Note: Descriptions are shown in the official language in which they were submitted.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
1
IMPROVED CONTAMINANT REMOVAL PROCESS
FIELD OF THE INVENTION
The present invention relates to an improved process for removing
contaminants, in particular to an improved removal of mercaptans in the
presence of other contaminants such as H2S and CO2, from non-aqueous
process streams, primarily gases but also including liquids.
BACKGROUND OF THE INVENTION
The removal of contaminants such as hydrogen sulfide (H2S),
carbon dioxide (CO2), carbon oxysulfide (COS), carbon disulfide (CS2),
disulfides and mercaptans, from a wide variety of feed streams, in
particular gaseous feed streams, is known to be important.
Such feed streams occur for example in the processing of natural
gas. The gas from natural gas fields mainly comprises methane and may
further comprise significant amounts of contaminants such as carbon
dioxide, readily up to 70% volume, hydrogen sulfide, mercaptans and
carbon oxysulfide. These contaminants may therefore need to be
removed to reduce further transportation costs as compressed gas, to
mitigate risk of corrosion in downstream systems and pipelines, to adjust
the heating value of the gas in order to suit the consumer needs, and/or to
enable the liquefaction of the natural gas into liquefied natural gas, LNG, a
means of transport of increasing use to bring natural gas from a remote
winning location to the gas consumption market.
Contaminant removal is also important in refinery streams such as
the liquefied petroleum gas (LPG) fraction, the off-gases from fluid
catalytic cracking (FCC), the hydrotreater off-gases, and the refinery gas
usually contain significant amounts of mercaptans and/or H25, and in
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
2
some also CO2 may be present. The distillation fractions intended for the
different fuel pools need to be desulfurized to alleviate the acid rain
problem caused by SOx emissions in the flue gas after combustion.
Several of these liquid fractions are therefore subjected to
hydrodesulfurization, a refinery step which converts the heavier sulfur
compounds into gaseous H25. In a so-called Claus plant, the H25 which is
collected from all these sources may then be converted into elemental
sulfur, which has become a product of commerce, for instance as
feedstock for the production of sulfuric acid.
Furthermore, contaminant removal may be important in the
production of synthesis gas, consisting primarily of H2, CO and possibly
also N2, a gas which is in various forms an important chemical building
block but also an important intermediate in the conversion of energy from
solid carbon containing streams, such as solid waste streams, tar sands,
oil shale and the like. It is usually desirable to remove mercaptans, H25
and CO2 from the synthesis gas, because mercaptans and H25 may be an
undesirable reactant therein and/or lead to emission of sulfur oxides (S0x)
upon combustion, undesirable in view of the acid rain concerns and
because the CO2 may for instance act as a disturbing inert in the
downstream process.
However, the simultaneous and efficient removal of mercaptans,
H25 and CO2 remains an important challenge, more so in view of the
increasingly stringent environmental and technical requirements and the
drive for a continuous cost reduction of gas processing plants.
The removal of contaminants from gases, and to a lesser extent
also from organic liquids such as for instance LPG streams, is typically
performed by washing the stream with an absorbent solution, usually at a
relatively low temperature to favour the absorption of the contaminant. A
major portion of the contaminant is absorbed in the absorbent solution,
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
3
and the rich absorbent solution is then routed to a regeneration step for
being regenerated. The lean absorbent solution from the regeneration
step is then usually cooled and recycled to the absorption step. A typical
absorbent solution contains absorbents of a basic nature, and amine
compounds have been preferred over alternatives such as hot potassium
carbonate. Even more preferred were alkanolamines. A conventional
absorbent is for instance 2-hydroxyethyl amine, also known as
monoethanol amine (MEA). The contaminants absorbed react with the
alkanolamine present in solution according to a reversible exothermic
reaction. With MEA, typically two molecules of MEA are required to absorb
one molecule of CO2. More complex, sterically hindered amines, including
tertiary amines, were found to provide improved stoichiometry.
These types of solvents which undergo a chemical reaction with the
contaminants and thereby form thermally regenerable salts, are often
called chemical solvents in this context. Alkanolamines derived from
primary, secondary and/or tertiary amines are the most widely used
chemical solvents. The most frequently used amines are derived from
ethanolamine, especially monoethanol amine (MEA), diethanolamine
(DEA), triethanolamine (TEA), diisopropanolamine (DIPA) and
methyldiethanolamine (MDEA). A disadvantage of such chemical solvents
is that in the regeneration step, the chemical bond between the solvent
and the contaminant needs to be broken which requires a large amount of
energy. Especially the regeneration of solutions of primary and secondary
alkanolamines consumes large volumes of steam. Since mercaptans are
weaker acids, their removal by chemical solvents will not be efficient when
H25 and CO2 are present because the latter react more quickly with the
alkanolamines. Therefore, mercaptans can react chemically with
alkanolamines but they are in competition with H25 and CO2.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
4
As an alternative to chemical solvents, so-called physical solvents
are often used for the capture of minor sulfuric components such as
mercaptans. Physical solvents rely on a physical absorption process, the
contaminants dissolve and thus are physically absorbed in the physical
solvent. The energy of the bond with the contaminant is typically smaller
than with most of the typical so-called chemical solvents. Typical physical
solvents are sulfolane and its derivatives, aliphatic acid amides, N-methyl-
2-pyrrolidone (NMP), methanol and mixtures of dialkylethers of
polyethylene glycols (Selexol , Union Carbide).
A mixture of a chemical and a physical solvent, so-called hybrid
solvents, can be employed as well. Such hybrid solvents can achieve very
low contaminant specifications in a single treating step through a
combination of bulk contaminant removal by the physical solvent and
stringent purification by the chemical solvent.
A well-known commercial hybrid solvent is the Sulfinol solvent
(Shell()) which is often used to treat refinery, natural, and synthesis gases.
The Sulfinol solvent is a mixture of sulfolane, an alkanolamine (either
DIPA or MDEA), and water. The solution with DIPA, referred to as
Sulfinol -D, is usually selected when complete removal of H25 and CO2
as well as deep removal of COS is required. The Sulfinol -D solvent is
used in most of the Sulfinol plants in operation worldwide. The solution
with MDEA, referred to as Sulfinol -M, is usually used for selective
removal of H25 in the presence of CO2 and is capable of partial removal of
COS. US 4,085,192 describes a process for the selective removal of H25
by using such a Sulfinol type solvent comprising an alkanolamine and
sulfolanes. The Sulfinol solvents shows a good absorption capacity for
H25, CO2 as well as mercaptans, however, it also shows co-absorption of
valuable hydrocarbons. Co-absorption of hydrocarbons should be avoided
to reduce hydrocarbon losses in the separated contaminants.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
US 2016/0288046 Al relates to processes for removal of acidic
sulfur constituents from fluid streams by using aqueous mixtures of an
amine constituent and a physical solvent. The amine constituents are
generally tertiary amines, such as notably 3-dimethylamino-1, 2-
5 propanediol (DMAPD), 3-diethylaminopropane-1,2-diol, 2-hydroxymethy1-
2-dimethylaminopropane-1,3-diol or 2-
hydroxymethy1-2-
diethylaminopropane-1,3-diol and sterically hindered amines such as 2-
hydroxymethy1-2-methylaminopropane-1,3-diol and 2-
methy1-2-
hydroxyethylaminopropanol. Preferred physical solvents include mono-
functional and di-functional alcohols having a dielectric constant of lower
than about 20, such as methoxytriglycol (MTG), methoxydiglycol (MDG),
ethoxytriglycol, butoxytriglycol and mixtures thereof or glycol ethers such
as methyl capped poly-ethylene glycol ethers and methyl capped poly-
propylene glycol. US 2016/0288046 Al is mainly concerned with the
removal of acidic sulfur constituents, in particular selective removal of H25
from a gas stream comprising other contaminants, for example N2, CO2,
CH4, C2H6, C3H8, H2, CO, H20, COS, HCN, NH3, 02, and/or mercaptans.
In US 2016/0288046 Al, it was found that the dielectric properties of the
amine constituent and the physical solvent was important. Hybrid solvents
with a low permittivity are most preferred. No mention is made about the
amount of co-absorption of hydrocarbons.
US 8,313,718 discloses a process for removing H2S and CO2 from
a hydrocarbon gas stream also containing a mercaptan by using an
absorption (scrubbing) composition comprising a solution of a chemical
solvent or physical solvent or mixture thereof and an effective amount of a
mercaptan inclusion compound, such as cyclodextrin, cal ixarene,
cucurbituril, a cryptand or mixtures thereof. Alkanolamines including for
example monoethanolamine (MEA), diethanolamine (DEA),
triethanolamine (TEA), diisopropylamine (DIPA), diglycolamine (DGA) and
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
6
methyldiethanolamine (MDEA) are mentioned as chemical solvents.
Cyclotetramethylene sulfone, aliphatic acid amides, N-alkylated
pyrrolidones such as N-methylpyrrolidone and corresponding piperidones,
methanol and mixtures of dialkylethers of polyethylene glycols are
mentioned as useful physical solvents. US 8,313,718 demonstrates that
the addition of such mercaptan inclusion compounds increases the
removal of mercaptans. A significant drawback of the process of US
8,313,718 is that it requires a composition with an extra ingredient on top
of the conventional hybrid solvent, which in turn creates extra raw material
costs. No mention is made about the amount of co-absorption of
hydrocarbons.
US 5,413,627 discloses a process for the selective removal of
sulfur compounds such as H25, COS, CS2 and thiols in the presence of at
least one of H2, N2, Ar, CO2, CO and aliphatic hydrocarbons using a
physical scrubbing agent. A heterocylic compound is used as a suitable
physical scrubbing agent which need to have five or more ring atoms, of
which 2 atoms are heteroatoms, one of which is nitrogen and the other of
which is oxygen or nitrogen. The nitrogen atom present in the ring is/are
either double bonded or single bonded but, if single bonded, the nitrogen
is organo-substituted. A wide variety of scrubbing agents are disclosed.
The physical scrubbing agent may further be used in a physically active
scrubbing agent composite with compounds selected from a long list of
various heterocycles. US 5,412,627 is mainly concerned with the removal
of sulfur compounds such as H25 in the presence of CO2 and not with the
effective simultaneous removal of mercaptans. No mention is made about
the amount of co-absorption of hydrocarbons.
US 2009/0299122 Al proposes a process for producing purified
hydrocarbon gas which does not incur the risk of significant losses of
hydrocarbon products. First the gas stream is partly purified by cooling the
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
7
gas stream and removing any solidified and/or liquefied contaminants.
The partly purified gas is then compressed and the compressed partly
purified gas is further contacted with an absorbing liquid. The absorbing
liquid may comprise an amine compound, such as alkanolamines, and
may further comprise a physical solvent. According to US 2009/0299122
Al suitable physical solvents include tetramethylene sulphone and
derivatives, amides of aliphatic carboxylic acids, N-alkyl pyrrolidone, in
particular N-methylpyrrolidine, N-alkyl piperidones, in particular N-methyl
piperidone, methanol, ethanol, ethylene glycol, polyethylene glycols,
mono- or di(C1-C4)alkyl ethers of ethylene glycol or polyethylene glycols. It
should be mentioned that US 2009/0299122 Al does not describe any
working examples. Therefore, US 2009/0299122 Al does not provide any
experimental evidence of an effective simultaneous removal of
mercaptans and other contaminants such as H25 and CO2 together with a
reduced risk of significant losses of hydrocarbon products.
In the prior art, the solvent N-methyl-2-pyrrolidone (NMP) is often
recommended as a physical solvent. However, NMP is under scrutiny
because of concerns over its potential health effects. NMP has
increasingly attracted attention as environmental regulators, first in
California and more recently in the European Union, have sought to
exercise control over the solvent primarily in markets where it represents
an inhalation hazard. Furthermore, NMP is now known to cause
reproductive toxicity (it is considered as being reprotoxic) and is being
labeled in the EU as "reprotoxic category 2" as from the 1st of December
2010. Formulations containing > 0.3 % of NMP have to be labelled as
such. Consequently, the use of the solvent is restricted to professional
users. NMP has been placed on the REACH "Substance of Very High
Concern" (SVHC) list and will expectedly, sooner or later, be put under
authorization or restriction.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
8
Therefore, there remains a need for a less toxic, more economical
and a more environmentally friendly absorbent solution which is able to
absorb mercaptans efficiently in the presence of other contaminants such
as H2S and CO2 while maintaining an excellent performance in a
conventional absorption-regeneration cycle and which further exhibits a
negligible co-absorption of hydrocarbons.
SUMMARY OF THE INVENTION
The inventors have now surprisingly found that it is possible to
provide an improved process fulfilling the above-mentioned needs.
The present invention provides a process comprising:
step a) contacting a feed stream comprising a contaminant with
an absorbent stream in a counter-current flow to produce a contaminant
depleted product stream depleted in the molar quantity of the contaminant
relative to the molar quantity of said contaminant in the feed stream, and a
contaminant enriched absorbent stream enriched in the molar quantity of
the contaminant relative to the molar quantity of said contaminant in the
absorbent stream; and
step b) treating the contaminant enriched absorbent stream to
form a gaseous stream comprising said contaminant and a regenerated
absorbent stream lean in the molar quantity of said contaminant relative to
the molar quantity of said contaminant in the contaminant enriched
absorbent stream;
wherein said absorbent stream comprises:
i. at least 15 weight
percent (wt.% herein after), relative
to the total weight of the absorbent stream, of at least one substituted 2-
pyrrolidone of general formula (I) [compound (A), herein after]:
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
9
H3c¨(cH4............., R1 0
n N __________________________________________ N)
H3C¨(CKr-- I \--R,
R2
M 0 formula (I)
wherein:
- each of R1 and R2, equal to or different from each other and at each
occurrence, is independently selected from H or CH3;
- each of R, equal to or different from each other and at each
occurrence, is selected from H or CH3;
- i is an integer in the range from 0 to 3;
- o is an integer in the range from 2 to 6; n is an integer in the range
from 0 to 4; m is an integer in the range from 0 to 4;
or,
ii. a mixture [mixture (M), herein after] comprising at
least one substituted 2-pyrrolidone of general formula (II) [compound (B),
herein after], herein below, and at least one amine compound of general
formula (III) [compound (C), herein after], herein below:
[ __________________________________ [R61 0
H3C C __ N)
R3 R5 1
P a formula (II)
-.,.
ri--,
R.: formula (III)
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
wherein:
- each of R', equal to or different from each other and at each
occurrence, is independently selected from H or CH3
- j is an integer in the range from 0 to 3
5 - each
of R3, R4, R5 and R6, equal to or different from each other and
at each occurrence, is independently selected from H or CH3;
- q is an integer in the range from 0 to 1; p is an integer in the range
from 1 to 6; with the proviso that when p =1 and q= 0 then R3 and
R4 are CH3 and when p + q = 2 then at least one of R3, R4, R5 or R6
10 is CH3;
- each of R7, R8 and R9, equal to or different from each other and at
each occurrence, is independently selected from H, C1_10 alkyl, C1-10
alkoxyalcohol or a C1_10 alkanol group; with the proviso that at least
one of R7, R8 or R9 is a C1_10 alkoxyalcohol or a C1_10 alkanol group.
The present invention further provides for a composition comprising
the mixture (M).
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 shows a schematic diagram of an apparatus suitable for
removing contaminants from a gas stream.
DETAILED DESCRIPTION
The present invention will be described in the following with respect
to particular embodiments and with reference to certain drawings but the
invention is not limited thereto but only by the claims. Any drawings
described are only schematic and are non-limiting. In the drawings, the
size of some of the elements may be exaggerated and not drawn on scale
for illustrative purposes. The dimensions and the relative dimensions do
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
11
not necessarily correspond to actual reductions to practice of the
invention.
Furthermore, the terms first, second, third and the like in the
description and in the claims, are used for distinguishing between similar
elements and not necessarily for describing a sequential or chronological
order. The terms are interchangeable under appropriate circumstances
and the embodiments of the invention can operate in other sequences
than described or illustrated herein.
Moreover, the terms top, bottom, over, under and the like in the
description and the claims are used for descriptive purposes and not
necessarily for describing relative positions. The terms so used are
interchangeable under appropriate circumstances and the embodiments
of the invention described herein can operate in other orientations than
described or illustrated herein.
The term "comprising", used in the claims, should not be interpreted
as being restricted to the means listed thereafter; it does not exclude other
elements or steps. It needs to be interpreted as specifying the presence of
the stated features, integers, steps or components as referred to, but does
not preclude the presence or addition of one or more other features,
integers, steps or components, or groups thereof. Thus, the scope of the
expression "a device comprising means A and B" should not be limited to
devices consisting only of components A and B. It means that with respect
to the present invention, the only relevant components of the device are A
and B. Accordingly, the terms "comprising" and "including" encompass the
more restrictive terms "consisting essentially of" and "consisting of".
As used in the foregoing and hereinafter, the following definitions
apply unless otherwise noted.
The term "alkyl" - alone or in combination means an alkane-derived
radical, for example, CF-G alkyl defines a straight or branched alkyl
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
12
radical having from F to G carbon atoms, e.g. C1-4 alkyl defines a straight
or branched alkyl radical having from 1 to 4 carbon atoms such as for
example methyl, ethyl, 1-propyl, 2-propyl, 1-butyl, 2-butyl, 2-methyl-2-
propyl, 2-methyl-1-propyl. An alkyl group may be a straight chain alkyl or
branched alkyl.
The term "alkanol" - alone or in combination means any alkyl group
comprising one or more hydroxyl (¨ OH) functional groups, for example
CF-G alkanol defines a straight or branched alkanol having from F to G
carbon atoms, e.g. C1_4 alkanol defines a straight or branched alkanol
having from 1 to 4 carbon atoms. An alkanol group may be monohydric,
dihydric, trihydric, or polyhydric and may be a straight chain alkanol or a
branched chain alkanol.
The term "alkoxyalcohol" - alone or in combination means an
organic compound of general formula X-O-Y, wherein X is an alkyl group
substituted by ¨0-Y and wherein Y is an alkanol group. For example, CF-G
alkoxyalcohol defines an alkoxyalcohol having from F to G carbon atoms
in total, e.g. C1-4 alkoxyalcohol defines an alkoxyalcohol group having from
1 to 4 carbon atoms such as for example ethoxyethanol, methoxyethanol,
ethoxymethanol, methoxymethanol.
The term "alkanolamine" is intended to denote an amine compound
comprising at least one alkanol group and wherein further substituents are
selected among H, alkyl or alkanol groups. In this context, a primary
alkanolamine comprises one alkanol group and two hydrogens bonded to
the nitrogen atom, a secondary alkanolamine comprises at least one
alkanol group and at least one hydrogen bonded to the nitrogen atom, a
tertiary alkanolamine comprises at least one alkanol group and no
hydrogen bonded to the nitrogen atom.
The term "at least one substituted 2-pyrrolidone of general formula
(I)" is intended to denote one or more than one substituted 2-pyrrolidone
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
13
of general formula (I). Mixtures of substituted 2-pyrrolidones of general
formula (I) can also be used for the purpose of the invention. The term "at
least one substituted 2-pyrrolidone of general formula (II)" is intended to
denote one or more than one substituted 2-pyrrolidone of general formula
(II). Mixtures of substituted 2-pyrrolidones of general formula (II) can also
be used for the purpose of the invention. The term "at least one amine
compound of general formula (III)" is intended to denote one or more than
one amine compound of general formula (III). Mixtures of amine
compounds of general formula (III) can also be used for the purpose of the
invention.
It is understood that the term "quantity" relates to the property of
being measurable in terms of amounts. The term "quantity" may denote
the mass or weight or moles or any physical or in any case numerical
parameter, unless otherwise specified, able to indicate at least, at quantity
level, the presence either of a contaminant or of a mixture of
contaminants.
Within the context of the present invention, the expression
"contacting a feed stream comprising a contaminant with an absorbent
stream in a counter-current flow" is intended to denote that an absorbent
stream is contacting a feed stream comprising a contaminant in such a
way that said absorbent stream and said feed stream are approaching
each other in an opposite direction. It is further understood that the angle
at which said streams approach each other may deviate from 180 .
Within the context of the present invention, at least one of the
streams is a mobile stream. Therefore, at least the following three
situations may occur: a static feed stream is contacted with a mobile,
flowing absorbent stream is; a mobile, flowing feed stream is contacted
with a static absorbent stream; or a mobile, flowing feed stream is
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
14
contacted with a mobile, flowing absorbent stream. The term "at least one
stream" is intended to denote one or more than one stream.
As discussed above, in the prior art, the solvent N-methyl-2-
pyrrolidone (NMP) is often recommended as a physical solvent. The
Applicants have now found that only specific substituted 2-pyrrolidones of
general formula (I) and of general formula (II), as detailed above,
characterised by having notably specific selections of o, n and m in
formula (I) and p and q in formula (II), as defined above, can provide
absorbent streams which are more efficient in removing mercaptans from
a feed stream compared to NMP, as evidenced by the examples below.
The Applicants believe that in terms of chemical structure, the
elongation of the chain attached to the nitrogen of the substituted 2-
pyrrolidone of formula (I) or (II) according to the present invention to a
total of at least four carbon atoms, appears to yield absorbent streams
which are more efficient in removing mercaptans from a feed stream. As a
further advantage, compounds with an elongated chain such as N-n-butyl-
2-pyrrolidone were found to be non-reprotoxic.
The Applicants have further found that the specific selection of o, n
and m in formula (I) and p and q in formula (II), as defined above, in terms
of chemical structure also restricts the chain length of the chain attached
to the nitrogen of the substituted 2-pyrrolidone of formula (I) or (II)
according to the present invention, and appears to yield absorbent
streams which maintain a low tendency towards co-absorption of
hydrocarbons. This brings the advantage that less valuable hydrocarbon
compounds are lost in the separated contaminants and further brings a
higher process selectivity towards the contaminants.
In a preferred embodiment of the process according to the present
invention, in compound (A) of general formula (I) i is an integer in the
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
range from 0 to 1, more preferably in compound (A) of general formula (I) i
is O.
In a preferred embodiment of the process according to the present
invention, each of R1 and R2 in compound (A) of general formula (I) are
5 H.
In a preferred embodiment of the process according to the present
invention, in compound (A) of general formula (I) o is an integer in the
range from 2 to 5, more preferably o is an integer in the range from 2 to 4,
even more preferably o is an integer selected from 2 or 3.
10 In a preferred embodiment of the process according to the present
invention, in compound (A) of general formula (I) n is an integer in the
range from 0 to 3, more preferably n is an integer in the range from 0 to 2,
even more preferably n is an integer selected from 0 or 1, most preferably
in compound (A) of general formula (I) n is 0.
15 In a preferred embodiment of the process according to the present
invention, in compound (A) of general formula (I) m is an integer in the
range from 0 to 3, more preferably m is an integer in the range from 0 to 2,
even more preferably m is an integer selected from 0 or 1, most preferably
in compound (A) of general formula (I) m is 0.
In a preferred embodiment of the process according to the present
invention, in compound (B) of general formula (II) j is an integer in the
range from 0 to 1, more preferably in compound (B) of general formula (II)
j is O.
In one embodiment of the process according to the present
invention, each of R5 and R6 in compound (B) of general formula (II) is H,
each of R3 and R4 in compound (B) of general formula (II) equal to or
different from each other and at each occurrence, is independently
selected from H or CH3, q is 1, p is an integer in the range from 1 to 6;
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
16
with the proviso that when p + q = 2 then at least one of R3, R4, R5 or R6 is
CH3.
In a preferred embodiment of the process according to the present
invention, each of R3, R4, R5 and R6 in compound (B) of general formula
(II) is H, q is 1 and p is an integer in the range from 2 to 6.
In a more preferred embodiment of the process according to the
present invention, each of R3, R4, R5 and R6 in compound (B) of general
formula (II) is H, q is 1 and p is 2, 3 or 4, more preferably p is 2.
In one embodiment of the process according to the present
invention, each of R7, R8 and R9 in compound (C) of general formula (III),
are independently selected from H, C1-5 alkyl, C1-5 alkoxyalcohol or C1-5
alkanol group; with the proviso that at least one of R7, R8 or R9 is a C1-5
alkoxyalcohol or C1-5 alkanol group.
In another embodiment of the process according to the present
invention, each of R7, R8 and R9 in compound (C) of general formula (III),
are independently selected from H, C1-5 alkyl or C1-5 alkanol group; with
the proviso that at least two of R7, R8 or R9 are a C1-5 alkanol group.
In yet another embodiment of the process according to the present
invention, each of R7, R8 and R9 in compound (C) of general formula (III),
are independently selected from C1-5 alkyl or C1-5 alkanol group; with the
proviso that at least one of R7, R8 or R9 is a C1-5 alkanol group.
In a preferred embodiment of the process according to the present
invention, each of R7 and R8 in compound (C) of general formula (III) are
an C1-5 alkanol group and R9 is selected from a C1-5 alkyl or C1-5
alkanol group.
In a more preferred embodiment of the process according to the
present invention, each of R7 and R8 in compound (C) of general formula
(III) are an C1_5 alkanol group and R9 is a C1-5 alkyl group. More preferably,
each of R7, and R8 are an C1-4 alkanol group and R9 is a C1-4 alkyl group.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
17
Even more preferably, each of R7, and R8 are an C1_3 alkanol group and R9
is a C1_3 alkyl group.
In compounds (A) of the absorbent stream as used in step a) of the
process according to the present invention, preferably R1 and R2 are H
and i is 0. Preferred compounds (A) are thus selected from those of
formula (I-a) herein below:
H3c¨(CH2).............., H 0
n N [II
1
H3C4CH2T: H ______________________________________
o (formula I-a)
wherein:
- o is an integer in the range from 2 to 4; n is an integer selected
from 0 or 1; m is an integer selected from 0 or 1.
Typical compounds (A) suitable for use in step a) of the process of
the present invention may include, but are not limited to,
dimethylaminopropy1-2-pyrrolidone (DMAP-2-
pyrrolidone),
dimethylaminoethy1-2-pyrrolidone
(DMAE-2-pyrrolidone),
diethylaminopropy1-2-pyrrolidone (DEAP-2-pyrrolidone) or a mixture of two
or more thereof.
More preferably, in compounds (A) comprised in the absorbent
stream as used in step a) of the process according to the present
invention, o is an integer in the range from 2 to 4; n is 0 and m is 0. More
preferred compounds (A) are thus selected from those of formula (I-b)
herein below:
H3C
NC [-I-N1\)
H
/ I
H3C 0
formula (I-b)
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
18
wherein:
- o is an integer in the
range from 2 to 4.
According to a preferred embodiment of the process according to
the present invention, the compounds (A) are selected from those of
formula (I-c) or formula (I-d) herein below:
H3C
\ 0
N-CH2-CH2-N)
/ \
H3C formula (I-c)
H3C
\ 0
N-CH2-CH2-CH2-N
/ \
H3C formula (I-d)
The inventors have found that compound (A) as specified herein,
as comprised in the absorbent stream in step a) of the process according
to the present invention, can simultaneously act as a physical solvent and
as a chemical solvent, thereby forming a so-called hybrid solvent on its
own. Without being bound to this theory, the inventors believe that the
pyrrolidone ring in compound (A) offers an increased ability to dissolve
mercaptans while the tertiary amine substituent on the pyrrolidone ring in
compound (A) can react with other contaminants such as CO2 and H2S.
The inventors have further found that when compound (A) is used
in the absorbent stream, a minimum amount of 15 wt.% of compound (A),
relative to the total weight of the absorbent stream, is required to achieve
an effective removal of mercaptans in the presence of other contaminants
such as H2S and CO2.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
19
In compounds (B) comprised in the mixture (M) as used in step a)
of the process according to the present invention, preferably R5 and R6
are H and j is 0. Preferred compounds (B) are thus selected from those of
formula (II-a) herein below:
o
Fi3c [71 '' C-N,)5
, ,
R3 H
P (formula II-a)
wherein:
- g is l
each of p is a
f R3 and tendinR4g,ear in thereind range
frentolym 2 to 6
selected from H or CH3;
-
Typical compounds (B) suitable for use in step a) of the process of
the present invention may thus include, but are not limited to, specific N-
alkyl 2-pyrrolidones such as N-n-butyl-2-pyrrolidone, N-sec-butyl-2-
pyrrolidone, N-isobuty1-2-pyrrolidone, N-tert-butyl-2-pyrrolidone, N-n-
penty1-2-pyrrolidone, N-sec-penty1-2-pyrrolidone, N-tert-penty1-
2-
pyrrolidone, N-3-penty1-2-pyrrolidone, N-isopenty1-2-pyrrolidone, N-
neopenty1-2-pyrrolidone, N-n-hexy1-2-pyrrolidone, N-
n-hepty1-2-
pyrrolidone, N-n-octy1-2-pyrrolidone, or a mixture of two or more thereof.
The inventors have found that in terms of chemical structure,
elongation of the carbon chain attached to the nitrogen of the pyrrolidone
ring in compound (B) as used in step a) according to the present
invention, to at least four carbon atoms yields absorbent streams which
are more effective in removing mercaptans from a feed stream compared
to NMP, as evidenced by the examples below and furthermore are non-
reprotoxic. The inventors have further found that compounds (B)
according to the present invention, in which the carbon chain attached to
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
the nitrogen of the pyrrolidone ring is restricted to at most 8 carbon atoms,
maintain a low tendency towards co-absorption of hydrocarbons.
More preferably, in compounds (B) comprised in the mixture (M) as
used in step a) of the process according to the present invention, R3, R4,
5 R5 and R6 are H and j is 0. More preferred compounds (B) are thus
selected from those of formula (II-b) herein below:
o
H3c [FC ] Y c-N\)5
H H
P (formula II-b)
10 wherein:
- p is an integer in the range from 2 to 6.
According to one embodiment of the process according to the
present invention, the compounds (B) are selected from those of formula
(II-c) to formula (II-g) herein below:
o
H3c¨cH2¨cH2¨CH2¨N
\)5 (formula II-c)
o
H3C--+CH2H-N
4 \)5 (formula II-d)
o
H3c H-CH2H¨N
5 \)5 (formula II-e)
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
21
o
H3c ¨FcH2-1¨N )6 \ ____________________________ (formula II-f)
o
H3C---ECH2H-N
7 \)5 (formula II-g)
According to a preferred embodiment of the process according to
the present invention, the compounds (B) are selected from those of
formula (II-c) to formula (II-e), as specified here above.
According to a more preferred embodiment, the compound (B) in
the absorbent stream as used in step a) of the process according to the
present invention, is a compound of formula (II-c):
o
H3c¨cH2¨cH2¨CH2¨N
\)5 (formula II-c)
In compounds (C) comprised in the mixture (M) as used in step a)
of the process according to the present invention, preferably each of R7,
R8 and R9, are independently selected from H, C1-4 alkyl, C1-4
alkoxyalcohol or C1-4 alkanol group; with the proviso that at least one of
R7, R8 or R9 is a C1-4 alkoxyalcohol or C1-3 alkanol group.
Typical compounds (C) suitable for use in step a) of the process of
the present invention may include primary, secondary and tertiary
alkanolamines such as, but not limited to, monoethanolamine (MEA),
diethanolamine (DEA), triethanolamine
(TEA),
monomethylmonoethanolamine (MMEA), diethylmonoethanolamine,
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
22
diisopropanollamine (DIPA), diglycolamine (DGA),
and
methyldiethanolamine (MDEA) or a mixture of two or more thereof.
Preferably, the compounds (C) comprised in the mixture (M) as
used in step a) of the process of the present invention are chosen among
tertiary alkanolamines such as notably, triethanolamine (TEA),
diethylmonoethanolamine and methyldiethanolamine (MDEA). More
preferably, the compounds (C) comprised in the absorbent stream as
used in step a) of the process of the present invention is
methyldiethanolamine (MDEA).
The inventors have found that even in the presence of a compound
(B) and comprised in a mixture (M) as used in step a) of the process
according to the present invention, tertiary alkanolamines rarely form
irreversible components with impurities. Furthermore, tertiary
alkanolamines comprised in a mixture (M) bring the advantage to be more
stable thermally and chemically under typical operating conditions and
usually they require less energy to be regenerated.
In certain embodiments, the compound (C) comprised in the
mixture (M) as used in step a) of the process according to the present
invention is diglycolamine (DGA).
A non-limitative example of a commercially available tertiary
alkanolamine is AdapT 100 which is available from the Eastman
Chemical Company.
It is further understood that all definitions and preferences as described for
compound (A) and compound (C) above equally apply for this
embodiment and all further embodiments, as described below.
As said above, the absorbent stream in step a) of the process
according to the present invention comprises at least 15 wt.%, relative to
the total weight of the absorbent stream, of at least one compound (A) of
general formula (I), as detailed above.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
23
Advantageously, the weight percent of the compound (A)
comprised in the absorbent stream as used in step a), relative to the total
weight of the absorbent stream, is equal to or greater than 20 wt.%,
preferably equal to or greater than 25 wt.%, preferably equal to or greater
than 30 wt.%, preferably equal to or greater than 35 wt.%, preferably
equal to or greater than 40 wt.%, preferably equal to or greater than 45
wt.%, preferably equal to or greater than 50 wt.%, preferably equal to or
greater than 55 wt.%, preferably equal to or greater than 60 wt.%,
preferably equal to or greater than 65 wt.%, preferably equal to or greater
than 70 wt.%, preferably equal to or greater than 75 wt.%, preferably
equal to or greater than 80 wt.%, preferably equal to or greater than 85
wt.%, preferably equal to or greater than 90 wt.%, preferably equal to or
greater than 95 wt.%, preferably equal to or greater than 98 wt.%.
It is further understood that, the upper value of the weight percent
of the compound (A) comprised in the absorbent stream as used in step
a), relative to the total weight of the absorbent stream, is not particularly
limited but is advantageously equal to or less than 99 wt.%, preferably
equal to or less than 95 wt.%, preferably equal to or less than 90 wt.%,
preferably equal to or less than 85 wt.%.
As explained above, the inventors have found that compounds (A)
can simultaneously act as a physical solvent and as a chemical solvent.
Therefore, compounds (A) can be used as a solvent as a part of the
absorbent stream or as an absorbent stream as such.
In one embodiment, the absorbent stream in step a) of the process
according to the present invention consists essentially of compounds (A)
of general formula (I), as detailed above.
For the purpose of the present invention, the expression "consists
essentially of" is intended to denote that any additional ingredient is
present in an amount of at most 5 wt.%, preferably at most 2 wt.%,
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
24
relative to the total weight of the absorbent stream. For example, any
additional ingredient, different from compound (A), as detailed above, is
present in an amount of at most 5 wt.%, preferably at most 2 wt.%,
relative to the total weight of the absorbent stream.
The inventors have further found that when the compound (A), as
detailed above, is further mixed with a compound (C) of general formula
(III), as detailed above, good results were also obtained.
According to a preferred embodiment, the absorbent stream in step
a) of the process according to the present invention comprises at least 15
wt.%, relative to the total weight of the absorbent stream, of at least one
compound (A) of general formula (I), as detailed above, and further
comprises at least one compound (C) of general formula (III), as detailed
above.
Advantageously, the at least one amine compound (C) of general
formula (III) is comprised in the absorbent stream in an amount
corresponding to a weight ratio of the compound (C) to the compound (A)
of at least 0.5:1, preferably at least 0.75:1, more preferably at least 1:1,
even more preferably at least 1.5:1, yet even more preferably at least
1.75:1. It is further understood that a weight ratio of the compound (C) to
the compound (A) in the absorbent stream is generally at most 5:1,
preferably at most 4:1, more preferably at most 3.5:1, more preferably at
most 3:1, more preferably at most 2.5:1.
According to certain embodiments of the present invention, the
absorbent streams as used in step a) of the process of the present
invention may further comprise water.
When water is present in the absorbent stream, then the water is
present in the absorbent stream in an amount of at least 0.5 wt.%,
preferably of at least 5 wt.%, preferably of at least 10 wt.%, more
preferably at least 15 wt.%, even more preferably at least 20 wt.%, and
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
even more preferably at least 22 wt.%, relative to the total amount of all
components of the absorbent stream.
It is further understood that the water is present in the absorbent
stream in an amount of at most 85 wt.%, preferably of at most 70 wt.%,
5 more preferably at most 60 wt.%, even more preferably at most 50 wt.%,
more preferably at most 40 wt.%, even more preferably at most 30 wt.%,
more preferably at most 28 wt.%, relative to the total amount of all
components of the absorbent stream.
According to a more preferred embodiment, the absorbent stream
10 in step a) of the process according to the present invention comprises
a
composition [composition (C), herein after], comprising at least 15 wt.% of
at least one compound (A) of general formula (I), as detailed above,
relative to the total weight of the composition (C), at least one compound
(C) of general formula (III), as detailed above, and water.
15
Advantageously, the water is comprised in composition (C) in an
amount corresponding to a weight ratio of the water to the compound (A)
of at least 0.25:1, preferably at least 0.3:1, more preferably at least 0.4:1,
even more preferably at least 0.6:1, yet even more preferably at least
0.9:1. It is further understood that the weight ratio of the water to the
20 compound (A) in the composition (C) is generally at most 4:1,
preferably
at most 3:1, more preferably at most 2.5:1, even more preferably at most
2:1, even more preferably at most 1.5:1, even more preferably at most
1.1:1.
Advantageously, the weight percent of the compound (A)
25 comprised in the composition (C) as used in step a), relative to the
total
weight of the composition (C), is advantageously equal to or greater than
18 wt.%, preferably equal to or greater than 20 wt.%, more preferably
equal to or greater than 21 wt.%, even more preferably equal to or greater
than 22 wt.%.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
26
It is further understood that, the upper value of the weight percent
of the compound (A) comprised in the composition (C) as used in step a),
relative to the total weight of the composition (C), is not particularly
limited
but is advantageously equal to or less than 50 wt.%, preferably equal to or
less than 40 wt.%, more preferably equal to or less than 35 wt.%, even
more preferably equal to or less than 30 wt.%, even more preferably equal
to or less than 29 wt.%, most preferably equal to or less than 28 wt.%.
Advantageously, the weight percent of the compound (C)
comprised in the composition (C) as used in step a), relative to the total
weight of the composition (C), is advantageously equal to or greater than
2 wt.%, preferably equal to or greater than 5 wt.%, preferably equal to or
greater than 10 wt.%, more preferably equal to or greater than 20 wt.%,
even more preferably equal to or greater than 30 wt.%, yet even more
preferably equal to or greater than 40 wt.%, most preferably equal to or
greater than 45 wt.%.
It is further understood that, the upper value of the weight percent
of the compound (C) comprised in the composition (C) as used in step a),
relative to the total weight of the composition (C), is advantageously equal
to or less than 85 wt.%, preferably equal to or less than 80 wt.%, more
preferably equal to or less than 75 wt.%, more preferably equal to or less
than 70 wt.%, more preferably equal to or less than 65 wt.%, even more
preferably equal to or less than 60 wt.%, yet even more preferably equal
to or less than 55 wt.%.
It is further understood that all definitions and preferences as
described for compound (B) and compound (C) above equally apply for
this embodiment and all further embodiments, as described below.
As said above, the absorbent stream in step a) of the process
according to the present invention may comprise a mixture (M), as
detailed above.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
27
According to one embodiment of the process according to the
present invention, the at least one compound (B) of general formula (II) is
advantageously comprised in the mixture (M) in an amount corresponding
to a weight ratio of the compound (B) to the compound (C) of at least
0.05:1, preferably at least 0.1:1, more preferably at least 0.2:1, more
preferably at least 0.3:1, more preferably at least 0.35:1, even more
preferably at least 0.4:1, yet even more preferably at least 0.45:1. It is
further understood that a weight ratio of the compound (C) to the
compound (A) in the mixture (M) is generally at most 2:1, preferably at
most 1.5:1, more preferably at most 1:1, more preferably at most 0.75:1
even more preferably at most 0.65:1, yet even more preferably at most
0.55:1.
In a preferred embodiment of the process according to the present
invention, the weight percent of the compound (B) comprised in the
mixture (M) as used in step a), relative to the total weight of the mixture
(M), is advantageously equal to or greater than 5 wt.%, preferably equal to
or greater than 10 wt.%, more preferably equal to or greater than 15 wt.%,
more preferably equal to or greater than 18 wt.%, more preferably equal to
or greater than 20 wt.%, even more preferably equal to or greater than 22
wt.%, yet even more preferably equal to or greater than 25 wt.%.
It is further understood that, the upper value of the weight percent
of the compound (B) comprised in the mixture (M) as used in step a),
relative to the total weight of the mixture (M), is advantageously equal to
or less than 50 wt.%, preferably equal to or less than 45 wt.%, more
preferably equal to or less than 40 wt.%, more preferably equal to or less
than 35 wt.%, more preferably equal to or less than 30 wt.%, even more
preferably equal to or less than 29 wt.%, most preferably equal to or less
than 28 wt.%.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
28
In a preferred embodiment of the process according to the present
invention, the weight percent of the compound (C) comprised in the
mixture (M) as used in step a), relative to the total weight of the mixture
(M), is advantageously equal to or greater than 2 wt.%, preferably equal to
or greater than 5 wt.%, preferably equal to or greater than 10 wt.%, more
preferably equal to or greater than 20 wt.%, even more preferably equal to
or greater than 30 wt.%, yet even more preferably equal to or greater than
40 wt.%, most preferably equal to or greater than 45 wt.%.
It is further understood that, the upper value of the weight percent
of the compound (C) comprised in mixture (M) as used in step a), relative
to the total weight of the mixture (M), is advantageously equal to or less
than 85 wt.%, preferably equal to or less than 80 wt.%, more preferably
equal to or less than 75 wt.%, more preferably equal to or less than 70
wt.%, more preferably equal to or less than 65 wt.%, even more preferably
equal to or less than 60 wt.%, yet even more preferably equal to or less
than 55 wt.%.
According to one embodiment, the absorbent stream as used in
step a) of the process according to the present invention consists
essentially of:
- 15 wt.% - 40 wt.% of at least one compound (A) of
general formula (II), as defined above,
- 30 wt.% - 65 wt.% of at least one compound (C) of
general formula (III), as defined above,
- 15 wt.% - 40 wt.% of water,
all wt.% are relative to the total weight of the absorbent stream.
It is further understood that in this embodiment, any additional
ingredient, different from compound (A), as detailed above, compound
(C), as detailed above, and water, is present in an amount of at most 5
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
29
wt.%, preferably at most 2 wt.%, relative to the total weight of the
absorbent stream.
Preferably, the mixture (M) as used in step a) of the process
according to the present invention further comprises water.
In this preferred embodiment of the process according to the
present invention, the weight percent of the compound (B) comprised in
the mixture (M) as used in step a), relative to the total weight of the
mixture (M), is advantageously equal to or greater than 5 wt.%, preferably
equal to or greater than 10 wt.%, more preferably equal to or greater than
15 wt.%, more preferably equal to or greater than 18 wt.%, more
preferably equal to or greater than 20 wt.%, even more preferably equal to
or greater than 22 wt.%.
It is further understood that the upper value of the weight percent of
the compound (B) comprised in the mixture (M) as used in step a), relative
to the total weight of the mixture (M), is advantageously equal to or less
than 50 wt.%, preferably equal to or less than 45 wt.%, more preferably
equal to or less than 40 wt.%, more preferably equal to or less than 35
wt.%, more preferably equal to or less than 30 wt.%, even more preferably
equal to or less than 28 wt.%.
In a preferred embodiment of the process according to the present
invention, the weight percent of the compound (C) comprised in mixture
(M) as used in step a), relative to the total weight of the mixture (M), is
advantageously equal to or greater than 2 wt.%, preferably equal to or
greater than 5 wt.%, preferably equal to or greater than 10 wt.%, more
preferably equal to or greater than 20 wt.%, even more preferably equal to
or greater than 30 wt.%, yet even more preferably equal to or greater than
40 wt.%, most preferably equal to or greater than 45 wt.%.
It is further understood that the upper value of the weight percent of
the compound (C) comprised in the mixture (M) as used in step a), relative
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
to the total weight of the mixture (M), is advantageously equal to or less
than 70 wt.%, preferably equal to or less than 65 wt.%, more preferably
equal to or less than 60 wt.%, even more preferably equal to or less than
55 wt.%.
5
According to one embodiment of the process according to the
present invention, the water is typically added to mixture (M) in an amount
corresponding to a weight ratio of the water to the compound (B) of at
least 0.25:1, preferably at least 0.3:1, more preferably at least 0.4:1, even
more preferably at least 0.6:1, yet even more preferably at least 0.9:1. It is
10 further understood that a weight ratio of the water to the compound (B)
in
the absorbent stream is generally at most 4:1, preferably at most 3:1,
more preferably at most 2.5:1, even more preferably at most 2:1, yet even
more preferably at most 1.1:1.
Good results were obtained when the weight percent of the
15 compound (B) comprised in the mixture (M) as used in step a), relative
to
the total weight of the mixture (M), is from 10-35 wt.%, the weight percent
of the compound (C) in the mixture (M) as used in step a), relative to the
total weight of the mixture (M), is between 30-65 wt.% and the weight
percent of the water comprised in the mixture (M) as used in step a),
20 relative to the total weight of the mixture (M), is from 15-40 wt.%.
According to a preferred embodiment, the absorbent stream as
used in step a) of the process according to the present invention
comprises:
- 10 wt.% - 35 wt.% of at least one compound (B) of
25 general formula (II), as defined above,
- 30 wt.% - 65 wt.% of at least one compound (C) of
general formula (III), as defined above,
- 15 wt.% - 40 wt.% of water,
all wt.% are relative to the total weight of the absorbent stream.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
31
According to another embodiment, the absorbent stream as used in
step a) of the process according to the present invention consist
essentially of:
- 10 wt.% - 35 wt.% of at least one compound (B) of
general formula (II), as defined above,
- 30 wt.% - 65 wt.% of at least one compound (C) of
general formula (III), as defined above,
- 15 wt.% - 40 wt.% of water,
all wt.% are relative to the total weight of the absorbent stream.
It is further understood that in this embodiment, any additional
ingredient, different from compound (B), as detailed above, compound
(C), as detailed above, and water, is present in an amount of at most 5
wt.%, preferably at most 2 wt.%, relative to the total weight of the
absorbent stream.
If desired, the absorbent stream may further comprise an
accelerator for the reaction of the absorbent stream with at least one of
the contaminants, in particular for the reaction with carbon dioxide. The
accelerator brings a clear improvement to the kinetics of the system,
absorption rates are faster, loading amounts are higher, absorbent
stream/feed stream ratios are lower, design of the plant may be smaller
and the regeneration heat requirement may be lower, resulting in less
cooling capacity. A lower absorbent stream/feed stream ratio, may result
in a smaller design of the plant, absorber as well as regenerator.
Alternatively, when using the same absorbent stream/feed stream ratio,
an often considerably larger amount of contaminants may be removed.
Advantageously, the weight percent of the accelerator as
comprised in the absorbent stream as used in step a), relative to the total
weight of the absorbent stream, is from 0.1 to 20 wt.%, preferably at least
0.5 wt.%, preferably at least 1 wt.%, preferably at least 1.5 wt.%,
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
32
preferably at least 2 wt.%, preferably at least 2.5 wt.%, and either
alternatively or in combination therewith at most 18 wt.% by weight,
preferably at most 16 wt.%, preferably at most 14 wt.%, preferably at most
12 wt.%, preferably at most 10 wt.%, preferably at most 9 wt.%, even
more preferably at most 8.5 wt.%..
Typical accelerators suitable for use in the absorbent stream as
used in step a) of the process of the present invention may thus include,
but are not limited to, amines, alcohols, ketones, vinyl alcohols, vinyl
ketones, ether alcohols, ether ketones, glycols, polyethylene glycols,
polypropylene glycols, ethylene glycolpropylene glycol copolymers, glycol
ethers, thioglycols, thioalcohols, sulfones, sulfoxide, N-alkylated 2-
pyrrolidones, N-alkylated piperidones, cyclotetramethylenesulfones, N-
al kylformam ides, N-al kylacetam ides, alkyl
phosphates, al kylene
carbonates and/or dialkyl carbonates. More preferred candidates are
monoethanol amine (MEA), diethanol amine (DEA), monomethyl
ethanolamine (MMEA), piperazine, 2-methylpiperazine, N-methyl
piperazine, N-ethyl piperazine, N-hydroxyethyl piperazine (HEP), N-(2-
aminoethyl) piperazine, homopiperazine, piperidine and morpholine. Most
preferred as accelerator is piperazine.
In general, the feed stream comprises at least one contaminant
selected from mercaptans, hydrogen sulfide (H2S), carbon dioxide (CO2),
carbon oxysulfide (COS), carbon disulfide, (CS2), and disulfides.
In particular, the feed stream comprises mercaptans and at least
one other contaminant selected from H2S, CO2, COS, CS2and disulfides.
We have found that mercaptans are removed efficiently from feed
streams even in the presence of other contaminants such as H2S and
CO2. The inventors have found that compound (B) as specified herein,
can act as a physical solvent while compound (C) as specified herein, can
act as a chemical solvent, thereby forming a so-called hybrid solvent. The
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
33
inventors believe that the pyrrolidone ring in compound (B) offers an
increased ability to dissolve mercaptans while the amine compound (C) is
able to react with other contaminants such as CO2 and H2S. Alternatively,
the compound (A) as specified herein, can simultaneously act as a
physical solvent and as a chemical solvent, thereby forming a so-called
hybrid solvent on its own. The inventors believe that the pyrrolidone ring in
compound (A) offers an increased ability to dissolve mercaptans while the
tertiary amine substituent on the pyrrolidone ring in compound (A) can
react with other contaminants such as CO2 and H2S.
It is understood that in the step a), the molar quantity of the
contaminant in the contaminant depleted product stream, relative to the
molar quantity of the contaminant in the feed stream, is reduced.
Furthermore, the molar quantity of the contaminant in the enriched
absorbent stream, relative to the molar quantity of the contaminant in the
absorbent stream, has been increased.
The molar quantity of the contaminant in the contaminant depleted
product stream, relative to the molar quantity of the contaminant in the
feed stream, is reduced by at least 5 /0, preferably at least 10 /0, more
preferably at least 20 /0, even more preferably at least 30 /0, yet even
more preferably at least 40 % and most preferably at least 50 /0.
The molar quantity of the contaminant in the enriched absorbent
stream, relative to the molar quantity of the contaminant in the absorbent
stream, has been increased by at least 5 /0, preferably at least 10%, more
preferably at least 20%, even more preferably at least 30 /0, yet even
more preferably at least 40 % and most preferably at least 50 /0.
The molar quantity of mercaptans in the contaminant depleted
product stream, relative to the molar quantity of mercaptans in the feed
stream, is reduced by at least 5 %, preferably at least 10 %, more
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
34
preferably at least 20 /0, even more preferably at least 30 /0, yet even
more preferably at least 40 % and most preferably at least 50 /0.
Typical feed streams as used in step a) of the process according to
the present invention, such as natural gas or refinery gas streams and
synthesis gas, may comprise valuable hydrocarbons such as olefins and
alkanes, for example methane, ethane, propane, butane, pentane,
hexane, heptane and octane.
It is understood that in the enriched absorbent stream produced in
step a) of the process of the present invention, the molar quantity of
hydrocarbons, relative to the molar quantity of the hydrocarbons in the
feed stream remains low.
In one embodiment of the process according to the present
invention, the feed stream comprises carbon dioxide, and the amount of
CO2 in the feed stream to step a) is at least 5 volume % [vol. /0, herein
after], preferably at least 10 vol.%, preferably at least 15 vol.%, preferably
at least 20 vol.%, preferably at least 25 vol.%, preferably at least 30 vol.%,
preferably at least 35 vol.%, preferably at least 40 vol.%, preferably at
least 45 vol.%, preferably at least 50 vol.%, preferably at least 55 vol.%,
preferably at least 60 vol.%, preferably at least 65 vol.%.
In one embodiment of the process according to the present
invention, the feed stream comprises hydrogen sulfide, and the amount of
hydrogen sulfide in the feed stream to step a) is at least 1 vol.%,
preferably at least 5 vol.%, preferably at least 8 vol.%, preferably at least
10 vol.%, preferably at least 12 vol.%, preferably at least 15 vol.%,
preferably at least 18 vol.%.
In one embodiment of the process according to the present
invention, the feed stream comprises carbon oxysulfide, and the amount
of carbon oxysulfide in the feed stream to step a) is at least 0.1 ppmv,
preferably at least 0.5 ppmv, preferably at least 1 ppmv, preferably at least
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
5 ppmv, preferably at least 10 ppmv, preferably at least 15 ppmv,
preferably at least 20 ppmv, preferably at least 30 ppmv, preferably at
least 40 ppmv, preferably at least 50 ppmv, preferably at least 100 ppmv,
preferably at least 150 ppmv, preferably at least 200 ppmv, preferably at
5 least
300 ppmv, preferably at least 400 ppmv, preferably at least 500
ppmv, preferably at least 1000 ppmv, preferably at least 1500 ppmv,
preferably at least 2000 ppmv, preferably at least 3000 ppmv, preferably
at least 4000 ppmv, preferably at least 5000 ppmv, preferably at least
6000 ppmv, preferably at least 7000 ppmv, preferably at least 8000 ppmv,
10
preferably at least 9000 ppmv, preferably at least 10000 ppmv. The
applicants have found that the absorbent streams according to the present
invention are particularly effective for removing mercaptans.
In a more preferred embodiment of the process according to the
present invention, the feed stream comprises mercaptans, and the
15 amount
of mercaptans in the feed stream to step a) is at least 0.1 parts
per million by volume [ppmv, herein after], preferably at least 0.5 ppmv,
preferably at least 1 ppmv, preferably at least 5 ppmv, preferably at least
10 ppmv, preferably at least 15 ppmv, preferably at least 20 ppmv,
preferably at least 30 ppmv, preferably at least 40 ppmv, preferably at
20 least
50 ppmv, preferably at least 100 ppmv, preferably at least 150 ppmv,
preferably at least 200 ppmv, preferably at least 300 ppmv, preferably at
least 400 ppmv, preferably at least 500 ppmv, preferably at least 600
ppmv, preferably at least 700 ppmv, preferably at least 800 ppmv,
preferably at least 900 ppmv, preferably at least 1000 ppmv, preferably at
25 least
1100 ppmv, preferably at least 1200 ppmv, preferably at least 1300
ppmv, preferably at least 1400 ppmv, preferably at least 1500 ppmv,
preferably at least 2000 ppmv, preferably at least 3000 ppmv, preferably
at least 4000 ppmv, preferably at least 5000 ppmv, preferably at least
6000 ppmv, preferably at least 7000 ppmv, preferably at least 8000 ppmv,
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
36
preferably at least 9000 ppmv, preferably at least 10000 ppmv. The
applicants have found that the absorbent streams according to the present
invention are particularly effective for removing mercaptans.
It is further understood that the term "mobile, flowing feed stream"
may refer to a liquid or a gaseous mobile, flowing feed stream.
In one embodiment of the process according to the present
invention, the feed stream is a gaseous, mobile, flowing feed stream
which is contacted with a liquid static absorbent stream. This embodiment
is clearly illustrated in the examples below which were performed on lab-
scale.
In another embodiment of the process according to the present
invention, the feed stream is a liquid or gaseous mobile, flowing feed
stream which is contacted with a liquid mobile, flowing absorbent stream.
This embodiment is clearly illustrated in absorption tower 1 in Figure 1,
which inherently includes the two stream separations in the tower top and
in the tower bottom.
In an embodiment of the process according to the present
invention, step a) is performed at a pressure of at least 1 bar gauge,
preferably at least 5 bar gauge, preferably at least 10 bar gauge, more
preferably at least 15 bar gauge, even more preferably at least 20 bar
gauge, yet more preferably at least 25 bar gauge. The applicants have
found that the absorbent streams according to the present invention are
particularly effective and efficient under these higher pressures as
specified.
In an embodiment of the process according to the present
invention, step a) is performed at a pressure of at most 150 bar gauge,
preferably at most 120 bar gauge, more preferably at most 100 bar gauge,
even more preferably at most 90 bar gauge. The applicants have found
that higher pressures do not bring much further improvements of
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
37
performance of the step a). The applicants have found that, if the feed
stream needs to be pressurized in order to prepare it for the step a), that
further compression energy to reach pressures above the specified levels
is usually not justified by the additionally obtainable benefits.
In an embodiment of the process according to the present
invention, step a) is performed at a temperature of at least 10 C,
preferably at least 20 C, more preferably at least 30 C, even more
preferably at least 40 C.
In an embodiment of the process according to the present
invention, step a) is performed at a temperature of at most 90 C,
preferably at most 80 C, more preferably at most 70 C, even more
preferably at most 65 C.
In one embodiment, the process according to the present invention
further comprises a step c) in which at least part of the regenerated
absorbent stream from step b) may be recycled as at least part of the
absorbent stream to step a).
In an embodiment of the process according to the present
invention, step b) comprises the step of stripping at least a portion of the
contaminant enriched absorbent stream obtained from step a) in counter-
current with a gaseous stream containing water vapour in a stripping
tower, to form a tower bottom liquid stream which is a first regenerated
absorbent stream for the recycling in step c). The applicants have found
that steam stripping as prescribed is a very efficient method for breaking
the bonds between the contaminant and the absorbent stream, and for
separating the contaminant from the absorbent stream.
In an embodiment of the process according to the present
invention, step b) comprises the step of flashing at least a portion of the
contaminant enriched absorbent stream obtained from step a) to a lower
pressure for releasing a gaseous stream containing the contaminant from
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
38
a liquid stream which is a second regenerated absorbent stream for the
recycling in step c). The applicants have found that flashing may be an
even more efficient method, as compared to steam stripping, for breaking
the bonds between the contaminant and the absorbent stream, and for
separating the contaminant from the absorbent stream. The applicants
prefer to combine a flashing step with a downstream regeneration step,
which preferably is a steam stripping step.
In an embodiment of the process according to the present
invention, the second regenerated absorbent stream is recycled to step a).
The applicants have found that the flashing step may produce a second
regenerated absorbent stream which is suitable for recycling in step c) to
step a). This brings the advantage that this part of the absorbent stream
does not necessarily need to be processed through a more thorough
regeneration step, such as by steam stripping, before it is suitable for
participating in the step a).
In an embodiment of the process according to the present
invention, in step b) a first regenerated absorbent stream and a second
regenerated absorbent stream are formed for the recycling in step c),
whereby the second regenerated absorbent stream contains more
contaminant than the first regenerated absorbent stream, preferably step
a) being performed in an absorption tower and the first regenerated
absorbent stream being recycled to a top liquid feed tray in the absorption
tower and the second regenerated absorbent stream being recycled to an
intermediate feed tray in the absorption tower located lower than the top
liquid feed tray. The applicants have found that this embodiment takes full
advantage of the presence of a flashing step, without increasing the risk
for contaminant breakthrough in the step a), because the feed stream in
contact with the second regenerated absorbent stream still has to pass
the zone where it is contacted with the first regenerated absorbent stream.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
39
It is further understood that all definitions and preferences as
described above for mixture (M) as used in step a) of the process
according to the present invention, equally apply for this embodiment and
all further embodiments, as described below.
Another aspect of the present invention is the mixture (M), as
detailed above.
Another aspect of the present invention is a composition comprising
the mixture (M), as detailed above.
A further aspect of the present invention is an absorbent stream
comprising the mixture (M), as detailed above.
It is another aspect of the present invention to provide a use of an
absorbent stream comprising the mixture (M), as detailed above or at
least 15 wt.% of at least one compound (A), as detailed above.
In another aspect, the present invention provides for a contaminant
enriched absorbent stream comprising at least one absorbed contaminant
selected from hydrogen sulfide (H2S), carbon dioxide (CO2), carbon
oxysulfide (COS), carbon disulfide (CS2), disulfides or mercaptans, the
absorbent stream comprising the mixture (M), as detailed above or at
least 15 wt.% of at least one compound (A), as detailed above.
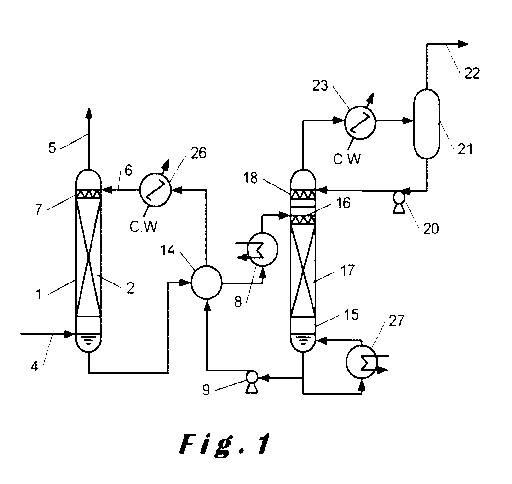
Fig. 1 is a schematic diagram of an apparatus for removing
contaminants according to the process of the present invention from a
gaseous feed stream. As shown in Fig. 1, gas feed is led to an absorption
tower 1 through a contaminant containing gas-feed inlet 4. In a packed
portion 2, the gaseous feed stream placed in the absorption tower 1 is
brought into contact in a counter flow with an absorbent stream fed from a
nozzle 7, and the contaminant is absorbed and removed from the gas by
the absorbent stream, and the contaminant depleted gas is discharged
through a contaminant depleted gas-discharge outlet 5. The absorbent
stream fed to the absorption tower 1 absorbs the contaminant, and is led
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
to a heat exchanger 14 and a heater 8 and heated and led to a
regeneration tower 15. In the regeneration tower 15, the absorbent stream
flows through a nozzle 16 and a packed portion 17 toward the lower
portion of the tower. During this time, contaminant is removed to
5 regenerate the absorbent stream. A part of the absorbent stream in the
bottom of the regenerator tower is routed over reboiler 27 to generate a
vapour stream for stripping the absorbent stream coming down the tower.
The regenerated absorbent stream is led by a pump 9 to the heat
exchanger 14 and an absorbent stream cooler 26 and fed back to the
10 absorption tower 1 through an absorbent stream feed inlet 6.
On the other hand, in the upper portion of the regeneration tower
15, the contaminant removed from the absorbent stream is brought into
contact with a reflux water fed from the nozzle 18, and cooled by a
regeneration tower reflux condenser 23, and, in a reflux drum 21, the
15 gaseous contaminant is separated from the reflux water formed by
condensing water vapour accompanying the contaminant, and may be led
to a contaminant recovery step through a recovered contaminant line 22.
The reflux water is fed by a reflux water pump 20 to the regeneration
tower 15.
EXAMPLES
The invention will be now described in more details with reference
to the following examples, whose purpose is merely illustrative and not
intended to limit the scope of the invention.
GENERAL PROCEDURE FOR DETERMINATION OF THE
ABSORPTION CAPACITIES
All experiments were performed using 30 g of an absorbent stream.
Lab-scale absorption experiments were carried out by contacting a feed
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
41
stream gas mixture comprising 1000 ppmv (parts per million by volume) n-
butane and 1000 ppmv methyl mercaptan (CH3SH) in nitrogen with the
various absorbent streams, at a temperature of 40 C and a pressure of 2
bar. The experimental absorption apparatus was comprising a double-
walled tubular absorber with a diameter of 7 mm, equipped with a
thermocouple and heated by a water bath. The pressure inside the
absorber was maintained at a constant pressure using a back-pressure
regulator mounted downstream of the tubular absorber. The gas flow rate
was 5.1 L.11-1, and the outlet gas passed through a condenser to avoid
evaporation of water from the absorber. When the feed stream gas
mixture was passed in an upward direction through the absorbent stream,
the resulting contaminant depleted product stream gas was depleted in n-
butane and methyl mercaptan until the contaminant enriched absorbent
stream became saturated. The composition of the outlet gas was
analysed at regular intervals using an Agilent 7890B Gas Chromatograph
(GC) equipped with a split injector and a flame ionization detector (290 C).
A capillary Rtx-Volatile Amine Column with dimensions of 60-meter x 0.32-
mm ID was employed. The GC oven temperature was set at 40 C and gas
samples were injected with a split ratio of 100:1. OpenLAB
Chromatography Data Systems software was used for data acquisition
and data processing. Once at room temperature, the absorber was
opened, contents transferred to a glass jar, and sampled for GC analysis
to determine the composition. The absorbent streams were evaluated in
five consecutive absorption¨regeneration cycles whereby regeneration
was performed by heating the solvents for 90 minutes at temperatures of
at least 80 C and a gas flow rate of 5.1 L h-1. Data of the first two cycles
were not incorporated into the analysis in order to make sure that the
absorption apparatus was fully equilibrated.
CA 03090928 2020-08-11
WO 2019/158591 PCT/EP2019/053555
42
To compare the performance of the different absorbent streams,
the concentration of methyl mercaptan and n-butane in the outlet gas was
followed in time. As long as the absorbent streams were not saturated, the
concentration of methyl mercaptan and/or n-butane in the outlet gas was
zero. Once the absorbent streams were nearing saturation, the outlet
concentrations of methyl mercaptan and/or n-butane (in ppmv) started to
increase (i.e. breakthrough). For the different absorbent streams, this
breakthrough took place at a different time. The solvent loadings were
approximated by integrating the areas above these respective
"breakthrough" curves (i.e. the ppmv concentration of methyl mercaptan or
n-butane in the outlet gas as a function of time (in minutes)) and by
subtracting the amount of gas used to fill the head space of the absorber.
The amount of absorbed methyl mercaptan and n-butane was calculated
by writing:
V 10-6 101325 1
n __________________________ * absorbed = AREAabove curve * 1000 * 60*
8.3143 * 273.15 m
wherein V is the flow rate of the gas phase (NL.11-1) and m the total mass
of absorbent stream (g).
The list of used materials according to the invention can be found in
Table 1.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
43
Table 1: List of products and description
Compound (A) according
to the invention Description
DMAP-2-pyrrolidone Dimethylaminopropy1-2-pyrrolidone
DMAE-2-pyrrolidone Dimethylaminoethyl -2-pyrrolidone
Compound (B) according
to the invention Description
N-tert-butyl-2-pyrrolidone N-tert-butyl-2-pyrrolidone
N-butyl-2-pyrrolidone N-butyl-2-pyrrolidone
N-octy1-2-pyrrolidone N-octy1-2-pyrrolidone
Compound (B) according
to the invention Description
MDEA Methyldiethanolamine
Reference compound Description
Sulfolane Sulfolane
2-Pyrrolidone 2-Pyrrolidone
N-methyl-2-pyrrolidone N-methyl-2-pyrrolidone
ABSORPTION PERFORMANCE
EXAMPLE 1-2
The absorbent stream of Examples 1-2 was a mixture (M) which
was prepared by mixing 25 wt.% of a compound (B), with 50 wt.% of
MDEA (i.e. compound (C)), and 25 wt.% of water. The absorption
performance data and the specific compounds (B) that were used are
summarized in Table 2 below.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
44
EXAMPLE 3-4
The absorbent stream of Examples 3-4 was a composition (C)
which was prepared by mixing 25 wt.% of a compound (A), with 50 wt.%
of MDEA (i.e. compound C), and 25 wt.% of water. The absorption
performance data and the specific compounds (A) that were used are
summarized in Table 2 below.
COMPARATIVE EXAMPLES 5-7
The absorbent streams of Comparative Examples 5 to 7 were
prepared according to the same procedure as Examples 1 to 2 except that
a reference compound was used which was not a compound (B)
according to general formula (II). Specifically, in Comparative Example 5,
sulfolane was used, in Comparative Example 6, 2-pyrrolidone was used
and in Comparative Example 7, N-methyl-2-pyrrolidone was used. The
absorption performance data of Comparative Example 5 were considered
as a reference since the absorbent stream comprising sulfolane is similar
to the well-known commercial Sulfinol -M solvent. The absorption
performance data are summarized in Table 2 below.
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
Table 2.
Name Reference or CH3SH concentration n-Butane
compound (A) (mmol. kg-1 solvent) concentration (mmol.
or compound kg-1 solvent)
(B) 1 2 3 1 2 3
Example 1 N-Butyl-2- 4.11 5.64 5.50 0.35 0.33
0.37
pyrrolidone
Example 2 N-Octy1-2- 4.09 4.13 N.D. 0.47 0.45
N.D.
pyrrolidone
Example 3 DMAP-2- 4.10 4.44 4.16 0.31 0.26
0.31
pyrrolidone
Example 4 DMAE-2- 4.12 4.21 4.13 0.34 0.25
0.35
pyrrolidone
Comparative Sulfolane 3.64 3.66 3.67 0.30 0.26 0.35
Example 5
Comparative 2-Pyrrolidone 3.17 3.00 3.05 0.23 0.20 0.28
Example 6
Comparative N-Methyl-2- 3.54 3.68 4.02 0.34 0.26 0.27
Example 7 pyrrolidone
For comparison purposes, it should be noted that the reference
absorbent solution of Comparative Example 5 comprising sulfolane, could
5 capture 3.64-3.67 mmol CH3SH and 0.26-0.35 mmol C4H10 per kg solvent
(Table 2). In the case of N-butyl-2-pyrrolidone (Example 1), CH3SH
loadings of between 4.11-5.64 mmol and C4H10 loadings of between 0.33-
0.37 mmol per kg solvent, were observed. Under the same conditions, the
results also indicate that the CH3SH absorption capacities of N-octy1-2-
10 pyrrolidone (Example 2), DMAP-2-pyrrolidone (Example 3), and DMAE-2-
pyrrolidone (Example 4) were comparable or superior to those of the
reference absorbent solution of Comparative Example 5 comprising
sulfolane. We inferred that these specific substitutions on the amide
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
46
nitrogen of the pyrrolidone ring positively impact the interactions of the
absorbent stream with the mercaptans while a low co-absorption of
hydrocarbons (such as n-butane) is maintained. Table 2 further indicates
that the CH3SH absorption values of the absorbent streams according to
the present invention (Examples 1-4) were comparable or superior to
those of the comparative absorbent solutions of Comparative Examples 5-
7.
EXAMPLE 8
The absorbent stream of Example 8 was a mixture (M) which was
prepared by mixing 30 wt.% of N-butyl-2-pyrrolidone (i.e. compound (B)),
with 50 wt.% of MDEA (i.e. compound (C)), and 25 wt.% of water. The
absorption performance data are summarized in Table 3 below.
COMPARATIVE EXAMPLE 9
The absorbent stream of Comparative Example 9 was prepared
according to the same procedure as Example 8 except that 30 wt.% of
sulfolane was used which was not a compound (B) according to general
formula (II). The absorption performance data are summarized in Table 3
below.
Table 3 shows typical absorption data (nabsorbed) for three
consecutive absorption-regeneration cycles for the reference absorbent
stream of Comparative Example 9 comprising 30 wt.% sulfolane and the
absorbent stream according to the invention comprising 30 wt.% of n-
butyl-2-pyrrolidone (i.e. compound B) (Example 8).
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
47
Table 3.
Name Reference or CH3SH concentration n-Butane
compound B (mmol. kg-1 solvent) concentration
(mmol.
kg-1 solvent)
1 2 3 1 2 3
Example 8 N-Butyl-2- 8.46 7.75 7.58 0.43 0.46
0.36
pyrrolidone
Comparative Sulfolane 5.22 4.91 5.35 0.33 0.33 0.37
Example 9
EXAMPLE 10
The absorbent stream of Example 10 was a mixture (M) which was
prepared by mixing 15 wt.% of N-butyl-2-pyrrolidone (i.e. compound (B)),
with 50 wt.% of MDEA (i.e. compound (C)), and 25 wt.% of water. The
absorption performance data are summarized in Table 4 below.
COMPARATIVE EXAMPLE 11
The absorbent stream of Comparative Example 11 was prepared
according to the same procedure as Example 10 except that 15 wt.% of
sulfolane was used which was not a compound (B) according to general
formula (II). The absorption performance data are summarized in Table 4
below.
Table 4 shows typical absorption data (nabsorbed) for three
consecutive absorption-regeneration cycles for the reference absorbent
stream of Comparative Example 11 comprising 15 wt.% sulfolane and the
absorbent stream according to the invention comprising 15 wt.% of n-
butyl-2-pyrrolidone (i.e. compound B) (Example 10).
CA 03090928 2020-08-11
WO 2019/158591
PCT/EP2019/053555
48
Table 4.
Name Reference or CH3SH concentration n-Butane
concentration
compound B (mmol. kg-1 solvent) (mmol. kg-1
solvent)
1 2 3 1 2 3
Example 10 N-Butyl-2- 4.76 5.11 4.61 0.33 0.36
0.40
pyrrolidone
Comparative Sulfolane 4.08 4.21 4.08 0.20 0.24 0.24
Example 11
The effect of a different concentration of compound B was studied
by varying the ratio of the different absorbent stream components
(Example 8 and 10) and by comparing the results with a reference
absorbent stream comprising sulfolane (Comparative Examples 9 and 11).
The absorbent solutions according to the invention comprising N-buty1-2-
pyrrolidone showed a consistently higher solubility of CH3SH compared to
the reference absorbent solutions of the Comparative Examples while the
co-absorption of n-butane remained low.