Note: Descriptions are shown in the official language in which they were submitted.
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
METHODS OF DIAGNOSING AND TREATING BASED ON
SITE-SPECIFIC TAU PHOSPHORYLATION
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional
Application No.
62/666,504, filed May 3, 2018, and U.S. Provisional Application No.
62/666,509, filed
May 3, 2018, the disclosures of which are incorporated herein by reference.
GOVERNMENTAL RIGHTS
[0002] This invention was made with government support under
N5065667 and N5095773 awarded by the National Institutes of Health. The
government has certain rights in the invention.
REFERENCE TO SEQUENCE LISTING
[0003] This application contains a Sequence Listing that has been
submitted in ASCII format via EFS-Web and is hereby incorporated by reference
in its
entirety. The ASCII copy, created on May 3, 2019, is named 623217_5T25.txt,
and is
24KB bytes in size.
BACKGROUND OF THE INVENTION
[0004] The microtubule-associated protein tau (MAPT or tau) plays
an
essential role in the morphology and physiology of neurons. Tau has six
different
isoforms of the full-length protein and undergoes a number of possible post-
translational
modifications including acetylation, glycosylation and phosphorylation.
Phosphorylation
is important for regulating the normal function of tau in axonal stabilization
and can
occur at over 80 different residues. However, excessive phosphorylation of tau
appears
to increase the probability of tau aggregating into intracellular insoluble
paired helical
filaments (PHF) and neurofibrillary tangles (NFT), which are primarily
composed of
hyperphosphorylated tau.
[0005] Intracellular neurofibrillary tangles in the cerebral cortex
are a
defining pathological feature of Alzheimer disease (AD) and correlate with the
onset of
1
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
clinical symptoms long after the appearance of extracellular amyloid-p (Ap)
plaques,
which begin to develop up two decades before symptom onset. In AD, soluble p-
tau and
unphosphorylated tau are increased by two-fold in the cerebrospinal fluid
(CSF). It has
been proposed that these changes reflect the effects of neuronal death
(neurodegeneration) passively releasing tau and NFT into the CSF. However, in
other
tauopathies with significant NFT pathology and neurodegeneration (e.g.
progressive
supranuclear palsy, frontotemporal lobar degeneration-tau), CSF levels of
soluble p-tau
and total tau do not increase. These observations suggest that Ap may trigger
a
process that leads to the unique tauopathy of AD, an idea that is supported by
cellular
and animal models. This concept is further supported by an increase in the
active
production of soluble tau in the presence of amyloid plaques in humans.
[0006] Although tau comprises a hallmark AD pathology and can be
measured in aggregated or soluble forms, important gaps remain in our
understanding
of how the post-translational modifications of this critical neuronal protein
lead to the
development of NFT and neurodegeneration in humans. For instance, the
relationship
of tau to amyloid-p plaques is unknown. Similarly, it is unknown what, if any,
pathophysiologic changes occur to tau during the preclinical and clinical
stages of AD.
As such, it is unclear to what extent, if any, tau can be used to stage
subjects prior to
the onset of symptoms associated with AD and guide treatment decisions.
[0007] Accordingly, there remains a need in the art for improved
methods
to quantify tau phosphorylation.
SUMMARY OF THE INVENTION
[0008] In an aspect, the present disclosure encompasses a method to
diagnose a subject as having an increased risk for conversion to mild
cognitive
impairment (MCI) due to Alzheimer's disease (AD). The method comprises (a)
providing
an isolated tau sample obtained from a subject and measuring, in the isolated
tau
sample, tau phosphorylation at one or more amino acid residue chosen from
T181,
T205 and T217 and optionally measuring total tau; and (b) diagnosing the
subject as
having an increased risk for conversion to MCI due to AD when the measured
phosphorylation level(s) significantly deviate from the mean in a control
population
2
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
without brain amyloid plaques as measured by PET imaging and/or A[342/40
measurement in CSF. Alternatively, or in addition to, using a measurement of
tau
phosphorylation at T181, T205 and/or T217, optionally with a measurement of
total tau,
a ratio calculated from the measured phosphorylation level(s), or a ratio
calculated from
the measured phosphorylation level(s) and total tau, may be used. A ratio
calculated
from the measured phosphorylation level(s) may be a ratio between p-T181 and p-
T205, p-T217 and p-T205, or p-T181 and p-T217. A ratio calculated from the
measured
phosphorylation level(s) and total tau may be a ratio between p-T181 and total
tau, p-
T205 and total tau, or p-T217 and total tau. Mathematical operations other
than a ratio
may also be used.
[0009] In another aspect, the present disclosure encompasses a
method
to stage a subject prior to the onset of mild cognitive impairment (MCI) due
to
Alzheimer's disease (AD). The method comprises (a) providing an isolated tau
sample
obtained from a subject and measuring, in the isolated tau sample, tau
phosphorylation
at one or more amino acid residue chosen from T181, T205 and T217 and
optionally
measuring total tau; and (b) diagnosing the subject as being a certain number
of years
from onset of MCI due to AD when the measured phosphorylation level(s)
significantly
deviate from the mean in a control population without brain amyloid plaques as
measured by PET imaging and/or A[342/40 measurement in CSF. Alternatively, or
in
addition to, using a measurement of tau phosphorylation at T181, T205 and/or
T217,
optionally with a measurement of total tau, a ratio calculated from the
measured
phosphorylation level(s), or a ratio calculated from the measured
phosphorylation
level(s) and total tau, may be used. A ratio calculated from the measured
phosphorylation level(s) may be a ratio between p-T181 and p-T205, p-T217 and
p-
T205, or p-T181 and p-T217. A ratio calculated from the measured
phosphorylation
level(s) and total tau may be a ratio between p-T181 and total tau, p-T205 and
total tau,
or p-T217 and total tau. Mathematical operations other than a ratio may also
be used.
[0010] In another aspect, the present disclosure encompasses a
method
to stage a subject after onset of Alzheimer's disease (AD) symptoms. The
method
comprises (a) providing an isolated tau sample obtained from a subject and
measuring,
3
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
in the isolated tau sample, tau phosphorylation at one or more amino acid
residue
chosen from T181, T205 and T217 and optionally measuring total tau; and (b)
diagnosing the subject as being a certain number of years after onset of MCI
due to AD
when the measured phosphorylation level(s) significantly deviate from the mean
in a
control population without brain amyloid plaques as measured by PET imaging
and/or
A[342/40 measurement in CSF. Alternatively, or in addition to, using a
measurement of
tau phosphorylation at T181, T205 and/or T217, optionally with a measurement
of total
tau, a ratio calculated from the measured phosphorylation level(s), or a ratio
calculated
from the measured phosphorylation level(s) and total tau, may be used. A ratio
calculated from the measured phosphorylation level(s) may be a ratio between p-
T181
and p-T205, p-T217 and p-T205, or p-T181 and p-T217. A ratio calculated from
the
measured phosphorylation level(s) and total tau may be a ratio between p-T181
and
total tau, p-T205 and total tau, or p-T217 and total tau. Mathematical
operations other
than a ratio may also be used.
[0011] In another aspect, the present disclosure encompasses a
method
for treating a subject in need thereof. The method comprises (a) providing an
isolated
tau sample obtained from a subject and measuring, in the isolated tau sample,
tau
phosphorylation at one or more amino acid residue chosen from T181, T205 and
T217
and optionally measuring total tau; and (b) administering a pharmaceutical
composition
to the subject when the measured phosphorylation level(s) significantly
deviate from the
mean in a control population without brain amyloid plaques as measured by PET
imaging and/or A[342/40 measurement in CSF. Alternatively, or in addition to,
using a
measurement of tau phosphorylation at T181, T205 and/or T217, optionally with
a
measurement of total tau, a ratio calculated from the measured phosphorylation
level(s),
or a ratio calculated from the measured phosphorylation level(s) and total
tau, may be
used. A ratio calculated from the measured phosphorylation level(s) may be a
ratio
between p-T181 and p-T205, p-T217 and p-T205, or p-T181 and p-T217. A ratio
calculated from the measured phosphorylation level(s) and total tau may be a
ratio
between p-T181 and total tau, p-T205 and total tau, or p-T217 and total tau.
Mathematical operations other than a ratio may also be used.
4
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0012] In another aspect, present disclosure encompasses a method
for
enrolling a subject into a clinical trial. The method comprises (a) providing
an isolated
tau sample obtained from a subject and measuring, in the isolated tau sample,
tau
phosphorylation at one or more amino acid residue chosen from T181, T205 and
T217
and optionally measuring total tau; and (b) enrolling the subject into a
clinical trial when
the measured phosphorylation level(s) significantly deviate from the mean in a
control
population without brain amyloid plaques as measured by PET imaging and/or
Ap42/40
measurement in CSF. Alternatively, or in addition to, using a measurement of
tau
phosphorylation at T181, T205 and/or T217, optionally with a measurement of
total tau,
a ratio calculated from the measured phosphorylation level(s), or a ratio
calculated from
the measured phosphorylation level(s) and total tau, may be used. A ratio
calculated
from the measured phosphorylation level(s) may be a ratio between p-T181 and p-
T205, p-T217 and p-T205, or p-T181 and p-T217. A ratio calculated from the
measured
phosphorylation level(s) and total tau may be a ratio between p-T181 and total
tau, p-
T205 and total tau, or p-T217 and total tau. Mathematical operations other
than a ratio
may also be used.
[0013] Other aspects and iterations of the invention are described
more
thoroughly below.
BRIEF DESCRIPTION OF THE FIGURES
[0014] The application file contains at least one photograph
executed in
color. Copies of this patent application publication with color photographs
will be
provided by the Office upon request and payment of the necessary fee.
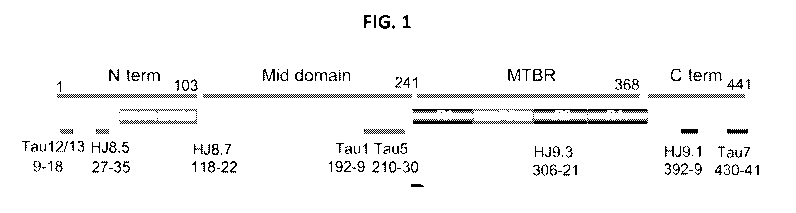
[0015] FIG. 1 is a schematic of the longest human tau isoform
(2N4R) and
epitopes of tau antibodies. The N-terminus, mid domain, MTBR, and C-terminus
are
identified for this isoform and will vary in a predictable way for other tau
isoforms (e.g.,
2N3R, 1NR4, 1N3R, ON4R, and ON3R).
[0016] FIG. 2 is a schematic showing the principle of the Parallel
Reaction
Monitoring experiment.
[0017] FIG. 3 shows data from a PRM screening of the mono-
phosphorylated tau sequence at 103-126 (ON isoform). A unique LC-MS/MS pattern
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
eluting closely to the unmodified peptide 103-126 and containing fragment
series
expected for phosphorylation at T111 (a), S113 (b) or T123 (c) was identified.
Hypothetical y ion fragments from each p-tau peptide are underlined on the
sequences.
The potential co-elution of the three putative mono-phosphorylated peptides
was
deconvoluted. Ion fragment y15 without phosphate is specific to the
phosphorylated
peptide on residue T111 (y15 (a)) and the y8 fragment with phosphate is
specific to p-
tau peptide on residue T123 (y8 (c)). Corresponding extracted ion
chromatograms (XIC)
are detected in low abundance above the limit of detection, supporting the
identification
of the two corresponding mono-phosphorylated tau peptides. In contrast, the
y15
fragment with phosphate, shared by pT111 and pS113 (y15 (a+b)), and the y8
fragment
without phosphate, shared by pS113 and pT123 (y8 (b+c)), are much more
abundant.
These signal differences support the existence of the tau peptide mono-
phosphorylated
at residue S113 (b) as the main specie of the pattern.
[0018] FIG. 4 shows data from a PRM screening of mono-
phosphorylated
tau sequence 68-126 (1N isoform) containing six potential phosphorylation
sites. Three
phosphorylation sites are shared by peptides containing residues 103-126 as
described
in FIG. 3 (d-f). Six LC-MS patterns were identified. The Y28 fragment carrying
phosphate, shared by pS68 (a) or pT69 (b), is found in the two LC-MS patterns
4 and 5.
This demonstrates the existence of the two phosphorylated peptides but
corresponding
LC-MS patterns cannot be strictly assigned without the detection of ion
fragment y29 to
differentiate pS68 and pT69. Specific fragments corresponding to pT71 (c) and
pT111
(d) are found in LC-MS patterns 6 and 1, respectively. Specific fragments for
pS113 (e)
and pT123 (f) (y15 with phosphate) are found in the LC-MS pattern 2. This
pattern
contained both y10 fragments with and without phosphate, suggesting the co-
elution of
these two phosphorylated peptides. Since y10 without phosphate has the major
signal
in comparison to y10 with phosphate, the degree of pT123 is likely lower than
pS113.
LC-MS pattern 3 is attributed to a minor conformer or LC artifact from the
phosphorylated peptide from pattern 4 as found for the non-phosphorylated
peptide.
[0019] FIG. 5 shows data from a PRM screening of mono-
phosphorylated
tau sequence 45-67 (1N and 2N isoforms). A strong signal from a conformer was
6
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
identified on the front of a non-phosphorylated peptide LC-MS pattern. Thus,
it was
predicted that corresponding LC-MS patterns for phosphorylated peptides would
also
have conformers separable by LC. Indeed, PRM scan interpretation led to the
detection
of pT50 (b) as the main phosphorylation site on this sequence (pattern 2).
Pattern 1,
with a similar fragmentation fingerprint, was attributed to a conformer of
pT50. pS46 (a),
able to differentiate signals from pT50 (b), was not detected, suggesting this
phosphorylation would be absent or low and co-eluted with other phosphorylated
peptides sharing similar non-specific fragments. Co-elution of y9, y15 without
phosphate, and y17 with phosphate in LC-MS pattern 3 identified a
phosphorylation on
residue T52 (c). Patterns 4/6 and 5/7 were respectively paired as conformers.
Thus, two
phosphorylated peptides could not be separated. Fragments found in these
patterns
were consistent with phosphorylation on one of the S61 (e), T63 (f), and S64
(g)
residues. MS/MS intensities were insufficient to identify the sites but at
least 2 of these
3 sites were likely phosphorylated on this sequence. Additionally, a minor y9
fragment
without phosphate was found in the shoulder of pattern 7, which could be
attributed to
minor phosphorylation on residue S56 (d).
[0020] FIG. 6 shows data from a PRM screening of mono-
phosphorylated
tau sequence 88-126 (2N isoform). 6 potential phosphorylation sites are
located in this
sequence and 6 LC-MS patterns were identified. Fragments found in pattern 1
and 2
were consistent with phosphorylated peptides at residues T111 (d) and S113
(e),
respectively. No specific fragment from the phosphorylated peptide at residue
T123 (f)
was found. Patterns 4 and 6 contained a low signal of the y29 fragment
matching with
phosphorylation on residue T101 (b) or T102 (c), but no specific fragment able
to
differentiate them was detected. Patterns 3 and 5 shared fragments found in
patterns 4
and 6 but in lower abundance, locating the phosphorylated residue at the N-
terminus on
residue G109. This could indicate the presence of an additional phosphorylated
peptide,
likely at residue T95 (a) or abundant conformers from peptides found in
patterns 4 and
6.
[0021] FIG. 7 shows a PRM scan of mono-phosphorylation tau sequence
68-87, containing 4 potential phosphorylation sites. 3 LC-MS patterns were
detected.
7
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
Patterns 2 and 3 were consistent with phosphorylation at residues S68 (a) or
T69 (b).
Pattern 1 contained both fragments compatible with the presence of two co-
eluted
phosphorylated peptides at T71 (c) and T76 (d). Comparison of y14 XIC with and
without phosphate in pattern 1 indicates pT71 (c) is more abundant than pT76
(d).
[0022] FIG. 8A, FIG. 8B, FIG. 8C, FIG. 80, FIG. 8E, FIG. 8F, FIG.
8G,
FIG. 8H, FIG. 81, FIG. 8J, FIG. 8K, and FIG. 8L show detection of
phosphorylation sites
in the mid-domain and C-terminus of brain p-tau protein.
[0023] FIG. 9A, FIG. 9B, FIG. 9C, FIG. 90, FIG. 89E, and FIG. 9F
show
phosphorylated peptide profiles from tau sequences 195-209 (SEQ ID NO: 38) and
212-
221 (SEQ ID NO: 64) are variable between the soluble brain fraction, normal
CSF, and
AD CSF tau protein. Brain soluble tau extracts are diluted as indicated to
approximately
match corresponding CSF tau level. Phosphorylated peptides on 195-209: in
brain
lysate, one signal corresponding to the co-elution of two phosphorylated
peptides
pS199 and pS202 is observed. In CSF, two additional signals are observed.
Fragment
analysis allowed the assignment of the signal on left to pT205. In AD CSF, the
two
signals are increased allowing the identification of specific fragments
assigning the
signal on the right to pS208. Phosphorylated peptides on 212-221: two signals
with
similar MS intensities corresponding to pT217 and pS214 are identified in
brain lysate.
In CSF, the signal corresponding to pT217 is the most intense while pS214 is
close to
the limit of detection, indicating a dramatic change in their relative
abundance in
comparison to the brain extract. In AD CSF, pT217 is significantly increased
due to
specific hyperphosphorylation.
[0024] FIG. 10A, FIG. 10B, FIG. 10C, FIG. 100, FIG. 10E, FIG. 10F,
FIG.
10G, FIG. 10H, and FIG. 101 show pT153, pT175 and pT231 phosphorylated
peptides
identified in CSF. AQUA internal standard signals are shown for pT175 and
pT231.
Fragmentation pattern of pT153 is similar to unmodified.
[0025] FIG. 11 shows phosphorylation abundance on T111 is higher in
the
CSF than the brain relative to S113 phosphorylation. In both brain and CSF,
the MS/MS
fragment y18 common to all mono-phosphorylated peptides on tau sequence 103-
126 is
detected. The relative abundance of the y15 fragment from p5113 (b) is
significantly
8
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
lower in CSF in comparison to brain extract. Inversely the y15 fragment from
pT111 (a)
is abundant in CSF and not detectable in brain soluble extract diluted to
match the AD
CSF tau level.
[0026] FIG. 12 shows relative abundance of tau phosphorylation
depends
on the biological extract and varies across the protein sequence. Comparison
of tau
phosphorylation abundance measured by MS in normal brain lysate, normal CSF
and
AD CSF extracted by immuno capture using HJ8.5 and Tau1. Circle area is
proportional
to site phosphorylation abundance. Red and green colors indicate an increase
or
decrease, respectively, in comparison to the brain soluble profile taken as
reference
(blue). Tau is c-terminally truncated in CSF, which explains the absence of
detection of
the C-terminal cluster of phosphorylation sites. Phosphorylation on T205 and
S208 is
specific to CSF (red X on Brain Soluble ¨ top).
[0027] FIG. 13 shows tau phosphorylation sites are differentially
modified
in brain, normal CSF and AD CSF. Measurements are the relative abundances of
the
phosphorylated signal compared to the corresponding non phosphorylated site
(HJ8.5+Tau1 IP-MS). Brain results are obtained from diluted lysates from 500x
to 8000x
factors to match the CSF tau level. Phosphorylations on T205 and S208 are
undetectable in brain tissue. Phosphorylation on T111 is undetectable in 500x
diluted
lysate but was detected in 10x diluted lysate with a corresponding abundance
of 0.02%.
Legend: ** indicates significance at p=0.01 level and * indicates significance
at p=0.05
level.
[0028] FIG. 14A, FIG. 14B, FIG. 14C, and FIG. 140 show antibody
effect
on phosphorylation ratio measurement by IP-MS. Brain lysate pool, non-AD (n=1)
and
AD CSF (n=1) pools were immunoprecipitated in parallel with antibodies against
tau N-
terminus projection domain (Tau13 or HJ8.5) or mid domain (HJ8.7, Tau1 or
Tau5).
Radar plots of phosphorylation ratios measured on main CSF sites (log10 scale)
are
shown in brain lysate (FIG. 14A, left panel), non-AD CSF (FIG. 14A, middle
panel)
and AD CSF (FIG. 14A, right panel). (FIG. 14A) Tau phosphorylation ratio
measurements on pT181/T181, pT231/T231 are consistent across antibodies
tested.
PS199/S199 is decreased by using Tau1 or Tau1+HJ8.5 in comparison to other
9
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
antibodies. (FIG. 14B) Low recovery of pS199 by Tau1 or Tau1+HJ8.5 IP
underestimate
pS199/S199 ratio measurements compared to other antibodies tested. Tau13,
HJ8.5,
and HJ8.7 antibodies indicate no significant changes of pS199 phosphorylation
ratio
between brain and CSF. Compared to brain, pS202/S202 CSF hypophosphorylation
(FIG. 14C) and pT217/pT217 hyperphosphorylation (FIG. 140) are evidenced
independently of the antibody used for IP-MS. The legend for FIG. 14B-D is the
same
and shown in FIG. 14C ¨ Brain (blue), nonAD CSF (green), and AD CSF (red).
[0029] FIG. 15 shows CSF incubation does not impact phosphorylation
rate measurement on tau.
[0030] FIG. 16A, FIG. 16B, FIG. 16C, FIG. 160, FIG. 16E, FIG. 16F,
and
FIG. 16G show amyloid plaques are strongly correlated with tau
hyperphosphorylation
but differ by site of phosphorylation. FIG. 16A Receiver operating
characteristics for
total tau (blue line, AUC=0.62) and site-specific phosphorylation ratios in
classifying
participants as having AR pathology based on Ap PiB-PET (SUVR cutoff of 1.25),
p-
T217 (yellow line) demonstrates a near perfect association with Ap pathology
(AUC=0.97), followed by p-T181 (AUC =0.89) and p-T205 (AUC = 0.74).
Standardized
(z-score) phosphorylation ratios are shown for p-T217 (FIG. 16B), p-T181,
(FIG. 16C),
p-5202 (FIG. 160), p-T205 (FIG. 16E) and total tau (FIG. 16F) levels by Ap PiB-
PET
quartiles (n=45, 47, 28, 30) for mutation carriers highlights site-specific
differences in
phosphorylation with increasing Ap PiB-PET levels: p-T217 and p-T181 increase
greatest with the initial increase in Ap PiB-PET amount and slow with the
highest levels
of AR PiB-PET, while p-T205 and total tau demonstrate a continued increase.
For p-
S202, there was a significant decrease in phosphorylation at the highest Ap
PiB-PET
quartiles relative to the lowest; ***- p-value < 0.001, **- p-value < 0.01
based on
Wilcoxon two sample test; the middle line represents the median, and the upper
and
lower notch = median +/- 1.58 * interquartile range/ square root(n-
observations), the
upper and lower whisker = largest observation greater/less than or equal to
upper/lower
hinge + 1.58 * IQR. FIG. 16G Bivariate correlations between cortical and sub-
cortical Ap
PiB-PET SUVR and site-specific phosphorylation for asymptomatic mutation
carriers
(n=139). The colors represent the correlation with positive correlations
(yellow-red) and
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
negative correlations (blue); all correlations represent statistically
significant values
surviving a false discovery rate (p <0.05) and are arranged by the strength of
the
correlations from top to bottom.
[0031] FIG. 17A, FIG. 17B, FIG. 17C, FIG. 170, FIG. 17E, and FIG.
17F
show longitudinal changes of different phosphorylated-tau sites are stage of
disease
specific and change in opposite directions as AD progresses. Individual, z-
transformed,
longitudinal changes in the ratio of phosphorylation of (FIG. 17A) p-T217,
(FIG. 17B) p-
T181 (FIG. 17C) total tau, (FIG. 170) p-T205, and (FIG. 17E) p-S202 for
mutation
carriers (black = asymptomatic mutation carriers, (n= 152), red = symptomatic
mutation
carriers (77)) and non-carriers (blue, (n=141)) across the estimated years to
symptom
onset (EYO). The vertical dashed line is the point of expected symptom onset,
the
green line represents the model estimated time when the rate of change for
each p-tau
isoform becomes greater for mutation carriers compared to non-carriers. (FIG.
17F)
Model estimated, longitudinal rates of change for each site of phosphorylation
where
standardized to the rates of non-carriers and plotted over EYO along with
amyloid PET
(red) and cognitive decline (yellow); the solid circles represent the point
when the rate of
change for each variable first becomes different for mutation carriers
compared to non-
carriers. This highlights the pattern of change for p-tau isoforms over the
course of the
AD spectrum and the close association between amyloid plaque growth and the
increase in p-T217 with plaques beginning to increase at -21 EYO and the
hyperphosphorylation of p-T217 (black) beginning at -21 EYO and the decline in
phosphorylation rate of these two sites with a decline in cognition (yellow
line). In
contrast, p-T205 (purple) continues increasing throughout disease progression
and total
tau levels (grey) increase at an increased rate near the time of symptom
onset.
[0032] FIG. 18A and FIG. 18B show phosphorylated-tau sites are
differentially related to brain hypometabolism and atrophy. FIG. 18A.
Bivariate
correlations between cortical and sub-cortical atrophy and site-specific
phosphorylation
ratios in asymptomatic mutation carriers (n= 152) demonstrates increases in
phosphorylation of p-T205 and p-T217, with smaller associations for p-T181.
Total tau
levels are associated with greater atrophy in multiple cortical and
subcortical regions.
11
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
FIG. 18B. Bivariate correlations between cortical and sub-cortical brain
metabolism
measured by FDG-PET and site-specific phosphorylation ratios in asymptomatic
mutation carriers (n= 143) demonstrates an increase in phosphorylation of p-
T205 is
associated with a decrease in most cortical and sub-cortical regions but not
for other p-
tau sites or tau.
[0033] FIG. 19A, FIG. 19B, FIG. 19C, and FIG. 190 show decreasing
phosphorylation at p-T217, p-T181 and p-T205 is associated with dementia and
cognitive decline. Individual estimated annualized rates of change of p-tau
isoforms and
total tau (y-axis) for mutation carriers were correlated with the annualized
change in
global cognitive function; the lines represent simple linear regression with
shaded area
representing 95% confidence interval. Each point represents an individual
level
correlation between measures. The linear regression was fit to those with no
dementia
(black, n= 47) and dementia (red, n= 25). A decline in p-T217 (FIG. 19A), r=
0.43(p=
0.02), p-T181 (FIG. 19B), r= 0.72 (p < .001) and p-T205 (FIG. 19C),
r=0.41(p=0.03)
phosphorylation rate was associated with cognitive decline after symptom onset
(red).
For total tau there was a trend suggesting an inverse correlation with
cognition (FIG.
190) but it was not significant.
[0034] FIG. 20 shows tau PET increases near symptom onset in DIAD
mutation carriers. The mean cortical standardized unit value ratio (SUVR), y-
axis, for
mutation carriers (red, n=12) and non-carriers (blue, n=9) over estimated
years to
symptom onset (EYO), x-axis, for those participants with a longitudinal CSF
evaluation
preceding the time of tau-PET. The plot shows that for mutation carriers there
is little
elevation in tau-PET until the point of estimated symptom onset (EYO =0). This
figure
shows that the neurofibrillary tangle (NFT) pathology detected by AV-1451
occurs much
later than the increase in multiple soluble phosphotau sites suggesting that
these
soluble markers of tau are likely a marker of NFT pathology, but rather might
predispose
to the development of the hyperphosphorylated, insoluble tau deposits
characteristic of
AD pathology.
[0035] FIG. 21A, FIG. 21B, FIG. 21C, FIG. 210, and FIG. 21E show
longitudinal change in tau and tau phosphorylation sites are differentially
related to
12
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
neurofibrillary tau (tau-PET) in dominantly inherited AD. Individual,
estimated rates of
change of phosphorylation and total tau (y-axis) leading up to the time of tau-
PET scan
(x-axis). The vertical line is an SUVR of 1.22 and represents a conservative
estimate of
the point when NFT tau-PET (a composite of multiple cortical and limbic
regions) is
considered elevated compared to non-carriers. The plots suggest that increases
in
soluble tau and p-T205 are associated with higher levels of aggregated tau,
whereas
the rate of phosphorylation at p-T217 and p-T181 decrease as levels of
aggregated tau
increase. These findings suggest that there are differences between increasing
levels of
tau and phosphorylation at different sites and may indicate that, in some
instances,
soluble p-tau is sequestered as the burden of hyperphosphorylated aggregates
increase
with the spreading of tau pathology. They also suggest that with the increase
in
aggregated tau there is a rise in soluble tau levels which could represent
either passive
or active release with greater burden of aggregated tau pathology.
[0036] FIG. 22 is an illustration showing tau pathology evolves
through
distinct phases in Alzheimer Disease. Measuring four different soluble tau
species and
insoluble tau in a group of participants with deterministic Alzheimer disease
mutations
we show over the course of 35 years (x-axis) tau related changes unfold (y-
axis) and
differ based on the stage of disease and other measurable biomarkers. A.
Starting with
the development of fibrillar amyloid pathology phosphorylation at position 217
(purple)
and 181 (blue) begins to increase. B. With the increase in neuronal
dysfunction (based
metabolic changes) phosphorylation at position 205 (green) begins to increase
along
with soluble tau (orange). C. Lastly, with the onset of neurodegeneration
(based on
brain atrophy and cognitive decline) tau PET tangles (red) begin to develop
while
phosphorylation of 217 and 181 begins to decrease. Together, this highlights
the
dynamic and diverging patterns of soluble and aggregated tau over the course
of the
disease and close relationship with amyloid pathology.
[0037] FIG. 23A, FIG. 23B, and FIG. 23C show quantitation of
phosphorylated tau isoforms in CSF. Sum of Extracted Ion Chromatograms from
Parallel Reaction Monitoring (PRM) analysis of tau phosphorylated peptides and
the
corresponding unmodified peptides in CSF. FIG. 23A, T181 monitoring using a
microLC
13
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
system. FIG. 23B, S199, S202, T205 (coeluted in framed signal) and T217
monitoring
using a nanoLC system. Endogenous signals (full blue line), 15N labeled
peptides (red
dotted line), AQUA peptides (green dotted line). FIG. 23C shows specific PRM
transitions, according to Biemann nomenclature for peptide fragmentation,
which
allowed identification of the three co-eluted monophosphorylated peptides
carrying
pS199, pS202 or pT205. Cps= Count per second.
[0038] FIG. 24A, FIG. 24B, FIG. 24C, FIG. 240 and FIG. 24E show CSF
tau phosphorylation on T217 is associated to amyloidosis status. FIG. 24A:
T217
phosphorylation significantly increases in participants having amyloidosis
(PiB-PET and
CSF A[342/40 ratio positive) compared to amyloid-negative controls with no or
mild
cognitive decline. FIG. 24B: ROC curves for the diagnosis of amyloid-positive
from
amyloid-negative participants using phosphorylation rate of T217, T181 by MS
and T181
by ELISA. FIG. 24C: pT217/T217 ratio comparison demonstrates the specific
phosphorylation on T217 in participants with amyloidosis. FIG. 240-E:
Comparison of
T217 phosphorylation with CSF A[342/40 changes measured by MS and to amyloid
plaque deposition measured by PiB-PET. FIG. 240: The extent of T217
hyperphosphorylation is not correlated with the decrease of CSF A[342 relative
to A[340.
The five conflicting cases (orange triangles, positive for PiB-PET and T217
hyperphosphorylation but negative for CSF A[3), were all slightly above the
0.12
threshold chosen to define the amyloid status, suggesting that they may result
from
insufficient sensitivity of the CSF amyloid assay. FIG. 24E: PiB-PET loading
(FBP Total
Cortical Mean) is correlated with T217 phosphorylation state in amyloid-
positive
participants. Cut-off value differentiating amyloid positive from amyloid
negative by PiB
is 0.18.
[0039] FIG. 25 shows CSF tau phosphorylation on T217 is independent
from cognitive status and is significantly modified in preclinical AD. Left
Panel: No
correlation exists between T217 phosphorylation and the cognitive profile
measured by
the clinical dementia rating sum of boxes (CDR-SB). Right Panel: Amongst
participants
with no cognitive decline (CDR-SB=0), T217 is already significantly hyper
phosphorylated in the amyloid-positive group.
14
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
DETAILED DESCRIPTION
[0040] Tau protein aggregation into neurofibrillary tangles in the
central
nervous system contributes to the etiology of certain neurodegenerative
disorders,
including Alzheimer's disease (AD). Though the mechanism of tau
destabilization is not
fully understood yet, tau protein has been found to be hyperphosphorylated in
tau
aggregates. Applicants have discovered that certain methods to quantify tau
phosphorylation at specific amino acid residues can be used to can track the
AD
process across its preclinical asymptomatic stages to symptomatic stages. FIG.
22
illustrates the dynamic pattern of tau phosphorylation measurable at T181,
T205 and
T217 created by the applicant's method in relation to years from onset of MCI
due to AD
and to the development of certain pathophysiological changes. The present
disclosure
encompasses use of the methods to quantify tau phosphorylation at specific
amino acid
residues to predict time to onset of mild cognitive impairment due to
Alzheimer's
disease, guide treatment decisions, select subjects for clinical trials, and
evaluate the
clinical efficacy of certain therapeutic interventions. Other aspects and
iterations of the
invention are described more thoroughly below.
I. Definitions
[0041] So that the present invention may be more readily
understood,
certain terms are first defined. Unless defined otherwise, all technical and
scientific
terms used herein have the same meaning as commonly understood by one of
ordinary
skill in the art to which embodiments of the invention pertain. Many methods
and
materials similar, modified, or equivalent to those described herein can be
used in the
practice of the embodiments of the present invention without undue
experimentation,
the preferred materials and methods are described herein. In describing and
claiming
the embodiments of the present invention, the following terminology will be
used in
accordance with the definitions set out below.
[0042] The term "about," as used herein, refers to variation of in
the
numerical quantity that can occur, for example, through typical measuring
techniques
and equipment, with respect to any quantifiable variable, including, but not
limited to,
mass, volume, time, distance, and amount. Further, given solid and liquid
handling
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
procedures used in the real world, there is certain inadvertent error and
variation that is
likely through differences in the manufacture, source, or purity of the
ingredients used to
make the compositions or carry out the methods and the like. The term "about"
also
encompasses these variations, which can be up to 5%, but can also be 4%,
3%,
2%,1 A, etc. Whether or not modified by the term "about," the claims include
equivalents to the quantities.
[0043] An antibody, as used herein, may be a complete antibody as
understood in the art, i.e., consisting of two heavy chains and two light
chains, or may
be any antibody-like molecule that has an antigen binding region, and
includes, but is
not limited to, antibody fragments such as Fab', Fab, F(ab')2, single domain
antibodies,
Fv, and single chain Fv. The term antibody also refers to a polyclonal
antibody, a
monoclonal antibody, a chimeric antibody and a humanized antibody. The
techniques
for preparing and using various antibody-based constructs and fragments are
well
known in the art. Means for preparing and characterizing antibodies are also
well known
in the art (See, e.g. Antibodies: A Laboratory Manual, Cold Spring Harbor
Laboratory,
1988; herein incorporated by reference in its entirety).
[0044] As used herein, the term "aptamer" refers to a
polynucleotide,
generally a RNA or DNA that has a useful biological activity in terms of
biochemical
activity, molecular recognition or binding attributes. Usually, an aptamer has
a molecular
activity such as binging to a target molecule at a specific epitope (region).
It is generally
accepted that an aptamer, which is specific in it binding to a polypeptide,
may be
synthesized and/or identified by in vitro evolution methods. Means for
preparing and
characterizing aptamers, including by in vitro evolution methods, are well
known in the
art. See, for instance US 7,939,313, herein incorporated by reference in its
entirety.
[0045] The term "Ap" refers to peptides derived from a region in
the
carboxy terminus of a larger protein called amyloid precursor protein (APP).
The gene
encoding APP is located on chromosome 21. There are many forms of Ap that may
have toxic effects: Ap peptides are typically 37-43 amino acid sequences long,
though
they can have truncations and modifications changing their overall size. They
can be
found in soluble and insoluble compartments, in monomeric, oligomeric and
aggregated
16
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
forms, intracellularly or extracellularly, and may be complexed with other
proteins or
molecules. The adverse or toxic effects of Ap may be attributable to any or
all of the
above noted forms, as well as to others not described specifically. For
example, two
such Ap isoforms include A[340 and A[342; with the A[342 isoform being
particularly
fibrillogenic or insoluble and associated with disease states. The term "A[3"
typically
refers to a plurality of Ap species without discrimination among individual Ap
species.
Specific Ap species are identified by the size of the peptide, e.g., A[342,
A[340, A[338 etc.
[0046] As used herein, the term "A[342/ A[340 value" means the
ratio of the
concentration of A[342 in a sample obtained from a subject compared to the
concentration of A[340 in the same sample.
[0047] "Ap amyloidosis" is clinically defined as evidence of Ap
deposition
in the brain. A subject that is clinically determined to have Ap amyloidosis
is referred to
herein as "amyloid positive," while a subject that is clinically determined to
not have Ap
amyloidosis is referred to herein as "amyloid negative." Ap amyloidosis likely
exists
before it is detectable by current techniques. Nonetheless, there are accepted
indicators
of Ap amyloidosis in the art. At the time of this disclosure, Ap amyloidosis
is typically
identified by amyloid imaging (e.g., PiB PET, fluorbetapir, or other imaging
methods
known in the art) or by decreased cerebrospinal fluid (CS F) A[342 or a
decreased CSF
A[342/40 ratio. [11C]PIB-PET imaging with mean cortical binding potential
(MCBP) score
> 0.18 is an indicator of Ap amyloidosis, as is cerebral spinal fluid (CS F)
A[342
concentration of about 1 ng/ml by immunoprecipitation and mass spectrometry
(IP/MS)).
Values such as these, or others known in the art, may be used alone or in
combination
to clinically confirm Ap amyloidosis. See, for example, Klunk W E et al. Ann
Neurol
55(3) 2004, Fagan A M et al. Ann Neurol, 2006, 59(3), Patterson et. al, Annals
of
Neurology, 2015, 78(3): 439-453, or Johnson et al., J. Nuc. Med., 2013, 54(7):
1011-
1013, each hereby incorporated by reference in its entirety. Subjects with Ap
amyloidosis may or may not be symptomatic, and symptomatic subjects may or may
not
satisfy the clinical criteria for a disease associated with Ap amyloidosis.
Non-limiting
examples of symptoms associated with Ap amyloidosis may include impaired
cognitive
function, altered behavior, abnormal language function, emotional
dysregulation,
17
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
seizures, dementia, and impaired nervous system structure or function.
Diseases
associated with AB amyloidosis include, but are not limited to, Alzheimer's
Disease
(AD), cerebral amyloid angiopathy, Lewy body dementia, and inclusion body
myositis.
Subjects with AB amyloidosis are at an increased risk of developing a disease
associated with AB amyloidosis.
[0048] A "clinical sign of AB amyloidosis" refers to a measure of
AB
deposition known in the art. Clinical signs of AB amyloidosis may include, but
are not
limited to, AB deposition identified by amyloid imaging (e.g. PiB PET,
fluorbetapir, or
other imaging methods known in the art) or by decreased cerebrospinal fluid
(CSF)
A[342 or A[342/40 ratio. See, for example, Klunk WE et al. Ann Neurol 55(3)
2004, and
Fagan AM et al. Ann Neurol 59(3) 2006, each hereby incorporated by reference
in its
entirety. Clinical signs of AB amyloidosis may also include measurements of
the
metabolism of A[3, in particular measurements of A[342 metabolism alone or in
comparison to measurements of the metabolism of other AB variants (e.g. A[337,
A[338,
A[339, A[340, and/or total A[3), as described in U.S. Patent Serial Nos.
14/366,831,
14/523,148 and 14/747,453, each hereby incorporated by reference in its
entirety.
Additional methods are described in Albert et al. Alzheimer's & Dementia 2007
Vol. 7,
pp. 170-179; McKhann et al., Alzheimer's & Dementia 2007 Vol. 7, pp. 263-269;
and
Sperling et al. Alzheimer's & Dementia 2007 Vol. 7, pp. 280-292, each hereby
incorporated by reference in its entirety. Importantly, a subject with
clinical signs of AB
amyloidosis may or may not have symptoms associated with AB deposition. Yet
subjects with clinical signs of AB amyloidosis are at an increased risk of
developing a
disease associated with AB amyloidosis.
[0049] A "candidate for amyloid imaging" refers to a subject that
has been
identified by a clinician as in individual for whom amyloid imaging may be
clinically
warranted. As a non-limiting example, a candidate for amyloid imaging may be a
subject
with one or more clinical signs of AB amyloidosis, one or more AB plaque
associated
symptoms, on one or more CAA associated symptoms, or combinations thereof. A
clinician may recommend amyloid imaging for such a subject to direct his or
her clinical
care. As another non-limiting example, a candidate for amyloid imaging may be
a
18
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
potential participant in a clinical trial for a disease associated with Ap
amyloidosis (either
a control subject or a test subject).
[0050] An "Ap plaque associated symptom" or a "CAA associated
symptom" refers to any symptom caused by or associated with the formation of
amyloid
plaques or CAA, respectively, being composed of regularly ordered fibrillar
aggregates
called amyloid fibrils. Exemplary Ap plaque associated symptoms may include,
but are
not limited to, neuronal degeneration, impaired cognitive function, impaired
memory,
altered behavior, emotional dysregulation, seizures, impaired nervous system
structure
or function, and an increased risk of development or worsening of Alzheimer's
disease
or CAA. Neuronal degeneration may include a change in structure of a neuron
(including molecular changes such as intracellular accumulation of toxic
proteins,
protein aggregates, etc. and macro level changes such as change in shape or
length of
axons or dendrites, change in myelin sheath composition, loss of myelin
sheath, etc.), a
change in function of a neuron, a loss of function of a neuron, death of a
neuron, or any
combination thereof. Impaired cognitive function may include but is not
limited to
difficulties with memory, attention, concentration, language, abstract
thought, creativity,
executive function, planning, and organization. Altered behavior may include,
but is not
limited to, physical or verbal aggression, impulsivity, decreased inhibition,
apathy,
decreased initiation, changes in personality, abuse of alcohol, tobacco or
drugs, and
other addiction-related behaviors. Emotional dysregulation may include, but is
not
limited to, depression, anxiety, mania, irritability, and emotional
incontinence. Seizures
may include but are not limited to generalized tonic-clonic seizures, complex
partial
seizures, and non-epileptic, psychogenic seizures. Impaired nervous system
structure
or function may include, but is not limited to, hydrocephalus, Parkinsonism,
sleep
disorders, psychosis, impairment of balance and coordination. This may include
motor
impairments such as monoparesis, hem iparesis, tetraparesis, ataxia, ballismus
and
tremor. This also may include sensory loss or dysfunction including olfactory,
tactile,
gustatory, visual and auditory sensation. Furthermore, this may include
autonomic
nervous system impairments such as bowel and bladder dysfunction, sexual
dysfunction, blood pressure and temperature dysregulation. Finally, this may
include
19
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
hormonal impairments attributable to dysfunction of the hypothalamus and
pituitary
gland such as deficiencies and dysregulation of growth hormone, thyroid
stimulating
hormone, lutenizing hormone, follicle stimulating hormone, gonadotropin
releasing
hormone, prolactin, and numerous other hormones and modulators.
[0051] As used herein, the term "subject" refers to a mammal,
preferably a
human. The mammals include, but are not limited to, humans, primates,
livestock,
rodents, and pets. A subject may be waiting for medical care or treatment, may
be
under medical care or treatment, or may have received medical care or
treatment.
[0052] As used herein, the term "healthy control group," "normal
group" or
a sample from a "healthy" subject means a subject, or group subjects, who
is/are
diagnosed by a physician as not suffering from Ap amyloidosis, or a clinical
disease
associated with Ap amyloidosis (including but not limited to Alzheimer's
disease) based
on qualitative or quantitative test results. A "normal" subject is usually
about the same
age as the individual to be evaluated, including, but not limited, subjects of
the same
age and subjects within a range of 5 to 10 years.
[0053] As used herein, the term "blood sample" refers to a
biological
sample derived from blood, preferably peripheral (or circulating) blood. The
blood
sample can be whole blood, plasma or serum, although plasma is typically
preferred.
[0054] The term "isoform", as used herein, refers to any of several
different
forms of the same protein variants, arising due alternative splicing of m RNA
encoding
the protein, post-translational modification of the protein, proteolytic
processing of the
protein, genetic variations and somatic recombination. The terms "isoform" and
"variant"
are used interchangeably.
[0055] Unless otherwise stated herein, the term "tau protein" or
"tau"
encompasses all tau isoforms, whether full-length, truncated, or post-
translationally
modified. In many animals, including but not limited to humans, non-human
primates,
rodents, fish, cattle, frogs, goats, and chicken, tau is encoded by the gene
MAPT. In
humans, there are six isoforms of tau that are generated by alternative
splicing of exons
2, 3, and 10 of MAPT. These isoforms range in length from 352 to 441 amino
acids.
Exons 2 and 3 encode 29-amino acid inserts each in the N- terminus (called N),
and
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
full-length human tau isoforms may have both inserts (2N), 1 one insert (1 N),
or no
inserts. All full-length human tau isoforms also have three repeats of the
microtubule
binding domain (called R). Inclusion of exon 10 at the C-terminus leads to
inclusion of a
fourth microtubule binding domain encoded by exon 10. Hence, full-length human
tau
isoforms may be comprised of four repeats of the microtubule binding domain
(exon 10
included) or three repeats of the microtubule binding domain (exon 10
excluded).
Human tau may or may not be post-translationally modified. For example, it is
known in
the art that tau may be phosphorylated, ubiquinated, glycosylated, and
glycated.
Accordingly, the term "human tau" encompasses the (2N, 3R), (2N, 4R), (1 N,
3R), (1 N,
4R), (ON, 3R), and (ON, 4R) isoforms, isoforms that are N- and/or C-terminally
truncated
species thereof, and all post-translationally modified isoforms. Alternative
splicing of the
gene encoding tau similarly occurs in other animals. In animals where the gene
is not
identified as MAPT, a homolog may be identified by methods well known in the
art.
[0056] A disease associated with tau deposition in the brain may be
referred to as a "tauopathy". Tauopathies known in the art include, but are
not limited to,
progressive supranuclear palsy, dementia pugilistica, chronic traumatic
encephalopathy,
frontotemporal dementia and parkinsonism linked to chromosome 17, Lytico-Bodig
disease, Parkinson-dementia complex of Guam, tangle- predominant dementia,
ganglioglioma and gangliocytoma, meningioangiomatosis, subacute sclerosing
panencephalitis, lead encephalopathy, tuberous sclerosis, Hallervorden-Spatz
disease,
lipofuscinosis, Pick's disease, corticobasal degeneration, argyrophilic grain
disease
(AGD), Frontotemporal lobar degeneration, Alzheimer's Disease, and
frontotemporal
dementia.
[0057] "Significantly deviate from the mean" refers to values that
are at
least 1 standard deviation, preferably at least 1.3 standard deviations, more
preferably
at least 1.5 standard deviations or even more preferably at least 2 standard
deviations,
above or below the mean.
[0058] The phrase "Ap and tau therapies" collectively refers to any
imaging
agent or therapeutic agent contemplated for, or used with, subjects at risk of
developing
21
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
AB amyloidosis or AD, subjects diagnosed as having AB amyloidosis, subjects
diagnosed as having a tauopathy, or subjects diagnosed as having AD.
II. Measuring total tau and tau phosphorylation in an isolated tau sample
[0059] Methods of the present disclose comprise providing an
isolated tau
sample obtained from a subject and measuring tau phosphorylation at one or
more
amino acid residue and optionally total tau.
(a) isolated tau sample
[0060] An isolated tau sample, as used herein, refers to a
composition
comprising tau, wherein tau has been purified from blood or cerebrospinal
fluid (CSF)
obtained from a subject. A subject is a mammal, preferably a human. CSF may be
obtained by lumbar puncture with or without an indwelling CSF catheter.
Multiple blood
or CSF samples contemporaneously collected from the subject may be pooled.
Blood
may be collected by veni-puncture with or without an intravenous catheter, or
by a finger
stick (or the equivalent thereof). Once collected, blood or CSF samples may be
processed according to methods known in the art (e.g., centrifugation to
remove whole
cells and cellular debris, use of additives designed to stabilize and preserve
the
specimen prior to analytical testing, etc.). Blood or CSF samples may be used
immediately or may be frozen and stored indefinitely.
[0061] In isolated tau samples of the present disclosure, tau has
been
either partially or completely purified from blood or CSF. Methods for
purifying tau from
blood or CSF are known in the art and include, but are not limited to,
selective
precipitation, size-exclusion chromatography, ion-exchange chromatography, and
affinity purification. Suitable methods concentrate both phosphorylated tau
and
unphosphorylated tau from blood or CSF.
[0062] In an exemplary embodiment, isolated tau samples of the
present
disclosure comprise tau that has been purified from blood or CSF by affinity
purification.
Affinity purification refers to methods that purify a protein of interest by
virtue of its
specific binding properties to an immobilized ligand. Typically, an
immobilized ligand is
a ligand attached to a solid support, such as a bead, resin, tissue culture
plate, etc.
22
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
Suitable ligands specifically bind both phosphorylated and unphosphoryated
tau. In one
example, a suitable ligand may bind an epitope within the mid domain of tau.
In another
example, a suitable ligand may bind an epitope within the N-terminus of tau,
preferably
within amino acids 1 to 35 of tau. In another example, a suitable ligand may
bind an
epitope within the MTBR of tau. In another example, a suitable ligand may bind
an
epitope within the C-terminus of tau. In still further embodiments, tau may be
affinity
purified from blood or CSF using two or more immobilized ligands. In one
example, an
immobilized ligand binds an epitope within the N-terminus of tau and another
immobilized ligand binds an epitope within the mid domain of tau. In another
example,
an immobilized ligand binds an epitope within the MTBR of tau and another
immobilized
ligand binds an epitope within the mid domain of tau. In another example, an
immobilized ligand binds an epitope within the C-terminus of tau and another
immobilized ligand binds an epitope within the mid domain of tau. In another
example,
an immobilized ligand binds an epitope within the C-terminus of tau and
another
immobilized ligand binds an epitope within the N-terminus of tau. In another
example,
an immobilized ligand binds an epitope within the MTBR of tau and another
immobilized
ligand binds an epitope within the N-terminus of tau. In another example, an
immobilized ligand binds an epitope within the MTBR of tau and another
immobilized
ligand binds an epitope within the C-terminus of tau. In each of the above
embodiments,
the ligand may be an antibody or an aptamer. Non-liming examples of suitable
antibodies are shown in FIG. 1.
[0063] An isolated tau sample may be used immediately or may be
stored
indefinitely by methods known in the art.
(b) tau phosphorylation at one or more amino acid residue
[0064] Phosphorylation of specific amino acids (i.e. "sites") in
tau results in
phosphorylated tau (p-tau) isoforms. Methods of the present disclosure provide
means
to measure the stoichiometry of phosphorylation at one or more specific amino
acids of
tau. In some embodiments, methods herein comprise measuring tau
phosphorylation at
one or more residue chosen from T111, S113, T181, S199, S202, S208, T153,
T175,
T205, S214, T217, and T231. In some embodiments, methods herein comprise
23
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
measuring tau phosphorylation at one or more residue chosen from T111, T181,
T205,
S208, S214, T217, and T231. In other embodiments, methods herein comprise
measuring tau phosphorylation at one or more residue chosen from T181, S214,
and
T217. In other embodiments, methods herein comprise measuring tau
phosphorylation
at one or more residue chosen from T181, T205, and T217. In other embodiments,
methods herein comprise measuring tau phosphorylation at one or more residue
that
includes S199. In other embodiments, methods herein comprise measuring tau
phosphorylation at one or more residue that includes S202. In other
embodiments,
methods herein comprise measuring tau phosphorylation at one or more residue
that
includes S199. In other embodiments, methods herein comprise measuring tau
phosphorylation at one or more residue that includes T181. In other
embodiments,
methods herein comprise measuring tau phosphorylation at one or more residue
that
includes T205. In other embodiments, methods herein comprise measuring tau
phosphorylation at one or more residue that includes T217. In other
embodiments,
methods herein comprise measuring tau phosphorylation at two or more residues
that
include T153 and T175. In other embodiments, methods herein comprise measuring
tau
phosphorylation at two or more residue chosen from T181, T205, and T217. In
other
embodiments, methods herein comprise measuring tau phosphorylation at three or
more residues that include T181, T205, and T217.
[0065] Applicants developed a highly sensitive and specific mass
spectrometry (MS) method using parallel reaction monitoring (PRM) to discover
tau
phosphorylation sites and initially quantify the abundance of phosphorylation
sites in
isolated tau proteins. However, the present disclosure is not limited to any
one particular
method to quantitatively assess site-specific phosphorylation of tau. Suitable
methods
should discriminate tau isoforms that differ only in the phosphorylation
status of a single
amino acid, discriminate p-tau isoforms that are phosphorylated at different
amino
acids, and quantify changes in phosphorylation occurring at specific sites
independently
from the global change in total tau. Three approaches to quantify changes in
phosphorylation stoichiometry occurring at specific sites independently from
the global
change in total tau are detailed in the examples: 1) relative comparison
between
24
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
phosphorylated peptide isomers, which can be used to estimate the relative
abundance
of each phosphorylated peptide sharing the same sequence; 2) normalizing
phosphorylated peptides with any peptide from the tau protein as reference;
and 3)
absolute quantitation using internal synthetic labeled standards for each
phosphorylated
and non-phosphorylated peptide, where absolute quantitation values for each
phosphorylated peptide is normalized with any absolute quantitation value
obtained for
any peptide from the tau protein. All three approaches use internal
normalization for
comparing relative phosphorylation changes for each site. Other methods known
in the
art may also be used.
[0066] In an exemplary embodiment, site-specific phosphorylation of
tau is
measured by high-resolution mass spectrometry. Suitable types of mass
spectrometers
are known in the art. These include, but are not limited to, quadrupole, time-
of-flight, ion
trap and Orbitrap, as well as hybrid mass spectrometers that combine different
types of
mass analyzers into one architecture (e.g., Orbitrap Fusion TM TribridTm Mass
Spectrometer from ThermoFisher Scientific). Additional processing of an
isolated tau
sample may occur prior to MS analysis. For example, tau may be proteolytically
digested. Suitable proteases include, but are not limited to, trypsin, Lys-N,
Lys-C, and
Arg-N. When affinity purification is used to produce an isolated tau sample,
digestion
may occur after eluting tau from the immobilized ligand or while tau is bound.
Following
one or more clean-up steps, digested tau peptides may be separated by a liquid
chromatography system interfaced with a high-resolution mass spectrometer. The
chromatography system may be optimized by routine experimentation to produce a
desired LC-MS pattern. A wide array of LC-MS techniques may be used to
quantitatively analysis site-specific tau phosphorylation. Non-limiting
examples include
selected-reaction monitoring, parallel-reaction monitoring, selected-ion
monitoring, and
data-independent acquisition. As stated above, all quantitative assessments of
site-
specific tau phosphorylation should account for global changes in total tau.
In an
exemplary embodiment, a mass spectrometry protocol outlined in the Examples is
used.
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
(c) total tau
[0067] "Total tau," as used herein refers to all tau isoforms in a
given
sample. Tau can be found in soluble and insoluble compartments, in monomeric
and
aggregated forms, in ordered or disordered structures, intracellularly and
extracellularly,
and may be complexed with other proteins or molecules. Accordingly, the source
of the
biological sample (e.g., brain tissue, CSF, blood, etc.) and any downstream
processing
of the biological sample will affect the totality of tau isoforms in a given
sample.
[0068] Total tau may be measured by monitoring abundance of
unmodified
tau peptides. For each phosphorylated tau site, a tau peptide sharing the
common
amino acid sequence with the phosphorylated peptide of interest may
preferentially be
used to measure total tau level, but any peptide from the tau sequence can be
used.
Tau peptides measurement can be performed by mass spectrometry and accuracy of
the measurement can be improved by using labeled internal standards as
reference.
Alternatively, total tau can be measured by immunoassays or other method
quantifying
tau concentration.
III. Methods to diagnose subjects prior to the onset of MCI due to AD to
diagnose
a subject's stage of AD
[0069] One aspect of the present disclosure encompasses methods to
diagnose subjects as having a high risk of conversion to mild cognitive
impairment due
to Alzheimer's disease, and to optionally stage or classify the subject in
terms of the
number of years of onset to MCI due to AD. Mild cognitive impairment (MCI) due
to
Alzheimer's disease (AD) refers to the symptomatic predementia phase of AD.
This
degree of cognitive impairment is not normal for age and, thus, constructs
such as age-
associated memory impairment and age-associated cognitive decline do not
apply. MCI
due to AD is a clinical diagnosis, and clinical criteria for the diagnosis of
MCI due to AD
are known in the art. See, for instance, Albert et al. Alzheimer's & Dementia,
2011, 7(3):
270-279. Cognitive testing is optimal for objectively assessing the degree of
cognitive
impairment for a subject. Scores on cognitive tests for subjects with MCI are
typically 1
to 1.5 standard deviations below the mean for their age and education matched
peers
on culturally appropriate normative data (i.e., for the impaired domain(s),
when
26
CA 03097667 2020-10-16
WO 2019/213612
PCT/US2019/030725
available). The designation of MCI is often supported by a global rating of
0.5 on the
Clinical Dementia Rating (CDR) scale. The CDR is a numeric scale used to
quantify the
severity of symptoms of dementia. Other suitable cognitive tests are known in
the art.
While suitable tests exist to assess the severity of cognitive impairment,
there is a need
in the art for a test that identifies subjects with a high degree of
confidence years before
the onset of MCI due to AD.
[0070] In
one embodiment, a method to diagnose a subject as having a
high risk of conversion to MCI due to AD may comprise (a) providing an
isolated tau
sample obtained from a subject and measuring, in the isolated tau sample, tau
phosphorylation at one or more amino acid residue chosen from chosen from
T111,
S113, T181, S199, S202, S208, T153, T175, T205, S214, T217, and T231, and
optionally measuring total tau; and (b) diagnosing the subject as having a
high risk of
conversion to MCI due to AD when the measured phosphorylation level(s)
significantly
deviate from the mean in a control population without brain amyloid plaques as
measured by PET imaging and/or A[342/40 measurement in CSF. In another
embodiment, a method to diagnose a subject as having a high risk of conversion
to MCI
due to AD may comprise (a) providing a first and a second isolated tau sample
obtained
from a subject and measuring, in each isolated tau sample, tau phosphorylation
at one
or more amino acid residue chosen from chosen from T111, S113, T181, S199,
S202,
S208, T153, T175, T205, S214, T217, and T231, and optionally measuring total
tau; (b)
calculating the change in the site-specific phosphorylation at each residue
measured
and optionally the change in total tau; and (c) diagnosing the subject as
having a high
risk of conversion to MCI due to AD when the calculated change(s)
significantly deviate
from the mean in a control population without brain amyloid plaques as
measured by
PET imaging and/or A[342/40 measurement in CSF. "Significantly deviate from
the
mean" refers to values that are at least 1 standard deviation, preferably at
least 1.3
standard deviations, more preferably at least 1.5 standard deviations or even
more
preferably at least 2 standard deviations, above or below the mean (i.e., 10-,
1.3a, 1.50-,
or 1.5a, respectively, where a is the standard deviation defined by the normal
distribution measured in a control population without brain amyloid plaques as
27
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
measured by PET imaging and/or A[342/40 measurement in CSF). In addition to
using a
threshold (e.g. at least 1 standard deviation above or below the mean), in
some
embodiment the extent of change above or below the mean may be used to
diagnose a
subject. An isolated tau sample can be obtained from a subject that may or may
not be
asymptomatic. An "asymptomatic subject" refers to a subject that does not show
any
signs or symptoms of AD. A subject may however exhibit signs or symptoms of AD
(e.g., memory loss, misplacing things, changes in mood or behavior, etc.,) but
not show
sufficient cognitive or functional impairment for a clinical diagnosis of mild
cognitive
impairment. In further embodiments, a subject may carry one of the gene
mutations
known to cause dominantly inherited Alzheimer's disease. In alternative
embodiments,
a subject may not carry a gene mutation known to cause dominantly inherited
Alzheimer's disease. Alzheimer's disease that has no specific family link is
referred to
as sporadic Alzheimer's disease.
[0071] Another aspect of the present disclosure encompasses methods
to
diagnose a subject's stage of Alzheimer's disease. In various embodiments, a
"stage of
AD" may be defined as an amount of time (e.g. 1, 2, 3,4, 5, 6, 7, 8, 9, 10,
11, 12
months, etc.) that has elapsed since the onset of MCI due to AD. Although
there are
criteria for a clinical diagnosis of AD, it is common in the clinical setting
for the timing of
symptom onset to be unknown for a given subject or for there to be a
questionable
diagnosis of either MCI or AD. As such, there is a need in the art for a test
that
objectively diagnoses a subject's stage of AD.
[0072] In one embodiment, a method to diagnose a subject's stage of
AD
may comprise (a) providing an isolated tau sample obtained from a subject and
measuring, in the isolated tau sample, tau phosphorylation at one or more
amino acid
residue chosen from chosen from T111, S113, T181, S199, S202, S208, T153,
T175,
T205, S214, T217, and T231, and optionally measuring total tau; and (b)
diagnosing the
stage of the subject's AD when the measured phosphorylation level(s)
significantly
deviate from the mean in a control population without brain amyloid plaques as
measured by PET imaging and/or A[342/40 measurement in CSF. In another
embodiment, a method to diagnose a subject prior to the onset of MCI due to AD
may
28
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
comprise (a) providing a first and a second isolated tau sample obtained from
a subject
and measuring, in each isolated tau sample, tau phosphorylation at one or more
amino
acid residue chosen from chosen from T111, S113, T181, S199, S202, S208, T153,
T175, T205, S214, T217, and T231, and optionally measuring total tau; (b)
calculating
the change in the site-specific phosphorylation at each residue measured and
optionally
the change in total tau; and (c) diagnosing the subject as being a certain
number of
years from onset of MCI due to AD when the calculated change(s) significantly
deviate
from the mean in a control population without brain amyloid plaques as
measured by
PET imaging and/or A[342/40 measurement in CSF. "Significantly deviate from
the
mean" includes values that are at least 1 standard deviation, preferably at
least 1.3
standard deviations or more preferably at least 1.5 standard deviations or
even more
preferably at least 2 standard deviations, above or below the mean (i.e., la,
1.3o-, 1.50-,
or 1.5a, respectively, where a is the standard deviation defined by the normal
distribution measured in a control population without brain amyloid plaques as
measured by PET imaging and/or A[342/40 measurement in CSF). In addition to
using a
threshold (e.g. at least 1 standard deviation above or below the mean), in
some
embodiment the extent of change above or below the mean may be used to
diagnose a
subject. An isolated tau sample can be obtained from a subject that may or may
not
have a clinical diagnosis of MCI due to AD, dementia, or AD. In further
embodiments, a
subject may carry one of the gene mutations known to cause dominantly
inherited
Alzheimer's disease. In alternative embodiments, a subject may not carry a
gene
mutation known to cause dominantly inherited Alzheimer's disease.
[0073] Alternatively or in addition to using a measurement of site-
specific
tau phosphorylation, optionally with a measurement of total tau, in any of the
above
embodiments, a ratio calculated from the measured phosphorylation level(s), or
a ratio
calculated from the measured phosphorylation level(s) and total tau, may be
used. Both
approaches are detailed in the examples. Mathematical operations other than a
ratio
may also be used. For instance, the examples use site-specific tau
phosphorylation
values in various statistical models (e.g., linear regressions, LME curves,
LOESS
curves, etc.) in conjunction with other known biomarkers (e.g. APOE e4 status,
age,
29
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
sex, cognitive test scores, functional test scores, etc.). Selection of
measurements and
choice of mathematical operations may be optimized to maximize specificity of
the
method. For instance, diagnostic accuracy may be evaluated by area under the
ROC
curve and in some embodiments, an ROC AUC value of 0.7 or greater is set as a
threshold (e.g., 0.7, 0.75, 0.8, 0.85, 0.9, 0.95, etc.).
[0074] Brain amyloid plaques in humans are routinely measured by
amyloid-positron emission tomography (PET). For instance, 11C-Pittsburgh
compound B
(PiB) PET imaging of cortical Ap-plaques is commonly used to detect Ap-plaque
pathology. The standard uptake value ratio (SUVR) of cortical PiB-PET reliably
identifies significant cortical AR-plaques and is used to classify subjects as
PIB positive
(SUVR 1.25) or negative (SUVR < 1.25). Accordingly, in the above embodiments,
a
control population without brain amyloid plaques as measured by PET imaging
may
refer to a population of subjects that have a cortical PiB-PET SUVR < 1.25.
Other
values of PiB binding (e.g., mean cortical binding potential) or analyses of
regions of
interest other than the cortical region may also be used to classify subjects
as PIB
positive or negative. Other PET imaging agents may also be used.
[0075] A control population without brain amyloid plaques as
measured by
A642/40 measurement in CSF may refer to a population of subjects that has an
A642/40 measurement of <0.12 when measured by mass spectrometry, as described
in
Patterson et al, Annals of Neurology, 2015.
[0076] In an exemplary embodiment, a method to diagnose a subject
as
having a high risk of conversion to MCI due to AD or a subject's stage of AD
may
comprise (a) providing an isolated tau sample obtained from a subject and
measuring,
in the isolated tau sample, tau phosphorylation at one or more amino acid
residue
chosen from T181, T205 and T217 and optionally measuring total tau; and (b)
diagnosing the subject as having a high risk of conversion to MCI due to AD,
or as
being a certain number of years from onset of MCI due to AD, or staging the
subject's
AD when the measured phosphorylation level(s) significantly deviate from the
mean in a
control population without brain amyloid plaques as measured by PET imaging
and/or
A642/40 measurement in CSF. FIG. 22 illustrates the dynamic pattern of tau
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
phosphorylation measurable at T181, T205 and T217 in an isolated tau sample in
relation to years from onset of MCI due to AD. Phosphorylation levels at T217
that
significantly deviate from the mean first occur about 21 years from onset of
MCI due to
AD; phosphorylation levels at T181 that significantly deviate from the mean
first occur
about 19 years from onset of MCI due to AD; an increase in total tau that
significantly
deviates from the mean first occurs about 17 years from onset of MCI due to
AD; and
phosphorylation levels at T205 that significantly deviate from the mean first
occur about
13 years from onset of MCI due to AD. Upon symptom onset (e.g., MCI due to
AD),
phosphorylation levels at T217 and T181 plateau and then decrease.
[0077] As noted above, additional mathematical operations may be
performed with the measurements of phosphorylation at T181, T205 and/or T217,
including but not limited to ratio between the measured phosphorylation
level(s) and
ratio between the measured phosphorylation level(s) and total tau. A ratio
calculated
from the measured phosphorylation level(s) may be a ratio between p-T181 and p-
T205, p-T217 and p-T205, or p-T181 and p-T217. A ratio calculated from the
measured
phosphorylation level(s) and total tau may be a ratio between p-T181 and total
tau, p-
T205 and total tau, or p-T217 and total tau.
[0078] In one example, a method of the present disclosure comprises
(a)
providing an isolated tau sample obtained from a subject and measuring tau
phosphorylation at (i) T217 and T205, (ii) T181 and T205, or (iii) T181, T205
and T217;
and (b) diagnosing the subject as being about 10 to about 25 years, or about
10 to
about 20 years from the onset of MCI due to AD when tau phosphorylation at
T217
and/or T181 is about 1.5a or above and tau phosphorylation at T205 is about
1.5a or
below, where a is the standard deviation defined by the normal distribution of
tau
phosphorylation at T217 and T205, T181 and T205, or T181, T205 and T217
measured
in a control population without brain amyloid plaques as measured by PET
imaging
and/or Ap42/40 measurement in CSF. In various embodiments, tau phosphorylation
at
T217 and/or tau phosphorylation at T181 may be about 1.3a, about 1.35a, about
1.4a,
about 1.45a, about 1.5a, about 1.6a, about 1.7a, about 1.8a, about 1.9a, about
2a, or
above 2a. In other embodiments, tau phosphorylation at T217 and/or tau
31
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
phosphorylation at T181 may be about 1.850-, about 1.9o-, about 1.950-, about
2a, about
2.10-, about 2.2a, about 2.3a, about 2.4a, about 2.5a or above 2.5a. In each
of the
above embodiments, tau phosphorylation at T205 may be about 1.3a, about 1.35a,
about 1.4a, about 1.45a, about 1.5a, about 1.51a, about 1.55a, about 1.6a,
about 1.7a,
about 1.8a, about 1.9a, about 2.0o-, or below 2.0o-. Alternatively, tau
phosphorylation at
T205 may be about 2.0o-, about 2.05a, about 2.10-, about 2.2a, about 2.3a,
about 2.4a,
about 2.5a, or below 2.5a. In a further example, tau phosphorylation at T217
and/or tau
phosphorylation at T181 about 2a or above and tau phosphorylation at T205 may
be
about 2a or less. In addition to using a threshold (e.g. at least 1 standard
deviation
above or below the mean), in some embodiment the extent of change above or
below
the mean may be used to diagnose a subject. In still further embodiments,
measured
levels of tau phosphorylation at T205 and at T181 and/or T217 may be used in
various
mathematical operations to improve the predictive power compared to each by
itself.
For instance, ratio(s) may be calculated from the measured phosphorylation
levels.
Mathematical operations other than a ratio may also be used.
[0079] In another example, a method of the present disclosure
comprises
(a) providing an isolated tau sample obtained from a subject and measuring
total tau
and tau phosphorylation at (i) T217 and T205, (ii) T181 and T205, or (iii)
T181, T205
and T217; and (b) diagnosing the subject as being about 10 to about 25 years,
or about
to about 20 years from the onset of MCI due to AD when the ratio of tau
phosphorylation at T217 to total tau and/or the ratio of tau phosphorylation
at T181 to
total tau is about 1.5a or above and the ratio of tau phosphorylation at T205
to total tau
is about 1.5a or below, where a is the standard deviation defined by the
normal
distribution of total tau and tau phosphorylation at T217 and T205, T181 and
T205, or
T181, T205 and T217 measured in a control population without brain amyloid
plaques
as measured by PET imaging and/or A[342/40 measurement in CSF. In various
embodiments, the ratio of tau phosphorylation at T217 to total tau and/or the
ratio of tau
phosphorylation at T181 to total tau may be about 1.3o-, about 1.350-, about
1.4o-, about
1.45a, about 1.5a, about 1.6a, about 1.7a, about 1.8a, about 1.9a, about 2a,
or above
2a. In other embodiments, the ratio of tau phosphorylation at T217 to total
tau and/or
32
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
the ratio of tau phosphorylation at T181 to total tau may be about 1.85a,
about 1.9a,
about 1.95a, about 2a, about 2.1a, about 2.2a, about 2.3a, about 2.4a, about
2.5a or
above 2.5a. In each of the above embodiments, the ratio of tau phosphorylation
at T205
to total tau may be about 1.3a, about 1.35a, about 1.4a, about 1.45a, about
1.50a,
about 1.55a, about 1.6a, about 1.7a, about 1.8a, about 1.9a, about 2.0o-, or
below 2a.
Alternatively, the ratio of tau phosphorylation at T205 to total tau may be
about 2.0o-,
about 2.05a, about 2.10-, about 2.2a, about 2.3a, about 2.4a, about 2.5a, or
below 2.5a.
In a further example, the ratio of tau phosphorylation at T217 to total tau
and/or the ratio
of tau phosphorylation at T181 to total tau may be about 2a or above and the
ratio of
tau phosphorylation at T205 to total tau may about 2a or less. In addition to
using a
threshold (e.g. at least 1 standard deviation above or below the mean), in
some
embodiment the extent of change above or below the mean may be used to
diagnose a
subject.
[0080] In another example, a method of the present disclosure
comprises
(a) providing an isolated tau sample obtained from a subject and measuring tau
phosphorylation at (i) T181 and T205, (ii) T217 and T205, or (iii) T181, T217
and T205;
and (b) diagnosing the subject as being about 15 years or less, or about 10
years or
less, from the onset of MCI due to AD when tau phosphorylation at the specific
sites
recited in (a)(i), (a)(ii) or (a)(iii) is about 1.5a or above, where a is the
standard deviation
defined by the normal distribution of tau phosphorylation at T217 and T205,
T181 and
T205, or T181, T205 and T217 measured in a control population without brain
amyloid
plaques as measured by PET imaging and/or A[342/40 measurement in CSF. In
various
embodiments, tau phosphorylation at the specific sites recited in (a)(i),
(a)(ii) or (a)(iii)
may be about 1.3a, about 1.35a, about 1.4a, about 1.45a, about 1.5a, about
1.6a,
about 1.7a, about 1.8a, about 1.9a, about 2a, or above 2a. In other
embodiments, tau
phosphorylation at the specific sites recited in (a)(i), (a)(ii) or (a)(iii)
may be about 1.85a,
about 1.9a, about 1.95a, about 2a, about 2.1a, about 2.2a, about 2.3a, about
2.4a,
about 2.5a or above 2.5a. In a further example, tau phosphorylation at the
specific sites
recited in (a)(i), (a)(ii) or (a)(iii) may be about 2a or above. In addition
to using a
threshold (e.g. at least 1 standard deviation above or below the mean), in
some
33
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
embodiment the extent of change above or below the mean may be used to
diagnose a
subject. In still further embodiments, measured levels of tau phosphorylation
at the
specific sites recited in (a)(i), (a)(ii) or (a)(iii) may be used in various
mathematical
operations to improve the predictive power compared to each by itself. For
instance,
ratio(s) may be calculated from the measured phosphorylation levels.
Mathematical
operations other than a ratio may also be used.
[0081] In another example, a method of the present disclosure
comprises
(a) providing an isolated tau sample obtained from a subject and measuring
total tau
and tau phosphorylation at (i) T181 and T205, (ii) T217 and T205, or (iii)
T181, T217
and T205; and (b) diagnosing the subject as being about 15 years or less, or
about 10
years or less, from the onset of MCI due to AD when the ratio of tau
phosphorylation at
the specific sites recited in (a)(i), (a)(ii) or (a)(iii) to total tau is
about 1.5a or above,
where a is the standard deviation defined by the normal distribution of total
tau and tau
phosphorylation at T217 and T205, T181 and T205, or T181, T205 and T217
measured
in a control population without brain amyloid plaques as measured by PET
imaging
and/or A[342/40 measurement in CSF. In various embodiments, the ratio of tau
phosphorylation at the specific sites recited in (a)(i), (a)(ii) or (a)(iii)
to total tau may be
about 1.3o-, about 1.350-, about 1.4o-, about 1.450-, about 1.50-, about 1.6o-
, about 1.7o-,
about 1.8a, about 1.9a, about 2a, or above 2a. In other embodiments, the ratio
of tau
phosphorylation at the specific sites recited in (a)(i), (a)(ii) or (a)(iii)
to total tau may be
about 1.850-, about 1.9o-, about 1.950-, about 2o-, about 2.10-, about 2.2o-,
about 2.3o-,
about 2.4a, about 2.5a or above 2.5a. In a further example, the ratio of tau
phosphorylation at the specific sites recited in (a)(i), (a)(ii) or (a)(iii)
to total tau may be
about 2a or above. In addition to using a threshold (e.g. at least 1 standard
deviation
above or below the mean), in some embodiment the extent of change above or
below
the mean may be used to diagnose a subject.
[0082] In another example, a method of the present disclosure
comprises
(a) providing a first and a second isolated tau sample obtained from a
subject, wherein
"first" and "second" refer to the order in which the samples were collected,
and
measuring tau phosphorylation at (i) T181 and T205, (ii) T217 and T205, or
(iii) T181,
34
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
T217 and T205; (b) calculating the change in the site-specific phosphorylation
at each
residue measured and optionally the change in total tau; and (c) diagnosing
the stage of
a subject's AD when the phosphorylation level at T181 and/or T217 decreases or
stays
the same and the phosphorylation level at T205 and optionally total tau
increases. The
first and the second isolated tau samples may be collected days, weeks, or
months
apart. Typically, tau phosphorylation at the specific sites recited in (a)(i),
(a)(ii) or (a)(iii)
will also be about 1.5a or above for both samples and , where a is the
standard
deviation defined by the normal distribution tau phosphorylation at T217 and
T205,
T181 and T205, or T181, T205 and T217 measured in a control population without
brain
amyloid plaques as measured by PET imaging and/or A[342/40 measurement in CSF.
In
still further embodiments, measured levels of tau phosphorylation at the
specific sites
recited in (a)(i), (a)(ii) or (a)(iii) may be used in various mathematical
operations to
improve the predictive power compared to each by itself. For instance,
ratio(s) may be
calculated from the measured phosphorylation levels. Mathematical operations
other
than a ratio may also be used.
[0083] In another example, a method of the present disclosure
comprises
(a) providing a first and a second isolated tau sample obtained from a
subject, wherein
"first" and "second" refer to the order in which the samples were collected,
and
measuring total tau and tau phosphorylation at (i) T181 and T205, (ii) T217
and T205, or
(iii) T181, T217 and T205; (b) calculating the change in the site-specific
phosphorylation
at each residue measured and the change in total tau; and (c) diagnosing the
stage of a
subject's AD when the phosphorylation level at T181 and/or T217 decreases or
stays
the same, the phosphorylation level at T205 decreases or stays the same, and
total tau
increases. The first and the second isolated tau samples may be collected
days, weeks,
or months apart. Typically, tau phosphorylation at the specific sites recited
in (a)(i),
(a)(ii) or (a)(iii) will also be about 1.5a or above for both samples and ,
where a is the
standard deviation defined by the normal distribution of tau phosphorylation
at T217 and
T205, T181 and T205, or T181, T205 and T217 measured in a control population
without brain amyloid plaques as measured by PET imaging and/or A[342/40
measurement in CSF. In still further embodiments, measured levels of tau
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
phosphorylation at the specific sites recited in (a)(i), (a)(ii) or (a)(iii)
may be used in
various mathematical operations to improve the predictive power compared to
each by
itself. For instance, ratio(s) may be calculated from the measured
phosphorylation
levels. Mathematical operations other than a ratio may also be used.
[0084] Methods for measuring tau phosphorylation and total tau are
described in Section II, and incorporated into this section by reference. For
instance,
using the protocol detailed for Examples 5-9, tau phosphorylation at T181,
T205 and
T217 indicated as percentage of ptau/tau ratio is 21.7 2.3, 0.34 0.13, and 1.2
0.66,
respectively, in a control population without brain amyloid plaques as
measured by PET
imaging, as measured in an isolated tau sample that was purified from CSF (see
Table
3, mutation non-carriers column). Accordingly, twice the standard deviation
above the
mean found for the mutation non-carrier population (i.e. 2a) for p-T181/T181,
p-
T205/T205 and p-T217/T217 is 43.4, 0.68, and 2.4, respectively. A skilled
artisan will
appreciate, however, that the absolute value may vary depending upon the
protocol and
the source/specifications of internal standards used for absolute
quantitation.
[0085] In a preferred embodiment, an isolated tau sample comprises
tau
that has been purified from blood or CSF by affinity purification and tau
phosphorylation
is measured by mass spectrometry. In another preferred embodiment, an isolated
tau
sample comprises tau that has been purified from blood or CSF by affinity
purification
using a ligand that specifically binds an epitope within the mid domain of
tau, and
optionally with a second ligand that specifically binds an epitope within the
N-terminus
of tau, and tau phosphorylation is measured by high resolution mass
spectrometry. In
another preferred embodiment, an isolated tau sample comprises tau that has
been
purified from blood or CSF by affinity purification using a ligand that
specifically binds an
epitope within the mid domain of tau, and optionally with a second ligand that
specifically binds an epitope within the MTBR or the C-terminus of tau, and
tau
phosphorylation is measured by high resolution mass spectrometry. In an
exemplary
embodiment, a mass spectrometry protocol outlined in the Examples is used.
36
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
IV. Methods of treatment
[0086] Another aspect of the present disclosure is a method for
treating a
subject in need thereof. The terms "treat," "treating," or "treatment" as used
herein,
refers to the provision of medical care by a trained and licensed professional
to a
subject in need thereof. The medical care may be a diagnostic test, a
therapeutic
treatment, and/or a prophylactic or preventative measure. The object of
therapeutic and
prophylactic treatments is to prevent or slow down (lessen) an undesired
physiological
change or disease/disorder. Beneficial or desired clinical results of
therapeutic or
prophylactic treatments include, but are not limited to, alleviation of
symptoms,
diminishment of extent of disease, stabilized (i.e., not worsening) state of
disease, a
delay or slowing of disease progression, amelioration or palliation of the
disease state,
and remission (whether partial or total), whether detectable or undetectable.
"Treatment" can also mean prolonging survival as compared to expected survival
if not
receiving treatment. Those in need of treatment include those already with the
disease,
condition, or disorder as well as those prone to have the disease, condition
or disorder
or those in which the disease, condition or disorder is to be prevented. In
some
embodiments, a subject receiving treatment is asymptomatic. An "asymptomatic
subject," as used herein, refers to a subject that does not show any signs or
symptoms
of AD. In other embodiments, a subject may exhibit signs or symptoms of AD
(e.g.,
memory loss, misplacing things, changes in mood or behavior, etc.,) but not
show
sufficient cognitive or functional impairment for a clinical diagnosis of mild
cognitive
impairment due to Alzheimer's disease. The phrase "mild cognitive impairment
due to
Alzheimer's disease" is defined in Section IV. A symptomatic or an
asymptomatic
subject may have Ap amyloidosis; however, prior knowledge of Ap amyloidosis is
not a
requisite for treatment. In still further embodiments, a subject may be
diagnosed as
having AD. In any of the aforementioned embodiments, a subject may carry one
of the
gene mutations known to cause dominantly inherited Alzheimer's disease. In
alternative
embodiments, a subject may not carry a gene mutation known to cause dominantly
inherited Alzheimer's disease.
37
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0087] In one embodiment, a method for treating a subject as
described
above may comprise (a) providing an isolated tau sample obtained from a
subject and
measuring, in the isolated tau sample, tau phosphorylation at one or more
amino acid
residue chosen from chosen from T111, S113, T181, S199, S202, S208, T153,
T175,
T205, S214, T217, and T231, and optionally measuring total tau; and (b)
administering
a pharmaceutical composition to the subject when the measured phosphorylation
level(s) significantly deviate from the mean in a control population without
brain amyloid
plaques as measured by PET imaging and/or A[342/40 measurement in CSF. In
another
embodiment, a method for treating a subject as described above may comprise
(a)
providing a first and a second isolated tau sample obtained from a subject and
measuring, in each isolated tau sample, tau phosphorylation at one or more
amino acid
residue chosen from chosen from T111, S113, T181, S199, S202, S208, T153,
T175,
T205, S214, T217, and T231, and optionally measuring total tau; (b)
calculating the
change in the site-specific phosphorylation at each residue measured and
optionally the
change in total tau; and (c) administering a pharmaceutical composition to the
subject
when the calculated change(s) significantly deviate from the mean in a control
population without brain amyloid plaques as measured by PET imaging and/or
A[342/40
measurement in CSF. "Significantly deviate from the mean" refers to values
that are at
least 1 standard deviation, preferably at least 1.3 standard deviations, more
preferably
at least 1.5 standard deviations or even more preferably at least 2 standard
deviations,
above or below the mean (i.e., la, 1.3o-, 1.50-, or 1.50-, respectively, where
a is the
standard deviation defined by the normal distribution measured in a control
population
without brain amyloid plaques as measured by PET imaging and/or A[342/40
measurement in CSF). In addition to using a threshold (e.g. at least 1
standard
deviation above or below the mean), in some embodiment the extent of change
above
or below the mean may be used as criteria for treating a subject.
[0088] Alternatively or in addition to using a measurement of site-
specific
tau phosphorylation, optionally with a measurement of total tau, in any of the
above
embodiments, a ratio calculated from the measured phosphorylation level(s), or
a ratio
calculated from the measured phosphorylation level(s) and total tau, may be
used. A
38
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
ratio calculated from the measured phosphorylation level(s) may be a ratio
between p-
T181 and p-T205, p-T217 and p-T205, or p-T181 and p-T217. A ratio calculated
from
the measured phosphorylation level(s) and total tau may be a ratio between p-
T181 and
total tau, p-T205 and total tau, or p-T217 and total tau. Mathematical
operations other
than a ratio may also be used. For instance, the examples use site-specific
tau
phosphorylation values in various statistical models (e.g., linear
regressions, LME
curves, LOESS curves, etc.) in conjunction with other known biomarkers (e.g.
APOE e4
status, age, sex, cognitive test scores, functional test scores, etc.).
[0089] Many imaging agents and therapeutic agents contemplated for,
or
used with, subjects at risk of developing Ap amyloidosis or AD, subjects
diagnosed as
having A amyloidosis, subjects diagnosed as having a tauopathy, or subjects
diagnosed as having AD, target a specific pathophysiological change. For
instance, Ap
targeting therapies are generally designed to decrease Ap production,
antagonize Ap
aggregation or increase brain Ap clearance; tau targeting therapies are
generally
designed to alter tau phosphorylation patterns, antagonize tau aggregation, or
increase NFT clearance; a variety of therapies are designed to reduce CNS
inflammation or brain insulin resistance, etc. The efficacy of these various
agents can
be improved by administering the agents to subjects that have certain tau
phosphorylation levels at T111, S113, T181, S199, S202, S208, T153, T175,
T205,
5214, T217, and T231, as measured by methods disclosed herein.
[0090] In an exemplary embodiment, the efficacy of imaging agents
and
therapeutic agents contemplated for, or used with, subjects at risk of
developing Ap
amyloidosis or AD, subjects diagnosed as having A amyloidosis, subjects
diagnosed
as having a tauopathy, or subjects diagnosed as having AD (collectively
referred to
herein as "Ap and tau therapies") can be improved by administering the Ap or
tau
therapy to subjects that have certain tau phosphorylation levels at T181, T205
and/or
T217, as measured by methods disclosed herein and illustrated, for example, in
FIG.
22. For instance, when tau phosphorylation at T217 is about 1.50- or above and
tau
phosphorylation at T181 and T205 is about 1.50- or below, preferred
therapeutic agents
may include those designed to prevent a subject from becoming amyloid positive
(e.g.,
39
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
amyloid targeting therapies designed to decrease Ap production, antagonize Ap
aggregation, etc). As another example, when tau phosphorylation at T217 and/or
T181
is about 1.5a or above and tau phosphorylation at T205 is about 1.5a or below,
preferred therapeutic agents may include those designed to prevent amyloid
deposition
from increasing or reduce a subject's existing plaque load. As another
example, when
tau phosphorylation at T217, T181 and T205 is about 1.5a or above, preferred
therapeutic agents may include those designed to prevent amyloid deposition
from
increasing, reduce a subject's existing plaque load, prevent tau aggregation,
or target
NFTs. As another example, when tau phosphorylation at T217, T181 and T205 is
about
1.5a or above, and tau phosphorylation at T217 or T181 is plateauing or
decreasing,
and total tau and/or tau phosphorylation at T205 is increasing, preferred
therapeutic
agents may include those designed to prevent amyloid deposition from
increasing,
reduce a subject's existing plaque load, prevent tau aggregation, or target
NFTs, as well
as those specific for subjects with AD. The details disclosed herein can
similarly be
used to administer therapeutic agents designed for other targets (e.g., CNS
inflammation, ApoE, etc.), including but not limited to those identified in
the following
paragraphs.
[0091] In one example, the present disclosure provides a method for
treating a subject having an increased risk of conversion to MCI due to AD,
the method
comprising (a) providing an isolated tau sample obtained from a subject and
measuring
tau phosphorylation at (i) T217 and T205, (ii) T181 and T205, or (iii) T181,
T205 and
T217; and (b) administering a pharmaceutical composition to the subject when
tau
phosphorylation at T217 and/or T181 is about 1.5a or above and tau
phosphorylation at
T205 is about 1.5a or below, where a is the standard deviation defined by the
normal
distribution of tau phosphorylation at T217 and T205, T181 and T205, or T181,
T205
and T217 measured in a control population without brain amyloid plaques as
measured
by PET imaging and/or A[342/40 measurement in CSF. In various embodiments, tau
phosphorylation at T217 and/or tau phosphorylation at T181 may be about 1.3a,
about
1.350-, about 1.4o-, about 1.450-, about 1.50-, about 1.6o-, about 1.7o-,
about 1.8o-, about
1.9a, about 2a, or above 2a. In other embodiments, tau phosphorylation at T217
and/or
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
tau phosphorylation at T181 may be about 1.85a, about 1.9a, about 1.95a, about
2a,
about 2.10-, about 2.2a, about 2.3a, about 2.4a, about 2.5a or above 2.5a. In
each of
the above embodiments, tau phosphorylation at T205 may be about 1.3a, about
1.35a,
about 1.4o-, about 1.450-, about 1.50-, about 1.510-, about 1.550-, about 1.6o-
, about 1.7o-,
about 1.8a, about 1.9a, about 2.0o-, or below 2.0o-. Alternatively, tau
phosphorylation at
T205 may be about 2.0o-, about 2.05a, about 2.10-, about 2.2a, about 2.3a,
about 2.4a,
about 2.5a, or below 2.5a. In a further example, tau phosphorylation at T217
and/or tau
phosphorylation at T181 about 2a or above and tau phosphorylation at T205 may
be
about 2a or less. In addition to using a threshold (e.g. at least 1 standard
deviation
above or below the mean), in some embodiment the extent of change above or
below
the mean may be used as criteria to treat a subject. In still further
embodiments,
measured levels of tau phosphorylation at T205 and at T181 and/or T217 may be
used
in various mathematical operations to improve the predictive power compared to
each
by itself. For instance, ratio(s) may be calculated from the measured
phosphorylation
levels. Mathematical operations other than a ratio may also be used.
[0092] In another example, the present disclosure provides a method
for
treating a subject having an increased risk of conversion to MCI due to AD,
the method
comprising (a) providing an isolated tau sample obtained from a subject and
measuring
total tau and tau phosphorylation at (i) T217 and T205, (ii) T181 and T205, or
(iii) T181,
T205 and T217; and (b) administering a pharmaceutical composition to the
subject
when the ratio of tau phosphorylation at T217 to total tau and/or the ratio of
tau
phosphorylation at T181 to total tau is about 1.5a or above and the ratio of
tau
phosphorylation at T205 to total tau is about 1.5a or below, where a is the
standard
deviation defined by the normal distribution of total tau and tau
phosphorylation at T217
and T205, T181 and T205, or T181, T205 and T217 measured in a control
population
without brain amyloid plaques as measured by PET imaging and/or A[342/40
measurement in CSF. In various embodiments, the ratio of tau phosphorylation
at T217
to total tau and/or the ratio of tau phosphorylation at T181 to total tau may
be about
1.3a, about 1.35a, about 1.4a, about 1.45a, about 1.5a, about 1.6a, about
1.7a, about
1.8a, about 1.9a, about 2a, or above 2a. In other embodiments, the ratio of
tau
41
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
phosphorylation at T217 to total tau and/or the ratio of tau phosphorylation
at T181 to
total tau may be about 1.85a, about 1.9a, about 1.95a, about 2a, about 2.1a,
about
2.2a, about 2.3a, about 2.4a, about 2.5a or above 2.5a. In each of the above
embodiments, the ratio of tau phosphorylation at T205 to total tau may be
about 1.3a,
about 1.35a, about 1.4a, about 1.45a, about 1.50a, about 1.55a, about 1.6a,
about
1.7a, about 1.8a, about 1.9a, about 2.0o-, or below 2a. Alternatively, the
ratio of tau
phosphorylation at T205 to total tau may be about 2.0o-, about 2.05a, about
2.10-, about
2.2a, about 2.3a, about 2.4a, about 2.5a, or below 2.5a. In a further example,
the ratio
of tau phosphorylation at T217 to total tau and/or the ratio of tau
phosphorylation at
T181 to total tau may be about 2a or above and the ratio of tau
phosphorylation at T205
to total tau may about 2a or less. In addition to using a threshold (e.g. at
least 1
standard deviation above or below the mean), in some embodiment the extent of
change above or below the mean may be used as criteria to treat a subject.
[0093] In another example, the present disclosure provides a method
for
treating a subject having an increased risk of conversion to MCI due to AD,
the method
comprising (a) providing an isolated tau sample obtained from a subject and
measuring
tau phosphorylation at (i) T181 and T205, (ii) T217 and T205, or (iii) T181,
T217 and
T205; and (b) administering a pharmaceutical composition to the subject when
tau
phosphorylation at the specific sites recited in (a)(i), (a)(ii) or (a)(iii)
is about 1.5a or
above, where a is the standard deviation defined by the normal distribution of
tau
phosphorylation at T217 and T205, T181 and T205, or T181, T205 and T217
measured
in a control population without brain amyloid plaques as measured by PET
imaging
and/or A[342/40 measurement in CSF. In various embodiments, tau
phosphorylation at
the specific sites recited in (a)(i), (a)(ii) or (a)(iii) may be about 1.3a,
about 1.35a, about
1.4a, about 1.45a, about 1.5a, about 1.6a, about 1.7a, about 1.8a, about 1.9a,
about
2a, or above 2a. In other embodiments, tau phosphorylation at the specific
sites recited
in (a)(i), (a)(ii) or (a)(iii) may be about 1.85a, about 1.9a, about 1.95a,
about 2a, about
2.10-, about 2.2a, about 2.3a, about 2.4a, about 2.5a or above 2.5a. In a
further
example, tau phosphorylation at the specific sites recited in (a)(i), (a)(ii)
or (a)(iii) may be
about 2a or above. In addition to using a threshold (e.g. at least 1 standard
deviation
42
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
above or below the mean), in some embodiment the extent of change above or
below
the mean may be used as criteria to treat a subject. In still further
embodiments,
measured levels of tau phosphorylation at the specific sites recited in
(a)(i), (a)(ii) or
(a)(iii) may be used in various mathematical operations to improve the
predictive power
compared to each by itself. For instance, ratio(s) may be calculated from the
measured
phosphorylation levels. Mathematical operations other than a ratio may also be
used.
[0094] In another example, the present disclosure provides a method
for
treating a subject having an increased risk of conversion to MCI due to AD,
the method
comprising (a) providing an isolated tau sample obtained from a subject and
measuring
total tau and tau phosphorylation at (i) T181 and T205, (ii) T217 and T205, or
(iii) T181,
T217 and T205; and (b) administering a pharmaceutical composition to the
subject
when the ratio of tau phosphorylation at the specific sites recited in (a)(i),
(a)(ii) or (a)(iii)
to total tau is about 1.5a or above, where a is the standard deviation defined
by the
normal distribution of total tau and tau phosphorylation at T217 and T205,
T181 and
T205, or T181, T205 and T217 measured in a control population without brain
amyloid
plaques as measured by PET imaging and/or A[342/40 measurement in CSF. In
various
embodiments, the ratio of tau phosphorylation at the specific sites recited in
(a)(i), (a)(ii)
or (a)(iii) to total tau may be about 1.3a, about 1.35a, about 1.4a, about
1.45a, about
1.5a, about 1.6a, about 1.7a, about 1.8a, about 1.9a, about 2a, or above 2a.
In other
embodiments, the ratio of tau phosphorylation at the specific sites recited in
(a)(i), (a)(ii)
or (a)(iii) to total tau may be about 1.85a, about 1.9a, about 1.95a, about
2a, about
2.10-, about 2.2a, about 2.3a, about 2.4a, about 2.5a or above 2.5a. In a
further
example, the ratio of tau phosphorylation at the specific sites recited in
(a)(i), (a)(ii) or
(a)(iii) to total tau may be about 2a or above. In addition to using a
threshold (e.g. at
least 1 standard deviation above or below the mean), in some embodiment the
extent of
change above or below the mean may be used as criteria to treat a subject.
[0095] In another example, the present disclosure provides a method
for
treating a subject with symptoms of AD, the method comprising (a) providing a
first and
a second isolated tau sample obtained from a subject, wherein "first" and
"second" refer
to the order in which the samples were collected, and measuring tau
phosphorylation at
43
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
(i) T181 and T205, (ii) T217 and T205, or (iii) T181, T217 and T205; (b)
calculating the
change in the site-specific phosphorylation at each residue measured and
optionally the
change in total tau; and (c) administering a pharmaceutical composition to the
subject
when the phosphorylation level at T181 and/or T217 decreases or stays the same
and
the phosphorylation level at T205 and optionally total tau increases. The
first and the
second isolated tau samples may be collected days, weeks, or months apart.
Typically,
tau phosphorylation at the specific sites recited in (a)(i), (a)(ii) or
(a)(iii) will also be
about 1.5a or above for both samples and , where a is the standard deviation
defined
by the normal distribution of tau phosphorylation at T217 and T205, T181 and
T205, or
T181, T205 and T217 measured in a control population without brain amyloid
plaques
as measured by PET imaging and/or A[342/40 measurement in CSF. In still
further
embodiments, measured levels of tau phosphorylation at the specific sites
recited in
(a)(i), (a)(ii) or (a)(iii) may be used in various mathematical operations to
improve the
predictive power compared to each by itself. For instance, ratio(s) may be
calculated
from the measured phosphorylation levels. Mathematical operations other than a
ratio
may also be used.
[0096] In another example, the present disclosure provides a method
for
treating a subject with symptoms of AD, the method comprising (a) providing a
first and
a second isolated tau sample obtained from a subject, wherein "first" and
"second" refer
to the order in which the samples were collected, and measuring total tau and
tau
phosphorylation at (i) T181 and T205, (ii) T217 and T205, or (iii) T181, T217
and T205;
(b) calculating the change in the site-specific phosphorylation at each
residue measured
and the change in total tau; and (c) administering a pharmaceutical
composition to the
subject when the phosphorylation level at T181 and/or T217 decreases or stays
the
same, the phosphorylation level at T205 decreases or stays the same, and total
tau
increases. The first and the second isolated tau samples may be collected
days, weeks,
or months apart. Typically, tau phosphorylation at the specific sites recited
in (a)(i),
(a)(ii) or (a)(iii) will also be about 1.5a or above for both samples and ,
where a is the
standard deviation defined by the normal distribution of tau phosphorylation
at T217 and
T205, T181 and T205, or T181, T205 and T217 measured in a control population
44
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
without brain amyloid plaques as measured by PET imaging and/or A[342/40
measurement in CSF. In still further embodiments, measured levels of tau
phosphorylation at the specific sites recited in (a)(i), (a)(ii) or (a)(iii)
may be used in
various mathematical operations to improve the predictive power compared to
each by
itself. For instance, ratio(s) may be calculated from the measured
phosphorylation
levels. Mathematical operations other than a ratio may also be used.
[0097] In each of the above embodiments, a pharmaceutical
composition
may comprise an imaging agent. Non-limiting examples of imaging agents include
functional imaging agents (e.g. fluorodeoxyglucose, etc.) and molecular
imaging agents
(e.g., Pittsburgh compound B, florbetaben, florbetapir, flutemetamol,
radionuclide-
labeled antibodies, etc.)
[0098] Alternatively, a pharmaceutical composition may comprise an
active pharmaceutical ingredient. Non-limiting examples of active
pharmaceutical
ingredients include cholinesterase inhibitors, N-methyl D-aspartate (NMDA)
antagonists,
antidepressants (e.g., selective serotonin reuptake inhibitors, atypical
antidepressants,
aminoketones, selective serotonin and norepinephrine reuptake inhibitors,
tricyclic
antidepressants, etc.), gamma-secretase inhibitors, beta-secretase inhibitors,
anti-Ap
antibodies (including antigen-binding fragments, variants, or derivatives
thereof), anti-
tau antibodies (including antigen- binding fragments, variants, or derivatives
thereof),
stem cells, dietary supplements (e.g. lithium water, omega-3 fatty acids with
lipoic acid,
long chain triglycerides, genistein, resveratrol, curcumin, and grape seed
extract, etc.),
antagonists of the serotonin receptor 6, p38a1pha MAPK inhibitors, recombinant
granulocyte macrophage colony-stimulating factor, passive immunotherapies,
active
vaccines (e.g. CAD106, AF20513, etc. ), tau protein aggregation inhibitors
(e.g.
TRx0237, methylthionimium chloride, etc.), therapies to improve blood sugar
control
(e.g., insulin, exenatide, liraglutide pioglitazone, etc.), anti-inflammatory
agents,
phosphodiesterase 9A inhibitors, sigma-1 receptor agonists, kinase inhibitors,
phosphatase activators, phosphatase inhibitors, angiotensin receptor blockers,
CB1
and/or CB2 endocannabinoid receptor partial agonists, [3-2 adrenergic receptor
agonists, nicotinic acetylcholine receptor agonists, 5-HT2A inverse agonists,
alpha-2c
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
adrenergic receptor antagonists, 5-HT 1A and 1D receptor agonists, Glutaminyl-
peptide
cyclotransferase inhibitors, selective inhibitors of APP production, monoamine
oxidase
B inhibitors, glutamate receptor antagonists, AMPA receptor agonists, nerve
growth
factor stimulants, HMG-CoA reductase inhibitors, neurotrophic agents,
muscarinic M1
receptor agonists, GABA receptor modulators, PPAR-gamma agonists, microtubule
protein modulators, calcium channel blockers, antihypertensive agents,
statins, and any
combination thereof
[0099] In another alternative, a pharmaceutical composition may
comprise
a kinase inhibitor. Suitable kinase inhibitors may inhibit a thousand-and-one
amino acid
kinase (TAOK), CDK, GSK-3[3, MARK, CDK5, Fyn, 5' adenosine monophosphate-
activated protein kinase (AMPK), Calcium-calmodulin kinase II, Cyclin-
dependent
kinase-5 (cdk5), Casein kinase 1 (CK1), Casein kinase 2 (CK2), Cyclic AMP-
dependent
protein kinase (PKA), Dual-specificity tyrosine-phosphorylation regulated
kinase 1A
(DYRK1A), Glycogen synthase kinase-3 (GSK-3), JNK, LRRK2, Microtubule affinity-
regulating kinase (MARK), MSK1, p35/41, p42/p44 mitogen-activated protein
kinases
(ERKs1/2), p38 mitogen-activated kinase (p38MAPK), p70S6 kinase, Phosphorylase
kinase, PKB/AKT, Protein kinase C (PKC), Protein kinase N (PKN), Prostate-
derived
sterile 20-like kinase 1 alpha/beta, 90 kDa Ribosomal S6 kinase (RSK1/2)
(PSK1/TAOK2), Prostate-derived sterile 20-like kinase 2 (PSK2/TAOK1), Stress-
activated protein kinase (SAPK) 1gamma, SAPK2a, SAPK2b, SAPK3, SAPK4, SGK1,
SRPK2, or Tau-tubulin kinase 1/2 (TTBK1/2).
[0100] In still another alternative, a pharmaceutical composition
may
comprise a phosphatase activator. As a non-limiting example, a phosphatase
activator
may increase the activity of protein phosphatase 1, 2A, 2B, or 5.
[0101] Methods for measuring tau phosphorylation and total tau are
described in Section II, and incorporated into this section by reference. In a
preferred
embodiment, an isolated tau sample comprises tau that has been purified from
blood or
CSF by affinity purification and tau phosphorylation is measured by mass
spectrometry.
In another preferred embodiment, an isolated tau sample comprises tau that has
been
purified from blood or CSF by affinity purification using a ligand that
specifically binds an
46
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
epitope within the mid domain of tau, and optionally with a second ligand that
specifically binds an epitope within the N-terminus of tau, and tau
phosphorylation is
measured by high resolution mass spectrometry. In another preferred
embodiment, an
isolated tau sample comprises tau that has been purified from blood or CSF by
affinity
purification using a ligand that specifically binds an epitope within the mid
domain of tau,
and optionally with a second ligand that specifically binds an epitope within
the MTBR or
the C-terminus of tau, and tau phosphorylation is measured by high resolution
mass
spectrometry. In an exemplary embodiment, a mass spectrometry protocol
outlined in
the Examples is used.
V. Clinical trials
[0102] Another aspect of the present disclosure is a method for
enrolling a
subject into a clinical trial, in particular a clinical trial for an AB or tau
therapy, provided
all other criteria for the clinical trial have been met. In one embodiment, a
method for a
method for enrolling a subject into a clinical trial may comprise (a)
providing an isolated
tau sample obtained from a subject and measuring, in the isolated tau sample,
tau
phosphorylation at one or more amino acid residue chosen from chosen from
T111,
S113, T181, S199, S202, S208, T153, T175, T205, S214, T217, and T231, and
optionally measuring total tau; and (b) enrolling the subject into a clinical
trial when the
measured phosphorylation level(s) significantly deviate from the mean in a
control
population without brain amyloid plaques as measured by PET imaging and/or
A[342/40
measurement in CSF. In another embodiment, a method for a method for enrolling
a
subject into a clinical trial may comprise (a) providing a first and a second
isolated tau
sample obtained from a subject and measuring, in each isolated tau sample, tau
phosphorylation at one or more amino acid residue chosen from chosen from
T111,
S113, T181, S199, S202, S208, T153, T175, T205, S214, T217, and T231, and
optionally measuring total tau; (b) calculating the change in the site-
specific
phosphorylation at each residue measured and optionally the change in total
tau; and
(c) enrolling the subject into a clinical trial when the calculated change(s)
significantly
deviate from the mean in a control population without brain amyloid plaques as
measured by PET imaging and/or A[342/40 measurement in CSF. The phrase "a
control
47
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
population without brain amyloid plaques as measured by PET imaging and/or
A[342/40
measurement in CSF" is defined in Section IV. "Significantly deviate from the
mean"
refers to values that are at least 1 standard deviation, preferably at least
1.3 standard
deviations, more preferably at least 1.5 standard deviations or even more
preferably at
least 2 standard deviations, above or below the mean (i.e., la, 1.3a, 1.50-,
or 1.50-,
respectively, where a is the standard deviation defined by the normal
distribution
measured in a control population without brain amyloid plaques as measured by
PET
imaging and/or A[342/40 measurement in CSF). In addition to using a threshold
(e.g. at
least 1 standard deviation above or below the mean), in some embodiment the
extent of
change above or below the mean may be used as criteria for enrolling a
subject.
[0103] Alternatively or in addition to using a measurement of site-
specific
tau phosphorylation, optionally with a measurement of total tau, in any of the
above
embodiments, a ratio calculated from the measured phosphorylation level(s), or
a ratio
calculated from the measured phosphorylation level(s) and total tau, may be
used. A
ratio calculated from the measured phosphorylation level(s) may be a ratio
between p-
T181 and p-T205, p-T217 and p-T205, or p-T181 and p-T217. A ratio calculated
from
the measured phosphorylation level(s) and total tau may be a ratio between p-
T181 and
total tau, p-T205 and total tau, or p-T217 and total tau. Mathematical
operations other
than a ratio may also be used. For instance, the examples use site-specific
tau
phosphorylation values in various statistical models (e.g., linear
regressions, LME
curves, LOESS curves, etc.) in conjunction with other known biomarkers (e.g.
APOE e4
status, age, sex, cognitive test scores, functional test scores, etc.).
[0104] The design of clinical trials for Ap and tau therapies can be
greatly
aided by the methods disclosed herein. Many clinical trials are designed to
test the
efficacy of imaging agents or therapeutic agents that target a specific
pathophysiological
change which occurs prior to the onset of AD symptoms. As discussed above in
Section V, the efficacy of these various agents can be improved by
administering the
agents to subjects that have certain site-specific tau phosphorylation levels,
as
measured by methods disclosed herein and illustrated. Similarly, clinical
trials enrolling
subjects with symptoms of AD (e.g., after the onset of MCI due to AD) would
also
48
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
benefit from being able to accurately stage an enrollee's AD status in order
to determine
if efficacy is associated with a particular stage of AD. Accordingly,
measuring tau
phosphorylation levels as described herein prior to enrolling a subject in a
clinical trial,
in particular into a treatment arm of a clinical trial, may result in smaller
trials and/or
improved outcomes. In some instances, methods described herein may be
developed
and used as a companion diagnostic for a therapeutic agent.
[0105] In an exemplary embodiment, a method for a method for
enrolling a
subject into a clinical trial may comprise (a) providing an isolated tau
sample obtained
from a subject and measuring, in the isolated tau sample, tau phosphorylation
at one or
more amino acid residue chosen from chosen from T181, T205, and T217, and
optionally measuring total tau; and (b) enrolling the subject into a clinical
trial when the
measured phosphorylation level(s) significantly deviate from the mean in a
control
population without brain amyloid plaques as measured by PET imaging and/or
A[342/40
measurement in CSF. In another exemplary embodiment, a method for a method for
enrolling a subject into a clinical trial may comprise (a) providing a first
and a second
isolated tau sample obtained from a subject and measuring, in each isolated
tau
sample, tau phosphorylation at one or more amino acid residue chosen from
T181,
T205, and T217, and optionally measuring total tau; (b) calculating the change
in the
site-specific phosphorylation at each residue measured and optionally the
change in
total tau; and (c) enrolling the subject into a clinical trial when the
calculated change(s)
significantly deviate from the mean in a control population without brain
amyloid plaques
as measured by PET imaging and/or A[342/40 measurement in CSF. The phrase "a
control population without brain amyloid plaques as measured by PET imaging
and/or
A[342/40 measurement in CSF" is defined in Section IV. "Significantly deviate
from the
mean" refers to values that are at least 1 standard deviation, preferably at
least 1.3
standard deviations, more preferably at least 1.5 standard deviations or even
more
preferably at least 2 standard deviations, above or below the mean (i.e., 10-,
1.3o-, 1.50-,
or 1.5a, respectively, where a is the standard deviation defined by the normal
distribution measured in a control population without brain amyloid plaques as
measured by PET imaging and/or A[342/40 measurement in CSF). In addition to
using a
49
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
threshold (e.g. at least 1 standard deviation above or below the mean), in
some
embodiment the extent of change above or below the mean may be used as
criteria for
enrolling a subject.
[0106] In one example, the present disclosure provides a method for
enrolling a subject into a clinical trial, the method comprising (a) providing
an isolated
tau sample obtained from a subject and measuring tau phosphorylation at (i)
T217 and
T205, (ii) T181 and T205, or (iii) T181, T205 and T217; and (b) administering
a
pharmaceutical composition to the subject when tau phosphorylation at T217
and/or
T181 is about 1.5a or above and tau phosphorylation at T205 is about 1.5a or
below,
where a is the standard deviation defined by the normal distribution of tau
phosphorylation at T217 and T205, T181 and T205, or T181, T205 and T217
measured
in a control population without brain amyloid plaques as measured by PET
imaging
and/or A[342/40 measurement in CSF. In various embodiments, tau
phosphorylation at
T217 and/or tau phosphorylation at T181 may be about 1.3a, about 1.35a, about
1.4a,
about 1.45a, about 1.5a, about 1.6a, about 1.7a, about 1.8a, about 1.9a, about
2a, or
above 2a. In other embodiments, tau phosphorylation at T217 and/or tau
phosphorylation at T181 may be about 1.850-, about 1.9o-, about 1.950-, about
2a, about
2.10-, about 2.2a, about 2.3a, about 2.4a, about 2.5a or above 2.5a. In each
of the
above embodiments, tau phosphorylation at T205 may be about 1.3a, about 1.35a,
about 1.4o-, about 1.450-, about 1.50-, about 1.510-, about 1.550-, about 1.6o-
, about 1.7o-,
about 1.8a, about 1.9a, about 2.0a, or below 2.0a. Alternatively, tau
phosphorylation at
T205 may be about 2.0a, about 2.05a, about 2.10-, about 2.2a, about 2.3a,
about 2.4a,
about 2.5a, or below 2.5a. In a further example, tau phosphorylation at T217
and/or tau
phosphorylation at T181 about 2a or above and tau phosphorylation at T205 may
be
about 2a or less. In addition to using a threshold (e.g. at least 1 standard
deviation
above or below the mean), in some embodiment the extent of change above or
below
the mean may be used as criteria for enrolling a subject. In still further
embodiments,
measured levels of tau phosphorylation at T205 and at T181 and/or T217 may be
used
in various mathematical operations to improve the predictive power compared to
each
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
by itself. For instance, ratio(s) may be calculated from the measured
phosphorylation
levels. Mathematical operations other than a ratio may also be used.
[0107] In another example, the present disclosure provides a method
for
enrolling a subject into a clinical trial, the method comprising (a) providing
an isolated
tau sample obtained from a subject and measuring total tau and tau
phosphorylation at
(i) T217 and T205, (ii) T181 and T205, or (iii) T181, T205 and T217; and (b)
administering a pharmaceutical composition to the subject when the ratio of
tau
phosphorylation at T217 to total tau and/or the ratio of tau phosphorylation
at T181 to
total tau is about 1.5a or above and the ratio of tau phosphorylation at T205
to total tau
is about 1.5a or below, where a is the standard deviation defined by the
normal
distribution of total tau and tau phosphorylation at T217 and T205, T181 and
T205, or
T181, T205 and T217 measured in a control population without brain amyloid
plaques
as measured by PET imaging and/or A[342/40 measurement in CSF. In various
embodiments, the ratio of tau phosphorylation at T217 to total tau and/or the
ratio of tau
phosphorylation at T181 to total tau may be about 1.3o-, about 1.350-, about
1.4o-, about
1.45a, about 1.5a, about 1.6a, about 1.7a, about 1.8a, about 1.9a, about 2a,
or above
2a. In other embodiments, the ratio of tau phosphorylation at T217 to total
tau and/or
the ratio of tau phosphorylation at T181 to total tau may be about 1.85a,
about 1.9a,
about 1.95a, about 2a, about 2.1a, about 2.2a, about 2.3a, about 2.4a, about
2.5a or
above 2.5a. In each of the above embodiments, the ratio of tau phosphorylation
at T205
to total tau may be about 1.3a, about 1.35a, about 1.4a, about 1.45a, about
1.50a,
about 1.55a, about 1.6a, about 1.7a, about 1.8a, about 1.9a, about 2.0a, or
below 2a.
Alternatively, the ratio of tau phosphorylation at T205 to total tau may be
about 2.0a,
about 2.05a, about 2.10-, about 2.2a, about 2.3a, about 2.4a, about 2.5a, or
below 2.5a.
In a further example, the ratio of tau phosphorylation at T217 to total tau
and/or the ratio
of tau phosphorylation at T181 to total tau may be about 2a or above and the
ratio of
tau phosphorylation at T205 to total tau may about 2a or less. In addition to
using a
threshold (e.g. at least 1 standard deviation above or below the mean), in
some
embodiment the extent of change above or below the mean may be used as
criteria for
enrolling a subject.
51
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0108] In another example, the present disclosure provides a method
for
enrolling a subject into a clinical trial, the method comprising (a) providing
an isolated
tau sample obtained from a subject and measuring tau phosphorylation at (i)
T181 and
T205, (ii) T217 and T205, or (iii) T181, T217 and T205; and (b) administering
a
pharmaceutical composition to the subject when tau phosphorylation at the
specific
sites recited in (a)(i), (a)(ii) or (a)(iii) is about 1.5a or above, where a
is the standard
deviation defined by the normal distribution of tau phosphorylation at T217
and T205,
T181 and T205, or T181, T205 and T217 measured in a control population without
brain
amyloid plaques as measured by PET imaging and/or A[342/40 measurement in CSF.
In
various embodiments, tau phosphorylation at the specific sites recited in
(a)(i), (a)(ii) or
(a)(iii) may be about 1.3a, about 1.35a, about 1.4a, about 1.45a, about 1.5a,
about
1.6a, about 1.7a, about 1.8a, about 1.9a, about 2a, or above 2a. In other
embodiments,
tau phosphorylation at the specific sites recited in (a)(i), (a)(ii) or
(a)(iii) may be about
1.85a, about 1.9a, about 1.95a, about 2o-, about 2.10-, about 2.2a, about
2.3a, about
2.4a, about 2.5a or above 2.5a. In a further example, tau phosphorylation at
the specific
sites recited in (a)(i), (a)(ii) or (a)(iii) may be about 2a or above. In
addition to using a
threshold (e.g. at least 1 standard deviation above or below the mean), in
some
embodiment the extent of change above or below the mean may be used as
criteria for
enrolling a subject. In still further embodiments, measured levels of tau
phosphorylation
at the specific sites recited in (a)(i), (a)(ii) or (a)(iii) may be used in
various mathematical
operations to improve the predictive power compared to each by itself. For
instance,
ratio(s) may be calculated from the measured phosphorylation levels.
Mathematical
operations other than a ratio may also be used.
[0109] In another example, the present disclosure provides a method
for
enrolling a subject into a clinical trial, the method comprising (a) providing
an isolated
tau sample obtained from a subject and measuring total tau and tau
phosphorylation at
(i) T181 and T205, (ii) T217 and T205, or (iii) T181, T217 and T205; and (b)
administering a pharmaceutical composition to the subject when the ratio of
tau
phosphorylation at the specific sites recited in (a)(i), (a)(ii) or (a)(iii)
to total tau is about
1.5a or above, where a is the standard deviation defined by the normal
distribution of
52
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
total tau and tau phosphorylation at T217 and T205, T181 and T205, or T181,
T205 and
T217 measured in a control population without brain amyloid plaques as
measured by
PET imaging and/or A[342/40 measurement in CSF. In various embodiments, the
ratio
of tau phosphorylation at the specific sites recited in (a)(i), (a)(ii) or
(a)(iii) to total tau
may be about 1.3a, about 1.35a, about 1.4a, about 1.45a, about 1.5a, about
1.6a,
about 1.7a, about 1.8a, about 1.9a, about 2a, or above 2a. In other
embodiments, the
ratio of tau phosphorylation at the specific sites recited in (a)(i), (a)(ii)
or (a)(iii) to total
tau may be about 1.85a, about 1.9a, about 1.95a, about 2a, about 2.1a, about
2.2a,
about 2.3a, about 2.4a, about 2.5a or above 2.5a. In a further example, the
ratio of tau
phosphorylation at the specific sites recited in (a)(i), (a)(ii) or (a)(iii)
to total tau may be
about 2a or above. In addition to using a threshold (e.g. at least 1 standard
deviation
above or below the mean), in some embodiment the extent of change above or
below
the mean may be used as criteria for enrolling a subject.
[0110] In another example, the present disclosure provides a method
for
enrolling a subject into a clinical trial, the method comprising, (a)
providing a first and a
second isolated tau sample obtained from a subject, wherein "first" and
"second" refer to
the order in which the samples were collected, and measuring tau
phosphorylation at (i)
T181 and T205, (ii) T217 and T205, or (iii) T181, T217 and T205; (b)
calculating the
change in the site-specific phosphorylation at each residue measured and
optionally the
change in total tau; and (c) enrolling the subject into the clinical trial
when the
phosphorylation level at T181 and/or T217 decreases or stays the same and the
phosphorylation level at T205 and optionally total tau increases. The first
and the
second isolated tau samples may be collected days, weeks, or months apart.
Typically,
tau phosphorylation at the specific sites recited in (a)(i), (a)(ii) or
(a)(iii) will also be
about 1.5a or above for both samples and , where a is the standard deviation
defined
by the normal distribution of tau phosphorylation at T217 and T205, T181 and
T205, or
T181, T205 and T217 measured in a control population without brain amyloid
plaques
as measured by PET imaging and/or A[342/40 measurement in CSF. In still
further
embodiments, measured levels of tau phosphorylation at the specific sites
recited in
(a)(i), (a)(ii) or (a)(iii) may be used in various mathematical operations to
improve the
53
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
predictive power compared to each by itself. For instance, ratio(s) may be
calculated
from the measured phosphorylation levels. Mathematical operations other than a
ratio
may also be used.
[0111] In another example, the present disclosure provides a method
for
enrolling a subject into a clinical trial, the method comprising (a) providing
a first and a
second isolated tau sample obtained from a subject, wherein "first" and
"second" refer to
the order in which the samples were collected, and measuring total tau and tau
phosphorylation at (i) T181 and T205, (ii) T217 and T205, or (iii) T181, T217
and T205;
(b) calculating the change in the site-specific phosphorylation at each
residue measured
and the change in total tau; and (c) enrolling the subject into the clinical
trial when the
phosphorylation level at T181 and/or T217 decreases or stays the same, the
phosphorylation level at T205 decreases or stays the same, and total tau
increases.
The first and the second isolated tau samples may be collected days, weeks, or
months
apart. Typically, tau phosphorylation at the specific sites recited in (a)(i),
(a)(ii) or (a)(iii)
will also be about 1.5a or above for both samples and , where a is the
standard
deviation defined by the normal distribution of tau phosphorylation at T217
and T205,
T181 and T205, or T181, T205 and T217 measured in a control population without
brain
amyloid plaques as measured by PET imaging and/or A[342/40 measurement in CSF.
In
still further embodiments, measured levels of tau phosphorylation at the
specific sites
recited in (a)(i), (a)(ii) or (a)(iii) may be used in various mathematical
operations to
improve the predictive power compared to each by itself. For instance,
ratio(s) may be
calculated from the measured phosphorylation levels. Mathematical operations
other
than a ratio may also be used.
[0112] Methods for measuring tau phosphorylation and total tau are
described in Section II, and incorporated into this section by reference. For
instance,
using the protocol detailed for Examples 5-9, tau phosphorylation at T181,
T205 and
T217 is 21.7 2.3, 0.34 0.13, and 1.2 0.66, respectively, in a control
population without
brain amyloid plaques as measured by PET imaging, as measured in an isolated
tau
sample that was purified from CSF (see Table 3, mutation non-carriers column).
Accordingly, twice the standard deviation above the mean found for the
mutation non-
54
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
carrier population (i.e. 2a) for p-T181, p-T205 and p-T217 is 43.4, 0.68, and
2.4,
respectively. A skilled artisan will appreciate, however, that the absolute
value may vary
depending upon the protocol.
[0113] In a preferred embodiment, an isolated tau sample comprises
tau
that has been purified from blood or CSF by affinity purification and tau
phosphorylation
is measured by mass spectrometry. In another preferred embodiment, an isolated
tau
sample comprises tau that has been purified from blood or CSF by affinity
purification
using a ligand that specifically binds an epitope within the mid domain of
tau, and
optionally with a second ligand that specifically binds an epitope within the
N-terminus
of tau, and tau phosphorylation is measured by high resolution mass
spectrometry. In
another preferred embodiment, an isolated tau sample comprises tau that has
been
purified from blood or CSF by affinity purification using a ligand that
specifically binds an
epitope within the mid domain of tau, and optionally with a second ligand that
specifically binds an epitope within the MTBR or the C-terminus of tau, and
tau
phosphorylation is measured by high resolution mass spectrometry. In an
exemplary
embodiment, a mass spectrometry protocol outlined in the Examples is used.
[0114] In each of the above embodiments, a subject may be enrolled
into
a treatment arm of the clinical trial. The "treatment" is defined in Section
V. Subjects
enrolled in the treatment arm of a clinical trial may be administered a
pharmaceutical
composition. In some embodiments, a pharmaceutical composition may comprise an
imaging agent. Non-limiting examples of imaging agents include functional
imaging
agents (e.g. fluorodeoxyglucose, etc.) and molecular imaging agents (e.g.,
Pittsburgh
compound B, florbetaben, florbetapir, flutemetamol, radionuclide-labeled
antibodies,
etc.). Alternatively, a pharmaceutical composition may comprise an active
pharmaceutical ingredient. Non-limiting examples of active pharmaceutical
ingredients
include cholinesterase inhibitors, N-methyl D-aspartate (NMDA) antagonists,
antidepressants (e.g., selective serotonin reuptake inhibitors, atypical
antidepressants,
aminoketones, selective serotonin and norepinephrine reuptake inhibitors,
tricyclic
antidepressants, etc.), gamma-secretase inhibitors, beta-secretase inhibitors,
anti-Ap
antibodies (including antigen-binding fragments, variants, or derivatives
thereof), anti-
CA 03097667 2020-10-16
WO 2019/213612
PCT/US2019/030725
tau antibodies (including antigen- binding fragments, variants, or derivatives
thereof),
stem cells, dietary supplements (e.g. lithium water, omega-3 fatty acids with
lipoic acid,
long chain triglycerides, genistein, resveratrol, curcumin, and grape seed
extract, etc.),
antagonists of the serotonin receptor 6, p38a1pha MAPK inhibitors, recombinant
granulocyte macrophage colony-stimulating factor, passive immunotherapies,
active
vaccines (e.g. CAD106, AF20513, etc. ), tau protein aggregation inhibitors
(e.g.
TRx0237, methylthionimium chloride, etc.), therapies to improve blood sugar
control
(e.g., insulin, exenatide, liraglutide pioglitazone, etc.), anti-inflammatory
agents,
phosphodiesterase 9A inhibitors, sigma-1 receptor agonists, kinase inhibitors,
phosphatase activators, phosphatase inhibitors, angiotensin receptor blockers,
CB1
and/or CB2 endocannabinoid receptor partial agonists, [3-2 adrenergic receptor
agonists, nicotinic acetylcholine receptor agonists, 5-HT2A inverse agonists,
alpha-2c
adrenergic receptor antagonists, 5-HT 1A and 1D receptor agonists, Glutaminyl-
peptide
cyclotransferase inhibitors, selective inhibitors of APP production, monoamine
oxidase
B inhibitors, glutamate receptor antagonists, AMPA receptor agonists, nerve
growth
factor stimulants, HMG-CoA reductase inhibitors, neurotrophic agents,
muscarinic M1
receptor agonists, GABA receptor modulators, PPAR-gamma agonists, microtubule
protein modulators, calcium channel blockers, antihypertensive agents,
statins, and any
combination thereof. In an exemplary embodiment, a pharmaceutical composition
may
comprise a kinase inhibitor. Suitable kinase inhibitors may inhibit a thousand-
and-one
amino acid kinase (TAOK), CDK, GSK-3[3, MARK, CDK5, or Fyn. In another
exemplary
embodiment, a pharmaceutical composition may comprise a phosphatase activator.
As
a non-limiting example, a phosphatase activator may increase the activity of
protein
phosphatase 2A.
[0115] In
each of the above embodiments, a subject may or may not be
symptomatic. An "asymptomatic subject," as used herein, refers to a subject
that does
not show any signs or symptoms of AD. Alternatively, a subject may exhibit
signs or
symptoms of AD (e.g., memory loss, misplacing things, changes in mood or
behavior,
etc.,) but not show sufficient cognitive or functional impairment for a
clinical diagnosis of
mild cognitive impairment. A symptomatic or an asymptomatic subject may have
Ap
56
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
amyloidosis; however, prior knowledge of AB amyloidosis is not a requisite for
treatment. In still further embodiments, a subject may have AD. In any of the
aforementioned embodiments, a subject may carry one of the gene mutations
known to
cause dominantly inherited Alzheimer's disease. In alternative embodiments, a
subject
may not carry a gene mutation known to cause dominantly inherited Alzheimer's
disease.
[0116] The following examples are included to demonstrate preferred
embodiments of the invention. It should be appreciated by those of skill in
the art that
the techniques disclosed in the examples that follow represent techniques
discovered
by the inventors to function well in the practice of the invention. Those of
skill in the art
should, however, in light of the present disclosure, appreciate that changes
may be
made in the specific embodiments that are disclosed and still obtain a like or
similar
result without departing from the spirit and scope of the invention.
Therefore, all matter
set forth or shown in the accompanying drawings is to be interpreted as
illustrative and
not in a limiting sense.
EXAMPLES
[0117] The following examples illustrate various iterations of the
invention.
Example 1 - Phosphorylation sites on tau protein in the normal non-AD human
brain
[0118] In order to determine phosphorylation sites of normal
soluble brain
tau, extracts from healthy controls were purified by immunocapture using Tau-1
and
HJ8.5 tau antibodies concentrating both phosphorylated and unphosphorylated
tau. The
tryptic digestion of brain tau isoforms generated 27 unmodified peptides that
were long
and hydrophobic enough to be detected by LC-MS (Barthelemy et al., 2016). 25
peptides contained serine and/or threonine as potential phosphorylation sites.
Several
peptides contained multiple potential phosphorylation sites (4-9) leading us
to consider
different mono-phosphorylated peptides potentially co-eluting together during
the LC
separation. After this, the PRM screening method described above was applied
to these
peptides.
57
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0119] Results from analysis of the projection domains in the tau
sequence
(103-126) revealed multiple p-tau peptides eluting into overlapping complex LC-
MS/MS
patterns (FIG. 3-7). We identified 3 phosphorylation sites from the ON
isoform, the
shortest isoform of the tau protein. Although the LC system did not have the
resolution
for differentiating the 3 phosphorylated peptides, fragment identification and
corresponding intensities were used to deconvolute a signal composed of one
major
phosphorylation site at residue S113, and two minor phosphorylation sites at
residues
T111 and T123 (FIG. 3). In contrast, when PRM screening tau sequences from the
longer 1N and 2N isoforms, further chromatographic separation was necessary to
simplify phosphorylated peptide elution patterns, especially when multiple
potential
mono-phosphorylation sites were predicted within the same peptide sequence
(FIG. 4-
6). LC separation allowed us to identify co-eluted, semi-specific MS/MS
fragments
which contained differentially phosphorylated residues. However, we have also
identified a few signals that could result from LC artifacts, likely due to
dual
conformations of the same peptide in solution (FIG. 5). This effect was
important for
peptide sequence containing amino acid residues 45-67 (in the ON isoform) for
both
unmodified and phosphorylated peptides (FIG. 5) and less prevalent for
sequences 68-
126 (1N) and 88-126 (2N) (FIG. 4 and 6).
[0120] Phosphorylation at residues S113, T111 and T123 were
confirmed
on peptide sequences 68-126 (1N) and 88-126 (2N). In all cases, the S113
signal was
the most abundant of the three with a phosphorylation rate of 0.2-0.5% (FIG. 4
and FIG.
6, Table 1). Phosphorylation at residues T50, T52 and S56 were clearly
identified on
the peptide sequence 45-67 (shared by 1N and 2N isoforms). On the same
peptide, LC-
MS signals indicated at least two of the three residues (S61, T63, and S64)
were
phosphorylated (FIG. 5). No specific signals were found to identify potential
phosphorylation at residue S46. Phosphorylation at residues S68 and T69 were
evidenced in sequences 68-126 (1N) and 68-87 (2N) but no specific fragments
were
detected to differentiate their LC-MS patterns. On the same 1N and 2N peptide
sequences, lower phosphorylation levels were detected on T71. 2N specific
58
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
phosphorylations on the T76, T101, and T102 residues were also identified
(summarized in FIG. 8).
[0121] Induction of trypsin missed cleavage was also considered for
the
screening of phosphorylated residues located after a tryptic site as reported
previously
(sites T175, T181, S212, T231 and S396) (Hanger et al. 1998). Our PRM
screening
successfully detected LC-MS patterns corresponding to phosphorylated tau
peptides
already described in normal brain tissue by Hanger et al (T181, S199, S202 and
S404.
FIG. 8). Corresponding LC-MS signals were high, suggesting that p-tau peptides
previously reported in normal human brains are likely the most abundant. Our
comparison of S199 and S202 phosphorylation indicated a much more prevalent
abundance of phosphorylation at S202 (FIG. 8). The use of an anti-Tau1
antibody for
tau extraction, associating with aa non-phosphorylated epitope on the 192-199
amino
acid sequence, could explain the low recovery of S199 phosphorylation in the
extract.
Given the abundance of S202 and S404 phosphorylation in the extract, we
searched for
the presence of di-phosphorylated peptides from sequences 195-209 and 386-406.
We
detected two LC-MS patterns with specific fragments corresponding to double
phosphorylation at S202/S199 and S202/S198 and one LC-MS pattern corresponding
to
a double phosphorylation at 396/404 (FIG. 8).
[0122] Additional screenings of soluble tau in brain extract
evidenced other
less abundant mono-phosphorylated peptides corresponding to phosphorylated
residues at T175, S214, T217, T231, and S396. Signals from fragments
corresponding
to a phosphorylated peptide at S184 or S185 were also detected at low levels
when the
mono-phosphorylated peptide sequence 181-190 was screened (FIG. 8). The search
for
mono-phosphorylation on the long sequence 407-438 discovered a pattern
consistent
with phosphorylation at residues S409 and S416 and at least one
phosphorylation on
the group of residues 5412/5413/T414.
[0123] Overall, we identified a minimum of 29 unique
phosphorylation sites
detectable in the soluble tau fraction extracted from normal non-AD brains
using the
PRM screening method (Table 1). 25 of them can be unambiguously assigned to
unique LC-MS signals and 4 additional phosphorylation sites were evidenced
without
59
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
assignment to the exact LC-MS patterns. These sites were located on three
clusters: a
minimum of 14 phosphorylated sites were located in the N-terminal projection
domain,
on the proline-rich domain in the middle of the sequence, and 6 on the C-
terminus
(FIG. 14).
Table 1 ¨ Brain/CSF pool phosphorylation rate comparison (HJ8.5+Tau1 IP-MS).
Brain
Brain
lysate Normal AD
Phosphorylated Peptide lysate
Isoform soluble
CSF CSF
site sequence soluble
(1m1 (500u1) (500u1)
(1m110X)
500X)
S46 45-67 1N/2N ? nd x x
T50 45-67 1N/2N 0.55 nd x x
T52 45-67 1N/2N 2.25 nd x x
S56 45-67 1N/2N 0.015 nd x x
S61/T63/S64
(2 sites 45-67 1N/2N 0.5/0.25 nd x x
minimum)
S68 68-126 1N 1.4/0.35 nd x x
68-87 2N 0.3/0.055 nd x x
T69 68-126 1N 1.4/0.35 nd x x
68-87 2N 0.3/0.055 nd x x
T71 68-126 1N 0.25 nd x x
68-87 2N 0.025 nd x x
T76 68-87 2N 0.015 nd x x
T95 88-126 2N ? nd x x
T101 88-126 2N 0.06-0.225 nd x x
T102 88-126 2N 0.06-0.225 nd x x
T111 103-126 ON 0.025 nd 0.855 8.155
68-126 1N 0.25 nd x x
88-126 2N 0.075 nd x x
S113 103-126 ON 0.25 nd x low
68-126 1N 0.535 nd x x
88-126 2N 0.595 nd x x
T123 103-126 ON 0.025 nd x x
68-126 1N 0.025 nd x x
88-126 2N X nd x x
T153 151-155 all X nd x
0.855
T175 mc 171-180 all nd 0.1*5 x 0.15
T181 mc 175-190 all nd 9.5*5 10.1*
13.3*
S184/S185 181-190 all 0.15 nd x x
S199 (1) 195-209 all 0.29 1.3*5 0.2*
0.2*
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
S202 195-209 all 3.9 9.7*5 2*555 1.5*
S199+S202 195-209 all 0.025 nd x x
S198+S202 195-209 all 0.015 nd x x
T205 195-209 all x x 2.3*55 4*55
S208 195-209 all x x 0.00155 0.00355
T212 210-221 all ? nd x
S214 212-221 all 0.14 0.085 0.04 0.07
T217 212-221 all 0.43 0.5*5 1.9*55 8.5*55
T231 mc 226-234 all nd 0.1*5 0.8*55 1.4*
S396 396-406 all 1.25 nd x x
S404 396-406 all 955 110* x x
S396+S404 mc 386-406 all nd nd x x
S409 407-438 all 0.35 nd x x
S411/S412/T413
(2 sites 407-438 all 1.25 nd x x
minimum)
S416 407-438 all 1.05 nd x x
Number of
29 9 12
unique sites
Values indicate phosphorylated/unphosphorylated ratio in %.
x: not detected.
*: measured using AQUA internal standards
nd: not determined
?: not confidently assigned
(1) likely underestimated due to lower recovery using Tau1 immunocapture.=
- value considered as normal/reference for the considered site
- hyperphosphorylation
- hyporphosphorylation
Example 2 - Phosphorylation sites on tau protein in the CSF
[0124] In comparison to the brain tau digest, CSF tau purification
using
tau-specific antibodies and digestion generated detectable peptides mainly
from the
mid-domain of the protein sequence (residues 150-221). Peptides were
detectable to a
lesser extent from the N-terminus, and almost no sequence was detectable from
the
microtubule binding repeat (MTBR) domain or the C-terminus of tau (Barthelemy
et al.,
2016; Sato et al., 2018). This difference in signal recovery may result from
tau
truncation during its release from neurons (Sato et al., 2018). The peptide
recovery of
this mid-domain was sufficient to monitor corresponding minor phosphorylated
isoforms
using the current PRM method. Conversely, a significant technological advance
in MS
61
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
method will be required to detect phosphorylated peptides in the MTBR and C-
terminal
domains.
[0125] PRM screening of tau phosphopeptides from normal control CSF
identified several phosphorylation sites in common with brain soluble tau at
T181 (not
shown), S199, S202 and T217 (FIG. 9). A low signal corresponding to pS214 was
also
detected (FIG. 9). A specific fragmentation pattern corresponding to pT205 was
identified in the CSF extract and was separated by chromatography from the
pS199/pS202 signals (FIG. 9).
[0126] To increase the probability of detecting additional
phosphorylated
sites in CSF tau, we analyzed CSF pools from AD patients with mild to moderate
dementia. We expected AD CSF pools would contain increased concentrations of
tau
as well as increased levels of phosphorylated tau. The same phosphorylated
residues
found in normal CSF (T181, S199, S202, T217 and T231) were detected in AD CSF.
Additionally, signals corresponding to pS113 and pT175 previously found in tau
from the
brain but not in the normal CSF were detected in AD CSF (FIG. 9, FIG. 10). A
LC-
MS/MS pattern containing specific fragments and distinct retention times from
S202/S199 phosphorylated peptides allowed the identification of a new
phosphorylated
peptide at S208. When we reexamined pS208 in normal CSF, we detected the
corresponding signal in low abundance. A LC-MS/MS pattern matching with a new
phosphorylated peptide at residue T153 was detected close to the level of
corresponding unphosphorylated peptide (FIG. 10). Careful reexamination of
brain
extract data suggested the presence of a low abundant signal corresponding to
this site.
Finally, we found specific signals corresponding to phosphorylation on the 103-
126
amino acid sequence from the ON isoform in AD CSF, indicating T111 as the main
phosphorylation site on this peptide, while S113 was barely phosphorylated
(FIG. 11).
Altogether, 12 phosphorylation sites were detected in CSF tau, two of which
were not
detectable in brain lysate (FIG. 12).
62
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
Example 3 - CSF p-tau abundance measurements highlight differences compared
to p-tau abundance in brain
[0127] In the brain, S404 was the most highly phosphorylated of the
examined phosphorylation sites (pS404/S404=110%, i.e. 52% of S404 is
phosphorylated). Thus, more than half of the brain tau was phosphorylated at
S404
compared to other highly phosphorylated sites at S202 and T181 (9.7% and 9.5%,
respectively). 1.3% of S199 was phosphorylated but this could be
underestimated due
to the inability of the Tau1 antibody used for extraction to bind its
corresponding
phosphorylated epitope (Liu et al., 1993). Phosphorylation on C-terminal sites
other
than S404 was found to be around 1%. On the N-terminus, T52, 568/T69, T50, and
S113 were also phosphorylated with abundances ranging from around 0.5% to 2%.
Other detected sites appeared to be phosphorylated at much lower levels
(<0.5%).
[0128] In addition to the unique detection of pT205 and pS208 in
CSF tau
but not brain, the relative phosphorylation abundance of certain other
phosphorylation
sites detected in brain tau were altered in comparison to control CSF tau
(FIG. 13).
Compared to brain, CSF tau phosphorylation was significantly lower at sites
pS199 (6-
fold decrease, p=0.008), pS202 (5 fold decrease, p=0.016), and pS214 (2 fold-
decrease, p=0.016). Conversely, CSF tau phosphorylation was significantly
higher at
sites pT217 (4-fold increase, p=0.016), pT231 (7-fold increase, p=0.016) and
pT111
from ON (16-fold increase). PT181 abundance was similar in the CSF and the
brain
(-10%). When measured in AD CSF, pT175 remained low (0.1-0.2% compared to 0.1%
in brain).
[0129] Brain and CSF tau have different truncation patterns: brain
tau
isoforms are mainly full length while CSF tau isoforms are truncated. Brain
and CSF tau
isoforms and corresponding peptides recovered after IP depend on the antibody
used
for the immunoprecipitation (Sato et al., 2018). Similarly, antibodies used to
immunoprecipitate tau could impact tau phosphorylation recovery. To evaluate
this
impact on p-tau recovery, we compared IP-MS results on phosphorylation rates
using
different antibodies (Tau13, HJ8.5, HJ8.7, Tau1 and Tau5) in addition to the
Tau1+HJ8.5 combination (FIG. 14). A significant decrease of pS199/S199 rate
was
63
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
observed when Tau1 or Tau1+HJ8.5 were used in brain and CSF. This suggests CSF
truncation between the N-terminus and the mid-domain could affect pS199
phosphorylation measurement compared to brain. PS199/S199 rate measured in
brain
and CSF with other antibodies appeared to be relatively similar.
Interestingly, some
reactivity was still observed for p5199 in brain extract and to a lesser
extent in CSF,
suggesting nonspecific binding of Tau1, though S199 phosphorylation is present
at the
end of the reported Tau1 epitope (192-199).
[0130] Since phosphatase activity during the post-mortem interval
(PM!)
before autopsy can decrease tau phosphorylation measured in brain extracts, we
investigated the impact of the PM! on phosphorylation rates commonly found in
CSF
and brain extracts. 10 brains samples (middle frontal gyrus) without tau
pathology
collected from participants with PM! ranging from 5 to 16 hours were analyzed.
None of
the brain extracts originally analyzed had a PM! of greater than 16 hours. We
did not
find significant association (Spearman test, 95% confidence interval, not
shown)
between PM! and phosphorylation rates measured on T181, S199, S202, S214,
T217,
T231 and S404 sites.
Example 4 - AD specific p-tau change in CSF
[0131] The increased CSF p-tau commonly reported in AD could be the
consequence of two potential effects: 1) the global increase of tau regardless
of its
phosphorylation status, or 2) increased hyperphosphorylation at specific
sites.
Normalizing p-tau signal or level using non-phosphorylated tau allows for
quantifying
changes in phosphorylation stoichiometry (i.e. hyper- or hypo-phosphorylation
compared to normal CSF or brain tau) occurring at specific sites independently
from the
global change in total tau. As we recently demonstrated, soluble tau
production is
increased in AD and correlates with amyloid plaques. Therefore, controlling
for not just
the amount, but the rate of phosphorylation is key to understand. pT181,
pT217, pT231,
pT205, pS208 and pS214 were hyperphosphorylated in AD CSF as was ON-specific
pT111 (FIG. 13). pT153 and pT175 were not detected in non-AD and likely
increased
slightly in AD. The sites showing significant hyperphosphorylation compared to
non-AD
were pT181 (p=0.010), pS214 (p=0.005), and pT217 (p=0.003). pT181, pS214, and
64
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
pT217 showed approximately 1.2-fold, 1.6-fold, and 4-fold increases in
phosphorylation
respectively. Interestingly, not all the monitored sites were
hyperphosphorylated:
p5199/S199 was not significantly changed and pS202/S202 was significantly
lower
(p=0.030) with an approximately 1.2-fold decrease.
[0132] Robustness of phosphorylation ratios was assessed by
incubating
CSF for 16 hours. Incubation did not significantly affect tau phosphorylation
ratio
differences observed between non-AD and AD CSF (FIG. 15). Moreover, absence of
kinase activity on CSF tau was confirmed by the non-detectability of
phosphorylation on
recombinant 15N-tau spiked in CSF after incubation (not shown).
Discussion for Examples 1-4
[0133] Technological advancement of PRM on p-tau measurement - We
report the most comprehensive qualitative and quantitative analyses of p-tau
in normal
brain tissue and CSF to date. Our approach contrasts with previous DDA studies
on tau
phosphorylation which may not have captured potential minor phosphorylation
sites.
However, our approach uses highly sensitive targeted-MS in PRM mode to detect
and
quantify minor phosphorylation. Phosphorylation site identification depends on
the
careful manual interpretation of LC-MS/MS patterns to identify co-elution of
specific ion
fragments for each examined phosphopeptide. In prior studies, brain tau
phosphorylation has been mainly investigated in insoluble extracts enriched in
hyperphosphorylated tau from PHF and, to date, the most detailed study has
reported 9
phosphorylation sites in normal human tau protein (Hanger et al. 2007). Our in-
depth
PRM data analysis detected more than 29 phosphorylated residues with a
majority
being very low in abundance. Indeed, the previously reported 9 sites were
amongst the
most abundant modifications in the protein. Application of PRM analysis to
normal CSF
tau, present in much lower abundance compared to the brain, led to the
detection of 9
phosphorylation sites initially, with 3 additional sites detected in AD CSF.
[0134] In addition to the significantly increased number of
phosphorylation
sites detected, we demonstrated how sensitive PRM screening enabled us to
quantitatively assess the tau phosphorylation rate or stoichiometry,
independent of
global tau concentration. This concept is frequently overlooked but critical
in assessing
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
changes in AD when absolute CSF p-tau concentration may increase solely due to
increase in global tau isoforms concentration, and not due to change of
relative
phosphorylated tau abundance. Phosphorylation rate measurement from this study
enabled for the first time the comparison of the degree and distribution of
phosphorylation rate changes (hyper- vs hypo-phosphorylation) across the
protein, in
different compartments (intracellular brain vs extracellular CSF), and in
different
pathological conditions (AD vs non-AD).
[0135] Some caveats of the PRM screening process are the low
throughput for discovery and the risk of missing p-tau species or other post-
translational
modification (PTM) not hypothesized in the study. Identifications from MS data
can be
further used in a larger validation or clinical cohort to design scheduled LC-
MS
methods, increasing multiplexing and throughput (Gillette and Carr, 2013).
Other
proteases such as AspN could provide a different set of tau and p-tau peptides
than
from trypsin digest, allowing for a better coverage of tau, i.e. the C-
terminal domain
(Hanger et al. 1998; Sato et al. 2018). The search could be further refined by
screening
additional doubly- or triply-phosphorylated tau peptides not considered in
this study,
although their abundance may be minimal unless there is biological
coordination of site
phosphorylation.
[0136] Identification of a cluster of phosphorylation sites on the
tau
projection domain - We found a cluster of previously undescribed
phosphorylated
residues on the N-terminus projection domain of tau containing alternative
splicing-
dependent peptides. Interestingly, this domain was not previously found to be
extensively phosphorylated in PHF in the insoluble human brain fraction (Funk
et al.,
2014; Hanger et al., 2007; Russell et al., 2016; Thomas et al., 2012). This
cluster was
also not extensively characterized in the recent comprehensive study performed
in
mouse tau protein in the murine brain (Morris et al., 2015). These
discrepancies could
be attributed to the difficulty of characterizing complex mixtures of
phosphorylated
peptides, distinguishable only by few specific MS/MS fragments and/or subtle
retention
time shifting on LC. For example, S46 is the only phosphorylated site
previously
reported in this domain in normal human tau (Hanger et al. 1998). However, we
did not
66
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
detect a specific signal for this species, which can be confounded by search
algorithms
with neighboring phosphorylated sites at T50 or T52, sharing a close
fragmentation
pattern. In this regard, manual inspection is essential to clearly interpret
and decipher
corresponding LC-MS/MS patterns for each phosphorylated site. Another possible
explanation of this discrepancy could be the relatively low abundance of the N-
terminal
domain in PHF in comparison to the MTBR domain, the mid-domain, and the C-
terminus (Mair et al., 2016).
[0137] This N-terminus projection domain has been recently assigned
as a
part of the dominant component of the repulsive barrier that prevents
neighboring
microtubules (associated to tau via the MTBR domain) from getting close to
each other
(Chung et al., 2016). This sequence contains numerous acidic residues and an
increase
in phosphorylation may contribute to increased global acidity, enforcing the
repulsive
barrier. Together with a variable amount of N-terminal extension induced by
alternative
splicing on exon 2-3, tau phosphorylation could regulate tau/tau N-terminal
interactions
and microtubule intermolecular distance.
[0138] Biological implications of different p-tau profiles in the
brain vs CSF
- Tau is mainly an intracellular protein that functions in microtubule
stability, and was
traditionally considered to only be released extracellularly upon nerve injury
or cell
death. However, recent studies have suggested that tau is secreted under
physiological
and pathological conditions in a regulated manner (Karch et al. 2012; Yamada
et al.
2014). By comparing soluble brain tau and CSF tau profiles in parallel to
intracellular
and extracellular tau profiles and metabolism in neuronal models, we have
recently
shown that tau secretion is an active process that involves different turnover
rates of tau
isoforms including truncated tau and p-tau (Sato et al., 2018). Understanding
the
association between this active secretion and phosphorylation of tau through
comparison between brain and CSF p-tau profiles provides potential insight
into AD
pathogenesis.
[0139] We speculate that p-tau isoforms enriched in the CSF have
less
affinity for microtubules and a higher likelihood to be secreted. Inversely, p-
tau isoforms
impoverished in the CSF could have more propensity to stay inside the neurons
and/or
67
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
avoid cleavage. Our results demonstrate that several phosphorylated residues
are
significantly enriched in the CSF compared to brain extracts, such as T217,
T231, T153
and T111. All are proline-directed sites, are potential substrates of GSK-3[3
protein
kinase, and may be subject to kinase-dependent regulation. Unlike pT217, pS214
was
not elevated in the CSF compared to the brain. This extracellular enrichment
of pT217
over pS214 agreed with kinetic differences we previously identified within
cells for these
isoforms (Sato et al., 2018), with pT217 having a shorter turnover rate than
unphosphorylated tau and tau-pS214. Alternatively, decreased phosphorylation
observed on some of the tau sites in brain extract compared to CSF could also
result
from partial dephosphorylation occurring during the PM! (Matsuo et al., 1994).
Though
such a decrease was not observed within the 5-16 hour PM! range, modification
of
phosphorylation ratios between death and this investigated interval cannot be
excluded.
Assessing the kinetics of dephosphorylation of these tau sites from brain
biopsies by
mass spectrometry would help to address the impact of this phenomenon on
CSF/brain
comparison results in the future. The consideration of potential brain
phosphatase bias
affecting brain tau phosphorylation measurement would support that CSF tau
phosphorylation status is more likely to reflect the in vivo tau
phosphorylation status in
neurons than brain extracts collected post mortem. However, in vivo tau
phosphorylation monitoring in CSF would be limited to sites detected mainly in
the tau
mid-domain due to CSF tau truncation.
[0140] Conversely, pS202 was significantly lower in the CSF and
pS199
and pS202 were not elevated in AD, indicating that these phosphorylations may
promote tau sequestration inside neurons. T181 is equally phosphorylated in
the CSF
and the brain. pT205 and pS208 were exclusively detected in CSF tau and not in
soluble tau protein in the brain. This is interesting considering that the
triple
phosphorylation at S202, T205 and S208 is recognized by the anti-AT8 antibody
(Malia
et al., 2016) commonly used to characterize Braak stages of tau aggregation in
brain
(Braak and Braak, 1995). Indeed, pS208 was detected by MS in PHF (Hanger et
al.
1998). pT205 and pS208 were absent in normal soluble brain tau in our study,
confirming the unlikeliness to detect immunoreactivity of AT8 in normal brain
tau.
68
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
Furthermore, a recent study implicates pT205 and pS208 as a combinational
phosphorylation pattern that, together with pS202, leads to tau self-
aggregation
(Despres et al., 2017). Exclusive presence of pT205 and pS208 in the CSF and
further
increased rates of phosphorylation at both sites in AD could indicate a
potential
protective clearance mechanism for neurons to remove these pathology-prone p-
tau
species from the cells. Simultaneous decrease of pS202 in AD CSF could also
correspond to an increased sequestration of associated isoforms when
aggregated with
pT205 and pS208 in enriched AT8-positive tangles in the AD brain.
Alternatively,
absence of phosphorylation on pT205 and pS208 in brain extracts could result
from
their specific degradation by phosphatases occurring during the PMI. The rapid
disappearance (<3 hours) of AT8 reactivity reported on tau, collected from
brain biopsy,
supports this idea (Matsuo et al., 1994). Thus, such phosphatase activity
efficiently
targeting pT205 and pS208 could also be seen as an additional mechanism
preventing
long half-life of AT8-positive material in neurons. The increase of pT205 and
pS208
found in AD CSF would then indicate potentially pathological decreasing of
this
protective mechanism. Finally, simultaneous decrease of pS202 in AD CSF could
also
correspond to an increased sequestration of associated isoforms when
aggregated with
pT205 and pS208 in enriched AT8-positive tangles in the AD brain.
[0141] We were unable to provide convincing detection of
phosphorylated
residues from the MTBR domain in the soluble fraction from normal brain.
Phosphorylation sites on the MTBR domain have been purported to reduce the
affinity
of tau to microtubules (Biernat et al., 1993). The absence of such
phosphorylation in
normal tau could support the abnormality of these modifications found in PHF
(Hanger
et al. 2007). CSF tau truncation restricts the examination of phosphorylation
changes in
vivo since the MTBR domain likely degraded within the cell after tau
truncation
(Kanmert et al., 2015), and so was not recovered by the immuno-capture method
used
in this study.
[0142] CSF p-tau rates as AD biomarkers - In addition to the
significantly
increased number of phosphorylation sites detected, we demonstrated how tau
phosphorylation rate or stoichiometry can be quantified independent of global
tau
69
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
concentration. This concept is frequently overlooked but critical in assessing
CSF tau
phosphorylation changes in AD when absolute CSF p-tau concentration may
increase
solely due to increased total tau concentration (i.e. pT181), and not due to
an increase
in the relative phosphorylation rate itself. Phosphorylation rate measurements
from this
study enabled for the first time the comparison of the degree and distribution
of
phosphorylation rate changes (hyper- vs hypo-phosphorylation) across the
protein, in
different compartments, (intracellular brain vs extracellular CSF) and in
different
pathological conditions (AD vs non-AD). This method could be used in the
future to look
at modifications of AD brain phosphorylations on numerous sites across brain
regions
and Braak stages as recently performed using immunochemistry (Neddens et al.
2018).
[0143] In this study, we demonstrated that tau in AD CSF is
generally
hyperphosphorylated in comparison to non-AD CSF. However, the degree of
hyperphosphorylation is site dependent. T111, T205, S208 and T217 were more
hyperphosphorylated than T181, which is the most commonly measured target used
as
a p-tau biomarker for AD (Fagan et al. 2009). We have also identified T153
phosphorylation that was exclusively found in AD CSF. Interestingly, the sites
found
hyperphosphorylated in AD CSF correspond to the sites already significantly
increased
in normal CSF compared to the brain (i.e., T111, T205, S208, and T217, and to
a lesser
extent T231). This would indicate AD pathology exacerbates cellular mechanisms
contributing to specific p-tau isoforms enrichment during tau release into the
CSF.
Overall, the high magnitude of change found on these sites lets us envision
their use as
sensitive biomarkers to detect AD. Our study relied on a limited number of CSF
pools
and future studies on larger CSF cohorts would better establish potential
relationships
between p-tau changes and brain amyloidosis and tau aggregation over the
course of
the disease. We can also ask whether there are any specific p-tau profile
changes in
other non-AD tauopathies such as PSP, CBD, and FTD. Alternatively, this method
could
be used to track different kinase activities in vivo and in vitro and may also
provide a
promising tool to assess new drugs targeting abnormal tau metabolism.
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
Methods for Examples 1-4
[0144] Brain soluble tau extraction - Brain and CSF studies
involving
participants were approved by the Washington University Human Studies
Committee
and the General Clinical Research Center. Written informed consent was
obtained from
all participants prior to inclusion in the study. Brain soluble tau was
extracted as
described previously (Sato et al., 2018). Briefly, frozen human brain tissues
from
controls without amyloid and tau pathologies as described before (Sato et al.,
2018)
were obtained from Knight Alzheimer's Disease Research Center (ADRC) at
Washington University School of Medicine (St. Louis, MO). Sarkosyl-soluble tau
was
separated from putative tau aggregates by ultracentrifugation as reported in
the
literature (Hanger et al. 1998) and pooled. Frozen human brain (200-400 mg,
frontal
regions) were homogenized in Tris-HCI buffer (25mM Tris-HCI, 150mM NaCI, 10mM
EDTA, 10mM EGTA, 1mMDTT, phosphatase inhibitor Cocktail 3, Roche Protease
Inhibitor, pH 7.4. Final 3.25mL/mg tissue) on ice. Homogenates were
centrifuged at 4 C
for 60 minutes at 11,000xg. The supernatant was solubilized in 1% Sarkosyl for
60min
and centrifuged for 2hrs at 100,000xg. Sarkosyl soluble fractions were pooled
and 50uL
fraction was diluted 10 times with 0.5% human plasma before
immunoprecipitation. For
brain/CSF comparison, brain lysate pool was diluted from 500 to 8000 times
before
immunopurification to match CSF tau levels.
[0145] CSF tau extraction - Human CSF was pooled from a cohort of
80
participants, including amyloid negative and cognitively normal (CDR=0)
controls (n=47,
age 60+) and amyloid positive and CDR>0 AD patients (n=33, age 60+). Five and
seven pools of 500uL CSF aliquots were generated from the control and AD
groups,
respectively. At time of initial collection, CSF was spun down at 1,000xg for
10min to
remove cell debris and immediately frozen at -80 C. Protease inhibitor
cocktail was
added during experiments. Tau was immunoprecipitated and desalted as
previously
described with some modifications (Sato et al., 2018). Briefly, CNBr-activated
Sepharose beads (GE Healthcare 17-0430-01) were crosslinked to antibodies Tau1
and
HJ8.5, separately at a concentration of 3mg antibody per g of beads. Samples
are
spiked with AQUA peptides (ThermoFisher Scientific) corresponding to 10 fmol
71
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
phosphorylated and 100 fmol unphosphorylated tau for each sequence of interest
per
mL of sample. Tau and p-tau concentration is calculated using these internal
standards.
Soluble tau was immunoprecipitated in detergent (1% NP-40), chaotropic reagent
(5mM
guanidine), and protease inhibitors (Roche Complete Protease Inhibitor
Cocktail). Anti-
Tau1 and HJ8.5 antibodies conjugated to sepharose beads were diluted 10 and 5-
fold,
respectively, in inactivated sepharose beads, and 30uL of 50% slurry of the
antibody
beads were rotated with the solution for 90min at room temperature. The beads
were
washed three times in 25mM triethyl ammonium bicarbonate buffer (TEABC, Fluka
17902). The bound tau was digested on-beads with 400ng MS grade trypsin
(Promega,
V5111) for 16 hours at 37 C. Digests were loaded onto TopTip C18 (Glygen,
TT2C18.96), desalted, and eluted per manufacturer's instructions. The eluted
peptides
were dried by vacuum centrifugation (CentriVap Concentrator Labconco) and were
resuspended in 25uL of a solution of 2% acetonitrile and 0.1% formic acid in
MS grade
water.
[0146] Mass spectrometry - A 5uL aliquot of the peptide
resuspension was
injected into nano-Acquity LC for MS analysis. The nano-Acquity LC (Waters
Corporation, Milford, MA) was fitted with HSS T3 75umx 100um, 1.8um column and
a
flow rate of 0.5 uL/m in of a gradient of solution A and B was used to
separate the
peptides. Solution A was composed of 0.1% formic acid in MS grade water and
solution
B was composed of 0.1% formic acid in acetonitrile. Peptides were eluted from
the
column with a gradient of 2% to 20% of solution B in 28 minutes, then 20% to
40%
solution B for another 13 minutes before ramping up to 85% solution B in
another 3
minutes to clean the column. The Orbitrap Fusion Lumos was equipped with a
Nanospray Flex electrospray ion source (Thermo Fisher Scientific, San Jose,
CA).
Peptide ions sprayed from a 10um SilicaTip emitter (New Objective, Woburn, Ma)
into
the ion¨source were targeted and isolated in the quadrupole. These were then
fragmented by HCD and ion fragments were detected in the Orbitrap (resolution
of
60,000, mass range 150-1200 m/z). Monitoring of hydrophilic peptides (SSRcalc
< 9, all
without leucine) for peptide profiling was performed on a HSS T3 300um x
100um,
72
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
1.8mm column at a flow rate of 4u1/min with an elution occurring with a 2%to
12%
solution B gradient and a spray operating on a 30mm SilicaTip emitter.
[0147] Principles of PRM for p-tau peptides discovery - Tau
peptides
containing hypothesized phosphorylation sites were generated by digestion of
protein
from a tau-enriched biological extract such as human brain soluble fraction.
These
peptides are screened by targeted-MS analysis using a quadrupole-orbitrap
instrument,
such as the Thermo Fisher Orbitrap Lumos. For each phosphorylated peptide, the
selection of targeted-MS parameters such as precursor mass, collision energy,
or
expected retention time is performed in silico and refined using biophysical
properties
measured for corresponding unmodified peptides that are much more abundant in
the
sample. For detecting phosphorylated peptides, the quadrupole is set to select
a mass
window including the hypothesized precursor mass. After precursor collision,
all
generated fragments are simultaneously measured in the Orbitrap over the time
of
chromatographic elution. Potentially phosphorylated peptides can be searched
in post
analysis of the generated data. Skyline software (MacCoss Lab, University of
Washington, WA) was used to extract LC-MS/MS data. The hypothetical peptide
being
screened is detected as a LC-MS/MS fingerprint constituted by the strict co-
elution of
the extracted masses from predicted MS/MS fragments (FIG. 2). The advantage of
the
PRM method over classic DDA or targeted-MS methods is its ability to use the
MS
instrument in the highest sensitive configuration possible and conserve
discovery
capability.
[0148] Maximum sensitivity of the Quadrupole-Orbitrap instrument
during
the screening is essential to detect minor ion fragments and facilitates the
identification
of phosphorylated peptide together with the localization of phosphorylated
sites.
Sensitivity of the PRM measurement depends mainly on the number of ions from
the
target of interest transferred into the Orbitrap analyzer. This number was
enhanced by
increasing the fill time used to acquire one MS/HRMS scan. The fill time
corresponds to
the time spent to accumulate the targeted precursor mass from the ion beam
into the
ion trap device. Accumulated precursors are then fragmented and product
fragments
are transferred into the Orbitrap to be analyzed. Thus, fill time is set to a
maximum
73
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
limited by the need for a sufficient MS scan rate to acquire enough data
points to
describe the chromatogram signal (i.e. 8-15 scans per chromatographic peak).
Fill time
for PRM screening on each investigated phosphorylated peptides was typically
set to
lsecond.
[0149] Fill time is also limited by risk of trap saturation. This
occurs when
too many ions are sampled within the same scan and transferred to the
Orbitrap,
leading to inaccurate mass measurement due to space charge effects. To avoid
such
saturation, narrow precursor isolation (0.7Da) together with appropriate
sample
purification (immuno purification) enriching the target over matrix were
chosen to
decrease the contribution of potential near-isobaric interferences.
[0150] Specificity of the PRM discovery experiment depends on the
resolving power of the LC-Q Orbitrap system. Resolving power can be improved
by
different analytical parameters, with the ultimate goal to obtain interference-
free LC-
MS/MS fingerprints for the targeted peptides. This limits the risk of
ambiguous fragment
assignment due to false positive signals. Sample purification preferentially
enriching p-
tau can also improve the specificity in the discovery of minor phosphorylated
tau
peptides. High chromatographic peak capacity and resolution limit the
likeliness of co-
elution. A narrow quadrupole isolation window for the precursor decreases the
probability of interference on the MS/MS spectrum together with limiting the
risk of trap
saturation as described above. High Orbitrap resolution and analyzer
calibration allow
the accurate extraction of mass fragments during data processing limiting, the
risk of
transitions interference (Gallien et al., 2012; Peterson et al., 2012). The
choice of
Orbitrap resolution (60k) is a balance between high resolution requiring more
acquisition
time and reasonable scan rates compatible with the chromatographic
acquisition.
[0151] Quantitative assessment of site specific phosphorylation
rate of tau
- To quantitatively assess the relative abundance of phosphorylation of
specific sites in
tau from the brain and CSF, we measured the extent of phosphorylation on each
site
detected. We used three methods for this purpose: 1) Relative comparison
between
phosphorylated peptide isomers: Signal comparison from transition ions are
used to
identify each phosphorylated site. This can estimate the relative abundance of
each
74
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
phosphorylated peptides sharing the same sequence. 2) Phosphorylated peptides
are
normalized with the non-phosphorylated peptide as reference: LC-MS/MS
transition
specific to each phosphorylated peptide is compared to the corresponding
transition
from the non-phosphorylated peptide. Each phosphorylated site ratio obtained
can be
compared across the protein sequence. This strategy may be biased by
difference in
fragmentation efficiency between the non-phosphorylated and the phosphorylated
peptides. This method cannot be applied when the phosphorylated sites are part
of a
tryptic missed cleavage. 3) Absolute quantitation using internal synthetic
labeled
standards (e.g. AQUA) for each phosphorylated and non-phosphorylated peptide:
Signals from phosphorylated and non-phosphorylated standards are used to
define an
internal ratio. This strategy takes into account the fragmentation specificity
of each
compared peptide but requires peptides synthesis for each monitored species.
[0152] In this study, except for those including missed trypsin
cleavage,
the second method was utilized to calculate phosphorylation rates. We also
applied the
third method (AQUA normalization) on a limited set of phosphorylated sites
found in
both brain and CSF extracts when synthetic phosphorylated peptides were
available
(i.e. T175, T181, S199, S202, T205, T217 and T231) and one site found only in
brain
(S404) (Table 1). For AQUA measurement, brain extracts were diluted 500 to
8000
times to be comparable to CSF tau levels. This dilution minimized the matrix
effect that
may results from significantly different ratio of AQUA internal standard to
tau peptide
levels in the brain versus CSF. The first method was used for initial
interpretation of the
complex LC-MS/MS patterns from p-tau sequence containing numerous
phosphorylated
residues.
[0153] Statistics - Data are represented as mean SD, unless
otherwise
specified. After confirming the normal distribution of the data, one-way ANOVA
followed
by post hoc analyses (Tukey test) were performed for comparing tau
phosphorylation
rates. Additional statistical analysis was completed using GraphPad version
8Ø1 (244)
from GraphPad Software Inc. Statistical significance between relevant groups
was
determined with a 2-tailed, unpaired, Mann-Whitney t-test. Significance was
evaluated
at the 0.01 and 0.05 level.
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
References for Examples 1-4
[0154] Alonso, A. del C., Zaidi, T., Novak, M., Grundke-lqbal, I.,
and lqbal,
K. (2001). Hyperphosphorylation induces self-assembly of T into tangles of
paired helical
filaments/straight filaments. PNAS 98, 6923-6928. doi:10.1073/pnas.121119298.
[0155] Augustinack, J. C., Schneider, A., Mandelkow, E.-M., and
Hyman,
B. T. (2002). Specific tau phosphorylation sites correlate with severity of
neuronal
cytopathology in Alzheimer's disease. Acta Neuropathol 103, 26-35.
doi:10.1007/s004010100423.
[0156] Barthelemy, N. R., Fenaille, F., Hirtz, C., Sergeant, N.,
Schraen-
Maschke, S., Vialaret, J., et al. (2016). Tau Protein Quantification in Human
Cerebrospinal Fluid by Targeted Mass Spectrometry at High Sequence Coverage
Provides Insights into Its Primary Structure Heterogeneity. J. Proteome Res.
15, 667-
676. doi:10.1021/acs.jproteome.5b01001.
[0157] Biernat, J., Gustke, N., Drewes, G., Mandelkow, E.-, and
Mandelkow, E. (1993). Phosphorylation of 5er262 strongly reduces binding of
tau to
microtubules: Distinction between PHF-like immunoreactivity and microtubule
binding.
Neuron 11, 153-163. doi:10.1016/0896-6273(93)90279-Z.
[0158] Braak, H., and Braak, E. (1995). Staging of alzheimer's
disease-
related neurofibrillary changes. Neurobiology of Aging 16, 271-278.
doi:10.1016/0197-
4580(95)00021-6.
[0159] Chung, P. J., Song, C., Deek, J., Miller, H. P., Li, Y.,
Choi, M. C., et
al. (2016). Tau mediates microtubule bundle architectures mimicking fascicles
of
microtubules found in the axon initial segment. Nature Communications 7,
12278.
doi:10.1038/ncomms12278.
[0160] Despres, C., Byrne, C., Qi, H., Cantrelle, F.-X., Huvent,
I.,
Chambraud, B., et al. (2017). Identification of the Tau phosphorylation
pattern that
drives its aggregation. PNAS 114, 9080-9085. doi:10.1073/pnas.1708448114.
[0161] Funk, K. E., Thomas, S. N., Schafer, K. N., Cooper, G. L.,
Liao, Z.,
Clark, D. J., et al. (2014). Lysine methylation is an endogenous post-
translational
76
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
modification of tau protein in human brain and a modulator of aggregation
propensity.
Biochem J 462, 77-88. doi:10.1042/BJ20140372.
[0162] Gallien, S., Bourmaud, A., Kim, S. Y., and Domon, B. (2014).
Technical considerations for large-scale parallel reaction monitoring
analysis. Journal of
Proteomics 100, 147-159. doi:10.1016/j.jprot.2013.10.029.
[0163] Gallien, S., Duriez, E., Crone, C., Kellmann, M., Moehring,
T., and
Domon, B. (2012). Targeted Proteomic Quantification on Quadrupole-Orbitrap
Mass
Spectrometer. Molecular & Cellular Proteomics 11, 1709-1723.
doi:10.1074/mcp.0112.019802.
[0164] Gillette, M. A., and Carr, S. A. (2013). Quantitative
analysis of
peptides and proteins in biomedicine by targeted mass spectrometry. Nature
Methods
10, 28-34. doi:10.1038/nmeth.2309.
[0165] Hanger, D. P., Betts, J. C., Loviny, T. L., Blackstock, W.
P., and
Anderton, B. H. (1998a). New phosphorylation sites identified in
hyperphosphorylated
tau (paired helical filament-tau) from Alzheimer's disease brain using
nanoelectrospray
mass spectrometry. J. Neurochem. 71, 2465-2476.
[0166] Hanger, D. P., Betts, J. C., Loviny, T. L. F., Blackstock,
W. P., and
Anderton, B. H. (1998b). New Phosphorylation Sites Identified in
Hyperphosphorylated
Tau (Paired Helical Filament-Tau) from Alzheimer's Disease Brain Using
Nanoelectrospray Mass Spectrometry. Journal of Neurochemistry 71, 2465-2476.
doi:10.1046/j.1471-4159.1998.71062465.x.
[0167] Hanger, D. P., Byers, H. L., Wray, S., Leung, K.-Y., Saxton,
M. J.,
Seereeram, A., et al. (2007). Novel Phosphorylation Sites in Tau from
Alzheimer Brain
Support a Role for Casein Kinase 1 in Disease Pathogenesis. Journal of
Biological
Chemistry 282, 23645-23654. doi:10.1074/jbc.M703269200.
[0168] Hasegawa, M., Morishima-Kawashima, M., Takio, K., Suzuki,
M.,
Titani, K., and lhara, Y. (1992). Protein sequence and mass spectrometric
analyses of
tau in the Alzheimer's disease brain. J. Biol. Chem. 267, 17047-17054.
77
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0169] lqbal, K., Alonso, A. del C., Chen, S., Chohan, M. 0., El-
Akkad, E.,
Gong, C.-X., et al. (2005). Tau pathology in Alzheimer disease and other
tauopathies.
Biochim. Biophys. Acta 1739, 198-210. doi:10.1016/j.bbadis.2004.09.008.
[0170] Kanmert, D., CantIon, A., Muratore, C. R., Jin, M.,
O'Malley, T. T.,
Lee, G., et al. (2015). C-Terminally Truncated Forms of Tau, But Not Full-
Length Tau or
Its C-Terminal Fragments, Are Released from Neurons Independently of Cell
Death. J.
Neurosci. 35, 10851-10865. doi:10.1523/JNEUROSCI.0387-15.2015.
[0171] Karch, C. M., Jeng, A. T., and Goate, A. M. (2012).
Extracellular
Tau Levels Are Influenced by Variability in Tau That Is Associated with
Tauopathies. J.
Biol. Chem. 287, 42751-42762. doi:10.1074/jbc.M112.380642.
[0172] Kbpke, E., Tung, Y. C., Shaikh, S., Alonso, A. C., lqbal,
K., and
Grundke-lqbal, I. (1993). Microtubule-associated protein tau. Abnormal
phosphorylation
of a non-paired helical filament pool in Alzheimer disease. J. Biol. Chem.
268, 24374-
24384.
[0173] Liu, W.-K., Moore, W. T., Williams, R. T., Hall, F. L., and
Yen, S.-H.
(1993). Application of synthetic phospho- and unphospho- peptides to identify
phosphorylation sites in a subregion of the tau molecule, which is modified in
Alzheimer's disease. Journal of Neuroscience Research 34, 371-376.
doi:10.1002/jnr.490340315.
[0174] Mair, W., Muntel, J., Tepper, K., Tang, S., Biernat, J.,
Seeley, W.
W., et al. (2016). FLEXITau: Quantifying Post-translational Modifications of
Tau Protein
in Vitro and in Human Disease. Anal. Chem. 88, 3704-3714.
doi:10.1021/acs.analchem.5b04509.
[0175] Malia, T. J., Teplyakov, A., Ernst, R., Wu, S.-J., Lacy, E.
R., Liu, X.,
et al. (2016). Epitope mapping and structural basis for the recognition of
phosphorylated
tau by the anti-tau antibody AT8. Proteins: Structure, Function, and
Bioinformatics 84,
427-434. doi:10.1002/prot.24988.
[0176] Matsuo, E.S., Shin, R-W., Billingsley, M.L., Van deVoorde,
A.,
O'Connor, M., Trojanowski, J.Q., et al. (1994) Biopsy-derived adult brain tau
is
78
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
phosphorylated at many of the same sites as Alzheimer's disease paired
helical
filament tau. Neuron 13, 989-1002.
[0177] Morris, M., Knudsen, G. M., Maeda, S., Trinidad, J. C.,
loanoviciu,
A., Burlingame, A. L., et al. (2015). Tau post-translational modifications in
wild-type and
human amyloid precursor protein transgenic mice. Nature Neuroscience 18, 1183-
1189. doi:10.1038/nn.4067.
[0178] Neddens, J., Temmel, M., Flunkert, S., Kerschbaumer, B.,
HoeIler
C., Loeffler, T. et al. (2018). Phosphorylation of different tau sites during
progression of
Alzheimer's disease. Acta Neuropathogica Communications 6, 52.
doi:10.1186/s40478-
018-0557-6
[0179] Peterson, A. C., Russell, J. D., Bailey, D. J., Westphall,
M. S., and
Coon, J. J. (2012). Parallel Reaction Monitoring for High Resolution and High
Mass
Accuracy Quantitative, Targeted Proteomics. Molecular & Cellular Proteomics
11,
1475-1488. doi:10.1074/mcp.0112.020131.
[0180] Russell, C. L., Mitra, V., Hansson, K., Blennow, K., Gobom,
J.,
Zetterberg, H., et al. (2016). Comprehensive Quantitative Profiling of Tau and
Phosphorylated Tau Peptides in Cerebrospinal Fluid by Mass Spectrometry
Provides
New Biomarker Candidates. Journal of Alzheimer's Disease 55, 303-313.
doi:10.3233/JAD-160633.
[0181] Sato, C., Barthelemy, N. R., Mawuenyega, K. G., Patterson,
B. W.,
Gordon, B. A., Jockel-Balsarotti, J., et al. (2018). Tau Kinetics in Neurons
and the
Human Central Nervous System. Neuron 97, 1284-1298.e7.
doi:10.1016/j.neuron.2018.02.015.
[0182] Sch011, M., Lockhart, S. N., Schonhaut, D. R., O'Neil, J.
P., Janabi,
M., Ossenkoppele, R., et al. (2016). PET Imaging of Tau Deposition in the
Aging
Human Brain. Neuron 89, 971-982. doi:10.1016/j.neuron.2016.01.028.
[0183] Thomas, S. N., Funk, K. E., Wan, Y., Liao, Z., Davies, P.,
Kuret, J.,
et al. (2012). Dual modification of Alzheimer's disease PHF-tau protein by
lysine
methylation and ubiquitylation: a mass spectrometry approach. Acta Neuropathol
123,
105-117. doi:10.1007/s00401-011-0893-0.
79
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0184] Villemagne, V. L., Fodero-Tavoletti, M. T., Masters, C. L.,
and
Rowe, C. C. (2015). Tau imaging: early progress and future directions. The
Lancet
Neurology 14, 114-124. doi:10.1016/S1474-4422(14)70252-2.
[0185] Wang, Y., and Mandelkow, E. (2016). Tau in physiology and
pathology. Nat Rev Neurosci 17, 22-35. doi:10.1038/nrn.2015.1.
[0186] Yamada, K., HoIth, J. K., Liao, F., Stewart, F. R., Mahan,
T. E.,
Jiang, H., et al. (2014). Neuronal activity regulates extracellular tau in
vivo. J. Exp. Med.
211, 387-393. doi:10.1084/jem.20131685.
Example 5: Cerebral amyloid pathology is associated with site-specific
differences in tau hyperphosphorylation
[0187] The standard uptake value ratio (SUVR) of cortical PiB-PET
reliably
identifies significant cortical AR-plaques and is used to classify subjects as
PIB positive
(Amyloid +, SUVR 1.25) or negative (Amyloid SUVR < 1.25). To explore the
relationship of amyloid plaques and soluble tau species, we compared the SUVR
of
cortical PiB-PET with CSF total tau and with phosphorylation of CSF tau at
multiple
sites (i.e. ratio of phosphorylated to unphosphoryated sites of tau). (FIG.
16A)
Phosphorylation of tau at Thr217 (p-T217) had a 97.2% area under the curve
(AUC)
(95% Confidence Interval (CI) of 0.94, 0.99)); phosphorylation of tau at
Thr181 (p-T181)
had an 89.1% AUC (CI 0.83, 0.94); phosphorylation of tau at Thr205 (p-T205)
had a
74.5% AUC (CI 0.69, 0.82); and total tau had a 72% AUC (CI 0.65, 0.79) to
classify
mutation carriers (MC) as having cortical Ap-plaques (i.e., Amyloid +). These
data
indicate that at the early stages of significant fibrillar Ap positive
plaques, an increase of
phosphorylation has already begun at specific sites linking these two
processes in time.
These data also demonstrate that an increase in the phosphorylated to
unphosphorylated ratio of T217 could serve as a sensitive diagnostic marker
for fibrillar
Ap plaque pathology.
[0188] We then compared the mean, standardized phosphorylation
ratios
of four phosphorylation sites and total tau levels by PiB-PET SUVR quartiles
to explore
the relationship between total Ap-plaque load and phosphorylation, FIG. 16B.
All
phosphorylation sites except 5er202 (p-5202) demonstrated increased
phosphorylation
CA 03097667 2020-10-16
WO 2019/213612
PCT/US2019/030725
with greater Af3-plaque pathology. Differences among the other three sites
were also
discovered. p-T217 and p-T181 showed the largest increases once plaques had
started
(quartiles 2-3), but the magnitude of these increases diminished with greater
plaque
burden; in contrast, the increases in phosphorylation at p-T205 and total tau
levels
continued to grow along with the Af3 plaques. These results suggest that the
events
initially leading to increased tau phosphorylation in AD are likely related to
Af3-plaque
pathology, potentially through regulation of distinct kinases and phosphatases
that are
phosphorylation site specific. Further, the data suggest that p-T217 could
serve as a
surrogate biomarker for fibrillar Af3-plaque pathology, identifying a
potentially unique
signature of Af3-related tau processing. Importantly, among mutation non-
carriers (NCs),
the only participants who showed an increase in phosphorylation at T217 were
those
who were PiB+ (SUVR > 1.25, n=4).
[0189] We
next assessed whether site-specific tau phosphorylation was
associated with the anatomical distribution of cerebral Af3-plaque pathology
by exploring
the correlations between specific tau phosphorylation sites and cortical and
sub-cortical
regions of amyloid plaque deposition as measured by PiB-PET SUVR, FIG. 16C.
Phosphorylation at T217, T181, and T205 positively correlated with Af3-plaques
throughout the brain, but phosphorylation at S202 was negatively correlated.
In the
precuneus, a region of early amyloid plaque deposition, correlations with tau
isoforms
were compared based on the strength of bivariate regression controlling for
age,
gender, and estimated years to symptom onset (EYO) and adjusted for multiple
comparisons. We found a rank order of correlations of p-T217 (3 =0.68, p < 10 -
30), p-
T181 (13 =0.46, p < 10 -6), p-T205 (13 =0.41, p < 10 -5), and total tau (13
=0.35, p <0.001)
with positive correlations with Af3-plaques. In contrast, p-S202 had an
inverse
correlation (13 = -0.47, p < 10-7), suggesting that phosphorylation at this
site is lowered
with increasing Af3 pathology. These relationships were present in pre-
symptomatic
MCs when there is little evidence of neurodegeneration, suggesting that the
increase in
phosphorylated tau in the CSF is not a consequence of passive release from
dying
neurons, but more likely an active process related to the presence of Af3-
plaque
pathology22.
81
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
Example 6: Disease stage and progression are associated with site-specific
differences in tau hyperphosphorylation and longitudinal rates of change
[0190] The certainty of disease onset and predictability of symptom
onset
of DIAD enables the staging of individuals based on EYO 9'10'30 (i.e. the age
of an
individual at the time of assessment relative to the age of onset of others
with the
mutation). We next determined whether there were temporal differences in the
pattern
of phosphorylation of CSF tau. This was done by estimating the differences in
the
amount and rate of change in phosphorylation over time between MCs and NCs
based
on EYO. There were two important findings. First, there was evidence that
increases in
total tau and phosphorylation at specific sites occurred in a discreet order:
Phosphorylation of T217 occurred around -21 EYO, and was followed by
phosphorylation of T181 around -19 EYO, then an increase in total tau around -
17 EYO,
and then phosphorylation of T205 around -13 EYO (FIG. 17A-F). The initial
increase in
phosphorylation at T217, and to a lesser extent at T181, occurred at a similar
time to
when A[3-plaques began to increase (-19 EYO). Second, the ratio of
phosphorylation of
T217 and T181 began to decline significantly near the time of symptom onset,
while
phosphorylation at T205 remained elevated and total tau levels continued to
increase.
Of note, the concentration of all unphosphorylated peptides increased with
disease
progression (data not shown) suggesting that the decrease in the
phosphorylation ratio
for T217 and T181 was not a result of a disproportionate rise in
unphosphorylated
peptides specifically spanning these sites. For S202 there was no significant
change in
phosphorylation over the course of the disease, FIG. 17E. These results
indicate that
phosphorylation of tau occurs differentially for each site, increasing or
decreasing at
specific sites by disease stage. These site specific changes suggest a cascade
of
changes in soluble tau that is more dynamic than previously realized, and that
tau does
not monotonically increase in phosphorylation states or ratios. The emergence
of Ap
amyloid plaques and the onset of clinical decline, separated by two decades,
mark two
important demarcations of stages during which soluble tau phosphorylation
changes.
82
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
Example 7: Neuroimaging markers of disease progression are associated with
site- specific differences in tau hyperphosphorylation
[0191] In addition to estimating the onset of symptoms using family
history
(EYO), disease progression in DIAD can also be estimated using neuroimaging
measures that track various components of disease progression, e.g. brain
atrophy and
metabolic decline. These measures have been shown to change at different
periods of
time before symptom onset, with declining cerebral metabolism (measured by
[F18]
fluorodeoxyglucose [FDG]-PET) occurring up to 18 years and brain atrophy
(determined
by MRI) occurring up to 13 years before symptom onset 28'31-33. This raises
the question
of whether these biomarkers are likewise correlated with tau phosphorylation
at specific
sites. To examine this, we performed bivariate cross-sectional correlations
between the
phosphorylation sites and total tau with imaging measurements from 34 cortical
and 6
subcortical brain regions, controlling for gender, age and EYO. We focused the
analyses on asymptomatic MCs in order to identify any associations at the
earliest
stages of disease progression. The phosphorylation state of tau at S202 was
not
included in these analyses given its lack of change over disease progression.
[0192] MRI - Hyperphosphorylation was inversely associated with
cortical
thickness in asymptomatic MCs: p-T205 was most strongly associated with a
decrease
in cortical and subcortical thickness throughout the brain, FIG. 18A, while p-
T217 and
total tau levels showed fewer regional associations and weaker correlations.
Hyperphosphorylation at p-T181 had the lowest overall correlation with
cortical atrophy,
restricted to the medial and lateral parietal lobes and medial dorsal-medial
frontal lobes.
This suggests that the initial rise in p-T205 at -13 EYO may be related to the
underlying
etiology of cortical atrophy, which we have previously shown to begin
approximately at -
13 EYO in the precuneus28.
[0193] FDG PET- In addition to cortical atrophy, a decline in
glucose
metabolism in neurons and glia is associated with disease progression in AD.
Therefore, we tested whether there were distinct associations between cortical
or
subcortical metabolic impairment and tau phosphorylation. In the asymptomatic
MCs,
phosphorylation at T205 was correlated with glucose hypo-metabolism throughout
the
83
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
cortex and sub-cortical regions, as measured by FDG-PET, FIG. 18B. There were
minimal associations identified for the other p-tau sites or total tau level
in asymptomatic
MCs.
[0194] Together, these results indicate that the underlying
processes
leading to neuronal impairment and neurodegeneration during asymptomatic
disease
progression, as measured by neuroimaging, are most closely correlated with p-
T205.
Recent studies have identified a protective role for p-T205 in the post-
synaptic terminals
in response to AR-induced post-synaptic cytotoxicity34 through the fyn-kinase
pathway.
It is possible that the association found here, when synaptic function is
declining, could
represent a protective process resulting in increased phosphorylation at T205.
Over
time, however, hyperphosphorylation could predispose tau to aggregation.
Whereas,
phosphorylation at T181 and T217 appears to be more strongly associated with
the
presence of cortical AR-plaque pathology and, potentially, occurs upstream of
T205
hyperphosphorylation and elevation of soluble unphosphorylated tau levels.
Example 8 - Cognitive decline is specifically and differentially associated
with
site-specific differences in tau hyperphosphorylation
[0195] Prior studies have shown that AD dementia is more closely
related
to neocortical NFT pathology than neocortical A pathology35, yet the
relationship
between soluble tau and cognition remains uncertain. Therefore, we assessed
the
longitudinal change in the soluble tau phosphorylation ratio and total tau
levels over
time in comparison to clinical outcomes36. We performed a mixed effect model
with
longitudinal cognitive performance on the neuropsychological composite as the
outcome, and change in CSF tau measures (derived from individual linear mixed
effect
models), time, and their interactions as the predictor, adjusting for age,
gender,
education, and familial relations. We tested all MCs (symptomatic and
asymptomatic)
for this analysis and found differential effects between phosphorylation site
and clinical
and cognitive decline. Non-phosphorylated tau monotonically increased with
worsening
cognition and phosphorylation at T217 and T181 decreased with worsening
cognition,
while pT205 demonstrated no change relative to cognitive decline.
84
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0196] As the phosphorylation ratios of T217 and T181 decreased,
cognitive decline accelerated (t value 2.35, p = 0.02 and 2.11, p = 0.04),
Table 3 and
FIG. 19. This suggests that decreased phosphorylation of T217 and T181, more
than
increased soluble tau, presents an important marker of cognitive decline.
[0197] These findings challenge the current paradigm that a
continuous
rise in CSF p-tau is associated with cognitive dysfunction. In this work, we
uncovered
two general patterns: for some sites, phosphorylation decreased significantly
as
cognitive decline began, whereas other sites showed a continuous increase or
no
change with disease progression (see increasing vs. decreasing rates in FIG.
19).
Additionally, the associations between other markers of disease progression
(Ap-
plaques, brain atrophy and metabolism) and total tau levels and
phosphorylation at
different sites which we identified further emphasize that the processes
leading to tau
phosphorylation in AD are likely multifactorial and are not equivalent.
Example 9 - Increases in unphosphorylated tau are correlated with cortical
NFTs
by tau PET, but canonical phospho-tau species are not
[0198] Recent tau-PET (18F AV-1451, or flortaucipir) studies with
DIAD
participants have suggested that NFT increase only occurs following the onset
of clinical
symptoms37'38. We tested the hypothesis that soluble p-tau is a marker of NFT
pathology while soluble unphosphorylated tau is passively released from dying
neurons14 as a measure of neurodegeneration. We explored the relationship
between
longitudinal change of CSF tau and p-tau isoforms leading up to the time when
tau- PET
was performed. In a limited number of participants (10 MCs and 4 NCs), a
single tau-
PET scan was performed within 72 hours of the CSF sample being obtained. For
these
individuals, CSF samples had also been obtained on previous visits (within 1-3
years)
allowing us to assess the ability of longitudinal change in soluble tau
measures to
predict tau- PET levels.
[0199] First, we confirmed that cortical NFT levels in MCs only
increased
near the time of symptom onset, FIG. 20, suggesting that in DIAD MCs, clinical
decline
begins when tau aggregates start to increase. Second, we found that the
longitudinal
increase in CSF unphosphorylated tau was associated with an elevated cortical
tau-
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
PET (p = 0.03) value, Table 4. There were trends for an increase in
phosphorylation for
T205 being associated with higher levels of tau-PET and, contrarily, that
decreasing
phosphorylation of T217, T181 and S202 were associated with increasing levels
of tau-
PET, FIG. 21. Together, these findings suggest that increasing
unphosphorylated tau in
the CSF, rather than p-tau, is more closely linked to the spread of NFT
pathology. In
contrast, a soluble p-tau species decline when aggregated tau is increasing
which could
represent a process of sequestration by hyperphosphorylated aggregates.
Table 2. Demographic, cerebrospinal fluid, neuroimaging and cognition measures
for
mutation carriers and non-carriers.
Mutation p-
Mutation Carriers non-
value
carriers
N Asymptomatic Symptomatic (N = 141)
(N = 152) .. (N = 77)
Age 370 34.4 8.9 46.2 9.2 38.5
12.2 <.0001
Female, n (%) 370 84 (55.3) 39 (50.7) 88
(62.4) 0.15
APOE E4, n (%) 370 48 (31.6) 23 (29.9) 51
(36.2) 0.67
EYO, MEAN SD 370 -13.4 8.7 3.42 3.47 -9.2
12.5 <.0001
Cortical PiB PET 304 1.76 0.89 2.82 1.27 1.06
0.17 <.0001
SUVR
*PiB +, n (%) 304 81 (60.9) 48 (96.0) 2
(1.65) <.0001
CSF p-T181 370 26.5 7.2 34.2 7.7 21.7
2.3 <.0001
(phospho/unphospho)
CSF T181 total (ng/ml) 370 0.14 0.09 0.30 0.19 0.088
<.0001
0.034
CSF p-T205 370 0.44 0.24 0.93 0.36 0.34
0.13 <.0001
(phospho/unphospho)
CSF T205 total (ng/ml) 370 0.003 0.003 0.011
0.008 0.002 <.0001
0.001
CSF p-T217 370 3.49 3.08 8.42 4.05 1.25
0.66 <.0001
(phospho/unphospho)
CSF T217 total (ng/ml) 370 0.015 0.018 0.054
0.047 0.004 <.0001
0.004
86
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
CSF p-T208 370 0.00030 0.0010
0.00008 <.0001
(phospho/unphospho) 0.0004 0.0006 0.00014
CSF p-S202 370 2.77 0.80 2.52 0.68 3.10
0.72 <.0001
(phospho/unphospho)
CSF S202 total 370 0.016 0.006 0.025 0.011 0.014 <.0001
(ng/ml) 0.005
Tau (ng/ml) 370 0.51 0.21 0.82 0.41 0.40
0.14 <.0001
Precuneus (mm) 344 2.37 0.15 2.10 0.24 2.38
0.14 <.0001
Cortical FOG PET 318 1.73 0.14 1.57 0.18 1.71
0.14 <.0001
SURV
Hippocampal volume 344 8863 970 7290
1214 8787 775 <.0001
(mm)
Cognitive Composite 356 -0.096 0.640 -1.67
0.85 -0.03 0.59 <.0001
(z-score)
EY0- estimated years to onset of symptoms; PiB- Pittsburgh compound B; p-
phosphorylated; S- serine; SUVR- standard uptake value ratio; T- threonine.
Table 3 Longitudinal change in global cognition as predicted by the
longitudinal change
of phosphorylated-tau sites and total tau levels in mutation carriers.
Outcome Predictor CSF Tau p-T181
estimate SE t p estimate SE .. t
value p
value value value
Change in Change 0.16 1.91 0.09 0.93 -2.01 0.38 -5.23
<
Cognition in CSF * .0001
time
p-T205 p-T217
estimate SE t p estimate SE .. t
value p
value value value
Change in Change -0.06 0.5 -0.13 0.9 -2.41 0.45 -
5.32 <
Cognition in CSF * .0001
time
87
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
Table 4 The longitudinal change of phosphorylation ratio and total tau were
predicted
for each individual that had a Tau-PET scan performed at the time of a follow-
up
cerebrospinal fluid examination (n=14 (10 mutation carriers, 4 non-carriers)
was used to
predict the cortical tau standardized unit value ratio (SUVR). The table
indicates that
only predictor of an increase of tau-PET in this limited population was
increasing levels
of soluble tau in the CSF, with an increase of 36 ng/m I associated with an
increase of 1
unit cortical SUVR.
tau (ng/ml) p-T181 p-T205
Predictor estimate SE p estimate SE p estimate SE
value value
value
Tau-PET age 0.02 0.02 0.26 0.03 0.02 0.12 0.03
0.02 0.22
SUVR Slope MC 43.8 20.01 0.05 -0.68 0.63 0.3 15.3
22.87 0.52
Slope NC 31.15 47.31 0.53 -0.45 1.88 0.82
21.38 54.76 0.7
p-T217 p-S202
Predictor estimate SE p estimate SE
value value
Tau-PET age 0.03 0.02 0.18 0.03 0.02 0.06
SUVR Slope MC -2.69 3.16 0.41 -10.28 5.74 .. 0.1
Slope NC 11.55 13.91 0.43 -12.17 17.81 0.51
Discussion for Examples 5-9
[0200] Although tau comprises a hallmark AD pathology and can be
measured in aggregated or soluble forms, important gaps remain in our
understanding
of how the post-translational modifications of this critical neuronal protein
leads to the
development of NFT2 and neurodegeneration in humans. Here we demonstrate how
patterns of phosphorylation of tau in the CSF vary over the course of AD
progression.
We add to the existing clinical literature the demonstration that in DIAD, the
process of
tau phosphorylation and release into the central nervous system is a dynamic
process
that: 1) begins once A[3-plaque burden (as measured by PiB-PET) is established
(decades prior to symptoms), and subsequently unfolds over a period of nearly
two
decades, during which time the different phosphorylation sites of the tau
protein are
phosphorylated in distinct phases in association with different markers of
disease
progression; and 2) decreases significantly in a site-dependent manner near
the time of
cognitive decline and the rise in aggregated tau (as measured by tau-PET).
Together,
88
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
these results indicate that this method of quantifying soluble phosphorylated/
unphosphorylated tau peptides can track the AD process across its preclinical
to
symptomatic stages, providing a signature of phospho-tau pathology in this
disease.
Moreover, they challenge the purported roles of tau/p-tau in DIAD, and
possibly AD in
general, and recapitulate in humans those findings from animal studies that
link AB
pathology to tau hyperphosphorylation21'23'25'39 and active cellular release
rather than a
consequence of release of dying neurons.
[0201] Although causality needs to be addressed in future studies,
the
contemporaneous increases in p-T217, p-T181 and PiB -PET suggest that the
widespread phosphorylation of tau levels in AD is closely linked to AB
pathology. This
hypothesis is consistent with recent work in AD transgenic mice20,21,23,34,40
and in Stable
Isotope Labeling Kinetics (SILK) which demonstrate that p-tau isoforms are
released
from cells in an active process that is increased in the presence of A[3-
plaques22. Our
results link AB pathology to a distinct change in soluble tau peptide
concentrations and
phosphorylation patterns, shedding light on the phenomenon in which
significant
elevation of p-tau occurs in AD but not in other neurodegenerative
tauopathies.16'17
These findings also yield important insights into potential therapeutic
targets as well as
biomarkers of early AD pathology prior to the onset of clinical symptoms.
[0202] Recent work has shown an increase and spread of neuritic tau
aggregates (paired helical filaments in dystrophic neurites) induced by NFT
isolates
from AD brains in AB transgenic mice, occurring well before established
somatic
NFTs20. It is possible that the very early increase in p-T217 and p-T181 may
reflect this
"early" tau aggregation in response to A[3-plaques and might explain the
global
association of PiB PET with these isoforms that we identified. Additionally,
this might
better explain the lack of clinical symptoms seen during this early elevation
in p-tau as it
occurs years before significant neurodegeneration. This work also proposes p-
T217 as
a very early biomarker with promising application as an indicator of
therapeutic
response in amyloid targeting therapies. This could be particularly important
in
prevention studies where surrogate markers of disease progression are vital to
identifying effective therapies.
89
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0203] Some common assumptions about the diagnostic roles of
soluble
tau and p-tau are called into question here. Specifically, the current
diagnostic
framework in AD emphasizes the presence of biomarkers representing AD specific
and
non-specific pathologies (e.g. A[3, p-tau and tau)14. Within this diagnostic
framework,
soluble p-tau and unphosphorylated tau are often presumed to be passively
released
from degenerating neurons, with p-tau associated with aggregated NFTs and
unphosphorylated tau associated with axonal degeneration. Alternatively, our
results in
DIAD suggest AD tauopathy could be defined as a modification of soluble tau
phosphorylation status (ratio of phosphorylation). Moreover, considering the
timings of
status changes at specific phosphorylation sites (21 to 13 years before
estimated
symptoms onset), this would also suggest that increased phosphorylation,
although a
marker of pathology, is not necessarily a marker of tau-related toxicity or
cell body
NFTs. In fact, our results indicate that for pT217 and pT181, rather than
continuing to
increase when NFT pathology is rapidly increasing37, there is a dramatic
decrease in
phosphorylation. One possible explanation for this is similar to what has been
observed
with soluble/aggregated A[341: that the dramatic increase of aggregated tau
sequesters
phosphorylated tau42 in the brain, decreasing CSF levels. However, a reduction
of tau
through proteostatic mechanisms cannot be excluded. In either case, our
finding of the
negative correlation between the phosphorylation ratios of T217 or T181 and
longitudinal cognitive decline highlights the importance of this event in the
disease
progression. Elucidating the cause for this decline could lead to a better
understanding
of the links between soluble tau and neuronal dysfunction and the use of CSF p-
tau/tau
in AD prognostication.
[0204] Given the role of kinases in the phosphorylation of tau,
this group of
enzymes is currently viewed as a potential target for AD therapeutics43. As we
have
demonstrated here that not all forms of p-tau are associated with markers of
disease
progression, and some may in fact work against it, certain kinase/phosphatase
activities
that result in p-tau alterations may not be detrimental, at least early in the
disease
process.
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0205] In summary, we have now demonstrated that in AD associated
with
autosomal dominant mutations, CSF tau hyperphosphorylation occurs very early
and
exhibits pattern of site-specific changes at different stages of the disease.
The
underlying mechanisms behind these findings will have important implications
understanding the disease and for tau-directed therapies for AD.
Material and Methods for Examples 5-9
[0206] Participants - Participants with at least 50% risk of
inheriting an
DIAD mutation from families with a confirmed genetic mutation in PSEN1, PSEN2
or
APP were enrolled in the Dominantly Inherited Alzheimer Network study (DIAN,
NIA
U19 AG032438) (dian.wustl.edu; clinicaltrials.gov number NCT00869817)44. All
procedures were approved by the Institutional Review Board (IRB) of Washington
University and conformed to local IRB and Ethics Committees where the study
was
being performed. The presence or absence of a DIAD mutation was determined
using
PCR-based amplification of the appropriate exon followed by Sanger sequencing.
At
each study visit, participants underwent comprehensive clinical assessments,
cognitive
testing, neuroimaging, and CSF studies; however, at each visit, each
participant may
not have completed all study procedures. The details of study structure and
assessments can be found in prior publications 1044. Follow-up intervals were
determined by clinical status (normal or impaired) of each participant and by
their
estimated years to symptom onset (EYO) and ranged from yearly to every three
years.
Data was obtained from quality-controlled data (yearly quality assessments for
irregular
results and missing data from January 26, 2009 to June 30, 2017) and included
370
participants (n=150 with longitudinal CSF evaluations with a median time
between visits
of 2.8 years).
[0207] Estimated Years to Symptom Onset (EYO) - In dominantly
inherited
AD there is near 100% penetrance, with age at symptom onset in mutation
carriers
being relatively consistent for each mutation and within each family. This
allows for the
designation of estimated years to symptom onset (EYO). EYO was defined as
follows: A
parental age at earliest symptom onset was established for each participant by
semi-
structured interview. The parental age at onset for each mutation was then
entered into
91
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
a database consisting of the combined symptom onset values from DIAN and from
prior
publications from DIAD cohorts. These were used to compute an average age of
onset
specific to each mutation29. The mutation-specific age of onset was subtracted
from
each participant's age at the time of clinical assessment to define the
individual's EYO.
When a specific mutation average age of onset was unknown, the parental or
proxy age
of onset was used to define EY029. For participants who were symptomatic at
baseline,
as assessed by a CDR >0, the reported age of actual symptom onset was
subtracted
from age at each clinical assessment to define EYO.
[0208] Clinical Assessments - Standardized clinical evaluations,
including
the use of a study partner, were performed for each participant. The Clinical
Dementia
Rating Scale (CDR) was used to indicate dementia stage. Participants were
rated as
cognitively normal (CDR=0) or having very mild dementia (CDR= 0.5), mild
dementia
(CDR= 1) or moderate dementia (CDR =2)45. Evaluating clinicians were blind to
genetic
status. A comprehensive neuropsychological battery assessing general cognitive
function, memory, attention, executive function, visuospatial function, and
language was
performed at each visit46. From these tests we developed a cognitive composite
that
reliably detects decline across the range of EYO and CDR47. The composite
represents
the average of the z scores from tests including episodic memory, complex
attention,
and processing speed and a general cognitive screen (Mini Mental State
Examination).
[0209] CSF Tau Analyses - CSF was collected via standard lumbar
puncture procedures (L4/L5) using an atraumatic Sprotte spinal needle (22Ga)
into two
13m1 polypropylene tubes. CSF was flash-frozen upright on dry ice. Samples
collected
in the United States were shipped overnight on dry ice to the DIAN biomarker
core
laboratory at Washington University, St. Louis, MO, USA, whereas samples
collected at
international sites were stored at -80 C and shipped quarterly on dry ice.
Upon arrival,
each sample was subsequently thawed, combined into a single polypropylene
tube, and
aliquoted (500p1 each) into polypropylene microcentrifuge tubes (#05-538-69C,
Corning
Life Science, Corning, NY, USA), after which they were re-flash frozen on dry-
ice and
stored at -80 C.
92
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0210] Each thawed CSF sample was mixed with 25p1 of a solution
containing 15N-441 tau internal standard (2.5ng per sample), 50mM Guanidine,
10%
NP-40 and 10X protease inhibitor cocktail (Roche). Tau was extracted by immune
capture using incubation under rotation at room temperature during 2 hours
with 20plof
sepharose beads cross-linked to Tau1 (tau epitope 192-199) and HJ8.5 (tau
epitope 27-
35) antibodies. Beads were spun by centrifugation, then rinsed three times
with lmlof
25mM TEABC. Samples were digested overnight at 37 C with 400 ng of trypsin
Gold
(Promega, Madison, WI). AQUA peptides (Life Technologies, Carlsbad, CA) were
spiked to obtain an amount of 5fmo1 per labeled phosphorylated peptide and
50fmo1 per
labeled unmodified peptide in each sample. The peptide mixture was loaded on
TopTip
C18 tips, washed with 0.1% Formic Acid (FA) solution and eluted with 60% ACN
0.1%
FA solution. Eluates were dried using a Speedvac and dried samples were stored
at -
80 C prior to analysis. Samples were resuspended in 25plof 2% ACN 0.1 A FA.
Extracts were analyzed by nanoLC-MS/HRMS using Parallel Reaction Monitoring
using
HCD fragmentation. NanoLC-MS/MS experiments were performed using a nanoAcquity
UPLC system (Waters, Mildford, Massachusetts) coupled to a Fusion Tribrid mass
spectrometer (Thermo Scientific, San Jose, California). 5plwas injected for
each
sample. Peptide separation was achieved at 60 C in 24 minutes on a Waters HSS
T3
column (75pm x 100mm, 1.8pm). Mobile phases were (A) 0.1% formic acid in water
and
(B) 0.1% formic acid in acetonitrile. Gradient used was: 0min-0.5% B; 7.5min-
5% B;
22min-18% B; then the column was rinsed 2 minutes with 95% B. Flow rate was
set at
700n1/min for 7.5min then 400n1/min for the rest of the analysis. Data were
acquired in
the positive ion mode at a spray voltage of 2200V (Nanospray Flex Ion Source,
Thermo
Scientific) and ion transfer tube set at 270 C. S-lens RF voltage was set at
60 V.
MS/HRMS transitions and were extracted using Skyline software (MacCoss lab,
University of Washington). CSF tau phosphorylation levels were calculated
using
measured ratios between MS/HRMS transitions of endogenous unphosphorylated
peptides and 15N labeled peptides from protein internal standard. Ratio of
phosphorylation on T181, S202, T205 and T217 were measured using the ratio of
the
MS/HRMS transitions from phosphorylated peptides and corresponding
93
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
unphosphorylated peptides. Each phosphorylated/unphosphorylated peptide
endogenous ratio was normalized using the ratio measured on the MS/HRMS
transitions of the corresponding AQUA phosphorylated/unphosphorylated peptide
internal standards.
[0211] Brain Imaging - Amyloid deposition, glucose metabolism, tau
(NFT)
PET and cortical thickness/subcortical volumes were assessed using 11C-PiB-
PET, 18F-
FDG-PET, 18F-AV-1451 (a.k.a flortaucipir) and volumetric T1-weighted MRI
scans,
respectively. Standard procedures were used to ensure consistency in the data
collection of all DIAN sites28. The 11C-PiB-PET scan consisted of 70 minutes
of dynamic
scanning after a bolus injection of -13 mCi of PiB with regional standard
uptake ratios
(SUVR) determined from the 40-70 minute timeframe. The 18F-FDG-PET scan
started
thirty minutes after a -5 mCi bolus injection and lasted thirty minutes. The
18F-AV-1451
data was acquired from the 80-100 minute window after bolus injection and were
converted to SUVRs. The Ti MR sequence was an accelerated magnetization-
prepared
rapid acquisition with gradient echo (MPRAGE) acquired on 3T scanners
(parameters:
TR=23000, TE=2.95, and 1.0x1.0x1.2mm3 resolution).
[0212] The PIB and FDG SUVRs from 34 cortical and 6 subcortical
regions of interests (ROls) were obtained using FreeSurfer software
(surfer.nmr.mgh.harvard.edu/). The SUVRs were processed with grey cerebellum
as
reference regions and ROI data were corrected for partial volume effects using
a
regional point spread function (RSF)48 in geometric transfer matrix framework.
[0213] Statistical analysis - Baseline characteristics of the
participants
were summarized as mean SD for continuous variables and n (column percent)
for
categorical variables. P-values for comparing the differences among
asymptomatic MC,
symptomatic MC and NC as defined at baseline are obtained using general linear
mixed
effects models (LME) for continuous variables and generalized linear mixed
effects
models, with a logistic link for categorical variables. All of the models
incorporated a
random family effect to account for the correlations on the outcome measures
between
participants within the same family. The cut point for baseline cortical PiB
PET SUVR is
94
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
chosen such that the difference in the longitudinal rate of change of cortical
PiB PET
between MC and NC first starts to differ significantly different from 0.
[0214] The cross-sectional relationship of the different tau
phosphorylation
sites with PiB, FDG, and Cortical thickness/Subcortical volume were evaluated
in all
asymptomatic MCs (CDR=0, n=152) using bivariate LME on each ROI. The models
included fixed effects of EYO, education, sex, and random intercepts at the
family level.
Compared with the simple correlation estimation method (Pearson or Spearman
correlation), the bivariate LME can adjust for covariates such as EYO as well
as
accounting for the correlation within the family cluster49'59. P-values for
testing the
correlations were corrected using the Benjamini Hochberg method51 to control
the false
discovery rate due to multiple testing.
[0215] For within-individual annual rate of change over the
longitudinal
follow up, the best linear unbiased predictors for each biomarker were
estimated using
LME, which were then plotted against baseline EYO to examine biomarkers
trajectories.
Linear or linear spline mixed effects models, where appropriate, were then
used to
determine the baseline EYO point from which MC became significantly different
from
NC in baseline level and the rate of change for each biomarker. The details of
the linear
spline mixed effects models can be found in a recent publication9. The linear
or linear
spline mixed effects models included the fixed effects of mutation group (MC
or NC),
baseline EYO, time since baseline and all possible two-way or three-way
interactions
among them. Sex, years of education, and APOE et/ status were considered as
covariates, but only those effects that were significant were retained in the
models.
Random effects included in the models are the random intercepts for family
clusters,
individual random intercept and random slope with unstructured covariance
matrix to
account for the within-subject correlation due to repeated measures. The
adjusted
difference in the mean level at baseline and difference in the rates of change
between
MC and NC were then tested using the approximate t-test derived from the
models to
determine the first EYO point where the difference became significant.
[0216] To visualize the difference in the rate of change among
total tau,
tau phosphorylation site, cortical PiB, and global cognition across the range
of EYO,
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
measures of MC were first standardized using the mean and standard deviation
of the
NC. The rate of change of each measure for each MC were then calculated using
LME,
and LOESS curves were fitted to visually represent the trajectories of the
standardized
rates of change over EYO.
[0217] The utility of baseline total tau and p-tau in predicting
longitudinal
cognitive decline were evaluated using LME. Fixed effects in the models
included
mutation group, baseline age, sex, APOE e4 status, time and all possible two-
way or
three-way interactions among them. Random effects in the models included the
random
intercepts for family clusters, individual random intercept and random slope
with
unstructured covariance matrix.
[0218] Linear regressions were used to examine whether the annual
rate
of change of tau and phospho-tau position for MC and NC, leading up to and
including
the point when the tau PET was performed, could predict tau PET SUVR,
controlling for
the effect of age. Due to the limited number of participants, a family cluster
was not
included.
[0219] All analyses were conducted using SAS 9.4 (SAS Institute
Inc.,
Cary, NC). A p-value <0.05 was considered to be statistically significant.
References for Examples 5-9
[0220] Goedert, M., Spillantini, M. G., Jakes, R., Rutherford, D. &
Crowther, R. A. Multiple isoforms of human microtubule-associated protein tau:
sequences and localization in neurofibrillary tangles of Alzheimer's disease.
Neuron 3,
519-526, doi:10.1016/0896-6273(89)90210-9 (1989).
[0221] Grundke-lqbal, I. et al. Abnormal phosphorylation of the
microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology.
Proceedings of the National Academy of Sciences of the United States of
America 83,
4913-4917, doi:10.1073/pnas.83.13.4913 (1986).
[0222] Wang, Y. & Mandelkow, E. Tau in physiology and pathology.
Nat
Rev Neurosci 17, 5-21, doi:10.1038/nrn.2015.1 (2016).
96
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0223] Kimura, T., Sharma, G., Ishiguro, K. & Hisanaga, S.-i.
Phospho-Tau
Bar Code: Analysis of Phosphoisotypes of Tau and Its Application to Tauopathy.
Frontiers in Neuroscience 12, doi:10.3389/fnins.2018.00044 (2018).
[0224] Crowther, R. A. Straight and paired helical filaments in
Alzheimer
disease have a common structural unit. Proceedings of the National Academy of
Sciences 88, 2288-2292, doi:10.1073/pnas.88.6.2288 (1991).
[0225] Fitzpatrick, A. W. P. et al. Cryo-EM structures of tau
filaments from
Alzheimer's disease. Nature 547, 185, doi:10.1038/nature23002 (2017).
[0226] Price, J. L., Davis, P. B., Morris, J. C. & White, D. L. The
distribution of tangles, plaques and related immunohistochemical markers in
healthy
aging and Alzheimer's disease. Neurobiology of aging 12, 295-312 (1991).
[0227] Qian, J., Hyman, B. T. & Betensky, R. A. Neurofibrillary
Tangle
Stage and the Rate of Progression of Alzheimer Symptoms: Modeling Using an
Autopsy
Cohort and Application to Clinical Trial Design. JAMA Neurol 74, 540-548,
doi:10.1001/jamaneuro1.2016.5953 (2017).
[0228] McDade, E. et al. Longitudinal cognitive and biomarker
changes in
dominantly inherited Alzheimer disease. Neurology 91, e1295-e1306,
doi:10.1212/wn1.0000000000006277 (2018).
[0229] Bateman, R. J. et al. Clinical and biomarker changes in
dominantly
inherited Alzheimer's disease. N Engl J Med 367, 795-804,
doi:10.1056/NEJMoa1202753 (2012).
[0230] Fagan, A. M. et al. Cerebrospinal fluid tau/beta-amyloid(42)
ratio as
a prediction of cognitive decline in nondemented older adults. Arch Neurol 64,
343-349,
doi:10.1001/archneur.64.3.noc60123 (2007).
[0231] Toledo, J. B., Xie, S. X., Trojanowski, J. Q. & Shaw, L. M.
Longitudinal change in CSF Tau and Abeta biomarkers for up to 48 months in
ADNI.
Acta Neuropathol 126, 659-670, doi:10.1007/s00401-013-1151-4 (2013).
[0232] Vandermeeren, M. et al. Detection of tau proteins in normal
and
Alzheimer's disease cerebrospinal fluid with a sensitive sandwich enzyme-
linked
immunosorbent assay. J Neurochem 61, 1828-1834 (1993).
97
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0233] Jack, C. R., Jr. et al. NIA-AA Research Framework: Toward a
biological definition of Alzheimer's disease. Alzheimers Dement 14, 535-562,
doi:10.1016/j.jalz.2018.02.018 (2018).
[0234] Jack, C. R., Jr. et al. A/T/N: An unbiased descriptive
classification
scheme for Alzheimer disease biomarkers. Neurology 87, 539-547,
doi:10.1212/wn1.0000000000002923 (2016).
[0235] Hu, W. T. et al. Reduced CSF p-Tau181 to Tau ratio is a
biomarker
for FTLD-TDP. Neurology 81, 1945-1952, doi:10.1212/01.wn1.0000436625.63650.27
(2013).
[0236] Ham pel, H. et al. Measurement of phosphorylated tau
epitopes in
the differential diagnosis of Alzheimer disease: a comparative cerebrospinal
fluid study.
Archives of general psychiatry 61, 95-102, doi:10.1001/archpsyc.61.1.95
(2004).
[0237] Price, J. L. & Morris, J. C. Tangles and plaques in
nondemented
aging and "preclinical" Alzheimer's disease. Ann Neurol 45, 358-368 (1999).
[0238] Ittner, L. M. et al. Dendritic Function of Tau Mediates
Amyloid-p
Toxicity in Alzheimer's Disease Mouse Models. Cell 142, 387-397,
doi:https://doi.org/10.1016/j.ce11.2010.06.036 (2010).
[0239] Cohen, A. D. et al. Early striatal amyloid deposition
distinguishes
Down syndrome and autosomal dominant Alzheimer's disease from late-onset
amyloid
deposition. Alzheimer's & dementia: the journal of the Alzheimer's
Association,
doi:10.1016/j.jalz.2018.01.002 (2018).
[0240] Maia, L. F. et al. Changes in Amyloid-p and Tau in the
Cerebrospinal Fluid of Transgenic Mice Overexpressing Amyloid Precursor
Protein.
Science Translational Medicine 5, 194re192-194re192 (2013).
[0241] Sato, C. et al. Tau Kinetics in Neurons and the Human
Central
Nervous System. Neuron 98, 861-864, doi:10.1016/j.neuron.2018.04.035 (2018).
[0242] Schelle, J. et al. Prevention of tau increase in
cerebrospinal fluid of
APP transgenic mice suggests downstream effect of BACE1 inhibition.
Alzheimer's &
Dementia: The Journal of the Alzheimer's Association 13, 701-709,
doi:10.1016/j.jalz.2016.09.005 (2017).
98
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0243] Zempel, H., Thies, E., Mandelkow, E. & Mandelkow, E. M.
Abeta
oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into
dendrites, Tau phosphorylation, and destruction of microtubules and spines. J
Neurosci
30, 11938-11950, doi:10.1523/jneurosci.2357-10.2010 (2010).
[0244] Saman, S. et al. Exosome-associated Tau Is Secreted in
Tauopathy Models and Is Selectively Phosphorylated in Cerebrospinal Fluid in
Early
Alzheimer Disease. Journal of Biological Chemistry 287, 3842-3849,
doi:10.1074/jbc.M111.277061 (2012).
[0245] Barthelemy, N. R. et al. Tau Protein Quantification in Human
Cerebrospinal Fluid by Targeted Mass Spectrometry at High Sequence Coverage
Provides Insights into Its Primary Structure Heterogeneity. Journal of
proteome
research 15, 667-676, doi:10.1021/acs.jproteome.5b01001 (2016).
[0246] Jin, M. et al. Soluble amyloid p-protein dimers isolated
from
Alzheimer cortex directly induce Tau hyperphosphorylation and neuritic
degeneration.
Proceedings of the National Academy of Sciences 108, 5819-5824 (2011).
[0247] Gordon, B. A. et al. Spatial patterns of neuroimaging
biomarker
change in individuals from families with autosomal dominant Alzheimer's
disease: a
longitudinal study. The Lancet. Neurology 17, 241-250, doi:10.1016/51474-
4422(18)30028-0 (2018).
[0248] Ryman, D. C. et al. Symptom onset in autosomal dominant
Alzheimer disease: a systematic review and meta-analysis. Neurology 83, 253-
260,
doi:10.1212/WNL.0000000000000596 (2014).
[0249] Fleisher, A. S. et al. Associations between biomarkers and
age in
the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-
sectional study. JAMA Neurol 72, 316-324, doi:10.1001/jamaneuro1.2014.3314
(2015).
[0250] Benzinger, T. L. et al. Regional variability of imaging
biomarkers in
autosomal dominant Alzheimer's disease. Proceedings of the National Academy of
Sciences of the United States of America 110, E4502-4509,
doi:10.1073/pnas.1317918110 (2013).
99
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0251] Quiroz, Y. T. et al. Cortical atrophy in presymptomatic
Alzheimer's
disease presenilin 1 mutation carriers. J Neurol Neurosurg Psychiatry 84, 556-
561,
doi:10.1136/jnnp-2012-303299 (2013).
[0252] Ridha, B. H. et al. Tracking atrophy progression in familial
Alzheimer's disease: a serial MRI study. Lancet Neurol 5, 828-834,
doi:10.1016/51474-
4422(06)70550-6 (2006).
[0253] Ittner, A. et al. Site-specific phosphorylation of tau
inhibits amyloid-
13 toxicity in Alzheimer's mice. Science 354, 904-908 (2016).
[0254] Arriagada, P. V., Growdon, J. H., Hedley-Whyte, E. T. &
Hyman, B.
T. Neurofibrillary tangles but not senile plaques parallel duration and
severity of
Alzheimer's disease. Neurology 42, 631-631, doi:10.1212/wn1.42.3.631 (1992).
[0255] Bateman, R. J. et al. The DIAN-TU Next Generation
Alzheimer's
prevention trial: Adaptive design and disease progression model. Alzheimers
Dement
13, 8-19, doi:10.1016/j.jalz.2016.07.005 (2017).
[0256] Quiroz, Y. T. et al. Association Between Amyloid and Tau
Accumulation in Young Adults With Autosomal Dominant Alzheimer Disease. JAMA
Neurol 75, 548-556, doi:10.1001/jamaneuro1.2017.4907 (2018).
[0257] Gordon, B. A. et al. Tau PET in autosomal dominant Alzheimer
disease: relationship to cognition, dementia and biomarkers. Brain : a journal
of
neurology Accepted for Publication (2019).
[0258] He, Z. et al. Amyloid-beta plaques enhance Alzheimer's brain
tau-
seeded pathologies by facilitating neuritic plaque tau aggregation. Nat Med
24, 29-38,
doi:10.1038/nm.4443 (2018).
[0259] Buerger, K. et al. CSF phosphorylated tau protein correlates
with
neocortical neurofibrillary pathology in Alzheimer's disease. Brain : a
journal of
neurology 129, 3035-3041, doi:10.1093/brain/aw1269 (2006).
[0260] Potter, R. et al. Increased in Vivo Amyloid-1342 Production,
Exchange, and Loss in Presenilin Mutation Carriers. Science Translational
Medicine 5,
189ra177-189ra177 (2013).
100
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
[0261] Yamada, K. et al. In Vivo Microdialysis Reveals Age-
Dependent
Decrease of Brain Interstitial Fluid Tau Levels in P301S Human Tau Transgenic
Mice.
The Journal of Neuroscience 31, 13110-13117, doi:10.1523/jneurosci.2569-
11.2011
(2011).
[0262] Medina, M. & Avila, J. Further understanding of tau
phosphorylation: implications for therapy. Expert review of neurotherapeutics
15, 115-
122, doi:10.1586/14737175.2015.1000864 (2015).
[0263] Morris, J. C. et al. Developing an international network for
Alzheimer research: The Dominantly Inherited Alzheimer Network. Clinical
investigation
2, 975-984, doi:10.4155/cli.12.93 (2012).
[0264] Morris, J. C. The Clinical Dementia Rating (CDR): current
version
and scoring rules. Neurology 43, 2412-2414 (1993).
[0265] Storandt, M., BaIota, D. A., Aschenbrenner, A. J. & Morris,
J. C.
Clinical and psychological characteristics of the initial cohort of the
Dominantly Inherited
Alzheimer Network (DIAN). Neuropsychology 28, 19-29, doi:10.1037/neu0000030
(2014).
[0266] Lim, Y. Y. et al. BDNF Va166Met moderates memory impairment,
hippocam pal function and tau in preclinical autosomal dominant Alzheimer's
disease.
Brain: a journal of neurology 139, 2766-2777, doi:10.1093/brain/aww200 (2016).
[0267] Su, Y. et al. Partial volume correction in quantitative
amyloid
imaging. Neuroimage 107, 55-64, doi:10.1016/j.neuroimage.2014.11.058 (2015).
[0268] Luo, J., D'Angelo, G., Gao, F., Ding, J. & Xiong, C.
Bivariate
correlation coefficients in family-type clustered studies. Biometrical Journal
57, 1084-
1109, doi:doi:10.1002/bimj.201400131 (2015).
[0269] Xiong, C. et al. Longitudinal relationships among biomarkers
for
Alzheimer disease in the Adult Children Study. Neurology 86, 1499-1506,
doi:10.1212/WNL.0000000000002593 (2016).
[0270] Benjamini, Y., and Hochberg, Y. . Controlling the false
discovery
rate: a practical and powerful approach to multiple testing. Journal of the
Royal
Statistical Society Series B, 289-300 (1995).
101
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
Example 10
[0271] Alzheimer disease (AD) is the leading cause of dementia
worldwide. Its diagnosis and treatment remain extremely challenging in absence
of
specific and early biomarkers. Recent developments of cerebrospinal fluid
(CSF)
biomarkers and brain Positron-Emission Tomography (PET) imaging provided
valuable
tools to detect the pathognomonic AD brain pathologies of amyloid-Ap plaques
and
neurofibrillary hyperphosphorylated tau tangles. Currently, the CSF profile of
AD
patients is characterized by decreased amyloid-Beta 42 (A[342) and increased
total and
phosphorylated tau (p-tau 181) measured with standard immuno-assays. This
profile
allows the discrimination of AD versus non-AD pathologies and the detection of
AD
process many years prior to cognitive symptoms or complaint. However, changes
in
CSF tau and p-tau are not specific of AD. Although the increased p-tau181 has
been
interpreted as a consequence of hyperphosphorylation due to tangle formation,
CSF p-
tau increases concomitantly with total tau. Brain studies indicate tau
phosphorylation
on numerous sites, but the diagnostic relevance of these additional
phosphorylation
sites in CSF has not been fully addressed. Mass spectrometry (MS)-based
methods
are more relevant than immunoassays to assess changes in phosphorylation
levels of
specific sites independently of total tau levels as they allow independent
quantification
of phosphorylated peptides and their corresponding unmodified counterparts.
Accordingly, correlations between phosphopeptide and unphosphorylated peptide
slopes can be compared to evaluate the rate of phosphorylation. To quantify
phosphorylated tau isoforms in CSF we used an innovative targeted high-
resolution MS
(HRMS) method targeting tau phosphorylated peptides in the mid-domain of the
protein
sequence, which is the most abundant domain in CSF and is phosphorylated on
numerous sites in brain tau and AD tau aggregates.
[0272] We analyzed tau phosphorylation on T181, S199, S202, T205
and
T217 in CSF using a cohort comprising cognitively normal individuals and
patients with
mild cognitive impairment stratified by their amyloid status based on CSF
A[342/40 ratio
and PET-PIB imaging. This validation allowed us to highlight the potential of
CSF pT217
102
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
for AD diagnosis and to establish correlations between pT217 and amyloidosis
underlying tau modification at an early stage of the disease
[0273] Participants ¨ Eighty-six participants cognitively normal
(CDR=0) or
with mild cognitive impairment were recruited from the Washington University
in Saint
Louis ADRC study previously reported by Patterson et al. 2015 with CSF and
amyloid
PiB-PET data available. This cohort included 29 amyloid positive and 47
amyloid
negative participants according to the results of PiB-PET (considered positive
above
0.18 used as cut-off) and CSF A42/A40 ratio measured by MS (considered
pathological below 0.12 used as cut-off) and 10 cases with conflicting results
between
PET-PIB and CSF profiles (5 with a PiB-PET(+)/CSF(-) and 5 with PiB-PET(-
)/CSF(+)
amyloidosis profile). Seven CSF samples were extracted in duplicate and one in
triplicate to assess variability.
[0274] CSF tau purification using immunoprecipitation ¨ 800 pl of
CSF
supernatant obtained after Af3 immuno-precipitation and storage at -80C was
used for
tau analysis. Thawed supernatants were spiked with 15N tau internal standard
(5 ng per
sample) and extracted using Tau1 immunoprecipitation. 5mM Guanidine, 1% NP-40
and protease inhibitor cocktail were added to the sample, then samples were
mixed 3
hours at room temperature with 20 pl of sepharose beads cross-linked to Tau1
antibody. Beads were precipitated then rinsed with 0.5 M Guanidine and TEABC
25mM.
Samples were digested with 400 ng of trypsin. AQUA peptides (Life
Technologies,
Carlsbad, California) were spiked to achieve individual quantity of 10 fmol
per labeled
phosphopeptide and 100 fmol per labeled peptide per sample. AQUA TPSLPpTPPTR
(pT217) substituted the missed cleavage version used in the discovery cohort.
Tryptic
peptides were loaded on TopTip C18 tips, washed with 0.1% FA solution and
eluted
with 60 AACN 0.1 AFA solution. Eluates were dried on speedvac. Samples were
stored
at -80C. Before LC-MS analysis, samples were resuspended in 25 pl 2 AACN 0.1 A
FA.
Extracts were analyzed by nanoLC-MS/HRMS.
[0275] Tau peptides and phosphorylated peptide quantitation
(Validation)
¨ Stable Isotope dilution MS quantitation using 15N labeled peptides was used
to
calculate absolute levels of unmodified peptides. Phosphorylation rate for
each site was
103
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
calculated by single point calibration comparing area ratio obtained on AQUA
unphosphorylated and phosphorylated peptides counterpart to the area ratio
measured
for corresponding endogenous peptides. Phosphorylated peptide level was
calculated
by combining unmodified peptide level counterpart and rate of phosphorylation
of the
corresponding site.
[0276] Statistics ¨ Statistical analyses, including comparison of
the slopes
of regression lines, were performed using the GraphPad Prism software (7.0).
Statistical significances between values obtained across investigated groups
were
calculated using non-parametric Mann-Whitney tests. Non-parametric Spearman's
rho
rank correlation coefficients were used to assess the correlations between two
series of
values. Statistical significance was defined by p<0.05.
[0277] Quantification of CSF tau phosphorylated peptides (Results)
¨
Increased phosphorylation of tau at T217 in AD was detected in a cohort from
the
Knight AD Research Center (ADRC) at Washington University in Saint Louis
comprising
86 participants with no cognitive complaint or mild cognitive impairment.
Consistent with
the results of PiB-PET and CSF A[342/A[340 ratio measured by MS, participants
were
stratified in 29 amyloid-positive, 47 amyloid-negative participants and 10
cases with
conflicting results between PET-PIB and CSF profile. To improve the assay
sensitivity,
immunopurification with the Tau1 antibody was performed. Isotopically labeled
versions
of both phosphorylated and unphosphorylated peptides were used to measure site
phosphorylation ratio. All phosphorylated peptides targeted were detected in
all
samples, with the exception of pS199 containing peptide not recovered by the
Tau1
antibody. In this cohort, we confirmed the positive diagnosis relevance of
pT217
biomarker at early stage of the disease. The pT217/T217 ratio clearly
separated
amyloid positive and negative groups (FIG. 24B and FIG. 24C, AUC 0.999). In
addition,
the measurement of pT181/T181 ratio discriminated the amyloid-positive and
amyloid-
negative groups (FIG. 24B, AUC 0.956). T217 and T181 phosphorylation ratios
were
also correlated, (r=0.524, p=0.0002), suggesting that the phosphorylation of
these sites
could result from a common pathway. However, the diagnosis sensitivity of
pT181 was
104
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
lower than that of pT217. Both phosphorylation ratios were better
discriminators than p-
tau(181) and Mau levels measured by ELISA (AUC 0.874 and 0.932 respectively).
[0278] Correlations between T217 hyperphosphorylation, amyloid
pathology and cognitive status (Results) ¨ We next determined the correlations
between
this new biomarker and the amyloid process. Results from the ADRC cohort
suggested
a strong relationship between T217 hyperphosphorylation and amyloid status.
Importantly, T217 phosphorylation rate was significantly correlated with the
extent of
PiB-PET measured by FBP Total Cortical Mean (FIG. 24E, r=0.60, p=0.001).
Moreover,
all the five conflicting cases with PiB-PET(+)/CSF(-) amyloidosis profiles
were
hyperphosphorylated on T217 (FIG. 240, E). In contrast, no significant
correlation was
found between T217 phosphorylation and CSF A[342/A[340 ratio. Discrepancy
between
PiB-PET and CSF A[342/A[340 could be attributed to an insufficient sensitivity
of the
CSF amyloid assay, as corresponding CSF A[342/A[340 MS ratios were close to
the
threshold of quantification (0.12 0.02, FIG. 24E). Among the five
participants with
opposite PiB-PET(-)/CSF(+) amyloidosis, two exhibited hyperphosphorylated T217
(FIG. 240, E). This suggests these cases highlighted by their high tau
hyperphosphorylation could have significant CSF Ap changes prior to amyloid
plaques
deposits detection. Combination of CSF A[342/A[340 ratio with T217
phosphorylation
rate confidently identifies amyloid-positive participants without PiB-PET data
even with
CSF A[342/A[340 ratio values in the intermediate range slightly above the
threshold (0.12
20%). Amongst participants with no cognitive complaint (CDR-SB=0), T217
phosphorylation was able to completely distinguish amyloid-positive (n=9) from
amyloid-
negative participants (n=26, AUC 1.00, FIG. 25), further supporting the
ability of this
marker to confidently identify preclinical AD participants. However, no
correlation was
observed between global cognitive performances measured with CDR-SB and T217
phosphorylation ratio in this population (FIG. 25).
[0279] Discussion ¨ Using innovative targeted-HRMS methods
simultaneously measuring low-abundant phosphorylated tau peptides and their
unmodified counterparts in CSF, we provide for the first-time direct evidence
of changes
in CSF tau phosphorylation rates concomitant with amyloid- changes and
distinct from
105
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
increased CSF tau concentration. This approach allows for studying AD-specific
tau
phosphorylation rates assessing hyper- and hypo-phosphorylation to indicate
underlying
abnormal metabolism.
[0280] The most striking result of the current study is the highly
specific
hyperphosphorylation of T217 in CSF tau from preclinical, mild and moderate AD
participants investigated in two independent and well-characterized cohorts.
Amyloid-
pathology, which likely occurs before AD tauopathy, and tau
hyperphosphorylation on
T217 were strongly associated throughout the disease stages. This supports the
hypothesis that amyloidosis can be related to tau phosphorylation changes. The
absence of participants with A[3-amyloidosis and normal T217 phosphorylation
in this
study suggests that this tau phosphorylation site could follow the amyloid
process. The
hyperphosphorylation of T217 could be a key player in the AD
pathophysiological
process and its role differed from other tau biomarkers. In our cohorts, the
increase in
the levels of CSF tau or p-tau, as assessed by ELISA, was less effective in
identifying
amyloidosis status than pT217/T217 ratio. The specificity of these AD tau
biomarkers
may improve the prediction of the risk of cognitive decline in individuals who
ultimately
progress to clinical AD. Thus, combining amyloidosis biomarkers with
pT217/T217 ratio
measurement to detect AD, should dramatically improve diagnosis of preclinical
AD.
Although T217 hyperphosphorylation is highly correlated to amyloid plaques
measured
with PiB-PET load than amyloid markers in the CSF, tau modification may not be
exclusively caused by the presence of fibrillary plaques. For the two
participants with
T217 hyperphosphorylation and low CSF AB 42/40 without brain amyloid load
(FIG.
24E), they could be cases with diffuse plaques or AB oligomeric formation
only, not
detected by PiB-PET but contributing to reduce CSF A[342 levels.
[0281] The wide-spread use of pT181 as an AD marker in clinics is
likely
due to its high level of detectability, but not necessarily high specificity.
Measurement of
pT181 highlights a slight change in its phosphorylation stoichiometry in AD,
compared
with non-AD dementia. The increased phosphorylation rate of T181 was even more
apparent in a well-characterized amyloid-positive group investigated in the
validation
cohort. In both studies, the ratio combining ELISA t-tau and p-tau181 failed
to
106
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
demonstrate the change of phosphorylation ratio occurring on T181, stressing
the
limitations of ELISA to monitor accurately these changes. Thus, the increasing
of pT181
broadly reported in AD CSF mainly results from the concomitant increasing of
tau
isoforms level rather than significant change on the T181 phosphorylation
stoichiometry.
Amyloid- AB pathology induces hyperphosphorylation on T181 but the resulting
modification appears less specific or significant in comparison to the
relative increasing
observed on T217.
[0282] Modifications of the phosphorylation stoichiometry observed
on
S199, S202 and T205 in AD CSF likely reflect specific changes in tau
phosphorylation
previously observed in AD brain. Indeed, pS202 and pT205 are part of the
doubly
phosphorylated epitope recognized by the AT8 antibody. AT8 binds to tau
aggregates
found in AD brain autopsies and the extent of AT8-immunoreactive aggregates
correlates with the severity of tauopathy (Braak stages). AT8 has no
reactivity for
normal tau or tau related to AD measured in CSF. Moreover, the Tau1 antibody
that
binds to normal tau at a non-phosphorylated epitope containing S199 has no
affinity for
AD tau aggregates. Together, these findings support a concomitant and abundant
hyperphosphorylation of S199, S202 and T205 in tau aggregates from AD brain.
However, controversy exists about the intrinsic property of such
phosphorylated tau
isoforms to promote tau aggregation in AD. Decreased amounts of S199 and S202
phosphorylation observed in mild and moderate AD CSF is consistent with an
accumulation of the corresponding phosphorylated tau isoforms in aggregates.
Furthermore, the detection of T205 phosphorylation mainly in moderate AD CSF
suggests an important role of this site in pathological mechanisms underlying
amyloid-
related tauopathy. Modification in 5202/T205 phosphorylation was not detected
in the
second cohort composed of preclinical and mild AD participant (data not
shown),
suggesting a dynamic process of tau phosphorylation on specific sites during
the
course of the disease.
[0283] The present findings could suggest an interplay between AD
amyloidosis and hyperphosphorylation of tau on T217 and, to a lesser extent,
on T181.
These sites are both substrates for the serine/threonine proline-directed
kinase GSK-3,
107
CA 03097667 2020-10-16
WO 2019/213612 PCT/US2019/030725
and the activation of GSK-3 by Ap oligomers has been proposed as a link
between
amyloid peptide and tau phosphorylation. The common and relatively well-
correlated
hyperphosphorylation on these two GSK-3 sites in patients with amyloidosis
could be
consistent with such a mechanism. Further studies designed to compare tau
phosphorylation rates in CSF and brain, including tau aggregates measured by
tau PET,
are likely to provide novel insights into AD pathophysiology and may identify
novel
therapeutic approaches. These findings point to a specific link between
amyloid plaques
and tauopathy in AD and provide a potential link in the cascade of molecular
events that
leads to AD. Thus, given the specificity of pT217 within the AD process, it
may represent
an important target for future therapeutic development and an interesting tool
to follow
potential effects of anti-amyloid drugs in limiting this abnormal tau
metabolism.
108