Note: Descriptions are shown in the official language in which they were submitted.
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
TISSUE PHANTOMS
CROSS-REFERNCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional Application No.
62/793,839 (filed January 17, 2019), titled "TISSUE PHANTOMS," the entire
contents of which are incorporated by reference herein.
TECHNICAL FIELD
[0002] The present disclosure relates to a tissue phantom, and in one
exemplary
embodiment, a breast tissue phantom. The phantom may be used to calibrate an
imaging device that is used to identify one or more of residual cancer cells,
precancerous cells, and satellite lesions in a surgical site such as a
surgical cavity or
in a tissue specimen removed from a surgical cavity, such as breast tissue
removed
during Breast Conserving Surgery ("BCS"). In addition, the disclosed tissue
phantoms may be used to train or educate surgeons (or others) to identify one
or
more of residual cancer cells, precancerous cells, and satellite lesions in a
surgical
site that become visible in a surgical site or in an excised tissue specimen
through
use of an imaging device. The device also may be used to identify potential
interactions between different types of imaging and/or contrast agents used
together
during surgery, such as BCS, to make residual cancer cells, precancerous
cells,
satellite lesions, and/or other malignant cells in surgical cavities, tissue
specimens,
lymph nodes, or other areas visible to the surgeon.
INTRODUCTION
[0003] Surgery is one of the oldest types of cancer therapy and is an
effective
treatment for many types of cancer. Oncology surgery may take different forms,
1
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
dependent upon the goals of the surgery. For example, oncology surgery may
include biopsies to diagnose or determine a type or stage of cancer, tumor
removal
to remove some or all of a tumor or cancerous tissue, exploratory surgery to
locate
or identify a tumor or cancerous tissue, debulking surgery to reduce the size
of or
remove as much of a tumor as possible without adversely affecting other body
structures, and palliative surgery to address conditions caused by a tumor
such as
pain or pressure on body organs.
[0004] In surgeries in which the goal is to remove the tumor(s) or
cancerous
tissue, surgeons often face uncertainty in determining if all cancer has been
removed. The surgical bed, or tissue bed, from which a tumor is removed, may
contain residual cancer cells, i.e., cancer cells that remain in the surgical
margin of
the area from which the tumor is removed. If these residual cancer cells
remain in
the body, the likelihood of recurrence and metastasis increases. Often, the
suspected presence of the residual cancer cells, based on examination of
surgical
margins of the excised tissue during pathological analysis of the tumor, leads
to a
secondary surgery to remove additional tissue from the surgical margin.
[0005] For example, breast cancer, the most prevalent cancer in women, is
commonly treated by breast conservation surgery (BCS), e.g., a lumpectomy,
which
removes the tumor while leaving as much healthy breast tissue as possible.
Treatment efficacy of BCS depends on the complete removal of malignant tissue
while leaving enough healthy breast tissue to ensure adequate breast
reconstruction,
which may be poor if too much breast tissue is removed. Traditionally, tumor
margins are visualized under standard white light (WL) in an operating room in
order
to determine the effectiveness of the BCS procedure.
2
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
[0006] Imaging devices may also be used to evaluate specimen tissue for the
presence of cancer cells. For example, imaging devices may be used to
determine
the amount of cancer cells, if any, remaining after a BCS procedure, thus
determining the efficacy of the procedure. Imaging devices may also be used to
provide guidance during the BCS procedure. Calibration of the imaging devices,
as
well as training and/or education of the persons using the imaging devices to
identify
residual cancer cells will contribute to the efficacy of removing residual
cancer cells
during BCS.
SUMMARY
[0007] The present disclosure may demonstrate one or more of the above-
mentioned desirable features. Other features and/or advantages may become
apparent from the description that follows.
[0008] In accordance with one aspect of the present disclosure, a tissue
phantom includes a first portion having the optical properties of healthy
tissue and a
second portion having the optical properties of cancerous tissue.
[0009] In accordance with another aspect of the present disclosure, a
method of
calibrating an optical instrument. The method including illuminating a tissue
phantom with excitation light from the optical instrument, detecting optical
emissions
emitted by the tissue phantom in response to illumination with the excitation
light,
and calibrating the optical instrument based upon the detected fluorescence.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010] The present disclosure can be understood from the following detailed
description either alone or together with the accompanying drawings. The
drawings
are included to provide a further understanding of the disclosed teachings and
are
3
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
incorporated in and constitute a part of this specification. The drawings
illustrate one
or more example embodiments of the present disclosure and together with the
description serve to explain various principles and operations.
[0011] FIG. 1A is an illustration of the conversion of ALA to PpIX in a
tumor cell;
[0012] FIG. 1B shows peak absorption and emission for PpIX;
[0013] FIGS. 2A ¨ 2D show components of a first embodiment of a tissue
phantom including a first embodiment of a diseased tissue portion of the
tissue
phantom in accordance with the present disclosure;
[0014] FIGS. 3A and 3B show images of the tissue phantom of FIGS. 2A-2D in
use;
[0015] FIG. 30 is an example of a dial to be used to cover the wells shown
in
FIGS. 2B and 3B;
[0016] FIG. 4 is an image of a second embodiment of a diseased portion to
be
used with the tissue phantom of FIGS. 2A-2D in accordance with the present
disclosure;
[0017] FIGS. 5A and 5B are images of a third embodiment of a diseased
portion
to be used with the tissue phantom of FIGS. 2A-2D in accordance with the
present
disclosure;
[0018] FIG. 6 is an image of a fourth embodiment of a diseased portion to
be
used with the tissue phantom of FIGS. 2A-2D in accordance with the present
disclosure;
[0019] FIGS. 7 and 8 are images of a second embodiment of a tissue phantom
in accordance with the present disclosure;
[0020] FIG.9 is an image of a third embodiment of a tissue phantom in
accordance with the present disclosure;
4
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
[0021] FIG. 10 is an image of a fourth embodiment of a tissue phantom in
accordance with the present disclosure;
[0022] FIG. 11 is a flowchart illustrating an example method for making the
tissue phantom shown in FIG. 10;
[0023] FIG. 12 is an image of a fifth embodiment of a tissue phantom in
accordance with the present disclosure;
[0024] FIG. 13 is an image of a lymph node containing Methylene Blue (MB);
[0025] FIG. 14 shows peak absorption and emission for MB;
[0026] FIGS. 15 and 16 are images of a first embodiment of a MB tissue
phantom in accordance with the present disclosure;
[0027] FIGS. 17 and 18 show images of a second embodiment of a MB tissue
phantom in accordance with the present disclosure;
[0028] FIG. 19 is an image of a third embodiment of a MB tissue phantom in
accordance with the present disclosure;
[0029] FIGS. 20A and 20B are images of a thin film tissue phantom that may
be
used with the MB tissue phantom of FIG. 19;
[0030] FIGS. 21A and 21B are flowcharts illustrating example methods of
using
a tissue phantom in accordance with the present disclosure.
DESCRIPTION OF VARIOUS EXAMPLE EMBODIMENTS
[0031] Tissue phantoms, as discussed herein, may be used to calibrate an
imaging device and/or to provide practice for an operating surgeon. For
example,
the tissue phantoms may represent and mimic the optical properties of "normal"
or
"healthy" tissue. Additionally, the tissue phantoms may include one or more
portions
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
that represent and mimic the optical properties of diseased or abnormal tissue
such
as "cancerous" tissue.
[0032] In alternative embodiments, the tissue phantom may be configured to
include more than one component of a tissue and can be used to calibrate an
imaging device or other device configured to distinguish one tissue component
from
another or locate one tissue component relative to another. In addition to
calibration,
the phantom can also be used for training purposes. For example, the phantom
may
be configured to represent human breast tissue and may contain different
components/tissue types such as adipose tissue, connective tissue, and
vasculature
and the phantom can be used to train surgeons to locate the blood or
vasculature
relative to the adipose or connective tissue with an imaging device.
[0033] In another example, the tissue phantom may be used to train new
users
of a fluorescence imaging device to correctly identify tissues based on their
fluorescence (e.g., identifying red tumor against green/pink
connective/adipose
tissue background). Or to show how certain tissues would fluoresce when imaged
with such a device (blood shows up dark red/black for example, which may not
be
intuitive to new users).
[0034] As noted above, in accordance with one aspect of the present
disclosure, the tissue phantom is configured to include a "normal" or
"healthy" tissue
portion and one or more "diseased" or "abnormal" tissue portions. The healthy
tissue
of the tissue phantom can be any type of tissue and the diseased tissue or
"target"
tissue of the tissue phantom can be chosen to mimic any disease found in the
particular type of healthy tissue modeled by the tissue phantom. The examples
provided herein discuss a breast tissue phantom having one or more areas of
diseased tissue, i.e., cancerous tissue or tumors. It should be understood
that these
6
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
examples are non-limiting examples only and that the concept of a tissue
phantom
comprising healthy and diseased tissue is applicable to many other types of
human
and animal tissues and their diseases. Although discussed herein with regard
to
breast tissue, it is possible to use the present disclosure as a guide to
create a tissue
phantom representative of any tissue having a disease based on the optical
properties for normal tissue and diseased tissue for the particular tissue and
disease
of interest. For example, knowing the absorption coefficient and reduced
scattering
coefficient of the chosen tissue type (for both normal and diseased tissues)
at the
wavelength that is being used for excitation would permit the creation of a
phantom
for a particular tissue having a particular disease as described herein. To
create
such a custom tissue phantom, information regarding how the tissue appears
when
imaged using a particular excitation light source and optical filter
combination would
be relied upon. For example, a fluorescence emission spectrum and/or
fluorescence
images of the tissues would provide the information needed. Examples of
diseased
tissue that have optical properties that may differ from the optical
properties of
healthy tissue include inflamed tissue (e.g., rheumatoid arthritis), fibrotic
tissue, and
ischemic tissue.
[0035] For example, in accordance with the present disclosure, tissue
phantoms
representative of healthy or diseased tissues that may be created in as
disclosed
herein may include: spinal cord, brain, skin, limbs (sarcoma), oral cavity,
prostate,
cervix, colon, thyroid, ovaries, lymph nodes, lungs, pancreas, esophagus,
muscle,
bone, cartilage, uterus, or vagina. This list is intended to provide examples
only and
is not intended to limit the range of possible phantoms created in accordance
with
the present disclosure. In exemplary embodiments of a phantom configured to
represent healthy tissue and abnormal or diseased tissue such as "cancerous
7
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
tissue," the "cancerous tissue" of the phantom contains a material that will
cause the
"cancerous tissue" of the phantom, when illuminated with excitation light
having a
known wavelength, to fluoresce or emit light having a wavelength which will
allow
detection/visualization of the "cancerous tissue" relative to the healthy
tissue. For
example, in some embodiments, a tissue phantom in accordance with the present
disclosure may comprise "healthy tissue" configured to fluoresce green when
illuminated with excitation light having a wavelength of between about 400 nm
and
about 450 nm. In addition, the tissue phantom may comprise "cancerous tissue"
configured to fluoresce red when illuminated with the same excitation light
having a
wavelength of between about 400 nm and about 450 nm. An example of the
material
included in the "cancerous tissue" of the phantom that fluoresces a red color
when
illuminated with excitation light having a wavelength between about 400 nm and
about 450 nm is the porphyrin PpIX. Alternatively, other fluorophores can be
used to
represent tissues that are different from healthy tissue, also referred to
herein as
"target tissue.- For example, indocyanine green (ICG), a green dye such as
Pacific
Green (https://www.thermofishercom/ca/en/homedife-science/cell-
analysis/fluorophores/pacific-cireen-dye.html), IRDye 8000W, or other
fluorophores
of interest may be used. In an example embodiment where blood or vasculature
is
the "target tissue," ICG may be used in the portion of the phantom that
represents
the blood or vasculature.
[0036] In the example breast tissue phantoms disclosed herein, PpIX has
been
selected as the fluorophore of interest. PpIX is a fluorescent molecule that,
when
excited by the appropriate excitation light, emits a red fluorescence. The
PpIX
molecule is naturally broken down by healthy tissue (non-cancerous tissue) in
a
patient to Heme. Thus, healthy tissue does not contain PpIX and therefore does
not
8
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
emit the red fluorescence. However, cancerous tissue is not able to process
PpIX
and, thus, the PpIX collects in the cancerous tissue. The PpIX collected in
cancer
cells, when excited by light emitted by an imaging device and having a
wavelength of
between about 400 nm and about 450 nm, fluoresces red, making the cancerous
tissue appear red to the imaging device. This allows a user of the imaging
device to
determine the presence or absence of cancerous cells based upon the
corresponding presence or absence of red fluorescence emitted by the PpIX
molecules.
[0037] As disclosed herein, a tissue phantom may be used with an imaging
device in order to determine the presence, location and/or amount of the
"cancerous"
tissue with respect to the "normal" tissue within the tissue phantom. Such
results
may then allow a user to calibrate the imaging device, if the concentration of
PpIX
within the tissue phantom is known by the user.
[0038] Exemplary devices, systems, and methods for detecting cancer cells
containing PpIX or other induced porphyrins during surgical intervention are
disclosed in U.S. Provisional Patent Application No. 62/625,983, filed
February 3,
2018 and entitled "Devices, Systems, and Methods for Tumor Visualization and
Removal," and in PCT/0A2019/000015, filed February 1,2019, entitled "Devices,
Systems, and Methods for Tumor Visualization and Removal" and published as
W02019/148,268 on August 8, 2019, the entire content of each of which is
incorporated herein by reference.
[0039] During use of the tissue phantom for calibration of an imaging
device, the
imaging device may be inserted at least partially within a tissue phantom,
such as a
breast tissue phantom in accordance with the present disclosure and emit a
desired
wavelength of light to illuminate the tissue phantom. Illumination with the
excitation
9
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
light causes the "cancerous tissue" within the tissue phantom to fluoresce, as
described above, thus making the cancerous tissue of the tissue phantom
visible to
the imaging device and to those observing the output of the imaging device. As
discussed above, the "cancerous" tissue within the tissue phantom may
fluoresce
due to the presence of PpIX (or another fluorescent dye) contained within
portions of
the phantom.
[0040] The "normal" or "healthy" tissue of the tissue phantom does not
include
PpIX (or another fluorophore) and, therefore, does not fluoresce in the same
manner, i.e., does not emit/reflect light at the same wavelength as the
"cancerous
tissue" when illuminated by the excitation light of the imaging device.
However, the
"normal" tissue of the tissue phantom is created to mimic normal healthy
tissue,
which autofluoresces when illuminated with the excitation light. Different
healthy
tissues emit different wavelengths of light in response to illumination by
excitation
light. Thus, when illuminating a tissue phantom with excitation light as
disclosed
herein, the different components of the phantom (healthy tissue, cancerous
tissue)
will emit different wavelengths of light in response. This allows the light
emitted from
the cancerous tissue to be distinguished from the light emitted by the healthy
tissue
of the tissue phantom and, thus, permits the surgeon to identify the presence
of
cancerous tissue and its location.
[0041] For example, for calibration of an imaging device configured to emit
excitation light of between about 400 nm ¨ 450 nm, the tissue phantom has
optical
properties that allow the phantom to mimic the emission response of tissue
illuminated with excitation light of between about 400 nm ¨ 450 nm. The
optical
properties of the tissue phantom can be narrowly tailored to mimic tissue
response
(of both healthy tissue and diseased tissue) to excitation by any range of
excitation
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
light. For example, the phantom can be formed to have optical properties that
allow it
to mimic tissue response to illumination by excitation light in the
ultraviolet/blue
range, near infrared range, and infrared range. For example, the present
disclosure
contemplates a tissue phantom having optical properties that mimic tissue
response
to illumination by excitation light in the following exemplary ranges: about
350 nm ¨
about 400 nm, about 400 nm ¨ about 450 nm, about 450 nm ¨ about 500 nm, about
500 nm ¨ about 550 nm, about 550 nm ¨ about 600 nm, about 600 nm ¨ about 650
nm, about 650 nm ¨ about 700 nm, about 700 nm ¨ about 750 nm, about 750 nm ¨
about 800 nm, about 800 nm ¨ about 850 nm, about 850 nm ¨ about 900 nm, about
900 nm ¨ about 950 nm, about 950 nm ¨ about 1000 nm, and/or various
combinations therefor. In certain non-limiting, exemplary embodiments
disclosed
herein, the tissue phantom is configured to respond to illumination with
excitation
light in the blue/violent range, for example 405 nm, in a manner the same or
substantially the same as human or animal tissue.
[0042] The tissue phantoms disclosed herein also can be used to help
identify an
optimum amount of PpIX to be collected in cancerous cells in order for the
fluorescence of the cancer cells to be detected by the imaging device and/or
the
surgeon. Using this information, it is possible to then determine the
appropriate
amount or dose of porphyrin-inducing composition that should be administered
to the
patient, for example prior to BCS, as well as the timing of the dosage. For
example,
as disclosed in U.S. Provisional Patent Application No. 62/625,967, filed
February 2,
2018 and entitled "Devices, Systems, and Methods for Tumor Visualization and
Removal," and in U.S. Provisional Patent Application No. 62/625,983, filed
February
3, 2018 and entitled "Devices, Systems, and Methods for Tumor Visualization
and
Removal," and in PCT/0A2019/000015, filed February 1,2019, entitled "Devices,
11
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
Systems, and Methods for Tumor Visualization and Removal" and published as
W02019/148,268 on August 8, 2019, the entire content of each of which is
incorporated herein by reference, the surgical subject or patient may be given
a
diagnostic dose (i.e., not a therapeutic dose) of a compound (imaging/contrast
agent) such as the pro-drug aminolevulinic acid (ALA). As understood by those
of
ordinary skill in the art, dosages of ALA less than 60 mg/kg are generally
considered
diagnostic while dosages greater than 60 mg/kg are generally considered
therapeutic. As disclosed herein, the diagnostic dosage of ALA may be greater
than
0 mg/kg and less than 60 kg/mg, between about 10 mg/kg and about 50 mg/kg,
between about 20 mg/kg and 40 mg/kg, and may be administered to the subject in
a
dosage of 5 mg/kg, 10 mg/kg, 15 kg/mg, 20 mg/kg, 25 mg/kg, 30 mg/kg, 35 mg/kg,
40 mg/kg, 45 mg/kg, 50 mg/kg, or 55 mg/kg. The ALA may be administered orally,
intravenously, via aerosol, via immersion, via lavage, and/or topically.
Although a
diagnostic dosage is contemplated for visualization of the residual cancer
cells,
precancer cells, and satellite lesions, it is within the scope of the present
disclosure
to use the disclosed devices, systems, and methods to provide guidance during
treatment and/or removal of these cells and/or lesions.
[0043] The ALA given to the patient induces porphyrin formation
(protoporphyrin
IX (PplX)) in tumor/cancer cells present in the patient (FIG. 1A shows the
conversion
of ALA to PpIX within a tumor cell) and, when the cells containing PpIX are
illuminated by the appropriate excitation light, an emission having a
wavelength that
appears as red fluorescence from cells containing the PpIX is captured by the
imaging device. These cells are then visible against the green fluorescence
emitted
by the healthy tissues (which have broken down the PpIX into Heme and, thus,
do
not fluoresce a red color), which enhances the red-to-green fluorescence
contrast
12
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
between the tumor/cancer tissue cells and normal tissue cells (e.g., collagen)
imaged with the device. ALA is non-fluorescent by itself, but PpIX emissions,
when
excited by excitation light having a wavelength of between 400 nm and about
450
nm, have wavelengths of about 630 nm, about 680 nm, and about 710 nm, with the
630 nm emission being the strongest. FIG. 1B illustrates the fluorescence
emission
of PpIX when excited with excitation light having a wavelength of 405 nm.
Alternatively, the endogenous fluorescent difference between tumor/cancer
cells or
precancer cells and normal/healthy cells may be used without an
imaging/contrast
agent.
[0044] In exemplary embodiments, the non-activated, non-targeted compound
configured to induce porphyrin in tumor/cancer cells, precancer cells, and/or
satellite
lesions is administered to a subject between about 15 minutes and about 6
hours
before surgery, about 1 hour and about 5 hours before surgery, between about 2
hours and about 4 hours before surgery, or between about 2.5 hours and about
3.5
hours before surgery. These exemplary time frames allow sufficient time for
the ALA
to be converted to porphyrins in tumor/cancer cells, precancer cells, and/or
satellite
lesions. The ALA or other suitable compound may be administered orally,
intravenously, via aerosol, via immersion, via lavage, and/or topically. Use
of the
tissue phantoms disclosed in the present application can determine at what
concentration PpIX must be present in the phantom and, thus, the cancer cells
of the
subject administered the ALA, in order for the fluorescence of the cancer
cells to be
detected by the imaging device and/or the surgeon. Using this information, it
is
possible to then determine the appropriate amount of porphyrin-inducing
composition
that should be administered to the patient as well as the timing of the
dosage, as
discussed above.
13
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
[0045] The tissue phantoms disclosed herein are configured to have the same
optical properties as human tissue that contains the target tissue or disease,
such as
cancer. Thus, for example, in the case of breast cancer, the tissue phantom
may
include a first portion that has the same optical properties as healthy
skin/normal
tissue, such as breast tissue. The tissue phantom also includes one or more
second
portions having the same optical properties as the diseased tissue, such as
cancerous breast tissue. Thus, the breast tissue phantom "mimics" or is an on-
the-
bench representation of a human breast with breast cancer.
[0046] The first portion of the tissue phantom may comprise any material
that is
sufficient to mimic the optical properties of "normal" or "healthy" tissue,
i.e., tissue
that is not cancerous, to provide the tissue phantom with optical
characteristics
substantially the same as the optical characteristics of normal tissue. In
some
embodiments, the first portion of the tissue phantom may be made from a
mixture of
tris buffer and gelatin. The tris buffer aids to maintain the pH and stability
of the
tissue phantom, and the gelatin aids to provide a consistency that mimics an
actual
human or animal tissue. More specifically, the gelatin mixture allows the
tissue
phantom to cure into a long-lasting, reproducible shape that mimics the
connective
tissue found in human breast tissue. In other embodiments, the first portion
of the
tissue phantom may be formed of silicone, agar, or organic tissues from
animals
including chicken, pork, or beef.
[0047] Additionally, the first portion of the tissue phantom may include
other
components such as, for example, elements representative of hemoglobin and
intralipid molecules. As discussed further below, the concentrations of the
hemoglobin and intralipid molecules may be manipulated to adjust the optical
properties of the tissue phantom such as scattering and absorption
coefficients,
14
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
isotropy, and/or turbidity. Sodium azide may also be added to the tissue
phantom to
provide an antibacterial agent that inhibits the growth of bacteria on the
tissue
phantom, thus increasing its longevity. Furthermore, the antibacterial agent
may
also help ensure that any visible fluorescence is not due to the presence of
bacteria
on or in the tissue phantom. The first portion of the tissue phantom may be
made of
one or more materials. In one example embodiment, the first portion of the
tissue
phantom may be homogenous throughout such that the tissue phantom comprises
no more than a 2% variation in a full spectrum reflectance measurement
throughout
the phantom.
[0048] In some embodiments, the optical properties of the first portion of
the
tissue phantom may be selected by varying hemoglobin and/or intralipid
concentrations. Hemoglobin concentrations may be varied to adjust the rate of
absorption of incoming photons from, for example, an excitation light source
of an
imaging device. Infralipid concentrations may be varied to adjust for
scattering of
incoming excitation and/or other light from the imaging device. Hemoglobin
and/or
intralipid concentrations may be adjusted so that the material(s) of the
tissue
phantom substantially match the absorption and emission coefficients of
"normal"
tissue, such as normal breast tissue.
[0049] In some embodiments, the concentration of hemoglobin may range from
approximately 0-50 m. When using the high end of this range, the tissue
phantom
may produce a visibly stronger red fluorescence, which may be used to mimic
the
optical properties of tissue when the hemoglobin absorbs less light. When
using the
low end of this range, the tissue phantom may produce a visibly weaker red
fluorescence, which may be used to mimic the optical properties of tissue when
the
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
hemoglobin absorbs more light. In some embodiments, the concentration of
intralipids may range from approximately 0-20%.
[0050] In one example embodiment, the tissue phantom includes a hemoglobin
concentration of 2.40 M and an intralipid concentration of 1.20% for use with
an
imaging device that emits excitation light at a wavelength of 405 nm. This
concentration of hemoglobin is below the normal concentration of hemoglobin in
breast tissue, which typically ranges from 15-40 M. The concentration of
hemoglobin may be below the normal concentration because less hemoglobin is
required in a tissue phantom (as compared with a patient's breast tissue) to
match
the desired absorption coefficient at 405 nm.
[0051] An example method for preparing tissue phantom material (healthy
tissue
material) is provided below:
Tris buffer
= Suspend 6.1g of Tris and 8.8g NaCI in 800mL of RO water in a 1000mL
Erlenmeyer flask.
= Dissolve 1.0g of sodium azide into the same flask for a resultant pH of
approximately 10.5.
= Using pH indicator strips, adjust the pH to 7.4 by adding 5mL of
hydrochloric acid
in 1mL increments for a total of 5mL.
= Adjust the total volume of the flask to 1000mL with RO water.
Tissue Phantom Material
= Add 50g of gelatin 300 bloom to 500mL of Tris Buffer in a 1000mL flask.
= Place the flask on a hot plate and heat until 5000 under constant
stirring with a
magnetic stir bar. Monitor the temperature with a thermometer or a thermal
gun.
= Once the gelatin is completely dissolved, remove the gelatin mixture from
the
heat source and cool 35 C on the lab bench under constant stirring.
= Prepare 0.0872g of hemoglobin and 32m1 of intralipid in a separate 25mL
flask.
Mix the hemoglobin into the intralipid until no clumping is visible.
16
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
= Once cooled, stir the hemoglobin mixture into the gelatin.
= Pour the gelatin mixture into an appropriate mould and set in the
refrigerator for
24 h before removing.
[0052] In accordance with the present teachings, the second portion(s) of
the
tissue phantom may include material configured to represent diseased tissue.
In
various exemplary embodiments, the tissue phantom includes material configured
to
represent diseased tissue, e.g., cancer in breast tissue. The material
configured to
represent diseased tissue may be incorporated into the tissue phantom in
various
ways, positioned within the healthy tissue portion of the phantom or separated
from
the healthy tissue portion of the phantom. For example, in one embodiment, the
material configured to represent the diseased tissue may be disposed
sporadically
throughout the tissue phantom. In other embodiments, the material configured
to
represent the diseased tissue may be disposed in one distinct location within
the
tissue phantom in order to represent, for example, a tumor. Each location of
the
diseased tissue within the tissue phantom may include portions with varying
concentrations of diseased tissue. In some embodiments, the tissue phantom may
be composed of 3D printed cells, for example cells or tissue that are printed
into
desired shapes with densities and optical properties that substantially match
those of
human tissue. In one example, the tissue phantom is a 3D printed ear.
[0053] In accordance with the present disclosure, a tissue phantom includes
a
healthy tissue portion and a diseased tissue portion. In one example
embodiment
shown in FIG. 2D, a tissue phantom 10 includes a body 11 having a first member
12
and a second member 17. First member 12 and/or second member 17 may be
hollow cylindrical or semi-spherical members with rounded outer edges. In the
embodiment shown, each member 12, 17 has been molded to have a hollow
interior.
17
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
However, it is also contemplated that first and second members 12, 17 may be
solid
members. The first member 12 and second member 17 fit together to form body 11
of the tissue phantom 10 as shown in FIG. 2D and together form the first
portion or
healthy tissue portion of the tissue phantom 10. When first and second members
12,
17 are positioned together, a cavity 35 is formed within body 11. Tissue
phantom 10
may be shaped and sized to represent a patient's breast. Tissue phantom may
alternatively be embodied as excised breast tissue (i.e., representative of a
lumpectomy specimen). In alternative embodiments, the tissue phantom may be
shaped and sized to represent other body parts. Although discussed herein with
regard human breasts and excised breast tissue, the phantoms of the present
disclosure may be formed to replicate any tissue body part, human or animal.
[0054] First member 12 and second member 17 may be attached together at
middle portion 15. In some embodiments, first member 12 and second member 17
are two distinct components. In other embodiments, first member 12 and second
member 17 form one unitary component. First member 12 and second member 17 of
phantom 10 may each comprise any material that is sufficient to mimic optical
properties of "normal" or "healthy" tissue, i.e., tissue that is not
cancerous, to provide
the phantom with optical characteristics substantially the same as the optical
characteristics of normal tissue. Thus, first member 12 and second member 17
may
separately or together form a portion of the phantom 10 that has the optical
properties of healthy tissue. In some embodiments, first member 12 and/or
second
member 17 may be formed of a mixture of tris buffer and gelatin. First member
12
and second member 17 may be formed of the same or different materials. In one
example embodiment, the material(s) of first member 12 and second member 17
may be homogenous throughout such that the healthy tissue portion of the
tissue
18
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
phantom 10 comprises no more than a 2% variation in a full spectrum
reflectance
measurement throughout the phantom.
[0055] In accordance with the present disclosure, the tissue phantom 10
includes
a diseased tissue or cancerous tissue portion. In the example embodiment of
FIGS.
2A-2D, the material configured to represent the diseased tissue may form a
part of
the tissue phantom separate from the healthy tissue part of the phantom. For
example, as shown in FIG. 20, a tray 20 is configured to contain the material
that
represents the diseased tissue of the phantom. As shown in FIG. 2B, the tray
20
may be positioned in the cavity 35 defined by the body 11 of the phantom. In
the
example embodiment of FIG. 2A, the second member 17 of tissue phantom 10 may
be configured to receive and support tray 20 and its contents, and tray 20 may
be
disposed within second member 17 of tissue phantom 10 as illustrated in FIG.
2B.
Although illustrated as being positioned in the second member 17 of phantom
10, it
is contemplated that tray 20 may be positioned in the first member 12 of the
phantom
10, or two trays 20 may be provided, one in each member 12, 17 of the tissue
phantom 10.
[0056] The tray may take on various shapes and configurations. An example
configuration of tray 20 is illustrated in FIG. 20. Tray 20 may have a rounded
donut
shape and may include one or more recesses 25 in a top surface thereof.
Recesses
25 may be circular in shape or may have any other shape sufficient to hold the
material configured to represent diseased tissue, which may be in solution
form as
discussed further below. Recesses 25 may be arranged uniformly around tray 20.
Alternatively, recesses 25 may be arranged sporadically around tray 20. In one
embodiment, tray 20 includes ten recesses 25.
19
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
[0057] Tray 20, including recesses 25, may be formed of a polymeric
material, for
example, polylactic acid (PLA). In some embodiments, tray 20 is coated,
printed,
and/or manufactured with a black paint, which acts an optical barrier between
recesses 25 and second member 17 of tissue phantom 10. Thus, wavelengths
emitted from an imaging device do not penetrate through recesses 25 and onto
second member 17. These embodiments may allow for a more accurate
determination of the optical properties detected in each recess 25, which may
be
used for calibration of an imaging device.
[0058] For calibration purposes, the size and location of recesses 25 may
be
dependent on their distance from the imaging device. In the example shown,
each
recess may have a diameter of 3.55 mm when the imaging device is intended to
be
disposed 2cm from tray 20 during testing/calibration. See, for example, FIG.
3A. FIG.
3B shows the tray 20 positioned in second member 17 of phantom 10 during
fluorescent imaging. As will be understood by those in the art, in order to
obtain
reproducible results for calibration and training purposes, it is necessary to
understand the distance between the excitation light source and the material
configured to represent diseased tissue. It is further contemplated that the
diameter
of recesses 25 may increase as the imaging device is disposed further away
from
tray 20. Furthermore, each recess 25 may be the same size, or recesses 25 may
be
of varying sizes.
[0059] One or more materials may be disposed within each recess 25. The
materials include a first composition configured to have the optical
properties of
diseased tissue. In addition, a second material configured to cause the
diseased
tissue material to fluoresce in response to illumination with excitation light
is
included. These materials may be in solid or liquid form, or a combination
thereof. In
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
accordance with one example embodiment, the first composition may include any
material that is capable of mimicking the optical properties of "cancerous"
tissue to
provide one or more portions of the phantom with optical characteristics
substantially
the same as the optical characteristics of cancerous tissue. The first
composition
may include, for example, agar, phosphate buffered saline (PBS), water,
agarose,
dimethyl sulfoxide (DMSO) and/or blood tissue. These components may be used to
hold the second material in suspension in the solution.
[0060] The second material may include PpIX and/or another fluorescent dye.
Each recess 25 may include a solution with a different concentration of PpIX
and/or
fluorescent dye, such that the concentration of PpIX or fluorophore in each
recess
differs from that of an adjacent recess 25. For example, a first recess may be
a
control that does not contain any PpIX or dye. A second recess may contain a
small
concentration of PpIX (and/or another fluorescent dye), a third recess may
contain a
relatively greater concentration of PpIX (and/or another fluorescent dye), a
fourth
recess may contain an even greater concentration of PpIX (and/or another
fluorescent dye), etc. In one example, tray 10 has ten recesses 25 such that
each
recess has one of the following concentrations of PpIX: 0.001 M, 0.005 M,
0.01
M, 0.05 M, 0.1 M, 0.5 OA, 1 OA, 5 OA, 10 OA, and 20 M.
[0061] The solutions disposed within each recess 25 may mimic the optical
properties of "cancerous tissue." Thus, tray 20 may form a portion of tissue
phantom
that has the optical properties of cancerous tissue.
[0062] The distal end of second member 17, where tray 20 is disposed, may
include an opening 30 through which an optical power meter may be inserted
into
the tissue phantom. Opening 30, without an optical power meter, can be seen in
21
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
FIGS. 2A and 2B. Opening 30 with an optical power meter positioned therein is
shown in FIG. 3B. In some embodiments, opening 30 may be a 1 x 1 cm square
hole. In other embodiments, opening 30 may be a round hole or any other shape
sufficient for any well-known optical power meter.
[0063] An opening 37 may also be created in the end of first member 12 to
allow
access to the interior of the tissue phantom and the cavity 35 defined
therein, to
permit an imaging device a field of view of the diseased portion of the tissue
phantom represented by the contents of the recesses in tray 20. An imaging
device
may be inserted at least partially through opening 37. Thus, opening 37 may be
of
sufficient size to receive at least a portion of an imaging device configured
to be
placed within a body lumen or cavity or surgical cavity. The imaging device
may pass
through opening 37 to access cavity 35 in any manner that maintains the
integrity of
tissue phantom 10 while providing the ability for the imaging device to
illuminate the
diseased tissue material in recesses 25 with excitation light and to receive
emissions
responsive to illumination by the excitation light from the diseased tissue
material in
recesses 25.
[0064] Once assembled, tissue phantom 10 includes first and second members
12, 17 attached together with tray 20 disposed within cavity 35 and positioned
on
second member 17. Additionally, a variety of solutions are disposed within
each
recess 25 of tray 20, such that each recess 25 has a different concentration
of, for
example, PpIX. An optical power meter may then be inserted at least partially
through cavity 30, and an imaging device may be inserted at least partially
through
cavity 35. The imaging device, once inserted within tissue phantom 10, will
then
emit an excitation light onto and/or within tissue phantom 10. As discussed
above,
any recess 25 that contains PpIX (or another fluorescent dye) will be
illuminated by
22
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
the excitation light and emissions, in response to the excitation light, from
the
material in the recesses will be received and captured by the imaging device.
Such
may allow a user to determine which concentrations of PpIX are detectable by
the
imaging device. The diseased tissue material in the recesses 25 may be excited
individually or in a group. To allow the material in each recess 25 to be
illuminated
with excitation light individually, a dial or cover 42 may be placed over the
recesses
25 such that only a single recess is visible at a time. An example dial 42 is
shown in
FIG. 30. An example of a plurality of recesses being illuminated at the same
time is
shown in FIG. 3B. The central square in the tissue phantom is an optical meter
and
the black surrounding the red recesses (or wells) is the material of the tray.
This
determination may then be used to calibrate the imaging device. The optical
power
meter inserted within cavity 30 measures the optical power of the imaging
device.
[0065] The imaging device may emit white light (WL), fluorescence (FL),
infrared
(IR), or a mixture thereof. In some embodiments, the imaging device may emit
excitation light at a wavelength from 350 nm to 450 nm and/or from 550 nm to
600
nm, and more specifically the imaging device may emit excitation light at a
wavelength of 405 nm and/or 572 nm.
[0066] When illuminated with a wavelength of, for example, 405 nm, first
member
12 and second member 17 will fluoresce in the same manner as healthy breast
tissue, which autofluoresces under 405 nm excitation light and appears green
in
fluorescent images. Thus, the parts of the phantom 10 made to mimic healthy
breast
tissue will, for example, fluoresce green when illuminated with 405 nm
excitation
light. However, when the solutions within each recess 25 that contain PpIX are
illuminated with a wavelength of, for example, 405 nm, the solutions will
fluoresce in
the same manner as cancerous breast tissue. Thus, the parts of the phantom 10
23
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
made to mimic cancerous tissue will, for example, fluoresce red when
illuminated
with 405 nm excitation light. This allows the cancerous tissue of the phantom
to be
distinguished from the healthy tissue of the phantom when both tissues are
illuminated with the same excitation light, thus allowing a user to determine
portions
in tissue phantom that include the cancerous tissue.
[0067] Different concentrations of PpIX in each solution, will emit
different
wavelength intensities of light from tissue phantom 10. This allows the light
emitted
from each unique solution to be distinguished from the light emitted from a
subsequent solution (with a different amount of PplX). Additionally, this
allows the
light emitted from first and second end portions 12, 17 (the healthy tissue)
to be
distinguished from the light emitted from recesses 25 (the cancerous tissue).
Therefore, a user can identify the presence of cancerous tissue and its
location
within tissue phantom 10.
[0068] Because the different concentrations of PpIX in each recess 25 emit
different intensities of light from tissue phantom 10, a user may use tissue
phantom
to calibrate an optical instrument. More specifically, an optical instrument
may be
used to illuminate tissue phantom 10 with excitation light, for example
excitation light
having a wavelength of 405 nm. As discussed above, a first solution disposed
in a
first recess 25 may have a first concentration of PpIX, and a second solution
disposed in a second recess 25 may have a second concentration of PpIX. The
first
solution may emit a different intensity of light from the second solution when
illuminated with the excitation light. A user may then calibrate the optical
instrument
by comparing the different concentrations of PpIX in the first and second
solutions
with the light emitted therefrom. Additionally or alternatively, a user may
compare
24
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
the amount of fluorescence from the first and second solutions with the
concentrations of PpIX in order to calibrate the optical instrument.
[0069] The imaging device may also include a camera in order to capture the
illuminated solution in recesses 25. Such results may then be displayed to a
user.
[0070] In other embodiments of a tissue phantom, it may be desirable to
remove
the optical barrier provided by the tray 20, in order to visualize the
"diseased tissue"
or fluorophore representing the diseased tissue against the background of the
"healthy
tissue" forming the phantom 10. This may be accomplished in various ways such
as,
for example, placing the "diseased tissue" or fluorophore directly on the
phantom
material mimicking the healthy tissue, placing the "diseased tissue" or
fluorophore on
an optically clear surface (such as a microscope well slide) and then placing
the clear
surface on the phantom's "healthy tissue," or by creating wells in the
phantom's
"healthy tissue" and placing the "diseased tissue" or fluorophore in the
wells.
[0071] In one example embodiment, shown in FIG. 4, the material forming the
"diseased tissue," 120 e.g., a fluorophore, can be placed directly on the
phantom
material that mimics the healthy tissue 105, for example using a pipette,
syringe, or
paintbrush. The phantom material will absorb liquid fluorophores if placed
directly on
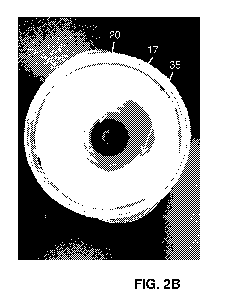
top. Alternatively, the fluorophore can be mixed with a solidifying agent such
as gelatin
to prevent this. A fluorescent image of a phantom 100 having healthy tissue
portion
105 painted with a PpIX and gelatin mixture (red/pink) to form the diseased
tissue
portions 120 is shown in FIG. 4.
[0072] In another example embodiment, shown in FIGS. 5A and 5B, a surface
or
container with an optically clear bottom, such as a glass microscope well
slide 130
(see FIG. 5A, containing PpIX in the right side of the top row) or plastic PCR
tube lids
140 (see FIG. 5B, containing 630 nm quantum dots in decreasing concentration)
is
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
used to position the "diseased tissue" or fluorophore representing the
diseased tissue
onto the phantom material representing the healthy tissue. This allows for
various
concentrations of the fluorophore to be imaged against the green background
(the
phantom material forming the "healthy tissue" of the phantom will fluoresce
green
when illuminated with 405 nm excitation light) to see the contrast between
each
concentration of the fluorophore and the background. In another embodiment,
tray 20
is transparent so that wavelengths of light emitted by a light source of the
imaging
device penetrate through recesses 25 and onto second member 17, i.e., against
the
healthy tissue of the phantom or the phantom background. These embodiments may
provide a realistic scenario for training purposes by allowing a determination
of the
amount of "diseased tissue" required, or percentage of PpIX or fluorophore
required,
to visualize the diseased tissue in situ.
[0073] In the example embodiment shown in FIG. 6, recesses or wells can be
created directly in the healthy tissue of the phantom material by cutting out
cylindrical
portions of the tissue phantom. This can be done, for example, using a punch
biopsy
tool (2-, 4-, 8-mm, or another diameter) to remove a cylindrical chunk of the
phantom
material. Then, the cylindrical recess or well can be filled with a
fluorophore. The
liquid fluorophore can be mixed with phantom material in liquid form and added
to
the well, to create a solid fluorescent well that will maintain its form over
time. A
phantom material concentration of greater than 50% will allow the mixture to
solidify
while not significantly affecting the fluorescent color of fluorophore mixed
into the
wells. With a stable fluorophore such as a quantum dot, this method can be
used to
create reproducibly fluorescent phantoms that maintain their fluorescence over
time.
FIG. 6 shows a fluorescent image of varying concentrations of PpIX mixed with
89%
26
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
phantom material and pipetted into wells created with a 4 mm punch biopsy
tool.
This image was captured after the mixture solidified.
[0074] In some embodiments, as shown in FIGS. 7 and 8, a tissue phantom 200
may be used to mimic the shape of a patient's breast, which may include a
breast
lumpectomy cavity. Such phantoms are designed to mimic the shape and
mechanical/elastic properties of a breast. These phantoms might come in a
variety of
different configurations.
[0075] In one example, the phantom may be formed as a full breast with a
solid
PpIX tumor inclusion. In this embodiment, a surgeon can palpate to locate the
tumor
as they would normally do prior to a surgery. The surgeon would then create an
incision/surgical cavity accordingly. The surgeon would image the resected
mass
and the cavity to ensure clear margins.
[0076] In another example, the phantom may be formed as a full breast with
a
pre-made cavity containing a lumpectomy sample. Such an embodiment of the
breast phantom would have a pre-made cavity and corresponding lumpectomy
sample, both with positive margins (i.e., both including diseased or
"cancerous"
tissue that a surgeon could image). The cavity size and shape would be
designed to
allow the surgeon to manipulate an imaging device in accordance with the
present
disclosure in a variety of ways. This would allow surgeons to practice imaging
at the
bottom and sides of a surgical cavity, as well as under (premade) skin flaps.
[0077] As discussed above, the target tissue may be made identifiable by
use a
fluorophore. Although the example embodiments describe the use of PpIX, other
fluorophores may be used. For example, a phantom in accordance with FIG. 7 can
be combined with ICG. These models would train the surgeon to image in
fluorescent (FL) mode and infrared (IR) mode, as well as to switch between
them
27
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
and observe the resulting images in real time. Fluorescent ICG "bars" similar
to the
ICG phantom would be placed within the cavity to mimic vasculature, both at
and
below the surface of the phantom.
[0078] In one example embodiment, a tissue phantom may include a first
healthy
tissue portion 305 and one or more target tissue portions 310, 315, 320. In
the
example shown in FIG. 9, the tissue phantom 300 includes solid ICG "bars" 310,
315, 320 to mimic vasculature or masses (target tissue) that contain ICG. The
tissue
phantom 300 may be a flat slab of the phantom tissue material with three ICG
bars
placed within: (A) a bar 310 directly at the top surface of the phantom 300,
(B) a bar
315 submerged 3 mm below the surface of the phantom 300, and (C) a bar 320 on
an angle such that one side of the bar is at the surface of the tissue phantom
300
and the other side of the bar is submerged 1 cm in the phantom material. This
third
bar 300 is also placed close to the end of the phantom 300 such that the slope
of the
bar can be visualized if the side of the phantom is imaged under IR
excitation.
[0079] In accordance with another aspect of the present disclosure, tissue
phantoms may incorporate diseased cells in the "diseased" portion of the
phantom.
For example, in one example embodiment of a breast tissue phantom, the portion
of
the phantom configured to mimic cancerous tissue comprises carcinoma cells. In
preparing this phantom, carcinoma cells were treated with 5-ALA, resuspended
in
tissue phantom material and injected under the surface of a normal or healthy
tissue
phantom to create a "tumor." The tumor was resected from the breast tissue
phantom, creating an excised tissue specimen or phantom lumpectomy. The
phantom lumpectomy and the surgical cavity were examined using widefield
fluorescence imaging. Residual carcinoma cells producing PpIX were visualized
in
the surgical cavity and at the margins of the excised lumpectomy. The image of
FIG.
28
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
illustrates an example embodiment of a breast tissue phantom 400 comprising
carcinoma cells in the diseased tissue portion 420 of the phantom. The PpIX-
containing carcinoma cells and phantom material may be injected into the
healthy
phantom tissue 405 to form discrete diseased portions or tumors 420 in phantom
400.
[0080] An example process of making the carcinoma tumors for inclusion in
the
healthy tissue of the tissue phantom is described below and shown in the
flowchart
of FIG. 11. Example cells lines that may be used include MDA-MB-468 (ATCC
HTB-132Tm); MDA-MB-231 (ATCC CRM-HTB-26Tm); MCF-7(ATCC HTB-22Tm);
BxPC3 (ATCC CRL-1687Tm); and 06 (ATCC CCL-107Tm).
Protocol
Prep
Cells
1. Supplement new media (RPM! or DMEM) with 10% heat-inactivated fetal bovine
serum, penicillin (0.05 mg/ml) and streptomycin (0.05 mg/ml).
2. Start a new cell line culture from liquid nitrogen stored cryovials in T25
plate
2.1. Use complete supplemented RPM! media for BxPC3 cell line.
2.2. Use complete supplemented DMEM for MDA-MB-468, MDA-MB-231, 06,
and MCF-7 cell lines.
3. Check cells daily to ensure they are growing and healthy. Follow ATCC
recommendations for splitting cells into T75 Flasks.
5-ALA
1. Prepare 6mM stock solution of ALA by dissolving 15.736mg of 5-ALA in 20mL
of
PBS.
2. For each T75 plate of BXPC3 cells, add 2mL of stock solution of ALA with
4mL of
warmed complete RPM! media [2mM]
3. For each T75 plate of the other cell lines, add 2mL of stock solution of
ALA with
4mL of warmed complete DMEM media [2mM].
4. Test the pH of the adjusted media to ensure that the pH is 7.4.
Treatment
1. Once cells are between 70-90% confluent in a T75 plate, aspirate the media
from
the wells and wash with 2mL of PBS.
2. Add 6mL of appropriate ALA media to the plates.
3. Incubate for 4hr5.
29
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
4. Keeping the Biosafety cabinet dark, aspirate the media from the flasks and
wash
with 2mL of PBS.
5. Detach the cells from the flasks with 2mL of warmed 0.05%Trypsin-EDTA. Wait
1-3 minutes for the cells to detach, the BxPC3 cells may require additional
time.
Monitor flasks closely to ensure that they do not sit in trypsin-EDTA for more
time
than necessary.
6. Deactivate the trypsin EDTA by adding 2mL of warmed complete ALA-free
media.
7. Suspend the cells in the flask and transfer to a labelled 15mL tube.
8. Centrifuge 15mL tubes at 500rpm for 5mins.
9. Resuspend the cells in 5mL of PBS.
10. Count cells
a) remove lOul of each tube and place in individual microfuge tubes.
b) dilute with lOul of trypan blue.
c) place 10uL in the hemocytometer
d) count cells
11. Centrifuge each 15mL tube at 500rpm for 5m ins.
12. Remove the 15mL tubes from the centrifuge and aspirate the supernatant.
[0081] In accordance with another aspect of the present disclosure tissue
phantoms may be used to determine a depth of healthy tissue through which
diseased tissue can be detected, for example by an imaging device as described
herein. A depth tissue phantom 500 is illustrated in FIG. 12. In accordance
with the
present teachings, a depth phantom 500 is a phantom that has been designed to
measure the fluorescence limit of detection of an imaging device in terms of
depth,
target size, and target concentration. This phantom 500 comprises a
microfluidics
chip 510 preloaded with PpIX at varying concentrations 520a, 520b, 520c, etc.
The
size of the wells 525 that contain the PpIX can vary within any range. An
example
range is 10 m-1 mm. The chip is placed in a tray and imaged. Then, a small
amount
of the phantom material designed for 405 nm wavelength excitation is poured to
cover the chip. Once the phantom material sets, the chip is imaged again. This
process would be repeated until no more fluorescence is visible from any of
the wells
at some depth.
[0082] As an alternative to pouring phantom material over the chip, one or
more
thin film phantoms may be used. The phantom material can be used to create
thin
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
films with thickness around 100 pm or less, by dehydrating the liquid phantom
material. This material can be used to mimic thin films of tissue to measure
the depth
below the tissue surface at which a fluorophore can still be detected using,
for
example, a depth phantom. See, for example, FIGS. 20A and 20B.
[0083] In accordance with another aspect of the present disclosure, a
tissue
phantom may be used to identify potential interactions between different types
of
imaging and/or contrast agents used together during surgery, such as BCS, to
make
residual cancer cells, precancerous cells, satellite lesions, and/or other
malignant
cells in surgical cavities, tissue specimens, lymph nodes, or other areas
visible to the
surgeon.
[0084] During breast cancer surgery, surgeons may perform a biopsy on the
sentinel lymph node(s) 600 surrounding the tumor. By using methods such as
blue
dye localization or radioactive tracers, surgeons can locate the sentinel
lymph nodes
to dissect them and determine if the cancer has metastasized from the original
tumor. In breast cancer surgery, typically, the blue dye is subcutaneously
injected in
the tumor, the surrounding tissue (pen-tumor), or near the nipple, so that it
may
diffuse through the lymphatic vessels and reach the nearby sentinel lymph
nodes,
where it accumulates to turn blue. This allows surgeons to visualize the
sentinel
lymph nodes using conventional white lighting and the unaided eye. An example
of
this is shown in the image of FIG. 13. One of the most common dyes for
surgical
purposes is Methylene Blue (MB). MB absorbs certain wavelengths of light,
often in
the 600-700 nm range. The peak absorption of MB is 665 nm as shown in FIG. 14.
[0085] MB is typically administered in 3-5 mL volumes at a 1%
concentration. It is
suspected that MB has a strong ability to absorb light in the 550-700 nm
wavelength
spectrum. Under 405 nm excitation, protoporphyrin IX's (PpIX's) peak emission
31
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
occurs at -635 nm, as shown in FIG. 1B, which overlaps with the absorption
spectra
of MB (or blue dye), potentially attenuating the PpIX fluorescence (FL)
emission due
to the spectral overlap. This spectral overlap is well known in the field of
intraoperative fluorescence imaging, however, there is a paucity of
information about
this issue in the area of (fluorescence imaging) during breast cancer surgery.
[0086] Tissue phantoms in accordance with the present disclosure can be
used to
obtain a better understanding of this phenomenon to better appreciate its
potential
effect (if any) on the fluorescence detection of (tumor) PpIX fluorescence
when MB
(or similar blue dye) is used intraoperatively for SLN detection during breast
cancer
surgery.
[0087] The phantom material "recipe" can be modified to incorporate dyes,
fluorophores, or other materials directly into the phantom material. In one
example
embodiment, a plurality of tissue phantoms were created, the phantoms having
increasing concentrations of Methylene Blue (MB) in the phantom tissue
material. A
plurality of wells or recesses were then created in each tissue phantom. The
wells in
the phantoms were also created with punch biopsy tools although other methods
can
be used. In these wells, a mixture of PpIX, methylene blue, and phantom
material
was pipetted to form solid phantoms.
[0088] To create the MB tissue phantom, MB and PpIX were diluted in phantom
material (PM), which was prepared as described below. When mixing PM with
PpIX,
for longevity and reproducibility, a proportion of PM >75% allows the mixture
to
solidify and minimizes the liquid content being absorbed by surrounding PM. A
combination of PpIX diluted with PM to 5 M PpIX and 90% PM proportion
produces
bright red FL when imaged.
32
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
[0089] As previously described with respect to other embodiments of a
tissue
phantom, the phantom material comprises two mixtures: a tris buffer and
gelatin
mixture. In preparing MB tissue phantoms, the formulation was adjusted to
include
methylene blue by combining cooled base phantom mixture with concentrated
stocks
of 100, 50, 10, 5, 1, and 0 M methylene blue, to dilute by a factor of 10,
which
achieved a final concentration of 10,5, 1, 0.5, 0.1, and 0 M methylene blue
phantom mixture. These mixtures were used to create 25 mL phantom samples with
a gradient of methylene blue concentrations. Three different MB phantom models
were created.
[0090] The first MB tissue phantom is designed to simulate the clinical
scenario
where, at the surgical margin, there is residual tumor (containing PplX) that
also
contains MB within the tumor, and/or is surrounded by normal tissue containing
MB.
This first MB tissue phantom was used to measure which MB concentrations (in
the
tissue and within the tumor) are able to decrease the FL signal from a single
concentration of PpIX. The phantoms 700 were designed as pucks, each with ten
wells 725 cut with a punch biopsy tool. This was repeated for 3 punch biopsy
sizes: 2
mm, 4 mm and 8 mm (The 8 mm phantoms had to be split into two "pucks" due to
the relative size of the wells and the pucks, FIG. 16). Six groups of these
phantoms
were made, one with each of the following concentrations of MB mixed into the
phantom tissue: 0, 0.1, 0.5, 1,5, 10 M (FIGS. 15 and 16). Pucks measured 55
mm
in diameter and 10 mm in thickness. The wells were filled as follows (the %PM
is the
proportion of the mixture that is PM) and allowed to set until solidified:
= b: PM ¨ represents background tissue phantom
= c: 5 M PpIX + water + 89% PM
= 1: 5 M PpIX + 0.01 OA MB + 89% PM
= 2: 5 M PpIX + 0.1 M MB + 89% PM
= 3: 5 M PpIX + 0.5 M MB + 89% PM
= 4: 5 M PpIX + 1 M MB+ 89% PM
33
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
= 5:5 M PpIX + 1.75 M MB+ 89% PM
= 6: 5 M PpIX + 2.5 M MB + 89% PM
= 7: 5 M PpIX + 5 OA MB + 89% PM
= 8: 5 OA PpIX + 10 OA MB + 89% PM
[0091] Each phantom was imaged under FL imaging using an imaging device as
described herein to determine the imaging effect of each concentration of MB
on PpIX
fluorescence.
[0092] A second MB tissue phantom 800 was created to measure how the FL
from various concentrations of PpIX is affected by a given concentration of
MB.
Examples of these tissue phantoms 800 are shown in FIGS. 17 and 18. Six
versions
of the second MB tissue phantoms were made with the same variation in MB
tissue
concentration as the first MB tissue phantom. The ten wells 825 cut in these
phantoms all contain phantom tissue material mixture and a concentration of MB
which matches the tissue, with one of nine PpIX concentrations. The wells were
filled as follows (the %PM is the proportion of the mixture that is PM):
= b: PM + MB (same [MB] as tissue) ¨ represents background tissue phantom
= 1: 50 M PpIX + MB + 89% PM (same [MB] as tissue)
= 2: 25 M PpIX + MB + 89% PM (same [MB] as tissue)
= 3: 10 M PpIX + MB + 89% PM (same [MB] as tissue)
= 4: 5 M PpIX + MB + 89% PM (same [MB] as tissue)
= 5: 2.5 M PpIX + MB + 89% PM (same [MB] as tissue)
= 6: 1 M PpIX + MB + 89% PM (same [MB] as tissue)
= 7: 0.5 M PpIX + MB + 89% PM (same [MB] as tissue)
= 8: 0.25 M PpIX + MB + 89% PM (same [MB] as tissue)
= 9: 0.1 M PpIX + MB + 89% PM (same [MB] as tissue)
[0093] A third MB tissue phantom 900 was made to test the effect of a thin
layer
of MB-containing tissue phantom covering normal tissue and tumor that do not
contain MB. The third MB tissue phantom comprises a first part 905 ¨ a tissue
phantom that does not contain MB and a second part comprising a thin film MB
tissue phantom 930 similar to the thin film tissue phantoms described above.
Each
34
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
first part tissue phantom 905 has ten wells 925 cut, all identically filled
with 51..1M
PpIX and 89% PM (see FIG. 18). The second part of the third MB tissue phantom
900 is a thin dehydrated MB tissue phantom film 930 (FIGS. 20A and 20B) which
were produced, each with one of nine concentrations of MB. The films 930 were
made by filling a 58-mm diameter dish with 3 mL of PM with the desired
concentration of MB and allowing to dehydrate at 210, -55% humidity for 48
hours.
These films are 100 +/- 25 pm in thickness as measured with a Mitutoyo
Digimatic
Micrometer 293-832-30. To replicate the scenario where a thin layer of MB is
on the
surface of a tumor with PpIX, a "pizza slice" shape was cut from each film and
placed over a portion of the phantom puck, fully covering a well.
[0094] The MB tissue phantoms discussed herein were used to test the effect
of
blue dyes on PpIX, with the concentrations of the blue dye and PpIX both
varied
using different phantom models to isolate the impact of each concentration of
blue
dye on each concentration of PpIX. Such an experiment can also be conducted
using phantoms that replace with PpIX with some other FL contrast agent such
as
ICG, IRDye800, or fluorescein. The blue dye can be replaced with another
(clinically
relevant) dye (such as for example Patent Blue V, Indigo Carmine, lsosulfan
Blue,
and specimen inking dyes such as, for example, India ink or other proprietary
formulations of specimen inking dyes) to understand the interaction of the
standard
of care dye/material on the desired fluorescence signal.
[0095] It will be appreciated by those ordinarily skilled in the art having
the benefit
of this disclosure that the present disclosure provides various exemplary
devices,
systems, and methods for tumor visualization. Further modifications and
alternative
embodiments of various aspects of the present disclosure will be apparent to
those
skilled in the art in view of this description.
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
[0096] Furthermore, the devices and methods may include additional
components
or steps that were omitted from the drawings for clarity of illustration
and/or
operation. Accordingly, this description is to be construed as illustrative
only and is
for the purpose of teaching those skilled in the art the general manner of
carrying out
the present disclosure. It is to be understood that the various embodiments
shown
and described herein are to be taken as exemplary. Elements and materials, and
arrangements of those elements and materials, may be substituted for those
illustrated and described herein, parts and processes may be reversed, and
certain
features of the present disclosure may be utilized independently, all as would
be
apparent to one skilled in the art after having the benefit of the description
herein.
Changes may be made in the elements described herein without departing from
the
spirit and scope of the present disclosure and following claims, including
their
equivalents.
[0097] It is to be understood that the particular examples and embodiments
set
forth herein are non-limiting, and modifications to structure, dimensions,
materials,
and methodologies may be made without departing from the scope of the present
disclosure.
[0098] Furthermore, this description's terminology is not intended to limit
the
present disclosure. For example, spatially relative terms¨such as "beneath,"
"below," "lower," "above," "upper," "bottom," "right," "left," "proximal,"
"distal," "front,"
and the like¨may be used to describe one element's or feature's relationship
to
another element or feature as illustrated in the figures. These spatially
relative terms
are intended to encompass different positions (i.e., locations) and
orientations (i.e.,
rotational placements) of a device in use or operation in addition to the
position and
orientation shown in the drawings.
36
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
[0099] For the purposes of this specification and appended claims, unless
otherwise indicated, all numbers expressing quantities, percentages or
proportions,
and other numerical values used in the specification and claims, are to be
understood as being modified in all instances by the term "about" if they are
not
already. Accordingly, unless indicated to the contrary, the numerical
parameters set
forth in the following specification and attached claims are approximations
that may
vary depending upon the desired properties sought to be obtained by the
present
disclosure. At the very least, and not as an attempt to limit the application
of the
doctrine of equivalents to the scope of the claims, each numerical parameter
should
at least be construed in light of the number of reported significant digits
and by
applying ordinary rounding technique.
[00100] Notwithstanding that the numerical ranges and parameters setting forth
the broad scope of the present disclosure are approximations, the numerical
values
set forth in the specific examples are reported as precisely as possible. Any
numerical value, however, inherently contains certain errors necessarily
resulting
from the standard deviation found in their respective testing measurements.
Moreover, all ranges disclosed herein are to be understood to encompass any
and
all sub-ranges subsumed therein.
[00101] It is noted that, as used in this specification and the appended
claims, the
singular forms "a," "an," and "the," and any singular use of any word, include
plural
referents unless expressly and unequivocally limited to one referent. As used
herein, the term "include" and its grammatical variants are intended to be non-
limiting, such that recitation of items in a list is not to the exclusion of
other like items
that can be substituted or added to the listed items.
37
CA 03127027 2021-07-16
WO 2020/148720
PCT/IB2020/050379
[00102] It should be understood that while the present disclosure has been
described in detail with respect to various exemplary embodiments thereof, it
should
not be considered limited to such, as numerous modifications are possible
without
departing from the broad scope of the appended claims, including the
equivalents
they encompass.
38