Note: Descriptions are shown in the official language in which they were submitted.
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
TITLE: FLASH CHEMICAL IONIZING PYROLYSIS OF HYDROCARBONS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application is a non-provisional of and claims the benefit of and
priority to US Ser.
No. 62/750,708, filed October 25, 2018. The priority document is incorporated
by reference.
BACKGROUND
[0002] The crude oil refining industry is ever in need of more efficient
and/or improved refining
techniques to obtain products from petroleum. Many crudes, including heavy
crude oil and many
crudes with a high "resid" yield from distillation, are difficult to refine
and have poor conversion
of the heavier hydrocarbon fractions, especially asphaltenes, to valuable
products. In a typical
refinery process, the crude must be washed with water to remove salts and
dehydrated in advance
of atmospheric and vacuum distillation. Distillation recovers the lighter,
valuable fractions of the
oil, e.g., butane and lighter products, gasoline blending components, naphtha,
kerosene, jet fuel,
and distillates, e.g., diesel and heating oil. The heavier components such as
medium and heavy
weight gas oil may be processed in cracking and/or alkylation units to obtain
LPG, gasoline, jet
fuel, diesel fuel, etc., whereas the resid, representing the heaviest
components such as resins and
asphaltene, may be processed in a coker to obtain coke and coker gas oil
and/or used as asphalt
base. Some of the heavier components may conventionally contain a small amount
of lube oil base
stock, which are relatively low viscosity high-carbon oils, however, the
conventional yields of base
stocks from petroleum are quite low, typically 0.5 ¨ 1 volume percent of the
crude oil. Processing
excessive amounts of resid such as in a delayed coker is undesirable and often
not economical.
[0003] The blending optimization of crude oils has been used in refinery
operations to increase
the refined margins and commercial value. For example, Li et al.,
"Distillation Yields and
Properties from Blending Crude Oils: Maxila and Cabinda Crude Oils, Maxila and
Daqing Crude
Oils," Energy & Fuels (2007) 2/ (2), 1145-1150 (DOT: 10.1021/ef060316d),
discloses the
optimized blending ratio was 3:7 for Maxila and Cabinda or Daqin crude oils,
and the distillation
yields (<520 C) were higher than theoretical. Demirbas et al., "Optimization
of crude oil refining
products to valuable fuel blends," Petroleum Science and Technology, 35:4, 406-
412, (2017) DOT: 10.1080/10916466.2016.1261162, discloses simulation software,
such as linear
programming modeling, to estimate and optimize the blending of crude oils,
especially cheaper
crude oils.
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[0004] Conversion of heavy crude fractions to lighter ones often requires
expensive catalysts that
need recovery, regeneration, and recycle to be economic. Moreover, expensive
catalysts may
require pretreatment of the feedstock to ensure catalyst poisons like sulfur
are removed.
Conversion is generally a downstream process, often applied to the least
possible quantity of
material after the more valuable, easily recoverable hydrocarbon fractions
have been recovered.
Conversion processes often need to operate at high pressure, with the addition
of external
hydrogen, and/or with long residence times, to maximize conversion and
minimize capital costs.
[0005] Frequently, the "upgraded" products are of poor quality and may still
require blending with
more valuable petroleum fractions, and even then, the blended products are
often only suitable for
use as fuel oil. In some instances, the heavier fractions and resid have been
simply disposed of,
and many places in the world are overrun with stores of such material that are
difficult to
economically process. The main product obtained from the resid is coke, which
often has low
value and entails difficult processing and handling operations. Hence,
refineries have a strong
incentive to minimize resid yields and coke production.
[0006] My earlier patent, US 10,336,946 B2, discloses a process for upgrading
heavy oil
comprising feeding to a reactor an emulsion of 100 parts by weight heavy oil,
5-100 parts by
weight water, and 1-20 parts by weight solid particulates comprising a mineral
support and an
oxide or acid addition salt of a Group 3 ¨ 16 metal, e.g., FeCl3 on NaCl-
treated clay, and spraying
the feed mixture in the reactor at a high temperature and low pressure.
Further improvements in
liquid oil yield and quality, especially in the conversion of asphaltenes to
saturates, especially
isomerates, and aromatics as reflected in a SARA analysis, are desired.
[00071 As reported in Amani et al., J Pet Environ Biotechnol 2017, 8:3 DOT:
10.4172/2157-
7463.1000330, in the refining of crude oil, great pains are taken in
pretreating the crude to remove
entrained water and salt before distillation. Sometimes the water and oil are
in the form of an
emulsion or rag that can be exceedingly difficult to break. Large sums are
spent to dewater and
desalt crude oil. Additionally, the crude oil is typically pre-heated prior to
distillation, but this must
be done very slowly and carefully to avoid forming coke or other deposits on
the heat transfer
surfaces that can result in fouling, especially in the case of heavy and/or
highly viscous crudes.
The industry is ever in search of ways to avoid or reduce the problems and
costs incident to
pretreating and preheating crude oil.
- 2 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[0008] Sulfur is an undesirable crude oil contaminant. Sour crude contains
more than 0.5 wt%
sulfur. Crude oil stabilization can remove some E125 before refining, but
organic sulfides generally
build up during refining and are removed downstream with the higher-boiling
constituents. There
is a need in the art for better ways to remove sulfides from crude oil. An
upstream pretreatment
method would be especially advantageous, so that sulfur could be removed to
provide a lower
level of sulfur in the higher-boiling, downstream refining products.
[0009] It is known from Hancsok, Jena et al., Importance of Isoparaffins in
the Crude Oil Refining
Industry, Chemical Engineering Transactions, 11, 41-47 (2007), that isomerates
such as
isoparaffins have the most advantageous performance properties in gasoline,
diesel fuel, and base
oils. However, isomerates are usually made in exacting downstream processes
such as benzene
saturating isomerization, catalytic hydrodewaxing of gas oils, selective
isomerization of
lubricating base oils, and so on. The industry would benefit from an
inexpensive way to distill or
otherwise process crude oil in such a manner to increase isomerate yields.
[00101 There remains a need for more efficient techniques and systems to
refine and process
petroleum and other hydrocarbons with ever higher yields of lighter, higher-
value hydrocarbon
products, while reducing the amount of resid and coke that must be handled. A
solution would
preferably be an upstream process to treat crude oil; minimize asphaltene and
coke yields; improve
saturates and/or aromatics yields; improve the quality of the saturates with
increased isomerates
production; improve lube oil base stock yields; minimize end product blending
requirements;
employ mild pressure conditions with a short residence time and high
throughput using
inexpensive chemical additives; reduce the need for feedstock pretreatment or
conditioning to
remove catalyst poisons; reduce the need for dewatering and/or desalting;
facilitate crude pre-
heating by minimizing fouling in the pre-heaters; and/or avoid adding
hydrogen.
SUMMARY
[00111 The present invention discloses a process applicant refers to herein as
"flash chemical
ionizing pyrolysis" or FCIP, and a liquid ionizing pyrolyzate or LIP produced
by the process. FCIP
can be used as a method to pretreat crude oil, optionally without dewatering,
to convert asphaltenes
from the crude, and form a resulting LIP with a reduced sulfide content,
increased isomerates
content, and other improvements detailed hereinbelow.
[0012] It has been quite unexpectedly found that, when the LIP is blended in a
relatively small
proportion with another oil stock comprising asphaltenes and the LIP blend is
thermally processed,
- 3 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
e.g., by distillation in an otherwise conventional manner, the amount of
valuable liquid oil products
that is recovered from the blends is substantially increased, whereas the
resid from the oil stock is
rather substantially reduced. Moreover, the resid has a surprisingly low
Conradson carbon residue,
and a viscosity ¨ it is readily pourable at 50 C ¨ suggesting a high lube oil
content. The LIP can
be used as a blend component either in a "front-end" process for crude oil
prior to or in conjunction
with distillation, or in a downstream process to upgrade a stream comprising
heavy gas oil, resins,
asphaltenes, resid, etc. When the LIP-modified feedstock is thermally
processed, such as in
atmospheric or vacuum distillation, or in FCIP, there is an unexpectedly low
resid yield and/or a
high liquid oil yield, e.g., in excess of theoretical. Thus, LIP-modified
crude can dramatically
reduce the amount of resid and coke that is produced in a refinery to a
greater extent than could be
attributed to the presence of the LIP as an ordinary low-resid blending
component.
[0013] Moreover, introducing the LIP into the feed to a pyrolysis process such
as FCIP also
synergistically improves the quality and/or yield of the pyrolyzate, e.g., the
LIP from FCIP of an
LIP-modified crude results in a synergistically lower sulfur content. The
present invention also
discloses a pyrolysis process and additive that has improved performance
relative to the disclosure
in my earlier US 10,336,946 B2.
[0014] Although not wishing to be bound by theory, these synergistic,
transformative properties
of the liquid ionizing pyrolyzate are believed to contain ionized species,
such as relatively stable
free radicals and hydrogen-rich donor compounds, that may inhibit aggregation
of maltenes and
asphaltenes in petroleum fractions and/or promote the formation of isomerates
and/or alkylates in
a manner consistent with hydrocracking, but at a lower range of temperatures
and near atmospheric
pressures. This is evidenced by an unexpected reduction of viscosity when the
LIP is added to
crude oil, and also by improved liquid oil yields from distillation and/or
pyrolysis of an LIP-crude
oil blend.
[0015] In one aspect, embodiments according to the present invention provide a
hydrocarbon
conversion process comprising: emulsifying water and an oil component with
finely divided solids
comprising a mineral support and an oxide and/or acid addition salt of a Group
3-16 metal
(preferably FeCl3 on an NaCl-treated clay); introducing the emulsion into a
flash chemical ionizing
pyrolysis (FCIP) reactor maintained at a temperature greater than about 400 C
up to about 600 C
and an absolute pressure up to about 1.5 atm to form a chemical ionizing
pyrolyzate effluent;
condensing a liquid ionizing pyrolyzate (LIP) from the effluent; combining a
feedstock oil with
- 4 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
the LIP to form a pyrolyzate-feedstock blend; and thermally processing the
blend at a temperature
above about 100 C.
[0016] In another aspect, embodiments according to the present invention
provide a flash chemical
ionizing pyrolysis (FCIP) process comprising the steps of: preparing a feed
emulsion comprising
100 parts by weight of an oil component, from about 1 to 100 parts by weight
of water, and from
about 1 to 20 parts by weight of finely divided solids comprising a mineral
support and an oxide
or acid addition salt of a Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-
treated clay); spraying
the feed emulsion in a flash pyrolysis reactor at a temperature from about 425
C to about 600 C;
collecting an effluent from the reactor; recovering a liquid ionizing
pyrolyzate (LIP) from the
effluent; and supplying a portion of the LIP as a portion of the oil component
in the feed emulsion
preparation step.
[00171 In a further aspect, embodiments according to the present invention
provide a hydrocarbon
refinery process comprising the steps of: combining a liquid ionizing
pyrolyzate (LIP) blend
component with a feedstock oil at a weight ratio from about 1:100 to about 1:1
to form an LIP
blend; preparing an emulsion comprising (i) a first portion of the LIP blend,
(ii) water, and (iii)
finely divided solids comprising a mineral support and an oxide or acid
addition salt of a Group 3
¨ 16 metal (preferably FeCl3 on an NaCl-treated clay); spraying the emulsion
in a flash pyrolysis
reactor at a temperature from about 425 C to about 600 C and a pressure from
about 1 to about
1.5 atm; collecting an effluent from the reactor; recovering a product LIP
from the effluent;
.. incorporating the product LIP as the LIP blend component in the LIP blend;
and distilling a second
portion of the LIP blend.
[0018] In a further aspect still, embodiments of the present invention provide
a hydrocarbon
refinery process comprising the steps of: preparing a feed emulsion comprising
(i) 100 parts by
weight of an oil component, (ii) from about 1 to 100 parts by weight of water,
and (iii) from about
.. 1 to 20 parts by weight finely divided solids comprising a mineral support
and an oxide or acid
addition salt of a Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-treated
clay); spraying the feed
emulsion in a flash pyrolysis reactor at a temperature from about 425 C to
about 600 C; collecting
an effluent from the flash pyrolysis reactor; recovering a liquid ionizing
pyrolyzate (LIP) from the
effluent; combining the recovered LIP with a feedstock oil comprising crude
oil or a petroleum
fraction selected from gas oil, resid, or a combination thereof to form a
pyrolyzate-feedstock blend;
- 5 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
distilling, cracking, visbreaking, and/or coking a first portion of the blend;
and supplying a second
portion of the blend as the oil component in the feed emulsion preparation
step.
[0019] In yet another aspect, embodiments according to the present invention
provide a crude oil
upgrading process comprising blending a liquid ionizing pyrolyzate (LIP) with
a heavy oil, and
thermally processing the blend at a temperature above about 100 C.
BRIEF DESCRIPTION OF THE DRAWINGS
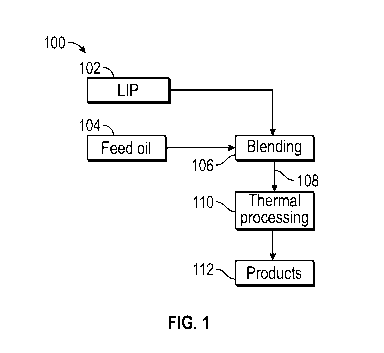
[00201 FIG. 1 shows a schematic flow diagram of thermally processing a blend
comprising a liquid
ionizing pyrolyzate (LIP) from flash chemical ionizing pyrolysis (FCIP),
according to
embodiments of the present invention.
.. [0021] FIG. 2 shows a simplified schematic flow diagram of a method for
preparing ferric chloride
(FeCl3) solids for FCIP, according to embodiments of the present invention.
[0022] FIG. 3 shows a more detailed flow diagram of the preferred method shown
in FIG. 2.
[0023] FIG. 4 shows a schematic flow diagram of a hydrocarbon conversion
process wherein an
LIP is combined with a feedstock oil to form an LIP blend and the LIP blend is
thermally
processed, according to embodiments of the present invention.
[0024] FIG. 5 shows a schematic flow diagram of a hydrocarbon refinery process
wherein LIP
from FCIP is blended with feed oil, desalted, heated, distilled, and
optionally supplied to the
emulsion preparation step for FCIP, according to embodiments of the present
invention.
[0025] FIG. 6 shows a schematic flow diagram of a hydrocarbon refinery process
wherein a first
.. portion of LIP from FCIP is blended with heavy products from distillation,
supplied to the
emulsion preparation step for FCIP, and a second portion is optionally
supplied to the distillation
step, according to embodiments of the present invention.
[0026] FIG. 7 shows a schematic flow diagram of an FCIP process for making the
LIP, according
to embodiments of the present invention.
.. [00271 FIG. 8 shows a schematic flow diagram of another FCIP process for
making the LIP,
according to embodiments of the present invention.
[0028] FIG. 9 shows a schematic flow diagram of a further FCIP process for
making the LIP,
according to embodiments of the present invention.
[0029] FIG. 10 shows chromatograms of the non-distilled, residual fraction
(>220 C) from the
LIP-diesel blend of Example 6 according to an embodiment of the present
invention, compared to
the residual fraction from the diesel alone.
- 6 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
DETAILED DESCRIPTION
[00301 The words and phrases used herein should be understood and interpreted
to have a meaning
consistent with the understanding of those words and phrases by those skilled
in the relevant art.
No special definition of a term or phrase is intended except where such a
special definition is
expressly set forth in the specification. The following definitions are
believed to be consistent with
their understanding by the skilled person, and are provided for the purpose of
clarification.
[0031] As used in the specification and claims, "near" is inclusive of "at."
The term "and/or" refers
to both the inclusive "and" case and the exclusive "or" case, whereas the term
"and or" refers to
the inclusive "and" case only and such terms are used herein for brevity. For
example, a component
comprising "A and/or B" may comprise A alone, B alone, or both A and B; and a
component
comprising "A and or B" may comprise A alone, or both A and B.
[0032] For purposes herein the term "alkylation" means the transfer of an
alkyl group from one
molecule to another, inclusive of transfer as an alkyl carbocation, a free
radical, a carbanion or a
carbene, or their equivalents.
[0033] For purposes herein, API refers to the American Petroleum Institute
gravity (API gravity),
which is a measure of the density of a petroleum product at 15.6 C (60 F)
compared to water at
4 C, and is determined according to ASTM D1298 or ASTM D4052, unless otherwise
specified.
The relationship between API gravity and s.g. (specific gravity) is API
gravity = (141.5/5.g.) -
131.5.
[0034] As used herein, the term "aqua regia" refers to any concentrated
mixture of hydrochloric
and nitric acids.
[0035] As used herein, "asphaltenes" refer to compounds which are primarily
composed of carbon,
hydrogen, nitrogen, oxygen, and sulfur, but which may include trace amounts of
vanadium, nickel,
and other metals. Asphaltenes typically have a C:H ratio of approximately
1:1.1 to about 1:1.5,
depending on the source. Asphaltenes are defined operationally as the n-
heptane (C7H16)-
insoluble, toluene (C6H5CH3)-soluble component of a carbonaceous material such
as crude oil,
bitumen, or coal. Asphaltenes typically include a distribution of molecular
masses in the range of
about 400 g/mol to about 50,000 g/mol, inclusive of aggregates.
[0036] For purposes herein the term "atmospheric distillation" means
distillation where an
uppermost stage is in fluid communication with the atmosphere or with a fluid
near atmospheric
pressure, e.g., less than 5 psig.
- 7 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[00371 For purposes herein, the abbreviation AET refers to "atmospheric
equivalent temperature"
of distillation, which is the temperature calculated from an observed vapor
temperature at a
pressure below atmospheric according to the Maxwell and Bonne11 equations as
described in
Annex A9 to ASTM D2892-18a.
[0038] For purposes herein the term "blending" means combining two or more
ingredients
regardless of whether any mixing is used.
[0039] For purposes herein the term "calcination" refers to heating a material
in air or oxygen at
high temperatures, e.g., at or above about 400 C.
[00401 For purposes herein the term "catalyst" means a substance that
increases the rate of a
chemical reaction usually but not always without itself undergoing any
chemical change. For
example, noble metal catalysts can become slowly poisoned as they contact
deleterious substances.
[0041] As used herein, "clay" refers to a fine-grained material comprising one
or more clay
minerals, i.e., a mineral from the kaolin group, smectite group (including
montmorillonite), illite
group, or chlorite group, or other clay types having a 2:1 ratio of
tetrahedral silicate sheets to
octahedral hydroxide sheets.
[0042] For purposes herein the term "coking" refers to the thermal cracking of
resid in an oil
refinery processing unit known as a "coker" that converts a heavy oil such as
the residual oil from
a vacuum distillation column into low molecular weight hydrocarbon gases,
naphtha, light and
heavy gas oils, and petroleum coke. Coking is typically effected at a
temperature of about 480 C.
[0043] For purposes herein the term "cracking" means the process whereby
complex organic
molecules are broken down into simpler molecules by the breaking of carbon-
carbon bonds in the
precursors. "Thermal cracking" refers to the cracking of hydrocarbons by the
application of
temperature, typically but not always 500-700 C and sometimes also pressure,
primarily by a free
radical process, and is characterized by the production of light hydrocarbon
gases, C4- C15 olefins
in moderate abundance, little aromatization, little or no branched chain
alkanes, slow double bond
isomerization, little or no skeletal isomerization, 13-scission of
alkylaromatics, and/or slow
cracking of naphthenes. "Catalytic cracking" refers to the cracking of
hydrocarbons in the presence
of a catalyst, typically but not always at 475-530 C that forms ionic species
on catalyst surfaces,
and is characterized by the production of little or no methane and/or ethane,
little or no olefins
larger than C4, some aromatization of aliphatic hydrocarbons, rapid skeletal
isomerization and
branched chain alkanes, rapid olefin isomerization, a-scission or dealkylation
of alkylaromatics,
- 8 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
and/or cracking of naphthenes and n-paraffins at comparable rates.
"Hydrocracking" refers to
cracking in the presence of hydrogen, typically but not always at 260-425 C
and using a
bifunctional catalyst comprising an acid support such as silica, alumina,
and/or zeolite, and a metal,
resulting in hydrogenation or saturation of aromatic rings and decyclization.
[0044] For purposes herein the term "crude oil" means an unrefined liquid
mixture of
hydrocarbons that is extracted from certain rock strata.
[0045] For purposes herein the term "desalting" means the removal of salt from
petroleum in a
refinery unit referred to as a "desalter" in which the crude oil is contacted
with water and separated
to remove the salt in a brine.
[0046] For purposes herein the term "distillation" means the process of
separating components or
substances from a liquid mixture by selective boiling and condensation.
[00471 For purposes herein, "distillation temperature" refers to the
distillation at atmospheric
pressure or the AET in the case of vacuum distillation, unless otherwise
indicated.
[0048] For purposes herein the term "emulsion" means a mixture of immiscible
liquids in a
discontinuous dispersed phase and a continuous phase, optionally including
dispersed solids.
[0049] For purposes herein the term "flash pyrolysis" means thermal reaction
of a material at a
very high heating rate (e.g., >450 C/s, preferably >500 C) with very short
residence time (e.g.,
<4 s, preferably <2 s).
[00501 For purposes herein the term "flash chemical ionizing pyrolysis" or
"FCIP" means flash
pyrolysis of a material in the presence of a chemical additive to promote
ionization and/or free
radical formation and is sometimes referred to as "catalytic pyrolysis" as
described in US
10,336,946 B2.
[00511 For purposes herein "finely divided" refers to particles having a major
dimension of less
than 1 mm, and a minor dimension of less than 1 mm. A particulate "fine" is
defined as a solid
-- material having a size and a mass which allows the material to become
entrained in a vapor phase
of a thermo-desorption process as disclosed herein, e.g., less than 250
microns.
[0052] For purposes herein the term "hydrocarbon" means a compound of hydrogen
and carbon,
such as any of those that are the chief components of petroleum and natural
gas. For purposes
herein the term "naphtha" refers to a petroleum distillate with an approximate
boiling range from
40 C to 195 C, a "kerosene" from greater than 195 C to 235 C, a "distillate"
from greater than
235 C to 370 C, a "gas oil" from greater than 370 C to 562 C.
- 9 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[0053] For purposes herein the term "hydrocarbon conversion" means the act or
process of
chemically changing a hydrocarbon compound from one form to another.
[0054] For purposes herein, "incipient wetness loading" refers to loading a
material on a support
by mixing a solution and/or slurry of the material with a dry support such
that the liquid from the
solution and/or slurry enters the pores of the support to carry the material
into the pores with the
slurry, and then the carrier liquid is subsequently evaporated. Although not
technically "incipient",
in the present disclosure and claims "incipient wetness loading" specifically
includes the use of a
volume of the solvent or slurry liquid that is in excess of the pore volume of
the support material,
where the liquid is subsequently evaporated from the support material, e.g.,
by drying.
[0055] For purposes herein, "limited solubility" means that a material mostly
does not dissolve in
water, i.e., not more than 50 wt% of a 5 g sample is digested in 150 ml
distilled water at 95 C in
12 h; and "acid soluble" means that a material mostly dissolves in aqueous
HC1, i.e., at least 50
wt% of a 5 g sample is digested in 150 ml of 20 wt% aqueous HC1 at 95 C in 12
h.
[0056] For purposes herein the term "liquid ionizing pyrolyzate" or "LIP"
refers to an FCIP
pyrolyzate that is liquid at room temperature and 1 atm, regardless of
distillation temperature. In
some embodiments, the LIP has blending characteristics indicative of the
presence of ionized
species and/or stable free radicals that can induce chemical and/or physical
rearrangement of
molecules or "normalization" in the blend components. For example, blending
the LIP with crude
containing asphaltenes results in viscosity changes that are more significant
than would be
predicted from conventional hydrocarbon blending nomographs, which is
consistent with
molecular rearrangement of the asphaltene molecules, including disaggregation.
Such an
unexpected viscosity reduction in turn produces unexpected increases in the
efficiencies of thermal
processes such as distillation, for example, employing the blend.
[00571 In some embodiments, the LIP has blending characteristics such that
when blended with a
specific blend oil, obtains a distillation liquid oil yield (<562 C) that is
greater than a theoretical
liquid oil yield, and/or obtains a total resid yield (>562 C) that is in an
amount less than a
theoretical resid yield, wherein the theoretical yields of the blend are
calculated as a weighted
average of the separate distillation of the LIP and blend oil alone, wherein
yields are determined
by atmospheric distillation in a 15-theoretical plate column at a reflux ratio
of 5:1, according to
ASTM D2892-18 up to cutpoint 400 C AET, and by vacuum potstill method
according to ASTM
- 10 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
D5236-18a above the 400 C cutpoint to cutpoint 562 C AET. Preferably, the LIP
has one, or
preferably more, or more preferably all, of the following oil blending
characteristics:
1) for a blend of Oil:LIP of 90:10, the liquid hydrocarbon yield, obtained
from distillation of the
blend up to a distillation temperature of 562 C, is equal to or greater than
1% (preferably at
least 1.5%) more than the theoretical yield, wherein the percentage is
absolute; and/or
2) for a blend of Oil:LIP of 90:10, a resid yield, obtained from the
distillation of the blend that is
decreased in an amount equal to or more than 1.5% (preferably at least 2.5%)
of the theoretical
resid yield, wherein the percentage is absolute; and/or
3) for a blend of Oil:LIP of 90:10, amounts of distillation of the blend
into a first fraction <290 C,
a second fraction 291-331 C, a third fraction 332-378 C, a fourth fraction 379-
440 C, and a
fifth fraction 441-531 C, are greater than theoretical amounts of the
respective fractions,
wherein the theoretical amounts of the blend fractions are calculated as
weighted averages of
the separate distillation of the LIP and blend oil alone; and/or
4) for a blend of Oil:LIP of 90:10, densities of fractions distilled into a
first fraction <290 C, a
second fraction 291-331 C, a third fraction 332-378 C, a fourth fraction 379-
440 C, and a fifth
fraction 441-531 C, are less than or equal to the densities in respective
fractions obtained from
distillation of the blend oil alone, preferably wherein the density in at
least one of the distilled
blend oil fractions is less than the density of the respective blend oil
fraction(s); and/or
5) for a blend of Oil:LIP of 80:20, the liquid hydrocarbon yield, obtained
from distillation of the
blend up to a distillation temperature of 562 C, is equal to or greater than
1.5% (preferably at
least 2.5%) more than the theoretical yield, wherein the percentage is
absolute; and/or
6) for a blend of Oil:LIP of 80:20, a resid yield, obtained from the
distillation of the blend that is
decreased in an amount equal to or more than 2.5% (preferably at least 4%) of
the theoretical
resid yield, wherein the percentage is absolute; and/or
7) for a blend of Oil:LIP of 80:20, amounts of distillation of the blend into
a first fraction <290 C,
a second fraction 291-331 C, a third fraction 332-378 C, a fourth fraction 379-
440 C, and a
fifth fraction 441-531 C, are greater than theoretical amounts of the
respective fractions,
wherein the theoretical amounts of the blend fractions are calculated as
weighted averages of
the separate distillation of the LIP and blend oil alone; and/or
8) for a blend of Oil:LIP of 80:20, densities of fractions distilled into a
first fraction <290 C, a
second fraction 291-331 C, a third fraction 332-378 C, a fourth fraction 379-
440 C, and a fifth
-11-
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
fraction 441-531 C, are less than or equal to the densities in respective
fractions obtained from
distillation of the blend oil alone, preferably wherein the density in at
least two, or more
preferably in at least three, of the blend fractions is less than the density
of the respective blend
oil fraction(s).
9) for a blend of Oil:LIP of 70:30, the liquid hydrocarbon yield, obtained
from distillation of the
blend up to a distillation temperature of 562 C, is equal to or greater than
2% (preferably at
least 3%) more than the theoretical yield, wherein the percentage is absolute;
and/or
10) for a blend of Oil:LIP of 70:30, a resid yield, obtained from the
distillation of the blend that is
decreased in an amount equal to or more than 3% (preferably at least 5%) of
the theoretical
resid yield, wherein the percentage is absolute; and/or
11) for a blend of Oil:LIP of 70:30, amounts of distillation of the blend into
a first fraction <290 C,
a second fraction 291-331 C, a third fraction 332-378 C, a fourth fraction 379-
440 C, and a
fifth fraction 441-531 C, are greater than theoretical amounts of the
respective fractions,
wherein the theoretical amounts of the blend fractions are calculated as
weighted averages of
the separate distillation of the LIP and blend oil alone; and/or
12) for a blend of Oil:LIP of 70:30, densities of fractions distilled into a
first fraction <290 C, a
second fraction 291-331 C, a third fraction 332-378 C, a fourth fraction 379-
440 C, and a fifth
fraction 441-531 C, are less than or equal to the densities in respective
fractions obtained from
distillation of the blend oil alone, preferably wherein the density in at
least two, or more
preferably in at least three, of the blend fractions is less than the density
of the respective blend
oil fraction(s).
[0058] As used herein, unless indicated, a "liquid oil" or "liquid product" or
"liquid hydrocarbon"
refers to the fraction(s) of petroleum from distillation that are normally
liquid at room temperature
and 1 atm obtained at distillation temperatures from 29 C to 562 C AET,
including gasoline
blending components, naphtha, kerosene, jet fuel, distillates, diesel, heating
oil, and gas oil;
whereas a "resid" or "heavy product" or "heavy hydrocarbon" refers to the
residual oil remaining
after distillation to 562 C AET, including resins, asphaltenes, and/or coke.
[0059] For purposes herein the term "oil" means any hydrophobic, lipophilic
chemical substance
that is a liquid at ambient temperatures.
[0060] All percentages are expressed as weight percent (wt%), based on the
total weight of the
particular stream or composition present, unless otherwise noted. All parts by
weight are per 100
- 12 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
parts by weight oil, adjusted for water and/or solids in the oil sample (net
oil), unless otherwise
indicated. Parts of water by weight include water added as well as water
present in the oil.
[0061] For purposes herein the term "pyrolysis" means decomposition brought
about by high
temperatures.
[0062] For purposes herein the term "ionizing pyrolyzate" means the oil
condensed or otherwise
recovered from the effluent of flash chemical ionizing pyrolysis.
[0063] Room temperature is 23 C and atmospheric pressure is 101.325 kPa unless
otherwise
noted.
[0064] For purposes herein, SARA refers to the analysis of saturates,
aromatics, resins, and
.. asphaltenes in an oil sample. SARA can be determined by IP 143 followed by
preparative HPLC
(IP-368) or Clay-Gel (ASTM D-2007), or by IATRO SCAN TLC-FID. For the purposes
of the
claims, in the event of a conflict, the results from ASTM D-2007 shall
control.
[0065] For purposes herein, the term "spray" means to atomize or otherwise
disperse in a mass or
jet of droplets, particles, or small pieces.
.. [0066] For purposes herein, sulfur in crude oil and pyrolyzates is
determined according to ASTM
D-4294. A "high sulfur" oil is one containing more than 0.5 wt% sulfur as
determined by ASTM
D-4294.
[00671 For purposes herein the term "thermal processing" means processing at
an elevated
temperature, e.g., above 100 C.
[0068] For purposes herein, viscosity is determined at 40 C and 100 s1, unless
otherwise stated,
or if the viscosity cannot be so determined at 40 C, the viscosity is measured
at higher temperatures
and extrapolated to 40 C using a power law equation.
[0069] Broadly, according to some embodiments of the invention, a process
comprises combining
a feedstock oil with a liquid ionizing pyrolyzate (LIP) to form a pyrolyzate-
feedstock blend. The
blend, quite unexpectedly, has a lower apparent viscosity at 40 C and/or at
100 C and a shear
rate of 100 s1 than predicted using API nomographs. The feedstock oil
preferably comprises
asphaltenes. The LIP is preferably prepared by flash chemical ionizing
pyrolysis (FCIP) as
described in various embodiments herein.
[00701 In some embodiments according to the invention, a process comprises
combining a
feedstock oil with a liquid ionizing pyrolyzate (LIP) to form a pyrolyzate-
feedstock blend; and
thermally processing the blend. In any embodiment, the process can recover a
light oil-enriched
- 13 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
hydrocarbon product, e.g., a hydrocarbon product having an enriched yield of
liquid hydrocarbons
boiling at a temperature below 562 C, relative to separate thermal processing
of the LIP and
feedstock oil, relative to separate thermal processing of the LIP and
feedstock oil, as determined
by atmospheric distillation in a 15-theoretical plate column at a reflux ratio
of 5:1, according to
ASTM D2892-18 up to cutpoint 400 C AET, and by vacuum potstill method
according to ASTM
D5236-18a above the 400 C cutpoint to cutpoint 562 C AET.
[0071] The feedstock oil may preferably be crude oil, which may be desalted or
preferably un-
desalted, but can also be, for example, gas oil, resid (atmospheric and/or
vacuum), and the like,
including mixtures or combinations.
[0072] The LIP is present in a sufficient amount to enhance light oil
enrichment. There is no upper
limit on the amount of LIP that can be used, but excessive amounts may not be
economical. The
pyrolyzate-feedstock blend can comprise the LIP in a weight ratio of about
1:100 to 1:1, preferably
from 1:100 to 1:2, more preferably from about 1:20 to 1:3, even more
preferably from about 1:10
to 1:4. Preferably, the percentages of LIP and feedstock oil total 100, i.e.,
the blend consists
essentially of or consists of the LIP and the feedstock oil.
[0073] The thermal processing is preferably distillation, e.g., atmospheric
and/or vacuum
distillation, and/or flash chemical ionizing pyrolysis (FCIP), which may
optionally be used to
produce the LIP, but the thermal processing can also be, for example, heating,
cracking (thermal
and/or catalytic), alkylation, visbreaking, coking, and so on, including
combinations in parallel
and/or series.
[0074] With reference to the embodiment of the invention shown in the
simplified schematic flow
diagram of FIG. 1, broadly, in process 100, a liquid ionizing pyrolyzate (LIP)
102 is combined
with a feed oil 104 in blending step 106. LIP 102 from any source can be used,
preferably from an
FCIP process as described herein, e.g., LIP 424 from FIG. 4, LIP 502 from FIG.
5, and/or LIP 604
from FIG. 6. The feed oil 104 can be any suitable hydrocarbon liquid, such as,
for example, crude
oil (including heavy crude oil), which can be desalted or un-desalted,
petroleum distillation
fractions (especially medium or heavy gas oil) or residue, waste oil, used
lube oil, etc. The resulting
LIP blend stream 108 is thermally processed in step 110 and light product(s)
112 are obtained,
depending on the nature of the thermal processing step 110. Thermal processing
step 110 may
comprise heating, distillation, cracking, alkylation, reforming, pyrolysis
such as FCIP, and the like,
including serial and/or parallel combinations thereof.
- 14 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[0075] The LIP 102 is produced from a flash chemical ionizing pyrolysis (FCIP)
process (see
FIGs. 7-9 discussed below), e.g., the process referred to as catalytic
pyrolysis in US 10,336,946
B2. In any embodiment, the FCIP preferably comprises the steps of preparing an
FCIP feed
emulsion comprising (i) an oil component, (ii) a water component, and (iii)
finely divided solids
comprising a mineral support and an oxide and/or acid addition salt of a Group
3 ¨ 16 metal
(preferably FeCl3 on an NaCl-treated clay), preferably 100 parts by weight of
the oil component,
from about 1 to 100 parts by weight of the water component, and from about 1
to 20 parts by
weight of the finely divided solids; spraying the FCIP feed emulsion in a
pyrolysis reactor,
preferably at a temperature from about 425 C to about 600 C, preferably 450 C
to 500 C;
collecting an effluent from the pyrolysis reactor; and recovering a product
LIP from the effluent.
[0076] In any embodiment, the FCIP feed emulsion may preferably comprise from
about 20 to
about 50 parts by weight of the water, and/or from about 5 to about 10 parts
by weight of the finely
divided solids, per 100 parts by weight LIP-feedstock blend or other feed oil.
[00771 In embodiments, the finely divided solids may preferably comprise or be
prepared as any
of those catalysts disclosed in my earlier patent, US 10,336,946 B2, which is
hereby incorporated
herein by reference in jurisdictions where permitted. For example, the finely
divided solids can
comprise clay and/or a derivative from a clay, such as montmorillonite, for
example, bentonite.
The mineral support can be any other mineral disclosed in the '946 patent,
including processed
drill cuttings, albite, and so on. The metal can comprise a Group 3 ¨ 16
metal, e.g., iron, lead, zinc,
or a combination thereof, preferably a Group 8 ¨ 10 metal, e.g., iron, cobalt,
nickel or the like. In
any embodiment, the finely divided solids may comprise an oxide and/or acid
addition salt of a
Group 8 ¨ 10 metal supported on clay, preferably FeCl3 on an NaCl-treated
clay.
[0078] Preferably, the finely divided solids comprise ferric chloride (FeCl3),
montmorillonite, and
a source of a salt that forms a eutectic with the FeCl3. The montmorillonite
is preferably a non-
swelling clay such as calcium bentonite, and the salt is preferably NaCl,
which may be provided
as sodium ions from treating the calcium bentonite with NaCl brine and
chloride ions provided by
or with the FeCl3. The finely divided solids are preferably the product of the
method comprising
the steps of: (a) treating iron with an aqueous mixture of hydrochloric and
nitric acids to form a
solids mixture of mixed valences of iron and iron chlorides, nitrites,
nitrites, oxides, and/or
hydroxides, preferably wherein the mixture has limited solubility in water and
is acid soluble, (b)
treating montmorillonite, preferably calcium bentonite, with brine, preferably
NaCl brine and
- 15 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
drying the treated montmorillonite; (c) combining the solids mixture with the
treated
montmorillonite to load the iron and/or iron chlorides, nitrites, nitrites,
oxides, and/or hydroxides
on the montmorillonite, preferably by incipient wetness or by adding an
aqueous slurry of the
solids mixture to the essentially dry montmorillonite; and (d) heat treating
the loaded
montmorillonite at a temperature above 400 C up to the FCIP temperature,
preferably 400 C to
425 C (see FIGs. 5-6 discussed below).
[0079] Preferably, the finely divided solids comprise FeCl3 derived from the
solids formed by the
treatment of iron, preferably an excess of iron, with an aqueous mixture of
hydrochloric and nitric
acids to form a solids mixture optionally of mixed valences of iron and iron
chlorides, nitrites,
nitrites, oxides, and/or hydroxides. The admixture of equal weights (1:1 by
weight) of iron and
aqua regia (HC1:H20:HNO3 at 3-6:2:1 by weight) forms FeCl3, which is
consistent with the dark
violet to black coloration of the solids that is observed. The aqua regia is
preferably slowly added
to the iron, or may be added in several aliquots, to avoid excessive heat
formation and reactant
vaporization since the reaction is exothermic. The proportion of iron may be
increased somewhat,
but too much iron may form insufficient FeCl3 as indicated by a generally
brown or rust color.
Greater proportions of aqua regia do not yield much if any benefit and thus
may lead to lower
yields of the solids mixture and/or excessive reagent costs. The admixture can
also contain
elemental iron, since the iron may be present in excess. Also, other iron
chlorides, nitrates, nitrites,
oxides, oxychlorides, hydroxides, or combinations and/or mixtures of these may
also be present.
For example, treatment of iron with aqua regia may in theory form the Fe(VI)
compound
hexachloroferrate. Further, since water is present, these compounds may be
hydrated to varying
degrees, e.g., especially upon slurrying with water, or decomposed by the
water.
[00801 The FeCl3 solids mixture preferably has limited solubility, e.g., less
than 50 wt% will
dissolve in hot water when mixed at a ratio of 1 g solids to 30 ml distilled
water, preferably less
than 40 wt%; and the FeCl3 solids mixture preferably is acid soluble, e.g.,
more than 50 wt% will
dissolve in 20 wt% aqueous HC1 when mixed at a ratio of 1 g solids to 30 ml
aqueous HC1,
preferably at least about 65 wt%. The solids mixture may be dried, e.g., in an
oven at a temperature
above 100 C, for example, 100 C to 150 C, and ground as needed. When the
solids mixture is
slurried in water and partially dissolved, the aqueous solution phase may
comprise an excess of
chloride ions, e.g., a molar ratio of chloride to total dissolved iron that is
greater than 3:1, such as
between 4 and 5 moles chloride per mole of solubilized iron. The aqueous phase
of the slurry may
- 16 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
also contain nitrite and/or nitrate in lesser amounts, e.g., 0.04-0.8 mole
nitrite per mole of dissolved
iron and/or 0.01-0.2 mole nitrate per mole of iron.
[00811 The montmorillonite support is preferably a non-swellable bentonite
such as calcium
bentonite. The bentonite is preferably treated with a brine to replace calcium
ions with sodium,
e.g., by treating the bentonite with 1 molar NaCl brine. The treated bentonite
may then be dried,
e.g., in an oven at a temperature above 100 C, for example, 100 C to 150 C,
and ground as needed
to prepare it for loading with the FeCl3 slurry by incipient wetness. The
loading is thus achieved
by mixing the FeCl3 slurry with the dried NaCl-treated bentonite, which may
form a paste. In this
mixture, Na ions in the bentonite may theoretically be displaced with iron
and/or iron complex
cations to form, e.g., possible species such as Fe(III)C12(-0-Si-bentonite)
and/or FeC15(-0-Si-
bentonite), or the like. The displaced Na ions can then theoretically react
with excess chloride from
the FeCl3 solids mixture slurry to form NaCl.
[0082] The mix of FeCl3 slurry and dried, NaCl-treated bentonite is then
preferably heat treated
or calcined. Heat treating the finely divided solids involves heating at a
temperature above 200 C,
such as from about 300 C up to 600 C, for a period of time from less than 1
minute up to 24 hours
or more, e.g., 1 to 16 hours. Heating at a temperature above 400 C for a
period of 4 to 6 hours is
preferred. High temperatures above 400 C are preferred to activate the solids,
and may result in
isolated Lewis and/or Bronsted acid sites in the bentonite being formed and/or
other hydrate
compounds, e.g., iron compound hydrates, may be dehydrated. Lower temperatures
may result in
.. insufficient activation or require longer periods of heating. Substantially
higher temperatures may
cause undesirable reaction, volatilization, and/or deactivation of the
chemical species in the solids.
Preferably, the heat treatment is at a temperature lower than the FCIP
temperature, which may
avoid premature reaction and/or deactivation of the solids material prior to
FCIP, more preferably
the heat treating is at a temperature of equal to or greater than 400 C up to
a temperature equal to
or less than 425 C.
[0083] Although not wishing to be bound by theory, it is believed salts or
ions present in the solids
material can form a eutectic mixture with one or more metal compounds or
reaction products
thereof, especially where the metal compound melts or boils at the heat
treatment temperature and
the eutectic mixture is non-volatile. For example, where the metal compound
includes FeCl3,
which has a normal boiling point of 315 C and is thus normally quite volatile
at 400 -425 C, the
presence of NaCl or another salt may form a eutectic mixture of FeCl3-NaCl
with substantially
- 17 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
lower volatility. This allows the FeCl3 to remain on the support during heat
treatment at 400 -
425 C and to be available as a reactant and/or catalyst at a higher pyrolysis
temperature. Other
iron compounds such as nitrates and/or nitrites may or may not decompose
during the heat
treatment step, e.g., to form iron oxides. In theory, similar eutectic systems
such as FeCl3-Na-
bentonite may also form. Also, the FeCl3 from the aqua regia treated iron has
unexpectedly limited
solubility in water suggesting that other complexes may be formed which could
also limit volatility
during heat pretreatment. As an example, the aqua regia-treated iron compounds
might form
covalent bonds with the bentonite, e.g., Fe(III)C12(-0-Si-bentonite), to limit
premature volatility.
[0084] The solids mixture of iron compounds or other FeCl3 source may be
loaded on the bentonite
in an amount from 1 mg/kg to 10 wt%, for example, from about 1000 mg/kg to 5
wt%, preferably
2-4 wt%, based on the total weight of the finely divided solids.
[0085] FIGs. 2 and 3 show the preparation of the finely divided solids in
exemplary embodiments
according to methods 200 and 300 for a laboratory or pilot plant scale
production quantities. In
the summarized method 200, brine 202, preferably NaCl brine, and
montmorillonite 206,
preferably bentonite, are admixed in support preparation step 207. Separately,
iron 222 is treated
with an aqueous mixture of HC1 and HNO3 in ferric chloride preparation step
225. The ferric
chloride is loaded on the support in step 232, and the mixture is heat treated
in step 234 prior to
use in FCIP step 238.
[0086] In the more detailed method 300 seen in FIG. 3, brine 302, preferably
1M sodium chloride,
is admixed in step 304 with calcium bentonite 306, preferably passing through
a 100 mesh screen.
Preferably, the weight ratio of Ca-bentonite to brine is 1:2. The mixture can
be stirred, e.g., for 1
h, and allowed to stand, e.g., for 16-24 h. In step 308, the excess brine is
discarded, e.g., by
decantation and/or filtration, and in step 310 the solids are dried, e.g.,
dried in an oven at 120-
130 C for 4-6 h. When the NaCl-bentonite is dry, it can be optionally ground
in step 312, e.g., to
pass through an 80 mesh screen.
[00871 Separately a reduced iron complex is prepared. In step 320, finely-
divided elemental iron
322, e.g., 100 mesh carbon steel shavings, are admixed with aqua regia 324,
preferably at
sub stoichiometric ratio where the moles of iron are greater than the total
moles of HC1 and HNO3,
e.g., at a weight ratio of 1:1 (Fe: aqua regia) where the aqua regia has a
weight ratio of nitric
acid:hydrochloric acid:water of about 1:3-6:2. The aqua regia is preferably
added in 3 aliquots
while stirring, and the temperature may increase, e.g., to about 95 C. In step
326, the solids can be
- 18 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
recovered from the aqueous phase, e.g., by filtration, water washing, and
drying, for example in
an oven at 100 C. The aqua-regia-treated Fe solids ("AR-Fe") at this point can
comprise a complex
mixture of iron chlorides, nitrates, nitrites, and oxides with the iron in
various valence states, e.g.,
Fe(0), Fe(II), Fe(III), and so on. The AR-Fe unexpectedly has a low fractional
solubility in water
so that no more than 40 wt%, preferably no more than about 35 wt% or 30 wt%,
dissolves and/or
digests in an aqueous mixture of 1 g AR-Fe in 30 ml total mixture (33.33 g/L)
at 100 C, but has a
high fractional solubility in 20 wt% aqueous hydrochloric acid such that at
least 90 wt%, preferably
at least about 95 wt % or 98 wt%, dissolves and/or digests in an aqueous
mixture of 1 g AR-Fe in
30 ml total mixture (33.33 g/L) at 100 C.
[0088] In step 328, the filtered solids can be ground, e.g., to pass a 100
mesh screen, and in step
330 slurried in water, e.g., at 4 weight percent solids. Then, in step 332 the
slurry from step 330 is
admixed with the dry, ground NaCl-bentonite from step 312, e.g., at a weight
ratio of 2:3 (slurry:
NaCl-bentonite) to load the AR-Fe on the NaCl-bentonite by incipient wetness.
The mixture from
step 332 is then dried and calcined, e.g., at 400 C for 2 h in step 334,
cooled and ground in step
336, e.g., to pass an 80 mesh screen, and recovered as the supported iron-
based solids 338.
[0089] In any embodiment of the invention, the FCIP process may comprise the
steps of: (a)
preparing an FCIP feed emulsion comprising 100 parts by weight of an oil
component, from about
1 to 100 parts by weight of a water component, and from about 1 to 20 parts by
weight of finely
divided solids comprising a mineral support and an oxide or acid addition salt
of a Group 3 ¨ 16
metal (preferably FeCl3 on an NaCl-treated clay); (b) spraying the FCIP feed
emulsion in a
pyrolysis reactor at a temperature from about 425 C to about 600 C, preferably
450 C to 500 C;
(c) collecting an effluent from the pyrolysis reactor; (d) recovering a
product LIP from the effluent;
I combining at least a portion of the product LIP with a feedstock oil to form
an LIP blend
comprising from 1 to 33.33 wt% of the product LIP; and (f) thermally
processing the LIP blend to
form a hydrocarbon product having an enriched yield of liquid hydrocarbons
boiling at a
temperature below 562 C, relative to separate thermal processing of the LIP
and feedstock oil,
relative to separate thermal processing of the LIP and feedstock oil, as
determined by atmospheric
distillation in a 15-theoretical plate column at a reflux ratio of 5:1,
according to ASTM D2892-18
up to cutpoint 400 C AET, and by vacuum potstill method according to ASTM
D5236-18a above
the 400 C cutpoint to cutpoint 562 C AET.
- 19 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[00901 Preferably, the FCIP process further comprises supplying at least a
portion of the LIP blend
as the oil component to the FCIP feed emulsion preparation step (a) wherein
the thermal processing
step (f) consists of or comprises the spraying of the FCIP feed emulsion into
the pyrolysis reactor
of step (b).
[0091] In any embodiment of the invention, the FCIP process may comprise the
steps of: (a)
preparing an FCIP feed emulsion comprising (i) 100 parts by weight of an oil
component
comprising a feedstock oil and optionally from 1 to 50 wt% of an LIP, e.g., 1
to 50 wt% LIP and
99 to 50 wt% feedstock oil, preferably 5 to 35 wt % LIP and 95 to 85 wt%
feedstock oil, more
preferably 10 to 30 wt% LIP and 90 to 70 wt% feedstock oil, based on the total
weight of the oil
component, preferably where the percentages of LIP and feedstock oil total
100, (ii) from about 1
to 100 parts by weight of a water component, preferably 1 to 30 parts by
weight water, and (iii)
from about 1 to 20 parts by weight finely divided solids comprising a mineral
support and an oxide
or acid addition salt of a Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-
treated clay); (b)
spraying the FCIP feed emulsion in a flash pyrolysis reactor at a temperature
from about 425 C to
about 600 C, preferably 450 C to 500 C; (c) collecting an effluent from the
pyrolysis reactor; (d)
recovering a product LIP from the effluent; and (e) optionally supplying a
portion of the product
LIP to the oil component in the feed emulsion preparation step (a).
[0092] While not wishing to be bound by theory, it is believed that hydrogen
radicals and/or
molecular hydrogen are generated in situ during flash pyrolysis by reaction
and/or catalysis of one
or more iron compound(s) and/or the support material. For example, where the
ionizing solids
comprise FeCl3 on an NaCl-treated clay, hydrogen may be formed primarily by
the decomposition
of FeCl3 vapor in the presence of steam, according to the following reactions:
2FeC13 <=> 2FeC12(s) + C12
C12 + 2H20 <=> 2HC10 + H2
[0093] Here, the formation of hydrogen may be favored due to an excess of
water (steam). Other
hydrogen generating reactions, including the water-gas
shift reaction
(CO+H20eCO2+H2), the reaction of FeCl2 with HC1, which may also be present in
this system,
the reaction of elemental iron and steam (Fe+H20v4'e0+H2), may occur to a
limited extent,
however, the residence time in the reactor, e.g. 0.1 to 2 seconds, and/or
temperature (-25 C -
600 C), may not be favorable for the reaction kinetics or equilibrium to form
hydrogen by these
mechanisms. Higher pyrolysis temperatures may not be favorable for hydrogen
generation and/or
- 20 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
may favor formation of undesirable byproducts such as HC1. Thus, the pyrolysis
is preferably
limited to 500 C to maximize in situ hydrogen formation, more preferably 480
C, e.g., 450 C-
480 C.
[0094] In addition to the chemical production of hydrogen radicals by
decomposition, FeCl3 per
.. se and bentonite can function as Lewis and/or Bronsted acids, and thus in
theory can initiate ionic
cracking reactions to form liquid ionizing pyrolyzate. Another possibility in
theory is that iron
compound(s) having higher oxidation states relative to FeCl3 may be formed
during the preparation
of the iron compounds with aqua regia and/or during heat treatment, e.g.,
hexachloroferrate ion
(Fe(VI)C13)3- which might also help form ions and/or free radicals to
propagate thermal and/or
catalytic cracking reactions.
[0095] While not wishing to be bound by theory, it is believed that FCIP using
the FeC13-NaC1-
bentonite solids system at low pressure and a specific range of temperatures
achieves extensive
conversion of heavy hydrocarbons such as asphaltenes and/or resins to lighter
hydrocarbons, and
removal of heteroatoms such as nitrogen, sulfur, metals, etc., by reactions
normally seen in high
pressure catalytic cracking and hydrocracking, e.g., isomerization, cracking,
dealkylation,
aromatic saturation, decyclization, etc. For example, there is evidence that
sulfur is both reduced,
presumably by hydrogen radicals, and oxidized, presumably by reaction with
HC10. The LIP
product is unexpectedly characterized by low noncondensable gas yield, e.g.,
only small quantities
of methane may be formed; the light products may be primarily C1-C6
hydrocarbons; small
quantities of or no C4+ olefins may be seen; and there may be significant
formation of branched
chain alkanes, isomerates, dealkylated aromatics, and naphthene cracking
products. At the same
time, the yield of coke can be minimized.
[0096] Liquid ionizing pyrolyzate (LIP) products obtained when a feedstock oil
is processed by
FCIP according to embodiments disclosed herein, especially when an oil with
high contents of
asphaltenes and/or resins is processed, include various medium-length
hydrocarbon fractions
having from about 12 to about 30 carbons, and various light oil fractions
having from about 6 to
12 carbons. The LIP is thus enriched in hydrocarbons similar to those seen in
catalytic and/or
hydrocracking products.
[00971 Additionally, the LIP from the FCIP disclosed herein has an
unexpectedly low viscosity
for its density, compared to other hydrocarbons, suggesting the presence of
relatively high levels
of isomerates. Moreover, blends of the LIP with other crude oils, heavy oils,
resids, and the like
-21 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
also have an unexpectedly low viscosity compared to conventional crude oil
blends. Applicant is
not bound by theory, but believes there may be ionized species in the LIP such
as stable radicals
that can inhibit asphaltene aggregation and/or decyclize asphaltenes, which is
reflected in a
significant reduction in coking tendency. The asphaltenes and other
hydrocarbon molecules
.. subjected to FCIP can form relatively stable free radical species, and can
also form hydrogen donor
species such as hydroaryl compounds. Some rearrangement of molecules appears
to occur at
ambient temperatures upon blending, whereas at moderate thermal processing
temperatures, e.g.,
100-250 C, the free radicals and hydrogen donors can facilitate conversion to
saturates, aromatics,
and lube oil base stock molecules, and reducing the amount of Conradson carbon
residue and coke
make.
[0098] In any case, when a feedstock oil is blended with the LIP, the
viscosity reduction and
reduced tendency to form coke results in unexpected improvements in thermal
processing. For
example, a crude-LIP blend can be heated more rapidly, e.g., during preheating
for feed to the
distillation column, since fouling from coke formation and deposition is
markedly reduced.
Distillation of a crude-LIP or resid-LIP blend results in liquid oil yields
that are substantially and
synergistically higher, and resid yields that are substantially and
synergistically lower, than could
be obtained by separate distillation of the LIP and crude or resid. Flash
pyrolysis of a crude-LIP
or resid-LIP blend, by FCIP as described herein, or otherwise, likewise
results in similarly
increased yields of liquid oil products and decreased yields of coke and also
noncondensable gases.
Unexpectedly, the resid from thermal processing of such LIP-modified blends
exhibits a
remarkably low viscosity, suggesting it contains an unusually high proportion
of lube oil base
stock. Moreover, the production of olefins by FCIP can be controlled by the
selection of
appropriate operational parameters, e.g., increasing the water content in the
emulsion feed to the
pyrolysis reactor and/or increasing the pyrolysis temperature can produce
relatively larger amounts
of olefins such as ethylene and propylene.
[0099] With reference to the embodiment of the invention shown in the
simplified schematic flow
diagram of FIG. 4, in FCIP process 400, feed oil 402 and liquid ionizing
pyrolyzate (LIP) from
stream 404 are optionally blended in step 406 or otherwise fed separately to
emulsification in step
408 with finely divided solids 410 and water 412. The emulsion from step 408
is supplied to FCIP
step 414. One or more effluents are separated in step 416 to obtain solids
418, water 420, LIP 422,
and noncondensable gas 424.
- 22 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[01001 The feed oil 402 can be any hydrocarbon liquid suitable for FCIP 414,
such as, for example,
crude oil, petroleum distillation fractions, especially medium or heavy gas
oil or residuum, waste
oil, used lube oil, etc. When the feed oil 402 is crude oil, it is
advantageously un-desalted since the
inorganic components do not appear to adversely impact FCIP 414 and much of
the inorganics can
be recovered with the solids from FCIP. Since the inorganics are removed in
FCIP process 400,
the load on the desalter associated with treatment of the crude oil for feed
to an atmospheric
distillation can be reduced by the amount fed to the FCIP process 400.
Moreover, the water content
of the crude oil does not impact the FCIP 414 since the feed is in the form of
an oil/water emulsion.
In fact, it is preferred to use the water or brine from desalting as all or
part of the water 412 for the
emulsion preparation, thereby reducing the load on the desalter and reducing
the amount of water
that must be added to the emulsion in step 408. Further, the salt may form a
eutectic mixture with
one or more of the other additive components, e.g., FeCl3, or otherwise
enhance the catalytic and/or
reactive activity of the finely divided solids.
[0101] The LIP 422 may optionally be supplied to the blending and/or emulsion
steps 406, 408
via stream 404 along with or in lieu of another LIP stream from another FCIP
source (e.g., see
FIGs. 3-4). The remaining LIP 424 can be optionally thermally processed by
heating, distillation,
cracking, visbreaking, coking, alkylation, reforming, etc. and/or directly
supplied as product(s). If
desired, water 420 recovered from the effluent may be recycled to the supply
412 and/or step 408
for the FCIP feed emulsion.
[0102] Preferably, a portion of the oil component in the FCIP feed emulsion
from step 408
comprises a recycled portion of the product LIP via line 404. If used, the LIP
can be used in the
blend in a weight proportion of LIP 404: feed oil 402 of from 1:100 to 1:1,
preferably in an amount
from 1 to 40 wt% based on the total weight of the oil components supplied to
the FCIP feed
emulsion step 208, e.g., 1 to 40 wt% product LIP and 99 to 60 wt% feed oil,
preferably 5 to 35 wt
% product LIP and 95 to 65 wt% feed oil, more preferably 10 to 30 wt% product
LIP and 90 to 70
wt% feed oil, based on the total weight of the oil component, preferably where
the percentages of
product LIP and feed oil in the LIP blend total 100.
[0103] One advantage of using emulsion 408 is that the oil, water, and finely
divided solids are
intimately mixed prior to vaporization of the oil and water, which are in
close contact with the
solids, and the solids are already well-dispersed in liquid, promoting
fluidization in the gas phase.
Another advantageous feature of the present invention is that in some
embodiments the emulsion
- 23 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
408 can have a viscosity that is lower, preferably an order of magnitude
lower, than the
corresponding oil components, which facilitates preparation, pumping,
spraying, conversion,
yield, etc., and can avoid adding solvent or diluent. For example, the feed
mixture may be an
emulsion having an apparent viscosity at 30 C and 100 s' at least 30% lower
than the oil
component alone. In embodiments, the emulsion has a viscosity of less than or
equal to about 50
Pa-s (50,000 cP) at 25 C, or less than or equal to about 20 Pa-s at 25 C, or
less than or equal to
about 300 mPa-s (300 cP) at 130 C, or less than about 250 mPa-s at 130 C.
Accordingly, the
emulsion may include heavy oil emulsified with water and the finely divided
solids to produce a
pumpable emulsion which facilitates adequate and uniform injection of the feed
mixture into the
pyrolysis chamber.
[0104] Also, in some embodiments the emulsion 408 can have a high stability
that inhibits
separation into oil or water phases and solids precipitation, which might
otherwise result in a
buildup of asphaltenes, wax, mineral particles, etc. The stability can
facilitate advance preparation
and storage of the emulsion 408. For example, the feed mixture 408 can be an
emulsion having an
electrical stability of equal to or greater than 1600 V, when determined
according to API 13B-2 at
130 C, preferably greater than 1800 V or even greater than 2000 V. If desired,
the emulsion may
further comprise an emulsifying agent such as a surfactant or surfactant
system. Preferably, the
emulsion is substantially free of added surfactant.
[0105] In some embodiments, the process comprises first mixing the feed oil
402 (or blend from
step 406) and the finely divided solids 410, and then mixing the water 412
with the mixture of the
oil and finely divided solids. Preferably, the process further comprises
passing (e.g., pumping) the
feed mixture through a line to the reactor, as opposed to mixing the oil,
water, and/or finely divided
solids together in the reactor 414, e.g., introducing them separately and/or
at a nozzle used for
spraying the mixture. In embodiments, the heavy oil is combined with the water
and the finely
divided solids to form the feed mixture at a temperature of about 25 C to
about 100 C, e.g., 30 C
to 95 C. The emulsion 408 may be fed to the FCIP reactor 414 at a relatively
high temperature to
minimize viscosity and enhance rapid heating in the pyrolysis chamber, but
below boiling, e.g.,
40 C to 60 C.
[0106] An exemplary process according to embodiments of the present invention
comprises the
steps of preparing the FCIP feed emulsion 408 comprising (i) 100 parts by
weight of the oil
component which comprises from 1 to 50 wt% of the LIP, preferably 5 to 40 wt%
LIP, based on
- 24 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
the total weight of the oil component, (ii) from about 1 to 100 parts by
weight of the water
component 412, and (iii) from about 1 to 20 parts by weight finely divided
solids 410 comprising
a mineral support and an oxide or acid addition salt of a Group 3 ¨ 16 metal
(preferably FeCl3 on
an NaCl-treated clay); spraying the FCIP feed emulsion from step 408 in a
pyrolysis reactor 414
at a temperature from about 425 C to about 600 C (preferably about 450 C to
about 500 C);
collecting effluent(s) 416 from the pyrolysis reactor 414; recovering a
product LIP 422, 424 from
the effluent 416; and optionally supplying a portion 404 of the product LIP
422 to the feed
emulsion preparation step 408 and/or optionally supplying the LIP portion 404
to the blending step
406. Higher amounts of water in the emulsion 408, e. g., more than 50 parts by
weight, tend to
produce more hydrocarbon gases, which may be preferred where olefin production
is preferred.
[01071 In embodiments, the absolute pressure in the FCIP reactor 414 is from
below atmospheric
or about atmospheric up to about 5 atm, or preferably up to about 3 atm, or
more preferably up to
about 2 atm, or especially up to about 1.5 atm (7-8 psig). For example, the
pressure in the FCIP
reactor 414 can be about 1 to 3 atm, preferably 1 to 1.5 atm. The higher
pressures are less preferred
since they require more expensive equipment to handle them and may inhibit
reactions necessary
for forming the conversion-promoting and/or coke-inhibiting components in the
product LIP 422.
[0108] The FCIP reactor 414 is operated and/or pyrolyzate exits from the
reactor 414 preferably
at a temperature between about 425 C and about 600 C, more preferably between
about 450 C
and about 500 C. The lower temperatures tend to favor more liquid hydrocarbon
products and less
gas, but total conversion may also be lower. Conversely, the higher
temperatures tend to favor
more conversion but hydrocarbon gas formation, including olefins, is greater
and liquid
hydrocarbon yield is less. The temperature depends on the hydrocarbon products
desired: for
greater liquid hydrocarbon yields, a temperature of 450 C to 500 C is
preferred, 450 C to 480 C
more preferred; for higher olefin and/or other light hydrocarbon yields, 500 C
to 600 C is
preferred.
[0109] In some embodiments, the heating of the reactor 414 and/or emulsion 408
can be direct by
contact with a hot gas such as a combustion effluent, and/or in indirect heat
exchange relationship
with the combustion gas or by using an electrical or induction heating. In
direct heating, the flue
gas preferably comprises less than about 3 vol% molecular oxygen, or less than
about 2 vol%
molecular oxygen, or less than about 1 vol% molecular oxygen.
- 25 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[01101 In some embodiments, the process comprises injecting the emulsion into
the reactor, e.g.,
using an atomizing nozzle, and in some embodiments the injection is into a
stream of combustion
flue gases or other hot gas in direct heat exchange to promote rapid heating
and mixing, e.g.,
countercurrently sprayed upstream against an oncoming flow of the combustion
gas, for example,
spraying the emulsion downwardly against an upward flow of the hot gas from
below. If desired
the combustion flue gases or other hot gas can be introduced into a lower end
of a reactor vessel
housing the pyrolysis zone, e.g., through a gas inlet through a side or bottom
wall of the reactor.
Regardless of heating mode, when sprayed downwardly into the reactor, the
residue and solids can
accumulate in the bottom of the reactor, and periodically or continuously
removed from the reactor,
for example, through an outlet for continuous or periodic removal of the
solids, e.g., using a rotary
valve in the outlet.
[01111 In some embodiments, especially where the feedstock oil is a heavy
crude oil or very heavy
crude oil, the pyrolyzate vapor phase preferably comprises a condensate upon
cooling having an
overall API gravity greater than 20 API or greater than 22.3 API or greater
than 26 API. In
some embodiments, the process further comprises cooling the pyrolyzate vapor
phase to form a
condensate, and collecting the condensate, wherein the condensate has an
overall API gravity
greater than 20 or greater than 22.3 .
[0112] In some embodiments, the pyrolyzate vapor phase comprises hydrocarbons
in an amount
recoverable by condensation at 30 C of at least about 70 parts (preferably 80
parts, more preferably
90 parts) by weight per 100 parts by weight of the oil in the feed mixture,
and especially greater
than 100 parts by weight liquid hydrocarbons per 100 parts by weight of the
oil. Liquid
hydrocarbon yields in excess of 100% of the feed oil are made possible by
incorporating hydrogen
and/or oxygen (from the water), especially hydrogen, into the product oil, and
minimizing gas and
residue formation. In some embodiments, the pyrolyzate vapor phase comprises
less than 5 vol%
of non-condensable (30 C) hydrocarbon gases based on the total volume of
hydrocarbons in the
pyrolyzate vapor phase (dry basis).
[0113] In embodiments, the feed oil 402 can be a crude oil, including heavy
crude oil, extra heavy
crude oil, tar, sludge, tank bottoms, spent lubrication oils, used motor
crankcase oil, oil based drill
cuttings, oil recovered from oil based drill cuttings, etc., including
combinations and mixtures
thereof. In embodiments, the feed oil has an API gravity of less than 22.3 API
or less than 20 API
or less than 10 API. In embodiments, the heavy oil has a viscosity greater
than 10,000 cP, or
- 26 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
greater than 50,000 cP, or greater than 100,000 cP, or greater than 300,000
cP, whereas the LIP
422 can have a viscosity less than 1000 cP, or less than 100 cP, or less than
30 cP.
[0114] As mentioned above, the feed oil need not be dewatered or desalted and
can be used with
various levels of aqueous and/or inorganic contaminants. Any water that is
present, for example,
means that less water needs to be added to form the emulsion 408 to obtain the
desired water:oil
ratio. The salts and minerals that may be present in crude oil do not appear
to adversely affect
results. These embodiments are particularly advantageous in being able to
process waste emulsions
or emulsions such as rag interface that is often difficult to break.
Considering that the industry
goes to great lengths to break emulsions into clean oil and water phases,
feeding such emulsions
in the feed mixture herein to the reactor can avoid the need to break such
emulsions altogether, or
at least reduce the volume of emulsion that must be separated. For example,
the rag layer that often
forms at the interface between the oil and water, that is often quite
difficult to separate, can be used
as a blend component in the feed emulsion step 408.
[0115] In some embodiments of the present invention, a hydrocarbon refinery
process comprises
the steps of: (a) combining an LIP with a feedstock oil to form an LIP blend
comprising from 1 to
50 wt% LIP and 99 to 50 wt% feedstock oil, preferably 5 to 35 wt % LIP and 95
to 65 wt%
feedstock oil, more preferably 10 to 30 wt% LIP and 90 to 70 wt% feedstock
oil, based on the total
weight of the oil component, preferably where the percentages of LIP and
feedstock oil total 100;
(b) preparing an FCIP feed emulsion comprising (i) 100 parts by weight of a
first portion of the
LIP blend, (ii) from about 1 to 100 parts by weight of a water component, and
(iii) from about 1
to 20 parts by weight finely divided solids comprising a mineral support and
an oxide or acid
addition salt of a Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-treated
clay); (c) spraying the
FCIP feed emulsion in a flash pyrolysis reactor at a temperature from about
425 C to about 600 C,
preferably 450 C to 500 C; (d) collecting an effluent from the flash pyrolysis
reactor; (e)
recovering a product LIP from the effluent; (f) incorporating at least a
portion of the product LIP
into the LIP blend; and (g) distilling a second portion of the LIP blend. The
feedstock oil preferably
comprises crude oil, more preferably un-desalted crude oil, e.g., the process
may further comprise
water washing to desalt the second portion of the LIP blend, and distilling
the desalted second
portion of the LIP blend in step (g).
[0116] In some embodiments of the present invention, a hydrocarbon refinery
process comprises
the steps of: (a) preparing an FCIP feed emulsion comprising (i) 100 parts by
weight of an oil
- 27 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
component, (ii) from about 5 to 100 parts by weight of a water component, and
(iii) from about 1
to 20 parts by weight finely divided solids comprising a mineral support and
an oxide or acid
addition salt of a Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-treated
clay); (b) spraying the
FCIP feed emulsion in a pyrolysis reactor at a temperature from about 425 C to
about 600 C,
preferably 450 C to 500 C; (c) collecting an effluent from the pyrolysis
reactor; (d) recovering
LIP from the effluent; (e) combining the recovered LIP with a feedstock oil
comprising a
petroleum fraction selected from medium weight gas oil, heavy gas oil, resid,
or a combination
thereof to form an LIP blend; and (0 distilling, cracking, visbreaking, and/or
coking the LIP blend.
Preferably, the oil component in the feed emulsion from the preparation step
(a) comprises the
.. petroleum fraction used in step (d), e.g., the feed emulsion from step (a)
may comprise the LIP
blend from the combining step (e).
[01171 With reference to the embodiment of the invention shown in the
simplified schematic flow
diagram of FIG. 5, a hydrocarbon refinery process 500 comprises combining a
liquid ionizing
pyrolyzate (LIP) 502 from FCIP 504 with a feed oil 506 in step 508 to form an
LIP blend
comprising the LIP. A first portion 520 of the LIP blend from 508 is supplied
for FCIP 504, and a
second portion 509 for distillation 514.
[0118] The LIP can be used in the blend in a weight proportion of LIP 502:
feed oil 502 of from
1:100 to 1:1, e.g., or from 1:20 to 1:2, preferably in an amount from 1 or 5
to 35 wt%, e.g., about
10 to 30 wt%, based on the total weight of the feed oil 506 and LIP 502
supplied to the blending
.. step 508. Lesser amounts of the LIP have diminishing improvement of the
blend, whereas higher
amounts may not be economically attractive.
[0119] Surprisingly, it has been found that a blend of the LIP and crude oil
can have a substantially
lower viscosity than would be expected from traditional API viscosity
prediction methods for
blends.
[01201 The first LIP blend portion 520 can be pyrolyzed in FCIP 504. In step
522, there is prepared
an FCIP feed emulsion comprising (i) 100 parts by weight of the first portion
520 of the LIP blend,
(ii) from about 1 to 100 parts by weight water, and (iii) from about 1 to 20
parts by weight finely
divided solids comprising a mineral support and an oxide or acid addition salt
of a Group 3 ¨ 16
metal (preferably FeCl3 on an NaCl-treated clay), e.g., from about 5 to about
50 parts by weight
.. of the water, and from about 1 to about 10 parts by weight of the finely
divided solids, per 100
parts by weight of the LIP blend. In step 504, the FCIP feed emulsion from 522
is injected,
- 28 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
preferably sprayed, in a pyrolysis reactor at a temperature from about 425 C
to about 600 C. An
effluent 530 is collected from the pyrolysis reactor, a product LIP 502 is
recovered from the
effluent, and at least a portion is incorporated into the LIP blend in step
508 as mentioned above.
[0121] Feed oil 524, which can be the same feed oil as 506 or another oil
source can optionally be
supplied to the emulsion step 522 along with or in lieu of stream 520. Where
blend stream 520 and
feed oil 524 are both used, they can optionally be blended together in a
vessel or line (not shown)
before the emulsion step 522. Preferably, the blend stream 520 is the
exclusive oil source for the
emulsion 522 fed to FCIP 504, i.e., feed oil 524 is not supplied to the
emulsion 522, thereby
avoiding a duplication of oil blending equipment.
[0122] The emulsion step 522 emulsifies the blend stream 520 and/or feed oil
524 with finely
divided solids 526 and water 528. The emulsion is pyrolyzed in FCIP step 504,
and separated in
step 530 to obtain solids 532, water 534, LIP 502, and noncondensable gas 536.
Use of the blend
stream 520 in this manner can facilitate pyrolysis by reducing fluid
viscosities, improving
emulsion stability, enhancing atomization, improving conversion, improving
liquid yield of LIP
502, and improving the isomerization and/or alkylation promoting qualities of
the product LIP
502, relative to the feed oil 506 and/or feed oil 524.
[0123] The second portion 509 of the LIP blend from 508 is fractionated in
distillation 514. In any
embodiment, the feed oil 506 may be a crude oil, preferably un-desalted crude
oil, preferably where
the process further comprises water washing in step 510 to desalt the second
portion 509 of the
LIP blend, preheating the crude in step 512, and distilling in step 514 to
obtain light and heavy
products 516, 518. In practice, the crude is often partially preheated to
reduce viscosity, desalted,
and then preheated to the distillation feed temperature. The distillation step
514 can include
atmospheric and/or vacuum distillation, with which the skilled person is
familiar.
[0124] Desalting 510 of the LIP blend portion 509 is facilitated due to lower
salt and water content,
synergistically lower viscosity and lower density, relative to the feed oil
506 by itself, and can thus
be separated from water or brine more readily than the crude. Because some of
the inorganic
contaminants are removed by FCIP 504 from the first portion 520, the load on
the desalter 510 is
likewise reduced. If desired, the water 536 for the desalting 510 may come
from the FCIP water
534, and/or the brine 538 may be supplied to water 528 for preparing the
emulsion in 522.
[0125] Heating 512 can likewise be improved by less tendency to form coke or
otherwise foul the
heat transfer surfaces, allowing a higher differential temperature to be
applied. To avoid this,
- 29 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
refineries often use a series of heaters, e.g., more than a dozen, to
incrementally raise the crude to
the desired temperature. The LIP blend may reduce the number of heaters
required. Also, the LIP
blend has an unexpectedly lower viscosity and may provide higher heat transfer
coefficients.
Finally, distillation 514 is improved by providing a higher yield of light
products 516, a lower
yield of heavy products 518, and improved quality of both the light and heavy
products 516, 518.
For example, the lighter products 516 tend to have an unexpectedly high
proportion of the type of
hydrocarbons normally obtained by isomerization and/or alkylation, which can
be reflected in a
lower density, lower viscosity, higher viscosity index, etc.
[0126] With reference to the embodiment according to the present invention
shown in the
simplified schematic flow diagram of FIG. 6, a hydrocarbon refinery process
600 is shown in
which (i) a blend of the heavy products 610 from distillation 612 and a
portion 602 of the product
LIP 604 is treated in FCIP 606 for improved conversion, liquid yield, and LIP
quality, and a
reduction in the amount of coke that is formed, relative to treatment of the
heavy products 610
alone and especially relative to conventional processing of the heavy products
610, e.g., in a
delayed coker; and/or (ii) a portion 616 of the product LIP 604 is supplied to
distillation 612 for
improved yield and quality of distillates, and a reduction in the yield of the
heavy products 610
and/or the amount of coke that is formed, relative to distillation of the feed
oil 618 alone.
[01271 Optionally, the feed oil 618 used for distillation 612 can be processed
for feed to the
distillation 602 in the manner as shown in FIG. 5 for the feed oil 506 in
process 500 that is fed to
distillation 514. In this arrangement, FIG. 5 can be seen as the front end or
pretreatment of the
crude supplied in a blend with the LIP to the distillation 514, 612, and FIG.
6 as a downstream
processing of the heavy products 518, 610 from distillation 514, 612. In other
words, processes
500 and 600 can be integrated where distillation 514 and 612 are equivalent,
light products 516
and 620 are equivalent, and heavy products 518 and 610 are equivalent. The
feed oil 618 is
preferably a washed, preheated crude oil, e.g., the oil from heating step 512
in FIG. 5.
[0128] A first portion 602 of LIP 604 from FCIP 606 can be blended in step 608
with heavy
products 610 from distillation 612. The blend and finely divided solids 613
are supplied with water
615 to the emulsion preparation step 614 for the FCIP 606.
[0129] A second portion 616 of the LIP 604 is optionally collected as a
product stream and/or
supplied to the distillation 612 for improved conversion of the feed oil 618
to light products 620
from the distillation, improved yield and quality of light products 620, and
decreased yield of
- 30 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
heavy products 610 and/or a reduced flow rate to resid processing 622. If
desired, the LIP in stream
616 may be blended in step 508 with the feed oil 618 (corresponding to feed
oil 506 in FIG. 5)
upstream from the desalting 510, heating 512, and so on. When the LIP 604
derived from the heavy
product 610 in FIG. 6 is supplied to the blending 508 in FIG. 5, the treatment
loop through line
520 to FCIP 504 and return from LIP 502 may or may not be used, and if used,
the processing rate
through FCIP 504 may be reduced in size relative to the flow scheme of FIG. 3
alone.
[01301 Effluent 624 from FCIP 606 is separated to recover LIP 604,
noncondensable gas 626,
water 628, and solids 630. Recovered water 628 may optionally be supplied for
re-use as the water
615 fed to the emulsion step 614 and/or water 528 (see FIG. 5).
[01311 With reference to FIG. 7, an apparatus 700 that may be used to prepare
the feed mixture in
accordance with some embodiments of the present invention comprises a mixing
tank 702A
equipped with an agitator 704A, which may be driven by motor 706A. If desired,
redundant pumps
708A, 710A can be provided with valved lines for selective recirculation and
transfer to an optional
holdup tank 712 and/or directly to reactor 714. If desired, an optional second
mixing train 716,
including mixing tank 702B, agitator 704B, motor 706B, and pumps 708B, 710B,
can be provided
to facilitate batch, semi-batch or continuous feed mixture preparation.
[0132] In batch operation, feed oil 718, water 720, and finely divided solids
722 are charged to
the mixing tank 702A (or 702B) in any order, preferably by transferring the
feed oil into the mixing
tank, then the finely divided solids, and then the water while maintaining
agitation via agitator
704A (or 704B) and/or providing agitation before and/or after each addition.
One of the pumps
708A, 710A (708B, 710B) can recirculate the mixture via valved line 711A
(711B) while agitating
to facilitate mixing. Once the mixture has been prepared, the pumps 708A, 710A
(708B, 710B)
can transfer the mixture to holding tank 712 via valved line 724A (724B), or
directly to FCIP
reactor 714 via valved lines 726A (726B) and 728.
[0133] If desired, the feed oil 718 may be heated or mixed with a hydrocarbon
diluent to reduce
viscosity and facilitate pumping and mixing. The water 720 and/or finely
divided solids 722 may
also be optionally heated to facilitate mixing. Also, if desired, the tanks
702A, 702B, 712 and the
associated lines and pumps may also be heated to keep the viscosity of the
mixture low; however,
the mixture in some embodiments has a lower viscosity than the feed oil 718,
so it may be possible
to maintain a lower temperature for the mixture or to avoid heating
altogether. Furthermore, the
mixing operation may be exothermic providing a source of heat in situ for the
mixture. Moreover,
-31-
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
the emulsion of the feed mixture is stable in some embodiments and so it may
be prepared in
advance, e.g., up to several days or more, and stored until use without phase
separation, before
transfer to the tank 712 and/or reactor 714. The emulsion can also be prepared
off-site and pumped
or trucked to the pyrolysis site. The feed mixture preparation apparatus shown
in FIG. 7 may be
used in or with any of the embodiments of the invention as shown in the other
figures.
[0134] In some embodiments, the feed mixture may be mixed using an in-line
mixer(s) and/or
produced in-situ within the FCIP reactor 714 by adding at least one of the
feed oil, water and/or
the finely divided solids directly into the FCIP reactor 714 and/or by the
addition of water and/or
addition of solids directly to the pyrolysis chamber, depending on the
composition of the feed oil
and the end use of the product LIP.
[0135] In some embodiments, the pyrolyzate vapor phase is condensable to form
an oil phase
lighter than the feed oil. In some embodiments the pressure in the FCIP
reactor 714 is sufficiently
low and the temperature sufficiently high such that the pyrolyzate exits the
reactor in the vapor
phase or primarily in the vapor phase, e.g. with at least 70 wt% of the
recovered hydrocarbons,
preferably at least 80 wt%, or at least 90 wt%, or at least 95 wt%, or at
least 98 wt%, or at least 99
wt% or at least 99.9 wt%, or 100 wt% of the recovered hydrocarbon exit the
reactor 146 in the
vapor phase, based on the total weight of the recovered hydrocarbons. In
general, the pyrolyzate
effluent 148 is primarily or mostly gas phase, comprised of hydrocarbons,
steam, and in the case
of direct heating, flue gases such as carbon dioxide or monoxide, nitrogen,
additional steam, etc.,
but may entrain relatively minor amounts of liquid droplets and/or small-
particle solids (fines) that
may be removed by filtration, cyclonic separation and/or condensation with the
recovered
hydrocarbons when they are subsequently condensed to produce the catalytic
pyrolysis oil product.
[0136] In an embodiment, the absolute pressure in the reactor 714 is from
about 1 to 1.5 atm
absolute, e.g. from about 1 atm to about 1.5 atm, or to about 1.1 atm, and the
pyrolyzate vapor 148
exits from the reactor at a temperature above 425 C, e.g., above 450 C, up to
about 480 C, up to
about 500 C, or up to about 600 C, e.g., 450 C-500 C, 450 C-480 C, or 500 C-
600 C.
[01371 The feed mixture from line 728 may be heated in the pyrolysis chamber
by hot gas 730,
e.g., combustion effluent or another gas at a temperature from about 300 C or
600 C up to about
1200 C, either in direct heat exchange relation via line 732 or indirect heat
exchange relation via
line 734. In practice only one arrangement is present in the apparatus 700,
either direct or indirect
heating. In embodiments the hot gas 730 comprises combustion gas from a fuel-
rich combustion,
- 32 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
e.g., comprising less than about 1 vol% molecular oxygen, or another effluent
having a sufficiently
low oxygen content to inhibit combustion in the reactor 714. In direct
heating, the hot gas 730 may
have a temperature from about 300 C to about 1200 C, and is contacted or
mixed directly with
the feed mixture or reaction products thereof, and the hot gas exits the FCIP
reactor 714 with the
pyrolyzate in effluent stream 736. In indirect heating, the hot gas 730,
preferably supplied at an
inlet temperature from about 600 C to about 1200 C, enters a heat exchanger
737 within the FCIP
reactor 714 and cooled gas 738 is collected from an outlet of the heat
exchanger. Solids 740
accumulating in the reactor 714 may be periodically or continuously removed
for disposal or for
recycling in the process (re-used as the finely divided solids and/or its
preparation), with or without
regeneration.
[0138] In embodiments, the effluent 736 with the product LIP exits the FCIP
reactor 714 at a
temperature greater than about 425 C, or greater than about 450 C. In
embodiments, the effluent
736 exits the process vessel at a chamber exit 24 at a temperature of about
600 C or below, or
below about 500 C. The effluent 736 from the reactor 714 can be processed as
desired, e.g., in
separator 742 to remove entrained fines 744 and/or in separator 746 to recover
water 748 and one
or more oil fractions, e.g., LIP 750, and to exhaust non-condensable gases
752. The separator 740
can comprise a cyclone separator, a filter such as a baghouse, an electric
precipitator, etc. Separator
746 can comprise condensers to recover condensate and gravity separation
devices, e.g., a
centrifuge or oil-water separator tank, to phase separate condensate
comprising oil and water
mixtures. Separator 746 can if desired optionally further include recovery of
light hydrocarbons,
e.g., hydrogen, methane, ethane, ethylene, propane, propylene, fuel gas, or
the like, using a
cryogenic process, membrane separators, and so on.
[0139] In embodiments, the FCIP reactor 714 comprises a turbulent environment,
and may contain
a bed of particulate inert solids (see FIG. 9), which may comprise silica,
alumina, sand, or a
combination thereof, and/or may include nonvolatile residues from previously
treated mixtures
such as ash, coke, and/or heavy hydrocarbons (i.e., having 40 carbons or
more). These residues
may collect and/or may be continuously or periodically removed from the FCIP
reactor 714. In
embodiments, the feed mixture in line 728 is fed to FCIP reactor 714 at a
point below a bed, thus
fluidizing the bed, and/or the feed mixture may enter just over the bed, e.g.,
downwardly directed
such as onto the bed or on an impingement plate (fixed or partially fluidized
bed) from which the
- 33 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
more volatile compounds rise immediately and the less volatile compounds are
converted to more
volatile compounds in the bed.
[01401 In embodiments, the combustion gases utilized as the hot gas 730 in any
of the processes
disclosed herein, especially in the direct heating embodiments, are sub-
stoichiometric with respect
to oxygen (oxygen lean/fuel rich) such that the concentration of molecular
oxygen 02 in the reactor
is less than about 1 vol%, or less than 0.1 vol%, or the combustion gas is
essentially free of
molecular oxygen. Accordingly, in embodiments, the pyrolysis reactor 714
comprises a reducing
atmosphere.
[01411 With reference to FIG. 8, a process 800 according to some embodiments
of the present
.. invention comprises a mixer and/or mixing tank 802 to combine feed oil 804,
water 806, and finely
divided solids 808 into an emulsion as described herein (cf. discussion of
FIG. 7). The emulsion
is transferred via pump 810 to FCIP reactor 812. An oxygen source 814 such as
air, oxygen or
oxygen-enriched air is combined with fuel 816 in combustion burner 818 to
supply combustion
effluent in line 820 to the reactor 812, as described herein (cf. discussion
of FIG. 7). Control system
.. 821 is provided to control the operating conditions of the FCIP reactor
812, e.g., by manipulation
or adjustment of the feed rate(s) and/or combustion rates to maintain the
pyrolysis zone at a
temperature, pressure and residence time to form an LIP vapor phase. In the
case of indirect
heating, cold gas 822 is recovered; otherwise the combustion gases are mixed
with the steam and
LIP vapors and recovered in effluent line 824. Solids 826 may be recovered
from the reactor 812
continuously or periodically.
[0142] The effluent from line 824 is processed in fines removal unit 828, to
separate fines 830,
optionally including any liquid droplets or other solids, and the remaining
vapor can optionally be
supplied directly to an oil or heavy oil reservoir recovery process (see Fig.
11 of US 2016/0160131
Al), or after conditioning to remove any undesirable components, supplement
any additional
components needed, compress to injection pressure, heat to the desired
injection temperature,
and/or cool to recover waste heat.
[0143] The remaining vapor can be cooled in exchanger 834 and hydrocarbon
condensate (LIP I)
836 recovered from separator 838. The process temperature in the exchanger 834
and separator
838 is preferably above the water dew point so that the condensate 836 is
essentially free of water,
e.g., less than 1 wt%. The vapors from separator 838 are then cooled in
exchanger 840 and
condensate 842 recovered from separator 844. The process temperature in the
exchanger 840 and
- 34 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
separator 844 is preferably below the water dew point so that the condensate
842 is a mixture of
water and oil, which can be further separated in separator 846, which can be a
centrifuge or gravity
settling tank, for example, to obtain oil product (LIP II) 848 and water 850.
The overhead vapor
from the separator 844 can be exhausted and/or used as a fuel gas, or it can
optionally be further
processed in exchanger 852 for cooling and separated in separator 854 into non-
condensable gases
856 and or product 858 comprised of one or more streams of hydrogen, methane,
ethane, ethylene,
propane, propylene, carbon dioxide, fuel gas, including combinations thereof.
The separator 854
can be any one or suitable combination of a cryogenic separator, membrane
separator, fractionator,
solvent extraction, pressure swing absorption, or the like.
[0144] With reference to FIG. 9, a process 900 comprises a reactor 902 that is
directly heated by
combustion gases supplied from burner 904 in combustion chamber 906 through
duct 908, which
can direct the combustion effluent through distributor 908a located to
fluidize the solids 909. Feed
mixture 910 can be prepared, for example, as described above (cf. discussion
of FIGs. 7-8). The
feed mixture 910 is supplied to nozzle 912 and forms a preferably conical
spray pattern 914 in the
reactor 902.
[0145] The nozzle 912 is directed downwardly and can be positioned near the
upper end of the
reactor, e.g., 1/3 of the way down from the top of the reactor toward the
bottom. The nozzle 912
is preferably designed and positioned so that the spray pattern 914 avoids
excessive impingement
on the inside surfaces of the reactor 902 that can lead to caking and/or
buildup of solids on the
walls. For example, the nozzle 912 can provide a conical spray pattern. The
feed mixture 910 is
thus introduced countercurrently with respect to the flue gas from combustion
chamber 906 to
promote mixing and rapid heating to facilitate the conversion and
volatilization of hydrocarbons.
[0146] The pyrolyzate vapor phase exits the reactor 902 together with the
combustion gas and
steam from the feed mixture water into duct 916. The upward flow rate of the
gases in the reactor
902 in some embodiments is sufficiently low to avoid excessive entrainment of
solid particulates.
The solid particulates can thus fall to the bottom of the reactor 902 and can
be periodically and/or
continuously withdrawn, e.g., via rotary valve 918, for disposal and/or
regeneration and recycle to
the slurry preparation. Regeneration can be effected in some embodiments by
contacting the solids
with an oxygen containing gas at high temperature to promote combustion of
hydrocarbon residue
and coke from the particles. In any embodiment, regeneration can be in situ in
reactor 902, e.g.,
by supplying oxidant gas into the solids bed 940 for combustion of coke.
- 35 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[0141 The gases from the reactor 902 in some embodiments are passed into
cyclone 920 for
removal of fines. Fines can be periodically and/or continuously withdrawn from
the cyclone 920,
e.g., via rotary valve 926. The solids-lean gases in some embodiments are then
passed through
condensers 922 and 924. The first condenser 922 preferably condenses
hydrocarbons, which have
a relatively higher boiling point than water, at a temperature above the water
dew point so that the
oil 928 (LIP I) has a low water content, e.g., essentially free of water so
that water separation is
not needed. The second condenser 924 preferably condenses the hydrocarbons and
water which
may be processed, if desired, in separator 932 to separate an oil phase 934
(LIP II) from a water
phase 936, e.g., by gravity settling, centrifuge, or the like. The recovered
water in this and any of
the other embodiments illustrated herein can, if desired, be recycled for
preparation of the feed
mixture to the FCIP reactor (cf. FIGs. 1, 4-8), the desalting 510 (FIG. 5),
and so on. Non-condensed
exhaust gases 938 are recovered overhead from the condenser 924.
EMBODIMENTS
[0148] The present invention provides, among others, the following preferred
embodiments:
1. A hydrocarbon refinery process comprising the steps of:
(a) combining a liquid ionizing pyrolyzate with crude oil to form an LIP-crude
blend comprising
the pyrolyzate in an amount from 10 to 20 wt% based on the total weight of the
HP-crude
blend;
(b) combining a first portion of the LIP-crude blend, water, and 1-4 wt% of a
finely divided solids
to obtain an emulsion comprising (i) 75-85 wt% of an oil phase, (ii) 5-15 wt%
of an aqueous
phase, and (iii) 3-10 wt% total solids, based on the total weight of the
emulsion, wherein the
finely divided solids comprise the product of combining FeCl3 of limited
solubility and NaCl-
treated bentonite and heat treating the combined FeCl3 and bentonite at a
temperature of 400 C
to 425 C;
(c) spraying the emulsion in a vapor phase of a flash chemical ionizing
pyrolysis reactor at a
temperature of 450-500 C;
(d) collecting an effluent from the pyrolysis reactor;
(e) recovering a crude oil pyrolyzate from the effluent;
(f) supplying the crude oil pyrolyzate from step (e) as the hydrocarbon
pyrolyzate in step (a);
(g) desalting a second portion of the LIP-crude blend from step (a);
(h) supplying brine recovered from step (g) as the water in step (b);
- 36 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
(i) preheating the desalted LIP-crude blend from step (g);
(j) atmospherically distilling the preheated LIP-crude blend from step (i) to
separate an
atmospheric resid from lower boiling hydrocarbon fractions; and
(k) vacuum distilling the atmospheric resid to separate a vacuum resid from
gas oil.
-- 2. A hydrocarbon refinery process comprising the steps of:
(a) combining a liquid ionizing pyrolyzate with resid to form an LIP-resid
blend comprising the
pyrolyzate in an amount from 10 to 20 wt% based on the total weight of the LIP-
resid blend;
(b) combining a first portion of the LIP-resid blend, water, and 1-4 wt% of a
finely divided solids,
wherein the finely divided solids comprises the product of combining FeCl3 of
limited
solubility and NaCl-treated bentonite and heat treating the combined FeCl3 and
bentonite at a
temperature of 400 C to 425 C, to obtain an emulsion comprising (i) 75-85 wt%
of an oil
phase, (ii) 5-15 wt% of an aqueous phase, and (iii) 3-10 wt% total solids,
based on the total
weight of the emulsion;
(c) spraying the emulsion in a vapor phase of a flash chemical ionizing
pyrolysis reactor at a
temperature of 450-500 C;
(d) collecting an effluent from the pyrolysis reactor;
(e) recovering a liquid ionizing pyrolyzate product from the effluent;
(I) supplying the liquid ionizing pyrolyzate product from step (e) as the
liquid ionizing pyrolyzate
in step (a);
.. (g) distilling a second portion of the LIP-resid blend from step (a) to
separate resid from lower
boiling hydrocarbon fractions;
(h) supplying a first portion of the resid from step (g) to the LIP-resid
blend in step (a); and
(i) optionally coking a second portion of the resid from step (g) to obtain
coker gas oil.
3. Finely divided solids for emulsion flash ionizing pyrolysis, comprising:
(a) NaCl-treated calcium bentonite;
(b) FeCl3;
(c) preferably wherein the finely divided solids are prepared according to the
process comprising
the steps of:
i. treating iron particles with an equal weight of aqua regia,
the aqua regia
comprising 3 parts by weight hydrochloric acid, 2 parts by weight water, and 1
part by weight nitric acid, to form a solids mixture;
- 37 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
ii. rinsing, drying, and grinding the solids mixture from (i);
iii. treating calcium bentonite with 1 M NaCl brine;
iv. rinsing, drying at 100-125 C, and grinding the treated bentonite from
(iii);
v. slurrying the solids mixture from (ii) in water to obtain a slurry
comprising 4 wt%
of the solids from (ii) by weight of the slurry;
vi. combining 2 parts by weight of the slurry from (v) with 3 parts by
weight of the
treated bentonite from (iv) to form a paste; and
vii. heat treating the paste from (vi) at a temperature of 400 C to 425 C
for a period
of 4-6 hours to obtain the solids; and
viii. grinding the solids from (vii) to form the finely divided solids.
Al. A hydrocarbon conversion process, comprising the steps of:
emulsifying water and an oil component with finely divided solids comprising a
mineral support
and an oxide and/or acid addition salt of a Group 3-16 metal (preferably FeCl3
on an NaCl-
treated clay);
introducing the emulsion into a flash chemical ionizing pyrolysis (FCIP)
reactor maintained at a
temperature greater than about 400 C up to about 600 C and a pressure up to
about 1.5 atm
to form an ionized pyrolyzate effluent;
condensing the ionized pyrolyzate from the effluent to recover a liquid
ionized pyrolyzate (LIP);
combining a feedstock oil with the LIP to form a pyrolyzate-feedstock blend;
and
thermally processing the blend at a temperature above about 100 C.
A2. The process of embodiment Al, wherein the solids comprise brine-treated
clay and an acid
addition salt of a Group 8 ¨ 10 metal, wherein the brine comprises a salt that
forms a eutectic with
the acid addition salt of the Group 8 ¨ 10 metal.
A3. The process of embodiment A2, wherein the clay comprises bentonite, the
brine comprises
sodium chloride, and the acid addition salt comprises FeCl3.
A4. The process of embodiment A3, comprising preparing the solids by a
method comprising
the steps of:
(a) contacting bentonite with the sodium chloride brine;
(b) contacting an excess of iron with an aqueous mixture of hydrochloric and
nitric acids to form
FeCl3 solids;
(c) loading the FeCl3 solids on the brine-treated bentonite; and
- 38 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
(d) calcining the loaded bentonite at a temperature below the FCIP
temperature.
A5. The process of any of embodiments Al to A4, further comprising the
steps of:
wherein the emulsion comprises (i) 100 parts by weight of the oil component,
preferably wherein
the oil component comprises the pyrolyzate-feedstock blend; (ii) from about 1
to 100
parts by weight of water, and (iii) from about 1 to 20 parts by weight of the
finely divided
solids; and
spraying the emulsion into the reactor, wherein the reactor temperature is
from about 425 C to
about 600 C, preferably 450 C to 500 C.
A6. The process of embodiment A5 wherein the finely divided solids comprise
the product of
.. the method comprising the steps of:
treating iron with an aqueous mixture of hydrochloric and nitric acids to form
a solids mixture of
FeCl3 optionally with mixed valences of iron and iron chlorides, nitrites,
nitrites, oxides,
and/or hydroxides, wherein the solids mixture has limited solubility;
treating montmorillonite with NaCl brine and drying the treated
montmorillonite;
combining a slurry of the solids mixture with the treated montmorillonite to
load the FeCl3 on the
montmorillonite; and
heat treating the loaded montmorillonite at a temperature above 400 C.
A7. The process of any of embodiments Al to A6, wherein the feedstock oil
comprises
hydrocarbons boiling at a temperature equal to or greater than 562 C, and
further comprising the
step of recovering a hydrocarbon product from the thermally processed blend,
the hydrocarbon
product having an enriched yield of liquid hydrocarbons boiling at a
temperature below 562 C,
relative to separate thermal processing of the LIP and feedstock oil, as
determined by atmospheric
distillation in a 15-theoretical plate column at a reflux ratio of 5:1,
according to ASTM D2892-18
up to cutpoint 400 C AET, and by vacuum potstill method according to ASTM
D5236-18a above
the 400 C cutpoint to cutpoint 562 C AET.
A8. The process of embodiment A7 wherein the feedstock oil is crude oil,
gas oil, resid, or a
mixture thereof.
A9. The process of any of embodiments Al to A8 wherein the thermal
processing comprises
pyrolysis, distillation, cracking, alkylation, visbreaking, coking, and
combinations thereof
A10. The process of any of embodiments Al to A9, further comprising supplying
at least a
portion of the pyrolyzate-feedstock blend as the oil component to the FCIP
feed emulsion
- 39 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
preparation step wherein the thermal processing step consists of or comprises
the spraying of the
FCIP feed emulsion into the flash pyrolysis reactor.
A11. A flash chemical ionizing pyrolysis (FCIP) process comprising the steps
of:
preparing a feed emulsion comprising (i) 100 parts by weight of an oil
component comprising a
liquid ionizing pyrolyzate (LIP) and a feedstock oil at a weight ratio of from
1:100 to 1:1,
(ii) from about 1 to 100 parts by weight of water, and (iii) from about 1 to
20 parts by
weight finely divided solids comprising a mineral support and an oxide or acid
addition
salt of a Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-treated clay);
spraying the feed emulsion in a flash pyrolysis reactor at a temperature from
about 425 C to
about 600 C;
collecting an effluent from the reactor;
recovering a product oil from the effluent; and
supplying a portion of the product oil as the LIP to the feed emulsion
preparation step.
Al2. A hydrocarbon refinery process comprising the steps of:
combining a liquid ionizing pyrolyzate (LIP) blend component with a feedstock
oil at a weight
ratio from about 1:100 to about 1:1 to form an LIP blend;
preparing an emulsion comprising (i) a first portion of the LIP blend, (ii)
water, and (iii) from
finely divided solids comprising a mineral support and an oxide or acid
addition salt of a
Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-treated clay);
spraying the emulsion in a flash pyrolysis reactor at a temperature from about
425 C to about
600 C and a pressure from about 1 to about 1.5 atm;
collecting an effluent from the reactor;
recovering a product LIP from the effluent;
incorporating the product LIP as the LIP blend component in the LIP blend; and
distilling a second portion of the LIP blend.
A13. The process of embodiment Al2, wherein the feedstock oil comprises crude
oil.
A14. The process of embodiment A13, wherein the feedstock oil comprises un-
desalted crude
oil wherein the process further comprises water washing to desalt the second
portion of the LIP
blend, and distilling the desalted second portion of the LIP blend.
A15. The process of embodiment A9 wherein the feedstock oil comprises crude
oil and further
comprising washing the LIP blend with wash water, recovering a solute-enriched
spent water
- 40 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
from the water washing step, recovering a desalted LIP blend, and heating the
desalted LIP blend
in advance of distillation of the LIP blend.
A16. A hydrocarbon refinery process comprising the steps of:
preparing a feed emulsion comprising (i) 100 parts by weight of an oil
component, (ii) from about
1 to 100 parts by weight of water, and (iii) from about 1 to 20 parts by
weight finely divided
solids comprising a mineral support and an oxide or acid addition salt of a
Group 3 ¨ 16
metal (preferably FeCl3 on an NaCl-treated clay);
spraying the feed emulsion in a flash pyrolysis reactor at a temperature from
about 425 C to about
600 C;
collecting an effluent from the flash pyrolysis reactor;
recovering a liquid ionizing pyrolyzate (LIP) from the effluent;
combining the recovered LIP with a feedstock oil comprising crude oil or a
petroleum fraction
selected from gas oil, resid, or a combination thereof to form a pyrolyzate-
feedstock blend;
distilling, cracking, visbreaking, and/or coking a first portion of the LIP
blend; and
supplying a second portion of the LIP blend as the oil component in the feed
emulsion preparation
step.
A17. The process of embodiment A16, wherein the LIP exhibits a SARA analysis
having
higher saturates and aromatics contents and a lower asphaltenes content than
the feedstock oil.
A18. The process of embodiment A16 or A17 wherein a proportion of the LIP in
the oil
component in the flash pyrolysis is effective to improve yield of liquid
hydrocarbons boiling at a
temperature below 562 C, relative to separate flash chemical ionizing
pyrolysis of the LIP and
feedstock oil, as determined by atmospheric distillation in a 15-theoretical
plate column at a reflux
ratio of 5:1, according to ASTM D2892-18 up to cutpoint 400 C AET, and by
vacuum potstill
method according to ASTM D5236-18a above the 400 C cutpoint to cutpoint 562 C
AET.
A19. The process of any of embodiments A16 to A18 wherein a proportion of the
LIP in the LIP
blend in the distillation, cracking, visbreaking, and/or coking step, is
effective to improve yield of
liquid hydrocarbons boiling at a temperature below 562 C, relative to separate
distillation,
cracking, visbreaking, and/or coking of the LIP and feedstock oil, as
determined by atmospheric
distillation in a 15-theoretical plate column at a reflux ratio of 5:1,
according to ASTM D2892-18
up to cutpoint 400 C AET, and by vacuum potstill method according to ASTM
D5236-18a above
the 400 C cutpoint to cutpoint 562 C AET.
-41 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
A20. A crude oil upgrading process, comprising:
blending a liquid ionizing pyrolyzate (LIP) with a heavy oil; and
thermally processing the blend at a temperature above about 100 C.
A21. The process of any of embodiments Al to A19 wherein the oil component
and/or the
feedstock oil comprise crude oil.
A22. The process of any of embodiments Al to A19 wherein the oil component
and/or the
feedstock oil comprise heavy crude oil.
A23. The process of any of embodiments Al to A19 wherein the oil component
and/or the
feedstock oil comprise diesel.
A24. The process of any of embodiments Al to A19 wherein the oil component
and/or the
feedstock oil comprise atmospheric resid.
A25. The process of any of embodiments Al to A19 wherein the oil component
and/or the
feedstock oil comprise vacuum resid.
Bl. A hydrocarbon conversion process, comprising the steps of:
combining a feedstock oil with a liquid ionizing pyrolyzate (LIP) to form an
LIP blend;
thermally processing the LIP blend; and
recovering a hydrocarbon product having an enriched yield of liquid
hydrocarbons boiling at a
temperature below 562 C, relative to separate thermal processing of the LIP
and feedstock
oil, as determined by atmospheric distillation in a 15-theoretical plate
column at a reflux
ratio of 5:1, according to ASTM D2892-18 up to cutpoint 400 C AET, and by
vacuum
potstill method according to ASTM D5236-18a above the 400 C cutpoint to
cutpoint
562 C AET.
B2. The process of embodiment B1 wherein the feedstock oil is crude oil,
gas oil, resid, or a
mixture thereof.
B3. The process of embodiment B1 or embodiment B2 wherein the thermal
processing
comprises emulsion flash chemical ionizing pyrolysis (FCIP), distillation,
cracking,
alkylation, visbreaking, coking, and combinations thereof, preferably FCIP
and/or
distillation.
B4. The process of embodiment B3 wherein the liquid ionizing pyrolyzate
(LIP) is produced
from emulsion flash chemical ionizing pyrolysis (FCIP) comprising the steps
of:
- 42 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
preparing an FCIP feed emulsion comprising (i) 100 parts by weight of an oil
component,
preferably wherein the oil component comprises the LIP blend; (ii) from about
5 to 100
parts by weight of a water component, and (iii) from about 1 to 20 parts by
weight of finely
divided solids comprising a mineral support and an oxide and/or acid addition
salt of a
Group 3-16 metal, preferably a Group 8-10 metal (preferably FeCl3 on an NaCl-
treated
clay);
spraying the FICP feed emulsion in a pyrolysis reactor at a temperature from
about 425 C to about
600 C, preferably 450 C to 500 C;
collecting an effluent from the pyrolysis reactor; and
recovering a product LIP from the effluent for use in the combining step to
form the LIP blend.
B5. The process of embodiment B4 wherein the finely divided solids
comprises FeCl3 and
montmorillonite, preferably wherein the finely divided solids comprises:
(i) FeCl3 derived from the solids recovered from the treatment of iron with an
aqueous mixture of
hydrochloric and nitric acids, the FeCl3 supported on a brine-treated
montmorillonite,
preferably NaCl brine-treated calcium bentonite, and/or
(ii) the product of the method comprising the steps of:
treating iron with an aqueous mixture of hydrochloric and nitric acids to form
a solids mixture of
FeCl3 optionally with mixed valences of iron and iron chlorides, nitrites,
nitrites, oxides,
and/or hydroxides, preferably wherein the solids mixture has limited
solubility;
treating montmorillonite, preferably calcium bentonite, with brine, preferably
NaCl brine;
combining a slurry of the solids mixture with the dried, treated
montmorillonite to load the FeCl3
on the montmorillonite; and
heat treating the loaded montmorillonite at a temperature above 400 C,
preferably 400 C to 425 C.
B6. An emulsion flash chemical ionizing pyrolysis (FCIP) process
comprising the steps of:
preparing an FCIP feed emulsion comprising 100 parts by weight of an oil
component, from about
5 to 100 parts by weight of a water component, and from about 1 to 20 parts by
weight of
finely divided solids comprising a mineral support and an oxide or acid
addition salt of a
Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-treated clay);
spraying the FICP feed emulsion in a flash pyrolysis reactor at a temperature
from about 425 C to
about 600 C;
collecting an effluent from the pyrolysis reactor;
- 43 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
recovering a product liquid ionizing pyrolyzate (LIP) from the effluent;
combining at least a portion of the product LIP with a feedstock oil to form
an LIP blend
comprising from 1 to 33.33 wt% of the product LIP; and
thermally processing the LIP blend to form a hydrocarbon product having an
enriched yield of
liquid hydrocarbons boiling at a temperature below 562 C, relative to separate
thermal
processing of the LIP and feedstock oil, relative to separate thermal
processing of the LIP
and feedstock oil, as determined by atmospheric distillation in a 15-
theoretical plate
column at a reflux ratio of 5:1, according to ASTM D2892-18 up to cutpoint 400
C AET,
and by vacuum potstill method according to ASTM D5236-18a above the 400 C
cutpoint
to cutpoint 562 C AET.
B7. The process of embodiment B6, further comprising supplying at least
a portion of the LIP
blend as the oil component to the FCIP feed emulsion preparation step wherein
the thermal
processing step consists of or comprises the spraying of the FCIP feed
emulsion into the flash
pyrolysis reactor.
B8. An emulsion flash chemical ionizing pyrolysis (FCIP) process comprising
the steps of:
preparing an FCIP feed emulsion comprising (i) 100 parts by weight of an oil
component
comprising a feedstock oil and from 1 to 33.33 wt% of a liquid hydrocarbon
pyrolyzate
(LIP), based on the total weight of the oil component, (ii) from about 5 to
100 parts by
weight of a water component, and (iii) from about 1 to 20 parts by weight
finely divided
solids comprising a mineral support and an oxide or acid addition salt of a
Group 3 ¨ 16
metal;
spraying the FCIP feed emulsion in a pyrolysis reactor at a temperature from
about 425 C to about
600 C;
collecting an effluent from the pyrolysis reactor;
recovering a product LIP from the effluent; and
optionally supplying a portion of the product LIP to the feed emulsion
preparation step.
B9. A hydrocarbon refinery process comprising the steps of:
combining a liquid ionizing pyrolyzate (LIP) with a feedstock oil to form an
LIP blend comprising
the LIP in an amount from 1 to 33.33 wt% based on the total weight of the LIP
blend;
preparing an FCIP feed emulsion comprising (i) 100 parts by weight of a first
portion of the LIP
blend, (ii) from about 5 to 100 parts by weight of a water component, and
(iii) from about
- 44 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
1 to 20 parts by weight finely divided solids comprising a mineral support and
an oxide or
acid addition salt of a Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-
treated clay);
spraying the FCIP feed emulsion in an emulsion flash chemical ionizing
pyrolysis reactor at a
temperature from about 425 C to about 600 C;
collecting an effluent from the flash pyrolysis reactor;
recovering a product LIP from the effluent;
incorporating at least a portion of the product LIP into the LIP blend; and
distilling a second portion of the LIP blend.
B10. The process of embodiment B9, wherein the feedstock oil comprises crude
oil, preferably
un-desalted crude oil wherein the process further comprises water washing to
desalt the second
portion of the LIP blend, and distilling the desalted second portion of the
LIP blend.
B11. The process of embodiment B9 wherein the feedstock oil comprises crude
oil and further
comprising washing the LIP blend with wash water, recovering a solute-enriched
spent water from
the water washing step, recovering a desalted LIP blend, and heating the
desalted LIP blend,
preferably in advance of distillation of the LIP blend.
B12. A hydrocarbon refinery process comprising the steps of:
preparing a feed emulsion comprising (i) 100 parts by weight of an oil
component, (ii) from about
5 to 100 parts by weight of a water component, and (iii) from about 1 to 20
parts by weight
finely divided solids comprising a mineral support and an oxide or acid
addition salt of a
Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-treated clay);
spraying the feed emulsion in a flash pyrolysis reactor at a temperature from
about 425 C to about
600 C;
collecting an effluent from the flash pyrolysis reactor;
recovering a liquid ionizing pyrolyzate (LIP) from the effluent;
combining the recovered LIP with a feedstock oil comprising a petroleum
fraction selected from
gas oil, resid, or a combination thereof to form an LIP blend; and
distilling, cracking, visbreaking, and/or coking the LIP blend.
B13. The process of embodiment B12 wherein the oil component in the feed
emulsion from the
preparation step comprises the petroleum fraction, preferably the LIP blend
from the combining
step.
- 45 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
B14. The process of any of embodiments B6 to B13 wherein the pressure in the
pyrolysis reactor
is from about 1 to 3 atm, preferably 1 to 1.5 atm.
B15. The process of any of embodiments B6 to B13 wherein the LIP blend
comprises the
feedstock oil and a proportion of the LIP effective to improve conversion in
the pyrolysis reactor
.. of the oil component to the LIP at an enriched yield of liquid hydrocarbons
boiling at a temperature
below 562 C, and/or an enriched yield of distillates, relative to separate
FCIP of the LIP and
feedstock oil, relative to separate thermal processing of the LIP and
feedstock oil, as determined
by atmospheric distillation in a 15-theoretical plate column at a reflux ratio
of 5:1, according to
ASTM D2892-18 up to cutpoint 400 C AET, and by vacuum potstill method
according to ASTM
D5236-18a above the 400 C cutpoint to cutpoint 562 C AET.
B16. The process of any of embodiments B6 to B13 wherein the LIP blend
comprises the LIP
in an amount from 1 to 33.33 percent and the feedstock oil in an amount from
99 to 66.67 percent,
by weight of the LIP blend, preferably from 5 to 25 percent LIP and from 95 to
75 percent
feedstock oil, more preferably from 10 to 20 percent LIP and from 90 to 80
percent feedstock oil.
B17. The process of any of embodiments B6 to B13 wherein the mineral support
comprises
montmorillonite, preferably bentonite, more preferably wherein the process
comprises treating
calcium bentonite with a sodium chloride brine and/or heat treating the
bentonite, preferably to a
temperature of 400 C to 425 C.
B18. The process of embodiment B17 wherein the finely divided solids comprises
FeCl3 and
NaCl-treated montmorillonite.
B19. The process of embodiment B17, wherein the finely divided solids
comprises the reaction
product of elemental iron with an aqueous mixture of hydrochloric acid and
nitric acid, preferably
wherein a molar ratio of the iron to the total hydrochloric and nitric acids
is from 1:2 to 2:1, a
molar ratio of the iron to water is from 1:2 to 2:1, and/or a molar ratio of
hydrochloric acid to nitric
acid is from 1:1 to 10:1, more preferably the reaction product of equal
weights of the iron and aqua
regia wherein the aqua regia comprises 3 parts by weight hydrochloric acid, 2
parts by weight
water, and 1 part by weight nitric acid.
B20. The process of embodiment B19, wherein the finely divided solids
comprises the reaction
product of the iron and the aqueous hydrochloric and nitric acids loaded on
NaCl-treated calcium
bentonite and heat treated, preferably to 400 C to 425 C.
- 46 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
B21. The process of any of embodiments B6 to B13, further comprising
preparation of the finely
divided solids according to a procedure comprising the steps of:
(a) reacting elemental iron with an aqueous mixture of hydrochloric acid
and nitric acid,
preferably wherein a molar ratio of the iron to the total hydrochloric and
nitric acids is from
1:2 to 2:1, a molar ratio of the iron to water is from 1:2 to 2:1, and/or a
molar ratio of
hydrochloric acid to nitric acid is from 1:1 to 10:1, more preferably the
reaction product of
equal weights of the iron and aqua regia wherein the aqua regia comprises 3
parts by weight
hydrochloric acid, 2 parts by weight water, and 1 part by weight nitric acid;
(b) treating calcium bentonite with NaCl brine;
(c) loading the reaction product from (a) on the treated bentonite from
(b), preferably by
incipient wetness, more preferably by drying the treated bentonite from (b),
slurrying the
reaction product from (a), and contacting the dried bentonite with the slurry;
(d) heat treating the bentonite loaded with the reaction product,
preferably by heating to a
temperature from 400 C to 425 C; and
(e) grinding the heat treated sodium bentonite, preferably to a size
passing a 60 mesh screen.
B22. The process of any of embodiments B1 to B21 wherein the oil component (if
present)
and/or the feedstock oil comprise crude oil.
B23. The process of any of embodiments B1 to B21 wherein the oil component (if
present)
and/or the feedstock oil comprise heavy crude oil.
B24. The process of any of embodiments B1 to B21 wherein the oil component (if
present)
and/or the feedstock oil comprise diesel.
B25. The process of any of embodiments B1 to B21 wherein the oil component (if
present)
and/or the feedstock oil comprise atmospheric resid.
B26. The process of any of embodiments B1 to B21 wherein the oil component (if
present)
and/or the feedstock oil comprise vacuum resid.
A hydrocarbon desulfurization process, comprising the steps of:
emulsifying water and a high sulfur oil component comprising a feedstock oil
with finely divided
solids comprising a mineral support and an oxide and/or acid addition salt of
a Group 3-
16 metal (preferably FeCl3 on an NaCl-treated clay);
- 47 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
introducing the emulsion into a flash chemical ionizing pyrolysis (FCIP)
reactor maintained at a
temperature greater than about 400 C up to about 600 C and a pressure up to
about 1.5 atm
to form an ionized pyrolyzate effluent;
condensing the ionized pyrolyzate from the effluent to recover a liquid
ionized pyrolyzate (LIP)
having a reduced sulfur content relative to the high sulfur oil component.
C2. The process of embodiment Cl, wherein the solids comprise brine-
treated clay and an acid
addition salt of a Group 8 ¨ 10 metal, wherein the brine comprises a salt that
forms a eutectic with
the acid addition salt of the Group 8 ¨ 10 metal.
C3. The process of embodiment C2, wherein the clay comprises bentonite,
the brine comprises
sodium chloride, and the acid addition salt comprises FeCl3.
C4. The process of embodiment C3, comprising preparing the solids by a
method comprising
the steps of:
(a) contacting bentonite with the sodium chloride brine;
(b) contacting an excess of iron with an aqueous mixture of hydrochloric and
nitric acids
to form FeCl3 solids;
(c) loading the FeCl3 solids on the brine-treated bentonite; and
(d) calcining the loaded bentonite at a temperature below the FCIP
temperature.
C5. The process of any of embodiments Cl to C4, further comprising:
wherein the emulsion comprises (i) 100 parts by weight of the oil component,
preferably wherein
the oil component comprises the pyrolyzate-feedstock blend; (ii) from about 1
to 100
parts by weight of water, and (iii) from about 1 to 20 parts by weight of the
finely divided
solids; and
spraying the emulsion into the reactor, wherein the reactor temperature is
from about 425 C to
about 600 C, preferably 450 C to 550 C.
C6. The process of embodiment C5 wherein the finely divided solids comprise
the product of
the method comprising the steps of:
treating iron with an aqueous mixture of hydrochloric and nitric acids to form
a solids mixture of
FeCl3 optionally with mixed valences of iron and iron chlorides, nitrites,
nitrites, oxides,
and/or hydroxides, wherein the solids mixture has limited solubility;
treating montmorillonite with NaCl brine and drying the treated
montmorillonite;
- 48 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
combining a slurry of the solids mixture with the treated montmorillonite to
load the FeCl3 on the
montmorillonite; and
heat treating the loaded montmorillonite at a temperature above 400 C.
C7. The process of any of embodiments Cl to C6, further comprising
combining the
.. feedstock oil with the LIP from the condensation step to form the oil
component for the
emulsifying step (preferably at weight ratio of 5-35 wt% LIP and 95-65 wt%
feedstock oil).
C8. The process of embodiment Cl, further comprising:
combining the feedstock oil with the LIP from the condensation step to form a
pyrolyzate-
feedstock blend; and
thermally processing the blend at a temperature above about 100 C.
C9. The process of embodiment C8, wherein the feedstock oil comprises
hydrocarbons boiling
at a temperature equal to or greater than 562 C, and further comprising the
step of recovering a
hydrocarbon product from the thermally processed blend, the hydrocarbon
product having an
enriched yield of liquid hydrocarbons boiling at a temperature below 562 C,
relative to separate
thermal processing of the LIP and feedstock oil, as determined by atmospheric
distillation in a 15-
theoretical plate column at a reflux ratio of 5:1, according to ASTM D2892-18
up to cutpoint
400 C AET, and by vacuum potstill method according to ASTM D5236-18a above the
400 C
cutpoint to cutpoint 562 C AET.
C10. The process of embodiment C9 wherein the feedstock oil is crude oil, gas
oil, resid, or a
mixture thereof.
C11. The process of any of embodiments C8 to C10 wherein the thermal
processing comprises
pyrolysis, distillation, cracking, alkylation, visbreaking, coking, and
combinations thereof
C12. The process of any of embodiments C8 to C11, further comprising supplying
at least a
portion of the pyrolyzate-feedstock blend as the oil component to the FCIP
feed emulsion
preparation step wherein the thermal processing step consists of or comprises
the spraying of the
FCIP feed emulsion into the flash pyrolysis reactor.
C13. A flash chemical ionizing pyrolysis (FCIP) process comprising the steps
of:
preparing a feed emulsion comprising (i) 100 parts by weight of an oil
component comprising a
liquid ionizing pyrolyzate (LIP) and a high sulfur feedstock oil at a weight
ratio of from
1:100 to 1:1, (ii) from about 1 to 100 parts by weight of water, and (iii)
from about 1 to
- 49 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
20 parts by weight finely divided solids comprising a mineral support and an
oxide or
acid addition salt of a Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-
treated clay);
spraying the feed emulsion in a flash pyrolysis reactor at a temperature from
about 425 C to
about 600 C;
collecting an effluent from the reactor;
recovering a product oil from the effluent, wherein the product oil has a
sulfur content lower than
sulfur content of the oil component; and
supplying a portion of the product oil as the LIP to the feed emulsion
preparation step.
C14. A hydrocarbon refinery process comprising the steps of:
combining a liquid ionizing pyrolyzate (LIP) blend component with a high
sulfur feedstock oil at
a weight ratio from about 1:100 to about 1:1 to form an LIP blend;
preparing an emulsion comprising (i) a first portion of the LIP blend, (ii)
water, and (iii) from
finely divided solids comprising a mineral support and an oxide or acid
addition salt of a
Group 3 ¨ 16 metal (preferably FeCl3 on an NaCl-treated clay);
spraying the emulsion in a flash pyrolysis reactor at a temperature from about
425 C to about
600 C and a pressure from about 1 to about 1.5 atm;
collecting an effluent from the reactor;
recovering a product LIP from the effluent;
incorporating the product LIP as the LIP blend component in the LIP blend; and
distilling a second portion of the LIP blend.
C15. The process of embodiment C14, wherein the feedstock oil comprises crude
oil.
C16. The process of embodiment C15, wherein the feedstock oil comprises un-
desalted crude
oil wherein the process further comprises water washing to desalt the second
portion of the LIP
blend, and distilling the desalted second portion of the LIP blend.
C17. The process of embodiment C11 wherein the feedstock oil comprises high
sulfur crude
oil and further comprising washing the LIP blend with wash water, recovering a
solute-enriched
spent water from the water washing step, recovering a desalted LIP blend, and
heating the
desalted LIP blend in advance of distillation of the LIP blend.
C18. A hydrocarbon refinery process comprising the steps of:
preparing a feed emulsion comprising (i) 100 parts by weight of an oil
component, (ii) from about
1 to 100 parts by weight of water, and (iii) from about 1 to 20 parts by
weight finely divided
- 50 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
solids comprising a mineral support and an oxide or acid addition salt of a
Group 3 ¨ 16
metal (preferably FeCl3 on an NaCl-treated clay);
spraying the feed emulsion in a flash pyrolysis reactor at a temperature from
about 425 C to about
600 C;
collecting an effluent from the flash pyrolysis reactor;
recovering a liquid ionizing pyrolyzate (LIP) from the effluent;
combining the recovered LIP with a high sulfur feedstock oil comprising crude
oil or a petroleum
fraction selected from gas oil, resid, or a combination thereof to form a
pyrolyzate-
feedstock blend;
distilling, cracking, visbreaking, and/or coking a first portion of the LIP
blend; and
supplying a second portion of the LIP blend as the oil component in the feed
emulsion preparation
step.
C19. The process of embodiment C18, wherein the LIP exhibits a SARA analysis
having
higher saturates and aromatics contents and a lower asphaltenes content than
the feedstock oil.
C20. The process of embodiment C18 or C19 wherein a proportion of the LIP in
the oil
component in the flash pyrolysis is effective to improve yield of liquid
hydrocarbons boiling at a
temperature below 562 C, relative to separate flash chemical ionizing
pyrolysis of the LIP and
feedstock oil, as determined by atmospheric distillation in a 15-theoretical
plate column at a reflux
ratio of 5:1, according to ASTM D2892-18 up to cutpoint 400 C AET, and by
vacuum potstill
method according to ASTM D5236-18a above the 400 C cutpoint to cutpoint 562 C
AET.
C21. The process of any of embodiments C18 to C20 wherein a proportion of the
LIP in the LIP
blend in the distillation, cracking, visbreaking, and/or coking step, is
effective to improve yield of
liquid hydrocarbons boiling at a temperature below 562 C, relative to separate
distillation,
cracking, visbreaking, and/or coking of the LIP and feedstock oil, as
determined by atmospheric
distillation in a 15-theoretical plate column at a reflux ratio of 5:1,
according to ASTM D2892-18
up to cutpoint 400 C AET, and by vacuum potstill method according to ASTM
D5236-18a above
the 400 C cutpoint to cutpoint 562 C AET.
EXAMPLES
[0149] Example 1A: Preparation of supported iron solids: Preferred finely
divided solids
according to the present invention were prepared by loading oxidized Fe
material containing FeCl3
on NaCl-treated calcium bentonite generally using the process 300 of FIG. 3.
The Fe was prepared
-51 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
by mixing with constant stirring 1 part by weight 100 mesh carbon steel
shavings with 1 part by
weight aqua regia (1 part by weight nitric acid, 3 parts by weight
hydrochloric acid, 2 parts by
weight water). The aqua regia was added in three aliquots (1 part each, i.e.,
1/3, 1/3, 1/3), and the
temperature increased to 95 C. The material dried considerably, leaving wet
solids. The oxidized
iron solids were washed with water, filtered, dried in an oven at 100 C, and
ground to pass a 100
mesh screen. The oxidized iron solids had a black or dark violet color
indicative of FeCl3.
[01501 The oxidized iron solids were analyzed by wet chemistry by sequential
digestion in hot
water, followed by digestion of the water-insoluble solids in 20 wt% HC1(aq),
and recovery of the
insoluble material which was not further analyzed. Initially, a 5 g sample of
the oxidized iron solids
was placed in 150 ml of 100 C water, and the water-insoluble solids remaining
were recovered
and weighed. The amount digested in the water was surprisingly only 1.4488 g,
or 28.98 wt%. The
filtrate was diluted to 1 L and the solute was found by spectrophotometry to
contain 11.32 wt%
total Fe consisting of 3.24 wt% Fe(II) and 8.08 wt% Fe(III), 32.79 wt%
chloride, 3.52 wt% nitrite,
and 1.17 wt% nitrate. The water-soluble fraction was thus determined to be
mostly chloride and
nitrite salts with some nitrate salts.
[0151] The water-insoluble fraction was then digested in 150 ml of 20% HC1 in
water, and 3.478
g went into solution, or 69.56 wt% of the initial oxidized iron sample. The
acid soluble fraction
was found to contain 62.23 wt% total Fe consisting of 7.04 wt% Fe(II) and
55.19 Fe(III), 51.18
wt% nitrate, and 0.2587 wt% nitrite. The acid soluble fraction was thus found
to contain mostly
ferric oxides and/or nitrates, with some ferrous iron and a small amount of
nitrite. From a relatively
small proportion of ferrous iron seen in the acid soluble fraction, it was
inferred that little or no
elemental iron was present. The acid insoluble fraction was just 1.46 wt% of
the original sample,
and appeared from its red color to be Fe(III) oxide, hematite. The wet
chemistry data are
summarized in Table 1.
.. [0152] A 100 mesh calcium bentonite was obtained commercially. A 1 M
aqueous NaCl brine was
prepared from distilled water and salt obtained commercially. The bentonite
was prepared by
mixing the as-received bentonite with the brine at a 1:2 weight ratio (1 part
by weight bentonite, 2
parts by weight brine), stirring for 1 hour, and then allowing the mixture to
sit for 16-24 hours.
The excess brine was removed, the NaCl-treated bentonite dried at 120-130 C
for 4 ¨ 6 hours, and
the dried material ground to pass through an 80 mesh screen. The dried NaCl-
bentonite had a
reddish-brown to dark violet color.
- 52 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
[0153]
TABLE 1. WET CHEMISTRY ANALYSIS OF IRON OXIDIZED BY AQUA REGIA
Total
Nitrate Nitrite
Iron, Fe(II), Fe(III), Chloride, (NO3-), (NO2-),
Sample Mass, g wt% wt% wt% wt%
Original Sample 5
Water Solubles 1.449 11.32 3.24 8.08 32.79
1.17 3.52
Acid Solubles 3.478 62.23 7.04 55.19 nd
51.18 0.2587
Acid Insolubles 0.073 nd nd nd nd
nd nd
nd = not determined
[0154] The 100 mesh oxidized iron was slurried at 1 part by weight oxidized
iron in 24 parts by
weight distilled water (4 wt% oxidized iron). Then 2 parts by weight of the
slurry were mixed with
3 parts by weight of the dried 80 mesh bentonite, the resulting paste dried at
400 C for 2 hours in
.. an oven, and the solids cooled and ground to pass a 60 mesh screen. This
oxidized Fe-bentonite,
or one prepared in a similar manner, was used in the following examples.
[0155] Example 1B: Preparation of supported iron solids: The finely divided
solids were
prepared as in Example 1A except 1 part by weight 100 mesh carbon steel
shavings was mixed
with 1 part by weight aqua regia comprising 1 parts by weight nitric acid, 6
parts by weight
hydrochloric acid, and 2 parts by weight water, and/or the bentonite was
treated with 2 molar NaCl
brine and was not rinsed with water prior to drying.
[0156] Example 2: Steady State Flash Chemical Ionizing Pyrolysis Tests: These
flash chemical
ionizing pyrolysis (FCIP) tests used a pilot plant scale reactor similar to
the direct-heating design
shown in FIG. 9, except that only one exchanger downstream from the cyclone
was used and there
were no solids discharged from the reactor. Instead, a bed of sand was placed
in the bottom of the
reactor and some solids accumulated on the sand during the test. The reactor
was heated by
combustion flue gas flowing into the side of the reactor near the bottom. A
slurry injection nozzle
pointed downwardly (countercurrent to the flue gases) was positioned 1/3 of
the way from the top
of the reactor toward the bottom to provide a conical spray pattern. The
reactor was equipped with
thermocouples in the combustion chamber, within the reactor, at the top of the
reactor, and in the
cyclone.
[01571 An emulsion of heavy crude (API <10 ) was prepared by heating the crude
oil to 70 C,
adding water and mixing with an overhead mixer for 10 minutes, then adding the
finely divided
solids, FeCl3 on NaCl-treated bentonite prepared in a manner similar to
Example 1A, and mixing
- 53 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
for another 5 minutes. The resulting emulsion was composed of 5 parts by
weight finely divided
solids, 30 parts by weight water (added water plus water in heavy oil sample),
and 65 parts by
weight oil (heavy oil less water and solids).
[0158] The reactor was heated up to operating temperature with combustion
gases only before the
slurry feed was started. The reactor was then brought to steady state over 1-2
hours at a reactor
temperature generally between 400 C and 600 C, the reactor outlet temperature
generally between
300 C and 400 C, and the cyclone temperature between 200 C and 300 C while
maintaining the
combustion at a steady rate between 1100 C and 1200 C, adjusting the emulsion
feed rate as
necessary to obtain the desired temperatures, and collecting the pyrolyzate
liquids from the
condenser. The recovered liquid ionizing pyrolyzate (LIP) was a low viscosity,
low-density ( API
> 30) liquid representing a recovery of 90 wt% of the oil from the slurry,
while non-condensable
gases represented just 4 wt% of the oil in the slurry.
[0159] Example 3: Flash Chemical Ionizing Pyrolysis with Maya Crude Oil-LIP
Blends: In this
example, flash chemical ionizing pyrolysis (FCIP) was conducted by the
following procedure. The
finely divided solids were the FeCl3 on NaCl-treated bentonite prepared in a
manner similar to
Example 1A and/or 1B. The emulsion was prepared with a commercial blender,
placed in a tank
heated at 90 C, pressurized at 2-8 kg/cm2 with inert gas, and fed to a nozzle
with a conical spray
pattern in a reactor measuring 8 in. diameter by 16 in. long. The reactor was
heated using a gas
burner, and a sand bed was placed in the reactor at the beginning of the test.
The effluent was
passed through a water-cooled condenser and the condensate was collected and
separated into oil,
water, and solids.
[01601 A 22 API Maya crude oil was used. The crude had a composition by
retort distillation of
71 wt% oil (0-520 C), 28 wt% heavy hydrocarbons (>520 to 800 C), and 1 wt%
inorganic solids.
The physical properties and distillation fractions are described below in
Table 2.
[0161] First, in Run 3-1, an emulsion was prepared as a baseline using 100%
crude, and subjected
to FCIP at 470 C. The FCIP product mix obtained a gas yield of 14%, an oil
("LIP-M1") yield of
69% (retort distillation <550-600 C), a resid yield of 11% (>600 C), and coke
yield of 6%,
expressed as percentages of the oil in the FCIP emulsion.
[0162] Then an emulsion was prepared in Run 3-2 as an example according to the
present
invention, using 90% of the crude and 10% of the LIP-M1 from the crude FCIP in
Run 3-1,
subjected to FCIP at 430 C. The yields were gas 7%, oil ("LIP-B1") 89%, and
coke 4%, expressed
- 54 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
as percentages of the oil in the FCIP emulsion. These represent yield
increases in the oil and
decreases in the resid, gas and coke, all to a greater extent than
theoretical.
[0163] Then another emulsion was prepared for Run 3-3 as another example
according to the
present invention, again using 90% of the crude and 10% of the LIP-M1 from the
crude FCIP in
Run 3-1, subjected to FCIP at 470 C, and the yields were gas 3%, oil ("LIP-
B2") 93%, and coke
4%, expressed as percentages of the oil in the FCIP emulsion. These likewise
represent yield
increases in the oil and decreases in the resid, gas and coke, all greater
than theoretical relative to
LIP-Ml. The crude oil, emulsions, and FCIP products had the characteristics
shown in Tables 2-
3.
.. [0164] It is considered that if the yields of FCIP of oil LIP-M1 alone is
assumed to be 100%, then
the theoretical oil LIP-B1/LIP-B2 yields from FCIP of the 90:10 blend of Maya
crude and LIP-
M1 would be (0.9*80.3) + (0.1*100) = 82.3wt%. However, the resulting yields of
89.3wt% of
LIP-B1 for FCIP at 430 C, and 93.17 of LIP-B2 for FCIP at 470 C (see Table
2), demonstrated
an unexpected synergy in FCIP thermal processing of the blends of Maya crude
and LIP-Ml.
Moreover, the improved quality of the LIP-B1 and LIP-B2, namely an increased
level of
isomerates, was demonstrated by the lower viscosities at 100 C and/or 40 C
and higher initial
boiling points, relative to the LIP-M1 product.
- 55 -
CA 03114476 2021-03-25
WO 2020/086948 PCT/US2019/058034
TABLE 2. MAYA CRUDE, BLENDS, AND FCIP CHARACTERIZATION
Property Unit Maya Run 3- Run 3-2 Run 3-3
Crude 1
FLASH CHEMICAL IONIZING PYROLYSIS
Emulsion Feed Composition
Oil (<600 C) wt% N/A 57.50 51.38 51.38
Heavy HC wt% N/A 22.68 20.26 20.26
LIP-M wt% N/A -- 9.04 9.04
Water wt% N/A 15.00 15.01 15.01
Finely divided solids wt% N/A 4.01 3.58 3.58
Other solids wt% N/A 0.81 0.73 0.73
Reactor Temperature C N/A 470 430 470
PRODUCT (LIP) YIELDS
Oil (<600 C) wt% N/A 80.38 89.3 93.17
Gas wt% N/A 13.57 6.63 3.05
Coke wt% N/A 6.05 4.07 3.78
OIL PHYSICAL PROPERTIES
Designation Crude LIP-M1 LIP-B1 LIP-B2
omq omq 22 35.60 35.60 35.60
Density g/cm3 0.92 0.847 0.847 0.847
Viscosity @40 C cP 459.20 13.30 14.43 11.76
Viscosity@100 C cP 58.68 11.85 7.05 6.45
Flash Point C 133 33.4 31.0 36.0
Initial Boiling Point C 155 100 108 145
Conradson carbon % CC 11.96 1 1 1
TABLE 3. MAYA CRUDE DISTILLATES CHARACTERIZATION
PROPERTY/FRACTION F-1 F-2 F-3 F-4 F-
5
Recovery, Weight % 13.2 11.1 18.4 25.9 0
Distillation Temp. ( C) <330 331-344 345-423 423-428 453-528
omq 52 39 35 31 X
Density (g/cm3) 0.77 0.83 0.85 0.87 X
Viscosity @ 50 C (cP) nd nd 9.63 10.35 X
Aniline Point ( C) 61 65 63 57 X
Flash Point ( C) 32 81 32 35 X
Initial Boiling Point ( C) 120 145 67 164 X
X = no product; nd = not determined
[0165] Example 4: Flash Chemical Ionizing Pyrolysis of Maya Crude: In Run 4,
an 8 API Maya
crude oil was subjected to FCIP to produce an LIP (LIP-B3) in a manner similar
to LIP-B2 in Run
3-3. SARA analyses of the crude and LIP showed the results in Table 4 below.
The LIP
- 56 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
unexpectedly had more than twice the saturates, and more than three times the
aromatics, slightly
less resins, and substantially lower asphaltenes, relative to the crude
starting material. This shows
that primarily the asphaltenes were converted to saturates and aromatics.
TABLE 4. SARA ANALYSES OF 8 API CRUDE AND
LIP FROM FCIP
Component 8 API Crude LIP-B3
Saturates, wt 4 10
Aromatics, wt% 12 40
Resins, wt% 37 36
Asphaltenes, wt% 47 14
[0166] Example 5: Desulfurization of Maya Crude Oil-LIP Blends in Flash
Chemical Ionizing
Pyrolysis. In this example, Maya crude (Run 5-1) and a mixture (Run 5-2) of 85
wt% Maya crude
and 15 wt% liquid ionizing pyrolyzate (an LIP-M from FCIP of the Maya crude)
were subjected
to FCIP in a manner similar to Examples 3 and 4, to study sulfur removal. In
FCIP, sulfur can be
removed by reduction of organic sulfur compounds by reactive hydrogen radicals
to produce H25,
and/or by oxidation of organic sulfur compounds by reaction with HOC1 to form
SO, compounds.
As determined by ASTM D4294, the Maya crude had an initial sulfur content of
4.4 wt%. When
the Maya crude by itself was subjected to FCIP in Run 5-1, the resulting LIP-
M2 had an ASTM
D4294 sulfur content of 2.7 wt%. However, when the 85:15 blend of Maya crude
and LIP-M2 was
subjected to FCIP under similar conditions in Run 5-2, the resulting LIP-B4
had an ASTM D4294
sulfur content of 1.5 wt%, demonstrating synergy in sulfur removal when the
blend was thermally
processed by FCIP. The results are listed in Table 5.
[01671 Example 6: Desulfurization of Texistepec Crude Oil-LIP Blends in Flash
Chemical
Ionizing Pyrolysis. In this example, Texistepec crude (Run 6-1) and a mixture
(Run 6-2) of 85 wt%
Texistepec crude and 15 wt% liquid ionizing pyrolyzate (an "LIP-T" from FCIP
of the Texistepec
crude) were subjected to FCIP in a manner similar to Example 5, to study
sulfur removal. In FCIP,
sulfur can be removed by reduction of organic sulfur compounds by reactive
hydrogen radicals to
produce H25, and/or by oxidation of organic sulfur compounds by reaction with
HOC1 to form
SO x compounds. As determined by ASTM D4294, the Texistepec crude had an
initial sulfur
content of 9.7 wt%. When the Texistepec crude by itself was subjected to FCIP
in Run 6-1, the
resulting LIP-Ti had an ASTM D4294 sulfur content of 6.6 wt%. However, when
the 85:15 blend
of Texistepec crude and LIP-Ti was subjected to FCIP under similar conditions
in Run 6-2, the
resulting LIP-B5 had an ASTM D4294 sulfur content of 5.4 wt%, again
demonstrating synergy in
- 57 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
sulfur removal when the blend was thermally processed by FCIP. The results are
also listed in
Table 5.
Table 5. FCIP Desulfurization of Crude and Crude-LIP Blends
FCIP Crude, wt% LIP, wt% FCIP Product ASTM D4294
Run Designation S content, wt%
N/A Maya, 100 N/A 4.4
5-1 Maya, 100 LIP-M2 2.7
5-2 Maya, 85 LIP-M2, 15 LIP-B4 1.5
N/A Texistepec, 100 -- N/A 9.7
6-1 Texistepec, 100 -- LIP-Ti 6.6
6-2 Texistepec, 85 LIP-T1, 15 LIP-B5 5.4
[0168] Example 7: Distillation of Maya Crude Oil-LIP Blends: In this example,
distillation of
100% Maya crude (22-23 API) was compared with distillation in an identical
manner of blends
of the Maya crude with 10, 20, and 30 wt% of a liquid ionizing pyrolyzate (LIP-
M3) obtained by
the flash chemical ionizing pyrolysis (FCIP) of the Maya crude in a manner
similar to Example 3.
The distillation comprised or was similar to atmospheric distillation in a 15-
theoretical plate
column at a reflux ratio of 5:1, according to ASTM D2892-18 up to cutpoint 400
C AET, and by
vacuum potstill method according to ASTM D5236-18a above the 400 C cutpoint to
cutpoint
562 C AET. Table 5 below lists the distillate yields and Conradson carbon
residue (CCR) of the
distillates from atmospheric and vacuum distillation. These data show that not
only were the liquid
yields synergistically higher for the crude-LIP blends, the quality of the
distillates was
unexpectedly improved, as reflected in the substantially lower CCRs of the
distillates from the
blends.
TABLE 6. DISTILLATE YIELDS AND CCR'S OF CRUDE, LIP, AND BLENDS
FCIP Maya LIP-M3, Distillation Conradson Carbon
Run Crude, wt% wt% Yield, wt% Residue, wt%
7-1 100 60 12
7-2 80 20 68 7.6
7-3 70 30 74 5
7-4 100 89 4
[0169] The characteristics of the selected fractions of distillation of the
Maya crude by itself are
similar to those presented in Example 3 and Table 3. The data obtained for
characteristics of
selected fractions of the distillation of the 90:10 and 80:20 Maya crude:LIP
blends are shown in
Tables 7 and 8 below. These data show that blending a liquid ionizing
pyrolyzate with a crude oil
can synergistically increase distillation oil yield and reduce coke and gas
yields in excess of
- 58 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
theoretical, even assuming the LIP blend component converts 100% to oil and 0%
to gas and coke.
Moreover, the quality of the recovered oil is also improved, for example, no F-
5 fraction was
obtained from the Maya crude distilled by itself, but was recovered in both
the 10 and 20% LIP
blends. The density of each of the fractions F-1 to F-5 in the blends is the
same or lower than the
Maya crude distillation, e.g., F-1 fraction was lighter as reflected in the
degrees API in the 10%
LIP distillation, while F-1, F-2, and F-3 in the 20% LIP distillation were
lighter (higher API
gravity).
TABLE 7.90% MAYA:10% LIP DISTILLATES CHARACTERIZATION
PROPERTY F-1 F-2 F-3 F-4 F-5
Recovery, Weight % 22.5 11.3 8.0 17.9 14.1
Distillation Temp. ( C) <342 343-383 384-404 405-440 441-497
omq 55 39 35 31 29
Density (g/cm3) 0.76 0.83 0.85 0.87 0.88
Viscosity @ 50 C (cP) 4.62 nd nd 14.67 nd
Aniline Point ( C) 60 64 62 59 58
Flash Point ( C) 32 52 88 49 54
Initial Boiling Point ( C) 125 220 240 125 130
nd = not determined
TABLE 8.80% MAYA:20% LIP DISTILLATES CHARACTERIZATION
PROPERTY F-1 F-2 F-3 F-4 F-5
Recovery, Weight % 19.1 9.4 14.5 20.5 14.6
Distillation Temp. ( C) <320 320-340 340-417 418-452 453-475
omq 62 45 35 33 31
Density (g/cm3) 0.73 0.8 0.85 0.86 0.87
Viscosity @ 50 C (cP) 5.13 nd nd 8.22 nd
Aniline Point ( C) 54 60 60 59 52
Flash Point ( C) 32 76 45 65 36
Initial Boiling Point ( C) 90 160 125 190 150
nd = not determined
[01701 The properties of the vacuum residuum from the distillation of the Maya
crude by itself,
.. the LIP by itself, and the 80:20 and 70:30 blends are listed in Table 9
below. These data show that
the resid is unexpectedly improved relative to that from the crude by itself
such that a delayed
coker is not needed or is only needed for a much lesser volume of coke
product. For example, the
low CCR values and low flow temperatures of the resid from the blends
indicates that the resid
can be used as a lube stock, which is a very valuable product compared to
resid from distillation
- 59 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
of the crude by itself. Moreover, if the resid is processed in a delayed
coker, the products from the
delayed coker are of much higher quality.
TABLE 9. CHARACTERISTICS OF RESID
FROM CRUDE, LIP, AND BLENDS
Resid Product From CCR, wt% Flow T, C
100% Maya Crude 30 >400
20% LIP/80% Crude 18 50
30% LIP/70% Crude 10 40
100% LIP 1 <0
[0171] Example 8: Diesel upgrading: Diesel fuel was obtained commercially and
blended with
an LIP obtained by FCIP of the diesel fuel at a weight ratio of 80:20
diesel:LIP. The blend and the
diesel were distilled from 58 C to 220 C similarly to the method of Example 5.
The product yields
are given in Table 10 below. The distillate yields for the fractions 1: 58-100
C, 2: 100-180 C, 3:
180-220 C, and residual (>220 C) are given in Table 11 below. The aniline
points, corresponding
to aromatics contents, are presented in Table 12.
TABLE 10. DIESEL AND LIP BLEND DISTILLATION
Product Initial Boiling Distillate Resid Gas, Resid
CCR,
Point, C (<220 C), wt% (>220 C), wt% wt% wt%
Diesel 58 54 44 2 0
80:20 blend* 60 83 16 1 0
Note: * = 80 wt% diesel fuel, 20 wt% LIP from FCIP of diesel fuel
TABLE 11. DIESEL AND BLEND DISTILLATION PRODUCT PROPORTIONS
Product 58-100 C, wt% 100-180 C, wt% 180-220 C, wt%
Diesel 9 49 42
80:20 blend* 20 41 39
Note: * = 80 wt% diesel fuel, 20 wt% LIP from FCIP of diesel fuel
TABLE 12. DIESEL/BLEND DISTILLATION PRODUCT ANILINE POINTS
Product Starting 1st Fraction 2nd Fraction 3rd
Fraction Residual
Material 58-100 C 100-180 C 180-220 C >220 C
Diesel 68 56 66 66 76
80:20 blend* 66 40 64 64 86
Note: * = 80 wt% diesel fuel, 20 wt% LIP from FCIP of diesel fuel
[0172] These data show that diesel can be upgraded to lower boiling products
in high yield by
FCIP and distillation of the LIP blend, with unexpected improvements in yield
and properties.
Notably, the residual material boiling above the 220 C cut point from the mix
had aniline point
- 60 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
of 86 a pour point of -5 C and a viscosity index of 253, compared to a pour
point of -4 C and a
viscosity index of 303 for the residual (>220 C) of the residual fraction
from distillation of the
diesel fuel by itself These data indicate the distillates and resid materials
from the diesel-LIP
mixtures have excellent properties for a solvent, e.g., for use in an oil-
based drilling fluid, or as
base stock oils.
TABLE 13. CHROMATOGRAM COMPARISON OF FIRST FRACTION (<100 C)
Relative response area (x 10-)
Retention time % Increase or
Alkane 1st Diesel 1st LIP-Diesel
(min) decrease
Fraction Mix Fraction
12.9 n-C10 3.320 5.04 51.81
14.4 n-C 11 5.036 5.99 18.86
15.6 n-C12 1.532 2.507 63.64
16.7 n-C13 1.210 2.248 85.79
17.8 n-C14 0.5030 1.412 180.72
18.8 n-C15 0.3740 7.758 107.43
20.1 n-C16 0.4754 0.4304 -9.47
25.3 n-C17 0.3850 0.2760 -28.31
[0173] Moreover, chromatographic analysis shows further unexpected results
comparing the
distillate fractions and the original diesel and diesel/LIP blend. The samples
were analyzed by GC-
MS of a 2 !IL sample at a concentration of 2 volume percent in methylene
chloride through an HP-
5MS SEMIVOL column of 30 m length and 0.25 mm ID with a temperature ramp from
50 C
initially held for 6 minutes up to 315 C at 15 C/minute. The original diesel
and the original blend
showed no significant difference and the chromatograms were virtually
identical. Chromatograms
of the first distillate fractions (<100 C) showed higher response areas for
the lower n-alkanes C10-
and lower response areas for the higher n-alkanes C16-17 from the blend
relative to the first
fraction from the diesel itself. These results are shown in Table 13.
15 [0174] Chromatograms of the second distillate fractions (100-180 C)
showed higher response
areas for the lower n-alkanes C10-13 and lower response areas for the higher n-
alkanes C14-17 from
the blend relative to the second fraction from the diesel itself. These
results are shown in Table 14.
TABLE 14. CHROMATOGRAM COMPARISON OF SECOND FRACTION (<100 C)
Relative response area (x 10-7)
Retention time % Increase or
Alkane 2nd Diesel 2nd LIP-Diesel
(min) decrease
Fraction Mix Fraction
12.9 n-C10 0.0789 2.27 2778.40
14.4 n-C 11 0.0517 5.49 963.70
15.6 n-C12 0.340 2.69 693.23
16.7 n-C13 0.408 3.68 803.31
- 61 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
17.8 n-C14 0.558 0.404 -27.62
18.8 n-C15 0.535 0.322 -39.80
20.1 n-C16 0.510 0.210 -58.86
25.3 n-C17 0.491 0.163 -66.91
[0175] Chromatograms of the third distillate fractions (180-220 C) showed
higher response areas
for the lower n-alkanes C10-12 and n-alkanes C14-17, and a lower response area
for the middle-range
n-alkane C13, from the blend, relative to the third fraction from the diesel
itself. These results are
shown in Table 15.
TABLE 15. CHROMATOGRAM COMPARISON OF THIRD FRACTION (<100 C)
Relative response area (x 10-)
Retention time % Increase or
Alkane 3rd Diesel 3rd LIP-Diesel
(min) decrease
Fraction Mix Fraction
12.9 n-C10 0.0650 0.623 858.89
14.4 n-C 11 0.449 2.75 512.73
15.6 n-C12 2.70 3.05 13.05
16.7 n-C13 4.42 3.58 -19.05
17.8 n-C14 4.55 7.12 56.56
18.8 n-C15 4.25 5.19 22.21
20.1 n-C16 4.84 5.02 3.66
25.3 n-C17 4.67 6.37 36.33
[0176] Chromatograms of the non-distilled, residual fractions (>220 C) showed
the residual from
the diesel itself was composed of primarily C12-17 hydrocarbons, whereas the
residual from the
blend was comprised of virtually no C12-16 alkanes and consisted almost
entirely of Ci7+
hydrocarbons. See the chromatograms shown in FIG. 10.
[01771 Example 9: FCIP with Texistepec/ Crude Oil-LIP Blends: In this example,
flash chemical
ionizing pyrolysis (FCIP) was conducted by the following procedure. The finely
divided solids
were the FeCl3 on NaCl-treated bentonite prepared in a manner similar to
Example 1A and/or 1B.
The emulsion was prepared with a commercial blender, placed in a tank heated
at 70-90 C,
pressurized at 2-8 kg/cm2 with inert gas, and fed to a nozzle with a conical
spray pattern in a reactor
measuring 8 in. diameter by 16 in. long. The reactor was heated using a gas
burner, and a sand bed
was placed in the reactor at the beginning of the test. The effluent was
passed through a water-
cooled condenser and the condensate was collected and separated into oil,
water, and solids.
[0178] An 8 API Texistepec crude oil having a viscosity of 144,400 cP at 40
C was used. The
crude had a composition by retort distillation of 46.1 wt% oil (0-600 C),
40.4 wt% heavy
- 62 -
CA 03114476 2021-03-25
WO 2020/086948
PCT/US2019/058034
hydrocarbons (>600 to 800 C), 8.1 wt% water, and 5.4 wt% inorganic solids.
First, in Run 9-1, a
baseline emulsion was prepared using all crude for the oil (0-600 C) and
heavy HC components,
14 wt% total water, and no finely divided solids other than the solids present
in the crude (5.4
wt%), and subjected to flash pyrolysis at 500-550 C. The product ("LIP-T3")
yield was just 55.2
wt% oil (<600 C), 8.4 wt% gas, and 36.4 wt% coke.
[0179] Then, in Run 9-2, an emulsion was prepared using all crude for the oil
and heavy oil
components, 16.2 wt% total water, and 3.8 wt% finely divided solids and
subjected to FCIP at
500-550 C. The FCIP product mix obtained a gas yield of 1.3 wt%, an oil ("LIP-
T4") yield of
87.7 wt%, and coke yield of 11 wt%, expressed as percentages of the oil in the
FCIP emulsion.
[01801 Then, in Run 9-3, an emulsion was prepared using 90 wt% of the crude
and 10 wt% of the
LIP-T4 from Run 9-2, similarly subjected to FCIP at 500-550 C. The yields
were gas 1.3 wt%,
oil ("LIP-B5") 95.2 wt%, and coke 3.5 wt%, expressed as percentages of the oil
in the FCIP
emulsion. These represent unexpected yield increases in the oil LIP-B5 and
decreases in the resid
and coke, all to a greater extent than theoretical (assuming the added LIP-T4
gives 100% oil and
0% coke yield). The results are summarized in Table 16.
- 63 -
CA 03114476 2021-03-25
WO 2020/086948 PCT/US2019/058034
TABLE 16. TEXISTEPEC, BLENDS, AND FCIP CHARACTERIZATION
Property Unit TXPC Run 9-1 Run 9-2 Run 9-3
FCIP EMULSION FEED COMPOSITION
Oil (<600 C) wt% 46.1 42.6 40.1 37.4
Heavy HC wt% 40.4 37.3 35.2 32.7
LIP-Ti wt% -- -- -- 10.0
Water wt% 8.1 15.2 16.2 12.7
Finely divided wt% -- -- 3.8 3.5
solids
Other solids wt% 5.4 4.9 4.7 3.7
Reactor C N/A 500-550 500-550 500-550
Temperature
PRODUCT YIELDS
Oil (<600 C) wt% N/A 55.2 87.7 95.2
Gas wt% N/A 8.4 1.3 1.3
Coke wt% N/A 36.4 11.0 3.5
PRODUCT OIL (LIP) PHYSICAL PROPERTIES
Oil Designation LIP-T3 LIP-T4 LIP-B5
omq omq 8 12 21 21
Density g/cm3 1.16 0.96 0.93 0.93
Viscosity @40 C cP 144,400 55 52.2 44.0
Viscosity@100 C cP 4,722 22.0 19.2 17.8
Flash Point C 204 78 75 85
Initial Boiling C 280 145 142 120
Point
Conradson carbon % CC 18.2 8.0 4.0 2.8
[0181] The invention has been described above with reference to numerous
embodiments and
specific examples. Many variations will suggest themselves to those skilled in
this art in light of
the above detailed description. All such obvious variations are within the
full intended scope of
the appended claims.
- 64 -