Note: Descriptions are shown in the official language in which they were submitted.
1
DESCRIPTION
TITLE OF INVENTION: LOW-SULFUR COAL PRODUCTION METHOD
BACKGROUND OF THE INVENTION
[0001]
The present invention relates to a low-sulfur coal
production method.
BACKGROUND ART
[0002]
In an iron manufacturing process, when coal is used
as a reducing material for iron ore, a part of sulfur
contained in the coal dissolves as a solid in iron obtained
by reducing the iron ore. If sulfur remains, toughness and
workability of steel deteriorates, so that a great amount
of effort has been made to remove sulfur from iron.
When coal is used as a heat source, a sulfur oxide is
mixed in an exhaust gas, so that a great amount of effort
has been required to remove a sulfur content from an
exhaust gas from the standpoint of prevention of air
pollution.
[0003]
From such background, the industrial value is high if
sulfur (sulfur content) in coal can be removed before the
coal is used.
[0004]
CA 03134533 2021-10-21
2
As a method of producing coal having a reduced sulfur
content (low-sulfur coal), the claim of Patent Literature 1
describes "a chemical desulfurization method for coal,
characterized in that an aqueous solution of caustic soda
or caustic potash alone, or an aqueous solution of a
mixture thereof is mixed with pulverized coal, and the
resultant mixture is heated and reacted at a high
temperature under an atmosphere of an oxygen gas or air or
a mixture thereof, thereby removing a sulfur content in the
coal."
CITATION LIST
PATENT LITERATURES
[0005]
Patent Literature 1: JP 3-275795 A
SUMMARY OF INVENTION
TECHNICAL PROBLEMS
[0006]
In producing low-sulfur coal by desulfurizing coal
(removing sulfur in coal), the conventional method had an
insufficient desulfurization effect in some cases.
An object of the present invention is therefore to
provide a low-sulfur coal production method having an
excellent desulfurization effect.
SOLUTION TO PROBLEMS
CA 03134533 2021-10-21
3
[0007]
The present inventors have made an intensive study
and as a result found that when the configuration described
below is employed, the foregoing object is achieved. The
invention has been thus completed.
[0008]
Specifically, the present invention provides the
following [11 to [11].
[1] A low-sulfur coal production method comprising
bringing coal into contact with a chemical agent which is a
mixed solution of hydrogen peroxide and acetic anhydride to
thereby remove sulfur in the coal.
[2] The low-sulfur coal production method according
to [1] above, wherein a molar ratio between the acetic
anhydride and the hydrogen peroxide (acetic
anhydride/hydrogen peroxide) is not less than 0.5 and not
more than 12Ø
[3] The low-sulfur coal production method according
to [1] or [2] above, wherein the acetic anhydride and the
hydrogen peroxide are mixed before the chemical agent is
brought into contact with the coal, and
wherein when 10 minutes or more have elapsed after
the acetic anhydride and the hydrogen peroxide are mixed,
the chemical agent is brought into contact with the coal.
CA 03134533 2021-10-21
4
[4] The low-sulfur coal production method according
to any one of [11 to [3] above, wherein a mass ratio
between the chemical agent and the coal (chemical
agent/coal) is not less than 1Ø
[5] The low-sulfur coal production method according
to any one of [1] to [4] above, wherein a temperature of
the chemical agent at a time of being brought into contact
with the coal is not less than 5 C.
[6] The low-sulfur coal production method according
to any one of [1] to [5] above, wherein a temperature of
the chemical agent at a time of being brought into contact
with the coal is not more than 30 C.
[7] The low-sulfur coal production method according
to any one of [1] to [6] above, wherein the coal comprises
sub-bituminous coal.
[8] The low-sulfur coal production method according
to any one of [1] to [7] above, wherein the coal that has
been brought into contact with the chemical agent is heat-
treated at a heat treatment temperature of not less than
150 C.
[9] The low-sulfur coal production method according
to [8] above, wherein a heating rate at which the coal that
has been brought into contact with the chemical agent is
heated to the heat treatment temperature is not less than
CA 03134533 2021-10-21
S
C/min.
[10] The low-sulfur coal production method according
to any one of [1] to [7] above, wherein the coal that has
been brought into contact with the chemical agent is
brought into contact with a hydrogen peroxide solution
having a temperature of not more than 40 C.
[11] The low-sulfur coal production method according
to [10] above,
wherein a concentration of the hydrogen peroxide
solution is not less than 2.0 massst, and
wherein a mass ratio between the hydrogen peroxide
solution and the coal (hydrogen peroxide solution/coal) is
not less than 1Ø
ADVANTAGEOUS EFFECTS OF INVENTION
[0009]
The present invention can provide a low-sulfur coal
production method having an excellent desulfurization
effect.
BRIEF DESCRIPTION OF THE DRAWINGS
[0010]
[FIG. 1] FIG. 1 is a graph showing a desulfurization
rate with respect to a mass ratio between a chemical agent
and coal (chemical agent/coal).
[FIG. 2] FIG. 2 is a graph (lower part) showing an
CA 03134533 2021-10-21
6
amount of peracetic acid generated with respect to a
temperature of a chemical agent, and a graph (upper part)
showing a desulfurization rate (solid line) and a carbon
yield (dashed line) with respect to a temperature of a
chemical agent.
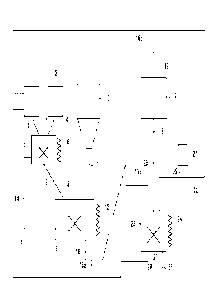
[FIG. 3] FIG. 3 is a schematic view showing an
example of a facility for producing low-sulfur coal.
DETAILED DESCRIPTION OF THE INVENTION
[0011]
[Low-sulfur Coal Production Method]
The low-sulfur coal production method of the
invention (hereinafter, also simply referred to as "the
method of the invention") is a low-sulfur coal production
method comprising bringing coal into contact with a
chemical agent which is a mixed solution of hydrogen
peroxide and acetic anhydride to thereby remove sulfur in
the coal.
[0012]
<Primary Treatment (Chemical Treatment)
First, described below is a primary treatment
(chemical treatment) in which coal is brought into contact
with a chemical agent which is a mixed solution of hydrogen
peroxide and acetic anhydride.
[0013]
CA 03134533 2021-10-21
7
Sulfur in coal is roughly classified into inorganic
sulfur (inorganic sulfur content) and organic sulfur
(organic sulfur content).
A typical example of inorganic sulfur is FeS2.
Examples of organic sulfur include: an aromatic sulfur
compound in which sulfur is present inside an aromatic ring
such as dibenzothiophene; an aliphatic sulfur compound such
as mercaptan. Of these, sulfur present inside an aromatic
ring constituting coal is known to be particularly
difficult to be removed.
[0014]
The present inventors studied various chemical agents
(desulfurization agents). As a result, it was found that
peracetic acid effectively acts on thiophene form sulfur
which is a component particularly difficult to be removed
among organic sulfurs in coal, thereby successfully
removing sulfur from coal or increasing an efficiency of
converting sulfur into an easily removable form. It is
assumed that by the action of peracetic acid, thiophene
form sulfur is oxidized to be, for example, sulf one form
sulfur or sulfide form sulfur, and a bond between carbon
and sulfur is relatively weakened to be easily cut off,
whereby the sulfur becomes easy to be separated.
[0015]
CA 03134533 2021-10-21
8
Meanwhile, peracetic acid is easy to decompose. In
the invention, therefore, a mixed solution of hydrogen
peroxide and acetic anhydride (hereinafter, also simply
referred to as "mixed solution") is used as a chemical
agent. The mixed solution generates peracetic acid which is
a reaction product of hydrogen peroxide and acetic
anhydride. The mixed solution as above is brought into
contact with coal.
[0016]
A reaction of hydrogen peroxide (H202) and acetic
anhydride ( (CH3C0)20) to obtain peracetic acid (CH3C00211)
and water (H20) is represented by Formula (I) below.
211202 + ( CH3C0) 20 itz> 2 CH3C002H + 1120 . . . ( I )
In Formula (I) above, an equilibrium state changes
depending on various conditions such as a temperature and a
mixing ratio of a chemical agent. Therefore, the
concentration of each component varies depending on the
combination of the conditions. Suitable conditions will be
described in detail below.
[0017]
When a chemical agent is brought into contact with
coal, inorganic sulfur which is easy to be removed
dissolves and leaches into the chemical agent in the form
of, for example, a sulfate ion. Similarly, a part of
CA 03134533 2021-10-21
9
organic sulfur is also oxidized and leaches into the
chemical agent in the form of, for example, a sulfate ion.
Coal is desulfurized (i.e., sulfur in coal is removed) in
this manner to thereby obtain coal having a reduced sulfur
content (low-sulfur coal).
[0018]
<Molar Ratio (Acetic Anhydride/Hydrogen Peroxide)
A molar ratio between acetic anhydride and hydrogen
peroxide (acetic anhydride/hydrogen peroxide) in a chemical
agent is preferably not less than 0.1 and more preferably
not less than 0.5 because peracetic acid which is a
reaction product can be formed in a proper amount and the
desulfurization effect can become more excellent.
Further, when the molar ratio (acetic
anhydride/hydrogen peroxide) is within the foregoing range,
acetic anhydride can be prevented from becoming excessive
with respect to hydrogen peroxide, and residual hydrogen
peroxide in the mixed solution can be minimized (as
described below, hydrogen peroxide decreases a carbon yield
of coal).
[0019]
The molar ratio (acetic anhydride/hydrogen peroxide)
is preferably not more than 15.0 and more preferably not
more than 12Ø When the molar ratio (acetic
CA 03134533 2021-10-21
10
anhydride/hydrogen peroxide) is within the foregoing range,
as in the above, peracetic acid which is a reaction product
can be formed in a proper amount, so that the
desulfurization effect can become more excellent. Further,
the generated peracetic acid is prevented from being
diluted with excessive acetic anhydride.
[0020]
The molar ratio (acetic anhydride/hydrogen peroxide)
is calculated as follows.
First, a molar amount [mol] of each component (acetic
anhydride or hydrogen peroxide) in a chemical agent is
represented by Formula (a) below. Therefore, the molar
ratio between acetic anhydride and hydrogen peroxide
(acetic anhydride/hydrogen peroxide) in the chemical agent
is calculated by Formula (b) below.
Molar amount = (Li x Ci)/(100 x Mi)
(a)
Molar ratio = (L1 x Cl x M2)/(L2 x C2 x M1)
(b)
Li: amount of i aqueous solution [g/h]
Ci: concentration of i aqueous solution [masst]
Mi: molecular weight of i [g/mol]
Here, i is 1 or 2, 1 is acetic anhydride and 2 is
hydrogen peroxide.
The molecular weight of acetic anhydride is assumed
to be 102, and the molecular weight of hydrogen peroxide is
CA 03134533 2021-10-21
11
assumed to be 34. The amount of an aqueous solution Li is
adjusted such that the desired molar ratio (acetic
anhydride/hydrogen peroxide) is obtained.
[0021]
<Elapsed Time After Mixing
The reaction (forward reaction) of Formula (I) above
has a relatively slow rate. Therefore, generation of
peracetic acid is insufficient immediately after acetic
anhydride and hydrogen peroxide are mixed in some cases.
The present inventors determined the quantities of
various reaction rates and found out that it takes about 10
minutes for the reaction of Formula (I) above to settle
into a steady state.
In the invention, therefore, it is preferable that
acetic anhydride and hydrogen peroxide are mixed before a
chemical agent is brought into contact with coal, and when
minutes or more have elapsed after this mixing, the
chemical agent is brought into contact with the coal. This
allows peracetic acid to be sufficiently generated, whereby
the desulfurization effect of removing sulfur in coal can
become more excellent. Further, this allows peracetic acid
hydrogen to be decreased, whereby decrease in a carbon
yield due to a reaction of hydrogen peroxide with coal can
be minimized.
CA 03134533 2021-10-21
12
The elapsed time after mixing is more preferably not
less than 20 minutes and even more preferably not less than
30 minutes and, at the same time, preferably not more than
120 minutes, more preferably not more than 90 minutes, and
even more preferably not more than 60 minutes.
[0022]
Mass Ratio (Chemical Agent/Coal)
The present inventors studied a mass ratio between a
chemical agent and coal (chemical agent/coal). In this
study, a chemical agent having a molar ratio between acetic
anhydride and hydrogen peroxide (acetic anhydride/hydrogen
peroxide) of 5.0 was used.
FIG. 1 is a graph showing a desulfurization rate with
respect to a mass ratio between a chemical agent and coal
(chemical agent/coal). As shown in the graph of FIG. 1, as
the amount of a chemical agent with respect to coal
increases, the desulfurization rate increases, so that the
desulfurization effect becomes more excellent. Therefore,
the mass ratio (chemical agent/coal) is preferably not less
than 0.5, more preferably not less than 1.0 and even more
preferably not less than 2Ø
[0023]
As shown in the graph of FIG. 1, when the amount of a
chemical agent becomes excessive with respect to the amount
CA 03134533 2021-10-21
13
of coal, the desulfurization rate barely changes. The mass
ratio (chemical agent/coal) is preferably not more than
100.0 and more preferably not more than 50.0 for the sake
of reducing the amount of a chemical agent used.
[0024]
When a mass of coal (solid content) before
desulfurization is Wi [kg], a sulfur content of coal (solid
content) before desulfurization is %S1 [mass%], a mass of
coal (solid content) after desulfurization is W2 [kg], and
a sulfur content of coal (solid content) after
desulfurization is %82 [masst], the desulfurization rate
[mass%) is defined by Formula (1) below.
Desulfurization rate [mass%] = 100 x
- (W2 x
x %Si) ) ... (1)
[0025]
Temperature of chemical agent
The present inventors also studied a temperature of a
chemical agent at the time of being brought into contact
with coal (hereinafter, also simply referred to as "a
temperature of a chemical agent"). In this study, a
chemical agent having a molar ratio between acetic
anhydride and hydrogen peroxide (acetic anhydride/hydrogen
peroxide) of 5.0 was used.
FIG. 2 provides a graph (lower part) showing an
CA 03134533 2021-10-21
14
amount of peracetic acid generated with respect to a
temperature of a chemical agent, and a graph (upper part)
showing a desulfurization rate (solid line) and a carbon
yield (dashed line) with respect to a temperature of a
chemical agent. The amount of peracetic acid generated is
an index obtained by setting a calculated value at the time
when the reaction contributing substances (hydrogen
peroxide and acetic anhydride) completely react to 1Ø
[0026]
As shown in the graphs (lower and upper parts) of
FIG. 2/ when the temperature of a chemical agent at the
time of being brought into contact with coal is high, the
amount of peracetic acid generated is large, and the
desulfurization rate is high, so that the desulfurization
effect becomes more excellent. In connection with this, the
temperature of a chemical agent is preferably not less than
C, more preferably not less than 10 C, even more
preferably not less than 20 C and particularly preferably
not less than 25 C.
[0027]
On the other hand, as shown in the graph (upper part)
of FIG. 2, the temperature of a chemical agent is
preferably not too high in order to maintain a high carbon
yield. Specifically, the temperature is preferably not more
CA 03134533 2021-10-21
15
than 40 C, more preferably not more than 35 C and even more
preferably not more than 30 C because the carbon yield can
become more excellent.
[0028]
When a carbon content of coal (solid content) before
desulfurization is %C1 [masst] and a carbon content of coal
(solid content) after desulfurization is %C2 [mass's], the
carbon yield [mass's] is defined by Formula (2) below.
Carbon yield [masst] = 100 x (W2 x %C2)/(1471 x tC1)
(2)
[0029]
The presumable reason why the carbon yield decreases
is described below.
Hydrogen peroxide and peracetic acid may become an
oxidizing agent which may destroy a skeleton of coal, and
in this case, the carbon yield unintentionally decreases
simultaneously with removal of sulfur. The present
inventors found, through a study, that peracetic acid first
causes cutting off of a bond between sulfur and carbon of
thiophene form sulfur, and thereafter destroy of a carbon
skeleton (carbon-carbon bond) occurs. The degree of destroy
of a carbon skeleton is low with peracetic acid and high
with hydrogen peroxide. In particular, it is remarkable
with hydrogen peroxide having a high temperature.
CA 03134533 2021-10-21
16
Therefore, by appropriately controlling a condition
when a chemical agent is brought into contact with coal
(for example, preventing the temperature of a chemical
agent from becoming too high, or appropriately adjusting
the mixing ratio of hydrogen peroxide in a mixed solution),
the thiophene form sulfur can be effectively removed while
the destroy of a carbon skeleton is minimized.
[0030]
<Coal>
While the coal used in the invention is not
particularly limited and a wide variety of coals can be
used, the coal preferably includes coal having a moderate
degree of coalification such as sub-bituminous coal, more
preferably includes sub-bituminous coal and even more
preferably is sub-bituminous coal.
When such coal is used, the desulfurization effect
tends to be more excellent than that in the case where coal
having a high degree of coalification such as anthracite
coal is used, and the carbon yield tends to be more
excellent than that in the case where coal having a low
degree of coalification such as brown coal is used.
The grain size (mean grain size) of coal used in the
invention is not particularly limited. For example, even
when the grain size of coal is on the order of several
CA 03134533 2021-10-21
17
millimeters, there is no significant change in
desulfurization performance. When the grain size of coal is
equal to or larger than this, a mild pulverization
treatment may be performed as necessary.
[0031]
The primary treatment (chemical treatment) for
desulfurizing coal was described above.
Next, two types of secondary treatments are described
as a treatment for further removing sulfur remaining in
coal having been desulfurized by the primary treatment.
[0032]
<Secondary Treatment (Heat Treatment)).
By the action of peracetic acid which is a reaction
product of hydrogen peroxide and acetic anhydride,
thiophene form sulfur which is difficult to be removed is
changed into an easily removable form; therefore, the
thiophene form sulfur can be removed by a heat treatment at
a relatively low temperature (about 150 C)
That is, it is preferable that a heat treatment is
further performed on coal which has been brought into
contact with a chemical agent because the desulfurization
effect can become more excellent. The heat treatment
temperature is preferably not less than 150 C, more
preferably not less than 250 C, and even more preferably
CA 03134533 2021-10-21
18
not less than 350 C.
[0033]
Note that a hydrocarbon-containing gas derived from
coal and generated by a heat treatment can be recovered and
used as a part of a gaseous fuel in an iron manufacturing
process. In consideration of performing a heat treatment
using, for example, exhaust heat generated at a factory
such as ironworks, a heat treatment at a temperature of up
to several hundreds Celsius is preferred.
[0034]
One example of a furnace for subjecting coal to a
heat treatment in iron manufacturing process is a coke
oven. The heat treatment temperature in a coke oven is
about 1000 to 1200 C, and the coke oven may be operated at
a temperature at or above 1200 C. Coal that has been
brought into contact with a chemical agent and desulfurized
may be introduced into a coke oven to produce low-sulfur
coke. While a hydrocarbon gas and a sulfur-containing gas
are generated in this case, the sulfur-containing gas can
be separately removed. The generated gas after the sulfur-
containing gas is removed can be reused as a fuel gas.
Among processes for subjecting coal to a heat
treatment, a process having the highest temperature is
probably substantially a process of producing coke. As a
CA 03134533 2021-10-21
19
result of experiments conducted by the present inventors,
it was confirmed that a sufficient desulfurization effect
was also exhibited even with a heat treatment temperature
in a coke oven.
Therefore, the heat treatment temperature is, for
example, not more than 1300 C.
[0035]
Coal that has been subjected to a heat treatment at
about 600 C is generally called semi-coke. Coal that has
been brought into contact with a chemical agent and
desulfurized can also be used in producing semi-coke. Since
semi-coke is generally inferior in strength to coke, it can
hardly be used as coke for a blast furnace, but it can be
used for other applications. In particular, semi-coke
containing less sulfur is useful as, for example, a heating
agent (carburizing material) used for heating in a
converter.
[0036]
It is preferable that a heating rate at which coal
that has been brought into contact with a chemical agent is
heated to the heat treatment temperature (hereinafter, also
simply referred to as "heating rate") is higher. This is
because a sulfur compound which has been changed into a
form allowing desulfurization by the action of a mixed
CA 03134533 2021-10-21
20
solution of hydrogen peroxide and acetic anhydride may be
resynthesized into thiophene form sulfur which is difficult
to desulfurize under heating, and this resynthesis is
suppressed. Specifically, the heating rate is preferably
not less than 10 C/min and more preferably not less than
20 C/min.
[0037]
While the upper limit of the heating rate is not
particularly limited, realization of an excessively high
heating rate is difficult for technical and industrial
(cost) reasons. Therefore, the heating rate is, for
example, not more than 100 C/min.
[0038]
<Secondary treatment (hydrogen peroxide treatment)
The present inventors found, through the study, that
for further desulfurizing coal that has been brought into
contact with a chemical agent, a treatment using low-
temperature hydrogen peroxide may be performed separately
from the above-described heat treatment.
When hydrogen peroxide acts on coal that has not been
subjected to the primary treatment (chemical treatment), as
described above, a carbon skeleton is destroyed, and the
carbon yield decreases. However, since a sulfur content
remaining in coal that has been subjected to the primary
CA 03134533 2021-10-21
21
treatment is in an easily removable form, the coal can be
easily additionally desulfurized with hydrogen peroxide.
That is, it is preferable that the coal that has been
brought into contact with the chemical agent is further
brought into contact with a hydrogen peroxide solution
having a low temperature.
[0039]
The temperature of a hydrogen peroxide solution is
preferably not more than 50 C and more preferably not more
than 40 C. The oxidizing ability of hydrogen peroxide
becomes increasingly strong as the temperature of the
hydrogen peroxide becomes high, and not only the
desulfurization effect but also the carbon yield tends to
decrease. When the temperature of a hydrogen peroxide
solution is within the above range, the desulfurization
effect is further excellent, and the carbon yield is also
good.
The lower limit thereof is not particularly limited,
and the temperature of a hydrogen peroxide solution is, for
instance, not less than 5 C.
(0040]
The concentration of a hydrogen peroxide solution
(the content of hydrogen peroxide in a hydrogen peroxide
solution) is preferably not less than 2.0 mass% and more
CA 03134533 2021-10-21
22
preferably not less than 3.0 mass% because the
desulfurization effect can become more excellent.
When the concentration of a hydrogen peroxide
solution is not less than 3.0 mazes, the effect thus
obtained is substantially constant regardless of the
concentration of a hydrogen peroxide solution. Therefore,
the upper limit thereof is not particularly limited, and
the concentration of a hydrogen peroxide solution is
preferably not more than 35.0 masst, for instance.
Hydrogen peroxide is often commercially available as
an aqueous solution of 30 to 35 mass% because it is easy to
decompose on the high concentration side. In the present
invention, such a commercially available aqueous solution
may be appropriately diluted and used.
[0041]
[Facility for Producing Low-sulfur Coal]
Next, an example in which the present invention is
implemented using a specific facility will be described
with reference to FIG. 3.
[0042]
FIG. 3 is a schematic view showing an example of a
facility for producing low-sulfur coal (hereinafter, also
simply referred to as "production facility").
The production facility shown in FIG. 3 has a
CA 03134533 2021-10-21
23
hydrogen peroxide storage tank 1 for storing hydrogen
peroxide and an acetic anhydride storage tank 3 for storing
acetic anhydride.
The hydrogen peroxide inside the hydrogen peroxide
storage tank 1 is supplied to a chemical agent mixing tank
via a hydrogen peroxide transport pipe 2. The acetic
anhydride inside the acetic anhydride storage tank 3 is
supplied to the chemical agent mixing tank 5 via an acetic
anhydride transport pipe 4. The hydrogen peroxide transport
pipe 2 and the acetic anhydride transport pipe 4 are each
provided with a suitable flow rate control device (not
shown), and the flow rates of the hydrogen peroxide and the
acetic anhydride can be controlled.
The chemical agent mixing tank 5 is provided with a
heating device 6 and a mixing device 7. The hydrogen
peroxide and the acetic anhydride supplied to the chemical
agent mixing tank 5 are heated to a predetermined
temperature using the heating device 6 as necessary and
mixed using the mixing device 7.
A chemical agent which is a mixed solution obtained
by mixing in the chemical agent mixing tank 5 is supplied
to a desulfurization treatment tank 9 via a chemical agent
transport pipe 8. The chemical agent transport pipe 8 is
provided with a suitable flow rate control device (not
CA 03134533 2021-10-21
24
shown), and the flow rate of the chemical agent can be
controlled.
The desulfurization treatment tank 9 is further
supplied with coal from a coal storage tank 10 for storing
coal via a coal transport pipe 11. The coal transport pipe
11 is provided with a suitable flow rate control device
(not shown), and the flow rate of the coal can be
controlled.
The desulfurization treatment tank 9 is provided with
a heating device 12. The heating device 12 controls the
chemical agent supplied from the chemical agent mixing tank
S and the coal supplied from the coal storage tank 10 to an
appropriate temperature as necessary. Further, the
desulfurization treatment tank 9 is provided with a mixing
device 13. The mixing device 13 mixes the chemical agent
and the coal well as necessary.
Thus, in the desulfurization treatment tank 9, the
coal is brought into contact with the chemical agent and
desulfurized, thereby obtaining coal with low sulfur
content (low-sulfur coal) (hereinafter, also referred to as
"chemical-treated coal")
[0043]
The desulfurization treatment tank 9 is provided with
discharge holes at two places. A chemical agent circulation
CA 03134533 2021-10-21
25
pipe 14 is provided at one discharge hole. Peracetic acid
may remain in a part of the chemical agent after use in
desulfurization of the coal. In this case, the chemical
agent may be flown back from the desulfurization treatment
tank 9 to the chemical agent mixing tank 5 and reused.
However, sulfur may leach into the chemical agent
after desulfurization. Reuse of the chemical agent into
which sulfur leaches may adversely affect desulfurization.
Therefore, a chemical agent discharge pipe 15 is connected
to the chemical agent circulation pipe 14, and a part or
all of the chemical agent after desulfurization can be
discharged through the chemical agent discharge pipe 15.
[0044]
A chemical-treated coal transport pipe 16 is provided
at the other discharge hole of the desulfurization
treatment tank 9. The chemical-treated coal transport pipe
16 is further branched into three pipes, i.e., a chemical-
treated coal discharge pipe 16a, a heat treatment device
connection pipe 16b and a hydrogen peroxide treatment
device connection pipe 16c.
The chemical-treated coal discharge pipe 16a
discharges the chemical-treated coal obtained in the
desulfurization treatment tank 9 without performing the
secondary treatment. The heat treatment device connection
CA 03134533 2021-10-21
26
pipe 16b transports the chemical-treated coal to a heat
treatment device 17. The hydrogen peroxide treatment device
connection pipe 16c transports the chemical-treated coal to
a hydrogen peroxide treatment device 23.
[0045]
First, the heat treatment device 17 will be
described.
When low-sulfur coal (chemical-treated coal) is
subjected to a heat treatment in the heat treatment device
17, sulfur is further volatilized, so that the
desulfurization proceeds further. The coal that has been
subjected to the heat treatment in the heat treatment
device 17 and has been further reduced in sulfur content
(hereinafter, also referred to as "heat-treated coal") is
taken out through a heat-treated coal discharge pipe 18 and
used for a predetermined use.
Further, the heat treatment device 17 is provided
with a heat treatment gas exhaust pipe 19. A gas generated
by a heat treatment may include a combustible gas. In this
case, the gas can be taken out through the heat treatment
gas discharge pipe 19 and used for a predetermined use.
[0046]
Next, the hydrogen peroxide treatment device 23 will
be described.
CA 03134533 2021-10-21
27
The hydrogen peroxide treatment device 23 is supplied
with the chemical-treated coal via the hydrogen peroxide
treatment device connection pipe 16c. In the hydrogen
peroxide treatment device 23, the chemical-treated coal is
subjected to the above-described secondary treatment
(hydrogen peroxide treatment).
The hydrogen peroxide treatment device 23 is supplied
with the hydrogen peroxide via a hydrogen peroxide supply
pipe 20. The hydrogen peroxide supply pipe 20 is connected
to the hydrogen peroxide storage tank 1. When the hydrogen
peroxide is diluted, water may be supplied from a dilution
water tank 21 through a dilution water supply pipe 22.
Another hydrogen peroxide storage tank (not shown) may be
provided exclusively for the hydrogen peroxide treatment
device 23.
The hydrogen peroxide treatment device 23 is provided
with a cooling device 24. The cooling device 24 controls a
temperature inside the hydrogen peroxide treatment device
23 to an appropriate temperature as necessary.
Further, the hydrogen peroxide treatment device 23 is
provided with a mixing device 25. The mixing device 25
mixes the hydrogen peroxide solution and the chemical-
treated coal well as necessary.
[0047]
CA 03134533 2021-10-21
28
The hydrogen peroxide treatment device 23 is provided
with discharge holes at two places.
A hydrogen peroxide circulation pipe 27 is provided
at one discharge hole. Hydrogen peroxide may remain in a
part of the hydrogen peroxide solution after use in
desulfurization of the coal (chemical-treated coal). In
this case, the hydrogen peroxide solution may be flown back
from the hydrogen peroxide treatment device 23 to the
hydrogen peroxide storage tank I and reused. A destination
of the flowback may be a separately provided hydrogen
peroxide storage tank (not shown) or the chemical agent
mixing tank 5.
However, sulfur may leach into the hydrogen peroxide
solution after desulfurization. Reuse of the hydrogen
peroxide solution into which sulfur leaches may adversely
affect desulfurization. Therefore, a hydrogen peroxide
discharge pipe 28 is connected to the hydrogen peroxide
circulation pipe 27, and a part or all of the hydrogen
peroxide solution after desulfurization can be discharged
through the hydrogen peroxide discharge pipe 28.
[0048]
A discharge pipe 26 is connected to the other
discharge hole of the hydrogen peroxide treatment device
23. Coal that has been further desulfurized inside the
CA 03134533 2021-10-21
29
hydrogen peroxide treatment device 23 (hereinafter, also
referred to as "hydrogen peroxide-treated coal") is taken
out through the discharge pipe 26 and used for a
predetermined use.
[0049]
Note that since the chemical-treated coal transported
to the heat treatment device 17 or the hydrogen peroxide
treatment device 23 is already reduced in sulfur content,
it may be taken out through the heat-treated coal discharge
pipe 18 or the discharge pipe 26 without being subjected to
the secondary treatment (heat treatment or hydrogen
peroxide treatment).
[0050]
Each part of the production facility described with
reference to FIG. 3 need not have a special specification,
and existing devices can be used as appropriate. For
example, the heat treatment device 17 may be a heat
exchanger using exhaust heat as a heat source, and it may
be a furnace such as a semi-coke oven or a coke oven.
[EXAMPLES]
[0051]
The present invention is specifically described below
with reference to examples. However, the present invention
should not be construed as being limited to the following
CA 03134533 2021-10-21
30
examples.
[0052]
<Examples 1 to 16 and Comparative Example 1
By using the production facility described with
reference to FIG. 3, a test was conducted in which coal was
desulfurized to produce low-sulfur coal by the method of
the present invention.
As the coal, at least one selected from the group
consisting of Coal A (sub-bituminous coal), Coal B (sub-
bituminous coal) and Coal C (semi-anthracite coal) was
used. The details of the coals used are shown in Table 1
below. The granularity of each coal was about 300 pm in a
mean grain size. With all coals, permeability of peracetic
acid is high, and the desulfurization, performance did not
vary greatly depending on the granularity.
[0053]
[Table 1]
Table 1
Industrial analysis value [mass% d.a.f.] Industrial analysis value [mass%
d.b.)
C _ H N S
V.M Ash
Coal A 78.5 4.6 0.8 0.2
38.2 6.8
_
Coal B 77.1 4.9 1.5 0.5
33.2 6.7
Coal C 82.1 1.2 1.4 2
9.4 8.1
[0054)
In Table 1 above, "d.a . f" indicates a dry ash free
basis, and means an analytical value of net coal excluding
moisture and ash.
CA 03134533 2021-10-21
31
"d.b." means an analysis value on a dry basis.
"V.M" means a content of volatile matter in
industrial analysis.
"Ash" means a content of ash in industrial analysis.
[0055]
Test conditions such as supply amounts (flow rates)
of coal/ hydrogen peroxide and acetic anhydride are shown
in Table 2 below.
In Examples 1 to 7 and Comparative Example 1, only
the above-described primary treatment (chemical treatment)
was performed. That is/ the coal after being brought into
contact with the chemical agent was taken out, and the
desulfurization rate and the carbon yield were determined.
In Examples 8 to 11, the above-described secondary
treatment (heat treatment) was further performed. That is,
after the primary treatment (chemical treatment), the coal
was further introduced into the heat treatment device
capable of raising the temperature to 1200 C and then
subjected to heat treatment under a nitrogen atmosphere,
and the desulfurization rate and the carbon yield after the
heat treatment were determined.
In Examples 12 to 16, the above-described secondary
treatment (hydrogen peroxide treatment) was further
performed. That is, after the primary treatment (chemical
CA 03134533 2021-10-21
32
treatment), the coal was further introduced into the
hydrogen peroxide treatment device and then subjected to
the hydrogen peroxide treatment, and the desulfurization
rate and the carbon yield after the hydrogen peroxide
treatment were determined.
[0056]
In the primary treatment, an aqueous solution having
a concentration of hydrogen peroxide of 35 mass% was used
as hydrogen peroxide. As acetic anhydride, acetic anhydride
having a purity of 99 mass% was used.
[0057]
[Table 2]
CA 03134533 2021-10-21
4=.
NO
NO
33
NJ
Table 2
Example
Example Example
Comparative
Example
Unit
1 2 3 4 5 _ 6 7 8 9 10 11 12
13 14 15 16 1
Coal A
g/h
100 , 50 0 100 100 100 100 100 100 100 100 100 100 100 100 100 100
Coal B
g/h 0 50 0 0 0 , 0 0 0 0 0 0 0 0 0
0 0 0
Coal Coal C
g/h 0 0 100 0 0 0 0 0 0 0 0 0 0
0 0 0 0
Total amount
9/h
100 100 100 100 100 100 100, 100 100 100 100 100 100 100 100 100
100
Hydrogen peroxide
g/h
50 100 50 95 50 20 240 50 50 , 50 50 50 50 50 50 50 300
Acetic anhydride
9/h 265 55 265 40 350 70 60 265 265 265 265 265 265 265 265 265 ,
0
Molar ratio
mol/mol 5.0 0.5 5.0 0.4 6.6 3.3 0.2 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.0 5.0
0.0
(acetic anhydride/hydrogen peroxide)
Chemical agent Elapsed time after mixing
min 30 60 30 30 8
30 30 30 30 30 30 30 30 30 30 30
and Mass ratio (chemical agent/coal)
g/g
3.2 1.6 3.2 1.4 4.0 0.9 3.0 3.2 3.2 3.2 3.2 3.2 3.2 3.2 3.2 3.2 3.0
primary treatment Chemical
agent temperature
t 20 22 20 20 17 20 35 20 , 20 20 20 20 20 20 20 20 20
(chemical treatment)
Desulfurization rate
mass% 52 51 50 48 51 45 58 52 52 52 52 52 52 52 52 52 28
(after primary treatment)
Carbon yield
mass% 96 93 97 96 94 96 90 96 96 96 96 96 96 96 96 96 96
(after primary treatment)
Heat treatment temperature t - - - - -
- - 150 1200 100 150 - - - - -
Heating rate
t/min - - - - - - - 20 25 10 5 - -
- - -
Secondary treatment Desulfurization rate
mass% - - - - - - - 65 68 53 61 - - - - -
(heat treatment) (after
secondary treatment)
Carbon yield
mass% -
- - - - 95 94 95 95 - - - -
(after secondary treatment)
Temperature of hydrogen peroxide solution t
- - - - - - - - - - -
20 40 45 30 30
Concentration of hydrogen peroxide solution mass% -
- - - - - - - - - - 35.0 35.0 5.0 1.5 3.0
Secondary treatment Mass ratio (hydrogen peroxide solution/coal)
g/g - - - - - - - - - - -
2.5 2.5 2.5 2.5 0.9
(hydrogen peroxide Desulfurization rate
mass% - - - - - - - - - - -
65 66 55 62 63
treatment) (after
secondary treatment)
Carbon yield
mass% - - - - - - - - - -
95 93 71 95 95
(after secondary treatment)
34
[0058]
<Summary of Test Results>
It was revealed that Examples 1 to 16 using a mixed
solution of hydrogen peroxide and acetic anhydride as a
chemical agent exhibited a higher desulfurization rate than
that of Comparative Example 1 in which such a solution was
not used, thus having a sufficient desulfurization effect.
The carbon yield was also good.
[0059]
The comparison between Example 1 and Example 4
revealed that Example 1 in which a molar ratio (acetic
anhydride/hydrogen peroxide) was 5.0 had a higher
desulfurization rate than that of Example 4 in which a
molar ratio (acetic anhydride/hydrogen peroxide) was 0.4,
thus having a more excellent desulfurization effect.
[0060]
The comparison between Example 1 and Example 5
revealed that Example 1 in which the elapsed time after
mixing of acetic anhydride and hydrogen peroxide was 30
minutes had a higher desulfurization rate than that of
Example 5 in which the time was 8 minutes, thus having a
more excellent desulfurization effect.
[0061]
The comparison between Example 1 and Example 6
CA 03134533 2021-10-21
35
revealed that Example 1 in which the mass ratio (chemical
agent/coal) was 3.2 had a higher desulfurization rate than
that of Example 6 in which the mass ratio (chemical
agent/coal) was 0.9, thus having a more excellent
desulfurization effect.
[0062]
The comparison between Example 1 and Example 7
0
revealed that Example 1 in which the temperature of the
chemical agent at the time of being brought into contact
with coal was 20 C had a better carbon yield than that of
Example 7 in which the temperature was 35 C.
[0063]
The desulfurization rates (after the secondary
treatment) of Examples 8 to 11 were equal to or higher than
the desulfurization rates (after the primary treatment) of
Examples 1 to 7.
The comparison between Example 8 and Example 10
revealed that Example 8 in which the heat treatment
temperature was 150 C had a higher desulfurization rate
(after the secondary treatment) than that of Example 10 in
which the heat treatment temperature was 100 C, thus having
a more excellent desulfurization effect.
The comparison between Example 8 and Example 11
revealed that Example 8 in which the heating rate at which
CA 03134533 2021-10-21
36
the temperature was raised to the heat treatment
temperature was 20 C/min had a higher desulfurization rate
(after the secondary treatment) than that of Example 11 in
which the heating rate was 5 C/min, thus having a more
excellent desulfurization effect.
[0064]
The desulfurization rates (after the secondary
treatment) of Examples 12 to 16 were equal to or higher
than the desulfurization rates (after the primary
treatment) of Examples 1 to 7.
The comparison between Example 12 and Example 14
revealed that .Example 12 in which the temperature of the
hydrogen peroxide solution was 20 C had a higher
desulfurization rate (after the secondary treatment) than
that of Example 14 in which the temperature was 45 C, thus
having a more excellent desulfurization effect.
The comparison between Example 12 and Example 15
revealed that Example 12 in which the concentration of the
hydrogen peroxide solution was 35.0 mass% had a higher
desulfurization rate (after the secondary treatment) than
that of Example 15 in which the concentration was 1.5
mass%, thus having a more excellent desulfurization effect.
The comparison between Example 12 and Example 16
revealed that Example 12 in which a mass ratio (hydrogen
CA 03134533 2021-10-21
37
peroxide solution/coal) was 2.5 had a higher
desulfurization rate (after the secondary treatment) than
that of Example 16 in which a mass ratio (hydrogen peroxide
solution/coal) was 0.9, thus having a more excellent
desulfurization effect.
REFERENCE SIGNS LIST
[0065]
1: Hydrogen peroxide storage tank
2: Hydrogen peroxide transport pipe
3: Acetic anhydride storage tank
4: Acetic anhydride transport pipe
5: Chemical agent mixing tank
6: Heating device
7: Mixing device
8: Chemical agent transport pipe
9: Desulfurization treatment tank
10: Coal storage tank
11: Coal transport pipe
12: Heating device
13: Mixing device
14: Chemical agent circulation pipe
15: Chemical agent discharge pipe
16: Chemical-treated coal transport pipe
16a: Chemical-treated coal discharge pipe
CA 03134533 2021-10-21
38
16b: Heat treatment device connection pipe
16c: Hydrogen peroxide treatment device connection
pipe
17: Heat treatment device
18: Heat-treated coal discharge pipe
19: Heat treatment gas exhaust pipe
20: Hydrogen peroxide supply pipe
21: Dilution water tank
22: Dilution water supply pipe
23: Hydrogen peroxide treatment device
24: Cooling device
25: Mixing device
26: Discharge pipe
27: Hydrogen peroxide circulation pipe
28: Hydrogen peroxide discharge pipe
CA 03134533 2021-10-21