Note: Descriptions are shown in the official language in which they were submitted.
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
COCHLEAR IMPLANT LOCALIZATION SYSTEM
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001]
This application includes subject matter related to subject matter
disclosed in U.S. Pat. App. No. 15/890,920, entitled COCHLEAR IMPLANT
LOCALIZATION SYSTEM and U.S. Pat. App. No. 15/890,949 entitled COCHLEAR
IMPLANT LOCALIZATION SYSTEM. The entire disclosures of each of the above
applications are incorporated herein by reference.
FIELD
[0002]
The present disclosure relates generally to a system for localizing a
tracked item, and particularly to a localization system using one or more
modalities for
localizing the item within a volume.
BACKGROUND
[0003]
This section provides background information related to the present
disclosure which is not necessarily prior art.
[0004]
A navigation system can be used to track and navigate an instrument
within a volume. For example, a navigation system can be used to track an
electromagnetic tracking device on an instrument during a surgical procedure.
The
tracking device is localized to determine its location.
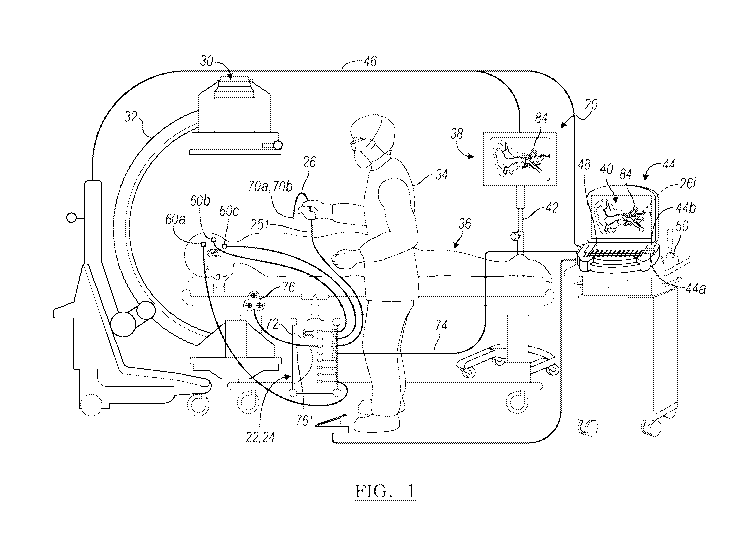
[0005]
Certain instruments, however, are not associated with a tracking
device.
Thus, certain instruments are not trackable with a navigation system.
Instruments that are not trackable may require direct inspection to be certain
of final
location. Moreover, it may be difficult to associate tracking hardware with
such
instruments due to various constraints.
SUMMARY
[0006]
This section provides a general summary of the disclosure, and is not
a comprehensive disclosure of its full scope or all of its features.
[0007]
A navigation system or combination of navigation systems can be
used to provide navigation for tracking an instrument. The instrument may be
an
instrument to assist in a procedure and/or may be a permanently implanted
device.
Permanently implantable devices may include generally known devices such as
cochlear implants, including the Contour Advance and/or Nucleus cochlear
implants
1
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
sold by Cochlear Americas having a place of business at Centennial, CO USA
Cochlear implants may include a plurality of electrodes spaced apart along a
length of
an implantable device. The implantable device may, therefore, be an implant.
The
implant may be connected to an external device for various purposes, such as
receiving, amplifying, and/or providing stimulation. Operation of cochlear
implants is
well known to one skilled in the art to receive audio signals and provide
stimulation
within a cochlea to replicate hearing.
[0008]
Navigation systems may include one or more types of navigation or
modalities of navigation to navigate a single instrument. The single
instrument can be
positioned within the patient and tracked. For example, both an
Electromagnetic (EM)
and Electropotential (EP) tracking systems can be used to navigate an
instrument
within a patient. Various EM navigation systems including those disclosed in
U.S. Pat.
No. 7,599,730 and 7,697,972, both incorporated herein by reference. Various EP
navigation systems include those disclosed in U.S. Patent Nos. 8,260,395 and
9,101,285, both incorporated herein by reference. Certain navigation systems
may
incorporate and/or operate with two types of navigation modalities, such as
both EM
and EP, and include those disclosed in U.S. Pat. Nos. 8,494,613 and 8,494,614,
incorporated herein by reference.
[0009]
A navigation system can generally include a localizer and a tracking
device. One skilled in the art will understand that the localizer can either
transmit or
receive a signal and the tracking device can also transmit or receive a signal
to allow
for a determination of a location of the tracking device associated with the
surgical
instrument. A surgical instrument can have associated therewith one or more
tracking
devices for navigation, as discussed herein. Also, each tracking device may be
used in
one or more modalities of navigation. For example, an instrument may include
an
electrode that can be used with an EP tracking system and can also be
associated or
moved relative to a tracking device that includes an EM coil to be used with
an EM
tracking system.
[0010]
An instrument may include one or more tracking devices to be used
with one or more navigation systems during a single procedure. In addition, a
method
can be used to register the two navigation systems during a single procedure.
The
registration of the two navigation systems can allow all or a determination of
a selected
number of points within one navigational domain to coordinate or correspond to
all or a
selected number of points in a second navigational domain.
2
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
[0011]
The instrument may also include one or more tracking devices that
may be used with a single selected navigation system of a single selected
modality. In
various embodiments, portions of an instrument (including an implant) may be
used as
tracking devices during an implantation procedure. The same portions may,
after
implantation, be repurposed as or re-tasked as augmenting portions.
[0012]
It is understood, that although the following disclosure specifically
relates to a cochlear implant that other types of implants may be used. A
cochlear
implant, as discussed herein, includes various portions that may be useful
during an
implantation procedure for navigation purposes, but may be repurposed after
implantation for stimulation of a patient. In other words, the cochlear
implant may have
portions (e.g. electrodes) that operate for a first function and are then
changed to a
different function. The cochlear implant may include a plurality of electrodes
that are
connected to a receiver and stimulator (R&S) for use. The R&S may receive a
signal
and provide a stimulation signal to the electrodes, during use after
implantation. Prior
to implantation, the electrodes may be used for tracking so that the hardware
of the
implant need not be altered or augmented. Standard construction of the
cochlear
implant, therefore, may be maintained. As discussed herein, various additional
tracking
devices may be added, however, to the cochlear implant.
[0013]
Further, it is understood, that the systems and methods disclosed
herein are not limited to use during implantation into a human, but may also
be useful
during navigation of an instrument relative to any non-living machine having
selected or
appropriate characteristics.
[0014] Further areas of applicability will become apparent from the
description provided herein. The description and specific examples in this
summary are
intended for purposes of illustration only and are not intended to limit the
scope of the
present disclosure.
DRAWINGS
[0015]
The drawings described herein are for illustrative purposes only of
selected embodiments and not all possible implementations, and are not
intended to
limit the scope of the present disclosure.
[0016] Fig. 1 is an environmental view of a navigation system;
[0017]
Fig. 2A is a detailed view of a subject and localizer portions, according
to various embodiments;
3
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
[0018]
Fig. 2B is a detailed cross-section view of the subject, according to
various embodiments;
[0019]
Fig. 3 is a detailed view of an instrument, according to various
embodiments;
[0020] Fig. 4
is an environmental view of the instrument during implantation in
the subject, according to various embodiments;
[0021]
Fig. 5 is a detailed view of an instrument, according to various
embodiments;
[0022]
Fig. 6 is a detailed environmental view of an instrument, according to
various embodiments;
[0023]
Figs. 7, 7A, and 7B is a flowchart illustrating a method of determining a
shape of an implantable device, and sub-routines thereof;
[0024]
Fig. 7A1 and 7A2 illustrate theoretical shapes based on capacitances
between multiple electrodes; and
[0025] Fig. 8
is a perspective view of an implantable device, according to
various embodiments.
[0026] Corresponding reference numerals indicate corresponding parts
throughout the several views of the drawings.
DETAILED DESCRIPTION
[0027]
Example embodiments will now be described more fully with reference
to the accompanying drawings.
[0028]
A navigation system 20 is illustrated in Fig. 1, and in further detail in
Fig. 2A, that may be a surgical navigation system. A first tracking system can
include
an electropotential (EP) tracking system 22. A second tracking system can
include an
electromagnetic (EM) tracking system 24. Appropriate EP tracking systems may
include those disclosed in U.S. Patent Nos. 8,260,395 and 9,101,285, both
incorporated herein by reference. Appropriate EM navigation systems include
those
disclosed in U.S. Pat. No. 7,599,730 and 7,697,972, both incorporated herein
by
reference. The first and second tracking systems 22, 24 can be used to track
one or
more instruments, such as a single surgical instrument 26. The surgical
instrument 26
can be any appropriate instrument, including a cochlear implant that is
positioned in a
cochlea of an inner ear of a subject. Other appropriate instruments may also
include
but not limited to, cardiac, brain, spinal, sacral, vagal, and gastric
electrode implants
4
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
such as a lead used as a part of an implantable medical device (IMD) for heart
rhythm
treatment, neurological treatment, ablation, or other appropriate purposes.
[0029]
The navigation system 20 may be operated with only a single one of
the tracking systems 22, 24, each provided in a selected separate modality.
The first
tracking system 22 being an EP modality tracking system and the second
tracking
system 24 being an EM modality tracking system. The navigation system 20,
therefore,
may be a single or multiple modalities tracking system.
[0030]
In certain procedures, having two tracking systems can be useful.
Exemplary procedures include placing a cochlear implant with one or more
electrodes
in a cochlea of a subject, such as a human subject or patient 36. In the
cochlea, an
electrode on an implant may be exposed to a conductive fluid for position
determination
with the EP tracking system 22. Also, a tracking device that may transmit or
receive an
EM signal may be tracked with the EM tracking system 24. Also, registration of
the EM
tracking system 24 to image data can be used to assist in illustrating a
position, such as
an anatomical position or anatomy, relative to the patient 36.
[0031]
Certain procedures also may be more easily tracked with the EP
tracking system 22 as opposed to the EM tracking system 24. For example, a
stylet
including an EM tracking device can be positioned through at least a portion
the implant
or delivery portion of the implant. In various procedures, however, the stylet
can be
removed from a portion of the implant to allow the implant to be substantially
less rigid
and more flexible. Once the stylet is removed from the implant the exact
position of the
implant may not be trackable with the EM tracking system 24. When the stylet
is
removed, the implant electrode can be tracked with the EP tracking system 22.
[0032]
The navigation system 20 used in the various procedures discussed
above or herein, can also include various components in addition to the
tracking
systems 22, 24, such as an imaging system 30. The imaging system 30 can be any
appropriate imaging system and is exemplary illustrated as a fluoroscopic C-
arm
system 32. Other imaging systems can include computed tomography (CT) imaging
systems, 0-Arm imaging system, magnetic resonance imaging (MRI) systems, and
positron emission tomography (PET) imaging systems. The imaging system 30 can
be
used by a surgeon 34 to image the patient 36 prior to (preoperatively), during
(intraoperatively), or after (postoperatively) a procedure. Imaging the
patient 36 can
create image data that can be viewed as an image 84 on a display device 38 or
a
display device 40. The display device 38, 40 can be provided alone, such as on
a
stand 42 or with a processing system as a part of a workstation or processing
system
5
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
44. The image data can be transferred from the imaging system 30 through a
data
transmission system 46, such as a wired or wireless transmission system, to
the display
devices 38, 40. Images may be selected and appropriate for a selected
procedure,
such as that of an inner ear or cochlea for a cochlear implant.
[0033] The
navigation system 20, also including the tracking systems 22, 24
can be incorporated or connected to the processor system 44. The processor
system
44 can include human input devices such as a keyboard 48, a joystick or mouse
50, a
foot pedal 52 or any other appropriate human input device. Each of the human
input
devices 48-52 can be connected with the processor system 44 or other systems,
such
as the imaging system 30, for control or actuation thereof. The processor
system may
further include a general purpose processor 44a that is configured to execute
instructions that are stored on a memory 44b, such as an intransatory memory
(e.g.
solid state memory, spinning hard-disc, optical readable disc). The processor
44a may
also be a special purpose processor, such as an ASIC.
[0034] The EP
tracking system 22 can include components to generate a
current in the patient 36. The EP tracking system can include or be based on
the
LocalisaTM intracardiac tracking system sold by Medtronic, Inc. having a place
of
business in Minneapolis, Minn. The EP tracking system 22 can also include
portions
disclosed in U.S. Patent Numbers 5,697,377 or 5,983,126 to Wittkampf,
incorporated
herein by reference
[0035]
Briefly, the EP tracking system 22 can include one or more axis
electrodes 60 (including 60a, 60b, 60c). In various embodiments the axis
electrodes
may be operated as pairs of axis electrodes. The axis electrodes 60 can also
be
referred to as a localizer, operable to generate or inject a current within a
volume, such
as the patient 36. The axis electrodes 60, however, may include a single axis
electrode
60a. It is understood, however, that two or more axis electrodes 60b and 60c,
may also
be provided. With two or more electrodes, temporal variations of injected
current may
be used to more finely resolve the location of the implantable device, as
discussed
further herein.
[0036] It is
also understood, that one or more pairs of axis electrodes may be
provided. For example, three pairs of axis electrodes may be used to generate
three
substantially orthogonal axes of current within the patient 36. The axis can
be defined
between selected axis electrode pairs, as discussed above, by an alternating
current
that is generated between any pair of the axis electrodes. For example, a
first pair of
6
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
axis electrodes can be positioned on a left and right side of the patient 36
to define an
X-axis when a current is generated between the two axis electrodes.
[0037]
The injected current from the one or more axis electrodes can be used
to determine or calculate a location of a tracking device 70. The tracking
device 70 can
include a first or EP tracking device 70a and a second or EM tracking device
70b. The
EP tracking system 22 can be used to track the EP tracking device 70a. The
first
tracking device 70a can sense voltages or related impedances in the patient 36
based
upon the induced current from the axis electrodes 60a or between pairs of axis
electrodes. The voltages can be related to a position of the first tracking
device 70a in
the patient 36.
[0038]
The axis electrodes 60a-60c can be driven with a generator in a
controller 72 that is connected via wires or wirelessly with the axis
electrodes 60a-64b.
The generator can provide the power to generate a selected current, such as an
alternating current in the patient 36. The controller 72 can also include a
connection for
the instrument 26 to communicate a signal from the tracking device 70 to the
controller
72. The connection with the instrument 26 can be wired or wireless, according
to
various embodiments. In addition, the controller 72 can include a processor
portion or
simply be a transmitter to transmit signals from the tracking device 70.
Signals can be
transmitted from the controller 72 to the processor system 44 with a
transmission
system 74. The transmission system 74 can be a wired or wireless transmission
system.
[0039]
The EM tracking system 24 can also be associated with the controller
72 or can be provided with a separate controller system. It will be understood
that
various separate circuitry portions may be provided in the controller 72 to
generate or
operate the EP tracking system 22 or the EM tracking system 24.
[0040]
The EM tracking system 24 includes an EM localizer 76 that can be
positioned relative to the patient 36. The EM tracking system can include the
AxiEMTm
electromagnetic tracking system sold by Medtronic Navigation, Inc. having a
place of
business in Colorado, USA. The localizer 76 can generate an electromagnetic
field that
is sensed by the EM tracking device 70b. Alternatively, the EM tracking device
70b can
generate a field that is sensed by the localizer 76.
[0041]
A localizer can be used as a part of a tracking system to determine the
location of the tracking device 70. For example, the localizer 76 can be
interconnected
with the controller 72 to transmit a signal to the processor system 44
regarding the
position of the EM tracking device 70b. The axis electrodes 60a-60c can be a
localizer
7
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
that induces axes of current in the patient 36 to localize the EP tracking
device 70a.
Accordingly, the localizer can refer to a portion of the tracking system which
can be
exterior or interior to the volume, such as the patient 36, that is used to
determine a
position of the tracking device 70.
[0042]
According to various embodiments, the localizer devices, including the
EM localizer 76 and the axis electrodes 60a-60c, can be used to define a
navigation
domain in a subject space. Subject space may refer to the physical space in
which the
instrument is moving during a selected procedure. The subject space may
include a
patient space of the patient 36. Patient space can be the physical space that
is being
operated on during the operative procedure. The patient space can also include
the
navigated space through which the surgical instrument 26 is being navigated.
Image
space can be defined by image data that is displayed as an image 84 on the
display
devices 38, 40. Image data can include any appropriate image data, such as
image
data of a cranium, inner ear, or other appropriate portion of the patient 36.
The image
data is used to generate or render the image 84 that is displayed on the
display devices
38, 40 and may also include atlas data. Atlas data can include statistical or
historical
data. The atlas data can be registered or morphed to the patient image data or
patient
space. It will be understood that atlas data may be used in an imageless
navigation
system. For example, an imageless navigation system may not require the
acquisition
of image data of the patient 36. The image data and atlas data may be stored
or
recalled from the memory 44b.
[0043]
The patient space can be registered to the image space of the image
data to allow a position of the instrument to be displayed (e.g. superimposed
as an icon
on the image 84) according to any appropriate technique, including those
discussed
herein. Generally, however, the patient space is registered to the image data
to allow
for displaying or superimposing an icon or representation of a tracked device,
for
example the surgical instrument 26, over the image 84 on the display device
38, 40.
Registration generally allows for a transformation of the image data to the
patient
space. Various registration techniques can include contour matching, fiducial
or point
matching, automatic registration, or any other appropriate registration. For
example,
various landmarks or fiducials can be identified in the image data and/or
image 84 and
the same fiducials or landmarks can be identified in the patient 36, such as
within the
inner ear. The image data can then be transformed to the patient space of the
patient
36 so that a proper position of a superimposed icon 26i can be shown relative
to the
8
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
image 84. Registration techniques can include those discussed in the U.S.
Patents
incorporated above.
[0044]
In addition, as discussed herein, the EP tracking system 22 can be
registered to the EM tracking system 24. The registration of the EP tracking
system 22
to the EM tracking system 24 can allow navigation of the EP tracking device
70a with
the image data 80. For example, the EP tracking system 22 may not form a
localization
geometry that is Euclidean to be efficiently mapped to Euclidean coordinates
of an
image of the subject 36. Thus, mapping or co-relating points between the EM
tracking
system 24 and the EP tracking system 22 allows for a point determined in the
EP
tracking system 22 to be translated to the EM tracking system 24 and,
therefore, to the
image 84. In various embodiments, co-registration may include co-locating an
EM
tracking device trackable with the EM tracking system 24 with an EP tracking
device
that is trackable by the EP tracking system 22. The co-location (e.g. placing
the EM
tracking device and the EP tracking device at the same position) may allow for
tracking
one point in space with both the EP tracking system 22 and the EM tracking
system 24
to allow for registration (i.e. translation) of the two navigation spaces.
[0045]The instrument 26 may include an implantable cochlear member or
cochlear implant electrode assembly 226 to be positioned within the patient
36. In
various embodiments, the cochlear implant 226, as illustrated in Fig. 2B and
Fig. 3, may
include an elongated body 230 that extends along a long axis. The elongated
body 230
may include a plurality of implant electrode or contacts, such as twenty-four
individual
electrode portions or contacts (also referred to individually as electrodes)
232i to
232xxiv, which is configured to be positioned within a cochlea 37 of the
subject. It is
understood that the cochlear implant 226 may include any appropriate number of
electrodes 232 and that twenty-four electrodes is merely exemplary. Also, the
cochlear
implant 226 may also be referred to as an array or assembly of electrode
contacts that
may contact a cochlea to transmit or deliver a stimulation signal from a
stimulator or
source, as discussed herein.
[0046]One or more of the electrodes 232 may be formed as solid pads or
members (e.g. biocompatible conductive pads (e.g. stainless steel, gold, etc.)
that have
a thickness that extends from the long axis of the body 230. The electrodes
may also
or alternatively be formed as coils of conductive material (e.g. copper, gold,
carbon,
etc.). The coils may be formed around a core (such as an air core) and the
core may
extend along an axis that is generally at an angle to and/or perpendicular to
the long
axis of the body 320 In various embodiments, at least a selected number, which
may
9
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
include all, of the electrodes may be formed as solid pads while others are
formed as
coils or lengths of wire. If formed as a coil of conductive material, the
coils may be
formed to have a geometry similar to the pads, as illustrated in Fig. 3, thus
formed on a
side or arc of the implantable member 226. Thus, when a coil is provided as
the
electrode 232, an outer most coil portion may contact the subject.
[0047] Each of the electrodes 232 may be connected with selected portions with
one or more connectors, communication or transmission lines, or cables 233.
Each of
the electrodes 232 may have a separate line or connection 233 that allows each
of the
electrodes 232 to be directly connected (e.g. wired) to an internal receiver
and
.. stimulator (R&S) 234, as illustrated in Fig. 2A, and/or to an external R&S
234a, as
discussed herein. Thus, the implant 226 may deliver a stimulation from the R&S
234,
234a to a selected portion of the subject 36 and/or transmit or receive other
selected
signals as discussed herein.
[0048] The R&S 234, 234a may be connected to the cochlear implant 226 at a
selected time to provide stimulation within the cochlea 37, as is generally
understood in
the art. Thus, the cochlear implant may be connected to the R&S 234 as shown
in
phantom 226'. The R&S 234, 234a may include any appropriate R&S such as the
receiver and stimulator portions and features included with the CochlearTM
Nucleus
Profile with slim Modiolar Electrode (CI532) implantable electrode system
and/or
CochlearTM Nucleus Kanso sound processor both sold by Cochlear Ltd. having a
place of business at Centennial, CO. The receiver may receive an external
stimulus
and provide an electrical stimulus through the one or more electrical
connectors 233 to
selected one or more of the electrodes 232. The connectors 233 may be
conductive,
and may include conductive wires or other conductive materials.
[0049]The R&S 234 may include an internal R&S 234, as illustrated in Fig. 2A,
and/or an external R&S 234a. The external R&S 234a may be substantially
identical to
the internal R&S 234 or include selected additional or alternative components.
In
various embodiments, the external R&S 234a may include portions that allow for
transdermal communication to an internal receiver, such as the internal R&S
234. The
internal portion may then transmit the signal along the communication line
226' in a
manner as discussed above, such as to the implantable electrode 226 via the
connectors 233. Accordingly, the R&S 234 may include an internal portion 234
and an
external R&S portion 234a. It is understood that the external portion 234a may
include
most or all of a power supply, a processor portion, and an audible or signal
receiving
.. portion and a transmitter to transmit to an internal R&S 234. The external
component
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
may include those portions, such as those described and further as described
in U.S.
Patent 4,352,960, incorporated herein by reference. Further the external
component
may include various additional transmission features such as transmitting a
signal to
the CAC 22, 24 such as to assist in navigation and placement of the implant
portion
226.
[0050] By providing the stimulation to the electrodes 232, external sound may
be
provided as stimulus to the subject 36 in an appropriate manner. During
implantation,
the cochlear implant 226 may be positioned through a small opening 36o, as
illustrated
in Fig. 2B and Fig. 4, into a cochlea 37 of the subject 36. The cochlear
implant 226
may be flexible in an appropriate manner to be positioned within the cochlea
37 to
achieve a shape of the internal space including a circular or internal
winding, of the
cochlea 37. Therefore, the cochlear implant 226 may include a selected
flexibility to
allow for implantation into the cochlea 37.
[0051] The opening 36o may include a small surgical opening that is only
slightly
larger than a diameter of the cochlear implant 226. For example, the cochlear
implant
226 may have an external maximum diameter of about 0.1 millimeters (mm) to
about 2
mm, including about 0.8 mm to about 0.1 mm, further including about 0.5 mm to
about
0.2 mm. Further, the cochlear implant 226 portion that includes the electrodes
232 may
taper from a maximum dimension to about 0.4mm to about 0.8mm. The opening 36o
may therefore have an internal diameter that is about 0.1 to about 0.8
millimeter greater
than the diameter of the cochlear implant 226. In various embodiments, the
opening
36o may have an internal diameter that is about 1`)/0 to about 300%, including
about
150%, greater than the external of the diameter of the cochlear implant 226.
The size
of the opening 36o, therefore, may not allow for direct visualization of the
cochlear
implant 226 as it is being positioned within the cochlea 37.
[0052] As discussed above, the navigation system 20 may allow for tracking of
selected portions of the cochlear implant 226. For example, an EM tracking
device 250
may be positioned at a distal end 254 of the cochlear implant 226. The EM
tracking
device 250 may be any appropriate EM tracking device, such as one including
one or
more coils of conductive material or wire. For example, the EM tracking device
250
may include three coils of wire, all on different axis, but having a common
origin. EM
tracking devices may be designed and configured to allow for a determination
of six
degree of freedom position (including location and orientation). Exemplary EM
tracking
devices include those disclosed in U.S. Patent No. 8,644,907, incorporated
herein by
reference.
11
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
[0053]The EM tracking device 250 may be tracked by the EM tracking system
24. As discussed above, the EM tracking system 24 may include the localizer 76
to
transmit or receive an EM field to allow for a determination of the EM
tracking device
70b, which may include one or more of the tracking device 250. The tracking
device
250 may be sized and configured to be positioned within an internal structure
and/or not
alter a generally understood external geometry of the cochlear implant 226
while
allowing for tracking of the cochlear implant 226.
[0054] Furthermore, it is understood that a plurality of the devices 250 may
be
positioned along a length of the cochlear implant 226. For example, a
plurality of the
devices 250 may be positioned spaced apart about 0.1 millimeter (mm) to about
1
centimeter, including about 0.2 mm apart along the length of the cochlear
implant 226.
The tracking devices may be spaced apart along the body 230, such as spaced
apart
along a longitudinal axis of the body 230. For example, each of a plurality of
these
devices 250 may be positioned substantially adjacent to each of the electrodes
232
along the length of the body 230. Therefore, a defined or substantially
precise position
of the length of the body 230 of the cochlear implant 226 may be determined.
Moreover, based upon registration of image data to the patient 36, the tracked
and
determined position of the cochlear implant 226 may be illustrated relative to
the image
84 on a selected one or more display devices 38, 40.
[0055]Accordingly, in various embodiments, the EM tracking device 250, or a
plurality of the devices 250, may be formed into the body 230 of the cochlear
implant
226 to allow for a tracking of one or more points along a length of the
cochlear implant
226. Tracking one or more of the devices 250 allows for a determination of a
positon of
the cochlear implant 226. The determined position of the cochlear implant 226
may
then be illustrated relative to the image 84 illustrated on the selected
display device 38,
40. Tracking the EM tracking device 250 with the EM tracking system 24 is
generally
understood in the art. As discussed above, the tracking device 250 may
generate or
sense an electromagnetic (EM) field while the localizer 76 operates in the
reverse of the
tracking device 250. A signal from the localizer 76 and/or the tracking device
250 is
transmitted to the processor system 44, directly or through the CAC 72.
Transmission
of the signal may be wireless or wired, such as with a transmission line 76'
from the
localizer 76 and/or an appropriate transmission system (wireless or wired)
from the EM
tracking device 250. In various embodiments, the connectors 236 of the
cochlear
implant 226 may be connected to the EM tracking system 24, such as the CAC 72,
to
transmit a signal from the EM tracking device 250.
12
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
[0056] The signal from the tracking device 250 is used to determine the
position
(including three-dimensional location and one or more orientation in the space
of the
tracking device 250 and, therefore, the instrument 26).
As discussed above,
registration of the image data to the subject may be performed such that
tracking of the
cochlear implant 226 may allow for illustration of its position relative to
the image 84.
[0057] With continuing reference to Figs. 2B, 3, and 4, the position of the
body
230 of the cochlear implant 226 may be determined by a plurality of the EM
tracking
devices 250, including a second EM tracking device 250' and a third EM
tracking device
250". The plurality of tracking devices, as noted above, can be used to show
or
determine a specific position of a plurality of points along the length of the
body 230.
Therefore, a specific shape, such as a coiled shape, may be determined based
on the
plurality of spaced apart tracking devices. This will be used to determine the
final
location and when a selected location has been achieved of the implant 226.
[0058] With additional reference to Fig. 5, in addition to, or alternatively
to,
providing the EM tracking device 70b as one or a plurality of the EM tracking
devices
250 in the implanted member 226, the EM tracking device 70b may be provided as
a
temporary member or removable member. In various embodiments, the cochlear
implant 226 may be positioned within a sleeve or flexible member, such as a
silicone or
other selected flexible material sleeve 270. The sleeve 270 may have a
terminal end
272 that covers the end of the implant 226. The sleeve 270 may further include
openings or gaps 274, such as a first gap 274a and a second gap 274b. The gaps
may
be separated by solid portions 276. The gaps 274 allow for access of the
electrodes
232 to a portion of the patient 36, such as within the cochlea 37. The sleeve
may be
positioned over the implant 226 before positioning it into the cochlear,
generally known
in the matter. The sleeve 270 may form a portion of the implant, in such an
instance.
[0059] A trackable stylet or guide wire 280 may be positioned within the
sleeve
270 and may extend a distance into the sleeve 270, including to a terminal end
272.
The guide wire 280 may include one or more EM tracking devices 70b such as a
tracking device 282' and/or more tracking devices 282". The tracking devices
282 may
include a plurality of tracking devices positioned along a length of the guide
wire 280.
For example, the tracking devices 282 may be spaced apart along a longitudinal
axis of
the guide wire 280. Therefore, the guide wire 280 may be positioned within the
sleeve
270 during implantation of and positioning of the implantable member 226.
[0060] Once the final location of the implant 226 is achieved, the guide wire
280
may then be removed. When the guide wire 280 is removed, the sleeve 270 may
13
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
remain in place with the cochlear implant 226. In various embodiments, the
guide wire
280 may be removed at any time prior to final implant location being achieved
to allow
the implantable member 226 to achieve a shape or position within the cochlea
37.
Because the guide wire 280 is removable, the tracking devices 282 need not be
permanently included with the implantable member 226. The removal of the guide
wire
280 also allows the cochlear implant 226 to be unchanged from a current
configuration,
but allows for tracking of at least a portion of the cochlear implant 226.
Having the
guide wire 280 with the implant while positioning it within the cochlea,
however, may
allow for tracking a position of the implantable member 226 during at least a
portion of
the implantation procedure.
[0061]The guide wire 280 may include one or more tracking devices 282. The
tracking devices 282 may include those that are formed around an exterior of
the guide
wire 280, such as having a plurality of coils of a conductive material (e.g.
wire) round
around and along an axis, such as a long axis of the guide wire 280. The coils
may be
formed of conductive material, such as wire, wrapped around an exterior
surface of the
guide wire 280. Further, the wire may be wrapped at a selected angle to
provide a
differentiation of tracking axes relative to the longitudinal axis of the
guide wire 280.
Selected wrapping configurations and trackable portions include those
disclosed in U.S.
Patent No. 8,644,907, incorporated herein by reference. The tracking members
282
may also or alternatively be individual coils of wire wound around a core
having a size
and shape (e.g. a diameter of about 0.1 mm to about 0.5 mm and length of about
0.5
mm to about 5.0 mm) to be positioned within the guide wire 280. The individual
coils
may be selectively placed within the guide wire 280 at selected positions,
such as near
a tip thereof and/or along its length. The tracking members 282, regardless of
configuration, may be used to track the trackable member 226 to offer a
determination
of the position of a plurality of points of the trackable member 226,
including a shape
thereof. However, the guide wire 280 may be removed from the sleeve 270 prior
to
ending a procedure on the patient 36. By removing the guide wire, the
implanted
member 226 is configured to be positioned within the subject without
permanently
including the tracking devices 280.
[0062]The implantable body 226 may alternatively, or in addition to the EM
tracking devices 70b, as discussed above, include the external sleeve 270. The
sleeve
270, discussed above and illustrated in Fig. 5, may be permanently implanted
with the
implantable device 226. However, a removable or tracking sleeve 290 may be
temporarily positioned over the implantable device 226 and/or over the sleeve
270.
14
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
The tracking sleeve 290, as illustrated in Fig. 3, may include one or more of
the EM
tracking devices 70b, such as a first EM tracking device 294' and a second
tracking
device 294". The EM tracking devices 294', 294" may be formed with (e.g.
molded
into) the removable sleeve 290. The removable sleeve 290 may be formed of an
elastic material and/or material that includes a substantially high
coefficient of friction
with the body 230. Therefore, the sleeve 290 may be maintained in a selected
position
relative to the implantable device 226 when positioning and moving the
implantable
device 226 into the patient 36. The removable sleeve 290, however, may be
removed
once the implantable device 226 is selectively positioned within the cochlea,
such as
with an open or frangible end.
[0063]As the implantable portion 226 is moved to and through the opening 36o
toward the cochlea 37, the tracking device 294', 294" may be used to track the
position
of the sleeve 290. As the sleeve 290 moves with the implantable portion 226,
the
position of the implantable portion 226 can also be determined. The sleeve
290,
formed of the material that remains substantially fixed relative to the
implantable body
230, the tracking devices 294', 294" in the sleeve 290 are used to track the
position of
the implantable device 226. The sleeve 290 may be formed of selected material
such
as a biocompatible silicone. The material of the sleeve 290 may fixedly engage
the
implantable device 226 to be held relative to the implantable device 226
during
implantation procedure. The sleeve 290, therefore, remains substantially fixed
and
does not move relative to the implantable member 226 more than an error
tolerance of
the tracking system 24 during implantation.
[0064] The tracking devices 294', 294" may be formed at discrete intervals
along
a length of the removable sleeve 290. Each of the tracking devices 294', 294"
has a
plurality of coils of conductive material for acting as EM tracking devices
70b. For
example, the coils may be formed relative to one another on a surface of the
sleeve
290. In various embodiments, the conductive material of the tracking devices
294' and
294" may be formed as windings around an exterior of the sleeve 290 along a
longitudinal axis of the sleeve 290. The tracking devices 294', 294" may also
be
formed or provided as small coils and/or printed on, and/or formed as wire
windings on
the sleeve, as discussed above. Accordingly, the tracking devices 294', 294"
may be
formed substantially similar to the formation of the tracking devices of the
guide wire
280. However, it is understood, that the tracking devices 294', 294" may be
formed
from individual and separate portions that are positioned or formed into a
surface or
wall of the sleeve 290.
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
[0065]After positioning the implantable member 226 in the cochlea 37, the
sleeve 290 may be removed from the implantable device 226. A tab or leash 298
may
be connected to the sleeve 290 and extend out through the opening 36o, as
illustrated
in Figs. 2B and 3, to be grasped by the user or surgeon 34. After positioning
the
implantable device 226 within the patient 36, the leash 298 may be grasped and
pulled
to remove the sleeve 290 from the implantable device 226. The sleeve 290 may
include a frangible or breakable portion, such as a perforated portion, such
as
perforation 302, at a distal end 290' of the sleeve 290. The perforation 302
may be
opened or perforated by pulling on the leash 298 and the sleeve 290 at the
perforation
engaging the distal end 254 of the implantable device 226. When engaging the
implantable member 226, the perforation may open to allow the sleeve 290 to be
withdrawn over the implantable device 226 after positioning the implantable
device 226
in the cochlea 37.
[0066] It is understood that other features may be provided to remove the
sleeve
290 from the implantable device 226 to make certain that the sleeve 290 need
not be
maintained within the subject 36 after positioning the implantable device 226.
For
example, the terminal end may be open and the sleeve 290 may include features,
e.g.
a high co-efficient of friction material, to maintain the sleeve 290 fixed
relative to the
cochlear implant 226 during implantation. It is understood, however, the
sleeve 290
may be maintained on the implantable device 226 during a lifetime of usage of
the
implantable device 226. The sleeve 290 may be formed of a biocompatible
material
similar to the sleeve 270. Moreover, it is understood that the sleeve 270 may
include
the tracking devices 294', 294" rather than providing the removable sleeve 290
as a
separate and second sleeve.
[0067]According to various embodiments, wireless or wired transmission of a
signal to the EM tracking system 24 may be made from the various EM tracking
devices
70b. The EM tracking system 24 may receive a tracking signal to allow for
determination of the location of the respective tracking devices and further
to determine
the tracked position of the instrument 26, including the implantable device
226. Various
portions of the implantable devices 226 may be repurposed or re-tasked from
the
tracking during implantation to use of the implantable device 226 to stimulate
the
cochlea after implantation. For example, the implantable device 226, as
discussed
above, includes one or more wires 236 that are interconnectable to the
plurality of
electrodes 232 on the implantable device 226. The tracking signal from the
respective
tracking devices may be transmitted along the wire 236 prior to connecting the
wire 236
16
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
from the cochlear implant 226 to the selected receiver and stimulator 234 that
may be
positioned with the patient 36, as is generally understood with a cochlear
implant.
Therefore, the wires 236 may be used both to transmit the signal from the
tracking
devices to the EM tracking systems 24 and later be disconnected from the EM
tracking
system 24 and connected to the receiver and stimulator 234 for operation of
the implant
member 226. As noted above, one or both of the internal R&S portion 234 and/or
the
external R&S 234a may transmit a signal from selected portions, such as the
coils 250
and/or the electrodes 232 for tracking. It is understood that the coils 250
and/or
electrodes 232 may communicate via signal through the internal R&S portion 234
and/or the external R&S 234a and, or alternatively, the connectors 233.
[0068] In addition to and/or alternatively to the EM tracking devices 70b, as
discussed above, the EP tracking devices 70a may be provided with the
implantable
device 226. As discussed above one or more axis electrodes 60a-60c may inject
a
current into the subject 36. The current injected may be produce a voltage or
impedance at one or more electrodes on the implantable device 226. For
example, the
implantable device 226, as discussed above, includes the one or more
electrodes 232,
where the electrodes 232 may be implant electrodes that are to stimulate the
cochlea
37 once connected to the R&S 234. The electrodes 232 may transmit or send a
signal
along the conductive members or wires 233. As discussed above, the wires 233
may
be connected to the EP tracking system 22 in a manner similar to connection to
the EM
tracking device 24. Accordingly, the wires 233 may be initially tasked or
purposed as
transmission wires for tracking the electrodes 232 and later may be connected
to the
stimulator and receiver for transmitting and/or receiving a signal from the
receiver
and/or stimulator associated with the patient 36. Similarly, the electrodes
232 may be
initially tasked or purposed as EP tracking device 70a. After implantation,
the
electrodes and wires 233 may be re-tasked or repurposed as simulation
electrodes for
the cochlear implant system.
[0069] In the EP tracking system 22, the axis electrodes 60a-60c, or any
appropriate number of axis electrodes 60, may inject a current into the
subject 36. A
voltage or impedance may be, due to the injected current, sensed at the one or
more
electrodes 232. With reference to Figs. 2A, 2B, and additional reference to
Fig. 5, the
implant member 226 may be positioned within the cochlea 37. The cochlea 37 may
include conductive or partially conductive fluids to allow a voltage to be
sensed by one
or more of the electrodes 232 based upon the injected current and from the
axis
17
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
electrodes 60. Although the axis electrodes 60a, 60b, and 60c are illustrated
it is
understood that more than three axis electrodes may be provided.
[0070]The axis electrodes each may inject a current, which may include an
alternating current. The alternating current generates a voltage or electrical
potential at
each of the electrodes 232 on the implant device 226. Each of the axis
electrodes 60a-
60c may inject a current at a selected frequency, where each frequency may be
different. Further, there may be a temporal or time differential between the
injected
current from each of the axis electrodes 60a-60c. In other words, the currents
injected
with the axis electrodes 60a-60c may be time or frequency multiplexed or
differentiated.
Therefore, the potential measured at each of the electrodes 232 may be
triangulated
relative to all of the individual axis electrodes 60a-60c. The relative
position of each of
the electrodes 232 to one another may be determined based upon the spaced
apart
position of each of the axis electrodes 60a-60c. This may allow the user 34 to
operate
the processor system 44 to determine a relative position of the implant device
226
during implantation. The user 34 may view the image 84 and the determined
position
of the implant device 226 may be displayed on the image 84.
[0071]The image 84 may be registered, as discussed above, to the implant
device 226. The EP tracking system 24 may be registered to the image 84 using
appropriate registration techniques, such as those discussed above. For
example, an
EP tracking device 70a may be positioned within the subject 36 at a known
landmark to
determine a registration to the image data by identifying the landmark on the
image 84.
Alternatively, or in addition thereto, one or more of the EM tracking devices
70b may be
positioned at a same or known position relative to the EP tracking device 70a
to allow
for registration of the EP tracking device 70a and the EM tracking device 70b
and,
therefore, registration to the image 84. A co-registration with the EM
tracking system
24 may be used to assist in registration, including the EP tracking system 22,
to the
image 84. For example, as discussed above, one or more of the EM tracking
devices
70b may be placed with one or more of the axis electrodes 60a-60c. Thus, the
location
of the axis electrodes 60a-60c of the EP tracking system 22 may be determined
with
the EM tracking system 24 and, therefore, the location of the EP tracking
device 70a
that is tracked with the EP tracking system 22 may have its determined
position
translated to the EM tracking system 24.
[0072] Registration In this way, the image 84 may be registered with EM
tracking
system 24, which may include a substantially Euclidean geometry, which the EP
tracking system may not include. As is understood by one skilled in the art,
the EP
18
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
tracking system including the current injected from the axis electrodes 60a-
60c may not
provide a purely Euclidean geometry of registration. It is understood,
however, that
exact registration may not be necessary if the user 34 selects to simply
identify and/or
understand the relative position of the electrodes 232 relative to one another
during the
implant procedure.
[0073] In addition, one or more of the EM tracking devices 70b may be
associated with one or more of the axis electrodes 60a-60c. In various
embodiments,
as noted above, rather than a single solid member (e.g. a metal plate) used as
the
electrode 232, a coil of wire or other conductive material may be used to form
the
electrode 232. The coiled wire, if selected, may have a thickness that extends
from a
surface of the implant electrode 232 towards a central axis of the electrode
232. The
outer most portion may act as the electrode pad of the electrode implant 232.
[0074]The coiled wire as the electrode may operate as the tracking device 70b
with the EM tracking system 24. The position of the axis electrodes 60a-60c
may,
therefore, be tracked with the EM tracking system 24. The position of the
electrodes
232 may be determined relative to the axis electrodes 60a-60c. This would
allow the
position of the electrodes 232, therefore, to be determined relative to a
position with the
EM tracking system 24. Thus, if the EM tracking system 24 is registered to the
image,
the position of the electrodes 232 may also be illustrated as an icon on the
image 84.
[0075] By understanding a position of each of the electrodes 232 relative to
one
another, on the implantable device 226, the user 34 may understand the
changing
position of the implantable device 226. The user 34 may therefore understand
and
determine whether the implantable device 226 is achieving a selected shape,
such as a
pre-determined shape of the cochlea 37. Moreover, the user 34 can, prior to a
procedure, understand and evaluate the image 84 of the subject 36 to
predetermine a
final desired orientation and position of the implant device 226. Therefore,
by
determining the relative position of the electrodes 232 of the implant device
226, the
user 34 may determine whether a preselected shape, including location and/or
orientation of the implant device 26, has been achieved.
[0076]Each of the electrodes 232 on the implantable portion 226 may be used to
receive a voltage at the electrode based upon the injected current from the
axis
electrodes 60a-60c. This information may be used to resolve or determine a
location
and orientation of the implant device 226. A selected specific shape of the
implantable
device 226, as discussed above, may be selected to assist in assuring that the
implantable device 226 is achieving the shape of the cochlea 37. To assist in
19
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
determining the shape of the implantable device 226, with or without a
determination of
a location of the implantable device 226 relative to the subject 36, a
relative
capacitance between each of the electrodes 232 may be determined. As discussed
herein, any first electrode and any second electrode of the electrodes 232 may
form an
arbitrary or selected pair of electrodes. A capacitance between each arbitrary
pair of
the electrodes 232 may be measured. A comparison of the capacitance between
each
arbitrary pair may be compared over time and at any instant in time to
determine a
relative distance between the arbitrary pair of electrodes. It is understood
that a relative
capacitance between any two of the electrodes of the implant electrode 232 may
also
be determined with a direct reading (such as with a volt meter) between the
two
electrodes (e.g. 232i and 232ii). Thus, an injection of a current from the
axis electrodes
60a-60c is not necessary for a determination of a comparison of capacitance.
[0077] Each of axis electrodes 60a-60c positioned on an external surface of
the
patient 36 may be used to generate a potential relative to each of the
electrodes 232 on
the implantable device 226. For example, the axis electrodes 60a-60c may be
used to
generate a relatively positive charge relative to the electrodes 232 on the
implantable
device 226. A determination of capacitance between any two of the electrodes
232
may be used to resolve and determine a shape of the implantable device 226
between
the two electrodes 232. As the distance between two respective electrodes
decreases,
the capacitance between the same two electrodes increases. For example, with
reference to Fig. 6, an initial distance between the electrode 232xxii and
232xxi may be
achieved when the implantable device 226 is substantially straight and being
initially
inserted into the patient 36. As the implantable device 226 achieves the coil
within the
cochlea 37, the distance between the two electrodes 232xxii and 232xxi may
change.
As the two electrodes 232xxii and 232xxi get closer together, as they begin to
coil
within the cochlea 37, the capacitance between the two electrodes may
increase.
Therefore, a measurement of capacitance between any two selected electrodes,
especially the electrode 232xxii and 232xxi, can be measured and compared over
time
to determine that they are becoming closer to one another.
[0078]The capacitance may be determined between any two of the electrodes,
therefore the capacitance between the electrode 232xxii can also be determined
relative to the electrode 232xx and any other of the electrodes to the
electrode 232ii.
Again, a change in capacitance between the electrodes 232xxii and all of the
other
electrodes may be measured and determined over time to assist in determining a
shape of the implantable device 226. The processor system 44, including the
memory
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
44b, may maintain a record of the changing capacitance over time and determine
a
shape of the implantable device 226, also over time. The determined shape may
then
be illustrated on the display device 38, 44 either alone or relative to the
image 84.
[0079] In addition to determining a capacitance between two of the electrodes
232 of the implantable device 226, as discussed above with the injection of
current from
the access electrodes 60a - 60c, a direct determination or sensing may also be
made.
As discussed above, the connectors 233 connect portions of the implantable
device
226, such as each of the electrodes 232, to selected systems. The connectors
233 may
connect to the selected tracking systems 22, 24 and/or the work station 44. It
is
understood that other appropriate measuring systems may also be included or
associated with the various tracking systems 22, 24 and/or the work station
44. For
example, a multi-meter may be used to directly connect the multi-meter to two
or more
of the electrodes 232. By connecting a selected meter to two or more of the
electrodes,
a direct calculation of a capacitance difference between two different
electrodes may be
determined. The measurement may be made between any or all pairs of the
electrodes
232 of the implantable device 226, as discussed above. Accordingly, it is
understood
that the capacitance difference between two of the electrodes 232 need not be
induced
by the axis electrodes 60a ¨ 60c, but may be determined directly. Moreover,
the EM
localizer 76 may also be used to generate a field that may induce a voltage or
capacitance difference between the electrodes 232 that may also be measured
with a
selected system, such as a selected multi-meter or other appropriate meter.
[0080] It is understood that each of the electrodes 232 on the implantable
device
226 may be measured relative to any other of the electrodes on the implantable
device
226. Therefore, the number of possible electrode pairs is also a function of
the number
of electrodes on the implantable device 226. Accordingly, the greater the
number of
electrodes the greater number of possible pair measurement may be made. The
greater the number of electrode pairs, the greater resolution in determining a
shape of
the implantable device 226.
[0081]As discussed above, a capacitance between various electrodes may be
determined, such as the electrodes 232 of the implantable device 226. The
implantable
device 226 including the selected number of electrodes 232, as noted above, or
any
appropriate number, may be used to determine a shape of the implantable device
226
in the patient 36, or any appropriate volume. The measurements may be based
upon a
shape determining algorithm and/or method, as discussed below, and as
illustrated in
the flowchart 360 in Fig. 7. To determine a shape of the implantable device
226 the
21
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
capacitance between selected pairs of the electrodes, as noted above, may be
determined and used to calculate the relative shape of the implantable device
226. It is
understood that the relative shape of the implantable device 226 may be
determined
relative to any selected fixed or known point (e.g. tracked position), such as
the location
of one or more of the tracking devices 250 associated with the implantable
device 226.
Accordingly, a shape of the implantable device 226 may be determined according
to
method 360 illustrated in Fig. 7 and may be determined relative to a selected
origin,
such as one or more of the tracking devices 250.
[0082] In various embodiments, the method 360 begins at start block 364 and
continues to determining a pairwise capacitance between selected electrodes
232 in
block 368. In determining a pairwise capacitance between selected electrodes
232 it is
understood that a capacitance may be determined between any two of the
electrodes
232. For example, capacitance between the electrode 232i and 232ii may be
determined as maybe a capacitance between electrode 232i and 232iii or 232i
and
234iv. Therefore, it is understood by one skilled in the art, pairwise
capacitance
between selected electrodes may include a determination of capacitance between
all
possible pairs of electrodes of the implantable device 226. Accordingly, the
number of
selected pairwise determinations may be based upon the number of electrodes
included in the implantable device 226.
[0083] It is further understood, the greater number of pairwise capacitance
determinations in block 368, the finer the determination of the shape of the
implantable
device 226 may be determined. For example, if a selected error or coarseness
of the
shape to be determined of the implantable device 226 is selected, a pairwise
calculation between each of the electrodes may only be between every other
electrode
or appropriate number, such as one-half of the electrodes, one-third of the
electrodes,
or the like. If a finer shape determination is chosen, then a pairwise
capacitance may be
measured and determined between each electrode 232 and all of the other
electrodes
232.
Nevertheless, the pairwise capacitance determination may be made for
electrodes along the length of the implantable device 226 for determining a
shape along
the length of the implantable device 226.
[0084]Once the pairwise capacitance measurements, from which the
determinations may be made, between selected electrodes are completed in block
368,
a calculated pairwise separation is made in block 372. The calculated pairwise
separation may be between any of the pairs of electrodes for which a pairwise
capacitance has been measured or determined in block 368. For example, a
pairwise
22
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
capacitance between all of the electrodes relative to each of the other
electrodes may
be made in block 368. The calculation of a pairwise separation may be made
based
upon the determined capacitance of the selected electrodes from block 368 in
block
372. The determination of the pairwise separation may be based upon a known
calculation or algorithm techniques such as direct computation, conformal
conversions,
auxiliary functions, and numerical approximations. Generally, the pairwise
separation
may be a distance or a radius (r) between two electrodes (i) and (j).
Accordingly, a
determination of the radius (r) between two electrodes may be illustrated or
determined
as rij. As discussed herein and illustrated in Figs 7A1 and 7A2, the radius r
may define a
circle relative to respective electrodes.
[0085]The determination of a shape of the implant device 226 may be
determined based upon the calculated pairwise separations in light of the
measured
pairwise capacitances. The determination of the shape of the implantable
device 226
may be made by initializing electrode relative positions (also referred to as
determining
an initial or initial guess position of the electrodes) in block 376.
Initialization of the
relative electrode positions may be made by selecting an origin electrode,
such as the
electrode 232i. It is understood that any appropriate electrode may be
selected as the
origin electrode and the electrode 232i is merely exemplary. An
initialization, however,
need not be with a single one of the electrodes 232. Rather, it may be assumed
that
the implant electrode device 226 is a straight line and the shape
determination
algorithm and method, as discussed herein, may proceed from such an
initialization.
[0086]Moreover, the shape of the implantable device 226 determined with a
method 360, as illustrated in the flowchart in Fig. 7, may be used to
calculate or
determine a selected fine shape of the implantable device 226. However, a
relative or
.. absolute position of the implantable device 226 may be determined with
alternative or
separate tracking devices, such as the tracking device 250. Accordingly, the
origin
electrode may be near one of more of the tracking devices associated or
connected
with the implantable device 226.
[0087] With continued reference to Fig. 7 and in additional reference to Fig.
7A
the initialization of the relative electrode positions in block 376 will be
described in
greater detail. Fig. 7A describes in greater detail the process of block 376.
Accordingly,
the method at 360 including block 376 includes the sub-steps or a sub-
algorithm in
block 376, as illustrated in Fig. 7A. As discussed above, a selection or
placement of a
single electrode at an origin position Pi is performed in block 380. With
continuing
reference to Fig. 7A and additional reference to Fig. 7A1 and 7A2, the
position of
23
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
electrodes are illustrated by dots and enumerated by Pi or Pi+i and Pi+N. It
is understood
that any appropriate number of the electrodes 232 may be determined and that
the
following discussion regarding the three electrodes is merely exemplary.
Moreover, it is
understood that the electrodes may have an initial or initiated position
determined as Pi
and a relative position may then be determined, as discussed further herein.
[0088]With reference to Fig. 7A1, it is illustrate that the electrodes are in
a
straight line. It is understood, however, that the electrodes may be
positioned in any
three-dimensional relative positon, as illustrated in Fig. 7A2. Moreover, it
is understood
that a position of the electrodes may be constrained by various constraints,
such as the
anatomy of the subject. Accordingly, a returned or determined relative
position of the
electrodes may be based upon or limited to constraints determined relative to
the
anatomy of the subject 36. For example, as illustrated in Fig. 7A2, only one
of the two
orientations may be determined as possible and illustrated to the user 34,
such as one
that illustrates a branch to the right from the origin Pi. For example, as
illustrated in Fig.
7A2, therefore, the electrode determined to be at the right (i.e. Pi+N(o) may
be
determined to be the real or proper electrode position while the one to the
left (i.e.
Pi+N(D) may be determined to not be the proper or correct possible electrode
position. As
discussed further herein, therefore, only one of a selected possible position
of
electrodes may be determined or returned or illustrated for a user.
[0089]The distance between the electrodes (as noted above defined by radius r)
may be based upon a measured capacitance between different electrodes.
Measured
capacitance may be based upon an applied voltage on the implantable device 226
between the various electrodes and the implantation system. As discussed
above, the
implantable device 226 includes the communication line 233 to a controller,
which may
be included with the processor system 44. Accordingly, a capacitance between
each of
the electrodes may be measured and determined by the controller.
[0090]As illustrated in Fig. 7A1 and 7Al2, a determined distance or radius
relative to each of the other electrodes. For example, the electrode Pi
includes a radius
ri(1) relative to the second electrode
The electrode Pi also includes a radius ri(2)
relative to the end electrode Pi+N. Similarly the electrodes Pi+i and Pi+N
include radii
relative to each of the other electrodes. It is understood, that only three
electrodes is
merely exemplary and for ease of the present discussion. As discussed further
herein,
the radii may be used to determine the relative positions of the electrodes.
[0091] Once the selection of a single electrode at the origin is performed in
block
380, a first nearest neighbor electrode may also be "placed". In placing the
first nearest
24
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
neighbor, it is understood to be a determination of a spacing based on the
measure
capacitance between the selected electrode and the neighbor, here the first
nearest
neighbor. The placement of the first nearest neighbor (where the two
electrodes are
-7õ
and $9`(4v) is in a manner such that the following equations in Table 1 are
satisfied.
Table 1
-
-7
4' 7 4' and
¨ -
26
Error may be present in an amount due to measurement error, instrument error
or an
arbitrary amount to make the fit, error is represented by "E". 11 is further
understood that
additional equations in a like manner may be provided or derived to calculate
the
positon of all of the electrodes measure, as discussed below.
[0092]The implantable device 226 is positioned within the patient, or at least
beginning to be positioned in the patient, and the determination of the
position of the
electrodes can assist in determining a shape of the implantable device 226
within the
subject 36. Accordingly, the method 360 assists in determining the position or
shape of
the implantable device 226. The placement of the electrodes and the initiation
of
placement in block 376, therefore, allows for the determined shape of the
implantable
device 226. The placement or possible determination of the location of each of
the
electrodes may also include a slight amount of error indicated by E.
Accordingly, the
calculated or checked position of the electrode may be the radius between the
electrode including an amount of error (e.g. plus or minus ( )c).
[0093]The determined position of the electrode may be between the origin or
first electrode Pi and any other electrodes, such as the electrode Pi+N. The
determination may then further include the placement of all neighbors or the
kth nearest
neighbor with a less than or equal to k+1 nearest neighbor constraint in block
386. As
discussed above, the constraints may include an amount of error to allow for a
determination of the placement of the electrode relative to one another.
Constrains may
also include the positions determined of the tracking devices 70. For example,
a shape
of the implantable device 226 may be limited or constrained by a tracked and
determined position of any selected tracking device. Additional constraints
may include
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
a selected or known rotational position of the electrodes in a shape relative
to the
patient or subject space. For example, as illustrated in Fig. 7A2 the
electrodes may
branch in a selected position, such as in a right or left direction. As the
implantable
device 226 is positioned in a selected portion of the patient 36, such as in
the cochlea
of the subject 36, the implantable device 226 may have a selected or known
curve
limitation. Accordingly, a constraint may include only returning or placing
electrodes that
curve in a selected direction, such as to the right as illustrated in Fig.
7A2. It is
understood that the curve may be any appropriate curve or constrained shape
and that
a curve to the right is merely exemplary. Moreover, it is understood that the
curve or
branch position may be in three-dimensional space relative to any of the
placed
electrodes.
[0094] Finally, after placing all of the electrodes the electrode relative
positions
may be returned in block 390. Again, it is understood that the returned
relative positions
may be initialized positions in block 376 and that the detail sub-algorithm or
steps
illustrated in Fig. 7A are included in the initialization of the electrode
relative position in
block 376. Moreover, the placement of the electrodes is based upon the
measured and
determined capacitance between the electrodes in a pairwise manner. As noted
above,
it is understood that the position between the electrodes or distance between
the
electrodes may be based upon a measurement of a capacitance between all of the
electrodes in a pairwise manner, or a selected number of the electrodes in a
pairwise
manner. The placement of electrodes may be based upon the process as discussed
above and illustrated in Fig. 7A and Fig. 7A1 and 7A2. The initialization of
the relative
electrode positions in block 376, however, may allow for a determination of a
shape of
the implantable device 226, as discussed further herein.
[0095]After initializing the electrode relative positions in block 376, as
discussed
above, adjustment of the electrode relative position to block 396 may occur.
It is
understood that adjusting the relative positions of block 396 is optional and
not required
for the method 360. Nevertheless, the adjustment of electrode relative
positions in block
396 may also include various sub-steps or algorithm, as illustrated and
discussed in
detail with reference to Fig. 7B. The sub-steps illustrated and discussed in
relation to
Fig. 7B is understood to include or be included in block 396 of the method
360. In
particular, the adjustment of the relative electrode position in block 396 may
account for
and redistribute error (c) as discussed above. In particular, as noted above,
the error
may be incorporated into the placed or selected position for the electrodes
323.
Accordingly, the error may not be initially or at first pass properly
distributed amongst
26
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
the electrodes. In other words, the error in a calculation, such as from a
measurement,
may be associated with one electrode more than another when determining the
position
of the electrode based upon the calculated radius relative to other pairwise
electrodes.
Redistributing the error may better identify the distance between pairwise
electrodes
and allow for a better determination of the shape of the implantable device
226.
[0096] Initially, in adjusting the electrode relative positions of block 396,
a
calculation of expected pairwise capacitances may be made in block 400. The
calculation of expected pairwise capacitances may be based upon generally
known
calculations such as direct computation, conformal conversions, auxiliary
functions, and
numerical approximations. The calculated pairwise capacitances may be based
upon
the initialized placement of the electrodes in block 376. Accordingly, a
calculation may
be based upon the placement, including distance between, the electrodes 232.
The
calculation of the expected pairwise capacitance may be performed by a
processor
system, such as the processor system 44 as discussed above. An algorithm may
calculate the expected pairwise capacitances based upon the calculation
formula
discussed above.
[0097]After calculating the expected pairwise capacitances in block 400, based
upon the initialized tree from block 376, a difference between the expected
capacitances and the measured capacitances may be made by receiving measured
pairwise capacitances in block 402. Then, calculating a function (F0) with the
measured
capacitances and difference to expected capacitances (Au) in block 404. The
function
(Fo) may be any appropriate function, such as a least-squares function such as
In particular, the function may be a sum of the differences
between the measured and the expected capacitances between each pair of
selected
electrodes 232. Again, it is understood, that the pairs of electrodes may be a
pair of
each one electrode to each of the other electrode of any selected number of
pairs of
electrodes. Further, as discussed above, the measured capacitance and/or
expected
capacitance is based upon a distance between the electrodes. Given that the
distance
between a pair, a circle with the distance as a radius from the first
electrode should
intersect with a circle with the distance as a radius from a second electrode,
as
illustrated in Fig. 7A1 and 7A2 of where the electrode actually lies in space
relative to
the other electrode in the pair. Accordingly, a difference between a measured
and
expected (as calculated as discussed above) capacitance may be due to a
measurement error or a calculation error. Accordingly, the error E may be
distributed
27
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
amongst the selected or initialized position of the electrodes to provide a
better fit and
therefore minimize the function Fo
[0098] Once the function is calculated or determined in block 404, the
function
may be optimized over electrode positions in block 406. As discussed above,
the error
may be included in a calculation or positioning of each of the electrode 232
in space as
discussed and illustrated in block 376. Accordingly, distributing or applying
more error
to one electrode or another may optimize the fit or reduce the error between
the
expected and measured position of the various electrodes 232. The error may,
therefore, be adjusted or moved between the different electrodes 232 of the
pairwise
calculation or determination in block 406.
[0099]The optimization of the function, including the distribution of error to
minimize the function (Fo below) may be done in a selected manner, such as
selectively
or randomly applying the error to different ones of the electrodes. In various
embodiments, the error redistribution and/or determined shape may be adjusted
by
generally known random movements algorithms (e.g. a Monte Carlo method), local
optimization methods (e.g. gradient descents), global optimization methods
(e.g.
parallel tempering), and pattern recognition methods (e.g. deep neural
networks). It is
understood, however, that other appropriate optimization algorithms may be
used.
[0100]Accordingly, once the function Fo is optimized in block 406, a
determination of the optimized or determined relative electrode position may
be made
in block 410. The determined relative electrode positions may then be output
in block
414. The output may be any appropriate output, such as a signal to the
navigation
system 22, 24, rendering a display, etc.
[0101]The relative electrode positions in block 316, including as adjusted,
may
be used to determine relative shape of the implantable device 226. As
discussed
above, the relative positions of the electrodes 232 that are fixed or
positioned along the
length of the implantable device 226 may be used to determine a relative shape
of the
implantable device 226. The adjusted electrode relative to position in block
396 may be
used to determine or output a relative shape of the implantable device in
block 420. The
shape of the implantable device 226, as determined based upon the initialized
electrode relative positions in block 376 and/or the option adjusted electrode
relative
positions in block 396, is a relative shape of the implanted device 226.
Generally, as
discussed above, the implantable device 226 may be positioned or initially
positioned
relative to the subject 36. Accordingly, the shape of the implantable device
226
28
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
determined in block 420 may be used to determine the shape of the implantable
device
226 within the patient 36.
[0102]The determined relative shape of the implantable device 226 relative to
the patient 36 may then be combined with position information of the
implantable device
226 to determine a position of the implantable device 226 relative to the
patient 36. A
combination of the shape determined in block 420 and the determined position
may be
combined in block 424. The determined position of the implantable device 226
may be
based upon various tracking devices such as the tracking device 70 (e.g.
tracking
device 250). As discussed above, the tracking devices 70 associated with the
implantable device 226 may be registered to the patient 36 and/or the image
84.
[0103] For example, the tracking device 250 associated with the implantable
device 226 may be used to determine location of at least one point or portion
of the
implantable device 226. Therefore, the position of at least one point on the
implantable
device 226 is known relative to the patient 36. The determined shape from
block 420
may be known relative to the tracking device 250. Accordingly, the shape of
the
implantable device 226 relative to the tracked location of the tracking device
250 is
known or combined in block 424. Again, the determined shape in block 420 and
the
combination of the shape and determined position in block 424 may be formed by
executing instructions with the processor system 44a using the tracking
information
from the tracking device 250 and the determined shape from block 420.
[0104] Once combined in block 424, a determined position and shape combined
in block 424 may be output in block 428. The output shape and position may be
displayed as an icon (e.g. 26i) on the display device 40 including the image
84. It is
understood that the icon may be displayed on other display devices, but may
generally
be illustrated as an icon relative to image data of the subject 36. For
example, as
discussed above, the tracked location of the tracking device 250 may be
registered to
the patient 36. The patient (defining patient space or subject space) may be
registered
to the image 84 (defining image space). Accordingly, as the tracked location
of the
tracking device 250 is known relative to the image 40 and the shape is
determined
relative to the tracking device 250 the determined shape of the implantable
device 226
may be displayed as an icon on the image 40. The user 34 may view the
determined
shape and position of the implantable device 226 on the image 40 for assisting
and
determining whether the implantable device 226 is properly located in a
selected or final
location.
29
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
[0105]As discussed above, it is understood that the method 360, including the
various sub-algorithms or portions as discussed above, may be provided as
algorithms
and instructions executed by the processing system 44 including the processor
44a.
The algorithm, as discussed above, may be executed by the processor 44a based
upon
instructions stored on the memory 44b. The processor system 44, therefore, may
allow
for a fast and efficient manner of determining the shape of the implantable
device 226
within the patient 36 while not directly viewable by the user 34 and/or
imageable by an
imaging system. Accordingly, the method 360 allows for the processor system 44
to
efficiently and quickly determine a shape of the implantable device 226 and a
position
of the shape of the implantable device 226 for use during a selected procedure
or
operation.
[0106]The shape of the implantable device 226 may also be otherwise
determined and/or estimated. For example, an estimate of the shape of the
implantable
device 226 may be made with a single EM tracker, such as the EM tracker 250 at
the
tip of the implantable device 226. The previously tracked positions of the EM
tracking
device 250 may be used to determine or estimate a shape of the implantable
device
226. The trace of previous and/or current positions of the EM tracking device
250 may
be displayed with or without a current determined position of the EM tracking
device
250 with the display device. In addition to or alternatively to the single
tracking device,
multiple tracking devices may have positions determined simultaneously and/or
over a
past period of time. The past "traced" positions may be determined as a shape
or a
best fit to the past determined positions may be made. Again, the determined
shape
may be displayed if selected. For example, the shape may be displayed as an
icon
superimposed on the image 84.
[0107] With continuing reference to Figs. 1-6 and additional reference to Fig.
8,
the implantable device 226 may include a plurality of the electrodes 232. Each
of the
electrodes may be formed as small coils, as discussed above, which may be
operated
in a selected field or emit a selected field to assist in tracking the
location of each of the
electrodes 232. As generally understood by one skilled in the art, the coils
may sense
an electromagnetic field and generate a signal based upon the sensed field.
Alternatively the coils may emit a field that is sensed by a receiver.
Regardless, the
location of each of the electrodes 232 may be determined.
[0108] In various embodiments, even if the electrodes 232 are solid or single
piece members, one or more of the electrodes 232 may be connected together. It
is
understood that each of the electrodes is connected to the communication line
233. The
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
communication line 233, as discussed above, may allow for communication with
the
R&S 234 and/or other systems, such as the EP or EM tracking system 22, 24. As
discussed above, signals from the electrodes 232 may be transmitted to the
respective
tracking systems 22, 24 for selected position determination.
[0109] Further a connection 450 may be formed between one or more of the
electrodes 232. For example, and merely for ease of the following discussion,
a first
connection 450a may be between the second electrode 232ii and the third
electrode
232iii to form a first electrode coil 460. Further, a connection 450b may be
formed
between the fourth electrode 232iv and the fifth electrode 232v to form a
second
electrode coil 462. A third or alternative connection may interconnect more
than two of
the electrodes 232, such as the connection 452 that interconnects the sixth
electrode
232 vi and the ninth electrode 232ix to form a third electrode coil 466. The
connection
452 may, therefore, not connect or be in contact with the electrodes between
the sixth
electrode 232vi and the ninth electrode 232ix. Further, the connections, such
as the
connection 450 and 452 may electrically connect the selected electrodes. In
this
manner, the first electrode coil 460 may be smaller than the third electrode
coil 466.
The larger electrode coil 466 may generate a stronger signal or higher signal-
to-noise
ratio than the smaller electrode coil 460.
[0110]The electrode to electrode connections 450, 452 may be formed in any
appropriate manner. For example, a permanent or breakable connection may be
formed between each of the electrodes such as with a wire or other conductive
manner.
Further, the connections 450, 452, according to various embodiments, may be
formed
on the sleeve 290 such as with a trace or other appropriate mechanism to
temporarily
connect selected ones of the electrodes 232, but are removable once the sleeve
290 is
removed from the implantable device 226. As discussed above, the sleeve 290
may be
positioned on the implantable device 226 for positioning the implantable
device 226
within the patient 36. The sleeve 290 may include traces that form the
connections 450,
452 between selected ones of the electrodes 232. The connections 450, 452 may
be
substantially passive, and not be used to generate or transmit a signal or
power or form
a potential relative to other portions. The connections 450, 452, as discussed
further
herein, may simply be used to connect to the selected ones of the electrodes
232. The
connections of the electrode 232 to the communication line 233 may be used to
transmit or receive a signal relative to the selected electrodes 232.
[0111]With continuing reference to Fig. 8, an implantable device 226a may be
substantially similar to the implantable device 226 illustrated in Fig. 3. The
implantable
31
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
device 226a, however, may not include the EM tracking device 250.
Nevertheless,
various portions of the implantable device 226 may be operated in combination
with the
EM tracking system 24 to provide for a determination of the position and/or
orientation
of the implantable device 226a. The implantable device 226a, including the
communication line 233, may communicate with the EM tracking system 24 to
communicate a signal to the processor system 44 to assist in determining a
location of
at least a portion of the implantable device 226, according to various
embodiments, as
discussed herein.
[0112]According to various embodiments, the connections 450a and 450b may
connect to respective electrodes, such as adjacent electrodes. It is
understood,
however, that the connection 450a and 450b may not connect to adjacent
electrodes or
may not connect only two electrodes. As discussed above the connection 452 may
connect more than one electrode. Nevertheless, a signal from one or more
electrode
coils may be transmitted. Although the connected electrodes may be referred to
herein
as coils, it is understood that the coils may be formed due to the respective
connections, such as the connection 450a between the electrodes 232ii and
232iii, and
a single from the coil portion may be transmitted on the communication line
233 to the
EM tracking system 24. The connection 450a in combination with the two
electrodes
232ii, 232iii forms the coil 460. Similarly the coil 462 is formed due to the
connection
450b and a single coil 466 may be formed due to the connection 452 between the
electrodes 232vi to 232ix. It is understood that the coil 466 may be an
effectively larger
coil relative to the coils 460, 462. It is further understood, other coils may
be formed by
connections between the other electrodes in the implantable device 226 and
that the
illustrated ones are merely for use in the current discussion.
[0113] The coil 460 and 462 may be operated as two separate coils with the EM
navigation system 24. The signal from the coils 460, 462 may be transmitted on
the
communication line 233 to the EM navigation system 24, as discussed above. The
coils
may operate in the EM navigation system 24 similar to other generally known
coils, as
discussed above, in EM navigation systems. Accordingly, one skilled in the art
will
understand that the coils 460, 462 may be operated to sense a field (e.g. have
a current
induced in the coil due to an externally generated field) and/or emit a field
to be sensed
by a receiver. A signal transmitted through the communication line 233 may be
used by
the EM navigation system 24 to transmit to the processor system 44 and/or any
appropriate processor system to determine a location of the coils 460, 462.
The
32
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
individual location of either of the coils 460, 462 may be determined based
upon the
received signal.
[0114] In an alternative and/or complementary operation, a differential signal
between the two coils 460, 462 may be used to determine the position and
orientation
of the two coils 460, 462. In other words, a signal regarding the difference
in the sensed
fields between two nearly adjacent or adjacent coils may be transmitted. This
differential signal may reduce or eliminate the effect of noise or
interference on the
transmitted signal.
[0115]Accordingly, it is understood, that the coils 460, 462 may be operated
in
more than one operational manner to assist in determining a position of one or
more of
the coils 460, 462. The position of the implantable device 226, however, may
be
determined based upon a signal from the coils 460, 462 either individually
and/or as a
differential signal between the two coils 460, 462. Accordingly, the
electrodes 232 may
be operated as at least a pair to form a coil that may be used with the EM
navigation
system 24 to assist in determining a location of the coils 460, 462. It is
further
understood that all of the electrodes 232 may be combined as selected pairs to
form
coils along the length of the implantable device 226.
[0116] Furthermore the coil 466 may be formed based upon a connection 452 of
more than two of the electrodes 232. It is further understood that any
selected number
of the electrodes 232 may be connected to form a coil of a selected size.
Therefore any
appropriate or a selected number, such as ten or twelve of the electrodes 232
may be
combined to form a coil. Regardless of the number of the electrodes connected,
however, the connected or large coil 466 may also transmit a signal on the
communication line 233 to the EM navigation system 24. The signal to the EM
navigation system may be used to determine a location of the coil 466 to the
determined location of the implantable device 226. Again, the coil 466 may
operate in a
manner substantially similar to a coil as discussed above in the EM navigation
system.
[0117]Therefore, one skilled in the art will understand that one or more coils
may
be formed in the implantable device 226 to be used by the EM navigation system
24 to
determine a position (e.g. a location and orientation) of the implantable
device 226
based upon a determined location and orientation of one or more of the coils
460, 462,
466.
[0118] In addition to the position information determined by the EM navigation
system 24 due to the coils 460, 462, 466, a shape of the implantable device
226 may
also be determined as discussed above. Accordingly, the position of the
implantable
33
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
device 226 may be used to determine a location relative to the subject 36, in
a manner
similar to using the tracking devices 250, as discussed above. However, the
tracking
devices 250 may be eliminated from the implantable device 226a and one or more
of
the electrodes 232 may be combined to form a selected coil, such as the coil
460, and
a shape of the implantable device 226a may be determined in a manner somewhat
similar to that as discussed above. Therefore a tracking device, such as the
tracking
device 250 may not be incorporated as a separate element relative to the
implantable
device 226a to determine a location of the implantable device 226a.
[0119]In addition, with reference to Fig. 3 and/or Fig. 8, each of the
electrodes
232 may communicate with the EM tracking system 24. The EM tracking system
including a localizer array may generate a field that is sensed or generates a
potential
difference across two electrodes, such as the first electrode 232i and the
second
electrode 232ii. Although, the two electrodes 232i, 232ii are not connected
with a
conductive member associated or formed by the implantable device 226, the
electrodes
may be in electrical communication due to the environment in which they are
placed.
For example, the electrodes 232 are positioned or may be positioned within the
subject
36. The subject 36 may include an amount of conductivity where a potential
difference
may be determined and transmitted to the EM tracking system 24. The induced
voltage
may be based upon the field emitted by the localizer the EM navigation system
24 and
may be used by the EM navigation system 24 to determine the position of the
tracking
device or the potential differential between the two electrodes 232i and
232ii. Again,
one skilled in the art will understand that the electrical connection between
the
electrodes 232i and 232ii may form or allow for the determination of a
potential
differential to allow for tracking or determining a location of the electrodes
similar to the
tracking devices 250 in the EM tracking system, as discussed above.
[0120]Accordingly, it is understood that tracking devices 250 may not need to
be
incorporated into the implantable device 226, such as the implantable device
226a.
Nevertheless the included electrode 232 may be used with the EM tracking
system 24
to determine a position (e.g. a location and orientation) of at least a
portion of the
implantable device 226. This position may be used alone and/or in combination
with a
determined shape of the implantable device 226, as also discussed above.
Moreover,
the position of the implantable device 226 may be illustrated as an icon
superimposed
on the image 84 of the subject 36 once the position and/or shape are
determined.
Therefore, the user 34 may be able to view the determined position and shape
of the
implantable device 226 during a procedure.
34
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
[0121]Accordingly, the implantable device 226 may be positioned within the
cochlea 37 to be tracked with selected tracking systems, such as the EP
tracking
system 22 or the EM tracking system 24. As discussed above, selected EM
tracking
devices 70b may be associated with the implantable device 226, either
incorporated
into the implantable device 226 or provided in the removable member 290
relative to
the implantable device 226. The EM tracking device 70b may be tracked with the
EM
tracking system 24 to assist in determining the position of the implantable
device 226
and a shape of the implantable device 226, such as within cochlea 37. In such
systems, the transmission line 233 may be repurposed or re-tasked from an
initial
transmission of tracking device signals, during implantation of the
implantable device
226, to transmitting simulation signals when connected with the receiver and
stimulator
as a part of the cochlear stimulation system.
[0122] In addition to, or alternatively to, the EM tracking device 70b, the
electrodes 232 of the implantable device 226 may be used in the EP tracking
system 24
to resolve various features of the implantable device 226. For example, the
shape of
the implantable device 226 may be determined based upon relative capacitance
between various electrodes 232 of the implantable device 226, as discussed
above.
Thus, a shape of the implantable device 226 may be determined when positioning
the
implantable device 226 in the cochlea 37.
Further, the electrodes 232 of the
implantable device 226 may be used with axis electrodes, such as the axis
electrodes
60a-60c, to determine a position of the implantable device 226 within the
patient 36.
The determined position of the implantable device 226 using the EP tracking
system 24
may be registered to the image 84 or may be illustrated or determined relative
to the
axis electrodes 60a-60c. It is understood by one skilled in the art that the
EP tracking
system 22 is not required to register to the image 84, but may be tracked
relative to the
axis electrodes 60a-60c positioned on the patient 36. In any case, the shape
and/or
position of the implantable device 226 may be determined by measuring the
voltage
and/or capacitance at the individual electrode 232 along the length of the
implantable
device 226.
[0123]
Further, the translation or distance between the respective EM
tracking devices and the EP tracking devices can be determined using selected
external or additional image modalities. For example, fluoroscopy can be used
to
determine a distance between two tracking devices if both of the tracking
devices are
radio opaque. Although it can be selected to eliminate or substantially reduce
the use
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
of ionizing radiation during a procedure, such as may be used in fluoroscopy,
fluoroscopy can be minimally used to determine certain information.
[0124]
Additional imaging systems can also be used to obtain information of
the patient 36 or information regarding the mapping or trackable devices.
Imaging
systems can include ultrasound (US), computed tomography (CT), magnetic
resonance
imaging (MRI), and other appropriate imaging techniques can be used. For
example,
an US system can be used to image or view the position of the selected
tracking device
within the patient 36. An US transducer can be used to view the tracked device
and
determine its position in the patient 36. Accordingly, selected imaging
systems can be
used to image the location of the instrument within the patient 36. As
discussed above,
this can also be used to determine a distance between two tracked devices
within the
patient 36, such as for translation or registration purposes between the two
tracking
systems 22, 24.
[0125] The R&S 234 may also include various wired or wireless
communication systems for communicating with one or both of the tracking
systems 22,
24. The implantable device 226, which may be placed in a cochlea of the
subject 36,
may be connected to the R&S 234 during implantation of the implant 226. A
signal
from any of the selected tracking devices 70a (e.g. electrode 232), 70b (e.g.
tracking
device 250), as discussed above, may be communicated to the R&S 234. The
signal
may relate to various sensed fields, capacitances, etc., as discussed above.
The signal
may be communicated wirelessly to the respective tracking systems 22, 24.
Thus, a
direct connection of the tracking devices 70a, 70b from the cochlear implant
226 to the
respective tracking systems 22, 24 may not be necessary as a communication may
be
made first to the R&S 234 then to the respective tracking systems 22, 24. The
wireless
transmission may be any appropriate transmission such as one using known
Bluetooth transmission protocols and hardware, a wireless network (e.g. local
area
network) hardware and/or protocol or scheme such as IEEE 802.11 wireless LAN
systems, or other appropriate communication systems and protocols.
[0126]As illustrated above, image data may be acquired of the subject. The
image data may be used to generate the image 84 for display on the display
device 40,
or any appropriate display device. In various embodiments the user 34 may view
the
image 84 and make a determination of a selected or predetermined shape of the
implantable device 226 once positioned in the patient 36. The user 34 may
enter or
save the predetermined shape in the memory 44b by entering the selected shape
or
predetermined shape with an input, such as the keyboard 48. It is understood
that the
36
CA 03090802 2020-08-07
WO 2019/157004
PCT/US2019/016764
selection or predetermination of a shape of the implantable device 226 may be
made at
any appropriate time and need not be made in an operating theater, as
illustrated in
Fig. 1.
[0127] Nevertheless, a predetermined shape may be transferred to the memory
44b for accessing with the processor 44a. Accordingly, the predetermined shape
of the
implant 226 may be illustrated relative to the image 84, such as with an icon
superimposed on the image 84. During the procedure, such as during positioning
the
implantable device 226 in the patient 36, the determined shape and/or
position, as
discussed above, of the implantable device 226 may be illustrated on the
display 40.
The user 34 may then compare the determined or tracked position and/or shape
of the
implantable device 226 relative to the predetermined position and/or shape.
Moreover,
the work station 44 may execute instructions stored on the memory device 44b
to make
a comparison, such as a fit comparison, of the determined shape and/or
position to the
predetermined shape and/or position. A percent or coefficient of matching of
the
present and tracked shape of the implantable device 226 relative to a
predetermined
shape may be displayed.
[0128] Moreover, the user 34 may make a determination (e.g. in real time)
based
upon the determined position and/or shape of the implantable device 226
displayed on
the display device 40 whether it is a selected shape. Accordingly, the user 34
may view
the display 40, or other appropriate selected display, during the procedure
relative to
the image 84. The user 34 may then view whether the shape of the image 84 is a
selected shape.
[0129]Accordingly, the tracking of the location of the implantable device 226
and
the method of determining the shape, as discussed above, may be used to
efficiently
perform a procedure to reduce procedure times and/or confirm or select
appropriate or
optimal locations for the implantable device 226.
[0130]
The foregoing description of the embodiments has been provided for
purposes of illustration and description. It is not intended to be exhaustive
or to limit the
invention. Individual elements or features of a particular embodiment are
generally not
limited to that particular embodiment, but, where applicable, are
interchangeable and
can be used in a selected embodiment, even if not specifically shown or
described.
The same may also be varied in many ways. Such variations are not to be
regarded as
a departure from the invention, and all such modifications are intended to be
included
within the scope of the invention.
37