Note: Descriptions are shown in the official language in which they were submitted.
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
POLYCRYSTALLINE METAL OXIDES WITH ENRICHED GRAIN
B OUNDARIE S
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application depends from and claims priority to U.S.
Patent Application No:
16/250,615 filed January 17,2019, U.S. Patent Application No: 16/250,762 filed
January 17,2019,
and U.S. Patent Application No: 16/250,622 filed January 17, 2019, the entire
contents of each of
which are incorporated herein by reference.
FIELD
[0002] Disclosed is polycrystalline metal oxide particle, methods of
manufacture thereof, and
electrochemical cells or batteries comprising the same.
BACKGROUND
[0003] Layered structure lithium nickelate (LiNi02)-based materials
have been developed for
Lithium-ion battery cathodes because they generally have lower cost, higher
capacity and higher
rate capability than the historically predominant LiCo02 cathode material.
However, pure LiNi02
materials exhibit poor electrochemical stability and cycling performance. To
address this, non-
nickel, elemental additives have been formulated into LiNi02 that stabilize
the structure improving
the cycling performance, but typically at the expense of discharge capacity.
As demands for energy
density have increased, research has focused on optimizing and reducing these
non-nickel
additives to capture the capacity of high Ni materials while at the same time
maintaining cycling
performance.
1
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
[0004]
As such, new materials are needed to address the demands for high
capacity materials
with long cycle life. The materials provided herein and methods of forming
such materials address
this need by maintaining high capacity over a long cycle life.
SUMMARY
[0005] The
following summary is provided to facilitate an understanding of some of the
innovative features unique to the present disclosure and is not intended to be
a full description. A
full appreciation of the various aspects of the disclosure can be gained by
taking the entire
specification, claims, drawings, and abstract as a whole.
[0006]
Provided are secondary particles that were found to have greatly reduced
impedance
growth when used as a cathode electrochemically active material in a Li-ion
secondary cell. It was
found that by selectively enriching grain boundaries between the crystallites
in the secondary
particles with a combination of Co and Al, that the improved impedance
profiles could be
achieved, and that these improved impedance profiles were achieved in many
compositionally
distinct electrochemically active materials.
[0007] As
such provided are particles that include a plurality of crystallites
comprising a first
composition comprising lithium, nickel, and oxygen; a grain boundary between
adjacent
crystallites of the plurality of crystallites and comprising a second
composition having the layered
a-NaFe02-type structure, a cubic structure, a spinel structure, or a
combination thereof; wherein a
concentration of aluminum in the grain boundary is greater than a
concentration of aluminum in
the crystallites, and wherein a concentration of cobalt in the grain boundary
is greater than a
concentration of cobalt in the crystallites. It was found in some aspects that
Al enrichment of grain
boundaries was non-uniform, incomplete or not achieved, but that when
manufacturing processes
as provided herein were utilized Al grain boundary enrichment could be
achieved, optionally Co
2
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
and Al grain boundary enrichment. As such, in some aspects, the Al is
substantially uniformly
distributed through the plurality of particles.
[0008] In some aspects, the amount of the Al in the grain boundaries
is 0.01 at% to 10 at% of
the total transition metal amounts in the remainder of the secondary particle.
Optionally, the
amount of the Co in the grain boundaries is 0 at% to 10 at% of the total
transition metal amounts
in the remainder of the secondary particle, optionally 0.1 at% to 10 at% of
the total transition metal
amounts in the remainder of the secondary particle. Optionally, the amount of
the Al in the grain
boundaries is 0.01 at% to 5 at% of the total transition metal amounts in the
remainder of the
secondary particle, and the amount of the Co in the grain boundaries is 0.01
at% to 10 at% of the
total transition metal amounts in the remainder of the secondary particle.
Optionally, the amount
of aluminum in the grain boundary is equal to or less than the amount of Co in
the grain boundary.
[0009] In some aspects, the plurality of crystallites has an a-NaFe02-
type layered structure, a
cubic structure, a spinel structure, or a combination thereof.
[0010] Optionally, the first composition, the second composition or
both, in any of the
forgoing or other aspects are defined by defined by Lii+xM02+y, wherein
¨0.1<x<0.3,
¨0.3y<0.3, and
wherein M comprises nickel at greater than or equal to 10 atomic percent.
Optionally, M comprises
an atomic percent of nickel greater than or equal to 75 at%. Optionally, the
overall grain boundary
comprises cobalt in an amount of about 2 at% to about 99 at%, and aluminum in
an amount of
about 2 at% to about 99 at%. Optionally, in the first composition, the second
composition, or both,
M further comprises an additional metal, wherein the additional metal is
present in an amount of
about 1 at% to about 90 at%; the additional metal is selected from the group
consisting of Mg, Sr,
3
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
Co, Al, Ca, Cu, Zn, Mn, V, Ba, Zr, Ti, Nb, Ta, Cr, Fe, Mo, W, Hf, B, and any
combination thereof,
whereby the one or more additional elements optionally reside in a Li layer, a
M layer, or both.
[0011] Optionally, the crystallites comprise cobalt, with the cobalt
concentration in the range
of 1 at% to about 50 at%, optionally, in the range of 1 at% to about 15 at%.
In some aspects, the
crystallites comprise Mn present in an amount of about 1 at% to about 60 at%,
and the grain
boundary comprises Mn present in an amount of about 1 at% to about 60 at%. In
other aspects, the
grain boundary comprises Ni, Co, and Al. Optionally, the concentration of Ni
in the grain boundary
is greater than 75 at%.
[0012] Some aspects of a particle include an outer coating on a
surface of the particle, the
outer coating comprising: an oxide of one or more elements selected from Al,
Zr, Y, Co, Ni, Mg,
and Li; a fluoride comprising one or more elements selected from Al, Zr, and
Li; a carbonate
comprising one or more elements selected from Al, Co, Ni, Mn, and Li; or a
phosphate or sulfate
comprising one or more elements selected from Al and Li.
[0013] In other aspects as provided herein are provided an
electrochemically active
polycrystalline secondary particle comprising: a plurality of crystallites,
the plurality of crystallites
comprising a first composition defined by Li i+xM02+y, wherein ¨0.1<x<0.3,
¨0.3<y<0.3, and
wherein M comprises nickel at greater than or equal to 80 atomic percent; and
a grain boundary
between adjacent crystallites of said plurality of crystallites and comprising
a second composition
optionally defined by Li1+xM02+y, wherein ¨0.1<x<0.3, ¨0.3<y<0.3 and
optionally having an a-
NaFe02-type layered structure, a cubic structure, or a combination thereof,
wherein a
concentration of aluminum in the grain boundary is greater than a
concentration of aluminum in
the crystallites, and optionally wherein a concentration of cobalt in the
grain boundary is greater
than a concentration of cobalt in the crystallites, and wherein the aluminum
is substantially
uniformly distributed through the grain boundary. Optionally, a concentration
of cobalt in the
4
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
crystallites is about 0 to about 17 atomic percent of M in the first
composition, and amount of
cobalt in the grain boundary is about 0 to about 10 atomic percent of M in the
crystallites,.
Optionally, M further comprises one or more elements selected from the group
consisting of Na,
K, Al, Mg, Co, Mn, Ca, Sr, Ba, Zn, Ti, Zr, Y, Cr, Mo, Fe, V, Si, Ga and B,
said one or more
elements residing in a Li layer, a M layer, or both, of the crystallites.
Optionally, M comprises an
atomic percent of nickel greater than or equal to 90 percent.
[0014] Also provided are electrochemical cells. The electrochemical
cells include a cathode
active material. The cathode active material optionally includes any of the
particles as provided
above or otherwise herein. The electrochemical cell is optionally
characterized by an impedance
growth at 4.2V less than 100% for greater than 100 cycles at 45 C, optionally
less than 100% for
greater than 200 cycles at 45 C.
[0015] Also provided are electrochemical cells, optionally secondary
cells, optionally lithium
ion secondary cells that include an anode, an electrolyte, and a cathode, the
cathode comprising
an electrochemically active cathode active material comprising a plurality of
particles, said
plurality of particles comprising a plurality of crystallites each comprising
a first composition
comprising lithium, nickel, and oxygen; a grain boundary between adjacent
crystallites of the
plurality of crystallites and comprising a second composition having a layered
a-NaFe02-type
structure, a cubic structure, a spinel structure, or a combination thereof;
wherein the
electrochemically active cathode active material has an initial discharge
capacity of 180 mAh/g or
greater; and wherein the electrochemical cell has an impedance growth at 4.2V
less than 50% for
greater than 100 cycles at 45 C. Electrochemical cells optionally are
characterized by an
impedance growth at 50% state of charge of less than 50% for greater than 200
cycles at 45 C,
optionally less than 120% for greater than 200 cycles at 45 C. In some
aspects, an electrochemical
cell is characterized by an impedance growth at 50% state of charge of less
than 50% for greater
5
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
than 200 cycles at 45 C. Optionally, each of the crystallites include
lithium, nickel, cobalt, and
oxygen. Optionally, the crystallites include Al, Mn, Mg, or combinations
thereof.
[0016] Optionally, the first composition, the second composition or
both, in any of the
forgoing or other aspects are defined by Li i+xM02+y, wherein
¨0.95<x<0.3,
¨0.3y<0.3, and
wherein M comprises nickel at greater than or equal to 80 atomic percent.
Optionally, M in a first
composition comprises an atomic percent of nickel greater than or equal to 75
at% relative to total
transition metal in the first composition. In a second composition, M is
optionally less than or
equal to 90 at% relative to total transition metal in the second composition.
Optionally, the overall
grain boundary comprises cobalt in an amount of about 2 at% to about 99 at%,
and aluminum in
an amount of about 2 at% to about 99 at%. Optionally, in the first
composition, the second
composition, or both, M further comprises an additional metal, wherein the
additional metal is
present in an amount of about 1 at% to about 90 at% relative to total metal in
the respective first
or second composition; the additional metal is selected from the group
consisting of Mg, Sr, Co,
Al, Ca, Cu, Zn, Mn, V, Ba, Zr, Ti, Nb, Ta, Cr, Fe, Mo, W, Hf, B, and any
combination thereof,
whereby the one or more additional elements optionally reside in a Li layer, a
M layer, or both.
[0017] Optionally, the crystallites comprise cobalt, with the cobalt
concentration in the range
of 1 at% to about 50 at%, optionally, in the range of 1 at% to about 15 at%%
relative to total
transition metal the first composition. In some aspects, the crystallites
comprise Mn present in an
amount of about 1 at% to about 60 at%, and the grain boundary comprises Mn
present in an amount
of about 1 at% to about 60 at%. In other aspects, the grain boundary comprises
Ni, Co, and Al.
Optionally, the concentration of Ni in the grain boundary is greater than 75
at%.
6
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
BRIEF DESCRIPTION OF THE DRAWINGS
[0018] The aspects set forth in the drawings are illustrative and
exemplary in nature and not
intended to limit the subject matter defined by the claims. The following
detailed description of
the illustrative aspects can be understood when read in conjunction with the
following drawings
and in which:
[0019] FIG. 1 is a schematic perspective view of of a cross-section
of a secondary particle as
provided according to some aspects as described herein;
[0020] FIG. 2 illustrates capacity fade for full cells employing
secondary particles according
to some aspects as provided herein;
[0021] FIG. 3 illustrates impedance growth for full cells employing
secondary particles
according to some aspects as provided herein;
[0022] FIG. 4 illustrates EDS mapping of secondary particles as
provided herein grain
boundary enriched with Al only or Al in the presence of Co;
[0023] FIG. 5 illustrates a scanning transmission electron micrograph
(STEM) image of a
small section of a secondary particle according to one aspect as provided
herein containing several
crystallites from and prepared by enriching the grain boundaries with both 1.9
at% Al and 4 at%
Co, and shows locations at which 3 EDS spot analyses were performed;
[0024] FIG. 6 illustrates the EDS spectra of the three spots indicted
in FIG. 5;
[0025] FIG. 7 illustrates a STEM image of a small section of a
secondary particle according
to one aspect as provided herein containing several crystallites from and
prepared by enriching the
grain boundaries with 1.9 at% Al in the absence of Co in the process solution,
and shows locations
at which 2 EDS spot analyses were performed;
[0026] FIG. 8 illustrates the EDS spectra of the three spots indicted
in FIG. 7;
7
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
[0027] FIG. 9 illustrates EDS mapping of secondary particles as
provided herein made by
non-aqueous processing for grain boundary enrichment of raw particles during
manufacture using
Al alone or Al in the presence of Co in the process solution;
[0028] FIG. 10 illustrates cycling capacity fade for cells formed
with a cathode incorporating
active material grain boundary enriched according to an aspect as provided
herein prepared with 0
at% Al in the process solution and with 0.5 at% Al in the process solution and
their dependence
on additional Co content;
[0029] FIG. 11 illustrates impedance growth for cells formed with a
cathode incorporating
active material grain boundary enriched according to an aspect as provided
herein prepared with 0
at% Al in the process solution and with 0.5 at% Al in the process solution and
their dependence
on additional Co content;
[0030] FIG. 12 illustrates a synergistic benefit observed from
inclusion of Al in addition to
Co enrichment in grain boundaries;
[0031] FIG. 13 illustrates cycling capacity fade and impedance growth
for cells with cathode
materials that were grain boundary enriched according to some aspects as
provided herein from 3
at% Co process application with varied Al levels;
[0032] FIG. 14 illustrates impedance growth at various Al/Co
enrichment atomic percent
ratios for cathode active materials as provided herein according to some
aspects;
[0033] FIG. 15 illustrates STEM and results for EDS analyses of 3
spots in a small section of
a secondary particle of NCA that was prepared as provided herein, being grain
boundary enriched
with Al in the presence of Co;
[0034] FIG. 16 illustrates cycling capacity fade for cells formed
with a cathode incorporating
control or grain boundary enriched NCA active material according to an aspect
as provided herein;
8
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
[0035] FIG. 17 illustrates impedance growth for cells formed with a
cathode incorporating
control or grain boundary enriched NCA active material according to an aspect
as provided herein;
[0036] FIG. 18 illustrates cycling capacity fade for cells formed
with a cathode incorporating
control or grain boundary enriched NCM active material according to an aspect
as provided herein;
and
[0037] FIG. 19 illustrates impedance growth for cells formed with a
cathode incorporating
control or grain boundary enriched NCM active material according to an aspect
as provided herein.
DETAILED DESCRIPTION
[0038] The following description of particular aspect(s) is merely
exemplary in nature and is
in no way intended to limit the scope of the disclosure, its application or
uses, which may of course
vary. The materials and processes are described with relation to the non-
limiting definitions and
terminology included herein. These definitions and terminology are not
designed to function as a
limitation on the scope or practice of the disclosure, but are presented for
illustrative and
descriptive purposes only. While the processes or compositions are described
as an order of
individual steps or using specific materials, it is appreciated that steps or
materials may be
interchangeable such that the description of the invention may include
multiple parts or steps
arranged in many ways as is readily appreciated by one of skill in the art.
[0039] It will be understood that, although the terms "first,"
"second," "third," etc. may be
used herein to describe various elements, components, regions, layers, and/or
sections, these
elements, components, regions, layers, and/or sections should not be limited
by these terms. These
terms are only used to distinguish one element, component, region, layer, or
section from another
element, component, region, layer, or section. Thus, unless specified
otherwise, "a first element,"
9
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
"component," "region," "layer," or "section" discussed below could be termed a
second (or other)
element, component, region, layer, or section without departing from the
teachings herein.
[0040] The terminology used herein is for the purpose of describing
particular aspects only
and is not intended to be limiting. As used herein, the singular forms "a,"
"an," and "the" are
intended to include the plural forms, including "at least one," unless the
content clearly indicates
otherwise. "Or" means "and/or." As used herein, the term "and/or" includes any
and all
combinations of one or more of the associated listed items. It will be further
understood that the
terms "comprises" and/or "comprising," or "includes" and/or "including" when
used in this
specification, specify the presence of stated features, regions, integers,
steps, operations, elements,
and/or components, but do not preclude the presence or addition of one or more
other features,
regions, integers, steps, operations, elements, components, and/or groups
thereof The term "or a
combination thereof' means a combination including at least one of the
foregoing elements.
[0041] Unless otherwise defined, all terms (including technical and
scientific terms) used
herein have the same meaning as commonly understood by one of ordinary skill
in the art to which
this disclosure belongs. It will be further understood that terms such as
those defined in commonly
used dictionaries, should be interpreted as having a meaning that is
consistent with their meaning
in the context of the relevant art and the present disclosure, and will not be
interpreted in an
idealized or overly formal sense unless expressly so defined herein.
[0042] Ni-based layered materials of the LiM02 type are dense,
polycrystalline agglomerates
of primary crystals. These are typically made using standard solid-state
processes at temperatures
in the range of 600 C to 900 C starting from a variety of precursor
materials. Precursor materials
are typically transition metal hydroxides (M(OH)2), lithium precursors (e.g.,
LiOH or Li2CO3), or
inorganic precursors for other dopants (e.g., hydroxides, carbonates,
nitrates). During heating of
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
the precursor mixture, polycrystalline LiM02 is formed along with the
expulsion of gases such as
H20, CO2 or NO2.
[0043] The result of the sintering action under the right conditions
and with the proper
precursors is the formation of a plurality of primary crystallites that are
formed into the larger
secondary particle that may serve as the electrochemically active material. It
was previously found
that the regions between these primary crystallites, the grain boundaries,
could be selectively
enriched with Co as is found in U.S. Patent No. 9,209,455. In the present
disclosure the inventors
have found that significant further improvements can be achieved by replacing
some of the
enriched Co in the grain boundaries with Al leading to further reductions in
impedance growth and
improved cycle life. It is understood that a synergistic relationship between
the Co and the Al in
the grain boundaries, particularly at levels of Co between 2 mole percent Co
and 5 mole percent
Co (relative to total M content in the crystallites) combined with lower
relative levels of Al
dramatically reduces impedance growth and improves the cycle life of an
electrochemical cell
employing the material as a cathode active material.
[0044] Accordingly, this disclosure provides improved electrochemically
active materials
such as those suitable for use in a positive electrode (cathode) for a Li-ion
secondary cell that,
relative to prior materials, reduce the rate of impedance growth and/or
capacity fade during
charge/discharge cycling of the battery. Also, provided are a variety of
methods for achieving high
discharge capacity cathode active materials that show reductions in impedance
growth and
capacity fade as they are cycled relative to the same materials but absent Co
and Al enrichment in
the grain boundaries.
[0045] The polycrystalline layered-structure lithiated metal oxides
as provided herein exhibit
enhanced electrochemical performance and stability. The compositions prevent
the performance
degradation of electrochemically cycled Ni-containing polycrystalline LiM02-
based materials,
11
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
while maintaining other desirable end-use article properties, e.g.,
electrochemical capacity of
rechargeable lithium-ion cathodes made from such layered metal oxides by
reducing the rate of
impedance growth during electrochemical cycling. Such Co and Al grain boundary
enriched
materials may be readily manufactured by calcining a green body formulation
including a LiOH
and a precursor hydroxide or carbonate to form particles with defined grain
boundaries and then
enriching the grain boundaries with a combination of Co and Al such that the
resulting particles
have grain boundaries where the concentration of Co and Al in the grain
boundary is greater than
prior to enrichment and optionally greater than within the primary
crystallites, the outer surfaces
of which define the edges of the grain boundaries in the secondary particle.
[0046] As such, provided are compositions, systems, and methods of making
and using
polycrystalline layered-structure lithiated metal oxides having Co and Al
enriched grain
boundaries in lithium-ion secondary cells as the means of achieving high
initial discharge capacity
and low impedance growth during cycling, thereby overcoming prior challenges
in high-nickel
formulations that may also have high discharge capacity (e.g., >205 mAh/g at
C/20).
[0047] The materials as provided include a particle comprising a plurality
of crystallites each
comprising a first composition. The particle formed of a plurality of
crystallites may be referred
to as a secondary particle. The particles as provided herein are uniquely
tailored to have grain
boundaries between the primary crystallites. Enriching these grain boundaries,
subsequent to their
formation, with a combination of Co and Al, optionally at particular relative
concentrations of Co
and Al, results in particles that provide for reduced impedance growth during
cycling, improving
performance and cycle life of a cell incorporating the particles as a
component of a cathode.
[0048] The particles are appreciated to include a grain boundary
formed of or including a
second composition, wherein a concentration of cobalt and aluminum, for
example, in the grain
boundary is greater than a concentration of cobalt and aluminum, for example,
in the primary
12
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
crystallite adjacent thereto. The concentration of Co and Al in the grain
boundary is optionally
greater than the average Co and Al concentration within the adjacent
crystallites on average. The
materials as provided herein are optionally relatively uniform in Co and/or Al
concentration (if
either is present at all) within the crystallites. Whether uniform or not, the
concentration of Co and
Al in the grain boundary is greater than the concentration of Co and Al,
individually or combined
as averaged within an adjacent crystallite. Optionally, the provided materials
include a further
outer coating layer may be disposed on an outer surface of the secondary
particle to provide a
coated secondary particle.
[0049] In some aspects of the presently provided particles, the first
composition includes
polycrystalline layered-structure lithiated metal oxides defined by
composition Li1+xM02+y and
optionally a cell or battery formed therefrom, where ¨0.1<x<0.3 and
¨0.3<y<0.3. In some aspects,
x is ¨0.1, optionally 0, optionally 0.1, optionally 0.2, or optionally 0.3.
Optionally, x is greater
than or equal to ¨0.10, ¨0.09, ¨0.08, ¨0.07, ¨0.06, ¨0.05, ¨0.04, ¨0.03,
¨0.02, ¨0.01, 0.00, 0.01,
0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, 0.10, 0.11, 0.12, 0.13, 0.14,
0.15, 0.16, 0.17, 0.18,
0.19, 0.20, 0.21, 0.22, 0.23, 0.24, 0.25, 0.26, 0.27, 0.28, 0.29, or 0.30. In
some aspects, y is ¨0.3,
optionally ¨0.2, optionally ¨0.1, optionally 0, optionally 0.1, optionally
0.2, or optionally 0.3.
Optionally, y is greater than or equal to ¨0.30, ¨0.29, ¨0.28, ¨0.27, ¨0.26,
¨0.25, ¨0.24, ¨0.23,
¨0.22, ¨0.21, ¨0.20, ¨0.19, ¨0.18, ¨0.17, ¨0.16, ¨0.15, ¨0.14, ¨0.13, ¨0.12,
¨0.11, ¨0.10, ¨0.09,
¨0.08, ¨0.07, ¨0.06, ¨0.05, ¨0.04, ¨0.03, ¨0.02, ¨0.01, 0.00, 0.01, 0.02,
0.03, 0.04, 0.05, 0.06,
0.07, 0.08, 0.09, 0.10, 0.11, 0.12, 0.13, 0.14, 0.15, 0.16, 0.17, 0.18, 0.19,
0.20, 0.21, 0.22, 0.23,
0.24, 0.25, 0.26, 0.27, 0.28, 0.29, or 0.3.
[0050] It is appreciated that in some aspects Li need not be
exclusively Li, but may be partially
substituted with one or more elements selected from the group consisting of
Mg, Sr, Na, K, and
Ca. The one or more elements substituting Li, are optionally present at 10
atomic % or less,
13
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
optionally 5 atomic % or less, optionally 3 atomic % or less, optionally no
greater than 2 atomic
percent, where percent is relative to total Li in the material.
[0051] M as provided in the first composition includes Ni. The amount
of Ni in the first
composition is optionally from 10 atomic percent to 100 atomic percent (at%)
of total M.
Optionally, the Ni component of M is greater than or equal to 75 at%.
Optionally, the Ni
component of M is greater than or equal to 80 at%. Optionally, the Ni
component of M is greater
than or equal to 85 at%. Optionally, the Ni component of M is greater than or
equal to 90 at%.
Optionally, the Ni component of M is greater than or equal to 95 at%.
Optionally, the Ni
component of M is greater than or equal to 75 at%, 76 at%, 77 at%, 78 at%, 79
at%, 80 at%, 81
at%, 82 at%, 83 at%, 84 at%, 85 at%, 86 at%, 87 at%, 88 at%, 89 at%, 90 at%,
91 at%, 92 at%, 93
at%, 94 at%, 95 at%, 96 at%, 97 at%, 98 at%, 99 at%, 99.5 at%, 99.9 at%, or
100 at%.
[0052] In some aspects, M in the first composotion is Ni alone or in
combination with one or
more additional elements. The additional elements are optionally metals.
Optionally, an additional
element may include or be one or more of Al, Mg, Co, Mn, Ca, Sr, Zn, Ti, Y,
Cr, Mo, Fe, V, Si,
Ga, or B. In particular aspects, the additional element may include Mg, Co,
Al, or a combination
thereof. Optionally, the additional element may be Mg, Al, V, Ti, B, or Mn, or
a combination
thereof. Optionally, the additional element is selected from the group
consisting of Mg, Al, V, Ti,
B, or Mn. Optionally, the additional element selected from the group
consisting of Mg, Co, and
Al. Optionally, the additional element selected from the group consisting of
Ca, Co, and Al. In
some aspects, the additional element is Mn or Mg, or both Mn and Mg.
Optionally, the additional
element is Mn, Co, Al, or any combination thereof Optionally the additional
element includes Co
and Mn. Optionally the additional element is Co and Al. Optionally the
additional element is Co.
[0053] An additional element of the first composition may be present
in an amount of about
1 to about 90 at%, specifically about 5 to about 80 at%, more specifically
about 10 to about 70
14
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
at% of M in the first composition. Optionally, the additional element may be
present in an amount
of about 1 to about 20 at%, specifically about 2 to about 18 at%, more
specifically about 4 to about
16 at%, of M in the first composition. In some illustrative examples, M is
about 75-100 at% Ni,
0-15 at% Co, 0-15 at% Mn, and 0-10 at% additional elements.
[0054] Within the polycrystalline material, each crystallite may have any
suitable shape,
which can be the same or different within each particle. Further, the shape of
each crystallite can
be the same or different in different particles. Because of its crystalline
nature, the crystallite may
be faceted, the crystallite may have a plurality of flat surfaces, and a shape
of the crystallite may
approximate a geometric shape. In some aspects, the crystallite may be fused
with neighboring
crystallites with mismatched crystal planes. The crystallite may optionally be
a polyhedron. The
crystallite may have a rectilinear shape, and when viewed in cross-section, a
portion of or an
entirety of the crystallite may be rectilinear. The crystallite may be square,
hexagonal, rectangular,
triangular, or a combination thereof
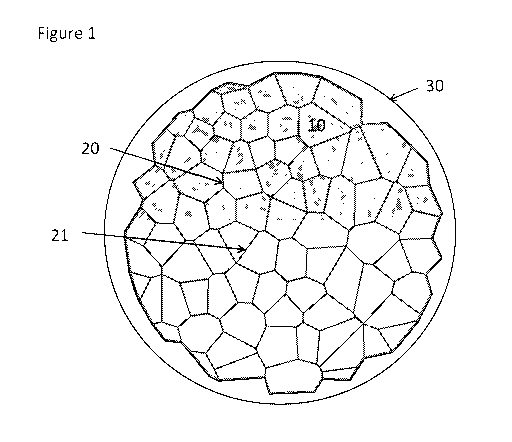
[0055] In particular aspects, a secondary particle has a Co and Al
enriched grain boundary,
optionally where the atomic percentage of Co and Al in the grain boundary is
higher than the
atomic percentage of Co and Al in the crystallites as averaged throughout.
Referring to FIG. 1 as
an exemplary illustration, the grain boundary 20, 21 is between adjacent
crystallites 10, and
includes the second composition. A second composition may be as described in
U.S. Pat. Nos.
9,391,317 and 9,209,455 with the exception that both Co and Al must
independently be enriched
in the grain boundary relative to the concentration of Co and Al each
independently in the
crystallites and, in some aspects, may provide synergistic effects in reducing
impedance or
improving cycle life due to the concentrations of Co and Al being within
certain concentration
ranges. The second composition optionally has the layered a-NaFe02-type
structure, a cubic
structure, or a combination thereof. As noted above, a concentration of Co and
Al in the grain
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
boundaries may be greater than a concentration of Co and Al in the
crystallites. An aspect in which
the grain boundaries have the layered a-NaFe02-type structure is specifically
mentioned. Another
aspect in which the grain boundaries with a-NaFe02-type structure with defects
is specifically
mentioned. Another aspect in which parts of the grain boundaries have a cubic
or spinel structure
is specifically mentioned.
[0056] The second composition as present in part or in whole in the
grain boundaries
optionally includes lithiated metal oxides defined by composition Li1+xM02+y,
where ¨0.1<x<0.3
and ¨0.3<y<0.3. Optionally a second composition and a first composition are
identical with the
exception of the presence of or increased concentration of Co and Al in the
second composition
relative to the first composition. In some aspects of the second composition,
x is ¨0.1, optionally
0, optionally 0.1, optionally 0.2, or optionally 0.3. Optionally, xis greater
than or equal to ¨0.10,
¨0.09, ¨0.08, ¨0.07, ¨0.06, ¨0.05, ¨0.04, ¨0.03, ¨0.02, ¨0.01, 0.00, 0.01,
0.02, 0.03, 0.04, 0.05,
0.06, 0.07, 0.08, 0.09, 0.10, 0.11, 0.12, 0.13, 0.14, 0.15, 0.16, 0.17, 0.18,
0.19, 0.20, 0.21, 0.22,
0.23, 0.24, 0.25, 0.26, 0.27, 0.28, 0.29, or 0.30. In some aspects, y is ¨0.3,
optionally ¨0.2,
optionally ¨0.1, optionally 0, optionally 0.1, optionally 0.2, or optionally
0.3. Optionally, y is
greater than or equal to ¨0.30, ¨0.29, ¨0.28, ¨0.27, ¨0.26, ¨0.25, ¨0.24,
¨0.23, ¨0.22, ¨0.21,
¨0.20, ¨0.19, ¨0.18, ¨0.17, ¨0.16, ¨0.15, ¨0.14, ¨0.13, ¨0.12, ¨0.11, ¨0.10,
¨0.09, ¨0.08, ¨0.07,
¨0.06, ¨0.05, ¨0.04, ¨0.03, ¨0.02, ¨0.01, 0.00, 0.01, 0.02, 0.03, 0.04, 0.05,
0.06, 0.07, 0.08, 0.09,
0.10, 0.11, 0.12, 0.13, 0.14, 0.15, 0.16, 0.17, 0.18, 0.19, 0.20, 0.21, 0.22,
0.23, 0.24, 0.25, 0.26,
0.27, 0.28, 0.29, or 0.3.
[0057] M as provided in the second composition includes Co and Al.
The amount of Ni, if
present, is optionally from 0.01 atomic percent to 99 atomic percent (at%) of
M. Optionally, M in
the second composition is free of Ni. Optionally, the amount (i.e. relative
concentration) of Ni in
the second composition is lower than the amount of Ni in the first composition
in relative atomic
16
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
percent (with respect to the respective composition in which the Ni is
present). Optionally, the Ni
component of M is less than or equal to 1 at%. Optionally, the Ni component of
M is less than or
equal to 5 at%. Optionally, the Ni component of M is less than or equal to 10
at%. Optionally, the
Ni component of M is less than or equal to 20 at%. Optionally, the Ni
component of M is less than
or equal to 75 at%. Optionally, the Ni component of M is less than or equal to
80 at%. Optionally,
the Ni component of M is less than or equal to 85 at%. Optionally, the Ni
component of M is less
than or equal to 90 at%. Optionally, the Ni component of M is less than or
equal to 95 at%.
Optionally, the Ni component of M is less than or equal to 75 at%, 76 at%, 77
at%, 78 at%, 79
at%, 80 at%, 81 at%, 82 at%, 83 at%, 84 at%, 85 at%, 86 at%, 87 at%, 88 at%,
89 at%, 90 at%, 91
at%, 92 at%, 93 at%, 94 at%, 95 at%, 96 at%, 97 at%, 98 at%, 99 at%, or 99.9
at%.
[0058] For the materials optionally as provided herein, the nominal
or overall formulated
composition of the secondary particles (for example, characterized by
Inductively Coupled Plasma
(ICP)), optionally the first composition, or optionally the second
composition, is defined by the
formula LiMO, wherein M is Ni and optionally one or more additional metals
that in the second
composition must include at least Co and Al. The mole fraction of Co and Al in
the first
composition, if present, as defines the composition of the crystallites is
lower than the mole
fraction of the total Co and Al independently or combined in the total
particle composition as
determined by ICP. The mole fraction of Co and Al independently or combined in
the first
composition can be zero. The mole fraction of Co and Al in the second
composition independently
or combined as defines the grain boundary is higher than the mole fraction of
Co and Al
independently or combined in the total particle as measured by ICP.
[0059] A second composition located within the grain boundaries
includes Co and Al,
optionally with the condition that the concentration of Co and Al
independently or combined in
the grain boundary is greater than the concentration of Co and Al
independently or combined in
17
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
the crystallites, optionally where the concentration of Co in the grain
boundary is greater than the
concentration of Co in the crystallites, and optionally where the
concentration of Al in the grain
boundary is greater than the concentration of Al in the crystallites. It was
found that using
processes that are capable of enriching Co and Al in the grain boundaries,
liquid solutions that
included amounts relative to the total transition metal of the first
composition to be enriched of Co
of at or between 0 at% and 8 at%, optionally at or between 3 at% and 5 at% Co
could be
supplemented with 0.01 at% to 10 at% Al, optionally 1.5 at% or less Al, and
create materials that
showed significantly reduced impedance growth during cycling, where the added
Co and Al are
incorporated into the grain boundaries of the secondary particle.
[0060] The volume fraction of grain boundaries within a given secondary
particle will vary
because the primary particle size distribution varies with variations in
overall composition and
synthetic conditions, and accordingly, the final concentration of Co and Al in
the second
composition can vary between different secondary particles and within
individual secondary
particles as well, while still always being greater than the concentrations of
Co and Al in the first
composition. It is thus most useful that the amount of Co and Al added to the
grain boundary be
defined relative to the first composition.
[0061] The provided amounts of Co and Al in the process solution are
considered average
amounts of Co and Al added to the secondary particles and distributed in the
grain boundaries of
the entire secondary particle and are presented relative to M of the first
composition. When making
the secondary particles as described herein it was found that virtually all of
the Co and Al in the
process solution was adhered to the particles prior to calcination. As such,
the amount of Co and
Al available for enriching the grain boundaries is the amount in the process
solution. Therefore,
when describing a process solution that is, for example, 1 at% Al and 2 at%
Co, this listed at% is
relative to the amount of M in the first composition prior to grain boundary
enrichment. As such,
18
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
the at% of Al and Co in the process solution as used herein is always relative
to total M in the
primary particles to be grain boundary enriched.
[0062] The amount of Al in the process solution is optionally 0.01
at% to 10 at%, optionally
9 at% or less, optionally 8 at% or less, optionally 7 at% or less, optionally
6 at% or less, optionally
5 at% or less, optionally 4 at% or less, optionally 3 at% or less, optionally
2 at% or less, optionally
1 at% or less, optionally 0.1 to 1 at%, optionally 0.5 to 1 at%. Optionally,
the amount of Al in the
process solution is at or less than an atomic percentage of 0.1, 0.2, 0.3,
0.4, 0.5, 0.6, 0.7, 0.8, 0.9,
1.0, 1.1, 1.2, 1.3, 1.4, or 1.5.
[0063] Optionally, the amount of Co in the process solution is
greater than 3 at% and up to 4
at%, and the amount of Al is less than 1 at%, optionally from 0.1 to 1 at%,
optionally 0.1 to less
than 1 at%. Optionally, the amount of Co in the process solution is 0.5 at% to
4 at%, and the
amount of Al is 0.01 at% to 10 at%.
[0064] In some aspects the amount of Co in the process liquid is
about 3 at%. At this
concentration of Co, the amount of Al is optionally less than 1 at%. Amounts
of Al of about 0.3
at% to 0.7 at%, optionally about 0.5 at% are optimal for reducing impedance
growth during
cycling. Optionally, the Al is distributed substantially uniformly among the
plurality of the
secondary particles.
[0065] In some aspects the amount of Co in the process liquid is
about 3.5 at%. At this amount
of Co, the amount of Al is optionally less than 1 at%. Amounts of Al of about
0.3 to 0.7 at%,
optionally about 0.5 at% are optimal for reducing impedance growth during
cycling. Optionally,
the Al is distributed substantially uniformly among the plurality of the
secondary particles.
[0066] In some aspects the amount of Co in the process liquid is
about 4 at%. At this
concentration of Co, the amount of Al is optionally less than 1.5 at%. Amounts
of Al of about 0.7
to 1.3 at%, optionally about 1.0 at% are optimal for reducing impedance growth
during cycling.
19
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
Optionally, the Al is distributed substantially uniformly among the plurality
of the secondary
particles.
[0067] In some aspects the amount of Co in the process liquid is
about 4.5 at%. At this
concentration of Co, the amount of Al is optionally less than 1 at%. Amounts
of Al of about 0.3 to
0.7, optionally about 0.5 at% are optimal for reducing impedance growth during
cycling.
Optionally, the Al is distributed substantially uniformly among the plurality
of the secondary
particles.
[0068] In some aspects the amount of Co in the process liquid is
about 3 at%. At this
concentration of Co, the amount of Al is optionally less than 1.5 at%. Amounts
of Al of about 0.5
to 1.3 at%, optionally about 1.0 at% are optimal for reducing impedance growth
during cycling.
Optionally, the Al is distributed substantially uniformly among the plurality
of the secondary
particles.
[0069] In some aspects the amount of Co in the process liquid is
about 3 at% to about 4 at%.
At this concentration of Co, the amount of Al is optionally less than 1 at%.
Amounts of Al of about
0.3 to 0.1.3, optionally about 0.5 at% or about 1.0 at% are optimal for
reducing impedance growth
during cycling. Optionally, the Al is distributed substantially uniformly
among the plurality of the
secondary particles.
[0070] In some aspects the amount of Co in the process liquid is
about 3 at%. At this
concentration of Co, the amount of Al is optionally less than 1.5 at%. Amounts
of Al of about 0.5
to 1.3 at% are optimal for reducing impedance growth during cycling.
Optionally, the Al is
distributed substantially uniformly among the plurality of the secondary
particles.
[0071] In some aspects the amount of Co in the process liquid is
about 4 at%. At this
concentration of Co, the amount of Al is optionally less than 1.0 at%. Amounts
of Al of about 0.5
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
to 0.7 at% are optimal for reducing impedance growth during cycling.
Optionally, the Al is
distributed substantially uniformly among the plurality of the secondary
particles.
[0072] As such, in some aspects, as the amount of Co increased from 3
at% to 4 at%, the
amount of Al that produces the most improved results moves from less than 1.3
at% to less than
0.7 at%. Optionally, the Al is distributed substantially uniformly among the
plurality of the
secondary particles.
[0073] Optionally, as exhibited by some aspects of the secondary
particles described herein,
the amount of Al relative to the amount of Co in the second composition is
equal to or less than
100 at% meaning that the amount of Al is optionally equal to or less than the
amount of Co.
Optionally, the amount of Al relative to Co is less than 90 at%, optionally
less than 80 at%,
optionally less than 70 at%, optionally less than 60 at%, optionally less than
50 at%, optionally
less than 40 at%, optionally less than 30 at%, optionally less than 20 at%,
optionally less than 10
at%, optionally less than 9 at%, optionally less than 8 at%, optionally less
than 7 at%, optionally
less than 6 at%, optionally less than 4 at%, optionally less than 3 at%,
optionally less than 2 at%,
optionally less than 1 at%. It was found that the amount of Co being greater
than the amount of Al
allows for a synergistic relationship that unexpectedly reduces impedance
growth relative to Co
alone at the same or greater concentrations.
[0074] Optionally, the Al present in the second composition or
throughout a portion or the
whole of the grain boundaries among the plurality of the secondary particles
is substantially
uniform. For example, when Al is introduced by the methods provided herein for
Al-only grain
boundary enrichment, the result as observed by EDS of the powder by standard
techniques is the
presence of "hotspots" rich in Al illustrating uneven or inefficient uptake of
Al into the grain
boundaries resulting in separate phases of Al. However, in the presence of Co
and Al at the
concentrations as described herein in the process solution, the result is a
much more uniform
21
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
distribution of Al illustrating the near or total absence of observed hotspots
by EDS. In some
aspects, the number and/or size of hotspots of Al is reduced by 50% or more,
optionally 60% or
more, optionally 70% or more, optionally 80% or more, relative to that
occurring with Al grain
boundary enrichment in the absence of Co co-enrichment.
[0075] Aluminum uniformity can also be assessed by comparing the EDS at two
magnifications. Table 2 of Example 1 shows aluminum concentration by EDS for a
wide area
(around 150 p.m X 150 m) as the first number in the table followed by a
second number which is
average EDS analyses of much narrower areas (approximately 1 p.m X 1 m)
centered on particles
with no apparent hotspots. When 4% cobalt is used in the process liquid, these
two numbers are
close together. However, when cobalt is not used, the non-hotspot narrow areas
have much less
aluminum compared to the wider area, showing that much more aluminum is
centered in hotspots
rather than being uniformly distributed among the plurality of secondary
particles.
[0076] In some aspects, M in a second composition further includes
one or more Ni
substituting elements (substitution element). The Ni-substituting elements are
optionally metals
and are not Co or Al as the presence of these elements results in the observed
synergistic reductions
in impedance growth. Optionally, a substituting element may include or be one
or more of Mg,
Mn, Ca, Sr, Zn, Ti, Zr, Hf, Y, Cr, Mo, W, Fe, V, Nb, Ta, Si, Ga, or B. A
substitution element of
the second composition may be present in an amount of about 1 to about 90 at%,
specifically about
5 to about 80 at%, more specifically about 10 to about 70 at% of the first
composition. Optionally,
the additional element may be present in an amount of about 1 to about 20 at%,
specifically about
2 to about 18 at%, more specifically about 4 to about 16 at%, of the first
composition.
[0077] Optionally, Li in the second composition need not be
exclusively Li, but may be
partially substituted with one or more Li-substitution elements selected from
the group consisting
of Mg, Sr, Na, K, and Ca. The one or more Li-substitution elements, are
optionally present at 10
22
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
atomic % or less, optionally 5 atomic % or less, optionally 3 atomic % or
less, optionally no greater
than 2 atomic percent, where percent is relative to total Li in the as-made
material.
[0078] The secondary particles as provided herein may be prepared by
synthesizing a green
body from at least two components, optionally in powder form. At least two
components may
include micronized (or non-micronized) lithium hydroxide or its hydrate and a
precursor
hydroxide(s) comprising nickel, and optionally one or more other elements, and
where the
precursor hydroxides are optionally obtained by co-precipitation processes. It
is appreciated that
the final overall composition (although not necessarily distribution) of the
elements in the final
particle may be adjusted by increasing or decreasing the relative amounts of
the precursor materials
in the formation of the green body. In some aspects, the lithium hydroxide or
its hydrate are
micronized. The two or more powders forming the green body may be combined and
shaken on
a paint shaker to thoroughly mix the precursors. The green body is then
calcined with a controlled
air or pure oxygen atmosphere to a maximum temperature. Calcining is
optionally preformed
following a heating curve. The calcined product may then be processed to form
a free-flowing
powder.
[0079] In some aspects, the precursor hydroxide may be a mixed metal
hydroxide. In some
aspects, the mixed metal hydroxide may include a metal composition of Ni, Co,
and Mg.
Optionally, the mixed metal hydroxide includes as a metal component 10 ¨ 100
at% Ni, 0 ¨ 15
at% Co, and 0 ¨ 5 at% Mg. Optionally the mixed metal hydroxide includes Ni
from 10-100 at%,
Co in the range of 0-30 at%, and Mn in the range of 0.1-80 at%. Optionally the
mixed metal
hydroxide includes Ni from 10-100 at%, Co in the range of 0-30 at%, and Al in
the range of 0-10
at%. Optionally, the metals of the mixed metal hydroxide is 92 at% Ni and 8
at% Co. Optionally,
the metals of the mixed metal hydroxide is 90 at% Ni, 8 at% Co, and 2 at% Mg.
Optionally, the
metals of the mixed metal hydroxide is 89 at% Ni, 8 at% Co, 3 at% Mg.
Optionally, the metals of
23
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
the mixed metal hydroxide is 91 at% Ni, 8 at% Co, and 1 at% Mg. Optionally,
the metal of the
mixed metal hydroxide is 100 at% Ni. For example, precursor hydroxide may be
made by a
precursor supplier, such as Hunan Brunp Recycling Technology Co. Ltd., using
standard methods
for preparing nickel-hydroxide based materials.
[0080] A secondary particle may be formed by a multi-step process whereby a
first material
particle is formed and calcined so as to establish the formation of defined
grain boundaries
optionally with the primary particles having a¨NaFe02 structure with few
defects. The particles
are then subject to a liquid process that applies Co and Al at the desired
concentration levels
followed by drying and then a second calcination so as to move the Co and Al
precipitated species
at the surface selectively into the grain boundaries to thereby form the
secondary particle having a
concentration of Co and Al in the grain boundaries that is higher than in the
crystallites. According
to methods of manufacturing a secondary particle that has a base of Ni, Co,
and Mg as provided
herein as an example, formation may include: combining a lithium compound, and
a hydroxide
precursor compound of one or more metals or metalloids (e.g. Ni, Co, and Mg
combined as
previously generated such as by a co-precipitation reaction) to form a
mixture; heat treating the
mixture at about 30 to about 200 C to form a dried mixture; heat treating the
dried mixture at about
200 to about 500 C for about 0.1 to about 5 hours; then heat treating at 600
C to less than about
800 C for about 0.1 to about 10 hours to manufacture the secondary particle.
A first calcination
maximum temperature is relative and specific to the material used in the
hydroxide precursor.
Optionally, in a first calcination, a maximum temperature may be at or less
than 850 degrees
Celsius, optionally at or less than 720 degrees Celsius, optionally at or less
than 715 degrees
Celsius, optionally at or less than 710 degrees Celsius, optionally at or less
than 705 degrees
Celsius, optionally at or less than 700 degrees Celsius. Optionally, the
maximum temperature of
the first calcination may be about 680 degrees Celsius or less. Optionally,
the maximum
24
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
temperature may be about 660 degrees Celsius or less. Optionally, the maximum
temperature may
be about 640 degrees Celsius or less. In yet other aspects, the maximum
temperature may be less
than about 700 degrees Celsius, about 695 degrees Celsius, about 690 degrees
Celsius, about 685
degrees Celsius, about 680 degrees Celsius, about 675 degrees Celsius, about
670 degrees Celsius,
about 665 degree Celsius, about 660 degrees Celsius, about 655 degrees
Celsius, about 650 degrees
Celsius, about 645 degrees Celsius, or about 640 degrees Celsius. The dwell
time at the maximum
temperature is optionally less than 10 hours. Optionally, the dwell time at
the maximum
temperature is less than or equal to 8 hours; optionally less than or equal to
7 hours; optionally less
than or equal to 6 hours; optionally less than or equal to 5 hours; optionally
less than or equal to 4
hours; optionally less than or equal to 3 hours; optionally less than or equal
to 2 hours.
[0081] After calcination, subsequent processing may include breaking
up the calcined
material with a mortar and pestle so that the resulting powder passes through
a desired sieve,
optionally a #35 sieve. The powder is optionally then jar milled in a 1 gallon
jar with a 2 cm drum
YSZ media for optionally 5 minutes or an adequate time such that the material
may pass through
optionally a #270 sieve.
[0082] The product of the first calcination or milled product may be
subsequently processed,
optionally in a method so as to result in enriched grain boundaries following
a second calcination.
A process to enrich grain boundaries within a primary particle may be
performed by methods or
using compositions as illustrated in U.S. Patent Nos. 9,391,317 and 9,209,455
with the exception
that the application process uses a liquid solution that includes a level of
Co and a level of Al,
optionally whereby the level is such to produce a synergistic enrichment of Co
and Al in the grain
boundaries of the secondary particle. The grain-boundary-enriching elements
may optionally be
applied by suspending the milled product in an aqueous slurry comprising Co,
Al, and a lithium
compound optionally at a temperature of about 60 degrees Celsius whereby the
Co and Al are
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
present in the aqueous solution at the concentrations as described herein. The
slurry may then be
spray dried to form a free-flowing powder which is then subjected to a second
calcination
optionally with a heating curve following a two ramp/dwell process. The first
two ramp/dwell
temperature profile may be from ambient (about 25 degree Celsius) to 450
degrees Celsius and
optionally at a rate of 5 degree Celsius per minute with a 1 hour hold at 450
degrees Celsius.
Subsequently, the second ramp/dwell may be from 450 degrees Celsius to a
maximum temperature
at a rate of 2 degree Celsius per minute with a 2 hour hold at the maximum
temperature. In some
aspects, the maximum temperature is less than about 725 degrees Celsius,
optionally at or about
700 degrees Celsius. In other aspects, the maximum temperature is about 725
degrees Celsius,
optionally 750 degrees Celsius.
[0083] By combining a first calcination with a maximum temperature as
described above with
a process to apply grain-boundary-enriching elements followed by a second
calcination also as
described above, it was found that the resulting particles could be used in a
cathode so as to produce
significantly improved reductions in impedance growth and/or capacity fade.
Such a combination
was found to result in additional cycle life and reduction in impedance
growth, significantly
improving the electrochemical performance of the material. As such, it is
appreciated that in some
aspects, a particle includes a plurality of crystallites with a first
composition including
polycrystalline layered-structure lithiated metal oxides defined by
composition Li i+xM02+y where
¨0.1<x<0.3 and ¨0.3<y<0.3. In some aspects x is ¨0.1, optionally 0, optionally
0.1, optionally
0.2, or optionally 0.3. Optionally x is greater than or equal to ¨0.10, ¨0.09,
¨0.08, ¨0.07, ¨0.06,
¨0.05, ¨0.04, ¨0.03, ¨0.02, ¨0.01, 0.00, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06,
0.07, 0.08, 0.09, 0.10,
0.11, 0.12, 0.13, 0.14, 0.15, 0.16, 0.17, 0.18, 0.19, 0.20, 0.21, 0.22, 0.23,
0.24, 0.25, 0.26, 0.27,
0.28, 0.29, or 0.30. In some aspects, y is ¨0.3, optionally ¨0.2, optionally
¨0.1, optionally 0,
optionally 0.1, optionally 0.2, or optionally 0.3. Optionally, y is greater
than or equal to ¨0.30,
26
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
¨0.29, ¨0.28, ¨0.27, ¨0.26, ¨0.25, ¨0.24, ¨0.23, ¨0.22, ¨0.21, ¨0.20, ¨0.19,
¨0.18, ¨0.17, ¨0.16,
¨0.15, ¨0.14, ¨0.13, ¨0.12, ¨0.11, ¨0.10, ¨0.09, ¨0.08, ¨0.07, ¨0.06, ¨0.05,
¨0.04, ¨0.03, ¨0.02,
¨0.01, 0.00, 0.01, 0.02, 0.03, 0.04, 0.05, 0.06, 0.07, 0.08, 0.09, 0.10, 0.11,
0.12, 0.13, 0.14, 0.15,
0.16, 0.17, 0.18, 0.19, 0.20, 0.21, 0.22, 0.23, 0.24, 0.25, 0.26, 0.27, 0.28,
0.29, or 0.3. The
crystallites have an amount of Ni of 10 atomic percent to 100 atomic percent
(at%) of the M
element. Optionally, the Ni component of M is greater than or equal to 75 at%.
Optionally, the Ni
component of M is greater than or equal to 80 at%. Optionally, the Ni
component of M is greater
than or equal to 85 at%. Optionally, the Ni component of M is greater than or
equal to 90 at%.
Optionally, the Ni component of M is greater than or equal to 95 at%.
Optionally, the Ni
component of M is greater than or equal to 75 at%, 76 at%, 77 at%, 78 at%, 79
at%, 80 at%, 81
at%, 82 at%, 83 at%, 84 at%, 85 at%, 86 at%, 87 at%, 88 at%, 89 at%, 90 at%,
91 at%, 92 at%, 93
at%, 94 at%, 95 at%, 96 at%, 98 at%, 99 at% or 100 at%. The M component may
include one or
more additional elements. The additional elements are optionally metals.
Optionally, an additional
element may include or be one or more of Al, Mg, Co, Mn, Ca, Sr, Zn, Ti, Y,
Cr, Mo, Fe, V, Si,
Ga, or B. In particular aspects, the additional element may include Mg, Co,
Al, or a combination
thereof. Optionally, the additional element may be Mg, Al, V, Ti, B, or Mn, or
a combination
thereof. Optionally, the additional element consists of Mg, Al, V, Ti, B, or
Mn. In some aspects,
the additional element is Mn or Mg, or both Mn and Mg. The additional element
of the first
composition may be present in an amount of about 1 to about 90 at%,
specifically about 5 to about
80 at%, more specifically about 10 to about 70 at% of the first composition.
Optionally, the
additional element may be present in an amount of about 1 to about 20 at%,
specifically about 2
to about 18 at%, more specifically about 4 to about 16 at%, of the first
composition. In some
illustrative examples, M is about 75-100 at% Ni, 0-15 at% Co, 0-15 at% Mn, and
0-10 at%
27
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
additional elements. The resulting secondary particles have grain boundaries
whereby the amount
of Co and the amount of Al are greater than in the crystallites.
[0084] The resulting particles optionally demonstrate reductions in
impedance growth relative
to particles with Co enriched grain boundaries alone, optionally at levels of
Co that were thought
to be insufficient to significantly improve cycling characteristics of
particles without Co
enrichment in the grain boundaries. When the resulting secondary particles are
the active material
of Li-ion cell cathodes, the cells cycled between 4.2V and 2.7V at 45 C
optionally exhibit
impedance growth in the fully charged (4.2V) state of less than 50% for
greater than 50 cycles,
optionally greater than 60 cycles, optionally greater than 70 cycles,
optionally greater than 80
cycles, optionally greater than 90 cycles, optionally greater than 100 cycles,
optionally greater than
110 cycles, optionally greater than 120 cycles, optionally greater than 130
cycles, optionally
greater than 140 cycles, optionally greater than 150 cycles, optionally
greater than 200 cycles.
[0085] In other aspects, the cells cycled between 4.2V and 2.7V at 45
C optionally exhibit
impedance growth in the fully charged (4.2V) state of less than 100% for
greater than 100 cycles,
optionally greater than 110 cycles, optionally greater than 120 cycles,
optionally greater than 130
cycles, optionally greater than 140 cycles, optionally greater than 150
cycles, optionally greater
than 160 cycles, optionally greater than 170 cycles, optionally greater than
180 cycles, optionally
greater than 190 cycles, optionally greater than 200 cycles, optionally
greater than 210 cycles,
optionally greater than 220 cycles.
[0086] In other aspects, the cells cycled between 4.2V and 2.7V at 45 C
optionally exhibit
impedance growth at 50% state of charge (SOC) of less than 50% for greater
than 200 cycles,
optionally of less than 40% for greater than 200 cycles, optionally of less
than 30% for greater
than 200 cycles.
28
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
[0087] An electrochemical cell as provided herein optionally uses as
an electrochemically
active material particles as provided herein optionally having an initial
discharge capacity of 180
mAh/g of the particles or greater, optionally 185 mAh/g, optionally 190 mAh/g,
optionally 195
mAh/g, optionally 200 mAh/g, optionally 210 mAh/g.
[0088] As shown in FIG. 1, disclosed is a particle comprising a crystallite
10 comprising a
first composition, and grain boundaries 20, 21 comprising a second
composition, wherein a
concentration of Al in the grain boundary is greater than a concentration of
Al in the crystallites
and a concentration of cobalt in the grain boundary is greater than a
concentration of cobalt in the
crystallite. The particle comprises a plurality of crystallites, and is
referred to as a secondary
particle. Optionally an outer layer illustrated at 30 in FIG. 1, such as a
passivation layer or a
protective layer, may be deposited on an outer surface of the particle. The
outer layer may fully
or partially cover the secondary particle. The layer may be amorphous or
crystalline. The layer
may comprise an oxide, a phosphate, a pyrophosphate, a fluorophosphate, a
carbonate, a fluoride,
an oxyfluoride, or a combination thereof, of an element such as Al, Tiõ B, Li,
or Si, or a
combination thereof. In some aspects the outer layer comprises a borate, an
aluminate, a silicate,
a fluoroaluminate, or a combination thereof. Optionally, the outer layer
comprises a carbonate.
Optionally, the outer layer comprises ZrO2, A1203, TiO2, A1PO4, A1F3, B203,
5i02, Li2O, Li2CO3,
or a combination thereof Optionally, an outer layer includes or is A1PO4 or
Li2CO3. The layer
may be deposited disposed by any process or technique that does not adversely
affect the desirable
properties of the particle. Representative methods include spray coating and
immersion coating,
for example.
[0089] Also provided are electrodes that include as a component of or
the sole
electrochemically active material a secondary particle as described herein. A
secondary particle as
provided herein is optionally included as an active component of a cathode. A
cathode optionally
29
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
includes a secondary particle disclosed above as an active material, and may
further include a
conductive agent and/or a binder. The conductive agent may comprise any
conductive agent that
provides suitable properties and may be amorphous, crystalline, or a
combination thereof. The
conductive agent may include a carbon black, such as acetylene black or lamp
black, a mesocarbon,
graphite, graphene, carbon fiber, carbon nanotubes such as single wall carbon
nanotubes or multi-
wall carbon nanotubes, or a combination thereof The binder may be any binder
that provides
suitable properties and may include polyvinylidene fluoride, a copolymer of
polyvinylidene
fluoride and hexafluoropropylene, poly(vinyl acetate), poly(vinyl butyral-co-
vinyl alcohol-co
vinyl acetate), poly(methylmethacrylate-co-ethyl acrylate), polyacrylonitrile,
polyvinyl chloride-
co-vinyl acetate, polyvinyl alcohol, poly(1-vinylpyrrolidone-co-vinyl
acetate), cellulose acetate,
polyvinylpyrrolidone, polyacrylate, polymethacrylate, polyolefin,
polyurethane, polyvinyl ether,
acrylonitrile-butadiene rubber, styrene-butadiene rubber, acryl onitrile-
butadi ene- styrene, tri-block
polymer of sulfonated styrene/ethylene-butylene/styrene, polyethylene oxide,
or a combination
thereof, for example.
[0090] The cathode may be manufactured by combining the particle as
described herein, the
conductive agent, and the binder in a suitable ratio, e.g., about 80 to about
98 weight percent of
the particle, about 1 to about 20 weight percent of the conductive agent, and
about 1 to about 10
weight percent of the binder, based on a total weight of the particle, the
conductive agent, and the
binder combined. The particle, the conductive agent, and the binder may be
suspended in a suitable
solvent, such as N-methylpyrrolidinone, and disposed on a suitable substrate,
such as aluminum
foil, and dried in air. It is noted that the substrate and the solvent are
presented for illustrative
purposes alone. Other suitable substrates and solvents may be used or combined
to form a cathode.
[0091] A cathode as provided herein when cycled with a MCMB 10-28
graphite anode, a
polyolefin separator and an electrolyte of 1 M LiPF6 in 1/1/1 (vol.)
EC/DMC/EMC with 1 wt. %
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
VC in a 2025 coin cell optionally demonstrates a significantly reduced
impedance growth relative
to materials with Co enrichment alone or no grain boundary enrichment. One
measure of
impedance growth may be obtained by high-rate cycling of the cell, with a
1C/1C charge/discharge
cycle interspersed in the cycling regime at 20 cycle intervals. The 1C charge
to 4.2V is followed
by a voltage hold during which the cell voltage is maintained at 4.2V until
the current decays to
C/10 rate, and then the cell is allowed to rest at open circuit for 5 minutes.
When the fully charged
cell is then discharged, the the voltage drop it undergoes in the first 10
seconds of discharge and
the 1C discharge rate are plugged into Ohm's law (V=IR) to calculate a DCR
(direct current
resistance) measurement of cell impedance. The impedance measurement plotted
against cycle
number results in a curve with a defined slope. The impedance slope is lower
when active particle
material has grain boundaries enriched with Co and Al as described herein
relative to particles
without such enrichment of grain boundaries or relative to particles having
grain boundaries
enriched only with Co. In some aspects, the impedance growth of cells is at or
less than 25% for
the first 50 cycles, optionally 50% or less over the first 100 cycles,
optionally 63% or less over the
first 125 cycles, optionally 75% or less over the first 150 cycles.
Optionally, the impedance growth
is at or less than 25% over 50 cycles, optionally 50% or less over 100 cycles,
optionally 63% or
less over 125 cycles, optionally 75% or less the first 150 cycles.
[0092] Also disclosed is a battery comprising the cathode. The
battery may be a lithium-ion
battery, a lithium-polymer battery, or a lithium battery, for example. The
battery may include a
cathode, an anode, and a separator interposed between the cathode and the
anode. The separator
may be a microporous membrane, and may include a porous film including
polypropylene,
polyethylene, or a combination thereof, or may be a woven or non-woven
material such a glass-
fiber mat. The anode may include a coating on a current collector. The coating
may include a
31
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
suitable carbon, such as graphite, coke, a hard carbon, or a mesocarbon such
as a mesocarbon
microbead, for example. The current collector may be copper foil, for example.
[0093] The battery also includes an electrolyte that may contact the
positive electrode
(cathode), the negative electrode (anode), and the separator. The electrolyte
may include an
organic solvent and a lithium salt. The organic solvent may be a linear or
cyclic carbonate.
Representative organic solvents include ethylene carbonate, propylene
carbonate, butylene
carbonate, trifluoropropylene carbonate, y-butyrolactone, sulfolane, 1,2-
dimethoxyethane, 1,2-
diethoxyethane, tetrahydrofuran, 3-methyl-1,3-dioxolane, methyl acetate, ethyl
acetate,
methylpropionate, ethylpropionate, dimethyl carbonate, diethyl carbonate,
ethyl methyl carbonate,
dipropyl carbonate, methylpropyl carbonate, propane sultone, or a combination
thereof In another
aspect the electrolyte is a polymer electrolyte.
[0094] Representative lithium salts useful in an electrolyte include
but are not limited to
LiPF6, LiBF4, LiAsF6, LiC104, LiCF3S03, Li(CF3S02)2N, LiN(S02C2F5)2, Li SbF6,
LiC(CF3S02)3,
LiC4F9S03, and LiA1C14. The lithium salt may be dissolved in the organic
solvent. A combination
comprising at least one of the foregoing can be used. The concentration of the
lithium salt can be
0.1 to 2.0M in the electrolyte.
[0095] The electrolyte may be a solid ceramic electrolyte.
[0096] The battery may have any suitable configuration or shape, and
may be cylindrical or
prismatic.
[0097] Various aspects of the present disclosure are illustrated by the
following non-limiting
examples. The examples are for illustrative purposes and are not a limitation
on any practice of
the present invention. It will be understood that variations and modifications
can be made without
departing from the spirit and scope of the invention.
32
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
EXAMPLES
Example 1: Polycrystalline 2D a-NaFe02-type layered structure particles with
grain boundary
enrichment with Al and Co.
[0098] Electrochemically active polycrystalline 2D a-NaFe02-type
layered structure particles
with or without differing types of grain boundary enrichment and each with
high nickel in the
cathode material were prepared.
[0099] A material having the composition Li1.o3Mgo.oiNio.92Coo.0802
was prepared dry mixing
252.1 g Li(OH)2 (dehydrated, micronized LiOH*H20 from FMC) 961.6
Nio.91Coo.o8Mgo.o10H)2
(custom made) in a 1 liter jar. The compounds were mixed by shaking ajar in a
paint shaker.
[00100] The mixed compounds were placed in an alumina crucible and
sintered. Sintering was
performed by heating at a rate of about 5 C per minute to about 450 C, and
held at about 450 C
for about two hours. The temperature was then raised at about 2 C per minute
to about 700 C,
and held for about six hours. The sample was then allowed to cool naturally to
room temperature.
The cooled sample was ground for about five minutes to break up any
agglomerates to provide
Li1.03Mgo.oiNio.92Coo.0802. The material was analyzed by XRD demonstrating an
a-NaFe02-type
structure.
[00101] Samples of Co and Al grain boundary enriched secondary
particles of 100 g each were
prepared with the above base material. Li, Co and Al nitrate salts were
dissolved in 100 g of H20
heated to 60 C. The amounts of Al and Co added were such to correspond to 1.9
at% and 4 at%,
respectively, relative to Ni+Co in the Li1.o3Mgo.oiNio.92Coo.0802 first
composition. The amount of
LiNO3 formulated were such that the final Li to transition metal + Al ratio
was 1.01.
[00102] 100 g of the Li1.03Mgo.oiNio.92Coo.0802 of as produced above
was added thereto and the
resulting slurry was stirred for 30 to 120 minutes. The slurry was then spray-
dried to yield a
powder. The resulting powder was placed in an alumina crucible and heated at a
rate of about 5
33
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
C per minute to about 450 C, and held at about 450 C for about one hour. The
temperature was
then raised at about 2 C per minute to about 700 C, and held for about two
hours. Each material
was then permitted to naturally cool to 100 C. The calcined materials were
first individually
ground in a mortar and pestle and then milled in ajar mill.
[00103] Overall, four materials grain-boundary-enriched with or without Al
and/or Co were
prepared using the procedures as described above with the final overall
compositions shown in
Table 1 based on their synthetic formulations.
Table 1:
Addition to
Overall Final Particle
Process Liquid
Material Description
Composition Co
Al
(at%)
(at%)
No Grain-Boundary Enriching
Process1 Lii.03Mgo.oiNio.92Coo.o802 0.0
0.0
No Grain-Boundary Enriching
Elements2 Lii.03Mgo.oiNio.92Coo.o802 0.0
0.0
Cobalt Only Litoi Mg0.01 Ni0.88Coo.1202 4.0
0.0
Aluminum Only Litoi Mg0.01 Ni0.90Coo.o78Alo.o1902
0.0 1.9
Cobalt and Aluminum Litoi Mg0.01 Ni0.86Co0.1 iAlo.o1902
4.0 1.9
1 control ¨ no treatment and no contact with processing liquid
2 control ¨ spray dry and calcination only no elements added to process liquid
[00104] The resulting materials as well as a control were each blended with
PVDF binder and
conductive carbon in a slurry of NMP solvent and coated onto an aluminum foil
current collector.
Similar coatings of selected materials were also prepared on Cu foil so that
they could be analyzed
for Al distribution by FIB-STEM-EDS without risk of measuring Al originating
from the foil.
Cathode electrodes were then punched out of the foil and combined with MCMB 10-
28 graphite
anodes, porous polypropylene separators and carbonate-based electrolytes in a
Li-ion "full" coin
34
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
cell format for electrochemical cycle life testing. The cathode electrodes
were also combined with
lithium metal anodes, porous polypropylene separators and carbonate-based
electrolytes in a "half'
coin cell format for electrochemical discharge capacity testing.
[00105] The full cells were cycled through a series of charge and
rapid discharge cycles at 45
C. A low rate discharge capacity and the impedance value were measured every
20
charge/discharge cycles. Some of the materials were further analyzed by EDS as
well as cross-
section TEM/EDX prior to use in cathode formation.
[00106] Figures 2 and 3 show the capacity fade for full cells cycling
at 45 C and the associated
impedance growth. Figure 2 shows that the "No Grain-Boundary-Enriching
Process" (no slurry,
spray dry, or 2nd calcination) and "No Grain-Boundary-Enriching Elements" (no
Co or Al solutes
in aqueous slurry, spray dry, and 2nd calcination) samples had capacity fade
at roughly the same
high rate. The "No Grain-Boundary-Enriching Elements" material was run through
the aqueous
slurry and subsequent calcination process but with only water devoid of grain-
boundary-enriching
elements. Figure 3 shows that the 2 materials also had similar rates of
impedance increase,
demonstrating that the aqueous immersion, spray drying and second calcination
process had no
significant impact. The aluminum-only sample showed a modest improvement over
the no-grain-
boundary-enriching elements- baseline but the most significant improvement was
observed for the
cobalt-only grain-boundary enrichment with 10% fade in 300 cycles.
[00107] The sample grain boundary (GB) enriched with both cobalt and
aluminum sample did
not show improvement in capacity fade over the cobalt-only. However, it did
have significantly
lower impedance growth as seen in Figure 3, having about 50% growth of
impedance at full state
of charge after 100 cycles, and about 115% impedance growth after 200 cycles.
The material GB-
enriched with both Co and Al provided 30% lower impedance growth than the
material GB-
enriched only with Co, and did so without using any additional Co.
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
[00108] Cobalt greatly improves the uptake of the aluminum as, without
the presence of Co in
the process solution, separate phases of lithiated alumina are observed as
indicated by EDS
mapping of the calcined materials and illustrated in Figure 4. The images
below of elemental maps
by EDS for Al (bright spots) show that the material formulated only with Al in
the process solution
had more intense and more numerous "hotspots" of Al than the material
formulated with Co
together with Al in the process solution, indicating that adding Co to the
process formulation
caused more uniform distribution of Al among the plurality of the secondary
particles, and
suggesting that the Co caused much more Al to be taken up by the particles
when they were
calcined.
[00109] The more uniform distribution and uptake of Al among the plurality
of the secondary
particles when it was formulated together with Co in the process solution was
also shown by
comparing the quantitative EDS results for Al analysis of the particles over a
large area (about 150
tm x 150 p.m) to those obtained by averaging 3 spot (about 1 tm x 1 p.m)
analyses obtained at
non-hotspot locations. Table 2 shows these comparisons for the Al-only-
formulated and the Al-
and Co-formulated cathode particles, and shows that the localized non-hotspot
analyses formulated
with Co and Al yielded Al content results similar to those obtained over a
broad area, indicating
that the Al was substantially uniformly distributed among the plurality of the
secondary particles.
In contrast, the Al-only formulated material had much lower non-hotspot Al
analysis results than
the broad area did, indicating that much of the Al was concentrated in hotspot
particles rather than
being uniformly distributed among the plurality of the secondary particles.
Table 2: Results for EDS analyses of cathode material powders.
Al/(Co+Ni) Atom Ratio
Process Liquid
Broad Area Non-Hotspot Area
1.9% Al, no Co 0.024 0.014
1.9% Al, 4% Co 0.022 0.021
36
CA 03126058 2021-07-07
WO 2020/149910 PCT/US2019/057630
[00110] Quantitative point analyses by STEM/EDS on thin lamellae of
the 2 materials
confirmed that inclusion of Co in the process formulation with Al promotes the
uptake of Al into
grain boundaries upon subsequent calcination. A secondary particle of each
material coated on
Cu foil as described above was sectioned by focused ion beam (FIB) milling to
yield a thin lamella
about 100 nm thick. Figure 5 shows a scanning transmission electron micrograph
(STEM) image
of a small section containing several crystallites from a particle prepared by
the grain-boundary-
enrichment process in which both 1.9 at% Al and 4 at% Co were formulated in
the process liquid,
and shows locations at which 3 EDS spot analyses were performed, locations 1
and 3 being at the
interiors of adjacent crystallites, and location 2 being at the intervening
grain boundary. Figure 6
shows the EDS spectra collected at the 3 locations marked in Figure 5.
Spectrum 2 at the grain
boundary shows a clear peak for Al at about 1.5 keV whereas spectrum 1 and
spectrum 3 at the
crystallite interiors do not. Spectrum 2 at the grain boundary also indicates
a higher ratio of Co to
Ni than is seen in spectrum 1 and spectrum 3 at the crystallite interiors, as
indicated by comparing
the 6.9 keV Co peak to the 8.3 keV Ni peak in each spectrum. Quantitative
results obtained by
integrating the Figure 6 spectra are shown in Table 3 illustrating that the
spectrum 2 grain boundary
location is enriched in both Al and Co.
Table 3: Results for 3 EDS point analyses of particles produced using a 1.9
at% Al, 4 at% Co
process liquid material.
1.9% Al, 4% Co in Process Liquid Co/(Co+Ni)
Al/(Co+Ni)
0 Ni Co Al Atom Ratio Atom
Ratio
Spectrum 1 66.28 30.38 3.34 ND 0.10 0.00
Spectrum 2 63.3 27.58 6.39 2.73 0.19 0.08
Spectrum 3 64.55 31.48 3.97 ND 0.11 0.00
ND: Not Detectable
37
CA 03126058 2021-07-07
WO 2020/149910 PCT/US2019/057630
0 1 1 1] The same type of STEM-EDS analyses were performed on a particle
that had been
prepared by the grain-boundary-enrichment process in which 1.9 at% Al was
present in the process
solution in the absence of Co, and a STEM image and locations of EDS point
analyses are shown
in Figure 7. The location of spectrum 1 is at a grain boundary and that of
spectrum 2 is at a
5 crystallite interior. Figure 8 shows the EDS spectra collected at the 2
locations marked in Figure
7, and neither spectrum indicates the presence of Al, and there is little
difference between their Co
peaks. Quantitative results obtained by integrating the Figure 8 spectra are
shown in Table 4, and
show that the grain boundary and bulk crystallite compositions are the same,
indicating little or no
Al uptake or grain boundary enrichment of the secondary particle.
10 Table 4: Results for 2 EDS point analyses of 1.9 at% Al only in the
process liquid.
1.9% Al in Process Liquid Co/(Co+N i)
Al/(Co+Ni)
0 Ni Co Al Atom Ratio Atom
Ratio
Spectrum 1 65.5 31.87 2.64 ND 0.08 0.00
Spectrum 2 65.09 32.21 2.7 ND 0.08 0.00
[00112] STEM-EDS point analyses of the type described above were
performed on a total of
11 grain boundary and 10 crystallite interior locations for the 1.9 at% Al, 4
at% Co grain boundary
enriched material, and 16 grain boundary and 7 crystallite interior locations
for the 1.9 at% Al-
only enriched material. Table 5 gives the averaged results for those analyses
in comparison to the
bulk formulated compositions of the materials, and shows that whereas applying
the liquid process
with Al only and then calcining results in little or no Al enrichment of grain
boundaries
(measurable quantity of Al detected in 3 of 16 locations), processing with
both Al and Co and then
calcining results in substantial grain boundary enrichment with both elements.
Table 5: Averaged EDS point analysis results for grain boundaries (second
composition) and
crystallite interiors (first composition) of 1.9 at% Al only or 1.9 at% Al, 4
at% Co co-enriched
materials.
38
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
formulated formulated average GB average GB # bulk
average average
coating Al/Ni Co/Ni # GB points Al/Ni Co/Ni
points bulk Al/Ni bulk Co/Ni
formulation atom ratio atom ratio analyzed atom ratio atom ratio analyzed
atom ratio atom ratio
1.9% Al, no Co 0.026 0.087 16 0.005 0.079 7 0.000
0.078
1.9% Al, 4% Co 0.026 0.130 11 0.037 0.164 10 0.000
0.094
GB: grain boundaries
bulk: crystallite interiors
Example 2: Grain boundary enrichment with both Al and Co via a nonaqueous
grain boundary
enrichment process.
[00113] Electrochemically active polycrystalline 2D a-NaFe02-type
layered structure particles
with differing types of grain boundary enrichment and each with high nickel in
the cathode
material were prepared via a nonaqueous grain boundary enrichment process. A
material with
composition Li i.o3Mgo.oiNio.92Coo.o802 was prepared by the method of Example
1. 30 g of this
material was then dispersed in 40 ml of methanol containing 1.9 at % Al as
dissolved nitrate salt
together with or without 4 at% Co nitrate, and with sufficient LiNO3 such that
the final Li to
transition metal + Al ratio was 1.01. The resulting slurry was rotary
evaporated to dryness, and
the recovered material then underwent the same calcination procedure that was
applied to spray-
dried material in Example 1. Figure 9 shows Al EDS maps of the calcined
materials, and shows
that, as was seen for aqueous-process materials in Example 1, the material
formulated only with
Al had more intense and more numerous "hotspots" of Al than the material
formulated with Co
together with Al. This result shows that the role of solution-processing with
Co in promoting the
more uniform uptake of Al by secondary particles is not an artifact of the
aqueous process, and
that non-aqueous deposition of grain-boundary-enriching elements by solvent
evaporation yields
the same result as aqueous deposition via acid-base precipitation.
Example 3: Synergistic benefit obtained by grain boundary enrichment with both
Al and Co.
39
CA 03126058 2021-07-07
WO 2020/149910 PCT/US2019/057630
[00114] Electrochemically active polycrystalline 2D a-NaFe02-type
layered structure particles
with differing types of grain boundary enrichment and each with high nickel in
the cathode
material were prepared from a Li1.o3Mgo.oiNio.92Coo.0802 base material via the
methods of Example
1. The Al and Co process formulations used to make these materials are shown
by the matrix in
Table 6.
Table 6: Al and Co levels in the process solution used to make Example 3 grain
boundary enriched
materials.
Co (at%)
Al (at%) 3 3.5 4 4.5 5
0 X X X X X
0.5 X X X X
1 X X
2 X
4 X
[00115] Li-ion coin cells were built with the materials enriched as in
Table 6 and cycled per
the methods of Example 1. Figure 10 illustrates cycling capacity fade for the
cells with 0 at% Al
grain-boundary-enriched materials and with 0.5 at% Al grain-boundary-enriched
materials and
their dependence on formulated Co content. The capacities of the various
materials decrease with
increased Co in the formulations, but their rates of capacity fade do not
differ greatly, being in the
4% to 6% range over 200 cycles, and are not substantially impacted by the
inclusion or absence of
0.5 at% Al in the formulations. However, Figure 11 shows that increasing the
Co level in the
formulation reduces impedance growth, with 4.2V impedance growth at 200 cycles
decreasing
from 250% to 130% as the formulated Co level is increased from 3 at% to 4 at%,
and that further
inclusion of 0.5 at% Al in the solution process formulation substantially
reduces impedance
growth at all levels of Co in the formulation, with impedance growth at 200
cycles dropping from
150% to 90% as the Co level in the solution process formulation is increased
from 3 at% to 4.5
at%. Figure 12 further shows that a synergistic benefit is observed from
inclusion of Al in the
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
solution process formulation, with impedance being reduced by a greater amount
than can be
obtained by only further increasing the Co concentration.
[00116] Figure 13 shows cycling capacity fade (left) and impedance
growth (right) for cells
with cathode materials grain boundary enriched from 3 at% Co solution process
formulation with
varied Al levels. The figure shows that increasing the formulated Al level
decreases the cathode
material's capacity without significantly impacting capacity fade (all cells
faded in the 6%-8%
range over 200 cycles), but that increasing the formulated Al level from 0 at%
to 1 at% reduces
4.2V impedance growth at 200 cycles from 200% to 120%, while even higher Al
levels cause
impedance growth to increase slightly. The impedance increases plotted in
Figure 13 are
summarized in Figure 14, which shows that for 3 at% Co in the process solution
together with Al,
minimum impedance growth is obtained when the process solution's formulated
atomic% ratio of
Al/Co is about 0.3-0.4.
Example 4: Al and Co grain boundary enrichment of an NCA material.
[00117] Two NCA materials of similar overall composition were made, one
having Co and Al
grain boundary enrichment and one not. In each case, a base material was made
by blending
hydroxide precursors together and firing in an oxygen atmosphere until a final
lithiated oxide was
formed and sintered.
[00118] Material 1: NCA base material having first composition
LiNio.93Coo.o4Alo.0302, grain
boundary-enriched with additional 4 at% Co, 0.6 at% Al relative to total M in
the first composition.
[00119] A precursor transition metal hydroxide was used for this
process. It contained at
transition metal 4 at% Co and 3 at% Al and the balance Ni. A micronized LiOH
powder was made
by placing 51 g of LiOH into a plastic jar with 500 g of Y-stabilized zirconia
1/4" spheres and
shaking on a paint shaker for 45 minutes. This micronized powder was then
transferred to another
41
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
plastic jar containing 190.15 g of the transition metal hydroxide precursor
and the two powders
were blended by shaking the jar on a paint shaker for a further 10 minutes.
After blending, the
roughly 240 g of blended powder was split between two crucibles and fired in
an oxygen
atmosphere by first ramping to 450 C at 5 C/min and soaking at temperature
for 2 hours, and
then ramping to 680 C at 2 C/min and soaking for 6 hours. When this heating
regime was over,
the furnace was allowed to cool to 130 C and the powder was removed and
placed into ajar mill.
The jar mill contained 1/2" drum media and was used to mill the powder for 2
minutes. The powder
was then sieved through a 270 mesh sieve. The material was analyzed by XRD
demonstrating an
a-NaFe02-type structure.
[00120] The powder was then coated with Co and Al by making a solution of
80 g water, 9.5
g cobalt nitrate (4 at% Co relative to total M in LiM02 base composition), 1.9
g aluminum nitrate
(0.6 at% Al relative to total M in LiM02 base composition), 2.7 g lithium
nitrate and heating to 60
C. To this was added 80 g of the previously prepared powder. The slurry was
allowed to stir for
25 minutes after which it was spray dried to remove the water from the slurry
and prepare a dry
powder. This powder was then fired in an air atmosphere by first heating to
450 C at 5 C/min
and soaking for 1 hour and then heating at 2 C /min to 700 C and soaking for
2 hours. The
furnace was then allowed to cool to 130 C and the powder removed from the
furnace and sieved
through a 270 mesh sieve. Figure 15 shows STEM micrographs of a thinly
sectioned secondary
particle of the thus-prepared material that was coated on Cu foil as described
in Example 1. Figure
15 also provides the Al/Ni and Co/Ni atomic ratio results for 3 EDS point
analyses of an interior
grain boundary (GB) and bulk areas of the adjacent primary particles, showing
that the grain
boundary is enriched with both Co and Al.
[00121] Material 2: comparative NCA base material having homogeneous
first composition
LiNi0.89Coo.o8Alo.0302
42
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
[00122] A precursor transition metal hydroxide was used for this
process. It contained 8 at%
Co and 3 at% Al and the balance Ni. A micronized LiOH powder was made by
placing 25.5 g of
LiOH into a plastic jar with 500 g of Y-stabilized zirconia 1/4" spheres and
shaking on a paint
shaker for 45 minutes. This micronized powder was then transferred to another
plastic jar
containing 95.1 g of the transition metal hydroxide and the two blended by
shaking on a paint
shaker for a further 10 minutes. After blending, the roughly 120 g of powder
was placed in one
crucible and fired in an oxygen atmosphere by first ramping to 450 C at 5
C/min and soaking at
temperature for 2 hours, and then ramping to 680 C at 2 C/min and soaking
for 6 hours. The
furnace was then allowed to cool to 130 C and the powder was removed and
placed into ajar mill.
The jar mill contained 1/2" drum media and was used to mill the powder for 2
minutes. The powder
was then sieved through a 270 mesh sieve. The material was analyzed by XRD
demonstrating an
a-NaFe02-type structure.
[00123] The above cathode materials 1 and 2 were assembled in coin
cells as described in
Example 1. The cells were 1C/1C cycled at 45 C, with 10 second discharge DCR
being measured
at 100% state of charge (SOC) for every cycle and at 50% SOC every 20th cycle.
The capacity fade
and impedance growth results for 2 cells Li-ion coin cells made with each
material are presented
in Figure 16 and Figure 17, respectively. Although the overall compositions of
the two materials
were nearly equivalent, the grain boundary-enriched material #1 contained Co
enriched between
bulk crystallites in the grain boundaries while material #2 contained an
equivalent overall amount
of Co uniformly distributed throughout the secondary particle. Material #1 had
only slightly higher
overall Al content than material #2, but the excess Al (-16 % more Al than
material #2) was all
concentrated in the grain boundaries. Both capacity fade and impedance growth
were much
improved for the GBE material when compared to the uniform-composition
material. Figure 16
shows that the grain boundary-enriched material #1, in addition to having
slightly higher capacity
43
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
(-1%) than the homogeneous material #2, had only 8% capacity fade in 200
cycles as compared
to >30% fade for material #2. Figure 17 shows that cells with grain boundary-
enriched material
#1 had 100% impedance growth at full SOC (4.2V) and 30% impedance growth at
50% SOC after
200 cycles, while cells with material #2 had >800% impedance growth at full
SOC and >200%
impedance growth at 50% SOC after 200 cycles.
Example 5: Al and Co grain boundary enrichment of an NCM material.
[00124] An NCM base material having first composition
LiNio.8C00.iMno.102 (NCM 811) was
prepared from co-precipitated precursor transition metal hydroxide containing
10 at% Co and 10
at% Mn and the balance Ni. A micronized LiOH powder was made by placing 87.7 g
of LiOH into
a plastic jar with 500 g of Y-stabilized zirconia 1/4" spheres and shaking on
a paint shaker for 45
minutes. This micronized powder was then transferred to another plastic jar
containing 335.7 g of
the precursor transition metal hydroxide and the two blended by shaking on a
paint shaker for a
further 10 minutes. After blending, the roughly 440 g of powder was split
between three crucibles
and fired in an oxygen atmosphere by first ramping to 450 C at 5 C/min and
soaking at
temperature for 2 hours, and then ramping to 770 C at 2 C/min and soaking
for 10 hours at 770
C. The furnace was then allowed to cool to 130 C and the powder was removed
and placed into
ajar mill. The jar mill contained 3/4" drum media and was used to mill the
powder for 2 minutes.
The powder was then sieved through a 270 mesh sieve.
[00125] The powder was then divided into base (no further treatment) or
grain-boundary-
enriched with Co and Al by making a solution of 200 g water, 11.9 g cobalt
nitrate (2 at% Co
relative to base composition), 3.1 g aluminum nitrate (0.4 at% Al), 3.4 g
lithium nitrate and heating
to 60 C. To this was added 200 g of the previously prepared lithiated
precursor powder. The slurry
was allowed to stir for 10 minutes after which it was spray dried to remove
the water from the
44
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
slurry and prepare a dry powder. This powder was then fired in an air
atmosphere by first ramping
to 450 C at 5 C/min and soaking at temperature for 1 hour, and then ramping
to 770 C at 2
C/min and soaking for 0.25 hour. The furnace was then allowed to cool to 130
C and the powder
was removed from the furnace and sieved through a 270 mesh sieve. The overall
composition of
the synthesized cathode powder was LiNio.o79Coo.iiMno.o9Alo.00602
[00126] The above base and grain boundary enriched NCM materials were
then assembled in
Li-ion coin cells which were cycled at 45 C by methods described in Examples
1 and 4. The
capacity fade and impedance growth results for 2 cells Li-ion coin cells made
with each material
are presented in Figure 18 and Figure 19, respectively. Both materials had
good cycling stability
(Figure 18), with the grain boundary-enriched material fading slightly less
(about 4% fade in 200
cycles vs. about 5% fade for the base material) and having a somewhat lower
initial capacity.
Nonetheless, the grain boundary enriched material had much lower impedance
growth than the
similar composition, homogeneous base material (Figure 19), having 45%
impedance growth at
100% SOC and 38% impedance growth at 50% SOC after 200 cycles, as compared to
225%
impedance growth at 100% SOC and 70% impedance growth at 50% SOC after 200
cycles for the
base material. These results demonstrate that grain boundary enrichment with
relatively low levels
of Al and Co is beneficial to NCM.
[00127] Various modifications, in addition to those shown and
described herein, will be
apparent to those skilled in the art of the above description. Such
modifications are also intended
to fall within the scope of the disclosure.
[00128] It is appreciated that all reagents are obtainable by sources
known in the art unless
otherwise specified.
[00129] Patents, publications, and applications mentioned in the
specification are indicative of
the levels of those skilled in the art to which the disclosure pertains. These
patents, publications,
CA 03126058 2021-07-07
WO 2020/149910
PCT/US2019/057630
and applications are incorporated herein by reference to the same extent as if
each individual
patent, publication, or application was specifically and individually
incorporated herein by
reference.
[00130] The foregoing description is illustrative of particular aspects
of the invention, but is
not meant to be a limitation upon the practice thereof
46