Note: Descriptions are shown in the official language in which they were submitted.
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
COMPOSITION AND METHOD FOR PREVENTING OR REDUCING LOW SPEED
PRE-IGNITION IN SPARK-IGNITED INTERNAL COMBUSTION ENGINES
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application is a continuation-in-part of co-pending U.S. patent
application Ser. No. 16/362,157, entitled "COMPOSITION AND METHOD FOR
PREVENTING OR REDUCING LOW SPEED PRE-IGNITION IN SPARK-IGNITED INTERNAL
COMBUSTION ENGINES", filed March 22, 2019, which claims priority from U.S.
Provisional Patent Application No. 62/647,186, entitled "COMPOSITION AND
METHOD FOR PREVENTING OR REDUCING LOW SPEED PRE-IGNITION IN SPARK-
IGNITED INTERNAL COMBUSTION ENGINES", filed March 23, 2018, and U.S.
Provisional Patent Application No. 62/767,686, entitled "COMPOSITION AND
METHOD
FOR PREVENTING OR REDUCING LOW SPEED PRE-IGNITION IN SPARK-IGNITED
INTERNAL COMBUSTION ENGINES", filed November 15, 2018, the contents which are
incorporated by reference.
TECHNICAL FIELD
[002] This disclosure relates to fuel and lubricant compositions for spark-
ignited engines and methods for preventing or reducing low speed pre-ignition
events
using the same.
BACKGROUND
[003] Turbocharged or supercharged engines (i.e., boosted internal
combustion engines) may exhibit an abnormal combustion phenomenon known as
stochastic pre-ignition or low-speed pre-ignition (or "LSPI"). LSPI is an
event that may
include very high pressure spikes, early combustion during an inappropriate
crank
angle, and knock. All of these, individually and in combination, have the
potential to
cause degradation and/or severe damage to the engine. However, because LSPI
1
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
events occur only sporadically and in an uncontrolled fashion, it is difficult
to identify
the causes for this phenomenon and to develop solutions to suppress it.
[004] Pre-ignition is a form of combustion that results in ignition of the air-
fuel
mixture in the combustion chamber prior to the desired ignition of the air-
fuel mixture
by the igniter. Pre-ignition has typically been a problem during high load
engine
operation since heat from operation of the engine may heat a part of the
combustion
chamber to a sufficient temperature to ignite the air-fuel mixture upon
contact. This
type of pre-ignition is sometimes referred to as hot-spot pre-ignition.
[005] More recently, intermittent abnormal combustion has been observed in
boosted internal combustion engines at low speeds and medium-to-high loads.
For
example, during operation of the engine at 3000 rpm or less, under load, with
a brake
mean effective pressure (BMEP) of at least 10 bar, low-speed pre-ignition
(LSPI) may
occur in a random and stochastic fashion. During low speed engine operation,
the
compression stroke time is longest.
[006] Previous studies have demonstrated that turbocharger use, engine
design, engine coatings, piston shape, fuel choice, and/or engine oil
additives may
contribute to an increase in LSPI events. Accordingly, there is a need for
fuel and
engine oil additive components and/or combinations that are effective to
reduce or
eliminate LSPI.
SUMMARY
[007] In one aspect, there is provided a fuel composition comprising (1)
greater
than 50 wt % of a hydrocarbon fuel boiling in the gasoline or diesel range and
(2) a
minor amount of a low-speed pre-ignition (LSPI)-reducing additive comprising
one or
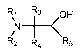
more of an amidine, or a beta-amino alkanol having the structure
Rµl R3 OH
IN 1 <
R2 R4 R5
, wherein Ri , R2, R3, and R4 are each independently selected
from hydrogen, aromatic ring, and a Ci-C20 alkyl group and R5 is hydrogen or
an
2
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
alcohol having the structure ¨(CH)R6-0H wherein R6 is hydrogen, a Ci-C10 alkyl
group,
or a Ci-C10 alkenyl group, or a salt thereof.
[008] In another aspect, there is provided a fuel concentrate comprising (1)
from 90 to 30 wt % of an organic solvent boiling in a range of from 65 C to
205 C and
(2) from 10 to 70 wt % of an additive component selected from one or more of
an
amidine, or a beta-amino alkanol having the structure
IR,i R3 OH
IN 1 <
R2 R4 R5
, wherein R1, R2, R3, and R4 are each independently selected
from hydrogen, aromatic ring, and a Ci-C20 alkyl group and R5 is hydrogen or
an
alcohol having the structure ¨(CH)R6-0H wherein R6 is hydrogen, a Ci-Cio alkyl
group,
or a Ci-Cio alkenyl group, or a salt thereof.
[009] In a further aspect, there is provided a method of reducing low-speed
pre-ignition events in an engine comprising (1) greater than 50 wt % of a
hydrocarbon
fuel boiling in the gasoline or diesel range and (2) a minor amount of a low-
speed pre-
ignition (LSPI)-reducing additive comprising one or more of a triazole, an
amidine, a
beta-amino alkanol having the structure
IR,i R3 OH
IN 1 <
R2 R4 R5
, wherein Ri , R2, R3, and R4 are each independently selected
from hydrogen and a Ci-C20 alkyl group and R5 is hydrogen or an alcohol having
the
structure ¨(CH)R6-0H wherein R6 is hydrogen, a Ci-Cio alkyl group, or a Ci-Cio
alkenyl
group, or a salt thereof.
DETAILED DESCRIPTION
Introduction
[010] In this specification, the following words and expressions, if and when
used, have the meanings ascribed below.
3
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
[011] "Gasoline" or "gasoline boiling range components" refers to a
composition containing at least predominantly C4-C12 hydrocarbons. In one
embodiment, gasoline or gasoline boiling range components is further defined
to refer
to a composition containing at least predominantly C4-C12 hydrocarbons and
further
having a boiling range of from about 100 F (37.8 C) to about 400 F (204 C). In
an
alternative embodiment, gasoline or gasoline boiling range components is
defined to
refer to a composition containing at least predominantly C4-C12 hydrocarbons,
having
a boiling range of from about 100 F (37.8 C) to about 400 F (204 C), and
further
defined to meet ASTM D4814.
[012] The term "diesel" refers to middle distillate fuels containing at least
predominantly C10-C25 hydrocarbons. In one embodiment, diesel is further
defined to
refer to a composition containing at least predominantly C10-C25 hydrocarbons,
and
further having a boiling range of from about 165.6 C (330 F) to about 371.1 C
(700 F).
In an alternative embodiment, diesel is as defined above to refer to a
composition
containing at least predominantly C10-C25 hydrocarbons, having a boiling range
of
from about 165.6 C (330 F) to about 371.1 C (700 F), and further defined to
meet
ASTM D975.
[013] The term "oil soluble" means that for a given additive, the amount
needed to provide the desired level of activity or performance can be
incorporated by
being dissolved, dispersed or suspended in an oil of lubricating viscosity.
Usually, this
means that at least 0.001% by weight of the additive can be incorporated in a
lubricating oil composition. The term "fuel soluble" is an analogous
expression for
additives dissolved, dispersed or suspended in fuel.
[014] The term "alkyl" refers to saturated hydrocarbon groups, which can be
linear, branched, cyclic, or a combination of cyclic, linear and/or branched.
[015] An "alkanol" is an alkyl group, as described herein, having a hydroxy
substituent (i.e., an ¨OH group).
4
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
[016] A "minor amount" means less than 50 wt % of a composition, expressed
in respect of the stated additive and in respect of the total weight of the
composition,
reckoned as active ingredient of the additive.
[017] An "analog" is a compound having a structure similar to another
compound but differing from it in respect to a certain component such as one
or more
atoms, functional groups, substructures, which are replaced with other atoms,
groups,
or substructures.
[018] A "homolog" is a compound belonging to a series of compounds that
differ from each other by a repeating unit. Alkanes are examples of homologs.
For
example, ethane and propane are homologs because they differ only in the
length of
a repeating unit (-CH2-). A homolog may be considered a specific type of
analog.
[019] A "derivative" is a compound that is derived from a similar compound
via a chemical reaction (e.g., acid-base reaction, hydrogenation, etc.). In
the context
of substituent groups, a derivative may be a combination of one or more
moiety. For
example, a phenol moiety may be considered a derivative of aryl moiety and
hydroxyl
moiety. A person of ordinary skill in the related art would know the metes and
bounds
of what is considered a derivative. The term "substituted" refers to a
substitution or
replacement of an atom or atoms of a compound. As an illustrative example, a
"substituted alkyl group" may refer to, among other things, an ethanol.
[020] An "engine" or a "combustion engine" is a heat engine where the
combustion of fuel occurs in a combustion chamber. An "internal combustion
engine"
is a heat engine where the combustion of fuel occurs in a confined space
("combustion
chamber"). A "spark ignition engine" is a heat engine where the combustion is
ignited
by a spark, usually from a spark plug. This is contrast to a "compression-
ignition
engine," typically a diesel engine, where the heat generated from compression
together with injection of fuel is sufficient to initiate combustion without
an external
spark.
Low Speed Pre-Ignition (LSPI)
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
[021] Low Speed Pre-Ignition (LSPI) is most or more likely to occur in direct-
injected, boosted (turbocharged or supercharged), spark-ignited (gasoline)
internal
combustion engines that, in operation, generate a brake mean effective
pressure level
of greater than 1000 kPa (10 bar) at engine speeds of from 1500 to 2500
rotations per
minute (rpm), such as at engine speeds of from 1500 to 2000 rpm. "Brake mean
effective pressure" (BMEP) is defined as the work accomplished during on
engine cycle,
divided by the engine swept volume, the engine torque normalized by engine
displacement. The word "brake" denotes the actual torque or power available at
the
engine flywheel, as measured on a dynamometer. Thus, BMEP is a measure of the
useful energy output of the engine.
[022] It has now been found that the fuel compositions or lubricating oil
compositions of this disclosure which are particularly useful in high pressure
spark-
ignited internal combustion engines and, when used in the high pressure spark-
ignited
internal combustion engines, will prevent or minimize engine knocking and pre-
ignition problems.
Primary LSPI-Reducing Additives
[023] The following are descriptions of primary additives that can be utilized
as a fuel or lubricant additive to reduce LSPI activity. Primary LSPI-reducing
additives
can be used as standalone additives and/or with other primary additive(s)
and/or with
of one or more secondary LSPI-reducing additive (described later). When more
than
one additive is used, the additives may be in salt form. Moreover, when two or
more
additives are used, there may be synergy between the two or more additives. In
general, these additives are fuel or oil soluble at concentrations needed to
achieved a
desired LSPI reduction level. Table 1 summarizes the primary additive types.
6
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
Table 1
Primary Additive Types
1. Amino Additives
Beta-amino alkanol
Amino acid
Amino ester
2. N=C-X Motif Additives
Amidine
Guanidine
Imidazole
Benzamidine
Benzamidazole
Aminobenzimidazole
3. Triazole Additives
4. Benzamidium Additives
5. Benzoxazole Additives
6. Amine Additives
Aromatic amine
Aliphatic amine
7
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
1. Amino Additives
n-Amino Alkanol
[024] The fuel additive or lubricating oil additive of this disclosure may be
a 13-
amino alkanol, a substituted 13-amino alkanol, a derivative thereof or an
acceptable salt
thereof. Useful 13-amino alkanols include those that can be represented by the
following general formula:
R1 R3 OH
;NI _______________________________ <
R2 R4 R5
Formula 1
wherein Ri, R2, R3, and R4 are each independently selected from hydrogen and a
Ci -
C20 alkyl (e.g., Ci-C6 alkyl) group; and two or more of Ri, R2, R3, and R4
optionally can
be bonded together to form a ring structure (e.g., a five-, six-, or seven-
membered
ring). In some embodiments, R1, R2, R3, and R4 may independently include one
or more
aromatic rings. R5 is hydrogen or an alcohol having the structure -(CH)R6-0H
wherein
R6 is hydrogen, a Ci-Cio alkyl group, or a CI -CI() alkenyl group. In some
embodiments,
R5 is hydrogen. In some embodiments, R5 is an alcohol having the structure -
(CH)R6-
OH wherein R6 is hydrogen, a Ci-Cio alkyl group, or a Ci-Cio alkenyl group.
In embodiments, the 13-amino alkanol is not the following:
/OH ne
-\/\./.\./
OH
0
In certain embodiments, the fuel composition does not comprise the following:
/OH
OH
0
In certain embodiments, the fuel concentrate does not comprise the following:
8
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
/OH
1;)
e
H2N........õ
OH
0 .
In certain embodiments, the beta-amino alkanol used in the method of
reducing low-speed-pre-ignition events in an engine is not the following:
/
/OH
,;)
o
H2N......,..õ.õ...õ...,
In certain embodiments, the low-speed pre-ignition (LSPI)-reducing additive
does not comprise the following:
/
/OH
oc)
e
H2N,............õ
OH
0 .
In certain embodiments, the amino alcohol is not the following:
/
/OH
,:)
e
H2N ..,.........
OH
0 .
[025] The 13-amino alkanol has at least 2 carbon atoms (e.g., from 4 to 30
carbon atoms, from 4 to 20 carbon atoms, from 4 to 16 carbon atoms, from 4 to
12
carbon atoms, from 5 to 30 carbon atoms, from 5 to 20 carbon atoms, from 5 to
16
carbon atoms, or from 5 to 12 carbon atoms).
[026] Representative examples of suitable 13-amino alkanols include
ethanolamine (Formula 1A), 1-amino-2-propanol (Formula 1B), alaninol (Formula
1C), 2-(methylamino)ethanol (Formula 1D), 2-(ethylamino)ethanol (Formula 1E),
2-
amino-2-methyl-1-propanol (Formula 1F), 2-amino-1-butanol (Formula 1G), 2-
amino-1-pentanol (Formula 1H), valinol (Formula 11), 2-amino-1-hexanol
(Formula
1.1), leucinol (Formula 1K), isoleucinol (Formula 1L), cycloleucinol (Formula
1M),
9
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
cyclohexylglycinol (Formula 1N), prolinol (Formula 10), 2-
(hydroxymethyl)piperidine
(Formula 1P), 2-aminocyclopentanol (Formula 1Q), 2-aminocyclohexanol (Formula
1R), aminoheptyl propanediol (3-(heptan-2-ylamino)propane-1,2-diol) (Formula
1T),
aminooctyl propanediol (3-(methyl(octyl)amino)propane-1,2-diol) (Formula 1U),
and
aminododecyl ethanol (2-(dodecyl(methyl)amino)ethan-1-ol) (Formula 1V). In
certain
embodiments, the 13-amino alkanol is aminoheptyl propanediol (3-(heptan-2-
ylamino)propane-1,2-diol) (Formula 1T). In certain embodiments, the 13-amino
alkanol is aminooctyl propanediol (3-(methyl(octyl)amino)propane-1,2-diol)
(Formula
1U). In certain embodiments, the 13-amino alkanol is aminododecyl ethanol (2-
(dodecyl(methyl)amino)ethan-1-ol) (Formula 1V). Representative structures are
shown below.
OH
OH H2Nj
H2N \__/OH H2N
Formula lA Formula 1B Formula 1C
H H
N \
.rNi OH H2N
OH
OH
Formula 1D Formula lE Formula 1F
H2N
OH
NH2 H2N.,-.
OH
OH
_
Formula 1G Formula 1H Formula 1I
NH2
NH2
NH2 ).OH
OH I.OH
Formula 1J Formula 1K Formula 1L
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
OH
H21\11
H2N
OH /OH
Formula 1M Formula 1N Formula 10
a OH
N R-NH2
OH
OH NH2
Formula 1P Formula 1Q Formula 1R
OH
H2N
HN;OH
OH
Formula 1S Formula 1T
Me OH
OH
Formula 1U
Me
HO N
Formula 1V
In certain embodiments, the 13-amino alkanol is not the following:
OH
HN
OH
11
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
In certain embodiments, the fuel composition does not comprise the following:
OH
HN
OH
In certain embodiments, the fuel concentrate does not comprise the following:
OH
HN
OH
In certain embodiments, the beta-amino alkanol used in the method of
reducing low-speed-pre-ignition events in an engine is not the following:
OH
HNjOH
In certain embodiments, the low-speed pre-ignition (LSPI)-reducing additive
does not comprise the following:
OH
HNjOH
In certain embodiments, the amino alcohol is not the following:
OH
HN
OH
In certain embodiments, the 13-amino alkanol is or comprises aminoheptyl
propanediol, with the proviso that it is not the following:
/OH
H2N
OH
0
12
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
In certain embodiments, the fuel composition comprises aminoheptyl
propanediol, with the proviso that it is not the following:
/
/OH
e
H2N.,.................
OH
O .
In certain embodiments, the fuel concentrate comprises aminoheptyl
propanediol, with the proviso that it is not the following:
/
/OH ,c)
e
H2N............õõõ.".........
OH
O .
In certain embodiments, the beta-amimo alkanol used in the method of
reducing low-speed-pre-ignition events in an engine is or comprises
aminoheptyl
propanediol, with the proviso that it is not the following:
/
/OH
,:)
e
H2N.,....õ......õ---........
OH
O .
In certain embodiments, the low-speed pre-ignition (LSPI)-reducing additive is
or comprises aminoheptyl propanediol, with the proviso that it is not the
following:
/
/OH
e
H2N.,...,..õ...õ,,,,......,
In certain embodiments, the amino alcohol is or comprises aminoheptyl
propanediol, with the proviso that it is not the following:
/
/OH no
0
H2N.,..........õ.õ,,,%,...,
OH
O .
13
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
Amino Acid
[027] The fuel additive or lubricating oil additive of this disclosure may be
an
aliphatic amino acid, a substituted aliphatic amino acid, or a derivative
thereof, or an
acceptable salt thereof. Useful amino acids include those that can be
represented by
the following general formula:
H
H 0
/\N 1 <
H
R OH
Formula 2
wherein R is an "aliphatic" or "aromatic" side chain. Amino acid side chains
can be
broadly classified as aromatic or aliphatic. An aromatic side chain includes
an aromatic
ring. Examples of amino acids with aromatic side chains include for example,
histidine
(Formula 2A), phenylalanine (Formula 2B), tyrosine (Formula 2C), tryptophan
(Formula 2D) and the like. Non-aromatic side chains are broadly grouped as
"aliphatic" and include, for example, alanine (Formula 2E), glycine (Formula
2F),
cysteine (Formula 2G), and the like.
[028] The amino acid(s) can be natural and/or non-natural a-amino acids.
Natural amino acids are those encoded by the genetic code, as well as amino
acids
derived therefrom. These include, for example, hydroxyproline (Formula 2H), y-
carboxyglutamate (Formula 21), and citrulline (Formula 2J). In this
specification, the
term "amino acid" also includes amino acid analogs and mimetics. Analogs are
compounds having the same general structure of a natural amino acid, except
that the
R group is not one found among the natural amino acids.
[029] Representative examples of analogs of naturally occurring amino acids
include homoserine (Formula 2K), norleucine (Formula 2L), homoproline (Formula
2M) and proline (Formula 2N). An amino acid mimetic is a compound that has a
structure different from the general chemical structure of an a-amino acid but
14
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
functions in a manner similar to one. The amino acid may be an L- or D-amino
acid.
Representative structures are shown below.
0 0
N...n)\I
( OH OH
NH2
HN NH2
Formula 2A Formula 2B
EN1
o 10 /
NH2
OH
NH2 OH
HO 0
Formula 2C Formula 2D
0
0
*LOH
H2NJL
NH2 OH
Formula 2E Formula 2F
0
0
1-\11....._AOH
HSOH
NH2 OH
Formula 2G Formula 2H
0 0
0 0
HOLOH
H2N)LNLOH
H
NH2
0 OH NH2
Formula 21 Formula 2J
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
0 0
HO(
OH .Y'LOH
NH2 NH2
Formula 2K Formula 2L
0
0
)LOH
C?(OH
NH
Formula 2M Formula 2N
Amino Ester
[030] The fuel additive or lubricating oil additive of this disclosure may be
an
amino ester, a substituted amino ester, or a derivative thereof, or an
acceptable salt
thereof. Amino esters can be derived from amino acids (as described above) and
alcohols. Amino esters and amino acids may be considered derivatives of each
other.
Useful amino esters include those that can be represented by the following
general
formula:
H
H 0
> 1 <
H
R ORi
Formula 3
wherein R is an aliphatic side chain and Ri is a carbon chain 1 to 20 carbon
atoms in
length, preferably 1 to 4 carbon atoms, in particular, methanol or ethanol,
preferably
methanol.
[031] The amino esters may include aromatic or aliphatic side chains.
Representative examples of amino esters include methyl alaninate (Formula 3A),
ethyl
alaninate (Formula 3B), methyl glycinate (Formula 3C), and ethyl glycinate
(Formula
3D). Representative structures are shown below.
16
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
0 0
/ )L0 0
NH2 NH2
Formula 3A Formula 3B
0 0
H2NJL H2N
0 0
Formula 3C Formula 3D
2. N=C-X Motif Additives
[032] A fuel additive or lubricating oil additive of this disclosure may have
a
N=C-X motif as shown in the generalized structure below
NR
Xi X2
Formula 4
wherein R is H, monovalent organic group, or monovalent heterorganic group
(described in greater detail below), Xi and X2 are independently H, C, N, 0,
or S and
wherein Xi or X2 independently includes one or more Ci-C20 alkyl group (e.g.,
Cl-C6
alkyl) or one or more aromatic ring. In some embodiments, Xi and X2 may
include a
cyclic structure (e.g., a five-, six-, or seven-membered ring). Cyclic
structures may be
aromatic or non-aromatic, as well as vary from being fully saturated to fully
unsaturated. Suitable examples of additives compatible with Formula 4 include
amidines, guanidines, imidazoles, benzamidines,
benzimidazoles, and
aminobenzimidazoles.
Amidine
17
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
[033] The fuel additive or lubricating oil additive of this disclosure may be
an
amidine, a substituted amidine, or a derivative thereof or an acceptable salt
thereof.
Useful amidines include those that can be represented by the following general
formula:
N R8
R5 .4.... ,....1.4.4.
N R7
I
R6
Formula 5
wherein R5, R6, R7 and Rs are each independently selected from hydrogen,
monovalent
organic groups, monovalent heterorganic groups (e.g., comprising nitrogen,
oxygen,
sulfur or phosphorus, in the form of groups or moieties that are bonded
through a
carbon atom and that do not contain acid functionality such as carboxylic or
sulfonic),
and combinations thereof; and wherein any two or more of R5, R6, R7 and Rs
optionally
can be bonded together to form a cyclic structure (e.g., a five-, six, or
seven-membered
ring). The cyclic structures may be aromatic or non-aromatic, as well as vary
from
being fully saturated to fully unsaturated. The organic and heterorganic
groups may
have from 1 to 10 carbon atoms (e.g., 1 to 6 carbon atoms).
[034] Representative examples of suitable amidines include 1,4,5,6-
tetrahydropyri midine (Formula 5A), 1,2-dimethy1-1,4,5,6-
tetrahydropyrimidine
(Formula 5B), 1,2-diethyl-1,4,5,6-tetrahydropyrimidine (Formula 5C), 1,5-
diazabicyclo[4.3.0]non-5-ene (DBN; Formula 5D), 1,8-diazabicyclo[5.4.0]-undeca-
7-
ene (DBU; Formula 5E), benzamidine (Formula 5F), benzimidazole (Formula 5G)
and
2-phenyl-1H-benzo[d]imidazole (Formula 5M). Representative structures are
shown
below.
N / N N H C) C
N N N
1 8
CA 03119923 2021-05-13
WO 2020/099953 PCT/IB2019/058057
Formula 5A Formula 5B Formula 5C
NH
NO
/ N r--) NH2
N N
Formula 5D Formula 5E Formula 5F
H
ON..õ
* N>
c)o..-- NE12 C 11 NH2
\---N N
Formula 5G Formula 5H Formula 51
S N11-12
cs)...-- NH2 (s)--- NH2 /\\---N \---N
Formula 5J Formula 5K Formula 5L
0 1\1\ *
N
H
Formula 5M
Guanidine
[035] The fuel additive or lubricating oil additive of this disclosure may be
a
guanidine, a substituted guanidine, or a derivative thereof, or an acceptable
salt
thereof. Useful guanidines include those that can be represented by the
following
general formula,
N,R13
R9,, N N ...... R12
1 1
R10 R11
Formula 6
19
CA 03119923 2021-05-13
WO 2020/099953 PCT/IB2019/058057
wherein R9, R10, R11, R12 and Ri 3 are each independently selected from
hydrogen,
monovalent organic groups, monovalent heterorganic groups (e.g., comprising
nitrogen, oxygen, sulfur or phosphorus, in the form of groups or moieties that
are
bonded through a carbon atom and that do not contain acid functionality such
as
carboxylic or sulfonic), and combinations thereof; and wherein any two or more
of R9,
Rio, R11, R12 and Ri 3 optionally can be bonded together to form a cyclic
structure (e.g.,
a five-, six, or seven-membered ring). The cyclic structures may be aromatic
or non-
aromatic, as well as vary from being fully saturated to fully unsaturated. The
organic
and heterorganic groups may have from 1 to 10 carbon atoms (e.g., 1 to 6
carbon
atoms).
[036] Representative examples of suitable guanidines include 1,1,3,3-
tetramethylguanidine (TMG; Formula 6A), 2-tert-butyl-1,1,3,3-
tetramethylguanidine
(BTMG; Formula 66), 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD; Formula 6C), 7-
methyl-
1,5,7-triazabicyclo[4.4.0]dec-5-ene (MTBD; Formula 6D) and 1,2-
diphenylguanidine
(Formula 61). Representative structures shown below.
NH I
Ny N
I I
Formula 6A Formula 6B
N
N
\ NLN /
\ NLN /
H I
Formula 6C Formula 6D
N
H a,
N N
0 N)¨
N N H2 I
H 0
CA 03119923 2021-05-13
WO 2020/099953 PCT/IB2019/058057
Formula 6E Formula 6F
H
N N R
Y'
NH
JLN-------r-OH * N
H2N
1
CH3 0 CF-I3
Formula 6G Formula 6H
0 NH2 00)
N N
H
Formula 61
lmidazoles
[037] The fuel additive or lubricating oil additive of this disclosure may be
an
imidazole, a substituted imidazole, or a derivative thereof, or an acceptable
salt
thereof. Suitable imidazoles include imidazole (Formula 7A), 1-methylimidazole
(Formula 7B), 1-ethylimidazole (Formula 7D), 1-propylimidazole (Formula 7E), 1-
n-
butylimidazole (Formula 7F), 1-decylimidazole, 1-dodecylimidazole, 2-
methylimidazole (Formula 7G), 2-ethylimidazole, 2-isopropylimidazole (Formula
7H),
4-methylimidazole (Formula 71), 1,2-dimethylimidazole (Formula 7J), 2-ethyl-
4(5)-
methylimidazole (Formula 7K), and 1-vinylimidazole (Formula 7L).
Representative
structures are shown below.
(-3
N Nl
N
N=i
H
Formula 7A Formula 7B Formula 7C
Nl N/ Nl
21
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
Formula 7D Formula 7E Formula 7F
H
µX zN N¨JN N
.-õ-=:- \
N H HN
Formula 7G Formula 7H Formula 71
H
zN
,
(51,1
) ____________________________ 11 N/
N
Formula 7J Formula 7K Formula 7L
3. Triazole Additives
[038] The fuel additive or lubricating oil additive of this disclosure may be
a
triazole, a substituted triazole, or a derivative thereof, or an acceptable
salt thereof.
Suitable triazoles include 1, 2, 3-triazole (Formula 8A), 5,6-
dimethylbenzotriazole
(Formula 88), 1, 2, 4-triazole (Formula 8C), piperidine-substituted triazole
(Formula
8D) and benzotriazole analog, for example, an alkyl-substituted benzotriazole,
such as
a methyl substituted benzotriazole (Formula 8E). Representative structures are
shown
below.
inNiFi
\IA ,N
N
N H
Formula 8A Formula 8B
HN(..._
NH NH
1\1
NN,N
N
Formula 8C Formula 8D
22
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
N
Formula 8E
4. Benzamidinium Additives
[039] The fuel additive or lubricating oil additive of this disclosure may be
a
benzamidinium, a substituted benzamidinium, or a derivative thereof, or an
acceptable
salt thereof. Useful benzamidinium additives include those that can be
represented
by the following general formula 9, wherein Ri, R2, and R3 are independently
Ci-C20
alkyl groups.
a
R
µ1\1( 2
R3
0
Formula 9
[040] Suitable benzamidiniums include N,N-dimethyl-N-octylbenzamidium-2-
oxide (Formula 9A). Representative structures are shown below.
= I
0
Formula 9A
23
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
e e
N N
. =
0 0
Formula 9B Formula 9C
5. Benzoxazole Additives
[041] The fuel additive or lubricating oil additive of this disclosure may be
a
benzoxazole, a substituted benzoxazole, or a derivative thereof, or an
acceptable salt
thereof. Suitable benzoxazoles include benzoxazole (Formula 10A) and 2-
aminobenzoxazole (Formula 10B). Representative structures are shown below.
ON( NH2
0 N
0
Formula 10A Formula 10B
6. Amine Additives
Aromatic amine
[042] The fuel additive or lubricating oil additive of this disclosure may be
an
aromatic amine, a substituted aromatic amine, or a derivative thereof, or an
acceptable
salt thereof. Aromatic amine additives can have the generalized structure
shown in
Formula 11-1 or 11-2,
R
rN N
X
X
Formula 11-1 Formula 11-2
24
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
wherein R is independently one or more H or Ci-C20 alkyl group and X is N
(e.g., R-N-
R) or 0 .
[043] Suitable aromatic amines include 2-methylquinolin-8-amine (Formula
11A). Representative structures are shown below.
NFi2
/
N
NH2 N
Formula 1 lA Formula 11B
N
H2N
Formula 11C
Aliphatic amine
[044] Suitable aliphatic amines are shown below.
HN
H2N
Formula 12A Formula 12B
HN/*/
H2
[10 N*
Formula 12C Formula 12D
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
Secondary LSPI-Reducing Additives
[045] The following are descriptions of secondary LSPI-reducing additives that
can be utilized as fuel or lubricating additives to reduce LSPI activity. In
general, a
secondary LSPI-reducing additive, a substituted secondary LSPI-reducing
additive, or
a derivative thereof will be used in their salt form and in combination with a
primary
additive to reduce LSPI activity. For example, 8-amino alkanol (primary
additive) and
aliphatic acid (secondary additive) can be combined and utilized as an LSPI
additive.
Table 2 lists the secondary additive types. Some additives can act as a
primary additive
and/or secondary additive.
Table 2
Secondary Additive Types
7. Acid Additives
Aliphatic acid
Unsaturated acid
Alkylaromatic acid
Aromatic acid
Hydroxy acid
Amino acid
8. Phenol Additives
9. 1, 3 Dicarbonyl Additives
1,3 Diketone
1,3 Ketoester
10. Hydroxamide Additives
11. Antioxidant Additives
12. Salicylate Additives
26
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
7. Acid Additives
Aliphatic Acid
[046] Aliphatic acids are non-aromatic carboxylic acids. Suitable aliphatic
acids
include mono-carboxylic acids having the following structure
0
R)LOH
Formula 13
wherein R is an aliphatic group having between 2 to 20 carbon atoms. The
aliphatic
group may be linear or branched and may contain heteroatoms.
[047] Suitable aliphatic acids include hexanoic acid (Formula 13A), heptanoic
acid (Formula 13B), octanoic acid (Formula 13C), nonanoic acid (Formula 13D),
decanoic acid (Formula 13E), undecanoic acid, lauric acid, myristic acid,
palmitic acid,
stearic acid, arachidic acid (C20), behenic acid (C22), 2-ethylbutyric acid
(Formula 13F),
3,3-dimethylbutyric acid, 2-methylpentanoic acid (C6), 2-methylhexanoic acid
(C7), 4-
methylhexanoic acid (C7), 5-methylhexanoic acid (C7), 2,2-dimethylpentanoic
acid (C7),
2-propylpentanoic acid (Cs), 2-ethylhexanoic acid (Formula 13G), 2-
methylheptanoic
acid (Cs), isooctanoic acid (Cs), 3,5,5-trimethylhexanoic acid (C9), 4-
methyloctanoic acid
(C9), 4-methylnonanoic acid, (C10), isodecanoic acid (C10), 2-butyloctanoic
acid (C12),
isotridecanoic acid (C13), 2-hexyldecanoic acid (C16), isopalmitic acid (C16),
isostearic
acid (Formula 13H), 3-cyclohexylpropionic acid, 4-cyclohexylbutyric acid
(Formula
131), and cyclohexanepentanoic acid. Representative structures are shown
below.
0 0
OH )(OH
Formula 13A Formula 13B
27
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
0 0
LOH OH
Formula 13C Formula 13D
0
0 LOH
OH
Formula 13E Formula 13F
0
OH
Formula 13G
0
OH
Formula 13H
0
a.):(
OH C))LOH
Formula 131 Formula 13J
Unsaturated Acid
[048] Suitable unsaturated acids include any organic acids that contain double
or triple carbon-carbon bond. Representative unsaturated acids include maleic
acid
(Formula 14A), fumaric acid (Formula 14B), as well as unsaturated fatty acids
such as
palmitoleic acid (Formula 14C) and oleic acid (Formula 14D). Representative
structures are shown below.
28
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
0 HO HO
)-)L OH
OH 0
Formula 14A Formula 14B
0
_
OH
Formula 14C
0
¨
OH
Formula 14D
Allwlaromatic Acid
[049] Suitable alkylaromatic acids include both mono-carboxylic acids and
dicarboxylic acids. The alkyl carboxylic acid may have 6 or more carbon atoms
(e.g., 6
to 24 carbon atoms, 6 to 20 carbon atoms, 8 to 24 carbon atoms, 8 to 20 carbon
atoms,
or even 8 to 18 carbon atoms). The alkyl moiety may be optionally substituted
with
one or more substituents such as hydroxy, alkoxy and carbonyl (e.g., aldehydic
or
ketonic) groups. Suitable examples of alkylaromatic acid include methylbenzoic
acid
(Formula 15A) and ethylbenzoic acid (Formula 15B). Representative structures
are
shown below.
0 0
1401 0H 0JLOH 0
OH
Formula 15A Formula 15B Formula 15C
Aromatic Acid
[050] Suitable aromatic acids include both mono-carboxylic acids and
dicarboxylic acids. The alkyl carboxylic acid may have 6 or more carbon atoms
(e.g., 6
29
CA 03119923 2021-05-13
WO 2020/099953 PCT/IB2019/058057
to 24 carbon atoms, 6 to 20 carbon atoms, 8 to 24 carbon atoms, 8 to 20 carbon
atoms,
or even 8 to 18 carbon atoms). The alkyl moiety may be optionally substituted
with
one or more substituents such as hydroxy, alkoxy and carbonyl (e.g., aldehydic
or
ketonic) groups. Suitable aromatic acids include benzoic acid (Formula 16A),
hydroxybenzoic acid (Formula 166), and tetralin carboxylic acid (Formula 16C).
Representative structures are shown below.
0 0H
0 0
OH
* OH
Formula 16A Formula 16B Formula 16C
Hydroxy Acid
[051] Suitable hydroxy acids include those that can be represented by the
following general formula:
OH 0
Formula 17
wherein n = 1 to 3. Suitable examples of hydroxy acid include glycolic acid
(Formula
17A), lactic acid (Formula 176), malic acid (Formula 17C), tartaric acid
(Formula 17D),
and citric acid (Formula 17E). Representative structures are shown below.
0 0
0 * HO1 L OH
HO OH JL
OH OH 0 OH
Formula 17A Formula 17B Formula 17C
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
OH 0
o oOH 0
HO(
OH
HO)LOH
0 OH OH
Formula 17D Formula 17E
Amino Acid
[052] Amino acids can be utilized as primary and/or secondary additives.
Suitable amino acids were previously described above.
8. Phenol Additives
Phenol
[053] Suitable phenols include, thymol (Formula 18A), eugenol (Formula
188), hydroquinone (Formula 18C), resorcinol (Formula 18D), cresol (Formula
18E)
and 2-methylquinolin-8-ol (Formula 18G). Representative structures are shown
below.
0 OH
HO . OH
0 HO
Formula 18A Formula 18B Formula 18C
OH
HO * OH 0
*
OH
Formula 18D Formula 18E Formula 18F
/
N
OH
Formula 18G
31
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
9. 1,3 Dicarbonyl Additives
1, 3 Diketone
[054] Suitable examples of 1,3 diketone compounds include acetylacetone
(Formula 19A)õ and curcumin (Formula 19B). Representative structures are shown
below.
))0 0
Formula 19A
o o/
HOI OH
I I
0 0
Formula 19B
1,3 Ketoester
[055] Suitable 1,3 ketoesters are shown below.
0
1::
0 0
c;1))
Formula 20A Formula 20B
10. Hydroxamide Additives
[056] A hydroxamide is a hydroxy derivative of an amide. Useful hydroxamides
include those that can be represented by the following general formula:
32
CA 03119923 2021-05-13
WO 2020/099953 PCT/IB2019/058057
0
R1--...N..--1--- R2
I
OH
Formula 21
wherein Ri and R2 are each independently selected from hydrogen or Ci-C20
(e.g., C3-
C12) alkyl group. Suitable hydroxamide includes hydroxy methylacetamide
(Formula
21A). Representative structures are shown below.
0 0 0
N N )LN=
1 I I
OH OH OH
Formula 21A Formula 21B Formula 21C
11. Antioxidant Additives
[057] Suitable antioxidants include both mono-carboxylic acids and
dicarboxylic acids. The alkyl carboxylic acid may have 6 or more carbon atoms
(e.g., 6
to 24 carbon atoms, 6 to 20 carbon atoms, 8 to 24 carbon atoms, 8 to 20 carbon
atoms,
or even 8 to 18 carbon atoms). The alkyl moiety may be optionally substituted
with
one or more substituents such as hydroxy, alkoxy and carbonyl (e.g., aldehydic
or
ketonic) groups. Suitable antioxidants include the following.
OH
0 OH
Formula 22A
33
CA 03119923 2021-05-13
WO 2020/099953 PCT/IB2019/058057
12. Salicylate Additives
Salicylate
[058] Suitable salicylates include 2-hydroxy-5-
(tetracosa-
1,3,5,7,9,11,13,15,17,19,21,23-dodecayn-1-yl)benzoic acid--dihydrogen (Formula
23E). Suitable salicylates are shown below.
0 0 0H o
0
101 C)
0 0
OH OH OH
Formula 23A Formula 23B Formula 23C
OH 0 OH 0
* OH
* OH
C12H25 C24H49
Formula 23D Formula 23E
Salts
[059] The salts of this disclosure may be prepared by conventional means, for
example, by mixing the primary additive with a suitable secondary additive in
an
aprotic solvent. The order in which one additive is added to the other is not
important.
The primary additive and secondary additive are usually mixed together in an
approximately equimolar ratio. An excess of the primary or secondary additive
component may be used. For example, the molar ratio of base relative to the
alkyl
carboxylic acid may be about 1.05:1 to 2:1 (e.g., 1.1:1 to 1.5:1).
Representative salts are
shown below.
34
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
0
CN (7)
Formula 24A
Formula 24B
H H
IN
;)
(H3C)2N N(CH3)2
Formula 24C
0
I
õ
0 iso-,1735
Formula 24D
0
(+
/Nh
oH
H H
Formula 24E
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
o
NH3
C,
Formula 24F
. 73 0
Me
/
N
- -5- +
,
Formula 24G
H
I. 0
Formula 24H
o
VOH -
0
OH
Formula 241
H
'1
0 0
...,...,....,õN
Formula 24J
36
CA 03119923 2021-05-13
WO 2020/099953 PCT/IB2019/058057
NZD0
Formula 24K
0
0
N
Formula 24L
I
0
Formula 24M
* I
Formula 24N
0-
0
Formula 240
37
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
OH
H
NZD
-
0 o
Formula 24P
H 0
I .
OH
N.,...õ,.......
Formula 24Q
H
. 1
Formula 24R
H 0
1 .
CN
N.õ,.....õ.õ"..., H.
Formula 24S
38
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
0
H
aNF
1
I
H ........,........õ0
Formula 24T
\
0 0
'H2N1,,,,N-----.
0
NN
Formula 24U
0 H
+1
0 N%0
N
* _
0
Formula 24V
OH 0 H
1+
101 0_ CrN
N
C12H25
Formula 24W
39
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
OH
H H
\ =/
N
)L
(H3 0)2 N N (CH3)2
0 0
Formula 24X
OH
e
()
I-12N
OH
0
Formula 24Y
Y+ 0
00
N
0
_
Formula 24Z
H OH
1+
0
c N CO2 (,)
C24H49
Formula 24AA
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
Fuel Compositions
[060] The compounds of the present disclosure may be useful as additives in
hydrocarbon fuels to prevent or reduce engine knock or pre-ignition events in
spark-
ignited internal combustion engines.
[061] The concentration of the compounds of the present disclosure in
hydrocarbon fuel may range from 25 to 5000 parts per million (ppm) by weight
(e.g.,
50 to 1000 ppm).
[062] The compounds of the present disclosure may be formulated as a
concentrate using an inert stable oleophilic (i.e., soluble in hydrocarbon
fuel) organic
solvent boiling in a range of 65 C to 205 C. An aliphatic or an aromatic
hydrocarbon
solvent may be used, such as benzene, toluene, xylene, or higher-boiling
aromatics or
aromatic thinners. Aliphatic alcohols containing 2 to 8 carbon atoms, such as
ethanol,
isopropanol, methyl isobutyl carbinol, n-butanol and the like, in combination
with the
hydrocarbon solvents are also suitable for use with the present additives. In
the
concentrate, the amount of the additive may range from 10 to 70 wt % (e.g., 20
to 40
wt %).
[063] In gasoline fuels, other well-known additives can be employed including
oxygenates (e.g., ethanol, methyl tert-butyl ether), other anti-knock agents,
and
detergents/dispersants (e.g., hydrocarbyl amines, hydrocarbyl
poly(oxyalkylene)
amines, succinimides, Mannich reaction products, aromatic esters of
polyalkylphenoxyalkanols, or polyalkylphenoxyaminoalkanes). Additionally,
friction
modifiers, antioxidants, metal deactivators and demulsifiers may be present.
[064] In diesel fuels, other well-known additives can be employed, such as
pour
point depressants, flow improvers, cetane improvers, and the like.
[065] A fuel-soluble, non-volatile carrier fluid or oil may also be used with
compounds of this disclosure. The carrier fluid is a chemically inert
hydrocarbon-
soluble liquid vehicle which substantially increases the non-volatile residue
(NVR), or
solvent-free liquid fraction of the fuel additive composition while not
overwhelmingly
contributing to octane requirement increase. The carrier fluid may be a
natural or
41
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
synthetic oil, such as mineral oil, refined petroleum oils, synthetic
polyalkanes and
alkenes, including hydrogenated and unhydrogenated polyalphaolefins, synthetic
polyoxyallwlene-derived oils, such as those described in U.S. Patent Nos.
3,756,793;
4,191,537; and 5,004,478; and in European Patent Appl. Pub. Nos. 356,726 and
382,159.
[066] The carrier fluids may be employed in amounts ranging from 35 to 5000
ppm by weight of the hydrocarbon fuel (e.g., 50 to 3000 ppm of the fuel). When
employed in a fuel concentrate, carrier fluids may be present in amounts
ranging from
20 to 60 wt % (e.g., 30 to 50 wt %).
Lubricating Oil Compositions
[067] The compounds of the present disclosure may be useful as additives in
lubricating oils to prevent or reduce engine knock or pre-ignition events in
spark-
ignited internal combustion engines.
[068] The concentration of the compounds of the present disclosure in the
lubricating oil composition may range from 0.01 to 15 wt % (e.g., 0.5 to 5 wt
%), based
on the total weight of the lubricating oil composition.
[069] The oil of lubricating viscosity (sometimes referred to as "base stock"
or
"base oil") is the primary liquid constituent of a lubricant, into which
additives and
possibly other oils are blended, for example to produce a final lubricant (or
lubricant
composition). A base oil, which is useful for making concentrates as well as
for making
lubricating oil compositions therefrom, may be selected from natural
(vegetable,
animal or mineral) and synthetic lubricating oils and mixtures thereof.
[070] Definitions for the base stocks and base oils in this disclosure are the
same as those found in American Petroleum Institute (API) Publication 1509
Annex E
("API Base Oil Interchangeability Guidelines for Passenger Car Motor Oils and
Diesel
Engine Oils," December 2016). Group I base stocks contain less than 90%
saturates
and/or greater than 0.03% sulfur and have a viscosity index greater than or
equal to
80 and less than 120 using the test methods specified in Table E-1. Group II
base stocks
contain greater than or equal to 90% saturates and less than or equal to 0.03%
sulfur
and have a viscosity index greater than or equal to 80 and less than 120 using
the test
42
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
methods specified in Table E-1. Group III base stocks contain greater than or
equal to
90% saturates and less than or equal to 0.03% sulfur and have a viscosity
index greater
than or equal to 120 using the test methods specified in Table E-1. Group IV
base
stocks are polyalphaolefins (PAO). Group V base stocks include all other base
stocks
not included in Group I, II, Ill, or IV.
[071] Natural oils include animal oils, vegetable oils (e.g., castor oil and
lard
oil), and mineral oils. Animal and vegetable oils possessing favorable thermal
oxidative
stability can be used. Of the natural oils, mineral oils are preferred.
Mineral oils vary
widely as to their crude source, for example, as to whether they are
paraffinic,
naphthenic, or mixed paraffinic-naphthenic. Oils derived from coal or shale
are also
useful. Natural oils vary also as to the method used for their production and
purification, for example, their distillation range and whether they are
straight run or
cracked, hydrorefined, or solvent extracted.
[072] Synthetic oils include hydrocarbon oil. Hydrocarbon oils include oils
such
as polymerized and interpolymerized olefins (e.g., polybutylenes,
polypropylenes,
propylene isobutylene copolymers, ethylene-olefin copolymers, and ethylene-
alphaolefin copolymers). Polyalphaolefin (PAO) oil base stocks are commonly
used
synthetic hydrocarbon oil. By way of example, PAOs derived from C8 to C14
olefins, e.g.,
C8, C10, C12, C14 olefins or mixtures thereof, may be utilized.
[073] Other useful fluids for use as base oils include non-conventional or
unconventional base stocks that have been processed, preferably catalytically,
or
synthesized to provide high performance characteristics.
[074] Non-conventional or unconventional base stocks/base oils include one
or more of a mixture of base stock(s) derived from one or more Gas-to-Liquids
(GTL)
materials, as well as isomerate/isodewaxate base stock(s) derived from natural
wax or
waxy feeds, mineral and or non-mineral oil waxy feed stocks such as slack
waxes,
natural waxes, and waxy stocks such as gas oils, waxy fuels hydrocracker
bottoms, waxy
raffinate, hydrocrackate, thermal crackates, or other mineral, mineral oil, or
even non-
43
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
petroleum oil derived waxy materials such as waxy materials received from coal
liquefaction or shale oil, and mixtures of such base stocks.
[075] Base oils for use in the lubricating oil compositions of present
disclosure
are any of the variety of oils corresponding to API Group I, Group II, Group
III, Group
IV, and Group V oils, and mixtures thereof, preferably API Group II, Group
III, Group IV,
and Group V oils, and mixtures thereof, more preferably the Group III to Group
V base
oils due to their exceptional volatility, stability, viscometric and
cleanliness features.
[076] Typically, the base oil will have a kinematic viscosity at 100 C (ASTM
D445) in a range of 2.5 to 20 mm2/s (e.g., 3 to 12 mm2/s, 4 to 10 mm2/s, or
4.5 to 8
mm2/s).
[077] The present lubricating oil compositions may also contain conventional
lubricant additives for imparting auxiliary functions to give a finished
lubricating oil
composition in which these additives are dispersed or dissolved. For example,
the
lubricating oil compositions can be blended with antioxidants, ashless
dispersants,
anti-wear agents, detergents such as metal detergents, rust inhibitors,
dehazing
agents, demulsifying agents, friction modifiers, metal deactivating agents,
pour point
depressants, viscosity modifiers, antifoaming agents, co-solvents, package
compatibilizers, corrosion-inhibitors, dyes, extreme pressure agents and the
like and
mixtures thereof. A variety of the additives are known and commercially
available.
These additives, or their analogous compounds, can be employed for the
preparation
of the lubricating oil compositions of the invention by the usual blending
procedures.
[078] Each of the foregoing additives, when used, is used at a functionally
effective amount to impart the desired properties to the lubricant. Thus, for
example,
if an additive is an ashless dispersant, a functionally effective amount of
this ashless
dispersant would be an amount sufficient to impart the desired dispersancy
characteristics to the lubricant. Generally, the concentration of each of
these additives,
when used, may range, unless otherwise specified, from about 0.001 to about 20
wt %,
such as about 0.01 to about 10 wt %.
44
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
EXAMPLES
[079] The following illustrative examples are intended to be non-limiting.
EXAMPLES 1-45
[080] The test compounds were blended in gasoline or lube oil and their
capacity for reducing LSPI events were determined using the test method
described
below.
[081] A GM 2.0 L LHU 4-cylinder gasoline turbocharged direct-injected engine
was used for LSPI testing. Each cylinder was equipped with a combustion
pressure
sensor.
[082] A six-segment test procedure was used to determine the number of LSPI
events that occurred under conditions of an engine speed of 2000 rpm and a
load of
275 Nm. The LSPI test condition is run for 28 minutes with each segment
separated by
an idle period. The first segment is used to condition the oil and the number
of LSPI
events are not counted. Each segment is slightly truncated to eliminate the
transient
portion. Each truncated segment typically has approximately 100,000 combustion
cycles (25,000 combustion cycles per cylinder). In total, the five truncated
segments
where LSPI events are counted have approximately 500,000 combustion cycles
(125,000 combustion cycles per cylinder). There may be instances of shortened
tests
in the event the engine cannot complete all six segments.
[083] LSPI-impacted combustion cycles were determined by monitoring peak
cylinder pressure (PP) and crank angle at 5% total heat release (A15). LSPI-
impacted
combustion cycles are defined as having both (1) a PP greater than five
standard
deviations than the average PP for a given cylinder and truncated segment and
(2) an
A15 greater than five standard deviations less than the average for a given
cylinder and
truncated segment.
[084] The LSPI frequency is reported as the number of LSPI-impacted
combustion cycles per million combustion cycles and is calculated as follows:
CA 03119923 2021-05-13
WO 2020/099953
PCT/IB2019/058057
LSPI Frequency = [(Total Number of LSPI Impacted Combustion Cycles in five
Truncated
Segments)/(Total Number of Combustion Cycles in five Truncated Segments)] x
1,000,000
[085] An additive associated with a test fuel and/or test lubricant that
reduces
the LSPI frequency, when compared to the corresponding baseline fuel and/or
baseline
lubricant, is considered an additive that mitigates LSPI frequency. The test
results are
set forth in Table 2.
46
TABLE 2
o
t..)
o
LSPI Activity
t..)
o
Additive Reference Drop in Ex. Base Base
(events/million o
Additive Component Concentr
(events/million LSPI Formula o
No. Fluid combustion
o
u,
ation combustion cycles) Activity (...)
cycles)
1000
1 Prolinol Fuel 108
268 60% 10
PPmw
1000
2 Prolinol Fuel 155
268 42% 10
PPmw
1000
3 DBU/2-ethylhexanoate Fuel 14
250 94% 24A
PPmw P
500 .
4 DBU/2-ethylhexanoate Fuel 28
225 87% 24A ,
,
PPmw
"
-4 250
DBU/2-ethylhexanoate Fuel 65 255
75% 24A 10;
"
PPmw ,
,
0
Tributylammonium/2- 1114
,
6 Fuel 166
265 37% 24B ,
ethylhexanoate PPmw
875
7 TMG/2-ethylhexanoate Fuel 52
217 76% 24C
PPmw
1473
8 DBU/isostearate Fuel 48
240 80% 24D
PPmw
Lube
9 DBU/isostearate 1.9 wt % 113
285 60% 24D 1-d
Oil
n
1-i
828 5
Prolino1/2-ethylhexanoate Fuel 193 316
39% 24E t..)
PPmw =
,-,
Lube
o
O-
11 Prolino1/2-ethylhexanoate 1.1 wt % 131 316
59% 24E u,
Oil
cee
o
u,
-4
Monosubstituted amine/2- 1114
0
12 Fuel 213
300 29% 24F t..)
ethylhexanoate PPmw
2
o
13 Aliquat/2-ethylhexanoate High Fuel 243
350 31% 24G O-
o
901
o
14 DBU/Proline Fuel 50
350 86% 24H o
u,
PPmw (...)
946
15 DBU/diketone Fuel 62
374 83% 24J
PPmw
1466
16 DBU/oleate Fuel 38
520 93% 24K
PPmw
1020
17 DBU/Toluene Fuel 74
531 86% 24L
PPmw
1108 P
18 DBU/Tetralin Carboxylate Fuel 106
540 80% 24M 2
PPmw ,
,
1020 rõ
cio 19 DBU/Phenoxide Fuel 36
600 94% 24N
rõ
PPmw 2
,
514 ,
-
20 DBU Fuel 78
419 81% 5E
,
,
PPmw
389
21 TMG Fuel 154
463 67% 6A
PPmw
2338
22 DBU/Phenol Fuel 218
462 53% 24W
PPmw
1814
23 DBU/SA analog Fuel 152
396 62% 240 1-d
PPmw n
1-i
2421 t..)
24 DBU Antioxidant Fuel 24
416 94% 24P
,-,
PPmw o
O-
u,
cio
o
u,
-4
1757 0
25 DBU/Hydroxy acid Fuel 159
367 57% 24Q t..)
o
PPmw t..)
o
O-
o
1577 o
26 DBU/Ketoester Fuel 10
510 98% 24R o
u,
PPmw (...)
1750
27 DBU/Hydroxamide Fuel 123
536 77% 24S
PPmw
1650
28 MorphGuam Fuel 119
478 75% 6F
PPmw
2213
29 TMG/antioxidant Fuel 24
498 95% 24X P
PPmw 2
,
,
.6. 676
o 30 Benzamidine Fuel
59 444 87% 5F
PPmw
"
'7
2461
5',
31 MorphGuam/2- ethylhexanoate Fuel 26
352 93% 241
PPmw
664
32 Benzamidazole Fuel 151
353 57% 5G
PPmw
383
33 lmidazole Fuel 112
330 66% 7A
PPmw
749
34 2-aminobenzimidazole Fuel 16
281 94% 6E 1-d
n
PPmw
1382
35 TMG/Ketoester Fuel 99
381 74% 24U t..)
=
PPmw
o
O-
u,
cio
o
u,
-4
1790 0
36 DBU/Ethyl Salicylate Fuel 34
472 93% 24V t..)
PPmw
t..)
o
N-(2,3-dihydroxypropyl)heptan-
O-
1000 o
37 2-aminium (AHPD)/2- Fuel 84
312 73% 24Y o
o
PPmw u,
ethylhexanoate
(...)
401.5
38 benzoxazole Fuel 197
344 43% 10A
PPmw
805.4
39 2-aminobenzoxazole Fuel 239
338 29% 10B
PPmw
N,N-dimethyl-N- 1665.7
40 Fuel 10
246 96% 9A
octylbenzamidinium-2-oxide ppmw
991.9 P
41 5,6-dimethylbenzotriazole Fuel 109 235
54% 8B PPmw ,
,
u, 1869.9
.
"
o 42 DBU/phenoxide
Fuel 0 215 100% 24Z
IV
PPMW 2
,
4162.2 ,
.
43 DBU/salicylate Fuel 46
197 77% 24AA
,
,
PPmw
1166.1
44 2-phenyl-1H-benzo[d]imidazole Fuel 121
192 37% 5M
PPmw
1725.5
45 1,3-diphenylguanidine Fuel 12
134 91% 61
PPmw
3-(heptan-2-ylamino)propane- 1126
46 Fuel 14
197 93% 1T
1,2-diol (AHPD) PPmw
1-d
n
1000
47 Prolinol Fuel 35
159 78% 10 5
PPmw ,..,
=
500
o
48 Prolinol Fuel 43
158 73% 10 O-
PPmw u,
cio
o
u,
-4
250
0
49 Prolinol Fuel 43 137
69% 10 t..)
PPmw
t..)
125
o
50 Prolinol Fuel 108 163
35% 10 O-
o
PPmw
o
o
u,
3-(methyl(octyl)amino)propane- 1345
(...)
51 Fuel 157 292
46% 1U
1,2-diol PPmw
2-(methyl(dodecyl)amino)ethan- 1506
52 Fuel 141 318
56% 1V
1-ol PPmw
1870
53 DBU 2-methylquinolin-8-olate Fuel 0 215
100% 24Z
PPmw
4162
54 DBU C24-salicylate Fuel 46 197
77% 24AA
PPmw
P
.
,
,
,,
,,
.
,,
'7
.
u,
,
,
IV
n
1-i
,..,
=
,z
-a
u,
c,
=
u,
-4