Note: Descriptions are shown in the official language in which they were submitted.
CA 03092366 2020-08-26
NON-INVASIVE NERVE STIMULATION
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Application Serial No.
15/912,058,
filed on March 5, 2018.
FIELD
[0002] This invention pertains to the activation of nerves by topical
stimulators to
control or influence muscles, tissues, organs, or sensation, including pain,
in humans
and mammals.
BACKGROUND INFORMATION
[0003] Nerve disorders may result in loss of control of muscle and other body
functions, loss of sensation, or pain. Surgical procedures and medications
sometimes
treat these disorders but have limitations. This invention pertains to a
system for offering
other options for treatment and improvement of function.
BRIEF DESCRIPTION OF THE DRAWINGS
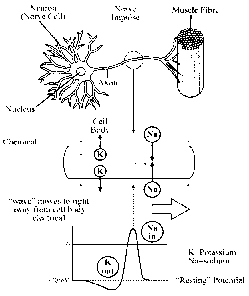
[0004] Fig. 1 is a depiction of a neuron activating a muscle by electrical
impulse.
[0005] Fig. 2 is a representation of the electrical potential activation time
of an
electrical impulse in a nerve.
[0006] Fig. 3 is a cross section of a penis.
[0007] Fig. 4 is an illustration of a Topical Nerve Stimulator/Sensor (TNSS)
component configuration including a system on a chip (SOC).
[0008] Fig. 5 is an illustration of the upper side of a Smart Band Aid
implementation of a TNSS showing location of battery, which may be of various
types.
[0009] Fig. 6 is an illustration of the lower side of the SBA of Fig. 5.
[0010] Fig. 7 is TNSS components incorporated into a SBA.
- 1 -
Date Recue/Date Received 2020-08-26
CA 03092366 2020-08-26
WO 2019/173084 PCT/1JS2019/019572
[0011] Fig. 8 is examples of optional neural stimulator and sensor chip sets
incorporated into a SBA.
[0012] Fig. 9 is examples of optional electrode configurations for a SBA.
[0013] Fig. 10 is an example of the use of TNSS with a Control Unit as a
System,
in a population of Systems and software applications.
[0014] Fig. 11 shows a method for forming and steering a beam by the user of a
plurality of radiators.
[0015] Fig. 12 is an exemplary beam forming and steering mechanism.
[0016] Fig. 13 illustrates exemplary Control Units for activating a nerve
stimulation device.
[0017] Fig. 14 are exemplary software platforms for communicating between the
Control Units and the TNSS, gathering data, networking with other TNSSs, and
external
cornmunications.
[0018] Fig. 15 represents TNSS applications for patients with spinal cord
injury.
[0019] Fig. 16 shows an example TNSS system.
[0020] Fig. 17 shows communications among the components of the TNSS
system of Fig. 16 and a user.
[0021] Fig. 18 shows an example electrode configuration for electric field
steering and sensing.
[0022] Fig. 19 shows an example of stimulating and sensing patterns of signals
in a volume of tissue.
[0023] Fig. 20 is a graph showing pulses applied to the skin.
[0024] Fig. 21 is a graph showing symmetrical and asymmetrical pulses applied
to the skin.
[0025] Fig. 22 is a cross-sectional diagram showing a field in underlying
tissue
produced by application of two electrodes to the skin.
[0026] Fig. 23 is a cross-sectional diagram showing a field in underlying
tissue
produced by application of two electrodes to the skin, with two layers of
tissue of
- 2 -
CA 03092366 2020-08-26
WO 2019/173084
PCT/US2019/019572
different electrical resistivity.
[0027] Fig. 24 is a cross-sectional diagram showing a field in underlying
tissue
when the stimulating pulse is turned off.
[0028] Fig. 25A is a system diagram of an example software and hardware
components showing an example of a Topical Nerve Stimulator/Sensor (TNSS)
interpreting a data stream from a control device in accordance with one
example.
[0029] Fig. 25B is a flow chart showing an example of a function of a master
control program in accordance with one example.
[0030] Fig. 26 is a block diagram of an example TNSS component configuration
including a system on a chip (SOC) in accordance with one example.
[0031] Fig. 27 is a flow diagram of the protocol for adaptive current control
in
accordance with one example.
[0032] Fig. 28 is a Differential Integrator Circuit used in the Adaptive
Current
Protocol in accordance with one example.
[0033] Fig. 29 is a table relating charge duration vs. frequency to provide
feedback to the Adaptive Current Protocol in accordance with one example.
[0034] Fig. 30 is a tibial patch or TNSS or SmartPad designed in a shape to
conform to the skin in accordance with one example.
[0035] Fig. 31 is a tibial patch or TNSS or SmartPad designed in a shape to
conform to the skin in accordance with other examples.
[0036] Fig. 32 is a skin patch that includes a SmartPad with TNSS design and
packaging in accordance with one example.
[0037] Fig. 33 illustrates other example locations for a patch.
[0038] Fig. 34 illustrates a cutaway view where a right foot plantar sock
patch is
affixed into the sole of a sock in accordance with one example.
[0039] Fig. 35 illustrates a cutaway view where a right foot plantar shoe
patch is
affixed into the sole of a shoe in accordance with one example
DETAILED DESCRIPTION
- 3 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[0040] A method for electrical, mechanical, chemical and/or optical
interaction
with a human or mammal nervous system to stimulate and/or record body
functions
using small electronic devices attached to the skin and capable of being
wirelessly
linked to and controlled by a cellphone, activator or computer network.
[0041] The body is controlled by a chemical system and a nervous system.
Nerves and muscles produce and respond to electrical voltages and currents.
Electrical
stimulation of these tissues can restore movement or feeling when these have
been
lost, or can modify the behavior of the nervous system, a process known as
neuro
modulation. Recording of the electrical activity of nerves and muscles is
widely used for
diagnosis, as in the electrocardiogram, electromyogram, electroencephalogram,
etc.
Electrical stimulation and recording require electrical interfaces for input
and output of
information. Electrical interfaces between tissues and electronic systems are
usually
one of three types:
[0042] a. Devices implanted surgically into the body, such as pacemakers.
These
are being developed for a variety of functions, such as restoring movement to
paralyzed
muscles or restoring hearing, and can potentially be applied to any nerve or
muscle.
These are typically specialized and somewhat expensive devices.
[0043] b. Devices inserted temporarily into the tissues, such as needles or
catheters, connected to other equipment outside the body. Health care
practitioners use
these devices for diagnosis or short-term treatment.
[0044] c. Devices that record voltage from the surface of the skin for
diagnosis
and data collection, or apply electrical stimuli to the surface of the skin
using adhesive
patches connected to a stimulator. Portable battery-powered stimulators have
typically
been simple devices operated by a patient, for example for pain relief. Their
use has
been limited by;
[0045] i. The inconvenience of chronically managing wires, patches and
stimulator, particularly if there are interfaces to more than one site, and
[0046] ii. The difficulty for patients to control a variety of stimulus
parameters
- 4 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
such as amplitude, frequency, pulse width, duty cycle, etc.
[0047] Nerves can also be stimulated mechanically to produce sensation or
provoke or alter reflexes; this is the basis of touch sensation and tactile
feedback.
Nerves can also be affected chemically by medications delivered locally or
systemically
and sometimes targeted to particular nerves on the basis of location or
chemical type.
Nerves can also be stimulated or inhibited optically if they have had genes
inserted to
make them light sensitive like some of the nerves in the eye. The actions of
nerves also
produce electrical, mechanical and chemical changes that can be sensed.
[0048] The topical nerve stimulator/sensor (TNSS) is a device to stimulate
nerves and sense the actions of the body that can be placed on the skin of a
human or
mammal to act on and respond to a nerve, muscle or tissue. One implementation
of the
TNSS is the Smart Band AIdTM (SBA). A system, incorporating a SBA, controls
neuro
modulation and neuro stimulation activities. It consists of one or more
controllers or
Control Units, one or more TNSS modules, software that resides in Control
Units and
TNSS modules, wireless communication between these components, and a data
managing platform. The controller hosts software that will control the
functions of the
TNSS. The controller takes inputs from the TNSS of data or image data for
analysis by
said software. The controller provides a physical user interface for display
to and
recording from the user, such as activating or disabling the TNSS, logging of
data and
usage statistics, generating reporting data. Finally, the controller provides
communications with other Controllers or the Internet cloud.
[0049] The controller communicates with the Neurostim module, also called
TNSS module or SBA, and also communicates with the user. In at least one
example,
both of these communications can go in both directions, so each set of
communications
is a control loop. Optionally, there may also be a control loop directly
between the TNSS
module and the body. So the system optionally may be a hierarchical control
system
with at least four control loops. One loop is between the TNSS and the body;
another
loop is between the TNSS and the controller; another loop is between the
controller and
- 5 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
the user; and another loop is between the controller and other users via the
cloud.
Each control loop has several functions including: (1) sending activation or
disablement
signals between the controller and the TNSS via a local network such as
Bluetooth; (2)
driving the user interface, as when the controller receives commands from the
user and
provides visual, auditory or tactile feedback to the user; (3) analyzing TNSS
data, as
well as other feedback data such as from the user, within the TNSS, and/or the
controller and/or or the cloud; (4) making decisions about the appropriate
treatment; (5)
system diagnostics for operational correctness; and (6) communications with
other
controllers or users via the Internet cloud for data transmission or exchange,
or to
interact with apps residing in the Internet cloud.
[0050] The control loop is closed. This is as a result of having both
stimulating
and sensing. The sensing provides information about the effects of
stimulation, allowing
the stimulation to be adjusted to a desired level or improved automatically.
[0051] Typically, stimulation will be applied. Sensing will be used to measure
the
effects of stimulation. The measurements sensed will be used to specify the
next
stimulation. This process can be repeated indefinitely with various durations
of each
part. For example: rapid cycling through the process (a-b-c-a-b-c-a-b-c);
prolonged
stimulation, occasional sensing (aaaa-b-c-aaaa-b-c-aaaa-b-c); or prolonged
sensing,
occasional stimulation (a-bbbb-c-a-bbbb-c-a-bbbb). The process may also start
with
sensing, and when an event in the body is detected this information is used to
specify
stimulation to treat or correct the event, for example, (bbbbbbbbb-c-a-
bbbbbbbb-c-a-
bbbbbbbbb). Other patterns are possible and contemplated within the scope of
the
application.
[0052] The same components can be used for stimulating and sensing
alternately, by switching their connection between the stimulating circuits
and the
sensing circuits. The switching can be done by standard electronic components.
In the
case of electrical stimulating and sensing, the same electrodes can be used
for both. An
electronic switch is used to connect stimulating circuits to the electrodes
and electric
- 6 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
stimulation is applied to the tissues. Then the electronic switch disconnects
the
stimulating circuits from the electrodes and connects the sensing circuits to
the
electrodes and electrical signals from the tissues are recorded.
[0053] In the case of acoustic stimulating and sensing, the same ultrasonic
transducers can be used for both (as in ultrasound imaging or radar). An
electronic
switch is used to connect circuits to the transducers to send acoustic signals
(sound
waves) into the tissues. Then the electronic switch disconnects these circuits
from the
transducers and connects other circuits to the transducers (to listen for
reflected sound
waves) and these acoustic signals from the tissues are recorded.
[0054] Other modalities of stimulation and sensing may be used (e.g. light,
magnetic fields, etc.) The closed loop control may be implemented autonomously
by an
individual TNSS or by multiple TNSS modules operating in a system such as that
shown
below in Fig 16. Sensing might be carried out by some TNSSs and stimulation by
others.
[0055] Stimulators are protocol controlled initiators of electrical
stimulation,
where such protocol may reside in either the TNSS and/or the controller and/or
the
cloud. Stimulators interact with associated sensors or activators, such as
electrodes or
MEMS devices.
[0056] The protocol, which may be located in the TNSS, the controller or the
cloud, has several functions including:
[0057] (1) Sending activation or disablement signals between the controller
and
the TNSS via a local network such as Bluetooth. The protocol sends a signal by
Bluetooth radio waves from the smartphone to the TNSS module on the skin,
telling it to
start or stop stimulating or sensing. Other wireless communication types are
possible.
[0058] (2) Driving the user interface, as when the controller receives
commands
from the user and provides visual, auditory or tactile feedback to the user.
The protocol
receives a command from the user when the user touches an icon on the
smartphone
screen, and provides feedback to the user by displaying information on the
smartphone
- 7 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
screen, or causing the smartphone to beep or buzz.
[0059] (3) Analyzing TNSS data, as well as other feedback data such as from
the
user, within the TNSS, and/or the controller and/or or the cloud. The protocol
analyzes
data sensed by the TNSS, such as the position of a muscle, and data from the
user
such as the user's desires as expressed when the user touches an icon on the
smartphone; this analysis can be done in the TNSS, in the smartphone, and/or
in the
cloud.
[0060] (4) Making decisions about the appropriate treatment. The protocol uses
the data it analyzes to decide what stimulation to apply.
[0061] (5) System diagnostics for operational correctness. The protocol checks
that the TNSS system is operating correctly.
[0062] (6) Communications with other controllers or users via the Internet
cloud
for data transmission or exchange, or to interact with apps residing in the
Internet cloud.
The protocol communicates with other smartphones or people via the internet
wirelessly; this may include sending data over the internet, or using computer
programs
that are operating elsewhere on the internet.
[0063] A neurological control system, method and apparatus are configured in
an
ecosystem or modular platform that uses potentially disposable topical devices
to
provide interfaces between electronic computing systems and neural systems.
These
interfaces may be direct electrical connections via electrodes or may be
indirect via
transducers (sensors and actuators). It may have the following elements in
various
configurations: electrodes for sensing or activating electrical events in the
body;
actuators of various modalities; sensors of various modalities; wireless
networking; and
protocol applications, e.g. for data processing, recording, control systems.
These
components are integrated within the disposable topical device. This
integration allows
the topical device to function autonomously. It also allows the topical device
along with
a remote control unit (communicating wirelessly via an antenna, transmitter
and
receiver) to function autonomously.
- 8 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[0064] Referring to Fig. 1, nerve cells are normally electrically polarized
with the
interior of the nerve being at an electric potential 70mV negative relative to
the exterior
of the cell. Application of a suitable electric voltage to a nerve cell
(raising the resting
potential of the cell from -70mV to above the firing threshold of -55mV) can
initiate a
sequence of events in which this polarization is temporarily reversed in one
region of
the cell membrane and the change in polarization spreads along the length of
the cell to
influence other cells at a distance, e.g. to communicate with other nerve
cells or to
cause or prevent muscle contraction.
[0065] Referring to Fig. 2, a nerve impulse is graphically represented from a
point of stimulation resulting in a wave of depolarization followed by a
repolarization that
travels along the membrane of a neuron during the measured period. This
spreading
action potential is a nerve impulse. It is this phenomenon that allows for
external
electrical nerve stimulation.
[0066] Referring to Fig. 3, the dorsal genital nerve on the back of the penis
or
clitoris just under the skin is a purely sensory nerve that is involved in
normal inhibition
of the activity of the bladder during sexual activity, and electrical
stimulation of this
nerve has been shown to reduce the symptoms of the Over Active Bladder.
Stimulation
of the underside of the penis may cause sexual arousal, erection, ejaculation
and
orgasm.
[0067] A Topical nerve stimulator/sensor (TN SS) is used to stimulate these
nerves and is convenient, unobtrusive, self-powered, controlled from a
smartphone or
other control device. This has the advantage of being non-invasive, controlled
by
consumers themselves, and potentially distributed over the counter without a
prescription.
[0068] Referring to Fig. 4, the TNSS has one or more electronic circuits or
chips
that perform the functions of: communications with the controller, nerve
stimulation via
electrodes 408 that produce a wide range of electric field(s) according to
treatment
regimen, one or more antennae 410 that may also serve as electrodes and
- 9 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
communication pathways, and a wide range of sensors 406 such as, but not
limited to,
mechanical motion and pressure, temperature, humidity, chemical and
positioning
sensors. One arrangement would be to integrate a wide variety of these
functions into
an SOC, system on chip 400. Within this is shown a control unit 402 for data
processing, communications and storage and one or more stimulators 404 and
sensors
406 that are connected to electrodes 408. An antenna 410 is incorporated for
external
communications by the control unit. Also present is an internal power supply
412, which
may be, for example, a battery. An external power supply is another variation
of the chip
configuration. It may be necessary to include more than one chip to
accommodate a
wide range of voltages for data processing and stimulation. Electronic
circuits and chips
will communicate with each other via conductive tracks within the device
capable of
transferring data and/or power.
[0069] In one or more examples, a Smart Band AidTM incorporating a battery and
electronic circuit and electrodes in the form of adhesive conductive pads may
be applied
to the skin, and electrical stimuli is passed from the adhesive pads into the
tissues.
Stimuli may typically be trains of voltage-regulated square waves at
frequencies
between 15 and 50Hz with currents between 20 and 100 mA. The trains of stimuli
are
controlled from a smartphone operated by the user. Stimuli may be either
initiated by
the user when desired, or programmed according to a timed schedule, or
initiated in
response to an event detected by a sensor on the Smart Band Aid TM or
elsewhere.
Another implementation for males may be a TNSS incorporated in a ring that
locates a
stimulator conductively to selected nerves in a penis to be stimulated.
[0070] Referring to Fig. 5, limited lifetime battery sources will be employed
as
internal power supply 412, to power the TNSS deployed in this illustration as
a Smart
Band Aid TM . These may take the form of Lithium Ion technology or traditional
non-toxic
Mn02 technologies. Fig. 5 illustrates different battery options such as a
printable
Manganese Oxide battery 516 and a button battery 518. A TNSS of different
shapes
may require different battery packaging.
-10-
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[0071] Fig. 6 shows an alternate arrangement of these components where the
batteries 616-618 are positioned on the bottom side of the SBA between the
electrodes
610 and 620. In this example, battery 616 is a lithium ion battery, battery
617 is a Mn02
battery and battery 618 is a button battery. Other types of batteries and
other battery
configurations are possible within the scope of this application in other
examples.
[0072] Aside from the Controller, the Smart Band Aid TM Packaging Platform
consists of an assembly of an adhesive patch capable of being applied to the
skin and
containing the TNSS Electronics, protocol, and power described above.
[0073] Referring to Fig. 7 is a TNSS deployed as a Smart Band AidTM 414. The
Smart Band Aid TM has a substrate with adhesive on a side for adherence to
skin, the
SOC 400 previously described in Fig. 4, or electronic package, and electrodes
408
disposed between the dermis and the adhesive surface. The electrodes provide
electrical stimuli through the dermis to nerves and other tissue and in turn
may collect
electrical signals from the body, such as the electrical signals produced by
muscles
when they contract (the electromyogram) to provide data about body functions
such as
muscle actions.
[0074] Referring to Fig. 8, different chips may be employed to design
requirements. Shown are sample chips for packaging in a TNSS in this instance
deployed as a SBA. For example, neural stimulator 800, sensor 802,
processor/communications 804 are represented. The chips can be packaged
separately
on a substrate, including a flexible material, or as a system-on-chip (SOC)
400. The
chip connections and electronics package are not shown but are known in the
art.
[0075] Referring to Fig. 9, SBAs with variations on arrangements of electrodes
are shown. Each electrode may consist of a plurality of conductive contacts
that give the
electrode abilities to adjust the depth, directionality, and spatial
distribution of the
applied electric field. For all the example electrode configurations shown,
901-904, the
depth of the electrical stimulation can be controlled by the voltage and power
applied to
the electrode contacts. Electric current can be applied to various electrode
contacts at
-11 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
opposite end of the SBA, or within a plurality of electrode contacts on a
single end of the
SBA. The phase relationship of the signals applied to the electrode contacts
can vary
the directionality of the electric field. For all configurations of
electrodes, the applied
signals can vary over time and spatial dimensions. The configuration on the
left, 901,
shows a plurality of concentric electrode contacts at either end of the SBA.
This
configuration can be used to apply an electric stimulating field at various
tissue depths
by varying the power introduced to the electrode contacts. The next
configuration, 902,
shows electrodes 404 that are arranged in a plurality of parallel strips of
electrical
contacts. This allows the electric field to be oriented perpendicular or
parallel to the
SBA. The next configuration, 903, shows an example matrix of electrode
contacts
where the applied signal can generate a stimulating field between any two or
more
electrode contacts at either end of the SBA, or between two or more electrode
contacts
within a single matrix at one end of the SBA. Finally, the next configuration
on the far
right, 904, also shows electrodes that are arranged in a plurality of parallel
strips of
electrical contacts. As with the second configuration, this allows the
electric field to be
oriented perpendicular or parallel to the SBA. There may be many other
arrangements
of electrodes and contacts.
[0076] One or more TNSSs with one or more Controllers form a System.
Systems can communicate and interact with each other and with distributed
virtualized
processing and storage services. This enables the gathering, exchange, and
analysis of
data among populations of systems for medical and non-medical applications.
[0077] Referring to Fig. 10, a system is shown with two TNSS units 1006, with
one on the wrist, one on the leg, communicating with its controller, a
smartphone 1000
or other control device. The TNSS units can be both sensing and stimulating
and can
act independently and also work together in a Body Area Network (BAN). Systems
communicate with each other over a communication bridge or network such as a
cellular network. Systems also communicate with applications running in a
distributed
virtualized processing and storage environment generally via the Internet
1002. The
- 12 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
purpose for communications with the distributed virtualized processing and
storage
[0078] environment is to communicate large amounts of user data for analysis
and networking with other third parties such as hospitals, doctors, insurance
companies,
researchers, and others. There are applications that gather, exchange, and
analyze
data from multiple Systems 1004. Third party application developers can access
TNSS
systems and their data to deliver a wide range of applications. These
applications can
return data or control signals to the individual wearing the TNSS unit 1006.
These
applications can also send data or control signals to other members of the
population
who employ systems 1008. This may represent an individual's data, aggregated
data
from a population of users, data analyses, or supplementary data from other
sources.
[0079] Referring to Fig. 11, shown is an example of an electrode array to
affect
beam forming and beam steering. Beam forming and steering allows a more
selective
application of stimulation energy by a TNSS to nerves and tissue. Beam
steering also
provides the opportunity for lower power for stimulation of cells including
nerves by
applying the stimulating mechanism directionally to a target. In the use of an
electrical
beam lower power demand lengthens battery life and allows for use of low power
chip
sets. Beam steering may be accomplished in multiple ways for instance by
magnetic
fields and formed gates. Fig. 11 shows a method for forming and steering a
beam by
the use of a plurality of radiators 1102 which are activated out of phase with
each other
by a plurality of phase shifters 1103 that are supplied power from a common
source
1104. Because the radiated signals are out of phase they produce an
interference
pattern 1105 that results in the beam being formed and steered in varying
controlled
directions 1106. Electromagnetic radiation like light shows some properties of
waves
and can be focused on certain locations. This provides the opportunity to
stimulate
tissues such as nerves selectively. It also provides the opportunity to focus
the
transmission of energy and data on certain objects, including topical or
implanted
electronic devices, thereby not only improving the selectivity of activating
or controlling
those objects but also reducing the overall power required to operate them.
-13-
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[0080] Fig. 12 is another example of a gating structure 1200 used for beam
shaping and steering 1202. The gating structure 1200 shows an example of an
interlocked pair of electrodes that can be used for simple beam forming
through the
application of time-varying voltages. The steering 1202 shows a generic
picture of the
main field lobes and how such beam steering works in this example. Fig. 12 is
illustrative of a possible example that may be used.
[0081] The human and mammal body is an anisotropic medium with multiple
layers of tissue of varying electrical properties. Steering of an electric
field may be
accomplished using multiple electrodes, or multiple SBAs, using the human or
mammal
body as an anisotropic volume conductor. Electric field steering will
discussed below
with reference to Figs. 18 and 19.
[0082] Referring to Fig. 13, the controller is an electronics platform that is
a
smartphone 1300, tablet 1302, personal computer 1304, or dedicated module 1306
that
hosts wireless communications capabilities, such as Near Field Communications,
Bluetooth, or Wi-Fi technologies as enabled by the current set of
communications chips,
e.g. Broadcom BCM4334, TI WiLink 8 and others, and a wide range of protocol
apps
that can communicate with the TNSSs. There may be more than one controller,
acting
together. This may occur, for example, if the user has both a smartphone
control app
running, and a key fob controller in his/her pocket/purse.
[0083] TNSS protocol performs the functions of communications with the
controller including transmitting and receiving of control and data signals,
activation and
control of the neural stimulation, data gathering from on board sensors,
communications
and coordination with other TNSSs, and data analysis. Typically the TNSS may
receive
commands from the controller, generate stimuli and apply these to the tissues,
sense
signals from the tissues, and transmit these to the controller. It may also
analyze the
signals sensed and use this information to modify the stimulation applied. In
addition to
communicating with the controller it may also communicate with other TNSSs
using
electrical or radio signals via a body area network.
- 14-
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[0084] Referring to Fig. 14, controller protocol executed and/or displayed on
a
smartphone 1400, tablet 1402 or other computing platform or mobile device,
will
perform the functions of communications with TNSS modules including
transmitting and
receiving of control and data signals, activation and control of the neuro
modulation
regimens, data gathering from on board sensors, communications and
coordination with
other controllers, and data analysis. In some cases local control of the neuro
modulation
regimens may be conducted by controller protocol without communications with
the
user.
[0085] Fig. 15 shows potential applications of electrical stimulation and
sensing
for the body, particularly for users who may suffer from paralysis or loss of
sensation or
altered reflexes such as spasticity or tremor due to neurological disorders
and their
complications, as well as users suffering from incontinence, pain, immobility
and aging.
Different example medical uses of the present system are discussed below.
[0086] Fig. 16 shows the components of one example of a typical TNSS system
1600. TNSS devices 1610 are responsible for stimulation of nerves and for
receiving
data in the form of electrical, acoustic, imaging, chemical and other signals
which then
can be processed locally in the TNSS or passed to the Control Unit 1620. TNSS
devices 1610 are also responsible for analysis and action. The TNSS device
1610 may
contain a plurality of electrodes for stimulation and for sensing. The same
electrodes
may be used for both functions, but this is not required. The TNSS device 1610
may
contain an imaging device, such as an ultrasonic transducer to create acoustic
images
of the structure beneath the electrodes or elsewhere in the body that may be
affected by
the neural stimulation.
[0087] In this example TNSS system, most of the data gathering and analysis is
performed in the Control Unit 1620. The Control Unit 1620 may be a cellular
telephone
or a dedicated hardware device. The Control Unit 1620 runs an app that
controls the
local functions of the TNSS System 1600. The protocol app also communicates
via the
Internet or wireless networks 1630 with other TNSS systems and/or with 3rd
party
-15-
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
software applications.
[0088] Fig. 17 shows the communications among the components of the TNSS
system 1600 and the user. In this example, TNSS 1610 is capable of applying
stimuli to
nerves 1640 to produce action potentials in the nerves 1640 to produce actions
in
muscles 1670 or other organs such as the brain 1650. These actions may be
sensed by
the TNSS 1610, which may act on the information to modify the stimulation it
provides.
This closed loop constitutes the first level of the system 1600 in this
example.
[0089] The TNSS 1610 may also be caused to operate by signals received from
a Control Unit 1620 such as a cellphone, laptop, key fob, tablet, or other
handheld
device and may transmit information that it senses back to the Control Unit
1620. This
constitutes the second level of the system 1600 in this example.
[0090] The Control Unit 1620 is caused to operate by commands from a user,
who also receives information from the Control Unit 1620. The user may also
receive
information about actions of the body via natural senses such as vision or
touch via
sensory nerves and the spinal cord, and may in some cases cause actions in the
body
via natural pathways through the spinal cord to the muscles.
[0091] The Control Unit 1620 may also communicate information to other users,
experts, or application programs via the Internet 1630, and receive
information from
them via the Internet 1630.
[0092] The user may choose to initiate or modify these processes, sometimes
using protocol applications residing in the TNSS 1610, the Control Unit 1620,
the
Internet 1630, or wireless networks. This software may assist the user, for
example by
processing the stimulation to be delivered to the body to render it more
selective or
effective for the user, and/or by processing and displaying data received from
the body
or from the Internet 1630 or wireless networks to make it more intelligible or
useful to
the user.
[0093] Fig. 18 shows an example electrode configuration 1800 for Electric
Field
Steering. The application of an appropriate electric field to the body can
cause a nerve
- 16-
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
to produce an electrical pulse known as an action potential. The shape of the
electric
field is influenced by the electrical properties of the different tissue
through which it
passes and the size, number and position of the electrodes used to apply it.
The
electrodes can therefore be designed to shape or steer or focus the electric
field on
some nerves more than on others, thereby providing more selective stimulation.
[0094] An example 10x10 matrix of electrical contacts 1860 is shown. By
varying
the pattern of electrical contacts 1860 employed to cause an electric field
1820 to form
and by time varying the applied electrical power to this pattern of contacts
1860, it is
possible to steer the field 1820 across different parts of the body, which may
include
muscle 1870, bone, fat, and other tissue, in three dimensions. This electric
field 1820
can activate specific nerves or nerve bundles 1880 while sensing the
electrical and
mechanical actions produced 1890, and thereby enabling the TNSS to discover
more
effective or the most effective pattern of stimulation for producing the
desired action.
[0095] Fig. 19 shows an example of stimulating and sensing patterns of signals
in a volume of tissue. Electrodes 1910 as part of a cuff arrangement are
placed around
limb1915. The electrodes 1910 are external to a layer of skin 1916 on limb
1915.
Internal components of the limb 1915 include muscle 1917, bone 1918, nerves
1919,
and other tissues. By using electric field steering for stimulation, as
described with
reference to Fig. 18, the electrodes 1910 can activate nerves 1919
selectively. An array
of sensors (e.g., piezoelectric sensors or micro-electro-mechanical sensors)
in a TNSS
can act as a phased array antenna for receiving ultrasound signals, to acquire
ultrasonic
images of body tissues. Electrodes 1910 may act as an array of electrodes
sensing
voltages at different times and locations on the surface of the body, with
software
processing this information to display information about the activity in body
tissues, e.g.,
which muscles are activated by different patterns of stimulation.
[0096] The SBA's ability to stimulate and collect organic data has multiple
applications including bladder control, reflex incontinence, sexual
stimulations, pain
control and wound healing among others. Examples of SBA's application for
medical
-17-
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
and other uses follow.
Medical uses
Bladder management
[0097] Overactive bladder: When the user feels a sensation of needing to empty
the bladder urgently, he or she presses a button on the Controller to initiate
stimulation
via a Smart Band Aid TM applied over the dorsal nerve of the penis or
clitoris. Activation
of this nerve would inhibit the sensation of needing to empty the bladder
urgently, and
allow it to be emptied at a convenient time.
[0098] Incontinence: A person prone to incontinence of urine because of
unwanted contraction of the bladder uses the SBA to activate the dorsal nerve
of the
penis or clitoris to inhibit contraction of the bladder and reduce
incontinence of urine.
The nerve could be activated continuously or intermittently when the user
became
aware of the risk of incontinence, or in response to a sensor indicating the
volume or
pressure in the bladder.
[0099] Erection, ejaculation and orgasm: Stimulation of the nerves on the
underside of the penis by a Smart Band AidTM (electrical stimulation or
mechanical
vibration) can cause sexual arousal and might be used to produce or prolong
erection
and to produce orgasm and ejaculation.
[00100] Pain control: A person suffering from chronic pain from a particular
region of the body applies a Smart Band AidTM over that region and activates
electrically
the nerves conveying the sensation of touch, thereby reducing the sensation of
pain
from that region. This is based on the gate theory of pain.
[00101] Wound care: A person suffering from a chronic wound or ulcer applies a
Smart Band Aid TM over the wound and applies electrical stimuli continuously
to the
tissues surrounding the wound to accelerate healing and reduce infection.
[00102] Essential tremor: A sensor on a Smart Band Aid TM detects the tremor
and triggers neuro stimulation to the muscles and sensory nerves involved in
the tremor
-18-
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
with an appropriate frequency and phase relationship to the tremor. The
stimulation
frequency would typically be at the same frequency as the tremor but shifted
in phase in
order to cancel the tremor or reset the neural control system for hand
position.
[00103] Reduction of spasticity: Electrical stimulation of peripheral nerves
can
reduce spasticity for several hours after stimulation. A Smart Band Aid TM
operated by
the patient when desired from a smartphone could provide this stimulation.
[00104] Restoration of sensation and sensory feedback: People who lack
sensation, for example as a result of diabetes or stroke use a Smart Band Aid
TM to
sense movement or contact, for example of the foot striking the floor, and the
SBA
provides mechanical or electrical stimulation to another part of the body
where the user
has sensation, to improve safety or function. Mechanical stimulation is
provided by the
use of acoustic transducers in the SBA such as small vibrators. Applying a
Smart Band
AidTM to the limb or other assistive device provides sensory feedback from
artificial
limbs. Sensory feedback can also be used to substitute one sense for another,
e.g.
touch in place of sight.
[00105] Recording of mechanical activity of the body: Sensors in a Smart Band
AidTM record position, location and orientation of a person or of body parts
and transmit
this data to a smartphone for the user and/or to other computer networks for
safety
monitoring, analysis of function and coordination of stimulation.
[00106] Recording of sound from the body or reflections of ultrasound waves
generated by a transducer in a Smart Band Aid TM could provide information
about body
structure, e.g., bladder volume for persons unable to feel their bladder.
Acoustic
transducers may be piezoelectric devices or MEMS devices that transmit and
receive
the appropriate acoustic frequencies. Acoustic data may be processed to allow
imaging
of the interior of the body.
Recordind of electrical activity of the body
[00107] Electrocardiogram: Recording the electrical activity of the heart is
widely
used for diagnosing heart attacks and abnormal rhythms. It is sometimes
necessary to
-19-
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
record this activity for 24 hours or more to detect uncommon rhythms. A Smart
Band
AidTM communicating wirelessly with a smartphone or computer network achieves
this
more simply than present systems.
[00108] Electromyogram: Recording the electrical activity of muscles is widely
used for diagnosis in neurology and also used for movement analysis. Currently
this
requires the use of many needles or adhesive pads on the surface of the skin
connected to recording equipment by many wires. Multiple Smart Band Aids TM
record
the electrical activity of many muscles and transmit this information
wirelessly to a
smartphone.
[00109] Recording of optical information from the body: A Smart Band Aid TM
incorporating a light source (LED, laser) illuminates tissues and senses the
characteristics of the reflected light to measure characteristics of value,
e.g.,
oxygenation of the blood, and transmit this to a cellphone or other computer
network.
[00110] Recording of chemical information from the body: The levels of
chemicals or drugs in the body or body fluids is monitored continuously by a
Smart
Band Aid TM sensor and transmitted to other computer networks and appropriate
feedback provided to the user or to medical staff. Levels of chemicals may be
measured
by optical methods (reflection of light at particular wavelengths) or by
chemical sensors.
Special populations of disabled users
[00111] There are many potential applications of electrical stimulation for
therapy
and restoration of function. However, few of these have been commercialized
because
of the lack of affordable convenient and easily controllable stimulation
systems. Some
applications are shown in the Fig. 15.
[00112] Limb Muscle stimulation: Lower limb muscles can be exercised by
stimulating them electrically, even if they are paralyzed by stroke or spinal
cord injury.
This is often combined with the use of a stationary exercise cycle for
stability. Smart
Band Aid TM devices could be applied to the quadriceps muscle of the thigh to
stimulate
these, extending the knee for cycling, or to other muscles such as those of
the calf.
- 20 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
Sensors in the Smart Band AIdTM could trigger stimulation at the appropriate
time during
cycling, using an application on a smartphone, tablet, handheld hardware
device such
as a key fob, wearable computing device, laptop, or desktop computer, among
other
possible devices. Upper limb muscles can be exercised by stimulating them
electrically,
even if they are paralyzed by stroke of spinal cord injury. This is often
combined with the
use of an arm crank exercise machine for stability. Smart Band Aid TM devices
are
applied to multiple muscles in the upper limb and triggered by sensors in the
Smart
Band Aids-n" at the appropriate times, using an application on a smartphone.
[00113] Prevention of osteoporosis: Exercise can prevent osteoporosis and
pathological fractures of bones. This is applied using Smart Band Aids TM in
conjunction
with exercise machines such as rowing simulators, even for people with
paralysis who
are particularly prone to osteoporosis.
[00114] Prevention of deep vein thrombosis: Electric stimulation of the
muscles
of the calf can reduce the risk of deep vein thrombosis and potentially fatal
pulmonary
embolus. Electric stimulation of the calf muscles is applied by a Smart Band
AIdTM with
stimulation programmed from a smartphone, e.g., during a surgical operation,
or on a
preset schedule during a long plane flight.
Restoration of function (Functional Electrical Stimulation) Lower limb
[00115] 1) Foot drop: People with stroke often cannot lift their forefoot and
drag
their toes on the ground. A Smart Band Aid TM is be applied just below the
knee over the
common peroneal nerve to stimulate the muscles that lift the forefoot at the
appropriate
time in the gait cycle, triggered by a sensor in the Smart Band Aid TM
[00116] 2) Standing: People with spinal cord injury or some other paralyses
can
be aided to stand by electrical stimulation of the quadriceps muscles of their
thigh.
These muscles are stimulated by Smart Band AidsTM applied to the front of the
thigh
and triggered by sensors or buttons operated by the patient using an
application on a
smartphone. This may also assist patients to use lower limb muscles when
transferring
from a bed to a chair or other surface.
-21 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[00117] 3) Walking: Patients with paralysis from spinal cord injury are aided
to
take simple steps using electrical stimulation of the lower limb muscles and
nerves.
Stimulation of the sensory nerves in the common peroneal nerve below the knee
can
cause a triple reflex withdrawal, flexing the ankle, knee and hip to lift the
leg, and then
stimulation of the quadriceps can extend the knee to bear weight. The process
is then
repeated on the other leg. Smart Band Aids TM coordinated by an application in
a
smartphone produce these actions.
Upper limb
[00118] Hand grasp: People with paralysis from stroke or spinal cord injury
have
simple hand grasp restored by electrical stimulation of the muscles to open or
close the
hand. This is produced by Smart Band AidsTM applied to the back and front of
the
forearm and coordinated by sensors in the Smart Band AidsTM and an application
in a
smartphone.
[00119] Reaching: Patients with paralysis from spinal cord injury sometimes
cannot extend their elbow to reach above the head. Application of a Smart Band
Aid TM
to the triceps muscle stimulates this muscle to extend the elbow. This is
triggered by a
sensor in the Smart Band Aid TM detecting arm movements and coordinating it
with an
application on a smartphone.
[00120] Posture: People whose trunk muscles are paralyzed may have difficulty
maintaining their posture even in a wheelchair. They may fall forward unless
they wear
a seatbelt, and if they lean forward they may be unable to regain upright
posture.
Electrical stimulation of the muscles of the lower back using a Smart Band Aid
TM allows
them to maintain and regain upright posture. Sensors in the Smart Band Aid TM
trigger
this stimulation when a change in posture was detected.
[00121] Coughing: People whose abdominal muscles are paralyzed cannot
produce a strong cough and are at risk for pneumonia. Stimulation of the
muscles of the
abdominal wall using a Smart Band Aid TM could produce a more forceful cough
and
prevent chest infections. The patient using a sensor in a Smart Band Aid TM
triggers the
- 22 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
stimulation.
[00122] Essential Tremor: It has been demonstrated that neuro stimulation can
reduce or eliminate the signs of ET. ET may be controlled using a TNSS. A
sensor on a
Smart Band Aid TM detects the tremor and trigger neuro stimulation to the
muscles and
sensory nerves involved in the tremor with an appropriate frequency and phase
relationship to the tremor. The stimulation frequency is typically be at the
same
frequency as the tremor but shifted in phase in order to cancel the tremor or
reset the
neural control system for hand position.
Non-medical Applications
Sports training
[00123] Sensing the position and orientation of multiple limb segments is used
to
provide visual feedback on a smartphone of, for example, a golf swing, and
also
mechanical or electrical feedback to the user at particular times during the
swing to
show them how to change their actions. The electromyogram of muscles could
also be
recorded from one or many Smart Band Aids TM and used for more detailed
analysis.
Gaming
[00124] Sensing the position and orientation of arms, legs and the rest of the
body produces a picture of an onscreen player that can interact with other
players
anywhere on the Internet. Tactile feedback would be provided to players by
actuators in
Smart Band Aids on various parts of the body to give the sensation of striking
a ball, etc.
Motion Capture for film and animation
[00125] Wireless TNSS capture position, acceleration, and orientation of
multiple
parts of the body. This data may be used for animation of a human or mammal
and has
application for human factor analysis and design.
Sample Modes of Operation
[00126] A SBA system consists of at least a single Controller and a single
SBA.
Following application of the SBA to the user's skin, the user controls it via
the
- 23 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[00127] Controller's app using Near Field Communications. The app appears on
a smartphone screen and can be touch controlled by the user; for 'key fob'
type
Controllers. The SBA is controlled by pressing buttons on the key fob.
[00128] When the user feels the need to activate the SBA s/he presses the "go"
button two or more times to prevent false triggering, thus delivering the
neuro
stimulation. The neuro stimulation may be delivered in a variety of patterns
of
frequency, duration, and strength and may continue until a button is pressed
by the user
or may be delivered for a length of time set in the application.
[00129] Sensor capabilities in the TNSS, are enabled to start
collecting/analyzing
data and communicating with the controller when activated.
[00130] The level of functionality in the protocol app, and the protocol
embedded
in the TNSS, will depend upon the neuro modulation or neuro stimulation
regimen being
employed.
[00131] In some cases there will be multiple TNSSs employed for the neuro
modulation or neuro stimulation regimen. The basic activation will be the same
for each
TNSS.
[00132] However, once activated multiple TNSSs will automatically form a
network of neuro modulation/stimulation points with communications enabled
with the
controller.
[00133] The need for multiple TNSSs arises from the fact that treatment
regimens may need several points of access to be effective.
Controlling the Stimulation
[00134] In general, advantages of a wireless TNSS system as disclosed herein
over existing transcutaneous electrical nerve stimulation devices include: (1)
fine control
of all stimulation parameters from a remote device such as a smartphone,
either directly
by the user or by stored programs; (2) multiple electrodes of a TNSS can form
an array
to shape an electric field in the tissues; (3) multiple TNSS devices can form
an array to
shape an electric field in the tissues; (4) multiple TNSS devices can
stimulate multiple
- 24 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
structures, coordinated by a smartphone; (5) selective stimulation of nerves
and other
structures at different locations and depths in a volume of tissue; (6)
mechanical,
acoustic or optical stimulation in addition to electrical stimulation; (7) the
transmitting
antenna of TNSS device can focus a beam of electromagnetic energy within
tissues in
short bursts to activate nerves directly without implanted devices; (8)
inclusion of
multiple sensors of multiple modalities, including but not limited to
position, orientation,
force, distance, acceleration, pressure, temperature, voltage, light and other
electromagnetic radiation, sound, ions or chemical compounds, making it
possible to
sense electrical activities of muscles (EMG, EKG), mechanical effects of
muscle
contraction, chemical composition of body fluids, location or dimensions or
shape of an
organ or tissue by transmission and receiving of ultrasound.
[00135] Further advantages of the wireless TNSS system include: (1) TNSS
devices are expected to have service lifetimes of days to weeks and their
disposability
places less demand on power sources and battery requirements; (2) the
combination of
stimulation with feedback from artificial or natural sensors for closed loop
control of
muscle contraction and force, position or orientation of parts of the body,
pressure
within organs, and concentrations of ions and chemical compounds in the
tissues; (3)
multiple TNSS devices can form a network with each other, with remote
controllers, with
other devices, with the Internet and with other users; (4) a collection of
large amounts of
data from one or many TNSS devices and one or many users regarding sensing and
stimulation, collected and stored locally or through the internet; (5)
analysis of large
amounts of data to detect patterns of sensing and stimulation, apply machine
learning,
and improve algorithms and functions; (6) creation of databases and knowledge
bases
of value; (7) convenience, including the absence of wires to become entangled
in
clothing, showerproof and sweat proof, low profile, flexible, camouflaged or
skin colored,
(8) integrated power, communications, sensing and stimulating inexpensive
disposable
TNSS, consumable electronics; (9) power management that utilizes both hardware
and
software functions will be critical to the convenience factor and widespread
deployment
- 25 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
of TNSS device.
[00136] Referring again to Fig. 1, a nerve cell normally has a voltage across
the
cell membrane of 70 millivolts with the interior of the cell at a negative
voltage with
respect to the exterior of the cell. This is known as the resting potential
and it is
normally maintained by metabolic reactions which maintain different
concentrations of
electrical ions in the inside of the cell compared to the outside. Ions can be
actively
"pumped" across the cell membrane through ion channels in the membrane that
are
selective for different types of ion, such as sodium and potassium. The
channels are
voltage sensitive and can be opened or closed depending on the voltage across
the
membrane. An electric field produced within the tissues by a stimulator can
change the
normal resting voltage across the membrane, either increasing or decreasing
the
voltage from its resting voltage.
[00137] Referring again to Fig. 2, a decrease in voltage across the cell
membrane to about 55 millivolts opens certain ion channels, allowing ions to
flow
through the membrane in a self-catalyzing but self-limited process which
results in a
transient decrease of the trans membrane potential to zero, and even positive,
known
as depolarization followed by a rapid restoration of the resting potential as
a result of
active pumping of ions across the membrane to restore the resting situation
which is
known as repolarization. This transient change of voltage is known as an
action
potential and it typically spreads over the entire surface of the cell. If the
shape of the
cell is such that it has a long extension known as an axon, the action
potential spreads
along the length of the axon. Axons that have insulating myelin sheaths
propagate
action potentials at much higher speeds than those axons without myelin
sheaths or
with damaged myelin sheaths.
[00138] If the action potential reaches a junction, known as a synapse, with
another nerve cell, the transient change in membrane voltage results in the
release of
chemicals known as neuro-transmitters that can initiate an action potential in
the other
- 26 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
cell. This provides a means of rapid electrical communication between cells,
analogous
to passing a digital pulse from one cell to another.
[00139] If the action potential reaches a synapse with a muscle cell it can
initiate an action potential that spreads over the surface of the muscle cell.
This
voltage change across the membrane of the muscle cell opens ion channels in
the
membrane that allow ions such as sodium, potassium and calcium to flow across
the
membrane, and can result in contraction of the muscle cell.
[00140] Increasing the voltage across the membrane of a cell below -70
millivolts
is known as hyper-polarization and reduces the probability of an action
potential being
generated in the cell. This can be useful for reducing nerve activity and
thereby
reducing unwanted symptoms such as pain and spasticity
[00141] The voltage across the membrane of a cell can be changed by creating
an electric field in the tissues with a stimulator. It is important to note
that action
potentials are created within the mammalian nervous system by the brain, the
sensory
nervous system or other internal means. These action potentials travel along
the
body's nerve "highways". The TNSS creates an action potential through an
externally
applied electric field from outside the body. This is very different than how
action
potentials are naturally created within the body.
Electric Fields that can cause Action Potentials
[00142] Referring to Fig. 2, electric fields capable of causing action
potentials
can be generated by electronic stimulators connected to electrodes that are
implanted surgically in close proximity to the target nerves. To avoid the
many issues
associated with implanted devices, it is desirable to generate the required
electric
fields by electronic devices located on the surface of the skin. Such devices
typically
use square wave pulse trains of the form shown in Fig. 20. Such devices may be
used instead of implants and/or with implants such as reflectors, conductors,
refractors, or markers for tagging target nerves and the like, so as to shape
electric
fields to enhance nerve targeting and/or selectivity.
- 27 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[00143] Referring to Fig. 20, the amplitude of the pulses "A", applied to the
skin,
may vary between 1 and 100 Volts, pulse width "t", between 100 microseconds
and 10
milliseconds, duty cycle (t/T) between 0.1% and 50%, the frequency of the
pulses within
a group between 1 and 100/sec, and the number of pulses per group "n", between
1 and
several hundred. Typically, pulses applied to the skin will have an amplitude
of up to 60
volts, a pulse width of 250 microseconds and a frequency of 20 per second,
resulting in
a duty cycle of 0.5%. In some cases balanced-charge biphasic pulses will be
used to
avoid net current flow. Referring to Fig. 21, these pulses may be symmetrical,
with the
shape of the first part of the pulse similar to that of the second part of the
pulse, or
asymmetrical, in which the second part of the pulse has lower amplitude and a
longer
pulse width in order to avoid canceling the stimulatory effect of the first
part of the pulse.
Formation of Electric Fields by Stimulators
[00144] The location and magnitude of the electric potential applied to the
tissues by electrodes provides a method of shaping the electrical field. For
example,
applying two electrodes to the skin, one at a positive electrical potential
with respect
to the other, can produce a field in the underlying tissues such as that shown
in the
cross-sectional diagram of Fig. 22.
[00145] The diagram in Fig. 22 assumes homogeneous tissue. The voltage
gradient is highest close to the electrodes and lower at a distance from the
electrodes.
Nerves are more likely to be activated close to the electrodes than at a
distance. For
a given voltage gradient, nerves of large diameter are more likely to be
activated than
nerves of smaller diameter. Nerves whose long axis is aligned with the voltage
gradient are more likely to be activated than nerves whose long axis is at
right angles
to the voltage gradient.
[00146] Applying similar electrodes to a part of the body in which there are
two
layers of tissue of different electrical resistivity, such as fat and muscle,
can produce a
field such as that shown in Fig. 23. Layers of different tissue may act to
refract and
direct energy waves and be used for beam aiming and steering. An individual's
tissue
- 28 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
parameters may be measured and used to characterize the appropriate energy
stimulation for a selected nerve.
[00147] Referring to Fig. 24, when the stimulating pulse is turned off the
electric
field will collapse and the fields will be absent as shown. It is the change
in electric field
that will cause an action potential to be created in a nerve cell, provided
sufficient voltage
and the correct orientation of the electric field occurs. More complex three-
dimensional
arrangements of tissues with different electrical properties can result in
more complex
three-dimensional electric fields, particularly since tissues have different
electrical
properties and these properties are different along the length of a tissue and
across it,
as shown in Table 1.
Table 1
Electrical Direction Average
Conductivity
(siemenslm)
Blood .65
Bone Along .17
Bone Mixed .095
Fat .05
Muscle Along .127
Muscle Across .45
Muscle Mixed .286
Skin (Dry) .000125
Skin (Wet) .00121
Modification of Electric Fields by Tissue
- 29 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[00148] An important factor in the formation of electric fields used to create
action potentials in nerve cells is the medium through which the electric
fields must
penetrate. For the human body this medium includes various types of tissue
including
bone, fat, muscle, and skin. Each of these tissues possesses different
electrical
resistivity or conductivity and different capacitance and these properties are
anisotropic. They are not uniform in all directions within the tissues. For
example, an
axon has lower electrical resistivity along its axis than perpendicular to its
axis. The
wide range of conductivities is shown in Table 1. The three-dimensional
structure and
resistivity of the tissues will therefore affect the orientation and magnitude
of the
electric field at any given point in the body.
Modification of Electric Fields by Multiple Electrodes
[00149] Applying a larger number of electrodes to the skin can also produce
more complex three-dimensional electrical fields that can be shaped by the
location of
the electrodes and the potential applied to each of them. Referring to Fig.
20, the pulse
trains can differ from one another indicated by A, UT, n, and f as well as
have different
phase relationships between the pulse trains. For example with an 8x8 array of
electrodes, combinations of electrodes can be utilized ranging from simple
dipoles, to
quadripoles, to linear arrangements, to approximately circular configurations,
to
produce desired electric fields within tissues.
[00150] Applying multiple electrodes to a part of the body with complex
tissue geometry will thus result in an electric field of a complex shape. The
interaction of electrode arrangement and tissue geometry can be modeled using
Finite Element Modeling, which is a mathematical method of dividing the
tissues
into many small elements in order to calculate the shape of a complex electric
field.
This can be used to design an electric field of a desired shape and
orientation to a
particular nerve.
[00151] High frequency techniques known for modifying an electric field, such
as
the relation between phases of a beam, cancelling and reinforcing by using
phase
- 30 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
shifts, may not apply to application of electric fields by TNSSs because they
use low
frequencies. Instead, examples use beam selection to move or shift or shape an
electric field, also described as field steering or field shaping, by
activating different
electrodes, such as from an array of electrodes, to move the field. Selecting
different
combinations of electrodes from an array may result in beam or field steering.
A
particular combination of electrodes may shape a beam and/or change the
direction of
a beam by steering. This may shape the electric field to reach a target nerve
selected
for stimulation.
Activation of Nerves by Electric Fields
[00152] Typically, selectivity in activating nerves has required electrodes to
be
implanted surgically on or near nerves. Using electrodes on the surface of the
skin to
focus activation selectively on nerves deep in the tissues, as with examples
of the
invention, has many advantages. These include avoidance of surgery, avoidance
of
the cost of developing complex implants and gaining regulatory approval for
them, and
avoidance of the risks of long-term implants.
[00153] The features of the electric field that determine whether a nerve will
be
activated to produce an action potential can be modeled mathematically by the
"Activating Function" disclosed in Rattay F., "The basic mechanism for the
electrical
stimulation of the nervous system", Neuroscience Vol. 89, No. 2, pp. 335-346
(1999).
The electric field can produce a voltage, or extracellular potential, within
the tissues that
varies along the length of a nerve. If the voltage is proportional to distance
along the
nerve, the first order spatial derivative will be constant and the second
order spatial
derivative will be zero. If the voltage is not proportional to distance along
the nerve, the
first order spatial derivative will not be constant and the second order
spatial derivative
will not be zero. The Activating Function is proportional to the second-order
spatial
derivative of the extracellular potential along the nerve. If it is
sufficiently greater than
zero at a given point it predicts whether the electric field will produce an
action potential
in the nerve at that point. This prediction may be input to a nerve signature.
-31 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[00154] In practice, this means that electric fields that are varying
sufficiently
greatly in space or time can produce action potentials in nerves. These action
potentials are also most likely to be produced where the orientation of the
nerves to the
fields change, either because the nerve or the field changes direction. The
direction of
the nerve can be determined from anatomical studies and imaging studies such
as MRI
scans. The direction of the field can be determined by the positions and
configurations
of electrodes and the voltages applied to them, together with the electrical
properties of
the tissues. As a result, it is possible to activate certain nerves at certain
tissue
locations selectively while not activating others.
[00155] To accurately control an organ or muscle, the nerve to be activated
must be accurately selected. This selectivity may be improved by using
examples
disclosed herein as a nerve signature, in several ways, as follows:
(1) Improved algorithms to control the effects when a nerve is stimulated, for
example, by measuring the resulting electrical or mechanical activity of
muscles
and feeding back this information to modify the stimulation and measuring the
effects again. Repeated iterations of this process can result in optimizing
the
selectivity of the stimulation, either by classical closed loop control or by
machine
learning techniques such as pattern recognition and artificial intelligence;
(2) Improving nerve selectivity by labeling or tagging nerves chemically; for
example, introduction of genes into some nerves to render them responsive to
light or other electromagnetic radiation can result in the ability to activate
these
nerves and not others when light or electromagnetic radiation is applied from
outside the body;
(3) Improving nerve selectivity by the use of electrical conductors to focus
an
electric field on a nerve; these conductors might be implanted, but could be
passive and much simpler than the active implantable medical devices
currently used;
- 32 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
(4) The use of reflectors or refractors, either outside or inside the body, is
used to
focus a beam of electromagnetic radiation on a nerve to improve nerve
selectivity. If these reflectors or refractors are implanted, they may be
passive
and much simpler than the active implantable medical devices currently used;
(5) Improving nerve selectivity by the use of feedback from the person upon
whom the stimulation is being performed; this may be an action taken by the
person in response to a physical indication such as a muscle activation or a
feeling from one or more nerve activations;
(6) Improving nerve selectivity by the use of feedback from sensors associated
with the TNSS, or separately from other sensors, that monitor electrical
activity
associated with the stimulation; and
(7) Improving nerve selectivity by the combination of feedback from both the
person or sensors and the TNSS that may be used to create a unique profile of
the user's nerve physiology for selected nerve stimulation.
[00156] Potential applications of electrical stimulation to the body, as
previously disclosed, are shown in Fig. 15.
[00157] Referring to Fig. 25A, a TNSS 934 human and mammalian interface
and its method of operation and supporting system are managed by a Master
Control
Program ("MCP") 910 represented in function format as block diagrams. It
provides
the logic for the nerve stimulator system in accordance to one example.
[00158] In one example, MCP 910 and other components shown in Fig. 25A
are implemented by one or more processors that are executing instructions. The
processor may be any type of general or specific purpose processor. Memory is
included for storing information and instructions to be executed by the
processor. The
memory can be comprised of any combination of random access memory ("RAM"),
read only memory ("ROM"), static storage such as a magnetic or optical disk,
or any
other type of computer readable media.
Master Control Program
- 33 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[00159] The primary responsibility of MCP 910 is to coordinate the activities
and communications among the various control programs, a Data Manager 920, a
User 932, and the external ecosystem and to execute the appropriate response
algorithms in each situation. The MCP 910 accomplishes electric field shaping
and/or beam steering by providing an electrode activation pattern to TNSS
device
934 to selectively stimulate a target nerve. For example, upon notification by
a
Communications Controller 930 of an external event or request, the MCP 910 is
responsible for executing the appropriate response, and working with the Data
Manager 920 to formulate the correct response and actions. It integrates data
from
various sources such as Sensors 938 and external inputs such as TNSS devices
934, and applies the correct security and privacy policies, such as encryption
and
HIPAA required protocols. It will also manage the User Interface (UI) 912 and
the
various Application Program Interfaces (APIs) 914 that provide access to
external
programs.
[00160] MCP 910 is also responsible for effectively managing power
consumption by TNSS device 934 through a combination of software algorithms
and
hardware components that may include, among other things: computing,
communications, and stimulating electronics, antenna, electrodes, sensors, and
power sources in the form of conventional or printed batteries.
Communications Controller
[00161] Communications controller 930 is responsible for receiving inputs
from the User 932, from a plurality of TNSS devices 934, and from 3rd party
apps
936 via communications sources such as the Internet or cellular networks. The
format of such inputs will vary by source and must be received, consolidated,
possibly reformatted, and packaged for the Data Manager 920.
[00162] User inputs may include simple requests for activation of TNSS devices
934 to status and information concerning the User's 932 situation or needs.
TNSS
devices 934 will provide signaling data that may include voltage readings,
TNSS 934
- 34 -
CA 03092366 2020-08-26
WO 2019/173084
PCT/US2019/019572
status data, responses to control program inquiries, and other signals.
Communications Controller 930 is also responsible for sending data and control
requests to the plurality of TNSS devices 934. 3rd party applications 936 can
send
data, requests, or instructions for the Master Control Program 910 or User 932
via the
Internet or cellular networks. Communications Controller 930 is also
responsible for
communications via the cloud where various software applications may reside.
[00163] In one example, a user can control one or more TNSS devices using a
remote fob or other type of remote device and a communication protocol such as
Bluetooth. In one example, a mobile phone is also in communication and
functions as
a central device while the fob and TNSS device function as peripheral devices.
In
another example, the TNSS device functions as the central device and the fob
is a
peripheral device that communicates directly with the TNSS device (i.e., a
mobile
phone or other device is not needed).
Data Manager
[00164] The Data Manager (DM) 920 has primary responsibility for the storage
and movement of data to and from the Communications Controller 930, Sensors
938,
Actuators 940, and the Master Control Program 910. The DM 920 has the
capability
to analyze and correlate any of the data under its control. It provides logic
to select
and activate nerves. Examples of such operations upon the data include:
statistical
analysis and trend identification; machine learning algorithms; signature
analysis and
pattern recognition, correlations among the data within the Data Warehouse
926, the
Therapy Library 922, the Tissue Models 924, and the Electrode Placement Models
928, and other operations. There are several components to the data that is
under its
control as disclosed below.
[00165] The Data Warehouse (DVV) 926 is where incoming data is stored;
examples of this data can be real-time measurements from TNSS devices 934 or
from
Sensors (938), data streams from the Internet, or control and instructional
data from
various sources. The DM 920 will analyze data, as described above, that is
held in the
- 35 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
DW 926 and cause actions, including the export of data, under MCP 910 control.
Certain decision making processes implemented by the DM 920 will identify data
patterns both in time, frequency, and spatial domains and store them as
signatures for
reference by other programs. Techniques such as EMG, or multi-electrode EMG,
gather a large amount of data that is the sum of hundreds to thousands of
individual
motor units and the typical procedure is to perform complex decomposition
analysis on
the total signal to attempt to tease out individual motor units and their
behavior. The
DM 920 will perform big data analysis over the total signal and recognize
patterns that
relate to specific actions or even individual nerves or motor units. This
analysis can be
performed over data gathered in time from an individual, or over a population
of TNSS
Users.
[00166] The Therapy Library 922 contains various control regimens for the TNSS
devices 934. Regimens specify the parameters and patterns of pulses to be
applied by
the TNSS devices 934. The width and amplitude of individual pulses may be
specified
to stimulate nerve axons of a particular size selectively without stimulating
nerve axons
of other sizes. The frequency of pulses applied may be specified to modulate
some
reflexes selectively without modulating other reflexes. There are preset
regimens that
may be loaded from the Cloud 942 or 3rd party apps 936. The regimens may be
static
read-only as well as adaptive with read-write capabilities so they can be
modified in real-
time responding to control signals or feedback signals or software updates.
Referring to
Fig. 3, one such example of a regimen has parameters A = 40 volts, t = 500
microseconds, T = 1 Millisecond, n = 100 pulses per group, and f = 20 per
second.
Other examples of regimens will vary the parameters within ranges previously
specified.
[00167] The Tissue Models 924 is specific to the electrical properties of
particular body locations where TNSS devices 934 may be placed. As previously
disclosed, electric fields for production of action potentials will be
affected by the
different electrical properties of the various tissues that they encounter.
Tissue Models
- 36 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
924 are combined with regimens from the Therapy Library 922 and Electrode
Placement Models 928 to produce desired actions. Tissue Models 924 may be
developed by MRI, Ultrasound or other imaging or measurement of tissue of a
body or
particular part of a body. This may be accomplished for a particular User 932
and/or
based upon a body norm. One such example of a desired action is the use of a
Tissue
Model 924 together with a particular Electrode Placement Model 928 to
determine how
to focus the electric field from electrodes on the surface of the body on a
specific deep
location corresponding to the pudendal nerve in order to stimulate that nerve
selectively
to reduce incontinence of urine. Other examples of desired actions may occur
when a
Tissue Model 924 in combination with regimens from the Therapy Library 22 and
Electrode Placement Models 928 produce an electric field that stimulates a
sacral
nerve. Many other examples of desired actions follow for the stimulation of
other
nerves.
[00168] Electrode Placement Models 928 specify electrode configurations that
the TNSS devices 934 may apply and activate in particular locations of the
body. For
example, a TNSS device 934 may have multiple electrodes and the Electrode
Placement Model 928 specifies where these electrodes should be placed on the
body
and which of these electrodes should be active in order to stimulate a
specific structure
selectively without stimulating other structures, or to focus an electric
field on a deep
structure. An example of an electrode configuration is a 4 by 4 set of
electrodes within
a larger array of multiple electrodes, such as an 8 by 8 array. This 4 by 4
set of
electrodes may be specified anywhere within the larger array such as the upper
right
corner of the 8 by 8 array. Other examples of electrode configurations may be
circular
electrodes that may even include concentric circular electrodes. The TNSS
device 934
may contain a wide range of multiple electrodes of which the Electrode
Placement
Models 928 will specify which subset will be activated. The Electrode
Placement
Models 928 complement the regimens in the Therapy Library 922 and the Tissue
Models 924 and are used together with these other data components to control
the
- 37 -
CA 03092366 2020-08-26
WO 2019/173084
PCT/US2019/019572
electric fields and their interactions with nerves, muscles, tissues and other
organs.
Other examples may include TNSS devices 934 having merely one or two
electrodes,
such as but not limited to those utilizing a closed circuit.
Sensor/Actuator Control
[00169] Independent sensors 938 and actuators 940 can be part of the TNSS
system. Its functions can complement the electrical stimulation and electrical
feedback that the TNSS devices 934 provide. An example of such a sensor 938
and
actuator 940 include, but are not limited to, an ultrasonic actuator and an
ultrasonic
receiver that can provide real-time image data of nerves, muscles, bones, and
other
tissues. Other examples include electrical sensors that detect signals from
stimulated
tissues or muscles. The Sensor/Actuator Control module 950 provides the
ability to
control both the actuation and pickup of such signals, all under control of
the MCP
910.
Application Program Interfaces
[00170] The MCP 910 is also responsible for supervising the various
Application Program Interfaces (APIs) that will be made available for 3rd
party
developers. There may exist more than one API 914 depending upon the specific
developer audience to be enabled. For example many statistical focused apps
will
desire access to the Data Warehouse 926 and its cumulative store of data
recorded
from TNSS 934 and User 932 inputs. Another group of healthcare professionals
may
desire access to the Therapy Library 922 and Tissue Models 924 to construct
better
regimens for addressing specific diseases or disabilities. In each case a
different
specific API 914 may be appropriate.
[00171] The MCP 910 is responsible for many software functions of the
TNSS system including system maintenance, debugging and troubleshooting
functions, resource and device management, data preparation, analysis, and
communications to external devices or programs that exist on the smart phone
or in
the cloud, and other functions. However, one of its primary functions is to
serve as a
- 38 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
global request handler taking inputs from devices handled by the
Communications
Controller 930, external requests from the Sensor Control Actuator Module
(950),
and 3rd party requests 936. Examples of High Level Master Control Program
(MCP)
functions are disclosed below.
[00172] The manner in which the MCP handles these requests is shown in
Fig. 25B. The Request Handler (RH) 960 accepts inputs from the User 932, TNSS
devices 934, 3rd party apps 936, sensors 938 and other sources. It determines
the
type of request and dispatches the appropriate response as set forth in the
following
paragraphs.
[00173] User Request: The RH 960 will determine which of the plurality of User
Requests 961 is present such as: activation; display status, deactivation, or
data input,
e.g. specific User condition. Shown in Fig. 25B is the RH's 960 response to an
activation request. As shown in block 962, RH 960 will access the Therapy
Library
922 and cause the appropriate regimen to be sent to the correct TNSS 934 for
execution, as shown at block 964 labeled "Action."
[00174] TNSS/Sensor Inputs: The RH 960 will perform data analysis over TNSS
934 or Sensor inputs 965. As shown at block 966, it employs data analysis,
which may
include techniques ranging from DSP decision-making processes, image
processing
algorithms, statistical analysis and other algorithms to analyze the inputs.
In Fig. 25B
two such analysis results are shown; conditions which cause a User Alarm 970
to be
generated and conditions which create an Adaptive Action 980 such as causing a
control feedback loop for specific TNSS 934 functions, which can iteratively
generate
further TNSS 934 or Sensor inputs 965 in a closed feedback loop.
[00175] 3rd Party Apps: Applications can communicate with the MCP 910, both
sending and receiving communications. A typical communication would be to send
informational data or commands to a TNSS 934. The RH 960 will analyze the
incoming application data, as shown at block 972. Fig. 25B shows two such
actions
that result. One action, shown at block 974 would be the presentation of the
- 39 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
application data, possibly reformatted, to the User 932 through the MCP User
Interface
912. Another result would be to perform a User 932 permitted action, as shown
at 976,
such as requesting a regimen from the Therapy Library 922.
[00176] Referring to Fig. 26, an example TNSS in accordance to one example is
shown. The TNSS has one or more electronic circuits or chips 2600 that perform
the
functions of: communications with the controller, nerve stimulation via
electrodes 2608
that produce a wide range of electric field(s) according to treatment regimen,
one or
more antennae 2610 that may also serve as electrodes and communication
pathways,
and a wide range of sensors 2606 such as, but not limited to, mechanical
motion and
pressure, temperature, humidity, chemical and positioning sensors. In another
example, TNSS interfaces to transducers 2614 to transmit signals to the tissue
or to
receive signals from the tissue.
[00177] One arrangement is to integrate a wide variety of these functions into
an
SOC, system on chip 2600. Within this is shown a control unit 2602 for data
processing, communications, transducer interface and storage and one or more
stimulators 2604 and sensors 2606 that are connected to electrodes 2608. An
antenna
2610 is incorporated for external communications by the control unit. Also
present is
an internal power supply 2612, which may be, for example, a battery. An
external
power supply is another variation of the chip configuration. It may be
necessary to
include more than one chip to accommodate a wide range of voltages for data
processing and stimulation. Electronic circuits and chips will communicate
with each
other via conductive tracks within the device capable of transferring data
and/or power.
[00178] The TNSS interprets a data stream from the control device, such as
that
shown in Fig. 25A, to separate out message headers and delimiters from control
instructions. In one example, control instructions contain information such as
voltage
level and pulse pattern. The TNSS activates the stimulator 2604 to generate a
stimulation signal to the electrodes 2608 placed on the tissue according to
the control
instructions. In another example the TNSS activates a transducer 2614 to send
a
- 40 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
signal to the tissue. In another example, control instructions cause
information such as
voltage level and pulse pattern to be retrieved from a library stored in the
TNSS.
[00179] The TNSS receives sensory signals from the tissue and translates them
to a data stream that is recognized by the control device, such as the example
in Fig.
25A. Sensory signals include electrical, mechanical, acoustic, optical and
chemical
signals among others. Sensory signals come to the TNSS through the electrodes
2608
or from other inputs originating from mechanical, acoustic, optical, or
chemical
transducers. For example, an electrical signal from the tissue is introduced
to the
TNSS through the electrodes 2608, is converted from an analog signal to a
digital
signal and then inserted into a data stream that is sent through the antenna
2610 to the
control device. In another example an acoustic signal is received by a
transducer 2614
in the TNSS, converted from an analog signal to a digital signal and then
inserted into a
data stream that is sent through the antenna 2610 to the control device. In
certain
examples sensory signals from the tissue are directly interfaced to the
control device
for processing.
[00180] An open loop protocol to control current to electrodes in known neural
stimulation devices does not have feedback controls. It commands a voltage to
be set,
but does not check the actual Voltage. Voltage control is a safety feature. A
stimulation pulse is sent based on preset parameters and cannot be modified
based on
feedback from the patient's anatomy. When the device is removed and
repositioned,
the electrode placement varies. Also the humidity and temperature of the
anatomy
changes throughout the day. All these factors affect the actual charge
delivery if the
voltage is preset.
[00181] In contrast, examples of the TNSS stimulation device have features
that
address these shortcomings using the Nordic Semiconductor nRF52832
microcontroller to regulate charge in a TNSS. The High Voltage Supply is
implemented
using a LED driver chip combined with a Computer controlled Digital
Potentiometer to
produce a variable voltage. A 3-1 step up Transformer then provides the
desired High
-41 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
Voltage, "VBOOST", which is sampled to assure that no failure causes an
incorrect
Voltage level as follows. The nRF52832 Microcontroller samples the voltage of
the
stimulation waveform providing feedback and impedance calculations for an
adaptive
protocol to modify the waveform in real time. The Current delivered to the
anatomy by
the stimulation waveform is integrated using a differential integrator and
sampled and
then summed to determine actual charge delivered to the user for a Treatment.
After
every pulse in a Stimulation event, this measurement is analyzed and used to
modify,
in real time, subsequent pulses.
[00182] This hardware adaptation allows a firmware protocol to implement the
adaptive protocol. This protocol regulates the charge applied to the body by
changing
VBOOST. A treatment is performed by a sequence of periodic pulses, which
insert
charge into the body through the electrodes. Some of the parameters of the
treatment
are fixed and some are user adjustable. The strength, duration and frequency
may be
user adjustable. The user may adjust these parameters as necessary for comfort
and
efficacy. The strength may be lowered if there is discomfort and raised if
nothing is felt.
The duration will be increased if the maximum acceptable strength results in
an
ineffective treatment.
[00183] A flow diagram in accordance with one example of the Adaptive
Protocol disclosed above is shown in Fig. 27. The Adaptive Protocol strives to
repeatedly and reliably deliver a target charge ("Qtarget") during a treatment
and to
account for any environmental changes. Therefore, the functionality of Fig. 27
is to
adjust the charge level applied to a user based on feedback, rather than use a
constant
level.
[00184] The mathematical expression of this protocol is as follows:
Qtarget = Qtarget (A * dS + B * dT), where A is the Strength Coefficient ¨
determined empirically, dS is the user change in Strength, B is the Duration
Coefficient
¨ determined empirically, and dT is the user change in Duration.
- 42 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[00185] The Adaptive Protocol includes two phases in one example: Acquisition
2700 and Reproduction 2720. Any change in user parameters places the Adaptive
Protocol in the Acquisition phase. When the first treatment is started, a new
baseline
charge is computed based on the new parameters. At a new acquisition phase at
2702, all data from the previous charge application is discarded. In one
example, 2702
indicates the first time for the current usage where the user places the TNSS
device on
a portion of the body and manually adjusts the charge level, which is a series
of charge
pulses, until it feels suitable, or any time the charge level is changed,
either manually or
automatically. The treatment then starts. The mathematical expression of this
function
of the application of a charge is as follows:
T * f
The charge delivered in a treatment is o
¨target = E 0..pulse
i=1
Where T is the duration; f is the frequency of "Rep Rate"; Qpuise (i) is the
measured charge delivered by Pulse (i) in the treatment pulse train provided
as a
voltage MON_CURRENT that is the result of a Differential Integrator circuit
shown in
Fig. 28 (i.e., the average amount of charge per pulse). The Nordic
microcontroller of
Fig. 28 is an example of an Analog to Digital Conversion feature used to
quantify
voltage into a number representing the delivered charge and therefore
determine the
charge output. The number of pulses in the treatment is T *f.
[00186] At 2704 and 2706, every pulse is sampled. In one example, the
functionality of 2704 and 2706 lasts for 10 seconds with a pulse rate of 20
Hz, which
can be considered a full treatment cycle. The result of phase 2700 is the
target pulse
charge of 0 ¨target.
[00187] Fig. 29 is a table in accordance with one example showing the number
of pulses per treatment measured against two parameters, frequency and
duration.
Frequency is shown on the Y-axis and duration on the X-axis. The Adaptive
Current
- 43 -
CA 03092366 2020-08-26
WO 2019/173084
PCT/US2019/019572
protocol in general performs better when using more pulses. One example uses a
minimum of 100 pulses to provide for solid convergence of charge data
feedback.
Referring to the Fig. 29, a frequency setting of 20Hz and duration of 10
seconds
produces 200 pulses, which is desirable to allow the Adaptive Current Protocol
to
reproduce a previous charge.
[00188] The reproduction phase 2720 begins in one example when the user
initiates another subsequent treatment after acquisition phase 2700 and the
resulting
acquisition of the baseline charge, Qtarget. For example, a full treatment
cycle, as
discussed above, may take 10 seconds. After, for example, a two-hour pause as
shown at wait period 2722, the user may then initiate another treatment.
During this
phase, the Adaptive Current Protocol attempts to deliver Qtarget for each
subsequent
treatment. The functionality of phase 2720 is needed because, during the wait
period
2722, conditions such as the impedance of the user's body due to sweat or air
humidity
may have changed. The differential integrator is sampled at the end of each
Pulse in
the Treatment. At that point, the next treatment is started and the
differential integrator
is sampled for each pulse at 2724 for purposes of comparison to the
acquisition phase
Qtarget. Sampling the pulse includes measuring the output of the pulse in
coulombs.
The output of the integrator of Fig. 28 in voltage, referred to as Mon_Current
2801, is a
direct linear relationship to the delivered charge in micro-coulombs and
provides a
reading of how much charge is leaving the device and entering the user. At
2726, each
single pulse is compared to the charge value determined in phase 2700 (i.e.,
the target
charge) and the next pulse will be adjusted in the direction of the
difference.
NUM PULSES = (T*f)
After each pulse, the observed charge, Qpuise(i), is compared to the expected
charge per pulse.
Qpuise(I)> Qtarget / NUM_PULSES ?
The output charge or "VBOOST" is then modified at either 2728 (decreasing) or
2730 (increasing) for the subsequent pulse by:
- 44 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
dV(i) = G[O ,target/NUM_PULSES- Qpulse(i)]
where G is the Voltage adjustment Coefficient ¨ determined empirically. The
process continues until the last pulse at 2732.
[00189] A safety feature assures that the VBOOST will never be adjusted higher
by more than 10%. If more charge is necessary, then the repetition rate or
duration
can be increased.
[00190] In one example, in general, the current is sampled for every pulse
during acquisition phase 2700 to establish target charge for reproduction. The
voltage
is then adjusted via a digital potentiometer, herein referred to as "Pot",
during
reproduction phase 2720 to achieve the established target_charge.
[00191] The digital Pot is calibrated with the actual voltage at startup. A
table is
generated with sampled voltage for each wiper value. Tables are also
precomputed
storing the Pot wiper increment needed for 1v and 5v output delta at each pot
level.
This enables quick reference for voltage adjustments during the reproduction
phase.
The tables may need periodic recalibration due to battery level.
[00192] In one example, during acquisition phase 2700, the minimum data set =
100 pulses and every pulse is sampled and the average is used as the
target_charge
for reproduction phase 2720. In general, less than 100 pulses may provide an
insufficient data sample to use as a basis for reproduction phase 2720. In one
example, the default treatment is 200 pulses (i.e., 20Hz for 10 seconds). In
one
example, a user can adjust both duration and frequency manually.
[00193] In one example, during acquisition phase 2700, the maximum data set =
1000 pulses. The maximum is used to avoid overflow of 32bit integers in
accumulating
the sum of samples. Further, 1000 pulses in one example is a sufficiently
large data
set and collecting more is likely unnecessary.
[00194] After 1000 pulses for the above example, the target_charge is
computed. Additional pulses beyond 1000 in the acquisition phase do not
contribute to
the computation of the target charge.
- 45 -
CA 03092366 2020-08-26
WO 2019/173084
PCT/US2019/019572
[00195] In one example, the first 3-4 pulses are generally higher than the
rest so
these are not used in acquisition phase 2700. This is also accounted for in
reproduction phase 2720. Using these too high values can result in target
charge
being set too high and over stimulating on the subsequent treatments in
reproduction
phase 2720. In other examples, more advanced averaging algorithms could be
applied
to eliminating high and low values.
[00196] In an example, there may be a safety concern about automatically
increasing the voltage. For example, if there is poor connection between the
device
and the user's skin, the voltage may auto-adjust at 2730 up to the max. The
impedance may then be reduced, for example by the user pressing the device
firmly,
which may result in a sudden high current. Therefore, in one example, if the
sample is
500mv or more higher than the target, it immediately adjusts to the minimum
voltage.
This example then remains in reproduction phase 2720 and should adjust back to
the
target current/charge level. In another example, the maximum voltage increase
is set
for a single treatment (e.g.,by). More than that should not be needed in
normal
situations to achieve the established target_charge. In another example, a max
is set
for VBOOST (e.g., 80V).
[00197] In various examples, it is desired to have stability during
reproduction
phase 2720. In one example, this is accomplished by adjusting the voltage by
steps.
However, a relatively large step adjustment can result in oscillation or over
stimulation.
Therefore, voltage adjustments may be made in smaller steps. The step size may
be
based on both the delta between the target and sample current as well as on
the actual
VBOOST voltage level. This facilitates a quick and stable/smooth convergence
to the
target charge and uses a more gradual adjustments at lower voltages for more
sensitive users.
[00198] The following are the conditions that may be evaluated to determine
the
adjustment step.
delta-mon_current = abs(sample_mon_current - target_charge)
- 46 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
If delta_mon_current > 500mv and VBOOST > 20V then step = 5V for
increase adjustments
(For decrease adjustments a 500mv delta triggers emergency decrease
to minimum Voltage)
If delta_mon_current > 200mv then step=1V
If delta_mon_current > 100mv and delta_mon_current > 5%*
sample_mon_current then step = 1V
[00199] In other examples, new treatments are started with voltage lower than
target voltage with a voltage buffer of approximately 10%. The impedance is
unknown
at the treatment start. These examples save the target_voltage in use at the
end of a
treatment. If the user has not adjusted the strength parameter manually, it
starts a new
treatment with saved target_voltage with the 10% buffer. This achieves target
current
quickly with the 10% buffer to avoid possible over stimulation in case
impedance has
been reduced. This also compensates for the first 3-4 pulses that are
generally higher.
[00200] As disclosed, examples apply an initial charge level, and then
automatically adjust based on feedback of the amount of current being applied.
The
charge amount can be varied up or down while being applied. Therefore, rather
than
setting and then applying a fixed voltage level throughout a treatment cycle,
implementations of the invention measure the amount of charge that is being
input to
the user, and adjust accordingly throughout the treatment to maintain a target
charge
level that is suitable for the current environment.
Location-Specific Patch
[00201] The duration of use and electronic effectiveness of the Topical Nerve
Stimulation and Sensor (TNSS) apparatus as disclosed in examples herein may be
further optimized by form factor according to specific location of skin
application.
Examples include the use of a patch incorporating a TNSS apparatus and
designed in
a shape to adhere to a specific location on a person's body, or in a shape to
be
- 47 -
CA 03092366 2020-08-26
WO 2019/173084
PCT/US2019/019572
incorporated into clothing to be in close proximity to a specific location on
a person's
body, to optimize the effectiveness of the TNSS.
[00202] In Fig. 30, a tibial patch or TNSS or "SmartPad" 100 in accordance
with
examples is designed in a shape to conform to the skin when affixed in the
location
below the ankle bone 110 to be effective at stimulating the tibial nerve; and
the shape
to be of one type for the left ankle, and of a similar but mirrored type for
the right ankle.
A SmartPad is more effective when the positive and negative electrodes are
placed
axially along the path of the nerve in contrast to transversely across the
path of the
nerve, which is not as effective.
[00203] In Fig. 31, a radial SmartPad 200 is designed in a shape to conform to
the skin when affixed in the location on the forearm to be electronically
effective at
stimulating the radial nerve 202; a median SmartPad 220 is designed in a shape
to
conform to the skin when affixed in the location on the forearm to be
electronically
effective at stimulating the median nerve 222; and an ulnar SmartPad 240 is
designed
to conform to the skin when affixed in the location on the forearm to be
electronically
effective at stimulating the ulnar nerve 242.
[00204] Each of the SmartPad shapes in Figs. 30 and 31 is designed to
minimize discomfort for the user when affixed in the target location.
[00205] In some examples, two or more of the radial 200, median 220 and ulnar
SmartPads 240 may be designed into a larger SmartPad with a shape to cover the
locations on the skin corresponding to the two or more of radial, median and
ulnar
nerve stimulation electrode pairs, such as a bracelet shape 250 surrounding
the
forearm, or a semi-bracelet 255 spanning one side of the forearm, or a
bracelet shape
with strap 260 surrounding the forearm and using a strap 265 to tighten to
maintain
placement of electrodes without the need for additional adhesives. In some
examples,
these combined SmartPads are designed in one shape for the left forearm, and a
similar but mirrored shape for the right forearm.
- 48 -
CA 03092366 2020-08-26
WO 2019/173084
PCT/US2019/019572
[00206] In Fig. 32, a skin patch 300 includes a SmartPad 340 with TNSS design
and packaging disclosed above. SmartPad 340 material is selected to be
disposable
after removal from the skin, for example paper, and is selected to inhibit
moisture
penetration and foreign material intrusion which might adversely affect the
performance
of the TNSS. SmartPad 340 is packaged before use between top outer packaging
310,
and bottom outer packaging 320. Top outer packaging incorporates one or more
of
writing 312, illustrations 314, and orientation mark 316, the orientation mark
316 being
useful for properly positioning the SmartPad 340 on the skin. Bottom outer
packaging
incorporates one or both of writing 322 and illustrations 324. SmartPad 340
may have
removable orientation marking 346, initially affixed to the outer surface of
the SmartPad
340, this marking designed to simplify proper orientation of the SmartPad onto
the
target location on the skin and designed to be removed by the user while
leaving the
SmartPad in place such that the distinctive marking 346 is no longer seen on
the user's
skin. SmartPad 340 may have supplementary adhesive pads 350 of sufficient size
and
efficacy to maintain adhesion when in use but minimize pulling force when
removing
the SmartPad 340; and adhesive pad covers 330, initially covering the
supplementary
adhesive pads 350 and covering the electrodes, the adhesive pad covers 330
being
removed before affixing the adhesives to the skin; folded pull tabs 332 to
facilitate
remove of the adhesive film covers 330. SmartPad 340 may have non-adhering tab
area 344, at one or both ends of the SmartPad, to facilitate grabbing of the
SmartPad
edge to begin removal of the SmartPad, opposite the adhesive film patches 350.
All
components of SmartPad 340 are coupled to the same substrate in one example.
[00207] Fig. 33 illustrates other example locations for a patch.
[00208] Fig. 34 illustrates a cutaway view where a right foot plantar sock
patch
530 is affixed into the sole 520 of a sock 510, using adhesive or stitches,
such that the
sock patch 530 is effective for stimulation through the sole of the user's
foot skin and
tissue to stimulate the plantar nerve.
- 49 -
CA 03092366 2020-08-26
WO 2019/173084 PCT/US2019/019572
[00209] In some examples, the sock patch uses a removable battery power
supply. In some examples, the sock patch uses a rechargeable battery power
supply
and has a recharging port on the sock. In some examples, the sock patch uses a
battery power supply with kinetic power converter.
[00210] Fig. 35 illustrates a cutaway view where a right foot plantar shoe
patch
630 is affixed into the sole 625 of a shoe 615, such that the shoe patch 630
is effective
for stimulation through the sole of the user's foot skin and tissue to
stimulate the plantar
nerve, particularly when wearing no intervening clothing layer such as a sock
which
reduces the effectiveness of the stimulation.
[00211] In some examples, shoe patch 630 uses a removable battery power
supply. In some examples, the shoe patch uses a rechargeable battery power
supply
and has a recharging port on the shoe. In some examples, the shoe patch uses a
battery power supply with kinetic power converter. In some examples, shoe
patch 630
is incorporated into the shoe 615 during manufacture of the shoe, the shoe
being
specifically designed for a wearer to use the integral TNSS device.
[00212] In some examples, shoe patch 630 is applied to an interior surface of
an
ordinary shoe 610 by the person intending to wear the shoe.
[00213] Skin patches designed for specific body locations use different
software
libraries for their operation, each of which is optimized for the skin patch
location and
using a model for the underlying skin, tissue and nerves. An example is a
sacral skin
patch which involves models for the skin, fat, muscle, bone and nerves
specific to the
sacrum location, as compared to an ulnar skin patch which involves models
which
involves models for the tibial nerve location.
[00214] Several examples are specifically illustrated and/or described herein.
However, it will be appreciated that modifications and variations of the
disclosed
examples are covered by the above teachings and within the purview of the
appended
claims without departing from the spirit and intended scope of the invention.
- 50 -