Note: Descriptions are shown in the official language in which they were submitted.
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
USE OF A PPAR-DELTA AGONIST IN THE TREATMENT OF FATTY ACID
OXIDATION DISORDERS (FAOD)
CROSS-REFERENCE
[0001] This application claims the benefit of U.S. Provisional Patent
Application No.
62/800,995 filed on February 4, 2019, which is incorporated herein by
reference in its entirety.
FIELD OF THE INVENTION
[0002] Described herein are methods of using a peroxisome proliferator-
activated receptor
delta (PPAR-delta) agonist in the treatment or prevention of fatty acid
oxidation disorders
(FAOD).
BACKGROUND OF THE INVENTION
[0003] Healthy mitochondria are vital to normal cellular activities.
Mitochondrial dysfunction
drives the pathogenesis of a wide variety of medical disorders, including
acute conditions and
chronic diseases. Distinct aspects of mitochondrial function, for example,
bioenergetics,
dynamics, and cellular signaling are well described and impairments in these
activities likely
contribute to disease pathogenesis. Impairments of mitochondrial function
result in a family of
disorders termed fatty acid oxidation disorders. PPAR-delta, a member of the
nuclear regulatory
superfamily of ligand-activating transcriptional regulators, is expressed
throughout the body.
PPAR-delta agonists induce genes related to fatty acid oxidation and
mitochondrial biogenesis.
PPAR-delta also has anti-inflammatory properties.
SUMMARY OF THE INVENTION
[0004] In one aspect, described herein are methods for treating a fatty acid
oxidation disorders
(FAOD) in a mammal comprising administering to the mammal with a fatty acid
oxidation
disorder (FAOD) a peroxisome proliferator-activated receptor delta (PPARo)
agonist compound.
[0005] In another aspect, described herein is a method for improving whole-
body fatty acid
oxidation in a mammal with a fatty acid oxidation disorder (FAOD) comprising
administering to
the mammal with a fatty acid oxidation disorder (FAOD) a peroxisome
proliferator-activated
receptor delta (PPARo) agonist compound.
[0006] In another aspect, described herein is a method of modulating
peroxisome proliferator-
activated receptor delta (PPARo) activity in a mammal with a fatty acid
oxidation disorder
(FAOD) comprising administering to the mammal with the fatty acid oxidation
disorder (FAOD)
a proliferator-activated receptor delta (PPARo) agonist compound.
- 1 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[0007] In some embodiments, modulating peroxisome proliferator-activated
receptor delta
(PPAIto) activity comprises activating peroxisome proliferator-activated
receptor delta (PPAR6).
[0008] In some embodiments, modulating peroxisome proliferator-activated
receptor delta
(PPAIto) activity comprises increasing peroxisome proliferator-activated
receptor delta (PPAIto)
activity.
[0009] In yet another aspect, described herein is a method for increasing
fatty acid oxidation
(FAO) in a mammal with a fatty acid oxidation disorder (FAOD) comprising
administering to the
mammal with the fatty acid oxidation disorder (FAOD) a proliferator-activated
receptor delta
(PPAIto) agonist compound.
[0010] In some embodiments, the peroxisome proliferator-activated receptor
delta (PPAIto)
agonist compound is administered to the mammal in an amount sufficient for
normalizing FAO
capacities in the mammal, up-regulating gene expression of any one of the
enzymes or proteins
involved in FAO, or a combination thereof
[0011] In some embodiments, normalizing FAO capacities in the mammal comprises
increasing
FAO capacities to sufficient levels for ameliorating or reducing the severity
of any one of
symptoms of any one of the fatty acid oxidation disorders described herein.
[0012] In one aspect, described herein is a method for treating a fatty acid
oxidation disorder
(FAOD) in a mammal comprising administering to the mammal with a FAOD a
peroxisome
proliferator-activated receptor delta (PPAIto) agonist compound.
[0013] In some embodiments, treating FAOD comprises improving whole-body fatty
acid
oxidation (FAO) in the mammal, improving the mammal's exercise tolerance,
decreasing pain,
decreasing fatigue, or a combination thereof.
[0014] In some embodiments, improving the mammal's exercise tolerance
comprises
increasing the distance walked in about a 12-minute walk test. In some
embodiments, the
distance walked in such a 12 minute walk test increases by at least about 1
meter, at least about 5
meters, at least about 10 meters, at least about 20 meters, at least about 30
meters, at least about
40 meters, at least about 50 meter, at least about 60 meters, at least about
70 meters, at least
about 80 meters, at least about 90 meters, at least about 100 meters, or more
than about 100
meters.
[0015] As used herein the term "about" means within 10% of the value.
[0016] In some embodiments, improving the mammal's exercise tolerance
comprises decreases
in heart rate during the about 12-minute walk test. In some embodiments, heart
rate decreases by
1 heart beat per minute, by 2 heart beats per minute, by 3 heart beats per
minute, by 4 heart beats
- 2 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
per minute, by 5 heart beats per minute, by at least about 10 heart beats per
minute, or by at least
about 20 heart beats per minute.
[0017] In some embodiments, improving the mammal's exercise tolerance
comprises decreases
in measured respiratory exchange ratios (RER).
[0018] In some embodiments, improving whole-body fatty acid oxidation in the
mammal
comprises increasing fatty acid oxidation (FAO) in the mammal. In some
embodiments,
increasing fatty acid oxidation (FAO) in the mammal comprises increases in the
amount of
exhaled '3CO2 from the mammal after consuming a meal comprising '3C-enriched
fatty acids. In
some embodiments, the amount of exhaled '3CO2 increases by at least about 5%,
about 10%,
about 15%, about 20%, about 25%, about 30%, about 40%, about 50%, about 60%,
about 70%,
about 80%,about 90%, or more than about 90% as compared to a mammal who is fed
a meal
comprising '3C-enriched fatty acids and not administered a PPARo agonist
compound. In some
embodiments, the amount of exhaled '3CO2 increases by at least about 5%, about
10%, about
15%, about 20%, about 25%, about 30%, about 40%, about 50%, about 60%, about
70%, about
80%,about 90%, or more than about 90% as compared to a mammal who is not fed a
meal
comprising '3C-enriched fatty acids. In some embodiments, the amount of
exhaled '3CO2
increases by at least about 5%, about 10%, about 15%, about 20%, about 25%,
about 30%, about
40%, about 50%, about 60%, about 70%, about 80%,about 90%, or more than about
90% as
compared to a mammal who is not fed a meal comprising '3C-enriched fatty acids
and not
administered a PPARo agonist compound.
[0019] In some embodiments, administration of the PPARo agonist compound to
the mammal
normalizes FAO capacities in the mammal, up-regulates gene expression of any
one of the
enzymes or proteins involved in FAO, increases the activity of an enzyme or
protein involved in
FAO, or a combination thereof.
[0020] In some embodiments, the peroxisome proliferator-activated receptor
delta (PPARo)
agonist compound is administered to the mammal in an amount sufficient for
increasing the
activity of mutated enzymes or proteins involved in FAO. In some embodiments,
the peroxisome
proliferator-activated receptor delta (PPARo) agonist compound is administered
to the mammal
in an amount sufficient for increasing the activity of mutated but
catalytically active enzymes or
proteins involved in FAO.
[0021] In some embodiments, the fatty acid oxidation disorder comprises
defects in the
enzymes or proteins involved in the entry of long-chain fatty acids into
mitochondria,
intramitochondrial 13-oxidation defects of long-chain fatty acids affecting
membrane bound
enzymes, 13-oxidation defects of short- and medium-chain fatty acids affecting
enzymes of the
- 3 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
mitochondrial matrix, defects in the enzymes or proteins involved with
electron transfer to the
respiratory chain from mitochondrial 13-oxidation, or a combination thereof.
[0022] In some embodiments, the fatty acid oxidation disorder (FAOD) comprises
carnitine
transporter deficiency, carnitine/acylcarnitine translocase deficiency,
carnitine palmitoyl
transferase deficiency Type 1, carnitine palmitoyl transferase deficiency Type
2, glutaric
acidemia Type 2, long-chain 3-hydroxyacyl CoA dehydrogenase deficiency, medium-
chain acyl
CoA dehydrogenase deficiency, short-chain acyl CoA dehydrogenase deficiency,
short-chain 3-
hydroxyacyl CoA dehydrogenase deficiency, trifunctional protein deficiency, or
very long-chain
acyl CoA dehydrogenase deficiency, or a combination thereof.
[0023] In some embodiments, the fatty acid oxidation disorder comprises
carnitine
palmitoyltransferase II (CPT2) deficiency, very long-chain Acyl-CoA
dehydrogenase (VLCAD)
deficiency, long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD) deficiency,
Trifunctional
Protein (TFP) Deficiency; or a combination thereof.
[0024] In another aspect, described herein is a method of increasing activity
of an enzyme or
protein of the mitochondrial fatty acid beta-oxidation pathway in a mammal
comprising
administering a PPAIto agonist compound to a mammal with a mutation or
deficiency in an
enzyme or protein of the mitochondrial fatty acid beta-oxidation pathway.
[0025] In yet another aspect, described herein is a method of increasing
activity of an enzyme
or protein of the mitochondrial fatty acid beta-oxidation pathway in a mammal
comprising
administering a PPAIto agonist compound to a mammal with a deficiency in the
activity of an
enzyme or protein of the mitochondrial fatty acid beta-oxidation pathway.
[0026] In some embodiments, the deficiency in the activity of the enzyme or
protein of the
mitochondrial fatty acid beta-oxidation pathway results from a mutation in any
one of the
enzyme or protein of the mitochondrial fatty acid beta-oxidation pathway.
[0027] In some embodiments, the enzyme or protein of the mitochondrial fatty
acid beta-
oxidation pathway is short-chain acyl-CoA dehydrogenase (SCAD), medium-chain
acyl-CoA
dehydrogenase (MCAD), long-chain 3-hydroxyacyl-CoA dehydrogenase (LCHAD), very
long-
chain acyl-CoA dehydrogenase (VLCAD), mitochondrial trifunctional protein
(TFP), carnitine
transporter (CT), Carnitine palmitoyltransferase I (CPT I), carnitine-
acylcarnitine translocase
(CACT), carnitine palmitoyltransferase II (CPT II), isolated long-chain L3-
hydroxyl-CoA
dehydrogenase, medium-chain L3-hydroxyl-CoA dehydrogenase, short-chain L3-
hydroxyl-CoA
dehydrogenase, medium-chain 3-ketoacylCoA thiolase, or long-chain 3-
ketoacylCoA thiolase
(LCKAT).
- 4 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[0028] In some embodiments, the mutation is K304E of MCAD; L540P, V174M,
E609K, or
combination thereof, of VLCAD; E510Q of TFP-alpha subunit (HADHA); R247C of
TFP-beta
subunit (HADHB); or a combination thereof.
[0029] In some embodiments, the mutation is a nucleotide mutation in the gene
encoding
VLCAD. In some embodiments, the mutation is 842C>A,848T>C, 865G>A, 869G>A,
881G>A,
897G>T, 898A>G, 950T>C, 956C>A, 1054A>G, 1096C>T, 1097G>A, 1117A>T, 1001 T>G,
1066A>G, 1076C>T,1153C>T, 1213G>C, 1146G>C, 1310T>C, 1322G>A, 1358G>A,
1360G>A, 1372T>C, 1258A>C, 1388G>A, 1405C>T, 1406G>A, 1430G>A, 1349G>A,
1505T>C, 1396G>T, 1613G>C, 1600G>A, 1367G>A, 1375C>T, 1376G>A, 1532G>A,
1619T>C, 1804C>A, 1844G>A, 1825G>A, 1844G>A, 1837C>G, or a combination
thereof.
[0030] In some embodiments, the mammal has one or more symptoms typically
associated
with a fatty acid oxidation disorder. In some embodiments, symptoms typically
associated with a
fatty acid oxidation disorder include, but are not limited to: elevated
creatine kinase (CPK)
levels, hepatic dysfunction, cardiomyopathy, hypoglycemia, rhabdomyolysis,
acidosis, decreased
muscle tone (hypotonia), muscle weakness, exercise intolerance, or
combinations thereof.
[0031] In some embodiments, the PPARo agonist binds to and activates the
cellular PPARo
and does not substantially activate the cellular peroxisome proliferator
activated receptors - alpha
(PPARa) and - gamma (PPARy). In some embodiments, the PPARo agonist compound
is a
phenoxyalkylcarboxylic acid compound. In some embodiments, the PPARo agonist
compound
is a phenoxyethanoic acid compound, phenoxypropanoic acid compound,
phenoxybutanoic acid
compound, phenoxypentanoic acid compound, phenoxyhexanoic acid compound,
phenoxyoctanoic acid compound, phenoxynonanoic acid compound, or
phenoxydecanoic acid
compound.
[0032] In some embodiments, the PPARo agonist compound is a phenoxyethanoic
acid
compound or a phenoxyhexanoic acid compound. In some embodiments, the PPARo
agonist
compound is an allyloxyphenoxyethanoic acid acid compound.
[0033] In some embodiments, the PPARo agonist is a compound selected from the
group
consisting of (Z)-[2-Methy1-4-[3-(4-methylpheny1)-3-[4-[3-(morpholin-4-
yl)propynyl]phenyl]allyloxy]-phenoxy]acetic acid; (E)- [2-Methy1-4-[3-[4-[3-
(pyrazol-1-y1)prop-
1-ynyl]phenyl]-3-(4-trifluoromethylpheny1)-allyloxy]phenoxy]acetic acid; (E)-
[4-[3-(4-
Fluoropheny1)-3-[4-[3-(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-
phenoxy]acetic
acid; (E)- [2-Methy1-4-[3-[4-[3-(morpholin-4-yl)propynyl]phenyl]-3-(4-
trifluoromethylphenyl)allyloxy]-phenoxy]acetic acid; (E)-[4-[3-(4-
Chloropheny1)-3-[4-[3-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid;
(E)4443-(4-
- 5 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
Chloropheny1)-34443-(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methylphenyl]-
propionic
acid; {443,3-Bis-(4-bromo-pheny1)-allyloxy]-2-methyl-phenoxy}-acetic acid; or
a
pharmaceutically acceptable salt thereof.
[0034] In some embodiments, the PPARo agonist is a compound selected from the
group
consisting of (Z)-[2-Methy1-4-[3-(4-methylpheny1)-3-[4-[3-(morpholin-4-
yl)propynyl]phenyl]allyloxy]-phenoxy]acetic acid; (E)- [2-Methy1-4-[3-[4-[3-
(pyrazol-1-y1)prop-
1-ynyl]phenyl]-3-(4-trifluoromethylpheny1)-allyloxy]phenoxy]acetic acid; (E)-
[4-[3-(4-
Fluoropheny1)-3-[4-[3-(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-
phenoxy]acetic
acid; (E)- [2-Methy1-4-[3-[4-[3-(morpholin-4-yl)propynyl]phenyl]-3-(4-
trifluoromethylphenyl)allyloxy]-phenoxy]acetic acid; (E)-[4-[3-(4-
Chloropheny1)-3-[4-[3-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid;
(E)4443-(4-
Chloropheny1)-34443-(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methylphenyl]-
propionic
acid; { 443 -Isobutoxy-5-(3 -morpholin-4-yl-prop-1 -yny1)-b enzyl sulfany1]-2-
methyl-phenoxy } -
acetic acid; {443-Isobutoxy-5-(3-morpholin-4-yl-prop-1-yny1)-phenylsulfanyl]-2-
methyl-
phenoxy}-acetic acid; {443,3-Bis-(4-bromo-pheny1)-allyloxy]-2-methyl-phenoxy}-
acetic acid;
2-[2-methy1-4-[[3-methy1-4-[[4-
(trifluoromethyl)phenyl]methoxy]phenyl]thio]phenoxy]-acetic
acid; (S)-4- [ci s-2,6-dim ethyl -4-(4-trifluoromethoxy-phenyl)piperazine-l-
sulfonyl] -indan-2-
carboxylic acid or a tosylate salt thereof (KD-3010); 4-butoxy-a-ethy1-3-[[[2-
fluoro-4-
(trifluoromethyl)benzoyl]amino]methy1]-benzenepropanoic acid (TIPP-204); 242-
methy1-4-[[[4-
methyl-2-[4-(trifluoromethyl)pheny1]-5-thiazolyl]methyl]thio]phenoxy]-acetic
acid (GW-
501516); 2- [2,6 dimethy1-44344-(methylthio)phenyl] -3 -oxo-1(E)-
propenyl]phenoxyl]-2-
methylpropanoi c acid (GF T-505); {2-methy1-4-[5-methy1-2-(4-trifluoromethyl-
pheny1)-2H-
[1,2,3]triazol-4-ylmethyl sylfany1]-phenoxy} -acetic acid; (R)-3-methy1-6-(2-
((5-methy1-2-(4-
(trifluoromethyl)pheny1)-1H-imidazol-1-yl)methyl)phenoxy)hexanoic acid; (R)-3 -
methyl-6-(2-
((5 -methyl-2-(6-(trifluoromethyl)pyri din-3 -y1)-1H-imi dazol-1-
yl)methyl)phenoxy)hexanoi c acid;
2-(2-methyl-4-(((2-(4-(trifluoromethyl)pheny1)-2H-1,2,3 -tri azol-4-
yl)methyl)thi o)phenoxy)aceti c
acid; and (R)-2-(4-((2-ethoxy-3-(4-
(trifluoromethyl)phenoxy)propyl)thio)phenoxy)acetic acid; or
a pharmaceutically acceptable salt thereof.
100351 In some embodiments, the PPARo agonist is a compound selected from the
group
consisting of PPARo agonist is a compound selected from the group consisting
of: (Z)42-
Methy1-4-[3-(4-methylpheny1)-3-[4-[3-(morpholin-4-y1)propynyl]phenyl]allyloxy]-
phenoxy]acetic acid; (E)- [2-Methy1-4- [3 -[4-[3 -(pyraz ol-1-yl)prop-1-
ynyl]phenyl] -3 -(4 -
trifluoromethylpheny1)-allyloxy]phenoxy]acetic acid; (E)4443-(4-Fluoropheny1)-
34443-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid; (E)-
[2-Methyl 4-[3-
- 6 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[4-[3-(morpholin-4-yl)propynyl]pheny1]-3-(4-trifluoromethylphenyl)allyloxy]-
phenoxy]acetic
acid; (E) -[4 -[3 -(4-Chloropheny1)-3-[4-[3-(morpholin-4-
yl)propynyl]phenyl]allyloxy]-2-methyl-
phenoxy]acetic acid; (E)4443-(4-Chloropheny1)-34443-(morpholin-4-
yl)propynyl]phenyl]allyloxy]-2-methylphenyl]-propionic acid; {443-Isobutoxy-5-
(3-morpholin-
4-yl-prop-1-yny1)-benzylsulfanyl]-2-methyl-phenoxy}-acetic acid; {443-
Isobutoxy-5-(3-
morpholin-4-yl-prop-1-yny1)-phenylsulfanyl]-2-methyl-phenoxy}-acetic acid; and
{4-[3,3-Bis-
(4-bromo-pheny1)-allyloxy]-2-methyl-phenoxy}-acetic acid; or a
pharmaceutically acceptable
salt thereof
[0036] In some embodiments, the PPARo agonist is (E)-[4-[3-(4-Fluoropheny1)-3-
[4-[3-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid
(Compound I) or a
pharmaceutically acceptable salt thereof.
[0037] In some embodiments, (E)4443-(4-Fluoropheny1)-34443-(morpholin-4-
yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid or a
pharmaceutically acceptable salt
thereof, is administered to the mammal at a dose of about 10mg to about 500mg,
about 50mg to
about 200mg, or about 75mg to about 125mg.
[0038] In some embodiments, the PPARo agonist is (E)-[4-[3-(4-Fluoropheny1)-3-
[4-[3-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid or a
pharmaceutically
acceptable salt thereof, and is administered to the mammal at a dose of about
10mg to about
500mg. In some embodiments, the PPARo agonist is (E)4443-(4-Fluoropheny1)-
34443-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid or a
pharmaceutically
acceptable salt thereof, and is administered to the mammal at a dose of about
50mg to about
200mg. In some embodiments, the PPARo agonist is (E)4443-(4-Fluoropheny1)-
34443-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid
(Compound I) or a
pharmaceutically acceptable salt thereof, and is administered to the mammal at
a dose of about
75mg to about 125mg.
[0039] In some embodiments, a PPARo agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof), is systemically administered to the mammal with a
fatty acid oxidation
disorder (FAOD). In some embodiments, the PPARo agonist is administered to the
mammal
orally, by injection or intravenously. In some embodiments, the PPARo agonist
is administered
to the mammal in the form of an oral solution, oral suspension, powder, pill,
tablet or capsule.
[0040] In one aspect, described herein is a pharmaceutical composition
comprising PPARo
agonist and at least one pharmaceutically acceptable excipient. In some
embodiments, the
pharmaceutical composition is formulated for administration to a mammal by
intravenous
administration, subcutaneous administration, oral administration, inhalation,
nasal administration,
- 7 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
dermal administration, or ophthalmic administration. In some embodiments, the
pharmaceutical
composition is formulated for administration to a mammal by intravenous
administration,
subcutaneous administration, or oral administration. In some embodiments, the
pharmaceutical
composition is formulated for administration to a mammal by oral
administration. In some
embodiments, the pharmaceutical composition is in the form of a tablet, a
pill, a capsule, a liquid,
a suspension, a gel, a dispersion, a solution, an emulsion, an ointment, or a
lotion. In some
embodiments, the pharmaceutical composition is in the form of a tablet, a
pill, or a capsule.
[0041] In one aspect, described herein is a method of treatment or prevention
of any one of the
fatty acid oxidation disorders (FAOD) described herein comprising
administering a
therapeutically effective amount of a PPAIto agonist to a mammal in need
thereof.
[0042] In any of the aforementioned aspects are further embodiments in which
the effective
amount of the PPAIto agonist (e.g. Compound 1, or a pharmaceutically
acceptable salt thereof),
is: (a) systemically administered to the mammal; and/or (b) administered
orally to the mammal;
and/or (c) intravenously administered to the mammal; and/or (d) administered
by injection to the
mammal; and/or (e) administered non-systemically or locally to the mammal.
[0043] In any of the aforementioned aspects are further embodiments comprising
single
administrations of the effective amount of the PPAIto agonist (e.g. Compound
1, or a
pharmaceutically acceptable salt thereof), including further embodiments in
which the PPAIto
agonist (e.g. Compound 1, or a pharmaceutically acceptable salt thereof), is
administered once
daily to the mammal or is administered to the mammal multiple times over the
span of one day.
In some embodiments, the PPAIto agonist (e.g. Compound 1, or a
pharmaceutically acceptable
salt thereof), is administered on a continuous dosing schedule. In some
embodiments, the
PPAIto agonist is administered on a continuous daily dosing schedule.
[0044] In any of the aforementioned aspects involving the treatment of a
disease or condition
are further embodiments comprising administering at least one additional agent
in addition to the
administration of a PPAIto agonist (e.g. Compound 1, or a pharmaceutically
acceptable salt
thereof). In various embodiments, each agent is administered in any order,
including
simultaneously.
[0045] In some embodiments, the at least one additional therapeutic is
ubiquinol, ubiquinone,
niacin, riboflavin, creatine , L-carnitine, acetyl-L-carnitine, biotin,
thiamine, pantothenic acid,
pyridoxine, alpha-lipoic acid, n-heptanoic acid, CoQ10, vitamin E, vitamin C,
methylcobalamin,
folinic acid, N-acetyl-L-cysteine (NAC), zinc, folinic acid/leucovorin
calcium, resveratrol, or a
combination thereof. In some embodiments, the at least one additional
therapeutic is an odd-
chain fatty acid, odd-chain fatty ketone, L-carnitine, or combinations
thereof. In some
- 8 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
embodiments, the at least one additional therapeutic is triheptanoin, n-
heptanoic acid, a
triglyceride, or a salt or thereof, or combinations thereof.
[0046] In any of the embodiments disclosed herein, the mammal is a human.
[0047] In some embodiments, the PPAIto agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof), is administered to a human. In some embodiments, the
PPAIto agonist
(e.g. Compound 1, or a pharmaceutically acceptable salt thereof), is orally
administered.
[0048] Articles of manufacture, which include packaging material, a compound
described
herein, or a pharmaceutically acceptable salt thereof, within the packaging
material, and a label
that indicates that a PPAIto agonist (e.g. Compound 1, or a pharmaceutically
acceptable salt
thereof), is used for modulating the activity of PPAIto, or for the treatment,
prevention or
amelioration of one or more symptoms of a fatty acid oxidation disorder (FAOD)
that would
benefit from modulation of PPAIto activity, are provided.
[0049] Other objects, features and advantages of the compounds, methods and
compositions
described herein will become apparent from the following detailed description.
It should be
understood, however, that the detailed description and the specific examples,
while indicating
specific embodiments, are given by way of illustration only, since various
changes and
modifications within the spirit and scope of the instant disclosure will
become apparent to those
skilled in the art from this detailed description.
BRIEF DESCRIPTION OF THE FIGURES
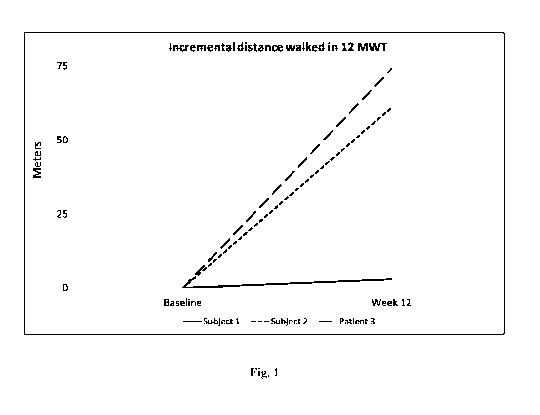
[0050] Figure 1 shows the effect of Compound 1 (50 mg once a day for 12 weeks)
on the 12-
minute walk test in patients with genetically diagnosed long chain FAOD with
symptoms of
myopathy.
DETAILED DESCRIPTION
[0051] Mitochondria are the main site for the oxidation of fatty acids and
triglycerides through
a series of four enzyme reactions called 13-oxidation. The 13-oxidation
pathway is a cyclic process
in which two carboxy-terminal carbon atoms are released from fatty acids as
acetyl-CoA units
each time a cycle is fully completed. The acetyl-CoA can enter the citric acid
cycle and the
electron carriers deliver the electrons to the electron transport chain. Fatty
acid oxidation (FAO)
both produces acetyl-CoA to fuel the tricarboxylic acid (TCA) cycle and
ketogenesis, and
reduces flavin adenine dinucleotide (to FADH2) and nicotinamide adenine
dinucleotide (to
NADH); these reduced products directly feed into the respiratory chain. As the
acyl-CoA gets
shorter, its physicochemical properties change. To be able to fully degrade
fatty acids, the 13-
oxidation machinery harbors different chain length¨specific enzymes. Inherited
defects for most
- 9 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
of the 13-oxidation enzymes have been identified and characterized (see for
example, S. M.
Houten, et at., The Biochemistry and Physiology of Mitochondrial Fatty Acid 13-
Oxidation and
Its Genetic Disorders. Annual Review of Physiology 2016 78:1, 23-44).
[0052] FAO is crucial for ATP production in muscle, particularly during
exercise. The sources
of fatty acids differ depending on the exercise intensity, with the
contribution of free fatty acids
increasing with exercise intensity. Mutations in any of the enzymes involved
in FAO, in some
cases, lead to a variety of clinical symptoms in particular during fasting and
in organs with high
energy needs. During infancy, patients, in some cases, present with cardiac
symptoms such as
dilated or hypertrophic cardiomyopathy and/or arrhythmias. Alternatively, FAO
defects, in some
cases, present as a milder, later (adult') onset disease, characterized by
exercise-induced
myopathy and rhabdomyolysis. Human inherited defects have been described for
almost all
enzymes and transporters involved in FAO
[0053] In most FAO defects, disease-causing mutations have been characterized
that result in
absent or non-functional protein, or variable levels of residual enzyme
activity. The PPARs
(PPAR-a, PPAR-6, PPAR-y) are known for their transcriptional regulation of
FAO. Activation of
PPARs, in some cases, trigger an up-regulation of gene expression of the
enzymes involved in
FAO resulting in an increase in residual enzyme activity and thereby
correction of FAO flux in
treated cells. This is the case for the defect in CPT2. CPT2 is an inner
mitochondrial membrane
enzyme involved in the transfer of long-chain fatty acids from cytosol to the
mitochondrial
matrix, in concert with its outer membrane counterpart, CPT1. Using the PPAR
agonist
bezafibrate, pharmacological enhancement of a deficient enzyme could be
achieved in cultured
patient fibroblasts carrying mild mutations of the CPT2 gene (Djouadi, F., et
at. Pediatr. Res.
54, 446-451, 2003). Bezafibrate is a pan-PPAR agonist with limited selectivity
for any of the
three receptor subtypes. In a follow up study (Djouadi, F., et at. I Clin.
Endocrinol. Metab. 90,
1791-1797, 2005) using cultured patient muscle cells, specific agonists of
PPAR 6 (GW 072)
and, to a lower extent, PPARa (GW 7647) stimulated FAO in control myoblasts.
However, when
tested in CPT2-deficient myoblasts, both bezafibrate and the PPAR 6 agonist
were able to restore
FAO, whereas the PPARa agonist had no effect. The PPAR 6 selective agonist
increased residual
CPT2 activity and normalized long-chain acylcarnitine production by deficient
cells. In some
embodiments, selective PPAR 6 agonists are therapeutic options for correction
of FAO defects.
[0054] Pharmacological rescue of residual enzyme activity, in some cases, is
potentially
extended to other FAO gene defects, such as VLCAD, because the PPAR signaling
pathway
controls several enzymes of the 13-oxidation pathway. For example, using the
PPARo agonist
compound MA-0211, improvements in fatty acid oxidation were observed in
fibroblasts derived
- 10 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
from patients with very long-chain Acyl-CoA dehydrogenase (VLCAD), long-chain
3-
hydroxylacyl-CoA dehydrogenase (LCHAD) and mitochondrial trifunctional protein
(TFP)
deficiencies were observed (see Goddeeris, M., et at., A Novel Small-Molecule
PPAR6
Modulator for the Treatment of Fatty Acid Oxidation Disorders. Poster Session
presented at
INFORM: International Network for Fatty Acid Oxidation Research and
Management; Rio de
Janeiro, Brazil, September 4, 2017).
[0055] Using the VLCAD deficient cell line FB833, the following PPARo agonist
compounds
were shown to increase VLCAD enzyme activity: 242-Methy1-4-[[[4-methy1-244-
(trifluoromethyl)pheny1]-5-thiazolyl]methyl]thio]phenoxy]acetic acid
(GW501516), [4-[[[243-
Fluoro-4-(trifluoromethyl)pheny1]-4-methyl-5-thiazolyl]methyl]thio]-2-
methylphenoxy]acetic
acid (GW0742 also known as GW610742), and [443-(4-Acety1-3-hydroxy-2-
propylphenoxy)propoxy]phenoxy]acetic acid (L-165,0411) (See figures 20 and 21
of
International publication no. W018093839).
[0056] In vitro studies with Compound 1 have demonstrated its ability to
elicit a dose-
dependent increase in fatty acid oxidation in human and rat muscle cell lines.
In addition,
Compound 1 treatment altered the expression patterns of several well-known
PPAR6 regulated
genes in pathways important for fatty acid metabolism (CPT lb) and
mitochondrial biogenesis
(PGC1a) in vivo.
[0057] In vitro studies with cultured fibroblasts obtained from symptomatic
patients with
FAOD due to very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency,
Compound 1
increased VLCAD enzymatic activity. In some embodiments, Compound 1 increases
the activity
of mutated but catalytically active enzymes and transporters in the FAO
pathway in subjects with
a FAOD. In some embodiments, Compound 1 increases the activity of mutated but
catalytically
active enzymes and transporters in the FAO pathway in symptomatic patients
with FAOD due to
very long-chain acyl-CoA dehydrogenase (VLCAD) deficiency. In some
embodiments,
Compound 1 improves whole-body fatty acid oxidation, and thus decreases
disease severity in
VLCAD patients.
[0058] Described herein, in some embodiments, are methods of pharmacological
rescue of
residual enzyme activity of enzymes involved in the fatty acid 13-oxidation
pathway. In some
embodiments, certain cells bearing mutations are expected to have some
residual enzymatic
activity. For example, in some embodiments, low residual enzymatic activity of
VLCAD is
observed in fibroblasts obtained from patients bearing missense mutations
(Goetzman ES.
Advances in the Understanding and Treatment of Mitochondrial Fatty Acid
Oxidation Disorders.
Curr Genet Med Rep . 2017;5(3):132-142). In some embodiment, described herein
are methods
-11-
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
of increasing residual enzyme activity of one or more enzymes involved in the
fatty acid 13-
oxidation pathway in a mammal with a FAOD comprising administering a PPAR6
agonist (e.g.
Compound 1, or a pharmaceutically acceptable salt thereof) to a mammal with a
FAOD. In some
embodiment, described herein are methods of increasing residual enzyme
activity of one or more
enzymes involved in the fatty acid 13-oxidation pathway in a mammal with a
FAOD by about 5%,
about 10%, about 15%, about 20%, about 25%, about 30%, about 35%, about 40%,
about 45%,
about 50%, about 55%, about 60%, about 75%, about 80%, about 95%, about 100%,
or more than
100% of the enzyme activity levels observed for a mammal without a FAOD
comprising
administering a PPAR6 agonist (e.g. Compound 1, or a pharmaceutically
acceptable salt thereof)
to a mammal with a FAOD.
[0059] In some embodiments, deficiencies in FAO capacities are measured by
comparing FAO
capacities of a mammal identified as having a FAOD to the FAO capacities of a
mammal without
a FAOD (i.e. a control). In some embodiments, described herein are methods of
increasing FAO
capacities in a mammal with a FAOD comprising administering a PPAR6 agonist
(e.g.
Compound 1, or a pharmaceutically acceptable salt thereof) to a mammal with a
FAOD. In some
embodiments, described herein are methods of increasing FAO capacities in a
mammal with a
FAOD by about 5%, about 10%, about 15%, about 20%, about 25%, about 30%, about
35%,
about 40%, about 45%, about 50%, about 55%, about 60%, about 75%, about 80%,
about 95%,
about 100%, or more than 100% of the levels observed for a mammal without a
FAOD. In some
embodiments, described herein are methods of increasing FAO capacities in a
mammal with a
FAOD to a level substantially similar to that observed for a mammal without a
FAOD comprising
administering a PPAR6 agonist (e.g. Compound 1, or a pharmaceutically
acceptable salt thereof)
to a mammal with a FAOD. In some embodiments, described herein are methods of
restoring (i.e.
normalizing) FAO capacities in a mammal with a FAOD to a level substantially
similar to that
observed for a mammal without a FAOD comprising administering a PPAR6 agonist
(e.g.
Compound 1, or a pharmaceutically acceptable salt thereof) to a mammal with a
FAOD.
[0060] In some embodiments, administration of a PPAR6 agonist (e.g. Compound
1, or a
pharmaceutically acceptable salt thereof), to a mammal with a FAOD restores
(i.e. normalizes) a
deficiency in the activity of one or more enzymes of proteins involved in the
fatty acid 13-
oxidation pathway. In some embodiments, restoring activity comprises
increasing the activity to
substantially similar levels observed in a mammal without a FAOD.
[0061] Described herein, in some embodiments, are methods and compositions for
treating a
fatty acid oxidation (FAO) disorder. In some embodiments, the FAO disorder is
caused by a
mutation in a gene involved in FAO. In some embodiments, the mutation causes
the gene to
- 12 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
encode a non-functional protein or a protein with reduced activity. In some
embodiments,
methods comprise administering a peroxisome proliferator-activated receptor
delta (PPAR6). In
some embodiments, administration of the PPAR6 increases the expression of the
gene involved
in FAO. In some embodiments, administration of the PPAR6 increases the
activity of the protein
involved in FAO.
[0062] Methods described herein, in some embodiments, comprise treating a FAO
disorder
caused by a mutation in a gene of interest. In some embodiments, the mutation
is a gene
mutation. In some embodiments, the mutation is a missense mutation, a nonsense
mutation, an
insertion, a deletion, a duplication, a frameshift mutation, a repeat
expansion, a splicing mutation,
or a whole gene deletion. In some embodiments, the FAO disorder is caused by
one or more
mutations in the gene of interest.
[0063] In some embodiments, the gene of interest is a gene involved in fatty
acid oxidation. In
some embodiments, the gene of interest encodes for a protein involved in fatty
acid oxidation. In
some embodiments, the gene of interest encodes for a protein that functions as
a carnitine shuttle.
In some embodiments, the gene of interest encodes for a protein that functions
in the fatty acid
oxidation cycle. In some embodiments, the gene of interest encodes for a
protein that functions as
an auxiliary enzyme. In some embodiments, the mutation in a gene of interest
encodes for a
protein with increased activity. In some embodiments, the mutation in a gene
of interest encodes
for a protein with reduced activity.
[0064] Methods described herein, in some embodiments, comprise treating a FAO
disorder
caused by a mutation in a gene of interest, wherein the gene of interest
encodes for a protein that
functions as a carnitine shuttle. Exemplary genes that encode for a protein
that functions as a
carnitine shuttle include, but not limited to, CPT1A, CPT1B, SLC25A20, CPT2,
and SLC22A5 . In
some embodiments, the mutation is in CPT1A. In some embodiments, the mutation
is in CPT1B.
In some embodiments, the mutation is in SLC25A20. In some embodiments, the
mutation is in
CPT2. In some embodiments, the mutation is in SLC22A5. In some embodiments,
the mutation is
in one or more genes selected from the group consisting of CPT1A, CPT1B,
SLC25A20, CPT2,
and SLC22A5 .
100651 CPT1A, also known as carnitine palmitoyltransferase 1A, encodes the CPT
1A protein.
CPT1B, also known as carnitine palmitoyltransferase 1B, encodes the CPT1B
protein. CTP1 is
an outer-mitochondrial-membrane protein and catalyzes the transesterification
of the acyl-CoA to
acylcarnitine. In some embodiments, a mutation is in CPT1A. In some
embodiments, a mutation
in CPT1A results in a decrease or loss of activity in CPT1A. In some
embodiments, a mutation is
in CPT1A comprising a sequence as set forth in NCBI Reference Number NM
001031847.2. In
- 13 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
some embodiments, a mutation in CPT1A is a mutation in a peptide sequence. In
some
embodiments, the mutation results in a missense substitution, a nonsense
substitution (*), a
coding silent substitution, deletion (del), an insertion (ins), or a
frameshift (fs). In some
embodiments, a mutation in CPT1A translates to amino acid positions in CPT 1A
selected from:
R123, C304, T314, R316, F343, R357, E360, A414, D454, G465, P479, L484, Y498,
G709, and
G710, wherein the amino acids correspond to positions 123, 304, 314, 316, 343,
357, 360, 414,
454, 465, 479, 484, 498, 709 and 710 of SEQ ID NO: 6. In some embodiments, a
mutation in
CPT1A translates to one or more different amino acid positions of SEQ ID NO:
6. In some
embodiments, the mutation in CPT1A, which translates to amino acid positions
in CPT1A
includes, but are not limited to, R123C, C304W, T314I, R316G, F343V, R357W,
E360G,
395de1, A414V, D454G, G465W, P479L, L484P, Y498C, G709E, and G710E.
[0066] In some embodiments, a mutation is in CPT1B. In some embodiments, a
mutation is in
CPT1B comprising a sequence as set forth in NCBI Reference Number NM 004377.3.
In some
embodiments, a mutation in CPT1B is a mutation in a peptide sequence. In some
embodiments,
the mutation results in a missense substitution, a nonsense substitution (*),
a coding silent
substitution, deletion (del), an insertion (ins), or a frameshift (fs). In
some embodiments, a
mutation in CPT1B translates to amino acid positions in CPT1B selected from:
166, G320, S427,
E531, and S664, wherein the amino acids correspond to positions 66, 320, 427,
531, and 664 of
SEQ ID NO: 7. In some embodiments, a mutation in CPT1B translates to one or
more different
amino acid positions of SEQ ID NO: 7. In some embodiments, the mutation in
CPT1B, which
translates to amino acid positions in CPT1B includes, but are not limited to,
I66V, G320D,
5427C, E531K, and 5664Y.
[0067] SLC25A20, also known as solute Carrier Family 25 Member 20 or carnitine
acylcarnitine translocase (CACT), encodes the CACT protein. CACT carries out
the transport of
acylcarnitines across the inner mitochondrial membrane in exchange for a free
carnitine
molecule. In some embodiments, a mutation is in SLC25A20 comprising a sequence
as set forth
in NCBI Reference Number NM 000387.6. In some embodiments, a mutation in
SLC25A20 is a
mutation in a peptide sequence. In some embodiments, the mutation results in a
missense
substitution, a nonsense substitution (*), a coding silent substitution,
deletion (del), an insertion
(ins), or a frameshift (fs). In some embodiments, a mutation in SLC25A20
translates to amino
acid positions in CACT selected from: R133, D231, and Q238, wherein the amino
acids
correspond to positions 133, 231, and 238 of SEQ ID NO: 8. In some
embodiments, a mutation
in SLC25A20 translates to one or more different amino acid positions of SEQ ID
NO: 8. In some
- 14 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
embodiments, the mutation in SLC25A20, which translates to amino acid
positions in CACT
includes, but are not limited to, R133W, D231H, and Q238R.
[0068] CPT2, also known as carnitine 0-palmitoyltransferase 2, encodes the
CPT2 protein.
CPT2 is a peripheral inner-mitochondrial-membrane protein and completes the
fatty acid
oxidation cycle by reconverting the acylcarnitine into an acyl-Co. In some
embodiments, a
mutation is in CPT2. In some embodiments, a mutation is in CPT2 comprising a
sequence as set
forth in NCBI Reference Number NM 000098.3. In some embodiments, a mutation in
CPT2 is a
mutation in a peptide sequence. In some embodiments, the mutation results in a
missense
substitution, a nonsense substitution (*), a coding silent substitution,
deletion (del), an insertion
(ins), or a frameshift (fs). In some embodiments, a mutation in CPT2
translates to amino acid
positions in CPT2 selected from: P50, S113, R151, Y210, D213, M214, P227,
R296, F383,
F448, Y479, R503, G549, Q550, D553, G600, P604, Y628, and R631, wherein the
amino acids
correspond to positions 50, 113, 151, 210, 213, 214, 227, 296, 383, 448, 479,
503, 549, 550, 553,
600, 604, 628, and 631 of SEQ ID NO: 9. In some embodiments, a mutation in
CPT2 translates
to one or more different amino acid positions of SEQ ID NO: 9. In some
embodiments, the
mutation in CPT2, which translates to amino acid positions in CPT2 includes,
but are not limited
to, P5OH, 5113L, R151Q, Y210D, D213G, M214T, P227L, R296Q, F383Y, F448L,
Y479F,
R503C, G549D, Q550R, D553N, G600R, P604S, Y6285, and R631C.
100691 SLC22A5, also known as solute carrier family 22 member 5, encodes OCTN2
protein.
OCTN2 functions to transport carnitine across the plasma membrane. In some
embodiments, a
mutation is in SLC22A5. In some embodiments, a mutation is in SLC22A5
comprising a sequence
as set forth in NCBI Reference Number NM 001308122.1. In some embodiments, a
mutation in
SLC22A5 is a mutation in a peptide sequence. In some embodiments, the mutation
results in a
missense substitution, a nonsense substitution (*), a coding silent
substitution, deletion (del), an
insertion (ins), or a frameshift (fs). In some embodiments, a mutation in
SLC22A5 translates to
amino acid positions in OCTN2 selected from: G12, G15, P16, F17, R19, L20,
S26, S28, N32,
A44, P46, C50, T66, R75, R83, S93, L95, G96, D115, D122, V123, E131, A142,
P143, V151,
R169, V175, M177, M179, L186, M205, N210, Y211, A214, T219, S225, R227, F230,
S231,
T232, G234, A240, G242, P247, R254, R257, T264, L269, S280, R282, W283, A301,
1312,
E317, 1348, W351, S355, Y358, S362, L363, P398, R399, S412, V439, T440, A442,
F443,
V446, Y447, V448, Y449, E452, P455, G462, S467, T468, S470, R471, L476, P478,
R488, and
L5075, wherein the amino acid corresponds to 12, 15, 16, 17, 19, 20, 26, 28,
32, 44, 46, 50, 66,
75, 83, 93, 95, 96, 115, 122, 123, 131, 142, 143, 151, 169, 175, 177, 179,
186, 205, 210, 211,
214, 219, 225, 227, 230, 231, 232, 234, 240, 242, 247, 254, 257, 264, 269,
280, 282, 283, 301,
- 15 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
312, 317, 348, 351, 355, 358, 362, 363, 398, 399, 412, 439, 440, 442, 443,
446, 447, 448, 449,
452, 455, 462, 467, 468, 470, 471, 476, 478, 488, and 507of SEQ ID NO: 10. In
some
embodiments, a mutation in SLC22A5 translates to one or more different amino
acid positions of
SEQ ID NO: 10. In some embodiments, the mutation in SLC22A5, which translates
to amino acid
positions in OCTN2 includes, but are not limited to 4 - 557de1, G125, G15W,
P16L, F17L,
R19P, L2OH, 22de1, 526N, S28I, N325, A44V, P46L, P46S, C50Y, T66P, R75P, R83L,
S93W,
L95V, G96A, D115G, 117 - 557del, D122Y, V123G, E131D, 132 - 557del, 140 -
557del,
A1425, P143L, V151M, R169P, R169Q, R169W, V175M, M177V, M179L, L186P, M205R,
N2105, Y211C, A214V, T219K, 5225L, R227H, F230L, S23 1F, T232M, G234R, A240T,
G242V, P247R, 254 - 557de1, R254Q, 256 - 557de1, R257W, T264M, T264R, L269P,
275 -557del, 5280F, 282 - 557de1, R282Q, W283C, W283R, 289 - 557de1, 295 -
557de1, A301D,
I312V, E317K, 319- 557de1, I348T, W351R, 5355L, Y358N, 5362L, L363P, 387-
557de1,
394de1, P398L, R399Q, R399W, 5412G, V439G, T440M, A442I, F443V, V446F, Y447C,
V448L, Y449D, E452K, P455R, G462V, 5467C, T468R, 5470F, R471C, R471H, R471P,
L476R, P478L, R488C, R488H, and L5075.
[0070] Methods described herein, in some embodiments, comprise treating a FAO
disorder
caused by a mutation in a gene of interest, wherein the gene of interest
encodes for a protein that
functions in the fatty acid oxidation cycle. Exemplary genes that encode for a
protein that
functions in the fatty acid oxidation cycle include, but not limited to,
ACADVL, ACADM,
ACADS, HADHA, HADHB, ECHS1, HADH, ACAA2, Ti,ACA ACADL, and ACAD9. In
some
embodiments, the mutation is in ACADVL. In some embodiments, the mutation is
in ACADM. In
some embodiments, the mutation is in ACADS. In some embodiments, the mutation
is in
HADHA. In some embodiments, the mutation is in HADHB. In some embodiments, the
mutation
is in ECHS1. In some embodiments, the mutation is in HADH. In some
embodiments, the
mutation is in ACAA2. In some embodiments, the mutation is in ACAT1. In some
embodiments,
the mutation is in ACADL. In some embodiments, the mutation is in ACAD9. In
some
embodiments, the mutation is in one or more genes selected from the group
consisting of
ACADVL, ACADM, ACADS, HADHA, HADHB, ECHS1, HADH, ACAA2, ACAT 1, ACADL, and
ACAD9.
[0071] ACADVL, also known as very long chain acyl-CoA dehydrogenase, encodes
the
VLCAD protein. VLCAD is a member of the aceyl-CoA dehydrogenase family and
metabolizes
aceyl-CoA's from long chain acyl CoA. In some embodiments, a mutation is in
ACADVL. In
some embodiments, a mutation is in ACADVL comprising a sequence as set forth
in SEQ ID NO:
11. Exemplary mutations in the nucleotide sequence include, but are not
limited to, 128G>A,
- 16 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
194C>T, 215C>T, 439C>T, 473C>A, 476A>G, 455G>A, 481G>A, 482C>T, 520G>A,
553G>A, 622G>A, 637G>C, 520G>A, 652G>A, 535G>T, 728T>G, A739G, 740A>C,
c.637G>A, 753-2A>C, 7790>T, 664G>C, 689C>T, 739A>C transversion, 842C>A,
848T>C,
865G>A, 869G>A, 881G>A, 897G>T, 898A>G, 950T>C, 956C>A, 1054A>G, 1096C>T,
1097G>A, 1117A>T, 1001T>G, 1066A>G, 1076C>T, 1153C>T, 1213G>C, 1146G>C,
1310T>C, 1322G>A, 1358G>A, 1360G>A, 1372T>C, 1258A>C, 1388G>A, 1405C>T,
1406G>A, 1430G>A, 1349G>A, 1505T>C, 1396G>T, 1613G>C, 1600G>A, 1367G>A,
1375C>T, 1376G>A, 1532G>A, 1619T>C, 1804C>A, 1844G>A, 1825G>A, 1844G>A, and
1837C>G. In some embodiments, the mutation in the nucleotide sequence is
842C>A,848T>C,
865G>A, 869G>A, 881G>A, 897G>T, 898A>G, 950T>C, 956C>A, 1054A>G, 1096C>T,
1097G>A, 1117A>T, 1001 T>G, 1066A>G, 1076C>T,1153C>T, 1213G>C, 1146G>C,
1310T>C, 1322G>A, 1358G>A, 1360G>A, 1372T>C, 1258A>C, 1388G>A, 1405C>T,
1406G>A, 1430G>A, 1349G>A, 1505T>C, 1396G>T, 1613G>C, 1600G>A, 1367G>A,
1375C>T, 1376G>A, 1532G>A, 1619T>C, 1804C>A, 1844G>A, 1825G>A, 1844G>A,
1837C>G, or a combination thereof. In some embodiments, a mutation in ACADVL
is a mutation
in a peptide sequence. In some embodiments, the mutation results in a missense
substitution, a
nonsense substitution (*), a coding silent substitution, deletion (del), an
insertion (ins), or a
frameshift (fs). In some embodiments, a mutation in ACADVL translates to amino
acid positions
in VLCAD selected from: P65, S72, P147, T118, Q119, A161, V134, G145, G208,
A213, E218,
L243, K247, T260, G222, T230, V283, G289, M300, R366, 1373, M334, 1356, A359,
R385,
K382, M437, G439, G441, 1420, R450, D466, R459, R511, L540, E609, R615, and
R613,
wherein the amino acids correspond to positions 65, 72, 147, 118, 119, 161,
134, 145, 208, 213,
218, 243, 247, 260, 222, 230, 283, 289, 300, 366, 373, 334, 356, 359, 385,
382, 437, 439, 441,
420, 450, 466, 459, 511, 540, 609, 615, and 613 of SEQ ID NO: 22. In some
embodiments, a
mutation in ACADVL translates to one or more different amino acid positions of
SEQ ID NO: 22.
In some embodiments, the mutation in ACADVL, which translates to amino acid
positions in
VLCAD includes, but are not limited to, G3D, P65L, 572F, P147S, T118N, Q119R,
G152D,
A121T, A161V, V134M, G1455, G168R, A173P, V174M, E178K, G179W, L203R, K207E,
K207T, A213T, T220M, G222R, T2301, K247Q, A281D, G289R, G250D, G254E, K259N,
M300V, V277A, M312V, R326C, R326H, I333F, M334R, I356V, A359V, R345W, D365H,
K382N, M437T, G401D, R413Q, D414N, F418L, G423E, R429W, R429Q, C437Y, R450H,
L462P, D466Y, R538P, E454K, R456H, R459W, R459Q, R511Q, L5621, R575Q, R615Q,
and
R613G. In some embodiments, the mutation in ACADVL, which translates to amino
acid
positions in VLCAD, is L540P, V174M, E609K, or combination thereof.
- 17 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[0072] ACADM, also known as medium-chain specific acyl-CoA dehydrogenase,
encodes the
MCAD protein. MCAD is a member of the aceyl-CoA dehydrogenase family and
metabolizes
aceyl-CoA's from medium chain acyl CoA. In some embodiments, a mutation is in
ACADM. In
some embodiments, a mutation is in ACADM comprising a sequence as set forth in
SEQ ID NO:
12. In some embodiments, a mutation in ACADM is a mutation in a peptide
sequence. In some
embodiments, the mutation results in a missense substitution, a nonsense
substitution (*), a
coding silent substitution, deletion (del), an insertion (ins), or a
frameshift (fs). In some
embodiments, a mutation in ACADM translates to amino acid positions in MCAD
selected from:
R53, Y67, 178, C116, T121, M149, T193, G195, R206, C244, S245, G267, R281,
G310, M326,
K329, S336, Y352, and 1375, wherein the amino acids correspond to positions
53, 67, 78, 116,
121, 149, 193, 195, 206, 244, 245, 267, 281, 310, 326, 329, 336, 352, and 375
of SEQ ID NO:
23. In some embodiments, a mutation in ACADM translates to one or more
different amino acid
positions of SEQ ID NO: 23. In some embodiments, the mutation in ACADM, which
translates to
amino acid positions in MCAD, includes, but are not limited to, R53C, Y67H,
I78T, 115 -116del, C116Y, T1211, M149I, T193A, G195R, R206L, C244R, 5245L,
G267R, R281T, G310R,
M326T, K329E, 5336R,Y352C, and I375T. In some embodiments, the mutation in
ACADM,
which translates to amino acid positions in MCAD is K304E.
[0073] ACADS, also known as short-chain specific acyl-CoA dehydrogenase,
encodes for the
SCAD protein. SCAD is a member of the aceyl-CoA dehydrogenase family and
metabolizes
aceyl-CoA's from short chain acyl CoA. In some embodiments, a mutation is in
ACADS. In some
embodiments, a mutation is in ACADS comprising a sequence as set forth in SEQ
ID NO: 13. In
some embodiments, a mutation in ACADS is a mutation in a peptide sequence. In
some
embodiments, the mutation results in a missense substitution, a nonsense
substitution (*), a
coding silent substitution, deletion (del), an insertion (ins), or a
frameshift (fs). In some
embodiments, a mutation in ACADS translates to amino acid positions in SCAD
selected from:
R46, G90, G92, R107, W177, A192, R325, S353, R380, and R383, wherein the amino
acids
correspond to positions 46, 90, 92, 107, 177, 192, 325, 353, 380, and 383 of
SEQ ID NO: 24. In
some embodiments, a mutation in ACADS translates to one or more different
amino acid
positions of SEQ ID NO: 24. In some embodiments, the mutation in ACADS, which
translates to
amino acid positions in SCAD, includes, but are not limited to, R46W, G905,
G92C, 104de1,
R107C, W177R, A192V, R325W, 5353L, R380W, and R383C.
[0074] HADHA, also known as hydroxyacyl-CoA dehydrogenase trifunctional
multienzyme
complex subunit alpha, encodes the protein MTPa. MTPa is a subunit of MTP,
which is located
at mitochondrial inner membrane and metabolizes long chain intermediates. In
some
- 18 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
embodiments, a mutation is in MTPa. In some embodiments, a mutation is in
HADHA
comprising a sequence as set forth in SEQ ID NO: 14. In some embodiments, a
mutation in
MTPa is a mutation in a peptide sequence. In some embodiments, the mutation
results in a
missense substitution, a nonsense substitution (*), a coding silent
substitution, deletion (del), an
insertion (ins), or a frameshift (fs). In some embodiments, a mutation in
HADHA translates to
amino acid positions in MTPa selected from: V282, 1305, L341, and E510,
wherein the amino
acids correspond to positions 282, 305, 341, and 510 of SEQ ID NO: 25. In some
embodiments, a
mutation in HADHA translates to one or more different amino acid positions of
SEQ ID NO: 25.
In some embodiments, the mutation in HADHA, which translates to amino acid
positions in
MTPa, includes, but are not limited to, V282D, 1305N, L341P, and E510Q. In
some
embodiments, the mutation in HADHA, which translates to amino acid positions
in MTPa is
E510Q.
[0075] HADHB, also known as hydroxyacyl-CoA dehydrogenase trifunctional
multienzyme
complex subunit beta, encodes the protein MT113. MTPf3 is a subunit of MTP. In
some
embodiments, a mutation is in MT113. In some embodiments, a mutation is in
HADHB
comprising a sequence as set forth in SEQ ID NO: 15. In some embodiments, a
mutation in
MTPf3 is a mutation in a peptide sequence. In some embodiments, the mutation
results in a
missense substitution, a nonsense substitution (*), a coding silent
substitution, deletion (del), an
insertion (ins), or a frameshift (fs). In some embodiments, a mutation in
HADHB translates to
amino acid positions in MTPf3 selected from: G59, R61, R117, L121, T133, D242,
R247, D263,
G280, P294, G301, and R444, wherein the amino acids correspond to positions
59, 61, 117, 121,
133, 242, 247, 263, 280, 294, 301, and 444 of SEQ ID NO: 26. In some
embodiments, a mutation
in HADHB translates to one or more different amino acid positions of SEQ ID
NO: 26. In some
embodiments, the mutation in HADHB, which translates to amino acid positions
in MT113,
includes, but are not limited to, G59D, R61C, R61H, R117G, L121P, T133P,
D242G, R247H,
259 - 270de1, D263G, G280D, P294L, P294R, G3015, and R444K. In some
embodiments, the
mutation in HADHB, which translates to amino acid positions in MTPf3 is R247C.
[0076] ECHS1, also known as enoyl-CoA hydratase, short chain, encodes the
Crotonase
protein, short chain protein. Crotonase functions to metabolize fatty acids
during fatty acid
oxidation to generate acetyl CoA. In some embodiments, a mutation is in
crotonase. In some
embodiments, a mutation is in ECHS1 comprising a sequence as set forth in SEQ
ID NO: 16. In
some embodiments, a mutation in crotonase is a mutation in a peptide sequence.
In some
embodiments, the mutation results in a missense substitution, a nonsense
substitution (*), a
coding silent substitution, deletion (del), an insertion (ins), or a
frameshift (fs). In some
- 19 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
embodiments, a mutation in ECHS1 translates to amino acid positions in
crotonase selected from:
A2, F33, R54, N59, 166, E77, G90, A132, A138, D150, A158, Q159, G195, C225,
K273, and
E281, wherein the amino acids correspond to positions 2, 33, 54, 59, 66, 77,
90, 132, 138, 150,
158, 159, 195, 225, 273, and 281 of SEQ ID NO: 27. In some embodiments, a
mutation in
ECHS1 translates to one or more different amino acid positions of SEQ ID NO:
27. In some
embodiments, the mutation in ECHS1, which translates to amino acid positions
in crotonase,
includes, but are not limited to, A2V, F335, R54H, N595, I66T, E77Q, G9OR,
A132T, A138V,
D150G, A158D, Q159R, G1955, C225R, K273E, and E281G.
[0077] HADH, also known as short-chain (S)-3-hydroxyacyl-CoA dehydrogenase,
encodes the
SCHAD protein, short chain protein. SCHAD functions in the beta oxidation of
short chain fatty
acids. In some embodiments, a mutation is in SCHAD. In some embodiments, a
mutation is in
HADH comprising a sequence as set forth in SEQ ID NO: 17. In some embodiments,
a mutation
in SCHAD is a mutation in a peptide sequence. In some embodiments, the
mutation results in a
missense substitution, a nonsense substitution (*), a coding silent
substitution, deletion (del), an
insertion (ins), or a frameshift (fs). In some embodiments, a mutation in HADH
translates to
amino acid positions in SCHAD selected from: A40, D57, and P258, wherein the
amino acids
correspond to positions 40, 57, and 258 of SEQ ID NO: 28. In some embodiments,
a mutation in
HADH translates to one or more different amino acid positions of SEQ ID NO:
28. In some
embodiments, the mutation in HADH, which translates to amino acid positions in
SCHAD,
includes, but are not limited to, A40T, D57E, and P258L.
[0078] ACAA2, also known as medium-chain 3-ketoacyl-CoA thiolase, encodes the
MCKAT
protein, short chain protein. MCKAT catalyzes ketoacyl-CoA. In some
embodiments, a mutation
is in SCHAD. In some embodiments, a mutation is in ACAA2 comprising a sequence
as set forth
in SEQ ID NO: 18. In some embodiments, a mutation in MCKAT is a mutation in a
peptide
sequence. In some embodiments, the mutation results in a missense
substitution, a nonsense
substitution (*), a coding silent substitution, deletion (del), an insertion
(ins), or a frameshift (fs).
In some embodiments, a mutation in ACAA2 translates to one or more different
amino acid
positions of SEQ ID NO: 29.
[0079] ACAT 1 , also known as acetoacetyl-CoA thiolase or acetyl-CoA
acetyltransferase 1,
encodes the acetyl-CoA acetyltransferase protein. Acetyl-CoA acetyltransferase
functions in
ketone body metabolism. In some embodiments, a mutation is in acetoacetyl-CoA
thiolase. In
some embodiments, a mutation is in ACAT /comprising a sequence as set forth in
SEQ ID NO:
19. In some embodiments, a mutation in acetoacetyl-CoA thiolase is a mutation
in a peptide
sequence. In some embodiments, the mutation results in a missense
substitution, a nonsense
- 20 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
substitution (*), a coding silent substitution, deletion (del), an insertion
(ins), or a frameshift (fs).
In some embodiments, mutation in ACAT1 translates to amino acid positions in
acetoacetyl-CoA
thiolase selected from: N93, G152, N158, G183, T297, A301, 1312, A333, G379,
and A380,
wherein the amino acids correspond to positions 93, 152, 158, 183, 297, 301,
312, 333, 379, and
380 of SEQ ID NO: 30. In some embodiments, a mutation in ACAT1 translates to
one or more
different amino acid positions of SEQ ID NO: 30. In some embodiments, the
mutation in ACAT1,
which translates to amino acid positions in acetoacetyl-CoA thiolase,
includes, but are not limited
to, 85de1, N935, G152A, N158D, G183R, T297M, A301P, I312T, A333P, G379V, and
A380T.
[0080] ACADL, also known as acyl-CoA dehydrogenase long chain, encodes the
LCAD
protein. LCAD catalyzes the beta oxidation of straight chain fatty acids. In
some embodiments, a
mutation is in LCAD. In some embodiments, a mutation is in ACADL comprising a
sequence as
set forth in SEQ ID NO: 20. In some embodiments, a mutation in LCAD is a
mutation in a
peptide sequence. In some embodiments, the mutation results in a missense
substitution, a
nonsense substitution (*), a coding silent substitution, deletion (del), an
insertion (ins), or a
frameshift (fs). In some embodiments, the mutation in ACADL translates to one
or more different
amino acid positions of SEQ ID NO: 31.
[0081] ACAD9, also known as acyl-CoA dehydrogenase family, member 9, encodes
the
ACAD9 protein. ACAD9 is a member of the ACAD family that act on fatty acids
comprising 14-
20 carbons. In some embodiments, a mutation is in ACAD9. In some embodiments,
a mutation is
in ACAD9 comprising a sequence as set forth in SEQ ID NO: 21. In some
embodiments, a
mutation in ACAD9 is a mutation in a peptide sequence. In some embodiments,
the mutation
results in a missense substitution, a nonsense substitution (*), a coding
silent substitution,
deletion (del), an insertion (ins), or a frameshift (fs). In some embodiments,
mutation in ACAD9
translates to amino acid positions in ACAD9 selected from: F44, R127, R193,
A220, S234,
R266, C271, G303, A326, V384, E413, R414, R417, R469W, R518, R532, and L606,
wherein
the amino acids correspond to positions 44, 127, 193, 220, 234, 266, 271, 303,
326, 384, 413,
414, 417, 469, 518, 532, and 606. In some embodiments, a mutation in ACAD9
translates to one
or more different amino acid positions of SEQ ID NO: 32. In some embodiments,
the mutation in
ACAD9, which translates to amino acid positions in ACAD9, includes, but are
not limited to,
F44I, R127K, R193W, A220V, 5234F, R266Q, C271G, G3035, A326T, V384M, E413K,
R414C, R417C, R469, R518H, R532W, and L606H.
[0082] Methods described herein, in some embodiments, comprise treating a FAO
disorder
caused by a mutation in a gene of interest, wherein the gene of interest
encodes for a protein that
functions as an auxiliary enzyme. Exemplary genes that encode for a protein
that functions as an
-21 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
auxiliary enzyme include, but not limited to, ECI 1, ECI2, DECR1, and ECH 1 .
In some
embodiments, the mutation is in ECI 1 . In some embodiments, the mutation is
in ECI2. In some
embodiments, the mutation is in DECR1 . In some embodiments, the mutation is
in ECH 1 . In
some embodiments, the mutation is in one or more genes selected from the group
consisting of
ECI 1, ECI2, DECR1, and ECH 1 .
[0083] ECI 1, also known as enoyl-CoA delta isomerase 1, encodes for the
protein DCI. DCI is
a mitochondrial enzyme involved in beta oxidation of unsaturated fatty acids.
In some
embodiments, a mutation is in DCI. In some embodiments, a mutation is in ECI1
comprising a
sequence as set forth in SEQ ID NO: 33. In some embodiments, a mutation in DCI
is a mutation
in a peptide sequence. In some embodiments, the mutation results in a missense
substitution, a
nonsense substitution (*), a coding silent substitution, deletion (del), an
insertion (ins), or a
frameshift (fs). In some embodiments, a mutation in ECI1 translates to one or
more different
amino acid positions of SEQ ID NO: 37.
[0084] ECI2, also known as enoyl-CoA delta isomerase 2, encodes for the
protein PECI. PECI
is a mitochondrial enzyme involved in beta oxidation of unsaturated fatty
acids. In some
embodiments, a mutation is in PECI. In some embodiments, a mutation is in ECI2
comprising a
sequence as set forth in SEQ ID NO: 34. In some embodiments, a mutation in
PECI is a mutation
in a peptide sequence. In some embodiments, the mutation results in a missense
substitution, a
nonsense substitution (*), a coding silent substitution, deletion (del), an
insertion (ins), or a
frameshift (fs). In some embodiments, a mutation in ECI2 translates to one or
more different
amino acid positions of SEQ ID NO: 38.
[0085] DECR1, also known as 2,4-dienoyl-CoA reductase, encodes for the protein
DECR.
DECR participates in the metabolism of unsaturated fatty enoyl-CoA esters
having double bonds
in both even- and odd-numbered positions. In some embodiments, a mutation is
in DECR. In
some embodiments, a mutation is in DECR1 comprising a sequence as set forth in
SEQ ID NO:
35. In some embodiments, a mutation in DECR is a mutation in a peptide
sequence. In some
embodiments, the mutation results in a missense substitution, a nonsense
substitution (*), a
coding silent substitution, deletion (del), an insertion (ins), or a
frameshift (fs). In some
embodiments, mutation in DECR1 translates to amino acid positions in DECR
selected from:
N148, Y199, S210, and K214, wherein the amino acids correspond to positions
148, 199, 210,
and 214 of SEQ ID NO: 35. In some embodiments, a mutation in DECR1 translates
to one or
more different amino acid positions of SEQ ID NO: 39. In some embodiments, the
mutation in
DECR1, which translates to amino acid positions in ACAD9, includes, but are
not limited to,
N148A, Y199A, 5210A, and K214A.
- 22 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[0086] ECH 1 , also known as enoyl-CoA hydratase 1, encodes for the protein
ECH1. ECH1
functions in the auxiliary step of the fatty acid oxidation pathway. In some
embodiments, a
mutation is in ECH1. In some embodiments, a mutation is in ECH 1 comprising a
sequence as set
forth in SEQ ID NO: 36. In some embodiments, a mutation in ECH1 is a mutation
in a peptide
sequence. In some embodiments, the mutation results in a missense
substitution, a nonsense
substitution (*), a coding silent substitution, deletion (del), an insertion
(ins), or a frameshift (fs).
In some embodiments, a mutation in ECH1 translates to one or more different
amino acid
positions of SEQ ID NO: 40.
[0087] Muscle tissue is soft tissue found in most animals comprising muscle
cells. Muscle
cells contain protein filaments that, in some cases, slide past one another
and produce a
contraction that changes both the length and shape of the muscle cell. Muscles
function to
produce force and motion. There are three types of muscles in the body: a)
skeletal muscle (the
muscle responsible for moving extremities and external areas of the bodies);
b) cardiac muscle
(the heart muscle); and c) smooth muscle (the muscle that is in the walls of
arteries and bowel).
[0088] The term "muscle cell" as used herein refers to any cell that
contributes to muscle
tissue. Myoblasts, satellite cells, myotubes, and myofibril tissues are all
included in the term
"muscle cells" and, in some embodiments, are treated using the methods
described herein.
Muscle cell effects, in some cases, are induced within skeletal, cardiac, and
smooth muscles.
[0089] Skeletal muscle, or voluntary muscle, is generally anchored by
tendons to bone and is
generally used to effect skeletal movement such as locomotion or in
maintaining posture.
Although some control of skeletal muscle is generally maintained as an
unconscious reflex (e.g.,
postural muscles or the diaphragm), skeletal muscles react to conscious
control. Smooth
muscle, or involuntary muscle, is found within the walls of organs and
structures such as
the esophagus, stomach, intestines, uterus, urethra, and blood vessels. Unlike
skeletal muscle,
smooth muscle is not under conscious control. Cardiac muscle is also an
involuntary muscle but
more closely resembles skeletal muscle in structure and is found only in the
heart. Cardiac and
skeletal muscles are striated in that they contain sarcomeres that are packed
into highly regular
arrangements of bundles. By contrast, the myofibrils of smooth muscle cells
are not arranged in
sarcomeres and therefore are not striated.
[0090] Skeletal muscle is further divided into two broad types: Type I (or
"slow twitch") and
Type II (or "fast twitch"). Type I muscle fibers are dense with capillaries
and are rich in
mitochondria and myoglobin, which gives Type I muscle tissue a characteristic
red color. Type I
muscle fibers, in some cases, carry more oxygen and sustain aerobic activity
using fats or
carbohydrates for fuel. Type I muscle fibers contract for long periods of time
but with little
- 23 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
force. Type II muscle fibers are subdivided into three major subtypes (Ha,
'Ix, and lib) that vary
in both contractile speed and force generated. Type II muscle fibers contract
quickly and
powerfully but fatigue very rapidly, and therefore produce only short,
anaerobic bursts of activity
before muscle contraction becomes painful.
[0091] Mitochondrial biogenesis is measured by mitochondrial mass and volume
through
histological section staining using a fluorescently labeled antibody specific
to the
oxidative-phosphorylation complexes, such as the Anti-OxPhox Complex Vd
subunit antibody
from Life Technologies or using mitochondrial specific dyes in live cell
staining, such as the
Mito-tracker probes from Life Technologies. Mitochondrial biogenesis, in some
cases, is also
measured by monitoring the gene expression of one or more mitochondrial
biogenesis related
transcription factors such as PGCla, NRF1, or NRF2 using a technique such as
QPCR.
[0092] In some aspects, PPAR6 agonist is administered in a therapeutically
effective amount to
a subject (e.g., a human). As used herein, the term "effective amount" or
"therapeutically
effective amount" refers to an amount of an active ingredient that elicits the
desired biological or
medicinal response, for example, reduction or alleviation of the symptoms of
the condition being
treated. In some embodiments of the invention, the amount of PPAR6 agonist
administered
varies depending on various factors, including, but not limited to, the weight
of the subject, the
nature and/or extent of the subject's condition, etc.
Compounds
[0093] A peroxi some proliferator activated receptor-delta (PPARo) agonist
compound is a fatty
acid, lipid, protein, peptide, small molecule, or other chemical entity that
binds to the cellular
PPARo and elicits a downstream response, namely gene transcription, either
native gene
transcription or a reporter construct gene transcription, comparable to
endogenous ligands such
as retinoic acid or comparable to a standard reference PPARo agonist such as
carbacyclin.
[0094] In an embodiment, a PPARo agonist is a selective agonist. As used
herein, a selective
PPARo agonist is viewed as a chemical entity that binds to and activates the
cellular PPARo and
does not substantially activate the cellular peroxisome proliferator activated
receptors alpha
(PPARa) and gamma (PPARy). As used herein, a selective PPARo agonist is a
chemical entity
that has at least a 10-fold maximum activation (as compared to endogenous
receptor ligand) with
a greater than 100-fold potency for activation of PPARo relative to either or
both of PPARa and
PPARy. In a further embodiment, a selective PPARo agonist is a chemical entity
that binds to
and activates the cellular human PPARo and does not substantially activate
either or both of
human PPARa and PPARy. In a further embodiment, a selective PPARo agonist is a
chemical
entity that has at least about a 10-fold, or about a 20-fold, or about a 30-
fold, or about a 40-fold,
- 24 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
or about a 50-fold, or about a 100-fold potency for activation of PPARo
relative to either or both
of PPARa and PPARy.
[0095] In some embodiments, a selective PPARo agonist compound contemplated
herein is
capable of simultaneously contacting the amino-acid residues at positions
VAL312, and ILE328
of PPARo (hPPAR6 numbering). In some embodiments, a selective PPARo agonist
compound is
capable of simultaneously contacting the amino-acid residues at positions
VAL298, LEU303,
VAL312, and ILE328 (hPPAR6 numbering).
[0096] "Activation" herein is defined as the abovementioned downstream
response, which in
the case of PPAR's is gene transcription. Gene transcription, in some cases,
is measured
indirectly as downstream production of proteins reflective of the activation
of the particular
PPAR subtype under study. Alternatively, an artificial reporter construct, in
some cases, is
employed to study the activation of the individual PPAR's expressed in cells.
The ligand binding
domain of the particular receptor to be studied, in some embodiments, is fused
to the DNA
binding domain of a transcription factor, which produces convenient laboratory
readouts, such as
the yeast GAL4 transcription factor DNA binding domain. The fusion protein, in
some cases, is
transfected into a laboratory cell line along with a Gal4 enhancer, which
effects the expression of
the luciferase protein. When such a system is transfected into a laboratory
cell line, binding of a
receptor agonist to the fusion protein will result in light emission.
[0097] A selective PPARo agonist, in some embodiments, exemplifies the above
gene
transcription profile in cells selectively expressing PPARo, and not in cells
selectively expressing
PPARy or PPARa. In an embodiment, the cells express human PPARo, PPARy, and
PPARa,
respectively.
[0098] In a further embodiment, a PPARo agonist has an EC50 value of less than
about 5 p.m as
determined by the PPAR transient transactivation assay described below. In an
embodiment, the
EC50 value is less than about 1 p.m. In another embodiment, the EC50 value is
less than about 500
nM. In another embodiment, the EC50 value is less than about 100 nM. In
another embodiment,
the EC50 value is less than about 50 nM.
[0099] The PPAR transient transactivation assay, in some cases, is based on
transient
transfection into human HEK293 cells of two plasmids encoding a chimeric test
protein and a
reporter protein respectively. The chimeric test protein, in some cases, is a
fusion of the DNA
binding domain (DBD) from the yeast GAL4 transcription factor to the ligand
binding domain
(LBD) of the human PPAR proteins. The PPAR-LBD moiety harbored in addition to
the ligand
binding pocket also has the native activation domain, allowing the fusion
protein to function as a
PPAR ligand dependent transcription factor. The GAL4 DBD will direct the
chimeric protein to
- 25 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
bind only to Gal4 enhancers (of which none existed in HEK293 cells). The
reporter plasmid
contained a Gal4 enhancer driving the expression of the firefly luciferase
protein. After
transfection, HEK293 cells expressed the GAL4-DBD-PPAR-LBD fusion protein. The
fusion
protein will in turn bind to the Gal4 enhancer controlling the luciferase
expression, and do
nothing in the absence of ligand. Upon addition to the cells of a PPAR ligand,
luciferase protein
will be produced in amounts corresponding to the activation of the PPAR
protein. The amount of
luciferase protein is measured by light emission after addition of the
appropriate substrate.
[00100] Cell Culture and Transfection: HEK293 cells, in some cases, are grown
in DMEM +
10% FCS. Cells, in some cases, are seeded in 96-well plates the day before
transfection to give a
confluency of 50-80 % at transfection. A total of 0.8 mg DNA containing 0.64
mg pM1a/gLBD,
0.1 mg pCMVbGal, 0.08 mg pGL2(Ga14)5,and 0.02 mg pADVANTAGE, in some cases,
are
transfected per well using FuGene transfection reagent according to the
manufacturer's
instructions. Cells, in some instances, are allowed to express protein for 48
hours followed by
addition of compound.
[00101] Plasmids: Human PPAR, in some cases, is obtained by PCR amplification
using
cDNA synthesized by reverse transcription of mRNA from human liver, adipose
tissue, and
plancenta, respectively. In some embodiments, amplified cDNAs is cloned into
pCR2.1 and
sequenced. The ligand binding domain (LBD) of each PPAR isoform, in some
cases, is
generated by PCR (PPAR: aa 128 ¨ C-terminus) and fused to the DNA binding
domain (DBD)
of the yeast transcription factor GAL4 by subcloning fragments in frame into
the vector pM1
(Sadowski et al. (1992), Gene 118, 137), generating the plasmids pM1aLBD,
pMlyLBD, and
pM16. Ensuing fusions, in some cases, is verified by sequencing. The reporter,
in some cases, is
constructed by inserting an oligonucleotide encoding five repeats of the GAL4
recognition
sequence (Webster et al. (1988), Nucleic Acids Res. 16, 8192) into the vector
pGL2 promotor
(Promega), generating the plasmid pGL2(GAL4)5. pCMVbGal, in some cases, is
purchased from
Clontech and pAD VANTAGE, in some cases, is purchased from Promega.
[00102] Compounds: Compounds, in some cases, are dissolved in DMSO and diluted
1:1000
upon addition to the cells. Compounds, in some cases, are tested in quadruple
in concentrations
ranging from 0.001 to 300 [tM. Cells, in some cases, are treated with compound
for 24 h
followed by luciferase assay. Each compound, in some cases, is tested in at
least two separate
experiments.
[00103] Luciferase assay: Medium including test compound, in some cases, is
aspirated and
100 11.1 PBS including 1 mM Mg and Ca, in some cases, is added to each well.
In some
embodiments, the luciferase assay is performed using the LucLite kit according
to the
- 26 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
manufacturer's instructions (Packard Instruments). Light emission, in some
cases, is quantified
by counting on a Packard LumiCounter. To measure P-galactosidase activity, 25
ml supernatant
from each transfection lysate, in some cases, is transferred to a new
microplate. In some
embodiments, 0-Galactosidase assays are performed in the microwell plates
using a kit from
Promega and read in a Labsystems Ascent Multiscan reader. The P-galactosidase
data, in some
cases, is used to normalize (transfection efficiency, cell growth, etc.) the
luciferase data.
[00104] Statistical methods: The activity of a compound, in some cases, is
calculated as fold
induction compared to an untreated sample. In some embodiments, for each
compound, the
efficacy (maximal activity) is given as a relative activity compared to
Wy14,643 for PPARa,
rosiglitazone for PPARy, and carbacyclin for PPAR6. The EC50 is the
concentration giving 50%
of maximal observed activity. ECso values, in some cases, is calculated via
non-linear regression
using GraphPad PRISM 3.02 (GraphPad Software, San Diego, CA).
[00105] In a further embodiment, a PPARo agonist has a molecular weight of
less than about
1000 g/mol, or a molecular weight of less than about 950 g/mol, or a molecular
weight of less
than about 900 g/mol, or a molecular weight of less than about 850 g/mol, or a
molecular weight
of less than about 800 g/mol, or a molecular weight of less than about 750
g/mol, or a molecular
weight of less than about 700 g/mol, or a molecular weight of less than about
650 g/mol, or a
molecular weight of less than about 600 g/mol, or a molecular weight of less
than about 550
g/mol, or a molecular weight of less than about 500 g/mol, or a molecular
weight of less than
about 450 g/mol, or a molecular weight of less than about 400 g/mol, or a
molecular weight of
less than about 350 g/mol, or a molecular weight of less than about 300 g/mol,
or a molecular
weight of less than about 250 g/mol. In another embodiment, a PPARo agonist
has a molecular
weight of greater than about 200 g/mol, or a molecular weight of greater than
about about 250
g/mol, or a molecular weight of greater than about 250 g/mol, or a molecular
weight of greater
than about 300 g/mol, or a molecular weight of greater than about 350 g/mol,
or a molecular
weight of greater than about 400 g/mol, or a molecular weight of greater than
about 450 g/mol, or
a molecular weight of greater than about 500 g/mol, or a molecular weight of
greater than about
550 g/mol, or a molecular weight of greater than about 600 g/mol, or a
molecular weight of
greater than about 650 g/mol, or a molecular weight of greater than about 700
g/mol, or a
molecular weight of greater than about 750 g/mol, or a molecular weight of
greater than about
800 g/mol, or a molecular weight of greater than about 850 g/mol, or a
molecular weight of
greater than about 900 g/mol, or a molecular weight of greater than about 950
g/mol, or a
molecular weight of greater than about 1000 g/mol. Any of the upper and lower
limits described
above in this paragraph, in some embodiments, are combined.
- 27 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[00106] In some embodiments, a PPAIto agonist is a PPAIto agonist compound
disclosed in any
of the following published patent applications: WO 97/027847, WO 97/027857, WO
97/028115,
WO 97/028137, WO 97/028149, WO 98/027974, WO 99/004815, WO 2001/000603, WO
2001/025181, WO 2001/025226, WO 2001/034200, WO 2001/060807, WO 2001/079197,
WO
2002/014291, WO 2002/028434, WO 2002/046154, WO 2002/050048, WO 2002/059098,
WO
2002/062774, WO 2002/070011, WO 2002/076957, WO 2003/016291, WO 2003/024395,
WO
2003/033493, WO 2003/035603, WO 2003/072100, WO 2003/074050, WO 2003/074051,
WO
2003/074052, WO 2003/074495, WO 2003/084916, WO 2003/097607, WO 2004/000315,
WO
2004/000762, WO 2004/005253, WO 2004/037776, WO 2004/060871, WO 2004/063165,
WO
2004/063166, WO 2004/073606, WO 2004/080943, WO 2004/080947, WO 2004/092117,
WO
2004/092130, WO 2004/093879, WO 2005/060958, WO 2005/097098, WO 2005/097762,
WO
2005/097763, WO 2005/115383, WO 2006/055187, WO 2007/003581, and WO
2007/071766
(each of which is incorporated for such PPAIto agonist compounds).
[00107] In some embodiments, a PPAIto agonist is a PPAIto agonist compound
disclosed in any
of the following published patent applications: W02014/165827; W02016/057660;
W02016/057658; W02017/180818; W02017/062468; and WO/2018/067860 (each of which
is
incorporated for such PPAIto agonist compounds).
[00108] In some embodiments, a PPAIto agonist is a PPAIto agonist compound
disclosed in any
of the following published patent applications: United States Patent
Application Publication Nos.
20160023991, 201 70226154, 20170304255, and 20170305894 (each of which is
incorporated
for such PPAIto agonist compounds).
[00109] In some embodiments, a PPAIto agonist compound is a
phenoxyalkylcarboxylic acid
compound. In some embodiments, the phenoxyalkylcarboxylic acid compound is a 2-
methylphenoxyalkylcarboxylic acid compound.
[00110] In some embodiments, a PPAIto agonist compound is a
phenoxyalkylcarboxylic acid
compound that is a phenoxyethanoic acid compound, phenoxypropanoic acid
compound,
phenoxypropenoic acid compound, phenoxybutanoic acid compound, phenoxybutenoic
acid
compound, phenoxypentanoic acid compound, phenoxypentenoic acid compound,
phenoxyhexanoic acid compound, phenoxyhexenoic acid compound, phenoxyoctanoic
acid
compound, phenoxyoctenoic acid compound, phenoxynonanoic acid compound,
phenoxynonenoic acid compound, phenoxydecanoic acid compound, or
phenoxydecenoic acid
compound. In some embodiments, a PPAIto agonist compound is a phenoxyethanoic
acid
compound or a phenoxyhexanoic acid compound. In some embodiments, a PPAIto
agonist
compound is a phenoxyethanoic acid compound. In some embodiments, the
phenoxyethanoic
- 28 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
acid compound is a 2-methylphenoxyethanoic acid compound. In some embodiments,
a PPARo
agonist compound is a phenoxyhexanoic acid compound.
[00111] In some embodiments, a PPARo agonist compound is a phenoxyethanoic
acid
compound, a ((benzamidomethyl)phenoxy)hexanoic acid compound, a
((heteroarylmethyl)phenoxy)hexanoic acid compound, a methylthiophenoxyethanoic
acid
compound, or an allyloxyphenoxyethanoic acid acid compound.
[00112] In some embodiments, a PPARo agonist compound is a
((benzamidomethyl)phenoxy)hexanoic acid compound.
[00113] In some embodiments, a PPARo agonist compound is a
((heteroarylmethyl)phenoxy)hexanoic acid compound. In some embodiments, a
PPARo agonist
compound is a ((imidazolylmethyl)phenoxy)hexanoic acid compound. In some
embodiments, a
PPARo agonist compound is an imidazol-1-ylmethylphenoxyhexanoic acid compound.
In some
embodiments, a PPARo agonist compound is a 6-(2-((2-pheny1-1H-imidazol-1-
yl)methyl)phenoxy)hexanoic acid.
[00114] In some embodiments, a PPARo agonist compound is an
allyloxyphenoxyethanoic acid
compound. In some embodiments, the allyloxyphenoxyethanoic acid compound is a
4-allyloxy-
2-methylphenoxy)ethanoic acid compound.
1001151 In some embodiments, a PPARo agonist compound is a
methylthiophenoxyethanoic
acid compound. In some embodiments, a PPARo agonist compound is a 4-
(methylthio)phenoxy)ethanoic acid compound.
[00116] In some embodiments, a PPARo agonist compound is a
phenoxyalkylcarboxylic acid
compound selected from the group consisting of: (Z)42-Methy1-443-(4-
methylpheny1)-3-[4-[3-
(morpholin-4-y1)propynyl]phenyl]allyloxy]-phenoxy]acetic acid; (E)42-Methy1-
4434443-
(pyrazol-1-y1)prop-1-ynyl]phenyl]-3-(4-trifluoromethylpheny1)-
allyloxy]phenoxy]acetic acid;
(E)-[4-[3-(4-Fluoropheny1)-3-[4-[3-(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-
methyl-
phenoxy]acetic acid (Compound 1); (E)42-Methy1-4434443-(morpholin-4-
yl)propynyl]phenyl]-
3-(4-trifluoromethylphenyl)allyloxy]-phenoxy]acetic acid; (E)-[4-[3-(4-
Chloropheny1)-3-[4-[3-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid;
(E)4443-(4-
Chloropheny1)-34443-(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methylphenyl]-
propionic
acid; 443 -Isobutoxy-5 -(3 -morpholin-4-yl-prop-1 -yny1)-b enzyl sulfany1]-2-
methyl-phenoxy } -
acetic acid; {443-Isobutoxy-5-(3-morpholin-4-yl-prop-1-yny1)-phenylsulfanyl]-2-
methyl-
phenoxy}-acetic acid; and {443,3-Bis-(4-bromo-pheny1)-allyloxy]-2-methyl-
phenoxy}-acetic
acid; (R)-3 -methyl-6424(5 -methyl-2-(4-(trifluoromethyl)pheny1)-1H-imi dazol-
1 -
- 29 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
yl)methyl)phenoxy)hexanoic acid; (R)-3-methy1-6-(2-((5-methy1-2-(6-
(trifluoromethyl)pyridin-3-
y1)-1H-imidazol-1-y1)methyl)phenoxy)hexanoic acid; (E)4443-(4-Fluoropheny1)-
34443-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid
(Compound 1); 2-{4-
[({242-Fluoro-4-(trifluoromethyl)pheny1]-4-methyl-1,3-thiazol-5-
ylImethyl)sulfanyl] -2-
methylphenoxy}-2-methylpropanoic acid (sodelglitazar; GW677954); 242-methy1-4-
[[3-methy1-
44[4-(trifluoromethyl)phenyl]methoxy]phenyl]thio]phenoxy]-acetic acid; 242-
methy1-4-[[[4-
methyl-2-[4-(trifluoromethyl)pheny1]-5-thiazolyl]methyl]thio]phenoxy]-acetic
acid (GW-
501516); [4-[[[243-Fluoro-4-(trifluoromethyl)pheny1]-4-methyl-5-
thiazolyl]methyl]thio]-2-
methylphenoxy]acetic acid (GW0742 also known as GW610742); 2-[2,6 dimethy1-
44344-
(methylthio)pheny1]-3-oxo-1(E)-propenyl]phenoxyl]-2-methylpropanoic acid
(elafibranor; GFT-
505); {2-methy1-4-[5-methy1-2-(4-trifluoromethyl-pheny1)-2H-[1,2,3]triazol-4-
ylmethylsulfanyl]-
phenoxy}-aceti c acid; and [4-({ (2R)-2-Ethoxy-3-[4-
(trifluoromethyl)phenoxy]propylIsulfany1)-
2-methylphenoxy]acetic acid (seladelpar; MBX-8025); (S)-4-[cis-2,6-dimethy1-4-
(4-
trifluoromethoxy-phenyl)piperazine-l-sulfony1]-indan-2-carboxylic acid or a
tosylate salt thereof
(KD-3010); (2s)-2-{4-butoxy-3-[({ [2-Fluoro-4-
(Trifluoromethyl)phenyl]carbonylIamino)methyl]benzylIbutanoic acid (TIPP-204);
[4-[3-(4-
Acety1-3-hydroxy-2-propylphenoxy)propoxy]phenoxy]acetic acid (L-165,0411); 2-
(4-{2-[(4-
Chlorobenzoyl)amino]ethylIphenoxy)-2-methylpropanoic acid (bezafibrate); or a
pharmaceutically acceptable salt thereof.
[00117] In another embodiment, a PPARo agonist is a 2-
methylphenoxyalkylcarboxylic acid
compound selected from the group consisting of (E)4443-(4-Fluoropheny1)-34443-
(morpholin-
4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid (Compound 1); 2-
{44({242-
Fluoro-4-(trifluoromethyl)pheny1]-4-methy1-1,3 -thi az ol-5-
ylImethyl)sulfanyl] -2-
methylphenoxy}-2-methylpropanoic acid (sodelglitazar; GW677954); 242-methy1-4-
[[3-methy1-
44[4-(trifluoromethyl)phenyl]methoxy]phenyl]thio]phenoxyFacetic acid; 242-
methy1-4-[[[4-
methyl-2-[4-(trifluoromethyl)pheny1]-5-thiazolyl]methyl]thio]phenoxy]-acetic
acid (GW-
501516); [4-[[[243-Fluoro-4-(trifluoromethyl)pheny1]-4-methyl-5-
thiazolyl]methyl]thio]-2-
methylphenoxy]acetic acid (GW0742 also known as GW610742); 2-[2,6 dimethy1-
44344-
(methylthio)pheny1]-3-oxo-1(E)-propenyl]phenoxyl]-2-methylpropanoic acid
(elafibranor; GFT-
505); {2-methy1-4-[5-methy1-2-(4-trifluoromethyl-pheny1)-2H-[1,2,3]triazol-4-
ylmethylsulfanyl]-
phenoxy}-aceti c acid; and [4-({ (2R)-2-Ethoxy-3-[4-
(trifluoromethyl)phenoxy]propylIsulfany1)-
2-methylphenoxy]acetic acid (seladelpar; MBX-8025).
[00118] In another embodiment, a PPARo agonist is a compound selected from the
group
consisting of (S)-4-[cis-2,6-dimethy1-4-(4-trifluoromethoxy-phenyl)piperazine-
1-sulfonyl]-indan-
- 30 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
2-carboxylic acid or a tosylate salt thereof (KD-3010); (2s)-2-{4-butoxy-
34({[2-Fluoro-4-
(Trifluoromethyl)phenyl]carbonylIamino)methyl]benzylIbutanoic acid (TIPP-204);
[443-(4-
Acety1-3-hydroxy-2-propylphenoxy)propoxy]phenoxy]acetic acid (L-165,0411); and
2-(4-{2-[(4-
Chlorobenzoyl)amino]ethylIphenoxy)-2-methylpropanoic acid (bezafibrate).
[00119] In another embodiment, a PPARo agonist is a compound selected from the
group
consisting of sodelglitazar; lobeglitazone; netoglitazone; and isaglitazone; 2-
(4-{2-[(4-
Chlorobenzoyl)amino]ethylIphenoxy)-2-methylpropanoic acid (bezafibrate); 2-[2-
methy1-44[3-
methyl-44[4-(trifluoromethyl)phenyl]methoxy]phenyl]thio]phenoxy]-acetic acid
(See WO
2003/024395); (S)-44cis-2,6-dimethy1-4-(4-trifluoromethoxy-phenyl)piperazine-1-
sulfonyl]-
indan-2-carboxylic acid or a tosyl ate salt thereof (KD-3010); 4-butoxy-a-
ethy1-3-[[[2-fluoro-4-
(trifluoromethyl)benzoyl]amino]methylFbenzenepropanoic acid (TIPP-204); 242-
methy1-4-[[[4-
methyl-2-[4-(trifluoromethyl)pheny1]-5-thiazolyl]methyl]thio]phenoxy]-acetic
acid (GW-
501516); 2-[2,6 dimethy1-44344-(methylthio)pheny1]-3-oxo-1(E)-
propenyl]phenoxyl]-2-
methylpropanoic acid (GFT-505); {2-methy1-4-[5-methy1-2-(4-trifluoromethyl-
pheny1)-2H-
[1,2,3]triazol-4-ylmethyl sylfany1]-phenoxy}-acetic acid; and [44{(2R)-2-
Ethoxy-3-[4-
(trifluoromethyl)phenoxy]propylIsulfany1)-2-methylphenoxy]acetic acid
(seladelpar; MBX-
8025).
[00120] In some embodiments, a PPARo agonist is (E)4443-(4-Fluoropheny1)-34443-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid
(Compound 1):
o
0 CH3
OrOH
0
[00121] An example of the chemical synthesis of (E)4443-(4-Fluoropheny1)-34443-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid is
found in Example
of PCT Application Pub. No. WO 2007/071766.
[00122] Compound 1 was tested on all three human PPAR subtypes (hPPAR):
hPPARa,
hPPARy, and hPPAR6 in vitro assays testing for such activity. Compound 1
exhibited a
significantly greater selectivity for PPAR6 over PPARa and PPARy (by at least
about 100-fold
and at least about 400-fold, respectively). In some cases, Compound 1 acts as
a full agonist of
PPAR6 and only a partial agonist for both PPARa and PPARy. In some cases,
Compound 1
-31-
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
demonstrates negligible activity on PPARa and/or PPARy in tranasctivation
assays testing for
such activity.
[00123] In some embodiments, Compound 1 did not show any human retinoid X
receptor
(hRXR) activity, or activity on the nuclear receptors FXR, LXRa or LXRp. as a
full agonist of
PPAR6 and only a partial agonist for both PPARa and PPARy.
[00124] In some embodiments, a PPARo agonist is (Z)- [2-Methy1-4-[3-(4-
methylpheny1)-3-[4-
[3-(morpholin-4-yl)propynyl]phenyl]allyloxy]-phenoxy]acetic acid:
H3C
0 CH3
OrOH
0
[00125] An example of the chemical synthesis of (Z)42-Methy1-443-(4-
methylpheny1)-3-[4-[3-
(morpholin-4-y1)propynyl]phenyl]allyloxy]-phenoxy]acetic acid is found in
Example 3 of PCT
Application Pub. No. WO 2007/071766.
[00126] In some embodiments, a PPARo agonist is (E)42-Methy1-4-[3-[4-[3-
(pyrazol-1-
y1)prop-1-ynyl]phenyl]-3-(4-trifluoromethylphenyl)-allyloxy]phenoxy]acetic
acid:
F3C
0 CH3
orOH
0
[00127] An example of the chemical synthesis of (E)-[2-Methy1-4-[3-[4-[3-
(pyrazol-1-y1)prop-
1-ynyl]phenyl]-3-(4-trifluoromethylpheny1)-allyloxy]phenoxy]acetic acid is
found in Example 4
of PCT Application Pub. No. WO 2007/071766.
[00128] In some embodiments, a PPARo agonist is (E)42-Methy1-4434443-
(morpholin-4-
y1)propynyl]phenyl]-3-(4-trifluoromethylphenyl)allyloxy]-phenoxy]acetic acid:
- 32 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
F3Co
0 CH3
oThrOH
0
[00129] An example of the chemical synthesis of (E)42-Methy1-4434443-
(morpholin-4-
y1)propynyl]phenyl]-3-(4-trifluoromethylphenyl)allyloxy]-phenoxy]acetic acid
is found in
Example 20 of PCT Application Pub. No. WO 2007/071766.
[00130] In some embodiments, a PPAIto agonist is (E)4443-(4-Chloropheny1)-
34443-
(morpholin-4-y1)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid:
CI
0 01 CH3
orOH
0
[00131] An example of the chemical synthesis of (E)4443-(4-Chloropheny1)-34443-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid is
found in Example
46 of PCT Application Pub. No. WO 2007/071766.
[00132] In some embodiments, a PPAIto agonist is (E)4443-(4-Chloropheny1)-
34443-
(morpholin-4-y1)propynyl]phenyl]allyloxy]-2-methylphenyl]-propionic acid:
N
CI o
0 CH3
OH
0
[00133] An example of the chemical synthesis of (E)4443-(4-Chloropheny1)-34443-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methylphenyl]-propionic acid is
found in Example
63 of PCT Application Pub. No. WO 2007/071766.
- 33 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[00134] In some embodiments, a PPAIto agonist is {443,3-Bis-(4-bromo-pheny1)-
allyloxy]-2-
methyl-phenoxy} -acetic acid:
Br Br
0 CH3
or0H
0
[00135] An example of the chemical synthesis of {443,3-Bis-(4-bromo-pheny1)-
allyloxy]-2-
methyl-phenoxy}-acetic acid is found in Example 10 of PCT Application Pub. No.
WO
2004/037776.
[00136] In some embodiments, a PPAIto agonist is {443-Isobutoxy-5-(3-morpholin-
4-yl-prop-
1-yny1)-benzylsulfany1]-2-methyl-phenoxy}-acetic acid:
CH3
u N
S CH3
oOH
0
[00137] An example of the chemical synthesis of {443-Isobutoxy-5-(3-morpholin-
4-yl-prop-1-
yny1)-benzylsulfany1]-2-methyl-phenoxy}-acetic acid is found in Example 9 of
PCT Application
Pub. No. WO 2007/003581.
[00138] In some embodiments, a PPAIto agonist is {443-Isobutoxy-5-(3-morpholin-
4-yl-prop-
1-yny1)-phenylsulfany1]-2-methyl-phenoxy}-acetic acid:
CH3
H3C
H3C 0
0j-LOH
[00139] An example of the chemical synthesis of {443-Isobutoxy-5-(3-morpholin-
4-yl-prop-1-
yny1)-phenylsulfany1]-2-methyl-phenoxy}-acetic acid is found in Example 35 of
PCT
Application Pub. No. WO 2007/003581.
- 34 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[00140] Accordingly, in an embodiment, a PPARo agonist is a compound selected
from the
group consisting of: (Z)42-Methy1-443-(4-methylpheny1)-3-[4-[3-(morpholin-4-
y1)propynyl]phenyl]allyloxy]-phenoxy] acetic acid; (E)- [2-Methyl -4- [3 -[4-
[3 -(pyrazol-1-yl)prop-
1-ynyl]pheny1]-3-(4-trifluoromethylpheny1)-allyloxy]phenoxy]acetic acid; (E)-
[4-[3-(4-
Fluoropheny1)-3-[4-[3-(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-
phenoxy]acetic
acid; (E)-[2-Methy1-4-[3-[4-[3-(morpholin-4-yl)propynyl]phenyl]-3-(4-
trifluoromethylphenyl)allyloxy]-phenoxy]acetic acid; (E)-[4-[3-(4-
Chloropheny1)-3-[4-[3-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid;
(E)4443-(4-
Chloropheny1)-34443-(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methylphenyl]-
propionic
acid; 443 -Isobutoxy-5-(3 -morpholin-4-yl-prop-1 -yny1)-b enzyl sulfanyl] -2-
methyl-phenoxy } -
acetic acid; {443-Isobutoxy-5-(3-morpholin-4-yl-prop-1-yny1)-phenylsulfanyl]-2-
methyl-
phenoxy}-acetic acid; and {443,3-Bis-(4-bromo-pheny1)-allyloxy]-2-methyl-
phenoxy}-acetic
acid; or a pharmaceutically acceptable salt thereof.
[00141] In a further embodiment, a PPAR6 agonist is (E)4443-(4-Fluoropheny1)-
34443-
(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid or a
pharmaceutically
acceptable salt thereof. In some embodiments, the PPAR6 agonist is (E)4443-(4-
Fluoropheny1)-
34443-(morpholin-4-yl)propynyl]phenyl]allyloxy]-2-methyl-phenoxy]acetic acid
sodium salt.
[00142] In a further embodiment, a PPAR6 agonist is Compound 1, Compound 2,
Compound 3,
Compound 4, Compound 5, Compound 6, Compound 7, Compound 8, Compound 9,
Compound
10, Compound 11, Compound 12, Compound 13, Compound 14, Compound 15, or
Compound
16, disclosed in Wu et at. Proc Natl Acad Sci USA March 28, 2017 114 (13)
E2563-E2570.
[00143] In a further embodiment, a PPAR6 agonist is (R)-3-methy1-6-(24(5-
methyl-2-(4-
(trifluoromethyl)pheny1)-1H-imidazol-1-y1)methyl)phenoxy)hexanoic acid, or (R)-
3 -methyl-6-(2-
((5 -m ethy1-2-(6-(trifluoromethyl)pyri din-3 -y1)-1H-imi dazol-1-yl)m
ethyl)phenoxy)hexanoi c acid,
or a pharmaceutically acceptable salt thereof
[00144] In a further embodiment, a PPAR6 agonist is (R)-3-methy1-6-(24(5-
methyl-2-(4-
(trifluoromethyl)pheny1)-1H-imidazol-1-y1)methyl)phenoxy)hexanoic acid, or a
pharmaceutically
acceptable salt thereof. In some embodiments, the PPAR6 agonist is the
hemisulfate salt of (R)-
3 -methyl-6-(2-((5 -methyl-2-(4-(trifluorom ethyl)pheny1)-1H-imi daz ol-1-
yl)methyl)phenoxy)hexanoic acid. In some embodiments, the PPAR6 agonist is the
meglumine
salt of(R)-3-methy1-6-(24(5-methyl-2-(4-(trifluoromethyl)pheny1)-1H-imidazol-1-
y1)methyl)phenoxy)hexanoic acid.
1001451 In a further embodiment, a PPAR6 agonist is (R)-3-methy1-6-(24(5-
methyl-2-(6-
(trifluoromethyl)pyridin-3-y1)-1H-imidazol-1-y1)methyl)phenoxy)hexanoic acid,
or a
- 35 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
pharmaceutically acceptable salt thereof. In some embodiments, the PPAR6
agonist is the
hemi sulfate salt of (R)-3-methy1-6-(2-((5-methy1-2-(6-
(trifluoromethyl)pyridin-3-y1)-1H-
imidazol-1-yl)methyl)phenoxy)hexanoic acid. In some embodiments, the PPAR6
agonist is the
meglumine salt of (R)-3-methy1-6-(24(5-methyl-2-(6-(trifluoromethyppyridin-3-
y1)-1H-
imidazol-1-y1)methyl)phenoxy)hexanoic acid.
[00146] In a further embodiment, a PPAR6 agonist is 2-(2-methy1-44(2-(4-
(trifluoromethyl)pheny1)-2H-1,2,3-triazol-4-y1)methyl)thio)phenoxy)acetic
acid, or a
pharmaceutically acceptable salt thereof.
[00147] In a further embodiment, a PPAR6 agonist is (R)-2-(44(2-ethoxy-3-(4-
(trifluoromethyl)phenoxy)propyl)thio)phenoxy)acetic acid, or a
pharmaceutically acceptable salt
thereof
[00148] The term "pharmaceutically acceptable salt" in reference to a PPAR6
agonist refers to a
salt of the PPAR6 agonist, which does not cause significant irritation to a
mammal to which it is
administered and does not substantially abrogate the biological activity and
properties of the
compound. Handbook of Pharmaceutical Salts: Properties, Selection and Use.
International
Union of Pure and Applied Chemistry, Wiley-VCH 2002. S.M. Berge, L.D. Bighley,
D.C.
Monkhouse, J. Pharm. Sci. 1977, 66, 1-19. P. H. Stahl and C. G. Wermuth,
editors, Handbook of
Pharmaceutical Salts: Properties, Selection and Use, Weinheim/Zurich:Wiley-
VCH/VHCA,
2002. In some embodiments, pharmaceutical salts typically are more soluble and
more rapidly
soluble in stomach and intestinal juices than non-ionic species and so are
useful in solid dosage
forms. Furthermore, because their solubility often is a function of pH,
selective dissolution in one
or another part of the digestive tract is possible and this capability, in
some cases, is manipulated
as one aspect of delayed and sustained release behaviors. Also, because the
salt-forming
molecule, in some cases, is in equilibrium with a neutral form, passage
through biological
membranes, in some cases, is adjusted.
[00149] In some embodiments, pharmaceutically acceptable salts are generally
prepared by
reacting the free base with a suitable organic or inorganic acid or by
reacting the acid with a
suitable organic or inorganic base. The term, in some embodiments, is used in
reference to any
compound of the present invention. Representative salts include the following
salts: acetate,
benzenesulfonate, benzoate, bicarbonate, bisulfate, bitartrate, borate,
bromide, calcium edetate,
camsylate, carbonate, chloride, clavulanate, citrate, dihydrochloride,
edetate, edisylate, estolate,
esylate, fumarate, gluceptate, gluconate, glutamate, glycollylarsanilate,
hexylresorcinate,
hydrabamine, hydrobromide, hydrochloride, hydroxynaphthoate, iodide,
isethionate, lactate,
lactobionate, laurate, malate, maleate, mandelate, mesylate, methylbromide,
methylnitrate,
- 36 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
methylsulfate, monopotassium maleate, mucate, napsylate, nitrate, n-
methylglucamine, oxalate,
pamoate (embonate), palmitate, pantothenate, phosphate/diphosphate,
polygalacturonate,
potassium, salicylate, sodium, stearate, subacetate, succinate, tannate,
tartrate, teoclate, tosylate,
triethiodide, trimethylammonium, and valerate. When an acidic substituent is
present, such as -
CO2H, in some cases, formation of ammonium, morpholinium, sodium, potassium,
barium,
calcium salt, and the like for use as the dosage form. When a basic group is
present, such as
amino, or a basic heteroaryl radical, such as pyridyl, in some cases,
formation of an acidic salt,
such as hydrochloride, hydrobromide, phosphate, sulfate, trifluoroacetate,
trichloroacetate,
acetate, oxalate, maleate, pyruvate, malonate, succinate, citrate, tartarate,
fumarate, mandelate,
benzoate, cinnamate, methanesulfonate, ethanesulfonate, picrate, and the like,
and include acids
related to the pharmaceutically acceptable salts listed in Berge, et at.,
Journal of Pharmaceutical
Sciences, Vol. 66(1), pp. 1-19 (1977).
Certain Terminology
[00150] Unless otherwise stated, the following terms used in this application
have the
definitions given below. The use of the term "including" as well as other
forms, such as
"include", "includes," and "included," is not limiting. The section headings
used herein are for
organizational purposes only and are not to be construed as limiting the
subject matter described.
[00151] The term "acceptable" with respect to a formulation, composition or
ingredient, as used
herein, means having no persistent detrimental effect on the general health of
the subject being
treated.
[00152] The term "modulate" as used herein, means to interact with a target
either directly or
indirectly so as to alter the activity of the target, including, by way of
example only, to enhance
the activity of the target, to inhibit the activity of the target, to limit
the activity of the target, or to
extend the activity of the target.
[00153] The term "modulator" as used herein, refers to a molecule that
interacts with a target
either directly or indirectly. The interactions include, but are not limited
to, the interactions of an
agonist, partial agonist, an inverse agonist, antagonist, degrader, or
combinations thereof In
some embodiments, a modulator is an antagonist. In some embodiments, a
modulator is a
degrader.
[00154] The terms "administer," "administering", "administration," and the
like, as used herein,
refer to the methods that in some cases enable delivery of compounds or
compositions to the
desired site of biological action. These methods include, but are not limited
to oral routes,
intraduodenal routes, parenteral injection (including intravenous,
subcutaneous, intraperitoneal,
intramuscular, intravascular or infusion), topical and rectal administration.
Those of skill in the
- 37 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
art are familiar with administration techniques that can be employed with the
compounds and
methods described herein. In some embodiments, the compounds and compositions
described
herein are administered orally.
[00155] The terms "co-administration" or the like, as used herein, are meant
to encompass
administration of the selected therapeutic agents to a single patient and are
intended to include
treatment regimens in which the agents are administered by the same or
different route of
administration or at the same or different time.
[00156] The terms "effective amount" or "therapeutically effective amount," as
used herein,
refer to a sufficient amount of an agent or a compound being administered,
which will relieve to
some extent one or more of the symptoms of the disease or condition being
treated. The result
includes reduction and/or alleviation of the signs, symptoms, or causes of a
disease, or any other
desired alteration of a biological system. For example, an "effective amount"
for therapeutic uses
is the amount of the composition comprising a compound as disclosed herein
required to provide
a clinically significant decrease in disease symptoms. An appropriate
"effective" amount in any
individual case is optionally determined using techniques, such as a dose
escalation study.
[00157] The terms "enhance" or "enhancing," as used herein, means to increase
or prolong
either in potency or duration a desired effect. Thus, in regard to enhancing
the effect of
therapeutic agents, the term "enhancing" refers to the ability to increase or
prolong, either in
potency or duration, the effect of other therapeutic agents on a system. An
"enhancing-effective
amount," as used herein, refers to an amount adequate to enhance the effect of
another
therapeutic agent in a desired system.
[00158] The term "pharmaceutical combination" as used herein, means a product
that results
from the mixing or combining of more than one active ingredient and includes
both fixed and
non-fixed combinations of the active ingredients. The term "fixed combination"
means that the
active ingredients, e.g. a compound described herein, or a pharmaceutically
acceptable salt
thereof, and a co-agent, are both administered to a patient simultaneously in
the form of a single
entity or dosage. The term "non-fixed combination" means that the active
ingredients, e.g. a
compound described herein, or a pharmaceutically acceptable salt thereof, and
a co-agent, are
administered to a patient as separate entities either simultaneously,
concurrently or sequentially
with no specific intervening time limits, wherein such administration provides
effective levels of
the two compounds in the body of the patient. The latter also applies to
cocktail therapy, e.g. the
administration of three or more active ingredients.
[00159] The terms "kit" and "article of manufacture" are used as synonyms.
- 38 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[00160] The term "subject" or "patient" encompasses mammals. Examples of
mammals include,
but are not limited to, any member of the Mammalian class: humans, non-human
primates such
as chimpanzees, and other apes and monkey species; farm animals such as
cattle, horses, sheep,
goats, swine; domestic animals such as rabbits, dogs, and cats; laboratory
animals including
rodents, such as rats, mice and guinea pigs, and the like. In one aspect, the
mammal is a human.
[00161] The terms "treat," "treating" or "treatment," as used herein, include
alleviating, abating
or ameliorating at least one symptom of a disease or condition, preventing
additional symptoms,
inhibiting the disease or condition, e.g., arresting the development of the
disease or condition,
relieving the disease or condition, causing regression of the disease or
condition, relieving a
condition caused by the disease or condition, or stopping the symptoms of the
disease or
condition either prophylactically and/or therapeutically.
Pharmaceutical Compositions
[00162] In some embodiments, the compounds described herein are formulated
into
pharmaceutical compositions. Pharmaceutical compositions are formulated in a
conventional
manner using one or more pharmaceutically acceptable inactive ingredients that
facilitate
processing of the active compounds into preparations that are used
pharmaceutically. Proper
formulation is dependent upon the route of administration chosen. A summary of
pharmaceutical
compositions described herein is found, for example, in Remington: The Science
and Practice of
Pharmacy, Nineteenth Ed (Easton, Pa.: Mack Publishing Company, 1995); Hoover,
John E.,
Remington's Pharmaceutical Sciences, Mack Publishing Co., Easton, Pennsylvania
1975;
Liberman, H.A. and Lachman, L., Eds., Pharmaceutical Dosage Forms, Marcel
Decker, New
York, N.Y., 1980; and Pharmaceutical Dosage Forms and Drug Delivery Systems,
Seventh Ed.
(Lippincott Williams & Wilkins1999), herein incorporated by reference for such
disclosure.
[00163] In some embodiments, the compounds described herein are administered
either alone or
in combination with pharmaceutically acceptable carriers, excipients or
diluents, in a
pharmaceutical composition. Administration of the compounds and compositions
described
herein, in some cases, are effected by any method that enables delivery of the
compounds to the
site of action. These methods include, though are not limited to delivery via
enteral routes
(including oral, gastric or duodenal feeding tube, rectal suppository and
rectal enema), parenteral
routes (injection or infusion, including intraarterial, intracardiac,
intradermal, intraduodenal,
intramedullary, intramuscular, intraosseous, intraperitoneal, intrathecal,
intravascular,
intravenous, intravitreal, epidural and subcutaneous), inhalational,
transdermal, transmucosal,
sublingual, buccal and topical (including epicutaneous, dermal, enema, eye
drops, ear drops,
intranasal, vaginal) administration, although the most suitable route, in some
instances, depends
- 39 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
upon for example the condition and disorder of the recipient. By way of
example only,
compounds described herein, in some cases, are administered locally to the
area in need of
treatment, by for example, local infusion during surgery, topical application
such as creams or
ointments, injection, catheter, or implant. The administration, in some cases,
is by direct injection
at the site of a diseased tissue or organ.
[00164] In some embodiments of the invention, a PPAR6 agonist is included
within a
pharmaceutical composition. As used herein, the term "pharmaceutical
composition" refers to a
liquid or solid composition, preferably solid (e.g., a granulated powder),
that contains a
pharmaceutically active ingredient (e.g., a PPAR6 agonist) and at least a
carrier, where none of
the ingredients is generally biologically undesirable at the administered
quantities.
[00165] Pharmaceutical compositions incorporating a PPAR6 agonist, in some
cases, take any
physical form that is pharmaceutically acceptable. Pharmaceutical compositions
for oral
administration are particularly preferred. In one embodiment of such
pharmaceutical
compositions, an effective amount of a PPAR6 agonist is incorporated.
[00166] In some cases, known methods of formulating pharmaceutical
compositions that are
typically used in the pharmaceutical sciences are followed. All of the usual
types of
compositions are contemplated, including, but not limited to, tablets,
chewable tablets, capsules,
and solutions. The amount of the PPAR6 agonist, however, is best defined as
the effective
amount, that is, the amount of the PPAR6 agonist that provides the desired
dose to the subject in
need of such treatment. Any of the PPAR6 agonists as described herein are
formulated in any
desired form of composition.
[00167] Capsules, in some cases, are prepared by mixing the PPAR6 agonist with
a suitable
diluent and filling the proper amount of the mixture in capsules. The usual
diluents include inert
powdered substances such as starch of many different kinds, powdered
cellulose, especially
crystalline and microcrystalline cellulose, sugars such as fructose, mannitol
and sucrose, grain
flours and similar edible powders.
[00168] Tablets, in some cases, are prepared by direct compression, by wet
granulation, or by
dry granulation. Their formulations usually incorporate diluents, binders,
lubricants, and
disintegrators, as well as the PPAR6 agonist. Typical diluents include, for
example, various
types of starch, lactose, mannitol, kaolin, calcium phosphate or sulfate,
inorganic salts such as
sodium chloride, and powdered sugar. Powdered cellulose derivatives are also
useful. Typical
tablet binders are substances such as starch, gelatin, and sugars such as
lactose, fructose, glucose,
and the like. Natural and synthetic gums are also convenient, including
acacia, alginates,
- 40 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
methylcellulose, polyvinylpyrrolidine, and the like. Polyethylene glycol,
ethylcellulose, and
waxes, in some cases, also serve as binders.
[00169] A lubricant in a tablet formulation, in some cases, help prevent the
tablet and punches
from sticking in the die. A lubricant, in some cases, is chosen from such
solids as talc,
magnesium and calcium stearate, stearic acid, and hydrogenated vegetable oils.
[00170] Tablet disintegrators are substances that swell when wetted to break
up the tablet and
release the compound. They include starches, clays, celluloses, aligns, and
gums. More
particularly, corn and potato starches, methylcellulose, agar, bentonite, wood
cellulose, powdered
natural sponge, cation-exchange resins, alginic acid, guar gum, citrus pulp,
and
carboxymethylcellulose, for example, in some cases, are used, as well as
sodium lauryl sulfate.
[00171] Enteric formulations are often used to protect an active ingredient
from the strongly
acidic contents of the stomach. Such formulations are created by coating a
solid dosage form
with a film of a polymer that is insoluble in acid environments, and soluble
in basic
environments. Exemplary films are cellulose acetate phthalate, polyvinyl
acetate phthalate,
hydroxypropyl methylcellulose phthalate, and hydroxypropyl methylcellulose
acetate succinate.
[00172] Tablets are often coated with sugar as a flavor and sealant. The PPAR6
agonists, in
some cases, are also be formulated as chewable tablets by using large amounts
of pleasant-tasting
substances, such as mannitol, in the formulation.
[00173] Transdermal patches, in some cases, are used. Typically, a patch
comprises a resinous
composition in which the active compound(s) will dissolve, or partially
dissolve, and is held in
contact with the skin by a film that protects the composition. Other, more
complicated patch
compositions are also in use, particularly those having a membrane pierced
with innumerable
pores through which the drugs are pumped by osmotic action.
[00174] In any embodiment where a PPAR6 agonist is included in a
pharmaceutical
composition, such pharmaceutical compositions, in some cases, are in a form
suitable for oral
use, for example, as tablets, troches, lozenges, aqueous or oily suspensions,
dispersible powders
or granules, emulsions, hard or soft capsules, or syrups or elixirs.
Compositions intended for oral
use, in some cases, are prepared according to any known method, and such
compositions, in
some cases, contain one or more agents selected from the group consisting of
sweetening agents,
flavoring agents, coloring agents, and preserving agents in order to provide
pharmaceutically
elegant and palatable preparations. Tablets, in some cases, contain the active
ingredient in
admixture with non-toxic pharmaceutically acceptable excipients that are
suitable for the
manufacture of tablets. These excipients include for example, inert diluents,
such as calcium
carbonate, sodium carbonate, lactose, calcium phosphate, or sodium phosphate;
granulating and
-41 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
disintegrating agents, for example, corn starch or alginic acid; binding
agents, for example,
starch, gelatin, or acacia; and lubricating agents, for example, magnesium
stearate, stearic acid,
or talc. The tablets, in some cases, are uncoated or they, in some cases, are
coated by known
techniques to delay disintegration and absorption in the gastrointestinal
tract and thereby provide
a sustained action over a longer period. For example, a time delay material
such as glyceryl
monostearate or glyceryl distearate, in some cases, is employed.
Methods of Dosing and Treatment Regimens
[00175] In one embodiment, a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof), is used in the preparation of medicaments for the
treatment of fatty acid
oxidation disorders (FAOD) in a mammal. Methods for treating any of the
diseases or conditions
described herein in a mammal in need of such treatment, involves
administration of
pharmaceutical compositions that include a PPAR6 agonist (e.g. Compound 1, or
a
pharmaceutically acceptable salt thereof), active metabolite, prodrug, in
therapeutically effective
amounts to said mammal.
[00176] In certain embodiments, the compositions containing the compound(s)
described herein
are administered for prophylactic and/or therapeutic treatments. In certain
therapeutic
applications, the compositions are administered to a patient already suffering
from a disease or
condition, in an amount sufficient to cure or at least partially arrest at
least one of the symptoms
of the disease or condition. Amounts effective for this use depend on the
severity and course of
the disease or condition, previous therapy, the patient's health status,
weight, and response to the
drugs, and the judgment of the treating physician. Therapeutically effective
amounts are
optionally determined by methods including, but not limited to, a dose
escalation and/or dose
ranging clinical trial.
[00177] In prophylactic applications, compositions containing a PPAR6 agonist
(e.g. Compound
1, or a pharmaceutically acceptable salt thereof), are administered to a
patient susceptible to or
otherwise at risk of a particular disease, disorder or condition. Such an
amount is defined to be a
"prophylactically effective amount or dose." In this use, the precise amounts
also depend on the
patient's state of health, weight, and the like. When used in patients,
effective amounts for this
use will depend on the severity and course of the disease, disorder or
condition, previous therapy,
the patient's health status and response to the drugs, and the judgment of the
treating physician.
In one aspect, prophylactic treatments include administering to a mammal, who
previously
experienced at least one symptom of the disease being treated and is currently
in remission, a
pharmaceutical composition comprising a PPAR6 agonist (e.g. Compound 1, or a
- 42 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
pharmaceutically acceptable salt thereof), in order to prevent a return of the
symptoms of the
disease or condition.
[00178] In certain embodiments wherein the patient's condition does not
improve, upon the
doctor's discretion the administration of a PPAR6 agonist (e.g. Compound 1, or
a
pharmaceutically acceptable salt thereof), is administered chronically, that
is, for an extended
period of time, including throughout the duration of the patient's life in
order to ameliorate or
otherwise control or limit the symptoms of the patient's disease or condition.
[00179] In certain embodiments wherein a patient's status does improve, the
dose of drug being
administered is temporarily reduced or temporarily suspended for a certain
length of time (i.e., a
"drug holiday"). In specific embodiments, the length of the drug holiday is
between 2 days and 1
year, including by way of example only, 2 days, 3 days, 4 days, 5 days, 6
days, 7 days, 10 days,
12 days, 15 days, 20 days, 28 days, or more than 28 days. The dose reduction
during a drug
holiday is, by way of example only, by about 10%-100%, including by way of
example only
about 10%, about 15%, about 20%, about 25%, about 30%, about 35%, about 40%,
about 45%,
about 50%, about 55%, about 60%, about 65%, about 70%, about 75%, about 80%,
about 85%,
about 90%, about 95%, and about 100%.
[00180] Once improvement of the patient's conditions has occurred, a
maintenance dose is
administered if necessary. Subsequently, in specific embodiments, the dosage
or the frequency of
administration, or both, is reduced, as a function of the symptoms, to a level
at which the
improved disease, disorder or condition is retained. In certain embodiments,
however, the patient
requires intermittent treatment on a long-term basis upon any recurrence of
symptoms.
[00181] In one aspect, a PPAR6 agonist (e.g. Compound 1, or a pharmaceutically
acceptable salt
thereof), is administered daily to humans with a FAOD in need of therapy with
a PPAIto agonist
(e.g. Compound 1, or a pharmaceutically acceptable salt thereof). In some
embodiments, a
PPAIto agonist (e.g. Compound 1, or a pharmaceutically acceptable salt
thereof), is administered
once-a-day. In some embodiments, a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof), is administered twice-a-day. In some embodiments, a
PPAR6 agonist
(e.g. Compound 1, or a pharmaceutically acceptable salt thereof), is
administered three times-a-
day. In some embodiments, a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof), is administered every other day. In some
embodiments, a PPAR6 (e.g.
Compound 1, or a pharmaceutically acceptable salt thereof), is administered
twice a week.
[00182] In some instances, a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof) is administered once per day, twice per day, three
times per day or more.
In some instances, a PPAR6 agonist (e.g. Compound 1, or a pharmaceutically
acceptable salt
- 43 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
thereof) is administered twice per day. A PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically acceptable salt thereof), in some embodiments, is
administered daily, every
day, every alternate day, five days a week, once a week, every other week, two
weeks per month,
three weeks per month, once a month, twice a month, three times per month, or
more. In some
embodiments, a PPAR6 agonist (e.g. Compound 1, or a pharmaceutically
acceptable salt thereof)
is administered twice daily, e.g., morning and evening. In some embodiments, a
PPAR6 agonist
(e.g. Compound 1, or a pharmaceutically acceptable salt thereof) is
administered for at least 1
day, 2 days, 3 days, 4 days, 5 days, 6 days, 1 week, 2 weeks, 3 weeks, 1
month, 2 months, 3
months, 4 months, 5 months, 6 months, 7 months, 8 months, 9 months, 10 months,
11 months, 12
months, 18 months, 2 years, 3 years, 4 years, 5 years, 10 years, or more. In
some embodiments, a
PPAR6 agonist (e.g. Compound 1, or a pharmaceutically acceptable salt thereof)
is administered
twice daily for at least or about 1 week, 2 weeks, 3 weeks, 1 month, 2 months,
3 months, 4
months, 5 months, 6 months, or more. In some embodiments, a PPAR6 agonist
(e.g. Compound
1, or a pharmaceutically acceptable salt thereof) is administered once daily,
twice daily, three
times daily, four times daily, or more than four times daily for at least or
about 1 week, 2 weeks,
3 weeks, 1 month, 2 months, 3 months, 4 months, 5 months, 6 months, or more.
[00183] In general, doses of a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof), employed for treatment of the diseases or conditions
described herein in
humans are typically in the range of from about 0.1 mg/kg to about 10 mg/kg of
body weight per
dose. In one embodiment, the desired dose is conveniently presented in a
single dose or in
divided doses administered simultaneously (or over a short period of time) or
at appropriate
intervals, for example as two, three, four or more sub-doses per day. In some
embodiments, a
PPAR6 agonist (e.g. Compound 1, or a pharmaceutically acceptable salt
thereof), is conveniently
presented in divided doses that are administered simultaneously (or over a
short period of time)
once a day. In some embodiments, a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof), is conveniently presented in divided doses that are
administered in equal
portions twice-a-day.
[00184] In some embodiments, a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof), is administered orally to the human at a dose from
about 0.1 mg to about
mg/kg of body weight per dose. In some embodiments, a PPAR6 agonist (e.g.
Compound 1,
or a pharmaceutically acceptable salt thereof), is administered to the human
on a continuous
dosing schedule. In some embodiments, a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically acceptable salt thereof), is administered to the human on a
continuous daily
dosing schedule.
- 44 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
1001851 The term "continuous dosing schedule" refers to the administration of
a particular
therapeutic agent at regular intervals. In some embodiments, continuous dosing
schedule refers
to the administration of a particular therapeutic agent at regular intervals
without any drug
holidays from the particular therapeutic agent. In some other embodiments,
continuous dosing
schedule refers to the administration of a particular therapeutic agent in
cycles. In some other
embodiments, continuous dosing schedule refers to the administration of a
particular therapeutic
agent in cycles of drug administration followed by a drug holiday (for
example, a wash out
period or other such period of time when the drug is not administered) from
the particular
therapeutic agent. For example, in some embodiments the therapeutic agent is
administered once
a day, twice a day, three times a day, once a week, twice a week, three times
a week, four times a
week, five times a week, six times a week, seven times a week, every other
day, every third day,
every fourth day, daily for a week followed by a week of no administration of
the therapeutic
agent, daily for a two weeks followed by one or two weeks of no administration
of the
therapeutic agent, daily for three weeks followed by one, two or three weeks
of no administration
of the therapeutic agent, daily for four weeks followed by one, two, three or
four weeks of no
administration of the therapeutic agent, weekly administration of the
therapeutic agent followed
by a week of no administration of the therapeutic agent, or biweekly
administration of the
therapeutic agent followed by two weeks of no administration of the
therapeutic agent. In some
embodiments, daily administration is once a day. In some embodiments, daily
administration is
twice a day. In some embodiments, daily administration is three times a day.
In some
embodiments, daily administration is more than three times a day.
[00186] The term "continuous daily dosing schedule" refers to the
administration of a particular
therapeutic agent every day at roughly the same time each day. In some
embodiments, daily
administration is once a day. In some embodiments, daily administration is
twice a day. In some
embodiments, daily administration is three times a day. In some embodiments,
daily
administration is more than three times a day.
[00187] In some embodiments, the amount of a PPAR6 agonist (e.g. Compound 1,
or a
pharmaceutically acceptable salt thereof), is administered once a day. In some
other
embodiments, the amount of a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof), is administered twice a day. In some other
embodiments, the amount of
a PPAR6 agonist (e.g. Compound 1, or a pharmaceutically acceptable salt
thereof), is
administered three times a day.
[00188] In certain embodiments wherein improvement in the status of the
disease or condition in
the human is not observed, the daily dose of a PPAR6 agonist (e.g. Compound 1,
or a
- 45 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
pharmaceutically acceptable salt thereof), is increased. In some embodiments,
a once-a-day
dosing schedule is changed to a twice-a-day dosing schedule. In some
embodiments, a three
times a day dosing schedule is employed to increase the amount of a PPAR6
agonist (e.g.
Compound 1, or a pharmaceutically acceptable salt thereof), that is
administered. In some
embodiments, the frequency of administration by inhalation is increased in
order to provide
repeat high Cmax levels on a more regular basis. In some embodiments, the
frequency of
administration is increased in order to provide maintained or more regular
exposure to a PPAR6
agonist (e.g. Compound 1, or a pharmaceutically acceptable salt thereof). In
some embodiments,
the frequency of administration is increased in order to provide repeat high
Cmax levels on a
more regular basis and provide maintained or more regular exposure to a PPAR6
agonist (e.g.
Compound 1, or a pharmaceutically acceptable salt thereof).
[00189] In any of the aforementioned aspects are further embodiments
comprising single
administrations of the effective amount of a PPARo agonist (e.g. Compound 1,
or a
pharmaceutically acceptable salt thereof), including further embodiments in
which the PPAR6
agonist, is administered (i) once a day; or (ii) multiple times over the span
of one day.
[00190] In any of the aforementioned aspects are further embodiments
comprising multiple
administrations of the effective amount of a PPARo agonist (e.g. Compound 1,
or a
pharmaceutically acceptable salt thereof), including further embodiments in
which (i) the PPAR6
agonist is administered continuously or intermittently: as in a single dose;
(ii) the time between
multiple administrations is every 6 hours; (iii) the PPAR6 agonist is
administered to the mammal
every 8 hours; (iv) the PPAR6 agonist is administered to the mammal every 12
hours; (v) the
PPAR6 agonist is administered to the mammal every 24 hours. In further or
alternative
embodiments, the method comprises a drug holiday, wherein the administration
of the PPAR6
agonist is temporarily suspended or the dose of the PPAR6 agonist being
administered is
temporarily reduced; at the end of the drug holiday, dosing of the PPAR6
agonist is resumed. In
one embodiment, the length of the drug holiday varies from 2 days to 1 year.
[00191] Generally, a suitable dose of a PPAR6 agonist, or a pharmaceutically
acceptable salt
thereof, for administration to a human will be in the range of about 0.1 mg/kg
per day to about 25
mg/kg per day (e.g., about 0.2 mg/kg per day, about 0.3 mg/kg per day, about
0.4 mg/kg per day,
about 0.5 mg/kg per day, about 0.6 mg/kg per day, about 0.7 mg/kg per day,
about 0.8 mg/kg per
day, about 0.9 mg/kg per day, about 1 mg/kg per day, about 2 mg/kg per day,
about 3 mg/kg per
day, about 4 mg/kg per day, about 5 mg/kg per day, about 6 mg/kg per day,
about 7 mg/kg per
day, about 8 mg/kg per day, about 9 mg/kg per day, about 10 mg/kg per day,
about 15 mg/kg per
day, about 20 mg/kg per day, or about 25 mg/kg per day). Alternatively, a
suitable dose of a
- 46 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
PPAR6 agonist, or a pharmaceutically acceptable salt thereof, for
administration to a human will
be in the range of from about 0.1 mg/day to about 1000 mg/day; from about 1
mg/day to about
400 mg/day; or from about 1 mg/day to about 300 mg/day. In other embodiments,
a suitable
dose of a PPAR6 agonist, or a pharmaceutically acceptable salt thereof, for
administration to a
human will be about 1 mg/day, about 2 mg/day, about 3 mg/day, about 4 mg/day,
about 5
mg/day, about 6 mg/day, about 7 mg/day, about 8 mg/day, about 9 mg/day, about
10 mg/day,
about 15 mg/day, about 20 mg/day, about 25 mg/day, about 30 mg/day, about 35
mg/day, about
40 mg/day, about 45 mg/day, about 50 mg/day, about 55 mg/day, about 60 mg/day,
about 65
mg/day, about 70 mg/day, about 75 mg/day, about 80 mg/day, about 85 mg/day,
about 90
mg/day, about 95 mg/day, about 100 mg/day, about 125 mg/day, about 150 mg/day,
about 175
mg/day, about 200 mg/day, about 225 mg/day, about 250 mg/day, about 275
mg/day, about 300
mg/day, about 325 mg/day, about 350 mg/day, about 375 mg/day, about 400
mg/day, about 425
mg/day, about 450 mg/day, about 475 mg/day, or about 500 mg/day. Dosages, in
some cases, are
administered more than one time per day (e.g., two, three, four, or more times
per day). In one
embodiment, a suitable dose of a PPAR6 agonist, or a pharmaceutically
acceptable salt thereof,
for administration to a human is about 100 mg twice/day (i.e., a total of
about 200 mg/day). In
another embodiment, a suitable dose of a PPAR6 agonist, or a pharmaceutically
acceptable salt
thereof, for administration to a human is about 50 mg twice/day (i.e., a total
of about 100
mg/day).
[00192] In some embodiments, the daily dosage or the amount of active in the
dosage form are
lower or higher than the ranges indicated herein, based on a number of
variables in regard to an
individual treatment regime. In various embodiments, the daily and unit
dosages are altered
depending on a number of variables including, but not limited to, the disease
or condition to be
treated, the mode of administration, the requirements of the individual
subject, the severity of the
disease or condition being treated, the identity (e.g., weight) of the human,
and the particular
additional therapeutic agents that are administered (if applicable), and the
judgment of the
practitioner.
[00193] Toxicity and therapeutic efficacy of such therapeutic regimens are
determined by
standard pharmaceutical procedures in cell cultures or experimental animals,
including, but not
limited to, the determination of the LD50 and the ED50. The dose ratio between
the toxic and
therapeutic effects is the therapeutic index and it is expressed as the ratio
between LD50 and
ED50. In certain embodiments, the data obtained from cell culture assays and
animal studies are
used in formulating the therapeutically effective daily dosage range and/or
the therapeutically
effective unit dosage amount for use in mammals, including humans. In some
embodiments, the
- 47 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
daily dosage amount of the PPAR6 agonist lies within a range of circulating
concentrations that
include the ED50 with minimal toxicity. In certain embodiments, the daily
dosage range and/or
the unit dosage amount varies within this range depending upon the dosage form
employed and
the route of administration utilized.
[00194] In some embodiments, following the administration of a therapeutically
effective dose
of the PPAR6 agonist to a subject, the no observed adverse effect level
(NOAEL) is at least 1, 10,
20, 50, 100, 500 or 1000 milligrams of the PPAR6 agonist per kilogram of body
weight (mpk). In
some examples, the 7-day NOAEL for a rat administered PPAR6 agonist is at
least about 200,
300, 400, 500, 600, 700, 800, 900, 1000, 1500 or 2000 mpk. In some examples,
the 7-day
NOAEL for a dog administered PPAR6 agonist is at least about 10, 20, 30, 40,
50, 60, 70, 80, 90,
100, 200, 500 mpk.
[00195] In some embodiments, methods for treating a fatty acid oxidation
disorder (FAOD) in a
mammal with a PPARo agonist compound described herein (e.g. Compound 1, or a
pharmaceutically acceptable salt thereof) results in improvements in one or
more outcome
measures. In some embodiments, outcomes measures include, but are not limited
to: patient
reported outcomes (PRO), exercise tolerance, whole body fatty acid oxidation
(e.g. 13CO2
production), blood acylcarnitines profiles, and blood inflammatory cytokines.
In some
embodiments, a baseline assessment is determined, typically prior to the
administration of a
PPARo agonist (e.g. Compound 1, or a pharmaceutically acceptable salt
thereof). Improvements
in outcome measures are assessed with repeated assessments taken during
treatment with a
PPARo agonist compound and a comparison against the baseline assessment and/or
any prior
assessment(s). In some embodiments, improvements are by at least or about 10%,
15%, 20%,
25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or
more
than 95%. In some embodiments, the PPARo agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof) described herein improvements are by at least or
about 0.5X, 1.0X, 1.5X,
2.0X, 2.5X, 3.0X, 3.5X, 4.0X, 5.0X, 6.0X, 7.0X, 8.0X, 9.0X, 10X, or more than
10X.
Improvements, in some embodiments, are compared to a control. In some
embodiments, a
control is an individual who does not receive a PPARo agonist (e.g. Compound
1, or a
pharmaceutically acceptable salt thereof). In some embodiments, the control is
an individual
who does not receive a full dose of a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof). In some embodiments, the control is baseline for the
individual prior to
receiving a PPAR6 agonist (e.g. Compound 1, or a pharmaceutically acceptable
salt thereof).
[00196] In some embodiments, patient reported outcomes (PRO) are measured with
questionnaires. In some embodiments, the questionnaire covers health concepts
related to the
- 48 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
disorder being treated. In some embodiments, the questionnaire covers health
concepts related to
the disorder being treated such as, but not, limited to: physical functioning,
bodily pain, role
limitations due to physical health problems, role limitations due to personal
or emotional
problems, emotional well-being, social functioning, energy/fatigue, and
general health
perceptions, including perceptions in change of health.
[00197] In some embodiments, outcome measures are assessed with tests that
assess exercise
tolerance. In some embodiments, exercise tolerance is assessed with exercise
tests. Exercise
tests include, but are not limited to, submaximal treadmill, walking tests
(e.g. without limitation,
6 minute; 12 minute walks), run tests, treadmill and ergometry exercise
testing. In some
embodiments, exercise tests are used in combination with the Borg Scale of
perceived exertion.
In some embodiments, exercise tests are performed according to guidelines set
forth by the
American Thoracic Society (ATS).
[00198] In some embodiments, the respiratory exchange ratio (RER) is measured
to assess
exercise tolerance. RER is the ratio between the amount of carbon dioxide
(CO2) produced in
metabolism and oxygen (02) used. In some mebodiments, the ratio is determined
by comparing
exhaled gases to room air.
[00199] PPAR agonists have demonstrated the ability to increase '3CO2
production in clinical
trials (Gillingham, M. B., et at., Journal of Inherited Metabolic Disease,
Volume 40, Issue 6,
Nov. 2017, 831-843; Riserus, U., et al. Diabetes 2008 Feb; 57(2): 332-339;
each of which is
incorporated for such protocols). In some embodiments, stable isotope methods
are used to
measure in vivo residual fatty acid oxidation capacity. Enrichment of 13CO2
only occurs by one
complete round of fatty acid oxidation. A representative protocol is as
follows. A fasting blood
sample is obtained after an overnight fast. Prior to breakfast, a resting
indirect calorimetry is
measured. Subjects are then given a meal (e.g a shake) containing 17-mg/kg 13C-
oleic acid.
Breath samples are collected prior to (time 0) and again hourly at 1, 2, 3, 4,
5, 6, 7, and 8 hours
following the 13C-oleic administration. 13C in breath samples are measured as
a ratio of 13c/12c
using the Delta Plus IRMS (Finnigan MAT, Bremen, Germany). Recovery is
calculated as 13C
divided by the dose of 13C administered. The amount of excess 13C in breath is
a measure of
residual fatty acid oxidation capacity in subjects with disorders of long-
chain fatty acid oxidation.
[00200] In some embodiments, improvements in fatty acid oxidation in subjects
with a FAOD
that are treated with a PPAR6 agonist compound described herein (e.g. Compound
1, or a
pharmaceutically acceptable salt thereof) are measured with a suitable 13CO2
breath sample test.
In some embodiments, a suitable 13CO2 breath sample test comprises the steps
of: 1) providing
the subject a meal comprising 13C-enriched fatty acid(s); 2) administering to
the subject a PPARo
- 49 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
agonist compound, or a pharmaceutically acceptable salt thereof, after the
consumption of the
meal; and 3) collecting breath samples from the subject at regular intervals
and measuring the
relative amount of 13CO2 to 12CO2 in the breath samples. In some embodiments,
the breath
samples are collected about every hour. In some embodiments, the meal is
enriched with a 13C
labeled fatty acid, wherein the fatty acid is butyric acid, caproic acid,
caprylic acid, capric acid,
lauric acid, myristic acid, palmitic acid, stearic acid, arachidic acid,
behenic acid, lignoceric acid,
caproleic acid, lauroleic acid, myristoleic acid, palmitoleic acid, oleic
acid, elaidic acid, vaccenic
acid, gadoleic acid, erucic acid, brassidic acid, nervonic acid, linoleic
acid, alpha-linolenic acid,
gamma-linolenic acid, columbinic acid, stearidonic acid, mead acid, dihomo-y-
linolenic acid,
arachidonic acid, eicosapentaenoic acid, docosapentaenoic acid, or
docosahexaenoic acid.
[00201] In some embodiments, described herein is a method for measuring whole-
body fatty
acid oxidation in a human with a fatty acid oxidation disorder (FAOD)
comprising: feeding the
human with a fatty acid oxidation disorder (FAOD) a meal comprising 13C-
enriched fatty acids
and measuring the amount of exhaled '3CO2 from the human, wherein the human
with a fatty
acid oxidation disorder (FAOD) is undergoing treatment with a PPAIto agonist
compound.
[00202] In some embodiments, described herein is a method for measuring
changes in whole-
body fatty acid oxidation in a human with a fatty acid oxidation disorder
(FAOD) comprising the
steps of: 1) providing a meal enriched with a 13C labeled fatty acid; 2)
administering to the
human a PPAIto agonist compound, or a pharmaceutically acceptable salt
thereof; and 3)
collecting breath samples from the human at regular intervals and measuring
for the content of
13CO2 in the breath samples.
[00203] In some embodiments, the amount of 13CO2 in breath samples is used as
a diagnostic to
guide treatment of the subject with a FAOD with a PPAR6 agonist compound. For
example, if a
subject or individual has a change in the amount of 13CO2 of at least a
specified percentage or
level following the administration of a PPAR6 agonist compound, the subject or
individual
continues the treatment using a PPAR6 agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof) described herein. In some embodiments, modest
increases in 13CO2 in
breath samples may necessitate an increase in the amount of PPAR6 agonist
compound that is
administered to the subject, an increase in the frequency of administering the
PPAR6 agonist
compound, or both.
[00204] In some instances, the change in the amount of 13CO2 is at least or
about 10%, 15%,
20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%,
95%, or
more than 95% compared to baseline. In some instances, the change occurs after
at least or about
1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 12
hours, 16 hours, 20 hours,
- 50 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
1 day, 2 days, 3 days, 4 days, 5 days, 6 days, 1 week, 2 weeks, 3 weeks, 1
month, 2 months, 3
months, 4 months, or more than 4 months after initiation of treatment with a
PPAR6 agonist
compound (e.g. Compound 1, or a pharmaceutically acceptable salt thereof) has
begun. In some
instances, a treatment regimen comprising a PPAR6 agonist compound (e.g.
Compound 1, or a
pharmaceutically acceptable salt thereof) is continued if the change in the
amount of 13CO2 is at
least or about 10%, 15%, 20%, 25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%,
70%, 75%,
80%, 85%, 90%, 95%, or more than 95% compared to baseline after at least or
about 1 hour, 2
hours, 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 12 hours, 16
hours, 20 hours, 1 day, 2
days, 3 days, 4 days, 5 days, 6 days, 1 week, 2 weeks, 3 weeks, 1 month, 2
months, 3 months, 4
months, or more than 4 months after initiation of treatment with a PPAR6
agonist compound
(e.g. Compound 1, or a pharmaceutically acceptable salt thereof) has begun. In
some instances,
the change is an increase in the levels of 13CO2.
1002051 In some embodiments, increases of amount of 13CO2 over time is
indicative of a
subject's responsive to the PPAR6 agonist compound (e.g. Compound 1, or a
pharmaceutically
acceptable salt thereof). In some instances, a subject is responsive to the
PPAR6 agonist
compound (e.g. Compound 1, or a pharmaceutically acceptable salt thereof) if
there is a change
in the amount of 13CO2 of at least or about 10%, 15%, 20%, 25%, 30%, 35%, 40%,
45%, 50%,
55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or more than 95% compared to
baseline in
13CO2 levels. In some instances, the change in the amount of 13CO2 occurs
after at least or about
1 hour, 2 hours, 3 hours, 4 hours, 5 hours, 6 hours, 7 hours, 8 hours, 12
hours, 16 hours, 20 hours,
1 day, 2 days, 3 days, 4 days, 5 days, 6 days, 1 week, 2 weeks, 3 weeks, 1
month, 2 months, 3
months, 4 months, or more than 4 months after administration of the PPARo
agonist (e.g.
Compound 1, or a pharmaceutically acceptable salt thereof) described herein.
In some instances,
a subject is responsive if the change in the amount of 13CO2 is at least or
about 10%, 15%, 20%,
25%, 30%, 35%, 40%, 45%, 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 95%, or
more
than 95% compared to baseline after at least or about 1 hour, 2 hours, 3
hours, 4 hours, 5 hours, 6
hours, 7 hours, 8 hours, 12 hours, 16 hours, 20 hours, 1 day, 2 days, 3 days,
4 days, 5 days, 6
days, 1 week, 2 weeks, 3 weeks, 1 month, 2 months, 3 months, 4 months, or more
than 4 months
after initiation of treatment with a PPAR6 agonist compound (e.g. Compound 1,
or a
pharmaceutically acceptable salt thereof) has begun. In some instances, the
change is an increase
in the amount of 13CO2 in the breath samples overt time.
-51 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
Combination Treatments
[00206] In certain instances, it is appropriate to administer a PPARo agonist
(e.g. Compound 1,
or a pharmaceutically acceptable salt thereof), in combination with one or
more other therapeutic
agents.
[00207] In one embodiment, the therapeutic effectiveness of a PPARo agonist
(e.g. Compound
1), or a pharmaceutically acceptable salt or solvate thereof, is enhanced by
administration of an
adjuvant (i.e., by itself the adjuvant has minimal therapeutic benefit, but in
combination with
another therapeutic agent, the overall therapeutic benefit to the patient is
enhanced). Or, in some
embodiments, the benefit experienced by a patient is increased by
administering a PPARo
agonist (e.g. Compound 1), or a pharmaceutically acceptable salt or solvate
thereof, with another
agent (which also includes a therapeutic regimen) that also has therapeutic
benefit.
[00208] In one specific embodiment, a PPARo agonist (e.g. Compound 1), or a
pharmaceutically acceptable salt or solvate thereof, is co-administered with a
second therapeutic
agent, wherein a PPARo agonist (e.g. Compound 1), or a pharmaceutically
acceptable salt or
solvate thereof, and the second therapeutic agent modulate different aspects
of the disease,
disorder or condition being treated, thereby providing a greater overall
benefit than
administration of either therapeutic agent alone.
[00209] In any case, regardless of the disease, disorder or condition being
treated, the overall
benefit experienced by the patient is simply additive of the two therapeutic
agents or the patient
experiences a synergistic benefit.
[00210] In certain embodiments, different therapeutically-effective dosages of
a PPARo agonist
(e.g. Compound 1), or a pharmaceutically acceptable salt or solvate thereof,
will be utilized in
formulating pharmaceutical composition and/or in treatment regimens when a
PPARo agonist
(e.g. Compound 1), or a pharmaceutically acceptable salt or solvate thereof,
is administered in
combination with one or more additional agent, such as an additional
therapeutically effective
drug, an adjuvant or the like. Therapeutically-effective dosages of drugs and
other agents for use
in combination treatment regimens is optionally determined by means similar to
those set forth
hereinabove for the actives themselves. Furthermore, the methods of
prevention/treatment
described herein encompasses the use of metronomic dosing, i.e., providing
more frequent, lower
doses in order to minimize toxic side effects. In some embodiments, a
combination treatment
regimen encompasses treatment regimens in which administration of a PPARo
agonist (e.g.
Compound 1), or a pharmaceutically acceptable salt or solvate thereof, is
initiated prior to,
during, or after treatment with a second agent described herein, and continues
until any time
during treatment with the second agent or after termination of treatment with
the second agent. It
- 52 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
also includes treatments in which a PPAIto agonist (e.g. Compound 1), or a
pharmaceutically
acceptable salt or solvate thereof, and the second agent being used in
combination are
administered simultaneously or at different times and/or at decreasing or
increasing intervals
during the treatment period. Combination treatment further includes periodic
treatments that start
and stop at various times to assist with the clinical management of the
patient.
[00211] It is understood that the dosage regimen to treat, prevent, or
ameliorate the condition(s)
for which relief is sought, is modified in accordance with a variety of
factors (e.g. the disease,
disorder or condition from which the subject suffers; the age, weight, sex,
diet, and medical
condition of the subject). Thus, in some instances, the dosage regimen
actually employed varies
and, in some embodiments, deviates from the dosage regimens set forth herein.
[00212] For combination therapies described herein, dosages of the co-
administered compounds
vary depending on the type of co-drug employed, on the specific drug employed,
on the disease
or condition being treated and so forth. In additional embodiments, when co-
administered with
one or more other therapeutic agents, a PPAIto agonist (e.g. Compound 1), or a
pharmaceutically
acceptable salt or solvate thereof, is administered either simultaneously with
the one or more
other therapeutic agents, or sequentially.
[00213] In combination therapies, the multiple therapeutic agents (one of
which is a PPAIto
agonist (e.g. Compound 1), or a pharmaceutically acceptable salt or solvate
thereof) are
administered in any order or even simultaneously. If administration is
simultaneous, the multiple
therapeutic agents are, by way of example only, provided in a single, unified
form, or in multiple
forms (e.g., as a single pill or as two separate pills).
[00214] A PPAIto agonist (e.g. Compound 1), or a pharmaceutically acceptable
salt or solvate
thereof, as well as combination therapies, are administered before, during or
after the occurrence
of a disease or condition, and the timing of administering the composition
containing a PPAIto
agonist (e.g. Compound 1), or a pharmaceutically acceptable salt or solvate
thereof, varies. Thus,
in one embodiment, Compound I, or a pharmaceutically acceptable salt or
solvate thereof, is used
as a prophylactic and are administered continuously to subjects with a
propensity to develop
conditions or diseases in order to prevent the occurrence of the disease or
condition. In another
embodiment, a PPAIto agonist (e.g. Compound 1), or a pharmaceutically
acceptable salt or
solvate thereof, is administered to a subject during or as soon as possible
after the onset of the
symptoms. In specific embodiments, a PPAIto agonist (e.g. Compound 1), or a
pharmaceutically
acceptable salt or solvate thereof, is administered as soon as is practicable
after the onset of a
disease or condition is detected or suspected, and for a length of time
necessary for the treatment
of the disease. In some embodiments, the length required for treatment varies,
and the treatment
- 53 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
length is adjusted to suit the specific needs of each subject. For example, in
specific
embodiments, a PPARo agonist (e.g. Compound 1), or a pharmaceutically
acceptable salt or
solvate thereof, or a formulation containing Compound I, or a pharmaceutically
acceptable salt or
solvate thereof, is administered for at least 2 weeks, about 1 month to about
5 years.
Exemplary Agents for use in Combination Therapy
[00215] In some embodiments, a PPARo agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt), is administered in combination with one or more additional
therapies used for
treating fatty acid oxidation disorders.
[00216] In certain embodiments, the at least one additional therapy is
administered at the same
time as a PPARo agonist (e.g. Compound 1), or a pharmaceutically acceptable
salt or solvate
thereof In certain embodiments, the at least one additional therapy is
administered less
frequently than a PPARo agonist (e.g. Compound 1), or a pharmaceutically
acceptable salt or
solvate thereof In certain embodiments, the at least one additional therapy is
administered more
frequently than a PPARo agonist (e.g. Compound 1), or a pharmaceutically
acceptable salt or
solvate thereof In certain embodiments, the at least one additional therapy is
administered prior
to administration of a PPARo agonist (e.g. Compound 1), or a pharmaceutically
acceptable salt
or solvate thereof. In certain embodiments, the at least one additional
therapy is administered
after administration of a PPARo agonist (e.g. Compound 1), or a
pharmaceutically acceptable salt
or solvate thereof.
[00217] In some embodiments, a PPARo agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt), is administered in combination with ubiquinol, ubiquinone,
niacin, riboflavin,
creatine , L-carnitine, acetyl-L-carnitine, biotin, thiamine, pantothenic
acid, pyridoxine, alpha-
lipoic acid, n-heptanoic acid, CoQ10, vitamin E, vitamin C, methylcobalamin,
folinic acid,
resveratrol, N-acetyl-L-cysteine (NAC), zinc, folinic acid/leucovorin calcium,
or a combination
thereof
[00218] In some embodiments, a PPARo agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt), is administered in combination with succinic acid, or salt
thereof, or
trisuccinylglycerol, or salt thereof. In some embodiments, a PPARo agonist
(e.g. Compound 1,
or a pharmaceutically acceptable salt), is administered in combination with a
compound
described in International PCT publication no. WO 2017/184583.
[00219] In some embodiments, a PPARo agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt), is administered in combination with an antioxidant.
- 54 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[00220] In some embodiments, a PPARo agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt), is administered in combination with an odd-chain fatty acid,
odd-chain fatty
ketone, L-carnitine, or combinations thereof
[00221] In some embodiments, a PPARo agonist (e.g. Compound 1, or a
pharmaceutically
acceptable salt), is administered in combination with triheptanoin, n-
heptanoic acid, a
triglyceride, or a salt or thereof, or combinations thereof
[00222] In some embodiments, a PPARo agonist is administered in combination
with a
Nicotinamide Adenine Dinucleotide (NAD+) pathway modulator. NAD+ plays many
important
roles within cells, including serving as an oxidizing agent in oxidative
phosphorylation which
generates ATP from ADP. Increasing cellular concentrations of NAD+ will
enhance the
oxidative capacity within mitochondria, thereby increasing nutrient oxidation
and boost energy
supply, which is a primary role of mitochondria. In some embodiments, the NAD+
modulator
targets Poly ADP Ribose Polymerase (PARP), Aminocarboxymuconate Semialdehyde
Decarboxylase (ACMSD) and N'-Nicotinamide Methyltransferase (NNMT).
Kits and Articles of Manufacture
[00223] Described herein are kits for treating treatment of fatty acid
oxidation disorders
(FAOD) in an individual comprising administering to said individual a PPAR6
agonist (e.g.
Compound 1, or a pharmaceutically acceptable salt thereof).
[00224] For use in the therapeutic applications described herein, kits and
articles of manufacture
are also described herein. In some embodiments, such kits include a carrier,
package, or container
that is compartmentalized to receive one or more containers such as vials,
tubes, and the like,
each of the container(s) including one of the separate elements to be used in
a method described
herein. Suitable containers include, for example, bottles, vials, syringes,
and test tubes. The
containers, in some cases, are formed from a variety of materials such as
glass or plastic.
[00225] The articles of manufacture provided herein contain packaging
materials. Examples of
pharmaceutical packaging materials include, but are not limited to, blister
packs, bottles, tubes,
inhalers, pumps, bags, vials, containers, syringes, bottles, and any packaging
material suitable for
a selected formulation and intended mode of administration and treatment. A
wide array of
formulations of the compounds and compositions provided herein are
contemplated as are a
variety of treatments for any treatment of fatty acid oxidation disorder
(FAOD) that benefits from
PPAR6 modulation.
[00226] The container(s) optionally have a sterile access port (for example
the container is an
intravenous solution bag or a vial having a stopper pierceable by a hypodermic
injection needle).
- 55 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
Such kits optionally comprise a compound with an identifying description or
label or instructions
relating to its use in the methods described herein.
[00227] A kit will typically include one or more additional containers, each
with one or more of
various materials (such as reagents, optionally in concentrated form, and/or
devices) desirable
from a commercial and user standpoint for use of a compound described herein.
Non-limiting
examples of such materials include, but not limited to, buffers, diluents,
filters, needles, syringes;
carrier, package, container, vial and/or tube labels listing contents and/or
instructions for use, and
package inserts with instructions for use. A set of instructions will also
typically be included.
[00228] In some embodiments, a label is on or associated with the container. A
label, in some
cases, is on a container when letters, numbers or other characters forming the
label are attached,
molded or etched into the container itself; a label, in some cases, is
associated with a container
when it is present within a receptacle or carrier that also holds the
container, e.g., as a package
insert. A label, in some cases, is used to indicate that the contents are to
be used for a specific
therapeutic application. The label, in some cases, indicates directions for
use of the contents, such
as in the methods described herein.
[00229] In certain embodiments, a pharmaceutical composition comprising a
PPAR6 agonist
(e.g. Compound 1, or a pharmaceutically acceptable salt thereof), is presented
in a pack or
dispenser device which, in some cases, contains one or more unit dosage forms.
The pack, in
some cases, for example contains metal or plastic foil, such as a blister
pack. The pack or
dispenser device, in some cases, is accompanied by instructions for
administration. The pack or
dispenser, in some cases, is also accompanied with a notice associated with
the container in form
prescribed by a governmental agency regulating the manufacture, use, or sale
of pharmaceuticals,
which notice is reflective of approval by the agency of the form of the drug
for human or
veterinary administration. Such notice, for example, in some cases, is the
labeling approved by
the U.S. Food and Drug Administration for prescription drugs, or the approved
product insert.
Compositions containing a compound provided herein formulated in a compatible
pharmaceutical carrier, in some cases, is also prepared, placed in an
appropriate container, and
labeled for treatment of an indicated condition.
EXAMPLES
[00230] The following examples are provided for illustrative purposes only and
not to limit the
scope of the claims provided herein.
- 56 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
Example 1: Cell lines and culture
[00231] Subjects. Skin biopsies for fibroblast culture are performed on a
clinical basis with
written informed consent from subjects and/or legal guardians. Fibroblast
cells with mutations in
any one of the genes and/or proteins associated with a fatty acid oxidation
disorder (FAOD) ae
obtained from patients' skin biopsies, while wild type (WT) fibroblast cells
are obtained from
healthy individuals.
[00232] Fibroblast cells, in some cases, are obtained from subjects with a
confirmed diagnosis
of a fatty acid oxidation disorder (FAOD) (e.g. MCAD, VLCAD, CPT1, CACT, CPT2,
LCHAD,
and/or mitochondrial TFP deficiencies or mutations) or they, in some cases,
are purchased is
available from commercial sources, e.g. from the Coriell Institute for Medical
Research (403
Haddon Avenue, Camden, New Jersey 08103).
[00233] Cell culture and treatments. Cells are grown in Dulbecco's Modified
Eagle Medium
(DMEM), Corning Life Sciences, Manassas, VA, containing high glucose levels or
in DMEM
devoid of glucose for 48-72 hr. Both media are supplemented with fetal bovine
serum,
glutamine, penicillin and/or streptomycin. In some experiments, fibroblasts
are incubated with N-
acetylcysteine, resveratrol, mitoQ, Trolox (a hydro-soluble analogue of
vitamin E), or
bezafibrate, prior to the analysis of parameters.
[00234] A PPARo agonist compound is dissolved in phosphate buffer saline, PBS,
as a stock
solution. Amounts are added appropriately directly to cell culture media in
flasks when the
cultures are about 85-90 confluent. The cultures are allowed to grow for 48 h
at 37 C, and then
harvested. Harvested cell pellets are stored at -80 C until immune and
enzymatic assays analyses.
lmL to 1.5 mL media samples are also stored at -80 C for acylcarnitines.
Example 2: Measurement of mitochondrial respiration.
[00235] Oxygen consumption rate (OCR) is measured with a Seahorse XFe96
Extracellular Flux
Analyzer (Sea horse Bioscience, Billerica, MA).
[00236] Briefly, the apparatus contains a fluoro-phore that is sensitive to
changes in oxygen
concentration, which enables it to accurately measure the rate at which
cytochrome c oxidase
(complex IV) reduces one 02 molecule to two H20 molecules during OXPHOS. Cells
are seeded
in 96-well Seahorse tissue culture microplates in growth media at a density of
80,000 cells per
well. To ensure equal cell numbers, cells are seeded in cell culture plates
pre-coated with Cell-
Tak, BD Biosciences, San Jose, CA. All cell lines are measured with four to
eight wells per cell
line. Then, the entire set of experiments is repeated. Before running the
Seahorse assay, cells are
incubated for 1 hour without CO2 in unbuffered DMEM. Initial OCR is measured
to establish a
- 57 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
baseline (basal respiration). Maximal respiration is also determined after the
injection of 300 nM
carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), Seahorse XF Cell
Mito Stress
Test Kit, Santa Clara, CA.
Example 3: ATP production assay
[00237] ATP production is determined by a bioluminescence assay using an ATP
determination
kit (ATPlite kit) from PerkinElmer Inc, Waltham, MA, according to the
manufacturer's
instructions.
Example 4: Western blotting.
[00238] Cells are grown in T175 flasks and, at 90-95% confluence, are
harvested by
trypsinization, pelleted and stored at -80 C for western blot. Protein
content in samples is
quantified for data normalization using DC' Protein Assay kit (Bio-Rad
Laboratories).
[00239] For cell lysates, pellets are re-suspended in 150-250 tL of RIPA
buffer with protease
inhibitor cocktail, Roche Diagnostics, Mannheim, Germany. Homogenates are kept
on ice for 30
min, shaken every 10 min, and centrifuged. Supernatants are used for western
blotting. For
mitochondria, pellets are re-suspended in 150-250 tL of 5 mM Tris buffer, pH
7.4, containing
250 mM sucrose, 2 mM EDTA, protease inhibitor cocktail, Roche Diagnostics,
Mannheim,
Germany, and 0.5 [tM trichostatin A, Sigma-Aldrich Co., St. Louis, MO,
homogenized and
centrifuged. The pellet is discarded and the supernatant centrifuged. The
resulting pellet
containing mitochondria is re-suspended in 50 mM Tris buffer, pH 7.4,
sonicated and centrifuged
again.
[00240] Cell lysates or mitochondria are used for western blotting as
previously described
(Goetzman, E. S. et at. Expression and characterization of mutations in human
very long-chain
acyl-CoA dehydrogenase using a prokaryotic system. Mol. Genet. Metab. 91, 138-
147, (2007)).
Briefly, 10 or 20 [tg of protein are loaded onto the gel. Following
electrophoresis, the gel is
blotted onto a nitrocellulose membrane, which is incubated with rabbit anti-
ND6 polyclonal
antibody (1:100), Santa Cruz Biotechnology, Dallas, TX, rabbit anti-NDUFV1
polyclonal
antibody (1:100), Santa Cruz Biotechnology, Dallas, TX, rabbit anti-ACAD9
antiserum (1:500),
Cocalico Biologicals Inc., PA, rodent anti-total OXPHOS cocktail antibody
(1:250), Abcam,
Cambridge, MA, mouse anti-mitofusin 1 (MFN1) monoclonal antibody (1:100),
Abcam,
Cambridge, MA, mouse anti-dynamin-related protein 1 (DRP1) monoclonal antibody
(1:100),
Abcam, Cambridge, MA, rabbit anti-very long-chain acyl-CoA dehydrogenase
(VLCAD)
antiserum (1:1,000), Cocalico Biologicals Inc., PA, rabbit anti-voltage-
dependent anion channel
- 58 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
1 (VDAC1) monoclonal antibody (1:1,000), Abeam, Cambridge, MA, mouse anti-
glucose-
related protein 75 (Grp75) monoclonal antibody (1:250), Abeam, Cambridge, MA,
rabbit anti-
glucose-related protein 78 (Grp78) polyclonal antibody (1:250), Abeam,
Cambridge, MA, mouse
anti-DNA damage inducible transcript 3 (DDIT3) monoclonal antibody (1:250),
Abeam,
Cambridge, MA, goat anti-inositol 1,4,5-trisphosphate receptor 3 (IP3R)
polyclonal antibody
(1:50), Santa Cruz Biotechnology, Dallas, TX, or IgG-HRP conjugated antibody,
Bio-Rad,
Hercules, CA. Staining of the membranes with Ponceau S, Sigma-Aldrich Co., St.
Louis, MO, or
mouse anti-0-actin monoclonal antibody (1:10,000), Sigma-Aldrich Co., St.
Louis, MO, or
mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) monoclonal
antibody
(1:15,000), Abeam, Cambridge, MA, is used to verify equal loading.
Example 5: Immunofluorescence Microscopy and Mitochondrial Membrane Potential
(AT)
[00241] Cells are incubated with the antibodies anti-VLCAD (1:1000), anti-Nrf2
(1:100) or anti-
NF-kB (1:1000) at 4 C overnight. After brief washing with TBST, cells are
incubated with
donkey anti-rabbit secondary antibody Alexa Fluor 488, from Invitrogen. Nuclei
are
immunostained with DAPI. The coverslips are then mounted using mounting media
before taking
images with an Olympus Confocal FluoroView1000 microscope at a magnification
of 60x.
Example 6: Fatty Acid Oxidation (FAO) Flux Analysis
[00242] Fatty acid oxidation (FAO) flux analysis is performed by quantifying
the production of
3H20 from 9,10-[3H]palmitate, PerkinElmer, Waltham, MA, conjugated to fatty
acid-free albumin
in fibroblasts cultured in a 24-well plate.
[00243] A representative non-limiting example of a FAO flux analysis is
described in Bennett,
M. J. Assays of fatty acid beta-oxidation activity. Methods Cell Biol 80, 179-
197, (2007)). In
some embodiments, 300,000 fibroblasts are plated per well in 6-well plates and
grown for 24
hours in DMEM with 10% fetal bovine serum. The growth media is then changed to
either the
same media or devoid of glucose and fibroblasts are grown as described for 48
hr. Subsequently,
cells are washed once with PBS and then incubated with 0.34 [tCi [9,10-
3H]oleate (45. 5
Ci/mmol; Perkin Elmer, Waltham, MA) in 50 nmol of oleate prepared in 0.5 mL
glucose-free
DMEM with 1 [t/m1 carnitine and 2 mg/ml a-cyclodextrin for 2 hours at 37 C.
Fatty acids are
solubilized with a-cyclodextrin as described (Watkins, P. A., Ferrell, E. V.
Jr., Pedersen, J. I. &
Hoefler, G. Peroxisomal fatty acid beta-oxidation in HepG2 cells. Arch Biochem
Biophys 289,
329-336 (1991)). After incubation, 3H20 released is separated from the oleate
on a column
containing 750 tL of anion exchange resin (AG 1 X 8, acetate, 100-200 Mesh,
BioRad,
- 59 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
Richmond, CA) prepared in water. After the incubation medium passes through
the column, the
plate is washed with 750 !IL of water which is also transferred to the column.
The resin is then
washed twice with 750 !IL of water. All eluates are collected in a
scintillation vial and mixed
with 5 mL of scintillation fluid (Eco-lite, MP), followed by counting in a
Beckman scintillation
counter in the tritium window. Assays are performed in quadruplicate with
triplicate blanks (cell
free wells). Standards contain a 50 !IL aliquot of the incubation mix with
2.75 mL of deionized
water and 5 mL of scintillation fluid.
Example 7: Cell Viability Assay
[00244] Cell viability is evaluated with a 3-(4,5-dimethylthiazol-2-y1)-5-(3-
carboxymethoxyp
heny1)-2-(4-sulfopheny1)-2H-tetrazolium (MTS) assay kit according to the
manufacturer's
instructions, Abcam, Cambridge, MA. The absorbance is read in the FLUOstar
Omega plate
reader at 490 nm.
Example 8: Apoptosis Assay
[00245] Apoptosis is evaluated with an Alexa Fluor 488 annexin V/Dead Cell
Apoptosis kit
according to manufacturer's instructions, Invitrogen, Grand Island, NY. The
kit contains annexin
V labeled with a fluorophore and propidium iodide (PI). Annexin V can identify
apoptotic cells
by binding to phosphatidylserine exposed on the outer leaflet of cell plasma
membrane while PI
stains dead cells by binding to nucleic acids. Fluorescence is determined in a
Becton Dickinson
FACSAria II flow cytometer, BD Biosciences, San Jose, CA.
Example 9: Determination of Acylcarnitine Levels
[00246] Acylcarnitine analysis is performed utilizing the appropriate tandem
mass spectrometry
(MS/MS) protocols.
Example 10: ETF Fluorescence Reduction ACAD Activity Assay
[00247] Enzyme assays used to measure ACAD enzyme activity at the picomoles
level in
tissues and in cell culture have been described. An assay protocol with the
key ingredient being
ETF (electron transfer flavoprotein) that is isolated from pig liver has been
published (Vockley et
at., Mammalian branched-chain acyl-CoA dehydrogenases: molecular cloning and
characterization of recombinant enzymes, Methods Enzymol. 2000; 324:241-58;
which is
incorporated by reference for such assay).
- 60 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
Example 11: Measurement of the Level of Expression of VLCAD
[00248] The effect of increasing amounts of PPARo agonist compound on ACADVL
gene
expression in VLCAD deficient or mutated cells is monitored using standard qRT-
PCR protocol.
Messenger RNA transcription levels of ACADVL (MIM: 609575) for the patient's
fibroblasts
cell lines with VLCAD deficiency untreated and treated with PPARo agonist
compound are
quantified via qRT-PCR with an Applied Biosystems StepOnePlus instrument using
TaqManTm
Gene Expression Master Mix (from ThermoFisher Scientific). The reference
sample is fibroblasts
with no VLCAD deficiency. Human GAPDH is used as an endogenous control.
Commercial
primers for ACADVL and GAPDH are used using and TaqManTm Gene Expression Assay
(ThermoFisher Scientific), which consists of a pair of unlabeled PCR primers
and a TaqMan
probe with a FAMTm or VIC(R) dye label on the 5'-end and minor groove binder
(MGB) and
non-fluorescent quencher (NFQ) on the 3'-end. The relative quantity RQ of the
samples is
compared between the reference sample, treated VLCAD deficiency cell lines
untreated and
treated with PPARo agonist compound.
Example 12: Combination Therapy
[00249] PPARo agonists can be used in combination with other therapies for
fatty acid oxidation
disorders (FAOD). In some embodiments, a PPARo agonist compound is
administered to an
individual with a FAOD in combination with one or more of the following:
ubiquinol,
ubiquinone, niacin, riboflavin, creatine , L-carnitine, acetyl-L-carnitine,
biotin, thiamine,
pantothenic acid, pyridoxine, alpha-lipoic acid, n-heptanoic acid,
triheptanoin, a triglyceride, or a
salt or thereof, CoQ10, vitamin E, vitamin C, methylcobalamin, folinic acid, N-
acetyl-L-cysteine
(NAC), zinc, folinic acid/leucovorin calcium.
[00250] Combination therapy is advantageous when efficacy is greater than
either agent alone or
when the dose required for either drug is reduced thereby improving the side
effect profile.
Example 13: Clinical Trial for Fatty Acid Oxidation Disorder
[00251] A non-limiting example of a fatty acid oxidation disorder (FAOD)
clinical trial in
humans is described below.
[00252] Purpose: The purposes of this study are: to assess the safety and
tolerability of 12
weeks treatment with Compound 1, or a pharmaceutically acceptable salt or
solvate thereof, in
subjects with FAOD; to investigate pharmacokinetics of Compound 1, or a
pharmaceutically
acceptable salt or solvate thereof, in subjects with FAOD treated with
Compound 1, or a
pharmaceutically acceptable salt or solvate thereof; to investigate the
pharmacodynamics effects
- 61 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
of Compound 1, or a pharmaceutically acceptable salt or solvate thereof, in
subjects with FAOD
treated with Compound 1, or a pharmaceutically acceptable salt or solvate
thereof
[00253] Intervention: Patients are administered 10-2000 mg of Compound 1, or a
pharmaceutically acceptable salt or solvate thereof, per day as single agent
or in combination. In
one cohort, subjects will receive 50 mg of Compound 1, or a pharmaceutically
acceptable salt or
solvate thereof, once daily for a total of 12 weeks. In another cohort,
subjects will receive 100
mg of Compound 1, or a pharmaceutically acceptable salt or solvate thereof,
once daily for a total
of 12 weeks. Other cohorts are contemplated.
[00254] Compound 1, or a pharmaceutically acceptable salt or solvate thereof,
will be packed in
bottles as capsules.
[00255] Detailed Description: Patients will be given Compound 1, or a
pharmaceutically
acceptable salt or solvate thereof, orally once a day.
[00256] Eligibility: 18 years and older with FAOD.
[00257] Inclusion Criteria: Confirmed diagnosis of one of the following:
carnitine
palmitoyltransferase II deficiency (CPT2), very long-chain Acyl-CoA
dehydrogenase deficiency
(VLCAD), long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency (LCHAD), or
trifunctional
protein deficiency (TFP).
[00258] A diagnostic acylcarnitine profile, in blood or cultured fibroblasts.
[00259] Genotyping with at least 1 allele that is not a stop codon or a frame
shift.
[00260] Have evidence of any one of the following clinical manifestations
despite therapy:
Chronic elevated Creatine Kinase (CPK) as evidenced by at least 2 blood CPK
levels above the
ULN obtained at least 3 months apart, history of cardiomyopathy, a clinical
event of
hypoglycemia, rhabdomyolysis, or exacerbation of cardiomyopathy within the 12
months
preceding enrollment.
[00261] Currently following a stable dietary regimen with avoidance of fasting
as documented
by a 3-day dietary record obtained during the screening period.
[00262] A stable treatment regimen for at least 30 days prior to enrollment.
[00263] Expected and willing to remain on stable diet and medication through
the study.
[00264] Ambulatory and able to perform the study exercise tests.
[00265] Adequate kidney function defined as an estimated glomerular filtration
rate (eGFR) >
60 mL/min/1.73 m2 using the Cockcroft-Gault formula.
[00266] Able to swallow capsules.
[00267] Exclusion Criteria: Subjects presenting with any of the following will
not be included
in the study:
- 62 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
[00268] - unstable or poorly controlled disease as determined by one or more
of the following:
echocardiogram with evidence of active or worsening cardiomyopathy at
screening; presence of
symptoms of acute rhabdomyolysis with elevations in serum CPK consistent with
acute
exacerbation of myopathy; evidence of acute crisis from their underlying
disease.
[00269] - currently taking anticoagulants.
[00270] - have motor abnormalities other than those related to the fatty acid
oxidation disorder
that could interfere with the outcome measures.
[00271] - treatment with an investigational drug within 1 month or within 5
half-lives,
whichever is longer.
[00272] - evidence of significant concomitant clinical disease that in the
opinion of the
Investigator may need a change in management during the study or could
interfere with the
conduct or safety of this study. (Stable well-controlled chronic conditions
such as controlled
hypertension (BP<140/90 mmHg) thyroid disease, well-controlled Type 1 or Type
2 diabetes
(HbAlc< 8%), hypercholesterolemia, gastroesophageal reflux, or depression
under control with
medication (other than tricyclic antidepressants), are acceptable provided the
symptoms and
medications would not be predicted to compromise safety or interfere with the
tests and
interpretations of this study).
[00273] - history of cancer with the exception of in situ skin cancer.
[00274] - have been hospitalized within the 3 months prior to screening for
any major medical
condition (as deemed by the primary investigator).
[00275] - any condition possibly reducing drug absorption (e.g., gastrectomy).
[00276] - history of clinically significant liver disease as evidenced by
elevations in ALT, GGT
or TB.
[00277] - positive hepatitis B surface antigen (HBsAg) or hepatitis C, or HIV
at screening.
[00278] - history of regular alcohol consumption exceeding 14 drinks/week (1
drink = 150 mL
of wine or 360 mL of beer or 45 mL of spirits) within 6 months of screening.
[00279] - any other severe acute or chronic medical or psychiatric condition
or laboratory
abnormality that in the opinion of the Investigator may increase the risk
associated with study
participation or investigational product administration or may interfere with
the interpretation of
study results.
[00280] Primary Outcome Measures: Safety Endpoints include: number and
severity of
adverse events. Absolute values, changes from baseline at Week 12 and
incidence of clinically
significant changes in: laboratory safety tests; electrocardiograms; supine
vital signs; evaluation
- 63 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
of events of special interest (rhabdomyolysis) and clinically significant
changes in laboratory
parameters of muscle injury including total CPK, adolase, and cardiac specific
troponin (cTn).
[00281] Pharmacokinetic Endpoints include: Compound 1 plasma concentrations
and
identification of metabolites using pooled plasma.
[00282] Pharmacodynamic Endpoints include: Absolute values and changes from
baseline at
Week 12 in: whole body fatty acid oxidation (13CO2 production) and blood
acylcarnitines
(UHPLC¨MS/MS method).
[00283] Secondary Outcome Measures: To assess the change from baseline
following 12 weeks
of treatment with Compound 1, or a pharmaceutically acceptable salt or solvate
thereof, in:
submaximal treadmill exercise tolerance; distance walked during a 12 minute
walk test; 36-Item
Short Form Survey (SF-36) total score and subscales (questions 3-12). Change
from baseline in
Fatigue Impact Scale score (every visit). Change from baseline in Brief Pain
Inventory (short
form) (every visit). Blood inflammatory cytokines (Multiplex Immunoassay for
sE-Selectin; GM-
CSF; ICAM-1/CD54; IFN alpha; IFN gamma; IL-1 alpha; IL-1 beta; IL-4; IL-6; IL-
8; IL-10; IL-
12p'70; IL-13; IL-17A/CTLA-8; IP-10/CXCL10; MCP-1/CCL2; MIP-1alpha/CCL3; MW-1
beta/CCL4; sP-Selectin; TNF alpha).
FAOD Clinical Trial Results with Compound 1
[00284] In general, Compound 1 was well tolerated among subjects that
participated in the
study.
[00285] Improvements in exercise capacity was observed in subjects that
received 50 mg of
Compound 1, or a pharmaceutically acceptable salt or solvate thereof, once
daily for a total of 12
weeks. Subjects were able to increase the distance walked during a 12-minute
walk test. Figure
1 shows the results of the impact of Compound 1 on the 12-minute walk test in
this group of
subjects. In this same group of subjects, decreases in heart rate were
observed during the last ten
minutes of exercise.
[00286] A trend towards increases in exhaled 13CO2 was observed in subjects
that received 50
mg of Compound 1, or a pharmaceutically acceptable salt or solvate thereof,
once daily for a total
of 12 weeks.
[00287] The examples and embodiments described herein are for illustrative
purposes only and
various modifications or changes suggested to persons skilled in the art are
to be included within
the spirit and purview of this application and scope of the appended claims.
- 64 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
Example 14: Sequences
Table 1. Carnitine Shuttle Genes
SEQ Gene NCBI Nucleotide Sequence
ID Reference
NO Number
1 CPT1A NM_001031 GGACCCGCCTCAGCCAATCCGCTGCTGCCGGCGTCGGGTGC
847.2 GCTCGGCCTCGCCCGCGGCCCTCCTTCCCCGGCTCCCGCTCG
CCGCTCGTTCACTCCACCGCCGCCGCCGCCGCCGCCGCTGC
CGCTGCCGCTGCCGCACCTCCGTAGCTGACTCGGTACTCTCT
GAAGATGGCAGAAGCTCACCAAGCTGTGGCCTTTCAGTTCA
CGGTCACTCCGGACGGGATTGACCTGCGGCTGAGCCATGAA
GCTCTTAGACAAATCTATCTCTCTGGACTTCATTCCTGGAAA
AAGAAGTTCATCAGATTCAAGAACGGCATCATCACTGGCGT
GTACCCGGCAAGCCCCTCCAGTTGGCTTATCGTGGTGGTGG
GCGTGATGACAACGATGTACGCCAAGATCGACCCCTCGTTA
GGAATAATTGCAAAAATCAATCGGACTCTGGAAACGGCCA
ACTGCATGTCCAGCCAGACGAAGAACGTGGTCAGCGGCGT
GCTGTTTGGCACCGGCCTGTGGGTGGCCCTCATCGTCACCA
TGCGCTACTCCCTGAAAGTGCTGCTCTCCTACCACGGGTGG
ATGTTCACTGAGCACGGCAAGATGAGTCGTGCCACCAAGAT
CTGGATGGGTATGGTCAAGATCTTTTCAGGCCGAAAACCCA
TGTTGTACAGCTTCCAGACATCGCTGCCTCGCCTGCCGGTCC
CGGCTGTCAAAGACACTGTGAACAGGTATCTACAGTCGGTG
AGGCCTCTTATGAAGGAAGAAGACTTCAAACGGATGACAG
CACTTGCTCAAGATTTTGCTGTCGGTCTTGGACCAAGATTAC
AGTGGTATTTGAAGTTAAAATCCTGGTGGGCTACAAATTAC
GTGAGCGACTGGTGGGAGGAGTACATCTACCTCCGAGGAC
GAGGGCCGCTCATGGTGAACAGCAACTATTATGCCATGGAT
CTGCTGTATATCCTTCCAACTCACATTCAGGCAGCAAGAGC
CGGCAACGCCATCCATGCCATCCTGCTTTACAGGCGCAAAC
TGGACCGGGAGGAAATCAAACCAATTCGTCTTTTGGGATCC
ACGATTCCACTCTGCTCCGCTCAGTGGGAGCGGATGTTTAA
TACTTCCCGGATCCCAGGAGAGGAGACAGACACCATCCAGC
ACATGAGAGACAGCAAGCACATCGTCGTGTACCATCGAGG
ACGCTACTTCAAGGTCTGGCTCTACCATGATGGGCGGCTGC
TGAAGCCCCGGGAGATGGAGCAGCAGATGCAGAGGATCCT
GGACAATACCTCGGAGCCTCAGCCCGGGGAGGCCAGGCTG
GCAGCCCTCACCGCAGGAGACAGAGTTCCCTGGGCCAGGTG
TCGTCAGGCCTATTTTGGACGTGGGAAAAATAAGCAGTCTC
TTGATGCTGTGGAGAAAGCAGCGTTCTTCGTGACGTTAGAT
GAAACTGAAGAAGGATACAGAAGTGAAGACCCGGATACGT
CAATGGACAGCTACGCCAAATCTCTACTACACGGCCGATGT
TACGACAGGTGGTTTGACAAGTCGTTCACGTTTGTTGTCTTC
AAAAACGGGAAGATGGGCCTCAACGCTGAACACTCCTGGG
CAGATGCGCCGATCGTGGCCCACCTTTGGGAGTACGTCATG
TCCATTGACAGCCTCCAGCTGGGCTATGCGGAGGATGGGCA
CTGCAAAGGCGACATCAATCCGAACATTCCGTACCCCACCA
GGCTGCAGTGGGACATCCCGGGGGAATGTCAAGAGGTTAT
AGAGACCTCCCTGAACACCGCAAATCTTCTGGCAAACGACG
TGGATTTCCATTCCTTCCCATTCGTAGCCTTTGGTAAAGGAA
TCATCAAGAAATGTCGCACGAGCCCAGACGCCTTTGTGCAG
CTGGCCCTCCAGCTGGCGCACTACAAGGACATGGGCAAGTT
TTGCCTCACATACGAGGCCTCCATGACCCGGCTCTTCCGAG
AGGGGAGGACGGAGACCGTGCGCTCCTGCACCACTGAGTC
- 65 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
ATGCGACTTCGTGCGGGCCATGGTGGACCCGGCCCAGACGG
TGGAACAGAGGCTGAAGTTGTTCAAGTTGGCGTCTGAGAAG
CATCAGCATATGTATCGCCTCGCCATGACCGGCTCTGGGAT
CGATCGTCACCTCTTCTGCCTTTACGTGGTGTCTAAATATCT
CGCTGTGGAGTCCCCTTTCCTTAAGGAAGTTTTATCTGAGCC
TTGGAGATTATCAACAAGCCAGACCCCTCAGCAGCAAGTGG
AGCTGTTTGACTTGGAGAATAACCCAGAGTACGTGTCCAGC
GGAGGGGGCTTTGGACCGGTTGCTGATGACGGCTATGGTGT
GTCGTACATCCTTGTGGGAGAGAACCTCATCAATTTCCACA
TTTCTTCCAAGTTCTCTTGCCCTGAGACGGGGATTATAAGTC
AAGGACCAAGTTCAGATACTTGAGACAAAGTGGAAAGTCT
CAGCATATGGAAACAAGGCCTTGGAGGAGACCATGGACAT
CACCAAGTTCATGTGCTGGGCTGGAAAGAAAAGCCTGTTGA
TTTTCACTTGCTGTGCATTTATTCATCCATTCCATTGCCTCAA
TGCTGAGAACAGTGCCTGACACATAAAAGATGCTCAATAAA
TATGTTAAAAGTAAAAAAAAAAAAAAAAAAAAAAAAAAA
AAAAAAAAA
2
CPT1B NM 004377. GGGGGTGGCTAGGCCTGAAGGACGTGGGGACACGGGCCAG
3
AGTGGCTGGCCCCACGCACGGACAGGAGTGAACCCGAGCT
GTGCCGACCAACCCCCAGGATGGCGGAAGCTCACCAGGCC
GTGGCCTTCCAGTTCACGGTGACCCCAGACGGGGTCGACTT
CCGGCTCAGTCGGGAGGCCCTGAAACACGTCTACCTGTCTG
GGATCAACTCCTGGAAGAAACGCCTGATCCGCATCAAGAAT
GGCATCCTCAGGGGCGTGTACCCTGGCAGCCCCACCAGCTG
GCTGGTCGTCATCATGGCAACAGTGGGTTCCTCCTTCTGCA
ACGTGGACATCTCCTTGGGGCTGGTCAGTTGCATCCAGAGA
TGCCTCCCTCAGGGGTGTGGCCCCTACCAGACCCCGCAGAC
CCGGGCACTTCTCAGCATGGCCATCTTCTCCACGGGCGTCT
GGGTGACGGGCATCTTCTTCTTCCGCCAAACCCTGAAGCTG
CTTCTCTGCTACCATGGGTGGATGTTTGAGATGCATGGCAA
GACCAGCAACTTGACCAGGATCTGGGCTATGTGTATCCGCC
TTCTATCCAGCCGGCACCCTATGCTCTACAGCTTCCAGACAT
CTCTGCCCAAGCTTCCTGTGCCCAGGGTGTCAGCCACAATT
CAGCGGTACCTAGAGTCTGTGCGCCCCTTGTTGGATGATGA
GGAATATTACCGCATGGAGTTGCTGGCCAAAGAATTCCAGG
ACAAGACTGCCCCCAGGCTGCAGAAATACCTGGTGCTCAAG
TCATGGTGGGCAAGTAACTATGTGAGTGACTGGTGGGAAGA
GTACATCTACCTTCGAGGCAGGAGCCCTCTCATGGTGAACA
GCAACTATTATGTCATGGACCTTGTGCTCATCAAGAATACA
GACGTGCAGGCAGCCCGCCTGGGAAACATCATCCACGCCAT
GATCATGTATCGCCGTAAACTGGACCGTGAAGAAATCAAGC
CTGTGATGGCACTGGGCATAGTGCCTATGTGCTCCTACCAG
ATGGAGAGGATGTTCAACACCACTCGGATCCCGGGCAAGG
ACACAGATGTGCTACAGCACCTCTCAGACAGCCGGCACGTG
GCTGTCTACCACAAGGGACGCTTCTTCAAGCTGTGGCTCTA
TGAGGGCGCCCGTCTGCTCAAGCCTCAGGATCTGGAGATGC
AGTTCCAGAGGATCCTGGACGACCCCTCCCCACCTCAGCCT
GGGGAGGAGAAGCTGGCAGCCCTCACTGCAGGAGGAAGGG
TGGAGTGGGCGCAGGCACGCCAGGCCTTCTTTAGCTCTGGA
AAGAATAAGGCTGCCTTGGAGGCCATCGAGCGTGCCGCTTT
CTTCGTGGCCCTGGATGAGGAATCCTACTCCTATGACCCCG
AAGATGAGGCCAGCCTCAGCCTCTATGGCAAGGCCCTGCTA
CATGGCAACTGCTACAACAGGTGGTTTGACAAATCCTTCAC
TCTCATTTCCTTCAAGAATGGCCAGTTGGGTCTCAATGCAG
AGCATGCGTGGGCAGATGCTCCCATCATTGGGCACCTCTGG
GAGTTTGTCCTGGGCACAGACAGCTTCCACCTGGGCTACAC
- 66 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
GGAGACCGGGCACTGCCTGGGCAAACCGAACCCTGCGCTC
GCACCTCCTACACGGCTGCAGTGGGACATTCCAAAACAGTG
CCAGGCGGTCATCGAGAGTTCCTACCAGGTGGCCAAGGCGT
TGGCAGACGACGTGGAGTTGTACTGCTTCCAGTTCCTGCCC
TTTGGCAAAGGCCTCATCAAGAAGTGCCGGACCAGCCCTGA
TGCCTTTGTGCAGATCGCGCTGCAGCTGGCTCACTTCCGGG
ACAGGGGTAAGTTCTGCCTGACCTATGAGGCCTCAATGACC
AGAATGTTCCGGGAGGGACGGACTGAGACTGTGCGTTCCTG
TACCAGCGAGTCCACAGCCTTTGTGCAGGCCATGATGGAGG
GGTCCCACACAAAAGCAGACCTGCGAGATCTCTTCCAGAAG
GCTGCTAAGAAGCACCAGAATATGTACCGCCTGGCCATGAC
CGGGGCAGGGATCGACAGGCACCTCTTCTGCCTTTACTTGG
TCTCCAAGTACCTAGGAGTCAGCTCTCCTTTCCTTGCTGAGG
TGCTCTCGGAACCCTGGCGTCTCTCCACCAGCCAGATCCCC
CAATCCCAGATCCGCATGTTCGACCCAGAGCAGCACCCCAA
TCACCTGGGCGCTGGAGGTGGCTTTGGCCCTGTAGCAGATG
ATGGCTATGGAGTTTCCTACATGATTGCAGGCGAGAACACG
ATCTTCTTCCACATCTCCAGCAAGTTCTCAAGCTCAGAGAC
GAACGCCCAGCGCTTTGGAAACCACATCCGCAAAGCCCTGC
TGGACATTGCTGATCTTTTCCAAGTTCCCAAGGCCTACAGCT
GAAGGTTGGAGAAATGCCAGCTGCCCTTTCGTCCCCACACT
GTGGAGGAAGGGACCTGTGGCAGCTCACAGGCATGAGGGG
TGGCCGTGCACAGGTGCCCAGGCTCCAAGGACAGCTCCGGC
AGCAGGTCCTCGCTGGGCAGATGCTGCTCCCTGAGGGCCCA
GGTGGTGGAGGTGGGGTTGGAGCAGGAAGGGAATTTTGAT
TTTTTTTTTTCTTGATAGATACTAATAAAAATAAGGCTGTGT
AATTTTCTCTCAGCCCTTAGGTACCTGTGTTTTGTTTGGGAA
CTCGGAGGCCCTCCCCCTCCCCCAGCTCAGACCACAGAGGT
GGCAAGAGAAGGGCTGAAGCTGGAAGACTGTTCATGAGGG
ACTTGTGTGACCTGCTTTGAAATGTGTGACTCTGCTGAGTGA
CGTAGGCTCTGAGATAGCTGTCCACGCCCACGTGTTTGCTT
GGAATAAATACTTGCCTCAGAACCTTCAAAAAAAAAAAAA
AAAAA
3 SLC25 NM 000387. GAAAGGTCGGCGGCGCCGGCACTGCAGCTGGGGCTGAGAA
A20 6 GCCAGGACGGCCCGAGAACTGACAGACGGAGTGACAGACG
GACTGACCATGGCCGACCAGCCAAAACCCATCAGCCCGCTC
AAGAACCTGCTGGCCGGCGGCTTTGGCGGCGTGTGCCTGGT
GTTCGTCGGTCACCCTCTGGACACGGTCAAGGTCCGACTGC
AGACACAGCCACCGAGTTTGCCTGGACAACCTCCCATGTAC
TCTGGGACCTTTGACTGTTTCCGGAAGACTCTTTTTAGAGAG
GGCATCACGGGGCTATATCGGGGAATGGCTGCCCCTATCAT
CGGGGTCACTCCCATGTTTGCCGTGTGCTTCTTTGGGTTTGG
TTTGGGGAAGAAACTACAACAGAAACACCCAGAAGATGTG
CTCAGCTATCCCCAGCTTTTTGCAGCTGGGATGTTATCTGGC
GTATTCACCACAGGAATCATGACTCCTGGAGAACGGATCAA
GTGCTTATTACAGATTCAGGCTTCTTCAGGAGAAAGCAAGT
ACACTGGTACCTTGGACTGTGCAAAGAAGCTGTACCAGGAG
TTTGGGATCCGAGGCATCTACAAAGGGACTGTGCTTACCCT
TATGCGAGATGTCCCAGCTAGTGGAATGTATTTCATGACAT
ATGAATGGCTGAAAAATATCTTCACTCCGGAGGGAAAGAG
GGTCAGTGAGCTCAGTGCCCCTCGGATCTTGGTGGCTGGGG
GCATTGCAGGGATCTTCAACTGGGCTGTGGCAATCCCCCCA
GATGTGCTCAAGTCTCGATTCCAGACTGCACCTCCTGGGAA
ATATCCTAATGGTTTCAGAGATGTGCTGAGGGAGCTGATCC
GGGATGAAGGAGTCACATCCTTGTACAAAGGGTTCAATGCA
GTGATGATCCGAGCCTTCCCAGCCAATGCGGCCTGTTTCCTT
- 67 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
GGCTTTGAAGTTGCCATGAAGTTCCTTAATTGGGCCACCCC
CAACTTGTGAGGCTGAAGGCTGCTCAAGTTCACTTCTGGAT
GCTGGAAGCTGTCGTTGAGGAGAAGGAGTAGTAAGCAGAA
CTAAGCAGTCTTGGAGGGCAAGGGGAGGGGAATGGTGAGA
TCCGAGCCCTGTGCATGGACTTGGTGAGACTGTTGCCTTAA
TGACATCCTGCACCGTGTATAACTTAGTGTGTCATTTTGAAA
CTTGAATTCATTCTTATCAATTTAAGGGATCTTAAAAGGATT
TGGAAATGGAACAAGTAGCTTCCAGACCAGATACTACCTGT
GGCAAGAATGCTGCCTACCAGTTAACTGCTGGTCCTACCAC
AGTCAAAGTATTCCTCATTAAAGAGAGAATCTCAGGTTCTC
ACTGGAGGCACTGTGCATATTTTCAACCAGATCACCAGGAG
CTGAGATCTTCTTCAGTCCCTAGCCAGGAATACCCATTTGAT
TTCCAGGGTGCCATCTAATCCTGGGCTGTACATGTGGATAT
GGACTTGAGGCCCACCTCTGTGTCCAAGTGGATTGAGCATA
TATGCCTAGGAGGAGATAGACTGTTAATCGTTGGATTTTGA
TTTTTTTTTTTTATGCCTGCAAATAATCAAAAGTAAAACTGG
AGTAGCCTAATTTTCTGGGAGCAGGTGGAGAACTTTCCCTC
CTACACAGTGAGGACAGTCCCAGTCTGCTGGGATAAGTGAG
AAAGCCCAGGGTGTAGGAAGGCCCTTTTTACATACTCTTTT
CTCATGAGAGCTCACTATTTTAACAATAAACAATAAACGTT
GTTTCTAATTTTT
4
CPT2 NM 000098. GGAGAAGTGCCTCAGGAGTCCTGACGCAGTGTCTTGGGCGC
3
TAACGGCGGCGGCGGCCTTGTGTTTAGACTCCAGAACTCCC
CACTTGCCGCGTTCTCGCCGCCGCAGGCTCCCGGGACGATG
GTGCCCCGCCTGCTGCTGCGCGCCTGGCCCCGGGGCCCCGC
GGTTGGTCCGGGAGCCCCCAGTCGGCCCCTCAGCGCCGGCT
CCGGGCCCGGCCAGTACCTGCAGCGCAGCATCGTGCCCACC
ATGCACTACCAGGACAGCCTGCCCAGGCTGCCTATTCCCAA
ACTTGAAGACACCATTAGGAGATACCTCAGTGCACAGAAGC
CTCTCTTGAATGATGGCCAGTTCAGGAAAACAGAACAATTT
TGCAAGAGTTTTGAAAATGGGATTGGAAAAGAACTGCATG
AGCAGCTGGTTGCTCTGGACAAACAGAATAAACATACAAG
CTACATTTCGGGACCCTGGTTTGATATGTACCTATCTGCTCG
AGACTCCGTTGTTCTGAACTTTAATCCATTTATGGCTTTCAA
TCCTGACCCAAAATCTGAGTATAATGACCAGCTCACCCGGG
CAACCAACATGACTGTTTCTGCCATCCGGTTTCTGAAGACA
CTCCGGGCTGGCCTTCTGGAGCCAGAAGTGTTCCACTTGAA
CCCTGCAAAAAGTGACACTATCACCTTCAAGAGACTCATAC
GCTTTGTGCCTTCCTCTCTGTCCTGGTATGGGGCCTACCTGG
TCAATGCGTATCCCCTGGATATGTCCCAGTATTTTCGGCTTT
TCAACTCAACTCGTTTACCCAAACCCAGTCGGGATGAACTC
TTCACTGATGACAAGGCCAGACACCTCCTGGTCCTAAGGAA
AGGAAATTTTTATATCTTTGATGTCCTGGATCAAGATGGGA
ACATTGTGAGCCCCTCGGAAATCCAGGCACATCTGAAGTAC
ATTCTCTCAGACAGCAGCCCCGCCCCCGAGTTTCCCCTGGC
ATACCTGACCAGTGAGAACCGAGACATCTGGGCAGAGCTC
AGGCAGAAGCTGATGAGTAGTGGCAATGAGGAGAGCCTGA
GGAAAGTGGACTCGGCAGTGTTCTGTCTCTGCCTAGATGAC
TTCCCCATTAAGGACCTTGTCCACTTGTCCCACAATATGCTG
CATGGGGATGGCACAAACCGCTGGTTTGATAAATCCTTTAA
CCTCATTATCGCCAAGGATGGCTCTACTGCCGTCCACTTTGA
GCACTCTTGGGGTGATGGTGTGGCAGTGCTCAGATTTTTTA
ATGAAGTATTTAAAGACAGCACTCAGACCCCTGCCGTCACT
CCACAGAGCCAGCCAGCTACCACTGACTCTACTGTCACGGT
GCAGAAACTCAACTTCGAGCTGACTGATGCCTTAAAGACTG
GCATCACAGCTGCTAAGGAAAAGTTTGATGCCACCATGAAA
- 68 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
ACCCTCACTATTGACTGCGTCCAGTTTCAGAGAGGAGGCAA
AGAATTCCTGAAGAAGCAAAAGCTGAGCCCTGACGCAGTT
GCCCAGCTGGCATTCCAGATGGCCTTCCTGCGGCAGTACGG
GCAGACAGTGGCCACCTACGAGTCCTGTAGCACTGCCGCAT
TCAAGCACGGCCGCACTGAGACCATCCGCCCGGCCTCCGTC
TATACAAAGAGGTGCTCTGAGGCCTTTGTCAGGGAGCCCTC
CAGGCACAGTGCTGGTGAGCTTCAGCAGATGATGGTTGAGT
GCTCCAAGTACCATGGCCAGCTGACCAAAGAAGCAGCAAT
GGGCCAGGGCTTTGACCGACACTTGTTTGCTCTGCGGCATC
TGGCAGCAGCCAAAGGGATCATCTTGCCTGAGCTCTACCTG
GACCCTGCATACGGGCAGATAAACCACAATGTCCTGTCCAC
GAGCACACTGAGCAGCCCAGCAGTGAACCTTGGGGGCTTTG
CCCCTGTGGTCTCTGATGGCTTTGGTGTTGGGTATGCTGTTC
ATGACAACTGGATAGGCTGCAATGTCTCTTCCTACCCAGGC
CGCAATGCCCGGGAGTTTCTCCAATGTGTGGAGAAGGCCTT
AGAAGACATGTTTGATGCCTTAGAAGGCAAATCCATCAAAA
GTTAACTTCTGGGCAGATGAAAAGCTACCATCACTTCCTCA
TCATGAAAACTGGGAGGCCGGGCATGGTGGCTCATGCCTGT
AATCCCAGCATTTTGAGAGGCTGAGGCGGGTGGATCACTTG
AGGTCAGGAGTTTGAGACCAACCTGGCCAACATGGTGAAA
CCTTGTCTCTACTAAAAATACAAAAATTAGCTGGGTGTGGT
GGCATGTGCCTATAATCCCAGCTACTTGGGAGGTTGAAGCA
GAATTGCTTGAACCCAGGAGGTGGAGGTTGCAGTGAGCTGA
GATCACACCACTGCACTCCGGCCTGGGCGACAGAGCGAGA
CTGTCTCAAAAAAACAAAAAAGAAAAAAAAACTGGGGCCT
GTGTAGCCAGTGGGTGCTATTCTGTGAAACTAATCATAAGC
TGCCTAGGCAGCCAGCTACAGGCTTGAGCTTTAAATTCATG
GTTTTAAAGCTAAACGTAATTTCCACTTGGGACTAGATCAC
AACTGAAGATAACAAGAGATTTAAGTTTTAAGGGCATTTAA
TCAGGAGGAAAGGTTTGGAAAACTAACTCAGGTGTATTTAT
TGTTTAAGCAGAAATAAAGTTTAATTTTTGCTTGAA
SLC22 NM_001308 CGCCTTCGCCGGCGCCGCTCTGCCTGCCAGCGGGGCGCGCC
A5 122.1 TTGCGGCCCAGGCCCGCAACCTTCCCTGGTCGTGCGCCCTA
TGTAAGGCCAGCCGCGGCAGGACCAAGGCGGCGGTGTCAG
CTCGCGAGCCTACCCTCCGCGGACGGTCTTGGGTCGCCTGC
TGCCTGGCTTGCCTGGTCGGCGGCGGGTGCCCCGCGCGCAC
GCGCAAAGCCCGCCGCGTTCCCCGACCCCAGGCCGCGCTCT
GTGGGCCTCTGAGGGCGGCATGCGGGACTACGACGAGGTG
ACCGCCTTCCTGGGCGAGTGGGGGCCCTTCCAGCGCCTCAT
CTTCTTCCTGCTCAGCGCCAGCATCATCCCCAATGGCTTCAC
CGGCCTGTCCTCCGTGTTCCTGATAGCGACCCCGGAGCACC
GCTGCCGGGTGCCGGACGCCGCGAACCTGAGCAGCGCCTG
GCGCAACCACACTGTCCCACTGCGGCTGCGGGACGGCCGCG
AGGTGCCCCACAGCTGCCGCCGCTACCGGCTCGCCACCATC
GCCAACTTCTCGGCGCTTGGGCTGGAGCCGGGGCGCGACGT
GGACCTGGGGCAGCTGGAGCAGGAGAGCTGTCTGGATGGC
TGGGAGTTCAGTCAGGACGTCTACCTGTCCACCATTGTGAC
CGAGCAAGACAGTGGGGCCTACAATGCTATGAAAAACAGG
ATGGGAAAGAAGCCTGCTCTCTGCCTTCCTGCCCAGTGGAA
CCTGGTGTGTGAGGACGACTGGAAGGCCCCACTCACAATCT
CCTTGTTCTTCGTGGGTGTGCTGTTGGGCTCCTTCATTTCAG
GGCAGCTGTCAGACAGGTTTGGCCGGAAGAATGTGCTGTTC
GTGACCATGGGCATGCAGACAGGCTTCAGCTTCCTGCAGAT
CTTCTCGAAGAATTTTGAGATGTTTGTCGTGCTGTTTGTCCT
TGTAGGCATGGGCCAGATCTCCAACTATGTGGCAGCATTTG
TCCTGGGGACAGAAATTCTTGGCAAGTCAGTTCGTATAATA
- 69 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
TTCTCTACGTTAGGAGTGTGCATATTTTATGCATTTGGCTAC
ATGGTGCTGCCACTGTTTGCTTACTTCATCCGAGACTGGCGG
ATGCTGCTGGTGGCGCTGACGATGCCGGGGGTGCTATGCGT
GGCACTCTGGTGGTTCATCCCTGAGTCCCCCCGATGGCTCAT
CTCTCAGGGACGATTTGAAGAGGCAGAGGTGATCATCCGCA
AGGCTGCCAAAGCCAATGGGATTGTTGTGCCTTCCACTATC
TTTGACCCGAGTGAGTTACAAGACCTAAGTTCCAAGAAGCA
GCAGTCCCACAACATTCTGGATCTGCTTCGAACCTGGAATA
TCCGGATGGTCACCATCATGTCCATAATGCTGTGGATGACC
ATATCAGTGGGCTATTTTGGGCTTTCGCTTGATACTCCTAAC
TTGCATGGGGACATCTTTGTGAACTGCTTCCTTTCAGCGATG
GTTGAAGTCCCAGCATATGTGTTGGCCTGGCTGCTGCTGCA
ATATTTGCCCCGGCGCTATTCCATGGCCACTGCCCTCTTCCT
GGGTGGCAGTGTCCTTCTCTTCATGCAGCTGGTACCCCCAG
ACTTGTATTATTTGGCTACAGTCCTGGTGATGGTGGGCAAG
TTTGGAGTCACGGCTGCCTTTTCCATGGTCTACGTGTACACA
GCCGAGCTGTATCCCACAGTGGTGAGAAACATGGGTGTGGG
AGTCAGCTCCACAGCATCCCGCCTGGGCAGCATCCTGTCTC
CCTACTTCGTTTACCTTGGTGCCTACGACCGCTTCCTGCCCT
ACATTCTCATGGGAAGTCTGACCATCCTGACAGCCATCCTC
ACCTTGTTTCTCCCAGAGAGCTTCGGTACCCCACTCCCAGAC
ACCATTGACCAGATGCTAAGAGTCAAAGGAATGAAACACA
GAAAAACTCCAAGTCACACAAGGATGTTAAAAGATGGTCA
AGAAAGGCCCACAATCCTTAAAAGCACAGCCTTCTAACATC
GCTTCCAGTAAGGGAGAAACTGAAGAGGAAAGACTGTCTT
GCCAGAAATGGCCAGCTTGTGCAGACTCCGAGTCCTTCAGT
GACAAAAGGCCTTTGCTGTTTGTCCTCTTGACCTGTGTCTGA
CTTGCTCCTGGATGGGCACCCACACTCAGAGGCTACATATG
GCCCTAGAGCACCACCTTCCTCTAGGGACACTGGGGCTACC
TACAGACAACTTCATCTAAGTCCTAACTATTACAATGATGG
ACTCAGCACCTCCAAAGCAGTTAATTTTTCACTAGAACCAG
TGAGATCTGGAGGAATGTGAGAAGCATATGCTAAATGTACA
TTTTAATTTTAGACTACTTGAAAAGGCCCCTAATAAGGCTA
GAGGTCTAAGTCCCCCACCCCTTTCCCCACTCCCCTCTAGTG
GTGAACTTTAGAGGAAAAGGAAGTAATTGCACAAGGAGTT
TGATTCTTACCTTTTCTCAGTTACAGAGGACATTAACTGGAT
CATTGCTTCCCCAGGGCAGGAGAGCGCAGAGCTAGGGAAA
GTGAAAGGTAATGAAGATGGAGCAGAATGAGCAGATGCAG
ATCACCAGCAAAGTGCACTGATGTGTGAGCTCTTAAGACCA
CTCAGCATGACGACTGAGTAGACTTGTTTACATCTGATCAA
AGCACTGGGCTTGTCCAGGCTCATAATAAATGCTCCATTGA
ATCTACTATTCTTGTTTTCCACTGCTGTGGAAACCTCCTTGC
TACTATAGCGTCTTATGTATGGTTTAAAGGAAATTTATCAG
GTGAGAGAGATGAGCAACGTTGTCTTTTCTCTCAAAGCTGT
AATGTGGGTTTTGTTTTATTGTTTATTTGTTTGTTGTTGTATC
CTTTTCTCCTTGTTATTTGCCCTTCAGAATGCACTTGGGAAA
GGCTGGTTCCTTAGCCTCCTGGTTTGTGTCTTTTTTTTTTTTT
TTTTAAAACAGAATCACTCTGGCAATTGTCTGCAGCTGCCA
CTGGTGCAAGGCCTTACCAGCCCTAGCCTCTAGCACTTCTCT
AAGTGCCAAAAACAGTGTCATTGTGTGTGTTCCTTTCTTGAT
ACTTAGTCATGGGAGGATATTACAAAAAAGAAATTTAAATT
GTGTTCATAGTCTTTCAGAGTAGCTCACTTTAGTCCTGTAAC
TTTATTGGGTGATATTTTGTGTTCAGTGTAATTGTCTTCTCTT
TGCTGATTATGTTACCATGGTACTCCTAAAGCATATGCCTCA
CCTGGTTAAAAAAGAACAAACATGTTTTTGTGAAAGCTACT
- 70 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
GAAGTGCCTTGGGAAATGAGAAAGTTTTAATAAGTAAAATG
ATTTTTTAAATAACAAAAAAAAAAAAAAAAAAAA
Table 2. Carnitine Shuttle Proteins
SEQ Protein Accession Amino Acid Sequence
ID Number
NO
6 Camitine P50416 MAEAHQAVAFQFTVTPDGIDLRLSHEALRQIYLSGLHSW
palmitoyltrans KKKFIRFKNGIITGVYPASPSSWLIVVVGVMTTMYAKIDPS
ferase 1A LGIIAKINRTLETANCMSSQTKNVVSGVLFGTGLWVALIV
(CPT1A) TMRYSLKVLLSYHGWMFTEHGKMSRATKIWMGMVKIFS
GRKPMLYSFQTSLPRLPVPAVKDTVNRYLQSVRPLMKEE
DFKRMTALAQDFAVGLGPRLQWYLKLKSWWATNYVSD
WWEEYIYLRGRGPLMVNSNYYAMDLLYILPTHIQAARAG
NAIHAILLYRRKLDREEIKPIRLLGSTIPLCSAQWERMFNTS
RIPGEETDTIQHMRDSKHIVVYHRGRYFKVWLYHDGRLL
KPREMEQQMQRILDNTSEPQPGEARLAALTAGDRVPWAR
CRQAYFGRGKNKQSLDAVEKAAFFVTLDETEEGYRSEDP
DTSMDSYAKSLLHGRCYDRWFDKSFTFVVFKNGKMGLN
AEHSWADAPIVAHLWEYVMSIDSLQLGYAEDGHCKGDIN
PNIPYPTRLQWDIPGECQEVIETSLNTANLLANDVDFHSFP
FVAFGKGIIKKCRTSPDAFVQLALQLAHYKDMGKFCLTY
EASMTRLFREGRTETVRSCTTESCDFVRAMVDPAQTVEQ
RLKLFKLASEKHQHMYRLAMTGSGIDRHLFCLYVVSKYL
AVESPFLKEVLSEPWRLSTSQTPQQQVELFDLENNPEYVS
SGGGFGPVADDGYGVSYILVGENLINFHISSKFSCPETDSH
RFGRHLKEAMTDIITLFGLSSNSKK
7 Camitine Q92523 MAEAHQAVAFQFTVTPDGVDFRLSREALKHVYLSGINSW
palmitoyltrans KKRLIRIKNGILRGVYPGSPTSWLVVIMATVGSSFCNVDIS
ferase 1B LGLVSCIQRCLPQGCGPYQTPQTRALLSMAIFSTGVWVTG
(CPT1B) IFFFRQTLKLLLCYHGWMFEMHGKTSNLTRIWAMCIRLLS
SRHPMLYSFQTSLPKLPVPRVSATIQRYLESVRPLLDDEEY
YRMELLAKEFQDKTAPRLQKYLVLKSWWASNYVSDWW
EEYIYLRGRSPLMVNSNYYVMDLVLIKNTDVQAARLGNII
HAMIMYRRKLDREEIKPVMALGIVPMCSYQMERMFNTTR
IPGKDTDVLQHLSDSRHVAVYHKGRFFKLWLYEGARLLK
PQDLEMQFQRILDDPSPPQPGEEKLAALTAGGRVEWAQA
RQAFFSSGKNKAALEAIERAAFFVALDEESYSYDPEDEAS
LSLYGKALLHGNCYNRWFDKSFTLISFKNGQLGLNAEHA
WADAPIIGHLWEFVLGTDSFHLGYTETGHCLGKPNPALAP
PTRLQWDIPKQCQAVIESSYQVAKALADDVELYCFQFLPF
GKGLIKKCRTSPDAFVQIALQLAHFRDRGKFCLTYEASMT
RMFREGRTETVRSCTSESTAFVQAMMEGSHTKADLRDLF
QKAAKKHQNMYRLAMTGAGIDRHLFCLYLVSKYLGVSS
PFLAEVLSEPWRLSTSQIPQSQIRMFDPEQHPNHLGAGGGF
GPVADDGYGVSYMIAGENTIFFHISSKFSSSETNAQRFGNH
IRKALLDIADLFQVPKAYS
8 Camitine 043772 MADQPKPISPLKNLLAGGFGGVCLVFVGHPLDTVKVRLQ
acylcamitine TQPPSLPGQPPMYSGTFDCFRKTLFREGITGLYRGMAAPII
translocase GVTPMFAVCFFGFGLGKKLQQKHPEDVLSYPQLFAAGML
(CACT) SGVFTTGIMTPGERIKCLLQIQASSGESKYTGTLDCAKKLY
QEFGIRGIYKGTVLTLMRDVPASGMYFMTYEWLKNIFTPE
GKRVSELSAPRILVAGGIAGIFNWAVAIPPDVLKSRFQTAP
- 71 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
PGKYPNGFRDVLRELIRDEGVTSLYKGFNAVMIRAFPANA
ACFLGFEVAMKFLNWATPN
9 Camitine P23786 MVPRLLLRAWPRGPAVGPGAPSRPLSAGSGPGQYLQRSIV
palmitoyltrans PTMHYQDSLPRLPIPKLEDTIRRYLSAQKPLLNDGQFRKTE
ferase 2 QFCKSFENGIGKELHEQLVALDKQNKHTSYISGPWFDMY
(CPT2) LSARDSVVLNFNPFMAFNPDPKSEYNDQLTRATNMTVSAI
RFLKTLRAGLLEPEVFHLNPAKSDTITFKRLIRFVPSSLSW
YGAYLVNAYPLDMSQYFRLFNSTRLPKPSRDELFTDDKA
RHLLVLRKGNFYIFDVLDQDGNIV SP SEIQAHLKYILSDSS
PAPEFPLAYLTSENRDIWAELRQKLMSSGNEESLRKVDSA
VFCLCLDDFPIKDLVHLSHNMLHGDGTNRWFDKSFNLIIA
KDGSTAVHFEHSWGDGVAVLRFFNEVFKDSTQTPAVTPQ
SQPATTDSTVTVQKLNFELTDALKTGITAAKEKFDATMKT
LTIDCVQFQRGGKEFLKKQKLSPDAVAQLAFQMAFLRQY
GQTVATYESCSTAAFKHGRTETIRPASVYTKRCSEAFVRE
PSRHSAGELQQMMVECSKYHGQLTKEAAMGQGFDRHLF
ALRHLAAAKGIILPELYLDPAYGQINHNVLSTSTLSSPAVN
LGGFAPVVSDGFGVGYAVHDNWIGCNVSSYPGRNAREFL
QCVEKALEDMFDALEGKSIKS
Organic 076082 MRDYDEVTAFLGEWGPFQRLIFFLLSASIIPNGFTGLSVFLI
cation/carnitin ATPEHRCRVPDAANLSSAWRNHTVPLRLRDGREVPHSCR
e transporter RYRLATIANFSALGLEPGRDVDLGQLEQESCLDGWEFSQD
(OCTN2) VYLSTIVTEWNLVCEDDWKAPLTISLFFVGVLLGSFISGQL
SDRFGRKNVLFVTMGMQTGFSFLQIFSKNFEMFVVLFVLV
GMGQISNYVAAFVLGTEILGKSVRIIFSTLGVCIFYAFGYM
VLPLFAYFIRDWRMLLVALTMPGVLCVALWWFIPESPRW
LISQGRFEEAEVIIRKAAKANGIVVPSTIFDPSELQDLSSKK
QQSHNILDLLRTWNIRMVTIMSIMLWMTISVGYFGLSLDT
PNLHGDIFVNCFLSAMVEVPAYVLAWLLLQYLPRRYSMA
TALFLGGSVLLFMQLVPPDLYYLATVLVMVGKFGVTAAF
SMVYVYTAELYPTVVRNMGVGVSSTASRLGSILSPYFVY
LGAYDRFLPYILMGSLTILTAILTLFLPESFGTPLPDTIDQM
LRVKGMKHRKTPSHTRMLKDGQERPTILKSTAF
Table 3. Fatty Acid Oxidation Cycle Genes
SEQ Gene NCBI Nucleotide Sequence
ID Reference
NO Number
11 ACADVL NM 00001 AGAGCTGGGTCAGAGCTCGAGCCAGCGGCGCCCGGAG
8.4 AGATTCGGAGATGCAGGCGGCTCGGATGGCCGCGAGCT
TGGGGCGGCAGCTGCTGAGGCTCGGGGGCGGAAGCTC
GCGGCTCACGGCGCTCCTGGGGCAGCCCCGGCCCGGCC
CTGCCCGGCGGCCCTATGCCGGGGGTGCCGCTCAGCTG
GCTCTGGACAAGTCAGATTCCCACCCCTCTGACGCTCT
GACCAGGAAAAAACCGGCCAAGGCGGAATCTAAGTCC
TTTGCTGTGGGAATGTTCAAAGGCCAGCTCACCACAGA
TCAGGTGTTCCCATACCCGTCCGTGCTCAACGAAGAGC
AGACACAGTTTCTTAAAGAGCTGGTGGAGCCTGTGTCC
CGTTTCTTCGAGGAAGTGAACGATCCCGCCAAGAATGA
CGCTCTGGAGATGGTGGAGGAGACCACTTGGCAGGGCC
TCAAGGAGCTGGGGGCCTTTGGTCTGCAAGTGCCCAGT
GAGCTGGGTGGTGTGGGCCTTTGCAACACCCAGTACGC
CCGTTTGGTGGAGATCGTGGGCATGCATGACCTTGGCG
TGGGCATTACCCTGGGGGCCCATCAGAGCATCGGTTTC
- 72 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
AAAGGCATCCTGCTCTTTGGCACAAAGGCCCAGAAAGA
AAAATACCTCCCCAAGCTGGCATCTGGGGAGACTGTGG
CCGCTTTCTGTCTAACCGAGCCCTCAAGCGGGTCAGAT
GCAGCCTCCATCCGAACCTCTGCTGTGCCCAGCCCCTGT
GGAAAATACTATACCCTCAATGGAAGCAAGCTTTGGAT
CAGTAATGGGGGCCTAGCAGACATCTTCACGGTCTTTG
CCAAGACACCAGTTACAGATCCAGCCACAGGAGCCGTG
AAGGAGAAGATCACAGCTTTTGTGGTGGAGAGGGGCTT
CGGGGGCATTACCCATGGGCCCCCTGAGAAGAAGATG
GGCATCAAGGCTTCAAACACAGCAGAGGTGTTCTTTGA
TGGAGTACGGGTGCCATCGGAGAACGTGCTGGGTGAG
GTTGGGAGTGGCTTCAAGGTTGCCATGCACATCCTCAA
CAATGGAAGGTTTGGCATGGCTGCGGCCCTGGCAGGTA
CCATGAGAGGCATCATTGCTAAGGCGGTAGATCATGCC
ACTAATCGTACCCAGTTTGGGGAGAAAATTCACAACTT
TGGGCTGATCCAGGAGAAGCTGGCACGGATGGTTATGC
TGCAGTATGTAACTGAGTCCATGGCTTACATGGTGAGT
GCTAACATGGACCAGGGAGCCACGGACTTCCAGATAG
AGGCCGCCATCAGCAAAATCTTTGGCTCGGAGGCAGCC
TGGAAGGTGACAGATGAATGCATCCAAATCATGGGGG
GTATGGGCTTCATGAAGGAACCTGGAGTAGAGCGTGTG
CTCCGAGATCTTCGCATCTTCCGGATCTTTGAGGGGAC
AAATGACATTCTTCGGCTGTTTGTGGCTCTGCAGGGCTG
TATGGACAAAGGAAAGGAGCTCTCTGGGCTTGGCAGTG
CTCTAAAGAATCCCTTTGGGAATGCTGGCCTCCTGCTA
GGAGAGGCAGGCAAACAGCTGAGGCGGCGGGCAGGGC
TGGGCAGCGGCCTGAGTCTCAGCGGACTTGTCCACCCG
GAGTTGAGTCGGAGTGGCGAGCTGGCAGTACGGGCTCT
GGAGCAGTTTGCCACTGTGGTGGAGGCCAAGCTGATAA
AACACAAGAAGGGGATTGTCAATGAACAGTTTCTGCTG
CAGCGGCTGGCAGACGGGGCCATCGACCTCTATGCCAT
GGTGGTGGTTCTCTCGAGGGCCTCAAGATCCCTGAGTG
AGGGCCACCCCACGGCCCAGCATGAGAAAATGCTCTGT
GACACCTGGTGTATCGAGGCTGCAGCTCGGATCCGAGA
GGGCATGGCCGCCCTGCAGTCTGACCCCTGGCAGCAAG
AGCTCTACCGCAACTTCAAAAGCATCTCCAAGGCCTTG
GTGGAGCGGGGTGGTGTGGTCACCAGCAACCCACTTGG
CTTCTGAATACTCCCGGCCAGGGCCTGTCCCAGTTATGT
GCCTTCCCTCAAGCCAAAGCCGAAGCCCCTTTCCTTAA
GGCCCTGGTTTGTCCCGAAGGGGCCTAGTGTTCCCAGC
ACTGTGCCTGCTCTCAAGAGCACTTACTGCCTCGCAAA
TAATAAAAATTTCTAGCCAGTCA
12 ACADIVI NM 00001 CTGCACCGCGCCGCAAGTCCCCCCACCGTTCAGCGCAA
6.5 CCGGGCCCTCCCAGCCCCGCCGCCGTCCCCCTCCCCCG
CCCTGGCTCTCTTTCCGCGCTGCGGTCAGCCTCGGCGTC
CCACAGAGAGGGCCAGAGGTGGAAACGCAGAAAACCA
AACCAGGACTATCAGAGATTGCCCGGAGAGGGGATGC
GACCCCTCCCCAGGTCGCAGCGACGGCGCACGCAAGG
GTCACGGAGCATGCGTTGGCTACCCGGCGCCGGGGACC
GCTGCCACCCCGCCTAGCGCAGCGCCCCGTCCTTCCGC
AGCCCAACCGCCTCTTCCCGCCCCGCCCCATCCCGCCC
ACGGGCTCCAGTGGGCGGGACCAGAGGAGTCCCGCGTT
CGGGGAGTATGTCAAGGCCGTGACCCGTGTATTATTGT
CCGAGTGGCCGGAACGGGAGCCAACATGGCAGCGGGG
TTCGGGCGATGCTGCAGGGTCCTGAGAAGTATTTCTCG
TTTTCATTGGAGATCACAGCATACAAAAGCCAATCGAC
- 73 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
AACGTGAACCAGGATTAGGATTTAGTTTTGAGTTCACC
GAACAGCAGAAAGAATTTCAAGCTACTGCTCGTAAATT
TGCCAGAGAGGAAATCATCCCAGTGGCTGCAGAATATG
ATAAAACTGGTGAATATCCAGTCCCCCTAATTAGAAGA
GCCTGGGAACTTGGTTTAATGAACACACACATTCCAGA
GAACTGTGGAGGTCTTGGACTTGGAACTTTTGATGCTT
GTTTAATTAGTGAAGAATTGGCTTATGGATGTACAGGG
GTTCAGACTGCTATTGAAGGAAATTCTTTGGGGCAAAT
GCCTATTATTATTGCTGGAAATGATCAACAAAAGAAGA
AGTATTTGGGGAGAATGACTGAGGAGCCATTGATGTGT
GCTTATTGTGTAACAGAACCTGGAGCAGGCTCTGATGT
AGCTGGTATAAAGACCAAAGCAGAAAAGAAAGGAGAT
GAGTATATTATTAATGGTCAGAAGATGTGGATAACCAA
CGGAGGAAAAGCTAATTGGTATTTTTTATTGGCACGTT
CTGATCCAGATCCTAAAGCTCCTGCTAATAAAGCCTTT
ACTGGATTCATTGTGGAAGCAGATACCCCAGGAATTCA
GATTGGGAGAAAGGAATTAAACATGGGCCAGCGATGT
TCAGATACTAGAGGAATTGTCTTCGAAGATGTGAAAGT
GCCTAAAGAAAATGTTTTAATTGGTGACGGAGCTGGTT
TCAAAGTTGCAATGGGAGCTTTTGATAAAACCAGACCT
GTAGTAGCTGCTGGTGCTGTTGGATTAGCACAAAGAGC
TTTGGATGAAGCTACCAAGTATGCCCTGGAAAGGAAAA
CTTTCGGAAAGCTACTTGTAGAGCACCAAGCAATATCA
TTTATGCTGGCTGAAATGGCAATGAAAGTTGAACTAGC
TAGAATGAGTTACCAGAGAGCAGCTTGGGAGGTTGATT
CTGGTCGTCGAAATACCTATTATGCTTCTATTGCAAAGG
CATTTGCTGGAGATATTGCAAATCAGTTAGCTACTGAT
GCTGTGCAGATACTTGGAGGCAATGGATTTAATACAGA
ATATCCTGTAGAAAAACTAATGAGGGATGCCAAAATCT
ATCAGATTTATGAAGGTACTTCACAAATTCAAAGACTT
ATTGTAGCCCGTGAACACATTGACAAGTACAAAAATTA
AAAAAATTACTGTAGAAATATTGAATAACTAGAACACA
AGCCACTGTTTCAGCTCCAGAAAAAAGAAAGGGCTTTA
ACGTTTTTTCCAGTGAAAACAAATCCTCTTATATTAAAT
CTAAGCAACTGCTTATTATAGTAGTTTATACTTTTGCTT
AACTCTGTTATGTCTCTTAAGCAGGTTTGGTTTTTATTA
AAATGATGTGTTTTCTTTAGTACCACTTTACTTGAATTA
CATTAACCTAGAAAACTACATAGGTTATTTTGATCTCTT
AAGATTAATGTAGCAGAAATTTCTTGGAATTTTATTTTT
GTAATGACAGAAAAGTGGGCTTAGAAAGTATTCAAGAT
GTTACAAAATTTACATTTAGAAAATATTGTAGTATTTGA
ATACTGTCAACTTGACAGTAACTTTGTAGACTTAATGGT
ATTATTAAAGTTCTITTTATTGCAGTTTGGAAAGCATTT
GTGAAACTTTCTGTTTGGCACAGAAACAGTCAAAATTT
TGACATTCATATTCTCCTATTTTACAGCTACAAGAACTT
TCTTGAAAATCTTATTTAATTCTGAGCCCATATTTCACT
TACCTTATTTAAAATAAATCAATAAAGCTTGCCTTAAAT
TATTTTTATATGACTGTTGGTCTCTAGGTAGCCTTTGGT
CTATTGTACACAATCTCATTTCATATGTTTGCATTTTGG
CAAAGAACTTAATAAAATTGTTCAGTGCTTATTATCAT
ATCTTTCTGTATTTTTTCCAGGAAATTTCATTACTTCGTG
TAATAGTGTATATTTCTTGTATTTACTATGATGAAAAAA
GGTCGTTTTAATTTTGAATTGAATAAAGTTACCTGTTCA
TTTTTTATTAGATATTTTAAAGACTTCAGAAAATATAAA
TATGAAATAATTTAAGAACCCAAA
- 74 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
13 ACADS NM 00001 ACTCCGGAACAGCGCGCTCGCAGCGGGAGGTCGCGAA
7.4 GCCTGGGACTGTGTCTGTCGCCCATGGCCGCCGCGCTG
CTCGCCCGGGCCTCGGGCCCTGCCCGCAGAGCTCTCTG
TCCTAGGGCCTGGCGGCAGTTACACACCATCTACCAGT
CTGTGGAACTGCCCGAGACACACCAGATGTTGCTCCAG
ACATGCCGGGACTTTGC CGAGAAGGAGTTGTTTCC CAT
TGCAGCCCAGGTGGATAAGGAACATCTCTTCCCAGCGG
CTCAGGTGAAGAAGATGGGCGGGCTTGGGCTTCTGGCC
ATGGACGTGCCCGAGGAGCTTGGCGGTGCTGGCCTCGA
TTACCTGGCCTACGCCATCGCCATGGAGGAGATCAGCC
GTGGCTGCGCCTCCACCGGAGTCATCATGAGTGTCAAC
AACTCTCTCTACCTGGGGCCCATCTTGAAGTTTGGCTCC
AAGGAGCAGAAGCAGGCGTGGGTCACGC CTTTCAC CA
GTGGTGACAAAATTGGCTGCTTTGC CCTCAGCGAAC CA
GGGAACGGCAGTGATGCAGGAGCTGCGTCCACCACCG
CC CGGGC CGAGGGCGACTCATGGGTTCTGAATGGAAC C
AAAGCCTGGATCACCAATGCCTGGGAGGCTTCGGCTGC
CGTGGTCTTTGCCAGCACGGACAGAGCCCTGCAAAACA
AGGGCATCAGTGC CTTC CTGGTC CC CATGC CAACGCCT
GGGCTCACGTTGGGGAAGAAAGAAGACAAGCTGGGCA
TCCGGGGCTCATCCACGGCCAACCTCATCTTTGAGGAC
TGTCGCATCCCCAAGGACAGCATCCTGGGGGAGCCAGG
GATGGGCTTCAAGATAGCCATGCAAACCCTGGACATGG
GCCGCATCGGCATCGCCTCCCAGGCCCTGGGCATTGCC
CAGACCGCCCTCGATTGTGCTGTGAACTACGCTGAGAA
TCGCATGGCCTTCGGGGCGC CC CTCAC CAAGCTC CAGG
TCATCCAGTTCAAGTTGGCAGACATGGCCCTGGCCCTG
GAGAGTGCCCGGCTGCTGACCTGGCGCGCTGCCATGCT
GAAGGATAACAAGAAGCCTTTCATCAAGGAGGCAGCC
ATGGCCAAGCTGGCCGCCTCGGAGGCCGCGACCGCCAT
CAGC CAC CAGGCCATC CAGATC CTGGGCGGCATGGGCT
ACGTGACAGAGATGCCGGCAGAGCGGCACTACCGCGA
CGCCCGCATCACTGAGATCTACGAGGGCACCAGCGAAA
TCCAGCGGCTGGTGATCGCCGGGCATCTGCTCAGGAGC
TAC CGGAGCTGAGCC CGCGGCGGACTGC CC CAGGACTG
CGGGAAGGCGCGGGAGC CAGGGGCCTC CAC C CCAACC
CCGGCTCAGAGACTGGGCGGCCCGGCGGGGGCTCCCTG
GGGACC CCAGATGGGCTCAGTGCTGC CAC CCAGATCAG
ATCACATGGGAATGAGGCCCTCCGACCATTGGCAGCTC
CGCCTCTGGGCCTTTCCGCCTCCTCACCACTGTGCCTCA
AGTTCCTCATCTAAGTGGCCCTGGCCTCCTGGGGGCGG
GGTTGTGGGGGGGCTGAGCGACACTCAGGGACACCTCA
GTTGTCCTCCCGCGGGCCCTGGTGCCCTGGCATGAAGG
CC CAGTGCGACAGGCC CTTGGTGGGGTCTGTCTTTTC CT
TGAGGTCAGAGGTCAGGAGCAGGGCTGGGGTCAGGAT
GACGAGGCCTGGGGTCCTGGTGTTGGGCAGGTGGTGGG
GCTGGGCCATGGAGCTGGC CCAGAGGC CC CTCAGCC CT
TTGTAAAGTCTGATGAAGGCAGGGGTGGTGATTCATGC
TGTGTGACTGACTGTGGGTAATAAACACAC CTGTCC CC
CA
14 HADHA NM 00018 AGAGGCGCTCTCCACTGCTGTCCTCTTCAGCTCAAGAT
2.5 GGTGGCCTGCCGGGCGATTGGCATCCTCAGCCGCTTTT
CTGCCTTCAGGATCCTCCGCTCCCGAGGTTATATATGCC
GCAATTTTACAGGGTCTTCTGCTTTGCTGAC CAGAAC CC
ATATTAACTATGGAGTCAAAGGGGATGTGGCAGTTGTT
CGAATTAACTCTCCCAATTCAAAGGTAAATACACTGAG
- 75 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
TAAAGAGCTACATTCAGAGTTCTCAGAAGTTATGAATG
AAATCTGGGCTAGTGATCAAATCAGAAGTGCCGTCCTT
ATCTCATCAAAGCCAGGCTGCTTTATTGCAGGTGCTGA
TATCAACATGTTAGCCGCTTGCAAGACCCTTCAAGAAG
TAACACAGCTATCACAAGAAGCACAGAGAATAGTTGA
GAAACTTGAAAAGTCCACAAAGCCTATTGTGGCTGCCA
TCAATGGATCCTGCCTGGGAGGAGGACTTGAGGTTGCC
ATTTCATGCCAATACAGAATAGCAACAAAAGACAGAA
AAACAGTATTAGGTACCCCTGAAGTTTTGCTGGGGGCC
TTACCAGGAGCAGGAGGCACACAAAGGCTGCCCAAAA
TGGTGGGTGTGCCTGCTGCTTTGGACATGATGCTGACT
GGTAGAAGCATTCGTGCAGACAGGGCAAAGAAAATGG
GACTGGTTGACCAACTGGTGGAACCCCTGGGACCAGGA
CTAAAACCTCCAGAGGAACGGACAATAGAATACCTAG
AAGAAGTTGCAATTACTTTTGCCAAAGGACTAGCTGAT
AAGAAGATCTCTCCAAAGAGAGACAAGGGATTGGTGG
AAAAATTGACAGCGTATGCCATGACTATTCCATTTGTC
AGGCAACAGGTTTACAAAAAAGTGGAAGAAAAAGTGC
GAAAGCAGACTAAAGGCCTTTATCCTGCACCTCTGAAA
ATAATTGATGTGGTAAAGACTGGAATTGAGCAAGGGA
GTGATGCCGGTTATCTCTGTGAATCTCAGAAATTTGGA
GAGCTTGTAATGACCAAAGAATCAAAGGCCTTGATGGG
ACTCTACCATGGTCAGGTCCTGTGCAAGAAGAATAAAT
TTGGAGCTCCACAGAAGGATGTTAAGCATCTGGCTATT
CTTGGTGCAGGGCTGATGGGAGCAGGCATCGCCCAAGT
CTCCGTGGATAAGGGGCTAAAGACTATACTTAAAGATG
CCACCCTCACTGCGCTAGACCGAGGACAGCAACAAGTG
TTCAAAGGATTGAATGACAAAGTGAAGAAGAAAGCTC
TAACATCATTTGAAAGGGATTCCATCTTCAGCAACTTG
ACTGGGCAGCTTGATTACCAAGGTTTTGAAAAGGCCGA
CATGGTGATTGAAGCTGTGTTTGAGGACCTTAGTCTTA
AGCACAGAGTGCTAAAGGAAGTAGAAGCGGTGATTCC
AGATCACTGTATCTTTGCCAGTAACACATCTGCTCTCCC
AATCAGTGAAATCGCTGCTGTCAGCAAAAGACCTGAGA
AGGTGATTGGCATGCACTACTTCTCTCCCGTGGACAAG
ATGCAGCTGCTGGAGATTATCACGACCGAGAAAACTTC
CAAAGACACCAGTGCTTCAGCTGTAGCAGTTGGTCTCA
AGCAGGGGAAGGTCATCATTGTGGTTAAGGATGGACCT
GGCTTCTATACTACCAGGTGTCTTGCGCCCATGATGTCT
GAAGTCATCCGAATCCTCCAGGAAGGAGTTGACCCGAA
GAAGCTGGATTCCCTGACCACAAGCTTTGGCTTTCCTGT
GGGTGCCGCCACACTGGTGGATGAAGTTGGTGTGGATG
TAGCGAAACATGTGGCGGAAGATCTGGGCAAAGTCTTT
GGGGAGCGGTTTGGAGGTGGAAACCCAGAACTGCTGA
CACAGATGGTGTCCAAGGGCTTCCTAGGTCGTAAATCT
GGGAAGGGCTTTTACATCTATCAGGAGGGTGTGAAGAG
GAAGGATTTGAATTCTGACATGGATAGTATTTTAGCGA
GTCTGAAGCTGCCTCCTAAGTCTGAAGTCTCATCAGAC
GAAGACATCCAGTTCCGCCTGGTGACAAGATTTGTGAA
TGAGGCAGTCATGTGCCTGCAAGAGGGGATCTTGGCCA
CACCTGCAGAGGGAGACATCGGAGCCGTCTTTGGGCTT
GGCTTCCCGCCTTGTCTGGGAGGGCCTTTCCGCTTTGTG
GATCTGTATGGCGCCCAGAAGATAGTGGACCGGCTCAA
GAAATATGAAGCTGCCTATGGAAAACAGTTCACCCCAT
GCCAGCTGCTAGCTGACCATGCTAACAGCCCTAACAAG
AAGTTCTACCAGTGAGCAGGCCTCATGCCTCGCTCAGT
- 76 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
CAGTGCACTAAC CC CAGCTGCCGGCAGTGCTGGTTCTC
CAACAGAGTGGTGTCTAGATTTATCAGAGTAACGAGAA
GACAAACTCCGGCACTGGGTTTGCTCCCTGATTAAAGT
GCCTTCAGCCAAGACCATCTCTCCCTCCTGGTGAAGTGT
GACTTCGAATTAGTTTGCACTTCCTGTTGGAAGGTAGA
GCCCACTGCTCATTGTATAAGCCCCGAGGCCTAGAGTG
GCAGCCAAGAGCCATCTGAAGCCACCTCTCTGCCTGTT
CCTCCCAAGAGGCCAGGGTGGCCAGGGGTGGTGAGGG
CAGTTCTGCACCCAGCCAAACACATAACAATAAAAACC
AAACTCTGTGTCAGCATCTTTGCCCTTCTGGTTTAAACG
CCTCCTTCAAAAAGCAATCTGGAAGAAAGCCCTGTGCT
TTGGGGGAGTAAGAATGTGTGTGCAGAATTCTAGGCAG
CAC CTTAGGGAGGGACTGGGATGAGAGAAAGTGGGAC
CTGGTGGGCTCAACCACACACACCTGTCTGTGCAGATG
CTTTGCCCAGGCTTCTCACCACGGTGTACCGGGATATTA
AACCTCTTTC CC CAGC CTGGA
15 HADHB NM 00018 ACTTGGACCTGAACCTTGCTCCGAGAGGGAGTCCTCGC
3.3 GGACGTCAGCCAAGATTCCAGAATGACTATCTTGACTT
ACC CCTTTAAAAATCTTC CCACTGCATCAAAATGGGC C
CTCAGATTTTCCATAAGACCTCTGAGCTGTTCCTCCCAG
CTACGAGCTGC CC CAGCTGTCCAGACCAAAACGAAGAA
GACGTTAGCCAAACCCAATATAAGGAATGTTGTGGTGG
TGGATGGTGTTCGCACTCCATTTTTGCTGTCTGGCACTT
CATATAAAGACCTGATGCCACATGATTTGGCTAGAGCA
GCGCTTACGGGTTTGTTGCATCGGACCAGTGTCCCTAA
GGAAGTAGTTGATTATATCATCTTTGGTACAGTTATTCA
GGAAGTGAAAACAAGCAATGTGGCTAGAGAGGCTGCC
CTTGGAGCTGGCTTCTCTGACAAGACTCCTGCTCACACT
GTCACCATGGCTTGTATCTCTGCCAACCAAGCCATGAC
CACAGGTGTTGGCTTGATTGCTTCTGGCCAGTGTGATGT
GATCGTGGCAGGTGGTGTTGAGTTGATGTCCGATGTCC
CTATTCGTCACTCAAGGAAAATGAGAAAACTGATGCTT
GATCTCAATAAGGCCAAATCTATGGGCCAGCGACTGTC
TTTAATCTCTAAATTCCGATTTAATTTCCTAGCACCTGA
GCTCCCTGCGGTTTCTGAGTTCTCCACCAGTGAGACCAT
GGGCCACTCTGCAGACCGACTGGCCGCTGCCTTTGCTG
TTTCTCGGCTGGAACAGGATGAATATGCACTGCGCTCT
CACAGTCTAGCCAAGAAGGCACAGGATGAAGGACTCC
TTTCTGATGTGGTACCCTTCAAAGTACCAGGAAAAGAT
ACAGTTACCAAAGATAATGGCATCCGTCCTTCCTCACT
GGAGCAGATGGCCAAACTAAAACCTGCATTCATCAAGC
CCTACGGCACAGTGACAGCTGCAAATTCTTCTTTCTTGA
CTGATGGTGCATCTGCAATGTTAATCATGGCGGAGGAA
AAGGCTCTGGCCATGGGTTATAAGCCGAAGGCATATTT
GAGGGATTTTATGTATGTGTCTCAGGATCCAAAAGATC
AACTATTACTTGGACCAACATATGCTACTCCAAAAGTT
CTAGAAAAGGCAGGATTGACCATGAATGATATTGATGC
TTTTGAATTTCATGAAGCTTTCTCGGGTCAGATTTTGGC
AAATTTTAAAGCCATGGATTCTGATTGGTTTGCAGAAA
ACTACATGGGTAGAAAAACCAAGGTTGGATTGCCTCCT
TTGGAGAAGTTTAATAACTGGGGTGGATCTCTGTCCCT
GGGACACCCATTTGGAGCCACTGGCTGCAGGTTGGTCA
TGGCTGCTGCCAACAGATTACGGAAAGAAGGAGGCCA
GTATGGCTTAGTGGCTGCGTGTGCAGCTGGAGGGCAGG
GCCATGCTATGATAGTGGAAGCTTATCCAAAATAATAG
ATCCAGAAGAAGTGACCTGAAGTTTCTGTGCAACACTC
- 77 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
ACACTAGGCAATGCCATTTCAATGCATTACTAAATGAC
ATTTGTAGTTCCTAGCTCCTCTTAGGAAAACAGTTCTTG
TGGCCTTCTATTAAATAGTTTGCACTTAAGCCTTGCCAG
TGTTCTGAGCTTTTCAATAATCAGTTTACTGCTCTTTCA
GGGATTTCTAAGCCACCAGAATCTCACATGAGATGTGT
GGGTGGTTGTTTTTGGTCTCTGTTGTCACTAAAGACTAA
ATGAGGGTTTGCAGTTGGGAAAGAGGTCAACTGAGATT
TGGAAATCATCTTTGTAATATTTGCAAATTATACTTGTT
CTTATCTGTGTCCTAAAGATGTGTTCTCTATAAAATACA
AACCAACGTGCCTAATTAATTATGGAAAAATAATTCAG
AATCTAAACACCACTGAAAACTTATAAAAAATGTTTAG
ATACATAAATATGGTGGTCAGCGTTAATAAAGTGGAGA
AATATTGGA
16 ECHS1 NM 00409 GGGCGAGGAGTCCAGAGAGCCATGGCCGCCCTGCGTGT
2.4 CCTGCTGTC CTGCGTCCGCGGCC CGCTGAGGC CC CCGG
TTCGCTGTCCCGCCTGGCGTCCCTTCGCCTCGGGTGCTA
ACTTTGAGTACATCATCGCAGAAAAAAGAGGGAAGAA
TAACACCGTGGGGTTGATC CAACTGAAC CGC CC CAAGG
CC CTCAATGCACTTTGCGATGGC CTGATTGACGAGCTC
AACCAGGCCCTGAAGACCTTCGAGGAGGACCCGGCCGT
GGGGGCCATTGTCCTCACCGGCGGGGATAAGGCCTTTG
CAGCTGGAGCTGATATCAAGGAAATGCAGAACCTGAGT
TTCCAGGACTGTTACTCCAGCAAGTTCTTGAAGCACTG
GGACCACCTCACCCAGGTCAAGAAGCCAGTCATCGCTG
CTGTCAATGGCTATGCCTTTGGCGGGGGCTGTGAGCTT
GCCATGATGTGTGATATCATCTATGCCGGTGAGAAGGC
CCAGTTTGCACAGCCGGAGATCTTAATAGGAACCATCC
CAGGTGCGGGCGGCACCCAGAGACTCACCCGTGCTGTT
GGGAAGTCGCTGGCGATGGAGATGGTCCTCACTGGTGA
CCGGATCTCAGCCCAGGACGCCAAGCAAGCAGGTCTTG
TCAGCAAGATTTGTCCTGTTGAGACACTGGTGGAAGAA
GCCATCCAGTGTGCAGAAAAAATTGCCAGCAATTCTAA
AATTGTAGTAGCGATGGCCAAAGAATCAGTGAATGCAG
CTTTTGAAATGACATTAACAGAAGGAAGTAAGTTGGAG
AAGAAACTCTTTTATTCAACCTTTGCCACTGATGACCGG
AAAGAAGGGATGACCGCGTTTGTGGAAAAGAGAAAGG
CCAACTTCAAAGACCAGTGAGAACCAGCTGCCCCTGCT
TCACACCTCTGCTTGGAGAGGACAAGTGCAGCCTGTCA
GTTTTAGAAGCAAGTAAATCATCCTCTTTTCAAGAGCA
GTGTCCGTGGTGTGCAGTTCCTCTCCAATTGCTGCGTGG
TCGTGGCCCGACCTCTCACGGCATGACAGCCTTCGTCA
CC CAGC CTGTGAGGGTC CTGACTGGAGCAC CTTCTAAA
TCTAAGATTCTGCTGAGGAGCC CC CGCTGGTCC CTCTG
GGCATGCTGTGCTCGGACGGAAAGCGGGGCCTGCGGGT
CCTTGTGTCCCTGCCGCTGAAGAATGGGGCTGCTCTGA
GGGAAACGCTGTCTGCTGCCTTCATACAGATGCTGATT
AAAGTGATAGCGATTCAGATTA
17 HADH NM_00118 CGTGTATACCCGCTCAACGCTGGGACGTTACAGCCAGG
4705.2 GCCAATGGGCAGAGCGGGACTCGAGGC CC CGC CC CCG
CCTTGTGGCGTCACGGGGACGCCGGGGGCGCGCGGGCT
GCAGGGCCGCGTAGGTCCCCGCCCCCAGAGTCTGGCTT
TCCGCGGCTGCCCGCCTCGCGCGTCTTCCCTGCCCGGGT
CTCCTCGCTGTCGCCGCCGCTGCCACACCATGGCCTTCG
TCACCAGGCAGTTCATGCGTTCCGTGTCCTCCTCGTCCA
CCGCCTCGGCCTCGGCCAAGAAGATAATCGTCAAGCAC
GTGACGGTCATCGGCGGCGGGCTGATGGGCGCCGGCAT
- 78 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
TGCCCAGGTTGCTGCAGCAACTGGTCACACAGTAGTGT
TGGTAGACCAGACAGAGGACATCCTGGCAAAATCCAA
AAAGGGAATTGAGGAAAGCCTTAGGAAAGTGGCAAAG
AAGAAGTTTGCAGAAAACCCTAAGGCCGGCGATGAATT
TGTGGAGAAGACCCTGAGCACCATAGCGACCAGCACG
GATGCAGCCTCCGTTGTCCACAGCACAGACTTGGTGGT
GGAAGCCATCGTGGAGAATCTGAAGGTGAAAAACGAG
CTCTTCAAAAGGCTGGACAAGTTTGCTGCTGAACATAC
AATCTTTGCCAGCAACACTTCCTCCTTGCAGATTACAAG
CATAGCTAATGCCACCACCAGACAAGACCGATTCGCTG
GCCTCCATTTCTTCAACCCAGTGCCTGTCATGAAACTTG
TGGAGGTCATTAAAACACCAATGACCAGCCAGAAGAC
ATTTGAATCTTTGGTAGACTTTAGCAAAGCCCTAGGAA
AGCATCCTGTTTCTTGCAAGGACACTCCTGGGTTTATTG
TGAACCGCCTCCTGGTTCCATACCTCATGGAAGCAATC
AGGCTGTATGAACGAGACTTCCAAACGTGTGGTGATTC
TAACTCGGGTTTGGGCTTTTCTTTAAAAGGTGACGCATC
CAAAGAAGACATTGACACTGCTATGAAATTAGGAGCCG
GTTACCCCATGGGCCCATTTGAGCTTCTAGATTATGTCG
GACTGGATACTACGAAGTTCATCGTGGATGGGTGGCAT
GAAATGGATGCAGAGAACCCATTACATCAGCCCAGCCC
ATCCTTAAATAAGCTGGTAGCAGAGAACAAGTTCGGCA
AGAAGACTGGAGAAGGATTTTACAAATACAAGTGATGT
GCAGCTTCTCCGGCTCTGAGAAGAACACCTGAGAGCGC
TTTCCAGCCAGTGCCCCGAGTGCCTGTGGGAATGCTCTT
TGGTCAGACATTCCCTCACACAGTACAGTTTAATAAAT
GTGCATTTTGATTGTAATCTATCGAAGTGATTATTACAC
CAGTTACAGCAGTAATAGATTCTCCATTAAGAAATAAT
TCCCTTTTTTAGTCTGTTCATTTCTGTGTATTTTCTAAAC
AGCTTTACACCCTTGGTGCCTTGGAGCAAACATGTTTTT
TGAACCTTGTCATTTTTGTGAAGAATTGCCTAGATTCCT
TCTCTCATCAACGGGAAAGTACTTCCTCTGAGAGTGCG
AGTGCACCATGCTCACTGTTGCTGCGTGGGAGAGTCAC
AAGCCACTGGCAAGCAAGTGGTATAGTCTGTGAAGCAC
TGCAGCGAGCAGCACCTGGATCTTGCCTTTATAAGAAC
ATTTTACTACCTGCAGCTTTGAGTCTTGCCCTACATTTT
GGGCATGACATAAGATGTGTCTTTATTCAGCTCGTCGT
GAAGATGCTGCTGCTGAATGGGTCAGCATATCTCTGTT
TGCATGGTTTGCAGGAGGTCGGTTTTCATGGTCATTCAG
TTCCACAGATCTGAATGATTACTGTCTGTCTGTGTCTTT
TTTCCATGAGAAATCACTGTTGCAAATTGCCTATAAATT
GACTCTACTAAAATAACAATGTTTCAGTCTGAAAATTT
GAATTGAAAAAAATGTATAATATAAAATTGTAATACAC
TCAAATGATTATAAAAGTAAAAGTTGGTAATTTAGGCA
GAAGCTAAAAA
18 ACAA2 NM 00611 AGCGTCCCCCACACCACAGACCCGCGCCGCCGACGACC
1.3 CAGCAGCCGCCATGGCTCTGCTCCGAGGTGTGTTTGTA
GTTGCTGCTAAGCGAACGCCCTTTGGAGCTTACGGAGG
CCTTCTGAAAGACTTCACTGCTACTGACTTGTCTGAATT
TGCTGCCAAGGCTGCCTTGTCTGCTGGCAAAGTCTCAC
CTGAAACAGTTGACAGTGTGATTATGGGCAATGTCCTG
CAGAGTTCTTCAGATGCTATATATTTGGCAAGGCATGTT
GGTTTGCGTGTGGGAATCCCAAAGGAGACCCCAGCTCT
CACGATTAATAGGCTCTGTGGTTCTGGTTTTCAGTCCAT
TGTGAATGGATGTCAGGAAATTTGTGTTAAAGAAGCTG
AAGTTGTTTTATGTGGAGGAACCGAAAGCATGAGCCAA
- 79 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
GCTCCCTACTGTGTCAGAAATGTGCGTTTTGGAACCAA
GCTTGGATCAGATATCAAGCTGGAAGATTCTTTATGGG
TATCATTAACAGATCAGCATGTCCAGCTCCCCATGGCA
ATGACTGCAGAGAATCTTGCTGTAAAACACAAAATAAG
CAGAGAAGAATGTGACAAATATGCCCTGCAGTCACAGC
AGAGATGGAAAGCTGCTAATGATGCTGGCTACTTTAAT
GATGAAATGGCACCAATTGAAGTGAAGACAAAGAAAG
GAAAACAGACAATGCAGGTAGACGAGCATGCTCGGCC
CCAAACCACCCTGGAACAGTTACAGAAACTTCCTCCAG
TATTCAAGAAAGATGGAACTGTTACTGCAGGGAATGCA
TCGGGTGTAGCTGATGGTGCTGGAGCTGTTATCATAGC
TAGTGAAGATGCTGTTAAGAAACATAACTTCACACCAC
TGGCAAGAATTGTGGGCTACTTTGTATCTGGATGTGAT
CCCTCTATCATGGGTATTGGTCCTGTCCCTGCTATCAGT
GGGGCACTGAAGAAAGCAGGACTGAGTCTTAAGGACA
TGGATTTGGTAGAGGTGAATGAAGCTTTTGCTCCCCAG
TACTTGGCTGTTGAGAGGAGTTTGGATCTTGACATAAG
TAAAACCAATGTGAATGGAGGAGCCATTGCTTTGGGTC
ACCCACTGGGAGGATCTGGATCAAGAATTACTGCACAC
CTGGTTCACGAATTAAGGCGTCGAGGTGGAAAATATGC
CGTTGGATCAGCTTGCATTGGAGGTGGCCAAGGTATTG
CTGTCATCATTCAGAGCACAGCCTGAAGAGACCAGTGA
GCTCACTGTGACCCATCCTTACTCTACTTGGCCAGGCCA
CAGTAAAACAAGTGACCTTCAGAGCAGCTGCCACAACT
GGCCATGCCCTGCCATTGAAACAGTGATTAAGTTTGAT
CAAGCCATGGTGACACAAAAATGCATTGATCATGAATA
GGAGCCCATGCTAGAAGTACATTCTCTCAGATTTGAAC
CAGTGAAATATGATGTATTTCTGAGCTAAAACTCAACT
ATAGAAGACATTAAAAGAAATCGTATTCTTGCCAAGTA
ACCACCACTTCTGCCTTAGATAATATGATTATAAGGAA
ATCAAATAAATGTTGCCTTAACTTCAGTTAATATTTTCC
TGTCATTTATATTTTTAAAAATTTTAAATTGTGATAAGA
TACACATTACATAAACTTTACCATCTTAACCCTTTTTTA
GCGTACAATTCACTGGTATTAAGTACATTCACATTTTTA
TACAAACATCCCCACTTTTTATCAACAGAACTTTTTCAG
TCACCACACATGGAAACAATAACTCCTGATTCTCCCAT
CCCCCATCCCCTGACAACCACCAGTGTATTTTGTTTCTA
TAAATTTGATGACTCGAGGTACCTCATAAGTGAAATTA
TAAATATCTGTCCTTTCGTGACTGGCTTATTTTACTTTA
CTTTATATAATGTTCTCAAGATTCATCCACCTTATGGTG
TAGCATGTGTCAGAATTTCCTTCTTTTTAAAGGCTGAAT
AATATTCTGCTGTGTGTATAAACCTTACTTCCTTCTTCC
CAGCTTAAAGGCCATCTTTCATCCTTTATTTTCTCCCTTT
AAAATGCCCCCACAACACTTCCATTGCTTTATTTGTCTG
TTCTAAGACTGGATATCTAGTAGGGCAAGGCCCTATTC
TTGTTAACTTCATCAAAGAGCCACTGGAAATTTTAATTA
AGATTAAATTGAATTTATGGGTTATACATTTATTGGGG
GGAAATTTTTTTTTTTTTTTTTGAGACAGAGTCTCGCTCT
GTCCTCCAGGCTGGAGTGCAGTGGCGCGATCTCAGCTT
ACTGCAAGCTCCGCCTCCTGGGTTCATGCCATTCTCCTG
CCTCAGCCTCCCCAGTAGCTGGGACTACAGGCGCCTGC
CACTACGCCCGGCTAATTTTTTGTATTTTTAGTAGAGAT
GGGGTTTCACCGTGTTAGCCAGGATGGTCTCGATCTCCT
GACCTCGTGATCCACCCGCCTCGGCCTCCCAAAGAAGT
GCTGGGATTACAGGCGTGAGCCACTGCACCCGGCCTTT
TTTTTTTTTTTTTGAGATAGCATCTTGCTCTGTCACCCAG
- 80 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
GCAGAATTGCAGTGGCACAGTCATGGCTCACTGAATAA
TAGATGTTAAATAATACTAGATGTTAAATAATAGTATC
ATAAGTACCTACACTGTTTCCTCAACCCTTTGCTTATAT
GGTTTCCTTCATTTGATTAAAAAGCTGAAGTGGCACAT
ACATCCCCCTTTCTGTCATAGAGAGGCAGATGACAAGC
GGCCTACCCACGGTTTGGGATAATGGACTAGTGGCAAC
AGGCAAGTCCAGCTTTTATTGTTTGGGATCCTTACTGAG
AAGCAGCAGGCTTCCTCTACTGTCATAAAAATATTAAA
AAGTAAGAGCCCTGTATAATTTCTCATAATAAAACAAT
GTTTTGCAGAACA
19 ACAT1 NM 00001 GGCCGCTAGGGGTGCGGGGTTGGGGAGGAGGCCGCTA
9.3 GTCTACGCCTGTGGAGCCGATACTCAGCCCTCTGCGAC
CATGGCTGTGCTGGCGGCACTTCTGCGCAGCGGCGCCC
GCAGCCGCAGCCCCCTGCTCCGGAGGCTGGTGCAGGAA
ATAAGATATGTGGAACGGAGTTATGTATCAAAACCCAC
TTTGAAGGAAGTGGTCATAGTAAGTGCTACAAGAACAC
CCATTGGATCTTTTTTAGGCAGCCTTTCCTTGCTGCCAG
CCACTAAGCTTGGTTCCATTGCAATTCAGGGAGCCATT
GAAAAGGCAGGGATTCCAAAAGAAGAAGTGAAAGAAG
CATACATGGGTAATGTTCTACAAGGAGGTGAAGGACAA
GCTCCTACAAGGCAGGCAGTATTGGGTGCAGGCTTACC
TATTTCTACTCCATGTACCACCATAAACAAAGTTTGTGC
TTCAGGAATGAAAGCCATCATGATGGCCTCTCAAAGTC
TTATGTGTGGACATCAGGATGTGATGGTGGCAGGTGGG
ATGGAGAGCATGTCCAATGTTCCATATGTAATGAACAG
AGGATCAACACCATATGGTGGGGTAAAGCTTGAAGATT
TGATTGTAAAAGACGGGCTAACTGATGTCTACAATAAA
ATTCATATGGGCAGCTGTGCTGAGAATACAGCAAAGAA
GCTGAATATTGCACGAAATGAACAGGACGCTTATGCTA
TTAATTCTTATACCAGAAGTAAAGCAGCATGGGAAGCT
GGGAAATTTGGAAATGAAGTTATTCCTGTCACAGTTAC
AGTAAAAGGTCAACCAGATGTAGTGGTGAAAGAAGAT
GAAGAATATAAACGTGTTGATTTTAGCAAAGTTCCAAA
GCTGAAGACAGTTTTCCAGAAAGAAAATGGCACAGTA
ACAGCTGCCAATGCCAGTACACTGAATGATGGAGCAGC
TGCTCTGGTTCTCATGACGGCAGATGCAGCGAAGAGGC
TCAATGTTACACCACTGGCAAGAATAGTAGCATTTGCT
GACGCTGCTGTAGAACCTATTGATTTTCCAATTGCTCCT
GTATATGCTGCATCTATGGTTCTTAAAGATGTGGGATTG
AAAAAAGAAGATATTGCAATGTGGGAAGTAAATGAAG
CCTTTAGTCTGGTTGTACTAGCAAACATTAAAATGTTGG
AGATTGATCCCCAAAAAGTGAATATCAATGGAGGAGCT
GTTTCTCTGGGACATCCAATTGGGATGTCTGGAGCCAG
GATTGTTGGTCATTTGACTCATGCCTTGAAGCAAGGAG
AATACGGTCTTGCCAGTATTTGCAATGGAGGAGGAGGT
GCTTCTGCCATGCTAATTCAGAAGCTGTAGACAACCTC
TGCTATTTAAGGAGACAACCCTATGTGACCAGAAGGCC
TGCTGTAATCAGTGTGACTACTGTGGGTCAGCTTATATT
CAGATAAGCTGTTTCATTTTTTATTATTTTCTATGTTAAC
TTTTAAAAATCAAAATGATGAAATCCCAAAACATTTTG
AAATTAAAAATAAATTTCTTCTTCTGCTTTTTTCTTGGT
AACCTTGAAAAGTTTGATACATTTTTGCATTCTGAGTCT
ATACTTATCGAAATATGGTAGAAATACCAATGTGTAAT
ATTAGTGACTTACATAAGTAGCTAGAAGTTTCCATTTGT
GAGAACACATTTATATITTTGAGGATTGTTAAAGGTCA
AGTGAATGCTCTTTATAGGTAATTTACATTTAGTAAATT
- 81 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
ACGGTAAATTAAATTACTTCTCTTTACAGTAAGAGTTG
GCTATTCTGGACAAACTAGCAGTGCTTCATATAATCAC
TCAAACCACAGTGTGTGCAGCAGTACTAGAAACAAGAC
AGAAGCCCATGTCCTCAGGGTCTAGAGTGGGGGCAATT
TCTTATAACCTCAACATTCAGGGTTGGGGGAGGTCAAG
CAGAAAACCCTGGAGTTTGGGCTCTGAATTACTATAGC
AGCATAGAGAGTGGGAAGGGAGGTAGAAACTGATATG
CTGAATGGATATATAAAAAAGGGAACAGATCACCACTT
CCAATACACGACAATGCCTGTTCTTAAGCAGGACAGAC
TGTAACAGAAGTATCTCGCATTGCATTTTATCTGGGAA
AAAAAAAAAAAAAAA
20 ACADL NM 00160 GTATTCCCTCGCGACCAGCCTGTGGCGTGGTTGGGGCT
8.4 CCGGAAGGGCGCGCGCGAGCGCTTTTTTGGGAGGACAC
CACAGGTGGACGCCTCAGCTGATCGTCCTCCCTCCCGG
GGACCCTGCCCCGAGTCGCCGAGTAGCCGCAGAGTCGC
CTCCGTCGCCCCGCCGCCCCTGTGTTTCGGACATGGCCG
CACGCCTTCTCCGAGGGTCCCTACGCGTCCTGGGCGGC
CACCGTGCGCCGCGCCAGCTGCCCGCCGCGCGATGTTC
TCATTCCGGAGGGGAAGAACGTCTAGAAACTCCTTCTG
CTAAAAAATTAACAGATATAGGAATTCGAAGAATCTTT
TCTCCAGAGCATGACATTTTCCGGAAAAGTGTAAGGAA
GTTTTTCCAAGAAGAAGTGATTCCTCATCACTCAGAAT
GGGAGAAAGCTGGAGAAGTAAGTAGGGAGGTTTGGGA
AAAAGCTGGAAAACAAGGACTGCTTGGTGTCAATATTG
CAGAGCATCTTGGTGGAATTGGAGGGGATCTGTACTCC
GCAGCTATTGTCTGGGAGGAGCAAGCTTATTCAAATTG
TTCAGGCCCAGGTTTTAGTATTCATTCAGGTATTGTCAT
GTCCTATATTACAAACCATGGCTCAGAAGAACAGATTA
AGCACTTTATTCCCCAGATGACTGCAGGCAAATGTATT
GGTGCAATAGCAATGACAGAGCCTGGAGCTGGAAGTG
ACTTACAGGGAATAAAAACAAATGCTAAAAAGGATGG
AAGTGACTGGATTCTCAATGGAAGCAAGGTGTTCATCA
GTAATGGGTCATTAAGTGATGTTGTGATTGTAGTTGCG
GTCACAAATCATGAAGCTCCCTCCCCTGCCCATGGTATT
AGCCTTTTTCTGGTGGAAAATGGAATGAAAGGATTTAT
CAAGGGACGAAAGCTACATAAAATGGGATTAAAAGCC
CAGGATACCGCAGAACTATTCTTTGAAGATATACGGTT
GCCAGCTAGTGCCCTACTTGGAGAAGAGAATAAAGGCT
TCTATTACATCATGAAAGAGCTTCCACAGGAAAGGCTG
TTAATTGCTGATGTGGCAATTTCAGCTAGTGAATTCATG
TTTGAAGAAACCAGGAACTATGTTAAACAAAGAAAAG
CTTTTGGCAAAACAGTTGCTCACCTACAGACAGTGCAA
CATAAATTAGCAGAATTAAAAACACATATATGTGTAAC
CCGAGCATTTGTGGACAACTGTCTCCAGCTGCATGAAG
CGAAACGTTTGGACTCCGCCACTGCTTGCATGGCGAAA
TATTGGGCATCTGAGTTACAAAATAGTGTAGCTTACGA
CTGTGTACAGCTCCATGGAGGTTGGGGATACATGTGGG
AGTACCCAATTGCAAAAGCTTATGTGGATGCCAGAGTT
CAGCCAATCTATGGTGGTACAAATGAAATAATGAAGGA
GCTGATTGCAAGAGAGATTGTCTTTGACAAGTAGACAT
CTGCCCACATCCTGGAGTCCTATTACAGCTAATCTCGTT
TTAAATCTGCTCAAGATAAAATGTAACTTGGAAAGCGA
GGAAACACTAAACATGTTTTTACCTGCTCTCTCTATAGA
GAAGGAAATAAAATATAAATATAAGATTAACACAGTG
GAAGGACAAATCTTTGAAGCCAAAATTCTAGTTTTCCA
ATATAAGGTTTAACTTACAGTTTTTTATGTAGCCAAAGG
- 82 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
TAAACGGTTTTCTGAATCTTGCCTAGGTGTTTCATTTAT
CTCTAAAATTCTAAAAAGCATAAATCATTCAAATCTTC
AAACCAAGGCAGAAATAATTTTATGTCGCTATAGTATA
AAAACATTAATAAGATAGCACATTGACTTTTAAAGGGA
AAAGTAAATATAACTTAGCATGTAAACTCATTTCGGCT
ACCATTTGCTCCAAATTCCCTAGAACAGTGGTTTTTACC
ACTGTACTCCAACCCCGTITTTAAGCAATGGAACTCTTT
CTTCAAACAAAAGCTTATGCAGAACATCTCTGTGAAAC
GCTGCTGAGTGAGAACTGCTTTCATTGAAGCTGGAAGC
CATCATACCTTACTGCCTTGAAACCCCTAGGACTCAGCT
AAGTATTTGCCTAACCCTGACCAGGGAATGCCTTGGTT
CTGTCAATTGCTGACATCTGAGAACACAGAATAATCCA
TCATTTTTAATTTCAAGATATTGGTACATTTTATAGGTA
TCAAAGCAATGGCTTTTCTTTTGCAACAGTTAATGTATT
TATTAACTTAATAATTACTTTATGTCTTCTATAAACCAG
GCTGTTAATACAATGATGACAAACAAAACTGGCAAGAT
CACTAAAAAATAAGTGAATAAACAAATAAGTAGTAAA
ATAAGGTAAGAAGTAAATATGTAAAAGAGATAATTTCA
AGCATAAGTGCAATGTAAATAATAAAGTAAGCATTTAA
AATTCAAAAGTGAGGAAATGACATTTGATTTAAGACTT
AAAAGTAATTACAAAAAATAAACCATTAATTTAAAGTA
21 ACAD9 NM 01404 ATCAGACGTGTGTGTGTCCCTGCGGCGCTAAGAAGGGG
9.5 AGACTGAGGCTGAGGCTGGGGAACATCGGGCAGCATG
AGCGGCTGCGGGCTCTTCCTGCGCACCACGGCTGCGGC
TCGTGCCTGCCGGGGTCTGGTGGTCTCTACCGCGAACC
GGCGGCTACTGCGCACCAGCCCGCCTGTACGAGCTTTC
GCCAAAGAGCTTTTCCTAGGCAAAATCAAGAAGAAAG
AAGTTTTCCCATTTCCAGAAGTTAGCCAAGATGAACTT
AATGAAATCAATCAGTTCTTGGGACCCGTGGAAAAATT
CTTCACTGAAGAGGTGGACTCCCGAAAAATTGACCAGG
AAGGGAAAATCCCAGATGAAACTTTGGAGAAATTGAA
GAGCCTAGGGCTTTTTGGGCTGCAAGTCCCAGAAGAAT
ATGGTGGCCTGGGCTTCTCCAACACCATGTACTCAAGA
CTAGGGGAGATCATCAGCATGGATGGGTCCATCACTGT
GACCCTGGCAGCGCACCAGGCTATTGGCCTCAAGGGGA
TCATCTTGGCTGGCACTGAGGAGCAGAAAGCCAAATAC
TTGCCTAAACTGGCGTCCGGGGAGCACATTGCAGCCTT
CTGCCTCACGGAGCCAGCCAGTGGGAGCGATGCAGCCT
CAATCCGGAGCAGAGCCACACTAAGTGAAGACAAGAA
GCACTACATCCTCAATGGCTCCAAGGTCTGGATTACTA
ATGGAGGACTGGCCAATATTTTTACTGTGTTTGCAAAG
ACTGAGGTCGTTGATTCTGATGGATCAGTGAAAGACAA
AATCACAGCATTCATAGTAGAAAGAGACTTTGGTGGAG
TCACTAATGGGAAACCCGAAGATAAATTAGGCATTCGG
GGCTCCAACACTTGTGAAGTCCATTTTGAAAACACCAA
GATACCTGTGGAAAACATCCTTGGAGAGGTCGGAGATG
GGTTTAAGGTGGCCATGAACATCCTCAACAGCGGCCGG
TTCAGCATGGGCAGCGTCGTGGCTGGGCTGCTCAAGAG
ATTGATTGAAATGACTGCTGAGTACGCCTGCACAAGGA
AACAGTTTAACAAGAGGCTCAGTGAATTTGGATTGATT
CAGGAGAAATTTGCACTGATGGCTCAGAAGGCTTACGT
CATGGAGAGTATGACCTACCTCACAGCAGGGATGCTGG
ACCAACCTGGCTTTCCCGACTGCTCCATCGAGGCAGCC
ATGGTGAAGGTGTTCAGCTCCGAGGCCGCCTGGCAGTG
TGTGAGTGAGGCGCTGCAGATCCTCGGGGGCTTGGGCT
ACACAAGGGACTATCCGTACGAGCGCATACTGCGTGAC
- 83 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
ACCCGCATCCTCCTCATCTTCGAGGGAACCAATGAGAT
TCTCCGGATGTACATCGCCCTGACGGGTCTGCAGCATG
CCGGCCGCATCCTGACTACCAGGATCCATGAGCTTAAA
CAGGCCAAAGTGAGCACAGTCATGGATACCGTTGGCCG
GAGGCTTCGGGACTCCCTGGGCCGAACTGTGGACCTGG
GGCTGACAGGCAACCATGGAGTTGTGCACCCCAGTCTT
GCGGACAGTGCCAACAAGTTTGAGGAGAACACCTACTG
CTTCGGCCGGACCGTGGAGACACTGCTGCTCCGCTTTG
GCAAGACCATCATGGAGGAGCAGCTGGTACTGAAGCG
GGTGGCCAACATCCTCATCAACCTGTATGGCATGACGG
CCGTGCTGTCGCGGGCCAGCCGCTCCATCCGCATTGGG
CTCCGCAACCACGACCACGAGGTTCTCTTGGCCAACAC
CTTCTGCGTGGAAGCTTACTTGCAGAATCTCTTCAGCCT
CTCTCAGCTGGACAAGTATGCTCCAGAAAACCTAGATG
AGCAGATTAAGAAAGTGTCCCAGCAGATCCTTGAGAAG
CGAGCCTATATCTGTGCCCACCCTCTGGACAGGACATG
CTGAGGCAGGGGACAGTGTCCCCTGCTACCGCCCGCCC
CTACCCATGGCCCGTTGCTGGATGACTGTTACTCTTTTT
TCAGAAGGTGTTGGGATTATCACAGGTTAAGCCTTTTG
TTCCCCGTCTGCACCTGAAGGGTTGTCGCCTGGCCTGG
GAGAGCCTCTTCCAGGTTTTGACCTGCAGGCAGTGCTC
TCTAACAGGACCATCACAGCTTCTGAACTGAGCCGGAG
AGAGAGAATGGAATTGCTGACCCCTGGAACTGGCGGGT
ATTCTGGTCATTGAGGAGACACCATAGTGGAAACTGGG
GCTTATGCTGCTGCCTCCAGGGTGTGAGGTGGGTGGGG
ACCTGTGTCAGGTGTGGATAGCCATTTCTGCTCAACCA
CACATTCTCTAAGAAACAGCTTGAAAGCTCTGTCTGGG
TCATTCATTTAAACTAGAAGCAGAGGCACTTAAAACAT
GTACCAGGAACCATTTAACAAAGAATATAAAATGTCAC
AATCTGTGTACTGTTA
Table 4. Fatty Acid Oxidation Cycle Proteins
SEQ Protein Accession Amino Acid Sequence
ID Number
NO
22 Very long P49748 MQAARMAASLGRQLLRLGGGS SRLTALLGQPRPGPARRP
chain acyl-CoA YAGGAAQLALDK SD SHP S DALTRKKPAKAE SKSFAVGMF
dehydrogenase KGQLTTDQVFPYPSVLNEEQTQFLKELVEPVSRFFEEVND
(VL CAD) PAKNDALEMVEETTWQGLKELGAFGLQVPSELGGVGLC
NTQYARLVEIVGMHDLGVGITLGAHQ SIGFKGILLFGTKA
QKEKYLPKLASGETVAAFCLTEPS SGSDAASIRTSAVP SP C
GKYYTLNGSKLWISNGGLADIFTVFAKTPVTDPATGAVK
EKITAFVVERGFGGITHGPPEKKMGIKASNTAEVFFDGVR
VP SENVLGEVGSGFKVAMHILNNGRFGMAAALAGTMRGI
IAKAVDHATNRTQFGEKIHNFGLIQEKLARMVMLQYVTE
SMAYMVSANMDQGATDFQIEAAISKIFGSEAAWKVTDEC
IQIMGGMGFMKEPGVERVLRDLRIFRIFEGTNDILRLFVAL
QGCMDKGKELSGLGSALKNPFGNAGLLLGEAGKQLRRR
AGLGSGLSLSGLVHPELSRSGELAVRALEQFATVVEAKLI
KHKKGIVNE QFLLQRLADGAIDLYAMVVVL SRA SRS L SE
GHPTAQHEKMLCDTWCIEAAARIREGMAALQ SDPWQQE
LYRNFKSISKALVERGGVVTSNPLGF
23 Medium-chain P11310 MAAGFGRCCRVLRSISRFHWRSQHTKANRQREPGLGFSF
acyl-CoA EFTEQQKEFQATARKFAREEIIPVAAEYDKTGEYPVPLIRR
- 84 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
dehydrogenase AWELGLMNTHIPENCGGLGLGTFDACLISEELAYGCTGV
(MCAD) QTAIEGNSLGQMPIIIAGNDQQKKKYLGRMTEEPLMCAYC
VTEPGAGSDVAGIKTKAEKKGDEYIINGQKMWITNGGKA
NWYFLLARSDPDPKAPANKAFTGFIVEADTPGIQIGRKEL
NMGQRCSDTRGIVFEDVKVPKENVLIGDGAGFKVAMGAF
DKTRPVVAAGAVGLAQRALDEATKYALERKTFGKLLVE
HQAISFMLAEMAMKVELARMSYQRAAWEVDSGRRNTY
YASIAKAFAGDIANQLATDAVQILGGNGFNTEYPVEKLM
RDAKIYQIYEGTSQIQRLIVAREHIDKYKN
24 Short-chain P16219 MAAALLARASGPARRALCPRAWRQLHTIYQSVELPETHQ
acyl-CoA MLLQTCRDFAEKELFPIAAQVDKEHLFPAAQVKKMGGLG
dehydrogenase LLAMDVPEELGGAGLDYLAYAIAMEEISRGCASTGVIMS
(SCAD) VNNSLYLGPILKFGSKEQKQAWVTPFTSGDKIGCFALSEP
GNGSDAGAASTTARAEGDSWVLNGTKAWITNAWEASAA
VVFASTDRALQNKGISAFLVPMPTPGLTLGKKEDKLGIRG
SSTANLIFEDCRIPKDSILGEPGMGFKIAMQTLDMGRIGIAS
QALGIAQTALDCAVNYAENRMAFGAPLTKLQVIQFKLAD
MALALESARLLTWRAAMLKDNKKPFIKEAAMAKLAASE
AATAISHQAIQILGGMGYVTEMPAERHYRDARITEIYEGT
SEIQRLVIAGHLLRSYRS
25 Mitochondrial P40939 MVACRAIGILSRFSAFRILRSRGYICRNFTGSSALLTRTHIN
trifunctional YGVKGDVAVVRINSPNSKVNTLSKELHSEFSEVMNEIWA
protein, alpha SDQIRSAVLISSKPGCFIAGADINMLAACKTLQEVTQLSQE
subunit AQRIVEKLEKSTKPIVAAINGSCLGGGLEVAISCQYRIATK
(MTPa) DRKTVLGTPEVLLGALPGAGGTQRLPKMVGVPAALDMM
LTGRSIRADRAKKMGLVDQLVEPLGPGLKPPEERTIEYLE
EVAITFAKGLADKKISPKRDKGLVEKLTAYAMTIPFVRQQ
VYKKVEEKVRKQTKGLYPAPLKIIDVVKTGIEQGSDAGYL
CESQKFGELVMTKESKALMGLYHGQVLCKKNKFGAPQK
DVKHLAILGAGLMGAGIAQVSVDKGLKTILKDATLTALD
RGQQQVFKGLNDKVKKKALTSFERDSIFSNLTGQLDYQG
FEKADMVIEAVFEDLSLKHRVLKEVEAVIPDHCIFASNTS
ALPISEIAAVSKRPEKVIGMHYFSPVDKMQLLEIITTEKTSK
DTSASAVAVGLKQGKVIIVVKDGPGFYTTRCLAPMMSEVI
RILQEGVDPKKLDSLTTSFGFPVGAATLVDEVGVDVAKH
VAEDLGKVFGERFGGGNPELLTQMVSKGFLGRKSGKGFY
IYQEGVKRKDLNSDMDSILASLKLPPKSEVSSDEDIQFRLV
TRFVNEAVMCLQEGILATPAEGDIGAVFGLGFPPCLGGPF
RFVDLYGAQKIVDRLKKYEAAYGKQFTPCQLLADHANSP
NKKFYQ
26 Mitochondrial P55084 MTILTYPFKNLPTASKWALRFSIRPLSCSSQLRAAPAVQTK
trifunctional TKKTLAKPNIRNVVVVDGVRTPFLLSGTSYKDLMPHDLA
protein, beta RAALTGLLHRTSVPKEVVDYIIFGTVIQEVKTSNVAREAA
subunit LGAGFSDKTPAHTVTMACISANQAMTTGVGLIASGQCDV
(MTP(3) IVAGGVELMSDVPIRHSRKMRKLMLDLNKAKSMGQRLSL
ISKFRFNFLAPELPAVSEFSTSETMGHSADRLAAAFAVSRL
EQDEYALRSHSLAKKAQDEGLLSDVVPFKVPGKDTVTKD
NGIRPSSLEQMAKLKPAFIKPYGTVTAANSSFLTDGASAM
LIMAEEKALAMGYKPKAYLRDFMYVSQDPKDQLLLGPT
YATPKVLEKAGLTMNDIDAFEFHEAFSGQILANFKAMDS
DWFAENYMGRKTKVGLPPLEKFNNWGGSLSLGHPFGAT
GCRLVMAAANRLRKEGGQYGLVAACAAGGQGHAMIVE
AYPK
27 Short-chain P30084 MAALRVLLSCVRGPLRPPVRCPAWRPFASGANFEYIIAEK
enoyl-CoA RGKNNTVGLIQLNRPKALNALCDGLIDELNQALKTFEEDP
- 85 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
hydratase AVGAIVLTGGDKAFAAGAD IKEMQNL SF QD CY S SKFLKH
(Crotonase) WDHLTQVKKPVIAAVNGYAFGGGCELAMMCDIIYAGEK
AQFAQPEILIGTIPGAGGTQRLTRAVGKSLAMEMVLTGDR
I SAQDAKQAGLV S KICPVETLVEEAIQ CAEKIA SN S KIVVA
MAKES VNAAFEMTLTEGSKLEKKLFYSTFATDDRKEGMT
AFVEKRKANFKDQ
28 Short-chain Q16836 MAFVTRQFMRSV S S S STA SA SAKKIIVKHVTVIGGGLMGA
(S)-3- GIAQVAAATGHTVVLVDQTEDILAKSKKGIEESLRKVAK
hydroxyacyl- KKFAENLKAGDEFVEKTLSTIATSTDAASVVHSTDLVVEA
CoA IVENLKVKNELFKRLDKFAAEHTIFASNTS SLQITSIANATT
dehydrogenase RQDRFAGLHFFNPVPVMKLVEVIKTPMTSQKTFESLVDFS
(SCHAD) KALGKHPVS CKDTPGFIVNRLLVPYLMEAIRLYERGDASK
EDIDTAMKLGAGYPMGPFELLDYVGLDTTKFIVDGWHE
MDAENPLHQP SP SLNKLVAENKFGKKTGEGFYKYK
29 Medium-chain P42765 MALLRGVFVVAAKRTPFGAYGGLLKDFTATDLSEFAAKA
3-ketoacyl- AL SAGKV S PETVD SVIMGNVLQ S S SDAIYLARHVGLRVGI
CoA thiolase PKETPALTINRLCGSGFQSIVNGCQEICVKEAEVVLCGGTE
(MCKAT) SMS QAPYCVRNVRFGTKLGSDIKLED SLWV SLTDQHVQL
PMAMTAENLAVKHKISREECDKYALQ S QQRWKAANDAG
YFNDEMAPIEVKTKKGKQTMQVDEHARPQTTLEQLQKLP
PVFKKDGTVTAGNASGVADGAGAVIIASEDAVKKHNFTP
LARIVGYFV SGCDPSIMGIGPVPAISGALKKAGLSLKDMD
LVEVNEAFAPQYLAVERSLDLDISKTNVNGGAIALGHPLG
GSGSRITAHLVHELRRRGGKYAVGSACIGGGQGIAVIIQ ST
A
30 Acetoacetyl - P24752 MAVLAALLRSGARSRSPLLRRLVQEIRYVERSYVSKPTLK
CoA thiolase EVVIVSATRTPIGSFLGSLSLLPATKLGSIAIQGAIEKAGIPK
(T2) EEVKEAYMGNVLQGGEGQAPTRQAVLGAGLPISTPCTTIN
KVCASGMKAIMMAS Q SLMCGHQDVMVAGGMESMSNVP
YVMNRGSTPYGGVKLEDLIVKDGLTDVYNKIHMGSCAE
NTAKKLNIARNEQDAYAINSYTRSKAAWEAGKFGNEVIP
VTVTVKGQPDVVVKEDEEYKRVDFSKVPKLKTVFQKEN
GTVTAANASTLNDGAAALVLMTADAAKRLNVTPLARIV
AFADAAVEPIDFPIAPVYAASMVLKDVGLKKEDIAMWEV
NEAFSLVVLANIKMLEIDPQKVNINGGAV SLGHPIGMSGA
RIVGHLTHALKQGEYGLASICNGGGGASAMLIQKL
31 Long-chain P28330 MAARLLRGSLRVLGGHRAPRQLPAARC SHSGGEERLETP
acyl-CoA SAKKLTDIGIRRIFSPEHDIFRKSVRKFFQEEVIPHHSEWEK
dehydrogenase AGEVSREVWEKAGKQGLLGVNIAEHLGGIGGDLYSAAIV
(LCAD) WEEQAYSNCSGPGF SIHSGIVMSYITNHGSEEQIKHFIPQM
TAGKCIGAIAMTEPGAGSDLQGIKTNAKKDGSDWILNGS
KVFISNGSLSDVVIVVAVTNHEAP SPAHGISLFLVENGMK
GFIKGRKLIAKMGLKAQDTAELFFEDIRLPASALLGEENKG
FYYIMKELPQERLLIADVAISASEFMFEETRNYVKQRKAF
GKTVAHLQTVQHKLAELKTHICVTRAFVDNCLQLHEAKR
LD SATACMAKYWA SELQN SVAYD CV QLHGGWGYMWE
YPIAKAYVDARVQPIYGGTNEIMKELIAREIVFDK
32 Acyl-CoA Q9H845 SGCGLFLRTTAAARACRGLVV STANRRLLRTSPPVRAFAK
dehydrogenase ELFLGKIKKKEVFPFPEVSQDELNEINQFLGPVEKFFTEEV
9 (ACAD9) D SRKIDQEGKIPDETLEKLKSLGLFGLQVPEEYGGLGFSNT
MYSRLGEIISMDGSITVTLAAHQAIGLKGIILAGTEEQKAK
YLPKLA SGEHIAAF CLTEPA S GS DAA S IRS RATL SEDKKHY
ILNGSKVWITNGGLANIFTVFAKTEVVDSDGSVKDKITAFI
VERDFGGVTNGKPEDKLGIRGSNTCEVHFENTKIPVENIL
GEVGDGFKVAMNILNSGRFSMGSVVAGLLKRLIEMTAEY
- 86 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
ACTRKQFNKRLSEFGLIQEKFALMAQKAYVMESMTYLTA
GMLDQPGFPDCSIEAAMVKVF SSEAAWQ CV S EALQ ILGG
LGYTRDYPYERILRDTRILLIFEGTNEILRMYIALTGLQHA
GRILTTRIHELKQAKVSTVMDTVGRRLRDSLGRTVDLGLT
GNHGVVHP S LAD SANKFEENTYCFGRTVETLLLRFGKTIM
EEQLVLKRVANILINLYGMTAVL SRA SRSIRIGLRNHDHE
VLLANTFCVEAYLQNLFSLSQLDKYAPENLDEQIKKVS QQ
ILEKRAYICAHPLDRTC
Table 5. Auxiliary Enzyme Genes
SEQ Gene NCBI Nucleotide Sequence
ID Reference
NO Number
33 ECI1 NM_00117 AGCCCGCGACCTTTATCCCGCGCGTTGCGGTCAAGATGGCG
8029.1 CTGGTGGCTTCTGTGCGAGTCCCGGCGCGCGTTCTGCTCCGC
GCGGGGGCCCGGCTCCCGGGCGCGGCCCTCGGGCGGACGG
AGCGGGCGGCCGGCGGCGGAGACGGCGCGCGGCGCTTCGG
GAGCCAGCGGGTGCTGGTGGAGCCGGACGCGGGCGCAGGG
GTCGCTGTGATGAAATTCAAGAACC CC CCAGTGAACAGCCT
GAGCCTGGAGTTTCTGACGGAGCTGGTCATCAGCCTGGAGA
AGCTGGAGAATGACAAGAGCTTCCGCGGTGTCATTCTGACC
TCGGACCGCCCGGGTGTCTTCTCGGCCGGCCTGGACCTGAC
GGAGATGTGTGGGAGGAGCCCCGCCCACTACGCTGGGTACT
GGAAGGCCGTTCAGGAGCTGTGGCTGCGGTTGTACCAGTCC
AACCTGGTGCTGGTCTCCGCCATCAACGGAGCCTGCCCCGC
TGGAGGCTGCCTGGTGGCCCTGACCTGTGACTACCGCATCC
TGGCGGACAACCCCAGGTTGAAAGACACCCTGGAGAACAC
CATCGGGCACCGGGCGGCGGAGCGTGCCCTGCAGCTGGGG
CTGCTCTTCCCGCCGGCGGAGGCCCTGCAGGTGGGCATAGT
GGACCAGGTGGTCCCGGAGGAGCAGGTGCAGAGCACTGCG
CTGTCAGCGATAGCCCAGTGGATGGCCATTCCAGACCATGC
TCGACAGCTGACCAAGGCCATGATGCGAAAGGC CA CGGC C
AGCCGCCTGGTCACGCAGCGCGATGCGGACGTGCAGAACTT
CGTCAGCTTCATCTCCAAAGACTCCATCCAGAAGTCCCTGC
AGATGTACTTAGAGAGGCTCAAAGAAGAAAAAGGCTAACG
ATTGGGCTGCCACAGGCTTACGGCCACACGTGCCCCTGTGG
GTCCCAGGGAGGTCTTAAACAAGGTATTTTTCAACTTAAAA
GTACTGCCAGCGTTTCATTTTGCAAAAAAAAAAAAAAAAAA
34 ECI2 NM 00611 ACCCCCGAGCCCCCGCAGCCCTAGAGCCGCCCAAGGGATGG
7.2 CGATGGCGTACTTGGCTTGGAGACTGGCGCGGCGTTCGTGT
CCGAGGTCACTAGTTTCCCGGTAGTTCAGCTGCACATGAAT
AGAACAGCAATGAGAGCCAGTCAGAAGGACTTTGAAAATT
CAATGAATCAAGTGAAACTCTTGAAAAAGGATCCAGGAAA
CGAAGTGAAGCTAAAACTCTACGCGCTATATAAGCAGGC CA
CTGAAGGACCTTGTAACATGCCCAAACCAGGTGTATTTGAC
TTGATCAACAAGGCCAAATGGGACGCATGGAATGCCCTTGG
CAGCCTGCCCAAGGAAGCTGCCAGGCAGAACTATGTGGATT
TGGTGTCCAGTTTGAGTCCTTCATTGGAATCCTCTAGTCAGG
TGGAGCCTGGAACAGACAGGAAATCAACTGGGTTTGAAACT
CTGGTGGTGACCTCCGAAGATGGCATCACAAAGATCATGTT
CAACCGGCCCAAAAAGAAAAATGCCATAAACACTGAGATG
TATCATGAAATTATGCGTGCACTTAAAGCTGCCAGCAAGGA
TGACTCAATCATCACTGTTTTAACAGGAAATGGTGACTATT
ACAGTAGTGGGAATGATCTGACTAACTTCACTGATATTCCC
- 87 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
CCTGGTGGAGTAGAGGAGAAAGCTAAAAATAATGCCGTTTT
ACTGAGGGAATTTGTGGGCTGTTTTATAGATTTTCCTAAGCC
TCTGATTGCAGTGGTCAATGGTCCAGCTGTGGGCATCTCCGT
CACCCTCCTTGGGCTATTCGATGCCGTGTATGCATCTGACAG
GGCAACATTTCATACACCATTTAGTCACCTAGGCCAAAGTC
CGGAAGGATGCTCCTCTTACACTTTTCCGAAGATAATGAGC
CCAGCCAAGGCAACAGAGATGCTTATTTTTGGAAAGAAGTT
AACAGCGGGAGAGGCATGTGCTCAAGGACTTGTTACTGAAG
TTTTCCCTGATAGCACTTTTCAGAAAGAAGTCTGGACCAGG
CTGAAGGCATTTGCAAAGCTTCCCCCAAATGCCTTGAGAAT
TTCAAAAGAGGTAATCAGGAAAAGAGAGAGAGAAAAACTA
CACGCTGTTAATGCTGAAGAATGCAATGTCCTTCAGGGAAG
ATGGCTATCAGATGAATGCACAAATGCTGTGGTGAACTTCT
TATCCAGAAAATCAAAACTGTGATGACCACTACAGCAGAGT
AAAGCATGTCCAAGGAAGGATGTGCTGTTACCTCTGATTTC
CAGTACTGGAACTAAATAAGCTTCATTGTGCCTTTTGTAGTG
CTAGAATATCAATTACAATGATGATATTTCACTACAGCTCTG
ATGAATAAAAAGTTTTGTAAAACAAGCTTAAGAATTCAAAA
AAAAAAAAAAAAAA
35 DECR1 NM 00135 GTTCTGGAGACTCAACATGAAGCTACCGGCCAGGGTTTTCT
9.2 TTACTCTGGGGTCCCGGCTGCCCTGTGGCCTCGCTCCTCGGA
GGTTTTTCAGTTATGGGACAAAAATATTATATCAAAACACT
GAAGCTTTGCAATCTAAATTCTTTTCACCTCTTCAAAAAGCG
ATGCTACCACCTAATAGTTTTCAAGGAAAAGTGGCATTCAT
TACTGGGGGAGGTACTGGCCTTGGTAAAGGAATGACAACTC
TTCTGTCCAGCCTAGGTGCTCAGTGCGTGATAGCCAGCCGG
AAGATGGATGTTTTGAAAGCTACCGCAGAACAAATTTCTTC
TCAAACTGGAAATAAGGTTCATGCAATTCAGTGTGATGTGA
GGGATCCTGATATGGTTCAAAACACTGTGTCAGAACTGATC
AAAGTTGCAGGACATCCTAATATTGTGATAAACAATGCAGC
AGGGAATTTTATTTCTCCTACTGAAAGACTTTCTCCTAATGC
TTGGAAAACCATAACTGACATAGTTCTAAATGGCACAGCCT
TCGTGACACTAGAAATTGGAAAACAACTAATTAAAGCACAG
AAAGGAGCAGCATTTCTTTCTATTACTACTATCTATGCTGAG
ACTGGTTCAGGTTTTGTAGTACCAAGTGCTTCTGCCAAAGC
AGGTGTGGAAGCCATGAGCAAGTCTCTTGCAGCTGAATGGG
GTAAATATGGAATGCGATTCAATGTGATTCAACCAGGGCCT
ATAAAAACCAAAGGTGCCTTTAGCCGTCTGGACCCAACTGG
AACATTTGAGAAAGAAATGATTGGCAGAATTCCCTGTGGTC
GCCTGGGGACTGTAGAAGAACTCGCAAATCTTGCTGCTTTC
CTTTGTAGTGATTATGCTTCTTGGATTAATGGAGCAGTCATT
AAATTTGACGGTGGAGAGGAAGTACTTATTTCAGGGGAATT
CAACGACCTGAGAAAGGTCACCAAGGAGCAGTGGGACACC
ATAGAAGAACTCATCAGGAAGACAAAAGGTTCCTAAGACC
ACTTTGGCCTTCATCTTGGTTACAGAAAAGGGAATAGAAAT
GAAACAAATTATCTCTCATCTTTTGACTATTTCAAGTCTAAT
AAATTCTTAATTAACAAACATTCATTGAATATGTATTATGTG
CCAGGCCAGTGATAGCCATTGTATATTCAAAGATAAATAAA
ATGAAATATAGTCCTTCAAAACATTAAAAAAAAAAAAAGG
AGGCATGGGGAGAGTAGGTAAAGGCTCCTCTTTACCTATTG
ATAGAGGTAAAAAGTACTTAGAAGTGCAGAGAGAACAGAT
CTTTGTGACTTGGAAAATCAGGAGAAACTCAATGGTGGCGG
TAGCATTTGAGTTACATAATATACTATACCTATATTAATAGG
GCCTAAAAGAAAGAAATTAGAGGATACACACTAAATATAA
TAGACTTTGCCTTTCCAGTATACTTTCTTTTCACTGGACTTGT
GAATTATCTTCTTTGGGTAACTCAGTATTAACTCAAACCTTT
- 88 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
AATTTTTACTAGGACCTATTTGTAGCCAGGCATTTTATTTAG
TACTGAATAAGCTATAGCCGTTGCCCTTTTTAAATTCATTAT
CTAGCAAGATAGTCAAACTTATAAATAATTATTTATGATAC
ATTGTGATAAGTATTATTCCAGCAGTATTTAAGTGTAGAGG
AGGAAGTAATTCATTCTGTCTCCAGAGTTTGGAGAATGTGA
TGCCTAAGAGATAGCATGCCATCCCAGCTGTAAAAGAAGAA
TAGATTTCTCTGGGTAAAAGAGGTAAAGAAAGCCTATAAAA
TATTTTTGTATATCATTTGATTAAATTTCATCTTTGGTTTGAC
TAATTTGTCATCCTGAAAATCAAATAATAATGAATCCAAAG
TCTCAAGTCTACAGAGCTATACTTTTGAGCCTATATTTTTAA
AATGTCCATTTTGCTTTCCCAGGAGTCAGTTACAACATGTTC
ACTAGACTGACTATCCCCATTGCCCAAGTTGACACAAGAGG
AAACCAGCTTCCATCTTACCTCATCTGAATAAATCTGCCACA
AGCCCATGGAAACCCCAATTAACATTGACAGTTAATTGTGT
ACATAAATTACATTTATTACATTTAATTGTGTATATATAGGG
GATGTTATAGGTTTGGAATAAGTGGCCCAACATTTCCAATT
ATACTGACTTTCACTGGGCTTTTTTTTAGGCTGTTGCACTTTT
TCTCCACATGCTTGCAATACAATACTCTCAAAATAAAACGC
AGACAGGTACCTAGTCTCCATTTTACCTTTAGTACTAATCCT
GTGTATTAGTCTGTTCTCATGCTGCTAATAAAGACATAACCC
AAACTGGGTAATTTATAAAAGAAAGAGGTTTCATTGACTCA
TAGTTCAGCATGGCTAGGGAGGCCTCACAATCATGGCAGAA
GGTGAGTGAGGAGCAAAGTCATGTCTTACGTGGTGGCACCC
AAGAGAGCTTGTGCAGGGGAACTCCCATTTATAAAACCATC
AGATCTCGTGAGACTTATTCACTATCACACTATTGTGTTGAT
ATTGTGTTCACACACCAATAATGATGGTTTATCACTCACTCC
ATTTCCAAACCCACCTTCCCACCCACCTCTCACCAAACACAC
AAAGACACACTCTTTCCCTCCACTGATTCCACCAGTATAGCC
ATATTTCTCTTTCTGGTTAAATTTATACTAAATGTTTACATTT
ATATAACTTAATAAATATTATTTITTTCCA
36
ECH1 NM 00139 GCAGTAGACGAAGGCGGCGGCGATGGCGGCGGGGATAGTG
8.3
GCTTCTCGCAGACTCCGCGACCTACTGACCCGGCGACTGAC
AGGCTCCAACTACCCGGGACTCAGTATTAGCCTTCGCCTCA
CTGGCTCCTCTGCACAAGAGGAGGCTTCCGGAGTAGCCCTC
GGTGAAGCCCCAGACCACAGCTATGAGTCCCTTCGTGTGAC
GTCTGCGCAGAAACATGTTCTGCATGTCCAGCTCAACCGGC
CCAACAAGAGGAATGCCATGAACAAGGTCTTCTGGAGAGA
GATGGTAGAGTGCTTCAACAAGATTTCGAGAGACGCTGACT
GTCGGGCGGTGGTGATCTCTGGTGCAGGAAAAATGTTCACT
GCAGGTATTGACCTGATGGACATGGCTTCGGACATCCTGCA
GCCCAAAGGAGATGATGTGGCCCGGATCAGCTGGTACCTCC
GTGACATCATCACTCGATACCAGGAGACCTTCAACGTCATC
GAGAGGTGCCCCAAGCCCGTGATTGCTGCCGTCCATGGGGG
CTGCATTGGCGGAGGTGTGGACCTTGTCACCGCCTGTGACA
TCCGGTACTGTGCCCAGGATGCTTTCTTCCAGGTGAAGGAG
GTGGACGTGGGTTTGGCTGCCGATGTAGGAACACTGCAGCG
CCTGCCCAAGGTCATCGGGAACCAGAGCCTGGTCAACGAGC
TGGCCTTCACCGCCCGCAAGATGATGGCTGACGAGGCCCTG
GGCAGTGGGCTGGTCAGCCGGGTGTTCCCAGACAAAGAGGT
CATGCTGGATGCTGCCTTAGCGCTGGCGGCCGAGATTTCCA
GCAAGAGCCCCGTGGCGGTGCAGAGCACCAAGGTCAACCT
GCTGTATTCCCGCGACCATTCGGTGGCCGAGAGCCTCAACT
ACGTGGCGTCCTGGAACATGAGCATGCTGCAGACCCAAGAC
CTCGTGAAGTCGGTCCAGGCCACGACTGAGAACAAGGAACT
GAAAACCGTCACCTTCTCCAAGCTCTGAGAGCCCTCGCGTC
CCAGGCCCCAGCCAGGGGGCCGGCCTTGTCCCGCCTCATCC
- 89 -
CA 03127495 2021-07-21
WO 2020/163240 PCT/US2020/016430
ACAGAAAGGGAGGATGGGCGATGACAGTTGTTTCTATGCCT
TCTGACCCAGTTTCCCAGTTTATAACTTTATGACAATGAGTT
TCTCAAGCCCAAGGCCTTATCTTCACCCCACAAACAATAAA
GCAAAGTAAAGAA
Table 6. Auxiliary Enzymes
SEQ Protein Accession Amino Acid Sequence
ID Number
NO
37 43, A2- P42126 MALVASVRVPARVLLRAGARLPGAALGRTERAAGGGDGAR
Enoyl -CoA RFGS QRVLVEPDAGAGVAVMKFKNPPVNS L SLEFLTELVIS LE
isomerase KLENDKSFRGVILTSDRPGVFSAGLDLTEMCGRSPAHYAGYW
1 (DCI) KAVQELWLRLYQSNLVLVSAINGACPAGGCLVALTCDYRILA
DNPRYCIGLNETQLGIIAPFWLKDTLENTIGHRAAERALQLGL
LFPPAEALQVGIVDQVVPEEQVQSTALSAIAQWMAIPDHARQ
LTKAMMRKATASRLVTQRDADVQNFVSFISKDSIQKSLQMYL
ERLKEEKG
38 43, A2- 075521 MAMAYLAWRLARRS CP S SLQVTSFPVVQLEIMNRTAMRASQ
Enoyl -CoA KDFENSMNQVKLLKKDPGNEVKLKLYALYKQATEGPCNMP
isomerase KPGVFDLINKAKWDAWNALGSLPKEAARQNYVDLVS SL SP S
2 (PECI) LESS SQVEPGTDRKSTGFETLVVTSEDGITKIMFNRPKKKNAIN
TEMYHEIMRALKAASKDDSIITVLTGNGDYYSSGNDLTNFTDI
PPGGVEEKAKNNAVLLREFVGCFIDFPKPLIAVVNGPAVGISV
TLLGLFDAVYASDRATFHTPFSHLGQSPEGCS SYTFPKIM SPA
KATEMLIFGKKLTAGEACAQGLVTEVFPDSTFQKEVWTRLKA
FAKLPPNALRISKEVIRKREREKLHAVNAEECNVLQGRWL SD
ECTNAVVNFLSRKSKL
39 2,4- Q16698 MKLPARVFFTLGSRLPCGLAPRRFFSYGTKILYQNTEALQSKF
Dienoyl- FSPLQKAMLPPNSFQGKVAFITGGGTGLGKGMTTLLS SLGAQ
CoA CVIASRKMDVLKATAEQISSQTGNKVHAIQCDVRDPDMVQN
reductase TV S ELIKVAGHPNIVINNAAGNFISPTERL SPNAWKTITDIVLN
(DECR) GTAFVTLEIGKQLIKAQKGAAFLSITTIYAETGSGFVVP SA SAK
AGVEAMSKSLAAEWGKYGMRFNVIQPGPIKTKGAF SRLDPT
GTFEKEMIGRIPCGRLGTVEELANLAAFLCSDYASWINGAVIK
FDGGEEVLISGEFNDLRKVTKEQWDTIEELIRKTKGS
40 A3,5- Q13011 MAAGIVASRRLRDLLTRRLTGSNYPGLSISLRLTGSSAQEEAS
A2,4- GVALGEAPDHSYESLRVTSAQKHVLHVQLNRPNKRNAMNK
Di enoyl- VFWREMVECFNKISRDADCRAVVISGAGKMFTAGIDLMDMA
CoA SD ILQPKGDDVARISWYLRDIITRYQETFNVIERCPKPVIAAVH
GGCIGGGVDLVTACDIRYCAQDAFFQVKEVDVGLAADVGTL
isomerase
QRLPKVIGNQSLVNELAFTARKMMADEALGSGLVSRVFPDKE
(ECH 1)
VMLDAALALAAEISSKSPVAVQSTKVNLLYSRDHSVAESLNY
VA SWNM SMLQTQDLVK SV QATTENKELKTVTF SKL
- 90 -