Note: Descriptions are shown in the official language in which they were submitted.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
1
OIL SANDS TAILINGS TREATMENT
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional
Application No.
62/535,392 filed 21 July 2017 and U.S. Application No. 62/583,371 filed 8
November 2017 the
entire disclosures of each of which are hereby incorporated by reference
herein.
TECHNIC AL FIELD
[0002] The present disclosure relates to dewatering and consolidation of
oil sands
tailings, e.g., tailings from the extraction of bitumen from oil sands solids.
Residual
hydrocarbons that may be present in the tailings can also be separated and
recovered.
BACKGROUND
[0003] Oil sands tailings are a waste by-product of extracting bitumen
from oil sands.
The tailings are typically discharged into large ponds which are growing
faster than processes to
remediate the tailings.
[0004] A simplified illustration of tailings production as a result of
bitumen extraction is
illustrated in Figure 1. As illustrated, oil sands are mined to extract
bitumen. Extraction of
bitumen from oil sands involves the use of significant amounts of energy and
heated water.
Approximately 9 barrels of water are required for every barrel of oil
produced. Water, sodium
hydroxide (NaOH) and other additives are mixed with the oil sands to form a
slurry. The NaOH
releases surfactants from the oil sands and improves bitumen recovery. The
slurry is conditioned
by mixing and/or shearing the slurry to detach bitumen from the oil sands
particles. Bitumen is
separated from water by aeration to form an oil containing froth that can be
skimmed off the
surface of the water. After further treatment to remove fines and process
water, the extracted
bitumen is sent to a refinery for upgrading. Process water, coarse sand
particles and mineral fine
particles are discharged into tailings ponds. The whole tailings is a complex
mixture of alkaline
water, dissolved salts, residual bitumen and other organics, surfactants and
solid particles, e.g.,
sand, clay, silt and trace metals, etc.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
2
[0005] Tailings ponds partially separate the solids from the water
through gravity so that
some of the surface water can be recycled into the extraction process. Four
layers are typically
formed in such tailings ponds. Figure 2 illustrates the layers of
sedimentation in a typical oil
sands tailings pond. As illustrated, the tailings pond includes a layer of
coarse sand (18), which
settles to the bottom of the pond relatively quickly. Water with some
entrained fines, e.g. solids
having a size of no more than 44 microns, sit on the surface (12) and is
reused in the process.
However, most of the fines (mainly silica and clay particles) form an
intermediate layer of so-
called fluid fine tailings (FFT) (14), which is also contaminated with
residual bitumen, organics,
solvents and chemicals used as processing aids in the bitumen extraction
process. Over time, this
mixture undergoes additional settling to form a distinct layer of so-called
mature fine tailings
(MFT) (16). It is believed that the presence of sodium chloride, the main
electrolyte present in
tailings water, stabilizes the sludge-like formation of MFT. See Kotlyar et
al, Clays and Clay
Minerals, 1996:44:121-131.
[0006] MFT contain about 30 wt% or more solids (on average) and has a
sludge-like
consistency that makes it difficult to handle and dewater. It has been
estimated that mature fine
tailings can be stable for centuries without further densification. See, e.g.,
Wang et al. Minerals
Engineering, 2014:58:113-131. Because of the scale of oil sands operations,
tailings ponds
covered an area of at least 175 km2 in 2011. The management of legacy tailings
ponds is a thus a
very large problem for the oil sands industry. And the problem continues to
grow as production
rates exceed remediation efforts.
[0007] In 2009, the Alberta Energy Regulator implemented Directive 074,
aimed at
reducing the inventory of fluid tailings and their conversion into trafficable
deposits. This
directive specified a minimal trafficable shear strength for consolidated
tailings. Shear strength
is the amount of force a soil can sustain. Sludge has a low shear strength and
cannot sustain any
weight or pressure; this soft material is therefore non-trafficable. A
trafficable landscape
requires a shear strength high enough so the ground can sustain people and
equipment without
sinking. The shear strength of fine tailings is mostly a function of water
content. An effective
shear strength of 5 kPa equates to approximately 65% solids and 35% water.
Removing water
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
3
from the fines is a pivotal first step to reclaiming the landscape. However,
an effective shear
strength of 5 kPa does not provide a trafficable surface.
[0008] Various approaches have been used to dewater and consolidate the
solids of MET.
Some approaches are summarized in a recent review and in a report from the
Council of
Canadian Academies. See Wang et al. Minerals Engineering 2014:58:113-131; and
Council of
Canadian Academies, Technological Prospects for Reducing the Environmental
Footprint of
Canadian Oil Sands, 2015. One method involves a freeze-thaw approach, where
IVIFT placed in
shallow pits is allowed to freeze in the winter. The following summer, it
thaws and evaporative
dewatering occurs. However, this method requires enormous surface areas and
would be highly
dependent on weather and season. Another method involves combining mature fine
tailings with
sand and gypsum to form so-called "consolidated tailings." However, the
composition of the
blend needs to be tightly controlled, or the mixture segregates. In addition,
a high proportion of
sand to fines (solids) is required, at least 4 to 1. Even so, the resulting
material still does not have
the necessary strength for land reclamation and requires containment dikes. So-
called "end pit
lakes" are also under consideration, but their ultimate environmental impact
is a major concern.
[0009] Other methods have attempted to treat IVIFT with the addition of
chemicals to
flocculate the fines and create a thickened paste that will solidify and
eventually dewater. For
example, polymers such as polyacrylamide and its derivatives can act as
flocculating agents and
have been a component disclosed in a number of patents and patent
applications. See US
4399039; EP 2477708 Al; EP 2349945 Al; US 20130112561 Al; US 20140150886 Al;
EP
2989163 Al. However, even when the resulting mixture is centrifuged, the
extent of dewatering
remains insufficient for reclamation purposes. Typically, solids contents of
about 55% are
achieved and it has been noted that the resulting solid paste has "roughly the
consistency of
toothpaste". (Council of Canadian Academies, Technological Prospects for
Reducing the
Environmental Footprint of Canadian Oil Sands, 2015.)
[0010] Hydrolyzing salts, salts that hydrolyze to produce hydroxide ions
when dissolved
in water, such as alum (aluminum sulfate, Al2(SO4)3) and ferric chloride
(FeCl3), have been used
in municipal water treatment plants to coagulate and settle fine mineral
particles. See, e.g.,
Gregory et al., J. Pure Appl. Chem. 2001:73:2017-2026. Lime (CaO) was
disclosed for use as a
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
4
coagulating agent for whole tailings. See U.S. 4,225,433. This patent
discloses that mixing the
hydrolyzing salt with whole tailings prior to settling can agglomerate fines
with coarse particles
with the result that the fines co-settle with the coarse particles.
[0011] Hydrolyzing salts work, in part, by reacting with water to form
hydroxides, which
precipitate from the water. In practice, these coagulants are overdosed and
the resulting rapid
precipitation of the metal hydroxide enmeshes and captures solid particles in
the form of a floc.
See, Duan et al., Advances in Colloid and Interface Science 2003:100-102:475-
502; Wang et al.,
Minerals Engineering 2014:58:113-131.
[0012] Although incorporation of fine particles into flocs allows for
settling under
gravity in applications such as water clarification, the structure of these
flocs is very open and
contains large amounts of water. See Hogg, Int. J. Min. Proc. 2000:58:223-236.
These flocs
were formed using polyacrylamide as a flocculating agent and do not have the
high solids
content desired for consolidated tailings.
[0013] Other approaches to dewatering tailings include treating tailings
with a solution of
a polymer and salt. See W02015/083069 and W02014/173624. W02015/083069
discloses
using a salt to lower the viscosity of a polymer solution which is believed to
improve contact of
mineral particles with the polymer. W02014/173624 discloses treating an
aqueous suspension
of mineral particles with a water soluble polymer and a calcium and/or
magnesium salt. Both
references disclose using low concentrations of salt.
[0014] However, there is a continuing need to manage and treat oil sands
tailings to
reduce such tailings and/or to dewater and consolidate solids in such tailings
and in a manner
preferable for land reclamation and remedi ati on
SUMMARY OF THE DISCLOSURE
[0015] Advantages of the present disclosure include processes to dewater
oil sands
tailings to produce high solids content materials.
[0016] These and other advantages are satisfied, at least in part, by a
process of
consolidating oil sands tailings. The process comprises treating the oil sands
tailings, which
includes fines and process water, with a highly water soluble salt.
Advantageously, the process
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
can include treating the oil sands tailings with the at least one highly water
soluble salt or
solution thereof and can optionally include either or both of at least one
polymer flocculant or
solution thereof and/or coarse particles, e.g., sand, to form a treated
tailings. The treated tailings
can include a consolidated material in the process water. The process water
can then be
advantageously separated from the consolidated material. The consolidated
material can be
transferred for further dewatering or disposal.
[0017] Implementations of the process of the present disclosure include,
for example, (i)
treating the oil sands tailings with at least one highly water soluble salt to
form a treated tailings
including a consolidated material in the process water, (ii) treating the oil
sands tailings with at
least one highly water soluble salt and at least one polymer flocculant to
form a treated tailings
including a consolidated material in the process water, (iii) treating the oil
sands tailings with at
least one highly water soluble salt thereof, and coarse particles to form a
treated tailings
including a consolidated material in the process water, and (iv) treating the
oil sands tailings with
at least one highly water soluble salt, at least one polymer flocculant and
coarse particles to form
a treated tailings including a consolidated material in the process water.
Each of these
implementations can include aqueous solutions of the salt and/or polymer
flocculant to treat the
tailings. Each of these implementations can include separating the process
water from the
consolidated material. Advantageously, the consolidated material has a density
greater than the
process water.
[0018] In practicing aspects of the processes, tailings that include
hydrocarbon, such as
tar, crude oil, heavy oil, or other hydrocarbon oil, bitumen, asphaltenes,
etc. or a combination
thereof, can be separated and recovered. The process can further comprise
treating the tailings
with a diluent to dilute the hydrocarbon and recovering the diluted
hydrocarbon.
Advantageously, the hydrocarbon separated from the tailings can contain a low
amount of fines
or has low minerals content, e.g., less than about 1 wt% or no more than about
0.5 wt% or no
more than about 0.1 wt%.
[0019] In practicing aspects of the processes of the present disclosure
and the various
embodiments thereof, the separated process water can include the at least one
highly water
soluble salt and the process can further comprise recovering at least a
portion of the separated
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
6
process water. In some embodiments, the process can further comprise recycling
at least a
portion of the recovered separated process water to treat additional oil sands
tailings. In other
embodiments, the process can further include purifying at least a portion of
the recovered
process water.
[0020] Yet another aspect of the present disclosure includes recovering
the consolidated
materials from the tailings. Advantageously, the processes of the present
disclosure can
consolidate the solids of the tailings to produce a consolidated material
having a solids content in
excess of about 45% by weight, e.g., a solids content of greater than about
50% and higher than
about 60%, 65%, 70% and 75% by weight.
[0021] Embodiments of the processes include one or more of the following
features
individually or combined. For example, the oil sands tailings can contain
about 5 wt% to 60
wt% solids, e.g., from between about 10 wt% to about 50 wt% solids. In some
embodiments, the
at least one highly water soluble salt can have a solubility in water (a
salt/water solubility) of at
least about 5 g/100 g at 20 C, e.g., at least about 10 g/100 g at 20 C. In
other embodiments, the
at least one highly water soluble salt is a non-hydrolyzing salt. In still
further embodiments, the
at least one highly water soluble salt can have a monovalent cation and can
include an
ammonium based salt, a phosphate based salt, or a sulfate based salt.
[0022] In certain embodiments, the treated tailings can have a salt-
tailings concentration
of at least 0.5 wt% of the at least one highly water soluble salt and
preferably no less than about
1 wt%, such as at least about 2 wt% and even greater than about 3 wt%, 4 wt%,
5 wt%, etc. of
the at least one highly water soluble salt. In some embodiments, the at least
one polymer
flocculant is a polyacrylamide or co-polymer thereof. The treated tailings can
have a polymer-
tailings concentration of the at least one polymer flocculant of no less than
about 0.001 wt%,
e.g., no less than about 0.003 wt%, 0.005 wt%, 0.01 wt% or 0.04 wt?/o. In
other embodiments,
the tailings also can be treated with coarse particles, e.g., sand, at a sand
to fines ratio of less than
4:1, e.g., between about 2.5:1.0 to 0.5:1 or between about 2.25:1 to about
0.75:1.
Advantageously, the polymer flocculant forms high density flocs, e.g., having
a density greater
than the process water, which facilitates separation and dewatering of the
consolidated solids.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
7
[0023] In various embodiments, treating the tailings can include
combining the oil sands
tailings with a solution including the at least one highly water soluble salt
and the at least one
polymer flocculant. In some embodiments, treating the tailings can include
combining a stream
of the oil sands tailing with a stream of a solution including the at least
one highly water soluble
salt and a separate stream of a solution including the at least one polymer
flocculant.
Alternatively, or in combination, treating the tailings can include combining
a stream of the oil
sands tailings with a stream of a solution including both the at least one
highly water soluble salt
and the at least one polymer flocculant. Coarse particles (sand) can also be
added to the oil
sands tailings or stream thereof and/or to any or all of the solution streams.
Advantageously, the
streams can be mixed inline and/or with the aid of an inline mixer. In certain
embodiments,
treating the oil sands tailings can be carried out at a temperature of no more
than 50 C, e.g., no
more than about 40 C or about 30 C. In other embodiments, treating the
composition includes
using a solution of one or more highly soluble salts sourced from a natural or
existing source
such as seawater or a body of hypersaline water.
[0024] In still further embodiments, the process water can be separated
from the
consolidated material by any one or more of decanting, filtering, vacuuming,
gravity draining,
etc. or combinations thereof. In various embodiments, separating the process
water from the
consolidated material can include mechanically dewatering the consolidated
material, e.g.,
mechanically dewatering the consolidated material by a dewatering screw. Once
separated, the
consolidated material can be transferred for further dewatering or disposal.
[0025] In practicing certain aspects of the processes of the present
disclosure and the
various embodiments thereof, the consolidated material formed in the treated
tailings according
to certain embodiments can result in a high solids content after mixing and/or
dewatering the
treated tailings in a short period of time. In some embodiments, the
consolidated material can
have a solids content of greater than about 50% and at least about 60%, 65%,
70 4), 75% and
80% by weight after mixing and/or dewatering. In certain embodiments a solids
content of at
least about 70 % is achieved within about one month of gravity draining after
treating the
tailings, e.g., within about two weeks or within about one week of gravity
draining after treating
the tailings.
CA 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
8
[0026] Additional advantages of the present invention will become readily
apparent to
those skilled in this art from the following detailed description, wherein
only the preferred
embodiment of the invention is shown and described, simply by way of
illustration of the best
mode contemplated of carrying out the invention. As will be realized, the
invention is capable of
other and different embodiments, and its several details are capable of
modifications in various
obvious respects, all without departing from the invention. Accordingly, the
drawings and
description are to be regarded as illustrative in nature, and not as
restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
[0027] Reference is made to the attached drawings, wherein elements
having the same
reference numeral designations represent similar elements throughout and
wherein:
[0028] Figure 1 schematically illustrates tailings production as a result
of bitumen
extraction.
[0029] Figure 2 is schematic illustration of a typical sedimentation of
an oil sands tailings
pond.
[0030] Figure 3 schematically illustrates an exemplary embodiment of a
process of
consolidating oil sands tailings.
[0031] Figure 4 is a picture of vials containing mature fine tailings
treated in various
ways one week after re-shaking and showing that treated MFT were destabilized
by processes
according to embodiments of the present disclosure.
[0032] Figure 5 is a picture of calibrated centrifuge tubes containing
mature fine tailings
treated in various ways and centrifuged at 3000 rpm for 30 seconds.
[0033] Figure 6 is a plot of volume of compacted slurry versus
centrifugation time of
various MFT samples treated with water, salt, or various solutions of salt and
polymer.
[0034] Figure 7 is a plot of volume of compacted slurry versus
centrifugation time of
various MFT samples treated with a salt, a polymer and various ratios of sand
to fines.
[0035] Figure 8 is another plot of volume of compacted slurry versus
centrifugation time
of various MFT samples treated with salt, polymer and sand.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
9
[0036] Figure 9 is a plot of calculated solids content versus time for
the two treated MFT
samples provided in Figure 8.
[0037] Figure 10 shows pictures of vials containing mature fine tailings
treated with an
ammonium salt solution including a polyacrylamide flocculant at the
concentrations indicated in
the figure.
[0038] Figures 11A and 11B are pictures of consolidated solids produced
according to a
process of the present disclosure. Figure 11A shows consolidated solids
collected and draining
and Figure 11B shows the consolidated solids and after being pressed between
paper towels.
[0039] Figure 12 shows pictures of vials containing mature fine tailings
treated with an
ammonium salt and a polyacrylamide flocculant and illustrate effects of
increasing salt
concentration and reducing polymer concentration under the conditions tested.
DETAILED DESCRIPTION OF THE DISCLOSURE
[0040] The present disclosure relates to treating oil sands tailings to
consolidate and
dewater the tailings. In certain embodiments, the oil sands tailings can
contain about 5 wt% to
60 wt% solids, e.g., from between about 10 wt% to about 50 wt% solids.
Advantageously, the
process of the present disclosure can consolidate the solids of the tailings
to produce
consolidated material having a solids content in excess of about 45% by
weight, e.g., a solids
content of greater than about 50% and higher than about 60%, 65%, 70% and 75%
by weight.
[0041] As described in the background section, oil sands tailings are a
waste by-product
of the process of extracting bitumen from oil sands and include process water,
sand, fines, and
residual bitumen. Oil sands tailings can be characterized as a suspension of
particulate solids in
an aqueous liquid and generically include fluid fine tailings and mature fine
tailings.
[00421 The terms fluid fine tailings and mature fine tailings are used
herein consistent
with the art recognized use of these terms in the oil sands industry (see,
e.g., Technical Guide for
Fluid Fine Tailings Management, Oil Sands Tailings Consortium, August 30,
2012). Hence,
fluid fine tailings (FFT) is a liquid suspension of oil sands fines in water
with a solids content
greater than 2 wt% but less than the solids content corresponding to the
Liquid Limit. The
Liquid Limit is the geotechnical water content defining the boundary between a
liquid and a
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
solid in soil mechanics, with equivalent remolded shear strength of Ito 2 kPa.
This state is
defined by a standard laboratory test (ASTM D4318-10; modified for use in oil
sands tailings
containing bitumen). Mature fine tailings (MFT) is a fluid fine tailings with
a solids content
greater than or equal to about 30 wt%. Solids content is used herein to mean
the mass of solids
divided by mass of (solids + bitumen + water) x 100 4). Solids includes sand,
clay, silt and other
solid particles contained in oil sands tailings (does not include bitumen).
[0043] The solids of oil sands tailings are classified according to
particle sizes. The term
fines as used herein is consistent with the Canadian oil sands classification
system and means
solid particles with sizes equal to or less than 44 microns (p.m). Sand is
considered solid
particles with sizes greater than 44 Inn. The fines in oil sands tailings are
comprised mostly of
silt and clay material. Oil sands deposits include a significant amount of
fines, e.g., 10-30 wt%.
The tailings from oils sands extraction can also include a significant amount
of fines by weight
(>5 wt%) as their solids content. Such tailings can include at least about 10
wt%, 20 wt%, 30
wt%, 40 wt%, 50 wt%, 60 wt%, 70 wt% or higher fines as their solids content.
MET typically
include over 90 wt% fines as its solids content.
[0044] The terms coagulation and flocculation are often used
interchangeably in the
literature. As used herein, however, coagulation means particle aggregation
brought about by the
addition of hydrolyzing salts, whereas flocculation means particle aggregation
induced by
flocculating polymers. Hydrolyzing salts undergo hydrolysis when added to
water to form metal
hydroxides, which precipitate from the solution, trapping fines and other
minerals in the
coagulating mass. Hydrolyzing salts typically have low solubility in water and
are used as
coagulants. Aggregation induced by flocculation, in contrast, is believed to
be the result of the
polymer binding to the particles thereby tying the particles together into a
so called floc causing
aggregation of the particles.
[0045] In practicing aspects of the present disclosure, oil sands
tailings, e.g., a suspension
of particulate solids in an aqueous liquid which include fines and process
water, can be
consolidated by treating the oil sands tailings with one or more highly water
soluble salt(s) or an
aqueous solution thereof to destabilize and consolidate solids in the
tailings, e.g., to destabilize
and consolidate fines in the tailings. Aggregation induced by the addition of
salts is believed to
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
11
be the result of destabilizing the particles suspended in the fluid by an
alteration or a shielding of
the surface electrical charge of the particles to reduce the inter-particle
repulsive forces that
prevent aggregation. The process water can then be separated from the
consolidated material.
Advantageously, the consolidated material has a solids content of at least 45%
by weight, e.g., a
solids content of greater than about 50% by weight.
[0046] Salts that are useful in practicing the present disclosure include
salts that are
highly soluble in water. A highly water soluble salt as used herein is one
that has a solubility in
water of greater than 2 g of salt per 100 g of water (i.e., a salt/water
solubility of 2g/100g) at 20
C. Preferably the highly water soluble salt has a water solubility of at least
about 5 g/100 g at
20 C, e.g., at least about 10 g/100 g of salt/water at 20 C.
[0047] In addition, the highly water soluble salts used in the processes
of the present
disclosure are preferably non-hydrolyzing. Hydrolyzing salts undergo
hydrolysis when added to
water to form metal hydroxides, which precipitate from the solution. Such
hydrolyzing salts are
believed to form open flocs with inferior solids content and cannot be readily
recycled for use
with additional tailings in continuous or semi-continuous processes. In
addition, hydrolyzing
salts typically have low solubility in water and are used at elevated
temperatures to ensure
sufficient solubility for aggregation, which is an energy intensive process.
See US 4,225,433
which discloses the use of lime as a coagulating agent at a temperature of 75
C.
[0048] Further, the highly water soluble salts are preferably not
carboxylate salts since
such organic acid salts tend to be more expensive than inorganic salts and can
be deleterious to
plant and/or animal life.
[0049] Highly water soluble salts that are not hydrolyzing and useful in
practicing
processes of the present disclosure include salts having a monovalent cation,
e.g., alkali halide
salts such as sodium chloride, potassium chloride; also salts with monovalent
cations such as
sodium nitrate, potassium nitrate, sodium and potassium phosphates, sodium and
potassium
sulfates, etc. are useful in practicing processes of the present disclosure.
Other monovalent
cationic salts useful in practicing processes of the present disclosure
include ammonium based
salts such as ammonium acetate (NI-14C2H302), ammonium chloride (NH4C1),
ammonium
bromide (NH4Br), ammonium carbonate ((NF14)2CO3), ammonium bicarbonate
(NH4FIC03),
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
12
ammonium nitrate (NH4NO3), ammonium sulfate ((NH4)2SO4), ammonium hydrogen
sulfate
(NRIHSO4), ammonium dihydrogen phosphate (NH4H2PO4), ammonium hydrogen
phosphate
((NH4)2HPO4), ammonium phosphate ((NH.4)3PO4), etc. Mixtures of such salts can
also be used.
[0050] Ammonium based salts are useful for practicing the present
disclosure since
residual ammonium based salts on the consolidated material after combining the
salt with the oil
sands tailings are not harmful to plant life. In fact, many of the ammonium
based salts are useful
as fertilizers and are in fact beneficial to plant life, e.g., ammonium
chloride, ammonium nitrate,
ammonium sulfate, etc. Many of the monovalent sulfate and phosphate salts are
also useful as
fertilizers. In certain embodiments of the present disclosure, the highly
water soluble salt or salts
used in the processes of the present disclosure can preferably be non-toxic
and beneficial to plant
life to aid in environmental remediation and the restoration of mine sites.
[0051] In one aspect of the present disclosure, treating oil sands
tailings with a highly
water soluble salt destabilizes and consolidates solids in the tailings. Such
a process can include
mixing the oil sands tailings, which includes fines and process water, with a
highly water soluble
salt to consolidate the fines, and separating the process water from the
consolidated fines to
produce a high solids content, e.g., at least 45% by weight. In certain
embodiments, the highly
water soluble salt is an ammonium based salt.
[0052] Highly water soluble salts that can be used in practicing the
present process can
also include salts having multivalent cations. Such salts include, for
example, divalent cation
salts such as calcium and magnesium cation salts, such as calcium chloride
(CaCl2), calcium
bromide (CaBr2), calcium nitrate (Ca(NO3)2), magnesium chloride (MgCl2),
magnesium bromide
(MgBr2), magnesium nitrate (Mg(NO3)2), magnesium sulfate (MgSO4); and
trivalent cation salts
such as aluminum and iron (III) cation salts, e.g., aluminum chloride (A1C13),
aluminum nitrate
(Al(NO3)3), aluminum sulfate (Al2(504)3), iron (III) chloride (FeCl3), iron
(III) nitrate
(Fe(NO3)3), iron (III) sulfate (Fe2(SO4)3, etc. As explained above, the highly
water soluble salts
used in the processes of the present disclosure are preferably non-
hydrolyzing. Many of the
multivalent cation salts are hydrolyzing and thus less preferred for the
reasons stated above.
Moreover, experimentation with multivalent salts showed increased fouling of
containers and
formation of less cohesive consolidated materials as compared to highly water
soluble salts
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
13
having monovalent cations. In addition, some multivalent salts, such as FeCl3
and Fe2(SO4)3, are
particularly corrosive and Fe2(SO4)3 is formed from oxidizing pyrite and
results in acid mine run-
off, which make such salts less preferable for use in processes of the present
disclosure.
[0053] It was counterintuitive and surprising that addition of highly
water soluble salts
that are not hydrolyzing, or solutions thereof, to oil sands tailings, such as
NUT, would
destabilize and consolidate fines in such tailings, since certain literature
indicated that sodium
chloride, a highly water soluble salt, is partly responsible for the
stabilization of tailings. A
minimum concentration of salt is required for stabilization, but once this
minimum concentration
has been reached, it was believed that the equivalent solids content of the
tailings is virtually
independent of salt concentration. See, e.g., Kotlyar et al., Clays and Clay
Minerals,
1996:44:121-131. However, we found that when a sufficiently high concentration
of the highly
water soluble is included in the treated tailings, the salt can destabilize
and consolidate solids in
the tailings. For a relatively short process times with a relatively low
energy input, the salt-
tailings concentration of the at least one highly water soluble salt should
preferably be at least
0.5 wt% and preferably no less than about 1 wt%, such as at least about 2 wt%
and even at least
about 3 wt%, 4 wt%, 5 wt%, etc. The term "salt-tailings concentration" as used
herein refers to
the concentration of the highly water soluble salt(s) in the treated tailings
and is determined by
taking the percentage of the mass of highly water soluble salt(s) divided by
the combined mass
of the salt(s) plus the tailings and any water used to dilute the salt(s). For
example, combining 1
part undiluted (i.e., neat) salt to 99 parts tailings by weight results in a
salt-tailings concentration
of 1 wt%. Alternatively, treating oil sands tailings with an equal weight of a
2 wt% solution of
the salt also results in a salt-tailings concentration of 1 wt% in the treated
tailings.
[0054] The highly water soluble salt(s) can be used to treat oil sands
tailings as a solid,
e.g., combining the salt as a powder with the tailings. Alternatively, the
salt can be used to treat
oil sands tailings as a solution, e.g., combining an aqueous salt solution
with the tailings. In
some aspects of the present disclosure, an aqueous solution of the highly
water soluble salt can
be prepared having a concentration of no less than about 1 wt%, e.g., greater
than about 2 wt%, 5
wt%, 10 wt%, 20 wt%, 30 wf)/0 and even as great as a 40 wt% or as an aqueous
salt slurry. The
oil sands tailings and salt solution or slurry should be mixed at a ratio
sufficient to destabilize the
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
14
tailings and/or cause consolidation of the solids therein. In one aspect of
the present disclosure,
the oil sands tailings and the salt solution are mixed at a ratio of between
5.0:1.0 and 1.0:5.0,
e.g., mixed at a ratio between 1.5:1.0 to 1.0:1.5 oil sands tailings to salt
solution.
[0055] In some embodiments of the present processes, it can be more
advantageous to
use a natural source of a highly soluble salt or salts such as in a natural
body of water including
such salts in sufficiently high concentration such as at least about 2 wt% and
even at least about
3 wt% or greater. For example, ocean or sea water can be used as a source of
highly soluble
salts, which can significantly improve the economics of the process under
certain conditions.
The vast majority of seawater has a salinity of between 31 g/kg and 38 g/kg,
that is, 3.1-3.8%.
On average, seawater in the world's oceans has a salinity of about 3.5% (35
g/L, 599 mM).
Seawater includes of a mixture of salts, containing not only sodium cations
and chlorine anions
(together totaling about 85% of the dissolved salts present), but also sulfate
anions and calcium,
potassium and magnesium cations. There are other ions present (such as
bicarbonate), but these
are the main components. Another natural source of highly soluble salts that
can be used as a
source of highly soluble salts includes a hypersaline body of water, e.g., a
hypersaline lake,
pond, or reservoir. A hypersaline body of water is a body of water that has a
high concentration
of sodium chloride and other highly soluble salts with saline levels
surpassing ocean water, e.g.,
greater than 3.8 wt% and typically greater than about 10 wt%. Such hypersaline
bodies of water
are located on the surface of the earth and also subsurface, which can be
brought to the surface as
a result of oil sands mining operations.
[0056] After treating the oil sands tailings with at least one highly
water soluble salt the
solids in the tailings can be consolidated such as by mixing followed by
gravity sedimentation in
a settling tank or by centrifugation to increase the rate of forming a
consolidated material in the
treated tailings. The consolidated material can be separated from the process
water by decanting,
filtration, electrofiltration, vacuuming, and/or by mechanical dewatering,
i.e., applying an
external force to the consolidated material. Once separated, the consolidated
material can be
transferred for further dewateiing or disposal.
[0057] The process of the present disclosure allows for large scale
treatment of oil sands
tailings in a continuous or semi-continuous process. For example, the process
water separated
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
from an initial tailings treatment can advantageously include a significant
amount of the one or
more highly water soluble salt(s). This separated process water, or at least a
portion thereof, can
then be recovered and recycled to consolidate the solids of additional oil
sands tailings by mixing
the recovered process water with additional oil sands tailings. The highly
water soluble salt(s) in
the recovered process water can be concentrated and/or additional highly water
soluble salt(s)
added to formulate a solution from the recovered process water for use in
treating additional oil
sands tailings.
[0058] Although highly water soluble salts can destabilize and
consolidate solids in the
tailings, it was found that the process could be significantly improved by
including one or more
polymer flocculant(s) to the process. Including a polymer flocculant to the
process of treating
tailings with a highly water soluble salt can significantly reduce the time
for consolidation of
fines.
[0059] In addition, the processes of the present disclosure can also
include treating oil
sands tailings with coarse particles, e.g., particles with sizes greater than
44 tm, such as sand, to
significantly increase the solids content. It is believed that use of coarse
particles such as sand
are needed to increase the solids content of the tailings to greater than
about 60% without use of
thermal treatments or long processing times. While treating oil sands tailings
with water soluble
salt(s) and coarse particles without polymer flocculant(s) can consolidate
solids in the tailings,
such a process leads to a loose consolidation.
[0060] Hence, implementation of the process of the present disclosure
include (i) treating
the oil sands tailings with at least one highly water soluble salt to form a
treated tailings
including a consolidated material in the process water, (ii) treating the oil
sands tailings with at
least one highly water soluble salt and at least one polymer flocculant to
form a treated tailings
including a consolidated material in the process water, (iii) treating the oil
sands tailings with at
least one highly water soluble salt thereof, and coarse particles to form a
treated tailings
including a consolidated material in the process water, and (iv) treating the
oil sands tailings with
at least one highly water soluble salt, at least one polymer flocculant and
coarse particles to form
a treated tailings including a consolidated material in the process water.
Each of these
implementations can include aqueous solutions of the salt and/or polymer
flocculant to treat the
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
16
tailings. In certain embodiments, the oil sands tailings can contain about 5
wt% to 60 wt%
solids, e.g., from between about 10 wt% to about 50 wt%. Each of these
implementations can
include separating the process water from the consolidated material.
Advantageously, the
consolidated material has a density greater than the process water. The
process water can be
readily separated from the consolidated material as, for example, by one or
more of decanting,
filtering, electrofiltering, cross-flow filtering, gravity draining, vacuuming
and other evaporating
techniques, etc. or combinations thereof and/or by one or more of a device for
dewateting
consolidated material such as a centrifuge, decanting centrifuge, dewatering
screw,
hydrocyclone, vacuum belt filter, filter press or pressing devices, etc. or
combinations thereof.
In addition, the separated consolidated material can be disposed or deposited
in a containment
structure which allows removal of released water from the consolidated
material.
[0061] Polymers that are useful in practicing the present disclosure
include water soluble
flocculating polymers such as polyacrylamides or copolymers thereof such as a
nonionic
polyacrylamide, an anionic polyacrylamide (APAM) such as a polyacrylamide-co-
acrylic acid,
and a cationic polyacrylamide (CPAM), which can contain co-monomers such as
acryloxyethyltrimethyl ammonium chloride (DAC), methacryloxyethyltrimethyl
ammonium
chloride (DMC), dimethyldiallyammonium chloride (DMDAAC), etc. Other water
soluble
flocculating polymers useful for practicing the present disclosure include a
polyamine, such as a
polyamine or quaternized form thereof, e.g., polyacrylamide-co-
dimethylaminoethylacrylate in
quaternized form, a polyethyleneimine, a polydiallyldimethyl ammonium
chloride, a
polydicyandiamide, or their copolymers, a polyamide-co-amine, polyelectrolytes
such as a
sulfonated polystyrenes can also be used. Other water soluble polymers such as
polyethylene
oxide and its copolymers, and polymers based on modified starch and other. The
polymer
flocculants can be synthesized in the form of a variety of molecular weights
(MW), electric
charge types and charge density to suit specific requirements. Advantageously,
the flocculating
polymer used in practicing processes of the present disclosure do not include
use of activated
polysaccharides or activated starches, i.e., polysaccharides and starches that
have been heat
treated, in sufficient amounts to lower the density of the floc to below the
density of the process
water from which they are separated. Such activated polysaccharides and
activated starches
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
17
when used in sufficiently high dosages tend to form low density flocs which
rise to the surface of
the treated tailings, which can hinder removal of solids in large scale
operations involving high
solids content and can also hinder dewatering of consolidated material.
[0062] The amount of polymer(s) used to treat tailings should preferably
be sufficient to
flocculate the solids in the tailings and any added sand. The amount of
polymer(s) used to treat
tailings can be characterized as a concentration based on the total weight of
the tailings or as a
dosage based on the weight percent of the solids in the tailings.
[0063] In some embodiments of the present disclosure, the concentration
of the one or
more polymer flocculant(s) in the treated tailings has a polymer-tailings
concentration of no less
than about 0.001 wt%, e.g., no less than about 0.003 wt%, 0.005 wt% or no less
than about 0.01
wt%. For relatively short processing times, consolidation of the fines/sand
mixture can be
obtained at polymer-tailings concentrations no less than about 0.04 wt%. The
term "polymer-
tailings concentration" as used herein refers to the concentration of the
flocculating polymer(s) in
the treated tailings and is determined by taking the percentage of the mass of
the polymer(s)
divided by the combined mass of the polymer(s) plus the tailings and any water
used to dissolve
the polymer(s). For example, combining 1 part undiluted (i.e., neat) polymer
to 9999 parts
tailings by weight results in a polymer-tailings concentration of 0.01 wt%.
Alternatively,
treating oil sands tailings with an equal weight of a 0.02 wt% solution of the
polymer also results
in a polymer-tailings concentration of 0.01 wt%. In certain embodiments, oil
sands tailings is
treated with at least one polymer flocculant to yield a polymer-tailings
concentration of no less
than about 0.02 wt%, such as no less than about 0.03 wt%, 0.04 wt%, 0.05 wt%,
and even at
least about 0.07 wt%, 0.09 wt%, 0.1 wt%, 0.2 wt%, etc. The amount of polymer
flocculant can
be used in greater concentrations. However, after certain high concentrations
it becomes
difficult to dissolve the flocculant, the solution becomes too viscous and the
process is less
economical.
[0064] In some embodiments of the present disclosure, the concentration
of the one or
more polymer flocculant(s) in the treated tailings has dosage (weight of the
flocculant(s) to
weight of the solids in the tailings) of no less than about 0.005 wt%, e.g.,
no less than about 0.01
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
18
wt% and preferably no less than about 0.015 wt%, 0.020 wt%, 0.025 wt%, 0.03
wt%, or 0.04
wt%.
[0065] It was observed that the amount of polymer flocculant can be
reduced if the salt-
tailings concentration is increased. While the reason for this effect is not
clear, a very low
polymer-tailings concentration of no less than about 0.001 wt%, e.g. no less
than about 0.003
wt%, 0.005, wt%, 0.01 wt%, 0.02 wt%, 0.03 wt%, 0.04 wt%, 0.05 wt %, for
example, can
achieve reasonably fast consolidation of solids in oil sands tailings if the
salt-tailings
concentration is increased.
[0066] Coarse particles useful for practicing processes according to the
present disclosure
are preferably sand and when used in treating tailings the amount of such
particles are preferably
in a sand to fines ratio (SFR ratio) of less than 4:1, e.g., between about
2.5:1.0 to 0.5:1 or
between about 0.75:1 and 2.25:1. The SFR ratio is calculated by determining
the amount of sand
added to an estimated amount of solid fines in the tailings on a weight basis.
It is believed that
the use of coarse particles facilitates packing of the consolidated fines
which advantageously
increases the solids content and can even form a jammed structure of
consolidated solids, i.e. a
structure in which generally individual particles of the consolidated solid
can no longer move
freely relative to other particles.
[0067] Treating oil sands tailings with at least one highly water soluble
salt and
optionally with either or both of at least one polymer flocculant and/or sand
can be carried out in
a number of ways. In certain embodiments, treating the oil sands tailings
includes combining
and/or mixing the various components. In addition, the at least one salt can
be added directly to
the tailings either as an undiluted powder or as a solution; the at least one
polymer flocculant can
be added directly to the tailings either as an undiluted material or as a
solution, and the sand can
be added to the tailings directly or with the salt and/or polymer or solutions
thereof. The salt and
polymer can be combined in a single solution, with or without sand, and
combined with the
tailings. The order of combining the salt, polymer and sand to the tailings
can give equivalent
results and optimization of the process will depend on the scale and equipment
used in the
process.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
19
[0068] However, it tends to be more convenient to first prepare one or
more solutions
including the one or more highly water soluble salt(s) and the one or more
polymer flocculant(s)
followed by combining the one or more solutions with the oil sands tailings
and sand. In certain
embodiments, an aqueous solution of one or more highly water soluble salt(s)
can be prepared
having a concentration of no less than about 0.5 wt% or 1 wt%, e.g., at least
about 2 wt%, 3
wt%, 4 wt?/o, 5 wt%, 6 wt%, 7 wt?/o, 10 wt%, 20 wt%, 30 wt% and even as great
as a 40 wt% or
as an aqueous salt slurry for use in treating the tailings. The one or more
polymer flocculant(s)
can also be included in the aqueous solution of the salt(s) and can have a
concentration of no less
than about 0.001 wt!/o, e.g. no less than about 0.003 wt%, 0.005 wt%, 0.01
wt%, 0.04 wt!/o, 0.05
wt %, 0.1 wt%, 0.2 wt%, 0.4 wt%, for example. The aqueous solution of the
highly water
soluble salt(s) and polymer flocculant(s) can be used to treat the oil sands
tailings and can be
combined with such tailings at a ratio of between 5.0:1.0 and 1.0:5.0, e.g.,
at a ratio between
1.5:1.0 to 1.0:1.5 of oil sands tailings to aqueous solution. Sand can be
combined with the
tailings before, during, or after combining the tailings with the solutions.
[0069] Because highly water soluble salts and polymer flocculants that
are preferably
water soluble are used in the process of the present disclosure, the
temperature of the treated
tailings need not be elevated above ambient to practice the process. In
certain embodiments,
treating the oil sands tailings according to the various embodiments herein
can be carried out at a
temperature of no more than 50 C, e.g., no more than about 40 C or about 30
C.
[0070] In practicing aspects of the present disclosure, oil sands
tailings, e.g., a suspension
of particulate solids in an aqueous liquid which include fines and process
water, can be
consolidated by treating the oil sands tailings with at least one highly water
soluble salt or
aqueous solutions thereof and can optionally include either or both of (i) at
least one polymer
flocculant, e.g., a water soluble flocculating polymer, or aqueous solutions
thereof, and/or (ii)
coarse particles, e.g., sand to form a treated tailings. Treating tailings in
this manner can cause
destabilization and consolidation of the solids, e.g., fines and sand, in the
treated tailings to form
a consolidated material, which can settle under gravity relatively quickly, in
the process water.
The process water can then be readily separated from the consolidated
material.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
[0071] The treated tailings and/or consolidated material can be further
dewatered to
further separate the process water from the consolidated material and, in some
instances, further
consolidate the solids. In some embodiments, the consolidated material formed
in the treated
tailings can be separated from the process water by any one or more of
decanting, filtering, e.g.,
electrofilteting, cross-flow filtering, gravity draining, vacuuming and other
evaporating
techniques, etc. or combinations thereof and/or by any one or more of a
mechanical dewatering,
i.e., applying an external force to the consolidated material, with a device
for dewatering
consolidated material such as by applying a centrifuge, decanting centrifuge,
dewatering screw,
hydrocyclone, filter press, pressing device, etc. or combinations thereof. In
one aspect of the
processes of the present disclosure, the process water can be separated from
the consolidated
material by passing a stream of treated tailings through a cross-flow filter,
e.g., a porous or
slotted pipe, which filters and dewaters the treated tailings stream to
separate the process water
from the consolidated material. In another aspect of the processes of the
present disclosure, the
process water can be separated from the consolidated material by gravity
draining to achieve a
solids content of at least about 700/o within about a month after treating the
tailings, e.g., within
about two weeks or within about one week of gravity draining after treating
the tailings. In still
further aspect of the processes of the present disclosure, the consolidated
material can be further
dewatered after separating from the treated composition by depositing the
separated consolidated
material in a thin lift deposition.
[0072] The consolidated material formed in the treated tailings can
advantageously have
a high solids content, e.g., a solids content of greater than about 50% and at
least about 60%,
65%, 70% and 75% by weight. In addition, the consolidated material formed in
the treated
tailings according to certain embodiments can result in a high solids content
after mixing and/or
dewatering the treated tailings in a short period. In embodiments of the
present disclosure, the
consolidated material can have a solids content of greater than about 50% and
at least about
60%, 65%, 70%, 75% and 80% by weight after mixing and/or dewatering. In
certain
embodiments a solids content of at least about 70 ()/0 is achieved within
about one month of
gravity draining after treating the tailings, e.g., within about two weeks or
within about one week
of gravity draining after treating the tailings.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
21
[0073] In an embodiment of the present disclosure, the process includes
mixing the oil
sands tailings with a highly water soluble salt, e.g., an ammonium based salt,
a water soluble
polymer, e.g., a polyacrylamide, and sand, e.g., in a sand to fines ratio of
between 0.75:1 and
2.25:1 to form a treated tailings including a consolidated material having a
high solids content,
e.g., a solids content of greater than about 50% by weight, e.g., at least
about 60 4), 65%, 70 wt%
or higher.
[0074] Another advantage of the processes of the present disclosure is
the recovery of
materials from tailings that include rare earth elements. For example, certain
tailings can include
valuable minerals that include rare earth elements. A rare earth element
(REE), as defined by
IUPAC, is one of a set of seventeen chemical elements in the periodic table,
specifically the
fifteen lanthanides, as well as scandium and yttrium. Scandium and yttrium are
considered rare
earth elements because they tend to occur in the same ore deposits as the
lanthanides and exhibit
similar chemical properties. Many of the REE are used in electronic devices,
magnets, high
performance coatings. Such REE include cerium (Ce), dysprosium (Dy), erbium
(Er), europium
(Eu), gadolinium (Gd), holmium (Ho), lanthanum (La), lutetium (Lu), neodymium
(Nd),
praseodymium (Pr), promethium (Pm), samarium (Sm), scandium (Sc), terbium
(Tb), thulium
(Tm), ytterbium (Yb) and yttrium (Y).
[0075] REE in aqueous fines are typically in the form of an ion or oxide.
For example,
zirconium can be present as zircon, ZrSiat, titanium can be present as the
minerals ilmenite,
leucoxene and rutile. Coal ash and coal cleaning wastes contain rare earth
elements. Fire clay
coal ash has unusually high concentrations of Yttrium and zirconium. Oil sands
tailings also
contain REE.
[0076] The processes of the present disclosure are useful in recovering
REE. It is
believed that in some tailings, REEs absorb on the surface of clays in
tailings. In other tailings,
REEs are included also among the solids of the tailings or are predominately
included among the
solids of the tailings. Absorbed REEs can be exchanged with the highly water
soluble salts of
the present disclosure, e.g., ammonium based salts due to an exchange of
ammonium ions for the
REE ions. REEs from the solids of the tailings can be obtained by leaching the
solids with acid
followed by extraction and precipitation or by caustic decomposition followed
by acid leaching.
CA 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
22
[0077] Another aspect of processes of the present disclosure includes
consolidating an
aqueous composition including fines and process water, e.g., tailings, which
include REE
materials by treating the composition with at least one highly water soluble
salt, e.g., an
ammonium based salt such as ammonium sulfate, to form a treated composition
including a
consolidated material in process water which includes the REE materials in the
process water
and/or among the consolidated materials. In one aspect of the present
disclosure, the treated
composition consolidates the fines and also separates REE materials from the
solids and into the
process water. The process water can then be separated from the consolidated
material and the
REE materials can be recovered from the separated process water. The REE
materials can be
recovered from the process water by precipitation, e.g., using oxalic acid, or
extraction. Other
methods for recovering REE from the process water include mineral processing
and physical
beneficiation, deep eutectic solvents/ionic liquids extraction, acid
dissolution, high temperature
phase separations, use of REE selective sorbents, photophoresis, in-situ brine
injection and
extraction, reactive grinding, etc. In other aspect of the present disclosure,
the treated
composition consolidates the fines and REEs are among the consolidated
materials. The process
water can then be separated from the consolidated material. The consolidated
material can then
be leached with acid, e.g., nitric acid, sulfuric acid, etc., followed by
extraction with solvent
and/or ion exchange resins and precipitated. Alternatively, the consolidated
material can then be
treated with a caustic reagent such as sodium hydroxide to decompose certain
of the materials to
form hydroxides of the REEs followed by leaching in acid, e.g., HCl.
[0078] The process of the present disclosure allows for large scale
treatment of oil sands
tailings in a continuous or semi-continuous process with further recovering,
recycling and
purifying at least a portion of the process water in the tailings. When non-
hydrolyzing, highly
water soluble salts are used in the processes of the present disclosure, the
process water separated
from an initial treated tailings can advantageously include a significant
amount of the one or
more highly water soluble salt(s) initially used to treat the tailings. In
certain embodiments, the
separated process water includes the at least one highly water soluble salt
and the process
includes recovering at least a portion of the separated process water;
recycling at least a portion
CA 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
23
of the recovered separated process water to treat additional oil sands
tailings; and/or purifying at
least a portion of the recovered process water.
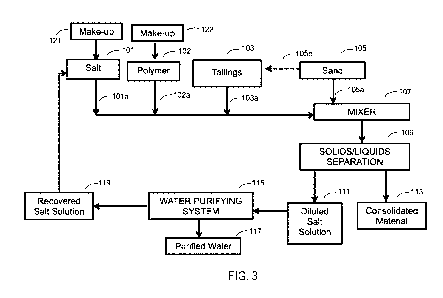
[0079] Figure 3 schematically illustrates such an exemplary continuous or
semi-
continuous process. As shown in the figure, oil sands tailings, e.g., the oil
sands tailings
containing about 5 wt% to 60 wt% solids, is treated with one or more highly
water soluble salt(s)
by combining a stream of the salt(s) (101a), which can be as an aqueous
solution, with a stream
of Tailings (103a). Optionally, the tailings can also be treated with one or
more polymer
flocculant(s) by combining a stream of the flocculants(s) (102a), which can be
as an aqueous
solution, with the Tailings stream (103a). Alternatively, the salts(s) and
flocculant(s) can be
combined together as a solution to treat the tailings as a stream thereof.
Coarse particles (sand)
can also be added to the oil sands tailings or stream thereof and/or to any or
all of the solution
streams.
[0080] The streams of salt(s) and polymer(s) can be sourced from holding
tanks 101 and
102 and the streams of Tailings and sand can be sourced from holding tanks 103
and 105,
respectively. Alternatively, the Tailings can be sourced from an oil sands
extraction operation.
[0081] For this embodiment, the stream of salt(s) (101a) and polymer(s)
(102a) and
Tailings stream (103a) are carried to mixing device 107 where a stream of sand
(105a) is added
and the combination mixed. Mixing device 107 can be an inline mixer, a mixing
tank, ribbon
mixer or other mixing device that can mix streams 101a, 102a, 103a and 105a.
For this
embodiment, the oil sands tailings are combined with the salt(s) and
polymer(s) as solutions
followed by addition of sand to treat the tailings. However, the order can be
changed, e.g., the
sand can be combined with the oil sands tailings (105b) followed by mixing
with the salt(s) and
polymer(s) solutions. The sand can be added as a wet or dry stream. In some
embodiments, the
combination of the streams in a line can cause sufficient mixing to eliminate
the need for a
separate mixing device, e.g., inline mixing, and the combined streams can be
carried directly to a
mechanical dewatering device to separate consolidated material from process
water and, in some
instances, to further consolidate the solids in the consolidated material.
[0082] As shown in the embodiment of Figure 3, after mixer 107, the
treated tailings,
which include a consolidated material and process water, is transferred to
dewatering device 109
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
24
to separate the process water from the consolidated material. Such dewatering
devices include,
for example, one or more of a decanting, filtering, electrofiltering, cross-
flow filtering, gravity
draining, or vacuuming device or combination thereof and/or by one or more of
a device for
dewatering consolidated material such as a centrifuge, decanting centrifuge,
dewatering screw,
hydrocyclone, vacuum belt filter, filter press or pressing devices, etc. or
combinations thereof.
[0083] Separated process water can be recovered and collected in tank 111
and separated
consolidated material can be recovered and collected in tank 113. For this
embodiment, the
recovered process water in tank 111 includes the process water from the
tailings diluted with
stream 101a and thus includes residual salt(s) from the one or more highly
water soluble salt(s)
and can possibly include residual polymer(s) form the one or more polymer
flocculant(s) as well
as contaminants from the tailings There are also highly water soluble salts
that are constituents
of the original tailings and these become part of the recovered process water.
In some
embodiments, the recovered process water in tank 111 can then be transferred
to a water
purifying system 115 to purify at least a portion of the recovered process
water which is
transferred to tank 117. Water purifying systems that can be used for
embodiments of the
processes of the present disclosure include reverse osmosis systems, vacuum
distillation systems,
electrodialysis, filtration systems, etc. The remaining, non-purified
recovered process water is
transferred to tank 119 to recover process water including the one or more
highly water soluble
salt(s) and highly water soluble salts that are constituents of the original
tailings. This
remaining, non-purified recovered process water can be recycled back to the
tailings treatment
process. For this embodiment, at least a portion of the non-purified recovered
process water can
be recycled back to holding tank 101 and deficiency in the concentration of
the salt(s) or
polymer(s) can be corrected by adding additional highly water soluble salt(s)
or polymer
flocculant(s) from one or more make-up tanks such as make-up tanks 121 and
122.
[0084] The process of the present disclosure can also include steps to
recover residual
hydrocarbon, e.g., tar, crude oil, heavy oil, or other hydrocarbon oil,
bitumen, asphaltenes, etc.
from the oil sands tailings. As explained earlier, oil sands tailings
typically include a low
amount of residual bitumen, e.g., MFT include about 1 to 2 wt% residual
bitumen. The oil sands
tailings can also include residual asphaltenes depending on the oil sands
extraction process as
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
well as other hydrocarbons. The process of the present disclosure can include
adding an organic
solvent (e.g., naphtha, kerosene or a C5.8 hydrocarbon, such as pentane,
hexane, heptane,
benzene, toluene, etc. or mixtures thereof) to dilute residual hydrocarbons
and form an organic
mixture and removing the organic mixture, e.g., diluted hydrocarbons, for
example.
Advantageously, the hydrocarbon separated from the tailings can contain a low
amount of fines
or has low minerals content, e.g., less than about 1 wt% or no more than about
0.5 wt% or no
more than about 0.1 wt%. The determination of fines content can be assessed by
detecting for
mineral matter content in the separated hydrocarbon by infrared spectroscopy,
x-ray diffraction,
ash content or by an equivalent method.
[0085] In addition, the consolidated solids can be recovered. The
recovered consolidated
solids can include residual highly water soluble salt(s) from the treatment of
the tailings. When
the salt used in treating the tailings is beneficial to plant life, such as an
ammonium based salt or
sulfate based salt or phosphate based salt, the residual salt can act as a
fertilizer with the
consolidated solids.
EXAMPLES
[0086] The following examples are intended to further illustrate certain
preferred
embodiments of the invention and are not limiting in nature. Those skilled in
the art will
recognize, or be able to ascertain, using no more than routine
experimentation, numerous
equivalents to the specific substances and procedures described herein.
[0087] Treatment of Tailings with Highly Water Soluble Salt.
[0088] For this set of experiments, five vials of MFT were prepared,
i.e., vials labeled A,
B, C, D, and E, shown in Figure 4. Each of the five vials included
approximately 5 grams of
MFT. The MFT were obtained from Alberta Innovates Corp., which obtained the
samples from
oil sands tailings ponds. The MFT had a sludge-like consistency with an
average solids content
of about 30 wt%.
[0089] Water was added to vial A in an equal weight of the MFT, which was
used as a
control for this experiment. The MFT in vials B and C were treated by adding
an equal weight
of a sodium chloride (NaC1) solution to MFT, e.g. vials B and C each contain a
1.0:1.0 ratio of
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
26
NaC1 salt solution to MFT, by weight. For vial B, a 25 wt% NaCl solution was
used and for vial
C, a 10 wt% NaCl salt solution was used as the salt solution. Thus the salt-
tailings concentration
for vial B was 12.5 wt% and for vial C was 5 wt%. An equal weight of an
ammonium chloride
(NH4C1) solution to MFT was added to vials D and E. For vial D, a 25 wt% NH4C1
solution was
used and for vial E, a 10 wt% NI-14C1 solution was used as the ammonium
chloride salt solution.
Thus the salt-tailings concentration for vial D was 12.5 wt% and for vial E
was 5 wt%.
[0090] The materials in the vials were then mixed by shaking and were
then centrifuged.
The vials were centrifuged at 3000 rpm for 30 minutes on a centrifuge. After
centrifugation, the
vials were re-shaken. The purpose of re-shaking the samples was to provide an
equal starting
point for all of the samples for comparisons. The samples were then allowed to
stand and
separate under gravity. It should be noted that in practicing certain aspects
of the process of the
present disclosure, the samples would be mixed and, optionally, centrifuged
but not re-shaken.
[0091] The appearance of vial A after standing for 10 minutes showed a
small degree of
settling, which indicating that dilution with water alone is useful but does
not significantly cause
consolidation of the solids. The vials containing salt solutions (Vials B, C,
D, and E) showed an
enhanced rate of settling, however, with the 10% NH4C1 solution showing
visible signs of
forming a clear supernatant. After 1 hour, the vials containing NH4C1
solutions (vials D, E) were
almost clear, while the vials with added NaCl solutions (vials B, C) were also
starting to clarify
as the mineral fines settled. In contrast, the control vial containing just
added water remained
murky with just a small degree of settling.
[0092] In order to demonstrate that the settled mineral fines were also
consolidated, the
vials were vigorously re-shaken and again allowed to stand. The salt
containing vials quickly
settled, while the water control vial only had a small degree of settling and
consolidation. This is
more clearly seen in a picture of the vials taken one week later, shown in
Figure 4. The water
control vial A contains some settled material and a large volume of an
unsettled and
unconsolidated material. There is a relatively small volume of what appears to
be clear water at
the top. (The clear water probably contains some dissolved salts from the MFT
itself). The vials
that were treated with added highly water soluble salts clearly showed just
two phases, however.
CA 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
27
A consolidated, settled sediment of fines at the bottom of the vials with a
clear water solution of
salts present as a supernatant can be seen.
[0093] The supernatant liquid above the consolidated solids in the vials
with added salt
solutions, together with water that remains dispersed between the mineral fine
particles in the
consolidated material, can be removed by decanting, centrifugation or other
methods known in
the art.
[0094] Treatment of Tailings with Highly Water Soluble Salt and/or Water
Soluble
Polymer.
[0095] For this set of experiment, four vials of MFT were prepared and
treated, i.e., vials
labeled A2, B2, C2, and D2. Each of the four vials included approximately 5
grams of MFT.
The MFT were obtained from Alberta Innovates Corp., which obtained the samples
from oil
sands tailings ponds. The MFT had a sludge-like consistency with an average
solids content of
about 30 wt%.
[0096] Each vial contained 50% MFT by weight and 50% by weight of an
added
solution. The solution added to vial A2 included 10% ammonium chloride and
0.1% of
polyacrylamide in water; the solution added to vial B2 included 10% ammonium
chloride and
0.1% of a cationic polyacrylamide in water; the solution added to vial C2
included 0.1% of
polyacrylamide without salt; and the solution added to vial D2 included 0.1%
of a cationic
polyacrylamide without salt.
[0097] After adding an equal weight of each of the solutions to MFT, the
vials A2
through D2 were centrifuged at 3000 rpm for 1 min on a LW Scientific
laboratory centrifuge.
After the vials were centrifuged, it was observed that the addition of polymer
alone was not
effective under the experimental conditions to cause significant consolidation
of the fines in the
MFT.
[0098] To provide a more quantitative measure of sedimentation rate and
extent of
compaction, centrifugation tests using various polymers were conducted in
calibrated centrifuge
tubes. Six samples were prepared by combining 5 ml of MFT and 5 ml of a
solutions to each
tube labeled A3, B3, C3, D3, E3 and F3 such that each tube contained 10 ml of
the combined
MFT and solution. The first tube (A3) had just 5 ml of water added to the 5 ml
of MET and was
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
28
used as a control tube. The second tube (B3) had just 5 ml of a 10% ammonium
chloride (AC)
solution added to 5 ml of MFT. The remaining four tubes (C3, D3, E3, F3) were
prepared by
adding 5 ml of a solution including 100/o ammonium chloride with 0.1 wt% of
either a
polyacrylamide (PAM) (C3), a polyacrylamide copolymer (cationic PAM1) (D3), an
anionic
polyacrylamide (anionic PAM) (E3), or a cationic polyacrylamide (cationic
PAM2) (F3),
respectively.
[0099] The tubes were then centrifuged at 3000 rpm, initially for 10
seconds. (Note that it
took about 20 seconds to reach this speed and approximately another 20 seconds
to come to a
halt after turning the motor off). As seen in the results of Figure 5, which
show the tubes after
centrifuging at 3000 rpm for 30 seconds, the addition of polymers to the
systems clearly sped-up
consolidation of the solids by aggregating and flocculating particles to give
a larger effective
mass. Tube C3 clearly gave the best result.
[00100] Centrifugation at 3000 rpm was continued for additional periods of
time and the
volume of compacted slurry vs. centrifugation time is plotted in Figure 6.
[00101] As demonstrated by the data in Figure 6, the water control showed
no
compaction, even after centrifuging at 3000 rpm for close to 500 seconds. The
ammonium
chloride alone, with no polymer additive, showed a much slower compaction rate
than those that
also used polymer. Apart from the tube containing water, all slurries showed
an initial
(relatively) fast rate of sedimentation. Addition of polymer PAM at a
concentration of 0.1 wt%
resulted in a very fast compaction of the MFT, with a partly consolidated mass
forming in the
first 10 seconds (at 3000 rpm). After the initial fast rate of sedimentation,
the rate of compaction
slowed significantly and the improved rate of compaction did little to affect
the solids content
obtained after centrifuging 8 min.
[00102] Solid contents of the samples were determined from the volume of
the compacted
slurry and the known weight of fines in the WT. For tube C3, containing
polymer PAM, the
solids content was calculated to be about 44%. This value was checked by
drying the slurry
from tube C3. That is, the consolidated material in the form of a slurry for
tube C3 was
separated from the supernatant liquid by decanting the liquid. The separated
slurry was then
removed from the tube and the wet mass weighed followed by drying and
reweighing the dried
CA 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
19
mass to determine the solids content. Drying the sample of C3 gave a dried
solids content of
about 46%.
[00103] Both estimates of the solid contents, volume measurement and
weight
measurement, are subject to some measurement error. For example, the
calculated values based
on volume can have measurement error due to some difficulty in accurately
measuring the
slurry/liquid boundary in the tube and assumptions concerning the specific
gravity of the
minerals, while the weighing experiments can have measurement error due to
carried-over free
water near the surface of the slurry when separating the supernatant liquid
from the slurry.
Nevertheless, these numbers are consistent and show the relative trend and
relative performance
of the various treatments of the MFT.
[00104] Treatment of Tailings with Highly Water Soluble Salt, Water
Soluble Polymer
and Coarse Particles.
[00105] Vial tests were performed using 5 ml of MFT to which was added 5
ml of a
solution of 10 wt% of ammonium chloride and 0.1 wt% of PAM. Sand was then
added to this
mixture. Three different ratios of sand to fines (SFR), 0.75/1, 1.5/1, 2.25/1,
respectively, were
used for each vial. The results after centrifuging for various periods of time
are summarized in
Figure 7.
[00106] Care was taken to avoid segregation of the fines and sand, as
there is some
immediate flocculation of the particles as MFT and NH4C1/PAM solution are
mixed. However,
the initial flocs appear to be open structures (low solids content) and good
mixing of the sand
and the initial flocs occurred as the polymer acts to tie coarse and fine
particles together, both
limiting segregation and speeding up compaction.
[00107] Treating MFT with the salt, polymer and sand quickly gave a
consolidated
material with the consistency of a wet solid. The calculated final solids
contents were about 62%,
about 75% and about 90% for SFR ratios of 0.75/1, 1.5/1 and 2.25/1,
respectively. All sand/fines
ratios are significantly less than 4:1 SFR ratio. For the 1.5/1 SFR sample,
the solids content
determined by drying was about 73%.
[00108] In addition, all three solids appeared to have formed a "jammed"
structure. They
all had a degree of mechanical integrity and had to be scraped out of the
tubes with some
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
difficulty, unlike the paste-like slurries obtained with the use of ammonium
chloride and/or
polymer alone.
[00109] The experiments with the two lowest SFR ratios were repeated.
Figure 8 shows
the slurry volume observed after centrifuging for various periods of time. The
results are slightly
different to those reported in Figure 7 (possibly as a result of jamming or
measurement errors),
but slurry volumes were again consistently reduced to 3.5 ml or less within 30
sec. The
calculated solids contents are shown in Figure 9. For an SFR ratio of 1.5/1,
the solids content
was calculated to be about 75%, while for a SFR ratio of 0.75/1, the solids
content was
calculated to be about 68%. After further centrifugation, the solids content
of the consolidated
material determined by drying were about 74% and about 67%, respectively.
[00110] Varying Salt and Salt Concentration
[00111] Additional experiments were carried out with various highly water
soluble salts
and in different concentrations and with and without sand to treat oil sands
tailings. A series of
salt/polymer solutions were prepared. All of the salt/polymer solutions
included 0.1 wt% of
polyacrylamide (PAM) but varied the type and concentration of the salt. For
example, a series of
10 wt%, 5 wt% and 2 wt% calcium chloride solutions each with 0.1 wt% of PAM
were prepared
and used to treat MFT. Other 10 wt%, 5 wt% and 2 wt% salt solutions of
ammonium sulfate,
potassium chloride, etc. were prepared each with 0.1 wt% of PAM. An equal
weight of a
particular salt/polymer solution was then combined with Mfg', with or without
sand, in a vial
followed by vigorous mixing. The vials were then centrifuged at 3000 rpm on a
LW Scientific
laboratory centrifuge for 30 seconds to form a consolidated material in the
form of a slurry.
After centrifugation, the supernatant liquid was separated from the
consolidated material by a
pipette. The consolidated material was then weighed, dried and reweighed to
determine a solids
content of the consolidated material. The various salts and their
concentrations which were used
to treat NET and the resultant solids content data are summarized in Tables 1
and 2 below.
Table 1: Solids content of MFT treated with an equal weight of a salt/PAM
solution without the
addition of sand and after centrifugation.
Salt 10% Concentration' 5% Concentration' 2%
Concentration3
(+ 0.1 wt% PAM)
CA 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
31
No Sand
Ferric Chloride (FeC13) 34.9 A) 35.6%
Aluminum Sulfate 33.1% 34.1%
(A1004)3)
Calcium Chloride 36.8% 37.1% 35.8 A)
(CaCl2)
Ammonium Sulfate 33.1% 31.8% 31.4%
(NH4SO4)
Potassium Chloride 35.4% 32.4% 33.5%
(KC1) õ_
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
32
Table 2: Solids content of MFT treated with an equal weight of a salt/PAM
solution with the
addition of sand (SFR ratio 1:1) after centrifugation.
Salt 10% Concentration' 5% Concentration2 2%
Concentration3
(+ 0.1 wt% PAM)
With Sand
Ferric Chloride 45.7% 52.8%
(FeCl3)
Aluminum Sulfate 51.4% 53.7%
(Al2(5003)
Calcium Chloride 58% 56.8% 56.1%
(CaCl2)
Ammonium Sulfate 53.6% 52.3% 53.5%
(NH4SO4)
=
Potassium Chloride 53.4% 52.5% 53.9%
(KCl)
1. The salt-tailings concentration was about 5 wt%.
2. The salt-tailings concentration was about 2.5 wt!/o.
3. The salt-tailings concentration was about 1 wt%.
[00112] Table 1 reports the solids content of dried consolidated material
following treating
of MFT with the various salt/polymer solutions without sand. After
centrifugation for just 30
seconds, the highly water soluble salts gave solids contents for the
consolidated materials in a
range between about 31%-37%. However, the use of highly water soluble salts
having a
multivalent cation such as the aluminum and ferric cations appeared to cause
fouling of the vial
walls and gave a less cohesive consolidated material as compared to highly
water soluble salts
having a monovalent cation under the tested conditions. In some tests using
salt concentrations
of 10%, the clarified water sitting on top of the consolidated materials were
removed using a
pipette and the wet solids pressed between paper towels. It was found that the
salts with
multivalent cations, aluminum chloride (AIC13), ferric chloride (FeCl3) and
calcium chloride
(CaCl2), which all gave significant deposits of a slimy material on the vial
walls, were less
cohesive than the pressed solids obtained using salts with monovalent cations,
such as the
ammonium salts NH4C1 and (NH4)2504.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
33
[00113] Table 2 reports the solids content of dried consolidated material
following treating
MFT with the various salt/polymer solutions and sand. Sand was added with a
1:1 sand to fines
ratio (i.e., 1.5 g of sand was added to the 5 gm of MFT having 30% solids to
give a 1:1 ratio of
the weight of sand to that of the solids in the MFT). After centrifugation for
just 30 seconds, the
highly water soluble salts gave solids contents for the consolidated materials
in a range between
about 46%-58%, which was significantly higher than the range of solids
contents without use of
sand. Although the solids content of the vials containing added sand is twice
those without sand,
the volume of the centrifuged slurry is about the same.
[00114] The data in Tables 1 and 2 show that addition of 2 wt% salt
solution to treat MFT
was as effective as a 10 wt% salt solution. That is, a 1 wt% salt-tailings
concentration was as
effective as a 5 wt% salt-tailings concentration. Since an equal weight of the
salt/polymer
solution was used to treat MFT, the salt concentration of the added salt in
the treated tailings is
one-half of the concentration in the salt/polymer solution, i.e., the added 2
wt% salt solution
provided a 1 wt% salt-tailings concentration and the 10 wt% salt solution
provided a 5 wt% salt-
tailings concentration. The salt-tailings concentration in treated MFT can be
achieved in a
number of ways. For ease of handling in the foregoing vial tests, it was
convenient to combine
equal weights of salt/polymer solutions to MFT. However, smaller amounts of
salt/polymer
solutions with higher concentrations thereof to give the same salt-tailings
concentration give
equivalent results of consolidated materials.
[00115] Centrifuging in flat-bottomed vials is not as effective in terms
of producing a high
solids material as using centrifuge tubes. It should be kept in mind that for
all sets of laboratory
vial and tube tests, there is always solution remaining in the voids between
the particles. It will
be shown later that the solids content of the consolidated material can easily
be increased from
the 46%-58% range by simply draining or the use of mechanical dewatering
methods known to
the art, such as filter presses, belt filters, dewatering sand screws,
decanting centrifuges,
hydrocyclones, etc.
[00116] Varying Salt Concentration and Polymer Concentration
[00117] When salt, polymer and sand are used together to treat tailings,
salt-tailings
concentrations in excess of 0.5 wt% and preferably no less than about 1%
should be used to
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
34
achieve reasonably fast consolidation of the solids in the tailings. In
addition, although a degree
of consolidation of the fines/sand mixture is obtained at polymer-tailings
concentrations as low
as 0.01 wt% for relatively short processing times, superior results are
obtained at polymer-
tailings concentrations of 0.05% and higher. These preferences were determined
by a set of vial
experiments, the results of which are illustrated in Figure 10. The top set of
vials shows results
obtained by adding 5 g of a 2 wt% ammonium sulfate ((N1-14)7504) solution
containing PAM to 5
g of MFT. Sand was also added to give a sand-to-fines ratio of 1:1 (i.e., 1.5
g of sand was
added). The amount of PAM in the solutions was varied between 0.1% (by weight)
and 0.02%
(by weight). The bottom set of vials show what is observed when a 1 wt% of the
ammonium
sulfate was used. The vials were centrifuged at 3000 rpm for 30 seconds to
accelerate settling.
[00118] It can be seen that for all the vials treated with the 1 wt%
(NH4)2SO4 solutions,
there is a degree of settling of the fines and sand, but the supernatant
liquid contains a significant
amount of suspended particles. In addition, visually there appears to be a
degree of segregation
of the sand and fines. In contrast, the MFT treated with a 2 wt% (NI-14)2504
solution containing
0.1 wt% PAM showed settled and compacted solids in contact with a clear
supernatant. As the
amount of polymer in the solution is reduced from vial A4 to E4, the clarity
of the supernatant
decreases, as more suspended particles remain in the liquid phase. Greater
clarity of the
supernatant liquid should be achievable at longer centrifuge times, but for
short processing times,
treating MFT to result in a salt-tailings concentration of no less than about
0.5 wt% and a
polymer-tailings concentration of no less than about 0.04 wt% are preferable.
[00119] The solids contents of the consolidated materials in each of the
vials shown in
Figure 10 was determined by drying, i.e., the centrifuged consolidated
material was separated
from its supernatant liquid, the wet mass weighed, dried and reweighed to
determine a solids
content. The solids content of the consolidated materials for the sets of
vials shown in Figure 10
are summarized in Table 3.
Ch 03070408 2020-01-17
WO 2019/018370
PCT/US2018/042450
Table 3: The solids content of centrifuged ammonium sulfate/PAM treated MFT as
determined
by separating and drying consolidated material
0.1% PAM 0.08% PAM 0.06% PAM 0.04% PAM 0.02% PAM
% Solids % Solids A) Solids % Solids %
Solids
2% (NH4)2SO4 60.3% 58.8% 58.1% 52.0% 48.5%
1% (NH4)2SO4 54.4% 57.2% 58.1% 56.3% 44.6%
[00120] It can be seen that for the 2 wt% (NI-14)2504 solution containing
0.1 wt% PAM, a
solids content of just over 60% was achieved. This decreased only slightly
when treating MFT
with solutions including PAM concentrations of 0.08 wt% and 0.06 wt%, but
significantly at
lower PAM concentrated solutions. Treating MFT with an equal weight of the
(NH4)2SO4
polymer solutions resulted in a salt-tailings concentration of about 1 wt% for
each of vials A4-
E4, and for vial A4, a polymer-tailings concentration of about 0.05 wt% PAM,
for vial B4 a
polymer-tailings concentration of about 0.04 wt% PAM, for vial C4 a polymer-
tailings
concentration of about 0.03 wt% PAM, for vial D4 a polymer-tailings
concentration of about
0.02 wt% PAM, and for vial E4 a polymer-tailings concentration of about 0.01%
PAM. For the
1 wt% (N114)2504 solutions, the solids content was very variable, reflecting
the problems with
segregation of coarse and fine particles in the consolidated materials in
these experiments.
[00121] Varying Order of Addition and Concentration
[00122] The salt-tailings concentration and polymer-tailings
concentrations in treated
MET can be achieved in a number of ways. Further, the order of combining the
highly water
soluble salt, water soluble flocculating polymer and coarse particles to the
tailings can give
equivalent results. This was shown by preparing a series of vials with MFT and
treating the
MFT by differing the order of salt (ammonium sulfate), polymer (PAM added as a
1 wt% stock
solution to the MFT to give a final polymer-tailings concentration of 0.1 wt%)
and sand and also
by adding the salt either as: (i) a 2 wt% solution, (ii) a solution including
2 wt% of the salt and 1
wt% of PAM, or (iii) an undiluted, dry powder. After treating the MFT with
salt, polymer and
sand in differing orders and differing concentrations of salt, the treated MFT
in the vials were
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
36
mixed and then centrifuged for 30 seconds at 3000 rpm. All of the so treated
MFT gave solids
contents in a range of 58 wt% to 62 wt%.
[00123] Insoluble Salts
[00124] Unlike highly water soluble salts, salts such as gypsum
(CaSO4.2H20) and lime
(CaO) that are used to coagulate wastewater have a very low water solubility
(less than 0.3
g/100g at 20 C) and work by a sweep coagulation mechanism. Largely insoluble
salts such as
gypsum and lime have also been used in treating wastewater and in attempts to
dewater WT.
These largely insoluble salts are ineffective in the process of the present
disclosure since they
produce a segregated slurry with poor cohesion. For example, if lime and water
are mixed, the
(hydrolyzed) lime remains largely insoluble. The amount of lime was 10% of the
total (lime +
water). If this 10% suspension with 0.1% PAM is added to MFT, with or without
sand, a clearly
segregated centrifuged material (3000 rpm, 30 sec) is obtained, as evident by
the layers of solids
that can be seen in the vials.
[00125] Large Scale Testing.
[00126] In initial large-scale work, about 100 lbs (approximately 45.4 kg)
of MFT was
treated with an equal weight of a 10% solution of ammonium chloride that
included 0.1% PAM.
These components were mixed together in a drum and a loose floc of fines was
formed. About
30 lbs (approximately 13.6 kg) of sand was then added, the amount of sand
being equal to the
calculated weight of fines present in the MFT (i.e., sand-to-fines solids
ratio of 1:1). After
mixing, the resulting consolidated sand/fines material has sufficient
integrity that it could be
collected with a paddle and a ball could be formed by pressing the
consolidated material by
hand. Drying a sample of the consolidated material established that it had a
solids content of
48.5%.
[00127] In addition, the consolidated material was suspended in what
appeared to be clear
liquid. Rather than attempting to centrifuge this material, a paper towel test
was conducted. A
ball of the consolidated slurry-like material was pressed a few times between
paper towels. The
towels were not fouled by fines. The final pressed disk had a solids content
of 74.5% and was
cohesive. This shows that a high solids content material can be obtained using
far less sand (1:1
SFR ratio) than presently used (4:1 SFR ratio) in composite tailings
technologies.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
37
[00128] In an additional large scale test, about 100 lbs (approximately
45.4 kg) of MFT
was mixed with a 10% solution of ammonium chloride containing 0.1% PAM in a
large ribbon
mixer. About 24 lbs (approximately 10.9 kg) of sand was then added, the
proportion of sand to
MFT solids in this run was somewhat less than in the work described above, a
SFR ratio of about
0.8:1. Mixing continued for a few minutes. The resulting consolidated slurry-
like material clearly
had two phases, a semi-solid like material in an apparently clear liquid. The
consolidated solids
and liquid was then poured out of the bottom of the mixer and the solids were
captured on a
metal sieve and the liquid quickly drained into a drum placed beneath.
[00129] A sample of the collected consolidated material was taken and
dried in an oven
overnight to determine its solids content. The solids content of the initially
gravity-drained
consolidated material which included fines and sand was determined to be 53%.
Another sample
of the collected consolidated material was pressed by hand between paper
towels. See Figure
11B. The solids content of this sample was 70%. The remaining collected
consolidated material
was allowed to drain overnight. Figure 11A shows consolidated solids collected
and draining.
The solids content of the consolidated material that was allowed to drain
overnight increased to
68% from the initially collected sample of 53%. After standing for a week, the
solids content of
the remaining collected consolidated material increased to 75%. Upon standing
for an additional
week, the drained material achieved a crumbly, relatively dry solid material
form. This shows
that a high solids content composite material can be formed according to
embodiments of the
present disclosure by simply mixing tailings with a highly water soluble salt,
polymer flocculant
and sand followed by gravity drainage.
[00130] The remaining collected consolidated material was allowed to
weather in the open
environment to further drain and dewater under gravity and natural evaporative
processes.
Within three months, the recovered treated tailings were still cohesive and
could still readily
support the weight of a person (over about 160 lbs) without deformation.
However, as with clay-
like soils, it could also be broken apart using ordinary tools.
[00131] The data of present disclosure show that oil sands tailings can be
treated with a
highly water soluble salt to consolidate the fines therein and also with such
a salt in combination
with a polymer flocculant and coarse particles, e.g., sand, to consolidate
fines in oil sands tailing.
Ch 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
38
Superior compaction rates and solids contents were provided by treating the
oil sands tailings
with the combination of at least one highly water soluble salt, at least one
polymer flocculant and
sand.
[00132] Further, the data indicates that use of a SFR ratio between about
1.5/1 to about
0.8:1 provided a solids content of at least about 70%, which solids appear to
have the mechanical
properties necessary to form a trafficable surface, in a relatively short
period of processing time,
e.g. within about one week under gravity draining. In addition, centrifugation
in a small lab
centrifuge (calculated to be approximately 1000 g centrifugal force) results
in a fast compaction
of the slurry, about 20 ¨ 40 seconds centrifugation time for slurries with an
SFR ratio greater
than about 1.5. Larger centrifuges having larger g forces (i.e., in a decanter
centrifuge) should
improve the rate and consolidation of fines.
[00133] A pilot-scale demonstration was conducted using a first holding
tank containing
NTT and a second holding tank containing a solution of highly soluble salt
(about 5% of
ammonium sulfate) and polymer (about 0.1 wt% of Non-ionic polyacrylamide). The
two
holding tanks were linked by pumps to a pipe. Sand at an SFR of about 1:1 was
added to the
NIFT. Then a stream of the MFT/sand and a stream of the salt solution were
combined and
mixed in-line through about 300 ft (about 91 m) of pipe. The resulting treated
tailings were then
emptied onto a wooden flume.
[00134] As the treated tailings emerged from the mixing pipe onto the
flume, it was
observed that the solids had already partially separated from liquids, which
appeared to be a
mostly clear stream with just a few suspended particles. The consolidated
material quickly
drained to give a material that could be pressed by hand to give a ball with a
solids content of
75%. The consolidated material was allowed to drain under gravity over a
couple of days to give
a material with a solids content in excess of 90%.
[00135] Increased Salt Concentration Allows for Lower Polymer
Concentration
[00136] When salt, polymer and sand are used together to treat tailings,
it was observed
that the polymer-tailings concentration can be reduced if the salt-tailings
concentration is
increased under certain circumstances. Thus, very low polymer-tailings
concentration can
achieve reasonably fast consolidation of solids in the tailings if the salt-
tailings concentration is
CA 03070408 2020-01-17
WO 2019/018370 PCT/US2018/042450
39
increased. Figure 12 illustrates that as the salt concentration increases,
less polymer flocculant is
needed to obtain clear supernatant solutions. For these tests, the polymer-
tailings concentration
increases from 0.01% to 0.05% in 0.01% increments from right to left while the
salt-tailings
concentration increases from 1% to 2% from top to bottom.
[00137] Only the preferred embodiment of the present invention and
examples of its
versatility are shown and described in the present disclosure. It is to be
understood that the
present invention is capable of use in various other combinations and
environments and is
capable of changes or modifications within the scope of the inventive concept
as expressed
herein. Thus, for example, those skilled in the art will recognize, or be able
to ascertain, using
no more than routine experimentation, numerous equivalents to the specific
substances,
procedures and arrangements described herein. Such equivalents are considered
to be within the
scope of this invention, and are covered by the following claims.