Note: Descriptions are shown in the official language in which they were submitted.
CA 03107950 2021-01-27
WO 2020/028692 PCT/US2019/044712
EXERCISE DEVICE AND METHODS OF USE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] The present application is a non-provisional of United States
Provisional Patent
Application Serial No. 62/713,372 titled "Exercise Device and Methods of Use",
filed August 1,
2018, which is incorporated herein as if set out in full.
BACKGROUND
[0002] Many of the largest and strongest muscles in the body act on, or attach
around, the hip
structure. This results in the hips and core being the primary power source
for many movements
in sports. In addition, the hip joint is a ball and socket joint and the
second most mobile joint
within the body. Due to the sitting demands of today's society as well as much
of our life and
activities being in the sagittal (straight forward/backward) plane, there is
often dysfunction in
strength and mobility around the hips.
[0003] Weakness of the hip and core have been linked to multiple injuries and
pain symptoms
of the lower extremity and lumbar spine. Hip weakness combined with poor trunk
control (core
stability) are considered the largest modifiable risk factor in non-contact
knee injury.
Specifically, weakness of hip abduction and external rotation results in what
is termed "dynamic
valgus". This position can be seen in athletes who lack appropriate
lumbopelvic and hip control
when performing foundational athletic movements like squatting, cutting or
landing from a jump.
[0004] Weakness of the hip musculature, particularly hip abductors and
external rotators, have
been associated with many lower extremity injuries. Poor lumbopelvic control
has been linked
to increased risk of ACL injury, patella tendinopathy/tendonitis, meniscus
injury, patellafemoral
pain syndrome, hip impingement and labral tears, Achilles
tendonapthy/tendonitis, and ankle
sprains. Due to the fact that the hip is a ball and socket joint, there are
multiple degrees of
freedom (planes of motion). The knee has less degrees of freedom and can
become stressed and
painful when there are faulty movements at the hip joint. The hip joint is
home to the body's
1
CA 03107950 2021-01-27
WO 2020/028692 PCT/US2019/044712
largest and strongest muscles in the entire body; because of this, much of the
power generated
for sports comes from the hip and pelvis region. Without proper mechanics of
motion and
stability, this power will be lost due to poor direction of force, poor energy
transfer and an
increased risk of injury.
[0005] What is needed is an exercise device that addresses the basic but
essential components
of proper hip and core function. Such a device should function as a movement
primer to
stimulate neuromotor control and activation of the essential muscles and
planes of motion that
are foundational to all athletes. While these exercises won't replace sport
specific warm-ups,
they should be the catalyst to increase blood flow, neuromuscular activation
and optimize
function within the key muscles of the hip and core in order to maximize the
remainder of the
warm-up in preparation for best performance.
SUMMARY
[0006] This Summary is provided to introduce a selection of concepts in a
simplified form that
are further described below in the Detailed Description. This Summary, and the
foregoing
Background, is not intended to identify key aspects or essential aspects of
the claimed subject
matter. Moreover, this Summary is not intended for use as an aid in
determining the scope of the
claimed subject matter.
[0007] The present disclosure provides various embodiments of an exercise
device and methods
of use. The exercise device includes a deformably resilient, continuous looped
cord, a sleeve
disposed coaxially about the cord, and first and second pads disposed
coaxially about the cord
and sleeve. In some embodiments, the cord is formed from latex tubing or
bands. The sleeve, in
particular embodiments, is formed from a nylon material. In various
embodiments, each pad is
formed from a high density closed cell foam. The first and second pads are
positioned to be the
outmost layer of the exercise device to engage the user's skin.
2
CA 03107950 2021-01-27
WO 2020/028692 PCT/US2019/044712
[0008] In various embodiments, the total length of the two pads equals or
approaches 100% of
the length of the cord. In some embodiments, the total length of the pads,
falls in the ratio of
65% to 100% of the length of the cord or various smaller ranges up to a ratio
of 95% to 100% of
the length of the cord.
[0009] In various embodiments, the exercise device is constructed to exhibit a
4:1 stretch ratio
with regard to the length of the sleeve. The 4:1 stretch ratio means that the
sleeve surrounding
the cord will stretch four times the length of the un-stretched cord. It is
contemplated, however,
that some embodiments of the exercise device will have a stretch ratio of 2:1
to 5:1 ratio.
[0010] In various embodiments, the color of the pad signifies a different
resistance. For
example, in one particular embodiment, blue signifies super heavy resistance,
yellow signifies
medium resistance, red signifies heavy resistance, and purple signifies light
resistance. In such
embodiments, the different colored pads denote cords having different lengths.
It is
contemplated that the variance in resistance can be provided by length of
cord, its thickness, type
of material comprising the cord, or a combination thereof.
[0011] These and other aspects of the present system and method will be
apparent after
consideration of the Detailed Description and Figures herein. It is to be
understood, however,
that the scope of the invention shall be determined by the claims as issued
and not by whether
given subject matter addresses any or all issues noted in the Background or
includes any features
or aspects recited in this Summary.
DRAWINGS
[0012] Non-limiting and non-exhaustive embodiments of the present invention,
including the
preferred embodiment, are described with reference to the following figures,
wherein like
reference numerals refer to like parts throughout the various views unless
otherwise specified.
3
CA 03107950 2021-01-27
WO 2020/028692 PCT/US2019/044712
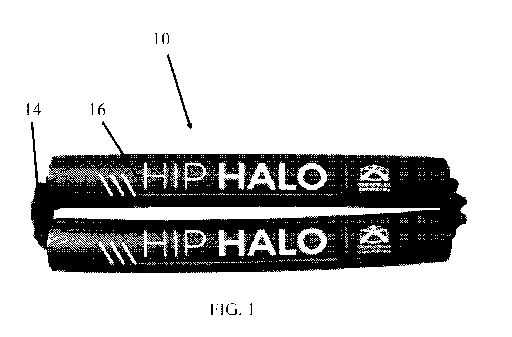
[0013] Figure 1 depicts a plan view of one embodiment of the exercise device
of the present
technology.
[0014] Figure 2 depicts a perspective view of embodiments of the exercise
device of the
present technology and further depicts one embodiment of the manner in which
the exercise
devices may be used.
[0015] Figure 3 depicts a perspective view of embodiments of the exercise
device of the
present technology and further depicts another embodiment of the manner in
which the exercise
devices may be used.
[0016] Figure 4 is a cross-sectional view of one embodiment of the exercise
device of the
present technology and depicts one manner in which the cord, sleeve, and pad
can be coaxially
disposed with respect to one another.
DETAILED DESCRIPTION
[0017] Embodiments are described more fully below with reference to the
accompanying
figures, which form a part hereof and show, by way of illustration, specific
exemplary
embodiments. These embodiments are disclosed in sufficient detail to enable
those skilled in the
art to practice the invention. However, embodiments may be implemented in many
different
forms and should not be construed as being limited to the embodiments set
forth herein. The
following detailed description is, therefore, not to be taken in a limiting
sense.
[0018] Embodiments of the exercise device 10 are depicted in Figures 1-4. The
exercise
device 10 includes a deformably resilient cord 12, a sleeve 14 disposed
coaxially about the cord
12, and a pair of pads 16 disposed coaxially about the cord 12 and sleeve 14.
In particular
embodiments, the cord 12 is formed to define a continuous loop. In at least
one embodiment, the
cord 12 has a circumferential length of approximately 30.5 inches in a relaxed
state. Various
embodiments of the cord 12 will have a circumferential length of approximately
24 inches to 28
4
CA 03107950 2021-01-27
WO 2020/028692 PCT/US2019/044712
inches. In some embodiments, the cord 12 is formed from latex tubing or bands.
In other
embodiments, the cord 12 is formed from an elastic fabric. In still other
embodiments, the cord
12 is provided in the form of a spring, which could be comprised of various
known materials
including metal or plastic. The sleeve 14, in particular embodiments, is
formed from a nylon
material. In a particular embodiment, the sleeve 14 is provided with a length
of approximately
89 inches. In various embodiments, the sleeve 14 will have a length of
approximately 65 inches
to 100 inches, depending on the length of cord 12 being used. In particular
embodiments, each
pad 16 is approximately 14.5 inches long. In various embodiments, the pad 16
will have a length
of approximately 11 inches to 14 inches or 12.5 to 13.25 inches, depending on
the length of cord
12 being used. Embodiments of the pad 16 are made of a high density closed
cell foam, having
an "A" density. Embodiments of the pad 16 may be formed to have a thickness of
approximately
7 mm and an internal channel having a diameter of approximately 24 millimeters
to 48
millimeters, depending on the thickness of the cord 12 being used. The pads 16
are positioned to
be the outmost layer of the exercise device 10 to engage the user's skin. In
some embodiments,
the pads 16 have a round or curvilinear cross-sectional shape. In various
embodiments, the
manner in which the cord 12, the sleeve 14, and pads 16 are coaxially coupled
with one another
permits the cord 12, the sleeve 14, and pads 16 to move with respect to one
another.
[0019] In various embodiments, such as that described above, the total length
of the two pads
16 are 29 inches or approximately 95% of the length of the 30.5 inch long
cord. It is
contemplated that the pads 16 could be shaped to provide a total length that
equals or approaches
100% of the length of the cord 12. In some embodiments, the total length of
the pads 16, falls in
the ratio of 95% to 100% of the length of the cord 12. In other embodiments,
the total length of
the pads 16, falls in the ratio of 90% to 100% of the length of the cord 12.
In yet other
embodiments, the total length of the pads 16 falls in the ratio of 85% to 100%
of the length of the
cord 12. In still other embodiments, the total length of the pads 16 falls in
the ratio of 80% to
100% of the length of the cord 12. In further embodiments, the total length of
the pads 16 falls
in the ratio of 75% to 100% of the length of the cord 12. In other
embodiments, the total length
CA 03107950 2021-01-27
WO 2020/028692 PCT/US2019/044712
of the pads 16 falls in the ratio of 70% to 100% of the length of the cord 12.
In further
embodiments, the total length of the pads 16 falls in the ratio of 65% to 100%
of the length of the
cord 12. It will be appreciated that the length ratios above will be more
optimal for use with
various exercises and less optimal with others when considering the advantages
of relatively long
pads 16, as described below.
[0020] In particular embodiments, the exercise device 10 is constructed to
exhibit a 4:1 stretch
ratio with regard to the length of the sleeve 16. The 4:1 stretch ratio means
that the sleeve 16
surrounding the cord 12 will stretch four times the length of the un-stretched
cord 12. With a
relatively long sleeve 16, taller users may perform a greater range of
exercises and not "bottom
out" the sleeve 16 by reaching the fully extended length of the sleeve 16
before completing the
desired movement. It is contemplated, however, that some embodiments of the
exercise device
will have a stretch ratio of 2:1 to 5:1 ratio. Accordingly, where the cord
measures 30.5
inches, for example, and the particular embodiment of exercise device 10 will
have a 3.5:1
stretch ratio, the sleeve length would be 106.75 inches. Similarly, where the
stretch ratio is to be
4:1, the sleeve 16 will be 122 inches in length. It is contemplated that the
exercise device 10 can
be configured for shorter users, such as children. In one such embodiment, the
total length of the
cord 12 could be 25 inches in total length. In embodiments where the exercise
device 10
includes a 100 inch sleeve 14, the stretch ratio would be 4:1. It is
contemplated that the pads 16
in such an embodiment would also be scaled to a shorter length of 11.75
inches.
[0021] In various embodiments, the color of the pad 16 signifies a different
resistance. For
example, in one particular embodiment, blue signifies super heavy resistance,
yellow signifies
medium resistance, red signifies heavy resistance, and purple signifies light
resistance. In such
embodiments, the blue pad 16 is combined with a cord 12 having a length of 27
inches. The red
pad 16 is combined with a cord 12 having a length of 26.5 inches. The yellow
pad 16 is
combined with a cord 12 having a length of 26.0 inches. The purple pad 16 is
combined with a
cord 12 having a length of 25.5 inches. It is contemplated that the variance
in resistance can be
provided by length of cord 12, its thickness, type of material comprising the
cord 12, or a
6
CA 03107950 2021-01-27
WO 2020/028692 PCT/US2019/044712
combination thereof The length of the cord 12 may also be provided to be 24
inches to 25
inches in length for smaller exercise devices 10 intended for smaller users,
such as children.
[0022] Testing of the exercise device 10 has demonstrated a number of
advantages to having
relatively high pad 16 to cord 12 length ratios, such as disclosed above. For
example, these
ratios allow the pads 16 to cover greater amounts of the sleeve 14 and reduce
the incidents of
engagement between the cord 12 or sleeve 14 and the user's skin when using the
exercise device
10. The ratios further help to create earlier resistance in the range of
motion during the
exercises. Where shorter pads 16 are used, the shape of the pads 16 tend to be
circular, but
longer pads 16 tend to be straighter. This helps provide tension earlier in
the range of motion
when the exercise device 10 is positioned around the ankles or thighs. The
earlier resistance
helps the pads 16 stay in place when using the exercise device 10.
[0023] Compared to fabric hip exercise devices, embodiments of the exercise
device 10
provide variable resistance. Most of the fabric hip exercise devices are
capable of a single
resistance. The only way to increase the resistance of the fabric hip exercise
devices is to make
the circumference of the device smaller or larger. The exercise device 10 is
also easily cleaned
after each use. This is one of the biggest issues in commercial settings. In
various embodiments,
the pads 16 are made of a high density closed cell foam. This allows the pads
16 to be cleaned
by wiping the pads 16 with a towel; whereas, fabric exercise deices require
laundering.
Moreover, with the exercise device 10, the length to tension relationship is
improved because the
fully stretched resistance is closer to the starting resistance (before being
stretched). Elongation
(how far the elastic member will stretch) is also improved.
[0024] Compared to latex products, embodiments of the exercise device 10
provides greater
durability. The biggest complaint with latex bands is that they are not
durable. In an outdoor
setting, most such devices don't last two weeks. UV rays break down latex. In
embodiments of
the exercise device 10, the sleeve 14 covers the latex tubing, which allows
for outdoor use. The
sleeve 14 also protects the latex tube from abrasions, cuts and other foreign
substances.
7
CA 03107950 2021-01-27
WO 2020/028692 PCT/US2019/044712
Additionally, the sleeve 14 serves as a barrier and protects users who are
allergic to latex. Even
where a latex allergy is not an issue, latex can easily irritate the skin.
[0025] Exercise device 10 is an effective tool to help promote proper movement
in the hips and
trunk. Its construction is more durable, comfortable, and sanitary (can easily
be wiped down
between participant uses) than other products. With multiple resistance
options and fluid tension
throughout the range of motion, it can easily be adapted for all strength
levels. The exercise
device 10 can be used to perform a wide variety of exercises that can be
performed nearly
anywhere.
[0026] Although the technology been described in language that is specific to
certain structures,
materials, and methodological steps, it is to be understood that the invention
defined in the
appended claims is not necessarily limited to the specific structures,
materials, and/or steps
described. Rather, the specific aspects and steps are described as forms of
implementing the
claimed invention. Since many embodiments of the invention can be practiced
without departing
from the spirit and scope of the invention, the invention resides in the
claims hereinafter
appended. Unless otherwise indicated, all numbers or expressions, such as
those expressing
dimensions, physical characteristics, etc. used in the specification (other
than the claims) are
understood as modified in all instances by the term "approximately." At the
very least, and not
as an attempt to limit the application of the doctrine of equivalents to the
claims, each numerical
parameter recited in the specification or claims which is modified by the term
"approximately"
should at least be construed in light of the number of recited significant
digits and by applying
ordinary rounding techniques. Moreover, all ranges disclosed herein are to be
understood to
encompass and provide support for claims that recite any and all subranges or
any and all
individual values subsumed therein. For example, a stated range of 1 to 10
should be considered
to include and provide support for claims that recite any and all subranges or
individual values
that are between and/or inclusive of the minimum value of 1 and the maximum
value of 10; that
is, all subranges beginning with a minimum value of 1 or more and ending with
a maximum
8
CA 03107950 2021-01-27
WO 2020/028692 PCT/US2019/044712
value of 10 or less (e.g., 5.5 to 10, 2.34 to 3.56, and so forth) or any
values from 1 to 10 (e.g., 3,
5.8, 9.9994, and so forth).
9