Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 323
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 323
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HETEROCYCLIC COMPOUNDS AS ADENOSINE ANTAGONISTS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional Application No.
62/534,176, filed
July 18, 2017, the contents of which are incorporated by reference in their
entirety.
FIELD OF THE INVENTION
[0002] This disclosure relates generally to therapeutics for treatment
mediated through a G-
protein¨coupled receptor (GPCR) signaling pathway and, more particularly, to
compounds that
inhibit an adenosine receptor (such as an A2A antagonist). The disclosure also
provides
pharmaceutically acceptable compositions comprising such compounds and methods
of using the
compounds or compositions in the treatment of a disease associated with a GPCR
signaling
pathway.
BACKGROUND OF THE INVENTION
10003] Adenosine receptors (ARs) are distributed throughout the body and are
responsible for
numerous biological functions. The seven trans-membrane G-protein-coupled
receptors
(GPCRs) have been divided into four different subtypes: A1, A2A, A2B, and A3.
The A2A and A2B
ARs stimulate activity of the adenylyl cyclase, inducing an increase of cAMP
levels. A2A ARs
have a distinct tissue localization, different biochemical pathways, and
specific pharmacological
profiles.
[0004] Adenosine is one of the human body's most important neuromodulators in
both the
central and the peripheral nervous systems. Adenosine is released from tumor
cells and its
concentration in the extracellular fluid of tumors can reach immunosuppressive
levels (Blay et al.
(1997), Cancer Res., 57(13), pp. 2602-5). The extracellular fluid of solid
carcinomas contains
immunosuppressive concentrations of adenosine. Id. This increase in adenosine
concentration is
a result of increases in CD73 (ecto-5'-nucleotidase) and CD39 (nucleoside
triphosphate
dephosphorylase) enzymes, which are responsible for directly catabolizing ATP
into adenosine.
These upregulations are triggered by hypoxia and the generation of HIF-la.
High levels of
adenosine around tumor cells act to regulate multiple immune cells (e.g., CD4+
T-cells and
cytotoxic CD8 T-cells) via activation of multiple adenosine receptor subtypes,
but particularly
1
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
A2A receptors, resulting the suppressing of pro-inflammatory activities and
upregulation of anti-
inflammatory molecules and immunoregulatory cells (Kumar et al. (2013),
Adenosine as an
endogenous immunoregulator in cancer pathogenesis: where to go? Purinergic
Signal., 9(2), pp
145-65 and Sitkowsky et al., Hostile, hypoxia-A2-adenosinergic tumor biology
as the next
barrier to overcome for tumor immunologists. Cancer Immunol. Res. 2(7), pp 598-
605; Ohta
(2016), A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment.
Frontiers in
Immunology., 7 article// 109, pp 1-11). It was demonstrated that chimeric
antigen receptor (CAR)
T cells upregulate A2ARs upon antigen-specific stimulation in vitro and in
vivo (Beavls (2017),
Targeting the Adenosine 2A Receptor Enhances Chimeric Antigen Receptor T Cell
Efficacy. J of
Clin Invest. 127 (3): pp 929-941).
[0005] Survival of cancer cells is dependent on their ability to avoid attack
by the immune
system. In addition, tumor cells can overtake the immune system to facilitate
tumor survival and
metastasis. Adenosine, whose concentration increases within hypoxic regions of
solid tumors,
has been recognized as being able to interfere with the recognition of tumor
cells by cytolytic
effector cells of the immune system. (Tuite and Riss (2013). Recent
developments in the
pharmacological treatment of Parkinson's disease. Expert Opin. Investig.
Drugs, 12(8) pp 1335-
52, Popoli et al. (2002). Blockade of striatal adenosine A2A receptor reduces,
through a
presynaptic mechanism, quinolinic acid-induced excitotoxicity: possible
relevance to
neuroprotective interventions in neurodegenerative diseases of the striatum,
./. Neurosci, 22(5)
pp. 1967-75, Gessi et al. (2011). Adenosine receptors and cancer. Biochim
Biophys Acta,
1808(5), pp. 1400-12).
[0006] Although all adenosine receptors now have an increasing number of
recognized
biological roles in tumors, the A2A and A3 subtypes appear promising targets
for therapeutic
development. In particular, activation of A2A receptors leads to
immunosuppressive effects,
which decreases anti-tumoral immunity and thereby encourages tumor growth.
[0007] The A2B receptor is another potential target for therapeutic
development
Autocrineiparacrine stimulation of A25 expressed on tumor cells is believed to
enhance their
metastatic potential and A2B blockade may reduce tumor metastasis in an immune-
independent
manner (Beavis et al. (2013). Blockade of A2A receptors potently suppresses
the metabolism of
CD73+ Tumors. Proc. Nail. Acad. Sci., 110(36) pp. 14711-6). A25 expression
also correlates with
relapse-free survival (RFS) in triple negative breast cancer suggesting that
this pathway may be
2
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
clinically relevant. AM blockade also has the potential to modulate the
immunosuppressive
properties of tumor-associated immune cells including dendritic cells and
myeloid-derived
suppressor cells (MDSCs) (Cekic et al. (2011). Adenosine A2B receptor blockade
slows growth
of bladder and breast tumors. J. Immunol. 188(1), pp. 198-205; Sorrentino et
al. (2015).
Myeloid-derived suppressor cells contribute to AM adenosine receptor-induced
VEGF
production and angiogenesis in a mouse melanoma model. Oneotarget 6(29), pp.
27478-89;
Iannone et al. (2013). Blockade of AM adenosine receptor reduces tumor growth
and immune
suppression mediated by myeloid-derived suppressor cells in a mouse model of
melanoma.
Neoplasia, 15(12), pp. 1400-9.
[0008] There remains a continuing need for new therapies for the treatment of
diseases and
disorders related to the adenosine signaling pathway.
BRIEF SUMMARY OF THE INVENTION
[0009] In one aspect, provided is a compound of the formula (I):
A
1
(1),
or a tautomer or isomer thereof, or a pharmaceutically acceptable salt of any
of the foregoing,
wherein A, B, RI and R2 are as detailed herein. In some embodiments, provided
is a compound
of formula (I), or a salt thereof.
[0010] In some embodiments, the compound of the formula (I), or a tautomer or
isomer thereof,
or a pharmaceutically acceptable salt of any of the foregoing, is of the
formula (11), (TIE) or (IV)
or a tautomer or isomer thereof, or a pharmaceutically acceptable salt of any
of the foregoing, as
detailed herein. In some embodiments, the compound of the formula (I), or a
salt thereof, is of
the formula (II), (11I) or (IV) or a salt of the foregoing, as detailed
herein.
[0011] In another aspect, provided is a method for any one or more of: (a)
treating a disease,
such as a proliferative disease, in an individual in need thereof; (b)
enhancing an immune
response in an individual in need thereof; (c) inhibiting tumor metastasis in
an individual in need
thereof; (d) modulating the activity of a G protein coupled receptor signaling
pathway in an
individual in need thereof; (e) modulating the activity of an adenosine
receptor, such as an A2A
3
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
receptor, in an individual in need thereof; and (f) increasing the activity of
a natural killer cell in
an individual in need thereof, wherein the method comprises administering to
the individual an
effective amount of a compound of formula (I), or a tautomer or isomer
thereof, or a
pharmaceutically acceptable salt of any of the foregoing. In some embodiments,
provided is a
method for any one or more of: (a) treating a disease, such as a proliferative
disease, in an
individual in need thereof; (b) enhancing an immune response in an individual
in need thereof;
(c) inhibiting tumor metastasis in an individual in need thereof; (d)
modulating the activity of a G
protein coupled receptor signaling pathway in an individual in need thereof;
(e) modulating the
activity of an adenosine receptor, such as an A2A receptor, in an individual
in need thereof; and
(f) increasing the activity of a natural killer cell in an individual in need
thereof, wherein the
method comprises administering to the individual an effective amount of a
compound of formula
(I), or a salt thereof. In one aspect, the compound of formula (I) or a salt
thereof is administered
to the individual in combination with another therapeutic agent. In some
embodiments, the
compound of formula (I) or a tautomer or isomer thereof, or a pharmaceutically
acceptable salt
of any of the foregoing is administered to the individual in combination with
another therapeutic
agent. In a further aspect of the methods, the compound of formula (I) or a
salt thereof is a
compound of the formula (II), (III) or (IV) or a salt of the foregoing. In
some embodiments, the
compound of formula (I) or a tautomer or isomer thereof, or a pharmaceutically
acceptable salt
of any of the foregoing is a compound of the formula (II), (III) or (IV), or a
tautomer or isomer
thereof; or a pharmaceutically acceptable salt of any of the foregoing.
[0012] Also provided are pharmaceutical compositions comprising (A) a compound
detailed
herein, such as a compound of formula (I) or a tautomer or isomer thereof; or
a pharmaceutically
acceptable salt of any of the foregoing, or a compound of formula (II) or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing, or a
compound of formula
(III) or a tautomer or isomer thereof, or a pharmaceutically acceptable salt
of any of the
foregoing, or a compound of formula (IV) or a tautomer or isomer thereof, or a
pharmaceutically
acceptable salt of any of the foregoing, and (B) a pharmaceutically acceptable
carrier or
excipient. In some embodiments, provided are pharmaceutical compositions
comprising (A) a
compound detailed herein, such as a compound of formula (I) or a salt thereof,
or a compound of
formula (II) or a salt thereof; or a compound of formula (III) or a salt
thereof, or a compound of
formula (IV) or a salt thereof; and (B) a pharmaceutically acceptable carrier
or excipient. Kits
4
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
comprising a compound detailed herein or a tautomer or isomer thereof, or a
pharmaceutically
acceptable salt of any of the foregoing and instructions for use are also
provided. Kits
comprising a compound detailed herein or a salt thereof and instructions for
use are also
provided. A compound detailed herein or a tautomer or isomer thereof, or a
pharmaceutically
acceptable salt of any of the foregoing is also provided for the manufacture
of a medicament for
the treatment of cancer. Compounds as detailed herein or a pharmaceutically
acceptable salt
thereof are also provided for the manufacture of a medicament for the
treatment of cancer.
BRIEF DESCRIPTION OF THE DRAWINGS
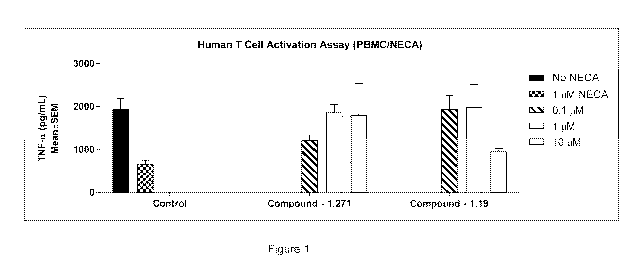
[0013] Figure 1 shows the effects of certain compounds on TNF-a production in
activated
human PBMCs.
100141 Figure 2 shows the effects of certain compounds on IFN-y production in
activated human
PBMCs.
DETAILED DESCRIPTION OF THE INVENTION
Definitions
[0015] For use herein, unless clearly indicated otherwise, use of the terms
"a", "an" and the like
refers to one or more.
[0016] "Alkenyl" as used herein refers to an unsaturated linear or branched
univalent
hydrocarbon chain or combination thereof, having at least one site of olefinic
unsaturation (i.e.,
having at least one moiety of the formula C) and having the number of carbon
atoms
designated (i.e., C2-C10 means two to ten carbon atoms). The alkenyl group may
be in "cis" or
"trans" configurations, or alternatively in "E" or "Z" configurations.
Particular alkenyl groups
are those having 2 to 20 carbon atoms (a "C2-C20 alkenyl"), having 2 to 8
carbon atoms (a "C2-C8
alkenyl"), having 2 to 6 carbon atoms (a "C-C6 alkenyl"), or having 2 to 4
carbon atoms (a "Cr
C4 alkenyl"). Examples of alkenyl include, but are not limited to, groups such
as ethenyl (or
vinyl), prop-1-enyl, prop-2-enyl (or allyl), 2-methylprop-1-enyl, but-2-
enyl, but-3-
enyl, buta-1,3-dienyl, 2-methylbuta-1,3-dienyl, homologs and isomers thereof,
and the like.
[0017] The term "alkyl" refers to and includes saturated linear and branched
univalent
hydrocarbon structures and combination thereof, having the number of carbon
atoms designated
CI-C10 means one to ten carbons). Particular alkyl groups are those having I
to 20 carbon
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
atoms (a "C1-C20 alkyl"). More particular alkyl groups are those having 1 to 8
carbon atoms (a
"C1-C8 alkyl"), 3 to 8 carbon atoms (a "C3-C8 alkyl"), 1 to 6 carbon atoms (a
"C1-C6 alkyl"), 1 to
carbon atoms (a "C1-05 alkyl"), or 1 to 4 carbon atoms (a "C1-C4 alkyl").
Examples of alkyl
include, but are not limited to, groups such as methyl, ethyl, n-propyl,
isopropyl, n-butyl, t-butyl,
isobutyl, sec-butyl, homologs and isomers of, for example, n-pentyl, n-hexyl,
n-heptyl, n-octyl,
and the like.
[0018] "Alkylene" as used herein refers to the same residues as alkyl, but
having bivalency.
Particular alkylene groups are those having 1 to 6 carbon atoms (a "CI-C6
alkylene"), 1 to 5
carbon atoms (a "C1-05 alkylene"), 1 to 4 carbon atoms (a "CI-Ca alkylene") or
1 to 3 carbon
atoms (a "CI-C3 alkylene"). Examples of alkylene include, but are not limited
to, groups such as
methylene (-CH2-), ethylene (-CH2CH2-), propylene (-CH2CH2CH2-), butylene
(-CH2CH2CH2CH2-), and the like.
[0019] "Alkynyl" as used herein refers to an unsaturated linear or branched
univalent
hydrocarbon chain or combination thereof, having at least one site of
acetylenic unsaturation
(i.e., having at least one moiety of the formula CC) and having the number of
carbon atoms
designated (i.e., C2-C10 means two to ten carbon atoms). Particular alkynyl
groups are those
having 2 to 20 carbon atoms (a "C2-C70 alkynyl"), having 2 to 8 carbon atoms
(a "C"-C8
alkynyl"), having 2 to 6 carbon atoms (a "C2-C6 alkynyl"), or having 2 to 4
carbon atoms (a "Cr
C4 alkynyl"). Examples of alkynyl include, but are not limited to, groups such
as ethynyl (or
acetylenyl), prop-I -ynyl, prop-2-ynyl (or propargyl), but-1-ynyl, but-2-ynyl,
but-3-ynyl,
homologs and isomers thereof, and the like.
[0020] The term "aryl" refers to and includes polyunsaturated aromatic
hydrocarbon groups.
Aryl may contain additional fused rings (e.g., from 1 to 3 rings), including
additionally fused
aryl, heteroaryl, cycloalkyl, and/or heterocyclyl rings. In one variation, the
aryl group contains
from 6 to 14 annular carbon atoms. Examples of aryl groups include, but are
not limited to,
phenyl, naphthyl, biphenyl, and the like.
[0021] The term "cycloalkyl" refers to and includes cyclic univalent
hydrocarbon structures,
which may be fully saturated, mono- or polyunsaturated, but which are non-
aromatic, having the
number of carbon atoms designated (e.g., C1-C10 means one to ten carbons).
Cycloalkyl can
consist of one ring, such as cyclohexyl, or multiple rings, such as adamantyl,
but excludes aryl
groups. A cycloalkyl comprising more than one ring may be fused, spiro or
bridged, or
6
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
combinations thereof. A preferred cycloalkyl is a cyclic hydrocarbon having
from 3 to 13
annular carbon atoms. A more preferred cycloalkyl is a cyclic hydrocarbon
having from 3 to 8
annular carbon atoms (a "C3-C8 cycloalkyl"). Examples of cycloalkyl include,
but are not
limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, 1-cyclohexenyl,
3-cyclohexenyl,
cycloheptyl, norbornyl, and the like.
100221 "Halo" or "halogen" refers to elements of the Group 17 series having
atomic number 9 to
85. Preferred halo groups include fluoro, chloro, bromo and iodo. Where a
residue is substituted
with more than one halogen, it may be referred to by using a prefix
corresponding to the number
of halogen moieties attached, e.g., dihaloaryl, dihaloalkyl, trihaloaryl etc.
refer to aryl and alkyl
substituted with two ("di") or three ("tri") halo groups, which may be but are
not necessarily the
same halo; thus 4-chloro-3-fluorophenyl is within the scope of dihaloaryl. An
alkyl group in
which each hydrogen is replaced with a halo group is referred to as a
"perhaloalkyl." A
preferred perhaloalkyl group is trifluoroalkyl (-CF3). Similarly,
"perhaloalkoxy" refers to an
alkoxy group in which a halogen takes the place of each H in the hydrocarbon
making up the
alkyl moiety of the alkoxy group. An example of a perhaloalkoxy group is
trifluoromethoxy
(-0CF3).
[0023] The term "heteroaryl" refers to and includes unsaturated aromatic
cyclic groups having
from 1 to 10 annular carbon atoms and at least one annular heteroatom,
including but not limited
to heteroatoms such as nitrogen, oxygen and sulfur, wherein the nitrogen and
sulfur atoms are
optionally oxidized, and the nitrogen atom(s) are optionally quaternized. A
heteroaryl group can
be attached to the remainder of the molecule at an annular carbon or at an
annular heteroatom.
Heteroaryl may contain additional fused rings (e.g., from 1 to 3 rings),
including additionally
fused aryl, heteroaryl, cycloalkyl, and/or heterocyclyl rings. Examples of
heteroaryl groups
include, but are not limited to, pyridyl, pyrimidyl, thiophenyl, furanyl,
thiazolyl, and the like.
Examples of heteroaryl groups also include, but are not limited to, pyridyl,
pyrimidyl,
thiophenyl, furanyl, thiazolyl, oxazolyl, isoxazolyl, thiophenyl, pyrrolyl,
pyrazolyl, 1,3,4-
oxadiazolyl, imidazolyl, isothiazolyl, triazolyl, 1,3,4-thiadiazolyl,
tetrazolyl, benzofuranyl,
benzothiophenyl, pyrazolopyridinyl, indazolyl, benzothiazolyl, benzooxazolyl
or
benzoimidazolyl and the like.
[0024] In one variation, a heteroaryl containing at least one additional fused
ring that is
nonaromatic (e.g., cycloakyl or heterocycly1) is attached to the parent
structure at an annular
7
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
atom of the additional ring. In another variation, a heteroaryl containing at
least one additional
ring that is nonaromatic (e.g., cycloakyl or heterocyclyl) is attached to the
parent structure at an
annular atom of the aromatic ring.
100251 The term "heterocycle" or "heterocyclyl" refers to a saturated or an
unsaturated non-
aromatic group having from 1 to 10 annular carbon atoms and from 1 to 4
annular heteroatoms,
such as nitrogen, sulfur or oxygen, and the like, wherein the nitrogen and
sulfur atoms are
optionally oxidized, and the nitrogen atom(s) are optionally quaterniza A
heterocyclyl group
may have a single ring or multiple condensed rings, but excludes heteroaryl
groups. A
heterocycle comprising more than one ring may be fused, spiro or bridged, or
any combination
thereof. In fused ring systems, one or more of the fused rings can be aryl,
cycloalyl or
heterocyclyl. Examples of heterocyclyl groups include, but are not limited to,
tetrahydropyranyl,
dihydropyranyl, piperidinyl, piperazinyl, pyrrolidinyl, thiazolinyl,
thiazolidinyl,
tetrahydrofuranyl, tetrahydrothiophenyl, 2,3-dihydrobenzo[b]thiophen-2-yl, 4-
amino-2-
oxopyrimidin-1(2H)-yl, and the like.
[0026] In one variation, a heterocyclyl containing at least one additional
ring (such as a fused
additional ring) that does not contain a heteroatom is attached to the parent
structure at an
annular atom of the additional ring. In another variation, a heterocyclyl
containing at least one
additional ring (such as a fused additional ring) that does not contain a
heteroatom is attached to
the parent structure at an annular atom of the ring containing a heteroatom.
[0027] "Oxo" refers to the moiety =0.
[0028] "Optionally substituted" unless otherwise specified means that a group
may be
unsubstituted or substituted by one or more (e.g., 1, 2, 3, 4 or 5) of the
substituents listed for that
group in which the substituents may be the same of different In one
embodiment, an optionally
substituted group has one substituent. In another embodiment, an optionally
substituted group
has two substituents. In another embodiment, an optionally substituted group
has three
substituents. In another embodiment, an optionally substituted group has four
substituents. In
some embodiments, an optionally substituted group has 1 to 2, 2 to 5, 3 to 5,
2 to 3, 2 to 4, 3 to 4,
1 to 3, 1 to 4 or 1 to 5 substituents.
100291 A "pharmaceutically acceptable carrier" refers to an ingredient in a
pharmaceutical
formulation, other than an active ingredient, which is nontoxic to a subject.
A pharmaceutically
acceptable carrier includes, but is not limited to, a buffer, excipient,
stabilizer, or preservative.
8
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
[0030] As used herein, "treatment" or "treating" is an approach for obtaining
beneficial or
desired results including clinical results. For example, beneficial or desired
results include, but
are not limited to, one or more of the following: decreasing symptoms
resulting from the disease,
increasing the quality of life of those suffering from the disease, decreasing
the dose of other
medications required to treat the disease, delaying the progression of the
disease, and/or
prolonging survival of individuals. In reference to cancers or other unwanted
cell proliferation,
beneficial or desired results include shrinking a tumor (reducing tumor size);
decreasing the
growth rate of the tumor (such as to suppress tumor growth); reducing the
number of cancer
cells; inhibiting, retarding or slowing to some extent and preferably stopping
cancer cell
infiltration into peripheral organs; inhibiting (slowing to some extent and
preferably stopping)
tumor metastasis; inhibiting tumor growth; preventing or delaying occurrence
and/or recurrence
of tumor; and/or relieving to some extent one or more of the symptoms
associated with the
cancer. In some embodiments, beneficial or desired results include preventing
or delaying
occurrence and/or recurrence, such as of unwanted cell proliferation.
[0031] As used herein, "delaying development of a disease" means to defer,
hinder, slow, retard,
stabilize, and/or postpone development of the disease (such as cancer). This
delay can be of
varying lengths of time, depending on the history of the disease and/or
individual being treated.
As is evident to one skilled in the art, a sufficient or significant delay
can, in effect, encompass
prevention, in that the individual does not develop the disease. For example,
a late stage cancer,
such as development of metastasis, may be delayed.
[0032] As used herein, an "effective dosage" or "effective amount" of compound
or salt thereof
or pharmaceutical composition is an amount sufficient to effect beneficial or
desired results. For
prophylactic use, beneficial or desired results include results such as
eliminating or reducing the
risk, lessening the severity of, or delaying the onset of the disease,
including biochemical,
histological and/or behavioral symptoms of the disease, its complications and
intermediate
pathological phenotypes presenting during development of the disease. For
therapeutic use,
beneficial or desired results include ameliorating, palliating, lessening,
delaying or decreasing
one or more symptoms resulting from the disease, increasing the quality of
life of those suffering
from the disease, decreasing the dose of other medications required to treat
the disease,
enhancing effect of another medication such as via targeting, delaying the
progression of the
disease, and/or prolonging survival. In reference to cancers or other unwanted
cell proliferation,
9
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
an effective amount comprises an amount sufficient to cause a tumor to shrink
and/or to decrease
the growth rate of the tumor (such as to suppress tumor growth) or to prevent
or delay other
unwanted cell proliferation. In some embodiments, an effective amount is an
amount sufficient
to delay development. In some embodiments, an effective amount is an amount
sufficient to
prevent or delay occurrence and/or recurrence. An effective amount can be
administered in one
or more administrations, in the case of cancer, the effective amount of the
drug or composition
may: (i) reduce the number of cancer cells; (ii) reduce tumor size; (iii)
inhibit, retard, slow to
some extent and preferably stop cancer cell infiltration into peripheral
organs; (iv) inhibit (i.e.,
slow to some extent and preferably stop) tumor metastasis; (v) inhibit tumor
growth; (vi) prevent
or delay occurrence and/or recurrence of tumor; and/or (vii) relieve to some
extent one or more
of the symptoms associated with the cancer. An effective dosage can be
administered in one or
more administrations. For purposes of this disclosure, an effective dosage of
compound or a salt
thereof, or pharmaceutical composition is an amount sufficient to accomplish
prophylactic or
therapeutic treatment either directly or indirectly. It is intended and
understood that an effective
dosage of a compound or salt thereof, or pharmaceutical composition may or may
not be
achieved in conjunction with another drug, compound, or pharmaceutical
composition. Thus, an
"effective dosage" may be considered in the context of administering one or
more therapeutic
agents, and a single agent may be considered to be given in an effective
amount if, in
conjunction with one or more other agents, a desirable result may be or is
achieved.
[0033] As used herein, the term "individual" is a mammal, including humans. An
individual
includes, but is not limited to, human, bovine, horse, feline, canine, rodent,
or primate. In some
embodiments, the individual is human. The individual (such as a human) may
have advanced
disease or lesser extent of disease, such as low tumor burden. In some
embodiments, the
individual is at an early stage of a proliferative disease (such as cancer).
In some embodiments,
the individual is at an advanced stage of a proliferative disease (such as an
advanced cancer).
[0034] Reference to "about" a value or parameter herein includes (and
describes) embodiments
that are directed to that value or parameter per se. For example, description
referring to "about
X" includes description of "X".
[00351 It is understood that aspects and variations described herein also
include "consisting"
and/or "consisting essentially of' aspects and variations.
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
Compounds
[0036] In one aspect, provided is a compound of the formula (I):
or a tautomer or isomer thereof, or a pharmaceutically acceptable salt of any
of the foregoing,
wherein:
A is 4-hydroxyphenyl optionally further substituted by R3, 4-hydroxy-2-pyridyl
optionally further substituted by R4, a naphthyl substituted by R4, a 9- or 10-
membered bicylic
heterocylyl optionally substituted by R4, or a 9- or 10-membered bicyclic
heteroaryl optionally
substituted by R4;
B is a phenyl optionally substituted by R3, C3-C6 cycloalkyl optionally
substituted by R4,
3- to 6-membered heterocyclyl optionally substituted by R4 or a 5- to 10-
membered heteroaryl
optionally substituted by R4;
RI is a hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C6 cycloalkyl,
3- to 6-
membered heterocyclyl, 5- to 10-membered heteroaryl, -(C1-C3 alkylene)(C3-C6
cycloalkyl),
-(C1-C3 alkylene)(3-6-membered heterocyclyl), -(Ci-C3 allqlene)(5-6-membered
heteroaryl),
-(Ci-C3 alkylene)(C6 aryl), -C(0)Ria, -C(0)OR', -C(0)NR11'Ric, NRRlc¨s(o)2R', -
(Ci-
C3 alkylene)C(0)NRIbe, -(C1-C3 alkylene)C(0)Rla or -(C1-C3
alkylene)NRlb.n"KW., wherein the
C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C6 cycloalkyl, 3- to 6-membered
heterocyclyl, 5-
to 10-membered heteroaryl, -(C1-C3 alkylene)(C3-C6 cycloalkyl), -(C1-C3
alkylene)(3-6-
membered heterocyclyl), -(C1-C3 alkylene)(5-6-membered heteroaryl), and
-(C1-C3 alkylene)(C6 aryl) of RI are independently optionally substituted by
R4;
each Rla is independently hydrogen, C1-C6 alkyl, C3-C6 cycloalkyl, 3-6-
membered
heterocyclyl, C6 aryl, 5-6-membered heteroaryl, -(C1-C3 alkylene)(C3-C6
cycloalkyl),
C3 alkylene)(3-6-membered heterocyclyl), -(C1-C3 alkylene)(C6 aryl) or -(C1-C3
alkylene)(5-6-
membered heteroaryl), wherein each of which is optionally substituted by
methyl, ethyl, halogen,
oxo, -CF3, -OH, -OCH3, -CN, -C(0)0CH3, -C(0)0C2H5, -NH2 or -NHCH3;
11
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
each Rib and Ric is independently hydrogen, C1-C6 alkyl, C3-C6 cycloalkyl, 3-6-
membered heterocyclyl, C6 aryl, 5-6-membered heteroaryl, -(C1-C3 alkylene)(C3-
C6
cycloalkyl), -(C1-C3 alkylene)(3-6-membered heterocyclyl), -(C1-C3
alkylene)(C6 aryl) or -(C1-
C3 alkylene)(5-6-membered heteroaryl), wherein each of which is optionally
substituted by
methyl, ethyl, halogen, oxo, -CF3, -OH, -OCH3, -CN, -C(0)OCH3, -C(0)0C2H5, -
NH2 or -
NHCH3;
or Rib and Ric are taken together with the nitrogen atom to which they are
attached to form a 3- to 6-membered heterocyclyl;
R2 is hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C6-C14 aryl, C5-C14
heteroaryl,
C3-C6 cycloalkyl, 3- to 6-membered heterocyclyl, -CN, halogen, -0R2, -SR2a, -
NR2bR2c,
-C(0)R2', -NR2bc(0)R2c _NR2ac(0)NR2bR2c _C(0)0R2', -C(0)0NR21'R2c or -
C(0)NR2bR2c,
wherein the C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C6-C14 aryl, C3-C6
cycloalkyl and 3- to
6-membered heterocyclyl of R2 are independently optionally substituted by R4;
each R2a is independently hydrogen, C1-C6 alkyl, C3-C6 cycloalkyl, 3- to 6-
membered
heterocyclyl, C6-aryl, 5- to 6-membered heteroaryl, -(C1-C3 a1kylene)N(C2H5)2,
-(Ci-
C3 a1kylene)(C3-C6 cycloalkyl), -(C1-C3 alkylene)(3-6-membered heterocyclyl), -
(Ci-
C3 alkylene)(5-6-membered heteroaryl) or -(C1-C3 alkylene)(C6 aryl), wherein
each of which is
optionally substituted by methyl, ethyl, halogen, oxo, -CF3, -OH, -OCH3, -CN, -
C(0)OCH3, -
C(0)0C2H5, -NH2 or -NHCH3;
each R2b and R2c is independently hydrogen, C1-C6 alkyl, C3-C6 cycloalkyl, 3-
to 6-
membered heterocyclyl, C6-aryl, 5- to 6-membered heteroaryl, -(C1-C3
alkylene)N(C2H5)2,
C3 alkylene)(C3-C6 cycloalkyl), -(C1-C3 alkylene)(3-6-membered
heterocyclyl), -(C1-C3 alkylene)(C6 aryl) or -(CI-C3 alkylene)(5-6-membered
heteroaryl),
wherein each of which is optionally substituted by methyl, ethyl, halogen,
oxo, -CF3, -OH, -
OCH3, -CN, -C(0)OCH3, -C(0)0C2H5, -NH2 or -NHCH3;
or R2b and R2c are taken together with the nitrogen atom to which they are
attached to form a 3- to 6-membered heterocyclyl;
each R3 is independently C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, halogen, -
CN, -0R5,
-SR5, -NR6R7, -NO2, -C=NH(OR5), -C(0)R5, -0C(0)R5, -C(0)0R5, -C(0)NR6R7,
-0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)0R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, -
NR5S(0)R6,
-C(0)NR5S(0)R6, -NR5S(0)2R6, -C(0)NR5S(0)2R6, -S(0)NR6R7, -S(0)2NR6R7, -
P(0)(0R6)
12
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/04 2 7 7
7
(OR7), C3-C6 cycloalkyl, 3-12-membered heterocyclyl, 5- to 10-membered
heteroaryl, C6-
C14 aryl, -(CI-C3 alkylene)CN, -(C1-C3 alkylene)0R5, -(C1-C3 alkylene)SR5, -
(C1-
C3 alkylene)NR6R7, -(C1-C3 alkylene)CF3, -(C1-C3 alkylene)NO2, -C=NH(0R5), -
(C1-
C3 alkylene)C(0)R5, -(C1-C3 alkylene)0C(0)R5, -(C1-C3 alkylene)C(0)0R5, -(Ci-
C3 alkylene)C(0)NR6R7, -(C1-C3 alkylene)0C(0)NR6R7, -(C1-C3
alkylene)NR5C(0)R6,
C3 alkylene)NR5C(0)0R6, -(Ci-C3 alkylene)NR5C(0)NR6R7, -(C1-C3
alkylene)S(0)R5, -(C1 -
C3 alkylene)S(0)2R5, -(C1-C3 alkylene)NR5S(0)R6, -C(0)(C J-C3
alky1ene)NR5S(0)R6, -(C1-
C3 alkylene)NR5S(0)2R6, -(C1-C3 alkylene)C(0)NR5S(0)2R6,
alkylene)S(0)NR6R7,
-(C1-C3 alkylene)S(0)2NR6R7, -(C1-C3 alkylene)P(0)(0R6)(01e), -(C1-C3
alkylene)(C3-C6
cycloalkyl), -(C1-C3 alkylene)(3-12-membered heterocyclyl), -(C1-C3
alkylene)(5- 10-membered
heteroaryl) or -(C1-C3 alkylene)(C6-C14 aryl), wherein each R3 is
independently optionally
substituted by halogen, oxo, -0R8, -NR8R9, -C(0)R8, -CN, -S(0)R8, -S(0)2R8, -
P(0)(0R8)(0R9),
-(C1-C3 alkylene)0R8, -(Ci-C3 alkylene)NR8R9, -(C1-C3 alkylene)C(0)R8,
-(C1-C3 alkylene)S(0)R8, -(Ci-C3 alkylene)S(0)2R8, -(C1-C3
alkylene)P(0)(0R8)(0R9), C3-C8
cycloalkyl, or C1-C6 alkyl optionally substituted by oxo, -OH or halogen;
each R4 is independently oxo or R3;
R5 is independently hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C6
cycloalkyl, C6-C14 aryl, 5-6-membered heteroaryl or 3-6-membered heterocyclyl,
wherein the
C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C6 cycloalkyl, C6-C14 aryl, 5-6-
membered
heteroaryl and 3-6-membered heterocyclyl of R5 are independently optionally
substituted by
halogen, oxo, -CN, -0R9, -NR9R1 , -P(0)(0R9)(0R1 ), phenyl optionally
substituted by halogen,
or C1-C6 alkyl optionally substituted by halogen, -OH or oxo;
R6 and R7 are each independently hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6
alkynyl,
C3-C6 cycloalkyl, C6-C14 aryl, 5-6-membered heteroaryl, -(C1-C3 alkylene)(C6
aryl) or 3-6
membered heterocyclyl, wherein the C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl,
C3-C6 cycloalkyl,
C6-C14 aryl, 5-6-membered heteroaryl, -(C1-C3 alkylene)(C6 aryl) and 3-6
membered
heterocyclyl of R6 and R7 are independently optionally substituted by halogen,
oxo, -CN, -0R9,
-NR9R1 or C1-C6 alkyl optionally substituted by halogen, -OH or oxo;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl optionally substituted by halogen, oxo, -0R9, -NR9R1 or
C1-C6
alkyl optionally substituted by halogen, oxo or -OH;
13
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
R8 and R9 in R3 are each independently hydrogen, Ci-C6 alkyl optionally
substituted by
halogen or oxo, C2-C6 alkenyl optionally substituted by halogen or oxo, or C2-
C6 alkynyl
optionally substituted by halogen or oxo;
or R8 and R9 in R3 are taken together with the atom to which they attached to
form
a 3-6 membered heterocyclyl optionally substituted by halogen, oxo or CI-Co
alkyl
optionally substituted by halogen or oxo; and
R9 and R1 in R5, R6 and R7 are each independently hydrogen, C1-C6 alkyl
optionally
substituted by halogen or oxo, C2-C6 alkenyl optionally substituted by halogen
or oxo, or C2-
C6 alkynyl optionally substituted by halogen or oxo;
or R9 and R1 in R5, R6 and R7 are taken together with the atom to which they
attached to form a 3-6 membered heterocyclyl optionally substituted by
halogen, oxo or
Ci-C6 alkyl optionally substituted by oxo or halogen.
[00371 in some embodiments, provided is a compound of the formula (I):
0),
or a salt thereof, wherein:
A is 4-hydroxyphenyl optionally further substituted by R3, 4-hydroxy-2-pyridyl
optionally further substituted by R4, or a 9- or 10-membered bicyclic
heteroaryl optionally
substituted by R4;
B is a phenyl optionally substituted by R3, or a 5- to 6-membered heteroaryl
optionally
substituted by R4;
RI is a hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C6 cycloalkyl,
3- to 6-
membered heterocyclyl, -C(0)R1a, -C(0)OR', -C(0)NR1bRic, or _NRibRic, wherein
the CI -C6
alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C6 cycloalkyl and 3- to 6-membered
heterocyclyl of R1
are independently optionally substituted by R4;
each lea is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
each RII) and lee is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or Rib and Ric are taken together with the nitrogen atom to which they are
attached to form a 3- to 6-membered heterocyclyl;
14
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
R2 is hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C6-C14 aryl, -CN,
halogen,
-0R2a, -NR2bR2c, -C(0)R2a, -C(0)0R2a, or -C(0)NR2bR2c, wherein the Ci-C6
alkyl, C2-C6 alkenyl,
C2-C6 alkynyl and C6-C14 aryl of R2 are independently optionally substituted
by R4;
each R2a is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
each R2b and R2c is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R21' and R2c are taken together with the nitrogen atom to which they are
attached to form a 3- to 6-membered heterocyclyl;
each R3 is independently CI-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, halogen, -
CN, -0R5,
-SR5, -NR6R7, -NO2, -C=NH(0R5), -C(0)R5, -0C(0)R5, -C(0)0R5, -C(0)NR6R7,
-0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)0R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, -
NR5S(0)R6,
-C(0)NR5S(0)R6, -NR5S(0)2R6, -C(0)NR5S(0)2R6, -S(0)NR6R7, -S(0)2NR6R7, -
P(0)(0R6)
(OR7), C3-C6 cycloalkyl, 3-1 2-membered heterocyclyl, 5- to 10-membered
heteroaryl, C6-
C14 aryl, -(C1-C3 alkylene)CN, -(C1-C3 alkylene)0R5, -(C1-C3 alkylene)SR5, -
(C1-
C3 alkylene)NR6R7, -(C1-C3 alkylene)CF3, -(C1-C3 alkylene)NO2, -C=NH(0R5), -
(C1-
C3 alkylene)C(0)R5, -(Ci-C3 alkylene)0C(0)R5, -(C1-C3 alkylene)C(0)0R5, -(Ci-
C3 alkylene)C(0)NR6117, -(C1-C3 alkylene)0C(0)NR6R7, -(C1-C3
alkylene)NR5C(0)R6, -(C1-
C3 alkylene)NR5C(0)0R6, -(C1-C3 alkylene)NR5C(0)NR6R7, -(C1-C3
alkylene)S(0)R5, -(C1-
C3 alkylene)S(0)2R5, -(Ci-C3 alkylene)NR5S(0)R6, -C(0)(C1-C3
a1kylene)NR5S(0)R6, -(Ci-
C3 alkylene)NR5S(0)2R6, -(C1-C3 alkylene)C(0)NR5S(0)2R6, -(C1-C3
alkylene)S(0)NR6R7,
-(C1-C3 alkylene)S(0)2NR6R7, -(C1-C3 alkylene)P(0)(0R6)(01e), -(C1-C3
alkylene)(C3-C6
cycloalkyl), -(C1-C3 alkylene)(3-1 2-membered heterocyclyl), -(C1-C3
alkylene)(5-1 0-membered
heteroaryl) or -(C1-C3 alkylene)(C6-Ci4 aryl), wherein each R3 is
independently optionally
substituted by halogen, oxo, -0R8, -NR8R9, -C(0)R8, -CN, -S(0)R8, -S(0)2R8, -
P(0)(0R8)(0R),
-(C1-C3 alkylene)0R8, -(Ci-C3 alkylene)NR8R9, -(C1-C3 alkylene)C(0)R8,
-(CI-C3 alkylene)S(0)R8, -(C1-C3 alkylene)S(0)2R8, -(C1-C3
alkylene)P(0)(0R8)(0R9), C3-C8
cycloalkyl, or C1-C6 alkyl optionally substituted by oxo, -OH or halogen;
each R4 is independently oxo or R3;
R5 is independently hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C6
cycloalkyl, C6-C14 aryl, 5-6-membered heteroaryl or 3-6-membered heterocyclyl,
wherein the
CI-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C6 cycloalkyl, C6-C14 aryl, 5-6-
membered
heteroaryl and 3-6-membered heterocyclyl of R5 are independently optionally
substituted by
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
halogen, oxo, -CN, -0R9, -NR91e, -P(0)(0R9)(010, phenyl optionally substituted
by halogen,
or CI-C6 alkyl optionally substituted by halogen, -OH or oxo;
R6 and R7 are each independently hydrogen, C1-C6 alkyl, C2-C6 alkenyl, C2-C6
alkynyl,
C3-C6 cycloalkyl, C6-C14 aryl, 5-6-membered heteroaryl or 3-6 membered
heterocyclyl, wherein
the C1-C6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl, C3-C6 cycloalkyl, C6-C14 aryl,
5-6-membered
heteroaryl and 3-6 membered heterocyclyl of R6 and R7 are independently
optionally substituted
by halogen, oxo, -CN, -0R9, -NR9R1 or C1-C6 alkyl optionally substituted by
halogen, -OH or
oxo;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl optionally substituted by halogen, oxo, -0R9, -NR9R1 or
C1-C6
alkyl optionally substituted by halogen, oxo or -OH;
R8 and R9 are each independently hydrogen, C1-C6 alkyl optionally substituted
by
halogen or oxo, C2-C6 alkenyl optionally substituted by halogen or oxo, or C2-
C6 alkynyl
optionally substituted by halogen or oxo;
or R8 and R9 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl optionally substituted by halogen, oxo or C1-C6 alkyl
optionally
substituted by halogen or oxo; and
R9 and le are each independently hydrogen, Ci-C6 alkyl optionally substituted
by
halogen or oxo, C2-C6 alkenyl optionally substituted by halogen or oxo, or C2-
C6 alkynyl
optionally substituted by halogen or oxo;
or R9 and le are taken together with the atom to which they attached to form a
3-
6 membered heterocyclyl optionally substituted by halogen, oxo or C1-C6 alkyl
optionally
substituted by oxo or halogen.
1100381 In some embodiments, the compound of formula (I) or a salt thereof is
other than a
compound selected from Table 1 or a salt thereof. In some embodiments, the
compound of
formula (I) or a tautomer or isomer thereof, or a pharmaceutically acceptable
salt of any of the
foregoing is other than a compound seletec from Table 1 or a tautomer or
isomer thereof, or a
pharmaceutically acceptable salt of any of the foregoing.
Table 1
Compound
No. Compound Name
16
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042 777
Ix 5-(1-ethyl-1H-indo1-5-y1)-6-(3-pyridinyl)-2-pyrazinamine
2x 5-( I -ethyl-1H-indo1-5-y1)-6-(2H-tetrazol- 5-yI)-2-pyrazinam in e
3x 246-amino-3-(1-ethy1-2-fluoro-1H-indo1-5-y1)-2-pyrazinyl]-4-
fluorophenol
4x 5-( I -ethy1-1H-indo1-5-y1)-6-(3-th i eny I )-2-pyrazinamine
5x 2- [6-amino-3-(1-ethy1-1H-indo1-5-yr1)-2-pyrazinyl]-6-fluorophenol
6x 5-(1-ethy1-1H-indo1-5-y1)-6-(1H-pyrazol-3-y1)-2-pyrazinamine
7x 246-amino-3-(1-ethy1-2-methy1-1H-indo1-5-y1)-2-pyrazinyl]-4-
fluorophenol
8x 546-amino-3-(1-ethy1-1H-indo1-5-y1)-2-pyrazinyl]-4(3H)-pyrimidinone
9x 5-(2-benzofurany1)-6-(4-pyridiny1)-2,3-pyrazinediamine
2-[6-amino-3-(1-ethy1-2-methy1-111-benzimidazol-5-y1)-2-pyraziny1]-4-
10x
fluorophenol
I I x 5-(i-ethyl-I H-indo1-5-y1)-6-(1H-pyrrol-2-y1)-2-pyrazinam inc
12x 2- [6-amino-3 -(1 -ethyl-1 H-indo1-5-y1)-2-pyraziny1]-4-fluorophenol
13x 346-amino-3-(1-ethy1-1H-indo1-5-y1)-2-pyrazinyli-2(1H)-pyridinone
14x 5-(1-ethy1-1H-i ndo1-5-y1)-6[5-fluoro-2-(phenylmethoxy)pheny1]-2-
pyrazinami ne
15x 246-amino-3-(1-ethy1-1H-benzimidazol-5-y1)-2-pyrazinyl]-4-
fluorophenol
I 6x 5-(1-ethy1-1H-indo1-5-y1)-6-(2-thieny1)-2-pyrazinamine
17x 2-[6-amino-3-(1-ethy1-1H-indo1-5-y1)-2-pyrazinyl]phenol
18x 5,6-bis(1-pheny1-1H-benzimidazol-2-y1)-2,3-pyrazinediamine
100391 In some embodiments, RI is a hydrogen, CI-C.6 alkyl, C2-C6 alkenyl, C2-
C6 alkynyl, C3-C6
cycloalkyl, 3- to 6-membered heterocyclyl, 5- to 10-membered heteroaryl, -
C(0)Ria, -C(0)0Ria,
-C(0)NRibRic, or _soy 2¨ la,
) K wherein the CI-C.6 alkyl, C2-C6 alkenyl, C2-C6 alkynyl,
C3-C6
cycloalkyl, 3- to 6-membered heterocyclyl, and 5- to 10-membered heteroaryl
are optionally
substituted with R4. In some embodiments, RI is hydrogen, C1-C6 alkyl or
¨C(0)R. In certain
embodiments, RI is hydrogen. In certain embodiments, RI is ¨C(0)Ria where RI
is Ci-C6 alkyl
(e.g., methyl) or C3-C6 cycloalkyl. It is understood that each RI may be
combined with each R2,
A and/or B the same as if each and every combination of RI with R2, A and/or B
were
specifically and individually listed.
17
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
[00401 In some embodiments R2 is selected from the group consisting of:
hydrogen, fluor ,
chloro, bromo, -CN, methyl, ethyl, isopropyl, propyl, tert-butyl, isopropenyl,
-0043, -
C(0)OCH3, -C(0)0H, -C(0)0NE2, -CH2NH2, -NH2, -NHCH3, -N(CH3)2, -OH, -CF3, -OM,
_., --..i'
µ,.....,..- .,,,:.,¨.s..,, ,,,,,,,..-L, 4.1/4...,.."....õ,. µ,. ,,,,, "v-
-"N.µ,..õ 41,.."----
, 5-.
NH2 OH
µ
jj ,LO ..,70 CN-- r----NIA N r 3"
..õ, Nr---71 µ \ ,,,, -,,/ .õ.._,N-,./ y N.
.1zr_
N r_ 0,0H 0 o 0
--....ii µ,- IN
\--r`i. -,..-:-=)- µzzir-1-"- µ = 01 '2 SS N-)
F.
H N H N
µ0µ0µ0
. ,
co
'-,..
H
40.. C N
N 2
--..
0.yeA . 14111) y
H N H N
N H
V N H V N H N H
V N H
,
0.
HO
',.. ,=-`
N 0
0,J 0
r)
yil_, .NH
-NH _ S ,zazi,, N H v0 ,21.2,..._, N H
V NH
18
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
1 OH ."-=0
N
---= N. `µ,.. "-... .=
1
The '`i\j'i ,-,' ..-- ,-..f---- =
(--- re. r''-'0 '' ---` Oy NH
r---
N,J ,r<) , 0 NH NH NH NH 0
'11i ' V
, ,
i H
N 0 N co 1 \
y 7 9 9 qv
v...0 ..,,O
,
1
,) 0
vo \---() \--O ,0 \--- 1 \--- µ,0
, , ,
0 O 0
4 N
H
,2(0
FE
----F
(-/ N F H
CN N N
r
: 0
rCil =-..
\-- \--6 µ,---
0 0 \
9 (r) g 71_5
\ 0 Nid
si s
19
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I H
N 0 N
, rN IA ri--77
s s s s
v ..s vs ,s
vs ---- \-- \--- , vs
, ,
F F
?---F;
9 0 \
re.13 reCrr.-- ralH N N N
H rj rCN¨ rj
\..
.s vs vs v.s vs
N N 0 F tan ON H
N
NH v_NH ,2,141
7 4 7 -4 7 -4 '
I
0 I
., 9 p ti,N)H
crN)H l',,,c5 <,D) p \ 0-1--
µ,..NH NH \NH µõNH NH µ,õNH v_NH v.,NH
NH õt NH
, '
spy
r.-N-r= (I\ ri-
'NH NH NH NH L s3 ri3
s, NH NH ,NH
µ,--- '?,---
µ' , NH
, , , ,
OH
F F
N
N N N
NH rai
rj rj rj rj
NH \,õNH ,aNH ,,..-NH ,,,NH NH v.,NH
, 1..
, -a- , .2. =
0 F 0 H
ON
N N
<ae(NH y_e NH ,,,õ. NH
'''-4.
, '
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
0 H
..,./...c. re-"N"'-- ----NH N
0 0\
-,- /-
\.
,---- \
..Y
0.---
/ H
N
N H .,-0) N 0 0
ry :
-.-, N--) r N ....,,,,-
\. `'2z.,-'0 \ 0 µ
,
i
..... r N
rk..,) (ND 0
_,- K.-0
r ,,,)
,..-.
, , ,
9
0
CON,) rNin_,,, _
rõ"C- , -,... 0
: I .......- L,..... -,.......ro oyi
o.yk
A, ,,,, NH NH
. `zzl:r0 ''2z. 0 `'zz.--0 '-t. v, N õ,... , ,
,
/
0 0
0...õ.1-...)
0y1\ 0,y1--3 0 0 0
N H ,z N H NH NH NH NH NH
It. - , , ,
, , ,
..""
0
i
N..,-= dith F r- N N
n r \N H 0,..,.)-1...i 0 1 .,.,-'-' (--)
k..µ,...---...õ/ 0 0 RIP
%.,õ...õ...-
N H N H N H NH NH NH
.'2,_7. '121.-'
, , , , , ,
OH
I
OH
N
46
? \ N
N N N
N,J
NH ,.. .õ NH NH NH NH _,. NH _.õ., N H N H
.1 'Ilt. \a" 1.- V'
, , . , ,
21
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
r--- 1
0 0 0 0
,.Ø..,
, .,-.
N
0,y,1 N,.I 0 0 HN -.-* HN HNI---
NH NH NH NH v-"L-0 ,N.....-0
'a 7
'a.
,.
,
/
HN A ....-0 r¨NH
)...s.s.:0
HN-X-3 HN HN "Q
cz2z() \Ao \v,0 µ70 EN N
0 HN HN---Cs/
0
r
NH
iiih I; F i 4N.,)õõ:1
L ,
HN HN-aj--- HN Ill HN --,
HN HN
, , , =
I H
OH N 0 0 N
OH
HNY 11\11Y
HN HN HN HN HN HNJ HN
--c) ,zza.,--.0 O 'O ,L4.--.0 õ.11...,-"Lo .1/4,-.0
,
OH
r----
0,.0
i -,:-
0 N 1 /
N..) 1¨NH 0 ,
0
y ....., N
HN HN HN
HN FIN HN HN
.2,( -, -- III,0 "-0 --7-0 µazt..O 'ir-0
\ ' , ,
22
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
i
Oz..,_ ,..0
--'3"
`-....
".0HN CY oj< .A -0 0-0 0
! . 0
.\----0 \------0 \---.0 \:-'-'L0 , VLO , ,
ip 0
/ /
r---N--
b
N..-
- N
J-,-....0
j.1.> ¨
--1 9 0--;-----/ o
0
. , . OF-1
r2
r
01101 F
N
/
...,0,/ N,,
,.)','N
aY
0 0 . 0 0 0
..,..0 0 ,,,,,,-
0 ,õ,..0
, ,
, 1
T 9 / iN1 N
yH 0 :H N
0 ...0FI N
0
0 0 0 0 0 0 0
N.
, r--- 1
0 0 0 0
/
5N --'-
----
H r--- Y .9
QyNH 0NH
1 i
,22(...õ, ,222.,""õ..0 , ,/,,NH
NNHz. , VNH
,
-5.
, ,
23
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
I / H
K1 0 N
N
? Ni
9 0 NH 0,NH 0 0NH Qz.,..õ-NH 0.,..õ.õNH
NH
,...õõ ---.1-
-µ -1 1
NH V 4.ec t,--
NH ,,z21..õ.NH NH NH NH `
,..___,NH
Y V
-,..... , , , 1-,N--
e,'`. 13
0.,õ.1\1H 0,..õNH C:t.õNH 0-....-NH 0NH 0.NH
NH NH NH v,NH NH ,.., ,..,,NH
, 1 , ,
/ /
v.Q p
rfiN.-- r-N1 . .0
Ia. N. rr-N >
-)) . = .`"-W
0..._,NH 0..,.NH 0_,...NH 0,õNH 0,..NH
NH .._,,NH i- ,....õõNH NH ,,NH
it'
...""
. and
N 0
HU'
N,...NH
NH
µ7 ; wherein the wavy lines denote attachment points to the parent
molecule.
100411 In some embodiments R.2 is selected from the group consisting of:
hydrogen, fluor ,
chloro, bromo, -CN, methyl, ethyl, isopropyl, propyl, tert-butyl, isopropenyl,
-OCH3, -
C(0)0CH3, -C(0)0H, -C(0)0NH2, -CH2NH2, -NH2, -NHCH3, -N(CH3)2, -OH, -CF3, -
0CF3,
¨0 r--NH
HNI ----
/NH
(v,Z)-- ji
).õ...).
2 2 2 7
N
..... õflp ...0 ,701H .410 õ,,,NO -',,,,. 1 -;;Cl.1
\ = = = = \. µ ,,.,
and
,
so
; wherein the wavy lines denote attachment points to the parent molecule.
24
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
[00421 In some embodiments R2 is selected from the group consisting of:
hydrogen, fluor ,
chloro, bromo, -CN, methyl, ethyl, isopropyl, propyl, tert-butyl, isopropenyl,
-0043, -
C(0)OCH3, -C(0)0H, -C(0)0NE2, -CH2NH2, -NH2, -NHCH3, -N(CH3)2, -OH, -CF3, -
0CF3,
N'IN1
V
NH-,..
r---\
1.----NN¨ 1 NH r¨r- ' r----r-OH
N--1 ,z. \ V N.¨/ NH NH NH NI ¨../ .,õ --
'7.---
V µ s. V
, , , ,
OH '.=0
F õ...0õ,1
40 11 F 1/101
r)
1,-----.N.-- ....õ---...O r)
µNH ..õ. NH NH v, N ,...) ,,It...õ, N ,.... ) ,atzõ. NH
,11( N H NH
..-- 4??.2. -
,
0 .
-'-` Y 7 9 n C \-1:3--1 7 K 9
\I
v. NH v....NH 0 v.-NH IFi ,122..r, NH NH ,v
NH ,v, NH ..z.t2:õ. NH v, NH
,
\ 0 N /
U
1,,,,Alli CiNil 01
, rN IA L:7 r
v_NH NH võ N H ,z2z...., 1.11H \c, NH L..õ.
NH v. NH NE-I
V V NH
, t , ,
OH F F
ri0 4N1-1
rj N
---- N
,--) N
--)
µ, NH ,a,21,õ NH ,,,.NH ,v,NH \. NH NH
,2z2.,,.NH
V
' , `2.= ,
N F 40 ON
szer, NH v,. NH ,,..,. NH
and µ2- ;
wherein the wavy lines denote attachment
points to the parent molecule.
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
[00431 In some embodiments R2 is selected from the group consisting of:
hydrogen, fluor ,
chloro, bromo, -CN, methyl, ethyl, isopropyl, propyl, tert-butyl, isopropenyl -
OCH3, -
C(0)OCH3, -C(0)0H, -C(0)0N112, -CH2NH2, -NH2, -N-HCH3, -N(CH3)2, -OH, -CF3, -
0CF3,
F
elõ.._,,..,N
o
0 0 -,,A
H..--
________________________________________________________ HN N
n 0 0 ...:> :_t
0 ,,,,,,..õ.õ0
,
CN H
N L.N
)
0õ-A 0 0 . 0 0 ,... 1 y
HN HN
\-L0 \/L0 \NH NH
t2L--- \-.-- NH
\--NH
\--.NH
, , -i- , . ,
HO
L. 0 N 0
''....---1. 0
I !
---- .------) n
N ---
,) 0.) ) C,N)
N --,,r0 0.,.... j 0..,,,l<
vNH ' NH
YLO 'ta(LO V NH
NH
i
,-N 0 oy,co
OA 00 0 0 0 i 0,,L)
,
,z.c.., NH v. NH ,7221/.., NH
NH
N H µ2....õ. NH , .., NH
X
, , , ''. , ,
0
/ N N
õ-N -.... õ--- -,---
1----\
(-).õ...1.,i,,NH,Jt 0-1-1-..) 0 1
0 0
-1
\
< NH NH NH NH NH NH--"'
z.
, , , .
= ,
,
26
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
4.H
\N 9H r,N
N N N
0,57 0 0 0,õ-1 0.,.)
(:),J 0,)
N/
NH ,., N1I-1 NH NH NH NH ,NH NH
'122.
, , , , '
..--
I
0 0 0 0
0
I=-, N ..,'
N N
0,J Cy 0 0 HN"... El HNV-- HN j<
\V
NH NH NH NH N. \V- \-V 411. lk2Nj
/
--NH -N
HNA
HN.,-0 HNLI) HN HN-L)---47 T-0
FIN -HN
0
r
F/ 1,:,,...1
--NH
I )
ij...i .--'"
HN 1. HN HN HN"..--
HN ----/ HN"all---
I H
OH N 0 0 N
y OH
HNY N
-I
HN HNy HN HN HN FiN HN'
lk.,---0 ,z2_,-,--0 ",22,.."--0 tt.,-.0 ,,t2...----0 ,-tc,L0 4..,10 \------0
27
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
----
OH 1 C-a
¨N/
7_0 y ivr\i/
f f H--'
HN' HN
HN HN HN HN N
µ.,-"-"--,0 ,,.<0 .1(--0 \...,-"0 ,,..----0
,
1
0 0
H N
,,,..---.0
and 1. ; wherein the wavy lines denote attachment points to the parent
molecule.
[00441 In some embodiments R2 is selected from the group consisting of:
hydrogen, fluor ,
chloro, bromo, -CN, methyl, ethyl, isopropyl, propyl, tert-butyl, isopropenyl;
-OM, -
C(0)0CH3, -C(0)0H, -C(0)0N-H2, -CH2NH.2, -I\III2, -MICH-. -N(CH3)2, -OH, -CF3,
-0C,F3,
I H
CriO :::N.TiO \c)
or Y Y .<?. 9 Y ?Y
___0 0 0 6 ,,o 0
V V V. lar \--' V Vo
V \---
Id --= --- p 6.-------'N
V V
0 O 0
r JO rzi,\F-- 1-4 N
N
i'N¨ _...),..,7ri_
r'j I 1)
28
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
VF
F
.,. d N F . CN
ra N
rr,,..- 1 1411)
and
, Tz.
, t
H
N N
.-_,:: --....-= =,õ
I
v0
; wherein the wavy lines denote attachment points to the parent molecule.
[00451 In some embodiments R2 is selected from the group consisting of:
hydrogen, fluor ,
chloro, bromo, -CN, methyl, ethyl, isopropyl, propyl, tert-butyl, isopropenyl -
OCH3, -
C(0)0CH3, -C(0)014, -C(0)0NIT2, -CH2N1I2, -NI-12, -NHCII3, -N(CII3)2, -OH, -
CF3, -0CF3,
F 0 \ 0 1
illo i r y 7 9. 9 g H Ni.c/ Lµ111 N
vs .õS
I H
N 0 N
p isi; -Ty) 0 ciii ,
rN rA r
S s S S S ft .,..S ) S S
.12?...õ v v S v v
V \---.
0 0
ri ra raNi H N
ri N
(ON -
VF
F
F . CN H
N N
N
ro I. ...
,...- 1
i ,ztcs
and "it. ;
wherein
, -'= ,
the wavy lines denote attachment points to the parent molecule.
29
CA 03070273 2020 - 01 - 16
WO 2019/018584 PCT/US2018/042777
100461 In some embodiments R2 is selected from the group consisting of:
hydrogen, fluoro,
chloro, bromo, -CN, methyl, ethyl, isopropyl, propyl, tert-butyl, isopropenyl,
-0C1-13, -
C(0)OCH3, -C(0)0H, -C(0)0NH2, -CH2NH2, -NH2, -NHCH3, -N(CH)2, -OH, -CF3, -
0CF3,
Oy NH N,N,,. Oy NH oy NH Oy NH Oy NH NH ONH Oy NH
v NH 421...õ.NH 5.,N,,, \,..#,NH v NH ,NH
NH v NH ,,-
ci,,,, NH
1 H (NJ
Ni) , oL. to riii
, i
ri rA
N
0 .._,NH 0.NH 0,U 0..,,N1H 0NH 0N1H
YNH 0 NH 0 =' -. --s --.
NH
5.(NH '7.
..v,NH v-INH NH i.r,NH ,z(NH NH
t).).
/
(I:, ro r-C1 rLiN". r
p
N,NH O. NH 0,,..,NH
-I 0,t,..,, NH
-C 0NH 0, NH
'S
NH ., ,NH
Y , ,NH
NH
`( NH _ NH
rx) r
0.,,NH N., NH N, NH
"
/NH NH
'V''NH
, Tt and ;
wherein the wavy lines denote
attachment points to the parent molecule.
100471 In some embodiments R2 is selected from the group consisting of:
hydrogen, fluoro,
chloro, bromo, -CN, methyl, ethyl, isopropyl, propyl, tert-butyl, isopropenyl,
-0C1-13, -
C(0)0C1I3, -C(0)0H, -C(0)0N1-I2, -Cl2N112, -NI12, -NHC1-13, -N(C143)2, -OH, -
C,F3, -0CF3,
r'N¨ rN,-
.,,N,,,
V-LO \X \ \- XO 10 \O 510 .2/(0 ,
, 5 ,
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
0'-'
0 H H
/
NH N NI/ OH
'2.2L 0 `/42X \ 0 YLO
i
r"
----C
0
0
Cl--N-- "Nr---=-\_._ 0
N,.) N
L.
µ and \
C) ;
wherein
the wavy lines denote attachment points to the parent molecule.
[00481 In some embodiments R2 is selected from the groups consisting of:
hydrogen, Now,
chloro, bromo, -CN, methyl, ethyl, isopropyl, propyl, tert-butyl, isopropenyl,
-OM, -
C(0)OCH3, -C(0)0H, -C(0)0NE2, -CH2NH2, -NH2, -NHCH3, -N(CH3)2, -OH, -CF3, -OM,
/
r- N
-,-
0 0'< OA 0 OL) TO cyõ1¨o
\-A0 \----Lo '',,Ao
'
o o
i b L
N_ F
r...,
0---L----/ 0 0 0 . =
\----Lo
,
, , 2 .
r ? ?
OH
i N V
0 0H;
A)
,..---N '''-')
,.-
0 0) 0 0 0
.\...,---0 ,,,,v0 ,,,,--0 ,z,,,,-.0
, , ,
31
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
01H FN
0
or- o o
111.
and ; wherein the wavy lines denote attachment points to the parent
molecule.
[0049] It is understood that each R2 may be combined with each RI, A and/or B
the same as if
each and every combination of R2 with RI, A and/or B were specifically and
individually listed.
In some embodiments R2 is hydrogen, CI-Co alkyl, -CN, halogen, -0R2a,
¨C(0)R2a,
-C(0)0NR2bR2c, -C(0)NR2bR2c, -NR2bR2c, -NR2bC(0)R2c, -NR2aC(0)NR2bR20, -SR2a
or -
C(0)0R2a. In some embodiments, R2 is hydrogen. In some embodiments, R2 is Ci-
C6 alkyl (e.g.,
methyl). In some embodiments, R2 is -CN. In some embodiments, R2 is halogen
(e.g., bromo).
In some embodiments, R2 is -0R20 and in certain aspects R2a is Ci-C6 alkyl
(e.g., methyl). In
some embodiments, R2 is C(0)R20 and in certain aspects R20 is CI-Co alkyl
(e.g., methyl) or C3-
C6 cycloalkyl. In some embodiments, R2 is C(0)0R20 and in certain aspects R20
is hydrogen or
CI-Co alkyl (e.g., methyl). In some embodiments, R2 is -C(0)1N1R2bR20 and in
certain aspects R21)
and R2 are independently hydrogen or CI-Co alkyl (e.g., methyl) or C3-C6
cycloalkyl. In some
embodiments, R2 is ).K _NR2bc(0,- 2c
and in certain aspects R21) and R2c are independently hydrogen
or CI-Co alkyl (e.g., methyl) or C3-C6 cycloalkyl. In some embodiments, R2
is _NR2ac(0)NR2bR2c and in certain aspects R2a is hydrogen or C1-C6 alkyl
(e.g., methyl) and R21'
and R2c are independently hydrogen or Ci-C6 alkyl (e.g., methyl) or C3-C6
cycloalkyl. In some
embodiments, R2 is -SR2a and in certain aspects R2a is Ci-C6 alkyl (e.g.,
methyl).
[0050] In some embodiments R2 is hydrogen, Ci-C6 alkyl, -CN, halogen, -0R2a, -
C(0)R2a, or -
C(0)0R2a. In some embodiments, R2 is hydrogen. In some embodiments, R2 is CI-
Co alkyl (e.g.,
methyl). In some embodiments, R2 is -CN or halogen (e.g., bromo). In some
embodiments, R2
32
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
is ¨0R2a and in certain aspects R2a is Ci-C6 alkyl (e.g., methyl). In some
embodiments, R2 is
C(0)R2a and in certain aspects R2a is Ci-C6 alkyl (e.g., methyl) or C3-C6
cycloalkyl. In some
embodiments, R2 is C(0)0R2 and in certain aspects R2a is hydrogen or C1-C6
alkyl (e.g.,
methyl).
[0051] In some embodiments, RI and R2 are each hydrogen. In one such
variation, le and R2 are
each hydrogen and A is 4-hydroxyphenyl optionally further substituted by R3 or
4-hydroxy-2-
pyridyl optionally further substituted by R4. In another variation, RI and R2
are each hydrogen
and B is unsubstituted phenyl. In another variation, R' and R2 are each
hydrogen and B is a 5- to
10-membered heteroaryl substituted by 1 to 3 R4 wherein each R4 is
independently oxo or R3. In
a further variation, RI and R2 are each hydrogen and A is an unsubstituted 9-
or 10-membered
bicyclic heteroaryl containing at least one annular nitrogen atom. In a
further variation, RI and
R2 are each hydrogen, A is a 9- or 10-membered bicyclic heteroaryl containing
at least one
annular nitrogen atom and which is optionally substituted by R4 and B is
unsubstituted phenyl or
a 5- to 10-membered heteroaryl substituted by Ito 3 R4 wherein each R4 is
independently oxo or
R3. In another embodiment, RI is hydrogen and R2 is C1-C6 alkyl, -CN, halogen
or -0R2a. In one
aspect, RI is hydrogen and R2 is bromo, methyl, -CN, -OH, -CONH2, -COOH or
methoxy.
[0052] In some embodiments, RI and R2 are hydrogen. In one such variation, R'
and R2 are
hydrogen and A is 4-hydroxyphenyl optionally further substituted by R3 or 4-
hydroxy-2-pyridyl
optionally further substituted by R4. In another variation, R' and R2 are
hydrogen and B is
unsubstituted phenyl. In another variation, R1 and R2 are hydrogen and B is a
5- to 6-membered
heteroaryl substituted by 1 to 3 R4 wherein each R4 is independently R3. In a
further variation, RI
and R2 are hydrogen and A is an unsubstituted 9- or 10-membered bicyclic
heteroaryl containing
at least one annular nitrogen atom. In a further variation, RI and R2 are
hydrogen, A is a 9- or 10-
membered bicyclic heteroaryl containing at least one annular nitrogen atom and
which is
optionally substituted by R4 and B is unsubstituted phenyl or a 5- to 6-
membered heteroaryl
substituted by I to 3 R4 wherein each R4 is independently R3. In another
embodiment, RI is
hydrogen and R2 is Ci-C6 alkyl, -CN, halogen or -0R2a. In one aspect, R' is
hydrogen and R2 is
bromo, methyl, -CN or methoxy.
[0053] In some embodiments, RI is hydrogen and R2 is -CN. In one such
variation, R' is
hydrogen, R2 is ¨CN, A is a 9- or 10-membered bicyclic heteroaryl containing
at least one
annular nitrogen atom (e.g., quinolinyl or indazoly1) and which is optionally
substituted by R4
33
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
and B is unsubstituted phenyl or a 5- to 6-membered heteroaryl (e.g.,
pyrazolyl, pyridyl or
pyridone) substituted by 1 to 3 R4 wherein each R4 is independently oxo or R3.
In some
embodiments, R' is hydrogen, R2 is Br, A is a 9- or 10-membered bicyclic
heteroaryl containing
at least one annular nitrogen atom (e.g., quinolinyl or indazolyl) and which
is optionally
substituted by R4 and B is unsubstituted phenyl or a 5- to 6-membered
heteroaryl (e.g., pyrazolyl,
pyridyl or pyridone) substituted by 1 to 3 R4 wherein each R4 is independently
oxo or R3. In
some embodiments, both le and R2 are hydrogen, A is a 9- or 10-membered
bicyclic heteroaryl
containing at least one annular nitrogen atom (e.g., quinolinyl or indazolyl)
and which is
optionally substituted by R4 and B is unsubstituted phenyl or a 5- to 6-
membered heteroaryl (e.g.,
pyrazolyl, pyridyl or pyridone) substituted by 1 to 3 R4 wherein each R4 is
independently oxo or
R3. In these variations R4 is independently oxo, methyl, methoxy, chloro or
¨CN.
[0054] In some embodiments, A is 4-hydroxyphenyl optionally further
substituted by R3, 4-
hydroxy-2-pyridyl optionally further substituted by R4, or a 9- or 10-membered
bicyclic
heteroaryl optionally substituted by R4.
[0055] In some embodiments, A is 4-hydroxyphenyl optionally further
substituted by R3 or 4-
hydroxy-2-pyridyl optionally further substituted by R4. In some embodiments, A
is 4-
hydroxyphenyl optionally further substituted by R3. In some embodiments, A is
4-hydroxy-2-
pyridyl optionally further substituted by R4. In some embodiments, A is a 9-
or 10-membered
bicyclic heteroaryl optionally substituted by R4. In some embodiments, A is a
9- or 10-
membered bicyclic heteroaryl optionally substituted by R4, wherein one ring is
saturated. In
some embodiments, A is a 9- or 10-membered bicyclic heteroaryl optionally
substituted by R4,
wherein both rings are unsaturated. In some embodiments, A is selected from
the group
consisting of benzimidazolyl, benzoxazolyl, benzothiazolyl, quinolinyl,
isoquinolinyl, indazolyl,
quinoxalinyl, quinazolinyl, cinnolinyl, naphthyridinyl and naphthyl. In some
embodiments, A is
selected from the group consisting of benzimidazolyl, benzoxazolyl,
benzothiazolyl, quinolinyl,
isoquinolinyl, indazolyl, quinoxalinyl, quinazolinyl, cinnolinyl,
naphthyridinyl and naphthyl,
each of which is optionally substituted by R4. In yet further embodiments, A
is a 9- or 10-
membered bicyclic heteroaryl optionally substituted by R4, comprising a first
and second ring,
wherein the first ring has a greater number of ring atoms than the second
ring. In certain
embodiments, the point of attachment of A to the parent molecule is on the
first ring having a
greater number of ring atoms. In other embodiments, the point of attachment of
A to the parent
34
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
molecule is on the second ring having a smaller number of ring atoms. In some
embodiments, A
is a 9- or 10-membered bicyclic heteroaryl optionally substituted by R4,
wherein the two rings
are selected from the group consisting of: a 5-membered ring and a 6-membered
ring or two 6-
membered rings.
[0056] In one aspect, when A is a 9- or 10-membered bicyclic heteroaryl
optionally substituted
by R4, A is an unsubstituted 9- or 10-membered bicyclic heteroaryl containing
at least one
annular nitrogen atom, a 9- or 10-membered bicyclic heteroaryl containing at
least two annular
nitrogen atoms and optionally substituted by R4 which R4 groups are connected
to the parent
structure via a carbon atom, or a 10-membered bicyclic heteroaryl optionally
substituted by R4.
[0057] In some embodiments, A is 4-hydroxyphenyl optionally further
substituted by R3 where
R3 is selected from the group consisting of halogen, -CN, -0R5, -SR5, -NR6R7, -
NO2, -C(0)R5,
-C(0)0R5, -C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6,
-NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6 cycloalkyl and C1-C6 alkyl optionally
substituted by
halogen. In some embodiments, A is 4-hydroxyphenyl further substituted by 1 to
3 R3 where
each R3 is independently selected from the group consisting of halogen, -CN, -
0R5, -SR5,
-NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -
0C(0)NR6R7,
-NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6 cycloalkyl and C1-C6 alkyl
optionally
substituted by halogen. In some embodiments, A is 4-hydroxyphenyl optionally
further
substituted by R3 where R3 is selected from the group consisting of halogen, -
0R5 and C1-C6
alkyl optionally substituted by halogen. In some embodiments, A is 4-
hydroxyphenyl further
substituted by 1 to 3 R3 where each R3 is independently selected from the
group consisting of
halogen, -0R5 and C1-C6 alkyl optionally substituted by halogen. In some
embodiments, A is 4-
hydroxyphenyl further substituted by 1 to 3 R3 where each R3 is independently
selected from the
group consisting of fluoro, chloro, -0-Ci-C6alkyl and Ci-C6 alkyl optionally
substituted by
halogen.
[0058] In some embodiments, A is 4-hydroxy-2-pyridyl optionally further
substituted by R4
where R4 is selected from the group consisting of halogen, -CN, -0R5, -SR5, -
NR6R7, -NO2,
-C(0)R5, -C(0)0R5, -C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -
NR5C(0)R6,
-NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6 cycloalkyl and C1-C6 alkyl optionally
substituted by
halogen. In some embodiments, A is 4-hydroxy-2-pyridyl optionally further
substituted by 1 to 3
R4, where each R4 is independently selected from the group consisting of
halogen, -CN, -0R5,
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
-SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5,
-0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6 cycloalkyl
and CI-
C6 alkyl optionally substituted by halogen. In some embodiments, A is 4-
hydroxy-2-pyridyl
further substituted by 1 to 3 R4 where each R4 is independently selected from
the group
consisting of halogen, ¨0R5 and C1-C6 alkyl optionally substituted by halogen.
In some
embodiments, A is 4-hydroxyphenyl further substituted by 1 to 3 R4 where each
R4 is
independently selected from the group consisting of fluoro, chloro, ¨0-CI-
C6alkyl and C1-C6
alkyl optionally substituted by halogen.
100591 In some embodiments, A is a 4-hydroxyphenyl or a 4-hydroxy-2-pyridyl
substituted with
1 to 3 R3 groups, which may be the same or different. In some of these
embodiments, A is
selected from the group consisting of:
CI CI HO
HO 401 HO HO HO 11101 HO
CI
CI CN CI
HO
HO HO HO is HO HO
CI CI
CI
, and ; wherein the wavy lines
denote attachment points to the parent molecule.
10060] In some embodiments, A is a 9- or 10-membered bicyclic heteroaryl
substituted with 0 to
3 R4 groups which may be the same or different, and which may be present on
either one ring or
both rings. In one such aspect, A is a 9- or 10-membered bicyclic heteroaryl
substituted with 0 to
3 R3 groups which may be the same or different, and which may be present on
either one ring or
both rings. In one such aspect, A is a 9- or 10-membered bicyclic heteroaryl
substituted with 1
R3 group. In another such aspect, A is a 9- or 10-membered bicyclic heteroaryl
substituted with 2
R3 groups, which may be the same or different. In another such aspect, A is a
9- or 10-
36
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
membered bicyclic heteroaryl substituted with 3 R3 groups, which may be the
same or different.
In some embodiments, A is selected from the group consisting of:
(R3)0 OH-1 /---- (R3)0-3 f-------N (R3)0.3 N--
HN / HN H NI /
/
I I
,--
N..--- ----
H
/
/j-0 (R3)0_3 iz--- N (R3)0_3 R3)0_3 f----N
(R3)0_3
N
1 /
I I N'
, , ,
. ,
......, N ''. / (R3)0-3
I I N ,
/ (R3)0-3 N 3
i '2_,........---- (R3)0_3 1
I..,....7,1i
, ' , ,
õ...--y(R3)0-3 ,,--k.,,,.../(R3)0_3 r N
I I II I --... ,,---.. 3
(R3)0_3 11-- (R3)0-3 NI I _A--- (R )0-3
N N N / N ,---
---õ,
N 'c -N
i
..-' N ,-- I -LJ
--
I ,
, ,
irs'..:µ-1.. 3e----N\---1 .1 (R3)0-3 (R3)0-3 ....,,,R3)0-3
--- (R )0-3 II ---(R )0-3 H ---(R)0-3 (---/ 00 --- i NH
N.,9--1\ N ,..- N 1 '''''41111P
N HN . itah HN _eight HN
, sal
N HN
>" 1.,== tepi ss
e = ,
r3)3 0 HN .."-
----z,--
i 1 , 0 i,
-3 \
---,-.(R3) -
N ,-- HN HN (R3)0_3 0
H1\11.9N_I I ..,-' ----
, , ,
,.../ (R3)0-3
0 N
-ZNH ...-'
0 i.,õ
---,,,-. -(R3)0-3 H (R3)03 (R3)0-3 rI\I 1 µ->< I1 ' N
1 I
..--- .--
N--.1')/
, and ,where R3, if
,
present, is attached at any available position on the bicyclic ring system. in
one aspect, at
37
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
least one R3 is present and is attached at a position on the ring bearing the
wavy line (on the
ring that is the attachment point of the bicyclic ring to the parent
molecule). In one aspect, at
least one R3 is present and is attached at a position on the ring that does
not bear the wavy
line (on the ring that is fused to the ring which is the attachment point of
the bicyclic ring to
the parent molecule).
100611 In some embodiments, A is a 9- or 10-membered bicyclic heteroaryl
substituted with 0 to
3 R3 groups which may be the same or different, and which may be present on
either one ring or
both rings. In some embodiments, A is selected from the group consisting of:
OH
(R3)o-1 ).,.. F---N (R3)0.3 /71 (R363 r (R3)o_3 frs (R3)
HNN,,,,i,õ/ N \_,.,).,.,../
NN71,/, N \\.....õ..µ,./ 0-3
I I I I
N-...õ....7,),-......-õ7-../ _.--,..,..;:-
.--y
H . . , , ,
ii-- N---- (R3)0-3 lb r- N ,
(R3)0-3 NJ..õ, (R3)0_3
N...õ..,--- (R3)o 3 -.......I.,....--V
_ 11 --------- ' - I - I Y
N / -..,.....,..<^y
, .
AR3)0-3
(R3)0.. N.,
- " r - (R3)o 3 (.\..-(R3)0-3 "(NR3)0-3
N ,--:,,s.N N ..../ õ....,..),, N.õ..,,,--., - N 1,
õ...(A
1 I ii 1 /N
HN--S.õ 0-4
(R3)0_3
C*1----(R3)o-3 rzz'12.---(R3)0-3 rA/F`3)0 3
'1'.....>(R3)0_3 r--/-
N
/ HN N.õ{-1,./
HNõ,..,,--õi yHN-- rN
_,.../9
(R3)0-3
-----N--/
,
I
N , ,. (1:3 )0.3
HN(R3)0_3 HN õ..,..,,...,..õ,...õ (R3)0 3 li A ''''i N
1 1 1 .,.1,./
N
and where
R3, if
present, is attached at any available position on the bicyclic ring system. In
one aspect, at
least one R3 is present and is attached at a position on the ring bearing the
wavy line (on the
38
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
ring that is the attachment point of the bicyclic ring to the parent
molecule). In one aspect, at
least one R3 is present and is attached at a position on the ring that does
not bear the wavy
line (on the ring that is fused to the ring which is the attachment point of
the bicyclic ring to
the parent molecule).
100621 In some embodiments, A is selected from the group consisting of:
OH . = HN i'iii. N F---N
. HN
......õ.
CI / 1 ===.... = = . . .
..-=."
N WI =
H === HO. = = 1111" ...= CI
N.¨. N:-----..
HN N HN HN HN HN
0101 . ..0 ..
IS.
. CI = = = = :o,
.
irS ir-S
HNI . N 0 .,,, N IP. =
==,/ == = c, .= = ** = .= c,
,
F3C H2N
\)7---S )7.---S >7---S ------c-S
N N N N µ N ,-= N c,I . c ,,-
-...õ. ... = = N N
-.-'' 0.
, ci= 5,5, c, = .,
r
I ......:õ. ..
N -:..... ,,..0 ........ =-,....
=== .40 N
,
.
= . . = . .......
,... cl= == =
=
. , , . ==,:.= ci
1 N ''`= --.,.. . = =-=-...= = I . = Ss.
,..,... I I
I . 1
%.,. N N .... ¨ N iiihh,,,.. . N
-,,,....
...--..-
100- . W. . I .
1
..." .111111 Ns,
CI . CI = = iSIS
.
1 , 1
39
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
= , `--,. = , '=- = = . ,,. CF3 ,.
= :===:.,
NI
Ni N dill N diet,=
= = Ni = .
I
= illir = .. 1. .. = 011.
=
CI CI WI = =.: CI = =.= CI = *. = F3C =
.==
9 9
0 = ..' Br III. = . .
tail r'N'N
N . 401 N .., N NI -µ'N- ., (N -.,
N Lik. = (N
-:.
N .... . .
I I 1 II
.....-'
illir = = . CI
-=,õ.. '-'0 Oy--,,o
I
HN HN . 40
-.,õ.
0 . . = Oy-,õ,
NH (-----NH 0
..., ,,
,
HN el N ,-- HN . si HN . .0 HN
1
I
0 =
--...
HN "--- N '- HN "--- ---. NH I,
HN . 40 N = =
a
1 1 1
ci
HO. gith
illiPs' N
1 N --yir
,and .
100631 In some embodiments. A is selected from the group consisting of:
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
OH 7.-.--=:' T----=-- rz----RP-N r----_-_N
HN HN HN 46,, HN
'-..
CI --e-lel (el HO1
ci 40 N
H . ,
---7----N ii---N/
HN N N N iiiil 0 N lab
1110 40 ir-
cl gip
, , , ,
I
NirSs 4119 WP
..--o --...,.
N AI N igith N40 N
cl CI
,
-.,.
CI N "---
401 N N,
1 Ilk.
I
N .,---
rr,-' N
NI
N NO. N Ni l" HN NI
116 i
40 ..--
111,- '`.
. '
,----0 0
-..,,,,-,----0 , ---- OyeN,
NH r------,H
HN HN doh N .,,--
q111-P N y HN .-,-'
---.. I HN 010
=
ci
N "--
HN 401 HN 401 III 1 , IIIP' N
I '
NJ
jv
and .
41
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HN, .....
[00641 In certain embodiments, A is selected from the group consisting of:
,
/--:---N F---N \>:-----:N 7--z---N I II
Ø
HN, HN10 HN0 0 .. N .
õ. .
.= . . . . 10 . .---
. CI = = = = =
== , , '
iHN .."-=
HN-A HN HN
sii .
. n N___
is
=.= c, ..
, and , wherein
the wavy lines denote attachment points to the parent molecule.
HN, = ...=
[0065] in certain embodiments, A is selected from the group consisting of:
,
. .
r----N r"-:7-N \)-----zN /7-------N
I '' I.
HN HN HN, 0 . N N at
401== . .......
wip = .
....is . : ,
c, = =.. cl
, = , ,
I
N .
lip . .
and ;
wherein the wavy- lines denote attachment points to the parent molecule.
I--IN "=-= 1
N
[00661 In some embodiments, A is . In some embodiments, A is . In
1 N_
N HN .
-..., ..õ..........
some embodiments, A is CI
. In sonic embodiments, A is = , In some
42
CA 03070273 2020-01-16
WO 2(119/(118584 PCT/US2018/042777
CI
embodiments, A is . In some embodiments, A is naphthyl substituted with
halogen, -CN, or hydroxy.
100671 It is understood that each description of A may be combined with each
description of RI
and/or R2 the same as if each and every combination were specifically and
individually listed.
For example, in one embodiment, A is as described in any of the embodiments,
aspects or
variations herein and le and R2 are each H. It is similarly understood that
each description of A
may be combined with each description of B (and further with each description
of le and R2) the
same as if each and every combination were specifically and individually
listed. For example, in
one aspect, it is understood that each description of A may be combined in one
aspect with a
variation in which RI and R2 are each hydrogen. In one aspect, it is
understood that each
description of A may be combined in one aspect with a variation in which RI
and R2 are each
hydrogen and B is a 5- to 10-membered heteroaryl optionally substituted by R4.
[0068] In some embodiments, B is an unsubstituted phenyl. In some embodiments,
B is a phenyl
optionally substituted by R3. In some embodiments, B is a phenyl substituted
by 1 to 3 R3 which
R3 groups may be the same or different. In other embodiments, B is a 5- to 10-
membered
heteroaryl optionally substituted by R4. In other embodiments, B is a 5- to 10-
membered
heteroaryl substituted by 1 to 3 R4 which R4 may be the same or different In
some
embodiments, the 5- to 10-membered heteroaryl of B is a 5-membered heteroaryl
selected from
the group consisting of furanyl, oxazolyl, thiophenyl, pyrazolyl, isoxazolyl,
1,3,4-oxadiazolyl,
imidazolyl, thiazolyl, isothiazolyl, triazolyl, 1,3,4-thiadiazoly1 and
tetrazolyl, which 5-membered
heteroaryl is optionally substituted by 1 to 3 R4 which R4 groups may be the
same or different.
In other embodiments, the 5- to 10-membered heteroaryl of B is a 6-membered
heteroaryl
selected from the group consisting of pyridyl, pyriclazinyland pyrimidinyl
which 6-membered
heteroaryl is optionally substituted to 1 to 3 R4 which R4 groups may be the
same or different. In
some embodiments, the 5- to 10-membered heteroaryl of B is a bicyclic
heteroaryl selected from
the group consisting of benzofuranyl, benzothiophenyl, pyrazolopyridinyl,
indazolyl,
benzothiazolyl, benzooxazolyl or benzoimidazolyl, each of bicyclic heteroaryl
is optionally
substituted by 1 to 3 R4 which R4 groups may be the same or different
43
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
[0069] In some embodiments, B is an unsubstituted phenyl. In some embodiments,
B is a phenyl
optionally substituted by R3. In some embodiments, B is a phenyl substituted
by 1 to 3 R3 which
R3 groups may be the same or different. In other embodiments, B is a 5- to 6-
membered
heteroaryl optionally substituted by R4. In other embodiments, B is a 5- to 6-
membered
heteroaryl substituted by 1 to 3 R4 which R4 may be the same or different In
some
embodiments, the 5- to 6-membered heteroaryl of B is a 5-membered heteroaryl
selected from
the group consisting of furanyl, oxazolyl, thiophenyl, pyrazolyl, isoxazolyl,
1,3,4-oxadiazolyl,
imidazolyl, thiazolyl, isothiazolyl, triazolyl, 1,3,4-thiadiazoly1 and
tetrazolyl, which 5-membered
heteroaryl is optionally substituted by 1 to 3 R4 which R4 groups may be the
same or different.
In other embodiments, the 5- to 6-membered heteroaryl of B is a 6-membered
heteroaryl selected
from the group consisting of pyridyl and pyrimidinyl which 6-membered
heteroaryl is optionally
substituted to 1 to 3 R4 which R4 groups may be the same or different.
[0070] In some embodiments of B in which B is a phenyl substituted by R3, such
as when B is a
phenyl substituted by 1 to 3 R3 which may be the same or different, each R3 of
B in one aspect is
independently selected from the group consisting of halogen, -0R5, -NR6R7, -
C(0)R5, C3-
C6 cycloalkyl and CI-C6 alkyl optionally substituted by halogen. In other
embodiments, each R3
of B is independently selected from the group consisting of halogen and C1-C6
alkyl optionally
substituted by halogen (e.g., CF3).
[0071] In some embodiments, B is a phenyl substituted with 1 to 3 halo groups
which may be the
same or different. In some embodiments, B is phenyl, fluoro-phenyl, di-fluoro-
phenyl, chloro-
phenyl, di-chloro-phenyl or (fluoro)(chloro)-phenyl. In some embodiments, B is
selected from
the group consisting of:
I
I I
F F CI CI ,
y-
=-.19-- =
CI 0 F F
44
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
F F CI õI S 0
F F 0 I F
0
. CI CI CI CI CI F
, , , , , ,
0 0 CI.
and F ; wherein the wavy lines denote
attachment points to the parent molecule.
[00721 in some embodiments, B is a phenyl substituted with I to 3 halo groups
which may be the
same or different. In some embodiments, B is phenyl, fluoro-phenyl, di-fluoro-
phenyl, chloro-
phenyl, di-chloro-phenyl or (fluoro)(chloro)-phenyl. In some embodiments, B is
selected from
the group consisting of:
410 F 0 1110 I.
F F CI 1110 4101 CI
, , ,
lb (11
F(1101 F1110F F F F CI 0 10
CI IP F F CI
,
s CI 0 F s I.
, and
F Oil CI CI F F CI F
,
01
CI
F ; wherein the wavy lines denote attachment points to the parent
molecule.
[00731 In some embodiments, B is a 5-membered heteroaryl substituted with 0 to
3 R4 groups
which may be the same or different. In some embodiments, B is a 5-membered
heteroaryl
substituted with 0 to 3 R3 groups which may be the same or different. In one
such aspect, B is a
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
5-membered heteroaryl substituted with I R3 group. In another such aspect, B
is a 5-membered
heteroaryl substituted with 2 R3 groups, which may be the same or different.
In another such
aspect, B is a 5-membered heteroaryl substituted with 3 R3 groups, which may
be the same or
different. In some embodiments, B is a 5-membered heteroaryl selected from the
group
CYN (R3)0_3 ..,,,O....)A
(R3) 'µ1--0 \\N / (R3)0_3))(-0 (R3)0.3-7
'
consisting of: \ ¨ 0-3 , ,
(R3)0-3 (R3)0-3 (R3)0-3 (R3)- N3 H
]:)A IDA (R3),(2- µ3
Wil3A
N / / WIDA 1--µ N I Y 14
(R3)0e '
b HN FIN HN¨N HµN¨N
, , ,
e
HjA 3 .-4 /
(R3)0-34'41 (R3)0-3-<'4 (R3)0-3-<-4 (R )03
1 - N ,
,
(R3)0-3
N N"\s' (\.S3,,,\ (7),,,\
(R3)0_34¨NH (R3)0.33--4 (R3)0-3-A 1 (R3)0-311 1 'S
, ,
(R3)0-3
N
N'Ny\ e rN N'N-11A
(R3)0_34-S 3 A.-
(R )0-3 0
(R3)0-3,s(:¨.NH HN¨N , N¨NH ,
and
, ,
N
W y--\\
wherein the wavy lines denote attachment points to the parent molecule.
[00741 In some embodiments, B is a 5-membered heteroaryl substituted with 0 to
3 12.3 groups
which may be the same or different. In some embodiments, B is a 5-membered
heteroaryl
(.=.-"A ,,,3A
selected from the group consisting of: (R3)0-34¨ , (R3)0_30 N ,
(R3)0-3
J:7A
(R3)0_3' \---N (R3)0.3 \µ--0 (R3)0_3"X b 1-1
N /
1\1
, .
. .
46
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
NY H
N
H I / N.N, ..,,, ,N
N )\
;:' (R3)0-34---I (R3)0-3'-"-\--=¨=1 (R3)0-311 /
(R3)0..3
(Ny..\\
(R3)0-3-'4Ni
'3 .-'-',____//
(R3)0-3-; (FR' )0-3 S
, ,
'
,7rA3)0-3,
(R3 N
(N,-\ N N' -y-..\ N N N
' -----TA WY\ (R) 3 0 (R3)0_3X-NF-1 IRJ¨NH
,0-3
; and (R3)0-3
wherein the wavy lines denote attachment points to the parent molecule.
, -¨S
[00751 in some embodiments, B is a 5-membered heteroaryl selected from the
group consisting
(3),, OA -----nA /
---0-A /N 1 of: HN HN /
/ / / 1 -.),,, \ '=-..
N N N N \ 0 \
/ / , / , / 0 0
, , ,
<L1A \ \ 0 \ 0
0 y,, cy\ ct 0t N
, , ,
47
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N / / N / / N / Nit'sjA
Ny\s"/ : NtY-\ '
0 / HN I-1'N N N
0 HN
,
N-N
1\1 (17)\ N-N N-N
N-N
/ HN-N / /
, , ,
:Ni.,õ,-\õ, ,FNII H
N
I / / .,,r\i,3_,--\,,
N-N I NN N \ /
/ HN-N /
,
1 1 I
111._.7A1 N \N / N \N / N'\N C u r, F.
`N
3 --
N,
N _1,1 \ Nµ
CI¨ /c_f F----Ur- ¨
,
'
N, ,\ /NI -N-\ kliA, hNlt H
N
-NH NH
NH
NH NH
N N N
----,--''N
\
NrN-N% N / NA -N% J,
N / N S
2-1 \----:----c .. NnN-Xs= N)..--------1(
, , , ,
48
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
S S S
S
S \ 1 \ / \ / \ / SyN.
,=S't
\\
N
= , , , , ,
,
_ ,S-\\ _....,õ..c,,,,,St
/ Nt))14 NI\ /
S
N ' ,N N'Ny\ N'N'y'\\ N,Ny\ N ,rysi, \
/=¨=0 N
\---NH \ ,
N. .,.\ N,
N N
---N'N'Y'''' ,-T---Nill ---------NII\I -----
</ -7,----\
SN-YN \ N-N
/ HN¨N
, , =
,
NY N N
NY
NA N N y)s% N
)ss -Y\ '
N-N IN-N N:,
HN¨N HN¨N / N¨NH , --S ,and
2.---S =
wherein the wavy lines denote attachment points to the parent molecule.
[00761 in some embodiments, 13 is a 5-membered heteroaryl selected from the
group consisting
-=-.
CIA \ ,
\ 0 \ \ \ \ 0
of: 0 0 0 0
,
\
0
, \--N
N N:.,0 /
49
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
;Ot Np)',, N,
N\ / \ / \ / Ne.)----\
hj://)\ 1\1,..)-A Fii\J 1
b 0
HN
,
H
H ,I-N-\_.:,,\ kipA
H/
µN /N N
,
, ,
H I I I
,N;),A ,N
N\ / ,N.D,, N \ / N \ / N\ / l'N-\,,
N\ / \_____ , ---- i
, ---N
N
N N
, , , , , ,
I
N,TA N
NH NH ,
N N N
N N'I\I-N% .1\1-'\ ),s,
------CY\KI ,--ZA ----",-----ITA NA" 7:---4 N\.____c¨ NN
,
)\ As
N N NI/ N S S S
\ /
, , , , ,
S S
-------/st i A,
NIS_K.)\
N N S
,
= '
,
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N NyNs
N
N'')\ N:41-tt tNz;;IA _OA W
\
µS µS 1
S
,N r,\ N
N --1-Ns ,N,7A N N' '-')--A, ,Nyi, ,N N
,_,-,
k../ N ' .....\NH
/ \\___ 'N '
.%
t...NH N \ ......e.
. , , ,
N
and 2S
--- ; wherein
the wavy lines denote attachment points to the parent molecule.
100771 In some embodiments, B is a pyridyl or pyrimidyl optionally substituted
by I to 3 R4,
which R4 may be the same or different. In some embodiments, B is a pyridyl or
pyrimidyl
optionally substituted by 1 to 3 halo groups which may be the same or
different. In some
-,,,,,N
embodiments, B is a 6-membered heteroaryl selected from the group consisting
of ,
ci:\ Fr, A
,,, .,.
ICC\
,D.AciNc-Ns ,
N .-' Al N F N N ----
F
, .
. ,
F
N N./-k-"-A
11.,,
Na-C-\\ F C""slAN , and N--;-. ;
wherein the wavy lines denote attachment points to
the parent molecule.
[00781 In some embodiments, B is a pyridyl or pyrimidyl optionally substituted
by I to 3 R4,
which R4 may be the same or different. In some embodiments, B is a pyridyl or
pyrimidyl
optionally substituted by 1 to 3 R3, which R3 may be the same or different. In
some
embodiments, B is a pyridyl or pyrimidyl optionally substituted by I to 3 halo
groups which may
be the same or different. In some embodiments. B is a 6-membered heteroaiy1
selected from the
51
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N O .,.." N i Nr'IAN
A NOA I ,.
..,...0 ,.,:...... N
group consisting of: ''.
H I 0 0 N
F
.naA L)As
N ,,,' N / a riN ,and
wherein F F (,,,,, and N -
, F , ,
wherein the wavy lines denote attachment points to the parent molecule.
[00791 In some embodiments. B is selected from the group consisting of: OS
,
IS/ O's AP CN -1
cOlA
F ' 0 N , , and ;
wherein the wavy lines denote
attachment points to the parent molecule.
[00801 In some embodiments, B is a bicycle heteroaryl optionally substituted
by 1 to 3 R4, which
may be the same or different, and which may be present on either one ring or
both rings. In some
0
embodiments, B is a bicylic heteroaryl selected from the group consisting of:,
,
Ny\ Ny\
/
* 0
or
N ..y.\
* NH
; wherein the wavy lines denote attachment points to the parent molecule.
52
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/04 2 7 7
7
"
I
[0081] In some embodiments, B is selected from the group consisting of: ,
N N
CrA,
\ 0 N
and
wherein the wavy lines denote attachment points to the parent molecule.
N
[0082] In some embodiments, B is . In some embodiments. B is 10))% In some
N,
N¨N
embodiments, B is / . In some embodiments, B is
[0083] It is understood that each description of B may be combined with each
description of le
and/or R2 the same as if each and every combination were specifically and
individually listed. It
is similarly understood that each description of B may be combined with each
description of A
(and further with each description of RI and R2) the same as if each and every
combination were
specifically and individually listed. For example, in one aspect, it is
understood that each
description of B may be combined in one aspect with a variation in which RI
and R2 are each
hydrogen. In one such variation, B is as defined in any variation herein, RI
and R2 are each
hydrogen and A is 4-hydroxyphenyl optionally further substituted by R3 or 4-
hydroxy-2-pyridyl
optionally further substituted by R4. In another variation, B is as defined in
any variation herein,
R' and R2 are as defined in any variation herein and A is 4-hydroxyphenyl
optionally further
substituted by R3 or 4-hydroxy-2-pyridyl optionally further substituted by R4.
In another
variation, B is as defined in any variation herein, RI and R2 are each
hydrogen and A is 9- or 10-
membered bicyclic heteroaryl (eg., quinolinyl or indazoly1) optionally
substituted by R4. In
another variation, B is as defined in any variation herein, RI and R2 are as
defined in any
variation herein and A is 9- or 10-membered bicyclic heteroaryl (eg.,
quinolinyl or indazolyl)
optionally substituted by R4.
[0084] In some embodiments, the compound of formula tl) is of the formula
(II):
53
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HO)R3)N R2
X
or a salt thereof, wherein RI, R2 and B are as defined for formula (I) or any
embodiment or
aspect or other variation thereof,
X is N, CH or CR3;
each R3 is independently halogen, -CN, -0R5, -SR'. -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or C1-C6 alkyl optionally substituted by
halogen;
each R5 is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl; and
nis 0,1, 2 or3.
100851 In some embodiments, provided is a compound of formula (II), or a
tautoiner or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
100861 In some embodiments of the compound of formula (II), R3 is selected
from the group
consisting of halogen, ¨0R5 and C1-C6 alkyl optionally substituted by halogen.
100871 In some embodiments, the compound of formula (I) is a compound of
formula (IIIa):
TO-X2
X2 N
(Ma),
or a salt thereof, wherein RI, R2 and B are as defined for formula (I);
each XI is independently 0, S, NH, NR4a, CH2, CHR4b, Cele, N, CH or CR41';
54
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
each X2 is independently NH, NR4a, cHR4b,R4b CH, CR4b or N;
each -7-7 is a single or double bond, provided that when XI=X2 is a double
bond,
X2=-X1 is a single bond and when X2=X1 is a double bond, X1=X2 is a single
bond;
R4a is CI-C6 alkyl;
each R4b is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or CI-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, CI-Co alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl,
provided the compound is other than a compound selected from Table 1 or a salt
thereof.
[0088] In some embodiments, provided is a compound of formula (Bla), or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
[0089] In some embodiments, the compound of formula (I) is a compound of
formula (IIIb):
X2¨x1
22R
or a salt thereof, wherein RI, R2 and B are as defined for formula (I);
each XI is independently 0, S, NH, NW'', CH2, CHR4b,R4b, N, CH or CR41';
each ,-,4b ch X2 is independently NH,
NR4a cR4btc. , CH2, CHR4b, CH, CR4b or N;
each 77- is a single or double bond;
R4a is C1-C6 alkyl;
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
each R4b is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or C1-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, CI-Co alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl,
provided the compound is other than a compound selected from Table 1 or a salt
thereof.
[0090] In some embodiments, provided is a compound of formula (llb), or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
[0091] In some embodiments, the compound of formula (I) is a compound of
formula (Mc):
X2-xl
C:\
N R2
0 X2
X
./.1R1
(11Ic),
or a salt thereof, wherein le, R2 and B are as defined for formula (I);
each Xi is independently 0, S. NH, NR4a, CH2, CHR4b, N, CH or CR4b;
each X2 is independently CH, CR4b or N;
R4a is C1-C6 alkyl;
each R41' is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or C1-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, CI-C6 alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl,
56
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042 777
provided the compound is other than a compound selected from Table 1 or a salt
thereof.
[0092] In some embodiments, provided is a compound of formula (Mc), or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
[0093] In some embodiments, the compound of formula (I) is a compound of
formula (IIIc-1):
x2,xi
.,N R2
')(2=
X =1 R1
X10 /
X1
(111c-1),
or a salt thereof, wherein RI and R2 are as defined for formula (I);
each XI and X2 are as defined for formula (Mc);
X4 is C or N;
provided the compound is other than a compound selected from Table 1 or a salt
thereof.
100941 In some embodiments, provided is a compound of formula (Inc-1), or a
tautomer or
isomer thereof, or a pharmaceutically acceptable salt of any of the foregoing.
[0095] In some embodiments, the compound of formula (I) is a compound of
formula (Inc-2):
xOL
n X2
X N R2
-')(2
R1
R3 (llic-2),
or a salt thereof, wherein RI, R2 and R3 are as defined for formula (I);
57
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
each X' and X2 are as defined for formula (111c);
provided the compound is other than a compound selected from Table 1 or a salt
thereof.
100961 In some embodiments, provided is a compound of formula (Inc-2), or a
tautomer or
isomer thereof, or a pharmaceutically acceptable salt of any of the foregoing.
100971 In some embodiments, the compound of formula (I) is a compound of
formula (Ind):
X2.. X1
X '
X2
I ;
X2, N R2
X2-
R1
(Ind),
or a salt thereof, wherein le, R2 and B are as defined for formula (1);
each XI is independently 0, S. NH, CH2, CHR4b, CR4bR4b, N, CH or CR4b;
each X2 is independently CH, CR4b or N;
each R4b is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or C1-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, CI-Co alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, CI-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl.
100981 In some embodiments, provided is a compound of formula (lid), or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
[0099] In some embodiments, the compound of formula (I) is a compound of
formula (Me):
58
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
X2
X2
x2-,, xl
0 /
X.1-X2
(me),
or a salt thereof, wherein R', R2 and B are as defined for formula (I);
each XI is independently 0, S. NH, NR', CH2, CHR4b, CR4bR4b, N, CH or CR41';
each X2 is independently 0, CH2, CHR4b, CR4bR4b, CH, Cleb or N;
each = is a single or double bond;
R4a is C1-C6 alkyl;
each R41) is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or C1-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl,
provided the compound is other than a compound selected from Table 1 or a salt
thereof.
[0100] In some embodiments, provided is a compound of formula (ffle), or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
101011 In some embodiments, the compound of formula (I) is a compound of
formula (MO:
59
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
x\2
x2 xl
X.17-7:X2N R2
or a salt thereof, wherein R', R2 and B are as defined for formula (I);
each XI is independently 0, S. NH, NR', CH2, CHR4b, CR4bR4b, N, CH or CR41';
each X2 is independently C, CH, CR41' or N;
each = is a single or double bond, provided that when X1=X2 is a double bond,
X=
7X1 is a single bond and when X2¨ ¨X1 is a double bond, X1=X2 is a single
bond;
R4a is C1-C6 alkyl;
each R41' is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or C1-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl,
provided the compound is other than a compound selected from Table I or a salt
thereof.
101021 In some embodiments, provided is a compound of formula (BM, or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
101031 In some embodiments, the compound of formula (I) is a compound of
formula (111g):
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
X2 -
/
X2
\
)\-:/`"-= 1
X
\
x = ---X-
N R2
R1
(IIIg),
or a salt thereof, wherein RI, R2 and B are as defined for formula (I);
each XI is independently 0, S, NH, NR', N, CH or CR4b;
each X2 is independently C, CH, CR4b or N;
R4a is C1-C6 alkyl;
each R41' is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or C1-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl,
provided the compound is other than a compound selected from Table 1 or a salt
thereof.
[0104] In some embodiments, provided is a compound of formula (Mg), or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
[0105] In some embodiments of the compound of formula (III), R41' is selected
from the group
consisting of halogen, ¨0R5 and C1-C6 alkyl optionally substituted by halogen.
[0106] In some embodiments of the compound of formula (III), one of XI is N,
and the other one
of XI is NR4a, and each X2 is CH or CR4b. In other embodiments of the compound
of formula
(III), one of XI is N, and the other one of XI is 0 or S. and each X2 is CH or
CR4b.
[0107] In some embodiments, the compound of formula (1) is a compound of
formula (IVa):
61
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
X3
X3-:- -X3
I ' .
'
X3' -J
R2
-X3 ``-, `',' ==`-''
I
B/'\N/\NR1
Fl (Jva)
or a salt thereof, wherein R', R2 and B are as defined for formula (I);
each X3 is independently NH, NR4, CH2, CHR4, CR4R4, CR4, CH, CD, 0 or N;
each = is a single or double bond;
each R4 is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, -(C1-C3 alkylene)( 6-membered aryl)
optionally substituted
by halogen or C1-C6 alkyl optionally substituted by halogen;
where each R5 is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl.
10108.1 In some embodiments, the compound of formula (1) is a compound of
formula (IVa):
,X3,
X3 -- --X3
XL-J ...,,,
...-)
X3 R2
I R1
B-N----. ''N-
H (1 Va)
or a salt thereof, wherein R', R2 and B are as defined for formula (I);
each X3 is independently NH, NR4, CH2, CHR4, CR4R4, CR4, CH or N;
each :-.-7- is a single or double bond;
62
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
each R4 is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or C1-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl.
[0109] In some embodiments, provided is a compound of formula (IVa), or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
[0110] In some embodiments, the compound of formula (I) is a compound of
formula (IVb):
X3
X3
x130
N R2
)(3-
./õR1
(IVb)
or a salt thereof, wherein R R2 and B are as defined for formula (I);
each X3 is independently NH, NR4, CH2, CHR4, CR4R4, CR4, CH or N;
each = is a single or double bond;
each R4 is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or CI-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6 membered
heterocyclyl.
[0111] In some embodiments, provided is a compound of formula (I'Vb), or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
[0112] In some embodiments, the compound of formula (I) is a compound of
formula (IVc):
63
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
3
X3 X3
I 0
(Th X3
R2
-s X3
R1
(IVc),
or a salt thereof, wherein RI, R2 and B are as defined for formula (I);
each X3 is independently CR4, CH or N;
each R4 is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR611.7, -NR5C(0)R6, -
NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or Ci-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6 membered
heterocyclyl.
[011.3] In some embodiments, provided is a compound of formula (IVc), or a
tautomer or isomer
thereof, or a pharmaceutically acceptable salt of any of the foregoing.
[011.4] In some embodiments of formula (IV), R4 is selected from the group
consisting of
halogen, ---OR5 and C1-C6 alkyl optionally substituted by halogen.
[011.5] In some embodiments, one X3 is N, and the remaining X3 are each CR4.
In some
embodiments, two of the X3 are N, and the remaining X3 are each CR4.
[0116] In some embodiments, the compound of formula (I) is a compound of
formula (IVc-1):
64
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
X3
N R2
_
X10
\Xl (lye-1),
or a salt thereof, wherein R.' and R2 are as defined for formula (I);
each XI is independently 0, S, NH, NR4a, N, CH or CR41';
X4 is C or N;
each X3 is as defined for formula (IVc)
R4a is Ci-C6 alkyl;
each R41' is independently halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -
C(0)0R5,
-C(0)NR6R7, -C(0)NR5S(0)2R6, -0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7,
-S(0)R5, -S(0)2R5, C3-C6 cycloalkyl, or C1-C6 alkyl optionally substituted by
halogen;
where each R5 is independently hydrogen, C1-C6 alkyl, or C3-C6 cycloalkyl; and
R6 and R7 are each independently hydrogen, Ci-C6 alkyl, or C3-C6 cycloalkyl;
or R6 and R7 are taken together with the atom to which they attached to form a
3-6
membered heterocyclyl.
1.01171 In some embodiments, provided is a compound of formula (IVc-1), or a
tautomer or
isomer thereof, or a pharmaceutically acceptable salt of any of the foregoing.
10118] In some embodiments, the compound of formula (I) is a compound of
formula (IVc-2):
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 777
x3
v3 ')(3
(-1
N
I U
X3, ,N R2
D1
R3 (1Vc-2),
or a salt thereof, wherein RI, R2 and R3 are as defined for formula (1);
each X3 is as defined for formula (lye);
101191 In some embodiments, provided is a compound of formula (1Vc-2), or a
tautomer or
isomer thereof, or a pharmaceutically acceptable salt of any of the foregoing.
101201 In some embodiments of a compound of formula (I), (IVa), (IVb), or
(IVc), A is
R402
No401 0403
rµ 1 "
R4 4
R4 6
R405 .-. K402, 401 404 0
, wherein R401, R R R 4--5 , and R406 are each independently R4.
In
some embodiments, R401, R402, R403, R404, R405, and R406 are each
independently halogen, -CN,
-0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR611.7, -C(0)NR5S(0)2R6, -
0C(0)R5,
-0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6 cycloalkyl,
or Ci-
C6 alkyl optionally substituted by halogen.
101211 In some embodiments of a compound of formula (I), (1Va), (IVb), or
(IVc), A is
R402
0401
1% I R4"
R404
R406
R405 , wherein R401, R402, R403, R404, R405, and R406 are each
independently halogen,
-CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -C(0)NR5S(0)2R6,
66
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
-0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6
cycloalkyl,
or Ci-C6 alkyl optionally substituted by halogen; and B is phenyl, optionally
substituted with R3.
101221 In some embodiments of a compound of formula (I), (IVa), (IVb), or
(IVc), A is
R402
No401 R403
R4o4
R406
R405 , wherein R401, R402, R403, R404, R405, and R406 are each
independently halogen,
-CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -C(0)NR5S(0)2R6,
-0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6
cycloalkyl,
or Ci-C6 alkyl optionally substituted by halogen; and B is 5- to 6-membered
heteroaryl,
optionally substituted with R4.
101231 In some embodiments of a compound of fomiula (I), (IVa), (IVb), or
(IVc), A is
R402
R4 1 L-3403
R404
R406
R405 R402, .-.403,
, wherein R4 1, R404, R4 5, and R406 are each independently
halogen,
-CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -C(0)NR5S(0)2R6,
-0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6
cycloalkyl,
or C1-C6 alkyl optionally substituted by halogen; and B is 5-membered
heteroaryl such as
furanyl, oxazolyl, thiophenyl, pyrazolyl, isoxazolyl, 1,3,4-oxadiazolyl,
imidazolyl, thiazolyl,
isothiazolyl, triazolyl, 1,3,4-thiadiazoly1 and tetrazolyl, each of which
optionally substituted with
R4.
[0124] In some embodiments of a compound of formula (I), (IVa), (Rib), or
(IVc), A is
R402
R401 R403
NI
R4 4
R4 6
R405 , wherein R401, R402, R403, R404, R405, and R406 are each
independently halogen,
-CN, -0R5, -5R5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -C(0)NR5S(0)2R6,
67
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
-0C(0)R, -0C(0)NR6R7, -NR:C(0)R6, -NR2C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6
cycloalkyl,
or C1-C6 alkyl optionally substituted by halogen; and B is selected from the
group consisting of:
\
N/
HN / / HN / /
,
-----et ---.
,
/ / \ 0 0 \ 0
, , , ,
,
0)4,,
Ot Ot N
A
N N \----0
N N ,-- 0 0 e\ ,--Y\O NCY\ WI\ 1\1 / hc /
1\13" \ 0 1
, , ,
b N:ery\s4 Fii\I ' H'N ' N N N
HN / / /
, ,
' ,
N¨N N¨N <( N¨N N¨N
HN¨N /
/ /
'
H H H
N N
/
HN-1(1 N¨N N\
68
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I 1 1
N\ / N\ / N\ / c
/ NA' ./N'N-)\ F3C
T'c.--/
NA N2_
N
, :;%, .., õ5....,2 q..._ ,õ
C1------U F---,C ----5-_-_-__J--
, ,
1-it
N N
, N N N
,
tN N,,,,,TA
(N\N.),
NH
N N NH ---NH
' ,
N N N N
N
-5--1.'NFI CTAN ------tZ A ,--N --)--N 1\1/7N-)\
\
,
S S S
S
S \ /s)(`F\45'
N-
,
StN S S
N' N,7,õ.\ ,NzsTA
N N N
NY\ ,',---0 N' '.'sTA
/
, , , ,
69
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N N -.)N4
- N c2 N-N ir
HN- N HN-N
=
N NINNirA N ,N yNis N
Ns N 1\j-N N-N N, N
N-NH and )---S
HN-N H N N
101251 In some embodiments of a compound of formula (I), (IVa), (IVb), or
(IVc), A is
R402
R403
R404
R4
R406
R405 , wherein R401, R402, R403, R404, R405, and R406 are each
independently R4. In
some embodiments, R4 1 402 40 404 5 , R, R3, R, - K 40,
and R4 6are each independently halogen, -CN,
-0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -C(0)NR5S(0)2R6, -
0C(0)R5,
-0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6 cycloalkyl,
or C1-
C6 alkyl optionally substituted by halogen.
101261 In some embodiments of a compound of formula (I), (IVa), (IVb), or
(IVc), A is
R402
R403
N
R4 4
R4
R4 5
R4 5 wherein R401, Run, R4o3, Rug%
=.405,
and R406 are each independently
halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -
C(0)NR5S(0)2R6,
-0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6
cycloalkyl,
or C1-C6 alkyl optionally substituted by halogen; and B is phenyl, optionally
substituted with R3.
101271 In some embodiments of a compound of formula (I), (IVa), (IVb), or
(IVc), A is
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042 777
R402
R4 3
N
R40,1
R4
R406
, R403, R404, - x405, R405 , wherein R401, R402 and R406 are each
independently
halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -
C(0)NR5S(0)2R6,
-0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6
cycloalkyl,
or C1-C6 alkyl optionally substituted by halogen; and B is 5- to 6-membered
heteroaryl,
optionally substituted with R4.
[0128] In some embodiments of a compound of formula (I), (Wa), (Wb), or (IVc),
A is
R402
R403
1\1
R404
R4
R406
R405 , wherein R401, R402, R403, R404, R405, and R406 are each
independently
halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -
C(0)NR5S(0)2R6,
-0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6
cycloalkyl,
or C1-C6 alkyl optionally substituted by halogen; and B is 5-membered
heteroaryl such as
furanyl, oxazolyl, thiophenyl, pyrazolyl, isoxazolyl, 1,3,4-oxadiazolyl,
imidazolyl, thiazolyl,
isothiazolyl, triazolyl, 1,3,4-thiadiazoly1 and tetrazolyl, each of which
optionally substituted with
R4.
[0129] In some embodiments of a compound of formula (I), (Wa), (IVb), or
(IVc), A is
R402
R4C)3
N
R40`
R40
R4D6
R405 , wherein R401, R402, R403, R404, K-405,
and R4 6 are each independently
halogen, -CN, -0R5, -SR5, -NR6R7, -NO2, -C(0)R5, -C(0)0R5, -C(0)NR6R7, -
C(0)NR5S(0)2R6,
-0C(0)R5, -0C(0)NR6R7, -NR5C(0)R6, -NR5C(0)NR6R7, -S(0)R5, -S(0)2R5, C3-C6
cycloalkyl,
or C1-C6 alkyl optionally substituted by halogen; and B is selected from the
group consisting of:
71
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HrIAN- 1 /N/ 1 i HN
i /
,
N N 0
/
---. \
N
N.,7),
\077A 10___,\ \ ,,TA \
N
, N N .0 .0 ,
Nrc\_)\ w\c) 1
N:7)----\\
0
N / N&\
N:DA Nt / / N: /
b NO-A Hi\J HN N 7 /N
HN i
ry)\ CY\
HN-N 7--N _________________________ N-N ,zz:( N-N N-N
/ / / ,
H H
N H
N
/
1-2.7,-\\
/ N-N N
HN-N /
' '
I 1 1
N
).N.D.,,N ,N
N \ / N \ / N \ / N õ N ,N,s
C-N-\ / 'N F^L, / 'N
, --Ki , ----i i --_.¨i
, ,
72
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N\ NA
C1-----C1- F----ty ,
H
H
Nt
N N
et 1 N,õ:1,A,
N N
N
4: NH
---K\ crAs,-NH NH
,
-5 ,
Ny.õ, N ,TA
(N\(N\N,, -NH ---N ---5---N 1\iN)1%
\
N N
n).% ./ '\'' -1\j)\ )\
_N. N NN
N N ci s S
r---- \---1---c NI N- N
S S S
S \ / el .,\ \ \
S \ / \ / \ /
\ I N
, , N ,
,õ\i, N / N / 4,N,,y,--\ ,Nyi,s
N NI, /
Ny\ , ,N N
N YIN' N' N' ' )\---0 N'NLY--\\ >---\ NH
\\,....N
\\--0 \\-NH / \ ,
, / ,
73
CA 03070273 2020-01-16
WO 2(119/(118584 PCT/US2018/042777
N N . N
N
y
'ii)N4 N if
N H N ¨ N / HN-N
= =
,N
N /)..Y\ N N N , N
ss
H1N¨N 1-11\1¨N , / N ¨N H S and
[0130] Also provided are salts of compounds referred to herein, such as
pharmaceutically
acceptable salts. The invention also includes any or all of the stereochemical
forms, including
any enantiomeric or diastereomeric forms, and any tautomers or other forms of
the compounds
described.
[0131] A compound as detailed herein may in one aspect be in a purified form
and compositions
comprising a compound in purified forms are detailed herein. Compositions
comprising a
compound as detailed herein or a salt thereof are provided, such as
compositions of substantially
pure compounds. In some embodiments, a composition containing a compound as
detailed
herein or a salt thereof is in substantially pure form. Unless otherwise
stated, "substantially pure"
intends a composition that contains no more than 35 % impurity, wherein the
impurity denotes a
compound other than the compound comprising the majority of the composition or
a salt thereof.
In some embodiments, a composition of substantially pure compound or a salt
thereof is
provided wherein the composition contains no more than 25 %, 20%, 15%, 10%, or
5% impurity.
In some embodiments, a composition of substantially pure compound or a salt
thereof is
provided wherein the composition contains or no more than 3 %, 2%, 1% or 0.5%
impurity.
[0132] Representative compounds are listed in Table 2. It is understood that
individual
enantiomers and diastereomers if not depicted and their corresponding
structures can be readily
determined therefrom. Compounds 1.180-1.185 are provided as reference
compounds.
74
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/04 2 7 7 7
Table 2
Compound Compound Structure
Structure
N
No. o.
-..,.....,.%
HO,n.,,,
.. I
N , HO., ,..,
,-----.;,;. I 1 -...-,
..,,xõ..,-...,...,,,.
CI 1.5 N
NH2
1 =::-.
1.1
I
,
-N"
N----s"NH2
I
I
CI
HO,---õ. HO
1
I
I N
1.2
.,--- --...,,,-, -:::, 1.6
CI
I
I
*--,. --1--""\"--------NNH 2
N NH2
HO
N HO
N,,,z,
1.7 I
1.3 I
.--,--., ....-1-........ õ;--,-...,
H N NH?
I
CI
HN- I HO Alm
,--- ,
I
. Nz.z., 1. 0
8
,----
4LIF 1 N
1.4 '''"='
I I
1 ,,,,. N,------N1-13 ,....... N
NH2
I
\
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
-z---N ,r----N
F-IN 1 HN
1.9 N 1.13 N
CI
,>=,..N.
N NH2 N NH,
-/----:--.-N ...,
I
HN N 40
1.10 0 N 1 ,'-:- 1.14 N Br
I I
(111101 N N H 2 1 10/ N N H 2
N
HO
I I I NN'
N so
.---=
1.11 I N
-, N 1. 15
1 . I
i
NNH2
N NH,
F
I i
N N
1.12 N
, -,- 1 . 16 14111 N
I I
N N H2 111101 1\1"-N N's N H 2
76
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
-..õ,
I CI
1.17 CI N
1 ..:::-. 1.21 ..,-,..,..-..--I N,...,..,
I I
.--7,... N NH2 0...õ---
-,. N. NH2
--.õ- _ 1
rN -
0 .. ., . .. -
I N ,
N
1 .--,-
1.18 I 1.22 N
...,1.--,
N N H2 I
<1--11'-N1N H2
v.---7-N
rr --
N,,-.,.. rr--N-J
I N ''''N
1.19 ,..õ--..,,,,, NCN 1.23 --....,},,,,, N.z...,
-r -N,'`' N- NH2 "1---::'"--..."-,--
--'-'N'N H2
I
j
`-...,....4-%
N I I
N
N
1.20 1 ',> 1.24
(
-:%"--,
N NH2 I
i [1.2.'")"... .-N NH2
y---0
77
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/0 4 2 7 7
7
r - I
1.25 -.-,,,,.....õ,---1 -..,,,N,...., 1.29
Na N
1 ' ''=,''..
0
.--, - -- -- -, - - - - = - - N.--NH2 re'-',;r`i N-- 'N.--.
I K 1 I 11 H
a
1 .N
1.26 ---...,
1 -,'-: 1.30
.---...,õ
7"----z:y."-"'N" NH2 ----
"NNH2
II
µ-6 N....,....
I Ii
N ---,
1.27 N .;-,,..;.;.....,,,,---õ. .--N 1.31..õ--
õNit
I
N NH2 N.--,.,
NH 2
I I
N''''.."":` Y-
N
1.28 =U-..,,,,... N .,..,,,.., 1.32 II N
1 ,..,..
---"-----, -----s.'N----'''NH2 - - - - " '-' = , ..."1
- - - - N NH2
s-,,,,,........--,-, -....,..
78
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 777
/
N..,. ,õ N N/71,,
1.33 1.37 1
0- N.-- N H2 -T-.----
---.¨...-NNH-,
I_.....
õ---- --....- =
N -- rr-----
1 1
N--..,-----N, Nõ,.,,..;:,-.
I
..,,..,,
1.38
ir---"'NN H2 Niz-y"--N.--N-NH-
11-,õ 0
ir 0
\ frk
N ).-N--,1 N,.
1.35 -::::,...õ,---I-,,,,...N.:. 1.39 '.-, I N
N NH2
N/N1--.TN-..---N H2
4----s rz---__N
HN µ,
I \,-;--- ---ii
1.36 ,c,,,--...,,,.N,
1.40
I I
i-------------- N----'..N. N H2 ff..- N-
---N' N.-'; NH2
-.....,...7- \--.;h1
79
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
/1-0 /-----_-__N
H N,
.--..---- --,
I , I
1.41 CI '-'-.-----'s.''-'' r\i`z= 1.45 CI
I I
N NH2 yNN H2
I
y,..0
,...,
rrs CI
N N HO ).-..
1.42 CI '-',.. 1 .:-. 1.46
I
N NH2 ______N
NH2
CI
0
I HO
N I
--;,,.....õ-.,,,...,. N,....
1.43 1.47
1 N-,== I
I N,7,...NH 2
..._:(' \ N NH2
I CI
HO,,,,...4-1,-õii
, I
1.44 -..,-...z.õ,....--õ,,,,õ Nõ....,..:,,,õ0...õ, 1.48 -
,,,,õj=,.,,, N,,z._
I
-'----"c-", ------N= N-->.---'' NH2
I N /
/C\:::1¨" N --..'µ'N= N H2
\ -
--...,-:-.---
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
CI CI
HO HO
1.49 -`:..,.,..----.õ.õ,..I Ni.,,_
1.53 I
-:.-.....,õ.õ--....õ,õ INI:...,
I I
N)::_)2( N NH2 0. -:-----.,
N NH2
N
CI CI
HO HO.,,4.õ1,
I
I I
1 N I\1
.50 ...- ..z...... -,'-_,.,-,.... ,. j..,.
/0- ...--..s.
N N NH2 1.54 0 r N NH2
/ =
CI CI
HO,.õ,,-1, HO
I
N., --,-õ---,,rx N.,
1
I -.- 1.55
.51 N N NH2
I
N-:"---,. N H2
1
-1-- IN
/
CI CI
HO ash HO
b,,,,
1.52 gill ,,,i, 1\1,,, 1.56 -.. ,, N.....,.
I
Ko---ir----- N-r.-- NH2
\ i
--- N N -
81
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
CI CI
HO.õ HO-......
I
1.57 -:,-,-:,õ:õ---.,.....,.I..N...,,,.
1.61
I H I
N e N NH2 N ----.N.-:- -
NI-1-, NJ(
N
CI CI
HO,.../, HO
"----)'"-
1.58 -,'õ, N. 1.62
H I
_ /0 _,---.1 'N"-r"---"NNH2
'IT 1\l';'-. NH2 N /I
N- /
CI CI
HO
HO oit -,---.)\
I
N 1.63
1.59 1 '.
I H I
ei N NH2 NN,17,--
--,,N-:'---,...NH2
N
CI CI
HO-,--7-, H0,6,,,,,,,
,s. I
1.60 .µ" --',---'.."--,-,-N1.-zz, 1.64 1 N
-:.-
H I I
N\N*---ri NN H2
Nõ.----,N,7-.NH2
N fi
\,,,,.......2., \\,...ij
82
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
C I CI
HOõ,,),õ.
,js.,,,. N I
1.65 1.69
I
\ I
NN NH2 N.,----r N NH2
\ 1 4N --N\
I ________________________________________________________________________
CI CI
Has.õ.,,õ.1õ... HO
I
-...,...,..õ---..,,,,N -N.1,,.1 .N
1.66 I 1.70 --....... ,:::,...,.
N ----...N..:'----....NH2
Ny ,..,.õ
Np--. / N NH2
I
4N----,õ
I ________________________________________________________________________
CI CI
HO 110 HOci
I
.õ NIN., N---.,..----õ,N.,.
1.67 1.71
N---- NH2 I
N I Ny'N- -'NH2
\ 1
iN
/
I ________________________________________________________________________
CI CI
HO HOõ,...L.,
z.
1.68 ....-..,..õ----..,....õ N..,,,. 1.72
'-NH2
N li '7.---(-
--"N-j,._ NH2
NI, li
N--\
FiN----' /
83
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
CI CI
HO1,,,i
N
, I
,.,-----,,õ,I
1 z:., z.-....-----s--õ,--
N.:.=,....
I
1.73 1.77
I
N, ----. --,---,,
1.--q"-7-71--NNH2
,),J.1 N NH2
iNI-----`',,
/
CI
CI
HO
HOõ,õ, -,....-.7k
I I
"-n,1\1_,.,
1.74 =====`..;õ,... ..----xl\iõ.z.z.õ 1.78
I
IN. -.7,,
Nõ -7'
-Thl NH2
_IN ''N NH2
CI CI
HO
N 1
- -,..
I1.75 1.79 -...õ-----",,,-N
e/N.'N N NH2 NI, ..-^,I.
__
NI\ N 12\IH
j
CI CI
HO N HOõ,,.,,,,,,.,,
'N,.
1.76 r N -.: 1.80
I
N, ,--,,I -:----. _____\
PõN.----,N-7..NH2
AIN N NH2
\ ....- I
/
84
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 777
CI CI
HO .1.., HOõ,,..,;>N,1
I
1.81 -ss....,-----..Nõ, N.z.,..z_ 1.85
,'N..,)===,_õ1 N,..,,,
H I \ I
N'Y''''NNH2 N.3,------1\INI-12
j 1
N NV
CI CI
HO,..õ.. 1 HO,,,
I
1.82 ,..x
1.86 `,--,,, , INI
N N .,
- i -"1-.. -,:%---.
<õ,\ ii N NH2 r N--.
NH2
N---\ N--"Nõ
CI CI
Ho, 1,.., HO,,,,,j1,,,õ
1.83
--:- 1.87
I
H -.., I
iNI.-----,NNH2 _ N N.--'-
'"NH2
---- i
N'i N
Cl Cl
I HO,,,,.7.-1..õ.1
I
1.84 ..,,,
1.88
-1,,
N -'1- NH2 ..
N,,,,..----.N-:----.NH2
"
---- II
N---\ N---\\
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 777
CI CI
HO,
1.89 ,..,,, N.,,_. 1.93
I I 1.
S.. .,"'-.. ==-N- õ
-fir N N n2 ,t2M-----'' N.-;.- -
NH2
cc
N if
N --j b---\
CI CI
HO.--.,. HO
1.90 .. ,..z.,...õ,..e.,N .,..,..:. 1.94 -..:z...,..,,
õ..,--õ,...õ, N.,.õ.,,,
I j
,s N NH
----,------ ''..- 2 I
----''''' ''' e'. NH2
---\\ j
N 0
CI CI
HO...õ,..,.::..),5 H 0 -õõ..,.....-
I I
: .
1.91 N ..,
1 N-`,,,
I
....;-,õ
N NH2 N
N NH2
N \S ---
CI CI
H 0 ---L,.. HO
I
1.92 -:-.,-,,,.,,,---, N.,.,,. 1.96 LJJN
1 ----
Nii.---"B ii-''''" 1\1 NH2 ....!7--- N
NH2
N i
86
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/04 2 7 7
7
CI
CI
HO HO.rt., õc
, .-1,,,
1.97
I I
NNN'NH2
N"Nr\NH2
i
\-...-;----,- )--'\
CI CI
HO -1,,.
I I
,,,-----õ,õN 1\1 --zk.,..,.,---
.
1.98
I _,... 1.102
0---,.. ..-----õ -õ,---.
Nix N N N H2 -'-.. N
.ININH2
N
t......d r--'\
CI CI
I I
1.99
1.103
N\
1\1----"N----.N H2 /NL-r--
N-N- NH2
µ¨NH
CI CI
HO.1, HO
I,
1.100 --.;zõ,.---,,,,1\k,,, 1.104
I
N. .---. -7-.
N./ N N NH2-.---T N NH2
87
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
CI CI
HO,õ,--L,
,--- 1 HO...rt. .,..sc
., I
I
-..-.-.:...,,,,,N,......z... -...-",--
1\4.--,-.
1.105 1.109
I
N._...----. -.------ ,,,-,,,.,
y-7- N '''NH2 <N--y--
"N, NH2
\ NH
,--N
N.
CI CI
HOC HO
'------'-'I '...,--
I
N., --,,---_, N,,z,
1.110
1.106
yi *N'N I
....._./N N-7-.NH2
...__/N-z-N. -NH2
,---NH rN\
CI CI
HOki
1.107 -,-;;;-,.õ----..õ,...N,z,,, 1.111
I N. ___
_..rt
ei---f------N--NNNH2 (N--- N-,-;--
-.NH2
.--N
---s
N.
CI CI
HO..,..õ,),, Hor
J 1.108 N 1.112
N
-- N NH2 N--zr"-
--NNH-)
---------N -
\
88
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
CI CI
HO HOõ,,õ),-.,,,
N , I
1.117
1.113
I z:.,
,,:,, I
N-----i- N NH2 N. .---... -.-----.
= ---'
).--S N 1Y N
NH2
..--N
N
CI
CI
HO 1,
HO
I---..--)N-.
1.118 1.114
NI. -7-. I
.1\1 NH2
\ S
...___
N=N--,IrN NH2
\--;--N
CI CI
HO-,--õ HO 40
....,..,
1.115 .....,,,,,- ITN 1.119 N
1
I I
N=-r"`
FeNNNI12
'-- N NH2
i N'1
----.NH ---0
'
CI
CI
HOõ,õksi
,..-.õ .,õN....,.
1.116 1.120
N, j,, -'t... I
N= ---r-- N--. NH2
Nr-N- NH2
N'\
i .--S
89
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 777
OH CI
I I
-..n1\1...z.,,
1.121 ..,
I 1 . 125
.- - ' - - - - '*. - = = = . '1 rs.r.--
NH2
,N-zz-r---'N''1\1"NH2
N i I
F
CI CI
HO,õ2,-,-.1--õ HO.,,,_47
I
I
1.122
-,-õ,N.....,
1.126
,,,,-,-õ
N'N--'-zr'NN NH2 -''-:.`- N.----
"NH2
7- S
F I
.,--
CI CI
HO HO
1.123 . , . N
1 --:-. 1 . 127
N'N'1"" N*-'--NH2NH-
1 z
,,,,,,,-NH .,---
''---- CI
I Cl
CI
HO HO..,.
N
1.124 .,6,
- . , I .õrs1.,,_
1.128 I ,
1_,--,z_T-----_N-----NH2
N N H2
..""
F
Cl
1
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
CI CI
HO k1 HOõ_õ;:---1,,,,,
I I
1.129 1 ---:.- 1.133
,:-,--õ
1 "-- N NH2
I F7-X-N-
'-'N-NH2
I
CI CI
HO...õ)--..,. H 0L.7.
I
I\1
.., ...
1.130 I 1.134 I
CI ... -
N-....-- NH2
I,
,---
---'--- r,-..,
FN NH2
I
r,--
CI CI
I _________________________________________________________________________
CI CI
HO,-., HO-..,
I
...I...., ,-..., N ,1\1õ..,.>
--,-- -.--- -z.....,
1.131 I . 1.135
`N--'":,---'--1 N--'-'-' NH2 ,-"-...----'-.1 N-'--
NH2
õ.---.1.::,-----
F F
CI I CI
HO ..,.- H 0...,,,7.---1,..,
I ,_ I
----, N
1 ., ,...-
..õ.,....,,,õ1,.. N.z.,.,
1 . 132 I 1.136
F ;.-,-õ
N N H2 FN"-rr I\1 N H2
F C I
91
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
CI CI
HO
' 1 HO
I
'--.. . N
1.137 1 --:- 1.141
F I I
I T `-'1,---N" N NH2
-1,,,;,,,- N
CI
CI CI
HO HO
N
1.13 8 1 --:., 1.142 . .........,..--...,,,_.= N -
I I 1
-:::',--,
N NH2 -r-----N'Nf> NH2
I
F F - N
CI CI
HO
.N.----;,--j".,, HO
,..._ I
1.139 -.....,...,....,,,. N......:µ,..
1.143 1-:-'- . N-:-.
I I
-N'NNH2 ----,---1 r'N.<'-'"NH2
1 N
F CI F'''------
CI CI
HO _.,,...1, HO, -,,.
..._ I
---, --.., ..1 N
...,,z,z,,õ...-..õ......õ. Nz,,,,,
1.140 1.144 'N...::,,-- -..õ-- ---:-.1
I I i
1\r/-'-- NH2 ..-- ,,
I ....,. ri---,-------- N.-
NH2
..---`-,..--
CI F N ...,:,-
õ,
92
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 777
CI
HO ,/ 1 I
1
CI N
1.145 N.,.....,
....-z...,õ,,....--..N.......z.,
1.150
I.....
,...-
N N
F
;-'=-='... N NH2 H
1
....N-;%.
CI
HO
1.146
HO =2:1:1
--õ, .--......õ,,..N.....,...,
N
CI 1.151
I õ....
.,-- :;-...
I
1111 1\(''`'NH2
-;:-.----,
fiN-s, N NH2
N1)-s-s.----/
O'''
HO ..õ-
N, I N Br 1
--;:-..---
I CI''''' 1 N
1.152
1.147
1
....2.õ
N NH2 0 N-
--...-"NH2
HO HO...õ....,7".N
..,-
N 0
.õ.õ,..õ.õ..,.,N.,,,....,
CI' 1 CI
1.148 1.153
I ,..,._.
1
N NH2 -',..1 N---
--N.-NH?
1 --`,-
I I
.,'
HO HO...,,,;;.:...N
...,...4-7,,,
CI CI
1.149
N- ,,, il,. N 1
1.154
1 I
N NH2
,
93
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
OH HON
1.155 N N
i .---, 1.160
--'''N' N----." N H2
N NH,
"....õ,../..0,-.'"
Has,,,,,.7,- .N
Ha.õ....õ,.....?-1,./.,
..õ,..k,..)1,õ,,,..N....,,,,
CI
1.156 I ...-,,,.. 1.161 1 N
'N's-ry.'"'W NH2 0 I
-,..
N NH2
F
_
HO,-7,..N HO .,,----
,=-,-...1-............õ. N.zz., õ0õ,...j..õ.......õN
1.157 1 1.162
Cr -
NN,in'syN'NNH2 o,I N1-----N'NH 2
N .- N
HO
"N
'µ.. N
CI i .z.:=. s'=-.....-^
1.158 I 1.163
.., .__,0
N ri I
,,- II F ....''''''v -s-,
N NH2
-Xi
HO,,,,,,-7....,
HO
.,=-' N ,.., I
I N
N. N ,.........õ...-,...,,, ,..,,z,...
CI i ===,-= C I
1.159 I 1.164 I
NI::=-=.. N -,--..\/0 _____,,,0
- \\ r,----N H2
H
94
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
HO 0 HO si
CI N N
1.165 I 1.170 CI
0 I
S.--.,.
\ / N NH2
\ / N NH2
HO 0 HO .
N N
CI 1 CI 1 ,.
1.166 I 1.171 I
0 S ..,-.-,.
\ \ / N NH2 / N NH2
HO 0 HO .
1.167 N CI N
CI is 1.172 I
S N NH2 N NH2
/
N
HO 0 HO .
N N
CI ).,., CI i ===µ:-.
1.168 1 1.173 I
S..;.-N,
\ / N NH2 N **'. N NH2
l(N--'
HO 0 N
S CI HO 40)
N
CI
1
1.169 I 1.174 I
\ 1 N NH2 1 N NH2
N ,=-'
F
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
HOõ.,,,.---.
CI
,õ....õN.:. j,.,,,, I N
CI'
1.175 I F .õ 1.180
'-`=----s.'N--/-s"'NH2
h -(''NT-- 'N__ ---
NH:,
I
N., .--' N...,........,...----
F
HO 1.176 0
I
j.
N ---N --- ,,,..,,..... N
N NH2 CI 1.181 CI 1 -=,-,-
I
N NH2
1! .
-..,,......--.
HO:ja,
I N N
1.177 1.182 CI ---;.,.
I I
'1\1 NH2 -,-"/--,N-----..1 N
NH2
.--' ..õ...õ../.,...::-.I.
HOõ,K2-,N a/Th
, -...,,z,,,,,,/ Nõ,, ,,,,,.N N
1.178 1.183 t .`
I I
0-.. .---..."N NH2 N NH2
i ..---
HN\_,;,,,..õ,.,
N--/---N"'
Nz...
1.179 HO 1.184
I I
alp --N.NNH2
i
96
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/0 4 2 777
H N 1,......õ
1.185 N
, =:',.., 1.190 .--.,õ N
, --:s-
I i
N N H 2 '''",1 N N H =-
,
1 ________________________________________________________________________
...,.,-........ .
....
1
H N
, ...)
1.186 ".---..1 :::" `-..,,.. N:.-.õ 1.191 ----- NCN
""--'1\1"-' N' N H2 1 '''= NNH2
II 1
- ' = - = .:.,'" -<:' --"'
/i-s
1
N \,....,---=`,...
N 1
, N
1.187 N
1.192
H I '...
110 N N H2 ""--, N N H2
I
1
Nirr 0
`,..).''`N,
N 1
N
1.188
1 1.193 -,:,,,,-,=1-,,, N,zz..,.....õ, C N
N..7-,.. N H2
6--------:-..------1\i" N H2
1
N
1.189 . N CN
- L N
, -:- 1194 ,----
-,..,_ 1 ,,
, -...-----
I I ,
...-.., 0 ---õ--,....
C 1\14 ' N N H2
.... ..- -- N
97
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
/7-0 frz7N
N HN
1.195 --,,,,,,-,,,,I,õ..1\1....CN 1.199 CI N
1 ---,,
I , I
li C N...----,N2-,NH2
- I ''N.
N NH2
1
.... (---
I
N, 0 N....,_;,2,,
Nk CN 1.200
1.196 1 =-,-
I
/ N
c N NH2
-NI N."- N---';-.NH2
\-0
-r--
=,...,.
r----:--N I
HN
,)---L,,,,..,,I--,,.,.õN., ,CN N
1.197 CI 1 N----- 1.201
I
/C11NNH2 1 N:NH,,
--7-N ---(31 ,
/
,..--
II rr'
N....-,-.
, I I
1.198 s-s.:,..,.....Trx-,õ,. N.,,,,.. 1.202
1 =-=,-
I I
....;-., -----= .7.,
-N NH2 N NH2
-=,..,,0 N-s.
-..
98
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
ii-------- 1.,....,0
N.õ-,..
HN -----
-,-;.--- =s-ii
1.203 -,.;,,.,....)--.,õ,õN.,,, 1.207
r -.---
N'' NH2
II
i'D''NNH2
N.,..,.;:,N -I, ----
I I
N
1.204 N
1 '-.-. 1.208 N
1 --,
I I
N NH2
N NH2
N - NH
N I
N
N
1.205 1 ,,- 1.209 N
N NH2
N-..=----..NH2 I
eiN-y
-.,.
HN. j, N
N
1.206 T 1
µ,.........,,,,Ni 1.210
1 -:-
I
N NH
N NH2 ,)
\ S
-,..--=-j
99
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 7 7 7
II I
N ,,, N el
N
1.211 I N 1.215
1 - 1 ---:.-
I 1
es-= N 's ;'-"-
1\17'1' NI-;.-- N H2 NN H2
N --
,,
õ.. 1
1 N
N 5
N
1.212 N 1.216
1 --'=:. 1
N --;,,,,. N N N H2
1\1'-- N N H2 N),_ j
t-- S
I rl
N 5 N
----
I
1.213 N 1.217 .-.. N
I
N N N H2 I
...õ
N N
1.214 N
1 --:- 1.218 Ne.-.
I 1
----- N N H2 N N H2
\ N
--
100
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/0 4 2 7 7 7
-....s.,
IJ
N N
1.219 1.223 __,. N..,....z.õ
1 N NH2 N-:-,
I I ,
.:;--...õ. r--"`',.-''N' NI-- N H 2
-..,...
I
1.220
N \ /
N
N..z.z...
.224
1 N 1
=== 1
I..4.--õ..,
.....;:s., N NH2
I / N NH2
L 0
r-'-'-
Nli µN lb
,,=,:'-`".1
===;:zõ.õ----,I N.z...,..s. N N,z,,
1.221
I . 1.225
....---..,
..--"----1 ----" N N H2 N;;. NH2
& N 'N,(D
Fi
..,..
N 0
NH N ...,,,..,;...,,,
I ,
1.222 1 1.226 N
-...õ.....õ..,õ..-",õ.,,, z:z....
N NH2 I
1 N H N NH2
1
0
101
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
-,,,...
0 I
HN
I 1.227 -,õ---,-----..,,,,õ N..,.,. 1.231 1
N NH2 N CN
F
r.--'---,N.
0. --., H
-1-- 0 N-
H ,r;..,,,
Nõ..,-1.-.-.,
1\1CN
1.228 -õ....(7,,..õ..,... N.,,,.... 1.232
I
N N H2 N NH2
O'-'---- I
.."--
,..,.
(NH I
N
HN ,,...
I N CN
1 -:..---
1.229 ...- ., N
i --=:.. 1.233
L11
N NH2
' N NH2
OM e
I 1
N
I ..-- N CN
1.230 N F N
i =-..,--"" 1.234
,===,.,, ..,,,---õ
N NH2 1 -=== N NH-,
F
102
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
I I
N =-.. N
0
N
1.235 1 ===z- 0
1 1.239 1 N''-.-)LOKile
.,.-..-...õ )1.,õ..v I
\ 0 H N NH2
--,
I rr'
N
A
HN,,
1.236 1 N.-==----NH2 0
I 1.240
,...;-..., 1.<\
\ 0 H ---===1 1\r'-"NFI2
...,....õ:õ.õ5-I...-
--....,
N N
..---- 1
1.237 N CN 1.241 ,,.., I
N OH
i -z:-:---"
I I
N NH 0 -- '--µ=N NH2
I
---."
1111.14
N..õ...õõ:. 0
1.238
.,-,J,,N)LOH
1.242 RP NI,,.
1. '-'---
1 CI
,:>-....õ
1_,,,,IN NH9 41 NIVH2
,---
_
103
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/04 2 7 7 7
-1-
NC HO".;:.7)1
1.243 N-`,-- 1.247 -z-
,,,,.,...--9-,,,,, NCN
I
N--;;-"" NH2
1
1
Ha.õ,,,..,--...,
I
,..,.....õ..,-;=,,.. ....
1.244 01 NxCN 1.248
,z,..õ,...._õ,,,,..õ...:jI hi,:...,,
I I
N N H2 ---' ''''''.::, N
NH2
I,
..,,........:õ--,--
HO. .,.----
-µkiNC
1.245 N CN
1.249 -:.-,,,..,-,,1 ,...,. NC N
1 \ 'N-';'-NH2
N---'''N H2
I,
-...,...,7,--
I
HO I
1.246 N 1.250 CI . ... e. - n---, N`-
's.'>
'N NH2 --
..N'. N H2
\--0
104
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
-1
NC HO'''' NI
-11-s, .,----. , N N CN
-k-
1 1.255 1.251
I
N NH2 'r-zr1\1---/--NH2
\ 0
---0
HO-
I
1.252 cl-,-.---'--,....1\1,,,.,,CN
1.256 N...
I I
-;]
N- NH2
----'y---N NJ H2
----0 \ -0
I.
NC
1.257
N CN r 1
1.253 , ====-:,----
I
.',,',1,, NCN
..3:-.., il '
--.. N NH2
C---y. '1\l';'-'..NH2
1 _________________________________________________________________________
F
-"'->--,
1
.r.
HO'''''''''' ,
1
"=-.. N
, ..-
1 1.258 - HN
1.254
Nc.)
\ 0 I
7s.*---r.'1\1NH:,
--0
,/
_
_
105
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/0 4 2 7 7
7
.........c,...,õ....F
H I
--..,
N 1
N .., j7.,..1 0
1.259 Nõ -CN 1.262 II
IN.....õõON
C
I N N'---/-." N H2
.7õ
--:-- 11 -- N NH-,
\ 6
-..,.. I OM e
ir-:---
N N ..--
-õ,,,N., .õNICN
1.260 1.263
I I
r4H2
}---0
\ 0
/
sp F
H
r. N.,,,,
H N
1.261 -,. I i\L,..,.. 1.264 I
1 --- 0 -sz.-.;.:,,,-,,,.,õ
Nts.,..,,CN
N N H2 I
/----------r-'--N`---NH2
106
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
F
0..y.----....
HN,,,,,..,..1 1 I
11101
F N
1.265 ---,...----,,..,õI N,,,.CN 1.268 LJIN S
Ii -z-;----
e N--N-I\1H2 I
-õ, ,-------..
7-0 \''''I
N NH2
0
i
F
õõ.,....F
l'I'.
....._ I
N
N,,,---), -,..y-7 .-- 1
1
'N:3.-.õ,.õN __NH
1.266 .'--, I N NH
--C1 -=:.---- 1.269 I I
"--r.'NNH2
\ = \ 0
ro
F
N._..._
N..,,,,..2õ...... HNI kip tahh
N
1.267
.`----,---,,.õ..N 1.270,...._,.0 , -:.--
I I
\ 0
0 N NH2
`,.
I `N,
N
NI
0
1.271 CI , N'=:-.
I 1.272 N
1 '1)LNH,
I
r,1 NNH2
- N
/c N NH2
107
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
`-.. N-
I
N HN1 ahh
0
1.273 * NXAN''''N'y 1.274 IV N CN
I H I I I
NH2 N - r *I N NH2
`.. "..
I I
N N
I. N r----N-
1.275 N 1.276 N N,.....)
I X I
I
N NH2 N NH2
r----.N
HN N
101 N C N
1.277 CI 1.278
I X I
0 111 ..., 0 N NH2 N NH2
I
\
\ 1
I
N N
1.279 Oli N 1.280 N
1
1
I
I
S , N NH2
c1,1N NH2
I..
..,
'',..
I
N N
,....-
LJLN N 0 Nx,"%N
1.281
I 1.282
I
---.. N NH2 ---- N NH2
\ 0 \ S
108
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
õ,.N \
I I
N N
4111 N ,/.%
1 . 283 N
1.284
I ;J., I
1101 N NH2 N NH
AO
\
i
'S
N N. 411 N 4111 N
1.285
1
1 1.286
1 4....
/j N NH2 / N N''
NH2
N -N -- N
i
N.¨ \
H13
14 i
N
N 1411 N
1.287
i a, 1.288
I ,),..
NH2
ctisi N NH2 ---.... N
-- N N
7_s
HN N
4111) NC
1.289 N
CI N 1.290
I I
SI N NH2
c r,1 Nx NH2
--N
\
NI \
NI
N
1.291 is 1.292 NxCN
N
1
........tz\ ' N NH2 CI
.......tyN's I N NH2
109
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042 7 7
7
I 1
N N
1.293 N
1.294 N
I I
....;¨,.. ...,- ...,
N N H2
N NH2
H N - N 1-1sN
N¨
H NI I
N
N
1.295 CI
1 , 1.296
c 1 N*
/ N N---'N." N H2
- N/ N N.--1......s. N H2
1
I..,-..
N I
N
N
I
1.297 C I , ::::-
1.298 N
, =::,===
....,..., I ,..,
N N H2
C Ns N H2
N - N
/
N¨ 1
N ¨
Hr4 HN
Si N )IIixN
1.299
1 -:....s, 1.300
...7..... .,,
/j N N H2 /N N"....µ.. NH2
N
/
1
r---z:N
I
N H N
N N
1.301 , 1302
1\ N H2 .
I , I
CZ, c 11 N ".."..' N H2
-- N
0
110
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
"",.. N-
I HNI
N
1.303 N 1.304 Cl 001 N
I
fJ N""--"NH2 / i N NH2
---N N-N
/
N
I
N
N N
1.305 CI I , -:.--
1.306
/ 1 N N H2
c fl N 'NH2
N-N --N
/
F3C
-,...
I
N
N
CI , --;=
1.307 I 1.308 N
....,--., CI , =-;=
/ i N NH2 I
N-N N NNH2
4
N N
N OH
1.309 C N CN I , -':-.=-="
I 1.310
..,,,,,-, I .......,
N NH2 / / N NH2
N-N N-N
/ i
Ti
'-=-,
I I
N
0 N
1.311 CI N N 0 NH2 1.312 CI
N NH2
N NH2
N-N N-N
/ /
111
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
s-,-, `,,
I I
N N
N OH N
1.313 CI 1.314 F , - : : .- = -
c l'1I\I , I
/ r H2 N
- N
-- N
/ / NH2
N-N
/
HN
I
0 N
N N
1.315 i ,--=.-..
I 1.316 CI Br
...,,......, I
N NH2 c r/4 N1 N H2
N-N N
/
'N. \
I I
N
0 N
j-( N
1.317 CI N
1 -N- NH2 1.318
N c NINH2 / iN1
- N
I ,.;,.....,
N -N NH2
--s
\
I
N N......
Hri
JJL N
CI ,
1.319 I 1.320 CI N
N NH2
,
/
,....,-,-..õ
...,. /
4-7.--NI 1\Ni-i2
N-N
c ":k........,--L-
0
I HN
N
0
1.321 CI
N
I 1.322 CI N
I
N N'NFI2 ..-7..õ
N-N
/
112
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HN
I
0 N
N ON 1.323 i ,s::,-,"
I 1.324 CI N Br
..,..1.-.õ
N NH2 / i N NH2
N¨N N¨N
/ /
I HN -"--=
N
01 N CN 0 N
1.325 1.326 ,I s.-
I ,
N '--, N---"NH2 ..-..--N N----N"NH2
7---S % !
...---N
--...N ...õ....
HN ',..
0 0
1.327 LJLN
1.328 N
1 -:-.=
I
N NH2 / i I N NH2
N¨N
/
N..
I '---,
N I
N
N
1.329 CI , -::.-==
I ..., 1.330 CI
I N'zs4'
1 N*--- NN H2 N
...;.====.,..
---- NH2
0 S N
I
===,.. N.
I
N HN
N CN Oil 1.332 N
1.331 CI
I -:.---- CI , -:-.=
.õ*õ..
=---- N NH2 N --- N
NH2
0 7¨S
113
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HN "==== ,..
I
0 N N
ill
1.333 , =k,
I 1.334 CI
N
N ----- N N H2 -7,,
N' N's- N NH2
7-- s
2-- s
I I
N N
1.335 CI N's=-
I 1.336 CI N
-;-...., ..õ..
N'N.-- N NH2
c r,1I N
--N
====, /7-0
I N
N
1.337 a 1 N o
N-Th 1.338 CI , N--":,
/ i N NH2
NN
/ N-N
/
I N
N
0 I
1.339 Ci N ..........õ -N. N----...,-
-...,
1.340 CI , -:--.
I
./' 1 N.".."'"NF-12 C-=
N '-- N N H2
i N - N 7¨s
Nfr-S
N'
N N
1.341 CI , --:, 1.342 CI , `=:,
...?...õ
,.."...'" NI N NH2 --4';µ..'NI N*...s.'NH2
===:;,,,.õ,-.
0 szks.õ,-Lo
114
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
'N..
NI F-0
N
N
1.343 1 CI 0 1.344 CI i N,;=
I
cr;,I1 N ii io ,....",
NH2
-N ,---S
'-, --,
I I
N N
SI 1.345 CI
N
I 1.346 CI
N
.....7. N NH2 N ---- N NH2
HN '-'=-=
HN .."-,
0 Olt 0
N
1
I 1 1.348
.347
I ,....
N "-- N NH2
--="*"N N NH2
7---S
\ \
I I
N N
1.349 Br N
1 '-',-_-= 1.350 Me0 1 N,.==
......-,.. I ..........,
/ I N NH2 / / N NH2
NN N-N
/ /
I I
N N
1.351 CI NX0H 1.352
CI N
i =::.-,
N NH2 N N NH2
/
115
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
NH
HN
0
1.353 1 N-:- 1.354 N
I I
,-,-......, ../}....,
/j N NH / i N NH2
NN N-N
/ /
'-..
I `N.
N I
N
1.355 CI
I N...... 1.356 CI N-:.,
..-,-,.. I
I N NH2 .......:-..,
NH2
N / N N-N
/
`-.. -...
I I
N N
N SI
1.357 ci
1.358 ci
N
I
/ N N-:;-."'s N H2 N
.1,...
.41 *.--; N
NH2
,
N ,.., N
I H ....'
N
410 N
1.359 CI i '-:.,-
I 1.360 41111 N
1 :.=-
N--- N NH2 ....:;:-...,
. 0 / i N NH2
N-N
/
i
..õ,N ....,N
H
N N
101 N
1.361 CI 1 ====-:, 1.362 14110 N CN
I I
.......,--,
/ / N NH2 / i N NI-
12
N-N N-N
/ /
116
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N I
N
410 1.363 a
N
I 1.364
CI N
, .....
.....-...õ_ I
N--- ..
NH2
41, NH N NH2 N '-", N
N
I
I
N N
N Br N CN
1.365 CI
I - 1.366.....--"
N NH2 / i N NH2
N-N N-N
i /
1
-....
- CF-
I --, J
N i
N
1.367 CI N" CN
I 1.368
CI 1'4 CN
...,.....õ.. I
N NH2 ...7.....
N NH2
1
`,.. ",..
I 1
N N
1.369 F3C N CN 1.370 CI N OH
, -`,..=-,"
I I
N NH2 N NH2
N-N
/
i
OH
fr. 0
0 isi 0H
I
ci `. I Nr3t,N N
1.371 1.372
__NH
N -.= N
I
/ .../.;--,..
N NH2
N-N
/
t
117
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
e
\ 110
NI \
I
N
1.373 1.374 N.,x c N
4111 NX NH F
I
CI
1 N NH2
/ 1 N NH2
N-1\1
/
`-.. "....
1 1
N N
1CN
1.375 F N 1.376 NNICN
I CI
I
N NH2 N NH2
F F
\
\ I
NI N
1.377 NxCN 1.378 NICN
CI
CI
I I
N NH2 N NH2
F F
\
I \
N
NI
NICN
1.379 CI 1.380 N/CN
I CI
I
N NH2 N NH2
F
118
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042 777
'--,
1 /---3
N
0 N
JL
1.381 F N
1 --- N1H2 1.382 CI N CN
/ i N NH2 -;-....,
- N NH2
N-N
/ N-N
/
F3C
-----S 1 --'
N N
1.383 CI N CN
, -z=.-.,""
I 1.384 CI N NH2
1
I......
/
.....;,, i N NI-12 / N N - NH2
/
N-N --- N
/
HN ri-----
N
01 N
1.383 CI
I `....
1.386 CI N
...7,., I
/
/ i N NH2 N 1\1-5'''' N H2
N-N
/
r------N
0
0
N
1.388
1.387
,
/ N NH2
I / N 1 N NH2
N-N -- RI
/
HN
i
0 N
1.389 N
, ==:, 1.390 N
N NH2 -;-.."..,
01 N NH2
119
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\ \
1.391 F N
1 1.392 N
I I 1
N NH2 N NH2
i HN
N
40 NI CN
1.393 F NICN 1.394 CI
I I
N NH2 / i N NH2
N-N
/
=-.
I N.
N
1410 N I
N
1.395 CI 1 -'.-
1.396
CI 1411) N CN
N I ...;-, I
N NH2 zS
N '' NH2
--S ---% 1
N
NI I
N
411 N Br 01 Nx Br
1.397 CI
I 1.398 CI
I I
/j N NH2 / 1 N NH2
N-N N-N
/ /
NI .-= I
N
1.399
F3C N
-.)...,, 1.400 CI 1411 N
1 '.'z' 0
N NH2 / 1 N N
N-N
/
120
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\
1 \
N I
N
4111 NICN0
1.401 CI
I 1.402
CI 41:1 NxCN
/ i N ill lb
S I
N-N N NH2
/ ---tiN
\ CI
I `.=..
N I
N
1.403 CI SI N,,,..1
I õ)..... 1.404 01 N
1 *-:==.
I
/J N NH2 1.,.,..õ
/ 1 N NH2
NN N- N
/
/
I CI
-µ.. CI
N I\
N
4111 NxCN
1.405 1.406 N
I1
I
/j N NH2
NN N NH2
-
/
CI
NI I
N
1.407 N CN 1.408 CI
N NH2
F
\
I N..-
N FIN 446.
NxCN lip N
1.409 CI 1.410
N NH2 /j N NH2
N -N
F /
121.
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N......
N._
Hni
HNI
N
1.411 CI , -=..., 1.412 N
I, -:-.=
..,;,---, I
N NH2 .....-..,
N NH2
N-N
/
N¨ N¨
Hni HM11
1.413 , Nµ,,,,,,CN 1.414 N
CI
I I
,-...-...õ ,,,,.....,
N NH2 N NH2
F
N¨ N¨
HNI 1 HNI
1.415 CI N
, -z.=-. 1.416 N.........,,,,
I I
,...f,-......,
Nõ NH-) N NH2
,_
F F
H2N
H2N
)i----S
N )T---S
N
1.417 N
CI , ",:- 1.418 N
N NH2 .
N NH2
N -N
/
H2N
)f-S
N c-S
N
1.419 N 1.420
N
N NH2
N F NH2
F
122
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
`-,..
cS I
N N
1.421 411 N 1.422 N
i --....,
CI I
NH2
/j N NH2
N-N
/
".. -`,..
I I
N
1.423 1.424 CI , ==,-,
N c I hi ,,,, I
/ "---"NH2 j NNH2
,c1,1
---N
F
-N.
I
N
1.425 CI N
---"I ==,-, N CN
1 I H
....1t,N ...,...
,,c11 1\l 1.426 NH2
N
0
CI
I I
N N
1.427 N.,..z,.,,./ 1.428 CI N.,..s.:...õ,,-
.._
N NH2 / 1,1 NNH2
--- N
'N.
i N-
N HNI
1
I 1.430
.429 CI
c
/ 11 N NH2
--N
/ rl 1\1NH2
--- N
123
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
`'...
I Pl"--
N HN
1
1.431 1.432
N,,...,., a 14111 N1,J
CI
I
,..--..,
/ i N NH2 /j N NH2
N-N NN
/ /
=
\ HN '`-=
I
N 0
N.,...,......,,-
1.433 CI 1.434
N,,,,.....,,,-....,,
..-,....,
---4-N"'NI N"--s-"NH2 / i N NH2
0 NN
/
., .,
I I
N N
1.435 Ne 1.436 CI Nx,A
1 1
N NH2
c NN NH2
-N
Ns.
I N____
N HNI
Nx..1:7 N
1.437
1 1.438 CI , ...{7
I
cll N NH2 /N N NH
-N -N
',..
I N.._
N HNI
I e, ,r1D
1.439 CI N 1. 0 N
440 ci
I
N NH2 / i N NE-i2
N-N N-N
/ /
_
124
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042 7 7
7
',.. HN
N ...s-,
I
N
0
e
1.441 CI 1.442 N
I I
.-.4.7"N N-..- NH2 / i N NH2
N1- N
0
/
".-... `-..
I I
N N __
1.443 N N.,/
, -s.---. -"- 1.444 CI N N N Hzra.-
I 1
...õ:-..õ
N NH2 / N N1-;'''' NH2
-4
-,.
I N-
N HNI
NrJ
I 1.446 CI Ix
1.445
c1"1 N'N H2 c ri N NH2
--- N --- N
I N-
N H NI N.....
N1,..,õ,,N.,,,,,..,-, 01
1.447 CI
I 1.448 CI
N NH2 / i NIN H2
N-N N-N
/ /
"=-, HN ***"-=
I
N0 0.,.....---....õ
1.449 CI N Ni-/-- 1.450
N,.....,..,..N.,.,..õ.--
NI".-N- NH2 / 1 N NH2
N-N
/
125
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HN
0 0 7 0.)õ,""
"...,...
NI
1.451
I 1.452 1 N,y,.NH
NS/ I
N N NH2 'N1
LJ N NH2
/
\ \
I I
4
N -y0 N
1.453
NI NH NI N H
CI
I 1.454
ci)i N NH2 c rlI N NH2
-- N -- N
N.- \
FIN1
NI
I
1.455 CI
I 1.456 0
N NH
--- I I
/cli NNF12 N NH2
N
NN
/
HN
N
Olki N_ NH I
1.457 CI
I 1.458
I
/, N NH2
CN.L, tc NH2
N-N
/ \
0
0 -",-
1\1
411
N
H
1.460 N.i4D1,. \\
1.459
I
I = No
I
/ 1 N NH2
N-N 0 N NH2
i
126
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/0 42777
I I
0 N .N,,J
1.461 CI N , NN =====.:,'"k=-=
N1H2 c
I , 1.462
N
I "0
/ --'s"..
--KJ
c NN' r's'NH2
--N
N-
HN' I N
N
NIY.,
1.463 CI 0
I 1.464 CI
-- i N:-=-'*"..L0
I
cll N NH2
N"...N.NH2
N
N-N
/
N.
HIN1 I N)
0 N
1.465 Cl 4111/ N..,µ,..õ,.-L.N..
I CI 0
1.466 N.......A,
1 N-
/ i N'''NH2 I ."--"7'N N-4-N"NH2
N-N
/
0
.
HN '"=-=
-^-..
,..,xL\O I
0 N 0....,.4
N-- o
1.467
I 1.468 N NH
, -1:.===,"
N NH2 ...,---,...
N NH2
N-N
/
i
....- N I
"-.... 0S
0 N
I
N
1.469 1.470 N NH
1 .......----
N NH I
CI
I ...,. / 11 NNH2
N NN H2
--- K1
/c --- N
127
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
0
N ¨ C)
NO
H NI oy I
1.471 1.472
c
N NH N NH
CI :-.N..,"
CI , c`:=,-'
I
/ I,IIN"-.....'NH2
--N
,;,-....,
/ i N NH2
/N-N
0
HO
N¨ 1110
I `..... N
HNI da6 0 N
1.473 1.474 0,,J
lip N NH N NH
/ I N-----'s N H2 '-' N NN H2
N-N S-,....õ....-Lo
/
I
0 0 NH "-..
HN "=== 1
N
0
1.475 N,.. NH 1.476 N F
I , I
NH2 X
N NH2
N-N
/
I I
N N
F
N CI F
N
1.477 1.478
I
I ..,.
cl,1 NNH2 / N 1\INH2
--N ¨RI
128
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N- N-..
HN' F
I
Ft F
N
N 0
1.479 CI
I :=:-..--' 1.480 CI 1 N CI
."......"
c / N NNH2
--N
/ I N NH2
N-N
/
N-
"---,
HN1 I
N
1411 1.481 CI N F
I 1.482 CI N CI
i
,.õ
/ i N"----s"NH2
.--"4:NI NNH2
N-N
/ '====.,.õ,--Lo
HN ."-=-= `...
I
0 N
HNA
1.483 i N F
I 1.484 N
/
1 --X-.0
..;.;-.õ I 1 N NH2
N NH,
N-N
/ F
0 CN
-,..
....joN I
--..
I N
HN
N
1.485 HN
1.486 IiIN,-L
1 "-- 0
N ...õ
CI 1 "="=""--.L0
/ NI N"--...F12
N N--"N'N H2
/
--N
/c --N
129
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042 777
0 H
N¨ C )
N 0,..s....õ.õ. N
HN
HN.) I ---... ====...v.,-,
N
HN
1.487 "r-
CI 1 N*kse'-LO 1.488
I ,... CI N
1 0
I
c/ N N "....-' NH2
--. KI
N NH2
N-N
/
r----
0 0 OH
r., a
,,...
N. I
HN,--1
1.489 FIN HN 1.490 N
,,,..,.....
CI 411 N N
.k..,..,õ"Lo CI 1 --== No
1 .-----N N-NH2
/j N NH2
/
HN "`-= HN "Pj a NH I
N
0
1.491 N 1.492 01 NI. ....1._,---
1 T'LO
1
N
/ i N NH2
...:-.....- N NH2
N
/
rz---N
HN N _
HN
I
1.493 CI N , <
I 1.494
CI N N
, z-:.---' =====
---- NNH2 I ....
\ S N NH2
-
130
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
`,..
I r--zN
N HN
411 1.495 CI Nõ.,,......".."
01 Nxi<
I 1.496 CI
I
N ---- N NH2 ."--- N NH2
----S \ S
r-----.N
0
NI
1 1\1...õ;.,.,,,,
N H2
1.497 1\/ NI
c N CI 1.498 ,.*õ,.......,..
CI
/
----1%/4
N NH2
0
F-0
N
S
1.499
N......,
CI
I 1.500
<,-..N., I
/ i N NH2
.,"*"7"'N N-5"-'
NH2
N-N
/ ===:-,.....,,,,L
0
N I
N
N..,,,,,,,,,,,..
1.501 CI
I 1.502 NI,A
"--- N NH2
/
`,..
I F---N
N I-IN
01 NIA NZ:17
1.503 a
I 1.504 Ci
I
N -N NH2 I\I--. N
NH2
7---S \ S
_
131
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N.__ N.
d I
N
1,1:7
1.505 ci N
I 1.506 CI
I N?
c / i;=J N NH2
"N- N NH2
\ 0
r-------N
0 -r-z--N
S
N27.)
1.507 CI
I / 1.508 CI 1 Ne , N NH2
=-="'N Nr NH,
N-N
/
0
fr-0 /7-0
N N
N1C N I.
1.509 CI
I I 1.510 CI
/ i N NH2 / i N NH2
N-N N-N
/ /
d 1
-
1.511 N N., 1-\/41
1 -z.,-," 1.512 CI N NH2
N N--/
I I
,-;.-....,
N NH2 ---- N NH2
\ 0
r-------N N.__
HN O
1.513 CI )N/ N NID
I 1.514 CI I I
õc"N N NH2
132
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
Nr----...N
Oy",,, s N.....
1 N 1.515 ...y,
CI 1.516 CI I I
/ 1 N NH2 / i N NH2
N-N N-N
/ /
pr---N 11-0
0 1.518
N 0,)0
NN --- N N
1.517 CI
I CI
I
'N 0 I I
N NH2
Oµo NN
/
g-0
N N ,y,0
Olt N NH #111 N NH
1.519 CI 1.520 CI
I X I I
io N NH2 NI--- N
NH2
,.--S
9
fr-S N-
N 4:5
i
Nx NH NINH
1.521 CI
I 1.522 CI
I
c1,1 N NH2 cr,i N NH
--N --11
ON /7.---N ra
N 0,)õ,1\ HN
1.523 CI Si N NH
1.524 N NH
I I CI
I
N / 1 N NH2 / NI NH2
I
µS S-N
133
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N¨ HN ..."-
HN1
1 0
H,.........113
1.525 CI , ==.====' '`===
i 1.526
,
N NH2 / 1 N NH2
N¨N
/
N I
N
0
1.527 ci , NT?0 1.528 I N CI , -:..----
"L-,
I
N.'. NH2 N N.'"?..N"NH2
0
li
..--
1.529 N 0..s.,õ,.0
1.530 CI NI 0
cr1 N' NH2
1 I
/ '
---"N
/ 1,:i N NH2
---N
*".s. F----N
I N
N HN
0
N
1.531 CI N 1 --",====0
I 1.532 , ==s---K-
I
NH2 / i N NH2
\ 0
N¨N
/
0 /
N.
HN 0 HN ==-.N
N 0 N
,- ---)
1.533 1 N:L.0 1.534 0
N1,...s..õ....0
a N NH2 N
- N NH2
\ \ S
0
134
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
fr--0
I
N
I
1.535 CI N NH
, -.----..'
I 1.536 N 0
N NH2 CI
I
N
p N NH2
,
(0.,..1
ff---0 0 CN
N 0 6N- LN)
N NH
1.537 ci ,N ...:-.--
NH2
I 1.538
N NH
c N / -5-s'
-N
CI
1
c'N N NH2
--IV
I
0 0..,..,-
.õ a.
N 0
I
1101
N y
1.539 N NH 1.540 HINI\j¨ 0
CI , ==:=-=-=
I 410 N NH
N .....?...s. I N NH2 CI I
N / / N"..".'NH2
/
N-N
1 /
HO
---) HN '-`, I
NH
'---, N
I 0 10
N 0,õy..-1 0 0
1.542 N NH
1.541 N NH , -z..=-=*-
CI , -,'-...,=-'
I , N I
NH2 N NH2
\ S
_.
135
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I 1
N N
N F
1.544 01 N CI
1.543 , ....-..-----
I CI
;:..... N NH2
\ 0 \ S
0
HN HN F F
F
F".....,e
F
N 0
1.545 c 1 N.'-`===)(F 1.546 CI
...,
/ NI EN1*-....µ"NH2
- K1
/ NI 1\1NH2
-- KI
0 ---.. N-
HN (3
N CI 40 1.547 CI i -':-,--'"
I 1.548 a N F
I
N
I N NH2 // N-r...s-NH2
N N-N
/ /
".. HN
1
N 0
1.549 CI N CI
I ...,. 1.550 N F
I....?.,
N N----s.'NH2 / 1 N NH2
N
/
1
HN A -...., .,3.,.. N
1
N
1.551 1.552 HN
N
I N
-7-.... CI , ''="=--""LO
/ 1 N NH2 I
s-N / N NNE-12
136
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
0
CN HN ( )
NI d46,
HN /7=---N N
iv WI
HN
N)
1.553 i OT'L 1.554
I. N
N I CI 1 :CLO
N NH2 I
S
/'NN NH2
---N
H
N 0 0
N 0
I
N
HN) N-
LJ
1.555 1.556 H 14
01 N
CI 1 'X'LO
I , HN
/ 1 CI 4110 Nõ-L0
N NH2
1
N N
/
N NH2
1
N
/
1-10......,
1. ) HN ."=-= 1
NH
N
141111
HN
..-I 0 HN
1.557 1.558 HN N
1 ---'-'"--LO
CI i N'=rLO I
I , ...?..,.,
N, N NH2
es' ri N NH2 ,---S
=-,c,õ,. ,-.13
".. ..,
I I
N N
411 N OH 01 N OH
1.559 CI
I 1.560 CI
NI,. 1
N NH2
N ----- N NH2
,.--S ,--S
,
137
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I
N N
SI N
1.561 CI N OH
I 1.562 CI I YOH
....,-1,,
N'N"-- N NH2 N N NH2
---S ,--- 0
I
-\
N HN
001 N OH Olt N OH
1.563 Cl
I 1.564 CI 1 X
N'N--- N NH2 N "===== N NH2
y--0
,--S
1
N._.... N.
Hnf Hisi
N OH 01 N OH
1.565 CI
I 1.566 CI
N,N-.. N NH2
I N_
N
1\r o NH _
--S
"--S
I
N...._ rt._
HN/ H14
4111 N OH 411 N OH
1.567 CI
1 1.568 CI N1 I
N -N NI-12 N'--- N NH2
---0
)--O
N...... I
N.¨.
HN1 Hni
1110 N OH
1.569 CI N OH
1 1.570 CI , -:.,=-="
I
/ i 1µ1"¨s"NH2 / N NNH2
N¨N ---
/
---------------------------------------------- I ............................
138
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
r-S ir-S
N N
y
1.371 CI N 0H
1 1.572 CI
-.
N ---- 1µ1.'INH2 Ns-. 1N.NH2
7.--S ,---S
/r-S frs
NIN
N OH N 1.573 CI
N 1 I 1.574 CI
1 OH
..*.,..
N'---- Nr NH2
N ---- N Vil
fl-S r-S
NIN
I Nx0H
1.575 C N OH
I
1
1 1.576 CI
N'N.--. N NH2 / i N NH2
,--0 N-N
/
NrS
N
N/OH i N/
1.577 CI 1.578 CI OH
i
NH2 N -=-= N'..- NH2
--N
c
7.--S
/7-0 fj-0
N N
ROH N,x01-1
1.579 CI
1 ,,,õ,1, 1.580 CI
N,N,.. I N NH2
N
N NH
,--S
,..-S
139
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/04 2 777
NIN
N OH N OH
1.581 CI
I -:.,--- 1.582
N '--- N N H
N'N.-- N 2 NH2
y-- 0
N
N
N OH
1.583 CI
1 --`;µ,"
1.584 N OH
CI 1
...;.=.....,
N NH2 I
N - N / (1 N
NH2
/ ---- N
N..
i
NI
N
1.585 CI N,CN
1 "`" 0
1.586 CI N OH
1 ' 0
N N
c N N1:.?N N " \\
H jJ
.
fr=S /7-- S
N N
N CN N CN
1.587 CI :".Y.- 0 1.588 CI , ,":.----
I õ.),.. , I
cil N FNIJ b
c r'l IN FNi
N-
1114
0 0
1.589 CI 1N 1:1(NH2 1.590
I
p 1Nr. iii
N ..,N
--"N
. H .
140
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N-..... ''µ.,
HN1 426 1
N
1.591 CI itill N CN
1.592 N CN
I CI
1101 NNF X3 N
`',.. N-
i
N H14 alb,
1.393 CI N,CN
1 ' p
1.594 CI IIIP N CN
1 '''.====" 0
A
NH 401 N¨Iii NH
/
A
1.595 CI N CN
X 1.596 CI NI CN
I I
N NH
H
A
N- P---
HI4 HN
0 NXCN 41111 N CN
1.597 CI
I 1.598 CI 1 I
/j N NH / i N NH
N-I\I
,6 ,
N-14
e"...
0
..... ...
, I
N N
SI CI NXC N 411 NXC N
CI
1.599 I 1.600 I
/ 14
i N NH / i N NH
N-N -1=1
/
a i e.,14
N
0 \
141
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N-
HN I
N
I* N CN
CI N CN
1.601 1 I 1.602 CI 1 I 0
/1 N NH 6,4
N-N N N *
/
N
\ F
N.
r-S
HIsi N
N CN
N
CI , I I 0
N N NH
1.604 CI
1.603 1 -1,
N
H a
F
"=..
110/11
Olt N CN
CI N
1.605 I I 1.606 HN
N NH N
N-N I
/ r/N N NH2
N
N
F F
N... Oil IP
I N.-
N Htsi
1.607
Si N HN 1.608
N HN
CI 1 srLO
I I
/f N NH2 c1,4 N NH2
N-N --N
/
142
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
F F
IP 1101
rS r=O
N
1.609 HN 1.610 HN
N N
I
===1-*L0
I
/ i N NH2 / 1 N NH2
N-N NN
/ /
F
F
0
11111
I N
N
1.611 NL
HN 1.612
1.1 CI
CI 1 1-O I
I
N =--... N NH2
--11
'-S
F F
NN,
I
01 I
1110
N N
1.613 411 N 0 1.614 el N 0
CI I I CI
1
N NH2 N .N'' NH2
N -N
-.--S
/
F F
1,1--
1101 Nr's
HN
Oil
1.615 1410 I N 0 1.616 CI 1 NI.,...,.,,0
C
/ 1 N NH2 / i N NH2
N - N N-N
/ /
143
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
F
N
Si I
F
1.617 I N=*" 0 1.618 CI N NH
C i -z..,=-
N N NH2
/ 1 N N H2 /
-- N
N -N
/
0 F F
`====, N..
I I
4111
N N
N NH SI 1.619 CI , =-=:-/-
I 1.620 CI N NH
I / 1 N NH2 N '--- N
NH2
N-N --S
/
F F
NI_
TS
HNI 446
0 410
W N NH
I N NH
1.622 CI , ==,----
1.621 CI
/ 1 N NH2 N NH2
N¨N N¨N
/
/
0 F
ir--0 1
N N
H H
N N N
1.623 CI
N
I
INH 1.624 1 CI =======:--- -,\( IS
/ 1 N NH2 / N N NH2
N-N -- K1
/
`N.
".. I
I
N
N
= N 11 N N H H
NN\c" N 0
1.625 ci I X 1) 0 1.626 CI
I ==zsr-
/j N NH2
N "--- N NH2
N-N
y-- s
/
144
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N-.
NrS
HI4
H H H H
N N N NNeN
1.627 a
1
1:-- 'C'o * 1.628 CI
I ..j., \\O 1401
/ 1 N NH2 / i N NH2
N-N
/ NN
/
,.. 'N.
NI 1\1
H
1 N N.,....õ,-., s,"...,.. 14
c
1.629 a --.1 N. 1.630 CI 1 N I I ..........,--..N..-
---...s. li N NI-17
N *---- N NH2
N..... fr-S
FIN N
H H
1.631 X a
I N N............... ,......,... y 1.632 CI 1 N
N. ....,
y N -
/ / N NH2 'N''' i N A L-,
/ 1s11-12
N-14
/ NN
/
Nir-0
Nil
H
1.633 1.634
1 NINõ,...,..,,,N,,,,.., N OH
a CI I
NH2 IN` I
N-I'l
c ti N El 401
i......N
--.
1
14
N
4111/ NOH 14111 N OH
1.635 CI
I . 1.636 CI I I
/ i NI 11 1101 N ---= N N
N-N
/ ,--S H
145
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N..._
N
1114 146
RP 1.637 N,y.,01-.1 Nx0I-i
0
I j...., 1.638 CI
I
/ / N / ill 110 i N El
411
N-N N-N
,
/
...0 ..
N I
N
N 1.639 CI
OH
I
1 1.640
CI NxCN
f'1
'N N NH2
/
N.._
Pi-
HNi erght, HN
WI NxON 1. N Br
1.641 CI
I 1.642 CI
HL
/, N NH2 / i N NH2
N-N N-N
/ /
N.._ N.._
FINI Hisl 446
1.643 CI 410 N CN
CI
1.644 lip N Br
I I I
Si N''''.."NH2 101 N NH2
N
lel 1 NxCN 14111
1.645 CI 1.646 CI NIBr
I
1110 N NH2 1101 N NH2
146
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
fl ¨S
NI Nii¨S
N CN N I
1.647 CI -:.----
1.648 CI , --%,"" Br
/ i N NH2 ,;:s.,.
/ 1 N NH2
N¨N N¨N
/ /
HN 4-0
N
N
1.649 CI , .=-=
I 1.650 CI N Br
, -:=.----
..-,...., I
N NH2 ..,1-,-....
N NH2
N¨N
/
fr 0
N I
N
N CN 1.651 CI , -',..-----
I 1.652 CI N CN
N NH2 ;.-
...,
N NH2
F
4---0 fr-0
NiN
N I Br N CN
I
1.653 CI 1.654 a , :.----
I
/ i N NH2 N
/ 1 NH2
N¨N N¨N
/ /
¨
HN s=-,
I
N CN
1.655 CI
I ===;---."
1.6 N 56 N CN
CI, =":-...,"
/ 1 N NH2 I
N¨N \I r\ 1 N NH2
/
147
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
HN .."=== I -N.'
N
0
1.657 N OH
1.658 N
I I
, .7......
N NH2 / / N NH2
N"N
/
',..
I
N N.,...,,.7--.)
1.659 1 NNH2 OH 1.660 ,,,.õ.. hN N
c .,...
/ Nlf:rs'.
---
rr-N. NH2
K1
N"N
/
- -N-.
I
HN N
CN N
1.661 CI N , =-:-.=-""
I 1.662 CI 1 1
,,,,...
/ 1 N N H2 / N N- NH2
N -- N
/
HN "==== I .N.µ
N
0
1.663 CI N CN
LJL
, ==,...--'
I 1.664 N CN
, ===":,--"
I
, ...7....,
N NH2 / i N NH2
N 'N
/
"--,
I rr'
N N.,,...)7,...1
1.665 1 NOH 1.666 .....,,N N.:L.,.
N NH2 411 NJNH2
148
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I
HN- N
1.667 CI N rN 1.668 CI N CN
i ==:===-""
, ===:-,'
I
...:;...õ
N NH2 c NII N".."..'
NH2
HN
I
0
N OH N OH
1.669 CI
1 -z......-" 1.670 , -,..s.--
N NH2 N NH2
N-N N-N
/ /
-'-,
I r%
N NI
.........,..NH2 N NI,...
1.671 1.672
IN ).......
c N I'N H2
--- <PZ."....s. N NH2
N
N-N
/
HN I
N
N CN N
OH
1.674 1.673 CI , .===-=-=
I CI
...:?.... ,
N / Ni N''''.." NH2
NH2
N`,..
-^-s.
I I
NI .....-'
1.675 F N OH
, ,..s...--=-'
I 1.676 LJL.N OH
-....--=-''
N NH2
.....?....õ
N NH2
149
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\
I
N
0 N,......õ7-..1
N
1.677 1 1. I678 ,..,---..-N N
c..,....s.,
I
,..,
/ tI/J NNH2
-- N
1110 NNH2
I
\
I \
N I
N
N OH
1.679 CI
1 ,=:--- 1.680 F N OH
, --":=,"
/ N NNH2 j
N NH
FI ...,--....,
2
1
\ CI
N N ,....-'
1.681 lJ.NOH
, -:.===-' 1.682 N
, =:..-
I I
1.,,,, ...7...,
N NH / i N NH2
F N -1\1
/
(CI
N......./.....1 N ,..-
1.683 ...õ...N N.,s.s.,..õCN 1.684 IJL.NCN
40 INI NH2 N NH2
N-N
/
1
(MCI
N.õ ....;......"-' -NI N
--,,,,.N..õ,N,,,_,CN
1.685
I 1.686 IJJN OH
i ==:=-="
er N..
s' NH2 ..;.r.,
N NH2
N-N N-N
/ /
-
150
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I
N N
1.687 IILN" NH,
, -:.., 2 1.688
I CI N NH 2
N NH2 ...:;,,
N NH2
`,..
I \
N I
N
N,s,.....õ.NH2
1.689 CI
1 1.690 IJLN" CN
-
...,--s, I
/ i N NH2 ......-......
N NH2
N-N
/ F
\
I \
N I
N
, NI,,....õ...CN
1.691 I 1.692 N CN
, -;.-----
N NH2 I
....;.-.õ
0N NH2
F CI
\ \
I I
N N
1.693 N CN
, 1.694 N CN
, -:---
II -;:.,=,,
. N NH2
N NH2
CI
CI
\ \
IIL,
I I
N N
N CN N CN
1.695
1.696
I --;---
.;.1,...õ ,..;....,
N NH2 N NH2
OMe
_
151
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\
i \
N I
N
N CN
1.697 I I 1.698 NXCN
i NH2 I
N NH2
F
F F F
\
I \
N I
N
N CN
1.699 I
....1 1.700 N CN
F F I
1
N NH2
N NH2
F F
\ =.,
I I
N N
NXCN
1.701 IL11.702 LJLN CN
I
F I CI ,-,....,
N NH2
N NH2
CI
CI
\ \
1 I
N N
N CN NXCN
1.703 I
1 1.704
F
N NH2 I N NH2
F
CI CI
\ \
I I
N N
1.705 N CN 1.706 LJLN CN
I
1: 1
1
N NH2 N NH2
CI F F CI
152
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\ \
I I
N N
N CN NXCN
1.707 I ....-I 1.708
I
)IXN NH2 N Nti2
CI
F F
P- P-
HN
HN
I
N CN CI NxCN
1. 709 CI 1. 710
I ...X N NH2
N NH2
F
F
N.....
11¨
HI4 HN
N CN NXCN
1.711 CI
1 1.712
I CI
I
N NH2 N NH2
CI CI
11--
11-
I-1N HN
411 N CN
N CN
CI
1.713 I X 1.714 CI
0 N NH2 I NX NH2
CI
Pi¨ N......
HN HNi
NxCN CN
CI CI
1.715 I 1. 716
I
N NH2 N NH2
F
OMe F
153
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N¨ Pi¨
HKI HN
N CN CI NxNH2CN
1.717 CI 1.718
1
1 X F
N NH2
F F N
F
N¨ N¨
H14 His
CN NXCN
1.719 CI IN1 1.720 CI
N NH2 N NH2
F CI
N¨
Pi¨
HNC HN
N CN )N CN
CI CI
1. 721 i X 1.722
CI
N Ni-i2 N NH2
1LJF i
Cl Cl
Pi¨
HN . M
PI¨
F
N CN
NXCN
1.723 i N----XNH 1
CI724 CI
I
2
N NH2
Cl CI F
P- P-
HN
HN
NXCN CI N CN
,
1.725 CI
i 1.726
I
N NH2
NXNH2
F CI
F
154
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N__.
H NI `,.
I
N
, N:z........,..CN
CI
1.727 I 1.728 = N CN
<,=-...,
N NH2 1
N .N' NH2
CI
t-O
F
N. N.
I i
N N
1.729 41 N CN 1.730 Oil N,....,.CN
I 1
N "-- N NH2 ...7..,
N:s- N NH2
-----S
I I
N N
1.731 N CN
1.732
I 1
.
--- N N1.4: ....,.....,
N. N NH2
\
\
N-C) N-S
N. N.
I I
N N
1.733 N CN
, ===`.-..e" 1.734 i Nzõ.../..-CN
I I
--;.-..., ....:-..,
*-N- N NH2 '--.. N NH2
N -NH N-11 \
I I
N N
1.735 41 i N,.,,,,,,-CN 1.736 SI N CN
,
N,... N-,----, N H2 1
N N NH2
155
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\ \
N N
1.737 411) N CN 1.738 411 N CN
. I
N..... I1 ei.. I N--- NH2 N NH2
\
I \
N, I
N
NxCN
1.739
I 1.740 01 N CN
N NH2 I I
N¨N N'! N NH2
i HN¨N
\.
I
1.741 N NxCN 1.742 N NICN
I I .,...
11,47y N N H2 N/\%:...:. j....'N N
NH2
N
\ `...
I I
N N
1.743 101 N CN 1.744 411 NCN
N
is,r,--. N NH2
I I
N:'.- NIX N NH2
N-NH N-NN
I I
N N
1.745 1411/ N C 1.746 4111 N CN
1 'IN I I
---- N N "--- N NH2
\ NH H2 \
N¨NH
156
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/04 2 7 7 7
`,.
i I -.....
N N
1.747 I. NCN 1.748 SI I F
N ON
I
N,'..- N NH2 /N-- N
NH2
\\
µN-NH N-NH
N- N_
FIN( Firri
1.749 410 a N CN 1.750 SO N CN
I CI
I
N NI/NH2 N ---- N 'NH2
t--0 ---S
N- N-
HN HN
1.751 Si C N CN 1.752 CI 40 N CN
i
I I
N "--- NNH2 *--"` N NH2
N-
N_ N-.
HNI Ht\i
1.753 a 40 N CN
I I 1.754
CI 01 N CN
---- N NH2 I I
\ ''''- N NH2
N-S \
N-NH
N-
N-
HN
HN
1.755 Ci 40 N CN
1.756 NICN
I CI
e=-.. I N NH2
---- N'ress--NH2
\
N-N\ N-0
157
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N¨ N¨
HN HN
1.757 CI N CN
, Cs..--," 1.758 N CN
,
e... N-;.----'N H2 CI
I
7.'N X
'-= N NH2
1\
N'S N-NH
N¨ N_
HN HN
1.759 Ci 01 N CN
1.760 CI 1. N CN
1 1
Nõ... NN H2 N/ 1 N NH2
N-N\ 'N-N
/
N¨ N¨
Hhi HN
1.761 Ci Si N CN 1.762 N CN
1 CI , ====-..."-
I
N/ I N N1-12 eliN NNH2
14N-N 1\1:---..
......
N¨ N...._
Hr\i FIN
1.763 i N,....s.,,CN 1.764 N CN
Ci
I
,N,... I NN H2
..'"'N/ N NH2
N\:.õ....j N,
sN-NH
=
N¨
N¨
HN
HN
1.765 c, 410 N CN
1.766 N CN
1 CI , -:.=-="
NN,.... N NH2
µN-N\
\ NH
158
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N¨ N_
1-114 HNI
1.767 CI 010 N CN 1.768 N CN
I CI , ....-=:--""
I
--,. N"----'NH2 .4.:-..s.
\ N, '--- N N H2
N¨NH µN¨NH
N¨ NN
HN' I
N
1.769 F 01 N CN 1.770 N CN
N,.... N NH2 I
N¨NH /1 N NH2
HN
I I
N N
1.771 N CN
, =%%-..,"" 1.772 N CN
I I
,,, N NH2 1.---.,
/ N / ; NH2
I i
HN N
/
--,.. `-..
I I
NJ N
N CN N CN
1.773 1 -:.----
I 1.774
.....;. I
/ N,. NH2
I / 1 N NH2
N NN
`N. `..
I I
N N N CN
N CN
1.775 , ==,-,--
I 1.776 , *-,---
N/ I N NH2
N/ I N ....,?...,
NH2
sN1 'NI
/ /
159
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I
N N
N CN N CN
1.777 ,
I 1.778
...,-;.õ. I
N1 I N N H2
N/ i N ....7...õ,
NH2
1\1 NN
I
N N
1.779 N CN
1.780 N CN
N/
..1,,,,, I N NH2
N/
N NH2
FIN HµN-N
`,... `,..
I I
N N
1.781 N CN 1.782 N CN
, --;====="-
...;.--,..
'-- N NH.2 ''"=== N NH2
\ 0 \ 0
N., \
I I
N N
1.783 N CN 1.784 N.' CN
, -.*:::=-="
I I
-;.---, ..../.;-...,
'"-- N NH2 .."-` N NH2
\ 0 \ 0
=
-`... ..\
I I
N N
1.785 N CN 1.786 N CN
, ,:-.-'"
,=-.....õ
"-- N NH
- N
-. ....;-...,
.,.. ---- NH2
\ S \ S
- -
160
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
...,
.õ.. i
i N
N
1.787TJL N CN 1.788 N CN
, ......,'"
1 1
I
-;......,
---. N V H ---- N NH2
\ S \ S
.,
.., 1
i
N N
1.789 =N CN 1.790 14111
NxCN
ta i
N---1 N NH2
-..../"".- N''...-"NH2
.,
i
N N
1.791. 41111 N CN 1.792 410 N CN
N I I
N... LL NNH2
t N NH2
--S
1---S
.., .,.
i 1
N N
1.793 1101) NCN 1.794 N C
Ix 1 XN
----. N NH2 "==== N N
I I
N N
1.795 1411 N CN 1.796 40 NXCN
........e.--. N NH2
Ix 1
"====== N NH2
\
N- N-S
161.
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
`.. `..
i 1
N N
1.797 i N...s.,..,...CN 1.798 41 N ON
I
N NH2 N.... 1 NNH2
,..*-...,
----
\ , ----
N--. N'S
N____ N......
HNs HN
1.799 CI N CN
,-:..=-,"
I 1.800
.,-...õ, I
;-,..-..
N NH2
i / i N.s. NH2
HN HN
N.__ N___.
HN HNI
NTCN N,y..-CN
1.801 CI
1 1.802 ci
/ i N NH2
N H2
N N
/ /
N._..
HN HN
411 1.803 CI N CN 1.804 N,T,CN
I ci
I
/ 1 N NNH2
N/ I ....1.,
NH2
N-N ,
N
/ /
N.__ N¨
H14 HN1
N CN N..........,.CN
1.805 ci
I -,-;--- 1.806
I
a
......,--.,
/ N NH2 .
N / N/ I N NH2
N 'N
162
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
P---
P----
HN
HN
51.807 CI
I X 1.808
CI 14111 N CN
N'! N NH2 I I
N-N Ns/ I N NH2
/ HN
P--- N.-.
HN HN 446
1.809 c a
, illti N CN 1.810 tio N CN
I I X
I
N'! N NH2 - N NH2
HN-N \ 0
P.- P-
HN HN
1.811 CI 14111 NXC N 1.812 N CN
I CI I 'I
,..
N NH2 "-.. N NH2
\ 0 \ 0
N
N¨
H14 .....
1-114 aah
N CN
IP I I
1.813 Ci 1.814 N CN
CI I I
---.. N NH2
\ 0 ..... N NH2
\ S
P--- P-
HN HN
1.815 0 Si NCN 1.816 NxCN
Ix CI
I
*".-- N NH2 --.. N NH2
\ S \ S
163
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N____
FIN N.__
HN
N
1.817 a 1 CN 1.818
Ci 410 N CN
---- N NH2 1 I
\ S N--- N NH2
%--0
N.__
HN NI__
HN
410 N CN
1.819 C 1.820 14111 N CN
N I I CI
I
N NH2
1--0 N'-- NNH2
---tS
N_
N-
FIN
el I
1.821 CI N CN 1.822 HN'
CI N CN
I
Nõ. N NH, I
-5---S 4 '-- N NH2
\
N-C)
N- N.
HNI hir\I
1.823 CI N CN
,=::-.."."
I 1.824
CI 14111 N CN
---iNõ. N.-----.NH2
\ õ
N`u N-C)
N- N_
HNFir\I
1.825 G N CN
, :=-.,"
I 1.826
CI N CN
,..13-..õ
`--. N NH2 "".= N NH2
\ , \ N ,
r\l' ---
164
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N¨
Hr4 1 '
N
1.827 G 01 N CN 1.828 101 N CN
I I
eµ- N NH2
NN,... N.,--
...NH2
N-S ..---S
I
N HNI
1.829 Si N: CI
CN 1.830 10 N CN
, -.....,"
I
...;;-õs I
N'N"--. N NH2 N
t-0 N' ." N NH2
t-S
HN' I
N
1.831 10 N CN 1.832 N CN
ICI , -::.-====="
N=1\1.--. N NH2 I
õ...N
IC- N NH2
--0 N ,./
`-,..
I `N.
I
1.833 N
1.834 N411 N CN
NH ."..,---,..
': N
2 ."- N NH2
N
.`-.. `..
I I
N N
1.835 N CN
, 4z,----- 1.83
IN NH2 6 N CN
CI , ==:,---
N,N's- N,N"-s. N NH2
I
---' I
,=-=--
165
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I
NI
Olt N CN IIIIIII N
CN
1.837 I I 1.838 C
N I
(14µ.. N NH2
I I
fr NN... N NH2
IN ..,
N
I I
N N
1.839 1411 N CN
1 1.840 NXCN
14IN. N NH2 N I
.'N.11...". N.'. NH2
I I
N N
1.841 1411) N CN
1.842 NXCN
I *): I
1 N"- N NH2 1 *".. N NH2
IxN NN
NN-'
N
N-..
I
1.843 SI N CN 1.844 : N C
r Ni==== N NH2
N H2
I I
..,,,-..,........, N
*N... I
I N
N
1.843 =N CN 1.846
LJç,N CN
1
I I
N,N`s= NI NH2
N.- N,. N NH2
166
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
., .õ.
1.847
N 411 N
NCN
1.848 N1 CN
IX I
1 NN N..;K1 ``... N NH2 1 ""===
N'... NH2
I I -
i 1
N N
411 N CN . 01 N, CN
1850
`s..-i'N-... I N'" NH2
1 . 849 I I
N
= N NH2
I
N N
"..
I 1.851 I. N CN
N
1 1.852 Si N CN
N
I ..:C
I
N .".== N NH2
N ."-- N NH2
õõ,11,:;.-..N
P--- P--
HN HN lath
SI N CN V N CN
1.854 CI
1.853 CI I NX I ,
it === N NH2 '',--
NINH2
I
N , N.N""
14- P-
HN HN
4101:1 N CN Sit N CN
1.856
CI
1.855 Ci I I I I
irN-s- N NH2 ,N
N -== N NH2
I
Q....4,,, N
167
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N- N_
HNI HN1
010 N CN
1.857 1.858 OOP N CN
1 CI
I
N-/-*.NNH2 N
( "=-= N NH2
.:..N
N N
N- N-
HNI HNI
1.859 el N CN I
1.860 el N CN
I Cl 1
N ""=== '...
N"."..s-NH-; --...,,N
N NH2
1!õ..,,,;::õN II
N-
N N-
H14 N CN Fin(
1.861 CI
,-:....--'
1 1.862 CI 1
N,y,CN
....131...
N NH2 µ..`" N NH2
1
N ----' N,
N
N-
Fir.1 HIV
N CN
1.863 CI , ...---
I 1.864
Cl III N CN
NH2 N
f ''= N"--N.NH2
N, ?"
N- N-
HIN/ HN'
1.865 CI 1411 N CN 1.866 N ON
N I CI
1 . '-'= N . NH2 -N .7...,,
..,..,-.,......;,I N N '-'= N NH2
I
..'
168
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HN HN1
01 N CN
1.867 CI I I 1.868 1.1 N CN
N..Nss, N NH2
I i NINH2
../ I
re
P- P-
H N HN
4111 1.869 CI CI
NXCN 4111 N CN
I 1.870 I I
,i N
1 "-=.. N NH2 i N. N NH2
1 N1,N fµr
L. P-
HN FIN
I. 1.871 CI CI
N CN 1411 N CN
I I 1.872
I I
."1N,....==== N NH2
N **'= N NH2
N
N. .,
FIN i
N
III NXCN
1.873 CI
I 1.874 N NC
N "=== N NH2
N ---. N
NH2
S
.., ,.,
I I
N N
NXCN N CN
1.875
I 1.876
X
I
/ i N NH2 / li N NH2
169
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
".. '`,..
I I
N I. N
N CN
N ON
1.877 1.878
I
N--.. N NH2 N---. 1 N'NH2
= S * N H
N_
I
N HI4
10 N CN N ,y,CN
1.879 , -:,--'
I 1.880 CI
...,..=,--.,
N"-- N NH2 .."*.= N NH2
41 0 S
N- N-
1-114 HIV
N CN N CN
1.881 CI
1 --`:-/ 1.882
N NH2 / N N
NH2
/NN 4i-rj
N- N.
HIN' HIN:
4110 N CN 411 N CN
1.883 CI
1 1.884 CI 1 I
N--- N NH2 N"- N
NH2
=rµill
= S
HNI I
N
4111 1.885 ci N CN 1.886 CI
1\1.........õ,...
I
N-.. N NH2 1 ,;(.,-.....
. 0 / I N
NH2
N-N
/
170
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N, N,
I I
N N
1.887 CI N.s.,,...,,,..,-.õ N
1.888 CI
I I
...p.õ
N'"N"NH2
N-N N-N
/ /
I I
N N
N,.....sõ,"N,-.
1.889 CI 1.890 Cl
1 I
-,-......, ...;......,
N NH2 N NH2
N-N N-N
/ /
I I
N N
1.891 CI N.,,.....,..-
1.892 Cl Ns.Issõ..,--N,
1 I
N NH2 N NH2
N-N N-N
/ /
I I
N N
N.,..õ...,..N.õ:,..,./...-'
1.893 CI 1.894 Cl
1 1
N NH2
N-N N-N
/ /
N, N,
I I
N N
N N
1.895 CI , 7."-
I 1.896 Cl 1 N..N----"--"--
1 ...-õ?..õ
ey'' les-NH2 N NH2
N-N N-N
/ /
171.
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N,... -s.
I I
N N
N 4110
1.897 CI 1 --..."--"-""'s,,, 1.898 CI N
I......., I
/ / N NH2 // N NH2
N¨N N¨N
/ /
"=-. N,
NI ii
N,
I .
1.899 CI , N .... 1.900 N
CI
.-
N NH2 / i I Ne NH2
N ¨N
/ N¨N
/
I I
N N
NILl N1,.., p
1.901 CI 1.902 CI
I I
N NH2
N¨N N¨N
/ /
I I
N N /
N
1.903 CI 1 N.904 CI
I
..).,,,
N NH2 / i N NH2
N¨N N¨N
/ /
-.... N,
I I
N N
N Ni.. N NJ
I i
1.905 CI 1.906 CI -:..--""
..,.
W.'s.' NH2 / i N NH2
N ¨ N N¨N
/ /
172
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
INI 1
N
010 Nx 0 4111 INxN.,....,.,.
I=
1.907 CI
1.908 CI
/ i N NH2 / i N NH2
N-N N-N
/ /
\ \
N N
lit NxCI
1.909 CI
Nx
I 1.910 CI
I
/j N NF
H2 / i N NH2
N-N N-N
/ /
\ \
i I
N N
F
F
IIIIII N Or 14110 NI-.1(F
1.911 CI
I 1.912 CI
I I
N NH2 / 1 N NH2
N-N N-N
/ /
---, *.
NI F .,FF , I
F N
SI x 411
1.913 CI
N 0
I 1.914 CI
N x0H
I
/ 1 N NH2 / 1 N NH2
N-N N-N
/ /
'. '..
I I
N N
0
1.915 CI I 1 1.916 CI SI IXN
N
/ 1 N NH2 /j N NH2
N-N N-N
/ /
173
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\ \
I 1
N
Y Y N 0
1.917 N
N 0 CI
I -:..., 1. 918 C I , =:--""
/
..,.., I / N NH2 N
...!",,.....
N H2
N-N N-N
/ /
\
1
Y
...
1
N N
N 0
1.919 CI
N?
I ===`....-- 1.920
..
N = NH2 / i N NH2
N-N N-N
/ /
\ I
1
P NI N._ _.-0
N
0
1.921 CI N
I -...."-," 1.922
C I N 0
I
N N H2
N NH2
N-N
/ N-N
/
1 \
N
? N
Y
N 0
1.923 CI N 0 , ====,===='
I 1.924 C I , ==-**;--""
N = NH2 / N = NH2
i
N-N N-N
/ /
1
N \
\ ...-= --... I
lel
i
N
Y N
1.925 N 0 1.926 C I N 0
...7õ
N NH2 / 1 N = NH2
N-N N-N
/ /
174
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/04 2 7 7
7
N/
N
y ,
N
1.927 CI
N 0
I =-=::.=," 1.928 CI N O
.2,, I
/ / N NH2
N NH2
N-N N-N
/
/
I
.--. --,
1 I
N
rA
1.929 CI N......,,,õ 0 ) N 0
I 1.930 CI , =====-,,'"
.
N NH2 / i N NH2
N - N N - N
/ /
`-..
I
(Ci N
N
N 0 153
1.931 I CI , =:==..-,--
1.932 N 0
/I i N NH2 .31.,-..õ
N - N N H2
/ N - N
/
,
I
I
(CI
N
N 0 N 0
1.933 CI
I -.:," 1.934 CI , .......".
...,:-..,
N NH2 / I N NH2
N - N N - N
/ /
. .
4,N)
`,. N '`..
I I
N
? N
r)
1.935 N 0 1.936 N 0
I CI , --;.=--'.
...,f,-..., I
/ 1 N NH2 .
N NH2
N - N
/ N - N
/
175
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
F F
d4--F
'===,
p-
NI ... N
141111 N 0 I
N
1.937 CI
1 1.938
I 1411 N 0
/ 1 N NH2 CI I X
NN
/ /j N NH2
NN
/
4
N F
''... `N.
I I 11I
N ::. 1
N
01111 N0 III
1.939 Cl 1.940 CI N 0
....1,
X
/ 1 N NH2 /j N NH2
NN N-N
/ /
H
CN
I I i
N daht N
r,,=== '
Lei 1.941 Cl N 0
1 1.942 N 0
Cl 410
I
/ 1 N NH2 I I
N-N /j N NH2
/ NN
/
I I
N N
14111 010
1.943 CI I N11 N S
1.944 CI I I
/ 1 N NH2 N NH2
N-N N-N
/ /
176
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I
Y
N S
N S
1.945 N Y N
CI
I 1.946 CI , -=...=-=-=""
N NH2 / / N NH2
N-N N-N
/ /
I
.9' 9
.....
,
N N
N S N S
1.947 CI
1 " 1.948 CI
,..;,....õ I
..;.---.,..
/ N NH2 N NH2
/
N-N N-N
/ /
\ \ 0
I
N
N S
1.949 1 CI -...`,.. '-' 1.950
CI N S
-;-,..... I
/ / N, NH2 r.....,
N-N
/ N-N
/
I \
\
t?
N
Y I
N
N S N/ S
1.951 CI , -:.----"
I 1.952 CI
/ / N NH2
N-N N-N
/ /
I
,.... 1
Y IliP
N
1.953 N S 1. 9 NI54 CI N S
, ---Z--"'"
CI
N--z..1 NH2 / I N NH2
N-N N-N
/ /
177
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I I -=-.. 9/
NI
y N
1.955
N S CI
I =-=::,="" 1.956 CI N S
.<,......., I
N NH2 ./.......,
N NH2
N-N N-N
/
/
I
--. --,
i 1
NN ..---A
N 1.958
...,..õS ) N, .r
1.957 CI
1 CI , -.:.,-----
õ
N NH2 N NH2
N-N N'N
/ /
s-,..
I
N
N S
1.959 I CI =::=-=,..," 1.960 N S
/I 1 N NH2 ....1,....,
N / i N NH2
-N
/ N -N
/
,
N-. ...
I
I
(13
N
N
N S 1.961 CI , -::-.,-".
I 1.962 CI , -zz.-,-' N S
N NH2 / ! N NH2
N-N N-N
/ /
. .
N)
\ N --s.
I I
N
ri N
rj
1.963 N S 1.964 N S
I CI , -::=,-."
..-7..., I
N NH2 ..;:"...,
N NH2
NI --- N
/ N-N
/
178
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
VF
F
I dN \ N
ii. N S I
N
1.965 CI
1 1.966
I 1410 N S
N NH2 CI I I
N-N
/ /j N NH2
NN
/
N F
\ \
I
NI
N ........,. 1
Of
lei N....,,s 0 Nx S
1.967 CI 1.968 CI
i 1
/ 1 N NH2 /j N NH2
NN N-N
/ /
H
\ op CN N N
4.; '......-- -.,
I I I
N aiht N
rks.,..
1.969 a iv N.S
1.970 0 N S
i ,./.L. ci I I
N NH2
NN / 1 N NH2
/ NN
/
\ `=,
I I
N N
N NH2 0 N 11111
1.971 CI IX 1.972 CI I ):
/ 1 N NH2 / i N NH2
N-N N-*N
/ /
179
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/04 2 7 7
7
N. N.
1 I
N 1 r
1.973 CI , -='-:.=-=," N N s-s
I 1.974 N
N N H CI , '
.
N NH2
N - N N - N
/ /
N. "..
I I
N
N N H
1.975 Cl , NNH '-s-s-"-
I 1.976 CI , :..=-=."
N NH2 N NH2
N - N N - N
/ /
N..
I N.
I
N
7 N N H
1.977 N
N N H CI , -k=-="
I 1.978 CI
..;.,..., I
....;.-.õ
N NH2
N - N N - N
/ /
`,.
,
4? P
......
1
N N
N N H N N H
1.979 CI
I :-:-.--,-- 1.980 CI
,-=..,.õ I ;.-.,,
N NH2
N - N
/ /
`-.
91 H I
1 'N. N
N I
N
?
N H
1.981 CI N
1 ..--:".--"" 1.982 CI N N H
i .-,N..;=,-'
-......-..., I
N NH2
N NH2
N - N
/ N - N
/
180
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\ I
\
,,, N
I
N I
N
4111 N1.983 CI 1.984 5 N NH
II CI
I
/; N NH2
/J NNH2
NN
/ N-N
/
I
41111 I
N
Si NxNH 410 NINH
1.985 CI
I 1.986 CI
I
/ 1 N NH2 /; N NH2
NN N-N
/ /
\ ce),,, N \
I I
N N
1411) N NH 5 N NH c
1.987 CI IX 1.988 CI I I
/ i N NH2 / 1 N NH2
NN N-N
/ /
s., \
I I
N N
4111 Nx NH 010 __NH
1.989 CI
I 1.990 CI
I
/ 1 N NH2 // N NH2
N-N N-N
/ /
"=-=..
\ I
(CINI N
1.991 101 N NH 1.992 CI SO N NH
CI
Ix HL
I 1
/ 1 N NH2 / 1 N NH2
NN N-N
/ /
181.
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/0 4 2 7
7 7
OH
---- --'.5
N I
N
NI
rj
1.993 H 1.994 N NH
N NH CI 1 ===:=-'"
CI ===-,-" I
...-,..
/ i N NH2 / i N NH2
/ /
N, I
I N
N I " ,..,.N..õ)
I
1.995 4111 N NH 1.996 CI NNH
,
C
/ 1 N NH2 / i N NH2
N -N N - N
/ /
VF
I
I
N N
P 1.998 CI N NH
N NH
1.997 I
I X / i N NH2
// N' NH2 N - N
/
NN
/
F 0 CN
NI 1111111 I
N
Olt N NH
1.999 Ci 2.000 N H
N
1 CI i -:.=,--
I I
/ i N NH2 N
N H2
NI- N
/ N -N
/
_ -
182
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
H ".
N Nõ.
--.. i
I N
2.001 Ci N NH =2.002 CI 4111
N.1õLo
i 1
/ 1 NX NH2 / 1 N NH2
N-N N-N
/
/
\ \
I I
N N
N,40 011:1 N
2.003 CI 10
i 2.004 CI 1 '110
i
/ i N NH2 /j N
NH2
N-N N-N
/ /
\ \
I I
N N
2.005 a 14111 NI,..xio
i 2.006 CI 01 N
I
/ i N NH2 / 1 N
NH2
N-N N-N
/ /
"s. 0
I \ HN
i 4 41
N
NI 1 1111) N
2.007 CI 1%1
2.008
CI 1 ''... 0
1
/ 1 N NH2 /j N NH2
N-N N-N
/ /
H
\ N \
01
I I
N c> N
Si ...
2.009 a N I (Nµ`) 2.010 CI SI
Nro
,
/, N NH2 / i N
NH2
N-N NN
/ /
183
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
0
\
Is11 C )
NI
11101
N
Si N SI I
2.011 CI 1 T.L0 2.012 N
0
i
/j N NH2 ..
/j N NH2
NN
/ N ¨N
/
0
'`.. /,: q
N N
I
NI
101 N 'N... ..,'
I
2.013 CI 0
I 2.014 4111 N
/j N NH2 I ...
N¨N / 1 N NH2
/ N¨N
/
\ \
1 r I
N N..,,,- N
2.015 CI Si Nõ...õ.,40
I 2.016 CI 4110 N.,..1,,,Ccf
/ 1 N NH2 / 1 N NH2
NN N¨N
/ /
N. \
I I
N N
el N ,I,C10:7
2.017 CI
I 2 411 N.018 CI 1 "):-
CfC:1)
I
/ i N NH2 /f N NH2
N¨N NN
/ /
I I r---\N--
N N N.,/
2.019 CI 4111 N CI 41111,..1õCI:03
I 2.020 N
i 'NILO
I
/ 1 N NH2 / 1 N NH2
NN N ¨N
/ /
184
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
'==-,
I `N,
I
,_ ,......,,...
2.021 CI N
1 ------ '0
I 2.022 CI NI....õ....,..,0
, I _
N NH2 / i N NH2
N-N N-N
/ /
---,
ro N-..
1
1
2.023 CI
I N......"-.. 2.024 CI N
1 CO
NH2
N-N N-N
/ /
. .
--... Oy.-.k....õ 0
I
N.,..*,..'
N
2.025 CI N o , r
, 2.026
a N
N NH2 1 ,===
/ NN i N NH2
-
/ N-N
i
I
--..
N NH Oy'
2.027 CI
1 -k-7 2.028 CI N N
, =-.."`;µ," "s-
.. I .,......,
N NH2 f i N NH2
N-N N-N
/ /
1
"..
I I
N 0
N NH N NH
2.029 CI
1 *-:::--- 2.030-."...---
1.-,-õ, I 1...,..,
N NH2 / i N NH2
N-N N-N
i /
-
185
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
\ \
i I
N
N NH
2.031 CI I I 2.032 CI N NH
I ==,---7
/ i N NH2 / i N NH2
N-N N-N
/ /
\ \
i I
N 00 N
0...õ....CNH
411 2.033 CI N NH
I T: 2.034 Ci 1 Nz NH
-:-.--'
/ i N NH2 / i N NH2
N-N N-N
/ /
1 o ---,
N 0
NI
0.y-Cj>N
N NH
1
2.035 CI -;':,---- 2.036
CI N NH
, -:.---'-
N NH2 ..-..?...,
N NH2
N - N
/ N-N
/
0
`,..
\
I
I
1110F
N 0 N 0
2.037 4111I N NH 2.038 N NH
CI
CI I 1
/ I N NH2 / / N NH2
N-N N-N
/ /
/
.." ",, N
.. -:=...
1 I
N 0,j,, N 0...---
,,..,..13.r--1
141 N NH N " NH
2.039 CI I X 2.040 ci
I --z.---"
/ i N NH2 / i N NH2
N-N N-N
/ /
186
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
r
,.. .5,N...,..,./ I NN.
(31,Y
I ,,
0 N
CIN H
2.041 N 2.042 CI , N=-=-
sz,/-NH
N
N NH2 / 1 N NH2
N-N N-N
/ /
1
OH
.,,
I N NT
0
CIN N HN ,..,.
2.043 2.044 N HN
/ 1 N NH2 / i N NH2
N-N
NN
i i
' \
1-N1.---)
NI N
0 . I
N 0.)
2.045 N NH 2.046
I CI N NH
,,,-;-., I
N NH2 N ..?=-...,
NH2
N-N
/ NI-N
/
I
OH
N. N
NI N
I
N 0.1 0.,J
2.047 2.048 N HN
N HN
I I ,
1 N NH2
N NH2
N-N
N-N /
/
-
187
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I
N N
---. ( 1/ \\
N
N I
I N
2.049 2.050 N NH
N NH CI , "
CI , ---:
N NH2
N NH2
N-N
N-N
/ /
r 1
0 0 0 0
.....õ
,
1411
I I
2.051 N 0 2.052 N 0
CI N NH N NH
,.----,-"-
I CI , --.:---
""
I
,4--, .,,,,,=,-,.
/ 1 N _ NH2 N NH2
N-N N-N
/ /
I I
N
HN N
H2N
N 0 N
2.053 CI , -zz.s,=-=
I 2.054 CI ,
I..,-...,
N NH2 N NH2
N-N N-N
/ /
I
HNJ I
N N
HN".<
N
2.055 CI 1 :":='--A0
I 2.056 CI
N NH2 N NH2
N-N N-N
/ /
188
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N-,
I
..-1:7
N
HN A H N N
N N
2.057 Cl 1 ''''z''''0
I 2.058 CI 1 "::=""-LO
I ..(,-,,
N''NH2 / i N NH2
N¨N N¨N
/ /
., ---..
I I
N N
HNX) HNC)
N 0
2.059 CI , N 0 -:.---"
I 2.060
I
N NH2 / i N NH2
N¨N N¨N
/ /
',..
I ..,
N ZNH I
HN N
H"Li N
2.061 2.062
N..,...,...õ"...,...,0
CI i %.17L.0 CI
I
/ i N NH2
N NH2
N¨N N¨N
/ /
=
/ 0
HN)..s'i f=-- \N
I
N I
N
HN''N'-')
2.063 CI , N'sN.-...-Vo
I 2.064
CI i N........õ....
/ / N NH, I
N NH2
N¨N
/ N¨N
/
/
,,,01
NI 101 F
I
HN N
HN
2.065 CNpI 2.066 I N1.,..,0 CI
I
N NH2
N NH2
N¨N
/ N¨N
/
189
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N r
N 1
HN.L.''' N
SIP N....,1,0 HNjõ
2.067 CI
1 2.068
CI 14111 N .,....0
N NH2 I
N-N / i N NH2
/ NN
/
OH
\.
I
HNY
0
N \
I
N N ...17-0 HN
2.069 CI 2.070
i 4111 INJ,,,,,co
CI
N NH2 I
N - N /j N NH2
/
N-14
/
\
I
4? .
9
N
N I
HN
HN
2.071 I. N ....,,,.,7
0 2.072
CI
I CI
/ 1 N NH2 / i N NH2
N -N
N - N
/ /
0 OH
HLN\s/
\ \ N
I I
N
HN,-, N
HN)
2.073 2.074
411 N0
CI CI
I I
/j N NH2 /j N NH2
/ /
190
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
i
N
...-- '..
0
NI -s. ''''..,="'
I
HN N
HN,-,
2.075 i NIo 2.076
CI i N_...,70
I Cl
NI-N / / N'e"NµNH2
/
N-N
/
r---
d 00
I I
N===-)... ,õN
HNI"'- `....
2.077 2.078 I
CI N
HN
I
N1\1H2 Cl N
I
N-N
/ / / N NH2
N-N
/
1
0-0
N-.
1
N
---.. HO
I
2.079 N 2080 . CI N
HN 1 0
I
1 N1,....õ....."...0
N NH2
I N-N
/ / N NH2 /
NN
/
\ \
I 1
oJ
N
o.." N
2.081 CI N1 0 N
I 2.082 CI
I
NNH2 / i N NH2
N-N NI-NI
/ /
191
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042 777
I I
N
N N A
Ok 0
N
2.083 CI 1 -'=-z-""-LO
I 2.084 CI
N NH2 / i N NH2
N-N N-N
/ /
I
o-,-0
N N
,....,,,,
2.085 CI N
1 "-,----- -0
I 2.086 CI N
.0
-,,,,-,-,õ 1
....,,:,,
N NH2 / i N NH2
N-N N-N
/ /
N
OC
0
, ....,....
2.087 CI N*.0
I 2.088
CI N
1 ".==-""-LO
I
N NH2 N NH2
NI -- N
/ N --N
/
\. /
I
N I
N
411) N
2.089 CI 1 '''''.a0
I 2.090
I
/ i N NH2
N NH2
N-N
/ N-N
/
0
F
NI
I
2.091 i CI No 2.092
CI
I I
N NH2 / i N NH2
N-N N-N
/ /
192
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
/
`-,- õ.0 "=-=., N
I I I
N N
2.093 CI , N.z,....õ,,---0 2.094 ci i N,,z...õ,..-
0
I I
N NH2
NI-N N-N
/ /
r
,,N,õ,,,- -=
`,... I
, N
N
N
0
2.095 , N,c) 2.096 o.57
0
CI
CI
I I
N NH2 / i NNH2
N-N N-N
/ /
---..
\ OH
I
,
N
N 0
0
2.097 2.098 ,
Ns.,,...z..."...-0
CI N
/ i N1-12
/ 1 NNH2
N-N
N-N /
/
`-,... `..
I
9 , HO
N N
0 0
2.099 N 2.100
CI i :-.,...õ-= , NI,..z.,,..70
I CI
I
N NH2 / i N NH2
N-N N-N
/ /
193
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/0 4 2 7
7 7
___?-01-1
CO
(NrJ
`,. '`
I
I
N N
0) 0
2.101 N 0 2.102
1
ci ="=.--. , Nz,..,,..0
CI
N ---'' NH? 1
N ¨N /J N NH2
/
N¨N
/
I
N
--- --.., 0/
---.. ."-=-=,--' "---..
I I
N N
2.103 0
2.104 0
N 0 N
, =-=.'":-.
CI
II .4.......,
N. NH2 / i N NH2
N¨N N¨N
/ /
0.,,..0 0 0
...-"J's.
I
--y ---N
---, `-µ,
I I
.105 N 2.106 2 N
TX 0
N...,..10 N
CI CI
I I
N NH2 / i N NH2
N¨N N¨N
/ /
,..
I H 1 r
N 0.,,N,.... N 0.).,, NH
N NF-I
2.107 I CI *-:.--- 2.108 CI N N
-,, I
..,;.....õ
N NH2 / i N NH2
N¨N N¨N
/ /
194
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I 7
N 0.1., NH N 0.).,. NH
2.109 CI I. N NH
Ix 2.110 CI 0110 N
NH
I 1
/ 1 N NH2 /; N NH2
W. N NV N
/ /
\ \
N Oy NH N 0....,,, NH
2. 1 1 1 1.1 N NH 2.112 41 N1' N H
CI I X CI I
/j N NH2 / 1 N NH2
N''''N
/ /
*`=..
, q... H
r ,srN 0
0..,,, NH
NY
Oy NH
2.113 N 1 N NH 2.114
CI
I X CI 1 N NH
N NH2 Ix
/ N¨N 1 N NH2
/ N¨N
\ /,
I
/-1/
N
N Oy NH N (*)..,õ NH
2.116 2.115
1 N x NH . N..,,. NH
CI CI
i I ....1.,,
/ i N NH2 /j N NH2
t\i". N N-"N
/ /
195
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N-.. ''..õ..."
0
N 0,..,õNH I
N 0..,,NH
2.117 N NH 2.118
I CI N NH
, .--;---7
N-N
/ N-N
/
/
I I
I
N NH N
0....,.NH
2.119 CI , -k----
I 2.120
N NH
,
N NH-.., I
-5,....õ
NI-N / i N NH2
/ N-N
/
LNj
1 rj N 0...,.NH
2.121 2.122 140 N NH
N NH CI , -:-./..
CI , ---z=-.--".
/ I N NH2
/ i N NH2
N-N
N-N /
/
`--,
N 0.,NH N 0.NH
2.123 N NH 2.124 N NH
, I
N NH2 ..,-.,
/ 1 N NH2
N-N
/ N-N
/
196
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N.
NIid,
0,..,..NH
N 0,..., NH
ci
2.125 NN H 2.126
:.---' N NH
,..>-=,., I
N NH2 -,....,
N / i N NH2
-N
/ N-N
/
0 /
(LJNI.--
tN)I
N.
il
0.--.NH N
0..,.NH
2.127 CI 2.128
NN H
ICI -:=2-,-'
..5...,
N-NI N/ I N NH2
/
N. 'IN
/
....
0"-NH N 0...., NH
2.129 2.130
I
CI N-:-7NH NN H
..;,.. I
N NH2 .,-.,
/ i N NH2
N-N
/ N-N
/
(0/
I
I I
N 2.131 0NH N 0....,NH
2.132
NN H NH
N
CI -:---'- CI =',-,-,"
I I
, .
/ I N NH2 / i N NH2
N-N N-N
/ /
197
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\ "
N N
2.133 CI N ,.,-= 2.134
N....szs,õ,,,,,_
I CI
,;:...,.
N NH2 N NH2
\ \
I I
N N
2.135 CI NIk 2.136
I CI
I
N NH2 N NH2
\ \
I i
N N
2.137 CI N.,..rõ,- 2.138 N,...N.
N- NH2 N NH2
\ \
N N
2.139 CI Nxi, 2.140
I CI
I
N NH2 N NH2
\ \
N N
2.141 CI NN.1.-1, 2.142 Nxifr
I
N NH2 N NH2
198
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
".. `,..
I I
N N
2.143 CI N
i ..."-------""-s.,,,, 2.144
CI N
1 ,..1;,.......
N NH2 N NH2
I I
N N
2.145 CI N 410 2.146 CI 1 N
I -....
N NH2 ..,
N NH2
",.. "...
I I
N N
2.147 CI Ne 2.148 I CI NI,,C.7
N NH I N NH
.2
I I
N N
2.149 CI NIL) 2.150
I CI
I
N NH.,
,_ N NH
I I
N N
NH
2.151 C I N Nr-2.õ
, :-....õ--
1 2.15,
CI N NID
-;.....--,... I
N NH2 N NH2
199
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
`-..
I I
NI
OH N
2.153 CI N N -.1 2.154 N 0
1 CI
I
N NH2 N---N" NH2
N-. OH NI
N-.
I I
N
2.155 CI N 0- 1 2.156 N F
I CI
I --*---
N NH2 ..---......
N NH2
I I
N N
7.157 CI N CI
, ==-,--- 2.158 N Br
I CI , -"-.0--'
..;:-..,.. I
N NH2 ,-,.--,
N NH2
I I ELF
N
NL
F
F
2.159 CI 1 N==--')<F 2.160 N 0
I
NI---µ" NH2 .......,
N NH2
I 1
N N
N 0
2.161 CI N OH
, -z:.----=- 2.162 I
-z=-...-/--
-;-,- I
N, NH2 ..,1-,-
.....
N NH2
_
200
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\ \
i I
r
N N
Y
CI
2.163 CI N.10 2.164 N./0
I
I
N NH2 N NH2
\ \
N 7 N
2.165 CI 1 N.10
2.166
CI Nõ..../0
I
N NH2 N NH2
*\
, NI
P
N
2.167 CI N IO
2.168 N,....10
I CI
I
N NH2 N NH2
LJ
1
,,,,
" ps\i/0
NI I
N
Y
2.169 1µ1,,x0 2.170 N.,10
NH2
CI CI
I I
N N NH2
\ 1
\.
r.N.õ..
N I
N
2.171 11,õ../0 2.172
CI
I CI
I
N NH2
N NH2
201
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
'N.
I
41111 (N
N
2.173 CI N 0
, -::::-..-'"
I 2.174
CI N 0
, ===:--=".
N, NH2 N NH2
-=-.. crõ), N N,..
I I r
N N (N,....,....-
2.175 N 0 2.176 N 0
CI , -=:=-=--
I I
..;;-,.. ....1,-;,..,
N NH2 N NH2
-s-,
I
N
irA N
2.177 CI N 0
, --**:,/
I 2.178
CI I N 0
====;,-."
...;....õ ...,,,,
N NH2 N NH2
I
N
N
2.179 N 0 2.180
CI N 0
, -`,..=-==="
CI , ==="::=,-""
II N NH2 ,...,/,..,
;f....., N NH2
0
s's. N...
I Ni
N (6--
2.181 N 0 2.182 N 0
CI , =-:,-""
I
<;.......
N NH2 N NH2
_
202
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
0 \ (0--
NI
NI
rj
0
2.183 N 0 2.184 N
CI
I
cl 1
1 I
N NH2
N NH2
VF
N,7,
d F
N N
r,...C.)
NI
2.185
1) N 2.186
CI NO
0 I
I N NH2
N NH2
F op CN
N.. '-.
I
01 NI
N
2.187 a N,,0 2.188 N 0
I As CI
I I
N NH2 N NH2
H
N N \
,..
NI
i
((5N i
aighh
N SI
2.189 iv N,,,0 2.190
a CI
-1
I I
* NA, NH2 N NH2
\, \
NI
NI
Y
2.191 CI N S
Ir 2.192 N S
ICI , =:,--'
I N,7.,N1-12
N NH2
203
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
\ \
7
i i
9
N N
2.193 CI 1 N S
-1:X 2.194
CI 1 N S
N NH2 N NH2
N. \
1
9 ,
N N
1 1
2.195 CI N S
...:X 2.196 N S CI c)
:-.1
N NH2 N NH2
0
i
1 i
N N
?
2.197 N S 2.198 N S
CI
1
=',X CI
I
:NI
N NH2 N NH2
\ 1
\
N I
Y
N
2.199 N S 2.200
CI
:,..I N S
i CI
I
N
1
NH2
N NH2
1
411 i
N N
y
2.201 CI 1 N S
'-.1 2.202
CI N S
-=::--"
N NH2LJ
N NH2
204
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
. 9 .
, , r--
N
2.203 N S 2.204 N.....r,.S
N
CI
I'NI CI
I .,-.1.,
N NH2 NH2
\ \
I I
(1:7
N
(L\ N
2.205 CI N,y,S 2.206 I N S
NH2 CI
I
N N NH2
I
15) r---C
N
N
2.207 N1 S 2.208 N NXNH2
S
CI
CI
1 I
N NH2
\ \
N
2.209 CI NXS 2.210 N S
I CI
I
I
N NH2 N NH2
0 \ ..=
\ N
I N
N
r) (,)
2.211 N S 2.212 N N
CI S
I
CI ''...-=-="'
I
- NI NH2
N NH2
205
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
F
N
I
N N
r.,..z.,..............,./
I
2.213 N
1) 2.?14
CI N S
N S I
CI , :::s. -..,"
I .....,.¨õ,
N NH2
...?..s.,
N NH2
N. N.
NI 111111 F
I
N siCN
2.215 CI N S
..x 2.216 N S
I
N NH2 ...;;¨,.
N NH2
H
Nr..."--,,,..,-=
2.217 N S 2.218 Nz.,.....õ,..NH2
CI
I 1
--õ:
N ' NH2 N".., NH2
I I
N N
I I
2.219 CI N NH
, :-.N.,...,"
I 2.220
CI N N
......., I
...:?..
N NH2 N., NH2
N.
N
r N
Y
2.221 CI N NH
, =-....-=""
I 2.222
CI I N NH
==zs,"-
...1,-...õ
...):-...õ
N NH2 N NH2
206
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
'N. \
i i
Y
2.223 CI NX NH 2.224 N NH
a ==,"".:¨.."
I CI
I
.,,,....õ
N NH2 N NH2
\ \
i
9 ,
9
N N
2.225 I CI N NH 2.226 N NH
CI
I
N NH2 1.-
;....,
N NH2
,
P ,
N N
Y
2.227 i CI N NH 2.228 N NH
a "=-:-. =""
:-...1 CI
NH2
I
N,,.,=...õ
N NH2
i \
N,
,,..?
? N
2.229 N NH 2.230 N NH
ICI CI I a == : .= -= - '
N NH2
N = NH2
1
-.. ,
N
1
Y Oil
N
2.231 N NH 2./31 N NH
CI a -':%.=-''
CI
I
I
'-=-1 ,
N = NH2
N NH2
207
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I \
,
N
2.233 CI N.,INH 2.234 N NH
I CI
I
-,X
N NH2 N NH2
\ \
I I
(4'
N (N N
2.235 CI N NH 1,,,
I 1 2.236
CI 1 N.syõ.NH
.,:l.õ
N NH2 N NH2
.-
1
1\1
N
¨/)
2.237 CI IN NH 2.238
CI NI NH
I
I
N NH2
N NH2
\
4NH
I
0
N
2.239 CI NI NH 2.240
I CI N...1 NH
N NH2 I
N NH2
O
0
\ N
I \ N
I
N
rj N
1)
2.241 N. NH 2.242
CI i ==:=,
I CI N NH
===X
N NH N NH2
208
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
F F
`,.
I
( Ile-F
N
2.243 CI NXNH 2.244 N
I. r)
N NH2
, =:-..--*
N NH2
===., 11,, F
Si
r's,...= N
2.243 CIN N NH 2.246 N NH
, ==:.
I CI , -:.=-=".
N NH2 N NH2
. .
H
".. 0 CN
-..... ..,..õ.N N,,
NI ream I
N r=--',..,.,L
2.247 ci RP N NH 2.248 CI N NH
I X
*
.x N NH2 I
N NH2
.'= ''..
I 1
N N
2.249 CI NI..z......,..,...0 2.250
Nz.....õ,...0
1 CI
,.-...., 1
N NH2 ..;-,...,..
N NH2
'N. `-''
-,.
I I
N N
2.251 CI N
1 IO
I 2.252
CI N
1 "Ii0
I
N NH2 N NH2
209
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/0 4 2
777
\ \
I i
N N
2.253 CI N
1 s-I.?0 2.254 N
I CI 1 "120
I
N NH2 N
NH2
s \
OH H
I \ N
I
N
N
2.255 Ci N..zz...õ,..0 2.256 lel N
I CI 1 '0
-;.-...., i
N NH2 0 N NH2
``,..
0 ...,
I I 0
N N NH
2.257 N.,...,..õ...0 2.258 4111:1 N
CI CI
I I
N NH2 110 N N H2
\ \ cO,N
I
I
110
N N
2.259 N 2.260 N
Cl 1 "s= 0
..
N NH2 , Nr NH2
/
0
, i N
N 262 2.261 ... N N,,,_,,--
. N
N 2 CI i ---..-1,-
'L0
Cl
I I
--- N NH2 0N NH2
210
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
I I
N .. N
2.263 CI N
I '...1--e 2.264
I CI
I
NH2 N NH2
`,-, `,..
I I
N N
2.265 CI N
1 4CP L
I 2.266
CI N
1
i),C11
I
N NH N NH
' .
."....
I r.--\N¨ --,,
N ,..,.N,/ I
N NI-
..=-==
2.267 CI N...,.....õ.,....0 2.268 I CI N.,.,,........õ..0
N NH2 .11,--...*
N NH2
. .
----..
I
N ND 1
N N ..õ-J
0
2.269 CI N
1 '`...'z"--X
I 2.270
N NH2
N NH2
1
"s. 0
-,, 'y'"
.".".=
N 1
2.271 ci Nõ..,c,,...õ..0 2.272 N.....õ...õ,0
I CI
I
NH2 ,7N.
N NH2
.............................................. I ............................
211
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
0 \
0.
fsl ail N
1-...
l ...õ--
2.273 kip) N 0 2.274 N NH
Ci -, CI
I ,. I
NI'
* N NH2 N NH
I I
N 0...--- N 0<
2.275 CI N N
, `===;=--' "-.. 2.276 N NH
-;.-...., I
[jJN NH2 N
NH2
==,,
I
Oyfj
N Oyb' N
2.277 CI N NH
, -::---' 2.278 IN NH
I CI , .:::-.,"
Ir...., *-,..,
N NH2 N NH2
I
I
0. .,./ED 0,,,-0
N N
2.279 CI N NH
, -zz's..-,7 2.280 N NH
N NH2 .e..õ
N N H2
0
\ ."...
I I
o.../Z0
N oydNH N
2.281 N NH 2.282 CI, N NH
CI , ,:.==-='"
II ......:-,
..,1-,..., N NH2
N NH2
212
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
/ 0
\ N
NI
2.283 N NH 2.284
N NH
I CI
I -I--
N NH2 N NH
F /
NI
110 NI
0 Offt)
2.285 ci N NH
-T- 2.286 N NH
I CI
N NH2
N NH2
=., Ni. r
I
N 0 I
NI ..
(3,r
2.287 N NH 2.288
CI
N NH
I CI
-,-).
N NH2 1
N NH2
OH
\
NI
I
2.289 CI N NH CIN 2.290 0.,,,>.
-"1"
I N NH
"=,-
N NI-12 I
N NH2
NI
0.5.) NI
0.:j
2.291 N NH C 2.292
I -* N NH
I CI
N NH2
N NH2
213
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
0 OH
\ N
NI \ N
I
2.293 CS)
2.294 N Cy
N===1/ NH N NH
CI
I :
CI
1 ...- .1.-
N NH2 N NH2
C-0 I
N
\ N
I EN)
N
2.295 N NH 2.296 0,J
1 CI N NH
N NH2 I
N NH2
r
N 0 0
r,
I I
N 0.,)
\ \ N
2.297 N NH 2.298 I
CI i ="=..---''
I , N 0
N.. NH2 N NH
CI
--.X.-
I
N NH2
I
00
\ 14111 NI
I NIT,o
2.299 N 0 2.300
CI
I
N NH
CI
..1"- N NH2
I
N NH2
214
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
`-. ...
I I
HNJ
N N
H2N
2.301 CI , N,..,..z../..0 2.302 N
I CI 1 '-'z'--0
I
N NH2 N NH2
-..õ
N
HN".< N
HN.-A
2.303 N CI 1 ''.1.-"".-LCI
I 2.304
N
CI
..
1 --":0
I
NNH2 NNH2
I
H N 1:7 I
N N
HNL)
2.305 N
1 ss=-'="Ao
I 2.306
CI
ci 1 N._.,...,..õõ.-0
I
N NH2 NNH2
Ns, 0
I ""N
N I
HNJ:::::1
2.307 Ci , Nõ:õ......µõ,..0 2.308 HN
I N
CI 1 -:'----"-LO
N NH2 I
N NH2
I _L7
N I
HN N
HN'L---/
2.309 N
1 '"..-"."*"..LO 2.310
CI
I CI
I
N NH2
N"-N-NH2
215
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
0
. F
\
NI N
HN
HN'a-'
2.311 CI N,..1,..o 2.312 41111
CI
I I
N NH2 . N NH2
/
I
\ ,f() \ N.,,
I
N N 1 ;
HN HN
2.313 N.,ivo 2.314
CI CI
I 1
N NH2 N NH2
r ,
... j,, HN
N,-
I
NI N
7
HN
2.315 N. 2.316 N...,...o ,....io CI
CI
1 I
N NH2 N NH2
OH
i
.. 5'
N
N HN
2.317 HN 2.318
CI
CI
I 1 o
N
N NH2 NH2
216
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
,
9 .... N
N I
HN N
HN)
2.319 2.320
I CI
I
N NH2
N NH
2
0
f....,,,,,OH
Ni--1
". ID
, I I
N i
HN N
2.321 2.322 HN
CI N
N NH2 I
N NH2
i
r.,N,,
N/
I I
,--
2.323 N HN. N 2.324 HN
CI
1 N CI
,..,z.,..0 i N.....zõ...õ--0
I I
NH2 N NH2
r ,
0,...0 0 0
.......
,
..."....j. ,N
-...s. -...,..
2.325 I 2.326 I
N N
HN HN
N 0 CI , N 0 -Z-.",--
CI , =-=µ,-,,".-
I I
NH2 N NH2
217
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I
N N
HO 0
2.327 CI i N...,:z..."0 2.328 N
I CI
I
N NH
2 NNH 2
I
oJ I
N N
2.329
1 *--::-**
I 2.330
ClCI 0 a N
1 '''."----A0
I
NNH2 -;==..,..õ
N NH
--,,
N A I
JD
N
0 0
2332
2.331 Cl N
i. N
I Cl 1 ''.:','""LO
I
N NH2 NNH2
I I
N N
OL> OCI
2.333 ci i N.s.,:z..õ,-0 2.334 N
I CI
I
N NH2 N NH2
',... "...
0 0
2.335 N 2.336 N
=
Cl 1 'scs.-A0
I CI i *-- O
--""'L
I
.,--,....., .....;......s.
N NH2 N NH2
_...
218
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
/ 0
". or-N\
I
1"--,/ "..
N
NI
Oa'''.
2.337 N....,p 2.338
CI 14,.,...0
I CI
I
N NH2 N NH2
411 F
f:(>1/
NI I
0 N 0
2.339 CI N,1õ,-.
0 2.340
I CI
I
'sr NH2
N NH2
oc:N i---
..
NI `=. r,N,,,,,-
I
N
2.341 c) 2.342 0)
CI
I CI
N NH2 I
N NH2
OH
I
Y 9''
N =-..
0
NI
2.343 N..I7-0 2.344 0
CI
CI
N NH2 I
N NH2
-=,,
NI
4? CI 1 ,--,
9
0 N
2.345 N.,,..x.-0 2.346 0
CI
1
N NH2 N NH2
219
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
H4NN)
r,r,OH
,.. r
N'...../
1
o)
N ..'
0 N
2.347 2.348
I
N NH2 N NH2
i I
(---1: N
`.. 0
1
.1" . ... ....
-,.....-
N I
2.349 0 2.350 N
0)
CI N
i .;.,...,..0 N
I
NNH2
NNH 2
r.
? N dy0
I .."..11
N
2.351 0 2.352 I .õN',..
CI N
0)
I ,
N..,..z.......-0
N NH2 CI
I
N----N-NH2
i
0 0
I H
LJJ NL 0N,
I
2.353 N 1 N 0 2.354 N NH
0 CI i -Z-z=-=""
I
CI ..,-,:z..7. ...,¨...s
N NH
2
I
N'NH2
220
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
"=,.
I r- ... ....--
N OyNH
NI
OyNH
2.355 CI I. N N
`== 2.356
CI 1 NINH
401 N NH2 N NH2
I
, 7 ,....
9
N OyNH I
N OyNH
2.357 N..1NH 2.358
NH2
N,..INFI
CI
I CI
I
N
N NH2
`===.
P
N (:)NH I
N NNH
2.359 N NH 2.360
CI
^=%1'. N NH
I CI
I
.='''
N NH2 NI NH2
LJ
0 /
tr.N.) <N)
,.. ...
1 1 Y
N OyNH N OyNH
2.361 2.362
CI Nõ...xNH
CI
I I
N NH2 N NH2
221
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
t\I 0 N
.., .NH Cf.rg\1H
2.363 2.364 --i-
N NH N NH
CI , ...=--,'.
I a , --:.-----
.õ,
N NH2 N NH2
N. /
IP 1 ---- N
I N
N 0,../,,)
2.365 N.,NH
2.366 N NH
N NH CI ,
CI , ====:-,-
..õ, N NH2
N NH
L NJ
. .
i
NI I)
N ONH
2.367 2.368 0,.,.NH
N NH N NH
CI=,-
I
N NH2 ,.....,
N NH2
N 0...,,NH N ONH
2.369 N NH 2.370 N NH
I
N NH2
N NH2
222
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N.
NI r 1 0NH N,NH
2.371 N NH 2.372
CI
-C N 401 N " NH
N NH2 . NNH2
(CY.-
ra
NI
NI
0;,NFI 0,..NH
1-
2.373 N NH 2.374 N NH
CI
I I
N NH2 N NH2
ni
NI
0.
0.,,,,NH .õNH
2.373 1 2.376
N NH
CI
CI N NH
N NH2 N NH2
/
= o,,
(0
14 ak, I
0 NH N FI
2.377
klIP N ,., NH 2.378 ON
a
I j,, CI N NH
0 N NH2 I :'Z
N NH2
rci,:yo,,
N._
,-. HI4
14 aghp, 0 NH
2.379 MP NNH 2.380 a II Ny:
ci
. N NH2 10/ N NH2
223
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N¨ N.._
HN1 1-114
2.381 CI N,,,..,...,,,,.
2.382 410 1 N.-1
I CI
reN.-NH2 io N NI-12
N_ N
.383 CI _
HN1 Hr\I
2 N......"..õ.....,,-
2.384 N..,..õ,õõ--:=,.....õ..,...,
I CI
I ,,---õ,
N' NH2 N NH2
N- N_
FIN HN1
1 N..,....,
rµJ.,..,..õõ.....s.,
2.385 CI 2.386 CI
0'N NH2 ..<,-,...,
N NH2
N- N-
HNI HINi
2.387 CI N.,...,.....,.--,'
2.388
I CI
N NH2 N NH2
.
N-
N-
HNI HN1
2.389 CI N
, "-'-' 2.390 N
I...>..,
N NH2 N NH2
224
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 777
N- N-
HIV HNI
2.391 CI N
--..."---'-'"--%. 2.392 N I.
I CI
I
N NH2 N NH2
I
N..... H N
2.393 N__
1114 I
CI VI N
2.394 Ne
, .... c,
,
40/ N NH2 N NH2
. .
N. N-
H NI HI\I
2.395 CI Si Nlf-3 2.396 NI-1D
1 CI
1
0 N NH2 N NH2
N- I N-
H14 HIV /
2.397 CI Si Nxf.D 2.398 N
N Ni
I CI
I --:----
lip N NH2 ...1-,--...,
N NH2
1_
Pis- N_
HN H NI'
OH
2.399 CI 1.I N 1µ1/'
I I 2.400 CI 1 N N/Y
401 N NH2 N NH2
I
N-... N-
H14 H Ni õ.,--
,..,....,-OH
41111 2.401 CI N 10I I 2.402 CI 1
401 N NH2 N .....:õ.-N,
NH2
I
225
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N....., N......
HI=1 HE4
lel 14110
2.403 CI N F
i I 2.404
CI N CI
i I
110 N NH2 ill N NH2
N._ N......
HNI 1-114
F
I. N Br
2.405 CI CI
I I 2.406 411 NsI)<F F
I
0 N NH2 411 N NH2
N. N.._
F
HN1 F,f.,F Ht4
4111 Olt
2.407 CI N 0
2.408
i I CI
N 1 1OH
101 N NH2 401 N NH2
N...... N._
HI4 HI4
1 r
N
2.409 CI 0
'-.1 2.410 Si Nx0
I CI
I
N NH2 /1101 N NH2
it... N.....
Ht4 HNI
011t N 0 410
2.411 CI N
i
I 2.412 CI i 10
. N NH2 SI N M-I2
226
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N¨ N¨
HNI
IV
9
2.413 a N? H
, --::,---
I 2.414 CI N 0
N = NH2 ...1-
?....
N NH2
N¨ H
HN
P H'
2.415
--- --..,..-
2.415 CI N 0
, .-Z-s..,"
I 2.416 Y
N 0
N NH2
N¨ I
N N.__ \
y
HNI
? HN
I 1
2.417 N 0 2.418 N 0
CI sk-----
CI , -:.=-=""
N = NH2 ..-,-
--.õ
N NH2
I
N
N¨ ---- -.. N_
Si
HN HN'
2.419 Y
N 0 2.420 N " 0
I
-;-,,,
N NH2 N NH2
N_ .."--"-"N /
N¨ 9
HNI
,....,õg HNI
2.421 N 0
2.422 N 0
CI , -1:-. -'
I CI , --=:-'"
N NH, I ,...f.,,
, N NH2
227
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N-_, p......
Ht4 MN
(N=
2.423 CI kli 1 N.10 )
2.424 CI Olt Nx0
I
/001 N NH2 N NH2
Pis-
15)
FIN 446 N..._
HI4
2.425 CI Mil I NIO
2.426
41i Nx0
CI
1111 N NH2 I
so N NH2
ri
N.-. N......
HNI CI H14 N.õ/
2.427 Nx0 2.428 CI r'N-
lel N cr,
CI
I
I N NH2 11101 N" NH2
N......
HIN1 N-.
2.429 CI H14
41111 I NIG,
2.430
1411/ I NO
CI
410 N NH2
N NH2
FF
F
1\1--
HN
2.431 CI ego i NI() 2.432 FIN
110 Olt N
N NH2
CI x0
I
*I N NH2
228
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N.....
1114 I
r,,,_/,-. FINN---
SI
2.433 Nx0 2.434 411 Nx0
CI
I CI
I
N NH2 ail N NH2
H
N N
N¨ * CN
HIV 44h,, Hrsi
2.435 WI NO, 2.436 NIO CI
I CI
0 NA NH2 I
N NH2
N.._ N¨
HAI 1114 alb,
2.437 CI Olt I\I,I
2.438 RP NxS
I I ..;.,1,. CI
0 N NH2 0 N NH2
N-
1,1---
Ht\I HN
NxS 4110 Y
,..x
2.439 CI
I 2.440 CI N
I
N NH2 0 N NH2
N¨.
HI4 H
aith
'9 t4N-
9
2.441 CI ILIF N S
I I 2.442 CI Si N S
I
..-X
Si N NH2 0 N NH2
229
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N-
P HNj\j- H
....Y.'
N.._ _....0
HNI ...--
2.443 CI N S
, -:::----"
I 2.444 N S
CI , -,'"z=-=""
.-).:-..,
I
N NH2 ...,:-..._
N NH2
i \
N- N N_
y
HNI
HN'
2.445 IN? 2.446 N S
CI1 --..-.---"
...,:-..õ I
N NH2
i
N- ,,N,, N...._
Hri Hni
4111
--N..-
2.447 N S 2.448 CI N S
, -...."
II ,=-.",
../..,
N NH2 N NH2
N-
Y N-
HNI
,
HNI
2.449 N S 2.450 N S
I CI
N NH) .r.....õ
N NH2
N- N-
HNI
IA
HNI
i'''N.
2.451 CI 1 N S )
2.452
Ci 1 N S
-:==-i"
..,-,
N NH2 N... NH2
-
230
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N¨
HrNI
r NIN-
2.453 ci 1 N S
=*--..--- 2.454 H
N S
N NH2 I
..p..s.
N N H2
I
N._
I¨ rii
HNI N 'ANN
HN
2.455 ci 1 N S
-..----- 2.456
CI
N S
-;.=-..., 1 -,..----'
N NH, I
,.
N NH2
2.457 2.458
..)'
N¨ N N¨ ..N
HNI
r-) HNI 3
2.459 N S 2.460 r-
1
ci ..,...-- a N S
-,1----,,, I
N NH2 ....,....õ
N NH2
i
F>I
N
HN
_
N¨ N'F
I
2.461 ci N S
1 2.462 HNI
I ri
N NH2 N S
CI
Ec:T
I
_4-Ns,
N NH2
I
231
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N rx,...3 F
.- N N
-.
HI4 Ht4 tam
14111
2.463 14S 2.464 MP N.,S
CI
I CI
I .,..,t,
N NH2 40 N NH2
H
N.._ * CN
N-. N
HN 4,06 HI4 erehh ....õ.... 1
2.465 kii N S 2.466 Igo N S
CI
1 A * N NH2 CI 1
ill 1\-NH2
N.- N-.
H14 aith Htµi kil aik l 1 N x
NH2 glij I
2.467 CI 2.468 CI N NH
I 1
SI N NH2 1101 N NH2
N._ N.._
HN HI
2.469 CI 411I N III
1 *=-= 2.470 4111 NH
I CI
I
io N'.. NH2 (10 te"NH2
!µ1--- N.-
HN H14 gab,
'=,....'"
I I
2.471 CI Si N ...y,. NH
2.472 MP N,.(y,.NH Cl õ;,..
N NH2 lb N NH2
232
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N. N.
1-114 HK
2.473 CI IMP N NH
Ix 2.474 CI 411 N9. ,1NH
I
401 N NH2 ip N NH2
FIN
9 HK ,,,,,,
g
2.475 CI 01 N NH
I 1 2.476 CI 11111P N___
I )
N NH2 401 N NH2
0 /
i
N..... tr.!) N-
HN Y
2.477 1.1 N FINNH 2.478 N.,,INH
CI
I CI
I
/110 N NH2 N NH2
\ 1
N.-.
N N
1114 dah N-.
I-IN
2.479 CI 114, 1 NINH 2.480
1111) N.õ..INH
CI
I
N NH2
0 N NH2
N-
HI4 Ail 0 Hr4 ait,
2
2.481 CI IIIP NINH
I 2.482
CI 111,I, 11 ININH
ill N NH2 110 N NH2
233
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
i NI N.-.
3\1--
HN
cr.,, H14 J
2.483 I. N.,INH 2.484 CI
N NH c
CI
1 Ix
/110 N NH2 110 N NH2
N..... N-...
H14 gagth
iA Htsi dais
(17-7
2.485 CI eip N NH
i 2.486 Illi NXNH
I CI
I
III N NH2 ill N NH2
N-
N.-.
HNI CI
abi
I HI4 ash,
2.487 RP N NH 2.488 111.1, 11 NXNH
CI
.1 1
ill N NH2 0 N NH2
N.- 1---\N-- O
N.-... N
HNI N../ .....
HI4 ahh
rj
2.489 CI lei N NH
X 2.490
Rill N NH
1 CI
I
illo N NH2 1
. N NH2
N-.
HI4 r alb, HI4 aik,
2.491 kill N NH 2.492 RP N NH
CI
CI
X
tip ( N NH2 N ' ---* N H2 110
234
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
VF
d N-
I
N- N HNi
HN
2.493
ri 1
CI N NH
2.494 CI N NH
1 -z."...--- I
..1-,--..,..
I N NH:-.=
N NH2
I.
011
N-
F
N- 0 H CN
N HIN1
2.495 )JLN NH 2.496 N NH
CI
O
,...,...,. I
N NH2 ,;...=,,
N NH2
. .
H N-
FIN i HIV
2.497 N Ni-1.--- 2.498
CI
i I
,.-....., ...;.-.....õ
N NH2 N NH2
1
N- N-
HN HN
2.499 CI N o
1 2.500
I
r\l"--NNH2
N NH2
. .
N- N-
HN HN
N
2.501 CI N 1 --'-'1I0
I 2.502
I
N NH2 N NH2
_...
235
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
H
N...... OT.,...;.N
I-114 N-
I-IN
2.503 CI N
1 .120 2.504
i ci Nro
N NH2 I
N NH2
H
Nz.-_ N N- 1\1/
1-IN,
V HN
4\-.)
2.505 1\1........õ,..,....0 2.506
CI
I CI ,,...0
N NH2
N-
N
HNk L,..,,,, NH
11101
H N
2.507 )jLNro
2.508
CI
I CI N
1 -=== 0
N NH2 1 ...-
N NH2
0,-,
N- cO,N
FIN N-
HN I
Nro
2.509 CI
I 2.510
N
CI I -=-= 0
N NH2 ...,
'N NH2
N-
r N-
HN
2.511 )LLN,...,,,,..0 2.512
CI
I CI
..;.....,_ I
N NH2 N NH2
_
236
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
jt"-- PL.-
HN aim 2.514 IIP
HN
2.513 ,,lib.
1111/ N
CI 1 `=(CCI::::,
I , CI
I
Si N, NH2 lio N.'.. NH2
P--
HN 14---
N
2.515 CI 1.1 N H N
I "s.X.C.: ) 2.516 01 N
I CI 1 ro
40, 1\r- NH2 I
401 N NH2
Pi- PI-
HN NID HN
0
2.517 C N
I 4111
i '-='=140
I 2.518N.,..x,C0
lbN-.... NH2 N'' NH2
N.... ro pi--
HN tab, ..õN.,.) HN NO---
2.519 CI Iv Nro
2.520 CI 4111 N
i O
1111 N NH2 IN NTC.'. NH2
P1--
HN N. ,,4 14---
HN arbit
2.521 4111 NIL 2.522
iv N,,, a
CI
I CI
I
110 N.' NH2 lio N NH2
237
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N-
HN :
446, Hisi Ail Oyi
11111 N 1,NH
2.523 CI 2.524 CI 114111
N N
1 .,;( 1 1
. N NH2 /10 N NH2
N-
Pis-
HI's1 ahh 0,,< HN cal OyA
2.525 CI 111 NNH 2.526 a IV N.NH
I .. I .õ.1s.
. N NH2 10 N NH2
N_ N-
HN gab, 0y1-23 FIN 4,66,
0...,,C)
N,,..x NH 1111
2.527 a
1 2.528
CI
1
101 N NH2 401 N NH2
N- N-
Hr4 0.õ1:22) HN OyCY
2.529 a 1. N,...,NH
2.530 N.,INH
I I ,,,,,I, CI
Si N NH2 1j2N NH2
N.- /
I-04 Ail 0.)õ,-0) N._
FIN1 alit 1:>1
2.531 a
Ill N NH
2.532 RI N NH
CI I
1111 N NH2 si N NH2
238
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
0 40 F
N.._ , N.
FIN ka 0,6\1- HI4 0
2.533 I4P N,NH 2.534 si N NH
CI
I I ,,
illoN NH2 lb N NH2
N/
N. ,,,.,N
_ N-
HN 0
2.535 01 N,,NH 2.536 SI N,,NH
CI
I ,
ISO N NH2 41) Nj NH2
(-- N
H-
N_ N..,.õ-
HI4
2.538 4.4, Oj
I4 Aabi 0.i
2.537 I.1 N,NH
CI 111 N NH
CI
X
40 N NH2 /110 N NH2
OH
N_
PI- FIN 05)
HN
2.540 1411 N NH
2.539 0
N NH CI
CI I X- I 1
0 N-- NH2
40 N NH2
239
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
\N...)
N _..... (N
HINI 0....,9 HNIN-
0,)
2.541 2.541
N NH
CI , '=-=:.=-=*" N NH
.;.-.,. I
N NH- .
' N NH2
OH
N...._. NO
N.___ N
HINj 0,) HIV 0.,,i
2.544
N NH N NH
2.543
CI
1 ===s,"" CI , -Z":--'"
N NH2 N NH2
1
(N... N
N
LN..". N.__ r,
N
_
HN1 0) . 1-IN1
2.545 2.546
N "- NH CI , -zz.---=".
CI
N
N NH2
NH2
r 1
0 0 0 0
.,.. ,
I.
=-.. N
N...._ N.__
2.547 HNI 0 2.548 FINI 0
Ni" NH N NH
1
--,...";=-=""
N 1=11-12 N NH2
240
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 777
N¨
N¨
H14
H,-. HN
N.
HN
2.549 CI N.s.':-7-o 2.550 i Ns.z.......,..-0
I CI
....,.-....õ I
N NH2 N NH2
N____
Fini
HN" HN'N----
H N ".<
2 N L
.551
1 --=----- 0 2.552 N
I CI 1 -=--.)-
CI -0
N NH2 N NH2
N
H N A ¨
H Ns
HN1N¨
H N ,L7
2.553 CI N, _.,...,=,,
1 s'-'-' -0 2.554 )JLN
I CI 1 .0
.;=,---.... I
N NH2 NI"'-N. NH2
N¨ N_
HN HIV
HN "1:1> H Nj:::1
, Nµz.z...õ,..,-0
2.555 ci 2.556 N0
I CI
I
N NH2
N¨
rzt N¨
HIV 0 HN" LIO
HN HN
N
1 z"-N-r"LO
2.558 CI N
1 ':-.--- '''''L
2.557 CIIO
N NH2 N NH2
241
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
i 0
N_ r \NI
HNI N-
HN '.1.-'3 HN1
2.559 N
, .-.;.,-.0 2.560 HN'')
I CI
I
N NH2
N_ N_
HNI 1110 F
HNI
HN HN
2.561 . µ,...,,,,D 2.562 N
CI
INI----'sNH2
NI''NH2
N (--
N-
HN1 I ; N- J.N...õ,,,..,
HN HNI
2.563 NI ,..,o 2.564 HN
CI
N NH2 I
N NH2
OH
N-
HNI
HNY N-
HN''
HN)cJ'
2.565 N
, .;::...,,.."0 2.566
Ci
I N
, ..,.--.:.õ.0
CI
N---s'NH2 I
N- N NI-12
HIV ? H 9 N-
HN N'
2.567 N 2.568 HN
1 NI,.....z,..0
CI
I CI
I
NI".-NH2 ...,-..õ
N NH2
242
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
õ0
r_...t,OH
N- '===,," N-
HNI HI\I
HN..,-
HN.-,
2.569 2.570
N 0 N
1 ....,...s,"0
CI , .-.1:-.7.
I CI
I
11---N'NH2 N NH2
ct,t I
N
....-. ',..
N_ 0
HN'
N
HN.,-=
HIN/
2.572 2.571 HN.."
N
1 ,..-..,_...õ/"0
CI
I CI N0
N NH2 1 *.L
N NH2
r
Nl 00
N
CiN
-
'...1.1
Hri
HN.,-
N...._.
2.573 2.574
0 HN,1
CI HN
1 N 0
N NH2 CI I <=:.-7.
I
NH2
1
0 0
N...._
HNI
N- HO
2.575 HNI 2.576 N
CI
1 ''''s".."(:)
HN
CI N
1 ..,-,.....õ.70 N NH2
I
NNH2
243
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N- N-
HN HN
J
o..,"
0
2.577 CI INI0
2.578 N
I CI
I
N.'-'NH2 N NH-,
N- N-
HN1 HN/
o----A
0"".<
N N L2.579 CI 1 ''''.."=-=A0
I 2.580 CI i "-''O
I
N1--"N-NH2
0N NH2
N_ N-
Hri
HNI
0.0
0
2.581 CI N10
2.582 CI N 0
.......õ. I
N NH2
N NH2
0
N_
N-
HN1 ,..,---H eCi
0
2.583 SI N 2.584 CI N
1 LO
I I
NH2 N NH2
0 N
/ 0
N- r- \N A N'
HNI1
0-"1\./ N-
,'- HN1
,---s-,-,)
2.585 2.586 0
CI i '-'=,-=-=-'7.
I
N NH2
N NH2
244
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
F
N._ N¨ xiNi
HN HN
0 11111 0
2.387 , NI.,-.,,.,,0 2.588
CI
CI N,-0=0
I
NH2 1
0N 'NH2
Lsz,INI r
N_
HN N_
0 Hhl
2.589 N 0 2.590 0
CI , -::=,"'"
N NH2 1
N."-ss"NH2
OH
N¨
Y
HN' N¨
O
HN'
2.391 N0 2.59' 0
CI
CI
N NH2 1
1 N'' N NH2
HN¨
r.1 ? N¨
'N../
0 HNI
2.593 N 2.594 0
N
CI
I CI 1 ...-.s...."0
I
N NH2
N NH2
245
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
i
r,....r,OH
N¨ N¨ NI-I
HN' HN' I
2.595 0 2.596 0
CI N
, .,..--z..../0 CI N
I I
N''.."'NH2 N NH2
Fli I
N- C"../0 N
,-- --,
HN N- 's-s--/-
1
e HNI
2.597 2.598 O'r
CI N
1 -,-....70 N
I C i 0
I
N''NH2 I
N'''..ss NH2
r
c
/ 00
N¨
HN'
2.599 N
2.600 H-
N 0 IV
I 0)
N NH2 CI N
, ..,-
....õ.".0
I
N NE-12
i
00
N H
N- 011) HNI-
0,.s.õN,
-1" -
2.601 Hr4 2.602 CI N NH
, '-z-,=-/-
0 I
CI N
i ..z,....."0 ...,-,..,
N NH2
1
N NH2
246
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N.....
r N-....
HN OyNH HN OyNH
2.603 CI 1411 N N
I I 2.604
CI i N NH
I
01 N NH2 N NH2
N..... 7 N-.
HN9H
Oy NH H14 0.y..NH
2.605 5 N NH 2.606
4110 N NH
CI IX CI 1 I
110 N NH2 10/ N NH2
N.....
9
c) HNN,NH
HN1\L 0NH
2.607 0 N NH 2.608
CI 410 N NH
i X CI
1
===1/
(110 N NH2 0 N NH2
0 i ../. ====..
N
N-
Y N......
Y
HN OyNH HNI OyNH
2.609 S 2.610 I N N H 0 NI NH
CI
I
[110 N NH2 ill N NH2
r- 1µ1/
N.....
Y N.....
Y
HN O HN
0NH .,..I-i
2.611 2.61 2 NN
lt N NH N NH
CI I ,(/ CI
1
(100 N NH2 N NH2
247
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
/
N._ 1101
HN,N-
HR1
2.613 N, NH
2.614 N NH
N NH CI , -=:-.7
CI1 -===;=-='"
N NH2
1 N N
y
N.._ N.__
H
Hr.1 0,NH Hr4
2.615 2.616 C.) NH
N NH N NH
CI
1 ---":-..-"- CI , --::,=-"--
N NH2
N__....
rA N.__
113
HN1 0...,_NH HN1 0..,õNH
2.617 N NH CI
2.618 N NH
1 :::-...7
CI , =====V
..7..... I
N NH2
N NH2
n
r---C
NI_
1 N...._.
HN1 0 NH HN'
O...,. NH
2.619 N NH 2.620
CI , -z=,-,/-
I CI N NH
"-==
0'N NH2
N NH2
248
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
0
rC..7..,'
r&
N-.....
N-__ HN 0,,NH
HN H 2.621
N --c 2.622I N NH
N NH CI , -:*::>="...
CI
I
N NH2
N NH2
/
N-.... (..--N
rs.../ N-._
r'..)
HN HN
0NH 0,.õNH
2.623 2.624
-' N NH N NH
CI , µ:.=-'
1 CI , z.s.:=,V
I
,,..
N NH2 N NH2
I
/
N.... N ¨
Hi N, NH HN
2.625 2.626 0NH
N NH
CI
N NH2 I ,
N NH2
N 0 N
,, I
......
..-..,., i
H N
PI¨ I
HN 0 NH
2.627 2.628 N CN
N NH CI , -:,---
CI -%=1" 1
N NH2 N NH
)
- i
249
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
L.
HNF aah HN gaih
2.629 CI Rip N ,y,,CN
2.630 ci tip i NxCN
0 N IIIFI 4p N NH
......----...,
N......
HN N.-
HN Ndiebt
I N/CN
CI 11.111 N CN
2.631 2.632 Cl
0 N Zis....-1 I I
N NH
I'''`....,k.....
-..,..
N.......
N- HN cal
HN araki
11111 N CN
RP 1 NxCN Cl
2.633 Cl
2.634 i X
1110 N NH401 N cyI)-1
--,...
...s.,
N
I
N-
HN ahh N.
HN ahh
Iti. N CN
CI
Nõ..e,-CN
2.635 I I 2.636 Cl
I* N 111 I ,...1,
lb
....,
250
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042 7 7 7
N-__ N -....
HN HN
CI ,N==%-.==-"C NI CI N CN
-1"...=-=,"
2.637 I 2.638
I
...7,...
N NH N NH
LID Ls 0
N_..._
HN' HNi
N CN N......
CI , ::====,'" N CN
I 2.639 1
,:;....., 2.640 CI LJL
..----
N NH ... ;.-
... ..,
N NH
\
N...._ N.._
1-114 HN,1
N CN N CN
CI , ::-..-=-='
2.641 1 2.642 1
.....,-......... ....,-....,
N NH N NH
1-....--....-.,. Is -,. I
t. Nj0J
1
N___ N¨._
HN FIN!
N ON
CI
X
I CI N CN
2.643
I
2.644 -5........,
N NH N NH
N..N
I I
251
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N- N_
HIV HNi
N CN N CN
CI i =`.-....'"
2.645 N = NH
2.646 ,-.--,,
N NH
L.
N CY
N
I I
N_ N_
HIV HN1
N CN N CN
CI i -:=-=--
I CI --:.==.-=-'"
2.647 N = NH 2.648 -,...-,,
N NH
L. L.
N N
N_
HR1 N-
HNI
N CN
I 1
2.649 N = NH 2.650 CI N CN...:?....
L--. N NH
ON
0
\ N
\
N- N-
1-114 HN1
N CN
CI i --,------ CI 1 -1:.*=-="'
2.651 I 2.652 I N CN
...,--,
N NH ..;-,
N NH
Oil 0
252
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HN ahh FIN gahh
qv i NxCN IV 1 NxCN
.).653 CI 2.654 CI
S
N y11 ill N ji.,,,,,,,,
e'''N. 0
N_...
HN
0111---
N CN HN gahh
CI 1 I RP 1 NICN
2.655 2.656 CI
110
N N NH
0)'Nj) 40 X
0 0
N-.. N-._
HN air. HN
It. 1 NxCN NICN
2.657 CI 2.658 CI
1
100 N X N NH
0 OH
N-.
P---
HN HN
N/CN 01111 1
NxCN
2.659 CI 2.660 CI
i
N NH
1--- 0 N X
0 0 0 0
N-- P--
Ht4 aah HN 446
11, 1 NICN CI lip N CN
2.661 CI 2.662 1 I
0 N , lj,\IF: ,A 1110 N r
0 0 0Cr'i3
253
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N.__ N-
HN gab,
HN1 ii4tik,
ell N CN 4.
CI 111 1 N/CN
2.663 I X 2.664 CI
* N r
411 N Z õC"I''''
N.
HN dizik, Htµi triiir,
WI NXCN 1111 N CN
2.665 CI
I 2.666 CI I I
* N r r\N..... N NH
0 0
N....,
NI_
HN ail, HN'
2.667 Cl aik,
kip N CN RP N CN
I X 2.668 CI I X
IS N NH fa.-...N, 401 N X ..,.,
tD"O'''..A...11
0 N
H
N-- N-
HNi 4,126 H14
2.669
RPII 1 NxCN NICN
CI 2.670 CI
I
110/ N NH L. N NH
Ce's NH2 dN"
H
N-.... N-
1-114 H14 ahh
NxCN IP 1 NxCN
2.671 CI
I 2.672 CI
N NH / [1110 N .21-,1 ,../z..
dN'Y 0 N
H H
254
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
Pi- N-
HN Ht4
4111 N CN illi N CN
2.673 CI
I X 2.674 CI I I
0 N NHA 10 N NHJD
00*N"."---1 ON
H H
N- N_
H14 gap, 1-114
kw N CN lel N CN
a CI
2.675 I X 2.676 I I
= N NH
N NH
.N''''''
H"==
ON iir\_.
N
N- N_
HI4 aak, H14
Ili N CN 110 N CN
2.677 a
I X 2.678 CI I I
= N 1\11H er 40 N NH *
H
0'14
Ce`N
H
N.._
HN FiNf ahh
2.679 a
I N1CN
2.680 CI lip N CN
N NH I I
ON"----(11 = N NH /7-...-..N
H N O'N''--- -----
H N
HN
H14
N CN ilo
2.681 Ci 1411
I I CZ\ .,0 2.682 CI NICN
I (:).µ .0
...- s-
40 0 N N, )
255
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N- N_
HN HN
2.683 ci N CN
I "'.----- 0 \ 0 2.684 ci N CN
N N ...%,;,...,
N
N N \ \
H H c /)
N
N_ N_
HNi , N/
FIN1 l
C
N CN C)
2.685 ci 2.686 N , _,CN ki
I CZ` CI ' 0
..7..., S 1 µ`s.
H N N ' µ`
H
N-
N_
H4 rah HNI1
2.687 ci N CN CC) 2.688 1 NC NJ
...)...., ....sµ I
g
0 N El
HO 1.0
N._ N-
I-1N FIN
2.689 ci N GNI ,TO
, 0.--,-'
I
2.690 CI N CN (-1
N N
H s., NN'
H
N._ 0 /
i N._ 0
HN
HN
2.691 CI N,,.....õ..CN 0 / 2.692 =
N CN 0
I ,>\---NH CI
1
, I ,õ NI-1
N N
H N N
H
256
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N-
HN N._ CN
N CN HN eiaby
CI I t ,,): ip =
2.693 N N'''''"e 2.694 N CN 0
LLJ
H . CI
HN 4i, I X
IP 0 Si N ,h.1
0.,
N._ CN N._ CN
NN HN ahh
N
2.695 ci N CN 0 0
i I ..õ)\-NH 2.696 CI tip N CN
N N I I
I-1 = N ri
N-
14- Hisi
HN
2.697 a N CN P' 2.698 CI 'SI N CN
N N
I X . I I
H /, N NH
N-N ..)
/
N¨ N....
HN HI4
Si N CN 11111 N
CN
2.699 CI 1 X 2.700 CI 1 X
/j N'. NH /j N
NH
NN /-= NN
...,..---...õ
/ /
N-.
N-...
HN H14
140 N CN
2.701 CI
I 2.702 CI 111110 N CN
I I/j N'''NH
N NH
N-N N-N
/
,
Lir
i...õ
257
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N.
Htsi Hi4
lel N CN 410 N CN
,
2.703 CI
I 2.704 I
X CI X
N NH / i N NH
N-N N-N (L0
, L.....õ,
,
N)
N.-
11 Isi tail N.-
14
It. N CN 1-1
CI
Si N CN
2.703 I 2.706 Cl
/ i NNH I I
/ i N NH
N-
/ N / ...)..õ
N.- N-.
Firsi 1114
0 N CN 14110 N CN
CI CI
2.707 I I 2.708 I I
/ 1 N NH /j N NH
N- N N-N
/
1NNO /
(0
0
N.-.
N-
FINi
H14 alb,
SI N CN
CI Lep 2.709 I X 2.710 CI
N CN I I
/j N NH
/j N NH
N-N N-N
/ ir N
/
(NUN I )
\ Nr
258
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N¨ N¨
HN1 HNI
01 CI
N ON ofti N CN
CI
2.711 I ): 2.712 I
// N NH / / N NH
N¨N L---..
/ I / &NJ
N
I
N¨ N¨
HNI HN;
140 N CN
C
I
CI 4110 N CN I
I
2.713 2.714
/j N NH // N'''..s.NH
NI ¨N
L--- N N ¨N
/ I Jj / I II
N
N'
I I
N¨ N¨
HNI HNI
141111 N CN in N CN
CI
CI I I I
2.715 / i N NH 2.716 / / N NH
/N¨N
NN/
C
N
I
N.__
HN
0 I N¨
HNI
N CN
CI 0 N ON
I CI
2.717 /j N NH 2.718 I
N¨N I\ /j N NH
/ N¨N L.
/
C5)
I N
259
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N- N.
HNI HNI
141111 N CN 40 N CN
CI CI
I I
2.719 / i N NH 2.720 / / Nr" NH
N-N l'...,
/ /
N N-N
0
N) \ N
\
N-
HNI N
14110 -
HNI
N CN
CI I I CI 2.721 / N NH 2.722 I
N CN
i
N-N
/
/ N-N
el /
1101
N. N-
HNI HNI
01 N CN 140 N CN
2.723
I 2.724 CI
I
/j N -I El lb / I NI"-N.-- NH
N-N N-N
/ /
N-
N.._ H NI
FINI
4111 N CN
el CI
2.725 CI
N CN
I 2.726 I
/ i N NH
// Nr"-NNNI-1
N-N
N-N /
CY)N-s6
/ d.'v
260
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N.....
HN HAI
0 NICN 2.727 CI
i 2.728 CI 111111 N CN
i I
N NH / / N NH
N-N J-,= ../'
/ 0 0
/
NN
0)*N'OH
1-114 1114
4111 2.729 CI N CN 5 NXCN
2.730 CI
HL i
/J N NH / i N NH
.,./.....
NN '''N
/ /
N-. N....
HN HN
4110 NXCN 141110 N CIA
2.731 CI
I 2.732 CI I X
/J N NH /,.. / i N NH
NN N-N
/
/
N- P--
HNI arah HN
(PI N CM SI N CN
2.733 CI
1 X 2.734 CI 1 I
N NH j,-:), / / N Z
N-N NN
/ Cd'-'0
/
N- N.....
H14 I HN
N CN 4111 N CN
2.735 a
1 X 2.736 CI 1 I
N NH _0
/ 1 N NH
NN
/
/
261
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/0 4 2 7 7
7
N._ N-
HN HN'
N CN SI 2.737 a
I --..-- 2.738 CI N CN
..,-,... I
/ 1 N NH
NN 0)µ'() * / i N NH
cNi)
/
N-
H14 N-
HN1
01 2.739 N CN CI
I 2 CI 0 N CN
.740
I
// N NH /j N'NH
N-N
(2).N.--* N-N
0..---NH 2
/ H /
=
N- N -
H Ni H Ni
14111 N CN el N ON
2.741 CI I X 2.742 CI
I
/j N NH / i N NH
N-N N -N
ON'
/ H / H
N- N....._
HNI H NI
0 2.743 N CN SI N CN
CI
I 2.744 CI
I
// N NH }, / / N NH A
N -N
0."-s= N /N-N
0-*=-=N
/ H H
N- N-
H HN
N1
SI N X C N 411 N CN
2.745 CI
I 2.746 CI
I
/; N NH ,..0 // N NH
N - N
0-".., N N - N --=-
ri"---"0
262
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N- N.
HNI
...I CN N CN
CE 41)
2.747 iN 2.748 CI
I
N NH
N-N
0,A,N / i NNH ....."...N..-
-
/ HN N-N ON.N..."-'-µ')
-,.
/ H
HNi HN
N III
2.749 CN a
1 2.750 ci N CN
/ i NX NH I
NN (:).-.'N * / i N'NH
/
H N-N (:)N-/- (
/ H N
N-
N-
H14 HN
N CN 41111 N CN
2.751 a 1 X 2.752 ci 0 n
/ i N NH ir:::Ns, ,;-..",.. ,S-'
N-N 6N,--%___ /)-- / i N rIl \>
/ H N N-N
/
N- N-
HN HN
Si N CN 010 N CN
2.753 CI 1 X 0 0 2.754 ci
i ,.., µµs.,?
/j N II ) 1.....-, ,S--
// N \
N-N N-N
/ /
N_ N-
HN1 FIN c), /
N CN , '
2.755 a
I -k---- a
.µ ,o 2.756 ci 410 NCN
1
N`N N / / N H H
/ N-N
/
_
263
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/04 2 7 7 7
N- N-
HN aht HN
%pi e il
2.757 a .I, Nµ,...,.,..CN 3-CO
Rµs 2.758 c, 410 N CN TO
1 0 '. \
N-N / / N' N"
/ N - N
/
N._ N-
HNI H NI
2.759 CI 1\1õ...=CI J-0 N,..;..........õC NO
/
I µ` N 2.760 CI
. ,sµ
NN
/ N - N
/
. .
HN
0 /
N F i N-'l 0
'
CI N':=-,-
CN
2.761 ci N CN 0 A 2.762 I
1 1.... .., j-NH
/ i N pi N-N H HN
N-N /
0 0
/
i
HIV HN
kit] N CN Ot_ 0
2.763 a
2.764 a
"I N ril =."'" ,-.. ."--
....,,,,-11-,N 0
N-N / 1 N iNil
H
/ N-N
/
N-
isi- _ _ /CN H NI
HN
ON
N r-r-i- 01 k 2.765 a CN
2.766 CI
N CN
I õ..... j NH
i H
N-N / i N N
/ H
N - N
/
i
264
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N-
HI4 HI4 46
Nx0H ulp N,y,AOH
2.767 CI
1 2.768 CI
1 ....
LJN NH 0 N NH
-)
IN.-. j\L-
HN HN
ill 1 1\110H 1.1 N OH
2
2.769 CI .770 CI i N_
_OH
N NH . N NH
,..."-,........
i
N.- N-
Ht4 46 HI4
2.771
ItIP N....yõ..OH 0 N ,y,.0 H
CI
i , 2.772 CI
i j.,...
N NH4110 N Ni(-1,
--,,, =......
N-.
N-.
11,4 4,riih
HI4 416
kill NI OH
CI 1 CI Ill N OH
ii
2.773 2.774 I
111 N c,1, j-i
110 N NH
i
L. L.
Ht alit, Ht 416
It. 1 NIOH iv N OH
2.773 CI
2.776 CI
1
0 N NH (1110 N'NH
LO
265
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
N._ N¨.
HN HN
N OH N OH
CI , ,--=:-.-,* CI , -.;=,-"
2.777 I 2.778 I
........, ........õ
N NH N NH
(-0
o L.
f---\*)
L----N
\
N¨ N¨
HN FIN
NOH
CI ,
N OH
2.779 1 2.780 CI I
.....-.1õ
N NH ,-).--õ
N NH
1 Y L'-.="''''''
I
N¨.... N.._
HN H Ne
N OH N OH
I
2.781 ,;-.", 2.782 .7,
N NH N NH
1--...
A j !N,IN
N
i 1
N¨ Nzz Fir.1
N OH N OH
CI , ---s.---'
CI , --"sz..,-"
I I N NH
...
2.783
N, NH 2.784
N
i
266
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N
HN-
' N..._
HNI
N OH
CI , =:-...-"" I N OH
I CI
,;-....-,
X
2.785 N NH
L-.. 2.786 N NH
(N.
CY
n
N
I N
N- N-
HNI Hf\I
N OH
N OH
CI , -zz,"" ,
I CI
I
2.787 ..,,,
'N NH 2.788 N NH
C-7"-N
N) 0
\---KI
\
N-_.
HI\I N-
H14
N OH
CI
I N OH
2.789 --;..,
N NH 2.790 CI
I :::=,""
N NH
0
4101
. . .
N-.
N- HI\I
HN1
N OH
N OH
2.791 CI , '-....-" 2.792
I..,...,.
-;.--, N NH
I
N NH
0
')"=-=... CYNI
267
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N.-
HI4
HI4
Si N OH
CI 14111 2.793 I I 2.794
N OH CI I I
0 N IXI-I illp N X ....õ
0
0 0
N. N......
H14 HI4
1401 N ,OH N/OH
2.795 CI 2.796 CI
I . I
110 N X N NH
0 OH LJ
N-- N..-
H14 H 14
Nx0H lei NI OH
2.797 CI 2.798 CI
I I
N NH / 10 N NH
N--- N.-._
HN H4
N/OH 01 N -OH
2.799 CI 2.800 CI
I I ,..,;..L.
N NH .A 10 N NH ,
0j.µ0 0)'.." 0C)
N.... N.-,
Hni Ht4 ail
N NO1-1 1111
Ci
2.801 I ..,..1 2.802 CI I IOH
N NM
00"--'"CIN,., 0 N NH õCr
268
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N- N.....
Ht\I aah,t Hri ail
Ili Nx0H tip N OH
2.803 CI
I 2.804 CI I I
* N tri r-\
N 1
N__
110 ,1-,1 *
0 0
N- 11---
FIN air, HN 446
tip MP
2.805 CI N OH =:1-'
I ,L,s 2.806 CI N OH
I I
= N 01,: le <=11/> 5
NNH ../..
H
N.-, N-
H14 alit H14
kW N OH Nx0H
2.807 CI
I N_
2.808 CI
I
IS N NH N NH
0 NH2 ON'
H
N-.... N.....
H14 F114 air.
Nx0H WI N OH
2.809 CI
I 2.810 CI
I I
N NH ." lb N NH
ON ON
H H
N.-. N.....
H14 HI4
N/OH Si
NIOH
2.811 CI
I 2.812 CI
N NH A N 7,1 xj
d's N 0 N
H H
269
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N-
N-
HNI arek, HNI
lip N OH 4111 N OH
2.813 ci
I X 2.814 CI
I I
* N r * N ri
0. "'N),
H e'N'
lir't\N,,
FINI dait HN
111/ N OH 14111 N OH
2.815 a
I X 2.816 CI I X
* N r õCf
(..,N N NH
. =`, *
H 0 N
H
N.- 11--
HNI ash, HN 44161
141 N OH RP N OH
2.817 ci .k.y."
I s
.5..L., 2.818 CI I I
= 110 N NH /-----N
O'INI'' 0
H N O''NN"'---- ----
H N
P--
N--
H14 HN
Si N OH 1410 N OH
2.820 2.819 CI
I CI
1 I
--- .,s- --- .S'
411 N N 0 N N )
N.._ N-..
His( HN1
2.821 CI Oli N OH
I I. R\ .0 2.822 CI Olt N OH
,,- ,s- I X
.µs.....0 .....N
usi N N \ 0 N 1.1 \ c
N
270
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N.__
/ !V_
HN1 N HN
/ ,'N
2.823 CI JJ1NX OH5/
2.824 N OH \
( JO
I R`s CI
ftJN
H N N µµ
H
N._ N.._
HNI FINI
/.825 CI N Oj--
H -C]
2.826 Of N OH
1 X % CI 1 X CZ`srQ
N N' \`
H µ-' 1101 N r b
,
N__ 0 /
HN
HN N__ 0
2.827 CI N....,...,OH 0 / 2.828 OH 0 4111
1 \-NH ci N NH
....,, I ...õ..i¨
N N
H N"----N
H
HN' N.__ CN
HN
CI
1 NYOH
2.829 N").NN'y 2.830 0 N, ,OH 0 *
H =
CI
HN id,h 1
NH
ip... 0 0 1\1N
H
0,_
, .
CN
HNP---
OH HN'
1 (NI
I N1 0 4 2.832 ci N-k--,"OH
2.831 ----/
/
i N vi
H
NN N N
/ H
\
N__
H N
N1
2.833 Ci N OH )> 2.834 CI N CN
1 --;=-=-
i
N N .;=,,,
H / / N NH
NN
i
271
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I
N N
40 I
2.835 NXCN 14111 N CN
CI
I 2.836 CI
I
/j N NH / i N NH
N-N ......----
.,,
/ /
\
I \
N
Il
el 01NXCN
N CN
2.837 CI
I 2.838 CI I I
/j N NH
/ i N NH
N--14 N-N
/
Lil / L-:=,,,,
I I
N N
14111 2.839 CI CI
NXCN 01111 N CN
I 2.840 I I
/J N NH / i N NH
NN L N-N
/ \:,.....,,
/
a.
\
NI \
I
N
lel NXCN
CI SI N CN
2.841 1 2.842 CI
/ i N NH I X
/ i 6 N NH
N-N
i
N
/ N-
272
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/0 4 2
777
... --,.
I I
N N
N CN N CN
2.843 0
I ....."
2.844
I
...),.., .:,...
/ I N NH / / Nõ NH
N-N 6 N-N
/ i
. .
N I
N
N CN
CI , ==:=/' N CN
2.845 I ..;,=-., N NH 2.846 CI , -=;.=-=-'
I
..-,..-..õ
/ 1 N NH
N
/-N N-N
/
LN-C)N tN,)
\
I I
N N
N CN N CN
X2.848
2.847 2.848 2.847 I
-;-.....õ
N NH / / N NH
N-N N-N
INN".1 N
/ Le /
N
1
N-.
`N. I
NI N
N CN CI N CN
II .;-......,
2.849 N N ..,-.....õ
2.850 / i N NH
H
N (..
N-N -N
/
/
C
N
i
273
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
''..
N I
N
N CN
CI , ==-,--"
I CI N CN
, =====.-.,..'
./..;-.õ
I
2.851 / i N NH 2.852 .....)....,
N NH
N-N
/ 5 N-N 1--,
i.s.
N
n
I N
`,...
I I ....,
N N
N CN N CN
I CI
2.853 ..-,-...,
/ / N NH 2.854 ...,......,
N-N 1---.. N-N (--,
i /
r. N
Nj 0
N
\
'-..
I
N......
I
N
r r,1 C 4
I N CN
2.855 / ;I.,.
'N NH 2.856 Ci , 1
i I
N-N / N NH
/
/ N-N
41111 /
lb
---,
I
N
N CN
1.857 I 2.858
N NH I
..f-?......
N-N / / N NH
/
lb /N-N OA-
_
274
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
N...
I
N \
I
N CN N
I
_.-õ....., 2.859 N CN
/ i N NH
2.860
I ,-,.-....õ
N¨N N NH
/ CY"..".1 / i
N N-N
N
I
\ \
I I
N N
N CN N CN
2.861 CI
I :::=-7 2.862 CI , ==-,==-='
,r;:.......
N NH / i N NH
NN....., .,-' N-N ==-===
0 0
/ /OOH
\ \
I I
N N
0 N CN N CN
2.863 CI
I 2.864 CI i ,..-=:-.""
I...p...,
// NNH / / NNH /
N --N OICYN.= N-N --"/
/ / 0 0
i I
N N
0111 N CN
2.865 CI N CN
i 2.866 CI , =zs..,"'
I
/j N NH /, / i N-,--..NH A
N-N =-=== -- =-=====
/ 0 0 /N-N 0 0
275
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
`,... `,...
NI I
N
N CN N CN
CI , ====:-.,"
2.867 CI
I =-=:,.-- 2.868
j:), NH
N-N N-N
/ 0..-NO /
I 1
N N
CN N CN
2.869 CI N
1
I 2.870 CI
/ / N X Os
NN H ,...^.N .....-
N -N
/ 00 N-N /\)
/ 0 0
`,...
I
N N
N CN N CN
2.871 ci
I ==-=-,--- 2.872 CI
/ I N NH ..,
NN C)() * / i N .......NH
....... c_N)
/ N-N /
/ 0 0
\ N
I I
N N
N CN
2.873 CI
1 -:,..?"
2.874 CI N CN
...-,..--.., I
N NH ..,--...,
N NH
N-N
/ 0...ssN''''
H /N-N
0======NH2
N... "=-,
I I
N N
N CN 1
2.875 CI .4.---"*.
2.876 CI N CN
I
)N NH / / N NH /
N-N
(:)N'''...." N-N
0....*N
/ H / -'1
H
276
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I
N N
SI N CN 0 N CN
2.877 CI I I 2.878 CI I I
N NH L. N NH ..4,
N-N N-N
/ 0....s.'N"1---
H / 0..."N
H
".. -s-..
I I
N N
iIiLSi NXCN NXCN
2.879 CI
I 2.880 CI
I
/ i N NH xj / i N NH
N-N ON N-N
/
H / H
.--, --.
i I
N N
NxCN 41111/ 1 N CN
2.881 CI 2.882 CI I X
/j N NH
N / i li". NH õCr
r
ON y - ri = - - - - a . N-N
N-..
/ CN
H
ss.
I
N N
N_ _CN Si N CN
2.883 ci 2.884 CI I I
I
/ 1 N NH
N-N '.-0 . N NH rt-=.N
d
/ N-N o'===Ni=-' %..... -
---
/ H N
N N
1.1 N CN lei N CN
2.885 CI I CZ\ ,0 2.886 CI
I .µ
....0
.,- '
/ i NI N S)>. / , N X N (:)
)
N-N N-N
/ /
277
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
I I
N N
2.887 CI 1 `.."-- 0 ,-, 2.888 CI
I N
Nr,5-... õS"' e_
i , \
N-N N-N N
/ /
rN
S,,r)N/
N CN -,.
I
/
2.889 CI
2.890 CI 1.1,..õ..,.CNc..)1 1 0)
--====-, ,S I -,.,...,_ ,\s-)
N-N N-N
/ /
--.
NI I
N
2.891 i
N CN c I X cZµ, 2.892 CI 1 R ¨r\ii
Frrµb ...", ;s.
/ N-N
/
--,
I o /
N
N
Ns..s.,.....õCN 0 /
2.893 CI
I ,.. j--NH 2.894
CI N CN 0 41
,---,..
/ 1 N N 1 I ,,i--NH
H / i N N
N-N N-N
/ I
,
==.
I
N "N, CN
N
=-. i N CN
CI I X N CN 0 .
2.895 2.896 CI
NH
HN I ..,.. j
/
RIP 0 / / NN
H
N-N
0õ /
278
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042 777
CN
Nil
(
\ N
¨/
2.897 cro i 411 2.898 ci
I
/
N-N
N-N
(-1\1
2.899 N
/ N
N -N
[0133] In some embodiments, provided herein are compounds described in Table
2, including
pharmaceutically acceptable salts thereof, and uses thereof. Isomers of
compounds of Table 2 are
also provided, as are compositions comprising a compound, or any isomer
thereof, in any ratio,
including racemic mixtures. Isotopic varients of the compounds are also
provided.
[0134] The embodiments and variations described herein are suitable for
compounds of any
formulae detailed herein, where applicable.
[0135] Representative examples of compounds detailed herein, including
intermediates and final
compounds according to the present disclosure are depicted herein. It is
understood that in one
aspect, any of the compounds may be used in the methods detailed herein,
including, where
applicable, intermediate compounds that may be isolated and administered to an
individual.
[0136] The compounds depicted herein may be present as salts even if salts are
not depicted and
it is understood that the present disclosure embraces all salts and solvates
of the compounds
depicted here, as well as the non-salt and non-solvate form of the compound,
as is well
understood by the skilled artisan. In some embodiments, the salts of the
compounds provided
herein are pharmaceutically acceptable salts. Where one or more tertiary amine
moiety is present
in the compound, the N-oxides are also provided and described.
[0137] Where tautomeric forms may be present for any of the compounds
described herein, each
and every tautomeric form is intended even though only one or some of the
tautomeric forms
may be explicitly depicted. The tautomeric forms specifically depicted may or
may not be the
predominant forms in solution or when used according to the methods described
herein.
279
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
[0138] The present disclosure also includes any or all of the stereochemical
forms, including any
enantiomeric or diastereomeric forms of the compounds described. The structure
or name is
intended to embrace all possible stereoisomers of a compound depicted, and
each unique
stereoisomer has a compound number bearing a suffix "a", "b", etc. All forms
of the compounds
are also embraced by the invention, such as crystalline or non-crystalline
forms of the
compounds. Compositions comprising a compound of the invention are also
intended, such as a
composition of substantially pure compound, including a specific
stereochemical form thereof,
or a composition comprising mixtures of compounds of the invention in any
ratio, including two
or more stereochemical forms, such as in a racemic or non-racemic mixture.
[0139] The invention also intends isotopically-labeled and/or isotopically-
enriched forms of
compounds described herein. The compounds herein may contain unnatural
proportions of
atomic isotopes at one or more of the atoms that constitute such compounds. In
some
embodiments, the compound is isotopically-labeled, such as an isotopically-
labeled compound of
the formula (I) or variations thereof described herein, where a fraction of
one or more atoms are
replaced by an isotope of the same element. Exemplary isotopes that can be
incorporated into
compounds of the invention include isotopes of hydrogen, carbon, nitrogen,
oxygen, phosphorus,
sulfur, chlorine, such as 2H, 3H, icy 13c5 14c 13N5 1505 1705 32P5 35s5 18,-.r
365 Cl. Certain isotope
labeled compounds (e.g. and '4C) are useful in compound or substrate tissue
distribution
study. Incorporation of heavier isotopes such as deuterium (2H) can afford
certain therapeutic
advantages resulting from greater metabolic stability, for example, increased
in vivo half-life, or
reduced dosage requirements and, hence may be preferred in some instances.
[0140] Isotopically-labeled compounds of the present invention can generally
be prepared by
standard methods and techniques known to those skilled in the art or by
procedures similar to
those described in the accompanying Examples substituting appropriate
isotopically-labeled
reagents in place of the corresponding non-labeled reagent.
[0141] The invention also includes any or all metabolites of any of the
compounds described.
The metabolites may include any chemical species generated by a
biotransformation of any of
the compounds described, such as intermediates and products of metabolism of
the compound,
such as would be generated in vivo following administration to a human.
280
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
[0142] Articles of manufacture comprising a compound described herein, or a
salt or solvate
thereof, in a suitable container are provided. The container may be a vial,
jar, ampoule,
preloaded syringe, i.v. bag, and the like.
101431 Preferably, the compounds detailed herein are orally bioavailable.
However, the
compounds may also be formulated for parenteral (e.g., intravenous)
administration.
101441 One or several compounds described herein can be used in the
preparation of a
medicament by combining the compound or compounds as an active ingredient with
a
pharmacologically acceptable carrier, which are known in the art. Depending on
the therapeutic
form of the medication, the carrier may be in various forms. In one variation,
the manufacture of
a medicament is for use in any of the methods disclosed herein, e.g., for the
treatment of cancer.
General synthetic methods
101451 The compounds of the invention may be prepared by a number of processes
as generally
described below and more specifically in the Examples hereinafter (such as the
schemes
provided in the Examples below). In the following process descriptions, the
symbols when used
in the formulae depicted are to be understood to represent those groups
described above in
relation to the formulae herein.
[0146] Where it is desired to obtain a particular enantiomer of a compound,
this may be
accomplished from a corresponding mixture of enantiomers using any suitable
conventional
procedure for separating or resolving enantiomers. Thus, for example,
diastereomeric
derivatives may be produced by reaction of a mixture of enantiomers, e.g., a
racemate, and an
appropriate chiral compound. The diastereomers may then be separated by any
convenient
means, for example by crystallization and the desired enantiomer recovered. In
another
resolution process, a racemate may be separated using chiral High Performance
Liquid
Chromatography. Alternatively, if desired a particular enantiomer may be
obtained by using an
appropriate chiral intermediate in one of the processes described.
[0147] Chromatography, recrystallization and other conventional separation
procedures may also
be used with intermediates or final products where it is desired to obtain a
particular isomer of a
compound or to otherwise purify a product of a reaction.
101481 Solvates and/or polymorphs of a compound provided herein or a
pharmaceutically
acceptable salt thereof are also contemplated. Solvates contain either
stoichiometric or non-
281
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
stoichiometTic amounts of a solvent, and are often formed during the process
of crystallization.
Hydrates are formed when the solvent is water, or alcoholates are formed when
the solvent is
alcohol. Polymorphs include the different crystal packing arrangements of the
same elemental
composition of a compound. Polymorphs usually have different X-ray diffraction
patterns,
infrared spectra, melting points, density, hardness, crystal shape, optical
and electrical properties,
stability, and/or solubility. Various factors such as the recrystallization
solvent, rate of
crystallization, and storage temperature may cause a single crystal form to
dominate.
[01491 in some embodiments, compounds of the formula (I) may be synthesized
according to
Scheme I. In some embodiments, compounds of the formula (I) may be synthesized
according to
Scheme I, 2, 3, 4, 5, or 6.
Scheme l
Br
OH
B, OH
N
Cl
N NH2 Pd complex B N NH2
OH
03c,
or
B, OH B
X N A' ."0 A N
X. I
B N NH2 Pd complex B N NH2
wherein A and B are as defined for formula (I), or any variation thereof
detailed herein; and X is
a leaving group (e.g., alkoxy or halogen).
282
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
Scheme 2
)1(
A -Sri(n-Bu)3 or
OH 0 -....,s- N Nr_-...- 0
B-Sn(n-Bu)3 or E3.., X N
o,, A-3'0
N .N `,-;--- '--,
, I 2 Lir OH -' ) 4
-).
Cl"---N.N'-N-N H2 Pd complex B----: NI NH2 B.'s:.N -
---..I NH2 Pd complex
"
1
Step-1 3 Step-2 Step-3
X
0 1`,1
A ,...N1 _.--z\-= õro
A N X 2
--....-- -,....--
,-- H--R2 A N R2 X -R1
A N,,y, R2
BN..---.. NI-12 _________________ . II
',...s....R1
B..-s..--N.----, NH2 Step-4 Step-5 B X-- -'N NH2 Step-6
B N N H
7 8 9 10
,
Scheme 3
X -3c
1 PYX-- B-Sn(n-Bu):3 or 10 -
i Ei S,
..õ.õ..N,,, X N,, A -Sn(n-Bu) or
CI,-"-:-N N H2 CINNH, Pd
complex
Pd complex C1'.--.N N H2
Step-1 .
Step-2 Step-3
1 3 5
)1(
Nsr:0
A N.,... A ,,...<,,N .,,...,., X H-R2 A '-sle-'-'N'sy--R2
x --R1 A __ ---- R2
Ã3,-,-:-N>s,N H2 Step-4 B N NH- Sep-5 B..--ks=N N H2
Step-6 BN,--L N ., R1
` t.--k'---N.
.z. = 7 3 9 10 H
Scheme 4
x 03c,
1 A-Sn(n-Bu)3 or 1
B
NR2 0..--,--N=,---r0 X N R2 A-0 A N R2
,T--...- y
CI X.--Nk NH2 ------------------------------------ ¨ --- x--
CI N NH2 Pd complex a N NH?
Step-1
1 3 Step-2 5
(Xis_
B-Sn(n-Bu)3 or 1
B
6 [3- '0 N R2
AN,,....- R2 R1-X A
B)..
Pd complex ---, ...ssN NI-12 Step-4 B N
N..,,
Step-3 7 H
3
283
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
Scheme 5
m:104
0 (IR3k3 .... N.....,
.._....
EI-Sn(n-B0 0 3 or it, CxN1I2
N B' 0
2 -iN,.. N ,.1,4
is I SeO2, t-BuO0ti H 'N1 5 N-.-
ZH s Z ..1 -- ---,,
CI N N1-12 SteP4 B N NH2 Step-2 B N ''NH2 Step-3
El N NI 12
1 3 4 6
X (R3)0,3 (R3)0.3
4 0 --- -).s 13.....r
7 H-R2 e'----14
2N zi..,N R X-R I \ ¨___.-N
/
N :LI N R2
Z ..- Y
.1L, ________________________
13- --NI N' R1
Step-4 Step-5 B NH2 Step-6
B N NH2 I-1
8 9 10
Scheme 6
rili ,Zi''
x
4 NH
9H 0 .....r0
N B-Sn(n-Bu)3 or ,e, N X N 0-2
6
2 B OH r- 1 4
1 .13,.,.N I NH2 r ):: I IN-
Pd complex B N NH2 Pd complex
Cl N NH2 Step-2
Step-1 Step-3
1 3 5
Nt N
N ,,, -..)
N N 0.=Nsr80
H-R2 t N2.,.N ,,,,. R2 X-
R1 N N R2
% 1 1 _______________ N N X 2.X..- .1r
I . 0.2.X X
Step-4 Step-5 ...,1 k Step-6
B NiLts1 -
R1
B N NH2 B N NH2
7 9B N NH2
11 H
wherein A, B, RI, R2 and R3 are as defined for formula (1), or any variation
thereof detailed
herein; X is a leaving group (e.g., alkoxy or halogen) and Z is a heteroatom
selected from 0, S or
NH. R3 groups may be the same or different, and which may be present on either
one ring or
both rings.
[0150] It is understood that General Synthetic Schemes 1-6 and present
synthetic routes
involving steps clearly familiar to those skilled in the art, wherein the
substituents described in
compounds of formula (1) herein can be varied with a choice of appropriate
starting materials
and reagents utilized in the steps presented.
284
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
Pharmaceutical Compositions and Formulations
[0151] Pharmaceutical compositions of any of the compounds detailed herein are
embraced by
this disclosure. Thus, the present disclosure includes pharmaceutical
compositions comprising a
compound as detailed herein or a pharmaceutically acceptable salt thereof and
a
pharmaceutically acceptable carrier or excipient. In one aspect, the
pharmaceutically acceptable
salt is an acid addition salt, such as a salt formed with an inorganic or
organic acid.
Pharmaceutical compositions may take a form suitable for oral, buccal,
parenteral, nasal, topical
or rectal administration or a form suitable for administration by inhalation.
[0152] A compound as detailed herein may in one aspect be in a purified form
and compositions
comprising a compound in purified forms are detailed herein. Compositions
comprising a
compound as detailed herein or a salt thereof are provided, such as
compositions of substantially
pure compounds. In some embodiments, a composition containing a compound as
detailed
herein or a salt thereof is in substantially pure form.
[0153] In one variation, the compounds herein are synthetic compounds prepared
for
administration to an individual. In another variation, compositions are
provided containing a
compound in substantially pure form. In another variation, the present
disclosure embraces
pharmaceutical compositions comprising a compound detailed herein and a
pharmaceutically
acceptable carrier. In another variation, methods of administering a compound
are provided.
The purified forms, pharmaceutical compositions and methods of administering
the compounds
are suitable for any compound or form thereof detailed herein.
[0154] A compound detailed herein or salt thereof may be formulated for any
available delivery
route, including an oral, mucosal (e.g., nasal, sublingual, vaginal, buccal or
rectal), parenteral
(e.g., intramuscular, subcutaneous or intravenous), topical or transdermal
delivery form. A
compound or salt thereof may be formulated with suitable carriers to provide
delivery forms that
include, but are not limited to, tablets, caplets, capsules (such as hard
gelatin capsules or soft
elastic gelatin capsules), cachets, troches, lozenges, gums, dispersions,
suppositories, ointments,
cataplasms (poultices), pastes, powders, dressings, creams, solutions,
patches, aerosols (e.g.,
nasal spray or inhalers), gels, suspensions (e.g., aqueous or non-aqueous
liquid suspensions, oil-
in-water emulsions or water-in-oil liquid emulsions), solutions and elixirs.
101551 One or several compounds described herein or a salt thereof can be used
in the
preparation of a formulation, such as a pharmaceutical formulation, by
combining the compound
285
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
or compounds, or a salt thereof, as an active ingredient with a
pharmaceutically acceptable
carrier, such as those mentioned above. Depending on the therapeutic form of
the system (e.g.,
transdermal patch vs. oral tablet), the carrier may be in various forms. In
addition,
pharmaceutical formulations may contain preservatives, solubilizers,
stabilizers, re-wetting
agents, emulgators, sweeteners, dyes, adjusters, and salts for the adjustment
of osmotic pressure,
buffers, coating agents or antioxidants. Formulations comprising the compound
may also
contain other substances which have valuable therapeutic properties.
Pharmaceutical
formulations may be prepared by known pharmaceutical methods. Suitable
formulations can be
found, e.g., in Remington 's Pharmaceutical Sciences, Mack Publishing Company,
Philadelphia,
PA, 20th ed. (2000), which is incorporated herein by reference.
[0156] Compounds as described herein may be administered to individuals in a
form of
generally accepted oral compositions, such as tablets, coated tablets, and gel
capsules in a hard or
in soft shell, emulsions or suspensions. Examples of carriers, which may be
used for the
preparation of such compositions, are lactose, corn starch or its derivatives,
talc, stearate or its
salts, etc. Acceptable carriers for gel capsules with soft shell are, for
instance, plant oils, wax,
fats, semisolid and liquid poly-ols, and so on. In addition, pharmaceutical
formulations may
contain preservatives, solubilizers, stabilizers, re-wetting agents,
emulgators, sweeteners, dyes,
adjusters, and salts for the adjustment of osmotic pressure, buffers, coating
agents or
antioxidants.
[0157] Any of the compounds described herein can be formulated in a tablet in
any dosage form
described, for example, a compound as described herein or a pharmaceutically
acceptable salt
thereof can be formulated as a 10 mg tablet.
[0158] Compositions comprising a compound provided herein are also described.
In one
variation, the composition comprises a compound or salt thereof and a
pharmaceutically
acceptable carrier or excipient. In another variation, a composition of
substantially pure
compound is provided.
Methods of Use
[0159] Compounds and compositions detailed herein, such as a pharmaceutical
composition
containing a compound of any formula provided herein or a salt thereof and a
pharmaceutically
acceptable carrier or excipient, may be used in methods of administration and
treatment as
286
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
provided herein. The compounds and compositions may also be used in in vitro
methods, such
as in vitro methods of administering a compound or composition to cells for
screening purposes
and/or for conducting quality control assays.
101601 Provided herein is a method of treating a disease in an individual
comprising
administering an effective amount of a compound of formula (I) or any
embodiment, variation or
aspect thereof (collectively, a compound of formula (I) or the present
compounds or the
compounds detailed or described herein) or a pharmaceutically acceptable salt
thereof, to the
individual. In some embodiments, provided herein is a method of treating a
disease mediated by
a G protein coupled receptor signaling pathway in an individual comprising
administering an
effective amount of a compound of formula (I), or a pharmaceutically
acceptable salt thereof, to
the individual. In some embodiments, the disease is mediated by a class A G
protein coupled
receptor. In some embodiments, the disease is mediated by a class B G protein
coupled receptor.
In some embodiments, the disease is mediated by a class C G protein coupled
receptor. In some
embodiments, the G protein coupled receptor is a purinergic G protein
receptor. In some
embodiments, the G protein coupled receptor is an adenosine receptor, such as
any of the A1,
A2A, A2B, and A3 receptors.
[0161] The present compounds or salts thereof are believed to be effective for
treating a variety
of diseases and disorders. For example, in some embodiments, the present
compositions may be
used to treat a proliferative disease, such as cancer. In some embodiments the
cancer is a solid
tumor. In some embodiments the cancer is any of adult and pediatric oncology,
myxoid and
round cell carcinoma, locally advanced tumors, metastatic cancer, human soft
tissue sarcomas,
including Ewing's sarcoma, cancer metastases, including lymphatic metastases,
squamous cell
carcinoma, particularly of the head and neck, esophageal squamous cell
carcinoma, oral
carcinoma, blood cell malignancies, including multiple myeloma, leukemias,
including acute
lymphocytic leukemia, acute nonlymphocytic leukemia, chronic lymphocytic
leukemia, chronic
myelocytic leukemia, and hairy cell leukemia, effusion lymphomas (body cavity
based
lymphomas), thymic lymphoma lung cancer, including small cell carcinoma,
cutaneous T cell
lymphoma, Hodgkin's lymphoma, non-Hodgkin's lymphoma, cancer of the adrenal
cortex,
ACTH-producing tumors, nonsmall cell cancers, breast cancer, including small
cell carcinoma
and ductal carcinoma, gastrointestinal cancers, including stomach cancer,
colon cancer,
colorectal cancer, polyps associated with colorectal neoplasia, pancreatic
cancer, liver cancer,
287
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
urological cancers, including bladder cancer, including primary superficial
bladder tumors,
invasive transitional cell carcinoma of the bladder, and muscle-invasive
bladder cancer, prostate
cancer, malignancies of the female genital tract, including ovarian carcinoma,
primary peritoneal
epithelial neoplasms, cervical carcinoma, uterine endometrial cancers, vaginal
cancer, cancer of
the vulva, uterine cancer and solid tumors in the ovarian follicle,
malignancies of the male
genital tract, including testicular cancer and penile cancer, kidney cancer,
including renal cell
carcinoma, brain cancer, including intrinsic brain tumors, neuroblastoma,
astrocytic brain
tumors, gliomas, metastatic tumor cell invasion in the central nervous system,
bone cancers,
including osteomas and osteosarcomas, skin cancers, including melanoma, tumor
progression of
human skin keratinocytes, squamous cell cancer, thyroid cancer,
retinoblastoma, neuroblastoma,
peritoneal effusion, malignant pleural effusion, mesothelioma, Wilms's tumors,
gall bladder
cancer, trophoblastic neoplasms, hemangiopericytoma, and Kaposi's sarcoma.
[0162] In some embodiments, the present compounds or salts thereof are used in
treatment of
tumors which produce high levels of ATP and/or adenosine. For example, in some
embodiments
the extracellular concentration of adenosine is 10-20 times higher in the
tumor compared to
adjacent tissue. In some embodiments, the present compounds or salts thereof
are used in
treatment of tumors that express high levels of an ectonucleotidase. In some
embodiments, the
ectonucleotidase is CD39. In some embodiments, the ectonucleotidase is CD73.
[0163] Also provided herein is a method of enhancing an immune response in an
individual in
need thereof comprising administering an effective amount of a compound of
formula (I), or a
pharmaceutically acceptable salt thereof, to the individual. Adenosine
receptors are known to
play an immunosuppressive role in cancer biology. High levels of adenosine
present in the
tumor microenvironment bind to adenosine receptors on immune cells to provide
an
immunosuppressive microenvironment. Specifically, binding of adenosine to the
A2A receptor
provides an immunosuppressive signal that inhibits T cell proliferation,
cytokine production and
cytotoxicity. The A2A receptor signaling has been implicated in adenosine-
mediated inhibition of
NK cell cytotoxicity, NKT cell cytokine production and CD4OL upregulation.
Therefore, use of
an A2A receptor antagonist, such as those provided herein, may reverse the
immunosuppressive
effect of adenosine on immune cells. In some embodiments, the immune response
is enhanced
by a compound of formula (I) or a salt thereof enhancing activity of natural
killer (NK) cells. In
some embodiments, the present compounds or salts thereof increase NK cell-
meditated
288
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
cytotoxicity. In some embodiments, the immune response is enhanced by
enhancing the activity
of CD8+T cells. In some embodiments, the present compounds or salts thereof
cause an
inflammatory response in the tumor microenvironment.
[0164] The present disclosure further provides a method of increasing the
activity of a natural
killer cell in an individual comprising administering an effective amount of a
compound of
formula (I), or a pharmaceutically acceptable salt thereof, to the individual.
In some of these
embodiments, the present compounds or salts thereof increase NK cell-meditated
cytotoxicity.
In some embodiments, a compound of formula (I) or a salt thereof increases the
number of NK
cells.
[0165] A compound of formula (I) or a salt thereof may be useful for
modulating the activity of
G protein receptor coupled signaling pathway proteins. In some embodiments, a
compound of
formula (I) or a salt thereof activates a G protein receptor coupled signaling
pathway protein (i.e.
is an agonist of a G protein receptor). In some embodiments, a compound of
formula (I) or a salt
thereof inhibits a G protein receptor coupled signaling pathway protein (i.e.,
is a G protein
receptor antagonist). In some embodiments, a compound of formula (I) or a salt
thereof is an
adenosine receptor antagonist. In some embodiments, a compound of formula (I)
or a salt
thereof is an antagonist of any of the A1, A2A, A7B, and A3 receptors.
[0166] Accordingly, also provided herein is a method of modulating the
activity of an A2A
receptor in an individual comprising administering an effective amount of a
compound of
formula (I), or a pharmaceutically acceptable salt thereof to an individual.
In some embodiments
a compound of formula (I) or a salt thereof is an A2A receptor antagonist. In
some embodiments,
a compound of formula (1) or a salt thereof reduces A2A receptor signaling by
at least 10%, 20%,
30%, 40%, 50%, 60%, 70%, 80%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or
99%.
In some embodiments, a compound of formula (I) or a salt thereof reduces A2A
receptor
signaling by 40-99%, 50-99%, 60-99%, 70-99%, 80-99%, 90-99%, or 95-99%. In
some of these
embodiments, a compound of formula (I) or a salt thereof binds to the A2A
receptor with an IC50
of less than 1 j.tM, less than 900 nM, less than 800 nM, less than 700 nM,
less than 600 nM, less
than 500 nM, less than 400 nM, less than 300 nM, less than 200 nM, less than
100 nM, less than
nM, less than 1 nM or less than 100 pM. In some embodiments, [compound x]
binds to the
A2A receptor with an IC50 of 500 nM to 100 pM, 400 nM to 100 pM, 300 nM to 100
pM, 200 nM
to 100 pM, or 100 nM to 100 pM.
289
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
[0167] Also provided herein is a method of modulating the activity of an AM
receptor in an
individual comprising administering an effective amount of a compound of
formula (I), or a
pharmaceutically acceptable salt thereof to an individual. In some embodiments
a compound of
formula (I) or a salt thereof is an A2B receptor antagonist. In some
embodiments, a compound of
formula (I) or a salt thereof reduces AM receptor signaling by at least 10%,
20%, 30%, 40%,
50%, 60%, 70%, 80%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%. In
some
embodiments, a compound of formula (I) or a salt thereof reduces Am receptor
signaling by 40-
99%, 50-99%, 60-99%, 70-99%, 80-99%, 90-99%, or 95-99%. In some of these
embodiments, a
compound of formula (1) or a salt thereof binds to the Am receptor with an
IC50 of less than 1
gM, less than 900 nM, less than 800 nM, less than 700 nM, less than 600 nM,
less than 500 nM,
less than 400 nM, less than 300 nM, less than 200 nM, less than 100 nM, less
than 10 nM, less
than 1 nM or less than 100 pM. In some embodiments, a compound of formula (I)
or a salt
thereof binds to the A2B receptor with an IC50 of 500 nM to 100pM, 400 nM to
100 pM, 300 nM
to 100 pM, 200 nM to 100 pM, or 100 nM to 100 pM.
[0168] Also provided herein is a method of modulating the activity of an A3
receptor in an
individual comprising administering an effective amount of a compound of
formula (I), or a
pharmaceutically acceptable salt thereof to an individual. In some embodiments
a compound of
formula (I) or a salt thereof is an A3 receptor antagonist. In some
embodiments, a compound of
formula (I) or a salt thereof reduces A3 receptor signaling by at least 10%,
20%, 30%, 40%, 50%,
60%, 70%, 80%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, or 99%. In some
embodiments, a compound of formula (I) or a salt thereof reduces A3 receptor
signaling by 40-
99%, 50-99%, 60-99%, 70-99%, 80-99%, 90-99%, or 95-99%. In some of these
embodiments, a
compound of formula (I) or a salt thereof binds to the A3 receptor with an
IC50 of less than 1
less than 900 nM, less than 800 nM, less than 700 nM, less than 600 nM, less
than 500 nM, less
than 400 nM, less than 300 nM, less than 200 nM, less than 100 nM, less than
10 nM, less than 1
nM or less than 100 pM. In some embodiments, a compound of formula (I) or a
salt thereof
binds to the A3 receptor with an IC50 of 500 nM to 100 pM, 400 nM to 100 pM,
300 nM to 100
pM, 200 nM to 100 pM, or 100 n114 to 100 pM.
101691 In some embodiments, the present invention comprises a method of
inhibiting tumor
metastasis in an individual in need thereof comprising administering a
compound of formula (I),
or a pharmaceutically acceptable salt thereof, to the individual. In some
embodiments, the
290
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
metastasis is to the lung, liver, lymph node, bone, adrenal gland, brain,
peritoneum, muscle, or
vagina. In some embodiments, a compound of formula (I) or a salt thereof
inhibits metastasis of
melanoma cells. In some embodiments, the present disclosure includes a method
of delaying
tumor metastasis comprising administering a compound of formula (I), or a
pharmaceutically
acceptable salt thereof, to the individual. In some of these embodiments, the
time to metastatic is
delayed by 1 month, 2 months 3 months, 4 months, 5 months, 6 months, 12
months, or more,
upon treatment with the compounds of the present invention.
101701 In some embodiments, a compound of formula (I) or a salt thereof is
used to treat an
individual having a proliferative disease, such as cancer as described herein.
In some
embodiments, the individual is at risk of developing a proliferative disease,
such as cancer. In
some of these embodiments, the individual is determined to be at risk of
developing cancer based
upon one or more risk factors. In some of these embodiments, the risk factor
is a family history
and/or gene associated with cancer. In some embodiments, the individual has a
cancer that
expresses a high level of a nucleotide metabolizing enzyme. In some
embodiments, the
nucleotide metabolizing enzyme is a nucleotidase, such as CD73 (ecto-5'-
nucleotidase,
Ecto5'NTase). In some of these embodiments, the individual has a cancer that
expresses a high
level of a nucleotidase, such as CD73. In any of these embodiments, the
nucleotide metabolizing
enzyme is an ecto-nucleotidase. In some embodiments, the ecto-nucleotidase
degrades
adenosine monophosphate. In some embodiments, the nucleotide metabolizing
enzyme is CD39
(ecto-nucleoside triphosphate diphosphohydrolase 1, E-NTPDase 1 ). In some of
these
embodiments, the individual has a cancer that expresses a high level of CD39.
In some
embodiments, the individual has a cancer that expresses a high level of an
adenosine receptor,
such as the A2A receptor.
Combination Therapy
10171.1 As provided herein, the presently disclosed compounds or a salt
thereof may activate the
immune system by modulating the activity of a G protein coupled receptor
signaling pathway,
for example acting as an A2A receptor antagonist, which results in significant
anti-tumor effects.
Accordingly, the present compounds or a salt thereof may be used in
combination with other
anti-cancer agents to enhance tumor immunotherapy. In some embodiments,
provided herein is a
291
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
method of treating a disease mediated by a G protein coupled receptor
signaling pathway in an
individual comprising administering an effective amount of a compound of
formula (I), or a
pharmaceutically acceptable salt thereof, and an additional therapeutic agent
to the individual. In
some embodiments, the disease mediated by a G protein coupled receptor
signaling pathway is a
proliferative disease such as cancer.
101721 In some embodiments, the additional therapeutic agent is a cancer
immunotherapy. In
some embodiments, the additional therapeutic agent is an immunostimulatory
agent. In some
embodiments, the additional therapeutic agent targets a checkpoint protein. In
some
embodiments, the additional therapeutic agent is effective to stimulate,
enhance or improve an
immune response against a tumor.
[0173] In another aspect, provided herein is a combination therapy in which a
compound of
formula (I) is coadministered (which may be separately or simultaneously) with
one or more
additional agents that are effective in stimulating immune responses to
thereby further enhance,
stimulate or upregulate immune responses in a subject. For example, provided
is a method for
stimulating an immune response in a subject comprising administering to the
subject a
compound of formula (I) or a salt thereof and one or more immunostimulatory
antibodies, such
as an anti-PD-1 antibody, an anti-PD-Li antibody and/or an anti-CTLA-4
antibody, such that an
immune response is stimulated in the subject, for example to inhibit tumor
growth. In one
embodiment, the subject is administered a compound of formula (I) or a salt
thereof and an anti-
PD-1 antibody. In another embodiment, provided is a method for stimulating an
immune
response in a subject comprising administering to the subject a compound of
formula (I) or a salt
thereof and one or more immunostimulatory antibodies or immunotherapy like
Chimeric antigen
receptor (CAR) T-cell therapy; immunostimulatory antibodies such as an anti-PD-
I antibody, an
anti-PD-Li antibody and/or an anti-CTLA-4 antibody, such that an immune
response is
stimulated in the subject, for example to inhibit tumor growth. In another
embodiment, the
subject is administered a compound of formula (1) or a salt thereof and an
anti-PD-Ll antibody.
In yet another embodiment, the subject is administered a compound of formula
(I) or a salt
thereof and an anti-CTLA-4 antibody. In another embodiment, the
immunostimulatory antibody
(e.g., anti-PD-1, anti-PD-L1 and/or anti-C'TLA-4 antibody) is a human
antibody. Alternatively,
the immunostimulatory antibody can be, for example, a chimeric or humanized
antibody (e.g.,
prepared from a mouse anti-PD-1, anti-PD-Li and/or anti-C'TLA-4 antibody). In
another
292
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
embodiment, the subject is administered a compound of formula (I) or a salt
thereof and CART-
cells (genetically modified T cells).
[0174] In one embodiment, the present disclosure provides a method for
treating a proliferative
disease (e.g., cancer), comprising administering a compound of formula (I) or
a salt thereof and
an anti-PD-1 antibody to a subject. In further embodiments, a compound of
formula (1) or a salt
thereof is administered at a subtherapeutic dose, the anti-PD-1 antibody is
administered at a
subtherapeutic dose, or both are administered at a subtherapeutic dose. In
another embodiment,
the present disclosure provides a method for altering an adverse event
associated with treatment
of a hyperproliferative disease with an immunostimulatory agent, comprising
administering a
compound of formula (I) or a salt thereof and a subtherapeutic dose of anti-PD-
1 antibody to a
subject. In certain embodiments, the subject is human. In certain embodiments,
the anti-PD-1
antibody is a human sequence monoclonal antibody
[0175] In one embodiment, the present invention provides a method for treating
a
hyperproliferative disease (e.g., cancer), comprising administering a compound
of formula (I) or
a salt thereof and an anti-PD-Li antibody to a subject. In further
embodiments, a compound of
formula (I) or a salt thereof is administered at a subtherapeutic dose, the
anti-PD-L1 antibody is
administered at a subtherapeutic dose, or both are administered at a
subtherapeutic dose. In
another embodiment, the present invention provides a method for altering an
adverse event
associated with treatment of a hyperproliferative disease with an
immunostimulatory agent,
comprising administering a compound of formula (I) or a salt thereof and a
subtherapeutic dose
of anti-PD-Li antibody to a subject. In certain embodiments, the subject is
human. In certain
embodiments, the anti-PD-Li antibody is a human sequence monoclonal antibody.
[0176] In certain embodiments, the combination of therapeutic agents discussed
herein can be
administered concurrently as a single composition in a pharmaceutically
acceptable carrier, or
concurrently as separate compositions each in a pharmaceutically acceptable
carrier. In another
embodiment, the combination of therapeutic agents can be administered
sequentially. For
example, an anti-CTLA-4 antibody and a compound of formula (I) or a salt
thereof can be
administered sequentially, such as anti-CTLA-4 antibody being administered
first and a
compound of formula (I) or a salt thereof second, or a compound of formula (1)
or a salt thereof
being administered first and anti-CTLA-4 antibody second. Additionally or
alternatively, an anti-
PD-1 antibody and a compound of formula (I) or a salt thereof can be
administered sequentially,
293
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
such as anti-PD-1 antibody being administered first and a compound of formula
(1) or a salt
thereof second, or a compound of formula (I) or a salt thereof being
administered first and anti-
PD-1 antibody second. Additionally or alternatively, an anti-PD-Li antibody
and a compound of
formula (I) or a salt thereof can be administered sequentially, such as anti-
PD-L1 antibody being
administered first and a compound of formula (I) or a salt thereof second, or
a compound of
formula (I) or a salt thereof being administered first and anti-PD-Li antibody
second.
[0177] Furthermore, if more than one dose of the combination therapy is
administered
sequentially, the order of the sequential administration can be reversed or
kept in the same order
at each time point of administration, sequential administrations can be
combined with concurrent
administrations, or any combination thereof
[0178] Optionally, the combination of a compound of formula (I) or a salt
thereof can be further
combined with an immunogenic agent, such as cancerous cells, purified tumor
antigens
(including recombinant proteins, peptides, and carbohydrate molecules), cells,
and cells
transfected with genes encoding immune stimulating cytokines.
[0179] A compound of formula (I) or a salt thereof can also be further
combined with standard
cancer treatments. For example, a compound of formula (I) or a salt thereof
can be effectively
combined with chemotherapeutic regimes. In these instances, it is possible to
reduce the dose of
other chemotherapeutic reagent administered with the combination of the
instant disclosure
(Mokyr et al. (1998) Cancer Research 58: 5301-5304). Other combination
therapies with a
compound of formula (I) or a salt thereof include radiation, surgery, or
hormone deprivation.
Angiogenesis inhibitors can also be combined with a compound of formula (I) or
a salt thereof
Inhibition of angiogenesis leads to tumor cell death, which can be a source of
tumor antigen fed
into host antigen presentation pathways.
[0180] In another example, a compound of formula (1) or a salt thereof can be
used in
conjunction with anti-neoplastic antibodies. By way of example and not wishing
to be bound by
theory, treatment with an anti-cancer antibody or an anti-cancer antibody
conjugated to a toxin
can lead to cancer cell death (e.g., tumor cells) which would potentiate an
immune response
mediated by CTLA-4, PD-1, PD-Li or a compound of formula (I) or a salt
thereof. In an
exemplary embodiment, a treatment of a hyperproliferative disease (e.g., a
cancer tumor) can
include an anti-cancer antibody in combination with a compound of formula (I)
or a salt thereof
and anti-CTLA-4 and/or anti-PD-1 and/or anti-PD-Li antibodies, concurrently or
sequentially or
294
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
any combination thereof, which can potentiate anti-tumor immune responses by
the host. Other
antibodies that can be used to activate host immune responsiveness can be
further used in
combination with a compound of formula (I) or a salt thereof.
[0181] In some embodiments, a compound of formula (I) or a salt thereof can be
combined with
an anti-CD73 therapy, such as an anti-CD73 antibody.
[0182] In some embodiments, a compound of formula a) or a salt thereof can be
combined with
an anti-CD39 therapy, such as an anti-CD39 antibody.
[0183] In yet further embodiments, a compound of formula (I) or a salt thereof
is administered in
combination another G protein receptor antagonist, such as an adenosine Al
and/or A3
antagonist.
Dosing and Method clAdministration
101841 The dose of a compound administered to an individual (such as a human)
may vary with
the particular compound or salt thereof, the method of administration, and the
particular disease,
such as type and stage of cancer, being treated. In some embodiments, the
amount of the
compound or salt thereof is a therapeutically effective amount.
[0185] The effective amount of the compound may in one aspect be a dose of
between about
0.01 and about 100 mg/kg. Effective amounts or doses of the compounds of the
invention may
be ascertained by routine methods, such as modeling, dose escalation, or
clinical trials, taking
into account routine factors, e.g., the mode or route of administration or
drug delivery, the
pharmacokinetics of the agent, the severity and course of the disease to be
treated, the subject's
health status, condition, and weight. An exemplary dose is in the range of
about from about 0.7
mg to 7 g daily, or about 7 mg to 350 mg daily, or about 350 mg to 1.75 g
daily, or about 1.75 to
7 g daily.
[0186] Any of the methods provided herein may in one aspect comprise
administering to an
individual a pharmaceutical composition that contains an effective amount of a
compound
provided herein or a salt thereof and a pharmaceutically acceptable excipient.
[0187] A compound or composition of the invention may be administered to an
individual in
accordance with an effective dosing regimen for a desired period of time or
duration, such as at
least about one month, at least about 2 months, at least about 3 months, at
least about 6 months,
or at least about 12 months or longer, which in some variations may be for the
duration of the
295
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
individual's life. In one variation, the compound is administered on a daily
or intermittent
schedule. The compound can be administered to an individual continuously (for
example, at least
once daily) over a period of time. The dosing frequency can also be less than
once daily, e.g.,
about a once weekly dosing. The dosing frequency can be more than once daily,
e.g., twice or
three times daily. The dosing frequency can also be intermittent, including a
'drug holiday' (e.g.,
once daily dosing for 7 days followed by no doses for 7 days, repeated for any
14 day time
period, such as about 2 months, about 4 months, about 6 months or more). Any
of the dosing
frequencies can employ any of the compounds described herein together with any
of the dosages
described herein.
[0188] The compounds provided herein or a salt thereof may be administered to
an individual
via various routes, including, e.g., intravenous, intramuscular, subcutaneous,
oral and
transdermal. A compound provided herein can be administered frequently at low
doses, known
as 'metronomic therapy,' or as part of a maintenance therapy using compound
alone or in
combination with one or more additional drugs. Metronomic therapy or
maintenance therapy
can comprise administration of a compound provided herein in cycles.
Metronomic therapy or
maintenance therapy can comprise intra-tumoral administration of a compound
provided herein.
[0189] In one aspect, the invention provides a method of treating cancer in an
individual by
parenterally administering to the individual (e.g., a human) an effective
amount of a compound
or salt thereof. In some embodiments, the route of administration is
intravenous, intra-arterial,
intramuscular, or subcutaneous. In some embodiments, the route of
administration is oral. In still
other embodiments, the route of administration is transdermal.
[0190] The invention also provides compositions (including pharmaceutical
compositions) as
described herein for the use in treating, preventing, and/or delaying the
onset and/or development
of cancer and other methods described herein. In certain embodiments, the
composition
comprises a pharmaceutical formulation which is present in a unit dosage form.
[0191] Also provided are articles of manufacture comprising a compound of the
disclosure or a
salt thereof, composition, and unit dosages described herein in suitable
packaging for use in the
methods described herein. Suitable packaging is known in the art and includes,
for example,
vials, vessels, ampules, bottles, jars, flexible packaging and the like. An
article of manufacture
may further be sterilized and/or sealed.
296
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
Kits
[0192] The present disclosure further provides kits for carrying out the
methods of the invention,
which comprises one or more compounds described herein or a composition
comprising a
compound described herein. The kits may employ any of the compounds disclosed
herein. In
one variation, the kit employs a compound described herein or a
pharmaceutically acceptable salt
thereof. The kits may be used for any one or more of the uses described
herein, and,
accordingly, may contain instructions for the treatment of cancer.
101931 Kits generally comprise suitable packaging. The kits may comprise one
or more
containers comprising any compound described herein. Each component (if there
is more than
one component) can be packaged in separate containers or some components can
be combined in
one container where cross-reactivity and shelf life permit.
[0194] The kits may be in unit dosage forms, bulk packages (e.g., multi-dose
packages) or sub-
unit doses. For example, kits may be provided that contain sufficient dosages
of a compound as
disclosed herein and/or a second pharmaceutically active compound useful for a
disease detailed
herein (e.g., hypertension) to provide effective treatment of an individual
for an extended period,
such as any of a week, 2 weeks, 3 weeks, 4 weeks, 6 weeks, 8 weeks, 3 months,
4 months, 5
months, 7 months, 8 months, 9 months, or more. Kits may also include multiple
unit doses of
the compounds and instructions for use and be packaged in quantities
sufficient for storage and
use in pharmacies (e.g., hospital pharmacies and compounding pharmacies).
[0195] The kits may optionally include a set of instructions, generally
written instructions,
although electronic storage media (e.g., magnetic diskette or optical disk)
containing instructions
are also acceptable, relating to the use of component(s) of the methods of the
present invention.
The instructions included with the kit generally include information as to the
components and
their administration to an individual.
[0196] The invention can be further understood by reference to the following
examples, which
are provided by way of illustration and are not meant to be limiting.
297
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
EXAMPLES
Synthetic Examples
Example Si. Synthesis of 4-(5-amino-3-phenylpyrazin-2-A-2-chlomphenol
(Compound No.
1.1)
cos o
B'
Step-1 40 9 B.OH H Step-3 Is HO
Step-2
N BS DMF Br I N1 CI
I OH
CI N NH, N NH2 RT, 18 h :1/41 tu 3.4
N NH;
- Pd(OAc)2. K3PO4 101 ,,..2 .. pd(pph3)4
dppt, Dioxane Na2CO3
100 deg C, 18 h Dioxane-water
(5:1)
80 deg C, 18 h
[0197] Step-1 Synthesis of 6-phenylpyrazin-2-amine: To a solution of 6-
chloropyrazin-2-
amine (1.00 g, 7.75 minol, 1 eq.) in 1,4-dioxane (30 mL) was added
phenylboronic acid (1.42g.
11.62 mmol, 1.5 eq.), K3PO4 (3.286 g, 15.50 mmol, 2 eq.), Pd(OAc)2 ( 0.086 g,
0.38 mmol, 0.05
eq.), 1,1'-bis(diphenylphosphino)ferrocene (0.214 g, 0.38 mmol, 0.05 eq.). The
reaction mixture
was deoxygenated using N2 atmosphere and the reaction mixture was heated at
100 C overnight
The reaction was monitored by TLC and LCMS and found to be complete after 18
h. The
reaction mixture was cooled to RT, filtered through Celite-bed and washed with
ethyl acetate (2
x 20 mL). The reaction mixture was diluted with water (50 mL) and extracted
with ethyl acetate
(2 x 50 mL). The separated organic layer was dried over sodium sulfate and
concentrated under
reduced pressure. The crude product was purified by Combif lash on silica gel
using CH3OH-
CH2C12 system as eluent to afford 1.10 g (63%) of 6-phenylpyrazin-2-amine.
LCtivIS: 172
[M-1-1]'.
[0198] Step-2: Synthesis of 5-bromo-6-phenylpyrazin-2-amine: To a solution of
6-
phenylpyrazin-2-amine (0.150 g, 0.877 mmol, 1 eq.) in DMF (3 mL) was added N-
bromosuccinimide (0.156g. 0.877 mmol, 1 eq.) and the reaction mixture was
stirred at RT for 1
h. The reaction was monitored by TLC and NMR After completion, the reaction
mixture was
diluted with water (50 mL) and extracted by ethyl acetate (2 x 20 mL).
Combined organic layer
was washed with water (5 x 20 mL) followed by brine and dried over anhydrous
sodium sulfate.
The solvent was evaporated under reduced pressure to get the crude product
which was purified
298
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
by CombiFlash on silica gel using Et0Ac-Hexane system as eluent to afford 100
mg (46%) of 5-
bromo-6-phenylpyrazin-2-amine. LC/MS: 251 [Wit
101991 Step-3: Synthesis of 4-(5-amino-3-phenylpyrazin-2-y1)-2-ehlorophenol:
To a solution
of 5-bromo-6-phenylpyrazin-2-amine (100 mg, 0.4 mmol, 1 eq.) in 1,4-dioxane-
water (6 mL,
5:1) was added 2-chloro-4-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-yl)phenol
I (121 mg, 0.48
mmol, 1.2 eq.), Na2CO3 (84 mg, 0.8 mmol, 2eq.), Pd(PPh3)4 ( 23 mg, 0.02 mmol,
0.05 eq.). The
reaction mixture was deoxygenated using N2 atmosphere and the reaction mixture
was allowed
to stir at 80 C overnight. The reaction was monitored by NMR and LCMS and
found to be
complete after 18 h. The reaction mixture was cooled to RT, diluted with water
(50 mL) and
extracted with Et0Ac (3 x 50 mL). Combined organic layer was washed with brine
(50 mL) and
dried over sodium sulfate. Removal of solvent under reduced pressure gave
crude which was
purified by SFC to afford 20 mg (17% ) of 445-amino-3-phenyipyrazin-2-y1)-2-
chlorophenol.
1..C/MS: 298 [M-1-11'. NMR (400 MHz, DMSO-d6) 67.90 (s, 1H), 7.45 (s, 4H),
7.20 (s, 1H),
6.90 (d, 1H), 7.78 (d, 1H), 6.58 (s, 2H).
[0200] Reference: W02014/209034 Al.
Example 52. Synthesis of 4-(5-amino-3-phenylpyrazin-2-y1)-2,6-dichlorophenol
(Compound No.
1.2)
t
0, 0
CI
01111 HO
CI Br N
CI
OH CI
N NH2 Pd(PPh3)4 N NH2
Na2CO3
Dioxane-water (4:1)
80 deg C, 18 h
[0201] To a solution of 5-bromo-6-phenylpyrazin-2-amine (100 mg, 0.40 mmol, 1
eq.) in 1,4-
dioxane-water (10 mL, 4:1) was added 2,6-dichloro-4-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)phenol (174 mg, 0.48 mmol, 1.2 eq.), Cs2CO3 (391 mg, 1.20 mmol, 3.0 eq.),
PdC12(dppf)CH2C12 complex (32 mg, 0.04 mmol, 0.05 eq.). The reaction mixture
was
299
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
deoxygenated using N2 atmosphere and the reaction mixture was allowed to stir
at 80 C
overnight The progress of reaction was monitored by TLC and LCMS and found to
be complete
after 18 h. The reaction mixture was cooled to RT, diluted with water (50 mL)
and extracted
using ethyl acetate (3 x 50 mL). Combined organic layer was washed with brine
(50 mL) and
dried over sodium sulfate. Removal of solvent under reduced pressure gave
crude which was
purified by SFC to afford 15 mg (11%) of 4-(5-amino-3-phenylpyrazin-2-yI)-2,6-
dichlorophenol.
LCMS: 332 [M+1]. Ili NMR (400 MHz, DMSO-d6) 86.28 (s, 2H), 6.64 (s, 1H), 7.10
(s, 211),
7.38 (s, 414), 7.90 (s, 114).
Example S3. Synthesis 61 N-(5-(3-chloro-4-hydroxypheny1)-6-phenylpyrazin-2-
yl)acetamide.
(Compound No. 1.3)
CI CI
HO 0 HO
0
N
I N's"' 0
, ==:,
N-----NH2 _________ DCM, Pyridine N N,Ic
H
[0202] To a solution of 4-(5-amino-3-phenylpyrazin-2-y1)-2-chlorophenol (200
mg, 0.67 mmol,
1.0 eq) in CH2C12 (20 mL) was added pyridine (79 mg, 1.01 mmol, 1.5 eq) and
acetyl chloride
(78 mg, 1.01 mmol, 1.5 eq). The reaction mixture was allowed to stir at room
temperature
overnight Progress of reaction was monitored by mc and LCMS and found to be
complete
after for 18 h. The reaction mixture was diluted with water (20 mL) and
extracted with ethyl
acetate (3 x 30 mL). The combined organic layer was washed with brine (30 mL)
and dried over
sodium sulfate. Removal of solvent under reduced pressure gave crude which was
purified by
reversed phase column chromatography to afford 10 mg (4%) of N-(5-(3-chloro-4-
hydroxypheny1)-6-phenylpyrazin-2-yl)acetamide.
LCMS: 340 [M+1].
Example S4. Synthesis of 5-(1H-indo1-5-y1)-6-phenylpyrazin-2-amine (Compound
No 1.4)
300
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
0 HN
Br N
N
1
N NH2 Na2CO3/Pd(PPh3)4 -)q NH2
dioxane/H20
(02031 To a solution of 5-bromo-6-phenylpyrazin-2-amine (100 mg, 0.4 mmol, 1
eq.) in 1,4
dioxane (5 mL): water (1 mL) was added 5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)-1H-
indole (77 mg, 0.48 mmol, 1.2 eq.), Na2CO3 (84 mg, 0.8 mmol, 2eq.), Pd(PPh3)4
( 23 mg, 0.02
mmol, 0.05 eq.). The reaction mixture was deoxygenated using N2 atmosphere and
the reaction
mixture was heated at 80 C for 18 h. The reaction was monitored by NMR and
LCMS. The
reaction mixture was diluted with water (50 mL) and extracted using ethyl
acetate (2 x 50 mL).
The separated organic layer was dried over sodium sulfate and concentrated
under reduced
pressure. The crude product was purified by Supercritical Fluid Chromatography
to afford 546-
amino-3-pyridy1)-6-phenyl-pyrazin-2-amine (20mg, 17.8%) as white solid.
LCMS: 287.1 (M+1)+.
111NMR (400 MHz, DMSO-d6) 11.00 s(1H), 7.98 s(1H), 7.62 s(1H), 7.59-7.18
m(7H),
6.95d(1H), 6.40 d(2H), 6.35 s(1H).
Example Si. Synthesis of 4-(5-amino-3-phenylpyrazin-2-y1)-2-tert-butylphenol.
(Compound No.
1.6)
B HO
Br N OH
,
1
401 Pd(PPh3)4 N NH2
Na2CO3
Dioxane-water (5:1)
80 deg C. 18 h
301
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
102041 To a solution of 5-bromo-6-phenylpyrazin-2-amine (100 mg, 0.4 mmol, 1
eq.) in 1,4-
dioxane-water (6 mL, 5:1) was added 2-tert-buty1-4-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)phenol (132 mg, 0.48 mmol, 1.2 eq.), Na2CO3 (84 mg, 0.8 mmol, 2eq.),
(PdC12(PPh3)2 ( 14
mg, 0.02 mmol, 0.05 eq.). The reaction mixture was deoxygenated with N2 and
the reaction
mixture was stirred at 80 C overnight The reaction was monitored by NMR and
LCMS and
found to be complete after 18 h. The reaction mixture was cooled to RT,
diluted with water (50
mL) and extracted with ethyl acetate (2 x 50 mL). Combined organic layer was
washed with
brine (20 mL) and dried over sodium sulfate. Removal of solvent under reduced
pressure gave
crude which was purified by SFC to afford 4-(5-amino-3-phenyl-pyrazin-2-y1)-2-
tert-butyl-
phenol (20 mg, 15.7%) as white solid.
LCMS: 320.2 [M+1]. '11NMR (400 MHz, DMSO-d6) 9.36 (s, 1H), 7.95 (s, 1H), 7.40-
7.23 (m,
5H), 7.15 (d, 1H), 6.80 (s, 1H), 6.63 (d, 1H), 6.40 (s, 2H), 1.03(s, 9H).
Example S6. Synthesis of 4-(5-amino-3-phenylpyrazin-2-yl)phenol. (Compound No.
1.7)
0
,7c\ ...B
0 = HO
Br N OH
401
N NF12 Pd(PPh3)4 N NH2
Na2CO3
Dioxane-water (5:1)
80 deg C, 18h
102051 To a solution of 5-bromo-6-phenylpyrazin-2-amine (100 mg, 0.4 mmol, 1
eq.) in 1,4-
dioxane-water (6 mL, 5:1) was added 4-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-
2-yl)phenol
(105.6 mg, 0.48 mmol, 1.2 eq.), Na2CO3 (84 mg, 0.8 mmol, 2eq.), PdC12(PPh3)2 (
15 mg, 0.02
mmol, 0.05 eq.). The reaction mixture was deoxygenated with N2 and the
reaction mixture was
stirred at 80 C overnight. The reaction was monitored by NMR and LCMS and
found to be
complete after 18 h. The reaction mixture was cooled to RT, diluted with water
(50 mL) and
extracted with ethyl acetate (2 x 50 mL). Combined organic layer was washed
with brine (20
mL) and dried over sodium sulfate. Removal of solvent under reduced pressure
gave crude
302
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
which was purified by SFC to afford 4-(5-amino-3-phenyl-pyrazin-2-y1) phenol
(35 mg, 33.33%)
as white solid.
LCMS: 264.1 [M+1]+. 114 NMR (400 MHz, DMSO-d6): 8 9.40 (brs, 1H), 7.98 (s,
1H), 7.40-7.20
(m, 5H), 7.00 (d, 2H), 6.60 (d, 2H), 6.20 (s, 1H).
Example S7. Synthesis of -1-(5-amino-3-(firran-2-yl)pyrazin-2-y1)-2-
chlorophenol. (Compound
No. 1.8)
OH
so ci
CI
________________ STEP 1 STEP 3
STEP 2
,OH 0B.0
0 B HO
NBS, DMF, PT Br N, N
I ClN.'N H2 Pd(PPN r.I
eNIN H2 DP 4: xPaPnhe3, 41120 \ rsNH2
________________ 4 N NH2 ______
I;?N'A
Toluene, H20 Na2CO3, 80 deg 0
Ethanol, 80 deg 18 h 18h
102061 Step-1 Synthesis of 6-(furan-2-yl)pyrazin-2-amine: To a solution of 6-
chloropyrazin-2-
amine (1.00 g, 7.75 mmol, 1 eq.) in a solution of toluene and ethanol (20 mL,
1:1) was added
furan-2-ylboronic acid (0.955 g, 8.52 mmol, 1.1 eq.), Na2CO3 (1.479 g, 13.95
mmol, 1.8 eq.) in
H20 (10 mL), Pd(PPh3)4 (0.223 g, 0.193 mmol, 0.025 eq.). The reaction mixture
was
deoxygenated using N2 atmosphere and the reaction mixture was heated at 100 C
for 18 h. The
reaction was monitored by NMR The reaction mixture was filtered through Celite
with wash of
ethyl acetate (2 x 20 mL). The reaction mixture was diluted with water (50 mL)
and extracted
using ethyl acetate (2 x 50 mL). The separated organic layer was dried over
sodium sulfate and
concentrated under reduced pressure. The crude product was purified by
CombiFlash on silica
gel to afford 1 .00 g (80%) of 6-(furan-2-yl)pyrazin-2-amine.
[0207] Step-2 Synthesis of 5-bromo-6-(furan-2-yl)pyrazin-2-amine: To a
solution of 6-(furan-
2-yl)pyrazin-2-amine (0.200 g, 1.24 mmol, 1 eq.) in DMF (4 mL) was added N-
bromosuccinimide (0.222 g, 1.24 mmol, 1 eq.). The reaction mixture was stirred
at room
temperature for 2 h. The reaction was monitored by TLC and NMR. The reaction
mixture was
diluted with water (50 mL) and extracted by ethyl acetate (2 x 20 mL). The
organic layer was
303
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
separated, washed with water (5 x 20 mL) followed by brine and dried over
anhydrous sodium
sulfate. The solvent was evaporated under reduced pressure to get the crude
product. The crude
product was purified by CombiFlash on silica gel to afford 70 mg (30%) of 5-
bromo-6-(furan-2-
yl)pyrazin-2-amine.
102081 Step-3 Synthesis of 4-(5-arnino-:Hfuran-2-yi)pyrazin-2-y1)-2-
chlorophenol: To a
solution of 5-bromo-6-(furan-2-yl)pyrazin-2-amine (70 mg, 0.29 mmol, 1 eq.) in
a solution of
1,4-dioxane and water (7 mL, 6:1) was added 2-chloro-4-(4,4,5,5-tetramethy1-
1,3,2-
dioxaborolan-2-yl)phenol (89 mg, 0.35 mmol, 1.2 eq.), Na2CO3 (62 mg, 0.585
mmol, 2 eq.),
Pd(PPh3)4( 8 mg, 0.007 mmol, 0.025 eq.). The reaction mixture was deoxygenated
using N2
atmosphere and the reaction mixture was heated at 80 C for 18 h. The reaction
was monitored by
NMR and LCMS. The reaction mixture was diluted with water (50 mL) and
extracted using ethyl
acetate (2 x 50 mL). The separated organic layer was dried over sodium sulfate
and concentrated
under reduced pressure. The crude product was purified by SFC to afford 30 mg
(36%) of 4-(5-
ainino-3-(furan-2-yl)pyrazin-2-y1)-2-chlorophenol.
LCMS: 288 [M-F1]1H NMR (400 MHz, CD30D) 8 6.42 (d, 1H), 6.50 (d, 1H), 6.60-
6.64 (m,
2H), 6.90(d, 1H), 7.00-7.02 (d, 1H), 7.20 (d, 1H), 6.60-6.62 (s, 1H), 7.85 (s,
1H), 10.20( bs, 1H).
Example SS. Synthesis of 5-(2-methyl-1H-benzo[dlimidazol-5-y1)-6-phenylpyrazin-
2-amine
(compound 1.9)
0
r
HN
Br N N
N
-:.--
N---'..'NH2 Pd(PPh3)2 C12, Na2CO3 I
0
Dioxane-water (5:1)
0 N --'-''NH2
90 C, 18 h
[0209] To a solution of 5-bromo-6-phenylpyrazin-2-amine (100 mg, 0.4 mmol, 1
eq.) in 1,4-
dioxane-water (6 mL, 5:1) was added 2-methy1-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)-
1H-benzo[d]imidazole (124mg, 0.48 mmol, 1.2 eq.), Na2CO3 (84 mg, 0.8 mmol,
2eq.) and
PdC12(PPh3)2 ( 14 mg, 0.02 mmol, 0.05 eq.). The reaction mixture was
deoxygenated with N2
304
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
and the reaction mixture was stirred at 90 C overnight. The reaction was
monitored by NMR
and LCMS and found to be complete after 18 h. The reaction mixture was cooled
to RT, diluted
with water (50 mL) and extracted with ethyl acetate (2 x 50 mL). Combined
organic layers were
washed with brine (20 mL) and dried over sodium sulfate. Removal of solvent
under reduced
pressure gave crude which was purified by SEC to afford the desired product as
white solid. (20
mg, 16.6%)
LCMS: 302.0 [M+1]+
1H NMR (400 MHz, DMSO-d6) d 12.08 (d, J = 18.42 Hz, 1H), 7.94 (s, 1H), 7.30-
7.25(m, 614),
6.96 - 7.10 (m, 1H), 6.49 (d, J = 9.65 Hz, 214), 2.43 (s, 314)
Example S9. S)inthesis of 5-(1H-benzo[dlimidazol-6-y9-6-phenylpyrazin-2-amine.
(Compound
No. 1.10)
0
/N
Br N ,
NNH2 Pd(PFt3)4 N NH2
Na2CO3
Dioxane-water (5:1)
80 deg C. 18 h
(02101 To a solution of 5-bromo-6-phenylpyrazin-2-amine (100 mg, 0.4 mmol, 1
eq.) in 1,4-
dioxane-water (6 mL, 5:1) was added 5-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-
2-y1)-1H-
benzimidazole (124 mg, 0.48 mmol, 1.2 eq.), Na2CO3 (84 mg, 0.8 mmol, 2eq.),
(PdC12(PPh)2
(14 mg, 0.02 mmol, 0.05 eq.). The reaction mixture was deoxygenated with N2
and the reaction
mixture was stirred at 80 C overnight The reaction was monitored by NMR and
LCMS and
found to be complete after 18 h. The reaction mixture was cooled to RT,
diluted with water (50
mL) and extracted with ethyl acetate (2 x 50 mL). Combined organic layer was
washed with
brine (20 mL) and dried over sodium sulfate. Removal of solvent under reduced
pressure gave
crude which was purified by Reverse phase column chromatography to afford 5-
(311-
benzimidazol-5-y0-6-phenyl-pyrazin-2-amine (20 mg, 18.18%) as white solid.
305
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
LCMS: 288.1 [M+1]+. 1HNMR (400 MHz, DMSO-d6) 12.40(s, 1H), 8.18 (s, 1H), 8.00
(s, 1H),
7.65 (s, 1H), 7.40-7.20 (m, 5H), 7.00 (s, 1H), 6.50 (s, 2H).
Example Si a Synthesis of 5-(5-amino-3-phenylpyrazin-2-y1)-2-
hydroxybenzonitrile. Compound
No. 1.11)
NC ON
HO = 13/\ - HO
Br N
Pd(PPh3)4,
Na2003.Dioxane:Water i\l"- NH2
18 h, 80 deg
[02111 To a solution of 4-benzy1-5-bromopyrimidin-2-amine (100 mg, 0.40 mmol,
1 eq.) in 1,4-
dioxane-water (10 mL, 5:1) was added 2-hydroxy-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
yl)benzonitrile (107 mg, 0.44 mmol, 1.2 eq.), Na2CO3 (84 mg, 0.80 mmol, 2.0
eq.), Pd(PPh3)4
(11 mg, 0.01 mmol, 0.025 eq.). The reaction mixture was deoxygenated using N2
atmosphere and
the reaction mixture was heated at 90 C for 18 h. The reaction was monitored
by NMR and
LCMS. The reaction mixture was diluted with water (50 mL) and extracted using
ethyl acetate (2
x 50 mL). The separated organic layer was dried over sodium sulfate and
concentrated under
reduced pressure. The crude product was purified by CombiFlash on silica gel
to afford 10 mg
(11 110) of 5-(5-amino-3-phenylpyrazin-2-0-2-hydroxybenzonitrile.
LCMS: 289 [M+1]+. 111 NMR (400 MHz, CD30D) 8 6.80 (d, 1H), 7.32-7.50 (m, 7H),
8.20 (s, 1H).
Example 511. Synthesis of 6-phenyl-5-(quinolin-6-yl)pyrazin-2-amine. (Compound
No. 1.12)
B,.
OH
Br N OH
,
,
N-----****-NH Pd(dppf)C12.DCM, 2M aq. N NH2
2 Na2CO3
90 C . 4h, Dioxane
[0212] To a stirred solution of quinolin-6-ylboronic acid (0.100g, 0.57 mmol,
1.2 equiv) and 5-
bromo-6-phenylpyrazin-2-amine (0.120 g, 0.48 mmol, 1.0 equiv) in dioxane (3
mL) was added
306
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
2M aqueous Na2CO3 (0.101 g, 0.96 mmol, 2.0 equiv, 0.5 mL). The reaction was
purged with N2
for 5 min. To this reaction mixture was added Pd(dppf)C12=DCM (0.020 g, 5 mol
%) and N2 was
purged again for 5 more mins. The reaction mixture was heated at 90 C for 4h.
The reaction
mixture was allowed to cool to RT and extracted using ethyl acetate (2 x 35
mL). The combined
organic layers were washed (brine), dried (anhydrous Na2SO4) and concentrated
under vacuum
to get the solid residue which was purified by normal phase silica gel flash
column
chromatography to get the desired product as off white solid (0.030 g, 21%)
LCMS: 299 (M-F1). NMR (400 MHz, DMSO-d6) 58.84 (d, J = 2.93 Hz, 1H), 8.22
(d, J =
8.31 Hz, 1H), 8.01 (s, 1H), 7.92 (s, 1H), 7.80 (d, J = 8.80 Hz, 1H), 7.42 -
7.60 (m, 2H), 7.20 -
7.41 (m, 4H), 6.73 (s, 2H).
Example S12. Synthesis of 5-(7-chloro-1H-benzo[d]imidazol-5-y1)-6-
phenylpyrazin-2-amine.
(Compound No. 1.13)
N 0
HN 4,06
Br N b. N
I CI CI
I 1
PdC12(dppf)DCM complex ,
Na2CO3, H20 N NH2
1,4-Dioxane,
100 deg 18h
102131 To a solution of 5-bromo-6-phenylpyrazin-2-amine (120 mg, 0.48 mmol, 1
eq.) in 1,4-
dioxane (8 mL): water (2 mL) was added 7-chloro-5-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-
y1)-1H-benzo[d]imidazole (146 mg, 0.52 mmol, 1.2 eq.), Na2CO3 (101 mg, 0.96
mmol, 2 eq.),
PdC12(dPPO=DCM complex ( 19 mg, 0.024 mmol, 0.05 eq.). The reaction mixture
was
deoxygenated using N2 atmosphere and the reaction mixture was heated at 80 C
for 18 h. The
reaction was monitored by NMR and LCMS. The reaction mixture was diluted with
water (30
mL) and extracted using ethyl acetate (2 x 50 mL). The separated organic layer
was dried over
sodium sulfate and concentrated under reduced pressure. The crude product was
purified by
reverse phase column chromatography to afford 15 mg (8%) of 5-(7-chloro-lii-
benzo[d]imidazol-5-y1)-6-phenylpyrazin-2-amine.
307
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
LCMS: 322 [M+1]+. NMR (400 MHz, DMSO) 5 6.60-6.80 (bs, 2H) 7.22-7.38 (m, 6H),
7.40
(s, 1H), 7.98 (s, 1H), 8.68 (bs, 1H).
Example S13. Synthesis of 3-bromo-6-phenyl-5-(quinolin-6-yl)pyrazin-2-amine.
(Compound No.
1.14)
NBS/AGN N Br
NNH2 RT 13 min N NH,
102141 To a solution of 6-phenyl-5-(quinolin-6-yl)pyrazin-2-amine (20 mg,
0.068 mmol, 1 eq.)
in acetonitrile (12 mL) at room temperature was added N-bromosuccinimide (12
mg, 0.068
mmol, 1 eq.) portion wise and the reaction mixture was allowed to stir at room
temperature.
Progress of reaction was monitored by TLC and was found to be complete after
13 minutes.
Reaction mixture was diluted with water and extracted with ethyl acetate (3 x
20 mL). Combined
organic layer was washed with water (3 x 20 mL) and dried over anhydrous
sodium sulfate.
Removal of solvent gave crude which was purified by reversed phase HPLC to
give 10 mg
(40%) 3-bromo-6-phenyl-5-(quinolin-6-yl)pyrazin-2-amine.
LCMS: 377 [M+1]+. NMR (400 MHz, DMSO-d6) 5 7.05 (brs, 2H), 7.25-7.40 (m, 5H),
7.45-
7.55 (m, 2H), 7.62 (d, 1H), 7.97 (s, 1H), 8.30 (d, 1H), 8.87 (s, 1H).
308
CA 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
Example SI4. Synthesis of 6-(4:/luoropheny1)-5-(quinolin-6-y1)pyrazin-2-amine.
(Compound No.
1.15)
OH
Bs.
Si OH
, Br
NBS, DMF
__________________________ ,
Br-7====NH2 PdC12(dppf)DCM complex, N.:.::NH2 RI, 2 h N NH2
STEP 1 Na2CO3 F STEP 2
Dioxane
OH
Api 'OH
Pd(PPh3)4,Na2CO3,
N NH,
Dioxane, 100 deg
48h.
STEP 3
[0215] Step 1: Synthesis of 6-(4-fluorophenyl)pyrazht-2-amine: To a solution
of 6-
bromopyrazin-2-amine (1.00 g, 5.780 mmol, 1 eq.) in 1,4 dioxane (20 mL) was
added 4-
fluorophenylboronic acid (1.213 g, 8.67 mmol, 1.5 eq.), Na2CO3 (1.225 g, 11.56
mmol, 2 eq.)
and PdC12(dppf).DCM complex ( 0.235 g, 0.289 mmol, 0.05 eq.). The reaction
mixture was
deoxygenated using N2 atmosphere and the reaction mixture was heated at 100 C
for 48 h. The
reaction was monitored by NMR. The reaction mixture was filtered through
Celite with wash of
ethyl acetate (2 x 50 mL). The reaction mixture was diluted with water (50 mL)
and extracted
using ethyl acetate (2 x 50 mL). The separated organic layer was dried over
sodium sulfate and
concentrated under reduced pressure. The crude product was purified by Combi-
Flash column
chromatography to afford 550 mg (50%) of 6-(4-fluorophenyl)pyrazin-2-a.mine.
[0216] Step 2: Synthesis of 5-bromo-6-(4-fluorophenyl)pyrazin-2-amine: To a
solution of 6-
(4-fluorophenyl)pyrazin-2-amine (0.530 g, 2.80 mmol, 1 eq.) in mixture of DMF
(5 mL) was
added N-bromosuccinimide (0.549 g, 3.08 mmol, 1 eq.). The reaction mixture was
stirred at
room temperature for 1 h. The reaction was monitored by TLC and NMR. The
reaction was
diluted with water (50 mL) and extracted by ethyl acetate (2 x 50 mL). The
organic layer was
separated, washed water (5 x 50 mL) and brine and dried over anhydrous sodium
sulfate. The
solvent was evaporated under reduced pressure to get the crude product. The
crude product was
309
Ch 03070273 2020-01-16
WO 2019/018584
PCT/US2018/042777
purified by Combi-Flash column chromatography to afford 250 mg (32%) of 5-
bromo-6-(4-
fluorophenyl)pyrazin-2-amine.
102171 Step 3: Synthesis of 6-(4-fluoropheny1)-:'.4-' (quhiolin-6-yi)pyrazin-2-
amine: To a
solution of 5-bromo-6-(4-fluorophenyl)pyrazin-2-amine (100 mg, 0.37 mmol, 1
eq.) in 1,4
dioxane (8 mL): water (2 mL) was added quinolin-6-ylboronic acid (77 mg, 0.44
mmol, 1.2 eq.),
Na2CO3 (79 mg, 0.74 mmol, 2eq.), Pd(PPh3)4 ( 21 mg, 0.018 mmol, 0.05 eq.). The
reaction
mixture was deoxygenated using N2 atmosphere and the reaction mixture was
heated at 100 C
for 48 h. The reaction was monitored by TLC and LCMS. The reaction mixture was
diluted with
water (20 mL) and extracted using ethyl acetate (2 x 50 mL). The separated
organic layer was
dried over sodium sulfate and concentrated under reduced pressure. The crude
product was
purified by supercritical fluid chromatography to afford 45 mg (38%) of 6-(4-
fluoropheriy1)-5-
(quiriolin-6-yl)pyrazin-2-amine.
LCMS: 317 [M+1]. NMR (400 MHz, DMSO-d6) 8 6.58 (bs, 2H), 7.30 (t, 2H), 7.60
(q, 1H),
8.02-8.20 (m, 4H), 8.40-8.50 (m, 2H), 8.60 (s, 1H), 8.98 (bs, 1H) .
Example S15. Synthesis of 6-phenyl-5-quinoxalin-6-yl-pyrazin-2-amine.
(Compound No. 1.16)
Br
V-0õ0-1¨
Brrc
40/ 0-7 91-1
N NH2 N Ail
_______________________________________ HO'B 11fiks N's=1 ___ Igo
õ.
N Pacl2dppf.DCM complex wp N.) Pd(PPh3)4, Na2003 11
KOAc, Dioxane 110 C, 12h DME, H20 N NH2
STEP-1 MW/30 mm, 120 C
STEP-2
[0218] Step 1: Synthesis of quhioxalin-6-ylboronk acid: To a solution of 6-
bromoquinoxaline
(500 mg, 2.39 mmol, 1 eq.) in 1,4-dioxane (10 mL) was added 5-(4,4,5,5-
Bis(pinacolato)diboron (729 mg, 2.87 mmol, 1.2 eq.), KOAc (469 mg, 4.78 mmol,
2 eq.), and
PdC12dppf=DCM complex ( 195 mg, 0.23 mmol, 0.1 eq.). The reaction mixture was
deoxygenated with N2 allowed stir at 80 C for 18 h. The reaction mixture was
cooled to RT,
diluted with water (50 mL) and extracted with ethyl acetate (2 x 50 mL).
Combined organic
layer was washed with brine (20 mL) and dried over sodium sulfate. Removal of
solvent under
reduced pressure gave crude which was purified by normal phase Combi-Flash
column
310
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
chromatography (0-100% EtOAC-Hexane) to afford quinoxalin-6-y 1 boronic acid
(350 mg, 85%)
as brown oil.
LCMS: 175 [M+1]
102191 Step 2: Synthesis of 6-phenyl-5-quinoxalin-6-yi-pyrazin-2-amine: To a
solution of 5-
bromo-6-phenyl-pyrazin-2-amine (100 mg, 0.40 mmol, 1 eq.) in DME-water (2 mL)
was added
quinoxalin-6-ylboronic acid (69.6 mg, 0.40 mmol, 1 eq.), Na2CO3(106 mg, 1.0
mmol, 2.5 eq.),
and Pd(PPh3)4 (13.86 mg, 0.01 mmol, 0.03 eq.). The reaction mixture was
deoxygenated with N2
and allowed stir at 120 C for 30 min under Microwave irradiation. The
reaction was monitored
by LCMS and found to be complete after 30 min. The reaction mixture was cooled
to RT, diluted
with water (20 mL) and extracted with ethyl acetate (2 x 50 mL). Combined
organic layer was
washed with brine (20 mL) and dried over sodium sulfate. Removal of solvent
under reduced
pressure gave crude which was purified by reversed phase column chromatography
to afford 6-
pheny1-5-quinoxalin-6-yl-pyrazin-2-amine (10 mg, 8 %) as a white solid.
LCMS: 300 [M+1]+. NMR (400 MHz, DMSO-d6): 8 8.56 (s, 2H), 8.00 (s, 1H),
7.98-7.80 (m,
2H), 7.65 (m, 1H), 7.40-7.20 (m, 5H), 6.80 (s, 2H).
Example S16. S)mthesis of 5-(8-chloroquinolin-6-y1)-6-phenylpyrazin-2-amine.
(Compound No.
1.17)
OH
B'OH
I
Br N
CI
CI
N NH2 ____________________________________________
PdC12(dppf)DCM complex, Na2CO3
N NH2
1,4 Dioxane:H20
100 deg, 48h
(02201 To a solution of 5-bromo-6-phenylpyrazin-2-amine (100 mg, 0.4 mmol, 1
eq.) in 1,4
dioxane (5 mL): water (1 mL) was added 8-chloroquinolin-6-ylboronic acid (99
mg, 0.48 mmol,
1.2 eq.), Na2CO3 (84 mg, 0.8 mmol, 2 eq.), and PdC12(dppO=DCM (16 mg, 0.02
mmol, 0.05 eq.).
The reaction mixture was deoxygenated using N2 atmosphere and the reaction
mixture was
heated at 80 C for 18 h. The reaction was monitored by NMR and LCMS. The
reaction mixture
was diluted with water (50 mL) and extracted using ethyl acetate (2 x 50 mL).
The separated
311
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
organic layer was dried over sodium sulfate and concentrated under reduced
pressure. The crude
product was purified by Supercritical Fluid Chromatography to afford 10 mg of
548-
chloroquinolin-6-yI)-6-phenylpyrazin-2-amine.
LCMS: 333 [M+1]+. NMR (400 MHz, DMSO-d6) 8 7.30-7.40 (m, 5H), 7.60 (m, 1H),
7.70 (s,
1H), 7.88(s, 1H), 8.00 (s, 1H), 8.30 (d, 111), 8.98 (d, 1H).
Example S17. Synthesis of 5-(benzo[dloxazol-5-y9-6-phenylpyrazin-2-amine.
(Compound No.
1.18)
o,
OH OH OH
HNO3/CH3COOH4CI 0 0_ NO2No2 Fe. N 40 NH2 1
Et0H, 1120
Step-1 Step-2 Step-3
Br Br Br Br
KOAc,
Step-4 PdC12oppf.DCM
complex
Dioxane, 80 C
Br Ni
I
0 io N NH2
SO
K2CO3,
N NH2 PdC12dppf.DCM complex
DME:H20, 100 C Step-5
[0221] Step 1: Synthesis of 4-bromo-2,-nitrophenol: To a solution of 6-
bromophenol (5 g,
28.90 mmo1,1 eq.) in acetic acid (10 mL) was added nitric acid (1 mL) drop
wise. Reaction
mixture was stirred at RT for 5 min. Progress of the reaction was monitored by
LCMS and TLC.
The reaction mixture was poured over ice, resulting in solid precipitates
which were filtered and
dried under vacuum to afford 4-bromo-2-nitrophenol (6 g, 95.23%) as yellow
solid.
LCMS: 175 [M+1]+
[0222] Step 2: Synthesis of 2-amino-4-bromophenol: To a solution of 4-bromo-2-
nitrophenol
(3 g, 13.76 mmol, 1 eq.) in ethanol:water (50 mL, 9:1) was added ammonium
chloride (2.1 g,
41.28 mmol, 3 eq.) and Iron powder( 2.3g, 41.28 mmol). The reaction was
stirred at 90 C for 2
h. Progress of the reaction was monitored by LCMS. The reaction mixture was
cooled to RT,
evaporated under reduced pressure to remove the solvent, diluted with water
(20 mL) and
312
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
extracted with ethyl acetate (2 x 50 mL). The combined organic layer was
washed with brine (20
mL) and dried over anhydrous sodium sulfate. Removal of solvent under reduced
pressure gave
crude material which was purified by flash chromatography to obtain the 2-
amino-4-
bromophenol (2 g, 80%) as brown solid.
LCMS: 188, 190 [M+1]+
102231 Step 3: Synthesis of 5-bromobenzo[d loxazole: A solution 2-amino-4-
bromophenol
(1.5, 7.90 mmol, 1 eq.) in methylorthoformate (10 mL) was stirred at 150 C
for 6 h. Progress of
the reaction was monitored by LCMS. The reaction mixture was cooled to RT,
evaporated under
reduced pressure to remove the solvent; diluted with water (20 mL) and
extracted with ethyl
acetate (2 x 50 mL). The combined organic layer was washed with brine (20 mL)
and dried over
anhydrous sodium sulfate. Removal of solvent under reduced pressure gave crude
material
which was purified by flash chromatography to obtain the 5-
bromobenzo[d]oxazole (1.2 g,
80.0%) as yellow solid.
1HNMR (400 MHz, DMSO-d6) 8.80 (s, 1H), 8.00 (s, 1H), 7.80 (d, 1H), 7.60 (d,
111).
[02241 Step 4: Synthesis of 5(4,4,5,5-tetramethyl-1,3,2-dioxaborollan-2-
Abenzo[d]oxazole:
To a solution of 5-bromobenzo[d]oxazole (1.2 g, 6.06 mmol, 1 eq.) in DMF (10
mL) was added
5-(4,4,5,5-Bis(pinacolato)diboron (1.68 g, 1.66 mmol, 1.1 eq.), KO Ac (1.7 g,
18.09 mmol, 3eq.),
and PdCl2(dppf).DCM complex ( 247 mg, 0.23 mmol, 0.05 eq.). The reaction
mixture was
deoxygenated with N2 and the reaction mixture was stirred at 80 C for 18 h.
The progress of the
reaction was monitored by LCMS. The reaction mixture was cooled to RT, diluted
with water
(50 mL) and extracted with ethyl acetate (2 x 50 mL). Combined organic layer
was washed with
brine (20 mL) and dried over sodium sulfate. Removal of solvent under reduced
pressure gave
crude which was purified by flash chromatography(0-100% Hexane-Et0Ae) to
afford 544,4,5,5-
tetrarnethyl-1,3,2-dioxaborolan-2-yl)benzo[d]oxazole (0.900g. 60%) as off
white solid.
LCMS: 245, 247 [M+1]
10225) Step 5: Synthesis of 5-(benzoldloxazol-5-y1)-6-phenylpyrazin-2-amine:
To a solution
of 5-bromo-6-phenylpyrazin-2-amine (100 mg, 0.40 mmol, 1 eq) in dioxane-water
(5:1 mL) was
added 5-(4,4,5,5-tetrarnethy1-1,3,2-dioxaborolan-2-yl)benzo[d]oxazole (122 mg,
0.48 mmol, 1.2
eq), Na2CO3 (84.8 mg, 0.80 mmol, 2.0 eq), and PdC12(dP0').DCM complex (16.32
mg, 0.02
mmol, 0.05 eq.). The reaction mixture was deoxygenated with N2 and stirred at
100 C for 12 h.
The progress of the reaction was monitored by LCMS. The reaction mixture was
cooled to RT,
313
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
diluted with water (20 mL) and extracted with ethyl acetate (2 x 50 mL).
Combined organic
layer was washed with brine (20 mL) and dried over anhydrous sodium sulfate.
Removal of
solvent under reduced pressure gave crude material which was purified by
reversed-phase
column chromatography to afford 5-(benzo[d]oxazol-5-y1)-6-phenylpyrazin-2-
amine (10 mg,
9.00%) as an off white solid.
LCMS: 289.1 (M+1). 1H1MR (400 MHz, DMSO-d6) 8.70(s, 1H), 8.50 (s, 111), 7.72-
7.56 (m,
2H), 7.40-7.20 (m, 6H), 6.60 (s, 2H).
Example Sl8A. Synthesis of 3-amino-5-phenyl-6-(quinolin-6-Apyrazine-2-
carbonitrile.
(Compound No. 1.19)
CuCN 1JLN Br N CN
N M P
N NH2 N NI-12
[0226] To a stirred solution of 6-phenyl-5-(quinolin-6-yl)pyrazin-2-amine
(0.220 g, 0.58 mmol,
1.0 eq) in NMP (1.5 mL) was added cuprous cyanide (0.155 g, 1.74 mmol, 3.0
eq). The reaction
mixture was allowed to stir at 170 C for lh. The progress of the reaction was
monitored by
LCMS. The reaction mixture was allowed to cool to RT and extracted using ethyl
acetate (3 x 50
mL). The combined organic layers were washed (brine), dried (anhydrous Na2SO4)
and
concentrated under vacuum to get the solid which was purified by normal phase
column
chromatography to get the desired product as an off white solid (0.020 g, 10
%).
LCMS: 324 (M+1r. 111 NMR (400 MHz, METHANOL-d4) 8 8.83 (d, J= 3.07 Hz, 1H),
8.25 (d,
J = 7.45 Hz, 1H), 7.95 (dõI = 2.19 Hz, 1H), 7.88 (d, J= 8.77 Hz, 1H), 7.65
(ddõI = 2.19, 8.77
Hz, 1H), 7.52 (dd, J = 4.38, 8.33 Hz, 1H), 7.40 - 7.47 (m, 2H), 7.36 (d, J=
7.45 Hz, 1H), 7.22 -
7.32 (m, 2H).
Example S1 8B. Alternative synthesis of 3-amino-5-pheny1-6-(quinolin-6-
yl)pyrazine-2-
earhonitrile. (Compound No. 1.19)
314
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
HO.. ..OH
NBS, DMF, 10 min Sr N..1 -MN ErOH
0 C
N
C: N Pci(FFh3)Ci2. _________ I ,Nhz
I NA's' NH2 P(I(4/"Pf)G'2 acm'
: Step 2 I
90 Dionne, water - 90 C, Dioxane, water
Ikt.- NH2
18 h step.' 16 h step 3
DMF
NaCN/CuCN
NeS/DMF N Br
N CN
X
Step 4 Step 5
=====. N NI-12
N NH2
[0227] Step 1: Synthesis of 6-phenylpyrazin-2-amine: To a stirred solution of
6-chloropyrazin-2-
amine (50g, 0.3861mo1) in dioxane:water (400 mL;100mL) was added
benzeneboronic acid
(56.4 g,0.46 mol). The reaction mixture was purged with nitrogen for 20 min
then charged
Na2CO3 (70.6g, 0.57 mol) and Pd(PPh3)C12 (13.5g, 0.01930 mol). The reaction
mixture was
again purged with nitrogen .The reaction mixture was stirred at RT for 10 min
followed by
heating at 90 C for 16 h. The reaction was monitored by TLC & LCMS. The
reaction mixture
was filter through celite and distilled. The reaction was diluted with water
and extracted with
ethyl acetate (3x 200 mL). The combined organic layers were washed (brine),
dried (anhydrous
Na2504) & concentrated under vacuum to get the solid which was purified by
column
chromatography over silica gel (100-200 mesh) [Ethyl acetate: Hexane (3:7) as
eluent] to get the
desired product (55 g, 83%).
LCMS: 172 [M+1]+. NMR (400 MHz, CHLOROFORM-d) 8 8.38 (s, 1H), 7.83 - 7.99 (m,
3H), 7.40 - 7.49 (m, 3H), 4.82 (br. s., 211)
[0228] Step 2: Synthesis of 5-bromo-6-phenylpyrazin-2-amine: To a stirred
solution of 6-
phenylpyrazin-2-amine (48g, 0.2803m01) in DMF was added NBS (49.9 g, 0.28 mol)
at 0 c
under nitrogen atmosphere. The reaction mixture was stirred at RT for 16 h.
The reaction was
monitored by TLC & LCMS. The reaction was diluted with water and extracted
with ethyl
acetate (3x 100 mL). The combined organic layers were washed (brine), dried
(anhydrous
Na2SO4) & concentrated under vacuum to get the solid which was purified by
column
chromatography silica gel (100-200 mesh) [Ethyl acetate: Hexane (1:4) as
eluent] to get the
desired product (38 g, 55%).
LCMS: 252 [M+2]+. 114 NMR (400 MHz, DMSO-d6) 8 7.68 (s, 111), 7.55 - 7.64 (m,
211), 7.40 -
7.51 (m, 311), 6.75 (br. s., 2H)
315
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
102291 Step 3: synthesis of 6-phenyl-5-(quinolin-6-yl)pyrazin-2-amine: To a
stirred solution of
5-bromo-6-phenylpyrazin-2-amine (38g, 0.1519mol) in dioxane:water (320 mL;
80mL) was
added quinolin-6-ylboronic acid (46.4g, 0.18 mol). The reaction mixture was
purged with
nitrogen for 20 min then charged with Na2CO3 (32.2g, 0.3038m01) and
Pd(dppf)C12 (6.19 g,
0.007 mol). The reaction mixture was again purged with nitrogen .The reaction
mixture was
stirred at RI for 10 min followed by heating at 90 C for 16 h. The reaction
was monitored by
TLC & LCMS. The reaction mixture was filtered through celite and distilled.
The reaction was
diluted with water and extracted with ethyl acetate (3x 200 mL). The combined
organic layers
were washed (brine), dried (anhydrous Na2SO4) & concentrated under vacuum to
get the solid
which was purified by column chromatography over basic alumina [Ethyl acetate:
Hexane (3:7)
as eluent] to get the desired product (31 g, 68%).
LCMS: 299 [M+1]+. 114 NMR (400 MHz, DMSO-d6) 8 8.83 (d, J = 3.07 Hz, 1H), 8.21
(d, J =
7.89 Hz, 1H), 8.02 (s, 1H), 7.93 (s, 1H), 7.80 (d, J = 8.33 Hz, 1H), 7.41 -
7.64 (m, 2H), 7.16 -
7.40 (m, 5H), 6.73 (s, 2H)
102301 Step 4: synthesis of 3-bromo-6-phenyl-5-(quinolin-6-yl)pyrazin-2-amine:
To a stirred
solution of 6-phenyl-5-(quinolin-6-y1) pyrazin-2-amine (21g, 0.07 mol) in DMF
was added NBS
(12.5g, 0.07 mol) at 0 c under nitrogen atmosphere. The reaction mixture was
stir at RI for 16h.
The reaction was monitored by TLC & LCMS. The reaction was diluted with water
and
extracted with ethyl acetate (3x 30 mL). The combined organic layers were
washed (brine), dried
(anhydrous Na2SO4) & concentrated under vacuum to get the solid which was
purified by
column chromatography over basic alumina [Ethyl acetate: Hexane (3:7) as
eluent] to get the
desired product (18 g, 69%).
LCMS: 377 [M+1]+. 114 NMR (400 MHz, CHLOROFORM-d) 8 8.88 (br. s., 1H), 8.11-
7.96 (m,
3H), 7.60-7.26(m, 7H), 5.23 (br. s., 2H).
102311 Step 5: Synthesis of 3-amino-5-phenyl-6-(quinolin-6-yl)pyrazine-2-
carbonitrile: To a
stirred solution of NaCN (1.56g, 0.03 mol) and CuCN (5.7g, 0.06 mol) in dry
DMF (150 mL)
was added 3-bromo-6-phenyl-5-(quinolin-6-yl)pyrazin-2-amine (12.0 g, 0.03 mol)
at 120 C. The
reaction mixture was stirred at 145 C for 12h. The reaction was monitored by
TLC & LCMS.
The reaction was distilled. The crude product was poured in ice-water the
solid precipitate out.
The reaction mixture pH was adjusted with aqueous ammonia and extracted with
ethyl acetate (3
x 100 mL). The combined organic layers were washed (brine), dried (anhydrous
Na2SO4) &
316
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
concentrated under vacuum to get the solid which was purified by column
chromatography using
basic alumina [Ethyl acetate: Hexane (1:1) as eluent] to get the desired
product (3.8 g, 34%).
LCMS: 354 [M+1]. H NMR (400 MHz, DMSO-d(;) 8 8.88 (d, J= 2.63 Hz, 1H), 8.29
(d, J=
7.89 Hz, 1H), 7.99 (s, 1H), 7.84 (d, J= 8.77 Hz, 1H), 7.58 (br. s., 2H), 7.47 -
7.54 (m, 2H), 7.35
- 7.42 (m, 3H), 7.27 - 7.34 (m, 2H)
Example S19. S)inthesis of 6-(5-methylfUran-2-y1)-.5-(quinolin-6-yl)pyrazin-2-
amine. (Compound
No. 1.20)
OH(N Br N
NBS, DI\IF 0 C, 1 h
I 0 OH
NH2 __________________________________________________________ cs(1-=- N:NH2
12(dppf).DCM complex, ) 0 Step 2
CKs'N PdC
-N-NH2 \ 0
Na2CO3, Dioxane:water
90"C, 18h Step 1
OH
LN
B4OH
,
,
PdC12(dppl).DCM complex,
K2CO3, Dioxane:water N NH2
\
85 ''C, 18 h Step 3 0
[0232] Step 1: Synthesis of 6-(5-methylfuran-2-yl)pyrazin-2-amine: To a
solution of 6-
chloropyrazin-2-amine (1.00 g, 7.75 mmol, 1 eq.) in 1,4-dioxane (20 mL) was
added 5-
methylfuran-2-ylboronic acid (1.074 g, 8.52 mmol, 1.1 eq.), Na2CO3 (1.23 g,
11.62 mmol, 1.5
eq.), and PdC12(dppO=DCM complex ( 0.316 g, 0.38 mmol, 0.05 eq.). The reaction
mixture was
deoxygenated using N2 atmosphere and the reaction mixture was heated at 90 C
for 18 h. The
progress of the reaction was monitored by LCMS. The reaction mixture was
filtered through
Celite and washed with ethyl acetate (2 x 20 mL). The filtrate was diluted
with water (50 mL)
and extracted using ethyl acetate (2 x 50 mL). The separated organic layer was
dried over
sodium sulfate and concentrated under reduced pressure. The crude product was
purified by flash
column chromatography to afford 6-(5-met.hylfuran-2-yl)pyrazin-2-amine (0.600
g, 43%).
LCMS: 176 [M+1]
[0233] Step 2: Synthesis of 5-bromo-6-(5-methylfuran-2-yl)pyrazin-2-amine: To
a solution
of 6-(5-methylfuran-2-yl)pyrazin-2-amine (300 mg, 1.69 mmol, 1 eq.) in DMF (5
mL) was
317
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
added NBS (301 mg, 1.69 mmol, 1 eq.) portion-wise at 0 C. The reaction mixture
was stirred at
0 C for 1 h. The reaction was monitored by TLC and NMR. The reaction was
diluted with water
(50 mL) and extracted by ethyl acetate (2 X 20 mL). The organic layer was
separated, washed
with water (5 X 20 mL) and brine and dried over anhydrous sodium sulfate. The
solvent was
evaporated under reduced pressure to get the crude product. The crude product
was purified by
flash column chromatography to afford 5-bromo-6-(5-methylfuran-2-yl)pyrazin-2-
amine (0.100
g, 22%).
LCMS: 254 [M+1]+
[0234] Step 3: Synthesis of 6-(5-methylfuran-2-y1)-5-(quinolin-6-yl)pyrazhi-2-
amine: To a
solution of 5-bromo-6-(5-methylfuran-2-yl)pyrazin-2-amine (100 mg, 0.39 mmol,
1 eq.) in 1,4-
dioxane (8 mL): water (1 mL) was added quinolin-6-ylboronic acid (74 mg, 0.43
mmol, 1.2 eq.),
K2CO3 (81 mg, 0.59 mmol, 1.5 eq.), and PdC12(dppf)=DCM complex (16 mg, 0.019
mmol, 0.05
eq.). The reaction mixture was deoxygenated using N2 atmosphere and the
reaction mixture was
heated at 100 C for 18 h. The reaction was monitored by NMR and LCMS. The
reaction
mixture was diluted with water (50 mL) and extracted using ethyl acetate (2 X
50 mL). The
separated organic layer was dried over anhydrous sodium sulfate and
concentrated under reduced
pressure. The crude product was purified by supercritical fluid chromatography
to afford 6-(5-
methylfuran-2-y1)-5-(quinolin-6-yl)pyrazin-2-amine (0.020 g, 16.80%).
LCMS: 303 [M+1] .114 NMR (400 MHz, DMSO-d6) 2.03 (s, 3H), 6.12 (s, 1H), 6.20
(s, 1H),
6.70 (bs, 2H), 7.50 (d, 1H), 7.64 (d, 1H), 7.90 (s, 1H), 7.99 (d, 1H), 8.00
(s, 1H), 8.38 (d, 1H),
8.90 (d, 1H).
Example S20. Synthesis of .5-(8-chloroquinolin-6-y1)-6-(5-methytfuran-2-
yl)pyrazin-2-amine.
(C7ompound No. 1.21)
9H
CI
I/ 'NI --=== E1,0 H
i N
NIS Ci
OH
NH2 ACN CI N NH2 POCl2(dppODCM complex,
PdC12(cIppODCM complex, 0 ' Ntf.k.NH
8tep-1 Na2CO3: Dioxanemater
Na2CO3, D
2iOxene=water \ 1
85 C. 18h Step-2 85 C. 16h 8tep4
[0235] Step-1: Synthesis of 6-chloro-5-iodopyrazin-2-amine: To a stirred
solution of 2-amino-
6-chloropyrazine (2.0 g, 1.0 eq., 15.50 mmol) in acetonitrile (20 mL) was
added MS (3.46 g, 1.0
eq., 15.50 mmol) at 0 C. The reaction was allowed to stir at RT. Progress of
the reaction was
318
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
monitored by TLC and LCMS. After completion of the reaction the solvent was
evaporated
under vacuum and the solid was extracted using ethyl acetate (3 x 100 mL). The
combined
organic layers were washed (brine), dried (anhydrous Na2SO4) and concentrated
under vacuum
to get the solid which was purified by normal phase column chromatography to
get the desired
product (1.7 g, 42%).
LCMS: 256 [M+1]+
[0236] Step-2: Synthesis of 6-chloro-5-(8-chloroquinolin-6-yl)pyrazin-2-amine:
To a stirred
solution of 6-chloro-5-iodopyrazin-2-amine ( 0.500 g, 1.95 mmol, 1.0 eq.) and
(8-
chloroquinolin-6-yl)boronic acid (0.487 g, 2.3 mmol, 1.2 eq.) in 1,4-dioxane
(4.0 mL) was added
sodium carbonate (0.307g, 2.9 mmol, 1.5 eq.) and 1 mL of water. The reaction
mixture was
purged with nitrogen and Pd(dppf)C12=DCM complex (0.084 g, 0.05 eq.) was
added. The
reaction mixture was again purged with nitrogen and heated at 85 C for 16 h.
Progress of the
reaction was monitored by TLC and LCMS. Reaction mixture was allowed to cool
to RT and
extracted using ethyl acetate (3 x 50 mL). The combined organic layers were
washed (brine),
dried (anhydrous Na2SO4) and concentrated under vacuum to get the solid which
was purified by
normal phase column chromatography to get the desired product (0.140 g, 25%).
LCMS: 291 [M+1]+
[0237] Step-3: Synthesis of 5-(8-chloroquinolin-6-yI)-6-(5-methylfuran-2-
yl)pyrazin-2-
amine: To a stirred solution of 6-chloro-5-(8-chloroquinolin-6-yl)pyrazin-2-
amine (0.100 g, 0.34
mmol, 1.0 eq.) and (5-methylfuran-2-yl)boronic acid ( 0.051 g, 0.41 mmol, 1.2
eq.) in1,4-
dioxane (3.0 mL) was added sodium carbonate (0.054 g, 0.54 mmol, 1.5 eq.) and
1 mL of water.
The reaction mixture was purged with nitrogen and Pd(dppf)C12=DCM complex
(0.014 g, 0.05
eq.) was added. The reaction mixture was again purged with nitrogen and heated
at 85 C for 16
h. Progress of the reaction was monitored by TLC and LCMS. Reaction mixture
was allowed to
cool to RT and extracted using ethyl acetate (3 x 25 mL). The combined organic
layers were
washed (brine), dried (anhydrous Na2SO4) and concentrated under vacuum to get
the solid which
was purified by normal phase column chromatography to get the desired product
(0.025 g, 14%).
LCMS: 337 [M+1]+. H NMR (400 MHz, DMSO-d6) d 9.00 (br. s., 1H), 8.45 (d, J =
7.89 Hz,
1H), 8.01 (br. s., 1H), 7.90 (s, 1H), 7.83 (br. s., 1H), 7.65 (br. s., 1H),
6.80 (br. s., 211), 6.38 (br.
s., 1H), 6.14 (br. s., 1H), 2.08 (s, 311).
319
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
Example 521. Synthesis of 6-(1H-pyrazol-1-y1)-5-(quinolin-6-yl)pyrazin-2-amine
(Compound No.
1.22)
OH
kN 410 B4OH
I N GNI
,
NNH2 _____________________________________________________ =
CI N NH2 ________________ Cs2CO3. DMF, 90 =C C I Pd(dppf)C12.DCM
complex
-N N NH2
Na2CO3, DME:H20, 120 C
[0238] Step 1: Synthesis of 5-iodo-6-(1H-pyrazol-1-yl)pyrazin-2-amine : To a
solution of 6-
chloro-5-iodopyrazin-2-amine (300 mg, 1.2 mmol, 1 eq.) in DMF (8 mL) was added
pyrazole
(159 mg, 2.3 mmol, 2.0 eq.) and Cs2CO3 (1.148 g, 3.5 mmol, 3.0 eq.). The
reaction mixture was
allowed to heat at 90 C for 18 h. The progress of the reaction was monitored
by TLC and
LCMS. The reaction mixture was diluted with water (30 mL) and extracted using
ethyl acetate (2
x 50 mL). The separated organic layer was dried over sodium sulfate and
concentrated under
reduced pressure. The crude product was purified by reverse phase column
chromatography to
afford the desired product (120 mg, 35%).
LCMS: 288 [M+1]
[0239] Step 2: Synthesis of 6-(1H-pyrazol-1-y1)-5-(quinolin-6-yl)pyrazin-2-
amine: To a solution
of 5-iodo-6-(1H-pyrazol-1-yl)pyrazin-2-amine (0.100 g, 0.34 mmol, 1 eq.) in
DME (4.0 mL) was
added boronic acid (0.071 g, 0.41 mmol, 1.2 eq.), Na2CO3 (0.047 g, 0.45 mmol,
1.3 eq.) and
PdC12(dppf)=DCM complex ( 0.014 g, 0.017 mmol, 0.05 eq.). The reaction mixture
was
deoxygenated using N2 atmosphere and the reaction mixture was heated at 120 C
for 1.5 h
under microwave irradiation. The reaction was monitored by TLC and LCMS. The
reaction
mixture was filtered through Celite. The filtrate was extracted with ethyl
acetate (2 x 25 mL).
The separated organic layer was dried over anhydrous sodium sulphate and
concentrated under
reduced pressure. The crude product was purified by reverse phase column
chromatography to
afford desired product (13 mg, 13%).
LCMS 289 [M+1]. NMR (400 MHz, DMSO-d6) 5 8.84 (d, J = 2.63 Hz, 1H), 8.23 (d, J
=
8.33 Hz, 111), 8.12 (s, 1H), 8.07 (d, J = 2.19 Hz, 1H), 7.74 - 7.84 (m, 2H),
7.56 (s, 1H), 7.49 (dd,
J = 4.17, 8.11 Hz, 1H), 7.30 (d, J = 7.02 Hz, 1H), 7.07 (s, 1H), 6.47 (br. s.,
1H).
320
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/04 2 7 7 7
Example 522. Synthesis of 3-amino-5-phenyl-6-(quinolin-6-yl)pyrazin-2-ol
(Compound No.
1.241)
NQ NJ
N Br N OH
Ay. NaOH, Dioxane..
N NH2 180 ()C, 30 min, MW NNH2
102401 To a stirred solution of 3-bromo-6-phenyl-5-(quinolin-6-yl)pyrazin-2-
amine (0.050 g,
0.13 mmol, 1.0 eq) in dioxane (1 mL) was added 10% aqueous NaOH solution (1
mL). The
resulting reaction mixture was heated at 180 C for 30 min under microwave
irradiation. The
reaction mixture was allowed to cool to RT. The solvent was evaporated under
vacuum to get the
solid which was purified by reversed phase column chromatography to get the
desired product as
yellowish green solid (0.018 g, 45 %).
LCMS: 315 (M+1)+,111 NMR: (400 MHz, DMSO-d6) 8 11.96 (br. s., 1H), 8.88 (s,
1H), 8.28 (d, J
= 7.45 Hz, 1H), 7.93 (br. s., 1H), 7.82 (d, J = 8.77 Hz, 1H), 7.52 (d, ./ =
3.95 Hz, 1H), 7.40 (d, J
= 9.21 Hz, 1H), 6.99 - 7.28 (m, 5H), 6.84 (br. s., 2H).
Example S23. Synthesis of 5-(7-ehloro-1H-benzimidazol-.5-y1)-6-(5-methylfitran-
2-yl)pyrazin-2-
amine (Compound No. 1.45)
OH
M OH
(10 HN
OH NBS. ACN. 04' C Br N
peipp= _______ = I NNH2 '111 Pd ci co ex.
(dPpfiCCI,I an rnp; CI
CI N"-;"'Nli =
nwecomp:ex. I
2 Na2CO3, DME:water \ 0 N NH2
Na2CO3-, DME:water N NH2
Step-2
120 C, 2n MW 120 C, 2h MW \ 0
S
Step-1 tep-3
10241] Step-1: Synthesis of 6-(5-methylfuran-2-yl)pyrazin-2-amine: 6-
chloropyrazin-2-amine
(1.0 g, 7.7 mmol, 1.0 eq.) and (5-methylfuran-2-yl)boronic acid (1.2 g, 9.3
mmol, 1.2 eq) was
dissolved in DME:water (10.0 mL, 8:2). The reaction mixture was deoxygenated
using nitrogen
followed by addition of Pd(PPh3)4 complex (0.045 g, 0.04 mmol, 0.05 eq.) and
sodium carbonate
(1.6 g, 15.4 mmol, 2.5 eq.). The reaction mixture was again purged with
nitrogen and heated at
150 C for 2 h under microwave irradiation. Progress of the reaction was
monitored by TLC and
321
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
LCMS. Reaction mixture was allowed to cool to RT and quenched by adding water
and extracted
using ethyl acetate (3 x 100 mL) The combined organic layers were washed
(brine), dried
(anhydrous Na2SO4) and concentrated under vacuum to get the solid which was
purified by silica
gel column chromatography to get the desired product (0.350 g, 24%)
LCMS: 176 [M+1]
102421 Step-2: Synthesis of 5-bromo-6-(5-methylfuran-2-yl)pyrazin-2-amine: To
a stirred
solution of 6-(5-methylfuran-2-yl)pyrazin-2-amine (0.7 g, 3.9 mmol, 1.0 eq.)
in ACN (3.0 mL)
was added N-bromosuccinimide (0.7 g, 3.9 mmol, 1.0 eq.) portionwise at 0 C.
Progress of the
reaction was monitored by TLC and LCMS. The reaction mixture was quenched by
adding cold
water and extracted using ethyl acetate (3 x 50 mL). The combined organic
layers were washed
with brine, dried with anhydrous Na2SO4 and concentrated under vacuum to get
the solid which
was purified by normal phase column chromatography to get the desired product
(0.140 g, 14%)
LCMS: 254 [M+1]
10243] Step-3: Synthesis of 5-(7-chloro-1H-benzimidazol-5-y1)-6-(5-methylfuran-
2-yl)pyrazin-
2-amine: 5-bromo-6-(5-methylfuran-2-yl)pyrazin-2-amine (0.140 g, 0.27 mmol,
1.0 eq.) and (7-
chloro-1H-benzo[d]imidazol-5-ypboronic acid (0.140 g, 0.35 mmol, 1.3 eq.) was
dissolved in
DME:water (10.0 mL, 8:2). The reaction mixture was deoxygenated using nitrogen
followed by
addition of Pd(dppf)C12.DCM complex (0.022 g, 5 mol%) and sodium carbonate
(0.088 g, 0.41
mmol, 1.5 eq.).The reaction mixture was again purged with nitrogen and allowed
to heat at 120
C for 2 h using microwave irradiation. Progress of the reaction was monitored
by TLC and
LCMS. Reaction mixture was allowed to cool to RT and quenched by adding water
and extracted
using ethyl acetate (3 x 30 mL) The combined organic layers were washed with
brine, dried with
anhydrous Na2SO4 and concentrated under vacuum to get the solid which was
purified by
reversed phase column chromatography to afford the desired product (4.9 mg,
6%)
LCMS: 326 [M+1r. NMR: (400 MHz, DMSO-d6) 8 12.78 (s,1H), 8.31 (s, 1 H), 7.84
(s, 1 H),
7.45 (br. s., 1 H), 7.19 (br. s., 1 H), 6.64 (br. s., 2 H), 6.07 (br. s., 2
H), 1.91 - 2.20 (m, 3 H).
Example S24. Synthesis of 5-(naphthalen-2-y1)-6-phenylpyrazin-2-amine
(Compound M. 1.185)
322
CA 03070273 2020-01-16
WO 2019/018584 PCT/US2018/042777
ErOH
011 Br N.
I N*.
Pd(dppf).Cl2 dcmcomplex, N NH2
Na2CO3, DME:water
120 C, 30 min MW
[0244] To a stirred solution of 5-bromo-6-phenylpyrazin-2-amine (0.100 g, 0.39
mmol, 1.0 eq.) )
in DME:water (5.0 mL, 4:1) was added naphthalen-2-ylboronic acid (0.089g,
0.51mmol, 1.3
eq.). The reaction mixture was deoxygenated using nitrogen gas, and
Pd(dppf)C12DCM complex
(0.016 g, 0.05 eq. 0.019 mmol) was added. The reaction mixture was again
purged with nitrogen
and heated at 120 C for 2 h under microwave irradiation. Progress of the
reaction was
monitored by TLC and LCMS. Reaction mixture was allowed to cool to RT and
quenched by
adding water and extracted using ethyl acetate (3 x 30mL) The combined organic
layers were
washed (brine), dried (anhydrous Na2SO4) and concentrated under vacuum to get
the solid which
was purified by normal phase column chromatography to get the desired product
(0.05 g,
43.10%)
LCMS: 298 [M+1]+. NMR: (400 MHz, DMSO-d6) 8 6.66 (s, 2 H) 7.17 - 7.33 (m, 4 H)
7.35
(br. s., 2 H) 7.40 - 7.50 (m, 2 H) 7.64 - 7.79 (m, 2 H) 7.82 (br. s., 1 If)
7.87 (s, 1 If) 8.00 (s, 1 H).
Example S25. Synthesis of .5-(1H-indazol-5-y1)-6-phenylpyrazin-2-amine
(Compound M. 1.270)
OH HNiJL
Br OH
N=-=
so NN H2 Pc1(dppf).C12 dcm complex,
Na2CO3. Dioxanewater N NH2
100 C, 18h
[0245] To a stirred solution of 5-bromo-6-phenylpyrazin-2-amine (0.1 g, 0.4
mmol, 1.0 eq.) and
(1H-indazol-5-yl)boronic acid (0.127 g, 0.44 mmol, 1.1 eq.) in dioxane (4 mL)
was added
Na2CO3 (0.085g, 0.8 mmol, 2.0 eq.) and 1 mL water. The reaction was purged
with N2 for 5 min.
To this reaction mixture was added with Pd(dppf)C12.DCM complex (0.016 g, 5
mol %) and N2
was purged again for another 5 min. The reaction mixture was heated at 100 C
for 18 h. The
reaction mixture was allowed to cool to RT and extracted using ethyl acetate
(3 x 35 mL). The
323
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 323
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 323
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: