Note: Descriptions are shown in the official language in which they were submitted.
PROCESS FOR EXTRACTING COMPOUNDS FROM DENDROBIUM NOBILE LINDL.
AND APPLICATION THEREOF
[0001] This application claims the priority to Chinese Patent Application No.
202010159498.1,
titled "PROCESS FOR EXTRACTING COMPOUNDS FROM DENDROBIUM NOBILE
LINDE AND APPLICATION THEREOF", filed on March 11, 2020 with the China
National
Intellectual Property Administration.
FIELD
[0002] The present disclosure belongs to the technical field of biotechnology,
and specifically
related to a process for extracting compounds from Dendrobium nobile Lindl.
and application
thereof.
BACKGROUND
[0003] In recent years, with the improvement of people's living standards, the
prevalence of
diabetes mellitus is increasing year by year. According to data released by
the International
Diabetes Federation (IDF) in 2017, about 425 million people (20-79 years old)
in the world suffer
from diabetes mellitus, of which 114 million are in China, ranking first in
the world. According to
this trend, by 2045, there will be 629 million people with diabetes mellitus
in the world. In
addition, the incidence of diabetes is as high as 12%, and it is tend to occur
more and more in
young people. Diabetes mellitus (DM) is a metabolic disorder caused by many
factors and
etiologies, which is characterized by chronic hyperglycemia. Its onset is
closely related to diet,
genetics, environmental factors and dysfunction in the immune system. Diabetes
mellitus is
mainly divided into three types, namely type I, type II and gestational
diabetes mellitus, in which
type H patients account for more than 90% of the total number of patients with
diabetes mellitus.
The precise molecular mechanism of its occurrence and development has not been
yet fully
understood. In recent years, many clinical experimental evidences have
disclosed that the
pathogenesis of type II diabetes mellitus is related to insulin resistance
caused by obesity and
inflammation.
- -
Date Recue/Date Received 2022-11-17
[0004] Inflammation is a series of immune responses of the body and cells to
harmful external
physical, chemical and biological irritation. The common manifestations of
these responses include
fever, redness, pain, and dysfunction. Generally speaking, inflammation is the
defensive response
of tissues and organs to injury or infection, which is beneficial to the body,
but a long-term
inflammatory response may induce the body to produce excessive and abnormal
responses, and
even induce many diseases, such as diabetes mellitus and atherosclerosis,
alzheimer's disease, and
cancer. At present, steroid hormones and non-steroids (aspirin, diclofenac,
ibuprofen, etc.) are often
used clinically to treat inflammation, but after be taken, it will produce a
series of toxic side effects
in the human body, such as liver and gastrointestinal tract damage and
cardiovascular system
damage. In addition, plant-derived drugs have slow and mild effects, strong
durability, low toxic
and side effects, and are not easily tolerated by the body. Therefore, it is
necessary to find
substances with hypoglycemic and anti-inflammatory activities from plants to
provide lead
compounds for the development of new drugs for diabetes mellitus and
inflammation.
SUMMARY
[0005] An object of the present disclosure is to provide a process for
extracting compounds from
Dendrobium nobile Lindl. and application thereof. According to the process in
the present
disclosure, the compounds of Formula I and II are extracted from the stems of
the medicinal plant
Dendrobium nobile Lindl., which can effectively inhibit the activity of
enzymes key to blood sugar
levels. When using LPS-induced RAW264.7 to produce NO as a model for
evaluation of anti-
inflammatory activity, the compounds of Formula I and II can inhibit the
production of NO, thereby
exhibiting anti-inflammatory activity.
[0006] The present disclosure provides a process for extracting compounds from
Dendrobium
nobile Lindl., comprising the following steps:
[0007] A) pulverizing Dendrobium nobile Lindl., leaching with 1-3 times volume
of ethanol
aqueous solution for 2-5 times, filtering the obtained leaching solution, and
combining and
concentrating the filtrate to obtain extract A;
[0008] B) preparing a suspension from the extract A and water, extracting with
petroleum ether,
16838106.2
34273/88
- 2 -
Date Recue/Date Received 2021-05-04
ethyl acetate and normal butanol in sequence, screening three extract liquids,
concentrating ethyl
acetate extract liquid to prepare extract B;
[0009] C) passing the extract B through a pressure-reduced column, performing
gradient elution
with a mixed eluent of petroleum ether and ethyl acetate, washing the column
with acetone to
collect fractions, concentrating and combining each of the obtained fractions,
to finally obtain 16
fractions recorded as Fr.1 - Fr.16;
100101 D) passing fraction Fr.13 through a reversed-phase column, performing
gradient elution
with 30-100% methanol aqueous solution to obtain 14 fractions recorded as
Fr.13-1 - Fr.13-14; and
100111 E) passing fraction Fr.13-7 through a sephadex column, eluting with a
mixed liquid of
methanol and chloroform to obtain 18 fractions recorded as Fr.13-7-1 - Fr.13-7-
18, combining
fractions Fr.13-7-15 - Fr.13-7-17, and purifying by semi-preparative HPLC to
obtain a compound
represented by Formula I at tR = 13.5 min and a compound represented by
Formula II at tR = 16.4
min;
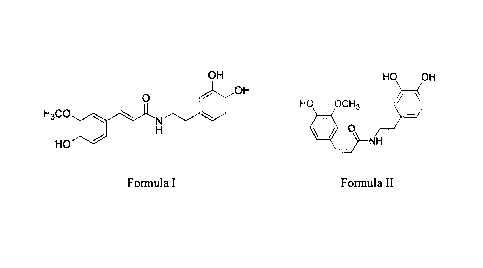
100121
OH HO OH
OH
41/
0 HO OCH3
H3CO,
NN-
I-1 0
HO NH
Formula I Formula II.
100131 Preferably, in step B), a volume ratio of the extract A to the water is
1 : (0.5-2);
100141 a volume ratio of the extract A to the petroleum ether is 1 : (0.5-2);
a volume ratio of the
extract A to the ethyl acetate is 1 : (0.5-2); a volume ratio of the extract A
to the normal butanol is
1 : (0.5-2).
100151 Preferably, in step C), the gradient elution with the mixed eluent of
petroleum ether and
ethyl acetate is specifically by uniformly reducing a volume ratio of
petroleum ether to ethyl acetate
from 20 : 1 to 0 : 1 within 80-120 hours, with a total time of the gradient
elution of 90-110 hours
16838106.2
34273/88
- 3 -
Date Recue/Date Received 2021-05-04
preferably, and more preferably 100 hours.
[0016] Preferably, in step C), each of the obtained fractions is concentrated
under reduced
pressure, and detected by spotting on a thin layer chromatography plate, and
similar fractions are
combined according to color visualization.
.. 100171 Preferably, in step D), the gradient elution is specifically by
eluting with methanol
aqueous solution having a mass concentration of 30%, 40%, 50%, 60%, 70%, 80%,
90%, and 100%
in sequence, with an equal elution time for each of elution gradients and a
total elution time of 24-
48 hours, more preferably 32-36 hours.
[0018] Preferably, in step E), a volume ratio of the methanol to the
chloroform is 1 : (0.5-2).
.. [0019] The present disclosure provides use of a compound represented by
Formula I and a
compound represented by Formula II extracted by the process described above in
the preparation
of a medicament for preventing and treating diabetes mellitus and relieving
inflammation.
[0020] Preferably, the medicament for preventing and treating diabetes
mellitus is an oi-
glucosidase activity inhibitor; and the medicament for relieving inflammation
is an inhibitor against
NO production.
100211 The present disclosure provides a pharmaceutical preparation,
comprising a compound
represented by Formula I or a compound represented by Formula II extracted by
the process
described above and a pharmaceutically acceptable adjuvant.
[0022] Preferably, a dosage form of the pharmaceutical preparation is a
tablet, a capsule, a pill,
a granule, a decoction, an ointment, a distillate formula, an oral liquid, a
dropping pill or asyrup.
[0023] The present disclosure provides a process for extracting compounds from
Dendrobium
nobile Lindl., comprising the following steps: A) pulverizing Dendrobium
nobile Lindl., leaching
with 1-3 times volume of ethanol aqueous solution for 2-5 times, filtering the
obtained leaching
solution, and combining and concentrating the filtrate to obtain extract A; B)
preparing a suspension
from the extract A and water, extracting with petroleum ether, ethyl acetate
and normal butanol in
sequence, and concentrating ethyl acetate extract liquid to prepare extract B;
C) passing the extract
B through a pressure-reduced column, performing gradient elution with a mixed
eluent of
petroleum ether and ethyl acetate, and then washing the column with acetone to
collect fractions,
16838106.2
34273/88
- 4 -
Date Recue/Date Received 2021-05-04
concentrating and combining each of the obtained fractions, to finally obtain
16 fractions recorded
as Fri - Fr.16; D) passing fraction Fr.13 through a reversed-phase column,
performing gradient
elution with 30-100% methanol aqueous solution to obtain 14 fractions recorded
as Fr.13-1 - Fr.13-
14; E) passing Fr.13-7 through a sephadex column, eluting with a mixed liquid
of methanol and
chloroform to obtain 18 fractions recorded as Fr.13-7-1 - Fr.13-7-18,
combining fractions Fr.13-7-
- Fr.13-7-17, and purifying by semi-preparative HPLC to obtain a compound
represented by
Formula I at tR = 13.5 min and a compound represented by Formula II at tR =
16.4 min. The
researches of the present disclosure have shown that the compounds represented
by Foimula I and
II can inhibit the activity of a-glucosidase. When using LPS-induced RAW264.7
to produce NO
10 as a model for evaluation of anti-inflammatory activity, the compounds
represented by Formula I
and II can inhibit the production of NO, thereby exhibiting anti-inflammatory
activity. This shows
that the compound can be used to prepare a food product and/or drug for
treating and/or preventing
diabetes mellitus and inflammatory.
15 BRIEF DESCRIPTION OF DRAWINGS
[0024] Hereinafter, in order to more clearly describe the technical solutions
in the embodiments
of the present disclosure or the existing technology, a brief introduction
will be made to the
drawings that need to be used in the description of the embodiments or the
existing technology. It
is obvious that the drawings described below are only some examples of the
present disclosure and
that for those skilled in the art, other drawings may also be derived from
them without inventive
effort.
[0025] FIG.1 is a 1H NMR spectrum of a compound represented by Formula I in
Example 1 of
the present disclosure;
[0026] FIG.2 is a 13C NMR+DEPT135 spectrum of a compound represented by
Formula I in
Example 1 of the present disclosure;
[0027] FIG.3 is a HSQC spectrum of a compound represented by Formula Tin
Example 1 of the
present disclosure;
[0028] FIG.4 is a 1H NMR spectrum of a compound represented by Formula II in
Example 1 of
the present disclosure;
16838106.2
34273/88
- 5 -
Date Recue/Date Received 2021-05-04
[0029] FIG.5 is a "C NMIR +DEPT135 spectrum of a compound represented by
Formula II in
Example 1 of the present disclosure; and
[0030] FIG.6 is a HSQC spectrum of a compound represented by Formula II in
Example 1 of the
present disclosure.
DETAILED DESCRIPTION
[0031] The present disclosure provides a process for extracting compounds from
Dendrobium
nobile Lindl., comprising the following steps:
[0032] A) pulverizing Dendrobium nobile Lindl., leaching with 1-3 times volume
of ethanol
aqueous solution for 2-5 times, filtering the obtained leaching solution, and
combining and
concentrating the filtrate to obtain extract A;
[0033] B) preparing a suspension from the extract A and water, extracting with
petroleum ether,
ethyl acetate and normal butanol in sequence, screening three extract liquids,
concentrating ethyl
acetate extract liquid to prepare extract B;
[0034] C) passing the extract B through a pressure-reduced column, performing
gradient elution
with a mixed eluent of petroleum ether and ethyl acetate, and then washing the
column with acetone
to collect fractions, concentrating and combining each of the obtained
fractions, to finally obtain
16 fractions recorded as Fr.1 - Fr.16;
[0035] D) passing fraction Fr.13 through a reversed-phase column, performing
gradient elution
with 30-100% methanol aqueous solution to obtain 14 fractions recorded as
Fr.13-1 - Fr.13-14; and
[0036] E) passing Fr.13-7 through a sephadex column, eluting with a mixed
liquid of methanol
and chlorofoim to obtain 18 fractions recorded as Fr.13-7-1 - Fr.13-7-18,
combining fractions
Fr.13-7-15 - Fr.13-7-17, and purifying by semi-preparative HPLC to obtain a
compound
represented by Formula I at tR = 13.5 min and a compound represented by
Formula II at tR = 16.4
min;
[0037]
16838106.2
34273/88
- 6 -
Date Recue/Date Received 2021-05-04
OH HO OH
O
0 IH HO OCH3
1-13C0N
* 0
HO NH
Formula I Formula H.
100381 Dendrobium nobile Lindl. is a plant belonging to orchidaceae dendrobium
Sw., also
known as Harba Dendrolii Nobilis, Dendrobium chrysanthum, Dendrobium
chrysanthum Wall, etc..
It is precious traditional Chinese medicine in China, always called as
"extremely precious herb",
and often used as medicine with its fresh or dry stems. Dendrobium nobile
Lindl. is mainly
distributed in the subtropical regions, such as Guizhou, Yunnan, and Guangxi
located in south of
the Changjiang River in China. In the present disclosure, it is preferred to
use the dry stems of the
Dendrobium nobile Lindl. as raw material to extracte.
100391 In the present disclosure, the dry stems of Dendrobium nobile Lindl.
are pulverized, and
leached with ethanol aqueous solution; the obtained leaching solution is
filtered and then combined
and concentrated to obtain extract A.
100401 In the present disclosure, the dry stems of Dendrobium nobile Lindl.
are preferably
pulverized to a particle size of 0.1-1 cm, and more preferably to 0.5-0.6 cm;
a volume ratio of
ethanol to water in the ethanol aqueous solution is preferably (15-20) : 1,
and more preferably (18-
19) :1; a volume of the ethanol aqueous solution is 1-3 times the volume of
the pulverized
Dendrobium nobile Lindl., and preferably 2 times the volume; the leaching is
preferably carried
out for 2-5 times, and more preferably 3-4 times; the leaching solution is
concentrated preferably
at a concentration ratio of (3-5) :1, and more preferably 4 : 1.
100411 In the present disclosure, after the extract A is obtained, the extract
A and water are
prepared into a suspension, and then the suspension is extracted with
petroleum ether, ethyl acetate
and normal butanol in sequence. The extraction is stopped when each extract
layer is extracted until
the extract liquid becomes colorless. The ethyl acetate extract liquid is
concentrated to prepare
extract B.
16838106.2
34273/88
- 7 -
Date Recue/Date Received 2021-05-04
100421 The polarity of petroleum ether, ethyl acetate and normal butanol
increases in sequence.
The normal butanol extract liquid may contain some highly polar glycoside
compounds. In a
specific experiment, the fraction with medium polarity, that is, the ethyl
acetate extract liquid is
selected. In the present disclosure, a volume ratio of the extract A to the
water preferably is 1: (0.5-
2), and more preferably 1 : (1-1.5); a volume ratio of the extract A to the
petroleum ether is 1 : (0.5-
2), and more preferably 1: (1-1.5). Specifically, in embodiments of the
present disclosure, a volume
ratio of extract A: water : petroleum ether is 1 : 1 : 1. A volume ratio of
the extract A to the ethyl
acetate is preferably 1 : (0.5-2), and more preferably 1 : (1-1.5).
Specifically, in embodiments of
the present disclosure, a volume ratio of extract A: water: ethyl acetate is
1: 1 : 1. A volume ratio
of the extract A to the normal butanol is 1 : (0.5-2), and more preferably 1
(1-1.5). Specifically, in
embodiments of the present disclosure, a volume ratio of extract A: water:
normal butanol is 1 :
1 : 1.
[0043] In the present disclosure, the ethyl acetate extract liquid is
concentrated preferably at a
concentration ratio of (2-5) :1, and more preferably (3-4) : 1.
[0044] In the present disclosure, after the extract B is obtained, the extract
B is passed through a
pressure-reduced column, and subjected to gradient elution with a mixed eluent
of petroleum ether
and ethyl acetate. Then the column is washed with acetone, to collect the
fractions. Each of the
obtained fractions is concentrated, and combined to finally obtain 16
fractions recorded as Fr.1 -
Fr.16.
[0045] In the present disclosure, the pressure-reduced column is silica gel
column
chromatography. The silica gel column is silica gel H with a particle size of
100-200 meshes. A
volume ratio of petroleum ether to ethyl acetate in their mixed solution is
(20-0) : 1. Specifically,
in embodiments of the present disclosure, the gradient elution is specifically
carried out as follows.
[0046] The volume ratio of petroleum ether to ethyl acetate in the mixed
solution is initially 20:
1, and with the progress of the gradient elution, the volume ratio is
uniformly reduced until it
decreased to 0 : 1. The gradient elution is performed preferably at a total
time of 80-120 hours,
more preferably 90-110 hours, and most preferably 100 hours. The gradient
elution is performed
preferably at a temperature of room temperature, i.e. 20-35 C, and more
preferably 25-30 C. A
volume of the eluate used in each gradient preferably is 3-4 L.
16838106.2
34273/88
- 8 -
Date Recue/Date Received 2021-05-04
100471 After the elution is completed, the column is washed with acetone, to
collect fractions
preferably once each 500 mL. Each of the obtained fractions is detected by
spotting it on a thin
layer chromatography (TLC) plate. Similar fractions are combined to obtain 16
fractions, recorded
as Fr.1 - Fr.16.
100481 In the present disclosure, each of the obtained fractions is detected
by spotting on a thin
layer chromatography (TLC) plate. The fractions with the same or similar main
points observed by
the naked eye are combined together to obtain a total of 16 fractions.
100491 After analysis by high performance liquid chromatography, it is showed
that the
characteristic peak of Fr.13 is relatively obvious. Fr.13 is passed through a
reversed-phase column
.. (in which the filler is C18), and subjected to gradient elution with 30%,
40%, 50%, 60%, 70%, 80%,
90%, and 100% of methanol aqueous solutions in sequence, with 100 mL collected
once. For each
gradient, 1.5 L of the mixed solution is used. Each of the obtained fractions
is detected by spotting
it on a thin layer chromatography (TLC) plate. The fractions with the same or
similar main points
observed by the naked eye are combined together to obtain a total of 14
fractions, recorded as Fr.13-
1 - Fr,13-14.
[0050] In the present disclosure, in the gradient elution, the elution time
for each gradient is equal;
the total elution time is preferably 24-48 hours, and more preferably 32-36
hours.
100511 The fraction Fr.13-7 is passed through a Sephadex LH-20 column, and
eluted with a
mixed solution of methanol and chloroform at room temperature at a rate of 2-3
seconds per drop,
with each 5 mL collected in one tube. Each of the obtained fractions is
concentrated under reduced
pressure, and detected by spotting it on a thin layer chromatography (TLC)
plate. The similar
fractions are combined together to obtain 18 fractions, recorded as Fr.13-7-1 -
Fr.13-7-18.
[0052] In the present disclosure, a volume ratio of the methanol and the
chloroform preferably is
1 : (0.5-2), and more preferablyl (1-1.5).
[0053] Fr.13-7-15 - Fr.13-7-17 are similar in spots. They are combined and
purified by semi-
preparative HPLC (a C18 chromatography column, eluted with 35% methanol-
water), to obtain the
compound represented by Formula I (tR = 13.5 min) and the compound of Formula
II (tR = 16.4
min).
16838106.2
34273/88
- 9 -
Date Recue/Date Received 2021-05-04
[0054]
OH HO OH
3' 4'
0 2çOH
HO OCH3
2
H3C0 3 7 = 1 5. 4 3 it
HO 4 6 6 1 9 NH
6 7 8
Formula I Formula II.
[0055] The present disclosure further provides use of a compound represented
by Formula I or a
compound represented by Formula II in the preparation of a medicament for
preventing and treating
diabetes mellitus and relieving inflammation. Preferably, the medicament for
preventing and
treating diabetes mellitus is an a-glucosidase activity inhibitor, and the
diabetes mellitus is type II
diabetes mellitus. The compounds represented by Formula I and Formula II in
the present
disclosure inhibit the activity of a-glucosidase, reduce the decomposition of
oligosaccharides in the
digestive tract, and delay the absorption of glucose by the intestine, thereby
reducing the risk of
postprandial glycemia, and achieving the effect of lowering blood sugar
levels.
[0056] In the present disclosure, the medicament for relieving inflammation is
an inhibitor
against NO production. The compound represented by Formula I or the compound
represented by
Formula II in the present disclosure can inhibit the production of NO, exhibit
anti-inflammatory
activity, and relieve symptoms of redness, swelling, fever, pain in body, etc.
[0057] The present disclosure further provides a pharmaceutical preparation,
comprising a
compound represented by Formula I or a compound represented by Formula II
extracted by the
process described above and a pharmaceutically acceptable adjuvants.
[0058] Preferably, a dosage form of the pharmaceutical preparation is an oral
preparation, and
more preferably a tablet, a capsule, a pill, a granule, a decoction, an
ointment, a distillate formula,
an oral liquid, a dropping pill or a syrup.
[0059] More preferably, the capsule is a hard capsule or a soft capsule. More
preferably, the tablet
is an oral tablet or a buccal tablet. More preferably, the oral tablet refers
to a tablet for oral
16838106.2
34273/88
- 10 -
Date Recue/Date Received 2021-05-04
administration. The medicaments in most of these tablets are absorbed by
gastrointestinal tract to
exert their effects, while the medicaments in some of the tablets act locally
in the gastrointestinal
tract. In some embodiments provided by the present disclosure, the oral tablet
is an ordinary
compressed tablet, a dispersible tablet, an effervescent tablet, a chewable
tablet, a coated tablet or
a sustained-release tablet.
[0060] The pharmaceutically acceptable adjuvant includes one selected from
fruit powders, an
edible essence, a sweetener, a sour agent, a filler, a lubricant, a
preservative, a suspending agent, a
food coloring, a diluent, an emulsifier, a disintegrating agent, a plasticizer
or a mixture of two or
more of them.
100611 The present disclosure provides a process for extracting compounds from
Dendrobium
nobile Lindl., comprising the following steps: A) pulverizing Dendrobium
nobile Lindl., leaching
with 1-3 times volume of ethanol aqueous solution for 2-5 times, filtering the
obtained leaching
solution, and combining and concentrating the filtrate to obtain extract A; B)
preparing a suspension
from the extract A and water, extracting with petroleum ether, ethyl acetate
and normal butanol in
sequence, and concentrating ethyl acetate extract liquid to prepare extract B;
C) passing the extract
B through a pressure-reduced column, performing gradient elution with a mixed
eluent of
petroleum ether and ethyl acetate, and then washing the column with acetone to
collect fractions,
concentrating and combining each of the obtained fractions, to finally obtain
16 fractions recorded
as Fr.1 - Fr.16; D) passing fraction Fr.13 through a reversed-phase column,
performing gradient
elution with 30-100% methanol aqueous solution to obtain 14 fractions recorded
as Fr.13-1 - Fr.13-
14; E) passing fraction Fr.13-7 through a sephadex column, eluting with a
mixed liquid of methanol
and chloroform to obtain 18 fractions recorded as Fr.13-7-1 - Fr.13-7-18,
combining fractions
Fr.13-7-15 - Fr.13-7-17, and purifying by semi-preparative HPLC to obtain a
compound
represented by Formula I at tR = 13.5 min and a compound represented by
Formula II at tR = 16.4
min. The researches of the present disclosure have shown that the compounds
represented by
Formula I and II can inhibit the activity of a-glucosidase. When using LPS-
induced RAW264.7 to
produce NO as a model for evaluation of anti-inflammatory activity, the
compounds represented
by Formula I and II can inhibit the production of NO, thereby exhibiting anti-
inflammatory activity.
This shows that the compound can be used to prepare a food product and/or drug
for treating and/or
16838106.2
34273/88
- 11 -
Date Recue/Date Received 2021-05-04
preventing diabetes mellitus and inflammatory.
100621 Hereafter, in order to further describe the present disclosure, the
process for extracting
compounds from Dendrobium nobile Lindl. and application thereof provided by
the present
disclosure will be described in detail in conjunction with examples, but it
should not be construed
as limiting the protection scope of the present disclosure.
Example 1
100631 Step 1: The dry stems of Dendrobium nobile Lindl. (13 kg) were
pulverized, and then
leached with 2 times the volume of ethanol aqueous solution for 3 times. The
obtained leaching
solution was filtered, and then combined and concentrated to prepare extract
A.
100641 Step 2: A suspension was prepared from extract A and water at a volume
ratio of 1 : 1,
and extracted with petroleum ether (extract A : water : petroleum ether = 1 :
1 : 1), ethyl acetate
(extract A: water :ethyl acetate = 1 : 1: 1), normal butanol ( extract A:
water :normal butanol = 1:
1 : 1) in sequence. The extraction was stopped when each extraction layer was
extracted until the
extract liquid became colorless. The ethyl acetate extract liquid was
concentrated to prepare extract
B.
100651 Step 3: The extract B was passed through a pressure-reduced column, and
subjected to
gradient elution with a mixed solution of petroleum ether and ethyl acetate
(20: 1 ¨> : 1, V/V).
Finally, the column was washed with acetone, with each 500 mL collected in one
bottle. Each of
the obtained fractions was detected by spotting it on a thin layer
chromatography (TLC) plate.
Similar fractions were combined to obtain 16 fractions, recorded as Fr.1 -
Fr.16.
100661 Step 4: Analysis by high performance liquid chromatography showed that
the
characteristic peak of Fr.13 was relatively obvious. Fr.13 was passed through
a reversed-phase
column, and subjected to gradient elution with 30%, 40%, 50%, 60%, 70%, 80%,
90%, and 100%
of methanol aqueous solution, with 100 mL collected in one bottle. For each
gradient, 1.5 L of the
mixed solution was used. Each of the obtained fractions was detected by
spotting it on a thin layer
chromatography (TLC) plate. The similar fractions were combined together to
obtain 14 fractions,
recorded as Fr.13-1 - Fr.13-14.
16838106.2
34273/88
- 12 -
Date Recue/Date Received 2021-05-04
100671 Step 5: Fr.13-7 was better in spot. Fr.13-7 was passed through a
sephadex column, and
eluted with 500 mL of a mixed solution with methanol : chloroform = 1 : 1,
with 6 mL collected in
one tube. After spotting on the plate, the similar fractions were combined
together to obtain 18
fractions, recorded as Fr.13-7-1 - Fr.13-7-18.
100681 Step 6: Fr.13-7-15 - Fr.13-7-17 were similar in spot. They were
combined together, and
then purified by semi-preparative HPLC (a C18 chromatography column, eluted
with 35%
methanol-water), to obtain compounds of Formula I and II (tR = 13.5 min and tR
= 16.4 min).
100691 The identification spectra of the compounds having the structures
represented by Formula
land Formula II are shown in spectrum FIGs.1-6.
[0070] The identification data of the structures represented by Formula I and
Formula II was as
follows: low resolution mass spectrometry miz was 352.4354 [M + Na]; the
molecular formula
was C18H19N05; the data in NMR(500 MHz) and 1.3C NMR(125 MHz) are shown in
Table 1.
[0071] Table 1 The NMR data of the compounds of Formula I and II (the solvent
was
deuterated methanol)
I II
position Oc, type off(J in Hz) Oc, type c5H(J in Hz)
1 128.2 128.5
2 111.5 7.12, brs,1H 113.9 7.36, brs,1H
3 149.9 148.6
4 149.3 148.5
5 116.5 6.79, d,1H (8.07) 115.8 6.73,
d,1H(8.2)
6 123.2 7.02, brd,1H 124.9 6.93,brd,1H (8.2)
(8.16)
7 142.0 7.43,d,1H (15.65) 138.4 6.61,d,1H (12.7)
8 118.7 6.40,d,1H (15.58) 121.6 5.81,d,1H(12.7)
9 169.2 170.3
132.1 132.0
2' 116.9 6.67,brs 116.8
6.64, brs
3' 146.3 146.3
4' 144.8 144.8
5' 116.4 6.69, d,1H (8.0)
116.4 6.66, d,1H(8.2)
6' 121.0 6.55,
brd,1H (8.0) 121.0 6.49, brd,1H(8.2)
7' 36.1 2.70, t,2H (7.34)
35.8 2.64,t,2H(7.51)
8' 42.5 3.46, t,2H (7.40)
42.4 3.39,t,2H(7.53)
3- OCH3 56.4 3.88,s,3H 56.4 3.83,s,3H
16838106.2
34273/88
- 13 -
Date Recue/Date Received 2021-05-04
[0072] Inhibition effects of the compounds of Formula I and II against a-
glucosidase
[0073] The test sample solution was prepared as follows. 450 [IL of the
prepared 2 U/ml a-
glucosidase solution (diluted with a PBS solution with pH = 6.8) was taken
into an EP tube. The
test compound was dissolved in DMSO (with a concentration of 2 mM), and then
45 I:IL of the
solution was added to the EP tube and shaken well. Four uniformly mixed test
solutions each with
a volume of 110 p.L were taken into a 96-well plate separately. (the negative
control group and
blank control group were prepared as follows: 4500_, of the prepared 2 U/ml a-
glucosidase solution
was taken into an EP tube, and then to the EP tube 45 III, of DMSO solution
was added, and shaken
well; four uniformly mixed test solutions each with a volume of 110 [IL were
taken into a 96-well
plate separately.)
[0074] After the 96-well plate was placed at 37 C for 15 minutes, each group
was added with 40
[IL of (2.5 mmol/L of 4-nitropheny1-13-D-glucopyranoside) PNPG solution; (the
blank control group
was added with 401.1L (0.1 mol/L) of PBS solution).
[0075] After the 96-well plate was placed at 37 C for 15 minutes, the OD
absorbance in each
well was measured by an enzyme reader set at a wavelength of 405 nm.
[0076] The inhibitory activity of the compound against a-glycosidase was
calculated using the
following formula.
[0077] Inhibition rate = (ODDmso - ODsample) / (ODDMSO - ODPBS) X 100%
[0078] After the compounds were diluted to several gradients in a two-fold
serial dilution way,
inhibition rates of the samples with different concentrations against a-
glucosidase were detected
by the same detection method, and their IC50 values were calculated by
GraphPad Prism 7 software.
[0079] The results are shown in Table 2. The compounds have a certain
inhibitory activity against
a-glucosidase, and are better than the positive control acarbose.
[0080] Table 2 Inhibitory activity of the compounds against ct-glucosidase
Test samples IC50 SD (j.1M)
Geni stein (Positive control) 8.54 0.69
16838106.2
34273/88
- 14 -
Date Recue/Date Received 2021-05-04
Acarbose (Positive control) 701.45+0.52
Compound I 22.98+1.46
Compound II 46.42+1.12
[0081] Evaluation of anti-inflammatory activity of the compounds of Formula I
and II
[0082] RAW264.7 (mouse mononuclear macrophage leukemia cells) were selected
out. 100 !IL
of these cells were seeded in a 96-well flat-bottom cell culture plate at a
concentration of 5x 104
cells/mL, and cultured under the condition of 37 C, 5% CO2 and a humidity
above 90%. After 24
.. h, the cells were added with 50 [IL of the prepared test compound solution,
and continued to be
cultured under this condition. After 1 h, 50 pt of the prepared LPS (a final
concentration of 500
ng/mL) solution was added. After 24 h, 100 j.iL of the supernatant from each
well was added to
another 96-well plate, and then 100 .1_, (40 mg/mL) of the Griess reagent was
added to each well,
and they are mixed well using the cross method. The absorbance in each well
was measured by an
.. enzyme reader at a wavelength of 540 nm and recorded. NO inhibition rates
were calculated
according to the following formula. The control group was indomethacin. The
negative control
group was DMSO. The test compounds were diluted to 5 concentration gradients
in a two-fold
serial dilution way. Plotting was carried out to calculate IC50 values of the
test compounds with the
concentrations of the test compounds on abscissa and their inhibition rates on
ordinate.
[0083] Inhibition rate (%) = (C2-C1) / (C2-Co) x 100%
100841 In the formula, Co, Ci, and C2 were the absorbance measured at 540 nm
of the blank
controlled group (without LPS added), the experimental group, and the negative
(with LPS added)
control group, respectively. The inhibition rates at each of concentrations
were calculated and a
curve of compound concentrations __ inhibition rate was plotted. Half
maximal inhibitory
concentrations (IC50 value) of the compounds against LPS-induced NO production
by RAW264.7
were calculated. The results are shown in Table 3. The compounds can
effectively inhibit the
amount of NO produced by RAW264.7 cells, exhibit a certain anti-inflammatory
activity, and have
significantly better effects than the positive control indomethacin.
[0085] Table 3 Inhibitory effects of the compounds against the production of
NO from
RAW264.7
16838106.2
34273/88
- 15 -
Date Recue/Date Received 2021-05-04
Test samples IC50 SD (p.M)
Indomethacin (Positive control) 52.30 2.11
Compound! 11.07 0.61
Compound II 10.43 1.17
100861 The above description is only the preferred embodiments of the present
disclosure. It
should be noted that for those skilled in the art, various improvements and
modifications may be
made without departing from the principle of the present disclosure, and these
improvements and
modifications should fall within the scope of protection of the present
disclosure.
16838106.2
34273/88
- 16 -
Date Recue/Date Received 2021-05-04