Note: Descriptions are shown in the official language in which they were submitted.
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
COMPOSITIONS AND METHODS FOR TREATING TRANSIENT BIOFILMS
Related Applications
This application claims priority to and the benefit of U.S. Provisional Patent
Application
62/755,113, filed November 2, 2018, the content of which is incorporated by
reference herein in its
entirety.
Field of the Invention
The invention generally relates to compositions of acetic acid and
hypochlorous acid for
treating transient biofilm infection, while maintaining the natural flora. The
invention relates to
compositions of acetic acid and hypochlorous acid for the treatment of oral
biofilm.
Background
Healthcare professionals have been relying on the use of hand disinfectants as
an alternative
or supplement to handwashing for some time now because they are less abrasive
and easier than
washing with antimicrobial soap and water. And when the Food and Drug
Administration
published an article barring consumers from purchasing and using certain
antibacterial soaps, the
rise of the use of hand disinfectants spread amongst the broader population.
Hand disinfectants or
hand sanitizers are generally gel, foam, or liquid solutions with an active
ingredient for killing
microorganisms. Most hand disinfectants include alcohol in the form of ethanol
or isopropanol.
It is readily known that alcoholic hand rubs significantly reduce the skin
flora count
immediately and up to three hours after disinfection. However, the skin flora
not only consists of
"bad" microbes, but also consists of microorganisms that are generally
nonpathogenic or
commensal, and are even beneficial to one's health. An example of such a
microbe is
Staphylococcus epidermidis. Much like the bacteria of the gut, the natural
skin flora plays an active
role in protecting the host. For example, resident skin flora inhibits the
growth of transient bacteria
and maintains the balance between the colonizing microorganisms. The natural
skin microbiota
plays an integral role in eliciting the host immune networks. (Br J Dermatol.
2008 Mar; 158(3):
442-455). As such, the significant reduction of resident skin flora for a
prolonged period of time
leaves the host defenseless against microbial infection.
When exposed to alcohol, Staphylococcus aureus and other transient bacteria
that survive
1
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
respond by producing a protective biofilm. When the natural skin microbes have
been eradicated
by alcohol, they are unable to protect against the colonization of such
pathogenic microbes.
Biofilms are known to impair cutaneous wound healing and reduce topical
antibacterial efficiency,
and they can also spread bacteria into the air. Microbial infections that
produce biofilms can pose
serious health problems. Scientists estimate that up to 80% of all infections
affecting mammals are
biofilm infections. Meanwhile, antibiotic resistant transient bacteria such as
MRSA (methicillin-
resistant Staphylococcus aureus) proliferate within the biofilm, leading to
many serious and deadly
infections. Dysbiosis of the natural skin microbiome predisposes the host not
only to disease and
infection, but also other inflammatory and immunological diseases. (Prescott
et al. World Allergy
Organization Journal (2017) 10:29).
Though, only a small number of transient bacteria cause biofilm infections,
the presence of
the natural microbes are important for fighting and protecting against those
harmful transient
microbes. There is a need for disinfectants that are not harmful to the
natural microbes of the skin.
Prior art disinfectant compositions have various shortcomings. Some eradicate
the natural
flora while treating transient bacteria. Some have long recovery times,
leaving the tissue
defenseless against pathogenic bacterial infection. Others are unable to
prevent pathogenic bacteria
infection.
Summary
Antimicrobial compositions comprising hypochlorous acid and an organic acid,
such as
acetic acid, as described herein are useful for treating transient microbes
without harming natural
microbial flora. For example, compositions comprising hypochlorous acid and
acetic acid are
useful for treating biofilms on skin while maintaining the homeostasis of the
natural skin
microbiota. Compositions of the invention are useful for disinfecting skin
without negatively
impacting the protective qualities of the bacteria that naturally exists on
skin. Compositions of the
invention are thus effective for hand disinfection. Compositions of
hypochlorous acid and acetic
acid are also effective for treatment of pathogenic transient microbes found
in nasal passages, the
vagina, and the oral cavity. Compositions of the invention reduce or eliminate
pathogenic bacteria
while maintaining homeostatis of the microbiota naturally present on skin. The
concentrations of
HOC1 and HAc are balanced in order to achieve a synergistic effect, resulting
in the antimicrobial
capabilities of the composition being greater than would be expected based on
the antimicrobial
2
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
properties of each component on its own. Acetic acid provides an important
buffering capacity that
allows optimal performance of hypochlorous acid, especially in environments in
which the
tendency is to drive pH to homeostatic levels. For example, in the oral cavity
the natural pH is
about 7.4 and acetic acid provides a buffering capacity in that environment to
allow optimal
activity of HOC. In addition, the hypochlorous acid modulates the toxicity of
the acetic acid and
provides an analgesic effect, allowing stronger compositions to be applied to
skin or other tissue
without adverse side effects, patient discomfort, or eradication of the
natural flora. Finally, acetic
acid is particularly effective against anaerobic bacteria, such as Pseudomona.
In addition to acetic
acid, other organic acids, such as ascorbic acid, lactic acid, formic acid,
malic acid, citric acid, uric
acid, and other carboxylic acids or sulfonic acids.
Various compositions are described herein, having different concentrations of
each acid
component for treatment of diverse types of tissues. Acetic acid
concentrations from about 0.1% to
about 5% are useful. Likewise, hypochlorous acid is found in different
concentrations in the
compositions depending on the type of tissue being treated. For example,
hypochlorous acid at 80
to 2500 ppm is useful for treating a biofilm infecting the root of a tooth,
while only 5-60 ppm is
needed for a dental mouthwash composition, and between 30-100 ppm is to
eliminate transient
biofilm of skin, while maintaining the natural flora. In general, hypochlorous
acid should be
present in a concentration of up to about 2500 ppm. Compositions of the
invention are particularly
effective at biofilm reduction due to the synergistic balance between
hypochlorous acid and acetic
acid, which gives the compositions the dual effect of surface, just beneath
surface, and deeper sub-
surface treatment of biofilm. In general, hypochlorous acid is able to act
rapidly at or near the
surface; whereas acetic acid takes longer time to act and therefore can act
below the tissue surface.
The disclosed acetic acid and hypochlorous acid compositions are effective
against
transient microflora on tissue or non-tissue surfaces without affecting the
natural biofilm.
Application of the compositions to the skin aids in combating microbial
infection by preventing
and treating transient bacterial infections, including biofilms, while
maintaining the natural
microbiome of the skin. Compositions of the invention are useful for hand
washing and
disinfecting surface area. Compositions of the invention are useful for
treating transient bacteria on
epidermal tissue. The disclosed acetic acid and hypochlorous acid compositions
are effective
against treating transient bacteria on dermal tissue.
Compositions of the invention are also useful for treating orally-associated
transient
3
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
biofilm. Compositions of the invention are effective in treating transient
biofilm on the teeth, gums,
gingiva, tongue and oral mucosa. Application of the disclosed compositions of
acetic acid and
hypochlorous acid to the teeth or oral mucosa treats transient biofilm
infection while not harming
the natural flora of the mouth. The inventive compositions are effective in
treating dental plaque,
tooth hypersensitivity, and dental disease. Application of a prophylactically
effective amount of the
disclosed acetic acid and hypochrlous acid compositions aids in preventing
orally-associate
biofilm. Compositions of acetic acid and hypochlorous acid are effective in
preventing dental
plaque biofilm. Additionally, the disclosed compositions of acetic acid and
hypochlorous acid are
effective in sterilizing a root canal.
Compositions of the invention may be provided as a gel or cream, which allows
longer
contact time with the tissue. Additionally, one or both of the components of
inventive compositions
can be encapsulated in a nanoparticle for controlled or delayed release. The
disclosed acetic acid
and hypochlorous acid compositions may also be provided as a dentifrice or
mouthwash.
The compositions described herein can be combined with various excipients and
carriers to
facilitate topical administration. Hypochlorous acid and acetic acid products
can take the form of
gels, creams, lotions, sprays, liquids, foams, powders, varnishes, paints, and
other delivery
formulations known in the art. Alternatively, the compositions provided can be
incorporated into
cloth or fibrous wipes, or applied to bandages, dressing, gauze, brushes, and
implants and permitted
to dry into a film.
The hypochlorous acid and acetic acid compositions described herein can also
be combined
with a fluoride-containing composition or a liquid polymer formulation.
Application of
compositions comprising acetic acid, hypochlorous acid and liquid polymer
formulation to an oral
surface helps to prevent transient biofilm infection. Compositions of the
invention can be applied to
adhesive strips. Application of the adhesive strips to the teeth or other oral
surface is effective in
treating and preventing transient biofilm infection.
In certain aspects, the invention includes a composition made up of acetic
acid and
hypochlorous acid. The acetic acid is present in concentrations greater than
about 0.1%, and
preferably less than about 5.0%. A preferred hypochlorous acid concentration
is between about 5
ppm and about 2500 ppm. In various embodiments the particular concentrations
of acetic acid and
hypochlorous acid depend on the intended treatment area and how deep beneath
the surface of the
tissue the treatment is desired.
4
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
In some embodiments, acetic acid is present in a concentration sufficient to
penetrate
beneath the surface of a tissue. In some embodiments, acetic acid
concentration is greater than
about 0.12%, and preferably greater than about 0.2%, and in some embodiments
it is about 0.5% or
more. The acetic acid may be encapsulated in a nanoparticle for controlled or
delayed release. In
some embodiments, the hypochlorous acid is present in a concentration
sufficient to treat biofilm
on and just beneath a surface of tissue and not eradicate the natural flora.
The composition may
further include a gel, cream, ointment, oil, liquid polymer, or fluoride.
In related aspects, the invention involves methods for treating a transient
biofilm in or on
tissue without harming the natural flora of the tissue. Methods include
applying to a tissue a
composition comprising acetic acid in a concentration sufficient to penetrate
tissue and
hypochlorous acid in an amount sufficient to remove transient biofilm on and
just beneath a surface
of the tissue. Methods include applying to teeth or oral surface a composition
comprising acetic
acid and hypochlorous acid to treat orally-associated biofilm and not harming
the natural flora.
Methods include applying a composition comprising acetic acid in a
concentration sufficient to
prevent transient biofilm on oral mucosa and on teeth.
In some embodiments, the tissue to be treated is the epidermis and the
hypochlorous acid
concentration is about 10-200 ppm. In still other embodiments, the tissue to
be treated is vaginal
tissue, and the hypochlorous acid concentration is about 5-100 ppm, and the
acetic acid
concentration is between about 0.1% and about 5.0%.
The acetic acid may be present in amounts sufficient to remove transient
biofilm beneath
the surface of the oral mucosa or the tooth root. The acetic acid may be
present in an amount from
about 0.1% to about 2.0%, and the hypochlorous acid may be present in a
concentration from about
ppm to about 250 ppm.
Brief Description of the Drawings
Figure 1 is a schematic showing an exemplary system for producing hypochlorous
acid
according to methods of the invention.
Figure 2 is a schematic showing a magnified view of the mixing device shown in
Figure
1.
Figure 3 is a schematic showing an internal view of the mixing chamber of the
mixing
device.
Figure 4 is a schematic showing a front view of the members that divide the
mixing
5
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
chamber into a plurality of sub-chambers. This view shows the apertures in the
members.
Figure 5 is a schematic showing a valve configured with measuring sensors for
switching
from a waste line to a product collection line.
Figure 6 is a schematic showing the valve in-line with the waste line and the
product
collection line.
Figure 7 is a schematic showing another exemplary system for producing
hypochlorous
acid according to methods of the invention. This system is configured for
automated use with
buffered deionized water. The buffer can either be included in the inflowing
water or can be
introduced through an injection port. The buffer may also be mixed during the
mixing process by
using NaOH in Na0C1 or separately injected and acetic acid or others similar
acids or bases.
Figure 8 is a graph of a calibration curve showing HOC1 concentration (ppm)
calculated
indirectly versus conductivity.
Figure 9 is a graph showing a spectrophotometric analysis of the produced
HOC1. The
gases generally produced during production of HOC1 are C102, C120 and C12, all
of which are
detectable in the visible range as yellow or yellow-red. The graph shows no
absorption from
colored gases in the produced HOC1.
Figure 10 is a graph showing the amount (parts per million (ppm)) of HOC1
initially
produced (T = 0) and its stability over time.
Figure 11 is a graph showing how the pH of the HOC1product changed over time.
Figure 12 is a graph showing the oxidation and reduction (redox) of the
HOC1product over
time.
Figure 13 shows an anti-microbial composition that includes an aqueous
solution of
hypochlorous acid encapsulated in a nanoparticle.
Figure 14 is an illustration of a method of making an anti-microbial
composition that
includes an aqueous solution of hypochlorous acid encapsulated in a
nanoparticle.
Figures 15-21 provide data on the reduction of various biofilms when exposed
to
compositions of acetic acid and hypochlorous acid at different concentrations,
compared with
commercially available biofilm treatments.
Figure 22 is a graph showing the killing effect of various concentrations of
HOC1 with
either 1% or 4% acetic acid.
Figure 23 is a graph showing the killing effect of HOC1 at 200 ppm and 500 ppm
with
6
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
either 1%, 2% or 4% acetic acid.
Detailed Description
Treatment of bacterial infection and biofilm is achieved using a synergistic
composition
comprising an organic acid, such as acetic acid, and hypochlorous acid. The
acetic acid component
is particularly effective for penetrating tissue, while the hypochlorous acid
is particularly effective
for treating biofilm on the outer surface of tissue. Acetic acid can penetrate
up to 2 mm or more
beneath the surface of a wound to treat otherwise difficult to reach biofilms.
Preferred compositions of the invention comprise hypochlorous acid in
synergistic
combination with acetic acid. It has been discovered that a balanced
composition in which a
biocidal amount of acetic acid (or an equivalent organic acid) optimized with
hypochlorous acid
achieves maximum therapeutic benefit, whether applied to a surface or in a
manner designed to
penetrate skin. For example, for surface applications, the relative amount of
acetic acid to
hypochlorous acid is lower than for penetrative applications. According to the
invention, surface
bacterial contamination or biofilm is sufficiently treated by hypochlorous
acid with a lower amount
of acetic acid; but for applications requiring deep penetration (e.g.,
wounds), the amount of acetic
acid must be increased. In that case, hypochlorous acid is used to moderate
the toxicity of the acetic
acid to surrounding tissue, while allowing the acetic acid to attack the
biofilm. In addition, it has
been discovered that the synergistic combination of acetic acid and
hypochlorous acid selectively
kill harmful biofilm while preserving beneficial biofilm. Compositions of the
invention comprise
balanced concentrations of acetic acid and hypochlorous acid in order to
selectively kill harmful
biofilm. Concentrations are also balanced in consideration of the application
to which they are
directed (e.g., surface treatment of a tooth or skin versus deep tissue
treatment of a wound or tooth
root). The application provides guidance on the synergistic effects of various
combinations of
acetic acid and hypochlorous acid. The skilled artisan can determine, based
upon the information
provided in the instant specification, the relative amounts of acetic acid and
hypochlorous acid
necessary for treatment of any bacterial infection or biofilm formation.
Treating both the surface-level and subdermal infections provides a dual
action treatment
that is particularly needed for wound care. Chronic wounds and eczema are
plagued by biofilm that
affects subsurface parts of the wound. These wounds frequently have S. aureus
infections which
typically exist on or near the surface and prevent the wound from closing and
healing. Also, P.
7
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
aeruginosa infection is often present, which is generally deeper, underneath
the surface of the
wound. When a deep infection is present, it is important to keep the wound
open and hydrated
while healing from the inside out, to prevent the wound from closing before
the deeper infection is
healed. Only treating the surface-level infection causes the wound to close
and trap the deeper
biofilm inside the tissue, which can lead to sepsis and other complications.
A feature of the invention is also seen in the modulation of hypochlorous acid
and acetic
acid. For example, increasing the concentration of either HOC1 or acetic acid,
while holding the
other constant, increases the bacterial killing effect of the combination. In
addition, increasing the
concentration of acetic acid increases the killing effect in a "dirty" sample,
i.e., one that contains
organics (such as blood, sputum and other organic compounds). Thus, in a dirty
sample, the
concentration of acetic acid is more important to modulate. Figure 22 shows
the killing effect of
various concentrations of HOC1 with either 1% or 4% acetic acid. Figure 23
shows that the biggest
jump in killing effect happens between 1% and 2% acetic acid. Both Figures 22
and 23 are the
result of applying the various concentrations of HOC1 and acetic acid to a
wound simulation model
of infection with Pseudomonas aeruginosa.
There are numerous applications of the invention to prevent and/or treat
biofilm. For
example, a frequent problem with diabetic foot ulcers, for example, is that
they close at the surface,
creating an open cavity beneath. The cavity will contain pus as part of the
immune system
response, which consists of debris, bacteria, and white blood cells. Pus
contained in a closed
compartment, particularly in a foot ulcer, can help spread the infection and
potentially cause sepsis.
In an open wound properly dressed, however, the pus will be discharged into
the wound bed and
the dressing. The bacteria that survive the pus environment inside a closed
wound can spread
infection. Therefore, removing the S. aureus infection without first or
simultaneously removing the
P. aeruginosa infection may not completely eradicate biofilm infection,
leading to early closure
and possibly sepsis.
Antimicrobial solutions, such as compositions provided herein, reduce
infection in the deep
areas of the wound bed and allow wound healing from the inside out, so that
the surface does not
heal faster than the inner wound. Acetic acid is present in an amount
sufficient to
disinfect a biofilm beneath the wound bed, and hypochlorous acid is present in
an amount
sufficient to disinfect the surface of the wound. The composition therefore
allows complete
disinfection of the wound to prevent premature closing and trapping a biofilm
beneath the
8
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
surface of a closed wound.
The disclosed compositions are particularly effective because balancing the
concentrations
of hypochlorous acid and acetic acid allows treatment of the surface level
biofilm and also the sub-
surface biofilm. The precise balance depends on the treatment site and the
amount of surface
penetration that is desired. The hypochlorous acid can be present in about 10
ppm up to about 500
ppm or more. Different uses and types of tissue may require higher or lower
concentrations. The
acetic acid may be present at about 0.25% up to about 2.0% or more, and
preferably about 1.0%.
By balancing the two components, the composition can have the dual effect of
treating at the
surface and beneath the surface of the tissue or wound.
Compositions consistent with the present disclosure can be created for a
variety of uses.
For example, a composition of relatively low acetic acid (as low as about
0.05%) and about 5-60
ppm of hypochlorous acid is a useful composition as a mouthwash for combating
infection in
dental tissue. The lower concentration of acetic acid is sufficient in
mouthwash compositions
because the microbial infection does not tend to penetrate deep within the
tissue.
For wound treatment, on the other hand, a composition may include a higher
concentration
of acetic acid (about 1.0%, about 2.0%, about 3.0%, about 4.0%, or about 5.0%)
to more
effectively treat the biofilm deep inside the tissue.
Other uses may require more or less of each component. For example, a
composition with
hypochlorous acid concentration of about 80-250 ppm is useful for flushing a
bladder, a treatment
that is often needed for patients with a urinary catheter to prevent infection
or blockage. A
composition having a hypochlorous acid concentration of about 15-60 ppm is
sufficient for
treatment of an infected lung.
In certain embodiments, the composition is in the form of a gel, which allows
longer
contact times with the wound. Rinsing with a solution may not be sufficient,
as the contact time of
the antiseptic will be very short. In many cases, to fully remove a biofilm,
the composition should
be in contact with it for a prolonged period of time, ranging from a few
seconds, to several minutes,
to an hour or more. The composition may be provided in a gel or cream, which
resists immediate evaporation or dispersal. Gels, creams, ointments, oils, and
other similar carriers
for topical administration are known in the art.
Additionally, for wound treatment, compositions in gel form have the benefit
of
maintaining moisture at the site of the wound. It is important to keep the
wound hydrated during
9
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
and after treatment with the compositions of the invention. The disclosed
compositions are mostly
water (generally 95% or more), allowing the wound to remain hydrated while the
antiseptic
ingredients of the composition fight infection in the wound and prevent new
infections from taking
hold. Maintaining hydration also prevents the wound from prematurely closing
and trapping
biofilm inside the tissue. Acetic acid is readily formulated in a gel because
acetic acid is not overly
reactive. Other organic acids can be used as well, and those that are less
reactive are desirable.
Slow-release compositions may be used as well. In some compositions, acetic
acid may be
encapsulated in lipid soluble nanoparticles, which allow the acetic acid to be
carried beneath the
surface of a wound before being released from the nanoparticle. Administration
with nanoparticles
of different properties allows the acetic acid to be released slowly over
time, prevent dispersal, and
provide other benefits to administration. Acetic acid is freely diffusing,
water soluble, and has a
high vapor pressure. These properties add to the difficulty of controlling
where the acetic acid goes.
Nanoparticle encapsulated acetic acid allows the composition to be more
precisely controlled.
Nanoparticles are described in greater detail below and shown in FIGS. 13 and
14.
In some embodiments, the composition includes some acetic acid that is free
from
nanoparticles and some acetic acid that is encapsulated within nanoparticles.
Alternatively, slow-
release formulations may be used on their own or in combination with other
instant-acting
formulations. For example, a wound may be treated with a composition of acetic
acid and
hypochlorous acid, and then treated with a composition of mainly acetic acid
encapsulated in
nanoparticles to provide ongoing release of acetic acid into the deep parts of
the wound after the
initial treatment.
Production of hypochlorous acid compositions
The basis of compositions and methods of the invention is the protonation of
the
hypochlorite ion (Off). Using HC1 or acetic acid (HAc) and Na0C1 as an
example, the protonation
is accomplished by introducing an acid (e.g., HC1) to the solution, which
results in the following
reaction occurring:
HC1 HOC1 Na0C1(aq) NaCl
(aq) (aq) (aq)
or
HAc HOC1 Na0C1(aq) NaA
(aq) (aq) (aq).
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
The hypochlorous acid in aqueous solution partially dissociates into the anion
hypochlorite
(0C1), thus in aqueous solution there is always an equilibrium between the
hypochlorous acid and
the anion (OC1). This equilibrium is pH dependent and at higher pH the anion
dominates. In
aqueous solution, hypochlorous acid, is also in equilibrium with other
chlorine species, in particular
chlorine gas, Clõ and various chlorine oxides. At acidic pH, chlorine gases
become increasingly
dominant while at neutral pH the different equilibria result in a solution
dominated by
hypochlorous acid. Thus, it is important to control exposure to air and pH in
the production of
hypochlorous acid.
Additionally, the concentration of protons (H+) affects the stability of the
product. The
invention recognizes that the proton concentration can be controlled by using
an acid that has a
lesser ability at a given pH to donate a proton (i.e., the acid can provide
buffering capacity). For
example, conducting the process with acetic acid instead of hydrochloric acid
is optimal when the
desired pH of the final solution is approximately the pKa of acetic acid. This
can be achieved by
mixing ratios in water of 250X or greater, meaning 1 part proton donor at 100%
concentration (e.g.,
HC1 or acetic acid) to 250 parts water.
In certain embodiments, methods of manufacturing HOC1 involve mixing together
in water
in an air-free environment, a compound that generates a proton (Hi) in water
and a compound that
generates a hypochlorite anion (OC1) in water to thereby produce air-free
hypochlorous acid. The
water may be tap water or purified water, such as water purchased from a water
purification
company, such as Millipore (Billerica, MA). Generally, the pH of the water is
maintained from
about 4.5 to about 9 during the method, however the pH may go above and below
this range during
the production process. Conducting methods of the invention in an air- free
environment prevents
the build-up of chlorine gases during the production process. Further,
conducting methods of the
invention in an air-free environment further stabilizes the produced HOC1.
Any compound that produces a hypochlorite anion (OC1) in water may be used.
Exemplary
compounds include Na0C1 and Ca(0C1)2. In particular embodiments, the compound
is Na0C1.
Any compound that produces a proton (Hi) in water may be used with methods of
the invention.
Exemplary compounds are acids, such as acetic acid, HC1 and H2SO4. In
particular embodiments,
the compound is HC1. In preferred embodiments, the compound is acetic acid
because it is a
weaker acid with a preferred pKa to HC1, meaning, it donates fewer protons
during the reaction
than HC1 and is able to maintain the preferred pH level better.
11
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
Mixing can be conducted in any type of vessel or chamber or fluidic system. In
certain
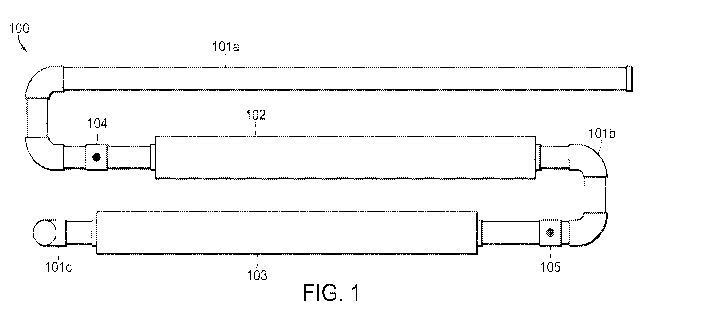
embodiments, a fluidic system 100 as shown in Figure 1 is used to perform
methods of the
invention. The system 100 includes a series of interconnected pipes 101a-c
with a plurality of
mixing devices 102 and 103 in-line with the plurality of pipes 101a-c. The
pipes and the mixing
devices can be interconnected using seals such that all air can be purged from
the system, allowing
for methods of the invention to be performed in an air-free environment. In
certain embodiments,
methods of the invention are also conducted under pressure. Making HOC1 in an
air-free
environment and under pressure allows for the production of HOC1 that does not
interact with
gases in the air (e.g., oxygen) that may destabilize the produced HOC1.
Pipes 101a-c generally have an inner diameter that ranges from about 5mm to
about 50
mm, more preferably from about 17 mm to about 21 mm. In specific embodiments,
the pipes 101a-
c have an inner diameter of about 21 mm. Pipes 101a-c generally have a length
from about 10 cm
to about 400 cm, more preferably from about 15 cm to about 350 cm. In certain
embodiments,
pipes 101a-c have the same length. In other embodiments, pipes 101a-c have
different lengths. In
specific embodiments, pipe 101a has a length of about 105 cm, pipe 101b has a
length of about 40
cm, and pipe 101c has a length of about 200 cm.
The pipes and mixers can be made from any inert material such that material
from the pipes
and mixers does not become involved with the reaction occurring within the
fluidic system.
Exemplary materials include PVC-U. Pipes are commercially available from Georg
Ficher AB. The
pipes and mixers can be configured to have a linear arrangement such that the
pipes and the mixers
are arranged in a straight line. Alternatively, the pipes and mixers can have
a non-linear
arrangement, such that the water must flow through bends and curves throughout
the process.
System 100 shows a non-linear configuration of the pipes 101a-c and mixers 102
and 103.
Pipe 101a is an inlet pipe that receives the water that will flow through the
system.
Generally, the water in pipes 101a-c is under a pressure of at least about 0.1
bar, such as for
example, 0.2 bar or greater, 0.3 bar or greater, 0.4 bar or greater, 0.5 bar
or greater, 0.7 bar or
greater, 0.9 bar or greater, 1.0 bar or greater, 1.2 bar or greater, 1.3 bar
or greater, or 1.5 bar or
greater. At such pressures, a turbulent water flow is produced, thus the
reagents are introduced to a
highly turbulent water flow which facilitates an initial mixing of the
reagents with the water prior
to further mixing in the mixing devices 102 and 103.
In order to control the pH during the production process, the incoming water
should have a
12
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
buffering capacity in the range of pH 3.5-9.0, more preferably from 6.0 and
8.0, to facilitate
addition of the compounds that generates the proton and the compound that
generates the
hypochlorite anion. The dissolved salts and other molecules found in most tap
waters gives the tap
water a buffering capacity in the range of pH 5.5-9.0, and thus tap water is a
suitable water to be
used with methods of the invention.
In certain embodiments, deionized water with the addition of known buffering
agents to
produce a water having a buffering capacity in the range of pH 3.5-9.0 is
used. On example of a
buffer in this particular range is phosphate buffer. For greater process
control and consistency,
using a formulated deionized water may be preferable to using tap water
because tap water can
change between locations and also over time. Additionally, using deionized
water with known
additives also ensures a stable pH of the incoming water flow. This process is
discussed in greater
detail below.
In particular embodiments, an initial pH of the water prior to addition of
either the
compounds that generates the proton or the compound that generates the
hypochlorite anion is at
least about 8.0, including 8.1 or greater, 8.2 or greater, 8.3 or greater, 8.4
or greater, 8.5 or greater,
8.6 or greater, 8.7 or greater, 8.8 or greater, 8.9 or greater, 9.0 or
greater, 9.5 or greater, 10.0 or
greater, 10.5 or greater, or 10.8 or greater. In specific embodiments, the pH
of the water prior to
addition of either the compound that generates the proton or the compound that
generates the
hypochlorite anion is 8.4.
Methods of making HOC1 include introducing to the water the compound that
generates the
proton and the compound that generates the hypochlorite anion in any order
(e.g., simultaneously
or sequentially) and in any manner (aqueous form, solid form, etc.). For
example, the compound
that generates the proton and the compound that generates the hypochlorite
anion are each aqueous
solutions and are introduced to the water sequentially, e.g., the compound
that generates the proton
may be introduced to the water first and the compound that generates the
hypochlorite anion may
be introduced to the water second.
System 100 is configured for sequential introduction of reagents to the water
flow, and the
process is described herein in which the compound that generates the proton is
introduced to the
water first and the compound that generates the hypochlorite anion is
introduced to the water
second. In certain embodiments, the compound that generates the proton and the
compound that
generates the hypochlorite anion are introduced to the water in small
aliquots, e.g, from about 0.1
13
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
mL to about 0.6 mL. The iterative and minute titrations make it possible to
control the pH in spite
of additions of acid (compound that generates the proton) and alkali (the
compound that generates
the hypochlorite anion). In certain embodiments, no more than about 0.6 mL
amount of compound
that generates the proton is introduced to the water at a single point in
time. In other embodiments,
no more than about 0.6 mL amount of the compound that generates the
hypochlorite anion is
introduced to the water at a single point in time.
To introduce the reagents to the water, pipe 101a includes an injection port
104 and pipe
101b includes an injection port 105. The injection ports 104 and 105 allow for
the introduction of
reagents to the water flow. In this embodiments, aqueous compound that
generates the proton is
introduced to the water in pipe 101a via injection port 104. The compound that
generates the
proton is introduced by an infusion pump that is sealably connected to port
104. In this manner, the
flow rate, and thus the amount, of compound that generates the proton
introduced to the water at
any given time is controlled. The infusion pump can be controlled
automatically or manually. The
rate of introduction of the compound that generates the proton to the water is
based upon the
incoming water quality (conductivity and pH level) and the pressure and the
flow of the incoming
water. In certain embodiments, the pump is configured to introduce about 6.5
liters per hour of
hydrochloric acid into the water. The introducing can be a continuous infusion
or in an intermittent
manner. Since the water is flowing though the pipes in a turbulent manner,
there is an initial mixing
of the compound that generates the proton with the water upon introduction of
the hydrochloric
acid to the water.
Further mixing occurs when the water enters the first mixing device 102. FIG.
2 shows a
magnified view of the mixing device 102 shown in FIG. 1. In the illustrated
embodiment, the
mixing device includes a length of about 5.5 cm and a diameter of about 5 cm.
One of skill in the
art will recognize that these are exemplary dimensions and methods of the
invention can be
conducted with mixing devices having different dimensions than the exemplified
dimensions.
Mixing device 102 includes a fluidic inlet 106 that sealably couples to pipe
101a and a fluidic
outlet 107 that sealably couples to pipe 101b. In this manner, water can enter
the mixing chamber
108 of device 102 from pipe 101a and exit the chamber 108 of device 102
through pipe 101b.
The mixing device 102 is configured to produce a plurality of fluidic vortexes
within the
device. An exemplary device configured in such a manner is shown in FIG. 3,
which is a figure
providing an internal view of the chamber 108 of device 102. The chamber 108
includes a plurality
14
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
of members 109, the members being spaced apart and fixed within the chamber
108 perpendicular
to the inlet and the outlet in order to form a plurality of sub-chambers 110.
Each member 109
includes at least one aperture 111 that allows fluid to flow there through.
FIG. 4 shows a front view
of the members 109 so that apertures 111 can be seen. The size of the
apertures will depend on the
flow of water and the pressure in the system.
Any number of members 109 may be fixed in the chamber 108, the number of
members
109 fixed in the chamber 108 will depend on the amount of mixing desired. FIG.
4 shows four
members 109a-d that are fixed in the chamber to produce four sub-chambers 110a-
d. The members
109 may be spaced apart a uniform distance within the chamber 108, producing
subchambers 110
of uniform size. Alternatively, the members 109 may be spaced apart at
different distances within
the chamber 108, producing sub-chambers 110 of different size. The members 109
are of a size
such that they may be fixed to an interior wall within the chamber 108. In
this manner, water
cannot flow around the members and can only pass through the apertures 111 in
each member 109
to move through mixing device 102. Generally, the members will have a diameter
from about 1 cm
to about 10 cm. In specific embodiments, the members have a diameter of about
3.5 cm.
A fluidic vortex is produced within each sub-chamber 110a-d. The vortices
result from
flow of the water through the apertures 111 in each member 109. Methods of the
invention allow
for any arrangement of the apertures 111 about each member 109. FIG. 4
illustrates non-limiting
examples of different arrangements of the apertures 111 within a member 109.
The apertures may
be of any shape. FIG. 4 illustrates circular apertures 111. In certain
embodiments, all of the
apertures 111 are located within the same place of the members 109. In other
embodiments, the
apertures 111 are located within different places of the members 109. Within a
single member
109, all of the apertures 111 may have the same diameter. Alternatively,
within a single member
110, at least two of the apertures 111 have different sizes. In other
embodiments, all of the
apertures 111 within a single member 110 have different sizes.
In certain embodiments, apertures 111 in a member 110 have a first size and
apertures 111
in a different member 110 have a different second size. In other embodiments,
apertures 111 in at
least two different members 110 have the same size. The size of the apertures
will depend on the
flow of water and the pressure in the system. Exemplary aperture diameters are
from about 1 mm to
about 1 cm. In specific embodiments, the apertures have a diameter of about 6
mm.
The solution enters mixing device 102 through inlet 106, which is sealably
mated with pipe
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
101a. The solution enters the chamber 108 and turbulent mixing occurs in each
of subchambers
110a-d as the solution pass through members 109a-d via the apertures 111 in
each member 109a-d.
After mixing in the final sub-chamber 110d, the water exits the chamber 108
via the fluidic outlet
107 which is sealably mated to pipe 101b.
The compound that generates the hypochlorite anion is next introduced to the
solution that
is flowing through pipe 101b via injection port 105. The compound that
generates the hypochlorite
anion is introduced by an infusion pump that is sealably connected to port
105. In this manner, the
flow rate, and thus the amount, of compound that generates the hypochlorite
anion introduced to
the water at any given time is controlled. The infusion pump can be controlled
automatically or
manually. The rate of introduction of the compound that generates the
hypochlorite anion to the
water is based upon properties of the solution (conductivity and pH level) and
the pressure and the
flow of the solution. In certain embodiments, the pump is configured to
introduce about 6.5 liters
per hour of compound that generates the hypochlorite anion into the solution.
The introducing can
be a continuous infusion or in an intermittent manner. Since the solution is
flowing though the
pipes in a turbulent manner, there is an initial mixing of the compound that
generates the
hypochlorite anion with the solution upon introduction of the compound that
generates the
hypochlorite anion to the solution.
Further mixing occurs when the solution enters the second mixing device 103.
Mixing
device 103 includes all of the features discussed above with respect to mixing
device 102.
Mixing device 103 may be configured the same or differently than mixing device
102, e.g., same or
different number of sub-chambers, same or different diameter of apertures,
same or different sizes
of sub-chambers, etc. However, like mixing device 102, mixing device 103 is
configured to
produce a fluidic vortex within each sub-chamber.
The solution enters mixing device 103 through an inlet in the device, which is
sealably
mated with pipe 101b. The solution enters the mixing chamber and turbulent
mixing occurs in each
sub-chamber of the mixing device as the solution pass through members in the
chamber via the
apertures in each member. After mixing in the final sub-chamber, the water
exits the chamber via
the fluidic outlet in the mixing device which is sealably mated to pipe 101c.
At this point, the reaction has been completed and the HOC1 has been formed.
The
production is controlled in-line by measuring pH and conductivity. The pH is
used in combination
with conductivity based on a pre-calibrated relation between the conductivity
and concentration of
16
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
HOC1 measured with spectrophotometry. The measured conductivity is a measure
of the solvent's
ability to conduct an electric current. Comparing the same matrix with
different known
concentrations of HOC1 and 0C1-, a calibration curve (FIG. 8) has been
established that is used in
combination with the pH meter to regulate the titrations and control the
process.
Pipe 101c can be connected to a switch valve 112 that switches between a waste
line 113
and a product collection line 114. Shown in FIGS. 5 and 6. The valve 112
includes the pH meter
and the conductivity measuring device. These devices measure the concentration
(ppm), purity, and
pH of the HOC1 being produced and provide feedback for altering such
properties of the produced
HOC1. Once the HOC1 being produced in pipe 101c meets the required
concentration, purity, and
pH, the valve 112 switches from the waste line 113 to the product collection
line 114 to collect the
desired product.
The HOC1 that has been produced in an air-free manner is collected and bottled
in an air-
free manner. Placing liquids into a bottle in an air-free manner is known in
the art. An exemplary
method includes placing an inflatable vessel (such as a balloon) into a
bottle. The inflatable vessel
is connected directly to the collection line 114 and the HOC1 is pumped
directed into the inflatable
vessel in the bottle without ever being exposed to air. Another method
involves filling
the bottles under vacuum. Another air-free filling method involves filling the
bottles in an
environment of an inert gas that does not interact with the HOC1, such as an
argon environment.
The produced hypochlorous acid is air-free and will have a pH from about 4.5
to about 7.5.
However, the pH of the produced HOC1 can be adjusted post production process
by adding either
acid (e.g., HC1) or alkali (e.g., Na0C1) to the produced hypochlorous acid.
For example, a pH of
between about 4.5 and about 7 is particularly suitable for the application of
reprocessing heat
sensitive medical instruments. Other applications, such as its use in non-
medical environments, for
example as in the processing of poultry and fish and general agricultural and
petrochemical uses,
the breaking down of bacterial biofilm and water treatment, may demand
different pH levels.
The process can be performed manually or in an automated manner. Fluidic
systems
described herein can be operably connected to a computer that controls the
production process. The
computer may be a PCL-logic controller system. The computer opens and closes
the valves for the
water inlet, the waste water outlet, and the product outlet according to the
feedback received from
the sensors in the system (e.g., conductivity, pH, and concentration of
product (ppm) being
produced). The computer can also store the values for the water pressures and
water amounts and
17
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
can adjust these according to the feedback received from the sensors regarding
the properties of the
HOC1 being produced. The computer can also control the infusion pumps that
inject the reagents
into the water for the production process.
The process can be performed iteratively in that pipe 101c can be attached to
a second
fluidic system and the produced HOC1 is then flowed through the second system
where the process
described above is repeated with the starting solution being HOC1 instead of
water. In this manner,
an increased yield of HOC1 is produced. Any number of fluidic systems may be
interconnected
with methods of the invention.
FIG. 7 is a schematic showing another exemplary system 200 for producing
hypochlorous
acid according to methods of the invention. System 200 is configured for
regulation of the pH of
the incoming water and injecting buffer for stability. In system 200, water is
introduced into pipe
201a. Pipe 201a has a pH meter 208 connected to it. pH meter 208 measures the
pH of the
incoming water. The pH meter 208 is connected to injection port 202.
The injection port 202 allows for the introduction of at least one buffering
agent to the incoming
water. The buffering agent is introduced by an infusion pump that is sealably
connected to port
202. In this manner, the flow rate, and thus the amount, of buffering agent
introduced to the water
at any given time is controlled. The infusion pump can be controlled
automatically or manually.
The rate of introduction of the buffering agent to the water is based upon the
incoming water
quality (conductivity and pH level), the buffer composition, and the pressure
and the flow of the
incoming water. The introducing can be a continuous infusion or in an
intermittent manner. Since
the water is flowing through the pipe 201a in a turbulent manner, there is an
initial mixing of the
buffering agent with the water upon introduction of the buffering to the
water. This initial mixing
may be sufficient to properly adjust the properties of the incoming water.
In certain embodiments, further mixing of the water and buffer is performed
prior to
conducting the process of producing the HOC1. In those embodiments, further
mixing occurs when
the water with buffering agent enters the first mixing device 203. Mixing
device 203 includes all of
the features discussed above with respect to mixing device 102. Mixing device
203 may be
configured the same or differently than mixing device 102, e.g., same or
different number of sub-
chambers, same or different diameter of apertures, same or different sizes of
subchambers, etc.
However, like mixing device 102, mixing device 203 is configured to produce a
fluidic vortex
within each sub-chamber.
18
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
The solution enters mixing device 203 through an inlet in the device, which is
sealably
mated with pipe 201a. The solution enters the mixing chamber and turbulent
mixing occurs in each
sub-chambers of the mixing device as the solution pass through members in the
chamber via the
apertures in each member. After mixing in the final sub-chamber, the water
exits the chamber via
the fluidic outlet in the mixing device which is sealably mated to pipe 202b.
The water has a pH of
at least about 8.0, preferably 8.4, and a buffering capacity of pH 5.5-9Ø
The process is now conducted as described above for producing HOC. The
compound that
generates the proton is next introduced to the water that is flowing through
pipe 201b via injection
port 204. The compound that generates the proton is introduced by an infusion
pump that is
sealably connected to port 204. In this manner, the flow rate, and thus the
amount, of compound
that generates the proton introduced to the water at any given time is
controlled. The infusion pump
can be controlled automatically or manually. The rate of introduction of the
compound that
generates the proton to the water is based upon properties of the water
(conductivity and pH level),
the buffer composition, and the pressure and the flow of the water.
In certain embodiments, the pump is configured to introduce from about 6.5
liters per hour to about
12 liters per hour of compound that generates the proton into the water. The
introducing can be a
continuous infusion or in an intermittent manner. Since the water is flowing
though the pipes in a
turbulent manner, there is an initial mixing of the compound that generates
the proton with the
water upon introduction of the hydrochloric acid to the water.
Further mixing occurs when the solution enters the second mixing device 205.
Mixing
device 205 includes all of the features discussed above with respect to mixing
device 102.
Mixing device 205 may be configured the same or differently than mixing device
203, e.g., same or
different number of sub-chambers, same or different diameter of apertures,
same or different sizes
of sub-chambers, etc. However, like mixing device 203, mixing device 205 is
configured to
produce a fluidic vortex within each sub-chamber.
The solution enters mixing device 205 through an inlet in the device, which is
sealably
mated with pipe 201b. The solution enters the mixing chamber and turbulent
mixing occurs in each
sub-chambers of the mixing device as the solution pass through members in the
chamber via the
apertures in each member. After mixing in the final sub-chamber, the water
exits the chamber via
the fluidic outlet in the mixing device which is sealably mated to pipe 201c.
The compound that generates the hypochlorite anion is next introduced to the
solution that
19
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
is flowing through pipe 201c via injection port 206. The compound that
generates the hypochlorite
anion is introduced by an infusion pump that is sealably connected to port
206. In this manner, the
flow rate, and thus the amount, of compound that generates the hypochlorite
anion introduced to
the water at any given time is controlled. The infusion pump can be controlled
automatically or
manually. The rate of introduction of the compound that generates the
hypochlorite anion to the
water is based upon properties of the solution (conductivity and pH level) and
the pressure and the
flow of the solution. In certain embodiments, the pump is configured to
introduce about 6.5-12
liters per hour of compound that generates the hypochlorite anion into the
solution. The amount
introduced depends on the desired concentration of HOC1 (ppm) and flow of
water through the
pipes. The introducing can be a continuous infusion or in an intermittent
manner. Since the solution
is flowing though the pipes in a turbulent manner, there is an initial mixing
of the compound that
generates the hypochlorite anion with the solution upon introduction of the
compound that
generates the hypochlorite anion to the solution.
Further mixing occurs when the solution enters the second mixing device 207.
Mixing
device 207 includes all of the features discussed above with respect to mixing
device 102.
Mixing device 207 may be configured the same or differently than mixing
devices 205 or 203, e.g.,
same or different number of sub-chambers, same or different diameter of
apertures, same or
different sizes of sub-chambers, etc. However, like mixing devices 205 and
203, mixing device 207
is configured to produce a fluidic vortex within each sub-chamber.
The solution enters mixing device 207 through an inlet in the device, which is
sealably
mated with pipe 201c. The solution enters the mixing chamber and turbulent
mixing occurs in each
sub-chambers of the mixing device as the solution pass through members in the
chamber via the
apertures in each member. After mixing in the final sub-chamber, the water
exits the chamber via
the fluidic outlet in the mixing device which is sealably mated to pipe 201d.
At this point, the reaction has been completed and the HOC1 has been formed.
The
produced HOC1 can be measured and collected as described above. Pipe 201d can
be connected to
a switch valve that switches between a waste line and a product collection
line. The valve includes
a pH meter and a conductivity measuring device. These devices measure the
concentration, purity,
and pH of the HOC1 being produced and provide feedback for altering such
properties of the
produced HOC1. Once the HOC1 being produced in pipe 201d meets the required
concentration,
purity, and pH, the valve switches from the waste line to the product
collection line to collect the
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
desired product.
In another embodiment, a deionizer is placed in-line with incoming water. The
deionizer
deionizes the water and then a buffering agent is added to the deionized
water. The production
process is then conducted as described for embodiments of system 200 to
produce water having a
pH of at least about 8, for example 8.4, and a buffering capacity of pH 6-8.
The HOC1 produced by the above process can be used in numerous different
applications,
for example medical, foodservice, food retail, agricultural, wound care,
laboratory, hospitality,
dental, delignification, or floral industries.
Wound care
In certain embodiments, compositions of the invention are used for wound care.
Wound
care involves treating damaged or broken skin, including abrasions,
lacerations, ruptures,
punctures, or burns. Particular wound care treatments involve treating
biofilms. Biofilms may form
when free floating microorganisms such as bacteria and fungus attach
themselves to a surface.
Biofilms are known to impair cutaneous wound healing and reduce topical
antibacterial efficiency
in healing or treating infected wounds. Other common health conditions related
to biofilms include
urinary tract infections, middle-ear infections, chronic wounds, and the
formation of dental plaque.
Cystic fibrosis, native valve endocarditis, otitis media, periodontitis, and
chronic prostatitis also
involve microorganisms that produce biofilms. Microorganisms commonly
associated with
biofilms include Candida albicans, coagulase-negative staphylococci,
Enterococcus, Klebsiella
pneumoniae, Pseudomonas aeruginosa, Staphylococcus aureus, and others.
Biofilms are often resistant to traditional antimicrobial treatments, and are
therefore a
serious health risk. The resistance of biofilms renders traditional antibiotic
and antimicrobial
treatments ineffective. Because biofilms can greatly reduce susceptibility to
antibiotics and
disinfectants, treatments are needed that are capable of breaking down
biofilms but that are not too
toxic to the patient.
Methods are provided for administration of a composition to an individual in
need of
treatment for a biofilm-associated infection. Methods of the invention include
prophylaxis, therapy,
or cure of a biofilm-associated infection. Methods include administration of
one or more unit doses
of a composition in a therapeutically or prophylactically effective amount for
treatment of an
existing biofilm-associated infection or prevention of establishment of a
biofilm- associated
21
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
infection in the individual. In some embodiments, spread of a biofilm-
associated infection to
another site in the individual is inhibited. In various embodiments, the
composition may be
administered parenterally, orally, locally, or topically. Compositions may be
applied by
intravenous, intra-muscular, or subcutaneous injection. In methods of the
invention, compositions
may be administered in a pharmaceutically acceptable carrier, examples of
which are discussed
below.
Treatment includes killing of microbes inhabiting the biofilm or removing a
biofilm,
inhibiting biofilm formation, and disrupting an existing biofilm. The
compositions disclosed herein
are particularly effective for treatment of microbial biofilms in or on a
wound. The composition
may be in the form of a topically administrable wound treatment composition
which comprises a
hypochlorous acid and acetic acid compound. The composition may be combined
with an
additional antimicrobial agent.
Compositions of the invention can be administered topically to a subject,
e.g., by the direct
laying on or spreading of the composition on the epidermal or epithelial
tissue of the subject. The
composition may be formulated as a liquid, powder, lotion, cream, gel, oil,
ointment, gel, solid,
semi-solid formulation, or aerosol spray. Such formulations may be produced in
a conventional
manner using appropriate carriers which are well known to a person skilled in
the art.
Suitable carriers for topical administration preferably remain in place on the
skin as a
continuous film, and resist being removed by perspiration or immersion in
water. The carrier may
include pharmaceutically-acceptable emollients, emulsifiers, thickening
agents, solvents, and the
like.
The composition may be provided as part of a wound dressing in which the
composition is
provided within the wound dressing or on the wound-contacting surface thereof.
A wound dressing
may be intended to be applied to a wound to be treated and which comprises a
substrate comprising
compositions in accordance with the invention. Such a dressing is particularly
convenient because
it delivers the composition of the invention to the wound to be treated and
simultaneously provides
a dressing therefor. The wound dressing may, for example, be fibrous, a foam,
a hydrocolloid, a
collagen, a film, a sheet hydrogel or a combination thereof. The wound
dressing may be in the form
of a layered dressing in which one or more layers of the dressing are formed
at least in part or one
or of; natural fibers, alginate, Chitosan, Chitosan derivatives, cellulose,
carboxymethyl-cellulose,
cotton, Rayon, Nylon, acrylic, polyester, polyurethane foam, hydrogels,
hydrocolloids, polyvinyl
22
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
alcohol, starch, a starch film, collagen, hylaronic acid and its derivatives,
biodegradable materials,
and other materials known in the art. Methods of the invention may further
comprise negative-
pressure wound therapy, as is known in the art. Such therapies involve
applying negative pressure
to the wound, such as with a vacuum dressing.
The composition may be administered in a single daily dose or in multiple
doses, e.g., 2, 3,
4, or more doses, per day. The total daily amount of composition may be about
0.01 mg, 0.1 mg, 1
mg, 2 mg, etc., up to about 1000 mg. In some embodiments, the total daily
amount of administered
is about 0.01 mg to about 1 mg, about 1 mg to about 10 mg, about 10 mg to
about 100 mg, about
100 mg to about 500 mg, or about 500 mg to about 1000 mg. The actual dosage
may vary
depending upon the specific composition administered, the mode of
administration, the type or
location of biofilm to be treated, and other factors known in the art. In some
embodiments a dosage
can also be selected so as to provide a predetermined amount of composition
per kilogram of
patient weight.
The use of the compound in conjunction with another known antimicrobial
treatment may
increase the efficacy of the antimicrobial agent. In some embodiments, methods
of the invention
further comprise administration (simultaneously or sequentially with
compositions of the
invention) of one or more doses of an antibiotic substance, including, but not
limited to,
ciproflaxin, ampicillin, azithromycin, cephalosporin, doxycycline, fusidic
acid, gentamycin,
linezolid, levofloxacin, norfloxacin, ofloxacin, rifampin, tetracycline,
tobramycin, vancomycin,
amikacin, deftazidime, cefepime, trimethoprim/sulfamethoxazole,
piperacillin/tazobactam,
aztreanam, meropenem, colistin, or chloramphenicol. In some embodiments,
methods of the
invention further comprise administration of one or more doses of an
antibiotic substance from an
antibiotic class including, but not limited to, aminoglycosides, carbacephem,
carbapenems, first
generation cephalosporins, second generatin cephalosporins, third generation
cephalosporins,
fourth generation cephalosporins, glycopeptides, macrolides, monobactam,
penicillins,
polypeptides, quinolones, sulfonamides, tetracyclines, lincosamides, and
oxazolidinones. In some
embodiments, methods of the invention comprise administration of a
nonantibiotic antimicrobial
substance, including but not limited to sertraline, racemic and stereoisomeric
forms of thioridazine,
benzoyl peroxide, taurolidine, and hexitidine.
23
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
Treating bio film on other tissues
Compositions of the invention can be used to treat biofilms affecting various
parts of the
body, or attached to various surfaces. In some embodiments, methods of the
invention comprise
administration of a therapeutically effective composition to an individual in
need thereof for
treatment of a biofilm-associated infection in the bladder, kidney, heart,
middle ear, sinuses, skin,
lung, a joint, subcutaneous tissue, soft tissue, vascular tissue, and/or the
eye. In other embodiments,
a therapeutically effective amount of composition is administered to an
individual in need thereof
for treatment of one or more of the following conditions associated with
biofilm: urinary tract
infection; chronic bacterial vaginosis; prostatitis; bacterial infection
stemming from diabetes, such
as a diabetic skin ulcer; pressure ulcer; venous catheter-associated ulcer; or
a surgical wound (e.g.,
a surgical site infection). In some embodiments, the biofilm is on the skin of
an individual. In some
embodiments, the biofilm is associated with a wound, including abrasions,
lacerations, ruptures,
punctures, burns, and chronic wounds. In some embodiments, the biofilm is
below the surface of
the skin, in subcutaneous tissue, such as a deep tissue wound or a surgical
site infection.
Treating biofilm on non-tissue surfaces
Other applications for treating biofilms are also envisaged. For example, the
composition of
the invention has application for the treatment of microbial biofilms on
surfaces, e.g. surfaces in
hospitals (such as operating rooms or patient care rooms) as well as other
surfaces (e.g., household
work surfaces). The invention also encompasses treating biofilms that form on
implanted medical
devices and prosthetics.
As is known in the art, implanted medical devices are susceptible to biofilm
formation,
including fungal biofilms and bacterial biofilms. Methods and compositions of
the invention can
also be used to treat biofilms that form on the surfaces of implanted medical
devices such as
catheters and prosthetics. Compositions of the invention can be applied to a
medical device pre-
implantation. Alternatively, the medical device can comprise a reservoir
containing the
composition, such that the composition can be released in a controlled manner
after implantation.
Methods for treating implanted medical devices can be found in U.S. Patents
5,902,283 and
6,589,591, and U.S. Patent Publication 2005/0267543, each of which is
incorporated by reference
herein in its entirety.
24
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
Dental treatment
In another embodiment of the invention, a method is provided for treating an
orally-
associated biofilm such as dental plaque. The invention provides methods for
oral plaque
prevention, treating oral plaque infection, treating tooth hypersensitivity,
sterilizing a root canal, or
treating a dental disease.
Methods of the invention comprise contacting an oral surface, such as teeth,
gums, gingiva,
or tongue, with a therapeutically effective amount of the composition. Some
methods of the
invention comprise prevention of an orally-associated biofilm by
administration of a
prophylactically effective amount of composition to an individual. The
composition may be
formulated as a dentrifice, such as toothpaste, for treatment or prevention of
dental plaque. In other
embodiments, the biofilm may be located on the tongue, oral mucosa, or gums.
In some
embodiments, the composition is formulated as a mouthwash. In some
embodiments, the
composition is formulated as a paint, foam, gel, or varnish, for example, in a
fluoride-containing
composition. In an embodiment, the composition is in the form or a gel or foam
in a mouthguard
that a patient wears for several minutes for fluoride treatment. In other
embodiments the
composition is contacted to an adhesive strips, which can be applied to the
teeth or other oral
surface. The composition may comprise a liquid polymer formulation, which is a
composition that
is preferably topically applied to a surface such as a tooth, to skin, to a
mucous membrane, and
which dries as a film adhering to that surface, in a manner which resists
removal under normal
conditions, such as eating or brushing, for applications to the teeth and oral
mucosa, or normal
washing and abrasion, when applied to skin. Alternatively, the composition may
be applied to
bandages, dressings, gauze, brushes, implants, etc. and permitted to dry into
a film in advance of its
administration to a patient.
Mastitis treatment
In another embodiment of the invention, compositions and methods are provided
for
treating mastitis. Mastitis is an inflammation tissue in the breast or udder
of a mammal. It is often
associated with bacterial infections such as Pseudomonas aeruginosa,
Staphylococcus aureus,
Staphylococcus epidermidis, Streptococcus agalactiae, Streptococus uberis, and
others. Some of
the bacteria known to cause mastitis also form biofilms, but not all mastitis
results from biofilm
formation.
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
Mastitis can occur in any mammal, such as humans, cows (dairy cattle), and
other animals.
Mastitis is a particular problem for dairy cattle. In cattle the condition
occurs when leukocytes are
released into the mammary gland, often as a response to bacteria in the teat
canal. Cows that are
repeatedly infected often must be culled to prevent widespread infection in
the herd. The loss of
milk from infected cows and the loss of cows and entire herds due to infection
results in large
economic losses for the dairy industry worldwide. In the United States, for
example, mastitis is
estimated to cost the dairy industry up to $2 billion each year.
Methods are provided for administration of a composition to a mammal in need
of
treatment for mastitis. Methods of the invention include prophylaxis, therapy,
or cure for mastitis.
In some embodiments, spread of mastitis to another quarter or to another
animal is inhibited.
The formulations, dosages, and routes of administration discussed above are
applicable to
these embodiments of the invention. For example, in various embodiments, the
composition may
be administered parenterally, orally, locally, or topically. Compositions may
be applied by
intravenous, intra-muscular, or subcutaneous injection. Compositions may be
applied by infusion
via a teat canal, as is known in the art. In methods of the invention,
compositions may be
administered in a pharmaceutically acceptable carrier, which may include
emollients, emulsifiers,
thickening agents, solvents, and the like.
The composition may be administered in a single daily dose or in multiple
doses, e.g., 2, 3,
4, or more doses, per day. The total daily amount of composition may be about
0.01 mg, 0.1 mg, 1
mg, 2 mg, etc., up to about 1000 mg. In some embodiments, the total daily
amount of administered
is about 0.01 mg to about 1 mg, about 1 mg to about 10 mg, about 10 mg to
about 100 mg, about
100 mg to about 500 mg, or about 500 mg to about 1000 mg. The actual dosage
may vary
depending upon the specific composition administered, the mode of
administration, and other
factors known in the art. The composition may be administered in conjunction
with another known
antimicrobial treatment such as an antibiotic.
Compositions can be administered topically to a cow's udder by directly
applying or
spreading the composition onto the udder or teat. The composition may be
formulated as a liquid,
powder, lotion, cream, gel, oil, ointment, gel, solid, semi-solid formulation,
or aerosol spray.
Methods of the invention may further comprise dipping a teat into the
composition. Teat dipping
can be used to treat an already infected udder or to prophylactically prevent
mastitis from
developing. The composition may be applied immediately before milking,
immediately after
26
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
milking, or both. Methods of teat dipping are known in the art, and are
described in more detail in
U.S. Patent 4,113,854, as well as U.S. Patent Publications 2003/0235560 and
2003/0113384, each
of which is incorporated by reference herein in its entirety. Methods may
further comprise use of a
teat sealant to create a physical barrier for the teat orifice after
administration of the composition.
In other embodiments, compositions can be provided via intramammary infusion.
Intramammary infusion involves forcing the antibiotic up through the teat
canal into the udder.
Infusion liquid may comprise a composition disclosed herein in combination
with a
pharmaceutically acceptable carrier such as canola oil. Prior to infusion, the
teat is cleaned, for
example with an alcohol swab. An antibiotic infusing device may include a
cannula sized and
shaped to fit into the teat canal. The cannula may be fully or partially
inserted through the streak
canal. Methods for infusion are known in the art and are described, for
example, in U.S. Patents
4,983,634 and 5,797,872, the entirety of each of which is incorporated by
reference herein.
Methods of the invention may further comprise administering antibiotics in
conjunction
with compositions of the invention, or in sequential doses before or after
administration of the
compositions.
Wounds and surgical uses
The compositions can be applied to prevent and treat biofilm on other types of
living tissue
as well. Tissue includes, for example, skin, mucus membranes, wounds, or
ostomies. As has been
described above, the composition is useful for wound treatment. Wounds include
bedsores, chronic
wounds, burns, pressure wounds, diabetes wounds, and other forms of skin
trauma. Wounds are
often susceptible to biofilm formation, which prevents healing and can lead to
chronic conditions.
Hypochlorous acid and acetic acid compositions can be used for debridement and
cleaning of
damaged tissue.
The compositions can also be used in a surgical setting, for treating skin
prior to or after an
operation. The composition prevents infection that would lead to biofilm
formation. At times, an
area requiring surgery such as a traumatic wound may already be at risk of
having developed a
biofilm. The hypochlorous acid composition can be used to disinfect the area
prior to surgical
incision, which would not only help treat the biofilm but also lessen the
likelihood of it spreading
to other tissue during surgery. The compositions may be used to disinfect any
surface within a
surgical operating field.
27
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
Other medical uses
In addition to wound care, HOC1 compositions of the invention can also be used
for non-
traumatic tissue treatment. They may be used for bladder irrigation, for
preventing or treating
bladder infections or catheter-associated urinary tract infections, and the
like. They may be
similarly used to treat infections in the aerodigestive tract such as sinus
and lung infections, or
infections in the oral cavity, pharynx, paranasal sinuses, sinonasal tract,
larynx, pyriform sinus, or
esophagus. They can be used to fight microbial growth that leads to infection
and to reduce
allergens that cause adverse immune responses. The compositions may also be
administered to the
gastrointestinal tract, including the stomach, intestines, and colon, to
combat microbial infections
such as gastroenteritis, Clostridium difficile infection, and small-intestine
bacterial overgrowth
(SIB 0).
In various other embodiments, the compositions can be administered in the form
of eye
drops to fight eye infections, or can be used to clean or store contact lenses
to prevent bacterial
growth and biofilm formation. In other embodiments, the composition can be
used as an oral rinse
or mouthwash to fight biofilm buildup in the oral cavity, or it can be used to
clean or store
dentures.
In addition to the use as an antiseptic to treat or prevent biofilms on living
tissue, the
compositions can be used as a disinfectant on other surfaces such as for use
in healthcare facilities,
food preparation, cooking utensils, and the like. Compositions can be used to
disinfect countertops,
hospital beds, or food preparation surfaces.
The compositions can be used to disinfect medical devices and surgical
instruments, for
example. Medical devices are often initially supplied as sterile, but may
require additional or
subsequent cleaning and disinfection or sterilization. Reusable medical
devices in particular must
be sterilized or disinfected prior to reuse. Compositions can be applied to
the medical device using
any known technique. For example, the composition can be applied by wiping or
spreading it onto
the surface of the device, by spraying an aerosol or mist form of the
composition onto the device,
by dipping the device into a vessel containing a volume of the composition, or
by placing the
device into a flow of the composition such as from a faucet. Additionally or
alternatively, medical
devices and surgical instruments may also be stored submerged in the
composition and removed at
the time of use.
Treatment with a hypochlorous acid composition disclosed herein can be done in
addition
28
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
to other known techniques such as autoclaving. Alternatively, the composition
can be applied
instead of autoclaving. Because heat sterilization is not useful for all
devices (e.g., some devices
contain delicate parts or electronics that cannot withstand high
temperatures), hypochlorous acid
compositions are a useful alternative, providing an effective way to sterilize
or disinfect such
devices.
The compositions can also be used to disinfect implants and prostheses before
introducing
them to the body. Such devices include orthopedic implants, wires, screws,
rods, artificial discs,
prosthetic joints, soft tissue fillers, pacemakers, intra-uterine devices,
coronary stents, ear tubes,
artificial lenses, dental implants, and many others known in the art.
The stabilized hypochlorous acid and acetic acid compositions described herein
are useful
for both biofilm prevention and biofilm removal on all of the surfaces and
tissues discussed herein.
Because biofilms make microbes far more resistant to traditional antimicrobial
agents, microbes
that form biofilms are more able to share and modify their resistance genes
and spread into the air
and surroundings. As a consequence of biofilm development, a simple infection
may become
chronic, antibiotics and antiseptics stop working, and new strains of
infections emerge.
Both acetic acid (or other organic acids) and hypochlorous acid, however, are
particularly
useful for treating and preventing biofilms. The HOC1 compositions disclosed
herein mimic the
natural disinfectant of the immune system. Therefore the compositions are not
susceptible to
microbial resistance. The combination of acetic acid and hypochlorous acid
reduce the likelihood
of development of antibiotic and antimicrobial resistance because the microbes
contained in the
biofilm are unable to being treated are unable to The compositions of acetic
acid and hypochlorous
acid disclosed herein are effective in treating and preventing biofilms,
without inducing
antimicrobial resistance. Additionally, they are non-toxic, do not sting, and
relieve itching.
The various embodiments and uses described above involve a variety of methods
of
administration, as would be understood in the art.
Hypochlorous acid compositions are particularly effective for transdermal
treatment due to
the small size of the HOC1 molecule. Hypochlorous acid is able to penetrate
epithelium and wound
surfaces, and so can generally reach deeper tissue layers without requiring
injection. This is
particularly useful for biofilm infection that forms beneath the top layer of
skin. Unlike many other
antimicrobial treatments, acetic acid and similar organic acids can penetrate
deeper layers of skin
without requiring an invasive delivery mechanism.
29
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
In some embodiments, however, it may be desirable to prevent the HOC1 from
penetrating
into the skin, and therefore the compositions can be combined with excipients,
carriers, emulsifiers,
polymers, or other ingredients, examples of which are discussed in U.S. Patent
Application
2016/0271171, which is incorporated herein by reference in its entirety.
In addition to topical use, wherein the compositions can be sprayed, wiped, or
rubbed onto
skin, in other embodiments the compositions may be injected into a particular
tissue requiring
treatment. The compositions can be ingested in capsule form for administration
to the
gastrointestinal tract. They can be supplied in slow-release or delayed-
release capsules. The
compositions can be provided as a suppository for insertion into the rectum or
vagina. The
compositions can be provided as a spray for release in the vagina. The
compositions can be
provided as a cloth for wiping male and female genitalia or buttocks.
In other embodiments, the compositions can be provided as a nasal spray for
treatment of
the aerodigestive tract, which can include treatment of allergic reactions,
sinus infections and the
like. The nasal spray may be in droplet, aerosol, gel, or powder form. The
buffered hypochlorous
acid and acetic acid composition can be combined with one or more of a
decongestant or an anti-
inflammatory or anti-histamine agent, as needed. The composition can be
aerosolized with a nasal
spray dispenser as is known in the art.
The nasal spray may also include a pharmaceutically acceptable carrier, such
as a diluent,
to facilitate delivery to the nasal mucosa. The carrier might be an aqueous
carrier such as saline.
The composition may be isotonic, having the same osmotic pressure as blood and
lacrimal fluid.
Suitable non-toxic pharmaceutically acceptable carriers are known to those
skilled in the art.
Various carriers may be particularly suited to different formulations of the
composition, for
example whether it is to be used as drops or as a spray, a nasal suspension, a
nasal ointment, a nasal
gel or another nasal form. Other additives, excipients, emulsifiers,
dispersing agents, buffering
agents, preservatives, wetting agents, consistency aids, and gelling agents
may be included as well.
Preferably, additives should be chosen that impart the desired characteristic
without reducing the
stability of the hypochlorous acid. Additives may aid in evenly administering
the composition over
the mucosa or for reducing or delaying the rate of absorption of the
composition.
The composition can be delivered by various devices known in the art for
administering
drops, droplets, and sprays. The nasal spray composition can be delivered by a
dropper, pipet, or
dispensing. Fine droplets, sprays, and aerosols can be delivered by an
intranasal pump dispenser or
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
squeeze bottle. The composition can also be inhaled via a metered dose
inhaler, such as a dry
powder inhaler or a nebulizer.
Controlled Release with Nanoparticle Encapsulation
Stable aqueous solutions of hypochlorous acid and/or acetic acid can be
encapsulated in
nanoparticles that allow controlled release of the acid from the
nanoparticles. Controlled release
allows persistent anti-microbial protection.
FIG. 13 shows an anti-microbial composition 1301 comprising an aqueous
solution 1303 of
hypochlorous acid encapsulated in a nanoparticle 1305. The aqueous solution
1303 of
hypochlorous acid is made by a method described herein to produce a solution
in which the acid
is stable. The stable hypochlorous acid solution 1303 is then encapsulated in
nanoparticle 1305.
The nanoparticle allows gradual release of the hypochlorous acid. Although not
depicted, acetic
acid can also be encapsulated in a nanoparticle for controlled release.
The nanoparticle may be any type of nanoparticle that provides controlled
release of the
acid from the nanoparticle. The nanoparticle may comprise a polymer, such as
an organic polymer.
Examples of polymers suitable for controlled-release nanoparticles include
acrylic acid,
carrageenan, cellulosic polymers (e.g., ethyl cellulose or hydroxypropyl
cellulose), chitosan,
cyclodextrins, gelatin, guar gum, high amylase starch, hyaluronic acid, locust
bean gum, pectin,
polyacrylamide, poly(D,L-lactide-co-glycolide acid), poly(lactic acid),
poly(xylitoladipate
salicylate), polyanhydride, poly(ethylene oxide), poly(ethyleneimine),
polyglycerol ester of a fatty
acid, polysaccharides, polyvinyl alcohol, povidone, sodium alginate, and
xanthan gum. For details
on the use of polymers to form controlled-release nanoparticles, see
Binnebose, et al., PLOS Negl
Trop Dis 9:e0004713 (2015); Campos, et al., Scientific Reports 5:13809 (2015);
Dasgupta et al.,
Mol. Pharmaceutics 12:3479-3489; Gao, et al., The Journal of Antibiotics
64:625-634, (2011); Lee,
et al., International Journal of Nanomedicine 11:285-297 (2016); and U.S.
Patent No. 8,449,916
(incorporated by reference). The nanoparticle may contain an alumino silicate
(such as a zeolite,
e.g., analcime, chabazite, clinoptilolite, heulandite, leucite,
montmorillonite, natrolite, phillipsite, or
stilbite ), calcium ammonium nitrate, hydroxyapatite (e.g., urea-modified
hydroxyapatite), metal
hydroxide, metal oxide, polyphosphate, or silicon compound (e.g., silicon
dioxide). The
nanoparticle may contain lipids, i.e., it may be a lipid nanoparticle. The
nanoparticle may include a
liposome. For details on the use of liposomes to form controlled-release
nanoparticles, see
31
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
Weiniger et al., Anaesthesia 67:906-916 (2012). The liposome may be multi-
lamellar. The
nanoparticle may contain a gel, sol-gel, emulsion, colloid, or hydrogel. For
details on the use of
hydrogels to form controlled-release nanoparticles, see Grijalvo et al.,
Biomater. Sci. 4:555 (2016).
The nanoparticle may contain a combination of formats, such as a hydrogel
encapsulated within a
liposome. The nanoparticle may have a coreshell structure. The nanoparticle
may be biodegradable.
Compositions of the invention may include an anti-metabolic agent. The anti-
metabolic agent may
be a metal ion. For example, the anti-metabolic agent may be zinc, copper, or
silver.
A nanoparticle that allows controlled release of hypochlorous acid or acetic
acid permits
diffusion of the acid to occur more slowly than the acid would diffuse from an
equal volume of
the same aqueous solution of the acid that is not encapsulated in a
nanoparticle. The controlled
release of hypochlorous acid or acetic acid may be due to permeability
characteristics of the
nanoparticle, e.g., a nanoparticle that is partially or poorly permeable to
the acid. A controlled-
release nanoparticle may be a nanoparticle that releases the acid due to
degradation of the
nanoparticle or impairment of its structural integrity in a time-dependent
manner. Release of the
acid from the nanoparticle may be triggered by environmental conditions, such
as pH, temperature,
light, pressure, redox conditions, or the presence of a particular chemical.
FIG. 14 is an illustration of a method 1401 of making an anti-microbial
composition that
includes an aqueous solution 1403 of hypochlorous acid encapsulated in a
nanoparticle 1405.
The method entails mixing 1411 in water in a chamber 1413 from which air has
been purged a
compound 1415 that generates a proton (H+) in water and a compound 1417 that
generates a
hypochlorite anion (Off) in water. The mixing 1411 produces an air-free
aqueous solution 1403 of
hypochlorous acid. The solution 1403 is then encapsulated 1421 in a
nanoparticle 1405. The
encapsulation may be performed in an air-free environment to produce a
composition that is
substantially free of air.
Compositions of acetic acid and hypochlorous acid for biofilm treatment
The disclosed formulations of acetic acid and hypochlorous acid are superior
for treating
biofilms on surfaces including skin or other tissue. The compositions use a
balanced formula where
the combination of acetic acid and hypocholorous acid provide greater
disinfecting qualities than
either substance alone. In fact the present invention recognizes that the
particular disclosed
combinations provide greater disinfecting power than would be expected by
adding the acetic acid
32
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
and hypochlorous acid. In other words, the compositions have been found to be
greater than the
sum of their parts. These benefits are shown in the accompanying data in FIGS.
15-21, which
demonstrate how the balanced compositions of acetic acid and hypochlorous acid
provide enhanced
disinfecting capabilities against biofilms and outperform all other products
on the market. The
differences in performance are shown across a wide range of concentrations.
Additionally, since acetic acid is toxic at high concentrations, the prior art
has taught away
from its use on skin or other tissue, except in trace amounts. Some of the
disclosed compositions
contain acetic acid at 2% or greater, and when in combination with HOC1 have
proven to be safe
and effective for treating skin and other tissues. The HOC1 in these
compositions has been found to
have a modulating effect of the acetic acid. This allows the compositions to
take advantage of the
antimicrobial properties of acetic acid without causing harm to the tissue.
Additionally, HOC1 has
an analgesic function, so it also allows higher concentrations of HAc to be
used on skin or other
tissue without causing excessive pain or discomfort to the patient.
FIG. 15 for example, shows a comparison of various concentrations of HOC1 and
acetic
acid against other commercially available antimicrobial compositions. Eight
different treatments
were tested, as listed along the x-axis. Each composition was exposed to a 24-
hour filter-grown S.
aureus biofilm, and the reduction in biofilm was measured in colony-forming
units per milliliter
(cfu/ml) and reported on a log scale along the y-axis. Measurements of the
reduction in biofilm
were recorded at 3 hours and 6 hours. Each column therefore has two bars, and
shows the effect of
each composition on the biofilm over time.
The first three columns show the results of 200ppm HOC1 with three different
concentrations of acetic acid (0.25%, 1.0%, and 2.0%, respectively). The
fourth column shows 1%
acetic acid alone. The next four columns show commercially available
antimicrobial products:
Prontosan; Octenilin; Pyrisept; and Microdacyn, which is a hypochlorous acid
composition.
The results show that all three combinations of acetic acid and hypochlorous
acid were
more effective against the biofilm than any of the other compositions. At 3
hours, test composition
A (200ppm HOC1 and 0.25% HAc) performed approximately as well as the
Prontosan, the current
market leader in biofilm treatment. It also far outperformed the 1% HAc or the
other commercially
available products. After 6 hours, however, composition A showed much greater
efficacy than even
Protosan.
Meanwhile, test composition B (200ppm HOC1 and 1.0% HAc) was even more
effective at
33
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
treating biofilm. Comparing composition B with the 1% HAc (in the fourth
column) shows the
unexpected benefit of the addition of HOC1. Despite having the same
concentration of acetic acid,
composition B far outperforms the 1% HAc alone at both 3 hours and 6 hours.
Composition C (200ppm HOC1 and 2.0% HAc) showed by far the greatest reduction
in
biofilm among the tested compositions. At both 3 and 6 hours, it was several
orders of magnitude
more effective than the commercially available products.
These data show that in addition to being more effective in reducing biofilm
than any of the
commercially available products, the compositions containing both acetic acid
and hypochlorous
acid were more effective than acetic acid alone (1% HAc) or hypochlorous acid
alone
(microdacyn), and those superior results cannot be explained merely by the
additive effect of the
two components. Without being bound by any particular mechanism, the data show
that the acetic
acid and hypochlorous acid combination provides a synergistic effect that
allows the composition
to be more effective than would otherwise be predicted based on the efficacy
of each component
alone.
FIGS. 16-19 show the effects of various compositions of HOC1 and HAc on P.
aeruginosa
biofilms. FIG. 16 shows a comparison of compositions having 1% acetic acid and
varying
concentrations of HOC1. Five different treatments were tested with HOC1 in
concentrations of
Oppm, 50ppm, 100ppm, 150ppm, and 200ppm. Each composition was exposed to a 24-
hour filter-
grown P. aeruginosa biofilm, and the reduction in biofilm was measured in
colony-forming units
per milliliter (cfu/ml) and reported on a log scale along the y-axis.
Measurements of the reduction
in biofilm were recorded at 2 hours and 4 hours.
As shown in the graph, the reduction at 2 hours was greater with higher
concentrations of
HOC1, with a particularly significant spike at 150ppm. At 4 hours, the spike
occurs at even lower
concentrations of HOC1.
FIG. 17 shows the effects on P. aeruginosa of different compositions where the
concentration of HOC1 is maintained at 100ppm and the percentage of acetic
acid varies from 25%
to 2%.
FIG. 18 shows the effects as both HOC1 and HAc increase.
FIGS. 19-21 show different compositions of HOC1 and HAc against S. aureus and
P.
aeruginosa under various conditions. The figures show the superior results
obtained with
combinations of hypochlorous acid and acetic acid, which demonstrate the
synergistic effect of
34
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
those two compounds.
The various disclosed formulations may be effective for treating biofilm
infections in
different types of tissue. For example, the 200ppm HOC1 and 0.25% HAc
composition is useful for
topical applications such as hand disinfection or mouth wash. This composition
is more effective
than other commercially available products at treating surface-level biofilms
as shown in FIG. 15.
For treating penetrating deeper into tissue, or for clearing particularly bad
biofilm infections or
invasive biofilms that have penetrated beneath the surface, a higher
percentage of
HAc may be used, such as the formulation of 200ppm HOC1 with 2% HAc. This
composition is
useful for treating infected wounds, preventing biofilm in wounds, treating
eczema, or treating
other infections. This formulation has been found to be effective for
combatting biofilms that have
formed in the root of teeth.
FIGS. 20-21 show additional data supporting the unexpected efficacy of acetic
acid and
hypochlorous acid compositions on various biofilms, particularly as compared
to prior art and
commercially available compositions. As the figures make clear, various
compositions that balance
in the concentrations of HOC1 and HAc in different ways provide an assortment
of disinfecting
compositions that can target different types of biofilms on different types of
tissue.
Compositions of hypochlorous acid and acetic acid for treatment of transient
bacteria while
maintaining natural flora
Another benefit of the disclosed HOC1 and HAc compositions is that while they
are
particularly effective at reducing pathogenic biofilms, they have been found
not to inhibit growth
of "good" or natural biofilms and other microbes that live symbiotically on
and in tissue. In fact the
present invention recognizes the disclosed compositions are effective for
immediately eradicating
transient microbes while maintaining levels of natural biofilm.
Tests were performed on the hands of healthy subjects to determine the
transient bacteria
killing effect of the disclosed compositions. The subjects washed their hands,
and dipped their
fingertips into Escherichia coli. The disclosed compositions were applied to
the subjects' hands by
rubbing the disclosed compositions into the skin to stimulate the removal of
the transient bacteria
on the skin. The same test was performed using prior art alcohol compositions.
The results
provided that the disclosed invention reduced the presence of the transient
bacteria by 99.98%,
where the prior art compositions reduced the presence of transient bacteria by
99.9%.
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
In some trials, the good biofilm was reduced at a lesser rate than the
pathogenic biofilm,
and it grew back faster than the target biofilm did. Specifically, tests were
performed on the hands
of 20 subjects. The disclosed compositions were applied to the subjects' hands
by rubbing the
disclosed compositions into the skin to stimulate the removal of the transient
bacteria on the skin.
In this trial the disclosed compositions were applied multiple times to the
hands and forearms of the
subjects over 5 minutes to maintain exposure to the disclosed compositions.
The same test was
performed using prior art alcohol compositions. Bacteria kills were measured
immediately after the
application and again 3 hours after application. The results provided
demonstrated that alcohol has
a 99.5% killing effect on the natural biofilm immediately after application
and 99.16% effect 3
hours after application. Surprisingly, the disclosed invention had only a 90%
killing effect on the
natural biofilm immediately after application, and a 99.16% killing effect on
the natural biofilm
immediately after application.
Surprisingly, the disclosed inventions compared to prior art alcohol-based
disinfectants,
were more effective at targeting the pathogenic biofilm infection without
damaging the good
microbes. Therefore, the disclosed compositions are effective at the targeted
treatment of biofilm
infections, without harming the body's natural flora.
Incorporation by Reference
Any and all references and citations to other documents, such as patents,
patent
applications, patent publications, journals, books, papers, web contents, that
have been made
throughout this disclosure are hereby incorporated herein by reference in
their entirety for all
purposes.
Equivalents
The invention may be embodied in other specific forms without departing from
the spirit or
essential characteristics thereof. The foregoing embodiments are therefore to
be considered in all
respects illustrative rather than limiting on the invention described herein.
36
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
Examples
Example 1: Product analysis
When spectrophotometry is expanded to also cover the visible range it is
possible to detect
colors. The gases generally produced during production of HOC1 are C102, C120
and C12, all of
which are detectable in the visible range as yellow or yellow-red. Tzanavaras
et al. (Central
European J. of Chemistry, 2007, 5(1)1-12). Data in FIG. 9 illustrates that the
HOC1produced by
methods on the invention shows no absorption from colored gases as shown by
the lack of colored
substance. It is known that HOC1produces a peak at 292 nm (Feng et al. 2007,
J.
Environ. Eng. Sci. 6, 277-284).
Example 2:
HOC1produced by the process described above was stored under heat stress at 40
C in
order to accelerate degradation using four different types of aqueous
solutions: (1) reagent grade
water (deionized water); (2) tap water; (3) reagent grade water with a
phosphate buffer; and (4) tap
water with a phosphate buffer. Characteristics of the HOC1 product were
monitored after the initial
reaction (T = 0); four weeks (T = 4); eight weeks (T = 8); and twelve weeks (T
= 12).
FIG. 10 is a graph showing the amount (parts per million (ppm)) of HOC1
initially
produced (T = 0) and its stability over time. The data show that the reagent
grade water (deionized
water) without phosphate buffer is the most stable over the twelve weeks,
showing the least amount
of product degradation from the initial amount produced. The deionized water
produces a much
more stable product than that produced using tap water. Additionally, and
surprisingly, the data
show that phosphate buffer may negatively impact amount of HOC1 product
produced.
FIG. 11 is a graph showing how the pH of the HOC1product changed over time. In
all
cases, the pH decreased over time, however, for all cases, the pH stayed in
the range of pH = 4 to
pH = 7 over the twelve weeks.
FIG. 12 is a graph showing the oxidation capacity of the HOC1product over
time. The data
show that the product retained oxidation capacity over the twelve weeks
regardless of the starting
water.
Example 3: Acetic acid compared to hydrochloric acid
Using the above described process, HOC1 was produced using hydrochloric acid
(HC1) and
37
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
acetic acid and thereafter stored under heat stress at 40C. The amount of HOC1
initially produced
was recorded (T = 0) and then the amount of HOC1product remaining after twelve
days was
recorded. Three batches of each were produced. The data for the HC1produced
HOC1 is shown in
Table 1. The data for the acetic acid produced HOC1 is shown in Table 2.
Table 1: HOC1produced with HC1
Batch Initial Initial
pH after 12 Amount of Amount pH
number amount pH Amount after days degradation change
(ppm) 12 days (ppm)
1 192 7.12 159 5.71 17.2% 19.8%
2 183 5.88 147 4.01 19.7% 31.8%
3 189 5.21 154 3.97 18.5% 23.8%
Table 2: HOC1produced with acetic acid
Batch Initial Initial
pH after 12 Amount of Amount pH
number amount pH Amount after days degradation change
(ppm) 12 days (ppm)
1 205 4.62 180 4.50 12.4% 2.7%
2 205 5.33 178 5.04 13.3% 5.4%
3 207 4.07 178 3.89 13.9% 4.6%
The data show that using acetic acid provides greater product stability, most
likely due to
greater stability in the pH. Without being limited by any particular theory or
mechanism of action,
it is believed that the different protonation capacity of acetic acid as
compared to hydrochloric acid,
i.e., acetic acid donates fewer protons to a liquid than hydrochloric acid,
results in greater HOC1
stability over time.
Example 4: Hand Disinfectant
Tests were performed on the hands of healthy subjects to determine the
transient bacteria
killing effect of the disclosed compositions.
In this experiment, 18 subjects washed their hands, and dipped their
fingertips into a
solution of Escherichia coli. Once the subjects' hands were dry, aliquots of
the disclosed
composition (5 mL of 160 ppm HOC1, 0.13% Hac) were applied to the subjects'
dry hands and
rubbed into the skin for 30 to stimulate the removal of the transient bacteria
on the skin, according
to Handrub procedure PN-EN 1500:2013-07. The same was done using 3 mL of the
reference
alcohol 2-propanol 60% v/v.
38
CA 03118352 2021-04-30
WO 2020/089689 PCT/IB2019/001177
The results provided that both the disclosed composition and the reference
alcohol reduced
the presence of the transient bacteria by a 4-log reduction.
In another experiment aliquots of the disclosed composition (3 mL of 0.03%
HOC, 0.13%
Hac) were applied to each of the hands of 20 subjects. For each of the
subjects, one hand was bare
and the other had a surgical glove covering it. The composition was rubbed
into the hands and
forearms for 5 minutes to maintain exposure to the compositions according to
Handrub procedure
PN-EN 12791:2005. The same was done using 3 mL of the reference alcohol propan-
l-ol, 60% v/v
for 3 minutes of maintained exposure.
Bacteria kills of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus
areus, and
Enterococcus hirae were measured immediately after the application and again 3
hours after
application. The results demonstrated that the reference alcohol reduced the
natural bacteria by 2.48
logs immediately after application and 2.16-log reduction 3 hours after
application. The disclosed
composition reduced the natural bacteria by a 0.69 logs 3 hours after
application and a 1.01-log
reduction immediately after application.
Surprisingly, the disclosed compositions of the present invention compared to
the prior art
alcohol-based disinfectants, were more effective at targeting transient
pathogenic bacteria without
damaging the natural bacteria. Therefore, the disclosed compositions are
effective at the targeted
treatment of pathogenic biofilm infections, without harming the body's natural
flora that protects
the skin.
39