Note: Descriptions are shown in the official language in which they were submitted.
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
MONONUCLEAR CELL DERIVED NK CELLS
Field of the Invention
[0001] The present disclosure relates to compositions, methods, and devices to
generate and
cultivate immune competent cells, especially as it relates to cord blood (CB)
or peripheral
blood (PB) NK cells from whole blood.
Back2round of the Invention
[0002] The background description includes information that may be useful in
understanding
the present disclosure. It is not an admission that any of the information
provided herein is
prior art or relevant to the presently claimed invention, or that any
publication specifically or
implicitly referenced is prior art.
[0003] All publications and patent applications herein are incorporated by
reference to the
same extent as if each individual publication or patent application were
specifically and
individually indicated to be incorporated by reference. Where a definition or
use of a term in
an incorporated reference is inconsistent or contrary to the definition of
that term provided
herein, the definition of that term provided herein applies and the definition
of that term in the
reference does not apply.
[0004] Natural killer (NK) cells constitute a group of innate immune cells,
which are often
characterized as cytotoxic lymphocytes that exhibit antibody dependent
cellular toxicity via
target-directed release of granulysin and perforin. Most NK cells have a
specific cell surface
marker profile (e.g., CD3 , CD56+, CD16+, CD57+, CD8+) in addition to a
collection of
various activating and inhibitory receptors. While more recently NK cells have
become a
significant component of certain cancer treatments, generation of significant
quantities of NK
cells (and especially autologous NK cells) has been a significant obstacle as
the fraction of
NK cells in whole blood is relatively low.
[0005] To obtain therapeutically meaningful quantities of NK and NK-like
cells, NK cells
can be generated from various precursor cells. For example, various stem cell
factors (SCF),
FLT3 ligand, interleukin (IL)-2, IL-7 and IL-15 have been reported in various
in vitro
approaches to induce and expand cord blood-derived cytokine-induced killer
(CIK) cells
(Anticancer Research 30: 3493-3500 (2010)). Similarly, CD34+ hematopoietic
cells can be
exposed to IL-12 and other agents as is reported in US 2018/0044636. In still
other
1
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
approaches, human hemangioblasts were sequentially exposed to two different
cytokine
cocktails as described in W02011/068896, and different cytokine cocktails were
used with
post-embryonic hematopoietic stem cells as taught in W02012/128622. While at
least some
of these methods provide a significant n-fold expansion of NK cells, methods
and reagents
for such expansion are both time and resource demanding. Still further, it
should be noted
that many of the known methods also require NK cell culture on a feeder cell
layer, which is
often problematic from a technical and a regulatory perspective.
[0006] In more simplified methods, acute myeloid leukemia (AML) cells can be
exposed to
TpoR agonists to so induce the AML cells to form NK cells. However, such
approach is
likely not viable as a source for therapeutic cell preparations. Alternative
methods have also
relied on culturing peripheral blood cells in the presence of various
interleukins, stem cell
factors, and FLT3 ligands as is disclosed in WO 2011/103882. In yet another
method, US
2013/0295671 teaches methods of stimulating already existing NK cells with
anti-CD16 and
anti-CD3 antibodies along with cytokines. While procedurally simpler, such
methods still
require elaborate manipulation of the cells and add significant costs due to
the specific
reagent required.
[0007] In still further known methods, US 10,125,351 describes use of cord
blood or
peripheral blood as a source of cells that are subject to density gradient
separation to isolate
nucleated cells that are then cultivated with a medium that contains
interferon, interleukin, a
CD3 antibody and human albumin. Most advantageously, such method is amenable
to
perfusion culture in a bioreactor and as such significantly reduces
operational difficulties.
Unfortunately, however, the yield of NK cells is relatively low.
[0008] Thus, even though various methods of generating significant quantities
of NK cells
are known in the art, all or almost all of them suffer from various
disadvantages.
Consequently, there is a need to provide improved systems and methods that
produce
significant quantities of NK cells, and especially autologous NK cells.
Moreover, improved
systems and methods will also allow for automation of cell culture and will
have substantially
reduced reagent requirements to render such methods clinically and
commercially viable.
Summary of The Invention
[0009] The inventors have discovered compositions, methods, and devices that
enable
generation and expansion of NK cells in a conceptually simple and efficient
manner.
2
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
Advantageously, NK cells can be generated from blood mononuclear cells (MNCs)
obtained
from cord or whole blood without isolating either CD34+ hematopoietic stem
cells (HSC) or
NK cells, and without the use of a feeder layer, preferably by an enrichment
process that uses
N-803 and optionally an anti-CD16 agonist antibody and an anti-CD3 antibody.
[0010] In one aspect of the inventive subject matter, the inventors
contemplate a method of
producing NK cells that includes a step of isolating from a biological fluid a
mixture of
mononuclear cells, a step of contacting the mixture of the mononuclear cells
with an anti-
CD16 antibody and N-803 to activate NK cells, and another step of sequentially
feeding the
activated NK cells with a medium containing N-803.
[0011] In most typical examples, the step of isolating the mixture of the
mononuclear cells is
performed using density gradient centrifugation, and/or the biological fluid
is whole blood or
cord blood. Therefore, the mixture of mononuclear cells will generally include
T cells, NK
cells, NKT cells, and double negative (DN) T cells. While not categorically
excluded, it is
generally preferred that the mixture of mononuclear cells is not further
processed to enrich
NK cells.
[0012] With respect to contemplated anti-CD16 antibodies it is generally
preferred that the
antibody is a monoclonal antibody with specificity to human CD16. Most
typically, the anti-
CD16 antibody is present at a concentration of between 0.05-0.5 mcg/ml, and/or
the N-803 is
present at a concentration of between 0.1-1.0 nM. Where desired, contemplated
methods
may also include a step of contacting the mixture further includes contacting
the mixture of
the mononuclear cells with an anti-CD3 antibody (e.g., at a concentration of
between 0.1-1.0
ng/ml).
[0013] In some embodiments, the mixture of the mononuclear cells contains
about 100-500 x
106 cells, and/or the step of contacting the mixture is performed in a volume
of between about
100-300m1 or at a cell density of about 1 x 106 cells/ml. Preferably, but not
necessarily, the
medium containing N-803 comprises human AB serum and/or NK MACSTM medium
(commercially available from Mileny Biotech, Friedrich-Ebert-StraBe 68, 51429
Bergisch
Gladbach, Germany) and hydrocortisone (0.1-5 uM). Moreover, it is contemplated
that the
step of sequentially feeding is performed about every 72 hours, and/or that
the step of
sequentially feeding is performed until a total cell number of about 0.5- 5.0
x 109 cells is
reached. Furthermore, the step of sequentially feeding the activated NK cells
may be
3
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
performed in single container and the step of contacting the mixture of the
mononuclear cells
may be performed in the same container.
[0014] In other embodiments, the step of sequentially feeding the activated NK
cells is
performed until NK cells are enriched to an at least 100-fold expansion,
and/or until NK cells
constitute at least about 80% or at least about 90% of all live cells.
[0015] Therefore, and viewed form a different perspective, the inventors also
contemplate a
method of expanding NK cells from a mixture of mononuclear cells that includes
a step of
providing a mixture of the mononuclear cells that contains equal or less than
5% NK cells. In
another step, the mixture of the mononuclear cells is then contacted with an
anti-CD16
antibody and N-803 to activate NK cells, and in a further step the activated
NK cells are fed
with a medium containing N-803.
[0016] Preferably, but not necessarily, the mixture of the mononuclear cells
is obtained from
whole blood or cord blood, or the mixture of the mononuclear cells is obtained
from an
MI-IC-matched autologous source relative to an individual that receives the NK
cells. In
typical examples, the mixture of the mononuclear cells that contains equal or
less than 3%
NK cells, and/or may further comprise T cells, NKT cells, and DN cells. With
respect to the
medium, anti-CD16 antibody, the N-803, and the anti-CD3 antibody, the same
considerations
as noted above apply.
[0017] Additionally, it is contemplated that the step of feeding comprises
sequentially
feeding at an interval of about every 72 hours, that the step of feeding is
performed until a
total cell number of about 0.5- 5.0 x 109 cells is reached, and/or that the
step of feeding the
activated NK cells is performed until NK cells are enriched to an at least 100-
fold expansion.
In further embodiments, it is contemplated that the step of feeding the
activated NK cells is
performed in an automated manner, preferably in a single container.
[0018] Thus, in yet another aspect of the inventive subject matter, the
inventors also
contemplate a method of expanding NK cells in an automated bioreactor. Such
method will
typically include a step of incubating a mixture of mononuclear cells in an
activation medium
containing N-803 and an anti-CD16 antibody for a time sufficient to activate
NK cells,
wherein the mixture of mononuclear cells is contained in a cell culture
container while
incubating the mixture. In another step, growth of the cells is measured while
the cells are in
the container, and the cells are automatically fed with a medium containing N-
803 according
4
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
to a predetermined schedule and/or a result from the step of measuring growth
of the cells. In
still another step, feeding the cells is terminated according to a
predetermined schedule and/or
a result from the step of measuring growth of the cells.
[0019] For example, suitable containers will have a volume of between about
200 ml and
about 2,500 ml, and/or the step of measuring growth of the cells is performed
through a wall
of the container (e.g., using optical measurements). Most preferably, the
activation medium
contains N-803 at a concentration of between 0.1-1.0 nM and an anti-CD16
antibody at a
concentration of between 0.05-0.5 mcg/ml. In other examples, the medium
containing N-803
contains N-803 at a concentration of between 0.1-1.0 nM. Most typically, the
time sufficient
to activate NK cells is between 24 hours and 96 hours, and the cells are fed
until a total cell
number of about 0.5- 5.0 x 109 cells is reached, and/or until NK cells are
enriched to an at
least 100-fold expansion.
[0020] In still another aspect of the inventive subject matter, the inventors
also contemplate a
cell culture container (e.g., having a volume of between about 200 ml and
about 2,500 ml)
that contains a medium with distinct types of immune competent cells. Most
preferably, the
medium contains NK cells in an amount of at least 80% of all live cells, NKT
cells in an
amount of equal or less than 10 % of all live cells, T cells an amount of
equal or less than 5 %
of all live cells, and DN T cells an amount of equal or less than 3 % of all
live cells.
Preferably, at least one wall of the container has an optically transparent
portion, and/or the
NK cells are present in an amount of at least about 90% of all live cells.
[0021] Various objects, features, aspects, and advantages will become more
apparent from
the following detailed description of preferred embodiments, along with the
accompanying
drawing in which like numerals represent like components.
Brief Description of The Drawin2
[0022] Fig.1 depicts an exemplary schematic illustrating a process starting
from cord blood
through isolation of CBMCs that then make up the seed for the
enrichment/expansion of NK
cells.
[0023] Fig.2 depicts exemplary details of a representative process in an
automated
environment (GMP in a box') and the schedule of addition of various
ingredients.
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
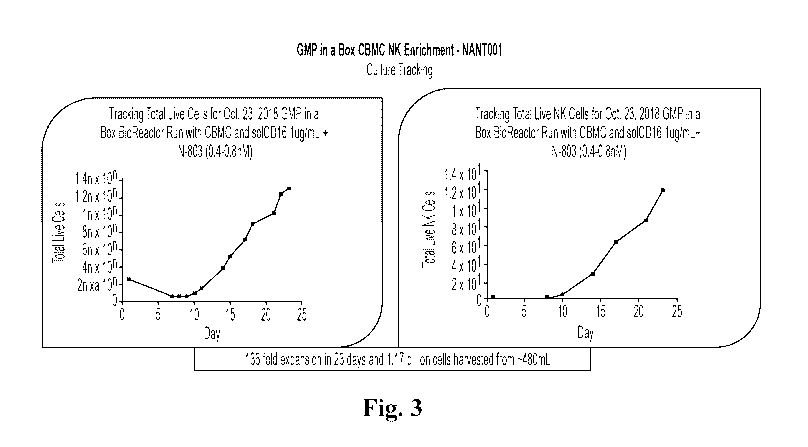
[0024] Fig.3 depicts exemplary results for enrichment kinetics of the process
of Fig. 2 for NK
cells by number and selected flow cytometry properties.
[0025] Fig.4 depicts exemplary results for kinetics of various cell
populations of the process
of Fig. 2 along results for with marker expression, especially significant
expression of the
majority of NK activation receptors.
Detailed Description
[0026] With the continuously increasing use of immune therapies in the
treatment of cancer,
production of sufficient quantities of NK cells, and especially autologous NK
cells as
therapeutic entities has become critical. Unfortunately, many of the current
methods require
use of feeder layers or differentiation of isolated CD34+ hematopoietic stem
cells (HSCs),
which is both time and resource intensive. Moreover, due to the various
manipulation steps
needed, such methods typically require human interaction and are prone to
contamination.
[0027] In an effort to improve production methods for NK cells, the inventors
have now
discovered various systems, compositions, and methods to generate
therapeutically
meaningful quantities (e.g., at least 0.5 x 109 NK cells) from a biological
fluid containing
mononuclear cells (e.g., whole blood, cord blood) in a simple and effective
manner that can
even be fully automated once the mononuclear cells are obtained as is
schematically
illustrated in Fig.!. Preferably, the bioreactor is a self-contained unit and
will have a central
processor and memory on board to execute a programmable protocol for various
activities
(e.g., operation of pumps for fluid movements, temperature and gas regulation,
image
processing, etc.) and to generate regulatory-ready reports, as well as a
microscope (or other
optical unit) for monitoring the cell culture.
[0028] For example, in one process contemplated herein, whole peripheral blood
or cord
blood is used as a starting material that is processed to obtain mononuclear
cells. Most
typically, processing can be done using conventional density gradient
centrifugation (e.g.,
using Ficoll-Paque PlusTM (a hydrophilic soluble polysaccharide, density 1.077
g/mL),
commercially available from GE Lifesciences). Once the mononuclear cells are
separated
from the centrifuge tube, the cells are washed and re-suspended in an
activation medium
(e.g., NK MACS supplemented with 10% human AB serum). The activation medium
further
comprises N-803 at a concentration of about 0.4 nM, and an anti-CD16 antibody
at a
concentration of about 1.0 mcg/ml.
6
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
[0029] Most typically, the mononuclear cells have a density of 1-2 x106
cells/ml in a total
volume of about 200 ml, and the cells and medium are in a single container.
After about 3-4
days, the cells are fed with fresh medium containing N-803, and further feed
cycles are
performed about every three days through recovery, rapid expansion, and
culture culmination
as exemplarily shown in Fig.2. Cells are harvested upon reaching a desired
quantity, typically
about 0.5- 5.0 x 109 total cells and/or upon reaching a desired expansion
(e.g. at least 100-
fold expansion). Notably, despite the apparent simplicity, the so obtained
cell culture contains
after about three weeks more than about 85% NK cells, with less than about 8%
NKT cells,
and with less than about 2.5% T cells, and less than about 1.2% double
negative (DN) T cells.
Moreover, it should be recognized that the entire culture process may be
performed in a
single container within a self-contained bioreactor, which substantially
reduces risk of
contamination and eliminates reagent and cell handling during the cultivation
step. Fig.3
depicts exemplary results for an NK production that yielded an about 136-fold
expansion of
NK cells in 23 days with a total of 1.17 x 109 cells harvested from a final
volume of about
480 ml. Fig.4 depicts further experimental data that illustrate cell
composition over time
tracking T cells, NK cells, NKT cells, DN cells (along with CD16 results; left
panel). Results
for the final phenotyping for the cells harvested in the process of Fig.2 and
Fig.3 are shown in
the right panel of Fig.4. As can be readily seen, the detected markers were
indicative of NK
cells.
[0030] With respect to suitable biological fluids, it is generally
contemplated that the fluids
could be autologous relative to the individual that will receive the NK cells
isolated in the
methods presented herein. Therefore, especially preferred biological fluids
include fresh
whole blood, cord blood (frozen or fresh), and cells separated in a
leukapheresis procedure.
However, it should be appreciated that the biological fluid may also be any
fluid that contains
NK cells (typically among other cell types). For example, suitable alternative
biological
fluids include whole blood from allogenic donors, which may or may not be
matched for a
compatible MHC type. Therefore, samples in a blood bank that approach
expiration date are
deemed suitable for use, as well as freshly donated whole or stored cord blood
by an
individual other than the NK cell recipient.
[0031] Likewise, it should be noted that the manner of isolating or enriching
mononuclear
cells may vary considerably, and the person of ordinary skill in the art will
be readily
apprised of the most suitable methods of isolation and enrichment. For
example, where the
7
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
biological fluid is whole blood or cord blood, it is preferred that the fluid
is processed via
gradient density centrifugation using any suitable medium (e.g., Ficoll-
Hypaque).
Alternatively, mononuclear cells may be obtained directly from the patient by
leukapheresis,
or the biological fluid may be subjected to removal of red blood cells using
antibodies. In still
further methods, mononuclear cells may be isolated using magnetic bead
separation where
the beads are coated or otherwise coupled to antibodies binding the
mononuclear cells.
[0032] Likewise, it should be recognized that the particular nature of the
medium for
activation and feeding need not be limited to NK MACS medium, but that all
media known
to support growth of NK cells are deemed suitable for use herein. Most
preferably, however,
defined media are used and may be supplemented with human AB serum.
[0033] Activation of the NK cells in the mixture of mononuclear cells is
preferably
performed with a combination of an anti-CD16 antibody and N-803, and
optionally an anti-
CD3 antibody. There are various sources for anti-CD16 antibodies known in the
art/commercially available, and particularly preferred anti-CD16 antibodies
have agonist
(activating) activity and are specific to human CD16. However, activators
other than anti-
CD16 antibodies are also deemed suitable for use herein include anti-CD16
antibody
fragments and fusion proteins with anti-CD16 antibody fragments. Additionally,
or
alternatively, contemplated activators also include CD314 or NKG2D, the
natural
cytotoxicity receptors CD335 (NKp46), CD336 (NKp44) and CD337 (NKp30), CD226
(DNAM-1), CD244 (2B4), members of the CD158 or killer immunoglobulin-like
receptor
(KIR) family that carry a short cytoplasmic tail (KIR2DS and KIR3DS) and
CD94/NKG2C,
among others.
[0034] Concentrations of the anti-CD16 antibody will typically follow those
already known
in the art for activation of NK cells. Therefore, suitable concentrations for
anti-CD16
antibodies will be between about 0.01-5.0 mcg/ml, and more typically between
about 0.01-
0.3 mcg/ml, or between about 0.05-0.5 mcg/ml, or between about 0.1-1.0 mcg/ml,
or
between about 1.0-5.0 mcg/ml. With respect to the duration of exposure to the
anti-CD16
antibody it is generally contemplated that the mixture of mononuclear cells is
exposed to only
a single, two, or there doses of the anti-CD16 antibody, most typically when
the mononuclear
cells are isolated and contacted with the activation medium for the first
(and/second, and/or
third) time. The person of ordinary skill in the art will be readily able to
recognize proper
schedule and dosage to achieve NK cell activation. Most typically, exposure of
the
8
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
mononuclear cells to the anti-CD16 antibody is contemporaneous with exposure
of the
mononuclear cells with the N-803. However, in less preferred embodiments,
exposure of the
mononuclear cells to the anti-CD16 antibody is sequentially to exposure of the
mononuclear
cells with the N-803 (with exposure of the mononuclear cells to the anti-CD16
antibody first
being the preferred sequence).
[0035] Where desired, activation may also include contacting the cells with
anti-CD3
antibody, typically at the same time of contacting the cells with anti-CD16
antibody. As
noted above, concentrations of the anti-CD3 antibody will typically follow
those already
known in the art for activation of NK cells. Therefore, suitable
concentrations for anti-CD3
antibodies will be between about 0.01-10.0 ng/ml, and more typically between
about 0.01-0.1
ng/ml, or between about 0.1-0.5 ng/ml, or between about 0.3-1.0 ng/ml, or
between about
1.0-5.0 ng/ml. Likewise, with respect to the duration of exposure to the anti-
CD3 antibody it
is generally contemplated that the mixture of mononuclear cells is exposed to
only a single,
two, or there doses of the anti-CD3 antibody, most typically when the
mononuclear cells are
isolated and contacted with the activation medium for the first (and/second,
and/or third)
time. The person of ordinary skill in the art will be readily able to
recognize proper schedule
and dosage to achieve NK cell activation.
[0036] With respect to N-803 it is contemplated that N-803 (an IL-15N72D:IL-
15RaSu/IgG1
Fc complex with human sequences; see US 2019/0023766, commercially available
from
ImmunityBio) is preferred as an agent in the activation and feed medium.
However, various
alternative agents with IL-15 activity are also deemed suitable for use
herein. In this context,
and without wishing to be bound by any theory or hypothesis, the inventors
contemplate that
N-803 enables growth and expansion of the NK cells by virtue of continuous
signaling. In
contrast, IL-15 as isolated cytokine has a very short lifespan and signaling
activity is typically
very short. This, where IL-15 as isolated cytokine is added to a growth
medium, the
signaling will be pulsed or intermittently. In contrast, where N-803 is
provided, stability of
IL-15 is dramatically extended and signaling is deemed continuous. Moreover,
it should be
recognized that N-803 also provides a physiological context (i.e., IL-15 R-
alpha chain) and a
N72D form that acts as a super agonist. Therefore, any stabilized IL-15
compound is also
expressly deemed suitable for use herein. In yet further contemplated aspects,
IL-15
(recombinant, recombinantly expressed, or isolated) and/or N-803 may be at
least in part
replaced or supplemented by TxM type fusion protein complexes, especially
preferred fusion
9
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
protein complexes are described in WO 2018/165208, which is incorporated by
reference
herein. For example, contemplated TxM type fusion protein complexes will
include at least
one additional cytokine selected from the group consisting of IL-7, IL-18, and
IL-21.
Therefore, and among other suitable choices, contemplated TxM fusion complexes
include an
IL-18/IL-7 TxM and/or IL-18/IL-21 TxM.
[0037] For example, all compounds and complexes that effect IL-15 signaling
are deemed
suitable for use herein so long as such compounds and complexes have a serum
half-life that
is longer than isolated/recombinant and purified IL-15 alone. Moreover, it is
generally
preferred that the stabilized IL-15 compounds will include at least portions
of human
sequences for IL-15 and/or IL-15 Ra. For example, suitable compounds include
P22339 (a
complex of IL-15 and the Sushi domain of IL-15Ra chain with a disulfide bond
linking the
IL-15/Sushi domain complex with an IgG1 Fc to augment its half-life; see
Nature, Scientific
Reports (2018) 8:7675), and XmAb24306, which is a IL-15/IL-15Ra-Fc heterodimer
(see
e.g., WO 2018/071919).
[0038] In further especially contemplated embodiments, the mixture of
mononuclear cells is,
after isolation from the biological fluid, placed into a cell culture
container together with the
medium containing the anti-CD16 (and optionally anti-CD3) antibody and N-803
to activate
the NK cells. Most preferably, the container is a cell culture flask with at
least one wall (or
portion thereof) that is transparent to light such that cell shape, staining,
and/or growth can be
observed with a microscope or other optical instrument. Thus, it should be
noted that the cells
can be continuously or periodically monitored in a bioreactor, and so obtained
measurements
(e.g., cell size, cell number, cell distribution, etc.) can be used to trigger
or modify an
automated feeding schedule in a control unit that is logically coupled to the
bioreactor. Most
typically, and as shown in Fig. 2, feeding fresh medium with N-803 can be
performed using a
predefined schedule, typically every three days, where preferably each feeding
will include
N-803 to maintain continuous signaling. While the specific volumes shown in
Fig.2 are
suitable for expanding the NK cells to cell densities consistent with cell
growth, it should be
appreciated that the volumes may be adjusted to accommodate particular growth
patterns. To
that end, it should also be appreciated that the feeding may be continuously
or that
predetermined volumes may be changed in response to the growth kinetic
observed in the
container.
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
[0039] In most cases, the yield of the NK cells at the end of the cultivation
will be typically
at least 80%, or at least 82%, or at least 85%, or at least 88%, or at least
90%, or at least 92%,
or at least 94% of all live cells with the remainder being NKT cells, DN T
cells, and T cells.
For example, remaining NKT cells will typically be equal or less than 10%, or
equal or less
than 8%, or equal or less than 7%, or equal or less than 6% of all live cells,
while remaining
T cells will typically be equal or less than 5%, or equal or less than 4%, or
equal or less than
3%, or equal or less than 2% of all live cells, and remaining DN T cells will
typically be
equal or less than 3%, or equal or less than 2%, or equal or less than 1.5 %,
or equal or less
than 1% of all live cells.
[0040] Therefore, and viewed from a different perspective, it should be
appreciated that the
systems and methods contemplated herein are capable of remarkably high
expansion of NK
cells, and typical expansions are at least 80-fold, or at least 100-fold, or
at least 120-fold, or
at least 130-fold, or at least 140-fold with respect to the number of NK cells
originally
present in the mixture of mononuclear cells. Such expansion is particularly
notable in view of
the very simple manner of activation and cultivating (one-pot process).
Indeed, once the
mixture of mononuclear cells is placed into the cell culture container, the
entire process con
continue within the same container and will be sustained by addition of media
only as
schematically shown in Fig.2. Thus, complex handling and expensive reagents
are entirely
avoided, and the risk for contamination is significantly reduced.
[0041] While not limiting to the inventive subject matter, it is therefore
contemplated that the
NK cells are expanded and/or activated in a culture environment that allows
for continuous
monitoring, continuous management of CO2 and 02 levels, and continuous
monitoring to
detect cell density (e.g., confluence). Among other options for such
environments, especially
preferred environments are automated cell culturing and harvesting devices as
are described,
for example, in WO 2015/165700. Such `GMB-in-a-box' systems beneficially allow
control
over feeding schedules, gas control, allow for real-time detection of cell
density, growth
(kinetics) and cell health, as well as dramatically reduce the possibility of
contamination due
to significantly reduced handling requirements.
[0042] In still further contemplated aspects, it should be noted that the
systems and methods
presented herein advantageously also allow generation of CD56dim and CD 5
6bright NK cells,
particularly where the NK cells are generated from peripheral blood. Depending
on further
culture conditions, CD56bright NK cells may then differentiate to CD56dim
cells. Such distinct
11
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
NK cell populations can then be employed as for distinct therapeutic options
due to their
distinct maturation and cytotoxicity profile. Additionally, it should be
appreciated that the
compositions, systems and methods will also be suitable to generate NKT cells
upon proper
stimulation and culture.
Examples
[0043] In view of the above, and as provided in more detail below, one
exemplary method
entailed isolating CBMCs or PBMCs by a single Ficoll centrifugation step,
which was
followed by incubation of the cells with about 0.4 nM N-803 and about 0.1
mcg/ml of an
anti-CD16 antibody (e.g., clone B73.1, commercially available from BD
Biosciences), and
optionally about 0.5 ng/ml of an anti-CD3 antibody in NK MACS media with 10%
human
AB serum. Typically 150 mL of CBMCs at a million cells/ml were used as the
starting
material with above reagents. Media was used for dilution with N-803 twice a
week with a
regimen of a 1:2 and 1:10 compared to existing volume with corresponding
concentration of
N-803 for a final concentration of 0.4 nM.
Materials: MNCs from Cord and Peripheral Blood, anti-CD16 antibody, BD
bioscience San
Diego CA; NK MACS medium with NK supplement, staining antibodies for
phenotyping
(aCD3, aCD16, aCD56, aNKp30, aNKp44, aNKp46, aNKG2A, aNKG2D, aTIGIT, aCD34,
aTRAIL, aCD57, aCXCR3, and aCCR5), Miltenyi Biotec San Diego, CA; Human AB
serum,
Access Biologicals, San Diego CA; N-803, GMP in a Box kit, Nantbio Inc Culver
City CA.
Methods: MNCs were freshly isolated from cord blood or peripheral blood. It
was washed
twice with complete NKMACS medium (NKMACS+ Supplements+ 10% hu-AB-serum).
MNCs were suspended in 150mL of medium with density of 1x10^6 cell/mL. 150mL
cell
suspension was supplemented with aCD16 antibody (1mcg/mL) and N-803 (0.4nM).
Further
GMP kit was installed in the box and protocol uploaded through VivaBio web
portal. Cells
suspension with complete cytokine and antibody were transferred to cell bag,
and 150mL cell
suspension was injected through cell injection port in Box-kit. GMP Box
started imaging and
cells were propagated according to steps written in protocol as mentioned in
Fig2. Cells in the
box were supplemented with 10X cytokine medium or with 2X cytokine medium in
alternate
fashion as described in Fig2. NK enrichment (phenotype for CD3, CD56, and CD16
expression) and cell health (cell number, viability, and cell density) were
monitored regularly
and plotted in graph as in Fig 3 and Fig 4a. Cells were harvested after
enrichment from the
12
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
box and measured for the expression of NK cell based receptors for its
complete
characterization as in Fig 4.
[0044] As used herein, the term "administering" a pharmaceutical composition
or drug refers
to both direct and indirect administration of the pharmaceutical composition
or drug, wherein
direct administration of the pharmaceutical composition or drug is typically
performed by a
health care professional (e.g., physician, nurse, etc.), and wherein indirect
administration
includes a step of providing or making available the pharmaceutical
composition or drug to
the health care professional for direct administration (e.g., via injection,
infusion, oral
delivery, topical delivery, etc.). Most preferably, the cells or exosomes are
administered via
subcutaneous or subdermal injection. However, in other contemplated aspects,
administration
may also be intravenous injection. Alternatively, or additionally, antigen
presenting cells may
be isolated or grown from cells of the patient, infected in vitro, and then
transfused to the
patient. Therefore, it should be appreciated that contemplated systems and
methods can be
considered a complete drug discovery system (e.g., drug discovery, treatment
protocol,
validation, etc.) for highly personalized cancer treatment.
[0045] The recitation of ranges of values herein is merely intended to serve
as a shorthand
method of referring individually to each separate value falling within the
range. Unless
otherwise indicated herein, each individual value is incorporated into the
specification as if it
were individually recited herein. All methods described herein can be
performed in any
suitable order unless otherwise indicated herein or otherwise clearly
contradicted by context.
The use of any and all examples, or exemplary language (e.g., "such as")
provided with
respect to certain embodiments herein is intended merely to better illuminate
the the full
scope of the present disclosure, and does not pose a limitation on the scope
of the invention
otherwise claimed. No language in the specification should be construed as
indicating any
non-claimed element essential to the practice of the claimed invention.
[0046] It should be apparent to those skilled in the art that many more
modifications besides
those already described are possible without departing from the full scope of
the concepts
disclosed herein. The disclosed subject matter, therefore, is not to be
restricted except in the
scope of the appended claims. Moreover, in interpreting both the specification
and the claims,
all terms should be interpreted in the broadest possible manner consistent
with the context. In
particular, the terms "comprises" and "comprising" should be interpreted as
referring to
elements, components, or steps in a non-exclusive manner, indicating that the
referenced
13
CA 03120695 2021-05-20
WO 2021/006875
PCT/US2019/040867
elements, components, or steps may be present, or utilized, or combined with
other elements,
components, or steps that are not expressly referenced. Where the
specification claims refers
to at least one of something selected from the group consisting of A, B, C
.... and N, the text
should be interpreted as requiring only one element from the group, not A plus
N, or B plus
N, etc.
14