Note: Descriptions are shown in the official language in which they were submitted.
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Topology Guided Ocular Lens Design
Cross Reference to Related Applications
This application claims priority to a co-pending U.S. Patent Application Ser.
No. 15/634,631
entitled "TOPOLOGY GUIDED OCULAR LENS DESIGN" filed June 27, 2017, the entire
contents of which are hereby incorporated by reference.
Background
Scleral lenses have been used to restore sight to those with injured or
diseased corneas and to
relieve discomfort from dry eye disorders. The incidence of dry eyes in the
general population is
estimated to be 15%, of which nearly 2 in 10 have symptoms severe enough to
significantly
impact their quality of life. Globally, this corresponds to 3% of the
worldwide population and
approximately 9,240,000 severe dry eye patients in the United States alone.
In addition, there are millions of people whose eyes are not normally dry but
feel dry after
wearing conventional contact lenses for an extended period of time.
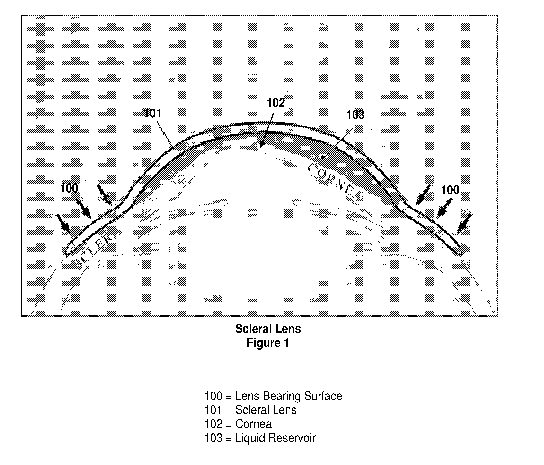
A scleral lens is a large contact lens that rests on the white scleral region
of the eye and is vaulted
over the cornea as shown in Figure 1. The gap 103 between the back-interior
surface of the lens
and cornea is typically filled with saline solution which acts like a liquid
bandage to soothe the
thousands of nerves on the corneal surface. In some applications, medication
can be added to, or
replace, the saline solution to assist in healing of an injured eye.
To ensure that the lens does not irritate the nerves on the scleral surface,
the shape of the bearing
surface 100, shown in Figure 1, must match the unique three-dimensional shape
of the patient's
sclera, including the regions normally covered by the eyelids.
1
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Unfortunately, there is currently no way to precisely measure scleral shape.
As a result, to find a
comfortable fitting lens, scleral lenses are manually selected from a set of
up to 2000 trial lenses
to find a suitable fit to the patient's scleral surface. This is an iterative,
expensive, and time-
consuming process which can take several weeks. If a close-fitting trial lens
can be found,
frequently it must be further modified to optimize fit.
If the patient has an abnormally shaped eye, due to an injury or disease for
example, as shown in
Figures 2a, 2b, 2c, 2d, fitting may not be possible because there is no trial
lens that conforms to
the shape of the irregular shaped bearing surface.
There is also a category of smaller diameter scleral lenses whose bearing
surface lies on both
sides of the limbus straddling the sclera and outermost regions of the cornea.
For injured eyes,
these lenses may be even more difficult to fit because they must conform to
injuries in both the
corneal and scleral regions of the bearing surface, as shown in Figures 2c and
2d.
Assuming a well-fitting trial lens can be found, the next step in the prior
art approach is to
determine the optical properties of the vaulted optics that needs to lie in
front of the patient's
cornea to properly focus light onto the retina.
It is important to emphasize that while a trial lens has no vision correction
optics, it must be
placed on the patient's eye and worn to enable design of the optics because
the fluid (typically
saline) that lies between the back surface of the scleral lens and front
surface of the cornea, alters
how light rays are bent at both the fluid-cornea and fluid-back- scleral-lens
boundaries.
With the trial lens now in place, the doctor or eye care practitioner performs
an optical refraction
(i.e. places different known lenses in front of the trial scleral lens) to
determine the optical power
of the scleral lens optics.
2
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Once the refraction is completed, then knowing the required optical power and
the bearing
surface shape of the best fitting trial lens, a patient specific custom
scleral lens can now be
manufactured.
A prior art attempt to measure scleral shape without iteratively interchanging
trial lenses is
described by Gemoules, U.S. Patent 7,862,176 B2 entitled "Method of Fitting
Rigid Gas-
permeable Contact Lenses from High Resolution Imaging". Gemoules' fitting
method is based
on using a digital acquisition device to acquire a two-dimensional cross
sectional sagittal image of
the eye which includes the sclera, as shown in Figure 3a. However, the eye is
not two
dimensional in shape, it is three-dimensional, as shown in Figures 5a, 5b and
5c, so a cross
sectional image is a poor approximation to a three-dimensional shape. This
limitation is further
illustrated by the injured eye shown in Figure 3b. Figure 3b shows multiple
independent
meridians in a quadrant over an injured region to enable the back-lens surface
to better conform to
eye surface topology. Each radial meridian can have different independent
spatial Z height values.
The cross-sectional sagittal image shown in Figure 3a could easily correspond
to a scan taken
across line 301-307 in Figure 3b, which does not reveal the presence of the
injury shown by scan
lines 302, 303,304, and 305, such scan lines also referred to as meridians. In
addition, and while
not addressed by Gemoules, attempts to approximate the three-dimensional shape
by acquiring
multiple independent two-dimensional scans around the eye has failed in the
past because the
spatial position of the eye moves between scans.
Svochak, U.S. Patent 7,296,890 B2 entitled "Contact Lens with Controlled
Shape," presents
means for creating a contact lens that sits on the cornea and whose back-
surface shape is defined
by four (4) base curves, effectively one curve per quadrant. This technique
for designing a scleral
lens bearing surface has multiple limitations. First, it cannot conform to
small injuries, protrusions
or irregular shapes within a region of a generally different shape, as shown
in Figures 2a, 2b, 2c,
and 2d herein. Second, the base curve of the cornea is almost always different
from that of the
sclera with the demarcation point being the limbus. A scleral lens that
straddles both regions must
conform to this complex change in curvature across the region boundaries (as
illustrated in Figure
at arrow 1003) and Svochak is only concerned with lenses conforming to the
cornea. Third, the
four-base-curve solution cannot follow all possible three-dimensional topology
changes in an eye.
3
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
If an eye or optimized well-fitting lens requires more than 4 base curves to
define its shape, as for
the injured eyes in Figures 2a-d, Svochak' s method is not applicable.
Sindt, U.S. Patent 9,551,885 B2 entitled "Prosthetic Lenses and Methods of
Making the Same"
describes methods of applying a foreign material to the surface of an eye to
obtain a physical
impression thereof. The impression is then used to determine the back surface
of a lens. This
procedure is highly invasive and may not be well tolerated by patients with
sensitive eyes.
4
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Summary of Preferred Embodiments
As a result, there is a need for a non-invasive method to design and
manufacture a custom fitting
scleral lens shaped to the surface of a patient's eye.
Given the limitations of the prior art, a first method is described to enable
the design of a scleral
lens bearing surface so that it follows the actual three-dimensional shape of
the sclera, without
the need of using trial lenses. The lens bearing or back surface that rests on
the eye is described
by a three-dimensional array of data points each representing an independently
measured x, y, z
location on the surface of the eye. This new capability is applicable for the
design of any
scleral lens, independent of whether the lens is to relieve dry eye symptoms
or to restore sight to
patients with injured or diseased corneas. Unlike Gemoules, who uses one
sagittal image to
create the back surface of the lens, or Svochak, who uses four base curves to
create the lens back
surface, in the approach described herein every data point on the lens bearing
surface can
correspond to a uniquely measured three-dimensional x, y, z value on the
patient's eye. Unlike
the prior art, lens design is not limited to four base curves, one per
quadrant, and the maximum
number of radial meridians used to design the lens is limited only by the
spatial-resolution of the
topographer and each meridian can be, and typically will be, different from
each other, as shown
in Figure 3b. Figure 3d is an actual three-dimensional, high-resolution, high
density scan of a
patient's eye showing the three-dimensional array of independently measured
data points on the
surface of the eye and the ability to conform to fine surface detail. Figure
3d was obtained using
the Bishop topographer shown in Figure 4. Figure 3c shows how this data can be
used to make a
contact lens whose back surface is shaped to the unique topology of a
patient's eye.
A second method is described to enable the design of just the scleral lens
optics without needing
to perform a refraction with a scleral lens placed on the eye. This is
applicable for those patients
that already have good vision, or use eyeglasses or contacts to obtain good
vision and want to
wear a scleral lens to relieve dry eye symptoms or for any other reason.
A third method is described is to enable the design of the entire scleral
lens, including the
bearing surface and optics without the need of a trial lens. In this
implementation, the bearing
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
surface of the patient specific scleral lens follows the true three-
dimensional shape of the
patient's eye without requiring the use of a trial lens to determine this
shape. This is applicable
for those patients that already have good vision, or use eyeglasses or
contacts to obtain good
vision and want to wear a scleral lens to relieve dry eye symptoms or for any
other reason.
A fourth method enables the 3D printing of a lens designed using the methods
described.
6
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Brief Description of the Drawings
Figure 1 shows the cross section of a scleral lens placed on an eye;
Figure 2a to 2d show injuries on the eye;
Figure 3a is a cross-sectional sagittal image of an eye;
Figure 3b is an image of an injured eye with multiple independent meridians in
a quadrant;
Figure 3c is a cross section of a lens showing that the front and back
surfaces can be
independently designed;
Figure 3d is a motion compensated scan of a human eye acquired by the Bishop
scanner;
Figure 4 is a picture of the Bishop Topographer;
Figure 5a is a three-dimensional model of a human eye, showing the front view;
Figure 5b is a three-dimensional model of a human eye, showing the side view;
Figure 5c is a three-dimensional topology map of a human eye, showing the
front view;
Figures 6, 7a and 7b show multiple gaze images of an eye stitched together to
create a
topology map and three-dimensional model of the entire eye;
Figure 8a shows central corneal and scleral data superimposed on a scanned
eye;
Figure 8b shows central corneal and scleral data superimposed on a topology
map of an eye;
Figure 9a shows the selected bearing surface of the eye superimposed on the
three-
dimensional model;
Figure 9b shows the selected bearing surface of the eye superimposed on the
topology map;
Figure 10a shows the central corneal surface of the eye superimposed on the
three-
dimensional model;
Figure 10b shows the central corneal surface of the eye superimposed on the
topology map;
Figure 10c is the side view of an eye model showing the lens bearing surface
shaped to the
topology of the eye;
Figure 11 shows a scleral lens with vaulted optics, transition region, and
bearing surface;
Figure 12a shows an eye with corrective eyeglasses;
Figure 12b shows a scleral lens placed on the eye;
Figure 13a shows optical rays traveling through eyeglasses into an eye;
Figure 13b shows optical rays traveling through a scleral lens into an eye;
Figure 14a and 14b shows relationship between eyeglass and scleral lens
optical rays;
7
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Figure 15 shows the visual field of an eye at a fixed gaze;
Figure 16 describes Snell's Law;
Figure 17 is an illustration of Snell's Law at air-cornea boundary;
Figure 18 is an illustration of Snell's Law at saline-cornea boundary;
Figures 19a and 19b show the first and second computer model required to
design scleral
lens optics;
Figure 20 is an enlarged section of FIG. 19b showing optical rays close to
eye;
Figure 21 is an enlarged superposition of FIG. 19a and 19b, showing the
relationship
between optical rays close to the eye;
Figure 22a is the three-dimensional representation of FIG. 19a;
Figure 22b is the three-dimensional representation of FIG. 19b;
Figure 22c is the superposition of FIG. 22a and FIG. 22b, showing the
relationship between
optical rays;
Figure 23a shows optical rays traveling through air into an eye;
Figure 23b shows optical rays traveling through a scleral lens into an eye;
Figure 24a and 24b shows relationship between optical rays with and without a
scleral lens
on the eye;
Figure 25a and 25b show the first and second computer model required to design
scleral lens
optics;
Figure 26 is an enlarged superposition of FIG. 25a and 25b, showing the
relationship
between optical rays close to the eye;
Figure 27a is the three-dimensional representation of FIG. 25a;
Figure 27b is the three-dimensional representation of FIG. 25b;
Figure 27c is the superposition of FIG. 27a and FIG. 27b, showing the
relationship between
optical rays; and
Figure 28 is a block diagram of a preferred embodiment of a system that may be
used to
implement the methods described herein.
8
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Detailed Description of the Preferred Embodiment
Historically, the three-dimensional shape of the scleral lens bearing surface
was obtained through
the use of trial lenses, as described above. However, new advances in eye
topology scanners
now provide a way to directly measure three-dimensional scleral shape.
Topographers such as,
for example, the one developed by Bishop et al., U.S. Patents 9,398,845 and
9,489,753 (both of
which are incorporated by reference herein), enable all regions of the sclera
to be scanned in
three dimensions, including the regions normally covered by the eyelids. A
view of the Bishop
Topographer is shown in Figure 4, and more technical details concerning its
operation are
contained in the referenced patents. Furthermore, the Bishop Topographer
compensates for eye
motion during all scanning operations. Figure 5 shows the three-dimensional
topology scan of a
human eye obtained using the Bishop Topographer. To expose the upper and lower
scleral
regions, a speculum was used to hold the eyelids open as the eye was scanned.
Figure 5a shows
the resulting front view, Figure 5b the resulting side view, and Figure Sc a
resulting contour map
with intensity proportional to height. Figures 5a, b, c were all generated
from a single 3 second
scan of the eye.
If for any reason, it is not desirable to use a speculum, the Bishop
Topographer can also acquire
and stitch together multiple scans of the eye, each acquired with the eye at a
different gaze to
expose a different region of the sclera, as shown in Figure 6. The topographer
can then combine
the scans into a single three-dimensional model from which the bearing surface
can be extracted.
The scanner compensates for all motion of the eye during and between scans.
Figure 7a shows
the height contour map and Figure 7b shows the three-dimensional model
generated by stitching
together the gaze scans shown in Figure 6.
For the purpose of the following discussion, we will use the three-dimensional
model of an eye
and the topology map obtained using a speculum with the Bishop Topographer
(U.S. Patent
9,398,845) as shown in Figures 5 through 12. However, any topographer, whether
it uses light
triangulation, light interference, OCT technology, pattern projection,
interferometry, or any other
means to scan the eye that enables the scleral lens bearing region to be
scanned in three
9
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
dimensions, without introducing artifacts due to eye motion, can be used to
implement the
methods and systems described herein.
To determine the scleral lens bearing surface the following steps are
performed:
1. Use an ocular topographer, such as the Bishop Topographer, to measure the
three-
dimensional topology of the patient's eye in the region that will be used to
design the
back surface of the lens. Figures 8a and 8b are an example of the bearing
region being in
the sclera as shown by arrow 801 in Figure 8a and arrow 802 in Figure 8b. The
topology
information, obtained as a set of data points, provided must be free from
motion blur to
represent the true shape of the eye. As shown in Figures 8a and 8b, each data
point
represents an independently measured x, y, z location on the surface of the
eye.
2. Define the location, width, and size of the lens bearing surface on the
eye. As an
example, Figures 9a and 9b show the bearing surface residing in the sclera as
indicated
by arrow 901 in Figure 9a, arrow 902 in Figure 9b and arrow 1001 in Figure
10c.
However, the bearing surface can also straddle the cornea and sclera or
contact the eye in
any location. Create the back surface of the lens from the bearing surface
information, so
that it follows the actual three-dimensional topology of the eye with a high
density of
sample points within the entire 360 degree bearing region, as shown in Figures
9a and 9b.
This method of design yields a back surface shaped like a molded impression of
the eye,
which may include injured and/or irregular regions on the bearing surface.
3. If the lens is to vault over the central corneal region, extract from a
three-dimensional
model, or three-dimensional topology map obtained from the topographer, the
maximum
height of the central corneal surface over the pupil relative to the scleral
lens bearing
surface, such as height H1 indicated by arrow 1002 in Figure 10c.
4. Design the back surface of the scleral lens optics, indicated by 1101 in
Figure 11, to vault
over the top of the cornea, creating a clearance distance 1107, so as to
ensure that the
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
back surface of the scleral lens does not touch the cornea. For a scleral lens
that vaults
over the cornea, clearing distances are typically in the range of 100 to 300
microns.
5. Locate scars or injuries in the scleral and/or corneal region, as shown in
Figures 2a, 2b, 2c,
and 2d, that could prevent the lens from properly and/or comfortably sitting
on the eye. If
necessary, elevate the back surface of the lens to vault over the injured
areas as indicated by
arrow 1102 in Figure 11. It is important to ensure that the topographer has
sufficient spatial
resolution to detect such abnormalities. Such scars or injuries can be
identified by an
operator upon examination of a video camera image (also provided by the
topographer), as
shown in Figures 2a, 2b, 2c, 2d, 3b, and/or by examination of the three-
dimensional topology
data.
6. For a vaulted scleral lens that contains fluid between the cornea and
central back surface
of the lens, use any naturally occurring low valley or valleys in the eye
topology under
the lens bearing surface, as indicated by arrow 1103 and/or create at least
one or more
small raised gaps under the lens back surface indicated by arrows 1104 and
1105 to allow
the free flow of tears in and out of the region covered by the lens. Such gaps
also prevent
excessive suction from forming between the lens and eye which if not prevented
could
make lens removal difficult. Such valleys can be identified upon manipulation
and
examination of the three-dimensional topology map, and or model, and or the
video
image.
While Figures 8 through 11 illustrate the lens back surface sitting on the
sclera, this same
technique for designing a lens back surface, in which:
= the entire lens bearing region of the eye is described by a three-
dimensional array of
independently measured data points provided by an ocular topographer;
= the spatial relationship between the data points is either compensated
for eye motion
during the scan and/or free of artifacts from motion blur;
= the topology of the eye, in the bearing region, is extracted to create
the back surface of
the lens; and
11
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
= each data point of the lens back surface represents an independently
measured x, y, z
location on the surface of the eye,
can be applied to any lens resting on any part of the eye.
With the lens bearing surface now designed, a method for designing scleral
lens optics, without
placing a series of trial lenses on the eye, will now be described.
Design of Patient Specific Scleral Lens Optics Without the Use of Trial Lenses
If a patient wants to wear a scleral lens to relieve dry eye or contact lens
induced dry eye
symptoms, and has good vision defined as producing a sharp image on their
retina without the
need for eyeglasses or corrective lenses, then the scleral lens must be
designed to maintain the
same quality of vision when applied to the eye as existed prior to its
application.
Alternatively, if the patient wears eyeglasses, or requires a corrective lens
placed in front of the
eye to produce a sharp image on their retina, then this vision correction
function can be
incorporated into the scleral lens optics. The scleral lens must be designed
such that when it is
placed on the eye, it recreates the same image on the retina as is formed by
the corrective lens
placed in front of the eye as shown in Figures 12a and 12b. Therefore, the
total light bending
properties between the object (candle in Figures 12a and 12b) and retina must
be the same for
both optical configurations illustrated in Figures 12a and 12b. Figures 13a
and 13b describe
these two optical configurations in more detail with the image projected onto
the retina being the
same again for both configurations. The only difference between Figures 13a
and 13b is what
happens outside the eye in front of the cornea. Since in both configurations
nothing within the
patient's eye changes and in both configurations a sharp image must be
projected onto the retina,
one can conclude that the optical rays within the cornea must be the same in
Figures 13a without
the scleral lens, as in Figure 13b with the scleral lens, to produce an in-
focus image on the retina.
Therefore, the optical rays only need to be matched up to the interior side of
the cornea as
illustrated in Figures 14a and 14b.
12
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Furthermore, it is reasonable to assume that these optical rays need only to
be matched over the
fixed gaze viewing angle of the eye which is typically +/- 10 degrees for text
and +/- 30 degrees
for shape, as given in Xthona, A., "Optimizing Image Quality in the
Radiologist's Field of
Vision", Barco Healthcare, 03 November 2015, and as illustrated in Figure 15.
The matching of optical rays is accomplished using Snell's law which governs
how light bends
when it transitions between different materials as illustrated in Figure 16.
Snell's Law is given
by:
L sincti= Jr sinar
where: L = index of refraction of the incident beam material
134= angle of incidence of the incident beam relative to a perpendicular line
drawn
to the entering surface
Jr = index of refraction of the refracted beam material
ar= angle of incidence of the refracted beam relative to a perpendicular line
drawn to the exiting surface
When a light ray enters the eye perpendicular to the corneal surface, the
angle of incidence (cti)
equals zero, and since (sin 0) =0, the light ray is not refracted (bent) and
passes straight through
the corneal surface. However, when an off axis light ray enters the corneal
surface it is bent
toward the perpendicular axis. As an example, when no scleral lens is applied
to the eye, a light
ray with an incident angle of 30 degrees entering the cornea (Jr = 1.376) from
air (L =1.00), will
be bent by 8.7 degrees to an angle of 21.3 degrees inside the cornea as
illustrated in Figure 17.
However, when a scleral lens is applied with saline solution between the back
surface of the
scleral lens and cornea, a 30-degree incident beam at the saline ¨ cornea
boundary is only bent a
total of 0.98 degrees, as illustrated in Figure 18, because there is only a
0.041 difference in the
index of refraction between saline (L= 1.335) and the cornea (L = 1.376).
Therefore, to place the
incoming light ray at the same place inside the cornea, most of the light
bending must be
performed by the scleral lens at the air-scleral-lens boundary.
13
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Designing a Scleral Lens With Vision Correction Optics
Prior to designing the scleral lens, one must first determine where in the
cornea the optical rays
are positioned by the corrective lens (eyeglass or refractive lens) to provide
good vision. This is
accomplished by tracing the optical rays from the source (candle 1401a in
Figure 14a) through
the corrective lens 1402, and through the front surface of the cornea 1403a to
a three-
dimensional reference surface 1404a located within the eye.
The exact position of reference surface 1404a is not important as long as it
is a plane or some
other known 3D shape entirely located within the eye. However, placing it
toward the front of
the eye within the cornea simplifies the calculations because the optical rays
do not need to be
traced past this point. Figure 14a shows the reference surface 1404a located
entirely within the
cornea.
The set of optical rays from the source to the reference surface created by
the corrective lens is
referred to as the "Reference Ray Set." and contains the following ray subsets
indicated in Figure
14a:
1. Source to corrective lens rays 1410a
2. Corrective lens to cornea rays 1411a
3. Cornea to reference surface rays 1412a
The degree of light bending by the corrective lens is determined using Snell's
law and the known
shape of the corrective lens 1402. The degree of light bending at the air-
corneal surface is
determined using Snell's law and the shape of the cornea 1403a provided by the
three-
dimensional topographer.
The goal is to design the scleral lens so that it places the optical rays from
the source at the same
approximate position within the cornea as did the corrective lens, within the
limits imposed by
Snell's law and the technology used to fabricate the three-dimensional scleral
lens surface shape.
14
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
To accomplish this goal the following steps are performed (with reference to
Figure 14b):
1. Retrace each optical ray in the Reference Ray Set back out of the eye
starting at the
interior corneal Reference Surface 1404b and stopping at the cornea-saline
fluid
boundary 1403b. Reference Surface 1404b in Figure 14b is the same Reference
Surface
as 1404a in Figure 14a redrawn for convenience. Cornea 1403b is the same
cornea as
1403a redrawn for convenience.
2. Snell's law is then applied at the cornea (1403b) ¨saline fluid (1405)
boundary to
determine how much each optical ray is bent at this boundary. The degree of
light
bending at the corneal-saline boundary is determined using Snell's law, the
index of
retraction of the cornea (T=1.376) and the shape of the cornea 1404b as
provided by the
three-dimensional topographer. Each optical ray then continues at its new
projected
angle through the saline fluid (1405) to the back surface of the scleral lens
1406.
3. Snell's law is then applied at the scleral lens (1406) back surface ¨
saline fluid (1405)
boundary and the scleral lens (1406) front surface ¨ air boundary. The shape
of the three-
dimensional front and back scleral lens surfaces are adjusted so that the
optical rays
exiting the front surface of the scleral lens (1406) retraces, as close as
possible, within the
limits of Snell's law, the equivalent rays in the Reference Ray Set existing
between the
source 1401a and corrective lens 1402, indicated by arrow 1410a in Figure 14a.
Snell's
law is applied using the index of retraction of the saline fluid (1=1.335).
When the design
is completed the goal is to match rays 1412a to 1412b inside the eye and match
rays
1410a and1410b outside the eye, as illustrated in Figures 14a and 14b.
This conceptual scleral lens optical design procedure can be implemented to
create actual scleral
lens optics to replace eyeglasses worn to correct for nearsightedness. Design
performance can be
evaluated by superimposing the reference ray set for the eyeglass
configuration onto the
corresponding optical rays for the scleral lens configuration. The front
surface of the scleral
lens will bend light more than the back surface for the same incident light
angle because the
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
difference in the index of refraction between the scleral lens material
(1.424) and saline (1.335)
on the back surface is 0.089 and the difference between air (1.00) and the
scleral lens material
(1.424) on the front surface is 0.424, a factor of 4.7 times greater. It is
for this reason that the
design example provided uses a spherical shape for the back surface and an
aspheric shape for
the front surface. However, more complex shapes can be used to achieve closer
matches to a
reference ray set if desired.
To design the scleral lens:
a. Create a first computer model (Figure 19a) containing an optical source
1901a, typically placed at infinity, a corrective lens 1902 that when placed
in front of a patient's eye improves their vision, a three-dimensional model
of the patient's corneal front surface 1903a, and a Reference Surface
1904a placed behind the cornea within the eye.
b. Trace optical rays from the source (1901a), through air, to the front
surface 1902F of the corrective lens 1902, using Snell's law.
c. Knowing the three-dimensional shape of the front (ray entering) surface
of
the corrective lens 1902F, apply Snell's law at the front surface air-lens
boundary to determine the path of the optical rays through the corrective
lens 1902.
d. Knowing the three-dimensional shape of the back (ray exiting) surface of
the corrective lens 1902B, apply Snell's law at the back-surface-air
boundary to determine the path of the optical rays from the back surface of
the corrective lens 1902 to the front surface of the cornea 1903a.
e. Determine the path of the optical rays from the front surface of the
cornea
1903a to a Reference Surface 1904a placed within the eye. Knowing the
three-dimensional shape of the front surface of the cornea, apply Snell's
16
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
law at the front surface air-cornea boundary and at any material
boundaries within the eye lying between the cornea and the Reference
Surface. The Reference Surface can be planar or curved. If the reference
surface is placed within the cornea, as drawn in Figure 19a then the only
boundary encountered will be the air-cornea boundary.
f. Store the path of the optical rays traveling from the source 1901a to
the
Reference Surface 1904a and refer to this set of rays as the Reference Ray
Set.
g. Create a second computer model, Figure 19b, containing the same
identical optical source 1901b, eye 1903b, and Reference Surface (1904b)
as in the first computer model, where optical source 1901b is identical to
optical source 1901a, eye 1903b is identical to eye 1903a, and Reference
Surface 1904b is identical to Reference Surface 1904a. Place the optical
source 1901b the same distance from the eye as in the first computer
model. Place the Reference Surface at the same location within the eye as
in the first computer model.
h. Place a scleral lens 1906 over the eye in the second computer model,
Figure 19b, filling the gap between the cornea and back surface of the
scleral lens with fluid 1905, typically saline.
i. Insert the three-dimensional optical rays from the Reference Ray Set
that
lie inside the eye between the cornea 1903a and Reference Surface 1904a
in the first computer model, Figure 19a, into the second computer model,
Figure 19b, placing the rays at the identical position within the eye 1903b
as in the first computer model. For the purpose of designing the scleral
lens it is now assumed that the rays originate at the Reference Surface
1904b and travel out of the eye 1903b, through the fluid 1905, through
17
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
scleral lens 1906 and out the front of the scleral lens toward the source
1901b.
j. Knowing the three-dimensional shape of the front surface of the cornea
1903b, the three-dimensional shape of the cornea-fluid boundary, the
index of refraction of the cornea (typically 1.376), and the index of
refraction of the fluid (typically 1.336 for saline), apply Snell's law to the
cornea- (saline) fluid boundary to determine the path of the optical rays
from the front surface of the cornea through the fluid 1905 to the back-
surface of the scleral lens 1906.
k. Adjust the height of the back surface of the scleral lens, indicated by
H2
(1107) in Figure 11, to vault over the cornea. Vaulting height is not
critical, but is typically less than 300 microns.
1. Apply Snell's law,
in the second computer model, to the front and back
surfaces of the scleral lens, surfaces 2006F and 2006B respectively, shown
in zoomed in view Figure 20, adjusting the three-dimensional shape of the
front and back surface of the scleral lens optics so that the angles and
positions of the scleral lens rays, 1908b shown in Figure 19b, approximate
as closely as possible, within the limits imposed by Snell's law, the path
traveled by the Reference Ray Set (1907a) between the source and
corrective lens in the first computer model, as shown by arrows 1908a in
Figure 19a. That is, 1908b 1908a.
Figure 21 is the superposition of the examples of Figures 19a and 19b zoomed
in around the
cornea and enlarged to show how well the optical rays from the Scleral Lens
design match the
Reference Ray Set from the corrective lens configuration. Optical rays are
shown entering the
eye at approximately 0, 10, and 20 degrees relative to a line perpendicular to
the front surface of
the cornea.
18
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Figure 22a is the three-dimensional representation of Figure 19a and is the
first three-
dimensional model that is needed by the computer to design the scleral lens.
Figure 22b, is the
three-dimensional representation of Figure 19b and is the second three-
dimensional model that is
needed by the computer to design the scleral lens.
Figure 22c is the superposition of the examples of Figures 22a and 22b showing
the alignment of
the ray bundles and more specifically how well the scleral lens is able to
duplicate the Reference
Ray Set. For the design example illustrated in Figures 19 through 22, the
front surface curvature
1902F of corrective lens 1902 in Figure 19 is 150mm and the back-surface
radius of curvature
1902B is 100mm. The scleral lens 2006 in Figure 20 has a back surface
spherical radius of
curvature 2006B equal to 10.64mm. The front surface of scleral lens 2006 is an
even Asphere
with a radius of 38.473mm, a conic of -4.75mm, 2nd order term of 0.058, 4th
order term of
0.000623, 6th order term of -0.0001804, 8th order term of 0.00003288, 10th
order term of -
2.947E-6, and 12th order term of 1.06E-7. The parameters used to specify an
aspheric surface are
described in an article by Czajkowski, A., entitled "Specifying an Aspheric
Surface," OPT 521 ¨
Report #2, December 14, 2007.
While there are numerous ray tracing and lens design programs on the market,
the design shown
in Figures 19 through 22, was generated by a ray tracing lens design program
called
"Opticstudio" produced by Zemax LLC, of Kirkland, WA.
Designing a Scleral Lens with No Vision Correction Optics
Having described how to incorporate corrective lens optics into a scleral lens
to eliminate the
need for eyeglasses, we will now describe how to design a scleral lens for
patients that do not
require corrective lens optics or eyeglasses, to produce a sharp image on
their retina, but want to
wear scleral lenses to relieve dry eye symptoms or for any other reason.
Figure 23a illustrates
the patient's eye focused on an object 2301a (typically placed at infinity).
It is assumed that the
patient sees the object clearly. Figure 23b shows a scleral lens 2304a placed
on the eye. The
scleral lens must be designed to maintain the same quality of vision when
applied to the eye as
19
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
was obtained prior to its application. The goal is to project the same image
onto the retina in
Figure 23b as in Figure 23a, within the limits of Snell's law. As explained
previously, and
referring to Figures 24a and 24b, this can be achieved by matching the optical
rays inside the eye
to a reference surface (2412a and 2412b). The reference surface can be placed
anywhere inside
the eye behind the surface of the cornea (in front of, within, or behind the
crystalline lens). In
addition to matching the optical rays inside the eye, the optical rays are
also matched outside the
eye prior to and after application of the scleral lens. Matching rays outside
the eye correspond to
matching reference rays 2410a to rays 2410b. Therefore, optical rays 2410a
2410b and 2412a
2412b within the limits imposed by Snell's law and the scleral lens
manufacturing process.
The precise steps required to design such a scleral lens will now be described
in the second
design example.
As in the first example, the back surface of the scleral lens will be made
spherical and the front
aspheric in shape. More complex shapes can be used to achieve closer matches
to the Reference
Ray Set if desired. To design the scleral lens:
a. Create a first computer model (Figure 25a) containing an optical source
2501a, preferably placed at infinity, the patient's eye (2502a) a three-
dimensional model of the patient's corneal front surface 2503a, obtained
from a topographer, and a Reference Surface 2504a placed behind the
cornea within the eye.
b. Trace optical rays from the source (2501a), through air, to the front
surface of the cornea 2503Fa.
c. Determine the path of the optical rays from the front surface of the
cornea
2503Fa to a Reference Surface 2504a placed within the eye. Knowing
the three-dimensional shape of the front surface of the cornea 2503Fa,
supplied by the topographer, apply Snell's law at the front surface air-
cornea boundary and at any material boundary within the eye lying
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
between the cornea and the Reference Surface 2504a. The Reference
Surface can be planar or curved.
d. Store the three-dimensional path of the optical rays traveling from the
source 2501a to the Reference Surface 2504a and refer to this set of rays
as the Reference Ray Set 2507a.
e. Create a second computer model, Figure 25b, containing the same
identical optical source 2501b, eye 2502b, and Reference Surface (1904b)
as in the first computer model, where optical source 2501b is identical to
optical source 2501a, eye 2502b is identical to eye 2502a, cornea 2503a is
identical to cornea 2503b, and Reference Surface 2504b is identical to
Reference Surface 2504a. Place the optical source 2501b the same
distance from the eye as in the first computer model. Place the Reference
Surface 2504b at the same location within the eye as in the first computer
model.
f. Place a scleral lens 2506 over the eye in the second computer model,
Figure 25b, filling the gap between the cornea and back surface of the
scleral lens with fluid 2505, typically saline.
g. Insert the three-dimensional optical rays from the Reference Ray Set that
lie inside the eye between the cornea 2503a and Reference Surface 2504a
in the first computer model, Figure 25a, into the second computer model,
Figure 25b, placing the rays in the identical location within the eye 2503b
as in the first computer model. For the purpose of designing the scleral
lens it is now assumed that the rays originate at the Reference Surface
2504b and travel out of the eye 2502b, through the front surface of the
cornea 2503Fb, through the fluid 2505, through scleral lens 2506 and out
the front of the scleral lens.
21
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
h. Knowing the three-dimensional shape of the front surface of the cornea
2503b, the three-dimensional shape of the cornea-fluid boundary, the
index of refraction of the cornea (typically 1.376), and the index of
refraction of the fluid (typically 1.336 for saline), apply Snell's law to the
cornea- (saline) fluid boundary to determine the path of the optical rays
from the front surface of the cornea 2503F through the fluid 2505 to the
back-surface of the scleral lens 2506.
i. Adjust the height of the back surface of the scleral lens, indicated by
H2
(1107) in Figure 11, to vault over the cornea. Vaulting height is not
critical, but is typically less than 300 microns.
j. Apply Snell's law, in the second computer model, to the front and back
surfaces of the scleral lens, surfaces 2606F and 2606B respectively,
(shown in zoomed in view Figure 26). This involves adjusting the three-
dimensional shape of the front and back surface of the scleral lens optics
so that the optical rays 2508b between the front surface of the scleral lens
and source, shown in Figure 25b, approximate as closely as possible,
within the limits imposed by Snell's law, the path traveled by the
Reference Ray Set between the source and cornea in the first computer
model, shown by rays 2508a in Figure 25a. Stop the matching of rays
2508b to 2508a at the front of the scleral lens.
Figure 26 is the superposition of Figures 25a and 25b zoomed in around the
cornea and enlarged
to show how well the optical rays from the Scleral Lens design match the
Reference Ray Set
computed without the scleral lens on the eye. Optical rays are shown emanating
0, 10, and 20
degrees from a source located at infinity.
Figure 27a shows the actual first three-dimensional computer model used to
calculate the
Reference Ray Set, with three optical ray bundles emanating 0, 10, and 20
degrees from the
22
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
source at infinity. Figure 27a is the three-dimensional drawing corresponding
to the two-
dimensional drawing shown in Figure 25a.
Figure 27b shows the actual second three-dimensional computer model used to
design the scleral
lens also with ray bundles emanating at 0, 10, and 20 degrees from the
infinity source. Figure
27b is the three-dimensional drawing corresponding to the two-dimensional
drawing shown in
Figure 25b.
Figure 27c is the superposition of Figures (27a and 27b showing how well the
optical rays from
the scleral lens design are able to duplicate the Reference Ray Set. For the
scleral lens design
shown in Figures 25 through 27, the back surface has a spherical radius of
curvature equal to
16.473mm. The front surface is an Even Asphere with a Radius of 8.866mm, a
conic of -0.053,
2nd order term of -1.727E-4, 4th order term of 1.251E-4, 6th order term of -
6.553E-5, 8th order
term of 9.107E-6, 10th order term of -4.023E-7 and 12th order term of 0Ø
While there are numerous ray tracing and lens design programs on the market,
the models shown
in Figures 25 through 27 were generated by a ray tracing lens design program
called Opticstudio
by Zemax LLC of Kirkland, WA. Again, it is emphasized that Figures 25 through
27 correspond
to the design of a real scleral lens.
Manufacturing the Scleral Lens
Once the scleral lens optics, bearing surface shape, and vaulting height are
specified such lenses
can be manufactured either by using a precision lathe or using a 3D printer.
An example of a
precision lathe is the "Nanoform X" manufactured by Ametek Precitech, Inc. of
Keene, NH. An
example of a precision 3D printer is the "Photonic Professional GT" by
Nanoscribe GmBH of
Eggenstein-Leopold Shafen, Germany.
In addition to the scleral lenses previously described herein, there exists a
class of scleral lenses
that incorporate a soft material, such as for example a silicone hydrogel, for
the bearing surface.
Such pliable materials, frequently referred to as "skirts" conform to the
shape of the eye in the
23
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
bearing region. A rigid optical lens, vaulting over the cornea, is supported
by the soft
conforming skirt. An example of a scleral lens with a soft skirt is
manufactured by SynergEyes,
Inc. of Carlsbad, CA.
Scleral lens optics designed using the procedure described herein can also be
combined with a
soft skirt or incorporated into a pliable bearing surface lens, thereby
greatly reducing the time
and complexity of such scleral lens design.
System Implementation
Figure 28 is a block diagram of a preferred embodiment of a system 2800 that
may be used to
implement the methods described herein.
This implementation uses a topographer 2810, a Digital Signal Processor (DSP)
/ computer
2820, a display 2830, and data storage 2840 to process and/or generate one or
more three-
dimensional model(s) 2850 of an eye and/or the resulting lens. The lens
model(s) may then be
provided to a precision lathe 2860 and/or 3D printer 2870 to produce a
physical lens.
As explained in the above-referenced U.S. Patent 9,398,845 by Bishop et al.,
the topographer
2810 may use a video camera and a line scan device to obtain a 3D model of an
eye. Other
topographers, as described in the above-referenced U.S. Patent 9,489,753 by
Bishop et al. use
Optical Coherent Topography to measure eye topology. Furthermore, there are
topographers that
project patterns onto the eye and measure pattern distortion to determine
corneal shape, such as
the Placido Disc topographers. Other topographers insert fluorescent dyes into
the eye and
project patterns onto the fluorescing material to determine the shape of the
eye. For the
applications described herein, any topographer that compensates for eye motion
during the scan
to provide motion corrected blur fee topology can be used. The topographer
2810 thus typically
includes a number of components (not shown in detail here) such as a two
dimensional (2D)
digital video camera to take a sequence of images of an eye including at least
one feature on the
eye. The camera may be a television camera or other digital camera capable of
capturing a
sequence of images. The topographer 2810 also includes a scanner that measures
distances to
24
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
the surface of the eye to produce a set of independently measured data points
in 3D space ¨ that
is, each "pixel" in the sequence of camera images is thus associated with an
x, y, z location on
the surface of the eye.
The DSP/computer 2820 may further include storage 2840, a display 2830 and /
or other
peripheral components.
The DSP/computer 2820 executes program code to perform some or all of the
steps of the
methods described herein for determining the design of a lens.
One or more three-dimensional models 2850 specifying a lens design may then be
provided as
output data files to a lens manufacturing machine (or process) such as the
precision lathe 2860 or
the 3D printer 2870.
It should be understood that many other arrangements of the programmable
and/or computer
controlled components are possible. For example, the DSP/computer 2820 shown
here may act
as both the computer for the topographer as well as the platform that executes
the lens design
method described herein. In other arrangements, the topographer 2810 may have
its own DSP
and/or computer arranged to operate on the output of the camera and the
scanner to produce
topology data points from the eye with a separate DSP/computer executing the
lens design
procedure. Scanned eye data from the topographer may be transferred in the
form of a data file
that is transferred over a network, or on a portable storage media such as a
memory stick, disk, or
magnetic tape, to the lens design computer. The precision lathe 2860 and/or 3D
printer 2870
may typically have their own processors and may be located remotely from the
DSP/computer
2820, and operate on 3D model designs provided to them in the form of a data
file that is
transferred over a network, or on a portable storage media such as a disk or
magnetic tape. The
DSP/computer may also directly control a local or remote precision lathe 2860
and/or 3D printer
2870 over a network connection. Still other arrangements are possible.
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
Conclusions
It can now be understood that a method for determining one or more
characteristics of a design
of a lens can include receiving, from an ocular topographer, an array of data
points from which at
least the three dimensional position of each data point can be extracted. The
data points are such
that each data point represents an independently measured x, y, z location on
a surface of an eye,
the spatial relationship accurately representing a true topology of the eye,
free from motion blur
artifacts that occur during acquisition of the data points, and a sampling
density of the data points
being sufficiently high to characterize anomalies in the eye. The method then
proceeds to
analyze the data points for determining an array of independent data points to
define the back
surface of the lens, such that the resulting lens is a contact lens that
conforms to or vaults over
said anomalies on the eye.
In some aspects, the data points can be further used for grouping together
independent data
points either as used from the topographer or as determined to define a lens
back surface into
meridians, the meridians being of any shape, with the meridians being
independent from each
other, and with a meridian data point density being sufficiently high so as to
characterize
anomalies anywhere in the eye that compromise lens comfort and fit.
The method may also include steps for determining one or more characteristics
of a design of a
lens, the lens comprising a front surface and a back surface, that involves
receiving, from an
ocular topographer, an array of data points from which at least the three
dimensional position of
each data point can be extracted with each data point used from the
topographer representing an
independently measured x, y, z location on a surface of an eye, the spatial
relationship between
the data points used from the topographer accurately representing a true
topology of the eye, free
from motion blur artifacts that occur during acquisition of the data points, a
sampling density of
the data points used from the topographer being sufficiently high to
characterize anomalies in the
eye that compromise lens comfort and fit; and determining, from the data
points used from the
topographer, a lens back-surface with quadrant or sub-division boundaries
defined by multiple
independent data points, with additional independent data points within each
quadrant or sub-
26
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
division that are not used to define the boundaries, and with a density of
independent data points
within each quadrant or sub-division being sufficiently high so as to
characterize anomalies
anywhere in the quadrant or sub-division that could compromise lens comfort
and fit, such that
the resulting lens is a contact lens that conforms to or vaults over said
anomalies on the eye.
The methods may also involve determining one or more characteristics of a
design of a lens,
comprising receiving, from an ocular topographer, an array of data points from
which at least
the three dimensional position of each data point of an eye can be extracted;
determining
characteristics of the lens including an optical region, a transition region,
and a bearing surface,
with the optical region focusing incoming light into an eye,the transition
region connecting the
optical region to the bearing surface, the bearing surface comprising a region
of the lens that
rests on a surface of an eye, the bearing surface further defined as an array
of independent data
points conforming to the three-dimensional data point positions of the eye
extracted from the
topographer, and with the resulting lens being a scleral lens that conforms to
or vaults over said
anomalies anywhere on the eye, such that
lens optics in the optical region are vaulted over a cornea of the eye to
create a fluid
reservoir between a back surface of the optics and the cornea,
the bearing surface resting either solely on a sclera and conforming to a
three-
dimensional shape of the sclera, or the bearing surface straddling a limbus,
such that the bearing
surface rests partially on and conforms to the three-dimensional shape of
sclera and rests
partially on and conforms to a three-dimensional shape of the cornea, and
containing:
at least one naturally occurring low valley in the eye topology under the lens
bearing
surface, and/or at least one raised gap formed in the bearing surface, to
allow free flow of tears in
and out of a region covered by the lens, with such valleys or gaps also
preventing excess suction
forming between the lens and eye which would otherwise make lens removal
difficult.
The method may further involve determining one or more characteristics of a
design of lens
optics without applying a trial lens to a patient's eye, by creating a first
computer model
containing an optical source, an eye with a three-dimensional model of a
corneal front surface of
the patient's eye as provided by a topographer, and a Reference Surface placed
behind the
corneal front surface within the eye, where the Reference Surface may be
planar or curved, and
27
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
further when the patient requires corrective lenses, or eyeglasses, to produce
a sharp image on
their retina,
inserting a corresponding corrective lens between the optical source and eye
in the first
computer model;
tracing rays from the optical source, through air, to a front surface of the
corrective lens;
using the three-dimensional shape of the front surface of the corrective lens,
applying
Snell's law at a front surface air-lens boundary to determine a path of the
optical rays within the
corrective lens; and
using the three-dimensional shape of a back surface of the corrective lens,
applying
Snell's law at a back-surface lens-air boundary to determine a path of the
optical rays as they
travel from the back surface of the corrective lens to the front surface of
the cornea; and
when the patient does not require corrective lenses, or eyeglasses, to produce
a sharp
image on their retina, then without inserting a corrective lens into the first
computer model:
tracing optical rays directly from the optical source, through air, to the
front surface of
the cornea;
determining a path of the optical rays from the front surface of the cornea to
a Reference
Surface placed within the eye, and using the three-dimensional shape of the
front surface of the
cornea and the Reference Surface, applying Snell's law at the front surface
air-cornea boundary
and at any material boundaries within the eye lying between the cornea and the
Reference
Surface;
storing the path of the optical rays traveling from the source to the
Reference Surface as a
Reference Ray Set;
creating a second computer model containing the same optical source, eye, and
Reference
Surface as in the first computer model, by placing the optical source the same
distance from the
eye as in the first computer model, and placing the Reference Surface at the
same location within
the eye as in the first computer model, and within the second computer model
further:
placing a scleral lens over the eye in the second computer model, and filling
a gap
between the cornea and back surface of the scleral lens with a model of a
fluid;
inserting a subset of optical rays from the Reference Ray Set that lie inside
the eye
between the cornea and Reference Surface in the first computer model into the
second computer
28
CA 03068530 2019-12-24
WO 2019/005557 PCT/US2018/038497
model, and placing the rays of this subset in the identical location within
the eye as in the first
computer model.
When the rays in the second computer model originate at the Reference Surface
and travel out of
the eye, the method(s) may further involve using the three-dimensional shape
of the front surface
of the cornea, the index of refraction of the cornea, and the index of
refraction of the fluid,
applying Snell's law to the cornea- fluid boundary to determine the path of
the optical rays as
they travel from the front surface of the cornea through the fluid to the back
surface of the scleral
lens; such that
when the first computer model contains a corrective lens:
applying Snell's law to the front and back surfaces of the scleral lens
optics,
adjusting the three-dimensional shape of the front and back surfaces of the
lens so that the optical
rays in the second computer model approximate, as closely as possible within
the limits specified
by Snell's Law, the optical rays in the first computer model, over a shared
region specified by
the Reference Ray Set between the optical source and corrective lens in the
first computer
model;
when the first computer model does not contain a corrective lens:
applying Snell's law to the front and back surfaces of the scleral lens
optics,
adjusting the three-dimensional shape of the front and back surfaces of the
lens so that the optical
rays in the second computer model approximate, within the limits specified by
Snell's Law, the
subset of the Reference Rays in the first computer model, over the shared
region specified by the
distance between the front surface of the scleral lens and optical source in
the second computer
model.
Therefore it should be understood that this patent is to be limited only by
the scope of the claims
that follow.
What is Claimed is:
29