Note: Descriptions are shown in the official language in which they were submitted.
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
1
COATED LITHIUM ION RECHARGEABLE BATTERY ACTIVE
MATERIALS
PRIORITY CLAIM
The present application claims priority under 35 U.S.C. 119(e) to U.S.
Provisional Patent Application Ser. No. 62/725,060, filed August 30, 2018,
titled
"COATED LITHIUM ION RECHARGEABLE BATTERY ACTIVE MATERIALS,"
which is incorporated by reference herein in its entirety.
TECHNICAL FIELD
The disclosure relates to a coated lithium ion rechargeable battery positive
electrode active material, methods of manufacturing such materials, and
lithium ion
rechargeable batteries containing such materials.
BACKGROUND
Many rechargeable batteries contain organic liquid electrolytes. Organic
liquid electrolytes are able to operate over a variety of voltages and have
other
advantages. However, organic liquid electrolytes can react with certain
positive
electrode active materials, generating gasses inside the battery.
Gasses cause
problems in batteries by interrupting the battery structure, often resulting
in a decrease
in battery capacity as the number of charge/discharge cycles increases
(capacity fade)
or failure of the battery to operate at all.
SUMMARY
The present disclosure provides a coated positive electrode active material
particle including an active material having the general chemical formula
AxMyEz(X04)q and a crystal structure, wherein A is an alkali metal or an
alkaline
earth metal, M includes cobalt (Co), E is located in the same structural
location as A
in the crystal structure and is a non-electrochemically active metal, a boron
group
element, or silicon (Si) or any alloys or combinations thereof, X is
phosphorus (P),
sulfur (S), or silicon (Si) or a combination thereof, 0<x1, y>0, z >0, q>0,
and the
relative values of x, y, z, and q are such that the general chemical formula
is charge
balanced. The coated positive electrode active material particle also includes
a
coating including A1203, ZrO2, TiO2, ZnO, B203, Mg02, La202, LiF and any
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
2
combinations thereof or LiM11304, where M' is Fe, Cr, Mn, Ni, V, or any alloys
or
combinations thereof
The above coated positive electrode active material particle may be further
characterized by one or more of the following additional features, which may
be
combined with one another or any other portion of the description in this
specification, including specific examples, unless clearly mutually exclusive:
i) A may be lithium (Li);
ii) M may further include cobalt (Co) in an alloy or combination with at least
one other electrochemically active metal;
iii-a) the at least one other electrochemically active material may include
iron
(Fe), chromium (Cr), manganese (Mn), nickel (Ni), vanadium (V), or titanium
(Ti);
iii-b) M may be a combination of Co and Fe;
iii-c) M may be a combination of Co and Cr;
iii-d) M may be a combination of Co, Fe, and Cr;
iv) z may be greater than 0;
iv-a) E may be Si;
iv-b) E may be a non-electrochemically active metal;
iv-b-1) the non-electrochemically active metal may be magnesium (Mg),
calcium (Ca) or strontium (Sr), or zinc (Zn), scandium (Sc), or lanthanum
(La), or any
alloys or combinations thereof.
iv-c) E may be a boron group element.
iv-c-1) the boron group element may be aluminum (Al) or gallium (Ga) or a
combination thereof;
v) LiM11304 may include a carbon layer;
vi) the coated positive electrode active material may further include a carbon
layer between the active material and the coating;
vi-a) the carbon layer may be integrally formed with the active material;
vii) the coating may be between and including 0.1 wt% and 20 wt% of the
coated particle;
viii) the active material may be an attritor-mixed active material.
The present disclosure also provides a first method of coating an active
material by applying a coating precursor solution to a particle of active
material and
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
3
heating the particle of active material with the coating precursor solution to
between
300 C and 600 C to form a coating on the active material. The active
material may
have the general chemical formula LixMyEz(X04)q and a crystal structure,
wherein A
is an alkali metal or an alkaline earth metal, M comprises cobalt (Co), E is
located in
the same structural location as A in the crystal structure and is a non-
electrochemically active metal, a boron group element, or silicon (Si) or any
alloys or
combinations thereof, X is phosphorus (P), sulfur (S), or silicon (Si) or a
combination
thereof, 0<x1, y>0, z >0, q>0, and the relative values of x, y, z, and q are
such that
the general chemical formula is charge balanced and the coating precursor
solution
comprises a coating precursor operable to form A1203, ZrO2, TiO2, ZnO, B203,
Mg02,
La202, LiF and any combinations thereof or a LiM1PO4 coating precursor
particle
where M' is Fe, Cr, Mn, Ni, V, or any alloys or combinations thereof.
The above first method may be further characterized by one or more of the
following additional features, which may be combined with one another or any
other
portion of the description in this specification, including specific examples,
unless
clearly mutually exclusive:
i) applying a coating precursor solution may include spray-drying the coating
precursor and the particle of active material;
i-a) spray-drying may include mixing the coating precursor solution and
particles of the active material to form a spray-drying solution and spray-
drying the
spray-drying solution.
ii) applying the coating precursor solution may include a hydrothermal method
including adding particles of the active material to the coating precursor
solution,
maintaining the solution at a hydrothermal coating temperature between and
including
70 C and 90 C, and drying the solution.
ii-a) the hydrothermal method may also include maintaining the solution at
the hydrothermal coating temperature for between and including 10 hours and 30
hours.
iii) the coating precursor solution may include an aqueous solvent;
iv) the coating precursor solution may include a non-aqueous solvent.
v) the coating precursor solution may include a solvent and a coating
precursor solute in a solvent:solute ratio of between 99.9:0.1 and 90:10;
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
4
vi) the coating precursor may include a metal or boron salt;
vi-a) the metal or boron salt may include an organic salt;
vii) heating may occur for a duration of 3-5 hours.
The present disclosure further provides a second method of coating an active
material, the method including combining a coating precursor particle with a
particle
of active material to form a dry unprocessed mixture and subjecting the dry
mixture to
high-speed mixing at between and including 8,000 rpm and 15,000 rpm. The
active
material may has the general chemical formula LixMyEz(X04)q and a crystal
structure,
wherein A is an alkali metal or an alkaline earth metal, M comprises cobalt
(Co), E is
located in the same structural location as A in the crystal structure and is a
non-
electrochemically active metal, a boron group element, or silicon (Si) or any
alloys or
combinations thereof, X is phosphorus (P) or sulfur (S) or a combination
thereof,
y>0, z >0, q>0, and the relative values of x, y, z, and q are such that the
general chemical formula is charge balanced and the coating precursor particle
comprises LiM1PO4, where Ml is Fe, Cr, Mn, Ni, V, or any alloys or
combinations
thereof.
The above second method may be further characterized by one or more of the
following additional features, which may be combined with one another or any
other
portion of the description in this specification, including specific examples,
unless
clearly mutually exclusive:
i) high-speed mixing may occur for between and including 5 minutes and 15
minutes.
The above first and second methods may both be further characterized by one
or more of the following additional features, which may be combined with one
another or any other portion of the description in this specification,
including specific
examples, unless clearly mutually exclusive:
i) A may be lithium (Li);
ii) M may further include cobalt (Co) in an alloy or combination with at least
one other electrochemically active metal;
ii-a) the at least one other electrochemically active material may include
iron
(Fe), chromium (Cr), manganese (Mn), nickel (Ni), vanadium (V), or titanium
(Ti);
ii-b) M may be a combination of Co and Fe;
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
ii-c) M may be a combination of Co and Cr;
ii-d) M may be a combination of Co, Fe, and Cr;
iii) z may be 0.
iii-a) E may be Si;
5 iii-b) E may be a non-electrochemically active metal;
iii-b-1) the non-electrochemically active metal may be magnesium (Mg),
calcium (Ca) or strontium (Sr), or zinc (Zn), scandium (Sc), or lanthanum
(La), or any
alloys or combinations thereof;
iii-c) E may be a boron group element;
iii-c-1) the boron group element may be aluminum (Al) or gallium (Ga) or a
combination thereof;
iii-d) X may be P.
iii-e) X may be S.
iii-f) X may be Si.
iv) LiM1PO4 may include a carbon layer;
v) the coated particle may have a carbon layer may between the active
material and the coating;
v-a) the carbon layer may be integrally formed with the active material;
vi) the coating may be between and including 0.1 wt% and 20 wt% of the
coated particle;
vii) the method may further include an attritor-mixing method to form the
active material, the attritor-mixing method including attritor-mixing
precursors of the
active material to form active material precursor particles having an average
size and
heating the stoichiometric amounts of the active material precursors to at
least a
temperature for at least a duration of time to form the active material.
vii-a) the active material precursors may include at least one hydroxide,
alkali
metal phosphate, non-metal phosphate, metal oxide, acetate, oxalate, or
carbonate;
vii-a-1) the hydroxide may include at least one of Li0H, Co(OH)2 Al(OH)3;
vii-a-2) the alkali metal phosphate may include at least one of LiH2PO4 or
Li2HPO4;
vii-a-3) the non-metal phosphate may include at least one of NH4H2PO4 or
(NH4)2HPO4;
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
6
vii-a-4) the metal oxide may include at least one of Cr203, CaO, Mg0, Sr0,
A1203, Ga203, Ti02, ZnO, Sc203, La203 or Zr02;
vii-a-5) the acetate may include Si(00CCH3)4;
vii-a-6) the oxalate may include FeC204,NiC204 or CoC204;
vii-a-7) the carbonate may include Li2CO3, MnCO3, CoCO3 or NiCO3,
vii-b) attritor-mixing may include placing balls and the active material
precursors in an attritor in a set w:w ratio;
vii-c) attritor-mixing may include placing a total volume of balls and active
material precursors in an attritor container that is no more than 75% of a
total volume
of the attritor container;
vii-d) attritor-mixing may occur until a particle size plateau is reached
vii-e) attritor-mixing may occur for no more than 10% longer than the duration
at which the particle size plateau is reached;
vii-f) attritor-mixing may occur for a duration of time sufficient to result
in a
yield in an active material yield plateau;
vii-g) attritor-mixing may occur for no more than 10% longer than a duration
of time sufficient to result in a yield in an active material yield plateau;
vii-h) attritor-mixing may occur for a duration of time sufficient to result
in an
active material capacity plateau;
vi-i) attritor-mixing may occur for no more than 10% longer than a duration
of time sufficient to result in an active material capacity plateau;
vii-j) attritor-mixing may occur for a mixing duration of time between and
including 10 hours and 12 hours.
vii-k) the active material precursor particles may have an average particle
size
of between and including 1 p.m and 700 m;
vii-1) the attritor-mixing method may also include filtering the active
material
precursor particles to remove particles over a set size;
vii-m) A is Li, M is Co or a Co alloy or combination, and X is P, and the
temperature is between and including 600 C and 800 C;
vii-n) heating during attritor-mixing may occur for a heating duration of time
between and including 6 hours and 24 hours;
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
7
vii-o) the attritor-mixing method may have a yield of between least 95% and
99.9%.
vii-p) the active material may have a purity of between 95% and 99.9%.
Any of the above methods may be used to prepare any of the above coated
positive electrode active materials unless clearly mutually exclusive.
The disclosure further provides an alkali metal or alkaline earth metal
rechargeable battery including an electrolyte including a liquid and an alkali
metal salt
or alkaline earth metal salt, a negative electrode including a surface that
contacts the
electrolyte, the negative electrode further including a negative electrode
active
material, a positive electrode including a surface that contacts the
electrolyte, the
positive electrode further including any positive electrode active material
described
above or elsewhere herein or prepared according to any of the above methods or
methods described elsewhere herein, an electronically insulative separator
between
the positive electrode and the negative electrode, and a casing surrounding
the
.. electrolyte, electrodes, and separator.
The above battery may be further characterized by one or more of the
following additional features, which may be combined with one another or any
other
portion of the description in this specification, including specific examples,
unless
clearly mutually exclusive:
i) the battery may further include a pressure application system that applies
pressure to at least a portion of the electrode surfaces contacting the
electrolyte;
i-a) the pressure application system may include a seal internal to the
battery
and a pressure application structure;
i-b) the pressure application structure may include plates and a clamp or
screw;
i-c) the pressure application structure may include a pressure bladder;
i-d) the battery may further include a gas relocation area;
i-e) the pressure application structure may apply pressure to at least 90% the
surfaces of the electrodes contacting the electrolyte;
i-f) pressure applied by the pressure application structure may not vary by
more than 5% between any points where the pressure is applied;
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
8
i-g) the pressure applied by the pressure application structure may be between
50 psi and 90 psi;
i-h) the pressure applied by the pressure application structure may be between
70 psi and 75 psi;
i-i) the electrolyte may include an organic liquid;
i-i-1) the organic liquid may include an organic carbonate;
i-i-l-A) the organic carbonate includes ethylene carbonate (EC) with dimethyl
carbonate (DMC), propylene carbonate (PC), diethyl carbonate (DEC), ethyl
methyl
carbonate (EMC), or any combinations thereof
i-i-2) the electrolyte may include a lithium salt;
i-i-2-A) the lithium salt may include LiPF6, LiBF4, lithium bisoxalato borate
(LiBOB), lithium difluorooxalato borate (LiDFOB), and lithium
trifluorosulfonylimide (LiTFSI), lithium perchlorate (LiC104), lithium
bis(fluorosulfonyl)imide (LiFSI), or any combinations thereof;
i-j) the electrolyte may include an ionic liquid;
i-j-1) the ionic liquid may include a nitrogen (N)-based ionic liquid;
i-j-1-A) the N-based ionic liquid may include an ammonium ionic liquid;
i-j-1-A-*) the ammonium ionic liquid may include NN-diethyl-N-methyl-N-
(2-methoxyethyl) ammonium;
i-j-l-B) the N-based ionic liquid may include an imidazolium ionic liquid;
i-j-l-B-*) the imidazolium ionic liquid may include ethyl methyl imidazolium
(EMIm), methyl propyl imidazolium, (PMIm), butyl methyl imidazolium (BMIm), or
1-ethy1-2,3-dimethylimidazolium, or any combinations thereof;
i-j-l-C) the N-based ionic liquids may include a piperidinium ionic liquid;
i-j-l-C-*) the piperidinium ionic liquid may include ethyl methyl piperidinium
(EMPip), methyl propyl piperidinium (PMPip), or butyl methyl piperidinium
(BMPip), or any combinations thereof
i-j-l-D) the N-based ionic liquid may include a pyrrolidinium ionic liquid;
i-j-l-D-*) the pyrrolidinium ionic liquid may include ethyl methyl
pyrrolidinium (EMPyr), methyl propyl pyrrolidinium (PMPyr), or butyl methyl
pyrrolidinium (BMPyr), or any combinations thereof;
i-j-2) the ionic liquid may include a phosphorus (P)-based ionic liquid;
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
9
i-j-2-A) the P-based ionic liquid may include a phosphonium ionic liquid;
i-j-2-A-*) the phosphonium ionic liquid includes PR3R' phosphonium,
wherein R is butyl, hexyl, or cyclohexyl, and R' is methyl or (CH2)13CH3, or
tributyl(methyl)phosphonium tosylate, or any combinations thereof;
x-j-3) the alkali metal salt my include LiF2NO4S2, LiCF2S03, LiNS02(F3)2,
LiNS02(F2CF3)2, LiC2F6NO4S2, or any combinations thereof.
ii) the negative electrode active material may include metal, carbon, a
lithium
or sodium titanate or niobate, or a lithium or sodium alloy.
BRIEF DESCRIPTION OF THE DRAWINGS
Embodiments of the present disclosure may be further understood through
reference
to the attached figures, in which like numerals represent like features. The
patent or
application file contains at least one drawing executed in color. Copies of
this patent
or patent application publication with color drawings will be provided by the
Office
upon request and payment of the necessary fee.
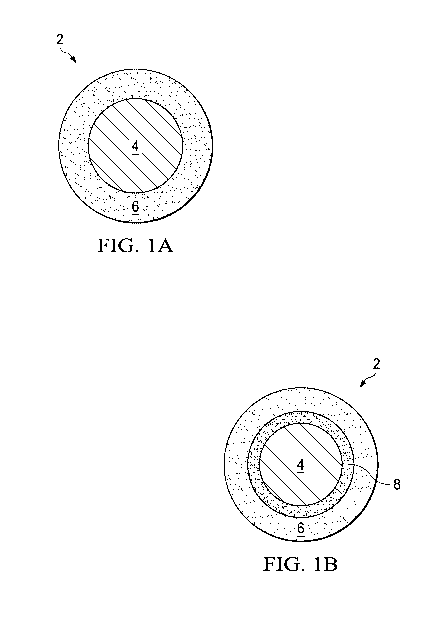
FIG. 1A is a schematic cross-sectional drawing of a particle of coated lithium
ion positive electrode active material.
FIG. 1B is a schematic cross-sectional drawing of a particle of coated lithium
ion positive electrode active material having a carbon layer.
FIG. 2 is an X-ray diffraction (XRD) profile of a multiple-substituted lithium
cobalt phosphate ( LiCoo s2Feo o976Cro o488Sio oo976PO4) positive electrode
active
material. Typical XRD patterns of the final product with trace of impurity are
marked
by *.
FIG. 3 is a representative energy-dispersive X-ray spectroscopy (EDX)
analysis of an iron (Fe), silicon (Si) and chromium (Cr)-containing positive
electrode
active material showing trace Si and Cr agglomeration. The scale bar in all
images is
10 pm.
FIG. 4 is a representative cross-sectional energy-dispersive X-ray
spectroscopy (EDX) analysis of a Fe, Cr and Si-containing positive electrode
active
material showing trace Cr impurities. The scale bar in the leftmost image is
10 um.
The scale bars in all other images is 5 p.m.
FIG. 5A and FIG. 5B are a pair of representative scanning electron
microscope (SEM) image of particles of positive electrode active material. The
scale
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
bar in FIG. 5A is 20 um. The scale bar in FIG. 5B image is 5 p.m.
FIG. 6 is a flow chart of a method of attritor-mixing precursors and heating
to
form an active material.
FIG. 7 is a schematic partially cross-sectional elevation drawing of an
attritor
5 suitable for use in the present disclosure.
FIG. 8 is a graph showing the effect of ball:precursor w:w ratio during
attritor
mixing on capacity of active material formed from the attritor-mixed
precursors.
FIG. 9A is a graph showing particle size distribution after attritor-mixing
for 6
hours with an 8:1 ball:precursor w:w ratio.
10 FIG. 9B is a graph showing the particle size distribution of the same
precursor
mixture as in FIG. 9A after attritor-mixing for 12 hours with an 8:1
ball:precursor
w:w ratio.
FIG. 10A is a flow chart of a coating method for forming a coated particle.
FIG. 10B is a flow chart of an alternative coating method for forming a coated
particle.
FIG. 11 is a battery including a coated active material according to the
present
disclosure.
FIG. 12 is a schematic cross-sectional drawing of a battery according to the
present disclosure.
FIG. 13 is a schematic drawing of a bottom portion of a battery according to
the present disclosure.
FIG. 14 is a photograph of a side of a screw-pressure battery according to the
present disclosure.
FIG. 15 is photograph of a side of an air-pressure battery according to the
present disclosure.
FIG. 16 is a graph showing cycling stability of batteries containing coated
positive electrode active materials according to the present disclosure and a
comparative uncoated positive electrode active material.
FIG. 17 is a scanning electron microscope (SEM) image of a lOwt% c-
LiFePO4-coated LiCoo.82Feo.o976Cro.0488Sio.00976PO4 positive electrode active
material.
LiFePO4 and LiCoo.82Feo.o976Cro.o488Sio.00976PO4 particles are labeled.
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
11
FIG. 18 is a graph of discharge capacity for LiF-coated
LiCoo.82Feo.o976Cro.o488Sio.00976PO4 at different wt% of LiF.
FIG. 19 is an XRD profile of a LiCoo.82Feo.o976Cro.o488Sio.00976PO4 active
material after 6 hours or 12 hours of attritor-mixing with a 6:1
ball:precursor w:w
.. ratio.
FIG. 20 is an XRD profile of a LiCoo.82Feo.o976Cro.o488Sio.00976PO4 active
material after 12 hours of attritor-mixing with an 8:1 ball:precursor w:w
ratio.
FIG. 21 is an XRD profile of a LiCoo.82Feo.o976Cro.o488Sio.00976PO4 active
material after 12 hours of attritor-mixing a with a 10:1 ball:precursor w:w
ratio.
FIG. 22 is an XRD profile of a LiCoo.82Feo.o976Cro.o488Sio.00976PO4 active
material after 12 hours of attritor-mixing a with a 12:1 ball:precursor w:w
ratio.
FIG. 23 is an XRD profile of a LiCoo.82Feo.o976Cro.o488Sio.00976PO4 active
material after 12 hours of attritor-mixing a with a 14:1 ball:precursor w:w
ratio.
DETAILED DESCRIPTION
The disclosure relates to a coated lithium ion rechargeable battery positive
electrode active material, methods of manufacturing such a material, and
lithium ion
rechargeable batteries containing such a material. The coating reduces
exposure of
the positive electrode active material to the electrolyte and thereby reduces
generation
of gasses in the battery.
Referring now to FIGs. 1A and 1B, a coated particle 2 of lithium ion
rechargeable battery positive electrode active material 4 has a coating 6. In
FIG. 1B,
a carbon layer 8 is present between the active material 4 and the coating 6.
Active Material
The active material 4 may have the general formula Ax MyEzPO4 and a crystal
structure, where 0<x1, y>0, and z>0, A is an alkali metal or an alkaline earth
metal,
M is cobalt (Co) alone or in an alloy or combination with another
electrochemically
active metal and E, when z>0, is located in the same structural location as A
in the
crystal structure and is a non-electrochemically active metal or a boron group
element
(Group 13, Group III), or Si, or any combinations or alloys thereof
The alkali metal (Group 1, Group I metal) in the active material may be
lithium (Li) sodium (Na), or potassium (K). The alkaline earth metal (Group 2,
Group IIA metal) may be magnesium (Mg) or calcium (Ca). The alkali metal or
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
12
alkaline earth metal may be present as a mobile cation or able to form a
mobile cation,
such as lithium ion (Lit), sodium ion (Nat), potassium ion (10, magnesium ion
(Mg2+), or calcium ion (Ca2+).
The electrochemically active metal is most commonly a transition metal, such
as a Group 4-12 (also referred to as Groups IVB-VIII, TB and JIB) metal.
Particularly
useful transition metals include those that readily exist in more than one
valence state.
Examples include iron (Fe), chromium (Cr), manganese (Mn), nickel (Ni),
vanadium
(V), and titanium (Ti).
The non-electrochemically active metals may affect the electrical or
electrochemical properties of the active material. For example, non-
electrochemically
active metals or boron group element or silicon (Si) may change the operating
voltage
of the active material, or increase the electronic conductivity of active
material
particles, or improve the cycle life or coulombic efficiency of an
electrochemical cell
containing the active material. Suitable non-electrochemically active metals
include
alkaline earth metals (Group 2, Group II metals) such as magnesium (Mg),
calcium
(Ca) or strontium (Sr), or zinc (Zn), scandium (Sc), or lanthanum (La), or any
alloys
or combinations thereof. Suitable boron group elements include aluminum (Al)
or
gallium (Ga) and combinations thereof
The alkali metal or alkaline earth metal, Co and electrochemically active
metal, non-electrochemically active metal or boron group element or Si, and
phosphate are present in relative amounts so that the overall active material
compound
or mixture of compounds is charge balanced. Example active materials include
LiCo0.9Foi11304, Li0.95Coo.85FeftiCrom5PO4, Li0.93Coo.84Fe0.1Crom5SiomPO4, and
LiCoo.82Feo.o976Cro.04885i0.00976PO4.
The active material compound or mixture of compounds are primarily present
in a crystalline, as opposed to an amorphous form, which may be confirmed via
XRD.
In particular, the active material may have an olivine crystal structure
similar to that
of lithium cobalt phosphate (LiCoPO4) regardless of whether other
electrochemically
active metals or non-electrochemically active metal or boron group element or
Si are
present. An example of an XRD pattern sufficient to confirm crystal structure
is
presented in FIG. 2.
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
13
If a carbon layer 8 is present, it may be observed via EDX or SEM. Such a
carbon layer 8 may be integrally formed on at least a portion of the exterior
surface of
a particle of active material 4. For example, portions of the carbon layer
that contact
the exterior surface of the particle of active material 4 may be covalently
bonded to
the active material 4. The carbon layer 8 may be at least 80% elemental carbon
(C).
The carbon layer 8 may be between and including 0.01 wt% to 10 wt% of the
total
coated particle 2.
Typically the active material 4 alone or with an carbon layer 8 will have a
particle size of between and including 1 p.m and 999 m, 1 p.m and 500 m, 1
p.m
and 100 m, 10 [tm and 999 m, 10 p.m and 500 m, or 10 p.m and 100 pm.
Coating
The coating 6 may include an electrochemically inactive material, such as a
metal or boron oxide, particularly A1203, ZrO2, TiO2, ZnO, B203, Mg02, La202,
or a
metal non-oxide, particularly a metal fluoride, such as LiF, and any
combinations
thereof. The coating 6 may also include an electrochemically active material,
such as
a non-cobalt-containing lithium metal phosphate material, particularly
LiM11304,
where M' is Fe, Cr, Mn, Ni, V, or any alloys or combinations thereof, such as
LiFePO4. The electrochemically active coating material may have a carbon layer
similar to the carbon layer 8 that may be present on the active material 4.
The relative amount of coating 6 to overall size of coated particle 2 may vary
depending on the coating used. In addition, there is generally a trade-off
between
specific capacity of a battery containing coated particle 2 and the cycle life
of such a
battery. Non-electrochemically active coatings 6 tend to better cover the
active
material 4 and reduce its reaction with the electrolyte, increasing cycle
life, but such
coatings contribute non-electrochemically active weight to the particle
decreasing
specific capacity of the battery. Electrochemically active coatings 6 may also
participate in an electrochemical reaction and increase the specific capacity
of a
battery containing coated particle 2, depending on the voltage range, leading
to better
performance such as high-rate properties of the battery. The electrochemically
active
coatings may reduce the side reaction between active material 4 and
electrolyte as
well, further increasing cycle life.
In general, coating 6 may be between and including 0.1 wt% and 20 wt%, 0.1
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
14
wt% and 10 wt%, 0.1 wt% and 5 wt%, 0.5 wt% and 20 wt%, 0.5 wt% and 10 wt%,
0.5 wt% and 5 wt%; 1 wt% and 20 wt%, 1 wt% and 10 wt%, or 1 wt% and 5 wt% of
the total coated particle 2.
Methods of Manufacturing a Coated Active Material
Although any active materials 4 as described above may be coated, active
materials 4 produced using an attritor-mixing method may be particularly
useful. An
attritor-mixing method may be usable to produce commercial-level quantities of
active material with no or low levels of impurities.
Active materials produced using attritor-mixing methods may have a purity of
at least 95%, at least 98%, at least 99%, or a purity in a range between and
including
any combinations of these values, as measured by XRD refinement, an example of
which is provided in FIG. 2. Impurities are typically in the form of unreacted
precursors or precursors that have reacted to form compounds other than the
active
material and crystalline impurities in amounts of 1% or greater of a given
crystalline
impurity compound may be detected using XRD. Non-crystalline impurities and
impurities in amounts of less than 1% may be detected using EDX, examples of
which are provided in FIG. 3 and FIG. 4.
Active materials formed by attritor-mixing, when used in an electrochemical
cell, may exhibit stable capacity, with a capacity fade of 50% or less, 40% or
less,
20% or less, 10% or less, 5% or less, 1% or less, or a range between and
including
any combinations of these values over 110 cycles at C/2 as compared to the
capacity
at the tenth cycle at C/2.
Active materials 4 formed by attritor mixing herein may be in the form of
particles that are, on average over the batch of particles, excluding
agglomerates, no
longer than 1 nm, 10 nm, 50 nm, 100 nm, 500 nm, or 999 nm, or any range
between
and including any combination of these values. Such particles are referred to
as
nanoparticles. Active materials formed using attritor-mixing methods may be in
the
form of particles that are, on average over the batch of particles excluding
agglomerates, no longer than 1 p.m, 10 p.m, 50 p.m, 100 p.m, 500 p.m, or 999
p.m, or
any range between and including any combination of these values. Such
particles are
referred to as microparticles.
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
Active materials particle may form agglomerates, in which case any
agglomerate is excluded from the average particle size discussed above.
However,
the agglomerate may itself be a nanoparticle or a microparticle. For example,
the
agglomerate may be a microparticle composed of nanoparticles of active
material.
5 Particle
and agglomerate size may be assessed using scanning electron
microscopy (SEM), an example of which is shown in FIGs. 5A and 5B.
Suitable precursors for use in manufacturing the active material will depend
on the specific active material to be produced. Typically the precursors are
in solid
form, as the methods disclosed herein are solid state manufacturing methods.
Wet
10
precursors or those available as hydrates or containing substantial humidity
may be
dried prior to use in the methods of the present disclosure. Common precursors
include metal hydroxides, such as Li0H, Co(OH)2 and Al(OH)3, alkali metal
phosphates, such as LiH2PO4 or Li2HPO4, alkaline earth metal phosphates, non-
metal
phosphates, such as NH4H2PO4, (NH4)2HPO4, metal oxides, such as Cr203, CaO,
15 Mg0,
Sr0, A1203, Ga203, Ti02, ZnO, 5c203, La203 or Zr02, acetates, such as
Si(00CCH3)4, and oxalates, such as FeC204, NiC204 or CoC204 (which are often
stored as a hydrate, which may be dried before use in the present methods), or
carbonates, such as Li2CO3, MnCO3, CoCO3 or NiCO3.
For active materials that have a carbon layer 8, carbon layer precursors may
also be included in the attritor-mixing methods described herein. Suitable
carbon
layer precursors include elemental carbon or carbon-containing materials, such
as
polymers, that are broken down to form a carbon coating.
Active materials, including those described above, may be manufactured from
precursors, including those described above using solid-state attritor-mixing
methods
that generally include attritor-mixing of at least non-coating precursors,
followed by
heating the mixture.
Attritor-mixing methods, alone or combined with coating methods, may be
used to form at least 1 kg, at least 2 kg, at least 3kg, at least 5 kg, at
least 10 kg, at
least 25 kg, at least 50 kg, at least 100 kg active material, or an amount
between and
including any two of these recited amounts (e.g. between and including lkg and
2 kg,
between and including 1 kg and 3 kg, between and including 1 kg and 5 kg,
between
and including 1 kg and 10kg, between 1 kg and 50 kg, between and including 1
kg
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
16
and 50 kg, between and including 1 kg and 100 kg, between 25 kg and 50 kg) per
batch.
Attritor-mixing methods, prior to particle size filtering, may have a yield of
at
least 80%, at least 85%, or at least 90%, at least 95%, or at least 99%, at
least 99.9%
or an amount between and including any two of these recited amounts per batch.
Yield is measured prior to particle size filtering to exclude effects directly
to the
particle size selected, rather than the active-particle forming reaction and
method.
For active materials having an carbon layer 8 , the carbon layer precursor may
be added prior to attritor-mixing, after attritor-mixing, but before heating,
or after
heating, depending largely on the carbon layer to be formed. Carbon layer
precursors
will typically be added prior to attritor-mixing. One of ordinary skill in the
art, using
the teachings of the present disclosure and, optionally, through conducting a
series of
simple experiments in which different carbon layer precursors are added at
different
stages of the methods, also optionally in different relative amounts, will be
able to
readily determine how to incorporate carbon layer formation steps into the
methods
disclosed herein.
Referring now to FIG. 6, the present disclosure provides an attritor-mixing
method 110 for manufacturing an active material. In step 120, wet or hydrate
precursors are dried. In step 130, precursors that are too large to fit in the
attritor
chamber or to be milled by the attritor are cut to a sufficiently small size.
Steps 120
and 130 may be performed in any order.
In step 140, stoichiometric amounts of precursors that will be attritor-mixed
are placed in the chamber of the attritor and attritor-mixed to form precursor
particles.
Although, typically, all active material precursors will be attritor-mixed,
some
precursors may be added after attritor-mixing.
The attritor used in step 140 may be any suitable attritor. An attritor is a
mixing apparatus having a container, an arm extending from the exterior of the
container through a lid of the container and into the interior of the
container, and at
least one and typically a plurality of paddles in the interior of the
container coupled to
the arm so that when the arm rotates in response to a rotational force applied
outside
of the container, the paddles rotate within the container. If a material is in
the
container, then it will be impacted by the paddles and its size will be
reduced by a
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
17
combination of friction and impact with the paddles or other materials in the
container.
An example attritor 200 suitable for use in methods of the present disclosure
is
depicted in FIG. 7. Attritor 200 includes a container 210, which has a lid
220.
Attritor 200 also includes couple 230, which attaches to an external source of
rotational force, such as a motor. Couple 230 is located at a first end of an
arm 240,
which is located exterior to the container 210. The arm 240 passes through a
guide
250 mounted on the lid 220 and through the lid 220 into the interior of the
container
210. At least one and, as depicted, typically a plurality of paddles 260 are
located in
the interior of the container 210 and are coupled to a portion of the arm 240
also in the
interior of the container 210.
The attritor 200 also includes a plurality of balls 270 (depicted as only two
balls for simplicity).
During operation of the attritor, the balls are also impacted by the paddles
and/or the material and help reduce the size of the precursors.
Balls used in step 140 may be of any size suitable to reduce the precursors to
a
set particle size within a set time. 19 mm diameter balls may work
particularly well,
and 12.7 mm diameter balls may also be suitable.
The balls may be made of any materials that do not react with the precursors
to a degree that reduces yield below 80% or produces impurities in an amount
of more
than 5% total impurities. Suitable materials for the balls include steel,
zirconium, or
tungsten. The balls may have an interior made of a different material with an
exterior
coating of a suitable material.
Although the balls contribute to reduction of precursor size, they also occupy
volume in the attritor chamber that might otherwise be occupied by precursors.
Accordingly, the proportion of balls to total precursors (w:w) may be limited
to the
smallest ratio that still allows an active material having the selected
particle size or
other set property to result from the overall method 110. For example, FIG. 8
shows
a comparison of capacity and ball:total precursors (w:w) such as might be used
to
select the proportion.
The particle size of precursors after attritor-mixing is typically 10 p.m or
less,
50 p.m or less, 100 p.m or less, 500 p.m or less, 600 p.m or less, or 750 p.m
or less and
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
18
any ranges between and including and combinations of these values, (e.g.
between
and including 1 p.m and 10 p.m, between and including 1 p.m and 50pm, between
and
including 10 p.m and 50 pm, between and including 1 p.m and 600 p.m). An
appropriate w:w ratio may vary depending on the precursors used, the size of
the
precursors prior to attritor mixing, the size of the balls, and the attritor
used, but one
of ordinary skill in the art, using the teachings of this disclosure, may
readily
determine the appropriate ball:precursor ratio by simply varying these
parameters
until an acceptable precursor particle size or other set property such as
capacity is
obtained.
The total volume of balls and precursors in the attritor should not have a
volume exceeding that specified by the attritor manufacturer. Typically, the
total
volume of balls and precursors is no more than 75% of the total volume of the
attritor
container, to allow sufficient room for the balls and precursors to move
during
mixing.
For any given set of precursors (at a selected pre-attritor-mixing size),
ball:precursor ratio, ball size, and attritor, there will be a reduction of
average
precursor particle size over time during attritor-mixing until a particle size
plateau is
reached. Once the particle size plateau is reached, any additional duration of
attritor-
mixing will not further reduce the average precursor particle size by more
than 10%,
as compared to the average precursor particle size at the duration of time
when the
particle size plateau is reached. The plateau may also readily be determined
by one of
ordinary skill in the art, using the teachings of this disclosure. Although
attritor-
mixing in step 140 may be continued after the particle size plateau is
reached,
typically step 140 will last only until the particle size plateau is reached,
no more than
10% longer than the duration at which the particle size plateau is reached, or
a
duration between and including these two times. Common mixing times to reach
plateau include 10-12 hours. Examples particle size distributions based on
mixing
duration that may be used to determine when plateau is reached are provided in
FIG.
9A and FIG. 9B.
Properties, such as yield or active material capacity, determined at least in
part
by particle size may also exhibit a plateau with respect to attritor-mixing
duration and
attritor-mixing duration may be set based on such an alternative plateau such
that the
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
19
attritor-mixing duration is only until the plateau is reached, no more than
10% longer
than the duration at which the plateau is reached, or a duration between and
including
these two times.
In some methods, it may be useful to control the temperature within the
attritor
during attritor-mixing. For example, some precursors may be temperature-
sensitive,
or it may be useful to limit reaction of the precursors to for the active
material during
attritor-mixing. If useful, the attritor may further contain a cooling system,
such as an
exterior cooling system or a cooling system located within the container, lid,
arm,
paddles, or any combinations of these. The cooling system may keep the
temperature
below a set temperature during step 140. Alternatively, or in addition, the
precursors
may be cooled prior to attritor-mixing in step 140. Also alternatively, or in
addition,
the attritor may include a thermometer to allow a ready determination of
whether the
precursors exceeded a set temperature during step 140, in which case they may
be
discarded or subjected to a quality control process.
After attritor-mixing in step 140, a stoichiometric amount of any precursors
not subjected to attritor-mixing is added to the attritor-mixed precursor
particles.
Next, in step 150, the attritor-mixed precursor particles are filtered to
exclude
particles above a set size, typically 101.tm, 50 p.m, or 100 p.m.
The filtered precursors are then heated in step 160 for a duration of time to
undergo a chemical reaction and form the active material. The temperature to
which
the precursors are heated may vary depending on the precursors and active
material.
The heating in step 50 may be a simple heating process, in which the
precursors are
heated to a set temperature and maintained at that temperature for the
duration of
time. The heating in step 160 may also be a more complicated, stepped process,
in
which the precursors are heated to one or more temperatures for one or more
times.
The rate at which heating in step 160 occurs may also be controlled to occur
at a
particular degrees per minute and step 160 may even include cooling followed
by
heating in the overall heating process.
For active materials containing lithium, cobalt, and phosphate, the maximum
temperature in heating step 50 may be at least 600 C, particularly between
and
including 600 C and 800 C, and may be attained through temperature increases
of
between 1 C/min and 10 C/min. The heating step may last for at least 6
hours, at
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
least 8 hours, at least 10 hours, or at least 12 hours, at least 18 hours,
least 24 hours
and ranges between and including and combinations of these values particularly
between and including 6 hours and 24 hours. Heating may occur under a reducing
or
inert atmosphere, such as a nitrogen (N2) atmosphere. Heating may be preceded
by a
5 purge at room temperature (25 C) under a reducing or inert atmosphere,
such as a
nitrogen atmosphere, for 1-4 hours, typically 3 hours.
After heating, in step 170 the material is cooled. Cooling may be a simple,
passive cooling process, an active cooling process, or a stepped process. The
material
may be maintained a particular temperatures for a duration of time. The rate
at which
10 cooling occurs may also be controlled to occur at a particular degrees
per minute and
step 170 may even include heating followed by cooling in the overall cooling
process.
The active material is present by the end of the cooling process 170.
Depending on the precursors and active material, the active material may often
be
present even at the end of heating in step 160. In some methods 110, the
heating
15 process 160 and the cooling process 170 may overlap to form one
continuous
heating/cooling process.
Finally, in step 180, the active material is filtered to exclude particles
above a
set size. For example, 25 p.m, 35 p.m, 38 p.m, 40 p.m, 501.tm, or 100 p.m.
It will be understood that attritor-mixing methods may practice only steps 140
20 and 160 (or step 160/170 in place of step 160 if heating and cooling
form one
continuous heating/cooling process). The other steps described in connection
with
method 110 are each independently omittable.
All or part of the steps of method 110 may be carried out in conditions that
limit humidity. For example, all or part of the steps of method 110 may be
carried out
in a dry room or in a water-exclusive atmosphere, such as an inert, hydrogen,
or
nitrogen atmosphere (although, for most active materials, this degree of
precaution is
not needed), or at ambient humidity of less than 25% or less than 10%.
Any active material 4, whether formed by attritor-mixing method 110 or any
other method, may have coating 6 applied using wet coating method 190a
illustrated
in FIG. 10A. In coating method 190a, in step 191, a coating precursor solution
is
formed. The solvent may be an aqueous solution or a non-aqueous solution. For
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
21
example, an alcohol, such as ethanol, may used as the solvent. The
solvent:solute
ratio may vary from 99.9:0.1 to 90:10.
The coating precursor may include particles of the coating material that are
smaller than the particle of active material 4. For example, the coating
precursor may
include particles of LiFePO4 having a carbon layer (c-LiFePO4). If the coating
precursor particles may have a longest average dimension no more than 0.8% or
1%
of the longest average dimension of the particles of active material 4 or
particles of
active material 4 with carbon layer 8.
The coating precursor may also include a compound that forms the coating 6
after heating. For example, the coating precursor may be a metal or boron salt
that
will form part of the coating 6. For example, the coating precursor may be an
organic
salt, such as C9H2103A1, or an inorganic salt, such as Al(NO3)3 if the coating
6 will
include A1203. If an organic compound precursor is used, the carbon and
hydrogen
components burn away in the heating steps that follow.
In step 192, the coating precursor solution is applied to particles of active
material 4 or active material 4 with carbon layer 8.
The precursor may be applied by a spray-drying method in which particles of
the active material are added to the coating precursor solution to form a
spray drying
solution. The spray-drying solution may be mixed prior to spray-drying, for
example
at a temperature between and including 50 C to 70 C or 55 C and 65 C.
Mixing
may occur for between and including 2 and 6 hours or 3 and 4 hours. The spray-
drying solution may be stirred while mixing. After the spray-drying solution
is
mixed, it may be spray-dried, for example at a temperature of between and
including
90 C and 110 C, or at least 100 C for an aqueous solution, or another
temperature
based on the evaporation temperature of the solvent for a non-aqueous
solution.
Spray-drying may take place under a nitrogen (N2) atmosphere.
Alternatively, in a hydrothermal method, the particles may simply be added to
the precursor solution to form a hydrothermal coating solutions which may be
maintained at a hydrothermal coating temperature prior to heating. The
hydrothermal
coating temperature may be between and including 70 C and 90 C or between 75
C
and 85 C. The hydrothermal coating solution may be maintained at the
hydrothermal
coating temperature for between and including 10 hours and 30 hours, 15 hours
and
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
22
25 hours, or 18 hours and 22 hours. The hydrothermal coating solution may be
stirred
during all or part of this time. The hydrothermal coating solution may then be
dried
and the precipitate heated, or drying may occur during heating.
In step 193, the particles with coating precursor are heated to a temperature
of
between and including 300 C and 600 C, 300 C and 500 C, 300 C and 450 C,
350 C and 600 C, 350 C and 500 C, 350 C and 450 C, 400 C and 600 C,
400
C and 500 C, or 400 C and 450 C, particularly 400 C. Heating lasts for
between
and including 3 and 5 hours, particularly 4 hours. The process is generally
not
sensitive to the rate at which temperature is increased during heating, but
the rate may
typically be between 1 C/min and 10 C/min. Heating may occur under a
reducing
or inert atmosphere, such as a nitrogen (N2) atmosphere. During heating,
coating 6
forms to produce coated particle 2.
In step 194, coated particles 2 are cooled, typically through passive cooling.
Any active material 4, whether formed by attritor-mixing method 110 or any
other method, may also have coating 6 applied using dry coating method 190b
illustrated in FIG. 10B. In method 190b, the coating precursor includes
particles of
the coating material that are smaller than the particle of active material 4.
For
example, the coating precursor may include particles of LiFePO4 having a
carbon
layer (c-LiFePO4). If the coating precursor particles may have a longest
average
dimension no more than 0.8% or 1% of the longest average dimension of the
particles
of active material 4 or particles of active material 4 with carbon layer 8.
In method 190b, in step 195 dry particles of the active material and the
coating
precursor are combined to form an unprocessed mixture. In step 196, the
unprocessed
mixture is subjected to high-speed mixing to produce coated particle 2. High-
speed
mixing is typically mixing at between and including 8,000 rpm and 15,000 rpm,
or
between and including 8,000 rpm and 10,000 rpm, such as mixing at 8,000 rpm,
9,000
rpm, or 10,000 rpm. Typical mixing times are between and including 5 minutes
and
15 minutes, 8 minutes and 12 minutes, and 9 minutes and 11 minutes, or 10
minutes.
All or part of the steps of methods 190a and 190b may be carried out in
conditions that limit humidity. For example, all or part of the steps of
methods 190a
and 190b may be carried out in a dry room or in a water-exclusive atmosphere,
such
as an inert, hydrogen, or nitrogen atmosphere (although, for most active
materials, this
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
23
degree of precaution is not needed), or at ambient humidity of less than 25%
or less
than 10%.
Batteries Containing Coated Active Materials
Coated active materials produced using the above method may be used in the
positive electrodes of batteries, such as battery illustrated in FIG. 11. The
battery 50
includes negative electrode (anode) 55, positive electrode (cathode) 60, and
organic
electrolyte 65 and a porous, electronically insulating separator (located in
electrolyte
65) that permits ionic, but not electronic conductivity within the battery
(not shown)
disposed in the organic liquid electrolyte between the negative electrode 55
and the
positive electrode 60.
The negative electrode 55 includes an active material. Suitable negative
electrode active materials include lithium metal, carbon, such as graphite,
lithium or
sodium titanates or niobates, and lithium or sodium alloys. The negative
electrode
may further include binders, conductive additives, and a current collector.
The positive electrode 60 includes a coated active material as disclosed
herein.
The positive electrode may further include any of fluorinated carbonates,
sulfolane
based organic solvents, a binder, conductive additives, and a current
collector.
The electrolyte 65 may include an organic liquid, such as an organic
carbonate, particularly organic carbonates, in particular, ethylene carbonate
(EC) with
dimethyl carbonate (DMC), propylene carbonate (PC), diethyl carbonate (DEC),
or
ethyl methyl carbonate (EMC), and any combinations thereof The electrolyte 65
may include a lithium salt suitable for use with an organic liquid, such as
LiPF6,
LiBF4, lithium bisoxalato borate (LiBOB), lithium difluorooxalato borate
(LiDFOB),
and lithium trifluorosulfonylimide (LiTFSI), lithium perchlorate (LiC104),
lithium
bis(fluorosulfonyl)imide (LiFSI) and any combinations thereof.
Suitable ionic liquids include cationic components that may include nitrogen
(N)-based ionic liquids. N-based ionic liquids include ammonium ionic liquids,
such
as N,N-diethyl-N-methyl-N-(2-methoxyethyl) ammonium. N-based ionic liquids
include imidazolium ionic liquids, such as ethyl methyl imidazolium (EMIm),
methyl
propyl imidazolium, (PMIm), butyl methyl imidazolium (BMIm), and 1-ethy1-2,3-
dimethylimidazolium. N-based ionic liquids further include piperidinium ionic
liquids, such as ethyl methyl piperidinium (EMPip), methyl propyl piperidinium
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
24
(PMPip), and butyl methyl piperidinium (BMPip). N-based ionic liquids
additionally
include pyrrolidinium ionic liquids, such as ethyl methyl pyrrolidinium
(EMPyr),
methyl propyl pyrrolidinium (PMPyr), butyl methyl pyrrolidinium (BMPyr).
Suitable cationic components of ionic liquids also include phosphorus (P)-
based ionic liquids. P-based ionic liquids include phosphonium ionic liquids,
such as
PR3R' phosphonium, where R is butyl, hexyl, or cyclohexyl, and R' is methyl or
(CH2)13CH3, or tributyl(methyl)phosphonium tosylate.
Any cationic components of ionic liquids, in any of those described above,
may be combined in any combinations in batteries of the present disclosure.
Ionic liquids may also include anionic components, in the form of other ionic
liquids, such as bis(fluorsulfonyl)imide-based (FSI) ionic liquids including 1-
ethy1-3-
methylimidazolium-bis(fluorsulfonyl)imide (EMI-F SI) and
N-methyl-N-
propylpyrrolidinium- bi s(fluorsulfonyl)imi de
(Py13-F SI),
bis(trifluoromethane)sulfonimide (TF SI), and
(bis(pentafluoroethanesulfonyl)imide)
(BETI). Anionic components of the ionic liquids may also include BF4 or PF6.
Any anionic components of ionic liquids, in any of those described above,
may be combined in any combinations in batteries of the present disclosure.
Suitable lithium salts for use with ionic liquids include LiF2NO4S2, LiCF2S03,
LiNS02(F3)2, LiNS02(F2CF3)2, or LiC2F6N04 S2, or any combinations thereof.
However, many salts increase the viscosity of the ionic liquid such that the
electrolyte
effectively loses ionic conductivity and the battery does not function well.
This effect
may increase as salt concentration increases. Some salts, such as the commonly
used
LiPF6, simply will not function as an electrolyte in an ionic liquid.
The electrolyte may further include any of a number of co-solvents in any
combinations.
Suitable co-solvents include fluorinated carbonates (FEMC),
fluorinated ethers, such as CF3CH2OCF2CHF2, nitriles, such as succinonitrile
or
adiponitrile, or sulfolane.
The electrolyte may also include any of a number of additives in any
combinations. Suitable additives include trimethylsilyl propanoic acid (TMSP),
trimethylsilyl phosphite (TMSPi), trimethylsilyl boric acid (TMSB),
trimethylboroxine, trimethoxyboroxine, or propane sultone.
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
The battery 55 may have particular structures to help control the formation of
gasses or to minimize the effects of any gasses that do form.
The battery 55 may apply a pressure to at least a portion of the surfaces of
the
electrodes contacting the electrolyte. This pressure is applied over 100% of
the
5 .. surfaces of the electrodes contacting the electrolyte, or over at least
90%, at least
95%, or at least 98% of the surfaces of the electrodes contacting the
electrolyte. The
pressure is sufficient to prevent or decrease the formation of gas in the
battery, or to
cause gas that is formed to move to an area of the battery not between the
surfaces of
the electrodes contacting the electrolyte.
10 In particular, batteries according to the present disclosure may apply a
pressure to the surfaces of the electrodes that is uniform and does not vary
by more
than 5% between any points where pressure is applied. The pressure may be at
least
50 psi, at least 60 psi, at least 70 psi, at least 75 psi at least 80 psi, at
least 90 psi, and
any range between and including any of the foregoing (e.g. between and
including 70
15 .. psi and 75 psi).
Referring now to FIGs. 12-15, an alkali metal or alkaline earth metal
rechargeable battery 50 as described herein may further include a casing 70
sufficient
to house and contain the electrodes 55, 60, the electrolyte 65 and the
separator, and
contacts 75 that, when connected via an electronically conductive connector,
allow
20 electric current to flow between the negative electrode 55 and the
positive electrode
60.
The alkali metal or alkaline earth metal rechargeable battery 50 may further
include a pressure application system that applies pressure to at least a
portion of the
surfaces of the electrodes 55 and 60 contacting the electrolyte 65. Pressure
application
25 systems may include internal seals along with a pressure application
structure, such as
plates (often the casing 70) and clamps, screws, pressure bladders, or other
such
structures that apply pressure to the plates or to the battery casing to
maintain pressure
within the battery. Pressure application systems may maintain pressure in a
sealed
portion of the battery, which likely inhibits the formation of gasses, but
does not cause
.. gasses to migrate once formed. Some batteries 50 may include a gas
relocation area,
to which the pressure application system tends to direct gasses once formed.
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
26
Seals, if present, may be formed from any material that is not reactive with
the
electrolyte, negative electrode, positive electrode, or other battery
components it
contacts. Although the some seal materials may exhibit some minimal
reactivity, the
material may be considered not reactive if its reactivity is sufficiently low
to avoid
seal failure, in an average battery having a given design, over a set number
of cycles,
such as at least 100 cycles, at least 200 cycles, at least 500 cycles, at
least 2000
cycles, at least 5000 cycles, at least 10,000 cycles, or a range between and
including
any combinations of these values, when cycled at C/2.
In addition, some pressure application systems may apply pressure constantly
once assembled. Other pressure application systems may be adaptable to apply
pressure on at set times, such as shortly prior to or during operation of the
battery or
both.
Although FIGs. 12-15 provide some specific pressure application systems,
one of ordinary skill in the art, using the teachings of this disclosure, may
design other
pressure application systems. In addition, although FIGs. 12-15 illustrate
pressure
application systems in use on a single pouch-type cell, a pressure application
system
may be used to apply pressure to multiple cells and cells of any format.
Furthermore,
although FIGs. 12-15 illustrate pressure applications systems in use on flat
cells, they
may be used on curved, bent, or other non-planar cell formats.
In FIGs. 12-14, the pressure application system includes ring seals 80 and
screws 85. This type of pressure application system, as shown, seals a portion
of the
alkali metal or alkaline earth metal rechargeable battery 50 in which the
electrodes 55
and 60 contact the electrolyte 65. The screws 85 apply pressure to the casing
70,
which is in the form of rigid plates. The casing 40 transfers the pressure to
the portion
of the battery 50 inside the ring seals 80, which are located in a groove 90
such that
there is pressure where the electrodes 55 and 60 contact the electrolyte 65
inside the
ring seals 80.
Many alternatives to this example may be envisioned and also used. For
instance, only a single seal may be used, the seal need not be located in a
groove, the
seal may have a shape other than a ring, and pressure applicators other than
screws
may be used.
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
27
In FIG. 15, the pressure application system includes air bladder 95, which
may be inflated to a set pressure that is transferred to the casing 70. As
depicted, this
pressure application system does not contain any seals and will force and
gasses that
do form to gas relocation areas 100, particularly when pressure is newly
applied to the
casing 70. Accordingly, this pressure application system is particularly well-
adapted
to apply pressure shortly before or during battery use or both.
Many alternatives to this example may also be envisioned and used. For
instance, air bladder 95 may be inflated with any other fluid, such as another
gas or a
liquid. The fluid in air bladder 95 may be selected, for example, to provide
insulative
or heat conduction properties.
Although not depicted, other batteries 50 of the present disclosure may attain
a
constant pressure on the electrodes 55 and 60 in contact with the electrolyte
65 simply
by pressurizing the electrolyte 65 when it is added to the battery, then
sealing the
casing 70 in a manner that retains pressure.
Uses of Batteries
Batteries containing coated active materials disclosed herein can be used in
many applications. For example, they may be standard cell format batteries,
such as
coin cells, jelly rolls, or particularly prismatic cells. Batteries disclosed
herein may be
used in portable consumer electronics, such as laptops, phones, notebooks,
handheld
gaming systems, electronic toys, watches, and fitness trackers. Batteries
disclosed
herein may also be used in medical devices, such as defibrillators, heart
monitors,
fetal monitors, and medical carts. Batteries disclosed herein may be used in
vehicles,
such as cars, light trucks, heavy trucks, vans, motorcycles, mopeds, battery-
assisted
bicycles, scooters, boats and ships, piloted aircraft, drone aircraft,
military land
transports, and radio-controlled vehicles. Batteries disclosed herein may also
be used
in grid storage or large scale energy supply applications, such as large grid
storage
units or portable energy supply containers. Batteries disclosed herein may be
used in
tools, such as handheld power tools.
Batteries disclosed herein may be connected in series or in parallel and may
be
used in connection with control or monitoring equipment, such as voltage,
charge, or
temperature monitors, fire suppression equipment, and computers programmed to
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
28
control battery usage or trigger alerts or safety measures if battery
conditions may be
unsafe.
EXAMPLES
The following examples are provided solely to illustrate certain principles
associated with the invention. They are not intended to nor should they be
interpreted
as disclosing or encompassing the entire breath of the invention or any
embodiments
thereof.
Example I: lw t% A1203 coating on LiCoo.82Fe0.0976Cr0.0488S10.00976PO4 by
hydrothermal method
0.8093 g C9H2103A1 was dissolved into 158 g ethanol under magnetic stirring.
Then 20 g LiCoo.82Feo.o976Cro.04885i0.00976PO4 was added and stirred at 60 C
for 4
hours. The mixed solution was then transferred into a 400 ml quartz autoclave
and
maintained at 80 C with continued stirring for 20 hours. After drying, the
precipitate
was heated at 350 C for 12 hours under N2 and naturally cooled. iwt% A1203
coated
positive electrode active material was produced.
The same process was repeated without stirring while the mixed solution.
Example 2: lwtY9 A1203 coating on LiCoo.82Feo.o976Cro.o488Sio.00976PO4 by
spray dray
method
0.8093 g C9H2103A1 was dissolved into 158 g ethanol under magnetic stirring.
Then 20 g LiCoo.82Feo.o976Cro.04885i0.00976PO4 was added and stirred at 60 C
for 4
hours. The mixed solution was then spray dried at 100 C under N2, followed by
heating the dry mixture at 350 C for 12 hours under N2 and naturally cooled.
lwt%
A1203 coated positive electrode material was produced.
Example 3: Comparative cycling stability
Coin-type half cells were prepared using 1 wt% positive electrode active
material from Examples 1 and 2 or uncoated
LiCoo.82Feo.o976Cro.04885i0.00976PO4 as a
comparison. Cycling stability results are presented in FIG. 16. All coated
materials
showed improved cycling stability as compared to the uncoated material.
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
29
Example 4: 1 Owt% c-LiFePO4 coating on LiCoo.82Feo.o976Cro.o488Sio.00976PO4 by
high
speed dry mixing method
2.22 g c-LiFePO4 particles (average diameter 300 nm) and 20 g
LiCoo.82Feo.o976Cro.o488Sio.00976PO4 having an average diameter of 38 p.m were
dry
mixed in a 50 ml mini NOBILTATm dry mill (NOB-130) (Hosokawa Micron Corp.,
Japan) at 9000 rpm for 10 min. FIG. 17 is a representative SEM image of the 10
wt%
c-LiFePO4-coated LiCoo.82Feo.o976Cro.o488Sio.00976PO4 obtained.
Example 5: 4wt% LiF coating on LiCoo.82Feo.o976Cro.o488Sio.00976PO4 by
hydrothermal
method with continued stirring
0.2572 g LiF and 6.4311 g LiCoo.82Feo.o976Cro.o488Sio.00976PO4 were mixed in
267 ml deionized water for 1 hour before transferring into a 400 ml quartz
autoclave
and maintained at 200 C with continued stirring for 15 hours. After cooling,
the
precipitate was centrifuged and washed three times with 150 ml deionized water
and
then washed and centrifuged in 150 ml ethanol. The residual powders were air
dried
at 80 C in air. The same procedure was repeated with different percentages of
LiF
from lwt% to 4wt%. FIG. 18 shows that the discharge capacity increased with
increasing LiF up to 4 wt%. Similar plateaus are expected with other coatings.
Example 6: Attritor-Mixed LiCoo.82Feo.o976Cro.o488Sio.00976P0 4 (6.1 ratio)
930 g of LiH2PO4, 675 g of Co(OH)2, 160 g of FeC204.2H20, 28.5 g of Cr203,
23 g of Cr(00CCH3)3, and 76.3 g of acetylene black having dimensions of less
than
500 1.tm were pre-dried at 120 C overnight under vacuum and then placed in an
attritor having container volume of 9.5 L, 11.3 kg of steel balls (6:1
ball:precursor
w:w ratio) with diameter of 19 mm were added. The attritor was operated at 400
rpm
for 6-12 hours. Attritor-mixed precursors were transferred to an oven then
heated to
700 C for 12 hours under N2 and naturally cooled in the oven. After heat
treatment,
about 1.4 kg of final product was obtained and then filtered through a 38 p.m
sieve.
XRD analysis of the resulting material is presented in FIG. 19. The XRD data
confirm that active material having the same structure as LiCoPO4 was produced
even
after only 6 hours of mixing.
Example 7: Attritor-Mixed LiCoo.82Feo.0976Cr0.0488S10.00976PO4 (8:1 ratio)
723 g of LiH2PO4, 525 g of Co(OH)2, 122 g of FeC204.2H20, 22.2 g of Cr203,
17.9 g of Cr(00CCH3)3, and 59.4 g of acetylene black having dimensions of less
than
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
5001.tm were firstly pre-dried at 120 C overnight under vacuum and then placed
in an
attritor having container volume of 9.5 L, 11.8 kg of steel balls (8:1
ball:precursor
w:w ratio) with diameter of 19 mm were added. The attritor was operated at 400
rpm
for 12 hours. Attritor-mixed precursors were transferred to an oven then
heated to
5 700 C for 12 hours under N2 and naturally cooled in the oven. After heat
treatment,
about 1.1 kg of final product was obtained and then filtered through a 38 p.m
sieve.
XRD analysis of the resulting material is presented in FIG. 20. The XRD data
confirm that active material having the same structure as LiCoPO4 was
produced.
Example 8: Attritor-Mixed LiCoo.82Feo.0976Cr0.0488S10.00976PO4 (1O.1 ratio)
10 578 g of LiH2PO4, 420 g of Co(OH)2, 97.6 g of FeC204.2H20, 17.7 g of
Cr203, 14.3 g of Cr(00CCH3)3, and 47.5 g of acetylene black having dimensions
of
less than 500 1.tm were firstly pre-dried at 120 C overnight under vacuum and
then
placed in an attritor having container volume of 9.5 L, 11.8 kg of steel balls
(10:1
ball:precursor w:w ratio) with diameter of 19 mm were added. The attritor was
15 operated at 400 rpm for 12 hours. Attritor-mixed precursors were
transferred to an
oven then heated to 700 C for 12 hours under N2 and naturally cooled in the
oven.
After heat treatment, about 0.9 kg of final product was obtained and then
filtered
through a 38 p.m sieve. XRD analysis of the resulting material is presented in
FIG.
21. The XRD data confirm that active material having the same structure as
LiCoPO4
20 produced.
Example 9: Attritor-Mixed LiCoo.82Feo.0976Cr0.0488S10.00976PO4 (1 2:1 ratio)
483 g of LiH2PO4, 351 g of Co(OH)2, 81.5 g of FeC204.2H20, 14.8 g of
Cr203, 12.0 g of Cr(00CCH3)3, and 39.8 g of acetylene black having dimensions
of
less than 500 1.tm were firstly pre-dried at 120 C overnight under vacuum and
then
25 .. placed in an attritor having container volume of 9.5 L, 11.8 kg of steel
balls (12:1
ball:precursor w:w ratio) with diameter of 19 mm were added. The attritor was
operated at 400 rpm for 12 hours. Attritor-mixed precursors were transferred
to an
oven then heated to 700 C for 12 hours under N2 and naturally cooled in the
oven.
After heat treatment, about 0.73 kg of final product was obtained and then
filtered
30 through a 38 p.m sieve. XRD analysis of the resulting material is
presented in FIG.
22. The XRD data confirm that active material having the same structure as
LiCoPO4
was produced.
CA 03109525 2021-02-11
WO 2020/047228 PCT/US2019/048768
31
Example 10: Attritor-Mixed LiCoo.82Feo.0976Cr0.0488S10.00976PO4 (14:1 ratio)
413 g of LiH2PO4, 301 g of Co(OH)2, 70 g of FeC204.2H20, 12.7 g of Cr203,
10.3 g of Cr(00CCH3)3, and 34 g of acetylene black having dimensions of less
than
5001.tm were firstly pre-dried at 120 C overnight under vacuum and then placed
in an
attritor having container volume of 9.5 L, 11.8 kg of steel balls (14:1
ball:precursor
w:w ratio) with diameter of 19 mm were added. The attritor was operated at 400
rpm
for 12 hours. Attritor-mixed precursors were transferred to an oven then
heated to 700
C for 12 hours under N2 and naturally cooled in the oven. After heat
treatment, about
0.62 kg of final product was obtained and then filtered through a 38 p.m
sieve. XRD
analysis of the resulting material is presented in FIG. 23. The XRD data
confirm that
active material having the same structure of LiCoPO4 was produced.
The above disclosed subject matter is to be considered illustrative, and not
restrictive, and the appended claims are intended to cover all such
modifications,
enhancements, and other embodiments which fall within the true spirit and
scope of
the present disclosure. Thus, to the maximum extent allowed by law, the scope
of the
present disclosure is to be determined by the broadest permissible
interpretation of the
following claims and their equivalents, and shall not be restricted or limited
by the
foregoing detailed description.