Note: Descriptions are shown in the official language in which they were submitted.
CA 03084963 2020-06-05
- 1 -
DESCRIPTION
Title of Invention: METHOD FOR MANUFACTURING ATOMIZED METAL
POWDER
Technical Field
[0001]
The present invention relates to a method for
manufacturing atomized metal powder. In particular, the
present invention can preferably be used for manufacturing
atomized metal powder containing iron-group constituents
(Fe, Ni, and Co) in a total amount of 76 at% or more in
terms of atomic fraction.
Background Art
[0002]
Conventionally, examples of a method for manufacturing
metal powder include an atomizing method. Examples of such
an atomizing method include a water atomizing method in
which high-pressure water jets (high-pressure water) are
ejected to a molten metal stream to obtain metal powder and
a gas atomizing method in which an inert gas, instead of
water jets, is ejected.
[0003]
In a water atomizing method, atomized metal powder is
obtained not only by separating a molten metal stream into
powdery metal (metal powder) with water jets ejected from,
for example, nozzles, but also by cooling the powdery metal
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 2 -
(metal powder) with the water jets. On the other hand, in a
gas atomizing method, atomized metal powder is usually
obtained by dropping powdery metal (metal powder), which has
been obtained by separating a molten metal stream into
powdery metal with an inert gas ejected through nozzles,
into a water tank or a flowing-water drum located under an
atomizing apparatus to cool the powdery metal.
[0004]
As a method for manufacturing metal powder, a water
atomizing method is superior to a gas atomizing method from
the viewpoint of high production capability and low cost.
In the case of a gas atomizing method, it is necessary to
use an inert gas when performing atomizing, and such a
method is inferior to a water atomizing method from the
viewpoint of atomizing energy. In addition, while metal
powder particles manufactured by using a gas atomizing
method have an almost spherical shape, metal powder
particles manufactured by using a water atomizing method
have irregular shapes. Therefore, a water atomizing method
has an advantage over a gas atomizing method in that, when
metal powder is formed into, for example, a motor core by
performing compaction forming, irregularly shaped metal
powder particles manufactured by using a water atomizing
method are more likely than spherically shaped metal powder
particles manufactured by using a gas atomizing method to be
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 3 -
entangled with each other to increase strength after
compaction has been performed.
[0005]
Nowadays, there is a demand for, for example, reducing
the iron loss and size of a motor core which is used for an
electric automobile or a hybrid automobile from the
viewpoint of energy saving. To date, such a motor core has
been manufactured by placing thin electrical steel sheets on
top of one another. However, nowadays, a motor core
manufactured by using metal powder, which has a high degree
of freedom for shape design, is receiving much attention.
To reduce the iron loss of such a motor core, using non-
crystalline (amorphous) metal powder is considered
effective. To manufacture amorphous metal powder, it is
necessary that atomized metal powder be rapidly cooled by
using a coolant to prevent crystallization while atomizing
high-temperature molten metal. In addition, it is necessary
to increase magnetic flux density to realize a reduction in
motor size and an increase in motor power along with a
reduction in iron loss. To increase magnetic flux density,
increasing iron-group constituent concentration (including
Ni and Co) is important, and there is a demand for soft
magnetic iron powder, which is an amorphous soft magnetic
metal powder for a motor core having an iron-group
constituent concentration of about 76 at% to 90 at%. In the
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 4 -
case where the iron-group constituent concentration is about
80 at%, it is considered necessary to perform cooling at a
cooling rate of 106 K/s or more to obtain amorphous metal
powder, and it is very difficult to realize both a reduction
in iron loss and an increase in magnetic flux density of
metal powder at the same time.
[0006]
In particular, one of the reasons why an increase in
cooling rate is suppressed is as follows. When high-
temperature molten metal is cooled with water, since water
is instantly vaporized at the time of contact between the
water and the molten metal to form vapor films around the
molten metal, direct contact between a surface to be cooled
and water is suppressed (film boiling occurs), which
suppresses an increase in cooling rate.
[0007]
To solve the problem of suppressed cooling due to vapor
films or film boiling when amorphous iron powder is
manufactured, investigations described in Patent Literature
1 through Patent Literature 11 have been conducted.
[0008]
For example, Patent Literature 1 describes a method for
manufacturing metal powder in which, when molten metal is
cooled so as to be solidified when being scattered to obtain
metal powder, the cooling rate until the molten metal is
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 5 -
solidified is set to be 105 K/s or more. It is indicated
that, in the case of the technique described in Patent
Literature 1, it is possible to achieve the cooling rate
described above by bringing the scattered molten metal into
contact with a cooling liquid flow which is formed by
swirling a cooling liquid along the inner wall surface of a
cylinder. In addition, it is indicated that it is
preferable that the flow rate of the cooling liquid flow
which is formed by swirling the cooling liquid be 5 m/s to
100 m/s.
[0009]
In addition, Patent Literature 2 describes a method for
manufacturing rapidly solidified metal powder. In the case
of the technique described in Patent Literature 2, molten
metal is rapidly solidified by feeding the molten metal onto
the inner peripheral surface of a swirling cooling liquid
layer which is formed by feeding the cooling liquid
circumferentially from the outside of the top end of the
cylindrical part of a cooling container having a cylindrical
inner peripheral surface so that the cooling liquid drops
while swirling along the inner peripheral surface of the
cylindrical part in such a manner that the swirling cooling
liquid layer having a hollow space at the center thereof is
centrifugally formed due to swirling. It is indicated that,
with this, it is possible to obtain high-quality rapidly
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 6 -
solidified powder with high cooling efficiency.
[0010]
In addition, Patent Literature 3 describes an apparatus
for manufacturing metal powder which uses a gas atomizing
method and which has gas jet nozzles for ejecting gas jets
onto molten metal flowing downward to separate the molten
metal into droplets and a cooling cylinder having an inner
peripheral surface along which a cooling liquid layer flows
downward while swirling. It is indicated that, in the case
of the technique described in Patent Literature 3, it is
possible to obtain rapidly solidified fine metal powder as a
result of molten metal being separated in two steps
consisting of one step utilizing gas-jet nozzles and the
other step utilizing a swirling cooling liquid layer.
[0011]
In addition, Patent Literature 4 describes a method for
manufacturing fine amorphous metal particles in which fine
amorphous metal particles are obtained by feeding molten
metal into a liquid cooling medium to form vapor films
covering the molten metal in the cooling medium and to
collapse the formed vapor films so that boiling occurs due
to spontaneous nucleation as a result of the molten metal
and the cooling medium being brought into direct contact
with each other and by utilizing a pressure wave due to
boiling to tear the molten metal into pieces so that the
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 7 -
molten metal is rapidly cooled to make amorphous metal. It
is indicated that it is possible to collapse the vapor films
covering the molten metal by controlling the temperature of
the molten metal which is fed into the cooling medium so
that, when the molten metal and the cooling medium being
brought into direct contact with each other, an interface
temperature is equal to or lower than a minimum film boiling
temperature and equal to or higher than a spontaneous
nucleation temperature or by performing ultrasonic
irradiation.
[0012]
In addition, Patent Literature 5 describes a method for
manufacturing fine particles in which a molten material is
made into fine particles and cooled so as to be solidified
by controlling the temperature of the molten material so
that the material is molten at a temperature equal to or
higher than the spontaneous nucleation temperature of a
cooling liquid medium when the molten material is fed into
the cooling liquid medium in the form of droplets or jets
and by controlling a relative velocity between the molten
material and the cooling liquid medium when the molten
material is fed into the cooling liquid medium to be 10 m/s
or more to forcibly collapse vapor films formed around the
molten material so that boiling occurs due to spontaneous
nucleation. It is indicated that, with this, it is possible
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 8 -
to manufacture fine particles or an amorphous material from
a material which is difficult to make into fine particles or
an amorphous material by using conventional methods.
[0013]
In addition, Patent Literature 6 describes a method for
manufacturing a functional member which has a process of
obtaining homogeneous functional fine particles of
polycrystalline or amorphous material without segregation by
dissolving a raw material to which a functional additive is
added in a base material and by feeding the molten mixture
into a cooling liquid medium so that the molten mixture is
made into fine particles due to vapor explosion and cooled
so as to be solidified while controlling the cooling rate
and a process of obtaining a functional member by
compressing the functional fine particles and fine particles
of the base material used as raw materials.
[0014]
Patent Literature 7 and Patent Literature 8 state that
it is possible to collapse vapor films formed around powder
particles, which have been obtained by atomizing a molten
material, by suctioning the particles into a suction pipe
disposed below a water atomizing device.
[0015]
Patent Literature 9 states that vapor films formed
around powder particles, which have been obtained by
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 9 -
atomizing a molten material, are collapsed by ejecting a
liquid at a pressure of 80 kgf/cm2 or higher so that the
particles collide with a cooling block disposed below a
water atomizing device.
[0016]
Patent Literature 10 states that covering vapor films
are removed by ejecting a second liquid from a device for
ejecting the second liquid, which is disposed below an
atomizing device, at an ejection pressure of the liquid of 5
MPa to 20 MPa to forcibly change the moving direction of a
fluid dispersion containing molten metal.
[0017]
Patent Literature 11 discloses an invention regarding
an iron-boron-based ferromagnetic material (permanent
magnet) containing a rare-earth metal and states that, when
performing pulverizing and manufacturing of an amorphous
material by using a water atomizing method, it is preferable
that a water pressure be 750 kgf/cm2 to 1200 kgf/cm2, that a
water temperature be 20 C or lower, and that the amount (kg)
of water for 1 kg of iron be 25 [-] to 45 [-].
Citation List
Patent Literature
[0018]
PTL 1: Japanese Unexamined Patent Application
Publication No. 2010-150587
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 10 -
PTL 2: Japanese Examined Patent Application Publication
No. 7-107167
PTL 3: Japanese Patent No. 3932573
PTL 4: Japanese Patent No. 3461344
PTL 5: Japanese Patent No. 4793872
PTL 6: Japanese Patent No. 4784990
PTL 7: Japanese Unexamined Patent Application
Publication No. 60-24302
PTL 8: Japanese Unexamined Patent Application
Publication No. 61-204305
PTL 9: Japanese Unexamined Patent Application
Publication No. 60-24303
PTL 10: Japanese Unexamined Patent Application
Publication No. 2007-291454
PTL 11: Japanese Unexamined Patent Application
Publication No. 2004-349364
Summary of Invention
Technical Problem
[0019]
In the case of the techniques described in Patent
Literature 1 through Patent Literature 3, it is intended to
remove vapor films formed around separated metal particles
by feeding molten metal into a cooling liquid layer formed
by swirling the cooling liquid. However, in the case where
the temperature of the separated metal particles is high,
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 11 -
since film boiling tends to occur in the cooling liquid
layer, and since the metal particles fed into the cooling
liquid layer move along with the cooling liquid layer, that
is, a relative velocity with respect to the cooling liquid
layer is small, there is a problem in that it is difficult
to avoid film boiling.
[0020]
In addition, in the case of the techniques described in
Patent Literature 1 through Patent Literature 6, since a gas
atomizing method is used to manufacture metal powder, and
since it is necessary to use a large amount of inert gas for
atomizing in a gas atomizing method, there is a problem of
an increase in manufacturing costs.
[0021]
The techniques described in Patent Literature 7 through
Patent Literature 10 relate to a water atomizing method. In
the case of the techniques described in Patent Literature 7
and Patent Literature 8, it is indicated that it is possible
to remove vapor films by suctioning powder. However, when
water exists around a high-temperature object, since water
is continuously vaporized to form vapor films due to heat
fed from the inside of the object, the water and the molten
metal are suctioned together with no change, and it is
difficult to remove the vapor films.
[0022]
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 12 -
Patent Literature 9 states that it is possible to
collapse vapor films by allowing molten metal which is
covered with vapor films to collide with a cooling block
disposed below an atomizing device. However, in the case
where a liquid is used for separation, since there is an
increase in the temperature of the liquid, vapor films tend
to be formed. In addition, since the ejection pressure
(pressure energy) of the liquid is utilized for separation,
there is insufficient energy for collapsing vapor films at
the time of collision with the cooling block. Even if vapor
films are collapsed, vapor films soon re-form as long as the
molten metal (powder) has a high temperature. Therefore, it
is necessary to always continue removing vapor films.
[0023]
In addition, Patent Literature 10 states that, it is
possible to remove vapor films by changing the moving
direction of a liquid dispersion containing molten metal
droplets, which have been formed by performing atomizing, by
using a liquid jet spray. However, in the case where the
temperature of the molten metal covered with vapor films is
excessively high when the moving direction is changed, the
molten metal may be covered with vapor films again due to
surrounding cooling water. On the contrary, in the case
where the temperature of the molten metal is excessively low
when the molten metal collides with a cooling block, there
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 13 -
may be a case where the molten metal solidifies and
crystallization progresses. In particular, in the case
where the content of iron-group elements (Fe + Co + Ni) is
large, since there is an increase in cooling start
temperature due to an increase in melting point, film
boiling tends to occur at the beginning of cooling.
Therefore, it may be said that an ejection pressure of a
liquid of about 5 MPa to 20 MPa is not sufficient.
[0024]
Patent Literature 11 relates to powder for a permanent
magnet and states that, to make powder pulverized and
amorphous, water pressure is set to be 750 kgf/cm2 to 1200
kgf/cm2, water temperature is set to be 20 C or lower, and
the amount of water for 1 kg of iron is set to be 25 L
(liters) to 45 L. Although it is not indicated that film
boiling or a vapor film is eliminated under these
conditions, controlling an ejection pressure to be 60 MPa or
higher incurs costs for a high-pressure pump and high-
pressure pipework, which results in an increase in product
price. In addition, although the amount of water for 1 kg
of iron is set to be 25 L to 45 L, it may be said that this
amount is not sufficient for a soft magnetic material having
a high iron-group constituent content.
[0025]
As described above in Background Art, a water atomizing
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 14 -
method is advantageous from the viewpoint of productivity
and the adhesiveness of particles. In addition, when rapid
cooling is performed to manufacture an amorphous material,
performing rapid cooling with water after having performed
gas atomizing is advantageous for the manufacture of an
amorphous material as in the case of Patent Literature 1
through Patent Literature 6. In the case of a water
atomizing method, since molten metal separated by performing
atomizing is covered with vapor films due to cooling water
that is used for atomizing, it is necessary to take further
measures exemplified by those described in Patent Literature
7 through Patent Literature 11. In particular, in the case
of such measures, there is an insufficient effect for
manufacturing an amorphous soft magnetic material containing
iron-group elements in a total amount of 76 at% or more.
[0026]
The present invention has been completed to solve the
problems described above, and an object of the present
invention is to provide a method for manufacturing atomized
metal powder having a high amorphous material fraction by
using a water atomizing method.
Solution to Problem
[0027]
The present inventors diligently conducted
investigations to solve the problems described above and, as
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 15 -
a result, solved the problems by focusing on collision
pressure instead of ejection pressure when atomized metal
powder is obtained by ejecting high-pressure water onto
molten metal to separate and cool the molten metal and by
controlling the state of water on the collision surface
between the molten metal and the high-pressure water. More
specifically, the present invention provides the following.
[0028]
[1] A method for manufacturing atomized metal powder in
which atomized metal powder having an amorphous material
fraction of 90% or more is obtained, the method including
ejecting high-pressure water so as to collide with a molten
metal stream flowing vertically downward, separating the
molten metal stream into metal powder, and cooling the metal
powder,
in which the high-pressure water collides with the
molten metal with a collision pressure of 20 MPa or higher,
and
in which a temperature of the molten metal and/or a
temperature of the high-pressure water are controlled so
that the high-pressure water is in a subcritical state or a
supercritical state on a collision surface with the molten
metal.
[0029]
[2] The method for manufacturing atomized metal powder
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 16 -
according to item [1], in which an average temperature of
the molten metal and the high-pressure water is 374 C or
higher at a time of collision between the high-pressure
water and the molten metal.
[0030]
[3] The method for manufacturing atomized metal powder
according to item [1] or [2], in which, when a flow rate of
the molten metal stream per unit time is defined as Qm
(kg/min) and an ejection rate of the high-pressure water per
unit time is defined as Qaq (kg/min), a mass ratio (Qaq/Qm)
is 35 or more.
[0031]
[4] The method for manufacturing atomized metal powder
according to any one of items [1] to [3], in which the
atomized metal powder contains iron-group constituents (Fe,
Ni, and Co) in a total amount of 76.0 at% or more in terms
of atomic fraction and Cu in an amount of 0.1 at% or more
and 2.0 at% or less in terms of atomic fraction.
[0032]
[5] The method for manufacturing atomized metal powder
according to any one of items [1] to [3], in which the
atomized metal powder contains iron-group constituents (Fe,
Ni, and Co) in a total amount of more than 82.5 at% and less
than 86 at% in terms of atomic fraction, at least two
selected from Si, P, and B, and Cu and has an average
Date Recue/Date Received 2020-06-05
86628475
- 17 -
particle size of 5 m or more.
[6] The method for manufacturing atomized metal
powder according to any one of items [1] to [5], in which the
subcritical state is represented by a pressure of 0.5 MPa to 22
MPa and a water temperature of higher than 150 C and lower than
374 C, and in which the supercritical state is represented by a
pressure of 22 MPa or higher and a water temperature of 374 C
or higher.
Advantageous Effects of Invention
[0033]
According to the present invention, it is possible to
manufacture atomized metal powder having an amorphous material
fraction of 90% or more. With this, by performing an
appropriate heat treatment after having performed forming on
the atomized metal powder obtained by using the present
invention, nanosized crystals are precipitated. In particular,
in the case where such powder is made of a soft magnetic
material having a high iron-group constituent content
(containing iron-group constituents (Fe, Ni, and Co) in a total
amount of 76 at% or more in terms of atomic fraction), by
performing an appropriate heat treatment after having performed
forming on such powder, it is possible to achieve both low iron
loss and high magnetic flux density. In such a manner, the
present invention can preferably be used for manufacturing any
conventionally known amorphous
Date Recue/Received Date 2020-07-14
CA 03084963 2020-06-05
- 18 -
soft magnetic material.
[0034]
Nowadays, in addition, as described in, for example,
Materia Japan, Vol. 41, No. 6, p. 392, the Journal of
Applied Physics 105, 013922 (2009), Japanese Patent No.
4288687, Japanese Patent No. 4310480, Japanese Patent No.
4815014, International Publication No. W02010/084900,
Japanese Unexamined Patent Application Publication No. 2008-
231534, Japanese Unexamined Patent Application Publication
No. 2008-231533, and Japanese Patent No. 2710938, hetero-
amorphous materials and nanocrystalline materials having a
high magnetic flux density have been developed. The present
invention can very advantageously be used when such a soft
magnetic material having a high iron-group constituent
concentration is manufactured by using a water atomizing
method. In particular, in the case where the iron-group
constituent concentration is more than 82.5 at%, or further,
more than 83.5 at%, it was difficult to increase an
amorphous material fraction by using conventional
techniques. However, by using the manufacturing method
according to the present invention, it is possible to
increase the amorphous material fraction to 90% or more
after atomizing has been performed. Moreover, it was very
difficult to control the amorphous material fraction to be
90% or more and an average particle size to be 5 m or more
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 19 -
by using conventional techniques. However, by using the
manufacturing method according to the present invention, it
is possible to control the amorphous material fraction to be
90% or more, even in the case where the average particle
size is increased. Since it is possible to control the
amorphous material fraction to be 90% or more and the
average particle size to be 5 m or more, there is a
significant increase in saturated magnetic flux density (Bs)
by performing an appropriate heat treatment after having
performed forming on atomized metal powder.
[0035]
In addition, although the present invention can
preferably be used to manufacturing atomized metal powder
having a high iron-group constituent concentration as
described above, by using the present invention as a method
for manufacturing atomized metal powder other than that
having a high iron-group constituent concentration, there is
an advantage in that it is possible to stably obtain
amorphous powder having a high particle size more easily
than before.
[0036]
Here, the term "amorphous material fraction" denotes a
value obtained by removing contaminants which are different
from metal powder from the obtained metal powder (soft
magnetic iron powder), by performing X-ray diffractometry to
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 20 -
determine halo peaks from amorphous materials (non-
crystalline materials) and diffraction peaks from crystals,
and by performing a calculation by utilizing a WPPD method.
The term "WPPD method" here is an abbreviation of "whole-
powder-pattern decomposition method". The WPPD method is
described in detail in Hideo Toraya: Journal of the
Crystallographic Society of Japan, vol. 30 (1988), No. 4,
pp. 253 to 258.
Brief Description of Drawings
[0037]
[Fig. 1] Fig. 1 is a schematic diagram of an example of
a manufacturing apparatus which can be used in a method for
manufacturing atomized metal powder according to the present
invention.
[Fig. 2] Fig. 2 is a schematic diagram illustrating an
example of manufacturing equipment for implementing the
manufacturing method according to the present invention.
[Fig. 3] Fig. 3 is a diagram illustrating the
relationship between the pressure, temperature, and state of
water.
[Fig. 4] Fig. 4 is a graph illustrating the
relationship between an amorphous material fraction and a
collision pressure.
[Fig. 5] Fig. 5 is a schematic diagram illustrating a
measurement configuration for determining the collision
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 21 -
pressure of molten metal by using a collision pressure
sensor.
[Fig. 6] Fig. 6 is a diagram illustrating a B-H diagram
obtained by using a VSM.
Description of Embodiments
[0038]
Hereafter, an embodiment of the present invention will
be described. Here, the present invention is not limited to
the embodiment below.
[0039]
Fig. 1 schematically illustrates an example of a
manufacturing apparatus which can be used in a method for
manufacturing atomized metal powder according to the present
invention. In Fig. 1, after molten metal 3 has been charged
into a tundish 2, the molten metal 3 flows downward through
a molten metal-injecting nozzle 4 due to the weight of the
molten metal 3. In addition, cooling water 20
(corresponding to high-pressure water) fed into a nozzle
header 5 is ejected through cooling nozzles 6. The cooling
water 20 collides with the molten metal (molten metal stream
flowing downward) and, as a result, the molten metal is
atomized, that is, separated into metal powder 8.
[0040]
Fig. 2 schematically illustrates an example of
manufacturing equipment for implementing the manufacturing
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 22 -
method according to the present invention. In the
manufacturing equipment illustrated in Fig. 2, atomized
metal powder is manufactured by controlling the temperature
of cooling water in a cooling water tank 15 by using a
cooling water-temperature controller 16, by transporting the
cooling water, whose temperature has been controlled, to a
high-pressure pump 17 for atomizing cooling water, by
transporting the cooling water from the high-pressure pump
17 for atomizing cooling water through pipework 18 for
atomizing cooling water to an atomizing apparatus 14
(corresponding to the manufacturing apparatus in Fig. 1),
and by ejecting the high-pressure water, which collides with
the molten metal stream flowing vertically downward, from
this atomizing apparatus 14 to separate the molten metal
stream into metal powder and to cool the metal powder.
[0041]
First, the present invention is characterized by
controlling a collision pressure to be 20 MPa or higher when
the cooling water 20 collides with the molten metal and the
state of the water to be a subcritical state of water or a
supercritical state of water on a collision surface. The
expression "supercritical state of water" denotes a state
which is represented by a temperature of 374 C or higher and
a pressure of 22 MPa or higher. The expression "subcritical
state of water" denotes a high-temperature and high-pressure
Date Recue/Date Received 2020-06-05
86628475
- 23 -
state which is close to a critical point and which is
exemplified by, as illustrated in Fig. 3, a state which is
represented by a temperature of higher than 150 C and lower
than 374 C and a pressure of 0.1 MPa or higher and lower than
22 MPa, a state which is represented by a temperature of 374 C
or higher and a pressure of 2 MPa or higher and lower than 22
MPa, and a state which is represented by a temperature of 250 C
or higher and lower than 374 C and a pressure of 22 MPa or
higher.
[0042]
In the manufacturing method according to the present
invention, the collision pressure of the cooling water 20 at
the time of collision with the molten metal is set to be 20 MPa
or more. The collision pressure is determined by using a
pressure sensor having a collision surface sensor whose
diameter is 2 mm when atomizing is not performed. To control
the collision pressure to be 20 MPa or more, it is necessary
that the ejection pressure of the cooling water 20 be more than
the collision pressure. To control the collision pressure so
that the maximum ejection pressure is 98 MPa, it is preferable
that the pressure control be performed by using an inverter
high-pressure pump. In addition, since there is a decrease in
ejection pressure in the case where the cooling water 20 is
spread out in a fan-like form, it is preferable that solid
stream-type nozzles
Date Recue/Received Date 2020-07-14
CA 03084963 2020-06-05
- 24 -
be used. In addition, since there is a decrease in ejection
pressure in the case where the distance between the cooling
nozzles 6 and the molten metal is increased, it is
preferable that the linear distance between the ejection
ports of the cooling nozzles 6 for the cooling water 20 and
the molten metal be 150 mm or less or more preferably 100 mm
or less.
[0043]
In addition, in the present invention, the temperature
of the molten metal and/or the temperature of the cooling
water are controlled so that the cooling water 20 is in a
subcritical state or a supercritical state on a collision
surface with the molten metal. It is possible to control
the temperature of the molten metal by controlling the
heating temperature of a melting furnace through high-
frequency output. In addition, by holding the molten metal
3 in the melting furnace after heating has been performed,
it is possible to control the temperature of the molten
metal 3 which is fed into the tundish 2.
[0044]
In the manufacturing method according to the present
invention, the temperature of the water on the collision
surface is defined as the average temperature of the molten
metal and the cooling water 20 (((molten metal temperature)
+ (cooling water temperature))/2). It is possible to
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 25 -
determine the molten metal temperature by using a non-
contact thermometer at an atomizing point. It is possible
to determine the temperature of the cooling water by using a
thermometer (not illustrated) for determining the water
temperature in the cooling water tank 15 illustrated in Fig.
2. In addition, in accordance with the relationship between
the pressure, the temperature, and the state of water
illustrated in Fig. 3, the collision pressure, the
temperature of the molten metal, and the temperature of the
cooling water 20 are controlled to achieve the average
temperature and the collision pressure with which the
cooling water is in a subcritical state or a supercritical
state. Here, since the temperatures of the molten metal and
the cooling water tend to fluctuate, the molten metal
temperature may be determined within the margin of error of
plus or minus 50 C, and the cooling water temperature may be
determined within the margin of error of plus or minus 5 C.
[0045]
Hereafter, the effects of the present invention will be
described.
[0046]
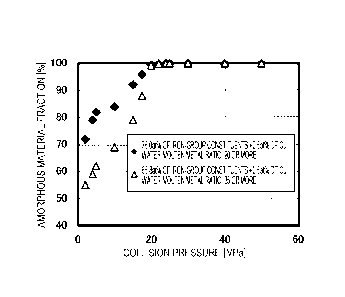
Fig. 4 is a graph illustrating the relationship between
an amorphous material fraction and a collision pressure.
The graph in Fig. 4 relates to a case where atomized metal
powder containing iron-group constituents (Fe, Ni, and Co)
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 26 -
in a total amount of 76.0 at% in terms of atomic fraction
(water-molten metal ratio(mass ratio: Qaq/Qm): 20) and Cu in
an amount of 0.5 at% is manufactured and a case where
atomized metal powder containing iron-group constituents
(Fe, Ni, and Co) in a total amount of 85.8 at% in terms of
atomic fraction (water-molten metal ratio: 35) and Cu in an
amount of 0.5 at% is manufactured. In addition, in the
graph in Fig. 4, in the case of a collision pressure of 20
MPa, the state of water was controlled to be a subcritical
state on the collision surface between the cooling water and
the molten metal. In the case of a collision pressure of 22
MPa or higher, that is, in the case of a collision pressure
of higher than 20 MPa, the state of water was controlled to
be a supercritical state on the collision surface between
the cooling water and the molten metal. In addition, in the
case of a collision pressure of lower than 20 MPa, the state
of water was controlled not to be either a subcritical state
or a supercritical state on the collision surface between
the cooling water and the molten metal.
[0047]
As indicated in Fig. 4, in the case where the collision
pressure is 20 MPa or higher, it is possible to achieve an
amorphous material fraction of 90% or more regardless of a
variation in the chemical composition of obtained atomized
metal powder, a variation in water-molten metal ratio, or
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 27 -
whether the state of the water is a subcritical state or a
supercritical state on a collision surface.
[0048]
In addition, when the manufacturing method according to
the present invention is implemented, it is preferable that
the average temperature of the molten metal and the cooling
water be 374 C or higher at the time of collision between
the cooling water (high-pressure water) and the molten
metal. By controlling the average temperature described
above to be 374 C or higher, there is an advantage in that
the state of water is brought close to a critical state and
that there is an increase in vapor density.
[0049]
When the flow rate of the molten metal stream per unit
time is defined as Qm (kg/min) and the ejection rate of the
cooling water (high-pressure water) per unit time is defined
as Qaq (kg/min), it is preferable that a mass ratio (Qaq/Qm)
be 35 or more. This is because, since there is a tendency
for an amorphous material fraction to increase in the case
where such a mass ratio is large, and since it is easy to
control the mass ratio in the case where the mass ratio is
35 or more, it is possible to achieve a sufficiently high
level of effect.
[0050]
The manufacturing method according to the present
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 28 -
invention can preferably be used for manufacturing atomized
metal powder containing iron-group constituents (Fe, Ni, and
Co) in a total amount of 76 at% or more in terms of atomic
fraction and Cu in an amount of 0.1 at% or more and 2 at% or
less in terms of atomic fraction. In the case where the
content of iron-group elements (Fe + Co + Ni) is large,
since there is an increase in cooling start temperature due
to an increase in melting point, film boiling tends to occur
at the beginning of cooling, which makes it difficult to
increase an amorphous material fraction to 90% or more by
using conventional methods. According to the present
invention, it is possible to increase an amorphous material
fraction, even in the case where the content of iron-group
elements (Fe + Co + Ni) is large. By using the
manufacturing method according to the present invention,
since it is possible to increase an amorphous material
fraction while increasing the content of iron-group elements
(Fe + Co + Ni), it is possible to increase magnetic flux
density. As a result, the manufacturing method according to
the present invention contributes to reducing the size of a
motor and to increasing motor power.
[0051]
Here, by controlling the chemical composition of the
molten metal to be within the range described above, the
chemical composition of the atomized metal powder is also
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 29 -
within the range described above.
[0052]
The manufacturing method according to the present
invention can preferably be used for manufacturing atomized
metal powder containing iron-group constituents (Fe, Ni, and
Co) in a total amount of more than 82.5 at% and less than
86.0 at% in terms of atomic fraction, at least two selected
from Si, P, and B, and Cu and having an average particle
size of 5 m or more. In the case where conventional
techniques are used for manufacturing atomized metal powder
containing iron-group constituents in significantly large
amounts, specifically, containing iron-group constituents
(Fe, Ni, and Co) in a total amount of more than 82.5 at% and
less than 86 at% in terms of atomic fraction, when an
average particle size is small, since it is easy to cool the
particles, it is possible to achieve an amorphous material
fraction larger than that achieved when the average particle
size is large. However, when the average particle size is 5
m or more, it is very difficult to increase the amorphous
material fraction to 90% or more. According to the present
invention, even when the average particle size is 5 m or
more, it is possible to increase the amorphous material
fraction to 90% or more. In addition, the upper limit of
the average grain diameter with which it is possible to
increase the amorphous material fraction to 90% or more by
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 30 -
using the present invention is 75 m as a rough guide.
Here, the particle size is determined by performing
classification utilizing a sieve method, and the average
particle size (D50) is calculated by using an integration
method. In addition, a laser diffraction/scattering
particle size distribution analyzer may also be used.
EXAMPLES
[0053]
Examples and comparative examples were implemented by
using the manufacturing equipment illustrated in Fig. 2 in
which the apparatus for manufacturing water-atomized metal
powder illustrated in Fig. 1 was installed.
[0054]
Molten metal 3, which has been prepared by melting a
raw material at a predetermined temperature by using a high-
frequency melting furnace or the like, is fed into a tundish
2. A molten metal-injecting nozzle 4 having a predetermined
molten metal-injecting nozzle diameter has been set in the
tundish 2 in advance. When the molten metal 3 is fed into
the tundish 2, the molten metal is extruded through the
molten metal-injecting nozzle 4 due to free drop or back
pressure and flows downward. Cooling water, which is
ejected through cooling water nozzles 6 with a predetermined
water pressure by using a high-pressure pump 17 for
atomizing cooling water, collides with the molten metal, so
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 31 -
that the molten metal is separated, pulverized, and cooled.
There may be a case where the cooling water has been stored
in a cooling water tank 15 in advance to control the water
temperature by using a cooling water-temperature controller
16 as needed. As the cooling water ejecting nozzles, solid
stream-type nozzles were used. A dozen cooling water
nozzles were arranged around the molten metal flowing
downward so as to make an angle of 30 with respect to the
vertical direction. Here, it is possible to realize the
effects of the present invention, even in the case where the
nozzles are arranged so as to make an angle of 50 to 600
with respect to the vertical direction. Before atomizing is
started, the collision pressure of the molten metal is
determined by using a collision pressure sensor 51 (refer to
Fig. 5). The collision pressure sensor 51 is arranged in a
direction perpendicular to the nozzle ejection direction to
confirm whether a predetermined collision pressure is
achieved. Here, although Fig. 5 illustrates not only a
configuration in which the cooling water is ejected onto the
molten metal but also a configuration in which the cooling
water is ejected onto the collision pressure sensor 51, this
is only for the purpose of description, and the collision
pressure is determined by using the collision pressure
sensor 51 before the molten metal is allowed to flow down.
Iron powder manufactured from the molten metal is collected
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 32 -
by using a hopper, dried, classified, and subjected to
evaluation regarding an amorphous material fraction. In the
case of an amorphous material fraction of 90% or more is
judged as satisfactory.
[0055]
When the manufacturing methods of the examples and the
comparative examples were implemented, soft magnetic
materials having the following chemical compositions were
prepared. "%" means "at%". (i) through (v) are Fe-based
soft magnetic row materials. (vi) is an Fe-Co-based soft
magnetic material. (vii) is an Fe-Co-Ni-based soft magnetic
material.
(i) Fe76%-Si9%-B10%-P5%
(ii) Fe78%-S19%-B9%-P4%
(iii) Fe80%-Si8%-B8%-P4%
(iv) Fe82.8%-B11%-P5%-Cu1.2%
(v) Fe84.8%-Si4%-B10%-Cu1.2%
(vi) Fe69.8%-Co15%-B10%-P4%-Cu1.2%
(vii) Fe69.8%-Ni1.2%-Co15%-B9.4%-P3.4%-Cu1.2%
Although (i) through (vii) were prepared so that each
of the materials had a corresponding one of the target
chemical compositions, in actual chemical compositions,
after having performed melting and atomizing, there were
errors within the margin of about plus or minus 0.3 at% or
impurities were contained in some cases. In addition, in
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 33 -
some cases, there was some variation in chemical composition
due to, for example, oxidation occurring in a melting
process or an atomizing process or after an atomizing
process.
[0056]
Examples 1 through 4 and comparative examples 1 through
3 were implemented under the conditions given in Table 1.
The average particle size and the amorphous material
fraction were evaluated by using the method described above.
From the results of the examples and the comparative
examples, it was clarified that an amorphous material
fraction of 90% or more was achieved in the case of all the
examples, which were within the range of the present
invention. In the case of the comparative examples, an
amorphous material fraction of 90% or more was not achieved.
[0057]
The atomized metal powder of examples 1 through 4 were
subjected to an appropriate heat treatment after having been
subjected to forming. With this, nanosized crystals were
precipitated. In addition, it was clarified that both low
iron loss and high magnetic flux density were achieved.
Specifically, such results were clarified by using the
following method.
[0058]
The sizes of the nanosized crystals were derived by
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 34 -
using the Scherrer equation after having performed
determination utilizing an XRD (X-ray diffractometer). In
the Scherrer equation, K denotes a shape factor (usually
assigned a value of 0.9), p denotes a full-width at half
maximum (expressed in units of radian), 0 is expressed by
the equation 20 = 52.505 (Fe110-plane), and T denotes a
crystal size.
T = KX/Pc050 (Scherrer equation)
In addition, the magnetic properties of the obtained
powder were investigated by using a VSM (vibrating sample
magnetometer), and, from the B-H diagram (Fig. 6) obtained
by using the VSM, the saturated magnetic flux density was
determined from point C (point F), the retaining force was
determined from point E, the magnetic permeability was
determined from the maximum slope of B, and the iron loss
was determined from the hysteresis area (C-D-F-G). Here,
the diagram in Fig. 6 is opened to the public by Japan
Science and Technology Agency (JST), which is one of the
National Research and Development Agencies, (URL:
https://www.jst.go.jp/pr/report/report27/grf2.html, as
searched on 16th November 2017)
[0059]
Date Recue/Date Received 2020-06-05
- 35 -
[Table 1]
Molten Cooling Water- Temperature Ejection
Iron-group
Atomizing
Example/ Metal Flow Water Molten of High- Pressure of Collision
Average Constituent Average Amorphous
Judgement
Start State of
Chemical Composition Particle Material
Comparative (Downward) Flow Metal
pressure High-pressure Pressure Temperature (A ratio of 90%
Temperature Water
[at%] Fe+Ni+Co Size Fraction
Example Rate Rate Ratio Water Water [MPa] [ C]
[ C]
[at%] [Jim] [%] or more is
[kg/min] [kg/min] [-]
[ C] [MPa] satisfactory.)
Example 1 15 300 20 10 90 20 1200 605
Subcritical (i) Fe76Si91310P5 76.0 32 93 Satisfactory
90 605 Subcritical (i) Fe76Si91310P5
76.0 33 99 Satisfactory
Example 2 15 420 28 10 90 20 1200 605
Subcritical (ii) Fe78Si9B9P4 78.0 33 97 Satisfactory
10 90 605 Subcritical
(iii) Fe8oSi8B8P4 80.0 35 94 Satisfactory
10 100 605 Supercritical
(i) Fe76Si91310P5 76.0 34 100 Satisfactory
10 100 605 Supercritical
(ii) Fe78Si9B9P4 78.0 29 99 Satisfactory
10 100 605 Supercritical
(iii) Fe8oSi8B8P4 80.0 29 97 Satisfactory
Example 3 12 420 35 10 100 23 1200 605
Supercritical (iv) Fe828B11P5Cu1 2 82.8 35 95
Satisfactory
P
10 100 605 Supercritical
(v) Fe848Si4B1oCu1 2 84.8 36 94 Satisfactory .
,..
10 100 605 Supercritical
(vi) Fess 8C015B1ORICUl 2 84.8 32 93 Satisfactory 0
.3
10 100 605 Supercritical
(vii) Fe698Ni1 2Col5B9 4P3 4CUl 2 86.0 35 92 Satisfactory g
,..
10 55 605 Vapor (i)
Fe76Si91310P5 76.0 43 83 Unsatisfactory 1,;
r.,
10 55 605 Vapor (ii)
Fe78Si9B9P4 78.0 44 62 Unsatisfactory ?
.
10 55 605 Vapor
(iii) Fe8oSi8B8P4 80.0 43 45 Unsatisfactory ?
Comparative
0
12 120 8 10 55 12 1200 605 Vapor (iv)
Fe82 8B11P5CU1 2 82.8 44 40 Unsatisfactory '
Example 1
10 55 605 Vapor (v)
Fe84 8Si4B1OCUl 2 84.8 45 38 Unsatisfactory
10 55 605 Vapor (vi)
Fe69 8C015B1ORICU1 2 84.8 42 35 Unsatisfactory
10 55 605 Vapor
(vii) Fe698Ni1 2Col5B94P3 4CUl 2 86.0 39 32 Unsatisfactory
10 60 605 Vapor (i)
Fe76Si91310P5 76.0 44 88 Unsatisfactory
10 60 605 Vapor (ii)
Fe78Si9B9P4 78.0 42 73 Unsatisfactory
10 60 605 Vapor
(iii) Fe8oSi8B8P4 80.0 39 63 Unsatisfactory
Comparative
12 420 35 10 60 15 1200 605 Vapor (vi)
Fe82 8B11P5CU1 2 82.8 43 53 Unsatisfactory
Example 2
10 60 605 Vapor (v)
Fe848Si4B1OCU1 2 84.8 46 52 Unsatisfactory
10 60 605 Vapor (vi)
Fe698C015B1ORICUl 2 84.8 43 46 Unsatisfactory
10 60 605 Vapor
(vii) Fe69 8Ni1 2C015139 4P3 4CU1 2 86.0 43 44
Unsatisfactory
Comparative 10
350 35 10 15 5 1200 605 Vapor (i) Fe76Si91310P5
76.0 38 5 Unsatisfactory
Example 3
Example 4 12 480 40 10 90 20 1200 605
Subcritical (i) Fe76Si91310P5 76.0 33 100 Satisfactory
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 36 -
In Table 1, the term "Atomizing Start Temperature"
denotes the temperature of the molten metal at the atomizing
point. The temperature of the molten metal at the atomizing
point was determined by using a non-contact thermometer.
[0060]
In Table 1, the term "Average Temperature" denotes a
value obtained by using the formula ((molten metal
temperature) + (cooling water temperature))/2. The molten
metal temperature at the atomizing point was determined by
using a non-contact thermometer at an atomizing point, and
the cooling water temperature was defined as the temperature
of water in the cooling water tank which was determined by
using a thermometer.
[0061]
In Table 1, the term "Water-Molten Metal Ratio" denotes
the mass ratio Qaq/Qm.
Reference Signs List
[0062]
2 tundish
3 molten metal
4 molten metal-injecting nozzle
nozzle header
6 cooling nozzle
8 metal powder
14 atomizing apparatus
Date Recue/Date Received 2020-06-05
CA 03084963 2020-06-05
- 37 -
15 cooling water tank
16 cooling water-temperature controller
17 high-pressure pump for atomizing cooling water
18 pipework for atomizing cooling water
20 cooling water
51 collision pressure sensor
Date Recue/Date Received 2020-06-05