Note: Descriptions are shown in the official language in which they were submitted.
1
MEDICAL ASSEMBLY INCLUDING FORCE-LIMITING DEVICE
TECHNICAL FIELD
[01] This document relates to the technical field of (and is not limited
to) an elongated
medical assembly including a force-limiting device (and method therefor).
BACKGROUND
[02] Known medical devices are configured to facilitate a medical procedure
and help
healthcare providers diagnose and/or treat medical conditions of sick
patients.
SUMMARY
[03] It will be appreciated that there exists a need to mitigate (at least
in part) at least one
problem associated with the existing (known) elongated medical assemblies
(also called
the existing technology). After much study of, and experimentation with, the
existing
(known) elongated medical assemblies, an understanding (at least in part) of
the problem
and its solution have been identified (at least in part) and are articulated
(at least in part) as
follows:
[04] During known transseptal catheterization procedures, physicians
identify the ideal
location to cross from the right atrium to the left atrium (of the heart) by
first tracking a
known medical device into the superior vena cava (of the heart) and then
dropping down
the known medical device onto the fossa ovalis. In order to do this procedure,
the known
medical device must have enough reach to make physical contact with the septum
(a
biological wall) while also contacting the free wall of the right atrium.
These two points of
contact (in the right atrium) may be utilized for the application of a tenting
force for
tenting the fossa ovalis, thereby allowing the physician the ability to cross
into the left
atrium. Without contacting the septum, crossing into the left atrium cannot
occur. Further,
insufficient contact with the septum may reduce the certainty and control in
the location of
the crossing (through the septum), and too much contact with the septum may
place
excessive force onto the septum, resulting in trauma and/or unintended
mechanical
puncture of the septum. In order to ensure an ideal amount of reach for the
known medical
device, the physician must precisely shape the known medical device before the
known
medical device is inserted into the patient (that is, while the known medical
device is
positioned outside of the patient); this may not always work out as intended
by the
physician resulting in removal of the known medical device from the interior
of the
patient, reshaping the known medical device, reinserting the known medical
device and
then reattempting the procedure (which is a waste of valuable procedure time
and/or may
lead to unwanted damage, etc.).
Date Recue/Date Received 2021-06-21
2
[05] In view of the above issues, it may be desirable to provide a medical
device having an
adjustment mechanism configured to adjust the reach (preferably this is done
automatically) while the medical device is utilized for applying and/or
maintaining a
contact force onto the septum (or for that matter a force that may be applied
to any
biological feature that the medical device might make contact with); in this
manner, the
adjustment mechanism for adjusting the reach (of the distal tip of the medical
device) may
provide (preferably, but not necessarily) a reduction in the need for the
physician to rely on
their professional estimation skills to adjust an amount of reach for the
distal tip of the
medical accessory (while the medical device is moved through the patient
toward a target
biological feature). In this manner, it may be possible to reduce the
application of a contact
force from the distal tip of the medical device to the biological wall while
the distal tip of
the medical device is moved toward the target biological wall of the patient.
[06] During known epicardial access procedures, physicians must pierce the
exterior
pericardium layer surrounding the heart while avoiding puncture of the
underlying
myocardium layer (also called the heart muscle tissue). The pericardium layer
surrounds
the heart similar to a sac. There are sites located on the underlying
epicardial surface of the
heart (that is, underneath the pericardium layer) that are a target for
catheter-based
therapies. This requires a delicate access procedure that may be analogous to
piercing
through a piece of saran wrap covering a steak without damaging the steak
itself. To add to
the complexity of the procedure, the heart is beating while the physician is
attempting to
gain access using their instruments. Placing too much force on the exterior
surface of the
pericardium layer if the heart with an access tool might inadvertently result
in unwanted
mechanical puncture of the myocardium layer, excessive ischemia to heart
muscle tissue,
and/or arrhythmias. Too little force applied onto the exterior surface of the
pericardium
layer might result in difficulties obtaining access and/or a lack of tactile
feedback provided
back to the physician (via their instruments).
[07] It may be desirable to provide a medical device having a distal tip
configured to
facilitate contact with a biological feature (such as the heart) and possess a
force-control
system (such as a dampening system, etc.) configured to ensure that excessive
force is not
placed on (applied to) the heart from the medical device, as the heart beats
and/or as the
physician manipulates the medical device for the application of force to the
exterior
pericardium layer of the heart (or other biological features while the medical
device is
moved, and positioned, toward a target biological feature).
Date Recue/Date Received 2021-06-21
3
[08] To mitigate, at least in part, at least one problem associated with
the existing
technology, there is provided (in accordance with a major aspect) an
apparatus. The
apparatus is for use with (is configured to use with) a biological feature of
a patient. The
apparatus includes and is not limited to (comprises) an elongated medical
assembly
including a distal section having a distal tip configured to be maneuvered to
contact, at
least in part, the biological feature of the patient. A force-limiting device
is positioned
proximate to, and is interactable with, the distal section. The force-limiting
device is
configured to limit, at least in part, an amount of a force to be applied from
the distal tip of
the distal section to the biological feature after (or once) the distal tip of
the distal section,
in use, contacts, at least in part, the biological feature of the patient. In
accordance with an
option, the force-limiting device includes a proximal section positioned
proximate to the
distal section, and being aligned with the distal section along a common axis
extending
between the proximal section and the distal section. The proximal section and
the distal
section are in fluid communication with each other. The force-limiting device
is
configured to maintain, at least in part, contact with the biological feature
of the patient
while the distal section is distally movable relative to the proximal section.
[09] To mitigate, at least in part, at least one problem associated with
the existing
technology, there is provided (in accordance with a major aspect) a method.
The method is
for using a distal section having a distal tip of an elongated medical
assembly for
contacting a biological feature of a patient. The method includes and is not
limited to
(comprises) maneuvering a distal section having a distal tip of an elongated
medical
assembly to contact, at least in part, the biological feature of the patient.
The method also
includes using a force-limiting device being positioned proximate to, and
being
interactable with, the distal section, for limiting, at least in part, an
amount of a force to be
applied from the distal tip of the distal section to the biological feature
after (or once) the
distal tip of the distal section, in use, contacts, at least in part, the
biological feature of the
patient.
[010] Other aspects are identified in the claims. Other aspects and
features of the non-
limiting embodiments may now become apparent to those skilled in the art upon
review of
the following detailed description of the non-limiting embodiments with the
accompanying
drawings. This Summary is provided to introduce concepts in simplified form
that are
further described below in the Detailed Description. This Summary is not
intended to
identify potentially key features or possible essential features of the
disclosed subject
matter, and is not intended to describe each disclosed embodiment or every
Date Recue/Date Received 2021-06-21
4
implementation of the disclosed subject matter. Many other novel advantages,
features,
and relationships will become apparent as this description proceeds. The
figures and the
description that follow more particularly exemplify illustrative embodiments.
BRIEF DESCRIPTION OF THE DRAWINGS
[011] The non-limiting embodiments may be more fully appreciated by reference
to the
following detailed description of the non-limiting embodiments when taken in
conjunction
with the accompanying drawings, in which:
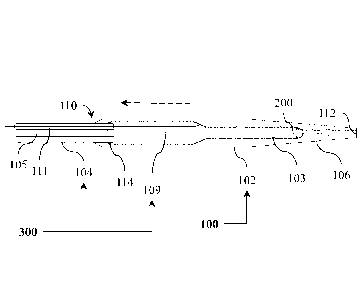
[012] FIG. 1 to FIG. 4 depict side views (FIG. 1 and FIG. 2) and cross-
sectional views
(FIG. 3 and FIG. 4) of embodiments of an elongated medical assembly; and
[013] FIG. 5 and FIG. 6 depict cross-sectional views of embodiments of the
elongated
medical assembly of FIG. 1; and
[014] FIG. 7 to FIG. 10 depict cross-sectional views of embodiments of the
elongated
medical assembly of FIG. 1; and
[015] FIG. 11 to FIG. 13 depict side views of embodiments of the elongated
medical
assembly of FIG. 1; and
[016] FIG. 14 and FIG. 15 depict side views of embodiments of the elongated
medical
assembly of FIG. 1; and
[017] FIG. 16 and FIG. 17 depict side views of embodiments of the elongated
medical
assembly of FIG. 1.
[018] The drawings are not necessarily to scale and may be illustrated by
phantom lines,
diagrammatic representations and fragmentary views. In certain instances,
details
unnecessary for an understanding of the embodiments (and/or details that
render other
details difficult to perceive) may have been omitted. Corresponding reference
characters
indicate corresponding components throughout the several figures of the
drawings.
Elements in the several figures are illustrated for simplicity and clarity and
have not been
drawn to scale. The dimensions of some of the elements in the figures may be
emphasized
relative to other elements for facilitating an understanding of the various
disclosed
embodiments. In addition, common, and well-understood, elements that are
useful in
commercially feasible embodiments are often not depicted to provide a less
obstructed
view of the embodiments of the present disclosure.
[019] LISTING OF REFERENCE NUMERALS USED IN THE DRAWINGS
medical assembly 100
Date Recue/Date Received 2021-06-21
5
movement length 101 applied force 302
distal section 102 biological feature 900
distal lumen 103 patient 902
proximal section 104 heart 903
proximal lumen 105 inferior vena cava 904
distal tip 106 right atrium 906
biasing device 108 superior vena cava 908
wire 109 ascending aorta 910
entrance portal 110 left atrium 912
control-wire lumen 111 descending aorta 914
exit portal 112 interatrial septum 916
leading portion 114 diaphragm 918
common axis 200 liver 920
movement direction 201 diastole condition 922
force-limiting device 300
DETAILED DESCRIPTION OF THE NON-LIMITING EMBODIMENT(S)
[020] The following detailed description is merely exemplary and is not
intended to limit
the described embodiments or the application and uses of the described
embodiments. As
used, the word "exemplary" or "illustrative" means "serving as an example,
instance, or
illustration." Any implementation described as "exemplary" or "illustrative"
is not
necessarily to be construed as preferred or advantageous over other
implementations. All
of the implementations described below are exemplary implementations provided
to
enable persons skilled in the art to make or use the embodiments of the
disclosure and are
not intended to limit the scope of the disclosure. The scope of the disclosure
is defined by
the claims. For the description, the terms "upper," "lower," "left," "rear,"
"right," "front,"
"vertical," "horizontal," and derivatives thereof shall relate to the examples
as oriented in
the drawings. There is no intention to be bound by any expressed or implied
theory in the
preceding Technical Field, Background, Summary or the following detailed
description. It
is also to be understood that the devices and processes illustrated in the
attached drawings,
and described in the following specification, are exemplary embodiments
(examples),
aspects and/or concepts defined in the appended claims. Hence, dimensions and
other
physical characteristics relating to the embodiments disclosed are not to be
considered as
limiting, unless the claims expressly state otherwise. It is understood that
the phrase "at
Date Recue/Date Received 2021-06-21
6
least one" is equivalent to "a". The aspects (examples, alterations,
modifications, options,
variations, embodiments and any equivalent thereof) are described regarding
the drawings.
It should be understood that the disclosure is limited to the subject matter
provided by the
claims, and that the disclosure is not limited to the particular aspects
depicted and
described. It will be appreciated that the scope of the meaning of a device
configured to be
coupled to an item (that is, to be connected to, to interact with the item,
etc.) is to be
interpreted as the device being configured to be coupled to the item, either
directly or
indirectly. Therefore, "configured to" may include the meaning "either
directly or
indirectly" unless specifically stated otherwise.
[021] FIG. 1 to FIG. 4 depict side views (FIG. 1 and FIG. 2) and cross-
sectional views
(FIG. 3 and FIG. 4) of embodiments of an elongated medical assembly 100. The
cross-
sectional views of FIG. 3 and FIG. 4 are taken along a cross-sectional line A-
A of FIG. 2.
[022] Referring to the embodiments as depicted in FIG. 1 to FIG. 4 (and
generally
applicable to all of the embodiments), the elongated medical assembly 100
includes a
distal section 102 having a distal tip 106 configured to be maneuvered to
contact, at least
in part, the biological feature 900 of the patient 902. A force-limiting
device 300 is
positioned proximate to, and is interactable with, the distal section 102. The
force-limiting
device 300 is configured to limit, at least in part, an amount of a force 302
(contact force)
to be applied from the distal tip 106 of the distal section 102 to the
biological feature 900
after (or once) the distal tip 106 of the distal section 102 contacts, at
least in part, the
biological feature 900 of the patient 902. An advantage of an embodiment of
the force-
limiting device 300 may include adjustment (reduction) of the application of
the force 302
from the distal tip 106 to the biological feature 900 while the distal tip 106
of the elongated
medical assembly 100 is moved along and toward a targeted biological wall (as
depicted in
FIG. 11 to FIG. 13). In this manner, an amount of the force 302 applied to
other biological
features might be kept to a minimum so as to avoid inadvertent damage to these
biological
features (that might be encountered) while the distal tip 106 of the elongated
medical
assembly 100 is moved along and toward the targeted biological wall (as
depicted in FIG.
11 to FIG. 13). For instance, an embodiment of the elongated medical assembly
100 may
be utilized for positioning a puncture device (known and not depicted) for
performing a
puncturing action for the formation of a puncture site (and/or dilating the
puncture site),
for the purpose of providing access to the left atrium of the heart during a
transseptal
catheterization procedure (along with an introducer assembly used for
epicardial access
procedures, if so desired). The elongated medical assembly 100 is configured
to provide a
Date Recue/Date Received 2021-06-21
7
force dampening effect (for instance, when used in the context of epicardial
access, and
not limited thereto, etc.) when the elongated medical assembly 100 is
manipulated by the
physician and/or is in contact with a beating heart, etc. In accordance with
an option, the
force-limiting device includes a proximal section positioned proximate to the
distal
section, and being aligned with the distal section along a common axis
extending between
the proximal section and the distal section. The proximal section and the
distal section are
in fluid communication with each other. The force-limiting device is
configured to
maintain, at least in part, contact with the biological feature of the patient
while the distal
section is distally movable relative to the proximal section.
[023] Referring to the embodiments as depicted in FIG. 1 to FIG. 4 (and
generally
applicable to all of the embodiments), the elongated medical assembly 100
includes
biocompatible material properties for desired performance (such as dielectric
strength,
thermal performance, electrical and/or thermal insulation, corrosion
resistance, water
resistance, heat resistance, etc.), for safe performance, for compliance with
industrial and
regulatory safety standards (or compatible for medical usage), etc. Reference
is made to
the following publication for consideration in the selection of a suitable
material: Plastics
in Medical Devices: Properties, Requirements, and Applications; 2nd Edition;
author:
Vinny R. Sastri; hardcover ISBN: 9781455732012; published: 21 November 2013;
publisher: Amsterdam [Pay s-B as]: Elsevier! William Andrew, [2014].
[024] Referring to the embodiments as depicted in FIG. 1 to FIG. 4 (and
generally
applicable to all of the embodiments), preferably, the force-limiting device
300 is
configured to limit, at least in part, an amount of a force 302 up to a
maximum applicable
force to be applied from the distal tip 106 of the distal section 102 to the
biological feature
900 after (or once) the distal tip 106 of the distal section 102 contacts, at
least in part, the
biological feature 900 of the patient 902.
[025] Referring to the embodiments as depicted in FIG. 1 to FIG. 4 (and
generally
applicable to all of the embodiments), there is provided a method for using
the distal
section 102 having the distal tip 106 of the elongated medical assembly 100
for contacting
the biological feature 900 of the patient 902. The method includes maneuvering
the distal
tip 106 of the distal section 102 of the elongated medical assembly 100 to
contact, at least
in part, the biological feature 900 of the patient 902. The method also
includes using a
force-limiting device 300 (that is positioned proximate to, and is
interactable with, the
distal section 102) for limiting, at least in part, an amount of a force 302
to be applied from
the distal tip 106 of the distal section 102 to the biological feature 900;
this is done,
Date Recue/Date Received 2021-06-21
8
preferably, after (or once) the distal tip 106 of the distal section 102
contacts, at least in
part, the biological feature 900 of the patient 902.
[026] Referring to the embodiments as depicted in FIG. 1 to FIG. 4, the force-
limiting
device 300 includes a synergistic combination of a proximal section 104 and a
biasing
device 108. The proximal section 104 is positioned proximate to the distal
section 102.
The proximal section 104 is (coaxially) aligned with the distal section 102
along a
common axis 200 extending between the proximal section 104 and the distal
section 102.
The proximal section 104 and the distal section 102 are in fluid communication
(internal
fluid communication) with each other. The biasing device 108 is positioned
between the
proximal section 104 and the distal section 102. The biasing device 108 may
include a
spring element, a bias spring structure, a mechanical bias, etc., and any
equivalent thereof.
The biasing device 108 is attached to the proximal section 104 and the distal
section 102.
[027] Referring to the embodiments as depicted in FIG. 1 and FIG. 2, the force-
limiting
device 300 is configured (preferably) to dynamically adjust (automatically
adjust) the
reach of the distal tip 106 of the distal section 102 (for the case where the
biasing device
108 is deployed) so that the distal section 102 is able to slide over a
section of the proximal
section 104, depending on the possible biological features that might be
encountered or
contacted while the surgeon inserts and moves the distal section 102 (of the
elongated
medical assembly 100) through the body of the patient 902 (as depicted in FIG.
11 to FIG.
13) toward a targeted biological feature. The biasing device 108 is configured
to maintain
(at least in part) the contact force within a defined window as the user
(physician) tracks
(moves) the distal section 102 (of elongated medical assembly 100) through the
patient.
The distal section 102 may be moved (at least in part) along the proximal
section 104; this
arrangement helps to maintain the contact force with the biological feature
(such as the
septum of the heart) by, preferably, dynamically adjusting an amount of reach
(of the distal
section 102) as (A) the distal section 102 interacts with (contacts) various
biological
tissues and/or (B) changes to the relative applied forces resulting in
inadvertent (unwanted)
contact with various biological tissues (while the distal section 102 is moved
toward the
targeted biological feature). The distal section 102 is moved (actuated) via
the biasing
device 108. In doing so, the application of excessive force to the biological
feature (such as
the heart) via the distal tip 106 may be reduced and/or (preferably) avoided.
[028] Referring to the embodiments as depicted in FIG. 1 and FIG. 2, the
biasing device
108 joins (attaches) the distal section 102 and the proximal section 104 with
each other.
The biasing device 108 is configured to control (specifically, automatically
control) the
Date Recue/Date Received 2021-06-21
9
relative displacement between the distal section 102 and the proximal section
104. The
biasing device 108 controls the force window within which the distal section
102 might be
moved (actuate within). The biasing device 108 may provide any equivalent of a
spring
constant (such as a spring connecting the distal section 102 and the proximal
section 104).
The spring constant is preferably about 0.25 Newtons per millimeter. It will
be appreciated
that any spring constant may be used for the biasing device 108 that is
attached (affixed) to
the distal section 102 and the proximal section 104. The biasing device 108 is
configured
to allow displacement of the distal section 102 relative to the proximal
section 104 when
the distal tip 106 encounters biological tissue (such as the interatrial
septum, the exterior of
heart, the pericardium layer, etc.) and experiences forces that might be
encountered during
a procedure (such as a transseptal/epicardial access procedure). The biasing
device 108 is,
preferably, not so stiff as to cause inadvertent damage to tissue while
facilitating actuation
(movement) of the distal section 102, and/or is not so compliant as to easily
bottom out
against the proximal section 104 during a typical procedure.
[029] Referring to the embodiments as depicted in FIG. 1 to FIG. 4 (and
generally
applicable to all of the embodiments), the distal section 102 has the distal
tip 106
configured to be maneuvered within, at least in part, the patient 902, and to
be positioned
proximate to (and, preferably, for contact with) the biological feature 900 of
the patient
902. The distal section 102 and the distal tip 106 are configured to be
maneuvered within
and along the patient 902. The proximal section 104 is positioned proximate
to, and is
movable relative to, the distal section 102. The proximal section 104 is
(coaxially) aligned
with the distal section 102 along a common axis 200 extending between the
proximal
section 104 and the distal section 102. The proximal section 104 is,
preferably, coaxially
aligned with the distal section 102. The proximal section 104 and the distal
section 102 are
in fluid communication with each other. The proximal section 104 and the
distal section
102 are independently movable, at least in part, relative to each along a
movement length
101. The proximal section 104 and the distal section 102 remain in fluid
communication
with each other while the proximal section 104 and the distal section 102 are
independently movable, at least in part, relative to each along the movement
length 101.
[030] Referring to the embodiments as depicted in FIG. 1 to FIG. 4 (and
generally
applicable to all of the embodiments), the distal section 102 defines
(preferably) a distal
lumen 103. The proximal section 104 defines (preferably) a proximal lumen 105.
The
distal lumen 103 of the distal section 102 and the proximal lumen 105 of the
proximal
Date Recue/Date Received 2021-06-21
10
section 104 are in fluid communication with each other (after the proximal
section 104 is
positioned proximate to the distal section 102).
[031] Referring to the embodiments as depicted in FIG. 1 and FIG. 2, the
distal section 102
defines an entrance portal 110. The distal section 102 defines an exit portal
112. The exit
portal 112 is spaced apart from the entrance portal 110. The proximal section
104 includes
a leading portion 114. The leading portion 114 (of the proximal section 104)
is configured
to be received (at least in part) within the entrance portal 110 of the distal
section 102
(leading into an interior cavity of the distal section 102). Once the leading
portion 114 (of
the proximal section 104) is received (at least in part) within the entrance
portal 110, the
leading portion 114 remains within the interior of the distal section 102 (by
devices known
and not depicted). In this manner, the distal section 102 interfaces with the
proximal
section 104. The distal section 102 (preferably) travels over (overlaps), at
least in part, the
proximal section 104. The distal section 102 is configured to be movable along
the
movement direction 201. The distal section 102 is configured to telescopically
extend from
the proximal section 104.
[032] Referring to the embodiments as depicted in FIG. 1 and FIG. 2, the
distal section 102
(distal shaft) and the proximal section 104 (proximal shaft) are constructed
in a manner
that allows them to displace relative to each other (preferably, for a
predetermined distance
or length). The distal section 102 and the proximal section 104 are configured
to be
movable relative to each other, at least for over the movement length 101
(also called a
coaxial movement length or an overlap length, etc.). The outer diameter of the
distal
section 102 is, preferably, from about 8.0 French to about 12.0 French. It
will be
appreciated that any outer diameter of the distal section 102 may be used.
Preferably, the
outer diameter of the distal section 102 is able to fit within the patient
anatomy and allow
relative movement with the proximal section 104. The inner diameter of the
distal section
102 is, preferably, about at least 0.927 millimeters. It will be appreciated
that any inner
diameter of the distal section 102 may be used. Preferably, the inner diameter
of the distal
section 102 has a size that allows conventional transseptal/epicardial devices
(guidewires,
needles, etc.) to be passed through. It will be appreciated that any length of
the proximal
section 104 may be used. Preferably, the length of the proximal section 104
may reach the
desired (targeted) patient anatomy from a chosen access site and facilitate
sufficient
relative displacement to the proximal section 104. The length of the distal
section 102 is,
preferably, about 4.0 centimeters. The material of the distal section 102 may
include high-
density polyethylene (HDPE) or any equivalent thereof. It will be appreciated
that any
Date Recue/Date Received 2021-06-21
11
biocompatible material can be used for the material of the distal section 102.
Preferably,
the material has sufficient stiffness for use in the selected procedure. The
distal section 102
may have a maximum displacement (over the proximal section 104 or overlaps, at
least in
part, with the proximal section 104) of about 2.0 centimeters. It will be
appreciated that
any maximum displacement of the distal section 102 may utilized. Preferably,
the total
displacement of the distal section 102 may travel relative to the proximal
section 104 to
facilitate shock absorption (preferably through the steps in the procedure).
Preferably, the
distal section 102 does not bottom out against the proximal section 104 during
the
procedure.
[033] Referring to the embodiments as depicted in FIG. 1 and FIG. 2, for
epicardial access,
the distal tip 106 is relatively soft and/or compliant, and may help to reduce
inadvertent
trauma caused by excessive force input. Steerable sheaths and dilators can
help with
positioning a transseptal access device against the septum; however, a
telescopic distal
section may further enable precise tenting with the distal tip 106 against a
desired
biological feature or location. The outer diameter of the distal tip 106 is,
preferably, about
1.4 millimeters. It will be appreciated that any outer diameter of the distal
tip 106 may be
used. Preferably, the outer dimeter of the distal tip 106 facilitates desired
performance
during transseptal/epicardial access procedures. The inner diameter of the
distal tip 106
ranges, preferably, from about 0.927 millimeters to about 0.953 millimeters.
It will be
appreciated that any inner diameter of the distal tip 106 may be used provided
the inner
diameter of the distal tip 106 facilitates desired performance during
transseptal/epicardial
access procedures. Preferably, the inner diameter of the distal tip 106 has a
size that allows
conventional transseptal/epicardial devices (guidewires, needles, etc.) to be
passed
through. The distal curve angle of the distal tip 106 may range from about 0.0
degrees to
about 66 degrees. It will be appreciated that the curvature applied to the
distal tip 106 may
be dependent on the chosen procedure. Transseptal devices typically have a
curvature
angle between about 45 degrees and about 66 degrees associated with them so
that they are
able to angle towards and point in the direction of the interatrial septum. On
the other
hand, epicardial access tools do not have any kind of distal curve angle
associated with
them. Alternatively, a steerable feature (known and not depicted) may be
employed where
the distal curvature may be changed (preferably dynamically) by the user.
Overall, the
distal curvature of the device should facilitate the chosen procedure.
[034] Referring to the embodiments as depicted in FIG. 1 and FIG. 2, the
proximal section
104 may include any hub compatible device (positioned at the proximal section)
having a
Date Recue/Date Received 2021-06-21
12
luer lock fitting and/or slip tip syringes, etc. A hub is not strictly
required for proper
functioning of the elongated medical assembly 100. Hubs make the elongated
medical
assembly 100 easier to use. Preferably, the elongated medical assembly 100 is
compatible
with conventional accessory devices such as syringes. The outer diameter of
the proximal
section 104 is, preferably, from about 5.0 French to about 8.5 French. It will
be
appreciated that any outer diameter of the proximal section 104 may be used.
Preferably,
the proximal section 104 may fit within the patient anatomy and allow relative
movement
with the distal section 102. The inner diameter of the proximal section 104
is, preferably,
from about 0.927 millimeters to about 1.83 millimeters. It will be appreciated
that any
inner diameter of the proximal section 104 may be used. Preferably, the inner
diameter of
the proximal section 104 has a size that allows conventional
transseptal/epicardial devices
(guidewires, needles, etc.) to be passed through. The length of the proximal
section 104 is,
preferably, from about 10 to about 95 centimeters. It will be appreciated that
any length of
the proximal section 104 may be used. Preferably, the length of the proximal
section 104
may reach the desired patient anatomy from a chosen access site. The material
of the
proximal section 104 may include high-density polyethylene (HDPE) or any
equivalent
thereof. It will be appreciated that any biocompatible material can be used
for the proximal
section 104. Preferably, the material has sufficient stiffness for use in the
selected
procedure.
[035] Referring to the embodiments as depicted in FIG. 3, and FIG. 4, the
biasing device
108 is configured to urge (normally urge) the distal section 102 to move to a
fully
extended position away from the proximal section 104. The biasing device 108
may
include an internal spring, and any equivalent thereof. The biasing device 108
is
configured to be compressed thereby shortening the overall length of the
distal section 102
and the proximal section 104 (in response to the distal tip 106 making contact
with a
biological feature 900, a biological wall or tissue, etc.). Contact between
the distal tip 106
of the distal section 102 and the biological feature 900 is depicted in FIG.
4. Cooperative
action between the distal section 102 and the proximal section 104 avoids, at
least in part,
application of excessive force to the biological feature 900 as the distal tip
106 of the distal
section 102 is moved toward and/or through the biological feature 900 (such as
the heart).
The distal section 102 and the proximal section 104 may be constructed to
accommodate
the largest patient anatomy that may be expected. The distal section 102 may
be moved to
shorten the overall length of the combination of the distal section 102 and
the proximal
section 104 via compression of the biasing device 108; this arrangement may
ensure that
Date Recue/Date Received 2021-06-21
13
enough reach may be maintained with the biological feature 900 or patient
anatomies,
etc.).
[036] Referring to the embodiment as depicted in FIG. 4, the distal section
102 is fully
compressed against or toward the proximal section 104. An input force
resulting from any
interaction with the biological feature 900 at the distal tip 106 may cause
the distal section
102 to move (back and forth) as the biasing device 108 may serve to dampen
potential
shocks imparted to the biological feature 900 from the distal tip 106.
[037] FIG. 5 and FIG. 6 depict cross-sectional views of embodiments of the
elongated
medical assembly 100 of FIG. 1
[038] Referring to the embodiments as depicted in FIG. 5 and FIG. 6, the force-
limiting
device 300 includes the proximal section 104 positioned proximate to the
distal section
102. The proximal section 104 is aligned (preferably coaxially aligned) with
the distal
section 102 along a common axis 200 extending between the proximal section 104
and the
distal section 102. The proximal section 104 and the distal section 102 are in
fluid
communication with each other. A control wire 109 extends to the distal
section 102 (and
is attached to the distal section 102). The proximal section 104 defines a
control-wire
lumen 111 configured to receive the control wire 109. The control wire 109 is
configured
to provide user control for the distal section 102. The proximal section 104
and the distal
section 102 are user controllable for independent relative movement between
the proximal
section 104 and the distal section 102.
[039] Referring to the embodiment as depicted in FIG. 5, it will be
appreciated that the
biasing device 108 is utilized for causing relative movement between the
distal section 102
and the proximal section 104. The distal section 102 is configured to be
manually
controlled by the user, thereby allowing for varying levels of protrusion of
the distal
section 102 relative to the proximal section 104 (if desired). The user may be
able to
telescope the distal section 102 out further from the proximal section 104 if
greater reach
(of the distal section 102) is needed when trying to tent the biological wall
(such as the
interatrial septum) with the distal tip 106. It will be appreciated that a
locking feature
(known and not depicted) may be added, which gives the user the ability to
lock the
position of the distal section 102 relative to the proximal section 104,
thereby no longer
allowing relative movement between the distal section 102 and the proximal
section 104.
[040] Referring to the embodiment as depicted in FIG. 6, it will be
appreciated that the
control wire 109 may be positioned outside of the proximal section 104.
Date Recue/Date Received 2021-06-21
14
[041] Referring to the embodiment as depicted in FIG. 5, the distal tip 106 of
the distal
section 102 and the proximal section 104 are protracted (moved together in
unison, more
or less by the user) toward the biological feature 900 so that eventually the
distal tip 106
may make contact (in a nice and easy or gentle manner) with the biological
feature 900.
The proximal section 104 is moved in response to the user moving the proximate
end
section (not depicted) of the proximal section 104 that sticks out from the
patient. The
distal tip 106 of the distal section 102 are moved in response to the user
moving the control
wire 109 (the control wire 109 extends from the proximate end section (not
depicted) of
the proximal section 104 that sticks out from the patient).
[042] Referring to the embodiment as depicted in FIG. 6, the distal tip 106 of
the distal
section 102 and the proximal section 104 are continued to be protracted (moved
together in
unison) toward the biological feature 900; in this manner, the distal tip 106
eventually
makes contact with the biological feature 900 (preferably in a gentle manner
that avoids
imparting unwanted damage to the biological feature 900). After contact is
made between
the distal tip 106 and the biological feature 900, the user should feel the
resistance to
movement of the distal section 102 (via the control wire 109) while the
resistance to
movement of the proximal section 104 is felt much less, so that the proximal
section 104
may continue to move freely toward the biological feature 900; in this case,
the user may
cognitively understand that the distal tip 106 has made an initial contact
with the biological
feature 900 (preferably without inadvertently striking the biological feature
900 with a
damaging force). A nice and easy movement of the distal section 102 is
preferred for
avoiding unwanted damage to the biological feature 900. The user may ponder
and
consider whether to back off and move the distal tip 106 of the distal section
102 (by
moving the control wire 109) away from the biological feature 900 (if so
desired, such as
when the user determines that the biological feature 900 is determined not to
be the desired
or targeted biological feature (this determination may be done by referring to
the display
device of a medical imaging system, known and not depicted). For instance,
this condition
might be desired when the user makes a determination that the biological
feature 900 is not
the desired biological feature (that should receive a tenting force), and that
it may be
necessary to continue moving the distal section 102 to another location
(preferably, to
position the distal section 102 proximate to a desired biological feature, for
instance).
However, for the case where the user makes a positive determination that the
biological
feature 900 is, in fact, the desired biological feature (that should receive a
tenting force),
then the user may apply the tenting force (such as the force 302) to the
biological feature
Date Recue/Date Received 2021-06-21
15
900. Application of the tenting force might be accomplished by moving the
proximal
section 104 toward the distal section 102 so that the proximal section 104 and
the distal
section 102 make contact with each other, and the tenting force may then be
transferred
from the user to the distal section 102 via the proximal section 104.
[043] FIG. 7 to FIG. 10 depict cross-sectional views of embodiments of the
elongated
medical assembly 100 of FIG. 1.
[044] Referring to the embodiments as depicted in FIG. 7 to FIG. 10, the force-
limiting
device 300 includes the proximal section 104 positioned proximate to the
distal section
102. The proximal section 104 is aligned (preferably, coaxially aligned) with
the distal
section 102 along a common axis 200 extending between the proximal section 104
and the
distal section 102. The proximal section 104 and the distal section 102 are in
fluid
communication (internal fluid communication) with each other. The proximal
section 104
and the distal section 102 are user controllable for independent relative
movement between
the proximal section 104 and the distal section 102.
[045] Referring to the embodiment as depicted in FIG. 7, the distal section
102 includes a
distal-proximate end section configured to extend from the patient after the
distal section
102 is inserted into the patient. The distal section 102 is movable in
response to a user
urging movement of (that is, an urging movement imparted to) the distal-
proximate end
section of the distal section 102. The proximal section 104 includes a
proximal-proximate
end section configured to extend from the patient after the proximal section
104 is inserted
into the patient. The proximal section 104 is movable in response to the user
urging
movement of (that is, an urging movement imparted to) the proximal-proximate
end
section of the proximal section 104.
[046] Referring to the embodiment as depicted in FIG. 7, the distal tip 106 of
the distal
section 102 and the proximal section 104 are protracted (moved together in
unison, more
or less by the user from the proximate end sections (not depicted) of the
distal section 102
and the proximal section 104 that stick out from the patient) toward the
biological feature
900 so that eventually the distal tip 106 may make contact (in a nice and easy
or gentle
manner) with the biological feature 900.
[047] Referring to the embodiment as depicted in FIG. 8, the distal tip 106 of
the distal
section 102 and the proximal section 104 continue to be protracted (moved
together in
unison) toward the biological feature 900 so that the distal tip 106
eventually makes
contact with the biological feature 900 (preferably, in a gentle manner that
avoids
imparting unwanted damage to the biological feature 900). After contact is
made between
Date Recue/Date Received 2021-06-21
16
the distal tip 106 and the biological feature 900, the user should feel the
resistance to
movement of the distal section 102 while the resistance to movement of the
proximal
section 104 is felt much less, so that the proximal section 104 may continue
to move freely
toward the biological feature 900; in this case, the user may cognitively
understand that the
distal tip 106 has made an initial contact with the biological feature 900
(preferably
without inadvertently striking the biological feature 900 with a damaging
force). A nice
and easy movement of the distal section 102 is preferred for avoiding unwanted
damage to
the biological feature 900. The user may ponder and consider whether to back
off and
move the distal tip 106 of the distal section 102 away from the biological
feature 900 (if so
desired, such as when the user determines that the biological feature 900 of
FIG. 8 is
determined not to be the (desired) targeted biological feature (this may be
determined with
assistance from a display device of a medical imaging system, known and not
depicted).
For instance, this condition might be desired when the user makes a
determination that the
biological feature 900 is not the desired biological feature (that should
receive a tenting
force), and that it may be necessary to continue moving the distal section 102
to another
location (preferably to position the distal section 102 may be positioned
proximate to a
desired biological feature as for the case depicted in FIG. 9, for instance).
[048] Referring to the embodiment as depicted in FIG. 9, the user has
determined that it is
necessary to continue movement of the distal tip 106 of the distal section 102
toward
another target, such as the biological feature 900, as depicted in FIG. 9 (on
the basis that
the biological feature 900 of FIG. 9 is a target biological feature that needs
to receive a
tenting force, or other treatment, etc.). The distal tip 106 of the distal
section 102 is
protracted (moved) toward the biological feature 900, and the biological
feature 900
becomes tented (due to the application of the force 302 from the distal tip
106 to the
biological feature 900); this is done while the proximal section 104 remains
relatively
unmoved (relative to the movement of the distal tip 106). As a result of this
relative
movement, the leading portion 114 of the proximal section 104 becomes
positioned away
from the distal tip 106 after (once) the distal section 102 is protracted
(moved) toward the
biological feature 900.
[049] Referring to the embodiment as depicted in FIG. 10, application of the
tenting force
(as depicted in FIG. 9) is no longer required or the medical procedure is
completed, etc.
and the distal tip 106 of the distal section 102 is retracted (moved) away
from the
biological feature 900, and the biological feature 900 becomes relaxed (due to
less or no
force applied from the distal tip 106 to the biological feature 900). As a
result, the leading
Date Recue/Date Received 2021-06-21
17
portion 114 of the proximal section 104 becomes positioned closer to the
distal tip 106
after (once) the distal section 102 is retracted (moved) away from the
biological feature
900.
[050] FIG. 11 to FIG. 13 depict side views of embodiments of the elongated
medical
assembly 100 of FIG. 1, in which there are depicted embodiments of a
transseptal
workflow.
[051] Referring to the embodiment as depicted in FIG. 10, the distal section
102 and the
proximal section 104 are initially placed (positioned) in the superior vena
cava 908 of the
heart 903. Other features of the heart 903 are also depicted, such as the
inferior vena cava
904, the right atrium 906, the ascending aorta 910, the left atrium 912 and
the descending
aorta 914. The distal section 102 is moved relative to (or over) the proximal
section 104 as
the distal tip 106 (of the distal section 102) is pressed against tissue.
Cooperative action
between the distal section 102 and the proximal section 104 helps to mitigate
inadvertent
trauma to tissue while maintaining (at least in part) contact between the
distal section 102
and the biological tissue (the biological feature 900); this may be performed,
preferably,
automatically where the biasing device 108 is deployed (as depicted in FIG.
3).
[052] Referring to the embodiment as depicted in FIG. 11, the distal section
102 and the
proximal section 104 are brought down from the superior vena cava 908 to drop
onto the
fossa ovalis (the interatrial septum 916). As the distal section 102 and the
proximal section
104 are removed from tighter vasculature, the distal section 102 is able to
move relative to
(such as telescope further out from) the proximal section 104 while the distal
section 102
maintains, advantageously, in contact with the tissue; this may be performed
automatically
for cases where the biasing device 108 is deployed (as depicted in FIG. 3).
[053] Referring to the embodiment as depicted in FIG. 12, the distal section
102 is moved
to tent against the fossa ovalis (the interatrial septum 916). The distal
section 102 is moved
relative to (such as, back and forth over) the proximal section 104 as the
user (not
depicted) manipulates the proximal section 104 while the heart 903 beats.
Cooperative
action between the distal section 102 and the proximal section 104 maintains
tissue contact
between the interatrial septum 916 and the distal tip 106 (of the distal
section 102) also
absorbs (to some extent) shock (forces), and may also help to mitigate
inadvertent tissue
trauma and/or unintentional mechanical crossing of the interatrial septum 916;
this may be
performed automatically (in accordance with a preferred embodiment) for the
case where
the biasing device 108 is deployed (as depicted in FIG. 3).
Date Recue/Date Received 2021-06-21
18
[054] FIG. 14 and FIG. 15 depict side views of embodiments of the elongated
medical
assembly 100 of FIG. 1.
[055] Referring to the embodiments as depicted in FIG. 14 and FIG. 15, the
distal tip 106 of
the distal section 102 is moved (along the movement direction 201); this is
done in such a
way that the distal tip 106 (of the distal section 102) tents against the
interatrial septum
916 (fossa ovalis) of the heart 903 (that is, the biological feature 900). As
the heart 903
beats, the distal section 102 is configured to move relative to (that is, move
over) the
proximal section 104 to maintain tissue contact; this may be performed
automatically for
the case where the biasing device 108 is deployed (as depicted in FIG. 3).
[056] Referring to the embodiment as depicted in FIG. 14, the interatrial
septum 916 is
shown moving further away from the distal tip 106, but the distal section 102
is able to
move relative to (that is, telescope over, along the movement direction 201)
the proximal
section 104 so that the distal tip 106 may maintain contact with the
interatrial septum 916.
[057] Referring to the embodiment as depicted in FIG. 15, the interatrial
septum 916 is
depicted moving closer to the distal tip 106 of the distal section 102 (the
dashed lines
depict the position of the interatrial septum 916 from the position as
depicted in FIG. 14).
To avoid placement of excessive mechanical force onto the interatrial septum
916 by the
distal section 102, the distal section 102 is configured to move (preferably,
automatically)
relative to (that is, over) the proximal section 104 (along the movement
direction 201); this
may be performed automatically for the case where the biasing device 108 is
deployed (as
depicted in FIG. 3). In this manner, the distal section 102 and the proximal
section 104
cooperate to dampen the force to be applied to the interatrial septum 916.
[058] FIG. 16 and FIG. 17 depict side views of embodiments of the elongated
medical
assembly 100 of FIG. 1.
[059] Referring to the embodiments as depicted in FIG. 15 and FIG. 16, the
medical
assembly 100 is utilized in an epicardial access procedure.
[060] Referring to the embodiment as depicted in FIG. 15, the heart 903 is
depicted in the
start (beginning) of the diastole phase (or the end of the systole phase) when
the ventricles
are empty. The diastole phase of the heartbeat occurs when the heart muscle
relaxes and
allows the chambers of the heart to receive (to fill with) blood. The distal
tip 106 (of the
distal section 102) is docked against the heart 903 (as the heart 903 beats).
In the end of the
systole phase, the heart 903 retracts away from the distal tip 106. To
maintain contact with
beating of the heart 903, the distal section 102 moves (telescopes over)
relative to the
proximal section 104; this is done in such a way that the distal section 102
advances
Date Recue/Date Received 2021-06-21
19
forwardly (along the movement direction 201); this may be performed
automatically for
the case where the biasing device 108 is deployed (as depicted in FIG. 3).
[061] Referring to the embodiment as depicted in FIG. 16, the heart 903 is
shown at the
start of the systole phase (or the end of the diastole phase) when the
ventricles (of the heart
903) are filled with blood and the heart 903 advances towards the distal tip
106. The
systole phase occurs when the heart muscle contracts to pump blood out from
the interior
of the heart. To prevent excessive mechanical force on the heart 903 via the
distal section
102, the distal section 102 is configured to retract (preferably,
automatically) over the
proximal section 104 along the movement direction 201; this may be performed
automatically for the case where the biasing device 108 is deployed (as
depicted in FIG.
3).
[062] The following is offered as further description of the embodiments, in
which any one
or more of any technical feature (described in the detailed description, the
summary and
the claims) may be combinable with any other one or more of any technical
feature
(described in the detailed description, the summary and the claims). It is
understood that
each claim in the claims section is an open-ended claim unless stated
otherwise. Unless
otherwise specified, relational terms used in these specifications should be
construed to
include certain tolerances that the person skilled in the art would recognize
as providing
equivalent functionality. By way of example, the term perpendicular is not
necessarily
limited to 90.0 degrees and may include a variation thereof that the person
skilled in the art
would recognize as providing equivalent functionality for the purposes
described for the
relevant member or element. Terms such as "about" and "substantially", in the
context of
configuration, relate generally to disposition, location, or configuration
that are either
exact or sufficiently close to the location, disposition, or configuration of
the relevant
element to preserve operability of the element within the disclosure which
does not
materially modify the disclosure. Similarly, unless specifically made clear
from its context,
numerical values should be construed to include certain tolerances that the
person skilled
in the art would recognize as having negligible importance as they do not
materially
change the operability of the disclosure. It will be appreciated that the
description and/or
drawings identify and describe embodiments of the apparatus (either explicitly
or
inherently). The apparatus may include any suitable combination and/or
permutation of the
technical features as identified in the detailed description, as may be
required and/or
desired to suit a particular technical purpose and/or technical function. It
will be
appreciated that, where possible and suitable, any one or more of the
technical features of
Date Recue/Date Received 2021-06-21
20
the apparatus may be combined with any other one or more of the technical
features of the
apparatus (in any combination and/or permutation). It will be appreciated that
persons
skilled in the art would know that the technical features of each embodiment
may be
deployed (where possible) in other embodiments even if not expressly stated as
such
above. It will be appreciated that persons skilled in the art would know that
other options
may be possible for the configuration of the components of the apparatus to
adjust to
manufacturing requirements and still remain within the scope as described in
at least one
or more of the claims. This written description provides embodiments,
including the best
mode, and also enables the person skilled in the art to make and use the
embodiments. The
patentable scope may be defined by the claims. The written description and/or
drawings
may help to understand the scope of the claims. It is believed that all the
crucial aspects of
the disclosed subject matter have been provided in this document. It is
understood, for this
document, that the word "includes" is equivalent to the word "comprising" in
that both
words are used to signify an open-ended listing of assemblies, components,
parts, etc. The
term "comprising", which is synonymous with the terms "including,"
"containing," or
"characterized by," is inclusive or open-ended and does not exclude
additional, unrecited
elements or method steps. Comprising (comprised of) is an "open" phrase and
allows
coverage of technologies that employ additional, unrecited elements. When used
in a
claim, the word "comprising" is the transitory verb (transitional term) that
separates the
preamble of the claim from the technical features of the disclosure. The
foregoing has
outlined the non-limiting embodiments (examples). The description is made for
particular
non-limiting embodiments (examples). It is understood that the non-limiting
embodiments
are merely illustrative as examples.
Date Recue/Date Received 2021-06-21