Note: Descriptions are shown in the official language in which they were submitted.
1
=
METHOD FOR PRODUCING SMELTER-GRADE ALUMINA FROM LOW-
.. GRADE HIGH-SILICON ALUMINIUM-CONTAINING RAW MATERIALS
The invention relates to the metallurgy sphere, in particular to acidic
methods for producing alumina, and can be used in processing low-grade high-
silicon aluminium-containing raw materials, including waste, such as ash from
coal combustion. Smelter-grade alumina and its semi-finished product -
aluminium hydroxide - have a wide range of industrial applications, primarily
for
the production of aluminium metal.
Alumina refineries worldwide produce high-quality smelter-grade alumina
mainly using the Bayer technology from low-silicon (Bayer) bauxite, in which
the A1203/SiO2 concentration ratio (alumina to silica ratio) is not lower than
3.
When the values of the alumina to silica ratio are in the range between 3 and
7, it
is necessary to use combined Bayer-sintering processes, which are more energy-
intensive ones. For high-silica aluminium-containing raw materials, for
example,
nepheline and kaolin, the industry uses only the sintering method, the energy
costs of which are approximately 5 times higher than those of the Bayer
process.
At the same time, acidic methods for the production of alumina from high-
silicon aluminium-containing raw materials are being developed. Among them,
the hydrochloric acid method is currently considered to be the most rational
one.
It is known that alumina can be produced from high silica bauxites by the
hydrochloric acid process, which includes the calcination of aluminium-
containing raw materials at a temperature of up to 700 C, their treatment
with
hydrochloric acid, the salting out of aluminium hexahydrate chloride
(A1C13-6H20) by saturating the clarified chloride liquor with gaseous hydrogen
Date Recue/Date Received 2021-05-13
2
chloride, the calcination of aluminium chloride to produce aluminium oxide
(alumina), the spent liquor pyrohydrolysis, and the rectification of absorbed
hydrochloric acid, including the return of hydrogen chloride at the stage of
acid
treatment and salting out in the form of an aqueous solution and gas,
respectively
(Elsner D., Jenkins D.H., and Sinha H.N. Alumina via hydrochloric acid
leaching
of high silica bauxites-process development. Light metals, 1984, p. 411-426).
According to the known method, only rectified hydrochloric acid is fed to
the raw materials processing area, which eliminates the circulation and
accumulation of impurities (for example, iron, sodium, potassium, calcium,
etc.)
in the acid cycle and as much as possible reduces their content in the
aluminium
chloride hexahydrate. The impurities are removed as oxides by complete
pyrohydrolysis of the spent liquor following the crystallisation of
AlC13.6H20.
Despite this, the phosphorus content in the end product is 1.5 times higher
than
the permissible limits for smelter-grade alumina.
The disadvantages of this method should also include very complex
equipment and process flow and a lot of expensive equipment to provide the
complete regeneration of hydrochloric acid, which entails high capital
expenditure for the construction of an alumina refinery using this technology.
The
area, in which complete pyrohydrolysis of the spent liquor after the
crystallisation
of AlC13.6H20 is implemented, is a very energy-intensive one and fuel costs
significantly increase the production cost.
In addition, alumina produced by the calcination of aluminium chloride
hexahydrate is fundamentally different from conventional smelter-grade alumina
by low particle strength, tendency to dusting, 1.5 to 3 times less bulk
density, and
completely different theological properties (very poor fluidity), which cause
problems during its transportation and in the course of electrolytic
production of
aluminium. When such alumina is calcined, it is almost impossible to
simultaneously achieve a low content of residual chlorine and a-phase, which
is
Date Recue/Date Received 2021-05-13
_________________ ¨ ______
3
one of the main requirements for smelter-grade alumina. In the event that the
feed
stock contains any phosphorus compounds, almost its entire volume will enter
the finished product, as indicated by the authors of the known method.
There is a known method for aluminium and iron extraction from
-5 aluminium ore (patent CA2684696 published on November 27, 2008); the
method includes the preparation of aluminium-containing raw materials
(kaolinitic argillite), the leaching thereof with 6-molar hydrochloric acid at
100-
110 C, the separation of the resulting suspension into solid and liquid
phases,
the distillation of the liquid phase and the washdown water of the solid phase
to
the extent of 90% including hydrogen chloride regeneration by rectification
and
its return to the leaching stage. The remaining 10% of the liquid phase are
neutralised with caustic alkali up to pH > 10 to produce aluminium chloride
liquor
and separate the iron oxide precipitate. The aluminium chloride liquor is
neutralised with hydrochloric acid up to pH = 3 4 and the aluminium is
separated
by liquid extraction and then converted to aluminium hydroxide and. oxide
(alumina).
This method also requires a very large amount of thermal energy to provide
the boiling-down of the entire flow of the liquor and washdown water after the
digestion of raw materials to an extent of 90% and a significant consumption
of
hydrochloric acid and caustic alkali to selectively recover iron and aluminium
from the liquors.
The closest to the claimed method is a combined acid and alkali method to
produce alumina by hydrochloric acid treatment of raw materials, including the
separation of silica sediment, the crystallisation of aluminium chloride
hexahYdrate from the clarified chloride liquor, followed by its calcination to
produce an intermediate alumina product, which the authors called 'raw' or
'crude'
alumina due to the significant content of iron and other impurities (with the
exception of silicon). This is followed by the leaching of the intermediate
alumina
Date Recue/Date Received 2021-05-13
_ __
4
product with alkaline spent liquor, the precipitation of the resulting
pregnant
liquor, the water washing, and subsequent calcination of the separated
aluminium
hydroxide, the boiling-down of the spent liquor, which left the precipitation
area
and the water, which was used to wash -down aluminium hydroxide, and the
formation of alkaline spent liquor returned to the area for leaching the
intermediate alumina product (Non-Ferrous Metal Production Reference.
Alumina Refining. Moscow: Metallurgiya, 1970, p. 236-237).
Essentially, processing of the intermediate alumina product is its alkaline
recrystallisation according to a simplified Bayer process flow, which is used
to
remove iron, phosphorus, and other impurities and produce smelter-grade
alumina both in terms of chemical composition and physical properties.
A serious drawback of the described flow is that iron, sodium, potassium,
calcium, magnesium, and other impurities delivered along with the raw
materials
are accumulated during the acid cycle, and under this method the problem is
solved by deep evaporation of the chloride liquor and chlorides
crystallisation to
the maximum possible extent under the circumstances. Following their
calcination with crude alumina, a significant amount of alkali metal chlorides
is
fed to the Bayer alkaline cycle; such chlorides will inevitably accumulate in
the
circulating flow and their removal is not provided for.
The disadvantages of the known method for producing alumina also
include the overall high energy costs and additional loss of alkali to an
amount of
36-37 kg/tonne of alumina. For these reasons, the method has found no
industrial
application.
The invention is based on the problem of developing a method to produce
smelter-grade alumina from low-grade (high-silicon) raw materials, which would
allow processing poor high-silicon ores and waste.
Date Recue/Date Received 2021-05-13
5
The technical result is to improve the quality of alumina and reduce energy
consumption when producing smelter-grade alumina from low-grade raw
materials, in other words, when processing poor high-silicon ores and wastes.
The problem can be solved, and the above technical result can be achieved
by the proposed method for producing smelter-grade alumina, which includes the
following stages:
extraction of aluminium-containing raw materials with hydrochloric acid,
separation of the resulting chloride slurry into waste silica precipitate and
clarified chloride liquor,
crystallisation of aluminium chloride hexahydrate from the clarified
chloride liquor,
aluminium chloride hexahydrate thermal decomposition into aluminium
oxide, followed by its calcination to produce crude alumina as an intermediate
product,
crude alumina leaching with the alkaline spent liquor and precipitation of
the resulting aluminate liquor,
water washing and subsequent calcination of the aluminium hydroxide
separated, and
boiling-down of the spent liquor, which left the precipitation area, and the
water, which was used to wash down aluminium hydroxide to form alkaline spent
liquor, which is returned to the intermediate alumina product leaching stage.
In addition, in order to optimise the process, the chloride ion concentration
in the intermediate alumina product should be maintained at a level of 0.2-5.0
wt.
%, the chloride ion concentration in the alkaline spent liquor should be
maintained at a level of 40-90 g/L, and the spent liquor, which left the
precipitation area (10-40 wt. % of the total flow), should be boiled-down
until the
chlorine-containing compound crystals to be removed from the process are
separated.
Date Recue/Date Received 2021-05-13
6
According to an embodiment, the smelter-grade alumina production
method includes the following stages:
milling of aluminium-containing raw materials, followed by their
extraction with hydrochloric acid, which is acidic spent liquor,
separation of the resulting chloride slurry into waste silica precipitate and
clarified chloride liquor,
crystallisation of aluminium chloride hexahydrate from the clarified
chloride liquor,
aluminium chloride hexahydrate thermal decomposition into aluminium
oxide, followed by its calcination to produce crude alumina as an intermediate
product,
crude alumina leaching with alkaline spent liquor and precipitation of the
resulting aluminate liquor and subsequent calcination of the separated
aluminium
hydroxide, while about 15% of the acidic spent liquor is subjected to
pyrohydrolysis, the chloride ion concentration in the crude alumina is
maintained
at 0.2-5.0%, the chloride ion concentration in the alkaline spent liquor is
maintained at a level of 40-90 g/L, the alkaline spent liquor after
precipitation in
the amount of 10-40 wt,% of the total flow is boiled down until the chlorine-
containing compound crystals to be removed from the process are separated.
According to the second embodiment, the smelter-grade alumina
production method includes the following stages:
milling of aluminium-containing raw materials, followed by their leaching
with hydrochloric acid spent liquor, which is acidic spent liquor,
separation of the resulting chloride slurry into waste silica precipitate,
which is dumped following its washing down with water, and clarified aluminium
chloride liquor, while the water, which was used for washdown purposes, is
supplied to the area to adiabatically absorb hydrogen chloride from the fumes
resulted from the calcination of aluminium chloride hexahydrate and those
Date Recue/Date Received 2021-05-13
7
resulted from the pyrohydrolysis process and the amount of washdown water is
determined by the amount of water for adiabatic absorption,
aluminium chloride hexahydrate crystallisation from the clarified
aluminium chloride liquor; after the crystals are separated, the resulting
spent
liquor is supplied to the rectification area, where the hydrogen chloride
concentration in the spent liquor is reduced to form hydrogen chloride gas,
which
is dried and then supplied to the salting-out area; the spent liquor
discharged from
the rectification area is divided into two unequal parts: a larger one is
supplied
directly to prepare spent liquor, the other part is supplied to remove
impurities by
pyrohydrolysis,
aluminium chloride hexahydrate thermal decomposition to form
aluminium oxide, which is subsequently calcined to produce crude alumina as an
intermediate product, while the calcination fumes are absorbed by water for
washing down the waste silica precipitate,
crude alumina leaching with the alkaline spent liquor according to the
Bayer process and precipitation of the resulting aluminate liquor,
water washing and subsequent calcination of the aluminium hydroxide
separated,
boiling-down of the spent liquor, which left the precipitation area, and the
water, which was used to wash down aluminium hydroxide to form the alkaline
spent liquor to be returned to the stage of leaching an intermediate alumina
product, and
the spent liquor is largely used for leaching aluminium-containing raw
materials and only a part of it is fed to remove impurities by pyrohydrolysis.
Both embodiments of the method ensure the achievement of the general
technical result that is increase in the quality of alumina and reduction in
energy
costs in the production of smelter-grade alumina from low-grade raw materials.
As additional measures, it is preferably to implement the following:
Date Recue/Date Received 2021-05-13
8
Attorney Ref.: 1671P041CA02
The spent liquor discharged from the precipitation area and the water,
which was used for washing down aluminium hydroxide, are boiled down in two
stages, with alkali metal carbonates being crystallised at the first stage and
alkali
metal chlorides being crystallised at the second stage.
Alkali metal chlorides, mainly sodium and potassium ones, are purified
and, in the form of an aqueous solution, subjected to membrane or diaphragm
electro lysis.
The chlorine and hydrogen formed in the course of membrane or
diaphragm electrolysis of the aqueous solution of alkali metal chlorides are
used
to synthesise hydrochloric acid, which is fed to extract the original
aluminium-
containing raw materials, and a part of the aqueous solution of alkali metal
hydroxides formed during membrane or diaphragm electrolysis of the aqueous
solution of alkali metal chlorides is mixed with the alkaline spent liquor,
which is
returned to the intermediate alumina product leaching stage.
A part of the alkali metal hydroxide solution resulted from membrane or
diaphragm electrolysis of the aqueous solution of alkali metal chlorides is
fed to
neutralise the silica precipitate.
In one aspect, this document discloses a method for producing smelter-
grade alumina from aluminium-containing raw materials comprising the
following stages: a milling of the aluminium-containing raw materials,
followed
by their extraction with hydrochloric acid represented by an acidic spent
liquor to
obtain a chloride slurry, a separation of the resulting chloride slurry into a
waste
silica precipitate and clarified chloride liquor, a crystallisation of an
aluminium
chloride hexahydrate from the clarified chloride liquor, a calcination of the
aluminium chloride hexahydrate to produce a crude alumina as an intermediate
product, a leaching of the crude alumina with an alkaline spent liquor to
obtain an
aluminate liquor as an intermediate product, a precipitation of an aluminum
hydroxide from the aluminate liquor to obtain an alkaline spent liquor and
aluminum hydroxide, followed by a calcination of the aluminum hydroxide,
wherein about 15 wt.% of the acidic spent liquor is subjected to
pyrohydrolysis,
the chloride ion concentration in the crude alumina is maintained at 0.2-5.0
wt.%,
Date Recue/Date Received 2023-08-31
8a
Attorney Ref.: 1671P041CA02
the chloride ion concentration in the alkaline spent liquor is maintained at
a level of 40-90 g/L, and 10-40 wt.% of the alkaline spent liquor after the
precipitation is boiled down to separate chlorine-containing compound crystals
that are removed from the process.
Drawing list
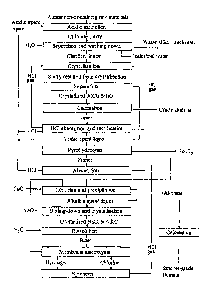
Fig. 1 - Schematic alumina production process flow.
The invention is illustrated by the schematic alumina production process
flow shown in Fig. 1, which clearly demonstrates the rational optimal
combination
of the acid and alkaline cycles of the technology both in terms of flows and
modes,
which, as a whole, provides the achievement of the technical result.
The crushed aluminium-containing raw materials, such as kaolin clay or
kaolin argillite, are subjected to acidic extraction (leaching) with
hydrochloric
acid spent liquor, for example, under autoclave conditions. After leaching,
the
slurry is separated into a precipitate containing about 90% silica (silica
residue)
Date Recue/Date Received 2023-08-31
9
and aluminium chloride liquor. The silica residue washed down with water is
dumped.
The water used to wash down the silica residue is supplied to the area to
adiabatically absorb hydrogen chloride (HCl) from the fumes resulted from the
calcination of the aluminium chloride hexahydrate (ACH, A1C13.6H20) and those
resulted from the pyrohydrolysis process. And the HC1 concentration in the
aluminium chloride liquor is brought to 17-19%. In the course of absorption,
due
to the large amount of heat released during the HC1 absorption, the aluminium
chloride liquor self-evaporates and all the water supplied for washing down
the ,
silica residue is removed from the cycle. Notably, the amount of silica
residue
washdown water is determined by the amount of water that can be evaporated at
= the adiabatic absorption stage.
Separated from the silica residue, the alumina chloride liquor is fed to the
crystallisation (salting-out) area, where the hydrogen chloride gas produced
by
rectification is bubbled through the liquor and the HC1 concentration in the
liquor
is brought to 32%, with most (¨ 95%) of aluminium precipitating as aluminium
chloride hexahydrate crystals. After the crystals (crystallised particles) are
separated, the resulting spent liquor is fed to the rectification area, where
the HC1
concentration in the spent liquor is reduced to almost 22-27% HC1) and a
gaseous
HC1 is formed, which is dried to a content of ¨ 5% H20 and supplied to the
salting-out area. Drying is carried out by cooling the gas with cooling water
to a
temperature of ¨ 35 C. The spent liquor discharged from the rectification
area
(contains 22-27% HC1) is divided into two unequal parts: a larger one is
supplied
directly to prepare acidic spent liquor; the other part is supplied to remove
impurities by pyrohydrolysis.
A proportion of the spent liquor fed for pyrohydrolysis is determined by
the permissible content of impurities in the spent liquor for leaching. The
proportion of spent liquor for pyrohydrolysis will approximately be 15%.
Date Recue/Date Received 2021-05-13
_______________________________________________________ _
_________________________
10
Notably, the content of impurities in the aluminium chloride liquor will
increase
by about 6 times compared to that in leaching the ore with pure hydrochloric
acid.
During pyrohydrolysis, all the free acid contained in the spent liquor and HC1
formed by hydrolysis of chlorides of the metals, including Al, Fe, Ca, Mg,
passes
into the gas phase. Pyrohydrolysis products will include fumes and
pyrohydrolysis cake consisting of iron oxide (Fe2O3) and partially Al, Ca, Mg
oxides, and other minor impurities. The fumes resulted from the pyrohydrolysis
contain regenerated HCl and are fed to the area to absorb HCl with the silica
residue washdown water.
The produced ACH is supplied to the calcination area to produce crude
alumina and fumes containing HCl). The fumes resulted from the calcination
process are delivered to the absorption stage, where absorption is carried out
= using the silica residue washdown water. In order to restore the losses,
fresh acid
is added to the spent liquor, which is fed to the leaching stage; also, it can
be
added by washing down of the ACH product supplied for rectification.
Fresh water is added for sanitary purification of fumes resulted from the
calcination and pyrohydrolysis processes (then it is used to wash down the
silica
residue).
The advantage of this process flow is that a significant part of the spent
liquor is used for leaching the ore and only a part of it is fed to remove
impurities
by pyrohydrolysis. There are no large and complex boiling-down and salt
rectification areas, the pyrohydrolysis area is minimised and not associated
with
the production of crude alumina but instead intended for partial removal of
impurities, which significantly reduces energy costs.
It should be noted that the crude alumina as per the prior art method
contains the minimum amount of impurities, including chlorides. In order to
achieve this in the prior art method, it is necessary to maintain that the
aluminium
chloride liquor contains the minimum permissible level of impurities, for
Date Recue/Date Received 2021-05-13
Ill
example, iron, as well as potassium, sodium, calcium, magnesium, etc. that are
fed with raw materials; for this, the acidic spent liquor should be purified
from
these impurities. The prior art method indicates that it is difficult to carry
out such
purification, for instance, from iron. The usual technique to do this is
pyrohydrolysis, notably, complete evaporation of the acidic spent liquor at a
temperature of up to 850 C as indicated in the similar method (Elsner D.,
Jenkins
D.H., and Sinha H.N. Alumina via hydrochloric acid leaching of high silica
bauxites - process development. Light metals, 1984, p. 423), so energy costs
are
very high in this case.
According to the claimed method, crude alumina is then delivered for
alkaline recrystallisation, which is based on the known Bayer process. The
product of the Bayer process is alumina, which is converted into smelter-grade
alumina by calcination.
To prepare the spent liquor, fresh caustic alkali is also fed, which
, 15 consumption is dependent on mechanical losses with waste mud and alumina
product, as well as alkali loss during its decaustification with metal
chlorides
(A1C13, FeC13, MgCl2, CaCl2) that the crude alumina contains. In this case,
NaCI
and KCI contained in the crude alumina just pass into solution and do not
cause
any loss of alkali.
A special feature of alkaline processing of crude alumina by the Bayer
method (as opposed to the natural bauxite processing method) is the fact that
due
to an extremely small amount of the mud formed, it requires little water to
wash
down the mud. Due to this, the water balance in the alumina production process
can be offset without boiling-down the entire flow of the spent liquor as the
amount of water added to wash down the hydrate approximately corresponds to
the amount of water removed along with alumina product. Moreover, it becomes
possible to recover heat by autoclave leaching using slurry-slurry heat
exchangers
Date Recue/Date Received 2021-05-13
12
without self-evaporation of the blow-off slurry since a small amount of mud
does
not require such a large quantity of condensate to wash down the mud.
The combined operations of deep boiling-down of apart of the spent liquor
and crystallisation are needed to remove chlorine from the Bayer cycle. The
solubility of NaC1 in caustic solutions is significantly reduced only in the
area
with high Na2O concentrations; therefore, we are talking about deep boiling-
down of a part of the spent liquor to a caustic alkali content of 25-33%
(Na2O).
The amount of spent liquor supplied to the boiling-down area is determined
by the permissible level of chloride accumulation in the Bayer process. The
higher the acceptable level of chlorides in the liquors is, the less
proportion of the
spent liquor to be fed to the boiling-down area and, accordingly, the amount
of
evaporated water (and heat energy consumption) will be (with the same content
of chlorides in the crude alumina).
According to the experience of the authors, the permissible level of
chlorides in the spent liquor used in the Bayer process is 90 g/L (for
chloride ion
C1-) in industrial conditions.
The crystallised sodium chloride and partially potassium chloride
separated after the boiling-down process are fed for the well-known diaphragm
or membrane electrolysis to release caustic alkali and hydrogen and chlorine
gases, from which hydrogen chloride gas is synthesised. The caustic alkali and
hydrogen chloride are returned to the acid and alkaline parts of the process,
respectively, to compensate for the inevitable loss of these agents.
Thus, the claimed method is a closed-loop process flow that makes it
possible to process low-grade (high-silicon) aluminium-containing raw
materials
to produce smelter-grade alumina.
Since crude alumina is an intermediate product but not a marketable one,
it is not necessary for the content of impurities of iron, potassium, sodium,
calcium, magnesium, etc. in it that are fed along with raw materials to be the
Date Recue/Date Received 2021-05-13
13
minimum allowable one. Therefore, the concentration of these impurities in the
acid cycle can be increased, which will reduce the chloride liquor evaporation
costs. To this end, at the crystallisation stage, it is advisable to most
fully and
quickly extract aluminium chloride hexahydrate into the solid phase using
simple
equipment and easily implemented process methods without worrying about the
purity of the crystallised ACH, which is fed to the calcination area to
produce
crude alumina. Moreover, there is no need for calcining the product deeply
during
the calcination in order to completely decompose the chlorides. On the one
hand,
this reduces calcination-related heat costs; on the other hand, this does not
create
conditions for the formation of a hardly soluble a-phase in the crude alumina.
Residual chlorine represented mainly by potassium, sodium, calcium, and
magnesium chlorides is extracted together with crude alumina to the acid phase
of the process flow, where it will inevitably accumulate. However, the
research
carried out by the authors has shown that the accumulation of chloride ion in
the
alkaline spent liquor to a level of 40-90 g/L causes no significant decrease
in the
performance of the Bayer process. In order to avoid further accumulation of
chlorine in the alkaline cycle of the process, a part of the spent liquor,
after
precipitation and in the amount of 10-40% of the total flow rate, is boiled-
down
until chlorine-containing compound crystals to be removed from the process are
separated. Laboratory experiments and cyclic process calculations have shown
that this method is sufficient to maintain the chloride ion concentration in
the
alkaline spent liquor at the required level and ensure the water balance of
the
Bayer process.
The selection method does not make it possible to determine an optimal
Combination of the operating parameters of such a multi-link cyclic
technology,
which the claimed method is. The authors solved this problem by using
specially
developed mathematical models of the mass heat balances of the process. At the
same time, the authors unexpectedly found that the consumption of energy in
the
Date Recue/Date Received 2021-05-13
14
form of fuel, heat, and electricity can be reduced, if we purposefully allow
the
accumulation of impurities in the acid and alkaline cycles of the process and
crude
alumina, which is an intermediate product passing from the acid cycle to the
alkaline one.
The numerical experiments performed according to the results of
optimisation iterative calculations based on the aforementioned mathematical
models found the following: if about 15% is fed to the pyrohydrolysis area,
the
content of impurities (iron, sodium, potassium, magnesium calcium, etc.) is
set at
an equilibrium level that does not reduce the recovery of aluminium from raw
materials to crude alumina, but leads to an increase in the concentration of
the
mentioned impurities in the aluminium chloride hexahydrate and further in the
crude alumina. However, when leaching the crude alumina in the alkaline cycle
of the process, the iron, calcium, and magnesium compounds immediately go into
an insoluble precipitate and are removed. In this case, it should be
considered that
the smaller the proportion of acidic spent liquor fed to the pyrohydrolysis
area is,
the lower the energy costs associated with fuel combustion in that area are.
The pyrohydrolysis-related costs can be reduced, if the crude alumina is
subjected to deep high-temperature calcination and washing down with water to
remove soluble chlorides prior to leaching in the Bayer cycle, as provided for
in
the prior art method. In this case, the chloride ion content in crude alumina
decreases to hundredths and tenths of a per cent, but the content of hardly
soluble
alpha alumina increases. Its alkaline processing is implemented through high-
temperature autoclave leaching, which, as a consequence, leads to increased
thermal energy consumption.
On the other hand, it is obvious that if the process temperature or the
intensity of heat and mass exchange in the calcination area are reduced, the
energy
consumption will significantly decrease there, but the chlorine content in the
crude alumina will increase and such chlorine in the form of chloride ion will
Date Recue/Date Received 2021-05-13
15
continue accumulating in the alkaline cycle of the process. Chlorine transfer
from
the acid cycle to the alkaline one will inevitably lead to losses of both
hydrochloric acid and caustic alkali. In the claimed method, these losses are
compensated by removing some potassium and sodium chlorides from the
alkaline cycle and their electrolytic processing to produce NaOH and chlorine
and hydrogen, gases from which HCl is synthesised. But such regeneration
requires thermal energy when evaporating potassium and sodium chlorides from
alkaline liquors and also electricity for electrolysing an aqueous solution of
these
chlorides.
However, the numerical experiments have shown that despite the
complexity of optimising the heat-and-mass balances of the process, the
mathematical models developed by the authors allow finding some non-obvious
alternative mutually linked combinations of process parameters in the acid and
alkaline cycles, in order to minimise energy consumption while maintaining the
required quality of the smelter-grade alumina product. This can be achieved,
when the chloride ion concentration in the crude alumina is maintained at a
level
of 0.2-5.0 wt.%, the chloride ion concentration in the alkaline spent liquor
is
maintained at a level of 40-90 g/L, and the spent liquor, which left the
precipitation area (10-40 wt.% of the total flow), is boiled-down, until the
chlorine-containing compound crystals to be removed from the process are
separated. The claimed method was implemented experimentally with the
presence of the above-mentioned optimal combination of process parameters.
Example
540 g of aluminium-containing raw materials (kaolin argillite) containing,
wt.%: A1203 27.1; SiO2 56.8; Fe2O3 2.0; Na2O 0.31; K20 <0.15; TiO2 0.48; CaO
0.45; MgO Ø27; P205; 0.05; 11.8, crushed to a particle size of <100 pm, were
mixed with 1,650 ml of 20% hydrochloric acid, placed in an autoclave and kept
under stirring for 3 h at 160 C. The resulting chloride slurry was separated
by
Date Recue/Date Received 2021-05-13
16
filtration, the solid precipitate (waste silica residue) was washed down with
water.
The clarified aluminium chloride liquor was bubbled with dry hydrogen chloride
gas at 70 C, until the separation of ACH crystals stopped. The crystallised
ACH
was separated from the spent liquor using a filter and calcined at 600 C to
produce crude alumina. The spent liquor was diluted with the silica residue
washdown water to free 20% HC1 to produce the acidic spent liquor, which was
fed to repeatedly extract kaolin argillite using the acid, and all the above
(cyclic)
operations were performed repeatedly.
After a total of 6 above cycles were performed, the content of the acidic
spent liquor components was stabilised as follows, %: A1C1320.5-21.5; FeCl3
3.9-
42; TiC12 0.001; CaCl2 0.4-0.48; NaC1 0.1-0.12; KC1 0.1-0.11. No reduction in
the rate of aluminium recovery from the aluminium-containing raw materials was
observed and it was 95.5-97.5 %. After each experiment, 15% volume of the
spent
liquor was replaced with pure hydrochloric acid (20% concentration) to
simulate
the removal of impurities from the cycle by pyrohydrolysis or by treatment
with
concentrated sulphuric acid to form low-soluble sulphates of the corresponding
metals.
The average composition of the resulting waste silica residue, wt.%, was
as follows: A1203 2.0; SiO2 90.5; Fe2O3 0.16; Na2O 0.2; K20 <0.15; TiO2 0.7;
CaO 0.12; MgO <0.025; P205; <0.02; 4.2.
Following the stabilisation of the acidic spent liquor composition, another
10 cyclic experiments were carried out; as a result of them, the authors
produced
crude alumina with the following composition, wt.%: A120386.0; SiO2 0.08;
Fe2032.9; Na2O 0.61; K20 <0.15; <TiO2 0.05; CaO 0.3; MgO <0.025; P205; 0.06;
C13,5 ; 7Ø
In order to produce smelter-grade alumina from crude alumina by alkaline
processing in the Bayer cycle, 500 g of crude alumina were dissolved for 2
hours
Date Recue/Date Received 2021-05-13
17
in an alkali green liquor with the following composition, g/L: A1203102.0;
Na2O
174.0; NaC163.3 in an autoclave at 150 'C.
The content of the resulting filtered green liquor was as follows, g/ L: A1203
167.3; Na2O 149.2; NaC1 57.7. The precipitation of the liquor carried out in
accordance with the Bayer technology resulted in the separation of alumina,
from
which the authors, after its washing down with hot water, %: and calcination
at
1,100 C, produced alumina with the following chemical composition, % A1203
98.7; SiO2 0.004; Fe2O3 0.008; Na2O 0.15; K20 0.01; TiO2 0.001; CaO 0.004;
MgO 0.0025; P205; 0.0007; V205 0.0002; Cr2O3 0.0003 0.02; C1-0.013.
In determining the physical and mechanical properties of this alumina
using standard methods, the authors found the following:
= - Al2O3
content 3 %
particle size distribution:
- size - 20 gm, 2.4 %
- size - 45 p.m, 8.8 %
- size + 125 gm, 3.9%.
specific surface area (BET) 74.2
m2/g
loss on ignition at 1,100 'V (LOT) 0.8%
humidity 0.8%,
hardness index 9%
bulk density 0.97
g/cm3
gradient 28.6
time, for which a 100 g weight can outflow from the funnel with a
2.2 min
2.4 mm hole and a bell angle and vertical line of 9 54' (Alcoa test)
Hydrogen fluoride (HF) adsorption capacity 23
mg/g,
Date Recue/Date Received 2021-05-13
18
The produced alumina fully complies with Russian (GOST 30558-98
'Smelter-Grade Alumina') and international requirements for 'sandy' smelter-
grade alumina despite the high content of chlorides in the alkaline cycle.
Due to the lack of published data on energy consumption in similar
methods, except for the similar method (Elsner D., Jenkins D.H., and Sinha
Alumina via hydrochloric acid leaching of high silica bauxites-process
development. Light metals, 1984, p. 411-426), the authors performed
calculations
on the consumption of heat and electricity to produce 1 kg of alumina and
compare the results with a view to comparing the energy saving in all the
technologies mentioned in this description of the invention. The results are
shown
below.
Energy
Quality of produced consumpt
Technology
alumina
ion
kJ/kg
Elsner D., Jenkins D.H., and Sinha H.N.
Alumina via hydrochloric acid leaching of
Not smelter-grade
37.1
high silica bauxites-process development.
Light metals, 1984, p. 411-426
Family member patent CA2684696 Not smelter-grade
56,3
Prior art - Non-Ferrous Metal Production
Reference. Alumina Refining. Moscow: Smelter-grade
46.2
Metallurgiya, 1970, p. 236-237).
Industrial method for sintering with
Smelter-grade
54,3
limestone and soda
Proposed method Smelter-grade
38.4
It is obvious that in terms of energy saving in processing high-silica raw
materials the proposed method is second only to family patent 1, which,
however,
Date Recue/Date Received 2021-05-13
19
cannot provide the production of smelter-grade alumina. Other family patents
require much higher energy consumption.
Energy saving specified for the claimed method can be optimally achieved
when about 15% of the acidic spent liquor is subjected to pyrohydrolysis, the
chloride ion concentration in the intermediate alumina product is maintained
at a
level of 0.2-5.0 wt.%, the chloride ion concentration in the alkaline spent
liquor
is maintained at a level of 40-90 g/L, and the spent liquor, which left the
precipitation area (10-40 wt.% of the total flow), is boiled-down, until the
chlorine-containing compound crystals to be removed from the process are
separated. The specified concentration and flow intervals were calculated on
the
basis of a mathematical model of the aggregate mass balance of the acid and
alkaline parts of the process. The total calculated energy consumption did not
exceed 41.2 kJ/kg at any combination of the operating parameters within the
declared intervals.
Although the description has some references to certain embodiments,
numerous modifications should be obvious to specialists in this art and are
not
limited strictly to the example, description, and process flow.
Date Recue/Date Received 2021-05-13