Note: Descriptions are shown in the official language in which they were submitted.
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
Method of Producing Hydrogen
CROSS-REFERENCE TO RELATED APPLICATIONS
[1] This application is related to U.S. Patent Application Nos. 16/707,046,
16/707,066 and
16/707,084, filed December 9, 2019.
TECHNICAL FIELD
[2] This invention generally relates to electrochemical reactors. More
specifically, this
invention relates to electrochemical reactors to produce syngas and hydrogen.
BACKGROUND
[3] Syngas (i.e., synthesis gas) is a mixture consisting primarily of
hydrogen, carbon
monoxide, and often carbon dioxide. It is used as intermediates to produce
various products,
such as synthetic natural gas, ammonia, methanol, hydrogen, synthetic fuels,
synthetic
lubricants. Syngas may be produced from almost any hydrocarbon feedstock, such
as natural
gas, coal, biomass, via steam reforming, dry reforming, partial oxidation, or
gasification. Syngas
is combustible and is often used in internal combustion engines or for
electricity generation
although its energy density is less than half of natural gas.
[4] Hydrogen in large quantities is needed in the petroleum and chemical
industries. For
example, large amounts of hydrogen are used in upgrading fossil fuels and in
the production
of ammonia or methanol or hydrochloric acid. Petrochemical plants need
hydrogen for
hydrocracking, hydrodesulfurization, hydrodealkylation. Hydrogenation
processes to increase
the level of saturation of unsaturated fats and oils also need hydrogen.
Hydrogen is also a
reducing agent of metallic ores. Hydrogen may be produced from electrolysis of
water, steam
reforming, lab-scale metal-acid process, thermochemical methods, or anaerobic
corrosion.
Many countries are aiming at a hydrogen economy.
[5] Clearly, there is continuing need and interest to develop methods and
systems to
produce these important gases.
1
Date Regue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
SUMMARY
[6] Further aspects and embodiments are provided in the following drawings,
detailed
description and claims. Unless specified otherwise, the features as described
herein are
combinable and all such combinations are within the scope of this disclosure.
[7] One aspect of the present invention is a method of producing hydrogen
including providing
a device, introducing a first stream that includes a fuel to the device,
introducing a second stream
that includes water to the device, reducing the water in the second stream to
hydrogen, and
extracting hydrogen from the device. The first stream and the second stream do
not come in
contact with each other in the device.
[8] In another method aspect, the first stream does not come in contact
with the hydrogen.
[9] In still another method aspect, the first stream and the second stream
are separated by an
electrolyte in the device.
[10] In a still further method aspect, the electrolyte is oxide ion
conducting and is solid state.
[11] In a yet still further method aspect, the electrolyte includes doped
ceria or where the
electrolyte comprises lanthanum chromite or a conductive metal or combination
thereof and a
material selected from the group consisting of doped ceria, YSZ, LSGM, SSZ,
and combinations
thereof. The lanthanum chromite includes undoped lanthanum chromite, strontium
doped
lanthanum chromite, iron doped lanthanum chromite, lanthanum calcium chromite,
or combinations
thereof. The conductive metal comprises Ni, Cu, Ag, Au, or combinations
thereof.
[12] In a yet still further method aspect, the electrolyte also conducts
electrons and where
the device includes no interconnect.
[13] In another method aspect, the device is tubular.
[14] In still another method aspect, the fuel comprises a hydrocarbon or
hydrogen or carbon
monoxide or combinations thereof.
[15] In a still further method aspect, the second stream includes hydrogen.
[16] In a yet still further method aspect, the first stream further
includes water or carbon
dioxide.
2
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759
PCT/US2020/0 13129
[171 In still yet another method aspect of the invention, the first stream
indudes the fuel
with little to no water.
[181 In another method aspect, the device is planar.
[19) In still another method aspect, the device includes multiple repeat units
separated by
interconnects. Each repeat unit includes two electrodes with an electrolyte
between the
electrodes.
[201 In a still further method aspect, the electrodes include fluid
channels or fluid dispersing
components and the interconnects include no fluid dispersing element.
[211 In a yet still further method aspect, the method of producing hydrogen
includes
introducing the first stream to a reformer before the first stream enters the
device.
[221 In still yet another method aspect of the invention, the reformer is a
steam reformer or an
autotherrnal reformer.
[231 In a yet still further method aspect, the method of producing hydrogen
includes
operating the device at a temperature of no less than 500 C.
[241 In another method aspect, the device includes a first electrode and a
second electrode
separated by an electrolyte. The first electrode or the second electrode
includes Ni or NiO and a
material selected from the group consisting of YSZ, CGO, SDC, SSZ, LSGM, and
combinations
thereof.
[251 In still another method aspect, the device includes a first electrode and
a second electrode
separated by an electrolyte. The first electrode includes doped or undoped
ceria and a material
selected from the group consisting of Cu, CuO, Cu2O, Ag, Ag2O, Au, Au20,
Au203, Pt, Pd, Ru, Rh,
stainless steel, and combinations thereof.
[26] In a still further method aspect, the first electrode comprises a
catalyst
BRIEF DESCRIPTION OF THE DRAWINGS
[27) The following drawings are provided to illustrate certain embodiments
described herein.
The drawings are merely illustrative and are not intended to limit the scope
of claimed
3
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
inventions and are not intended to show every potential feature or embodiment
of the claimed
inventions. The drawings are not necessarily drawn to scale; in some
instances, certain elements
of the drawing may be enlarged with respect to other elements of the drawing
for purposes of
illustration.
[28] Fig. 1A illustrates an electrochemical (EC) gas producer, according to
an embodiment of
this disclosure.
[29] Fig. 16 illustrates an EC gas producer, according to an embodiment of
this disclosure.
[30] Fig. 2A illustrates a tubular EC gas producer, according to an
embodiment of this
disclosure.
[31] Fig. 28 illustrates a cross section of a tubular EC gas producer,
according to an
embodiment of this disclosure.
[32] Fig. 3A illustrates a cross section of a multi-tubular EC gas
producer, according to an
embodiment of the disclosure;
[33] Fig. 38 illustrates a cross section of a multi-tubular EC gas
producer, according to an
embodiment of the disclosure;
[34] Fig. 3C illustrates a cross section of a multi-tubular EC gas
producer, according to an
embodiment of the disclosure;
[35] Fig. 30 illustrates a cross section of an EC gas producer, according
to an embodiment of
the disclosure;
[36] Fig. 4A illustrates a portion of a method of manufacturing an EC gas
producer using a
single point [MR source, according to an embodiment of the disclosure;
[37] Fig. 48 illustrates a portion of a method of manufacturing an EC gas
producer using a
ring-lamp EMR source, according to an embodiment of the disclosure;
[38] Fig. 4C illustrates a portion of a method of manufacturing an EC gas
producer using a
single point [MR source, according to an embodiment of the disclosure;
[39] Fig. 4D illustrates a portion of a method of manufacturing an EC gas
producer using a
tubular [MR source, according to an embodiment of the disclosure;
[40] Fig. 5A illustrates a first step in a tape casting method to form a
tubular or multi-tubular EC
gas producer, according to an embodiment of the disclosure;
4
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759
PCT/US2020/0 13129
[41] Fig. 58 illustrates steps 2-4 in a tape casting method to form a
tubular or multi-tubular EC
gas producer, according to an embodiment of the disclosure;
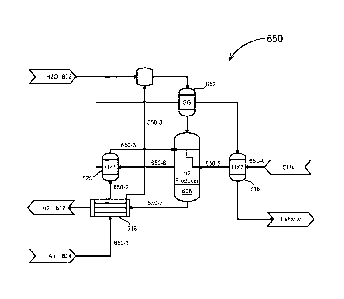
[42] Fig. 6A, illustrates an example of a hydrogen production system 600
with no external
heat source, according to an embodiment of the disclosure;
[43] Fig. 68 illustrates an alternative hydrogen production system with no
external heat
source, according to an embodiment of the disclosure;
[44] Fig. 7 illustrates a fuel cell component, according to an embodiment
of the disclosure;
[45] Fig. 8 schematically illustrates two fuel cells in a fuel cell stack,
according to an
embodiment of the disclosure;
[46] Fig. 9A illustrates a perspective view of a fuel cell cartridge (FCC),
according to an
embodiment of the disclosure;
[47] Fig. 98 illustrates a perspective view of a cross-section of a fuel
cell cartridge (FCC),
according to an embodiment of the disclosure;
[48] Fig. 9C illustrates cross-sectional views of a fuel cell cartridge
(FCC), according to an
embodiment of the disclosure;
[49] Fig. 9D illustrates top view and bottom view of a fuel cell cartridge
(FCC), according to an
embodiment of the disclosure;
[50] Fig. 10A illustrates a cross-sectional view of a TFC, according to an
embodiment of the
disclosure;
[51] Fig. 1013 illustrates a cross-sectional view of a TFC, according to an
embodiment of the
disclosure;
[52] Fig. 10C illustrates a cross-sectional view of a TFC, according to an
embodiment of the
disclosure;
[53] Fig. 11A illustrates a cross-sectional view of a TFC comprising a
support, according to an
embodiment of the disclosure;
[54] Fig. 118 illustrates a cross-sectional view of a TFC comprising a
support, according to an
embodiment of the disclosure;
[55] Fig. 11C illustrates a cross-sectional view of a TFC comprising a
support, according to an
embodiment of the disclosure;
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759
PCT/US2020/0 13129
[56] Fig. 12A illustrates an impermeable interconnect 1202 with a fluid
dispersing
component 1204, according to an embodiment of the disclosure;
[57] Fig. 128 illustrates an impermeable interconnect 1202 with two fluid
dispersing
components 1204, according to an embodiment of the disclosure;
[58] Fig. 12C illustrates segmented fluid dispersing components 1204 of
similar shapes but
different sizes on an impermeable interconnect 1202, according to an
embodiment of the
disclosure;
[59] Fig. 12D illustrates segmented fluid dispersing components 1204 of
similar shapes and
similar sizes on an impermeable interconnect 1202, according to an embodiment
of the
disclosure;
[60] Fig. 12E illustrates segmented fluid dispersing components 1204 of
similar shapes and
similar sizes but closely packed on an impermeable interconnect 1202,
according to an
embodiment of the disclosure;
[61] Fig. 12F illustrates segmented fluid dispersing components 1204 of
different shapes
and different sizes on an impermeable interconnect 1202, according to an
embodiment of the
disclosure;
[62] Fig. 12G illustrates an impermeable interconnect 1202 and fluid
dispersing component
segment 1204, according to an embodiment of the disclosure;
[63] Fig. 12H illustrates an impermeable interconnect and fluid dispersing
component
segment, according to an embodiment of the disclosure;
[64] Fig. 121 illustrates an impermeable interconnect and fluid dispersing
component
segments, according to an embodiment of the disclosure;
[65] Fig. 123 illustrates an impermeable interconnect 1202 and a fluid
dispersing component
segment 1204, according to an embodiment of the disclosure;
[66] Fig. 12K illustrates a fluid dispersing component 1204, according to
an embodiment of the
disclosure;
[67] Fig. 13A illustrates a template 1300 for making channeled electrodes,
according to an
embodiment of the disclosure;
6
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759
PCT/US2020/0 13129
[68] Fig. 133 is a cross-sectional view of a half cell between a first
interconnect and an
electrolyte, according to an embodiment of the disclosure;
[69] Fig. 13C is a cross-sectional view of a half cell between a second
interconnect and an
electrolyte, according to an embodiment of the disclosure;
[70] Fig. 13D is a cross-sectional view of a half cell between a first
interconnect and an
electrolyte, according to an embodiment of the disclosure;
[71] Fig. 13E is a cross-sectional view of a half cell between a second
interconnect and an
electrolyte, according to an embodiment of the disclosure;
[72] Fig. 14A schematically illustrates segments of fluid dispersing
components in a first
layer, according to an embodiment of the disclosure;
[73] Fig. 1413 schematically illustrates fluid dispersing components in a
first layer along with a
second layer, according to an embodiment of the disclosure;
[74] Fig. 14C schematically illustrates fluid dispersing components in a
first layer along with a
second and third layer, according to an embodiment of the disclosure;
[75] Fig. 14D schematically illustrates fluid dispersing components in a
first layer along with a
second layer, according to an embodiment of the disclosure;
[76] Fig. 15 is an illustrative example of an electrode having dual
porosities, according to an
embodiment of the disclosure;
[77] Fig. 16 illustrates a system for integrated deposition and heating
using electromagnetic
radiation (EMR), according to an embodiment of the disclosure;
[78] Fig. 17 is a scanning electron microscopy image; and
[79] Fig. 18 schematically illustrates an example of a half cell in an EC
reactor.
DETAILED DESCRIPTION
Overview
[80] Embodiments of methods, materials and processes described herein are
directed
towards electrochemical reactors. Electrochemical reactors include solid oxide
fuel cells, solid
oxide fuel cell stacks, electrochemical gas producers, electrochemical
compressors, solid
state batteries, or solid oxide flow batteries.
7
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[81] Electrochemical gas producers can be used to produce syngas, hydrogen or
other gasses
for use as a fuel or feedstock for fuel cells, ammonia production, fertilizer
production,
hydrogenation reactions, Bosch reactions or other applications. The disclosure
herein describes
methods to produce hydrogen with a device. The device can be an
electrochemical gas producer
and can be planar or tubular in shape.
Definitions
[82] The following description recites various aspects and embodiments of the
inventions
disclosed herein. No particular embodiment is intended to define the scope of
the invention. Rather,
the embodiments provide non-limiting examples of various compositions and
methods that are
included within the scope of the claimed inventions. The description is to be
read from the
perspective of one of ordinary skill in the art. Therefore, information that
is well-known to the
ordinarily skilled artisan is not necessarily included.
[83] The following terms and phrases have the meanings indicated below, unless
otherwise
provided herein. This disclosure may employ other terms and phrases not
expressly defined
herein. Such other terms and phrases shall have the meanings that they would
possess within
the context of this disclosure to those of ordinary skill in the art. In some
instances, a term or
phrase may be defined in the singular or plural. In such instances, it is
understood that any term
in the singular may include its plural counterpart and vice versa, unless
expressly indicated to
the contrary.
[84] As used herein, the singular forms "a," "an," and "the" include plural
referents unless
the context clearly dictates otherwise. For example, reference to "a
substituent" encompasses
a single substituent as well as two or more substituents, and the like. As
used herein, "for
example," "for instance," "such as," or "including" are meant to introduce
examples that
further clarify more general subject matter. Unless otherwise expressly
indicated, such
examples are provided only as an aid for understanding embodiments illustrated
in the present
disclosure and are not meant to be limiting in any fashion. Nor do these
phrases indicate any
kind of preference for the disclosed embodiment.
8
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[85] As used herein, compositions and materials are used interchangeably
unless otherwise
specified. Each composition/material may have multiple elements, phases, and
components.
Heating as used herein refers to actively adding energy to the compositions or
materials.
[86] The term "in situ" in this disclosure refers to the treatment (e.g.,
heating) process being
performed either at the same location or in the same device of the forming
process of the
compositions or materials. For example, the deposition process and the heating
process are
performed in the same device and at the same location, in other words, without
changing the
device and without changing the location within the device. For example, the
deposition process
and the heating process are performed in the same device at different
locations, which is also
considered in situ.
[87] In this disclosure, a major face of an object is the face of the
object that has a surface area
larger than the average surface area of the object, wherein the average
surface area of the object is
the total surface area of the object divided by the number of faces of the
object. In some cases, a
major face refers to a face of an item or object that has a larger surface
area than a minor face. In
the case of planar fuel cells or non-SIS type fuel cells, a major face is the
face or surface in the
lateral direction.
[88] As used herein, lateral refers to the direction that is perpendicular
to the stacking direction
of the layers in a non-SIS type fuel cell. Thus, lateral direction refers to
the direction that is
perpendicular to the stacking direction of the layers in a fuel cell or the
stacking direction of the
slices to form an object during deposition. Lateral also refers to the
direction that is the spread of
deposition process.
[89] In this disclosure, a liquid precursor of a substance refers to a
dissolved form
containing the substance, such as a salt in an aqueous solution. For example,
a copper salt
dissolved in an aqueous solution is considered a liquid precursor of copper.
Copper particles
suspended/dispersed (not dissolved) in a liquid are not considered liquid
precursors of copper.
[90] As used herein, CGO refers to Gadolinium-Doped Ceria, also known
alternatively as
gadolinia-doped ceria, gadolinium-doped cerium oxide, cerium(IV) oxide,
gadolinium-doped,
GDC, or GCO, (formula Gd:Ce02). CGO and GDC are used interchangeably unless
otherwise
specified.
9
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[91] Syngas (i.e., synthesis gas) in this disclosure refers to a mixture
consisting primarily of
hydrogen, carbon monoxide and carbon dioxide.
[92] In this disclosure, absorbance is a measure of the capacity of a
substance to absorb
electromagnetic radiation (EMR) of a wavelength. Absorption of radiation
refers to the energy
absorbed by a substance when exposed to the radiation.
[93] As used herein, ceria refers to cerium oxide, also known as ceric oxide,
ceric dioxide, or
cerium dioxide, is an oxide of the rare-earth metal cerium. Doped ceria refers
to ceria doped
with other elements, such as samaria-doped ceria (SDC), or gadolinium-doped
ceria (GDC or
CGO).
[94] As used herein, chromite refers to chromium oxides, which includes all
the oxidation
states of chromium oxides.
[95] As used herein, "little to no water" refers to a water content no greater
than 1 g/m3 or no
greater than 200 mg/m3 or no greater than 50 mg/m3.
[96] An interconnect in an electrochemical device (e.g., a fuel cell) is often
either metallic or
ceramic that is placed between the individual cells or repeat units. Its
purpose is to connect
each cell or repeat unit so that electricity can be distributed or combined.
An interconnect is
also referred to as a bipolar plate in an electrochemical device. An
interconnect being an
impermeable layer as used herein refers to it being a layer that is
impermeable to fluid flow.
For example, an impermeable layer has a permeability of less than 1 micro
darcy, or less than
1 nano darcy.
[97] In this disclosure, an interconnect having no fluid dispersing element
refers to an
interconnect having no elements (e.g., channels) to disperse a fluid. A fluid
may comprise a gas
or a liquid or a mixture of a gas and a liquid. Such fluids may include one or
more of hydrogen,
methane, ethane, propane, butane, oxygen, ambient air or light hydrocarbons
(i.e., pentane,
hexane, octane). Such an interconnect may have inlets and outlets (i.e.,
openings) for materials
or fluids to pass through.
[98] In this disclosure, the term "microchannels" is used interchangeably with
microfluidic
channels or microfluidic flow channels.
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759
PCT/US2020/0 13129
[991 In this
disclosure, sintering refers to a process to form a solid mass of material by
heat or pressure, or a combination thereof, without melting the material to
the extent of
liquefaction. For example, material particles are coalesced into a solid or
porous mass by
being heated, wherein atoms in the material particles diffuse across the
boundaries of the
particles, causing the particles to fuse together and form one solid piece. In
this disclosure
and the appended claims, Tsinter refers to the temperature at which this
phenomenon begins to
take place.
[100] As used herein, the term "pore former" is intended to have a relatively
broad meaning.
"Pore former" may be referring to any particulate material that is included in
a composition
during formation, which may partially or completely vacate a space by a
process, such as
heating, combustion or vaporizing. As used herein, the term "electrically
conductive
component" is intended to refer to components in a fuel cell, such as
electrodes and
interconnects, that are electrically conductive.
[101] For illustrative purposes, the production of solid oxide fuel cells
(SOFCs) will be used as
an example system herein to describe the various embodiments. As one in the
art recognizes
though, the methodologies and the manufacturing processes described herein are
applicable to
any electrochemical device, reactor, vessel, catalyst, etc. Examples of
electrochemical devices or
reactors includes electrochemical (EC) gas producer electrochemical (EC)
compressor, solid
oxide fuel cells, solid oxide fuel cell stack, solid state battery, or solid
oxide flow battery. In an
embodiment, an electrochemical reactor comprises solid oxide fuel cell, solid
oxide fuel cell
stack, electrochemical gas producer, electrochemical compressor, solid state
battery, or solid
oxide flow battery. Catalysts include Fischer Tropsch (FT) catalysts or
reformer catalysts.
Reactor/vessel includes FT reactor or heat exchanger.
Electrochemical (EC) Gas Producer
[1021 Fig. 1A illustrates an electrochemical (EC) gas producer 100, according
to an
embodiment of this disclosure. EC gas producer device 100 comprises first
electrode 101,
electrolyte 103 a second electrode 102. First electrode 101 is configured to
receive a fuel and
no oxygen 104. Second electrode 102 is configured to receive water or nothing
as denoted by
arrow 105. Device 100 is configured to simultaneously produce hydrogen 107
from second
11
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
electrode 102 and syngas 106 from first electrode 101. In an embodiment, 104
represents
methane and water or methane and carbon dioxide entering device 100. In other
embodiments, 103 represents an oxide ion conducting membrane. In an
embodiment, first
electrode 101 and second electrode 102 may comprise Ni-YSZ or NiO-YSZ. Arrow
104
represents an influx of hydrocarbon and water or hydrocarbon and carbon
dioxide. Arrow 105
represents an influx of water or water and hydrogen. In some embodiments,
electrode 101
comprises Cu-CGO further optionally comprising CuO or Cu2O or combinations
thereof.
Electrode 102 comprises Ni-YSZ or NiO-YSZ. Arrow 104 represents an influx of
hydrocarbon
with little to no water, with no carbon dioxide, and with no oxygen, and 105
represents an
influx of water or water and hydrogen. Since water provides the oxide ion
(which is
transported through the electrolyte) needed to oxidize the hydrocarbon/fuel at
the opposite
electrode, water is considered the oxidant in this scenario.
[103] Fig. 18 illustrates an EC gas producer 110, according to an embodiment
of this
disclosure. EC gas producer device 110 comprises first electrode 111, second
electrode 112,
and electrolyte 113 between the electrodes. The first electrode 111 is
configured to receive a
fuel and no oxygen 104, wherein second electrode 112 is configured to receive
water or
nothing. In some embodiments, 113 represents a proton conducting membrane, 111
and 112
represent Ni-barium zirconate electrodes. Hydrogen 107 is produced from second
electrode
112 and syngas 106 is produced from first electrode 111.
[104] In this disclosure, no oxygen means there is no oxygen present at first
electrode 101,
111 or at least not enough oxygen that would interfere with the reaction.
Also, in this disclosure,
water only means that the intended feedstock is water and does not exclude
trace elements or
inherent components in water. For example, water containing salts or ions is
considered to be
within the scope of water only. Water only also does not require 100% pure
water but includes
this embodiment. In embodiments, the hydrogen produced from second electrode
102, 112 is
pure hydrogen, which means that in the produced gas phase from the second
electrode,
hydrogen is the main component. In some cases, the hydrogen content is no less
than 99.5%. In
some cases, the hydrogen content is no less than 99.9%. In some cases, the
12
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
hydrogen produced from the second electrode is the same purity as that
produced from
electrolysis of water.
[105] In an embodiment, first electrode 101, 111 is configured to receive
methane and water
or methane and carbon dioxide. In an embodiment, the fuel comprises a
hydrocarbon having a
carbon number in the range of 1-12, 1-10 or 1-8. Most preferably, the fuel is
methane or
natural gas, which is predominantly methane. hi an embodiment, the device does
not generate
electricity. In an embodiment, the device comprises a mixer configured to
receive at least a
portion of the first electrode product and at least a portion of the second
electrode product.
The mixer may be configured to generate a gas stream in which the hydrogen to
carbon oxides
ratio is no less than 2, or no less than 3 or between 2 and 3.
[106] In an embodiment, first electrode 101, 111 or second electrode 102, 112,
or both the
first electrode 101, 111 and second electrode 102, 112 comprise a catalyst and
a substrate,
wherein the mass ratio between the catalyst and the substrate is no less than
1/100, or no less
than 1/10, or no less than 1/5, or no less than 1/3, or no less than 1/1. In
an embodiment, the
catalyst comprises nickel oxide, silver, cobalt, cesium, nickel, iron,
manganese, nitrogen, tetra-
nitrogen, molybdenum, copper, chromium, rhodium, ruthenium, palladium, osmium,
iridium, or
platinum, or combinations thereof. In an embodiment, the substrate comprises
gadolinium,
Ce02, Zr02, Si02, Ti02, steel, cordierite (2Mg0-2A1203-5902), aluminum
titanate (Alin05), silicon
carbide (SiC), all phases of aluminum oxide, yttria or scandia-stabilized
zirconia (YSZ), gadolinia
or samaria-doped ceria, or combinations thereof. In some embodiments, first
electrode 101,
111 or second electrode 102, 112, or both the first electrode 101, 111 and
second electrode
102, 112, comprise a promoter wherein the promoter is selected from the group
consisting of
Mo, W, Ba, K, Mg, Fe, and combinations thereof. In an embodiment, an anode
(e.g., the first
electrode or the second electrode) comprises a catalyst, wherein the catalyst
is selected from
the group consisting of nickel, iron, palladium, platinum, ruthenium, rhodium,
cobalt, and
combinations thereof.
[107] In some embodiments, the electrodes and electrolyte form a repeat unit.
A device may
comprise two or more repeat units separated by interconnects. In a preferred
embodiment, the
interconnects comprise no fluid dispersing element. In an embodiment, first
electrode 101,
13
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
111 or second electrode102, 112, or both the first electrode 101, 111 and
second electrode
102, 112, comprise fluid channels. Alternatively, the first electrode 101, 111
or second electrode
102, 112, or both the first electrode 101, 111 and second electrode 102, 112,
comprise fluid
dispersing components.
[108] Also discussed herein is an assembly method comprising forming a first
electrode
101, 111, forming a second electrode 102, 112, and forming an electrolyte 103,
113 between
the electrodes, wherein the electrodes and electrolyte are assembled as they
are formed.
Forming may comprise material jetting, binder jetting, inkjet printing,
aerosol jetting, or
aerosol jet printing, vat photopolymerization, powder bed fusion, material
extrusion, directed
energy deposition, sheet lamination, ultrasonic inkjet printing, or
combinations thereof. The
electrodes and electrolyte may form a repeat unit. The method may further
comprise
forming two or more repeat units and forming interconnects between the two or
more repeat
units. The assembly method may further comprise forming fluid channels or
fluid dispersing
components in the first electrode 101, 111 or the second electrode 102, 112,
or both the
first electrode 101, 111 and second electrode 102, 112. The forming method may
comprise
heating in situ. In a preferred embodiment, the heating comprises [MR. [MR may
comprise
one or more of UV light, near ultraviolet light, near infrared light, infrared
light, visible light,
laser or electron beam.
[109] The first electrode 101, 111 is configured to receive a fuel and no
oxygen, wherein the
second electrode 102, 112 is configured to receive water only or nothing,
wherein the device is
configured to simultaneously produce hydrogen from the second electrode 102,
112 and syngas
from the first electrode 101, 111.
[110] Further discussed herein is a method comprising providing a device
comprising a first 101,
111 electrode, a second electrode 102, 112, and an electrolyte 103, 113
between the electrodes,
introducing a fuel without oxygen to the first electrode 101, 111, introducing
water only or nothing
to the second electrode 102, 112 to generate hydrogen, extracting hydrogen
from the second
electrode 102, 112, and extracting syngas from the first electrode 101, 111.
In preferred
embodiments, the fuel comprises methane and water or methane and carbon
14
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
dioxide. In preferred embodiments, the fuel comprises a hydrocarbon having a
carbon number in
the range of 1-12 or 1-10 or 1-8.
[111] In an embodiment, the method comprises feeding at least a portion of the
extracted
syngas to a Fischer-Tropsch reactor. In an embodiment, the method comprises
feeding at least
a portion of the extracted hydrogen to the Fischer-Tropsch reactor. In an
embodiment, the at
least portion of the extracted syngas and the at least portion of the
extracted hydrogen are
adjusted such that the hydrogen to carbon oxides ratio is no less than 2, or
no less than 3, or
between 2 and 3.
[112] In an embodiment, the fuel is directly introduced into the first
electrode 101, 111 or
water is directly introduced into the second electrode 102, 112, or both the
first electrode
101, 111 and second electrode 102, 112. In an embodiment, the first electrode
101, 111 or
second electrode 102, 112, or both the first electrode 101, 111 and second
electrode 102,
112, comprise a catalyst and a substrate, wherein the mass ratio between the
catalyst and
the substrate is in no less than 1/100, or no less than 1/10, or no less than
1/5, or no less
than 1/3, or no less than 1/1. In preferred embodiments, the catalyst
comprises nickel oxide,
silver, cobalt, cesium, nickel, iron, manganese, nitrogen, tetra-nitrogen,
molybdenum, copper,
chromium, rhodium, ruthenium, palladium, osmium, iridium, platinum, or
combinations
thereof. In preferred embodiments, the substrate comprises gadolinium, Ce02,
Zr02, Si02,
Ti02, steel, cordierite (2Mg0-2A1203-5Si02), aluminum titanate (Al2Ti05),
silicon carbide (SiC),
all phases of aluminum oxide, yttria or scandia-stabilized zirconia (YSZ),
gadolinia or samaria-
doped ceria, or combinations thereof.
[113] In an embodiment, the method comprises applying a potential difference
between the first
electrode 101, 111 and the second electrode 102, 112. In an embodiment, the
method comprises
using the extracted hydrogen in one of the following reactions, or
combinations thereof: Fischer-
Tropsch (FT) reaction, dry reforming reactions, Sabatier reaction catalyzed by
nickel, Bosch
reaction, reverse water gas shift reaction, electrochemical reaction to
produce electricity,
production of ammonia and/or fertilizer, electrochemical compressor for
hydrogen storage or
fueling hydrogen vehicles, or hydrogenation reactions.
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[114] The gas producer is not a fuel cell and does not generate electricity,
in various
embodiments. Electricity may be applied to the gas producer at the anode and
cathode in
some cases. In other cases, electricity is not needed.
[115] Herein disclosed is a device comprising a first electrode, a second
electrode, and an
electrolyte between the electrodes, wherein the first electrode and the second
electrode comprise
a metallic phase that does not contain a platinum group metal when the device
is in use, and
wherein the electrolyte is oxide ion conducting. In an embodiment, wherein the
first electrode
comprises Ni or NiO and a material selected from the group consisting of YSZ,
CGO, samaria-
doped ceria (SDC), scandia-stabilized zirconia (SSZ), LSGM, and combinations
thereof. In an
embodiment, the first electrode is configured to receive a fuel and water or a
fuel and carbon
dioxide. In an embodiment, said fuel comprises a hydrocarbon or hydrogen or
carbon monoxide
or combinations thereof.
[116] In an embodiment, the first electrode comprises doped or undoped ceria
and a material
selected from the group consisting of Cu, CuO, Cu2O, Ag, Ag2O, Au, Au20,
Au203, stainless steel,
and combinations thereof. In an embodiment, the first electrode is configured
to receive a fuel
with little to no water. In an embodiment, said fuel comprises a hydrocarbon
or hydrogen or
carbon monoxide or combinations thereof. In an embodiment, the second
electrode comprises Ni
or NiO and a material selected from the group consisting of yttria-stabilized
zirconia (YSZ), ceria
gadolinium oxide (CGO), samaria-doped ceria (SIX), scandia-stabilized zirconia
(SSZ), lanthanum
strontium gallate magnesite (LSGM), and combinations thereof. In an
embodiment, the second
electrode is configured to receive water and hydrogen and configured to reduce
the water to
hydrogen. In an embodiment, the electrolyte comprises doped ceria or wherein
the electrolyte
comprises lanthanum chromite or a conductive metal or combination thereof and
a material
selected from the group consisting of doped ceria, YSZ, LSGM, SSZ, and
combinations thereof.
In an embodiment, the lanthanum chromite comprises undoped lanthanum chromite,
strontium
doped lanthanum chromite, iron doped lanthanum chromite, lanthanum calcium
chromite, or
combinations thereof. In an embodiment, the conductive metal comprises Ni, Cu,
Ag, Au, or
combinations thereof.
16
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[117] In an embodiment, the first electrode 101, 111 or second electrode 102,
112 or both
the first electrode 101, 111 and second electrode 102, 112 comprise fluid
channels.
Alternatively, the first electrode 101, 111 or second electrode 102, 112 or
both the first
electrode 101, 111 and second electrode 102, 112 comprise fluid dispersing
components. In an
embodiment, the electrodes and electrolyte 103, 113 form a repeat unit and
wherein a device
comprises multiple repeat units separated by interconnects. In an embodiment,
the
interconnects comprise no fluid dispersing elements. In an embodiment, the
electrodes 101,
102, 111, 112 and electrolyte 103, 113 may be planar. Fluid dispersing
components or fluid
channels in the electrodes function to distribute fluids, e.g., reactive gases
(such as methane,
hydrogen, carbon monoxide, air, oxygen, steam etc.), in an electrochemical
reactor. As such,
traditional interconnects with channels are no longer needed. The design and
manufacturing of
such traditional interconnects with channels is complex and expensive.
According to this
disclosure, the interconnects are simply impermeable layers that conduct or
collect electrons,
having no fluid dispersing elements.
[118] In an embodiment, the device comprises no interconnect. In an
embodiment, the
electrolyte 103, 113 conducts oxide ions and electrons. In an embodiment, the
electrodes 101,
102, 111, 112 and the electrolyte 103, 113 are tubular. In some embodiments,
the
electrochemical reactions at the anode and the cathode are spontaneous without
the need to
apply potential/electricity to the reactor. In such cases, the interconnect is
no longer needed,
which significantly simplifies the device. In such cases, the electrolyte in
the device conducts
both oxide ions and electrons.
[119] In an embodiment, the device comprises a reformer upstream of the first
electrode
101, 111, wherein the first electrode 101, 111 comprises Ni or Ni0 or a
combination thereof. In
an embodiment, the reformer is a steam reformer or an autothermal reformer. In
an
embodiment, the device is configured to operate at a temperature no less than
500 C, or no
less than 600 C, or no less than 700 C.
[120] In an embodiment, the electrodes and the electrolyte are tubular with
the first
electrode being outermost and the second electrode being innermost, wherein
the first
electrode comprises doped or undoped ceria and a material selected from the
group consisting
17
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
of Cu, CuO, Cu2O, Ag, Ag2O, Au, Au20, Au203, stainless steel, and combinations
thereof. In an
embodiment, the electrodes and the electrolyte are tubular with the first
electrode being
outermost and the second electrode being innermost, wherein the second
electrode is configured
to receive water and hydrogen.
[121] Herein also disclosed is a device comprising a first electrode, a second
electrode, and an
electrolyte between the electrodes, wherein the first electrode comprises
doped lanthanum
chromium oxide and doped or undoped ceria, wherein the second electrode
comprises Ni or NiO
and a material selected from the group consisting of YSZ, CGO, Samaria-doped
ceria (SDC),
Scandia-stabilized zirconia (SSZ), LSGM, ceria, and combinations thereof, and
wherein the
electrolyte is oxide ion conducting. In an embodiment, the electrolyte
comprises YSZ, CGO, LSGM,
SSZ, SDC, ceria, or combinations thereof. In an embodiment, the device is
planar. In an
embodiment, the device is tubular.
[122] Further discussed herein is a method of making a device, comprising
forming a first
electrode, forming a second electrode, and forming an electrolyte between the
electrodes,
wherein the first electrode comprises doped lanthanum chromium oxide and doped
or undoped
ceria, wherein the second electrode comprises Ni or NiO and a material
selected from the group
consisting of YSZ, CGO, Samaria-doped ceria (SDC), Scandia-stabilized zirconia
(SSZ), LSGM,
ceria, and combinations thereof, and wherein the electrolyte is oxide ion
conducting. In an
embodiment, the electrolyte comprises YSZ, CGO, LSGM, SSZ, SDC, ceria, or
combinations
thereof. In an embodiment, said forming comprises material jetting, binder
jetting, inkjet
printing, aerosol jetting, or aerosol jet printing, vat photopolymerization,
powder bed fusion,
material extrusion, directed energy deposition, sheet lamination, or
ultrasonic inkjet printing, or
combinations thereof. In an embodiment, the forming comprises extrusion, dip
coating,
spraying, spin coating, brush coating, pasting, or combinations thereof. In an
embodiment, the
forming comprises heating using an electromagnetic radiation source or a
furnace.
[123] Discussed herein is a method of making a device, comprising forming a
first electrode,
forming a second electrode, and forming an electrolyte between the electrodes,
wherein the first
electrode and the second electrode comprise a metallic phase that does not
contain a platinum
group metal when the device is in use, and wherein the electrolyte is oxide
ion
18
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
conducting. In an embodiment, the electrodes and electrolyte are assembled as
they are
formed. In an embodiment, said electrodes and electrolyte form a repeat unit
and said method
comprises forming said multiple repeat units and forming interconnects between
the repeat
units. In an embodiment, the interconnects comprise no fluid dispersing
element. In an
embodiment, the method comprises forming fluid channels or fluid dispersing
components in
the first electrode or the second electrode or both the first electrode and
the second electrode.
[124] In an embodiment, the first electrode comprises Ni or NiO and a material
selected from
the group consisting of YSZ, CGO, samaria-doped ceria (SDC), scandia-
stabilized zirconia (SSZ),
LSGM, and combinations thereof. In an embodiment, the first electrode
comprises doped or
undoped ceria and a material selected from the group consisting of Cu, CuO,
Cu2O, Ag, Ag2O,
Au, Au20, Au203, stainless steel, and combinations thereof. In an embodiment,
the second
electrode comprises Ni or NiO and a material selected from the group
consisting of YSZ, CGO,
samaria-doped ceria (SDC), scandia-stabilized zirconia (SSZ), LSGM, ceria, and
combinations
thereof. In an embodiment, the electrolyte comprises YSZ, CGO, LSGM, SSZ, SDC,
ceria, or
combinations thereof.
[125] In an embodiment, the forming comprises material jetting, binder
jetting, inkjet
printing, aerosol jetting, aerosol jet printing, vat photopolymerization,
powder bed fusion,
material extrusion, directed energy deposition, sheet lamination, ultrasonic
inkjet printing, or
combinations thereof. In an embodiment, the method comprises heating in situ.
In an
embodiment, the heating comprises electromagnetic radiation (EMR). In an
embodiment, EMR
comprises UV light, near ultraviolet light, near infrared light, infrared
light, visible light, laser,
electron beam, or combinations thereof. In an embodiment, EMR is provided by a
xenon
lamp. In an embodiment, the electrodes and the electrolyte are planar. In an
embodiment,
the device comprises no interconnect. In an embodiment, the electrolyte
conducts oxide ions
and electrons.
[126] In an embodiment, the forming comprises a) depositing a composition on a
substrate to
form a slice; b) drying the slice using a non-contact dryer; c) heating the
slice using
electromagnetic radiation (EMR) or conduction or both. In an embodiment, the
method
comprises repeating steps a)-c) to produce the device slice by slice. In an
embodiment, the
19
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
method comprises comprising d) measuring the slice temperature T within time t
after the last
exposure of the EMR without contacting the slice, wherein t is no greater than
5 seconds, or no
greater than 4 seconds, or no greater than 3 seconds, no greater than 2
seconds, or no greater
than 1 second. In an embodiment, the method comprises e) comparing T with
Tsinter, wherein
Tsinter is no less than 45% of the melting point of the composition if the
composition is non-
metallic; or wherein Tsinter is no less than 60% of the melting point of the
composition if the
composition is metallic. In an embodiment, the method comprises e) comparing T
with Tsinter,
wherein Tsinter is previously determined by correlating the measured
temperature with
microstructure images of the slice, scratch test of the slice, electrochemical
performance test of
the slice, dilatometry measurements of the slice, conductivity measurements of
the slice, or
combinations thereof. In an embodiment, the method comprises heating the slice
using [MR or
conduction or both in a second stage if T is less than 90% of Tsinter.
[127] In an embodiment, drying takes place for a period in the range of no
greater than 5
minutes, or no greater than 3 minutes, or no greater than 1 minute, or from 1
s to 30 s, or from 3 s
to 10 s. In an embodiment, the non-contact dryer comprises infrared heater,
hot air blower,
ultraviolet light source, or combinations thereof.
[128] As an example, all the layers of an EC gas producer are formed and
assembled via
printing. The materials for making the anode, cathode, electrolyte, and the
interconnect,
respectively, are made into an ink form comprising a solvent and particles
(e.g., nanoparticles).
The ink optionally comprises a dispersant, binder, plasticizer, surfactant, co-
solvent, or
combinations thereof. For the anode and the cathode of a gas producer, NiO and
YSZ particles
are mixed with a solvent, wherein the solvent is water (e.g., de-ionized
water) or an alcohol
(e.g., butanol) or a mixture of alcohols. Organic solvents other than alcohols
may also be used.
For the electrolyte, YSZ particles are mixed with a solvent, wherein the
solvent is water (e.g.,
de-ionized water) or an alcohol (e.g., butanol) or a mixture of alcohols.
Organic solvents other
than alcohols may also be used. For the interconnect, metallic particles (such
as, silver
nanoparticles) are dispersed or suspended in a solvent, wherein the solvent
may include water
(e.g., de-ionized water), organic solvents (e.g., mono-, di-, or tri-ethylene
glycols or higher
ethylene glycols, propylene glycol, 1,4-butanediol or ethers of such glycols,
thiodiglycol, glycerol
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
and ethers and esters thereof, polyglycerol, mono-, di-, and tri-ethanolamine,
propanolamine,
N,N-dimethylformamide, dimethyl sulfoxide, dimethylacetamide, N-
methylpyrrolidone, 1,3-
dimethylimidazolidone, methanol, ethanol, isopropanol, n-propanol, diacetone
alcohol,
acetone, methyl ethyl ketone, propylene carbonate), and combinations thereof.
For a barrier
layer, CGO particles may be dissolved, dispersed or suspended in a solvent,
wherein the
solvent is water (e.g., de-ionized water) or an alcohol (e.g., butanol) or a
mixture of alcohols.
Organic solvents other than alcohols may also be used. CGO is used as barrier
layer for LSCF.
YSZ may also be used as a barrier layer for LSM.
Tubular and Multi-Tubular EC Gas Producers
[129] Fig. 2A illustrates (not to scale) a tubular EC gas producer 200,
according to an
embodiment of this disclosure. Tubular EC gas producer 200 includes an inner
tubular structure
202, an outer tubular structure 204, and an electrolyte 206 disposed between
the inner and
outer tubular structures 202, 204, respectively. In some embodiments,
electrolyte 206 may
instead comprise a membrane. Tubular gas producer 200 further includes a void
space 208 for
fluid passage.
[130] Fig. 2B illustrates (not to scale) a cross section of a tubular EC gas
producer 200,
according to an embodiment of this disclosure. Tubular EC gas producer 200
includes a first
inner tubular structure 202, a second outer tubular structure 204, and an
electrolyte 206
between the inner and outer tubular structures 202, 204. In some embodiments,
electrolyte 206
may be referred to as a membrane. Tubular gas producer 200 further includes a
void space 208
for fluid passage.
[131] In an embodiment, inner tubular structure 202 comprises an electrode.
Inner tubular
structure 202 may be an anode or a cathode. In an embodiment, inner tubular
structure 202 may
be porous. Inner tubular structure 202 may comprise Ni or NiO and a material
selected from the
group consisting of YSZ, CGO, samaria-doped ceria (SDC), scandia-stabilized
zirconia (SSZ),
LSGM, and combinations thereof. Inner tubular structure 202 may comprise doped
or undoped
ceria and a material selected from the group consisting of Cu, CuO, Cu20, Ag,
Ag20, Au, Au20,
Au203, stainless steel, and combinations thereof. In an embodiment, outer
tubular structure 204
comprises an electrode. Outer tubular structure 204 may be an anode or a
21
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
cathode. Outer tubular structure 204 may comprise Ni or Ni0 and a material
selected from the
group consisting of YSZ, CGO, samaria-doped ceria (SDC), scandia-stabilized
zirconia (SSZ),
LSGM, and combinations thereof. Outer tubular structure 204 may comprise doped
or
undoped ceria and a material selected from the group consisting of Cu, CuO,
Cu20, Ag, Ag2O,
Au, Au20, Au203, stainless steel, and combinations thereof. It should be noted
that the listing
of materials above is not limiting.
[132] In embodiments, electrolyte 206 comprises doped ceria or wherein the
electrolyte
comprises lanthanum chromite or a conductive metal or combination thereof and
a material
selected from the group consisting of doped ceria, YSZ, LSGM, SSZ, and
combinations
thereof. In an embodiment, the lanthanum chromite comprises undoped lanthanum
chromite,
strontium doped lanthanum chromite, iron doped lanthanum chromite, lanthanum
calcium
chromite, or combinations thereof. In an embodiment, the conductive metal
comprises Ni, Cu,
Ag, Au, or combinations thereof. Electrolyte 206 is be oxide ion conducting.
In some cases,
electrolyte 206 is both oxide ion and electronically conducting. In some
embodiments, the
producer 200 further comprises one or more interconnects.
[133] Fig. 3A illustrates a cross section of a multi-tubular EC gas producer
300, according to an
embodiment of the disclosure. EC gas producer 300 comprises an inner electrode
302, an outer
electrode 304, and an electrolyte 306 between the electrodes 302, 304. In some
embodiments,
electrolyte 306 is referred to as a membrane. The inner electrode 302
comprises multiple tubular-
like void spaces 308 joined in the radial direction. Void spaces 308 allow for
fluid passage. Void
spaces 308 may also be referred to as fluid passages. The multi-tubular
structure 300 comprises
multiple fluid passages 308 in the axial direction of the tubular structure
300. The cross-section
of void spaces 308 may be circular-like, oval-like or other similar shapes.
The cross-sections of
spaces 308 may be irregular shaped as illustrated in Fig. 3A. Producer 300 has
a cross section
having a length and a width, wherein the length is at least 2 times the width
and the cross
section is orthogonal to the axial direction of the tubular. Multi-tubular
structure 300 is comprised
of multiple individual tubular structures 309 (denoted by a dotted line).
(1.343 Inner electrode 302 in producer 300 may be of unitary construction and
has no brazed or
soldered part. In an embodiment, the producer 300 is of unitary construction
and has no
22
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
brazed or soldered part. In an embodiment, the electrolyte 306 is oxide ion
conducting and is
solid state. In an embodiment, the electrolyte comprises a material previously
listed herein for
electrolyte 206 in tubular reactor 200. In embodiments, the electrodes 302,
304 may comprise
one or more materials previously listed herein for tubular structures 202, 204
in tubular reactor
200. In some embodiments, the producer 300 further comprises one or more
interconnects.
[135] Fig. 3B illustrates a cross section of a multi-tubular EC gas producer
320, according to an
embodiment of the disclosure. Gas producer has a rectangular-like shape cross-
section. EC gas
producer 320 comprises an inner electrode 302, an outer electrode 304, and an
electrolyte 306
between the electrodes 302, 304. In some embodiments, a membrane may be used
in place of
electrolyte 306. The inner electrode 302 comprises multiple void spaces 308
joined in the radial
direction of the tubular-like void spaces 308. Void spaces 308 allow for fluid
passage. The
multiple tubular structure 320 comprises multiple fluid passages 308 in the
axial direction of the
tubular structure 320. The cross-section of void spaces 308 may be circular-
like, oval-like,
square-like, hexagonal-like, triangular-like or other similar shapes in a
random or regular
fashion. Producer 320 has a cross section having a length and a width, wherein
the length is at
least 2 times the width and the cross section is orthogonal to the axial
direction of the tubular.
[1363 Inner electrode 302 in producer 320 may be of unitary construction and
have no brazed
or soldered part. Producer 320 may be of unitary construction and have no
brazed or soldered
part. In an embodiment, the electrolyte 306 is oxide ion conducting. In
embodiments, the
electrolyte may comprise one or more materials previously listed herein for
electrolyte 206 in
tubular reactor 200. In embodiments, the electrodes 302, 304 may comprise one
or more
materials previously listed herein for tubular structures 202, 204 in tubular
reactor 200. In
some embodiments, the producer 320 further comprises one or more
interconnects.
[1373 Fig. 3C illustrates a cross section of a multi-tubular EC gas producer
340, according to an
embodiment of the disclosure. Gas producer 340 has a rectangular-like shape
cross-section. EC gas
producer 340 comprises an inner electrode 302, an outer electrode 304, and an
electrolyte 306
between the electrodes 302, 304. In some embodiments, electrolyte 306 is
referred to as a
membrane. The inner electrode 302 comprises multiple void spaces 308 joined in
the axial direction
of the tubular. Void spaces 308 allow for fluid passage. The multiple tubular
structure
23
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
340 comprises multiple fluid passages 308 in the axial direction of the
tubular structure 340. The
cross-section of void spaces 308 may be square-like or rectangular-like as
shown in Fig. 3C or
other similar shapes in a regular fashion wherein the cross-sectional area of
each void space is
substantially identical. Producer 340 has a cross section having a length and
a width, wherein
the length is at least 2 times the width and the cross section is orthogonal
to the axial direction
of tubular.
[138] Inner electrode 302 in producer 340 may be of unitary construction and
have no brazed
or soldered part. Producer 340 may be of unitary construction and have no
brazed or soldered
part. In an embodiment, the electrolyte 306 is oxide ion conducting. In
embodiments, the
electrolyte may comprise one or more materials previously listed herein for
electrolyte 206 in
tubular reactor 200. In embodiments, the electrodes 302, 304 may comprise one
or more
materials previously listed herein for tubular structures 202, 204 in tubular
reactor 200. In
some embodiments, the producer 340 further comprises one or more
interconnects.
[139] Fig. 30 illustrates a cross section of an EC gas producer 360, according
to an
embodiment of the disclosure. Gas producer 360 has a rectangular-like shape
cross-section. EC
gas producer 360 is similar to gas producer 340 in Fig. 3C, except that the
fluid passage 380 is
single as shown in Fig. 30.
Manufacture of Tubular and Multi-Tubular EC Gas Producers
[140] Further discussed herein is a method of making a tubular EC gas producer
as illustrated by
device 200, 300, 320, 340, and 360, which are mere examples of some tubular
designs. At least
three methods are discussed herein regarding how to make the first tubular:
extrusion method,
substrate method, and the process as shown in Fig. SA-58.
[141] In an embodiment, a method of making a tubular EC gas producer comprises
forming
a first tubular structure by extrusion. In some embodiments, the first tubular
structure is an
inner electrode 202. The method further comprises depositing a layer on the
outer cylindrical
surface of the first tubular structure 202, wherein the layer comprises an
electrolyte 206, and
depositing a second tubular structure 204 over the electrolyte 206, wherein
the electrolyte 206
is oxide ion conducting. In an embodiment, the first tubular structure 202 and
the second
tubular structure 204 comprise a metallic phase that does not contain a
platinum group metal
24
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
when the device is in use. In an embodiment, the device comprises no
interconnect and
wherein the electrolyte is electronically conducting.
[142] In another manufacturing method embodiment, a method comprises extruding
an inner
tubular structure 202; sintering the inner tubular structure 202 in a furnace
or with EMR to
form a first electrode; coating the outer surface of the inner tubular
structure 202 with an
electrolyte material; sintering the electrolyte material in a furnace or EMR
to form an electrolyte
206; coating the electrolyte 206 with an electrode material; sintering the
electrode material in a
furnace or using electromagnetic radiation (EMR) to form an outer tubular
structure 204
wherein the outer tubular structure 204 is a second electrode. In an
embodiment, outer tubular
structure 204 comprises doped or undoped ceria and a material selected from
the group
consisting of Cu, CuO, Cu2O, Ag, Ag2O, Au, Au20, Au203, stainless steel, and
combinations
thereof; and is sintered using EMR. In an embodiment, the method further
comprises reducing
the outer tubular structure 204 or reducing the inner tubular structure 202 or
both tubular
structures 202, 204. These methods describe an "inside out" method wherein the
first extruded
layer is the inner electrode layer.
[143] The following method describes an "outside in" method wherein the first
layer formed is
the outer tubular structure 204 or outer electrode layer. The method comprises
extruding an
outer tubular structure 204; sintering the outer tubular structure 204 in a
furnace or with EMR to
form a first electrode; coating the inner surface of the outer tubular
structure 204 with an
electrolyte material; sintering the electrolyte material in a furnace or EMR
to form an electrolyte
206; coating the inner surface of electrolyte 206 with an electrode material;
sintering the
electrode material in a furnace or using electromagnetic radiation (EMR) to
form an inner tubular
structure 202 wherein the inner tubular structure 202 is a second electrode.
In an embodiment,
inner tubular structure 202 comprises doped or undoped ceria and a material
selected from the
group consisting of Cu, CuO, Cu2O, Ag, Ag2O, Au, Au20, Au203, stainless steel,
and combinations
thereof; and is sintered using EMR. In an embodiment, the method further
comprises reducing
the outer tubular structure 204 or reducing the inner tubular structure 202 or
both tubular
structures 202, 204.
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[144] In an embodiment, the coating steps for use in the "inside out" and
"outside in"
methods comprise dip coating, spraying, ultrasonic spraying, spin coating,
brush coating,
pasting, or combinations thereof. The electromagnetic radiation comprises UV
light, near
ultraviolet light, near infrared light, infrared light, visible light, laser,
electron beam, microwave
or combinations thereof. In an embodiment, electromagnetic radiation is
provided by a xenon
lamp. In some embodiments, the device may comprise one or more interconnects.
In an
embodiment, the inner tubular structure 202 and the outer tubular structure
204 comprise one
or more fluid channels or one or more fluid dispersing components or both
fluid channels and
fluid dispersing components.
[145] In another embodiment, the inner tubular structure 202 or the outer
tubular structure
204 may be formed from particulates and not from liquid precursors, especially
when the inner
tubular structure 202 or the outer tubular structure 204 comprises doped or
undoped ceria and
a material selected from the group consisting of Cu, CuO, Cu20, Ag, Ag20, Au,
Au20, Au203,
stainless steel, and combinations thereof. The particulates are suspended in a
liquid before
being deposited or coated, such as dip coating, spraying, spin coating, brush
coating, pasting,
or combinations thereof. In such cases, the inner tubular structure 202 or the
outer tubular
structure 204 is sintered using electromagnetic radiation (EMR).
[146] In other embodiments, a first tubular-like substrate is provided. The
tubular substrate
is substantially in a desired shape of an EC gas producer. In a first
embodiment, a first
electrode material is deposited on the outside of the tubular substrate. The
first electrode
material is sintered to form an inner electrode 202. An electrolyte material
is then deposited
on the inner electrode layer 202 surface. The electrolyte material is the
sintered to form an
electrolyte 206. A second electrode material is then deposited on the
electrolyte 206. The
second electrode material is then sintered to form an outer electrode 204.
This method may
be described as an "inside out substrate method" wherein the first layer
formed on the
substrate is the inner electrode layer 202 followed by the electrolyte 206
layer then the outer
electrode layer 204. The first and second electrodes may be an anode or
cathode. Sintering
may comprise thermal or EMR sintering.
26
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[147] In another similar method, a tubular-like substrate is provided. A first
electrode
material is deposited on the inside of the tubular substrate. The first
electrode material is
sintered to form an outer electrode 204. An electrolyte material is then
deposited on the
outer electrode layer 204 surface. The electrolyte material is the sintered to
form an
electrolyte 206. A second electrode material is then deposited on the
electrolyte 206. The
second electrode material is then sintered to form an inner electrode 202.
This method may
be described as an "outside in substrate method" wherein the first layer
formed on the
substrate is the inner electrode layer 202 followed by the electrolyte 206
layer then the outer
electrode layer 204. The first and second electrodes may be an anode or
cathode. Sintering
may comprise thermal or EMR sintering.
[148] In some embodiments, the substrate may then be removed once the final
electrode is
formed. The substrate may be removed by physical means. The substrate may be
dissolved
and removed by means of a solvent. In some methods, the substrate may be
comprised of a
low melting material such as a polymer, wherein the substrate may be melted or
gasified and
removed during any one of a thermal sintering step. For example, the substrate
may comprise
a combustible material such that during one of the thermal sintering steps the
substrate is
burned away.
[149] In an embodiment, the first tubular (inner or outer) and the electrolyte
are sintered in
an oven separately. In an embodiment, the first tubular (inner or outer) and
the electrolyte are
co-sintered in an oven, which means that the first tubular is coated with the
electrolyte
material before being sintered. The second tubular (outer or inner) is
deposited on the
electrolyte and then sintered using EMR, wherein the second tubular comprises
doped or
undoped ceria and a material selected from the group consisting of Cu, CuO,
Cu2O, Ag, Ag2O,
Au, Au20, Au203, stainless steel, and combinations thereof. Figs. 4A-40
illustrate various
arrangements to sinter a tubular using an EMR source. The EMR source and the
tubular may
move relative to one another, e.g., in the axial direction or in a spiraling
trajectory, to ensure
the entire surface of the tubular (inner or outer) is sintered by sufficiently
exposing it to the
EMR source. In an embodiment, the EMR source is a xenon lamp, such as a
circular xenon
lamp, a long tubular xenon lamp, a point tubular xenon lamp.
27
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
(1503 Figs. 4A-40 illustrate sintering methods and systems for manufacturing
tubular EC gas
producers using EMR. Fig. 4A illustrates a portion of a method of manufacture
400 of an EC
gas producer using a single point EMR source, according to an embodiment of
the disclosure.
The EMR source (e.g., a xenon lamp) 402 and the tubular structure 404 can move
relative to
one another. As shown in Fig. 4A, the single point EMR 402 may rotate around
the tubular
structure 404 (e.g., in a spiral-like trajectory) in either direction as
denoted by arrow 406.
Alternatively, the tubular structure 404 may rotate around the single point
EMR 402. In
another embodiment, the tubular structure 404 may rotate around its own axis
408 or move in
an up or down direction 410 along its own long axis or a combination thereof.
The single point
EMR source 402 may also move in an up or down direction 412.
[151] Fig. 4B illustrates a portion of a method of manufacture 420 of an EC
gas producer
using a ring-lamp EMR source, according to an embodiment of the disclosure. As
shown in Fig.
4B, a circular ring-like lamp (e.g., xenon lamp) 422 is shown as the EMR
source with a hollow
circle in the center. The tubular structure 404 is placed in the center of the
circular ring lamp
422. In some embodiments, the tubular structure 404 may move up or down 410 or
rotate 408
around its own axis while the ring lamp is 422 held in a stationary manner. In
other
embodiments, tubular structure 404 may be held in a stationary manner while
ring lamp 422
may move along the length of tubular structure 404. Ring lamp 422 may move in
an up or down
424 manner or in a manner which it rotates (426) on its own axis to assure
complete and
thorough sintering. In other embodiments, both the tubular structure 402 and
the ring lamp 422
may both be able to move relative to each other to ensure the entire tubular
structure 404 is
thoroughly and completely sintered. Fig. 4A-4B illustrate embodiments where
the outer surface
of the tubular structure 404 is sintered via EMR. These methods may be used to
sinter anodes,
cathodes, electrolytes and other components of tubular EC gas producers.
[152] Figs. 4C-4D illustrate the embodiment wherein the inner surface of the
tubular structure
404 is sintered via EMR. Fig. 4C illustrates a portion of a method of
manufacturing 440 of an EC gas
producer using a single point EMR source, according to an embodiment of the
disclosure. Fig. 4C
illustrates a single point EMR source (e.g., a xenon lamp) 402 that is placed
inside a tubular
structure 404. In a first embodiment, the tubular structure 404 may be held in
a
28
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
stationary manner while the single point EMR source may be moved in an up or
down 412
manner. In a preferred embodiment, single point EMR source 402 may irradiate
substantially
equally in all directions. In another embodiment, single point EMR source may
be held in a
stationary manner while tubular structure 404 may be moved in an up or down
direction 410 or
rotated 408 about its own axis. In another embodiment, the tubular structure
404 and the single
point EMR source 402 both move relative to one another such that the entire
inner surface of
the tubular structure 404 is thoroughly and substantially sintered.
[153] Fig. 4D illustrates a portion of a method of manufacturing 460 of an EC
gas producer
using a tubular EMR source, according to an embodiment of the disclosure. Fig.
3E40
illustrates a cylindrical lamp as the EMR source (e.g., a tubular xenon lamp)
462 that is
placed inside the tubular structure 404 to be sintered. The length of the lamp
in this case is
such that the entire inner surface of the tubular structure 404 may be
sintered without the
tubular lamp 462 and the tubular structure 404 needing to move relative to one
another. In
one embodiment, tubular lamp 462 may be held in a stationary manner while
tubular
structure 404 may be moved over the lamp 462. Tubular structure 404 may be
moved in an
up or down manner 464. For example, unsintered tubular structure 404 may be
moved over
tubular lamp 462 into a specified position, remain in this position until
sufficient radiation is
carried out and tubular structure 404 is substantially sintered, then moved in
an up or down
direction as denoted by arrow 464 off of the tubular lamp 462 for the next
manufacturing
step. In another embodiment, unsintered tubular structure 404 may be held in a
stationary
position while tubular lamp EMR source 462 is moved into the tubular structure
404. Tubular
lamp 462 may be moved in an up or down fashion as denoted by arrows 464. The
tubular
structure 404 may be formed using any suitable method, such as the methods
discussed
herein. For the embodiments of Fig. 4C-4D, coating and sintering take place on
the inner
surface of the tubular structure 404.
[154] Many variations are possible for sintering as illustrated in Figs. 4A-
4D. For example, an
outer tubular structure 204 may be formed and thermally sintered in a furnace
to form an anode or
a cathode. An electrolyte material may then be coated on the inner surface of
the outer tubular
structure 204 and then sintered in a furnace or using a single point EMR 402
or
29
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
tubular lamp EMR 462 inside of the tubular structure to form an electrolyte
206. Another
electrode material may then be coated on the inner surface of the electrolyte
206 and then
sintered in a furnace or using EMR source 402, 462 to form an inner tubular
structure 202 such
as an anode or cathode. For example, for a copper, gold, or silver-containing
anode, the inner
electrode is sintered using an EMR source. For example, for Ni or Ni0-
containing anode, the
inner electrode is sintered in a furnace or by an EMR source.
[155] In some embodiments, a combination of an EMR source inside of a tubular
electrode 202,
204 or electrolyte 206 and an EMR source on the outside of a tubular electrode
202, 204 or
electrolyte 206 may be used simultaneously to sinter. For example, a tubular
EMR source 462 and
a ring-like EMR source 422 may be used in the same sintering device to sinter
sequentially or
simultaneously.
[156] Figs. 5A-5I3 Illustrates another method to form the first tubular or
multi-tubular in EC
gas producers. Fig. SA illustrates a first step in a tape casting method 500
to form a tubular or
multi-tubular EC gas producer, according to an embodiment of the disclosure.
In a first step,
supports 504 are placed onto a substrate 502, wherein the height of the
supports 504 is
preconfigured to ensure a desirable thickness of the tubular electrode 506 on
the bottom side.
The substrate 502 and the supports 504 may be made of metal, glass, plastic,
wood, or any
suitable material as known in the art. An electrode material 506 in a
dispersion or slurry form is
deposited on the substrate 502 between the supports 504. The term slurry will
be used in the
description, but a dispersion may also be used interchangeably. One or more
spacers 508 are
then placed on top of the slurry 506 and rested on supports 504. View 501 is
an overhead view
or top view further illustrating and showing an example of how the substrate
502, supports
504, electrode material 506 and spacers 508 may be arranged.
[157] Fig. 513 illustrates steps 2-4 in a tape casting method 500 to form a
first tubular or a first
multi-tubular in an EC gas producer, according to an embodiment of the
disclosure. In step 2
additional slurry 510 is deposited to cover the spacer(s) 508 and the
previously deposited slurry
506. A blade, such as a doctor blade, may be used to scrape across the top of
the additional slurry
510 to ensure a suitable thickness of the tubular electrode on the top side.
In a preferred
embodiment, the slurry contains mainly organic solvent.
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[158] Step 3 illustrated in Fig. 5B includes immersing the substrate 502,
supports 504,
spacer(s) 508, the first slurry 506 and second slurry 510 are immersed in
deionized water to
allow phase inversion of the slurry to take place. Phase inversion is a form
of precipitation
when the slurry comprising a lower polarity organic solvent is placed into
higher polarity
deionized water. The components of the slurry precipitate out as a result
since the
components are not compatible with water.
[159] The substrate 502 and supports 504 are then removed from the slurry 506,
510 as a
whole after the phase inversion. The slurry 506, 510 is allowed to dry (e.g.,
in ambient air) to
remove excess deionized water. Then the spacers 508 are removed, e.g., being
pulled out from
either end. The electrode material 506, 510 is sintered to form the first
tubular electrode 512
with fluid passages 514. The spacers 508 may have any regular or irregular
shape as desired,
such as circular, oval-like, square-like, diamond-like, trapezoidal,
rectangular, triangular,
pentagonal, hexagonal, octagonal or other various cross-sectional shapes or
combinations
thereof. If the spacers 508 have a rectangular cross section, the multiple
joined tubular fluid
passages 514 will have a rectangular cross section as fluid passage 514 in the
inner electrode
512 as illustrated in Fig. 3C. As also can be seen in Figs. 3C-3D, the inner
electrode 302 has a
cross section having a length and a width, wherein the length is at least 2
times the width and
the cross section is orthogonal to the axial direction of the tubular.
Similarly, the reactor has a
cross section having a length and a width, wherein the length is at least 2
times the width and
the cross section is orthogonal to the axial direction of the tubular.
[160] In an embodiment, the method illustrated in step 4 in Fig. 5B further
comprises coating
the outer surface of the first tubular electrode 512 with an electrolyte
material. The electrolyte
material may then be sintered to form an electrolyte 516 in a furnace or by
using
electromagnetic radiation. Step 4 further comprises coating the electrolyte
516 with a second
electrode material. The second electrode material may be sintered in a furnace
or using
electromagnetic radiation to form a second outer tubular electrode 518. In an
embodiment,
the second electrode material comprises doped or undoped ceria and a material
selected from
the group consisting of Cu, CuO, Cu20, Ag, Ag20, Au, Au20, Au203, stainless
steel, and
combinations thereof; and it is sintered using EMR to form the second outer
tubular electrode
31
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
518. In an embodiment, the method comprises reducing the second outer tubular
electrode 518
or reducing the first inner tubular electrode 512 or both.
[161] In an embodiment, the coating step comprises dip coating, spraying,
ultrasonic
spraying, spin coating, brush coating, pasting, or combinations thereof. In an
embodiment,
electromagnetic radiation comprises UV light, near ultraviolet light, near
infrared light, infrared
light, visible light, laser, electron beam, microwave, or combinations
thereof. In an
embodiment, electromagnetic radiation is provided by a xenon lamp. In an
embodiment, the
first tubular electrode 512 has a cross section having a length and a width,
wherein the length
is at least 2 times the width and the cross section is orthogonal to the axial
direction of the
tubular fluid passage 514. In an embodiment, the EC gas producer comprises no
interconnect.
Operation of EC Gas Producer
[162] Disclosed herein is a method comprising providing a device comprising a
first electrode,
a second electrode, and an electrolyte between the electrodes, introducing a
first stream to the
first electrode, introducing a second stream to the second electrode,
extracting hydrogen from
the second electrode, wherein the first electrode and the second electrode
comprise a metallic
phase that does not contain a platinum group metal when the device is in use.
In an
embodiment, the electrolyte is oxide ion conducting. In an embodiment, the
device is operated
at a temperature no less than 500 C, or no less than 600 C, or no less than
700 C. In an
embodiment, the first stream comprises a fuel and water or a fuel and carbon
dioxide. In an
embodiment, said fuel comprises a hydrocarbon or hydrogen or carbon monoxide
or
combinations thereof. In an embodiment, the first stream is directly
introduced into the first
electrode or the second stream is directly introduced into second electrode or
both.
[163] In an embodiment, the first stream comprises a fuel with little to no
water. In an
embodiment, the fuel comprises a hydrocarbon or hydrogen or carbon monoxide or
combinations
thereof. In an embodiment, the second stream consists of water and hydrogen.
[164] In an embodiment, the method comprises providing a reformer upstream of
the first
electrode, wherein the first stream passes through the reformer before being
introduced to the first
electrode, wherein the first electrode comprises Ni or NiO. In an embodiment,
the reformer is a
steam reformer or an autothermal reformer.
32
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[165] In an embodiment, the method comprises using the extracted hydrogen in
one of
Fischer-Tropsch (FT) reactions, dry reforming reactions, Sabatier reaction
catalyzed by
nickel, Bosch reaction, reverse water gas shift reaction, electrochemical
reaction to
produce electricity, production of ammonia, production of fertilizer,
electrochemical
compressor for hydrogen storage, fueling hydrogen vehicles or hydrogenation
reactions or
combinations thereof.
[166] Herein disclosed is a method of producing hydrogen comprising providing
a EC gas
producer device, introducing a first stream comprising a fuel to the device,
introducing a
second stream comprising water to the device, reducing the water in the second
stream to
hydrogen, and extracting hydrogen from the device, wherein the first stream
and the second
stream do not come in contact with each other in the device. In an embodiment,
the first
stream does not come in contact with the hydrogen. In an embodiment, the first
stream and
the second stream are separated by a membrane in the device. In an embodiment,
the fuel
comprises a hydrocarbon or hydrogen or carbon monoxide or combinations
thereof. In an
embodiment, the second stream comprises hydrogen. In an embodiment, the first
stream
comprises the fuel and water or the fuel and carbon dioxide. In an embodiment,
the first
stream comprises the fuel with little to no water.
Hydrogen Production System
[167] Further discussed herein is a hydrogen production system comprising a
fuel source; a
water source; a hydrogen producer; wherein the fuel source and the water
source are in fluid
communication with the producer and wherein the fuel and the water do not come
in contact
with each other in the producer. The system may not include an external heat
source. In an
embodiment, the fuel and the water do not come in contact with each other in
the system. In an
embodiment, the producer comprises a first electrode, a second electrode, and
an electrolyte
between the first and second electrodes; wherein the fuel source is in fluid
communication with
the first electrode and the water source is in fluid communication with the
second electrode. In
an embodiment, the fuel source provides heat for the hydrogen producer and the
hydrogen
producer has no additional heat source.
33
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
(1.683 In an embodiment, the electrolyte comprises YSZ, CGO, LSGM, SSZ, SDC,
ceria,
lanthanum chromite, or combinations thereof or wherein the electrolyte
comprises doped or
undoped ceria and optionally a material selected from the group consisting of
YSZ, LSGM, SSZ,
and combinations thereof. In an embodiment, the lanthanum chromite comprises
undoped
lanthanum chromite, strontium doped lanthanum chromite, iron doped lanthanum
chromite,
lanthanum calcium chromite, or combinations thereof. The electrolyte may
further comprise any
material listed for electrolyte 206 in the "Tubular and Multi-Tubular EC Gas
Producers" section
herein. In an embodiment, the electrolyte comprises doped ceria or wherein the
electrolyte
comprises lanthanum chromite or a conductive metal or combination thereof and
a material
selected from the group consisting of doped ceria, YSZ, LSGM, SSZ, and
combinations thereof.
In an embodiment, the lanthanum chromite comprises undoped lanthanum chromite,
strontium
doped lanthanum chromite, iron doped lanthanum chromite, lanthanum calcium
chromite, or
combinations thereof. In an embodiment, the conductive metal comprises Ni, Cu,
Ag, Au, or
combinations thereof.
[1693 In an embodiment, the first electrode and the second electrode comprise
Ni or NiO and a
material selected from the group consisting of YSZ, CGO, samaria-doped ceria
(SDC), scandia-
stabilized zirconia (SSZ), LSGM, and combinations thereof. In an embodiment,
the first electrode
comprises doped or undoped ceria and a material selected from the group
consisting of Cu, CuO,
Cu2O, Ag, Ag2O, Au, Au20, Au203, stainless steel, and combinations thereof;
wherein the second
electrode comprises Ni or NiO and a material selected from the group
consisting of YSZ, CGO,
samaria-doped ceria (SDC), scandia-stabilized zirconia (SSZ), LSGM, and
combinations thereof.
The first electrode and second electrode may comprise any material listed for
the inner tubular
structure 202 or outer tubular structure 204 in the "Tubular and Multi-Tubular
EC Gas Producers"
section herein.
[170] In an embodiment, the system comprises an oxidant source and a boiler,
wherein the boiler is
in fluid communication with the oxidant source, the water source, and the
producer. In an
embodiment, the boiler is in thermal communication with the producer, fuel
input into the producer,
the oxidant, the water, or combinations thereof. In an embodiment, the boiler
is configured to
receive exhaust from the first electrode of the producer and to feed steam
into
34
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
the second electrode of the producer. In an embodiment, the fuel is partially
oxidized in the
producer and further oxidized in the boiler. In an embodiment, the system
comprises a steam
turbine between the boiler and the producer and in fluid communication with
the boiler and the
producer.
[171] In an embodiment, water is reduced in the producer to generate hydrogen.
In an
embodiment, the system comprises a condenser configured to receive exhaust
from the second
electrode of the producer and to recycle water to the boiler and to output
hydrogen. In an
embodiment, the condenser is in thermal communication with the fuel. In an
embodiment, the
system comprises a desulfurization unit between the fuel source and the
producer and in fluid
communication with the fuel source and the producer. In an embodiment, the
producer is
configured to have a fuel inlet temperature no greater than 1000 C or no
greater than 900 C or
from 800 C to 850 C. In an embodiment, the producer is configured to have a
fuel outlet
temperature no less than 600 C.
[172] Fig. 6A, illustrates an example of a hydrogen production system 600 with
no external
heat source, according to an embodiment of the disclosure. The system 600
comprises a water
source 602, an air/oxidant source 604, a fuel (e.g., methane) source 606, a
hydrogen producer
608, and a boiler 610. The system 600 generates hydrogen 612 and exhaust. The
hydrogen
producer 608 comprises an anode and a cathode separated by an electrolyte. The
anode and
cathode receive fuel and water respectively and the fuel and water do not come
in contact with
each other in the producer 608. In various cases, the fuel and water do not
come in contact
with each other in the entire system 600. The heating burden is fully met by
the system itself
with no need for any external heat source. For example, the boiler 610 heats
the fuel input
stream into the producer 608, the producer 608, the oxidant 604, and the water
602. The
producer 608 in operation has a fuel inlet temperature of no greater than 1000
C or no greater
than 900 C or from 800 C to 850 C and has a fuel outlet temperature no less
than 600 C.
[173] The fuel exits the fuel source 606 as stream 600-1, passes through a
desulfurization unit
614 and becomes stream 600-2. Stream 600-2 enters the condenser 616 and
functions as the
coolant for the condenser 616 and exits as stream 600-3, which is pre-heated
fuel. Stream 600-3
enters a heat exchanger (HX2) 618 and is further heated by the exhaust stream
600-6
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
from the boiler 610 to a proper temperature and enters the producer 608 as
stream 600-4.
Stream 600-4 is received by the anode in the producer 608 and is partially
oxidized and then
exits the producer 608 as stream 600-5. Stream 600-5 is introduced into the
boiler 610 and
further oxidized by an oxidant in the boiler 610, generating heat as a result.
The exhaust from
the boiler 610 exits as stream 600-6, passes through heat exchanger HX2 618 to
heat the fuel
input into the producer 608 and becomes stream 600-7. Stream 600-7 heats the
producer 608
to ensure proper operation temperatures for the producer 608 and becomes
stream 600-19.
Stream 600-19 passes through heat exchanger HX1 620 to heat the oxidant and
exits as
stream 600-20. Stream 600-20 passes through heat exchanger HX3 622 to heat the
water and
exits as stream 600-21.
[174] Water exits the water source as stream 600-8, passes through a pump and
becomes
stream 600-9. Stream 600-9 is heated in heat exchanger HX3 622 by stream 600-
20 and
becomes stream 600-10. Stream 600-10 enters the boiler 610 and becomes steam
(stream 600-
11) by the heat generated from the oxidation reactions in the boiler 610.
Stream 600-11 passes
through a turbine 624 and becomes stream 600-12. The turbine 624 is utilized
to power the
pump. Stream 600-12 enters the hydrogen producer 608 and is received by the
cathode of the
producer 608. Water/steam is reduced to hydrogen at the cathode. A mixture of
steam and
hydrogen exits the producer 608 as stream 600-13. Stream 600-13 enters the
condenser 616
and is cooled by the unheated fuel (stream 600-2). Water drops out of the
mixture and is
recycled from the condenser as stream 600-18. Stream 600-18 joins stream600- 9
and reenters
the boiler 610 after passing through heat exchangers HX3 622. Hydrogen exits
the condenser
as 616 stream 600-14.
[175] Air exits the oxidant source as stream 600-15 and passes through an air
cleaner 626,
where particulates and/or oxides are removed, and becomes stream 600-16.
Stream 600-16 is
heated in heat exchanger HX1 620 by stream 600-19 and becomes stream 600-17.
Stream 600-
17 enters the boiler 610 and reacts with stream 600-5 to further oxidize the
fuel and generate
heat. The reaction products exit the boiler 610 as stream 600-6.
[176] Fig. 68 illustrates an alternative hydrogen production system 650 with
no external heat
source, according to an embodiment of the disclosure. Steam generator (SG) 652
serves similar
36
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
functions as the boiler 610 in system 600 in Fig. 6A. Air enters the condenser
as stream 650-1
and is used as coolant in the condenser 616. Air stream 650-2 is then heated
in heat exchanger
HX1 620 before entering the hydrogen producer 608 as stream 650-3 and mixing
with the anode
output stream. The fuel enters as stream 650-4 and is heated in heat exchanger
HX2 618 by the
exhaust before entering the hydrogen producer 608 as stream 650-5. The fuel is
oxidized in the
anode of the hydrogen producer 608, becomes anode output stream, and is
further oxidized by
air to become exhaust 650-6. The exhaust provides thermal energy to the heat
exchangers (HX1
620 and HX2 618) and to SG 652 to produce steam from water. Steam enters the
hydrogen
producer 608 and is reduced to hydrogen at the cathode. The cathode output
stream 650-7 is
introduced to the condenser 616. Water from the condenser 616 is recycled as
stream 650-8
and hydrogen is extracted from the condenser 616.
Fuel Cell
[177] A fuel cell is an electrochemical apparatus that converts the chemical
energy from a
fuel into electricity through an electrochemical reaction. As mentioned above,
there are many
types of fuel cells, e.g., proton-exchange membrane fuel cells (PEMFCs), solid
oxide fuel cells
(SOFCs). A fuel cell typically comprises an anode, a cathode, an electrolyte,
an interconnect,
optionally a barrier layer and/or optionally a catalyst. Both the anode and
the cathode are
electrodes. The listings of material for the electrodes, the electrolyte, and
the interconnect in
a fuel cell are applicable in some cases to the EC gas producer and the EC
compressor. These
listings are only examples and not limiting. Furthermore, the designations of
anode material
and cathode material are also not limiting because the function of the
material during
operation (e.g., whether it is oxidizing or reducing) determines whether the
material is used
as an anode or a cathode.
[178] Figs. 7-8 illustrate various embodiments of the components in a fuel
cell or a fuel cell stack.
In these embodiments, the anode, cathode, electrolyte, and interconnect are
cuboids or rectangular
prisms.
[179] Fig. 7 illustrates a fuel cell component, according to an embodiment of
the disclosure.
Layer 701 schematically illustrates an anode, layer 702 represents a cathode,
layer 703
37
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
represents an electrolyte, layers 704 represents barrier layers, layer 705
represents a catalyst and
layer 706 represents an interconnect.
[1801 Fig. 8 schematically illustrates two fuel cells in a fuel cell stack,
according to an
embodiment of the disclosure. The two fuel cells are denoted "Fuel Cell 1" and
"Fuel Cell 2".
Each fuel cell in Fig. 8 comprises an anode layer 801, cathode layer 802,
electrolyte layer 803,
barrier layers 804, catalyst layer 805 and interconnect layer 806. Two fuel
cell repeat units or
two fuel cells form a stack as illustrated. As is seen, on one side
interconnect 806 is in contact
with the largest surface of cathode 802 of fuel cell 2 (or fuel cell repeat
unit) and on the
opposite side interconnect 806 is in contact with the largest surface of
catalyst 805 (optional) or
the anode 801 of bottom fuel cell 2 (or fuel cell repeat unit). These repeat
units or fuel cells are
connected in parallel by being stacked atop one another and sharing an
interconnect in between
via direct contact with the interconnect rather than via electrical wiring.
This kind of
configuration illustrated in Fig. 8 contrasts with segmented-in-series (SIS)
type fuel cells.
Cathode
[181] In some embodiments, the cathode comprises perovskites, such as LSC,
LSCF or LSM.
In some embodiments, the cathode comprises one or more of lanthanum, cobalt,
strontium or
manganite. In an embodiment, the cathode is porous. In some embodiments, the
cathode
comprises one or more of YSZ, nitrogen, nitrogen boron doped graphene,
La0.6Sr0.4Co0.2Fe0.803, SrCo0.55c0.503, BaFe0.75Ta0.2503, BaFe0.875Re0.12503,
Ba0.5La0.125Zn0.375Ni03, Ba0.75Sr0.25Fe0.875Ga0.12503, BaFe0.125Co0.125,
Zr0.7503. In
some embodiments, the cathode comprises LSCo, LCo, LSF, LSCoF, or a
combination thereof.
In some embodiments, the cathode comprises perovskites LaCo03, LaFe03, LaMn03,
(La,Sr)Mn03, LSM-GDC, LSCF-GDC, LSC-GDC. Cathodes containing LSCF are suitable
for
intermediate-temperature fuel cell operation.
[1821 In some embodiments, the cathode comprises a material selected from the
group
consisting of lanthanum strontium manganite, lanthanum strontium ferrite, and
lanthanum
strontium cobalt ferrite. In preferred embodiments, the cathode comprises
lanthanum strontium
manganite.
38
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
Anode
[183] In some embodiments, the anode comprises copper, nickel-oxide, nickel-
oxide-YSZ,
NiO-GDC, NiO-SDC, aluminum doped zinc oxide, molybdenum oxide, lanthanum,
strontium,
chromite, ceria, perovskites (such as, LSCF [La{1-x}Sr{x}Co{1-y}Fe{y}03] or
LSM [La{1-
x}Sr{x}Mn03], where x is usually in the range of 0.15-0.2 and y is in the
range of 0.7 to 0.8).
In some embodiments, the anode comprises SDC or BZCYYb coating or barrier
layer to reduce
coking and sulfur poisoning. In an embodiment, the anode is porous. In some
embodiments,
the anode comprises a combination of electrolyte material and
electrochemically active
material or a combination of electrolyte material and electrically conductive
material.
[184] In a preferred embodiment, the anode comprises nickel and yttria
stabilized zirconia. In a
preferred embodiment, the anode is formed by reduction of a material
comprising nickel oxide and
yttria stabilized zirconia. In a preferred embodiment, the anode comprises
nickel and gadolinium
stabilized ceria. In a preferred embodiment, the anode is formed by reduction
of a material
comprising nickel oxide and gadolinium stabilized ceria.
Electrolyte
[185] In an embodiment, the electrolyte in a fuel cell comprises stabilized
zirconia (e.g., YSZ,
YSZ-8, Yo.i6Zro.8402). In an embodiment, the electrolyte comprises doped
LaGa03, (e.g., LSGM,
La0.9SraiGaosMg0.203). In an embodiment, the electrolyte comprises doped
ceria, (e.g., GDC,
Gdo.2Ceo.802). In an embodiment, the electrolyte comprises stabilized bismuth
oxide (e.g., BVCO,
Bi2Vo.9Cuo.10s.35)=
[186] In some embodiments, the electrolyte comprises zirconium oxide, yttria
stabilized
zirconium oxide (also known as YSZ, YSZ8 (8m01e% YSZ)), ceria, gadolinia,
scandia, magnesia or
calcia or a combination thereof. In an embodiment, the electrolyte is
sufficiently impermeable to
prevent significant gas transport and prevent significant electrical
conduction; and allow ion
conductivity. In some embodiments, the electrolyte comprises doped oxide such
as cerium oxide,
yttrium oxide, bismuth oxide, lead oxide, lanthanum oxide. In some
embodiments, the electrolyte
comprises perovskite, such as, LaCoFe03 or LaCo03 or Ce0.9Gdo.302(GDC) or
Ceo.gSmo.102
(SDC, samaria doped ceria) or scandia stabilized zirconia or a combination
thereof.
39
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[187] In some embodiments, the electrolyte comprises a material selected from
the group
consisting of zirconia, ceria, and gallia. In some embodiments, the material
is stabilized
with a stabilizing material selected from the group consisting of scandium,
samarium,
gadolinium, and yttrium. In an embodiment, the material comprises yttria
stabilized
zirconia.
Interconnect
[188] In some embodiments, the interconnect comprises silver, gold, platinum,
A1S1441, ferritic
stainless steel, stainless steel, lanthanum, chromium, chromium oxide,
chromite, cobalt, cesium,
Cr203, or a combination thereof. In some embodiments, the anode comprises a
LaCr03 coating
on Cr203 or NiCo204 or MnCo204 coatings. In some embodiments, the interconnect
surface is
coated with Cobalt and/or Cesium. In some embodiments, the interconnect
comprises ceramics.
In some embodiment, the interconnect comprises lanthanum chromite or doped
lanthanum
chromite. In an embodiment, the interconnect comprises a material further
comprising metal,
stainless steel, ferritic steel, crofer, lanthanum chromite, silver, metal
alloys, nickel, nickel oxide,
ceramics, or lanthanum calcium chromite, or a combination thereof.
Catalyst
[189] In various embodiments, the fuel cell comprises a catalyst, such as,
platinum, palladium,
scandium, chromium, cobalt, cesium, Ce02, nickel, nickel oxide, zinc, copper,
titania, ruthenium,
rhodium, MoS2, molybdenum, rhenium, vanadium, manganese, magnesium or iron or
a
combination thereof. In various embodiments, the catalyst promotes methane
reforming
reactions to generate hydrogen and carbon monoxide such that they may be
oxidized in the fuel
cell. Very often, the catalyst is part of the anode, especially nickel anode
which has inherent
methane reforming properties. In an embodiment, the catalyst is between 1%-5%,
or 0.1% to
10% by mass. In an embodiment, the catalyst is used on the anode surface or in
the anode. In
various embodiments, such anode catalysts reduce harmful coking reactions and
carbon
deposits. In various embodiments, simple oxide versions of catalysts or
perovskite may be used
as catalysts. For example, about 2% mass Ce02 catalyst is used for methane-
powered fuel cells.
In various embodiments, the catalyst may be dipped or coated on the anode. In
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
various embodiments, the catalyst is made by an additive manufacturing machine
(AMM) and
incorporated into the fuel cell using the AMM.
[190] The unique manufacturing methods discussed herein have described the
assembly of
ultra-thin fuel cells and fuel cell stacks. Conventionally, to achieve
structural integrity, the fuel
cell has at least one thick layer per repeat unit. This may be the anode (such
as an anode-
supported fuel cell) or the interconnect (such as an interconnect-supported
fuel cell). As
discussed above, pressing or compression steps are typically necessary to
assemble the fuel
cell components to achieve gas tightness and/or proper electrical contact in
traditional
manufacturing processes. As such, the thick layers are necessary not only
because traditional
methods (like tape casting) cannot produce ultra-thin layers but also because
the layers must
be thick to endure the pressing or compression steps. The preferred
manufacturing methods
of this disclosure have eliminated the need for pressing or compression. The
preferred
manufacturing methods of this disclosure have also enabled the making of ultra-
thin layers.
The multiplicity of the layers in a fuel cell or a fuel cell stack provides
sufficient structural
integrity for proper operation when they are made according to this
disclosure.
[191] Herein disclosed is a fuel cell comprising an anode no greater than 1 mm
or 500
microns or 300 microns or 100 microns or 50 microns or no greater than 25
microns in
thickness. The cathode no greater than 1 mm or 500 microns or 300 microns or
100 microns or
50 microns or no greater than 25 microns in thickness. The electrolyte no
greater than 1 mm or
500 microns or 300 microns or 100 microns or 50 microns or 30 microns in
thickness. In an
embodiment, the fuel cell comprises an interconnect having a thickness of no
less than 50
microns. In some cases, a fuel cell comprises an anode no greater than 25
microns in
thickness, a cathode no greater than 25 microns in thickness, and an
electrolyte no greater
than 10 microns or 5 microns in thickness. In an embodiment, the fuel cell
comprises an
interconnect having a thickness of no less than 50 microns. In an embodiment,
the
interconnect has a thickness in the range of 50 microns to 5 cm.
[192] In a preferred embodiment, a fuel cell comprises an anode no greater
than 100 microns in
thickness, a cathode no greater than 100 microns in thickness, an electrolyte
no greater than 20
microns in thickness, and an interconnect no greater than 30 microns in
thickness. In a more
41
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
preferred embodiment, a fuel cell comprises an anode no greater than 50
microns in thickness, a
cathode no greater than 50 microns in thickness, an electrolyte no greater
than 10 microns in
thickness, and an interconnect no greater than 25 microns in thickness. In an
embodiment, the
interconnect has a thickness in the range of 1 micron to 20 microns.
[193] In a preferred embodiment, the fuel cell comprises a barrier layer
between the anode and
the electrolyte, or a barrier layer between the cathode and the electrolyte,
or both barrier layers. In
some cases, the barrier layers are the interconnects. In such cases, the
reactants are directly
injected into the anode and the cathode.
[194] In an embodiment, the cathode has a thickness of no greater than 15
microns, or no
greater than 10 microns, or no greater than 5 microns. In an embodiment, the
anode has a
thickness no greater than 15 microns, or no greater than 10 microns, or no
greater than 5
microns. In an embodiment, the electrolyte has a thickness of no greater than
5 microns, or no
greater than 2 microns, or no greater than 1 micron, or no greater than 0.5
micron. In an
embodiment, the interconnect is made of a material comprising metal, stainless
steel, silver,
metal alloys, nickel, nickel oxide, ceramics, lanthanum chromite, doped
lanthanum chromite, or
lanthanum calcium chromite. In an embodiment, the fuel cell has a total
thickness of no less
than 1 micron.
[195] Also discussed herein is a fuel cell stack comprising a multiplicity of
fuel cells, wherein
each fuel cell comprises an anode no greater than 25 microns in thickness, a
cathode no greater
than 25 microns in thickness, an electrolyte no greater than 10 microns in
thickness, and an
interconnect having a thickness in the range from 100 nm to 100 microns. In an
embodiment,
each fuel cell comprises a barrier layer between the anode and the
electrolyte, or a barrier layer
between the cathode and the electrolyte, or both barrier layers. In an
embodiment, the barrier
layers are the interconnects. For example, the interconnect is made of silver.
For example, the
interconnect has a thickness in the range from 500 nm to 1000 nm. In an
embodiment, the
interconnect is made of a material comprising metal, stainless steel, silver,
metal alloys, nickel,
nickel oxide, ceramics, or lanthanum calcium chromite.
[196] In an embodiment, the cathode has a thickness of no greater than 15
microns, or no
greater than 10 microns, or no greater than 5 microns. In an embodiment, the
anode has a
42
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
thickness of no greater than 15 microns, or no greater than 10 microns, or no
greater than 5
microns. In an embodiment, the electrolyte has a thickness of no greater than
5 microns, or no
greater than 2 microns, or no greater than 1 micron, or no greater than 0.5
micron. In an
embodiment, each fuel cell has a total thickness of no less than 1 micron.
[197] Further discussed herein is a method of making a fuel cell comprising
(a) forming an
anode no greater than 25 microns in thickness, (b) forming a cathode no
greater than 25
microns in thickness, and (c) forming an electrolyte no greater than 10
microns in thickness.
In an embodiment, steps (a)-(c) are performed using additive manufacturing. In
various
embodiments, said additive manufacturing employs one or more of extrusion,
photopolymerization, powder bed fusion, material jetting, binder jetting,
directed energy
deposition or lamination.
[198] In an embodiment, the method comprises assembling the anode, the
cathode, and the
electrolyte using additive manufacturing. In an embodiment, the method
comprises forming an
interconnect and assembling the interconnect with the anode, the cathode, and
the electrolyte.
[199] In preferred embodiments, the method comprises making at least one
barrier layer. In
preferred embodiments, the at least one barrier layer is used between the
electrolyte and the
cathode or between the electrolyte and the anode, or both. In an embodiment,
the at least one
barrier layer also acts as an interconnect.
[200] In preferred embodiments, the method comprises heating the fuel cell
such that
shrinkage rates of the anode, the cathode, and the electrolyte are matched. In
some
embodiments, such heating takes place for no greater than 30 minutes,
preferably no greater
than 30 seconds, and most preferably no greater than 30 milliseconds. When a
fuel cell
comprises a first composition and a second composition, wherein the first
composition has a first
shrinkage rate and the second composition has a second shrinkage rate, the
heating described in
this disclosure preferably takes place such that the difference between the
first shrinkage rate
and the second shrinkage rate is no greater than 75% of the first shrinkage
rate.
[201] In a preferred embodiment, the heating employs electromagnetic radiation
(EMR). In
various embodiments, EMR comprises UV light, near ultraviolet light, near
infrared light, infrared
light, visible light, laser, electron beam. Preferably, heating is performed
in situ.
43
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[202] Also disclosed herein is a method of making a fuel cell stack comprising
a multiplicity of
fuel cells, the method comprising: (a) forming an anode no greater than 25
microns in thickness in
each fuel cell, (b) forming a cathode no greater than 25 microns in thickness
in each fuel cell, (c)
forming an electrolyte no greater than 10 microns in thickness in each fuel
cell, and (d) producing
an interconnect having a thickness of from 100 nm to 100 microns in each fuel
cell.
[203] In an embodiment, steps (a)-(d) are performed using AM. In various
embodiments, AM
employs one or more of processes of extrusion, photopolymerization, powder bed
fusion, material
jetting, binder jetting, directed energy deposition or lamination.
[204] In an embodiment, the method of making a fuel cell stack comprises
assembling the
anode, the cathode, the electrolyte, and the interconnect using AM. In an
embodiment, the
method comprises making at least one barrier layer in each fuel cell. In an
embodiment, the at
least one barrier layer is used between the electrolyte and the cathode or
between the
electrolyte and the anode or both. In an embodiment, the at least one barrier
layer also acts
as the interconnect.
[205] In an embodiment, the method of making a fuel cell stack comprises
heating each fuel
cell such that shrinkage rates of the anode, the cathode, and the electrolyte
are matched. In an
embodiment, such heating takes place for no greater than 30 minutes, or no
greater than 30
seconds, or no greater than 30 milliseconds. In a preferred embodiment, said
heating
comprises one or more of electromagnetic radiation (EMR). In various
embodiments, [MR
comprises UV light, near ultraviolet light, near infrared light, infrared
light, visible light, laser,
electron beam. In an embodiment, heating is performed in situ.
[206] In an embodiment, the method comprises heating the entire fuel cell
stack such that
shrinkage rates of the anode, the cathode, and the electrolyte are matched. In
some
embodiments, such heating takes place for no greater than 30 minutes, or no
greater than 30
seconds, or no greater than 30 milliseconds.
[207] Herein discussed is a method of making an electrolyte comprising (a)
formulating a
colloidal suspension, wherein the colloidal suspension comprises an additive,
particles having a
range of diameters and a size distribution, and a solvent; (b) forming an
electrolyte comprising the
colloidal suspension; and (c) heating at least a portion of the electrolyte;
wherein
44
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
formulating the colloidal suspension is preferably optimized by controlling
the pH of the colloidal
suspension, or concentration of the binder in the colloidal suspension, or
composition of the
binder in the colloidal suspension, or the range of diameters of the
particles, or maximum
diameter of the particles, or median diameter of the particles, or the size
distribution of the
particles, or boiling point of the solvent, or surface tension of the solvent,
or composition of the
solvent, or thickness of the minimum dimension of the electrolyte, or the
composition of the
particles, or combinations thereof.
[208] Herein discussed is a method of making a fuel cell comprising (a)
obtaining a cathode
and an anode; (b) modifying the cathode surface and the anode surface; (c)
formulating a
colloidal suspension, wherein the colloidal suspension comprises an additive,
particles having a
range of diameters and a size distribution, and a solvent; (d) forming an
electrolyte comprising
the colloidal suspension between the modified anode surface and the modified
cathode surface;
and (e) heating at least a portion of the electrolyte; wherein formulating the
colloidal
suspension comprises controlling pH of the colloidal suspension, or
concentration of the binder
in the colloidal suspension, or composition of the binder in the colloidal
suspension, or the
range of diameters of the particles, or maximum diameter of the particles, or
median diameter
of the particles, or the size distribution of the particles, or boiling point
of the solvent, or
surface tension of the solvent, or composition of the solvent, or thickness of
the minimum
dimension of the electrolyte, or the composition of the particles, or
combinations thereof. In
various embodiments, the anode and the cathode are obtained via any suitable
means. In an
embodiment, the modified anode surface and the modified cathode surface have a
maximum
height profile roughness that is less than the average diameter of the
particles in the colloidal
suspension. The maximum height profile roughness 900 refers to the maximum
distance
between any trough 902 and an adjacent peak 904 of an anode surface or a
cathode surface as
illustrated in Fig. 9. In various embodiments, the anode surface and the
cathode surface are
modified via any suitable means.
[209] Further disclosed herein is a method of making a fuel cell comprising
(a) obtaining a
cathode and an anode; (b) formulating a colloidal suspension, wherein the
colloidal suspension
comprises an additive, particles having a range of diameters and a size
distribution, and a
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
solvent; (c) forming an electrolyte comprising the colloidal suspension
between the anode and
the cathode; and (d) heating at least a portion of the electrolyte; wherein
formulating the
colloidal suspension comprises controlling pH of the colloidal suspension, or
concentration of
the binder in the colloidal suspension, or composition of the binder in the
colloidal suspension,
or the range of diameters of the particles, or maximum diameter of the
particles, or median
diameter of the particles, or the size distribution of the particles, or
boiling point of the
solvent, or surface tension of the solvent, or composition of the solvent, or
thickness of the
minimum dimension of the electrolyte, or the composition of the particles, or
combinations
thereof. In various embodiments, the anode and the cathode are obtained via
any suitable
means. In an embodiment, the anode surface in contact with the electrolyte and
the cathode
surface in contact with the electrolyte have a maximum height profile
roughness that is less
than the average diameter of the particles in the colloidal suspension.
[210] In a preferred embodiment, the solvent comprises water. In a preferred
embodiment,
the solvent comprises an organic component. The solvent may comprise ethanol,
butanol,
alcohol, terpineol, diethyl ether 1,2-dimethoxyethane (DME (ethylene glycol
dimethyl ether),
1-propanol (n-propanol, n-propyl alcohol), or butyl alcohol or a combination
thereof. In some
embodiments, the solvent surface tension is less than half of water's surface
tension in air. In
an embodiment, the solvent surface tension is less than 30 mN/m at atmospheric
conditions.
[211] In some embodiments, the electrolyte is formed adjacent to a first
substrate or the
electrolyte is formed between a first substrate and a second substrate. In
some embodiments, the
first substrate has a maximum height profile roughness that is less than the
average diameter of
the particles. In some embodiments, the particles have a packing density
greater than 40%, or
greater than 50%, or greater than 60%. In an embodiment, the particles have a
packing density
close to the random close packing (RCP) density.
[212] Random close packing (RCP) is an empirical parameter used to
characterize the
maximum volume fraction of solid objects obtained when they are packed
randomly. A
container is randomly filled with objects, and then the container is shaken or
tapped until the
objects do not compact any further, at this point the packing state is RCP.
The packing fraction
is the volume taken by a number of particles in a given space of volume. The
packing fraction
46
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
determines the packing density. For example, when a solid container is filled
with grain,
shaking the container will reduce the volume taken up by the objects, thus
allowing more grain
to be added to the container. Shaking increases the density of packed objects.
When shaking
no longer increases the packing density, a limit is reached and if this limit
is reached without
obvious packing into a regular crystal lattice, this is the empirical random
close-packed density.
[213] In some embodiments, the median particle diameter is between 50 nm and
1000 nm, or
between 100 nm and 500 nm, or approximately 200 nm. In some embodiments, the
first
substrate comprises particles having a median particle diameter, wherein the
median particle
diameter of the electrolyte may be no greater than 10 times and no less than
1/10 of the
median particle diameter of the first substrate. In some embodiments, the
first substrate
comprises a particle size distribution that is bimodal having a first mode and
a second mode,
each having a median particle diameter. In some embodiments, the median
particle diameter in
the first mode of the first substrate is greater than 2 times, or greater than
5 times, or greater
than 10 times that of the second mode. The particle size distribution of the
first substrate may
be adjusted to change the behavior of the first substrate during heating. In
some embodiments,
the first substrate has a shrinkage that is a function of heating temperature.
In some
embodiments, the particles in the colloidal suspension may have a maximum
particle diameter
and a minimum particle diameter, wherein the maximum particle diameter is less
than 2 times,
or less than 3 times, or less than 5 times, or less than 10 times the minimum
particle diameter.
In some embodiments, the minimum dimension of the electrolyte is less than 10
microns, or
less than 2 microns, or less than 1 micron, or less than 500 nm.
[214] In some embodiments, the electrolyte has a gas permeability of no
greater than 1
millidarcy, preferably no greater than 100 microdarcy, and most preferably no
greater than 1
microdarcy. Preferably, the electrolyte has no cracks penetrating through the
minimum dimension
of the electrolyte. In some embodiments, the boiling point of the solvent is
no less than 200 C,
or no less than 100 C, or no less than 75 C. In some embodiments, the boiling
point of the
solvent is no greater than 125 C, or no greater than 100 C, or no greater
than 85 C, no
greater than 70 C. In some embodiments, the pH of the colloidal suspension is
no less than 7,
or no less than 9, or no less than 10.
4/
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
(2153 In some embodiments, the additive comprises polyethylene glycol (PEG),
ethyl cellulose,
polyvinylpyrrolidone (PVP), polyvinyl butyral (PVB), butyl benzyl phthalate
(BBP), polyalkylene glycol
(PAG) or a combination thereof. In an embodiment, the additive concentration
is no greater than
100 mg/cm3, or no greater than 50 mg/cm3, or no greater than 30 mg/cm3, or no
greater than 25
mg/cm3.
[216] In an embodiment, the colloidal suspension is milled. In an embodiment,
the colloidal
suspension is milled using a rotational mill wherein the rotational mill is
operated at no less than
20 rpm, or no less than 50 rpm, or no less than 100 rpm, or no less than 150
rpm. In an
embodiment, the colloidal suspension is milled using zirconia milling balls or
tungsten carbide
balls wherein the colloidal suspension is milled for no less than 2 hours, or
no less than 4 hours,
or no less than 1 day, or no less than 10 days.
(2171 In some embodiments, the particle concentration in the colloidal
suspension is no greater
than 30 wt%, or no greater than 20 wt%, or no greater than 10 wt%. In other
embodiments,
the particle concentration in the colloidal suspension is no less than 2 wt%.
In some
embodiments, the particle concentration in the colloidal suspension is no
greater than 10 vol%,
or no greater than 5 vol%, or no greater than 3 vol%, or no greater than 1
vol%. In an
embodiment, the particle concentration in the colloidal suspension is no less
than 0.1 vol%.
[218] In a preferred embodiment, the electrolyte is formed using an additive
manufacturing
machine (AMM). In a preferred embodiment, the first substrate is formed using
an AMM. In a
preferred embodiment, the heating comprises the use of electromagnetic
radiation (EMR)
wherein the EMR comprises one or more of UV light, near ultraviolet light,
near infrared light,
infrared light, visible light or laser. In a preferred embodiment, the first
substrate and the
electrolyte are heated to cause co-sintering. In a preferred embodiment, the
first substrate, the
second substrate, and the electrolyte are heated to cause co-sintering. In an
embodiment, the
EMR is controlled to preferentially sinter the first substrate over the
electrolyte.
[2193 In an embodiment, the electrolyte is compresses after heating. In an
embodiment, the
first substrate and the second substrate apply compressive stress to the
electrolyte after
heating. In an embodiment, the first substrate and the second substrate that
are applying
compressive stress are the anode and cathode of a fuel cell. In some
embodiments, the
48
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
minimum dimension of the electrolyte is between 500 nm and 5 microns or
between 1 micron and
2 microns.
[220] The detailed discussion described herein uses the production of solid
oxide fuel cells
(SOFCs) as an illustrative example. As one in the art recognizes, the
methodology and the
manufacturing process described herein are applicable to all fuel cell types.
As such, the
production of all fuel cell types is within the scope of this disdosure.
Reactor Cartridge
[221] In various embodiments, an electrochemical (EC) reactor is formed into a
cartridge
form. The discussion herein uses fuel cell or fuel cell stack as an example.
The cartridge design
is applicable to other electrochemical reactors, such as EC gas producer, EC
compressor, flow
battery. In various embodiments, the fuel cell stack is configured to be made
into a cartridge
form, such as an easily detachable flanged fuel cell cartridge (FCC) design.
Fig. 9A illustrates a
perspective view of a fuel cell cartridge (FCC) 900, according to an
embodiment of the
disclosure. FCC 900 comprises a rectangular shape as illustrated in Fig. 9A.
Other form factors
are possible such as square-like, cylindrical-like, hexagonal-like or
combinations thereof. The
form factor may depend on the application where the FCC may be used such as in
industrial,
home, automotive or other applications. FCC 900 also comprises holes for bolts
902 to secure
the FCC in a system or in series with other FCCs, or both. FCC cartridge 900
housing may be
comprised of aluminum, steel, plastic, ceramics, or a combination thereof. FCC
900 comprises a
top interconnect 904.
[222] Fig. 98 illustrates a perspective view of a cross-section of a fuel cell
cartridge (FCC) 900,
according to an embodiment of the disclosure. FCC 900 comprises holes for
bolts 902, cathode
layer 906, barrier layer 908, anode layer 910, gas channels 912 in the
electrodes (anode and
cathode), electrolyte layer 914, an air heat exchanger 916, fuel heat
exchanger 918 and top
interconnect 904. Air heat exchanger 916 and fuel heat exchanger 918 combined
form an
integrated multi-fluid heat exchanger. In some embodiments, there is no
barrier layer between
the cathode 906 and the electrolyte 914. FCC 900 comprises a second
interconnect 920, such as
between anode layer 910 and fuel heat exchanger 918. FCC 900 further comprises
openings 922,
924 for fuel passages.
49
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[2231 Fig. 9C illustrates cross-sectional views of a fuel cell cartridge
(FCC), according to an
embodiment of the disclosure. FCC 900 in Fig. 9C comprises electrical bolt
isolation 926, anode
910, seal 928 that seals anode 910 from air flow, cathode 906 and seal 930
that seals cathode
906 from fuel flow. The bolts may be isolated electrically with a seal as
well. In various
embodiments, the seals may be dual functional seal (DFS) comprising YSZ
(yttria-stabilized
zirconia) or a mixture of 3YSZ (3 mol% Y,03 in ZrO2) and 8YSZ (8 mol% Y203 in
ZrO2). In some
embodiments, the DFS is impermeable to non-ionic substances and electrically
insulating. In
some embodiments, the mass ratio of 3YSZ/8YSZ is in the range of from 10/90 to
90/10. In
some embodiments, the mass ratio of 3YSZ/8YSZ is about 50/50. In some
embodiments, the
mass ratio of 3YSZ/8YSZ is 100/0 or 0/100.
[224] Fig. 90 illustrates top view and bottom view of a fuel cell cartridge
(FCC), according to an
embodiment of the disclosure. FCC 900 comprises holes for bolts 902, air inlet
932, air outlet 934,
fuel inlet 922, fuel outlet 924, bottom 936 and top interconnect 904 of FCC
900., Fig. 9D further
illustrates the top view and bottom view of an embodiment of FCC 900, in which
the length of the
oxidant side of FCC 900 is shown 10, the length of the fuel side of FCC 900 is
shown Lf, the width
of the oxidant (air inlet 932) entrance is shown Wo, and the width of the fuel
inlet 922 is shown
Wf. In Fig. 90, two fluid exits are shown (air outlet 934 and fuel outlet
924). In some
embodiments, the anode exhaust and the cathode exhaust may be mixed and
extracted through
one fluid exit. In some cases, bottom 936 is an interconnect and 932, 934,
922, 924 are openings
for fluid passage, e.g., in the direction perpendicular to the lateral
direction.
[2251 Disclosed herein is a fuel cell cartridge (FCC) 900 comprising an anode
910, a cathode 906,
an electrolyte 914, at least one interconnect, a fuel entrance on a fuel side
of the FCC 900, an
oxidant entrance on an oxidant side of the FCC, at least one fluid exit,
wherein the fuel entrance
has a width of Wf, the fuel side of the FCC has a length of Lf, the oxidant
entrance has a width of
Wo, the oxidant side of the FCC has a length of Lo, wherein Wf/Lf is in the
range of 0.1 to 1.0, or
0.1 to 0.9, or 0.2 to 0.9, or 0.5 to 0.9, or 0.5 to 1.0 and W0/1.0 is in the
range of 0.1 to 1.0, or
0.1 to 0.9, or 0.2 to 0.9, or 0.5 to 0.9, or 0.5 to 1Ø
[2261 In some embodiments, the air and fuel entrances and exits are on one
surface of the FCC
900 wherein the FCC 900 comprises no protruding fluid passages on said
surface. In some
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
embodiments, the surface is smooth with a maximum elevation change of no
greater than 1
mm, or no greater than 100 microns, or no greater than 10 microns.
[227] In some embodiments, an FCC 900 comprises a barrier layer between the
electrolyte and
the cathode, or between the electrolyte and the anode, or both. In an
embodiment, the FCC
comprises a dual functional seal (DFS) that is impermeable to non-ionic
substances and electrically
insulating. In some embodiments, the DFS comprises YSZ (yttria-stabilized
zirconia) or a mixture
of 3YSZ (3 mol% Y203 in ZrO2) and 8YSZ (8 mol% Y203 in Zr02).
[228] In some embodiments, the interconnect comprises no fluid dispersing
element and the
anode and cathode comprise fluid dispersing components. In some embodiments,
the
interconnect comprises no fluid dispersing element while the anode and cathode
comprise fluid
channels.
[229] In some embodiments, a fuel cell cartridge (FCC) 900 comprising an
anode, a cathode,
an electrolyte, an interconnect, a fuel entrance, an oxidant entrance, at
least one fluid exit,
wherein the entrances and exit are on one surface of the FCC and the FCC
comprises no
protruding fluid passage on the surface. In some embodiments, the surface may
be smooth
with a maximum elevation change of no greater than 1 mm, or no greater than
100 microns,
or no greater than 10 microns.
[230] In some embodiments, the FCC 900 comprises a DFS that is impermeable to
non-ionic
substances and electrically insulating. In an embodiment, the interconnect
comprises no fluid
dispersing element and the anode and cathode comprise fluid dispersing
components. In an
embodiment, the interconnect comprises no fluid dispersing element and said
anode and cathode
comprise fluid channels.
[231] In an embodiment, the FCC 900 is detachably fixed to a mating surface
and not soldered
nor welded to said mating surface. In an embodiment, the FCC is bolted to or
pressed to the
mating surface. The mating surface comprises a matching fuel entrance,
matching oxidant
entrance, and at least one matching fluid exit.
[232] Further disclosed herein is an assembly comprising a fuel cell cartridge
(FCC) and a
mating surface, wherein the FCC comprises an anode, a cathode, an electrolyte,
an interconnect,
a fuel entrance on a fuel side of the FCC, an oxidant entrance on an oxidant
side
51
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
of the FCC, at least one fluid exit, wherein the fuel entrance has a width of
Wf, the fuel side of
the FCC has a length of Lf, the oxidant entrance has a width of W., the
oxidant side of the FCC
has a length of L., wherein Wf/Lf is in the range of 0.1 to 1.0, or 0.1 to
0.9, or 0.2 to 0.9, or 0.5
to 0.9, or 0.5 to 1.0 and W0/L. is in the range of 0.1 to 1.0, or 0.1 to 0.9,
or 0.2 to 0.9, or 0.5 to
0.9, or 0.5 to 1.0, wherein the FCC is detachably fixed to the mating surface.
[233] In some embodiments, said entrances and exits are on one surface of the
FCC and
wherein the FCC comprises no protruding fluid passage on said surface. The
surface may
be smooth with a maximum elevation change of no greater than 1 mm, or no
greater than
100 microns, or no greater than 10 microns.
[234] In an embodiment, said interconnect comprises no fluid dispersing
element and said
anode and cathode comprise fluid dispersing components. In an embodiment, said
interconnect comprises no fluid dispersing element and said anode and cathode
comprise
fluid channels.
[235] Discussed herein is a method comprising pressing or bolting together a
fuel cell
cartridge (FCC) and a mating surface. The method excludes welding or soldering
together the
FCC and the mating surface, wherein the FCC comprises an anode, a cathode, an
electrolyte, an
interconnect, a fuel entrance on a fuel side of the FCC, an oxidant entrance
on an oxidant side
of the FCC, at least one fluid exit, wherein the fuel entrance has a width of
Wf, the fuel side of
the FCC has a length of Lf, the oxidant entrance has a width of Wo, the
oxidant side of the FCC
has a length of L., wherein Wf/Lf is in the range of 0.1 to 1.0, or 0.1 to
0.9, or 0.2 to 0.9, or 0.5
to 0.9, or 0.5 to 1.0 and W0/1.0 is in the range of 0.1 to 1.0, or 0.1 to 0.9,
or 0.2 to 0.9, or 0.5
to 0.9, or 0.5 to 1.0, wherein the FCC and the mating surface are detachable.
[236] In an embodiment, said entrances and exit are on one surface of the FCC
wherein
the FCC comprises no protruding fluid passage on said surface. The surface is
smooth with a
maximum elevation change of no greater than 1 mm, or no greater than 100
microns, or no
greater than 10 microns. In an embodiment, said interconnect comprises no
fluid dispersing
element and said anode and cathode comprise fluid dispersing components. In an
embodiment, said interconnect comprises no fluid dispersing element and said
anode and
cathode comprise fluid channels.
52
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[237] Herein disclosed is a fuel cell cartridge (FCC) comprising a fuel cell
and a fuel cell
casing, wherein the fuel cell comprises an anode, a cathode and an
electrolyte, wherein at least
a portion of the fuel cell casing is made of the same material as the
electrolyte. In an
embodiment, the electrolyte is in contact with the portion of the fuel cell
casing made of the
same material. In an embodiment, the electrolyte and the portion of the fuel
cell casing are
made of a DFS, wherein the DFS comprises 3YSZ (3 mol% Y203 in Zr02) and 8YSZ
(8 mol% Y203
in Zr02), wherein the mass ratio of 3YSZ/8YSZ is in the range of from 100/0 to
0/100 or from
10/90 to 90/10 and wherein the DFS is impermeable to non-ionic substances and
electrically
insulating. In an embodiment, the mass ratio of 3YSZ/8YSZ is about 50/50 or
40/60 or 60/40 or
30/70 or 70/30 or 20/80 or 80/20.
[238] In an embodiment, said fuel cell casing comprises a fuel entrance and
fuel passage for the
anode, an oxidant entrance and oxidant passage for the cathode, and at least
one fluid exit. In an
embodiment, the entrances and at least one exit are on one surface of the FCC
wherein the FCC
comprises no protruding fluid passage on the surface. In an embodiment, the
fuel cell casing is in
contact with at least a portion of the anode.
[239] In an embodiment, the FCC comprises a barrier layer between the
electrolyte and the
cathode and between the fuel cell casing and the cathode. In an embodiment,
the FCC comprises an
interconnect, wherein the interconnect comprises no fluid dispersing element
and said anode and
cathode comprise fluid dispersing components. In an embodiment, the FCC
comprises an
interconnect, wherein the interconnect comprises no fluid dispersing element
and said anode and
cathode comprise fluid channels.
[240] In an embodiment, the FCC is detachably fixed to a mating surface and
not soldered nor
welded to said mating surface. In an embodiment, said mating surface comprises
matching fuel
entrance, matching oxidant entrance, and at least one matching fluid exit.
[241] Also discussed herein is a DFS comprising 3YSZ (3 mol% Y203 in Zr02) and
8YSZ (8 mol%
Y203 in Zr02), wherein the mass ratio of 3YSZ/8YSZ is in the range of from
10/90 to 90/10 and
wherein the DFS is impermeable to non-ionic substances and electrically
insulating. In an
embodiment, the mass ratio of 3YSZ/8YSZ is about 50/50 or 40/60 or 60/40 or
30/70 or 70/30
53
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
or 20/80 or 80/20. In an embodiment, the DFS is used as an electrolyte in a
fuel cell or as a
portion of a fuel cell casing, or both.
[242] Further disclosed herein is a method comprising providing a DFS in a
fuel cell system,
wherein the DFS comprises 3YSZ (3 mol% Y203 in Zr02) and 8YSZ (8 mol% Y203 in
Zr02), wherein
the mass ratio of 3YSZ/8YSZ is in the range of from 100/0 to 0/100 or from
10/90 to 90/10 and
wherein the DFS is impermeable to non-ionic substances and electrically
insulating. In an
embodiment, the mass ratio of 3YSZ/8YSZ is about 50/50 or 40/60 or 60/40 or
30/70 or 70/30 or
20/80 or 80/20.
[243] In an embodiment, the DFS is used as electrolyte or a portion of a fuel
cell casing or both in
the fuel cell system. The portion of a fuel cell casing may be the entire fuel
cell casing. The portion
of a fuel cell casing is a coating on the fuel cell casing. The electrolyte
and said portion of a fuel cell
casing are in contact.
[244] Disclosed herein is a fuel cell system comprising an anode having six
surfaces, a
cathode having six surfaces, an electrolyte, and an anode surround in contact
with at least
three surfaces of the anode, wherein the electrolyte is part of the anode
surround and said
anode surround is made of the same material as the electrolyte. In an
embodiment, said same
material is a DFS comprising 3YSZ (3 mol% Y203 in Zr02) and 8YSZ (8 mol% Y203
in Zr02),
wherein the mass ratio of 3YSZ/8YSZ is in the range of from 100/0 to 0/100 or
from 10/90 to
90/10 and wherein the DFS is impermeable to non-ionic substances and
electrically insulating.
In an embodiment, the mass ratio of 3YSZ/8YSZ is about 50/50 or 40/60 or 60/40
or 30/70 or
70/30 or 20/80 or 80/20. In an embodiment, the anode surround is in contact
with five
surfaces of the anode.
[245] In an embodiment, the fuel cell system comprises a barrier layer between
the cathode and
a cathode surround, wherein the barrier layer is in contact with at least
three surfaces of the
cathode, wherein the electrolyte is part of the cathode surround and said
cathode surround is made
of the same material as the electrolyte.
[246] In an embodiment, the fuel cell system comprises fuel passage and
oxidant passage in the
anode surround and the cathode surround. In an embodiment, the fuel cell
system comprises an
interconnect, wherein the interconnect comprises no fluid dispersing element
and
54
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
said anode and cathode comprise fluid dispersing components. In an embodiment,
the fuel cell
system comprises an interconnect, wherein the interconnect comprises no fluid
dispersing element
and said anode and cathode comprise fluid channels.
Tubular Design
[247] In various cases, the electrochemical reactors as discussed in this
disclosure are tubular.
The discussion in this section takes tubular fuel cell (TFC) as an example of
a tubular
electrochemical reactor. The tubular design is applicable to other types of
electrochemical
reactors, e.g., the EC gas producers, the EC compressors, or flow batteries.
Herein disclosed is a
tubular fuel cell (TFC) comprising an internal cathode, an external anode, an
electrolyte placed
between the anode and the cathode, and an interconnect. In some embodiments of
a TFC, an
electrolyte is considered as a membrane. The cross section of the cathode is a
rounded non-
circular shape with no sharp corner, wherein the cross section is orthogonal
to the longitudinal
axis of the TFC, wherein said interconnect is in contact with the cathode but
not with the anode
and said interconnect has a contacting surface configured to contact an anode
of an adjacent
TFC, wherein the anode has a contacting surface configured to contact an
interconnect of
another adjacent TFC and a non-contacting surface.
[248] In an embodiment, the TFC comprises a barrier layer between the cathode
and the
electrolyte or between the anode and the electrolyte or both. In an
embodiment, the rounded non-
circular shape comprises rounded rectangle, rounded square, rounded hexagon,
rounded trapezoid,
rounded parallelogram, rounded pentagon, rounded triangle, rounded octagon,
oval, ellipsoid, or
rounded irregular shape or combinations thereof.
[249] In an embodiment, the ratio of the area of the contacting surface of the
interconnect
over the area of the non-contacting surface of the anode is no greater than 1,
or no greater
than 0.75, or no greater than 0.5. In an embodiment, the ratio of the area of
the contacting
surface of the interconnect over the area of the non-contacting surface of the
anode is no
greater than 0.3, or no greater than 0.1, or no greater than 0.05.
[250] In an embodiment, the thickness of the cathode is in the range of from
about 10 microns
to about 1,000 microns; or from about 50 to about 150 microns; or from about
90 to about 110
microns; or about 100 microns. In an embodiment, the thickness of the anode is
in
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
the range of from about 1 micron to about 50 microns; or from about 5 microns
to about 25
microns; or from about 8 microns to about 12 microns; or about 10 microns. In
an
embodiment, the thickness of the electrolyte is in the range of from about 100
nm to about
microns; or from about 500 nm to about 5 microns; or from about 800 nm to
about 2
microns; or about 1 micron. In an embodiment, the thickness of the barrier
layer is in the
range of from about 100 nm to about 10 microns; or from about 500 nm to about
5 microns;
or from about 800 nm to about 2 microns; or about 1 micron. In an embodiment,
the
thickness of the interconnect is in the range of from about 10 microns to
about 1000 microns;
or from about 50 microns to about 500 microns; or from about 80 microns to
about 200
microns; or about 100 microns.
[251] In an embodiment, the TFC has a length L and the cross section has a
characteristic
length of W, wherein the ratio of L/W is no less than 1. In an embodiment, the
ratio of L/W is no
less than 2 or no less than 10 or no less than 100.
[252] In an embodiment, the TFC comprises a support in the cathode. In an
embodiment, the
support is in contact with the cathode. In an embodiment, the support is an
integral part of the
cathode. In an embodiment, the support and the cathode are made from the same
material. In
an embodiment, the support and the cathode are made from different materials.
In an
embodiment, the electrolyte is impermeable to fluids. In an embodiment, the
cathode and the
anode are porous.
[253] Also discussed herein is a fuel cell stack comprising a multiplicity of
tubular fuel cells
(TFCs), wherein each of said TFCs comprises an internal cathode, an external
anode, an
electrolyte placed between the anode and the cathode, and an interconnect,
wherein a cross
section of the cathode is a rounded non-circular shape with no sharp corner,
wherein the cross
section is orthogonal to the longitudinal axis of the TFC, wherein said
interconnect is in contact
with the cathode but not with the anode and said interconnect has a contacting
surface
configured to contact an anode of an adjacent TFC, wherein said anode has a
contacting surface
configured to contact an interconnect of another adjacent TFC and a non-
contacting surface.
[254] In an embodiment, each of said TFCs comprises a barrier layer between
the cathode and
the electrolyte or between the anode and the electrolyte or both. In an
embodiment, said
56
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
rounded non-circular shape comprises rounded rectangle, rounded square,
rounded hexagon,
rounded trapezoid, rounded parallelogram, rounded pentagon, rounded triangle,
rounded
octagon, oval, ellipsoid, rounded irregular shape.
[255] In an embodiment, the ratio of the area of the contacting surface of the
interconnect
over the area of the non-contacting surface of the anode is no greater than 1,
or no greater than
0.75, or no greater than 0.5. In an embodiment, the ratio of the area of the
contacting surface
of the interconnect over the area of the non-contacting surface of the anode
is no greater than
0.3, or no greater than 0.1, or no greater than 0.05.
[256] In an embodiment, each of said TFCs has a length L and the cross section
has a
characteristic length of W, wherein the ratio of L/W is no less than 1 or no
less than 2 or no less
than 10 or no less than 100.
[257] In an embodiment, each of said TFCs comprises a support in the cathode.
In an
embodiment, the support is in contact with the cathode. In an embodiment, the
support is an
integral part of the cathode. In an embodiment, the support and the cathode
are made from the
same material.
[258] Herein disclosed is a tubular fuel cell (TFC) comprising an internal
anode, an external
cathode, an electrolyte placed between the anode and the cathode, and an
interconnect,
wherein a cross section of the anode is a rounded non-circular shape with no
sharp corner,
wherein the cross section is orthogonal to the longitudinal axis of the TFC,
wherein the
interconnect is in contact with the anode but not with the cathode and the
interconnect has a
contacting surface configured to contact a cathode of an adjacent TFC, wherein
the cathode has
a contacting surface configured to contact an interconnect of another adjacent
TFC and a non-
contacting surface.
[259] In an embodiment, the rounded non-circular shape comprises rounded
rectangle,
rounded square, rounded hexagon, rounded trapezoid, rounded parallelogram,
rounded
pentagon, rounded triangle, rounded octagon, oval, ellipsoid, rounded
irregular shape or
combination thereof. In an embodiment, the ratio of the area of the contacting
surface of the
interconnect over the area of the non-contacting surface of the cathode is no
greater than 1, or
no greater than 0.75, or no greater than 0.5, no greater than 0.3, or no
greater than 0.1, or no
57
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
greater than 0.05. In an embodiment, the TFC comprises a barrier layer between
the cathode and
the electrolyte or between the anode and the electrolyte or both.
[260] Figs. 10A-10C illustrate different aspect ratios of fuel cells and how
that may be
connected in multi-tubular fuel cell (TFC) units comprising two or more TFCs.
The TFCs
comprise rounded edges. Fig. 10A illustrates a cross-sectional view of a TFC
1000, according to
an embodiment of the disclosure. TFC 1000 comprises an internal cathode layer
1002, barrier
layer 1004, electrolyte layer 1006, an external anode layer 1008, an
interconnect 1010 and a
fluid passage 1012. In some cases, the barrier layer 1004 is placed between
anode 1008 and
electrolyte 1006. In some cases, two barrier layers are placed (1) between
cathode 1002 and
electrolyte 1006 and (2) between anode 1008 and electrolyte 1006. Interconnect
1010 is in
contact with cathode 1002 but not with anode 1008. The top surface of
interconnect 1010 is
configured to be contact with the anode 1008 of an adjacent TFC. Anode 1008
has a contacting
surface on the bottom configured to be in contact with interconnect 1010 of
another adjacent
TFC. Anode 1008 has a non-contacting surface on both sides in the
configuration as shown in
Figs. 10A-10C. In this example in Fig. 10A, the TFCs 1000 have a rounded
rectangular shape
that are connected by interconnects 1010 on the short end of the rectangular
shape.
[261] Fig. 10B illustrates a cross-sectional view of a TFC 1020, according to
an embodiment of the
disclosure. TFCs 1020 are similar in construction to TFCs 1000 but are
connected by interconnects
1010 on the long side of the rectangular shape.
[262] Fig. 10C illustrates a cross-sectional view of a TFC 1040, according to
an embodiment of
the disclosure. TFCs 1040 in Fig. 10C are similar in construction to TFCs in
Figs. 10A-10B but
comprise a rounded square-like shape wherein the length of the sides are
substantially the
same. The TFCs 1040 are further connected by interconnects 1010.
[263] In alternative embodiments, the anode 1008 may be configured to be
internal and the
cathode 1002 may be external. In some cases, the barrier layer may be placed
between
cathode and electrolyte. In some cases, two barrier layers are placed (1)
between cathode and
electrolyte and (2) between anode and electrolyte. All the other
configurations and features as
discussed above are applicable in this embodiment as well.
58
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[264] In some embodiment, the TFC may further comprise one or more supports in
the
cathode layer as shown in Figs. 11A-11C. Fig. 11A illustrates a cross-
sectional view of a TFC
1100 comprising a support, according to an embodiment of the disclosure. TFC
1100 comprises
a cathode 1002, barrier layer 1004, electrolyte 1006, anode layer 1008,
interconnect 1010 and
at least one fluid passage 1012. The shape and design of how the TFCs 1100 are
arranged is
similar to TFCs in Fig. 10A. TFCs 1100 further comprise one or more supports.
The supports may
be in any shape, number, size, and material as suitable. In some cases, the
supports 1102 are
made from the same material as the internal electrode layer such as cathode
layer 1002. In
some cases, the supports 1104 are made from a material different from the
internal electrode
layer suach as cathode 1002 material. For example, an inert material in
relation to the fuel cell.
In some cases, the supports may be made from more than one material. In an
embodiment, the
one or more supports 1102, 1104 are in contact with the cathode 1002. In an
embodiment, the
one or more supports 1102, 1104 are integral parts of the cathode. In an
embodiment, the one
or more supports 1102, 1104 are made as an integral part of the cathode.
[265] Fig. 11B illustrates a cross-sectional view of a TFC 1120 comprising a
support, according
to an embodiment of the disclosure. The shape and design of how the TFCs 1120
are arranged
is similar to TFCs in Fig. 108. TFCs 1120 further comprise one or more
supports. The support
1102 may be a linear shaped support of the same material as the inner
electrode such as the
cathode 1002. The support 1104 may be a linear shaped support 1104 not
constructed of the
same material. The support 1106 may be an oval or circular-like shaped support
constructed of
the same material as the inner electrode, such as cathode 1002. The support
1108 may be an
oval or circular-like shaped support not constructed of the same material as
the inner electrode,
such as cathode 1002. As seen in Fig. 118, TFCs may comprise linear shaped
supports 1102,
1104 and circular-shaped supports 1106, 1108.
[266] Fig. 11C illustrates a cross-sectional view of a TFC 1140 comprising a
support, according
to an embodiment of the disclosure. The shape and design of how the TFCs 1140
are arranged is
similar to TFCs in Fig. 10C. TFCs 1140 further comprise one or more supports.
In this example, all
of the supports 1106, 1108 may be circular-like or oval-like shaped though
linear shaped supports
1102, 1104 may also be used.
59
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[267] In some embodiments, the inner electrode may be an anode layer 1008 in
TFCs 1100,
1120, 1140. The supports 1102, 1104, 1106, 1108 may be constructed of the same
material of
the inner anode layer or not constructed of the anode layer 1008 or a
combination thereof.
[268] Herein discussed is a method comprising placing a fluidic mixture
between two tubular
fuel cells (TFCs), wherein said two TFCs have a gap with a minimum distance of
no greater
than 1 mm; heating the fluidic mixture such that the two TFCs are connected;
wherein the
fluidic mixture has a viscosity of no greater than 1000 centipoise. In an
embodiment, the fluidic
mixture has a viscosity of no greater than 500 centipoise or no greater than
300 centipoise or
no greater than 200 centipoise or no greater than 100 centipoise or no greater
than 50
centipoises. In an embodiment, the minimum distance of the gap is no greater
than 500
microns, or no greater than 300 microns, or no greater than 200 microns, or no
greater than
100 microns, or no greater than 50 microns.
[269] In an embodiment, placing the fluidic mixture comprises aerosol jetting,
material
jetting, ink jet printing or combinations thereof. In an embodiment, the
fluidic mixture
comprises a fluid and a solid and wherein heating the fluidic mixture causes
the fluid to
dissipate and the solid to remain. In an embodiment, heating the fluidic
mixture causes it to
solidify. In an embodiment, the heating comprises the use of electromagnetic
radiation (EMR).
In an embodiment, EMR comprises UV light, near ultraviolet light, near
infrared light, infrared
light, visible light, laser, electron beam, microwave, or combinations
thereof. In an
embodiment, the heating comprising oven heating, furnace heating, kiln
heating, plasma
heating, hot surface heating, or combinations thereof. In an embodiment, the
heating is
accomplished via conduction, convection, radiation, or combinations thereof.
In an
embodiment, said heating causes sintering, co-sintering, annealing,
densification,
solidification, evaporation, drying, or combinations thereof.
[270] In an embodiment, the fluidic mixture comprises gold, silver, platinum,
nickel, iron,
steel, stainless steel, chromium, cobalt, carbon, or inconel. In an
embodiment, the fluidic
mixture comprises material used for an electrode in the fuel cells or material
used for an
interconnect in the fuel cells or both.
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[271] In an embodiment, each of the TFCs comprises an internal cathode, an
external anode,
an electrolyte placed between the anode and the cathode, and an interconnect,
wherein a cross
section of the cathode is a rounded non-circular shape with no sharp corner,
wherein the cross
section is orthogonal to the longitudinal axis of the TFC, wherein the
interconnect is in contact
with the cathode but not with the anode and said interconnect has a contacting
surface
configured to contact an anode of an adjacent TFC, wherein said anode has a
contacting surface
configured to contact an interconnect of another adjacent TFC and a non-
contacting surface.
[272] In an embodiment, each of the TFCs comprises an internal anode, an
external cathode,
an electrolyte placed between the anode and the cathode, and an interconnect,
wherein a cross
section of the anode is a rounded non-circular shape with no sharp corner,
wherein the cross
section is orthogonal to the longitudinal axis of the TFC, wherein the
interconnect is in contact
with the anode but not with the cathode and said interconnect has a contacting
surface
configured to contact a cathode of an adjacent TFC, wherein the cathode has a
contacting
surface configured to contact an interconnect of another adjacent TFC and a
non-contacting
surface.
[273] Also discussed herein is a method comprising applying contact paste on a
first tubular fuel
cell (TFC) and placing a second TFC in contact with the contact paste on the
opposite side of the
first TFC, wherein each of the first TFC and second TFC comprises an internal
cathode, an external
anode, an electrolyte placed between the anode and the cathode, and an
interconnect, wherein a cross section of the cathode is a rounded non-circular
shape with no
sharp corner, wherein the cross section is orthogonal to the longitudinal axis
of the TFC,
wherein the interconnect is in contact with the cathode but not with the anode
and said
interconnect has a contacting surface configured to contact an anode of an
adjacent TFC,
wherein the anode has a contacting surface configured to contact an
interconnect of another
adjacent TFC and a non-contacting surface.
[274] In an embodiment, the contact paste is applied via dipping, coating,
spreading,
spraying, airbrushing, spray pyrolysis, or painting or combinations thereof.
In an embodiment,
the contact paste comprises gold, silver, platinum, nickel, iron, steel,
stainless steel, chromium,
cobalt, carbon, or Inconel or combinations thereof. In an embodiment, the
contact paste
61
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
comprises material used for an electrode in the fuel cells or material used
for an interconnect in the
fuel cells or both. In an embodiment, the TFC comprises a barrier layer
between the cathode and
the electrolyte or between the anode and the electrolyte or both.
[275] In an embodiment, the rounded non-circular shape comprises rounded
rectangle,
rounded square, rounded hexagon, rounded trapezoid, rounded parallelogram,
rounded
pentagon, rounded triangle, rounded octagon, oval, ellipsoid, or rounded
irregular shape. In an
embodiment, the ratio of the area of the contacting surface of the
interconnect over the area of
the non-contacting surface of the anode is no greater than 1, or no greater
than 0.75, or no
greater than 0.5. In an embodiment, the ratio of the area of the contacting
surface of the
interconnect over the area of the non-contacting surface of the anode is no
greater than 0.3, or
no greater than 0.1, or no greater than 0.05. In an embodiment, the TFC has a
length L and
wherein the cross section has a characteristic length of W, wherein the ratio
of L/W is no less
than 1, or no less than 2 or no less than 10 or no less than 100.
[276] In an embodiment, the TFC comprises a support in the cathode. In an
embodiment, the
support is in contact with the cathode. In an embodiment, the support is an
integral part of the
cathode. In an embodiment, the support and the cathode are made from the same
material.
[277] In an embodiment, the method comprises heating the contact paste. In an
embodiment, the heating comprises the use of electromagnetic radiation (EMR).
In an
embodiment, [MR comprises UV light, near ultraviolet light, near infrared
light, infrared
light, visible light, laser, electron beam or combinations thereof. In an
embodiment, the
heating comprising oven heating, furnace heating, kiln heating, plasma
heating, hot surface
heating, or combinations thereof. In an embodiment, said heating is
accomplished via
conduction, convection, radiation, or combinations thereof. In an embodiment,
said heating
causes sintering, co-sintering, annealing, densification, solidification,
evaporation, drying, or
combinations thereof.
[278] Further discussed herein is a method comprising applying contact paste
on a first
tubular fuel cell (TFC) and placing a second TFC in contact with the contact
paste on the
opposite side of the first TFC, wherein each of the first TFC and second TFC
comprises an
internal anode, an external cathode, an electrolyte placed between the anode
and the cathode,
62
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
and an interconnect, wherein a cross section of the anode is a rounded non-
circular shape with
no sharp corner, wherein the cross section is orthogonal to the longitudinal
axis of the TFC,
wherein said interconnect is in contact with the anode but not with the
cathode and said
interconnect has a contacting surface configured to contact a cathode of an
adjacent TFC,
wherein said cathode has a contacting surface configured to contact an
interconnect of another
adjacent TFC and a non-contacting surface.
[279] In an embodiment, the contact paste is applied via dipping, coating,
spreading,
spraying, painting, or combinations thereof. In an embodiment, the contact
paste comprises
gold, silver, platinum, nickel, iron, steel, stainless steel, chromium,
cobalt, carbon, or Inconel or
combinations thereof. In an embodiment, the contact paste comprises material
used for an
electrode in the fuel cells or material used for an interconnect in the fuel
cells or both. In an
embodiment, the TFC comprises a barrier layer between the cathode and the
electrolyte or
between the anode and the electrolyte or both. In an embodiment, the ratio of
the area of the
contacting surface of the interconnect over the area of the non-contacting
surface of the anode
is no greater than 1, or no greater than 0.75, or no greater than 0.5, or no
greater than 0.3, or
no greater than 0.1, or no greater than 0.05. In an embodiment, the TFC has a
length L and
wherein the cross section has a characteristic length of W, wherein the ratio
of VW is no less
than 1, or no less than 2 or no less than 10 or no less than 100.
[280] In an embodiment, the TFC comprises a support in the anode. In an
embodiment, the
support is in contact with the anode. In an embodiment, the support is an
integral part of the
anode. In an embodiment, the support and the anode are made from the same
material.
[281] In an embodiment, the method comprises heating the contact paste. In an
embodiment,
the heating comprises the use of electromagnetic radiation (EMR). In an
embodiment, EMR
comprises UV light, near ultraviolet light, near infrared light, infrared
light, visible light, laser,
electron beam, microwave. In an embodiment, said heating comprising oven
heating, furnace
heating, kiln heating, plasma heating, hot surface heating, or combinations
thereof. In an
embodiment, the heating is accomplished via conduction, convection, radiation,
or combinations
thereof. In an embodiment, said heating causes sintering, co-sintering,
annealing, densiflcation,
solidification, evaporation, drying, or combinations thereof.
63
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
Integrated Heat Exchanger
[282] Disclosed herein is an electrochemical (EC) reactor, such as a EC gas
producer or a
solid oxide reactor (SOR), comprising a first electrode, a second electrode,
an electrolyte
between the first and second electrodes, and a first heat exchanger, wherein
the first heat
exchanger is in fluid communication with the first electrode. The minimum
distance between
the first electrode and the first heat exchanger is no greater than 10 cm. In
some
embodiments, the minimum distance is no greater than 5 cm. In other
embodiments, the
minimum distance is no greater than 1 cm. In still other embodiments, the
minimum distance
is no greater than 5 mm. In even still other embodiments, the minimum distance
is no greater
than 1 mm. In an embodiment, the EC reactor comprises a second heat exchanger,
wherein
the second heat exchanger is in fluid communication with the second electrode.
The minimum
distance between the second electrode and the second heat exchanger no greater
than 10 cm.
In some embodiments, the minimum distance is no greater than 5 cm. In other
embodiments,
the minimum distance is no greater than 1 cm. In still other embodiments, the
minimum
distance is no greater than 5 mm. In even still other embodiments, the minimum
distance is
no greater than 1 mm.
[283] In one embodiment, the first heat exchanger is adjacent to the first
electrode, or
alternatively wherein the second heat exchanger is side-by-side or adjacent to
the second
electrode. The one or more heat exchangers may be placed side-by-side the
components in an
EC reactor, or on top of or below the components (i.e., electrodes) of an EC
reactor. Fig. 98 is
an illustrative example where an integrated multi-fluid heat exchanger
comprising 916 and 918
is at the bottom of a repeat unit/stack in a fuel cell separated only by an
interconnect layer 920
from the anode 910. In this case, the minimum distance between the heat
exchanger and the
repeat unit/stack is only the thickness of the interconnect, which is 1 mm or
less, 0.5 mm or
less, 200 microns or less, or in the range of about 100 nm to about 100
microns. In some
embodiments, the first heat exchanger and the second heat exchanger are the
same heat
exchanger, wherein the heat exchangers form a multi-fluid heat exchanger. The
EC reactor may
comprise a solid oxide fuel cell, solid oxide flow battery, electrochemical
gas producer, or
electrochemical compressor. The EC reactor may comprise a reformer upstream of
the first
64
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
electrode or a reformer in contact with the first electrode or a reformer in
the first heat
exchanger. The EC reactor may comprise two or more repeat units separated by
interconnects,
wherein each repeat unit comprises a first electrode, a second electrode, and
an electrolyte.
Each repeat unit may comprise at least one heat exchanger adjacent to the
repeat unit.
[284] Herein also disclosed is an EC reactor, such as a solid oxide reactor
(SOR), comprising a
stack and a heat exchanger. The stack has a stack height and comprises
multiple repeat units
separated by interconnects, wherein each repeat unit comprises a first
electrode, a second
electrode, and an electrolyte between the first and second electrodes. The
heat exchanger is in
fluid communication with the stack and wherein the minimum distance between
the stack and
the heat exchanger is no greater than 2 times the stack height, or no greater
than the stack
height, or no greater than half the stack height. The heat exchanger may be
adjacent to the
stack. The heat exchanger comprises at least three fluid inlets and at least
three fluid channels,
wherein each of the at least three fluid channels has a minimum dimension of
no greater than
30 mm. The stack or the heat exchanger may further comprise a reformer. The
reformer may
be built into the stack or the heat exchanger. In an embodiment, the
interconnect comprises no
fluid dispersing element and the electrodes comprise fluid dispersing
components or fluid
channels.
[285] In an embodiment, the EC reactor is in the form of a cartridge (such as
that illustrated in
Figs. 9A-9D). The cartridge may comprise a fuel entrance on a fuel side of the
cartridge, an
oxidant entrance on an oxidant side of the cartridge, at least one fluid exit,
wherein the fuel
entrance has a width of Wf, the fuel side of the cartridge has a length of Lf,
the oxidant entrance
has a width of W., the oxidant side of the cartridge has a length of L.,
wherein Wf/Lf is in the
range of 0.1 to 1.0, 0.1 to 0.9, 0.2 to 0.9, 0.5 to 0.9, or 0.5 to 1.0 and
Wo/Lo is in the range of
0.1 to 1.0, 0.1 to 0.9, 0.2 to 0.9, 0.5 to 0.9, or 0.5 to 1Ø In some
embodiments, the entrances
and exit are on one surface of the cartridge wherein the cartridge comprises
no protruding fluid
passage on the surface. The cartridge may be detachably fixed to a mating
surface and not
soldered nor welded to the mating surface. The cartridge may be bolted to or
pressed to the
mating surface. The mating surface may comprise a matching fuel entrance,
matching oxidant
entrance, and at least one matching fluid exit.
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
(2863 Further disclosed herein is a EC reactor cartridge, such as a solid
oxide reactor
cartridge (SORC), comprising a first electrode, a second electrode, an
electrolyte between the
first and second electrodes, and a heat exchanger, wherein said heat exchanger
is in fluid
communication with the first electrode or the second electrode or both. The
minimum
distance between the heat exchanger and the first electrode or the second
electrode is no
greater than 10 cm, or no greater than 5 cm, or no greater than 1 cm, or no
greater than 5
mm, or no greater than 1 mm.
(2873 In an embodiment, the EC reactor cartridge comprises a reformer upstream
of the first
electrode or a reformer in contact with the first electrode or a reformer in
the heat exchanger.
The EC reactor cartridge may comprise a fuel entrance on a fuel side of the
cartridge, an
oxidant entrance on an oxidant side of the cartridge, at least one fluid exit,
wherein the fuel
entrance has a width of Wf, the fuel side of the cartridge has a length of Lf,
the oxidant
entrance has a width of W., the oxidant side of the cartridge has a length of
L.. The ratio of
Wf/Lf is in the range of 0.1 to 1.0, 0.1 to 0.9, 0.2 to 0.9, 0.5 to 0.9, or
0.5 to 1.0 and the ratio
of WA is in the range of 0.1 to 1.0, 0.1 to 0.9, 0.2 to 0.9, 0.5 to 0.9, or
0.5 to 1Ø The
entrances and exit may be on one surface of the cartridge and wherein the
cartridge comprises
no protruding fluid passage on the surface. The EC reactor cartridge may be
detachably fixed to
a mating surface and not soldered nor welded to the mating surface.
(2883 Discussed herein is a method of forming an EC reactor, such as a solid
oxide reactor
(SOR), comprising forming a first electrode in a device, forming an
electrolyte in the same
device, forming a second electrode in the same device, and forming a heat
exchanger in the
same device, wherein the electrolyte is between the first electrode and the
second electrode and
is in contact with the electrodes. The heat exchanger may be in fluid
communication with the
first electrode or the second electrode or both. The forming method may
comprise one or more
of material jetting, binder jetting, inkjet printing, aerosol jetting, aerosol
jet printing, vat
photopolymerization, powder bed fusion, material extrusion, directed energy
deposition, sheet
lamination, ultrasonic inkjet printing, direct (dry) powder deposition, or
combinations thereof.
Preferably, the forming is accomplished by inkjet printing.
66
Date Regue/Date Received 2022-12-08
CA 03126966 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
(2893 In an embodiment, the method of forming an EC reactor further comprises
heating the
EC reactor. The heating may be performed in situ. The heating may be performed
using
electromagnetic radiation (EMR). The method of forming an EC reactor may
further comprise
forming multiple repeat units and interconnects between the repeat units,
wherein a repeat
unit comprises the first electrode, the electrolyte, and the second electrode.
In an
embodiment, forming the repeat units and the interconnects take place in the
same device. In
a preferred embodiment, the method comprises heating the repeat units and the
interconnects
in situ using [MR. In a preferred embodiment, the method further comprises
forming a
reformer. The reformer may be formed in the same device.
(2903 In an embodiment, the interconnects in the EC reactor comprise no fluid
dispersing
element. In an embodiment, the method of forming an EC reactor comprises
forming a first
template while forming the first electrode, wherein the first template is in
contact with the first
electrode; removing at least a portion of the first template to form channels
in the first electrode.
The method further comprises forming a second template while forming the
second electrode,
wherein the second template is in contact with the second electrode; removing
at least a portion
of the second template to form channels in the second electrode. In an
embodiment, the first
electrode comprises fluid dispersing components (FDC) or fluid channels;
wherein the second
electrode comprises fluid dispersing components (FDC) or fluid channels. (2913
In an
embodiment, the EC reactor, such as an SOR, is formed into a cartridge. The
cartridge comprises
a fuel entrance on a fuel side of the cartridge, an oxidant entrance on an
oxidant side of the
cartridge, at least one fluid exit, wherein the fuel entrance has a width of
Wf, the fuel side of the
cartridge has a length of Lf, the oxidant entrance has a width of Wo, and the
oxidant side of the
cartridge has a length of Lo. The ratio of Wf/Lf may be in the range of 0.1 to
1.0, 0.1 to 0.9, 0.2
to 0.9, 0.5 to 0.9, or 0.5 to 1.0 and the ratio of Wo/Lo is in the range of
0.1 to 1.0, 0.1 to 0.9, 0.2
to 0.9, 0.5 to 0.9, or 0.5 to 1Ø In an embodiment, the entrances and exit
are on one surface of
the cartridge and said cartridge comprises no protruding fluid passage on said
surface. In an
embodiment, the cartridge is detachably fixed to a mating surface and not
soldered nor welded
to the mating surface. The cartridge may be bolted to or pressed to the mating
surface. In an
embodiment, the method comprises forming a reformer upstream of the
67
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
first electrode or a reformer in contact with the first electrode or a
reformer in the heat
exchanger. The reformer may be formed in the same device.
[292] Also disclosed herein is a method comprising forming an EC reactor stack
and a heat
exchanger, wherein the stack having a stack height comprises multiple repeat
units separated by
interconnects, wherein each repeat unit comprises a first electrode, a second
electrode, and an
electrolyte between the first and second electrodes. The heat exchanger may be
in fluid
communication with the stack and wherein the minimum distance between the
stack and the heat
exchanger is no greater than 2 times the stack height, or no greater than the
stack height, or no
greater than half the stack height.
[293] In an embodiment, the EC reactor stack, such as an SOR, and the heat
exchanger are
formed in the same device. The method may comprise forming the stack and the
heat exchanger
into a cartridge. The cartridge may be detachably fixed to a mating surface
and not soldered nor
welded to the mating surface.
[294] Further discussed herein is a method comprising forming an EC reactor,
such as a
SOR, comprising a first electrode, a second electrode, an electrolyte between
the first and
second electrodes, and a heat exchanger. The heat exchanger may be in fluid
communication
with the first electrode or the second electrode or both. The minimum distance
between the
heat exchanger and the first electrode or the second electrode is no greater
than 10 cm, no
greater than 5 cm, no greater than 1 cm, no greater than 5 mm, or no greater
than 1 mm. In
some cases, the electrodes, the electrolyte, and the heat exchanger are formed
in the same
device. The method in some cases also comprises forming the EC reactor into a
cartridge. The
cartridge may be detachably fixed to a mating surface and not soldered nor
welded to the
mating surface.
[295] Disclosed herein is a method comprising forming an EC reactor cartridge
comprising
forming a first electrode, forming a second electrode, forming an electrolyte
between the first and
second electrodes, and forming a heat exchanger. In an embodiment, the heat
exchanger is in
fluid communication with the first electrode or the second electrode or both.
In an embodiment,
the electrodes, the electrolyte, and the heat exchanger are formed in the same
device. In an
embodiment, the method comprises forming a reformer upstream of the first
68
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
electrode or a reformer in contact with the first electrode or a reformer in
the heat exchanger. In
an embodiment, the reformer is formed in the same device.
Fischer Tropsch
[296] The method and system of this disclosure are suitable for making a
catalyst or a catalyst
composite, such as a Fischer-Tropsch (FT) catalyst or catalyst composite.
Disclosed herein is a
Fischer-Tropsch (FT) catalyst composite comprising a catalyst and a substrate,
wherein the mass
ratio between the catalyst and the substrate is in no less than 1/100, or no
less than 1/10, or no
less than 1/5, or no less than 1/3, or no less than 1/1. In an embodiment, the
catalyst
comprises Fe, Co, Ni, or Ru. The substrate comprises A1203, ZrO2, SiO2, TiO2,
Ce02, modified
A1203, modified ZrO2, modified Si02, modified TiO2, modified Ce02, gadolinium,
steel, cordierite
(2Mg0-2A1203-5Si02), aluminum titanate (AI2TiO5), silicon carbide (SiC), all
phases of aluminum
oxide, yttria or scandia-stabilized zirconia (YSZ), gadolinia or samaria-doped
ceria, or
combinations thereof. In an embodiment, the catalyst composite comprises a
promoter wherein
the promoter comprises noble metals, metal cations, or combinations thereof.
The promoter
may comprise B, La, Zr, K, Cu, or combinations thereof. In an embodiment, the
catalyst
composite comprises fluid channels or alternatively fluid dispersing
components.
[297] The FT reactor/system of this disclosure is much smaller than
traditional FT
reactors/systems (e.g., 3 - 100 times smaller or 100+ times smaller for the
same FT product
generation rate). The high catalyst to substrate ratio is not achievable by
traditional methods to
make FT catalysts. As such, in some embodiments, the FT reactor/system is
miniaturized
compared to traditional FT reactors/systems.
[298] Also discussed herein is a method comprising depositing a FT catalyst to
a substrate to
form a FT catalyst composite, wherein said depositing comprises material
jetting, binder
jetting, inkjet printing, aerosol jetting, or aerosol jet printing, vat
photopolymerization, powder
bed fusion, material extrusion, directed energy deposition, sheet lamination,
ultrasonic inkjet
printing, or combinations thereof. In an embodiment, the mass ratio between
the catalyst and
the substrate is in no less than 1/100, or no less than 1/10, or no less than
1/5, or no less than
1/3, or no less than 1/1. In preferred embodiments, the deposition method
comprises forming
fluid channels or alternatively fluid dispersing components in the catalyst
composite.
69
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[299] Further discussed herein is a system comprising a Fischer-Tropsch (FT)
reactor
containing a FT catalyst composite comprising a catalyst and a substrate,
wherein the mass
ratio between the catalyst and the substrate is in no less than 1/100, or no
less than 1/10, or
no less than 1/5, or no less than 1/3, or no less than 1/1. In an embodiment,
the catalyst
comprises Fe, Co, Ni, or Ru. In an embodiment, the substrate comprises A1203,
Zr02, 5102,
Ti02, Ce02, modified A1203, modified Zr02, modified Si02, modified Ti02,
modified Ce02,
gadolinium, steel, cordierite (2Mg0-2A1203-5Si02), aluminum titanate
(AI2Ti05), silicon carbide
(SiC), all phases of aluminum oxide, yttria or scandia-stabilized zirconia
(YSZ), gadolinia or
samaria-doped ceria, or combinations thereof. In an embodiment, the catalyst
composite
comprises a promoter.
[300] As an example, a FT catalyst composite is formed via printing. The
catalyst and the
substrate/support are made into an ink form comprising a solvent and particles
(e.g.,
nanoparticles). The ink optionally comprises a dispersant, a binder, a
plasticizer, a surfactant, a co-
solvent, or combinations thereof. The ink may be any kind of suspension. The
ink may be treated
with a mixing process, such as ultrasonication or high shear mixing. In some
cases, an iron ink is in
an aqueous environment. In some cases, an iron ink is in an organic
environment. The iron ink may also include a promoter. The substrate/support
may be a
suspension or ink of alumina, in an aqueous environment or an organic
environment. The
substrate ink may be treated with a mixing process, such as ultrasonication or
high shear
mixing. In some cases, the substrate ink comprises a promoter. In some cases,
the promoter is
added as its own ink, in an aqueous environment or an organic environment. In
some cases,
the various inks are printed separately and sequentially. In some cases, the
various inks are
printed separately and simultaneously, for example, through different print
heads. In some
cases, the various inks are printed in combination as a mixture.
[301] As an example, an exhaust from the fuel cell comprises hydrogen, carbon
dioxide, water,
and optionally carbon monoxide. The exhaust is passed over a FT catalyst
(e.g., an iron catalyst)
to produce synthetic fuels or lubricants. The FT iron catalyst has the
property to promote water
gas shift reaction or reverse water gas shift reaction. The FT reactions take
place at a
temperature in the range of 150-350 C and a pressure in the range of one to
several
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
tens of atmospheres (e.g., 15 atm or 10 atm or 5 atm or 1 atm). Additional
hydrogen may be
added to the exhaust stream to reach a hydrogen to carbon oxides ratio (carbon
dioxide and
carbon monoxide) of no less than 2 or no less than 3 or between 2 and 3.
Fluid Dispersing Component
[302] Fig. 12A illustrates an impermeable interconnect 1202 with a fluid
dispersing
component 1204, according to an embodiment of the disclosure. Fig. 12B
illustrates an
impermeable interconnect 1202 with two fluid dispersing components 1204,
according to an
embodiment of the disclosure. The fluid dispersing components 1204 are in
contact with both
sides (major faces) of interconnect 1202. As such, the interconnect is shared
between two
repeat units in an electrochemical reactor, such as in a EC gas producer.
Fluid dispersing
components 1204 function to distribute fluids, e.g., reactive gases (such as
methane,
hydrogen, carbon monoxide, air, oxygen, etc.), in an electrochemical reactor.
As such,
traditional interconnects with channels are no longer needed. The design and
manufacturing of
such traditional interconnects with channels is complex and expensive.
According to this
disclosure, the interconnects are simply impermeable layers that conduct or
collect electrons,
having no fluid dispersing elements.
[303] Figs. 12C-F schematically illustrate segmented fluid dispersing
components 1204 on
top of impermeable interconnect 1202, according to embodiments of the
disclosure. Such
segments may have different compositions, shapes, densities, porosities, pore
sizes, pore
shapes, permeabilities, or combinations thereof. The segments may be
discontinuous. Fig.
13C illustrates segmented fluid dispersing components 1204 of similar shapes
but different
sizes on an impermeable interconnect 1202. Fig. 13D illustrates segmented
fluid dispersing
components 1204 of similar shapes and similar sizes on an impermeable
interconnect 1202,
according to an embodiment of the disclosure. Fig. 12E illustrates segmented
fluid dispersing
components 1204 of similar shapes and similar sizes but closely packed on an
impermeable
interconnect 1202, according to an embodiment of the disclosure. Fig. 12F
illustrates
segmented fluid dispersing components 1204 of different shapes and different
sizes on an
impermeable interconnect 1202, according to an embodiment of the disclosure.
It is also
11
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
contemplated that these segments have different compositions, densities,
porosities, pore
sizes, pore shapes, permeabilities, or combinations thereof.
[304] Figs. 12G-I schematically illustrates an impermeable interconnect 1202
with fluid
dispersing component 1204, according to embodiments of the disclosure. Further
illustrated
are different fluid inlet and out designs. The fluid dispersing components may
have varying
density, porosity, pore size, pore shape, composition, or permeability, or
combinations thereof,
in different portions (e.g., in the lateral direction or perpendicular to the
lateral
direction). Such variabilities provide control and adjustability of the fluid
flow in the fluid
dispersing component. Fig. 12G illustrates an impermeable interconnect 1202
and fluid
dispersing component 1204, according to an embodiment of the disclosure. Fig.
12H
illustrates an impermeable interconnect 1202 and fluid dispersing component
1204, according
to an embodiment of the disclosure. Fig. 121 illustrates an impermeable
interconnect 1202
and fluid dispersing component 1204, according to an embodiment of the
disclosure. 1206
and 1208 in Figs. 12G-1 represent different inlet and outlet designs,
according to
embodiments of the disclosure. The interconnect 1202 has matching inlet and
outlet for each
configuration. In Fig. 121, 1206 represents a fluid inlet and 1208 represents
a fluid outlet.
The fluid flow is denoted by arrows 1210. Fig. 123 illustrates an impermeable
interconnect
1202 and a fluid dispersing component 1204, according to an embodiment of the
disclosure.
Further illustrated in Fig. 123 are alternative fluid flow designs as shown by
the arrows. For
example, the fluid may flow from left to right across the fluid dispersing
component; or the
fluid may flow from front to back across the fluid dispersing component.
[305] Fig. 12K illustrates a fluid dispersing component 1204, according to an
embodiment of the
disclosure. Fluid dispersing component 1204 design comprises four corners
labeled A, B, C, and D.
Location A comprises fluid flow inlet 1212. Location B comprises fluid flow
outlet 1214.
[306] Discussed herein is an electrochemical reactor (e.g., a fuel cell)
comprising an
impermeable interconnect having no fluid dispersing element, an electrolyte, a
fluid dispersing
component (FDC) between the interconnect and the electrolyte. In an
embodiment, the fuel cell
comprises two FDC's. The two FDC's may be symmetrically placed in contact with
the interconnect
on its opposing side or opposing major faces. As such, the interconnect is
shared
12
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
between the two repeat units in the electrochemical reactor, each repeat unit
comprising one of
the two FDC's. The FDC may be a foam, open cell foam, or comprises a lattice
structure.
[307] In a preferred embodiment, the FDC is segmented wherein the segments
have
different compositions, materials, shapes, sizes, densities, porosities, pore
sizes, pore shapes,
permeabilities, or combinations thereof. The shapes of the segments may
comprise pillar,
hollow cylinder, cube, rectangular cuboid, trigonal trapezohedron,
quadrilateral frustum,
parallelepiped, triangular bipyramid, tetragonal anti-wedge, pyramid,
pentagonal pyramid,
prism, or combinations thereof.
[308] In some embodiments, the FDC has varying density, porosity, pore size,
pore shape,
permeability, or combinations thereof wherein the density, porosity, pore
size, pore shape, or
permeability or combination thereof is controlled. In some embodiments, the
density, porosity,
pore size, pore shape, or permeability or combination thereof, is controlled
to adjust flow of a
fluid through the FDC. In other embodiments, the density, porosity, pore size,
pore shape, or
permeability or combination thereof is controlled to cause uniform fluid flow
from a first point in
the FDC to a second point in the FDC. The fluid flow pattern may be adjusted
as desired. For
example, it does not need to be uniform. The fluid flow may be increased or
decreased
according to the reactivities of the FDC or reaction rates of the fluid in the
various portions of
the FDC. Alternatively and/or in combination, the fluid flow may be increased
or decreased
according to the fluid flow rates to an anode or a cathode in the various
portions of the FDC.
Alternatively and/or in combination, the fluid flow may be increased or
decreased according to
the reaction rates in an anode or a cathode related to or in contact with the
various portions of
the FDC.
[309] In an embodiment, density is higher in the center of the FDC. In an
embodiment,
density is lower in the center of the FDC. In an embodiment, porosity or
permeability or pore
throat size is lower toward the center of the FDC. In an embodiment, porosity
or permeability
or pore throat size is higher toward the center of the FDC.
[310] In an embodiment, at least a portion of the FDC is part of an anode or
part of a cathode.
In a preferred embodiment, the FDC is an anode or a cathode. In an embodiment,
the impermeable
interconnect has a thickness of no greater than 10 microns, or no greater than
1
73
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
micron, or no greater than 500 nm. In a preferred embodiment, the impermeable
interconnect
comprises inlets and outlets for fluids. In a preferred embodiment, the fluids
comprise reactants for
the fuel cell.
[311] Herein also disclosed is a method of making a fuel cell comprising (a)
forming an
impermeable interconnect having no fluid dispersing element; (b) forming an
electrolyte; (c)
forming a fluid dispersing component (FOC); and (d) placing the FDC between
the interconnect
and the electrolyte.
[312] In an embodiment, the FDC is formed by creating a multiplicity of
segments and
assembling the segments. The segments have different compositions, materials,
shapes, sizes,
densities, porosities, pore sizes, pore shapes, permeabilities, or
combinations thereof wherein
the shapes comprise a pillar, hollow cylinder, cube, rectangular cuboid,
trigonal trapezohedron,
quadrilateral frustum, parallelepiped, triangular bipyramid, tetragonal anti-
wedge, pyramid,
pentagonal pyramid, prism, or combinations thereof. The FOC may be a foam,
open cell foam;
or comprises a lattice structure.
[313] In preferred embodiments, the method of forming the FOC comprises
varying density,
porosity, pore size, pore shape, permeability, or combinations thereof. In an
embodiment, the
method comprises controlling the density, porosity, pore size, pore shape,
permeability, or
combinations thereof of the FDC. The method may comprise controlling density,
porosity, pore
size, pore shape, permeability, or combinations thereof of the FDC to adjust
flow of a fluid
through the FDC. The method may comprise controlling density, porosity, pore
size, pore
shape, permeability, or combinations thereof of the FDC to cause uniform fluid
flow from a first
point in the FDC to a second point in the FDC. The method may comprise
controlling density,
porosity, pore size, pore shape, permeability, or combinations thereof of the
FDC to cause
patterned fluid flow from a first point in the FDC to a second point in the
FDC.
[314] The fluid flow pattern may be adjusted as desired. For example, it does
not need to be
uniform. The fluid flow may be increased or decreased according to the
reactivities of the FDC or
reaction rates of the fluid in the various portions of the FDC. Alternatively
and/or in
combination, the fluid flow may be increased or decreased according to the
fluid flow rates to an
anode or a cathode in the various portions of the FDC. Alternatively and/or in
combination,
14
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
the fluid flow may be increased or decreased according to the reaction rates
in an anode or a
cathode related to or in contact with the various portions of the FOC.
[315] In an embodiment, step (c) comprises varying composition of material
used to form the
FDC. In an embodiment, step (c) comprises varying particles size used to form
the FDC. In an
embodiment, step (c) comprises heating different portions of the FDC to
different
temperatures. In an embodiment, said heating comprises electromagnetic
radiation (EMR). In
an embodiment, EMR comprises one or more of UV light, near ultraviolet light,
near infrared
light, infrared light, visible light, laser or electron beam.
[316] In an embodiment, steps (a)-(d) or steps (b)-(d) are performed using
additive
manufacturing (AM). In various embodiments, AM comprises extrusion,
photopolymerization,
powder bed fusion, material jetting, binder jetting, directed energy
deposition or lamination or
combinations thereof.
[317] In an embodiment, the method of forming the FDCs comprises heating the
fuel cell
such that shrinkage rates of the FDC and the electrolyte are matched or such
that shrinkage
rates of the interconnect, the FDC, and the electrolyte are matched. In a
preferred
embodiment, the heating comprises EMR. In an embodiment, EMR comprises UV
light, near
ultraviolet light, near infrared light, infrared light, visible light, laser
or electron beam or
combinations thereof. In a preferred embodiment, heating is performed in situ.
In preferred
embodiments, heating takes place for no greater than 30 minutes, or no greater
than 30
seconds, or no greater than 30 milliseconds.
[318] In a preferred embodiment, at least a portion of the FOC is part of an
anode or part of
a cathode. In a preferred embodiment, the FDC is an anode or a cathode. In
preferred
embodiments, the impermeable interconnect has a thickness of no greater than
10 microns, or
no greater than 1 micron, or no greater than 500 nm. Preferably, the
impermeable
interconnect comprises inlets and outlets for fluids. More preferably, the
fluids comprise
reactants for the fuel cell.
Channeled Electrodes
[319] Disclosed herein is a method comprising providing a template wherein the
template is in
contact with an electrode material; and removing at least a portion of the
template to form
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
channels in the electrode material, such as in an EC gas producer. Fig. 13A
illustrates a
template 1300 for making channeled electrodes, according to an embodiment of
the
disclosure. Such templates may be removed by oxidation, melting, vaporization,
reduction, or
any suitable means, either after the electrochemical reactor is made or at the
start of the
utilization of the reactor.
[320] In an embodiment, the channeled electrode material comprises NiO, YSZ,
GDC, LSM,
LSCF, or combinations thereof. The channeled electrode material may comprise
any material
previously described herein for a cathode or anode. In an embodiment,
providing a template
comprises printing the template or precursors that assemble to form the
template. Providing a
template comprises polymerizing one or more monomers or a photo-initiator, or
both. In an
embodiment, the method comprises curing monomers and/or oligomers, through
internal or
external techniques. In various embodiments, internal techniques include
polymerization by
free radical molecular initiation, and/or initiation by in situ
reduction/oxidation. In various
embodiments, external techniques include photolysis, exposure to ionizing
radiation,
(ultra)sonication and thermal decomposition to form the initiator species. In
a preferred
embodiment, said curing comprises UV curing. In an embodiment, the method
comprises
adding a polymerizing agent, wherein the polymerizing agent comprises a photo-
initiator. In
an embodiment, the polymerizing agent is printed on top of the monomer or
printed within
each slice of the monomer.
[321] In an embodiment, providing a template comprises dispersing metal oxide
particles in a
monomer ink before printing the template. In an embodiment, the metal oxide
comprises NiO,
CuO, LSM (lanthanum strontium manganite), LSCF (lanthanum strontium cobalt
ferrite), GDC
(gadolinium doped ceria), SDC (samaria-doped ceria), or combinations thereof.
In an embodiment,
said monomer comprises alcohol, aldehyde, carboxylic acid, ester, and/or ether
functional groups.
In an embodiment, said template comprises NiO, Cu(I)0, Cu(II)0, an organic
compound, a
photopolymer, or combinations thereof.
[322] In an embodiment, removing at least a portion of the template comprises
heating,
combustion, solvent treatment, oxidation, reduction, or combinations thereof.
In an embodiment,
the combustion leaves no deposits and is not explosive. In an embodiment, the
16
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
reduction takes place in a metal oxide and produces porous template. In an
embodiment, the
method of providing a template comprises heating in situ.
[3231 In an embodiment, the template and electrode material are printed slice
by slice and a
second slice is printed atop a first slice before the first slice is heated,
wherein the heating removes
at least a portion of the template. In an embodiment, the heating comprises
EMR. In an
embodiment, [MR comprises one or more of UV light, near ultraviolet light,
near infrared light,
infrared light, visible light, laser, electron beam.
[324] In an embodiment, the channels and the electrode material form an
electrode layer. In
an embodiment, the channels have regular trajectories within the electrode
layer. For example,
the channels are parallel to one another. The channels may run from one end,
edge, or corner
of the electrode layer to the opposite end, edge or corner. The channels may
run from one
end, edge or corner of the electrode, turn 90 degrees to another end, edge or
corner. The
channels have random trajectories within the electrode layer. For example, the
channels may
have tortuous trajectories with no regularities. The channels may have more
than one entry
point and more than one exit point. The more than one entry point and the more
than one exit
point are distributed across the electrode layer. The entry points and the
exits points of the
channels in the electrode layer may be on any side of the electrode layer,
including the top
surface or side and the bottom surface or side.
[325] In some embodiments, the volume fraction of the template in the
electrode layer is in the
range of 5%-95%, or 10%-90%, or 20%-80%, or 30%-70%, or 40%-60%. The volume
fraction of
the channels in the electrode layer is in the range of 10%-90%, or 20%-80%, or
30%-70%, or
40%-60%. The total effective porosity of the electrode layer with channels is
preferably in the
range of 20%-80%, or 30%-70%, or 40%-60%. Such total effective porosity of the
electrode layer
with channels is no less than the porosity of the electrode material. The
tortuosity of the electrode
layer with channels is no greater than the native tortuosity of the electrode
material.
[326] In preferred embodiments, the gas channels span the height of the
electrode layer. The
gas channels may occupy a height that is less than that of the electrode
layer. As an example, the
electrode layer is about 50 microns thick. In an embodiment, the gas channel
width is no less than
microns. In an embodiment, the gas channel width is no less than 100 microns.
17
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[327] Also discussed herein is a method comprising (a) printing a first
template and a first
electrode material to form a first electrode layer, wherein the first template
is in contact with
the first electrode material; (b) printing an electrolyte layer; (c) printing
a second template
and a second electrode material to form a second electrode layer, wherein the
second
template is in contact with the second electrode material; and (d) printing an
interconnect. In
a preferred embodiment, the steps are performed in any sequence. In a
preferred
embodiment, the method comprises repeating steps (a)-(d) in any sequence to
form a stack
or a repeat unit of a stack.
[328] In an embodiment, the method comprises (e) removing at least a portion
of the first
template and of the second template to form channels in the first and second
electrode layers.
In an embodiment, the removing comprises heating, combustion, solvent
treatment, oxidation,
reduction, or combinations thereof. In an embodiment, the removing takes place
in situ.
Removing may take place after a stack or a repeat unit of a stack is printed.
Removing may
take place when a stack is initiated to operate. In an embodiment, the
printing takes place
slice by slice and a second slice is printed atop a first slice before the
first slice is heated,
wherein the heating removes at least a portion of the template. The printing
step comprises
material jetting, binder jetting, inkjet printing, aerosol jetting, or aerosol
jet printing, or
combinations thereof.
[329] Further discussed herein is a method comprising (a) printing a first
electrode layer; (b)
printing an electrolyte layer; (c) printing a second electrode layer; and (d)
printing an
interconnect. In an embodiment, the printing comprises material jetting,
binder jetting, inkjet
printing, aerosol jetting, or aerosol jet printing. In a preferred embodiment,
the steps are
performed in any sequence. In a preferred embodiment, the method comprises
repeating
steps (a)-(d) in any sequence to form a stack or a repeat unit of a stack.
Also disclosed herein
is a method comprising aerosol jetting or aerosol jet printing an electrode
layer, or an
electrolyte layer, or an interconnect, or combinations thereof.
[330] Fig. 138 is a cross-sectional view of a half cell between a first
interconnect and an
electrolyte, according to an embodiment of the disclosure. The stack in Fig.
138 comprises a
bottom/first interconnect 1301, an optional layer that contains the bottom
interconnect
78
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
material and first electrode material 1302, first electrode segments 1303,
first filler materials that
form a first template 1304 and electrolyte 1305.
[331] Fig. 13C is a cross-sectional view of a half cell between a second
interconnect and an
electrolyte, according to an embodiment of the disclosure. The half cell
comprises an
electrolyte 1305, second electrode segments 1306, filler materials that forms
a second
template 1307 and a top/second interconnect 1308. The views shown in Fig. 138
and Fig. 13C
are perpendicular to one another.
[332] Fig. 13D is a cross-sectional view of a half cell between a first
interconnect and an
electrolyte, according to an embodiment of the disclosure. The half cell
comprises a bottom
interconnect 1301, an optional layer that contains the bottom interconnect
material and first
electrode material 1302, first electrode segments 1303, first filler materials
that forms a first
template 1304, electrolyte 1305 and optional shields 1409 for the first filler
materials when the first
electrode is heated and/or sintered.
[333] Fig. 13E is a cross-sectional view of a half cell between a second
interconnect and an
electrolyte, according to an embodiment of the disclosure. The half cell
comprises an
electrolyte 1305, second electrode segments 1306, filler materials that forms
a second
template 1307, top interconnect 1308 and optional shields for the second
filler materials when
the top interconnect is heated and/or sintered. The views shown in Fig. 13D
and Fig. 13E are
perpendicular to one another.
[334] In some embodiments, there is a layer between 1307 and 1308 (not shown)
that
contains the top interconnect material and second electrode material. In some
embodiments,
1305 represents an electrolyte with a barrier for the first electrode or for
second electrode.
1309 represents optional shields for the first fillers when the first
electrode is heated/sintered.
1310 represents optional shields for the second fillers when the top
interconnect is
heated/sintered. In some instances, electrolyte 1305 or electrolyte-barrier
layer is in contact
with the first electrode and the second electrode continuously along its
opposing major faces.
The shapes of the electrode segments and the fillers in these cross-sectional
views are only
representative and not exact. They may take on any regular or irregular
shapes. The fillers
and/or templates are removed when the electrochemical reactor is made (e.g., a
fuel cell stack
79
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
or a gas producer), for example, via heating in a furnace. Or alternatively,
they are removed
when the electrochemical reactor is initiated into operation via hot gas/fluid
passing through,
using the effects of oxidation, melting, vaporization, gasification,
reduction, or combinations
thereof. These removed fillers and/or templates become channels in the
electrodes. In various
embodiments, multiple tiers of channels are present in an electrode. For an
illustrative
example, an electrode is 25 microns thick with a multiplicity of channels
having a height of 20
microns. For another illustrative example, an electrode is 50 microns thick
with a multiplicity
of channels in 2 tiers, each tier of channels having a height of 20 microns.
In various
embodiments, the fillers comprise carbon, graphite, graphene, cellulose, metal
oxides,
polymethyl methacrylate, nano diamonds, or combinations thereof.
[335] In an embodiment, a unit in an electrochemical reactor comprising an
interconnect, a
first electrode, an electrolyte, and a second electrode is made via this
method: providing the
interconnect, depositing a first electrode material in segments on the
interconnect, sintering
the first electrode material, depositing a first filler material between the
first electrode material
segments, depositing additional first electrode material to cover the filler
material, sintering the
additional first electrode material and forming the first electrode,
depositing an electrolyte
material on the first electrode, sintering the electrolyte material to form
the electrolyte,
depositing a second electrode material on the electrolyte such that a
multiplicity of valleys are
formed in the second electrode material, sintering the second electrode
material to form the
second electrode, depositing a second filler material in the valleys of the
second electrode,
depositing a second interconnect material to cover the second electrode and
the second filler
material, and sintering the second interconnect material. In various
embodiments, deposition is
performed using inkjet printing or ultrasonic inkjet printing. In various
embodiments, sintering
is performed using electromagnetic radiation (EMR). In some cases, the first
and second filler
materials absorb little to no EMR; the absorption is so minimal that the
filler materials have no
measurable change. In some cases, shields are deposited to cover the first
filler material or the
second filler material or both so that the heating and/or sintering process
for the layer on top
does not cause measurable change in the first filler material or the second
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
filler material or both. In some cases, the shields comprise YSZ, SIX, SSZ,
CGO, NiO-YSZ, Cu,
CuO, Cu2O, LSM, LSCF, lanthanum chromite, stainless steel, LSGM, or
combinations thereof.
Dual Porosity Electrodes
[336] Figs. 14A-0 illustrates various embodiments of electrodes having dual
porosities with one,
two or three layers shown in detail that may be used in electrochemical
reactors such as EC gas
producers. Fig. 14A schematically illustrates segments of fluid dispersing
components in a first
layer, according to an embodiment of the disclosure. First layer 1400
comprises fluid dispersing
component segments 1402. Segments 1402 may have different compositions,
shapes, densities,
porosities, pore sizes, pore shapes, permeabilities, or combinations thereof.
Volume fraction of
channels (VFc) relative to layer 1400 containing the channels is also shown.
Herein discussed is
an electrode in an EC reactor comprising a material and channels, wherein the
material and
channels form a first layer in the electrode having a first layer porosity.
The material has a
material porosity. The channels have a volume fraction VFc, which is the ratio
between the
volume of the channels and the volume of the first layer. The first layer
porosity refers to the
average porosity of the first layer as a whole. The first layer porosity is at
least 5% greater than
the material porosity. The VFc is in the range of 0-99%, or 1-30%, or 10-90%,
or 550%, or 3-
30%, or 1-50%. The VFc is no less than 5%, or 10%, or 20%, or 30%, or 40%, or
50%.
[337] Fig. 148 schematically illustrates fluid dispersing components in a
first layer along with a
second layer in an electrode, according to an embodiment of the disclosure.
Electrode
embodiment in Fig. 14B shows a first layer 1404 of fluid dispersing component
segments 1405
and a second layer 1406. The segments, as shown in Fig. 148, may have
different compositions,
shapes, densities, porosities, pore sizes, pore shapes, permeabilities, or
combinations thereof.
The electrode comprises a second layer wherein the second layer has a second
layer porosity.
The second layer porosity refers to the average porosity of the second layer
as a whole. In an
embodiment, said second layer porosity is no greater than the first layer
porosity or the second
layer porosity is no less than the first layer porosity. The second layer 1406
may comprise the
same material as in the first layer. The second layer 1406 may also comprise
variabilities in
compositions, shapes, densities, porosities, pore sizes, pore shapes,
permeabilities, or
combinations thereof in the lateral direction or perpendicular to the lateral
direction.
81
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[338] Fig. 14D schematically illustrates fluid dispersing components in a
first layer 1408
along with a second layer 1412, according to an embodiment of the disclosure.
The
electrode embodiment in Fig. 140 is similar to the embodiment in Fig. 1413.
The electrode
in Fig. 140 comprises a first layer 1408 further comprising fluid dispersing
component
segments 1410, wherein segments 1410 may have different compositions, shapes,
densities,
porosities, pore sizes, pore shapes, permeabilities, or combinations thereof.
The second
layer 1412 may comprise the same material as in the first layer. The second
layer 1412 may
also comprise variabilities in compositions, shapes, densities, porosities,
pore sizes, pore
shapes, permeabilities, or combinations thereof in the lateral direction or
perpendicular to
the lateral direction.
[339] Fig. 14C schematically illustrates fluid dispersing components in a
first layer along with
a second and third layer, according to an embodiment of the disclosure.
Electrode embodiment
in Fig. 14C comprises a first layer 1414, second layer 1416 and a third layer
1418. In an
embodiment, the second layer and the third layer are on two sides of the first
layer. In an
embodiment, the second layer and the third layer are in continuous contact
with two sides of
the first layer. First layer 1414 may comprises segments 1420 that have
different
compositions, shapes, densities, porosities, pore sizes, pore shapes,
permeabilities, or
combinations thereof. The second layer or the third layer may comprise the
same material as
in the first layer. The second layer or the third layer may also comprise
variabilities in
compositions, shapes, densities, porosities, pore sizes, pore shapes,
permeabilities, or
combinations thereof in the lateral direction or perpendicular to the lateral
direction.
[340] In an embodiment, the material porosity of the first, second or third
layer is in the range of
20-60%, in the range of 30-50%, in the range of 30-40% or in the range of 25-
35%. In an
embodiment, the material porosity is no less than 25%, or 35%, or 45%.
[341] In an embodiment, the electrode has a thickness of no greater than 10
cm, or 5 cm, or 1
cm. In an embodiment, the electrode has a thickness of no greater than 8 mm,
or 5 mm, or 1 mm.
In an embodiment, the electrode has a thickness of no greater than 100
microns, or 80 microns, or
60 microns.
82
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[342] In an embodiment, contribution to the permeability of the first layer
from the channels is
greater than contribution to the permeability of the first layer from the
material. In an
embodiment, no less than 50%, or 70%, or 90% of the permeability of the first
layer is due to the
permeability of the channels. In an embodiment, permeability of the material
in the first layer is no
greater than 50%, or no greater than 10%, or no greater than 1%, or no greater
than 0.001% of
the permeability of the channels in the first layer.
[343] Herein disclosed is a method of making an electrically conductive
component (ECC) of
an electrochemical reactor (e.g., a fuel cell) comprising: (a) depositing on a
substrate a first
composition comprising a first pore former with a first pore former volume
fraction VFpl; (b)
depositing on the substrate a second composition comprising a second pore
former with a
second pore former volume fraction VFp2, wherein said first composition and
second
composition form a first layer in the ECC; and (c) heating the first layer
such that the first pore
former and the second pore former become empty spaces. In an embodiment, said
VFp1 is in
the range of 0-100%, or 10-90%, or 30-70%, or 50-100%, or 90-100%. In an
embodiment,
the VFp2 is in the range of 0-100%, or 0-70%, or 25-75%, or 30-60%. In an
embodiment, the
heating comprises reduction reactions or oxidation reactions, or both
reduction and oxidation
reactions.
[344] Fig. 15 is an illustrative example of an electrode having dual
porosities, according to an
embodiment of the disclosure. Fig. 15 shows EC component 1500 comprising a
channeled
electrode having dual porosities. Device 1500 comprises an anode gas inlet
1501, an anode gas
outlet 1502, a cathode gas inlet 1503, and a cathode gas outlet 1504. Exploded
view 1505 is a
view of a portion of a cathode layer. View 1506 is a closer view of the
cathode wherein view
1506 represents a slice through the cathode layer that is composed of cathode
1507. Cathode
1507 is a porous cathode that is formed using micro pore formers. Channels
1508 represents
channels formed from macro pore formers.
[345] In an embodiment, (a) and (b) are accomplished via printing, or via
extrusion, or via
additive manufacturing (AM), or via tape casting, or via spraying, or via
deposition, or via
sputtering, or via screen printing. In an embodiment, said additive
manufacturing comprises
83
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
extrusion, photopolymerization, powder bed fusion, material jetting, binder
jetting, directed
energy deposition, lamination.
[346] In an embodiment, the first pore former and the second pore former are
the same.
In an embodiment, the first pore former and the second pore former are
different. In an
embodiment, said first pore former or second pore former has an average
diameter in the
range of 10 nm to 1 mm or 100 nm to 100 microns or 500 nanometers to 50
microns. In an
embodiment, said first pore former or second pore former has a size
distribution. In an
embodiment, said first pore former or second pore former comprises carbon,
graphite,
polymethyl methacrylate (PMMA), cellulose, metal oxides, or combinations
thereof.
[347] In an embodiment, the method comprises repeating (a) and (b) to form a
second layer
in the [CC; and heating the second layer. In an embodiment, heating the second
layer takes
places at the same time as heating the first layer. In an embodiment, heating
the second layer
takes places at a different time as heating the first layer. In an embodiment,
heating the second
layer and heating the first layer have at least a portion of overlapping time
period. In an
embodiment, the method comprises repeating (a) and (b) to form a third layer
in the ECC; and
heating the third layer. In an embodiment, the second layer and the third
layer are on two sides
of the first layer. In an embodiment, heating the first, second, and third
layers is simultaneous.
Alternatively, the first, second, and third layers are heated at different
times. In an embodiment,
heating of the first, second, and third layers has overlapping time periods.
In an embodiment,
the first, second, or third layer is heated more than once.
[348] In an embodiment, at least a portion of the empty spaces caused by the
second pore former
or the first pore former or both become channels in the first layer. In an
embodiment, the channels
have a volume fraction VFc, which is the ratio between the volume of the
channels and the volume
of the first layer. In an embodiment, said VFc is in the range of 0-99% or 1-
30% or 10-90% or 5-
50% or 3-30% or 1-50%. In an embodiment, said VFc is no less than 5% or 10% or
20% or 30% or
40% or 50%.
[349] In an embodiment, VFpl is different from VFp2. In an embodiment, said
first layer has
dual porosities, a material porosity and a layer porosity. In an embodiment,
the material
84
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
porosity is in the range of 20-60%, or 30-50%, or 30-40%, or 25-35%. In an
embodiment, the
material porosity is no less than 25% or 35% or 45%.
[350] In an embodiment, the ECC has a thickness of no greater than 10 cm or 5
cm or 1 cm.
In an embodiment, the ECC a thickness of no greater than 8 mm or 5 mm or 1 mm.
In an
embodiment, the ECC has a thickness of no greater than 100 microns or 80
microns or 60
microns.
[351] In an embodiment, the first layer comprises channels and material after
(c), wherein
contribution to the permeability of the first layer from the channels is
greater than contribution
to the permeability of the first layer from the material. In an embodiment, no
less than 50% or
70% or 90% of the permeability of the first layer is due to the permeability
of the channels. In
an embodiment, permeability of the material in the first layer is no greater
than 50% or no
greater than 10% or no greater than 1% or no greater than 0.001% of the
permeability of the
channels in the first layer.
[352] Herein discussed is a method comprising: (a) providing a first material
to an additive
manufacturing machine (AMM); (b) providing a second material to the AMM; (c)
mixing the first
material and the second material into a mixture; and (d) forming said mixture
into a part. In an
embodiment, said first material or second material is a gas, or liquid, or
solid, or gel.
[353] In an embodiment, said additive manufacturing comprises extrusion,
photopolymerization, powder bed fusion, material jetting, binder jetting,
directed energy
deposition, lamination. In an embodiment, said AM comprises direct metal laser
sintering
(DMLS), selective laser sintering (SLS), selective laser melting (SLM),
directed energy
deposition (DED), laser metal deposition (LMD), electron beam (EBAM), or metal
binder jetting.
In an embodiment, steps (c) and (d) take place continuously.
[354] In an embodiment, step (c) comprises varying the ratio of the first
material and the
second material in the mixture. In an embodiment, the ratio of the first
material and the
second material in the mixture is varied in situ. In an embodiment, the ratio
of the first
material and the second material in the mixture is varied in real time. In an
embodiment, the
ratio of the first material and the second material in the mixture is varied
continuously. In an
embodiment, the ratio of the first material and the second material in the
mixture is varied
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
according to a composition profile. In an embodiment, the ratio of the first
material and the
second material in the mixture is varied according to a manual algorithm, a
computational
algorithm, or a combination thereof. In an embodiment, the ratio of the first
material and the
second material in the mixture is varied by controlling material flow rates or
pumping rates.
[355] In an embodiment, step (d) comprises placing said mixture in a pattern
on a substrate.
In an embodiment, step (d) comprises placing said mixture according to pre-
defined
specifications.
[356] In an embodiment, the formed part has varying properties. In an
embodiment, the
properties comprise strength, weight, density, electrical performance,
electrochemical performance, or combinations thereof. In various embodiments,
he formed part
possesses superior properties, such as strength, density, weight, electrical
performance, or
electrochemical performance, or combinations thereof, when compared with a
similar part formed
by a different process.
[357] In an embodiment, step (d) comprises depositing said mixture on a
substrate. In an
embodiment, mixing takes place prior to deposition, during deposition, or
after deposition. In an
embodiment, mixing takes place in the AMM or in the air or on the substrate.
In an embodiment,
mixing takes place via advection, dispersion, diffusion, melting, fusion,
pumping, stirring, heating,
or combinations thereof.
[358] Herein disclosed is an additive manufacturing machine (AMM) comprising:
(a) a first
material source; (b) a second material source; and (c) a mixer configured to
mix the first
material and the second material into a mixture; wherein said AMM is
configured to form said
mixture into a part. In an embodiment, said first material or second material
is a gas, or liquid,
or solid, or gel.
[359] In an embodiment, said AMM is configured to perform extrusion,
photopolymerization,
powder bed fusion, material jetting, binder jetting, directed energy
deposition, or lamination. In
an embodiment, said AMM is configured to perform direct metal laser sintering
(DMLS), selective
laser sintering (SLS), selective laser melting (SLM), directed energy
deposition (DED), laser metal
deposition (LMD), electron beam (HAM), or metal binder jetting.
86
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[360] In an embodiment, said mixer is configured to mix the first material and
the second
material continuously while the AMM forms said mixture into a part. In an
embodiment, said
mixer is configured to vary the ratio of the first material and the second
material in the mixture.
In an embodiment, said mixer is configured to vary the ratio of the first
material and the
second material in the mixture in situ. The mixer may be configured to vary
the ratio of the first
material and the second material in the mixture in real time. In an
embodiment, the mixer can
be configured to vary the ratio of the first material and the second material
in the mixture
continuously. In an embodiment, the mixer is configured to vary the ratio of
the first material
and the second material in the mixture according to a composition profile. In
an embodiment,
the mixer is configured to vary the ratio of the first material and the second
material in the
mixture according to a manual algorithm, a computational algorithm, or a
combination thereof.
In an embodiment, said mixer is configured to vary the ratio of the first
material and the
second material in the mixture by controlling material flow rates or pumping
rates.
[361] In an embodiment, said AMM is configured to place said mixture in a
pattern on a
substrate. In an embodiment, said AMM is configured to place said mixture
according to pre-
defined specifications.
[362] In an embodiment, the formed part has varying properties. In an
embodiment, the
properties comprise strength, weight, density, electrical performance,
electrochemical performance, or combinations thereof. In various embodiments,
he formed part
possesses superior properties, such as strength, density, weight, electrical
performance, or
electrochemical performance, or combinations thereof, when compared with a
similar part formed
using a different apparatus.
[363] In an embodiment, the AMM is configured to deposit said mixture on a
substrate. In an
embodiment, mixing takes place prior to deposition, during deposition, or
after deposition. In an
embodiment, mixing takes place in the AMM or in the air or on the substrate.
In an embodiment,
mixing takes place via advection, dispersion, diffusion, melting, fusion,
pumping, stirring, heating,
or combinations thereof.
87
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
Integrated Deposition and Heating
[364] Disclosed herein is a method comprising depositing a composition on a
substrate slice by
slice (this may also be described as line-by-line deposition) to form an
object; heating in situ the
object using electromagnetic radiation (EMR); wherein said composition
comprises a first
material and a second material, wherein the second material has a higher
absorbance of EMR
than the first material. In various embodiments, heating may cause an effect
comprising drying,
curing, sintering, annealing, sealing, alloying, evaporating, restructuring,
foaming or
combinations thereof. In some embodiments, the EMR has a peak wavelength
ranging from 10
to 1500 nm and a minimum energy density of 0.1 Joule/crn2 wherein the peak
wavelength is on
the basis of irradiance with respect to wavelength. In some embodiments, the
EMR comprises
one or more of UV light, near ultraviolet light, near infrared light, infrared
light, visible light,
laser or electron beam.
[365] Fig. 16 illustrates a system for integrated deposition and heating using
electromagnetic radiation (EMR), according to an embodiment of the disclosure.
The system
1600 may be used to assemble an electrochemical reactor such as a fuel cell or
EC gas
producer. Fig. 16 further illustrates system 1600 an object 1603 on a receiver
1604 formed
by deposition nozzles 1601 and EMR 1602 for heating in situ, according to an
embodiment of
this disclosure. Receiver 1604 may be a platform that moves and may further
receive
deposition, heat, irradiation, or combinations thereof. Receiver 1604 may also
be referred to
as a chamber wherein the chamber may be completely enclosed, partially
enclosed or
completely open to the atmosphere.
[366] In some embodiments, the first material comprises yttria-stabilized
zirconia (YSZ), 8YSZ
(8m01% YSZ powder), yttrium, zirconium, gadolinia-doped ceria (GDC or CGO),
samaria-doped
ceria (SDC), scandia-stabilized zirconia (SSZ), lanthanum strontium manganite
(LSM), lanthanum
strontium cobalt ferrite (LSCF), lanthanum strontium cobaltite (LSC),
lanthanum strontium
gallium magnesium oxide (LSGM), nickel, NiO, NiO-YSZ, Cu-CGO, Cu2O, CuO,
cerium, copper,
silver, crofer, steel, lanthanum chromite, doped lanthanum chromite, ferritic
steel, stainless steel
or combinations thereof. In other embodiments, the first material comprises
YSZ, SSZ, CGO,
SDC, NiO-YSZ, LSM-YSZ, CGO-LSCF, doped lanthanum chromite, stainless steel or
88
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
combinations thereof. In some embodiments, the second material comprises
carbon, nickel
oxide, nickel, silver, copper, CGO, SDC, NiO-YSZ, NiO-SSZ, LSCF, LSM, doped
lanthanum
chromite ferritic steels or combinations thereof. The first material may
comprise any electrode
material previously disclosed herein.
[367] In some embodiments, object 1603 comprises a catalyst, a catalyst
support, a catalyst
composite, an anode, a cathode, an electrolyte, an electrode, an interconnect,
a seal, a fuel cell, an
electrochemical gas producer, an electrolyser, an electrochemical compressor,
a reactor, a heat
exchanger, a vessel or combinations thereof.
[368] In some embodiments, the second material may be deposited in the same
slice as the
first material. In other embodiments, the second material may be deposited in
a slice
adjacent another slice that contains the first material. In some embodiments,
said heating
may remove at least a portion of the second material. In preferred
embodiments, said
heating leaves minimal residue of the second material such that there is no
significant
residue that would interfere with the subsequent steps in the process or the
operation of the
device being constructed. More preferably, this leaves no measurable reside of
the portion of
the second material.
[369] In some embodiments, the second material may add thermal energy to the
first material
during heating. In other embodiments, the second material has a radiation
absorbance that is at
least 5 times that of the first material; the second material has a radiation
absorbance that is at
least 10 times that of the first material; the second material has a radiation
absorbance that is
at least 50 times that of the first material or the second material has a
radiation absorbance that
is at least 100 times that of the first material.
[370] In some embodiments, the second material may have a peak absorbance
wavelength no
less than 200 nm, or 250 nm, or 300 nm, or 400 nm, or 500 nm. In other
embodiments, the first
material has a peak absorbance wavelength no greater than 700 nm, or 600 nm,
or 500 nm, or
400 nm, or 300 nm. In other embodiments, the [MR has a peak wavelength no less
than 200
nm, or 250 nm, or 300 nm, or 400 nm, or 500 nm.
[371] In some embodiments, the second material may comprise carbon, nickel
oxide, nickel,
silver, copper, CGO, NIO-YSZ, LSCF, LSM, ferritic steels, other metal oxides
or combinations
89
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
thereof. In some cases, the ferritic steel is Crofer 22 APU. In some
embodiments, the first
material comprises YSZ, CGO, NiO-YSZ, LSM-YSZ, other metal oxides or
combinations thereof.
In an embodiment, the second material comprises LSCF, LSM, carbon, nickel
oxide, nickel,
silver, copper, or steel. In some embodiments, carbon comprises graphite,
graphene, carbon
nanoparticles, nano diamonds or combinations thereof. The second material may
comprise any
electrode material previously disclosed herein.
[372] In some embodiments, the deposition method comprises material jetting,
binder jetting,
inkjet printing, aerosol jetting, aerosol jet printing, vat
photopolymerization, powder bed fusion,
material extrusion, directed energy deposition, sheet lamination, ultrasonic
inkjet printing or
combinations thereof.
[373] In some embodiments, the deposition method further comprises one or more
of the steps
of controlling distance from the EMR to the receiver, EMR energy density, EMR
spectrum, EMR
voltage, EMR exposure duration, EMR exposure area, EMR exposure volume, EMR
burst
frequency, EMR exposure repetition number. In an embodiment, the object does
not change
location between the deposition and heating steps. In an embodiment, the EMR
has a power
output of no less than 1 W, or 10 W, or 100 W, or 1000 W.
[374] Also disdosed herein is a system comprising at least one deposition
nozzle, an
electromagnetic radiation (EMR) source and a deposition receiver, wherein the
deposition
receiver is configured to receive EMR exposure and deposition at the same
location. In some
cases, the receiver is configured such that it receives deposition for a first
time period, moves to
a different location in the system to receive EMR exposure for a second time
period.
[375] The following detailed description describes the production of solid
oxide fuel cells
(SOFCs) for illustrative purposes. As one in the art recognizes, the
methodology and the
manufacturing processes are applicable to all fuel cell types. As such, the
production of all fuel
cell types is within the scope of this disclosure.
Additive Manufacturing
[376] Additive manufacturing (AM) refers to a group of techniques that join
materials to
make objects, usually slice by slice or layer upon layer. AM is contrasted to
subtractive
manufacturing methodologies, which involve removing sections of a material by
machining,
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
cutting, grinding or etching away. AM may also be referred to as additive
fabrication, additive
processes, additive techniques, additive layer manufacturing, layer
manufacturing or freeform
fabrication. Some examples of AM are extrusion, photopolymerization, powder
bed fusion,
material jetting, binder jetting, directed energy deposition, lamination,
direct metal laser
sintering (DMLS), selective laser sintering (SLS), selective laser melting
(SLM), directed energy
deposition (OED), laser metal deposition (LMD), electron beam (EBAM) and metal
binder
jetting. A 3D printer is a type of AM machine (AMM). An inkjet printer or
ultrasonic inkjet
printer are additional examples of AMMs.
[377] In a first aspect, the invention is a method of making an
electrochemical reactor such
as a EC gas producer or a fuel cell comprising: (a) producing an anode using
an AMM; (b)
creating an electrolyte using the AMM; and (c) making a cathode using the AMM.
In preferred
embodiments, the anode, the electrolyte and the cathode are assembled into a
fuel cell
utilizing an AMM in addition to other steps that are not completed using an
AMM. In a
preferred embodiment, the fuel cell is formed using only the AMM. In other
embodiments,
steps (a), (b), and (c) exclude tape casting and screen printing. In an
embodiment, the
method of assembling a fuel cell with an AMM excludes compression in
assembling. In other
embodiments, the layers are deposited one on top of another in a step-wise
manner such that
assembling is accomplished at the same time as deposition. The methods
described herein are
useful in making planar fuel cells. The methods described herein are also
useful in making fuel
cell, wherein electrical current flow is perpendicular to the electrolyte in
the lateral direction
when the fuel cell is in use.
[378] In an embodiment, the interconnect, the anode, the electrolyte, and the
cathode are
formed layer on layer, for example, printed layer on layer. It is important to
note that,
within the scope of the invention, the order of forming these layers can be
varied. In other
words, either the anode or the cathode can be formed before the other.
Naturally, the
electrolyte is formed so that it is between the anode and the cathode. Barrier
layer(s),
catalyst layer(s) and interconnect(s) are formed so as to lie in the
appropriate position
within the fuel cell to perform their functions.
91
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[3791 In some embodiments, each of the interconnect, the anode, the
electrolyte and the
cathode have six faces. In preferred embodiments, the anode is printed on the
interconnect
and is in contact with the interconnect; the electrolyte is printed on the
anode and is in contact
with the anode; the cathode is printed on the electrolyte and is in contact
with the electrolyte.
Each print may be sintered, for example, using EMR. As such, the assembly
process and the
forming process are simultaneous, which is not possible with conventional
methods.
Moreover, with the preferred embodiment, the needed electrical contact and gas
tightness are
also achieved at the same time. In contrast, conventional fuel cell assembly
processes
accomplish this via pressing or compression of the fuel cell components or
layers. The pressing
and compression processes can cause cracks in the fuel cell layers that are
undesirable.
[380] In some embodiments, the AM method comprises making at least one barrier
layer
using the AMM. In preferred embodiments, the at least one barrier layer may be
located
between the electrolyte and the cathode or between the electrolyte and the
anode or both.
In other embodiments, the at least one barrier layer may be assembled with the
anode, the
electrolyte and the cathode using the AMM. In some embodiments, no barrier
layer is
needed or utilized in the fuel cell.
[3811 In some embodiments, the AM method comprises making an interconnect
using the
AMM. In other embodiments, the interconnect may be assembled with the anode,
the
electrolyte and the cathode using the AMM. In some embodiments, the AMM forms
a catalyst
and incorporates said catalyst into the fuel cell.
[3821 In some embodiments, the anode, the electrolyte, the cathode and the
interconnect are
made at a temperature above 100 C. In some embodiment, the AM method
comprises heating
the fuel cell, wherein said fuel cell comprises the anode, the electrolyte,
the cathode, the
interconnect and optionally at least one barrier layer. In some embodiments,
the fuel cell
comprises a catalyst. In some embodiments, the method comprises heating the
fuel cell to a
temperature above 500 C. In some embodiments, the fuel cell is heated using
one or both of
[MR or oven curing.
[3831 In a preferred embodiment, the AMM utilizes a multi-nozzle additive
manufacturing
method. In a preferred embodiment, the multi-nozzle additive manufacturing
method
92
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
comprises nanoparticle jetting. In some embodiments, a first nozzle delivers a
first material, a
second nozzle delivers a second material, a third nozzle delivers a third
material. In some
embodiments, particles of a fourth material are placed in contact with a
partially constructed
fuel cell and bonded to the partially constructed fuel cell using a laser,
photoelectric effect,
light, heat, polymerization or binding. In an embodiment, the anode, the
cathode or the
electrolyte comprises a first, second, third or fourth material. In preferred
embodiments, the
AMM performs multiple AM techniques. In various embodiments, the AM techniques
comprise
one or more of extrusion, photopolymerization, powder bed fusion, material
jetting, binder
jetting, directed energy deposition or lamination. In various embodiments, AM
is a deposition
technique comprising material jetting, binder jetting, inkjet printing,
aerosol jetting, or aerosol
jet printing, vat photopolymerization, powder bed fusion, material extrusion,
directed energy
deposition, sheet lamination, ultrasonic inkjet printing or combinations
thereof.
[384] Further described herein is an AM method of making a fuel cell stack
comprising: (a)
producing an anode using an additive manufacturing machine (AMM); (b) creating
an electrolyte
using the AMM; (c) making a cathode using the AMM; (d) making an interconnect
using the AMM;
wherein the anode, the electrolyte, the cathode, and the interconnect form a
first fuel cell; (e)
repeating steps (a)-(d) to make a second fuel cell; and (f) assembling the
first fuel cell and the
second fuel cell into a fuel cell stack.
[385] In some embodiments, the first fuel cell and the second fuel cell are
formed from the
anode, the electrolyte, the cathode and the interconnect utilizing the AMM. In
an embodiment,
the fuel cell stack is formed using only the AMM. In other embodiments, steps
(a)-(f) exclude
one or both of tape casting and screen printing.
[386] In some embodiments, the AM method comprises making at least one barrier
layer using
the AMM. In some embodiments, the at least one barrier layer is located
between the electrolyte
and the cathode or between the electrolyte and the anode or both for the first
fuel cell and the
second fuel cell.
[387] In some embodiments, steps (a)-(d) are performed at a temperature above
100 C. In
other embodiments, steps (a)-(d) are performed at a temperature in the range
of 100 C to 500
93
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
C. In some embodiments, the AMM makes a catalyst and incorporates said
catalyst into the fuel
cell stack.
[388] In some embodiments, the AM method comprises heating the fuel cell
stack. In an
embodiment, the AM method comprises heating the fuel cell stack to a
temperature above
500 C. In some embodiments, the fuel cell stack is heated using EMR and/or
oven curing. In
some embodiments, the laser has a laser beam, wherein the laser beam is
expanded to create
a heating zone with uniform power density. In some embodiments, the laser beam
is
expanded by utilizing one or more mirrors. In some embodiments, each layer of
the fuel cell
may be cured separately by EMR. In some embodiments, a combination of one or
more fuel
cell layers may be cured together by EMR. In some embodiments, the first fuel
cell is EMR
cured, assembled with the second fuel cell, and then the second fuel cell is
EMR cured. In
other embodiments, the first fuel cell is assembled with the second fuel cell,
and then the
first fuel cell and the second fuel cell are cured separately by EMR. In some
embodiments,
the first fuel cell and the second fuel cell may be cured separately by EMR,
and then the first
fuel cell is assembled with the second fuel cell to form a fuel cell stack. In
some
embodiments, the first fuel cell is assembled with the second fuel cell to
form a fuel cell
stack, and then the fuel cell stack may be cured by EMR.
[389] Also discussed herein is an AM method of making a multiplicity of fuel
cells
comprising (a) producing a multiplicity of anodes simultaneously using an
additive
manufacturing machine (AMM); (b) creating a multiplicity of electrolytes using
the AMM
simultaneously; and (c) making a multiplicity of cathodes using the AMM
simultaneously. In
preferred embodiments, the anodes, the electrolytes and cathodes are assembled
into fuel
cells utilizing the AMM simultaneously. In other preferred embodiments, the
fuel cells are
formed using only the AMM.
[390] In some embodiments, the method comprises making at least one barrier
layer using
the AMM for each of the multiplicity of fuel cells simultaneously. The at
least one barrier layer
may be located between the electrolyte and the cathode or located between the
electrolyte
and the anode, or both. In preferred embodiments, the at least one barrier
layer may be
assembled with the anode, the electrolyte and the cathode using the AMM for
each fuel cell.
94
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[391] In some embodiments, the method comprises making an interconnect using
the AMM
for each of the multiplicity of fuel cells simultaneously. The interconnect
may be assembled with
the anode, the electrolyte and the cathode using the AMM for each fuel cell.
In other
embodiments, the AMM forms a catalyst for each of the multiplicity of fuel
cells simultaneously
and incorporates said catalyst into each of the fuel cells. In other
embodiments, heating each
layer or heating a combination of layers of the multiplicity of fuel cells
takes place
simultaneously. The multiplicity of fuel cells may include two or more fuel
cells.
[392] In preferred embodiments, the AMM uses two or more different nozzles to
jet or print
different materials at the same time. For a first example, in an AMM, a first
nozzle deposits an
anode layer for fuel cell 1, a second nozzle deposits a cathode layer for fuel
cell 2 and a third
nozzle deposits an electrolyte for fuel cell 3, at the same time. For a second
example, in an AMM,
a first nozzle deposits an anode for fuel cell 1, a second nozzle deposits a
cathode for fuel cell 2, a
third nozzle deposits an electrolyte for fuel cell 3 and a fourth nozzle
deposits an interconnect for
fuel cell 4, at the same time.
[393] Disclosed herein is an additive manufacturing machine (AMM) comprising a
chamber
wherein manufacturing of fuel cells takes place. Said chamber is able to
withstand temperatures
of at least 100 C. In an embodiment, said chamber enables production of the
fuel cells. The
chamber enables heating of the fuel cells in situ as the components of the
fuel cell are being
deposited.
[394] In some embodiments, the chamber may be heated by laser, electromagnetic
waves/electromagnetic radiation (EMR), hot fluid or a heating element
associated with the
chamber, or combinations thereof. The heating element may comprise a heated
surface,
heating coil or a heating rod. In other embodiments, said chamber may be
configured to apply
pressure to the fuel cells inside. The pressure may be applied via a moving
element associated
with the chamber. The moving element may be a moving stamp or plunger. In some
embodiments, said chamber may be configured to withstand pressure. the chamber
may be
configured to be pressurized or depressurized by a fluid. The fluid in the
chamber may be
changed or replaced when needed.
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[395] In some cases, the chamber may be enclosed. In some cases, the chamber
may be
sealed. In some cases, the chamber may be open to ambient atmosphere or to a
controlled
atmosphere. In some cases, the chamber may be a platform without top and side
walls.
[396] Referring to Fig. 16, system 1600 comprises deposition nozzles or
material jetting
nozzles 1601, EMR source 1602 (e.g., xenon lamp), object being formed 1603,
and chamber or
receiver 1604 as a part of an AMM. As illustrated in Fig. 16, the chamber or
receiverl 604 is
configured to receive both deposition from nozzles and radiation from EMR
source 1602. In
various embodiments, deposition nozzles 1601 may be movable. In various
embodiments, the
chamber or receiver 1604 may be movable. In various embodiments, EMR source
1602 is
movable. In various embodiments, the object comprises a catalyst, a catalyst
support, a
catalyst composite, an anode, a cathode, an electrolyte, an electrode, an
interconnect, a seal, a
fuel cell, an electrochemical gas producer, an electrolyser, an
electrochemical compressor, a
reactor, a heat exchanger, a vessel or combinations thereof.
[397] AM techniques suitable for this disclosure comprise extrusion,
photopolymerization,
powder bed fusion, material jetting, binder jetting, directed energy
deposition and lamination. In
some embodiments, extrusion may be used for AM. Extrusion AM involves the
spatially controlled
deposition of material (e.g., thermoplastics). Extrusion AM may also be
referred to as fused
filament fabrication (FFF) or fused deposition modeling (FDM) in this
disclosure.
[398] In some embodiments, AM comprises photopolymerization
stereolithography
(SLA)) for the process of this disclosure. SLA involves spatially-defined
curing of a photoactive
liquid (a "photoresin"), using a scanning laser or a high-resolution projected
image, and
transforming the photoactive liquid into a crosslinked solid.
Photopolymerization can produces
parts with details and dimensions ranging from the micrometer- to meter-
scales.
[399] In some embodiments, AM comprises powder bed fusion (PBF). PBF AM
processes build
objects by melting powdered feedstock, such as a polymer or metal. PBF
processes begin by spreading
a thin layer of powder across a build area. Cross-sections are then melted a
layer at a time, most often
using a laser, electron beam or intense infrared lamps. In some embodiments,
PBF of metals may use
selective laser melting (SLM) or electron beam melting (EBM). In other
embodiments, PBF of polymers
may use selective laser sintering (SLS). In various embodiments,
96
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
SLS systems may print thermoplastic polymer materials, polymer composites or
ceramics. In
various embodiments, SLM systems may be suitable for a variety of pure metals
and alloys,
wherein the alloys are compatible with rapid solidification that occurs in
SLM.
[400] In some embodiments, AM may comprise material jetting. AM by material
jetting may
be accomplished by depositing small drops (or droplets) of material with
spatial control. In
various embodiments, material jetting is performed three dimensionally (3D),
two
dimensionally (20) or both. In preferred embodiments, 3D jetting is
accomplished layer by
layer. In preferred embodiments, print preparation converts the computer-aided
design
(CAD), along with specifications of material composition, color, and other
variables to the
printing instructions for each layer. Binder jetting AM involves inkjet
deposition of a liquid
binder onto a powder bed. In some cases, binder jetting is combined with other
AM
processes, such as for example, spreading of powder to make the powder bed
(analogous to
SLS/SLM) and inkjet printing.
[401] In some embodiments, AM comprises directed energy deposition (DED).
Instead of
using a powder bed as discussed above, the DEO process uses a directed flow of
powder or a
wire feed, along with an energy intensive source such as laser, electric arc
or electron beam.
In preferred embodiments, DED is a direct-write process, wherein the location
of material
deposition is determined by movement of the deposition head which allows large
metal
structures to be built without the constraints of a powder bed.
[402] In some embodiments, AM comprises lamination AM or laminated object
manufacturing (LOM). In preferred embodiments, consecutive layers of sheet
material are
consecutively bonded and cut in order to form a 3D structure.
[403] Traditional methods of manufacturing a fuel cell stack can comprise over
100 steps.
These steps may include, but not limited to, milling, grinding, filtering,
analyzing, mixing,
binding, evaporating, aging, drying, extruding, spreading, tape casting,
screen printing,
stacking, heating, pressing, sintering and compressing. The methods disclosed
herein describe
manufacturing of a fuel cell or fuel cell stack using one AMM.
[4043 The AMM of this disclosure preferably performs both extrusion and ink
jetting to
manufacture a fuel cell or fuel cell stack. Extrusion may be used to
manufacture thicker layers
97
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
of a fuel cell, such as, the anode and/or the cathode. Ink jetting may be used
to manufacture thin
layers of a fuel cell. Ink jetting may be used to manufacture the electrolyte.
The AMM may operate
at temperature ranges sufficient to enable curing in the AMM itself. Such
temperature ranges are
100 C or above, 100- 300 C or 100 - 500 C.
[405] As a preferred example, all layers of a fuel cell are formed and
assembled via printing.
The material for making the anode, cathode, electrolyte and the interconnect,
respectively,
may be made into an ink form comprising a solvent and particles (e.g.,
nanoparticles). There
are two categories of ink formulations ¨ aqueous inks and non-aqueous inks. In
some cases,
the aqueous ink comprises an aqueous solvent (e.g., water, deionized water),
particles,
dispersant and a surfactant. In some cases, the aqueous ink comprises an
aqueous solvent,
particles, dispersant, surfactant but no polymeric binder. The aqueous ink may
optionally
comprise a co-solvent, such as an organic miscible solvent (methanol, ethanol,
isopropyl
alcohol). Such co-solvents preferably have a lower boiling point than water.
The dispersant
may be an electrostatic dispersant, steric dispersant, ionic dispersant, or a
non-ionic
dispersant, or a combination thereof. The surfactant may preferably be non-
ionic, such as an
alcohol alkoxylate or an alcohol ethoxylate. The non-aqueous ink may comprise
an organic
solvent (e.g., methanol, ethanol, isopropyl alcohol, butanol) and particles.
[406] For example, CGO powder is mixed with water to form an aqueous ink
further
comprising a dispersant and a surfactant but with no polymeric binder added.
The CGO
fraction based on mass (herein expressed as weight % (wt%)) is in the range of
10 wt% to 25
wt%. For example, CGO powder is mixed with ethanol to form a non-aqueous ink
further
comprising polyvinyl butaryl added with the CGO fraction in the range of 3 wt%
to 30 wt%.
For example, LSCF is mixed with n-butanol or ethanol to form a non-aqueous ink
further
comprising polyvinyl butaryl with the LSCF fraction in the range of 10 wt% to
40 wt%. For
example, YSZ particles are mixed with water to form an aqueous ink further
comprising a
dispersant and surfactant but with no polymeric binder added. The YSZ fraction
is in the range
of 3 wt% to 40 wt%. For example, Ni0 particles are mixed with water to form an
aqueous ink
further comprising a dispersant and surfactant but with no polymeric binder
added with the
NiO fraction in the range of 5 wt% to 25 wt%.
98
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
(4073 As an example, for the cathode of a fuel cell, LSCF or LSM particles are
dissolved in a
solvent, wherein the solvent is water or an alcohol (e.g., butanol) or a
mixture of alcohols.
Organic solvents other than alcohols may also be used in other examples. As an
example, LSCF
is deposited (e.g., printed) into a layer. A xenon lamp may be used to
irradiate the LSCF layer
with EMR to sinter the LSCF particles. The xenon flash lamp may be a 10 kW
unit applied at a
voltage of 400V and a frequency of 10 Hz for a total exposure duration of 1000
ms.
[408] For example, for the electrolyte, YSZ particles are mixed with a
solvent, wherein the
solvent is water (e.g., de-ionized water) or an alcohol (e.g., butanol) or a
mixture of alcohols.
Organic solvents other than alcohols may also be used in other examples. For
the interconnect,
metallic particles (e.g., silver nanoparticles) are dissolved in a solvent,
wherein the solvent may
comprise water (e.g., de-ionized water) and an organic solvent. The organic
solvent may
comprise mono-, di-, or tri-ethylene glycols or higher ethylene glycols,
propylene glycol, 1,4-
butanediol or ethers of such glycols, thiodiglycol, glycerol and ethers and
esters thereof,
polyglycerol, mono-, di-, and tri-ethanolamine, propanolamine, N,N-
dimethylformamide,
dimethyl sulfoxide, dimethylacetamide, N-methylpyrrolidone, 1,3-
dimethylimidazolidone,
methanol, ethanol, isopropanol, n-propanol, diacetone alcohol, acetone, methyl
ethyl ketone or
propylene carbonate, or combinations thereof. For a barrier layer in a fuel
cell, CGO particles are
dissolved in a solvent, wherein the solvent may be water (e.g., de-ionized
water) or an alcohol.
The alcohol may comprise methanol, ethanol, butanol or a mixture of alcohols.
Organic solvents
other than alcohols may also be used. CGO may be used as barrier layer for
LSCF. YSZ may also
be used as a barrier layer for LSM. In some cases, for the aqueous inks where
water is the
solvent, no polymeric binder may be added to the aqueous inks.
[409] The manufacturing process of a conventional fuel cell sometimes
comprises more than
100 steps and utilizing dozens of machines. According to an embodiment of this
disclosure, a
method of making a fuel cell comprises using only one AMM to manufacture a
fuel cell, wherein
the fuel cell comprises an anode, electrolyte and a cathode. In preferred
embodiments, the fuel
cell comprises at least one barrier layer, for example, between the
electrolyte and the cathode,
or between the electrolyte and the cathode, or both. The at least one barrier
layer is preferably
also made by the same AMM. In preferred embodiments, the AMM may also
99
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
produce an interconnect and assembles the interconnect with the anode,
cathode, at least one
barrier layer and the electrolyte. Such manufacturing methods and systems are
applicable not
only to making fuel cells but also for making other types of electrochemical
devices. The
following discussion uses fuel cells as an example, but any reactor or
catalyst is within the scope
of this disclosure.
[410] In various embodiments, a single AMM makes a first fuel cell, wherein
the fuel cell
comprises an anode, electrolyte, cathode, at least one barrier layer and an
interconnect. In
various embodiments, a single AMM makes a second fuel cell. In various
embodiments, a single
AMM is used to assemble a first fuel cell with a second fuel cell to form a
fuel cell stack. In
various embodiments, the production of fuel cells using an AMM is repeated as
many times as
desired. A fuel cell stack comprising two or more fuel cells is thus assembled
using an AMM. In
some embodiments, the various layers of the fuel cell are produced by an AMM
above ambient
temperature. For example, the temperatures may be above 100 C, in the range
of 100 C to
500 C or in the range of 100 C to 300 C. In various embodiments, a fuel
cell or fuel cell stack
is heated after it is assembled. In some embodiments, the fuel cell or fuel
cell stack is heated at
a temperature above 500 C. In preferred embodiments, the fuel cell or fuel
cell stack is heated
at a temperature in the range of 500 C to 1500 C.
[411] In various embodiments, an AMM comprises a chamber where the
manufacturing of
fuel cells takes place. This chamber may be able to withstand high temperature
to enable the
production of the fuel cells wherein the high temperature is at least 300 C,
at least 500 C, at
least 1000 C or at least 1500 C. In some cases, this chamber may also enable
the heating of
the fuel cells to take place in the chamber. Various heating methods may be
applied, such as
laser heating/curing, electromagnetic wave heating, hot fluid heating or one
or more heating
elements associated with the chamber. The heating element may be a heating
surface, heating
coil or a heating rod and is associated with the chamber such that the content
in the chamber
is heated to the desired temperature range. In various embodiments, the
chamber of the AMM
may also be able to apply pressure to the fuel cell(s) inside. For example, a
pressure may be
applied via a moving element, such as a moving stamp or plunger. In various
embodiments, the
chamber of the AMM is able to withstand pressure. The chamber can be
pressurized or
100
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
depressurized as desired by a fluid. The fluid in the chamber can also be
changed or replaced as
needed.
[4121 In preferred embodiments, a fuel cell or fuel cell stack is heated using
EMR. In other
embodiments, the fuel cell or fuel cell stack may be heated using oven curing.
In other
embodiments, the laser beam may be expanded (for example, by the use of one or
more
mirrors) to create a heating zone with uniform power density. In a preferred
embodiment, each
layer of the fuel cell may be cured by EMR separately. In preferred
embodiments, a combination
of fuel cell layers may be EMR cured separately, for example, a combination of
the anode, the
electrolyte, and the cathode layers. In some embodiments, a first fuel cell is
EMR cured,
assembled with a second fuel cell, and then the second fuel cell is EMR cured.
In an
embodiment, a first fuel cell is assembled with a second fuel cell, and then
the first fuel cell and
the second fuel cell are EMR cured separately. In an embodiment, a first fuel
cell is assembled
with a second fuel cell to form a fuel cell stack, and then the fuel cell
stack is EMR cured. A fuel
cell stack comprising two or more fuel cells may be EMR cured. The sequence of
laser
heating/curing and assembling is applicable to all other heating methods.
[413] In preferred embodiments, an AMM produces each layer of a multiplicity
of fuel cells
simultaneously. In preferred embodiments, the AMM assembles each layer of a
multiplicity of
fuel cells simultaneously. In preferred embodiments, heating each layer or
heating a
combination of layers of a multiplicity of fuel cells takes place
simultaneously. All the discussion
and all the features described herein for a fuel cell or a fuel cell stack are
applicable to the
production, assembling and heating of the multiplicity of fuel cells. In
preferred embodiments, a
multiplicity of fuel cells may be 2 or more 20 or more, 50 or more,80 or more,
100 or more, 500
or more, 800 or more, 1000 or more, 5000 or more or 10,000 or more.
Treatment Process
(4141 Herein disclosed is a treatment process that comprises one or more of
the following
effects: heating, drying, curing, sintering, annealing, sealing, alloying,
evaporating,
restructuring, foaming or sintering. A preferred treatment process is
sintering. The treatment
process comprises exposing a substrate to a source of electromagnetic
radiation (EMR). In
some embodiments, EMR is exposed to a substrate having a first material. In
various
101
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
embodiments, the EMR has a peak wavelength ranging from 10 to 1500 nm. In
various
embodiments, the EMR has a minimum energy density of 0.1 Joule/cm2. In an
embodiment,
the EMR has a burst frequency of 10.4-1000 Hz or 1-1000 Hz or 10-1000 Hz. In
an
embodiment, the EMR has an exposure distance of no greater than 50 mm. In an
embodiment,
the EMR has an exposure duration no less than 0.1 ms or 1 ms. In an
embodiment, the EMR is
applied with a capacitor voltage of no less than 100V. For example, a single
pulse of EMR is
applied with an exposure distance of about 10 mm and an exposure duration of 5-
20 ms. For
example, multiple pulses of EMR are applied at a burst frequency of 100Hz with
an exposure
distance of about 10 mm and an exposure duration of 5-20 ms. In some
embodiments, the
EMR consists of one exposure. In other embodiment, the EMR comprises no
greater than 10
exposures, or no greater than 100 exposures, or no greater than 1000
exposures, or no
greater than 10,000 exposures.
[415] In various embodiments, metals and ceramics are sintered almost
instantaneously
(milliseconds for 10 microns) using pulsed light. The sintering temperature
may be
controlled to be in the range of 100 C to 2000 C. The sintering temperature
may be tailored
as a function of depth. In one example, the surface temperature is 1000 C and
the sub-
surface is kept at 100 C, wherein the sub-surface is 100 microns below the
surface. In some
embodiments, the material suitable for this treatment process includes yttria-
stabilized zirconia
(YSZ), SYSZ (8m01% YSZ powder), yttrium, zirconium, gadolinia-doped ceria (GDC
or CGO),
samaria-doped ceria (SDC), scandia-stabilized zirconia (SSZ), lanthanum
strontium manganite
(LSM), lanthanum strontium cobalt ferrite (LSCF), lanthanum strontium
cobaltite (LSC),
lanthanum strontium gallium magnesium oxide (LSGM), nickel, NiO, NiO-YSZ, Cu-
CGO, Cu2O,
CuO, cerium, copper, silver, crofer, steel, lanthanum chromite, doped
lanthanum chromite,
ferritic steel, stainless steel, or combinations thereof. This treatment
process may be suitable
for any electrode or electrolyte material previously listed herein.
[416] This treatment process is applicable in the manufacturing process of a
fuel cell. In
preferred embodiments, a layer in a fuel cell (i.e., anode, cathode,
electrolyte, seal, catalyst, etc)
is treated using processes described herein to be heated, cured, sintered,
sealed, alloyed,
foamed, evaporated, restructured, dried or annealed or combinations thereof.
In preferred
102
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
embodiments, a portion of a layer in a fuel cell is treated using processes
described herein to be
heated, cured, sintered, sealed, alloyed, foamed, evaporated, restructured,
dried, annealed, or
combinations thereof. In preferred embodiments, a combination of layers of a
fuel cell are
treated using processes described herein to be heated, cured, sintered,
sealed, alloyed, foamed,
evaporated, restructured, dried, annealed or combinations thereof, wherein the
layers may be a
complete layer or a partial layer.
[417] The treatment process of this disclosure is preferably rapid, with the
treatment duration
varied from microseconds to milliseconds. The treatment duration may be
accurately
controlled. The treatment process of this disclosure may produce fuel cell
layers that have no
cracks or have minimal cracking. The treatment process of this disclosure
controls the power
density or energy density in the treatment volume (the volume of an object
being treated) of
the material being treated. The treatment volume may be accurately controlled.
In an
embodiment, the treatment process of this disclosure provides the same energy
density or
different energy densities in a treatment volume. In an embodiment, the
treatment process of
this disclosure provides the same treatment duration or different treatment
durations in a
treatment volume. In an embodiment, the treatment process of this disclosure
provides
simultaneous treatment for one or more treatment volumes. In an embodiment,
the treatment
process of this disclosure provides simultaneous treatment for one or more
fuel cell layers or
partial layers or combination of layers. In an embodiment, the treatment
volume is varied by
changing the treatment depth.
[4183 In an embodiment, a first portion of a treatment volume is treated by
electromagnetic
radiation of a first wavelength; a second portion of the treatment volume is
treated by
electromagnetic radiation of a second wavelength. In some cases, the first
wavelength is the same
as the second wavelength. In some cases, the first wavelength is different
from the second
wavelength. In an embodiment, the first portion of a treatment volume has a
different energy
density from the second portion of the treatment volume. In an embodiment, the
first portion of a
treatment volume has a different treatment duration from the second portion of
the treatment
volume.
103
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
(4193 In an embodiment, the EMR has a broad emission spectrum so that the
desired effects
are achieved for a wide range of materials having different absorption
characteristics. In this
disclosure, absorption of electromagnetic radiation (EMR) refers to the
process, wherein the
energy of a photon is taken up by matter, such as the electrons of an atom.
Thus, the
electromagnetic energy is transformed into internal energy of the absorber,
for example,
thermal energy. For example, the EMR spectrum extends from the deep
ultraviolet (UV) range
to the near infrared (IR) range, with peak pulse powers at 220 nm wavelength.
The power of
such EMR is on the order of Megawatts. Such EMR sources perform tasks such as
breaking
chemical bonds, sintering, ablating or sterilizing.
(4203 In an embodiment, the EMR has an energy density of no less than 0.1, 1,
or 10
Joule/cm'. In an embodiment, the EMR has a power output of no less than 1 watt
(W), 10 W,
100 W, 1000 W. The EMR delivers power to the substrate of no less than 1 W, 10
W, 100 W,
1000 W. In an embodiment, such EMR exposure heats the material in the
substrate. In an
embodiment, the EMR has a range or a spectrum of different wavelengths. In
various
embodiments, the treated substrate is at least a portion of an anode, cathode,
electrolyte,
catalyst, barrier layer, or interconnect of a fuel cell.
(4213 In an embodiment, the peak wavelength of the EMR is between 50 and 550
nm or
between 100 and 300 nm. In an embodiment, the absorption of at least a portion
of the
substrate for at least one frequency of the EMR between 10 and 1500 nm is no
less than 30% or
no less than 50%. In an embodiment, the absorption of at least a portion of
the substrate for at
least one frequency between 50 and 550 nm is no less than 30% or no less than
50%. In an
embodiment, the absorption of at least a portion of the substrate for at least
one frequency
between 100 and 300 nm is no less than 30% or no less than 50%.
[4223 Sintering is the process of compacting and forming a solid mass of
material by heat or
pressure without melting it to the point of liquefaction. In this disclosure,
the substrate under
EMR exposure is sintered but not melted. In preferred embodiments, the EMR
comprises one or
more of UV light, near ultraviolet light, near infrared light, infrared light,
visible light, laser,
electron beam, microwave. In an embodiment, the substrate is exposed to the
EMR for no less
than 1 microsecond, no less than 1 millisecond. In an embodiment, the
substrate is exposed to
104
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
the EMR for less than 1 second at a time or less than 10 seconds at a time. In
an embodiment,
the substrate is exposed to the EMR for less than 1 second or less than 10
seconds. In an
embodiment, the substrate is exposed to the EMR repeatedly, for example, more
than 1 time,
more than 3 times, more than 10 times. In an embodiment, the substrate is
distanced from the
source of the EMR for less than 50 cm, less than 10 cm, less than 1 cm, or
less than 1 mm.
[423] In some embodiments, after EMR exposure a second material is added to or
placed on to
the first material. In various cases, the second material is the same as the
first material. The
second material may be exposed to EMR. In some cases, a third material may be
added. The third
material is exposed to EMR.
[424] In some embodiments, the first material comprises YSZ, 8YSZ, yttrium,
zirconium, GDC,
SDC, LSM, LSCF, LSC, nickel, Ni0 or cerium or a combination thereof. The
second material may
comprise graphite. In some embodiments, the electrolyte, anode, or cathode
comprises a
second material. In some cases, the volume fraction of the second material in
the electrolyte,
anode, or cathode is less than 20%, 10%, 3%, or 1%. The absorption rate of the
second
material for at least one frequency (e.g., between 10 and 1500 nm or between
100 and 300 nm
or between 50 and 550 nm) is greater than 30% or greater than 50%.
[425] In various embodiments, one or a combination of parameters may be
controlled,
wherein such parameters include distance between the EMR source and the
substrate, the
energy density of the EMR, the spectrum of the EMR, the voltage of the EMR,
the duration
of exposure, the burst frequency and the number of EMR exposures. Preferably,
these
parameters are controlled to minimize the formation of cracks in the
substrate.
[426] In an embodiment, the EMR energy is delivered to a surface area of no
less than 1 mm2,
or no less than 1 cm2, or no less than 10 cm2, or no less than 100 cm2. In
some cases, during
EMR exposure of the first material, at least a portion of an adjacent material
is heated at least
in part by conduction of heat from the first material. In various embodiments,
the layers of the
fuel cell (e.g., anode, cathode, electrolyte) are thin. Preferably they are no
greater than 30
microns, no greater than 10 microns, or no greater than 1 micron.
[427] In some embodiments, the first material of the substrate is in the form
of a powder, sol
gel, colloidal suspension, hybrid solution or sintered material. In various
embodiments, the
105
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
second material may be added by vapor deposition. In preferred embodiments,
the second
material coats the first material. In preferred embodiments, the second
material reacts with
light, (e.g. focused light), as by a laser, and sintered or annealed with the
first material.
Advantages
[428] The preferred treatment process of this disclosure enables rapid
manufacturing of fuel
cells by eliminating traditional, costly, time consuming, expensive sintering
processes and
replacing them with rapid, in situ methods that allow continuous manufacturing
of the layers of
a fuel cell in a single machine if desired. This process also shortens
sintering time from hours
and days to seconds or milliseconds or even microseconds.
[429] In various embodiments, this treatment method is used in combination
with manufacturing
techniques like screen printing, tape casting, spraying, sputtering, physical
vapor deposition and
additive manufacturing.
[430] This preferred treatment method enables tailored and controlled heating
by tuning [MR
characteristics (such as, wavelengths, energy density, burst frequency, and
exposure duration)
combined with controlling thicknesses of the layers of the substrate and heat
conduction into
adjacent layers to allow each layer to sinter, anneal, or cure at each desired
target
temperature. This process enables more uniform energy applications, decreases
or eliminates
cracking, which improves electrolyte performance. The substrate treated with
this preferred
process also has less thermal stress due to more uniform heating.
Particle Size Control
[431] Without wishing to be limited by any theory, we have unexpectedly
discovered that the
sintering process may require much less energy expenditure and much less time
than what is
traditionally needed if the particle size distribution of the particles in a
material is controlled to
meet certain criteria. In some cases, such particle size distribution
comprises D10 and 090,
wherein 10% of the particles have a diameter no greater than 010 and 90% of
the particles have
a diameter no greater than 090, wherein 090/010 is in the range of from 1.5 to
100. In some
cases, such particle size distribution is bimodal such that the average
particle size in the first mode
is at least 5 times the average particle size in the second mode. In some
cases, such
106
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
particle size distribution comprises 050, wherein 50% of the particles have a
diameter no greater
than 050, wherein D50 is no greater than 100 nm. The sintering processes
utilize
electromagnetic radiation (EMR), or plasma, or a furnace, or hot fluid, or a
heating element, or
combinations thereof. Preferably, the sintering processes utilize
electromagnetic radiation (EMR).
For example, without the processes as disclosed herein, an EMR source just
sufficient enough to
sinter a material has power capacity P. With the processes as disclosed
herein, the material is
sintered with EMR sources having much less power capacity, e.g., 50% P or
less, 40% P or less,
30% P or less, 20% P or less, 10% P or less, 5% P or less.
[432] Herein disclosed is a method of sintering a material comprising mixing
particles with a
liquid to form a dispersion, wherein the particles have a particle size
distribution comprising D10
and 090, wherein 10% of the particles have a diameter no greater than 010 and
90% of the
particles have a diameter no greater than 090, wherein 090/010 is in the range
of from 1.5 to
100; depositing the dispersion on a substrate to form a layer; and treating
the layer to cause at
least a portion of the particles to sinter.
[433] In some embodiments, the particle size distribution is a number
distribution determined
by dynamic light scattering. Dynamic light scattering (DLS) is a technique
that can be used to
determine the size distribution profile of small particles in a dispersion or
suspension. In the
scope of DLS, temporal fluctuations are typically analyzed by means of the
intensity or photon
auto-correlation function (also known as photon correlation spectroscopy or
quasi-elastic light
scattering). In the time domain analysis, the autocorrelation function (ACF)
usually decays
starting from zero delay time, and faster dynamics due to smaller particles
lead to faster
decorrelation of scattered intensity trace. It has been shown that the
intensity ACE is the
Fourier transformation of the power spectrum, and therefore the DLS
measurements can be
equally well performed in the spectral domain.
[434] In an embodiment, the particle size distribution is determined by
transmission electron
microscopy (TEM). TEM is a microscopy technique in which a beam of electrons
is transmitted
through a specimen to form an image. In this case, the specimen is most often
a suspension on a
grid. An image is formed from the interaction of the electrons with the sample
as the beam is
transmitted through the specimen. The image is then magnified and focused onto
an imaging
107
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
device, such as a fluorescent screen or a sensor such as a scintillator
attached to a charge-
coupled device.
[435] Herein disclosed is a method of sintering a material comprising mixing
particles with a
liquid to form a dispersion, wherein the particles have a particle size
distribution comprising
050, wherein 50% of the particles have a diameter no greater than 050, wherein
050 is no
greater than 100 nm; depositing the dispersion on a substrate to form a layer;
and treating the
layer to cause at least a portion of the particles to sinter. In various
embodiments, 050 is no
greater than 50 nm, or no greater than 30 nm, or no greater than 20 nm, or no
greater than
nm, or no greater than 5 nm. In an embodiment, the layer has a thickness of no
greater
than 1 mm or no greater than 500 microns or no greater than 300 microns or no
greater than
100 microns or no greater than 50 microns.
[436] In some embodiments, depositing comprises material jetting, binder
jetting, inkjet
printing, aerosol jetting, or aerosol jet printing, vat photopolymerization,
powder bed fusion,
material extrusion, directed energy deposition, sheet lamination, ultrasonic
inkjet printing, or
combinations thereof. In some embodiments, said liquid comprises water and at
least one
organic solvent having a lower boiling point than water and miscible with
water. In some
embodiments, said liquid comprises water, a surfactant, a dispersant and no
polymeric binder. In
some embodiments, said liquid comprises one or more organic solvents and no
water. In some
embodiments, the particles comprise Cu, CuO, Cu20, Ag, Ag20, Au, Au20, Au203,
titanium, yttria-
stabilized zirconia (YSZ), 8YSZ (8m01% YSZ powder), yttrium, zirconium,
gadolinia-doped ceria
(GDC or CGO), samaria-doped ceria (SDC), scandia-stabilized zirconia (SSZ),
lanthanum
strontium manganite (LSM), lanthanum strontium cobalt ferrite (LSCF),
lanthanum strontium
cobaltite (LSC), lanthanum strontium gallium magnesium oxide (LSGM), nickel
(Ni), NiO, Ni0-
YSZ, Cu-CGO, cerium, crofer, steel, lanthanum chromite, doped lanthanum
chromite, ferritic
steel, stainless steel, or combinations thereof. The particles may comprise
any material
previously listed herein for electrodes or electrolyte.
[437] In some embodiments, wherein the particles have a bi-modal particle size
distribution such
that the average particle size in the first mode is at least 5 times the
average particle size in the
second mode. In some embodiments, 010 is in the range of from 5 nm to 50 nm or
from
108
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
nm to 100 nm or from 5 nm to 200 nm. In some embodiments, D90 is in the range
of from 50 nm
to 500 nm or from 50 nm to 1000 nm. In some embodiment, 090/D10 is in the
range of from 2 to
100 or from 4 to 100 or from 2 to 20 or from 2 to 10 or from 4 to 20 or from 4
to 10.
[438] In some embodiments, the method comprises drying the dispersion after
depositing. In
some embodiments, drying comprises heating the dispersion before deposition,
heating the
substrate that is contact with the dispersion, or combination thereof. Drying
may take place for a
time period in the range of 1 ms to 1 min or 1 s to 30 s or 3 s to 10 s. In
some embodiments, the
dispersion may be deposited at a temperature in the range of 40 C to 100 C or
50 C to 90 C or
60 C to 80 C or about 70 C.
[439] In some embodiments, treating comprises the use of electromagnetic
radiation (EMR),
or a furnace, or plasma, or hot fluid, or a heating element, or combinations
thereof. In some
embodiments, the EMR comprises UV light, near ultraviolet light, near infrared
light, infrared
light, visible light, laser, electron beam or microwave or a combination
thereof. In an
embodiment, the EMR consists of one exposure. In other embodiments, the EMR
has an
exposure frequency of 104-1000 Hz or 1-1000 Hz or 10-1000 Hz. In an
embodiment, the EMR
has an exposure distance of no greater than 50 mm. In an embodiment, the EMR
has an
exposure duration no less than 0.1 ms or 1 ms. In an embodiment, the EMR is
applied with a
capacitor voltage of no less than 100V.
EXAMPLES
[440] The following examples are provided as part of the disclosure of various
embodiments of
the present invention. As such, none of the information provided below is to
be taken as limiting
the scope of the invention.
Example 1. Making an EC reactor stack.
[441] Example 1 is illustrative of the preferred method of making an EC
reactor stack,
e.g., a fuel cell stack. The method uses an AMM model no. 0012323 from
Ceradrop and an
EMR model no. 092309423 from Xenon Corp. An interconnect substrate is put down
to start
the print.
109
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
[442] As a first step, an anode layer is made by the AMM. This layer is
deposited by the AMM as a
slurry A, having the composition as shown in the table below. This layer is
allowed to dry by
applying heat via an infrared lamp. This anode layer is sintered by
irradiating it with an
electromagnetic pulse from a xenon flash tube for 1 second.
[443] An electrolyte layer is formed on top of the anode layer by the AMM
depositing a slurry B,
having the composition shown in the table below. This layer is allowed to dry
by applying heat
via an infrared lamp. This electrolyte layer is sintered by irradiating it
with an electromagnetic
pulse from a xenon flash tube for 60 seconds.
[444] Next a cathode layer is formed on top of the electrolyte layer by the
AMM depositing a
slurry C, having the composition shown in the table below. This layer is
allowed to dry by
applying heat via an infrared lamp. This cathode layer is sintered by
irradiating it with an
electromagnetic pulse from a xenon flash tube for 1/2 second.
[445] An interconnect layer is formed on top of the cathode layer by the AMM
depositing a slurry
D, having the composition shown in the table below. This layer is allowed to
dry by applying heat
via an infrared lamp. This interconnect layer is sintered by irradiating it
with an electromagnetic
pulse from a xenon flash tube for 30 seconds.
[446] These steps are then repeated 60 times, with the anode layers being
formed on top of the
interconnects. The result is a fuel cell stack with 61 fuel cells.
Composition of Slurries
Slurry Solvents Particles
A 100% isopropyl alcohol 10 wt% Ni0-8YSZ
100% isopropyl alcohol 10 wt% 8YSZ
100% isopropyl alcohol 10 wt% LSCF
100% isopropyl alcohol 10 wt% lanthanum chromite
Example 2. LSCF in ethanol.
[447] Mix 200 ml of ethanol with 30 grams of LSCF powder in a beaker.
Centrifuge the mixture and
obtain an upper dispersion and a lower dispersion. Extract and deposit the
upper
110
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
dispersion using a 3D printer on a substrate and form a LSCF layer. Use a
xenon lamp (10 kW) to
irradiate the LSCF layer at a voltage of 400V and a burst frequency of 10 Hz
for a total exposure
duration of 1,000 ms.
Example 3. CGO in ethanol.
[448] Mix 200 ml of ethanol with 30 grams of CGO powder in a beaker.
Centrifuge the
mixture and obtain an upper dispersion and a lower dispersion. Extract and
deposit the upper
dispersion using a 3D printer on a substrate and form a CGO layer. Use a xenon
lamp (10 kW)
to irradiate the CGO layer at a voltage of 400V and a burst frequency of 10 Hz
for a total
exposure duration of 8,000 ms.
Example 4. CGO in water.
[449] Mix 200 ml of deionized water with 30 grams of CGO powder in a beaker.
Centrifuge the
mixture and obtain an upper dispersion and a lower dispersion. Extract and
deposit the upper
dispersion using a 3D printer on a substrate and form a CGO layer. Use a xenon
lamp (10 kW) to
irradiate the CGO layer at a voltage of 400V and a burst frequency of 10 Hz
for a total exposure
duration of 8,000 ms.
Example 5. NiO in water.
[450] Mix 200 ml of deionized water with 30 grams of Ni0 powder in a beaker.
Centrifuge the
mixture and obtain an upper dispersion and a lower dispersion. Extract and
deposit the upper
dispersion using a 3D printer on a substrate and form a NiO layer. Use a xenon
lamp (10 kW) to
irradiate the Ni0 layer at a voltage of 400V and a burst frequency of 10 Hz
for a total exposure
duration of 15,000 ms.
Example 6. Sintering results.
[451] Fig. 17 is a scanning electron microscopy image (side view). Fig. 17
illustrates an
electrolyte (YSZ) 1701 printed and sintered on an electrode (NiO-YSZ) 1702.
The scanning
electron microscopy image shows the side view of the sintered structures,
which demonstrates
gas-tight contact between the electrolyte and the electrode, full
densification of the electrolyte,
and sintered and porous electrode microstructures.
1 1 1
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
Example 7. Fuel cell stack configurations.
[452] A 48-Volt fuel cell stack has 69 cells with about 1000 Watts of power
output. The fuel
cell in this stack has a dimension of about 4 cm x 4 cm in length and width
and about 7 cm in
height. A 48-Volt fuel cell stack has 69 cells with about 5000 Watts of power
output. The fuel
cell in this stack has a dimension of about 8.5 cm x 8.5 cm in length and
width and about 7
cm in height.
Example 8. Channeled Electrodes/Fluid Dispersing Components.
[453] Fig. 18 schematically illustrates an example of a half cell in an EC
reactor. As shown in
Fig. 18, half cell 1700 comprises interconnect 1801. Interconnect 1801
comprises doped
lanthanum chromite. Half cell 1800 comprises anode segments 1802 that are
printed on
interconnect 1801. The anode segments are composed of NiO-YSZ. Anode segments
1802 are
sintered using EMR (see Example 1). Half cell 1800 comprises filler material
that is deposited
between anode segments 1802. The filler material is polymethyl methacrylate
(PMMA). Half
cell 1800 includes shields 1804 that are printed on filler materials 1803 that
are composed of
YSZ. Additional anode material 1806 is printed to cover anode segments 1802
and shields
1804 followed by sintering using EMR. The additional anode material is NiO-
YSZ. Electrolyte
1805 is printed on additional anode material 1806 and sintered using EMR.
Electrolyte 1805 is
YSZ. A barrier layer (not shown) composed of CGO is further printed on the
electrolyte and
sintered using EMR. A layer of cathode (not shown) composed of LSCF is printed
on the CGO
barrier and sintered. Cathode segments (not shown) composed of LSCF are
printed on this
layer and sintered. These segments form valleys and filler PMMA is deposited
to fill these
valleys (not shown). Shields composed of YSZ are printed on the fillers (not
shown). Doped
lanthanum chromite is printed to cover the shields and cathode segments and
then sintered
to form another interconnect (not shown). The fillers are removed by furnace
heating and
channeled electrodes are produced or fluid dispersing components are formed
between
electrolyte and interconnect (not shown).
[454] It is to be understood that this disclosure describes exemplary
embodiments for
implementing different features, structures, or functions of the invention.
Exemplary
embodiments of components, arrangements, and configurations are described to
simplify the
112
Date Recue/Date Received 2022-12-08
CA 03126466 2021-07-09
WO 2020/146759 PCT/US2020/0 13129
present disclosure; however, these exemplary embodiments are provided merely
as examples and
are not intended to limit the scope of the invention. The embodiments as
presented herein may
be combined unless otherwise specified. Such combinations do not depart from
the scope of the
disclosure.
[455] Additionally, certain terms are used throughout the description and
claims to refer to
particular components or steps. As one skilled in the art appreciates, various
entities may refer
to the same component or process step by different names, and as such, the
naming
convention for the elements described herein is not intended to limit the
scope of the invention.
Further, the terms and naming convention used herein are not intended to
distinguish between
components, features, andjor steps that differ in name but not in function.
[456] While the disclosure is susceptible to various modifications and
alternative forms,
specific embodiments thereof are shown by way of example in the drawings and
description.
It should be understood, however, that the drawings and detailed description
are not
intended to limit the disclosure to the particular form disclosed, but on the
contrary, the
intention is to cover all modifications, equivalents and alternatives falling
within the spirit and
scope of this disclosure.
113
Date Recue/Date Received 2022-12-08