Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
HARD METAL HAVING TOUGHNESS-INCREASING MICROSTRUCTURE
The present invention relates to a nanoscale or ultrafine cemented carbide
including
tungsten carbide, an additional metal carbide phase that is in a cubic crystal
structure, and a binder metal phase, to a process for the preparation thereof,
and to
the use thereof for preparing tools and wear parts. Further, the present
invention
relates to a component prepared from the cemented carbide described.
Cemented carbides are metal matrix composite materials in which hard materials
in
the form of small particles are cemented together by a matrix of metal.
Cemented
.. carbides are employed predominantly in applications in which materials
having a high
wear resistance and hardness while showing a high strength are required. Thus,
cemented carbides are used, for example, as a cutting material for tools (such
as
turning tools, drills, and milling tools) and as wear-resistant matrices,
e.g., in
reforming or punching tools. However, conventional cemented carbides have the
drawback of having a very low fracture toughness, which significantly limits
their
applicability. Conventionally, increasing the fracture toughness is possible
by
increasing the content of binder metal, which results in a decrease of
hardness,
however. Ideally, a tool made of a cemented carbide should have a high
hardness
while also having a high fracture toughness.
US 5,593,474 describes a sintered body of a composite material comprising a
plurality of regions of a first metal carbide; and a plurality of regions of a
second
metal carbide, the first metal carbide having a larger particle size than the
second
metal carbide.
DE 10 2004 051 288 addressed the object or providing a polycrystalline powder
of
hard material with improved hardness while the toughness is maintained. This
object is achieved by a polycrystalline powder of hard material consisting of
polycrystalline grains of hard material, which are made of crystals of
carbides,
nitrides and/or carbonitrides of the transition metals of groups 4, 5 and 6
(titanium,
vanadium and chromium groups) of the Periodic Table.
WO 2017/186468 relates to a cemented carbide comprising a phase of hard
material grains and a phase of a heterogeneously distributed binder metal,
wherein
Date recue/Date received 2024-01-19
CA 03114969 2021-03-31
- 2 -
said hard material grains have an average grain size within a range of from 1
nm
to 1000 nm, and said heterogeneously distributed binder metal is present in
the
form of binder islands within the cemented carbide that have an average size
0.1 pm to 10 pm, and an average distance between neighboring binder islands of
from 1 pm to 7 pnn.
EP 1 526 189 describes a cemented carbide comprising WC, a binder phase based
on Co, Ni or Fe, and a gamma phase in which said gamma phase has an average
particle size of less than 1 pm. Said gamma phase is prepared from
presynthesized
mixed carbides in the form (Me,W)C.
CN 103540823 describes a cemented carbide composition comprising from 40 to
50% by weight of WC, from 5 to 10% by weight of vanadium carbide, from 3 to
8% by weight of chromium carbide, from 5 to 9% by weight of titanium carbide,
from 6 to 11% by weight of tantalum carbide, from 2 to 5% by weight of niobium
carbide, and from 12 to 18% by weight of cobalt. The particle size of said WC
is
within a range of from 0.1 to 0.8 pm.
EP 1 557 230 relates to a cemented carbide body comprising from 10 to 12% by
weight of cobalt, less than 3% by weight of tantalum carbide, from 1 to 5.5%
by
weight of niobium carbide, and from 3 to 5% by weight of titanium carbide, the
balance being WC. Said WC has a particle size of from 0.4 to 1.5 pm,
especially
from 0.8 to 1.5 pm.
US 4,698,266 discloses a cutting tool comprising a maximum of 70% by weight of
WC, and from 5 to 10% by weight of a cobalt binder phase, the rest of the
composition being formed by metal carbides selected from the group consisting
of
TiC, TaC, NbC, HfC, and mixtures thereof. The average grain size of said WC is
from 0.9 to 1.3 pm.
Even if some solution approaches are already offered in the prior art, there
is still no
commercial solution for cemented carbides that have both a high hardness and
wear
resistance, and a high fracture toughness.
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 3 -
Therefore, it is the object of the present invention to provide a cemented
carbide that
pas an improved combination of hardness and fracture toughness, and preferably
is
accessible in a simple way.
Surprisingly, it has been found that this object is achieved by providing a
nanoscale
or ultrafine cemented carbide based on tungsten carbide, which further
includes a
metal carbide phase that is in a cubic crystal structure at room temperature.
Therefore, the present invention first relates to a cemented carbide,
comprising
a) a tungsten carbide phase having an average grain size of from 0.05
to 0.5
pm;
b) an additional metal carbide phase; and
c) a binder metal phase,
wherein said additional metal carbide phase is in a cubic crystal structure at
room
temperature, and wherein the proportion of said additional metal carbide phase
in
said cemented carbide is at least 4% by volume, based on the total volume of
the
cemented carbide, and wherein the average grain size was determined by the
linear-
intercept technique according to ISO 4499-2.
The conversion from percent by volume to percent by weight, or the conversion
from
percent by weight to percent by volume, is effected according to the following
formulas:
1
Tni = Vi ' pi = __
E021 = Pi)
m, 1
vi ¨ ____________________________________ = 7n.
Pi E( _________________________________________ r)
Pi
wherein m represents the mass proportion, vi represents the volume proportion,
and
p, represents the density of the respective component.
The cemented carbide according to the invention is a nanoscale or ultrafine
cemented
carbide, whose classification is effected in accordance with ISO 4499-2.
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 4 -
Within the scope of the present invention, "cemented carbide" describes a
sintered
composite material. Said additional metal carbide phase, which is in a cubic
crystal
structure at room temperature, i.e., at 25 C within the scope of the present
invention, is hereinafter referred to interchangeably as a "cubic metal
carbide".
The cemented carbide according to the invention has a high hardness and a high
fracture toughness. The problem that the fracture toughness decreases as the
hardness of the cemented carbide increases, i.e., that the material becomes
brittle
and friable, which occurs in conventional cemented carbides, was not observed
in
the case of the cemented carbide according to the invention. Without being
bound
by theory, it is considered that the positive properties of the cemented
carbide
according to the invention can be attributed, in particular, to the
combination of the
small grain size of the tungsten carbide and the presence of the cubic metal
carbide
phase. Therefore, the tungsten carbide used in the cemented carbide according
to
the invention has an average grain size of from 0.05 to 0.5 pm, preferably
from 0.05
to 0.23 pm, more preferably from 0.05 to 0.09 pm, as determined by the linear-
intercept technique according to ISO 4499-2.
In a further preferred embodiment, the metal carbide phase that is in a cubic
crystal
structure at room temperature is selected from the group consisting of
titanium
carbide, tantalum carbide, niobium carbide, hafnium carbide, zirconium
carbide,
mixtures thereof, and mixed carbides of these compounds.
Preferably, the metal carbide phase used in the cemented carbide according to
the
invention has an average grain size of from 0.3 to 4.0 pm, preferably from 0.5
to 1.5
pm, as determined by the linear-intercept technique according to ISO 4499-2.
Surprisingly, it has been found that a particularly advantageous relationship
of
hardness and fracture toughness could be achieved if the metal carbide phases
present in the cemented carbide according to the invention are homogeneously
distributed. Therefore, an embodiment is preferred in which the metal carbide
phase
contained in the cemented carbide is in a periodically repeated distribution
with an
average distance of from 0.5 to 10 pm, preferably from 1 to 3 pm. Said average
distance can be determined by linear analysis (linear-intercept technique) on
electron
micrographs of sections, and relates to the distance from grain center to
grain center.
Without being bound by theory, the particularly homogeneous distribution of
the
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 5 -
metal carbide phase in the cemented carbide according to the invention is
attributed,
inter alia, to the use of a tungsten carbide powder having the above mentioned
grain
sizes.
It has been found advantageous if powders having a particular particle size
are used
as starting materials for the tungsten carbide and the cubic metal carbide,
wherein
a "starting material" within the scope of the present invention means an
unsintered
powder. Therefore, in a preferred embodiment, a tungsten carbide powder having
an
average particle size dgET of from 0.05 to 0.30 pm, preferably from 0.05 to
0.25 pm,
more preferably from 0.05 to 0.2 pm, is used as a starting material. The
average
particle size clui is calculated from the specific surface area of the
starting material
as determined according to BET (BET surface area) by converting it according
to the
formula dBET = 6/(BET surface area * density). The specific surface area can
be
determined by the BET method according to DIN ISO 9277. The density
corresponds
to the physical density of the pure solid and can be extracted from the
literature, the
density of tungsten carbide usually being stated as 15.7 g/cm3.
As the starting material for the cubic metal carbide, there is preferably
employed a
cubic metal carbide powder having an average particle size dBET of from 0.3 to
5 pm,
more preferably from 0.4 to 1 pm, as determined according to the BET surface
area
of the starting material, and by conversion according to the formula CIBET =
6/(BET
surface area * density). The physical density of the respective cubic carbide
is to be
used as said density. The values can be extracted from the literature.
In a preferred embodiment, the binder metal is a compound selected from the
group
consisting of cobalt, iron, nickel, and mixtures thereof. More preferably, the
binder
metal is cobalt. In a further preferred embodiment, the binder metal is a
mixture
consisting of iron, cobalt, and nickel, in which the proportion of the
respective metals
in the mixture is more than lobo by mass.
Surprisingly, it has been found that the sole addition of the cubic metal
carbides as
described above has no influence on grain growth during the production
process, so
that grain growth inhibitors may be optionally added to the cemented carbide
according to the invention for reducing grain growth during the production
process
thereof. Therefore, an embodiment is preferred in which the cemented carbide
further includes grain growth inhibitors, preferably those selected from the
group
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 6 -
consisting of vanadium carbide, chromium carbide, mixtures thereof, and mixed
carbides of such compounds. The proportion of grain growth inhibitor in the
cemented carbide is preferably from 0.05 to 6% by volume, based on the total
volume of the cemented carbide.
Within the scope of the present invention, it has been found advantageous if
the
proportion of tungsten carbide in the cemented carbide according to the
invention
does not exceed a proportion of 95% by volume. Therefore, an embodiment is
preferred in which the proportion of tungsten carbide in the cemented carbide
according to the invention is from 40 to 80% by volume, based on the total
volume
of the cemented carbide. In this way, a sufficient hardness and fracture
toughness
of the cemented carbide can be ensured.
Further, it has been found advantageous to limit the proportion of binder
metal in
the cemented carbide. Therefore, an embodiment is preferred in which the
proportion
of binder metal in the cemented carbide according to the invention is not more
than
40% by volume, preferably from 10 to 32% by volume, respectively based on the
total volume of the cemented carbide.
Surprisingly, it has been found that the hardness of the cemented carbide can
be
Increased while the fracture toughness is maintained, if the volume proportion
of the
additional metal carbide phase in the cemented carbide according to the
invention
comprises at least 4% by weight. Therefore, an embodiment is preferred in
which
the proportion of the additional metal carbide phase is from 4 to 30% by
volume,
preferably from 10 to 20% by volume, alternatively from 25 to 37% by volume,
respectively based on the total volume of the cemented carbide.
In a particularly preferred embodiment, the cemented carbide according to the
invention has the following composition:
i) from 40 to 90% by volume of tungsten carbide phase; and
ii) from 10 to 32% by volume of binder metal phase; and
balance: additional metal carbide phase;
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 7 -
wherein the proportion of the additional metal carbide phase is at least 4% by
volume, based on the total volume of the cemented carbide, and wherein said
percent by volume are respectively based on the total volume of the cemented
carbide and sum up to 100% by volume, optionally considering further
components,
such as grain growth inhibitors.
Conventional cemented carbides have the disadvantage that although the
hardness
is increased by reducing the content of binder metal, the fracture toughness
decreases. At the same time, an undesirable increase of thermal conductivity
may
occur. Surprisingly, it has been found that the cemented carbide according to
the
invention has an advantageous thermal conductivity. In a preferred embodiment,
the
cemented carbide according to the invention has a thermal conductivity of less
than
50 W/m*K, preferably less than 40 W/m*K, as determined by the laser flash
technique at 40 C.
In addition to an advantageous thermal conductivity, the cemented carbide
according
to the invention is further characterized by an improved fracture toughness.
Therefore, an embodiment is preferred in which the cemented carbide according
to
the invention has a fracture toughness of more than 8.0 MPa*m1/2, as
determined
from Vickers hardness impressions according to the Palmquist method as
described
in Shetty et al., Journal of Materials Science 20 (1985), pp. 1873 to 1882.
The present invention further relates to a process for the preparation of a
cemented
carbide according to the invention, comprising:
i) providing a powder mixture, including
a) a tungsten carbide powder having an average particle size dBET of from 0.05
to 0.3 pm, preferably from 0.05 to 0.25 pm, more preferably from 0.05 to
0.2 pm;
b) an additional metal carbide powder that is in a cubic crystal structure at
room temperature (25 C) and has an average particle size dBET of from 0.3
to 5 pm; and
c) a binder metal powder; and
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 8 -
ii) forming and sintering the mixture.
The average particle size dBET is determined as described above from the BET
surface area and conversion according to the formula deEr = 6/(BET surface
area *
density).
The proportion of said additional cubic metal carbide powder in the powder
mixture
is selected for the cemented carbide obtained to have a proportion of at least
4% by
volume of the cubic metal carbide phase, based on the total volume of the
cemented
carbide.
The binder metal powders as stated above are preferably used.
1 0 In a preferred embodiment, said forming and sintering of the mixture is
performed
to obtain a cemented carbide body. Said cemented carbide body may be, for
example, a component.
In a preferred embodiment, the sintering within the scope of the process
according
to the invention is effected at a temperature of from 1150 to 1550 C. In this
way,
the cemented carbide according to the invention is accessible by a process
that can
be realized simply in industry.
Within the scope of the present invention, it has been surprisingly found
that, for
preparing the cemented carbide according to the invention, it is not necessary
to use
presynthesized mixed carbides of the form (Me,W)C, as described in the prior
art.
Rather, the cemented carbide according to the invention can be prepared from
the
pure metal carbides or mixtures thereof.
The cemented carbide according to the invention is suitable, in particular,
for use in
fields of application in which a high hardness and at the same time a good
fracture
toughness are required. Therefore, the present invention further relates to
the use
of the cemented carbide according to the invention for the production of
tools.
Preferably, the tools are tools with defined and undefined cutting edges, and
tools
for the machining of all kinds of materials.
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 9 -
The present invention further relates to a component obtained by forming the
cemented carbide according to the invention. Preferably, the component is
selected
from the group consisting of drills, solid carbide cutters, indexable inserts,
saw teeth,
forming dies, sealing rings, extrusion punches, press dies, and wear parts.
The present invention is further explained by means of the following Examples,
which
are by no means to be understood as limiting the idea of the invention.
mpj
Example 1:
As the starting powder, a WC powder having a dBFT value of 90 nm, a cobalt
metal
powder having a dBET value of 205 nm, a TIC powder having a dBET value of 610
nm,
a TaC powder having a dBET value of 370 nm, a Cr3C2 powder having a dBET value
of
430 nm, and a VC powder having a dBET value of 350 nm were used. A 200 g
mixture
of 62.7% by volume (77% by weight) WC, 15.9% by volume (11% by weight) Co,
12.9% by volume (5% by weight) TiC, 4.4% by volume (5% by weight) TaC, 1.9%
by volume (1% by weight) Cr3C2, and 2.2% by volume (1% by weight) VC was
ground in n-heptane in a ball mill for 48 hours. The dispersion of cemented
carbide
obtained was dried and pressed uniaxially with a pressing power of 300 MPa
into
rectangular test specimens with a green density of > 50% of the density to be
expected for a solid body (theoretical density). The test specimens were
compacted
under vacuum at a temperature of 1450 C and with a holding time of 30 min to
above 95% of the theoretical density, followed by a final compaction under an
argon
atmosphere at the same temperature (Sinter-HIP technology). The test specimens
proved to be completely dense under an optical microscope. The porosity
according
to ISO 4505 corresponded to > A02, BOO, COO. The Vickers hardness was
.. determined to be 1770 HV10, and the fracture toughness (Kic) was calculated
by
measuring the crack lengths and using the formula of Shetty (Shetty 1985 -
Indentation fracture of WC-Co cermets, see reference above) to be 9.5
MPa*m1/2.
The thermal conductivity (TC) was determined to be 29 W/m*K (measurement at
40 C by the laser flash technique).
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 10 -
Table 1 shows the characteristics determined as compared to a cemented carbide
having a composition without additions of cubic metal carbide, but with an
otherwise comparable content of binder metal.
Example 2:
As the starting powder, a WC powder having a cl8F-1- value of 90 nm, a cobalt
metal
powder having a dBET value of 205 nm, a TiC powder having a dBET value of 610
nm,
a TaC powder having a dBET value of 370 nm, a Cr3C2 powder having a dBET value
of
430 nm, and a VC powder having a dgET value of 350 nm were used. A 200 g
mixture
of 68.9% by volume (80.6% by weight) WC, 16% by volume (10.6% by weight)
Co, 4% by volume (2.6% by weight) TiC, 7% by volume (4.3% by weight) TaC,
1.9% by volume (0.9% by weight) Cr3C2, and 2.2% by volume (1% by weight) VC
was ground in n-heptane in a ball mill for 48 hours. The dispersion of
cemented
carbide obtained was dried and pressed uniaxially with a pressing power of 300
MPa
into rectangular test specimens with a green density of > 50% of the density
to be
.. expected for a solid body (theoretical density). The test specimens were
compacted
under vacuum at a temperature of 1450 C and with a holding time of 30 mm n to
above 95% of the theoretical density, followed by a final compaction under an
argon
atmosphere at the same temperature (Sinter-HIP technology). The test specimens
proved to be completely dense under an optical microscope. The porosity
according
to LSO 4505 corresponded to > A02, BOO, COO. The Vickers hardness was
determined to be 1690 HV10, and the fracture toughness (Kic) was calculated by
measuring the crack lengths and using the formula of Shetty (Shetty 1985 -
Indentation fracture of WC-Co cermets, see reference above) to be 9.7
MPa*m1/2.
The thermal conductivity (TC) was determined to be 39 W/rn*K (measurement at
40 C by the laser flash technique).
Table 1 shows the characteristics determined as compared to the
characteristics
from Example 1.
Table 1: Composition and achieved hardness, fracture toughness, and thermal
conductivity of nanoscale or ultrafine cemented carbides having a content of
binder
metal of 16 - 0.2% by volume with and without additions of cubic metal
carbide
(MeC) of 17 and 110/c by volume, respectively.
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 11 -
Comp.
Ex. without MeC WC Co TiC TaC Cr3C2 VC hardness 1783
20 HV10
. by. weight 89.1... 10 0 o 0.b.d.r3
Kic 8.9 1V1Pa*m1/2
% by yo I u me . 83..7 TC 50 Wirel<
hardness 1770 20 HV10
Ex, 1 17% by volume Kic 9.5
MPa*m1/2
________________ MeC WC Co TiC TaC Cr3C2 VC TC 29 Wfm*k
11". '.=62b:y w3e
, % byvoIume -62.7 15-.9 12.9 4.4
hardness 1690 20 HV10
Ex. 2 11% by volume K1c 9.7
MPa*m1/2
MeC WC Co TiC TaC Cr3C2 VC TC 39 Wfm*K
weight Xft6 10 2.6. ,.. 0.9,
%byvulurne 68.9
Example 3:
As the starting powder, a WC powder having a dBET value of 90 nm, a cobalt
metal
powder having a dBET value of 205 nm, a TIC powder having a dBET value of 610
nm,
a TaC powder having a clorr value of 370 nm, a Cr3C2 powder having a dpur
value of
430 nm, and a VC powder having a cIBET value of 350 nm were used. A 200 g
mixture
of 68.5% by volume (79.1% by weight) WC, 10% by volume (6.5% by weight)
Co, 10.1% by volume (3.7% by weight) TiC, 9% by volume (9.6% by weight) TaC,
1.2% by volume (0.6% by weight) Cr3C2 and 1.2% by volume (0.5% by weight)
VC was ground in n-heptane in a ball mill for 44 hours. The dispersion of
cemented
carbide obtained was dried and pressed uniaxially with a pressing power of 300
MPa
Into rectangular test specimens with a green density of > 50% of the density
to be
expected for a solid body (theoretical density). The test specimens were
compacted
under vacuum at a temperature of 1460 C and with a holding time of 30 min to
above 95% of the theoretical density, followed by a final compaction under an
argon
atmosphere at the same temperature (Sinter-HIP technology), The test specimens
proved to be completely dense under an optical microscope. The porosity
according
to ISO 4505 corresponded to > A02, BOO, COO. The Vickers hardness was
determined to be 2020 HV10, and the fracture toughness (Ki.c) was calculated
by
measuring the crack lengths and using the formula of Shetty (Shetty 1985 -
Indentation fracture of WC-Co cermets, see reference above) to be 8.5
MPa*m1/2.
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 12 -
The thermal conductivity was determined to be 35 Wim*k (measurement at 40 C
by the laser flash technique).
Table 2 shows the characteristics determined as compared to a cemented carbide
having a composition without additions of cubic metal carbide, but with an
otherwise comparable content of binder metal.
Table 2: Composition and achieved hardness, fracture toughness, and thermal
conductivity of nanoscale or ultrafine cemented carbides having a content of
binder
metal of 10 0.2% by volume with and without additions of cubic metal carbide
(MeC).
Comp. hardness 2010
20
without MeC WC Co TiC TaC Cr3C2 VC
Ex. HV10
V0 by weigh: 3i60 p 0.6 0.3 Kw. 8.0 MPa*mi"
% by volume p7.9 10 00 1.3 0.8 TC 61 Wirn*K
19 % by volume Ex. 3 WC Co TiC TaC Cr3C2 VC
hardness 2020 20
MeC HV10
by weight! 79.1. a;ki' 9.60.6 0.5 Kw 8.5 MPa*m1/2
% :by volume õ ; :68.5 : 10:0 :1.2 TC 35 Win-i*K
As can be seen from Tables 1 and 2, the cemented carbides of the invention
according to the Examples have a fracture toughness that is improved over that
of
conventional cemented carbides, and a lower thermal conductivity without
adversely affecting the Vickers hardness of the cemented carbides according to
the
invention within the accepted tolerance of 20 HV10.
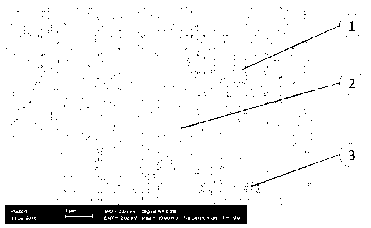
Figure 1 shows a scanning electron micrograph of a cemented carbide according
to the invention, which shows the periodically repeated distribution of the
additional metal carbide phase with an average distance of about 1 to 3 pm.
The
picture was recorded on an electron microscope with an EsB detector having an
acceleration voltage of 2 kV and a 10,000 x magnification. The numbers
represent:
Date Recue/Date Received 2021-03-31
CA 03114969 2021-03-31
- 13 -
1 - tungsten carbide phase
2 - cubic metal carbide phase
3 - binder metal phase
Date Recue/Date Received 2021-03-31