Note: Descriptions are shown in the official language in which they were submitted.
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
1
INHALER
FIELD OF THE INVENTION
This invention pertains in general to the field of medicament inhalers, and
more particularly to dry powder inhalers. Even more particularly, the
invention pertains
to a medicament inhaler comprising an agitator comprising an interactor.
BACKGROUND OF INVENTION
Inhalers have been widely used in the pharmaceutical field for treatment of
respiratory and/or other diseases. Numerous drugs, medications and other
substances
are inhaled into the lungs using the inhalers for rapid absorption of the drug
etc. in the
blood stream and for local action in the lung.
Inhaled drugs fall into two main categories, one being in the form of liquids,
including suspensions, and the other being powders. The choice of liquids or
powders
depends on the characteristics of the drugs, medications, etc. to be inhaled.
The most common type of inhaler is the pressurized metered-dose inhaler. In
this type of inhaler medication is most commonly stored in solution in a
pressurized
canister that contains a propellant, although it may also be a suspension. The
canister is
attached to a plastic, hand-operated actuator. On activation, the metered-dose
inhaler
releases a fixed dose of medication in aerosol form.
Another kind of inhaler is a nebulizer, which supplies medication as an
aerosol
created from an aqueous formulation.
The kind referred to herein is yet another type, in the form of a dry powder
inhaler. A dry powder inhaler releases a pre-metered, capsuled, dose or a
device-
metered dose of powdered medication that is inhaled through the inhaler.
Inhalers with a
device-metered dose of powdered medication are normally inhalers with a
medication
reservoir containing powdered medication, from which metered doses are
withdrawn
through the use of different dose metering arrangements, the doses then being
inhaled.
Dry powder inhalers need to deliver a particle size that is predominantly
below
5 microns, and preferably between 1 micron and 3.3 microns, for maximum
effectiveness. However, such small particles are often very cohesive due to
high surface
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
2
energy. Agglomeration may be worsened by moisture and / or when the medication
comprises more than one active substance, since the different active
substances may
have such properties as to form agglomerations with each other or with
pharmaceutical
carriers etc. Agglomeration of small particles is a problem which results in
the active
particles leaving the inhaler as large agglomerates.
EP0237507 relates to a device in powder inhalators intended to be used for
local administration of drugs to the respiratory tract and lungs of a patient.
Not only moisture can cause the dry powder to clump together, also static
electricity could make the dose stick to the walls and parts of the dose
metering system.
This can make the dose amount inconsistent or cause part of the dose stick in
a dose
administering location. This may lead to users receiving uneven amounts of
medicament per dose, and in extreme cases, it could even at least partially
clog the
inhaler.
As such, there exists a need for an improved dry powder inhaler device in
which effective and satisfactory dispersion of the dry powder is obtained and
which
inhaler efficiently facilitates deaggregation and dispersion and provides an
even
medicament amount per dose.
SUMMARY OF INVENTION
Accordingly, the present invention preferably seeks to mitigate, alleviate or
eliminate one or more of the above-identified deficiencies in the art and
disadvantages
singly or in any combination and solves at least the above mentioned problems
by
providing a dry powder inhaler comprising: at least one air inlet, at least
one air outlet,
and an air channel between the at least one air inlet and the at least one air
outlet; at
least one medicament reservoir; a dosage mechanism for arranging at least one
dose of a
medicament from the at least one medicament reservoir between the air channel
and the
air outlet such that the at least one dose may be delivered upon inhalation at
the air
outlet, wherein the dosage mechanism comprises a dose disc with at least one
cavity,
wherein the dose disc may be rotated between a dose collecting position
wherein the
cavity is positioned in the medicament reservoir, and a dose administering
position
wherein the cavity lies underneath the air channel; at least one agitator
located inside the
at least one reservoir, comprising an interactor, wherein said interactor
interacts with the
dose disc orcavity, such that the dose disc rotation transfers energy to the
agitator,
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
3
which will release energy as movement during the dose disc movement from the
dose
collecting position to the dose administering position.
Further advantageous embodiments are disclosed below and in the appended
patent claims.
BRIEF DESCRIPTION OF THE DRAWINGS
These and other aspects, features and advantages of which the invention is
capable will be apparent and elucidated from the following description of
embodiments
of the present invention, reference being made to the accompanying drawings,
in which
Fig. 1 is a cross sectional view along a longitudinal axis of an inhaler in
the
dose administering position according to one embodiment of the present
invention;
Fig. 2 is a perspective and cross sectional view of the inhaler in Fig. 1,
wherein
air inlets are omitted from Fig. 2 for clarity;
Fig. 3 is a cross sectional view along a longitudinal axis of the inhaler in
Fig. 1
wherein the air and air/medicament flows through the inhaler are disclosed;
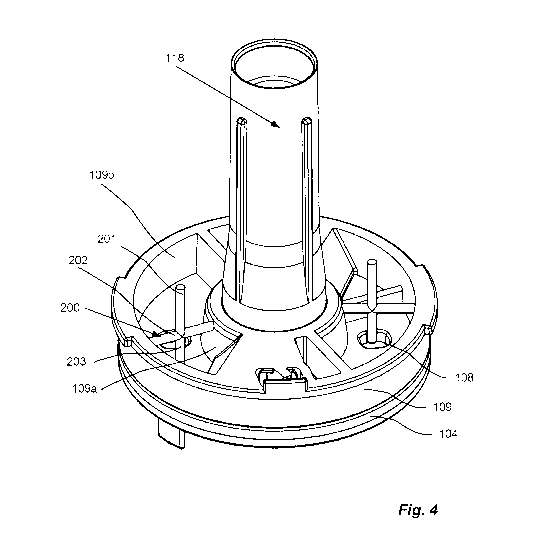
Fig. 4 show a detailed view of an agitator located inside a reservoir above
the
dose disc according to one embodiment for use in the inhaler in Fig. 1;
Fig. 5 show a detailed view agitator located inside a reservoir above the dose
disc according to one embodiment for use in the inhaler in Fig. 1;
Fig. 6 show a detailed view agitator located inside a reservoir above the dose
disc according to one embodiment for use in the inhaler in Fig. 1;
Fig. 7 show a detailed view agitator located inside a reservoir above the dose
disc according to one embodiment for use in the inhaler in Fig. 1;
Fig. 8 show a detailed view of an embodiment of the agitator for use in the
inhaler in Fig. 1;
Fig. 9 show a detailed view agitator located inside a reservoir above the dose
disc according to one embodiment for use in the inhaler in Fig. 1;
Fig. 10 shows partial views of the embodiments of the agitator in in Fig. 4
(10a) and Fig. 8(10b); and
Figs. lla-d shows detailed partial views of different axis supports.
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
4
DETAILED DESCRIPTION OF EMBODIMENTS
The following description focuses on embodiments of the present invention
applicable to a medicament inhaler 100, and in particular to a dry powder drug
inhaler
with more than one medicament reservoir, such as two medicament reservoirs.
However, it will be appreciated that the invention is not limited to this
application but
may be applied to many other inhalers having an inlet and an outlet, as well
as a
medicament reservoir.
Fig. 1 and 2 illustrate a dry powder drug inhaler 100. The dry powder drug
inhaler 100 comprises air inlets 101 and an air outlet 102. The outlet 102 is
arranged in
a zone of a proximal end 128 of the dry powder drug inhaler 100 while the
inlets 101
are arranged at a zone in an opposite distal end 129 of the dry powder drug
inhaler 100.
The outlet 102 is arranged centrally along the longitudinal axis of the dry
powder drug
inhaler 100. The inlets 101 may be arranged at the periphery of the dry powder
inhaler
100 in a radial position in relation to the longitudinal axis of the dry
powder drug
inhaler 100, such that the inlets 101 lead inhaled air transversally and
radially towards
the central portion of the dry powder inhaler 100.
Although not illustrated in Figs. 1 and 2, the inlets 101 may also be
positioned
with a direction that is parallel to the central axis of the dry powder
inhaler 100.
The number of inlets and outlets may be different from what is disclosed in
Figs. 1 and 2. The number of inlets may for example be adjusted in accordance
with
needs and specific inhaler design such that a number of smaller air inlets,
for reducing
pressure fall over the inhaler, are arranged circumferentially on the dry
powder inhaler
100. In a similar manner the number of air outlets may be adjusted in
accordance with
needs and specific inhaler design.
The different parts of the dry powder inhaler 100 may be manufactured in a
suitable material, such as injection moldable plastics, such as
thermoplastics.
The dry powder inhaler 100 comprises three major parts in the form of (i) an
upper proximal reservoir housing 103 with an inhalation chimney 112, (ii) a
dosage
mechanism 118 comprising a dose disc 104 having at least one cavity 108, a
mixing and
deaggregation chamber 106 adjacent to the at least one cavity 108, and a
conduit 116
extending distally from the chamber 106, and (iii) a lower distal twister 105
which in
some embodiments may comprise a floor disc 114. The reservoir housing 103 and
the
twister 105 cooperate so as to house the dosage mechanism 118 and the floor
disc 114
in between housing 103 and twister 105. The chimney 112 of the reservoir
housing 103
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
cooperates with the conduit 116 of the dosage mechanism 118 such that the dose
disc
104 may be rotated between a dose administering position and a dose collecting
position
when the reservoir housing 103 is rotated. The floor disc 114 is connected to
twister 105
so that floor disc 114 only moves when twister 105 is rotated as will be
described
5 further below. This may be accomplished by connecting the floor disc 114
and the
twister 105 via interconnecting grooves and ribs, or letting the twister 105
extend
longitudinally around the floor disc 114 as disclosed for example in Fig. 1.
Preferably,
the rotation of the dose disc 104 has two end positions corresponding to the
dose
administering position and the dose collecting position in its relation with
the reservoir
housing 103 in a known manner.
The dose administering position is illustrated in Fig. 1 and 2. In the dose
administering position, the inlets 101 are in communication with the mixing
and
deaggregation chamber 106 via air channels 107. The air channels 107 direct
the flow of
air from inlets 101 initially downwards onto cavities 108 in the dose disc
104. Hence, in
the dose administering position the cavities 108 may lie underneath and in
line with the
air channels 107, in particular in line with a longitudinal section 130 of the
air channels
107. In addition, in the dose administering position the cavities 108 may be
arranged
flush with a transversal section 132 of a respective air channel 107 and may
be arranged
partially in line with a longitudinal section 130 of a respective air channel
107. The
combination of an air flow A from channels 107 and the medicament M from
cavities
108 then flows radially to the chamber 106 via the transversal section of the
air channel
132 as will be described further below with respect to Fig. 5 and 6. When the
dose disc
104 is rotated into a dose collecting position (not shown), the chamber 106
and the
cavities 108 are rotated away from communication with the inlets 101 and air
channels
107. Instead, the cavities 108 are rotated into medicament reservoir 109 and
medicament reservoir 110 disclosed in Fig. 2, wherein the cavities 108 may
collect a
medicament housed in the reservoirs 109 and 110. The medicament contained in
the
medicament reservoir 109 may be a medicament different from the medicament
contained in the medicament reservoir 110. Due to the presence of two
reservoirs 109
and 110, the inhaler 100 may deliver two substances in one inhalation, the two
substances otherwise being incompatible meaning that these two substances
would not
be possible to be comprised in one joint reservoir. Thus, the dry powder
inhaler device
100 can effectively and satisfactorily disperse two dry powders and can
administer a
medicament comprising two or more substances which are incompatible in a
mixture or
are preferably stored in separate reservoirs for other reasons.
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
6
For single medicament delivery, only one medicament reservoir 109 is
required. If so, the dry powder inhaler 100 only comprises one medicament
reservoir
109 or the same medicament is filled in the two medicament reservoirs 109.
It is possible to arrange the dose disc 104 and the cavities 108 thereof such
that
when a first set of two cavities 108 lie underneath the air channels 107, i.e.
in a dose
administering position, a second set of two cavities 108 are positioned in the
medicament reservoirs 109. In this arrangement the inhaler has two medicament
reservoirs, two air inlets, and one dose disc with four cavities.
Additionally, the
distribution of the cavities 108 on the dose disc 104 is such that the dose
disc 104 may
be rotated in one direction only meaning that when the second set of two
cavities 108 lie
underneath and in line with the air channels 107, the first set of cavities
108 are
positioned in the medicament reservoirs 109, 110 respectively. It is also
possible for the
dose disc 104 to be rotated in a first direction so that cavities 108 lie
underneath the air
channels 107 in a dose administering position, and then for the dose disc 104
to be
rotated in the opposite direction into the dose collecting position, and
thereafter again
for the dose disc to be rotated in the first direction back into the dose
administering
position. When the dose disc 104 is rotated in a first direction into the dose
administering position and the opposite direction into the dose collecting
position, the
dose disc 104 may have rotational stops in the dose administering position and
the dose
collecting position, respectively, to ensure accurate alignment of the
cavities 108 under
air channels 107 and positioning in the medicament reservoirs 109, 110
respectively.
It is also envisioned that an inhaler provided with more than two, such as
three,
four, five, or six, reservoirs 109 with the same arrangement of inlets,
outlets, air
channels, dose disc, cavities etc., is within the ambit of the present
invention. For
example, the inhaler 100 may have three medicament reservoirs 109, three air
inlets
101, and a dose disc with three cavities 108. Alternatively, the inhaler 100
may have
four medicament reservoirs 109, four air inlets 101, and a dose disc with four
cavities
108. It is preferred however that the inhaler 100 have two air inlets 101, two
air
channels 107, one air outlet 102, two medicament reservoirs 109, 110, and one
dose
disc 104 with two cavities 108.
The air channels 107 have a first proximal conformation 134 as disclosed in
the
embodiment in Fig. 1. The proximal conformation 134 is such that the air
channels 107
start at inlets 101 and extend downstream (during inhalation) in a central and
transversal
direction, where after they bend downwards at a right angle (90 degrees) to
longitudinal
sections 130 of the air channel 107 extending in a longitudinal and distal
direction
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
7
before connecting to transversal sections 132 of the air channel 107 via a
second distal
convention 131. In this way, when medicament lies in the cavities 108, the
medicament
is arranged flush with the transversal sections of the air channel 132 and the
air flow
direction will facilitate initial deaggregation of the medicament from the
cavities 108.
This facilitates that the medicament in the cavities 108 will be dispersed
into the air
flow and enters into the chamber 106.
The reservoirs 109 may be provided with medicament scrapers 113 as
illustrated in Figs. 4 to 9. The scrapers 113 are suspended at the bottom of
the reservoirs
109 such that they bear upon the dose disc 104. The scrapers 113 will pass
over the
cavities 108 of the dose disc 104 so that excessive medicament is removed from
the
cavities 108 to ensure correct dose volume. The scrapers 113 will also aid in
compacting medicament in the cavities 108, which will improve retention of
medicament in cavities 108 when the dose disc has been rotated into the dose
administering position. Since the scrapers 113 are suspended at the bottom of
the
reservoirs 109 they will scrape the upper proximal surface of the dose disc
104, when
the dose disc 104 is rotated between the dose administering position and dose
collecting
position. Preferably, each reservoir 109 has a number of scrapers evenly
distributed
along the bottom of the reservoirs 109, 110. In this way the scrapers do not
only aid in
obtaining correct dose volume and dose compacting but also aid in distributing
medicament at the bottom of the reservoirs 109. The number of scrapers per
reservoir
109 could for example be selected in the interval of 1 to 6, such as 2 to 4,
such as 3. It is
also envisioned that the scrapers are arranged in an uneven distribution in
the reservoirs
109 if certain reservoirs are configured such that an uneven distribution of
the scrapers
will have a beneficial effect on the medicament distribution along the bottom
of the
reservoirs 109.
The inhaler 100 further comprises at least one agitator 200 located inside the
at
least one reservoir 109, comprising an interactor 201. The interactor 201
interacts with
the dose disc 104 and/or cavity 108, such that the dose disc 104 rotation
transfers
energy to the agitator 200. The interactor can also interact with the powder.
The agitator
200 will release energy as movement during the dose disc movement, from the
dose
collecting position to the dose administering position. The released movement
energy
helps agitating and homogenizing the medicament inside the dosage mechanism
and
provide a homogeneous dose in the cavity 108.
Referring to figures 4 to 10, the agitator 200 further comprises an axis 202,
which is arranged radially inside the reservoirs 109, between the inner wall
109a and
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
8
outer wall 109b of said reservoirs 109. In this way, the interactor 201 can be
attached to
said axis 202 and comprise means for mixing the medicament. Such means may be
in
the form of pegs 203, wheels 206, wings or flaps or other shapes suitable for
dispersing
the medicament.
Referring to figures 4 and 10a, the axis 202 is fixedly attached to the inner
wall
109a and/or the outer wall 109b inside the reservoirs 109. The agitator 200
further
comprises the interactor 201 in form of a peg 203 attached to the axis 202.
The peg 203
protrudes perpendicularly to the axis 202 towards the dose disc 104, and the
peg 203 is
longer than the perpendicular distance from the axis 202 to the dose disc 104.
The peg
203, or the peg 203 and the axis 202, is made from an elastic material such as
rubber or
elastic plastic, and interacts with the dose disc 104 and/or cavity 108 during
dose disc
movement to retain and release energy as movement.
During dose disc 104 movement, the peg 203 will be forced to flex by the
movement of the dose disc 104, whereby it will retain energy, which will be
released
burst-wise, as a snapping motion against the dose disc 104. This snapping
motion of the
peg 203 will transfer the energy retained by the interactor 201 to the
medicament, the
medicament reservoir 109 and dose disc 104, and will help dislodge and evenly
disperse
the medicament, thus helping to load a homogeneous dose in the cavity 108.
This burst-
wise release of energy not only agitates and mixes the medicament in contact
with the
peg 203, but facilitates a homogenizing effect for a large part of the
medicament
reservoir 109.
By having a second peg 203 attached to the axis 202, protruding
perpendicularly to the axis 202, towards the distal end of the inhaler, this
second peg
203 will also flex, providing an even more efficient release of the retained
energy, thus
helping to disperse the medicament.
Referring to for instance figure 7, the agitator 200 further may comprise axis
supports 204 fixedly attached to the inner wall 109a and the outer wall 109b
of the
medicament reservoir 109. The axis support 204 comprises an indentation 205
wherein
the axis 202 is being held, such that it is allowed to rotate along its
central axis,
perpendicular to the inner wall 109a and outer wall 109b.
Referring to the example of figure 7, the interactor 201 further comprises a
wheel 206 projecting radially from the axis 202, wherein the wheel 206
comprises
mixing means 207, here cogs 208, protruding radially from the wheel 206, and
wings
209, and the radius of the wheel 206 is the distance from the axis 202 to the
dose disc
104 or the radius being such that the cogs 208 rests upon the dose disc 104 in
such
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
9
manner that the wheel will rotate from friction with the dose disc 104, thus
helping to
disperse the medicament.
The cogs 208 and/or wings 209 may be made from an elastic material, such as
rubber or elastic plastic, such that they can flex and burst-wise release
energy as
movement.
It has been found that interactors 201 comprising a wheel 206 are very
efficient
at dispersing the medicament and providing even loading of the medicament in
the
cavity 108.
In the example of figure 11b, the axis support 204 may further comprise the an
elastic member 210, in fig. 1 lb illustrated by an elastic 0-ring 211 placed
in the circular
indentation 205 and around said axis 202. The axis 202 is fixedly attached to
the elastic
member 210, for instance by friction. Rotating the axis 202 around its central
axis will
build up energy in the interactor 201, and the interactor 201 will release
said energy by
rotating the axis 202 along its central axis in the counter direction (if
energy was
retained by clockwise rotation, energy will be released as counter-clockwise
rotation).
Referring to the example of figure 5, the interactor 201 comprises at least
one
peg 203 attached to the axis 202 and axis supports 204 may comprise an elastic
member
210, here an 0-ring 211. The at least one peg protrudes perpendicularly to the
axis 202
towards the dose disc 104, and the peg 203 is significantly longer (such as 25
to 50 %
longer) than the perpendicular distance from the axis 202 to the dose disc
104. The axis
202 is locked in a position by the 0-ring 211, such that the peg 203 scrapes
against the
dose disc 104 and vibrates and jumps during rotation of the dose disc 104.
When the
peg 203 tip reaches the dose cavity 108, the peg 203 will snap into the cavity
108,
creating further movement within the reservoir 109, whereby the medicament is
dispersed even loading of the medicament in the cavity 108 is provided.
Referring to the example of figure 9, the interactor 201 further comprises a
wheel 206 projecting radially from the axis 202 and circular axis supports
204. When
the dose disc is rotated, the wheel 206 will rotate, thus dispersing the
medicament and
provide even loading of the medicament in the cavity 108.
If the axis supports 204 were to comprise an elastic member 210, such as an 0-
ring, the wheel 206 would rotate and apply tension to the elastic member 210
during
dose disc 104 movement, and intermittently release the built up tension by
rotating in
counter direction, thus effectively disperse the medicament and provide even
loading of
the medicament in the cavity 108.
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
Referring to figures 11c and d, the axis supports 204 may be U-shaped or
comprise elongated indentations 205, holding the axis 202 such that the axis
202 is
allowed to both rotate along its central axis and also slide along the
indentation 205
towards or away the dose disc 104 in a direction in a direction parallel to
the rotational
5 axis of the dose disc 104.
Referring to the example of figure 6, the interactor 201 further comprises a
wheel 206 projecting radially from the axis 202 and the axis supports 204 are
U-shaped
or comprises elongated indentations 205. Here the wheel 206 comprises cogs 208
protruding both radially and at right angles to the plane of the wheel 206 to
promote
10 mixing.
Since the axis supports 204 comprises elongated indentations 205, the axis 202
is allowed to move along the indentation 205, towards or away the dose disc
104 in a
direction in a direction parallel to the rotational axis of the dose disc 104.
The radius of
said wheel 206 is in the range from shortest perpendicular distance between
the axis 202
and the dose disc 104 to the longest perpendicular distance between the axis
202 and the
dose disc 104, such that dose disc 104 movement will rotate the wheel 206.
When the interactor 201 interacts with the dose disc 104, cavity 108 or
powder,
the dose disc 104 movement will retain and release energy as movement.
However, due
to the elongated indentation 205, the wheel 206 may also jump towards and away
from
the dose disc 104. Thus, more modes of movement are allowed, which will help
to
disperse the medicament and provide even loading of the medicament in the
cavity 108.
This is especially the case when cogs 208 are made from an elastic material,
or
the an elastic member 210, such as a such as a flat spring 212, is placed in
the elongated
indentation 205. The flat-spring 212 will thus retain energy if the axis 202
is pushed in
the direction away from the dose disc 104, and releases said energy by pushing
the axis
202 towards the dose disc 104. The flat-spring 212 will also ensure that the
wheel 206
interacts with the wheel 206 by pushing it towards it.
Referring to the example of figure 9 and 11d, the interactor 201 comprises a
wheel 206 projecting radially from the axis 202 and the axis supports 204 are
U-shaped
or comprises elongated indentations 207. The wheel 206 comprises cogs 208
protruding
at right angles to the plane of the wheel 206, and the radius of the wheel 206
is the
shortest perpendicular distance between the axis 202 and the dose disc 104.
During
movement of the dose disc 104, the dose disc 104, cavity 108 or powder will
interact
with the wheel 206, making it rotate and jump towards and away from the dose
disc
104, through the allowed axis movement along the indentation in the axis
support 204.
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
11
This movement will help disperse the medicament and providing even loading of
the
medicament in the cavity 108.
To summarize, figures lla-d shows various exemplary embodiments of the
axis supports 204.
The axis supports 204 in Fig. 1 la features a circular indentation 205 for
holding the axis 202 of the agitator 200, allowing the axis 202 to rotate
freely around its
central axis.
Fig llb shows an axis support 204 with a circular indentation 205 for holding
the axis 202 of the agitator 200, also comprising an elastic member 210 placed
in the
indentation 205, here an 0-ring 211 fixedly attached to the axis support 204
and axis
202. The 0-ring 211 builds up tension if the axis 202 rotates along its
central axis, and
rotates the axis 202 back to its initial position when tension is released.
Fig llc shows a U-shaped axis supports 204, comprising an elongated
indentation 205 for holding the axis 202. Thus, not only allowing the axis 202
to rotate
around its central axis, but also to move along the indentation 205.
Fig lld shows an axis support 204 with an elongated indentation 205 for
holding the axis 202 of the agitator 200, also comprising an elastic member
210. Here a
flat spring 212, fixedly attached to one end of the axis support 204. The slat
spring 212
builds up tension if the axis 202 moves along the indentation 205 towards the
flat spring
212, and pushed the axis 202 back to its initial position when tension is
released.
Furthermore, referring to the example of figure 7, the interactor 201 may
comprise extra pegs 203, cogs 207, wings 209 or protrusions, such as the wings
209
located on the wheel 206 of figure 9, helping to dissipate the built up energy
in the
interactor 201, thus more effectively dispersing the medicament.
The agitator 200 may be made from any suitable material. Suitable materials
include injection moldable plastics, such as thermoplastics, preferably
flexible plastics,
such as nylons, polyethylene (PE), polypropylene (PP), polystyrene (PS) and
polyvinyl
chloride (PVC) or other suitable flexible materials such rubber and synthetic
rubber.
The skilled reader will appreciate that the disclosed invention is not limited
to
inhalers featuring shown in figure 1, but may be implemented in any inhaler
with a
rotating movement to enhance loading homogeneous doses of medicament in a
cavity
108.
The cavity 108 may have circular shape or a substantially circular shape
having
a diameter substantially corresponding the diameter or the air channel 107, in
particular
the longitudinal section 130 and/or the transversal section 131.
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
12
This arrangement means for example that the reservoirs 109, 110 may
comprise a dry powder medicament in the form of a micronized formulation or a
carrier
based formulation, or mixtures thereof The inhaler 100 may then for example
comprise
a dry powder medicament in form of a micronized formulation in the first
reservoir 109
and a free-flowing dry powder medicament in form of a carrier based
formulation in the
second reservoir 110.
Depending on the medicament to be administered, and the formulation thereof,
the cavities 108 may take the form of a single circular shape when viewed from
directly
above or below the inhaler 100 as illustrated by the semi-circular shape of
cavities 108
in Fig. 2. Other medicaments which tend to aggregate more may form an
undesirable
"plug" in the cavity 108 which is not readily dispersible during inhalation.
Then it may
be preferable to make several cavities 108 each having a relatively smaller
diameter
than a single circular shape as illustrated in Figs. 8. The several smaller
cavities will
continue to lie underneath one of the air channels 107 which remains unchanged
in size
and shape. An inhaler with several smaller cavities lying underneath one air
channel 107
also allows for delivery of a smaller amount of powder. This feature also adds
the
possibility to combine or adapt the inhaler 100 for deliverance of micronized
formulations and/or carrier based formulations.
The chimney 112 does not necessarily have to be directed upwardly; it can just
as well be directed downwardly or to the sides, whereby the outlet 102 is
instead
positioned at the bottom or on the sides, respectively. Additionally, the
chimney 112
does not have to be generally tubular, but could be bent or sinus-shaped,
depending on
where on the inhaler 100 it is preferred to position the outlet 102. For flow
characteristics and dose reliability and maintenance, it is however preferred
to have it
directed upwardly and generally tubular with optional diverters. The general
shapes of
the conduit 116 and the chimney 112 may also be such as to have differences in
cross-
sectional area, such as is present in a cone-shaped chimney. In this way, the
flow
velocity in the conduit and chimney may be regulated so as to help in
deaggregation at
chosen parts.
During use of the inhaler 100 the user will then simply rotate the upper
housing
103 in one direction and thus the dose disc 104 into a dose collecting
position if the
dose disc is in a dose administering position. Thereafter, the upper housing
103 and the
dose disc 104 are rotated preferably into the opposite direction to reach the
dose
administering position. If the dose disc 104 is already in the dose collecting
position
then of course the first rotation into the dose collecting position may be
omitted. During
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
13
these rotations, the agitator 200 will disperse the medicament evenly,
allowing the
scraper 113 to uniformly fill the cavities 108 of the dose disc 104 in the
reservoirs 109.
After the dose disc 104 has been rotated into the dose administering position
the cavities
108 are filled with medicament and lie underneath the air channels 107. Then
the user
puts his/her mouth at outlet 102 and inhales. During inhalation air A will
enter the
inhaler 100 through inlets 101 and flow through air channels 107 to disperse
and carry
therewith the medicament(s) M from the cavities 108 in a radial direction in
accordance
with the arrows shown in Fig. 3. The air/medicament flow AM will then enter
the
chamber 106. In the chamber 106, the air/medicament flows AM from the air
channels
107 and cavities 108 will cross each other or coincide with each other, such
that
deaggregation of the medicaments M will increase which may increase dose
uniformity
since the need for diverters then is decreased. The flow characteristics, such
as jet
stream formation, will also increase. This feature allows the possibility to
combine or
adapt the inhaler 100 for deliverance of micronized formulations and/or
carrier based
formulations. Of course, it is also possible to combine the feature of
crossing or
coinciding flows from the two air channels 107 with diverters, even though the
need
thereof is decreased. Thereafter, the air/medicament flow AM ¨ now comprising
air/medicament flows from both air channels 107 and cavities 108, will go up
through
the conduit 116, the inhaler chimney 112, and finally through outlet 102 into
the lungs
of the user. A similar sequence of steps is then repeated the next time the
inhaler 100 is
required i.e. the user rotates the upper housing 103 and thus the dose disc
104 into a
dose collecting position to fill the cavities 108 with a medicament(s), then
the user
rotates the dose disc 104 back into the dose administering position and
inhales at outlet
102 as described immediately above. During these movements, the agitator 200
will
disperse the medicament evenly, allowing the scraper 113 to uniformly fill the
cavities
108 of the dose disc 104 in the reservoirs 109.
The structure of, and functional relationship between, the cavities 108,
reservoirs 109 and the separate dose collecting and dose administering
positions allows
for no risk of multiple dosing by the user. In use the medicaments remain in
the cavities
108 until inhalation. If inhalation is not commenced or is no longer required
by the user,
the cavities 108 carrying the medicaments may be rotated back into the
reservoirs
109,110.
Although, the present invention has been described above with reference to
specific embodiments, it is not intended to be limited to the specific form
set forth
herein. Rather, the invention is limited only by the accompanying claims.
CA 03123725 2021-06-16
WO 2020/165390
PCT/EP2020/053846
14
In the claims, the term "comprises/comprising" does not exclude the presence
of other elements or steps. Furthermore, although individually listed, a
plurality of
means, elements or method steps may be implemented by e.g. a single unit or
processor.
Additionally, although individual features may be included in different
claims, these
may possibly advantageously be combined, and the inclusion in different claims
does
not imply that a combination of features is not feasible and/or advantageous.
In
addition, singular references do not exclude a plurality. The terms "a", "an",
"first",
"second" etc. do not preclude a plurality. Reference signs in the claims are
provided
merely as a clarifying example and shall not be construed as limiting the
scope of the
claims in any way.