Note: Descriptions are shown in the official language in which they were submitted.
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
LIQUID DOSAGE FORM FOR TOPICAL APPLICATION
CROSS-REFERENCE TO RELATED APPLICATION
This application claims the benefit of priority from U.S. Provisional
Application Serial
No. 62/612,853, filed January 2, 2018, the contents of which are incorporated
herein by
reference.
FIELD OF THE INVENTION
The present invention relates to novel topical compositions comprising at
least one
penetration enhancer that promotes permeation of pharmaceutically active agent
through nail
or skin.
BACKGROUND OF THE INVENTION
Onychomycosis is a fungal infection of nail that affects 10% of the general
population.
The most common cause of Onychomycosis is dermatophyte, which probably account
for more
than 85% of all cases. Onychomycosis is difficult to treat and has a high
recurrence rate.
Treatment for onychomycosis is commercially available in oral or topical
dosage form, which
involves terbinafine (Lamisin, itraconazole (Sopranoe), ciclopirox (Penlae),
and
amorolfine (Locery1 ). Lamisil , marketed by Novartis, is very effective
against
onychomycosis administrated systemically, however, oral administration is
associated with
side effects such as liver toxicity.
Topical therapy is an alternative to oral therapy to diminish the side
effects. Topical
application of medications on nails allows active agents to penetrate through
nail plate, reach
nail bed, and eventually enter the systemic circulation bypassing the first-
pass metabolism.
Commercially available nail lacquer like Penlac and Loceryl have been
developed for nail
fungal infection, but the treatment times are relatively long and cure rate
are relatively low
either compared with oral administration. Probably, the poor drug penetration
is the bottleneck
of these products. The 1% Lamisil cream is for treating skin fungal infection
instead,
terbinafine, being an effective antifungal agent, is not available in topical
dosage form yet. The
unmet medical need urges the development of effective topical therapies for
nail fungal
infection.
1
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
The nail comprises the nail plate, the nail matrix, and the nail bed. The nail
plate is the
hard, thin, translucent, slight elastic structure sitting on the nail bed,
composed of keratin. The
nail plate is made of dead keratinized cells which is formed by the cell
division of nail matrix.
The penetration of the drug through the nail plate, where the medication
applies on, is the key
determining the effectiveness of the topical therapy. The keratin binding
affinity of drugs
correlate to its penetration through the keratin matrix in nail plate. Low
keratin binding
efficiency contributes to increased permeability, which is a desirable
physiochemical property
of drugs as higher proportion of administrated drug can be targeted to the
lower nail layer.
Many antifungal drugs are reported to bind strongly to keratin and thus
developing
formulations that dwindle the interactions between the drugs and keratin is
necessary.
SUMMARY OF THE INVENTION
The present invention relates to novel liquid topical compositions comprising
at least one
penetration enhancer that promotes permeation of pharmaceutically active agent
through nail
or skin. In particular, the invention relates to pharmaceutical formulations
of but not limited to
terbinafine for treating onychomycosis.
BRIEF DESCRIPTION OF THE DRAWINGS
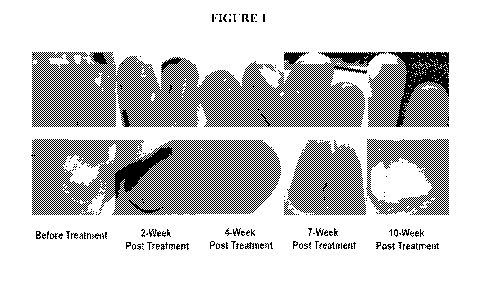
Figure 1 shows a human case study of the Terbinafine Lacquer formulation 11 on
treating
fingernail ony chomy co s is .
Figure 2 shows the cumulative amount of minoxidil penetrated-time curve.
DETAILED DESCRIPTION OF THE INVENTION
The detailed description is merely exemplary in nature and is not intended to
limit
application and uses. The following examples further illustrate the present
invention without,
however, limiting the scope of the invention thereto. Various changes and
modifications can
be made by those skilled in the art on the basis of the description of the
invention, and such
changes and modifications are also included in the present invention.
In one embodiment, the present invention provides a liquid topical composition
comprising a matrix comprising an effective amount of a pharmaceutically
active agent and
one or more penetration enhancer having a combined hydrophilic- lipophilic
balance of about
2
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
1 to about 16, wherein the matrix is a liquid such as solution, suspension,
emulsion and lacquer.
In other embodiment of the invention, the pharmaceutically active agent is
such as
antifungal agents, hair growth promoting agents, anesthetic agents,
nonnarcotic analgesics
such as the nonsteroidal anti-inflammatory agent (NSAIDS), erectile
dysfunction agents,
female sexual dysfunction agents, antihistamine and anti-cold agents, cough
suppressant
agents, respiratory disorder agents, antiemetic agents, oral hygiene agents,
antagonists of
Calcitonin Gene Related Peptide (CGRP) receptors, drugs for hormone
replacement,
Alzheimer's disease agent, caffeine and caffeine salt compounds and
corticosteroid. In
particular embodiment of the invention, pharmaceutically active agent is such
as terbinafine
hydrochloride, efinaconzole, minoxidil, sildenafil, tadalafil, vardenafil,
cetirizine, donepezil,
galantamine, rivastigmine, tacrine, and memantine.
In another embodiment of the invention, the liquid topical composition further
comprises
enhancers. Enhancers are selected from among PEG-8 beeswax, PEG-75 stearate,
pegoxo1-7
stearate, propylene glycol monocaprylate, propylene glycol monolaurate,
propylene glycol
monostearate, propylene glycol dioleate, 2-hydroxypropyl stearate, 2-
hydroxypropyl laurate,
propylene glycol oleate, propylene glycol distearate, propylene glycol
dicaprylate, propylene
glycol dilaurate, polypropylene glycol (17) dioleate, propyleneglycol
monolaurate, propylene
glycol monomyristate, dipropylene glycol dipelargonate, polypropylene glycol
monobutyl
ether oleate, propylene glycol dipelargonate, propylene glycol didecanoate,
dipropylene glycol
dipelargonate, propylene glycol bis(9,10-epoxystearate), propylene glycol
monoisostearate,
propylene glycol diundecanoate, glycol monoethyl ether, diethylene glycol
monobutyl ether,
oleoyl macrogolglycerides, lauroyl macrogolycerides, stearoyl
macrogolgylycerides,
caprylocaproyl macrogolglycerides, triglycerides medium-chain, polyglycery1-3
diisostearate,
polyglyceryl oleate, ethylene glycol paimitostearate, dissopropyl adipate, di-
n-butyl adipate,
dimethyl adipate, dimethyl isosorbide and diethylene glycol monoethyl ether.
In a particular
embodiment, liquid topical composition of the invention contains one or more
penetration
enhancers in an amount of from about 0.1% by weight to about 20%, about 0.1%
by weight to
about 15%, or about 1% by weight to about 10% by weight of the liquid dosage
form.
The inventive liquid topical composition further comprises a secondary
absorption
enhancer which is selected from among glycerol and terpenes.
In a still further embodiment, the inventive liquid topical composition
comprises a
3
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
surfactant, a humectant, an emulsifier, a solubilizing agent, a solvent, a
base polymer, a diluent,
an antioxidant, a preservative and optionally a film forming agent.
The invention also provides a method of drug delivery applying the topical
composition
of the invention topically on the skin of a subject in need thereof, wherein
at least a fraction of
1% to 80% or greater of the drug is delivered to and absorbed by the skin or
nail and absorbed
systemically.
Pharmaceutical Active Agent
The pharmaceutical active agent is such as antifungal agents, hair growth
promoting
agents, anesthetic agents, nonnarcotic analgesics such as NSAIDS, erectile
dysfunction agents,
female sexual dysfunction agents, antihistamine and anti-cold agents, cough
suppressant
agents, respiratory disorder agents, antiemetic agents, oral hygiene agents,
antagonists of
CGRP receptors, drugs for hormone replacement, Alzheimer's disease agent,
caffeine and
caffeine salt compounds and corticosteroid. For example, the antifungal agent
is terbinafine,
terbinafine hydrochloride, butenafine, butenafine hydrochloride, efinaconazole
or other
pharmaceutically acceptable salts thereof; the hair growth promoting agent is
minoxidil or its
pharmaceutically acceptable salts thereof; the anesthetic agent is lidocaine
(xylocaine),
procaine, benzocaine or its pharmaceutically acceptable salts thereof; the
nonnarcotic
analgesics such as NSAIDS is acetaminophen, Ibuprofen, ketoprofen,
indomethacin, aspirin
(low dose for cardiovascular), naproxen sodium, ketorolac, diclofenac,
meloxicam, piroxicam
or its pharmaceutically acceptable salts thereof; the erectile dysfunction
agent is sildenafil,
tadalafil, vardenafil or its pharmaceutically acceptable salts thereof; the
female sexual
dysfunction agent is sildenafil, tadalafil, vardenafil or its pharmaceutically
acceptable salts
thereof; the antihistamine and anti-cold agent is cetirizine hydrochloride,
loratadine,
chlorcyclizine hydrochloride, chlorpheniramine maleate, dextrochlorpheniramine
maleate,
dexbrompheniramine maleate, diphenhydramine citrate, diphenhydramine
hydrochloride,
doxylamine succinate, ketotifen fumarate, phenindamine tartrate, pheniramine,
pyrilamine
maleate, triprolidine hydrochloride, thonzylamine hydrochloride, clemastine
fumarate or other
pharmaceutically acceptable salts thereof; the cough suppressant agent is
menthol, camphor,
dextromethorphan hydrobromide, guaifenesin, codeine phosphate, codeine or its
pharmaceutically acceptable salts thereof; the respiratory disorder agent is
pseudoephedrine
4
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
hydrochloride, phenylephrine hydrochloride, guaifenesin, dextromethorphan
hydrobromide,
ephedrine or its pharmaceutically acceptable salts thereof; the antiemetic
agent is granisetron,
ondansetron, AZ-001 (Staccato prochlorperazine, proprietary product of
Alexza), AZ-004
(Staccato loxapine; proprietary product of Alexza), Levadex
(dihydroergotamine,
proprietary product of Allergan, Inc.), ZelrixTM (sumatriptan; proprietary
product of NuPathe
Inc.), VR-147 (proprietary product of Vectura), ROX-828 (ketorolac
tromethamine containing
6% lidocaine, proprietary product of ROXRO PHARIVIA, Inc.), COL-144
(lasmiditan,
proprietary product of Colucid Pharmaceuticals), BF-1(proprietary product of
Biofrontera),
diphenhydramine, scopolamine or other pharmaceutically acceptable salts
thereof; the drug for
hormone replacement is estradiol, testosterone or its pharmaceutically
acceptable salts thereof;
the Alzheimer's agent is donepezil, galantamine, rivastigmine, tacrine,
memantine or its
pharmaceutically acceptable salts thereof; and the corticosteroid is
triamcinolone acetonide or
its pharmaceutically acceptable salts thereof.
Enhancer
The present invention comprises one or more enhancers. The enhancer is
selected from
among PEG-8 beeswax, PEG-75 stearate, pegoxo1-7 stearate, propylene glycol
monocaprylate,
propylene glycol monolaurate, propylene glycol monostearate, propylene glycol
dioleate, 2-
hydroxypropyl stearate, 2-hydroxypropyl laurate, propylene glycol oleate,
propylene glycol
distearate, propylene glycol dicaprylate, propylene glycol dilaurate,
polypropylene glycol (17)
dioleate, propyleneglycol monolaurat
e, propylene glycol monomyristate, dipropylene glycol dipelargonate,
polypropylene glycol
monobutyl ether oleate, propylene glycol dipelargonate, propylene glycol
didecanoate,
dipropylene glycol dipelargonate, propylene glycol bis(9,10-epoxystearate),
propylene glycol
monoisostearate, propylene glycol diundecanoate, glycol monoethyl ether,
diethylene glycol
monobutyl ether, oleoyl macrogolglycerides, lauroyl macrogolycerides, stearoyl
macrogolgylycerides, caprylocaproyl macrogolglycerides, triglycerides medium-
chain,
polyglycery1-3 diisostearate, polyglyceryl oleate, ethylene glycol
paimitostearate, dissopropyl
adipate, di-n-butyl adipate, dimethyl adipate, dimethyl isosorbide and
diethylene glycol
monoethyl ether. The liquid topical composition further comprises a secondary
absorption
enhancer such as glycerol and terpenes. The liquid topical composition of the
invention further
5
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
contains one or more penetration enhancers in an amount of from about 0.1% by
weight to
about 20%, about 0.1% by weight to about 15%, or about 1% by weight to about
10% by
weight of the liquid dosage form.
Surfactant
The liquid topical composition further comprises the surfactant. The
surfactant is such as
anionic, cataionic, nonionic, amphoteric, saturated and unsaturated higher
aliphatic acid salts
including but not limited to sodium laurate, sodium stearate, sodium oleate,
and sodium
linolenate, long-chain alkyl sulfate salts, alkylbenzenesulfonic acids such as
hexylbenzenesulfonic acid, octylbenzenesulfonic acid dodecylbenzenesulfonic
acid and their
salts thereof, polyoxyalkylene alkyl ether sulfate salts, polyoxyalkylene
alkenyl ether sulfate
salts, the salts of polyoxyethylene alkyl sulfate esters, the salts of the
alkyl esters of
sulfosuccinic acid, polyoxyalkylene sulfosuccinate salts, the salts of the
alkyl esters of
polyoxyalkylene sulfosuccinic acid, the alkali metal salts of the
polyoxyalkylene-modified
dimethylpolysiloxane esters of sulfosuccinic acid, polyoxyalkylene alkylphenyl
ether sulfate
salts, long-chain alkanesulfonic acid salts, long-chain alkylsulfonates,
polyoxyethylene
alkylphenyl ether sulfate salts, polyoxyalkylene alkyl ether acetate salts,
long-chain alkyl
phosphate salts, polyoxyalkylene alkyl ether phosphate salts, acylglutamate
salts, alpha-
acylsulfonate salts, long-chain alkylsulfonate salts, alkylarylsulfonate
salts, long-chain alpha-
olefinsulfonate salts, alkylnaphthalenesulfonate salts, long-chain
alkanesulfonic acid salts,
long-chain alkyl or alkenyl sulfate salts, long-chain alkylamide sulfate
salts, long-chain alkyl
or alkenyl phosphate salts, alkylamide phosphate salts, alkyloylalkyltaurate
salts, N-acylamino
acid salts, sulfosuccinate salts, alkyl alkyl ether carboxylate salts, amide
ether carboxylate salts,
the salts of esters of alpha-sulfofatty acids, alanine derivatives, glycine
derivatives, and
arginine derivatives; salts can be exemplified by alkali-metal salts such as
the sodium salt and
potassium salt, alkanolamine salts such as the triethanolamine salt, and the
ammonium salt, the
sodium salt; alkyltrimethylammonium chloride, stearyltrimethylammonium
chloride,
lauryltrimethylammonium chloride, cetyltrimethylammonium chloride, beef tallow
alkyltrimethylammonium chloride, behenyltrimethylammonium
chloride,
octyltrimethylammonium hydroxide, dodecyltrimethylammonium hydroxide,
stearyltrimethylammonium bromide, behenyltrimethylammonium
bromide,
6
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
distearyldimethylammonium chloride,
dicocoyldimethylammonium chloride,
dioctyldimethylammonium chloride, di(POE)oleylmethylammonium (2E0) chloride,
benzalkonium chloride, alkylbenzalkonium chloride, alkyldimethylbenzalkonium
chloride,
benzethonium chloride, stearyldimethylbenzylammonium chloride, lanolin-derived
quaternary
ammonium salts, diethylaminoethylamide of stearic acid,
dimethylaminopropylamide of
stearic acid,
behenamidopropyldimethylhydroxypropylammonium chloride,
stearoylcolaminoformylmethylpyridinium chloride, cetylpyridinium chloride,
tall oil
alkylbenzylhydroxyethylimidazolinium chloride, and benzylammonium salts;
phospholipids,
such as lecithin, phosphatidylethanolamine, phosphatidic acid,
phosphatidylinositol,
phosphatidylserine, phosphatidylcholine, phosphatidylglycerol, sphingomyelin,
and
cardiolipin, and the hydrogenates of the preceding. Particularly preferred are
the hydrogenated
natural lecithins as yielded by the hydrogenation of, for example, soy
lecithin, egg yolk lecithin,
corn lecithin, cottonseed oil lecithin, rapeseed lecithin; polyoxyalkylene
ethers,
polyoxyalkylene alkyl ethers, polyoxyalkylene fatty acid esters,
polyoxyalkylene fatty acid
diesters, polyoxyalkylene resin acid esters, polyoxyalkylene (hardened) castor
oils,
polyoxyalkylene alkylphenols, polyoxyalkylene alkylphenyl ethers,
polyoxyalkylenephenyl
phenyl ethers, polyoxyalkylene alkyl esters, polyoxyalkylene alkyl esters,
sorbitan fatty acid
esters, polyoxyalkylene sorbitan alkyl esters, polyoxyalkylene sorbitan fatty
acid esters,
polyoxyalkylene sorbitol fatty acid esters, polyoxyalkylene glycerol fatty
acid esters,
polyglycerol alkyl ethers, polyglycerol fatty acid esters, sucrose fatty acid
esters, fatty acid
alkanolamides, alkyl glucosides, polyoxyalkylene fatty acid bisphenyl ethers,
polypropylene
glycol, polyether-modified silicones such as polyoxyalkylene-modified
diorganopolysiloxanes,
polyglycerol-modified silicones, glycerol-modified silicones, saccharide-
modified silicones,
perfluoropolyether-type surfactants, polyoxyethylene-polyoxypropylene block
copolymers,
alkyl polyoxyethylene-polyoxypropylene block copolymer ethers and mixtures
thereof.
Humectant
The liquid topical composition of the invention further comprises the
humectant. The
humectant is such as sorbitol, mineral oil, vegetable oil, glycerol, betaine,
guanidine, urea,
glycolic acid, glycolate salts, ammonium glycolate, quaternary alkyl ammonium
glycolate,
lactic acid, lactate salts, ammonium lactate, quaternary alkyl ammonium
lactate, aloe vera, aloe
7
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
vera gel, allantoin, urazole, alkoxylated glucose, hyaluronic acid, lactamide
monoethanolamine, acetamide monoethanolamine and derivatives, esters, salts
and mixtures
thereof, collagen, gelatin, aloe vera, hyaluronic acid or volatile water-
soluble solvents, such as
ethanol or propylene glycol.
Emulsifier
The liquid topical composition of the invention further comprises the
emulsifier. The
emulsifier is such as ethyl alcohol, isopropyl alcohol, ethyl carbonate, ethyl
acetate, benzyl
alcohol, benzyl benzoate, propylene glycol, 1,3-butylene glycol, oils such as
cottonseed,
groundnut, corn, germ, olive, castor and sesame oils, glycerol,
tetrahydrofuryl alcohol,
polyethylene glycols and fatty acid esters of sorbitan, and mixtures thereof;
carbomer,
hydroxypropyl cellulose, sodium lauryl sulfate; glycerin fatty acid esters
(monoglycerides,
MG); mono- and di-glycerides (MG & DG) such as Grindsted HV 4OTM, Poem
J2O21TM;
distilled monoglycerides; citric acid esters of MG (CMG); diacetyl tartaric
acid esters of mono-
and di-glycerides (DMEMs) such as Panodan AL 10Tm; polyglycerol esters of
fatty acids
(PGE); polyglycerol polyricinoleate (PGPR); sorbitan esters of fatty acids
suth as Palsgaard
7463TM; sucrose esters of fatty acids; calcium stearoyl lactylates; sodium
stearoyl lactylates;
lecithin including enzyme digested lecithin; caseinates such as sodium
caseinates including
Alanate 191TM; and diacetyl tartaric acid esters of mono- and di-glycerides
(DATEMs).
Solubilizing Agent
The liquid topical composition of the invention further comprises the
solubilizing agent.
The solubilizing agent is such as citric acid, ethylenediamine-tetraacetate,
sodium meta-
phosphate, succinic acid, urea, cyclodextrin, polyvinylpyrrolidone,
diethylammonium-ortho-
benzoate, and micelle-forming solubilizers such as TWEEN and spans such as
TWEEN 800;
polyoxyethylene sorbitan fatty acid ester, polyoxyethylene n-alkyl ethers, n-
alkyl amine n-
oxides, polyoxamers, organic solvents, such as acetone, phospholipids,
cyclodextrin, triacetin,
triethylcitrate, ethyl oleate, ethyl caprylate, sodium lauryl sulfate, sodium
doccusate, vitamin
E TPGS, dimethylacetamide, N-methylpyrrolidone, N-hydroxyethylpyrrolidone,
polyvinylpyrrolidone, hydroxypropylmethyl cellulose, hydroxypropyl
cyclodextrins, ethanol,
n-butanol, isopropyl alcohol, cholesterol, bile salts, polyethylene glycol 200
to 600, glycofurol,
8
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
transcutol, propylene glycol, and dimethyl isosorbide, miglyol, glycerin and
glycerol.
Solvent
The liquid topical composition of the invention further comprises the solvent.
The solvent
is such as methylene chloride, beta-cyclodextrin, dichloromethane; oily
excipients or solvents
are vegetable or animal oils, such as sunflower oil or cod liver oil; aqueous
or alcoholic
solutions such as water, ethanol, sugar solutions or mixtures thereof;
physiological saline
solution such as glycerol; alcohols such as methanol, ethanol, propanol,
isopropanol; sugar
solutions such as glucose or mannitol solutions or mixtures thereof; aromatic
hydrocarbon
solvents such as benzene, chlorobenzene, toluene and xylene; ether solvents
such as diethyl
ether, tert-butylmethyl ether, tetrahydrofuran, dimethoxyethane, dioxane and
THF; aliphatic
hydrocarbon solvents; ester solvents such as ethyl acetate; ketone solvents;
chlorinated
hydrocarbon solvents dichloromethane, chloroform, and 1,2-dichloroethane,
acetonitrile; and
an organic solvent such as 1,3 -dimethy1-2-imidazolidinone,
dimethylformamide, N-
dimethylacetamide, N-methylpyrrolidine, dimethylsulfoxide, pyridine,
nitromethane, and
mixtures thereof.
Base polymer
The liquid topical composition of the invention further comprises the base
polymer. The
base polymer is such as polysaccharide-based polymers, such as guar, xanthan
and/or their
derivatives; hydrophobic base polymers such as SIS (styrene/isoprene/styrene)-
triblock
copolymers, SBS (styrene/butadiene/styrene)-triblock copolymers, SBR
(copolymers of
styrene and butadiene), synthetic and/or natural polyisoprenes, polyamide,
polyester, co-
polyester, polyurethane and/or mixtures thereof are also possible as further
matrices; water-
soluble polymers, plant base polymers such as gum arabic, tragacanth gum,
galacian, guar gum,
carob gum, karaya gum, carragbeein, pectin, agar, quince seed (Marumero) algae
colloid
(seaweed extract), starch (rice, corn, potato, wheat), glycyrrhinic acid;
microorganism base
polymers such as xanthane gum, dextran, succinoglutan, pullulan; animal base
polymers such
as collagen, caseine, albumin, gelatin; starch base polymers such as
carboxymethyl starch,
methythydroxypropyl starch; cellulose base polymers such as methyl cellulose
nitro cellulose,
ethyl cellulose, methythydroxypropyl cellulose, hydroxyethyl cellulose, sodium
cellulose
9
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
sulfate, hydroxypropyl cellulose, sodium carboxymethyl cellulose (CMC),
crystalline
cellulose, cellulose powder; alginate base polymers such as sodium alginate,
alginate
propylene glycol esters; vinyl base polymers such as a polyvinyl alcohol,
polyvinylmethyl
ether, polyvinylpyrrolidone carboxyvinyl polymer (Carbopol), alkyl modified
carboxyvinyl
.. polymer, polyoxyethylene base polymers such as polyethylene glycol 2000,
4000, 6000; acryl
base polymers such as polyacrylates or salt thereof, polyoxyethylene
polyoxypropylene
copolymer brae polymer, sodium polyacrylate, polyethylene acrylate, polyacryl
amide,
polyethylene imine, and cationic polymer.
Diluent
The liquid topical composition of the invention further comprises the diluent.
The diluent
is such as water, saline, finger's solutions, dextrose solution; calcium
carbonate, sodium
carbonate, lactose, calcium phosphate or sodium phosphate; granulating and
disintegrating
agents such as corn starch or alginic acid; binding agents such as starch
gelatin, acacia,
microcrystalline cellulose or polyvinyl pyrrolidone; dicalcium phosphate,
calcium sulfate,
lactose, sorbitol, sucrose, inositol, cellulose, kaolin, mannitol, sodium
chloride, dry starch, and
powdered sugar.
Antioxidant
The liquid topical composition of the invention further comprises the
antioxidant. The
antioxidant is such as sodium bisulfite, butylated hydroxytoluene (BHT),
butylated
hydroxyanisole (BHA), propyl gallate, vitamin E, hydroquinone,
hydroxycoumarins,
ethanolamine, lecithin, cephalin, ascorbic acid, malic acid, sorbitol,
phosphoric acid, bisulfite,
sodium metabisulfite, thiodipropionic acid and its esters, and
dithiocarbamates.
Film Forming Agent
The liquid topical composition of the invention optionally comprises the film
forming
agent. The film forming agent is such as polyvinylpyrrolidone,
polyvinylpolypyrrolidone,
sodium alginate, carboxymethylcellulose, hydroxypropyl methylcellulose,
acrylate,
acrylamide and methacrylate.
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
Preservative
The liquid topical composition of the invention further comprises the
preservative. The
preservative is such as methyl hydroxy benzoate, propyl hydroxy benzoate,
chlorocresol,
benzoic acid and phenyl mercuric nitrate.
Method of Preparation
The liquid topical composition of the present invention is prepared using the
known
methods or by adopting specific conditions suitable for the ingredients
employed.
Examples of the liquid topical compositions are the amount of the ingredients
are listed
below, but not limited:
Terbinafine Lacquer Formulation
Ratio (w/w
Ingredients
%)
Terbinafine HC1 8.00-12.00
Polyvinylpyrrolidone K 30 0.40-0.60
Isopropyl Myristate 0.00-3.00
Propylene glycol 0.00-3.00
Polysorbate 80 0.00-3.00
Sorbitan Oleate 0.00-3.00
Diethylene Glycol Monoethyl Ether 0.00-6.00
Caprylocaproyl Polyoxylglycerides 0.00-6.00
Propylene Glycol Monolaurate 0.00-6.00
Polyglyceryl Dioleate 0.00-6.00
Medium-Chain Triglycerides 0.00-6.00
Diisopropyl Adipate 0.00-6.00
Benzyl Alcohol 0.40-0.60
Ethyl alcohol, anhydrous 74.00-91.00
11
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
Minoxidil Solution Formulation
Ratio (w/w
Ingredients
%)
Minoxidil 4.00-6.00
Ethanol, Anhydrous 20.00-35.00
Propylene Glycol 38.00-58.00
Caprylocaproyl Polyoxylglycerides 0.00-3.60
Diethylene Glycol Monoethyl
0.00-8.40
Ether
BHT 0.00-0.12
Water 12.00-18.00
EXAMPLES
Selected embodiments of the invention will be described in further detail with
reference
to the following experimental and comparative examples. These examples are for
illustrative
purposes only and are not intended to limit the scope of the invention.
EXAMPLE 1: TERBINAFINE LACQUER FORMULATION
Terbinafine Lacquers were prepared according to the components and amounts
shown
in Table 1.
Table 1
Example (% by weight)
Formulations
1A 1B 1C 1D 1E 1F 1G
Terbinafine HC1 10.00 10.00 10.00 10.00 10.00 10.00 ..
10.00
Polyvinyl-
0.50 0.50 0.50 0.50 0.50 0.50 0.50
pyrrolidone K 30
Isopropyl Myristate
Propylene glycol
Polysorbate 80 2.50 --
12
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
Sorbitan Oleate -- 2.50 -- -- -- -- --
Diethylene Glycol
-- -- 5.00 -- -- -- --
Monoethyl Ether
Caprylocaproyl
-- -- -- -- 5.00 -- --
Polyoxylglycerides
Propylene Glycol
-- -- -- 5.00 -- -- --
Monolaurate
Polyglyceryl
-- -- -- -- -- 5.00 --
Dioleate
Medium-Chain
-- -- -- -- -- -- 5.00
Triglycerides
Diisopropyl
Adipate
Benzyl Alcohol 0.50 0.50 0.50 0.50 0.50 0.50
0.50
Ethyl alcohol,
89.00 84.00 84.00 84.00 84.00 84.00 84.00
anhydrous
Table 1 (Continued)
Example (% by weight)
Formulations
1H 1! 1J 1K
Terbinafine HC1 10.00 10.00 10.00 10.00
Polyvinyl-pyrrolidone K 30 0.50 0.50 0.50 0.50
Isopropyl Myristate -- 2.50 2.50 2.00
Propylene glycol -- 2.50 -- 2.00
Polysorbate 80 -- -- -- --
Sorbitan Oleate -- -- -- --
Diethylene Glycol
Monoethyl Ether
Caprylocaproyl
-- -- 5.00 5.00
Polyoxylglycerides
13
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
Propylene Glycol
Monolaurate
Polyglyceryl Dioleate -- -- -- --
Medium-Chain
Triglycerides
Diisopropyl Adipate 5.00 -- -- --
Benzyl Alcohol 0.50 0.50 0.50 0.50
Ethyl alcohol, anhydrous 84.00 84.00 81.50 80.00
EXAMPLE 2: MINOXIDIL SOLUTION FORMULATION
Minoxidil solutions were prepared according to the components and amounts
shown in
Table 2.
Table 2
Ingredients 2A 2B 2C 2D
Minoxidil 5.00 5.00 5.00 5.00
Ethanol, Anhydrous 25.00 30.00 25.00 30.00
Propylene Glycol 45.00 50.00 45.00 50.00
Caprylocaproyl
3.00 -- 3.00 --
Polyoxylglycerides
Diethylene Glycol
7.00 -- 7.00 --
MonoethylEther
BHT -- -- -- --
Water 15.00 15.00 15.00 15.00
Total (%w/w) 100.00 100.00 100.00 100.00
Table 2 (Continued)
Ingredients 2E 2F 2G 2H
Minoxidil 5.00 5.00 5.00 5.00
Ethanol, Anhydrous 25.00 30.00 30.00 25.00
14
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
Propylene Glycol 45.00 50.00 50.00 45.00
Caprylocaproyl
3.00 3.00
Polyoxylglycerides
Diethylene Glycol
7.00 7.00
Monoethyl Ether
BHT 0.10 0.10
Water 14.90 14.90 15.00 15.00
Total (%w/w) 100.00 100.00 100.00 100.00
EXAMPLE 3: EFFECT OF PENETRATION ENHANCERS IN TRANSDERMAL
PENETRATION OF TERBINAFINE
A Franz diffusion cell system was used to assess the influence of penetration
enhancers
on the formulation of terbinafine lacquer for penetrating mouse skin. The test
drugs shown in
Table 1 were applied on mouse skin and allowed to diffuse for 20 hours. The
cumulative
amount of terbinafine penetrated at the end of 20th hour with regard to
various enhancers in
the formulations was shown in Table 3. The data in Table 3 showed that all the
enhancers
promoted the amount of penetrated terbinafine across mouse skin.
Table 3
Cumulative Amount of Terbinafine
Penetration Enhancers
Penetrated (ag/cm2)
1A 0
1B 343.46
1C 0.04
1D 1541.45
1E 0.44
1F 775.86
1G 449.13
1H 0.16
11 2904.86
CA 03085973 2020-06-16
WO 2019/135123 PCT/IB2018/059683
1J 1586.25
EXAMPLE 4: CASE STUDY: FINGERNAIL ONYCHOMYCOSIS TREATED WITH
THE INVENTION
A Chinese female onychomycosis patient was treated with the formulation 1K
once
daily for 10 weeks. Images in Figure 1 show that fungal infections of
fingernails were almost
completely cured after 10 weeks.
EXAMPLE 5: EFFECT OF PENETRATION ENHANCERS IN TRANSDERMAL
PENETRATION OF MINOXIDIL
A Franz diffusion cell system was used to assess the influence of penetration
enhancers
on the formulation of minoxidil solution for penetrating mouse skin. The test
drug containing
enhancers (formulation 2H) and no enhancer (formulation 2G) were applied on
mouse skin
and allowed to diffuse for 20 hours. The cumulative amount of minoxidil
penetrated-time
curve was shown in Figure 2. It is shown that enhancers promoted the
penetration rate of
.. minoxidil and it was increased up to around 3.5 times the maximum at 18th
hour.
16