Note: Descriptions are shown in the official language in which they were submitted.
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
METHODS FOR NEEDLE IDENTIFICATION ON
AN ULTRASOUND DISPLAY SCREEN
RELATED APPLICATIONS
The present application claims priority to U.S. Serial Number 16/233,680 filed
on December 27, 2018, which is incorporated herein by reference in its
entirety.
FIELD
The present invention relates generally to needle assemblies for use in
medical procedures, and more particularly, to a method for identifying a
portion of a
needle of a needle assembly (such as the distal tip thereof) on a display
screen.
BACKGROUND
Detection of anatomical objects using medical imaging is an essential step for
many medical procedures, such as regional anesthesia nerve blocks, and is
becoming the standard in clinical practice to support diagnosis, patient
stratification,
therapy planning, intervention, and/or follow-up. Various systems based on
traditional approaches exist for anatomical detection and tracking in medical
images, such as computed tomography (CT), magnetic resonance (MR), ultrasound,
and fluoroscopic images.
For example, ultrasound imaging systems utilize sound waves with
frequencies higher than the upper audible limit of human hearing. Further,
ultrasound imaging systems are widely used in medicine to perform both
diagnosis
and therapeutic procedures. In such procedures, sonographers perform scans of
a
patient using a hand-held probe or transducer that is placed directly on and
moved
over the patient.
Certain ultrasound systems may be used in combination with needles having
active (i.e. electrically-powered) transducers, which require an electrical
connection
to a power source. Such needles, however, can often be difficult to locate on
an
ultrasound display screen. Particularly, for anesthesiologists, it is often
difficult to
locate the needle tip on the ultrasound display during peripheral nerve block
(PNB)
procedures (both single shots and continuous).
Accordingly, the present disclosure is directed to a method for identifying a
portion of a needle of a needle assembly, such as the distal tip, on a display
screen
of an autonomous ultrasound imaging system and/or an add-on system to the
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
autonomous ultrasound imaging system that addresses the aforementioned issues.
SUMMARY
Objects and advantages of the invention will be set forth in part in the
following description, or may be obvious from the description, or may be
learned
through practice of the invention.
In one aspect, the present invention is directed to a method for identifying a
needle of a needle assembly on a display screen. The method includes
receiving,
via a needle assembly of the needle assembly, data signals from the autonomous
ultrasound imaging system. The data signals include information relating to a
plurality of ultrasound waves generated by an ultrasound probe of the
autonomous
ultrasound imaging system. The method also includes generating, via the needle
transducer of the needle assembly, a location signal for at least one portion
of the
needle based on the data signals from the autonomous ultrasound imaging
system.
Further, the method includes modifying, via a processor of the needle
assembly, at
least one characteristic of the location signal so as to improve visibility of
the
location signal on the display screen, wherein the modified location signal is
displayed on a display screen during use of the needle assembly so as to
locate the
at least one portion of the needle.
In one embodiment, generating the location signal for the portion of the
needle based on the data signals from the autonomous ultrasound imaging system
may include determining, via the processor the needle assembly, a threshold
for the
data signals and identifying, via the processor, a plurality of peak
amplitudes within
the data signals based on when the data signals exceed the threshold. In such
embodiments, determining the threshold for the data signals may include
determining a baseline noise for the data signals and subsequently determining
the
threshold for the data signals by eliminating the baseline noise therefrom.
In another embodiment, generating the location signal for the portion of the
needle based on the data signals from the autonomous ultrasound imaging system
may further include determining a meta-frame repeat period of the data
signals,
determining a time offset for the data signals based on the meta-frame repeat
period, anticipating a future frame rate of the autonomous ultrasound imaging
system based on the time offset, and signaling to the needle transducer of the
needle assembly to flash so as to display the location signal at the at least
one
2
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
portion of the needle on the display screen in anticipation of the future
frame rate.
In such embodiments, determining the meta-frame repeat period of the data
signals may include receiving the plurality of peak amplitudes, storing the
plurality of
peak amplitudes, determining a time frame between the stored plurality of peak
amplitudes, maintaining a record of the time frames between each of the
plurality of
peaks, applying an arithmetic correlation to the record of the time frames,
and/or
determining the meta-frame repeat period of the autonomous ultrasound imaging
system based on the record of the time frames.
In further embodiments, the method may include pulsing the location signal at
a known pulse rate using the known pulse rate to extract the location signal
from
ultrasound signal noise. In additional embodiments, modifying the
characteristic(s)
of the location signal may include collecting multiple pulsed location signals
and
processing the collected pulsed location signals via at least one of filtering
the
collected pulsed location signals, transforming one or more of the collected
pulsed
location signals, or removing outliers from the collected pulsed location
signals. In
addition, in one embodiment, the characteristic(s) of the location signal may
include,
for example, color, shape, size, brightness, intensity, rate of flashing,
and/or
echogenicity.
Thus, in particular embodiments, the location signal may include a
periodically flashing marker and/or a reflective marker coinciding with the at
least
one portion of the needle. For example, in one embodiment, the portion of the
needle may include a distal end of the needle.
In another aspect, the present disclosure is directed to a needle assembly for
use with an autonomous ultrasound imaging system. The needle assembly includes
a needle having a proximal end and a distal end adapted to be inserted into a
patient. The needle assembly also includes a needle transducer mounted to an
exterior surface of the needle and is electrically coupled to a power source.
The
needle transducer is configured to receive data signals from the autonomous
ultrasound imaging system which contain information relating to a plurality of
ultrasound waves generated by an ultrasound probe of the autonomous ultrasound
imaging system. The needle assembly further includes at least one processor
configured to perform one or more operations, including but not limited to,
generating a location signal for at least one portion of the needle based on
the data
signals from the autonomous ultrasound imaging system and modifying at least
one
3
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
characteristic of the location signal so as to improve visibility of the
location signal
on the display screen, wherein the modified location signal is displayed on a
display
screen during use of the needle assembly so as to locate the at least one
portion of
the needle. It should be further understood that the needle assembly may
include
any of the additional features and/or steps described herein.
These and other features, aspects and advantages of the present invention
will become better understood with reference to the following description and
appended claims. The accompanying drawings, which are incorporated in and
constitute a part of this specification, illustrate embodiments of the
invention and,
together with the description, serve to explain the principles of the
invention.
BRIEF DESCRIPTION OF THE DRAWINGS
A full and enabling disclosure of the present invention, including the best
mode thereof, directed to one of ordinary skill in the art, is set forth in
the
specification, which makes reference to the appended figures, in which:
FIG. 1 illustrates a perspective view of one embodiment of an imaging
system according to the present disclosure;
FIG. 2 illustrates a block diagram one of embodiment of a controller of an
imaging system according to the present disclosure;
FIG. 3 illustrates a schematic diagram of one embodiment of a needle
assembly according to the present disclosure, particularly illustrating the
needle
assembly communicating with an autonomous ultrasound imaging system and/or an
add-on system of the autonomous ultrasound imaging system;
FIG. 4 illustrates a perspective view of a portion of one embodiment of a
distal end of a needle assembly according to the present disclosure,
particularly
illustrating the location for a transducer and corresponding wire, wherein the
location
for the transducer is an embedded flat portion within the needle wall;
FIG. 5 illustrates a perspective view of a portion of another embodiment of a
distal end of a needle assembly according to the present disclosure,
particularly
illustrating the location for a transducer and corresponding wire, wherein the
location
for the transducer is a flat portion that extends to the distal end of the
needle;
FIG. 6 illustrates a perspective view of a portion of still another embodiment
of a distal end of a needle assembly according to the present disclosure,
particularly
illustrating the location for a transducer and corresponding wire, wherein the
location
4
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
for the transducer is a recess within the wall of the needle;
FIG. 7 illustrates a perspective view of a portion of yet another embodiment
of a distal end of needle assembly according to the present disclosure,
particularly
illustrating a flexible printed circuit board mounted onto an exterior surface
of the
needle;
FIG. 8 illustrates a perspective view of a portion of another embodiment of a
distal end of needle assembly according to the present disclosure,
particularly
illustrating a flexible printed circuit board mounted with a recess of the
needle so as
to electrically connect a needle transducer at the distal end to a power
source;
FIG. 9 illustrates a perspective view of a portion of still another embodiment
of a distal end of needle assembly according to the present disclosure,
particularly
illustrating a plurality of needle transducers radially spaced around a
circumference
of the needle;
FIG. 10 illustrates a perspective view of a portion of yet another embodiment
of a distal end of needle assembly according to the present disclosure,
particularly
illustrating a plurality of needle transducers mounted along a length of the
needle;
FIG. 11 illustrates a perspective view of another embodiment of a distal end
of a needle assembly according to the present disclosure, particularly
illustrating a
conduit assembly mounted onto an exterior surface of the needle so as to
electrically connect a transducer at the distal end to a power source;
FIG. 12 illustrates a flow chart of one embodiment of a method for identifying
a needle of a needle assembly on a display screen according to the present
disclosure;
FIG. 13 illustrates a sample image from a display screen according to the
present disclosure, particularly illustrating a location marker generated by a
needle
assembly reflected thereon;
FIG. 14 illustrates a graph of one embodiment of data signals received from
the ultrasound imaging system by a needle assembly according to the present
disclosure, particularly illustrating a predetermined threshold set with
respect to the
data signals as a function of a signal-to-noise ratio of the data signals
FIG. 15 illustrates a flow diagram of one embodiment of a system for
identifying a needle of a needle assembly on a display screen according to the
present disclosure;
FIG. 16 illustrates a flow diagram of one embodiment for determining a meta-
5
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
frame repeat period of an autonomous ultrasound imaging system according to
the
present disclosure; and
FIG. 17 illustrates a graph of amplitude (y-axis) versus time (x-axis) that
includes one embodiment of a plurality of ultrasound bursts according to the
present
disclosure.
DETAILED DESCRIPTION
Reference will now be made in detail to one or more embodiments of the
invention, examples of the invention, examples of which are illustrated in the
drawings. Each example and embodiment is provided by way of explanation of the
invention, and is not meant as a limitation of the invention. For example,
features
illustrated or described as part of one embodiment may be used with another
embodiment to yield still a further embodiment. It is intended that the
invention
include these and other modifications and variations as coming within the
scope and
spirit of the invention.
Referring now to the drawings, FIGS. 1-3 illustrate a medical imaging system
10 for scanning, identifying, and navigating anatomical objects of a patient
according to the present disclosure. As used herein, the anatomical object(s)
22
and surrounding tissue described herein may include any anatomical structure
and/or surrounding tissue of a patient. For example, in one embodiment, the
anatomical object(s) 22 may include one or more nerves or nerve bundles. More
specifically, in another embodiment, the anatomical object(s) 22 may include
an
interscalene brachial plexus of the patient, which generally corresponds to
the
network of nerves running from the spine, formed by the anterior ram i of the
lower
four cervical nerves and first thoracic nerve. As such, the surrounding tissue
of the
brachial plexus generally corresponds to the sternocleidomastoid muscle, the
middle scalene muscle, the anterior scalene muscle, and/or similar.
It should be understood, however, that the system of the present disclosure
may be further used for any variety of medical procedures involving any
anatomical
structure in addition to those relating to the brachial plexus. For example,
the
anatomical object(s) 22 may include upper and lower extremities, as well as
compartment blocks. More specifically, in such embodiments, the anatomical
object(s) 22 of the upper extremities may include interscalene muscle,
supraclavicular muscle, infraclavicular muscle, and/or axillary muscle nerve
blocks,
6
CA 03124801 2021-06-23
WO 2020/139649
PCT/US2019/067055
which all block the brachial plexus (a bundle of nerves to the upper
extremity), but at
different locations. Further, the anatomical object(s) 22 of the lower
extremities may
include the lumbar plexus, the Iliac fascia, the femoral nerve, the sciatic
nerve, the
adductor canal, the popliteal, the saphenous, and/or similar. In addition, the
anatomical object(s) 22 of the compartment blocks may include the intercostal
space, transversus abdominis plane, and thoracic paravertebral space, and/or
similar.
In addition, as shown, the imaging system 10 may correspond to an
autonomous ultrasound imaging system or any other suitable imaging system that
can benefit from the present technology. In addition, as shown, an additional
add-
on system 15 may also be used in conjunction with the autonomous ultrasound
imaging system, which will be discussed in more detail herein. Further, as
shown,
the imaging system 10 may generally include a controller 12 having one or more
processor(s) 14 and associated memory device(s) 16 configured to perform a
variety of computer-implemented functions (e.g., performing the methods and
the
like and storing relevant data as disclosed herein), as well as a display
screen 18
configured to display an image 20 of an anatomical object 22 or the
surrounding
tissue to an operator. In addition, the imaging system 10 may include a user
interface 24, such as a computer and/or keyboard, configured to assist a user
in
generating and/or manipulating the display screen 18. Further, as shown, the
add-
on system 15 may also include an additional display screen 17.
Additionally, as shown in FIG. 2, the processor(s) 14 may also include a
communications module 26 to facilitate communications between the processor(s)
14 and the various components of the imaging system 10, e.g. any of the
components of FIG. 1. Further, the communications module 26 may include a
sensor interface 28 (e.g., one or more analog-to-digital converters) to permit
signals
transmitted from one or more probes (e.g. such as an ultrasound probe 30
and/or
the needle transducer 35) to be converted into signals that can be understood
and
processed by the processor(s) 14. It should be appreciated that the various
probes/sensors described herein may be communicatively coupled to the
communications module 26 of the controller 12 using any suitable means. For
example, as shown in FIG. 2, the ultrasound probe 30 may be coupled to the
sensor
interface 28 via a wired connection. However, in other embodiments, the
ultrasound
probe 30 may be coupled to the sensor interface 28 via a wireless connection,
such
7
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
as by using any suitable wireless communications protocol known in the art. As
such, the processor(s) 14 may be configured to receive one or more sensor
signals
from the ultrasound probe 30.
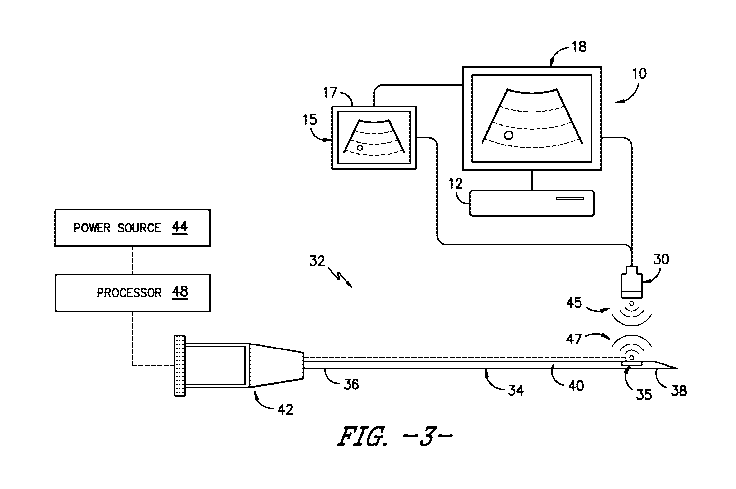
Referring now to FIG. 3, a side view of one embodiment of a needle
assembly 32 according to the present disclosure that can be used in
combination
with the autonomous ultrasound imaging system 10 is illustrated. More
specifically,
as shown, the needle assembly 32 includes a needle 34 having a proximal end 36
and a distal end 38 adapted to be inserted into a patient and a needle
transducer
35, which is mounted to an exterior surface 40 of the needle 34, e.g. at the
distal
end 38 thereof. However, in additional embodiments, it should be understood
that
the needle transducer 35 may be located at any location along the needle 4. In
addition, as shown, the needle assembly 32 may also include at least one
processor
48 configured to process information relating to the various components of the
needle assembly 32. For example, as shown, the processor 48 may be configured
to receive, at least, data signals 45 from the ultrasound probe 30 i.e.
relating to
ultrasound waves generated by the ultrasound probe 30 of the autonomous
ultrasound imaging system 10. Further, as shown, the processor 48 may be
configured to send, at least, data signals 47 from the needle transducer 35
i.e.
relating to a location thereof. Moreover, the needle 34 may also include a
needle
hub 42 at its proximal end 36. Moreover, the needle transducer 35 may be
coupled
to a power source 44, e.g. through the needle hub 42, that provides electrical
power
to the needle transducer 35.
As used herein, the term "processor" refers not only to integrated circuits
referred to in the art as being included in a computer, but also refers to a
controller,
a microcontroller, a microcomputer, a programmable logic controller (PLC), a
field-
programmable gate array (FPGA), an Application-Specific Integrated Circuit
(ASIC),
and other programmable circuits. As such, the processors 14, 45 described
herein
are also configured to compute advanced control algorithms and communicate to
a
variety of Ethernet or serial-based protocols (Modbus, OPC, CAN, etc.).
Furthermore, in certain embodiments, the processors 14, 45 may communicate
with
a server through the Internet for cloud computing in order to reduce the
computation
time and burden on the local device.
Additionally, the memory device(s) described herein may generally include
memory element(s) including, but not limited to, computer readable medium
(e.g.,
8
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
random access memory (RAM)), computer readable non-volatile medium (e.g., a
flash memory), a floppy disk, a compact disc-read only memory (CD-ROM), a
magneto-optical disk (MOD), a digital versatile disc (DVD) and/or other
suitable
memory elements. Such memory device(s) may generally be configured to store
suitable computer-readable instructions that, when implemented by the
processors
14, 45, configure the processors 14, 45 to perform the various functions as
described herein.
In addition, the needle transducer 35 may be any suitable transducer now
known or later developed in the art. For example, in one embodiment, the
transducer 35 may be a piezoelectric (PZT) transducer. Alternatively, the
transducer 35 may be a capacitive micromachined ultrasonic transducer (CMUT).
In yet another embodiment, the transducer(s) 30 may also include
Polydimethylsiloxane (PDMS) transducers and/or photoacoustic transducers.
Referring now to FIGS. 4-6, perspective views of different embodiments of
the needle 34 of the needle assembly 32 are illustrated. More specifically,
FIG. 4
illustrates a perspective view of one embodiment of the distal end 38 of the
needle
34 according to the present disclosure, particularly illustrating the location
for the
needle transducer 35 on a flat portion 49 of the needle 34 and the
corresponding
wire(s) within a longitudinal groove 51 of the needle 34. Alternatively, FIG.
5
illustrates a perspective view of one embodiment of the distal end 38 of the
needle
34 according to the present disclosure, particularly illustrating the location
for the
needle transducer 35 atop a flat portion that extends to the distal end 38 of
the
needle 34 and the corresponding wire(s) also within a longitudinal groove 51.
In still
another embodiment, FIG. 6 illustrates a perspective view of yet another
embodiment of the distal end 38 of the needle 34 according to the present
disclosure, particularly illustrating the location for the needle transducer
35 within
the recess 54 of the needle 34 and the corresponding wire(s) also within a
longitudinal groove 51.
Referring now to FIGS. 7-11, various example needle assemblies 32 are
provided according the present disclosure. FIG. 7 illustrates a detailed view
of one
embodiment of the needle assembly 32 according to the present disclosure,
particularly illustrating a flexible printed circuit board 46 being utilized
to electrically
connect the power source 44 and the needle transducer 35 is illustrated. FIG.
8
illustrates a perspective view of a portion of another embodiment of the
distal end 38
9
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
of the needle 34 according to the present disclosure, particularly
illustrating the
flexible printed circuit board 46 mounted with the recess 54 of the needle 34
so as to
electrically connect the needle transducer 35 to the power source 44. FIG. 9
illustrates a perspective view of a portion of still another embodiment of the
distal
end 38 of the needle 34 according to the present disclosure, particularly
illustrating a
plurality of needle transducers 30 radially spaced around a circumference of
the
needle 34. FIG. 10 illustrates a perspective view of a portion of yet another
embodiment of the distal end 38 of the needle 34 according to the present
disclosure, particularly illustrating a plurality of needle transducers 30
mounted
along a length of the needle 34. FIG. 11 illustrates yet another perspective
view of
one embodiment of the distal end 38 of the needle 34 according to the present
disclosure, particularly illustrating a conduit assembly 56 for receiving the
associated wires for connecting the needle transducer 35 to the power source
44.
More specifically, as shown in FIGS. 7 and 8, the flexible printed circuit
board
46 may be mounted on the exterior surface 40 of the needle 34 and may extend
from the proximal end 36 to the distal end 38. Thus, as shown, the flexible
printed
circuit board 46 is configured to electrically connect the needle transducer
35 to the
power source 44. In one embodiment, the flexible printed circuit board 46 may
include, for example, a flexible base 50 having a plurality of conductive
tracks 52 or
traces printed thereon. As such, the flexible base 50 can easily flex with the
shape
of the needle 34 so as to be effectively mounted onto the exterior surface 40
of
needle 34. For example, in certain embodiments, the conductive tracks 52 may
be
printed onto the flexible base 50 via screen printing, flexography, gravure
printing,
offset lithography, inkjet printing, additive manufacturing (e.g. 3D printing)
and/or
.. any other suitable printing process. In another embodiment, the flexible
base 50
may be omitted.
In several embodiments, the various components of the flexible printed circuit
board 46 may be printed on the exterior surface 40 of needle 34 via the
additive
manufacturing process. In such embodiments, the additive manufacturing process
may include, for example, directed energy deposition, direct laser deposition,
or any
other suitable additive manufacturing process. By using additive
manufacturing, the
various components of the flexible printed circuit board 46 can be printed
onto the
needle 34 in thin layers so as not to disturb the overall efficacy of the
needle 34 in
puncturing the necessary tissue of the patient. For example, in one
embodiment,
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
the conductive tracks 52 may have a predetermined thickness ranging from about
0.01 millimeters (mm) to about 0.05 mm. As used herein, terms of degree, such
as
"about," are meant to encompass a range of +/- 10% from the value set forth.
In
addition, in such embodiments, the conductive traces 52 may be narrow, such as
from about 0.10 millimeter (mm) up to about 0.25 mm. Further, in certain
embodiments, ground planes can be used to enclose the signal trace to achieve
better noise immunity.
In addition to being mounted at the distal end 38 of the needle 34, it should
also be understood that the needle transducer 35 may also be mounted at any
suitable location on the needle 34. Further, as shown in FIGS. 3-8, the needle
transducer 35 may be mounted on one side of the needle 34. In such
embodiments, during operation, the user of the needle assembly 32 must orient
the
needle transducer 35 towards the ultrasound probe 30 of the ultrasound imaging
system 10. In another embodiment, as shown in FIG. 9, the needle assembly 32
may include a plurality of needle transducers 30 spaced along the length of
the
needle 34. In alternative embodiments, as shown in FIG. 8, the needle assembly
32
may include multiple needle transducers 30 spaced radially around the needle
34.
In such embodiments, orientation of the needle 34 is not relevant (i.e. the
needle
assembly 32 is not direction sensitive) as the ultrasound probe can easily
view one
of the radially spaced transducers 30 due to the various radial positions.
Referring back to FIG. 11, rather than utilizing the flexible printed circuit
board 46 illustrated in FIG. 7, the needle assembly 32 may include the conduit
assembly 56 secured to the exterior surface 40 of the needle 34 from the
proximal
end 36 to the distal end 38. In such embodiments, the needle assembly 32 may
also include at least one electrically-conductive cable 60 extending through
the
conduit assembly 56 (e.g. extending loosely through the conduit assembly 56
rather
than being printed to the surface of the needle 34) so as to electrically
connect the
needle transducer 35 to the power source 44 of the ultrasound imaging system
10.
In such embodiments, the conduit assembly 56 may be constructed of metal
tubing,
polymer shrink tubing, or any other suitable tubing material. It should be
understood
that the conduit assembly 56 may define a single lumen 58 or any number of
additional lumens such as a double lumen and the lumens may be outside of the
needle 34 or inside of the needle 34.
In additional embodiments, the electrically-conductive cable(s) 60 may
11
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
include a single core wire, a coaxial cable, or any other suitable cable or
wire. For
example, in one embodiment, the electrically-conductive cable(s) 60 may
include a
solid- or multi-strand wire, such as an insulated wire of a small gauge (e.g.
in the
order of 40AWG or smaller). In another embodiment, the electrically-conductive
cable(s) 60 may include a coaxial cable of a small gauge (e.g. in the order of
40AWG or smaller) so as to provide a better noise immunity environment. In
such
embodiments, the lumen 58 of the conduit assembly 56 may be up to about 0.5
mm,
such as about 0.25 mm.
It should also be understood that interconnection of the various electrical
connections described herein (e.g. the flexible printed circuit board 46
and/or the
conduit assembly 56/cables 60) and the needle transducer 35 can be achieved
via a
variety of methods, including for example via soldering or using a conductive
epoxy
joint, i.e. with or without a polychlorinated biphenyl (PCB) interface, which
can be
used to wire bond to the device rather than connecting directly to the
wire/cable.
Referring now to FIG. 12, a flow diagram of one embodiment of a method for
identifying a needle of a needle assembly on a display screen is illustrated
according to the present disclosure. In general, the method 100 will be
described
herein with reference to the autonomous ultrasound imaging system 10 and the
needle assembly 32 shown in FIGS. 1-11 and 13-16. However, in other
embodiments, the method 100 may be used in connection with any other suitable
autonomous ultrasound imaging system and needle assembly configuration. It
should be appreciated that, although FIG. 12 depicts steps or functions
performed in
a particular order for purposes of illustration and discussion, the steps
discussed
herein are not limited to any particular order or arrangement. One skilled in
the art,
using the disclosures provided herein, will appreciate that various steps or
functions
of the methods disclosed herein can be omitted, rearranged, combined, and/or
adapted in various ways without deviating from the scope of the present
disclosure.
In one embodiment, it should be understood that the method 100 may include
inserting the needle 34 of the needle assembly 32 into a patient, generating,
e.g. via
the ultrasound probe 30 and/or the needle assembly 32, ultrasound waves that
include the needle 34, and then subsequently generating, via the display
screen, an
image of the needle 34 inserted within the patient based on the ultrasound
waves.
In other words, the needle assembly 32 is configured to generate its own
ultrasound
waves to trick the ultrasound imaging system 10 into thinking the waves are
12
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
reflected signals from the ultrasound probe 30.
Thus, as shown at 102 in FIG. 12, the method 100 includes receiving, via the
processor 48 of the needle assembly 32, data signals 45 from the autonomous
ultrasound imaging system 10 that include, for example, information relating
to
ultrasound waves generated by the ultrasound probe 30. In several embodiments,
the method 100 may include monitoring the data signals 45 from the autonomous
ultrasound imaging system 10 in real-time.
Referring still to FIG. 12, as shown at 104, the method 100 also includes
generating, via the needle transducer 35 of the needle assembly 35, a location
signal 68 for at least one portion of the needle 34 based on the data signals
45 from
the autonomous ultrasound imaging system 10. More specifically, as shown in
FIG.
14, the processor(s) 48 may be configured to determine a threshold 70 for the
received data signals 45. Thus, as shown, the processor(s) 48 may also be
configured to identify a plurality of peak amplitudes 72 within the data
signals 45,
e.g. based on when the data signals exceed the threshold 70.
Referring back to FIG. 12, as shown at 106, the method 100 also includes
modifying, via the processor 48, at least one characteristic of the location
signal 68
so as to improve visibility of the location signal on the display screen,
wherein the
modified location signal 68 is displayed on the display screen during use of
the
needle assembly so as to locate the at least one portion of the needle 34. For
example, in one embodiment, the modified characteristic(s) of the location
signal 68
as described herein may include, for example, color, shape, size, brightness,
intensity, rate of flashing, echogenicity, and/or other suitable
characteristic of the
signal 68. For example, as shown in FIG. 13, the location signal 68 is shown
on the
display screen 18 as being illuminated at the distal end 38 of the needle 34.
Thus,
in particular embodiments, the location signal 68 may include a periodically
flashing
marker, a reflective marker coinciding with the at least one portion of the
needle 34,
and/or any other suitable distinctive marker at the distal end 38 of the
needle 34.
In certain embodiments, the processor 48 is configured to pulse the location
signal 68 at a known pulse rate and use the known pulse rate to extract the
location
signal 68 from ultrasound signal noise. In other words, by pulsing the
location signal
68 at the known pulse rate, the signal-to-noise ratio of the location signal
68 can be
increased as compared to other data signals such that it can be easily
extracted and
modified. As such, the processor 48 can easily modify the characteristic(s) of
the
13
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
location signal 68 by extracting multiple pulsed location signals 68 from
ultrasound
noise and processing the collected pulsed location signals 68. In such
embodiments, processing the collected pulsed location signals 68 may include,
for
example, filtering the collected pulsed location signals 68, transforming one
or more
of the collected pulsed location signals 68, and/or removing outliers from the
collected pulsed location signals 68. Thus, the processor 48 can then modify
and/or
replace the location signal 68 with a different marker to improve
visibility/contrast/shape on the display screen.
In additional embodiments, as shown in FIG. 3, the display screen 18 may be
part of the autonomous ultrasound imaging system 10. In alternative
embodiments,
as shown, the display screen 17 may be part of the add-on system 15 to the
autonomous ultrasound imaging system 10.
The method 100 of the present disclosure may be better understood with
respect to FIGS. 15 and 16. For example, as shown in FIG. 15, a flow diagram
of
one embodiment of process for identifying the needle 34 of the needle assembly
32
on a display screen according to the present disclosure is illustrated. FIG.
16
illustrates a flow diagram of one embodiment for determining a meta-frame
repeat
period of an autonomous ultrasound imaging system according to the present
disclosure. FIG. 17 illustrates a graph of amplitude (y-axis) versus time (x-
axis) that
includes one embodiment of a plurality of ultrasound bursts 114 according to
the
present disclosure. For example, for certain ultrasound machines, the meta-
frame
rate 110 (e.g. T) generally includes a collection of sub-frames 112 (e.g. t1,
t2, t3,
etc.). Therefore, in such embodiments, the meta-frame rate 110 may be equal to
the number of frames per a specific time period (e.g. seconds). In this
instance, the
meta-frame rate 110 is equal to three frames per second, assuming T equals one
second. In other ultrasound machines, the sub-frames may all be equal (e.g.
T=t1=t2=t3. In such embodiments, the meta-frame rate is synonymous with the
frame rate.
Referring particularly to FIG. 15, the processor(s) 48 described herein
receives the data signals 45 from the transducer 35. As shown at 74, the
processor(s) 48 can then process the data signals 45, e.g. using various
analog-to-
digital converters, filtering, etc. In addition, as shown, the processor(s) 48
may then
time stamp 76 the data signals 45, e.g. using a clock 78 or similar.
As shown at 80 and 82, the processor(s) 48 can then determine a baseline
14
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
noise for the data signals 45 as well as the peak amplitudes of the data
signals 45.
Thus, as shown at 84, the processor(s) 48 can then set the threshold 70 by
accounting for the baseline noise 80 and considering the peak amplitudes 82.
In
certain embodiments, as shown, a gain 86 can eventually be applied to the
received
data signals 45, i.e. after the peak amplitudes 82 have been determined. Thus,
as
shown at 88, the processor(s) 48 can then select the trigger points
corresponding
the to the peak amplitudes 82. In addition, as shown at 90, the processor(s)
48 is
also configured to determine a meta-frame repeat period of the data signals
45. In
such embodiments, as shown, the processor(s) 48 may also time stamp 76 the
baseline noise 80, the peak amplitudes 82, the meta-frame repeat periods 90,
and/or the trigger points 88.
More specifically, as shown in FIG. 16, various process steps for determining
the meta-frame repeat period (frame rate) 90 of the data signals 45 according
to the
present disclosure are illustrated. As shown, in one embodiment, the
processor(s)
48 is configured to receive and store the trigger points 88 in a memory device
83. In
particular embodiments, as shown, the memory device 83 may periodically
discard
old data 85. In addition, as shown, the processor(s) 48 may also determine,
e.g. via
difference calculator 87, a time frame (see e.g. t1, t2, t3 in FIG. 17)
between the
stored trigger points 88 (peak amplitudes 82). Thus, as shown, the
processor(s) 48
may also maintain a record of the time frames between each of the peaks 82,
e.g.
via sorted store 89. In certain embodiments, the processor(s) 48 may also
optionally apply an arithmetic correlation (e.g. a binary correlation) to the
record of
the time frames. Accordingly, the processor(s) 48 can then determine the meta-
frame spacing 90 (e.g. Tin FIG. 17) of the autonomous ultrasound imaging
system
10 based on the record of the time frames. As such, the present disclosure can
be
used with any brand of ultrasound imaging system having different meta-frames
and/or sub-frames.
Referring back to FIG. 15, the processor(s) 48 is further configured to add a
time offset 92 for the data signals 45 based on the meta-frame repeat period
90. In
other words, by adding the time offset 92, the processor(s) 48 is configured
to
anticipate a future frame rate of the autonomous ultrasound imaging system 10.
Thus, as shown at 94, the processor(s) 48 is configured to trigger a
transmission
event. More specifically, as shown at 96, the processor(s) 48 is configured to
signal
to the needle transducer 35 of the needle assembly 32 to pulse or flash so as
to
CA 03124801 2021-06-23
WO 2020/139649 PCT/US2019/067055
display the location signal 68 on the needle 34 on the display screen.
This written description uses examples to disclose the invention, including
the
best mode, and also to enable any person skilled in the art to practice the
invention,
including making and using any devices or systems and performing any
incorporated methods. The patentable scope of the invention is defined by the
claims, and may include other examples that occur to those skilled in the art.
Such
other examples are intended to be within the scope of the claims if they
include
structural elements that do not differ from the literal language of the
claims, or if they
include equivalent structural elements with insubstantial differences from the
literal
languages of the claims.
16