Note: Descriptions are shown in the official language in which they were submitted.
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
PROCESS TO RECOVER AMMONIUM BICARBONATE FROM WASTEWATER
FIELD OF THE INVENTION
[002] The present invention relates generally to a process, a method, and a
system for the
recovery and concentration of dissolved ammonium bicarbonate from a wastewater
containing
ammonia (NH3) using gas separation, condensation, and filtration, each at
controlled operating
temperatures. The present Invention also relates generally to a process, a
method, and a system
for the recovery and concentration of dissolved ammonium bicarbonate from a
wastewater
containing ammonia (NH3) using gas separation, condensation, and
crystallization, each at
controlled operating temperatures. Wastewaters may contain dissolved ammonia
as ammonium
ion and as dissolved ammonia gas; as well as dissolved carbon dioxide as
bicarbonate and
carbonate ions and as dissolved carbon dioxide gas. The word "ammonia" will be
used generally
to refer to any dissolved form of ammonia. The present invention also relates
to a process, a
method, and a system for the production of a nitrogen rich fertilizer from an
ammonia (NH3)
containing wastewater using a process, method and/or system comprising the
concentration of
dissolved ammonium bicarbonate using gas separation and condensation, followed
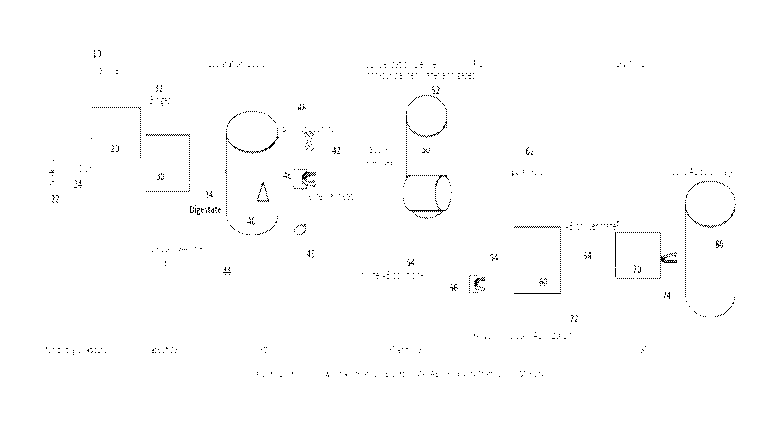
by
crystallization of concentrated ammonium bicarbonate, all at controlled
operating temperatures.
1
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
More specifically, the present invention relates to a process, method, and
system to produce,
from a wastewater containing ammonia (NH3), an organic solid containing high
concentrations
of nitrogen which could be utilized as a component ingredient in a nitrogen
rich, organic
fertilizer product. The process, method and system of the present invention is
a useful
improvement over existing technologies for the removal of ammonia from
wastewaters because
the present invention: converts NH3-N into ammonia gas but does not utilize
any chemicals to
increase pH, captures the ammonia gas in the form of a stable salt but does
not utilize industrial
acids to react with the ammonia, and produces a solid-fertilizer product with
minimal use of
energy.
[003] The present invention allows for the production/synthesis of organic N-
fertilizer,
ammonium bicarbonate (AB) derived from wastewaters, sludges and solids
containing ammonia
(NH3) or ammonia and carbon dioxide (CO2), without the use of chemical
additives.
BACKGROUND OF THE INVENTION
[004] Anaerobic digestion is a common unit operation employed in the treatment
of
wastewaters containing organics and nitrogenous compounds including
industrial, municipal and
agricultural wastewaters. The resulting solid/liquid slurry from an anaerobic
digester has a high-
solids portion and a low-solids portion. For example, the digestate produced
from dairy
wastewater, contains a high-solids portion comprising largely cellulosic
solids and a low-solids
portion containing concentrations of dissolved carbon dioxide and dissolved
ammonia nitrogen
as well as salts and both suspended and dissolved organics. The dissolved
ammonia nitrogen in
the digestate presents significant environmental issues if left untreated,
such as, when the
digestate is land applied, discharged to a body of water, or sent to a holding
pond or lagoon.
2
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
Potential adverse air and water impacts include: ammonia (toxic to fish,
irritating to human eyes
and lungs) will be lost both to the air and water; ammonia will be
biologically oxidized either in
water or soil and chemically oxidized in the air, in either case forming gases
that are irritants and
can form ozone or greenhouse gases, etc. Most often regulations for ammonia
release are
designed to prevent excess nutrient input to surface waters which may cause
eutrophication.
[005] Effective treatment technologies are needed for agricultural and
industrial waste streams
that may release ammonia to the environment. For example, anaerobic digester
digestate is often
high in ammonia and their sources are required to remove ammonia nitrogen to
avoid excessive
nitrogen discharges.
[006] One well-established technology for treatment of digestate is air
stripping which uses hot
air and/or steam to strip ammonia from the wastewater creating a liquid stream
comprising
substantially less dissolved ammonia and a heated gas containing the stripped
ammonia.
Formation of a solid precipitate containing ammonia, ammonium bicarbonate, and
ammonium
carbonate, during the air stripping process may foul the air stripping
substrates causing
operational and maintenance issues and thus is not desired. An increase of the
pH of the
wastewater shifts the equilibrium for ammonia away from dissolved ionized
ammonium and
more to ammonia gas. Accordingly, increased removal of the ammonia from
digestate using air
stripping is commonly achieved with chemical addition. Examples of chemicals
used to increase
pH include calcium, sodium or magnesium hydroxide. The stripped ammonia is
absorbed into
an acid solution. The use of acids is highly effective for ammonia recovery
and could also be
effective in producing a concentrated ammonium salt product using subsequent
unit operations.
On the other hand, treatment utilizing industrial chemicals to raise pH with a
stripping process
and for absorption of ammonia, such as, for example, for a treatment system
for a dairy waste
3
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
which produces a nutrient rich solid for use as a fertilizer, has the
unavoidable consequence
stemming from the use of such chemicals that any reusable end product cannot
be certified as an
"organic" product.
[007] Stripper exhaust gas containing ammonia is sometimes released to the
atmosphere
although regulations typically require that it is further processed to capture
the nitrogen. For
example, U.S. Patent No. 7,811,455 (Burke) describes a process for use of
biogas rather than air
and reclaiming ammonia from stripper exhaust gases in the form of ammonium
bicarbonate by
blending the CO2 in the digester biogas with the stripper gas and then
precipitating and
recovering ammonium bicarbonate with the added benefit of lowering CO2 in the
biogas. One
of the main drawbacks with that process is that the use of chemicals to raise
the pH in the
stripper precludes certification of the ammonium bicarbonate solids and any
solids created from
the ammonium bicarbonate solids as "organic" fertilizer. Another drawback is
the inefficiency
associated with processing large volumes of gas, including precipitation of
ammonium
bicarbonate in the gas phase.
[008] There is a need for a waste treatment technology that converts
wastewater containing
nutrients into beneficial use materials that can be certified as organic.
There is a need for a
wastewater treatment technology that can remove dissolved ammonia nitrogen,
react the
resulting gaseous ammonia with carbon dioxide to re-form dissolved ammonium
bicarbonate,
concentrate the dissolved ammonium bicarbonate, and then capture the ammonium
bicarbonate
in crystalline form. There is a need for an improved manure treatment system
comprising
anaerobic digestion that does not utilize expensive, hazardous, chemicals to
raise the pH of
digestate, chemicals that present significant handling and storage issues.
There is a need for an
4
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
improved wastewater treatment system that effectively removes and recovers
nitrogen in the
form of ammonium bicarbonate without any chemical addition.
SUMMARY OF THE INVENTION
[009] Applicants have invented a new process, system, and method for treating
wastewater that
satisfies these needs. While the invention will be described in connection
with certain
embodiments, it will be understood that the invention is not limited to those
embodiments. To
the contrary, the invention includes all alternatives, modifications and
equivalents as may be
included within the spirit and scope of the present invention.
[010] High-ammonia and ammonium containing wastewaters are produced in many
industrial
and municipal processes. Agriculture is one of the largest sources of these
wastewaters, in
particular in the form of products from the anaerobic digestion of organic
waste. Examples of
sources of this waste are animal manure, meat processing, dairy processing,
and silage.
Ammonia (NH3) that is not captured in a stable chemical form is a potential
source of air and
water pollution.
[011] The present invention provides a way to remove a substantial portion of
the ammonia
nitrogen within a liquid wastewater and capture the nitrogen in the form of
crystallized
ammonium bicarbonate using a series of unit operations operated under
specified temperatures
and without the use of chemicals to raise pH. The resulting ammonium
bicarbonate solid is high
in nitrogen content and could be used in combination with other materials to
create a nitrogen
rich organic fertilizer product.
[012] In one embodiment, the present invention includes at least three
components:
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
1) removal of the ammonia from the waste (including without limitation
sludges, semi-
solids, and solids and liquids) without the use of chemicals at a temperature
of at least
80 degrees Celsius thereby converting the ammonia to gaseous form;
2) mixing of the gaseous ammonia with carbon dioxide and water vapor at a
temperature
of between about 20 and 50 degrees Celsius causing the formation of dissolved
ammonium carbonate and ammonium bicarbonate in a liquid condensate and
concentrating the dissolved ammonium carbonate and ammonium bicarbonate using
reverse osmosis also operating at a temperature of between about 20 and 50
degrees
Celsius; and
3) crystallizing the concentrated dissolved ammonium carbonate and ammonium
bicarbonate at a temperature below 35 degrees Celsius such that the reverse
osmosis
concentrate becomes saturated with dissolved ammonium bicarbonate and ammonium
carbonate which depends upon the concentrations of ammonium carbonate and
ammonium bicarbonate to form solid ammonium bicarbonate and ammonium
carbonate.
[013] In a second embodiment using partial condensation of digester gases to
eliminate the
need for a filter (reverse osmosis), the present invention includes at least
three components:
1) removal of the ammonia from the waste (including without limitation
sludges, semi-
solids, and solids and liquids) without the use of chemicals at a temperature
of at least
80 degrees Celsius thereby converting the ammonia to gaseous form;
2) staged condensation and concentration of the gas containing gaseous
ammonia,
carbon dioxide, and water vapor, at a temperature at least 80 degrees Celsius
to
remove a significant amount of water vapor in liquid condensate form causing
6
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
formation of a concentrated gas, followed by the formation of a concentrated
dissolved ammonium bicarbonate liquid solution using absorption operating at a
temperature of between about 20 and 50 degrees Celsius; and
3) crystallizing the concentrated dissolved ammonium carbonate and ammonium
bicarbonate at a temperature below 35 degrees Celsius such that the
concentrated
dissolved ammonium carbonate and ammonium bicarbonate becomes saturated with
dissolved ammonium bicarbonate and ammonium carbonate which depends upon the
concentrations of ammonium carbonate and ammonium bicarbonate to form solid
ammonium bicarbonate and ammonium carbonate.
[014] The present invention is not limited to any one specific method or
process to remove
ammonia nitrogen from the waste but rather includes numerous alternatives
provided the
operating temperatures for the components are followed. Stripping animal
manure digestate at a
temperature of at least 60 degrees Celsius and preferably at a temperature of
at least 80 degrees
Celsius, for example, is one way to remove ammonia nitrogen from animal waste
creating an
exhaust gas containing ammonia gas. Examples of other ways to remove ammonia
nitrogen
from wastewaters and create an ammonia-containing gas include dryers, and
filtration devices
with membrane modules and heat sources.
[015] Once the dissolved ammonia is removed from the waste and is in gaseous
form, the
present invention includes two paths for the creation of a concentrated
solution of ammonium
carbonate and ammonium bicarbonate, the first using condensation to convert
gaseous ammonia,
CO2 and water vapor to a liquid solution containing ammonium carbonate and
ammonium
bicarbonate followed by filtration, the second using staged condensation to
remove a significant
amount of water vapor from the gas by partial condensation causing formation
of a concentrated
7
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
gas, followed by the formation of a concentrated dissolved ammonium
bicarbonate liquid
solution using absorption of ammonia from said gas. The present invention then
uses
crystallization to form solid ammonium bicarbonate and ammonium carbonate.
[016] When using condensation and reverse osmosis, the present invention
includes condensing
the gaseous ammonia with carbon dioxide and water vapor at a temperature of
between about 35
and 50 degrees Celsius causing the formation of dissolved ammonium carbonate
and ammonium
bicarbonate in a liquid condensate. Depending upon the characteristics of the
waste and the
preceding treatment processes, the amount of carbon dioxide within the ammonia-
containing gas
may be enough to convert substantially all of the ammonia into ammonium
carbonate and
ammonium bicarbonate without addition of carbon dioxide. Digested dairy
manure, for
example, put through a stripping process operated at greater than 80 degrees
Celsius should
create an exhaust gas containing enough carbon dioxide for the conversion of
substantially all of
the gaseous ammonia to dissolved ammonium carbonate and ammonium bicarbonate.
The net
result of the process is to recover ammonium bicarbonate and ammonium
carbonate from the
mixture of materials constituting the digestate as a high purity solution of
ammonium
bicarbonate and ammonium carbonate in the condensate. The formation of the
ammonium
carbonate and ammonium bicarbonate without the use of chemicals in the ammonia
removal
step, and without an outside source for carbon dioxide permits organic
certification of the
eventually created nitrogen rich solids. If additional carbon dioxide is
required, the organic
certification can still be used if the carbon dioxide is from non-synthetic
sources. While organic
fertilizers have a significant financial advantage over non-organic
fertilizers, the present
invention is not limited solely to a process, method, or system resulting in
organic products. The
8
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
present invention also includes addition of carbon dioxide from outside
sources resulting in
products unable to be certified as organic.
[017] The dissolved ammonium carbonate and ammonium bicarbonate in the
resulting
condensate liquid is then concentrated. A two-stage reverse osmosis unit
operating between
about 20 and 50 degrees Celsius, for example concentrates dissolved ammonium
carbonate and
ammonium bicarbonate to about 10 times the ammonium concentration of the
condensate, but
the increase could be anywhere from 1.5 to 20 times the concentration of the
ammonium
concentration in the condensate. More preferably, a two-stage reverse osmosis
unit operating
between about 35 and 50 degrees Celsius, concentrates dissolved ammonium
carbonate and
ammonium bicarbonate more efficiently.
[018] When using condensation and absorption, the present invention includes
condensing the
gaseous ammonia is a staged manner at a temperature of at least 80 degrees
Celsius in the first
stage to remove a significant amount of water vapor in liquid condensate form
causing formation
of a concentrated ammonia and carbon dioxide gas. Removing the water vapor and
concentrating the ammonia and carbon dioxide gas at this point instead of
condensing the gas
with all the water vapor eliminates the need to concentrate the ammonium
bicarbonate solution
after condensation using filters. It may eliminate the need, cost, and
maintenance expense of a
filtering unit process, potentially providing a more efficient and cost
effective overall process.
The concentrated ammonia and carbon dioxide gas is then treated using
absorption (an
absorption column, for example) operating at a temperature of between about 20
and 50 degrees
Celsius to form a concentrated dissolved ammonium bicarbonate liquid solution.
[019] The liquid containing concentrated dissolved ammonium carbonate and
ammonium
bicarbonate is then cooled to less than about 35 degrees Celsius to saturate
the reverse osmosis or
9
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
absorption concentrate and form solid ammonium bicarbonate and ammonium
carbonate which
is stable and high in nitrogen content. Moreover, resulting solids are
substantially free of
phosphorous. The solid ammonium bicarbonate can be stored and/or combined with
other
materials to create nitrogen rich fertilizer.
[020] Although the detailed chemistry for the formation of ammonium
bicarbonate from
ammonia and carbon dioxide is complex, the reactions provide predictable
behaviors at
temperatures and pressures accessible under normal industrial and agricultural
conditions. At
biological pH about 8, ammonium bicarbonate is stable in solutions below about
50 degrees
Celsius and rapidly decomposes in solutions above about 80 degrees Celsius, as
displayed in
Table 2 below. The present invention utilizes the varying stability and
solubility of ammonium
bicarbonate at different temperatures and pressures a) to drive substantially
all of the dissolved
ammonium out of the wastewater and into gaseous form (which occurs at a
temperature of about
80 degrees Celsius (Table 2) and is complete at a temperature of about 90
degrees Celsius (Table
1)), b) so that it can be condensed with carbon dioxide and water vapor and
concentrated at a
lower temperature (at a temperature of between about 35 and 50 degrees
Celsius) where
ammonium bicarbonate is stable, thereby converting the ammonia to dissolved
ammonium
bicarbonate in a concentrated liquid form, and c) so that the concentrated
dissolved ammonium
bicarbonate can be solidified at a lower temperate (less than about 20 degrees
Celsius).
[021] The following table shows how the solubility (and stability) of ammonium
bicarbonate
varies with temperature. Weast, R.C. (ed.) Handbook of Chemistry and Physics.
69th ed. Boca
Raton, FL: CRC Press Inc., 1988-1989; Perry's Chemical Engineers' Handbook,
6th Edition,
McGraw Hill, 1997.
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
[022] Table 1. Variation of solubility of different substances (mostly
inorganic compounds) in
water with temperature, under 1 atmosphere pressure. Units of solubility are
given in grams per
100 grams of water (g/100g)
Temperature (Degrees C) Range
Substance Formula 10 15 20 30 40 50 60 70 80 90 100
Ammonium
NH4HCO3 16.1 21.7 28.4 36.6 59.2 109 dec
bicarbonate
Ammonium (xTur õ,-, TT
_1=11-14)2lAJ3.1-12lJ 10 dec
carbonate
Ammonium
NH4NO3 150 192 242 297 344 421 499 580 740 871
nitrate
Ammonium
(NH4)2504 73 75.4 78.1 81.2 84.3 87.4 94.1 103
sulfate
dec = decomposition of compound at specified temperature
Table 2. Fraction of ammonia-N in the form of ammonia gas at conditions of pH
and
temperature.
f = 1/(1+10^(pKa-pH))
T F\
T K T C pKa pH 7 8 9 10 11 pKa from National
Research Council
. Ammonia. University Park Press,
300 27 9.20 80 0.01 0.06 0.39 0.86 0.98 Baltimore, MD
(1979).
322 49 8.57 120 0.03 0.21 0.73 0.96 1.00
333 60 8.29 140 0.05 0.34 0.84 0.98 1.00
344 71 8.02 160 0.09 0.49 0.90 0.99 1.00
355 82 7.78 180 0.14 0.63 0.94 0.99 1.00
BRIEF DESCRIPTION OF THE DRAWINGS
[023] The accompanying drawings, which are incorporated in and constitute a
part of this
specification, illustrate embodiments of the invention and, together with the
general description
of the invention given above and the detailed description of an embodiment
given below, serve
to explain the principles of the present invention. Similar components of the
devices are
similarly numbered for simplicity.
11
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
[024] Figure 1 is a schematic drawing of one embodiment of the invention for
the treatment of
cattle manure (e.g., from a CAFO) comprising solids separation, anaerobic
digestion, stripping,
condensation, concentration using reverse osmosis, and crystallization.
[025] Figure 2 is a schematic drawing of another embodiment of the invention
for the treatment
of dairy manure (e.g., from a CAFO) comprising solids separation, anaerobic
digestion, ultra
filtration with heating and membrane filtration, condensation, concentration
using reverse
osmosis, crystallization, and storage.
[026] Figure 3 is a schematic drawing of another embodiment of the invention
for the treatment
of an ammonia-containing wastewater (e.g., from layer manure) comprising heat
drying,
condensation of ammonia water, dissolution of carbon dioxide into the ammonia
water using
membrane filters, concentration of ammonium bicarbonate, crystallization, and
storage.
[027] Figure 4 is a schematic drawing of another embodiment of the invention
for the treatment
of cattle manure (e.g., from a CAFO) comprising solids separation, anaerobic
digestion,
stripping, absorption, concentration using reverse osmosis, and
crystallization. The carbon
dioxide dissolved in solution within the anaerobic digester's digestate, which
derives directly
from the cattle manure waste, is supplemented by carbon dioxide removed from
the biogas.
[028] Figure 5 is a schematic drawing of another embodiment of the invention
for the treatment
of livestock manure (e.g., from a CAFO) comprising solids separation,
anaerobic digestion,
stripping, condensation, absorption, and crystallization. The carbon dioxide
dissolved in solution
within the anaerobic digester's digestate, which derives directly from the
livestock manure
waste, may be supplemented by carbon dioxide removed from the biogas.
12
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
DETAILED DESCRIPTION OF THE INVENTION
[029] The accompanying drawings, which are incorporated in and constitute a
part of this
specification, illustrate embodiments of the invention and, together with the
general description
of the invention given above and the detailed description of an embodiment
given below, serve
to explain the principles of the present invention. Similar components of the
devices are
similarly numbered for simplicity.
[030] Figure 1 is a process flow schematic drawing of one embodiment of the
invention for the
treatment of cattle manure (e.g., from a CAFO) comprising solids separation,
anaerobic
digestion, stripping, condensation, concentration, and crystallization. In the
process according to
Figure 1, there is no chemical addition to adjust pH prior to, or in, the
stripping process. The
present invention excludes the use of pH adjustment chemicals. In the process
according to
Figure 1, there is also no external supply of carbon dioxide. The carbon
dioxide dissolved in
solution within the anaerobic digester's digestate, which derives directly
from the cattle manure
waste, is the sole source for carbon dioxide in the process.
[031] As depicted in Figure 1, raw manure 10 with or without associated dairy
waste generated
at the CAFO is transported to a solids separation unit/process 20 (it being
understood that a
mixing or holding tank/vessel could be used prior to solids separation and/or
can be used for
solids separation). The solids separation unit/process may be a single stage
or chamber unit or it
could be a series of stages or chambers for coarse solids separation and
intermediate solids
separation.
[032] The slurry/effluent 24 from the solids separation unit 20 is input into
an anaerobic
digester 30 which digests much, preferably most, of the dissolved organics and
small organic
particulates to produce biogas 32 and an effluent digestate 34.
13
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
[033] The effluent digestate 34 from the anaerobic digester 30 contains
residual solids,
dissolved salts and organics, and concentrations of dissolved ammonia and
carbon dioxide. The
present invention collects the ammonia and carbon dioxide and captures them in
a subsequent
multistage process to re-form solid ammonium bicarbonate. Each stage of the
subsequent
multistage process operates at different temperatures to take advantage of the
solubility
properties of ammonium bicarbonate for its concentration in dissolved form and
then its
formation as a nitrogen rich solid.
[034] The temperature of digestate 34 out of a typical anaerobic digester
treating cattle manure
is about 35 degrees Celsius. For the process of the invention, the digestate
needs to be heated to
greater than about 80 degrees Celsius for treatment in the stripper 40.
[035] The stripper operating at a temperature of greater than about 80 degrees
Celsius, without
any chemical addition to increase pH, removes dissolved ammonia and dissolved
carbon dioxide
from the digestate 34 creating exhaust vapor 42 containing water vapor,
gaseous carbon dioxide,
and gaseous ammonia. Vapor 42 will also contain traces of organic volatiles
and semi-volatiles.
In Figure 1, footnote 1 denotes the vapor is constant composition for
continuous operation and
varies during a batch process - H20, CO2, and NH3 evolve with traces of
organic volatiles and
semi volatiles. The treated water and solids 44 out of the stripper can be
further treated for
application to land or water using current treatment technologies. The
temperature of the
stripper 40 can be maintained using a heat exchanger 46. The vapor 42 created
by stripping the
digestate 34 in that first stage, the separation stage, is then treated in a
second condensation and
concentration stage to create a concentrated dissolved ammonium bicarbonate
solution.
[036] Condenser 50 and reverse osmosis filter 60 are then used to treat vapor
42 at a
temperature of between about 35 degrees Celsius and 50 degrees Celsius. A
pressure control
14
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
valve 48 can be used between the stripper 40 and the condenser 50 to maintain
a differential
between the two. In Figure 1, footnote 2 denotes pressure control valve is set
to maintain
differential between distillation unit and condenser - Condenser temperature,
T, must be less than
50 degrees Celsius to keep NH4 and HCO3 in solution, while distillation
temperature must be
greater than 80 degrees Celsius to convert to NH3 and CO2. Operating the
condenser 50
between about 35 and 50 degrees Celsius allows the water vapor, ammonia, and
carbon dioxide
to form dissolved ammonium bicarbonate. Maintaining between about 35 and 50
degrees
Celsius in the condenser 50, and a pH less than 9, prevents precipitation of
dissolved ammonium
bicarbonate or ammonium carbonate and keeps it in dissolved form. The
temperature of the
condenser 50 can be maintained using a heat exchanger 56. The non-condensed
water and gases
52 exiting the condenser 50 can be discharged to the atmosphere. In Figure 1,
footnote 3 denotes
AB solution in condenser is distillate of feed to stripping device.
[037] Following the condenser 50, and operating at about the same temperature
as the
condenser 50, the effluent ammonium bicarbonate solution 54 is treated in a
reverse osmosis
filter 60. Reverse osmosis filter 60 removes water thereby concentrating the
ammonium
bicarbonate in the solution. The resulting concentrated effluent 64 out of the
reverse osmosis
filter 60 contains about 50-100 times the concentration of dissolved ammonium
bicarbonate in
the digestate 34. In Figure 1, footnote 5 denotes AB concentrate is
supersaturated relative to
temperature of crystallizer. Control of the reverse osmosis temperature avoids
precipitation of
the ammonium bicarbonate on the membrane while achieving a concentration
sufficient for
saturation at the temperature in the crystallizer. The permeate 62 is a clean
water than can be
reused or discharged.
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
[038] The concentrated effluent 64 out of the reverse osmosis filter 60 is
then treated at a
temperature of less than about 35 degrees Celsius in stage three using a
crystallizer 70. It is
understood that lower temperatures, e.g., 20 degrees Celsius, could be used in
the crystallizer
depending upon the concentrations of dissolved ammonium carbonate and ammonium
bicarbonate in the reverse osmosis concentrate. Solid crystals of ammonium
bicarbonate are
grown in the crystallizer 70 under controlled conditions, separated from the
liquid fraction to
produce an ammonium-salt 74 which may be dried, pelletized or granulated to
form a final
product. In some embodiments, a portion of the saturated ammonium bicarbonate
supernatant is
recycled 72 to the reverse osmosis filter 60, after it is heated to the
required temperature in a heat
exchanger 56. In Figure 1, footnote 4 denotes heat exchange on recirculation
liquid to minimize
size of heat exchanger 56 to match temperature of reactor liquid.
[039] Due to the unique sequence of the preceding unit operations, the
resulting ammonium salt
may be dried and packaged for commercial distribution as a specialized
nitrogen fertilizer, that is
high-purity, phosphorus free, and certified USDA organic. The resulting
product is high-purity
and phosphorous free due to the two purification operations, namely, 1) the
distillation process
which removes ammonia and separates it from salts that are left behind in the
distillation unit's
liquid effluent, and 2) the crystallization process which removes solid
ammonium bicarbonate
from other contaminants including traces of phosphorous containing salts. If
synthetic chemicals
are not used in obtaining the solids or liquid digestate, the ammonium
bicarbonate product will
have the potential for designation as organic (USDA 2012) fertilizer. The USDA
designation is
of economic importance as the price of organic fertilizer expressed as dollars
per pound
ammonia nitrogen, is materially higher than that of chemical (non-organic)
fertilizers that are
equally uniform, high purity, and concentrated sources of NH3-N. As with
synthetic fertilizer,
16
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
the material is nearly odorless, and has low transport and application costs
relative to manure and
digestate. If the ammonia is captured with an industrial acid or is derived
from application of
caustic or other industrial alkali ¨ it will not qualify as organic
fertilizer. The ammonium salt
according to the invention resolves this conflict by (1) producing ammonia gas
thermally with no
chemical addition, and (2) using the carbon dioxide found in the digestate to
recover the
ammonia from the digestate to form an organic fertilizer, ammonium
bicarbonate.
[040] The ammonium salt 74 can be stored 80 for use on or off site.
[041] Another embodiment of the invention for a wastewater that utilizes
solids reduction prior
to membrane separation of ammonia is shown in Figure 2. In such instances,
stage 1 of the
foregoing described process can be modified to remove solids (effluent
suspended solids of 0.1%
or less) so that a membrane separation device could be employed to separate
the gases water
vapor, carbon dioxide, and ammonia from the digestate liquid.
[042] As depicted in Figure 2, raw manure 110 with or without associated dairy
waste
generated at the CAFO is transported to a solids separation unit/process 120
(it being understood
that a mixing or holding tank/vessel could be used prior to solids separation
or for the
separation). The solids separation unit/process 120 may be a single stage or
chamber unit or it
could be a series of stages or chambers. In Figure 2, the footnotes 1-4 denote
the following:
[1] - mesophilic digester, 35 C
- digested dairy manure, typical ammonia nitrogen is 1000 ppm
- AB is ammonium bicarbonate. Calculated from NH3-N and MW ratio
- AB concentration is 1% of saturation at 35 C
- digested manure is high in TSS
- No pH adjustment by either chemical addition or CO2 removal
17
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
[2] - gas from Separation Device has 20x concentration of ammonia as
input
- NH3-N is 60% NH3 at 80 C, 34% NH3 at 60 C, and 4% NH3 at 20
- Temperature must be about 80 C or higher to convert NH4+ to NH3
- AB concentration is about 5% of saturation at 80 C
- Nearly all the TSS is removed by the uF
[3] - Condensate < 50 C to convert dissolved NH3 and CO2 to dissolved
AB, and
>35 C to avoid precipitation in lines or RO
- AB concentrate from RO is about 81% of saturation at 50 C
- pH must be less than 9 to avoid carbonate formation and precipitation
- Recycle of liquor from crystallizer to RO has about the same
concentration
as the RO concentrate, and must be heated to the RO temperature
[4] Solids from crystallizer are high-purity, certifiable organic N-
fertilizer
[043] The output/effluent from the solids separation unit 124 is input into an
anaerobic digester
130 which digests much, preferably most, of the dissolved organics and small
organic
particulates to produce biogas 132 and an effluent digestate 134.
[044] The temperature of digestate 134, about 35 degrees Celsius, is heated to
greater than
about 80 degrees Celsius for treatment in stage 1, as described in detail
below. Here again, as for
the previous embodiment, the invention excludes the addition of chemicals to
increase pH and
also excludes the addition of carbon dioxide from a non-organic source
(preferably, the carbon
dioxide used in the process comes directly from the waste being treated). For
the embodiment
shown in Figure 2, an input vapor similar to that created in the foregoing
embodiment shown in
Figure 1 containing water vapor, gaseous carbon dioxide, and gaseous ammonia,
is created using
a different unit process than shown in Figure 1. In Figure 2, the separation
of the gaseous
18
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
ammonia and gaseous carbon dioxide from the digestate 134 is accomplished
using a membrane
device 143 instead of a stripper. The membrane passes gases, such as water
vapor, ammonia,
and carbon dioxide, but not liquid water. It therefore performs the same gas-
separation function
as the separation device shown in stage 1 of Figure 1.
[045] As shown in Figure 2, the digestate 134 is treated for solids removal
prior to stage 1, the
ultrafilter 136, and prior to gas separation in the membrane device 143. An
ultra filter 136 is
shown in Figure 2 for the solids removal it being understood that other solids
removal methods
producing the equivalent result of fine solids removal, for example passing
only solids of less
than 0.5 micron, are included within the scope of the invention. The
concentrated solids 138
from the ultra filter 136 can be mixed with the solids from the initial solids
separation step, or
processed as a high phosphorus solid product. The ultra filter 136 removes a
substantial portion
of the total suspended solids in the digestate. The low suspended-solids (0.1%
or less) digestate
137 is then treated in the gas-separation process of the invention which in
this embodiment
includes use of membrane device 143. The temperature of the low-solids
digestate 137 is raised
to at least about 80 degrees Celsius using a heat exchanger 146. Membrane
device 143 includes
a hydrophobic membrane that allows gas molecules to pass, such as water vapor,
ammonia, and
carbon dioxide, but not the liquid and its contaminants. The preceding uF is
required to remove
solids and organic material that might otherwise foul the hydrophobic
membrane. Vapor 142
will also contain traces of organic volatiles and semi-volatiles. The treated
water and solids 149
out of the membrane device 143 can be further treated for application to land
or water using
current treatment technologies.
[046] The vapor 142 created from the digestate using the membrane device 143
in that first
stage, the separation stage, is then treated in stage 2 and stage 3 using
condensation and
19
CA 03123802 2021-06-16
WO 2020/131116
PCT/US2018/067247
concentration, respectively, followed by crystallization, similar to the
embodiment shown in
Figure 1.
[047] Condenser 150 and reverse osmosis filter 160 are used to condense vapor
142 and
concentrate its condensate 154 at a temperature of between about 35 and 50
degrees Celsius, to
hold stable ammonium bicarbonate in solution. The effluent ammonium
bicarbonate solution
154 out of the condenser 150 contains the dissolved ammonium bicarbonate from
the ammonia
and carbon dioxide of the digestate 134. The non-condensed water and gases 152
exiting the
condenser 150 can be discharged to the atmosphere.
[048] Following the condenser 150, and operating at about the same temperature
as the
condenser 150, the effluent ammonium bicarbonate solution 154 is treated in a
reverse osmosis
filter 160. Reverse osmosis filter 160 removes water thereby concentrating the
ammonium
bicarbonate in the solution. The resulting concentrated effluent 164 out of
the reverse osmosis
filter 160 contains about 10 times the concentration of dissolved ammonium
bicarbonate in the
condenser effluent 154. The permeate 162 is a clean water than can be reused
or discharged.
[049] The concentrated effluent 164 out of the reverse osmosis filter 160 is
then treated at a
temperature of less than about 35 degrees Celsius, preferably less than 20
degrees Celsius, in
stage 3 using a crystallizer 170. Solid crystals of ammonium bicarbonate are
grown in the
crystallizer 170 under controlled conditions, separated from the liquid
fraction to produce an
ammonium-salt 174 which may be dried, pelletized or granulated to form a final
product. In
some embodiments, a portion of the saturated ammonium bicarbonate supernatant
is recycled
172 to the reverse osmosis filter 160.
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
[050] A resulting ammonium salt 174 solid, having physical and chemical
properties as stated
above for the first embodiment will result. The ammonium salt can be stored
180 for use on or
off site.
[051] Yet another embodiment of the invention using an external source for
carbon dioxide is
shown in Figure 3. Such an embodiment could be used for wastes that do not
contain the carbon
dioxide needed to convert the ammonia to ammonium bicarbonate. Examples of
such wastes
include waste not processed using anaerobic digestion, such as high-solids
manure or other
organic waste. In the embodiment shown in Figure 3, as compared to the
embodiment shown in
Figure 1, stage 1 and stage 2 are modified. In Figure 3, stage 1 comprises a
dryer 247 in place of
a stripper and stage 2 includes the addition of membrane modules 253 with a
source of carbon
dioxide 255 along with a condenser 250 and a reverse osmosis filter 260. In
Figure 3, the
footnotes 1-4 denote the following:
[1] - dryer exhaust to 2-stage condenser
- ammonia water at 2x exhaust ammonia concentration, temperature between
20 and 35 C
[2] - ammonia stabilized with CO2 as acid
- P adjusted to provide CO2 to stabilize ammonia water in effluent
- CO2 flow rate equals CO2 as HCO3 in effluent liquid.
[3] - Ammonium bicarbonate at 20 C in cystallizer
- Mother liquor recycled to RO, and must be heated to the RO temperature
[4] - inject compressed vent gas (CO2, H20, NH3) into ammonia water
feed line.
Here again, as for the previous embodiments, the invention excludes the
addition of chemicals to
increase pH. For the embodiment shown in Figure 3, a solution of ammonium
bicarbonate is
21
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
created and crystallized as in the foregoing embodiments shown in Figure 1 and
2. In Figure 3,
the separation of the gaseous ammonia from the waste 234 is performed using a
dryer 247, an
ammonia water is created using condensers 250, and gaseous carbon dioxide is
contacted with
the ammonia water solution using membrane device 253 to create a solution of
ammonium
bicarbonate.
[052] As depicted in Figure 3, the waste (such as layer manure) 234 is treated
in stage 1 of the
process of the invention which in this embodiment includes use of dryer 247.
The temperature
of the dryer is at least about 80 degrees Celsius. Dryer 247 operates at a
sufficiently high
temperature that the ammonia in the waste is converted to gas and removed with
the water vapor.
The dryer functions as a separation device in a manner analogous to the
distillation process 40 in
Figure 1. The exhaust vapor 242 from the dryer 247 contains water vapor and
gaseous
ammonia and lower than desired concentrations of carbon dioxide. In this
embodiment, it is
assumed that there is an insufficient amount of carbon dioxide in the waste to
react with and
convert substantially all of the gaseous ammonia into dissolved ammonium
bicarbonate and thus,
additional carbon dioxide is required. The dried waste 244 out of the dryer
247 can be processed
further into solid products such as fertilizer, animal feed supplement, or
fuel.
[053] The vapor 242 created using the dryer 247 in stage 1, the separation
stage, is then treated
in stage 2 using condensation, carbon dioxide addition, and concentration.
[054] Figure 3 shows an example of a two-step condenser 250 to create an
ammonia water 258
from the dryer exhaust gas 242. The first step removes about one half of the
water and nearly no
ammonia (NH3) and the second step is complete condensation producing ammonia
water at
about 50 degrees Celsius or less. The concentration of the dissolved ammonia
in the condensate
22
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
will be about twice that in the dryer vapor, for example about 0.5% by weight.
The non-
condensed water and gases 252 exiting the condenser 250 can be discharged to
the atmosphere.
[055] The ammonia water 258 is then treated in a membrane device 253 where an
external
source of gaseous carbon dioxide 255 is added. The gaseous carbon dioxide
passes through the
membrane, dissolves into the ammonia water, and reacts to create a solution of
ammonium
bicarbonate 254. For example, the solution of ammonium bicarbonate may be 2.3%
by weight
ammonium bicarbonate at about pH 6.5 ¨ 8.5, depending on the amount of CO2
added and the
temperature.
[056] The ammonium bicarbonate 254 is then treated in a reverse osmosis filter
260. Reverse
osmosis filter 260 removes water thereby concentrating the ammonium
bicarbonate in the
solution. The resulting concentrated effluent 264 out of the reverse osmosis
filter 260 contains
about 20 times the concentration of ammonia in the dryer gas. The permeate 262
is a clean water
than can be reused or discharged. Stage 2 which includes the condenser 250,
the membrane
device 253 and the reverse osmosis filter 260 operate at a temperature of
between about 35
degrees Celsius and 50 degrees Celsius.
[057] The dissolved ammonium bicarbonate solution 264 is then treated in stage
3 using
crystallization, similar to the embodiments shown in Figures 1 and 2. The
concentrated effluent
264 out of the reverse osmosis filter 260 is cooled to a temperature of less
than about 35 degrees
Celsius in stage 3 using a crystallizer 270. Solid crystals of ammonium
bicarbonate are grown in
the crystallizer 270 under controlled conditions, separated from the liquid
fraction to produce an
ammonium-salt 274 which may be dried (such as using dryer 285), pelletized or
granulated to
form a final product.
23
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
[058] A resulting ammonium salt solid having physical and chemical properties
as stated above
for the first and second embodiments will result. However, the certification
as an organic product
is contingent upon use of carbon dioxide produced organically. If synthetic
carbon dioxide is
used, the ammonium bicarbonate product cannot be designated as an organic
fertilizer.
[059] Carbon dioxide produced by fermentation of either animal waste or
agricultural material
(for example to produce ethanol) is certifiably organic; and is readily
available from agricultural
sources to assure that the carbon dioxide is neither synthetic nor
contaminated with synthetic
carbon dioxide.
[060] Figure 4 is a process flow schematic drawing of a variation on the
embodiment of the
invention shown in Figure 1 for the treatment of cattle manure (e.g., from a
CAFO) comprising
solids separation, anaerobic digestion, stripping, absorption, concentration,
and crystallization.
In the process according to Figure 4, there is no chemical addition to adjust
pH prior to, or in, the
stripping process. The present invention excludes the use of pH adjustment
chemicals. In the
process according to Figure 4, there is also no external supply of carbon
dioxide. The carbon
dioxide dissolved in solution within the anaerobic digester's digestate, which
derives directly
from the cattle manure waste, is supplemented by carbon dioxide from the
biogas to assure
maintenance of CO2 in the water to stabilize the ammonia in the absorber
column. Figure 4
shows the biogas 32 processed in a CO2 removal device 35 to provide CO2 to
provide
carbonated water for capture of ammonia as ammonium bicarbonate. For example,
the device
could be a pressure swing adsorption device which is commonly used to separate
gases, such as
CH4 and CO2, with materially different properties. In Figure 4, footnotes 1-5
denote the
following:
24
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
[1] - vapor is constant composition for continuous operation and varies
during a batch
process. H20, CO2, and NH3 evolve with traces of organic volatiles and semi-
volatiles.
[2] - pressure control valve is set to maintain differential between
stripper unit and
absorber. Absorber temperature, T, must be less than 50 C to keep NH4 and
HCO3 in solution, while stripper temperature must be greater than 80 C to
convert
to NH3 and CO2.
[3] - AB solution in absorber is formed from Digester biogas
[4] - HX on recycled stripper gas to match temperature of stripper liquid.
[5] - AB concentrate is supersaturated relative to temperature of
crystallizer.
[061] As depicted in Figure 4, raw manure 10 with or without associated dairy
waste generated
at the CAFO is transported to a solids separation unit/process 20 (it being
understood that a
mixing or holding tank/vessel could be used prior to solids separation and/or
can be used for
solids separation). The solids separation unit/process may be a single stage
or chamber unit or it
could be a series of stages or chambers for coarse solids separation and
intermediate solids
separation.
[062] The slurry/effluent 24 from the solids separation unit 20 is input into
an anaerobic digester
30 which digests much, preferably most, of the dissolved organics and small
organic particulates
to produce biogas 32 and an effluent digestate 34.
[063] The effluent digestate 34 from the anaerobic digester 30 contains
residual solids, dissolved
salts and organics, and concentrations of dissolved ammonia and carbon
dioxide. The present
invention collects the ammonia and carbon dioxide and captures them in a
subsequent multistage
process to form solid ammonium bicarbonate. Each stage of the subsequent
multistage process
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
operates at different temperatures to take advantage of the solubility
properties of ammonium
bicarbonate for its concentration in dissolved form and then its formation as
a nitrogen rich solid.
[064] The temperature of digestate 34 out of a typical anaerobic digester
treating cattle manure
is about 35 degrees Celsius. For the process of the invention, the digestate
needs to be heated to
greater than about 80 degrees Celsius for treatment in the stripper 40.
[065] The stripper operating at a temperature of greater than about 80 degrees
Celsius, without
any chemical addition to increase pH, uses gas (biogas, CH4, CO2, air, etc) to
remove dissolved
ammonia and dissolved carbon dioxide from the digestate 34 creating exhaust
vapor 42
containing water vapor, gaseous carbon dioxide, and gaseous ammonia. Vapor 42
will also
contain traces of organic volatiles and semi-volatiles. The treated water and
solids 44 out of the
stripper can be further treated for application to land or water using current
treatment
technologies. The temperature of the stripper 40 can be maintained using a
heat exchanger 46 to
heat the recycled stripper gas 45 from the absorber. The vapor 42 created by
stripping the
digestate 34 in that first stage, the separation stage, is then treated with a
cold water stream 53
saturated with CO2, in an absorption stage 50 to create a dissolved ammonium
bicarbonate
solution. Ammonia is removed from the vapor distillate, producing recycled
stripper gas 45
which is heated in heat exchanger 46 prior to entry at the bottom of stripper
40.
[066] Absorber 50 and reverse osmosis filter 60 are used to treat vapor 42 at
a temperature of
between about 35 degrees Celsius and 50 degrees Celsius. A pressure control
valve 48 can be
used between the stripper 40 and the absorber 50 to maintain a differential
between the two.
Operating the absorber 50 between about 35 and 50 degrees Celsius allows the
water vapor,
ammonia, and carbon dioxide to form dissolved ammonium bicarbonate.
Maintaining between
about 35 and 50 degrees Celsius in the absorber 50, and a pH less than 9,
prevents precipitation
26
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
of dissolved ammonium bicarbonate or ammonium carbonate and keeps it in
dissolved form.
Temperature of the absorber 50 can be maintained by control of the flow and
temperature of the
cold water 53.
[067] Following the absorber 50, and operating at about the same temperature
as the absorber
50, the effluent ammonium bicarbonate solution 54 is treated in a reverse
osmosis filter 60.
Reverse osmosis filter 60 removes water thereby concentrating the ammonium
bicarbonate in the
solution. The resulting concentrated effluent 64 out of the reverse osmosis
filter 60 contains
about 50-100 times the concentration of dissolved ammonium bicarbonate in the
digestate 34.
Control of the reverse osmosis temperature is critical to avoid precipitation
of the ammonium
bicarbonate on the membrane while achieving a concentration sufficient for
saturation at the
temperature in the crystallizer. The permeate 62 is a clean water than can be
reused or
discharged.
[068] The concentrated effluent 64 out of the reverse osmosis filter 60 is
then treated at a
temperature of less than about 20 degrees Celsius in stage three using a
crystallizer 70. Solid
crystals of ammonium bicarbonate are grown in the crystallizer 70 under
controlled conditions,
separated from the liquid fraction to produce an ammonium-salt 74 which may be
dried,
pelletized or granulated to form a final product. In some embodiments, a
portion of the saturated
ammonium bicarbonate supernatant is recycled 72 to the reverse osmosis filter
60, after it is
heated to the required temperature in heat exchanger 56.
[069] Due to the unique sequence of the preceding unit operations, the
resulting ammonium salt
may be dried and packaged for commercial distribution as a specialized
nitrogen fertilizer, that is
high-purity, phosphorus free, and certified USDA organic. The ammonia recovery
step is the
equivalent of distillation. This allows nearly no salt (including phosphorus
salts) carry over to
27
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
the input to reverse osmosis. Crystallization is another purification step, so
that "high-purity,
phosphorus free" product is achieved. If synthetic chemicals are not used in
obtaining the solid
AB or liquid digestate, the ammonium bicarbonate product will have the
potential for
designation as organic (USDA 2012) fertilizer. The USDA designation is of
economic
importance as the price of organic fertilizer expressed as dollars per pound
ammonia nitrogen, is
materially higher than that of chemical (non-organic) fertilizers that are
equally uniform, high
purity, and concentrated sources of NH3-N. As with synthetic fertilizer, the
material is nearly
odorless, and has low transport and application costs relative to manure and
digestate. If the
ammonia is captured with an industrial acid or is derived from application of
caustic or other
industrial alkali ¨ it will not qualify as organic fertilizer. The ammonium
salt according to the
invention resolves this conflict by (1) producing ammonia gas thermally with
no chemical
addition, and (2) using the carbon dioxide found in the digestate to recover
the ammonia from
the digestate to form an organic fertilizer, ammonium bicarbonate.
[070] The ammonium salt 74 can be stored 80 for use on or off site.
[071] Figure 5 is a process flow schematic drawing of a variation on the
embodiment of the
invention shown in Figure 1 for the treatment of livestock manure (e.g., from
a Controlled
Animal Feeding Operation, CAFO) comprising solids separation, physical
influent conditioning,
anaerobic digestion, stripping, condensation (concentration), absorption, and
crystallization. In
the Figure 5 process, there is no chemical addition to adjust pH prior to, or
in, the stripping
process. The present invention excludes the use of pH adjustment chemicals. In
the process
according to Figure 5, there is also no external supply of carbon dioxide. The
carbon dioxide,
which derives directly from the livestock manure waste, is supplemented by
carbon dioxide from
the biogas to assure maintenance of CO2 in the water to stabilize the ammonia
in the absorber
28
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
column. Figure 5 shows the biogas 32 processed in a CO2 removal device 35 to
provide CO2 to
for capture of ammonia as ammonium bicarbonate. For example, the device could
be a pressure
swing adsorption device which is commonly used to separate gases, such as CH4
and CO2, with
materially different properties. In Figure 5, footnotes 1-4 denote the
following:
[1] - vapor is constant composition for continuous operation and varies
during a
batch process. H20, CO2, and NH3 evolve with traces of organic volatiles and
semi-
volatiles.
[2] - pressure control valve(s) is set to maintain differentials between a)
the stripper
unit and the condenser, and b) the condenser and the absorber. Absorber
temperature, T,
must be less than 50 C to keep NH4 and HCO3 in solution, while stripper
temperature
must be greater than 80 C to convert to NH3 and CO2.
[3] - AB solution in absorber is formed from Digester biogas
[4] - AB concentrate is supersaturated relative to temperature of
crystallizer.
[072] As depicted in Figure 5, raw livestock manure 10 is transported to a
solids separation and
physical conditioning unit/process 20 (it being understood that a mixing or
holding tank/vessel
could be used prior to solids separation and/or can be used for solids
separation). The solids
separation unit/process may be a single stage or chamber unit or it could be a
series of stages or
chambers for coarse solids separation, intermediate solids separation, and
physical mixing and
conditioning. Physical conditioning may include dilution, grinding, mixing,
heating etc.,
depending on the specific livestock manure processed; dilution may be
necessary for some
manure to provide the appropriate consistency and concentration for the AD,
grinding and
mixing may be needed to help solubilize and make more available the organic
content for
29
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
digestion, heating may be needed for the anaerobic digester influent but may
also be used to
sterilize and prevent biological competition during digestion.
[072] The slurry/effluent 24 from the solids separation unit 20 is input into
an anaerobic
digester 30 which digests much, preferably most, of the dissolved organics and
small organic
particulates to produce biogas 32 and an effluent digestate 34.
[073] The effluent digestate 34 from the anaerobic digester 30 contains
residual solids,
dissolved salts and organics, and concentrations of dissolved ammonia and
carbon dioxide. The
present invention collects the ammonia and carbon dioxide and captures them in
a subsequent
multistage process to form solid ammonium bicarbonate. Each stage of the
subsequent
multistage process operates at different temperatures to take advantage of the
solubility
properties of ammonium bicarbonate for its concentration in dissolved form and
then its
formation as a nitrogen rich solid.
[074] The temperature of digestate 34 out of a typical anaerobic digester
treating livestock
manure is about 35 degrees Celsius. For the process of the invention, the
digestate needs to be
heated to greater than about 80 degrees Celsius for treatment in the stripper
40.
[075] The stripper operating at a temperature of greater than about 80 degrees
Celsius, without
any chemical addition to increase pH, creates exhaust vapor 42 containing
water vapor, gaseous
carbon dioxide, and gaseous ammonia. Vapor 42 will also contain traces of
organic volatiles and
semi-volatiles. The treated water and solids 44 out of the stripper can be
further treated for
application to land or water using current treatment technologies. The water
vapor 42 created by
stripping the digestate 34 in that first stage, the separation stage, is then
condensed in the
condenser. The condenser is operated as a single or multistage unit to
condense the water vapor
at a high temperature, to separate water from the gaseous ammonia and CO2
effectively
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
concentrating them in the gas. The amount of ammonium carbonate and ammonium
bicarbonate
concentration in the concentrated gas is at least 2 times greater than in the
gas before treatment
with condensation and could be as high as 100 times to 1000 times higher. The
high temperature
condensed water 55 is removed from the condenser and may be channeled back to
the stripper to
reclaim any re-dissolved ammonia and carbon dioxide, may be discharged from
the process, may
be used as seed liquid in the absorber, or may be recycled to combine with the
fresh livestock
manure entering the digester.
[076] Absorber 50 is used to treat vapor 142 at a temperature of between about
20 degrees
Celsius and 50 degrees Celsius. Pressure control valves 48 can be used between
the stripper 40,
the condenser, and the absorber 50 to maintain proper differential pressure
between the unit
processes. Operating the absorber 50 between about 20 and 50 degrees Celsius
allows the water
vapor, ammonia, and carbon dioxide to form dissolved ammonium bicarbonate.
Maintaining
between about 20 and 50 degrees Celsius in the absorber 50, and a pH less than
9, prevents
precipitation of dissolved ammonium bicarbonate or ammonium carbonate and
keeps it in
dissolved form. Temperature of the absorber 50 can be controlled with a heat
exchanger 56 and
by regulating the temperature of the carbon dioxide. Since a majority of the
water is condensed
and removed from the vapor phase prior to the absorber, the amount of water
used to generate
the concentrated AB solution is minimized and controlled. The ammonia and
carbon dioxide
gasses continue to absorb and form an AB solution in the controlled volume of
water until they
reach close to saturation at the selected temperature between 20 and 50
degrees Celsius.
[077] The concentrated effluent 64 out of the absorber is then treated at a
temperature of less
than about 20 degrees Celsius in stage four using a crystallizer 70. Solid
crystals of ammonium
bicarbonate are grown in the crystallizer 70 under controlled conditions,
separated from the
31
CA 03123802 2021-06-16
WO 2020/131116 PCT/US2018/067247
liquid fraction to produce an ammonium-salt 74 which may be dried, pelletized
or granulated to
form a final product. The more dilute AB solution, following this
crystallization process, is
returned to the absorber as seed liquid to dissolve more ammonia and carbon
dioxide as AB
under the higher temperature conditions, between 20 and 50 degrees Celsius.
[078] The embodiment of the invention shown in Figure 5 may increase the
overall efficiency
by potentially eliminating the need for any other concentrating unit process,
specifically the RO,
instead performing the concentration through removal of water vapor.
Substantial reductions in
capital cost, energy costs, operating costs, and maintenance costs could all
be realized with that
embodiment of the invention. The potential for product losses may also be
reduced by
eliminating or reducing the reverse osmosis, especially since reverse osmosis
operates at
relatively high pressures. A safer process may also created by eliminating or
reducing the high
pressure reverse osmosis, it being understood that the present invention also
includes variations
with the addition of a reverse osmosis step.
[079] The embodiment of the invention shown in Figure 5 may eliminate the need
for any other
concentrating unit process, specifically the RO, instead performing the
concentration through
removal of water vapor in the partial condenser and capturing the dissolved AB
in the absorber.
Substantial reductions in capital cost, energy costs, operating costs, and
maintenance costs may
be realized with that embodiment of the invention. While the present invention
has been
illustrated by description of various embodiments and while those embodiments
have been
described in considerable detail, it is not the intention of applicant to
restrict or in any way limit
the scope of the appended claims to such details. Additional advantages and
modifications will
readily appear to those skilled in the art. The invention in its broader
aspects is therefore not
limited to the specific details and illustrative examples shown and described.
Accordingly,
32
CA 03123802 2021-06-16
WO 2020/131116
PCT/US2018/067247
departures may be made from such details without departing from the spirit or
scope of
applicants' invention.
33