIHPVEEEDA GSGIPARFSGSGSGTD1-(I'LNIHPVEEE
ATYY VAATYY
(SEQ ID NO: 24) (SEQ ID NO: 32)
CDR3: CQQSNEDPYT CDR3: CQQNNEDPLT
SEQ ID NO: 8) (= SEQ ID NO: 9)
FR4: FGGGTKLEIK FR4: FGAGTLLELK
(SEQ ID NO: 25) (SEQ ID NO: 33)
In some embodiments of any aspect of the present invention, the inhibitor of
C5a activity
is an oligonucleotide that specifically binds to C5, or to C5a, or to a C5a
receptor. In further
embodiments, the oligonucleotide is a nucleic acid aptamer. The nucleic acid
aptamer may be
selected from the group consisting of DNA-aptamers, D-RNA aptamers, and L-RNA
aptamers
(e.g., SpiegelmersTm).
In some embodiments of any aspect of the present invention, the inhibitor of
C5a activity
reduces expression of C5 protein or a C5a receptor protein. In further
embodiments, said
inhibitor of C5a activity that reduces expression of C5 protein or a C5a
receptor protein is an
oligonucleotide selected from the group consisting of antisense DNA, antisense
RNA, siRNA,
and miRNA.
In some embodiments of any aspect of the present invention, the C5a receptor
is C5aR
and/or C5L2. In preferred embodiments of any aspect of the present invention,
the C5a receptor
is C5aR (also known as CD88 or C5aR1).
In some embodiments of any aspect of the present invention, the inhibitor of
C5a activity
is selected from the group consisting of:
(a) 1FX-1, INab708, MEDI-7814, ALXN-1007, or NOX-D21, or an antigen-binding
fragment
thereof;
CA 03066689 2019-12-09
WO 2018/234118 39
PCT/EP2018/065676
(b) an antibody or an antigen-binding fragment thereof, wherein said antibody
or antigen-
binding fragment thereof competes with one of the antibodies indicated under
(a) for
binding to C5a;
(c) Eculizumab, ALXN1210, ALXN5500, or LFG316, or an antigen-binding fragment
thereof;
(d) an antibody or an antigen-binding fragment thereof, wherein said antibody
or antigen-
binding fragment thereof competes with one of the antibodies indicated under
(c) for
binding to C5;
(e) Coversin or RA101495;
(f) an antibody or an antigen-binding fragment thereof or protein or
macrocyclic peptide
wherein said antibody or antigen-binding fragment thereof or macrocyclic
peptide competes
with one of the or protein or peptides indicated under (e) for binding to C5;
(g) Zimura;
(h) an antibody or an antigen-binding fragment thereof or an aptamer, wherein
said antibody or
antigen-binding fragment thereof or aptamer competes with Zimura for binding
to C5;
(i) AMY-201 or Mirococept;
(j) an antibody or an antigen-binding fragment thereof or a protein wherein
said antibody or
antigen-binding fragment thereof or protein competes with one of the proteins
indicated
under (i) for binding to C3b;
(k) Bikaciomab;
(1) an antibody or an antigen-binding fragment thereof, wherein said antibody
or antigen-
binding fragment thereof competes with Bikaciomab for binding to Factor B;
(m) Lamp alizumab ;
(n) an antibody or an antigen-binding fragment thereof, wherein said antibody
or antigen-
binding fragment thereof competes with Lampalizumab for binding to Factor D;
(o) ALN-CC5;
(p) Avacopan or a compound according to formula II or III or PMX-53 or a
compound
according to formula IV;
(q) an antibody or an antigen-binding fragment thereof, wherein said antibody
or antigen-
binding fragment thereof competes with avacopan or PMX-53 for binding to C5aR;
(r) clone S5/1 or clone 7H110, or an antigen-binding fragment thereof; and
(s) an antibody or an antigen-binding fragment thereof, wherein said antibody
or antigen-
binding fragment thereof competes with one of the antibodies indicated under
(r) for binding
to C5aR.
CA 03066689 2019-12-09
WO 2018/234118 40
PCT/EP2018/065676
In some embodiments of any aspect of the present invention, the cutaneous,
neutrophilic, inflammatory disease is
- an auto-inflammatory disease (more precisely: a cutaneous, neutrophilic,
auto-
inflammatory disease); or
- an autoimmune disease with cutaneous inflammation (more precisely: an
autoimmune
disease with cutaneous, neutrophilic inflammation).
In some embodiments of any aspect of the present invention, the cutaneous,
neutrophilic, inflammatory disease is an auto-inflammatory disease selected
from the group
consisting of hidradenitis suppurativa (HS); Pyoderrna gangrenosum (PG); PAPA
(pyogenic
arthritis, PG and acne); PASH (PG, acne and hidradenitis suppurativa); PAPASH
(pyogenic
arthritis, acne, PG and hidradenitis suppurativa); Sweet syndrome (SS);
subcorneal pustular
dermatosis (SPD); epidermolysis bullosa acquisita, erythema elevatum diutinum
(EED);
neutrophilic panniculitis; bowel-associated dermatosis-arthritis syndrome
(BADAS); and
SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) syndrome.
In some embodiments of any aspect of the present invention, the cutaneous,
neutrophilic, inflammatory disease is HS or a HS-related disease selected from
the group
consisting of Pyoderma gangrenosum (PG); PAPA (pyogenic arthritis, PG and
acne); PASH
(PG, acne and hidradenitis suppurativa); PAPASH (pyogenic arthritis, acne, PG
and
hidradenitis suppurativa); Sweet syndrome (SS); and subcorneal pustular
dermatosis (SPD).
In some embodiments of any aspect of the present invention, the cutaneous,
neutrophilic, inflammatory disease is an autoimmune disease with cutaneous
inflammation
selected from the group consisting of rheumatoid neutrophilic dermatosis;
familial
Mediterranean fever, cryopyrin-associated disorders, gout, and Schnitzler
syndrome.
In some embodiments of the first or third aspect of the present invention, the
compound
is to be administered at a dose of 800 mg once per week or at a dose of 800 mg
twice per week.
In further embodiments of the first or third aspect,
- the inhibitor of C5a activity is a compound specifically binding to
C5a (preferably selected
from the group consisting of IFX-1, INab708, MEDI-7814, ALXN-1007, NOX-D21,
and
an antigen-binding fragment thereof; more preferably the inhibitor of C5a
activity is
selected from the group consisting of IFX-1, INab708, MEDI-7814, ALXN-1007 and
an
antigen-binding fragment thereof; even more preferably, the inhibitor of C5a
activity is
selected from the group consisting of IFX-1 and an antigen-binding fragment
thereof; most
preferably the inhibitor of C5a activity is 1FX-1); and
- the cutaneous, neutrophilic, inflammatory disease is hidradenitis
suppurativa (HS); and
CA 03066689 2019-12-09
WO 2018/234118 41
PCT/EP2018/065676
- the compound is to be administered at a dose of 800 mg once per week or
at a dose of 800
mg twice per week.
In further embodiments of the first or third aspect, the inhibitor of C5a
activity is to be
administered intravenously. In further embodiments of the first or third
aspect, the inhibitor of
C5a activity is to be administered twice per week at a dose of 800 mg in the
first week of
treatment and once per week at a dose of 800 mg in the second and subsequent
weeks of
treatment. In further embodiments of the first or third aspect, the total
duration of treatment is
between 5 and 12 weeks (e.g. 5 weeks, 6, weeks, 7 weeks, 8 weeks, 9 weeks, 10
weeks, 11
weeks, or 12 weeks).
In some embodiments of the second aspect of the present invention, the
compound is
administered at a dose of 800 mg once per week or at a dose of 800 mg twice
per week. In
further embodiments of the second aspect,
- the inhibitor of C5a activity is a compound specifically binding to C5a
(preferably selected
from the group consisting of IFX-1, INab708, MEDI-7814, ALXN-1007, NOX-D21,
and
an antigen-binding fragment thereof; more preferably the inhibitor of C5a
activity is
selected from the group consisting of IFX-1, INab708, MEDI-7814, ALXN-1007 and
an
antigen-binding fragment thereof; even more preferably, the inhibitor of C5a
activity is
selected from the group consisting of IFX-1 and an antigen-binding fragment
thereof; most
preferably the inhibitor of C5a activity is IFX-1); and
- the cutaneous, neutrophilic, inflammatory disease is hidradenitis
suppurativa (HS); and
- the compound is administered at a dose of 800 mg once per week or at a
dose of 800 mg
twice per week.
In further embodiments of the second aspect, the inhibitor of C5a activity is
administered intravenously. In further embodiments of the second aspect, the
compound is
administered twice per week at a dose of 800 mg in the first week of treatment
and once per
week at a dose of 800 mg in the second and subsequent weeks of treatment. In
further
embodiments of the second aspect, the total duration of treatment is between 5
and 12 weeks
(e.g. 5 weeks, 6, weeks, 7 weeks, 8 weeks, 9 weeks, 10 weeks, 11 weeks, or 12
weeks).
Pharmaceutical compositions and Modes of Administration
In the practice of any aspect of the present invention, a compound (e.g. an
inhibitor of
C5a activity described herein) or a pharmaceutical composition comprising the
compound may
be administered to a patient by any route established in the art which
provides a sufficient level
of the compound in the patient. It can be administered systemically or
locally. Such
CA 03066689 2019-12-09
WO 2018/234118 42
PCT/EP2018/065676
administration may be parenterally, transmucosally, e.g., orally, nasally,
rectally,
intravaginally, sublingually, submucosally, transdermally, or by inhalation.
Preferably,
administration is parenteral, e.g., via intravenous or intraperitoneal
injection, and also
including, but is not limited to, intra-arterial, intramuscular, intradermal
and subcutaneous
administration. If the compound described herein (e.g. an inhibitor of C5a
activity described
herein) or a pharmaceutical composition comprising the compound is
administered locally, it
can be injected directly into the organ or tissue to be treated.
Pharmaceutical compositions adapted for oral administration may be provided as
capsules or tablets; as powders or granules; as solutions, syrups or
suspensions (in aqueous or
non-aqueous liquids); as edible foams or whips; or as emulsions. Tablets or
hard gelatine
capsules may comprise lactose, starch or derivatives thereof, magnesium
stearate, sodium
saccharine, cellulose, magnesium carbonate, stearic acid or salts thereof.
Soft gelatine capsules
may comprise vegetable oils, waxes, fats, semi-solid, or liquid polyols etc.
Solutions and syrups
may comprise water, polyols and sugars.
An active agent intended for oral administration may be coated with or admixed
with a
material that delays disintegration and/or absorption of the active agent in
the gastrointestinal
tract (e.g., glyceryl monostearate or glyceryl distearate may be used). Thus,
the sustained
release of an active agent may be achieved over many hours and, if necessary,
the active agent
can be protected from being degraded within the stomach. Pharmaceutical
compositions for
oral administration may be formulated to facilitate release of an active agent
at a particular
gastrointestinal location due to specific pH or enzymatic conditions.
Pharmaceutical compositions adapted for transdermal administration may be
provided
as discrete patches intended to remain in intimate contact with the epidermis
of the recipient
for a prolonged period of time. Pharmaceutical compositions adapted for
topical administration
may be provided as ointments, creams, suspensions, lotions, powders,
solutions, pastes, gels,
sprays, aerosols or oils. For topical administration to the skin, mouth, eye
or other external
tissues a topical ointment or cream is preferably used. When formulated in an
ointment, the
active ingredient may be employed with either a paraffinic or a water-miscible
ointment base.
Alternatively, the active ingredient may be formulated in a cream with an oil-
in-water base or
a water-in-oil base. Pharmaceutical compositions adapted for topical
administration to the eye
include eye drops. In these compositions, the active ingredient can be
dissolved or suspended
in a suitable carrier, e.g., in an aqueous solvent. Pharmaceutical
compositions adapted for
topical administration in the mouth include lozenges, pastilles and
mouthwashes.
CA 03066689 2019-12-09
WO 2018/234118 43
PCT/EP2018/065676
Pharmaceutical compositions adapted for nasal administration may comprise
solid
carriers such as powders (preferably having a particle size in the range of 20
to 500 microns).
Powders can be administered in the manner in which snuff is taken, i.e., by
rapid inhalation
through the nose from a container of powder held close to the nose.
Alternatively, compositions
adopted for nasal administration may comprise liquid carriers, e.g., nasal
sprays or nasal drops.
These compositions may comprise aqueous or oil solutions of the active
ingredient.
Compositions for administration by inhalation may be supplied in specially
adapted devices
including, but not limited to, pressurized aerosols, nebulizers or
insufflators, which can be
constructed so as to provide predetermined dosages of the active ingredient.
Pharmaceutical
compositions may also be administered via the nasal cavity to the lungs.
Pharmaceutical compositions adapted for rectal administration may be provided
as
suppositories or enemas. Pharmaceutical compositions adapted for vaginal
administration may
be provided as pessaries, tampons, creams, gels, pastes, foams or spray
formulations.
Pharmaceutical compositions adapted for parenteral administration include
aqueous and
non-aqueous sterile injectable solutions or suspensions, which may contain
antioxidants,
buffers, bacteriostats and solutes that render the compositions substantially
isotonic with the
blood of an intended recipient. Other components that may be present in such
compositions
include water, alcohols, polyols, glycerine and vegetable oils, for example.
Compositions
adapted for parenteral administration may be presented in unit-dose or multi-
dose containers,
for example sealed ampules and vials, and may be stored in a freeze-dried
(lyophilized)
condition requiring only the addition of a sterile liquid carrier, e.g.,
sterile saline solution for
injections, immediately prior to use. Extemporaneous injection solutions and
suspensions may
be prepared from sterile powders, granules and tablets.
In a preferred embodiment, a compound described herein (e.g. an inhibitor of
C5a
activity described herein) is formulated in accordance with routine procedures
as a
pharmaceutical composition adapted for intravenous administration to human
beings.
Typically, compositions for intravenous administration are solutions in
sterile isotonic aqueous
buffer. Where necessary, the composition may also include a solubilizing agent
and a local
anesthetic such as lidocaine to ease pain at the site of the injection.
Generally, the ingredients
are supplied either separately or mixed together in unit dosage form, for
example, as a dry
lyophilized powder or water-free concentrate in a hermetically-sealed
container such as an
ampule or sachette indicating the quantity of active agent. Where the
composition is to be
administered by infusion, it can be dispensed with an infusion bottle
containing sterile
pharmaceutical grade water or saline. Where the composition is administered by
injection, an
CA 03066689 2019-12-09
WO 2018/234118 44
PCT/EP2018/065676
ampule of sterile saline can be provided so that the ingredients may be mixed
prior to
administration.
In another embodiment, for example, a compound (e.g. an inhibitor of C5a
activity
described herein) or a pharmaceutical composition comprising the compound can
be delivered
in a controlled-release system. For example, the compound may be administered
using
intravenous infusion, an implantable osmotic pump, a transdermal patch,
liposomes, or other
modes of administration. In one embodiment, a pump may be used (see Sefton
(1987) CRC
Grit. Ref Biomed. Eng. 14: 201; Buchwald et al. (1980) Surgery 88:507; Saudek
et al. (1989)
N. Eng. J. Med. 321: 574). In another embodiment, the compound can be
delivered in a vesicle,
in particular a liposome (see Langer (1990) Science 249:1527-1533; Treat et
al. (1989) in
Liposomes in the Therapy of Infectious Disease and Cancer, Lopez-Berestein and
Fidler (eds.),
Liss, N.Y., 353-365; WO 91/04014; U.S. 4,704,355). In another embodiment,
polymeric
materials can be used (see Medical Applications of Controlled Release (1974)
Langer and Wise
(eds.), CRC Press: Boca Raton, Fla.; Controlled Drug Bioavailability, Drug
Product Design and
Performance, (1984) Smolen and Ball (eds.), Wiley: N.Y.; Ranger and Peppas
(1953) J.
Macromol. Sci. Rev. Macromol. Chem. 23: 61; see also Levy et al. (1985)
Science 228:190;
During et al. (1989) Ann. NeuroL 25: 351; Howard et al. (1989) J. Neurosurg.
71: 105).
In yet another embodiment, a controlled release system can be placed in
proximity of
the therapeutic target, i.e., the target cells, tissue or organ, thus
requiring only a fraction of the
systemic dose (see, e.g., Goodson (1984) 115-138 in Medical Applications of
Controlled
Release, vol. 2). Other controlled release systems are discussed in the review
by Langer (1990,
Science 249: 1527-1533).
In a specific embodiment, it may be desirable to administer a compound
described
herein (e.g. an inhibitor of C5a activity described herein) or a
pharmaceutical composition
comprising the compound locally to the area in need of treatment. This may be
achieved by, for
example, and not by way of limitation, local infusion during surgery, topical
application, e.g.,
in conjunction with a wound dressing after surgery, by injection, by means of
a catheter, by
means of a suppository, or by means of an implant, said implant being of a
porous, non-porous,
or gelatinous material, including membranes, such as silastic membranes, or
fibers.
Selection of the preferred effective dose will be determined by a skilled
artisan based
upon considering several factors which will be known to one of ordinary skill
in the art. Such
factors include the particular form of the pharmaceutical composition, e.g.
polypeptide or
vector, and its pharmacokinetic parameters such as bioavailability,
metabolism, half-life, etc.,
which will have been established during the usual development procedures
typically employed
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
in obtaining regulatory approval for a pharmaceutical compound. Further
factors in considering
the dose include the condition or disease to be prevented and or treated or
the benefit to be
achieved in a normal individual, the body mass of the patient, the route of
administration,
whether administration is acute or chronic, concomitant medications, and other
factors well
5 known to affect the efficacy of administered pharmaceutical agents. Thus
the precise dosage
should be decided according to the judgment of the practitioner and each
patient's
circumstances, e.g., depending upon the condition and the immune status of the
individual
patient, according to standard clinical techniques.
10 EXAMPLES
The following Examples are provided for further illustration of the invention.
i.e
invention, however, is not limited thereto, and the following Examples merely
show the
practicability of the invention on the basis of the above description.
15 1. METHODS
1.1 Preparation of Zymosan A stock solution and Zymosan A-activated
plasma (ZAP)
Zymosan A was dissolved to 2 mg/ml in 50 ml sterile saline and boiled for 1 h
at 100 C.
After centrifugation, supernatant was discarded and the pellet was resuspended
in 50 ml sterile
saline. After a second centrifugation step, pellet was resuspended in 5 ml
sterile saline to obtain
20 .. a 20 mg/ml stock solution. Stock solution was aliquoted and stored at -
20 C until use. To
activate the plasma, Zymosan A stock solution and 100 ill plasma were mixed
and incubated at
37 C for 30 min. After incubation, tubes were centrifuged and the supernatant
was aliquoted
and stored at -20 C until use.
25 1.2 CD 1 lb assay using rhC5a or ZAP as stimulants
Human whole blood was stimulated with rhC5a or ZAP. To test the blocking
activity of
11-X-1 and irrelative control IgG4 on rhC5a, the antibodies were diluted to
final Ab/Ag molar
ratios of 1:1 and 0.5:1. To test the blocking activity of IFX-1 on eC5a, IFX-1
was diluted to
reach final Ab/Ag molar ratios of approximately 4:1/3:1/2:1/1:1/0.5:1. Blood
only with buffer
30 served as a non-stimulation control to assess the baseline CD1lb
expression. Blood with
antibody alone was used to determine the effects on CD1 lb expression of the
antibody under
non-stimulated condition. The complete mixture (Ab/Ag/blood) was incubated at
37 C for 20
min to evaluate C5a-induced up-regulation of CD11b. After addition of anti-
mouse
CD11b:FITC samples were incubated for 30 min on ice to minimize background
staining.
CA 03066689 2019-12-09
WO 2018/234118 46
PCT/EP2018/065676
Granulocytes were gated and mean fluorescence intensity (MFI) of FITC labeled
(CD 1 lb
expressing) granulocytes was examined by flow cytometer.
1.3 CD I lb assay using rhC5a or zymosan A in the whole blood
Human blood was stimulated with rhC5a or zymosan A, and the complete mixture
(Ab/Ag/blood) was incubated at 37 C for 20 min to stimulate the C5a-induced up-
regulation of
CD11b. After incubation, 2 1 of anti-mouse CD 1 lb:FITC or isotype:FITC
control was added
and samples were incubated for 30 min on ice to minimize background staining.
After lysis,
cells were analyzed using flow cytometer. On the FSC/SSC dot-plot,
granulocytes were gated
and mean fluorescence intensity (MFI) of F1TC labeled (CD1 lb expressing)
granulocytes was
examined for the whole sample set.
1.4 Cytokine IL-8 ELISA
Human IL-8 ELISA was performed as recommended in the instruction manual under
section "Assay procedure" (eBioscience Inc., San Diego, CA). Briefly, coating
was performed
overnight at 4 C using 100 p.1 lx capture antibody. Plates were blocked using
200 pl lx assay
diluents at RT for 1 h. Standard stock solutions were diluted with lx assay
diluents to the desired
concentration, followed by 6 serial 1:2 dilutions. Sample supernatants were
diluted as required
in lx assay diluents. According to the "Assay procedure", 100 1.11 of standard
dilutions and
sample dilutions were added to the coated plate and incubated at RT for 1 h,
followed by the
incubation with 100 11x detection antibody (RT, 1 h) and 100 jt1 lx avidin-
HRP (RT, 30 min).
Color development was performed with 100 I TMB substrate solution at RT for
10 min in the
dark and was terminated with 100 1A1 stop solution. Absorbance was read out
within 30 min
using the plate reader at 450 nrn. Zero standard value (blank) was subtracted
from all standards
and samples. Cytokine concentration of samples was calculated using a log(x) /
log(y) standard
curve of included standard samples.
1.5 C5a ELISA
Purified anti-human C5a monoclonal antibody (InflaRx GmbH, Jena, Germany) was
coated overnight with a final concentration of 0.5 pg/mL on the ELISA plate.
After blocking
with the assay diluent (lx PBS with 0.05% Tween 20 and 2% heat-inactivated
FBS), calibration
samples (recombinant human C5a, Sigma, Taufkirchen, Germany) and samples
diluted in assay
diluent were incubated for 90 minutes at room temperature. Mouse anti-human
C5/C5a
antibody clone 561 (Hycult Biotech, Uden, The Netherlands) diluted to 2 g/mL
in assay
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
47
diluent was applied as the primary detection antibody for a 60-minute
incubation at room
temperature, followed by a 30-minute incubation with the secondary horseradish
peroxidase
labeled antibody (goat anti-mouse IgG2a polyclonal antibody, SouthernBiotech,
Birmingham,
USA) diluted to 0.05 g/mL in assay diluent. Color development was performed
with
tetramethylbenzidine substrate solution (TMB, Biozol, Eching, Germany) and was
stopped with
3.7 N sulfuric acid. The OD was read as the absorbance of 450 nm by Tecan
Infinite 200
reader with Tecan MagellanTM (Tecan Group, Maennedorf, Switzerland). The in-
house
developed C5a ELISA was validated according to the EMA guideline on
bioanalytical method
validation.
Intra-assay and inter-assay precision tested with five different
concentrations showed a
coefficient of variance (CV) of 0.65 % to 4.96% and 1.50 % to 4.88 % for six
and 18 repetitions,
respectively. Recovery analysis of the spiked recombinant human C5a in buffer
resulted in
recoveries of 86.98 1.20% (mean SD) at the lower limit of quantification
and 91.50 3.29
% at the upper limit of quantification. No cross-reactivity for C3, C3a and C4
and cross-
reactivity of < 0.01% for C5b-6 was detected. Human IgG4 antibodies did not
interfere with
the assay. The mean C5a level in citrate plasma from 20 human volunteers is
17.08 ng/mL
6.96 ng/mL with a range from 7.52 ng/mL to 30.17 ng/mL.
1.6 Measurements of complement activation products
Concentrations of complement activation products C3a, C5a and membrane attack
complex C5b-9 were measured by ELISA. C3a ELISA (BD OptEIATm Human C3a ELISA
Kit,
BD Bioscience, Germany) was conducted according to the manufacturer
instruction. C5b-9
concentration was determined using the C5b-9 ELISA validated by InflaRx based
on the BD
OptEIAlm Human C5b-9 ELISA Set (BD Bioscience). C5a concentration was measured
using
the C5a ELISA established and validated by InflaRx described above.
1.7 Statistical analysis
All results were expressed as the mean standard deviation. Statistical
differences
between groups, after baseline correction, were calculated by One-Way-ANOVA,
including
Tukey's multiple comparison test or by the students t-test for two groups. The
p value of 0.05
was used in the calculation to determine whether there were any significant
differences between
any two groups. Creating of graphs and statistical analysis were performed
with GraphPad
PRISM V6.05 (CA, USA).
CA 03066689 2019-12-09
WO 2018/234118 48
PCT/EP2018/065676
2. PRECLINICAL RELEVANT DATA
2.1 Neutrophils activation by C5a and the blocking effect of IFX-1
As CD lb up-regulation is a sensitive hallmark for neutrophil activation, CD1
lb levels
on neutrophils were employed to evaluate the neutrophil activation. The human
whole blood
model was used to assess the blocking activity of IFX-1 to recombinant human
C5a (rhC5a) in
this study. Human whole blood was incubated with buffer, antibody alone, rhC5a
alone, or
combinations of different antibody concentration and rhC5a. After incubation,
cells were
stained with anti- mouse CD11b:FITC and CD11b MFI was analysed by flow
cytometry
checking for the activation levels of blood neutrophils. As shown in Figure 1,
recombinant
.. human C5a strongly stimulates the CD1 lb up-regulation on human
neutrophils. This effect can
be completely blocked in presence of the anti-human C5a antibody IFX-1. This
inhibition is
highly specific and the irrelevant human IgG4 antibody did not show any
blocking activity.
As a source for endogenous C5a (eC5a), zymosan-activated plasma (ZAP) was used
to
stimulate the blood neutrophils. The amount of eC5a in ZAP was measured using
a commercial
C5a ELISA Kit. The data presented here (Figure 2) point out that eC5a in ZAP
induced
comparable levels of CD1lb up-regulation to rhC5a. The presence of IFX-1
significantly
decreased the CD1 lb expression on human neutrophils, even at an Ab:Ag molar
ratio of 0.5:1.
The overall blocking activity of IFX- 1 to ZAP-induced CD1 lb up-regulation
ranged from
100% to 82% depending on the Ab:Ag ratio. Despite the presence of high levels
of eC3a and
other complement activation products in ZAP, IFX-1 could specifically block
CD1lb
upregulation =up to 100%. It can therefore be concluded that eC5a is the sole
driver for
neutrophil activation upon ZAP stimulation, and IFX-1 can completely block it.
2.2 C5a blockade attenuates zymosan-induced inflammatory responses in
the human whole
blood
Zymosan A, as an active fungus cell wall component, can induce strong
inflammatory
responses in human whole blood as characterized by the activation of
neutrophils with elevated
cytokines and chemokines levels. In this study, human whole blood was spiked
with zymosan
A in the presence or absence of IFX-1, and CD1 lb expression on blood
neutrophils was
measured by flow cytometric analysis. As shown in Figure 3, CD! lb on blood
neutrophils was
strongly upregulated at the presence of zymosan in human whole blood. The
zymosan-
stimulated CD1lb upregulation can be suppressed by 79% ¨ 93% depending on the
concentration of IFX-1 added. As a positive control, the CD1 lb up-regulation
stimulated by
rhC5a was 100% blocked by IFX-1. Therefore, it is affirmative that the CD 1 lb
up-regulation
CA 03066689 2019-12-09
WO 2018/234118 49
PCT/EP2018/065676
on blood neutrophils upon zymosan A stimulation is caused primarily by eC5a.
In addition, it
can be concluded that eC5a, once generated in the whole blood by zymosan A,
binds to IFX-1
first, thereby blocking its access to its natural receptors.
In the same experimental set-up, IL-8 levels were measured and used to assess
the
inflammatory response. IL-8 concentrations after various doses of zymosan A
stimulation
ranged from 458 pg/ml to 3218 pg/ml in the absence of IFX-1. As shown in
Figure 4, the
presence of IFX-1 significantly reduced IL-8 generation upon stimulation with
various
concentrations of zymosan A and the reduction rate up to 54% was observed.
Thus, in the whole
blood setting of inflammation, zymosan-induced inflammatory responses are
largely dependent
on the presence of C5a.
3. CLINICAL RELEVANT DATA
3.1 DATA OBTAINED FROM CLINICAL SAMPLES
3.1.1 Complement activation in HS patients
A total of 54 patients with HS and 14 healthy volunteers were enrolled in the
study.
Patients are under follow-up in the Outpatient Department of Immunology of
Infectious
Diseases of the ATTIKON University Hospital, Greece. The study was approved by
the Ethics
Committee of the hospital. Written informed consent was provided by all
patients. Diagnosis
of HS was based on the following criteria: a) onset early after puberty; b)
presence of
subcutaneous nodules in areas of skin rich in apocrine glands; and c) a
compatible history of
recurrent drainage of pus from the affected areas.
Circulating concentrations of complement factors C3a and C5a as well as
membrane
attack complex sC5b-9 were determined in the plasma of 54 patients and of 14
healthy controls
as well as in the pus of seven patients. As shown in Figure 5, circulating C5a
was significantly
greater in patient plasma than in control plasma (P<0.01), and the differences
of C3a and C5b-
9 between patients and controls were of similar significance. Therefore, it
can be concluded
that systemic complement activation occurs in HS. Given the essential role of
complement
activation in the innate and adaptive immunity, the inventors assumed that
targeting of
complement activation could be a new therapeutic strategy for the treatment of
HS.
However, from the above results it was not clear which one of C3a, C5a or C5-
9b or
other complement activation products would be the most promising target for
this new
therapeutic strategy and whether it would be sufficient to target only one of
these factors or
whether two or more factors involved in complement activation have to be
targeted.
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
3.1.2 Blocking of CD1 1 b upregulation on blood neutrophils induced by HS
plasma
To determine the role of C5a in the HS plasma sample on the neutrophil
activation, the
HS plasma samples with high levels of C5a were chosen and assessed by
employing the human
whole blood model. As shown in Figure 6, in contrast to the control plasma
samples with low
5 C5a levels (Ctrl 008 and Ctrl 012), HS plasma samples (Pat. 088 and Pat.
092) with high levels
of C5a strongly upregulated CD1 lb expression on blood neutrophils.
Recombinant human C5a
was used as the positive control, while the plasma from healthy volunteers was
chosen as the
negative control. The CD1 lb upregulation induced by HS plasma can be 100%
suppressed by
IFX-1, indicating that C5a is the most important activator in the HS plasma to
initiate neutrophil
10 activation. From these novel results the inventors concluded that
blockade of C5a in HS patients
is sufficient to achieve a strong suppression on neutrophil activation.
3.2 DATA OBTAINED FROM CLINICAL TRIAL
3.2.1 Trial Design
15 An open label Phase II trial in 11 patients with moderate to severe
hidradenitis
suppurativa was conducted in Department of Internal Medicine, ATTIKON
University
Hospital, Greece.
Primary objective of the trial was to explore the safety and tolerability of
IFX-1
administered over 8 weeks. Secondary objectives of the trial were to assess
the
20 pharmacokinetics and pharmacodynamics of IFX-1 as well as to generate
preliminary data on
the efficacy of IFX-1 on clinical endpoints (e.g., HiSCR, DLQI, VAS for
disease status, VAS
for pain, HS-PGA, modified Sartorius Score) to generate further hypotheses.
The enrolled
patients were treated with 800 mg IFX-1 twice in the first week and once a
week thereafter for
the total 8-week treatment; i.e. IFX-1 was administered in nine intravenous
doses of 800 mg
25 11-X-1 on days 1, 4, 8, 15, 22, 29, 36, 43, and 50. All patients were
followed up for 12 additional
weeks.
Inclusion Criteria at Screening:
1. Male or female patients? 18 years old
30 2. Written informed consent
3. Diagnosis of HS for at least 1 year
4. HS lesions in at least 2 distinct anatomic areas, one of which is Hurley
Stage 11 or
III
5. Total AN (abscesses and nodules) count >3
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
51
6. Patients with either primary or secondary failure of biological treatment
or are not
eligible for treatment with other biologicals
NOTE: a primary failure is defined as an at least 12 week treatment with a
biological
compound without effect and a secondary failure as achieving an initial
response
after at least 12 week treatment with a biological compound followed by a
relapse.
7. Failure of previous antimicrobial treatments
Exclusion Criteria at Screening:
1. Body weight above 150 kg or body weight below 60 kg
2. Has a draining fistula count of greater than 30 at baseline
3. Surgical management planned within the next 24 weeks
4. Occurrence of a flare-up of HS leading to intravenous antimicrobial
treatment within
the last 14 days
5. Any other disease and condition that is likely to interfere with evaluation
of study
product, outcome assessment or satisfactory conduct of the study
a) Active infection
b) Severe congestive heart failure (i.e., NYHA Class IV)
c) Depression
d) History of systemic lupus erythematosus or rheumatoid arthritis
e) Any immunodeficiency disease
I) Active hematological or solid malignant tumor
g) Patients must not have had any other active skin disease or condition
(e.g.,
bacterial, fungal, or viral infection) that may have interfered with
assessment of
HS.
6. One of the following abnormal laboratory results
a) White blood cell count < 2,500/mm3
b) Neutrophil count < 1000/mm3
c) Serum creatinine > 3 x Upper Normal Limit (UNL)
d) Total bilirubin >2 x UNL
e) Alanine-AminotTansferase (ALAT) > 2x UNL
f) Positive screening test for Hepatitis B, Hepatitis C, or HIV 1/2
7. Prior administration of any biological compound in the last 3 months
8. Intake of corticosteroids defined as daily intake of prednisone or
equivalent more
than 1 mg/kg for the last three weeks;
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
52
9. Intake of immunosuppressive drugs within the past 30 days (e.g.,
cyclosporine,
tacrolimus)
10. General exclusion criteria
a) Pregnant (in women of childbearing potential an urine pregnancy test has to
be
performed) or breast-feeding women
b) Women with childbearing potential (defined as within two years of their
last
menstruation) not willing to practice appropriate contraceptive measures
(e.g.,
implanon, injections, oral contraceptives, intrauterine devices, partner with
vasectomy, abstinence) while participating in the trial
c) Participation in any interventional clinical trial within the last three
months
d) Known intravenous drug abuse
e) Employee at the study site, spouse/partner or relative of any study staff
(e.g.,
investigator, sub-investigators, or study nurse) or relationship to the
sponsor
3.2.2 Clinical Trial Findings
IFX-1 is well tolerated by HS patients. There were no drug-related serious
adverse
events reported over the treatment period.
A commonly used efficacy parameter in the Hidradenitis Suppurativa Clinical
Response
(HiSCR). HiSCR is defined by the status of three types of lesions (defining
criteria): abscesses
(fluctuant, with or without drainage, tender or painful), inflammatory nodules
(tender,
erythematous, pyogenic granuloma lesion) and draining fistulas (sinus tracts,
with
communications to skin surface, draining purulent fluid). The proposed
definition of responders
to treatment (HiSCR achievers) is: (i) at least a 50% reduction in ANs, (ii)
no increase in the
number of abscesses, and (iii) no increase in the number of draining fistulas
from baseline.
HiSCR has been validated recently as a responsive and clinically meaningful
endpoint of the
inflammatory manifestation of HS (Kimball and others, 2014).
The HiSCR response over the treatment period of 8 weeks was investigated in
this study,
and 8 out of 11 patients already treated up to Day 56 responded, which
represents a response
rate of 72.7% and a 95% confidence interval of 43% to 91%. To compare these
results with
historical data a literature search was performed to detect placebo controlled
clinical studies
that used HiSCR as an efficacy parameter. The following Table 4 summarizes the
five studies
that were completed recently:
53
Table 4. Completed clinical studies using HiSCR as an efficacy parameter.
Compound N Placebo Comment
responder
n(%)
Adalimumabl 13 2 (15%) Post hoc analysis of Phase II trial.
Only subgroup of patients with Hurley III
Adalimumabl 70 15 (21%) Study313, subgroup of patients with
Hurley
In
Adalimumabl 76 13 (17%) Study810, subgroup of patients with
Hurley
IH
Anakinra2 10 3 (30%) All patients. 6 of 10 patients had
Hurley Ill
MABp13 10 1 (10%) Anti-TNFa treatment failures
Humira EMA assessment report:
_As ses sment_Report_-_Variation/human/000481AVC500195564.pdf
2 Anakinra Study (Tzanetakou and others, 2016).
3 Press release XBiotech
In total, 179 patients have been treated in the placebo group of these studies
with a
response rate of 19.0% with a 95%-confidence interval of 14% to 25%. As both
confidence
intervals (e.g., the historical placebo patients and the patients treated with
1FX-1) are not
overlapping, a significant treatment effect of IFX-1 can be concluded.
Photographic documentation of the affected areas confirmed these findings by a
highly
reduced inflammation on the skin, as evidenced by the visual reduction of
inflammatory
swollenness and redness post treatment.
Thus, anti-05a represents a powerful anti-inflammatory agent in the disease
setting of
HS. This clinical finding demonstrates that blockade of C5a is highly
effective to reduce the
activation of neutrophils thereby effectively alleviating cutaneous
neutrophilic inflammatory
disorders.
Date recue/Date received 2023-06-09
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
54
4. BLOCKING THE CD11B UPREGULATION INDUCED BY ACTIVATED COMPLEMENT
FACTOR IN HIDRADENITIS SUPPURATIVA PATIENTS VIA C5A-05AR AXIS INHIBITION
4.1 PURPOSE
The purpose of the following study was to demonstrate the blockade of
Hidradenitis
suppurativa (HS) patient plasma-induced CD1 lb upregulation on the surface of
neutrophils by
anti-human C5a monoclonal antibody IFX-1, anti-human C5a receptor C5aR (CD88)
antibodies, and a C5aR antagonist as well as a C5aR inhibitor.
4.2 ASSAY PRINCIPLE
Accumulation of neutrophils at the site of inflammation is dependent on the
expression
of adhesion molecules, including CD1 lb (also known as integrin alpha M)
(Larson and
Springer, 1990; Carlos and Harlan, 1990). Upregulation and mobilizing of CD1
lb/CD18 from
intracellular pools to the surface of neutrophils is essential for the rolling
action and migration
of human neutrophils (Smith et al., 1989). Enhanced expression of CD1 lb/CD18
therefore
reflects an inflammatory triggering event. The human CD 1 lb assay is
conducted using flow
cytometry to detect FITC-conjugated anti-CD 1 lb antibody on the surface of
neutrophils.
Activated complement products, especially elevated endogenous C5a (eC5a) in HS
patient
plasma samples can strongly upregulate CD1lb expression through the binding of
C5a to its
receptor C5aR (CD88) on neutrophils. Consequently, blockade of the C5a-05aR
axis is
expected to abolish or attenuate CD1 lb upregulation on the surface of
neutrophils.
As the first anti-human C5a monoclonal antibody introduced into clinical
development
IFX-1 has been demonstrated to control the inflammatory responses that lead to
tissue and organ
damage. This antibody is currently being evaluated in a Phase IIb study for
patients with
moderate or severe Hidradenitis suppurativa. It specifically and directly
neutralizes the
terminal complement anaphylatoxin C5a and blocks its harmful effects as the
key inflammatory
mediator in both acute and chronic inflammatory diseases (Klos et al., 2009;
Guo and Ward,
2005; Riedemann et al., 2017).
C5a exerts its effects through interacting with the high-affinity C5a
receptors (C5aR and
C5L2) (Guo and Ward, 2005). C5aR belongs to the rhodopsin family of G-protein-
coupled
receptors with seven transmembrane segments, while C5L2 is not G-protein-
coupled. It is
generally understood that C5a-05aR signaling is very important in the
pathogenesis of
proinflanunatory outcomes (Ward, 2009). Therefore, targeting the C5aR is
another strategy for
inhibiting complement-dependent inflammatory diseases. A series of small
molecules derived
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
from the C-terminus of C5a were developed as C5aR antagonists. Among them, the
lead
compound cyclic hexapeptide PMX-53 (AcF-PDP(D-Cha)WRD (Finch et al., 1999) was
shown
to attenuate injury in numerous animal models of inflammation following
intravenous,
subcutaneous, intraperitoneal, and oral administration (Proctor et al.
2006).With their structural
5
similarity to C5a, such antagonists compete with C5a for the C5a receptors on
neutrophils
(March et al., 2004). In addition, anti-05aR antibodies could block the
binding of C5a to C5aR,
thereby reducing the accumulation and activation of myeloid-derived suppressor
cells and
neutrophils (Markiewski et al., 2008). Two commercially available monoclonal
anti-05aR
antibodies were tested in this study, clones S5/1 and 7H110. They were raised
in mouse against
10 a
synthetic peptide comprising the N-terminal extracellular domain of C5aR (Metl-
Asn31) and
the recombinant human C5aR (Metl-Va1350), respectively, and both antibodies
were described
as neutralizing antibodies. Avacopan (CCX168) is an orally-administered small
molecule drug
candidate that selectively inhibits the complement C5a receptor (C5aR), and is
being developed
for inflammatory and autoimmune diseases (Bekker et al., 2008; Jayne et al.,
2017)
15
The inhibitory effect of these blocking agents that target the C5a-05aR axis
was
monitored via flow cytometry for the blockade of CD 1 lb upregulation on
neutrophils.
4.3 EXPERIMENTAL DETAILS
4.3.1 Samples
20
According to the consensus definition and diagnostic criteria of the
Hidradenitis
Suppurativa Foundation 2009, plasma samples of two HS patients (Pat. 088 &
Pat. 092) and
two healthy controls (Ctrl 009 & Ctrl 010) were included in this study. The
complement system
was activated in the pathogenesis of HS as manifested by the elevated levels
of C3a, C5a and
C5b-9 (see Table 5 below). The C5a levels of patients 088 and 092 (93.77 ng/mL
and 70.01
25
ng/mL, respectively) are significantly higher than those of healthy controls
(21.02 ng/mL and
11.77 ng/mL).
Table 5. Concentrations of complement factors measured in plasma samples of
the study
objects
= Patient ID Matrix C5a Ing/m11 C3a Wink]
C5b-9 [ngintil
Pat. 088 plasma 93.77 13177.50 >max
Pat. 092 plasma 70.01 8801.00 276.12
Ctrl 009 plasma 21.02 1818.68 122.76
Ctrl 010 plasma 11.77 2625.40 129.52
56
4.3.2 Reagents
= AnalaR water, VWR (Darmstadt, Germany), Cat. No. 102923C, NORMAPUR for
analysis,
sterile filtered
= ACD, Sigma Aldrich (Taufkirchen, Germany), Cat. No. C3821-50ML
= Reagents for flow cytometer
o FACS Flow Sheat Fluid, BD Bioscience (NJ, USA), Cat. No. 342003
o FACS Shutdown solution, BD Bioscience (NJ, USA), Cat. No. 334224
o FACS Clean solution, BD Bioscience (NJ, USA), Cat. No. 340345
o rat anti-mouse CD11b:FITC, BD Bioscience (NJ, USA) Cat. No. 553310, 0.5
mg/mL
o 10x FACS Lysing solution, BD Bioscience (NJ, USA), Cat. No. 349202
Working
solution: lx FACS Lysing solution (1:10 diluted in AnalaR water)
o Staining buffer: 1% heat-inactivated FBS + 0.1% sodium azide in lx PBS
solution
= 143S, Thermo Fisher Scientific (Darmstadt, Germany), Cat. No. 10099133
heat-inactivation: 56 C, 30 min
= PBS powder, Sigma Aldrich (Tauflcirchen, Germany), Cat. No. P3813-10PAK
= sodium azide, VWR (Darmstadt, Germany), Cat. No. 1.06688.0250
= recombinant human C5a (rhC5a), Hycult Biotech (Uden, Netherlands), Cat.
No. HC2101,
expressed in E. coli, dissolved in sterile AnalaR water
= 0.9% sterile sodium chloride (saline), B.Braun (Melsungen, Germany), Cat.
No. 3200950
= IFX-1, anti-human C5a antibody applied as control, InflaRx (Jena,
Germany), 10 mg/mL
TM
in PBS + 0.05% Tween80
= PMX-53, bio-techne (Wiesbaden-Nordenstadt, Germany), Cat. No. 5473
= anti-05aR (CD88) antibody Clone S5/1, Hycult Biotech (Uden, Netherlands),
Cat. No.
HIVI2094
= anti-05aR (CD88) antibody Clone 7H110, biomol (Hamburg, Germany), Cat.
No. C2439-
60N
= Avacopan, MedKoo Biosciences Inc. (Morrisville, USA), Cat. No. 319575
= human blood (immediate use) from healthy donor containing 12% ACD
= human plasma pool (citrate plasma) from Jena University Hospital.
4.3.3 Equipment
= Flow Cytometer (FACS Canto II with DIVA software V6.1.2)
Date recue/Date received 2023-06-09
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
57
4.3.4 Procedures
a) Human CD1 lb Potency Assay (Flow Cytometric Assay)
Two patient plasma samples Pat. 088 and Pat. 092 (5 L) were incubated with
fresh
human blood (60 L, as source of neutrophils) in the absence or presence of
C5a-05aR axis
blockers (anti-05a antibody IFX-1, anti-05aR antibodies clone S5/1 and clone
7H110, C5aR
antagonist PMX-53, and C5aR inhibitor Avacopan; 10 L) in a total volume of
100 L. Two
control plasma samples (Ctrl 009 and Ctrl 010, 5 L) prepared according to the
procedure
applied to the patient samples served as controls for unspecific activation.
Blood with only
saline (40 lit) or normal human plasma pool (huPP 5 L + saline 35 L) served
as the non-
stimulated control to define the baseline expression of CD1 lb. Blood sample
with normal
human plasma pool and spiked with recombinant human C5a (rhC5a) mimicked the
stimulated
condition. All samples were incubated at 37 C for 20 min to activate CD! lb
upregulation. After
cooling on ice, 2 .1., of FITC-conjugated anti-mouse CD1 lb antibody was
added to the samples.
The labeled samples were kept on ice in the dark for another 30-mM to minimize
the
background signal. Red blood cells were then lysed with lx FACS Lysing
solution at room
temperature for 10 min. 2 mL Staining buffer was used to wash the remaining
cells twice. After
centrifugation at 2500 rpm for 3 min, cells were resuspended in 0.5 rnL
Staining buffer and
ready for FACS analysis. A gate was set on the FSC vs. SSC plot to allow
analysis only of cells
with the size of neutrophils.
The percentage of blocking activity (BA) of the C5a-05aR axis blockade test
material
was calculated using the formula below. Where MFI is the mean fluorescence
intensity emitted
from the CD11b-bound FITC on neutrophils.
BA (%) = (MB Patient plasma ¨ MH Test material spiked patient plasma) (MFI
Patient plasma ¨ MB huPP) X 100
b) Statistical analysis
Graphs were created with GraphPad Prism 7 (CA, USA).
4.4 RESULTS AND DISCUSSIONS
Expression of Integrin CD1 lb on Neutrophils
CD1 lb expression on neutrophils was evaluated with plasma samples from two
healthy
blood donors (Ctrl 009 and Ctrl 010) and two diagnosed HS patients (Pat. 088
and Pat. 092).
The mean fluorescence intensity (MFI) of the Ctrl- samples was 2156.9 114.3,
which falls
within the non-stimulated CD1 lb baseline expression range (MFI < 3500). In
contrast, a 2.3 to
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
58
3.9-fold elevation of CD1lb expression was induced either by HS patient
samples (Pat. 088,
Pat. 092) or by 20 nM recombinant human C5a (rhC5a) (Figures 8, 9, 10 and 11;
Tables 6, 7
and 8). These data suggest that the significantly upregulated CD1lb expression
was mediated
by the inflammatory factors in HS patients. It is postulated that the
complement activation
products, especially C5a, play a major role in the CD1lb upregulation (Table
5).
Table 6. Blocking activity of anti-05aR antibodies clones S5/1 and 7H110, and
C5aR
antagonist PMX-53 on complement factor-induced CD1lb upregulation.
ID Description
CD1lb (mean MFI) Blocking activity (%)1
A Saline 2049.5
B1 huPP 2166.5
B2 huPP I Clone S5/1 (50 nM) 2247
B3 huPP I Clone 7H110 (50 nM) 2401
B4 huPP I PMX 53 (50 nM) 2053.5
D3 huPP I rhC5a (20 nM) 6329.5
E7 huPP I rhC5a I Clone S5/1 (50 nM) 3288.5 73.05
E8 huPP I rhC5a I Clone 7H110 (50 nM) 3385 70.73
E9 huPP I rhC5a I PMX 53 (50 nM) 4235.5 50.30
Cl Ctrl 010 2102.5
C2 Ctrl 009 2018.5
DI Pat. 088 7918
El Pat. 088 I Clone S5/1 (50 nM) 3736.5 72.70
E2 Pat. 088 I Clone 7H110 (50 nM) 3564.5 75.69
E3 Pat. 088 I PMX 53 (50 nM) 4728.5 55.46
D2 Pat. 092 7206.5
E4, Pat. 092 I Clone S5/1 (50 nM) 3555 75.86
ES Pat. 092 I Clone 7H110 (50 nM) 3393.5 78.67
E6 Pat. 092 I PMX 53 (50 nM) 4657.5 56.69
4õ
CA 03066689 2019-12-09
WO 2018/234118
PCT/EP2018/065676
59
Table 7. Blocking activity of anti-05a antibody IFX-1 and C5aR antagonist PMX-
53 on
complement factor-induced CD11b upregulation.
ID Description
CD1lb (mean MFI) BlueklAkactivity (%)-1
A Saline 2516.5
B1 huPP 2324.5
B2 huPP I PMX 53 (20 p,M) 2218.5
/77777
B3 huPP I IFX-1 (20 nM) 2352
1)3 huPP I rhC5a (20 nM) 8332.5
ES huPP I rhC5a I PMX 53 (20 M) 1851 107.88
E6 huPP I rhC5a I IFX-1 (20 nM) 2067 104.29
Cl Ctrl 010 2241
C2 Ctrl 009 2265.5
D1 Pat. 088 8955
El Pat. 0881 PMX 53 (20 j.tM) 2027.5 104.48
E2 Pat. 088 I IFX-1 (20 nM) 2209 101.74
1)2 Pat. 092 7120.5
E3 Pat. 092 I PMX 53 (20 M) 1912.5 108.59
E4 Pat. 092 I IFX-1 (20 nM) 2147 103.70
Inhibition of CD1 lb upregulation by anti-human C5aR monoclonal antibodies,
C5aR
antagonist and C5aR inhibitor
As shown in Figure 8, rhC5a (20 nM) and two HS patient plasma samples with
high
levels of eC5a (Pat. 088 and Pat. 092) strongly upregulated CD1lb expression
on blood
neutrophils, while the presence of blocking antibodies targeting C5aR with a
final concentration
of 50 nM could significantly attenuate CD1 lb expression driven by either
rhC5a or HS patient
plasma samples. High blocking activities ranging from 71% to 79% were achieved
by using
anti-05aR antibodies, clone 7H110 and clone S5/1 (Figure 8, Tables 9 and 6).
In contrast, the
C5aR antagonist PMX-53, which is a small hexapeptide, was not as effective as
the C5aR-
specific monoclonal antibodies. The blocking activities rendered by the same
concentration of
PMX-53 (50 nM) were within 50% to 57% (Figure 9A, Tables 9 and 6). To examine
whether
abolishment of CD 1 lb upregulation can be achieved via C5aR inhibition, a
high concentration
of PMX-53 (20 iiM) was further evaluated for the blocking activities under the
same
experimental set-up. As a result, the CD1 lb upregulation induced by HS
patient plasma samples
CA 03066689 2019-12-09
WO 2018/234118 60
PCT/EP2018/065676
as well as by rhC5a was completely abrogated in the presence of high levels of
PMX-53 (Figure
9B, Tables 9 and 7). Similar data were achieved in the presence of Avacopan, a
C5aR inhibitor
(Figure 11, Tables 8 and 9). The blocking activities in the presence of 100
iiM Avacopan were
within 77% to 80%, whereas the CD1lb upregulation induced by HS patient plasma
was
completely blocked in the presence of 500 gM Avacopan (Figure 11, Tables 8 and
9).
These results suggested that the C5a/C5aR axis is predominantly responsible
for the
CD1 lb upregulation on blood neutrophils, and that C5aR can serve as a
potential target for
blocking C5a activities.
Table 8. Blocking activity of C5aR inhibitor Avacopan on complement factor-
induced
CD1lb upregulation.
ID Description
CIAO (meakN1FJ) Blocking activity (%
A Saline 3767
131 huPP 3407
132 Ctrl 010 3208.5
B3 Ctrl 009 3125.5
ClPat. 088 11717.5
DI Pat. 088 I Avacopan (500 M) 3027 104.57
D2 Pat. 088 I Avacopan (100 i.tM) 5289 77.35
C2 Pat. 092 11214.5
D3 Pat. 092 I Avacopan (500 iuM) 3081.5 104.17
1)4 Pat. 092 I Avacopan (100114) 5000 79.60
6, 1,1A
CA 03066689 2019-12-09
WO 2018/234118 61
PCT/EP2018/065676
Table 9. Summarized blocking activities (%) of all C5a-05aR axis blockade test
molecules
on CD11b upregulation induced by HS patient plasma- or rhC5a-spiked healthy
human
plasma.
Test Material Target Blocking Activity (%)
Pat. 088 101.74
IFX-1
C5a Pat. 092 103.70
20 nM
rhC5a-spiked huPP 104.29
Pat. 088 72.70
Clone S5/1
C5aR Pat. 092 75.86
50 nM
rhC5a-spiked huPP 73.05
Pat. 088 75.69
Clone 7H110
C5aR Pat. 092 78.67
50 nM
rhC5a-spiked huPP 70.73
Pat. 088 55.46
PMX-53
C5aR Pat. 092 56.69
50 nM
rhC5a-spiked huPP 50.30
Pat. 088 104.48
PMX-53
C5aR Pat. 092 108.59
20 pM
rhC5a-spiked huPP 107.88
Avacopan Pat. 088 77.35
C5aR
100 pM Pat. 092 79.60
Avacopan Pat. 088 104.57
C5aR
500 pM Pat. 092 104.17
lFX-1. the anti-human C5a monoclonal antibody, blocked the HS-induced CD1lb
upregulation completely
By employing the same experimental set-up described above, as would be
expected,
rhC5a and HS patient plasma samples (Pat. 088 and Pat. 092) strongly activated
CD1lb
expression on blood neutrophils, and addition of IFX-1 with a concentration as
low as 20 nM
could completely abolish the CD1 lb upregulation (Figure 10). These results
indicate that IFX-
1 is more efficient in inhibiting C5a/C5aR-driven inflammatory responses.
CA 03066689 2019-12-09
WO 2018/234118 62
PCT/EP2018/065676
4.5 CONCLUSION
Taken together, our results show that the C5a-targeted approach, i.e. the
application of
anti-05a monoclonal antibody IFX-1 abolishes C5a-mediated CD1 lb upregulation
effectively.
Furthermore, the anti-05aR antibodies, the C5aR antagonist as well as the C5aR
inhibitor also
exhibited a strong blocking activity under the same experimental conditions.
Thus, targeting
C5aR with blocking antibodies or antagonists represents an alternative
strategy to block
C5a/C5aR axis under inflammatory conditions such as HS.
REFERENCES
Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. 2011. Mouse neutrophils are
professional antigen-presenting cells programmed to instruct Thl and Th17 T-
cell
differentiation. Int Immunol 23(5):317-326.
Bekker, P. et al. Characterization of Pharmacologic and Pharmacokinetic
Properties of
CCX168, a Potent and Selective Orally Administered Complement 5a Receptor
Inhibitor, Based on Preclinical Evaluation and Randomized Phase 1 Clinical
Study.
PLoS One 11, e0164646, doi:10.1371/journal.pone.0164646 (2016).
Braun-Falco M, Kovnerystyy 0, Lohse P, Ruzicka T. 2012. Pyoderma gangrenosum,
acne, and
suppurative hidradenitis (PASH)--a new autoinflammatory syndrome distinct from
PAPA syndrome. J Am Acad Dermatol 66(3):409-415.
Carlos, T. M. & Harlan, J. M. Membrane proteins involved in phagocyte
adherence to
endothelium. Immunol Rev 114, 5-28 (1990).
Cugno M, Borghi A, Marzano AV. 2017. PAPA, PASH and PAPASH Syndromes:
Pathophysiology, Presentation and Treatment. Am J Clin Dermatol.
Cugno M, Gualtierotti R, Meroni PL, Marzano AV. 2018. Inflammatory Joint
Disorders and
Neutrophilic Dermatoses: a Comprehensive Review. Clinic Rev Allerg Immunol
54:269-281, doi:10.1007/s12016-017-8629-0
Czermak BJ, Sarma V. Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H,
Friedl
HP, Ward PA. 1999. Protective effects of C5a blockade in sepsis. Nat Med
5(7):788-
Finch, A. M. et al. Low-molecular-weight peptidic and cyclic antagonists of
the receptor for
the complement factor C5a. J Med Chem 42, 1965-1974, doi:10.1021/jm9806594
(1999)
Guo RF, Riedemann NC, Sun L, Gao H, Shi KX, Reuben JS, Sarma VJ, Zetoune FS,
Ward PA.
2006. Divergent signaling pathways in phagocytic cells during sepsis. J
Immunol
177(2):1306-1313.
Guo RF, Ward PA. 2005. Role of C5a in inflammatory responses. Annu Rev Immunol
23:821-
852, doi:10.1146/annurev.immuno1.23.021704.115835 (2005).
Huber-Lang MS, Sarma JV, McGuire SR, Lu KT, Guo RF, Padgaonkar VA, Younkin EM,
Laudes II, Riedemann NC, Younger JG and others. 2001. Protective effects of
anti-05a
peptide antibodies in experimental sepsis. FASEB J 15(3):568-570.
Huber-Lang MS, Younkin EM, Sarma JV, McGuire SR, Lu KT, Guo RF, Padgaonkar VA,
Curnutte JT, Erickson R, Ward PA. 2002. Complement-induced impairment of
innate
immunity during sepsis. J Immunol 169(6):3223-3231.
Jayne, D. R. W. et al. Randomized Trial of C5a Receptor Inhibitor Avacopan in
ANCA-
Associated Vasculitis. J Am Soc Nephrol 28, 2756-2767,
doi:10.1681/ASN.2016111179 (2017).
CA 03066689 2019-12-09
WO 2018/234118 63
PCT/EP2018/065676
Jemec GB. 2004. Medical treatment of hidradenitis suppurativa. Expert Opin
Pharmacother
5(8):1767-1770.
Jemec GB, Heidenheim M, Nielsen NH. 1996. The prevalence of hidradenitis
suppurativa and
its potential precursor lesions. J Am Acad Dermatol 35(2 Pt 1):191-194.
Kaplan MJ. 2013. Role of neutrophils in systemic autoimmune diseases.
Arthritis Res Ther
15(5):219.
Kimball AB, Jemec GB, Yang M, Kageleiry A, Signorovitch JE, Okun MM, Gu Y,
Wang K,
Mulani P, Sundaram M. 2014. Assessing the validity, responsiveness and
meaningfulness of the Hidradenitis Suppurativa Clinical Response (HiSCR) as
the
clinical endpoint for hidradenitis suppurativa treatment. Br J Dermatol
171(6):1434-
1442.
Klos, A. et al. The role of the anaphylatoxins in health and disease. Mol
Immunol 46, 2753-
2766, doi:10.1016/j.molimm.2009.04.027 (2009).
Kurzen H, Kurokawa I, Jemec GB, Emtestam L, Sellheyer K, Giamarellos-
Bourboulis EJ, Nagy
I, Bechara FG, Sartorius K, Lapins J and others. 2008. What causes
hidradenitis
suppurativa? Exp Dermatol 17(5):455-456; discussion 457-472.
Larson, R. S. & Springer, T. A. Structure and function of leukocyte integrins.
Immunol Rev
114, 181-217 (1990).
Lima AL, Karl I, Giner T, Poppe H, Schmidt M, Presser D, Goebeler M, Bauer B.
2016.
Keratinocytes and neutrophils are important sources of proinflammatory
molecules in
hidradenitis suppurativa. Br J Dermatol 174(3):514-521.
March, D. R. et al. Potent cyclic antagonists of the complement C5a receptor
on human
polymorphonuclear leukocytes. Relationships between structures and activity.
Mol
Pharmacol 65, 868-879, doi:10.1124/mo1.65.4.868 (2004).
Markiewski, M. M. et al. Modulation of the antitumor immune response by
complement. Nat
Immunol 9, 1225-1235, doi:10.1038/ni.1655 (2008).
Marzano AV. 2016. Hidradenitis suppurativa, neutrophilic dermatoses and
autoinflammation:
what's the link? Br J Dermatol 174(3):482-483.
Marzano AV, Ceccherini I, Gattomo M, Fanoni D, Caroli F, Rusmini M, Grossi A,
De Simone
C, Borghi OM, Meroni PL and others. 2014. Association of pyoderma gangrenosum,
acne, and suppurative hidradenitis (PASH) shares genetic and cytokine profiles
with
other autoinflammatory diseases. Medicine (Baltimore) 93(27):e187.
Nemeth T, Mocsai A. 2012. The role of neutrophils in autoimmune diseases.
Immunol Lett
143(1):9-19.
Nemeth T, Mocsai A, Lowell CA. 2016. Neutrophils in animal models of
autoimmune disease.
Semin Immunol 28(2):174-186.
Pawaria S, Ramani K, Maers K, Liu Y, Kane LP, Levesque MC, Biswas PS. 2014.
Complement
component C5a permits the coexistence of pathogenic Th17 cells and type I TEN
in
lupus. J Immunol 193(7):3288-3295.
Prat L, Bouaziz JD, Wallach D, Vignon-Pennamen MD, Bagot M. 2014. Neutrophilic
dermatoses as systemic diseases. Clin Dermatol 32(3):376-388.
Proctor, L. M., Woodruff, T. M., Sharma, P., Shiels, I. A. & Taylor, S. M.
Transdermal
pharmacology of small molecule cyclic C5a antagonists. Adv Exp Med Biol 586,
329-
345, doi:10.1007/0-387-34134-X_22 (2006).
Revuz J. 2009. Hidradenitis suppurativa. J Eur Acad Dermatol Venereol
23(9):985-998.
Riedemann NC, Guo RF, Neff TA, Laudes IJ, Keller KA, Sarma VJ, Markiewski MM,
Mastellos D, Strey CW, Pierson CL and others. 2002. Increased C5a receptor
expression
in sepsis. J Clin Invest 110(1):101-108.
Riedemann, N. C. et al. Controlling the anaphylatoxin C5a in diseases requires
a specifically
targeted inhibition. Clin Immunol 180, 25-32, doi:10.1016/j.clim.2017.03.012
(2017).
CA 03066689 2019-12-09
WO 2018/234118 64
PCT/EP2018/065676
Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune
FS, Gerard
NP, Cianflone K, Kohl J and others. 2008. Functional roles for C5a receptors
in sepsis.
Nat Med 14(5):551-557.
Slade DE, Powell BW, Mortimer PS. 2003. Hidradenitis suppurativa: pathogenesis
and
management. Br J Plast Surg 56(5):451-461.
Smith, C. W., Marlin, S. D., Rothlein, R., Toman, C. & Anderson, D. C.
Cooperative
interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in
facilitating
adherence and transendothelial migration of human neutrophils in vitro. J Clin
Invest
83, 2008-2017, doi:10.1172/JCI114111 (1989).
Strainic MG, Shevach EM, An F, Lin F, Medof ME. 2013. Absence of signaling
into CD4(+)
cells via C3aR and C5aR enables autoinductive TGF-betal signaling and
induction of
Foxp3(+) regulatory T cells. Nat Immunol 14(2):162-171.
Tzanetakou V. Kanni T, Giatrakou S, Katoulis A, Papadavid E, Netea MG,
Dinarello CA, van
der Meer JW, Rigopoulos D, Giamarellos-Bourboulis EJ. 2016. Safety and
Efficacy of
Anakinra in Severe Hidradenitis Suppurativa: A Randomized Clinical Trial. JAMA
Dermatol 152(1):52-59.
Ward PA. 2009. Functions of C5a receptors. J Mol Med (Berl) 87(4):375-378,
doi:10.1007/s00109-009-0442-7 (2009).
Wollina U, Koch A, Heinig B, Kittner T, Nowak A. 2013. Acne inversa
(Hidradenitis
suppurativa): A review with a focus on pathogenesis and treatment. Indian
Dermatol
Online J 4(1):2-11.
Xu R, Wang R, Han G, Wang J, Chen G, Wang L, Li X, Guo R, Shen B, Li Y. 2010.
Complement C5a regulates IL-17 by affecting the crosstalk between DC and
gammadelta T cells in CLP-induced sepsis. Eur J Immunol 40(4):1079-1088.