Note: Descriptions are shown in the official language in which they were submitted.
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
Artificial turf infill and artificial turf
Description
The invention relates to artificial turf infill and an artificial turf system
with an artificial
turf and artificial turf infill.
Artificial turf or artificial grass is a surface that is made up of fibers and
is used to
replace grass. The structure of the artificial turf is designed such that the
artificial
turf has an appearance that resembles grass. Artificial turf is typically used
as a
surface for sports such as soccer, American football, rugby, tennis and golf,
or for
playing fields or exercise fields. Furthermore, artificial turf is frequently
used for
landscaping applications.
Artificial turf may be manufactured using techniques for manufacturing
carpets. For
example, artificial turf fibers, which have the appearance of grass blades,
may be
tufted or attached to a backing. Oftentimes, artificial turf infill is placed
between the
artificial turf fibers.
Artificial turf infill is a granular material that covers the bottom portion
of the artificial
turf fibers. The use of artificial turf infill may have a number of
advantages. For
example, artificial turf infill may help the artificial turf fibers stand up
straight. Artificial
turf infill may also absorb impact from walking or running and provide an
experience
similar to that of being on real turf. The artificial turf infill may also
help keep the
artificial turf carpet flat and in place by weighing it down.
Even though the artificial turf infills known from the art are constantly
being further
developed, rubber granulate or recycled (e.g., from car tires) rubber
granulate is still
most commonly used as artificial turf infill. The most commonly used rubbers
are
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
2
styrene-butadiene rubber (SBR) and ethylene propylene diene monomer (EPDM),
both of which can be generated from recycled rubber (post-consumer waste or
post-
industrial waste) or virgin material. Recycled rubbers are cost-effective as
they are
derived from existing products that have reached the end of their service
life. Even
though recycling of, e.g., used car tires to make artificial turf infill
particles has an
environmentally friendly aspect, concerns have arisen lately about the
potential
health effects of substances released by the granulate in synthetic turf
sports fields
with vulcanized (either by peroxide or sulphur vulcanization) recycled rubber
infill.
Further, in the hot season, when the outside surfaces are subjected to severe
heat,
rubber-based artificial turf infill materials tend to heat to 20-40 C above
the ambient
temperature.
It is therefore the purpose of the invention to provide an improved turf
infill material
which is environmentally friendly and less likely to release potentially
health-
affecting substances.
The invention provides for an improved turf infill and an artificial turf with
the
improved turf infill. The problem is solved by the features as specified in
the
independent claims. Embodiments of the invention are given in the dependent
claims. The embodiments and examples described herein can freely be combined
with each other unless they are mutually exclusive.
In one aspect, the invention provides for a turf infill comprising a mixture
of cork
particles, wherein the cork particles are coated with a polymer and/or resin
component, and rubber particles, wherein the rubber particles are coated with
a
polymer and/or resin component.
The particles of cork and rubber may be of any suitable shape, including
granules,
gravel, grains and combinations thereof, and in various dimensions thereof.
The coating may be applied to the cork particles and rubber particles using
any
suitable method, and such methods are well known in the art. The cork
particles
may be coated, e.g., in a flow reactor or batch reactor, separately from the
rubber
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
3
particles, or both (rubber particles and cork particles) may be coated
simultaneously, e.g., in the same flow reactor or batch reactor. Methods for
coating
the particles are disclosed for example in WO 2017/153261, which is hereby
incorporated in its entirety by reference herein.
It is intended that the polymer and/or resin component of the coatings does
not
show any environmental toxicity. Possible polymer and/or resin components are
selected from the group consisting of polyurethane (PU), polyvinyl butyral
(PVB),
acrylic resin, acrylate monomers, methacrylates, methyl acrylates and blends
thereof.
The use of cork particles as part of the mixture of the inventive turf infill
may be
beneficial, as cork is a renewable raw material derived from the bark of the
cork oak
(Quercus suber) from sustainably managed sources, and the particles can also
be
recycled from leftover material, e.g., from bottle cork production.
Furthermore, cork
is not known to have any environmental toxicity, has insulating properties
with low
heat absorption when exposed to sunlight and has elastic features. Hence cork
particles as part of the mixture of the inventive turf infill may be
beneficial, even
though the ultraviolet radiation (U.V.) resistance and the mechanical
stability/resistance are limited.
The coating of the cork particles may be beneficial, as the coating may weigh
down
the relatively light cork particles. This can reduce the tendency of cork to
float away
during heavy rainfall or be blown away by wind and may reduce the unmixing of
particles of different weight. The coating can comprise one or more layers.
The
layers can have the same or different thicknesses. The polymer and/or resin
component of the layers of the coating may be the same or different. In
addition,
coating may have the effect that coated cork particles cling together or are
honed by
friction during particle movement and the abrasion and wear is thus reduced
and the
mechanical stability may be increased.
The coating of the known rubber particles may be beneficial, as the coating
may
prevent the release of possibly health-affecting substances. Hence, rubber
particles
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
4
recycled even from car tires, e.g., in the shape of granules, can be used for
the
inventive infill material. The coating can comprise one or more layers. The
layers
can have the same or different thicknesses. The polymer and/or resin component
of
the layers of the coating may be the same or different.
The combination of cork particles and rubber particles may be beneficial in
many
aspects, as both cork particles and rubber particles can be recycled from
previously
manufactured materials, rubber particles from, e.g., car tires and cork
particles from,
e.g., leftover material from bottle cork production. In addition, cork is a
renewable
raw material. Furthermore, the heating up of the artificial turf to high
temperatures
(i.e., to temperatures well above the ambient temperature), caused by the
heating
up of rubber particles, may be reduced due to the insulating properties of
cork
particles. Further, the coating of the cork particles and the rubber
particles, which
may, e.g., have a higher coefficient of friction than rubber or cork and/or
may be
slightly sticky, may slow the segregation (unmixing) of particles over time
due to
different weights or sizes.
In one embodiment of the invention, the weight percentage ratio of the cork
particles
to the rubber particles is between 1 : 4 and 1 : 8, in particular between 1 :
5.5 and
1 : 6.5.
This proportion of weight percentage is beneficial, as the bulk density and/or
poured
density (freely settled bulk density) of the cork particles is much lighter
than that of
the rubber particles. Therefore, in order to obtain an optimal weight
distribution of
cork particles and rubber particles, the weight percentage ratio can be chosen
to be
between 1 : 4 and 1 : 8. This weight percentage ratio range is beneficial as
it may
provide that the size and the surface areas of both the cork and rubber
particles are
within the same range; thus the friction resistance between the particles,
e.g. cork
particles and rubber particles, is basically homogenous. In order to obtain an
approximately even number of cork particles and rubber particles, the weight
percentage ratio can be chosen to be between 1 : 5.5 and 1 : 6.5. Due to this
optimized weight percentage ratio, it may also be possible to achieve the
optical
perception that the particles are homogeneous.
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
According to one embodiment, it is envisaged that the infill is configured
such that
the
poured density of the coated cork particles is between any one of the
5 following: 90 g/dm3 and 180 g/dm3, 100 g/dm3 and 150 g/dm3, and 125
g/dm3
and 135 g/dm3; and the
poured density of the coated rubber particles is between any one of the
following: 400 g/dm3 and 650 g/dm3, 450 g/dm3 and 600 g/dm3, and 530
g/dm3 and 550 g/dm3.
In one embodiment of the invention, the polymer and/or resin component of the
coating of the cork particles and/or rubber particles is polyurethane.
A polyurethane coating may be beneficial as fully reacted polyurethane polymer
is
considered to be chemically inert and thus environmentally friendly and may be
produced as a hard, abrasion-resistant and durable coating, which may seal the
rubber granule.
Alternatively, the polymer and/or resin component of the coating of the cork
particles
and/or rubber particles is polyvinyl butyral.
The use of PVB as a coating may be beneficial, as PVB is a resin which can be
prepared from polyvinyl alcohol by reaction with butyraldehyde, and can be
acquired
from remnants during production of laminated glass or can be recycled from
laminated glass. PVB may be used as a protective layer around the particles.
In
addition, PVB has good adhesion to rubber and cork, may be sticky, and is
tough
and flexible.
In one embodiment of the invention, the coating of the cork particles
comprises
fillers, in particular barium sulphate (barite), calcium carbonate (chalk),
talc, quartz
silica, other silicates, other oxides (such as iron oxides), hydro oxides,
hollow glass
spheres, organic fillers or a combination thereof.
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
6
The use of fillers can be advantageous, as the fillers are able to increase
the weight
of the coating and may thus increase the overall poured density of the coated
cork
particles. Thus, as the weight of the coated particles increases, the risk of
coated
cork floating away during heavy rainfall or being blown away by strong wind is
further reduced. It is envisaged that the coating may comprise between 0.1
wt.%
and 60 wt.% of fillers.
In one embodiment, it is envisaged that the coating of the cork particles
comprises
barium sulphate (barite) and/or calcium carbonate (chalk) as fillers, to
increase the
total weight of the artificial turf infill.
Barium sulphate and calcium carbonate are particularly advantageous, as they
have
a high density ¨ e.g., calcium carbonate has a density of 2.7 g/cm3 and barium
sulphate has a density of between 4.0 and 4.5 g/cm3. They are also relatively
cheap
materials and may be used to provide a dense coating.
According to one embodiment, the coating of the cork particles and/or rubber
particles comprises particles selected from the group consisting of colored
pigments, copper(II) sulfate particles, silver particles, chitosan particles
or mixtures
thereof.
The colored pigments may be inorganic, such as iron oxide pigments, chromium
oxide pigments and/or cobalt oxide pigments, or organic pigments. Further, the
colored pigments may be infrared-reflective pigments, which are beneficial due
to
their ability to reflect infrared light. This may reduce the heating of the
artificial turf
infill. Further, as the infrared-reflective colored pigments may be contained
solely in
the applied coating, the costs for the comparably expensive and precious
pigments,
being merely on the surface of the cork particles and/or rubber particles, is
reduced.
Copper(II) sulfate particles and/or chrome particles and/or iron oxide
particles may
be further beneficial due to their color, relatively low manufacturing costs
and/or
antibacterial properties. Other antibacterial components that may be used are
silver
and/or chitosan particles, both of which have natural antibacterial
properties.
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
7
According to one embodiment of the invention, the overall layer thickness of
the
coating of the cork particles and/or rubber particles is between 0.1 pm and 1
mm, or
between 0.5 pm and 750 pm, or between 10 pm and 150 pm. The coating of the
cork particles and/or rubber particles may each comprise one or more (sub-
)layers.
The (sub-)layers may have the same or different thicknesses; however, the sum
of
the individual layer thicknesses is between 0.1 pm and 1 mm. In one
embodiment,
the overall layer thickness of the coating of the cork particles and/or rubber
particles
is between 10 pm and 150 pm.
It is contemplated that the particles can be coated with one layer or with
multiple
layers. To increase the likelihood that the particles are fully encapsulated
and thus
no possibly health-affecting substances may be released, it may be beneficial
to
coat the particles two or more times. Alternatively or in addition the use of
multiple
layers comprising colored particles may have the advantageous effect of
providing a
more intense and saturated coloring of the coated infill particles.
According to one embodiment, the size of the coated cork particles is between
any
one of the following: 0.03 mm and 3.5 mm, and 0.3 mm and 2.5 mm; and the size
of
the coated rubber particles is between any one of the following: 0.03 mm and
3.5
mm, and 0.3 mm and 2.5 mm.
This configuration allows for a well-adjusted particle size distribution for
artificial turf.
Furthermore, the (natural) particle size distribution within each range allows
the
particles to be packed more densely.
It is feasible that the turf infill further comprises microporous zeolite
mineral
particles. The microporous zeolite mineral particles have pores that form
openings
on their outer surface. Hence, the use of the microporous zeolite mineral as
an infill
material is advantageous, as the particles are able to regulate the presence
of water
and may thus provide for a cooling effect of the surface of the artificial
turf. Hence,
increased playing comfort can be achieved when the outside temperatures are
high.
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
8
The microporous zeolite mineral particle may be selected from the group
consisting
of chabazite, erionite, mordenite, clinoptilolite, faujasite, phillipsite,
zeolite A, zeolite
L, zeolite Y, zeolite X and ZSM-5. The zeolite used may thus be natural or
obtained
by synthesis.
Since artificial turf infill may be used to modify an artificial turf carpet
to have more
earth-like properties, a microporous zeolite, with a Mohs hardness above 3
and/or a
strong absorbent power and/or a color that approximately resembles one of the
well-
known surface colors (e.g., red, brown, green), may preferably be used. The
most
preferred microporous zeolite mineral may be of the chabazite and/or
clinoptilolite
and/or mordenite type.
It is envisaged that the particle size of the microporous zeolite mineral
particles is
between any one of the following: 0.5 mm and 3.5 mm, and 1.0 mm and 2.5 mm;
and the weight percentage ratio of the microporous zeolite mineral particles
to the
cork particles and the rubber particles is between 2: 7 and 4: 7, in
particular
between 2.5: 7 and 3.5: 7.
For the microporous zeolite mineral particles, it may be envisaged that the
outer
surface of at least some microporous zeolite mineral particles is partly
covered with
a polyurethane coating. Hereby it may be feasible that 20% to 99% of the outer
surface of a microporous zeolite mineral particle is partly covered with a
polyurethane coating.
For this embodiment, it may be provided that the partial covering is applied
on each
side of each microporous zeolite mineral particle, but that there are gaps
(holes) in
the covering enclosing the particles. The partial coating may be advantageous,
since water can be absorbed and/or released by the microporous zeolite mineral
particles through their pores which are not covered by the polyurethane
coating. The
polyurethane coating may be advantageous, as the polymerization reaction may
be
controlled, as the polyurethane educts are smaller than many fully polymerized
polymers, such as polymers in a molten plastic mass. Thus, the surface and the
micropores of the microporous zeolite mineral particles may be wetted with the
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
9
educts, e.g., with a liquid polyurethane reaction mixture, and when the
desired
degree of wetting or the desired penetration depth into the pores is achieved,
the
polymerization can be initialized. Further, the reacted polyurethane polymer
is
chemically inert.
In addition, since the microporous zeolite mineral particles may be partly
coated with
the polyurethane coating, natural abrasion and wear of the microporous zeolite
mineral during use may be reduced, since the polyurethane coating may provide
for
a harder and thus protective surface compared with that of uncoated
microporous
zeolite mineral particles. It may also be advantageous that the Mohs hardness
of
polyurethane coating can be chosen to be higher or much higher than the Mohs
hardness of the microporous zeolite mineral particles. It may be thus
beneficial that
the Mohs hardness of the polyurethane coating is at least one Mohs unit higher
than
the Mohs hardness of the selected microporous zeolite mineral particles.
The gaps in the coating of the inventive infill material may result during the
manufacture of the infill material, since during the mixing, e.g., in a flow
reactor or a
batch reactor or a tumbler, the microporous zeolite mineral particles and a
liquid
polyurethane reaction mixture are mixed and while they are being mixed a
solidification reaction is initiated. During the mixing and while the
solidification takes
place, the microporous zeolite mineral particles may physically touch and
interact
with each other, thereby causing collision defects such as gaps in the coating
and
leaving the surface of the microporous zeolite particles partly uncovered.
Thus,
water may still be absorbed and released by the microporous zeolite mineral
particles in those areas in which the pores of the microporous zeolite
minerals are
not covered by the polyurethane coating.
It is further envisaged for this embodiment that the polyurethane coating may
extend
over some of the pores, forming respective covers of the pore openings,
wherein the
polyurethane coating may coat a portion of the inner surface of the covered
pores in
the region of the cover. Alternatively, the polyurethane coating may extend
for a
distance into some of the pores, forming reinforcements, with the
reinforcements
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
forming openings that provide a passage for sorbing water into the pores or
desorbing water from the pores.
For alternative partial coatings of the microporous zeolite mineral, the
coating may
5 penetrate a slight distance, for example between 0.2 pm and 500 pm,
preferably
between 1 pm and 150 pm and most preferred between 10 pm and 100 pm, into the
surface pores and thus may interfere with the pores in a form-locking manner;
this
may increase the hold of the coating on the microporous zeolite mineral
particles
and at the same time the overall stability and hardness of the microporous
zeolite
10 mineral particles may be increased.
Further, since the infill material is preferably produced by mixing the
microporous
zeolite mineral particles with a liquid polyurethane reaction mixture and
initializing
the solidification reaction during the mixing, the microporous zeolite mineral
particles
may have substantially the same amount of their outer surface covered by the
coating relative to the total outer surface. Substantially the same amount
means that
the outer surface of each of the partly coated microporous zeolite mineral
particles
may be coated between 20% and 99%, preferably between 50% and 98% and most
preferred between 70% and 99% with the polyurethane coating. Further, since
the
outer surface of each of the partly coated microporous zeolite mineral
particles may,
with the exception of the gaps, be essentially fully coated, fine dusts may be
bound.
The polyurethane coating of the microporous zeolite mineral particles may be
based
on a liquid polyurethane reaction mixture, which may be a dispersion or
solution,
comprising
¨ at least one isocyanic prepolymer with a totally blocked isocyanic
functionality;
¨ a hydroxyl component, wherein the hydroxyl component is selected from
the group of polyether polyol or polyester polyol; and
¨ at least one catalyst selected from the group consisting of linear or cyclic
tertiary amines such as triethylenediamine or 1,4-
Diazabicyclo[2.2.2]octane; cyclic amines such as 1,8-
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
11
Diazabicyclo(5.4.0)undec-7-ene (DBU); and inorganic compounds such
as sodium hydroxide (NaOH), chromium(III) oxide and zinc oxide (Zn0).
The use of an above-described liquid polyurethane reaction mixture may be
advantageous, because it is a reaction mixture that can be solidified or cured
(e.g.,
by cross-linking) under heat and/or by adding water. Therefore, the zeolite
mineral
particles and the liquid polyurethane reaction mixture may be mixed and
solidified
simultaneously in a batch reactor, a continuous reactor or a tumbler,
resulting in the
partial polyurethane coating or the coating with gaps. Further, the resulting
polyurethane coating may be a waterborne polyurethane coating.
Alternatively, the polyurethane coating of the microporous zeolite mineral
particles
may be based on a liquid polyurethane reaction mixture comprising
i. an NCO-terminal polymer, one or more components selected from a
pre-polymer, a polymeric isocyanate, an oligomeric isocyanate or a
monomer, such as
a. aromatic diisocyanate of the group of toluene diisocyanate and/or
methylene-2,2-diisocyanate; or
b. aliphatic diisocyanate of the group hexamethylene diisocyanate,
isophorone diisocyanate and/or 1,4-cyclohexyl diisocyanate; and
ii. a hydroxyl component, wherein the hydroxyl component is
selected
from the group of polyether polyol or polyester polyol.
The use of an above-described liquid polyurethane reaction mixture may be
.. advantageous, because it is a reaction mixture that may be solidified or
cured (e.g.,
by cross-linking) by adding a catalyst, e.g., a secondary amine catalyst; a
tertiary
amine catalyst, such as triethylenediamine or, e.g., 1,4-
Diazabicyclo[2.2.2]octane;
cyclic amines such as 1,8-Diazabicyclo(5.4.0)undec-7-ene (DBU) or a metal
organic
catalyst; and water. Therefore, the zeolite mineral particles and the liquid
polyurethane reaction mixture may be mixed and solidified simultaneously in a
controlled manner in a batch or continuous reactor, resulting in the partial
polyurethane coating or the coating with holes.
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
12
In one embodiment of the invention, the polyurethane coating of the
microporous
zeolite mineral particles is a reaction product of a liquid polyurethane
reaction
mixture comprising
¨ 0.1 ¨ 80 wt.% of an NCO-terminal prepolymer,
¨ 0.001 ¨ 0.5 wt.% of 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU) and
¨ 0.001 ¨ 20.0 wt.% water,
and optionally at least one further component selected from the group
consisting
of
= 0.001 ¨ 30.0 wt.% colored pigments,
= 0.001 ¨3.0 wt.% copper(II) sulfate,
= 0.05 ¨ 60 wt.% flame retardants,
= 0.01 ¨ 1.0 wt.% U.V. absorbers,
= 0.01 ¨ 60 wt.% filler and
= 0.01 ¨ 1.0 wt.% rheology additive,
wherein the amounts by weight add up to 100 wt.% and the amounts by weight are
based on the total weight of the polyurethane coating.
The NCO-terminal prepolymer of this embodiment may be an NCO-terminal
isocyanic prepolymer, which is a (oligomeric) urethane having at least one
free
isocyanate group, and which may be obtained by reacting polyisocyanates with
polyols. The NCO-terminal prepolymer of this embodiment may have an NCO-
content of 5.5% to 11.5%, preferably an NCO-content of 6.5% to 10.5% or most
preferred an NCO-content of 8.0% to 9.0%. Further, the NCO-terminal prepolymer
of this embodiment may have a curing time in air of 35 hours to 45 hours. A
suitable
prepolymer is known under the trade name P2440 (Polytex Sportbelage
Produktions
GmbH). The prepolymer may be mixed with the microporous zeolite particles in a
batch or continuous reactor, and the solidification reaction may be
initialized (during
the mixing) by adding water and/or a catalyst. The prepolymer may be
advantageously used for a moisture-curing 1K system, in which the reaction
(formation of urea groups) may be started with water and/or a catalyst, here
1,8-
diazabicyclo(5.4.0)undec-7-ene (DBU). DBU may be particularly useful as a
catalyst
for a 1K system, as it may be soluble in water. In addition, the curing rate
of the
polyurethane formation may be controlled by the addition of the DBU-water
mixture,
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
13
time and temperature so that, for example, the polyurethane coating may only
penetrate the pores to a certain depth or the curing takes place very quickly.
Correspondingly, curing may also be achieved such that the individual coated
zeolite particles do not stick to each other.
The polyurethane coating of the microporous zeolite mineral particles may
further
comprise a rheology additive that is adapted to induce a thixotropic flow
behavior in
one of the above-described liquid polyurethane reaction mixtures.
Adding a rheology additive with thixotropic capabilities may be advantageous
in
order to achieve a controllable viscosity-increasing thixotropic flow behavior
(i.e.,
liquid or fluid) of the polyurethane coating while applying it (e.g., by
mixing the liquid
polyurethane reaction mixture with the microporous zeolite mineral particles,
to the
surface of the microporous zeolite mineral particles) in order to control or
reduce the
depth of penetration of the liquid polyurethane reaction mixture into the
pores
contained in the surfaces of the microporous zeolite mineral particles.
Suitable rheology additives may be, e.g., fumed silica (e.g., synthetic,
hydrophobic,
amorphous silica), also known as pyrogenic silica, made from flame pyrolysis
of
silicon tetrachloride or from quartz sand vaporized in a 3000 C electric arc,
hydrophobic fumed silica, bentonite, acrylates, or a combination of the
aforementioned additives.
It may be further beneficial to charge at least some of the microporous
zeolite
mineral particles with a salt solution.
Charging the microporous zeolite mineral particles with salt ions may allow
for an
increased water adsorption and/or water desorption effect.
This incorporation of salt into the microporous zeolite mineral particles
allows a
synergy to operate between the following properties: the adsorption,
absorption and
release of water of the microporous zeolite mineral particles, and the ability
to lower
the freezing temperature of the water. Actually, in the presence of humidity,
the
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
14
microporous mineral particle may be in a position to adsorb and/or absorb this
humidity in order to prevent, on the one hand, the surface formation of a
layer of
slippery frost, in the case of a negative temperature, and on the other hand,
the
agglomeration of the turf infills.
In the context of using the microporous mineral particles on outside
artificial turf
surfaces that are subjected to severe heat, the coated rubber and coated cork
particles in combination with the microporous mineral particles, which are
loaded
with salt and water, may allow further increased release of the water and the
maintenance of relative humidity at the surface of said turf. Thus, on a turf
surface
subjected to severe heat, when it is refreshed by watering, the microporous
mineral
loaded with salt adsorbs and/or absorbs the water and then continuously
releases
those water molecules by desorption. This continuous release of the water by
the
microporous mineral prevents rapid evaporation of the water after the surface
is
watered and allows a lower than ambient temperature to be maintained at the
level
of the field surface. Said microporous mineral loaded with salt thus further
reduces
the amount of watering usually necessary to refresh a turf surface.
In a further aspect, the invention relates to turf infill, as described above,
for an
artificial turf comprising an artificial turf carpet, wherein the artificial
turf carpet
comprises multiple artificial turf fiber tufts, and wherein the turf infill is
configured for
scattering between the multiple artificial turf fiber tufts of the artificial
turf.
In a further aspect, the invention relates to an artificial turf system
comprising an
artificial turf, wherein the artificial turf comprises an artificial turf
carpet, wherein the
artificial turf carpet comprises multiple artificial turf fiber tufts, and a
turf infill, as
described above. The turf infill may be scattered, distributed or brushed
between the
multiple artificial turf fiber tufts of the artificial turf.
It is understood that one or more of the aforementioned embodiments of the
invention may be combined as long as the combined embodiments are not mutually
exclusive.
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
Below, the following embodiments of the invention are explained in greater
detail, by
way of example only, making reference to the drawings, in which
Fig. 1 illustrates an example of artificial turf with an artificial turf
carpet and
5 turf infill scattered between the artificial turf fiber tufts;
Fig. 2 shows a detail from Fig. 1, where the alignment of cork particles and
rubber particles is enlarged;
Fig. 3 illustrates the coated cork particles and rubber particles in sectional
views;
10 Fig. 4 illustrates the coated cork particles and rubber particles in
sectional
views, wherein the coating of the cork particles contains fillers and
the coating of the rubber granules comprises two layers;
Fig. 5 shows a turf infill comprising coated cork particles, coated rubber
particles and microporous zeolite particles;
15 Fig. 6 illustrates a sectional view of a microporous zeolite mineral
particle,
which is partially coated with a polyurethane coating;
Fig. 7 illustrates a section of the sectional view of Fig. 6; and
Fig. 8 illustrates a partially coated microporous zeolite mineral particle.
Like-numbered elements in these figures either are equivalent elements or
perform
the same function. Elements that have been discussed previously will not
necessarily be discussed in later figures if the function is equivalent.
In Fig. 1, an artificial turf 600 with artificial turf carpet 500 is shown.
The artificial turf
carpet 500 contains a backing 502. The artificial turf carpet 500 is a tufted
artificial
turf carpet, which is formed by artificial turf fiber tufts 504 that are
tufted into the
backing 502. The artificial turf fiber tufts 504 are tufted in rows. There is
row spacing
506 between adjacent rows of tufts. The artificial turf fiber tufts 504 also
extend a
distance above the backing 502. The distance that the fibers 504 extend above
the
backing 502 is the pile height 508. In Fig. 1, it can be further seen that the
artificial
turf carpet 500 has been installed by placing or attaching it to the ground
510 or to a
floor. As can be further seen, the turf infill 100 has been spread out on the
surface
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
16
and distributed between the artificial turf fiber tufts 504. The turf infill
100 comprises
coated cork particles (here granules) and coated rubber particles (here
granules).
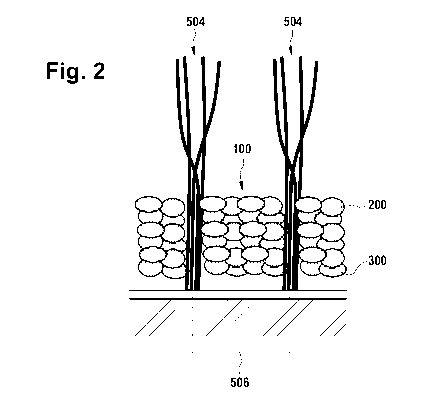
In Fig. 2 a detail from Fig. 1 is shown to visualize the turf infill
comprising cork
particles 200, wherein the cork particles 200 are coated with a polymer and/or
resin
component, and rubber particles 300, wherein the rubber particles 300 are
coated
with a polymer and/or resin component. The cork particles 200 and the rubber
particles 300 both have a coating, which may, e.g., have a higher coefficient
of
friction than rubber or cork by itself or may be slightly sticky. The coating
may slow
the segregation (unmixing) over time due to different weights or sizes of the
different
granules. In order to obtain an optimal weight distribution of the coated cork
particles 200 and the coated rubber particles 300, the weight percentage ratio
may
be chosen to be between 1 : 4 and 1 : 8.
In Fig. 3 the coated cork particles 200 and the coated rubber particles 300 of
the turf
infill are depicted. The coating 202 of the cork particles 200 is manufactured
from a
polymer and/or resin component, which may be polyurethane or polyvinyl
butyral.
The coating 302 of the rubber particles 300 is also manufactured from a
polymer
and/or resin component, which may be polyurethane or polyvinyl butyral.
Fig. 4 depicts the coated cork particles 200 and the coated rubber particles
300 of
the turf infill. As shown, the coating 202 of the cork particles 200 may
comprise
fillers, here barium sulphate particles 204 and calcium carbonate particles
205. It
shall be understood that it is also feasible that either only barium sulphate
particles
204 or only calcium carbonate particles 205 may be used as fillers. The use of
both
described fillers can be advantageous, as these fillers may be able to
increase the
weight of the coating 202 and may thus increase the overall poured density of
the
coated cork particles 200. As further shown, the coating 302 of the rubber
particles
300 may be comprised of two layers, an inner layer 302a and an outer layer
302b.
The double-layer coating may prevent possibly health-affecting substances from
being released by the rubber particles 300.
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
17
Fig. 5 depicts a turf infill 100 comprising microporous zeolite mineral
particles 400;
cork particles 200, wherein the cork particles 200 are coated with a polymer
and/or
resin component; and rubber particles 300, wherein the rubber particles 300
are
coated with a polymer and/or resin component. The microporous zeolite mineral
particles 400 may be uncoated or partially coated, as depicted in Figures 6 to
8.
Fig. 6 shows a microporous zeolite mineral particle after it has been
partially coated
with a polyurethane coating 420 and may be used as part of an infill material.
As
can be seen, at least some parts of the surface of the coated microporous
zeolite
mineral particle 400 may not be covered by the polyurethane coating 420. In
Fig. 6,
a dotted circle is also indicated, the schematic content of which is enlarged
in Fig. 7.
As shown in the enlarged sectional view in Fig. 7, the microporous zeolite
mineral
particle 400, which contains pores 411, has been partially coated with a
polyurethane coating 420. The polyurethane coating 420 may be formed by
providing microporous zeolite mineral particles 400 and a liquid polyurethane
reaction mixture in a batch reactor, tumbler or continuous reactor.
Simultaneous
mixing and initialization of the solidification reaction may lead to the
desired partial
coating of the polyurethane coating 420 on the surface of the microporous
zeolite
mineral particle 400. The partial coating may result from microporous zeolite
mineral
particles 400 colliding while being mixed with the initialized liquid
polyurethane
reaction mixture. Since the initialization of the solidification reaction may
take place
simultaneously, uncovered spaces (e.g., gaps or holes), created by collisions,
may
remain on the surface of the microporous zeolite mineral particles 400.
Further, as
shown in Fig. 7, the polyurethane coating 420 may coat a portion of the inner
surface of the microporous zeolite mineral particle 400 in the region of the
cover.
The coating 420 may penetrate with a slight distance, for example between 0.2
pm
and 500 pm, preferably between 1 pm and 150 pm and most preferred between 10
pm and 100 pm, into the surface pores 411 and thus may interfere with the
pores
411 in a form-locking manner; this will increase the hold of the coating on
the
microporous zeolite mineral particle 400 and at the same time the overall
stability
and hardness of the microporous zeolite mineral particle 400 may be increased.
Further, since the partially coated microporous zeolite mineral particle 400
is
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
18
preferably produced by mixing the microporous zeolite mineral particles with a
liquid
polyurethane reaction mixture and initializing the solidification reaction
during the
mixing, the microporous zeolite mineral particles may have substantially the
same
amount of their outer surface covered by the coating relative to the total
outer
surface. Substantially the same amount means that the outer surface of each
microporous zeolite mineral particle is covered between 20% and 99%,
preferably
between 50% and 98% or most preferred between 70% and 99%, with the
polyurethane coating 420. Since the outer surface of each microporous zeolite
mineral particle 400 may, with the exception of the gaps, be essentially fully
coated,
fine dusts may be bound. The polyurethane coating 420 may comprise a rheology
additive. The rheology additive may be added in order to achieve thixotropic
flow
behavior of the liquid polyurethane reaction mixture during mixing of the
liquid
polyurethane reaction mixture with the microporous zeolite mineral particle
400.
Fig. 8 depicts a partly coated microporous zeolite mineral particle 400. The
microporous zeolite mineral particle 400 has pores 411 that form openings on
the
outer surface of the microporous zeolite mineral particles 400. As shown, the
outer
surface of the microporous zeolite mineral particle 400 may be partly coated
with a
polyurethane coating, wherein the coating extends over most of the pores 411,
thereby forming covers over the openings. The polyurethane coating 420 may
have
been formed by providing a plurality of microporous zeolite minerals particles
400
and a liquid polyurethane reaction mixture in a batch reactor, tumbler or
continuous
reactor. The microporous zeolite mineral particles 400 may be mixed with a
liquid
polyurethane reaction mixture (here comprising 0.1 ¨80 wt.% of an NCO-terminal
prepolymer and optionally at least one further component selected from the
group
consisting of 0.001 wt.% to 30.0 wt.% colored pigments, 0.001 wt.% to 3.0 wt.%
copper(II) sulfate, 0.05 wt.% to 60 wt.% flame retardants, 0.01 wt.% to 1.0
wt.% U.V.
absorbers, 0.01 wt.% to 60 wt.% filler and 0.01 wt.% to 1.0 wt.% rheology
additive)
and the solidification reaction initialized during the mixing by adding a
catalyst and
water (here comprising 0.001 wt.% to 0.5 wt.% of 1,8-diazabicyclo(5.4.0)undec-
7-
ene (DBU) and 0.001 wt.% to 20.0 wt.% water). Simultaneous mixing and
initialization of the solidification reaction may lead to the desired partial
coating of
the polyurethane coating 420 on the surface of the microporous zeolite mineral
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
19
particle 400. The partial coating may result from collisions of microporous
zeolite
mineral particles 400 while being mixed with the initialized liquid
polyurethane
reaction mixture. Since the initialization of the solidification reaction may
take place
simultaneously, uncovered spaces (e.g., gaps or holes), created by collisions
or by
the formation of reinforcements, may remain on the surface of the microporous
zeolite mineral particles 400. As indicated in Fig. 8, it may be feasible to
cover 75%
to 99% of the outer surface of the microporous zeolite mineral particle 400
with a
polyurethane coating 420.
CA 03118800 2021-05-05
WO 2020/120799
PCT/EP2019/085381
List of Reference Numerals
5
100 turf infill
200 cork particles
202 coating of the cork particles
10 203 fillers comprised in the coating of the cork
particles
204 barium sulphate particles
205 calcium carbonate particles
300 rubber particles
302 coating of the rubber particles
15 400 microporous zeolite mineral particles
411 microporous zeolite mineral particle pores
420 polyurethane coating of microporous zeolite
mineral particles
430 cover
20 500 artificial turf carpet
502 backing
504 artificial turf fiber tufts
506 row spacing between adjacent rows of tufts
508 pile height
510 ground or floor
600 artificial turf