Note: Descriptions are shown in the official language in which they were submitted.
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
COMPOSITIONS AND METHODS FOR TREATING THE EYE
FIELD OF THE INVENTION
The present invention relates to compositions comprising one or more extracts
and/or
compounds having retinol-like activity and properties and methods of using the
compositions
to treat the eye.
BACKGROUND OF THE INVENTION
"Dry eye is a multifactorial disease of the ocular surface characterized by a
loss of
homeostasis of the tear film, and accompanied by ocular symptoms, in which
tear film
instability and hyperosmolarity, ocular surface inflammation and damage, and
neurosensory
abnormalities play etiological roles." Craig, J.P. et al. TFOS DEWS II
definition and
classification report. Ocul Surf 2017; 15: 276-283. Dry eye can result from
abnormal or
inadequate tear formation, and deficiency in mucin secretion (i.e.,
keratoconjunctivitis sicca).
Dry eye symptoms can be manifest as a result of various underlying disorders
such as
autoimmune disorders that damage lacrimal (i.e., tear-producing) glands, such
as rheumatoid
arthritis, Sjogren's syndrome, systemic lupus erythematosus, and systemic
sclerosis and
sarcoidosis. Dry eye can also be induced following eye surgery, such as Lasik0
surgery.
Dry eye is estimated to affect more than 13 million individuals in the United
States.
Regardless of the underlying pathology, dry eye commonly involves the rapid
breakdown of the pre-ocular tear film, resulting in dehydration of the exposed
outer surface.
Normal tear formation is required to keep the cornea and conjunctiva moist,
and this in turn
helps to prevent ulceration of both, as well as to maintain corneal
transparency. In addition,
tears facilitate movement of the eyelid over the eye surface (e.g., blinking)
and removal of
foreign substances from the eye. Tears also normally contain lysozyme which is
useful in
preventing infection in the eye. Dry eye can be associated with mild
discomfort to severe
pain in the eye. When it occurs for prolonged periods of time, it can cause
blurred vision,
grittiness and/or burning sensation, and itchiness. If the condition is
allowed to persist
without treatment, it can further lead to corneal ulcers and/or scarring.
Dry eye symptoms include eye pain or fatigue, increased blinking, and
bloodshot
eyes. Further, bacteria may enter through a scratch and cause infection, and
if the scratch is
deep enough it can even affect the vision of the person. In addition to
eyestrain, causes of dry
1
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
eye include Sjogren's syndrome, Stevens-Johnson syndrome, burns and injury to
the eye, and
side effects of hypotensive drugs, tranquilizers, eyedrops for treating
glaucoma, and other
such drugs.
Tear film is the bodies' natural defense against dry eye. The tear film
contains ocular
mucins and is essential for maintaining the homeostasis of the wet ocular
surface. Mucins are
produced, among other places, by corneal epithelial cells in the eye. Mucins
are
glycoproteins expressed by epithelial tissues of mucosal surfaces. They
protect tissues by
functioning as antioxidants and providing lubrication. Mucin genes associated
with the tear
film include MUC1, MUC2, MUC4, MUC5AC, MUC5B, MUC7 and MUC16.
Mucin is also useful as an antimicrobial, for general wound healing, and is
essential
for overall eye health maintenance.
There is therefore a need for an ophthalmic pharmaceutical composition that
would
promote and/or improve the production and/or release of mucin from and/or in
the cornea.
The present inventors have discovered compounds and/or extracts having retinol-
like
properties and/or benefits which can induce, promote and/or improve
production/release/delivery/excretion of mucin from and/or in the cornea.
Accordingly, an aspect of the present invention relates to compositions
comprising a
safe and effective amount of one or more compounds and/or extracts having
retinol-like
properties and/or benefits to induce, promote and/or improve the
production/release/delivery/excretion of mucin from and/or in the cornea.
Another aspect of the present invention relates to compositions comprising a
safe and
effective amount of one or more compounds and/or extracts having retinol-like
properties
and/or benefits which induce, promote and/or improve
production/release/delivery/excretion
of mucin from and/or in the cornea, which compositions can be administered to
patients
having a MUC5AC concentration in tears lower than 6 (or about 6), optionally 8
(or about 8),
nanograms per milligram of proteins, such that the concentration of MUC5AC in
the tears is
raised to (or, is made to be) equal to or greater than 8 (or about 8)
nanograms to 15 (or about
15) nanograms, optionally from 9 (or about 9) nanograms to 12 (or about 12)
nanograms, per
milligram of proteins.
In certain embodiments, the above-described concentration of MU5AC in the
tears
(i.e., equal to or greater than 8 (or about 8) nanograms to 15 (or about 15)
nanograms,
optionally from 9 (or about 9) nanograms to 12 (or about 12) nanograms, per
milligram of
proteins), resulting from the compounds and/or extracts having retinol-like
properties and/or
2
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
benefits which induce, promote and/or improve
production/release/delivery/excretion of
mucin from and/or in the cornea, is maintained for a period of up to, at
least, about 2 hours,
optionally about 4 hours, optionally about 6 hours, optionally about 8 hours,
optionally about
hours, optionally about 12 hours, or optionally from about 12 to about 24
hours.
5 Concentrations of MUC5AC in tears detailed above are determined using the
Uchino
Method (described below in the definitions).
Another aspect of the present invention relates to compositions comprising a
safe and
effective amount of one or more compounds and/or extracts having retinol-like
properties
and/or benefits which induce, promote and/or improve
production/release/delivery/excretion
10 of mucin from and/or in the cornea for treating dry eye.
Another aspect of the present invention relates to methods of preventing
and/or
treating (e.g., reducing) eye symptoms associated with dry eye and/or
resulting from
decreased or low-level production/release/delivery/excretion of mucin from
and/or in the
cornea by administering compositions comprising a safe and effective amount of
one or more
compounds and/or extracts having retinol-like properties and/or benefits which
induce,
promote and/or improve production/release/delivery/excretion of mucin from
and/or in the
cornea.
Another aspect of the present invention relates to methods of promoting
healing or
increasing the rate of healing of wounds in and/or on the eye (e.g., non-dry
eye associated,
eye trauma, postoperative surgical or nonspecific wounds) of a patient by
administering
compositions comprising a safe and effective amount of one or more compounds
and/or
extracts having retinol-like properties and/or benefits which induce, promote
and/or improve
production/release/delivery/excretion of mucin from and/or in the cornea
(i.e., which increase
production/release/delivery/excretion of mucin from and/or in the cornea, in
certain
embodiments, beyond the concentration level of mucin produced by such patient
without (or
absent) administration of the compositions comprising a safe and effective
amount of one or
more compounds and/or extracts having retinol-like properties and/or benefits
which induce,
promote and/or improve production/release/delivery/excretion of mucin from
and/or in the
cornea).
Another aspect of the present invention relates to methods for improving the
antimicrobial properties in tears (or the tear film of the eye) of a patient
by administering
compositions comprising a safe and effective amount of one or more compounds
and/or
extracts having retinol-like properties and/or benefits which induce, promote
and/or improve
3
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
production/release/delivery/excretion of mucin from and/or in the cornea
(i.e., which increase
production/release/delivery/excretion of mucin from and/or in the cornea, in
certain
embodiments, beyond the concentration level of mucin produced by such patient
without (or
absent) the administration of the compositions comprising a safe and effective
amount of one
or more compounds and/or extracts having retinol-like properties and/or
benefits which
induce, promote and/or improve production/release/delivery/excretion of mucin
from and/or
in the cornea).
SUMMARY OF THE INVENTION
The present invention relates to methods for
producing/releasing/delivering/excreting mucin from and/or in the cornea
(optionally,
in a patient in need of such production/release/deliverance/excretion of
mucin)
comprising the step of administering a composition comprising:
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
L
"ss
(I)
wherein -
the dotted lines represent simple or double bound; optionally one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; optionally a methyl (-
CH3) or methylene (=CH2) moiety; a carbonated chain, linear, ringed, or
branched, saturated or unsaturated, comprising from 1 to 20 carbon atoms;
4
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
optionally from 1 to 10 carbon atoms; optionally 6 carbon atoms; optionally an
aromatic moiety, optionally a phenyl moiety; optionally 2-methyl -prop-1,3-
diene; and
ii) optionally, an ophthalmologically acceptable carrier.
The present invention relates to methods for maintaining the concentration of
MU5AC in tears within the range of equal to or greater than 8 nanograms to 15
nanograms
per milligrams of protein, comprising the step of administering a composition
comprising:
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
L
ORi
(I)
wherein -
the dotted lines represent simple or double bound; optionally one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; optionally a methyl (-
CH3) or methylene (=CH2) moiety; a carbonated chain, linear, ringed, or
branched, saturated or unsaturated, comprising from 1 to 20 carbon atoms;
optionally from 1 to 10 carbon atoms; optionally 6 carbon atoms; optionally an
aromatic moiety, optionally a phenyl moiety; optionally 2-methyl -prop-1,3-
diene; and
ii) optionally, an ophthalmologically acceptable carrier.
5
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
The present invention relates to methods for treating a patient having
decreased or
low-level production/release/delivery/excretion of mucin from and/or in the
cornea
comprising the step of topically administering to the eye the patient a
composition
comprising:
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; optionally one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; optionally a methyl (-
CH3) or methylene (=CH2) moiety; a carbonated chain, linear, ringed, or
branched, saturated or unsaturated, comprising from 1 to 20 carbon atoms;
optionally from 1 to 10 carbon atoms; optionally 6 carbon atoms; optionally an
aromatic moiety, optionally a phenyl moiety; optionally 2-methyl -prop-1,3-
diene; and
ii) optionally, an ophthalmologically acceptable carrier.
The present invention relates to methods for preventing or treating the
symptoms
associated with dry eye comprising the step of topically administering to a
patient (optionally,
in a patient need of such prevention or reduction in dry eye symptoms) a
composition
comprising:
6
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; optionally one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; optionally a methyl (-
CH3) or methylene (=CH2) moiety; a carbonated chain, linear, ringed, or
branched, saturated or unsaturated, comprising from 1 to 20 carbon atoms;
optionally from 1 to 10 carbon atoms; optionally 6 carbon atoms; optionally an
aromatic moiety, optionally a phenyl moiety; optionally 2-methyl -prop-1,3-
diene;
ii) one or more demulcents or soothing agents; and
iii) optionally, an ophthalmologically acceptable carrier.
The present invention relates to methods for promoting healing or increasing
the rate of healing of wounds in and/or on the eye of a patient (optionally,
in a patient
need of such eye wound healing), comprising the step of administering
compositions
(i.e., which increase production/release/delivery/excretion of mucin from
and/or in the
cornea, in certain embodiments, beyond the concentration level of mucin
produced by
such patient without (or absent) administration of the compositions comprising
a safe
and effective amount of one or more compounds and/or extracts having retinol-
like
properties and/or benefits) comprising:
7
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; preferably one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; preferably a methyl (-
CH3) or methylene (=CH2) moiety;
a represent a carbonated chain, linear, ringed, or branched, saturated or
unsaturated, comprising from 1 to 20 carbon atoms; preferably from 1 to 10
carbon atoms; more preferably 6 carbon atoms; preferably an aromatic moiety,
preferably a phenyl moiety; preferably 2-methyl -prop-1,3-diene.
ii) optionally, an ophthalmologically acceptable carrier.
The present invention relates to methods for improving the antimicrobial
properties in tears (or, the tear film of the eye) of a patent (optionally, in
a patient
need of such antimicrobial properties), comprising the step of administering
compositions (i.e., which increase production/release/delivery/excretion of
mucin
from and/or in the cornea, in certain embodiments, beyond the concentration
level of
mucin produced by such patient without (or absent) administration of the
compositions comprising a safe and effective amount of one or more compounds
and/or extracts having retinol-like properties and/or benefits) comprising:
8
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; preferably one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; preferably a methyl (-
CH3) or methylene (=CH2) moiety;
a represent a carbonated chain, linear, ringed, or branched, saturated or
unsaturated, comprising from 1 to 20 carbon atoms; preferably from 1 to 10
carbon atoms; more preferably 6 carbon atoms; preferably an aromatic moiety,
preferably a phenyl moiety; preferably 2-methyl -prop-1,3-diene.
ii) optionally, an ophthalmologically acceptable carrier.
BRIEF DESCRIPTION OF THE FIGURES
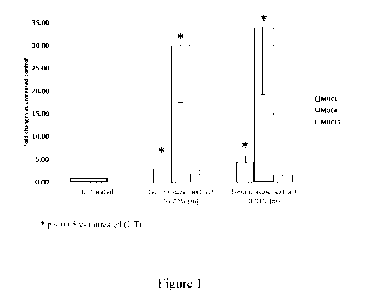
Figure 1 depicts bar graphs showing lemon aspen extract induced MUC1, MUC4 and
MUC16 gene expression in corneal epithelial cells.
Figure. 2 depicts bar graphs showing lemon aspen extract induced Mucin-1
secretion
in corneal epithelial cells.
DETAILED DESCRIPTION OF INVENTION
9
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
It is believed that one skilled in the art can, based upon the description
herein, utilize
this invention to its fullest extent. The following specific embodiments can
be construed as
merely illustrative, and not limitative of the remainder of the disclosure in
any way
whatsoever.
The compositions of the present invention can comprise, consist of, or consist
essentially of the elements, steps and limitations of the invention described
herein, as well
any of the additional or optional ingredients, components, or limitations
described herein.
The term "comprising" (and its grammatical variations) as used herein is used
in the
inclusive sense of "having" or "including" and not in the exclusive sense of
"consisting only
of" The terms "a" and "the" as used herein are understood to encompass the
plural as well as
the singular.
Unless defined otherwise, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
the invention
belongs. Also, all publications, patent applications, patents, and other
references mentioned
herein are incorporated by reference in their entirety to the extent that they
are not
inconsistent with this specification. As used herein, all percentages are by
weight of the total
composition unless otherwise specified
As used herein, the terms "cornea" or "corneal" is, includes and/or relates
to, the
transparent front part of the eye that covers the iris, pupil, and anterior
chamber, the layers of
which transparent front part include the corneal epithelium layer (comprising
corneal
epithelial cells), Bowman's layer (also known as the anterior limiting
membrane), Corneal
stroma (also substantia propria), Descemet's membrane (also posterior limiting
membrane),
and Corneal endothelium (simple squamous or low cuboidal monolayer, approx. 5
lam thick,
of mitochondria-rich cells).
As used herein, the phrase "decreased or low-level
production/release/delivery/excretion of mucin from and/or in the cornea"
means a
concentration of MUC5AC which is less than the concentration of MUC5AC in the
tears of a
normal (i.e., non-diseased) person, or, in certain embodiments, less than 6,
optionally less
than 8, nanograms per milligram of proteins, as determined using the method
described in
Uchino Y, Uchino M, Yokoi N, et al. Alteration of Tear Muein SAC in Office
Workers Using
Visual Display Terminals: The Osaka Study. JAAL4 Ophthairnot n2014;132(8):985-
992.
That method (the Uchino Method) is reproduced below:
a MUC5AC Concentration in Tears
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
The concentration of the secreted Illi1Cill MI.TC5AC in the tear samples was
quantified by enzyme-linked immunoassay (E907561-Iu; tiSCN Life Science). (See
Maker .AV, Katabi N, Gomm M. et al. Pancreatic cyst fluid and serum EMICill
levels
predict dysplasia in intraduetal papillary mucitions neoplasms of the
pancreas. Ann
Surg Oncol. 2011;18(1):199-206.) All samples were analyzed according to the
manufacturer's guidelines. Absorbance was measured at 450 rim, and the
standard
solutions in the kit were recombinant hutnan MIJC5AC. A protein assay reagent
kit
(13CA Protein Assay Kit; Pierce) was used to determine the protein
concentration in
the tear samples. The MUC5AC concentration was normalized to the tear protein
content and expressed as MUC5A.0 protein (nanograms) per tear total protein
(milligrams).
As used herein, a composition that is "essentially free" of an ingredient
means the
composition that has about 2% or less of that ingredient by weight based on
the total weight
of the composition. Preferably, a composition that is essentially free of an
ingredient has
about 1% or less, more preferably about 0.5% or less, more preferably about
0.1% or less,
more preferably about 0.05 or less, more preferably about 0.01% or less by
weight based on
the total weight of composition of the ingredient. In certain more preferred
embodiments, a
composition that is essentially free of an ingredient is free of the
ingredient, i.e. has none of
that ingredient in the composition.
As used herein, "ophthalmologically acceptable" means that the ingredients
which the
term describes are suitable for use in contact with tissues (e.g., the soft
tissues of the eye or
periorbital skin tissues) without undue toxicity, incompatibility,
instability, irritation, allergic
response, and the like. As will be recognized by one of skill in the art,
ophthalmologically
acceptable salts are acidic/anionic or basic/cationic salts.
As used herein, the term "safe and effective amount" means an amount of
disclosed
the extract, compound or of the composition sufficient to induce, promote
and/or improve the
production/release/delivery/excretion of mucin from and/or in one or more
layer of the
cornea, but low enough to avoid serious side effects. The safe and effective
amount of the
compound, extract, or composition will vary with e.g. the age, health and
environmental
exposure of the end user, the duration and nature of the treatment, the
specific extract,
ingredient, or composition employed, the particular pharmaceutically-
acceptable carrier
utilized, and like factors.
The term "retinol-like properties and/or benefits" means the properties and/or
benefits
induced by retinol.
11
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
In certain embodiments, the present invention as disclosed herein may be
practiced in
the absence of any compound or element (or group of compounds or elements)
which is not
specifically disclosed herein.
In general, IUPAC nomenclature rules are used herein and according to the
following
term definitions.
The term "C1-8 alkyl," whether used alone or as part of a substituent group,
refers to a
saturated aliphatic branched or straight-chain monovalent hydrocarbon radical
having from 1-
8 carbon atoms. For example, "C1-8a1ky1" specifically includes the radicals
methyl, ethyl, 1-
propyl, 2-propyl, 1-butyl, 2-butyl, tert-butyl, 1-butyl, 1-pentyl, 2-pentyl, 3-
pentyl, 1-hexyl, 2-
hexyl, 3- hexyl, 1-heptyl, 2-heptyl, 3-heptyl, 1-octyl, 2-octyl, 3-octyl and
the like. Said term
may also refer to the corresponding alkyldiyl radical. Alkyl and alkyldiyl
radicals may be
attached to a core molecule via a terminal carbon atom or via a carbon atom
within the chain.
Similarly, any number of substituent variables may be attached to an alkyl or
alkyldiyl radical
when allowed by available valences.
The term "C1-4a1ky1," whether used alone or as part of a substituent group,
refers to a
saturated aliphatic branched or straight-chain monovalent hydrocarbon radical
or alkyldiyl
linking group having a specified number of carbon atoms, wherein the radical
is derived by
the removal of one hydrogen atom from a carbon atom and the alkyldiyl linking
group is
derived by the removal of one hydrogen atom from each of two carbon atoms in
the chain.
The term "C1-4a1ky1" refers to a radical having from 1-4 carbon atoms in a
linear or branched
arrangement. For example, "C1-4a1ky1" specifically includes the radicals
methyl, ethyl, 1-
propyl, 2-propyl, 1-butyl, 2-butyl, tert- butyl, 1-butyl, and the like. Alkyl
and alkyldiyl
radicals may be attached to a core molecule via a terminal carbon atom or via
a carbon atom
within the chain. Similarly, any number of substituent variables may be
attached to an alkyl
or alkyldiyl radical when allowed by available valences.
The term "C2-4a1keny1" refers to an alkenyl radical having from 2-4 carbon
atoms.
For example, specifically includes the radicals ethenyl, propenyl, ally' (2-
propenyl), butenyl
and the like. As described above, an alkenyl radical may be similarly attached
to a core
molecule and further substituted where indicated.
The term "halo" as such or in combination with other terms means halogen atom,
such as fluoro, chloro, bromo or iodo.
The term "substituted," refers to a core molecule in which one or more
hydrogen
atoms have been replaced with that amount of substituents allowed by available
valences.
12
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Substitution is not limited to the core molecule, but may also occur on a
substituent radical,
whereby the radical becomes a linking group.
The term "independently selected" refers to two or more substituents that may
be
selected from a substituent variable group, wherein the selected substituents
may be the same
or different.
The term "dependently selected" refers to one or more substituent variables
that are
specified in an indicated combination for substitution in a core molecule
(e.g. variables that
refer to groups of substituents appearing in a tabular list of compounds).
Acceptable salts from inorganic bases include, for example, sodium or
potassium
.. salts, and the like. Acceptable salts from organic bases include, for
example, salts formed
with primary, secondary, or tertiary amines, and the like.
Compounds/Extracts Exhibiting Retinol-Like Bioactivity and/or Properties
The present invention comprises compounds and/or extracts having retinol-like
properties and/or benefits for use in treating dry eye including, selected
from or selected from
the group consisting of one or more of: botanical extracts, or sources of
extracts, from plants
of the genus Acronychia, Licaria, Calendula and/or Trigonella; bacterial
extracts, or sources
of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
ORi
(I)
wherein -
the dotted lines represent simple or double bound; optionally one of the
dotted line
is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; optionally a methyl (-CH3)
or
methylene (=CH2) moiety; a carbonated chain, linear, ringed, or branched,
saturated or unsaturated, comprising from 1 to 20 carbon atoms; optionally
from 1
13
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
to 10 carbon atoms; optionally 6 carbon atoms; optionally an aromatic moiety,
optionally a phenyl moiety; optionally 2-methyl -prop-1,3-diene.
Acronychia, Licaria, Calendula and/or Trigonella Botanical Plant Extract(s)
In certain embodiments, the compounds/extracts exhibiting retinol-like
properties
and/or benefits are, or comprise, extracts, or sources of extracts, of plant
Acronychia, Licaria,
Calendula and/or Trigonella. The Acronychia, Licaria, Calendula or Trigonella
extracts, or
sources of such extracts, are obtained from plants of the genus Acronychia,
Licaria,
Calendula or Trigonella.
Plants of the genus Acronychia, from which extracts useful in the present
invention
are, obtained include, for example, Acronychia aberrans, Acronychia acidula
(also referred
herein as lemon aspen), Acronychia acronychioides, Acronychia acuminate,
Acronychia
baeuerlenii, Acronychia chooreechillum, Acronychia crassipetala, Acronychia
eungellensis,
Acronychia imperforate, Acronychia laevis, Acronychia laurifolia, Acronychia
littoralis,
Acronychia oblongifolia, Acronychia octandra, Acronychia parviflora,
Acronychia
pauciflora, Acronychia pedunculata, Acronychia pub escens, Acronychia species
(Batavia
Downs), Acronychia sub erosa, Acronychia vestita, Acronychia wilcoxiana, and
combinations
of two or more thereof In one embodiment, the extract used in the present
invention dis
obtained from Acronychia acidula.
Plants of the genus Licaria, from which extracts useful in the present
invention are
obtained, include, for example, Licaria vernicosa, Licaria brittoniana,
Licaria cane/la,
Licaria cubensis, Licaria velutina and Licaria triandra, and combinations of
two or more
thereof There are about 40 species of genus Licaria reported and are endemic
to Central and
South Americas. In one embodiment, the extract used in the present invention
is obtained
from Licaria vernicosa.
There are about 15-20 species of genus Calendula reported and are found in
southwestern Asia, western Europe, Macaronesia, and the Mediterranean. Plants
of the genus
Calendula, from which extracts useful in the present invention are obtained,
include, for
example, Calendula arvensis (field marigold); Calendula maderensis (Madeiran
marigold);
and Calendula officinalis (pot marigold) and combinations of two or more
thereof In one
embodiment, the extract used in the present invention is obtained from
Calendula officinalis.
14
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Plants of the genus Trigonella include 36 known species Plants of the genus
Trigonella, from which extracts useful in the present invention are obtained,
include, for
example, Trigonella foenum-graecum, Trigonella balansae, Trigonella
corniculata,
Trigonella maritima, Trigonella spicata, Trigonella caerulea, Trigonella
occulta, Trigonella
polycerata, Trigonella Calliceras, Trigonella Cretica and combinations of two
or more
thereof Trigonella foenum-graecum or herb fenugreek is the best-known member
of the
genus Trigonella. In one embodiment, the extract used in the present invention
is obtained
from Trigonella foenum-graecum.
In certain embodiments, the extract used in the present invention is a mixture
of
extracts obtained from plants of the genus Acronychia, Licaria, Calendula
and/or Trigonella.
Extracts of Licaria vernicosa useful in the present invention can be obtained
from the
Baruch S. Blumerg Institute, Doylestown, PA (formerly known as IVHR). In
certain
embodiments, one extract comes from the woody part of the plant (E2) and the
second extract
was from the roots of the plant (E3). Within the nomenclature of IHVR
collection, the two
extracts are labeled as IHVR 40256 G10 = X-005348-001E002 and as IHVR 40256
E10 =
X-005346-001M002 respectively. The woody parts and the roots of Licaria
vernicosa (Mez)
Kosterm. can be collected from Guyana. 504.3 g of dried, ground woody plant
material can
be extracted with an ample methanol, which can be dried under vacuum to afford
10.54 g of
crude methanol extract (E2) for X-005348-001E002. 403.8 g of dried, ground
root material
can be extracted with ample methanol, which can be dried under vacuum to
afford 18.11 g of
crude methanol extract (E3) for X-005346-001M002.
In certain embodiments, the Acronychia and/or Licaria extracts useful in the
present
invention comprise compounds having the Formula II:
oR,
R3 R2 (II)
wherein:
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
RI is selected from the group consisting of CI-Cm alkyl, C2-C2o alkenyl, C2-
C2o alkynyl, and
C3-C8 cycloalkyl or aryl;
R2 is selected from the group consisting of hydrogen, hydroxyl, Ci-C6 alkyl,
C2-C6 alkenyl,
C2-C6 alkynyl, C3-C8 cycloalkyl or aryl, ¨OCI-C6 alkyl, ¨0C2-C6 alkenyl, ¨0C2-
C6 alkynyl, cycloalkyl or aryl, thiol, ¨SCI-C6alkyl, ¨SC2-C6 alkenyl, ¨SC2-
C6 alkynyl, -C8 cycloalkyl or aryl, ¨NR4C -C6 alkyl, ¨NR4C2-C6 alkenyl,
¨NR4C2-
C6 alkynyl, and ¨NR4C3-C8cycloalkyl or aryl;
R3 is selected from ¨CO2H, ¨0O2R4 or an isosteric equivalent of a carboxy
group, wherein
R4 is Ci-C6 alkyl, C2-C6 alkenyl, C3-C8 cycloalkyl or aryl; and
Y is ¨(CH2¨CH2)¨, ¨(CH=CH)¨, or
or a ophthalmologically acceptable salt thereof
In certain embodiments, the Acronychia and/or Licaria extracts useful in the
present
invention comprises compounds having the Formula II:
oRi
R3 Y R2 (II)
wherein:
RI is selected from the group consisting of C5-C16 alkyl, C5-C16 alkenyl, and
C5-Ci6 alkynyl,
more preferably C5-Ci6 alkenyl, including, for example, farnesyl;
R2 is selected from the group consisting of hydrogen, hydroxyl, ¨OCI-C6alkyl,
¨0C2-
C6 alkenyl, ¨0C2-C6 alkynyl, ¨0C3-C8 cycloalkyl, more preferably hydrogen,
hydroxyl, ¨
OC i-C6 alkyl, even more preferably hydrogen or ¨OCI-C3 alkyl;
R3 is selected from ¨CO2H, ¨0O2R4 wherein R4 is Ci-C6 alkyl, or an isosteric
equivalent of
a carboxy group; and
Y is ¨(CH2¨CH2)¨ or ¨(CH=CH)¨;
or a ophthalmologically-acceptable salt thereof
In certain embodiments, at least one of the compounds of Formula II is present
in the
extract the Acronychia and/or Licaria at a concentration equal to or greater
than 1% (or about
16
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
1%) to about 20%, or optionally from about 7% (or about 7%) to about 10% (or
about 10%),
by weight of the Acronychia and/or Licaria extract.
In certain embodiments, the compounds of Formula II useful in the present
invention
are in the form of an acid or alkylester selected from (or, selected from the
group consisting
of) 3-(4-farnesyloxypheny1)-propionic acid, 3-(4-farnesyloxy-3-hydroxypheny1)-
propionic
acid, 3-(4-farnesyloxy-3-methoxypheny1)-propionic acid, alkylesters thereof,
in particular
ethyl esters thereof, and combinations of two or more thereof
In certain embodiments, the compound of Formula II useful in the present
invention is
3-(4-farnesyloxypheny1)-propionic acid and/or its ethyl ester.
In certain embodiments, the compound of Formula II useful in the present
invention is
3-(4-farnesyloxy-3-hydroxypheny1)-propionic acid and/or its ethyl ester.
In certain embodiments, the compound of Formula II useful in the present
invention is
3-(4-farnesyloxy-3-methoxypheny1)-propionic acid and/or its ethyl ester.
Compounds and extracts derived from Acronychia species are described in US
9,220,928, which patent is herein incorporated by reference in entirety.
In certain embodiments, the 3-(4-farnesyloxypheny1)-propionic acid and/or its
ethyl
ester is present in the extract the Acronychia and/or Licaria at a
concentration equal to or
greater than 1% (or about 1%) to about 20%, or optionally from about 7% (or
about 7%) to
about 10% (or about 10%), by weight of the Acronychia and/or Licaria extract.
Any of a variety of extracts of Acronychia and/or Licaria may be used for
embodiments where the method comprises applying such an extract. The extract
may be
obtained from any part of the plant such as the fruit, the seed, the bark, the
leaf, the flower,
the roots and the wood.
In certain embodiments, the extract is obtained from the fruit of the plant.
Suitable
extracts of Acronychia or Licaria fruit, seed, bark, leaves, flower, root, and
wood may be
17
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
obtained using conventional methods including, but not limited to, direct
extraction of
material from the biomass by grinding, macerating, pressing, squeezing,
mashing,
centrifuging, and/or processes such as cold percolation,
agitation/distillation, microwave
assisted extraction, supercritical/subcritical CO2 compressed gas extraction
with or without
.. polar modifiers, pressurized solvent extraction, accelerated solvent
extraction, pressurized or
normal hot water extraction, surfactant assisted pressurized hot water
extraction, oil
extraction, membrane extraction, Soxhlet extraction, the gold finger
distillation/extraction
and/or processes disclosed, for example, in US Pat. Nos. 7442391, 7473435, and
7537791 to
Integrated Botanical Technologies, LLC, incorporated herein by reference, and
the like, or by
other methods such as solvent extraction, and the like. Any of a variety of
solvents including
polar solvents, non-polar solvents, or combinations of two or more thereof may
be used in
methods of comprising solvent extraction.
Suitable polar solvents include polar inorganic solvents such as water and the
like,
polar organic solvents such as alcohols and corresponding organic acids, for
example CI-Cs
alcohols including methanol, ethanol, propanol, butanol, and the like and
organic acids,
including acetic acid, formic acid, propanoic acid, and the like, polyols and
glycols, including
Ci-C8polyols/glycols and the like, and combinations of two or more thereof
Suitable non-
polar solvents include non-polar organic solvents such as alkanes, including
CI-Cs alkanes,
cycloalkanes, including CI-Cs alkanes, alkyl ethers, including CI-Cs alkyl
ethers, Petroleum
ethers, ketones, including CI-Cs ketones, methylene chloride, ethyl acetate,
xylene, toluene,
chloroform, vegetable oil, mineral oil and the like. In another embodiment
extraction may be
obtained by non-polar solvents described above or supercritical fluid
extraction with or
without a polar modifier such as C i-C8 alcohols, water, Ci-C8polyols/glycols
or Ci-C8
organic acids.
In one embodiment, the extract comprises an extract of Acronychia acidula. In
another embodiment, the extract of the invention comprises a combination of
polar and non-
polar extracts of from Acronychia acidula fruit. In another embodiment, the
extract of the
invention comprises alcoholic or glycolic extracts of Acronychia acidula
fruit.
In one embodiment, the extract comprises an extract of Licaria vernicosa. In
another
embodiment, the extract of the invention comprises a combination of polar and
non-polar
extracts of from Licaria vernicosa wood or Licaria vernicosa root. In another
embodiment,
the extract of the invention comprises alcoholic extracts of Licaria vernicosa
wood or Licaria
vernicosa root.
18
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
In another embodiment, the extract of the invention comprises a polar extract
prepared by extracting from fruit of Acronychia acidula, wood of Licaria
vernicosa, or root
of Licaria vernicosa using a polar solvent comprising water, Ci-Cs alcohols,
C1-C8 polyols,
C1-C8 glycols, and combinations of two or more thereof. In certain
embodiments, the extract
is extracted using one or more C1-C4 alcohols, C1-C4 polyols, and/or Ci-C4
glycols. In certain
embodiments, the extract is prepared using a solvent comprising methanol,
ethanol, or a
combination thereof with or without presence of water. In another embodiment,
the extract is
a polar extract extracted from Acronychia acidula fruit using a combination of
alcohol and
water. In yet another embodiment, the extract is a polar extract extracted
from the ground
.. wood of Licaria vernicosa, or ground root of Licaria vernicosa using
methanol.
In yet another embodiment, the extract comprises a non-polar extract prepared
by
extracting from Acronychia acidula fruit, Licaria vernicosa wood, or Licaria
vernicosa root
using a non-polar solvent comprising one or more Ci-Cs alkanes, Ci-Cs
cycloalkanes, Ci-Cs
alkyl ethers, C1-C8 alkyl esters and/or chloroform, more preferably one or
more C1-C8
alkanes, Ci-Cs alkyl esters and/or chloroform. In yet another embodiment, the
non-polar
extract is extracted from Acronychia acidula fruit, Licaria vernicosa wood, or
Licaria
vernicosa root using hexanes, ethyl acetate, chloroform or mixtures of two or
more thereof
In yet another embodiment, the non-polar extract is extracted from Acronychia
acidula fruit
using ethyl acetate.
In one embodiment, the extract comprises an extract of Calendula officinalis .
In
another embodiment, the extract of the invention comprises a combination of
polar and non-
polar extracts of Calendula officinalis petals. In another embodiment, the
extract of the
invention comprises a non-polar extract of Calendula officinalis petals.
In one embodiment, the extract comprises an extract of Trigonella foenum-
graecum.
In another embodiment, the extract of the invention comprises a combination of
polar and
non-polar extracts of Trigonella foenum-graecum leaves. In another embodiment,
the extract
of the invention comprises a non-polar extract of Tr/gone/la foenum-graecum
leaves.
In yet another embodiment, the Calendula and/or Tr/gone/la extract is a non-
polar
extract prepared using a non-polar solvent comprising one or more Ci-Cs
alkanes, Ci-Cs
cycloalkanes, Ci-Cs alkyl ethers, Ci-Cs alkyl esters and/or chloroform, more
preferably one
or more Ci-Cs alkanes, Ci-Cs alkyl esters and/or chloroform.
19
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
In yet another embodiment, Calendula and/or Trigonella extract is a non-polar
extract
prepared using hexanes, ethyl acetate, chloroform, or mixtures of two or more
thereof In yet
another embodiment, the extract is a non-polar extract prepared using ethyl
acetate.
In one embodiment, the botanical extracts may be obtained via extraction of
cell
cultures of various plants, including cell cultures of the genera Acronychia,
Licaria,
Calendula, or Trigonella. The cell cultures which are extracted to obtain
botanical extracts
for use in the invention may be of any form including suspension cell cultures
and the like.
Extracts of Calendula officinalis and of Trigonella foenum-graecum can be
obtained
from Caithness Biotechnologies Ltd, UK
(http://www.caithnessbiotechnologies.com/). These
extracts are part of The Phytotitre Natural Product Library available to
everyone for purchase.
Alternatively, the extracts can be obtained using a preparation method
described by Caithness
Biotechnologies Ltd. as non-polar and prepared with a mixture of methanol and
methylene
chloride. For detailed description please consult webpage
http://caithnessbiotechnologies.com/contact.html. In a typical extraction, a
preweighed dried
powdered biomass is suspended and stirred in a mixture of methanol/methylene
chloride (1:1)
over night at ambient temperature. The suspension is then filtered, filtrate
is dried under
reduced pressure to a residue free of solvents.
In certain embodiments, the extract of the Acronychia, Licaria, Calendula,
and/or
Trigonella is present in the compositions of the present invention in an
amount of from about
0.001% to about 10%, optionally, from about 0.001% to about 5%, or,
optionally, from about
0.01% to about1%, by weight of the composition.
.. Bacterial Extract(s) of the genus Actinomyces exhibiting Retinol-like
Properties and/or
Benefits
In certain embodiments, the compounds/extracts exhibiting retinol-like
properties
and/or benefits are, or comprise bacterial extract(s) of the genus
Actinomyces. Bacteria of the
genus Actinomyces include many species fully characterized, as well as some of
them which
are not well characterized, for example one species collected from USA and
labeled as
species A5640. A sample of this bacteria is collected, grown in culture and
made into an
extract. The extract is a part of natural products collection now in control
of the Baruch S.
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Blumerg Institute, Doylestown, PA (formerly known as the Institute of
Hepatitis Virus
Research Labs (IHVR)). Within the nomenclature of IHVR collection, the extract
is labeled
as IHVR 39565 F7.
In one embodiment, the extract used in the present invention is obtained from
Actinomyces species with a capacity to produce similar chemical composition as
is produced
by extract A5640. In another embodiment, the bacteria are collected in the USA
and the
strain is identical to previously assigned species A5640.
In certain embodiments, the extract of Actinomyces is present in the
composition of
the present invention in an amount of from 0.001% to 10%, optionally, from
0.001% to 5%,
or, optionally, from 0.01% to 1%, by weight of the composition.
Compound(s) of Formula (I) Exhibiting Retinol-like Bioactivity and/or
Properties.
In certain embodiments, the compounds/extracts exhibiting retinol-like
properties
and/or benefits are, or comprise the compounds of Formula (I).
R2 0
ORi
(I)
wherein -
the dotted lines represent simple or double bound; optionally one of the
dotted line
is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; optionally a methyl (-CH3)
or
methylene (=CH2) moiety; a carbonated chain, linear, ringed, or branched,
saturated or unsaturated, comprising from 1 to 20 carbon atoms; optionally
from 1
to 10 carbon atoms; optionally 6 carbon atoms; optionally an aromatic moiety,
optionally a phenyl moiety; preferably 2-methyl -prop-1,3-diene.
21
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
In certain embodiments, the compounds of Formula (I) include the corresponding
salts of such metal ions as, but not limited to, Li, Na, I( , Ca2 , or Mg2 .
In certain embodiments, the compounds of Formula I are:
0
OH
(1)
(2E,4E,6E)-7-(1,1,2,2,3,3-hexamethy1-2,3-dihydro-1H-inden-5-y1)-3-methylocta-
2,4,6-
trienoic acid
or
OH
(2) 0
4-(1-(1,1,2,2,3,3-hexamethy1-2,3-dihydro-1H-inden-5-yl)vinyl)benzoic acid and,
in each
case, their derivatives that display retinoid-like activity. These compounds
are referred
referenced in Examples 6 and 7 below as Compound 1 and Compound 2,
respectively.
The present invention is directed to compounds of Formula I such as (2E,4E,6E)-
7-
(1,1,2,2,3,3-hexamethy1-2,3-dihydro-1H-inden-5-y1)-3-methylocta-2,4,6-trienoic
acid and 4-
(1-(1,1,2,2,3,3-hexamethy1-2,3-dihydro-1H-inden-5-yl)vinyl)benzoic acid and
their
derivatives that display retinoid-like activity and mixtures thereof
Mixtures of any of the above the compounds/extracts/extract sources exhibiting
retinol-like properties and/or benefits may also be used.
Compounds and extracts of Formula I are described in US Patent Publication
2019/0091122, which patent is herein incorporated by reference in entirety.
In certain embodiments, the compound(s) of Formula I is present in the
composition
of the present invention in an amount of from about 0.0001% to about 20%,
optionally, from
about 0.001% to about 10%, optionally, from about 0.01% to about 5%, or
optionally from
22
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
about 0.2 to about 2%, by weight of the composition. In yet another
embodiment, the
compound(s) of Formula I is present in the composition of the present
invention in an amount
of from about 0.0001 to about 1%, optionally from about 0.001 to about 1%, or
optionally
from about 0.01 to about 1%, by weight of the composition.
Compositions The present inventors have discovered that compounds and/or
extracts
having retinol-like properties and/or benefits can promote and/or improve
delivery/excretion
of mucin from corneal epithelial cells.
Permeation Enhancer
In certain embodiments, the compositions of the present invention optionally
comprise a permeation enhancer.
Suitable permeation enhancers include (selected from or selected from the
group
consisting of) either alone or in combination, surfactants such as saponins,
polyoxyethylene,
polyoxyethylene ethers of fatty acids such as polyoxyethylene 4-, 9-, 10-, and
23-lauryl ether,
polyoxyethylene 10-and 20-cetyl ether, polyoxyethylene 10-and 20-stearyl
ether, sorbitan
monooleate, sorbitan monolaurate, polyoxyethylene monolaurate, polyoxyethylene
sorbitans
such as polyoxyethylene sorbitan monolaurate, decamethonium, decamethonium
bromide,
and dodecyltrimethylammonium bromide; chelators such natural polyacids (e.g.,
citric acid),
phosphate salts (e.g., disodium pyrophosphate), phosphonates, bisphosphonates
(e.g.,
etridronic acid), aminocarboxylic acids (e.g., ethylenediaminetetraacetic acid
(EDTA) and
disodium EDTA) and ethylenediamine-N,N-disuccinic acid (EDDS)); bile salts and
acids
such as cholic acid, deoxycholic acid, glycocholic acid, glycodeoxycholic
acid, taurocholic
acid, taurodeoxycholic acid, sodium cholate, sodium glycocholate,
glycocholate, sodium
deoxycholate, sodium taurocholate, sodium glycodeoxycholate, sodium
taurodeoxycholate,
chenodeoxycholic acid, and urosdeoxycholic acid; fusidic acid derivatives,
glycyrrhizic acid,
and ammonium glycyrrhizide, with saponin EDTA, fusidic acid, polyoxyethylene 9-
lauryl
ether, polyoxyethylene 20-stearylether, glycocholate, or mixtures of any of
the above.
The concentration of permeation enhancer administered should be the minimum
amount needed to sufficiently increase absorption of the compound and/or
extract through the
mucous or other barrier membranes of the eye. Generally, concentrations
ranging from
0.01% (or about 0.01%), optionally, from 0.05% (or about 0.05%), optionally,
from 0.1% (or
about 0.1%), optionally, from 0.15% (or about 0.15%), optionally, from 0.2%
(or about
23
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
0.2%), optionally, from 0.25% (or about 0.25%) to 2% (or about 2%),
optionally, to 2.5% (or
about 2.5%), optionally, to 3% (or about 3%), optionally, to 3.5%, (or about
3.5%),
optionally, to 4% (or about 4%), optionally, to 4.5% (or about 4.5%),
optionally, to 5% (or
about 5%), optionally, to 5.5% (or about 5.5%), optionally, to 6% (or about
6%), optionally,
to 6.5% (or about 6.5%), optionally, to 7% (or about 7%), optionally, to 7.5%
(or about
7.5%), optionally, to 8% (or about 8%), optionally, to 8.5% (or about 8.5%),
optionally, to
9% (or about 9%), optionally, to 9.5% (or about 9.5%), optionally, to 10% (or
about 10%),
optionally, to 10.5% (or about 10.5%), optionally, to 11% (or about 11%),
optionally, to
11.5% (or about 11.5%), optionally, to 12% (or about 12%), optionally, to
12.5% (or about
12.5%), optionally, to 13% (or about 13%), optionally, to 13.5% (or about
13.5%),
optionally, to 14% (or about 14%), optionally, to 14.5% (or about 14.5%),
optionally, to 15%
(or about 15%), optionally, to 15.5% (or about 15.5%), optionally, to 16% (or
about 16%),
optionally, to 16.5% (or about 16.5%), optionally, to 17% (or about 17%),
optionally, to
17.5% (or about 17.5%), optionally, to 18% (or about 18%), optionally, to
18.5% (or about
.. 18.5%), optionally, to 19% (or about 19%), optionally, to 19.5% (or about
19.5%),
optionally, to 20% (or about 20%), of the total composition (w/v), are useful
in the
compositions of the present invention.
Ophthalmologically Acceptable Carrier
The compositions of the present invention also comprise an aqueous, oil-in-
water
emulsion, or water-in-oil emulsion carrier. The carrier is ophthalmologically
acceptable.
Useful oil-in-water and water-oil-carriers can be found in US Patent
Publication
20030165545A1 and US Patents 9480645, 8828412 and 8496976, each of which
patent
.. documents are herein incorporated by reference in its entirety.
The ophthalmologically acceptable carrier (or, compositions of the present
invention)
may optionally comprise one or more additional excipients and/or one or more
additional
active ingredients. Examples of such optional components are described below.
Excipients commonly used in ophthalmic compositions include, but are not
limited to,
demulcents, tonicity agents, preservatives, chelating agents, buffering agents
(other than and
in addition to the organic acids of the present invention), and surfactants.
Other excipients
comprise solubilizing agents, stabilizing agents, comfort-enhancing agents,
polymers,
24
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
emollients, pH-adjusting agents (other than and in addition to the organic
acids of the present
invention), and/or lubricants. Any of a variety of excipients may be used in
the compositions
of the present invention including water, mixtures of water and water-miscible
solvents, such
as vegetable oils or mineral oils comprising from 0.5% to 5% non-toxic water-
soluble
polymers, natural products, such as agar and acacia, starch derivatives, such
as starch acetate
and hydroxypropyl starch, and also other synthetic products such as polyvinyl
alcohol,
polyvinylpyrrolidone, polyvinyl methyl ether, polyethylene oxide, and
preferably cross-
linked polyacrylic acid and mixtures thereof
Demulcents or soothing agents used with embodiments of the present invention
include, but are not limited to, cellulose derivatives (such hydroxyethyl
cellulose, methyl
cellulose, hypromellose or mixtures thereof), hyaluronic acid, tamarind seed
extract, glycerin,
polyvinyl pyrrolidone, polyethylene oxide, polyethylene glycol, propylene
glycol and
polyacrylic acid and mixtures thereof. In certain embodiments, one or more of
hyaluronic
acid, propylene glycol, tamarind seed extract, glycerin and/or polyethylene
glycol 400 are the
demulcents or soothing agents. In certain embodiments, the demulcent or
soothing agent is
selected from hyaluronic acid, tamarind seed extract or mixtures thereof
Compositions of the present invention are ophthalmologically suitable for
application
to a subject's eyes. The term "aqueous" typically denotes an aqueous
formulation wherein
the excipient is > about 50%, more preferably > about 75% and in particular >
about 90% by
weight water. In certain embodiments, the compositions of the present
invention are
essentially free of compounds which irritate the eye. In certain embodiments,
the
compositions of the present invention are essentially free of free fatty acids
and CI to C4
alcohols. In certain embodiments, the compositions of the present invention
are comprise
less than 40% (or about 40%), optionally, less than 35% (or about 35%),
optionally, less than
30% (or about 30%), optionally less than 25% (or about 25%), optionally, less
than 20% (or
about 20%), optionally, less than 15% (or about 15%), optionally less than 10%
(or about
10%), or optionally, less than 5% (or about 5%), by weight of the total
composition, of a non-
alcohol, organic excipient or solvent. These drops may be delivered from a
single dose
ampoule which may preferably be sterile and thus render bacteriostatic
components of the
formulation unnecessary. Alternatively, the drops may be delivered from a
multi-dose bottle
which may preferably comprise a device which extracts any preservative from
the
composition as it is delivered, such devices being known in the art.
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
In certain embodiments, the compositions of the present invention are
isotonic, or
slightly hypotonic in order to combat any hypertonicity of tears caused by
evaporation and/or
disease. This may require a tonicity agent to bring the osmolality of the
formulation to a
level at or near 210-320 milliosmoles per kilogram (mOsm/kg). The compositions
of the
.. present invention generally have an osmolality in the range of 220-320
mOsm/kg, or,
optionally, have an osmolality in the range of 235-300 mOsm/kg. The ophthalmic
compositions will generally be formulated as sterile aqueous solutions.
The osmolality of the compositions of the present invention may be adjusted
with
tonicity agents to a value which is compatible with the intended use of the
compositions. For
example, the osmolality of the composition may be adjusted to approximate the
osmotic
pressure of normal tear fluid, which is equivalent to about 0.9 w/v % of
sodium chloride in
water. Examples of suitable tonicity adjusting agents include, without
limitation, sodium,
potassium, calcium and magnesium chloride; dextrose; glycerin; propylene
glycol; mannitol;
sorbitol and the like and mixtures thereof In one embodiment, a combination of
sodium
chloride and potassium chloride are used to adjust the tonicity of the
composition.
The compositions of the present invention can also be used to administer
pharmaceutically active compounds. Such compounds include, but are not limited
to,
glaucoma therapeutics, pain relievers, anti-inflammatory and anti-allergy
medications, and
anti-microbials. More specific examples of pharmaceutically active compounds
include
betaxolol, timolol, pilocarpine, carbonic anhydrase inhibitors and
prostglandins;
dopaminergic antagonists; post-surgical antihypertensive agents, such as para-
amino
clonidine (apraclonidine); anti-infectives such as ciprofloxacin,
moxifloxacin, and
tobramycin; non-steroidal and steroidal anti-inflammatories, such as naproxen,
diclofenac,
nepafenac, suprofen, ketorolac, tetrahydrocortisol and dexamethasone; dry eye
therapeutics
such as PDE4 inhibitors; and anti-allergy medications such as H1/H4
inhibitors, H4
inhibitors, olopatadine or mixtures thereof
It is also contemplated that the concentrations of the ingredients comprising
the
formulations of the present invention can vary. A person of ordinary skill in
the art would
understand that the concentrations can vary depending on the addition,
substitution, and/or
subtraction of ingredients in a given formulation.
26
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
In certain embodiments, the compositions of the present invention may have a
pH
which is compatible with the intended use, and is often in the range of 4 (or
about 4) to 10 (or
about 10), optionally between 6 (or about 6) to 8 (to about 8), optionally
between 6.5 (or
about 6.5) to 7.5 (or about 7.5), or optionally between 6.8 (or about 6.8) to
7.2 (or about 7.2).
In certain embodiments, a variety of conventional buffers may be employed,
such as
phosphate, borate, citrate, acetate, histidine, tris, bis-tris and the like
and mixtures thereof
Borate buffers include boric acid and its salts, such as sodium or potassium
borate.
Potassium tetraborate or potassium metaborate, which produce boric acid or a
salt of boric
acid in solution, may also be employed. Hydrated salts such as sodium borate
decahydrate
can also be used. Phosphate buffers include phosphoric acid and its salts; for
example,
M2HPO4and MH2PO4, wherein M is an alkali metal such as sodium and potassium.
Hydrated salts can also be used. In one embodiment of the present invention,
Na2HPO4.7H20 and NaH2P02.H20 are used as buffers. The term phosphate also
includes
compounds that produce phosphoric acid or a salt of phosphoric acid in
solution.
Additionally, organic counter-ions for the above buffers may also be employed.
The
concentration of buffer generally varies from about 0.01 to 2.5 w/v % and more
preferably
varies from about 0.05 to about 0.5 w/v %.
In certain embodiments, the viscosity of the compositions of the present
invention
range from about 1 to about 500 cps, optionally from about 10 to about 200
cps, or optionally
from about 10 to about 100 cps, when measured using a TA Instrument AR 2000
rheometer.
The TA Instrument AR 2000 rheometer should be used with the AR2000 flow test
method of
the TA Rheological Advantage software with a 40 mm steel plate geometry; the
viscosity
ranges should be obtained by measuring steady state flow controlling shear
rate from 0 sec'
to 200 sec 1.
In certain embodiments, the compositions of the present invention are useful
as, and
in the form of, eye-drop solution, eye wash solution, contact lens lubricating
and/or rewetting
solution, spray, mist or any other manner of administering a composition to
the eye.
The compositions of the present invention may also be useful as, and in the
form of,
packing solutions for contact lenses. In certain embodiments, as packing
solutions, the
compositions of the present invention may be sealed in blister packaging and,
also, suitable
for undergoing sterilization.
27
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Examples of blister packages and sterilization techniques are disclosed in the
following references which are hereby incorporated by reference in their
entirety, U.S. Pat.
Nos. D435,966; 4,691,820; 5,467,868; 5,704,468; 5,823,327; 6,050,398,
5,696,686;
6,018,931; 5,577,367; and 5,488,815. This portion of the manufacturing process
presents
another method of treating the ophthalmic devices with anti-allergic agent,
namely adding
anti-allergic agents to a solution prior to sealing the package, and
subsequently sterilizing the
package. This is the preferred method of treating ophthalmic devices with anti-
allergic
agents.
Sterilization can take place at different temperatures and periods of time.
The
preferred sterilization conditions range from about 100 C for about 8 hours to
about 150 C
for about 0.5 minute. More preferred sterilization conditions range from about
115 C for
about 2.5 hours to about 130 C for about 5.0 minutes. The most preferred
sterilization
conditions are about 124 C for about 18 minutes.
When used as packing solutions, the compositions of the present invention may
be
water-based solutions. Typical packing solutions include, without limitation,
saline solutions,
other buffered solutions, and deionized water. In certain embodiments, the
packing solution
is an aqueous solution of deioinized water or saline solution containing salts
including,
without limitation, sodium chloride, sodium borate, sodium phosphate, sodium
hydrogenphosphate, sodium dihydrogenphosphate, or the corresponding potassium
salts of
the same. These ingredients are generally combined to form buffered solutions
that include
an acid and its conjugate base, so that addition of acids and bases cause only
a relatively
small change in pH. In certain embodiments, the pH of the packing solution is
as described
above. The buffered solutions may additionally include 2-(N-
morpholino)ethanesulfonic acid
(MES), sodium hydroxide, 2,2-bis(hydroxymethyl)-2,2',2"-nitrilotriethanol,
n-tris(hydroxymethyOmethy1-2-aminoethanesulfonic acid, citric acid, sodium
citrate, sodium
carbonate, sodium bicarbonate, acetic acid, sodium acetate, ethylenediamine
tetraacetic acid
and the like and combinations thereof Preferably, the solution is a borate
buffered or
phosphate buffered saline solution or deionized water. The particularly
preferred solution
contains about 500 ppm to about 18,500 ppm sodium borate, most particularly
preferred
about 1000 ppm of sodium borate.
If any ingredients incorporated into the packing solutions are subject to
oxidative
degradation, agents that stabilize packing solutions containing such
ingredients may be
28
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
added. Such "oxidative stabilization agents" include but are not limited to
chelants such as
EDTA, Dequest, Desferal, silica, chitin derivatives such as chitosan,
cellulose and its
derivatives, and N,N,N',N',N", N"-hexa(2-pyridy1)-1,3,5-
tris(aminomethyl)benzene, and
certain macrocyclic ligands such as crown ethers, ligand containing knots and
catenands.
See, David A. Leigh et al Angew. Chem Int. Ed., 2001, 40, No. 8, pgs. 1538-
1542 and Jean-
Claude Chambron et al. Pure & Appl. Chem., 1990, Vol. 62, No. 6, pgs. 1027-
1034.
Oxidative stabilization agents may include other compounds that inhibit
oxidations such as
those selected from the group consisting of 2,2',2",6,6',6"-Hexa-(1,1-
dimethylethy1)4,4',4"-
[(2,4,6-trimethy1-1,3,5-benzenetriy1)-trismethylenel-triphenol (Irganox 1330),
1,3,5tris[3,5-
di(1,1-dimethylethy1)4-hydroxybenzyll-1H,3H,5H-1,3,5-triazine-2,4,6-trione,
pentaerythrityl
tetrakis[343,5-di(1,1-dimethylethyl)-4-hydroxyphenyll -propionate], octadecy1-
3-[3,5-di(1,1-
dimethylethyl)-4-hydroxyphenyll-propionate, tris[2,4-di(1,1-dimethylethyl)-
phenyll-
phosphite, 2,2'-di(octalecyloxy)-5,5'-spirobi(1,3,2-dioxaphosphorinane),
dioctadecyl
disulphide, didodecy1-3,3'-thiodipropionate, dioctadecy1-3,3'-
thiodipropionate,
butylhydroxytoluene, ethylene bis[3,3-di[3-(1,1-dimethylethyl)-4-
hydroxyphenyllbutyratel
and mixtures thereof The preferred oxidative stabilization agents are
diethylenetriaminepentaacetic acid ("DTPA"), or salts of DTPA such as
CaNa3DTPA,
ZnNa3DTPA, and Ca2DTPA. See, U.S. App. Pat. No. 60/783,557 filed on, March 17,
2006,
entitled "Methods for Stabilizing Oxidatively Unstable Pharmaceutical
Compositions" and its
corresponding non-provisional filing which are hereby incorporated by
reference in their
entirety. In certain embodiments, the concentration of oxidative stabilization
agents in the
solution be from about 2.5 umoles/liter to about, 5000 umoles/liter,
optionally, from about
20 umoles/liter to about 1000 umoles/liter, optionally from about 100
umoles/liter to about
1000 umoles/liter, or optionally from about 100 umoles/liter to about 500
umoles/liter.
In particular embodiments, the compositions of the present invention are
formulated
for administration at any frequency of administration, including once a week,
once every five
days, once every three days, once every two days, twice a day, three times a
day, four times a
day, five times a day, six times a day, eight times a day, every hour, or
greater frequency.
Such dosing frequency is also maintained for a varying duration of time
depending on the
therapeutic needs of the user. The duration of a particular therapeutic
regimen may vary from
one-time dosing to a regimen that extends for months or years. One of ordinary
skill in the
art would be familiar with determining a therapeutic regimen for a specific
indication.
29
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
The composition and products containing such compositions of this invention
may be
prepared using methodology that is well known by an artisan of ordinary skill.
EXAMPLES
Any compositions of the present invention as described in following examples
illustrate specific embodiments of compositions of the present invention, but
are not intended
to be limiting thereof. Other modifications can be undertaken by the skilled
artisan without
departing from the spirit and scope of this invention.
The following test methods were used in the Examples:
Example 1
Compositions of Lemon aspen extract showed an increase of MUC1, MUC4 and
MUC16 gene expressions in human epicorneal 3D tissues when treated in the
medium.
EpiCorneal 3D human tissues were purchased from MatTek Company (Ashland, MA,
USA). Upon receiving, epicorneal 3D human tissues, they were incubated in
MatTek assay
medium overnight following the manufacturer's instruction. The epicorneal 3D
human
tissues were divided into three treatment groups with at least three tissues
per group. Lemon
aspen extract were added, respectively, into the culture medium containing the
human
epicorneal tissues of two of the treatment groups to produce media
concentrations of 0.001%
or 0.01% (w/v), respectively. The epicorneal tissues in all four treatment
groups were
allowed to incubate for two days. The extract of lemon aspen used in the and
extracted and
supplied by Southern Cross Botanicals (Knockrow Nsw, Australia. After the two
days'
incubation, gene expressions of mucin 1 (MUC1), mucin 4 (MUC4) and mucin 16
(MUC16)
were analyzed. After two days' incubation, the human epicorneal 3D tissues
were cut in
halves and half of the tissues were lysed in 350 ut lysis buffer, consisting
of 100 parts RLT
buffer (RNeasy Mini kit, Qiagen, Valencia, CA), to one part 2-mercaptoethanol.
RNA was
extracted from the solutions using the RNeasy Mini Kit (Qiagen, Valencia, CA)
according to
manufacturer's instructions and RNA was eluted in 25 iL RNase-free water.
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Reverse transcription (RT) was performed using the Applied Biosystems High
Capacity
Reverse Transcription Kit (ThermoFisher Scientific, Bridgewater, NJ). Gene
expression
assays sold under the tradename TAQMAN for mucin-1 (MUC1), mucin-4 (MUC4) and
mucin-16 (MUC16) polymerase (RNA) II polypeptide A (POLR2A), and Master Mix
were
purchased from ThermoFisher Scientific (Bridgewater, NJ). qPCR analysis was
performed
using TaqMan Master Mix (ThermoFisher Scientific, Bridgewater, NJ), and run
on a real
time PCR system sold under the tradename QUANTSTUDIO 7 Flex System
(ThermoFisher
Scientific, Bridgewater, NJ). The expression of the MUC1, MUC4 and MUC16 genes
were
normalized against the expression of the human POLR2A housekeeping gene. The
fold
changes were calculated in comparison to the untreated control (UT) and two-
tailed two-
sample Student t-tests (Microsoft Office Excel 2007; Microsoft, Redmond, WA,
USA) were
performed. Results are shown in Figure 1.
31
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Example 2
Compositions of Lemon aspen extract showed an increase of mucin-1 secretion in
human epicorneal 3D tissues when treated in the medium.
EpiCorneal 3D human tissues were purchased from MatTek Company (Ashland, MA,
USA). Upon receiving, epicorneal 3D human tissues, they were incubated in
MatTek assay
medium overnight following the manufacturer's instruction. The epicorneal 3D
human
tissues were divided into three treatment groups with at least three tissues
per group. Lemon
aspen extract were added, respectively, into the culture medium containing the
human
epicorneal tissues of two of the treatment groups to produce media
concentrations of 0.001%
or 0.01% (w/v), respectively. The epicorneal tissues in all four treatment
groups were
allowed to incubate for two days. After the two days, culture media were
collected for
measuring mucin 1 secretion using human mucin-1 (CA15-3) enzyme-linked
immunosorbent
assay (ELISA) kit (EHMUC1, ThermoFisher Scientific, Bridgewater, NJ) following
the
manufacturer's protocol. To assess activity, the colorimetric change was
measured using a
microplate reader (SpectraMax M2E, Molecular Devices, Sunnyvale, CA, USA).
This assay
employs the standard enzyme-linked immunoassay technique, so there is a linear
correlation
between Mucin-1 concentration in the sample and the colorimetric change. A
standard curve
was generated, with the Mucin-1 concentration on the x-axis and absorbance on
the y-axis to
indicate corresponding Mucin-1 concentration. Results are shown below in
Figure 2.
Example 3
Solutions can be prepared containing one or more compounds and/or extract
having
retinol-like properties and/or benefits of the present invention as shown in
Examples 3-7.
Table 1 illustrates the components of such formulations (as illustrated in
formulations
3A-3D), which components can be incorporated as described below using
conventional
mixing technology.
32
CA 03107726 2021-01-26
WO 2020/021480 PCT/IB2019/056344
Table 1
3A 3B 3C 3D
Useful for Relief of Useful for Relief of Useful for Relief of Useful for
Relief of
Dry Eye Irritation Dry Eye Irritation Dry Eye Irritation Dry Eye
Irritation
for Contact Lenses for Contact Lenses
INGREDIENT /0w/w amount /0w/w amoun /0w/w amount /0w/w amount
per t per per
per
batch batch batch
batch
(gms) (gms) (gms)
(gms)
Sodium 0.20 2.0 0.30 3.0 0.15 1.5 0.15
1.5
Hyaluronate
Lemon Aspen 0.10 1.0 0.01 0.10 0.10 1.0 0.01
0.10
Extract
Polysorbate 80 1.0 10.0 0.2 2.0 1.0 10.0 0.2
2.0
Polysorbate 20 5.0 50.0 1.0 10.0 2.0 20.0 1.0
10.0
Polyethylene 0.25 2.5 0.25 2.5 0 0 0
0
Glycol 400
Boric Acid 0.60 6.0 0.60 6.0 0.60 6.0 0.60
6.0
Sodium Borate 0.05 0.50 0.05 0.50 0.05 0.50 0.05
0.50
Sodium
Chloride*
Potassium 0.10 1.0 0.10 1.0 0.10 1.0 0.10
1.0
Chloride
33
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Calcium 0.006 0.06 0.006 0.06 0.006 0.06 0.006
0.06
Chloride
Dihydrate
Magnesium 0.006 0.06 0.006 0.06 0.006 0.06 0.006
0.06
Chloride
Sodium Chlorite 0.014 0.14 0.014 0.14 0.014 0.14 0.014
0.14
Dihydrate
Polyquaternium 0.0015 0.015 0.0015 0.015 0.0015 0.015
0.0015 0.015
42
(33%aqueous)
Sodium Chlorite 0.014 0.14 0.014 0.14 0.014 0.14 0.014
0.14
Dihydrate
1N Sodium
Hydroxide
solution**
1N Hydrochloric
Acid solution**
Purified
Water***
total 100.00 1000.0 g 100.00 % 1000.0 100.00 % 1000.00 100.00 %
1000.00
cyo
* can be adjusted to tonicity of 280-290 mOsm/Kg
** can adjust to pH 7.2
*** optionally, q.s to 100%w/w
For Examples 3A-3D: The Sodium Hyaluronate can be supplied by CONTIPRO A.S.
(DOLNI, DOBROUC, CZECH REPUBLIC)
For Examples 3A-3D: The Lemon Aspen Extract (AbacrossTM Acronychia acidula
fruit
extract) can be supplied by SOUTHERN CROSS BOTANICALS (KNOCKROW NSW,
AUSTRALIA).
34
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
For Examples 3A-3D: The Polysorbate 20 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 3A-3D: The Polysorbate 80 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 5A-5B: The Polyethylene Glycol 400 can be supplied by Clariant
Produkte
(BURGKIRCHEN, GERMANY).
For Examples 3A-3D: The Boric Acid can be supplied by Merck KGaA (DARMSTADT,
GERMANY).
For Examples 3A-3D: The Sodium Borate can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 3A-3D: The Sodium Chloride can be supplied by Caldic (DUSSELDORF,
GERMANY).
For Examples 3A-3D: The Potassium Chloride can be supplied by Merck KGaA
(DARMSTADT, GERMANY).
For Examples 3A-3D: The Calcium Chloride Dihydrate can be supplied by Merck
KGaA
(DARMSTADT, GERMANY).
For Examples 3A-3D: The Magnesium Chloride can be supplied by KGaA (DARMSTADT,
GERMANY).
For Examples 3A-3D: The Polyquaternium-42 (33% aqueous) can be supplied by DSM
BIOMEDICAL (BERKELEY, CA, USA).
For Examples 3A-3D: The Sodium Chlorite Dihydrate can be supplied by Oxychem
(WICHITA, KS, USA)
For Examples 3A-3D: The 1N Sodium Hydroxide can be supplied by VWR (RADNER,
PA,
USA).
For Examples 3A-3D: The 1N Hydrochloric acid can be supplied by VWR (RADNER,
PA,
USA).
Solution 3A can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 10 g of Polysorbate 80 and 50 g of Polysorbate 20.
The solution
is mixed until both are fully mixed and dissolved.
3. To the above is added 1.0 g of Lemon Aspen Extract. The solution is
mixed until the
Lemon Aspen Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
5. To the solution of Step 4 is added 2.0 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
6. The following ingredients are next added sequentially, allowing for each
to dissolve
before adding the next: 2.5 grams Polyethylene Glycol 400, 6.0 grams Boric
acid, 0.05gram
Sodium Borate, 1.0 gram Potassium Chloride, 0.06 gram Calcium Chloride
Dihydrate, 0.06
gram Magnesium Chloride, and 0.0015 grams Polyquaternium-42 (aqueous).
7. While continuing to mix, 0.14gram Sodium Chlorite Dihydrate is added and
mixed to
dissolve.
8. The tonicity of the formula is determined and adjusted to 280 mOsm/Kg
with Sodium
Chloride.
9. The pH of the formula is adjusted to pH of 7.2 using the 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
10. The solution is brought to 1000.0 grams using Purified Water USP and
mixed for 10
minutes to be fully uniform.
11. The solution is filtered using a 0.22 micron filter.
Solution 3B can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 2 g of Polysorbate 80 and 10 g of Polysorbate 20.
The solution
is mixed until both are fully mixed and dissolved.
3. To the above is added 0.1 g of Lemon Aspen Extract. The solution is
mixed until the
Lemon Aspen Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the solution of Step 4 is added 3.0 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
36
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
6. The following ingredients are next added sequentially, allowing for
each to dissolve
before adding the next: 2.5 grams Polyethylene Glycol 400, 6.0 grams Boric
acid, 0.05 gram
Sodium Borate, 1.0 gram Potassium Chloride, 0.06 gram Calcium Chloride
Dihydrate, 0.06
gram Magnesium Chloride, and 0.0015 grams Polyquaternium-42 (aqueous).
7. While continuing to mix, 0.14 gram Sodium Chlorite Dihydrate is added
and mixed to
dissolve.
8. The tonicity of the formula is determined and adjusted to 280 mOsm/Kg
with Sodium
Chloride.
9. The pH of the formula is adjusted to pH of 7.2 using the 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
10. The solution is brought to 1000.0 grams using Purified Water USP and
mixed for 10
minutes to be fully uniform.
11. The solution is filtered using a 0.22 micron filter.
Solution 3C can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 10 g of Polysorbate 80 and 20 g of Polysorbate 20.
The solution
is mixed until both are fully mixed and dissolved.
3. To the above is added 1.0 g of Lemon Aspen Extract. The solution is
mixed until the
Lemon Aspen Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the solution of Step 4 is added 1.5 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
6. The following ingredients are next added sequentially, allowing for each
to dissolve
before adding the next: 6.0 grams Boric acid, 0.05 gram Sodium Borate, 1.0
gram Potassium
Chloride, 0.06 gram Calcium Chloride Dihydrate, 0.06 gram Magnesium Chloride
and
0.0015 grams Polyquaternium-42 (aqueous).
37
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
7. While continuing to mix, 0.14 gram Sodium Chlorite Dihydrate is added
and mixed to
dissolve.
8. The tonicity of the formula is determined and adjusted to 280 mOsm/Kg
with Sodium
Chloride.
9. The pH of the formula is adjusted to pH of 7.2 using the 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
10. The solution is brought to 1000.0 grams using Purified Water USP and
mixed for 10
minutes to be fully uniform.
11. The solution is filtered using a 0.22 micron filter.
Solution 3D can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 2 g of Polysorbate 10 and 50 g of Polysorbate 20.
The solution
is mixed until both are fully mixed and dissolved.
3. To the above is added 0.1 g of Lemon Aspen Extract. The solution is
mixed until the
Lemon Aspen Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the solution of Step 4 is added 1.5 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
6. The following ingredients are next added sequentially, allowing for each
to dissolve
before adding the next: 6.0 grams Boric acid, 0.05 gram Sodium Borate, 1.0
gram Potassium
Chloride, 0.06 gram Calcium Chloride Dihydrate, 0.06 gram Magnesium Chloride
and
0.0015 grams Polyquaternium-42 (aqueous).
7. While continuing to mix, 0.14 gram Sodium Chlorite Dihydrate is added
and mixed to
.. dissolve.
38
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
8. The tonicity of the formula is determined and adjusted to 280 mOsm/Kg
with Sodium
Chloride.
9. The pH of the formula is adjusted to pH of 7.2 using the 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
10. The solution
is brought to 1000.0 grams using Purified Water USP and mixed for 10
minutes to be fully uniform.
11. The solution is filtered using a 0.22
micron filter.
Example 4
Table 2 illustrates the components of formulations of the present invention
(as
illustrated in formulations 4A-4D), which components can be incorporated as
described
below using conventional mixing technology.
Table 2
4A 4B 4C 4D
Useful for Relief Useful for Relief of
Useful for Relief of Useful for Relief of
of Dry Eye Dry Eye Irritation
Dry Eye Irritation Dry Eye Irritation
Irritation for Contact Lenses
for Contact Lenses
INGREDIENT %w/w amount %w/w amount %w/w amount %w/w amount per
per per batch per batch batch (gms)
batch (gms) (gms)
(gms)
Sodium 0 0 0 0 0.120 1.20 0.120 1.20
Hyaluronate
Tamarind Seed 0 0 0 0 0.200 2.00 0.200 2.00
Polysaccharide
39
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Lemon Aspen 0.10 1.0 0.10 1.0 0.01 0.10 0.01
0.10
Extract
Polysorbate 80 1.0 10.0 1.0 10.0 0.2 2.0 0.2 2.0
Polysorbate 20 5.0 50.0 5.0 50.0 1.0 10.0 1.0
10.0
Polyethylene 0 0 0 0 0.25 2.5 0.25 2.5
Glycol 400
Glycerin 0.25 2.5 0.25 2.5 0.25 2.5 0.25 2.5
Hypromellose E3 0.198 1.98 0.198 1.98 0.198 1.98
0.198 1.98
2910
Boric Acid 0.40 4.0 0.40 4.0 0.40 4.0 0.40 4.0
Sodium Borate 0.022 0.22 0.022 0.22 0.022 0.22
0.022 0.22
Disodium 0.027 0.27 0.027 0.27 0.027 0.27 0.027
0.27
Phosphate
Sodium Citrate 0.40 4.0 0.40 4.0 0.40 4.0 0.40 4.0
Dihydrate
Sodium
Chloride*
Potassium 0.10 1.0 0.10 1.0 0.10 1.0 0.10 1.0
Chloride
50% Aqueous 0.057 0.57 0.057 0.57 0.057 0.57 0.057
0.57
Solution of
Sodium Lactate
Magnesium 0.013 0.13 0.013 0.13 0.013 0.13 0.013
0.13
Chloride
Glucose 0.0036 0.036 0.0036 0.036 0.0036
0.036 0.0036 0.036
Glycine 0.0000 0.0002 0.0000 0.0002 0.00002 0.0002 0.00002 0.0002
2 2
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Ascorbic Acid 0.0000 0.0001 0.0000 0.0001 0.00001
0.0001 0.00001 0.0001
1 1
Disodium Edetate 0.01 0.1 0.05 0.5 0.01 0.1 0.05
0.5
Polyquaternium 0.0030 0.030 0.0015 0.015 0.0030
0.030 0.0015 0.015
42 (33%aqueous)
Sodium Chlorite 0.014 0.14 0.014 0.14 0.014 0.14
0.014 0.14
Dihydrate
1N Sodium
Hydroxide
solution**
1N Hydrochloric
Acid solution**
Purified
Water***
total 100.00 1000.00 100.00 1000.00 g 100.00
1000.00 g 100.00 1000.00
* can be adjusted to tonicity of 280-290 mOsm/Kg
** can adjust to pH 7.2
*** optionally, q.s to 100%w/w
For Examples 4C -4D: The Sodium Hyaluronate can be supplied by CONTIPRO A.S.
(DOLNI, DOBROUC, CZECH REPUBLIC)
For Examples 4C-4D: The Tamarind Seed Polysaccharide cab be supplied by INDENA
(MILANO, ITALY).
For Examples 4A-4D: The Lemon Aspen Extract (AbacrossTM Acronychia acidula
fruit
extract) can be supplied by SOUTHERN CROSS BOTANICALS (KNOCKROW NSW,
AUSTRALIA).
For Examples 4A-4D: The Polysorbate 20 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 4A-4D: The Polysorbate 80 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
41
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
For Examples 6C-6D: The Polyethylene Glycol 400 can be supplied by Clariant
Produkte
(BURGKIRCHEN, GERMANY).
For Examples 4A-4D: The Boric Acid can be supplied by Merck KGaA (DARMSTADT,
GERMANY).
For Examples 4A-4D: The Sodium Borate can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 4A-4D: The Sodium Chloride can be supplied by Caldic (DUSSELDORF,
GERMANY).
For Examples 4A-4D: The Potassium Chloride can be supplied by Merck KGaA
(DARMSTADT, GERMANY).
For Examples 4A-4D: The Hypromellose E3 2910 can be supplied by DOW CHEMICAL
(PLAQUEMINE, LOUISIANA, USA).
For Examples 4A-4D: The Glycerin can be supplied by Emery Oleochemicals GmbH
(DUSSELDORF, GERMANY).
For Examples 4A-4D: The Disodium Phosphate can be supplied by Merck KGaA
(DARMSTADT, GERMANY).
For Examples 4A-4D: The Sodium Citrate can be supplied by Merck KGaA
(DARMSTADT, GERMANY).
For Examples 4A-4D: The Sodium Lactate can be supplied as Sodium Lactate (50%
aqueous) by Merck KGaA (DARMSTADT, GERMANY).
For Examples 4A-4D: The Glucose can be supplied by Roquette Freres (LASTREM,
FRANCE).
For Examples 4A-4D: The Glycine can be supplied by Merck KGaA (DARMSTADT,
GERMANY).
For Examples 4A-4D: The Ascorbic Acid can be supplied by DSM NUTRITIONAL
Products (DRAKEMYRE, SCOTLAND, UK).
For Examples 4A-4D: The Polyquaternium 42 can be supplied as Polyquaternium 42
(33%
aqueous) by DSM BIOMEDICAL, (BERKELEY, CA).
For examples 4A-4D: The Disodium Edetate can be supplied by Merck NV/SA
(OVERUSE,
BELGIUM).
For Examples 4A-4D: The 1N Sodium Hydroxide can be supplied by VWR (RADNER,
PA,
USA).
For Examples 4A-4D: The 1N Hydrochloric acid can be supplied by VWR (RADNER,
PA,
USA).
For Examples 6A-6D: The Sodium Chlorite Dihydrate can be supplied by Oxychem
(WICHITA, KS, USA)
42
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Solution 4A can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 10 g of Polysorbate 80 and 50 g of Polysorbate 20.
The solution
.. is mixed until both are fully mixed and dissolved.
3. To the above is added 1.0 g of Lemon Aspen Extract. The solution is
mixed until the
Lemon Aspen Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the above is added 1.98 g of Hypromellose E3 Premium. The solution is
mixed
until the Hypromellose E3 Premium dissolved.
6. The following ingredients are next added sequentially, allowing each to
dissolve
before adding the next: 2.50 grams Glycerin, 4.0 grams Boric acid, 0.22 gram
Sodium
Borate, 0.27gram Disodium Phosphate, 4.00 grams Sodium Citrate Dihydrate, 1
gram
Potassium Chloride, 0.57 gram Sodium Lactate (50% aqueous), 0.13 gram
Magnesium
Chloride, 0.036 gram Glucose, 0.0002 gram Glycine, 0.0001 gram Ascorbic acid,
0.10 gram
Disodium Edetate, 0.030 gram Polyquaternium-42 (33%aqueous), and 0.14 gram
Sodium
Chlorite.
7. The tonicity of the solution is determined and adjusted to 280 mOsm with
Sodium
Chloride.
8. The pH of the solution is measured and adjusted to 7.2 with 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
9. The solution is brought to a volume of 1,000.00 grams with Purified
Water and mixed
for 10 minutes.
10. The solution is filtered using a 0.22 micron filter.
Solution 4B can be prepared as follows:
43
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 10 g of Polysorbate 80 and 50 g of Polysorbate 20.
The solution
is mixed until both are fully mixed and dissolved.
3. To the above is added 1.0 g of Lemon Aspen Extract. The solution is
mixed until the
Lemon Aspen Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the above is added 1.98 g of Hypromellose E3 Premium. The solution is
mixed
until the Hypromellose E3 Premium dissolved.
6. The following ingredients are next added sequentially, allowing each to
dissolve
before adding the next: 2.50 grams Glycerin, 4.0 grams Boric acid, 0.22 gram
Sodium
Borate, 0.27gram Disodium Phosphate, 4.00 grams Sodium Citrate Dihydrate, 1
gram
Potassium Chloride, 0.57 gram Sodium Lactate (50% aqueous), 0.13 gram
Magnesium
Chloride, 0.036 gram Glucose, 0.0002 gram Glycine, 0.0001 gram Ascorbic acid,
0.05 gram
Disodium Edetate, 0.015 gram Polyquaternium-42 (33%aqueous), and 0.14 gram
Sodium
Chlorite.
7. The tonicity of the solution is determined and adjusted to 280 mOsm with
Sodium
Chloride.
8. The pH of the solution is measured and adjusted to 7.2 with 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
9. The solution is brought to a volume of 1,000.00 grams with Purified
Water and mixed
for 10 minutes.
10. The solution is filtered using a 0.22 micron filter.
Solution 4C can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 2 g of Polysorbate 80 and 10 g of Polysorbate
20. The solution
is mixed until both are fully mixed and dissolved.
44
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
3. To the above is added 0.1 g of Lemon Aspen Extract. The solution is
mixed until the
Lemon Aspen Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the solution of Step 4 is added 1.2 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
6. To the above is added 2.0 grams of Tamarind Seed Polysaccharide. The
solution is
mixed to fully dissolve the Tamarind Seed Polysaccharide.
7. To the above is added 1.98 g of Hypromellose E3 Premium. The solution is
mixed
until the Hypromellose E3 Premium dissolved.
8. The following ingredients are next added sequentially, allowing each to
dissolve
before adding the next: 2.50 grams Polyethylene Glycol 400, 2.50 grams
Glycerin, 4.0 grams
Boric acid, 0.22 gram Sodium Borate, 0.27gram Disodium Phosphate, 4.00 grams
Sodium
Citrate Dihydrate, 1 gram Potassium Chloride, 0.57 gram Sodium Lactate (50%
aqueous),
0.13 gram Magnesium Chloride, 0.036 gram Glucose, 0.0002 gram Glycine, 0.0001
gram
Ascorbic acid, 0.10 gram Disodium Edetate, 0.030 gram Polyquaternium-
42(33%aqueous),
and 0.14 gram Sodium Chlorite.
9. The tonicity of the solution is determined and adjusted to 280 mOsm with
Sodium
Chloride.
10. The pH of the solution is measured and adjusted to 7.2 with 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
11. The solution is brought to a volume of 1,000.00 grams with Purified
Water and mixed
for 10 minutes.
12. The solution is filtered using a 0.22 micron filter.
Solution 4D can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
2. To the above is added 2 g of Polysorbate 80 and 10 g of Polysorbate 20.
The solution
is mixed until both are fully mixed and dissolved.
3. To the above is added 0.1 g of Lemon Aspen Extract. The solution is
mixed until the
Lemon Aspen Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the solution of Step 4 is added 1.2 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
6. To the above is added 2.0 grams of Tamarind Seed Polysaccharide. The
solution is
mixed to fully dissolve the Tamarind Seed Polysaccharide.
7. To the above is added 1.98 g of Hypromellose E3 Premium. The solution is
mixed
until the Hypromellose E3 Premium dissolved.
8. The following ingredients are next added sequentially, allowing each to
dissolve
before adding the next: 2.50 grams Polyethylene Glycol 400, 2.50 grams
Glycerin, 4.0 grams
Boric acid, 0.22 gram Sodium Borate, 0.27gram Disodium Phosphate, 4.00 grams
Sodium
Citrate Dihydrate, 1 gram Potassium Chloride, 0.57 gram Sodium Lactate (50%
aqueous),
0.13 gram Magnesium Chloride, 0.036 gram Glucose, 0.0002 gram Glycine, 0.0001
gram
Ascorbic acid, 0.10 gram Disodium Edetate, 0.015 gram Polyquaternium-42
(33%aqueous),
and 0.14 gram Sodium Chlorite.
9. The tonicity of the solution is determined and adjusted to 280 mOsm with
Sodium
Chloride.
10. The pH of the solution is measured and adjusted to 7.2 with 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
11. The solution is brought to a volume of 1,000.00 grams with Purified
Water and mixed
for 10 minutes.
12. The solution is filtered using a 0.22 micron hydrophilic filter.
46
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Example 5
Table 3 illustrates the components of formulations of the present invention
(as
illustrated in formulations 5A and 5B), which components can be incorporated
as described
below using conventional mixing technology.
Table 3
5A 5B
Useful for Relief of Useful for Relief of
Dry Eye Irritation Dry Eye Irritation
INGREDIENT /ow/w amount /0w/w amount
per batch per
(gms) batch
(gms)
Sodium 0.20 2.0 0.10 1.0
Hyaluronate
Tamarind Seed 0 0 0.20 2.0
Polysaccharide
Lemon Aspen 0.01 0.1 0.10 1.0
Extract
Polysorbate 80 0.2 2.0 1.0 10.0
Polysorbate 20 1.0 10.0 5.0 50.0
Polyethylene 0.25 2.5 0.25 2.5
Glycol 400
Boric Acid 0.60 6.0 0.60 6.0
Sodium Borate 0.06 0.60 0.06 0.60
47
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Sodium
Chloride*
Super refined 0.625 6.25 0.625 6.25
Castor Oil
Lumuluse 0.50 5.0 0.50 5.0
GRH-40
Ethyl linolenate 0.10 1.0 0 0
Retinyl 0.05 0.5 0 0
PaImitate
Polyquaternium 0.0045 0.045 0.0045 0.045
42
(33% aqueous)
1N Sodium
Hydroxide
solution**
1N
Hydrochloric
Acid solution**
Purified
Water***
Total 100.00 1000.0 g 100.00 1000.0 g
cyo cyo
* can be adjusted to tonicity of 280-290 mOsm/Kg
** can adjust to pH 7.2
*** optionally, q.s to 100%w/w
For Examples 5A-5B: The Sodium Hyaluronate can be supplied by CONTIPRO A.S.
(DOLNI, DOBROUC, CZECH REPUBLIC)
48
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
For Examples 5A-5B: The Lemon Aspen Extract (AbacrossTM Acronychia acidula
fruit
extract) can be supplied by SOUTHERN CROSS BOTANICALS (KNOCKROW NSW,
AUSTRALIA).
For Examples 5A-5B: The Polysorbate 20 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 5A-5B: The Polysorbate 80 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 5A-5B: The Polyethylene Glycol 400 can be supplied by Clariant
Produkte
(BURGKIRCHEN, GERMANY).
For Examples 5A-5B: The Boric Acid can be supplied by Merck KGaA (DARMSTADT,
GERMANY).
For Examples 5A-5B: The Sodium Borate can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 5A-5B: The Sodium Chloride can be supplied by Caldic (DUSSELDORF,
GERMANY).
For Examples 5A-5B: The 1N Sodium Hydroxide can be supplied by VWR (RADNER,
PA,
USA).
For Examples 5A-5B: The 1N Hydrochloric acid can be supplied by VWR (RADNER,
PA,
USA).
For Examples 5A-5B: The Lumuluse GRH-40 is can be supplied by is supplied by
VANTAGE (GURNEE, IL, USA).
For Examples 5A-5B: The Super refined Castor Oil can be supplied by CRODA
(EDISON,
NJ, USA).
For Examples 5A-5B: The Ethyl linolenate can be supplied by SIGMA-ALDRICH (ST.
LOUIS, MO, USA).
For Examples 5A-5B: The Retinyl PaImitate can be supplied by SIGMA-ALDRICH
(ST.
LOUIS, MO, USA).
For Examples 5A-5B: The Polyquaternium-42 (33% aqueous) can be supplied by DSM
BIOMEDICAL (BERKELEY, CA, USA).
For Example 5B: The Tamarind Seed Polysaccharide can be supplied by INDENA
(MILAN,
ITALY).
Solution 5A can be prepared as follows:
1. To a 50 ml beaker is added 5.0 grams of Lumuluse GRH-40
2. While mixing, 6.25 grams of Super refined Castor Oil is added.
49
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
3. Next is added 1 gram of Ethyl linolenate and 0.5 gram of
Retinyl PaImitate to mix until uniform.
4. In a separate 1500 ml beaker is added 500 grams of Purified Water.
5. To the above is added 2 g of Polysorbate 80 and 10 g of Polysorbate 20.
The solution
is mixed until both are fully dissolved.
6. To the above is added 0.1 gram of Lemon Aspen Extract. The solution is
mixed until
the Lemon Aspen Extract is dissolved.
7. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
8. To the solution in step 7 is added 2.0 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
9. The following ingredients are next added sequentially, allowing for each
to dissolve
before adding the next: 2.5 grams Polyethylene Glycol 400, 6.0 grams Boric
acid, 0.06gram
Sodium Borate, and grams of Polyquaternium-42 (33% aqueous).
10. The contents of step 3 are added and mixed until uniform using a
homogenizer.
11. The tonicity of the formula is determined and adjusted to 280 mOsm/Kg
with Sodium
Chloride.
12. The pH of the formula is adjusted to pH of 7.2 using the 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
13. The solution is brought to 1000.0 grams using Purified Water USP and
mixed for 10
minutes until fully uniform.
14. The solution is filtered using a 0.22 micron filter.
Solution 5B can be prepared as follows:
1. To a 50 ml beaker is added 5.0 grams of Lumuluse GRH-40
2. While mixing, 6.25 grams of Super refined Castor Oil is added.
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
3. The uniform solution is set aside for future use.
4. In a separate 1500 ml beaker is added 500 grams of Purified Water.
5. To the above is added 10 g of Polysorbate 80 and 50 g of Polysorbate 20.
The solution
is mixed until both are fully dissolved.
6. To the above is added 1.0 gram of Lemon Aspen Extract. The solution is
mixed until
the Lemon Aspen Extract is dissolved.
7. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
8. To the solution in step 7 is added 1.0 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
9. Next, 2.0 grams of Tamarind Seed Polysaccharide is added. The solution
is mixed to
fully dissolve the Tamarind Seed Polysaccharide.
10. The following ingredients are next added sequentially, allowing for
each to dissolve
before adding the next: 2.5 grams Polyethylene Glycol 400, 6.0 grams Boric
acid,
0.06gram Sodium Borate, and 0.045 grams of Polyquaternium-42 (33% aqueous).
11. The contents of step 3 are added and mixed until uniform using a
homogenizer.
12. The tonicity of the formula is determined and adjusted to 280 mOsm/Kg
with Sodium
Chloride.
13. The pH of the formula is adjusted to pH of 7.2 using the 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
14. The solution is brought to 1000.0 grams using Purified Water USP and mixed
for 10
minutes until fully uniform.
15. The solution is filtered using a 0.22 micron filter.
Example 6
Table 4 illustrates the components of formulations of the present invention
(as
.. illustrated in formulations 6A and 6B), which components can be
incorporated as described
below using conventional mixing technology.
51
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Table 4
6A 6B
Useful for Relief of Useful for Relief of
Dry Eye Irritation Dry Eye Irritation
for Contact Lenses
INGREDIENT /ow/w amount /0w/w amount
per per
batch batch
(gms) (gms)
Sodium 0.30 3.0 0.15 1.5
Hyaluronate
Actinomyces 5.0 50.0 0 0
Extract
Compound 1 0 0 5.0 50.0
****
Polysorbate 80 1.0 10.0 1.0 10.0
Polysorbate 20 10.0 100.0 7.5 75.0
Polyethylene 0.25 2.5 0 0
Glycol 400
Boric Acid 0.60 6.0 0.60 6.0
Sodium Borate 0.05 0.50 0.05 0.50
Sodium
Chloride*
Potassium 0.10 1.0 0.10 1.0
Chloride
Calcium 0.006 0.06 0.006 0.06
Chloride
Dihydrate
Magnesium 0.006 0.06 0.006 0.06
Chloride
52
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Sodium 0.014 0.14 0.014 0.14
Chlorite
Dihydrate
Polyquaternium 0.0015 0.015 0.0015 0.015
42
(33%aqueous)
Sodium 0.014 0.14 0.014 0.14
Chlorite
Dihydrate
1N Sodium
Hydroxide
solution**
1N
Hydrochloric
Acid solution**
Purified
Water***
Total 100.00 1000.0 g 100.00 1000.00
* adjust to tonicity of 280-290 mOsm/Kg
** adjust to pH 7.2
*** q.s to 100%w/w
**** (2E,4E,6E)-7-(1,1,2,2,3,3-hexamethy1-2,3-dihydro-1H-inden-5-y1)-3-
methylocta-2,4,6-
trienoic acid
For Examples 6A-6B: The Sodium Hyaluronate can be supplied by CONTIPRO A.S.
(DOLNI, DOBROUC, CZECH REPUBLIC)
For Example 6A: The Actinomyces species A5640 Extract (IHVR collection
bacteria extract
labeled under IHVR collection nomenclature as IHVR_39565J7), can be supplied
by
Baruch S. Blumerg Institute, Doylestown, PA.
For Example 6B: Compound 1 can be supplied by Sigma-Aldrich.
For Examples 6A-6B: The Polysorbate 20 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
53
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
For Examples 6A-6B: The Polysorbate 80 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 8A: The Polyethylene Glycol 400 can be supplied by Clariant
Produkte
(BURGKIRCHEN, GERMANY).
For Examples 6A-6B: The Boric Acid can be supplied by Merck KGaA (DARMSTADT,
GERMANY).
For Examples 6A-6B: The Sodium Borate can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 6A-6B: The Sodium Chloride can be supplied by Caldic (DUSSELDORF,
GERMANY).
For Examples 6A-6B: The Potassium Chloride can be supplied by Merck KGaA
(DARMSTADT, GERMANY).
For Examples 6A-6B: The Calcium Chloride Dihydrate can be supplied by Merck
KGaA
(DARMSTADT, GERMANY).
For Examples 6A-6B: The Magnesium Chloride to be supplied can KGaA (DARMSTADT,
GERMANY).
For Examples 6A-6B: The Polyquaternium-42 (33% aqueous) can be supplied by DSM
BIOMEDICAL (BERKELEY, CA, USA).
For Examples 6A-6B: The Sodium Chlorite Dihydrate can be supplied by Oxychem
(WICHITA, KS, USA)
For Examples 6A-6B: The 1N Sodium Hydroxide can be supplied by VWR (RADNER,
PA,
USA).
For Examples 6A-6B: The 1N Hydrochloric acid can be supplied by VWR (RADNER,
PA,
USA).
Solution 6A can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 10 g of Polysorbate 80 and 100 g of Polysorbate
20. The
solution is mixed until both are fully mixed and dissolved.
3. To the above is added 50.0 g of Actinomyces Extract. The solution is
mixed until the
Actinomyces Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
54
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
5. To the solution of Step 4 is added 3.0 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
6. The following ingredients are next added sequentially, allowing for each
to dissolve
before adding the next: 2.5 grams Polyethylene Glycol 400, 6.0 grams Boric
acid,
0.05 gram Sodium Borate, 1.0 gram Potassium Chloride, 0.06 gram Calcium
Chloride
Dihydrate, 0.06 gram Magnesium Chloride, and 0.0015 grams Polyquaternium-42
(aqueous).
7. While continuing to mix, 0.14 gram Sodium Chlorite Dihydrate is added
and mixed to
dissolve.
8. The tonicity of the formula is determined and adjusted to 280 mOsm/Kg
with Sodium
Chloride.
9. The pH of the formula is adjusted to pH of 7.2 using the 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
10. The solution is brought to 1000.0 grams using Purified Water USP and
mixed for 10
minutes to be fully uniform.
11. The solution is filtered using a 0.22 micron filter.
Solution 6B can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 10 g of Polysorbate 80 and 75 g of Polysorbate 20.
The solution
is mixed until both are fully mixed and dissolved.
3. To the above is added 50.0 g of Compound I. The solution is mixed until
the
Compound I is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the solution of Step 4 is added 1.5 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
6. The following ingredients are next added sequentially, allowing for each
to dissolve
before adding the next: 6.0 grams Boric acid, 0.05 gram Sodium Borate, 1.0
gram
Potassium Chloride, 0.06 gram Calcium Chloride Dihydrate, 0.06 gram Magnesium
Chloride and 0.0015 grams Polyquaternium-42 (aqueous).
7. While continuing to mix, 0.14 gram Sodium Chlorite Dihydrate is added
and mixed to
dissolve.
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
8. The tonicity of the formula is determined and adjusted to 280 mOsm/Kg
with Sodium
Chloride.
9. The pH of the formula is adjusted to pH of 7.2 using the 1N Sodium
Hydroxide and/or
1N Hydrochloric acid.
10. The solution is brought to 1000.0 grams using Purified Water USP and mixed
for 10
minutes to be fully uniform.
11. The solution is filtered using a
0.22 micron filter.
Example 7
Table 5 illustrates the components of formulations of the present invention
(as
illustrated in formulations 7A and 7B), which components can be incorporated
as described
below using conventional mixing technology.
Table 5
7A 7B
Useful for Useful for Relief
Relief of Dry of Dry Eye
Eye Irritation Irritation
for Contact
Lenses
INGREDIENT /ow/w amoun /0w/w amount
t per per
batch batch
(gms) (gms)
Sodium 0.120 1.20 0.120 1.20
Hyaluronate
Tamarind Seed 0.200 2.00 0.200 2.00
Polysaccharide
Actinomyces 5.0 50.0 0 0
Extract
Compound 2 0 0 0.5 5.0
****
56
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Polysorbate 80 1.0 10.0 1.0 10.0
Polysorbate 20 10.0 100.0 3.0 30.0
Polyethylene 0.25 2.5 0.25 2.5
Glycol 400
Glycerin 0.25 2.5 0.25 2.5
Hypromellose 0.198 1.98 0.198 1.98
E3 2910
Boric Acid 0.40 4.0 0.40 4.0
Sodium Borate 0.022 0.22 0.022 0.22
Disodium 0.027 0.27 0.027 0.27
Phosphate
Sodium Citrate 0.40 4.0 0.40 4.0
Dihydrate
Sodium
Chloride*
Potassium 0.10 1.0 0.10 1.0
Chloride
50% Aqueous 0.057 0.57 0.057 0.57
Solution of
Sodium Lactate
Magnesium 0.013 0.13 0.013 0.13
Chloride
Glucose 0.0036 0.036 0.0036 0.036
Glycine 0.0000 0.0002 0.0000 0.0002
2 2
Ascorbic Acid 0.0000 0.0001 0.0000 0.0001
1 1
Disodium 0.01 0.1 0.05 0.5
Edetate
Polyquaternium 0.0030 0.030 0.0015 0.015
42
(33%aqueous)
57
CA 03107726 2021-01-26
WO 2020/021480 PCT/IB2019/056344
Sodium 0.014 0.14 0.014 0.14
Chlorite
Dihydrate
1N Sodium
Hydroxide
solution**
1N
Hydrochloric
Acid solution**
Purified
Water***
Total 100.00 1000.0 100.00 1000.00
0 g
* adjust to tonicity of 280-290 mOsm/Kg
** adjust to pH 7.2
*** q.s to 100.00% volume
****4-(1-(1,1,2,2,3,3-hexamethy1-2,3-dihydro-1H-inden-5-yl)vinyl)benzoic acid
For Examples 7A-7B: The Sodium Hyaluronate can be supplied by CONTIPRO A.S.
(DOLNI, DOBROUC, CZECH REPUBLIC)
For Examples 7A-7B: The Tamarind Seed Polysaccharide can be supplied by INDENA
(MILANO, ITALY).
For Example 7A: The Actinomyces species A5640 Extract (IHVR collection
bacteria extract
labeled under IHVR collection nomenclature as IHVR_39565J7), can be supplied
by
Baruch S. Blumerg Institute, Doylestown, PA.
For Example 7B: Compound 2 can be supplied by Sigma-Aldrich.
For Examples 7A-7B: The Polysorbate 20 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 7A-7B: The Polysorbate 80 can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 7A-7B: The Polyethylene Glycol 400 can be supplied by Clariant
Produkte
(BURGKIRCHEN, GERMANY).
58
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
For Examples 7A-7B: The Boric Acid can be supplied by Merck KGaA (DARMSTADT,
GERMANY).
For Examples 7A-7B: The Sodium Borate can be supplied by Merck KGaA
(DARMSTADT,
GERMANY).
For Examples 7A-7B: The Sodium Chloride can be supplied by Caldic (DUSSELDORF,
GERMANY).
For Examples 7A-7B: The Potassium Chloride can be supplied by Merck KGaA
(DARMSTADT, GERMANY).
For Examples 7A-7B: The Hypromellose E3 2910 can be supplied by DOW CHEMICAL
(PLAQUEMINE, LOUISIANA, USA).
For Examples 7A-7B: The Glycerin to be supplied can Emery Oleochemicals GmbH
(DUSSELDORF, GERMANY).
For Examples 7A-7B: The Disodium Phosphate can be supplied by Merck KGaA
(DARMSTADT, GERMANY).
For Examples 7A-7B: The Sodium Citrate can be supplied by Merck KGaA
(DARMSTADT, GERMANY).
For Examples 7A-7B: The Sodium Lactate can be supplied as Sodium Lactate (50%
aqueous) by Merck KGaA (DARMSTADT, GERMANY).
For Examples 7A-7B: The Glucose can be supplied by Roquette Freres (LASTREM,
FRANCE).
For Examples 7A-7B: The Glycine can be supplied by Merck KGaA (DARMSTADT,
GERMANY).
For Examples 7A-7B: The Ascorbic Acid can be supplied by DSM NUTRITIONAL
Products (DRAKEMYRE, SCOTLAND, UK).
For Examples 7A-7B: The Polyquaternium 42 can be supplied as Polyquaternium 42
(33%
aqueous) by DSM BIOMEDICAL, (BERKELEY, CA).
For examples 7A-7B: The Disodium Edetate can be supplied by Merck NV/SA
(OVERUSE,
BELGIUM).
For Examples 7A-7B: The 1N Sodium Hydroxide can be supplied by VWR (RADNER,
PA,
USA).
For Examples 7A-7B: The 1N Hydrochloric acid can be supplied by VWR (RADNER,
PA,
USA).
59
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
For Examples 7A-7B: The Sodium Chlorite Dihydrate can be supplied by Oxychem
(WICHITA, KS, USA)
Solution 7A can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 10 g of Polysorbate 80 and 100 g of Polysorbate
20. The
solution is mixed until both are fully mixed and dissolved.
3. To the above is added 50 g of Actinomyces Extract. The solution is mixed
until the
Actinomyces Extract is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the solution of Step 4 is added 1.2 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
6. To the above is added 2.0 grams of Tamarind Seed Polysaccharide. The
solution is
mixed to fully dissolve the Tamarind Seed Polysaccharide.
7. To the above is added 1.98 g of Hypromellose E3 Premium. The solution is
mixed
until the Hypromellose E3 Premium dissolved.
8. The following ingredients are next added sequentially, allowing each to
dissolve
before adding the next: 2.50 grams Polyethylene Glycol 400, 2.50 grams
Glycerin,
4.0 grams Boric acid, 0.22 gram Sodium Borate, 0.27gram Disodium Phosphate,
4.00
grams Sodium Citrate Dihydrate, 1 gram Potassium Chloride, 0.57 gram Sodium
Lactate (50% aqueous), 0.13 gram Magnesium Chloride, 0.036 gram Glucose,
0.0002
gram Glycine, 0.0001 gram Ascorbic acid, 0.10 gram Disodium Edetate, 0.030
gram
Polyquaternium-42(33%aqueous), and 0.14 gram Sodium Chlorite.
9. The tonicity of the solution is determined and adjusted to 280 mOsm with
Sodium
Chloride.
10. The pH of the solution is measured and adjusted to 7.2 with 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
11. The solution is brought to a volume of 1,000.00 grams with Purified
Water and mixed
for 10 minutes.
12. The solution is filtered using a 0.22 micron filter.
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Solution 7B can be prepared as follows:
1. To a 1500 ml beaker is added 800 grams of Purified Water USP.
2. To the above is added 10 g of Polysorbate 80 and 30 g of Polysorbate 20.
The solution
is mixed until both are fully mixed and dissolved.
3. To the above is added 5.0 g of Compound I. The solution is mixed until
the
Compound I is dissolved.
4. The solution is filtered through a 0.45micron filter and returned to a
1500 ml beaker.
5. To the solution of Step 4 is added 1.2 grams of Sodium Hyaluronate. The
solution is
mixed to fully dissolve the Sodium Hyaluronate.
6. To the above is added 2.0 grams of Tamarind Seed Polysaccharide. The
solution is
mixed to fully dissolve the Tamarind Seed Polysaccharide.
7. To the above is added 1.98 g of Hypromellose E3 Premium. The solution is
mixed
until the Hypromellose E3 Premium dissolved.
8. The following ingredients are next added sequentially, allowing each to
dissolve
before adding the next: 2.50 grams Polyethylene Glycol 400, 2.50 grams
Glycerin,
4.0 grams Boric acid, 0.22 gram Sodium Borate, 0.27gram Disodium Phosphate,
4.00
grams Sodium Citrate Dihydrate, 1 gram Potassium Chloride, 0.57 gram Sodium
Lactate (50% aqueous), 0.13 gram Magnesium Chloride, 0.036 gram Glucose,
0.0002
gram Glycine, 0.0001 gram Ascorbic acid, 0.10 gram Disodium Edetate, 0.015
gram
Polyquaternium-42 (33%aqueous), and 0.14 gram Sodium Chlorite.
9. The tonicity of the solution is determined and adjusted to 280 mOsm with
Sodium
Chloride.
10. The pH of the solution is measured and adjusted to 7.2 with 1N Sodium
Hydroxide
and/or 1N Hydrochloric acid.
11. The solution is brought to a volume of 1,000.00 grams with Purified
Water and mixed
for 10 minutes.
12. The solution is filtered using a 0.22 micron hydrophilic filter.
61
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
Embodiments of the Present Invention
1. A method for producing/releasing/delivering/excreting mucin from and/or
in the
cornea (optionally, in a patient in need of such
production/release/deliverance/excretion of mucin) comprising the step of
administering a composition comprising:
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I) :
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; optionally one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; optionally a methyl (-
CH3) or methylene (=CH2) moiety; a carbonated chain, linear, ringed, or
branched, saturated or unsaturated, comprising from 1 to 20 carbon atoms;
optionally from 1 to 10 carbon atoms; optionally 6 carbon atoms; optionally an
aromatic moiety, optionally a phenyl moiety; optionally 2-methyl -prop-1,3-
diene; and
ii) optionally, an ophthalmologically acceptable carrier.
2. The method according to embodiment 1(or, any of the following
embodiments),
wherein the compound and/or extract having retinol-like properties and/or
benefits is
62
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
a botanical extract, or source of extracts, from plants of the genus
Acronychia and/or
Licaria.
3. The method according to embodiments 1 and/or 2 (or, any of the following
embodiments), wherein the compound and/or extract having retinol-like
properties
and/or benefits is a botanical extract, or source of extracts, from plants of
the genus
Acronychia.
4. The method according to anyone of, or a combination of, embodiments 1 ¨
3 (or, any
of the following embodiments), wherein the botanical extract, or source of
extracts,
from plants of the genus Acronychia is selected from the group consisting of
Acronychia aberrans, Acronychia acidula, Acronychia acronychioides, Acronychia
acuminate, Acronychia baeuerlenii, Acronychia chooreechillum, Acronychia
crassipetala, Acronychia eungellensis, Acronychia imperforate, Acronychia
laevis,
Acronychia laurifolia, Acronychia littoralis, Acronychia oblongifolia,
Acronychia
octandra, Acronychia parviflora, Acronychia pauciflora, Acronychia
pedunculata,
Acronychia pub escens, Acronychia species (Batavia Downs), Acronychia sub
erosa,
Acronychia vestita, Acronychia wilcoxiana, and combinations of two or more
thereof
5. The method according to anyone of, or a combination of, embodiments 1 ¨
4 (or, any
of the following embodiments), wherein the botanical extract, or source of
extracts,
from plants of the genus Acronychia is Acronychia acidula.
6. The method according to anyone of, or a combination of, embodiments 1 ¨
5 (or, any
of the following embodiments), wherein the botanical extract of Acronychia
and/or
Licaria comprises from about 1% to about 20%, by weight of the extract, of the
compound of formula II
ORI
R, .
wherein:
RI is selected from the group consisting of Ci-C2o alkyl, C2-C2o alkenyl, C2-
C2o alkynyl, and C3-C8 cycloalkyl or aryl;
63
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
R2 is selected from the group consisting of hydrogen, hydroxyl, Ci-C6 alkyl,
C2-
C6 alkenyl, C2-C6alkynyl, C3-C8 cycloalkyl or aryl, ¨OCI-C6 alkyl, ¨0C2-
C6 alkenyl, ¨0C2-C6alkynyl, ¨0C3-C8 cycloalkyl or aryl, thiol, ¨SC i-C6alkyl,
¨
SC2-C6alkenyl, ¨SC2-C6alkynyl, ¨SC3-C8cycloalkyl or aryl, ¨NR4CI-C6 alkyl,
¨NR4C2-C6alkenyl, ¨NR4C2-C6alkynyl, and ¨NR4C3-C8cycloalkyl or aryl;
R3 is selected from ¨CO2H, ¨0O2R4 or an isosteric equivalent of a carboxy
group,
wherein R4 is Ci-C6 alkyl, C2-C6 alkenyl, C3-C8 cycloalkyl or aryl; and
Y is ¨(CH2¨CH2)¨, ¨(CHH)¨, or ¨(C)¨.
7. The method according to anyone of, or a combination of, embodiments 1 ¨
6 (or, any
of the following embodiments), wherein the botanical extract ofAcronychia
and/or
Licaria comprises from about 1% to about 20%, by weight of the extract, of the
compound of Formula II
OR'
R3 R, (II) .
wherein:
RI is selected from the group consisting of C5-C16 alkyl, C5-C16 alkenyl, and
C5-
C16 alkynyl, more preferably C5-Ci6alkenyl, including, for example, farnesyl;
R2 is selected from the group consisting of hydrogen, hydroxyl, ¨OCI-C6alkyl,
¨
0C2-C6 alkenyl, ¨0C2-C6alkynyl, ¨0C3-C8 cycloalkyl, more preferably hydrogen,
hydroxyl, ¨OCI-C6 alkyl, even more preferably hydrogen or ¨OCI-C3 alkyl;
R3 is selected from ¨CO2H, ¨0O2R4 wherein R4 is Ci-C6 alkyl, or an isosteric
equivalent of a carboxy group; and
Y is ¨(CH2¨CH2)¨ or ¨(CHH)¨.
8. The method according to anyone of, or a combination of, embodiments 1 ¨
7 (or, any
of the following embodiments), wherein the compound of formula (II) are in the
form
of an acid or alkylester selected from 3-(4-farnesyloxypheny1)-propionic acid,
3-(4-
farnesyloxy-3-hydroxypheny1)-propionic acid, 3-(4-farnesyloxy-3-methoxypheny1)-
propionic acid, alkylesters thereof and combinations of two or more thereof
64
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
9. The method according to according to anyone of, or a combination of,
embodiments 1
¨ 8 (or, any of the following embodiments), wherein the compound of Formula II
useful in the present invention is 3-(4-farnesyloxypheny1)-propionic acid
and/or its
ethyl ester.
10. The method according to anyone of, or a combination of, embodiments 1 ¨
9 (or, any
of the following embodiments), wherein the compound and/or extract having
retinol-
like properties and/or benefits is a botanical extract, or source of extracts,
from plants
of the genus Licaria.
11. The method according to anyone of, or a combination of, embodiments 1 ¨
10 (or, any
of the following embodiments), wherein the botanical extract, or source of
extracts,
from plants of the genus Licaria is selected from the group consisting of
Licaria
vernicosa, Licaria brittoniana, Licaria cane/la, Licaria cub ensis, Licaria
velutina and
Licaria triandra, and combinations of two or more thereof.
12. The method according to anyone of, or a combination of, embodiments 1 ¨
11 (or, any
of the following embodiments), wherein the botanical extract, or source of
extracts,
from plants of the genus Licaria is Licaria vernicosa.
13. The method according to anyone of, or a combination of, embodiments 1 ¨
12 (or, any
of the following embodiments), wherein the compound and/or extract having
retinol-
like properties and/or benefits is a bacterial extract, or source of extracts,
of the genus
Actinomyces.
14. The method according to anyone of, or a combination of, embodiments 1 ¨
13 (or, any
of the following embodiments), wherein the compound and/or extract having
retinol-
like properties and/or benefits is a bacterial extract, or source of extracts,
of the
Actinomyces species A5640.
15. The method according to anyone of, or a combination of, embodiments 1 ¨
14 (or, any
of the following embodiments), wherein the compound and/or extract having
retinol-
like properties and/or benefits comprises the compound of Formula (I):
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; optionally, one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; a carbonated chain, linear,
ringed, or branched, saturated or unsaturated, comprising from 1 to 20 carbon
atoms.
16. The method according to anyone of, or a combination of, embodiments 1 ¨
15 (or, any
of the following embodiments), wherein the compound of Formula I is selected
from
(2E,4E,6E)-7-(1,1,2,2,3,3-hexamethy1-2,3-dihydro-1H-inden-5-y1)-3-methylocta-
2,4,6-trienoic acid and 4-(1-(1,1,2,2,3,3-hexamethy1-2,3-dihydro-1H-inden-5-
yl)vinyl)benzoic acid and their derivatives that display retinoid-like
activity and
mixtures thereof
17. A method for maintaining the concentration of MU5AC in tears within the
range of
equal to or greater than 8 nanograms to 15 nanograms per milligrams of
protein,
(optionally in a patient in need of such maintenance) comprising the step of
administering a composition comprising:
i) a safe and effective amount of a compound and/or extract having
retinol-like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
66
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; preferably a methyl (-
CH3) or methylene (=CH2) moiety;
a represent a carbonated chain, linear, ringed, or branched, saturated or
unsaturated, comprising from 1 to 20 carbon atoms; and
ii) optionally, an ophthalmologically acceptable carrier.
18. A method for treating a patient having decreased or low-level
production/release/delivery/excretion of mucin from and/or in the cornea
comprising
the step of topically administering to the eye the patient a composition
comprising:
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; preferably one of the
dotted
line is a double bound;
67
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; preferably a methyl (-
CH3) or methylene (=CH2) moiety;
a represent a carbonated chain, linear, ringed, or branched, saturated or
unsaturated, comprising from 1 to 20 carbon atoms; preferably from 1 to 10
carbon atoms; more preferably 6 carbon atoms; preferably an aromatic moiety,
preferably a phenyl moiety; preferably 2-methyl -prop-1,3-diene.
ii) optionally, an ophthalmologically acceptable carrier.
19. A method for preventing or treating the symptoms associated with dry
eye comprising
the step of topically administering (optionally, to a patient in need of such
prevention
or treatment) a composition comprising:
i) a safe and effective amount of a compound and/or extract having
retinol-like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; preferably one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; preferably a methyl (-
CH3) or methylene (=CH2) moiety;
68
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
a represent a carbonated chain, linear, ringed, or branched, saturated or
unsaturated, comprising from 1 to 20 carbon atoms; preferably from 1 to 10
carbon atoms; more preferably 6 carbon atoms; preferably an aromatic moiety,
preferably a phenyl moiety; preferably 2-methyl -prop-1,3-diene;
ii) one or more demulcents or soothing agents; and
iii) optionally, an ophthalmologically acceptable carrier.
20. A method for promoting healing or increasing the rate of healing of
wounds in and/or
on the eye (optionally, of a patient in need of such promoted healing or
increased
healing rate) comprising the step of administering compositions, comprising:
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; preferably one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; preferably a methyl (-
CH3) or methylene (=CH2) moiety;
a represent a carbonated chain, linear, ringed, or branched, saturated or
unsaturated, comprising from 1 to 20 carbon atoms; preferably from 1 to 10
carbon atoms; more preferably 6 carbon atoms; preferably an aromatic moiety,
preferably a phenyl moiety; preferably 2-methyl -prop-1,3-diene.
ii) optionally, an ophthalmologically acceptable carrier.
69
CA 03107726 2021-01-26
WO 2020/021480
PCT/IB2019/056344
21. A method for improving the antimicrobial properties in tears
(optionally, of a patient
in need of such improved properties in tears), comprising the step of
administering
compositions, comprising:
i) a safe and effective amount of a compound and/or extract having retinol-
like
properties and/or benefits for use in treating dry eye selected from one or
more
of: botanical extracts, or sources of extracts, from plants of the genus
Acronychia, Licaria, Calendula and/or Trigonella; bacterial extracts, or
sources of extracts, of the genus Actinomyces; and compounds of Formula (I):
R2 0
A ORi
(I)
wherein -
the dotted lines represent simple or double bound; preferably one of the
dotted
line is a double bound;
RI represent an H, a carbonated chain, linear, cyclic, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms;
R2 represent a carbonated chain, linear, cyclic ringed, or branched, saturated
or
unsaturated, comprising from 1 to 20 carbon atoms; preferably a methyl (-
CH3) or methylene (=CH2) moiety;
a represent a carbonated chain, linear, ringed, or branched, saturated or
unsaturated, comprising from 1 to 20 carbon atoms; preferably from 1 to 10
carbon atoms; more preferably 6 carbon atoms; preferably an aromatic moiety,
preferably a phenyl moiety; preferably 2-methyl -prop-1,3-diene.
ii) optionally, an ophthalmologically acceptable carrier.