Note: Descriptions are shown in the official language in which they were submitted.
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
PARTICLES FOR USE IN ACOUSTIC STANDING WAVE PROCESSES
BACKGROUND
[0001] The
present disclosure relates to particles in the micrometer or nanometer
range, which can be used with ultrasonically generated acoustic waves,
including
traveling and standing waves, to achieve trapping, concentration, and/or
transport of
the microparticles and nanoparticles to a target location.
[0002]
Acoustophoresis is the separation of materials using acoustics, such as
acoustic standing waves. Acoustic standing waves can exert forces on particles
in a
fluid when there is a differential in a parameter of the particles and fluid
that can be
influenced by acoustics, including density and/or compressibility, otherwise
known as
the acoustic contrast factor. The pressure profile in a standing wave contains
areas
of local minimum pressure amplitudes at standing wave nodes and local maxima
at
standing wave anti-nodes. Depending on their density and compressibility, the
particles can be trapped at the nodes or anti-nodes of the standing wave.
Generally,
the higher the frequency of the standing wave, the smaller the particles that
can be
trapped.
[0003] At a
micro scale, for example with structure dimensions on the order of
micrometers, conventional acoustophoresis systems tend to use half or quarter
wavelength acoustic chambers, which at frequencies of a few megahertz are
typically
less than a millimeter in thickness, and operate at very low flow rates (e.g.,
plimin).
Such systems are not scalable since they benefit from extremely low Reynolds
number, laminar flow operation, and minimal fluid dynamic optimization.
[0004] At the
macro-scale, planar acoustic standing waves have been used in
separation processes. However, a single planar wave tends to trap the
particles or
secondary fluid such that separation from the primary fluid is achieved by
turning off
or removing the planar standing wave. Planar waves also tend to heat the media
where
the waves are propagated due to the energy dissipation into the fluid that is
involved
with generating a planar wave and the planar wave energy itself. The removal
of the
planar standing wave may hinder continuous operation. Also, the amount of
power
that is used to generate the acoustic planar standing wave tends to heat the
primary
fluid through waste energy, which may be disadvantageous for the material
being
processed.
1
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
BRIEF DESCRIPTION
[0005] In
various embodiments, methods are disclosed herein for moving particles
within a host or primary fluid to a desired location using acoustic standing
waves. The
particles are placed within an acoustophoretic device, and an ultrasonic
transducer is
used to concentrate, trap, and/or move the particles as desired. The particles
can also
be used to interact or react with other particles or cells in the host or
primary fluid.
Sometimes, the structure of the particles can be changed upon exposure to the
acoustic wave.
[0006]
Disclosed herein in various embodiments are methods for concentrating
particles in a primary fluid at a first location, comprising: flowing a fluid
mixture
comprising the particles and the primary fluid through an acoustophoretic
device. The
acoustophoretic device comprises: an acoustic chamber through which the fluid
mixture flows; and an ultrasonic transducer including a piezoelectric material
that can
be driven to create an acoustic wave in the acoustic chamber. The ultrasonic
transducer is driven to create the acoustic wave, thus concentrating the
particles at
the nodes and antinodes of the standing wave, with negative contrast factor to
the
anti-nodes and positive contrast factor materials accumulating at the nodes.
[0007] The
acoustic wave can be a multi-dimensional acoustic standing wave, a
planar acoustic standing wave, a combination of a multi-dimensional acoustic
standing
wave and a planar acoustic standing wave, or an acoustic traveling wave.
[0008] In some
embodiments, the particles contain a payload. The payload can be
a virus, a nucleic acid, a cytokine, a pharmaceutical molecule, a liquid, a
gas, or
mixtures thereof. After moving the particles to the first location, the
payload can be
released.
[0009] The
particles can be microparticles or nanoparticles. The particles may be
solid, cellular, hollow, multilayer or a foam.
[0010] The
particles can be made of one or more polymeric materials, ionomers,
ceramics, or glass. Examples
of polymeric materials include polyethylene,
polypropylene, polystyrene, divinylbenzene, poly methyl methacrylate,
polysaccharide, polylactic acid (PLA), and poly(lactic-co-glycolic acid)
(PLGA).
[0011]
Particles may also be produced from agarose and polyhyaluronic acid.
These particles will dissolve in vivo, thus causing no deleterious issues with
the
patient.
2
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
[0012] The
particles can be formed from multiple layers of polymeric materials. In
some embodiments, the particles may be hollow, made of glass, and/or have an
ablative polymer coating an exterior surface of the glass. The ablative
polymer may
be a polysaccharide that is functionalized with an antigen, antibody, or
protein.
[0013] In other
embodiments, the particles comprise: a liquid core; and a lipid shell
encapsulating the liquid core. The liquid in the liquid core may comprise a
perfluorocarbon. The perfluorocarbon may be perfluoropentane, perfluorohexane,
perfluorooctane, perfluorooctyl bromide, perfluorodichlorooctane, or
perfluorodecalin.
[0014] The
lipid shell can be formed from dipalmitoylphosphatidylcholine (DPPC),
1 ,2-palmitoyl-phosphatidic acid (DPPA), a lipid-polyethylene glycol
conjugate, or a
complex of a lipid with albumin. The lipid shell can be functionalized with
streptavidin,
biotin, avidin, or an antibody.
[0015] Also
disclosed herein are particles, comprising: a liquid core; and a lipid
shell encapsulating the liquid core.
[0016] The
liquid in the liquid core may comprise a perfluorocarbon. The
perfluorocarbon may be perfluoropentane, perfluorohexane, perfluorooctane,
perfluorooctyl bromide, perfluorodichlorooctane, or perfluorodecalin. The
lipid shell
may be
formed from dipalmitoylphosphatidylcholine (DPPC), 1 ,2-palmitoyl-
phosphatidic acid (DPPA), a lipid-polyethylene glycol conjugate, or a complex
of a lipid
with albumin. The lipid shell can be functionalized with streptavidin, biotin,
avidin, or
an antibody.
[0017] A
process known as acoustic droplet vaporization (ADV) can be used to
generate a phase shift of the liquid core of such particles from liquid to gas
using an
acoustic wave. The vapor pressure of the liquid is a function of temperature,
and is
not necessarily based upon the liquid chemistry. Any liquid that has a normal
boiling
point near or below the body temperature can be used for these processes.
Fluorocarbons may be utilized in these processes because of their low toxicity
and
high contrast factor.
[0018] A spacer
may be placed in between the particle and the antigen, antibody,
or protein. The spacer is typically a polyethylene glycol (PEG) molecule that
allows for
less charged interference from the particle when materials are binding to the
functionalized molecule on the surface of the particle.
[0019] These
materials may also be utilized for the transduction of cells, for
example by sonoporation. The bubbles are acoustically cavitated near a cell
wall and
3
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
create oscillations that contribute to opening a passage in the cell wall.
Collapsing
bubbles via acoustically induced cavitation can produce jets of fluid that
contribute to
opening cell walls.
[0020] In another configuration, these bubbles can contain a therapeutic
agent.
Thus, when the bubbles are broken via the acoustic excitement, the jetting
material is
a therapeutic and enters the cell during this process. The therapeutic can be
a small
molecule, a large molecule, or a piece of genetic material utilized in
modifying the DNA
of the target cell.
[0021] More generally, the particles described herein can be used as an
agent to
cause a change to a second material when the particles are impinged upon by
acoustic
waves. For example, the particles may be used to increase the contrast factor
of the
second material, which is a factor in increasing acoustophoretic efficiency.
As another
example, liquids may be delivered by the particles to cause changes to cell
barriers in
operations such as sonoporation.
[0022] Also discussed herein are techniques and devices for generating
material
clusters that can be used to improve gravity or buoyancy separation and
collection
efficiency of the materials. Improved, continuous, acoustophoresis devices
using
improved fluid dynamics are also discussed, as well as control of the devices
for
desired performance. The materials can be preferentially trapped in or
released
from/through the acoustic wave, depending on various parameters and
characteristics
of the acoustic wave and/or materials, including, for example, the contrast
factor of the
material.
[0023] These and other non-limiting characteristics are more particularly
described
below.
BRIEF DESCRIPTION OF THE DRAWINGS
[0024] The following is a brief description of the drawings, which are
presented for
the purposes of illustrating the exemplary embodiments disclosed herein and
not for
the purposes of limiting the same.
[0025] FIG. 1 is a micrograph of particles in accordance with the present
disclosure.
[0026] FIG. 2A is a Scanning Electron Microscope (SEM) photograph of a
solid
particle.
[0027] FIG. 2B is an SEM photograph of a cellular particle.
[0028] FIG. 2C is a micrograph of a hollow particle.
4
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
[0029] FIG. 20
is an illustration of a particle having a solid core and an exterior
layer.
[0030] FIG. 2E
is an illustration of a hollow particle having material within the core,
and an exterior layer that can be ablated to release the material within the
core.
[0031] FIG. 2F
is an illustration of a hollow particle having a payload, and a shell
surrounding the payload.
[0032] FIG. 3
is a schematic illustration of a particle comprising a liquid core and a
lipid shell.
[0033] FIG. 4
is a schematic illustration of several particles being aligned / grouped
with each other.
[0034] FIG. 5A
is a graph showing number of particles versus particle diameter for
diameters of 0.6 microns to 1.25 microns, for initial droplets and droplets
after
incubation with NeutrAvidine. The y-axis is linear and runs from 0 to 3.0x108
at
intervals of 1.0x108. The x-axis is in microns, and runs from 0.6 to 1.2 at
intervals of
0.2.
[0035] FIG. 5B
is a graph showing number of particles versus particle diameter for
diameters of 1.25 microns to 2.25 microns, for initial droplets and droplets
after
incubation with NeutrAvidine. The y-axis is linear and runs from 0 to 8.0x108
at
intervals of 2.0x108. The x-axis is in microns, and runs from 1.4 to 2.2 at
intervals of
0.2.
[0036] FIG. 5B
is a graph illustrating droplet size distribution in accordance with the
present disclosure.
[0037] FIG. 6
illustrates a process for preparing particles that contain a payload,
and the subsequent release of that payload, in accordance with the present
disclosure.
[0038] FIG. 7
is a depiction of a traveling wave in accordance with the present
disclosure.
[0039] FIG. 8
is a depiction of a standing wave in accordance with the present
disclosure.
[0040] FIG. 9
is a front cross-sectional view of an acoustophoretic device in which
the methods of the present disclosure can be used.
[0041] FIG. 10
is an exterior perspective view of the acoustophoretic device of FIG.
9.
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
[0042] FIG. 11
is a cross-sectional diagram of an ultrasonic transducer of the
present disclosure. An air gap is present within the transducer, and no
backing layer
or wear plate are present.
[0043] FIG. 12
is a cross-sectional diagram of another ultrasonic transducer
suitable for use in the present disclosure. An air gap is present within the
transducer,
and a backing layer and wear plate are present.
DETAILED DESCRIPTION
[0044] The
present disclosure may be understood more readily by reference to the
following detailed description of desired embodiments and the examples
included
therein. In the following specification and the claims which follow, reference
will be
made to a number of terms which shall be defined to have the following
meanings.
[0045] Although
specific terms are used in the following description for the sake of
clarity, these terms are intended to refer only to the particular structure of
the
embodiments selected for illustration in the drawings, and are not intended to
define
or limit the scope of the disclosure. In the drawings and the following
description
below, it is to be understood that like numeric designations refer to
components of like
function.
[0046] The
singular forms "a," "an," and "the" include plural referents unless the
context clearly dictates otherwise.
[0047] As used
in the specification and in the claims, the term "comprising" may
include the embodiments "consisting of" and "consisting essentially of." The
terms
"comprise(s)," "include(s)," "having," "has," "can," "contain(s)," and
variants thereof, as
used herein, are intended to be open-ended transitional phrases, terms, or
words that
require the presence of the named ingredients/components/steps and permit the
presence of other ingredients/components/steps. However, such description
should
be construed as also describing compositions, articles, or processes as
"consisting of"
and "consisting essentially of" the enumerated ingredients/components/steps,
which
allows the presence of only the named ingredients/components/steps, along with
any
impurities that might result therefrom, and
excludes other
ingredients/components/steps.
[0048]
Numerical values in the specification and claims of this application should
be understood to include numerical values which are the same when reduced to
the
same number of significant figures and numerical values which differ from the
stated
6
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
value by less than the experimental error of conventional measurement
technique of
the type described in the present application to determine the value.
[0049] All
ranges disclosed herein are inclusive of the recited endpoint and
independently combinable (for example, the range of "from 2 grams to 10 grams"
is
inclusive of the endpoints, 2 grams and 10 grams, and all the intermediate
values).
[0050] The term
"about" can be used to include any numerical value that can vary
without changing the basic function of that value. When used with a range,
"about"
also discloses the range defined by the absolute values of the two endpoints,
e.g.
"about 2 to about 4" also discloses the range "from 2 to 4." The term "about"
may refer
to plus or minus 10% of the indicated number.
[0051] A
statement that a value exceeds (or is more than) a first threshold value is
equivalent to a statement that the value meets or exceeds a second threshold
value
that is slightly greater than the first threshold value, e.g., the second
threshold value
being one value higher than the first threshold value in the resolution of a
relevant
system. A statement that a value is less than (or is within) a first threshold
value is
equivalent to a statement that the value is less than or equal to a second
threshold
value that is slightly lower than the first threshold value, e.g., the second
threshold
value being one value lower than the first threshold value in the resolution
of the
relevant system.
[0052] It
should be noted that many of the terms used herein are relative terms.
For example, the terms "upper" and "lower" are relative to each other in
location, e.g.
an upper component is located at a higher elevation than a lower component in
a given
orientation, but these terms can change if the device is flipped. The terms
"inlet" and
"outlet" are relative to a fluid flowing through them with respect to a given
structure,
e.g. a fluid flows through the inlet into the structure and flows through the
outlet out of
the structure. The terms "upstream" and "downstream" are relative to the
direction in
which a fluid flows through various components, e.g. the flow fluids through
an
upstream component prior to flowing through the downstream component. It
should
be noted that in a loop, a first component can be described as being both
upstream of
and downstream of a second component.
[0053] The
terms "horizontal" and "vertical" are used to indicate direction relative
to an absolute reference, e.g. ground level. However, these terms should not
be
construed to require structures to be absolutely parallel or absolutely
perpendicular to
each other. For example, a first vertical structure and a second vertical
structure are
7
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
not necessarily parallel to each other. The terms "top" and "bottom" or "base"
are
used to refer to surfaces where the top is always higher than the bottom/base
relative
to an absolute reference, e.g. the surface of the earth. The terms "upwards"
and
"downwards" are also relative to an absolute reference; upwards is always
against the
gravity of the earth.
[0054] The
present application refers to "the same order of magnitude." Two
numbers are of the same order of magnitude if the quotient of the larger
number
divided by the smaller number is a value of at least 1 and less than 10.
[0055] The term
"virus" refers to an infectious agent that can only replicate inside
another living cell, and otherwise exists in the form of a virion formed from
a capsid
that surrounds and contains DNA or RNA, and in some cases a lipid envelope
surrounding the capsid.
[0056] The term
"crystal" refers to a single crystal or polycrystalline material that is
used as a piezoelectric material.
[0057] The
present disclosure refers to "microparticles." This term refers to
particles having an average particle diameter of 1 micrometer ( m) to 1000 m.
[0058] The
present disclosure refers to "nanoparticles." This term refers to particles
having an average particle diameter of 1 nanometer (nm) to less than 1000 nm.
[0059] Some of
the materials discussed herein are described as having an average
particle diameter. The average particle diameter is defined as the particle
diameter at
which a cumulative percentage of 50% (by volume) of the total number of
particles are
attained. In other words, 50% of the particles have a diameter above the
average
particle size, and 50% of the particles have a diameter below the average
particle size.
The size distribution of the particles may include a Gaussian distribution,
with upper
and lower quartiles at 25% and 75% of the stated average particle size, and
all
particles being less than 150% of the stated average particle size. Any other
type of
distribution may be provided or used. It is noted that the particles do not
have to be
spherical. For non-spherical particles, the particle diameter is the diameter
of a
spherical particle having the same volume as the non-spherical particle.
[0060]
Particles may be described herein as having a "core" and "shell" structure.
In such particles, the core will be made of a liquid or gas, and the shell
will be made
of one or more layers of a relatively solid material (relative to the core).
The shell and
the core can be distinguished by their phase of matter. The term "particle" is
meant to
refer to any type of individual structure that may be suspended in a fluid
such as a
8
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
liquid or gas and may be in any phase, e.g., solid, liquid or gas and
combinations
thereof.
[0061] "Organic" and "inorganic" materials are referred to herein. For
purposes of
the present disclosure, an "organic" material is made up of carbon atoms
(often with
other atoms), whereas an "inorganic" material does not contain carbon atoms.
[0062] The present disclosure may refer to temperatures for certain process
steps.
In the present disclosure, the temperature usually refers to the temperature
attained
by the material that is referenced, rather than the temperature at which the
heat source
(e.g. furnace, oven) is set. The term "room temperature" refers to a range of
from 68 F
(20 C) to 77 F (25 C).
[0063] The present disclosure relates to particles that are used in
conjunction with
acoustophoretic devices. The acoustophoretic device generates acoustic waves
that
can be used in various ways. For example, the acoustic waves can be used to
move
the particles to a desired location, or to change certain properties of the
particles, or
to enhance reaction of the particles with other particles (such as biological
cells). The
particles can be microparticles or nanoparticles, as desired. The particles
will first be
discussed herein, then the acoustophoretic devices themselves. Various methods
and
reactions that can be performed using the particles with the acoustophoretic
devices
will also be discussed.
[0064] Particles
[0065] As discussed above, the particles are generally microparticles or
nanoparticles. The particles may be spherical in shape, as shown in FIG. 1
with
reference numeral 100. However, their shape can vary. For example, the
particles
could be ellipsoidal or elongated along a longitudinal axis.
[0066] The particles may be, for example, solid, cellular, hollow, or a
foam. A solid
particle does not contain any voids or cavities, and a solid particle 200 is
illustrated in
FIG. 2A. A cellular particle contains voids / cavities in its interior, and
has passages
from the exterior of the particle to those voids / cavities (analogous to an
open-cell
foam). A cellular particle 204 is illustrated in FIG. 2B, with voids /
cavities 206 visible
from the exterior. A hollow particle is illustrated in FIG. 2C. The hollow
particle 210
has one or more large voids or cavities 212 within a solid exterior surface
214. A foam
contains multiple voids / cavities, each void being completely surrounded by
solid
material (also known as a closed-cell foam).
9
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
[0067] In
particular embodiments, the particles may be made of inorganic
materials, organic materials, or combinations thereof. Such materials may
include
polymers, ionomers, ceramics, glass, and other materials.
[0068] Polymers
that may be utilized for the manufacture of the particles discussed
herein include polyolefins such as polyethylene and polypropylene. The
polyethylene
may be a linear low density polyethylene, a high density polyethylene, a low
density
polyethylene, or an ultra-high molecular weight polyethylene. The polyethylene
or
polypropylene materials may be polymerized with a catalyst such as a peroxide
catalyst, a Ziegler¨Natta catalyst or a metallocene catalyst.
[0069] Other
polymers that may be utilized in manufacture of the particles include
polystyrene, divinyl benzene, polymethyl methacrylate (PMMA), polysaccharides
such
as agarose and agar, poly lactic acid (PLA), and poly(lactic-co-glycolic acid)
(PLGA).
[0070] These
polymers may be utilized to make up the bulk of the particles.
microparticles or nanoparticles. The polymers may also be utilized in various
combinations to make particles out of multiple layers (e.g. multilayer
particles).
Different polymers can be used to obtain the desired effect for the particles.
For
example, making particles out of multiple different layers can be used to
obtain both a
desired density and a desired acoustic contrast factor, or to obtain a desired
behavior
or interaction for the particle.
[0071] As one
example, a polystyrene bead may be created in an aqueous
suspension and then freeze-dried to obtain a foam particle. When the freeze-
dried
foam particle is suspended in water, small bubbles may form on its surface,
resulting
in a foam particle with a relatively solid core and nano-bubbles trapped in
cavities on
the surface of the foam particle.
[0072] As
another example, a polymethyl methacrylate core may be coated with a
PLA or PLGA polymer that forms an exterior surface for specialized drug
delivery or
interaction with biological cells. The resulting particle may be considered a
solid
particle or a foam particle (depending on the construction of the polymethyl
methacrylate core), and may have a negative or positive contrast factor
depending
upon the density of the composite particle and the speed of sound in the
composite
particle. This example is illustrated in FIG. 20. The particle 220 has a PMMA
core
222 with a PLA or PLGA coating 224.
[0073] As
another example, the exterior layer of the particle may be useful for
causing biological interaction / reaction of the particle. For example, the
exterior layer
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
may permit the particle to be used for affinity binding. As another example,
the particle
could be a hollow particle with an exterior layer that is made from an
ablative material
(e.g. a material that melts or dissolves). This structure would permit
materials held in
the core of the hollow particle to be released after a certain period of time
or exposure
to sufficient heat or other energy, which would permit the particles to travel
to a desired
target or location. This example is illustrated in FIG. 2E. The particle 230
has a core
232 with an exterior layer 234 made of the ablative material. Material 236 is
present
within the core.
[0074] In some
embodiments, the acoustic contrast factor of the particle can be
changed. For example, hollow glass particles could be coated with an ablative
polymer, such as a polysaccharide that is functionalized with antigens or
antibodies or
other protein or biological moieties. The particles could begin a process with
a first
acoustic contrast factor, and then be changed to a second acoustic contrast
factor by
removal of the ablative polymer.
[0075]
[0076] In some
embodiments, the particles of the present disclosure have a
positive acoustic contrast factor. Such particles can be trapped at the nodes
of an
acoustic standing wave. In other embodiments, the particles of the present
disclosure
have a negative acoustic contrast factor. These particles will be trapped at
the anti-
nodes of an acoustic standing wave. If the particle changes in the acoustic
contrast
factor while in a processing system or in vivo, the particle could then
migrate from a
node to an anti-node if the particle changes from a positive contrast factor
to a negative
contrast factor and vice versa if a particle changes from a negative contrast
factor to
a positive contrast factor.
[0077] In some
embodiments, the particles of the present disclosure contain a
payload. The payload may include a primary, secondary, tertiary and/or more
materials that are delivered by the particles to a specific area or cell
population.
Examples of materials that can be delivered as a payload include a virus, a
nucleic
acid, a cytokine (such as an interleukin), a pharmaceutical molecule, a
liquid, or a gas,
or mixtures of such materials. These payloads can be delivered to a desired
target or
location (by acoustic co-location) and then release the payload. That the
payload
would affect a target at the desired location, for example causing a change in
the
morphology, biochemistry or other attribute of the targeted material. This
example is
11
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
illustrated in FIG. 2F. The particle 240 is hollow, with a solid shell 242
surrounding a
core 244 that contains a payload 246.
[0078] In
addition, the particles of the present disclosure could also be affected by
an outside force such as a magnetic, electromagnetic, dielectric, ultrasonic
or other
type of energy. By affecting the particles with an outside energy source, the
particles
may be activated upon reaching certain process steps (e.g. an affinity
binding) or a
specific part of a host's anatomy (e.g. to destroy a tumor located within a
patient's
body).
[0079] In some
further embodiments, the particles are of a core-shell structure, with
a liquid core encapsulated by a lipid shell. In more particular embodiments,
the liquid
in the liquid core is a perfluorocarbon (PFC). The term "perfluorocarbon", as
used in
the present disclosure, refers to molecules in which all of the hydrogen atoms
have
been replaced with a halogen, and a majority of the halogen atoms are fluorine
atoms.
For purposes of the present disclosure, "halogen" refers to fluorine,
chlorine, and
bromine. Specific examples of PFCs include perfluoropentane (PFP),
perfluorohexane (PFH), perfluorooctane (PFO), perfluorooctyl bromide (PFOB,
C8F17Br), perfluorodichlorooctane (PFDCO, 08F16012), or perfluorodecalin (PFD,
CioF18).
[0080] These
PFC liquids have unique properties. The PFC liquids are denser than
water, have low surface tension and have low viscosity. The PFC liquids also
have a
high capacity to absorb oxygen and nitrogen. Perfluorocarbon liquids have a
low
speed of sound, are highly chemically inert, and are biocompatible. Table 1,
below,
shows various physical and acoustic properties of various PFC liquids which
may be
used in particles, along with other polymers for comparison. It is noted that
the
compressibility of the PFC liquids is very high compared to biological cells.
Table 1.
Compoun Densit Speed Boilin Contras Specifi Surface Compressibilit
d y of g t Factor c Tensio Y
(kg/m3) Soun Point Gravity n
d ( C) (g/mL) (mN/m)
(m/s)
PFP 1 600 477 29 -1.59 1.6 9 27.46x101
(Perfluoro
pentane)
12
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
Compoun Densit Speed Boilin Contras Specifi Surface Compressibilit
d y of g t Factor c Tensio y
(kg/m3) Soun Point Gravity n
d ( C) (g/mL) (mN/m)
(m/s)
PFH 1670 548 57 -
1.44 1.63 12 19.93x101
(Perfluoro
hexane)
PFOB 1920 630 141 -0.55 1.9 16 13.12x1010
(Perfluoro
octyl
bromide)
PMMA 2700 0.299 1.18
Polystyren 2350 0.22 1.06
e
Cell 1060 1600 3.68x101
[0081] Specific
examples of lipids that can be used to form the lipid shell include
dipalmitoylphosphatidylcholine (DPPC), 1,2-palmitoyl-phosphatidic acid (DPPA),
1,2-
dipalmitoyl-sn-glycero-3-phosphoethanolamine (DPPE), and 1,2-distearoyl-sn-
glycero-3-phosphoethanolamine (DSPE). These lipids can also be used in a lipid-
polyethylene glycol conjugate, or a complex of a lipid with albumin (such as
bovine
serum albumin or human serum albumin). The lipid shell can be functionalized
with
streptavidin, biotin, avidin, an antibody, or other functionalized moieties.
[0082] This
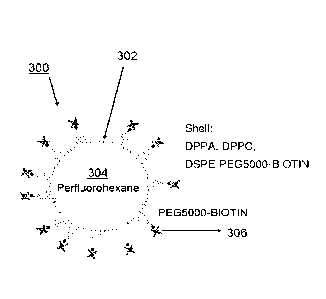
structure is illustrated in FIG. 3. The particle 300 is made of a lipid shell
302 that surrounds a liquid core 304, in this example perfluorohexane. The
shell can
be made of DPPA, DPPC, or a functionalized lipid-glycol conjugate, here
labeled as
DSPE-PEG5000-BIOTIN. Also illustrated is an avidin derivative 306 that binds
to the
biotin of the lipid shell.
[0083] The
lipid shell is used to attach the particle to another molecule, and for
protection of the liquid core. These lipid-PFC particles are believed to be
able to
produce transient changes in the permeability of cell membranes after
ultrasonic-
induced cavitation while reducing cellular damage. They may enable tissue-
specific
or site-specific intracellular delivery of genetic materials, both in vitro
and in vivo. They
can be used to enhance the efficacy of gene delivery, for use as a non-viral
vector
system.
[0084]
Generally, a PFC liquid and a lipid solution are combined to make a liquid
core with a lipid shell. The PFC liquid is dispersed in another solution to
form droplets.
An emulsifier may be added to the solution, to prevent the droplets from
coalescing.
13
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
In some embodiments, phospholipids are used as the emulsifier/surfactant. A
PFC
liquid is dispersed by different methods depending upon the size of droplets
desired
for the application. To create small nanometer-sized droplets, ultrasonic
agitation may
be used. To create larger droplets, a vial shaker may be used to agitate the
liquid
mixture.
[0085] In some
embodiments, a lipid solution consists of several different lipid
materials in solution. The procured lipids are stored in a freezer at about -
20 C. At this
temperature, the lipids are in a solid state. The lipids may be taken out of
the freezer
and left at room temperature for about 20 minutes before use. This is done to
bring
the lipids to gel state. Since lipids generally do not dissolve in water,
propylene glycol
may be used to dissolve them. It is desirable to not dissolve all the lipids
at once in the
propylene glycol, as putting all the lipids at a time may result into
formation of white
clumps in the solution. The solubility of the each lipid material should be
compared
and the lipid material with maximum solubility should be dissolved first in
the propylene
glycol and so on. Since, the solubility of the lipids are a function of
temperature of the
solution, the solution should be maintained at a temperature above the
transition
temperature of the lipids. Table 2 is an example of a lipid composition.
Table 2.
Lipids
Total lipid 1 mg/ml
concentration
Mol wt (gm) Molar Avanti No of
ratio catalog
carbons
information
DPPA 670.87 11 16
DPPC 734.04 82 16
DPPE-PEG-5000 5744 0 880200 16
DSPE-PEG-2000 2805.49 0 880120 18
DSPE-PEG-2000- 3070 0 880129 18
BIOTIN
DSPE-PEG-5000- 5670 7 18
BIOTIN
V (stock lipid DPPA (mg) DPPC DSPE-
PEG-5000-BIOTIN (mg)
volume), mL (mg)
0.69 5.61 3.70
1.38 11.22 7.40
2.06 16.84 11.10
2.75 22.45 14.80
3.44 28.06 18.50
14
CA 03087498 2020-06-30
WO 2019/135843 PCT/US2018/063698
60 4.13 33.67 22.20
70 4.82 39.28 25.90
80 5.50 44.89 29.60
90 6.19 50.51 33.30
100 6.88 56.12 37.00
110 7.57 61.73 40.70
120 8.26 67.34 44.40
[0086] One representative process for creating a lipid solution is as
follows. First,
the propylene glycol is heated to the maximum transition temperature of the
lipid blend
for mixing. Next, the lipid material with maximum solubility is added to the
heated
propylene glycol. The lipid material and propylene glycol are then mixed in a
bath
sonicator. Sequentially, lipids of lower solubility are added into the
propylene glycol
mixture while in the bath sonicator.
[0087] A mixture of glycerol and buffer solution may be prepared
simultaneously.
The glycerol and buffer solution is heated to the maximum transition
temperature.
Once the lipid-propylene glycol solution is translucent (free of white clumps)
in the
sonicator, the lipid-glycol solution is mixed with the glycerol-buffer
solution. The
resulting mixture is homogenized with a homogenizer operating at 3000 rpm. The
homogenization is performed for about one hour. During the homogenization
process,
the temperature is maintained at the maximum transition temperature of the
lipids.
[0088] The prepared lipid solution is filtered to remove any possible
contaminants
such as dust, undissolved lipid clumps, etc. The filtering process may be
performed
with a hydrophilic syringe filer. The filters are soaked in the same
temperature batch
prior to use. In some embodiments, a 2.0 micron filter is used. In other
embodiments,
a 0.8 micron filter is used. In yet other embodiments, a 0.45 micron filter is
used. In
some embodiments, a combination of filters may be used.
[0089] The lipid solution is then mixed with the PFC liquid in a narrow
vessel to
create core-shell particles. The PFC liquid is first placed into a vessel and
the lipid
solution is poured on top. To make smaller sized droplets, the amount of PFC
liquid
in the vessel should be minimal. As the ratio of PFC liquid volume to lipid
solution
volume increases, the size of the formed droplet increases until it reaches a
plateau
for a given sonication power. It is to be noted that the PFC liquids are low
strength as
they have low surface tension values. Therefore, the sonication amplitude
should be
selected appropriately and the input of ultrasonic waves should be done in a
pulsed
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
mode rather than in a continuous mode. The tip of a horn sonicator assembly
should
be placed at the interface of two liquid solutions. To avoid formation of
bubbles/foam
the horn should be sufficiently inside the solution. Here, the aim is to
prepare a droplet
solution, so the narrow vessel is submerged in a transparent low temperature
bath.
The transparent low temperature bath is made, for example, by making a
supersaturated solution of salt and then storing the salt solution in the
freezer at -20 C.
The sonication produces smaller beads.
[0090] In one
example, the lipid solution may comprise about 1 mL propylene glycol
+ 1 mL glycerol + 8 mL buffer solution + lipid blend of 10 mg. 9 mL of the
lipid solution
may be combined with about 1 mL of PFC solution. The Lipid-PFC solution may be
sonicated. For a 0.5 inch probe and 750 watt sonicator, a PFC solution
utilizing 30%
PFP is sonicated for about 3 seconds on and about 10 seconds off until a total
sonication time of about 15 seconds is reached. A PFC solution utilizing 40%
PFH is
sonicated for about 3 seconds on and about 10 seconds off until a total
sonication time
of about 15 seconds is reached. A PFC solution utilizing 50% PFOB is sonicated
for
about 3 seconds on and about 10 seconds off until a total sonication time of
about 15
seconds is reached. The sonication produces a droplet solution.
[0091] To
prepare larger sized droplets, the quantity of PFC liquid is increased and
the power input of the sonicator is reduced drastically. In another non-
limiting
exemplary embodiment, 500 microliters of PFC and 2 mL of lipid solution may be
placed in a 3 mL vial. The vial may then be shaken in a vial mixer at 4800 rpm
for 30
seconds. The prepared droplet suspension may have some microbubbles. In cases
where microbubbles are present, the solution may be centrifuged.
[0092] The
binding efficiency of these PFC-lipid particles can be tested by adding
NeutrAvidine to the droplet solution. NeutrAvidine is a deglycosylated version
of
avidin, with a mass of approximately 60,000 daltons. Like avidin itself,
NeutrAvidine
is a tetramer with a strong affinity for biotin (Kd = 10-15 M). Because the
carbohydrates are removed, though, undesired lectin binding is reduced to
undetectable levels, yet biotin binding affinity is retained. NeutrAvidine
also has a
near-neutral pl (pH 6.3), minimizing non-specific interactions with the
negatively-
charged cell surface or with DNA/RNA. Neutravidine still has lysine residues
that
remain available for derivatization or conjugation. Alternatively, If a
binding complex
is present (e.g. avidin-biotin), aggregates may be formed. This
aggregation
phenomenon may be one way to skew the droplet population towards a larger
size.
16
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
This mechanism is illustrated in FIG. 4. Nine PFC-lipid particles 300 are
illustrated on
the left-hand side, with the lipid shell surrounding the liquid PFH core. The
lipids
include a biotin complex 306. Upon exposure to avidin or similar molecule, the
particles aggregate into a larger particle 310.
[0093] In one
experiment, 5 mL of the droplet solution was taken and incubated
with 100 microliters of 5 mg/mL NeutrAvidine solution. This combination
solution was
left for one hour, and size measurement was done from the original droplet
solution
and from the droplet solution incubated with NeutrAvidine.
[0094] FIG. 5A
is a graph showing the size distribution of droplets having a size of
0.6 microns to 1.25 microns. FIG. 5B is a graph showing the size distribution
of
droplets having a size of 1.25 microns to 2.25 microns. The thin line is for
the droplet
solution without added NeutrAvidine. The thicker line is for the droplet
solution
incubated with NeutrAvidine. As seen here, the number of particles of a given
size
was greater when NeutrAvidine was added, or put another way the line was
shifted to
the right (e.g. greater particle sizes).
[0095]
Polymeric particles may also be produced through a continuous and
discontinuous phase emulsion where there is an aqueous phase and a
discontinuous
monomer phase. The reaction vessel for the emulsion may also contain
surfactants
and free radical initiators. As the emulsion is stirred, it is heated and free
radical
initiators are introduced into the emulsion. This causes the monomer particles
to
polymerize and thus gives a microparticles mixture of polymerized
microparticles in
the aqueous phase. This process allows for uniform size particles. An example
of this
process is styrene monomer dispersed in an aqueous phase with an octylphenol
ethoxylate, a non-ionic surfactant, where benzoyl peroxide is introduced into
the
reaction vessel while the emulsion is stirred and heated.
[0096]
Microparticles may also be produced using a technique of electro
hydrodynamic spraying (EHDS) where a polymeric fluid is sprayed into a gas
mixture
such that the atomization of the liquid stream while it is being sprayed
allows for very
fine particle size generation. The polymer may be seated before it is
introduced into
the spray nozzle. Also, the polymer may be the reaction result of a dual or
multicomponent mixture that is mixed prior to the spray nozzle and polymerizes
as it
moves through the spray nozzle and into the gas or gas mixture gas mixture.
The gas
may be an inert gas such as nitrogen or argon. The gas mixture may be air or
other
gas blends such as helium / oxygen and nitrogen! oxygen mixtures. The EHDS
system
17
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
is typically a physical process caused by the electric force applied to the
surface of the
liquid.
[0097]
Microparticles and nanoparticles may also be produced by simple spray
drying of a polymeric liquid or a polymeric liquid that is carried in an
aqueous or solvent
base.
[0098] The
medium or primary fluid in which the particles are used may also be
modified to increase the differentiation between the particles and the primary
fluid.
[0099] FIG. 6
illustrates a exemplary process 600 for creating and loading a
payload into micro/nanoparticles and the release of that payload, described in
more
detail in Xu et al. "Hollow hierarchical hydroxyapatite/Au/polyelectrolyte
hybrid
microparticles for multi-responsive drug delivery," J. Mater. Chem. B. 2014,
2, 6500-
6507 which is herein incorporated by reference in its entirety. First at 602,
Na2CO3 and
Ca(NO3)2 are combined to form CaCo3 template microparticles 603. Next, a
(Cal 0(PO4)6(OH)2, HAP) ("HAP") coating 606 is applied to the CaCo3 core 603
in a
hydrothermal reaction at 604. HAP is used widely in the biomedical field due
to its
biocompatibility and biodegradability. Following the creation of the HAP layer
606,
particles having the CaCo3 core 603 and HAP coating 606 are then subject to a
layer-
by-layer (LbL) technique to incorporate polyectrolytes 608. Such
polyelectrolytes
include (aliphatic poly(urethane-amine)(PUA) and sodium
poly(styrenesulfonate)(PSS). After the LbL coating 605, gold nanoparticles
(AuNPs)
610, are loaded into the microparticles via electrostatic interaction. The
AuNPs 610
help to slow the release of a payload loaded into a hollow particle.
[00100] A hollow HAP particle 612 is formed by removing the CaCo3 core 603
with
a chemical etching solution step 611, for example, acetic acid. The hollow HAP
particle
612 is then loaded with payload 614 for payload delivery. Once the loaded
particle 616
reaches a desired destination, the payload 614 may be released from the hollow
particle carrier 612. Release / activation 620 of the payload 614 may be
facilitated
with a change in environmental temperature, pH, or in response to near
infrared
irradiation (NIR).
[00101] Devices and Systems
[00102] In general, the particles of the present disclosure may be manipulated
with
acoustic waves. The acoustic wave that may be utilized for manipulation of the
microparticles and nanoparticles may be an acoustic standing wave such as a
18
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
multidimensional acoustic standing wave, a planar standing wave, or
combination of
a multidimensional acoustic standing wave and a planar wave.
[00103] FIG. 7 illustrates an acoustic traveling wave 700. Acoustic waves are
a type
of longitudinal waves that propagate by means of adiabatic compression and
decompression in a medium. The wave 700 includes a crest 702. The crest 702
moves in the direction of propagation 704.
[00104] An acoustic traveling wave 700 may change the contrast factor of the
microparticles and nanoparticles when they are processed in an acoustic
system. In
other words, the contrast factor of the microparticles nanoparticles that are
processed
by a traveling acoustic wave may be different from the microparticles and
nanoparticles when they are processed by an acoustic standing wave.
[00105] A combination of multiple traveling waves may generate an acoustic
standing wave when each wave traveling in opposite directions creating a
superposition of the waves. FIG. 8 illustrates an acoustic standing wave
system 800
that creates an acoustic standing wave 801. The system is comprised of a
reflector
plate 804 and an ultrasonic transducer 802. Excitation frequencies typically
in the
range from hundreds of kHz to tens of MHz are applied by the transducer 802.
One or
more standing waves are created between the transducer 802 and the reflector
804.
The standing wave is the sum of two propagating waves that are equal in
frequency
and intensity and that are traveling in opposite directions, e.g. from the
transducer to
the reflector and back. The propagating waves destructively interfere with
each other
and thus generate the standing wave. Point A on the medium moves from a
maximum
positive to a maximum negative displacement over time. The diagram only shows
one-
half cycle of the motion of the standing wave pattern. The motion would
continue and
persist, with point A returning to the same maximum positive displacement and
then
continuing its back-and-forth vibration between the up to the down position.
Position
A, having a maximum displacement is known as an anti-node. Note that point B
on
the medium is a point that never moves. Point B is a point of no displacement.
Such
points are known as nodes.
[0100] A fluid
medium carrying particles 806 (microparticles or nanoparticles)
disclosed above may flow in a direction 805 though an acoustic chamber /
acoustic
standing wave system 800. The standing wave 801 produced may trap the
particles
806 against the fluid flow 805. Particles having a positive contrast factor
would be
trapped at a pressure node, while particles having a negative contrast factor
would be
19
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
trapped at an anti-node. Put another way, the particles are concentrated at a
first
location or a desired location. If the particles carry a payload, that payload
may be
released. That release may occur for example after passage of time (e.g. the
shell
dissolves or melts), or upon exposure to an outside energy source, or as
previously
described herein.
[0101] The
acoustic devices discussed herein may operate in a multimode or
planar mode. Multimode refers to generation of acoustic waves by an acoustic
transducer that create acoustic forces in three dimensions. The multimode
acoustic
waves, which may be ultrasonic, are generated by one or more acoustic
transducers,
and are sometimes referred to herein as multi-dimensional or three-dimensional
acoustic standing waves. Planar mode refers to generation of acoustic waves by
an
acoustic transducer that create acoustic forces substantially in one
dimension, e.g.
along the direction of propagation. Such acoustic waves, which may be
ultrasonic,
that are generated in planar mode are sometimes referred to herein as one-
dimensional acoustic standing waves.
[0102] The
acoustic devices may be used to generate bulk acoustic waves in a
fluid/particle mixture. Bulk acoustic waves propagate through a volume of the
fluid,
and are different from surface acoustic waves which tend to operate at a
surface of a
transducer and do not propagate through a volume of a fluid.
[0103] The
acoustic transducers may be composed of a piezoelectric material.
Such acoustic transducers can be electrically excited to generate planar or
multimode
acoustic waves. The three-dimensional acoustic forces generated by multimode
acoustic waves include radial or lateral forces that are unaligned with a
direction of
acoustic wave propagation. The lateral forces may act in two dimensions. The
lateral
forces are in addition to the axial forces in multimode acoustic waves, which
are
substantially aligned with the direction of acoustic wave propagation. The
lateral
forces can be of the same order of magnitude as the axial forces for such
multimode
acoustic waves. The acoustic transducer excited in multimode operation may
exhibit
a standing wave on its surface, thereby generating a multimode acoustic wave.
The
standing wave on the surface of the transducer may be related to the mode of
operation of the multimode acoustic wave. When an acoustic transducer is
electrically
excited to generate planar acoustic waves, the surface of the transducer may
exhibit
a piston-like action, thereby generating a one-dimensional acoustic standing
wave.
Compared to planar acoustic waves, multimode acoustic waves exhibit
significantly
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
greater particle trapping activity on a continuous basis with the same input
power. One
or more acoustic transducers may be used to generate planar and/or multi-
dimensional acoustic standing waves. In some modes of operations, multimode
acoustic waves generate an interface effect that can hold back or retain
particles of a
certain size, while smaller particles can flow through the multimode acoustic
waves.
In some modes of operation, planar waves can be used to deflect particles at
certain
angles that are characteristic of the particle size.
[0104]
Acoustophoresis is the separation of materials using acoustic waves. An
implementation discussed herein provides a low-power, no-pressure-drop, no-
clog,
solid-state approach to particle separation from fluid dispersions. The
scattering of
the acoustic field off the particles creates secondary acoustic forces that
draw particles
together. The multimode operation results in a three-dimensional acoustic
radiation
force, which acts as a three-dimensional trapping field. The acoustic
radiation force
is proportional to the particle volume (e.g., the cube of the radius) when the
particle is
small relative to the wavelength. The acoustic radiation force is proportional
to
frequency and the acoustic contrast factor. The acoustic radiation force
scales with
acoustic energy (e.g., the square of the acoustic pressure amplitude). For
harmonic
excitation, the sinusoidal spatial variation of the force is what drives the
particles to the
stable positions within the standing waves. When the acoustic radiation force
exerted
on the particles is stronger than the combined effect of fluid drag force and
buoyancy/gravitational force, the particle is trapped within the acoustic
standing wave
field. The action of the lateral and axial acoustic forces on the trapped
particles results
in formation of tightly packed clusters through concentration, clustering,
clumping,
agglomeration and/or coalescence of particles that, when reaching a critical
size, settle
continuously through enhanced gravity for particles heavier than the host
fluid or rise
out through enhanced buoyancy for particles lighter than the host fluid.
Additionally,
secondary inter-particle forces, such as Bjerkness forces, aid in particle
agglomeration.
[0105] The
following discussion is directed towards biological cells, which can be
considered as particles for purposes of acoustophoretics. Most biological cell
types
present a higher density and lower compressibility than the fluid medium in
which they
are suspended, so that the acoustic contrast factor between the cells and the
medium
has a positive value. As a result, the axial acoustic radiation force (ARF)
drives the
cells towards the standing wave pressure nodes. The axial component of the
acoustic
21
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
radiation force drives the cells, with a positive contrast factor, to the
pressure nodes,
whereas cells or other particles with a negative contrast factor are driven to
the anti-
nodes. The radial or lateral component of the acoustic radiation force is the
force that
traps the cells. The radial or lateral component of the ARF is larger than the
combined
effect of fluid drag force and gravitational force.
[0106] For a
cell to be trapped in the multi-dimensional ultrasonic standing wave,
the force balance on the cell can be assumed to be zero, and, therefore, an
expression
for lateral acoustic radiation force FLRF is FLRF = FD + FB, where FD is the
drag force
and FB is the buoyancy force. For a cell of known size and material property,
and for
a given flow rate, this equation can be used to estimate the magnitude of the
lateral
acoustic radiation force.
[0107] One
theoretical model that is used to calculate the acoustic radiation force
is based on the formulation developed by Gor'kov. The primary acoustic
radiation
force FA is defined as a function of a field potential U, FA = -V" (II ) ,
which is affected
by the acoustic pressure p, the fluid particle velocity u, the ratio of cell
density pp to
fluid density pf, the ratio of cell sound speed cp to fluid sound speed cf,
and the volume
of the biological cell Vo.
[0108]
Gor'kov's theory may be limited to particle sizes that are small with respect
to the wavelength of the sound fields in the fluid and the particle, and it
also may not
take into account the effect of viscosity of the fluid and the particle on the
radiation
force. Additional theoretical and numerical models have been developed for the
calculation of the acoustic radiation force for a particle without any
restriction as to
particle size relative to wavelength. These models also include the effect of
fluid and
particle viscosity, and therefore are a more accurate calculation of the
acoustic
radiation force. The models that were implemented are based on the theoretical
work
of Yurii llinskii and Evgenia Zabolotskaya as described in AIP Conference
Proceedings, Vol. 1474-1, pp. 255-258 (2012). Additional in-house models have
been
developed to calculate acoustic trapping forces for cylindrical shaped
objects, such as
the "hockey pucks" of trapped particles in the standing wave, which closely
resemble
a cylinder.
[0109]
Desirably, the ultrasonic transducer(s) generates a multi-dimensional
standing wave in the fluid that exerts a lateral force on the suspended
particles to
accompany the axial force. Typical results published in literature state that
the lateral
22
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
force is two orders of magnitude smaller than the axial force. In contrast,
the
technology disclosed in this application provides for a lateral force to be of
the same
order of magnitude as the axial force. However, in certain embodiments
described
further herein, the device uses both transducers that produce multi-
dimensional
acoustic standing waves and transducers that produce planar acoustic standing
waves. The lateral force component of the total acoustic radiation force (ARF)
generated by the ultrasonic transducer(s) of the present disclosure is
significant and
is sufficient to overcome the fluid drag force at linear velocities of up to 1
cm/s, and to
create tightly packed clusters, and is of the same order of magnitude as the
axial force
component of the total acoustic radiation force.
[0110] The
acoustic standing wave is a three-dimensional acoustic field, which, in
the case of excitation by a rectangular transducer, can be described as
occupying a
roughly rectangular prism volume of fluid. The transducer can be configured to
face
a reflector or boundary to permit generation of a standing wave therebetween.
The
transducer can be configured to face another transducer, both of which are
operated
to generate a standing wave therebetween. The transducer can be configured to
face
an acoustically absorbent material to permit generation of a traveling wave.
[0111] In some
examples, the rectangular prism includes two opposing faces
defined by the transducer and the reflector, an adjacent pair of opposing
faces
composed of the walls of the device, and a final opposing pair of faces that
may define
a flow channel entrance and exit. The acoustic wave generated by the
transducer and
the reflector create an interface or barrier region interface near the flow
channel
entrance, e.g., located near the upstream face of the acoustic standing wave
field,
generating an "acoustic barrier or edge effect". This location is also
referred to as an
upstream interface region. The acoustic barrier can prevent particles with
certain
characteristics, such as a high acoustic contrast factor, for example, from
passing
through the acoustic wave generated by the transducer and the reflector.
[0112] The
particles that are retained or blocked by the acoustic barrier may be
captured in a chamber, such as a column, or returned to a holding device, such
as a
bioreactor. A circulating flow motion can be generated next to the acoustic
barrier by
a primary recirculation stream and can be optimized with acoustic chamber
geometry
variations to improve system efficiency.
[0113] FIG. 9
and FIG. 10 are views of an acoustophoretic device that can be used
with the particles of the present disclosure. FIG. 9 is a front cross-
sectional view, and
23
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
FIG. 10 is an exterior perspective view. Notably, this embodiment is
specifically
designed such that it can be fabricated with clean machining techniques, using
Class
VI materials (medical device grade HDPE, for example), or even as single or
welded
injection molded part. In this manner, this embodiment is an example of a
single-use
device, which is gamma-stable. The devices are flushed to remove bioburden and
then gamma-irradiated (generally from 25-40 kGy) to sterilize any potential
contamination that could destroy a healthy cell culture, such as that present
in a
perfusion bioreactor.
[0114]
Referring first to FIG. 9, in this device 700, the inlet port 710 and the
collection port 770 are both located at the top end 718 of the device, or on
the top wall
776 of the device. The outlet port 730 is located at a bottom end 716 of the
device.
Here, the inlet port 710 and the outlet port 730 are both on a first side 712
of the
device. The inlet flow path 751 is in the form of a channel 755 that runs from
the inlet
port downwards towards the bottom end and past the outlet port, the channel
being
separated from the acoustic chamber 750 (here, the separation occurring by an
internal wall 756). Fluid will flow downwards in the channel, then rise
upwards into the
acoustic chamber 750. The bottom wall 720 of the acoustic chamber is a sloped
planar
surface that slopes down towards the outlet port 730. The location of the
ultrasonic
transducers 760 are shown here as two squares, between the top end and the
bottom
end of the device. The collection flow path 753 is located above the
transducers.
[0115]
Referring now to FIG. 10, the device 700 is shown as being formed within a
three-dimensional rectangular housing 706. It can be seen that the outlet port
730 at
the bottom end 716 of the device is located on a front wall 775. Again, the
collection
port 770 and the inlet port 710 are located on a top wall 776. A viewing
window 708
made of a transparent material is present in the front wall. Through that
viewing
window, it can be seen that the ultrasonic transducers are mounted in the rear
wall
778 of the device housing 706. The viewing window acts as a reflector to
generate
the multi-dimensional acoustic standing waves.
[0116] The
device 700 can be used to cause cells and particles to react with each
other, with the particles delivering payloads to the cells in roughly the area
around the
transducers 760, where acoustic waves are present. The cells can then exit
through
outlet port 730, while other fluid exits through collection port 770.
[0117] The
particles can also interact with the cells and perform negative or positive
selection depending upon the functionalization on the surface of the particle
and the
24
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
desired cells to be selected. The functionalized portion of the particle will
bind with the
receptors on the surface of the target cells such that the cells may be
removed or
retained in the system.
[0118] FIG. 11
is a cross-sectional view of an ultrasonic transducer 81 according
to an example of the present disclosure, which is used in the acoustic
filtering device
of the present disclosure. Transducer 81 is shaped as a disc or a plate, and
has an
aluminum housing 82. The aluminum housing has a top end and a bottom end. The
transducer housing may also be composed of plastics, such as medical grade
HDPE
or other metals. The piezoelectric element is a mass of perovskite ceramic,
each
consisting of a small, tetravalent metal ion, usually titanium or zirconium,
in a lattice of
larger, divalent metal ions, usually lead or barium, and 02- ions. In this
example, a
PZT (lead zirconate titanate) piezoelectric element 86 defines the bottom end
of the
transducer, and is exposed from the exterior of the bottom end of the housing.
The
piezoelectric element is supported on its perimeter by a small elastic layer
98, e.g.
epoxy, silicone or similar material, located between the piezoelectric element
and the
housing. Put another way, no wear plate or backing material is present.
However, in
some embodiments, there is a layer of plastic or other material separating the
piezoelectric element from the fluid in which the acoustic standing wave is
being
generated. The piezoelectric element / crystal has an exterior surface (which
is
exposed) and an interior surface as well. In particular embodiments, the
piezoelectric
element / crystal is an irregular polygon, and in further embodiments is an
asymmetrical irregular polygon.
[0119] Screws
88 attach an aluminum top plate 82a of the housing to the body 82b
of the housing via threads. The top plate includes a connector 84 for powering
the
transducer. The top surface of the PZT piezoelectric element 86 is connected
to a
positive electrode 90 and a negative electrode 92, which are separated by an
insulating material 94. The electrodes can be made from any conductive
material,
such as silver or nickel. Electrical power is provided to the PZT
piezoelectric element
86 through the electrodes on the piezoelectric element. Note that the
piezoelectric
element 86 has no backing layer or epoxy layer. Put another way, there is an
interior
volume or an air gap 87 in the transducer between aluminum top plate 82a and
the
piezoelectric element 86 (e.g. the housing is empty). A minimal backing 58 (on
the
interior surface) and/or wear plate 50 (on the exterior surface) may be
provided in
some embodiments, as seen in FIG. 12.
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
[0120] The
transducer design can affect performance of the system. A typical
transducer is a layered structure with the ceramic piezoelectric element
bonded to a
backing layer and a wear plate. Because the transducer is loaded with the high
mechanical impedance presented by the standing wave, the traditional design
guidelines for wear plates, e.g., half wavelength thickness for standing wave
applications or quarter wavelength thickness for radiation applications, and
manufacturing methods may not be appropriate. Rather, in one embodiment of the
present disclosure, the transducers do not have a wear plate or backing,
allowing the
piezoelectric element to vibrate in one of its eigenmodes with a high 0-
factor, or in a
combination of several eigenmodes. The vibrating ceramic piezoelectric
element/disk
is directly exposed to the fluid flowing through the fluid cell.
[0121] Removing
the backing (e.g. making the piezoelectric element air backed)
also permits the ceramic piezoelectric element to vibrate at higher order
modes of
vibration with little damping (e.g. higher order modal displacement). In a
transducer
having a piezoelectric element with a backing, the piezoelectric element
vibrates with
a more uniform displacement, like a piston. Removing the backing allows the
piezoelectric element to vibrate in a non-uniform displacement mode. The
higher
order the mode shape of the piezoelectric element, the more nodal lines the
piezoelectric element has. The higher order modal displacement of the
piezoelectric
element creates more trapping lines, although the correlation of trapping line
to node
is not necessarily one to one, and driving the piezoelectric element at a
higher
frequency will not necessarily produce more trapping lines.
[0122] The
reflector may be of a nonplanar type such as a faceted reflector. The
reflector may also be another transducer that may have a planar or nonplanar
surface.
In some examples, two opposing transducers are used to generate an acoustic
wave,
such as an acoustic standing wave therebetween.
[0123] In some
embodiments of the acoustic filtering device of the present
disclosure, the piezoelectric element may have a backing that minimally
affects the 0-
factor of the piezoelectric element (e.g. less than 5%). The backing may be
made of
a substantially acoustically transparent material such as balsa wood, foam, or
cork
which allows the piezoelectric element to vibrate in a higher order mode shape
and
maintains a high 0-factor while still providing some mechanical support for
the
piezoelectric element. The backing layer may be a solid, or may be a lattice
having
holes through the layer, such that the lattice follows the nodes of the
vibrating
26
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
piezoelectric element in a particular higher order vibration mode, providing
support at
node locations while allowing the rest of the piezoelectric element to vibrate
freely.
The goal of the lattice work or acoustically transparent material is to
provide support
without lowering the 0-factor of the piezoelectric element or interfering with
the
excitation of a particular mode shape.
[0124] Placing
the piezoelectric element in direct contact with the fluid also
contributes to the high 0-factor by avoiding the dampening and energy
absorption
effects of the epoxy layer and the wear plate. Other embodiments of the
transducer(s)
may have wear plates or a wear surface to prevent the PZT, which contains
lead,
contacting the host fluid. This may be desirable in, for example, biological
applications
such as separating blood, biopharmaceutical perfusion, or fed-batch filtration
of
mammalian cells. Such applications might use a wear layer such as chrome,
electrolytic nickel, or electroless nickel. Chemical vapor deposition may be
used to
apply a layer of poly(p-xylylene) (e.g. Parylene) or another polymer. Organic
and
biocompatible coatings such as silicone or polyurethane are also usable as a
wear
surface. Thin films, such as a PEEK film, can also be used as a cover of the
transducer
surface exposed to the fluid with the advantage of being a biocompatible
material. In
one embodiment, the PEEK film is adhered to the face of the piezo-material
using
pressure sensitive adhesive (PSA). Other films can be used as well.
[0125] In some
embodiments, for applications such as oil/water emulsion splitting
and others such as perfusion, the ultrasonic transducer has a nominal 2 MHz
resonance frequency. Each transducer can consume about 28 W of power for
droplet
trapping at a flow rate of 3 GPM. This translates to an energy cost of 0.25 kW
hr/ m3.
This is an indication of the very low cost of energy of this technology. Each
transducer
may be powered and controlled by a dedicated driver, which may include an
amplifier,
or multiple transducers may be driven by a single driver. In other
embodiments, the
ultrasonic transducer uses a square piezoelectric element, for example with
1"x1"
dimensions.
Alternatively, the ultrasonic transducer can use a rectangular
piezoelectric element, for example with 1"x2.5" dimensions. Power dissipation
per
transducer was 10 W per 1"x1" transducer cross-sectional area and per inch of
acoustic standing wave span in order to get sufficient acoustic trapping
forces. For a
4" span of an intermediate scale system, each 1"x1" square transducer consumes
40
W. The larger 1"x2.5" rectangular transducer uses 100W in an intermediate
scale
system. The array of three 1"x1" square transducers would consume a total of
120 W
27
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
and the array of two 1"x2.5" transducers would consume about 200 W. Arrays of
closely spaced transducers represent alternate potential embodiments of the
technology. Transducer size, shape, number, and location can be varied as
desired
to generate desired multi-dimensional acoustic standing wave patterns.
[0126] The
size, shape, and thickness of the transducer determine the transducer
displacement at different frequencies of excitation, which in turn affects
separation
efficiency. Typically, the transducer is operated at frequencies near the
thickness
resonance frequency (half wavelength). Gradients in transducer displacement
typically result in more trapping locations for the cells/biomolecules. Higher
order
modal displacements generate three-dimensional acoustic standing waves with
strong
gradients in the acoustic field in all directions, thereby creating equally
strong acoustic
radiation forces in all directions, leading to multiple trapping lines, where
the number
of trapping lines correlate with the particular mode shape of the transducer.
[0127] The
lateral force of the acoustic radiation force generated by the transducer
can be increased by driving the transducer in higher order mode shapes, as
opposed
to a form of vibration where the crystal effectively moves as a piston having
a uniform
displacement. The acoustic pressure is proportional to the driving voltage of
the
transducer. The electrical power is proportional to the square of the voltage.
The
transducer is typically a thin piezoelectric plate, with electric field in the
z-axis and
primary displacement in the z-axis. The transducer is typically coupled on one
side by
air (e.g., the air gap within the transducer) and on the other side by the
fluid mixture of
the cell culture media. The types of waves generated in the plate are known as
composite waves. A subset of composite waves in the piezoelectric plate is
similar to
leaky symmetric (also referred to as compressional or extensional) Lamb waves.
The
piezoelectric nature of the plate typically results in the excitation of
symmetric Lamb
waves. The waves are leaky because they radiate into the water layer, which
result
in the generation of the acoustic standing waves in the water layer. Lamb
waves exist
in thin plates of infinite extent with stress free conditions on its surfaces.
Because the
transducers of this embodiment are finite in nature, the actual modal
displacements
are more complicated.
[0128] The
transducers are driven so that the piezoelectric element vibrates in
higher order modes of the general formula (m, n), where m and n are
independently 1
or greater. Generally, the transducers will vibrate in higher order modes than
(2,2).
Higher order modes will produce more nodes and antinodes, result in three-
28
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
dimensional standing waves in the water layer, characterized by strong
gradients in
the acoustic field in all directions, not only in the direction of the
standing waves, but
also in the lateral directions. As a consequence, the acoustic gradients
result in
stronger trapping forces in the lateral direction.
[0129] In
embodiments, the voltage signal driving the transducer can have a
sinusoidal, square, sawtooth, pulsed, or triangle waveform; and have a
frequency of
50 kHz to 10 MHz. The voltage signal can be driven with pulse width
modulation,
which produces any desired waveform. The voltage signal can also have
amplitude
or frequency modulation start/stop capability to eliminate streaming.
[0130] The
transducers are used to create a pressure field that generates acoustic
radiation forces of the same order of magnitude both orthogonal to the
standing wave
direction and in the standing wave direction. When the forces are roughly the
same
order of magnitude, particles of size 0.1 microns to 300 microns will be moved
more
effectively towards "trapping lines", so that the particles will not pass
through the
pressure field and continue to exit through the collection ports of the
filtering device.
Instead, the particles will remain within the acoustic chamber to be recycled
back to
the bioreactor.
[0131] In
biological applications, all of the parts of the system (e.g., the bioreactor,
acoustic filtering device, tubing fluidly connecting the same, etc.) can be
separated
from each other and be disposable. Acoustophoretic separators can provide
improved
performance over centrifuges and filters, by permitting better separation of
the CHO
cells without lowering the viability of the cells. The transducers may also be
driven to
create rapid pressure changes to prevent or clear blockages due to
agglomeration of
CHO cells. The frequency of the transducers may also be varied to obtain
optimal
effectiveness for a given power.
[0132] The
techniques and implementations described herein may be used for
integrated continuous automated bioprocessing. As a non-limiting example, CHO
mAb processing may be carried out using the techniques and apparatuses
described
herein. Control can be distributed to some or all units involved in the
bioprocessing.
Feedback from units can be provided to permit overview of the bioprocess,
which may
be in the form of screen displays, control feedbacks, reporting, status
reports and other
information conveyance. Distributed processing permits a high degree of
flexibility in
achieving a desired process control, for example by coordinating steps among
units
and providing a batch executive control.
29
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
[0133] The bioprocessing can be achieved with commercially available
components, and obtain 100% cell retention. Cell density can be controlled via
an
external cell bleed based on a capacitance signal. The perfusion device
utilizing an
acoustic wave system can be implemented with biocompatible materials, and may
include gamma sterilized and single use components. The processing system also
permits ultrasonic flow measurement, which is noninvasive, and is capable of
operating with high viscosity fluids. The system can be implemented with
single use
sterile connectors and a simple graphical user interface (GUI) for control.
[0134] The
acoustic wave system includes a sweeping flow that is induced below
the acoustic chamber. An acoustic standing wave can act as a barrier for
particulates
in the fluid to permit a clarified stream to be passed and extracted. The
recirculation
loop can be implemented with high fluid velocity and with a low shear rate.
The fluid
velocity through the acoustic field can be lower than the fluid velocity
through the
recirculation loop, which may help to improve separation with low shear
forces.
[0135] Positive
and negative selection of cells may also be performed using various
particles. For instance, the negative selection of TCR positive T cells is a
process
where functionalized particles bind with TCR positive T cells such that the
TCR
positive T cell is removed from the system. TCR positive T cells are
deleterious to
processes such as chimeric antigen receptor T cell therapies (CAR ¨ T).
[0136] A
positive selection process may also be utilized for specific cells where
modified T-cells are selected by appropriately functionalized particles such
that they
are culled from a cell culture to then subsequently be utilized in a cellular
therapy.
[0137] The
methods, systems, and devices discussed above are examples.
Various configurations may omit, substitute, or add various procedures or
components
as appropriate. For instance, in alternative configurations, the methods may
be
performed in an order different from that described, and that various steps
may be
added, omitted, or combined. Also, features described with respect to certain
configurations may be combined in various other configurations. Different
aspects and
elements of the configurations may be combined in a similar manner. Also,
technology
evolves and, thus, many of the elements are examples and do not limit the
scope of
the disclosure or claims.
[0138] Specific
details are given in the description to provide a thorough
understanding of example configurations (including implementations). However,
configurations may be practiced without these specific details. For example,
well-
CA 03087498 2020-06-30
WO 2019/135843
PCT/US2018/063698
known processes, structures, and techniques have been shown without
unnecessary
detail to avoid obscuring the configurations. This description provides
example
configurations only, and does not limit the scope, applicability, or
configurations of the
claims. Rather, the preceding description of the configurations provides a
description
for implementing described techniques. Various changes may be made in the
function
and arrangement of elements without departing from the spirit or scope of the
disclosure.
[0139] Also,
configurations may be described as a process that is depicted as a
flow diagram or block diagram. Although each may describe the operations as a
sequential process, many of the operations can be performed in parallel or
concurrently. In addition, the order of the operations may be rearranged. A
process
may have additional stages or functions not included in the figure.
[0140] Having
described several example configurations, various modifications,
alternative constructions, and equivalents may be used without departing from
the
spirit of the disclosure. For example, the above elements may be components of
a
larger system, wherein other structures or processes may take precedence over
or
otherwise modify the application of the invention. Also, a number of
operations may
be undertaken before, during, or after the above elements are considered.
Accordingly, the above description does not bound the scope of the claims.
31