Note: Descriptions are shown in the official language in which they were submitted.
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
SYSTEMS, METHODS, AND DEVICES FOR FALLOPIAN TUBE DIAGNOSTICS
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application is a continuation in part of, and claims the benefit
of priority to, U.S.
Patent Application Serial No. 15/053,568, filed February 25, 2016, entitled
"Methods and Devices
for Fallopian Tube Diagnostics," which is a continuation-in-part of U.S.
Patent Application Serial
No. 14/764,710, filed on July 30, 2015, entitled "Methods and Devices for
Fallopian Tube
Diagnostics," which is a national stage application of International Patent
Application Serial No.
PCT/U52014/014472, filed February 3, 2014, entitled "Methods and Devices for
Fallopian Tube
Diagnostics," which claims priority to U.S. Provisional Patent Application
Serial No. 61/873,753,
filed September 4, 2013, entitled "Everting Catheter for Fallopian Tube
Diagnostics," and U.S.
Provisional Patent Application Serial No. 61/759,783, filed February 1, 2013,
entitled "Methods
and Devices for Fallopian Tube Diagnostics," the entire disclosures of which
applications are
expressly incorporated by reference herein.
[002] This application is a nonprovisional application of, and claims the
benefit of priority
to, U.S. Provisional Application Serial No. 62/546,791, filed August 17, 2017,
entitled "Devices
for Fallopian Tube Diagnostics," and U.S. Provisional Application Serial No.
62/660,512, filed
April 20, 2018, entitled "Methods and Devices for Fallopian Tube Diagnostics,"
the entire
disclosures of which applications are expressly incorporated by reference
herein.
FIELD
[003] The present disclosure generally relates to Fallopian tube
diagnostics, and in particular
to systems, devices, and methods that accommodate the anatomical difficulties
associated with
navigation of body lumens, including the Fallopian tube, for tissue sample
collection.
BACKGROUND
[004] Ovarian cancer is a significant disease in women, in which 1 out of
72 women in the
United States may be diagnosed with this illness during her lifetime. In 2012,
over 22,000 women
in the United States were diagnosed with ovarian cancer. Early detection of
ovarian cancer may
be difficult due to a lack of effective screening tests, such that ovarian
cancer may not be diagnosed
until the disease has reached advanced stages, limiting treatment options.
1
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
[005] Screening for ovarian cancer may typically include a surgical
procedure for obtaining
cell samples for diagnosis. For example, because the ovaries are intra-
abdominal, laparoscopic or
open surgery (laparotomy) may be performed to access the ovaries. Any surgical
procedure
increases a risk to the patient, including but not limited to experiencing an
adverse reaction, and/or
requiring significant recovery time. Additionally, an ovary biopsy may expose
the patient to
additional risk of potentially spreading diseased (e.g., cancerous) cells.
[006] Thus, there exists a need for devices and processes to allow samples
to be obtained
from a Fallopian tube for evaluation of ovarian cancer in a less invasive and
controlled fashion
and, particularly without the need for a skin incision. There further exists a
need for securing a
sample of representative cells from the Fallopian tube with a catheter to
screen for early stage
cancers.
[007] It is with respect to these and other considerations that the present
improvements may
be useful.
SUMMARY
[008] This Summary is provided to introduce a selection of concepts in a
simplified form
that are further described below in the Detailed Description. This Summary is
not intended to
necessarily identify key features or essential features of the claimed subject
matter, nor is it
intended as an aid in determining the scope of the claimed subject matter.
[009] According to an exemplary embodiment of the present disclosure, a
device for
Fallopian tube diagnostics may include a tube having a distal end and a
balloon having a first end
coupled to the distal end of the tube. The balloon may be disposed in the tube
in a first, inverted
position, may be movable to a second, everted position, and may be extendable
a distance distal
of the tube distal end such that a surface of the balloon is contactable with
an inner surface of the
Fallopian tube. A push wire may have a distal end coupled to a second end of
the balloon. The
balloon may be movable from the first inverted position to the second everted
position by actuation
of the push wire. A surface of the balloon may include a plurality of surface
features for collection,
tetelition, ur both, of a tissue sample of the inner surface of the Fallopian
tube.
[010] In various of the foregoing and other embodiments of the present
disclosure, the
surface features may include a plurality of wrinkles formed in the surface of
the balloon, and may
have at least one of plurality of edges, micro-ridges, or overlapping
material, or combinations
thereof. A plurality of wrinkles may be formable in the balloon surface. A
plurality of wrinkles
in the balloon surface may be formed in the balloon surface, and may be
configured to retain at
least a portion of the tissue sample after contacting the inner surface of the
Fallopian tube. The
surface features may be etched in the surface of the balloon. A portion of the
surface of the balloon
may be embossed to form a plurality of peaks and valleys. The plurality of
surface features may
2
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
improve adhesion of the tissue sample to the balloon surface compared to the
balloon surface
without the surface features. The balloon may be inflatable for moving the
balloon from the first
inverted position to the second everted position. A filament may be attached
to the push wire, the
filament may be disposed within the balloon in the first inverted position,
and the filament may be
extendable from the balloon in the second everted position.
[011] According to an exemplary embodiment of the present disclosure, a
system for
collecting a tissue sample in a body lumen may include a tube having a distal
end and a balloon
having a first end coupled to the distal end of the tube and a second end
coupled to a distal end of
a push wire. The balloon may be positionable in a first, inverted state. The
push wire may be
configured to advance to evert the balloon to a second, everted state, such
that the balloon extends
out of the distal end of the tube. A surface of the balloon may be configured
in the second, everted
state, to contact an inner surface of the body lumen for transference of the
tissue sample to the
balloon surface. The balloon surface may include a plurality of surface
features for collection,
retention, or both, of the tissue sample.
[012] In various of the foregoing and other embodiments of the present
disclosure, the
surface features may include a plurality of wrinkles formed in the surface of
the balloon, having
at least one of a plurality of edges, micro-ridges, or overlapping material,
or combinations thereof.
A plurality of wrinkles may be formable in the balloon surface. A plurality of
wrinkles in the
balloon surface may be configured to retain at least a portion of the tissue
sample after contacting
the inner surface of the body lumen. The surface features may be etched in the
surface of the
balloon. The plurality of surface features may improve adhesion of the tissue
sample to the balloon
surface compared to the balloon surface without the surface features.
[013] According to an exemplary embodiment of the present disclosure, a
method for
collecting a tissue sample in a body lumen may include providing a tube having
a distal end and a
balloon having a first end coupled to the distal end of the tube and a second
end coupled to a distal
end of a push wire. The balloon may be positioned in a first, inverted state.
The push wire may
be advanced to evert the balloon to a second, everted state, such that the
balloon extends out of the
distal end of the tube. A balloon surface may contact in the second, everted
state, an inner surface
of the body lumen. The balloon surface may include a plurality of surface
features for collection,
retention, or both, of the tissue sample.
[014] In various of the foregoing and other embodiments of the present
disclosure, the
surface features may include a plurality of wrinkles formed in the surface of
the balloon, and may
have at least one of a plurality of edges, micro-ridges, or overlapping
material, or combinations
thereof. A plurality of wrinkles may be formable in the balloon surface. A
plurality of wrinkles
in the balloon surface may be configured to retain at least a portion of the
tissue sample after
3
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
contacting the inner surface of the body lumen. The plurality of surface
features may improve
adhesion of the tissue sample to the balloon surface compared to the balloon
surface without the
surface features.
[015] According to an exemplary embodiment of the present disclosure, a
device for
Fallopian tube diagnostics may include a tube having a distal end, and a
balloon having a first end
coupled to the distal end of the tube. The balloon may be disposed in the tube
in a first, inverted
position, may be movable to a second, everted position, and may be extendable
a distance distal
of the tube. An extending portion may have a proximal end coupled to a second
end of the balloon.
The extending portion may be disposed within the balloon in the first inverted
position, and may
be extendable from the second end of the balloon in the second everted
position.
[016] In various of the foregoing and other embodiments of the present
disclosure, the
extending portion may be any of a filament, suture, or string, or combinations
thereof. At least a
portion of the filament, suture, or string, or combinations thereof, may be
braided. The extending
portion may be formed of one or more filaments having a color. The colors of
the one or more
filaments of the extending portion may provide for a contrasting
visualization. The extending
portion may include one or more knots or indicia for one or both of visual and
tactile feedback.
The extending portion may be a braided filament configured to collect and
retain a tissue sample
in response to extending from the balloon in the second everted position. A
push wire may have
a distal end coupled to the second end of the balloon and the proximal end of
the extending portion.
The balloon and the extending portion may be movable from the first inverted
position to the
second everted position by actuation of the push wire.
[017] According to an exemplary embodiment of the present disclosure, a
system for
collecting a tissue sample in a body lumen may include a tube having a distal
end, and a balloon
may have a first end coupled to the distal end of the tube and a second end.
An extending portion
may be attached to the second end of the balloon. The balloon and the
extending portion may be
positionable in a first, inverted state. The balloon and the extending portion
may be configured to
advance to a second, everted state, such that the balloon and the extending
portion may extend out
of the distal end of the tube. The extending portion may be disposed within
the balloon in the first
inverted position, and may be extendable from the balloon in the second
everted position into the
body lumen.
[018] In various of the foregoing and other embodiments of the present
disclosure, the
extending portion may be any of a filament, suture, or string, or combinations
thereof. At least a
portion of the filament, suture, or string, or combinations thereof, may be
braided. The extending
portion may be formed of one or more filaments having a color. The colors of
the one or more
filaments of the extending portion may provide for a contrasting
visualization. The extending
4
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
portion may include one or more knots or indicia for one or both of visual and
tactile feedback.
The extending portion may be a braided filament configured to collect and
retain a tissue sample
in response to extending from the balloon in the second everted position into
the body lumen. A
push wire may have a distal end coupled to the second end of the balloon and
the proximal end of
the extending portion. The balloon and the extending portion may be movable
from the first
inverted position to the second everted position by actuation of the push
wire.
[019] According to an exemplary embodiment of the present disclosure, a
method for
collecting a tissue sample in a body lumen may include providing a tube having
a distal end, and
a balloon having a first end coupled to the distal end of the tube and a
second end. An extending
portion may be attached to the second end of the balloon. The balloon and the
extending portion
being may be positioned in a first, inverted state. The balloon may be
advanced to a second,
everted state, such that the balloon and the extending portion may extend out
of the distal end of
the tube. The extending portion may be disposed within the balloon in the
first inverted position,
and may be extendable from the balloon in the second everted position into the
body lumen.
[020] In various of the foregoing and other embodiments of the present
disclosure, the tissue
sample may be collected by the extending portion extendable from the balloon
in the second
everted position into the body lumen. The extending portion may be any of a
braided filament,
braided suture, or braided string, or combinations thereof. The extending
portion may be formed
of one or more filaments having a color. The colors of the one or more
filaments of the extending
portion may provide for a contrasting visualization. The extending portion may
be a braided
filament and may be configured to collect and retain a tissue sample in
response to extending from
the balloon in the second everted position into the body lumen. A push wire
may have a distal end
coupled to the second end of the balloon and the proximal end of the extending
portion, and may
be actuated to move the balloon and the extending portion from the first
inverted position to the
second everted position.
[021] According to exemplary embodiments of the present disclosure,
devices, systems, and
methods for Fallopian tube diagnostics may include a tube having a distal end
and a proximal end,
and a sheath disposed coaxial to the tube. A balloon may have a first end
coupled to the distal end
of the tube and a second end, and the sheath may extend over the balloon. The
sheath may provide
column strength to the balloon as the balloon moves from a first, inverted
position to a second,
everted position, into the Fallopian tube. The sheath may minimize balloon
collapse as the balloon
is everted into the Fallopian tube. The sheath may protect the everted balloon
or an extended
portion, or both after cell collection during removal from the patient. A
sheath knob may connect
the sheath to the tube. The sheath knob may be configured to lock the sheath
to the tube to
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
minimize relative movement. The sheath knob may be configured to unlock the
sheath from the
tube for adjusting the sheath relative to the balloon and the tube.
[022] According to exemplary embodiments of the present disclosure,
devices, systems, and
methods for Fallopian tube diagnostics may include one or more markers for
visualization. A first
marker may be disposed on a tube, and may indicate a position of the tube
relative to the sheath,
or sheath knob. The first marker may indicate positioning of the sheath
relative to the tube as a
preparation step to cover at least a portion of a balloon in a second, everted
position. The first
marker may indicate positioning of the sheath relative to the tube for initial
advancement of the
balloon into the Fallopian tube. In response to at least a portion of the
balloon in the second,
everted position, the sheath may be moved in a proximal direction to expose at
least the portion of
the balloon. A second marker may be disposed on the tube, and may indicate a
position of the
tube relative to the sheath or sheath knob. The second marker may indicate
positioning of the
sheath relative to the tube as a retraction marker, for visualization that the
sheath covers the everted
balloon and/or extending portion after cell collection to protect the
collected cells. The second
marker may be disposed at a proximal portion of the tube. A third marker may
be disposed on a
tube, and may be at a distal end of the tube relative to a connection point of
the balloon and the
tube. The third marker may visually indicate an end of the tube, to confirm a
balloon and/or
extending portion extension or positioning in the Fallopian tube. The one or
more markers may
be formed as a score line, a coating substance, or band of material, or
combinations thereof. The
one or more markers may improve or standardize balloon positioning and
extension into the
Fallopian tube. A seal may be disposed around a push wire and positioned
relative to a pressurized
chamber 116. The push wire may be movable relative to the seal for advancing
through the tube
to actuate the balloon between a first, inverted position and a second,
everted position. In response
to a leak formation between the push wire and the conical seal, a seal may be
adjustable to maintain
pressure for moving the balloon between a first inverted position and a second
everted position.
[023] According to exemplary embodiments of the present disclosure,
devices, systems, and
methods for Fallopian tube diagnostics may include that at least a portion of
the sheath may be
translucent, transparent, or otherwise see-through. At least a portion of the
tube may be
translucent, transparent, or otherwise see-through. At least a portion of the
balloon may be
translucent, transparent, or otherwise see-through. The tube may include a
transparent portion and
an opaque portion. The opaque portion may be disposed at a proximal end of the
tube. The
transparent portion of the tube may be more flexible than the opaque portion
of the tube. The
transparent portion of the tube may extend along the length and along an inner
diameter of the
opaque portion of the tube. An extending portion may be connected to the
balloon and may be
disposed within the balloon in the first, inverted position, and may extend
from the balloon in the
6
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
=
second, everted position. The extending portion may be visible through the
balloon, the tube, and
the sheath when in the first, inverted position. The balloon may be inflatable
by an opaque, or
otherwise visible or detectable fluid for visibility to move from the first,
inverted position, to the
second, everted position.
[024] According to exemplary embodiments of the present disclosure,
devices, systems, and
methods for Fallopian tube diagnostics may include a handle including a gear
mechanism for
actuation of the push wire. The gear mechanism may include a plurality of
gears and operable by
a drive wheel. The gear mechanism may include a step-down ratio for additional
control of balloon
movement. The drive wheel and gear mechanism may provide for uniform movement
of the
balloon during movement between the first inverted position and the second
everted position. In
response to extending the push wire to its proximal end, the handle may
include a limit mechanism
for providing audible or tactile feedback to a user. A pawl may be engageable
with one or more
gears, for stopping gear rotation. A pawl may be biased toward a gear rack by
a spring. The pawl
may engage with and slide over teeth of the gear rack for providing audible or
tactile feedback the
user. The teeth may have a steeper slope on a first side and a more moderate
slope on a second
side.
BRIEF DESCRIPTION OF THE DRAWINGS
[025] Non-limiting embodiments of the present disclosure are described by
way of
example with reference to the accompanying figures, which are schematic and
not intended to be
drawn to scale. In the figures, each identical or nearly identical component
illustrated is typically
represented by a single numeral. For purposes of clarity, not every component
is labeled in
every figure, nor is every component of each embodiment shown where
illustration is not
necessary to allow those of ordinary skill in the art to understand the
disclosure. In the figures:
[026] FIG. I illustrates a cross-sectional view of a Fallopian tube with
the uterotubal
junction (UTJ) that connects the uterus to the ovaries;
[027] FIGS. 2A-2D illustrate exemplary embodiments ot a sequential
insertion of an
insertion catheter into a Fallopian tube in accordance with the present
disclosure;
[028] FIG. 3 illustrates a schematic of a hysteroscope for deploying an
exemplary
embodiment of a catheter in accordance with the present disclosure;
[029] FIG. 4 illustrates an exemplary embodiment of a proximal introducer
catheter in
accordance with the present disclosure;
[030] FIG. 5A illustrates a cross-sectional view of an exemplary embodiment
of an everting
sleeve with a distal elastic balloon tip in a deflated state in accordance
with the present disclosure;
7
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
[031] FIG. 5B illustrates a cross-sectional view of the everting sleeve
with a distal elastic
balloon tip of FIG. SA in an inflated state in accordance with the present
disclosure;
[032] FIG. 6A illustrates a cross-sectional view of an exemplary embodiment
of an everting
balloon with an outer construction sleeve in a deflated state in accordance
with the present
disclosure;
[033] FIG. 6B illustrates a cross-sectional view of the everting balloon
with an outer
construction sleeve of FIG. 6A in an inflated state in accordance with the
present disclosure;
[034] FIG. 6C illustrates an exemplary embodiment of an inflation of the
everting balloon
with an outer construction sleeve of FIGS. 6A-6B;
[035) FIG. 7A illustrates a cross-sectional view of an exemplary
embodiment of an everting
sleeve and elastic balloon with an inelastic delivery balloon in a deflated
state in accordance with
the present disclosure;
[036] FIG. 7B illustrates a cross-sectional view of the everting sleeve and
elastic balloon
with an inelastic delivery baBoon of FIG. 7A in an inflated state in
accordance with the present
disclosure;
[037] FIG. 7C illustrates an exemplary embodiment of an inflation of the
everting sleeve
and elastic balloon with an inelastic delivery balloon of FIGS. 7A-7B;
[038] FIG. 8A illustrates a cross-sectional view of an exemplary embodiment
of an everting
sleeve and elastic balloon with an irrigation lumen in a deflated state in
accordance with the present
disclosure;
[039] FIG. 8B illustrates a cross-sectional view of the everting sleeve and
elastic balloon
with an irrigation lumen of FIG. 8A in an inflated state in accordance with
the present disclosure;
[040] FIG. 9A illustrates a cross-sectional view of an exemplary embodiment
of an everting
balloon catheter in a deflated state in accordance with the present
disclosure;
[041] FIG. 9B illustrates a cross-sectional view of the evening balloon
catheter of FIG. 9A
in an inflated state in accordance with the present disclosure;
[042] FIG. 9C is an exemplary embodiment of a spiral filament in accordance
with the
present disclosure;
[043] FIG. 10A illustrates a cross-sectional view of an exemplary
embodiment of an
everting balloon catheter in a deflated state in accordance with the present
disclosure;
[044] FIG. 10B illustrates a cross-sectional view of the everting balloon
catheter of FIG.
10A in an inflated state in accordance with the present disclosure;
[045] FIG. 11A illustrates a cross-sectional view of an exemplary
embodiment of an
everting balloon catheter in a deflated state in accordance with the present
disclosure;
8
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
[046] FIG. 11B illustrates a cross-sectional view of the everting balloon
catheter of FIG.
HA in an inflated state in accordance with the present disclosure;
[047] FIGS. 11C-11D illustrate cross-sectional views of an exemplary
embodiment of an
everting balloon catheter in accordance with the present disclosure;
[048] FIGS. 11E-11F illustrate cross-sectional views of an exemplary
embodiment of an
evening balloon catheter in accordance with the present disclosure;
[049] FIG. 12A illustrates a cross-sectional view of another exemplary
embodiment of an
everting balloon catheter in a deflated state in accordance with the present
disclosure;
[050] FIG. 12B illustrates a cross-sectional view of the everting balloon
catheter of FIG.
12B in an inflated state in accordance with the present disclosure;
[051] FIG. 13A illustrates a cross-sectional view of another exemplary
embodiment of an
everting balloon catheter in a deflated state in accordance with the present
disclosure;
[052] FIG. 13B illustrates a cross-sectional view of the everting balloon
catheter of FIG.
13A in an inflated state in accordance with the present disclosure;
[053] FIG. 14A illustrates a cross-sectional view of another exemplary
embodiment of an
everting balloon catheter in a deflated state in accordance with the present
disclosure;
[054] FIG. 14B illustrates a cross-sectional view of the everting balloon
catheter of FIG.
14A in an inflated state in accordance with the present disclosure;
[055] FIG. 15A illustrates a cross-sectional view of an exemplary
embodiment of an
everting balloon spiral cannula in a deflated state in accordance with the
present disclosure;
[056] FIG. 1511 illustrates the everting balloon spiral cannula of FIG. 15A
in an inflated
state in accordance with the present disclosure;
[057] FIG. 16A illustrates a cross-sectional view of an exemplary
embodiment of an
everting distal arc balloon cannula in a deflated state in accordance with the
present disclosure;
[058] FIG. 16B illustrates the everting distal arc balloon cannula of FIG.
16A in an inflated
state in accordance with the present disclosure;
[059] FIG. 17A illustrates a cross-sectional view of another exemplary
embodiment of an
everting balloon catheter in a deflated state in accordance with the present
disclosure;
[060] FIG. 17B illustrates the evening balloon catheter of FIG. 17A in an
inflated state in
accordance with the present disclosure;
[061] FIG. 18 illustrates an exemplary embodiment of an extending element
in accordance
with the present disclosure;
[062] FIG. 19 illustrates an exemplary embodiment of an extending portion
in a retracted
state after cell collection in accordance with the present disclosure;
9
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
[063] FIG. 20 illustrates the separate extending portion of FIG. 19 in a
deployed state in
accordance with the present disclosure;
[064] FIG. 21A illustrates a cross-sectional side view of an exemplary
embodiment of a ball
tip everting balloon catheter prior to deployment of the balloon in accordance
with the present
disclosure;
(065] FIG. 2111 illustrates a cross-sectional side view of an exemplary
embodiment of the
ball tip everting balloon catheter of FIG. 21A in a deployed state in
accordance with the present
disclosure;
[066] FIGS. 22A-22C illustrate an exemplary embodiment of an everting
balloon exiting
from a catheter in accordance with the present disclosure;
[067] FIG. 23A illustrates a cross-sectional side view of an exemplary
embodiment of a
balloon tip catheter in accordance with the present disclosure;
[068] FIG. 23B illustrates the balloon tip catheter of FIG. 23A in
accordance with the
present disclosure;
[069] FIG. 23C illustrates the balloon tip catheter of FIG. 23A in
accordance with the
present disclosure;
[070] FIG. 24 illustrates a cross-sectional side view of an exemplary
embodiment of a
balloon tip catheter in accordance with the present disclosure;
[071] FIG. 25 illustrates a side view of an exemplary embodiment of a
balloon tip catheter
in accordance with the present disclosure;
[072] FIG. 26A illustrates a cross-sectional view of an exemplary
embodiment of a handle
of the catheter of FIG. 25 in accordance with the present disclosure;
[073] FIG. 26B is a detail view illustrating an exemplary embodiment of a
gear system in
the handle portion of the catheter of FIG. 26A in accordance with the present
disclosure;
[074] FIG. 26C illustrates a perspective view of an exemplary embodiment of
a linear rack
ratcheting assembly in accordance with the present disclosure;
[075] FIG. 26D illustrates a side view of an exemplary embodiment of a drop
key-click of
the linear rack ratcheting assembly of FIG. 26C in accordance with the present
disclosure;
[076] FIG. 26E illustrates a side view of an exemplary embodiment of a gear
jam in
accordance with the present disclosure;
[077] FIG. 27 illustrates a cross-sectional side view of an exemplary
embodiment of a
balloon tip catheter in accordance with the present disclosure;
[078] FIG. 28 illustrates a cross-sectional side view of an exemplary
embodiment of a
balloon tip catheter in accordance with the present disclosure;
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
[079] FIGS. 29A-29C are a series of side perspective views of an exemplary
embodiment
of a steerable balloon tip using guide wires in accordance with the present
disclosure;
[080] FIG. 30 illustrates a side perspective view of an exemplary
embodiment of a balloon
catheter and lead balloon tip in accordance with the present disclosure;
[081] FIG. 31 illustrates a side perspective view of an exemplary
embodiment of a balloon
catheter with a flexible guide wire in accordance with the present disclosure;
[082] FIG. 32 illustrates an exemplary embodiment of a balloon prior to
inversion of the
balloon into a catheter in accordance with the present disclosure;
[083] FIG. 33 illustrates a cross-sectional side view of an exemplary
embodiment of a
balloon tip catheter with a sheath and the balloon of FIG. 32 inverted in
accordance with the
present disclosure;
[084] FIG. 34A illustrates a side perspective view of an exemplary
embodiment of a string
filament with a series of printed indicia in accordance with the present
disclosure;
[085] FIG. 34B illustrates a side perspective view of an exemplary
embodiment of a string
filament with a series of knots as indicia in accordance with the present
disclosure;
[086] FIG. 35 illustrates an eversion of an exemplary embodiment of a
balloon in
accordance with the present disclosure;
[087] FIG. 36A illustrates a cross sectional view of an exemplary
embodiment of a balloon
in accordance with the present disclosure; and
[088] FIG. 36B illustrates a cross sectional view of an exemplary
embodiment of a balloon
in accordance with the present disclosure.
DETAILED DESCRIPTION
[089] The present disclosure is not limited to the particular embodiments
described herein.
The terminology used herein is for the purpose of describing particular
embodiments only, and is
not intended to be limiting beyond the scope of the appended claims. Unless
otherwise defined,
all technical terms used herein have the same meaning as commonly understood
by one of
ordinary skill in the art to which the disclosure belongs.
[090] Where a range of values is provided, it is understood that each
intervening value, to
the tenth of the unit of the lower limit unless the context clearly dictates
otherwise, between the
upper and lower limits of that range is also specifically disclosed. Each
smaller range between
any stated value or intervening value in a stated range and any other stated
or intervening value
in that stated range is encompassed within the disclosure. The upper and lower
limits of these
smaller ranges may independently be included or excluded in the range, and
each range where
either, neither or both limits are included in the smaller ranges is also
encompassed within the
disclosure, subject to any specifically excluded limit in the stated range.
Where the stated range
11
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
includes one or both of the limits, ranges excluding either or both of those
included limits are
also included in the disclosure.
[091] As used herein, the singular forms "a," "an," and "the" are intended
to include the
plural forms as well, unless the context clearly indicates otherwise. It will
be further understood
that the terms "comprises" and/or "comprising," or "includes" and/or
"including" when used
herein, specify the presence of stated features, regions, steps elements
and/or components, but do
not preclude the presence or addition of one or more other features, regions,
integers, steps,
operations, elements, components and/or groups thereof.
[092] As described above, a challenge in effectively testing for early
stage cancers (e.g.,
ovarian cancer) in women may include obtaining biopsy samples without
undergoing a surgical
procedure. Anatomically, the ovaries are in close proximity to the fimbria at
the region of the
distal opening or os of the Fallopian tube. Eggs released by the ovary may be
gathered by the
fimbria and transported through the Fallopian tube to the uterus. With ovarian
cancer, cells may
be deposited in the Fallopian tube, which may eventually migrate into the
uterus. Cell samples
obtained from the uterus may detect ovarian malignancy; however, the incidence
of migration of
ovarian cancer cells into the uterus may be too low to render uterine sampling
a reliable diagnostic
test for ovarian malignancy.
[093] A higher number of cancer cells may migrate to or originate in the
Fallopian tube,
which may be concentrated in the distal portion of the tube, near the distal
os. The ability to test
cells in the Fallopian tube for malignancy may be of clinical value for the
early detection and
treatment of such cancers. It is understood that early detection screening may
be performed that
detects migrating cancerous cells.
[094] The Fallopian tube is extremely fragile and may be prone to
perforation in a medical
procedure. As such, safe introduction of a diagnostic device into the
Fallopian tube may be
difficult with known devices. Referring now to FIG. 1, a Fallopian tube 1 of a
patient may extend
from a proximal os 3 to a uterus, connecting at a uterotubal junction (UTJ) 2,
to a distal os 5 and
connecting to ovaries 6. A perforation may occur at the UTJ 2, which is a
constriction occurring
distal to the proximal os 3 (e.g., opening) of the Fallopian tube. For
example, in some patients the
UTJ 2 may be approximately 1 cm distal of the proximal os 3. In some patients,
the body lumen
size at this constriction may be as small as approximately 0.3 mm or 0.5 mm,
while the body lumen
size of the Fallopian tube adjacent to the UTJ may be approximately 1 mm.
[095] According to exemplary embodiments, systems and methods of the
present
disclosure may engage an interior wall of a Fallopian tube and may remove
cells therefrom for
diagnostic purposes. Devices and processes may be provided for collecting such
cells in a less
invasive procedure that in some embodiments occur without cutaneous incision.
Although the
12
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
description refers to sample collection and diagnostics of Fallopian tubes, it
is understood that
systems and methods of sample collection and diagnostics may be applicable to
any other body
lumens, tubes, and ducts, including but not limited to a bile duct, hepatic
duct, cystic duct,
pancreatic duct, lymphatic vessels, and circulatory vessels in accordance with
the present
disclosure.
[096] Embodiments of an exemplary catheter for Fallopian tube diagnostics
may be
provided for the performance of less invasive procedures including any of the
following: (1) access
to the proximal os of the Fallopian tube via an intrauterine approach; (2)
advancement of an
introducer catheter to cannulate and form a fluid tight seal with the proximal
os; (3) use of a second
catheter inside the introducer catheter to track the length of the Fallopian
tube out into the
abdominal cavity; (4) inflation of a balloon at the end of the second catheter
with retraction of the
second catheter until the balloon seals the distal os of the Fallopian tube
(retraction of the second
catheter may result in contact with the intraluminal surface of the Fallopian
tube to dislodge cells
for improved sampling); and/or (5) irrigation of the Fallopian tube and
recovery of the irrigation
fluid for cytology or cell analysis.
[097] Exemplary embodiments of a catheter for Fallopian tube diagnostics
for minimally
invasive procedures may include any of the following: (1) access to the
proximal os of the
Fallopian tube via an intrauterine approach; (2) advance of an introducer
catheter to cannulate the
proximal os; (3) use of a second catheter inside the introducer catheter to
track inside the Fallopian
tube. An inflated balloon at the end of the second catheter may be advanced
across the proximal
portion of the Fallopian tube and may be everted further into the Fallopian
tube; (4) the balloon
may contact the intraluminal surface of the Fallopian tube and may dislodge
cells for sampling;
and/or (5) the balloon may be removed and inserted into a vial for cell
collection and subsequent
processing.
[098] Embodiments of an exemplary catheter may be configured for insertion
into the
Fallopian tube (see FIG. 1). The Fallopian tube has a curvature (e.g., having
a tortuous pathway),
and the soft tissue of the tube may be collapsible, thereby resulting in
multiple constrictions as
passage is attempted. As described above, this may be particularly true at the
uterotubal junction
(UTJ), which may be muscular and therefore more prone to perforation by
insertion of medical
instruments. In some patients, the UTJ may also present a downward bend with a
lumen size at
the constriction that may be as small as approximately 0.3 mm or 0.5 mm, while
the body lumen
size of the Fallopian tube adjacent to the UTJ may be approximately 1 mm.
[099] In at least one embodiment of the present disclosure, an elongated
balloon that is
initially inverted into a catheter lumen may be deployable. The balloon may
partially evert to enter
a proximal end of the Fallopian tube, e.g., the UTJ, thereby cannulating the
proximal os. The
13
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
balloon may evert upon pressurization of the balloon from inside the catheter
so that an unrolling
mechanism of the eversion creates a path through the Fallopian tube regardless
of tortuosity or
constriction in the Fallopian tube. In some embodiments, the balloon may evert
by a push wire
advancement, which may be in concert with pressurization. A great majority of
the length of the
balloon may be substantially inelastic, such that the balloon does not
substantially expand and
dilate the Fallopian tube as it everts. Balloon expansion may burst or
otherwise damage or injure
the Fallopian tube. However, exemplary embodiments may also incorporate an
elastic distal
balloon end expandable to seal the distal os upon retraction of the distal
balloon. In embodiments,
the device may have a balloon having a sufficient rigidity to cannulate the
Fallopian tube and
sufficient flexibility for navigation through the tortuous path of the
Fallopian tube to minimize
potential damage or injury. In some embodiments, the device may include
support elements for
cannulating the Fallopian tube so that the balloon may not collapse at the
proximal os.
[0100] Exemplary embodiments of systems and methods of the present
disclosure may
include positioning, and deployment of, a distal end of a catheter. In some
embodiments, a catheter
distal end may be deliverable to a proximal end of the Fallopian tube by a
hysteroscope. In some
embodiments, the hysteroscope may be an exemplary hysteroscope (e.g., FIG. 3).
Regardless of
the mode of deployment, a retracted portion of a catheter may be extendable to
contact the interior
wall of the Fallopian tube. It has been surprisingly found that the act of
extending a portion of the
catheter may remove a sufficient sampling of cells and/or tissue from the
Fallopian tube wall to
perform histological and/or cytological evaluation. For example, at least a
portion of a length of
the balloon may contact the Fallopian tube for sample collection. In some
embodiments, a majority
of the length of the balloon may be substantially inelastic such that the
balloon does not
substantially expand and dilate the body lumen (e.g., Fallopian tube) as it
everts. In some
embodiments, the balloon may be sized such that the body lumen does not expand
or dilate as the
balloon everts. As described above, balloon expansion may burst or injure the
subject's body
lumen. According to some embodiments and as discussed above with regard to the
exemplary
balloon catheter, the balloon may be extendable by eversion from a catheter
only longitudinally
into the body lumen such that the balloon does not substantially expand and
dilate the lumen as
the balloon everts or is extended into the body lumen (e.g., the Fallopian
tube). In some
embodiments, the balloon may be extendable longitudinally into the body lumen,
where a diameter
of an inflated balloon may be up to approximately 10-15% greater than a
diameter of a Fallopian
tube. Radial expansion of the balloon may be limited or controlled by the
majority of the length
of the balloon being substantially inelastic. It is appreciated that portions
of a balloon that are not
intended to be inserted within a lumen structure can be elastomeric and
therefore may be
expandable in diameter and compliant rather than substantially inelastic. Such
a hybrid balloon
14
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
may be well-suited in embodiments when a seal is desired with the UTJ.
Exemplary of situations
when a seal is desired may include irrigation of the lumen, filling the lumen
with an imaging
contrast, diagnosing obstructions, and/or topical contact with a therapeutic
agent, such as a
chemotherapeutic or an antibiotic.
[0101] It has also been surprisingly found that withdrawal of an extended
portion of a balloon
may remove still more cells. In some embodiments, the extended portion may be
retracted prior to
catheter removal so as to preclude dispersal of dislodged Fallopian tube cells
to surrounding tissue.
In some embodiments, a slidable sheath may be deployable to protect the
collected sample. Upon
catheter removal the extended portion may deposit at least a portion of the
collected sample (e.g.,
lumina] cells) via contact with a microscope slide or other diagnostic
substrate, for testing for
abnormal cells (e.g., cancerous cells). In some embodiments, a dye may be
releasable in the
Fallopian tube for identifying abnormal and potentially cancerous cells.
[0102] Referring now to FIGS. 24-2D, an inverted inelastic sleeve 12 and an
attached distal
elastic balloon 14 may be insertable through an introduction catheter .10 that
may reside in the
working channel 22 of an operative hysteroscope 20 (FIG. 3), and used to
cannulate the proximal
os of the Fallopian tube 1, as shown in FIG. 2A. At FIG. 2B, the balloon may
be inflated to evert
the sleeve 12 the length of the Fallopian tube 1 and distend the distal
elastic balloon 14. At FIG.
2C, the balloon may be retracted proximally at least partially to seal the
distal os 18 of the Fallopian
tube 1, after full advancement of the inverted elastic sleeve 12 and inflation
of the elastic balloon
14. FIG. 2D illustrates the introduction of saline for irrigation along the
length of the Fallopian
tube 1 between the introducer catheter 10 and the everted sleeve 12.
Retraction of the inflated
elastic balloon 14 seals the opening of the distal os. Subsequent collection
of the irrigation fluid
obtains cell samples from substantially the entire length of the Fallopian
tube 1 for cell analysis in
the detection of ovarian cancer or other medical conditions. In an embodiment,
a dye may be
present in the irrigation fluid that is introduced in the Fallopian tube for
identifying and/or
differentiating abnormal and potentially cancerous cells. An illustrative
example of a dye may
include a fluorescent imaging agent attached to a modified type of folic acid,
which may act as a
homing device searching for ovarian cancer cells to attach onto. In some
embodiments, a
multispectral fluorescent camera may illuminate the detected cells, visually
identifying their
location, e.g., by a monitor. For ovarian cancer cells to grow and divide, the
cells need large
amounts of the vitamin (folic acid). Special receptors on the surface of the
cancer cells seize the
vitamin, and whatever is attached to it, and pull it inside.
[0103] The catheter 10 described above, and in greater detail below may be
introduced into
.the uterus of a patient using an operating hysteroscope 20, an example of
which is shown in FIG.
3. An operating hysteroscope 20 may include one or more working channels. One
channel may
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
provide irrigation to distend the uterus and allow endoscopic visualization,
and one or more
additional working channels 22 may allow instruments and/or catheters to be
advanced distally of
the hysteroscope. A proximal introducer catheter 10 (see, e.g., FIGS. 2A and
FIG. 4) may be
advanceable through a working channel of the operating hysteroscope 20, and
may be used to
cannulate the proximal os of a Fallopian tube. A balloon 24 on the proximal
introducer catheter
/0 may be inflated to occlude the proximal os (e.g., FIG. 4), and the everting
sleeve catheter may
be advanceable through the proximal introducer catheter 10 into the proximal
portion of the
Fallopian tube. The sleeve/balloon element 14 may be fully everted, and the
inflated balloon tip
may be pulled back to seal the distal os. Irrigation may be introduced via a
port 11, and aspirated
via the irrigation port 11 on the proximal introducer catheter 10, to collect
the sample. Irrigation
may also be introduced through both the everting sleeve catheter and the
proximal introducer
catheter, followed by aspiration through one or both ports (11, 13) of the
proximal introducer
catheter.
[0104J In embodiments of the catheter 10, the sleeve 12 of the everting
sleeve catheter may
be a flexible, elongated, substantially inelastic tube with an elastic balloon
tip 14 attached to its
distal end, see FIG. 5A and 5B. The inelastic tube 12 may have multiple ridges
15 disposed along
its length extending externally of the tube when the tube has been everted or
extended/deployed,
such as illustrated in FIG. 5B. Prior to deployment, the ridges may extend
inwardly, as the tube
is inverted, as illustrated in FIG. 5A. With the ridges extending externally,
as in FIG. 5B, the
ridges may be exposed to the luminal surface of the Fallopian tube when the
sleeve is fully everted.
These ridges may increase the ability of the sleeve to gather cells upon
balloon retraction, e.g., by
additional surface area, and/or frictional contact. In some embodiments, the
outer surface of the
everted inelastic balloon may be covered with fabric or otherwise textured, as
described below,
which may increase cell dislodgment and improve cell collection during balloon
retraction.
[0105] FIGS. 6A-6C illustrate an exemplary embodiment of an everting sleeve
catheter 10A
which may provide protection of a bond between a balloon 14A and a sleeve 17
of the everting
sleeve catheter 10A during deployment. The everting sleeve catheter 10A of
FIGS. 6A-6C may
include an elongated, elastic balloon attachable to a distal tip of the
everting sleeve catheter. A
substantially inelastic sleeve 17, slightly shorter in length than the elastic
balloon 14, may be
attached to the elastic balloon 14 at the distal tip of the catheter, and may
be invertible so that in
an undeployed state, the inelastic sleeve 17 is positioned inside the elastic
balloon 14. In response
to eversion of the balloon/sleeve combination 14A, 17 the inelastic sleeve 17
may emerge from a
double wall 19 of the catheter 10A, so that a portion of the elastic balloon
14A in an extended
position is internal to the inelastic sleeve 17, e.g., the inelastic sleeve 17
is disposed on the outside
of the elastic balloon 14A and may constrict the elastic balloon 14A along its
length, e.g., a
16
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
majority of its length, to prevent the elastic balloon 14 from expanding and
potentially rupturing
the Fallopian tube during the time that the everting sleeve is being advanced
through the Fallopian
tube. Upon full balloon/sleeve eversion, the distal elastic balloon may
inflate to approximately 3x
¨ 5x the diameter of the sleeve, for occlusion of the distal os upon
retraction of the catheter with
concomitant pullback of the inflated balloon. In some embodiments, the
catheter may contain a
port IL to allow irrigation to occur between the balloon and the inelastic
sleeve 17.
[0106] FIGS. 7A-7C illustrate an exemplary embodiment of an everting sleeve
catheter 10b
including a concentric double walled catheter, and the eversion of three
layers are attached to the
distal catheter tip. An elongated inelastic balloon 21 may be attached to a
distal tip of the inner
catheter 23, and the balloon 21 may lie within an inner catheter lumen 25. An
elongated elastic
balloon 14B, which in some embodiments may be equal in length to the inelastic
balloon 21, may
be attached to a distal tip of an outer wall 27 of catheter 10b. The balloon
1413 may be disposed
inside the inelastic balloon 21. An inelastic sleeve 29, which in some
embodiments may be shorter
in length than the elastic balloon 14B, may be attached to the distal tip of
the outer catheter wall
27. The sleeve 29 may be disposed inside the elastic balloon 14B in an
undeployed state.
Pressurization of the inner catheter 23 may evert the inelastic balloon 21,
which may deliver the
elastic balloon 14B and outer inelastic sleeve 29. Following full eversion of
all three layers,
pressurization between the walls of the inner catheter and outer catheter may
inflate the elastic
balloon 14B. The inelastic sleeve 29 may constrict the elastic balloon 14B
along the majority of
its length. A distal, un-constricted tip of the balloon 14T may expand to form
the occlusion
element. This may be advantageous to decrease friction in the system during
the eversion process.
For example, the inelastic balloon 21 may deliver the elastic balloon 14B and
inelastic sleeve 29.
The elastic balloon 14B may not undergo expansion until fully everted. In this
manner, the elastic
balloon 14B may avoid frictional contact with the wall of the inelastic sleeve
29 during eversion,
which may be advantageous in facilitating deployment, e.g., when working with
small diameter
catheters for traversing the Fallopian tube.
[0107] FIGS. 8A-8B illustrate an exemplary embodiment of an everting sleeve
catheter 10C
including an inelastic sheath 29A having a small lumen 31 for irrigation, with
the sheath 29A
connectable to a third port I IA used for fluid irrigation and aspiration to
obtain cytology samples.
As noted above, in some embodiments, the irrigation fluid may contain a dye
for identification of
abnormal and potentially cancerous cells.
[0108] Another exemplary embodiment according to the present disclosure is
shown in FIGS.
9A-9C and 10A-10B. An elongated balloon 32 including an extending portion 34,
e.g., an
expandable member, attachable to a distal end of the balloon 32 may be
inverted into the lumen
36 of a catheter 30. In an inverted, e.g., undeployed state, the extending
portion 34 may lie inside
17
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
the elongated balloon 32. In some embodiments, the extending portion 34 may be
a spiral of one
or more loops of filament 38. The filament that forms the extending portion 34
may be formed
from a variety of materials illustratively including a monofilament polymer
material such as Nylon
or polypropylene, fluoropolymers, or polylactic acid; metal such as stainless
steel titanium, or
platinum; or a superelastic metal such as Nitinol, or combinations thereof. In
some embodiments
a fiducial marker may be included on the filament and/or balloon and
deliverable to the Fallopian
tube (not shown) to facilitate subsequent return to the situs of cell
sampling. It may be appreciated
that the extending portion may also have alternative configurations, such as
an expandable
member. The extending portion 34 e.g., expandable member, may contain a
plurality of outwardly
oriented bristles 40 formed of polymer or metal (see FIG. 18). In some
embodiments, the
extending portion 34 may be included as an elongated strand 38 of material
that curls, spreads or
fans out 42, balls up 44 to a predetermined shaped when released from being
constrained inside
the catheter (FIGS. 11A-11F or FIGS. 14A-14B), or combinations thereof. In
some
embodiments, the extending portion 34, e.g., expandable member, may be formed
of a compressed
polymer foam that self-expands upon release into a wet environment (FIGS. 12A-
12B). Upon
pressurizing the catheter adjacent to the proximal os, the balloon 32 may
evert so as to urge the
inverted portion outward into the extended position and into contact with the
Fallopian tube inner
wall cells. In some embodiments, upon full balloon eversion, the extending
portion 34 may be
extended out of the distal os of the Fallopian tube and into the abdominal
cavity. In some
embodiments, the extending portion 34 may have an expanded outer diameter of
approximately 5-
15 mm.
[0109] An advantage of the extending portion 34 having a plurality of
bristles is that there
may be added surface area on which a sample (e.g., cells and/or tissue) is
collectable, including
areas that are not likely to be exposed to shear forces when the device is
retracted back within the
catheter. Cell collection may therefore be maximized, as well as minimizing an
amount of cells
that are wiped off when the device is pulled through the Fallopian tube or
into a sheath, as seen in
FIGS. 18-20. In those embodiments in which the extending portion has a greater
surface area, the
cell collection may increase per linear unit of Fallopian tube so engaged
under like pressurization
conditions, as compared to a contourless extending portion.
[0110] In still other embodiments of a catheter in accordance with the
present disclosure, the
extending portion, e.g., expandable member, may form any number of shapes and
contours. For
example, multiple filaments 42 may be attached to the distal end of the
balloon 32 that splay out
upon balloon eversion to form a brush 42 (FIGS. 11A-11B). In some embodiments,
a braided
string or suture 43 may be extendable distally of the balloon 32 upon eversion
(see FIGS. 11C-
11D), and in other embodiments, the braided suture may be formed of various
materials and/or
18
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
may be one or more colors for visual confirmation of extension of the suture
43 (see FIGS. 11E-
11F). A polymer foam structure 46 may be compressed inside the balloon 32, and
may self-expand
in response to balloon 32 eversion and exposure to a fluid environment (FIGS.
12A-12B). An
elastic or inelastic balloon 48 may be disposed on the distal end of the
inelastic sleeve balloon 32
(FIGS. 13A-13B). Alternatively or additionally, embodiments may include an
everting balloon
having a superelastic wire coil (FIGS. 14A-14B), a spiral everting balloon 50
(FIGS. 15A-15B),
an everting distal arc balloon 52 (FIGS. 16A-16B), or a long elastic filament
of polymer or metal
that gathers into a three-dimensional structure upon balloon eversion, such as
an inner lumen 54
(FIGS. 17A-17B), and expandable member 34 having a plurality of outwardly
oriented bristles 40
(FIG. 18), or combinations thereof. It may be appreciated that any of these
embodiments of a
catheter extending portion as an expandable member or otherwise may include a
fiducial marker
as a navigation aid for a medical professional to navigate back to a desired
situs in the Fallopian
tube. For example, a marker may be deliverable to a desired location in a
Fallopian tube, e.g.,
through an inner lumen 54, or by a balloon 32. Such markers are known to the
art and illustratively
include radio-opacity markers, isotopic markers, and radiofrequency markers.
In still other
embodiments, a biodegradable extending portion or a permanent extending
portion may be
severable from the catheter. In still other embodiments, the extending portion
may deliver a
therapeutic agent such as a chemotherapeutic drug, antibiotic, anti-
inflammatory, or combinations
thereof, of the Fallopian tube tissue.
[0111] When the catheter is retracted back into the working channel of the
hysteroscope, cells
may be dislodged from at least a portion of the entire length of the inner
surface of the Fallopian
tube. In some embodiments, the extending portion may be inverted back within
the balloon by
reducing the gas pressure within the balloon, and reinverting the balloon
within the catheter tip
region, so as to shield collected cells with the catheter tip region internal
bore. In other
embodiments, the extending portion and balloon, in either a deflated state or
remaining inflated,
may be retractable back within a sheath without the balloon being reinverted.
For example, as
shown in FIG. 19, an extending portion 34, e.g., an expandable filament 38
including a plurality
of bristles 40, may be protected during removal from the patient by a sheath
162 (see FIG. 23A).
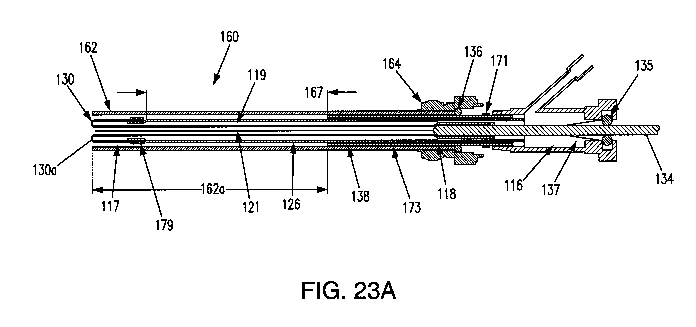
[0112] An extending portion 34, e.g., an expandable filament 38 as shown in
FIGS. 18-20,
may be attached to an end of the inverting balloon. In some embodiments, an
extending portion
34, e.g., expandable coil, may be connected to the push wire (see FIG. 23A).
In some
embodiments, the extending portion may be connected to a distal end of the
push wire 134. In
some embodiments, an extending portion 34 (e.g., spiral) may be a collection
device passed
through an inner lumen that may expand upon reaching the distal end into the
Fallopian tube. It
may be appreciated that cells may be collectable from a specific portion of
the Fallopian tubes, for
19
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
example the fimbria, and then protected by a sheath 162 so as to minimize
potential for distal cells
to be wiped off by the inner surface of the proximal Fallopian tube as the
device is removed.
[0113] In some embodiments, friction between an outer surface of the
extending portion 34,
e.g., an expandable filament 38, and an inner lining of the Fallopian tube is
sufficient to dislodge
cells and adhere such cells to the expandable member, even in embodiments
having a contourless
extending portion. For example, an expanded spiral at the distal end of the
balloon may contact
the fimbria at the distal end of the Fallopian tube to collect cell samples.
Since the Fallopian tube
increases in inner diameter as it proceeds from its proximal to its distal
end, expansion of the
extending portion 34, (e.g., by the expandable filament 38) may maximize
obtained cell samples
at the distal end of the Fallopian tube (e.g., fimbrial portion of the
Fallopian tube).
[0114] The elongated balloon and the extended portion may in some
embodiments be
retractable into the working channel of the hysteroscope to avoid loss of cell
samples as the
hysteroscope is removed from the patient. An elastomer seal at the proximal
end of the working
channel of the hysteroscope may seal against an outer surface of the catheter.
This seal may act
to deter the catheter from sliding from a desired position within the working
channel of the
hysteroscope, or from sliding completely out of the working channel. A mark on
the catheter body
may indicate a length of retraction necessary to ensure that the elongated
balloon and distal spiral
are fully within the hysteroscope working channel. Upon removal of the
hysteroscope from the
patient, in some embodiments, a syringe containing saline solution may be
attached to the Luer
fitting at a proximal end of the working channel. Saline may be used to flush
cells gathered by the
elongated balloon and expanding spiral into a test tube. It may be appreciated
that the cells
collected by the expandable member may be collected for testing by
conventional techniques and
may be prepared for cytological, molecular or genetic examination.
[0115] In some embodiments, an inner lumen 54 may be formed of a material
having
sufficient rigidity to maintain an opening in the lumen. For example, the
inner lumen 54 may be
sufficiently rigid to withstand a pressure of the balloon as it is inflated
and everted. In
embodiments, the inner lumen 54 may be formed of a metal, composite, or
polymer, or
combinations thereof, including a polyethylene terephthalate (PET) material
and may be attached
to the catheter, as shown in FIGS. 17A-17B. The eversion process follows that
of the
aforementioned embodiments having a push wire that does not include a lumen.
This embodiment
may also include an inflation sideport and a proximal seal 33 that may allow
the balloon 32 to be
everted while maintaining an orifice through the inner lumen 54 in fluid
communication between
the hysteroscope and the patient body tissue. Once everted, the inner lumen 54
may provide a
pathway through which a separate extending portion may be passed, or a
surgical instrument
package or visualization device may be passed. In some embodiments, various
agents may be
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
brought into contact with the lumen via the pathway. These agents and
rationales therefore may
illustratively include microbubbles to serve as acoustic contrast agents,
contrast dyes for various
forms of spectroscopic imaging, or therapeutics for treating cells or killing
cancerous cells, or
combinations thereof. Therapeutics may illustratively include antibodies
specific to cancerous
cells and carrying a chemotherapeutic or radio-isotope, chemotherapeutics,
radio-isotopic seeds,
antibiotics, antifungals, or combinations thereof.
[0116] FIGS. 21A-21B illustrate cross-sectional views of an exemplary
embodiment of a ball
tip everting balloon catheter 120 in accordance with the present disclosure. A
spherical ball 122
may be attached to the distal end of a spring tip 124 affixed to a tube, or
catheter 126. It is
understood that "tube" and "catheter" 126 may be used interchangeably. The
spherical ball 122
may he provided to negotiate through a patient's UTJ to minimize and/or avoid
inadvertent
penetration through the UTJ sidewalls. The spring tip 124 may allow the distal
end with the ball
122 to flex around corners and navigate through the UTJ. The spring tip 124
and spherical ball
122 may have an open lumen 128 extendable through the spring tip 124 and the
spherical ball 122.
The spherical ball 122 on the spring tip 124 may be approximately 0.8 ¨ 1.0 mm
in diameter, and
the hollow spring tip 124 may have a length of approximately 1.5 cm and an
outer diameter of
approximately 0.6 mm. The hollow spring tip 124 may be formed of a metal
(stainless steel or
superelastic metal, e.g., Nitinol) coil spring sheathed on the outside with
thin walled polymer heat
shrink tubing, made of nylon, PET (polyethylene terephthalate), or similar
material. In some
embodiments, the spring tip 124 may be a metal coil spring co-extruded into a
tubular polymer
body. The hollow spring tip 124 may also be a flexible polymer tube, and in
some embodiments
may be made of nylon, Polyethylene terephthalate (PET), polyether block amide,
or similar
materials. An everting balloon 130 may lie inside the hollow spring tip 124.
The everting balloon
130 may extend proximally inside the main lumen 132 of the introduction
catheter 126 (e.g., a
generally flexible tubular structure) or cannula (e.g., a generally rigid
tubular structure).
[0117] The proximal end of the everting balloon 130 may be attached to a
push rod 134
passable through a seal 135 on the proximal end of the catheter 126 or
cannula. In operational use
on a patient, the flexible ball tip 122 may be manually advanced through the
UTJ. Once passage
of the flexible ball tip 122 and spring tip 124 through the UTJ occurs, the
push rod 134 may be
advanced through the seal 135 of the previously pressurized introduction
catheter 126 or cannula.
Advancement of the push rod 134 may cause a controlled eversion of the balloon
130 out of the
hollow spring tip 124, through the length of the Fallopian tube.
[01181 According to some embodiments, a seal 137 may be disposed within the
tube/catheter
shaft 126 through which the push wire 134 passes as the push wire 134 actuates
the balloon (see
FIGS. 21B, 23A). In some embodiments, the seal 137 may be a conical seal
disposed between a
21
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
pressurized chamber 116 and the push wire 134. It is noted that the terms
"push wire" and "push
rod" are used herein synonymously. The conical seal 137 may allow the push
wire 134 to advance
through the catheter 126 to actuate the balloon 130 between an inverted
position and an everted
position while maintaining pressure in the catheter 126. Various embodiments
of the present
disclosure may provide an adjustable seal 135, disposed proximal to the
conical seal 137. In
response to a leak forming between the push wire 134 and the conical seal 137,
the adjustable seal
135 may be adjusted to maintain the pressure required to move the balloon
between the first
inverted position and the second everted position. The adjustable seal 135 may
be a rotating
hemostasis valve, e.g., a device for maintaining seals between coaxial
devices, and adjustable by
knob 133. In some embodiments, a hemostasis valve may be used as seal 135. The
hemostasis
valve may include a compressible gasket to provide a desired degree of
sealing.
[0119] The knob 133 may be rotatably adjustable to adjust the seal 135. In
use, a user may
be able to adjust the knob 133 to tighten or loosen the knob 133. By
tightening the knob 133, the
seal 135 may be compressed, thereby collapsing around the push wire 134. The
rotatable knob
133 may provide the user with improved control over the seal and the ability
to react if there are
any leaks from the conical seal 137.
[0120] In embodiments, the elongated balloon may be initially inverted into
a catheter lumen
during assembly, e.g., the balloon may be turned inside out during assembly.
The balloon may be
pressurized to deploy, so that the balloon everts and "unrolls" into the
Fallopian tube. The
unrolling mechanism of the eversion may track through the Fallopian tube
regardless of tortuosity
or constriction in the Fallopian tube. A great majority of the length of the
balloon may be
substantially inelastic, e.g., up to 100% of the length of the balloon, such
that the balloon may not
substantially expand and dilate as it everts, e.g., so the Fallopian tube may
not expand or dilate as
the balloon everts. In other embodiments, a portion of a distal end of a
balloon may be expandable
into the fimbriated end of the Fallopian tube (e.g., see FIGS. 5-8). Balloon
overexpansion may
burst or injure the Fallopian tube.
[0121] An exemplary process common to the various embodiments of devices
may include
the deployment of the distal end of a catheter. In some embodiments, a
catheter distal end may be
delivered to a proximal end of the Fallopian tube by a conventional
hysteroscope. Regardless of
the mode of deployment, a retracted portion of the balloon inside of the
catheter shaft 126 may be
extendable from within the catheter shaft 126 into contact with an interior
wall of the Fallopian
tube. It has been surprisingly found that the act of extending the portion may
abrade a sufficient
amount of cells and/or tissue from the Fallopian tube wall to perform
histological evaluation. This
is observed for planar surfaces of a balloon of seemingly non-abrasive
character. While a
roughened surface texture on the balloon may be included for contacting the
Fallopian tube wall
22
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
in some embodiments, the surface of the inelastic balloon portion may be
sufficient to dislodge a
sufficient amount of cells and/or tissue for statistically meaningful
histological evaluation
regardless of whether the balloon is fully inflated or partially deflated and
crinkled. It has also
been surprisingly found that withdrawal of the extended portion may removes
still more cells. In
other embodiments, the extended portion may be retracted prior to catheter
removal so as to
preclude dispersal of dislodged Fallopian tube cells to surrounding tissue.
Upon catheter removal,
contacting the exposed portion of the extended portion, now covered in cells
with a microscope
slide or other diagnostic substrate, may be sufficient to test for abnormal
cells and in particular
cancerous cells.
[0122] The catheter 126 described above, and in greater detail be/ow may be
introduced into
the uterus of a patient using an operating hysteroscope 20, an example of
which is shown in FIG.
3. An operating hysteroscope 20 may include one or more working channels. One
working
channel may provide irrigation to distend the uterus and allow endoscopic
visualization, and one
or more additional working channels may allow instruments and/or catheters to
be advanceable
distally of the hysteroscope. The catheter 126 (e.g., FIGS. 21A and 21B) may
be advanceable
through the working channel of the operating hysteroscope, and may cannulate
the proximal os of
a Fallopian tube. The everting balloon 130 may be advanced through the
proximal catheter 126
into the proximal portion of the Fallopian tube.
[0123] FIGS. 22A-22C illustrate an exemplary embodiment of an everting
balloon 130
exiting from a flexible tip 152 with a spherical ball 122 in accordance with
an embodiment of the
disclosure. The nylon flexible tip 152 and spherical ball 122 may be
configured to pass through
the patient UTJ for the deployment of the everted balloon 130 in the Fallopian
tube. In an
embodiment, a ball tip everting balloon catheter 150 may be configured with
approximately a 0.9
mm ball tip on approximately 0.66 mm dia. X 18 mm long tip. In some
embodiments, the tip may
be formed of nylon. In some embodiments, a 4 Fr catheter with a 0.64 mm
diameter balloon that
may evert through and beyond the tip.
[0124] FIGS. 23A-23B illustrate a cross-sectional side view of a balloon
tip catheter, or
device, 160 in accordance with the present disclosure. In some embodiments, a
balloon 130 may
have an outer diameter of approximately 0.8 - 1.0 mm, and may have an initial
everted length of
approximately 1-3 cm, e.g., approximately 1.2-1.5 cm extending out of the
distal end of the
catheter 126 or cannula. The balloon 130 may be fully evertible into the
Fallopian tube, e.g.,
extending approximately 7-12cm. The balloon 130 may be securable to a distal
end of the catheter
shaft or tube 126, as indicated at reference numeral 117, and a push wire 134,
as indicated at
reference numeral 118. For example, the distal end 118 of the push wire 134
may form an end of
the balloon 130. In embodiments, the balloon 130 may be bonded to the distal
end 118 of the push
23
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
wire 134. The push rod or wire 134 may actuate the balloon 130 from an
inverted position in the
catheter 126 to an everted position when an interior of the balloon, between
the catheter 126 and
the balloon 130 and indicated by reference numeral 119, is pressurized. In
embodiments, an
everted position may include at least a portion of the balloon 130 extending
beyond the distal end
of the tube 126. In some embodiments, the balloon 130 may be initially
partially everted and fixed
to the catheter 126, forming a rounded end 130a. In some embodiments, the
balloon 130 may be
inflatable with fluid to a pressure of approximately 14-24 atm (206-353 psi).
[0125] In some embodiments, as described above, the device 160 may include
a sheath 162.
The sheath 162 may be coaxial with the catheter 126. The sheath 162 may be
slidably adjustable
relative to the catheter 126 to cover at least a first length of the balloon
130 extending outward
from the distal end of the tube 126 in an everted position. The sheath 162 may
form a physical
barrier between the balloon 130 and the interior of the scope to protect the
balloon. For example,
an initial length, e.g., approximately 1.5 cm of the balloon 130, may be
extended from the catheter
126 during insertion through the scope. As the balloon is actuated (e.g., via
the push wire 134
and/or balloon pressurization), the sheath 162 may protect the balloon in at
least one of the inverted
position, a partially everted position, or a fully everted position, or
combinations thereof.
[0126] The sheath may also act to provide column strength to the balloon as
it is everted. In
some embodiments, a portion of the sheath 162 may be at least partially
translucent, optically
transparent, or combinations thereof, as indicated at reference numeral 162a.
In embodiments, the
transparent portion 162a of the sheath 162 may at least partially overlap with
a transparent portion
167 of the catheter 126. For example, a medical professional may be able to
visualize the balloon
130 (e.g., to confirm positioning and/or full balloon extension) with the
hysteroscope 20 through
at least a portion of the sheath 162 and/or the catheter 126. In some
embodiments, the catheter
may include a sheath knob 164 located at a proximal end of the sheath 162 to
connect the sheath
162 to the tube 126.
[0127] The pressurized balloon 130 may have a rounded end 130a for
atraumatic cannulation
of the proximal os and advancement within the Fallopian tube and a degree of
flexibility along the
balloon 130 length. The balloon 130 may have sufficient column strength to
allow the balloon
130 to be manually advanced through the UTJ, for example, with a push wire
134, under at least
a partial pressure or no pressure. In some embodiments, the balloon 130 may be
constructed of a
thin-walled polymer material, such as polyethylene terephthalate (PET),
polyethylene, Nylon,
polymer, or a similar material. The balloon 130 may have a wall thickness from
approximately
0.0001 to 0.001 inches and in some embodiments between approximately 0.00019
and 0.00031
inches. In some embodiments, the balloon 130 may have a thickness of less than
0.005 inches.
The material and/or thickness of the balloon may be important characteristics
of the balloon
24
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
impacting how the balloon acts as it is deployed and with respect to cell
collection. For example,
too thin of a balloon wall may result in the balloon lacking sufficient column
strength (acting more
compliant or elastic as desired), or too thick of a balloon wall may result in
the balloon resisting
everting or everting in an inconsistent manner. The thickness of the material
may affect the
contouring, wrinkling of the balloon surface to the extent the surface
features are created or
enhanced by the act of inverting, when loading the balloon in the catheter,
which in turn may affect
the ability to collect and retain cells. The material of the balloon may also
impact whether the
balloon may adhere or tend to stick to itself during eversion or after being
deflated and withdrawn
with the catheter.
[0128] In some embodiments, a first marker 171 may be disposed on at least
a portion of
catheter 126. The first marker 171 may be a preparation marker, indicating a
desired position of
the sheath knob 164. When the sheath knob is aligned with the first marker
171, the proximal end
of the sheath 162 may be a reference point for the medical professional for
balloon extension
during preparation and initial cannulation of the balloon 130 into the
Fallopian tube. In
embodiments, at least a portion of the catheter 126, e.g., a proximal portion
connected to the
transparent portion 167, may be formed of a metal such as stainless steel, or
other materials such
as composites, or polymers, or combinations thereof. The first marker 171 may
indicate to a user
an appropriate location of male luer lock fitting, or sheath knob, 164 with
respect to the balloon
130 within the sheath 162, so that the sheath 162 may be extended distally an
initial length as a
preparation step to cover, for example, approximately 10 to 20 mm length of
everted balloon 130
that is used to access the proximal os before the balloon is completely
everted.
[0129] When in position at the proximal os, the sheath may be pulled back
from the first
marker 171 to the original position, exposing the partially everted balloon
tip for accessing and
placement in the Fallopian tube. In embodiments, the sheath 162 may be
extendable along a
longitudinal axis to a point beyond the distal end of the catheter 126. When
the sheath 162 is
extended distally of the catheter 126, a distal tip of the sheath 162 may be
an indicator for balloon
advancement. The first marker 171 may include a score line, a coating
substance, or a selectively
oxidized region. In some embodiments, the first marker 171 may be an opaque
band of material
(e.g., including but not limited to polymer, or metal, or combinations
thereof) attachable to at least
a portion of the catheter 126 (e.g., metal portion, or hypotube 138) using,
for example, an adhesive,
bonding, or welding process. Such a preparation marker may allow the medical
professional to
know how far to deploy the balloon 130 in the initial preparation step,
thereby improving the ease
of use of the device by eliminating the need for an outside measuring tool and
improving the safety
of the procedure by eliminating any guesswork or eyeballing on the part of the
user.
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
[0130] In some embodiments markers may be incrementally spaced apart in
known
predetermined distances from each other such that a medical professional may
use the markers as
a visual counter or measuring device to verify an approximate length of
balloon that has been
everted. It is appreciated that any inner cannula or catheter described herein
may include indicia
as described for assistance in navigating patient anatomy.
101311 In some embodiments, a second marker 173 may be disposed on the
catheter 126, e.g.,
a metal portion 138, to indicate a desired location of sheath knob 164 to
confirm that the sheath
162 covers the deployed everting portion (balloon, suture, etc.) during device
removal into the
hysteroscope 20. For example, the second marker 173 may be a retraction
marker. This may allow
the user to visualize and confirm that the balloon 130 is fully protected by
the sheath 162 during
the removal process to avoid loss of cells collected on the balloon and/or
extended portion. When
the hysteroscopic view is obscured, for example, by blood or tissue in the
distension fluid,
additional user visualization by the second marker 173 may be advantageous.
The second marker
173 may be formed by the same techniques used to form the first marker 171.
The second marker
173 may also be included on any inner cannula or catheter described herein.
[0132] In some embodiments, a portion 167 of the catheter 126 and/or distal
portion of sheath
162 may have a transparent section along its length or a portion that is
translucent, optically
transparent, or a combination thereof under use conditions. According to
embodiments of the
present disclosure, the tube or catheter 126 may include at least one visual
marker. In other
embodiments, the visual marker on the catheter 126 may comprise a third marker
179 disposed on
the catheter 126. The third marker 179 may be located near or at the distal
end of the catheter 126
shaft where the balloon 130 is connected to the catheter 126. In some
embodiments, the third
marker 179 may be radio opaque. The third marker 179 may visually indicate to
a user the end of
the catheter 126 shaft, thereby improving control of the catheter 126. The
ability to visualize the
end of the catheter 126 may be desirable during cannulation, when the balloon
130 is advanced
beyond sheath 162 into the Fallopian tube. The third marker 179 may allow a
user to visualize the
distal end of the catheter 126 as thc cannulation step progresses. The user
may be able to see when
the cannulation step is complete, e.g., when the third marker 179 aligns with
the end of the sheath
162 at the os, thereby improving ease of use. The third marker 179 may be
formed by the same
techniques used to form the first marker 171 and/or the second marker 173. The
third marker 179
may be provided in an easy to see color, for example black or blue.
[0133] In some embodiments, a string, braid, and/or suture 121 may be
extendable distally of
the balloon 130 as the balloon 130 everts in the form of an extendable portion
of the balloon 130.
In some embodiments, the string or suture may be attached to the distal end of
the push rod or to
the balloon tip, by bonding or adhesive, e.g., at reference numeral 118. In an
inverted position of
26
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
the balloon 130, the string, braid, and/or suture 121 may be positioned
internal to the balloon 130,
e.g., within the tube of the catheter 126 as shown in FIG. 23A. Upon eversion
of the balloon 130,
= e.g., by actuation of the push rod, the string, braid, and/or suture may
extend to a position that
becomes the exterior to the balloon, either extending distally from the distal
tip of the balloon 130,
or extending proximally from the balloon tip alongside the exterior of the
balloon.
[02341 In some embodiments, at least a portion of the string, braid, and/or
suture 121, e.g., as
indicated by reference numeral 43 in FIGS. 11C-11D, may be braided. The
braided string or
suture 43, 121 may comprise one or more filaments. The string or suture 43,
121 may be
extendable when the balloon 32, 130 is everted. In some embodiments, the
string or suture 43,
121 may be a plurality of braids. In some embodiments, the string or suture
43, 12/ may be formed
of one or more colors, e.g., to improve visualization for the medical
professional to confirm that
the balloon is everting properly (see FIGS. 11E-11F). For example, the colors
may be visualized
through the scope as the balloon is everted and the string or suture 43, 121
is pushed out with
balloon. Since the balloon 32, 130 is everting, the string or suture 43, 121
within the interior of
the balloon 32, 130 may advance out of the balloon at approximately twice the
distance as the
balloon everts (e.g., as the balloon everts 1 mm, approximately 2 mm of string
or suture is exposed
beyond the distal end of the inner cannula/tube. The colors may determine
positioning of the
suture or string 43, 121 in the Fallopian tube for sample collection. In some
embodiments, the
string or suture 43, 121 may include one or more regions having printed
indicia, or color variations
along the length thereof, or combinations thereof. In some embodiments, the
string or suture 43,
121 may include one more knots along its length in predetermined spaced known
increments to
provide further visual or tactile feedback to the medical professional (see
FIGS. 34A-34B). The
colors and/or knots may be placed at incremental distances from each other, so
that a count of the
colors or knots may be translated to an approximate amount of distance/length
that the suture or
string 43, 121 (and to some extent the length of the balloon 130) has been
everted.
[0135] In some embodiments, the balloon material may be treated to change
the surface
properties of an exterior surface of the balloon 130. Processes such as plasma
or corona treatment
may increase surface receptiveness to various substances that illustratively
include subject cells,
inks, coatings, adhesives, laminates, and paints, or combinations thereof.
Surface treatment may
enhance wettability creating a surface with hydrophilic properties, or
discourage wetting creating
a surface with hydrophobic properties. Surface treatment may be used to
improve the adhesion
properties of the balloon surface, to create a surface in which cells are more
likely to adhere
compared to an untreated surface.
[0136] Surface treatments may also be used to prepare the balloon surface
for printing indicia
on the surface, e.g., including PAD printing. PAD printing (also called
tampography) is a printing
27
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
process that may transfer a 2-D image onto a 3-D object. Indicia printed on
the balloon surface
may serve as preparation markers for the user. These preparation markers may
allow the user to
know the length of the balloon 130 prior to deployment of the balloon 130,
thereby improving the
ease of use of the device by eliminating the need for an outside measuring
tool and improving the
safety of the procedure by eliminating any guesswork or eyeballing on the part
of the user.
[01371 In addition to marking for visualization purposes, the balloon 130
may also be treated
with a process that increases surface area such as the application of a
nanofiber or micropillar
surface (e.g., including but not limited to ULTRA-WEB from Corning), which
may improve cell
collection yield and/or retention compared to a balloon with little or no
surface treatment. The
suture or string 121 may include similar surface treatment features as a way
to enhance cell
collection and retention.
[0138] In various embodiments, the balloon 130 may be formed of a material
such that the
balloon 130 is capable of moving between the inverted and everted positions
without excessive
deployment pressures, yet rigid enough so that the balloon does not
excessively radially expand
during eversion. The material may also allow for wrinkles, overlapping
material, or micro ridges,
or a combination thereof to be formed on the balloon surface during
manufacturing and/or
assembly, for example by polymer deformation. Such wrinkles, overlapping
material, or micro-
ridges may be created on a normally smooth (contourless) balloon surface
material, or may
enhance a balloon surface material that already includes one or more surface
features. The
wrinkles, overlapping material, or micro ridges formed in the balloon material
may remain during
balloon eversion and/or inversion, e.g., the balloon surface may be
plastically deformed. Wrinkles,
overlapping material, and/or micro ridges may improve cell collection of the
balloon 130. For
example, cells may be removed from the Fallopian tube during balloon eversion
and/or may be
captured within the wrinkles as the balloon 130, so that when the balloon 130
is retracted into the
sheath 162 and the catheter 126 is removed with the scope, cells may be
retained within the
wrinkles of the balloon 130. Relieving pressure in the balloon, to deflate or
partially deflate the
balloon, prior to retraction, may act to increase or reform wrinkling on the
balloon surface and
further improve cell collection and/or retention. In some embodiments, the
surface of the balloon
130 may be roughened, or otherwise adjusted, to increase a surface area.
According to various
embodiments, the balloon may be made of polyethylene terephthalate (PET),
polyethylene, nylon,
a fluoropolymer, or a perfluoropolymer, or other similar suitable material.
[0139] In some embodiments, a surface area of the balloon 130, e.g., the
surface for
contacting the inner surface of the body lumen (Fallopian tube) may include
additional surface
features. In some embodiments, a balloon surface that is relatively smooth
because of the material
characteristics may be modified to include wrinkling and added surface area,
e.g., by processes
28
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
employed during manufacture or packaging that impart surface features to the
balloon surface that
are retained during use of the device. In other embodiments, a balloon
material surface that is
maintained relatively free of any contouring, may still be able to collect and
retain cells just
through the mechanism of everting and engaging the tissue lumen with the
balloon and then
(optionally deflating and) retracting the balloon along the tissue wall, as
described above. In some
embodiments, a surface of the balloon 130 may be embossed to impart micro
ridges having peak-
to-valley heights of from approximately 0.1 to 500 microns through a variety
of conventional
techniques that illustratively include plate-to-plate, roll-to-plate and roll-
to-roll. In some
embodiments, the peaks and valleys may be configured to be large enough to
provide additional
surface area but small enough to minimize the potential of peaks and valleys
locking together. For
example, peaks and valleys in the balloon surface area may interlock during
inversion/eversion
such that balloon movement may be impeded. It may therefore be advantageous to
configure the
peaks and valleys to have a profile to minimize potential interlock.
[0140j In some embodiments, a polymer surface of the balloon 130 may be
etched. Etching
may be accomplished by a variety of conventional techniques including but not
limited to solvent,
chemical, laser, or plasma exposure. Etching may be advantageous to increase a
surface area
without incurring the stressing on the balloon of having embossing tool
contact. This feature may
improve cell collection of the balloon by increasing surface area and creating
micro-edges that are
normal to the axis of the balloon as it is removed. In some embodiments, as
above, polymers
having low surface energies and/or having a limited ability to crinkle/wrinkle
at any balloon
thickness upon embossing and/or etching are nonetheless operative herein for
cell biopsy as the
opposing contacting surfaces have sufficient glide to allow the balloon to
evert smoothly, while
having enough surface area to dislodge and retain cells. Low surface energy
polymers in embossed
or etched form may include fluoropolymers, perfluoropolymers, polyalkylenes,
polypyromellitimide (Kapton H), or polystyrene, or combinations thereof.
[0141] In some embodiments, etching or embossing may be formed on a balloon
surface in
concentrated portions of the balloon, e.g., as indicia. For example, balloon
markings may provide
a visual indication for the medical professional to determine an extension of
the balloon into the
Fallopian tube. Concentrated etchings and/or embossing may be visible by the
medical
professional, e.g., potentially eliminating a need for a separately attached
marker or other indicia.
A marker formed as a portion of the balloon may be advantageous to minimize
and/or avoid
potential detachment.
[0142] The balloon 130 may be translucent, optically transparent, or a
combination thereof.
In some embodiments, the balloon 130 may be at least partially opaque to
enhance visibility during
use. In some embodiments an opaque fluid may be mixed in the inflation fluid
to control color of
29
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
the balloon and to further enhance visibility of the balloon. The amount of
the opaque fluid added
to the inflation fluid may control the level of translucence or opacity of the
balloon. In some
embodiments, the fluid may be rendered opaque or otherwise detectable through
the inclusion of
colloidal or suspended particulate or microbubbles released within the fluid.
Colloidal or
suspended particulate operative herein include without limitation,
polymethylmethacrylate, mica,
barium sulfate, starch, and combinations thereof.
[0143] The length of the fully everted balloon 130 may extend to
approximately 7-12 cm
within the lumen (e.g., Fallopian tube), such that when fully everted, the
balloon 130 may extend
within the patient's Fallopian tube, following the successful advancement of
at least a portion of
length of everted balloon through the UTJ. Eversion of the balloon 130 may be
performed in a
controlled manner, e.g., by advancing a push rod 134 through a fluid tight
seal 135, at the proximal
end of the catheter 126. As described above, at least a portion 167 of the
catheter 126 may be
transparent or translucent, so that movement of the balloon 130 may be
viewable through the
hysteroscope through which the catheter 126 is inserted, thereby providing the
user with a direct
view of the insertion procedure. The catheter 126 may be constructed of
polymers such as Nylon,
polyether block amide, polyurethane, PET (polyethylene terephthalate),
polyethylene, or polyvinyl
chloride (PVC), with or without polymer or metal coil or braid reinforcement,
or combinations
thereof.
[0144] In some embodiments, the transparent or translucent portion 167 of
the catheter 126
may be at least approximately 1 cm in length for visualization of the balloon
deployment through
the hysteroscope view. Providing a transparent or translucent portion 167 that
is of an adequate
length may ensure visualization of the balloon deployment while providing
sufficient catheter
column strength for Fallopian tube cannulation. In embodiments, the
transparent portion 167 of
the catheter 126 may have a length relative to an opaque portion, e.g., a
metal hypotube portion
138, to balance desired column strength and support to the catheter 126 with
visualization at the
distal end. In some embodiments, the transparent portion 167 may extend to a
proximal end of the
device, within the metal portion 138. It is understood that materials used to
form the transparent
portion 167 of the catheter 126 may have lower column strength than a metal
hypotube portion
138. This balance may improve ease of use (e.g., by visualization of the
distal end) and control of
the device (e.g., by having sufficient stiffness to enable placement of the
device at the ostium of
the Fallopian tube and maintain position throughout the procedure).
[0145] In some embodiments, a balloon 130, when everted at least partially
out of the catheter
126 or cannula, may not remain straight. Rather, the balloon 130 may assume an
undesired curved
configuration, either a single "C" curve, or an "S" curve, that may be
difficult to use to cannulate
the proximal os of the Fallopian tube, and to advance the balloon through the
UTJ. The extended
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
length of everted balloon 130 may be straightened out or maintained straight
by use of an outer
sheath 162 that lies coaxial about the exterior of catheter 126 or cannula,
and may assist in
providing column strength and cover of the partially everted balloon tip. At
least a portion of
sheath 162 and/or catheter 126 may be transparent 167, e.g., 167 of FIG. 23A,
so that movement
of the balloon 130 may be viewable through the hysteroscope through which the
catheter is
inserted, thereby providing a user with a direct view of the insertion
process. Similar to catheter
126, the sheath 162 may be constructed of polymers such as Nylon, polyether
block amide,
polyurethane, PET (polyethylene terephthalate), polyethylene, or polyvinyl
chloride (PVC), with
or without polymer or metal coil or braid reinforcement, or combinations
thereof. The sheath may
be alignable with respect to the catheter and/or the balloon, thereby
providing column strength to
the balloon. In response to cannulation of the balloon through the UTJ into
the Fallopian tube, the
sheath may support the balloon from outside of the proximal to minimize and/or
prevent collapse
as the balloon is further everted after navigating the UTJ. The sheath 162 may
also protect the
sample (e.g., cells) collected on the balloon and/or extended portion. For
example, the sheath 162
may protect the balloon in an everted position after contacting an inner
surface of the Fallopian
tube. In some embodiments, the sheath 162 and balloon with or without extended
portion may be
retracted coaxially with the inner lumen of the sheath to extend the sheath
over the everted balloon,
and extended portion, if included, subsequent to cell collection. In some
embodiments, the sheath
162 may remain stationary relative to the balloon 130 and/or the catheter 126,
so that the balloon
130 is received in the sheath 162 subsequent to cell collection. As the
balloon 130 is withdrawn
into the sheath and removed from the patient, the sheath may protect the cells
to minimize and/or
prevent loss of the sample collection by providing a barrier from distention
fluid in the uterus or
irrigation fluid in the Fallopian tube or uterus. For example, the balloon
subsequent to cell
collection may otherwise be exposed to environmental conditions that may
render the sample
collection unusable, and/or otherwise wash the cells from the balloon and
extended portion.
[0146] FIG. 35 illustrates an exemplary embodiment of a linear eversion of
a balloon 130 in
accordance with the present disclosure. In embodiments, one end of the balloon
may be fixed to
inner cannula/tube at point X (e.g., reference numeral 117 as illustrated in
FIG. 23A) and the other
end of the balloon may be movable at point Y (e.g., reference numeral 118 as
illustrated in FIG.
23A). The balloon 130 may evert from the position shown in Step 1 to the
position shown in Step
2 to the position shown in Step 3. In the eversion process, points A, B, and C
move towards the
left side of the diagram, e.g., extend distal of the distal end of the device
160. As the balloon 130
unrolls/everts at/toward the left side of the diagram, point A may move from
the inside surface of
the balloon to the outside surface. In practice, the balloon 130 that has been
partially, or initially,
everted during the preparation step may be advanced further into the proximal
end of the Fallopian
31
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
tube. Further eversion (extension) of the balloon (in total up to the full
length of the Fallopian
tube, approximately 7-12cm) may be accomplished by further rotation of a drive
wheel 204 (see
FIG. 25). The balloon 130 may then be deflated by relieving pressure in the
inflation device. The
balloon 130 may then be retracted from the Fallopian tube. Because the
Fallopian tube is a potential
space, the Fallopian tube tissue may tend to collapse around the balloon.
Because the balloon fills
the Fallopian tube, the balloon surface area may be substantially equivalent
to the surface area
inside the Fallopian tube. This surface area may optimize tissue collection
from the inside of the
Fallopian tube. While deflation of the balloon may be desirable prior to
retraction, it may be
possible in some embodiments to retract a balloon/extended portion from a
Fallopian tube without
first deflating the balloon and still retaining cells collected thereon. For
example, the balloon 130
in an inflated andJor a deflated state may be retracted within the sheath 162
while retaining a
sufficient amount of cells on the surface of the balloon 130 for testing.
Alternatively, the balloon
may be repeatedly inflated and deflated while extended in the Fallopian tube,
so that each time the
balloon contacts Fallopian tube walls, more cells may be collected and/or
retained by the balloon.
[0147] As mentioned, to further aid tissue collection, wrinkles or other
surface features may
be added to the surface of the balloon. Wrinkles may form as the balloon
deflates to create multiple
edges and/or overlapping material, to aid in cell collection. Edges may work
in a manner similar
to the edges of a curette or edges of jaws in a biopsy forceps. Similar to
these features on other
collection devices, edges formed by the wrinkled balloon may focus a contact
force on the
anatomical wall in order to collect cells.
[0148] The balloon deployment device in accordance with the present
disclosure may then be
removed from the working channel of the hysteroscope and from the patient.
Once the device is
removed from the patient, cells may be removed from the balloon by dipping the
balloon and/or
the extending portion (if used) into a cytopreservative and stirring in order
to agitate the cells.
Alternatively, balloon, extending portion, and/or sheath may be cut off and
placed into a
cytological preservative. In some embodiments a sheath may be extendable and
deployable over
the balloon as the balloon is deflated and removed to protect tissue samples
collected on the
balloon surface.
[0149] FIG. 24 illustrates a cross-sectional side view of a balloon tip
catheter 160' including
a superelastic push rod 175 and spiral carrier 176. The spiral carrier may
minimize and/or
eliminate the need to extend the push rod backwards, e.g., outside of a
handle, for the full length
of the push rod in accordance with embodiments of the disclosure. The push rod
175 may be
constructed of a superelastic material such as Nitinol (nickel-titanium
compound) wire. At least a
portion of a length of push rod 175 may be coiled into a spiraling tubular
carrier 176, which may
be made of polyethylene or polytetrafluoroethylene (Teflon). The outer spiral
diameter of the
32
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
carrier may be approximately 8 cm, rendering the proximal operating length of
the catheter handle
much more compact. The spiral carrier 176 may be attached to a proximal seal
135 on the catheter
by a flexible strap 177. In some embodiments, the flexible strap 177 may be
constructed of
polymer or silicone rubber material. In some embodiments the push rod 175 may
have a diameter
of approximately 0.025", or some other thin diameter, which may be
disadvantageous for purposes
of gripping the wire and push it forward through the seal 135. A flexible grip
178 may be included
that slides freely on the push rod 175, but upon compression between the thumb
and forefinger,
may provide a grip for push rod 175 advancement. The flexible grip 178 may be
an elliptical
cross-section frame that may be made of polyvinyl chloride, silicone rubber,
or combinations
thereof, or similar flexible compound. In some embodiments, the flexible grip
may have inner
dimensions of approximately 2 cm in length, 1 cm in width, and 3 mm in height,
and may have a
wall thickness of approximately 2 mm. Holes in the proximal and distal faces
of the grip may be
a slip fit with the push rod 175.
[0150J FIG. 25 illustrates an exemplary embodiment of a balloon tip
catheter 200 configured
with handle 202. In embodiments, the handle 202 may be included in the device
160 as illustrated
in FIG. 23A. The handle 202 may house a gear mechanism 220 (see FIGS. 26A-
26B), also
referred to herein as an actuator. The handle 202 may be in mechanical
communication with a
push wire 134, 206 and may control actuation of the push wire 134, 206, which
in turn may control
actuation of the balloon 130 between an inverted position and an everted
position. Handle 202
may include a drive wheel 204 for advancement and retraction of the push wire
134, 206, in which
the balloon 130 may evert linearly (e.g., gradually unfold or unroll from the
inside out.). The drive
wheel 204 may be made of polymer material including but not limited to ABS.
The outer edge of
the drive wheel 204 may include notches, or a knurl pattern, to facilitate
gripping the wheel during
operation of the catheter 200. The outer edge of the drive wheel 204 may
include multiple features
shaped like arrow heads that facilitate gripping and/or may indicate correct
direction of travel of
the drive wheel. A top surface of the drive wheel 204 may have an arrow molded
into it for
indication of a correct direction in which to turn in order to evert the
balloon. The opposite side
of the drive wheel 204 may include a square boss 222 insertable into a drive
gear 224. In some
embodiments, the gear mechanism 220 may include a step-down gearing that
provides a reduced
amount of extension of the push wire 134, 206 relative to a given rotational
distance travelled by
the drive wheel 204 (i.e., the drive wheel 204 must be turned more of a
distance to accomplish the
same extended length of balloon everted, than if step-down gears were not
included or a different
ratio of step-down was included). The resultant effect may be to have a finer
control over the
eversion of the balloon 130 as the drive wheel 204 is turned.
33
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
[0151] The catheter 200 may retain the balloon 130 in a shaft 210 (which
may at least partially
be formed of a stainless steel tube and/or a Nylon tube), a sheath 212, and/or
a sheath knob 214.
For balloon advancement, the balloon 130 and shaft 210 may be pressurized with
an inflation
device (such as inflation device 172 of FIG. 23C) that is attachable to an
extension tube 168, 216,
or to luer 218, of the handle 202 (see FIGS. 26A-26B). Once the catheter
device 200 is
pressurized, a user may rotate the drive wheel 204 causing a push wire 134,
206 to advance.
Although in some embodiments, the balloon 130 may evert under pressurization
without a drive
wheel advancement of the push wire 134, 206, it is understood that the drive
wheel may allow for
smooth, slow, controlled advancement of the balloon, thereby minimizing or
avoiding potential
perforation of the Fallopian tube. The sheath knob 214 may allow the sheath
162, 212 to be used
as an introducer as the sheath 162, 212 locks onto the body of the catheter
126, 210. The sheath
knob 214 may be compliant enough to allow the user to move the sheath 162, 212
when needed,
for example to the pre-extended portion of the balloon and to move the pre-
extended portion of
the balloon into the Fallopian tube. In embodiments, the sheath knob 214 may
be tight enough
such that unintended balloon or catheter movement may be minimized and/or
prevented.
[0152] FIG. 26A is a cross-sectional view of the handle portion of FIG. 25,
and FIG. 26B is
a detail view of an exemplary embodiment of an internal handle gear mechanism
220 in accordance
with the present disclosure. The handle 202 may also have an extension tube
168, 216 that is
attached to a luer 218 in the handle body, e.g., for attaching one or more
additional tools or devices
such as inflation device 172 (see also FIG. 23C). The gear mechanism or
actuator 220 may be in
mechanical communication with the push wire 134, 206, and may control
actuation of the push
wire 134, 206, which in turn may control actuation of the balloon 130 between
the inverted position
and the everted position. In some embodiments, the gear mechanism or actuator
220 may include
a plurality of gears operating enmeshed to have a step-down ratio. According
to various
embodiments, the handle gear mechanism 220 may include a drive wheel 204,
which allows
controlled actuation of the gear mechanism 220 and single user operation. The
loop "A" as shown
in FIG. 26A may be included in the handle 202 and the feature for positioning
a finger "B" may
allow for a user to hold the handle 202 in more than one position and may
allow for comfortable
use of the device no matter the hand size of the user. For example, if the
palm of the user's hand
is on the top of the handle "C", the fingers of the hand may wrap around the
inside of the loop "D"
for a small hand, or the outside of the loop "E" for a large hand.
[0153] In some embodiments, the drive wheel 204 may have a square boss
insertable into
square hole 222 in the drive gear 224. The drive wheel 204, operable by a
medical professional,
may be rotatable so that the square boss may cause drive gear 224 to rotate.
In embodiments, the
drive gear 224 may be rotatable in a direction indicated by arrow 224A by the
drive wheel 204
34
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
(see FIG. 26B). The drive gear 224 may engage an idler gear 226 and first gear
228, causing these
gears to rotate. For example, the first gear 228 may rotate in a direction
indicated by arrow 228A,
which may be a direction opposite of arrow 224A. Likewise, the idler gear 226
may rotate second
gear 230 in a direction indicated by arrow 230A, and third gear 232, in a
direction indicated by
arrow 232A in response to rotation of the second gear 230. The push wire 206
may extend between
surfaces on and between each of the four gears (224, 228, 230, 232), which may
each rotate as
shown by arrows 224A, 228A, 230A, 232A in FIG. 26B during advancement of
balloon 130 (e.g.,
in a distal direction) via the push wire 206. In some embodiments, gear
surfaces may be formed
of a material having a high coefficient of friction such as natural or
silicone rubber, or
polyurethane.
[0154] The balloon 130 may be advanceable until a proximal end of the push
wire 134, 206
passes between the drive gear 224 and may be in mechanical communication with
first gear 228.
Once the push wire 134, 206 has passed beyond the gear mechanism 220, further
rotation of the
drive wheel 204 may not advance the balloon 130 further. The absence of the
push wire 134, 206
in the gears 224, 228, 230, 232 may be felt by the user as a tactile indicator
of the balloon 130
being fully everted. The gear mechanism 220 by being in mechanical
communication with the
push wire 206 may allow for fine, precise, and controllable movement for the
deployment and/or
retraction of the balloon 130 through eversion and inversion, respectively. As
mentioned, the drive
wheel may provide for slow and uniform movement for minimizing a potential of
perforating the
Fallopian tube, or inability to navigate the Fallopian tube. The gear
mechanism 220 may be a 4 to
1 gear ratio, or a 2 to 1 gear ratio, and it is understood that any other gear
ratios may be used to
provide control of the advancement of the balloon. A gear ratio may be
configured to provide
slow gear rotation. This may ensure that the deployment speed of the balloon
is controlled (e.g.,
slow and uniform) across users, thereby increasing safety by reducing the risk
of adverse events
such as perforation.
[0155] In some embodiments, to provide feedback to the physician regarding
the end of
balloon deployment, the internal handle gear mechanism 220 or actuator may
include a limit
mechanism on the gears for limiting the advancement of the push wire and/or a
unidirectional
balloon movement. In some embodiments, the limit mechanism may include at
least one of a hard
stop, a gear jam, a rack and pawl gear, a linear gear, or a drop key-click in
mechanism. At a
predefined maximum extension, a pawl 242 may engage with one or more gears
(e.g., gears 224,
228, 230, 232) as shown in FIG. 26E, to form a gear jam. The pawl 242 may be
activated to stop
further advancement of the balloon 130. In embodiments, the pawl 242 may be
any mechanism
configured to engage with one or more gears. For example, at a predefined push
wire extension,
the pawl 242 may rotate around a pivot point to engage with one or more gears,
causing a jam and
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
preventing further rotation. Alternatively, a rack and pawl gear, a linear
gear, or a drop key-click-
in mechanism (FIG. 26D) may be employed to stop advancement of the balloon and
in some
embodiments may be disposed in the handle (see FIG. 26A, detail "F").
Referring to FIG. 26C,
an exemplary ratchet mechanism for linear motion is shown. Ratchets are
mechanisms that serve
to limit motion to only one direction. A ratchet may have three main parts: a
linear gear rack 233,
a pawl 235 (e.g., a "click"), and a base or mount 237. The edges on one side
of the teeth 239, 239'
on the linear rack may have a steep slope while the other edges of the rack's
teeth may have a
moderate or gradual slope. For example, edges on one side of the teeth 239,
239' may be steeper
than edges on another side of the teeth 239, 239'. In some embodiments, a
steeper slope may have
an angle of approximately 600-900, e.g., as indicated at 239a, 239a', and a
more moderate slope
may have an angle of approximately 10 -50 , e.g., as indicated at 239b, 239b'.
The pawl 235 may
contact the linear gear rack 233. When the linear rack is linearly moved in a
first direction, the
pawl 235 may slide over the teeth 239 without restricting the natural motion
of the device. When
the direction of motion is reversed to a second direction, the pawl 235 may
contact the steep slope
on the gear tooth 239 to impede motion. The pawl 235 may be biased downward by
a spring into
the linear gear rack 233. In some embodiments, a spring, e.g., a torsional
spring, may be disposed
at a pivot point 236, e.g., at a first end of pawl 235, for pivotable rotation
of the second end of the
pawl 235. In some embodiments, a spring, e.g., a linear spring, may be
disposed at a second end
of the pawl 235, as indicated by reference numeral 223, to bias the pawl 235
towards the gear teeth
239. The linear gear rack 233 and pawl 235 may be typically mounted in a fixed
relationship to
one another on a mount 237, with the rack sliding in relation to the mount and
the pawl 235 having
a pivot connection to the mount. In some embodiments, the device may include a
manual knob or
push button switch to overcome the spring bias on the pawl 235 to allow for
the lifting of the pawl
235 from the set of teeth on the linear gear.
[0156] A limit may be set on the ratcheting action of the linear gear rack
233 in the gear
mechanism 220 of FIGS. 26A-26B to set a limit on the advancement of the push
wire 206, e.g.,
as shown at "F'. During advancement of the push wire 206, a pawl 238 may be
biased toward
from linear gear rack 233 as shown in detail "F" of FIG. 26A. In some
embodiments, the pawl
238 may be pivotal about a point, as indicated by reference numeral 221.
Linear gear rack 233
may be directly attached to the end of the push wire 206 away from the balloon
130 in the handle
202. Advancement of the push wire 206 may be automatically stopped when pawl
238 meets stop
243, which may be greater in height than teeth 239'. A manual knob or push
button switch 205
as illustrated in FIG. 25 may be actuatable by a user to overcome a spring
bias on the pawl 238 to
allow for the lifting of the pawl 238 from the linear gear rack 233 and for
retraction of the push
wire 206 and the attached balloon 130. In FIG. 26D, in another embodiment
different from the
36
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
ratcheting action of the linear gear rack 233, the push wire 206 may be
continuously and smoothly
advanced and coiled around deployment wheel 245 until pawl 235 reaches and
engages with detent
247 to stop further advancement of the balloon 130. In FIG. 26E, the pawl 235
may act as a gear
jam when an extension limit of the balloon 130 is reached.
[0157] The sequence of steps used to enter and track through the Fallopian
tube may be
described with the embodiment of FIG. 23A. When it is desired to cross the UTJ
with a length
(e.g., approximately 15 mm) of an everted balloon 130, the outer sheath 162
may be placed in
apposition with the proximal os of the Fallopian tube, without entering the
proximal os. The outer
sheath 162 may support the initial length of everted balloon 130 until it
enters the proximal os. A
portion of the balloon, e.g., a short length, of pressurized everted balloon
130 exiting the supportive
outer sheath 162 may have sufficient column strength to be manually
advanceable through the
UTJ, whereas an unsupported length (e.g., without the sheath) of the everted
balloon 130 may not
contain sufficient rigidity by itself. As such, an everted/everting balloon
130 may buckle upon
attempted advancement through the proximal os and UTJ without a sheath. In
some embodiments,
crossing the UTJ with a length of the everted balloon (e.g., 15 mm), may
occur. This initial
cannulation length may support keeping the Fallopian tube open even if a spasm
occurs, which
may occur in this area of the Fallopian tube. It is also understood that other
cannulation lengths
may be utilized to maintain an open Fallopian tube.
[0158] In some embodiments, the sheath 162 may be compatible with standard
hysteroscopes
having a working channel, e.g., 5F. A sheath 162 may be used in an exemplary
system as a balance
to provide a wall thickness great enough to impart sufficient column strength
to the sheath and thin
enough to maintain a sheath inner diameter large enough to accommodate the
balloon 130. This
balance may improve cell collection efficiency, e.g., by having an inner
diameter sufficient to
retain the balloon 130 without inadvertently removing (scraping) cells from
the balloon surface.
It is understood that the balloon 130 may be retained within the sheath 162 in
an inflated state
and/or a deflated state.
[0159] As mentioned, a male luer lock fitting, or sheath knob, 164
including a Tuohy-Borst
seal 136 connector may be included at the proximal end of the sheath 162. A
Tuohy-Borst adapter
that includes seal 136 is a medical device used for creating seals between
devices and attaching
catheters to other devices. The Touhy-Borst seal 136 may be tightened to have
a slip fit with the
catheter or cannula holding the sheath 162 in place. The sheath knob 164 may
mate with a female
luer lock fitting, if present, at an instrumentation port, on the working
channel of the hysteroscope
20. Referring back to FIG. 3, the male luer lock or sheath knob 164 may be
connectable to the
instrumentation port 23 so that the catheter 126 and/or sheath 162 may move
with the hysteroscope
20. In some embodiments, the instrumentation port 23 may further include a
seal for the catheter
37
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
126 to extend through. When these respective luer fittings are connected, the
tip of the sheath 162
may protrude out of the distal end of the hysteroscope, e.g., approximately 2-
3 cm. The sheath
162 may also protect a portion of a balloon 130 everted during device
preparation (e.g., a length
approximately 1.5 cm) from injury as the catheter 126 is advanced through the
working channel
of the hysteroscope. A stainless steel tube, e.g., hypotube 138, may be at
least a portion the inner
cannula 126 to provide sufficient rigidity and/or column strength to minimize
or prevent kinking
of the portion protruding from the proximal end of the hysteroscope working
channel. In some
embodiments, the hypotube 138 may be sized having approximately 0.050" OD x
0.004" wall
thickness for sufficient rigidity.
[01601 In some embodiments, the hypotube 138 may ensure that the handle 202
is
undisturbed, or does not fall out of the working channel of the hysteroscope
20 when a medical
professional releases the device during a procedure. The sheath 162 may be
coaxial with the tube
or catheter 126 and may be slidably adjustable to cover at least a first
length of the balloon
extending outward from the distal end in the evened position. The sheath 162
may form a physical
barrier and may protect the balloon in at least one of the inverted position,
a partially everted
position, and/or a fully everted position and may serve to protect collected
cells from dislodgement
during transit out of the patient body.
[0161] As mentioned, at least a portion of each of the sheath 162, a
portion of the tube or
catheter 126, and/or the balloon 130 may be translucent, optically
transparent, or a combination
thereof, to facilitate visual feedback of relative positions of the
aforementioned device components
during deployment and retraction. It is understood that a hysteroscope 20 may
be well suited for
visual observation of a cell collection with the device. Translucency and/or
transparency of a
device component may be dependent on the observational wavelengths. By way of
example
thermoplastic materials can appear clear under visible light, yet are opaque
to other portions of the
electromagnetic spectrum.
[0162] FIG. 23C illustrates a balloon tip catheter 160 of FIG. 23A with a
tubing reservoir, or
extension tube 168, and inflation device 172 in accordance with an exemplary
embodiment of the
disclosure. It is understood that in some embodiments the extension tube 168
may be similar to
extension tube 216 as shown in FIG. 26A. The extension tube 168, 216 may be
configured to
withstand pressurization. Pressurization of the balloon 130 by fluid injection
may be performed
using a syringe device, such as the exemplary inflation device 172. Rotation
of a threaded plunger
shaft through a releasable lock may increase and maintain pressure in the
inflation device 172,
while a pressure gauge 174 provided with the inflation device 172 may allow
for control of input
pressure. In some embodiments, the balloon tip catheter 160 may provide for a
one-person
operation of the device. A length of pressure tubing, or extension tube 168,
216, may be added
38
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
between the inflation device 172 and the inflation port 166 on the device. The
extension tube 168,
216 may be constructed of polymers such as polyurethane or polyvinyl chloride
(PVC), with or
without polymer or metal coil or braid reinforcement. The extension tube 168,
216 may contain
an amount of intrinsic elasticity, while the everting balloon may be generally
inelastic. At full
pressurization of the balloon 130, the extension tube 168, 216 may impart a
fluid capacitance to
the system. A small volume of fluid may be containable in the everted balloon,
and this volume
may be further subtracted by the volume occupied by the push rod 134 (e.g.,
which moves into the
balloon 130 as it is being everted). The resultant everted balloon volume may
be small compared
with the larger volume in the pressure tubing 168, which may allow the balloon
130 to evert to its
full length without significant decrease in pressure, once the balloon tip
catheter 160 has been
pressurized.
[0163] A length of an extension tube 168, 216 may be added between the
inflation device 172
and the inflation port 166 on the device in response to positioning a stopcock
valve 170 in a
location proximal, or away, from the device and the hysteroscope 20. For
example, as shown in
FIG. 25, luer 218 may connect pressure tubing 168, 216, for connection with a
stopcock valve
170. In embodiments, a stopcock valve 170 may be disposed at an end of
extension tube 168, 216
for connection with a luer 219 and the inflation device 172. In some
embodiments, the stopcock
valve 170 may be connected to the luer 218. The stopcock valve 170 may be
closed following
pressurization, and the inflation device 172 may be removed from the
examination field, prior to
insertion and eversion of the balloon 130. This one-operator procedure may be
less cumbersome
and more efficient during a medical procedure. It is also understood that in
some embodiments,
the luer 219 may be connectable to the inflation device 172 without a stopcock
valve 170.
[0164] As described above with respect to FIGS. 23A-23C, the everting
balloon 130 may be
extendable a total distance of approximately 7 cm distal to the tip of the
catheter, in order to pass
through the entire length of the Fallopian tube. The everting balloon 130 may
form a toroidal
shape at the end 130a as it exits the catheter tip, and the everted portion
may include a double
walled configuration. A toroidal shape may be an atraumatic shape for
minimizing or avoiding
damage during extension into the Fallopian tube. Thus, for example, the push
rod 134 advances
forward a distance of approximately 14 cm in order to yield an everted balloon
length of 7 cm.
This length of push rod may initially extend backwards from the proximal end
of the catheter 126,
directly into the face of the operator, making its use cumbersome. The push
rod may also be
susceptible to contamination of the sterile device due to its length as it may
extend into a
physician's working space during a procedure. For example, the proximal end of
the long push
rod 134 may contact the physician's face or surgical mask during use.
Therefore, it may be
desirable to provide a push rod system that does not have to extend backwards
for the full length
39
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
of the push rod 134. The superelastic push rod 175 and carrier design of FIG.
24 and the balloon
tip catheter 200 configured with handle 202 of FIG. 25 may contain the push
rod and minimize
and/or avoid the need to extend the push rod back towards the user.
[0165] FIG. 27 illustrates a cross-sectional side view of an exemplary
everted balloon tip
catheter 180 including a tube 182 having diameter smaller than the inflated
diameter of the everting
balloon 130 for insertion into the patient's UTJ in accordance with the
present disclosure. The
tube 182 may straighten a portion of the balloon tip 163. In some embodiments,
the tube 182 may
extend distally to the tip of the cannula. In embodiments, the tube 182 may
have an approximately
0.0005" ¨ 0.001" wall thickness (e.g., being a "thin-walled" tube), and may
extend approximately
1.5 cm distal to the tip of the cannula. The tube 182 may have a thickness and
resiliency sufficient
to support the balloon 130, to maintain a position of the balloon tip 163
(e.g., maintain a straight
position). In some embodiments, the tube 182 diameter may be smaller than the
balloon 130
diameter, so that the balloon 130 may retain flexibility and compressibility.
This flexibility may
be beneficial to allow the balloon 130 to be advanced through the UTJ. In some
embodiments, the
balloon 130 may include the tube 182 to support and/or straighten the balloon.
In embodiments,
the tube 182 may have a 0.033" OD x 0.001" wall x 1.5 cm long.
[0166] FIG. 28 illustrates a cross-sectional side view of an everted
balloon tip catheter 190
including one or more flexible polymer monofilament string and/or suture 192
as an extending
portion attached to the distal end of the cannula or catheter 126. The strands
192 may extend into
everting balloon tip 163, thereby supporting and keeping the tip straight for
insertion into the
patient's UTJ in accordance with an embodiment of the present disclosure. In
some embodiments,
the one or more flexible polymer monofilament string and/or suture 192 may
extend into the
balloon tip 163 (e.g., approximately 1.5 cm). The monofilament 192 may be
formed of nylon,
polypropylene, or other flexible polymer material, or combinations thereof.
The monofilament
strands may have a diameter of approximately 0.006" ¨ 0.012". In some
embodiments, the balloon
130 may have approximately a 0.033" (.8 mm) OD with a 0.008" diameter
monofilament 192
inside an approximately 1.5 cm long everted balloon tip.
[0167] FIGS. 29A-29C illustrate a steerable balloon tip 252 for an everted
balloon catheter
250 using guide wires in accordance with an exemplary embodiment of the
present disclosure. As
shown in FIG. 29A a steerable balloon tip 252 may be controllable by a right
direction guide wire
254 and a left direction guide wire 256. In FIG. 29B the right guide wire 254
may be manipulated
(e.g., pulled, as shown by the arrow 255) to steer the balloon tip 252 to the
right. Conversely, in
FIG. 29C, the left guide wire 256 may be manipulated, (e.g., pulled, as shown
by the arrow 257)
to steer the balloon tip 252 to the left. It is noted that additional guide
wires may be included to
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
provide movement in the Z-plane in addition to movement in the X-Y plane
achieved with the pair
of guide wires as shown.
[0168] FIG. 30 illustrates a side perspective view of a balloon catheter
260 having a smaller
diameter lead balloon tip 262 at the distal end of the everted balloon 130 in
accordance with an
exemplary embodiment of the present disclosure. The smaller diameter lead
balloon tip 262 may
be dimensioned so as to gradually expand the opening at the constriction of
the UTJ, while being
flexible with blunted edges so as not to perforate the walls at the UTJ.
[0169] FIG. 31 illustrates a side perspective view of a balloon catheter
270 with a flexible
guide wire 272 on the tip of the balloon 30 in accordance with an exemplary
embodiment of the
present disclosure. The flexible guide wire may lead the balloon catheter 220
through the UTJ
into the Fallopian tube.
[0170] In embodiments, a portion of the everted balloon may be treated with
fluoropolymer,
silicone, and like material coatings, or combinations thereof, lubricating the
surface at the lead
portion of the balloon catheter, which may enter the constricted portions of
the Fallopian tube (e.g.,
the UTJ).
[0171] FIG. 32 illustrates a partial side perspective view of a striped
balloon 130S prior to
inversion of the striped balloon 130S into the catheter or cannula of FIG. 32
in accordance with
an embodiment of the disclosure. The indicia 131 on the balloon provide a
visual feedback
indicator of the progress of the balloon eversion. In a specific embodiment,
the indicia 131 may
be approximately 1 mm wide and spaced at approximately I cm increments along
the entire length
of the balloon 130S. Alternative spacing of the strips or other visual markers
on the balloon may
be spaced closer together for finer positional feedback, or further apart for
coarser feedback. Other
visual markers of length of eversion may include sinusoidal indicia with a
known length of
periodicity. It is also appreciated that indicia of length may also include
differently colorized
segments of a known length.
[0172] FIG. 33 illustrates a cross-sectional side view of a balloon tip
catheter 280 configured
with striped balloon 130S in accordance with an exemplary embodiment of the
present disclosure.
As shown in FIG. 33, the indicia 131 of the striped everting balloon 130S may
be coupled with a
transparent distal section 167 of the cannula or catheter 126 to provide
visual feedback of balloon
eversion. In some embodiments, the indicia may be pad printed or scribed with
an indelible marker
in a highly visible color. In some embodiments, the indicia 131 may be
approximately 1 mm wide,
spaced approximately in 0.5 cm increments along the entire length of the
balloon. Fad printing
(also called tampography) is a printing process for transferring a 2-D image
onto a 3-D object.
Other patterns may be used instead of, or in addition to, indicia 131 on the
surface of the balloon
130S. For example, indicium 131 on the balloon 130S may be spaced apart (e.g.,
approximately
41
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
0.5 cm), and dots may also be added in the remaining intervals between the
indicia 131. Each
indicium 131 that comes into view in the transparent distal section 167 may
indicate a successful
eversion of a length of a balloon 130S (e.g., 0.25 cm, as the push rod is
advanced approximately
twice the length for a corresponding approximate length of balloon eversion
(e.g., 0.5 cm). Indicia
131 of different thicknesses may be used, as well as different colored
indicia, or a different number
of indicia, or combinations thereof, in the same fashion described for the
stripe and dot
combination. In some embodiments, color coded sections may be added to the
balloon 130S to
indicate the extent of the balloon eversion.
[0173] Additional embodiments of feedback markers, which may be externally
visible to the
physician on the outside of the patient's body, for the extent of positive
balloon eversion. In
some embodiments, a knotted string or braided sutures as an extending portion
may be adhered
to the distal end of the push rod or tip of the balloon, and may be spaced in
known increments to
provide tactile feedback as to balloon eversion progress. The knotted string
or braided sutures
may allow for visualization of the forward movement of the balloon as it is
everted. The knotted
string or sutures may be radio opaque. The string may have color coded zones
for providing
visual feedback to the operator. To enhance visualization of the knotted
string or braided
sutures, the sutures, indicia, or color-coded zones may be provided in a
highly contrasting color
from the catheter and anatomy. In some embodiments, the braided surface of the
sutures may
assist with collection and/or retention of cells due to the texture and folds
of the braid. For
example, tissue.and/or cells may become embedded in the texture and/or folds
of the braiding.
In some embodiments shown in FIG. 34A, a string 140 may be pad printed with
indicia 131 in a
similar manner to the balloon as noted above in FIGS. 32 and 33. FIG. 34B
illustrates a string
140' with a series of knots or sutures 142. The balloon 130 may be at least
partially transparent
to enhance visibility of string, indicia, knots, or sutures.
[0174] In some embodiments, the string as an extending portion may be
braided as shown in
FIGS. 11C and 11D. The braided string, knots, or sutures may also provide an
additional cell
collection surface. In some embodiments, cells may be collected and retained
within the
braiding of the suture 43, which may be advantageous over cells collected only
on a suture
surface. Cells collected within the braiding of the suture 43 may be less
likely to be
inadvertently removed or wiped away during retraction of the suture 43 and/or
balloon 32, as
cells may be collected between braiding, thereby providing protection of the
collected cells.
[0175] In some embodiments, different strands of the string or suture may
be formed of
different colors, shades, or thicknesses, relative to other strands, as shown
in FIGS. 11E and
11F. For example, as shown in a three-strand suture in FIG. 11E, strands 47
and 49 of suture 46
may be a different color or darker shade as compared to strand 51.
Alternatively, as shown in
42
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
FIG. 11F, strands 47' and 51' of suture 46' may be a different color or
lighter shade as
compared to strand 49'. The strands 47, 47', 49, 49', 51, 51' may be formed of
a selected color
along an entire length, so that when in a braiding pattern, a medical
professional may be able to
visualize a color contrast, or distinction, along the length of the braid in
predetermined segment
lengths. For example, a first strand 47, 47' may extend on an outer portion of
the suture 46, 46'
(e.g., braid) for a length Li, Ll ' every third portion, a second strand 49,
49' may extend on an
outer portion of the suture 46, 46' for a length L2, L2' every third portion,
and a third strand 51,
51' may extend on an outer portion of the suture 46, 46' for a length L3, L3'
every third portion.
For visual clarity, the strands are shown of like thickness, although it is
understood that the
strings, knots, sutures may be any thickness, and may be equal thicknesses, or
different
thicknesses. In other embodiments, the fibers within a given strand may have a
color difference
relative to the remainder of the strand.
[0176] An advantage of varying the appearance of strands along a length of
the suture 46,
46' is that the appearance of the string or suture may vary along the length,
providing feedback
to the operator that the respective string or suture is moving and the balloon
is everting. For
example, a medical professional may be able to visualize movement of the
suture by the color
contrast of the suture 46, 46'. String or sutures may also be treated with
surface modifications
such as plasma, corona, or nanofiber surface application to modify surface
properties thereof.
Additionally, the braided string, knotted string, or sutures may also provide
additional tensile
strength for the balloon in that the string or sutures may act to absorb and
dissipate forces acting
on the balloon, thereby reducing the risk of the balloon detachment.
[0177] Additional feedback mechanisms may include filling the balloon 130
with agitated
saline and visualizing air bubbles with ultrasound, and a sinusoidal pattern
for the balloon, where
the distances between maximums of a sinusoidal wave define an incremental
distance of balloon
eversion.
[0178] Navigation within the Fallopian tube and the indication of a clear
path or obstructions
may be provided by release of microbubbles from the tip of the balloon or from
the distal end of
the tube that the balloon everts from. Travel of the microbubbles may be
trackable using imaging,
such as ultrasound, to ascertain where a clear path exists. In instances of an
obstruction 251, e.g.,
an occlusion or a constriction, the microbubbles may bunch up, or congregate,
when the
microbubbles are impeded. In response to detecting a grouping of microbubbles,
a medical
professional may be able to ascertain an obstruction. FIG. 36A illustrates a
release of a stream of
microbubbles 249 from a tip of the balloon 130 in the Fallopian tube 1 where
no constrictions or
obstructions are present, as evident by the steady continuous line of
microbubbles 249. In some
embodiments, microbubbles may be delivered through an inner lumen 54 of a
balloon, as shown
43
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
in FIGS. 17A-17B. The frequency or spacing of the microbubbles 249 may be
controllable for
finer measurements than with an air source that is modulated on or off, where
the air is introduced
to the fluid injected into the balloon 130. FIG. 36B illustrates a Fallopian
tube 1 with a tubular
constriction or obstruction 251, where the tubular constriction or obstruction
251 may impede a
flow of microbubbles 249 and the microbubbles 249 begin to congregate, or
bunch up, at the point
of the constriction or obstruction 251. The bunching of the microbubbles 249
may provide a visual
indication to the user where the constriction or obstruction 251 is in the
Fallopian tube 1. In
response to a detected obstruction 251, a medical professional may perform
additional imaging,
such as ultrasound, to determine where the balloon stopped.
[01791 The present disclosure further provides various methods for
collecting cells from a
lumen of a subject using embodiments of the catheter described above. Methods
may include
using a catheter including at least a tube, a balloon (with or without an
extending portion)
secured to a distal end of the tube, a push wire that actuates said balloon
between an inverted
position within the tube and an evened position extending beyond the distal
end, and a slidable
sheath coaxial with the tube, everting a first portion (approximately I to 2
cm according to some
embodiments) of the balloon distally beyond the distal end of the tube to the
preselected
distance, positioning the sheath and everted first portion of the balloon
proximate to the lumen of
a subject, or combinations thereof.
[0180] The balloon may be inflated, or otherwise pressurized, for initial
eversion of the
balloon to occur. For example, by pressurizing the balloon, column strength
may be provided to
the balloon, allowing it to evert when a push wire is advanced. The sheath
knob may be
advanced to the first marker on the hypotube and/or catheter. The balloon may
be everted to the
point at the distal tip of the sheath. The distal tip of the sheath, and the
pre-extended balloon,
may be placed proximal to the ostium of the Fallopian tube. The sheath may be
held in place by
maintaining the sheath knob in a selected position, and the balloon and
catheter may be further
advanced, so that an initial portion of an everted balloon is inserted into
the proximal os.
[0181] A medical professional may rotate a drive wheel for further eversion
of the balloon
and/or the suture as an extending portion. The drive wheel may be rotated
until the balloon
and/or suture is partially or fully everted. In some embodiments, a final
everted length (e.g.,
approximately 7-12cm) may be approximately equivalent to half of push wire
travel. When the
balloon and/or the suture is fully everted, the distal end of the push wire
may remain in the
catheter and may not contact the Fallopian tube.
[0182] The inflated balloon as fully everted in the Fallopian tube may fill
the potential space
of the Fallopian tube, contacting an inner surface of the Fallopian tube. The
surface area contact
may transfer cells onto the balloon surface. The balloon may be deflated while
everted in the
44
CA 03070679 2020-01-21
WO 2019/040094 PCT/US2018/000229
Fallopian tube, so that wrinkles in the balloon surface may capture cells
collected on the balloon
surface. In some embodiments, the balloon may be cycled between inflated and
deflated while
everted, for potentially increasing cell collection on the balloon surface and
within the balloon
surface features. In some embodiments, the suture may extend from the fully
everted balloon,
further collecting cells on the suture.
[0183] When cell collection on the balloon surface and/or the suture is
complete, the
medical professional may retract the handle of the device while holding the
sheath in place, so
that the everted balloon and/or the suture may be retracted within the sheath.
A marker on the
tube of the catheter when aligned with the sheath knob may provide an
indication that the full
length of the balloon/extending portion has been retracted with the sheath.
The sheath may
protect the collected cells on the balloon surface and/or the suture, for
removing the device from
the working channel of the hysteroscope.
[0184] By inserting the everted first portion of the balloon into the
lumen, and further
everting the balloon into the lumen using the push wire, cells may be
collected on the balloon.
Some embodiments of the method may also include adjusting a speed of the
further everting step
relative to the inserting the everted first portion of the balloon into the
lumen step. A marker on
the tube of the catheter when aligned with the sheath knob may provide an
indication that the full
length of the balloon/extending portion has been retracted with the sheath.
[0185] Any patents or publications mentioned in this specification are
herein incorporated by
reference to the same extent as if each individual publication was
specifically and individually
indicated to be incorporated by reference. The foregoing description is
illustrative of particular
embodiments of the disclosure, but is not meant to be a limitation upon the
practice thereof.
[0186] Numerous specific details have been set forth herein to provide a
thorough
understanding of the embodiments. It will be understood by those skilled in
the art, however, that
the embodiments may be practiced without these specific details. In other
instances, well-known
operations, components, and circuits have not been described in detail so as
not to obscure the
embodiments. It can be appreciated that the specific structural and functional
details disclosed
herein may be representative and do not necessarily limit the scope of the
embodiments.
[0187] Some embodiments may be described using the expression "coupled" and
"connected" along with their derivatives. These terms are not intended as
synonyms for each
other. For example, some embodiments may be described using the terms
"connected" and/or
"coupled" to indicate that two or more elements are in direct physical or
electrical contact with
each other. The term "coupled," however, may also mean that two or more
elements are not in
direct contact with each other, but yet still co-operate or interact with each
other.
CA 03070679 2020-01-21
WO 2019/040094
PCT/US2018/000229
[0188] It
should be noted that the methods described herein do not have to be executed
in
the order described, or in any particular order. Moreover, various activities
described with
respect to the methods identified herein can be executed in serial or parallel
fashion.
46