Note: Descriptions are shown in the official language in which they were submitted.
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
CLAY CONTROL ADDITIVE FOR WELLBORE FLUIDS
REFERENCE TO RELATED APPLICATIONS
[0001] The present application is a Patent Cooperation Treaty (PCT)
application that
claims priority to co-pending US Provisional Patent Application No.
62/669,871,
entitled "Clay Control Additive for Wellbore Fluids," filed 10 May 2018, the
contents
of which are incorporated herein by reference.
FIELD OF THE INVENTION
[0002] Embodiments of the invention relate to one or more environmentally
friendly
liquid chemical agents used to prevent clay from swelling or disintegrating
when
exposed to water containing lower electrolyte concentration than the resident
water
surrounding the clay. More particularly, the invention relates to
environmentally
friendly liquid chemical agents used to stabilize clays and shales during the
drilling,
completion and fracturing of wells for the production of oil, gas and other
fluids from
subterranean geological formations or used to stabilize clays in geological
formations
where produced and other water are injected for disposal or for enhanced oil
recovery.
The invention also relates to environmentally friendly chemical agents used to
reverse
the effects of swelling, if swelling of clay particles has already occurred.
BACKGROUND OF THE INVENTION
[0003] Water-based fluids have been used for many years to drill, complete,
stimulate
and fracture subterranean formations in the search for oil, gas and other
formation
fluids. In hydraulic fracturing, water is introduced into downhole formation
via a
drilled well bore lined with a metal casing. The subterranean geological
formation,
believed to contain oil, gas and other fluids. is penetrated using a
specialized
explosive charge device to perforate the metal casing and create a crack or
penetration
into the shale formation. This completion step is followed later by the high-
pressure
injection of water containing one or more chemical additives with and without
the
addition of a proppant. The flow rate and pressure are at least sufficient to
create and
extend a fracture into a desired portion of the geological formation. While
other fluids
may be used in the completion process, the vast majority of completion fluids
are
water-based.
1
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
[0004] The presence of clay in an oil and gas producing formation presents
problems
for production of oil, gas and other fluids from the subterranean geological
formation.
Many clays are inert in the formation and do not interfere or disrupt the flow
of oil &
gas. Clay minerals include an assortment of chemical and physical structures.
In oil &
gas producing formations, clay minerals such as: kaolinite, smectite, illite,
chlorite are
abundant in the geological formations where oil and gas are found. Shales are
fine-
grained rocks that form from the compaction of silt and clay-sized particles.
It is
estimated that sixty percent of the Earth's crust consists of shale, which is
the primary
source rock for most of the conventional hydrocarbon deposits in the world.
Clays are
naturally occurring layered minerals formed by weathering and decomposition of
igneous rock. The oil and gas production zones that contain clays have been
dehydrated over geologic time by overburden pressure. The amount of pressure
applied to the clay, which results in the thickness of the clay platelets, is
dictated by
the amount of overburden that exists above the zone of interest as in the
depth of the
oil and gas producing zone in the well.
[0005] Clays dispersed throughout oil & gas producing formations may be
described
as stacked platelets having, for example, a net positive charge associated
with the four
short dimensional sides of each clay platelet and a net negative charge
associated with
the two long dimensional faces of each clay platelet. In other embodiments,
the
number of sides of each clay platelet can vary, including more or less than
four sides
for each clay platelet. For example, in an embodiment, the clay platelet can
have six
sides associated with a net positive charge and two faces (i.e., top face and
bottom
face) having a net negative charge. It is generally understood that the
concept of
surface charge may be used to understand the mechanisms involved in the
swelling
inhibition of clay particles. The net negative charge on the platelet face is
typically
balanced mainly by sodium ions, although other inorganic cations may also be
present
in minor amounts. The cations, or charge-balancing ions, associate with the
platelet
faces and are termed "exchangeable" as they are readily substituted with other
cations.
[0006] When clay and water are mixed, water penetrates between platelets,
forcing
the platelets to move apart, an action characterized as "swelling." The
cations on the
platelet face begin to diffuse away from the platelet faces. At the deep
subterranean
2
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
depths, typically associated with oil & gas production, the geological over-
burden
forces out the inter-layer water to minimum concentrations. When the clay is
exposed
to water under pressure, and at ineffective levels of cationic mineral ions,
swelling
and solids disintegration can occur.
[0007] In order to inhibit the swelling phenomenon, minimization of the
hydratable
surface area of the clay is necessary. This can be accomplished by
flocculating and
decreasing the surface charge density, or by increasing the ionic strength of
the water
phase, or both. By allowing cations with small charge-to-surface area ratios
to
associate with the clay particle (clay platelet), the effective strength of
the solution
will also have the same effect. Often the salt-containing water is used in the
water-
based fluids. The salt can be potassium chloride (KC1), which can convert the
clay to
a less swellable form by potassium cation exchange with the sodium (Na+) ions
present on the clay surfaces. Other salts can be used and can include calcium
chloride,
ammonium chloride, and the like, typically dissolved in the water-based
drilling and
stimulation fluids used in well completion and well fracturing.
[0008] While certain salts may be effective in protecting the clay-containing
geological formation, several problems are associated with their use, for
example: (1)
the amount of salt material needed for preparing an effective water-based
fluid may
be high, and it is often difficult to dissolve the solid salt components in
the completion
fluid in the quantities required, or in the time allotted; (2) in
environmentally sensitive
areas, there may be limits on the amount of chloride permitted; (3) the
presence of
high concentrations of salt may interfere with the function and performance of
other
chemical additives in the stimulation fluid, such as, for example, water-
viscosifying
agents and friction reducers. The hydration and performance of many
viscosifying and
friction reducing agents are inhibited by such salts.
[0009] For example, a fracturing (frac) tank usually can contain 500 bbls (80
m3 or
21,000 gallons) of water, and can require 1,591 kg (3,507 lbs) of KC1 to mix
the one
frac tank to form a 2% KC1 solution, which can be a typical suitable
concentration for
a working fluid. The KC1 can be mixed in the water prior to adding the other
chemical
additives, which can take approximately one-half hour to form the KC1 solution
for
each frac tank. There is also considerable man power required to mix the KC1
into
solution. In addition to the labor costs, there is also the risk of injury. In
addition,
3
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
there are safety concerns so the 2% KC1 solution must be contained, as an
uncontrolled release of this liquid into the environment can cause damage to
local
flora and fauna.
[0010] There is a need for a clay control agent that can function at a low
concentration and can be environmentally acceptable. There is also a need for
an
effective chemical agent to control clay swelling and to reduce the hydration
and
migration of clay particles in all or substantially all of the clay
constituents in a
heterogenous clay / shale formation while operating under increasingly
stringent
environmental guidelines.
[0011] The invention and embodiments thereof, described in the present
application,
meet these needs.
SUMMARY
[0012] The present invention relates, generally, to a clay stabilizer for
providing
improved clay inhibition in drilling, completion and well fracture fluids,
wherein the
improvement is the addition of a clay-stabilizing agent, comprising an
effective
amount of a combination of a quaternary amine-treated starch, in water, which
is
capable of cation exchange and polymeric enveloping of clay/shale particles.
The clay
stabilizer of this invention provides a universal protection against clay
swelling and
clay fines migration, regardless of the clay present in the formation.
[0013] The embodiments of the present invention are capable of restoring
permeability in subterranean geological formations that have been previously
damaged, such as through the introduction of untreated or poorly treated water
or
aqueous based drilling or completion fluids. The clay stabilizer can be used
without
prior knowledge of or the need to determine the clay types in the formation,
and
variations of the clay stabilizer blends may be used in water-based drilling
fluids, well
fracturing fluids, acidizing fluids, or can be used alone to treat previously
damaged
wellbores to restore permeability therein.
[0014] In a broad aspect of the invention, the clay stabilizer comprises
effective
amounts of one or more quaternary amine functionalized starch polymers that
are
capable of cation exchange within the one or more clay types within the
geological
4
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
formation. Each of the quaternary amine functionalized starch polymers can
have a
similar or different molecular weight and configuration relative to each of
the other
quaternary amine functionalized starch polymers, with the balance being water.
The
quaternary amine-functionalized starch polymer of the invention can have an
average
molecular size of up to 2,000 repeating units and may have a molecular weight
of up
to 1 million repeating units.
[0015] Another object of the invention is to provide clay / shale stabilizing
functioning over a wide pH range of at least 4.0 to 13Ø
[0016] Embodiments of the invention provide advantages over the prior art. The
clay
stabilizer of the invention provides a clay-stabilizing agent which is
substantially odor
free, and poses little to no threat to the environment by eliminating the use
of harmful
chemical compounds in its production. The clay stabilizer of the invention
provides a
clay-stabilizing agent that is at least as effective as the most effective
prior art agent,
and is at least as cost effective as the prior art agents. Other objects and
advantages of
the present invention will become apparent upon consideration of the ensuing
description.
DRAWINGS
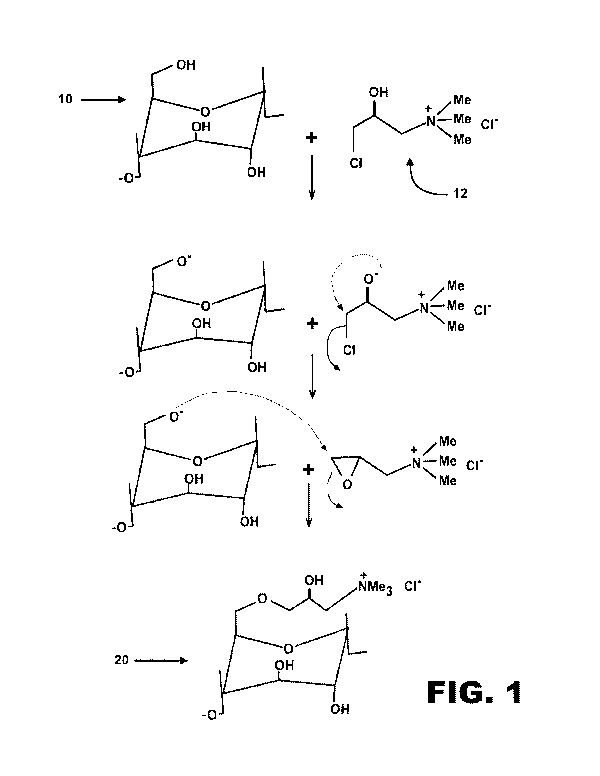
[0017] FIG. 1 depicts an embodiment of a cationization reaction usable in the
present
invention.
[0018] FIG. 2 depicts the results of a capillary suction test (CST) on several
clay
control agents and mixtures thereof, as described below.
[0019] FIG. 3 depicts the results of a CST test on several clay control agents
with and
without a cactus mucilage additive, as described below.
[0020] FIG. 4 depicts the results of a CST on several clay control agents at
pH levels
of between 4 and 13, as described below.
[0021] FIG. 5 depicts the results of a CST on several clay control agents with
and
without a cactus mucilage additive, as described below.
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
[0022] FIG. 6 depicts the results of a Fines Migration Test on several clay
control
agents and mixtures thereof, as described below.
DETAILED DESCRIPTION
[0023] The present invention is a clay control treatment for the common types
of clay,
including layered silicates, oxides, amorphous / allophones, and chained
silicates.
Typically, a clay particle is about one nanometer in thickness and up to
several
nanometers in width. Each layer is comprised of fused sheets of octahedral
Al+3,
Mg+2, or Fe+3 oxides and sheets of tetrahedral Si+4 oxides. If a clay mineral
contains one tetrahedral sheet and is bonded to one octahedral sheet, it is
known as a
1:1 clay. An octahedral sheet bonded to two tetrahedral sheets is known as a
2:1 clay.
The clays are classified as expanding (smectite) and non-expanding clays
(illite and
micas) on the basis of the sheet where isomorphous substitution takes place.
Clay
minerals are colloids and are characterized by a small particle size and large
surface
area. The surfaces carry charges which influences their ability to attract or
repulse
charge ions to or from surfaces. Clay minerals have large cation exchange
capacities,
this enables them to be modified to enhance sorption of organic and anionic
contaminants.
[0024] When these subterranean oil and gas formations containing clay
particles are
exposed to fresh or lower salinity water-based drilling fluids or water-based
completion fluids, such as in well fracture operations, the clays may
osmotically
absorb water from the water-based drilling fluid and, in turn, swell. The
swelling of
the clay induces stresses, loss of mechanical strength, and can lead to shale
failure or
disintegration of the shale, which may lead to plugging of the pore spaces
that exist or
were created during the formation fracturing operations, which potentially
reduces the
flow of oil and gas from within the zone, into the well bore and up to the
surface. The
production of oil, gas and fluids can be restricted by the presence of clays
or other
fines capable of migrating within the formation. These fines tend to migrate
as the oil,
gas and fluids flow from the reservoir rock into the wellbore. The fines
encounter
constrictions in the capillary flow channels of the reservoir formation and
bridge off
the flow channel, thus severely reducing the flow of oil, gas and fluids into
the
wellbore.
6
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
[0025] Water-based drilling fluids and water, used in the completion and well
fracturing operations, has been shown to disturb the fine clay particles. The
clay
particles are generally subcategorized as water-swelling and water-fracturing
particles. The water-swelling clay particles are generally comprised of
smectite clay,
and the non-swelling, water-fracturing clay particles are comprised of illite
clay.
These two general subcategories of clay particles are a major cause of
formation
damage and reduced flow or loss in production of oil, gas and fluids from
subsurface
geological reservoir formations. Smectite clays are of the type 2:1 and
frequently
occur in drilling and completion situations. Sodium saturated smectite swells
macroscopically, which causes instability of shales during drilling operations
and
during well completions. The wellbore may collapse as a result of this
swelling, or the
production zone in the oil and gas-producing reservoir can be shut off
[0026] The cations present at the clay platelet faces begin to diffuse away
from the
platelet faces. Further, the amount of water contained within the platelets is
dependent
upon the pressure under which the clay is located. In oil and gas producing
formations, much of the water between the platelets has been squeezed out and
forms
spaces, wherein each space is only a few molecules thick with the water bound
to the
internal clay surfaces and the cations associated with the clay structure. The
water
sensitive clay can be located on the surface or subsurface in geological
formations.
The chemical agents described herein minimize swelling and migration of clay
fines
found in clay containing formations and have demonstrated an ability to
substantially
reverse the effects of swelling, if swelling has already occurred.
[0027] The effect of water on clay particles occurs generally by three
mechanisms:
(1) surface hydration through bonding of water molecules to oxygen atoms on
the
surface of the clay platelets, (2) ionic hydration through hydration of the
interlayer
cations with surrounding shells of water molecules, and (3) osmotic hydration
that
occurs in some clays after they are completely surface and ionically hydrated.
[0028] While all clays experience hydration, smectite and illite clays exhibit
varying
degrees of ionic hydration. When surface hydration occurs, the osmotic
absorption
results in two distinctly different problems. One problem includes swelling in
the case
of smectitic clays, where the clay platelets expand due to water uptake. The
other
problem is fracture in the case of illitic clays, where dispersion of clay
particles
7
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
occurs. Swelling is less common in harder shales, due to lower smectite
content.
However, high downhole stresses can lead to a fracturing of the illite
containing shale,
especially if accompanied by high pore pressures. Once the illitic clays
fracture, the
resulting disintegrated particles can travel through the oil & gas producing
formation
and lodge in pore channels reducing and/or plugging the flow of fluids from
within
the formation into the well bore. Most fractures are not bounded by barriers
of
sufficient strength to contain the fracture, e.g., contain height growth. The
majority of
fractures are radial from the well bore. The natural and man-made fractures
within the
formation can be damaged by contact with fresh water or water absent of any
clay
control chemical additives.
[0029] It has been found that potassium (K+) is much better at creating
electrostatic
links between negatively charged faces of the stacked clay platelets than the
abundant
Na+, therefore allowing less osmotic migration of water to occur between the
clay
platelets. Monovalent cations have lower hydration energies than divalent
cations, and
ions with smaller radii have greater hydration energies than those with larger
radii.
Large radii monovalent cations, like K+, have the lowest hydration energies,
and
small divalent cations have the highest hydration energies, such as Magnesium
(Mg+2). Low hydration energy correlates with minimized swelling of clays.
[0030] Evaluating the performance of clay stabilizers on swelling clays is
commonly
performed using a capillary suction test (CST). This test can be used to
examine the
sensitivity of various formation solids samples and also to compare the
performance
of any clay stabilizer on a physical clay sample. The CST column is packed
with a 60
mesh (between 300 and 340 micron) clay sample, and the time for the sample to
imbibe 2 cm through the filter paper and the shale is recorded as CST time in
seconds.
Shorter CST time indicates less water is adsorbed by the clay particles, and
the more
inhibitive the clay stabilizer is on a particular sample, and the longer the
time, the less
inhibitive the clay stabilizer. The CST thus measures the ability to limit
swelling
clays.
[0031] For evaluating the performance of clay stabilizers on migrating or
fracturing
clays, their permeability can be measured on an apparatus that can consist of
a one
foot column that is packed with 20/40 mesh sand packed and crushed clay or
shale
sample. Without the clay stabilizer, the permeability decreases from roughly
130
8
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
Darcies to about 70 Darcies after flowing at 80 ml (2.7 fl oz.)/min for 30
minutes.
Using a clay stabilizer in the water flowing through the apparatus, the
permeability
remains constant over the course of the test, indicating that the clay fines
were
immobilized.
[0032] The clay stabilizers, used to prevent swelling clay interference, are
more
typically ion exchange driven, where the clay stabilizers to prevent fines
migration are
more typically polymeric types.
[0033] Embodiments of the present invention comprise a mixture of constituents
applicable for use in heterogeneous shale/clay formations as a clay stabilizer
to
minimize swelling and migration of fines within the formation.
[0034] More particularly, the clay stabilizer usable in the present invention
can
comprise an aqueous-blended cationic starch polymer composition that can be
used as
an additive in other wellbore fluids or can be used alone as a treatment for
the
wellbore, typically in matrix stimulation. The clay stabilizer can contain
effective
amounts of one or more commercial starch quaternary amine-functionalized
starch
polymers, with an average molecular size of up to 2,000 repeating units that
are
capable of cation exchange within one or more clay types, which exist in the
homogeneous and heterogeneous formations.
[0035] As used herein, a starch is a polysaccharide that is comprised of
repeating
glucose units. The starch molecule has a basic chemical formula of (C6H1005)n.
The
glucose units can be arranged in a straight chain manner identified as
Amylose, or in a
highly branched manner identified as Amylopectin. The starch is cooked in
water to
prepare it for commercial use. Cooking affects the starch granule's structure
and
properties. Starch is later modified to provide various properties including:
viscosity
development and stability; mitigate degradation and set back; freeze thaw
stability;
heat, acid and shear stability; ionic strength and flocculation;
emulsification, and
texture. Chemical derivatization of the starch can create a starch derivative
with either
a cationic, anionic, or nonionic charge. The chemical derivatization
introduces special
functionalities.
[0036] FIG. 1 depicts an embodiment of the invention in which the active
ingredient
of the clay stabilizer comprises a quaternary amine functionalized starch
polymer. An
9
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
example of a preferred quaternary amine reagent is an aqueous solution of
monomer
(3-chloro-2-hydroxypropyl) trimethylammonium chloride (CHPTAC) 12. This
chemical may be used to modify starch polymers, depicted here as an anhydrous
glucose unit 10, into quaternary ammonium compounds 20. The most important
chemical building block of the starch is the anhydroglucose unit (AGU). Each
glucose
unit has three hydroxyl (OH-) functions available for etherification with
CHPTAC.
Under suitable reaction conditions, all hydroxyls can be etherified. In one
embodiment of a wet process method, the reaction can be tested in a laboratory
method for forming a cationic starch by reacting the CHPTAC with a starch
product
that involves adding 500 grams (17.6 oz) of distilled water into a 1-liter
Erlenmeyer
flask. Next, five (5) grams (0.18 oz) of the CHPTAC (quaternerizing agent) can
be
added into the water in the Erlenmeyer flask, followed by the addition of 60
grams
(2.1 oz) of unmodified industrial grade corn starch. To the mixture, 5 grams
(0.18 oz)
of Sodium Sulfate (Na2SO4) can be added; and then, the mixture can be mixed
well in
a blender for approximately 7-minutes. To the mixture in the blender, 2 grams
(0.07
oz) of quick lime (CaO) can be added, and the mixture can be mixed in blender
for an
additional 3 minutes. Then, the mixture can be poured from the blender into a
1-liter
(0.26 gal) beaker, and the beaker can be placed into a hot water bath with
water
temperature of approximately 45 C for about 6-hours. At the end of the 6-
hours, the
mixture can be neutralized to a pH of 6 to 8 with a 10N Hydrochloric acid
(HC1).
Quaternary ammonium compounds are cationic, meaning they carry a positive
electrical charge. Cationic starch polymers are useful as flocculants and as
clay
control agents.
[0037] The embodiment set forth above is only one embodiment of the wet
process
method. Other embodiments can include varying amounts of the ingredients,
varying
times for blending and mixing, and varying temperatures for the hot water
baths, as
well as varying lengths of time for placing the mixture in the hot water
baths.
Depending upon the type of starch used as a starting material, the wet process
to
produce a cationic starch suitable for this invention involves a starch such
as derived
from corn, potato, or wheat with a moisture content of between 12% and 20%.
Beginning with 200 grams (0.44 lbs) of starch that has been air dried to the
moisture
levels stated add from 12 to 18 grams (0.42 ¨ 0.63 oz) of CHPTAC. To this
mixture
add 5.5 to 8.5 grams (0.19 ¨ 0.28 oz) of a 30% solution of sodium hydroxide.
Follow
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
the first addition of sodium hydroxide with a second addition of 17 to 34
grams (0.60-
1.20 oz) of a 10% sodium hydroxide solution. Between 165 to 192 grams (5.8 ¨
6.8
oz) of additional water can be added to the mixture to obtain a total quantity
in the
blend. The total amount of water can be reduced to increase the concentration
however, due to the chemistry, a minimum quantity of water or moisture in the
starch
is always needed for the cationization reaction. If the water content gets too
low, the
slurry cannot be stirred properly or pumped efficiently. Quaternary ammonium
compounds are cationic, meaning they carry a positive electrical charge.
Cationic
polymers are known to be useful as flocculants and as clay control agents.
[0038] Polymers with cationic groups demonstrate increased water solubility as
a
result of the highly water soluble quaternary ammonium group. A chemical
process of
reacting a quaternary amine with a starch polymer, preferably amylopectin, can
produce the quaternary functionalized starch polymer. Cationization is
measured by
the "degree of substitution" (DS). The degree of substitution indicates the
average
number of hydroxyl groups on each AGU. In theory, the three OH sites available
in
each glucose unit for reaction means the maximum obtainable value for starch
is 3.
The degree of substitution is expressed as moles of reagent per AGU. When
determining the actual obtained ("practical") DS, only the polymer bound
portion is
considered. The DS value is used for comparison purposes. In starch
cationization,
practical DS substitution of 0.50 and higher can be obtained. Depending upon
the type
and origin of the starch, biopolymers contain greater or smaller quantities of
protein
nitrogen.
[0039] Under alkaline conditions, this protein nitrogen is partially soluble
in the
reaction medium and is separated when the polymer is washed. To determine the
yield
correctly, the percentage of protein nitrogen, which is insoluble under the
cationization conditions, must be deducted from the nitrogen content
determined after
washing. Without this correction, the yield values would be overstated. It is
also
possible to use a starch slurry directly from native starch production. In
this case,
minimum water concentration is given and each subsequent addition of reagents
causes further dilution of the starch slurry. In order to avoid undesirable
losses in
reaction efficiency and production capacity, it is advantageous to prevent
further
dilution. The quaternary functionalized starch polymer can function with
varying DS;
11
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
however, it is preferred that the starch polymer have a high degree of
substitution, for
example, greater than 0.30 degrees. The higher degree of substitution
demonstrates an
improved binding to the clay particles.
[0040] In an embodiment of the invention, an aqueous slurry of cationic starch
can be
used, and the starch can be an amylose cationic starch. The starch may be
obtained
from any conventional source, including potato, corn, waxy corn, red milo,
white
milo, wheat, duckweed, and tapioca, and may be pearl or lightly thinned. In
addition,
the starch may have been oxidized, hydroxyalkylated, acid modified, enzyme
converted or various combinations thereof Higher molecular weight starches are
preferred in embodiments of the present invention because of greater strength
than
low molecular weight starches. The starches from various sources may be
blended
and subsequently chemically treated to produce a formulated starch of a
particular
capability. It is important for the starch to contain cationic functionality
to enhance
the clay control performance. In an embodiment of the present invention, the
starch
can be pre-cooked and modified with cationic substitution of tertiary amine or
quaternary amine groups to give the starch an overall cationic charge to
function
effectively as a clay control additive.
[0041] Non-limiting examples of a cationic starch polymer which may be used as
a
clay control additive in water-based completion, stimulation and well fracture
operations, include, but are not limited to: AquaBloc 330AW from Aquasol or;
ChargeMasterTm L-340 or ChargeMasterTm L-360 from Grain Processing Corporation
or; Sta-LokTM 280 from Tate & Lyle or; C BondTM cationic starches from Cargill
or:
ViscoStarTM 4630, ViscoStarTM 4620 from International Starch Trading A/S;
WespolyTM from Western Polymer Corporation or: GlucoPlusTM C+2F, GlucoPlusTM
C+2P, GlucoPlusTM C+3F, GlucoPlusTM C+3P from Chemstar or; RediBondTM 2038,
RediBondTM 5327, RediBondTM 5330A, OptiproTM 650, Cato from Ingredion.
[0042] Evaluating chemical additives for performance as a clay control
additive is
commonly done using a capillary suction time (CST) test 30, as shown in FIG.
2. The
CST test has been widely used to study the colloidal properties of clay
suspensions.
The petroleum industry uses the CST test to characterize clay-containing
shales and to
optimize the electrolyte or ionic salt concentration in drilling fluids for
minimizing its
negative effects in such shale formations. In the CST test, the more
flocculated the
12
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
rock-water slurry, the more permeable the core sample and the shorter the time
interval required to complete the test. Therefore, the shorter the CST time,
the smaller
the normalized value for a system, and the better the inhibition of shale
swelling in the
individual core sample.
[0043] When conducting the CST test, it is common to use a highly water
sensitive
clay sample, a sample with over 10% smectite, and expose this clay sample to
fresh or
salt water without any clay control treatment in the water. The CST value
reported is
considered the blank or untreated benchmark value. The lowered CST values
reported
for different clay control additives provide a range to quickly and easily
identify the
most effective clay control additive.
[0044] FIG. 2 depicts the results of a CST test performed using the preferred
cationic
starch polymer of this invention 202, and comparing the result to a blank
untreated
sample 200, as well as a sample treated with an industry leading clay control
additive
for use in well completion and well fracture operations 204. One of the
leading clay
control chemical additives today comprises a 50% to 70% aqueous solution of
choline
chloride. Choline chloride is a quaternary ammonium salt with a chloride
anion. The
chemical name is (2-hydroxyethyl) trimethyl ammonium chloride. Choline
chloride is
typically supplied with a pH from 7 to 9 with a specific gravity of 1.07 to
1.091. It is
preferred today over the use of a tetramethyl ammonium chloride (TMAC) due to
the
fact it has been determined by researchers to be more environmentally
friendly.
Choline chloride is produced in large volumes for use as a clay control
additive in
completion and hydraulic fracturing, also as an important additive in animal
feed and
for forming deep eutectic solvents.
[0045] While choline chloride is the most preferred and used choline salt for
clay
control other suitable choline carboxylate counterion salts where the
carboxylate
counterion is of the general formula R1C00-, where R1 is an alkyl group,
alkenyl
group, alkynyl group, an aryl group, an alkaryl group, an aralkyl group,
alkenylaryl
group, aralkenyl group, alkynylaryl group, aralkynyl group hetero atom
analogs,
where the hetero atom is selected from a group consisting of boron, nitrogen,
oxygen,
fluorine, phosphorus, sulfur, chlorine, bromine, iodine, and mixture or
combinations
thereof A non-exhaustive list of exemplary examples of choline carboxylate
counterion salts include choline formate, choline acetate, choline propanate,
choline
13
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
butanate, choline pentanate, choline hexanate, choline lactate, choline
citrate, choline
tartrate, choline itaconate, and mixtures and combinations thereof
[0046] In a clay control test, the blank or untreated clay sample reported a
CST value
of 40 seconds when exposed to fresh water. Using the preferred cationic starch
polymer at a 3% active concentration at a treated at a rate of 2-gallons per
thousand
gallons of water (approximately 2,000 mg/1) it reported a CST value of 14.4
seconds,
a 178% improvement in clay control as compared to fresh water alone. When
compared to choline chloride at the same treatment rate, the choline chloride
CST
value was recorded as 17.1 seconds. This demonstrates the cationic starch
polymer of
this invention providing a 16% improvement in clay control when compared to
the
use of the 70% aqueous choline chloride at the same treatment rate of 2-
gallons per
thousand gallons. The use of the cationic starch polymer offers an improved
alternative to the use of choline chloride and avoids introducing a chloride
salt into
the environment.
[0047] In an embodiment, the invention may also comprise a unique clay control
supplement made from a plant-derived, natural chemical additive that, alone or
when
combined with the cationic starch polymer, can further improve clay control
results.
The plant-derived, natural chemical additive is a mucilage extract of a cactus
known
for its mucilage production. Mucilage is a thick gelatinous substance produced
by
various plants, including cactus, which contains protein and polysaccharides
and is
similar to plant gums.
[0048] In particular, the Opuntia genus produces a complex carbohydrate with a
great
capacity to absorb water. A preferred plant to obtain cactus mucilage is the
Opuntia
ficus-indica, commonly referred to as the prickly pear cactus or nopal. The
prickly
pear cactus is commonly found in the southwestern United States and is
considered an
invasive weed which is difficult to control and is intruding onto productive
grazing
land for animals causing problems for farmers and ranchers. The prickly pear
cactus is
found in many regions around the world. In Mexico it is commonly found and
cultivated in tree-like proportions. The mucilage is a thick, gummy substance
that
resembles an industrial hydrocolloid. When water enters the mucilage it
swells,
producing a unique surface-active property to the solution.
14
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
[0049] The mucilage contains varying proportions of many sugar residues,
including
1-arabinose, d-galactose, 1-rhamnose, and d-xylose, as well as galacturonic
acid. The
molecular weight of the mucilage has been reported as different values,
probably due
to differences in extraction techniques and the possibility of contaminants.
The
molecular weight can range from as little as 3,000 up to over 1,000,000
daltons. The
sugar residues have a demonstrated capacity to interact with metals and
cations. The
mucilage content is found primarily in the cactus cladodes. In some regions,
farmers
use cactus mucilage as a flocculant to purify drinking water by removing
harmful
metal constituents and reducing the turbidity of the water. The mucilage has
the
potential to precipitate ions and clay particles from aqueous solutions. The
precipitation of clay particles reduces the migration of clay fines within the
pores of
the geological formation. Just as has been discovered how polyacrylamides and
polysaccharides can be used as soil additives to improve the physical
properties of
soil, including infiltration of water, so can cactus mucilage.
[0050] Returning to FIG. 2, it can be seen that the mucilage extract from the
prickly
pear cactus performs well as a clay control agent alone 206 and in combination
with
the cationic starch polymer 208 and the choline chloride 210. For example, a
blank
untreated sample of clay in fresh water yields a CST value of 40 seconds. When
the
same clay sample is treated with 2 gallons per thousand gallons of cactus
mucilage
extract in fresh water, the CST value drops to 15.7 seconds. The lower the CST
value
the better the clay control performance. When the clay sample is treated at 2
gallons
per thousand gallons (GPT) with the 70% choline chloride it reports a CST
value of
17.1 seconds. The cactus mucilage extract reports approximately an 8%
improvement
in CST value.
[0051] In an embodiment, by combining the aqueous cactus mucilage solution
with a
cationic starch polymer at a 90:10 ratio (90 parts cationic starch polymer to
10 parts
cactus mucilage) and added to the fresh water containing the same sample of
water
sensitive clay as used in the earlier tests, the CST is reported as 14.6
seconds, nearly a
15% improvement when compared to the industry leading clay control chemical,
the
70% choline chloride solution. By using a blend comprising of 5% active
aqueous
solution of cactus mucilage and 35% active aqueous solution of choline
chloride the
CST is reported as 15.8 seconds, a 7.6% improvement. The result of comparative
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
testing demonstrates how the extract of the cactus mucilage measurably
improves the
performance of clay control in water-based completion and fracture fluids.
[0052] Turning now to FIG. 3, the CST results are shown here in descending
order of
CST values (i.e., ascending order of efficacy). The blank CST run is
eliminated in this
graph for clarity. Shown are the standard 70% choline chloride additive 204,
an
additive of 5% cactus mucilage and 35% choline chloride 210, a 100% cactus
mucilage additive 206, an additive of 90% cactus mucilage and 10% cationic
starch
polymer 208, and finally an additive of 3% cationic starch polymer 202.
[0053] In another embodiment, further testing of clay control performance
included
the combination of the preferred cationic starch polymer with aqueous
electrolytes
such as: potassium formate, potassium chloride, and sodium chloride. In all
tests it
was demonstrated the addition of as little as 0.5% of cationic starch polymer
added to
a aqueous electrolyte solution improved the clay control performance
sufficiently
allowing for the reduction of the concentration of electrolyte. For example,
it is
typical for oil & gas operators to use a 2% by weight solution (approximately
20,000
mg/L) of potassium chloride (KC1) in water to inhibit water-sensitive clay. By
comparison, the cationic starch polymer of this invention functions
satisfactorily as a
clay control with as little as approximately 200 mg/L of active material in
water, an
improvement of 100 times.
[0054] Turning now to FIG. 4, the results of further CST testing demonstrate
that the
quaternary amine cationic starch polymer of this invention, either alone in a
5%
concentration 400 or at 5% concentration in combination with the 35%
concentration
of choline chloride 402, retains most of its functionality as a clay control
additive at
pH level of between 4 and 13. Importantly, these results also demonstrate that
a small
amount of the starch additive permits a drastic reduction in the amount of
salt additive
with an only modest corresponding reduction in clay control efficacy.
[0055] Turning now to FIG. 5, the results of further CST testing demonstrates
that the
cactus extract of this invention, in a 5% concentration in combination with a
10%
potassium formate 502 is superior to a 5% quaternary amine cationic starch of
this
invention in combination with 25% choline chloride 501 and a 70% choline
chloride
503.
16
CA 03099774 2020-11-09
WO 2019/217908
PCT/US2019/031848
[0056] Turning now to FIG. 6, the quaternary starch of this invention has, in
addition
to reducing clay swelling, demonstrated superior performance in reducing the
migration of clay fines. This is especially true when a formation contains
illitic type
clay, which tends to fragment in presence of low salinity water or in the
presence of
water containing a lower dissolved solids concentration than the shale's
resident
water. The fragmenting of the clay causes small particles or "fines" to be
dispersed in
the water. Fines migration causes suspended particles in the production fluid
to bridge
over the pore spaces near the wellbore, causing formation damage and reducing
productivity.
[0057] FIG. 6 shows the ability of the quarternary starch of this invention to
agglomerate or flocculate the fine particles, stabilizing them within the
formation and
preventing migration through the wellbore. The results of a 30 minutes fines
migration test are shown utilizing a blank (water) run 600, a run with a 5%
concentration of the quaternary anime cationic starch (at 0.5 GPT) 602, and a
run with
a 70% concentration of choline chloride (also at 0.5 GPT) 604. Crucially, this
test
shows that the choline chloride additive by itself may actually worsen fines
migration
at some points. The quarternary starch of the invention, alone and in
combination with
choline chloride or potassium formate, demonstrates an improvement in reducing
fines migration in low-salinity water compared to choline chloride alone.
[0058] While various embodiments usable within the scope of the present
disclosure
have been described with emphasis, it should be understood that within the
scope of
the appended claims, the present invention can be practiced other than as
specifically
described herein.
17