Note: Descriptions are shown in the official language in which they were submitted.
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
METHOD OF MANUFACTURING A BLOOD PUMP
FIELD OF THE INVENTION
100011 This invention relates to an intravascular blood pump for
percutaneous
insertion into a patient's blood vessel, in particular to be advanced into the
patient's
heart, and in particular a method of manufacturing the intravascular blood
pump.
BACKGROUND OF THE INVENTION
[0002] An intravascular blood pump designed to be inserted
percutaneously into
.. a patient's blood vessel, such as a femoral or axillary artery or vein, may
be advanced
into the patient's heart to act as a left ventricular assist device or right
ventricular assist
device. The blood pump may, thus, be referred to also as an intracardiac blood
pump.
An intravascular blood pump typically comprises a catheter and a pumping
device
attached to a distal end of the catheter. The catheter may contain supply
lines, such as an
electric line and a purge line. Throughout this disclosure, the term "distal"
will refer to
directions away from a user and towards the heart, whereas the term "proximal"
will
refer to directions towards a user.
[0003] The pumping device may comprise an electric motor and an
impeller
coupled to a rotor of the electric motor for rotation of the impeller about an
axis of
rotation. During operation of the blood pump, the impeller conveys blood from
a blood
flow inlet to a blood flow outlet of the blood pump, for instance through a
flow carmula.
The pump rate depends on the size of the pumping device. In particular, the
efficiency
of the electric motor included in the pumping device is highly dependent on
the limited
space. However, it is desirable to reduce the size of the pumping device, in
particular its
diameter, because of anatomical limitations for insertion into a blood vessel.
[0004] While the flow cannula and the catheter usually are
sufficiently flexible
for following the anatomical path of the blood vessel, the pumping device is
rigid. Thus,
in order to facilitate navigation of the blood pump through the patient's
blood vessel
- 1 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
into the patient's heart, it would be desirable to reduce not only the
diameter but also the
length of the rigid pumping device. Furthermore, a relatively long pumping
device
causes relatively strong kinks at the interface between the pumping device and
the flow
cannula and the catheter, respectively, during advancing the blood pump
through a
curved blood vessel, which may lead to kinks or breaking.
[0005] In known intravascular blood pumps having a micro motor for
driving
the impeller of the blood pump, e.g. the blood pump disclosed in WO 98/44619
Al, the
stator or at least stator parts of the electric motor are encapsulated in a
casting
compound, such as a polymer material, e.g. epoxy. According to a method of
manufacturing the micro motor disclosed in WO 98/44619 Al, the stator parts of
the
motor are placed on a mandrel, which is then inserted into a mold cavity. A
casting
compound is injected into the mold cavity to encapsulate the stator parts and
to form a
housing of the pumping device.
[0006] The described injection-molding process may be carried out in a
vacuum
.. atmosphere, which requires long production cycles, during which the
respective mold is
occupied until the casting compound is cured, which typically takes from about
one
hour to about 24 hours. Thus, a large number of molds has to be provided for
being able
to increase the number of produced pieces. However, the molds are expensive
and have
to be cleaned after each cycle. Furthermore, a release agent, such as a
silicone, is
usually necessary, which has to be removed from the finished products. The
resulting
electric motor has a relatively thick plastic housing, which serves as a
corrosion
protection as desired but does not add further value to the function of the
blood pump.
To the contrary, the plastic housing increases the diameter of the pumping
device and is
heat insulating, which may lead to undesired heating of the electric motor
during
operation of the blood pump.
SUMMARY OF THE INVENTION
[0007] It is therefore an object of the present invention to provide
an
intravascular blood pump having small outer dimensions while at the same time
having
- 2 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
an efficient electric motor for providing an increased pump rate, as well as a
fast and
cost-effective method of manufacturing such intravascular blood pump.
[0008] This object is achieved according to the present invention by a
method of
manufacturing an intravascular blood pump and a respective intravascular blood
pump
having the features of the independent claims. Preferred embodiments and
further
developments of the invention are specified in the claims dependent thereon.
[0009] According to an aspect of the invention, a method of
manufacturing an
intravascular blood pump is provided. In particular, an intravascular blood
pump as
outlined above is to be manufactured, which comprises a pumping device
including an
impeller and an electric motor for driving the impeller. The electric motor
includes a
stator and a rotor, wherein the rotor is rotatable about an axis of rotation
and coupled to
the impeller so as to be able to cause rotation of the impeller. For
manufacturing the
intravascular blood pump, a molding base, which is sized and shaped for
receiving
stator components thereon, is provided. The stator components, such as a coil
winding
and possibly other stationary components, are placed on the molding base. An
outer
sleeve, which may be considered as the outermost of the stator components, is
then
placed on the molding base, and thereby over the other stator components
already
placed on the molding base, to thereby form at least a portion of an outer
surface of the
blood pump and to form an interspace between the molding base and the outer
sleeve in
which the stator components are disposed. A casting compound, such as a
polymer
material, in particular a resin like epoxy, is then injected into said
interspace via the
molding base to fix the stator components inside, i.e. radially inwards
relative to the
outer sleeve.
[0010] By the manufacturing method according to the present invention
an
intravascular blood pump can be made which has an outer sleeve forming at
least a
portion of an outer surface of the pumping device, wherein stator components,
like the
coil winding, are fixed inside the outer sleeve by means of a casting
compound. The
stator components are fixed by the casting compound, i.e. they are secured
against
relative movement to each other, in particular also relative to the outer
sleeve. Stator
components which are fully surrounded by the casting compound, such as the
coil
- 3 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
winding, are encapsulated by the casting compound and the electrically active
components are well insulated on both sides against blood and purge fluids to
avoid any
leakage current or electro corrosion.
100111 According to the method, the outer sleeve may be regarded to
form a
housing of the pumping device (also denoted as pump casing below) by forming
at least
a portion of the outer surface of the pumping device. The housing, more
specifically the
outer sleeve, defines an outer surface of the blood pump, more specifically
the pumping
device, at least in an area in which the blood pump has a maximum outer
diameter.
Thus, in contrast to known intravascular blood pumps, the outer surface or
housing is
not formed by the casting compound but by the outer sleeve. The casting
compound is
completely disposed within the boundaries defined by the inner diameter of the
outer
sleeve. The outer sleeve provides a fluid tight barrier against blood or other
fluids to
protect the stator components from corrosion. The outer sleeve may also serve
as a soft
iron magnet yoke as will be described below.
100121 In other words, the mold for injecting the casting compound is
formed by
the outer sleeve, i.e. the pump casing itself forms the mold or, more
specifically, a first
casing section of the pump casing is formed by the mold. The first casing
section will be
connected with a second casing section at a later time to complete the pump
casing and,
in particular, to complete a housing for the motor. Thus, the method of
manufacturing
according to the present invention does not need expensive molds, which are
occupied
during curing the casting compound. The casting compound is directly injected
into the
inside of the pumping device, more specifically into the interspace formed
between the
outer sleeve and the molding base. No cleaning of the finished product is
required since
the casting compound is only in the inside of the outer sleeve, unlike in
known injection
molding processes where typical casting compounds like epoxy may stick to
undesired
areas on the outer surface of the product and require removal of excess
casting
compound. This may be particularly relevant if delicate structures on the
outer surface
of the pumping device shall be free of casting compound, such as a groove for
a sensor
or the like. Furthermore, since the product does not have to be removed from a
mold
cavity, the method does not require any release agents, and there is no need
for
removing a release agent from the surface of the finished product. Being able
to work
- 4 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
without any release agent also reduces the risk of any undesirable
contamination, which
could lead to a long term break down of the desired insulation of the casting
compound.
At the same time, other components of the blood pump, in particular stationary
components, including stationary components which are not necessarily part of
the
electric motor but which are located also inside the outer sleeve, can be
easily fixed by
the casting compound, e.g. the end of a purge line.
[0013] In contrast to known molds for an injection molding process,
the molding
base used in the method according to the present invention may be an easy to
manufacture and cheap piece and can be produced e.g. by injection molding or
other
techniques, like rapid prototyping, lathing, or the like. The molding base can
be fowled
as a disposable piece so that there is no need to wait for the casting
compound to cure
before another pumping device can be produced. The molding base, in particular
if
produced by injection-molding, may comprise a plastic material like
polyethylene (PE),
polypropylene (PP), polytetrafluoroethylene (PTFE), or other plastic materials
suitable
to withstand the injection molding process. PTFE allows for particularly easy
removal
of the molding base from the product after curing.
[0014] Forming the outer surface of the pumping device by the outer
sleeve
rather than by a casting compound has the further advantage that the outer
diameter of
the pumping device can be reduced because there is no additional casting
compound
that surrounds the pumping device to form a pump casing. For instance, the
pump
casing may have an outer dimension of 18 F (French) or smaller (i.e. an outer
diameter
of 6 mm or smaller). Despite the small dimensions, a pump rate of up to 5.5
liters per
minute may be achieved. By reducing the amount of plastic material, heat
transfer away
from the pumping device may be improved because of the reduced plastic
insulation.
Furthermore, this may reduce the likelihood of breakage of the blood pump, in
particular at interfaces between the pumping device and a catheter and a flow
cannula,
respectively, because stress peaks at these interfaces may be reduced.
[0015] The aforementioned advantages and effects become particularly
effective
if the outer sleeve comprises a magnetically conductive material to form a
yoke (back
iron) of the electric motor. Thus, the outer sleeve does not only fonn a
casing without a
- 5 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
further function but serves as the yoke for closing the magnetic flux of the
electric
motor. In particular, the outer sleeve may comprise or may be made of a metal
or metal
alloy, such as a ferritic alloy, e.g. a FeCrAl alloy. The outer surface of the
sleeve may be
covered with a respective oxide. It will be appreciated that the outer sleeve
may
comprise any suitable biocompatible magnetically conductive material. A metal
material has the further advantage that heat dissipation is increased compared
to a
plastic material and may provide increased structural stability.
[0016] According to a particularly preferred embodiment, the first
stator part to
be placed on the molding base may be an inner sleeve such that the interspace
for
injecting the casting compound is formed between the inner sleeve and the
outer sleeve.
The inner sleeve then forms a cavity for receiving the rotor of the electric
motor. In a
preferred embodiment, the inner sleeve is made of a ceramic material, such as
zirconia,
or more preferably alumina toughened zirconia (ATZ). After placing the inner
sleeve on
the molding base, other stator parts may be placed on the molding base, more
specifically on the inner sleeve, e.g. a coil winding, a bearing, a printed
circuit board, an
electric line, a purge line, etc.
[0017] By providing an inner sleeve made of a ceramic material a fluid
tight
enclosure of the cavity, in which the rotor is disposed, can be created. The
ceramic
material is diffusion resistant against the purge fluid. Thus, effective
corrosion
protection of the stator, in particular electric stator parts like the coil
winding, can be
achieved. Since in this preferred embodiment the ceramic sleeve rather than
the inner
surface of the casting compound forms the cavity for the rotor, corrosion
protection
does not depend on the precision of the injection molding process, but the
ceramic
material of the inner sleeve forms a secure barrier against the purge fluid.
[0018] Apart from the sealing properties of the ceramic material, the
ceramic
inner sleeve provides the advantage that it can be manufactured with very
small
manufacturing tolerances. Thus, e.g. by placing the coil winding on the
ceramic sleeve
before injection molding, the dimensions of the coil winding, in particular
the inner
diameter and, thus, the outer diameter, can be defined and adjusted very
precisely. The
ceramic sleeve is substantially rigid and good to handle and may improve
handling of
- 6 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
the coil winding once the coil winding is placed on the sleeve. For instance,
the coil
winding may be placed on the molding base together with the inner sleeve. The
ceramic
material allows for a very small wall thickness of the inner sleeve, which is
important
for not increasing the overall diameter of the pumping device and for
maintaining a
small air gap between the stationary coil and the rotating magnet in order to
ensure a
high motor efficiency and low core temperature.
[0019] The casting compound is preferably fed through the molding base
and
into said interspace between the molding base and the outer sleeve. That
means, the
molding base may be provided as a socket for the injection molding process and
the
molding base may have a port and one or more supply lines which are configured
to
supply the casting compound into the outer sleeve, more specifically into the
interspace
between the molding base and the outer sleeve or preferably into the
interspace between
the inner sleeve and the outer sleeve. In particular, the casting compound
will not be
supplied to the outside of the outer sleeve.
[0020] Injecting the casting compound may be carried out in a low-pressure
atmosphere, in particular vacuum, in which the interspace is substantially
evacuated.
This may aid in drawing the casting compound into the interspace and
distributing the
casting compound all-over within the interspace and to fix or encapsulate the
stator
components in the interspace and to the outer sleeve.
[0021] In order to compensate for shrinking of the casting compound during
a
curing process, an excess amount of casting compound may be fed into and
through the
interspace into a reservoir. In other words, a riser may be provided to
prevent bubbles or
cavities due to shrinkage during curing of the casting compound. The reservoir
is
preferably placed outside the outer sleeve and connected to the interspace.
This
connection will be removed along with the reservoir after curing is completed.
It will be
appreciated that this is the only exception for casting compound to be fed to
the outside
of the outer sleeve. Nevertheless, in any case, the casting compound will not
come into
contact with the outer surface of the outer sleeve.
[0022] In one embodiment, the molding base may comprise a pin,
preferably a
metal pin, arranged along a central longitudinal axis of the molding base and
protruding
- 7 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
from the molding base. The pin may be sized and shaped to receive e.g. a purge
line,
which extends from the pumping device. The pin may form a portion of the
molding
base having the smallest diameter. Thus, a metal pin may improve stability of
the
molding base. The pin may be coated, e.g. with the plastic material used for
the rest of
the molding base. In other words, the pin may form a central core of the
molding base.
[0023] Before the outer sleeve is placed on the molding base, an
electric line
may be connected, e.g. soldered, to at least one of the stator components,
preferably the
coil winding. The established electrical connection will then be fixed, in
particular
encapsulated, also by the casting compound. The electrical connection may be
located
on the ceramic inner sleeve described above.
[0024] Generally, the molding base in the method of manufacturing a
blood
pump according to the present invention may be denoted as molding inlay. The
molding
base may be formed as a mandrel. The mandrel is configured to receive the
stator
components and the outer sleeve thereon and may serve for centering all parts
placed
thereon. The molding base may have a substantially cylindrical body. More
generally,
the molding base has a convex body, in particular in contrast to concave
molding
cavities.
[0025] The molding base preferably has a shoulder, wherein a portion
of the
molding base with first outer diameter may correspond to a cavity for the
rotor of the
electric motor, and a portion of the molding base with second outer diameter,
smaller
than the first outer diameter, may correspond substantially to a central shaft
of the rotor
or a central aperture of a bearing, in particular a journal bearing.
[0026] In the above described method, the casting compound is cured
after being
injected into the interspace, wherein the molding base is preferably separated
from a
casting compound source before curing the casting compound. Thus, the molding
base
with the casting compound and the parts of the pumping device can be removed
from an
injection station and stored for curing. No mold is occupied, in particular if
the molding
base is a disposable piece. A plurality of molding bases may be placed on a
support
after the injection-molding process and stored for curing, while the injection
process
- 8 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
may be continued for further products. The method of the present invention is,
thus,
suitable for cost-effective mass production.
[0027] After the casting compound has been cured, the molding base is
removed. No cleaning is required since the casting compound is disposed only
in the
interspace fixing the stator components and securing them in and to the outer
sleeve.
For improving precision of the injection-molding, the outer sleeve may be
sealed
against the molding base, e.g. by applying adhesive or glue at possible
leakages before
injecting the casting compound into the interspace, to prevent casting
compound to leak
out of the outer sleeve. Adhesive may also be applied at locations inside the
outer sleeve
to prevent casting compound to flow into certain stator compounds, such as a
bearing.
[0028] The outer sleeve together with the stator components fixed
therein form a
first casing section. The cavity inside the outer sleeve in which the molding
base is
located during the manufacturing process will form a cavity for the rotor of
the electric
motor, in particular a magnet, which will be inserted into the cavity.
Furthermore, a
second casing section will be mounted against the first casing section so as
to complete
the housing for the motor, and an impeller may be coupled to a shaft connected
to the
rotor and extending out of the motor housing. Finally, a flow cannula forming
a blood
flow inlet and a blood flow outlet, a catheter, and other parts may be added
to complete
the intravascular blood pump.
100291 In use, a purge fluid will be directed through the rotor cavity from
proximal to distal and exit the motor housing where the rotor shaft extends
out of the
motor housing. In addition, purge fluid may seep through leaks, if any, at the
interface
between the first and second casing sections into the patient's blood. While
this would
not be critical, the purge fluid might further leak through micro cracks in
the casting
compound resulting from shrinkage of the casting compound during curing, and
might
reach the coil windings of the stator, which should be avoided. Therefore,
according to a
preferred embodiment, at least one sealing ring is provided between the outer
sleeve and
the inner sleeve of the stator components so as to form a seal there between
and protect
the coil winding contained in the interspace between the outer and inner
sleeves
accordingly against ingress of purge fluid.
- 9 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
[0030] If such sealing ring is provided prior to injecting and curing
the molding
compound in the interspace, molding compound may come in contact with the
sealing
ring and thereby negatively affect the sealing characteristics of the sealing
ring. In this
case, it is advantageous to provide a second sealing ring axially in row with
the first
sealing ring so that the second sealing ring protects the first sealing ring
against the
molding compound.
[0031] Since the first sealing ring may deteriorate over time due to
its contact
with the purge fluid, it is further preferred to dispose a sealing ring also
between the
first and second casing sections when mounting the second casing section
against the
first casing section so as to seal the first sealing ring against the rotor
cavity and, thus,
against the purge fluid.
[0032] Alternatively, instead of providing the afore-mentioned one or
two or
even three sealing rings, the first casing section may be mounted to the
second casing
section using a liquid sealing material between the first and second casing
sections at an
interface with the inner sleeve. The liquid sealing material completely fills
all spaces
and preferably wets and attaches to all surfaces in such spaces. Once the
liquid sealing
material is dry, the first casing section is sealingly connected to the second
casing
section at the interface with the inner sleeve, thereby preventing any purge
fluid from
reaching the casting compound surrounding the coil windings. Preferably, the
dried
liquid sealing material is elastic. For instance, an elastomeric material may
be used as
the liquid sealing material which ¨ when cured and dry ¨ provides elastic
properties in
order to provide proper sealing functions and also to compensate for shrinkage
of the
sealing material during curing.
BRIEF DESCRIPTION OF THE DRAWINGS
[0033] The foregoing summary, as well as the following detailed
description of
preferred embodiments, will be better understood when read in conjunction with
the
appended drawings. For the purpose of illustrating the present disclosure,
reference is
- 10-
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
made to the drawings. The scope of the disclosure is not limited, however, to
the
specific embodiments disclosed in the drawings. In the drawings:
Fig. 1 schematically shows an intravascular blood pump inserted into a
patient's heart.
Fig. 2 shows a cross-section through an intravascular blood pump according to
a first
embodiment.
Fig. 3a to 3f schematically show steps of a method of manufacturing a first
casing
section for the intravascular blood pump according to the first embodiment.
Fig. 4 shows a cross-section through the molding base used in the method of
manufacturing the first casing section for the intravascular blood pump
according to the
first embodiment with stator components placed thereon.
Figs. 5a to 5f schematically show steps of a method of manufacturing a first
casing
section for the intravascular blood pump according to a second embodiment.
Fig. 6 shows a cross-section through the molding base used in the method of
manufacturing the first casing section for the intravascular blood pump
according to the
second embodiment with stator components placed thereon.
Fig. 7 shows a cross-section through an intravascular blood pump according to
the
second embodiment comprising the first casing section shown in Fig. 6.
DETAILED DESCRIPTION
100341 In Fig. 1 is illustrated an intravascular blood pump P inserted into
a
patient's heart H. More specifically, the blood pump P comprises a pumping
device 1
attached to a catheter 5 by means of which the pumping device 1 is inserted
into the left
ventricle LV of the patient's heart H to pump blood from the left ventricle LV
into the
aorta AO. The shown application is only an exemplary application, and the
blood pump
P of the present invention is not limited to this application. For instance,
reverse
applications for the right ventricle RV may be envisioned. The blood pump P is
percutaneously inserted e.g. via a femoral access or an axillary access and is
advanced
-11-
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
through the aorta AO into the heart H. The blood pump P is placed such that a
blood
flow outlet 2 is disposed outside the patient's heart H in the aorta AO, while
a blood
flow inlet 3 which is in flow communication with a flow carmula 4 is disposed
inside
the left ventricle LV. An impeller is provided in the pumping device 1 to
cause the
blood flow from the blood flow inlet 3 to the blood flow outlet 2, and
rotation of the
impeller is caused by an electric motor disposed in the pumping device 1 as
will be
explained in more detail below.
[0035] Fig. 2 shows a cross-sectional view through the pumping device
1
according to a first embodiment along a central longitudinal axis L, which is
coincident
with an axis of rotation of the rotor 7 and the impeller 6. More specifically,
the rotor 7
and the impeller 6 are arranged on a common shaft 8 which extends along the
axis of
rotation. The rotor 7 of the electric motor is formed as a peinianent magnet
and is
disposed inside a cavity 22 of the pump casing. In order to cause rotation of
the rotor 7,
a coil winding 9 as part of a stator of the electric motor surrounds the rotor
7 and is
controllable so as to cause rotation of the rotor 7. The impeller 6 is coupled
to the rotor
7 via the shaft 8 such that rotation of the rotor 7 causes rotation of the
impeller 6 to
thereby draw blood into the blood flow inlet 3 and through the flow cannula 4
out of the
blood flow outlet 2 as indicated by the arrows in Fig. 2.
[0036] The shaft 8 is rotatably supported by a distal bearing 12 and
aproximal
bearing 11, both of which may be formed as a journal bearing as shown in Fig.
2. The
bearings 11, 12 and the shaft 8 may be formed of a ceramic material. However,
other
types of bearings, such as ball bearings, may be used for rotatably supporting
the shaft
8. The bearings may be axial bearings or radial bearings or combined axial and
radial
bearings. A purge fluid is supplied through the bearings 11, 12 and the cavity
22 in
which the rotor 7 is located by means of a purge line 15. The purge line 15
extends
through the catheter 5 and is connected to the proximal bearing 11 in a fluid
tight
manner. In this way, the purge fluid does not come into contact with
electrical
components of the pumping device 1 but only flows through the proximal bearing
11,
into the cavity 22 and through the distal bearing 12.
- 12-
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
100371 In order to provide a secure barrier to protect the electrical
components,
in particular the coil winding 9, from corrosion and short circuits caused by
the purge
fluid, the cavity 22 for the rotor 7 may be formed by an inner sleeve 14,
which is made
of a ceramic material. The ceramic inner sleeve 14 is attached to the proximal
bearing
11 in a fluid tight manner and is resistant against diffusion of the purge
fluid. The
ceramic inner sleeve 14 is so well defined also with a smooth inner surface
that in
another configuration of the blood pump some blood can be allowed to enter the
pump
instead of the purge fluid without clotting or blood destruction. Further
corrosion
protection is established by a casting compound 18, which fixes the stator
components
of the pumping device 1 and fills an interspace 19 between the inner sleeve 14
and an
outer sleeve 13. In particular, the coil winding 9 is encapsulated in the
casting
compound 18. The casting compound 18 also provides additional fixation for the
electrical connections 16 (i.e. a printed circuit board PCB) with the motor
cable 10 as
well as the purge line 15. The casting compound 18 may be a polymer material
like a
resin, preferably a two-component epoxy, and more preferably a two-component
epoxy
with a heat conducting and electrically insulating filler.
100381 The outer sleeve 13 defines the outer surface and the outer
dimensions of
a part of the pumping device I. Thus, a first section of the casing of the
pumping device
1 is defined by the outer sleeve 13 enclosing the aforementioned components,
in
particular the stator components fixed by the casting compound 18. It will be
appreciated that the outer sleeve 13 also forms a stator component, which is
magnetically active. The outer sleeve 13 is made of a biocompatible
magnetically
conductive material, such as a suitable metal alloy, and serves as a yoke for
the
magnetic flux of the electric motor. The metal outer sleeve 13 also allows for
good
dissipation of heat caused by operation of the electric motor. The outer
surface of the
outer sleeve 13 may include a groove 21 for receiving a line with a sensor 20.
A hub 17
is attached to the distal end of the outer sleeve 13 and forms an attachment
area for the
flow cannula 4. The hub 17 is preferably made of the same material as the
outer sleeve
13 and accommodates the distal bearing 12 and the impeller 6. The blood flow
outlet 2
is foimed in the hub 17, such that heat transfer away from the distal bearing
12 is
possible.
-13-
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
[0039] The outer sleeve 13 may have a length of about 7 mm to about 30
mm,
preferably about 10 mm to about 20 mm, more preferably about 10 mm to about 15
mm. The outer sleeve 13 may have an outer dimension of 18 F (French) or
smaller
(outer diameter of 6 mm or smaller). Despite the small dimensions, a pump rate
of up to
5.5 liters per minute may be achieved.
100401 Now referring to Figs. 3a to 3f, steps of a method of
manufacturing the
aforementioned first casing section of the intravascular blood pump as shown
in Fig. 2
is described. First, as shown in Fig. 3a, the ceramic inner sleeve 14 may be
provided
and attached to the ceramic end piece 11 which includes the proximal bearing
as
explained above. Attaching the ceramic inner sleeve 14 to the ceramic end
piece 11 may
be realized e.g. by bonding or by means of one or preferably two or more
preferably
three consecutive sealing rings (not shown). That is, one sealing ring may be
provided
on the cavity-side of a main sealing ring in order to protect the main sealing
ring from
contact with purge fluid during use of the blood pump, whereas another sealing
ring
may be provided on the opposite side of the main sealing ring so as to protect
the main
sealing ring against casting compound which might enter through the interface
between
the ceramic inner sleeve 14 and the ceramic end piece 11 during the
manufacturing
process of the first casing section, as will be explained further below.
100411 A pre-wound coil winding 9 is then placed on the sleeve 14 as
shown in
Fig. 3b. A molding base 30 is provided (Fig. 3c), which is formed as a mandrel
sized
and shaped for receiving the stator components of the pumping device 1. The
coil
winding 9 with the ceramic sleeve 14 is placed on the molding base 30 as shown
in Fig.
3d. Alternatively, the coil winding 9 may be placed on the inner sleeve 14
after the inner
sleeve 14 has been placed on the molding base 30. It will be appreciated that
alternatively the inner sleeve 14 (i.e. the steps shown in Figs. 3a and 3b)
may be
omitted, and the coil winding 9 may be placed directly on the molding base 30
without
the inner sleeve 14. In order to protect the proximal bearing, i.e. to avoid
that casting
compound enters the bearing and contaminates the bearing surface, the proximal
bearing may be sealed, as will explained further in connection with Fig.4. The
motor
cable 10 is electrically connected to the coil winding 9, in particular
soldered, as shown
in Fig. 3e. Moreover, the purge line 15 is attached to the end piece 11 (not
shown here).
- 14 -
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
[0042] Then, the outer sleeve 13 is placed over the molding base 30 to
form an
outer surface of the pumping device 1. Casting compound, such as epoxy, is
then
injected into the outer sleeve 13, more specifically into the interspace 19
formed
between the inner sleeve 14 and the outer sleeve 13 containing the coil
winding 9 to
encapsulate the coil winding 9. In order to compensate for shrinkage during
curing, a
riser 19a (see Fig. 4) with a reservoir (not shown) may be provided for the
casting
compound. After finishing the injection-molding step, which may be carried out
in a
vacuum, the molding base 30 with the injected casting compound may be stored
for
curing.
[0043] In the meantime, further pumping devices may be produced in the same
manner and stored for curing. No expensive molds are needed which are occupied
during the curing process, which requires long production cycles. The molding
base 30
is formed as a cheap disposable plastic piece and can be easily removed from
the
finished product. No release agent is needed. The casting compound does not
come into
contact with delicate parts of the pumping device. Thus, no cleaning of
delicate surface
structures, e.g. the aforementioned groove 21, is required.
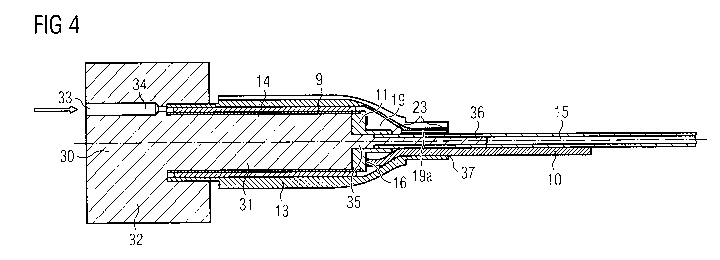
[0044] Fig. 4 shows a cross-section through the molding base 30 with
all desired
stator components, including the outer sleeve 13, placed thereon before
injecting the
casting compound 18. In particular, the ceramic inner sleeve 14 connected to
the
proximal bearing 11 and carrying the coil winding 9 is disposed on the molding
base 30.
However, it will be appreciated that the inner sleeve 14 may be omitted, and
the coil
winding 9 may be placed directly on the molding base 30. The motor cable 10
has been
connected, in particular soldered, to the electrical connections 16 of the
coil winding 9,
i.e. the PCB. The purge line 15 is secured to the end piece 11 so as to be in
fluid
communication with the cavity 22 for the rotor 7 (see Fig. 2). The metal outer
sleeve 13
covers all components and encloses the interspace 19 into which the casting
compound
will be injected. An interior surface of a proximal end of the end piece 11
and an
external surface of a distal end of the purge line 15 may be sealed against
each other by
an adhesive 23 to prevent leakage of the casting compound 18 from the
interspace 19
into the outer sleeve during the injection process. As can be seen in Fig. 2,
the purge
line 15 and the motor cable 10 extend through a proximal end of the outer
sleeve 13,
-15-
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
which will be attached to a distal end of the catheter 5. Accordingly, an
interior surface
of a proximal end of the outer sleeve 13 and external surfaces of the purge
line 15 and
motor cable 10 may be sealed against each other by an adhesive 23 to prevent
leakage
of the casting compound 18 from the interspace 19 to the surroundings during
the
injection process.
[0045] The molding base 30 is produced as an injection-molded
disposable
plastic piece and has a substantially cylindrical body portion 31 to form a
mandrel. In
particular, the body portion 31 is sized and shaped to receive the ceramic
inner sleeve
14 thereon, or in other words to correspond to the cavity 22 for receiving the
rotor 7 in
the finished product. A socket portion 32 includes an injection port 33 and a
supply line
34 for feeding the casting compound 18 into the interspace 19 between the
inner sleeve
14 and the outer sleeve 13. At an end of the molding base 30 opposite the
socket portion
32, a reduced diameter portion 35 extends from the main body portion 32 to
receive the
bearing 11. Further, a pin portion 36 extends from the portion 35 to receive
the purge
line 15 thereon. Although shown as an integral portion, the pin portion 36 may
be
formed as a metal pin for increased stability. The molding base 30 may be made
e.g. of
polytetrafluorethylene, polyethylene or polypropylene.
[0046] The casting compound 18 is injected into the injection port 33
as
indicated by the arrow. An excess amount of casting compound 18 may be
injected into
and through the interspace 19 into a reservoir (not shown) via a riser 19a to
compensate
for shrinkage of the casting compound 18 during curing. The reservoir may be
attached
to the riser 19a at a proximal surface 37 of the outer sleeve 13. After the
casting
compound 18 has cured, the molding base 30 is removed, if applicable after
removing
the reservoir, and the first casing section is finished. No cleaning of the
outer surface of
the outer sleeve 13 is necessary because the casting compound 18 does not come
into
contact with the outer surface.
[0047] Thereafter, referring to Fig. 2 again, the rotor 7, i.e. the
magnet, is
mounted in the cavity 22 of the pump casing 1 along with the hub 17 and the
distal
bearing 12, and the impeller 6 is coupled to the rotor 7. The hub 17
constitutes a second
casing section and forms together with the first casing section the housing
for the rotor
- 16-
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
7. In order to prevent that purge fluid can reach the cured casting compound
and
possibly migrate through micro cracks in the casting compound towards the coil
winding 9 during use of the blood pump, a liquid sealing material 40 is
provided
between the first and second casing sections, more specifically between the
inner sleeve
14 and the hub 17, and dried. In doing so, the hub 17 is placed vertically
with its
proximal end standing up, the liquid sealing material 40 is filled into an
inner
circumferential groove 17a of the hub 17 so as to fill the groove only partly,
and the
first casing section is placed onto the hub 17 such that a distal end of the
inner sleeve 14
reaches into the liquid sealing material 40. The liquid sealing material is
then cured to
form the seal. Preferably, the liquid sealing material 40 is an elastomeric
material so that
the seal has elastic properties. Finally, the cannula 4 and the catheter 5 are
attached to
the pump casing 1. It will be appreciated that not all of the aforementioned
method steps
may be included in the method of the present invention, or other steps may be
carried
out if necessary as will be appreciated by a person skilled in the art.
Likewise, the order
of some of the described method steps may be changed if necessary.
[0048] Figs. 5 to 7 relate to a second embodiment which differs from
the first
embodiment only in that the (dried) liquid sealing material 40 is omitted and
a number
of sealing rings 40a to 40c are used instead. Accordingly, Figs. 5a to 5f,
which
schematically illustrate the steps of manufacturing the first casing section
for the
intravascular blood pump according to the second embodiment, differ from Figs.
3a to
3f only in that in the step described in relation to Fig. 5b a first polymeric
sealing ring
40a and a second polymeric sealing ring 40b are provided consecutively at the
bottom
or distal end of the coil winding 9. Thus, as can be seen in Fig. 6 showing a
cross-
section through the molding base 30 with all stator components placed thereon
prior to
injecting the casting compound 18 in the interspace 19, the two sealing rings
40a, 40b
form a seal at the bottom of the molding base 30. In this second embodiment,
the
casting compound is supplied to the cavity 19 through the riser 19a, as
indicated by the
arrow, after the interspace 19 has been evacuated. Thus, the casting compound
will
reach the first sealing ring 40a, but not the second sealing ring 40b.
- 17-
CA 03094838 2020-09-23
WO 2019/180221
PCT/EP2019/057272
Then, when the hub 17 is mounted to the first casing section, a third sealing
ring 40c
may be placed in the hub 17 such it contacts the inner sleeve 14 and thereby
forms a
seal between the hub 17 and the first casing section. This way, purge fluid
flowing
through the cavity 22 during use of the blood pump is prevented from reaching
the
second sealing ring 40b. Thus, the second sealing ring 40b is completely
protected from
both sides and can provide a proper sealing function over a long time.
- 18 -