Note: Descriptions are shown in the official language in which they were submitted.
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
ETHER COMPOUNDS AND USES THEREOF
INCORPORATION BY REFERENCE TO ANY PRIORITY APPLICATIONS
[0001] Any
and all applications for which a foreign or domestic priority claim is
identified, for example, in the Request as filed with the present application,
are hereby
incorporated by reference, including U.S. Provisional Application No.
62/550489, filed
August 25, 2017.
BACKGROUND
Field
[0002]
Compounds, methods of making such compounds, pharmaceutical
compositions and medicaments comprising such compounds, and methods of using
such
compounds to treat, prevent or diagnose diseases, disorders, or conditions
associated with
protein malfunction are provided.
Description of the Related Technology
[0003]
Protein levels are highly regulated in response to physiological cues. In
cells, much of protein degradation is carried out by the ubiquitin-proteasome
system (UPS).
Aberrant protein function, and/or protein imbalance is a hallmark of many
disease states. For
example, dynamic modulation of key intracellular signaling proteins within the
immune
system is required to maintain proper balance of pro-inflammatory and anti-
inflammatory
mediators or cytokines.
Some cytokines promote inflammation (pro-inflammatory
cytokines), whereas other cytokines suppress the activity of the pro-
inflammatory cytokines
(anti-inflammatory cytokines). For example, IL-4, IL-10, and IL-13 are potent
activators of
B lymphocytes, and also act as anti-inflammatory agents. They are anti-
inflammatory
cytokines by virtue of their ability to suppress genes for pro-inflammatory
cytokines such as
interleukin 1 (IL-1), interleukin 6 (IL-6), tumor necrosis factor alpha
(TNFa), and
chemokines.
[0004]
Unregulated activities of these mediators can lead to the development of
serious inflammatory conditions. For example, autoimmune diseases arise when
immune
system cells (lymphocytes, macrophages) become sensitized against the "self"
Lymphocytes, as well as macrophages, are usually under control in this system.
However, a
-1-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
misdirection of the system toward the body's own tissues may happen in
response to still
unexplained triggers. One hypothesis is that lymphocytes recognize an antigen
which
mimics the "self' and a cascade of activation of different components of the
immune system
takes place, ultimately leading to tissue destruction. Genetic predisposition
has also been
postulated to be responsible for autoimmune disorders.
[0005] TNFa, IL-6, and IL-1 are pro-inflammatory cytokines that
mediate
inflammatory responses associated with infectious agents and other cellular
stresses.
Overproduction of these cytokines is believed to underlie the progression of
many
inflammatory diseases including rheumatoid arthritis (RA), Crohn's disease,
inflammatory
bowel disease, multiple sclerosis, endotoxin shock, osteoporosis, Alzheimer's
disease,
congestive heart failure, and psoriasis among others.
[0006] Recent data from clinical trials support the use of protein
antagonists of
cytokines, for example soluble TNFa receptor fusion protein (etanercept) or
the monoclonal
TNFa antibody (infliximab), for the treatment of rheumatoid arthritis, Crohn's
disease,
juvenile chronic arthritis and psoriatic arthritis. Thus, the reduction of pro-
inflammatory
cytokines such as TNFa, IL-6, and IL-I has become an accepted therapeutic
approach for
potential drug intervention in these conditions.
[0007] Moreover, IL-2 is now FDA approved for the treatment of renal
cancer
and melanoma patients, with durable, complete remissions achieved with IL-2 up
to 148
months. However, the short half-life of IL-2 in serum requires that large
amounts of IL-2 be
injected to achieve therapeutic levels. Many attempts have been made to
minimize side
effects of systemic IL-2 treatment, for example, introducing IL-2 directly
into the tumor,
though this complicates treatment, and has largely been unsuccessful.
[0008] Local delivery of cytokines is appealing compared to systemic
delivery for
a variety of reasons. It takes advantage of the natural biology of cytokines
that have evolved
to act locally in a paracrine or autocrine fashion. Local expression also
dramatically
minimizes many of the side effects of systemic delivery of cytokines. Thus,
compounds and
methods to increase local expression of IL-2 would be better tolerated than
high dose IL-2
treatment, which would expand therapeutic utility of strategies that increase
IL-2.
[0009] Additional targets include several candidate genes involved in
apoptosis
and cell survival, including casein kinase la (CK1a) and the zinc-finger
transcription factors
-2-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
aiolos, helios, and ikaros. Aiolos, helios, and ikaros are transcription
factors whose
expression is restricted to lymphoid lineages. For example, aiolos binds to
the Bc1-2
promoter, and also interacts with the Bc1-2 and Bc1-XL proteins to promote
cell survival.
Upregulation of aiolos expression, for example, can reduce apoptosis of HIV-1
infected cells.
[0010] Likewise, expression of aiolos in lung and breast cancers
predicts
significantly reduced patient survival. Aiolos decreases expression of a large
set of
adhesion-related genes, disrupting cell-cell and cell-matrix interactions,
facilitating
metastasis. Aiolos may also function as an epigenetic driver of lymphocyte
mimicry in
certain metastatic epithelial cancers. Similarly, aberrant ikaros and helios
expression may
promote Bc1-XL expression, driving the development of hematopoietic
malignancies. Thus,
down-regulation of aiolos, ikaros, and/or helios may reduce or eliminate
metastasis.
[0011] Casein kinase la (CK1a) is a component of the P-catenin-
degradation
complex and a critical regulator of the Wnt signaling pathway, and its
ablation induces both
Wnt and p53 activation. Schittek and Sinnberg, Mot. Cancer. 2014, /3, 231;
Cheong and
Virshup, I Biochem. Cell Biol. 2011, 43, 465-469; Elyada et at., Nature 2011,
470, 409-413.
CK la phosphorylates 13-catenin, which is subsequently further phosphorylated
by GSK-30.
This destabilizes 13-catenin and marks the protein for ubiquitination and
proteosomal
degradation. Thus, CKla functions as a molecular switch for the Wnt pathway.
Amit et at.,
Genes Dev. 2002, 16, 1066-1076. CKla is critical for embryogenesis and plays
an important
role in tissue development and response to DNA damage, at least partly
coordinated with
p53. Elyada et at., Nature 2011, 470, 409-413; Schneider et at., Cancer Cell
2014, 26, 509-
520. Levine and Oren, Nat. Rev. Cancer 2009, 9, 749-758.
[0012] Indeed, CK la also phosphorylates p53, which inhibits binding
to MDM2
(a p53 inhibitor) and stabilizes p53' s binding interactions with the
transcriptional machinery.
Huart, et al., I Biol. Chem. 2009, 284, 32384-32394. Thus, inhibiting CKla
activity
increases cellular levels of p53.
[0013] One mechanism to disrupt protein drivers of disease is to
decrease the
cellular concentration of these proteins. For example, regulated proteolytic
degradation of
cellular proteins is essential to normal cell function. Hijacking this
process, by targeting
specific disease-related proteins, presents a novel mechanism for the
treatment of disease.
The irreversible nature of proteolysis makes it well-suited to serve as a
regulatory switch for
-3-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
controlling unidirectional processes. For example, Ikaros is a transcriptional
repressor of IL-
2 expression. Accordingly, a reduction in Ikaros protein levels leads to
enhanced IL-2
expression in activated T cells. IL-2 therapy is currently being evaluated for
a wide array of
clinical indications, including for treatment of systemic lupus erythematousus
(SLE), wound
healing, and immune-oncology.
SUMMARY OF THE INVENTION
[0014] Some embodiments provide a compound of Formula (I), or a
pharmaceutically acceptable salt thereof:
(Ri)m 0 x
Qx 2
0 0
)n H 0
(I)
[0015] In some embodiments, Qi can be CH2, 0, NR2, S, or a bond.
[0016] In some embodiments, Q2 can be CH2 or a bond.
[0017] In some embodiments, X can be CH2 or CO.
[0018] In some embodiments, Xi can be hydrogen, deuterium, methyl, or
fluoro.
1
Y2
I I
Y3
[0019] In some embodiments, Ring B can be Y 4 ,
wherein Yi is N or
CR3A, Y2 is N or CR3B, Y3 is N or CR3C, Y4 is N or CR3D, and Y5 is N or CR3E.
[0020] In some embodiments, each Ri can be independently deuterium,
hydroxyl,
halogen, nitro, a substituted or unsubstituted amino, a substituted or
unsubstituted Ci-C6
alkoxy, a substituted or unsubstituted Ci-C6 alkyl, a substituted or
unsubstituted C2-C6
alkenyl, a substituted or unsubstituted C3-C8 cycloalkyl, a substituted or
unsubstituted 3 to 10
membered heterocyclyl, a substituted or unsubstituted C6-Cio aryl, a
substituted or
unsubstituted 5 to 10 membered heteroaryl, or L-Y.
[0021] In some embodiments, R2 can be hydrogen, deuterium, a
substituted or
unsubstituted C i-C6 alkyl, a substituted or unsubstituted C2-C6 alkenyl,
acyl, or ¨(502)-C i-C6
alkyl.
-4-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0022] In some embodiments, each of R3A, R3B, R3C, R3D, and R3E can be
independently hydrogen, deuterium, hydroxyl, halogen, nitro, a substituted or
unsubstituted
amino, a substituted or unsubstituted Ci-C6 alkoxy, a substituted or
unsubstituted Ci-C6
alkyl, a substituted or unsubstituted C2-C6 alkenyl, a substituted or
unsubstituted C3-C8
cycloalkyl, a substituted or unsubstituted 3 to 10 membered heterocyclyl, a
substituted or
unsubstituted alkoxyalkyl, a substituted or unsubstituted cycloalkylalkyl, a
substituted or
unsubstituted heterocyclylalkyl, a substituted or unsubstituted aralkyl, a
substituted or
unsubstituted heteroaralkyl, or L-Y.
[0023] In some embodiments, m can be 0, 1, 2, or 3.
[0024] In some embodiments, n can be 1, 2, 3, or 4. In some
embodiments, n can
be 1 or 2.
[0025] In some embodiments, L can be ¨Z1-(R4)t-Z2¨; ¨Z1-(R4-0-R4)t-
Z2¨;
¨Z1(R4-NH-R4)t-Z2¨; ¨Z1-(R4-(NHCO)-R4)t-Z2¨; ¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨; or
¨Z1-
(R4-(CONH)-R4)t-Z2¨.
[0026] In some embodiments, Zi can be ¨CH2NH(C0)¨; ¨NH¨; ¨0¨; ¨CH2¨;
¨NH(C0)¨; ¨(CO)NH¨; ¨CH2NH¨; ¨(CO)NHCH2¨; ¨CH2CH2NH¨; ¨NHCH2¨; or
¨NHCH2CH2¨.
[0027] In some embodiments, Z2 can be ¨NH¨; ¨0¨; ¨CH2¨; ¨NH(C0)¨;
¨(CO)NH¨; ¨CH2NH¨; ¨NHCH2¨; ¨(CO)NHCH2¨; ¨CH2CH2NH¨; ¨CH2NH(C0)¨.
[0028] In some embodiments, each R4 can be independently an
unsubstituted C1-
C6 alkylene.
[0029] In some embodiments, t can be 1, 2, 3, 4, 5, or 6.
[0030] In some embodiments, Y can be
N C)NI.D
H 11
I I
NNN
0 ,
wherein Y can be derivatized to attach
to L.
[0031] In some embodiments, at least one of Yt, Y2, Y3, Y4, and Y5 can
be carbon
(bonded to one of R3A, R3B, R3C, R3D, and R3E, respectively, e.g., CR3A, CR3u,
CR3c, CR3D,
and/or CR3E).
-5-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0032] In some embodiments, when Qi can be CH2 or a bond, then one or
more
of R3A, R3B, R3C, R3D, and R3E cannot be hydrogen. In other embodiments, when
Ri can be
L-Y, none of R3A, R3B, R3C, R3D, and R3E can be L-Y. In still other
embodiments, when Qi is
a bond, then m is not 0. In some embodiments, when Qi is a bond, Ri is L-Y. In
some
embodiments, when Qi can be a bond, Xi is hydrogen or methyl, and Q2 is CH2;
then m is
not 0. In other embodiments, when Qi can be a bond, Xi can be hydrogen or
methyl, and Q2
can be CH2; then one of R1, R3A, R3B, R3C, R3D, and R3E is L-Y. In some
embodiments, when
Q2 can be a bond, Qi can be a bond or CH2.
[0033] Some embodiments provide a compound of Formula (I):
(Ri)m 0
Xi
/ 2
0 0
)n H 0
(I)
or a pharmaceutically acceptable salt thereof, wherein: Qi is CH2, 0, NR2, S,
or a bond; Q2 is
CH2 or a bond; X is CH2 or C=0; Xi is hydrogen, deuterium, or fluoro; Ring B
is
Y2
I I
Y3 \,Y5
4 ,
wherein Yi is N or CR3A; Y2 is N or CR3B; Y3 is N or CR3C; Y4 is N or CR36;
Y5 is N or CR3E; each Ri is independently deuterium, hydroxyl, halogen, nitro,
a substituted
or unsubstituted amino, a substituted or unsubstituted Ci to C6 haloalkyl, a
substituted or
unsubstituted Ci-C6 alkoxy, a substituted or unsubstituted Ci-C6 alkyl, a
substituted or
unsubstituted C2-C6 alkenyl, a substituted or unsubstituted C3-C8 cycloalkyl,
a substituted or
unsubstituted 3 to 10 membered heterocyclyl, a substituted or unsubstituted C6-
Cio aryl, a
substituted or unsubstituted 5 to 10 membered heteroaryl, or L-Y; R2 is
Hydrogen,
deuterium, a substituted or unsubstituted Ci-C6 alkyl, a substituted or
unsubstituted C2-C6
alkenyl, acyl, or -(502)-Ci-C6 alkyl; each R3A, R3B, R3C, R3D, and R3E is
independently
hydrogen, deuterium, hydroxyl, halogen, nitro, a substituted or unsubstituted
amino, a
substituted or unsubstituted Ci to C6 haloalkyl, a substituted or
unsubstituted Ci-C6 alkoxy, a
substituted or unsubstituted Ci-C6 alkyl, a substituted or unsubstituted C2-C6
alkenyl, a
-6-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
substituted or unsubstituted C3-C8 cycloalkyl, a substituted or unsubstituted
3 to 10
membered heterocyclyl, a substituted or unsubstituted alkoxyalkyl, a
substituted or
unsubstituted cycloalkylalkyl, a substituted or unsubstituted
heterocyclylalkyl, a substituted
or unsubstituted aralkyl, a substituted or unsubstituted heteroaralkyl, or L-
Y; m is 0, 1, 2, or
3; n is 1, 2, or 3; L is ¨Z1-(R4-0-R4)t-Z2¨; ¨Zi(R4-NH-R4)t-Z2¨; ¨Zi-(R4-
(NHCO)-R4)t-Z2¨; ¨
Zi-(R4-(NHC(0)NH)-R4)t-Z2¨; or ¨Zi-(R4-(CONH)-R4)t-Z2¨; Zi is ¨NH¨; ¨0¨;
¨CH2¨; ¨
NH(C0)¨; ¨(CO)NH¨; ¨CH2NH¨;
¨NHCH2¨;
¨CH2NH(C0)¨, or ¨NHCH2CH2¨; Z2 is ¨NH¨; -0-; ¨CH2¨; ¨NH(C0)¨; or ¨(CO)NH¨;
¨CH2NH¨; ¨NHCH2¨; or ¨NHCH2CH2¨; each R4 is independently an unsubstituted Ci-
C6
H 0
>1\i'g NN N
alkylene; t is 1, 2, 3, 4, 5, or 6; and Y is 0
=
wherein Y is derivatized to attach to L; and wherein at least one of Yi, Y2,
Y3, Y4, and Y5 is
respectively R3A, R3B, R3C, R3D, or R3E; when Qi is CH2 or a bond, then one or
more of R3A,
R3B, R3C, R3D, or R3E is not hydrogen; when Ri is L-Y, none of R3A, R3B, R3C,
R3D, and R3E is
L-Y; and when Qi is a bond, then m is not 0.
[0034] In
some embodiments, X can be CH2. In some embodiments, X can be
C=0.
[0035] In
some embodiments, Xi can be hydrogen. In other embodiments, Xi can
be deuterium. In still other embodiments, Xi can be methyl. In some
embodiments, Xi can
be fluoro.
[0036] In
some embodiments, Qi can be NR2. In some embodiments, R2 can be
hydrogen. In some embodiments, R2 can be a substituted or unsubstituted Ci-C6
alkyl. In
some embodiments, R2 can be an unsubstituted Ci-C6 alkyl. In some embodiments,
R2 can
be acyl. In some embodiments, R2 can be -(S02)-Ci-C6 alkyl. In some
embodiments, R2 can
be methyl.
[0037] In
some embodiments, Qi can be CH2. In some embodiments, Qi can be
0. In some embodiments, Qi can be S. In some embodiments, Qi can be a bond. In
some
embodiments, when Qi is a bond, then m is not 0. In some embodiments, Q2 can
be CH2. In
some embodiments, Q2 can be a bond. In some embodiments, when Q2 can be a
bond, Qi
can be a bond or CH2.
-7-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0038] In some
embodiments, n can be 1. In some embodiments, n can be 2. In
some embodiments, n can be 3. In some embodiments, m can be 1. In some
embodiments,
m can be 2. In some embodiments, m can be 3. In some embodiments, m can be 0.
[0039] In some
embodiments, each Ri can be independently halogen, a
substituted or unsubstituted amino, a substituted or unsubstituted Cl-C6
alkoxy or a
substituted or unsubstituted Cl-C6 alkyl. In
some embodiments, each Ri can be
independently halogen, an unsubstituted amino, an unsubstituted Cl-C6
haloalkyl, an
unsubstituted Ci-C 6 alkoxy or unsubstituted Ci-C 6 alkyl.
[0040] In some
embodiments, each Ri can be independently fluoro, chloro, ¨NH2,
¨NH(CH3), ¨N(CH3)2, ¨CF3, ¨OCH3, ¨OCH2CH3, ¨OCH(CH3)2, ¨CH3, ¨CH2CH3 or
¨CH(CH3)2.
[0041] In some embodiments, Ring B can
be selected from:
R3A R3A R3A
N
R3B R3B / \ N/ \ R3 R3B / \ R3B --....e......"
R3c R3c c
R3D R3D R3D R3D , R3D ,
, , ,
R3A)...
R3A)..._..... N N
R3B--5/.........(-?-L. R3B ---..N
Nzzz(N N ' \ R3E
N--- R
\. 30 3E
mi
rN3c R3D R3D R3D R3D ,
, , , ,
N
).
....R...?........
R3B / \
R3E D,
3c
Nzz-N R3E )-%-- N
' `3C r--.
R3D and R3
D ,
R3D .
[0042] In some
embodiments, each Of R3A, R3B, R3C, R3D, and R3E can be
independently hydrogen, deuterium, hydroxyl, halogen, nitro, a substituted or
unsubstituted
amino, a substituted or unsubstituted Cl-C6 alkoxy, a substituted or
unsubstituted Cl-C6
alkyl, a substituted or unsubstituted C2-C6 alkenyl, a substituted or
unsubstituted C3-C8
cycloalkyl, a substituted or unsubstituted 3 to 10 membered heterocyclyl, a
substituted or
unsubstituted alkoxyalkyl, a substituted or unsubstituted cycloalkylalkyl, a
substituted or
unsubstituted heterocyclylalkyl, a substituted or unsubstituted aralkyl or a
substituted or
unsubstituted heteroaralkyl.
-8-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0043] In some embodiments, each of R3A, R3B, R3C, R3D, and R3E can be
independently hydrogen, hydroxyl, halogen, nitro, an unsubstituted amino, an
unsubstituted
Ci-C6 haloalkyl, an unsubstituted Ci-C6 alkoxy, an unsubstituted Ci-C6 alkyl,
a substituted or
unsubstituted C3-C8 cycloalkyl, a substituted or unsubstituted 3 to 10
membered
heterocyclyl, a substituted or unsubstituted alkoxyalkyl, a substituted or
unsubstituted
cycloalkylalkyl, a substituted or unsubstituted heterocyclylalkyl, a
substituted or
unsubstituted aralkyl or a substituted or unsubstituted heteroaralkyl.
[0044] In some embodiments, each of R3A, R3B, R3C, R3D, and R3E can be
independently hydrogen, hydroxyl, halogen, nitro, an unsubstituted amino, an
unsubstituted
Ci-C6 haloalkyl, an unsubstituted Cl-C6 alkoxy, an unsubstituted Cl-C6 alkyl,
an
unsubstituted C3-C8 cycloalkyl, an unsubstituted 3 to 10 membered
heterocyclyl, an
unsubstituted cycloalkylalkyl, unsubstituted 3 to 10 membered
heterocyclylalkyl,
unsubstituted aralkyl or unsubstituted 5 to 10 membered heteroaralkyl.
[0045] In some embodiments, each of R3A, R3B, R3C, R3D, and R3E can be
independently hydrogen, halogen, an unsubstituted Ci-C6 haloalkyl, an
unsubstituted Ci-C6
alkoxy, an unsubstituted Ci-C6 alkyl, an unsubstituted 3 to 10 membered
heterocyclyl, or an
unsubstituted 3 to 10 membered heterocyclylalkyl.
[0046] In some embodiments, one of R3A, R3B, R3C, R3D, and R3E can be
halogen,
an unsubstituted Ci-C6 haloalkyl, an unsubstituted Ci-C6 alkoxy, an
unsubstituted Ci-C6
alkyl, an unsubstituted 3 to 10 membered heterocyclyl, or an unsubstituted 3
to 10 membered
heterocyclylalkyl and the other of R3A, R3B, R3C, R3D, and R3E are hydrogen.
[0047] In some embodiments, one of R3A, R3B, R3C, R3D, and R3E can be
fluoro,
chloro, -CF3, -OCH3, an unsubstituted Ci-C6 alkyl, an unsubstituted 3 to 10
membered
heterocyclyl, or an unsubstituted 3 to 10 membered heterocyclylalkyl and the
other of R3A,
R3B, R3C, R3D, and R3E are hydrogen.
[0048] In some embodiments, one of R3A, R3B, R3C, R3D, and R3E can be
-9-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
NH
JVVVµ
C µ2C N
0
H , or , and the other of R3A, R3B, R3C, R3D, and R3E are
hydrogen.
[0049] In some embodiments, one Ri can be L-Y. In some embodiments,
one of
R3A, R3B, R3C, R3D, and R3E can be L-Y. In some embodiments, Y3 can be C-L-Y.
[0050] In some embodiments, L can be ¨Z1-(R4)t-Z2¨. In other
embodiments,
L can be ¨Z1-(R4-0-R4)t-Z2¨. In still other embodiments, L can be ¨Zi(R4-NH-
R4)t-Z2¨. In
some embodiments, L can be Z1-(R4-(NHCO)-R4)t-Z2¨. In some embodiments, L can
be ¨
Z1-(R4-(CONH)-R4)t-Z2¨. In other embodiments, L can be ¨Z1-(R4-(NHC(0)NH)-R4)t-
Z2¨.
[0051] In some embodiments, Zi can be ¨CH2NH(C0)¨. In other
embodiments,
Zi can be ¨NH¨. In still other embodiments, Zi can be ¨0¨. In some
embodiments, Zi can
be ¨CH2¨. In other embodiments, Zi can be ¨NH(C0)¨. In still other
embodiments, Zi can
be ¨CH2NH¨. In some embodiments, Zi can be ¨NHCH2¨. In other embodiments, Zi
can be
¨(CO)NH¨. In still other embodiments, Zi can be ¨NHCH2CH2¨. In some
embodiments, Zi
can be ¨(CO)NHCH2¨. In still other embodiments, Zi can be ¨CH2CH2NH¨.
[0052] In some embodiments, Z2 can be ¨NH¨. In other embodiments, Z2
can be
¨0¨. In still other embodiments, Z2 can be ¨CH2¨. In some embodiments, Z2 can
be
¨NH(C0)¨. In other embodiments, Z2 can be ¨(CO)NH¨. In still other
embodiments, Z2 can
be ¨CH2NH¨. In some embodiments, Z2 can be ¨NHCH2¨. In other embodiments, Z2
can be
¨(CO)NH¨. In still other embodiments, Z2 can be ¨NHCH2CH2¨. In some
embodiments, Z2
can be ¨(CO)NHCH2¨. In other embodiments, Z2 can be ¨CH2CH2NH¨. In still other
embodiments, Z2 can be ¨CH2NH(C0)¨.
[0053] In some embodiments, each R4 can be independently an
unsubstituted C1-
C4 alkylene. In some embodiments, each R4 can be independently an
unsubstituted Ci-C2
alkylene.
[0054] In some embodiments, t can be 1. In some embodiments, t can be
2. In
some embodiments, t can be 3. In some embodiments, t can be 4. In some
embodiments, t
can be 5. In some embodiments, t can be 6.
-10-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
[0055] In
some embodiments, the compound of Formula (I) is selected from:
00 H
......N:0 00 H 00 H 0 0 H
io N'. =
0 N........N:CD
o/
0 0 0 0
0 40 40
c,
40 ISI
r-,,, N N rThs1
13.) oj Oj Oj
00 H 00 H
N/ 0 N..=
0õ,...0õ,,...,,N
0 61-13 Hosr:L, 0 H 0 lµi,g
N N N
40 8, H H
Si
oj oj
, ,
00 H
.....N..1.,<0
00 H /
._....Njo
H 0
N .. =
0
H 0
0
8 0
H H
01
r-,N
(30) N
H
00 H
_....N..;.7.0
0
0 IS
H,,,J1,
---...-----:-N 0 0....õ.....N N
H 0 101 H
N,g
NNN
8 H H
, or a pharmaceutically
acceptable salt of any of the foregoing.
[0056]
Some embodiments provide a pharmaceutical composition comprising a
compound of Formula (I), or a pharmaceutically acceptable salt thereof; and a
pharmaceutically acceptable excipient.
-11-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0057] Some embodiments provide a method of inhibiting the activity of
a
cytokine, comprising contacting a cell with an effective amount of a compound
of Formula
(I), or a pharmaceutically acceptable salt thereof Other embodiments provide
the use of a
compound of Formula (I), or a pharmaceutically acceptable salt of any of the
foregoing, for
inhibiting the activity of a cytokine, comprising contacting a cell with an
effective amount of
a compound of Formula (I), or a pharmaceutically acceptable salt of any of the
foregoing.
Still other embodiments provide the use of a compound of Formula (I), or a
pharmaceutically
acceptable salt of any of the foregoing, for the manufacture a medicament for
inhibiting the
activity of a cytokine, comprising contacting a cell with an effective amount
of a compound
of Formula (I), or a pharmaceutically acceptable salt of any of the foregoing.
In some
embodiments, the cytokine is selected from: IL-113, IL-2, IL-6, and TNFa. In
some
embodiments, the cytokine is TNFa. In some embodiments, the cell is a cancer
cell.
[0058] Some embodiments provide a method of inhibiting the activity of
aiolos,
comprising contacting a cell with an effective amount of a compound of Formula
(I), or a
pharmaceutically acceptable salt thereof. Other embodiments provide the use of
a compound
of Formula (I), or a pharmaceutically acceptable salt of any of the foregoing,
for inhibiting
the activity of aiolos, comprising contacting a cell with an effective amount
of a compound
of Formula (I), or a pharmaceutically acceptable salt of any of the foregoing.
Still other
embodiments provide the use of a compound of Formula (I), or a
pharmaceutically
acceptable salt of any of the foregoing, for the manufacture a medicament for
inhibiting the
activity of aiolos, comprising contacting a cell with an effective amount of a
compound of
Formula (I), or a pharmaceutically acceptable salt of any of the foregoing. In
some
embodiments, the cell is a cancer cell.
[0059] Some embodiments provide a method of inhibiting the activity of
ikaros,
comprising contacting a cell with an effective amount of a compound of Formula
(I), or a
pharmaceutically acceptable salt thereof. Other embodiments provide the use of
a compound
of Formula (I), or a pharmaceutically acceptable salt of any of the foregoing,
for inhibiting
the activity of ikaros, comprising contacting a cell with an effective amount
of a compound
of Formula (I), or a pharmaceutically acceptable salt of any of the foregoing.
Still other
embodiments provide the use of a compound of Formula (I), or a
pharmaceutically
acceptable salt of any of the foregoing, for the manufacture a medicament for
inhibiting the
-12-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
activity of ikaros, comprising contacting a cell with an effective amount of a
compound of
Formula (I), or a pharmaceutically acceptable salt of any of the foregoing. In
some
embodiments, the cell is a cancer cell.
[0060] Some embodiments provide a method of inhibiting the activity of
helios,
comprising contacting a cell with an effective amount of a compound of Formula
(I), or a
pharmaceutically acceptable salt thereof. Other embodiments provide the use of
a compound
of Formula (I), or a pharmaceutically acceptable salt of any of the foregoing,
for inhibiting
the activity of helios, comprising contacting a cell with an effective amount
of a compound
of Formula (I), or a pharmaceutically acceptable salt of any of the foregoing.
Still other
embodiments provide the use of a compound of Formula (I), or a
pharmaceutically
acceptable salt of any of the foregoing, for the manufacture a medicament for
inhibiting the
activity of helios, comprising contacting a cell with an effective amount of a
compound of
Formula (I), or a pharmaceutically acceptable salt of any of the foregoing. In
some
embodiments, the cell is a cancer cell.
[0061] Some embodiments provide a method of inhibiting the activity of
CK-1 a,
comprising contacting a cell with an effective amount of a compound of Formula
(I), or a
pharmaceutically acceptable salt thereof. Other embodiments provide the use of
a compound
of Formula (I), or a pharmaceutically acceptable salt of any of the foregoing,
for inhibiting
the activity of CK-1 a, comprising contacting a cell with an effective amount
of a compound
of Formula (I), or a pharmaceutically acceptable salt of any of the foregoing.
Still other
embodiments provide the use of a compound of Formula (I), or a
pharmaceutically
acceptable salt of any of the foregoing, for the manufacture a medicament for
inhibiting the
activity of CK-1 a, comprising contacting a cell with an effective amount of a
compound of
Formula (I), or a pharmaceutically acceptable salt of any of the foregoing. In
some
embodiments, the cell is a cancer cell.
[0062] In some embodiments, the cell is a small cell lung cancer cell,
a non-small
cell lung cancer cell, a breast cancer cell, a prostate cancer cell, a head
and neck cancer cell,
a pancreatic cancer cell, a colon cancer cell, a rectal cancer cell, a
teratoma cell, an ovarian
cancer cell, an endometrial cancer cell, a brain cancer cell, a retinoblastoma
cell, a leukemia
cell, a skin cancer cell, a melanoma cell, a squamous cell carcinoma cell, a
liposarcoma cell,
a lymphoma cell, a multiple myeloma cell, a testicular cancer cell, a liver
cancer cell, an
-13-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
esophageal cancer cell, a kidney carcinoma cell, an astrogliosis cell, a
relapsed/refractory
multiple myeloma cell, or a neuroblastoma cell.
[0063] Some embodiments provide a method of treating, ameliorating, or
preventing a disease, disorder, or condition associated with a protein in a
subject, the protein
selected from a cytokine, aiolos, ikaros, helios, CK 1 a, and combinations of
any of the
foregoing; the method comprising administering a therapeutically effective
amount of a
compound of Formula (I), or a pharmaceutically acceptable salt thereof, or a
pharmaceutical
composition comprising a compound of Formula (I), or a pharmaceutically
acceptable salt
thereof, to the subject. Other embodiments provide the use of a compound of
Formula (I), or
a pharmaceutically acceptable salt of any of the foregoing, for treating,
ameliorating, or
preventing a disease, disorder, or condition associated with a protein
selected from a
cytokine, aiolos, ikaros, helios, CK 1 a, and combinations of any of the
foregoing. Still other
embodiments provide the use of a compound of Formula (I), or a
pharmaceutically
acceptable salt of any of the foregoing, for the manufacture a medicament for
treating,
ameliorating, or preventing a disease, disorder, or condition associated with
a protein
selected from a cytokine, aiolos, ikaros, helios, CK 1 a, and combinations of
any of the
foregoing.
[0064] In some embodiments, the disease, disorder, or condition is a
cancer
selected from a hematological malignancy and a solid tumor In some
embodiments, the
disease, disorder, or condition is a cancer selected from small cell lung
cancer, non-small cell
lung cancer, breast cancer, prostate cancer, head and neck cancer, pancreatic
cancer, colon
cancer, rectal cancer, teratoma, ovarian cancer, endometrial cancer, brain
cancer,
retinoblastoma, leukemia, skin cancer, melanoma, squamous cell carcinoma,
liposarcoma,
lymphoma, multiple myeloma, testicular cancer, liver cancer, esophageal
cancer, kidney
carcinoma, astrogliosis, relapsed/refractory multiple myeloma, and
neuroblastoma.
[0065] In some embodiments, the disease, disorder, or condition is
selected from
inflammation, fibromyalgia, rheumatoid arthritis, osteoarthritis, ankylosing
spondylitis,
psoriasis, psoriatic arthritis, inflammatory bowel diseases, Crohn's disease,
ulcerative colitis,
uveitis, inflammatory lung diseases, chronic obstructive pulmonary disease,
and Alzheimer's
disease. In some embodiments, the disease, disorder, or condition is selected
from
-14-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
fibromyalgia, rheumatoid arthritis, osteoarthritis, ankylosing spondylitis,
psoriasis, psoriatic
arthritis, Crohn's disease, and ulcerative colitis.
[0066] In some embodiments, the protein is a cytokine. In some
embodiments,
the cytokine is selected from: IL-113, IL-2, IL-6, and TNFa. In some
embodiments, the
cytokine is TNFa. In some embodiments, the protein is aiolos. In some
embodiments, the
protein is ikaros. In some embodiments, the protein is helios. In some
embodiments, the
protein is CKla.
[0067] Any of the features of an embodiment is applicable to all
embodiments
identified herein. Moreover, any of the features of an embodiment is
independently
combinable, partly or wholly with other embodiments described herein in any
way, e.g., one,
two, or three or more embodiments may be combinable in whole or in part.
Further, any of
the features of an embodiment may be made optional to other embodiments. Any
embodiment of a method can comprise another embodiment of a compound, and any
embodiment of a compound can be configured to perform a method of another
embodiment.
BRIEF DESCRIPTION OF THE DRAWINGS
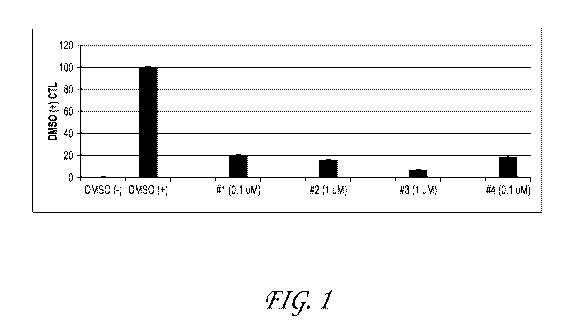
[0068] Fig. 1 represents the activity against IL-1-beta in peripheral
blood
mononuclear cells (PBMCs), plated in 96 well plates and pretreated with
compound for 1
hour and then induced with 100ng/mL LPS for 18-24 hrs. Cytokines in the media
were
measured according to MesoScale protocol. Negative control wells were treated
with
DMSO. Compound activity is measured as a percentage of LPS-induced activity.
[0069] Fig. 2 represents the activity against IL-6 in peripheral blood
mononuclear
cells (PBMCs), plated in 96 well plates and pretreated with compound for 1
hour and then
induced with 100ng/mL LPS for 18-24 hrs. Cytokines were measured according to
MesoScale protocol. Negative control wells were treated with DMSO. Compound
activity
was measured as a percentage of LPS-induced activity.
[0070] Fig. 3 represents the activity against TNFa in peripheral blood
mononuclear cells (PBMCs), plated in 96 well plates and pretreated with
compound for 1
hour and then induced with 100ng/mL LPS for 18-24 hrs. Cytokines in the media
were
measured according to MesoScale protocol. The negative control wells were
treated with
DMSO. Compound activity is measured as a percentage of LPS-induced activity.
-15-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0071] Fig. 4 represents Anti-CD3-induced IL-2 secretion in PBMCs. 1
pg/mL
anti-CD3 (OKT-3) antibody in PBS coated onto 96-well plates overnight at 4 C.
Approximately, 550,000 PBMCs were added to each well, followed by addition of
DMSO
only (control) or Compound 1. Induction was measured after 24 hrs as fold
difference from
the DMSO stimulated control.
[0072] Fig. 5A represents a Western Blot from Jurkat cells treated
with Control
(DMSO only), or the indicated concentration of Compound 1. Cells were lysed
using RIPA
Buffer (Pierce) and a Western Blot was performed using anti-casein kinase 1
alpha, (CK1-a)
and anti-f3-actin antibodies. Fig. 5B represents a Western Blot from Jurkat
cells treated with
Control (DMSO only), or the indicated concentration of Compound 1, 2, 3, or 4.
Cells were
lysed using RIPA Buffer (Pierce) and a Western Blot was performed using anti-
ikaros and
anti-f3-actin antibodies.
DETAILED DESCRIPTION
[0073] Some embodiments provide a compound of Formula (I), or a
pharmaceutically acceptable salt thereof:
(Ri)m 0
Xi
/ 2
0 0
)n H 0
(I)
[0074] In some embodiments, Qi can be CH2, 0, NR2, S, or a bond. In
some
embodiments, when Qi is a bond, then m is not 0. In some embodiments, Q2 can
be CH2 or a
bond. In some embodiments, when Q2 can be a bond, Qi can be a bond or CH2.
[0075] In some embodiments, X can be CH2 or C=0. In some embodiments,
X
can be CH2. In some embodiments, X can be C=0.
[0076] In some embodiments, Xi can be hydrogen, deuterium, methyl, or
fluor .
In some embodiments, Xi can be hydrogen. In other embodiments, Xi can be
fluoro. In still
other embodiments, Xi can be methyl. In some embodiments, Xi can be deuterium.
-16-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
Y)112z2.
2
I I
Y3 --....,Y5
[0077] In some embodiments, Ring B can be Y4 ,
wherein Yi is N or
CR3A; Y2 is N or CR3B; Y3 is N or CR3C; Y4 is N or CR3D; Y5 is N or CR3E. In
some
embodiments, Ring B can be selected from:
R3A R3A R3A
N
R3B R3B / \ N / \ R3B / \ R3B---
..e$.
R3D R3o R3D
R3D R3D R3D R3D , R3D ,
, , ,
R3A/\....... N
N R3E R3 N
R3B N
--.... R30 ---\(µ /
R3E
mi )--D
rN3D R3D R3D R3D R3D ,
, , , ,
R3A\ jµ
N
....R.?.........
R3B / \
R3E
R30 R3D
R3 E R3c)---N
, R3D and R3D
. In some embodiments,
,
Ring B is a phenyl group. In other embodiments, Ring B is a pyridyl group,
such as a 2-
pyridyl, 3-pyridyl, or 4-pyridyl group.
[0078] In
some embodiments, m can be 0, 1, 2, or 3. In some embodiments, m
is 1. In some embodiments, m is 2. In some embodiments, m is 3.
[0079] In
some embodiments, each Ri can independently be deuterium, hydroxyl,
halogen, nitro, a substituted or unsubstituted amino, a substituted or
unsubstituted Ci-C6
alkoxy, a substituted or unsubstituted Ci-C6 alkyl, a substituted or
unsubstituted C2-C6
alkenyl, a substituted or unsubstituted C3-C8 cycloalkyl, a substituted or
unsubstituted 3 to 10
membered heterocyclyl, a substituted or unsubstituted C6-Cio aryl, a
substituted or
unsubstituted 5 to 10 membered heteroaryl, or L-Y. In some embodiments, Ri can
be
deuterium. In some embodiments, Ri can be hydroxyl. In some embodiments, Ri
can be
halogen, for example, fluoro, chloro, or bromo. In some embodiments, Ri can be
nitro. In
some embodiments, Ri can be a substituted amino, for example, a (Ci-C6
alkyl)amino, a (3 to
membered heterocyclyl)amino or a (C6-Cio aryl)amino. In some embodiments, Ri
can be
-17-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
an unsubstituted amino. In some embodiments, Ri can be a substituted Ci-C6
alkyl which is
an unsubstituted Ci-C6 haloalkyl, for example, halomethyl, haloethyl, halo-n-
propyl,
haloisopropyl, halo-n-butyl, haloisobutyl, halo-sec-butyl, halo-t-butyl,
halopentyl (straight-
chained or branched), or halohexyl (straight-chained or branched). In some
embodiments, Ri
can be an unsubstituted Ci-C6 fluoroalkyl. In some embodiments, Ri can be an
unsubstituted
Ci-C6 chloroalkyl. In some embodiments, Ri can be an unsubstituted Ci-C6
haloalkyl
including both fluorine and chlorine. In some embodiments, Ri can be a
substituted or
unsubstituted Ci-C6 alkoxy, for example, methoxy, ethoxy, n-propoxy,
isopropoxy, n-butoxy,
isobutoxy, sec-butoxy, t-butoxy, pentoxy (straight-chained or branched), or
hexoxy (straight-
chained or branched). In some embodiments, Ri can be an unsubstituted Ci-C6
alkoxy, for
example, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec-
butoxy, t-
butoxy, pentoxy (straight-chained or branched), or hexoxy (straight-chained or
branched). In
some embodiments, Ri can be a substituted or unsubstituted Ci-C6 alkyl, for
example,
methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, t-butyl,
pentyl (straight-
chained or branched), or hexyl (straight-chained or branched). In some
embodiments, Ri can
be an unsubstituted Ci-C6 alkyl, for example, methyl, ethyl, n-propyl,
isopropyl, n-butyl,
isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or branched), or hexyl
(straight-chained
or branched). In some embodiments, Ri can be a substituted or unsubstituted C2-
C6 alkenyl,
for example, methylene, ethylene, n-propylene, isopropylene, n-butylene,
isobutylene, sec-
butylene, t-butylene, pentylene (straight-chained or branched), or hexylene
(straight-chained
or branched). In some embodiments, Ri can be an unsubstituted C2-C6 alkenyl,
for example,
methylene, ethylene, n-propylene, isopropylene, n-butylene, isobutylene, sec-
butylene, t-
butylene, pentylene (straight-chained or branched), or hexylene (straight-
chained or
branched). In some embodiments, Ri can be a substituted or unsubstituted C3-C8
cycloalkyl,
for example, a C3-C8 monocyclic cycloalkyl or a C6-C8 bridged, fused, or spiro
bicyclic
cycloalkyl. In some embodiments, Ri can be an unsubstituted C3-C8 Cs
cycloalkyl, for
example, a C3-C8 monocyclic cycloalkyl or a C6-C8 bridged, fused, or spiro
bicyclic
cycloalkyl, such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl,
cyclooctyl, octahydropentalene, decahydronaphthalene, bicyclo[4.2.0]octane,
and
bicyclo[3.1.1]heptane. In some embodiments, Ri can be a substituted or
unsubstituted 3 to
membered heterocyclyl, for example, a 3 to 8 membered monocyclic heterocyclyl,
a 6 to 8
-18-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
membered bridged, fused, or spiro bicyclic heterocyclyl, or a 3 to 8 membered
nitrogen-
containing heterocyclyl. In some embodiments, Ri can be an unsubstituted 3 to
10
membered heterocyclyl, for example, a 3 to 8 membered monocyclic heterocyclyl,
a 6 to 8
membered bridged, fused, or spiro bicyclic heterocyclyl, or a 3 to 8 membered
nitrogen-
containing heterocyclyl. In some embodiments, Ri can be a substituted or
unsubstituted C6-
C10 aryl, for example, a phenyl or naphthyl. In some embodiments, Ri can be an
unsubstituted C6-Cio aryl such as phenyl or naphthyl. In some embodiments, Ri
can be a
substituted or unsubstituted 5 to 10 membered heteroaryl, for example, a 5
membered
heteroaryl, a 6 membered heteroaryl, a 10 membered heteroaryl, or a 5 to 10
membered
heteroaryl with one or two nitrogen atoms. In some embodiments, Ri can be an
unsubstituted 5 to 10 membered heteroaryl, for example, a 5 membered
heteroaryl, a 6
membered heteroaryl, a 10 membered heteroaryl, or a 5 to 10 membered
heteroaryl with one
or two nitrogen atoms. In some embodiments, Ri can be L-Y, for example:
0
õIon NH
N N
I I
0
0
N
HO
Nõ....--LeLN
I I
0
0
i
>2H 0 110 r11 N
I I
0
H *
N
>,4
H 0 I 0 NN/ N
I I
0
-19-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
H H
0
-s N N N
0
H H *
N = 0,NyN,
H 0 I
N
0
N C)N NH
H 0 I j
I I
0
HO
N
I I
0
HO
ofo
I I
0
N N NrNH
HO
0 >2µii NreLN
I I
0
HO
>2=1,ig rsINN =
0
I I
0 , or
N N.ArslrEll'v
HO
>2=11 401 NeLN =
0
I I
0 , where
"*"
represents the point of connection of the L-Y moiety to the rest of the
molecule. In some
embodiments, when Ri can be L-Y, none of R3A, R3B, R3C, R3D, and R3E can be L-
Y.
[0080] In some embodiments, R2 can be Hydrogen, deuterium, a
substituted or
unsubstituted C i-C6 alkyl, a substituted or unsubstituted C2-C6 alkenyl,
acyl, or ¨(S02)-Ci-C6
alkyl. In some embodiments, R2 can be hydrogen. In some embodiments, R2 can be
-20-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
deuterium. In some embodiments, R2 can be a substituted or unsubstituted Ci-
C6, for
example, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, t-
butyl, pentyl
(straight-chained or branched), or hexyl (straight-chained or branched). In
some
embodiments, R2 can be an unsubstituted Ci-C6, for example, methyl, ethyl, n-
propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or
branched), or hexyl
(straight-chained or branched). In some embodiments, R2 can be a substituted
or
unsubstituted C2-C6 alkenyl, for example, methylene, ethylene, n-propylene,
isopropylene, n-
butylene, isobutylene, sec-butylene, t-butylene, pentylene (straight-chained
or branched), or
hexylene (straight-chained or branched). In some embodiments, R2 can be an
unsubstituted
C2-C6 alkenyl, for example, methylene, ethylene, n-propylene, isopropylene, n-
butylene,
isobutylene, sec-butylene, t-butylene, pentylene (straight-chained or
branched), or hexylene
(straight-chained or branched). In some embodiments, R2 can be acyl, for
example, a Ci-C6
alkyl carbonyl such as acetyl (ethan-l-one), propan-l-one, or 3-methylbutan-1-
one. In some
embodiments, R2 can be ¨(S02)-Ci-C6 alkyl, for example, ¨(S02)-methyl, ¨(S02)-
ethyl, ¨
(S02)-n-propyl, ¨(S 02)-i sopropyl, ¨(S 02)-n-butyl, ¨(S 02)-i sobutyl, ¨(S02)-
sec-butyl, ¨
(S02)-t-butyl, ¨(S02)-pentyl (straight-chained or branched), or ¨(S02)-hexyl
(straight-
chained or branched).
[0081] In
some embodiments, n can be 1, 2, or 3. In some embodiments, n can be
1 or 2. In some embodiments, n can be 1. In some embodiments, n can be 2.
[0082] In
some embodiments, each of R3A, R3B, R3C, R3D, and R3E can be
independently Hydrogen, deuterium, hydroxyl, halogen, nitro, a substituted or
unsubstituted
amino, a substituted or unsubstituted Ci-C6 alkoxy, a substituted or
unsubstituted Ci-C6
alkyl, a substituted or unsubstituted C2-C6 alkenyl, a substituted or
unsubstituted C3-C8
cycloalkyl, a substituted or unsubstituted 3 to 10 membered heterocyclyl, a
substituted or
unsubstituted alkoxyalkyl, a substituted or unsubstituted cycloalkylalkyl, a
substituted or
unsubstituted heterocyclylalkyl, a substituted or unsubstituted aralkyl, a
substituted or
unsubstituted heteroaralkyl, or L-Y. In some embodiments, one or more of R3A,
R3B, R3C,
R3D, and R3E can be hydrogen. In some embodiments, one or more of R3A, R3B,
R3C, R3D, and
R3E can be deuterium. In some embodiments, one or more of R3A, R3B, R3C, R3D,
and R3E can
be hydroxyl. In some embodiments, one or more of R3A, R3B, R3C, R3D, and R3E
can be
halogen, for example, fluoro, chloro, or bromo. In some embodiments, one or
more of R3A,
-21-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
R3B, R3C, R3D, and R3E can be nitro. In some embodiments, one or more of R3A,
R3B, R3C,
R3D, and R3E can be a substituted amino, for example, a (Ci-C6 alkyl)amino, a
(3 to 10
membered heterocyclyl)amino or a (C6-Cio aryl)amino. In some embodiments, one
or more
of R3A, R3B, R3C, R3D, and R3E can be an unsubstituted amino. In some
embodiments, one or
more of R3A, R3B, R3C, R3D, and R3E can be a substituted Cl-C6 alkyl which is
an
unsubstituted C haloalkyl, for example, halomethyl, haloethyl, halo-n-
propyl,
haloisopropyl, halo-n-butyl, haloisobutyl, halo-sec-butyl, halo-t-butyl,
halopentyl (straight-
chained or branched), or halohexyl (straight-chained or branched). In some
embodiments,
one or more of R3A, R3B, R3C, R3D, and R3E can be an unsubstituted Cl-C6
fluoroalkyl. In
some embodiments, one or more of R3A, R3B, R3C, R3D, and R3E can be an
unsubstituted
Ci-
C6 chloroalkyl. In some embodiments, one or more of R3A, R3B, R3C, R3D, and
R3E can be an
unsubstituted Cl-C6 haloalkyl including both fluorine and chlorine. In some
embodiments,
one or more of R3A, R3B, R3C, R3D, and R3E can be a substituted or
unsubstituted Cl-C6
alkoxy, for example, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy,
isobutoxy, sec-
butoxy, t-butoxy, pentoxy (straight-chained or branched), or hexoxy (straight-
chained or
branched). In some embodiments, one or more of R3A, R3B, R3C, R3D, and R3E can
be an
unsubstituted Cl-C6 alkoxy, for example, methoxy, ethoxy, n-propoxy,
isopropoxy, n-butoxy,
isobutoxy, sec-butoxy, t-butoxy, pentoxy (straight-chained or branched), or
hexoxy (straight-
chained or branched). In some embodiments, one or more of R3A, R3B, R3C, R3D,
and R3E can
be a substituted or unsubstituted Cl-C6 alkyl, for example, methyl, ethyl, n-
propyl, isopropyl,
n-butyl, isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or branched),
or hexyl (straight-
chained or branched). In some embodiments, one or more of R3A, R3B, R3C, R3D,
and R3E can
be an unsubstituted Cl-C6 alkyl, for example, methyl, ethyl, n-propyl,
isopropyl, n-butyl,
isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or branched), or hexyl
(straight-chained
or branched). In some embodiments, one or more of R3A, R3B, R3C, R3D, and R3E
can be a
substituted or unsubstituted C2-C6 alkenyl, for example, methylene, ethylene,
n-propylene,
isopropylene, n-butylene, isobutylene, sec-butylene, t-butylene, pentylene
(straight-chained
or branched), or hexylene (straight-chained or branched). In some embodiments,
one or
more of R3A, R3B, R3C, R3D, and R3E can be an unsubstituted C2-C6 alkenyl, for
example,
methylene, ethylene, n-propylene, isopropylene, n-butylene, isobutylene, sec-
butylene, t-
butylene, pentylene (straight-chained or branched), or hexylene (straight-
chained or
-22-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
branched). In some embodiments, one or more of R3A, R3B, R3C, R3D, and R3E can
be a
substituted or unsubstituted C3-C8 cycloalkyl, for example, a C3-C8 monocyclic
cycloalkyl or
a C6-C8 bridged, fused, or spiro bicyclic cycloalkyl. In some embodiments, one
or more of
R3A, R3B, R3C, R3D, and R3E can be an unsubstituted C3-C8 cycloalkyl, for
example, a C3-C8
monocyclic cycloalkyl or a C6-C8 bridged, fused, or spiro bicyclic cycloalkyl,
such as
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl,
cycloheptyl, cyclooctyl,
octahydropentalene, decahydronaphthalene, bicyclo[4.2.0]octane, and
bicyclo[3.1.1]heptane.
In some embodiments, one or more of R3A, R3B, R3C, R3D, and R3E can be a
substituted or
unsubstituted 3 to 10 membered heterocyclyl, for example, a 3 to 8 membered
monocyclic
heterocyclyl, a 6 to 8 membered bridged, fused, or spiro bicyclic
heterocyclyl, or a 3 to 8
membered nitrogen-containing heterocyclyl. In some embodiments, one or more of
R3A, R3B,
R3C, R3D, and R3E can be an unsubstituted 3 to 10 membered heterocyclyl, for
example, a 3 to
8 membered monocyclic heterocyclyl, a 6 to 8 membered bridged, fused, or spiro
bicyclic
heterocyclyl, or a 3 to 8 membered nitrogen-containing heterocyclyl. In some
embodiments,
one or more of R3A, R3B, R3C, R3D, and R3E can be a substituted or
unsubstituted alkoxyalkyl,
for example, a Ci-C4 alkoxy, as described herein and a Ci-C4 alkyl, as
described herein. In
some embodiments, one or more of R3A, R3B, R3C, R3D, and R3E can be an
unsubstituted
alkoxyalkyl, for example, a Ci-C4 alkoxy, as described herein and a Ci-C4
alkyl, as described
herein, such as methoxymethyl, ethoxymethyl, or ethoxypropyl. In some
embodiments, one
or more of R3A, R3B, R3C, R3D, and R3E can be a substituted or unsubstituted
cycloalkylalkyl,
for example, a C3-C8 monocyclic cycloalkyl or a C6-C8 bridged, fused, or spiro
bicyclic
cycloalkyl and a Ci-C6 alkyl, as described herein. In some embodiments, one or
more of
R3A, R3B, R3C, R3D, and R3E can be an unsubstituted cycloalkylalkyl, for
example, a C3-C8
monocyclic cycloalkyl or a C6-C8 bridged, fused, or spiro bicyclic cycloalkyl
and a Ci-C6
alkyl, as described herein, such as cyclopropylmethyl, cyclobutylmethyl,
cyclopentylethyl, or
cyclohexylethyl. In some embodiments, one or more of R3A, R3B, R3C, R3D, and
R3E can be a
substituted or unsubstituted heterocyclylalkyl, for example, a 3 to 8 membered
monocyclic
heterocyclyl, a 6 to 8 membered bridged, fused, or spiro bicyclic
heterocyclyl, or a 3 to 8
membered nitrogen-containing heterocyclyl, and a Ci-C6 alkyl, as described
herein. In some
embodiments, one or more of R3A, R3B, R3C, R3D, and R3E can be an
unsubstituted
heterocyclylalkyl, for example, a 3 to 8 membered monocyclic heterocyclyl, a 6
to 8
-23-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
membered bridged, fused, or spiro bicyclic heterocyclyl, or a 3 to 8 membered
nitrogen-
containing heterocyclyl, and a Ci-C6 alkyl, as described herein, such as
pyrrolidinylmethyl,
piperidinylmethyl, piperazinylmethyl, or morpholinomethyl. In some
embodiments, one or
more of R3A, R3B, R3C, R3D, and R3E can be a substituted or unsubstituted
aralkyl, for
example, a C6-Cio aryl such as phenyl or naphthyl and a Ci-C6 alkyl, as
described herein. In
some embodiments, one or more of R3A, R3B, R3C, R3D, and R3E can be an
unsubstituted
aralkyl, for example, a C6-Cio aryl such as phenyl or naphthyl and a Ci-C6
alkyl, as described
herein, such as benzyl or phenethyl. In some embodiments, one or more of R3A,
R3B, R3C,
R3D, and R3E can be a substituted or unsubstituted heteroaralkyl, for example,
a 5 membered
heteroaryl, a 6 membered heteroaryl, a 10 membered heteroaryl, or a 5 to 10
membered
heteroaryl with one or two nitrogen atoms, and a Ci-C6 alkyl, as described
herein. In some
embodiments, one or more of R3A, R3B, R3C, R3D, and R3E can be an
unsubstituted
heteroaralkyl, for example, a 5 membered heteroaryl, a 6 membered heteroaryl,
a 10
membered heteroaryl, or a 5 to 10 membered heteroaryl with one or two nitrogen
atoms, and
a Ci-C6 alkyl, as described herein, such as pyridinylmethyl,
pyrimidinylmethyl, or
imidazolomethyl. In some embodiments, one of R3A, R3B, R3C, R3D, and R3E can
be L-Y, for
example:
0
õIon NH
N N
I I
0
0
ON*
H 0
õg
õNIeLN
0
0
i
>2H 0 110 r11 N
I I
0
H *
N
>,4
H 0 I 0 NN/ N
I I
0
-24-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
H H
N ONyrµl*
110 I 0
N N N
II
0
H H *
N
H 0 I 0 >,,.N ,..1g NN"N
= 0
NH
H 0
>2sii rsieLN =
I I
0
H 0
õNõg N'NN
I I
0
H 0
>21Ig Nõ,,---õeL.N
=
I I
0
NH
H 0
0
>2v,g 411 NreLN =
I I
0
N ON0e=yN,v
H 0
>2=1,ig e=INN =
0
I I
0 , or
o
>,N,g
H rN, 41, INnrEN1*
N NN 0
II
0 , where
"*"
represents the point of connection of the L-Y moiety to the rest of the
molecule.
[0083] In some embodiments, when any one of R3A, R3B, R3C, R3D, and
R3E can be
L-Y, Ri cannot be L-Y. In some embodiments, R3A cannot be hydrogen. In some
embodiments, R3B cannot be hydrogen. In some embodiments, R3C cannot be
hydrogen. In
-25-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
some embodiments, R3D cannot be hydrogen. In some embodiments, R3E cannot be
hydrogen.
[0084] In some embodiments, L can be ¨Z1-(R4)t-Z2¨; ¨Z1-(R4-0-R4)t-
Z2¨;
¨Z1(R4-NH-R4)t-Z2¨; ¨Z1-(R4-(NHCO)-R4)t-Z2¨; or ¨Z1-(R4-(CONH)-R4)t-Z2¨. In
some
embodiments, L can be ¨Z1-(R4-0-R4)t-Z2¨. In
other embodiments, L can be
¨Z1(R4-NH-R4)t-Z2¨. In still other embodiments, L can be ¨Z1-(R4-(NHCO)-R4)t-
Z2¨. In
some embodiments, L can be Z1-(R4-(CONH)-R4)t-Z2¨. In other embodiments, L can
be
¨Z1-(R4)t-Z2¨. In still other embodiments, L can be ¨Z1-(R4-(NHC(0)NH)-R4)t-
Z2¨.
[0085] In some embodiments, each R4 can be independently an
unsubstituted C1-
C6 alkylene, for example, methylene, ethylene, n-propylene, isopropylene, n-
butylene,
isobutylene, sec-butylene, t-butylene, pentylene (straight-chained or
branched), or hexylene
(straight-chained or branched). In some embodiments, each R4 group is the
same. In some
embodiments, each R4 group is different.
[0086] In some embodiments, t can be 1, 2, 3, 4, 5, or 6. In some
embodiments, t
can be 1. In some embodiments, t can be 2. In some embodiments, t can be 3. In
some
embodiments, t can be 4. In some embodiments, t can be 5. In some embodiments,
t can be
6.
[0087] Some embodiments of L are shown in Table A, below.
Table A
Zi Z2
¨Z1-(R4-0-R4)t-Z2¨ ¨CH2NH(C0)¨ ¨NH¨
1
¨Z1-(R4-0-R4)t-Z2¨ ¨NH¨ ¨NH¨
1
¨Z1-(R4-0-R4)t-Z2¨ ¨0¨ ¨NH¨
1
¨Z1-(R4-0-R4)t-Z2¨ ¨CH2¨ ¨NH¨
1
¨Z1-(R4-0-R4)t-Z2¨ ¨NH(C0)¨ ¨NH¨
1
¨Z1-(R4-0-R4)t-Z2¨ ¨CH2NH¨ ¨NH¨
1
¨Z1-(R4-0-R4)t-Z2¨ ¨NHCH2¨ ¨NH¨
1
¨Z1-(R4-0-R4)t-Z2¨ ¨CH2NH(C0)¨ ¨0¨
1
¨Z1-(R4-0-R4)t-Z2¨ ¨NH¨ ¨0¨
1
¨Z1-(R4-0-R4)t-Z2¨ -0- -0-
1
-26-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
-Z 1-(R4 - 0 -R4)t-Z 2- -CH2- -0- 1
-Z1-(R4-0-R4)t-Z 2- -1\11-1(C0)- -0-
1
-Z1-(R4-0-R4)t-Z 2- -CH2N1-1- -0- 1
-Z1-(R4-0-R4)t-Z 2- -NEICH2- -0- 1
-Z1-(R4-0-R4)t-Z 2- -CH2N1-1(C0)- -CH2-
1
-Z1-(R4-0-R4)t-Z 2- -NH- -CH2-
1
-Z1-(R4-0-R4)t-Z 2- -0- -CH2-
1
-Z1-(R4-0-R4)t-Z 2- -CH2- -CH2-
1
-Z1-(R4-0-R4)t-Z 2- -1\11-1(C0)- -CH2-
1
-Z1-(R4-0-R4)t-Z 2- -CH2N1-1- -CH2-
1
-Z1-(R4-0-R4)t-Z 2- -NEICH2- -CH2-
1
-Z1-(R4-0-R4)t-Z 2- -CH2N1-1(C0)- -1\11-
1(C0)- 1
-Z1-(R4-0-R4)t-Z 2- -NH- -1\11-
1(C0)- 1
-Z1-(R4-0-R4)t-Z 2- -0- -1\11-
1(C0)- 1
-Z1-(R4-0-R4)t-Z 2- -CH2- -1\11-
1(C0)- 1
-Z1-(R4-0-R4)t-Z 2- -1\11-1(C0)- -1\11-
1(C0)- 1
-Z1-(R4-0-R4)t-Z 2- -CH2N1-1- -1\11-
1(C0)- 1
-Z1-(R4-0-R4)t-Z 2- -NEICH2- -1\11-
1(C0)- 1
-Z1-(R4-0-R4)t-Z 2- -CH2N1-1(C0)- -(CO)N1-
1- 1
-Z1-(R4-0-R4)t-Z 2- -NH- -(CO)N1-
1- 1
-Z1-(R4-0-R4)t-Z 2- -0- -(CO)N1-
1- 1
-Z1-(R4-0-R4)t-Z 2- -CH2- -(CO)N1-
1- 1
-Z1-(R4-0-R4)t-Z 2- -1\11-1(C0)- -(CO)N1-
1- 1
-Z1-(R4-0-R4)t-Z 2- -CH2N1-1- -(CO)N1-
1- 1
-Z1-(R4-0-R4)t-Z 2- -NEICH2- -(CO)N1-
1- 1
-Z1-(R4-0-R4)t-Z 2- -CH2N1-1(C0)- -CH2N1-
1- 1
-Z1-(R4-0-R4)t-Z 2- -NH- -CH2N1-1-
1
-Z1-(R4-0-R4)t-Z 2- -0- -CH2N1-1-
1
-Z1-(R4-0-R4)t-Z 2- -CH2- -CH2N1-1-
1
-27-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
-Z 1-(1t4 - 0 -R4)t-Z 2- -NH(C 0)- -CH2NH-
1
-Z 1 -(R4 - 0 -R4)t-Z 2- -CH2NH- -CH2NH-
1
-Z 1 -(R4 - 0 -R4)t-Z 2- -NHCH2- -CH2NH-
1
-Z 1 -(R4 - 0 -R4)t-Z 2- -CH2NH(C 0)- -NHCH2-
1
-Z 1 -(R4 - 0 -R4)t-Z 2- -NH- -NHCH2-
1
-Z 1 -(R4 - 0 -R4)t-Z 2- -0- -NHCH2-
1
-Z 1 -(R4 - 0 -R4)t-Z 2- -CH2- -NHCH2-
1
-Z 1 -(R4 - 0 -R4)t-Z 2- -NH(C 0)- -NHCH2-
1
-Z 1 -(R4 - 0 -R4)t-Z 2- -CH2NH- -NHCH2-
1
-Z 1 -(R4 - 0 -R4)t-Z 2- -NHCH2- -NHCH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH(C 0)- -NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH- -NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -0- -NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2- -NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH(C 0)- -NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH- -NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NHCH2- -NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH(C 0)- -0-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH- -0- 1
-Z 1(1t4 -NH-R4)t-Z 2- -0- -0- 1
-Z 1(1t4 -NH-R4)t-Z 2- -CH2- -0- 1
-Z 1 (R4 -NH-R4)t-Z 2- -NH(C 0)- -0- 1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH- -0- 1
-Z 1 (R4 -NH-R4)t-Z 2- -NHCH2- -0- 1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH(C 0)- -CH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH- -CH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -0- -CH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2- -CH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH(C 0)- -CH2-
1
-28-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
-Z1 (R4 -NH-R4)t-Z2- -CH2NH- -CH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NHCH2- -CH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH(C 0)- -NH(C 0)-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH- -NH(C 0)-
1
-Z 1 (R4 -NH-R4)t-Z 2- -0- -NH(C 0)-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2- -NH(C 0)-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH(C 0)- -NH(C 0)-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH- -NH(C 0)-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NHCH2- -NH(C 0)-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH(C 0)- -(CO)NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH- -(CO)NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -0- -(CO)NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2- -(CO)NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH(C 0)- -(CO)NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH- -(CO)NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NHCH2- -(CO)NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH(C 0)- -CH2NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH- -CH2NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -0- -CH2NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2- -CH2NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH(C 0)- -CH2NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH- -CH2NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NHCH2- -CH2NH-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH(C 0)- -NHCH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH- -NHCH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -0- -NHCH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2- -NHCH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -NH(C 0)- -NHCH2-
1
-Z 1 (R4 -NH-R4)t-Z 2- -CH2NH- -NHCH2-
1
-29-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
-Z 1(t4 -NH-R4)t-Z 2- -NEICH2- -NEICH2- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2NI-1(C 0)- -NH-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -NH- -NH-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -0- -NH-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2- -NH-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -1\11-1(C 0)- -NH-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2N1-1- -NH-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -NEICH2- -NH-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2NI-1(C 0)- -0-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -NH- -0- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -0- -0- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2- -0- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -1\11-1(C 0)- -0-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2N1-1- -0- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -NEICH2- -0- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2NI-1(C 0)- -CH2-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -NH- -CH2-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -0- -CH2-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2- -CH2-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -1\11-1(C 0)- -CH2-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2N1-1- -CH2-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -NEICH2- -CH2-
1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2NI-1(C 0)- -1\11-
1(C 0)- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -NH- -1\11-
1(C 0)- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -0- -1\11-
1(C 0)- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2- -1\11-
1(C 0)- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -1\11-1(C 0)- -1\11-
1(C 0)- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -CH2N1-1- -1\11-
1(C 0)- 1
-Z 1-(R4 -(NE1C 0)-R4)t-Z 2- -NEICH2- -1\11-
1(C 0)- 1
-30-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
-Z1-(R4-(NHCO)-R4)t-Z2- -CH2NH(C0)- -(CO)N1-
1- 1
-Z1-(R4-(NHCO)-R4)t-Z2- -NH- -(CO)N1-
1- 1
-Z1-(R4-(NHCO)-R4)t-Z2- -0- -(CO)N1-
1- 1
-Z1-(R4-(NHCO)-R4)t-Z2- -CH2- -(CO)N1-
1- 1
-Z1-(R4-(NHCO)-R4)t-Z2- -NH(C0)- -(CO)N1-
1- 1
-Z1-(R4-(NHCO)-R4)t-Z2- -CH2NI-1- -(CO)N1-
1- 1
-Z1-(R4-(NHCO)-R4)t-Z2- -NEICH2- -(CO)N1-
1- 1
-Z1-(R4-(NHCO)-R4)t-Z2- -CH2NH(C0)- -CH2NI-1-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -NH- -CH2NI-1-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -0- -CH2NI-1-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -CH2- -CH2NI-1-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -NH(C0)- -CH2NI-1-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -CH2NI-1- -CH2NI-1-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -NEICH2- -CH2NI-1-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -CH2NH(C0)- -NEICH2-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -NH- -NEICH2-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -0- -NEICH2-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -CH2- -NEICH2-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -NH(C0)- -NEICH2-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -CH2NI-1- -NEICH2-
1
-Z1-(R4-(NHCO)-R4)t-Z2- -NEICH2- -NEICH2-
1
-Z1-(R4-(CONH)-R4)t-Z2- -CH2NH(C0)- -NH-
1
-Z1-(R4-(CONH)-R4)t-Z2- -NH- -NH-
1
-Z1-(R4-(CONH)-R4)t-Z2- -0- -NH-
1
-Z1-(R4-(CONH)-R4)t-Z2- -CH2- -NH-
1
-Z1-(R4-(CONH)-R4)t-Z2- -NH(C0)- -NH-
1
-Z1-(R4-(CONH)-R4)t-Z2- -CH2NI-1- -NH-
1
-Z1-(R4-(CONH)-R4)t-Z2- -NEICH2- -NH-
1
-Z1-(R4-(CONH)-R4)t-Z2- -CH2NH(C0)- -0-
1
-31-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
-Z1-(R4-(CONE1)-R4)t-Z2- -NH- -0- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -0- -0- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2- -0- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -1\11-1(C0)- -0-
1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2N1-1- -0- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -NEICH2- -0- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2N1-1(C0)- -CH2-
1
-Z1-(R4-(CONE1)-R4)t-Z2- -NH- -CH2-
1
-Z1-(R4-(CONE1)-R4)t-Z2- -0- -CH2-
1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2- -CH2-
1
-Z1-(R4-(CONE1)-R4)t-Z2- -1\11-1(C0)- -CH2-
1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2N1-1- -CH2-
1
-Z1-(R4-(CONE1)-R4)t-Z2- -NEICH2- -CH2-
1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2N1-1(C0)- -1\11-
1(C0)- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -NH- -1\11-
1(C0)- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -0- -1\11-
1(C0)- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2- -1\11-
1(C0)- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -1\11-1(C0)- -1\11-
1(C0)- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2N1-1- -1\11-
1(C0)- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -NEICH2- -1\11-
1(C0)- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2N1-1(C0)- -(CO)N1-
1- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -NH- -(CO)N1-
1- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -0- -(CO)N1-
1- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2- -(CO)N1-
1- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -1\11-1(C0)- -(CO)N1-
1- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2N1-1- -(CO)N1-
1- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -NEICH2- -(CO)N1-
1- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -CH2N1-1(C0)- -CH2N1-
1- 1
-Z1-(R4-(CONE1)-R4)t-Z2- -NH- -CH2N1-1-
1
-32-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -0- -CH2N1-1-
1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -CH2- -CH2N1-1-
1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -1\11-1(C 0)- -CH2N1-
1- 1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -CH2N1-1- -CH2N1-1-
1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -NEICH2- -CH2N1-1-
1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -CH2NI-1(C 0)- -
NEICH2- 1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -NH- -NEICH2-
1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -0- -NEICH2-
1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -CH2- -NEICH2-
1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -1\11-1(C 0)- -NEICH2-
1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -CH2N1-1- -NEICH2-
1
-Z 1- (R4 -(C ONE1)-R4)t-Z 2- -NEICH2- -NEICH2-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -CH2NI-1(C 0)- -NH-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -NH- -NH-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -0- -NH-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -CH2- -NH-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z 2- -1\11-1(C 0)- -NH-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -CH2N1-1- -NH-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -NEICH2- -NH-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -CH2NI-1(C 0)- -0-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -NH- -0- 1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -0- -0- 1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -CH2- -0- 1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z 2- -1\11-1(C 0)- -0-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -CH2N1-1- -0- 1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -NEICH2- -0- 1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -CH2NI-1(C 0)- -CH2-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -NH- -CH2-
1
-Z 1- (R4 -(NE1C (0)N1-1)-R0t-Z2- -0- -CH2-
1
-33-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2- -CH2-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NH(C 0)- -CH2-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2NH- -CH2-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NHCH2- -CH2-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2NH(C 0)- -NH(C 0)-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NH- -NH(C 0)-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -0- -NH(C 0)-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2- -NH(C 0)-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NH(C 0)- -NH(C 0)-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2NH- -NH(C 0)-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NHCH2- -NH(C 0)-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2NH(C 0)- -(CO)NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NH- -(CO)NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -0- -(CO)NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2- -(CO)NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NH(C 0)- -(CO)NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2NH- -(CO)NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NHCH2- -(CO)NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2NH(C 0)- -CH2NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NH- -CH2NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -0- -CH2NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2- -CH2NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NH(C 0)- -CH2NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2NH- -CH2NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NHCH2- -CH2NH-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2NH(C 0)- -NHCH2-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -NH- -NHCH2-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -0- -NHCH2-
1
-Z 1- (R4 -(NHC (0)NH)-R4)t-Z 2- -CH2- -NHCH2-
1
-34-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NH(C0)- -NHCH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2NH- -NHCH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NHCH2- -NHCH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2NH(C0)- -NH-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NH- -NH-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -0- -NH-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2- -NH-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NH(C0)- -NH-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2NH- -NH-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NHCH2- -NH-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2NH(C0)- -0-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NH- -0- 1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -0- -0- 1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2- -0- 1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NH(C0)- -0- 1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2NH- -0- 1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NHCH2- -0- 1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2NH(C0)- -CH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NH- -CH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -0- -CH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2- -CH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NH(C0)- -CH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2NH- -CH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NHCH2- -CH2-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2NH(C0)- -NH(C0)-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NH- -NH(C0)-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -0- -NH(C0)-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -CH2- -NH(C0)-
1
-Z 1-(R4-(NHC (0)NH)-R4)t-Z 2- -NH(C0)- -NH(C0)-
1
-35-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
L Zi Z2 t
¨Z 1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2NH¨
¨NH(C0)¨ 1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NHCH2¨
¨NH(C0)¨ 1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2NH(C0)¨
¨(CO)NH¨ 1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NH¨
¨(CO)NH¨ 1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨0¨
¨(CO)NH¨ 1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2¨
¨(CO)NH¨ 1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NH(C0)¨
¨(CO)NH¨ 1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2NH¨
¨(CO)NH¨ 1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NHCH2¨
¨(CO)NH¨ 1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2NH(C0)¨ ¨CH2NH¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NH¨ ¨CH2NH¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨0¨ ¨CH2NH¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2¨ ¨CH2NH¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NH(C0)¨ ¨CH2NH¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2NH¨ ¨CH2NH¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NHCH2¨ ¨CH2NH¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2NH(C0)¨ ¨NHCH2¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NH¨ ¨NHCH2¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨0¨ ¨NHCH2¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2¨ ¨NHCH2¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NH(C0)¨ ¨NHCH2¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨CH2NH¨ ¨NHCH2¨
1
¨Z1-(R4-(NHC(0)NH)-R4)t-Z2¨ ¨NHCH2¨ ¨NHCH2¨
1
[0088] In some embodiments of Table A, each R4 can independently be a
Ci-C4
alkylene, for example, methylene, ethylene, n-propylene, isopropylene, n-
butylene,
isobutylene, or sec-butylene. In some embodiments of Table A, each R4 is
methylene. In
some embodiments of Table A, each R4 is ethylene. In some embodiments of Table
A, each
R4 is n-propylene. In some embodiments of Table A, each R4 is n-butylene. In
some
-36-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
embodiments of Table A, each R4 can be the same. In some embodiments of Table
A, each
R4 can be different. For example, one R4 can be methylene and the other R4 can
be ethylene;
one R4 can be methylene and the other R4 can be n-propylene; one R4 can be
methylene and
the other R4 can be n-butylene; one R4 can be ethylene and the other R4 can be
n-propylene;
or one R4 can be ethylene and the other R4 can be n-butylene.
[0089] In some embodiments, Y can be
N ()NO
H 0
>'N'g NN N
8
, wherein Y can be derivatized to attach to L.
As used herein, the phrase "Y is derivatized to attach to L" is used as would
be understood
by one having ordinary skill in the art. For example, when Y is derivatized to
attached to L,
Y can be:
N
H 0 I ii H H 0 I ii
>'N 'g N N N >'N 'g NN N
0 0
N * C)1\11D
H 0 0
)1,
NN N *-S NNN
0 0 ,
or
N C)Nt.D
H 0
*,N,ii 101 NN*N
8
, wherein * represents the point of attachment
to the L group.
[0090] In
some embodiments, at least one of Yi, Y2, Y3, Y4, and Y5 is carbon
(e.g., CR3A, CR3u, CR3c, CR3D, and/or CR3E). In some embodiments, one of Yi,
Y2, Y3, Y4,
and Y5 is carbon. In some embodiments, two of Yi, Y2, Y3, Y4, and Y5 are
carbon. In some
embodiments, three of Yi, Y2, Y3, Y4, and Y5 are carbon. In some embodiments,
four of Yi,
Y2, Y3, Y4, and Y5 are carbon. In some embodiments, all five of Yi, Y2, Y3,
Y4, and Y5 are
carbon.
[0091] In
some embodiments, when Qi is CH2, then one or more of R3A, R3B, R3C,
R3D, and R3E cannot be hydrogen. In some embodiments, when Qi is a bond, then
one or
more of R3A, R3B, R3C, R3D, and R3E cannot be hydrogen. In some embodiments,
when Ri is
-37-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
L-Y, none of R3A, R3B, R3C, R3D, and R3E can be L-Y. In some embodiments, when
Qi is a
bond, Xi is hydrogen or methyl, and Q2 is CH2; then one of R1, R3A, R3B, R3C,
R3D, and R3E is
L-Y. In some embodiments, when Q2 can be a bond, Qi can be a bond or CH2.
[0092] In some embodiments, each Ri can be independently halogen (for
example, fluor , chloro, or bromo), a substituted or unsubstituted amino (for
example, -NH2,
dimethylamino, diethylamino, isopropylethylamino, phenylamino, or
benzylamino), an
unsubstituted Ci-C6 haloalkyl (for example, -CF3, -CHF2, -CH2F, -CH2CF3, or -
CH2CH2CF3), a substituted or unsubstituted Ci-C6 alkoxy (for example, (for
example,
methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec-butoxy, t-
butoxy, pentoxy
(straight-chained or branched), or hexoxy (straight-chained or branched)), or
a substituted or
unsubstituted Ci-C6 alkyl (for example, methyl, ethyl, n-propyl, isopropyl, n-
butyl, isobutyl,
sec-butyl, t-butyl, pentyl (straight-chained or branched), or hexyl (straight-
chained or
branched)). In some embodiments, each Ri can be independently halogen (for
example,
fluor , chloro, or bromo), an unsubstituted amino, an unsubstituted Ci-C6
haloalkyl, an
unsubstituted Ci-C6 alkoxy (for example, (for example, methoxy, ethoxy, n-
propoxy,
isopropoxy, n-butoxy, isobutoxy, sec-butoxy, t-butoxy, pentoxy (straight-
chained or
branched), or hexoxy (straight-chained or branched)), or unsubstituted Ci-C6
alkyl (for
example, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, t-
butyl, pentyl
(straight-chained or branched), or hexyl (straight-chained or branched)).
[0093] In some embodiments, each Ri can be independently fluoro,
chloro, -NH2,
-NH(CH3), -N(CH3)2, -CF3, -OCH3, -OCH2CH3, -OCH(CH3)2, -CH3, -CH2CH3 or
¨CH(CH3)2.
[0094] In some embodiments, Ring B can be selected from:
-38-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
R3A R3A R3A
N
R3B R3B / \ N/ \ R3 R3B / \ R3B --....e......"
R3c R3o c
R3D R3D R3D R3D , R3D ,
, , ,
R3A)...
R3A)..._..... N N
R3B--5/.........(-?-L. R3B ---..N
N
Nzzz(N
mi
R3 c R30 3E
r--.3c R3D R3D R3D R3D ,
, , , ,
N
).
....R.2.........
R3B / \
)---N R3E 0,
r--.3c R3
R3 Nzz-N R3E
' `3C R3D ,and R3D
[0095] In some
embodiments, each of R3A, R3B, R3C, R3D, and R3E can be
independently hydrogen, deuterium, hydroxyl, halogen (for example, fluoro,
chloro, or
bromo), nitro, a substituted or unsubstituted amino (for example, -NH2,
dimethylamino,
diethylamino, isopropylethylamino, phenylamino, or benzylamino), a substituted
or
unsubstituted Ci-C6 alkoxy (for example, (for example, methoxy, ethoxy, n-
propoxy,
isopropoxy, n-butoxy, isobutoxy, sec-butoxy, t-butoxy, pentoxy (straight-
chained or
branched), or hexoxy (straight-chained or branched)), or a substituted or
unsubstituted Ci-C6
alkyl (for example, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-
butyl, t-butyl,
pentyl (straight-chained or branched), or hexyl (straight-chained or
branched)), a substituted
or unsubstituted C2-C6 alkenyl (for example, ethylene, n-propylene,
isopropylene, n-
butylene, isobutylene, sec-butylene, t-butylene, pentylene (straight-chained
or branched), or
hexylene (straight-chained or branched)), a substituted or unsubstituted C3-C8
cycloalkyl (for
example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, or
cyclooctyl), a
substituted or unsubstituted 3 to 10 membered heterocyclyl (for example a 3 to
8 membered
monocyclic heterocyclyl containing one or two heteroatoms selected from oxygen
and
nitrogen, or a 6 to 8 membered bicyclic heterocyclyl containing one or two
heteroatoms
selected from oxygen and nitrogen), a substituted or unsubstituted alkoxyalkyl
(for example,
methoxymethyl, ethoxyethyl, or methoxy-t-butyl), a substituted or
unsubstituted
cycloalkylalkyl (for example, a C3-C8 cycloalkyl group such as cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl, or cyclooctyl, connected to the rest of
the compound
-39-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
via a Ci-C3 alkyl group such as methyl, ethyl, n-propyl, or isopropyl), a
substituted or
unsubstituted heterocyclylalkyl (for example, for example a 3 to 8 membered
monocyclic
heterocyclyl containing one or two heteroatoms selected from oxygen and
nitrogen,
connected to the rest of the compound via a Ci-C3 alkyl group such as methyl,
ethyl, n-
propyl, or isopropyl), a substituted or unsubstituted aralkyl (for example,
phenyl or naphthyl,
connected to the rest of the compound via a Ci-C3 alkyl group such as methyl,
ethyl, n-
propyl, or isopropyl), or a substituted or unsubstituted heteroaralkyl, for
example, a five, six,
or ten membered heteroaryl group containing either one oxygen, one nitrogen,
one oxygen
and one nitrogen, two nitrogens, or three nitrogens, connected to the rest of
the compound
via a Ci-C3 alkyl group such as methyl, ethyl, n-propyl, or isopropyl).
[0096] In some embodiments, each of R3A, R3B, R3C, R3D, and R3E can be
independently Hydrogen, deuterium, hydroxyl, halogen (for example, fluoro,
chloro, or
bromo), nitro, a substituted or unsubstituted amino (for example, ¨NH2,
dimethylamino,
diethylamino, isopropylethylamino, phenylamino, or benzylamino), an
unsubstituted Ci-C6
haloalkyl (for example, ¨CF3, ¨CHF2, ¨CH2F, ¨CH2CF3, or ¨CH2CH2CF3), an
unsubstituted
Ci-C6 alkoxy (for example, methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy,
isobutoxy,
sec-butoxy, t-butoxy, pentoxy (straight-chained or branched), or hexoxy
(straight-chained or
branched)), or unsubstituted Ci-C6 alkyl (for example, methyl, ethyl, n-
propyl, isopropyl, n-
butyl, isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or branched), or
hexyl (straight-
chained or branched)), an unsubstituted C2-C6 alkenyl (for example, ethylene,
n-propylene,
isopropylene, n-butylene, isobutylene, sec-butylene, t-butylene, pentylene
(straight-chained
or branched), or hexylene (straight-chained or branched)), an unsubstituted C3-
C8 cycloalkyl
(for example, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl,
or cyclooctyl),
an unsubstituted 3 to 10 membered heterocyclyl (for example a 3 to 8 membered
monocyclic
heterocyclyl containing one or two heteroatoms selected from oxygen and
nitrogen, or a 6 to
8 membered bicyclic heterocyclyl containing one or two heteroatoms selected
from oxygen
and nitrogen), an unsubstituted alkoxyalkyl (for example, methoxymethyl,
ethoxyethyl, or
methoxy-t-butyl), an unsubstituted cycloalkylalkyl (for example, a C3-C8
cycloalkyl group
such as cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, or
cyclooctyl,
connected to the rest of the compound via a Ci-C3 alkyl group such as methyl,
ethyl, n-
propyl, or isopropyl), an unsubstituted heterocyclylalkyl (for example, for
example a 3 to 8
-40-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
membered monocyclic heterocyclyl containing one or two heteroatoms selected
from oxygen
and nitrogen, connected to the rest of the compound via a Ci-C3 alkyl group
such as methyl,
ethyl, n-propyl, or isopropyl), an unsubstituted aralkyl (for example, phenyl
or naphthyl,
connected to the rest of the compound via a Ci-C3 alkyl group such as methyl,
ethyl, n-
propyl, or isopropyl), or unsubstituted heteroaralkyl (for example, a five,
six, or ten
membered heteroaryl group containing either one oxygen, one nitrogen, one
oxygen and one
nitrogen, two nitrogens, or three nitrogens, connected to the rest of the
compound via a Ci-C3
alkyl group such as methyl, ethyl, n-propyl, or isopropyl).
[0097] In some embodiments, each of R3A, R3B, R3C, R3D, and R3E can be
independently hydrogen, halogen (for example, fluoro, chloro, or bromo), an
unsubstituted
Ci-C6 haloalkyl (for example, ¨CF3, ¨CHF2, ¨CH2F, ¨CH2CF3, or ¨CH2CH2CF3), an
unsubstituted Ci-C6 alkoxy (for example, methoxy, ethoxy, n-propoxy,
isopropoxy, n-
butoxy, isobutoxy, sec-butoxy, t-butoxy, pentoxy (straight-chained or
branched), or hexoxy
(straight-chained or branched)), an unsubstituted Ci-C6 alkyl (for example,
methyl, ethyl, n-
propyl, isopropyl, n-butyl, isobutyl, sec-butyl, t-butyl, pentyl (straight-
chained or branched),
or hexyl (straight-chained or branched)), an unsubstituted 3 to 10 membered
heterocyclyl
(for example a 3 to 8 membered monocyclic heterocyclyl containing one or two
heteroatoms
selected from oxygen and nitrogen, or a 6 to 8 membered bicyclic heterocyclyl
containing
one or two heteroatoms selected from oxygen and nitrogen), or an unsubstituted
3 to 10
membered heterocyclylalkyl (for example a 3 to 8 membered monocyclic
heterocyclyl
containing one or two heteroatoms selected from oxygen and nitrogen, or a 6 to
8 membered
bicyclic heterocyclyl containing one or two heteroatoms selected from oxygen
and nitrogen,
connected to the rest of the compound by a methyl, ethyl, n-propyl, isopropyl,
n-butyl,
isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or branched), or hexyl
(straight-chained
or branched) group).
[0098] In some embodiments, one of R3A, R3B, R3C, R3D, and R3E can be
halogen(for example, fluoro, chloro, or bromo), an unsubstituted Ci-C6
haloalkyl, an
unsubstituted Ci-C6 alkoxy (for example, methoxy, ethoxy, n-propoxy,
isopropoxy, n-
butoxy, isobutoxy, sec-butoxy, t-butoxy, pentoxy (straight-chained or
branched), or hexoxy
(straight-chained or branched)), an unsubstituted Ci-C6 alkyl (for example,
methyl, ethyl, n-
propyl, isopropyl, n-butyl, isobutyl, sec-butyl, t-butyl, pentyl (straight-
chained or branched),
-41-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
or hexyl (straight-chained or branched)), an unsubstituted 3 to 10 membered
heterocyclyl
(for example a 3 to 8 membered monocyclic heterocyclyl containing one or two
heteroatoms
selected from oxygen and nitrogen, or a 6 to 8 membered bicyclic heterocyclyl
containing
one or two heteroatoms selected from oxygen and nitrogen), or an unsubstituted
3 to 10
membered heterocyclylalkyl (for example a 3 to 8 membered monocyclic
heterocyclyl
containing one or two heteroatoms selected from oxygen and nitrogen, or a 6 to
8 membered
bicyclic heterocyclyl containing one or two heteroatoms selected from oxygen
and nitrogen,
connected to the rest of the compound by a methyl, ethyl, n-propyl, isopropyl,
n-butyl,
isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or branched), or hexyl
(straight-chained
or branched) group) and the other of R3A, R3B, R3C, R3D, and R3E are hydrogen.
[0099] In
some embodiments, one of R3A, R3B, R3C, R3D, and R3E can be fluor ,
chloro, -CF3, -OCH3, an unsubstituted Ci-C6 alkyl (for example, methyl, ethyl,
n-propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or
branched), or hexyl
(straight-chained or branched)), an unsubstituted 3 to 10 membered
heterocyclyl (for
example a 3 to 8 membered monocyclic heterocyclyl containing one or two
heteroatoms
selected from oxygen and nitrogen, or a 6 to 8 membered bicyclic heterocyclyl
containing
one or two heteroatoms selected from oxygen and nitrogen), or an unsubstituted
3 to 10
membered heterocyclylalkyl (for example a 3 to 8 membered monocyclic
heterocyclyl
containing one or two heteroatoms selected from oxygen and nitrogen, or a 6 to
8 membered
bicyclic heterocyclyl containing one or two heteroatoms selected from oxygen
and nitrogen,
connected to the rest of the compound by a methyl, ethyl, n-propyl, isopropyl,
n-butyl,
isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or branched), or hexyl
(straight-chained
or branched) group) and the other of R3A, R3B, R3C, R3D, and R3E are hydrogen.
[0100] In
some embodiments, one of R3A, R3B, R3C, R3D, and R3E can be selected
from:
OH 3'2CN `1(
NH , H ,
or
, and the other of R3A, R3B, R3C, R3D, and R3E can be hydrogen.
-42-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0101] In some embodiments, one Ri can be L-Y. In some embodiments,
one of
R3A, R3B, R3C, R3D, and R3E can be L-Y. In some embodiments, Y3 can be C-L-Y.
[0102] In some embodiments, L can be ¨Z1-(R4-0-R4)t-Z2¨. In
some
embodiments, L can be ¨Zi(R4-NH-R4)t-Z2¨. In some embodiments, L can be Z1-(R4-
(NHCO)-R4)t-Z2¨. In some embodiments, L can be ¨Zi-(R4-(CONH)-R4)t-Z2¨. In
other
embodiments, L can be ¨Zi-(R4-(NHC(0)NH)-R4)t-Z2¨. In some embodiments of this
paragraph, t is 1. In some embodiments of this paragraph, t is 2.
[0103] In some embodiments, Zi can be ¨NH¨. In some embodiments, Zi
can be
¨0¨. In some embodiments, Zi can be ¨CH2¨. In some embodiments, Zi can be
¨NH(C0)¨.
In some embodiments, Zi can be ¨CH2NH¨. In some embodiments, Zi can be
¨NHCH2¨. In
some embodiments, Z2 can be ¨NH¨. In some embodiments, Z2 can be ¨0¨. In some
embodiments, Z2 can be ¨CH2¨. In some embodiments, Z2 can be ¨NH(C0)¨. In some
embodiments, Z2 can be ¨(CO)NH¨. In some embodiments, Z2 can be ¨CH2NH¨. In
some
embodiments, Z2 can be ¨NHCH2¨. In some embodiments of this paragraph, Zi and
Z2 are
the same. In some embodiments of this paragraph, Zi and Z2 are different. In
some
embodiments of this paragraph, when Zi can be ¨NH¨, Z2 can be ¨NH¨. In some
embodiments of this paragraph, when Zi can be ¨0¨, Z2 can be ¨0¨. In some
embodiments
of this paragraph, when Zi can be ¨CH2¨, Z2 can be ¨CH2¨. In some embodiments
of this
paragraph, when Zi can be ¨NH(C0)¨, Z2 can be ¨NH(C0)¨. In some embodiments of
this
paragraph, when Zi can be ¨CH2NH¨, Z2 can be ¨CH2NH¨. In some embodiments of
this
paragraph, when Zi can be¨CH2NH(C0)¨, Z2 can be ¨NH¨. In some embodiments of
this
paragraph, when Zi can be¨CH2NH(C0)¨, Z2 can be ¨0¨. In some embodiments of
this
paragraph, when Zi can be¨CH2NH(C0)¨, Z2 can be ¨CH2¨. In some embodiments of
this
paragraph, when Zi can be¨CH2NH(C0)¨, Z2 can be ¨NH(C0)¨. In some embodiments
of
this paragraph, when Zi can be¨CH2NH(C0)¨, Z2 can be ¨CH2NH¨.
[0104] In some embodiments, each R4 can be independently an
unsubstituted C1-
C4 alkylene, for example, methylene, ethylene, n-propylene, isopropylene, n-
butylene, sec-
butylene, or t-butylene. In some embodiments, each R4 can be independently an
unsubstituted Ci-C2 alkylene, such as methylene or ethylene.
-43-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0105] In some
embodiments, t can be 1. In some embodiments, t can be 2. In
some embodiments, t can be 3. In some embodiments, t can be 4. In some
embodiments, t
can be 5. In some embodiments, t can be 6.
[0106] In some
embodiments, the compound of Formula (I) is selected from:
00 H 0 0 H 0 0 H
....1µ..z1No SI N'.= 0 N..= IIjN.. =
0 0 0
110 101 CI
40 10
(1µ1 (N N
0) 0) C.)
, , ,
0 0 H 0 0 H 00 H
=ININ.,,,D ININ.,,,D
N ..= N ..= 10 NI..
o/
N/
N/
0 0 6113 0 H
10 0
rN rN rN
0) 0) 0)
, , ,
0 o H
0 0 H .....N.:.,0
.._..NyIN,,o is N...
N ..=
H 0 0 N OON 0
I
-...õ....õ-N,g N,..---õN...,-,LN " o
8 H H
0 0
rN
.0) N
H
-44-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
00 H
H ..=
0 N . NN
H 0 1 H
.vNI,g
NN N C) (P1 N
0
6 H H
(101
rN
0J
,
00 H
yIN.,,,c)
0 o H
....Nsio
0
0S 0
LI 0 0 I H 0
,..,..11.g N.----.NN
8 H H
NH2
,
00 H 0 0 H
F
_...N../N,,,,,o 0 0 0 H
....:1...T.0 NI 0 0
0 HN)NNIH
*
0 0
SL
0 0 ,s
0-11
N)*/\/(3 0 N 0 Nr....-.)
H 0 0
00 H 0 0
0 N .......7
No
00 H 00 H
_....N_N:o 0 0 0
0 0 0 0
r0 NK. I
0 NH2 5 Nj o o
, , , N ,
-45-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
0 0 H
._....N..z1Ne.õ*0
00 H 0
.......NyINseo H
CI
0 N N
so 0 Y I
el Ny,,...
NAN/\) NH
-....,
0
0
0 N
H H
,====\rõ..-
S,
N)L):11 No NN 11 Oi I -NH
OX
H H
, ,
00 H 00 H
110 N"ZIN/r
0
0
0
00 H
110 0 ._....:1õ......:;::.0
0
N---1 r"-0 N---1
1........23 101 Nj 1.........õ.0
, , ,
0 0 H
......N..x.1 0
0 0,µ H
0 N-0
N HN
0 II
HN N NH
50 5 5 H
1µ1. 0
S
Oil N
I
N111===,../\,,,,0 0
c,,
H 0
, ,
-46-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
0
F 1:3tEN41
N .. = 0
00 H
0 .......N. N;c1
H
1110 NyN
0110 y,,..... /
N
N 0
0 0
ilAm) 0 NH
le
0,.._
0""
...;S.,
NH N
..-"j< I........,,0
00
N .. = t Er1_0
0 0 0 H
H F
.....11.......N......*0
laS 0 0 N N
Y 1
Illi Ny.,.., NX
HN N NH
......1,
NH 0
0 1110 100 H
(1110 0
..õ.N,......õ..-
iS S
0'.. NH 0*I I
X N.---IL}10 0
H
0 0 H 00
H
......N..x.1 0
>r" H N
N .. = 0
H2N HN ===.s , _0,0 0......õN
...:
1 0
0 NH 0
0 HN
r----
0 01
1 ir'i 0
N]\NN
0 H N-----..)
0
0 0 0 0 H
F N
N . = = c"co N .. = 0
N N
0
HN),N..7...,NH 0
HN)1õ.N....,NH
* 0 . 5 50 0 0
.õNõ.........õ.
ICipl 0-WI I
N,JL,...NI.,,..",-..õ.....õ,,0 0
N..--Li....õ/".....,A 0
H H
-47-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
0 o H
>r
0 0 H HN 0
====.5-,1. ON
=
NH 0
N = = =
HN
o
N
and
N
; or a
pharmaceutically acceptable salt of any of the foregoing.
[0107] Some embodiments of compounds of Formula (I) are shown in Table
B,
below.
Table B
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
C=0 H CH2 CH2 orCR3 CR3 CR3
CR3 CR3
bond
CH2 or
C=0 H 0 CR3 CR3 CR3 CR3 CR3
bond
CH2 or
C=0 H NR2 CR3 CR3 CR3 CR3 CR3
bond
C=0 H CM orCR3 CR3 CR3 CR3
CR3
bond
C=0 H a bond CH2 orCR3 CR3 CR3
CR3 CR3
bond
C=0 methyl CH2 CH2 orCR3 CR3 CR3
CR3 CR3
bond
C=0 methyl 0 CM or CR3 CR3 CR3 CR3
CR3
bond
C=0 methyl NR2 CH2 orCR3 CR3 CR3 CR3 CR3
bond
C=0 methyl S CH2 orCR3 CR3 CR3 CR3
CR3
bond
C=0 methyl a bond CH2 orCR3 CR3
CR3 CR3 CR3
bond
C=0 D CH2 CH2 orCR3 CR3 CR3
CR3 CR3
bond
CH2 or
C=0 D 0 CR3 CR3 CR3 CR3 CR3
bond
-48-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 or
C=0 D NR2 CR3 CR3 CR3 CR3 CR3
bond
CM or
C=0 D S CR3 CR3 CR3 CR3 CR3
bond
C=0 D a bond CH2 orCR3 CR3 CR3
CR3 CR3
bond
C=0 fluoro CH2 CH2 orCR3 CR3 CR3 CR3
CR3
bond
CM or
C=0 fluoro 0 CR3 CR3 CR3 CR3 CR3
bond
C=0 fluoro NR2 CH2 orCR3 CR3 CR3 CR3
CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CM or
C=0 fluoro S CR3 CR3 CR3 CR3 CR3
bond
C=0 fluoro a bond CH2 orCR3 CR3
CR3 CR3 CR3
bond
CH2 H CH2 CH2 orCR3 CR3 CR3 CR3
CR3
bond
CH2 or
CH2 H 0 CR3 CR3 CR3 CR3 CR3
bond
CH2 H NR2 CH2 orCR3 CR3 CR3 CR3
CR3
bond
CM or
CH2 H S CR3 CR3 CR3 CR3 CR3
bond
CH2 H a bond CH2 orCR3 CR3 CR3
CR3 CR3
bond
CH2 methyl CH2 CH2 orCR3 CR3 CR3 CR3
CR3
bond
CH2 methyl 0 CM or CR3 CR3 CR3 CR3
CR3
bond
CH2 methyl NR2 CH2 orCR3 CR3 CR3 CR3 CR3
bond
CH2 methyl S CH2 or CR3 CR3 CR3 CR3
CR3
bond
CH2 methyl a bond CH2 orCR3 CR3
CR3 CR3 CR3
bond
CH2 D CH2 CH2 orCR3 CR3 CR3 CR3
CR3
bond
-49-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CM or
CH2 D 0 CR3 CR3 CR3 CR3 CR3
bond
CH2 D NR2 CH2 orCR3 CR3 CR3 CR3 --
CR3
bond
CM or
CH2 D S CR3 CR3 CR3 CR3 CR3
bond
CH2 D a bond CH2 orCR3 CR3 CR3 --
CR3 -- CR3
bond
CH2 fluoro CH2 CH2 orCR3 CR3 CR3 CR3
CR3
bond
CH2 fluoro 0 CM or CR3 CR3 CR3 CR3 --
CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 fluoro NR2 CH2 orCR3 CR3 CR3 CR3
CR3
bond
CH2 fluoro S CM or CR3 CR3 CR3 CR3 --
CR3
bond
CH2 fluoro a bond CH2 orCR3 CR3
CR3 CR3 CR3
bond
C=0 H CH2 CH2 orN CR3 CR3 CR3 --
CR3
bond
CH2 or
C=0 H 0 N CR3 CR3 CR3 CR3
bond
C=0 H NR2 CH2 orN CR3 CR3 CR3 --
CR3
bond
CM or
C=0 H S N CR3 CR3 CR3 CR3
bond
C=0 H a bond CH2 orN CR3 CR3 CR3 --
CR3
bond
C=0 methyl CH2 CH2 orN CR3 CR3 CR3 CR3
bond
C=0 methyl 0 CM or N CR3 CR3 CR3
CR3
bond
C=0 methyl NR2 CH2 orN CR3 CR3 CR3 CR3
bond
C=0 methyl S CH2 or N CR3 CR3 CR3 --
CR3
bond
C=0 methyl a bond CH2 orN CR3 CR3
CR3 CR3
bond
-50-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
C=0 D CH2 CH2 orN CR3 CR3 CR3 --
CR3
bond
CM or
C=0 D 0 N CR3 CR3 CR3 CR3
bond
CH2 or
C=0 D NR2 N CR3 CR3 CR3 CR3
bond
CM or
C=0 D S N CR3 CR3 CR3 CR3
bond
C=0 D a bond CH2 orN CR3 CR3 CR3
CR3
bond
C=0 fluoro CH2 CH2 orN CR3 CR3 CR3
CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CM or
C=0 fluoro 0 N CR3 CR3 CR3 CR3
bond
C=0 fluoro NR2 CH2 orN CR3 CR3 CR3
CR3
bond
CM or
C=0 fluoro S N CR3 CR3 CR3 CR3
bond
C=0 fluoro a bond CH2 orN CR3 CR3
CR3 CR3
bond
CH2 H CH2 CH2 orN CR3 CR3 CR3 --
CR3
bond
CH2 or
CH2 H 0 N CR3 CR3 CR3 CR3
bond
CH2 H NR2 CH2 orN CR3 CR3 CR3
CR3
bond
CM or
CH2 H S N CR3 CR3 CR3 CR3
bond
CH2 H a bond CH2 orN CR3 CR3 CR3 --
CR3
bond
CH2 methyl CH2 CH2 orN CR3 CR3 CR3
CR3
bond
CH2 methyl 0 CH2 or N CR3 CR3 CR3 --
CR3
bond
CH2 methyl NR2 CH2 orN CR3 CR3 CR3 CR3
bond
CH2 methyl S CH2 or N CR3 CR3 CR3 CR3
bond
-51-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 methyl a bond CH2 orN CR3 CR3
CR3 CR3
bond
CH2 D CH2 CH2 orN CR3 CR3 CR3
CR3
bond
CM or
CH2 D 0 N CR3 CR3 CR3 CR3
bond
CH2 D NR2 CH2 orN CR3 CR3 CR3
CR3
bond
CM or
CH2 D S N CR3 CR3 CR3 CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 D a bond CH2 orN CR3 CR3 CR3
CR3
bond
CH2 fluoro CH2 CH2 orN CR3 CR3 CR3
CR3
bond
CH2 fluoro 0 CM or N CR3 CR3 CR3
CR3
bond
CH2 fluoro NR2 CH2 orN CR3 CR3 CR3
CR3
bond
CH2 fluoro S CM or N CR3 CR3 CR3
CR3
bond
CH2 fluoro a bond CH2 orN CR3 CR3
CR3 CR3
bond
C=0 H CH2 CH2 orCR3 N CR3 CR3
CR3
bond
CH2 or
C=0 H 0 CR3 N CR3 CR3 CR3
bond
C=0 H NR2 CH2 orCR3 N CR3 CR3
CR3
bond
CM or
C=0 H S CR3 N CR3 CR3 CR3
bond
C=0 H a bond CH2 orCR3 N CR3
CR3 CR3
bond
C=0 methyl CH2 CH2 orCR3 N CR3 CR3 CR3
bond
C=0 methyl 0 CH2 or CR3 N CR3 CR3
CR3
bond
C=0 methyl NR2 CH2 orCR3 N CR3 CR3 CR3
bond
-52-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
C=0 methyl S CM or CR3 N CR3 CR3
CR3
bond
C=0 methyl a bond CH2 orCR3 N CR3
CR3 CR3
bond
C=0 D CH2 CH2 orCR3 N CR3 CR3
CR3
bond
CM or
C=0 D 0 CR3 N CR3 CR3 CR3
bond
C=0 D NR2 CH2 orCR3 N CR3 CR3
CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CM or
C=0 D S CR3 N CR3 CR3 CR3
bond
C=0 D a bond CH2 orCR3 N CR3
CR3 CR3
bond
C=0 fluoro CH2 CH2 orCR3 N CR3 CR3
CR3
bond
CM or
C=0 fluoro 0 CR3 N CR3 CR3 CR3
bond
C=0 fluoro NR2 CH2 orCR3 N CR3 CR3
CR3
bond
CM or
C=0 fluoro S CR3 N CR3 CR3 CR3
bond
C=0 fluoro a bond CH2 orCR3 N CR3
CR3 CR3
bond
CH2 H CH2 CH2 orCR3 N CR3 CR3
CR3
bond
CH2 or
CH2 H 0 CR3 N CR3 CR3 CR3
bond
CH2 H NR2 CH2 orCR3 N CR3 CR3
CR3
bond
CM or
CH2 H S CR3 N CR3 CR3 CR3
bond
CH2 H a bond CH2 orCR3 N CR3
CR3 CR3
bond
CH2 methyl CH2 CH2 orCR3 N CR3 CR3
CR3
bond
CH2 methyl 0 CH2 or CR3 N CR3 CR3
CR3
bond
-53-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 methyl NR2 CH2 orCR3 N CR3 CR3 CR3
bond
CH2 methyl S CM or CR3 N CR3 CR3
CR3
bond
CH2 methyl a bond CH2 orCR3 N CR3
CR3 CR3
bond
CH2 D CH2 CH2 orCR3 N CR3 CR3
CR3
bond
CM or
CH2 D 0 CR3 N CR3 CR3 CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 D NR2 CH2 orCR3 N CR3 CR3
CR3
bond
CM or
CH2 D S CR3 N CR3 CR3 CR3
bond
CH2 D a bond CH2 orCR3 N CR3
CR3 CR3
bond
CH2 fluoro CH2 CH2 orCR3 N CR3 CR3
CR3
bond
CH2 fluoro 0 CM or CR3 N CR3 CR3
CR3
bond
CH2 fluoro NR2 CH2 orCR3 N CR3 CR3
CR3
bond
CH2 fluoro S CM or CR3 N CR3 CR3
CR3
bond
CH2 fluoro a bond CH2 orCR3 N CR3
CR3 CR3
bond
C=0 H CH2 CH2 orCR3 CR3 N CR3
CR3
bond
CH2 or
C=0 H 0 CR3 CR3 N CR3 CR3
bond
C=0 H NR2 CH2 orCR3 CR3 N CR3
CR3
bond
CH2 or
C=0 H S CR3 CR3 N CR3 CR3
bond
C=0 H a bond CH2 orCR3 CR3 N CR3
CR3
bond
C=0 methyl CH2 CH2 orCR3 CR3 N CR3 CR3
bond
-54-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
C=0 methyl 0 CM or CR3 CR3 N CR3
CR3
bond
C=0 methyl NR2 CH2 orCR3 CR3 N CR3 CR3
bond
C=0 methyl S CM or CR3 CR3 N CR3
CR3
bond
C=0 methyl a bond CH2 orCR3 CR3 N
CR3 CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
C=0 D CH2 CH2 orCR3 CR3 N CR3
CR3
bond
CM or
C=0 D 0 CR3 CR3 N CR3 CR3
bond
C=0 D NR2 CH2 orCR3 CR3 N CR3
CR3
bond
CM or
C=0 D S CR3 CR3 N CR3 CR3
bond
C=0 D a bond CH2 orCR3 CR3 N CR3
CR3
bond
C=0 fluoro CH2 CH2 orCR3 CR3 N CR3
CR3
bond
CM or
C=0 fluoro 0 CR3 CR3 N CR3 CR3
bond
C=0 fluoro NR2 CH2 orCR3 CR3 N CR3
CR3
bond
CM or
C=0 fluoro S CR3 CR3 N CR3 CR3
bond
C=0 fluoro a bond CH2 orCR3 CR3 N
CR3 CR3
bond
CH2 H CH2 CH2 orCR3 CR3 N CR3
CR3
bond
CH2 or
CH2 H 0 CR3 CR3 N CR3 CR3
bond
CH2 H NR2 CH2 orCR3 CR3 N CR3
CR3
bond
CH2 or
CH2 H S CR3 CR3 N CR3 CR3
bond
CH2 H a bond CH2 orCR3 CR3 N CR3
CR3
bond
-55-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 methyl CH2 CH2 orCR3 CR3 N CR3
CR3
bond
CH2 methyl 0 CM or CR3 CR3 N CR3 --
CR3
bond
CH2 methyl NR2 CH2 orCR3 CR3 N CR3 CR3
bond
CH2 methyl S CM or CR3 CR3 N CR3
CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 methyl a bond CH2 orCR3 CR3 N
CR3 CR3
bond
CH2 D CH2 CH2 orCR3 CR3 N CR3 --
CR3
bond
CM or
CH2 D 0 CR3 CR3 N CR3 CR3
bond
CH2 D NR2 CH2 orCR3 CR3 N CR3 --
CR3
bond
CM or
CH2 D S CR3 CR3 N CR3 CR3
bond
CH2 D a bond CH2 orCR3 CR3 N -- CR3 -
- CR3
bond
CH2 fluoro CH2 CH2 orCR3 CR3 N CR3
CR3
bond
CH2 fluoro 0 CM or CR3 CR3 N CR3 --
CR3
bond
CH2 fluoro NR2 CH2 orCR3 CR3 N CR3
CR3
bond
CH2 fluoro S CM or CR3 CR3 N CR3 --
CR3
bond
CH2 fluoro a bond CH2 orCR3 CR3 N
CR3 CR3
bond
C=0 H CH2 CH2 orN N CR3 CR3 --
CR3
bond
CH2 or
C=0 H 0 N N CR3 CR3 CR3
bond
C=0 H NR2 CH2 orN N CR3 CR3 --
CR3
bond
CH2 or
C=0 H S N N CR3 CR3 CR3
bond
-56-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
C=0 H a bond CH2 orN N CR3 CR3
CR3
bond
C=0 methyl CH2 CH2 orN N CR3 CR3 CR3
bond
C=0 methyl 0 CH2 or N N CR3 CR3
CR3
bond
C=0 methyl NR2 CH2 orN N CR3 CR3 CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
C=0 methyl S CH2 or N N CR3 CR3
CR3
bond
C=0 methyl a bond CH2 orN N CR3
CR3 CR3
bond
C=0 D CH2 CH2 orN N CR3 CR3
CR3
bond
CH2 or
C=0 D 0 N N CR3 CR3 CR3
bond
C=0 D NR2 CH2 orN N CR3 CR3
CR3
bond
CH2 or
C=0 D S N N CR3 CR3 CR3
bond
C=0 D a bond CH2 orN N CR3 CR3
CR3
bond
C=0 fluoro CH2 CH2 orN N CR3 CR3
CR3
bond
CH2 or
C=0 fluoro 0 N N CR3 CR3 CR3
bond
C=0 fluoro NR2 CH2 orN N CR3 CR3
CR3
bond
CH2 or
C=0 fluoro S N N CR3 CR3 CR3
bond
C=0 fluoro a bond CH2 orN N CR3
CR3 CR3
bond
CH2 H CH2 CH2 orN N CR3 CR3
CR3
bond
CH2 or
CH2 H 0 N N CR3 CR3 CR3
bond
CH2 H NR2 CH2 orN N CR3 CR3
CR3
bond
-57-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 or
CH2 H S N N CR3 CR3 CR3
bond
CH2 or
CH2 H a bond N N CR3 CR3 CR3
bond
CH2 or
CH2 methyl CH2 N N CR3 CR3 CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 or
CH2 methyl 0 N N CR3 CR3 CR3
bond
CH2 or
CH2 methyl NR2 N N CR3 CR3 CR3
bond
CH2 or
CH2 methyl S N N CR3 CR3 CR3
bond
CH2 or
CH2 methyl a bond N N CR3 CR3 CR3
bond
CH2 or
CH2 D CH2 N N CR3 CR3 CR3
bond
CH2 or
CH2 D 0 N N CR3 CR3 CR3
bond
CH2 or
CH2 D NR2 N N CR3 CR3 CR3
bond
CH2 or
CH2 D S N N CR3 CR3 CR3
bond
CH2 or
CH2 D a bond N N CR3 CR3 CR3
bond
CH2 or
CH2 fluoro CH2 N N CR3 CR3 CR3
bond
CH2 or
CH2 fluoro 0 N N CR3 CR3 CR3
bond
CH2 or
CH2 fluoro NR2 N N CR3 CR3 CR3
bond
CH2 or
CH2 fluoro S N N CR3 CR3 CR3
bond
CH2 or
CH2 fluoro a bond N N CR3 CR3 CR3
bond
CH2 or
C=0 H CH2 N CR3 CR3 CR3 N
bond
CH2 or
C=0 H 0 N CR3 CR3 CR3 N
bond
-58-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 or
C=0 H NR2 N CR3 CR3 CR3 N
bond
CH2 or
C=0 H S N CR3 CR3 CR3 N
bond
C=0 H a bond CH2 orN CR3 CR3 CR3 -- N
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
C=0 methyl CH2 CH2 orN CR3 CR3 CR3 N
bond
C=0 methyl 0 CH2 or N CR3 CR3 CR3 -- N
bond
C=0 methyl NR2 CH2 orN CR3 CR3 CR3 N
bond
C=0 methyl S CH2 or N CR3 CR3 CR3 N
bond
C=0 methyl a bond CH2 orN CR3 CR3
CR3 N
bond
C=0 D CH2 CH2 orN CR3 CR3 CR3 N
bond
CH2 or
C=0 D 0 N CR3 CR3 CR3 N
bond
C=0 D NR2 CH2 orN CR3 CR3 CR3 -- N
bond
CH2 or
C=0 D S N CR3 CR3 CR3 N
bond
C=0 D a bond CH2 orN CR3 CR3 CR3 -- N
bond
C=0 fluoro CH2 CH2 orN CR3 CR3 CR3 N
bond
CH2 or
C=0 fluoro 0 N CR3 CR3 CR3 N
bond
C=0 fluoro NR2 CH2 orN CR3 CR3 CR3 N
bond
CH2 or
C=0 fluoro S N CR3 CR3 CR3 N
bond
C=0 fluoro a bond CH2 orN CR3 CR3
CR3 N
bond
CH2 H CH2 CH2 orN CR3 CR3 CR3 N
bond
-59-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 or
CH2 H 0 N CR3 CR3 CR3 N
bond
CH2 or
CH2 H NR2 N CR3 CR3 CR3 N
bond
CH2 or
CH2 H S N CR3 CR3 CR3 N
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 or
CH2 H a bond N CR3 CR3 CR3 N
bond
CH2 or
CH2 methyl CH2 N CR3 CR3 CR3 N
bond
CH2 or
CH2 methyl 0 N CR3 CR3 CR3 N
bond
CH2 or
CH2 methyl NR2 N CR3 CR3 CR3 N
bond
CH2 or
CH2 methyl S N CR3 CR3 CR3 N
bond
CH2 or
CH2 methyl a bond N CR3 CR3 CR3 N
bond
CH2 or
CH2 D CH2 N CR3 CR3 CR3 N
bond
CH2 or
CH2 D 0 N CR3 CR3 CR3 N
bond
CH2 or
CH2 D NR2 N CR3 CR3 CR3 N
bond
CH2 or
CH2 D S N CR3 CR3 CR3 N
bond
CH2 or
CH2 D a bond N CR3 CR3 CR3 N
bond
CH2 or
CH2 fluoro CH2 N CR3 CR3 CR3 N
bond
CH2 or
CH2 fluoro 0 N CR3 CR3 CR3 N
bond
CH2 or
CH2 fluoro NR2 N CR3 CR3 CR3 N
bond
CH2 or
CH2 fluoro S N CR3 CR3 CR3 N
bond
CH2 or
CH2 fluoro a bond N CR3 CR3 CR3 N
bond
-60-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
C=0 H CH2 CH2 orCR3 N CR3 N CR3
bond
CH2 or
C=0 H 0 CR3 N CR3 N CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 or
C=0 H NR2 CR3 N CR3 N CR3
bond
CM or
C=0 H S CR3 N CR3 N CR3
bond
C=0 H a bond CH2 orCR3 N CR3 N
CR3
bond
C=0 methyl CH2 CH2 orCR3 N CR3 N CR3
bond
C=0 methyl 0 CM or CR3 N CR3 N CR3
bond
C=0 methyl NR2 CH2 orCR3 N CR3 N CR3
bond
C=0 methyl S CM or CR3 N CR3 N CR3
bond
C=0 methyl a bond CH2 orCR3 N CR3
N CR3
bond
C=0 D CH2 CH2 orCR3 N CR3 N CR3
bond
CM or
C=0 D 0 CR3 N CR3 N CR3
bond
C=0 D NR2 CH2 orCR3 N CR3 N CR3
bond
CM or
C=0 D S CR3 N CR3 N CR3
bond
C=0 D a bond CH2 orCR3 N CR3 N
CR3
bond
C=0 fluoro CH2 CH2 orCR3 N CR3 N CR3
bond
CH2 or
C=0 fluoro 0 CR3 N CR3 N CR3
bond
C=0 fluoro NR2 CH2 orCR3 N CR3 N CR3
bond
CH2 or
CO fluoro S CR3 N CR3 N CR3
bond
-61-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 or
CO fluoro a bond CR3 N CR3 N CR3
bond
CH2 or
CH2 H CH2 CR3 N CR3 N CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 or
CH2 H 0 CR3 N CR3 N CR3
bond
CH2 or
CH2 H NR2 CR3 N CR3 N CR3
bond
CH2 or
CH2 H S CR3 N CR3 N CR3
bond
CH2 or
CH2 H a bond CR3 N CR3 N CR3
bond
CH2 or
CH2 methyl CH2 CR3 N CR3 N CR3
bond
CH2 or
CH2 methyl 0 CR3 N CR3 N CR3
bond
CH2 or
CH2 methyl NR2 CR3 N CR3 N CR3
bond
CH2 or
CH2 methyl S CR3 N CR3 N CR3
bond
CH2 or
CH2 methyl a bond CR3 N CR3 N CR3
bond
CH2 or
CH2 D CH2 CR3 N CR3 N CR3
bond
CH2 or
CH2 D 0 CR3 N CR3 N CR3
bond
CH2 or
CH2 D NR2 CR3 N CR3 N CR3
bond
CH2 or
CH2 D S CR3 N CR3 N CR3
bond
CH2 or
CH2 D a bond CR3 N CR3 N CR3
bond
CH2 or
CH2 fluoro CH2 CR3 N CR3 N CR3
bond
CH2 or
CH2 fluoro 0 CR3 N CR3 N CR3
bond
CH2 or
CH2 fluoro NR2 CR3 N CR3 N CR3
bond
-62-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 fluoro S CM or CR3 N CR3 N CR3
bond
CH2 fluoro a bond CH2 orCR3 N CR3
N CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
C=0 H CH2 CH2 orN CR3 N CR3
CR3
bond
CH2 or
C=0 H 0 N CR3 N CR3 CR3
bond
C=0 H NR2 CH2 orN CR3 N CR3
CR3
bond
CM or
C=0 H S N CR3 N CR3 CR3
bond
C=0 H a bond CH2 orN CR3 N CR3
CR3
bond
C=0 methyl CH2 CH2 orN CR3 N CR3 CR3
bond
C=0 methyl 0 CM or N CR3 N CR3
CR3
bond
C=0 methyl NR2 CH2 orN CR3 N CR3 CR3
bond
C=0 methyl S CM or N CR3 N CR3
CR3
bond
C=0 methyl a bond CH2 orN CR3 N CR3
CR3
bond
C=0 D CH2 CH2 orN CR3 N CR3
CR3
bond
CM or
C=0 D 0 N CR3 N CR3 CR3
bond
C=0 D NR2 CH2 orN CR3 N CR3
CR3
bond
CM or
C=0 D S N CR3 N CR3 CR3
bond
C=0 D a bond CH2 orN CR3 N CR3
CR3
bond
C=0 fluoro CH2 CH2 orN CR3 N CR3
CR3
bond
CH2 or
C=0 fluoro 0 N CR3 N CR3 CR3
bond
-63-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 or
C=0 fluoro NR2 N CR3 N CR3 CR3
bond
X Xi Qi Q2 Y1 Y2 Y3 Y4 Y5
CH2 or
C=0 fluoro S N CR3 N CR3 CR3
bond
CH2 or
CO fluoro a bond N CR3 N CR3 CR3
bond
CH2 or
CH2 H CH2 N CR3 N CR3 CR3
bond
CH2 or
CH2 H 0 N CR3 N CR3 CR3
bond
CH2 or
CH2 H NR2 N CR3 N CR3 CR3
bond
CH2 or
CH2 H S N CR3 N CR3 CR3
bond
CH2 or
CH2 H a bond N CR3 N CR3 CR3
bond
CH2 or
CH2 methyl CH2 N CR3 N CR3 CR3
bond
CH2 or
CH2 methyl 0 N CR3 N CR3 CR3
bond
CH2 or
CH2 methyl NR2 N CR3 N CR3 CR3
bond
CH2 or
CH2 methyl S N CR3 N CR3 CR3
bond
CH2 or
CH2 methyl a bond N CR3 N CR3 CR3
bond
CH2 or
CH2 D CH2 N CR3 N CR3 CR3
bond
CH2 or
CH2 D 0 N CR3 N CR3 CR3
bond
CH2 or
CH2 D NR2 N CR3 N CR3 CR3
bond
CH2 or
CH2 D S N CR3 N CR3 CR3
bond
CH2 or
CH2 D a bond N CR3 N CR3 CR3
bond
CH2 or
CH2 fluoro CH2 N CR3 N CR3 CR3
bond
-64-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 fluoro 0 CM or N CR3 N CR3
CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 fluoro NR2 CH2 orN CR3 N CR3
CR3
bond
CH2 fluoro S CM or N CR3 N CR3
CR3
bond
CH2 fluoro a bond CH2 orN CR3 N CR3
CR3
bond
C=0 H CH2 CH2 orCR3 CR3 N CR3 N
bond
CM or
C=0 H 0 CR3 CR3 N CR3 N
bond
C=0 H NR2 CH2 orCR3 CR3 N CR3 N
bond
CM or
C=0 H S CR3 CR3 N CR3 N
bond
C=0 H a bond CH2 orCR3 CR3 N CR3
N
bond
C=0 methyl CH2 CH2 orCR3 CR3 N CR3 N
bond
CH2 or
X X1 Qi Y1 Y2 Y3 Y4 Y5
bond
C=0 methyl 0 CM or CR3 CR3 N CR3 N
bond
C=0 methyl NR2 CH2 orCR3 CR3 N CR3 N
bond
C=0 methyl S CM or CR3 CR3 N CR3 N
bond
C=0 methyl a bond CH2 orCR3 CR3 N
CR3 N
bond
C=0 D CH2 CH2 orCR3 CR3 N CR3 N
bond
CM or
C=0 D 0 CR3 CR3 N CR3 N
bond
C=0 D NR2 CH2 orCR3 CR3 N CR3 N
bond
CH2 or
C=0 D S CR3 CR3 N CR3 N
bond
-65-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
CH2 or
C=0 D a bond CR3 CR3 N CR3 N
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 or
C=0 fluoro CH2 CR3 CR3 N CR3 N
bond
CH2 or
C=0 fluoro 0 CR3 CR3 N CR3 N
bond
CH2 or
C=0 fluoro NR2 CR3 CR3 N CR3 N
bond
CH2 or
C=0 fluoro S CR3 CR3 N CR3 N
bond
CH2 or
C=0 fluoro a bond CR3 CR3 N CR3 N
bond
CH2 or
CH2 H CH2 CR3 CR3 N CR3 N
bond
CH2 or
CH2 H 0 CR3 CR3 N CR3 N
bond
CH2 or
CH2 H NR2 CR3 CR3 N CR3 N
bond
CH2 or
CH2 H S CR3 CR3 N CR3 N
bond
CH2 or
CH2 H a bond CR3 CR3 N CR3 N
bond
CH2 or
CH2 methyl CH2 CR3 CR3 N CR3 N
bond
CH2 or
CH2 methyl 0 CR3 CR3 N CR3 N
bond
CH2 or
CH2 methyl NR2 CR3 CR3 N CR3 N
bond
CH2 or
CH2 methyl S CR3 CR3 N CR3 N
bond
CH2 or
CH2 methyl a bond CR3 CR3 N CR3 N
bond
CH2 or
CH2 D CH2 CR3 CR3 N CR3 N
bond
CH2 or
CH2 D 0 CR3 CR3 N CR3 N
bond
CH2 or
CH2 D NR2 CR3 CR3 N CR3 N
bond
-66-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
CH2 or
CH2 CR3 CR3 N CR3
bond
X Xi Qi Q2 Yi Y2 Y3 Y4 Y5
CH2 CH2 or a bond CR3 CR3 N CR3
bond
CH2 fluoro CH2 CH2 orCR3 CR3 N CR3
bond
CH2 or
CH2 fluoro 0 CR3 CR3 N CR3
bond
CH2 fluoro NR2 CH2 orCR3 CR3 N CR3
bond
CH2 or
CH2 fluoro CR3 CR3 N CR3
bond
CH2 fluoro a bond CH2 orCR3 CR3 N
CR3
bond
[0108] In some embodiments of Table B, R2 can be hydrogen. In some
embodiments of Table B, R2 can be a substituted or unsubstituted C1-C6 alkyl,
for example,
methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, t-butyl,
pentyl (straight-
chained or branched), or hexyl (straight-chained or branched). In some
embodiments of
Table B, R2 can be an unsubstituted C1-C6 alkyl, for example, methyl, ethyl, n-
propyl,
isopropyl, n-butyl, isobutyl, sec-butyl, t-butyl, pentyl (straight-chained or
branched), or hexyl
(straight-chained or branched). In some embodiments of Table B, R2 can be
acyl, for
example, ¨(C=0)¨methyl, ¨(C=0)-ethyl, ¨(C=0)-n-propyl, ¨(C=0)-isopropyl,
¨(C=0)-n-
butyl, ¨(C=0)-isobutyl, ¨(C=0)-sec-butyl, ¨(C=0)-t-butyl, ¨(C=0)-pentyl
(straight-chained
or branched), or ¨(C=0)-hexyl (straight-chained or branched). In some
embodiments of
Table B, R2 can be ¨(S02)-C1-C6 alkyl, for example, ¨(S02)-methyl, ¨(S02)-
ethyl, ¨(S02)-n-
propyl, ¨(S 02)-i sopropyl, ¨(S 02)-n-butyl, ¨(S 02)-i sobutyl, ¨(S 02)- sec-
butyl, ¨(S02)-t-butyl,
¨(S02)-pentyl (straight-chained or branched), or ¨(S02)-hexyl (straight-
chained or
branched).
-67-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0109] In some
embodiments, a compound of Formula (I) is selected from:
o o o o
o s
SI N-c-riEio
1.1 N-c-NH Si No o N-c\WI CI
0 0
0
0 0 0 0
40 lel lel 1.1
R 3 c R 3 c
R 3 c R 3 c
o o
o r s 0 H
N 0
Ni-NH N--N1-0 ONi_
NH 0 N-i-NI.CC)
0 0 0 0 0
0 0 0
0 0
lei 0
0 0
R3c R3C
R3c R3C
0 H 0 0
No 0 0
101 N¨c\IFI 0
N¨c¨NH Ni-NH 101 Ni-NH
0 00 0 00 0 0
0 0
OP 0 pp 3B IS
R'
0
. s
. s3B
R3C R3C R3C
R3C
0
0 S
0 0 0 0 H
1\1
N¨c¨ N¨cl11-10
NH
NH [10
0 NH
0 0 0 0
0
D IS ,õ OP
D OP
0
. s3B . x3B . s3B
,õ
R3C R3C R3C . x3B
R3C
0 0 H 0
0
0 N¨i_ 0 S
0 Ni_o
NH
0 N-c---NH NH 0
0 0 0 o o 0
0 0 0
pp313 pp313 D,
pp313 . , 40
40 40
. ,
. s3B
. ,
R3c R3
R3
R3
-68-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
Ri 0
0 R1 0
S Ri 0 R1 0 H
N
Ni N¨cmi 0 0 N¨\
0 1110 ¨NH i¨NH
0 0 0 0 0 00 0 0
0 0 0 0
R3c R3c R3c R3
Ri 0 R1 0 Ri 0 H
N R1 0
N¨c.ill 0 N¨c_. o
NH
N¨c_. o
0 0 0 0
0 00 NH
0 0
0 0
R30
R30 R30 R30
R1 0
0 0 Ri 0 0 Ri
Ri 0 S S
NH
Ni¨NH
0 0 0 i¨NH ¨NH
0 0 0 0 0
0 0
0
0 R3B 140
R3B 410
R3
R3c R3c R3
0 0 0i 0
R1 Ri Ri S Ri
0 N¨clo N¨c-
mi 0
N¨c¨NH0 N¨NH
0 0
0 0 0 0 0 0
R3B R3B Is pp 141
3B pp 0 411)
0 Is
Is R3B
R30 R30 R30 R30
R1 0 H
1\1 R1 0 0 H
Ri rNõI 0
R1
0 N¨c_ /.0 11) N¨___ o
NH NH N
0 0 ¨--NH
0 0 0 0 00
R3B 4110
D 35 D 40
00
po 41
lx
"
R3c "35
. ,35
R3C
R3c R3C
-69-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
0 0 0 0
S
0 NQ Lo 0 NiC2.0 * N_c_ 0
N-clio
0 \ N N
O 0 \ 0 \
0 0
0
0 0 0
R3C
R3C R3c R3C
0 0
0 S 0
IN-I 0
Ni___No * Ni_ o 0 N_ /Lo
N >7---N
o 0 \ o
o o
0 0
0 0
R3c R3c
R3c
R3c
0 H
N-cN 0 0 0 OTh
_No NL0 0 N¨c.:0 0 N¨c_No
O 0 \ 0 00 0 0 \ 0 \ 0 \
0 0 D 0 0
I µ3B R3B
R3c R3c R3c
R3c
0 S 0 0 0 H
_c_00 0 N j_c_o
N
0 N¨c_N-0 N¨c-II0 N
0 \ N N
0 0 00 \ 0 00 \ 0 \
0
D 0 D 0
D 0
0
.µ3B .µ3B .µ3B
D
R3c R3c R3c
. s3B
R3c
0 0 H
0 N 0
S
0 N4/L0 0 N0 0 N¨c_NO 0 N¨N/L0
0 \ 0 0 \ 0 0 00 \
0 00 0 \
D3B . s 0
D3B D3B D 0 0
0
.,3B
. s
. s
R3c R3
R3
R3
-70-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
R1 0 0,1 R1 0 STh R1 0 Ri 0 H
,1
No N¨c_No N¨cro N¨c_
N1No
0 \ 0 \ 0 \
0 0 0 0 0 \ 0
0 0 0 0
R3c R3c R3c R3c
0 H R1 0
Ri 0 Ri R1 0 rN
:5 N¨c.:0 Ni_N/Lo N¨__
N¨c_N/L
\
0 \ 0 0
0 \ 0 0 \
0
0 0 0 0
R3c
R3
R3cR3
R1 0 0 R1 0 Ri 0 0
0 Ri
S rs
N No
N ¨c_No
i¨N
0 0 0 \ 0 00
0 0 \
0 0 \ 0
0
0
0 R3B 40
R3B 40
R3c
R3cR3 R3 R3
0 0
0 0
R1 R1 0 Ri S R1
(1101 No N¨c_No 0 N¨c-
\1 0
0 \ 0 \
0 \
!ç
\
R3B
140
R3B
le I R3B 140 0
. s3B
R3B . s3B. s3B
R3C R3C
R3C R3C
H
0
R1 N R1 0
0 H
Ri NTh R1 0
0 N¨c_No 0 N¨,L
N¨c_No
N¨i_r\i/Lo
0 \ Cf./ \
0 0 0 00 \ 0 00 \
R3B 0
R3B
3B pp 0
0
po 0
. ,
. ,
R3c . ,3B
. ,3B
R3c
R3c R3c
In some embodiments of this paragraph, Ri can be fluoro. In some embodiments
of this
paragraph, Ri can be chloro. In some embodiments of this paragraph, Ri can be
hydroxyl.
In some embodiments of this paragraph, Ri can be ¨NH2. In some embodiments of
this
-71-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
paragraph, Ri can be ¨CF3, ¨CHF2, or ¨CH2F. In some embodiments of this
paragraph, Ri
can be an unsubstituted Ci-C6 alkoxy, such as methoxy, ethoxy, n-propoxy,
isopropoxy, n-
butoxy, isobutoxy, sec-butoxy, t-butoxy, pentoxy (straight-chained or
branched), or hexoxy
(straight-chained or branched). In some embodiments of this paragraph, Ri can
be an
unsubstituted Ci-C6 alkyl, such as methyl, ethyl, n-propyl, isopropyl, n-
butyl, isobutyl, sec-
butyl, t-butyl, pentyl (straight-chained or branched), or hexyl (straight-
chained or branched).
In some embodiments of this paragraph, Ri can be an unsubstituted C3-C8
cycloalkyl, such as
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
In some
embodiments of this paragraph, Ri can be an unsubstituted 3 to 10 membered
heterocyclyl,
for example, a monocyclic heterocyclyl, a bridged heterocyclyl, or a fused
heterocyclyl,
including groups such as pyrrolidine, piperidine, piperazine, and morpholine.
In some
embodiments of this paragraph, R3B can be hydroxyl. In some embodiments of
this
paragraph, R3B can be fluoro. In some embodiments of this paragraph, R3B can
be chloro. In
some embodiments of this paragraph, R3B can be a substituted or unsubstituted
amino, for
example, -NH2, dimethylamino, diethylamino, isopropylethylamino, phenylamino,
or
benzylamino. In some embodiments of this paragraph, R3B can be ¨CF3, ¨CHF2, or
¨CH2F.
In some embodiments of this paragraph, R3B can be an unsubstituted Ci-C6
alkoxy, such as
methoxy, ethoxy, n-propoxy, isopropoxy, n-butoxy, isobutoxy, sec-butoxy, t-
butoxy, pentoxy
(straight-chained or branched), or hexoxy (straight-chained or branched).
In some
embodiments of this paragraph, R3B can be an unsubstituted Ci-C6 alkyl, such
as methyl,
ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, t-butyl, pentyl
(straight-chained or
branched), or hexyl (straight-chained or branched). In some embodiments of
this paragraph,
R3B can be an unsubstituted C3 -C 8 cycloalkyl, such as cyclopropyl,
cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, and cyclooctyl. In some embodiments of this
paragraph, R3c can be
a substituted or unsubstituted C3-C8 cycloalkyl, such as cyclopropyl,
cyclobutyl, cyclopentyl,
cyclohexyl, cycloheptyl, and cyclooctyl. In some embodiments of this
paragraph, R3c can be
an unsubstituted C 3 -C 8 cycloalkyl, such as cyclopropyl, cyclobutyl,
cyclopentyl, cyclohexyl,
cycloheptyl, and cyclooctyl. In some embodiments of this paragraph, R3c can be
a
substituted or unsubstituted 3 to 10 membered heterocyclyl, for example, a
monocyclic
heterocyclyl, a bridged heterocyclyl, or a fused heterocyclyl, including
groups such as
pyrrolidine, piperidine, piperazine, and morpholine. In some embodiments of
this paragraph,
-72-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
R3C can be an unsubstituted 3 to 10 membered heterocyclyl, for example, a
monocyclic
heterocyclyl, a bridged heterocyclyl, or a fused heterocyclyl, including
groups such as
pyrrolidine, piperidine, piperazine, and morpholine. In some embodiments of
this paragraph,
R3C can be a substituted or unsubstituted 5 to 10 membered heteroaryl, for
example, a 5 or 6
membered heteroaryl containing at least one nitrogen, such as pyrrole,
imidazole, oxazole,
thiazole, pyridine, or pyrimidine. In some embodiments of this paragraph, R3C
can be an
unsubstituted 5 to 10 membered heteroaryl, for example, a 5 or 6 membered
heteroaryl
containing at least one nitrogen, such as pyrrole, imidazole, oxazole,
thiazole, pyridine, or
pyrimidine.
[0110]
Compounds of Formula (I) can be provided in the form of
pharmaceutically acceptable salts, solvates, or tautomers, thereof
[0111]
Some embodiments provide a pharmaceutical composition comprising a
compound of Formula (I), or a pharmaceutically acceptable salt thereof and a
pharmaceutically acceptable excipient. In
some embodiments, the pharmaceutical
composition also contains at least one pharmaceutically acceptable inactive
ingredient. The
pharmaceutical composition can be formulated for intravenous injection,
subcutaneous
injection, oral administration, buccal administration, inhalation, nasal
administration, topical
administration, transdermal administration, ophthalmic administration, or otic
administration.
The pharmaceutical composition can be in the form of a tablet, a pill, a
capsule, a liquid, an
inhalant, a nasal spray solution, a suppository, a suspension, a gel, a
colloid, a dispersion, a
solution, an emulsion, an ointment, a lotion, an eye drop, or an ear drop.
[0112] In
some embodiments, the pharmaceutical composition is formulated as a
gel, salve, ointment, cream, emulsion, or paste for topical application to the
skin.
[0113]
Some embodiments provide a method of inhibiting the activity of a
cytokine, comprising contacting a cell with an effective amount of a compound
of Formula
(I), or a pharmaceutically acceptable salt thereof. In some embodiments, the
cytokine is
selected from: IL-113, IL-2, IL-6, and TNFa. In some embodiments, the cytokine
is TNFa.
In some embodiments, the cell is a cancer cell.
[0114]
Some embodiments provide a method of inhibiting the activity of aiolos,
comprising contacting a cell with an effective amount of a compound of Formula
(I), or a
pharmaceutically acceptable salt thereof
-73-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0115] Some embodiments provide a method of inhibiting the activity of
ikaros,
comprising contacting a cell with an effective amount of a compound of Formula
(I), or a
pharmaceutically acceptable salt thereof. In some embodiments, the cell is a
cancer cell.
[0116] Some embodiments provide a method of inhibiting the activity of
helios,
comprising contacting a cell with an effective amount of a compound of Formula
(I), or a
pharmaceutically acceptable salt thereof. In some embodiments, the cell is a
cancer cell.
[0117] [0120] Some embodiments provide a method of inhibiting the
activity
of CK-1 a, comprising contacting a cell with an effective amount of a compound
of Formula
(I), or a pharmaceutically acceptable salt thereof. In some embodiments, the
cell is a cancer
cell.
[0118] In some embodiments, the cell is a small cell lung cancer cell,
a non-small
cell lung cancer cell, a breast cancer cell, a prostate cancer cell, a head
and neck cancer cell,
a pancreatic cancer cell, a colon cancer cell, a rectal cancer cell, a
teratoma cell, an ovarian
cancer cell, an endometrial cancer cell, a brain cancer cell, a retinoblastoma
cell, a leukemia
cell, a skin cancer cell, a melanoma cell, a squamous cell carcinoma cell, a
liposarcoma cell,
a lymphoma cell, a multiple myeloma cell, a testicular cancer cell, a liver
cancer cell, an
esophageal cancer cell, a kidney carcinoma cell, an astrogliosis cell, a
relapsed/refractory
multiple myeloma cell, or a neuroblastoma cell.
[0119] In some embodiments, inhibiting the activity of a protein can
include
decreasing the activity of the protein by 20-50%, by 30-70%, by 40-90%, or any
value in
between. For example, inhibiting the activity of a protein can include
decreasing the activity
of the protein by 10%, 15%, 20%, 25%, 30%, 35%, 40% 45%, 50%, 55%, 60%, 65%,
70%,
75%, 80%, 85%, 90%, 95%, 99%, or any value in between.
[0120] Some embodiments provide a method of treating, ameliorating, or
preventing a disease, disorder, or condition associated with a protein in a
subject, the protein
selected from a cytokine, aiolos, ikaros, helios, CK la, and combinations of
any of the
foregoing; the method comprising administering a therapeutically effective
amount of a
compound of Formula (I), or a pharmaceutically acceptable salt thereof, or a
pharmaceutical
composition comprising a compound of Formula (I), or a pharmaceutically
acceptable salt
thereof, to the subject.
-74-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0121] In some embodiments, the disease, disorder, or condition is a
cancer
selected from a hematological malignancy and a solid tumor In some
embodiments, the
disease, disorder, or condition is a cancer selected from small cell lung
cancer, non-small cell
lung cancer, breast cancer, prostate cancer, head and neck cancer, pancreatic
cancer, colon
cancer, rectal cancer, teratoma, ovarian cancer, endometrial cancer, brain
cancer,
retinoblastoma, leukemia, skin cancer, melanoma, squamous cell carcinoma,
liposarcoma,
lymphoma, multiple myeloma, testicular cancer, liver cancer, esophageal
cancer, kidney
carcinoma, astrogliosis, relapsed/refractory multiple myeloma, and
neuroblastoma.
[0122] In some embodiments, the disease, disorder, or condition is
selected from
inflammation, fibromyalgia, rheumatoid arthritis, osteoarthritis, ankylosing
spondylitis,
psoriasis, psoriatic arthritis, inflammatory bowel diseases, Crohn's disease,
ulcerative colitis,
uveitis, inflammatory lung diseases, chronic obstructive pulmonary disease,
and Alzheimer's
disease. In some embodiments, the disease, disorder, or condition is selected
from
fibromyalgia, rheumatoid arthritis, osteoarthritis, ankylosing spondylitis,
psoriasis, psoriatic
arthritis, Crohn's disease, and ulcerative colitis.
[0123] In some embodiments, the protein is a cytokine. In some
embodiments,
the cytokine is selected from: IL-113, IL-2, IL-6, and TNFa. In some
embodiments, the
subject is known to possess wild-type IL-113, IL-2, IL-6, and TNFa. In some
embodiments,
the subject is known to overexpress one or more of IL-113, IL-2, IL-6, and
TNFa. In some
embodiments, the subject is known to possess a mutant form of IL-113, IL-2, IL-
6, and/or
TNFa.
[0124] In some embodiments, the cytokine is TNFa. In some embodiments,
the
subject is known to possess wild-type TNFa. In some embodiments, the subject
is known to
overexpress TNFa. In some embodiments, the subject is known to possess a
mutant form of
TNFa. In some embodiments, the protein is aiolos. In some embodiments, the
subject is
known to possess wild-type aiolos. In some embodiments, the subject is known
to
overexpress aiolos. In some embodiments, the subject is known to possess a
mutant form of
aiolos. In some embodiments, the protein is ikaros. In some embodiments, the
subject is
known to possess wild-type ikaros. In some embodiments, the subject is known
to
overexpress ikaros. In some embodiments, the subject is known to possess a
mutant form of
ikaros. In some embodiments, the protein is helios. In some embodiments, the
subject is
-75-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
known to possess wild-type helios. In some embodiments, the subject is known
to
overexpress helios. In some embodiments, the subject is known to possess a
mutant form of
helios. In some embodiments, the protein is CK 1 a. In some embodiments, the
subject is
known to possess wild-type CK 1 a. In some embodiments, the subject is known
to
overexpress CKla. In some embodiments, the subject is known to possess a
mutant form of
CKla.
[0125] Other objects, features, and advantages of the compounds,
methods, and
compositions described herein will become apparent from the following detailed
description.
It should be understood, however, that the detailed description and the
specific examples,
while indicating specific embodiments, are given by way of illustration only,
since various
changes and modifications within the spirit and scope of the instant
disclosure will become
apparent to those skilled in the art from this detailed description
Definitions
[0126] Unless defined otherwise, all technical and scientific terms
used herein
have the same meaning as is commonly understood by one of ordinary skill in
the art. All
patents, applications, published applications and other publications
referenced herein are
incorporated by reference in their entirety unless stated otherwise. In the
event that there are
a plurality of definitions for a term herein, those in this section prevail
unless stated
otherwise. As used in the specification and the appended claims, the singular
forms "a,"
"an" and "the" include plural referents unless the context clearly dictates
otherwise. Unless
otherwise indicated, conventional methods of mass spectroscopy, NMR, HPLC,
protein
chemistry, biochemistry, recombinant DNA techniques and pharmacology are
employed.
The use of "or" or "and" means "and/or" unless stated otherwise. Furthermore,
use of the
term "including" as well as other forms, such as "include", "includes," and
"included," is not
limiting. As used in this specification, whether in a transitional phrase or
in the body of the
claim, the terms "comprise(s)" and "comprising" are to be interpreted as
having an open-
ended meaning. That is, the terms are to be interpreted synonymously with the
phrases
"having at least" or "including at least." When used in the context of a
process, the term
"comprising" means that the process includes at least the recited steps, but
may include
additional steps. When used in the context of a compound, composition, or
device, the term
-76-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
"comprising" means that the compound, composition, or device includes at least
the recited
features or components, but may also include additional features or
components.
[0127] The section headings used herein are for organizational
purposes only and
are not to be construed as limiting the subject matter described.
[0128] The terms "co-administration" and similar terms as used herein
are broad
terms, and are to be given their ordinary and customary meaning to a person of
ordinary skill
in the art (and are not to be limited to a special or customized meaning), and
refer without
limitation to administration of the selected therapeutic agents to a single
patient, and are
intended to include treatment regimens in which the agents are administered by
the same or
different route of administration or at the same or different time.
[0129] The terms "effective amount" and "therapeutically effective
amount" are
broad terms, and are to be given their ordinary and customary meaning to a
person of
ordinary skill in the art (and are not to be limited to a special or
customized meaning), and
refer without limitation to a sufficient amount of an agent or a compound
being administered
which will relieve to some extent one or more of the symptoms of the disease
or condition
being treated. The result can be reduction and/or alleviation of the signs,
symptoms, or
causes of a disease, or any other desired alteration of a biological system.
For example, an
"effective amount" for therapeutic uses is the amount of the composition
comprising a
compound as disclosed herein required to provide a clinically significant
decrease in disease
symptoms. An appropriate "effective" amount in any individual case may be
determined
using techniques, such as a dose escalation study. Where a drug has been
approved by the
U.S. Food and Drug Administration (FDA) or a counterpart foreign medicines
agency, a
"therapeutically effective amount" optionally refers to the dosage approved by
the FDA or its
counterpart foreign agency for treatment of the identified disease or
condition.
[0130] As used herein, any "R" group(s) such as, without limitation,
R2, R3, R4,
Rs, R6, R9, and Rio represent substituents that can be attached to the
indicated atom. An R
group may be substituted or unsubstituted. If two "R" groups are described as
being "taken
together" the R groups and the atoms they are attached to can form a
cycloalkyl, aryl,
heteroaryl, or heterocycle. For example, without limitation, if R2 and R3, or
R2, R3, or R4,
-77-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
and the atom to which it is attached, are indicated to be "taken together" or
"joined together"
R2
I
it means that they are covalently bonded to one another to form a ring: R3
[0131] Whenever a group is described as being "substituted" or
"optionally
substituted" that group may be unsubstituted or substituted with one or more
of the indicated
substituents. Likewise, when a group is described as being "unsubstituted or
substituted" if
substituted, the substituent may be selected from one or more the indicated
substituents. If
no substituents are indicated, it is meant that the indicated or "substituted"
group may be
individually and independently substituted with one or more group(s)
individually and
independently selected from alkyl, alkenyl, alkynyl, cycloalkyl, aryl,
heteroaryl, heterocyclyl,
aralkyl, heteroaralkyl, (heterocyclyl)alkyl, hydroxy, protected hydroxyl,
alkoxy, aryloxy,
acyl, cyano, halogen, ester, nitro, silyl, haloalkyl, haloalkoxy, an
unsubstituted amino, a
substituted amino, and protected derivatives thereof.
[0132] As used herein, "Ca to Cb" in which "a" and "b" are integers
refer to the
number of carbon atoms in an alkyl, alkenyl or alkynyl group, or the number of
carbon atoms
in the ring of a cycloalkyl, aryl, heteroaryl or heterocyclyl group. That is,
the alkyl, alkenyl,
alkynyl, ring of the cycloalkyl, ring of the aryl, ring of the heteroaryl or
ring of the
heterocyclyl can contain from "a" to "b", inclusive, carbon atoms. Thus, for
example, a "Ci
to C4 alkyl" group or a "Ci-C4 alkyl" group refers to all alkyl groups having
from 1 to 4
carbons, that is, CH3-, CH3CH2-, CH3CH2CH2-, (CH3)2CH-, CH3CH2CH2CH2-,
CH3CH2CH(CH3)- and (CH3)3C-. Likewise, for example, a heterocyclyl group may
contain
from "a" to "b", inclusive, total atoms, such as a 3 to 10-membered
heterocyclyl group,
which includes 3 to ten total atoms (carbon and heteroatoms). If no "a" and
"b" are
designated with regard to an alkyl, alkenyl, alkynyl, cycloalkyl, aryl,
heteroaryl or
heterocyclyl group, the broadest range described in these definitions is to be
assumed.
[0133] As used herein, "alkyl" refers to a straight or branched
hydrocarbon chain
that comprises a fully saturated (no double or triple bonds) hydrocarbon
group. The alkyl
group may have 1 to 20 carbon atoms (whenever it appears herein, a numerical
range such as
"1 to 20" refers to each integer in the given range; e.g., "1 to 20 carbon
atoms" means that
the alkyl group may consist of 1 carbon atom, 2 carbon atoms, 3 carbon atoms,
etc., up to and
including 20 carbon atoms, although the present definition also covers the
occurrence of the
-78-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
term "alkyl" where no numerical range is designated). The alkyl group may also
be a
medium size alkyl having 1 to 10 carbon atoms. The alkyl group could also be a
lower alkyl
having 1 to 6 carbon atoms. The alkyl group of the compounds may be designated
as "Ci-C4
alkyl" or similar designations. By way of example only, "Ci-C4 alkyl"
indicates that there
are one to four carbon atoms in the alkyl chain, i.e., the alkyl chain is
selected from methyl,
ethyl, propyl, isopropyl, n-butyl, isobutyl, sec-butyl, and t-butyl. Typical
alkyl groups
include, but are in no way limited to, methyl, ethyl, propyl, isopropyl,
butyl, isobutyl, tertiary
butyl, pentyl, and hexyls. The alkyl group may be substituted or
unsubstituted.
[0134] As used herein, "alkenyl" refers to an alkyl group, as defined
herein, that
contains in the straight or branched hydrocarbon chain one or more double
bonds. An
alkenyl group may be unsubstituted or substituted.
[0135] As used herein, "alkynyl" refers to an alkyl group as defined
herein, that
contains in the straight or branched hydrocarbon chain one or more triple
bonds. An alkynyl
group may be unsubstituted or substituted.
[0136] As used herein, "alkylene" refers to a straight-chained or
branched alkyl
group forming bonds to connect molecular fragments via their terminal carbon
atoms.
Examples include but are not limited to methylene (-CH2-), ethylene (-CH2CH2-
), propylene
(-CH2CH2CH2-), and butylene (-CH2CH2CH2CH2-). An alkylene group can be
substituted by
replacing one or more hydrogen of the alkylene group with a sub stituent(s)
listed under the
definition of "substituted."
[0137] As used herein, "cycloalkyl" refers to a completely saturated
(no double or
triple bonds) mono- or multi- cyclic hydrocarbon ring system. When composed of
two or
more rings, the rings may be joined together in a fused fashion. Cycloalkyl
groups can
contain 3 to 10 atoms in the ring(s) or 3 to 8 atoms in the ring(s). Typical
cycloalkyl groups
include, but are in no way limited to, cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl,
cycloheptyl, and cyclooctyl. A cycloalkyl group may be unsubstituted or
substituted.
[0138] As used herein, "cycloalkyl" refers to all carbon ring systems.
Such
systems can be unsaturated, can include some unsaturation, or can contain some
aromatic
portion, or be all aromatic. Cycloalkyl group can contain from 3 to 30 carbon
atoms. A
cycloalkyl group may be unsubstituted or substituted.
-79-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0139] As
used herein, "cycloalkylalkyl" refers to an -(alkylene)-R radical where
R is cycloalkyl as defined above.
Examples include, but are not limited to,
cyclopropylmethyl and cyclohexylmethyl. A cycloalkylalkyl group may also be
referred to
as, for example, a (Ci-C6 alkyl)-cycloalkyl group. A cycloalkylalkyl group may
be
unsubstituted or substituted.
[0140] As
used herein, "aryl" refers to a carbocyclic (all carbon) monocyclic or
multicyclic aromatic ring system (including, e.g., fused, bridged, or spiro
ring systems where
two carbocyclic rings share a chemical bond, e.g., one or more aryl rings with
one or more
aryl or non-aryl rings) that has a fully delocalized pi-electron system
throughout at least one
of the rings. The number of carbon atoms in an aryl group can vary. For
example, the aryl
group can be a C6-C14 aryl group, a C6-Cio aryl group, or a C6 aryl group.
Examples of aryl
groups include, but are not limited to, benzene, naphthalene, and azulene. An
aryl group
may be substituted or unsubstituted.
[0141] As
used herein, "aralkyl" refers to an -(alkylene)-R radical where R is aryl
as defined above. Examples include, but are not limited to, benzyl and
phenethyl. An
aralkyl group may also be referred to as, for example, a (Ci-C6 alkyl)-aryl
group. An aralkyl
group may be substituted or unsubstituted.
[0142] As
used herein, "heteroaryl" refers to a monocyclic or multicyclic
aromatic ring system (a ring system having a least one ring with a fully
delocalized pi-
electron system) that contain(s) one or more heteroatoms, that is, an element
other than
carbon, including but not limited to, nitrogen, oxygen, and sulfur, and at
least one aromatic
ring. The number of atoms in the ring(s) of a heteroaryl group can vary. For
example, the
heteroaryl group can contain 4 to 14 atoms in the ring(s), 5 to 10 atoms in
the ring(s) or 5 to
6 atoms in the ring(s). Furthermore, the term "heteroaryl" includes fused ring
systems where
two rings, such as at least one aryl ring and at least one heteroaryl ring, or
at least two
heteroaryl rings, share at least one chemical bond. Examples of heteroaryl
rings include, but
are not limited to, furan, furazan, thiophene, benzothiophene, phthalazine,
pyrrole, oxazole,
benzoxazole, 1,2,3-oxadiazole, 1,2,4-oxadiazole, thiazole, 1,2,3-thiadiazole,
1,2,4-
thiadiazole, benzothiazole, imidazole, benzimidazole, indole, indazole,
pyrazole,
benzopyrazole, isoxazole, benzoisoxazole, isothiazole, triazole,
benzotriazole, thiadiazole,
tetrazole, pyridine, pyridazine, pyrimidine, pyrazine, purine, pteridine,
quinoline,
-80-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
isoquinoline, quinazoline, quinoxaline, cinnoline, and triazine. A heteroaryl
group may be
substituted or unsubstituted.
[0143] As used herein, "heteroaralkyl" refers to an -(alkylene)-R
radical where R
is heteroaryl as defined above. Examples include, but are not limited to,
methylpyridyl and
methylpyrimidyl. A heteroaralkyl group may also be referred to as, for
example, a (Ci-C6
alkyl)-heteroaryl group. A heteroaralkyl group may be substituted or
unsubstituted.
[0144] As used herein, "heterocyclic" or "heterocycly1" refers to
three-, four-,
five-, six-, seven-, eight-, nine-, ten-, up to 18-membered monocyclic,
bicyclic, and tricyclic
ring system wherein carbon atoms together with from 1 to 5 heteroatoms
constitute said ring
system. A heterocycle may optionally contain one or more unsaturated bonds
situated in
such a way, however, that a fully delocalized pi-electron system does not
occur throughout
all the rings. The heteroatoms are independently selected from oxygen, sulfur,
and nitrogen.
A heterocycle may further contain one or more carbonyl or thiocarbonyl
functionalities, so as
to make the definition include oxo-systems and thio-systems such as lactams,
lactones, cyclic
imides, cyclic thioimides, and cyclic carbamates. When composed of two or more
rings, the
rings may be joined together in a fused fashion. Additionally, any nitrogens
in a heterocycle
may be quaternized. Examples of such "heterocyclic" groups include but are not
limited to,
1,3-dioxin, 1,3-dioxane, 1,4-dioxane, 1,2-dioxolane, 1,3-dioxolane, 1,4-
dioxolane, 1,3-
oxathiane, 1,4-oxathiin, 1,3-oxathiolane, 1,3-dithiole, 1,3-dithiolane, 1,4-
oxathiane,
tetrahydro-1,4-thiazine, 2H-1,2-oxazine, maleimide, succinimide, barbituric
acid,
thiobarbituric acid, dioxopiperazine, hydantoin, dihydrouracil, trioxane,
hexahydro-1,3,5-
triazine, imidazoline, imidazolidine, isoxazoline, isoxazolidine, oxazoline,
oxazolidine,
oxazolidinone, thiazoline, thiazolidine, morpholine, oxirane, piperidine N-
oxide, piperidine,
piperazine, pyrrolidine, pyrrolidone, pyrrolidione, 4-piperidone, pyrazoline,
pyrazolidine, 2-
oxopyrrolidine, tetrahydropyran, 4H-pyran, tetrahydrothiopyran,
thiamorpholine,
thiamorpholine sulfoxide, thiamorpholine sulfone, and their benzo-fused
analogs (e.g.,
benzimidazolidinone, tetrahydroquinoline, 3,4-methylenedioxypheny1).
Heterocyclyl groups
may be substituted or unsubstituted.
[0145] As used herein, "heterocyclylalkyl" refers to an -(alkylene)-R
radical
where R is heterocyclyl as defined above. Examples include, but are not
limited to,
methylpyrrolidinyl and methylpiperidinyl. A heterocyclylalkyl group may also
be referred to
-81-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
as, for example, a (Ci-C6 alkyl)-heterocycly1 group. A heterocyclylalkyl group
may be
substituted or unsubstituted.
[0146] As
used herein, "alkoxy" refers to the formula ¨OR wherein R is an alkyl
as defined above. A non-limiting list of alkoxys is methoxy, ethoxy, n-
propoxy, n-butoxy,
isobutoxy, sec-butoxy, and tert-butoxy. An alkoxy may be substituted or
unsubstituted.
[0147] As
used herein, "alkoxyalkyl" refers to an alkyl as defined above which is
substituted with one or two alkoxy groups as defined above. Examples include,
but are not
limited to methoxyethyl, ethoxyethyl, and methoxypropyl. An alkoxyalkyl group
may be
substituted or unsubstituted.
[0148] As
used herein, "acyl" refers to a hydrogen, alkyl, alkenyl, alkynyl, or
aryl, as defined above, connected as substituents via a carbonyl group, as
defined herein.
Examples include formyl, acetyl, benzoyl, and acryl, with preferred acyl
groups being Ci-C6
alkyl carbonyl groups. An acyl may be substituted or unsubstituted.
[0149] As
used herein, "hydroxyalkyl" refers to an alkyl group in which one or
more of the hydrogen atoms are replaced by a hydroxy group. Exemplary
hydroxyalkyl
groups include but are not limited to, 2-hydroxyethyl, 3-hydroxypropyl, 2-
hydroxypropyl,
and 2,2-dihydroxyethyl. A hydroxyalkyl may be substituted or unsubstituted.
[0150] As
used herein, "haloalkyl" refers to an alkyl group in which one or more
of the hydrogen atoms are replaced by a halogen (e.g., mono-haloalkyl, di-
haloalkyl, and tri-
haloalkyl). Such groups include but are not limited to, chloromethyl,
fluoromethyl,
difluoromethyl, trifluoromethyl and 1-chloro-2-fluoromethyl, 2-fluoroisobutyl.
A haloalkyl
may be substituted or unsubstituted.
[0151] As
used herein, "haloalkoxy" refers to an alkoxy group in which one or
more of the hydrogen atoms are replaced by a halogen (e.g., mono-haloalkoxy,
di-haloalkoxy
and tri- haloalkoxy).
Such groups include but are not limited to, chloromethoxy,
fluoromethoxy, difluoromethoxy, trifluoromethoxy and 1-chloro-2-fluoromethoxy,
2-
fluoroisobutoxy. A haloalkoxy may be substituted or unsubstituted.
[0152] As
used herein, "aryloxy" refers to ¨OR, in which R is an aryl, as defined
above, such as but not limited to phenyl. An aryloxy may be substituted or
unsubstituted.
-82-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0153] The term "ester" refers to a "-C(=0)0R" group in which R can
be, for
example, alkyl, alkenyl, alkynyl, aryl, heteroaryl, heterocyclyl, or
cycloalkyl. An ester may
be substituted or unsub stituted.
[0154] The term "unsubstituted amino" as used herein refers to a ¨NH2
group.
[0155] The term "substituted amino" as used herein refers to a ¨NRaRb
group,
wherein Ra and Rb are independently selected from hydrogen, alkyl, aryl,
heteroaryl,
cycloalkyl, heterocyclyl, aralkyl, heteroaralkyl, cycloalkylalkyl, and
heterocyclylalkyl, as
defined herein; and not more than one of wherein Ra and Rb can be hydrogen.
[0156] As used herein, the term "hydroxy" refers to a ¨OH group.
[0157] A "cyano" group refers to a "-CN" group.
[0158] A "carbonyl" group refers to a CO group.
[0159] The term "halogen atom" or "halogen" as used herein, means any
one of
the radio-stable atoms of column 7 of the Periodic Table of the Elements, such
as, fluorine,
chlorine, bromine, and iodine.
[0160] In all of the definitions described herein, the terms used to
define a new
term are as previously defined herein.
[0161] Where the numbers of substituents is not specified (e.g.,
haloalkyl), there
may be one or more substituents present. For example "haloalkyl" may include
one or more
of the same or different halogens. As another example, "Ci-C3 alkoxyphenyl"
may include
one or more of the same or different alkoxy groups containing one, two, or
three atoms.
[0162] As used herein, the abbreviations for any protective groups,
amino acids
and other compounds, are, unless indicated otherwise, in accord with their
common usage,
recognized abbreviations, or the IUPAC-IUB Commission on Biochemical
Nomenclature
(See, Biochem. 11:942-944 (1972)).
[0163] The terms "protecting group" and "protecting groups" as used
herein refer
to any atom or group of atoms that is added to a molecule in order to prevent
existing groups
in the molecule from undergoing unwanted chemical reactions. Examples of
protecting
group moieties are described in T. W. Greene and P. G. M. Wuts, Protective
Groups in
Organic Synthesis, 3. Ed. John Wiley & Sons, 1999, and in J.F.W. McOmie,
Protective
Groups in Organic Chemistry Plenum Press, 1973, both of which are hereby
incorporated by
reference for the limited purpose of disclosing suitable protecting groups.
The protecting
-83-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
group moiety may be chosen in such a way, that they are stable to certain
reaction conditions
and readily removed at a convenient stage using methodology known from the
art. A non-
limiting list of protecting groups include benzyl; substituted benzyl; alkyl
carb onyl s (e.g., t-
butoxycarbonyl (BOC), acetyl, or isobutyryl); arylalkylcarbonyls (e.g.,
benzyloxycarbonyl or
benzoyl); substituted methyl ether (e.g., methoxymethyl ether); substituted
ethyl ether; a
substituted benzyl ether; tetrahydropyranyl ether; silyl ethers (e.g.,
trimethylsilyl,
triethylsilyl, triisopropylsilyl, t-butyldimethylsilyl, or t-
butyldiphenylsilyl); esters (e.g.,
benzoate ester); carbonates (e.g., methoxymethylcarb onate); sulfonates (e.g.,
to syl ate or
mesylate); acyclic ketal (e.g., dimethyl acetal); cyclic ketals (e.g., 1,3-
dioxane or 1,3-
dioxolanes); acyclic acetal; cyclic acetal; acyclic hemiacetal; cyclic
hemiacetal; cyclic
dithioketal s (e.g., 1,3 -dithi ane or 1,3 -dithi ol ane); and tri arylm ethyl
groups (e.g., trityl;
monomethoxytrityl (MNITr); 4,4'-dimethoxytrityl (DMTr); or 4,4',4"-
trimethoxytrityl
(TMTr)).
[0164] "Leaving group" as used herein refers to any atom or moiety
that is
capable of being displaced by another atom or moiety in a chemical reaction.
More
specifically, in some embodiments, "leaving group" refers to the atom or
moiety that is
displaced in a nucleophilic substitution reaction. In some embodiments,
"leaving groups" are
any atoms or moieties that are conjugate bases of strong acids. Examples of
suitable leaving
groups include, but are not limited to, tosylates and halogens. Non-limiting
characteristics
and examples of leaving groups can be found, for example in Organic Chemistry,
2d ed.,
Francis Carey (1992), pages 328-331; Introduction to Organic Chemistry, 2d
ed., Andrew
Streitwieser and Clayton Heathcock (1981), pages 169-171; and Organic
Chemistry, 5th ed.,
John McMurry (2000), pages 398 and 408; all of which are incorporated herein
by reference
for the limited purpose of disclosing characteristics and examples of leaving
groups.
[0165] The term "pharmaceutically acceptable salt" as used herein is a
broad
term, and is to be given its ordinary and customary meaning to a person of
ordinary skill in
the art (and is not to be limited to a special or customized meaning), and
refers without
limitation to a salt of a compound that does not cause significant irritation
to an organism to
which it is administered and does not abrogate the biological activity and
properties of the
compound. In some embodiments, the salt is an acid addition salt of the
compound.
Pharmaceutical salts can be obtained by reacting a compound with inorganic
acids such as
-84-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
hydrohalic acid (e.g., HC1 or HBr), H2SO4, HNO3, and H3PO4. Pharmaceutical
salts can also
be obtained by reacting a compound with an organic acid such as aliphatic or
aromatic
carboxylic or sulfonic acids, for example formic acid, AcOH, propionic acid,
glycolic acid,
pyruvic acid, malonic acid, maleic acid, fumaric acid, trifluoroacetic acid,
benzoic acid,
cinnamic acid, mandelic acid, succinic acid, lactic acid, malic acid, tartaric
acid, citric acid,
ascorbic acid, nicotinic acid, methanesulfonic acid, ethanesulfonic acid, p-
toluensulfonic
acid, salicylic acid, stearic acid, muconic acid, butyric acid, phenylacetic
acid, phenylbutyric
acid, valproic acid, 1,2-ethanedisulfonic acid, 2-hydroxyethanesulfonic acid,
benzenesulfonic
acid, 2-naphthalenesulfonic acid, or naphthalenesulfonic acid. Pharmaceutical
salts can also
be obtained by reacting a compound with a base to form a salt such as an
ammonium salt, an
alkali metal salt, such as a Li, Na, or a K salt, an alkaline earth metal
salt, such as a Ca, Mg,
or Al salt, a salt of organic bases such as dicyclohexylamine, N-methyl-D-
glucamine,
tris(hydroxymethyl)methylamine, a C1-C7 alkylamine, cyclohexylamine,
dicyclohexylamine,
triethanolamine, ethylenediamine, ethanolamine, diethanolamine,
triethanolamine,
tromethamine, and salts with amino acids such as arginine and lysine; or a
salt of an
inorganic base, such as Al(OH)3, Ca(OH)2, KOH, Na2CO3, NaOH, or the like.
[0166] Some embodiments provide pharmaceutically acceptable salts of
Formula
(II). In some embodiments, the salt is selected from the group consisting of
hydrochloride,
sulfate, hemisulfate, acetate, fumarate, malate, and citrate.
[0167] The term "solvate" as used herein is a broad term, and is to be
given its
ordinary and customary meaning to a person of ordinary skill in the art (and
is not to be
limited to a special or customized meaning), and refers without limitation to
mean that the
solvent is complexed with a compound in a reproducible molar ratio, including,
but not
limited to, 0.5:1, 1:1, or 2:1. Thus, the term "pharmaceutically acceptable
solvate," refers to
a solvate wherein the solvent is one that does not cause significant
irritation to an organism
to which it is administered and does not abrogate the biological activity of
the compound.
[0168] Some embodiments provide solvates of Formula (I). In
some
embodiments, the solvent in the solvate is selected from water, ethanol, and
acetone, or
combinations thereof.
[0169] It is understood that, in any compound described herein having
one or
more chiral centers, if an absolute stereochemistry is not expressly
indicated, then each
-85-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
center may independently be of R-configuration or S-configuration or a mixture
thereof.
Thus, the compounds provided herein may be enantiomerically pure,
enantiomerically
enriched, or may be stereoisomeric mixtures, and include all diastereomeric,
and
enantiomeric forms. In addition it is understood that, in any compound
described herein
having one or more double bond(s) generating geometrical isomers that can be
defined as E
or Z, each double bond may independently be E or Z a mixture thereof
Stereoisomers are
obtained, if desired, by methods such as, stereoselective synthesis and/or the
separation of
stereoisomers by chiral chromatographic columns. Likewise, it is understood
that, in any
compound described, all tautomeric forms are also intended to be included.
[0170]
Wherever a substituent is depicted as a di-radical (i.e., has two points of
attachment to the rest of the molecule), it is to be understood that the
substituent can be
attached in any directional configuration unless otherwise indicated. Thus,
for example, a
substituent depicted as ¨AE¨ or E
includes the substituent being oriented such
that the A is attached at the leftmost attachment point of the molecule as
well as the case in
which A is attached at the rightmost attachment point of the molecule.
[0171] It
is to be understood that where compounds disclosed herein have
unfilled valencies, then the valencies are to be filled with hydrogens and/or
deuteriums.
[0172] It
is understood that the compounds described herein can be labeled
isotopically or by another other means, including, but not limited to, the use
of chromophores
or fluorescent moieties, bioluminescent labels, or chemiluminescent labels.
Substitution with
isotopes such as deuterium may afford certain therapeutic advantages resulting
from greater
metabolic stability, such as, for example, increased in vivo half-life or
reduced dosage
requirements. Each chemical element as represented in a compound structure may
include
any isotope of said element. For example, in a compound structure a hydrogen
atom may be
explicitly disclosed or understood to be present in the compound. At any
position of the
compound that a hydrogen atom may be present, the hydrogen atom can be any
isotope of
hydrogen, including but not limited to hydrogen-1 (protium), hydrogen-2
(deuterium), and
hydrogen-3 (tritium). Thus, reference herein to a compound encompasses all
potential
isotopic forms unless the context clearly dictates otherwise.
-86-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0173] It is understood that the methods and formulations described
herein
include the use of salts, solvates, hydrates, and conformers of compounds of
preferred
embodiments, as well as metabolites and active metabolites of these compounds
having the
same type of activity. A conformer is a structure that is a conformational
isomer.
Conformational isomerism is the phenomenon of molecules with the same
structural formula
but different conformations (conformers) of atoms about a rotating bond. In
specific
embodiments, the compounds described herein exist in solvated forms with
pharmaceutically
acceptable solvents such as water, ethanol, or the like. In other embodiments,
the
compounds described herein exist in unsolvated form. Solvates contain either
stoichiometric
or non-stoichiometric amounts of a solvent, and may be formed during the
process of
crystallization with pharmaceutically acceptable solvents such as water,
ethanol, or the like.
Hydrates are formed when the solvent is water, or alcoholates are formed when
the solvent is
alcohol. In addition, the compounds provided herein can exist in unsolvated as
well as
solvated forms. In general, the solvated forms are considered equivalent to
the unsolvated
forms for the purposes of the compounds and methods provided herein. Compounds
of
Formula (I) can also be provided as, for example, amorphous forms, milled
forms and nano-
particulate forms.
[0174] Likewise, it is understood that the compounds described herein,
such as
compounds of preferred embodiments, include the compound in any of the forms
described
herein (e.g., pharmaceutically acceptable salts, crystalline forms, amorphous
form, solvated
forms, enantiomeric forms, tautomeric forms, and the like).
Dosing Regimes
[0175] In some embodiments, about 1 mg to about 5 grams of a compound
of
Formula (I) is administered each day. In some embodiments, about 2 mg to about
2 grams of
a compound of Formula (I) is administered each day. In some embodiments, the
amount of a
compound of Formula (I) administered each day is, or is about, 5 mg to 1 gram;
10 mg to 800
mg; 20 mg to 600 mg; 30 mg to 400 mg; 40 mg to 200 mg; 50 mg to 100 mg; or any
amount
in between.
[0176] In some embodiments, about 1 mg to about 5 grams of a compound
of
Formula (I) is administered each week. In some embodiments, about 2 mg to
about 2 grams
of a compound of Formula (I) is administered each week. In some embodiments,
the amount
-87-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
of a compound of Formula (I) administered each week is, or is about, 5 mg to 1
gram; 10 mg
to 800 mg; 20 mg to 600 mg; 30 mg to 400 mg; 40 mg to 200 mg; 50 mg to 100 mg;
or any
amount in between.
[0177] In some embodiments, about 1 mg to about 5 grams of a compound
of
Formula (I) is administered each cycle of treatment. In some embodiments,
about 2 mg to
about 2 grams of a compound of Formula (I) is administered each cycle of
treatment. In
some embodiments, the amount of a compound of Formula (I) administered each
cycle of
treatment is, or is about, 5 mg to 1 gram; 10 mg to 800 mg; 20 mg to 600 mg;
30 mg to 400
mg; 40 mg to 200 mg; 50 mg to 100 mg; or any amount in between.
[0178] In some embodiments, a compound of Formula (I) is administered
at least
once per day; twice per day; three times per day; or four times per day. In
some
embodiments, a compound of Formula (I) is administered at least once per week;
twice per
week; three times per week; or four times per week. In some embodiments, each
cycle of
treatment lasts 1 day; 2 days; 3 days; 4 days; 5 days; 6 days; 7 days; 8 days;
9 days; 10 days;
11 days; 12 days; 13 days; 14 days, or any time in between. In some
embodiments, each
cycle of treatment has at least 1 day; 2 days; 3 days; 4 days; 5 days; 6 days;
7 days; 8 days; 9
days; 10 days; 11 days; 12 days; 13 days; or 14 days, between administrations
of a compound
of Formula (I).
[0179] In some embodiments, a compound of Formula (I) is provided
intravenously over about 10 min; about 20 min; about 30 min; about 1 hour;
about 1.5 hrs;
about 2 hrs; about 2.5 hrs; about 3 hrs; about 3.5 hrs; about 4 hrs, or any
time in between.
EXAMPLES
[0180] Additional embodiments are disclosed in further detail in the
following
examples, which are not in any way intended to limit the scope of the claims.
[0181] Characterization of the compounds disclosed herein was
performed with
Bruker AV-500 and DRX-500 NMR spectrometers and a Perkin Elmer PE-SCIEX API-
150
mass spectrometer.
[0182] In the synthetic procedures described herein, "workup and
purification"
refers to combining organic layers after an aqueous phase extraction, washing
with brine,
drying over Na2SO4, filtering, concentrating, and purified by silica gel
chromatography with
-88-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
the indicated solvent system. The workup and purification may also include an
optional
washing step with 10% aq. Na2S203/ sat. aq. NaHCO3 (1:1) prior to the wash
with brine.
Example 1
Compound 1: (S)-3 -(4 -((4-(morpholinomethyl)b enzyl)oxy)-1-oxoi soindolin-2 -
yl)azepane-
2 7-dione
o 0 H
0 0 H
101 NI..
0 0 H 1.1
101
OH 1.2 40 e 1.4 l 1.3
CHO
)C'
00 H
0 0 H
0 0
NI'.
Co)
=1.5
CHO Compound 1
Lo
[0183] To a solution of 4-(diethoxymethyl)benzaldehyde (2.43 g, 11.67 mmol)
in
Me0H (40 mL) at 0 C was added NaBH4 (886.7 mg, 23.34 mmol). The suspension was
stirred at RT for 3 h. The solvent was then removed, and the residue was
diluted with water
and extracted with DCM. The combined organic layers were dried over Na2SO4,
filtered,
concentrated, and the residue purified by silica gel chromatography with Et0Ac
in pet. ether
(10% to 23%) to give (4-(diethoxymethyl)phenyl)methanol 1.1 (2.21 g, 90%
yield) as a
colorless oil. MS (ESI) m/z 165.1 [M-42] +.
[0184] To a solution of compound 1.2 (323 mg, 1.24 mmol) in THF (20 mL) at
0 C was added 1.1 (326.5 mg, 1.55 mmol) followed by PPh3 (650.4 mg, 2.48
mmol). A
solution of DEAD (431.9 mg, 2.48 mmol) in THF (1 mL) was added dropwise and
the
suspension was stirred at RT for 16 h. The solvent was removed, and the
residue was
purified by silica gel chromatography using Et0Ac in pet. ether (40% to 100%)
to give
compound 1.3 (373 mg, 66.7% yield) as a white solid. MS (ESI) m/z 407.1 [M-
45]t
-89-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0185] To a solution of compound 1.3 (473 mg, 1.05 mmol) in THF (10
mL) at
RT was added 4 M HC1 (1.31 mL), and the reaction was stirred at RT for 30 min.
The
solvent was removed, and the residue was dried under vacuum to give compound
1.4 (397
mg, 100% yield) as a white solid. MS (ESI) m/z 379.1 [M+1]+.
[0186] To a solution of compound 1.4 (396.9 mg, 1.05 mmol) in
fluorobenzene/DMSO (30 mL/5 mL, 1 drop water in DMSO) was added Dess-Martin
reagent
(1.12 g, 2.63 mmol). The suspension was heated at 80 C for 18 h. Additional
Dess-Martin
reagent (550 mg) was added and the mixture was heated at 80 C for 5 h. The
mixture was
cooled to RT and filtered. The filtrate was added to sat. aq. Na2S203. The
suspension was
stirred at 0 C for 5 min then extracted with DCM. Workup and purification by
silica gel
chromatography using Et0Ac in pet. ether (40-100%) provided compound 1.5 (184
mg, 45%
yield) as a white solid. MS (ESI) m/z 393.1 [M+1]+.
[0187] To a solution of compound 1.5 (76 mg, 0.194 mmol) in DCM (10
mL) was
added morpholine (25.3 mg, 0.291 mmol) followed by NaBH(OAc)3 (82.3 mg, 0.384
mmol).
The mixture was stirred at RT for 48 h, concentrated, purified by prep-TLC
using Et0Ac
then further purified by prep-HPLC (5 M C18 column, 0.1% TFA in H20, 0.1% TFA
in
ACN, 5%-95% 0.1%TFA in ACN) to afford Compound 1(30.7 mg, 21.7% yield) as a
white
solid. 1H NMR (DMSO-d6, 400 MHz) 6: 10.70 (s, 1 H), 7.49-7.45 (m, 3H), 7.35-
7.31 (m,
4H), 5.24-5.21 (m, 3H), 4.47 (s, 2H), 3.57 (s, 4H), 3.47 (s, 2H), 3.08 (t, J=
11.2 Hz, 1 H),
2.59-2.54 (m, 1H), 2.35 (s, 5H), 2.12-1.97 (m, 2H), 1.86-1.74 (m, 1H). MS
(ESI) m/z 464.1
[M+H]t
Example 2
Compound 2: (S)-3 -(4-((4-((2,3 -dihydro-4H-b enzo[b ] [1,4] oxazin-4-
yl)methyl)b enzyl)oxy)-
1-oxoi soindolin-2-yl)azepane-2, 7-dione
o 0 H
0 0 H
1101 NI-
H
CNI,. o 0
0
1.5
- 40
40
N Compound 2
CHO
So
-90-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0188] To a solution of compound 1.5 (90 mg, 0.229 mmol) in AcOH (4
mL) at
RT was added 3,4-dihydro-2H-benzo[b][1,4]oxazine (46.5 mg, 0.344 mmol)
followed by
NaBH(OAc)3 (145.6 mg, 0.687 mmol). The mixture was stirred at RT for 3 h, the
solvent
removed, and the residue purified by prep-TLC using Et0Ac in pet. ether (1:2)
then further
purified by prep-HPLC as previously described to afford Compound 2 (45.0 mg,
39% yield)
as a white solid. 1-EINMR (DMSO-d6, 400 MHz) 6: 10.69 (s, 1 H), 7.49-7.45 (m,
3H), 7.35-
7.31 (m, 4H), 6.70-6.63 (m, 3H), 6.51 (t, J= 7.2 Hz, 1H), 5.23 -5.19 (m, 3H),
4.47 (d, J= 8.8
Hz, 4H), 4.21 (t, J= 3.6 Hz, 2H), 3.39-3.35 (m, 2H), 3.06 (t, J= 13.2 Hz, 1
H), 2.57 (d, J=
18.0 Hz, 1H), 2.36-2.29 (m, 1H), 2.09-1.99 (m, 2H), 1.85-1.74 (m, 1H). MS
(ESI) m/z 512.2
[M+H]
Example 3
Compound 3: ((S)-3-(4-((3-chloro-4-(morpholinomethyl)benzyl)oxy)-1-
oxoisoindolin-2-
yl)azepane-2,7-dione
= =
0 3.1 -
o 0 H
0 H
N1,.. 1\zi Nõ,\I;
H =
0
0
OH
3.2 40
CI 101 c 3.4
3.3
O 0 CHO
0 0 H 00 H
401
(0)
0 0
3.5
411 Compound 3
ci CI
CHO NTh
[0189] To a solution of methyl 3-chloro-4-formylbenzoate (500 mg, 2.52
mmol)
in methanol (10 mL) at RT was added 2,2-dimethoxypropane (393.6 mg, 3.78 mmol)
followed by p-Ts0H (48 mg, 0.252 mmol). The mixture was refluxed for 16 h, the
solvent
was removed, the residue diluted with Et0Ac (10 mL), washed with sat. aq.
NaHCO3, dried
over Na2SO4, filtered, and concentrated to give methyl 3-chloro-4-
(dimethoxymethyl)
benzoate (540 mg, 87.8% yield) as a yellow oil. 1-EINMR (DMSO-d6, 300 MHz) 6
7.94 (s, 2
H), 7.71 (d, J= 8.4 Hz, 1H), 5.60 (s, 1H), 3.87 (s, 3H), 3.31 (d, J= 0.9 Hz,
6H).
-91-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0190] To a solution of methyl 3-chloro-4-(dimethoxymethyl)benzoate
(480 mg,
2.05 mmol) in THF (20 mL) at 0 C was added LiA1H4 (3.074 mL, 1 M in THF). The
suspension was stirred at RT for 2 h. To the reaction was slowly added water
(1 mL) then
10% NaOH (2 mL) followed by water (1 mL). The suspension was filtered, and the
filtrate
was extracted with Et0Ac. The combined organic layers were dried over Na2SO4,
filtered
and concentrated to give crude (3-chloro-4-(dimethoxymethyl)phenyl) methanol
3.1 (380
mg) as a yellow oil, which was used directly in the next step without further
purification. 11-1
NMR (CDC13, 300 MHz) 6 7.60 (d, J = 8.1 Hz,1 H), 7.39 (s, 1H), 7.26 (d, J= 7.2
Hz, 1H),
5.63 (s, 1H), 4.67 (s, 2H), 3.38 (d, J= 0.9 Hz, 6H).
[0191] To a solution of compound 3.1 (264 mg, 1.22 mmol) in THF (15
mL) at
0 C was added compound 3.2 (260 mg, 1.0 mmol) followed by PPh3 (524.6 mg, 2.0
mmol).
A solution of DEAD (348.3 mg, 2.0 mmol) in THF (1 mL) was added dropwise and
the
suspension was stirred at RT for 16 h. The solvent was removed, and the
residue was
purified by silica gel chromatography with Et0Ac in pet. ether (40% to 100%)
to give
compound 3.3 (262 mg, 39% yield) as a white solid. MS (ESI) m/z 427.1 [M-31]+.
[0192] To a solution of compound 3.3 (262 mg, 0.572 mmol) in THF (10
mL) at
RT was added 4 M HC1 (0.72 mL). The reaction was stirred at RT for 1 hour. The
solvent
was removed, and the residue was dried under vacuum to give compound 3.4
(235.7 mg,
100% yield) as a white solid. MS (ESI) m/z 413.0 [M+1]+.
[0193] To a solution of compound 3.4 (235.7 mg, 0.572 mmol) in
fluorobenzene/DMSO (30 mL/5 mL, 1 drop water in DMSO) was added Dess-Martin
reagent
(607 mg, 1.43 mmol). The suspension was heated at 80 C for 18 h then cooled
to RT.
Additional Dess-Martin reagent (303 mg) was added and the mixture was heated
at 80 C for
h and cooled to RT. The suspension was filtered, and the filtrate was added to
sat. aq.
Na2S203 (30 mL). The suspension was stirred at 0 C for 5 min then extracted
with DCM.
Workup and purification by silica gel chromatography using Et0Ac in pet. ether
(20% to
80%) provided compound 3.5 (72 mg, 30% yield) as a white solid. MS (ESI) m/z
427.0
[M+1]
[0194] To a solution of compound 3.5 (54 mg, 0.127 mmol) in DCM (5 mL)
at
RT was added morpholine (22.1 mg, 0.254 mmol) followed by NaBH(OAc)3 (80.8 mg,
0.38
mmol). The mixture was stirred at RT for 24 h. The solvent was removed, and
the residue
-92-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
was purified by prep-TLC using Et0Ac/petroleum ether (2:1) then by prep-HPLC,
as
previously described, to afford Compound 3 (19.9 mg, 32% yield) as a white
solid. NMR
(DMSO-d6, 400 MHz) 6: 10.70 (s, 1 H), 7.57 (s, 1H), 7.53-7.45 (m, 3H), 7.33
(t, J = 6.8 Hz,
2H), 5.27 (s, 2H), 5.20 (d, J = 5.2 Hz, 1H), 4.49 (s, 2H), 3.59-3.56 (m, 6H),
3.11-3.03 (m,
1H), 2.58 (d, J= 17.2 Hz, 1H), 2.42 (s, 4H), 2.38-2.33 (m, 1H), 2.14-1.99 (m,
2H), 1.86-1.76
(m, 1H). MS (ESI) m/z 498.1 [M+H]t
Example 4
Compound 4: (S)-3 -(4- { [p-(4-Piperidyl)phenyl]methoxy I -2-i soindolinoy1)-
2,7-azepanedione
0 o H
N ..=
0
[0195] To
a solution of ethyl 4-iodobenzoate (750 mg, 2.72 mmol), tert-butyl 4-
(4,4, 5,5 -tetram ethyl-1,3 ,2-di oxab orol an-2-y1)-5,6-di hy dropyri dine-
1(21/)-carb oxyl ate (923
mg, 2.99 mmol) in DMF (50 mL) at RT were added [1,1'-
bis(diphenylphosphino)ferrocene]
dichloropalladium(II) (400 mg) and K2CO3 (1.12 g, 8.16 mmol). The suspension
was stirred
at 85 C for 16 hrs. The mixture was filtered through a pad of Celite. The
filtrate was
diluted with water and extracted with Et0Ac. Workup and purification with
petroleum
ether/Et0Ac (8:1) provided 4-tert-butyl 4'-ethyl 1,2,3,6-tetrahydro-[1,1'-
bipheny1]-4,4'-
dicarboxylate (720 mg, 80% yield) as a white solid.
[0196] To
a solution of 4-tert-butyl 4'-ethyl 1,2,3,6-tetrahydro-[1,1'-bipheny1]-
4,4'-dicarboxylate (720 mg, 2.18 mmol) in Et0H (20mL) was added palladium on
activated
carbon (80 mg) at RT. The mixture was stirred at RT for 3 hrs. The mixture was
filtered
through a pad of Celite and the filtrate was concentrated to obtain the crude
product, which
was purified by silica gel chromatography eluting with petroleum ether/Et0Ac
(7:1) to give
ethyl 4-(4-(tert-butoxycarbonyl)cyclohexyl)benzoate (680 mg, 93% yield) as a
colorless oil.
[0197] To
a solution of ethyl 4-(4-(tert-butoxycarbonyl)cyclohexyl)benzoate (680
mg, 2.04 mmol) in THF (15mL) at 0 C was added LAH (1.0 M in THF, 1.63 mL,
4.08
-93-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
mmol) dropwise. The mixture was stirred at RT for 2 hrs. The reaction was
quenched with
Na2SO4 decahydrate (5.0 g). After stirring at RT for 1 hour, the mixture was
filtered,
concentrated, and purified by silica gel chromatography eluting with Me0H in
DCM (3%) to
give tert-butyl 4-(4-(hydroxymethyl)phenyl)piperidine-1- carboxylate (410 mg,
69% yield)
as a white solid.
[0198] To a solution of tert-butyl 4-(4-
(hydroxymethyl)phenyl)piperidine-1-
carboxylate (186 mg, 0.64 mmol), (S)-4-hydroxy-2-(2-oxoazepan-3-yl)isoindolin-
1-one (200
mg, 0.51 mmol), triphenylphosphine (524 mg, 1.024 mmol) in THF (10 mL) at 0 C
was
added DEAD (178 mg, 1.024 mmol) dropwise. The mixture was stirred at RT for 16
hrs then
concentrated to give the crude product which was purified by prep-HPLC (5 M
C18
column, 0.1% TFA in H20, 0.1% TFA in ACN, 5%-95% 0.1%TFA in ACN) to afford (5)-
tert-butyl 4-(4-(((1-oxo-2-(2-oxoazepan-3-yl)isoindolin-4-y1)
oxy)methyl)phenyl)piperidine-
l-carboxylate (200 mg, 56% yield) as a white solid. MS (ESI) m/z 478.1 [M+H-
13u]+.
[0199] To a solution of (S)-tert-butyl 4-(4-(((1-oxo-2-(2-oxoazepan-3-
yl)isoindolin-4-y1) oxy)methyl)phenyl)piperidine-l-carboxylate (200 mg, 0.375
mmol) in
fluorobenzene/DMSO (10 mL/1mL, 1 drops water in DMSO) at RT was added Dess-
Martin
reagent (397 mg, 0.94 mmol). The mixture was heated to 80 C for 18 hrs. The
mixture was
then cooled to 0 C and quenched with sat. aqueous sodium thiosulfate solution
(25 mL).
After stirring at 0 C for 15 min, the mixture was extracted with DCM. Workup
and
purification with Et0Ac/pet. ether (20% to 80%) provided (S)-tert-butyl 4-(4-
(((2-(2,7-
dioxoazepan-3-y1)-1-oxoi soindolin-4-yl)oxy)methyl) phenyl)piperi dine-l-carb
oxyl ate (32
mg, 15% yield) as a white solid.
[0200] To a solution of (5)-tert-butyl 4-(4-(((2-(2,7-dioxoazepan-3-
y1)-1-
oxoisoindolin-4-y1) oxy)methyl)phenyl)piperidine-l-carboxylate (110 mg, 0.2
mmol) in
DCM (4 mL) was added TFA (1 mL). The mixture was stirred at RT for 2 hrs then
a sat.
NaHCO3 solution was added to reach pH = 7, and the mixture was extracted with
DCM. The
combined organic phases were dried over anhydrous Na2SO4, filtered, and
concentrated to
give the crude product which was purified by prep-HPLC as previously described
to afford
Compound 4 (2.5 mg, 6.2% yield) as a white solid. 'El NMR (DMSO-d6, 400 MHz)
6: 10.71
(s, 1 H), 8.64 (s, 1H), 8.40 (s, 1H), 7.49 (m, 3H), 7.31 (m, 4H), 5.23 (m,
3H), 3.12 (s, 2H),
-94-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
2.85 (m, 2H), 2.55 (m, 3H), 2.32 (m, 1H), 2.12 (m, 4H), 2.07 (m, 3H). MS (ESI)
m/z 448.0
[M+H]t
Example 5
Compound 5: (S)-3 -(4- { [p-(Aminomethyl)phenyl]methoxy -2-i soindolinoy1)-2,7-
azepanedione trifluoroacetic acid
o H
N =
0
TFA
NH2
[0201] To a solution of tert-butyl (3 -
(4-((4-((3 -(N-(tert-
butyl)sulfamoyl)phenyl)amino)-5- methylpyrimidin-2-
yl)amino)phenoxy)propyl)carbamate
(120 mg, 0.205 mmol) in DCM (8 mL) at RT was added TFA (2 mL). The mixture was
stirred for 2 hrs. The
solvent was evaporated to give 3-((2-((4-(3-
aminopropoxy)phenyl)amino)-5-methylpyrimidin-4-yl)amino)-N-(tert-
butyl)benzenesulfonamide (102 mg, crude) which was used directly in the next
step without
further purification. MS (ESI) m/z 485.1 [M+H]
[0202] To
a solution of (S)-4-hydroxy-2-(2-oxoazepan-3-yl)isoindolin-1-one (700
mg, 2.69 mmol), tert-butyl 4-(hydroxymethyl)benzylcarbamate (804 mg, 3.37
mmol), and
triphenylphosphine (1.41g, 5.38 mmol) in THF (10 mL) at RT was added a
solution of
DEAD (938.7 mg, 5.38 mmol) in THF (1 mL). The mixture was stirred at RT for 16
hrs.
The solvent was evaporated, and the residue was purified by silica gel
chromatography
eluting with Me0H in DCM (from 0% to 7%) to give (S)-tert-butyl 4-(((1-oxo-2-
(2-
oxoazepan-3-yl)isoindolin-4-yl)oxy)methyl)benzyl carbamate (674 mg, 52% yield)
as a
white solid. MS (ESI) m/z 480.2 [M+H]t
[0203] To
a solution of (S)-tert-butyl 4-(((1-oxo-2-(2-oxoazepan-3-yl)isoindolin-
4-y1) oxy)methyl)benzylcarbamate (674 mg, 1.41 mmol) in fluorobenzene/DMSO (30
mL/5
mL, 1 drops H20 in DMSO) at RT was added Dess-Martin reagent (1.49 g, 3.52
mmol). The
mixture was stirred at 80 C for 16 hrs. The mixture was cooled to RT and
filtered. The
filtrate was added to a cooled sat. aqueous sodium thiosulfate solution (30
mL) and stirred at
-95-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
0 C for 5 min then extracted with DCM. Workup and purification with
Et0Ac/petroleum
ether from 40% to 100% provided (S)-tert-butyl 4-(((2-(2,7-dioxoazepan-3-y1)-1-
oxoisoindolin-4-y1) oxy)methyl)benzylcarbamate (320 mg, 46% yield) as a white
solid. MS
(ESI) m/z 494.2[M+H]t
[0204] To a solution of (S)-tert-butyl 4-(((2-(2,7-dioxoazepan-3-y1)-1-
oxoisoindolin-4-y1) oxy)methyl)benzylcarbamate (22 mg, 0.0446 mmol) in DCM (2
mL) was
added TFA (0.5 mL) at RT. The mixture was stirred at RT for 2 hrs. The solvent
was
evaporated to give the desired product which was lyophilized to afford
Compound 5 (17.6
mg, 78% yield) as a white solid. MS (ESI) m/z 394.1 [M+1]+. 1-E1 NMR (400 MHz,
DMSO-
d6) 6 10.71 (s, 1H), 8.18 (s, 1H), 7.55 (d, J = 7.6 Hz, 2H), 7.48-7.45 (m,
3H), 7.33-7.30 (m,
2H), 5.30 (s, 2H), 5.23 (dd, J = 4.8, 12.4 Hz, 1H), 4.48 (s, 2H), 4.05 (d, J =
5.2 Hz, 2H),
3.11-3.05 (m, 1H), 2.60-2.53 (m, 1H), 2.34-2.31 (m, 1H), 2.11-1.99 (m, 2H),
1.85-1.80 (m,
1H).
Example 6
Compound 6: (S)-3 -(4- { [p-( { 2- [3 -(p- {5-Methy1-4-[m-(tert-
butylaminosulfonyl)
phenylamino1-2-pyrimidinylamino}phenoxy)propylaminolacetylamino}methyl)phenyll
methoxy } -2-i soindolinoy1)-2, 7-azepanedione
o H
0
101 N ..=
0 HN,kNNH
0 110 110
N)L.k110 011
0
[0205] To a solution of (S)-3-(4-((4-(aminomethyl)benzyl)oxy)-1-
oxoisoindolin-
2-yl)azepane-2,7-dione (112 mg, 0.284 mmol) in DCM (5 mL) at RT was added TEA
(24.2
mg, 0.175 mmol) followed by 2-bromoacetyl chloride (45 mg, 0.284mmo1). The
mixture
was stirred for 1 hour. The solvent was evaporated, and the residue was
purified by silica gel
chromatography eluting with Et0Ac/petroleum ether from 20% to 100% to give (S)-
2-
bromo-N-(4-(((2-(2,7-dioxoazepan-3-y1)-1-oxoisoindolin-4-
yl)oxy)methyl)benzyl)acetamide
(89 mg, 61% yield) as a white solid. MS (ESI) m/z 514.1 [M+H]
-96-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0206] To a solution of (S)-2-bromo-N-(4-(((2-(2,7-dioxoazepan-3-y1)-1-
oxoisoindolin-4-y1) oxy)methyl)benzyl)acetamide (45 mg, 0.0877 mmol) in DMF (5
mL) at
RT was added K2CO3 (24.2 mg, 0.175 mmol) followed by 3-((2-((4-(3-
aminopropoxy)phenyl)amino)-5-methylpyrimidin-4-yl)amino)-N-(tert-
butyl)benzenesulfonamide (45 mg, 0.0877 mmol). The mixture was heated at 50 C
for 2
hrs. The solvent was evaporated, and the residue was purified by prep-HPLC as
previously
described to afford Compound 6 (12.6 mg, 16% yield) as a white solid. MS (ESI)
m/z 917.7
[M+H] NMR
(400 MHz, DMSO-d6) 6 10.71 (s, 1H), 8.77 (s, 1H), 8.53 (s, 1H), 8.12 (d,
J= 8.0 Hz, 2H), 7.89 (s, 1H), 7.55-7.41 (m, 9H), 7.31-7.27 (m, 4H), 6.77 (d,
J= 8.8 Hz, 2H),
5.23-5.18 (m, 3H), 4.44 (s, 2H), 4.31 (d, J = 5.6 Hz, 2H), 3.96 (t, J= 6.4 Hz,
2H), 3.09-3.01
(m, 2H), 2.74-2.67 (m, 2H), 2.58-2.53 (m, 2H), 2.32-2.26 (m, 1H), 2.11 (s,
3H), 2.04-1.97(m,
3H), 1.87-1.84 (m, 3H), 1.12 (s, 9H).
Example 7
Compound 7: (S)-3-(6-Fluoro-4-{ [p-(morpholinomethyl)phenyl]methoxy } -2-i
soindolinoy1)-
2,7-azepanedione
o H
0
401
Ci
[0207] To
a cooled (-15 C) solution of 5-fluoro-2-methylbenzoic acid (1.0 g, 6.5
mmol) in sulfuric acid (8 mL) at RT was added nitric acid (0.44 mL) dropwise.
The mixture
was stirred for 1 hour then warmed to 0 C stirred for 1 hour. The mixture was
poured
slowly into ice water and extracted with Et0Ac. The combined organic layers
were washed
with brine, dried over anhydrous Na2SO4, filtered, and concentrated to give 5-
fluoro-2-
methy1-3-nitrobenzoic acid (830 mg, crude) which was used in the next step
without further
purification.
[0208] To
a solution of 5-fluoro-2-methyl-3-nitrobenzoic acid (830 mg crude) in
Me0H (8 mL) at 0 C was added thionyl chloride (1 mL) dropwise. The mixture
was then
-97-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
heated to reflux for 3 hrs. The solvent was evaporated to give the crude
product which was
purified by silica gel chromatography using petroleum ether/Et0Ac (100:1 to
50:1) to afford
methyl 5-fluoro-2-methyl-3-nitrobenzoate (530 mg, 59% yield) as a yellow
solid.
[0209] To a solution of methyl 5-fluoro-2-methyl-3-nitrobenzoate (530
mg, 2.66
mmol) in Me0H (6 mL) at RT was added Pd/C (400 mg). The suspension was stirred
at RT
for 3 hrs under Hz. The suspension was filtered through a pad of Celite and
the filtrate was
concentrated to give methyl 3-amino-5-fluoro-2-methylbenzoate (420 mg, crude)
which was
used in the next step without further purification.
[0210] To methyl 3-amino-5-fluoro-2-methylbenzoate (420 mg, 2.28 mmol)
in
water at 0 C was dropwise added sulfuric acid (5 mL) followed sodium nitrate
(165 mg,
2.39 mmol, in 2.5 mL water). After stirring at 0 C for 2.5 hrs, the mixture
was dropwise
added into sulfuric acid (50% in water) at 100 C for 20 min then the mixture
was cooled to
RT and extracted with Et0Ac. The combined organic layers were washed with
brine, dried
over anhydrous Na2SO4, filtered, and concentrated to give methyl 5-fluoro-3-
hydroxy-2-
methylbenzoate which was used in the next step without further purification.
[0211] To a solution of methyl 5-fluoro-3-hydroxy-2-methylbenzoate
(320 mg,
1.74 mmol) in DMF (6 mL) 0 C was added sodium hydride (60%, 84 mg, 2.1 mmol).
The
mixture was stirred at this temperature for 15 min then iodomethane was added.
After
stirring at 0 C for 15 min, the mixture was warmed to RT and stirred for 1
hour. The
reaction was quenched with water and extracted with tert-butyl methyl ether.
The combined
organic layers were washed with brine, dried over anhydrous Na2SO4, filtered,
and
concentrated to afford methyl 5-fluoro-3-methoxy-2-methylbenzoate (327 mg
crude) which
was used in the next step without further purification.
[0212] To a solution of methyl 5-fluoro-3-methoxy-2-methylbenzoate
(5.4 g, 30.8
mmol) in carbon tetrachloride (40 mL) at RT was added NBS (8.2 g, 46.3 mmol)
and 2,2'-
azobis(2-methylpropionitrile) (2.0 mg, 12.3 mmol). The mixture was refluxed
overnight, the
solvent was evaporated, and the crude product was purified by silica gel
chromatography
(petroleum ether/Et0Ac, 100:1 to 20:1) to afford methyl 2-(bromomethyl)-5-
fluoro-3-
methoxybenzoate (4.6 g, 59% yield) as a white solid.
[0213] To a solution of (S)-3-aminoazepan-2-one (56 mg, 0.43 mmol) and
TEA
(72 mg, 0.72 mmol in DMF (2 mL) was added a solution of methyl 2-(bromomethyl)-
5-
-98-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
fluoro-3-methoxybenzoate (100 mg, 0.36 mmol) in DIVIF (2 mL). The mixture was
stirred at
50 C for 3 hrs, concentrated, and washed with Et0Ac to afford (S)-6-fluoro-4-
methoxy-2-
(2-oxoazepan-3-yl)isoindolin-1-one (100 mg, 95% yield) as a white solid. MS
(ESI) m/z
293.0 [M+H]t
[0214] To a solution of (S)-6-fluoro-4-methoxy-2-(2-oxoazepan-3-
yl)isoindolin-
1-one (100 mg, 0.34 mmol) in DCM (6 mL), boron tribromide (515 mg, 2.05 mmol
in 2 mL
DCM) was added at 0 C. After stirring at this temperature for 15 min, the
mixture was
warmed to RT and stirred for 3 hrs. The reaction was quenched by water at 0
C,
concentrated, and purified by silica gel chromatography (DCM/Me0H, 100:1 to
20:1) to
afford (S)-6-fluoro-4-hydroxy-2-(2-oxoazepan-3-yl)isoindolin-1-one (20 mg, 21%
yield) as a
white solid. MS (ESI) m/z 279.0 [M+H]t
[0215] To a solution of (S)-6-fluoro-4-hydroxy-2-(2- oxoazepan-3-
yl)isoindolin-
1-one (250 mg, 0.90 mmol) and (4-(diethoxymethyl)phenyl)Me0H (284 mg, 1.35
mmol),
triphenylphosphine (472 mg, 1.8 mmol) in THF (2.5 mL), was added DEAD (373 mg,
1.80
mmol) at 0 C. The mixture was stirred for 15 min, warmed to RT and stirred
for 1 hr, the
solvent was evaporated and the crude product was purified by silica gel
chromatography
(DCM/Me0H, 100:1 to 50:1) to afford (S)-4-((4-(diethoxymethyl)benzyl)oxy)-6-
fluoro-2-(2-
oxoazepan-3-yl)isoindolin-1-one (310 mg, 73% yield) as a white solid.
[0216] To a solution of (S)-4-((4-(diethoxymethyl)benzyl)oxy)-6-fluoro-
2-(2-
oxoazepan-3-y1) isoindolin-l-one (120 mg, 0.303 mmol) in fluorobenzene/DMSO
(24 mL/6
mL) was added Dess-Martin periodinane reagent (321.3 mg, 0.758 mmol). The
mixture was
stirred at 80 C for 16 hrs. After cooling to RT, Dess-Martin reagent (160 mg)
was added.
The suspension was heated to 80 C for another 16 hrs. After cooling to RT,
the reaction was
quenched with sat. aqueous sodium thiosulfate solution (15 mL) and stirred for
5 min. The
resulting mixture was extracted with DCM. Workup and purification with Et0Ac
in
petroleum ether from 10% to 69% to give (S)-4-(((2-(2,7- dioxoazepan-3-y1)-6-
fluoro-l-
oxoisoindolin-4-yl)oxy)methyl)benzaldehyde (45 mg, 36% yield) as a white
solid. MS (ESI)
m/z 411.1 [M+H]t
[0217] To a solution of (S)-4-(((2-(2,7-dioxoazepan-3-y1)-6-fluoro-1-
oxoisoindolin-4-yl)oxy)methyl)benzaldehyde (30 mg, 0.076 mmol) in DCM (4 mL)
at RT
was added morpholine (13 mg, 0.152 mmol) followed by sodium
triacetoxyborohydride (32
-99-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
mg, 0.152 mmol). The mixture was stirred at RT for 16 hrs then the solvent was
evaporated.
The residue was purified by prep-TLC (Et0Ac) to give the crude product which
was purified
by prep-HPLC as previously described to afford Compound 7 (5.5 mg, 15% yield)
as a white
solid. MS (ESI) m/z 481.8 [M+H]t1H NMR (400 MHz, DMSO-d6) 6 10.72 (s, 1H),
7.45 (d,
J = 7.2 Hz, 2H), 7.34 (d, J = 7.6 Hz, 2 H), 7.30 (d, J= 8.1 Hz, 1 H), 7.11 (d,
J= 7.2 Hz, 1 H),
5.32-5.19 (m, 1 H), 5.24 (s, 2 H), 4.44 (s, 2H), 3.57 (s, 4H), 3.47 (s, 2H),
3.21-3.06 (m, 1H),
2.59-2.57 (m, 1H), 2.34 (s, 5H), 2.02-1.96 (m, 2H), 1.82-1.75 (m, 1H).
Example 8
Compound 8: (S)-3-{44(p-Morpholinocarbonylphenyl)methoxy]-2-isoindolinoy1}-2,7-
azepanedione
0 0 H
N = = =
0
101
0
[0218] To
a solution of 4-(hydroxymethyl)benzoic acid (500 mg, 3.28 mmol) in
DMF (5 mL) was added morpholine (326 mg, 3.94 mmol), followed by HOBt (678.9
mg,
4.92 mmol), EDAC=HC1 (944 mg, 4.92 mmol) and DIEA (846.2 mg, 6.56 mmol). The
mixture was stirred at RT for 16 hrs. The reaction was diluted with water (5
mL) and
extracted with DCM. Workup and purification with Et0Ac/petroleum ether from
30% to
90% provided (4-(hydroxymethyl)phenyl)(morpholino)methanone (520 mg, 72%
yield) as a
colorless oil. MS (ESI) m/z 221.1 [M+H]t
[0219] To
a solution of (S)-4-hydroxy-2-(2-oxoazepan-3-yl)isoindolin-1-one (100
mg, 0.385 mmol), (4-(hydroxymethyl)phenyl)(morpholino)methanone (85 mg, 1.55
mmol)
and triphenylphosphine (201 mg, 0.77 mmol) in THF (3 mL) at 0 C was added a
solution of
DEAD (134 mg, 0.77 mmol) in THF (1 mL) dropwise. The mixture was stirred at RT
for 16
hrs. The solvent was evaporated, and the residue was purified by prep-TLC
(Et0Ac) to give
(S)-4-((4-(morpholine-4-carbonyl)benzyl)oxy)-2-(2-oxoazepan-3-yl)isoindolin-1-
one (135
mg, 76% yield) as a white solid. MS (ESI) m/z = 333.1 [M+1]+.
-100-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0220] To a solution of (S)-4-((4-(morpholine-4-carbonyl)benzyl)oxy)-2-
(2-
oxoazepan-3-yl)isoindolin-1-one (135 mg, 0.292 mmol) in fluorobenzene/DMSO (12
mL/2
mL, 1 drop H20 in DMSO) was added Dess-Martin periodinane (309.6 mg, 0.73
mmol). The
suspension was stirred at 80 C overnight, cooled to RT, filtered, and the
filtrate was added to
sat. aqueous sodium thiosulfate solution (10 mL). After stirring at 0 C for 5
min, the
mixture was extracted with DCM. The combined organic layers were washed with
10%
sodium thiosulfate /sat. NaHCO3 (1:1), and brine, dried over anhydrous Na2SO4,
filtered, and
concentrated to give the crude product which was purified by prep-TLC (Et0Ac)
to afford
Compound 8 (52.5 mg, 38% yield) as a white solid. MS (ESI) m/z 333.1 [M+H] 1-H
NMR
(400 MHz, DMSO-d6) 6 10.68 (s, 1H), 7.57 (d, J= 8.0 Hz, 2H), 7.51-7.44 (m,
3H), 7.33 (d, J
= 8.0 Hz ,2H), 5.31 (s, 2H), 5.23 (dd, J =5 .2, 12.4 Hz ,1H), 4.49 (s, 2H),
3.60 (s, 6H), 3.11-
3.03 (m, 1H), 2.60-2.54 (m, 1H), 2.38-2.29 (m, 1H), 2.12-1.98 (m, 2H), 1.86-
1.76 (m, 1H).
Example 9
Compound 9: (S)-3 -(4- { [m-(Aminomethyl)phenyl]methoxy } -2-i soindolinoy1)-
2,7-
azepanedione trifluoroacetic acid
o H
N =
0
T FA
NH2
[0221] To a solution of (S)-4-hydroxy-2-(2-oxoazepan-3-yl)isoindolin-1-
one (150
mg, 0.577 mmol), 3-(hydroxymethyl)benzonitrile (154 mg, 1.15 mmol) and
triphenylphosphine (378 mg, 1.44 mmol) in THF (3.5 mL) at RT was added DEAD (
251
mg, 1.44 mmol). The mixture was stirred for 30 min. The solvent was
evaporated, and the
residue was purified by prep-TLC (DCM/Me0H, 100:1 to 30:1) to give (S)-3-(((1-
oxo-2-(2-
oxoazepan-3-yl)isoindolin-4-yl)oxy)methyl)benzonitrile (235 mg, 81% yield) as
a white
solid. MS (ESI) m/z 376.2 [M+H].
[0222] To a solution of (S)-3-(((1-oxo-2-(2-oxoazepan-3-yl)isoindolin-
4-y1)
oxy)methyl) benzonitrile (200 mg, 0.48 mmol) in fluorobenzene/DMSO (15 mL/2
mL) at RT
was added Dess-Martin periodinane (508 mg, 1.2 mmol). The mixture was heated
to 80 C
overnight cooled to RT, and 20 mL of sat. sodium thiosulfate solution was
added, and the
-101-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
mixture was extracted with DCM. Workup provided the crude product which was
washed
with tert-butyl methyl ether to afford (S)-3-(((2-(2,7-dioxoazepan-3-y1)-1-
oxoisoindolin-4-
yl)oxy)methyl) benzonitrile (120 mg, 58% yield) as a white solid. MS (ESI) m/z
390.1
[M+H]t
[0223] To
a solution of (S)-3-(((2-(2,7-dioxoazepan-3-y1)-1-oxoisoindolin-4-
yl)oxy)methyl)benzonitrile (130 mg, 0.334 mmol) in THF (8 mL) at RT was added
di-tert-
butyl dicarbonate (146 mg, 0.668 mmol) and Raney Ni (60 mg). After degassing
and
purging with Hz, the mixture was stirred at RT overnight. The suspension was
filtered
through a Celite pad. The filtrate was concentrated and purified by prep-TLC
(Et0Ac) to
give (S)-tert-butyl 3 -
(((2-(2,7-di oxoazepan-3 -y1)-1-oxoi soindolin-4-yl)oxy)methyl)
benzylcarbamate as a white solid (150 mg, 59%).
[0224] To a solution of (S)-tert-butyl 3-(((2-(2,7-dioxoazepan-3-y1)-1-
oxoisoindolin-4-yl)oxy) methyl) benzylcarbamate (75 mg, 0.152 mmol) in DCM (2
mL) at
RT was added TFA (1 mL). The mixture was stirred at RT for 30 min. The solvent
was
evaporated, and the residue was lyophilized to give Compound 9 (40 mg, 66%
yield) as a
white solid.MS (ESI) m/z 394.2 [M+H] 1-H NMR (400 MHz, DMSO-d6) 6 10.73 (s,
1H),
8.18 (s, 3H), 7.58-7.43 (m, 5H), 7.34 (d, J= 7.2 Hz, 2H), 5.28-5.23 (m, 3H),
4.80 (m, 2H),
4.10-4.07 (m, 2H), 3.08-3.06 ( m, 1H), 2.60-2.55 ( m, 1H), 2.32-2.29 (m, 1H),
2.12-1.99 (m,
2H), 1.80-1.77 (m, 1H).
Example 10
Compound 10: (S)-3 -(4- { [m-(Morpholinomethyl)phenyl]methoxy -2-i
soindolinoy1)-2,7-
azepanedione
0 o H
N = = =
0
0
110 N()
[0225] To
a solution of (S)-4-hydroxy-2-(2-oxoazepan-3-yl)isoindolin-1-one (160
mg, 0.615 mmol), 1,3-phenylenedimethanol (170 mg, 1.23 mmol) and
triphenylphosphine
(402 mg, 1.538 mmol) in THF (8 mL), was added DEAD (268 mg, 1.53 mmol) at RT.
The
mixture was stirred at RT for 30 min. The solvent was evaporated, and the
residue was
-102-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
purified by prep-TLC (DCM/Me0H, 100:1 to 30:1) to give (S)-443-
(hydroxymethyl)benzyl)oxy)-2-(2-oxoazepan-3-yl)isoindolin-1-one (156 mg, 59%
yield) as a
white solid. MS (ESI) m/z 381.2 [M+H]t
[0226] To a solution of (S)-443-(hydroxymethyl)benzyl)oxy)-2-(2-
oxoazepan-3-
y1) isoindolin-l-one (138 mg, 0.363 mmol) in fluorobenzene/DMSO (4 mL/1 mL) at
RT was
added Dess-Martin periodinane (462 mg, 1.09 mmol). After stirring at 80 C
overnight, the
mixture was cooled to RT, and 20 mL of sat. sodium thiosulfate solution was
added
followed, and the mixture was extracted with DCM. Workup provided the crude
product
which was washed with tert-butyl methyl ether to afford (S)-34(2-(2,7-
dioxoazepan-3-y1)-1-
oxoisoindolin-4-yl)oxy)methyl)benzaldehyde (85 mg, 60% yield) as a white
solid. MS (ESI)
m/z 391.2 [M+HIP .
[0227] To a solution of (S)-3-(((2-(2,7-dioxoazepan-3-y1)-1-
oxoisoindolin-4-
yl)oxy)methyl) benzaldehyde (80 mg, 0.204 mmol) and morpholine (36 mg, 0.408
mmol) in
DCM (4 mL) at RT was added NaBH(OAc)3 (87 mg, 0.408 mmol). The mixture was
stirred
overnight. The solvent was evaporated, and the residue was purified by prep-
TLC using
DCM/Me0H (10:1) to give Compound 10 (30.5 mg, 32% yield) as a white solid.MS
(ESI)
m/z 464.2[M+H]t 1-H NMR (400 MHz, DMSO-d6) 6 10.73 (s, 1H), 7.49-7.27 ( m,
7H),
5.27-5.22 (m, 3H), 4.47 (s, 2H), 3.56 (s, 4H), 3.48 (s, 2H), 3.12-3.05 (m,
1H), 2.58 (d, J=
16.8 Hz, 1H), 2.34 ( s, 5H), 2.12-2.06 ( m, 1H), 2.04-1.99 ( m, 1H), 1.82-1.7
8 ( m, 1H).
Example 11
Compound 11: (S)-3 -(4- { [p-(Morpholinomethyl)phenyl]methoxy -3 -oxo-2H-i
soindo1-2-
oy1)-2, 7-azepanedione
o H
Ci
o 0
[0228] To a solution of 3-methoxyphthalic acid (3.0 g, 15.306 mmol) in
THF (24
mL) at RT was added acetic anhydride (10 mL). The mixture was stirred at 80 C
for 3 hrs.
-103-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
The solvent was evaporated to give 4-methoxyisobenzofuran-1,3-dione (2.72 g,
quant. yield)
as a white solid. MS (ESI) m/z = 178.9 [M+H]t
[0229] To a solution of (S)-3-aminoazepan-2-one (1.96 g, 15.28 mmol)
in
AcOH/ACN (28 mL/20 mL) at RT was added 4-methoxyisobenzofuran-1,3-dione (2.71
g,
15.28 mmol). The mixture was stirred at 85 C overnight then cooled to RT.
Sodium acetate
(3.13 g, 38.20 mmol) was added followed by AcOH (12 mL). The mixture was
stirred at 85
C overnight. The solvent was evaporated then the residue was diluted with
water (30 mL)
and stirred at RT for 30 min. The resulting suspension was filtered and the
filter cake was
washed with Et0Ac to give (S)-4-methoxy-2-(2-oxoazepan-3-yl)isoindoline-1,3-
dione (2.154
g, 49% yield) as a white solid. MS (ESI) m/z =289.1 [M+H].
[0230] To a solution of (S)-4-methoxy-2-(2-oxoazepan-3-yl)isoindoline-
1,3-dione
(2.1 g, 7.292 mmol) in DCM (40 mL) at 0 C was added tribromoborane (3.4 mL)
in DCM
(10 mL) dropwise. After stirring at RT for 3 hrs, the mixture was diluted with
ice-water and
extracted with DCM. The combined organic layers were washed with brine, dried
over
anhydrous Na2SO4, filtered, and concentrated to give the crude product which
was washed
with Et0Ac to afford (S)-4-hydroxy-2-(2-oxoazepan-3-yl)isoindoline-1,3-dione
(1 g, 50%
yield) as a light yellow solid. MS (ESI) m/z =275.0 [M+H]t
[0231] To a solution of (S)-4-hydroxy-2-(2-oxoazepan-3-yl)isoindoline-
1,3-dione
(300 mg, 1.095 mmol), 1,4-phenylenedimethanol (272 mg, 1.971 mmol) and
triphenylphosphine (574 mg, 2.190 mmol) in THF (5 mL) at 0 C was added DEAD
(381
mg, 2.19 mmol). The mixture was stirred at RT overnight. The solvent was
evaporated and
the crude product was purified by silica gel chromatography using
Et0Ac/petroleum ether
from 20% to 96% to give (S)-4-((4-(hydroxymethyl) benzyl)oxy)-2-(2-oxoazepan-3-
yl)isoindoline-1,3-dione (168 mg, 39% yield) as a white solid. MS (ESI) m/z
=395.1
[M+H]t
[0232] To a solution of (S)-4-((4-(hydroxymethyl)benzyl)oxy)-2-(2-
oxoazepan-3-
yl)isoindoline-1,3-dione (140 mg, 0.355 mmol) in 1,2-dichloroethane/DMS0 (12
mL/2 mL)
at RT was added Dess-Martin reagent (753 mg, 1.775 mmol). The mixture was
stirred at 80
C overnight. The mixture was cooled to RT and filtered, and the filtrate was
quenched with
sat. sodium thiosulfate solution (15 mL). After stirring for 10 min, the
mixture was extracted
with DCM. Workup and purification with Et0Ac/petroleum ether from 20% to 96%
to
-104-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
afford (S)-4-(((2-(2, 7-dioxoazepan-3 -y1)-1,3 -dioxoi soindolin-4-
yl)oxy)methyl)b enzaldehyde
(95 mg, 54% yield) as a yellow solid.
[0233] To a solution of (S)-44(2-(2,7-dioxoazepan-3-y1)-1,3-
dioxoisoindolin-4-
yl)oxy)methyl)benzaldehyde (75 mg, 0.1847 mmol) in DCM (3 mL) at RT was added
morpholine (32 mg, 0.3694 mmol) and sodium triacetoxyborohydride (78 mg,
0.3694 mmol)
and the mixture stirred overnight. The solvent was evaporated and the crude
product was
purified by prep-TLC using DCM/Me0H (10:1) to give Compound 11(42 mg, 48%
yield) as
a white solid. MS (ESI) m/z = 477.8 [M+H]t 1-E1 NMR (DMSO-d6, 400 MHz)o: 10.84
(s,
1H), 7.82-7.35 (m, 7H), 5.35 (s, 2H), 5.19-5.15 (m, 1H), 3.60-3.50 (m, 4H),
3.50-3.40 (m,
2H), 3.14-3.07 (m, 1H), 2.70-2.60 (m, 1H), 2.35 (s, 4H), 2.19-1.87 (m, 4H).
Example 12
Compound 12: (S)-3 -(4- { [p-(Morpholinomethyl)phenyl]methoxy -2-i
soindolinoy1)-2,5-
pyrrolidinedione
o o
101 N = = =
0
0
[0234] To a stirred solution of (9-4-amino-2-((tert-
butoxycarbonyl)amino)-4-
oxobutanoic acid (2.0 g, 8.6 mmol) in THF (10 mL) and Me0H (10 mL) at 0 C was
added
(trimethylsilyl)diazomethane solution (2 M in hexane, 6.5 mL) dropwise. The
mixture was
stirred at 0 C for 1 hour then concentrated to give (9-methyl 4-amino-2-
((tert-
butoxycarbonyl)amino)-4-oxobutanoate (crude) as a colorless oil.
[0235] To a stirred solution of (9-methyl 4-amino-2-((tert-
butoxycarbonyl)amino)-4- oxobutanoate (8.6 mmol) in DCM (6 mL) at RT was added
TFA
(3 mL). After stirring overnight, the mixture was concentrated to give (9-
methyl 2,4-
diamino-4-oxobutanoate, which was used directly for the next step.
[0236] To a solution of (9-methyl 2,4-diamino-4-oxobutanoate (8.6
mmol) and
methyl 2-(bromomethyl)-3-methoxybenzoate (2.2 g, 8.6 mmol) in DNIF (20 mL) was
added
-105-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
TEA (3.6 mL). After stirring at 50 C for 5 hrs, the mixture was diluted with
water and
extracted with Et0Ac. Workup and purification with Et0Ac/pet. ether (20% to
80%)
provided (9-methyl 4-amino-2-(4-methoxy-1-oxoisoindolin-2-y1)-4-oxobutanoate
(1.2 g,
48% yield) as a yellow solid. MS (ESI) m/z = 293.0 [M+H]t
[0237] To a solution of (9-methyl 4-amino-2-(4-methoxy-1-oxoisoindolin-
2-y1)-
4-oxobutanoate (1.2 g, 4.1 mmol) in DCM (12 mL) at 0 C was added boron
tribromide (2.3
mL) dropwise. The mixture was stirred at RT for 2 hrs and quenched by water
and Me0H.
The solvent was evaporated, and the residue was purified by silica gel
chromatography
(DCM/Me0H, 20:1) to give (9-4-amino-2-(4-hydroxy-1- oxoisoindolin-2-y1)-4-
oxobutanoic
acid (3 g, crude) as a yellow solid. MS (ESI) m/z = 265.0 [M+H]t
[0238] To a solution of (9-4-amino-2-(4-hydroxy-1-oxoisoindolin-2-y1)-
4-
oxobutanoic acid (3 g crude) in THF (15 mL) and Me0H (15 mL) was added
(trimethylsilyl)diazomethane solution (2 M in hexane, 6.8 mL) at 0 C
dropwise. The
mixture was stirred at 0 C for 1.5 hrs and concentrated. The residue was
purified by silica
gel chromatography (DCM/Me0H, 10 :1) to give (9-methyl 4-amino-2-(4-hydroxy-1-
oxoisoindolin-2-y1)-4-oxobutanoate (470 mg, 43% yield) as a white solid. MS
(ESI) m/z =
279.0 [M+H]t
[0239] To a solution of (9-methyl 4-amino-2-(4-hydroxy-1-oxoisoindolin-
2-y1)-
4-oxobutanoate (300 mg, 1.08 mmol), (4-(morpholinomethyl)phenyl)methanol (335
mg, 1.62
mmol) and triphenylphosphine (567 mg, 2.16 mmol in THF, 8 mL) at 0 C was added
DEAD
(376 mg, 2.16 mmol) and the mixture was stirred at RT overnight. The solvent
was
evaporated, and the residue was purified by prep-TLC (DCM/Me0H, 10 :1) to give
(9-
methy1-4-amino-2-(44(4-(morpholinomethyl)b enzyl)oxy)-1-oxoi soindolin-2-y1)-4-
oxobutanoate (150 mg, 30% yield) as a white solid. MS (ESI) m/z = 468.1 [M+H]t
[0240] To a solution of (9-
methy1-4-amino-2-(44(4-
(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-2-y1)-4-oxobutanoate (100 mg,
0.21 mmol)
in THF (6 mL) at RT was added lithium hydroxide (11 mg, 0.42 mmol). The
mixture was
stirred for 3 hrs then diluted with water and adjusted pH to 3 with 1 N HC1.
Workup and
purification with by prep-TLC (DCM/Me0H, 10 :1) to afford Compound 12 (13 mg,
14%
yield) as a yellow solid. MS (ESI) m/z = 453.9 [M+H]t 1-EINMR (400 MHz, DMSO-
d6) 6
11.49 (brs, 1H), 7.51-7.44 (m, 3H), 7.34-7.30 (m, 4H), 5.27 (t, J= 8.0 Hz,
1H), 5.23 (s, 2H),
-106-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
4.61 (d, J= 17.2 Hz, 1H), 4.27 (d, J= 17.2 Hz, 1H), 3.57 (t, J= 4.0 Hz, 4H),
3.46 (s, 2H),
2.94 (d, J= 7.6 Hz, 2H), 2.34 (s, 4H).
Example 13
Compound 13: (S)-3 -(4- { [p-( { 3 -[3 -(p-{ 5-Methy1-44m-(tert-
butylaminosulfonyl)phenylamino]-2-pyrimidinylamino}phenoxy)propyll
ureidoImethyl)phenyl]methoxy -2-i soindolinoy1)-2, 7-azepanedione
o H
101 N
0
N N
T
*I 0 0
NAN) NH
H H
0311NH
0)<
[0241] To a solution of tert-butyl (3 -
(4-((4-((3 -(N-(tert-
butyl)sulfamoyl)phenyl)amino)-5- methylpyrimidin-2-
yl)amino)phenoxy)propyl)carbamate
(100 mg, 0.171 mmol) in dry DCM (8 mL) at 0 C was added TFA (2 mL). The
reaction was
stirred at RT for 16 hrs. The solvent was evaporated, and the residue was
dried to give the
amine TFA salt (100 mg, crude). The amine TFA salt was dissolved in THF (5 mL)
and
TEA (34.5 mg, 0.342 mmol) was added at RT followed by 4-nitrobenzyl
chloroformate (34.2
mg, 0.171 mmol). The mixture was stirred for 1 hour. The solvent was
evaporated to give 4-
nitrophenyl (3 -
(44(44(3 -(N-(tert-butyl)sulfamoyl)phenyl)amino)-5-methylpyrimidin-2-
yl)amino)phenoxy)propyl) carbamate (30 mg, crude) which was used directly for
the next
step. MS (ESI) m/z 650.2[M+H]t
[0242] To a solution of (S)-tert-butyl 4-(((2-(2,7-dioxoazepan-3-y1)-1-
oxoisoindolin-4-y1) oxy)methyl)benzylcarbamate (23 mg, 0.046 mmol) in DCM (4
mL) at
RT was added TFA (1 mL). The solvent was evaporated, and the residue was dried
to give
the amine TFA salt (30 mg, crude) as a yellow gum. The amine TFA salt was
dissolved in
THF (5 mL) and TEA (10 mg, 0.092 mmol) was added at RT followed by a
suspension of 4-
nitrophenyl (3 -
(44(44(3 -(N-(tert-butyl)sulfamoyl)phenyl)amino)-5-methylpyrimidin-2-
yl)amino)phenoxy)propyl)carbamate (30 mg, 0.046 mmol) in THF (1 mL). The
reaction was
-107-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
stirred at 60 C for 3 hrs. The solvent was evaporated, and the residue was
purified by silica
gel chromatography eluting with Me0H/DCM from 0% to 9% to give Compound 13
(22.3
mg, 53% yield) as a white solid. MS (ESI) m/z 903.7[M+H]t NMR
(400 MHz, DMSO-
d6) 6 10.72 (s, 1H), 8.82 (s, 1H), 8.57 (s, 1H), 8.15-8.12 (m, 2H), 7.89 (s,
1H), 7.59 (s, 1H),
7.54-7.41 (m, 7H), 7.31-7.25 (m, 4H), 6.79 (d, J = 8.8 Hz, 2H), 6.37 (t, J=
6.0 Hz, 1H), 6.06
(t, J = 5.2 Hz, 1H), 5.23-5.19 (m, 3H), 4.45 (s, 2H), 4.21 (d, J= 6.4Hz, 2H),
3.91 (t, J= 5.6
Hz, 2H), 3.19-3.15 (m, 2H), 3.09-3.02 (m, 1H), 2.58-2.54 (m, 1H), 2.35-2.31
(m, 1H), 2.11
(s, 3H), 2.04-1.97 (m, 1H), 1.82-1.78 (m, 3H), 1.12 (s, 9H).
Example 14
Compound 14: (S)-3-(4-{[p-(Morpholinomethyl)phenoxy]methy1}-2-isoindolinoy1)-
2,7-
azepanedione
o H
Ci
0
[0243] To a solution of (5)-1-oxo-2-(2-oxoazepan-3-yl)isoindoline-4-
carbaldehyde (910 mg, 3.35 mmol) in THF (25 mL) at RT was added sodium
borohydride
(254 mg, 6.70 mmol). The mixture was stirred for 2 hrs. The reaction was
concentrated to
give the crude product which was purified by silica gel chromatography eluting
with
Me0H/DCM (1:10) to give (S)-4-(hydroxymethyl)-2-(2-oxoazepan-3-y1) isoindolin-
l-one
(539 mg, 59% yield) as a white solid. MS (ESI) m/z=275.1[M+H].
[0244] To
a solution of (S)-4-(hydroxymethyl)-2-(2-oxoazepan-3-y1) isoindolin-
1-one (539 mg, 1.97 mmol), 4-hydroxybenzaldehyde (288 mg, 2.36 mmol) and
triphenylphosphine (1.03 g, 3.93 mmol) in THF (30 mL) at 0 C was added DEAD
(685 mg,
3.93 mmol). The mixture was stirred for 15 min then warmed to RT and stirred
overnight.
The solvent was evaporated to give the crude product, which was purified by
silica gel
chromatography eluting with Me0H/DCM from 0% to 5% to afford (S)-441-oxo-2-(2-
-108-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
oxoazepan-3-yl)isoindolin-4-yl)methoxy)benzaldehyde (520 mg, 70% yield) as a
white solid.
MS (ESI)m/z 379.1 [M+H]
[0245] To
a solution of (S)-4((1-oxo-2-(2-oxoazepan-3-yl)isoindolin-4-y1)
methoxy) benzaldehyde (520 mg, 1.38 mmol) in fluorobenzene/DMSO (24 mL/4mL, 1
drop
water in DMSO) was added Dess-Martin reagent (1.46 g, 3.44 mmol). The
suspension was
heated at 80 C for 18 hrs. The suspension was cooled to RT then added to sat.
aqueous
sodium thiosulfate solution (20 mL) at 0 C and stirred for 5 min then
extracted with DCM.
Workup and purification with Et0Ac/petroleum ether from 20% to 96% to give (S)-
442-
(2,7-dioxoazepan-3-y1)-1-oxoisoindolin-4-yl)methoxy)benzaldehyde (174.2 mg,
32% yield)
a white solid. MS (ESI) m/z 393.1 [M+H]t
[0246] To
a solution of (S)-442-(2,7-dioxoazepan-3-y1)-1-oxoisoindolin-4-y1)
methoxy) benzaldehyde (100 mg, 0.255 mmol) in THF (5 mL) at RT was added
morpholine
(88 mg, 1.02 mmol), followed by sodium cyanoborohydride (64 mg, 1.02 mmol).
The
mixture was stirred for 2 hrs. The solvent was evaporated, and the residue was
purified by
prep-HPLC as previously described to give Compound 14 (22.1 mg, 19% yield) as
a white
solid. MS (ESI) m/z = 498.1 [M+H]. NMR
(DMSO-d6, 400 MHz) 6: 10.76 (s, 1H),
7.73-7.71 (m, 2H), 7.55 (t, J = 7.6 Hz, 1H), 7.23 (d, J= 8.4 Hz, 2H), 7.01 (d,
J= 8.4 Hz, 2H),
5.25 (s, 2H), 4.63 (s, 2H), 3.55 (s, 4H), 3.36 (s, 2H), 3.13-3.06 (m, 1H),
2.59-2.55 (m, 1H),
2.32 (s, 4H), 2.17-1.97 (m, 3H), 1.86-1.76 (m, 1H).
Example 15
Compound 15: (S)-3 -(5- { [p-(Morpholinomethyl)phenyl]methoxy -2-i
soindolinoy1)-2, 7-
azepanedione
o H
=N =
0
Sc?
[0247] To
a solution of methyl 4-methoxy-2-methylbenzoate (10 g, 55.56 mmol)
in carbon tetrachloride (150 mL) at RT was added NB S (10 g, 55.57 mmol) and
2,2'-
azobis(2-methylpropionitrile) (4.0 mg, 9.43 mmol). The mixture was stirred at
reflux 6 hrs.
The solvent was evaporated to give the crude product which was purified by
silica gel
-109-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
chromatography (Et0Ac/petroleum ether, 1:20 to 1:5) to give methyl 2-
(bromomethyl)-4-
methoxybenzoate (13.6 g, 94% yield) as a white solid.
[0248] To a solution of (S)-3-aminoazepan-2-one (1.6 g, 12.4 mmol) in
DIVIF (16
mL) at RT was added TEA (2.9 mL, 20.6 mmol) and methyl 2-(bromomethyl)-4-
methoxybenzoate (2.7 g, 10.3 mmol) in 4 mL DIVIF. The mixture was stirred at
85 C
overnight. The solvent was evaporated and the crude product was purified by
silica gel
chromatography (DCM/Me0H, 50:1) to give (5)-5-methoxy-2-(2-oxoazepan-3-
yl)isoindolin-
1-one (1.39 g, 49% yield) as a white solid. MS (ESI) m/z 275.0 [M+H]t
[0249] To a solution of (S)-5-methoxy-2-(2-oxoazepan-3-yl)isoindolin-1-
one (1.0
g, 3.65 mmol) in DCM (12 mL) at 0 C was added boron tribromide (2.0 mL, 21.89
mmol, in
4 mL DCM) dropwise. The mixture was stirred at this temperature for 30 min
then warmed
to RT for 2 hrs and quenched by water and Me0H. The solvent was evaporated,
and the
residue was purified by silica gel chromatography (DCM/Me0H, 20:1) to give (5)-
5-
hydroxy-2-(2-oxoazepan-3-yl)isoindolin-1-one (1.7 g crude) as a white solid.
MS (ESI) m/z
261.0 [M+H]t
[0250] To a solution of (5)-5-hydroxy-2-(2-oxoazepan-3-yl)isoindolin-1-
one (500
mg, 1.92 mmol), 1,4-phenylenedimethanol (1.0 g, 7.68 mmol) and
triphenylphosphine (2.0 g,
7.68 mmol) in THF (2 mL) at RT was added DEAD ( 1.3 mg, 7.68 mmol) at RT. The
mixture was stirred for 2 hrs. The solvent was evaporated, and the residue was
purified by
silica gel chromatography (DCM/Me0H, 50:1 to 20:1) to give (5)-5-((4-
(hydroxymethyl)benzyl)oxy)-2-(2-oxoazepan-3-yl)isoindolin-1-one (90 mg, 12%
yield) as a
white solid. MS (ESI) m/z 381.1 [M+H]t
[0251] To a solution of (5)-5-((4-(hydroxymethyl)benzyl)oxy)-2-(2-
oxoazepan-3-
yl)isoindolin-1-one (60 mg, 0.157 mmol) in fluorobenzene/DMSO (6.0 mL/1.5 mL)
at RT
was added Dess-Martin periodinane (268 mg, 0.631 mmol). After stirring at 80
C
overnight, the mixture was cooled to RT then 20 mL of sat. sodium thiosulfate
solution was
added, and the mixture was stirred at RT for 5 min. Workup and purification
with by prep-
TLC using hexanes/Et0Ac (1:1) provided (5)-4-(((2-(2,7-dioxoazepan-3-y1)-1-
oxoisoindolin-5-yl)oxy )methyl)benzaldehyde (15 mg, 16% yield) as a white
solid. MS
(ESI) m/z 393.0 [M+H]t
-110-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0252] To
a solution of (S)-4-(((2-(2,7-dioxoazepan-3-y1)-1-oxoisoindolin-5-
yl)oxy) methyl)benzaldehyde (15 mg, 0.038 mmol) and morpholine (7 mg, 0.076
mmol) in
DCM at RT was added sodium triacetoxyborohydride (16 mg, 0.076 mmol). The
mixture
was stirred for 28 hrs. The solvent was evaporated, and the crude product was
purified by
prep-TLC using DCM/Me0H (10:1) to give Compound 15 (6.5 mg, 38% yield) as a
white
solid. MS (ESI) m/z 464.2 [M+H]t 1E1 NMR (400 MHz, DMSO-d6) 6 10.71 (s, 1H),
7.64
(d, J = 8.4 Hz, 1H), 7.43 (d, J = 8.0 Hz, 2H), 7.34 (d, J= 8.0 Hz, 2H), 7.27 (
s, 1H), 7.13 (d,
J= 8.8 Hz, 1H), 5.23-5.19 (m, 3H), 4.48-4.47 (m, 2H), 3.58-3.56 (m, 4H), 3.47
(s, 2H), 3.08-
3.06 ( m, 1H), 2.60-2.51 (m, 1H), 2.35 (s, 4H), 2.27-2.24 (m, 1H), 2.10-2.00
(m, 2H), 1.80-
1.77(m, 1H).
Example 16
Compound 16: (S)-6-(4- { [p-(Morpholinomethyl)phenyl]methoxy -2-i
soindolinoy1)-1,4-
oxazepane-3,5-dione
o o H
N ..tNN,")
0/
0
[0253] To
a solution of 3-hydroxy-2-methylbenzoic acid (20 g, 0.13 mmol) in
Me0H (240 mL) at at 0 C was added thionyl chloride dropwise, then the mixture
was stirred
at reflux for 3 hrs. The solvent was evaporated, and the residue was purified
by silica gel
chromatography (petroleum ether/Et0Ac, 20:1 to 5:1) to give methyl 3-hydroxy-2-
methylbenzoate (18.7 g, 86% yield) as a yellow solid.
[0254] To
a solution of methyl 3-hydroxy-2-methylbenzoate (18.7 g, 0.11 mol)
and imidazole (19.0 g, 0.25 mol) in DMF (28 mL) at RT was added tert-
butyldimethylsilyl
chloride (20.4 g, 0.12 mol). After stirring at 60 C for 3 hrs, the mixture
was cooled to RT
and extracted with tert-butyl methyl ether. The combined organic layers were
dried over
anhydrous Na2SO4, filtered, and concentrated to
give -- methyl -- 3 -((tert-
-111-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
butyldimethylsilyl)oxy)- 2-methylbenzoate (32 g crude) as a yellow solid which
was used in
the next step without further purification.
[0255] To
a solution of 3-((tert-butyldimethylsilyl)oxy)-2-methylbenzoate (5.0 g,
17.8 mmol) in carbon tetrachloride (30 mL) at RT was added NBS (4.7 g, 26.8
mmol) and
2,2'-azobis(2-methylpropionitrile) ( 1.1 g, 7.1 mmol). The suspension was
stirred at reflux
for 5 hrs. The solvent was evaporated, and the residue was purified by silica
gel
chromatography (petroleum ether/Et0Ac, 100:1 to 50:1) to give methyl 2-
(bromomethyl)-3-
((tert-butyldimethylsily1) oxy)benzoate (5.8 g, 90% yield) as a yellow oil.
[0256] To
a solution of methyl 2-(bromomethyl)-3-((tert-butyldimethylsilyl)oxy)
benzoate (490 mg, 1.4 mmol) in DMF (6 mL) at RT was added TEA (0.4 mL, 2.8
mmol) and
(S)-6-amino-1,4-oxazepan-5-one (200 mg, 1.5 mmol, in 2 mL of DMF). The mixture
was
stirred at RT for 4 hrs, then heated to 80 C overnight. The mixture was
concentrated, and
the residue was diluted with THF (10 mL). TBAF (200 mg, 0.76 mmol) was added,
and the
mixture was stirred at reflux for 2 hrs. The solvent was evaporated and the
residue purified
by silica gel chromatography (DCM/Me0H, 50:1 to 20:1) to give (S)-6-(4-hydroxy-
1-
oxoisoindolin-2-y1)-1,4- oxazepan-5-one (183 mg, 50% yield) as a white solid.
MS (ESI)
m/z 263.0 [M+H]t
[0257] To
a solution of (S)-6-(4-hydroxy-1-oxoisoindolin-2-y1)-1,4-oxazepan-5-
one (230 mg, 0.87 mmol), 1,4-phenylenedimethanol (182 mg, 1.31 mmol) and
triphenylphosphine (568 mg, 2.17 mmol) in THF (2.5 mL) at RT was added DEAD
(377 mg,
2.17 mmol). The mixture was stirred at RT for 3 hrs. The solvent was
evaporated and the
crude product was purified by silica gel chromatography (DCM/Me0H, 100:1 to
20:1) to
give (S)-
6-(444-(hy droxym ethyl)b enzyl)oxy)-1-oxoi s oindol in-2-y1)-1,4 -oxazep an-5
-one
(110 mg, 33% yield) as a white solid. MS (ESI) m/z 383.1 [M+H]t
[0258] To a solution of (S)-6-(444-(hydroxymethyl)benzyl)oxy)-1-
oxoisoindolin-2-y1)-1,4-oxazepan-5-one (110 mg, 0.288 mmol) in ACN/DMSO (8
mL/2 mL)
at RT was added Dess-Martin periodinane (732 mg, 1.72 mmol). The mixture was
stirred at
80 C for 4 hrs, then cooled to RT, and 20 mL of a sat. sodium thiosulfate
solution was added
followed by stirring for 5 min. The mixture was extracted with DCM. Workup and
purification with DCM then DCM/Et0Ac, 5:1 to 1:1 to give (S)-44(2-(3,5-dioxo-
1,4-
-112-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
oxazepan-6-y1)-1-oxoisoindolin-4-y1) oxy)methyl)benzaldehyde (58 mg, 51%
yield) as a
white solid. MS (ESI) m/z 395.0 [M+H]t
[0259] To
a solution of (S)-4-(((2-(3,5-dioxo-1,4-oxazepan-6-y1)-1-oxoisoindolin-
4-yl)oxy)methyl)benzaldehyde (58 mg, 0.147 mmol) and morpholine (26 mg, 0.294
mmol)
in DCM (1.5 mL) at RT was added sodium triacetoxyborohydride (64 mg, 0.294
mmol). The
mixture was stirred at RT for 4 hrs then concentrated. The crude product was
purified by
prep-TLC using DCM/Me0H (10:1) to give Compound 16 (19 mg, 20% yield) as a
white
solid. MS (ESI) m/z 465.9 [M+H]t 1H NMIt (400 MHz, DMSO-d6) 6 11.11 (s ,1H),
7.48-
7.44 ( m, 3H), 7.35-7.32 (m, 4 H), 5.67-5.66 (m, 1H), 5.24 (s, 2H), 4.71 (d,
J= 17.2 Hz, 1H),
4.55 (d, J = 10.4 Hz, 2 H), 4.40-4.35 ( m, 2H), 4.06-4.05 ( m, 1H), 3.57-3.55
( m, 4H), 3.47 (
s, 2H), 2.35 (s, 4H).
Example 17
Compound 17: 3-(4-{dp-({243-(p-{5-Methy1-44m-(tert-
butylaminosulfonyl)phenylamino]-
2-pyrimidinylamino}phenoxy)propylamino]acetylamino}methyl)phenyl]methoxy}-2-
isoindolinoy1)-2,6-piperidinedione
o o
110 0
0 HN,kNNH
* 0 110
N)L.k1 0*11
0
[0260] To
a solution of 3-hydroxy-2-methylbenzoic acid (20 g, 0.13 mol) in
Me0H (240 mL) at 0 C was added thionyl chloride (30 mL, 0.41 mol) dropwise.
The
mixture was stirred at reflux for 3 hrs. The solvent was evaporated, and the
residue was
purified by silica gel chromatography using petroleum ether/Et0Ac (20:1 to
5:1) to give
methyl 3-hydroxy-2-methylbenzoate (18.7 g, 86% yield) as a yellow solid.
[0261] To
a solution of methyl 3-hydroxy-2-methylbenzoate (18.7 g, 0.11 mol)
and imidazole (19.0 g, 0.25 mol) in DMF (28 mL) at RT, tert-butyldimethylsilyl
chloride
(20.4 g, 0.12 mol) was added. After stirring at 60 C for 3 hrs, the mixture
was cooled to RT
and extracted with tert-butyl methyl ether. The combined organic layers were
dried over
anhydrous Na2SO4, filtered, and concentrated to
give methyl 3 -((tert-
-113-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
butyldimethylsilyl)oxy)-2-methylbenzoate (32 g crude) as a yellow solid which
was used in
the next step without further purification.
[0262] To a solution of methyl 3-((tert-butyldimethylsilyl)oxy)-2-
methylbenzoate
(3.0 g, 10.7 mmol) in carbon tetrachloride (22 mL) at RT was added NBS (2.9 g,
16.0 mmol)
and 2,2'-azobis(2-methylpropionitrile) (701 mg, 4.3 mmol). The mixture was
stirred at 80 C
for 5 hrs. The solvent was evaporated to give the crude product which was
purified by silica
gel chromatography (petroleum ether/Et0Ac, 30:1) to give methyl 2-
(bromomethyl)-3-((tert-
butyldimethylsilyl)oxy)benzoate (3.7 g 97%) as a yellow oil.
[0263] To a solution of methyl 2-(bromomethyl)-3-((tert-
butyldimethylsily1)
oxy)benzoate (1.0 g, 2.77 mmol) and TEA (0.8 mL, 5.54 mmol) in DNIF (6 mL) at
RT was
added tert-butyl 2,5-diamino-5-oxopentanoate (670 mg, 3.32 mmol ) in DNIF (4
mL). The
mixture was stirred for 2 hrs then heated to 80 C and stirred overnight. The
solvent was
evaporated to give the crude product which was purified by silica gel
chromatography
(DCM/Me0H, 100:1 to 50:1) to afford tert-butyl 5-amino-4-(4-hydroxy-1-
oxoisoindolin-2-
y1)-5-oxopentanoate (820 mg, 88% yield) as a white solid. MS (ESI) m/z 279.0
[M+H-56]t
[0264] To a solution of tert-butyl 5-amino-4-(4-hydroxy- 1 -
oxoisoindolin-2-y1)-5-
oxopentanoate (450 mg, 1.34 mmol), 4-(hydroxymethyl)benzonitrile (360 mg, 2.69
mmol)
and triphenylphosphine (1.4 g, 5.36 mmol) in THF (2 mL) at RT was added DEAD (
932 mg,
5.36 mmol) dropwise. The mixture was stirred at RT for 4 h. The solvent was
evaporated
and the crude product was purified by silica gel chromatography (DCM/Me0H,
100:1 to
20:1) to give tert-butyl 5-amino-4-(4-((4-cyanobenzyl)oxy)-1-oxoisoindolin-2-
y1)-5-
oxopentanoate (290 mg, 39% yield) as a white solid. MS (ESI) m/z 394.0 [M+H-
56]t
[0265] To a solution of tert-butyl 5-amino-4-(4-((4-cyanobenzyl)oxy)-1-
oxoisoindolin-2-y1)-5-oxopentanoate (200 mg, 0.45 mmol) in DCM (6 mL) at RT
was added
TFA (1.5 mL). The mixture was stirred at RT for 5 hrs then concentrated to
give 5-amino-4-
(4-((4-cyanobenzyl)oxy)-1-oxoisoindolin-2-y1)-5-oxopentanoic acid TFA salt
(250 mg
crude) as a white solid, which was used for the next step without further
purification. MS
(ESI) m/z 394.0 [M+H]t
[0266] To a solution of 5-amino-4-(4-((4-cyanobenzyl)oxy)-1-
oxoisoindolin-2-
y1)-5-oxopentanoic acid TFA salt (250 mg, 0.45 mmol) in ACN (8 mL) at RT was
added CDI
(291 mg, 1.80 mmol). The mixture was stirred at 95 C for 6 hrs. The solvent
was
-114-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
evaporated to give the crude product which was purified by silica gel
chromatography
(DCM/Me0H, 100:1 to 50:1) to give 4-(((2-(2,6-dioxopiperidin-3-y1)-1-
oxoisoindolin-4-
yl)oxy) methyl)benzonitrile (130 mg 77%) as a white solid. MS (ESI) m/z 376.0
[M+H]
[0267] To
a solution of 4-(((2-(2,6-dioxopiperidin-3-y1)-1-oxoisoindolin-4-
yl)oxy) methyl)benzonitrile (130 mg, 0.346 mmol) and di-tert-butyl dicarbonate
(0.16 mL,
0.693 mmol) in DCM (4 mL) and THF (4 mL) at RT was added Raney-Ni (80 mg). The
mixture was stirred at RT for 16 hrs under H2 then Me0H (4 mL) was added, and
the mixture
was heated to 80 C for 5 hrs. After cooling to RT, the suspension was
filtrated through a
Celite pad and the filtrate was concentrated to give the crude product, which
was purified by
silica gel chromatography using DCM/Me0H (50:1 to 20:1) to afford tert-butyl 4-
(((2-(2,6-
dioxopiperidin-3-y1)-1-oxoisoindolin-4-yl)oxy)methyl)benzylcarbamate (100 mg,
60% yield)
as a white solid.MS (ESI) m/z 380.0 [M+H-100]t
[0268] To a solution of tert-butyl 4-(((2-(2,6-dioxopiperidin-3-y1)-1-
oxoisoindolin-4-yl)oxy)methyl)benzylcarbamate (100 mg, 0.208 mmol) in DCM (4
mL) at
RT was added TFA (2 mL). The mixture was stirred at RT overnight. The solvent
was
evaporated to give 3-(4-((4-(aminomethyl)benzyl)oxy)-1-oxoisoindolin-2-
yl)piperidine-2,6-
dione TFA salt (80 mg crude) as a white solid, which was used for the next
step without
further purification. MS (ESI) m/z 380.0 [M+H]
[0269] To
a solution of tert-butyl 243-(4((4((3-(N-(tert-butyl)sulfamoyl)
phenyl)amino)-5-methylpyrimidin-2-yl)amino)phenoxy)propyl)amino) acetate (70
mg, 0.117
mmol) in DCM ( 4 mL) at RT was added TFA ( 1.5 mL) and the mixture was stirred
at RT
overnight. The solvent was evaporated, and the residue was dissolved in DMF (4
mL) then 3-
(4-((4-(aminomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione (80
mg, 0.162
mmol), DIEA (63 mg, 0.486 mmol), HOBt (33 mg, 0.243 mmol) and EDAC=HC1 (47 mg,
0.243 mmol) were added. The mixture was stirred at RT for 18 hrs. The solvent
was
evaporated and the crude product was purified by prep-HPLC as previously
described to give
Compound 17(11.3 mg, 10% yield) as a white solid. MS (ESI) m/z 904.3 [M+H]t
NIVIR
(400 MHz, DMSO-d6) 6 10.98 (s, 1H), 8.79 (s, 1H), 8.54 (s, 1H), 8.32 (t, J=
6.0 Hz, 1H),
8.15 ( s, 2H), 7.89 ( s, 1H), 7. 58-7.40 (m, 9H), 7.32-7.26 (m, 4H), 6.78 (d,
J= 8.8 Hz, 2H),
5.19 ( s, 2H), 5.12-5.07 ( m, 1H), 4.41-4.21 ( m, 4H), 3.96 (t, J= 6.4 Hz,
2H), 3.16 (s, 2H),
-115-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
2.93-2.86 (m, 1H), 2.65-2.62 (m, 2H), 2.59-2.51 (m, 1H), 2.46-2.41 (m, 1H),
2.11 (s, 3H),
2.09-1.95 (m, 1H), 1.90-1.80 (m, 2H), 1.12 (s, 9H).
Example 18
Compound 18: (S)-3-(6-Fluoro-4-{ [p-({ 3 -[3 -(p- {5-methy1-44m-(tert-
butylaminosulfonyl)phenylamino]-2-pyrimidinylamino}phenoxy) propyl]ureido
methyl)
phenyllmethoxy -2-isoindolinoy1)-2,6-piperidinedione
o 0
N
0
11,11
0 0
N)LN) NH
H H
S
CY NH
[0270] To a solution of methyl 5-fluoro-3-hydroxy-2-methylbenzoate
(3.2 g, 17.4
mmol) and imidazole (2.9 g, 43.5 mmol) in DMF (6 mL) at RT was added tert-
butyldimethylsily1 chloride (3.1 g, 20.8 mmol). The mixture was stirred at 60
C for 1 hr,
cooled to RT and extracted with tert-butyl methyl ether, dried over anhydrous
Na2SO4,
concentrated to give methyl 3-((tert-butyldimethylsilyl)oxy)-5-fluoro-2-
methylbenzoate
(5.2 g crude) as a yellow oil, which was used for the next step without
further purification.
[0271] To a solution of methyl 3-((tert-butyldimethylsilyl)oxy)-5-
fluoro-2-
methylbenzoate (3.2g, 10.7 mmol) in carbon tetrachloride (30 mL) at RT was
added NBS
(2.9 g, 16.1 mmol) and 2,2'-azobis(2-methylpropionitrile) ( 700 mg, 4.3 mmol
). The
mixture was stirred at 80 C for 6 h. The solvent was evaporated to give the
crude product
which was purified by silica gel chromatography (petroleum ether/Et0Ac, 10:1)
to give
methyl 2-(bromomethyl)-3-((tert-butyldimethylsily1) oxy)-5-fluorobenzoate (4.0
g, quant.
yield) as a yellow oil.
[0272] To a solution of methyl 2-(bromomethyl)-3-((tert-
butyldimethylsilyl)oxy)-
5-fluorobenzoate (1.0 g, 2.66 mmol) and TEA (0.7 mL, 5.32 mmol) in DMF (6 mL)
at RT
was added (S)-tert-butyl 4,5-diamino-5-oxopentanoate (696 mg, 2.92 mmol) in 4
mL DMF.
The mixture was stirred for 2 h then heated to 80 C overnight. The solvent
was evaporated
-116-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
to give the crude product which was purified by silica gel chromatography
(DCM/Me0H,
50:1 to 20:1) to give (S)-tert-butyl 5-amino-4-(6-fluoro-4-hydroxy-l-
oxoisoindolin-2-y1)-5-
oxopentanoate (1.0 g crude) as a white solid. MS (ESI) m/z 297.0 [M+H-56]t
[0273] To a solution of (S)-tert-butyl 5-amino-4-(6-fluoro-4-hydroxy-1-
oxoisoindolin-2-y1)-5-oxopentanoate (500 mg, 1.42 mmol), 4-
(hydroxymethyl)benzonitrile
(283 mg, 2.13 mmol) and triphenylphosphine (930 mg, 3.55 mmol) in THF (4 mL)
at RT was
added DEAD (617 mg, 3.55 mmol). The mixture was stirred at RT for 2 hrs. The
solvent
was evaporated, and the residue was purified by silica gel chromatography
(DCM/Me0H,
100:1 to 50:1) to give (S)-tert-butyl 5-amino-4-(4-((4-cyanobenzyl)oxy)-6-
fluoro-1-
oxoisoindolin-2-y1)-5-oxopentanoate (350 mg, 52% yield) as a yellow solid. MS
(ESI) m/z
412.0 [M+H-56]+.
[0274] To
a solution of (S)-tert-butyl 5-amino-4-(444-cyanobenzyl)oxy)-6-
fluoro-1- oxoisoindolin-2-y1)-5-oxopentanoate (350 mg, 0.75 mmol) in DCM (4
mL) at RT
was added TFA (4 mL). The mixture was stirred at RT overnight. The solvent was
evaporated to give (5)-5-amino-4-(444-cyanobenzyl)oxy)-6-fluoro-1-
oxoisoindolin-2-y1)-5-
oxopentanoic acid (400 mg crude) as a yellow solid, which was used for the
next step
without further purification. MS (ESI) m/z 412.0 [M+H]t
[0275] To
a solution of (S)-5-amino-4-(4-((4-cyanobenzyl)oxy)-6-fluoro-1-
oxoisoindolin- 2-y1)-5-oxopentanoic acid (400 mg, 0.75 mmol) in ACN (10 mL) at
RT was
added CDI (485 mg, 2.99 mmol). The mixture was stirred at 95 C for 3.5 hrs.
The solvent
was evaporated to give the crude product, which was purified by silica gel
chromatography
(DCM/Me0H, 100:1 to 50:1) to give (S)-4-(((2-(2,6-dioxopiperidin-3-y1)-6-
fluoro-1-
oxoisoindolin-4-yl)oxy)methyl)benzonitrile (200 mg 68%) as a white solid. MS
(ESI) m/z
394.0 [M+H]t
[0276] To a solution of (S)-4-(((2-(2,6-dioxopiperidin-3-y1)-6-fluoro-1-
oxoisoindolin-4-yl)oxy)methyl)benzonitrile (200 mg, 0.51 mmol) and di-tert-
butyl
dicarbonate (166 mg, 0.76 mmol) in THF (6 mL) at RT was added Raney-Ni (80
mg). The
mixture was stirred under H2 overnight. The suspension was filtered and the
filtrate was
concentrated and purified by silica gel chromatography (DCM/Me0H, 100:1 to
20:1) to give
(S)-tert-butyl 4-
(((2-(2, 6-di oxopiperi din-3 -y1)-6-fluoro-1-oxoi soindolin-4-
-117-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
yl)oxy)methyl)benzylcarbamate (90 mg, 35% yield) as a white solid. MS (ESI)
m/z 398.0
[M+H-100]+.
[0277] To
a solution of (S)-tert-butyl 4#(2-(2,6-dioxopiperidin-3-y1)-6-fluoro-1-
oxoisoindolin-4-yl)oxy)methyl)benzylcarbamate (70 mg, 0.146 mmol) in DCM (2
mL) at RT
was added TFA (0.5 mL). The mixture was stirred at RT for 2 hrs. The solvent
was
evaporated to give (S)-3-(4-((4-(aminomethyl)benzyl)oxy)-6-fluoro-1-
oxoisoindolin-2-
yl)piperidine-2,6-dione (80 mg crude) as a white solid, which was used for the
next step
without further purification. MS (ESI) m/z 398.0 [M+H]t
[0278] To
a solution of (S)-3-(4-((4-(aminomethyl)benzyl)oxy)-6-fluoro-1-
oxoisoindolin-2-yl)piperidine-2,6-dione (80 mg, 0.146 mmol) and TEA (45 mg,
0.438 mmol)
in THF at RT was added 4-nitrophenyl (3-
(4-((4-((3-(N-(tert-
butyl)sulfamoyl)phenyl)amino)-5-methylpyrimidin-2-
yl)amino)phenoxy)propyl)carbamate
(90 mg, 0.138 mmol). After stirring for 15 min, TEA (103 mg, 1.022 mmol) was
added, and
the reaction was stirred for 2 hrs. The solvent was evaporated to give the
crude product
which was purified by prep-HPLC as previously described to afford Compound 18
(60.9 mg,
46% yield) as a white solid. MS (ESI) m/z 908.3 [M+H]t NMR
(400 MHz, DMSO-d6) 6
10.96 (s, 1H), 8.76 (s, 1H), 8.52 (s, 1H), 8.14-8.12 (m, 2H), 7.88 (s, 1H),
7.55-7.40 (m, 7H),
7.26-7.24 (m, 3H), 7.11-7.08 (m, 1H), 6.79 (d, J= 8.8 Hz, 2 H), 6.36 (t, J=
5.6 Hz, 1H), 6.04
(t, J = 5.6 Hz, 1H), 5.20 ( s, 2 H), 5.11-5.06 ( m, 1H), 4.36 (d, J= 17.2 Hz,
1H), 4.23-4.19
(m, 3H), 3.91 (t, J= 6.0 Hz, 2 H), 3.19-3.14 (m, 2H), 2.89-2.82 (m, 1H), 2.58-
2.50 ( m, 1H),
2.45-2.40 ( m, 1H), 2.11 (s, 3H), 1.99-1.89 (m, 1H), 1.82-1.79 (m, 2H), 1.12
(s, 9H).
Example 19
Compound 19: 2- [(S)-2,7-Dioxo-3 -azepany1]-4- { [p-
(morpholinomethyl)phenyl]methoxy } -1-
oxo-5-i soindolinecarb onitrile
0 0 H
N
0
-118-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0279] To a solution of 3-hydroxy-2-methylbenzoic acid (100.0 g, 660
mmol) in
dry Me0H (700 mL) at 0 C was added thionyl chloride (156.0 g, 1316 mmol). The
reaction
was heated to 70 C for 3 hrs. The reaction was cooled to RT and the solvent
was
evaporated. The residue was diluted with water and extracted with Et0Ac.
Workup and
purification with Et0Ac in petroleum ether from 0% to 8% to give methyl 3-
hydroxy-2-
methylbenzoate (102 g, 93% yield) as a white solid.
[0280] NBS (53.6 g, 301.2 mmol) was added to a solution of methyl 3-
hydroxy-
2-methylbenzoate (50.0 g, 301.2 mmol) in DCM (800 mL) at -78 C. The mixture
was
stirred at -78 C for 1 hour then concentrated. The crude product was purified
by silica gel
chromatography eluting with Et0Ac in petroleum ether (from 0% to 8%) to give
methyl 4-
bromo-3-hydroxy-2-methylbenzoate (15.0 g, 21% yield) as a colorless solid.
[0281] To a solution of methyl 4-bromo-3-hydroxy-2-methylbenzoate
(10.0 g,
40.82 mmol) in DMF (50 mL) was added zinc cyanide (480 mg, 40.82 mmol) and
tetrakis(triphenylphosphine)palladium (240 mg, 2.04 mmol). The mixture was
heated to 100
C for 5 hrs. The reaction was cooled to RT and the solvent was evaporated. The
residue
was diluted with water and extracted with Et0Ac. Workup and purification with
Et0Ac in
petroleum ether (from 0% to 15%) to give methyl 4-cyano-3-hydroxy-2-
methylbenzoate (2.1
g, 27% yield) as a white solid.
[0282] To a solution of methyl 4-cyano-3-hydroxy-2-methylbenzoate (2.1
g, 11.0
mmol) and imidazole (1.5 g, 22.0 mmol) in DMF (6 mL) at RT was added tert-
butyldimethylsily1 chloride (1.98 g, 13.2 mmol). After stirring at 60 C for 1
hour, the
solution was cooled to RT and extracted with tert-butyl methyl ether, dried
over anhydrous
Na2SO4, filtered, concentrated to give methyl 3-((tert-butyldimethylsilyl)oxy)-
4-cyano-2-
methylbenzoate (3.2 g crude) as a yellow oil, which was used for the next step
without
further purification.
[0283] To a solution of methyl 3-((tert-butyldimethylsilyl)oxy)-4-
cyano-2-
methylbenzoate (3.2g, 10.5 mmol) in carbon tetrachloride (50 mL) at RT was
added NBS
(2.43 g, 13.64 mmol) and 2,2'-azobis(2-methylpropionitrile) (690 mg, 4.2
mmol). The
suspension was stirred at 80 C for 5 hrs. The solvent was evaporated to give
the crude
product which was purified by silica gel chromatography (petroleum
ether/Et0Ac, 10:1) to
-119-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
give methyl 2-(bromomethyl)-3 -((tert- butyldimethylsilyl)oxy)-4-cyanobenzoate
(3.5 g 87%
) as a colorless oil.
[0284] To a solution of methyl 2-(bromomethyl)-3-((tert-
butyldimethylsilyl)oxy)-
4-cyanobenzoate (2.65 g, 6.92 mmol) and TEA (1.39 g, 113.8 mmol) in DMF (20
mL) at RT
was added (S)-3-aminoazepan-2-one (1.06 g, 8.3 mmol). The mixture was stirred
for 2 hrs
then heated to 50 C overnight. After cooling to RT, tetrabutylammonium
fluoride (2.61 g,
8.3 mmol) was added. The mixture was heated to 50 C for 1 hour. The solvent
was
evaporated to give the crude product which was purified by silica gel
chromatography
(DCM/Me0H, 50:1 to 20:1) to afford (S)-4-hydroxy-1-oxo-2-(2- oxoazepan-3-
yl)isoindoline-5-carbonitrile (1.3 g, crude) as a yellow solid.
[0285] To a solution of (S)-4-hydroxy-1-oxo-2-(2-oxoazepan-3-
yl)isoindoline-5-
carbonitrile (1.0 g, 3.5 mmol), 4-(bromomethyl)benzaldehyde (900 mg, 4.6 mmol)
in
DMF(515 mL) was added K2CO3 970 mg, 7.1 mmol). The mixture was heated to 50 C
for
2h. The solvent was evaporated to give the crude product, which was purified
by silica gel
chromatography (DCM/Me0H, 50:1 to 20:1) to afford (S)-4-((4-formylbenzyl)oxy)-
1-oxo-2-
(2- oxoazepan-3-y1) isoindoline-5-carbonitrile (600 mg, 42% yield) as a yellow
solid. MS
(ESI) m/z 404.2 [M+H]t
[0286] To a solution of (S)-4((4-formylbenzyl)oxy)-1-oxo-2-(2-
oxoazepan-3-y1)
isoindoline-5-carbonitrile (980 mg, 2.44 mmol) in ACN /DMSO (12 mL/2 mL) at RT
was
added Dess-Martin periodinane (2.6 g, 6.1 mmol). The mixture was stirred at 80
C
overnight. The mixture was cooled to RT and 100 mL of a sat. sodium
thiosulfate solution
was added followed by stirring for 5 min. The mixture was extracted with DCM.
Workup
and purification with DCM/ACN, 5:1 to 3:1 provided (S)-2-(2,7-dioxoazepan-3-
y1)-4-((4-
formylbenzyl)oxy)-1-oxoisoindoline-5-carbonitrile (600 mg, 59% yield) as a
white solid.
MS (ESI) m/z 418.0 [M+H].
[0287] To a solution of (S)-2-(2,7-dioxoazepan-3-y1)-4-((4-
formylbenzyl)oxy)-1-
oxoisoindoline-5-carbonitrile (700mg, 1.68 mmol) and morpholine(292mg, 3.36
mmol) in
DCM (15 mL) at RT was added sodium triacetoxyborohydride (850mg, 4.2 mmol).
The
mixture was stirred for 3 hrs then concentrated. The residue was purified by
silica gel
chromatography (DCM/ACN, 3:1 to 1:1) to afford Compound 19 (800 mg, 57% yield)
as a
white solid. MS (ESI) m/z 489.2 [M+H]t 1-E1 NMR (400 MHz, DMSO-d6) 6 10.79 (s,
1H),
-120-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
7.91 (d, J = 7.6 Hz, 1H), 7.54-7.46 (m, 3H), 7.36 (d, J= 8.4 Hz, 2H), 5.47(s,
2H), 5.29-5.25
(m, 1H), 4.86 (t, J= 2.4 Hz, 2H), 3.58-3.56 (m, 4H), 3.47(s, 2H), 3.14-3.06
(m, 1H), 2.58 (d,
J= 16.4 Hzõ 1H), 2.40-2.34 (m, 5H), 2.13-2. 00 (m, 2H), 1.83-1.81 (m, 1H).
Example 20
Compound 20: (S)-3 -(4-{ [p-({ 3 -[3 -(p- {5-Methy1-4-[m-(tert-
butylaminosulfonyl)
phenylamino]-2-pyrimidinylamino}phenoxy)propyl]ureidoImethyl)phenyl]methoxy -2-
isoindolinoy1)-2,6-piperidinedione
o 0 H
101 N
0
N-
el I I
0 0
NAN7\)
40 NH
H H
Co
)S
CV NH
[0288] To a solution of (S)-tert-butyl 4#(2-(2,6-dioxopiperidin-3-y1)-
1-
oxoisoindolin-4-y1) oxy)methyl)benzylcarbamate (100 mg, 0.2 mmol) in DCM (4
mL) at RT
was added TFA (1 mL). The mixture was stirred for 1 hour then the solvent was
evaporated
to give (S)-3-(4-((4-(aminomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-
2,6-dione
TFA salt which was used for the next step without further purification. MS
(ESI) m/z =
380.0 [M+H]t
[0289] To a solution of (S)-3-(4-((4-(aminomethyl)benzyl)oxy)-1-
oxoisoindolin-
2-y1) piperidine-2,6-dione TFA salt (0.2 mmol) in DCM (4 mL) at RT was added
TEA (41
mg, 0.4 mmol) and 4-nitrophenyl carbonochloridate (50 mg, 0.24 mmol). After
stirring for 2
hrs, the mixture was concentrated to afford (S)-4-nitrophenyl 4-(((2-(2,6-
dioxopiperidin-3-
y1)-1-oxoisoindolin-4-yl)oxy)methyl)benzylcarbamate, which was used for the
next step.
[0290] To a solution of tert-butyl (3-(4-((443-(N-(tert-
butyl)sulfamoyl)phenyl)
amino)-5-methylpyrimidin-2-yl)amino)phenoxy)propyl)carbamate (120 mg, 0.2
mmol) in
DCM (2 mL) was added TFA (1 mL). The mixture was stirred at RT for 1 hr then
the
solvent was evaporated to
give 3 -((2-((4-(3 -aminoprop oxy)phenyl)amino)-5-
-121-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
methylpyrimidin-4-yl)amino)-N-(tert-butyl)benzenesulfonamide, which was used
for the
next step. MS (ESI) m/z = 485.1 [M+H]t
[0291] To
a solution of phenyl (S)-4-nitrophenyl 4#(2-(2,6-dioxopiperidin-3-y1)-
1-oxoisoindolin-4-yl)oxy)methyl)benzylcarbamate (0.2 mmol) in THF (4 mL) was
added
TEA (0.2 mL) followed by a solution of 34(2-((4-(3-aminopropoxy)phenyl)amino)-
5-
methylpyrimidin-4-yl)amino)-N-(tert-butyl)benzenesulfonamide (0.2 mmol) in DCM
(4 mL)
and the mixture was stirred at RT overnight. The solvent was evaporated and
the residue
was purified by silica gel chromatography (DCM/Me0H, 10:1) and prep-HPLC as
previously described to afford Compound 20 (11.5 mg, 6.5% yield) as a white
solid.MS
(ESI) m/z = 890.3 [M+H]. NMR
(400 MHz, DMSO-d6) 6 10.94 (s, 1 H), 8.76 (s, 1 H),
8.52 (s, 1 H), 8.11-8.14 (m, 2 H), 7.89 (s, 1 H), 7.39-7.78 (m, 8 H), 7.24-
7.31 (m, 4 H), 6.79
(d, J = 8.8 Hz, 2 H), 6.35 (t, J = 6.0 Hz, 1 H), 6.03 (t, J = 5.6 Hz, 1 H),
5.19 (s, 2 H), 5.09
(dd, J = 4.8, 13.2 Hz, 1 H), 4.19-4.41 (m, 4 H), 3.91 (t, J= 6.0 Hz , 2 H),
3.16 (q, J= 6.4 Hz
, 2 H), 2.85-2.94 (m, 1 H), 2.53-2.58 (m, 1 H), 2.42-2.49 (m, 1 H), 2.11 (s, 3
H), 1.95-1.99
(m, 1 H), 1.77-1.84 (m, 2H), 1.12 (s, 9 H).
Example 21
Compound 21: (S)-3-(6-Fluoro-4- { [p-({ 2- [3 -(p- {5-methy1-44m-(tert-
butylsulfonylamino)
phenylamino1-2-pyrimidinylamino}phenoxy)propylaminolacetylamino}methyl)phenyll
methoxy } -2-i soindolinoy1)-2, 7-azepanedione
o H
0 HN)NNH
1. 0 I. 10 ill
N)L.k110 Oi I
0
[0292] To
a solution of (S)-3-aminoazepan-2-one (2.0 g, 15.9 mmol) and TEA
(2.8 mL, 19.9 mmol) in DMF (30 mL) at RT was added methyl 2-(bromomethyl)-3-
((tert-
butyldimethylsilyl)oxy)-5-fluorobenzoate (5.0 g, 13.3 mmol ) in 10 mL DNIF.
The mixture
was stirred for 2 hrs, heated to 50 C overnight, and cooled to RT. TBAF (2.4
g, 9.31 mmol)
was added and the mixture was heated to 70 C for 1 hr. The solvent was
evaporated and the
residue was purified by silica gel chromatography (DCM/Me0H, 100:1 to 30:1) to
give (5)-
-122-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
6-fluoro-4-hydroxy-2-(2-oxoazepan-3-y1) isoindolin-l-one (3.1 g, 83% yield) as
a yellow
solid. MS (ESI) m/z 279.0 [M+H]
[0293] To a solution of (S)-6-fluoro-4-hydroxy-2-(2-oxoazepan-3-y1)
isoindolin-
1-one (320 mg, 1.15 mmol), tert-butyl 4-(hydroxymethyl)benzylcarbamate (409
mg, 1.72
mmol) and triphenylphosphine (602 mg, 2.30 mmol) in THF (2 mL) at RT was added
DEAD
( 400 mg, 2.30 mmol) and the mixture was stirred for 30 min. The solvent was
evaporated
and the residue was purified by silica gel chromatography (DCM/Me0H, 100:1 to
30:1) to
give (S)-tert-butyl 4-(((6-fluoro-1-oxo-2-(2-oxoazepan-3 -yl)i soindolin-4-
yl)oxy)methyl)
benzylcarbamate (430 mg, 75% yield) as a yellow solid. MS (ESI) m/z 398.1 [M+H-
100]t
[0294] To a solution of (S)-tert-butyl 4-(((6-fluoro-1-oxo-2-(2-
oxoazepan-3-y1)
isoindolin-4-yl)oxy)methyl)benzylcarbamate (200 mg, 0.40 mmol) in ACN/DMSO (4
mL/1
mL) at RT was added Dess-Martin periodinane (426 mg, 1.00 mmol). The mixture
was
stirred at 80 C overnight. After cooling to RT, 20 mL of sat. sodium
thiosulfate solution was
added, and the mixture was extracted with DCM. Workup provided the crude
product which
was washed with tert-butyl methyl ether to afford (S)-tert-butyl 4-(((2-(2,7-
dioxoazepan-3-
y1)-6-fluoro-1-oxoisoindolin-4-yl)oxy)methyl)benzylcarbamate (100 mg, 50%
yield) as a
white solid. MS (ESI) m/z 412.0 [M+H-100]t
[0295] To a solution of (S)-tert-butyl 4-(((2-(2,7-dioxoazepan-3-y1)-6-
fluoro-1-
oxoisoindolin-4-yl)oxy)methyl)benzylcarbamate (61 mg, 0.117 mmol) in DCM (4
mL) at RT
was added TFA (1 mL). The reaction was stirred at RT for 1 hour. The solvent
was
evaporated to give the amine TFA salt as a yellow gum.
[0296] The amine TFA salt was dissolved in DMA (1 mL) and 2-((3-(4-((4-
((3-
(N-(tert-butyl)sulfamoyl)phenyl)amino)-5-methylpyrimidin-2-
yl)amino)phenoxy)propyl)
amino)acetic acid (76 mg, 0.141 mmol) was added, followed by HOBt (23.7 mg,
0.176
mmol), EDAC=HC1 (34.1 mg, 0.176 mmol) and DIEA (30.0 mg, 0.234 mmol). The
reaction
was stirred at RT for 10 hrs. The solvent was evaporated, and the residue was
purified by
prep-HPLC as previously described to afford Compound 21(10.8 mg, 10.2% yield)
as a
white solid. MS (ESI) m/z 936.4[M+H]t 1-E1 NMR (400 MHz, DMSO-d6) 6 10.69 (s,
1H),
8.75 (s, 1H), 8.51 (s, 1H), 8.29 (t, J= 6.0 Hz, 1H), 8.14-8.11 (m, 2H), 7.88
(s, 1H), 7.54-7.23
(m, 10H), 7.09-7.07 (m, 1H), 6.77 (d, J= 4.8 Hz, 2H), 5.20-5.17 (m, 3H), 4.40
(s, 2H), 4.30
-123-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
(d, J = 6.0 Hz, 2H), 3.96 (t, J = 2.4 Hz, 2H), 3.15 (s, 2H), 3.09-3.00 (m,
1H), 2.67-2.54 (m,
3H), 2.32-2.25 (m, 3H), 2.11 (s, 3H), 2.05-1.96 (m, 2H), 1.84-1.71 (m, 3H),
1.12 (s, 9H).
Example 23
Compound 22: (S)-3-[5-(Aminomethyl)-4- { [p-(morpholinomethyl)phenyl]methoxy }
-2-
i soindolinoy1]-2,7-azepanedione ditrifluoroacetic acid
0 o H
0
H2N
= 2H
[0297] To
a solution of (S)-2-(2,7-dioxoazepan-3-y1)-4-((4-(morpholinomethyl)
benzyl)oxy)-1-oxoisoindoline-5-carbonitrile (700 mg, 1.43 mmol) and di-tert-
butyl
dicarbonate (625 mg, 2.87 mmol) in DMF (10 mL)/THF (15 mL) at RT was added
Raney-Ni
(500 mg). The mixture was stirred at RT for 48 hrs under Hz. The suspension
was filtered
through a Celite pad and concentrated to give the crude product which was
purified by silica
gel chromatography (DCM/CAN, 3:1 to 1:1) to afford (S)-tert-butyl((2-(2,7-
dioxoazepan-3-
y1)-44(4-(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-5-y1) methyl)carbamate
(800 mg,
85% yield) as a white solid. MS (ESI) m/z 593.1 [M+H]t
[0298] To a solution of (S)-tert-butyl ((2-(2,7-dioxoazepan-3-y1)-4-((4-
(morpholinomethyl) benzyl)oxy)-1-oxoisoindolin-5-yl)methyl)carbamate (70 mg,
0.118
mmol) in DCM (3 mL) at RT was added TFA (1.0 mL). The mixture was stirred for
1 hour.
The solvent was evaporated to afford Compound 22 (70 mg, 83% yield) as a white
solid. MS
(ESI) m/z 493.2 [M+H]t NMR
(400 MHz, DMSO-d6) 6 10.75 (s, 1H), 10.56 (s, 1H),
8.29 (s, 3H), 7.64-7.52 (m, 6H), 5.36(s, 2H), 5.28-5.24 (m, 1H), 4.85-4.73 (m,
2H), 4.39 (s,
2H), 4.14(s, 2H), 3.97 (s, 2H), 3.65 (s, 2H), 3.24-3.01 (m, 4H), 2.61-2.57 (m,
2H), 2.37-2.32
(m, 1H), 2.15-2.03 (m, 2H), 1.87-1.78 (m, 1H).
-124-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
Example 24
Compound 23: (S)-3 -[5-({ 3 -[3-(p- { 5-Methy1-44m-(tert-butyl
aminosulfonyl)phenylamino] -2-
pyrimi dinyl amino}phenoxy)propyllurei doImethyl)-4- { [p-
(morpholinomethyl)phenyll
methoxy -2-i soindolinoy1]-2,6-piperidinedione
Ii o o H
>rH N 0
HN ot) ON
S<
rNH 0
HN
=
I
N
[0299] To
a solution of methyl 2-(bromomethyl)-3-((tert-butyldimethylsilyl)oxy)-
4-cyanobenzoate (3.5 g, 9.096 mmol) and TEA (1.84 g, 18.2 mmol) in DMF (15 mL)
at RT
was added (S)-tert-butyl 4,5-diamino-5-oxopentanoate (2.6 g, 10.91 mmol ). The
mixture
was stirred for 2 hrs then heated to 50 C overnight.
After cooling to RT,
tetrabutylammonium fluoride (4.3 g, 13.6 mmol) added. The mixture was heated
to 50 C for
1 hr. The solvent was evaporated to give the crude product which was purified
by silica gel
chromatography (DCM/Me0H, 50:1 to 20:1) to give (S)-tert-butyl 5-amino-4-(5-
cyano-4-
hydroxy-l-oxoisoindolin-2-y1)-5-oxopentanoate (1.1 g, yield;34% yield) as a
yellow solid.
[0300] To a solution of (S)-tert-butyl 5-amino-4-(5-cyano-4-hydroxy-1-
oxoi soindolin-2-y1)-5-oxopentanoate (200 mg, 0.56
mmol), 4-(4-
(chloromethyl)benzyl)morpholine (188 mg, 0.835 mmol) in DMF (5 mL) was added
K2CO3
(156 mg, 1.13 mmol) and the mixture was heated to 50 C for 2 hrs. The solvent
was
evaporated and the residue was purified by silica gel chromatography
(DCM/Me0H, 50:1 to
20:1) to afford (S)-tert-butyl 5 -amino-4-(5 -cy ano-4-((4-(morphol
inomethyl)b enzyl)oxy)-1-
oxoi soindolin-2-y1)-5-oxopentanoate (240 mg, 80% yield) as a yellow solid.
[0301] To a solution of (S)-tert-butyl 5-amino-4-(5-cyano-4-((4-
(morpholinomethyl)benzyl) oxy)-1-oxoisoindolin-2-y1)-5-oxopentanoate (240 mg,
0.44
mmol) in DCM (5 mL) at RT was added TFA (2 mL). The mixture was stirred
overnight and
concentrated to afford (5)-5 -amino-4-(5 -cy ano-444-(morphol inomethyl)b
enzyl)oxy)-1-
-125-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
oxoisoindolin-2-y1)-5-oxopentanoic acid (200 mg, crude) as a yellow oil, which
was used for
the next step without further purification.
[0302] To a solution of (S)-5-amino-4-(5-cyano-4-((4-
(morpholinomethyl)benzyl)
oxy)-1-oxoisoindolin-2-y1)-5-oxopentanoic acid (200 mg, 0.41 mmol) in ACN (10
mL) at RT
was added CDI (200 mg, 1.22 mmol). The mixture was heated to 50 C for 2 hrs.
The
solvent was evaporated, and the residue was purified by silica gel
chromatography
(DCM/Me0H, 50:1 to 10:1) to give (S)-2-(2,6-dioxopiperidin-3-y1)-4-((4-
(morpholinomethyl)benzyl)oxy)-1-oxoisoindoline-5-carbonitrile (100 mg, 52%
yield) as a
white solid. MS (ESI) m/z 475.2 [M+H]t 1E1 NMR (400 MHz, DMSO-d6) 6 11.05 (s,
1H),
7.92 (d, J = 7.6 Hz, 1H), 7.54 (d, J = 8 Hz, 1H), 7.47 (d, J = 8 Hz, 2H), 7.36
(d, J = 8 Hz,
2H), 5.45(s, 2H), 5.18-5.14 (m, 1H), 4.85-4.66 (m, 2H), 3.58-3.56 (m, 4H),
3.47(s, 2H), 2.96-
2.89 (m, 1H), 2.65-2.60 (m, 1H), 2.47-2.44 (m, 1H), 2.36-2.33 (m, 4H), 2.05-
1.99 (m, 1H).
[0303] To a solution of (S)-2-(2,6-dioxopiperidin-3-y1)-4-((4-
(morpholinomethyl)
benzyl)oxy)-1-oxoisoindoline-5-carbonitrile (600 mg, 1.265 mmol) and di-tert-
butyl
dicarbonate (550 mg, 2.53 mmol) in DMF (5 mL) and Me0H (10 mL) at RT was added
Raney-Ni (300 mg). The mixture was stirred at RT for 16 hrs under Hz. The
suspension was
filtered through a Celite pad and concentrated to give the crude product which
was purified
by silica gel chromatography (DCM/Me0H, 50:1 to 20:1) to afford (S)-tert-butyl
((2-(2,6-
dioxopiperidin-3-y1)-4-((4-(morpholinomethyl)benzyl)oxy)-1-oxoisoindolin-5-
yl)methyl)
carbamate (400 mg, 55% yield) as a white solid. MS (ESI) m/z 579.1 [M+H]t
[0304] To a solution of (S)-tert-butyl ((2-(2,6-dioxopiperidin-3-y1)-4-
((4-
(morpholinomethyl) benzyl)oxy)-1-oxoisoindolin-5-yl)methyl)carbamate (250 mg,
0.435
mmol) in DCM (4 mL) at RT was added TFA (1.0 mL). The mixture was stirred at
RT for 1
hour. The solvent was evaporated to give (S)-3-(5-(aminomethyl)-4-((4-
(morpholinomethyl)
benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione (250 mg, 81% yield) as a
white solid.
MS (ESI) m/z 479.2 [M+H] 1E1 NMIt (400 MHz, DMSO-d6) 6 11.02 (s, 1H), 10.37
(s, 1H),
8.25 (s, 3H), 7.61-7.59 (m, 3H), 7.55-7.53 (m, 3H), 5.32(s, 2H), 5.17-5.12 (m,
1H), 4.78-4.55
(m, 2H), 4.36 (s, 2H), 4.14(d, J= 4.4 Hz, 2H), 3.91 (s, 2H), 3.71-3.60 (m,
2H), 3.26-3.09 (m,
4H), 2.96-2.91 (m, 1H), 2.66-2.61 (m, 1H), 2.46-2.42 (m, 1H), 2.04-1.99 (m,
1H).
[0305] To a solution of (S)-3-(5-(aminomethyl)-44(4-
(morpholinomethyl)benzyl)
oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione (150 mg, 0.21 mmol) in formic
acid (5 mL)
-126-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
at RT was added formaldehyde (1 mL, 40%). The mixture was heated to 100 C for
1 hour.
The solvent was evaporated, and the residue was purified by prep-HPLC as
previously
described to give (S)-3-(5-((dimethylamino)methyl)-444-
(morpholinomethyl)benzyl)oxy)-
1-oxoisoindolin-2-yl)piperidine-2,6-dione (80 mg, 79% yield) as a white solid.
MS (ESI)
m/z 507.2 [M+H]t 1H NMR (400 MHz, DMSO-d6) 6 11.05 (s, 1H), 10.91 (s, 1H),
10.01 (s,
1H), 7.68-7.54 (m, 6H), 5.37(s, 2H), 5.17-5.06 (m, 2H), 4.85-4.78 (m, 1H),
4.65-4.61 (m,
1H), 4.38 (s, 4H), 3.96 (s, 2H), 3.67 (s, 2H), 3.24-2.92 (m, 4H), 2.83-2.61
(m, 7H), 2.50-2.42
(m, 1H), 2.07-2.01 (m, 1H).
[0306] To a solution of (S)-3-(5-(aminomethyl)-444-
(morpholinomethyl)benzyl)
oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-dione (80 mg, 0.112 mmol) in THF (5
mL) was
added TEA (113 mg, 1.12 mmol). The mixture was stirred at RT for 10 min then 4-
nitrophenyl (3 -(44443 -(N-(tert-butyl)sulfamoyl)phenyl)amino)-5 -
methylpyrimidin-2-y1)
amino)phenoxy)propyl)carbamate (80 mg, 0.124 mmol) was added, followed by TEA
(90
mg, 0.9 mmol). After stirring at RT for lh, the mixture was concentrated and
the residue was
purified by prep-HPLC as previously described to afford Compound 23 (40 mg,
36% yield)
as a white solid. MS (ESI) m/z 989.4 [M+H]t 1E1 NMR (400 MHz, DMSO-d6) 6 11.01
(s,
1H), 8.79 (s, 1H), 8.54 (s, 1H), 8.13 (s, 2H), 7.90 (s, 1H), 7.57-7.33 (m,
11H), 6.79 (d, J = 8
Hz, 2H), 6.36-6.33 (m, 1H), 6.16-6.13 (m, 1H), 5.16-5.09(m, 3H), 4.65-4.54 (m,
2H), 4.33
(d, J = 4 Hz, 2H), 3.93-3.90 (m, 2H), 3.56 (s, 4H), 3.46 (s, 2H), 3.18-3.15
(m, 2H), 2.97-2.88
(m, 1H), 2.62-2.58 (m, 1H), 2.34 (s, 4H), 2.12 (s, 3H), 2.01-1.92 (m, 1H),
1.89 (s, 1H), 1.82-
1.79 (m, 2H), 1.12 (s, 9H).
Example 25
Compound 24: (S)-3 -(4-{ [p-({ 2-[3 -(p- {5-Methy1-4-[m-(tert-
butylaminosulfonyl)
phenylamino1-2-pyrimidinylamino}phenoxy)propylaminolacetylamino}methyl)phenyll
methoxy } -2-i soindolinoy1)-2,6-piperidinedione
o o
N...410
,k
0
HN NNH
* 0 100 100
N)L.k110
0
-127-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
[0307] To
a solution of 2-((3-(44(44(3-(N-(tert-butyl)sulfamoyl)phenyl)amino)-
5-methylpyrimidin-2-yl)amino)phenoxy)propyl)amino)acetic acid (54 mg, 0.1
mmol) and
(S)-3-(4-((4-(aminomethyl)benzyl)oxy)-1-oxoisoindolin-2-yl)piperidine-2,6-
dione (0.1
mmol) in DMF (2 mL) at RT was added DIEA (26 mg, 0.2 mmol), HOBt (20 mg, 0.15
mmol) and EDAC=HC1 (29 mg, 0.15 mmol) and the mixture was stirred overnight.
The
solvent was evaporated, and the residue was purified by prep-TLC (DCM/Me0H,
10:1) to
afford Compound 24 (6.0 mg, 6.7% yield) as a white solid. MS (ESI) m/z = 904.3
[M+H]t
NMR (400 MHz, DMSO-d6) 6 10.95 (s, 1H), 8.76 (s, 1H), 8.52 (s, 1H), 8.48 (s,
1H),
8.11-8.14 (m, 2H), 7.89 (s, 1H), 7.40-7.54 (m, 8H), 7.27-7.31 (m, 4H), 6.78
(d, J= 9.2 Hz,
2H), 5.20 (s, 2H), 5.09 (dd, J= 5.2, 13.2 Hz, 1H), 4.21-4.41 (m , 4H), 3.96
(t, J= 6.0 Hz ,
2H), 2.85-2.94 (m, 1H), 2.76 (t, J= 6.0 Hz , 2H), 2.53-2.58 (m, 1H), 2.40-2.45
(m, 2H), 2.11
(s, 3H), 1.95-1.98 (m, 2H), 1.87-1.90(m, 2H), 1.12 (s, 9H).
Example 26
Compound 25: (S)-3-(6-Fluoro-4- { [p-({ 2- [3 -(p- {5-methy1-44m-(tert-
butylaminosulfonyl)
phenylamino]-2-pyrimidinylamino phenoxy)propylamino] acetylamino
methyl)phenyl]
methoxy -2-i soindolinoy1)-2,6-piperidinedione
o o
N = = = - 0
0 HN)NNH
0
0,41)-µ11
0
[0308] To
a solution of methyl 5-fluoro-3-hydroxy-2-methylbenzoate (3.2 g, 17.4
mmol) and inidazole (2.9 g, 43.5 mmol) in DMF (6 mL) at RT was added tert-
butyldimethylsily1 chloride (3.1 g, 20.8 mmol). The mixture was stirred at 60
C for 1 hour
then cooled to RT and extracted with tert-butyl methyl ether. The combined
organic layers
were dried over anhydrous Na2SO4, filtered, and concentrated to give methyl 3-
((tert-
butyldimethylsilyl)oxy)-5-fluoro-2-methylbenzoate (5.2 g crude) as a yellow
oil which was
used for the next step without further purification.
[0309] To
a solution of methyl 3-((tert-butyldimethylsilyl)oxy)-5-fluoro-2-
methylbenzoate ( 3.2g, 10.7 mmol) in carbon tetrachloride (30 mL) at RT was
added NBS
-128-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
(2.9 g, 16.1 mmol) and 2,2'-azobis(2-methylpropionitrile) (700 mg, 4.3 mmol).
The mixture
was stirred at 80 C for 6 hrs. The solvent was evaporated to give the crude
product which
was purified by silica gel chromatography (petroleum ether/Et0Ac, 10:1) to
afford methyl 2-
(bromomethyl)-3-((tert-butyldimethylsilyl)oxy)-5-fluorobenzoate (4.0 g, quant.
yield) as a
yellow oil.
[0310] To
a solution of methyl 2-(bromomethyl)-3-((tert-butyldimethylsilyl)oxy)-
5-fluorobenzoate (1.0 g, 2.66 mmol) and TEA (0.7 mL, 5.32 mmol) in DMF (6 mL)
at RT
was added tert-butyl 4,5-diamino-5-oxopentanoate (696 mg, 2.92 mmol) in 4 mL
DNIF. The
mixture was stirred for 2 hrs, then heated to 80 C overnight. The solvent was
evaporated to
give the crude product which was purified by silica gel chromatography
(DCM/Me0H, 50:1
to 20:1) to afford tert-butyl 5-amino-4-(6-fluoro-4-hydroxy-1-oxoisoindolin-2-
y1)-5-
oxopentanoate (1.0 g crude) as a white solid. MS (ESI) m/z 297.0 [M+H-56]t
[0311] To a solution of tert-butyl 5-amino-4-(6-fluoro-4-hydroxy-1-
oxoisoindolin-2-y1)-5-oxopentanoate (500 mg, 1.42 mmol), 4-
(hydroxymethyl)benzonitrile
(283 mg, 2.13 mmol) and triphenylphosphine (930 mg, 3.55 mmol) in THF (4 mL)
at RT was
added DEAD ( 617 mg, 3.55 mmol). The mixture was stirred at RT for 2 hrs. The
solvent
was evaporated, and the residue was purified by silica gel chromatography
(DCM/Me0H,
100:1 to 50:1) to give tert-butyl 5-amino-4-(4-((4-cyanobenzyl)oxy)-6-fluoro-1-
oxoisoindolin-2-y1)-5-oxopentanoate (350 mg, 52% yield) as a yellow solid. MS
(ESI) m/z
412.0 [M+H-56]+.
[0312] To
a solution of tert-butyl 5-amino-4-(4-((4-cyanobenzyl)oxy)-6-fluoro-1-
oxoisoindolin-2-y1)-5-oxopentanoate (350 mg, 0.75 mmol) in DCM (4 mL) at RT
was added
TFA (4 mL). The mixture was stirred at RT overnight. The solvent was
evaporated to give
-amino-4-(4-((4-cyanob enzyl)oxy)-6-fluoro-l-oxoi soindolin-2-y1)-5-
oxopentanoic acid
(400 mg, crude) as a yellow solid which was used for the next step without
further
purification. MS (ESI) m/z 412.0 [M+H]t
[0313] To a solution of 5-amino-4-(4-((4-cyanobenzyl)oxy)-6-fluoro-1-
oxoisoindolin-2-y1)-5-oxopentanoic acid (400 mg, 0.75 mmol) in ACN (10 mL) at
RT was
added CDI (485 mg, 2.99 mmol). The mixture was stirred at 95 C for 3.5 hrs.
The solvent
was evaporated to give the crude product which was purified by silica gel
chromatography
(DCM/Me0H, 100:1 to 50:1) to afford 4#(2-(2,6-dioxopiperidin-3-y1)-6-fluoro-1-
-129-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
oxoisoindolin-4-yl)oxy)methyl)benzonitrile (200 mg, 68%) as a white solid. MS
(ESI) m/z
394.0 [M+H]t
[0314] To a solution of 44(2-(2,6-dioxopiperidin-3-y1)-6-fluoro-1-
oxoisoindolin-4-y1) oxy)methyl)benzonitrile (200 mg, 0.51 mmol) and di-tert-
butyl
dicarbonate (166 mg, 0.76 mmol) in THF (6 mL) at RT was added Raney-Ni (80
mg). The
mixture was stirred at RT under H2 overnight. The suspension was filtered
through a Celite
pad and the filtrate was concentrated to give the crude product which was
purified by silica
gel chromatography (DCM/Me0H, 100:1 to 20:1) to afford tert-butyl 4-(((2-(2,6-
dioxopiperidin-3-y1)-6-fluoro-1-oxoisoindolin-4-yl)oxy)methyl)benzylcarbamate
(90 mg,
35% yield) as a white solid.MS (ESI) m/z 398.0 [M+H-100]t
[0315] To
a solution of tert-butyl 44(2-(2,6-dioxopiperidin-3-y1)-6-fluoro-1-
oxoisoindolin-4-yl)oxy) methyl)benzylcarbamate (80 mg, 0.161 mmol) in DCM (4
mL) at
RT was added TFA (1 mL). The reaction was stirred for 1 hour. The solvent was
evaporated, and the residue was dried to give the amine TFA salt as a yellow
gum.
[0316] The
amine TFA salt was dissolved in DMA (1 mL) and 2-((3-(4-((4-((3-
(N-(tert-butyl)sulfamoyl)phenyl)amino)-5-methylpyrimidin-2-
yl)amino)phenoxy)propyl)
amino)acetic acid (109 mg, 0.161 mmol) was added, followed by HOBt (32.6 mg,
0.242
mmol), EDAC=HC1 (46.5 mg, 0.242 mmol) and DIEA (41.5 mg, 0.322 mmol) and the
mixture was stirred at RT for 10 hrs. The solvent was evaporated, and the
residue was
purified by prep-HPLC as previously described to afford Compound 25 (23.9 mg,
16% yield)
as a white solid. MS (ESI) m/z 922.3[M+H]t NMR
(400 MHz, DMSO-d6) 6 10.96 (s,
1H), 8.75 (s, 1H), 8.52 (s, 1H), 8.29 (t, J = 6.4 Hz, 1H), 8.14-8.11 (m, 2H),
7.88 (s, 1H),
7.55-7.23 (m, 10H), 7.10-7.08 (m, 1H), 6.77 (d, J = 5.2 Hz, 2H), 5.19 (s, 2H),
5.08 (dd, J =
8.4, 13.6 Hz, 1H), 4.37-4.18 (m, 4H), 3.95 (t, J = 6.4 Hz, 1H), 3.16 (s, 2H),
2.93-2.83 (m,
1H), 2.66-2.57 (m, 3H), 2.11 (s, 3H), 2.02-1.93 (m, 1H), 1.86-1.79 (m, 3H),
1.12 (s, 9H).
Example 27
Compound 26: (S)-3 -(4- {2[p-(Morpholinomethyl)phenyl]ethoxy -2-i
soindolinoy1)-2,7-
azepanedione
-130-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
0 0 H
N .= =
0
r0
1401 N)
[0317] To a solution of methyl 4-(2-methoxy-2-oxoethyl)benzoate (5 g,
0.24 mol)
in THF (25 mL) at 0 C was added lithium aluminium hydride (1M solution in
THF, 36 mL)
dropwise. The mixture was stirred at 0 C for 2 hrs then the reaction was
quenched by
Na2SO4decahydrate, diluted with Et0Ac and filtered. The filtrate was washed
with brine,
dried over anhydrous Na2SO4, filtered, and concentrated to give 2-(4-
(hydroxymethyl)phenyl)ethanol (3 g, crude) as a yellow oil. 'HNMR (400 MHz,
DMSO-d6)
6 7.13-7.23 (m, 4H), 5.08 (t, J = 5.6 Hz, 1H), 4.61 (t, J= 5.2, 8.4 Hz, 1H),
4.44 (d, J = 7.0
Hz, 2H), 3.54-3.59 (m, 2H), 2.69 (t, J = 7.2 Hz, 2H).
[0318] To a stirred solution of 2-(4-(hydroxymethyl)phenyl)ethanol (3
g, crude,
19.7 mmol) in chloroform (30 mL) was added manganese dioxide (6.9 g, 79 mmol).
The
mixture was stirred at 70 C overnight then filtered and concentrated. The
residue was
purified by silica gel chromatography (petroleum/Et0Ac, 5:1) to give 4-(2-
hydroxyethyl)benzaldehyde (1.3 g, 38% over 2 steps) as a yellow oil. 1-H NMR
(300 MHz,
CDC13) 6 9.90 (s, 1H), 7.76 (d, J = 8.0 Hz, 2H), 7.34 (d, J= 8.0 Hz, 2H), 3.84
(t, J= 6.4 Hz,
2H), 2.88 (t, J = 6.4 Hz, 2H).
[0319] To a stirred solution of 4-(2-hydroxyethyl)benzaldehyde (1.3 g,
8.67
mmol) in DCM (20 mL) was added tosyl chloride (2.5 g, 13 mmol) and TEA (4.8
mL). The
mixture was stirred at RT overnight then evaporated and purified by silica gel
chromatography (petroleum/Et0Ac, 5:1) to give 4-formylphenethyl 4-
methylbenzenesulfonate (2 g, 77% yield) as a white solid.
[0320] To a stirred solution of (S)-4-hydroxy-2-(2-oxoazepan-3-
yl)isoindolin-1-
one (250 mg, 0.96 mmol) and 4-formylphenethyl 4-methylbenzenesulfonate (392
mg, 1.15
mmol) in ACN (5 mL) at RT was added K2CO3 (400 mg, 2.88 mmol). After stirring
at 80 C
overnight, the mixture was concentrated, and the residue was purified by
silica gel
chromatography (DCM/Me0H, 50:1) to give (S)-4-(2-((1 -oxo-2-(2-oxoazep an-3 -
-131-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
yl)isoindolin-4-yl)oxy)ethyl)benzaldehyde (100 mg, 27% yield) as a colorless
oil. MS (ESI)
m/z = 393.1 [M+H]
[0321] To a solution of (S)-4-(2((1-oxo-2-(2-oxoazepan-3-yl)isoindolin-
4-y1)
oxy)ethyl)benzaldehyde (100 mg, 0.25 mmol) in 1,2-dichloroethane (6 mL) and
DMSO (1
mL) at RT was added Dess-Martin reagent (530 mg, 1.25 mol). The mixture was
stirred at
80 C overnight then cooled to RT and filtered. The filtrate was quenched with
sat. sodium
thiosulfate solution and extracted with DCM. Workup and purification by prep-
TLC
(Et0Ac) to give (S)-4-(242-(2,7-dioxoazepan-3-y1)-1-oxoisoindolin-4-y1)
oxy)ethyl)
benzaldehyde (40 mg, 38% yield) as a white solid. MS (ESI) m/z = 407.0 [M+H]t
[0322] To a solution of (S)-4-(242-(2,7-dioxoazepan-3-y1)-1-
oxoisoindolin-4-y1)
oxy)ethyl)benzaldehyde (40 mg, 0.1 mmol) and morpholine (26 mg, 0.3 mmol) in
DCM (5
mL) at RT was added sodium triacetoxyborohydride (106 mg, 0.5 mmol). The
mixture was
stirred overnight then concentrated. The residue was purified by prep-HPLC as
previously
described to afford Compound 26 (18 mg, 38% yield) as a white solid. MS (ESI)
m/z =
478.1 [M+H]t 1-E1 NMR (400 MHz, DMSO-d6) 6 10.72 (s, 1H), 7.45 (t, J = 8.0 Hz,
1H),
7.23-7.30 (m, 6H), 5.22 (dd, J = 4.8, 12.4 Hz, 1H), 4.36 (s, 2H), 4.32 (t, J=
6.8 Hz, 2H), 3.55
(t, J= 4.4 Hz, 4H), 3.41 (s, 2H), 3.03-3.08 (m, 3H), 2.59-2.60 (m, 1H), 2.29-
2.32 (m, 5H),
1.99-2.10 (m, 2H), 1.74-1.84 (m, 1H).
Example 28
Compound 27: (S)-3 -[5-({ 3 -[3 -(p- {5-Methy1-44m-(tert-
butylaminosulfonyl)phenylamino]-2-
pyrimidinylamino}phenoxy)propyl]ureidoImethyl)-4- { [p-
(morpholinomethyl)phenyl]
methoxy -2-i soindolinoy1]-2, 7-azepanedione
0 o H
HN A30 ON
S<
NH 0
110
HN
0
N N
[0323] To a solution of (S)-3-(5-(aminomethyl)-444-
(morpholinomethyl)benzyl)
oxy)-1-oxoisoindolin-2-yl)azepane-2,7-dione (50 mg, 0.084 mmol) in THF (5 mL)
at RT was
-132-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
added TEA (68 mg, 0.672 mmol) followed by a suspension of 4-nitrophenyl
(3444(44(3-(N-
(tert-butyl)sulfamoyl)phenyl)amino)-5-methylpyrimidin-2-
yl)amino)phenoxy)propyl)
carbamate (54 mg, 0.084 mmol) in THF (1 mL). The mixture was stirred for 3
hrs. The
solvent was evaporated, and the residue was purified by silica gel
chromatography eluting
with DCM/Me0H from 0% to 10% to give the crude product (70 mg) as a white gum,
which
was further purified by prep-HPLC as previously described to afford Compound
27 (27.7
mg, 33% yield) as a white solid. MS (ESI) m/z 1003.4[M+H]t 1-14 NMR (400 MHz,
DMSO-d6) 6 10.70 (s, 1H), 8.76 (s, 1H), 8.53 (s, 1H), 8.13 (s, 1H), 7.90 (s,
1H), 7.59-7.38
(m, 9H), 7.34 (d, J= 8.0 Hz, 2H), 6.79 (d, J= 8.8 Hz, 2H), 6.34 (t, J = 6.0
Hz, 1 H), 6.13 (t, J
= 5.6 Hz, 1H), 5.23 (dd, J = 4.8, 12.0 Hz, 1H), 5.18 (s, 2H), 4.65 (s, 2H),
4.33 (d, J= 5.2 Hz,
2H), 3.92 (t, J= 6.0 Hz, 2H), 3.58-3.55 (m, 4H), 3.47 (s, 2H), 3.20-3.15 (m,
2H), 3.12-3.04
(m, 1H), 2.60-2.56 (m, 1H), 2.35-2.31 (m, 5H), 2.12 (s, 3H), 2.08-1.99 (m,
1H), 1.86-1.78
(m, 4H), 1.12 (s, 9H).
PBMC Assays
[0324] Frozen primary blood mononuclear cells (PBMCs) were purchased
from
AllCells. Cells were quick thawed, washed once with RPMI-1640/10% FBS.
[0325] 1% Penicillin/1% Streptomycin and plated in 96 well plates at
200,000
cells per well. Cells were pretreated with DMSO only, or Compound 1 for 1 hour
and then
induced with 100 ng/mL lipopolysaccharide (LPS) for 18-24 hrs. The supernatant
was
analyzed for IL-1 beta, IL-6, and TNFa, using Meso Scale assay according to
manufacturer's
protocol. The negative control wells were treated with DMSO.
[0326] For the IL-2 analysis, 96 well plates were precoated with 1
g/mL anti-
human CD3 antibody (OKT3, eBioscience Inc.). After washing with PBS, Compound
1 was
added (50 L/well) followed by PBMCs diluted at 3 ¨ 4 million cells/mL (150
L/well).
Plates were incubated for 24 hr and the supernatants collected for Mesoscale
IL-2 analysis.
[0327] Compound activity was measured as fold difference from the DMSO
control. IL-113 activity is shown in Figure 1; IL-6 activity is shown in
Figure 2; TNFa
activity is shown in Figure 3; and IL-2 activity is shown in Figure 4.
Additional data for
compounds (shown as percent inhibition) at 10 M is shown in Table 1; at 1 M
in Table 2;
-133-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
and at 0.1 uM in Table 3. Additional data for IL-activity (measured as fold-
change in
activity) is shown in Tables 4-6.
Table 1
Compound IL-1B IL-6 TNFa
No. % Inhibition % Inhibition % Inhibition
3 93 77 94
12 99.6 100 99
14 65 42 83
15 81 50 87
16 52 78 75
17 100 100 100
18 83 45 89
21 11 7 30
24 23 1 23
28 5 2 5
Table 2
Compound IL-1B IL-6 TNFa
No. % Inhibition % Inhibition % Inhibition
1 88 71 93
2 86 67 90
3 93 79 93
4 86 67 90
6 92 76 83
12 23 0 32
13 51 59 84
14 76 37 82
15 59 10 65
16 24 5 27
17 75 35 75
18 80 49 83
19 65 43 73
20 43 5 57
21 6 8 20
22 74 27 80
23 79 32 88
24 7 9 10
25 8 32 25
26 81 8 87
-134-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
27 62 4 72
28 6 5 9
29 2 11 11
Table 3
Compound IL-1B IL-6 TNFa
No. % Inhibition % Inhibition % Inhibition
1 83 63 89
2 72 43 81
3 91 72 91
4 72 43 81
5 64 37 55
6 68 42 62
7 71 55 62
8 73 56 63
9 74 51 62
10 52 27 48
11 21 12 21
19 22 12 41
20 30 1 43
22 69 23 78
23 35 23 48
25 0 3 13
26 78 5 82
27 50 5 60
29 0 11 12
Table 4
IL-2
Compound 10 uM
Compound Fold
No. Change
12 0.7
13 0.1
14 2.3
15 2.1
16 0.2
17 0.1
18 3.3
-135-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
21 1
24 1
28 1.9
Table 5
IL-2
Compound at 1 uM
Compound Fold
No. Change
1 0.8
2 2.3
3 0.7
4 2.3
2.1
6 2.5
7 1.1
8 2.3
9 1.6
2.1
11 1.9
12 1
13 0.3
14 2.2
1.9
16 0.7
17 1.5
18 3.2
19 2.4
1.4
21 0.9
22 2.4
23 1.3
24 1
1
26 2.7
27 1.5
28 1.5
29 0.9
Table 6
-136-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
IL-2
Compound at 0.1 uM
Compound Fold
No. Change
1 2.1
2 1.3
3 2
4 1.3
1.2
6 1
7 1
8 1.8
9 1.6
1.9
11 1.2
19 1.5
0.9
22 2
23 0.9
0.9
26 2.6
27 1.3
29 0.9
Western Blot Analysis
[0328] Western Blot Protocol: Jurkat cells were grown in RPMI 1640
supplemented with streptomycin, penicillin and 10% fetal bovine serum.
[0329] Jurkat cells were cultured at approximately 106 cells per mL,
DMSO or
the indicated compound at the indicated concentration was added to the cells
and allowed to
incubate for the indicated period. Whole cell extracts were prepared with RIPA
Reagent
according to manufacturer's protocol (Pierce). Briefly, ¨5 x 106 cells were
washed once in
PBS, the cell pellet was resuspended in RIPA solution and allowed to incubate
for 10 min at
room temperature. Cell debris was removed by centrifugation and the cleared
whole cell
lysate was transferred to a new tube for further analysis.
[0330] For Western blot analysis, whole cell extracts were separated
on 4-12%
SDS-polyacrylamide gels, transferred to nitrocellulose and probed with the
indicated primary
-137-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
antibodies. Membranes were subsequently washed and probed with the appropriate
horseradish peroxidase (HRP)-conjugated secondary antibody. The signal was
detected
using the WesternBright Sirius Reagent (Advansta). Results are shown in FIG.
5A and FIG.
5B.
[0331] The following antibodies were used in these studies:
Beta-actin: Mouse anti-b-Actin was obtained from Cell Signaling (8H10D10).
CKla goat polyclonal antibody: Santa Cruz Biotechnology, sc-6477 (Santa Cruz,
CA)
Ikaros rabbit monoclonal antibody: Cell Signaling, #9034, D10E5 (Danver, MA)
Donkey anti-goat IgG-HRP: Santa Cruz Biotechnology, sc-2056 (Santa Cruz, CA)
Goat anti-rabbit IgG-HRP: Cell Signaling, #7074 (Danver, MA)
Goat anti-mouse IgG-HRP: Sigma, A4416 (St. Louis, MO)
Cell Viability Assay
[0332] Molm-13 and MV-4-11 cells were cultivated in RPMI-1640 (10% FBS
/
1% pen-strep) and were plated in white walled 96-well plates at 20,000
cells/well.
[0333] Cells were treated with compound at the indicated concentration
or
DMSO (control) and the cultures were incubated for 3 days at 37 C and 5% CO2.
Following
the incubation period, 100 tL of CellTiterGlow (CTG) reagent (CellTiter-Glo
Luminescent
Cell Viability Assay, Promega (Madison, WI)) was added to each well. Following
a 10 min
incubation with shaking, luminescence was measured using a Victor Wallac
Luminometer.
[0334] Molm-13 cellular proliferation activity is shown in Tables 7-9.
MV-4-11
cellular proliferation activity in shown in Tables 10 and 11.
Table 7
Molm-13 Proliferation
Compound at 10 uM
Compound 0
Inhi /0 bition
No.
1 37
2 30
3 29
40
6 91
8 16
-138-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
9 34
10 38
18 99
Table 8
Molm-13 Proliferation
Compound at 1 uM
Compound
% Inhibition
No.
1 15
2 5
3 1
13 29
14 35
20 99
22 56
23 30
26 66
27 37
Table 9
Molm-13 Proliferation
Compound at 0.1 uM
Compound
% Inhibition
No.
1 30
20 93
22 31
26 21
Table 10
MV-4-11 Proliferation
Compound at 10 uM
Compound
% Inhibition
No.
1 5
2 16
-139-
CA 03072543 2020-02-10
WO 2019/040274
PCT/US2018/045626
3 20
4 0
3
6 76
7 0
8 0
9 10
35
11 0
12 35
13 50
14 0
99
16 99
17 98
18 99
19 0
88
21 97
22 4
23 79
24 80
90
26 23
27 36
Table 11
MV-4-11 Proliferation
Compound at 1 uM
Compound . . .
% Inhibition
No.
1 2
2 6
3 0
4 0
5 1
6 0
7 0
-140-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
8 0
9 2
1
11 0
12 0
13 0
14 0
38
16 0
17 31
18 99
19 0
77
21 0
22 2
23 8
24 44
54
26 2
27 10
[0335] While the disclosure has been illustrated and described in
detail in the
drawings and foregoing description, such illustration and description are to
be considered
illustrative or exemplary and not restrictive. The disclosure is not limited
to the disclosed
embodiments. Variations to the disclosed embodiments can be understood and
effected by
those skilled in the art in practicing the claimed disclosure, from a study of
the drawings, the
disclosure and the appended claims.
[0336] All references cited herein are incorporated herein by
reference in their
entirety. To the extent publications and patents or patent applications
incorporated by
reference contradict the disclosure contained in the specification, the
specification is
intended to supersede and/or take precedence over any such contradictory
material.
[0337] Unless otherwise defined, all terms (including technical and
scientific
terms) are to be given their ordinary and customary meaning to a person of
ordinary skill in
the art, and are not to be limited to a special or customized meaning unless
expressly so
defined herein. It should be noted that the use of particular terminology when
describing
-141-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
certain features or aspects of the disclosure should not be taken to imply
that the terminology
is being re-defined herein to be restricted to include any specific
characteristics of the
features or aspects of the disclosure with which that terminology is
associated.
[0338] Where a range of values is provided, it is understood that the
upper and
lower limit, and each intervening value between the upper and lower limit of
the range is
encompassed within the embodiments.
[0339] Terms and phrases used in this application, and variations
thereof,
especially in the appended claims, unless otherwise expressly stated, should
be construed as
open ended as opposed to limiting. As examples of the foregoing, the term
'including'
should be read to mean 'including, without limitation,' including but not
limited to,' or the
like; the term 'comprising' as used herein is synonymous with 'including,'
containing,' or
'characterized by,' and is inclusive or open-ended and does not exclude
additional, unrecited
elements or method steps; the term 'having' should be interpreted as 'having
at least,' the
term 'includes' should be interpreted as 'includes but is not limited to;' the
term 'example' is
used to provide exemplary instances of the item in discussion, not an
exhaustive or limiting
list thereof; adjectives such as 'known', 'normal', 'standard', and terms of
similar meaning
should not be construed as limiting the item described to a given time period
or to an item
available as of a given time, but instead should be read to encompass known,
normal, or
standard technologies that may be available or known now or at any time in the
future; and
use of terms like 'preferably,' preferred,"desired,' or 'desirable,' and words
of similar
meaning should not be understood as implying that certain features are
critical, essential, or
even important to the structure or function of the invention, but instead as
merely intended to
highlight alternative or additional features that may or may not be utilized
in a particular
embodiment of the invention. Likewise, a group of items linked with the
conjunction 'and'
should not be read as requiring that each and every one of those items be
present in the
grouping, but rather should be read as 'and/or' unless expressly stated
otherwise. Similarly,
a group of items linked with the conjunction 'or' should not be read as
requiring mutual
exclusivity among that group, but rather should be read as 'and/or' unless
expressly stated
otherwise.
[0340] With respect to the use of substantially any plural and/or
singular terms
herein, those having skill in the art can translate from the plural to the
singular and/or from
-142-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
the singular to the plural as is appropriate to the context and/or
application. The various
singular/plural permutations may be expressly set forth herein for sake of
clarity. The
indefinite article "a" or "an" does not exclude a plurality. A single
processor or other unit
may fulfill the functions of several items recited in the claims. The mere
fact that certain
measures are recited in mutually different dependent claims does not indicate
that a
combination of these measures cannot be used to advantage. Any reference signs
in the
claims should not be construed as limiting the scope.
[0341] It will be further understood by those within the art that if a
specific
number of an introduced claim recitation is intended, such an intent will be
explicitly recited
in the claim, and in the absence of such recitation no such intent is present.
For example, as
an aid to understanding, the following appended claims may contain usage of
the
introductory phrases "at least one" and "one or more" to introduce claim
recitations.
However, the use of such phrases should not be construed to imply that the
introduction of a
claim recitation by the indefinite articles "a" or "an" limits any particular
claim containing
such introduced claim recitation to embodiments containing only one such
recitation, even
when the same claim includes the introductory phrases "one or more" or "at
least one" and
indefinite articles such as "a" or "an" (e.g., "a" and/or "an" should
typically be interpreted to
mean "at least one" or "one or more"); the same holds true for the use of
definite articles
used to introduce claim recitations. In addition, even if a specific number of
an introduced
claim recitation is explicitly recited, those skilled in the art will
recognize that such recitation
should typically be interpreted to mean at least the recited number (e.g., the
bare recitation of
"two recitations," without other modifiers, typically means at least two
recitations, or two or
more recitations). Furthermore, in those instances where a convention
analogous to "at least
one of A, B, and C, etc." is used, in general such a construction is intended
in the sense one
having skill in the art would understand the convention (e.g., "a system
having at least one of
A, B, and C" would include but not be limited to systems that have A alone, B
alone, C
alone, A and B together, A and C together, B and C together, and/or A, B, and
C together,
etc.). In those instances where a convention analogous to "at least one of A,
B, or C, etc." is
used, in general such a construction is intended in the sense one having skill
in the art would
understand the convention (e.g., "a system having at least one of A, B, or C"
would include
but not be limited to systems that have A alone, B alone, C alone, A and B
together, A and C
-143-
CA 03072543 2020-02-10
WO 2019/040274 PCT/US2018/045626
together, B and C together, and/or A, B, and C together, etc.). It will be
further understood
by those within the art that virtually any disjunctive word and/or phrase
presenting two or
more alternative terms, whether in the description, claims, or drawings,
should be understood
to contemplate the possibilities of including one of the terms, either of the
terms, or both
terms. For example, the phrase "A or B" will be understood to include the
possibilities of
"A" or "B" or "A and B."
[0342] All numbers expressing quantities of ingredients, reaction
conditions, and
so forth used in the specification are to be understood as being modified in
all instances by
the term 'about.' Accordingly, unless indicated to the contrary, the numerical
parameters set
forth herein are approximations that may vary depending upon the desired
properties sought
to be obtained. At the very least, and not as an attempt to limit the
application of the doctrine
of equivalents to the scope of any claims in any application claiming priority
to the present
application, each numerical parameter should be construed in light of the
number of
significant digits and ordinary rounding approaches.
-144-