Note: Descriptions are shown in the official language in which they were submitted.
CA 03090459 2020-08-05
[DESCRIPTION]
[INVENTION TITLE]
METHOD FOR PRODUCING FUNCTIONAL CRYSTALLINE SWEETENER
[TECHNICAL FIELD]
The present invention relates to a method for preparing a crystalline
functional sweetener,
and relates to a method for preparing an allulose crystal for raising the
crystallization yield and
increasing the particle size by controlling the content of impurities
converted from allulose in the
process of preparing the crystalline functional sweetener, for example,
allulose crystal.
[BACKGROUND ART]
General saccharides represented by sugar and starch sugar form the biggest
market of
about 65 trillion won in the world, but as consumer's needs for health-
oriented functional and
premium products are strengthened around the world, the market of functional
sweeteners such as
sugar alcohols including xylitol, oligosaccharides including
fructooligosaccharide, functional
saccharides including crystalline fructose, and sweeteners including sucralose
or aspartame, etc.
has been grown.
The sweetener is the generic term for seasoning and food additives providing
sweet taste.
Sugar, glucose, fructose, etc. among numerous sweeteners are distributed the
most widely as
natural components, and are the most widely used for preparing processed food.
However, as the
negative aspects of sugar such as cavity, obesity, diabetes, etc. become more
prominent, the
alternative functional sweetener for sugar have been received attention
worldwide.
Recently, there is an allulose as an alternative saccharide which can
substitute for sugar or
fructose as a functional sweetener. The allulose can be prepared by chemical
or biological methods,
but the processes of purification and concentration are required, since
allulose is contained at a
low amount of the product. However, as the concentrated syrup has a limited
application, the need
for crystalline powder is high. It is difficult to crystallize the allulose
due to its low crystallinity.
Therefore, a method for preparing an allulose crystal for raising the
crystallization yield
and increasing the particle size by minimizing the content of impurities
comprised in an allulose
solution for crystallization or production of impurities in a preparation
process of allulose, and
controlling the content of impurities converted from allulose has been
urgently needed.
1
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
[DISCLOSURE]
[TECHNICAL PROBLEM]
The present invention relates to a method for preparing an allulose crystal
for increasing
the crystallization yield and the particle size by controlling the content of
impurities or production
of impurities contained in a solution for preparing a crystal.
In addition, the present invention is to provide a preparation method of
allulose crystal
which can produce an allulose having uniform particle size by properly
controlling the crystal
particle growth, thereby reducing the loss in the recovery process and
increasing the crystallization
yield for the higher productivity.
In addition, the present invention is to provide a composition for
crystallizing an allulose
in which the content of allulose conversion material (Impurity-S) is
controlled in a specific range
of content, thereby increasing the crystallization yield with uniform particle
size and reducing the
loss in the recovery process.
[TECHNICAL SOLUTION]
The present invention relates to a composition for crystallization of allulose
for providing
uniform particle size and enhancing the crystallization yield by reducing the
loss in the recovery
process, in which the content of allulose conversion material (Impurity-S) is
controlled at the
specific content range, and a method for preparing an allulose crystal using
the same. In addition,
the present invention relates to a method for preparing an allulose crystal
for raising the
crystallization yield and increasing the particle size by controlling the
content of impurities or the
production of impurities contained in the solution for preparing the crystal.
Since the allulose is more unstable, as pH is lower and the temperature is
higher (FIG. 2,
FIG. 3), the content of allulose is changed in the actual production process,
particularly the
concentration step. This problem lowered the purity of high-purity allulose,
and thus, largely
affected the crystallization step. It was confirmed that the content of
allulose conversion material
(Impurity) produced additionally become higher, as the content of allulose was
decreased in this
process actually, and this component largely affected the crystallization of
allulose. The inventors
found that when the content of Impurity-S in the various allulose conversion
materials was
contained, this might act as an inhibitor for the growth of allulose crystal
particle, thereby largely
2
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
affecting the particle size of crystal particle and crystallization yield.
Therefore, the present invention may prevent to reduce the particle size of
allulose, and
provide the allulose having uniform particle size for performing the allulose
crystallization process,
by controlling the content of impurities (Impurity-S) to be specific content
or lower in the step
before and after the concentration following the high purity separation
process. In addition, the
growth of the particle with uniform particle size may reduce the loss in the
recovery process and
enhance the crystallization yield, thereby increasing the productivity.
In the allulose syrup which is a raw material used for the allulose
crystallization process,
various allulose conversion materials which are impurities other than allulose
produced in the
process of preparing the allulose may be included. Or, the allulose conversion
material may be
produced in the allulose crystallization process. By regulating (controlling)
the specific conversion
material (hereinafter, Impurity-S) in the conversion material lower than
specific amount, for
example, 2wt/wt% or lower, the shape, structure and size, crystal purity,
crystal production rate,
and crystallization yield of allulose crystal particle can be enhanced. The
Impurity-S acts as an
inhibitor preventing the growth of crystal particle of allulose, and thus
reduces the crystallization
yield. In the present invention, a method for increasing the particle size of
allulose crystal and the
yield can be increased by controlling the production process of allulose under
the condition that
allulose conversion material is not produced.
The allulose conversion material (Impurity-S) may be a material having 10 to
600 m/z, 10
to 550 m/z, 10 to 500 m/z, 10 to 450 m/z, 10 to 400 m/z, 20 to 600 m/z, 20 to
550 m/z, 20 to 500
m/z, 20 to 450 m/z, 20 to 400 m/z, 30 to 600 m/z, 30 to 550 m/z, 30 to 500
m/z, 30 to 450 m/z, 30
to 400 m/z, 40 to 600 m/z, 40 to 550 m/z, 40 to 500 m/z, 40 to 450 m/z, 40 to
400 m/z, 50 to 600
m/z, 50 to 550 m/z, 50 to 500 m/z, 50 to 450 m/z, or 50 to 400 m/z of the
ratio of mass/quantity of
electric charge measured by LC/MS analysis, or a material having the maximum
peak at the elution
time 31 2min measured by HPLC analysis. The LC/MS analysis is to analyze a
material obtained
by separating the material having the maximum peak at the elution time 31
2min time measured
by HPLC analysis.
In addition, the allulose conversion material (Impurity-S) may be modified
products of
allulose, polymers of the modified product of allulose, or an intermediate
substance produced or
conversed during the degradation of allulose. The lower limit of the molecular
weight of the
allulose conversion material (Impurity-S) may be 0.2 times or more, 0.3 times
or more, 0.4 times
3
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
or more, 0.5 times or more, 0.6 times or more, 0.7 times or more, 0.8 times or
more, 0.9 times or
more, 1 time or more, 1.1 times or more, 1.2 times or more, 1.3 times or more,
1.4 times or more,
1.5 times or more, 1.6 times or more, 1.7 times or more, 1.8 times or more,
1.9 times or more, or
twice or more than the molecular weight of allulose. The upper limit of the
molecular weight of
the allulose conversion material (Impurity-S) may be 10 times or less, 9 times
or less, 8 times or
less, 7 times or less, 6 times or less, 5 times or less, 4 times or less, 3
times or less, 2 times or less,
lower than 1.5 times, 1.5 times or less, 1.4 times or less , 1.3 times or
less, 1.2 times or less, 1.1
times or less, 1 time or less, 0.9 times or less, 0.8 times or less, 0.7 times
or less, 0.6 times or less,
0.5 times or less, 0.4 times or less, 0.3 times or less, or 0.2 times or less
than the molecular weight
.. of allulose. The allulose conversion material (Impurity-S) may have a
molecular weight in a range
set by a combination of the lower limit and the upper limit value. For
example, the molecular
weight of the allulose conversion material (Impurity-S) may modified products
of allulose,
polymer of modified product of allulose, or an intermediate substance produced
or conversed
during the degradation of allulose having a molecular weight of 0.4 times or
more to 10 times, 0.5
times or more to 10 times, 0.53 times or more to 10 times, 0.4times or more to
9times, 0.5times or
more to 9times, 0.53times or more to 9times, 0.4times or more to 8times,
0.5times or more to
8times, 0.53times or more to 8times, 0.4times or more to 7times, 0.4times or
more to 6times,
0.4times or more to 5times, 0.4times or more to 4times, 0.4times or more to
3times, 0.4times or
more to 2times, 0.4times or more to less than 1.5times, 0.4times or more to
1.5times or less,
0.4times or more to 1.4times, 0.4times or more to 1.3times, 0.4times or more
to 1.2times, 0.4times
or more to 1.1times, 0.4times or more to ltime, 0.4times or more to 0.9times,
0.4times or more to
0.8times, 0.4times or more to 0.7times, 0.4times or more to 0.6times, 0.4times
or more to 0.5times,
0.5times or more to 7times, 0.53times or more to 7times, 0.4times or more to
6times, 0.5times or
more to 6times, 0.5times or more to 5times, 0.5times or more to 4times,
0.5times or more to 3times,
.. 0.5times or more to 2times, 0.5times or more to less than 1.5times,
0.5times or more to 1.5times
or less, 0.5times or more to 1.4times, 0.5times or more to 1.3times, 0.5times
or more to 1.2times,
0.5times or more to 1.1times, 0.5times or more to ltimes, 0.5times or more to
0.9times, 0.5times
or more to 0.8times, 0.5times or more to 0.7times, 0.5times or more to
0.6times, 0.53times or more
to 6times, 0.4times or more to 5times, 0.5times or more to 5times, 0.53times
or more to 5times,
0.4times or more to 4times, 0.5times or more to 4times, 0.53times or more to
4times, 1.5times or
more to lOtimes, 2times or more to lOtimes, 2times or more to 4times of the
molecular weight of
4
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
allulose, and preferably, 0.4 times or more to 4 times of the molecular weight
of allulose.
In an embodiment, as the allulose conversion material (Impurity-S) is
continuously
exposed by external stress, for example, high temperature or acidic condition,
allulose denatured
polymer as the allulose conversion material (Impurity-S) may be converted into
the allulose
.. denatured polymer (tetramer analogue of allulose) having the molecular
weight similar to dimer.
This is because the allulose is easily denatured by external stress and thus,
the dehydration and
condensation reactions are randomly repeated with allulose or allulose
conversion material,
resulting from the mechanism that it is converted into the denatured polymer.
Alternatively, the
allulose conversion material (Impurity-S) may be an intermediate substance
produced or
conversed during the degradation of allulose.
Specifically, the component detected in the molecular weight of 341 m/z is the
component
with increased content, as the raw material of crystallization containing
allulose is treated severely
and the material of dimer-like structure of allulose denatured by dehydration
or condensation
reaction. As the result of inferring the structure by LC-MS analysis, it can
be predicted that the
material has the chemical formula of C12H22011, and is the allulose denatured
polymer. It was
confirmed that the content of allulose denatured polymers (tetramer analogues
of allulose) having
the molecular weight similar to dimer of allulose denatured polymer of
C25H28011, C24H42021
or C24H44022 was increased together with the material of dimer-like structure
of allulose, as the
thermal treatment was proceeded. This could be considered that the allulose
was easily denatured
by external stress, for example, thermal treatment, the dehydration and
condensation reactions
were randomly repeated with allulose or allulose conversion material, thereby
being converted into
the materials.
Specifically, as a result of an LC-MS analysis, the detected components in the
allulose
conversion material (Impurity-S) may contain an intermediate material (Furan
aldehyde
intermediate) produced during the decomposition of hexose such as allulose to
HMF by
dehydration reaction, [C61-11206 +Nal+ in which the Na + ion is bonded to the
allulose, or [C6111206
+Nal+ in which the Na + ion is bonded to the allulose dimer molecule.
In addition, as a result of inferring the chemical structure through LC-MS
analysis, the
allulose conversion material may contain a compound of molecular formula
CxHyOz, wherein x
may be an integer from 3 to 15, 3 to 14, 3 to 13, 3 to 12, 4 to 15, 4 to 14, 4
to 13, 4 to 12, 5 to 15,
5 to 14, 5 to 13, or 5 to 12, y may be an integer from 1 to 15, 1 to 14, 1 to
13, 1 to 12, 2 to 15, 2 to
5
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
14, 2 to 13, 2 to 12, 3 to 15, 3 to 13, 3 to 12, 4 to 15, 4 to 14, 4 to 13, or
4 to 12, and z may be an
integer from 1 to 10, 1 to 9, 1 to 8, 1 to 7, or 1 to 6.
For example, the allulose conversion material may contain a compound having
molecular
formula of C5H403, C5H604, C511803, C5H402, C5H1003, C611405, C6H1003,
C611404, C6H603,
C61180, C611405, C6H604, C611404, C611403, C611803, C1111806, C12111205, or
C12111005.
Specifically, the allulose conversion material may contain one or more kinds
of
compounds selected from the group consisting of levulinic acid (4-
oxopentanoic), furfural,
Hydroxymethylfurfural (HMF), y-Hydroxyvaleric acid (GVB), 2,5-Dimethylfuran,
2,5-
furandicarboxylic acid (FDCA), 5-hydroxymethy1-2-furoic acid, 2,5-
formylfurancarboxylic acid,
2,5-Furandicarbaldehyde, 2,5-bis-(hydroxymethyl)furan, bis(5-formy1-2-
furfuryl) ether, 2-Furoic
acid, 3-Furoic acid, 5-Hydroxyfurfural, 2,5-Dihydro-2,5-dimethoxyfuran, (2R)-5-
0xotetrahydro-
2-furancarboxylic acid, 2,5-formylfuran carboxylic acid, 5,5'-Methylenedi(2-
furoic acid), and
bis(5-methyl furfuryl) ether.
According to the present invention, it is to provide a method for removing or
reducing the
content of conversion material (Impurity-S) contained in the allulose syrup
that is a raw material
used for the allulose crystallization process, by performing the production,
separation and/or
purification process of allulose under the condition that the allulose
conversion material is not
produced. Accordingly, the crystal shape and crystallization yield can be
enhanced by reducing
the content of Impurity-S in the raw material of crystallization and lowering
the content of
impurities which prevents the crystal growth.
Specifically, the content of conversion material (Impurity-S) may be
controlled by a
method for preventing or reducing the production of Impurity-S, or removing or
reducing the
produced Impurity-S. In one embodiment of the present invention, as the method
for controlling
the production process of allulose under the condition that the production of
allulose conversion
material is inhibited or reduced, the allulose crystal particle size may be
increased and the crystal
can be formed in the shape close to quadrate, and the allulose yield can be
enhanced. More
specifically, the allulose crystal particle growth and yield can be enhanced,
when the content of
allulose conversion material (Impurity-S) component in the crystallization
undiluted solution as
2wt/wt% or lower.
The method for inhibiting or reducing the production of Impurity-S may be
achieved by
controlling the condition that the allulose conversion material is not
produced, particularly the
6
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
condition of production process of allulose such as control of pH,
temperature, conductivity
particularly in the concentration process. In addition, the method for
removing or reducing the
produced Impurity-S may use a method for performing an activated carbon
treatment or a method
of secondary crystallization by re-dissolving the crystal obtained in the
primary crystallization,
etc., and a method for removing impurities in the allulose syrup may be used.
Specifically, the method of controlling the production or content of
impurities may be
conducted with one or more methods among the following methods.
As one embodiment, one embodiment of the method for inhibiting or reducing the
production of allulose conversion material (Impurity-S) in the allulose
production process may be
a method for performing the allulose production process under pH 4 or higher
and/or 70 C or
lower temperature. Specifically, since it is relatively stable under the
condition of pH 4 to 7 or pH
4 to 6 and the temperature of 70 C or lower, preferably 60 C or less, it is
preferable to consistently
manage it not to be exposed by external stress, by managing the temperature of
reaction solution
not to be over 70 C, preferably over 60 C, in the allulose production process
like decoloring, ion
purification, high purity separation, etc., and particularly performing the
concentration process as
divided into 2 steps or more.
To inhibit or reduce the production of Impurity-S in the allulose production
process may
be performed for the allulose fraction obtained in SMB chromatography
separation process under
the temperature condition of 40 to 70 C or lower, and selectively, the
concentration process may
be conducted as divided into at least 2 steps or more. For example, when the
concentration process
is conducted as divided into 2 steps, the allulose syrup may be concentrated
to be 30 ¨ 50 Bx
concentration, and the primary concentrate may be secondarily concentrated to
be 60 ¨ 85 Bx
concentration again, and preferably an activated carbon treating process may
be further comprised
between the primary concentration process and the secondary concentration
process, thereby
removing the Impurity -S comprised in concentrates or reducing the content.
In another embodiment, the method for removing or reducing the content of
conversion
material (Impurity-S) comprised in the allulose syrup which is a raw material
for allulose
crystallization is to act as impurities by treating an activated carbon, or to
remove it by adsorbing
high molecular or small molecular organic matter, colored ionic material or
protein, etc., which
7
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
can induce the denaturation of allulose.
In detail, the content of conversion material (Impurity-S) may be removed or
reduced by
performing a process of treating an activated carbon before concentrating the
allulose fraction
obtained by conducting SMB chromatography separation process for the allulose
reaction solution
obtained from a substrate. The activated carbon process may be performed
additionally, after
performing an ion purification process of allulose fraction obtained in SMB
chromatography
separation process.
The activated carbon process may perform a solid-liquid separation process for
the
reaction solution comprising the activated carbon after contacting an
activated carbon to the
allulose solution and reacting them at the temperature of 40 to 50 C for 0.5
to 5 hours, thereby
collecting a remainder, and the impurities may be removed as filtration
residues. The filtration
may be carried out by using a filtration equipment like a filter press.
In the activated carbon reaction process, stirring may be carried out
selectively, and the
stirring rate of reaction solution may be 5 to 500rpm, preferably 50 to
300rpm. The stirring rate
may be properly selected in consideration of dispersion degree of activated
carbon and expense
cost for stirring. The contacting time of activated carbon and reaction
solution may be properly
selected in consideration of dispersion degree of activated carbon and
efficiency of removal of
impurities, etc., and for example, may be 0.5 to 5 hours, preferably 0.5 to 2
hours, and when the
contacting time is short, the removal of impurities, for example, decoloring
may be not sufficiently
achieved, and when the contacting time is long, destruction and browning of
major components
may be caused.
The activated carbon used for the activated carbon treatment process may be
derived from
Carboniferous system or Lignocellulosic system, and may remove the impurities
selectively
according to the pore diameter size of activated carbon.
As additional one embodiment, the method for inhibiting or reducing the
production of
allulose conversion material (Impurity-S) of allulose crystallization
composition is to carry out
recrystallization. The content of allulose conversion material (Impurity-S)
may be removed or
reduced in the primary crystallization process, by inputting it into the
secondary crystallization
process, after conducting the primary crystallization of allulose solution
passed through the high
purity separation and concentration processes and dissolving the allulose
crystal recovered by
8
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
removing the supernatant in the primary crystallization undiluted solution for
dehydration in water
again.
Accordingly, one embodiment of the present invention provides a method for
controlling
the content of allulose conversion material (Impurity-S) comprised in the
allulose composition for
crystallization as 2wt/wt% or less on the basis of content of solid of
composition.
The method may be performed by controlling one or more kinds of conditions
selected
from the group consisting of pH condition and temperature condition, and the
pH condition may
be in the range of pH 4 to 7, or the temperature condition may be 70 C or
lower.
The allulose composition for crystallization may be prepared by treating the
reaction
solution containing allulose by SMB chromatography separation process and
concentrating the
obtained allulose solution at the temperature condition of 40 to 70 C or
lower. The concentration
process may be performed as divided into at least 2 steps, and it may be
performing the primary
concentration of allulose solution to be 30-50Bx concentration, and the
secondary concentration
of the primary concentrate to be 60-85Bx concentration again. An activated
carbon treating
process may be further conducted before performing the concentration process.
Another embodiment of the present invention relates to a composition for
crystallizing an
allulose comprising the content of allulose conversion material (Impurity-S)
of 2wt/wt% or lower,
1.9wt/wt% or lower, 1.8wt/wt% or lower, 1.7wt/wt% or lower, 1.6wt/wt% or
lower, 1.5wt/wt% or
lower, 1.4wt/wt% or lower, 1.3wt/wt% or lower, 1.2wt/wt% or lower, 1.1wt/wt%
or lower,
1.0wt/wt% or lower, 0.9wt/wt% or lower, 0.8wt/wt% or lower, 0.7wt/wt% or
lower, 0.65wt/wt%
or lower, 0.6wt/wt% or lower, 0.5wt/wt% or lower, 0.4wt/wt% or lower,
0.3wt/wt% or lower,
0.2wt/wt% or lower, 0.1wt/wt% or lower, preferably 1.0wt/wt% or lower on the
basis of total solid
content of composition of 100wt/wt%, and it is more preferable not to include
impurities.
Preferably, the composition for crystallizing an allulose may comprise the
content of
allulose of 90 wt/wt% or more, 91 wt/wt% or more, 92 wt/wt% or more, 93 wt/wt%
or more, 94
wt/wt% or more, or 95 wt/wt% or more, on the basis of total solid content of
composition of
100wt/wt%.
The viscosity of allulose composition for crystallization may be 2 cps to 200
cps at the
temperature of composition of 45 C, and the conductivity may be 1,000uS/cm or
lower, for
9
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
example 0.01 to 1,000uS/cm, preferably 30 uS/cm or lower, for example 0.1 to
30 uS/cm. The
conductivity of composition for crystallizing the allulose is preferable as
lower. The conductivity
of allulose syrup is the value measured on the basis of content of solid of 30
Bx.
The allulose solution for crystallization may have the content of solid of 60
or more to 85
Bx or less, for example higher than 60 Bx to 80 Bx, 65 to 85 Bx, 65 to 80 Bx,
or 68 to 85 Bx.
One embodiment of the present invention relates to a method for preparing an
allulose
crystal by using the allulose solution for crystallization, and more
specifically, a method for
preparing an allulose crystal comprising a step of providing a composition for
crystallizing an
allulose comprising the content of allulose conversion material (Impurity-S)
of 2wt/wt% or less,
1.9wt/wt% or less, 1.8wt/wt% or less, 1.7wt/wt% or less, 1.6wt/wt% or less,
1.5wt/wt% or less,
1.4wt/wt% or less, 1.3wt/wt% or less, 1.2wt/wt% or less, 1.1wt/wt% or less,
1.0wt/wt% or less,
0.9wt/wt% or less, 0.8wt/wt% or less, 0.7wt/wt% or less, 0.65wt/wt% or less,
0.6wt/wt% or less,
0.5wt/wt% or less, 0.4wt/wt% or less, 0.3wt/wt% or less, 0.2wt/wt% or less,
0.1wt/wt% or less,
preferably 1.0wt/wt% or less on the basis of total content of solid of
composition of 100wt/wt%,
and a step of preparing an allulose crystal by cooling the allulose aqueous
solution.
In one specific embodiment of the present invention, the method for preparing
an allulose
crystal may comprise a step of secondary ion purification of allulose fraction
obtained in SMB
chromatography separation process, a step of concentrating the ion purified
allulose fraction, and
a step of obtaining an allulose crystal and allulose crystallization mother
liquor by crystallizing an
allulose from the concentrate, and selectively, may further comprise a
recovery process, washing
process and drying process of allulose crystal.
In addition, the content of allulose conversion material (Impurity-S) may be
reduced or
removed by treating the solution which the allulose fraction obtained in SMB
chromatography
separation process itself, or the solution of ion purifying the allulose
fraction, before the
concentration step, with an activated carbon. In addition, the content of
allulose conversion
material (Impurity-S) may be reduced or removed by performing the primary
crystallization after
concentrating the allulose solution for crystallization and performing the
secondary crystallization
by dissolving the obtained crystal.
In one specific embodiment of the present invention, the method for preparing
the allulose
crystal may comprise a step of the secondary ion purification of allulose
fraction obtained by
treating the allulose-containing reaction solution prepared from the substrate
by SMB
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
chromatography separation process and a step of concentrating the ion purified
allulose fraction,
or may comprise a process of ion purification treating the allulose fraction
obtained by treating by
SMB chromatography separation process, an activated carbon treating process,
or both of activated
carbon treating process and ion purification process.
The step of providing the allulose composition for crystallization relates to
a method for
preparing an allulose crystal, to comprise the content of allulose conversion
material (Impurity-S)
of 2wt/wt% or less, 1.9wt/wt% or less, 1.8wt/wt% or less, 1.7wt/wt% or less,
1.6wt/wt% or less,
1.5wt/wt% or less, 1.4wt/wt% or less, 1.3wt/wt% or less, 1.2wt/wt% or less,
1.1wt/wt% or less,
1.0wt/wt% or less, 0.9wt/wt% or less, 0.8wt/wt% or less, 0.7wt/wt% or less,
0.65wt/wt% or less,
0.6wt/wt% or less, 0.5wt/wt% or less, 0.4wt/wt% or less, 0.3wt/wt% or less,
0.2wt/wt% or less,
0.1wt/wt% or less, preferably 1.0wt/wt% or less, on the basis of total content
of solid comprised
in the composition for crystallization or not to comprise the allulose
conversion material (Impurity-
S).
The preparation method of allulose crystal according to the present invention
may have
the crystallization yield of allulose of 45% or more, preferably 48% or more,
50% or more, 53%
or more, 54% or more, more preferably 55% or more, 56% or more, 57% or more,
58% or more,
59% or more, or 60% or more.
The controlling the content of allulose conversion material may be performed
by
controlling one or more kinds selected from the group consisting of pH
condition and temperature
of allulose solution, and the pH control may be achieved in pH 4 to 7 range,
pH 4.5 to 7, or pH 5
to 7, preferably pH 5 to 7, and the temperature control may be achieved by
controlling it in the
range of 80 C or lower, 75 C or lower, 70 C or lower, preferably 30 to 70 C or
lower, 30 to 69 C,
to 65 C or 30 to 60 C.
Since the allulose is unstable as pH is low and the temperature is high, the
content of
25
allulose is changed in the concentration step in the actual production
process. This problem
lowered the purity of high purity of allulose, and therefore, largely affected
the crystallization step.
It was confirmed that the content of specific allulose conversion material
(Impurity) produced
additionally as the content of allulose was decreased in this process
actually, and this component
largely affected the crystallization of allulose. It was confirmed that when
the content of
30
component of Impurity-S in the various allulose conversion materials was over
2%, this might act
11
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
as major barrier factor for the growth of allulose crystal particle, and
thereby largely affect the
particle size of crystal particle and crystallization yield.
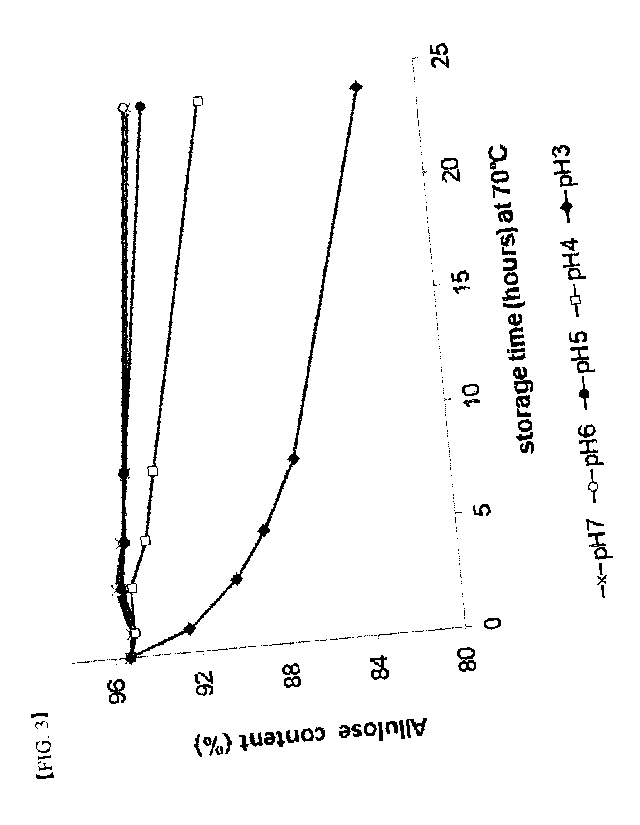
Specifically, as shown in FIG. 1 and FIG. 2, as the storage temperature was
higher, the
content of allulose was decreased and the content of allulose conversion
material (Impurity-S) was
increased. As shown in FIG. 3 and FIG. 4, as pH was lower at the temperature
of 70 C, the content
of allulose was decreased and the production of allulose conversion material
was increased.
The allulose composition for crystallization according to the present
invention may be a
reactant containing the allulose obtained by a biological or chemical method,
an allulose fraction
obtained by isolating the reactant with SMB chromatography, or a concentrate
concentrating the
allulose fraction. Before conducting the concentration process for preparing
the allulose
concentrate, ion purification and/or activated carbon treating process may be
further performed,
and the concentration process may be performed divided into at least 2 steps.
The reactant
containing the allulose may be obtained from a fructose substrate by a
biological or chemical
method, and preferably may be prepared by using an allulose converting enzyme
or a
microorganism producing the enzyme as the biological method.
For the allulose reaction solution, a separation process of allulose
conversion reactant
comprising ion purification and simulated moving bed (SMB) chromatograph
separation process.
In specific one embodiment, the allulose conversion reactant is separated as
allulose fraction
having higher allulose content than the converted reactant and fructose
raffinate by performing the
ion purification and SMB chromatograph separation process, and the allulose
fraction is input into
the crystallization process through the allulose concentration process.
The content of allulose in the allulose solution to collect the allulose
crystal should be
comprised at the high concentration as the supersaturated state, but as the
content of allulose of
allulose converted reactant was low, direct crystallization could not be
conducted, and to increase
the content of allulose before the crystallization step, the purification and
concentration process
by the desirable level should be carried out.
The method for obtaining the composition may perform the concentration process
at the
temperature of high purity allulose solution of 90 C or less, 85 C or less, 80
C or less, 75 C or
less, 70 C or less, lower than 70 C, for example, 40 to 70 C or less, and
specifically perform it by
using a thin film evaporator or multiple effect evaporator. In one specific
embodiment of the
12
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
present invention, the step of concentrating the purified allulose solution
may be performed at the
temperature condition of 40 to 70 C or less. When the temperature of
concentrate is higher than
70 C, the thermal denaturation of D-allulose could be caused, and thus, the
allulose conversion
material (Impurity-S) according to the present invention may be produced or
increased. In addition,
since the temperature of reactant was rapidly increased by heat of
vaporization as proceeding the
concentration, it should be rapidly concentrated as maintaining the
temperature of concentrate as
70 C or less.
Specifically, the concentration process of allulose fraction obtained in the
SMB
chromatography separation process may be performed by various methods, and it
may be carried
out so as that the content of solid of concentrate is 70 Brix or more. For
example, the allulose
fraction obtained by the simulated moving bed separation method (for example,
20-30 wt% of
solid content) may be concentrated by the solid content of 60 Brix or more by
the concentration
process. The composition for crystallizing the allulose according to the
present invention may have
the solid content of 60 to 85 Bx or less, for example, higher than 60 Bx to 85
Bx, 65 to 85 Bx, 70
to 85 Bx, 75 to 85 Bx, higher than 60 Bx to 83.5 Bx, 65 to 83.5 Bx, 70 to 83.5
Bx, or 75 to 83.5
Bx.
As one embodiment, the composition for crystallization may be an allulose
fraction
obtained by performing the simulated moving bed (SMB) chromatography
separation process by
using a column chromatograph filled with cation exchange resin in which
calcium active group is
attached, and specifically, may be an allulose fraction obtained by obtaining
the allulose converted
reactant which converted the fructose-containing raw material to allulose by
using a biological
catalyst and performing the activated carbon treating, ion purification and
simulated moving bed
(SMB) chromatography separation processes of allulose converted reactant. The
allulose fraction
may be obtained in the SMB chromatograph separation process itself or
collected by passing
through the ion purification process. The fructose content of fructose-
containing raw material is
85wt% or more on the basis of total content of solid of 100wt%, and it may use
a biological catalyst
having the allulose conversion rate of allulose conversion reaction of 15% to
70%.
For the allulose fraction obtained in the SMB chromatography separation
process, ion
purification and/or activated carbon treatment process may be further
conducted, before
13
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
performing the concentration process.
The method for preparing the allulose crystal according to the present
invention may
control the temperature and concentration of solution of allulose concentrates
to crystallizing it,
and specifically, the supersaturated condition required for crystallization
may be maintained by
reducing the temperature of allulose solution or changing the concentration of
D-allulose in the D-
allulose solution. In one specific embodiment of the present invention, the
crystallization progress
may be monitored by observing the sample collected at a constant interval in
the crystallization
step with the naked eye or microscope, or analyzing the concentration of sugar
in the supernatant
collected by centrifugation of sample, and according to the result, the
temperature or concentration
of D-allulose may be controlled. For preparing the allulose crystal, when the
solution of allulose
concentrates are cooled and crystallized, the crystal growth may be induced by
performing
temperature rising and cooling repeatedly, after rapidly cooling in the
temperature range of 10 to
25 C through a heat exchanger.
The method for preparing the allulose crystal according to the present
invention may
control the temperature and concentration of solution of allulose concentrates
to crystallizing it,
and specifically, the supersaturated condition required for crystallization
may be maintained by
reducing the temperature of allulose solution or changing the concentration of
D-allulose in the D-
allulose solution. The method for preparing the allulose crystal according to
the present invention
may be performed by various methods, preferably by a cooling method. One
embodiment of
cooling method according to the present invention may produce a crystal by
inducing a
supersaturated state by cooling the allulose solution by the temperature range
of 35 to 10 C. It is
better to maintain the cooling rate as 0.01 to 20 C/min, and when the cooling
rate is low, the time
for forming a crystal is long and thus the productivity may be low, and when
the cooling rate is
high, a small particle size of crystal is formed, and thus the recovery of
crystal may be difficult.
The preparation method of allulose crystal may comprise a step of producing a
crystalline
nucleus in an allulose solution comprising 90 wt/wt% or more allulose and
having 60 to 85 Brix
and 1,000 uS/cm or lower of conductivity, and a step of growing a crystal by
lowering the
temperature of the solution.
Specifically, the preparation method of allulose crystal may comprise a step
of producing
14
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
a crystalline nucleus by slowly stirring an allulose solution comprising 90
wt/wt% or more allulose
and having 60 to 85 Brix at the temperature of 20 to 40 C, or 30 to 40 C, for
example 35 C, and a
step of growing a crystal by lowering the temperature of the solution. The
method may be further
comprise a step of redissolving microcrystals produced during cooling by
increasing the
temperature of solution in the range of 30 to 35 C, one or more times. The
preparation method of
allulose crystal may further comprise a process of adding a seed. The seed
addition step and
redissolving step may be comprised in the preparation method of allulose
crystal respectively
selectively, or both steps may be comprised.
Generally, it is known that the bigger the size of allulose crystal is, the
better the properties
are and the more the use convenience is increased, and for preparing the big
size of crystal, a seed
crystal classified by a transferring process and a main crystallization
process should be performed
all, but the crystallization process according to the present invention can
easily prepare a relatively
big sized of crystal with high yield by only one step process.
In addition, the crystallization process may perform a process of dissolving a
microcrystal
by increasing the temperature range of 30 to 35 C of solution to redissolve
the microcrystal
formed in the cooling of the crystal growing process. In the crystallization
process according to
the present invention, the crystal growing process and microcrystal dissolving
process may be
repeated and carried out one or more times.
In the process for preparing the crystal, a seed may be further added in a
purpose of
increasing the crystal producing rate and size.
In specific one embodiment according to the present invention, the allulose
crystal may
be prepared by growing the crystal by cooling the temperature by 10 C as
decreasing the
temperature by 1 C per hour, after producing a small amount of crystal nucleus
by stirring at the
temperature of 35 C an allulose solution which comprises 90 wt/wt% or more of
allulose and has
60 to 85 Brix of total solid content, and optionally, by further including a
step of dissolving the
microcrystal by increasing the temperature of solution by 30 to 35 C for
redissolving the
microcrystal produced during the cooling at least one or more time, to prepare
the allulose crystal.
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
The method for preparing the allulose crystal according to the present
invention may
further comprise a step of recovering the allulose crystal collected in the
crystallization step with
various solid-liquid separation methods, for example, centrifugation, a step
of washing with
deionized water, and a step of drying. The drying step may be performed in a
fluidized bed drier
or vacuum drier, but not limited thereto.
An allulose crystal can be prepared by the method of cooling the allulose
composition for
crystallization according to the present invention. The allulose composition
for crystallization is
same as aforementioned.
The allulose comprised in the allulose crystal may be 94 wt/wt% or more, 95
wt/wt% or
more, 96 wt/wt% or more, 97 wt/wt% or more, 98 wt/wt% or more, or 99 wt/wt% or
more, based
on 100 wt/wt% of total solid content.
Herein, "purity of crystal" means the purity of allulose crystal. The
properties including
the purity of crystal of the present invention may be obtained by methods such
as for example, X-
ray powder diffraction analysis, differential scanning calorimetry (DSC)
analysis, infrared
spectroscopic (FTIR) analysis, HPLC analysis, LC/MS analysis, etc., and the
purity may be
specifically analyzed by HPLC chromatography.
The allulose crystal according to one embodiment of the present invention may
be an
allulose crystal having an X-ray spectroscopy which has a peak at angles of
diffraction (20) of
15.24, 18.78, and 30.84 0.2 in the X-ray spectroscopy. In one embodiment of
the present
invention, the X-ray spectroscopy may be the an allulose crystal having an X-
ray spectroscopy
which has a peak at angles of diffraction (20) of 15.24, 18.78, 30.84 and
28.37 0.2 , angles of
diffraction (20) of 15.24, 18.78, 30.84 and 31.87 0.2 , or angles of
diffraction (20) of 15.24,
18.78, 30.84 and 47.06. The angles of diffraction having the peak in the X-ray
spectroscopy of the
allulose crystal are the result of X-ray diffraction analysis by selecting and
representing the upper
(Relative Intensity %) major peaks and morphology specific peaks.
The allulose crystal according to the present invention may be obtained by
various
crystallization methods, but the characteristics may be measured with allulose
crystals prepared
by a cooling method.
The allulose crystal according to the present invention may have the Tm
temperature of
125.8 C 5 C or enthalpy of melting (AH) of 200 to 220J/g, according to DSC
analysis, and the
16
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
Tm may be 125.8 C 3 C. The differential scanning calorimetry analysis (DSC)
is operated
according to the temperature gradient, and it measures the energy provided to
maintain the
temperature increase of allulose powder sample. It could be predicted that the
higher the thermal
capacity is in the DSC analysis of crystal, the more difficult it is easily
dissolved, and the higher
the thermal capacity and the more narrow the width of endothermic peak are,
the crystal is formed
more homogeneous and the harder.
Another embodiment of the present invention is an allulose crystal prepared
with the
composition for allulose crystallization, and may be an allulose having one or
more kinds of
characteristics selected from the group consisting of the following (1) to
(5):
(1) an X-ray spectroscopy which has a peak at angles of diffraction (20) of
15.24, 18.78,
and 30.84 0.2 in the X-ray spectroscopy,
(2) a Tm temperature of 125.8 C 5 C according to a differential scanning
calorimetry
analysis (DSC),
(3) an enthalpy of melting (LH) of 200 to 220 J/g according to a differential
scanning
calorimetry analysis,
(4) a mean long diameter of 350pm or more, preferably 350 to 2,000 pm, and
(5) a ratio of long diameter length (micrometer) to short diameter of the
crystal (=long
diameter/short diameter) in the range of 1.0 to 8Ø
The allulose crystal according to the present invention, the mean short
diameter (minor
diameter) of crystal may be 50 to 1,000pm, preferably 50 to 500pm, and the
mean long diameter
(major diameter) may be 350pm or more, preferably 350 to 2,000 pm, more
preferably 400 pm or
more to 2,000 pm.
In addition, the ratio of long diameter length (micrometer) to short diameter
of the crystal
(=long diameter/short diameter) of allulose crystal according to the present
invention may be 1.0
to 8.0, 1.0 to 6.9, 1.0 to 6.0, 1.0 to 5.5, 1.0 to 5.0, 1.1 to 8.0, 1.1 to
6.9, 1.1 to 6.0, 1.1 to 5.5, 1.1 to
5.0, 1.3 to 8.0, 1.3 to 6.9, 1.3 to 6.0, 1.3 to 5.5, 1.3 to 5.0, 1.5 to 8.0,
1.1 to 6.9, 1.5 to 6.0, 1.5 to
5.5, 1.5 to 5.0, 2.0 to 8.0, 2.0 to 6.9, 2.0 to 6.0, 2.0 to 5.5, 2.0 to 5Ø
By the result of XRD pattern analysis of powder of allulose crystal according
to the present
17
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
invention, the allulose crystal according to the present invention is a pure
crystal particle, and has
a structure of rectangle hexahedron or close thereto. It is more preferable,
since the uniformity and
solidity of crystal is increased, as the crystal structure of the present
invention closes to a cubic
system.
In addition, as the crystal prepared in the crystallization process of
allulose is
homogeneous, the strength of crystal is increased and particle breakage is
minimized, and thereby
the particle-size distribution becomes homogeneous, and therefore the
flowability may be
enhanced. On the other hand, when the uniformity is low, it may be micronized
by breakage of
crystal particles at the drying and transferring stages, and may be relatively
easily dissolved, and
thus it affects the quality of product negatively.
The allulose crystal of the present invention has better flowability than
micronized powder,
and is stable during storage because it is not likely to be caked, and has the
characteristic in that
distribution and treatment are easy. In addition, the allulose powder has
lower calories than sugar,
and the sweetness is similar to sugar, and thus it may be used for easily and
advantageously carry
out the preparation of mixed sweeteners, solid mixed sweeteners, chocolate,
chewing gum, instant
juice, instant soup, granules, tablets, etc. Furthermore, the allulose crystal
powder may be used by
comprised in various kinds of compositions such as food and beverages,
favorite dainty, feed,
cosmetics, drugs, etc.
[EFFECT OF THE INVENTION]
The method for preparing an allulose crystal according to the present
invention can
prevent reducing the particle size of allulose by controlling the content of
allulose conversion
material (Impurity-S) content comprised in a solution for preparing the
crystal and can produce an
allulose having uniform particle size by properly controlling the crystal
particle growth. In addition,
by growing the particle as homogeneous size, the loss in the recovery process
can be reduced and
the crystallization yield can be enhanced, thereby increasing the
productivity.
[BRIEF DESCRIPTION OF DRAWINGS]
FIG. 1 is a graph showing the change of content of allulose, when the allulose
syrup of
pH 5 and 70 Brix concentration was stored by temperature.
FIG. 2 is a graph showing the change of content of allulose conversion
material, when the
allulose syrup of pH 5 and 70 Brix concentration was stored by temperature.
18
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
FIG. 3 is a graph showing the change of content of allulose, when the allulose
syrup of
different pH and 70 Brix concentration was stored at the temperature of 70 C.
FIG. 4 is a graph showing the change of content of allulose conversion
material, when the
allulose syrup of different pH and 70 Brix concentration was stored at the
temperature of 70 C.
FIG. 5 is an optical microscopic photograph of allulose powder obtained in
Example 5
measured at magnification X100.
FIG. 6 is a scanning electron microscope (SEM) photograph of allulose powder
obtained
in Example 5 measured at magnification X50.
FIG. 7 is an infrared spectroscopy (IR) spectrum of allulose crystal obtained
in Example
5.
[DETAILED DESCRIPTION OF THE EMBODIMENTS]
The present invention will be described in more detail by the following
examples.
However, the following examples are desirable examples of the present
invention, and the present
invention is not limited thereto.
Example 1: Allulose crystal preparation
The allulose syrup was prepared from the fructose substrate with the
substantially same
biological method as the preparation method disclosed in Korean laid-open
patent application no.
2014-0054997. After desalting the allulose syrup by passing through the column
at the room
temperature filled with the cation exchange resin, anion exchange resin and
resin mixed of cation
and anion exchange resins at the rate of 2 times (1-2 times) ion exchange
resin volume per hour
to remove impurities like colored and ion components, etc., the high purity of
allulose solution
was separately collected by using the chromatography filled with the Ca2+ type
of ion exchange
resin.
The high-purity syrup of allulose containing 97wt/wt% of allulose with 35 Bx
(w/w%)
was obtained through the high purity separation process (SMB) and
concentrated, thereby
preparing the allulose syrup for crystallization containing 97wt/wt% of
allulose with 81 Bx (w/w%)
and the conductivity of 12 uS/cm. The conductivity of allulose syrup was the
value measured on
the basis of the solid content of 30 Bx.
19
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
The concentrated allulose syrup for crystallization was cooled slowly from the
temperature of 35 C of the supersaturated state to the temperature of 10 C, to
grow the crystal. At
this time, the process of adding the allulose seed, and after producing a
small amount of crystal
nucleus by slowly stirring at the temperature of 35 C, growing the crystal by
decreasing the
.. temperature by 1 C per hour, and dissolving the microcrystal by increasing
the temperature of
solution to the range of 30 to 35 C for redissolving the microcrystal produced
in the cooling of
the crystal growing process. The crystal growing process and microcrystal
dissolving process were
repeated at least one or more times to perform the crystallization. The
allulose crystal produced
herein was recovered by drying after removing the mother liquor by centrifuged
dehydration and
washing the crystal obtained by the primary crystallization with cooling
water.
The content of allulose and content of allulose conversion material (Impurity-
S) of raw
material for crystallization, and the purity of allulose crystal were analyzed
under the following
analysis conditions.
Analysis column: Biolad Aminex HPX-87C column
Mobile phase: water
Flow rate: 0.6m1/min
Column temperature: 80 C
Detector: RI detector
As the result of HLPC analysis, the content of allulose conversion material
(Impurity S)
in aqueous allulose solution for crystallization was 0.4 wt/wt%, and the
content of allulose was
97.0 wt/wt%.
The yield of allulose crystal prepared by the method was 63.6%. The yield was
represented
as a percentage of the weight of recovered allulose crystal powder to the
weight of solid of raw
material allulose syrup for crystallization.
Examples 2 and 3: Allulose crystal preparation
By performing the substantially same method as the allulose preparation of
Example 1,
the high-purity syrup of allulose comprising 96.6 wt/wt% of allulose was
obtained at the
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
concentration of 35 Bx (w/w%) in Example 2, the high-purity syrup of allulose
comprising 95.8
wt/wt% of allulose was concentrated to obtaine 35 Bx (w/w%) of syrup in
Example 3, and the
high-purity syrup of allulose comprising 95.5 wt/wt% of allulose was
concentrated to obtain35 Bx
(w/w%) of syrup in Example 6. By concentrating the allulose solutions, Example
2 prepared the
allulose syrup of 81 Bx (w/w%) for crystallization including 96.6 wt/wt%
allulose with 81 Bx
(w/w%) and the conductivity of 14 uS/cm, Example 3 prepared the allulose syrup
of 81 Bx (w/w%)
for crystallization including 95.8 wt/wt% allulose with 81 Bx (w/w%) and the
conductivity of 14
uS/cm, and in Example 6, the allulose syrup for crystallization including 95.5
wt/wt% allulose
with 81 Bx (w/w%) and the conductivity of 12 uS/cm was prepared. The
conductivities of the
allulose syrup were values measured on a solid content basis of 30 Bx.
According to the same crystallization method as Example 1, the concentrated
allulose
syrup was crystallized and the crystals were washed with cooling water, and
dried to recover the
crystals.
According to the same method as Example 1, The content of allulose and content
of
allulose conversion material (Impurity-S) of raw material for crystallization,
and the purity of
allulose crystal were analyzed and the result was shown in the following Table
1.
Specifically, the yield of the allulose crystals prepared from the allulose
syrup for
crystallization in Example 2 (the content of the allulose conversion material
of 0.3 wt/wt%, and
the content of the allulose of 96.6wt/wt%) was 61.9%, the yield of the
allulose crystals prepared
from the allulose syrup for crystallization in Example 3 (the content of the
allulose conversion
material of 0.5 wt/wt%, and the content of the allulose of 95.8wt/wt%) was
61.6%, and the yield
of the allulose crystals prepared from the allulose syrup for crystallization
in Example 6 (the
content of the allulose conversion material of 0.25 wt/wt%, and the content of
the allulose of
95.5wt/wt%) was 62.1%.
Example 4: Allulose crystal preparation
The high purity alluose syrup including 97.0 wt/wt% allulose was obtained at
the
concentration of 35 Bx (w/w%) by the high purity separation process (SMB) by
conducting the
substantially same method as the allulose preparation of Example 1.
To minimize the impurities contained in the allulose syrup, it was treated by
using the
21
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
appropriate activated carbon at the temperature of 40 C for 30 min, and then
filtered. The allulose
syrup after treating the activated carbon was concentrated to be 81 Bx (w/w%),
thereby preparing
the allulose syrup for crystallization including 97.3 wt/wt% of allulose with
81 Bx (w/w%) and the
conductivity of 10 uS/cm.
The concentrated allulose syrup was cooled slowly from the temperature of 35 C
at the
supersaturated state to the temperature of 10 C, to grow the crystal. Then,
the process of adding
the allulose seed, and after producing a small amount of crystal nucleus by
slowly stirring at the
temperature of 35 C, growing the crystal by decreasing the temperature by 1 C
per hour, and
dissolving the microcrystal by increasing the temperature of solution to the
ranges of 30-35 C to
redissolve the microcrystal produced in the cooling in the crystal growing
process was carried out.
The crystal growing process and microcrystal dissolving process were repeated
at least one or
more times to perform the crystallization. The allulose crystal produced
herein was recovered by
drying after removing the mother liquor by centrifuged dehydration and washing
the crystal
obtained by the primary crystallization with cooling water.
Example 5: Allulose crystal preparation
The high purity alluose syrup including 97.0 wt/wt% allulose was concentrated
to obtain
the concentration of 35 Bx (w/w%) by the high purity separation process (SMB)
by conducting
the substantially same method as the allulose preparation of Example 1.
The allulose syrup was concentrated and the high purity allulose syrup
including 97 wt/wt%
allulose based on the solid content of 100 wt/wt% was concentrated at the
concentration of 81 Bx
(w/w%), thereby preparing the allulose syrup for crystallization having the
conductivity of 8 uS/cm.
According to the same crystallization method as Example 1, the concentrated
allulose syrup was
crystallized and the crystals were washed with cooling water, and dried to
recover the crystals.
The obtained primary crystal was dissolved in water, thereby preparing the
allulose
dissolved solution of 81.2 Bx, and as the result of analyzing the allulose
dissolved solution by the
HPLC analysis of Example 1, the content of allulose conversion material
(Impurity S) was 0.07
wt/wt% and the content of allulose was 99.5 wt/wt%.
22
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
The secondary crystallization process was performed with the prepared allulose
dissolved
solution as a raw material of secondary crystallization process by
substantially same method as the
primary crystallization method. The secondary crystal prepared herein was
recovered by drying
after removing the mother liquor by centrifuged dehydration and washing the
crystal obtained by
the secondary crystallization with cooling water. The yield of secondary
crystal was 62.5%.
[Table 1]
Classification Content of allulose of Content of Impurity-S of Crystallization
crystallization undiluted crystallization undiluted yield (%)
solution (%) solution (%)
Example 1 97.0 0.4 63.6
Example 2 96.6 0.3 61.9
Example 3 95.8 0.5 61.6
Example 4 97.3 0.2 62.0
Example 5 99.5 0.07 62.5
Example 6 95.5 0.25 62.1
As shown in the Table 1, it was confirmed that the allulose crystallization
yields of
Examples 1 to 3 were over 60%, on the other hand, Comparative Example 1 could
not obtain
proper crystal when the content of allulose conversion material (Impurity-S)
was more than 2
wt/wt%, although the allulose content of crystallization undiluted solution
was high, and the
crystallization yield was drastically decreased due to small crystals.
Comparative Example 1: Allulose crystal preparation when the content of the
allulose conversion material exceeds 2wt/wt%
In order to determine the yield of the allulose crystals when the content of
the allulose
conversion material in the crystallization solution was more than 2wt/wt%,
acidic pH condition or
heat treatment condition was applied to the crystallization solution for
secondary crystallization in
Example 5 to trigger the formation of the allulose conversion material.
Specifically, the starting material for secondary crystallization was prepared
by dissolving
the allulose crystal obtained by performing the primary crystallization in
Example 5 in water, and
then be heat-treated at the conditions of pH 3.5 and the temperature of 80 C
for 3 hours. According
to the same crystallization method as Example 1, the concentrated allulose
syrup was crystallized
and the crystals were washed with cooling water, and dried to recover the
crystals.
23
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
According to the same method as Example 1, the content of allulose and content
of
allulose conversion material (Impurity-S) of raw material for crystallization,
and the purity of
allulose crystal were analyzed and the result was shown in the following Table
2.
Comparative Examples 2 and Example 7 to 8: Attulose crystal preparation method
by the content of allulose conversion material
The starting material for secondary crystallization was prepared by dissolving
the allulose
crystal obtained by performing the primary crystallization in Example 5 in
water, and then be
treated at the conditions of pH 4.5 and the temperature of 70 C for 6, 13 or
24 hours. The content
of allulose and content of allulose conversion material (Impurity-S) of raw
material for
crystallization were shown in Table 2.
The method of proceeding crystallization using the prepared crystallization
solution was
carried out in substantially the same method as in Example 1. Specifically, in
Comparative
Example 2 and Examples 7 to 8, the allulose crystallization processes were
performed using
different allulose crystallization solution with different heat treatment
time, having different
allulose content and the allulose conversion material shown in Table 2.
According to the same method as Example 1, the content of allulose and content
of
allulose conversion material (Impurity-S) of raw material for crystallization,
and the purity of
allulose crystal were analyzed and the result was shown in the following Table
2.
[Table 2]
Classification Content of allulose of Content of Impurity-S of
Crystallization
crystallization undiluted crystallization
undiluted yield (%)
solution (%) solution (%)
Comparative Example 1 97.0 2.1 42.4
Example 7 98.5 0.65 58.8
Example 8 96.2 1.50 53.1
Comparative Example 2 93.3 3.40 29.1
In case of Comparative Example 2, since the purity of allulose was low and the
content of
allulose conversion material was high, the growth of crystal particles was not
gone well and
microcrystals were produced, and thus dehydration and washing of crystals were
very difficult. In
24
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
case of Comparative Example 1, it was confirmed that the growth of crystal
particle size was not
gone well and microcrystals were produced as the content of allulose
conversion material was
higher, as same as Comparative Examples 2. In case of Example 7 and Example 8,
it was confirmed
that the content of the allulose conversion material was lower than 2wt/wt%,
indicating a high
crystal yield compared to that of the comparative example.
Experimental Example 1:
LC-MS analysis of allulose conversion material (Impurity S)
(1) Analysis of allulose conversion material (Impurity S) of Example 2
The impurities fraction isolated in the peak at the elution time 31 2 min was
directly
collected in HPLC analysis of allulose syrup for crystallization used in
Example 2, and the solution
diluted during the separation fraction was freeze-dried and concentrated at
approximately 100
times concentration used for the analysis. The molecular weight of allulose
conversion material
(Impurity S) measured by LC/MS analysis by conducting the analysis of
molecular weight of
impurities with the liquid chromatograph/mass analyzer (LC/MS system, model
name: LTQ,
manufacturer: Thermo Finnigan, USA) was the material having the range of 300
to 400 m/z (ratio
of mass/quantity of electric charge).
(2) Analysis of allulose conversion material (Impurity S) of Comparative
Examples
2 and Examples 7 to 8
According to the substantially same as the LC-MS analysis method, the thermal
treated
crystallization undiluted solutions used in Comparative Examples 2 and
Examples 7 to 8 was used.
For the changes of molecular weight of allulose and allulose conversion
material according to the
thermal treatment, LC-MS analysis was conducted, and the result of analysis of
content of allulose
and content of allulose conversion material (%) comprised in the
crystallization undiluted solutions
used in Comparative Examples 2 and Examples 7 to 8 was shown in the following
Table 3.
The following Table 3 is data of LC-MS analysis of allulose syrup by thermal
treatment
time (Example 7, Example 8, Comparative Examples 2), and the numerical values,
which the
values of area of peak detected by each molecular weight (m/z) were converted
into a percentage,
.. were shown in the table. The molecular weight of 179.1 m/z in Row 1 of
Table 3 was allulose.
Rows 4, 8 and 10 in the following Table 3 represented that the content of
allulose conversion
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
material (Impurity S) was increased after the thermal treatment, and the other
Rows represented
that the content of allulose conversion material (Impurity S) was decreased
after the thermal
treatment.
[Table 3]
Row m/z Example 5 Example 7 Example 8 Comparative Formula Finder
Example 2 Result
1 179.1 56.68 38.53 31.65 24.75 C6H1206
2 225.1 9.27 6.26 5.12 4.26 C7H1408
3 251.1 0.36 1.05 1.09 1.18 C9H1608
4 341.1 6.03 16.68 18.99 19.71 C12H22011
359.1 21.31 14.66 11.50 8.42 C12H24012
6 387.1 1.37 3.44 3.91 4.76 C13H24013
7 485.1 0.04 0.06 0.17 0.61 C18H30015
8 503.2 0.44 2.11 5.53 9.89 C25H28011
9 665.2 0.06 0.16 0.60 2.03 C24H42021
683.2 4.13 16.01 18.50 18.51 C24H44022
11 711.2 0.02 0.02 0.15 0.56 C25H44023
12 827.3 0.01 0.02 0.06 0.35 C37H48021
13 845.3 0.09 0.60 1.37 1.92 C30H54027
14 1007.3 0.01 0.13 0.90 2.31 C36H64032
5
As shown in the analysis result, it was confirmed that the content of allulose
was lowered
and the content of impurities was raised in the molecular weight analysis as
the thermal treatment
time was increased. The molecular weight of 179.1 m/z in Row 1 of the Table 3
was allulose, and
it was confirmed that the numerical value of peak area value was decreased
after the thermal
10 treatment. On the other hand, it could be confirmed that the component
detected in the molecular
weight of 341 m/z (Table Row 5) was the component increased as thermal
treating the
crystallization undiluted solution containing allulose, and the material of
Dimer-like structure that
allulose was modified by dehydration or condensation reaction. As the result
of inferring the
structure by LC-MS analysis, it could be predicted that it was the material
having the chemical
formula of Cl2H22011, and the allulose denatured polymer. It was confirmed
that the content of
allulose denatured polymers (tetramer analogues of allulose) having the
molecular weight similar
to dimer of allulose denatured polymer of C25H28011, C24H42021 or C24H44022 as
the
thermal treatment was proceeded additionally. This could be considered that
the allulose was easily
denatured by external stress, for example, acidic pH and/or thermal treatment
in Comparative
Examples 1 or 2 and the dehydration and condensation reactions were randomly
repeated with
26
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
allulose or allulose conversion material, thereby being converted into the
above materials.
(3) Analysis of the allulose conversion material of Example 3
In the HPLC analysis of the allulose syrup for crystallization used in Example
3, the
separated impurity fraction was directly obtained at a peak of the elution
time of 31 +/- 2 minutes,
and the fraction diluted during the HPLC analysis was lyophilized and
concentrated about 100
times, and used for analysis.
- Name of analyzer: Ultimate-3000 ISQ EC (Thermo Fisher)
- Analytical column: Bio-rad Aminex HPX-87C
- Column temperature: 80 C
- Flow rate: 0.3mL/min
- Solvent: distilled water
- Injectino volume: 5p2
As a result of LC/MS analysis of the allulose conversion material, there were
peaks near
at 55.22 m/z, 60.24 m/z, 74.14 m/z, 79.25 m/z, 82.22 m/z, 83.23 m/z, 109 m/z,
117 m/z, 124.26m/z,
127.1m/z, 141.5m/z, 144 m/z, 163.23 m/z, 203.16 m/z, and 365.16 m/z, and the
main peaks were
near at 127m/z, 163m/z, 198.2 to 203m/z, and 365m/z.
Therefore, the allulose conversion material is a derivative material from
allulose which is
a molecule composed of C, H, and 0 having 5 to 12 carbon atoms (C), has a
molecular weight
charge value of 50 m/z or more to 400 m/z or less, contains HMF and levulinic
acid components,
and includes a derivative material containing a furan structure. Specifically,
in the case of the 163
m/z peak, it is considered that an intermediate substance (Furan aldehyde
intermediate) from the
process in which hexose such as allulose is decomposes to HMF by dehydration
reaction. In the
case of a peak of 198.2 to 203 m/z, it is considered to be a [C6141206 +Nal+
molecule in which a
Na + ion is bonded to an allulose molecule, and in the case of 365m/z peak, it
is considered to be
[C6111206 +Nal+ molecule in which a Na + ion is bonded to an allulose dimer
molecule.
Based on the LC/MS analysis results of the allulose conversion material, the
compounds
included in the allulose conversion material are shown in Table 4 below.
27
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
[Table 4]
name Formula molecular weight
(g/mol)
levulinic acid(4-oxopentanoic ) C5 H8 03 116.1
furfural C5 H4 02 96.08
HMF C6 H6 03 126.1
y-Hydroxyvaleric acid GVB C5 H10 03 118.13
2,5-Dimethylfuran C6 H8 0 96.13
2,5-Furandicarboxylic acid C6 H4 05 156.09
5-hydroxymethy1-2-furoic acid C6 H6 04 142.1
2,5-formylfurancarboxylic acid C6 H4 04 140
2,5-Furandicarbaldehyde C6 H4 03 124
2,5-bis-(hydroxymethyl)furan C6 H8 03 128
bis(5-formy1-2-furfuryl) ether C12 H10 05 234
2,5-Furandicarboxylic acid C6 H4 05 156
2-Furoic acid C5 H4 03 112
5-Hydroxyfurfural C5 H4 03 112
3-Furoic acid C5 H4 03 112
2,5-Dihydro-2,5-dimethoxyfuran C6 H10 03(C6H1203) 130 (132)
(2R)-5-0xotetrahydro-2-furancarboxylic acid C5 H6 04(C6H804) 130 (132)
2,5-formylfurancarboxylic acid(140) C6 H4 04 138
5,5'-Methylenedi(2-furoic acid) (dimer form) C11 H8 06 236 (234)
bis(5-methyl furfuryl) ether (-OH form) C12 H12 05 236 (234)
(4) LC/MS analysis of 5-HMF
LC/MS analysis of 5-HMF was performed to confirm that 5-HMF was included in
the
alululose conversion material.
As a 5-HMF analysis sample, a standard substance (SIGMA-ALDRICH, CAS Number
67-47-0) was purchased and used.
As a result, the molecular weight m/z values of the structures which can be
generated from
5-HMF by charge transfer, elimination and dehydration in aqueous solution
state were measured,
28
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
having the peaks at 79.09 m/z, 109m/z, 124.22 m/z, 127m/z, 144.15 m/z etc,
which are partially
identical to the result of LC/MS analysis result of the allulose conversion
material, iindicating that
5-HMF was contained in the allulose conversion material.
Experimental Example 2: Allulose stability analysis
In order to test the effect by temperature of allulose and allulose conversion
material, the
allulose syrup including 97 wt/wt% of allulose of Example 1 was divided and
placed in the same
amount of 30g each and stored in constant-temperature water baths of different
temperatures each
other, and sampled by time, thereby analyzing the content changes, and the
result was shown in
FIG. land FIG. 2.
FIG. 1 is the graph showing the content changes of allulose, when the 70% Brix
concentration of allulose syrup of pH 5 was stored by temperature. FIG. 2 is
the graph showing
the content changes of allulose conversion material, when the 70% Brix
concentration of allulose
syrup of pH 5 was stored by temperature. As shown in FIG. 1 and FIG. 2, as the
storage
temperature was higher, the content of allulose was decreased and the content
of allulose
conversion material (Impurity-S) was increased.
In addition, to test the effect according to pH of allulose and allulose
conversion material,
after controlling the syrup of 97.0% allulose content of Example 1 at
respectively different pH by
using caustic soda and hydrochloric acid solution, it was stored at the same
temperature (70 C)
and sampled by time, thereby analyzing the content changes, and the result was
shown in FIG. 3
and FIG. 4.
FIG. 3 is the graph showing the content changes of allulose, when the 70% Brix
concentration of allulose syrup of different pH was stored at the temperature
of 70 C. FIG. 4 is
the graph showing the content changes of allulose conversion material, when
the 70% Brix
concentration of allulose syrup of different pH was stored at the temperature
of 70 C. As shown
in FIG. 3 and FIG. 4, as the pH was lower at the temperature of 70 C, the
content of allulose was
decreased and the content of allulose conversion material (Impurity-S) was
increased.
Accordingly, since the allulose was unstable as pH was lower and temperature
was higher,
the content of allulose was changed in the actual production process,
particularly concentration
29
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
step. This problem lowered the purity of high purity of allulose, and
therefore, largely affected the
crystallization step. It was confirmed that the content of specific allulose
conversion material
(Impurity) produced additionally as the content of allulose was decreased in
this process actually,
and this component largely affected the crystallization of allulose. It was
confirmed that when the
content of component of Impurity-S in the various allulose conversion
materials was over 2%, this
might act as major barrier factor for the growth of allulose crystal particle,
and thereby largely
affect the particle size of crystal particle and crystallization yield.
Example 3: Analysis of allulose crystal characteristics
(1): Analysis of crystal particle size distribution
The particle size distribution of allulose crystal obtained in Example 5 was
confirmed by
using standard sieves by Mesh. The Mesh sizes of standard sieves were 20, 30,
40, 60, 80, 100mesh,
and the size distribution of crystal particle was measured by the hole-sizes
of standard sieves.
The hole-sizes of standard sieves by each mesh were 850, 600, 425, 250, 180,
and 150pm.
100g of each sample was collected and put in standard sieves by mesh size, and
passed through
the standard sieves by adding vibration. The percentage values were described
in Table 5 by
measuring the weight of samples remained in sieves by each mesh size. In the
following Table 5,
the particle size distribution by each mesh was represented by wt% of particle
with numerical
values.
[Table 51
Mesh size (mesh) 100 mesh pass 100meshi 80mesh T 60 meshi 40 meshi 30 meshi
20 meshi
Particle size(ym) =150 150< 180< 250< 425< 600< 850<
Example 5 0.9 2.6 5.9 20.2 70.0 0.4 0
As shown in the Table 5, it was confirmed that the allulose crystal of Example
5 exhibited
very narrow distribution converging into 90.2 wt% of the particle
distribution, and the allulose
crystal of Example 3 exhibited the most distribution in 401, but the particle
distribution was widely
spread as evenly distributed in 801, 601, 401, and 301. It was confirmed that
the hard crystal
particle having low ratio of long diameter/short diameter as Example 5 had
relatively low content
of micronized products and uniform distribution of particle size. In addition,
the particle having
high ratio of long diameter/short diameter and low homogeneity may be
micronized by particle
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
breakage in the drying and transferring processes and the particle size may be
heterogeneous,
thereby having the wide range of particle size distribution.
(2) Analysis of crystal form and crystal particle size
The optical microscopic photographs of allulose crystals obtained in Example 5
measured
by magnification X100 were shown in FIG. 5. The scanning microscopic
photograph(SEM) of
allulose crystals obtained in Example 5 measured by magnification X100 were
shown in FIG. 6.
In addition, the long diameters (height) and short diameters (width) for 9
samples of
allulose crystals obtained in Example 5 were measured, and the particle
diameter ratio (=long
diameter/short diameter) was obtained and shown in the following Table 6.
Specifically, for 5
crystals, the ratio of length of long diameter (pm) was shown, on the basis of
short diameter length
(pm) as 1.
[Table 6]
Crystals Example 5
#1 1.3
#2 1.5
#3 1.2
#4 1.2
#5 2.1
#6 1.7
#7 1.7
#8 1.4
#9 2.4
average 1.6
As shown in FIG. 6, the allulose crystal of the present invention had a
rectangle
hexahedron or crystal structure close thereto. The ratio of long diameter
length (pm) to the short
31
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
diameter length (pm) of 1 of crystals in the Table 5 showed that average 1.6
in Example 5. Example
formed the crystal form of rhombic system close to quadrate as each crystal
side was grown
homogeneously. In addition, it was confirmed that the ratio of long
diameter/short diameter tended
to be reduced, as the crystal side was grown homogenously. This was suggested
that since other
5 components except for allulose acted as impurities disrupting the crystal
growth of pure allulose,
as the allulose purity in raw material for crystallization was low, they
affected the crystal shape.
(3) Differential scanning calorimetry (DSC) analysis
The DSC analysis of allulose crystals obtained in Example 5 was performed
under the
specific DSC analysis conditions.
Equipment name: DSC [differential scanning calorimetry]
Manufacturer: Perkin Elmer
Method: 30 to 250 C, 10 C/min temperature rising, N2 gas purge
(standard method: refer to ASTM D 3418)
The result of DSC analysis of allulose crystal was shown in the following
Table 7.
[Table 7]
Classification Tm( C) AH(J/g)
Example 5 127.89 207.5
As the result of DSC analysis, the crystal in Example 5 had the highest Tm
value, and the
highest thermal capacity. It could be predicted that as the thermal capacity
was higher in the DSC
analysis of crystal, it was not easily dissolved, and as the thermal capacity
was higher and the
width of endothermic peak was narrower, the crystal was formed homogeneously
and firmly. In
consideration of thermal capacity and endothermic peak enthalpy values of
Example 5, it was
confirmed that the crystal of Example 5 was formed relatively more
homogeneously and fianly.
(4) Infrared absorption (IR) spectrum analysis
To confirm the prepared allulose crystal, the infrared absorption (IR)
spectrum analysis
was carried out for the crystals of Example 5, under the measuring conditions.
32
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
Analysis equipment: TENSOR II with Platinum ATR, manufacturer; Bruker (German)
Detector: highly sensitive photovoltaic MCT detector with liquid nitrogen
cooling.
Scan number of times: 64 scans at 20 kHz
Scan range: 800 - 4,000 cm-1 and averaged at 4 cm-1 resolution.
According to the result of infrared absorption (IR) spectrum analysis for the
allulose
crystal according to the present invention, the allulose crystal had unique
structural characteristic
as the allulose molecule included functional groups ¨OH, and C-O-C, C-C, C-OH,
etc. in the
allulose molecular structure. It demonstrated that the crystals of Example 5
were identical allulose
crystals. The IR analysis spectrum was shown in FIG. 7.
(5) X-ray diffraction (XRD) analysis
The X-ray diffraction analysis was performed according to the following
specific analysis
conditions, for the allulose crystals obtained in Example 5, and the result of
X-ray diffraction
analysis of allulose crystals obtained in Example 5 was shown in Table 8 by
selecting the higher
(Relative Intensity %) five peaks and morphology specific peaks.
Analysis equipment: D/MAX-2200 Ultima/PC
Manufacturer: Rigaku International Corporation (Japan)
X-ray sauce system target: sealed tube Cu
Tube voltage: 45 kV / Tube current: 200 mA
Scan range: 5 to 80 20
Step size: 0.019
Scan speed: 5 /min
[Table 8]
Angle 2-Theta degree Relative Intensity %
18.78 100.0
15.24 97.6
28.37 9.5
30.84 18.8
31.87 9.0
47.06 4.1
33
Date Recue/Date Received 2020-08-05
CA 03090459 2020-08-05
As shown in the Table 8, it was confirmed that the allulose crystal obtained
in Example 5
had specific peaks in 15.24, 18.78 and 30.84; 15.24, 18.78, 30.84, and 28.37;
15.24, 18.78, 30.84
and 31.87; 15.24, 18.78, 30.84 and 47.06; or 15.24, 18.78, 30.84, 28.37, 31.87
and 47.06; of 20
values in the powder X-ray spectroscopy.
34
Date Recue/Date Received 2020-08-05