Note: Descriptions are shown in the official language in which they were submitted.
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
1
FERRITIC STAINLESS STEEL
This invention relates to a stabilized ferritic stainless steel having good
corrosion resistance, good weldability and enhanced high temperature strength
for use in high temperature service in components used in applications such as
automotive exhaust systems, fuel cells and other energy sector applications,
appliances, furnaces and other industrial high temperature systems.
The most critical point in developing ferritic stainless steel is how to take
care
of carbon and nitrogen elements. These elements have to be bound to
carbides, nitrides or carbonitrides. The elements used in this type of binding
are called stabilizing elements. The common stabilizing elements are niobium
and titanium. The requirements for stabilization of carbon and nitrogen can be
diminished for ferritic stainless steels where for instance the carbon content
is
very low, less than 0.01 weight %. However, this low carbon content causes
requirements for the manufacturing process. The common AOD (Argon-
Oxygen-Decarburization) producing technology for stainless steels is not any
more practical and, therefore, more expensive producing methods shall be
used, such as the VOD (Vacuum-Oxygen-Decarburization) producing
technology.
Intermetallic Laves phase particles, which may form in ferritic stainless
steel,
increase the high temperature strength of the steel provided that the
particles
remain small and stable in the operating temperatures. Additionally, Laves
phase particles, precipitated inside grains and on grain boundaries, also
inhibit
grain growth. Alloying of a balanced combination of niobium, silicon and
titanium in ferritic stainless steel promotes precipitation of intermetallic
Laves
phase and stabilizes the phase by increasing the dissolution temperature of
precipitates.
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
2
The microstructure formed in the weld depends on the chemical composition of
weld metal. When a sufficient amount of titanium is used in the stabilization
of
the interstitial elements carbon and nitrogen, the compounds formed during the
stabilization, such as TiN, produce an equiaxed, fine grained structure in
welds.
The equiaxed, fine grained structure improves the ductility and toughness of
the welds. Unwanted columnar grains can cause hot cracking as impurities may
segregate to the weld centreline. Large columnar grains also decrease the
toughness of the weld.
The EP patent EP2922978B describes ferritic stainless steel having excellent
corrosion and sheet forming properties, characterized in that the steel
consists
of in weight percentages 0.003 - 0.035% carbon, 0.05- 1.0% silicon, 0.1 -0.8
% manganese, 20 - 21.5 % chromium, 0.05 - 0.8 % nickel, 0.003 -0.5 %
molybdenum, 0.2 - 0.8 % copper, 0.003 - 0.05 % nitrogen, 0.05 - 0.15 %
titanium, 0.25% - 0.8 % niobium, 0.03 - 0.5 % vanadium, 0.010- 0.04 %
aluminium, and the sum C+N less than 0.06 %, the remainder being iron and
inevitable impurities, wherein the ratio (Ti+Nb)/(C+N) is higher or equal to
8,
and less than 40, and the ratio Tieq/Ceq=(Ti+ 0.515*Nb +0.940*V)/(C+0.858*N)
is higher or equal to 6, and less than 40.
The EP patent 1818422 describes a niobium stabilized ferritic stainless steel
having, among others, less than 0.03 weight % carbon, 18 - 22 weight %
chromium, less than 0.03 weight % nitrogen and 0.2 - 1.0 weight % niobium. In
accordance with this EP patent the stabilization of carbon and nitrogen is
carried out using only niobium.
The EP patent application 2163658 describes a ferritic stainless steel with
sulfate corrosion resistance containing less than 0.02 % carbon, 0.05-0.8 %
silicon, less than 0.5 % manganese, 20-24 % chromium, less than 0.5 % nickel,
0.3-0.8 % copper, less than 0.02 % nitrogen, 0.20-0.55 % niobium, less than
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
3
0.1 % aluminium and the balance being iron and inevitable impurities. In this
ferritic stainless only niobium is used in the stabilization of carbon and
nitrogen.
The WO publication 2012046879 relates to a ferritic stainless steel to be used
for a separator of a proton-exchange membrane fuel cell. A passivation film is
formed on the surface of the stainless steel by immersing the stainless steel
in
a solution containing mainly hydrofluoric acid or a liquid mixture of
hydrofluoric
acid and nitric acid. The ferritic stainless steel contains carbon, silicon,
manganese, aluminium, nitrogen, chromium and molybdenum in addition to iron
as the necessary alloying elements. All other alloying elements described in
the reference WO 2012046879 are optional. As described in the examples of
this WO publication the ferritic stainless steel having a low carbon content
is
produced by vacuum smelting, which is a very expensive manufacturing
method.
EP1083241 describes niobium stabilized ferritic chromium steel strip, produced
from a steel having specified molybdenum, silicon and tin contents and
containing a cubic iron-niobium phase as the sole intermetallic phase at high
temperature. A niobium stabilized ferritic 14% chromium steel strip is
produced
from a steel of composition (by wt.) 0.02% C, 0.002-0.02% N, 0.05-1% Si,
greater than 0 to 1% Mn, 0.2-0.6% Nb, 13.5-16.5% Cr, 0.02-1.5% Mo, greater
than 0 to 1.5% Cu, greater than 0 to 0.2% Ni, greater than 0 to 0.020% P,
greater than 0 to 0.003% S, greater than 0.005 to 0.04% Sn, balance Fe and
impurities, the Nb, C and N contents satisfying the relationship Nb/(C + N)
9.5, by: (a) reheating before hot rolling at 1150-1250 (preferably 1175)
degrees
C; (b) coiling at 600-800 (preferably 600) degrees C; (c) cold rolling,
optionally
after pre-annealing; and (d) final annealing at 800-1100 (preferably 1050)
degrees C for 1-5 (preferably 2) min. An independent claim is also included
for
a niobium stabilized 14% chromium ferritic steel sheet obtained by the above
process.
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
4
EP1170392 describes ferritic stainless steel comprising all three of Co, V,
and
B, having a Co content of about 0.01 mass% to about 0.3 mass%, a V content
of about 0.01 mass% to about 0.3 mass%, and a B content of about 0.0002
mass% to about 0.0050 mass%, and having superior secondary working
embrittleness resistance and superior high temperature fatigue
characteristics.
Further components are (in mass%): 0.02% or less of C, 0.2 to 1.0% Si, 0.1 to
1.5% Mn, 0.04% or less of P, 0.01% or less of S, 11.0 to 20.0% of Cr, 0.1 to
1.0% Ni, 1.0 to 2.0% Mo, 1.0% or less of Al, 0.2 to 0.8% of Nb, 0.02% or less
of
N and optionally 0.05 to 0.5% Ti, Zr or Ta, 0.1 to 2.0% Cu, 0.05 to 1.0% W,
0.001 to 0.1% Mg and 0.0005 to 0.005% Ca.
US patent 4726853 concerns a strip or sheet of ferritic stainless steel,
usually
in the annealed state, the final annealing operation then being followed in
most
cases by a finishing and cold-working pass or "skin pass", producing a degree
of elongation of less than 1%, intended in particular for the production of
exhaust pipes and manifolds. The composition of the strip or sheet is as
follows
(% by weight):
(C+N)<0.060-Si<0.9-Mn<1
Cr 15 to 19-Mo<1-Ni<0.5-Ti<0.1-Cu<0.4-S<0.02-P<0.045
Zr=0.10 to 0.50 with Zr between 7 (C+N)-0.1 and 7 (C+N)+0.2 Nb between 0.25
and 0.55 if Zr7 (C+N) and between 0.25+7 (C+N)-Zr and 0.55+7 (C+N)-Zr if
Zr<7 (C+N)
Al 0.020 to 0.080; other elements and Fe: balance.
EP0478790 describes a heat-resistant ferritic stainless steel improved in low-
temperature toughness, prevented from undergoing high-temperature weld
cracking, and useful as a material of a passage of automobile exhaust gas,
particularly a passage exposed to high temperature between an engine and a
converter, which steel comprises up to 0.03 % of carbon, 0.1 to 0.8 % of
silicon,
0.6 to 2.0 % of manganese, up to 0,006 % of sulfur, up to 4 % of nickel, 17.0
to
25.0 % of chronium, 0.2 to 0.8 % of niobium, 1.0 to 4.5 % of molybdenum, 0.1
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
to 2.5 % of copper, up to 0.03 % of nitrogen, and optionally a necessary
amount of at least one of aluminum, titanium, vanadium, zirconium, tungsten,
boron and REM, wherein the manganese to sulfur ratio is 200 or above, [Nb] =
Nb % - 8(C % + N %) 0.2,
5 and Ni % + Cu % 4,
the balance being iron and inevitable impurities in the production process.
EP2557189 describes ferritic stainless steel sheet for an exhaust part which
has little deterioration in strength even if undergoing long term heat history
and
is low in cost, excellent in heat resistance and workability characterized by
containing, characterized by containing, by mass%, C: less than 0.010%, N:
0.020% or less, Si: over 0.1% to 2.0%, Mn: 2.0% or less, Cr: 12.0 to 25.0%,
Cu:
over 0.9 to 2%, Ti: 0.05 to 0.3%, Nb: 0.001 to 0.1%, Al: 1.0% or less, and B:
0.0003 to 0.003%, having a Cu/(Ti+Nb) of 5 or more, and having a balance of
Fe and unavoidable impurities.
The object of the present invention is to eliminate some drawbacks of the
prior
art and to achieve a ferritic stainless steel having good corrosion
resistance,
improved weldability and enhanced high temperature strength, which steel is
stabilized by niobium, titanium and vanadium and is produced using AOD
(Argon-Oxygen-Decarburization) technology. The essential features of the
present invention are enlisted in the appended claims.
The chemical composition of the ferritic stainless steel according to the
invention consists of in weight % 0.003 - 0.035 % carbon, 0.05- 1.0% silicon,
0.10 - 0.8 % manganese, 18 -24 % chromium, 0.05 - 0.8 % nickel, 0.003 -2.5
% molybdenum, 0.2 - 0.8 % copper, 0.003 - 0.05 % nitrogen, 0.05 - 1.0 %
titanium, 0.05 - 1.0 % niobium, 0.03 - 0.5 % vanadium, 0.01 - 0.04 %
aluminium, and the sum C+N less than 0.06 %, the rest being iron and evitable
impurities occupying in stainless steels, in such conditions that the sum of
(C+N) is less than 0.06 % and the ratio (Ti+Nb)/(C+N) is higher or equal to 8,
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
6
and less than 40, and the ratio (Ti + 0.515*Nb +0.940*V)/(C+0.858*N) is higher
or equal to 6, and less than 40, and 5.8*Nb + 5*Ti*Si is higher or equal to
3.3.
The ferritic stainless steel according to the invention is produced using AOD
(Argon-Oxygen-Decarburization) technology.
The effects and the content, in weight % if nothing else mentioned, of each
alloying element are discussed in the following:
Carbon (C) decreases elongation and r-value and, preferably, carbon is
removed as much as possible during the steel making process. The solid-
solution carbon is fixed as carbides by titanium, niobium and vanadium as
described below. The carbon content is limited to 0.035 %, preferably to 0.03
%, but having at least of 0.003 % carbon.
Silicon (Si) is used to reduce chromium from slag back to melt. Some silicon
remainders in steel are necessary to make sure that reduction is done well. In
the solid solution, silicon boosts formation of Laves phases and stabilizes
Laves phase particles at higher temperatures. Therefore, the silicon content
is
less than 1.0 %, but at least 0.05 %.
Manganese (Mn) degrades the corrosion resistance of ferritic stainless steel
by
forming manganese sulphides. With low sulphur (S) content the manganese
content is less than 0.8 %, preferable less than 0.65 %, but at least 0.10 %.
Chromium (Cr) enhances oxidation resistance and corrosion resistance. In
order to achieve corrosion resistance comparable to steel grade EN 1.4301,
the chromium content must be 18 ¨ 24 %, preferably 20 ¨ 22 %.
Nickel (Ni) is an element favourably contributing to the improvement of
toughness, but nickel has sensitivity to stress corrosion cracking (SCC). In
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
7
order to consider these effects the nickel content is less than 0.8 %,
preferably
less than 0.5 % so that the nickel content is at least 0.05 %.
Molybdenum (Mo) enhances corrosion resistance but reduces elongation to
fracture. The molybdenum content is less than 2.5 %, but at least 0.003%. For
applications in highly corrosive environments with low acidic pH-values the
molybdenum content is preferably less than 2.5% but at least 0.5%. For
applications in less corrosive environments with neutral or high pH-values >4,
the more preferable range is 0.003% - 0.5% molybdenum.
Copper (Cu) improves corrosion resistance in acidic solutions, but high copper
content can be harmful. The copper content is thus less than 0.8 %, preferably
less than 0.5 %, but at least 0.2 %.
Nitrogen (N) reduces elongation to fracture. The nitrogen content is less than
0.05 %, preferably less than 0.03 %, but at least 0.003 %.
Aluminium (Al) is used to remove oxygen from melt. The aluminium content is
less than 0.04 %.
Titanium (Ti) is very useful because it forms titanium nitrides with nitrogen
at
very high temperatures. Titanium nitrides prevent grain growth during
annealing and welding. In welds, titanium alloying promotes the formation of
equiaxed, fine grained structure. Titanium is the cheapest element of chosen
stabilization elements titanium, vanadium and niobium. Therefore, using
titanium for stabilization is an economic choice. The titanium content is less
than 1.0 %, but at least 0.05 %. The more preferable range is 0.07% - 0.40%
titanium.
Niobium (Nb) is used to some extent to bind carbon to niobium carbides. With
niobium the recrystallization temperature can be controlled. Niobium
stimulates
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
8
precipitation of Laves phases particles and has positive effect their
stability at
high temperatures. Niobium is the most expensive element of chosen
stabilization elements titanium, vanadium and niobium. The niobium content is
less than 1.0 %, but at least 0.05 %.
Vanadium (V) forms carbides and nitrides at lower temperatures. These
precipitations are small and major part of them is usually inside grains.
Amount
of vanadium needed to carbon stabilization is only about half of amount of
niobium needed to same carbon stabilization. This is because vanadium atomic
weight is only about a half of niobium atomic weight. Vanadium is economic
choice for stabilization element since vanadium is cheaper than niobium.
Vanadium also improves toughness of steel. The vanadium content is less than
0.5 %, but at least 0.03 % preferably 0.03 - 0.20 %.
The invention is described below in further detail with reference to the
attached
drawings, of which
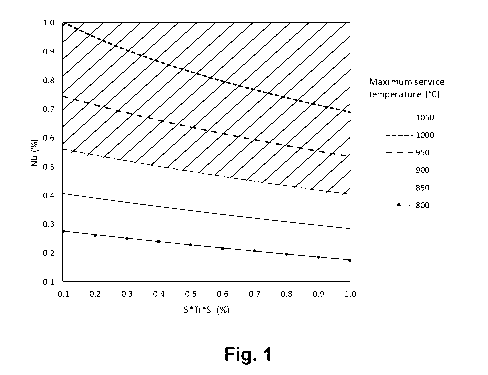
Figure 1 is a graph showing the combination of Ti, Nb and Si content,
resulting
in enhanced high temperature mechanical properties in a material according to
the present invention,
Figure 2 is a micrograph showing a typical microstructure used for determining
the chemical composition of Laves phase particles by energy dispersive
spectrometry (EDS),
Figure 3 is a micrograph showing a coarse-grained, columnar structure formed
in the weld in autogenous welding when the steel does not have a sufficient
amount of titanium, (a) cross-section transverse to the weld, and (b) cross-
section in the plane of welded sheet, and
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
9
Figure 4 is a micrograph of a fine-grained, equiaxed structure formed in the
weld in autogenous welding when the steel has a sufficient amount of titanium.
Using all three stabilization elements, titanium, niobium and vanadium in the
ferritic stainless steel according to the invention, it is possible to achieve
an
atomic lattice which is practically interstitially free. That means that
essentially
all carbon and nitrogen atoms are bound with stabilization elements. When a
sufficient amount of titanium is used in the stabilization of the interstitial
elements carbon and nitrogen, the compounds formed during the stabilization,
such as TiN, promote formation of equiaxed and fine grain structure in welds.
The equiaxed, fine grained structure improves the ductility and toughness of
welds. A sufficient titanium content therefore prevents forming coarse
columnar
structure in welds. Columnar grains can cause hot cracking as impurities could
segregate to the weld centreline. Large columnar grains can also decrease the
toughness of the weld. Using additionally sufficient Ti, Si and Nb content, it
is
possible to achieve ferritic stainless steel with enhanced mechanical
properties
at high temperatures. The combinations of Ti, Nb and Si contents resulting in
enhanced high temperature mechanical properties in the present invention are
shown in figure 1. The region is determined by having 5.8*Nb + 5*Ti*Si greater
than or equal to 3.3.
Several stainless steel alloys were prepared for testing the ferritic
stainless
steel of the invention. During the preparation every alloy was melted, cast
and
hot-rolled. The hot-rolled plate was further annealed and pickled before cold-
rolling. Then the cold-rolled sheet at the final thickness was again annealed
and pickled. The table 1 further contains the chemical compositions of the
reference materials EN 1.4509 and EN 1.4622.
Alloy C Si Mn P 5 Cr Ni Mo Ti Nb Cu
V Al
A 0.018 0.41 0.34 0.03 0.001 20.9 0.2 0.0 0.22 0.62 0.41 0.05 0.03 0.02
0.021 0.43 0.33 0.03 0.001 20.9 0.2 0.0 0.25 0.78 0.38 0.05 0.03 0.02
0.021 0.59 0.32 0.03 0.001 20.7 0.2 0.0 0.27 0.78 0.38 0.06 0.04 0.02
0.020 0.75 0.33 0.03 0.001 20.8 0.2 0.0 0.27 0.78 0.38 0.06 0.03 0.02
0.024 0.71 0.32 0.03 0.001 21.0 0.2 0.0 0.20 0.81
0.41 0.05 0.03 0.02
0.020 0.58 0.32 0.03 0.001 20.9 0.2 0.0 0.19 0.96 0.39 0.06 0.03 0.02
0.019 0.59 0.31 0.03 0.001 20.8 0.2 1.0 0.22 0.81 0.39 0.06 0.03 0.02
0.020 0.59 0.30 0.03 0.001 20.9 0.2 2.0 0.20 0.79 0.38 0.06 0.03 0.02
EN 1.4509 0.015 0.5 0.5 0.03 0.001 18.0 0.2 0
0.12 0.4 0.2 0 0 0.02
1-d
EN 1.4622 0.015 0.5 0.4 0.03 0.001 20.8 0.2 0
0.17 0.4 0.4 0.07 0 0.02
Table 1: Chemical compositions
1-d
cio
c:,
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
11
From table 1 it is seen that the alloy A has smaller amount of niobium and
silicon compared to the other alloys from B to H. The alloys B, C and D have
the same amount of niobium, while the amount of silicon is increasing
gradually
from the alloy B to C and to alloy D. The alloy E has essentially the same
chemical composition as the alloy D except for small variations in the amounts
of silicon, titanium and niobium. The alloy F has essentially the same amount
of
silicon as the alloy C, while the niobium content of alloy F is the highest
among
all alloys from A to H. The alloys G and H contain also molybdenum in addition
to silicon, titanium and niobium. All alloys A - H are triple stabilized with
titanium, niobium and vanadium in accordance with the invention.
When using niobium, titanium and vanadium in the stabilization of the
interstitial elements carbon and nitrogen in the ferritic stainless steel of
the
invention, the compounds which are generated during the stabilization, are
such as titanium carbide (TiC), titanium nitride (TiN), niobium carbide (NbC),
niobium nitride (NbN), vanadium carbide (VC) and vanadium nitride (VN). In
this stabilization a simple formula is used to evaluate the amount and the
effect
of stabilization as well as the role of the different stabilization elements.
The connection between the stabilization elements titanium, niobium and
vanadium is defined by a formula (1) for a stabilization equivalent (Tieq)
where
the content of each element is in weight A:
Tieq = Ti + 0.515*Nb + 0.940*V (1)
Respectively, the connection between of the interstitial elements carbon and
nitrogen is defined by a formula (2) for an interstitial equivalent (Cep)
where the
contents of carbon and nitrogen are in weight A:
Cep = C 0.858*N (2)
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
12
The ratio Tieq/Ceq is used as one factor for determining the disposition for
sensitization, and the ratio Tieq/Ceq is higher or equal to 6 and the ratio
(Ti+Nb)/(C+N) higher or equal to 8 for the ferritic stainless steel of the
invention
in order to avoid the sensitization. The EP patent EP292278B gives additional
information regarding sensitization to grain boundary corrosion. In this
document it is shown that stabilization against intergranular corrosion is
successful if Tieq/Ceq is higher or equal to 6 and (Ti+Nb)/(C+N) higher or
equal
to 8.
The enhanced high temperature strength of invented steel is ensured by fine
dispersion of thermodynamically stable Laves phase particles. The alloying of
Nb, Ti and Si must be carefully balanced in order to obtain an optimal
microstructure for high service temperatures. The correct alloying promotes
precipitation of Laves phase particles and raises their dissolution
temperature.
The Laves phase particles are formed quickly in exposure to temperatures in
the range from 650 to 850 C. Figure 2 illustrates intergranular and
intragranular precipitates observed in the alloys A to H when the material was
exposed to the temperature of 800 C for 30 minutes. Chemical composition of
precipitated particles was determined by means of by energy dispersive
spectrometry (EDS). The results in table 2 reveal that particles formed in the
steel of invention are Laves phase precipitates. According to table 2, the
chemical composition of precipitated particles in the steel of invention
follows
the model A2B, where A is a combination of Fe and of Cr and B is a
combination of Nb, Si and Ti. According to EDS measurements given in table
2, the chemical formula of the Laves phase particles is
(Fea8Cro.2)2(Nbo.705i0.25Tio.05). The number of Fe, Cr, Nb, Si and Ti atoms in
the
molecule depend on alloying and on heat cycles experienced by the material.
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
13
EDS chemical composition (at.%)
Analysis point Fe Nb Cr Si Ti
1 52.6 24.9 10.6 8.9 1.5
2 52.3 25.1 10.5 8.9 1.6
3 53.5 23.8 11.1 8.6 1.5
4 52.7 24.8 10.6 8.8 1.6
52.6 23.0 10.5 8.6 1.5
6 52.9 24.8 10.6 8.8 1.5
7 53.0 24.5 10.7 8.7 1.7
8 53.0 24.3 10.9 8.8 1.6
9 52.3 24.5 10.8 8.8 1.8
52.7 24.5 10.7 9.0 1.7
Average 52.8 24.4 10.7 8.8 1.6
Table 2: Chemical composition of 10 Laves phase particles in the steel of the
invention according to energy dispersive spectrometry (EDS).
5
A balanced combination of silicon, niobium and titanium ensures that the steel
contains sufficient amount Laves phase particles in high service temperatures
above 900 C. The connection between the Laves phase forming elements
titanium, niobium and silicon is defined by a formula (3) for a Laves phase
10 equivalent number Lep where the content of each element is in weight VO:
Lep = 5.8*Nb + 5*Ti*Si (3)
The Laves phase equivalent number Lep is higher or equal to 3.3 for the
ferritic
stainless steel of the invention in order to guarantee enhanced high
temperature strength properties. Laves phase equivalent corresponds to the
lower boundary of the region indicated order to guarantee enhanced high
temperature strength properties. For higher service temperatures above 950 C,
Laves phase equivalent number Lep is higher or equal to 4.5.
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
14
The values for ratios Tieq/Ceq, (Ti+Nb)/(C+N) and the value of equivalent Lep
are
calculated in table 3 for the alloys A to H. The values of table 3 show that
the
alloys A - H and the reference materials have favourable values for both the
ratios Tieq/Ceq and (Ti+Nb)/(C+N). Instead, only the alloys A - H, have
favourable values for the Laves phase equivalent number Lep in accordance
with the invention.
Alloy Tieq/Ceq (Ti+Nb)/(C+N) Lep
Invention A 16.6 22.0 4.0
18.6 25.6 5.1
19.3 25.9 5.3
20.2 27.1 5.5
16.0 22.8 5.5
20.0 28.9 6.1
20.0 27.7 5.3
19.3 27.0 5.2
Reference EN 1.4509 10.1 14.9 2.3
EN 1.4622 11.7 16.3 2.3
Table 3: Values for the ratios Tieq/Ceq, (Ti+Nb)/(C+N) and the Laves phase
equivalent number Lep.
The dissolution of precipitated Laves phase determines the upper limit for the
service temperature for the ferritic stainless steels of the invention. The
dissolution temperature was calculated using thermodynamic simulation
software Thermo-Calc version 2018b for the alloys of table 1. The results are
presented in table 4. The values for the dissolution temperature are
favourable
and above the target service temperature of 900 C for the alloys A - H. The
dissolution temperatures are unfavourably below the target temperature of
900 C for the reference materials.
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
Alloy Tsol ( C)
Invention A 929
986
1003
1013
1009
1039
1009
1009
Reference EN 1.4509 849
EN 1.4622 839
Table 4: The temperature at which the strengthening Laves phase particles
dissolve under sustained exposure. A value above T=900 C is considered
5 satisfactory.
The elevated temperature tensile strength of all alloys listed in the table 1
was
determined according to the elevated temperature tensile testing standard EN
ISO 10002-5. The results for tests performed at T=950 C and T=1000 C are
10 presented in table 5.
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
16
All Rm at 950 C Rm at 1000 C
oy
(MPa) (MPa)
Invention A 31 25
34 26
32 27
33 26
31 22
31 28
41 32
47 36
Reference EN 1.4509 26 18
EN 1.4622 24 18
Table 5: The tensile strength measured according to EN ISO 12002-5. Rm
value above 30 MPa at 950 C and above 20 MPa at 1000 C is considered
satisfactory.
The mechanical strength Rm is considered insufficient when Rm < 30MPa at
950 C or Rm < 20 MPa at 1000 C. The results in the table 5 show that the
steels in accordance with the invention satisfy these requirements whereas the
reference materials EN 1.4509 and EN 1.4622 do not satisfy these
requirements.
As corrosion resistance is the most important property of stainless steel, the
pitting corrosion potential of all the alloys listed in the table 1 was
determined
potentiodynamically. The alloys were wet ground with 320 mesh and allowed to
repassivate in air at ambient temperature for at least 24 hours. The pitting
potential measurements were done in naturally aerated aqueous 1.2 wt-%
NaCI-solution (0.7 wt-% Cl-, 0.2 M NaCI) at room temperature of about 22 C.
The polarization curves were recorded at 20 mV/min using crevice-free flushed-
port cells (Avesta cells as described in ASTM G150) with an electrochemically
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
17
active area of about 1 cm2. Platinum foils served as counter electrodes. KCI
saturated calomel electrodes (SCE) were used as reference electrodes. The
average value of six breakthrough pitting potential measurements for each
alloy was calculated and is listed in table 2.
The results in table 6 show that the ferritic stainless steel of the invention
has
better pitting corrosion potential than the reference steel EN 1.4509. The
pitting
corrosion potential of the alloys A ¨ F is essentially in the same with the
reference steel EN 1.4622, whereas the pitting corrosion potential of Mo-
alloyed alloys G and H is superior to that of the reference material EN
1.4622.
All Corrosion
oy
potential (mV)
Invention A 428
452
465
484
465
486
659
1000
Reference EN 1.4509 303
EN 1.4622 411
Table 6: Pitting corrosion potential for the alloys A ¨ H and for the
reference
materials.
The equiaxial, fine grained structure of welds is ensured if a sufficient
amount
of titanium is used for stabilization. The compounds formed by titanium in the
liquid weld metal, such as TiN, act as nucleation sites for heterogenous
solidification resulting in equiaxed, fine grained structure in welds. The
other
elements used for stabilization, vanadium and niobium, do not form compounds
CA 03122043 2021-06-03
WO 2020/127275 PCT/EP2019/085663
18
that will act as nucleation sites in the liquid metal. Therefore, a coarse-
grained
weld with columnar grain structure results if the amount of titanium is not
sufficiently high enough. The coarse-grained, columnar structure can cause hot
cracking as impurities may segregate to the weld centreline. Large columnar
grains also decrease the toughness of the weld. The problem is particularly
serious in autogenous welding, where the chemical composition of weld metal
cannot be changed by the welding additives. The influence of the stabilization
method on the weld structure is well-known and is discussed in detail for
example in the journal article published by W. Gordon and A. Van Bennecom
(W. Gordon & A. van Bennekom. Review of stabilisation of ferritic stainless
steels. Materials Science and Technology, 1996. Vol. 12, no. 2, pp. 126-131).
Figure 3 shows an illustrative example of coarse-grained, columnar weld
structure obtained in autogenous welding when insufficient amount of titanium
is alloyed in the steel. Figure 4 shows an example of fine-grained, equiaxial
weld structure obtained in autogenous welding when sufficient amount of
titanium was alloyed in the steel. The alloys A-H according to the invention
and
the reference materials EN 1.4509 and 1.4622 have favourable amount of
titanium in order to produce fine-grained equiaxial weld structure in
autogenous
welding.