Note: Descriptions are shown in the official language in which they were submitted.
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
AURICULAR STIMULATION DEVICE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This
application claims the benefit of U.S. Provisional Patent Application
Serial No. 62/539,178, filed on July 31, 2017, the entire contents of which
are
incorporated by reference herein.
TECHNICAL FIELD
[0002] The
present disclosure relates to treatment devices, and diagnostic and
therapeutic systems and, more specifically, to auricular stimulation devices,
diagnostic
and therapeutic systems and methods of use thereof
BACKGROUND
[0003] The
auricular branch of the vagus nerve (also known as Alderman's nerve or
Arnold's nerve) is located in the ear and supplies sensory innervation to the
skin of the
ear canal, tragus, and auricle. The auricular branch reaches the surface of
the ear and
divides into two branches. The first joins the posterior auricular nerve and
the second is
distributed to the skin on the back of the ear or auricle and to the posterior
part of the ear
canal.
[0004]
Stimulation of the auricular branch of the vagus nerve has been shown to have
diagnostic and therapeutic benefits. For example, various studies have shown
that
stimulation of the auricular branch of the vagus nerve can be used to treat
seizures, atrial
fibrillation, depression, diabetes, endotoxemia, myocardial infarction, and
tinnitus (see
references cited in the appendix).
1
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0005] In view of at least the foregoing benefits of stimulating the
auricular branch of
the vagus nerve, there is a continuing need for more effective auricular
stimulation
devices.
SUMMARY
[0006] The present disclosure provides an auricular stimulation device
having at least
two surface electrodes biased towards each other and offset from one other. In
aspects
described herein, the auricular stimulation device is configured for
positioning about the
ear of a patient with one or both of the electrodes in close proximity to
and/or overlaying
an auricular branch of the vagus nerve such that an electric field between the
two
electrodes passes through innervation that is connected to the auricular
branch. The
electrodes are configured to transcutaneously stimulate the auricular branch
of the vagus
nerve when activated by a stimulation circuit. The auricular stimulation
device stimulates
the auricular branch of the vagus nerve non-invasively.
[0007] The present disclosure further provides a method of treating a
patient using
the auricular stimulation device. The stimulation device can be used for
treating patients
with various conditions, including, but not limited to, high blood pressure,
depression,
high blood glucose level, and tinnitus.
[0008] The present disclosure further provides a diagnostic and therapeutic
system
having the auricular stimulation device, a smart device and a monitoring
device. In
aspects described herein, the auricular stimulation device of the system can
be controlled
by the smart device. Additionally, in aspects described herein, the smart
device controls
the auricular stimulation device based on biomarker information received from
the
2
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
monitoring device; information specific to the patient, such as age,
musculoskeletal
stability, etc.; and/or instructions received via a user input, such as
instructions received
via graphical user interface corresponding to an app. Further, in aspects
described herein,
the monitoring device can be an implantable sensor, such as the type
configured to
monitor and transmit a blood glucose level, cellular- and/or enzyme-related
information,
or any type of device external to the patient, such as a heart rate and
respiratory rate
monitor.
[0009]
According to one aspect of the present disclosure, a stimulation device
includes a first electrode disposed on a first arm, a second electrode
disposed on a second
arm, a biasing member configured to urge a portion of the first arm towards a
portion of
the second arm, and a stimulation circuit in operative communication with the
first and
second electrodes. The second electrode is in opposition to the first
electrode and offset
from the first electrode. One or both of the first and second electrodes is
urged towards
the other electrode to form a tissue clamping configuration. The stimulation
circuit is
configured for generating a stimulation signal for actuating one or both of
the first and
second electrodes for stimulating a nerve in close proximity to (and/or
within) tissue
clamped between the first and second electrodes. The first and second
electrodes can be
positioned such that the nerve does not have to be in the clamped tissue. In
embodiments,
the first and/or second electrodes are configured to generate electric field
lines that may
be straight and/or curved lines configured to pass through auricular tissue
and electrically
stimulate vagus nerve innervation within the auricle of the clamped ear.
[0010] In some
embodiments, a controller may be in operative communication with
the stimulation circuit for controlling operation of the stimulation circuit.
3
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0011] In
certain embodiments, the stimulation device may further comprise a
housing. The controller and the stimulation circuit may be disposed within the
housing.
The housing may be configured to be positioned about an ear of a patient.
[0012] In some
embodiments, the stimulation device may further comprise an
adjustment mechanism partially disposed within the housing and including the
biasing
member. The adjustment mechanism may enable the position of one or both of the
arms
to be changed for enabling the stimulation device to fit and conform to a
variety of ears.
[0013] In
certain embodiments, the stimulation device may further comprise a control
interface for receiving one or more control signals from an external device
for controlling
the stimulation circuit. The external device may be a smart device.
[0014] In
embodiments, the stimulation device may further comprise a power source
in operative communicative with the stimulation circuit. The power source may
be a
rechargeable power source.
[0015] In some
embodiments, the stimulation device may further comprise a memory
in operative communication with the controller. The memory may be configured
for
storing usage data and operating parameters of the stimulation device.
[0016] In
embodiments, the nerve may be the vagus nerve and the stimulation device
may be configured to be positioned about an ear for stimulating an auricular
branch of the
vagus nerve.
[0017] In
certain embodiments, one or both of the first and second arms is configured
to move between one or more bent configurations and an unbent configuration.
4
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0018]
According to yet another aspect of the present disclosure, a diagnostic and
therapeutic system comprises a smart device and a stimulation device in
operative
communication with the smart device. The stimulation device is configured to
receive
one or more control signals from the smart device. The stimulation device
includes a first
electrode disposed on a first arm, a second electrode disposed on a second
arm, a biasing
member configured to urge a portion of the first arm towards a portion of the
second arm,
and a stimulation circuit in operative communication with the first and second
electrodes.
The second electrode is in opposition to the first electrode and offset from
the first
electrode. One or both of the first and second electrodes is urged towards the
other
electrode to form a tissue clamping configuration. The stimulation circuit is
configured
for generating a stimulation signal after receiving the one or more control
signals for
actuating one or both of the first and second electrodes for stimulating a
nerve in within
the auricle of the ear that is between the first and second electrodes.
[0019] In
certain embodiments, the smart device may be in operative communication
with one or both of a memory and a database storing a plurality of treatment
regimens
corresponding to a plurality of conditions.
[0020] In some
embodiments, the system may further comprise a monitoring device
in operative communication with one or both of the smart device and the
stimulation
device. The monitoring device may transmit biomarker information to one or
both of the
smart device and the stimulation device.
[0021] In
embodiments, the smart device may determine one or more conditions of a
patient using the biomarker information. The smart device may determine one or
more
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
treatment regimens from the plurality of treatment regimens for treating the
one or more
conditions of the patient.
[0022] In certain embodiments, the smart device may include one or more
apps
having a corresponding graphical user interface for receiving one or more user
inputs for
controlling one or more operating parameters of the stimulation device.
[0023] According to yet another aspect of the present disclosure, a method
of
treatment by stimulating a nerve is provided. The method includes clamping
tissue
between first and second electrodes of a stimulation device, the first
electrode being
disposed opposite from the second electrode, and offset from the second
electrode; and
actuating a stimulating circuit of the stimulation device to generate and
deliver a
stimulation signal to one or both of the first and second electrodes for
stimulating a nerve
within the auricle of the clamped ear.
[0024] In aspects, the tissue may be ear tissue and the nerve may be an
auricular
branch of the vagus nerve.
[0025] The method may further involve monitoring biomarker information and
determining a treatment regimen in accordance with the biomarker information.
[0026] The method may further comprise changing one or more stimulation
parameters of the stimulation device in accordance with biomarker information
received
by a monitoring device. The monitoring device may be external to a patient
being treated.
The monitoring device may be an implantable sensor.
6
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0027] The
method may further involve controlling the stimulating circuit of the
stimulation device by one or more controllers. The one or more controllers may
be in a
smart device in operative communication with the stimulation device.
[0028] The
method may further include controlling the one or more controllers by
user input via a graphical user interface.
[0029]
Further, to the extent consistent, any of the aspects or features described in
the
present disclosure may be used in conjunction with any or all of the other
aspects or
features described herein.
[0030] Other
aspects, features, and advantages will be apparent from the description,
the drawings, and the claims that follow.
BRIEF DESCRIPTION OF THE DRAWINGS
[0031] Various
aspects of the present disclosure are described hereinbelow with
reference to the drawings, which are incorporated in and constitute a part of
this
specification, wherein:
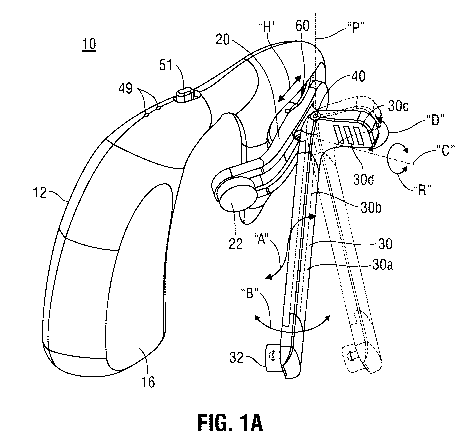
[0032] FIG. 1A
is a perspective view of one embodiment of a stimulation device
configured to stimulate the auricular branch of the vagus nerve in accordance
with the
present disclosure, the stimulation device including an outer probe, the outer
probe
illustrated in an unbent configuration;
7
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0033] FIG. 1B is a perspective view of the stimulation device of FIG. 1A
with the
outer probe thereof illustrated in a bent configuration;
[0034] FIG. 2 is a perspective view, with parts separated, of the
stimulation device of
FIGS. 1A and 1B;
[0035] FIG. 3 is a cross-sectional view taken along section line 3-3 of
FIG. 1B;
[0036] FIG. 4 is a cross-sectional view taken along section line 4-4 of
FIG. 1B;
[0037] FIG. 5 is a schematic view of a system configured to stimulate the
auricular
branch of the vagus nerve in accordance with the present disclosure;
[0038] FIG. 6 is a flowchart of an exemplary method of diagnosing and
treating a
condition with a stimulation device configured to stimulate the auricular
branch of the
vagus nerve in accordance with the present disclosure;
[0039] FIG. 7 is a flowchart of an exemplary treatment method of
stimulating an
auricular branch of the vagus nerve, such as with the stimulation device of
FIGS. 1A and
1B, in accordance with the present disclosure; and
[0040] FIG. 8 is a perspective view of another embodiment of a stimulation
device
configured to stimulate the auricular branch of the vagus nerve in accordance
with the
present disclosure.
DETAILED DESCRIPTION
[0041] Embodiments of the present disclosure are now described in detail
with
reference to the drawings in which like reference numerals designate identical
or
8
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
corresponding elements in each of the several views. As used herein, the term
"clinician"
refers to a doctor, a nurse, or any other care provider and may include
support personnel.
As used herein, the term "electrode" is defined herein as a single electrode
or an array of
electrodes.
[0042]
Referring now to FIGS. 1A and 1B, one embodiment of a StimClipTM or
auricular stimulation device 10 is shown in accordance with the present
disclosure. The
stimulation device 10 includes a housing 12, a first or inner arm 20 (e.g., a
probe), and a
second or outer arm 30 (e.g., a probe). The housing 12 is shaped and
configured for being
secured over the ear of a patient (see FIG. 5). As seen in FIG. 2, the housing
12 of
stimulation device 10 includes an outer flat portion 14, a middle flat portion
15, and an
outer shell-shaped portion 16. Two triangular spacers 14A and 15A are provided
in
proximity to outer flat portion 14 and middle flat portion 15 to prevent
housing portions
14 and 15 from abutting against each other.
[0043] With
continued reference to FIGS. 1A-3, the housing 12 of stimulation device
supports an adjustment mechanism 60 for horizontally and rotationally
adjusting the
position of the probes 20, 30 for fitting or conforming the stimulation device
10 to ears
having a variety of sizes and shapes. In particular, as indicated by arrows
"H," adjustment
mechanism 60 is slidably movable relative to housing 12 through mounting slot
64 of
housing 12 to selectively axially move probes 20, 30 relative to housing 12.
Further,
adjustment mechanism 60 defines a central axis "C" therethrough about which
probes 20,
30 selectively rotate relative to housing 12 and adjustment mechanism 60, as
indicated by
arrows "R." The stimulation device 10 is configured to be disposed on the left
ear of a
patient; however, in embodiments, a stimulation device 10 may be configured
(e.g., as a
9
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
mirror image of the left ear configuration) to be disposed on the right ear of
the patient
either separately or in conjunction with a stimulation device 10 disposed on
the left ear of
a patient. In addition, the stimulation device 10 may come in a variety of
sizes to be
disposed on the ear of an infant, a child, and/or an adult. The adjustment
mechanism 60
also secures the inner and outer probes 20, 30 to the housing 12 and in a
fixed position
relative to housing 12 upon tightening of a mounting nut 65 of adjustment
mechanism 60.
[0044] In
particular, the adjustment mechanism 60 of the stimulation device 10
includes a mounting shaft 62 that is selectively slidably secured, as
indicated by arrows
"H," and selectively rotatably secured, as indicated by arrows "R," in a
mounting slot 64
defined by the outer shell-shaped portion 16 of the housing 12. Allowing the
mounting
shaft 62 to selectively slide along and/or selectively rotate within the
mounting slot 64
enables the housing 12 and the probes 20, 30 of the stimulation device 10 to
be fitted or
conformed to ears having a variety of sizes and shapes. As detailed herein,
rotation of the
probes 20, 30 relative to housing 12 is limited by movement of pin 68 through
a
predetermined arc length defined by opposite ends of pin slot 67. Once a
suitable size
and/or comfort is established mounting nut 65 can be threadably rotated or
tightened on
mounting shaft 62 of adjustment mechanism 60 to fix the adjustment mechanism
60 and
the probes 20, 30 relative to the housing 12.
[0045] The
mounting shaft 62 of adjustment mechanism 60 includes an inner
segment 63 that extends inward and receives a mounting nut 65 thereabout to
secure the
mounting shaft 62 of adjustment mechanism 60 within the mounting slot 64 of
the
housing 12. The mounting shaft 62 also includes outer segment 66 that extends
outward
and defines a pin slot 67 that receives a pin 68 to secure the inner probe 20
to the outer
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
segment 66 of the mounting shaft 62. Pin slot 67 is configured to limit
rotational
movement of the inner and outer probes 20, 30 relative to housing 12 such that
pin 68 is
configured to abut opposite ends of pin slot 67 to prevent further rotational
movement of
the probes 20, 30 in a given direction.
[0046] With
continued reference to FIGS. 2 and 3, the inner and outer probes 20, 30
of the stimulation device 10 are pivotally mounted to one another by a pivot
40 of the
stimulation device 10 that defines pivot axis "P." The pivot 40 includes a
pivot pin 41
and a biasing member 42 that urges the inner and outer probes 20, 30 towards
one
another and into a tissue clamping position as shown in FIG. 3. The biasing
member 42
may be any suitable spring (e.g., a torsion spring) disposed about the pivot
pin 41.
[0047] The
inner probe 20 of the stimulation device 10 includes a first or inner
electrode 22. The outer probe 30 of stimulation device 10 includes a second or
outer
electrode 32 that opposes the inner electrode 22 and is offset from the inner
electrode 22.
[0048] The
outer probe 30 of the stimulation device 10 includes an elongate body 30a
that is formed of a flexible material and supports one or more flexible wires
30b that
extend through elongate body 30a to facilitate flexing and/or bending of
elongate body
30a, as indicated by arrows "A," between an unbent configuration (FIG. 1A) and
one or
more bent configurations (FIG. 1B). The elongate body 30a and/or flexible
wires 30b of
outer probe 30 may be formed of any suitable polymeric and/or metallic
material. While
outer probe 30 may be bent in any number of configurations to accommodate
different
user comforts, ear sizes, ear shapes, etc., flexible wires 30b are configured
to maintain
outer probe 30 fixed in a respective configuration until subsequently bent to
a different
11
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
configuration. The outer probe 30 further includes a rigid foot 30c that
extends transverse
to the elongate body 30a of the outer probe 30. The foot 30c may include a
finger grip
30d that may include any suitable surface texturing such as ridges, knurling,
etc. The foot
30c is actuatable a user's finger, as indicated by arrows "D," to facilitate
pivotal
movement of outer probe 30 relative to inner probe 20 about pivot axis "P."
[0049] The
biasing member 42 of pivot 40 urges the inner and outer probes 20, 30
towards one another, such that the inner and outer electrodes 22, 32 of the
inner and outer
probes 20, 30, respectively, are urged towards one another into the tissue
clamping
configuration to secure the stimulation device 10 to tissue supported between
the inner
and outer electrodes 22, 32. Specifically, a force is created by the biasing
member 42 of
the pivot 40 that urges the electrodes 22, 32 towards each other to clamp or
clasp tissue
between the electrodes 22, 32, and also secure the stimulation device 10 on
the ear of a
patient. The electrodes 22, 32 are positioned to overlay outer ear tissue
(e.g., the auricle)
innervated by the auricular branch of the vagus nerve when the stimulation
device 10 is
clamped to the ear.
[0050] With
continued reference to FIG. 3, the outer electrode 32 of the outer probe
30 is offset from the inner electrode 22 of the inner probe 20. Offsetting the
inner and
outer electrodes 22, 32 provides improved stimulation of the auricular branch
of the
vagus nerve when both electrodes 22, 32 are activated and when one of the two
electrodes 22, 32 is activated, as compared to having the electrodes 22, 32
directly
oppose one another.
12
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0051] With
continued reference to FIGS. 2-4, one or both of the inner and outer
probes 20, 30 and/or the housing 12 of the stimulation device 10 may house a
circuit
board 50 of the stimulation device 10 with associated electronics for
coordinating the
activation of the electrodes 22, 32 of the inner and outer probes 20, 30,
either separately
or together. The circuit board 50 may have several elements including, but not
limited to,
a rechargeable power source 52, a stimulation circuit 54, electrode outputs 56
(FIG. 2) in
operative communication with the stimulation circuit 54, a control interface
58, and a
memory 59. The circuit board 50 may also include a power switch 51 and a
corresponding status indicator 49 (FIG. 1) that extends from the housing 12
for being
accessible by a patient or clinician. The circuit board 50 may also include an
over-
current protection circuit (not shown) and/or an over-heating protection
circuit (not
shown). The status indicator 49 of the circuit board 50 indicates whether the
power
switch 51 is in the "on" or "off' position. For example, the status indicator
49 can be an
LED that is illuminated when the power switch 51 is in the "on" position and
not
illuminated when the power switch 51 is in the 'off' position.
[0052] The
circuit board 50 of the stimulation device 10 also includes a controller 55,
such as a microcontroller, for controlling the operation of the stimulation
device 10. The
controller 55 can be programmed with a variety of operating protocols and
parameters.
The operating protocols and parameters can be stored in the memory 59 or in a
memory
of controller 55. The memory 59 and/or the controller's memory store data,
such as data
logged during usage of the stimulation device 10 including, but not limited
to, duration of
use, time of day of use, and operating parameters of the stimulation circuit
54.
13
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0053] One or
more elements of the circuit board 50 of the stimulation device 10 may
be integrated into an application-specific integrated circuit (ASIC) or
controller 55. By
integrating and packaging elements of the circuit board 50 into and ASIC or
controller
55, the wiring and/or size of one or both of the inner and outer probes 20, 30
may be
reduced. In addition, the integration of elements into an ASIC or controller
55 may
increase the battery life of the stimulation device 10. Alternatively,
elements of the circuit
board 50 and the controller 55 can be housed in a separate unit other than the
stimulation
device 10, and wirelessly or by wired connection communicate with the
stimulation
circuit 54 of the stimulation device 10. For example, the stimulation circuit
54 can be
powered and controlled inductively via inductive coupling by control circuitry
positioned
in proximity to the stimulation device 10.
[0054] The
rechargeable power source 52 of the circuit board 50 may be a battery or
other device suitable for providing power to elements of the circuit board 50
and/or
controller 55. The rechargeable power source 52 may be recharged through
direct contact
with an external power source (e.g., via a selectively removable power chord
that may
couple to a port such as a USB (not shown) supported by housing 12) or may be
inductively charged by positioning a suitable power source adjacent the
respective inner
or outer probe 20, 30. The rechargeable power source 52 may be in
communication with
an induction coil 53 that receives power by inductive coupling from an
external power
supply (not shown) for recharging the power source 52. In some embodiments,
the
rechargeable power source 52 may include one or more photovoltaics to enable
recharging by light energy.
14
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0055] The
stimulation circuit 54 of the circuit board 50 is configured to generate
stimulation signals, such as pulses, oscillations, sinusoidal waveforms,
square
waveforms, triangular waveforms, etc., when activated, and to transmit the
stimulation
signals via the electrode outputs 56 to one or both of the electrodes 22, 32
of the inner
and outer probes 20, 30, respectively. The stimulation circuit 54 can be an
oscillator or
other electronic element configured to produce a signal or pulses which can
stimulate the
auricular branch of the vagus nerve to treat a predetermined condition. In
some
embodiments, the circuit board 50 may have a circuit configured to sense when
the
electrodes 22, 32 are clamped to tissue, such as by an impedance measurement,
and to
prevent actuation of the stimulation circuit 54 when tissue is not clamped
between the
electrodes 22, 32.
[0056] Each of
the electrode outputs 56 of the circuit board 50 connected to the
stimulation circuit 54 is in communication with a respective one of the
electrodes 22, 32
of the inner and outer probes 20, 30 to deliver a stimulation signal, such as,
for example,
a waveform or a series of pulse bursts, generated by the stimulation circuit
54 to the
electrodes 22, 32. The stimulation signal causes one or both of the electrodes
22, 32 to
transcutaneously stimulate the auricular branch of the vagus nerve within the
tissue. In
certain embodiments, the electrodes 22, 32 may sense when they are in contact
with
tissue, for instance, an ear of a patient, and provide a tissue sensing signal
via the
electrode outputs 56 to the stimulation circuit 54 or the controller 50. If
the stimulation
circuit 54 or the controller 55 does not receive the tissue sensing signal
from one or both
electrodes 22, 32, the stimulation circuit 54 will not be actuated.
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0057] Besides the controller 55 of the circuit board 50, the stimulation
circuit 54 of
the circuit board 50 can also be controlled by an external device via the
control interface
58 as detailed below. The controller 55 or external device can enable fine
control of the
operating parameters of the stimulation circuit 54, including, but not limited
to, the
duration, the amplitude, the frequency, the type of stimulation or oscillation
waveform, or
burst rate of the pulses, etc. Additionally or alternatively, the stimulation
device 10 may
be provided without the controller 55 such that one or more elements of the
circuit board
50 are controlled by an external controller or an external control mechanism,
such as by
the controller 122 (FIG. 5) of the smart device 120.
[0058] The stimulation circuit 54 and other elements of the circuit board
50,
including the controller 55, can be controlled or programmed using an app
running on a
smart device 120 (FIG. 5) or other electronic device (e.g., external
controller). The app
through the control interface 58, for example, can receive and transmit
control signals
wirelessly (e.g., WIFI signals) and program and/or control the stimulation
circuit 54
and/or the controller 55 in order for the stimulation device 10 to generate a
stimulation
signal for treating the predetermined condition by stimulating the auricular
branch of the
vagus nerve. The stimulation device 10 can be connected via the control
interface 58 or
other communication circuitry to the internet or other network for receiving
the control
signals. In this case, the stimulation device 10 can be an internet of things
(IoT) device
configured to be remotely controlled via a network connection.
[0059] The control interface 58 of the circuit board 50 may be a wireless
transmitter/receiver in wireless communication with the smart device 120 or
external
controller. The wireless communication may be radio frequency, optical, WIFI,
16
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
BLUETOOTH (an open wireless protocol for exchanging data over short distances
(using short length radio waves) from fixed and mobile devices, ZigBee (a
specification
for a suite of high level communication protocols using small, low-power
digital radios
based on the IEEE 802.15.4-2003 standard for wireless personal area networks
(WPANs)), etc.
[0060] As
stated above, the control interface 58 of the circuit board 50 may link to a
smart device 120. Through the link, the control interface 58 may transfer data
from the
memory 59 and/or real-time data from the stimulation circuit 54 to the smart
device 120.
The controller 55 of the circuit board 50 may also receive control signals
from the smart
device 120 via the communication link for controlling the stimulation circuit
54 of the
circuit board 50. The smart device 120 through the app may visually or audibly
present
data from the stimulation device10 to a clinician in real-time or other
individual,
including the patient. For example, a GUI of the app can provide visual
information,
such as the type of condition being treated and the operating parameters of
the
stimulation circuit 54 of the circuit board 50, such as the frequency and
amplitude of the
stimulation signal generated by the stimulation circuit 54.
[0061] With
reference to FIG. 5, a system 100 is provided which can include the
features described herein above and other features, and configured for
stimulating the
auricular branch of the vagus nerve and treating a condition in accordance
with the
present disclosure. The system 100 includes an auricular stimulation device 10
(see
FIGS. 1A and 1B), a monitoring device 110, and a smart device 120. The
auricular
stimulation device 10 can be the device described above with reference to
FIGS. 1A-4, or
it can be any stimulation device for stimulating the auricular branch of the
vagus nerve,
17
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
e.g., auricular stimulation. The monitoring device 110 is any monitoring
device suitable
for measuring, monitoring, and/or determining biomarker information of a
patient, such
as heart rate, respiration rate, electro-dermal activity, neural activity,
EEG, EKG, glucose
level, cholesterol, blood pressure, levels of cytokines present, and/or other
physiological
measurements or molecular or enzyme-related information corresponding to the
patient.
For example, the monitoring device 110 may be an implanted sensor within the
patient
for measuring the patient's blood glucose level. The monitoring device 110 may
also be
a wearable device, such as a smart watch, or a finger pulse oximeter which
monitors and
determines the heart rate and respiration rate of a patient.
[0062] The
smart device 120 of the system 100 may be, but not limited to, a
smartphone, a portable computer, a tablet, a fixed computer, or a wearable
device
connected to a network, such as the internet, and/or operating under a
communications
protocol, such as BLUETOOTHTm. In some embodiment, the smart device 120 may be
an external controller configured to communicate wirelessly or via a wired
connection
and control the stimulation device 10.
[0063] With
continued reference to FIG. 5, the communications links between the
smart device 120 and the stimulation device 10 and monitoring device 110 of
the system
100 can be wireless or non-wireless. A processor 124 of the smart device 120
receives
biomarker information from the monitoring device 110. The biomarker
information can
be used by the smart device 120 to determine the type of stimulation signal
configured to
treat the patient's condition. The processor 124 then communicates with a
controller 122
of the smart device 122 to transmit control signals to the stimulation device
10, either
wirelessly or non-wirelessly. The control signals are received by the
controller interface
18
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
58 and are used to control the stimulation circuit 54 for generating the
processor-
determined stimulation signal.
[0064] As
stated above, the smart device 120 of the system 100 may also receive data
from the memory 59 of the stimulation device 10, such as previous usage data,
operating
parameters of the stimulation device 10 during a previous treatment session or
cycle, etc.
which can aid the processor 124 to determine the most effective stimulation
signal and
associated operating parameters of the stimulation circuit 54 for treating the
patient.
Alternatively or additionally, user input 126 can be received by the smart
device 120 via
a GUI of an app to operate the stimulation circuit 54 based on user-selected
operating
parameters.
[0065] With
reference to FIG. 6, an exemplary method 200 of treating a condition is
described in accordance with the present disclosure with reference to the
system 100
detailed above. Other methods of treatment are contemplated and envisioned
using the
stimulation device 10 and system 100 described herein. For example, as shown
the
stimulation device 10 is configured for attachment to the left ear of a
patient; however,
the stimulation device 10 may be configured as the "mirror image" of the
device shown,
and attached to the right ear of the patient. Alternatively, two stimulation
devices 10 may
be configured as "mirror images" of each other, and each attached to a
respective ear of a
patient and simultaneously or sequentially used to stimulate the auricular
vagus
innervation bilaterally, as detailed below.
[0066]
Initially, with continued reference to FIG. 6, the stimulation device 10 and
the
monitoring device 110 of the system 100 are attached to the patient such that
the
19
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
stimulation device 10 is positioned to stimulate the vagus nerve, for example,
the
auricular branch of the vagus nerve in the ear of the patient (step 202), and
the
monitoring device 110 is positioned to monitor and determine biomarker
information of
the patient, for example, the heart rate and/or the respiration rate of the
patient (step 204).
Where the monitoring device 110 is an implantable device, such as a glucose
monitoring
implantable device, the monitoring device 110 includes transmission circuitry
for
transmitting the biomarker information, such as blood glucose level to the
processor 124
of the smart device 120.
[0067] With
the stimulation device 10 and the monitoring device 110 in position, the
smart device 120 can be linked to the stimulation device 10 and the monitoring
device
110 (step 206). The smart device 120 can be in wireless communication with the
stimulation device 10 and the monitoring device 110; however, in embodiments,
the
smart device 120 may be physically linked or hardwired to the stimulation
device 10 and
the monitoring device 110. In certain embodiments, the smart device 120 is
linked to the
stimulation device 10 and the monitoring device 110 prior to the stimulation
device 10
and the monitoring device 110 being in position.
[0068] When
the stimulation device 10 of the system 100 is positioned about the ear,
the stimulation device 10 may sense tissue properties via the inner and/or
outer electrode
22, 32 of the inner and outer probes 20, 30 and internally calibrate one or
more operating
parameters in response to the sensed tissue properties. The operating
parameters can also
be calibrated or initially determined using the initial biomarker information
received from
the monitoring device 110, and/or user input 126 (step 208). The initial
biomarker
information refers to information received prior to stimulating the auricular
branch of the
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
vagus nerve. The initial biomarker information received by the smart device
120 from the
monitoring device 110 can be used to establish a pre-stimulated state of the
patient (step
210). That is, the state of the patient prior to stimulation of the auricular
branch of the
vagus nerve. This state corresponds to the patient having a condition which
necessitates
the patient be treated by stimulating the auricular branch of the vagus nerve.
Therefore, it
is the objective of the treatment method to stimulate the vagus nerve to treat
the patient
and adjust the patient's pre-stimulated state to a post-stimulated state which
is healthier
than the pre-stimulated state.
[0069] Based on at least the initial biomarker information, the smart
device 120 of the
system 100 can access one or more databases or a memory which correlates the
initial
biomarker information to a plurality of conditions, and determine one or more
conditions
of the patient, such as high blood pressure, high blood glucose level, high
temperature,
etc., based on the initial biomarker information. Therefore, the smart device
120 is
configured to diagnose or determine one or more conditions of the patient
using the initial
biomarker information.
[0070] Once one or more conditions are determined by the smart device 120,
the
smart device can access one or more additional databases or the same
databases, or a
memory, which correlate a plurality of conditions with a plurality of
treatment regimens
or protocols. All of the databases referred to herein can be accessed by the
smart device
120 or other computing device via a network connection, such as the internet.
[0071] For example, the smart device 120 of the system 100 can access a
variety of
treatment regimens stored within one or more databases stored in a remote
location (i.e.,
21
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
cloud-based network architecture). The databases can be stand-alone databases
or data
structures stored in a remote server or other computing device. After
accessing the
plurality of treatment regimens stored within the one or more databases, the
smart device
120 or other computing device selects the treatment regimen that is most
suitable for
treating a patient having the determined condition(s) of the patient. For
example, if the
initial biomarker information indicates the patient has a high glucose level,
the smart
device 120 or other computing device selects the treatment regimen which has
been
previously determined to be effective in treating patients with a high glucose
level. The
treatment regimen selected can be tailored or adjusted based on other
information gleaned
from the patient's biomarker information or other information related to the
patient,
including, but not limited to, blood pressure and heart rate of the patient,
musculoskeletal
stability, age of the patient, medical history, prescription medication(s)
administered to
the patient, etc. The treatment regimen can also be manually selected or
tailored by a
clinician, and communicated to the smart device 120 via the user input 126
(e.g., via a
graphical user interface) or other controller in operative communication with
the
stimulation device 10.
[0072] Each
treatment regimen can include, but not limited to, the operating
parameters of the stimulation circuit 54, such as, the type of waveform and
corresponding
characteristics (e.g., frequency and amplitude), the duration of the treatment
session, and
the number of treatment sessions.
[0073] After
the treatment regimen is selected or determined, and/or adjusted or
tailored to the patient being treated, either by a clinician, the smart device
120 or other
computing device, the controller 122 of the smart device 120 or other
controller in
22
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
operative communication with the stimulation device 10 transmits a control
signal to the
controller interface 58 of the stimulation device 10 to begin the treatment
session and
treat the patient in accordance with the treatment regimen (step 220).
[0074] The
control signal may include parameters for the desired stimulation in
accordance with the treatment regimen. In response to receiving the control
signal via the
controller interface 58, the controller 55 controls the stimulation circuit 54
to generate
and deliver a waveform, a series of pulses or other stimulating signals to the
inner and/or
outer electrodes 22, 32 via the electrode outputs 56 to transcutaneously
stimulate the
vagus nerve, i.e., the auricular branch of the vagus nerve.
[0075] As the
stimulation device 10 non-invasively stimulates the vagus nerve, the
smart device 120 receives and monitors biomarker information, e.g., heart rate
and/or
respiration rate of the patient, via the monitoring device 110, continuously
in real-time or
at pre-set intervals (step 230). In some embodiments, the controller interface
58 of the
stimulation device 10 can also receive the biomarker information from the
monitoring
device 10.
[0076] In
response to the stimulation, the smart device 120 may detect a change (or
detect no change, or detect no significant therapeutic change) in the
biomarker
information received from the monitoring device 110 (step 240). If no change
or no
significant therapeutic change is determined in the biomarker information, the
smart
device 120 sends a control signal to the auricular stimulation device 10 to
change or
adjust the stimulation parameters (step 242). The method then proceeds to step
220 where
23
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
the patient is stimulated with the stimulation device 10 using the new
stimulation
parameters.
[0077] For
example, when no change or no significant therapeutic change is detected
in the biomarker information in step 240, the smart device 120 may increase
the duration,
amplitude, frequency, and/or burst rate of the pulses or other signal
parameters, change
the type of waveform, etc. until a desired or noticeable therapeutic change is
detected in
the biomarker information (step 244). These stimulation parameters are then
maintained
(step 250) and stimulation continues in step 220.
[0078]
However, before continuing with stimulation using the new stimulation
parameters in step 220, the system may check to determine if the temperature
and current
of the stimulation device 10 are within acceptable ranges (step 256). The
stimulation
device 10 is turned off if the temperature and/or current are outside
acceptable ranges
(step 258).
[0079] If in
step 244, a change is detected in the biomarker information of the patient
and the biomarker information is within an acceptable range, for instance,
heart rate
and/or the respiration rate of the patient is in the normal range, the smart
device 120 may
control the stimulation device 10 to cease the operation of the stimulation
circuit 54 (step
250) and conclude the treatment session. That is, if it is determined by the
smart device
120 that the stimulation treatment was effective in bringing the initial
biomarker
information of the pre-stimulated state of the patient within an acceptable
range, the
stimulation treatment session is finished.
24
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0080] The
data acquired during the treatment session, including the stimulation
parameters which were effective in treating the patient's condition can be
stored in
memory 59, smart device 120 or other computing device (steps 252, 254). The
stimulation device 10 or smart device 120 may also be programmed to operate
the
stimulation circuit 54 in a future treatment session using the stimulation
parameters that
brought the initial biomarker information within the normal or accepted range.
[0081] The
stimulation parameters can also be transmitted to a remote server and
stored in a data structure or in the one or more databases for being accessed
by clinicians
as a set of treatment parameters for a given condition. Hence, over time, a
"smart"
database or artificial intelligence (AI) system is built having a plurality of
sets of
treatment parameters corresponding to the treatment of a plurality of
conditions and
patient characteristics (e.g., age, musculoskeletal stability, etc.); that is,
the database or
AT system can eliminate or shorten the treatment sessions for many patients
since the
most optimum treatment and stimulation parameters for a plurality of
conditions will be
known in advance.
[0082] In some
embodiments, the smart device 120 of the system 100 may provide
visual and/or audible feedback to the patient and/or a clinician before,
during, and/or after
a treatment session.
[0083] With
reference to FIG. 7, an exemplary treatment method 300 of stimulating a
vagus nerve is disclosed using pulses as the stimulation signal in accordance
with the
present disclosure and with reference to the stimulation device 10 of FIGS. 1A-
4.
Initially, the stimulation device 10 is attached to the ear of a patient such
that the inner
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
and outer electrodes 22, 32 of the inner and outer probes 20, 30,
respectively, are
positioned in opposition and offset from one another (step 310). The patient
or a third
party, such as a clinician, may position the stimulation device 10 on the ear
of a patient.
When the stimulation device 10 is positioned on the ear of the patient, the
inner and outer
electrodes 22, 32 are positioned about the auricular branch of the vagus
nerve.
[0084] With
the stimulation device 10 positioned and the power switch 51 has been
set to the "on" position to allow operation of the stimulation device 10, the
stimulation
circuit 54 is activated to deliver pulses to the electrode outputs 56 which
are transmitted
from the inner and/or outer electrode 22, 32 of the inner and outer probes 20,
30,
respectively. The stimulation circuit 54 may detect when the stimulation
device 10 is
attached to the tissue, e.g., an ear, and self-activate or by control of the
smart device 120
to deliver therapeutic pulses to the auricular branch of the vagus nerve (step
320). For
example, a circuit may be completed between the inner and outer electrodes 22,
32 as
tissue is clamped therebetween.
[0085] The
offset between the inner and outer electrodes 22, 32 of the inner and outer
probes 20, 30, respectively, may prevent the circuit from being completed when
the
stimulation device 10 is in a clamped configuration with no tissue disposed
between the
inner and outer electrodes 22, 32. After interposed tissue is detected and
before the
therapeutic pulses are delivered, the stimulation circuit 54 may calibrate to
tissue between
the inner and outer electrodes 22, 32 by sending calibrating pulses from one
of the inner
or outer electrodes 22, 32 to the other and determining a resistance of tissue
between the
inner and outer electrodes 22, 32 (step 330). The resistance between the inner
and outer
26
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
electrodes 22, 32 may be used to determine one of the parameters of the
therapeutic
pulses to be delivered.
[0086] After
activation of the stimulation circuit 54, the stimulation circuit 54
generates and delivers pulses to the inner and/or outer electrodes 22, 32 for
a
predetermined amount of time or until tissue is not detected between the two
electrodes
(e.g., the user removed the stimulation device 10 from the ear) (step 340). As
the
stimulation circuit 54 delivers pulses to the inner and/or outer electrodes
22, 32, the
memory 58 may record data of the delivered pulses including, but not limited
to,
calibration data, time tissue detected, duration of tissue detection, and
parameters of the
pulses delivered (step 350).
[0087] As the
stimulation circuit 54 delivers pulses, the stimulation device 10, e.g.,
the controller 50 may conduct safety checks (step 370). For example, the
controller 50 or
circuitry may verify whether the current being drawn from the power source 52
is below
a maximum allowed current level. If the current being drawn from the power
source 52
exceeds the maximum allowed current, the controller 50 may cease the operation
of the
stimulation circuit 54. Additionally or alternatively, the controller 50 or
circuitry may
monitor the temperature of the stimulation device 10, such that if the
temperature of the
stimulation device 10 exceeds a maximum allowed temperature, the controller 50
may
cease operation of the stimulation circuit 54. This treatment method may be
repeated
multiple times over an extended period of time allowing for out-patient
procedures to be
completed between visits to a clinician's office.
27
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
[0088] During
or after the delivery of pulses to the auricular branch of the vagus
nerve by the stimulation device 10, the stimulation device 10 may link with a
smart
device 120 which receives data from the memory 59 including information
related to one
or more treatment sessions, such as, for example, operating parameters of the
stimulation
circuit 54 (step 360). The smart device 120 may be the patient's smart device
and
transmit the data to a clinician. Additionally or alternatively, the smart
device 120 may
be a clinician's such that at periodic visits, the clinician links to the
stimulation device 10
to receive data from the memory 59 of the stimulation device 10. The clinician
may
analyze the data and update or change one or more operating parameters of the
stimulation circuit 54 to update or change one or more characteristics of the
pulses
generated and delivered by the stimulation circuit 54. The clinician may use
the smart
device 120, e.g., in the clinician's office and/or remotely via a network
connection, to
connect with the stimulation device 10 and update or change the operating
parameters of
the stimulation circuit 54 to update or change one or more characteristics of
the pulses.
[0089] Turning
now to FIG. 8, another embodiment of a StimClipTM or auricular
stimulation device 410 is illustrated that is substantially similar to
stimulation device 10
and is only described herein to the extent necessary to describe the
differences in
operation and construction of stimulation device 410. In general, stimulation
device 410
includes a housing 412, a first or inner arm 420 (e.g. probe), a second or
outer arm 430
(e.g., probe), and an adjustment mechanism 460 that couples inner and outer
probes 420,
430 to housing 412 and enables horizontal and rotational adjustment of probes
420, 430
relative to housing 412, as indicated by arrows "H" and "R," respectively. The
inner and
outer probes 420, 430 are pivotally coupled together about pivot axis "P" to
selectively
28
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
clamp electrodes 422, 432 of respective inner and outer probes 420, 430 to an
ear while
the housing 412 is supported on the ear. More specifically, the outer probe
430 pivots
relative to first probe 420 about pivot axis "P" to move probes 420, 430
between a
clamped position and an unclamped position, as indicated by arrows "B."
[0090] The
outer probe 430 of the stimulation device 410 includes a foot 430a that
facilitates pivotal movement of outer probe 430 about pivot axis "P," as
detailed above
with respect to outer probe 30 of the stimulation device 410. The outer probe
430 further
includes an arched or pre-bent body 430b and a head 430c that supports the
electrode 432
of the outer probe 430. The foot 430a, pre-bent body 430b, and the head 430c
of the outer
probe 430 are formed of rigid material to prevent flexing of outer probe 430.
[0091] As can
be appreciated, securement of any of the components of the presently
disclosed apparatus can be effectuated using known securement techniques such
welding,
crimping, adhesion, fastening, etc.
[0092] Persons
skilled in the art will understand that the structures and methods
specifically described herein and shown in the accompanying figures are non-
limiting
exemplary embodiments, and that the description, disclosure, and figures
should be
construed merely as exemplary of particular embodiments. It is to be
understood,
therefore, that the present disclosure is not limited to the precise
embodiments described,
and that various other changes and modifications may be effected by one
skilled in the art
without departing from the scope or spirit of the disclosure. Additionally,
the elements
and features shown or described in connection with certain embodiments may be
combined with the elements and features of certain other embodiments without
departing
29
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
from the scope of the present disclosure, and that such modifications and
variations are
also included within the scope of the present disclosure. Accordingly, the
subject matter
of the present disclosure is not limited by what has been particularly shown
and
described.
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
APPENDIX
The following references are incorporated herein by reference:
= George, R., Sonnen, A., Upton, A., Salinsky, M., Ristanovic, R., Bergen,
D.,
Mirza, W., Rosenfeld, W., Nari-Toku, D., Manon-Espaillat, R. and Barolat, G.,
1995, "A randomized controlled trial of chronic vagus nerve stimulation for
treatment of medically intractable seizures," Neurology, 45(2), pp.224-230.
= Morris, G.L. and Mueller, W.M., 1999, "Long-term treatment with vagus
nerve
stimulation in patients with refractory epilepsy," Neurology, 53(8), pp.1731-
1731.
= Labar, D., Murphy, J., Tecoma, E. and E VNS Study Group, 1999, "Vagus
nerve
stimulation for medication-resistant generalized epilepsy," Neurology, 52(7),
pp.1510-1510.
= Sackeim, H.A., Rush, A.J., George, M.S., Marangell, L.B., Husain, M.M.,
Nahas,
Z., Johnson, C.R., Seidman, S., Giller, C., Haines, S. and Simpson, R.K.,
2001,
"Vagus nerve stimulation (\,7NSTM) for treatment-resistant depression:
efficacy,
side effects, and predictors of outcome," Neuropsychopharmacology, 25(5),
pp.713-728.
= Nemeroff, C.B., Mayberg, H.S., Krahl, SE., McNamara, J., Frazer, A.,
Henry,
T.R., George, M.S., Charney, D.S. and Brannan, S.K., 2006, "VNS therapy in
treatment-resistant depression: clinical evidence and putative neurobiological
mechanisms," Neuropsychopharmacology, 31(7), p.1345.
= Nahas, Z., Marangell, L.B., Husain, M.M., Rush, A.J., Sackeim, H.A.,
Lisanby,
S.H., Martinez, J.M. and George, M.S., 2005, "Two-year outcome of vagus nerve
31
CA 03071665 2020-01-30
WO 2019/028000
PCT/US2018/044568
stimulation (VNS) for treatment of major depressive episodes," The Journal of
clinical psychiatry, 66(9), pp.1097-1104.
= De Ridder, D., Vanneste, S., Engineer, N.D. and Kilgard, M.P., 2014,
"Safety and
efficacy of vagus nerve stimulation paired with tones for the treatment of
tinnitus:
a case series," Neuromodulation: Technology at the Neural Interface, 17(2),
pp.170-179.
= Stavrakis S, Humphrey MB, Scherlag BJ, Hu Y, Jackman WM, Nakagawa H,
Lockwood D, Lazzara R, Po SS (Mar 2015), "Low-level transcutaneous electrical
vagus nerve stimulation suppresses atrial fibrillation," J Am Coll Cardiol.
65:
867-75.
= Hein E, Nowak M, Kiess 0, Biermann T, Bayerlein K, Kornhuber J, Kraus T
(May 2013), "Auricular transcutaneous electrical nerve stimulation in
depressed
patients: a randomized controlled pilot study," J Neural Transm (Vienna). 120:
821-7.
= Huang F, Dong J, Kong J, Wang H, Meng H, Spaeth RB, Camhi S, Liao X, Li
X,
Zhai X, Li S, Zhu B, Rong P (June 2014), "Effect of transcutaneous auricular
vagus nerve stimulation on impaired glucose tolerance: a pilot randomized
study,"
BMC Complement Altern Med. 14: 203.
= Kreuzer PM, Landgrebe M, Resch M, Husser 0, Schecklmann M, Geisreiter F,
Poeppl TB, Prasser SJ, Hajak G, Rupprecht R, Langguth B (September 2014),
"Feasibility, safety and efficacy of transcutaneous vagus nerve stimulation in
chronic tinnitus: an open pilot study," Brain Stimul. 7: 740-7.
32