Note: Descriptions are shown in the official language in which they were submitted.
REPLACEMENT MITRAL VALVES
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] The present application claims priority on U.S. Application Serial No.
16/012,666, filed June 19,
2018, which is a continuation-in-part of International Patent Application No.
PCT/US2018/14902, filed
January 23,2018, titled "REPLACEMENT MITRAL VALVES", which claims priority to
U.S. Provisional
Application No. 62/513,877, filed June 1, 2017 and to U.S. Provisional Patent
Application No. 62/449,498,
filed January 23, 2017, and titled "REPLACEMENT MITRAL VALVES".
[0002] This application may also be related to International Patent
Application No. PCT/U52016/032550,
filed May 13, 2016, titled "REPLACEMENT MITRAL VALVES", to U.S. Patent
Application No.
14/170,388, filed January 31,2014, titled "SYSTEM AND METHOD FOR CARDIAC VALVE
REPAIR
AND REPLACEMENT," now U.S. Patent No. 8,870,948, and to U.S. Patent
Application No. 14/677,320,
filed April 2, 2015, titled "REPLACEMENT CARDIAC VALVES AND METHODS OF USE AND
MANUFACTURE".
[0003] [Intentionally left blank].
BACKGROUND
[0004] The mitral valve lies between the left atrium and the left ventricle of
the heart. Various diseases
can affect the function of the mitral valve, including degenerative mitral
valve disease and mitral valve
prolapse. These diseases can cause mitral stenosis, in which the valve fails
to open fully and thereby
obstructs blood flow, and/or mitral insufficiency, in which the mitral valve
is incompetent and blood flows
passively in the wrong direction.
[0005] Many patients with heart disease, including those with mitral valve
problems, are intolerant of the
trauma associated with open-heart surgery. Age or advanced illness may have
impaired the patient's ability
to recover from the injury of an open-heart procedure. Additionally, the high
costs associated with open-
heart surgery and extra-corporeal perfusion can make such procedures
prohibitive.
[0006] Patients in need of cardiac valve repair or cardiac valve replacement
can be served by minimally
invasive surgical techniques. In many minimally invasive procedures, small
devices are manipulated
within the patient's body under visualization from a live imaging source like
ultrasound, fluoroscopy, or
endoscopy. Minimally invasive cardiac procedures are inherently less traumatic
than open procedures and
may be performed without extra-corporeal perfusion, which carries a
significant risk of procedural
complications.
[0007] Minimally invasive aortic valve replacement devices, such as the
Medtronic Corevalve or the
Edwards Sapien, deliver aortic valve prostheses through small tubes which may
be positioned within the
heart through the aorta via the femoral artery or through the apex of the
heart. However, the mitral valve
- 1 -
Date Recue/Date Received 2022-07-12
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
differs from the aortic valve in that the shape and anatomy immediately
surrounding the valve varies greatly
from one side of the valve to the other. Moreover, current cardiac valve
prostheses are not designed to
function effectively within the mitral valve. Further, current cardiac valve
prostheses delivered via a
minimally invasive device are often difficult to place correctly within the
native valve, difficult to match in
size to the native valve, and difficult to retrieve and replace if initially
placed incorrectly.
[0008] These and other deficiencies in existing approaches are described
herein.
SUMMARY OF THE DISCLOSURE
[0009] In general, in one embodiment, a prosthetic mitral valve includes an
anchor assembly, a strut frame,
and a plurality of replacement leaflets secured to the annular strut frame.
The anchor assembly includes a
ventricular anchor, an atrial anchor, and a central portion therebetween. The
ventricular anchor and the atrial
anchor are configured to flare radially outwards relative to the central
portion. The annular strut frame is
disposed radially within the anchor assembly and is attached to the anchor
assembly at a plurality of
attachment locations that are positioned between the central portion and an
atrial-most edge of the anchor
assembly. The central portion is configured to align with a native valve
orifice and the ventricular anchor
and the atrial anchor are configured to compress native cardiac tissue
therebetween.
[0010] This and other embodiments can include one or more of the following
features. An atrial end of the
strut frame can be attached to the anchor assembly. Atrial tips of the strut
frame can be attached to the
anchor assembly. An atrial end of the strut frame can be flared radially
outwards. A flare of the strut frame
can be configured to substantially conform to a flare of the atrial anchor. A
ventricular end of the strut frame
can be spaced away from the anchor assembly. The ventricular end of the strut
frame can be spaced away
from the anchor assembly by a radial distance of 1-15mm. The anchor assembly
and the strut frame can be
configured to self-expand from a constrained configuration to an expanded
configuration. The strut frame
can be attached to the anchor assembly with a plurality of rivets. Each of the
plurality of attachment
locations can be radially aligned with tips of the atrial anchor. The
plurality of attachment locations can each
be part of the anchor assembly that extends further radially inwards than a
remaining portion of the anchor
assembly. The anchor assembly can comprise a plurality of diamond-shaped
cells. The plurality of
attachment locations can be positioned at a mid-point of the outermost atrial
diamond-shaped cells. The strut
frame can include a plurality of linear struts and v-shaped connectors
therebetween. The anchor assembly
can form a substantially hour-glass shape.
[0011] In general, in one embodiment, a prosthetic mitral valve includes an
anchor assembly, an annular
strut frame, and a plurality of replacement leaflets secured to the annular
strut frame. The anchor assembly
includes a ventricular anchor, an atrial anchor, and a central portion
therebetween. The ventricular anchor
and the atrial anchor are configured to flare radially outwards relative to
the central portion. Further, the
anchor assembly comprises a plurality of diamond-shaped cells. The annular
strut frame is disposed radially
within the anchor assembly and is attached to the anchor assembly at a
plurality of attachment locations that
- 2 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
are positioned at a mid-point of the outermost atrial diamond-shaped cells
between the central portion and an
atrial-most edge of the anchor assembly.
[0012] This and other embodiments can include one or more of the following
features. An atrial end of the
strut frame can be attached to the anchor assembly. Atrial tips of the strut
frame can be attached to the
anchor assembly. An atrial end of the strut frame can be flared radially
outwards. A flare of the strut frame
can be configured to substantially conform to a flare of the atrial anchor. A
ventricular end of the strut frame
can be spaced away from the anchor assembly. The ventricular end of the strut
frame can be spaced away
from the anchor assembly by a radial distance of 1-15mm. The anchor assembly
and the strut frame can be
configured to self-expand from a constrained configuration to an expanded
configuration. The strut frame
can be attached to the anchor assembly with a plurality of rivets. Each of the
plurality of attachment
locations can be radially aligned with tips of the atrial anchor. The
plurality of attachment locations can each
be part of the anchor assembly that extends further radially inwards than a
remaining portion of the anchor
assembly. The strut frame can include a plurality of linear struts and v-
shaped connectors therebetween. The
anchor assembly can form a substantially hour-glass shape.
[0013] In general, in one embodiment, a prosthetic mitral valve includes an
anchor assembly, an annular
strut frame, and a plurality of replacement leaflets secured to the annular
strut frame. The anchor assembly
further includes a ventricular anchor, an atrial anchor, and a central portion
therebetween. The ventricular
anchor and the atrial anchor are configured to flare radially outwards
relative to the central portion. Further,
the atrial anchor includes a plurality of atrial cells and the ventricular
anchor includes a plurality of
ventricular cells. The annular strut frame is disposed radially within the
anchor assembly. A first plurality of
the atrial cells are positioned radially inwards relative to a second
plurality of the atrial cells such that the
first plurality of cells attach the strut frame to the anchor assembly.
[0014] This and other embodiments can include one or more of the following
features. The central portion
can be configured to align with a native valve orifice, and the ventricular
anchor and the atrial anchor can be
configured to compress native cardiac tissue therebetween. An atrial end of
the strut frame can be attached
to the anchor assembly. Atrial tips of the strut frame can be attached to the
anchor assembly. An atrial end
of the strut frame can be flared radially outwards. A flare of the strut frame
can be configured to
substantially conform to a flare of the atrial anchor. A ventricular end of
the strut frame can be spaced away
from the anchor assembly. The ventricular end of the strut frame can be spaced
away from the anchor
assembly by a radial distance of 1-15mm. The anchor assembly and the strut
frame can be configured to
self-expand from a constrained configuration to an expanded configuration. The
strut frame can be attached
to the anchor assembly with a plurality of rivets. The first plurality of
atrial cells can end in disconnected
apexes. The disconnected apexes can be radially aligned with outer-most tips
of the second plurality of atrial
cells. The first plurality of atrial cells can be angled at approximately 70-
80 degrees relative to the axis that
extends through the central portion. The second plurality of atrial cells can
be angled at approximately 20-30
- 3 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
degrees relative to the axis that extends through the central portion. The
annular strut frame can flare radially
outwards at 70-80 degrees relative to the axis that extends through the
central portion.
[0015] In general, in one embodiment, a prosthetic mitral valve includes an
anchor assembly, an annular
strut frame, and a plurality of replacement leaflets secured to the annular
strut frame. The anchor assembly
includes a ventricular anchor, an atrial anchor, and a central portion
therebetween. The ventricular anchor
and the atrial anchor are configured to flare radially outwards relative to
the central portion. Further, the
atrial anchor includes a plurality of atrial cells. The annular strut frame is
disposed radially within the anchor
assembly. A first plurality of the atrial cells are interior disconnected
apexes and the second plurality of
atrial cells are outermost atrial cells. The first plurality positioned
radially inwards relative to a second
plurality of the atrial cells such that the first plurality of cells attach
the strut frame to the anchor assembly.
[0016] This and other embodiments can include one or more of the following
features. The central portion
can be configured to align with a native valve orifice. The ventricular anchor
and the atrial anchor can be
configured to compress native cardiac tissue therebetween. An atrial end of
the strut frame can be attached
to the anchor assembly. Atrial tips of the strut frame can be attached to the
anchor assembly. An atrial end
of the strut frame can be flared radially outwards. A flare of the strut frame
can be configured to
substantially conform to a flare of the atrial anchor. A ventricular end of
the strut frame can be spaced away
from the anchor assembly. The ventricular end of the strut frame can be spaced
away from the anchor
assembly by a radial distance of 1-15mm. The anchor assembly and the strut
frame can be configured to
self-expand from a constrained configuration to an expanded configuration. The
strut frame can be attached
to the anchor assembly with a plurality of rivets. The disconnected apexes can
be radially aligned with outer-
most tips of the second plurality of atrial cells. The first plurality of
atrial cells can be angled at
approximately 70-80 degrees relative to an axis that extends through the
central portion. The second
plurality of atrial cells can be angled at approximately 20-30 degrees
relative to the axis that extends through
the central portion. The annular strut frame can flare radially outwards at 70-
80 degrees relative to the axis
that extends through the central portion.
[0017] In general, in one embodiment, a prosthetic mitral valve includes a
valve support assembly that
includes a ventricular anchor and an atrial anchor. The valve support assembly
has a plurality of slots
therethrough. The prosthetic mitral valve further includes a plurality of
replacement leaflets. Each leaflet
has a leaflet arm extending through one of the plurality of slots. The
prosthetic mitral valve further includes
a plurality of commissure plates. Each commissure plate is circumferentially
and axially aligned with one of
the plurality of slots to form a commissure attachment mechanism. Each
commissure plate further includes a
plurality of channels in the sides thereof. The at least one suture is
positioned at each commissure
attachment mechanism and is wrapped around a portion of the valve support
assembly, through the plurality
of indents, and around the commissure plate.
[0018] This and other embodiments can include one or more of the following
features. The valve support
assembly can include an anchor assembly that includes the ventricular and
atrial anchors and an annular strut
- 4 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
frame that includes the plurality of slots. The annular strut frame can be
positioned radially within the
anchor assembly. The plurality of slots can be in a portion of the strut frame
that extends past the anchor
assembly in the ventricular direction. The anchor assembly can further include
a central portion, and the
ventricular and atrial anchors can flare radially outwards relative to the
central portion. The plurality of
channels can extend from the sides of each commissure plate towards a center
of the plate. The plurality of
channels can be substantially straight. There can be between 6 and 12 channels
in each commissure plate.
Each of the slots can be in an axially extending strut. Arms of the leaflets
can extend through the plurality of
slots. The arms can be further be wound around an outer perimeter of an inner
strut frame of the valve
support assembly. The plurality of slots can be positioned equidistance around
a circumference of the valve
support assembly. Each of the plurality of slots can be positioned towards a
ventricular end of the valve
support assembly. The valve support assembly can be configured to self-expand
from a constrained
configuration to an expanded configuration. Atrial edges of the leaflets can
be sewn around an inner
circumference of the valve support assembly. Each of the leaflets further
includes a leaflet protector thereon.
The leaflet protector can be made of a lubricious fabric and can be configured
to protect the respective leaflet
from an inner circumference of the valve support assembly.
[0019] In general, in one embodiment, a prosthetic mitral valve includes a
valve support assembly. The
valve support assembly includes an anchor assembly having a ventricular anchor
and an atrial anchor and an
annular strut frame positioned radially within the anchor assembly. The
annular strut frame includes a
plurality of slots therethrough. The prosthetic mitral valve further includes
a plurality of replacement
leaflets. Each leaflet has a leaflet arm extending through one of the
plurality of slots. The prosthetic mitral
valve further includes a plurality of commissure plates. Each commissure plate
is circumferentially and
axially aligned with one of the plurality of slots to form a commissure
attachment mechanism. Each
commissure plate further includes a plurality of channels in the sides
thereof.
[0020] This and other embodiments can include one or more of the following
features. The prosthetic mitral
valve can include at least one suture at each commissure attachment mechanism.
The at least one suture can
be positioned around the strut frame, through the plurality of indents, and
around the commissure plate. The
plurality of slots can be in a portion of the strut frame that extends past
the anchor assembly in the ventricular
direction. The anchor assembly can further include a central portion, and the
ventricular and atrial anchors
can be flared radially outwards relative to the central portion. The plurality
of channels can extend from the
sides of each commissure plate towards a center of the plate. The plurality of
channels can be substantially
straight. There can be between 6 and 12 channels in each commissure plate.
Each of the slots can be in an
axially extending strut. The arms of the leaflets can extend through the
plurality of slots. The arms can be
further be wound around an outer perimeter of the strut frame. The plurality
of slots can be positioned
equidistance around a circumference of the strut frame. Each of the plurality
of slots can be positioned
towards a ventricular end of the strut frame. The valve support assembly can
be configured to self-expand
from a constrained configuration to an expanded configuration. Atrial edges of
the leaflets can be sewn
- 5 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
around an inner circumference of the strut frame. Each of the leaflets can
further include a leaflet protector
thereon. The leaflet protector can be made of a lubricious fabric and can be
configured to protect the leaflet
from an inner circumference of the valve support assembly.
[0021] In general, in one embodiment, a prosthetic mitral valve includes a
valve support assembly, a
plurality of leaflets secured to the valve support assembly, and a plurality
of retention hooks. The valve
support assembly includes a ventricular anchor, a central portion, and an
atrial anchor. The valve support
assembly is configured to self-expand from a collapsed configuration to an
expanded configuration. The
plurality of retention hooks are attached to the ventricular anchor. Each of
the retention hooks curves
radially outwards to point in an atrial direction when the valve support
assembly is in the expanded
configuration. Each retention hook has a ratio of radius of curvature to
thickness of greater than 4:1.
[0022] This and other embodiments can include one or more of the following
features. Each of the plurality
of retention hooks can be configured to point at an angle of 50 -80 relative
to a central longitudinal axis of
the prosthetic mitral valve. The angle can be approximately 65'. A radius of
curvature of each of the
plurality of retention hooks can be between 3-5mm. A thickness of each
retention hooks can be between
0.8mrn and 1.6mm. The plurality of retention hooks can be integral with the
valve support assembly. The
valve support assembly can include an anchor assembly that further includes
the ventricular and atrial
anchors and the central portion and an annular strut frame positioned radially
within the anchor assembly.
The plurality of retention hooks can be attached to the anchor assembly. The
central portion can be
configured to align with a native valve orifice, and the ventricular anchor
and the atrial anchors can be
configured to compress native cardiac tissue therebetween. The valve support
assembly can include a
plurality of diamond-shaped cells. Each of the retention hooks can extend from
an apex of an interior
diamond-shaped cell. A retention hook can extend from each apex in a
circumferential line around the
prosthetic mitral valve except at positions closest to leaflet attachment
points.
[0023] In general, in one embodiment, a prosthetic mitral valve includes a
valve support assembly, a
plurality of leaflets secured to the valve support assembly, and a plurality
of retention hooks. The valve
support assembly includes a ventricular anchor, a central portion, and an
atrial anchor. Each of the retention
hooks is attached to the ventricular anchor and curves radially outwards to
point in an atrial direction. Each
retention hook has a ratio of radius of curvature to thickness of greater than
4:1 and points at an angle of 10 -
40 relative to a central longitudinal axis of the prosthetic mitral valve.
[0024] This and other embodiments can include one or more of the following
features. The angle can be
approximately 65 . A radius of curvature of each of the plurality of retention
hooks can be between 3-5mm.
A thickness of each retention hooks can be between 0.8mm and 1.6mm. The
plurality of retention hooks can
be integral with the valve support assembly. The valve support assembly can
include an anchor assembly
that further includes the ventricular and atrial anchors and the central
portion and an annular strut frame
positioned radially within the anchor assembly. The plurality of retention
hooks can be attached to the
anchor assembly. The central portion can be configured to align with a native
valve orifice, and the
- 6 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
ventricular anchor and the atrial anchors can be configured to compress native
cardiac tissue therebetween.
The valve support assembly can include a plurality of diamond-shaped cells.
Each of the retention hooks can
extend from an apex of an interior diamond-shaped cell. A retention hook can
extend from each apex in a
circumferential line around the prosthetic mitral valve except at positions
closest to leaflet attachment points.
[0025] In general, in one embodiment, a replacement mitral valve includes a
self-expandable valve support
assembly that includes a ventricular anchor, a central portion, and an atrial
anchor. The valve support
assembly has a self-expanded configuration in which the ventricular anchor and
the atrial anchor are flared
radially outward relative to the central portion. The atrial anchor has a
larger diameter than the ventricular
anchor when the valve assembly is in the self-expanded configuration. The
replacement mitral valve further
includes a plurality of replacement leaflets secured to the valve assembly.
[0026] This and other embodiments can include one or more of the following
features. The ventricular
anchor can have outer diameter of less than 55mm. The atrial anchor can have
diameter that is 3-10% larger
than diameter of ventricular anchor. The valve support assembly can include an
anchor assembly that
includes the central portion and ventricular and atrial anchors. The valve
support assembly can further
include an annular strut frame positioned radially within the anchor assembly.
The anchor assembly can be
made of a plurality of diamond-shaped cells joined together. The valve support
assembly can be configured
to self-expand from a constrained configuration to an expanded configuration.
The anchor assembly can be
configured to foreshorten when transitioning from the constrained
configuration to the expanded
configuration. The anchor assembly can be configured to take on an hour-glass
shape. Tips of the atrial
anchor can point in a ventricular direction. The atrial and ventricular
anchors can be configured to compress
native cardiac tissue therebetween. The atrial anchor can include a plurality
of atrial tips and the ventricular
anchor can include a plurality of ventricular tips. There can be more
ventricular tips than atrial tips.
[0027] In general, in one embodiment, a replacement mitral valve includes a
valve support assembly that
includes a ventricular anchor, a central portion, and an atrial anchor. The
valve support assembly has a self-
expanded configuration in which the ventricular anchor and the atrial anchor
are flared radially outward
relative to the central portion. The atrial anchor has a diameter that is 3-
10% larger than a diameter of the
ventricular anchor. The replacement mitral valve further includes a plurality
of replacement leaflets secured
to the valve assembly.
[0028] This and other embodiments can include one or more of the following
features. The ventricular
anchor can have outer diameter of less than 55mm. The valve support assembly
can include an anchor
assembly including the central portion and ventricular and atrial anchors. The
valve support assembly can
further include an annular strut frame positioned radially within the anchor
assembly. The anchor assembly
can be made of a plurality of diamond-shaped cells joined together. The valve
support assembly can be
configured to self-expand from a constrained configuration to an expanded
configuration. The anchor
assembly can be configured to foreshorten when transitioning from the
constrained configuration to the
expanded configuration. The anchor assembly can be configured to take on an
hour-glass shape. Tips of the
- 7 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
atrial anchor can point in a ventricular direction. The atrial and ventricular
anchors can be configured to
compress native cardiac tissue therebetween. The atrial anchor can include a
plurality of atrial tips and the
ventricular anchor can include a plurality of ventricular tips. There can be
more ventricular tips than atrial
tips.
[0029] In general, in one embodiment, a prosthetic mitral valve includes an
anchor assembly that includes a
ventricular anchor, an atrial anchor, and a central portion therebetween. The
anchor assembly is configured
to compress native cardiac tissue between the ventricular anchor and the
atrial anchor. An annular strut frame
is disposed radially within the anchor assembly and attached thereto. The
prosthetic mitral valve further
includes a plurality of replacement leaflets secured to the annular strut
frame. The anchor assembly and
annular strut frame are configured to self expand from a collapsed
configuration to an expanded
configuration. The anchor assembly is configured to foreshorten along a
central axis of the prosthetic mitral
valve when expanding from the collapsed configuration to the expanded
configuration. The annular strut
frame is configured to be substantially nonforeshortening along the central
axis when expanding from the
collapsed configuration to the expanded configuration.
[0030] This and other embodiments can include one or more of the following
features. The anchor
assembly can include a plurality of diamond-shaped cells. The ventricular
anchor can include a plurality of
struts and v-shaped connecting members. The ventricular anchor and atrial
anchors can flare radially
outwards relative to the central portion when in the expanded configuration.
The anchor assembly can be
configured to foreshorten by 20-30% when self-expanding from the collapsed
configuration to the expanded
configuration.
[0031] In general, in one embodiment, a prosthetic mitral valve includes an
anchor assembly that includes a
ventricular anchor, an atrial anchor, and a central portion therebetween. The
anchor assembly is configured
to compress native cardiac tissue between the ventricular anchor and the
atrial anchor. An annular strut
frame is disposed radially within the anchor assembly such that the annular
strut frame is spaced radially
away from the central portion of the anchor assembly. The prosthetic mitral
valve further includes a plurality
of replacement leaflets secured to the annular strut frame.
[0032] This and other embodiments can include one or more of the following
features. The annular strut
frame can be spaced radially away from the central portion by 2-3mm. The
annular strut frame can be flared
at an atrial end. Atrial tips of the annular strut frame can be attached to
the anchor assembly. A portion of
the anchor assembly can be pulled radially inwards relative to a remainder of
the anchor assembly so as to
attach to the annular strut frame.
[0033] In general, in one embodiment, a prosthetic mitral valve includes a
valve assembly that includes a
ventricular anchor, a central portion, and an atrial anchor. The anchor
assembly is configured to expand
from a collapsed configuration to an expanded configuration. The atrial anchor
includes a plurality of atrial
cells forming peaks and valleys around a circumference thereof, and the
ventricular anchor includes a
plurality of ventricular cells forming peaks and valleys around a
circumference thereof. A plurality of
- 8 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
replacement leaflets are secured to the valve assembly. A plurality of
retention hooks are attached only to
the ventricular anchor. Each of the plurality of retention hooks is positioned
in a valley between the
ventricular cells when the valve assembly is in the expanded configuration.
[0034] This and other embodiments can include one or more of the following
features. The plurality of
retention hooks can curve to point in the atrial direction when the anchor
assembly is in the expanded
configuration. The valve assembly can be configured to self-expand. The
plurality of retention hooks can
point at an angle of 500-800 relative to a horizontal axis of the prosthetic
mitral valve. The plurality of
retention hooks can be positioned in every valley except valleys closest to
leaflet attachment points.
[0035] In general, in one embodiment, a prosthetic mitral valve includes an
anchor assembly that includes a
ventricular anchor, a central portion, and an atrial anchor. The anchor
assembly configured to expand from a
collapsed configuration to an expanded configuration. The atrial anchor
includes a plurality of atrial cells at
an atrial edge of the atrial anchor, and the ventricular anchor includes a
plurality of ventricular cells at a
ventricular edge of the ventricular anchor. The number of ventricular cells is
divisible by 2, and the number
of atrial cells is divisible by 3. An annular strut frame is positioned within
the anchor assembly and includes
a plurality of struts connected by connection members. Three of the struts
include commissure attachment
points. The three commissure attachment points are spaced equally around a
circumference of the annular
strut frame. Three replacement leaflets are secured to the annular strut frame
at the commissure attachment
points.
[0036] This and other embodiments can include one or more of the following
features. There can be 30
ventricular cells, 15 atrial cells, and 15 struts. There can be 24 ventricular
cells, 12 atrial cells, and 12 struts.
There can be more ventricular cells than atrial cells. The number of
ventricular cells can also be divisible by
3.
[0037] In general, in one embodiment, a prosthetic mitral valve includes a
valve support assembly, a
plurality of leaflets, and a plurality of retention hooks. The valve support
assembly includes a ventricular
anchor, a central portion, and an atrial anchor. The valve support assembly is
configured to self-expand from
a collapsed configuration to an expanded configuration. The plurality of
leaflets are secured to the valve
support assembly, and the plurality of retention hooks are attached to the
ventricular anchor. Each of the
retention hooks curves radially outwards to point in an atrial direction when
the valve support assembly is in
the expanded configuration, and each retention hook has a ratio of radius of
curvature to thickness of 4:1 or
greater.
[0038] This and other embodiments can include one or more of the following
features. The ratio can be
between 4:1 and 8:1. Each of the plurality of retention hooks can be
configured to point at an angle of 10-40
degrees relative to a central longitudinal axis of the prosthetic mitral
valve. The angle can be approximately
28 . A radius of curvature of each of the plurality of retention hooks can be
less than 4mrn. A radius of
curvature of each of the plurality of retention hooks can be between 2mm-4mm.
A thickness of each of the
plurality of retention hooks can be less than 1.6mm. A thickness of each
retention hooks can be between
- 9 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
0.25mm and 1min. A ratio of width to thickness of each retention hook can be
between 0.3:1 and 1:1. Each
hook can be configured to engage approximately 3-10mm of mitral valve tissue
when the valve support
assembly is in the expanded configuration. The plurality of retention hooks
can be integral with the valve
support assembly. The valve support assembly can include an anchor assembly
including the ventricular and
atrial anchors and the central portion and an annular strut frame positioned
radially within the anchor
assembly. The plurality of retention hooks can be attached to the anchor
assembly. The central portion can
be configured to align with a native valve orifice, and the ventricular anchor
and the atrial anchors can be
configured to compress native cardiac tissue therebetween. The valve support
assembly can include a
plurality of diamond-shaped cells, and each of the retention hooks can extend
from an apex of an interior
diamond-shaped cell. A retention hook can extend from each apex in a
circumferential line around the
prosthetic mitral valve except a position closest to a leaflet attachment
point.
[0039] In general, in one embodiment, a prosthetic mitral valve includes a
valve support assembly, a
plurality of leaflets, and a plurality of retention hooks. The valve support
includes a ventricular anchor, a
central portion, and an atrial anchor. The plurality of leaflets are secured
to the valve support assembly, and
the plurality of retention hooks are attached to the ventricular anchor. Each
of the retention hooks curves
radially outwards to point in an atrial direction, and each retention hook has
a ratio of radius of curvature to
thickness of greater than 4:1 and points at an angle of 10 -40 relative to a
central longitudinal axis of the
prosthetic mitral valve.
[0040] This and other embodiments can include one or more of the following
features. A ratio of width to
thickness of each retention hook can be between 0.3:1 and 1:1. Each hook can
be configured to engage
approximately 3-10mm of mitral valve tissue when the valve support assembly is
in the expanded
configuration. A radius of curvature of each of the plurality of retention
hooks can be less than 4mm.
BRIEF DESCRIPTION OF THE DRAWINGS
[0041] The novel features of the invention are set forth with particularity in
the claims that follow. A better
understanding of the features and advantages of the present invention will be
obtained by reference to the
following detailed description that sets forth illustrative embodiments, in
which the principles of the
invention are utilized, and the accompanying drawings of which:
[0042] Figures 1A-1C show an exemplary mitral valve prosthesis. Figures 1A-1B
show the mitral valve
prosthesis in an expanded configuration. Figure 1C shows a portion of the
expanded anchor assembly in 2D.
[0043] Figures 2A-2E show another exemplary mitral valve prosthesis. Figures
2A-2B show the mitral
valve prosthesis in an expanded configuration. Figure 2C shows a portion of
the expanded anchor assembly
in 2D. Figure 2D shows the expanded annular strut frame. Figure 2E shows a 2D
pattern (pre-expanded)
for the strut frame.
[0044] Figure 3A-3C show another exemplary mitral valve prosthesis. Figures 3A-
3B show the mitral valve
prosthesis in an expanded configuration. Figure 3C shows the expanded annular
strut frame.
[0045] Figures 4A-4C show another exemplary mitral valve prosthesis in an
expanded configuration.
- 10-
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
[0046] Figures 5A-5C show an exemplary nonforeshortening anchor assembly in
the expanded
configuration.
[0047] Figures 6A-6C show another exemplary nonforeshortening anchor assembly
in the expanded
configuration.
[0048] Figures 7A-7B show an exemplary anchor assembly before it is heat-set
into an hour-glass shape.
[0049] Figures 8A-8G show another exemplary mitral valve prosthesis. Figures
8A-8C show the mitral
valve prosthesis in the expanded configuration. Figures 8D-8E show the
expanded anchor assembly. Figure
8F shows a portion of the expanded anchor assembly in 2D. Figure 8G shows a 2D
pattern (pre-expanded)
for the anchor assembly.
[0050] Figures 9A-9D show another exemplary nonforeshortening anchor assembly.
Figures 9A-9C show
the anchor assembly in the expanded configuration. Figure 9D shows a 2D
pattern (pre-expanded) for the
anchor assembly.
[0051] Figure 10 shows an exemplary valve assembly including the anchor
assembly, strut frame, skirt, and
leaflets.
[0052] Figures 11A-11E show one exemplary mechanism of attaching leaflets to
the strut frame. Figure
11A shows an exemplary commissure plate. Figure 11B shows a cross-sectional
view of leaflets extending
between the two commissure plates. FIG. 11C shows a valve assembly having a
strut with a series of holes
therein for attachment of leaflets to the valve assembly. Figure 11D shows a
cross-sectional view of the
leaflets and commissure plates attached to the strut. Figure lE shows a close-
up of a portion of the strut with
holes therein.
[0053] Figure 12 shows another exemplary mechanism of attaching leaflets to
the strut frame.
[0054] Figures 13A-13B show deployment of a ventricular anchor of an exemplary
mitral valve prosthesis
out of a sheath.
[0055] Figure 14 is a cross-section showing another exemplary mechanism of
attaching leaflets.
[0056] Figure 15A-15C show another exemplary mechanism of attaching leaflets.
Figure 15A shows a
secondary member including a slot. Figure 15B is a cross-sectional view
showing the leaflets passed through
the slot of the secondary member and around a strut of the strut frame. Figure
15C shows alignment of the
secondary member and the strut.
[0057] Figures 16A-16D show a holder including an exemplary mitral valve
prosthesis with a skirt or
covering.
[0058] Figures 17A-17J show an exemplary method of deploying a valve
prostheses.
[0059] Figures 18A-18E show another exemplary mechanism of attaching leaflets
to the strut frame. Figure
18A shows a plate attached to a valve assembly to attach the leaflets thereto.
Figures 18B is a cross-sectional
view showing the leaflets attached between the plate and a strut. Figure 18C
is a top view of the exemplary
mechanism. Figure 18D shows the plate positioned over two leaflets and a strut
of the valve assembly.
Figure 18E shows the plate attached to the strut frame.
-11-
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
[0060] Figure 19 shows a method of sewing leaflets around the circumference of
the strut frame.
[0061] Figures 20A-20Q show another exemplary mitral valve prosthesis. Figure
20A shows the exemplary
mitral valve prosthesis in the expanded configuration. Figures 20B-20C show
the expanded prosthesis
without the leaflets or skirt for clarity. Figures 20D-20G show the expanded
anchor assembly. Figure 20H
shows the atrial end of the expanded valve prosthesis. Figure 201 shows the
ventricular end of the expanded
valve prosthesis. Figure 20J is a 2D view of the (unexpanded) anchor assembly.
Figure 20K shows the
expanded strut frame. Figure 20L is a 2D view of the (unexpanded) strut frame.
Figure 20M is a side view
of the strut frame. Figure 20N is a top (atrial) view of the strut frame.
Figure 200 is another view of the
expanded anchor assembly. Figures 20P-20Q are additional view of the expanded
prosthesis without the
leaflets or skirt for clarity.
[0062] Figure 21 shows an exemplary leaflet for use with the mitral valve
prostheses described herein.
[0063] Figure 22 shows the inflow edges of the leaflets sewn to the strut
frame
[0064] Figures 23A-23C show an exemplary mitral valve prosthesis with a skirt
covering thereon.
[0065] Figures 24A-24C show an exemplary mitral valve prosthesis with a first
set of dimensions.
[0066] Figures 25A-25C show an exemplary mitral valve prosthesis with a second
set of dimensions.
[0067] Figure 26 shows an exemplary mandrel for shaping a skirt.
[0068] Figures 27A-27Q show another exemplary method of attaching leaflets to
a mitral valve prosthesis.
Figure 27A shows a strut frame with a slot in the strut and a first suture
positioned therearound. Figure 27B
shows a second suture positioned therearound. Figure 27C shows a third suture
positioned therearound.
Figure 27D shows the alignment of leaflet protectors. Figure 27E shows the
positioning of the leaflets such
that they are flush with one another. Figures 27F-2711 show placement of the
leaflet arms through the slot in
the strut frame. Figure 271 shows separation of the two leaflets to attach at
additional commissure points.
Figure 27J shows an inflow view of the leaflets after they have been attached
at the commissure attachment
points. Figure 27K shows an outflow view of the leaflets after they have been
attached at the commissure
attachment points. Figure 27L shows the arms of the leaflets wrapped around
the strut frame. Figure 27M
shows the leaflet protectors wrapped inside of the strut frame. Figure 27N
shows alignment of the leaflet
arms with the strut frame. Figure 270 shows placement of the plate over the
strut frame. Figure 27P shows
wrapping of two sutures around the plate. Figure 27Q shows wrapping of the
final suture around the plate to
attach the leaflets to the strut frame.
[0069] Figure 28 shows placement of an exemplary valve prosthesis in the
native mitral valve orifice.
[0070] Figures 29A-29E show a mitral valve prosthesis with a delivery system
attachment mechanism.
Figure 29A shows the expanded valve assembly with pins therein. Figure 29B
shows a close-up of a pin.
Figure 29C shows slots in the skirt to allow for access to the pins. Figure
29D shows a 2D (unexpanded)
view of the anchor assembly with pins. Figure 29E shows a close-up of a pin
with dimensions.
[0071] Figure 30 shows another exemplary mitral valve prosthesis with a skirt
thereon.
[0072] Figures 31A-31C show exemplary hooks for a mitral valve prosthesis.
- 12 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
[0073] Figure 32 shows an engagement radius R of hooks on a mitral valve
prosthesis.
[0074] Figure 33 shows an exemplary plate for leaflet attachment.
[0075] Figure 34 shows another exemplary plate for leaflet attachment.
[0076] Figure 35 shows an exemplary tubular pre-formed skirt.
[0077] Figures 36A-36B show an exemplary anchor assembly and strut frame with
a skirt extending
thereover.
[0078] Figures 37A-37B show additional exemplary mitral valve prostheses
without the leaflets or skirt.
- 13 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
DETAILED DESCRIPTION
[0079] This disclosure includes replacement heart valves (also referred to
herein as prosthetic heart valves),
methods of manufacturing replacement heart valves, including subassemblies
thereof, and methods of using
replacement heart valves. This disclosure describes the prostheses in the
context of replacement mitral
valves, but it is conceivable that the prostheses herein can be used or
modified to be used as other
replacement heart valves. In some embodiments, the replacement heart valves
are self-orienting replacement
mitral valves configured to be delivered using minimally invasive techniques.
[0080] The replacement heart valves described herein include an anchor
assembly that includes an atrial
anchor (e.g., configured to be placed on an atrial side of a mitral valve
annulus), a ventricular anchor (e.g.,
configured to be placed on a ventricular side of a mitral valve annulus), and
a central portion positioned
axially between the atrial and ventricular anchors. The anchor assembly is
adapted to collapse to a delivery
or collapsed configuration and expand to an expanded configuration. The
replacement heart valves also
include a strut frame secured to at least one of the central portion, the
ventricular anchor, or the atrial anchor
for attaching a plurality of replacement leaflets thereto. The strut frame can
be configured to deform and
collapse as the rest of the anchor assembly is collapsed. The struts of the
strut frame extend towards and/or
past the ventricular anchor.
[0081] The replacement heart valves described herein are configured to be
secured in the native valve
orifice by sandwiching the cardiac orifice between ventricular and atrial
anchors, which are larger in
diameter than the valve orifice, by applying an axial compressive force from
the anchors, a radial force from
the center portion outward against the cardiac orifice, and/or by using hooks
or barbs that extend into the
tissue of the orifice.
[0082] Further, the replacement heart valves described herein can be delivered
to a cardiac valve orifice,
such as the mitral valve, by using minimally invasive techniques to access the
cardiac valve. In some
embodiments, the mitral valve prostheses can be delivered through a
transatrial route, i.e., by making a small
incision in the patient's body and passing the prosthesis through the apex of
the heart to, for example, the
mitral valve. In other embodiments, the mitral valve prostheses can be
delivered through the transseptal
route, i.e., through the venous system and into the left atrium via a
transseptal puncture. In both the
transatrial and transseptal delivery methods, the distal-most anchor can be
delivered to the ventricle while the
proximal-most anchor can be delivered to the atrium.
[0083] Figures 1A-1C show an exemplary mitral valve prosthesis 100 in an
expanded configuration. The
portion of the replacement valve prosthesis 100 in Figure 1 may be referred to
as a prosthesis subassembly,
which includes an anchor assembly 101 and a strut frame 105, but excludes
leaflets and any skirts that may
be incorporated into the final replacement valve. Anchor assembly 101 includes
an atrial anchor 102, a
ventricular anchor 104, and a central portion 103 therebetween. In this
embodiment, atrial anchor 102 is
configured and adapted to be disposed on an atrial side of a mitral valve
orifice, and ventricular anchor 104 is
- 14 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
configured and adapted to be disposed on a ventricle side of the mitral valve
orifice. Further, the central
portion 103 can be configured to be situated in the mitral valve orifice. In
some embodiments, the central
portion 103 has a diameter that is substantially the same size as the native
mitral valve annulus (i.e., it is not
designed to be larger than the annulus).
[0084] In some embodiments, the anchor assembly 101 and/or strut frame 105 can
be made of wire, such as
a shape memory metal wire (e.g., a nitinol). In other embodiments, the anchor
assembly and/or strut frame
can be laser cut from one or more tubes, such as a shape memory metal tube
(e.g., nitinol). For example, the
anchor assembly 101 can be laser cut from a first hypotube while the strut
frame 105 can be laser cut from a
second hypotube of smaller diameter. The anchor assembly 101 can be cut, for
example, from a 9-12mm
diameter tube, such as a lOmm tube, while the strut frame 105 can be cut, for
example, from a 7-9mm
diameter tube, such as an 8mm tube.
[0085] The valve prosthesis 100 can be configured to expand (e.g., self-
expand) from a collapsed or
constrained (delivery) configuration to an expanded (treatment) configuration.
In the expanded configuration
shown in Figures 1A-1B, the atrial anchor 102 and ventricular anchor 104
extend radially outward from
central portion 103, and are considered to flare outward relative to central
portion 103. The atrial anchor 102
and ventricular anchor 104 can also be considered flanged relative to central
portion 103. The flared
configuration of atrial and ventricular anchors 102 and 104 relative to
central portion 103 is described in the
context of a side view of the anchor assembly, as can be best seen in Figure
1B. In some embodiments, the
flared configuration of the two anchors 102, 104 and the central portion 103
define a general hour-glass
shape in a side view of the anchor assembly 101. That is, the anchors 102, 104
can be flared outwards
relative to the central portion 103 and then curved or bent to point at least
partially back in the axial
direction. It should be understood, however, that an hour-glass configuration
is not limited to symmetrical
configuration.
[0086] The anchor assembly 101 can be configured to expand circumferentially
and foreshorten axially as
the valve prosthesis 100 expands from the collapsed delivery configuration to
the expanded treatment
configuration. For example, as shown in Figures 1A-1C, the anchor assembly 101
can be made of a plurality
of cells 111 that are each configured to expand circumferentially and
foreshorten axially upon expansion of
the anchor assembly 101. As shown best in Figure 1C, the cells 111 can each be
diamond-shaped. Further,
the cells 111 can be interconnected and configured such that every diamond
apex 117 is connected to another
diamond apex 117 except at the atrial or ventricular tips 112, 114 of the
assembly 101. The anchor assembly
101 can include, for example, three circumferential rows of diamond cells 111.
For example, the atrial
anchor 102 can comprises one row of diamond-shaped cells 111 extending
circumferentially, the central
portion 103 can comprise one row of diamond-shaped cells 111 extending
circumferentially, and the
ventricular anchor 104 can comprise one row of diamond-shaped cells extending
circumferentially 111.
[0087] The strut frame 105 can be configured to expand circumferentially, but
maintain the same axial
dimension (i.e., be non-foreshortening) as the valve prosthesis 100 expands
from the collapsed delivery
- 15 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
configuration to the expanded treatment configuration. By being non-
foreshortening, the strut frame 105 can
advantageously ensure that less strain is placed on the leaflets during
delivery and/or packing. Thus, while
the anchor assembly 101 is designed to be foreshortening, the strut frame 105
is designed so as to be
substantially non-foreshortening. As can be best seen in Figure 1B, the strut
frame 105 can include a
plurality of longitudinally extending struts 151 and interconnecting v-shaped
members 153. Further, in some
embodiments, and again as shown in Figures 1A-1B, the strut frame 105 can have
fewer v-shaped members
151 extending circumferentially around the diameter thereof than the cells 111
of the anchor assembly 101,
such as half the number. Further, the strut frame 105 can flare at radially
outwards at the atrial end, e.g., to
conform to the flare of the atrial anchor 102.
[0088] The strut frame 105 and the anchor assembly 101 can be coupled together
with coupling members,
such as rivets. In some embodiments, and as shown in Figures 1A-1B, the atrial
tips 129 of the strut frame
105 can be coupled to the atrial tips 112 of the anchor assembly 101. Where
there are fewer v-shaped
members 151 in the strut frame 105 than cells 111 in the anchor assembly 101
(as shown in Figure 1B), the
strut frame 105 can attach to every other atrial tip 112 on the anchor
assembly 101.
[0089] The radially inner surfaces of strut frame 105 can substantially define
the perimeter of a central
opening 106. Replacement leaflets, which are not shown in Figures 1A-1B for
clarity, can be secured to the
strut frame 105 and can be disposed at least partially in the central opening
106. The leaflets are configured
to control blood flow therethrough.
[0090] In some embodiments, the valve 100 can include hooks 188 or barbs to
help anchor the assembly in
the mitral valve orifice. As shown in Figures 1A-1C, in one embodiment, the
hooks 188 can be on the
ventricular most tips 114 of the ventricular anchor 104.
[0091] Figures 2A-2E show another exemplary valve prosthesis 200. The valve
prosthesis 200 is similar to
valve prosthesis 100 and can include many of the same features as valve
prosthesis 100, such as an anchor
assembly 201 (having atrial anchor 202, a ventricular anchor 204, and a
central portion 203) and a strut
frame 205. In contrast to the prosthesis 100, the cells 211 of the anchor 201
are not connected together at
every interior apex 217. Rather, the middle row of cells 211 can be
disconnected at every other atrial apex
219 at the atrial side. As a result, there can be fewer atrial tips 212 than
ventricular tips 214, and the atrial-
most cells can be truncated or v-shaped (i.e., straddling each disconnected
apex 219 and corresponding
diamond-shaped cell). For example, there can be 15 atrial tips 212 (and 15 v-
shaped cells 211 at the atrial
end) and 30 ventricular tips 214 (and 30 diamond-shaped cells at the
ventricular end). Advantageously,
because the atrial tips 212 are larger/wider than the ventricular tips 214,
the atrial tips 212 can be more
flexible to allow the atrial anchor 202 to conform to the tissue. The atrial
apexes 219 can be radially aligned
with the atrial tips 212 and can be positioned approximately mid-way along the
diamond-shaped cells at the
atrial tips 212 along the central longitudinal axis (as noted above, the
outermost cells can also be considered
v-shaped, particularly in 2D, as the inner cell and apex 219 sit within the
outer larger diamond, making it a v-
shape).
- 16 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
[0092] In some embodiments, each of the atrial apexes 219 can have a rivet
hole therein for connection to
the atrial tips 229 of the strut frame 205. Further, in some embodiments (and
as shown in Figures 2A and
2B), the atrial apexes 219 can all be bent slightly radially inwards towards
the strut frame 205 (e.g., further
radially inwards than the rest of the anchor assembly 201 so as to meet the
strut frame 205). The atrial
apexes 219 can be radially aligned with the atrial tips 212 of the atrial
anchor 202. Further, the apexes 219,
when bent radially inwards, can effectively act as an integrated suspension,
holding the central portion 203
and ventricular anchor 204 radially outwards relative to, and spatially
separated from, the strut frame 205.
For example, the ventricular anchor 204 can be separated from the strut frame
205 by a radial distance of, for
example, 1-15mm, such as 2-11mm, such as approximately 3mm. Further, the
central portion 203 can be
separated from the strut frame 205 by a radial distance of, for example, 2-
3mm. This separation of the
ventricular anchor 204 and/or the central portion 203 can advantageously
isolate the leaflets from the anchor
assembly 201 on the ventricular side where the greatest amount of distortion
is placed on the anchor
assembly 201.
[0093] Further, in this embodiment, the strut frame 205 and anchor assembly
201 can be attached at a
central point of the atrial anchor 202 (i.e., at apexes 219) rather than at
the outer-most or atrial-most tips 212
of the atrial anchor 202. By attaching the inner strut frame 205 to the anchor
assembly 201 at a mid-point of
the atrial anchor 202 rather than at the atrial tips 212, less torque or
torsion is applied to the strut frame 205
as the atrial anchor 202 conforms to the tissue, thereby helping to ensure
that the leaflets maintain their
required position.
[0094] As shown best in Figures 2D and 2E, the strut frame 205 can include a
plurality of struts 221 and v-
shaped members 223 (so as to be substantially non-foreshortening as described
with respect to strut frame
105). In this embodiment, there are four v-shaped members 223 extending
axially between each pair of
struts 221. The two ventricular-most v-shaped members 223 and the atrial-most
v-shaped member 223 all
point in the atrial direction. The last v-shaped member 223 points in the
ventricular direction. Having a v-
shaped member 223 that points in the ventricular direction can add to the
stiffness of the strut frame 205.
Additionally, having the last v-shaped member 223 point towards the atrium
reduces the length of the struts
and reduces the number of vertices that are pointed into the ventricle (to
reduce trauma to the ventricle). The
atrial tips 229 of the strut frame 205 can be formed by the vertex of the "V"
shape. Each atrial tip 229 can
include a rivet hole therein for connection to the anchor assembly 201.
Further, the strut frame 205 can
include a flare at the atrial end thereof to enable the strut frame to meet
the apexes 219 and/or to conform to
the flare of the atrial anchor 202. Further, in some embodiments (and as shown
in Figure 2D), the flare at the
atrial end of the strut frame 205 can include relatively flexible members 209
or zig-zag features therein. The
flexible members 209 can be configured to allow the atrial flare to easily
fold up during packing/delivery.
[0095] In some embodiments, the number of ventricular cells or ventricular
tips 214 in valve 200 (or any
valve described herein) can be divisible by both 2 and 3. For example, there
can be 18, 24, or 30 ventricular
cells or tips 214. Because the number of ventricular tips 214 is divisible by
2, there can be half as many
- 17 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
atrial tips 212. Further, by having the number of cells divisible by 3, the
three attachment points for the three
leaflets (e.g., struts 221a,b,c) of the strut frame 205 can be even spaced
around the circumference of the
central opening 206. Increasing the number of ventricular tips/cells (e.g.,
from 18 cells to 30 cells) in any
given design means that the total amount of required circumferential expansion
of each individual cell
decreases, thereby allowing the longitudinal lengths of the cells to be
shorter, decreasing the overall length of
the packed assembly (i.e., during delivery). In some embodiments, the cells
have a length of between 4 and
6mm and a width of between 0.2 and 0.4mm when collapsed, e.g., before
expansion. With these dimensions,
the packed assembly can be, for example, 30-40mm, such as 32-35mm. Further, in
some embodiments, the
cell dimensions are chosen such that the ratio of width to length yields no
more than 8-10% sheathing strain
when the anchor assembly is retracted into the catheter for delivery.
[00961 Figures 3A-3C show another exemplary valve prosthesis 300. Valve
prosthesis 300 is similar to
valve prosthesis 200 (with anchor assembly 301 similar to assembly 201). The
strut frame 305, however,
includes reduced thickness members 310 in the atrial flare rather than
flexible members 209. The reduced
thickness members 310 can have a smaller diameter than the rest of the strut
frame 305. The reduced
thickness members 310, similar to the flexible members 209, can allow for
easier bending at the flare of the
strut frame 305, thereby permitting easy packing.
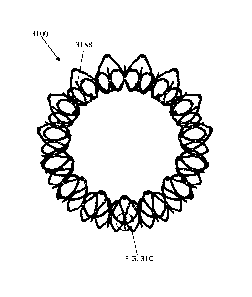
[0097] Figures 4A-4C show another exemplary valve prosthesis 400. The valve
400 is similar to valves
100-300 except that the attachment point between the strut frame 405 and the
anchor assembly 401 is at the
ventricular end of the strut frame 405. To connect the anchor assembly 401 to
the ventricular end of the strut
frame 405, connecting members 494 extend from the anchor assembly 401 (e.g.,
from the central portion 403
or the ventricular anchor 404) to the ventricular tips of the strut frame 405.
The connecting members 494
can be integral, for example, with the anchor assembly 401 and then riveted to
the strut frame 405. In some
embodiments, as shown in Figures 4A-4C, the connecting members 414 can be a
single longitudinal
member. In other embodiments, the connecting members 494 can be cells or tips
414 of the ventricular
anchor 404 that are pulled radially inwards (e.g., every other tip 414 of the
ventricular anchor 404 can be
pulled inwards). Further, in some embodiments, an additional layer of cells
can be coupled or riveted to the
ventricular anchor 404 to tune the rigidity thereof. As shown in Figures 4A-
4C, the atrial end of the strut
frame 405 can still be flared at an angle, e.g., to substantially confirm to
the flare of the atrial anchor 402 of
the anchor assembly 401.
[0098] Figures 8A-8G show another exemplary valve prosthesis 800 including
valve assembly 801
and strut frame 805. Valve prosthesis 800 is similar to valve prosthesis 200
except that valve
prosthesis 800 has a greater curvature on the flare of the ventricular anchor
804, which can help
improve retention force in some embodiments. For example, the ventricular
anchor 804 can flare at
an initial angle of 5 -15 , such as 10 , relative to a horizontal plane
through the central portion 803
(and/or 75 -95 , such as 80 , relative to a central axis through the
prosthesis 800). Additionally, the
- 18 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
anchor assembly 801 includes a plurality of barbs or hooks 888 extending from
the ventricular
anchor 804. Positioning the hooks on the ventricular anchor 804 advantageously
helps hold the
prosthesis in place, as the ventricular side of the mitral valve undergoes the
highest pressure. The
hooks 888 are positioned in the valleys between the ventricular tips 814.
Further, each hook 888,
when the anchor assembly 801 is in the expanded configuration, is curved
backwards to point at
least partially in the atrial direction.
[0099] Figures 20A-20Q show another exemplary valve prosthesis 2000 including
a valve assembly 2001
and a strut frame 2005. Prosthesis 2000 is similar to valve prosthesis 800
except that that the tips 2014 of the
ventricular anchor 2004, after flaring radially outwards at the angle of 5-15
relative to the horizontal plane
2020, can curve away from the horizontal axis 2020 to point substantially in
the axial (ventricular) direction,
such as at an angle of 60-70 , such as 67 relative to the horizontal plane
2020. The curvatures of the two
portions can be between 2mm and 8mm, respectively. Similarly, the atrial
anchor 2002 can extend at an
initial angle of 20 -30 , such as approximately 26 , relative to the
horizontal plane 2020 through the central
portion 2003. The tips 2012 of the atrial cells can then curve away from the
axis 2020 to point more in the
axial (atrial) direction, such as at an angle of 60-70 , such as 67 relative
to the horizontal plane 2020. The
curvatures of the two portions can be between 2mm and 8mm, respectively.
Further, the atrial apexes 2019
with the rivet holes therein can extend at an angle of approximately 50-70 ,
such as 60 relative to the axis
2020, to meet and affix to the strut frame 2050. Similarly, the atrial tips
2029 of the strut frame 2005 can
flare out at approximately 70 -80 relative to the horizontal axis 2020 so as
to substantially conform to the
flare of the atrial apexes 2019. There can be 30 atrial cells along a single
circumference and only 15
ventricular cells.
[0100] Further, as is best shown in Figures 20K-20N, the strut frame 2005 is
different from the strut frame
805 in that the strut frame 2205 does not include flexible members (e.g., zig-
zag features) in the flare at the
atrial end of the strut frame 2005. Rather, the connecting member on the
anchoring structure can be made
more compliant. Like strut frame 205, the strut frame 2005 includes a
plurality of struts 2021 and v-shaped
member 2023 so as to be non-foreshortening. In strut frame 2005, however,
there are five v-shaped members
2023 extending between each pair of struts 2021 rather than four. The extra v-
shaped member 2023 is
positioned proximate to the atrial-most v-shaped member 2023 and extends from
the struts 2023 in the atrial
direction. The extra v-shaped member can advantageously add circumferential
strength to the strut frame
2005. The strut frame 2005 can further include one or more slots 2733 in the
struts 2021 to allow for
attachment of leaflets, as described below.
[0101] The anchor assembly 2001 also includes barbs or hooks 2088 that,
similar to hooks 888, are
positioned between the ventricular tips 2014 in the valleys and are curved
backwards towards the atrial end.
Further, in some embodiments, and as shown at Figures 200-20Q, the hooks 2088
can be positioned between
every ventricular cell 2011 (e.g., in the valleys) except those valleys
closest to the commissure attachment
- 19 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
points. At those points, one or more (such as one, two, or three) of the hooks
2088 can be removed so as to
prevent interference with the commissures and/or leaflets when the prosthesis
is in the collapsed
configuration.
[0102] In some embodiments, such as for the anchor assembly 2000, the atrial
anchor 2002 can have a
larger diameter than the ventricular anchor 2004. Having a larger atrial
anchor 2002 than a ventricular
anchor 2004 allows the anchors 2002, 2004 to grip tissue while preventing the
ventricular anchor 2004 from
impeding flow to the aortic valve. That is, as shown in Figure 28, if the
ventricular anchor is too large, then
the Left Ventricular Outflow Tract (LVOT) 2828 may be obstructed and restrict
flow through the adjacent
aortic valve 2829. In some embodiments, for example, the atrial anchor 2002
can have a diameter that is 3-
10% larger than the diameter of the ventricular anchor 2004. The ventricular
anchor 2004 can thus be less
than 55mm, such as less than or equal to 54mm, such as less than or equal to
52mm.
[0103] As described above, the number of ventricular cells or ventricular tips
in any of the valves described
herein can be divisible by both 2 and 3. For example, as shown in Figure 37A,
there can be 30 ventricular
tips and 15 atrial tips. As another example, there can be 24 ventricular tips
and 12 atrial tips, as shown in
Figure 37B.
[0104] In some embodiments, the prostheses described herein can be made in a
plurality of different sizes so
as to fit within a range of native valve orifice sizes. For example, referring
to Figures 24A-24C, in some
embodiments, a valve prosthesis 2400 can have an atrial anchor 2402 with an
outer diameter of 54mm, a
ventricular anchor 2404 with an outer diameter of 52mm, and a central portion
2403 with an outer diameter
of 36mm. Further, the strut frame 2405 can have an inner diameter of 27mm-
30mm, such as approximately
29mm. A total height of the prosthesis 2400 can be, for example, 22-28mm, such
as 26mm. In contrast, the
valve prosthesis 2500 of Figures 25A-25C can have a larger diameter to fit
within a larger native valve
orifice. For example, the atrial anchor 2502 can have an outer diameter of
59mm, the ventricular anchor can
have an outer diameter of 54mm, and the central portion 2503 can have an outer
diameter of 40mm. The
strut frame 2505, like the strut frame 2405, can have an inner diameter of
27mm-30mm, such as 29mm. To
compensate for the increased diameter of the valve assembly 2501 relative to
the strut frame 2505, the
disconnected atrial apexes 2519 can be pulled further radially inwards (for
example, the disconnected atrial
apexes 2519 can be pulled downwards in an s-shape to reach further inwards). A
total length of the
expanded valve 2500 can be 28-29mm. Further, in order to maintain low packing
strain, the sheathed or
packed length of the valve 2500 can be longer than the packed length of the
valve 2400. For example, the
packed length of valve 2400 can be 32mm-35mm while the packed length of valve
2500 can be 34mm-
37niln.
[0105] Anchor assemblies 101-401, 801, 2001, 2401, and 2501 all foreshorten
upon expansion (due to their
cellular design). For example, the anchor assemblies can foreshorten by 20%-
30%. In contrast, the
corresponding strut frames 105-405, 805, 2005, 2405, and 2505 maintain
substantially the same axial length.
- 20 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
[0106] In some embodiments, the prosthesis can be designed such that the
entire prosthesis does not
foreshorten during expansion. Having the prosthesis not foreshorten
advantageously allows the packed
length to be much shorter, such as less than 35mm, such than 30mm, or less
than 25mm.
[0107] For example, FIGS. 5A-5C show an anchor assembly 501 that includes a
plurality of struts 505 and
circumferential v-shaped connectors 507 that do not substantially foreshorten
upon expansion. The anchor
assembly 501 forms a primarily hour-glass shape in the expanded configuration.
Further, the atrial end
includes flexible members 519 (e.g., zig-zag members) to aid in conforming to
the native orifice.
[0108] Figures 6A-6C show another exemplary nonforeshortening anchor assembly
601 with a plurality of
struts 605 and circumferential v-shaped connectors 607. In this embodiment,
the ventricular anchor 604 is
curled inwards at the tips to minimize interaction with the native ventricular
anatomy.
[0109] Figures 9A-9D show another exemplary nonforeshortening anchor assembly
901 with a plurality of
struts 905 and circumferential v-shaped connectors 907. In this embodiment,
there are 5 v-shaped connectors
907 extending between each set of struts 905. The ventricular ends of the
ventricular anchor 904, like
ventricular anchor 604, curl in at the tips to minimize interaction with the
native anatomy. Further, the struts
905 each include a flexible portion 929 (e.g., zig-zag or serpentine section)
that extends from the atrial tips to
the central portion 903. The flexible portions 929 aid in conforming the
atrial anchor 902 to the native
orifice. In this embodiment, the strut frame (which can be any strut frame
described herein) can be
configured to attached mid-way along the atrial anchor 902, such as rivet
location 939. Advantageously, by
attaching the strut frame at the atrial anchor (i.e., rather than the
ventricular anchor), the strut frame can be
less prone to distortion that can occur when the ventricular anchor is
expanded during delivery.
[0110] Various hook or barb mechanisms can be used with any of the valves
described herein. For example,
the barb or hook can be riveted to the anchor assembly, can be laser cut from
the assembly, and/ can be
formed as part of a v-shaped feature of the anchor assembly. The hook or barb
mechanisms can be designed
such that they point radially outwards during deployment (i.e., not into the
tissue) and do not engage with
tissue until fully released, thereby preventing interference with the
deployment. This can be achieved, for
example, by using a hook having the proper radius of curvature to thickness
ratio.
[0111] In some embodiments, the hooks can be on the ventricular most tips of
the ventricular anchor, as
shown in Figures 1A-1C. In other embodiments, the hooks can be in the valleys
(i.e., between petals or tips,
as shown in Figures 8A-8G and 20A-N). For example, the hooks can be placed in
valleys on the ventricular
anchor (e.g., from an apex of an interior diamond-shaped cell). When
positioned between valleys on the
ventricular anchor, the hooks can curve around and point in the atrial
direction at an angle of 40 -90 , e.g.,
50 -80 , e.g., 57-67 , such as about 62 relative to a horizontal axis of the
device (or up to 50 , e.g., 10 -40 ,
such as 23 -33 , such as about 28 relative to a central longitudinal axis of
the device). This angle can
advantageously allow the hooks to point in the atrial direction to dig into
tissue.
[0112] Referring to Figures 31A-31C, each hook 3188 on implant 3100 (which can
be any implant
described herein) can arc along an angle a of between 99 -119 , such as 104 -
114 , such as approximately
- 21 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
109. Further, each hook 3188 can have a ratio of radius of curvature RC to
thickness T of 4:1 or greater.
Having a ratio of radius of curvature RC to thickness T of 4:1 or greater
ensures that the hooks 3188 can
bend from their curved configuration to lay flat during collapse (e.g., for
delivery). In some embodiments, a
radio of width W to thickness T of each hook 3188 can be between 0.3:1 and
1:1, such as between 0.4:1 and
0.6:1. Having a ratio within this range advantageously ensures that the hooks
3188 don't twist or bend
sideways when collapsed or laid flat (e.g., for delivery of the implant).
[0113] In some embodiments, the ratio of radius of curvature RC to thickness T
is between 4:1 and 10:1,
such as between 5:1 and 9:1. The radius of curvature RC can, for example, be
less than 4mm, such as
between 2mm and 4rnm, such as between 2.5mm and 3.5mm, such as approximately
3mm. The thickness T
of the hook can be less than 1.6mm, such as between 0.25mm and lmm, such as
between 0.3mm and 0.5mm,
such as between 0.39nun and 0.45mm, such as approximately 0.42mm. The width W
of the hook can be
between 0.1mm and 0.4mm, such as between 0.2mm and 0.3mrn, such as
approximately 0.22mm.
[0114] In one exemplary embodiment, the radius of curvature of the hook is
3mm, the thickness of the hook
is 0.42mm, and the width of the hook is 0.22mm. The ratio of radius of
curvature to thickness is therefore
approximately 7.1:1, and the ratio of width to thickness is therefore 0.5:1.
[0115] Referring to Figure 32, in some embodiments, the hooks 3288 on an
implant 3200 (which can be any
implant described herein) can be configured to engage tissue (e.g., extend
within tissue of the native mitral
valve annulus) at a radius R of between 2-20mm, such as 3-10mm, such as
approximately 3.4mm. Having a
radius of engagement within this range advantageously ensures that the hooks
3288 can engage with tissue
even when the native valve is not circular while ensuring that the hooks 3288
do not interfere with the
adjacent aortic valve. For example, the diameter D1 of the implant 3200 at the
connection of each hook 3288
(e.g., radially inner most point of the hooks) can be 30-50mm, such as 40-
48mm, such as approximately
45mm. The diameter D2 of the implant 3200 at the tip of each hook 3288 (e.g.,
the radially outer most point
of the hook) can be 40-60mm, such as 45-55mm, such as 52mm.
[0116] In some embodiments, the hooks can be riveted to the anchor assembly.
In other embodiments (as
shown in Figures 7A-7B), the hooks can be tabs that are flared out from the
anchor assembly.
[0117] In some embodiments, as shown in Figures 9A-9D, a portion of the anchor
assembly 901 can include
a portion that is pointed radially outwards to act as a hook or barb in the
tissue. For example, one set of the
v-shaped circumferential members 907 can be bent to point outwards. The bent v-
shaped members can be
positioned, for example, on the inner diameter of the ventricular anchor 904
pointing towards the atrium.
[0118] Any of the valve prostheses described herein can include a fabric cover
and/or skirt on one or more
portions of the device. For example, referring to Figures 16A-16D (valve is
shown in a holder for clarity), a
covering or skirt 1616 can be sewn along the inner diameter of the atrial
anchor 1616 and the flare of the
strut frame 1605 and down the entire inner diameter of the strut frame 1605.
This skirt 1616 can thus
provide a smooth entrance for blood into the leaflets 1622. Further, the skirt
1616 can extend along the
entire outer diameter of the anchoring assembly and then around the tips of
the ventricular anchor 1604. In
- 22 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
some embodiments, the skirt 1616 can be a single piece while in other
embodiments, the skirt 1616 can be
made of a plurality of pieces.
[0119] In some embodiments, as shown in Figure 20A, the skirt 2016 can leave
the ventricular tips of the
ventricular anchor 2004 uncovered. In other embodiments, as shown in Figure
30, the skirt 3006 can be
wrapped fully around the ventricular tips of the ventricular anchor 3004.
[0120] In some embodiments, the skirt, or a portion of the skirt, can be knit
in a three-dimensional shape,
e.g., an hour glass shape, to help maintain a consistent seal of the skirt
against the prosthesis and to help pack
the skirt-covered prosthesis during delivery. For example, as shown in Figures
23A-23C, the skirt 2316 can
be cut in an hour-glass shape and configured to cover all of the exposed
sections of the valve on the atrial
side (leaving only the ventricular side of the ventricular anchor and the
outer diameter of the strut frame
uncovered).
[0121] Further, in some embodiments, and as shown in Figures 23A-23C, the
skirt 2316 can be cut in a saw-
tooth pattern on the ventricular side to mimic the pattern of cells that
extend to the outermost diameter of the
ventricular anchor. Cutting the skirt in such a manner can help pack the
ventricular anchor into the delivery
device by reducing the packed diameter of the ventricular anchor.
[0122] Referring to Figure 35, a skirt 3516 can be pre-formed as a tubular
three-dimensional structure. The
skirt 3516 can have a wide section 3535 configured to form around the outside
or external surface of the
anchor assembly and a narrow section 3553 configured to form against the
inside or internal surface of the
strut frame. The central section 3552 between the wide section 3535 and the
narrow section 3552 can have a
tapered diameter. The skirt 3515 can be wrapped around the anchor assembly and
strut frame such that the
first end 3554 is positioned at the tips of the ventricular anchor, the wide
portion 3535 conforms to the
outside of the anchor assembly, the tapered central section 3552 extends
between the atrial anchor and the
atrial end of the strut frame, and the narrow section 3553 is folded or
everted into the strut frame so as to
conform to the interior surface of the strut frame (with the second end 3555
positioned at the ventricular end
of the strut frame). As shown, the first end 3554 can have a saw-tooth pattern
so as to mimic the points of
the cells forming the ventricular anchor and/or the second end 3555 can have a
saw-tooth pattern so as to
mimic the apexes at the ventricular end of the strut frame. In some
embodiments, the skirt 3516 can have
pre-formed cuts (e.g., laser cuts) to provide access to hooks, commissure
attachment mechanisms, delivery
system attachment mechanisms, or other elements of the underlying frame.
[0123] Figure 36 shows a skirt 3616 that is formed and wrapped around the
valve similar to as described
with respect to skirt 3516. In this embodiment, however, the first and second
ends 3654, 3655 are not in a
saw-tooth configuration. Rather, the end 3654 is straight and ends at the tips
of the ventricular anchor 3604
while the end 3655 is straight and is wrapped around the tips of the
ventricular end of the strut frame 3605.
It should be understood that one or both of the ends 3655, 3654 could be saw-
tooth, one or both could be
wrapped, and/or or one or both could end at the tips. Further, the skirt 3655
can be sewn to the frame (e.g.,
with polyethylene sutures) at the edges thereof.
- 23 -
[0124] Referring to Figure 26, if a skirt is knit or otherwise formed in a
three-dimensional shape, an inner
mandrel 2626 can be used (i.e., the skirt can be knit or formed over the
mandrel). After the skirt has been
formed around the mandrel 2626, the mandrel 2626 can dissolve or otherwise
break apart to leave the
formed skirt. In some embodiments, a woven fabric, such as a polyester weave,
can be used to form the
skirt over the mandrel 2626. In other embodiments, a polyurethane layer can be
painted or otherwise
applied over the mandrel 2626. The polyurethane can advantageously create
fewer wrinkles than a woven
fabric. If a polyurethane layer is used, a flap of material may be added in
order to create some give in the
skirt as the valve is packed and/or unpacked. Further, in some embodiments,
one or more polyurethane
layers can be added after the skirt is sewn onto the frame (e.g., to fill in
any holes caused by sewing the
skirt to the frame the material).
[0125] The skirts described herein can be made of polyethylene terephalate
(PET), polyester, or PET with
a polyurethane dispersion.
[0126] The skirt can advantageously help block blood flow from one side of the
valve over the other. The
skirt can also help prevent the anatomy from having an adverse interaction
with the frame itself.
[0127] In some embodiments, a coupler can be used to connect the strut frame
to the anchor assembly.
Rivets herein are an example of a coupler. The locations where components are
secured to one another
may be referred to as a coupling herein. Coupling also refers to the two
components that are secured
together. Riveting as used herein is an example of a method that plastically
deforms a coupler to secure
two or more components together at a coupling. Coupling and rivets are
described further in U.S. Patent
Application No. 14/677,334, filed April 2, 2015, titled "REPLACEMENT CARDIAC
VALVES AND
METHODS OF USE AND MANUFACTURE".
[0128] In some embodiments, the valve prostheses have been shown without
leaflets for clarity. It is to
be understood that each of the embodiments described herein can include
replacement leaflets 1022a,b,c
attached thereto, as shown in Figure 10. An exemplary leaflet 2122 is shown in
Figure 21. The leaflet can
include an outflow (or free) edge 2191 configured to float within the strut
frame, an inflow edge 2193
configured to be sewn to the strut frame, and two arms 2195a,b. A plurality of
sewing holes 2197 can
provide for sewing of the leaflet 2122 to the strut frame. Thus, as shown in
Figure 19, the outer
circumference of the leaflets at the inflow edges can be sewn to the strut
frame and/or to the skirt covering
the skirt frame. That is, while the commissures or edges of the leaflets can
be attached as described above,
the inflow edges of the leaflets can be sewn all around the circumference of
the strut frame.
[0129] Further, the leaflets can be attached to any of the valve prosthesis
designs in a variety of different
ways.
[0130] For example, referring to Figures 11A-11F, two commissure plates
1010a,b can be used to
sandwich the arms of the leaflets 1022a,b therebetween. The leaflets 1022a,b
can then be sewn together
(and to the plates 1010a,b) with one or more suture 1011 through holes
1013a,bc. After being sewn
together, the joined leaflets and commissure plates can then be attached to
the strut frame 1105 through,
for example, a series of
- 24 -
Date Recue/Date Received 2022-07-12
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
holes 1113a,b,c in one of the struts 1121 using a suture 1017 (which can be
the same or different than suture
1011). The commissure plates 1010a,b can be made, for example, of stainless
steel or plastic.
Advantageously, the commissure plates 1010a,b can apply compression to the
leaflets 1022a,b and distribute
strains along the length of the commissure plates, thereby reducing tearing or
strain propagation through the
tissue.
[0131] Another exemplary mechanism for leaflet attachment is shown in Figure
12. Here, rather than using
two commissure plates, a single u-shaped plate 1110 with a set of holes on
either side can be used. In
contrast to the commissure plates 1010a,b, the plate 1110 can place a fixed
amount of compression on the
leaflets that are sandwiched therebetween.
[0132] Additional exemplary mechanisms for leaflet attachment are shown in
Figures 14 and 15A-15C. In
the version of Figure 14, the arms of two leaflets 1022a,b are pulled through
a slot 1333 that is part of a strut
1321 of the strut frame. A secondary member 1313 having a width greater than
the width of the slot 1313 is
placed against both arms leaflets, and then the arms of the leaflets 1022a,b
are wrapped around the secondary
member 11313 and attached together with a suture 1311 or staple. The secondary
member 1313 can be
coupled to the strut frame, for example with a rivet. In a similar embodiment,
shown in Figures 15A-15C,
the leaflets 1022a,b can be passed through a slot in a secondary member 1515
and then wrapped around a
strut 1521 of the strut frame. Advantageously, the mechanisms of Figures 14
and 15A-C evenly distribute
high stress areas of leaflet along the length of strut 1312 or riveted slot
1321. The load distribution along the
given length of these members decrease stresses in comparison to attachment
methods where many stress
concentrations are created i.e. sutures.
[0133] Another exemplary mechanism for leaflet attachment is shown in Figures
18A-18E. In this
embodiment, a plate 1818 including a plurality of holes 1819 can be positioned
on the outside of the strut
frame 1805. Further, the strut frame 1805 can include a slot 1833
therethrough. The arms of the leaflets
1822a,b can then be extended through the slot 1833 and flattened against the
outer surface of the strut frame
1805. The plate 1818 can be placed against the arms of the leaflets 1822a,b
and then sutured to the arms of
the leaflets, e.g., through the holes 1819. The arms of the leaflets 1822a,b
can thus be sandwiched between
the plate 1818 and the strut frame 1805. In some embodiments, the suture is
attached to a skirt or fabric layer
on the strut frame 1805 rather than directly to the strut frame.
[0134] Another exemplary mechanism for leaflet attachment is shown in Figures
27A-27R. In this
embodiment, a plate 2727 with a plurality of channels 2773 (or open slots or
indents) in the sides thereof can
be positioned on the outside of the strut frame 2705. The channels 2773 can
extend diagonally towards the
center of the plate 2727. There can be two or more channels 2773, such as
between 6 and 12 channels 2773,
such as ten channels 2773. Further, the frame 2705 can include three slots
2733 therethrough (one for each
attachment point) that are positioned equidistant from one another around the
circumference of the strut
frame 2705. The slots 2733 can be positioned within a strut 2721 at the
ventricular end. To attach the
leaflets 2722a,b to the frame 2705, a first suture 2772a can first be threaded
between the frame 2705 and skirt
- 25 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
2716 fabric and around the slot 2733. The first suture 2772a can then be slid
distally towards the ventricular
tips 2777 of the strut frame 2705 (Figure 27A). At Figure 27B, a second suture
2772b is threaded similarly
to the first suture 2772a. At Figure 27C, a third suture 2772c is pierced
through the fabric just distal to the
slot 2733 from the outside and back, wrapping the third suture 2772c around
the frame 2705. At Figure 27D,
two leaflets 2722a,b can be aligned, and leaflet protectors 2773 (e.g., made
of a lubricious fabric, such as a
polyester weave) can be placed along the outward-facing side of each arm
2795a,b of the leaflets 1022a,b.
At Figure 27E, the arms 2795a,b and leaflet protectors 2773a,b of the leaflets
1022a,b can remain flush. As
shown in Figures 27G-I, the leaflet arms 2795a,b can be slid through the slot
2733. As shown in Figure 27F,
the arms 2795a,b can be positioned at approximately a 90 degree angle relative
to the slot 2733. As shown at
Figure 27G, each arm 2795a,b can be slid through the slot 2733 until the
beginning of the bump 2778 on the
arm 2795a,b is flush with the inside of the slot 2733 (to do so, the inflow
edges 2793 can be folded inward
towards one another and the central axis. At Figure 27H, the arms 2795a,b can
be at approximately 90
degrees relative to the slot 2733 after being pulled therethrough. At Figure
271, the two leaflets 2722a,b can
be separated, and, at Figures 27J and 27K, the process can be repeated for
each of the other slots and
attachment points (e.g., two additional slots/leaflet attachment points). As
shown at Figures 27L and 27M,
the leaflet arms 2795a,b can be folded away from one another, and the leaflet
protectors 2773a,b can be
folded away from one another. As shown at Figure 27N, the edges of each arm
2795a,b can be placed
horizontal to the outflow plane and the side/vertical edges can be parallel
with the strut members 2721. At
Figure 270, the plate 2727 can be placed onto the leaflet arms and aligned
with the slot 2733. The vertical
edges 2761 of the retaining plate 2727 can be aligned parallel with the
vertical strut members 2721. The top
2762 of the retaining plate 2727 can be aligned with the outflow tips 2777 of
the strut frame 2705. The
center of the retaining plate 2727 can be aligned with the center of the slot
2733. At Figure 27P, the first
suture 2772a can be wound around the top set of indents 2773a,b in the plate
2727 and the third suture 2772c
can be wound around the bottom set of indents 2773i,j. At Figure 27Q, the
second suture 2772b can be
woven around the plate 2727 into the remaining indents 2773c-h in a crisscross
pattern (dotted lines
represent suture on the backside of the plate 2727). The process can be
repeated at each of the commissure
attachment points. The sutures can advantageously help prevent translation of
the plate 2727 relative to the
slot 2733 and frame 2705. Further, the plate 2727 and slot 2733 can
advantageously securely attach the
leaflets 2722 to the frame 2705 without damaging the frame 2705, leaflets
2722a,b, and/or skirt 2716.
[0135] Figures 33-34 show additional plate embodiments that are similar to
plate 2727. Referring to Figure
33A, the plate 3327 is similar to plate 2727 except that the indents 3373 are
longer and have a different angle
that the indents 2773 of plate 2727. Thus, the top sent of indents 3373a, b in
the plate 3427 are at an angle of
approximately 90 degrees relative to the longitudinal axis 3333 of the device.
Indents 3373c,d are angled
upwards at 30-60 degrees, such as approximately 45 degrees relative to the
longitudinal axis 3333. Indents
3373e,f are angled downwards at 30-60 degrees, such as approximately 45
degrees, relative to the
longitudinal axis 3333. Indents 3373g,h are angled upwards again at 30-60
degrees, such as approximately
- 26 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
45 degrees, relative to the longitudinal axis 3333, and intents 3373i are at
an angle of approximately 90
degrees relative to the longitudinal axis 3333. Further, each of the intents
3373c-h extends 15-30%, such as
20-25% of the width of the plate 3327.
[0136] Referring to Figure 34A, the plate 3427 is similar to plate 2727 except
that the indents 3473 are all at
an angle of substantially 90 degrees relative to the longitudinal axis 3433.
Further, the inner edge of each of
the indents 3473c-h has a substantially circular shape. The indents 3473c-h
each extend approximately 10%-
25%, such as 15%-20% of the width of the plate 3427.
[0137] In some embodiments, referring to Figure 22, once the arms of the
leaflets 2222 are attached to the
strut frame 2205, the inflow edges can be sewn to the strut frame 2205. An
exemplary sewing line 2525
(close to the rivets 2527 at the atrial end of the strut frame 2205) is shown
in Figure 22.
[0138] In some embodiments, a valve prosthesis as described herein can include
a delivery system
attachment mechanism. For example, as shown in Figures 2A-2B, the atrial tips
212 can each have a pin 215
extending therefrom (e.g., in the ventricular direction) around which tethers
from a delivery system can be
wound.
[0139] Another delivery system attachment mechanism is shown in Figures 29A-
29E. The atrial tips 2912
each have a pin 2915 extending therefrom (e.g., in the ventricular direction).
Each pin can be, for example,
0.030 inches long and approximately 0.012 inches thick. Further, as shown in
Figure 29E, the skirt 2916 can
have slots 2985 therein that are aligned with the pins 2915. The slots 2985
can allow for the passage of the
tethers therethrough (i.e., to provide access to the pins 2195).
[0140] An exemplary method of delivering a valve prosthesis 1700 (which can be
any of the valves
prostheses described herein) after attachment to the tethers of the delivery
system is shown in Figures 17A-
17J. At Figures 17A and 17B, the valve is packed inside of a sheath such that
the tips of the ventricular
anchor 1704 point towards the ventricular end (i.e., away from the central
portion 1703) and the tips of the
atrial anchor 1702 point towards the atrial end (i.e., away from the central
portion 1703). The valve 1700 can
be delivered, e.g., transseptally, to the native annulus in this packed
positioned. At Figures 17C-E, the
ventricular anchor 1704 is partially deployed, i.e., to allow the ventricular
anchor 1704 to begin to flare
outwards. In this embodiments, barbs on the device point radially outwards
rather than towards the atrium
during the initial deployment steps. At Figures 17F and G, the valve is pulled
1-3cm towards the atrium to
seat the ventricular anchor 1704 on the ventricular side of the annulus. At
Figure 17H, the ventricular anchor
1704 is fully deployed, allowing the barbs to extend into the tissue. At this
point, the strut frame 1705
(holding leaflets) is also fully exposed. At Figure 171, the atrial anchor
1702 is partially released to allow
the anchor 1702 to drop against the wall of the atrium. At Figure 17J, the
atrial anchor 1702 is fully released,
and the valve 1700 is seated in place.
[0141] The valve prostheses described herein can advantageously pack to a very
low packing length, such as
less than 4cm, less than 3.8cm, less than 3.6cm, less than 3.2cm, or less than
3.0cm for delivery with a 32
- 27 -
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
French catheter. This low axial packing length advantageously allows the
prostheses to be delivered
transseptally, e.g., be easily maneuvered around the bend through the septum.
[0142] Further, the cells and/or v-shaped patterns of the valve prostheses
described herein can be
specifically designed so as to ensure that the ventricular side doesn't flare
out when delivered. For example,
by making the atrial anchor flexible (e.g., with flexible members), the
ventricular anchor is less likely to
hook around when delivered. As another example, the radius of the valve (the
anchor or the strut frame) can
be tuned and/or the valve can be made more flexible in specific areas (of the
anchor or the strut frame) so as
to ensure that the valve is less prone to hooking/flaring out when delivered.
That is, referring to Figures 13A
and 13B, in one embodiment, a change in the radius of curvature in region 1401
will yield a change in the
deployment angle 0 of the ventricular anchor in region 1402. Decreasing the
curvature in region 1401 will
make the frame less prone to wrapping around the catheter tip when the
ventricular anchor is exposed from
the catheter. In another embodiment, by making region 1401 flexible, but
leaving the remaining portions of
the ventricular and atrial anchors relatively stiff, the deployment angle 0 in
region 1402 is less prone to
wrapping around the catheter tip when the ventricular anchor is exposed from
the catheter tip.
[0143] The valve prostheses described herein can advantageously avoid
interference with blood flow
through the valve. For example, the skirting and shape of the nitinol on the
inflow (or atrial) portion of the
valve can be contoured to provide smooth approach to the valve orifice. This
helps decrease the risk of any
turbulent flow or pockets of stagnant blood. As another example, the
attachment point between the inner
strut and the outer frame can be adjusted longitudinally to change the
relative obstruction of the inner strut
with blood flow and the ventricular sub-valvular apparatus. As yet another
example, the skirting can be
selectively applied to areas only in which there is a risk of blood escaping
between the prosthesis and the
anatomy. By allowing some cells to be open, particularly on the ventricular
anchoring member, there is less
impedance to flow.
[0144] Any of the valve features or structural details of any device
embodiment described herein can be
incorporated or combined with any of the other embodiments herein. For
example, the central members
described herein are not limited in use with the anchor assemblies and strut
frames in the specific
embodiment, but can be replaced with any of the features described in any
other embodiment.
[0145] In use, when the devices described herein can be used as mitral valve
replacements. In some
embodiments, when the replacement heart valve has been delivered near the
mitral valve, the ventricular
anchor can be deployed first in a cardiac chamber, such as the ventricle, and
retracted to a seated position
against the valve orifice, such as the mitral valve orifice. Then the center
portion and atrial anchor portion
may be deployed in another cardiac chamber, such as the atrium, wherein the
expansion and reconfiguration
of the atrial anchor and the central portion sandwiches the valve orifice
securely between the anchors that
have been deployed on either side of the annulus. Other exemplary aspects of
the methods of delivery
described in U.S. Pat. No. 8,870,948, issued October 28, 2014, in
International Patent Application No.
PCT/US2016/032546, filed May 13, 2016, titled "CARDIAC VALVE DELIVERY DEVICES
AND
- 28 -
SYSTEMS," and in U.S. Provisional Patent Application Nos. 62/424,021 and
62/424,051, both filed
November 18, 2016 and titled "CARDIAC VALVE DELIVERY DEVICES AND SYSTEMS".
[0146] When a feature or element is herein referred to as being "on" another
feature or element, it can be
directly on the other feature or element or intervening features and/or
elements may also be present. In
contrast, when a feature or element is referred to as being "directly on"
another feature or element, there
are no intervening features or elements present. It will also be understood
that, when a feature or element
is referred to as being "connected", "attached" or "coupled" to another
feature or element, it can be directly
connected, attached or coupled to the other feature or element or intervening
features or elements may be
present. In contrast, when a feature or element is referred to as being
"directly connected", "directly
attached" or "directly coupled" to another feature or element, there are no
intervening features or elements
present. Although described or shown with respect to one embodiment, the
features and elements so
described or shown can apply to other embodiments. It will also be appreciated
by those of skill in the art
that references to a structure or feature that is disposed "adjacent" another
feature may have portions that
overlap or underlie the adjacent feature.
[0147] Terminology used herein is for the purpose of describing particular
embodiments only and is not
intended to be limiting of the invention. For example, as used herein, the
singular forms "a", "an" and
"the" are intended to include the plural forms as well, unless the context
clearly indicates otherwise. It will
be further understood that the terms "comprises" and/or "comprising," when
used in this specification,
specify the presence of stated features, steps, operations, elements, and/or
components, but do not preclude
the presence or addition of one or more other features, steps, operations,
elements, components, and/or
groups thereof. As used herein, the term "and/or" includes any and all
combinations of one or more of the
associated listed items and may be abbreviated as "1'.
[0148] Spatially relative terms, such as "under", "below", "lower", "over",
"upper" and the like, may be
used herein for ease of description to describe one element or feature's
relationship to another element(s)
or feature(s) as illustrated in the figures. It will be understood that the
spatially relative terms are intended
to encompass different orientations of the device in use or operation in
addition to the orientation depicted
in the figures. For example, if a device in the figures is inverted, elements
described as "under" or "beneath"
other elements or features would then be oriented "over" the other elements or
features. Thus, the
exemplary term "under" can encompass both an orientation of over and under.
The device may be
otherwise oriented (rotated 90 degrees or at other orientations) and the
spatially relative descriptors used
herein interpreted accordingly. Similarly, the terms "upwardly", "downwardly",
"vertical", "horizontal"
and the like are used herein for the purpose of explanation only unless
specifically indicated otherwise.
[0149] Although the terms "first" and "second" may be used herein to describe
various features/elements
(including steps), these features/elements should not be limited by these
terms, unless the context indicates
otherwise. These terms may be used to distinguish one feature/element from
another feature/element.
Thus,
- 29 -
Date Recue/Date Received 2022-07-12
CA 03103294 2020-12-09
WO 2019/246096 PCT/US2019/037729
a first feature/element discussed below could be termed a second
feature/element, and similarly, a second
feature/element discussed below could be termed a first feature/element
without departing from the teachings
of the present invention.
[0150] Throughout this specification and the claims which follow, unless the
context requires otherwise, the
word "comprise", and variations such as "comprises" and "comprising" means
various components can be
co-jointly employed in the methods and articles (e.g., compositions and
apparatuses including device and
methods). For example, the term "comprising" will be understood to imply the
inclusion of any stated
elements or steps but not the exclusion of any other elements or steps.
[0151] As used herein in the specification and claims, including as used in
the examples and unless
otherwise expressly specified, all numbers may be read as if prefaced by the
word "about" or
-approximately," even if the term does not expressly appear. The phrase
"about" or "approximately" may be
used when describing magnitude and/or position to indicate that the value
and/or position described is within
a reasonable expected range of values and/or positions. For example, a numeric
value may have a value that
is +/- 0.1% of the stated value (or range of values), +/- 1% of the stated
value (or range of values), +/- 2% of
the stated value (or range of values), +/- 5% of the stated value (or range of
values), +/- 10% of the stated
value (or range of values), etc. Any numerical range recited herein is
intended to include all sub-ranges
subsumed therein.
[0152] Although various illustrative embodiments are described above, any of a
number of changes may be
made to various embodiments without departing from the scope of the invention
as described by the claims.
For example, the order in which various described method steps are performed
may often be changed in
alternative embodiments, and in other alternative embodiments one or more
method steps may be skipped
altogether. Optional features of various device and system embodiments may be
included in some
embodiments and not in others. Therefore, the foregoing description is
provided primarily for exemplary
purposes and should not be interpreted to limit the scope of the invention as
it is set forth in the claims.
[0153] The examples and illustrations included herein show, by way of
illustration and not of limitation,
specific embodiments in which the subject matter may be practiced. As
mentioned, other embodiments may
be utilized and derived there from, such that structural and logical
substitutions and changes may be made
without departing from the scope of this disclosure. Such embodiments of the
inventive subject matter may
be referred to herein individually or collectively by the term "invention"
merely for convenience and without
intending to voluntarily limit the scope of this application to any single
invention or inventive concept, if
more than one is, in fact, disclosed. Thus, although specific embodiments have
been illustrated and
described herein, any arrangement calculated to achieve the same purpose may
be substituted for the specific
embodiments shown. This disclosure is intended to cover any and all
adaptations or variations of various
embodiments. Combinations of the above embodiments, and other embodiments not
specifically described
herein, will be apparent to those of skill in the art upon reviewing the above
description.
- 30 -