Note: Descriptions are shown in the official language in which they were submitted.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
1
HYDROGEL
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Provisional Application
Serial Number
62/942,52, filed December 2, 2019, and Netherlands Application Serial Number
N2024749, filed
January 24, 2020; the contents of each of which is incorporated by reference
herein in its entirety.
BACKGROUND
[0002] Polymer or hydrogel-coated substrates are used in many
technological applications. In
one example, implantable medical devices can be coated with biologically inert
polymers. In
another example, a wound dressing may be coated with a thin hydrogel layer. In
yet another
example, polymer or hydrogel coated substrates may be used for the preparation
and/or analysis of
biological molecules. Some molecular analyses, such as certain nucleic acid
sequencing methods,
involve the attachment of nucleic acid strands to a polymer or hydrogel-coated
surface of a
substrate.
INTRODUCTION
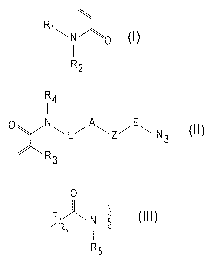
[0003] Disclosed herein is a hydrogel comprising a dendritic core having
from 2 arms to 30
arms, for example 2-20, or 2-10, a first acrylamide monomer incorporated into
each arm of the
dendritic core, the first acrylamide monomer having a structure:
N-N
R2
wherein R1 and R2 are independently selected from the group consisting of an
alkyl, an alkylamino,
an alkylamido, an alkylthio, an aryl, a glycol, and optionally substituted
variants thereof; and a
second acrylamide monomer incorporated into each arm of the dendritic core,
the second
acrylamide monomer having a structure:
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
2
Fiti =
A
'NNL7Z7 N3
wherein R3 is hydrogen or an alkyl; R4 is hydrogen or an alkyl; L is a linker
including a linear chain
of 2 atoms to 20 atoms selected from the group consisting of carbon, oxygen,
and nitrogen and
optional substituents on the carbon and any nitrogen atoms in the chain; A is
an N substituted
0
N ________________________________
R5
amide haying a structure , where R5 is hydrogen or an alkyl; E is a linear
chain
of 1 atom to 4 atoms selected from the group consisting of carbon, oxygen and
nitrogen, and
optional substituents on the carbon and any nitrogen atoms in the chain; and Z
is an optional
nitrogen containing heterocycle.
[0004] The first acrylamide monomer can be /V,N-dimethylacrylamide.
[0005] The dendritic core optionally contains a thiocarbonylthio group in
each arm. The
thiocarbonylthio group can be selected from the group consisting of a
dithiobenzoate, a
trithiocarbonate, and a dithiocarbamate. The dendritic core can be selected
from the group
consisting of 3,5-Bis(2-dodecylthiocarbonothioylthio-1-oxopropoxy)benzoic
acid, 1,1,1-
Tris Rdodecylthiocarbonothioylthio)-2-methylpropionatelethane, and
Pentaerythritol tetrakisp-
(dodecylthiocarbonothioylthio)-2-methylpropionate].
[0006] The dendritic core may include an atom transfer radical
polymerization initiator in
each arm. The dendritic core can be selected from the group consisting of
Bis[2-(2'-
bromoisobutyryloxy)ethyl]disulfide, 2-Bromoisobutyric anhydride, Ethylene
bis(2-
bromoisobutyrate), Pentaerythritol tetrakis(2-bromoisobutyrate),
Dipentaerythritol hexakis(2-
bromoisobutyrate), and 1,1,1-Tris(2-bromoisobutyryloxymethyl)ethane.
[0007] The dendritic core may include a multi-functional central
molecule; and a plurality of
atom transfer radical polymerization mono-initiators attached to the multi-
functional central
molecule. The atom transfer radical polymerization mono-initiator can be
selected from the group
consisting of 2-azidoethyl 2-bromoisobutyrate, poly(ethylene glycol) methyl
ether 2-
bromoisobutyrate, 2-(2-Bromoisobutyryloxy)ethyl methacrylate, Dodecyl 2-
bromoisobutyrate, 2-
Hydroxyethyl 2-bromoisobutyrate, 1-(Phthalimidomethyl) 2-bromoisobutyrate, and
Propargyl 2-
bromoisobutyrate.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
3
[0008] The dendritic core can include a nitroxide mediated
polymerization initiator in each
arm. In a particular example, the dendritic core is selected from the group
consisting of 1,3,5-
tris((4-(1-((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)benzyl)oxy)benzene
and 1,3,5-tris((3,5-
bis((4-(1-((2,2,6,6-tetramethylpiperidin-1-
yl)oxy)ethyl)benzyl)oxy)benzyl)oxy)benzene.
[0009] The dendritic core can include: a multi-functional central molecule;
and a plurality of
nitroxide mediated polymerization mono-initiators attached to the multi-
functional central
molecule. Each of the plurality of nitroxide mediated polymerization mono-
initiators can have a
I ),1
structure selected from the group consisting of:
o
t,
, and ; and wherein I is
optionally
selected from the group consisting of , and CI
[0010] The first acrylamide monomer and the second acrylamide monomer
can form a block
copolymer, a random copolymer, a statistical copolymer, or an alternating
copolymer in each arm
of the dendritic core.
[0011] The second acrylamide monomer is optionally azido acetamido
pentyl acrylamide.
[0012] It is to be understood that any features of the hydrogel disclosed
herein may be
combined together in any desirable manner and/or configuration to achieve the
benefits as
described in this disclosure, including, for example, to generate a polymeric
hydrogel that exhibits
suitable sequencing performance even after being exposed to dry storage at
room temperature (e.g.,
from about 18 C to about 25 C).
[0013] Also disclosed is a flow cell comprising a substrate; a multi-arm
polymeric hydrogel
on the substrate, the multi-arm polymeric hydrogel including: a dendritic core
having from 2 arms
to 30 arms; a first acrylamide monomer incorporated into each arm of the
dendritic core, the first
acrylamide monomer having a structure:
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
4
R1
NN N
R2
wherein R1 and R2 are independently selected from the group consisting of an
alkyl, an alkylamino,
an alkylamido, an alkylthio, an aryl, a glycol, and optionally substituted
variants thereof; and a
second acrylamide monomer incorporated into each arm of the dendritic core,
the second
.. acrylamide monomer having a structure:
RI 4
0 NNN I/7 A'*Z7 N3
R3
wherein R3 is hydrogen or an alkyl; R4 is hydrogen or an alkyl; L is a linker
including a linear chain
of 2 atoms to 20 atoms selected from the group consisting of carbon, oxygen,
and nitrogen and
optional substituents on the carbon and any nitrogen atoms in the chain; A is
an N substituted
0
R5
amide having a structure , where R5 is hydrogen or an alkyl; E is a linear
chain
of 1 atom to 4 atoms selected from the group consisting of carbon, oxygen and
nitrogen, and
optional substituents on the carbon and any nitrogen atoms in the chain; and Z
is an optional
nitrogen containing heterocycle.
[0014] The substrate optionally includes a plurality of depressions
separated by interstitial
.. regions, and wherein the hydrogel is positioned within each of the
depressions.
[0015] The flow cell optionally further comprises amplification primers
grafted to the
hydrogel.
[0016] The substrate optionally includes a channel, wherein the hydrogel
is optionally
positioned in the channel. The flow cell further optionally comprises
amplification primers
grafted to the hydrogel.
[0017] The first acrylamide monomer and the second acrylamide monomer
may form a
random copolymer in each arm of the dendritic core; or the first acrylamide
monomer and the
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
second acrylamide monomer may form a statistical copolymer in each arm of the
dendritic core; or
the first acrylamide monomer and the second acrylamide monomer may form an
alternating
copolymer in each arm of the dendritic core; or the first acrylamide monomer
and the second
acrylamide monomer may form a block copolymer in each arm of the dendritic
core.
5 [0018] The first acrylamide monomer is optionally /V,N-
dimethylacrylamide.
[0019] The second acrylamide monomer is optionally azido acetamido
pentyl acrylamide.
[0020] It is to be understood that any features of the flow cell may be
combined together in
any desirable manner. Moreover, it is to be understood that any combination of
features of the
flow cell and/or of the hydrogel may be used together, and/or combined with
any of the examples
disclosed herein to achieve the benefits as described in this disclosure,
including, for example,
improved sequencing metrics.
[0021] Also disclosed herein is a method comprising incorporating a
copolymer into each arm
of a multi-arm dendritic core component having from 2 arms to 30 arms, wherein
the copolymer
includes a first acrylamide monomer and a second acrylamide monomer, and
wherein: the first
acrylamide monomer has a structure:
R1 N
R2
wherein R1 and R2 are independently selected from the group consisting of an
alkyl, an alkylamino,
an alkylamido, an alkylthio, an aryl, a glycol, and optionally substituted
variants thereof; and the
second acrylamide monomer has a structure:
RI 4
0
L7 A E N3
R 3
wherein R3 is hydrogen or an alkyl; R4 is hydrogen or an alkyl; L is a linker
including a linear chain
of 2 atoms to 20 atoms selected from the group consisting of carbon, oxygen,
and nitrogen and
optional substituents on the carbon and any nitrogen atoms in the chain; A is
an N substituted
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
6
0
N ________________________________
R5
amide having a structure , where R5 is hydrogen or an alkyl; E is
a linear chain
of 1 atom to 4 atoms selected from the group consisting of carbon, oxygen and
nitrogen, and
optional substituents on the carbon and any nitrogen atoms in the chain; and Z
is an optional
nitrogen containing heterocycle.
[0022] The incorporating may involve polymerizing a mixture of the first
acrylamide
monomer and the second acrylamide monomer in the presence of the multi-arm
component.
[0023] The incorporating may involve forming a block copolymer in the
presence of the
multi-arm component by: i) polymerizing a first block with the first
acrylamide monomer in the
presence of the multi-arm component to form a modified multi-arm component;
and then
polymerizing a second block with the second acrylamide monomer in the presence
of the modified
multi-arm component; or ii) polymerizing a first block with the second
acrylamide monomer in the
presence of the multi-arm component to form a modified multi-arm component;
and then
polymerizing a second block with the first acrylamide monomer in the presence
of the modified
multi-arm component.
[0024] The incorporating may involve reversible addition-fragmentation
chain transfer
polymerization or atom transfer radical polymerization or nitroxide mediated
polymerization. The
disclosure also refers to a wound dressing and a medical device comprising the
hydrogel.
[0025] It is to be understood that any features of the method may be
combined together in any
desirable manner. Moreover, it is to be understood that any combination of
features of the method
and/or features of the flow cell and/or of the hydrogel may be used together,
and/or combined with
any of the examples disclosed herein to achieve the benefits as described in
this disclosure,
including, for example, controlling a molecular weight distribution of the
hydrogel.
[0026] The disclosure also includes the following clauses:
1. A hydrogel, comprising:
a dendritic core having from 2 arms to 30 arms;
a first acrylamide monomer incorporated into each arm of the dendritic core,
the first
acrylamide monomer having a structure:
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
7
R1
NN N
R2
wherein R1 and R2 are independently selected from the group consisting of an
alkyl, an alkylamino,
an alkylamido, an alkylthio, an aryl, a glycol, and optionally substituted
variants thereof; and
a second acrylamide monomer incorporated into each arm of the dendritic core,
the second
acrylamide monomer having a structure:
RI 4
0 NNN I/7 A'*Z7 N3
R3
wherein R3 is hydrogen or an alkyl; R4 is hydrogen or an alkyl; L is a linker
including a linear chain
of 2 atoms to 20 atoms wherein each atom is independently selected from the
group consisting of
carbon, oxygen, and nitrogen and optional substituents on any carbon and any
nitrogen atoms in
0
R5
the chain; A is an N substituted amide having a structure , where R5 is
hydrogen
or an alkyl; E is a linear chain of 1 atom to 4 atoms wherein each atom is
independently selected
from the group consisting of carbon, oxygen and nitrogen, and optional sub
stituents on any carbon
and any nitrogen atoms in the chain; and Z is an optional nitrogen containing
heterocycle.
2. The hydrogel as defined in clause 1, wherein the first acrylamide monomer
is NN-
dimethylacrylamide.
3. The hydrogel as defined in clause 1 or 2, wherein the dendritic core
contains a
thiocarbonylthio group in each arm.
4. The hydrogel as defined in clause 3, wherein the thiocarbonylthio group is
selected
from the group consisting of a dithiobenzoate, a trithiocarbonate, and a
dithiocarbamate.
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
8
5. The hydrogel as defined in clause 3, wherein the dendritic core is selected
from the
group consisting of 3,5-Bis(2-dodecylthiocarbonothioylthio-1-
oxopropoxy)benzoic acid, 1,1,1-
Tris Rdodecylthiocarbonothioylthio)-2-methylpropionatelethane, and
Pentaerythritol tetrakisp-
(dodecylthiocarbonothioylthio)-2-methylpropionate].
6. The hydrogel as defined in any of the preceding clauses, wherein the
dendritic core
includes an atom transfer radical polymerization initiator in each arm.
7. The hydrogel as defined in clause 6, wherein the dendritic core is selected
from the
group consisting of Bis[2,-(21-bromoisobutyryloxy)ethylldisulfide, 2-
Bromoisobutyric anhydride,
Ethylene bis(2-bromoisobutyrate), Pentaerythritol tetrakis(2-
bromoisobutyrate), Dipentaerythritol
hexakis(2-bromoisobutyrate), and 1,1,1-Tris(2-bromoisobutyryloxymethyl)ethane.
8. The hydrogel as defined in any of the preceding clauses wherein the
dendritic core
includes:
a multi-functional central molecule; and
a plurality of atom transfer radical polymerization mono-initiators attached
to the multi-
functional central molecule.
9. The hydrogel as defined in clause 8, wherein the atom transfer radical
polymerization
mono-initiator is selected from the group consisting of 2-azidoethyl 2-
bromoisobutyrate,
poly(ethylene glycol) methyl ether 2-bromoisobutyrate, 2-(2-
Bromoisobutyryloxy)ethyl
methacrylate, Dodecyl 2-bromoisobutyrate, 2-Hydroxyethyl 2-bromoisobutyrate, 1-
(Phthalimidomethyl) 2-bromoisobutyrate, and Propargyl 2-bromoisobutyrate.
10. The hydrogel as defined in any of the preceding clauses wherein the
dendritic core
includes a nitroxide mediated polymerization initiator in each arm.
11. The hydrogel as defined in clause 10, wherein the dendritic core is
selected from the
group consisting of 1,3,5-tris((4-(1-((2,2,6,6-tetramethylpiperidin-1-
yl)oxy)ethyl)benzyl)oxy)benzene and 1,3,5-tris((3,5-bis((4-(1-((2,2,6,6-
tetramethylpiperidin-1-
yl)oxy)ethyl)benzyl)oxy)benzyl)oxy)benzene.
12. The hydrogel as defined in any of the preceding clauses wherein the
dendritic core
includes:
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
9
a multi-functional central molecule; and
a plurality nitroxide mediated polymerization mono-initiators attached to the
multi-
functional central molecule.
13. The hydrogel as defined in clause12, wherein each of the plurality
nitroxide mediated
polymerization mono-initiators has a structure selected from the group
consisting of:
, I N = /
t
, o=NN ,and
I ' =
,
; and wherein I is selected from the group consisting of
CI
,and
14. The hydrogel as defined in any of the preceding clauses, wherein the first
acrylamide
monomer and the second acrylamide monomer form a block copolymer, a random
copolymer, a
statistical copolymer, or an alternating copolymer in each arm of the
dendritic core.
15. The hydrogel as defined in any of the preceding clauses wherein second
acrylamide
monomer is azido acetamido pentyl acrylamide.
16. A flow cell, comprising:
a substrate; and
a hydrogel according to any of the preceding clauseson the substrate.
17. The flow cell as defined in clause 16, wherein the substrate includes a
plurality of
depressions separated by interstitial regions, and wherein the hydrogel is
positioned within each of
the depressions.
18. The flow cell as defined in clause 16 or 17, further comprising
amplification primers
grafted to polymeric hydrogel.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
19. The flow cell as defined in any of the clauses16-18 wherein the substrate
includes a
channel, and wherein the hydrogel is positioned in the channel.
20. The flow cell as defined in any of the clauses16-19 wherein:
5 the first acrylamide monomer and the second acrylamide monomer form a
random
copolymer in each arm of the dendritic core; or
the first acrylamide monomer and the second acrylamide monomer form a
statistical
copolymer in each arm of the dendritic core; or
the first acrylamide monomer and the second acrylamide monomer form an
alternating
10 copolymer in each arm of the dendritic core; or
the first acrylamide monomer and the second acrylamide monomer form a block
copolymer in each arm of the dendritic core.
21. A method, comprising:
incorporating a copolymer into each arm of a multi-arm dendritic core
component having
from 2 arms to 30 arms, wherein the copolymer includes a first acrylamide
monomer and a second
acrylamide monomer, and wherein:
the first acrylamide monomer has a structure:
N
R2
wherein R1 and R2 are independently selected from the group consisting of an
alkyl, an alkylamino,
an alkylamido, an alkylthio, an aryl, a glycol, and optionally substituted
variants thereof; and
the second acrylamide monomer has a structure:
RI 4
0 A 77 E N3
R3
wherein R3 is hydrogen or an alkyl; R4 is hydrogen or an alkyl; L is a linker
including a linear chain
of 2 atoms to 20 atoms wherein each atom is independently selected from the
group consisting of
carbon, oxygen, and nitrogen and optional substituents on any carbon and any
nitrogen atoms in
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
11
0
R5
the chain; A is an N substituted amide having a structure , where R5 is
hydrogen
or an alkyl; E is a linear chain of 1 atom to 4 atoms wherein each atom is
independently selected
from the group consisting of carbon, oxygen and nitrogen, and optional sub
stituents on any carbon
and any nitrogen atoms in the chain; and Z is an optional nitrogen containing
heterocycle.
22. The method as defined in clause 21 wherein the incorporating involves
polymerizing
a mixture of the first acrylamide monomer and the second acrylamide monomer in
the presence of
the multi-arm component.
23. The method as defined in clause 21 or 22 , wherein the incorporating
involves
forming a block copolymer in the presence of the multi-arm component by:
i) polymerizing a first block with the first acrylamide monomer in the
presence of the
multi-arm component to form a modified multi-arm component; and
then polymerizing a second block with the second acrylamide monomer in the
presence of
the modified multi-arm component; or
ii) polymerizing a first block with the second acrylamide monomer in the
presence of the
multi-arm component to form a modified multi-arm component; and
then polymerizing a second block with the first acrylamide monomer in the
presence of the
modified multi-arm component.
24. The method as defined in any of the clauses 21-23 wherein the
incorporating
involves reversible addition-fragmentation chain transfer polymerization or
atom transfer radical
polymerization or nitroxide mediated polymerization.
25. A medical device coated with a hydrogel according to any of the
clausesl-15.
26. A wound dressing coated with a hydrogel according to any of the
clausesl-15.
27. A substrate coated with a hydrogel according to any of the clauses 1-15
for use in
sequencing analysis.
28. The hydrogel according to any of the clauses 1-15 having the structure
(10):
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
12
S S'CH2(CH2)10CH3
H3C(H2C)10H2C,
s=<S0
(õ0
so o
0 0 40
640 sS
Sµ
r/S CH2(CH2)10CH 3
H 3C(H2C)i 01u
wherein:
N3
NH
0-
m n
or the structure:
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
13
HO
H(
wherein:
0 =,
NH
or the structure:
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
14
J
, (
r
-N
wherein:
0
NH
(:)
NH
depicted in Fig -1B
or Fig-1C in the description.
29. The hydrogel according to any of the clauses 1-15, 28 having a
dispersity oflower
than 2.5, in particular between 1.8 and 2.0, preferably lower than 1.7, more
preferably lower than
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
1.3.
30. Hydrogel according to any of the clauses 1-15, wherein the dendritic
core has a
multi-functional central molecule selected from the group consisting of phenyl
group, benzoic acid,
5 pentraerythritol, and a phosphazene group.
31. Hydrogel according to any of the clauses 1-15, wherein the dendritic
core has 2
arms, 3 arms, 4 arms, 6 arms or 8 arms.
10 32. The method as defined in any of the clauses 22-24 wherein the
multi-arm
component comprises a multi-functional central molecule and each arm thereof
comprises a
thiocarbonylthio group or an initiator selected from the group consisting of
atom transfer radical
polymerization (ATR) initiator and a nitroxide mediated polymerization mono-
initiator.
15 33. The method as defined in any of the clauses 22-24, 32, wherein
the second
acrylamide monomer comprises an azide group, wherein the method additionally
comprises at least
one of:
attaching the multi-arm polymeric hydrogel to the surface of a substrate by
reaction of an
azide group of the formed multi-arm polymeric hydrogel;
attaching a primer to an arm of the multi-arm polymeric hydrogel by reaction
of an azide
group of the formed multi-arm polymeric hydrogel; and
cross-linking of the formed multi-arm polymeric hydrogel 10 by reaction of an
azide group
of the formed multi-arm polymeric hydrogel.
BRIEF DESCRIPTION OF THE DRAWINGS
[0027] Features of examples of the present disclosure will become
apparent by reference to
the following detailed description and drawings, in which like reference
numerals correspond to
similar, though perhaps not identical, components. For the sake of brevity,
reference numerals or
features having a previously described function may or may not be described in
connection with
other drawings in which they appear.
[0028] Fig. lA is a chemical formula illustrating the formation of one
example of a hydrogel,
e.g., a multi-arm polymeric hydrogel;
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
16
[0029] Fig. 1B is a chemical structure illustrating another example of a
multi-arm polymeric
hydrogel;
[0030] Fig. 1C is a chemical structure illustrating still another
example of a multi-arm
polymeric hydrogel;
[0031] Fig. 2A is atop view of an example of a flow cell;
[0032] Fig. 2B is an enlarged, and partially cutaway view of an example
of a flow channel of
the flow cell including an example of the multi-arm polymeric hydrogel
positioned in the flow
channel;
[0033] Fig. 2C is an enlarged, and partially cutaway view of an example
of a flow channel of
the flow cell including an example of the multi-arm polymeric hydrogel
positioned in depressions
formed in the flow channel;
[0034] Fig. 3 is a bar graph depicting, in one example, the dispersity
(El) for an example
multi-arm polymeric hydrogel and a comparative example polymeric hydrogel;
[0035] Fig. 4 includes graphs (labeled A, B, and C) which depict, in one
example, the effects
of dry staging on the example multi-arm polymeric hydrogel and the comparative
example
polymeric hydrogel;
[0036] Fig. 5A is a graph depicting, in one example, the quality metric
percentage for flow
cells including the example multi-arm polymeric hydrogel and for comparative
flow cells including
the comparative example polymeric hydrogel;
[0037] Fig. 5B is a graph depicting, in one example, the percentage of pre-
phasing for flow
cells including the example hydrogel and for comparative flow cells including
the comparative
example hydrogel;
[0038] Fig. 6A is a graph depicting, in one example, the error rate for
each sequencing cycle
for a first read for flow cells including the example multi-arm polymeric
hydrogel and the
comparative flow cells including the comparative example polymeric hydrogel;
[0039] Fig. 6B is a graph depicting, in one example, the error rate for
each sequencing cycle
for a second read for flow cells including the example hydrogel and the
comparative flow cells
including the comparative example hydrogel;
[0040] Fig. 7A is a bar graph depicting, in one example, the mean
fluorescence intensity after
a first or initial sequencing cycle (Cl) for flow cell lanes including the
example multi-arm
polymeric hydrogel and comparative flow cell lanes including the comparative
example polymeric
hydrogel;
[0041] Fig. 7B is a bar graph depicting, in one example, the mean error
rate for flow cell lanes
including the example multi-arm polymeric hydrogel and comparative flow cell
lanes including the
comparative example polymeric hydrogel;
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
17
[0042] Fig. 8A is a graph depicting, in one example, the percentage of
duplicate reads
detected during sequencing cycles for an example flow cell lane including the
example multi-arm
polymeric hydrogel and a comparative flow cell lane including the comparative
example polymeric
hydrogel; and
[0043] Fig. 8B is a graph depicting, in one example, the percentage of pad
hopping detected
during sequencing cycles for a flow cell lane including the example multi-arm
polymeric hydrogel
and a comparative flow ls including the comparative example polymeric
hydrogel.
DETAILED DESCRIPTION
[0044] A hydrogel is disclosed herein. One example of the hydrogel
described herein is a
polymeric hydrogel. The hydrogel disclosed herein may be a multi-arm polymeric
hydrogel.
Examples of the hydrogel include a dendrimeric core. During preparation of the
hydrogel
disclosed herein, the number of arms of the dendrimeric core may define the
degree of branching
of the polymer, hence providing control of the cross-linking. In other words,
the cross-linking state
of the hydrogel is fixed and constrained, depending upon the dendrimeric core
that is used.
Moreover, any cross-linking between branches may be adjusted through the
monomer choice. The
ability to adjust multiple parameters (e.g., initiator concentration, transfer
agents, etc.) enables
more control over dispersity (e.g., relative to a free radical polymerization
process), and thus the
resulting product has a relatively narrow molecular weight distribution (e.g.,
dispersity is less than
or equal to 5, or in some instances, is <4, or <2.5, or <1.7, or <1.3). As
such, the hydrogel can be
consistently produced from one batch to the next batch. Dispersity as referred
to in this invention
is defined as the ratio between NI, and M., wherein M., is the weight average
molecular weight and
M. is the number average molecular weight of the hydrogel. The NI, and M. can
be determined
using Gel Permeation Chromotography.
[0045] Moreover, examples of the hydrogel exhibit suitable sequencing
performance even
after being exposed to dry storage at room temperature (e.g., from about 18 C
to about 25 C).
Undesirable intra-molecular and inter-molecular interactions of polymer
strands, e.g., during dry
storage, may deleteriously affect the downstream sequencing performance. In
some
implementations, the acrylamide unit of the hydrogels disclosed herein
includes functional groups
that may at least reduce hydrogen bonding between polymer strands, and thus
may enable the
hydrogel to be dry stored without having a negative effect on downstream
sequencing
performance.
[0046] Definitions
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
18
[0047] It is to be understood that terms used herein will take on their
ordinary meaning in the
relevant art unless specified otherwise. Several terms used herein and their
meanings are set forth
below.
[0048] The singular forms "a", "an", and "the" include plural referents
unless the context
clearly dictates otherwise.
[0049] The terms comprising, including, containing and various forms of
these terms are
synonymous with each other and are meant to be equally broad.
[0050] The terms top, bottom, lower, upper, on, etc. are used herein to
describe the flow cell
and/or the various components of the flow cell. It is to be understood that
these directional terms
are not meant to imply a specific orientation, but are used to designate
relative orientation between
components. The use of directional terms should not be interpreted to limit
the examples disclosed
herein to any specific orientation(s).
[0051] As used herein, "alkyl" refers to a straight or branched
hydrocarbon chain that is fully
saturated (i.e., contains no double or triple bonds). The alkyl group may have
1 to 20 carbon
atoms. Example alkyl groups include methyl, ethyl, propyl, isopropyl, butyl,
isobutyl, tertiary
butyl, pentyl, hexyl, and the like. As an example, the designation "Cl-C6
alkyl" indicates that
there are one to six carbon atoms in the alkyl chain, i.e., the alkyl chain is
selected from the group
consisting of methyl, ethyl, propyl, iso-propyl, n-butyl, isobutyl, sec-butyl,
t-butyl, pentyl, and
hexyl.
[0052] As used herein, "alkylamino" refers to an alkyl group in which one
or more of the
hydrogen atoms are replaced by an amino group, where the amino group refers to
an -NRaRb
group, where Ra and RI, are each independently selected from a Cl-C6 alkyl, C2-
C6 alkenyl, C2-C6
alkynyl, C3-C7 carbocycle, C6-C10 aryl, a 5-10 membered heteroaryl, and a 5-10
membered
heterocycle.
[0053] As used herein, "alkylamido" refers to an alkyl group in which one
or more of the
hydrogen atoms are replaced by a C-amido group or an N-amido group. A "C-
amido" group refers
to a "-C(=0)N(RaRb)" group in which Ra and Rb can independently be selected
from the group
consisting of alkyl, alkenyl, alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl,
aryl, heteroaryl,
heteroalicycle, aralkyl, or (heteroalicycle)alkyl. An "N-amido" group refers
to a
.. group in which R and Ra can independently be selected from the group
consisting of alkyl, alkenyl,
alkynyl, cycloalkyl, cycloalkenyl, cycloalkynyl, aryl, heteroaryl,
heteroalicycle, aralkyl, or
(heteroalicycle)alkyl. Any alkylamido may be substituted or unsubstituted.
[0054] As used herein, "alkylthio" refers to RS-, in which R is an
alkyl. The alkylthio can be
substituted or unsubstituted.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
19
[0055] As used herein, "alkenyl" refers to a straight or branched
hydrocarbon chain
containing one or more double bonds. The alkenyl group may have 2 to 20 carbon
atoms.
Example alkenyl groups include ethenyl, propenyl, butenyl, pentenyl, hexenyl,
and the like.
[0056] As used herein, "alkyne" or "alkynyl" refers to a straight or
branched hydrocarbon
chain containing one or more triple bonds. The alkynyl group may have 2 to 20
carbon atoms.
[0057] As used herein, "aralkyl" and "aryl(alkyl)" refer to an aryl
group connected, as a
sub stituent, via a lower alkylene group. The lower alkylene and aryl group of
an aralkyl may be
substituted or unsubstituted. Examples include but are not limited to benzyl,
2-phenylalkyl, 3-
phenylalkyl, and naphthylalkyl.
[0058] The term "aryl" refers to an aromatic ring or ring system (i.e., two
or more fused rings
that share two adjacent carbon atoms) containing only carbon in the ring
backbone. When the aryl
is a ring system, every ring in the system is aromatic. The aryl group may
have 6 to 18 carbon
atoms. Examples of aryl groups include phenyl, naphthyl, azulenyl, and
anthracenyl. Any aryl
may be a heteroaryl, with at least one heteroatom, that is, an element other
than carbon (e.g.,
nitrogen, oxygen, sulfur, etc.), in ring backbone.
[0059] As used herein, the term "attached" refers to the state of two
things being joined,
fastened, adhered, connected or bound to each other, either directly or
indirectly. For example, a
nucleic acid can be attached to a functionalized polymer by a covalent or non-
covalent bond. A
covalent bond is characterized by the sharing of pairs of electrons between
atoms. A non-covalent
bond is a physical bond that does not involve the sharing of pairs of
electrons and can include, for
example, hydrogen bonds, ionic bonds, van der Waals forces, hydrophilic
interactions and
hydrophobic interactions.
[0060] An "azide" or "azido" functional group refers to -N3.
[0061] A "block copolymer" is a copolymer formed when two or more
monomers cluster
together and form blocks of repeating units. Each block should have at least
one feature which
is/are not present in adjacent blocks. Specific examples of block copolymers
will be described
further below.
[0062] As used herein, "carbocycle" means a non-aromatic cyclic ring or
ring system
containing only carbon atoms in the ring system backbone. When the carbocycle
is a ring system,
two or more rings may be joined together in a fused, bridged or spiro-
connected fashion.
Carbocycles may have any degree of saturation, provided that at least one ring
in a ring system is
not aromatic. Thus, carbocycles include cycloalkyls, cycloalkenyls, and
cycloalkynyls. The
carbocycle group may have 3 to 20 carbon atoms. Examples of carbocycle rings
include
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclohexenyl, 2,3-dihydro-
indene,
bicyclo[2.2.2loctanyl, adamantyl, and spiro[4.4]nonanyl. Any of the
carbocycles may be
heterocycles, with at least one heteroatom in ring backbone.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
[0063] As used herein, "cycloalkyl" refers to a completely saturated (no
double or triple
bonds) mono- or multi- cyclic hydrocarbon ring system. When composed of two or
more rings, the
rings may be joined together in a fused fashion. Cycloalkyl groups can contain
3 to 10 atoms in
the ring(s). In some examples, cycloalkyl groups can contain 3 to 8 atoms in
the ring(s). A
5 cycloalkyl group may be unsubstituted or substituted. Example cycloalkyl
groups include
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
[0064] As used herein, "cycloalkenyl" or "cycloalkene" means a
carbocycle ring or ring
system having at least one double bond, wherein no ring in the ring system is
aromatic. Examples
include cyclohexenyl or cyclohexene and norbornenyl or norbornene.
10 [0065] As used herein, "cycloalkynyl" or "cycloalkyne" means a
carbocycle ring or ring
system having at least one triple bond, wherein no ring in the ring system is
aromatic. An example
is cyclooctyne. Another example is bicyclononyne.
[0066] "Dendritic core" as used herein refers to the center of the
hydrogel. The dendritic core
is a synthetic polymer with a branching, and in some instances treelike,
structure. The dendritic
15 core can have anywhere from 2 arms (branches) to 30 arms.
[0067] The term "depositing," as used herein, refers to any suitable
application technique,
which may be manual or automated, and, in some instances, results in
modification of the surface
properties. Generally, depositing may be performed using vapor deposition
techniques, coating
techniques, grafting techniques, or the like. Some specific examples include
chemical vapor
20 deposition (CVD), spray coating (e.g., ultrasonic spray coating), spin
coating, dunk or dip coating,
doctor blade coating, puddle dispensing, flow through coating, aerosol
printing, screen printing,
microcontact printing, inkjet printing, or the like.
[0068] As used herein, the term "depression" refers to a discrete
concave feature in a substrate
or a patterned resin having a surface opening that is at least partially
surrounded by interstitial
region(s) of the substrate or the patterned resin. Depressions can have any of
a variety of shapes at
their opening in a surface including, as examples, round, elliptical, square,
polygonal, star shaped
(with any number of vertices), etc. The cross-section of a depression taken
orthogonally with the
surface can be curved, square, polygonal, hyperbolic, conical, angular, etc.
As examples, the
depression can be a well or two interconnected wells. The depression may also
have more
complex architectures, such as ridges, step features, etc.
[0069] The term "each," when used in reference to a collection of items,
is intended to
identify an individual item in the collection, but does not necessarily refer
to every item in the
collection. Exceptions can occur if explicit disclosure or context clearly
dictates otherwise.
[0070] As used herein, the term "flow cell" is intended to mean a vessel
having a chamber
(e.g., a flow channel) where a reaction can be carried out, an inlet for
delivering reagent(s) to the
chamber, and an outlet for removing reagent(s) from the chamber. In some
examples, the chamber
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
21
enables the detection of the reaction that occurs in the chamber. For example,
the chamber can
include one or more transparent surfaces allowing for the optical detection of
arrays, optically
labeled molecules, or the like.
[0071] As used herein, a "flow channel" or "channel" may be an area
defined between two
bonded components, which can selectively receive a liquid sample. In some
examples, the flow
channel may be defined between a patterned or non-patterned substrate and a
lid, and thus may be
in fluid communication with one or more depressions defined in the patterned
resin. The flow
channel may also be defined between two patterned or non-patterned substrate
surfaces that are
bonded together.
[0072] As used herein, "heteroalicyclic" or "heteroalicycle" refers to
three-, four-, five-, six-,
seven-, eight-, nine-, ten-, up to 18-membered monocyclic, bicyclic, and
tricyclic ring system
wherein carbon atoms together with from 1 to 5 heteroatoms constitute said
ring system. A
heteroalicyclic ring system may optionally contain one or more unsaturated
bonds situated in such
a way, however, that a fully delocalized pi-electron system does not occur
throughout all the rings.
The heteroatoms are independently selected from oxygen, sulfur, and nitrogen.
A heteroalicyclic
ring system may further contain one or more carbonyl or thiocarbonyl
functionalities, so as to
make the definition include oxo-systems and thio-systems such as lactams,
lactones, cyclic imides,
cyclic thioimides, and cyclic carbamates. The rings may be joined together in
a fused fashion.
Additionally, any nitrogens in a heteroalicyclic may be quaternized.
Heteroalicycle or
heteroalicyclic groups may be unsubstituted or substituted. Examples of such
"heteroalicyclic" or
"heteroalicycle" groups include 1,3-dioxin, 1,3-dioxane, 1,4-dioxane, 1,2-
dioxolane, 1,3-
dioxolane, 1,4-dioxolane, 1,3-oxathiane, 1,4-oxathiin, 1,3-oxathiolane, 1,3-
dithiole, 1,3-dithiolane,
1,4-oxathiane, tetrahydro-1,4-thiazine, 2H-1,2-oxazine, maleimide,
succinimide, barbituric acid,
thiobarbituric acid, dioxopiperazine, hydantoin, dihydrouracil, trioxane,
hexahydro-1,3,5-triazine,
imidazoline, imidazolidine, isoxazoline, isoxazolidine, oxazoline,
oxazolidine, oxazolidinone,
thiazoline, thiazolidine, morpholine, oxirane, piperidine N-oxide, piperidine,
piperazine,
pyrrolidine, pyrrolidone, pyrrolidione, 4-piperidone, pyrazoline,
pyrazolidine, 2-oxopyrrolidine,
tetrahydropyran, 4H-pyran, tetrahydrothiopyran, thiamorpholine, thiamorpholine
sulfoxide,
thiamorpholine sulfone, and their benzo-fused analogs (e.g.,
benzimidazolidinone,
tetrahydroquinoline, 3,4- methylenedioxyphenyl).
[0073] As used herein, "heteroaralkyl" and "heteroaryl(alkyl)" refer to
a heteroaryl group
connected, as a substituent, via a lower alkylene group. The lower alkylene
and heteroaryl group
of heteroaralkyl may be substituted or unsubstituted. Examples include 2-
thienylalkyl, 3-
thienylalkyl, furylalkyl, thienylalkyl, pyrrolylalkyl, pyridylalkyl,
isoxazolylalkyl, and
imidazolylalkyl, and their benzo-fused analogs.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
22
[0074] A "(heteroalicyclic)alkyl" refers to a heterocyclic or a
heteroalicyclic group connected,
as a substituent, via a lower alkylene group. The lower alkylene and
heterocycle or a heterocycle
of a (heteroalicyclic)alkyl may be substituted or unsubstituted. Examples
include but are not
limited tetrahydro-2H-pyran-4-yl)methyl, (piperidin-4-yl)ethyl, (piperidin-4-
yl)propyl, (tetrahydro-
2H-thiopyran-4-yl)methyl, and (1 ,3-thiazinan-4-yl)methyl.
[0075] As used herein, "hydroxy" or "hydroxyl" refers to an ¨OH group.
[0076] The term "gycol" refers to the end group ¨(CH2).0H, where n
ranges from 2 to 10. As
specific examples, the glycol may be an ethylene glycol end group ¨CH2CH2OH, a
propylene
glycol end group ¨CH2CH2CH2OH, or a butylene glycol end group ¨CH2CH2CH2CH2OH.
[0077] As used herein, the term "interstitial region" refers to an area,
e.g., of a substrate,
patterned resin, or other support that separates depressions. For example, an
interstitial region can
separate one depression of an array from another depression of the array. The
two depressions that
are separated from each other can be discrete, i.e., lacking physical contact
with each other. In
many examples, the interstitial region is continuous whereas the depressions
are discrete, for
example, as is the case for a plurality of depressions defined in an otherwise
continuous surface. In
other examples, the interstitial regions and the features are discrete, for
example, as is the case for a
plurality of trenches separated by respective interstitial regions. The
separation provided by an
interstitial region can be partial or full separation. Interstitial regions
may have a surface material
that differs from the surface material of the depressions defined in the
surface. For example,
depressions can have a polymer and a first primer set therein, and the
interstitial regions can have a
polymer and a second primer set thereon. For another example, depressions of
an array can have
beads therein while the interstitial regions do not have beads thereon.
[0078] As used herein, a "nucleotide" includes a nitrogen containing
heterocyclic base, a
sugar, and one or more phosphate groups. Nucleotides are monomeric units of a
nucleic acid
sequence. In ribonucleic acids RNA, the sugar is a ribose, and in
deoxyribonucleic acids DNA, the
sugar is a deoxyribose, i.e. a sugar lacking a hydroxyl group that is present
at the 2' position in
ribose. The nitrogen containing heterocyclic base (i.e., nucleobase) can be a
purine base or a
pyrimidine base. Purine bases include adenine (A) and guanine (G), and
modified derivatives or
analogs thereof Pyrimidine bases include cytosine (C), thymine (T), and uracil
(U), and modified
derivatives or analogs thereof The C-1 atom of deoxyribose is bonded to N-1 of
a pyrimidine or
N-9 of a purine. A nucleic acid analog may have any of the phosphate backbone,
the sugar, or the
nucleobase altered. Examples of nucleic acid analogs include, for example,
universal bases or
phosphate-sugar backbone analogs, such as peptide nucleic acid (PNA).
[0079] A "patterned resin" refers to any polymer that can have
depressions defined therein.
Specific examples of resins and techniques for patterning the resins will be
described further
herein.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
23
[0080] As used herein, the "primer" is defined as a single stranded
nucleic acid sequence (e.g.,
single strand DNA or single strand RNA). Some primers, referred to herein as
amplification
primers, serve as a starting point for template amplification and cluster
generation. Other primers,
referred to herein as sequencing primers, serve as a starting point for DNA or
RNA synthesis. The
5' terminus of the primer may be modified to allow a coupling reaction with a
functional group of
a polymer or with a bead surface. The primer length can be any number of bases
long and can
include a variety of non-natural nucleotides. In an example, the sequencing
primer is a short
strand, ranging from 10 to 60 bases, or from 20 to 40 bases.
[0081] The term "substrate" refers to a structure upon which various
components of the flow
cell (e.g., the hydrogel, primer(s), etc.) may be added. The substrate may be
a wafer, a panel, a
rectangular sheet, a die, or any other suitable configuration. The substrate
is generally rigid and is
insoluble in an aqueous liquid. The substrate may be inert to a chemistry that
is used to modify the
depressions or that is present in the depressions. For example, a substrate
can be inert to chemistry
used to form the polymer, to attach the primer(s), etc. The substrate may be a
single layer
structure, or a multi-layered structure (e.g., including a support and a
patterned resin on the
support). Examples of suitable substrates will be described further herein.
[0082] Multi-Arm Polymeric Hydrogel
[0083] One example of the hydrogel described herein is a multi-arm
polymeric hydrogel. The
.. multi-arm polymeric hydrogel includes a dendritic core having from 2 arms
to 30 arms; a first
acrylamide monomer incorporated into each arm of the dendritic core, the first
acrylamide
monomer having a structure:
R1
R2
wherein R1 and R2 are independently selected from the group consisting of an
alkyl, an alkylamino,
an alkylamido, an alkylthiol, an aryl, a glycol, and optionally substituted
variants thereof; and a
second acrylamide monomer incorporated into each arm of the dendritic core,
the second
acrylamide monomer having a structure:
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
24
RI 4
N L. 7A7 E N3
"3
wherein R3 is hydrogen or an alkyl; R4 is hydrogen or an alkyl; L is a linker
including a linear chain
of 2 atoms to 20 atoms selected from the group consisting of carbon, oxygen,
and nitrogen and
optional substituents on the carbon and any nitrogen atoms in the chain; A is
an N substituted
0
N ________________________________
R5
amide having a structure , where R5 is hydrogen or an alkyl; E is a linear
chain
of 1 atom to 4 atoms selected from the group consisting of carbon, oxygen and
nitrogen, and
optional substituents on the carbon and any nitrogen atoms in the chain; and Z
is an optional
nitrogen containing heterocycle.
[0084] The multi-arm polymeric hydrogel may be prepared by incorporating
a copolymer
into each arm of a multi-arm component having from 2 arms to 30 arms, wherein
the copolymer
includes the first acrylamide monomer and the second acrylamide monomer. The
incorporation of
the acrylamide monomers into the multi-arm component may be statistical,
random, alternating, or
in block. The incorporation of the acrylamide monomers into the multi-arm
component may be
accomplished by a variety of techniques including reversible addition-
fragmentation chain transfer
(RAFT) polymerization, atom transfer radical polymerization (ATRP), nitroxide
mediated radical
(NMP) polymerization in combination with RAFT or ATRP, NMP with an additional
cross-linking
step, cobalt-mediated polymerization, group transfer polymerization (GTP),
ring opening
polymerization (ROP), or any other polymerization process that either directly
or indirectly yields
the multi-arm architecture and the incorporation of the acrylamide monomers
(statistically,
randomly, alternatingly, or in block) into each arm. As one example of an
indirect process, NMP
may be followed by RAFT polymerization.
[0085] Fig. lA depicts an example of RAFT polymerization to generate one
example of the
hydrogel, which in this example is a multi-arm polymeric hydrogel 10.
[0086] In the example shown in Fig. 1A, the dendritic core 12 includes a
central
molecule/compound 13 and arms 14 (or branches) that extend from the central
molecule/compound
13. The dendritic core 12 may be any multi-functional component that enables a
controlled
polymerization mechanism, which leads to a defined arm length in the polymer
structure and an at
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
least substantially uniform arm length between polymer structures. In an
example, the arms of the
dendritic core 12 are identical to each other.
[0087] The central molecule/compound 13 of the dendritic core 12 may be
any multi-
functional molecule, such as macrocycles (e.g., cyclodextrins, porphyrins,
etc.), extended pi-
5 systems (e.g., perylenes, fullerenes, etc.), metal-ligand complexes,
polymeric cores, etc. Some
specific examples of the central molecule/compound 13 of the dendritic core 12
include a phenyl
group, benzoic acid, pentraerythritol, a phosphazene group, etc.
[0088] As mentioned, the dendritic core 12 includes arms 14 that extend
from the central
molecule/compound 13.
10 [0089] In one example, the dendritic core 12 contains a
thiocarbonylthio group in each arm
12, and thus is a reversible addition-fragmentation chain transfer agent (a
RAFT agent). This
example of the dendritic core 12 may have from 2 arms to 30 arms, each of
which includes the
thiocarbonylthio groups at or near the end of each arm. In some examples, the
dendritic core 12
including the thiocarbonylthio groups has 2 arms, 3 arms, 4 arms, 6 arms, or 8
arms.
15 [0090] Each RAFT agent includes the thiocarbonylthio group (S=C-S)
with substituents R and
Z that impact the polymerization reaction kinetics and the degree of
structural control. As
examples, the thiocarbonylthio group in each arm 14 of the dendritic core 12
may be selected from
R
the group consisting of a dithiobenzoate: , a trithiocarbonate:
Z r R
Z2
, and a dithiocarbamate: . The dendritic
core
20 12 shown in Fig. lA includes a trithiocarbonate group in each arm 14.
[0091] The R-group in the RAFT agent is a free radical leaving group,
and the Z-group(s)
control C=S bond reactivity and influence the rate of radical addition and
fragmentation.
[0092] In some examples, the dendritic core 12 including the
thiocarbonylthio group in each
arm 14 has an R-group configuration, where the central molecule 13 is the
leaving group during
25 the chain transfer process. Two examples of the dendritic core having
the R-group RAFT agent
configuration are:
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
26
P h
S
phS S Ph
P
where Ph is a phenyl group, and
4111
41111 S
S
In other examples, the dendritic core 12 including the thiocarbonylthio group
in each arm 14 has a
Z-group configuration. In these examples, the reactive polymeric arms 14 are
detached from the
central molecule/compound 13 during growth, and to undergo chain transfer,
again react at the
central molecule/compound 13. One example of the dendritic core having a Z-
group RAFT
configuration is:
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
27
S s
0
s
o 0
S s
0 0
[0093] In an example, the dendritic core 12 including the
thiocarbonylthio group in each arm
14 is selected from the group consisting of 3,5-Bis(2-
dodecylthiocarbonothioylthio-1-
oxopropoxy)benzoic acid:
S CH3 CH3
CI i (C1-12)10012, .k70 arbi 01(L. S õCH CH
S S 3
0 VI 0
0 OH
(an example of a 2-arm dendritic core); 1,1,1-Tris
(dodecylthiocarbonothioylthio)-2-
methylpropionatelethane:
0
CH3
Ci-i(C 112)10C WS Syko""%aelCSNw.SC (( CH3
I e I I
S H3C CH3 cH s
0
(CH, )10CH3
H3C; c s
(an example of a 3-arm dendritic core); and Pentaerythritol tetrakis[2-
(dodecylthiocarbonothioylthio)-2-methylpropionate]:
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
28
113C CH 3 SH
S...CH2(CH2)10CH3
S
0 0
CH3(CH2)ioCH( y A 0/1 %.*-0)1)(S I2) -0H3
s H3C CH3 H3C C1.-1 s
O'''.)(S'''-'Ss*CH2(CH2)icsCH3
H3C Cft,
(an example of a 4-arm dendritic core).
[0094] An example of the dendritic core 12 including a phosphazene ring
as the central
molecule/compound 13 is:
OR
RO P ¨OR
IN;
MN' OR
A
NA
¨0 ¨P ' P
A
N N
P
0
where each R is a trithiocarbonyl group. This is one example of dendritic core
12 including 30
arms.
[0095] Still another example of the dendritic core 12 including the
thiocarbonylthio group in
each arm 14 can be generated through the RAFT polymerization of acrylamide
with N,N'-
methylenebis(acrylamide) (BisAM) as a cross-linker with 3-
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
29
(((Benzylthio)carbonothioyl)thio)propanoic acid, followed by chain extension
with different levels
of acrylamide.
[0096] In another example, the dendritic core 12 includes an atom
transfer radical
polymerization (ATRP) initiator in each arm 14. This example dendritic core 12
may have from 2
arms to 30 arms, each of which includes the ATRP initiator at or near the end
of each arm 14. In
some examples, the dendritic core including the ATRP initiator has 2 arms, 3
arms, 4 arms, 6 arms,
or 8 arms.
[0097] In some examples, the dendritic core 12 including the atom
transfer radical
polymerization (ATRP) initiator is a multi-functional initiator. In these
examples, the dendritic
core 12 may be selected from the group consisting of Bis[2-(2'-
bromoisobutyryloxy)ethyl]disulfide, 2-Bromoisobutyric anhydride, Ethylene
bis(2-
bromoisobutyrate), Pentaerythritol tetrakis(2-bromoisobutyrate),
Dipentaerythritol hexakis(2-
bromoisobutyrate), and 1,1,1-Tris(2-bromoisobutyryloxymethypethane.
[0098] In other examples, mono-functional initiators are attached to a
non-ATRP multi-
functional central molecule to generate the dendritic core 12 including the
atom transfer radical
polymerization (ATRP) initiator in each arm. The non-ATRP multi-functional
central molecule
may be any example of the multi-functional central molecule 13 set forth
herein.
[0099] Examples of the ATRP mono-initiators include 2-azidoethyl 2-
bromoisobutyrate,
poly(ethylene glycol) methyl ether 2-bromoisobutyrate (of varying molecular
weights), 2-(2-
Bromoisobutyryloxy)ethyl methacrylate, Dodecyl 2-bromoisobutyrate, 2-
Hydroxyethyl 2-
bromoisobutyrate, 1-(Phthalimidomethyl) 2-bromoisobutyrate, Propargyl 2-
bromoisobutyrate, or
the like. These mono-initiators may be attached to any example of the central
molecules/compounds 13 disclosed herein to form the dendritic core 12
including the atom transfer
radical polymerization (ATRP) initiator in each arm.
[00100] In still another example, the dendritic core 12 includes a
nitroxide (aminooxyl)
mediated polymerization (NMP) initiator in each arm 14. This example dendritic
core may have
from 2 arms to 30 arms, each of which includes the NMP initiator at or near
the end of each arm
14. In some examples, the dendritic core including the NMP initiator has 2
arms, 3 arms, 4 arms, 6
arms, or 8 arms.
[00101] In some examples, the dendritic core 12 including the NMP initiator
is a multi-
functional initiator. As example, the multi-functional initiator (I) may be:
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
OVVY
OA
*
=
"MY
OVVV
0
ro
L J
or
Different nitroxide end group(s) may be attached to each arm of these
initiators, such as 2,2,6,6-
.
=
Tetramethylpiperidin-l-yl)oxyl (TEMPO): (where I is the multi-
functional
initiator), di-t-butyl nitroxide:
(where I is the multi-functional initiator), 1,1,3,3-
Pr.")
5
tetraethylisoindolin-N-oxyl tetraethylisoindoline nitroxide: (where I is
the multi-
1, =
functional initiator), 2,2,5-Trimethy1-4-pheny1-3-azahexane-3-nitroxide
(TIPNO):
(where I is the multi-functional initiator), N-tert-butyl-N41-diethylphosphono-
(2,2-
\
dimethylpropyOlnitroxide (SG1):
(where I is the multi-functional initiator).
In an example, the dendritic core 12 may be selected from the group consisting
of 1,3,5-tris((4-(1-
10 ((2,2,6,6-tetramethylpiperidin-1-yl)oxy)ethyl)benzyl)oxy)benzene:
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
31
C..
0
-
1'
and 1,3,5-tris((3,5-bis((4-(1-((2,2,6,6-tetramethylpiperidin-1-
yl)oxy)ethyl)benzyl)oxy)benzyl)oxy)benzene:
cto
cto =
ooc
= (..0k) ,o,N
1
9 [
[00102] In other examples, a plurality of mono-functional NMP initiators is
attached to a non-
NMP multi-functional central molecule to generate the dendritic core 12
including the NMP
initiator in each arm. The non-NMP multi-functional central molecule may be
any example of the
multi-functional central molecule 13 set forth herein. Examples of the NMP
mono-initiators
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
32
N(,'
include , and . It is to be
understood
that any of the nitroxide end group(s) described herein may be attached to the
NMP mono-initiator.
In an example, each of the plurality of nitroxide mediated polymerization mono-
initiators attached
to the non-NMP multi-functional central molecule has a structure selected from
the group
N
'0
consisting of: , and
N,
; wherein I is selected from the group consisting of
, and .
Any of these mono-initiators may be attached to any
example of the central molecules/compounds 13 disclosed herein to form the
dendritic core 12
including the NMP initiator in each arm.
[00103] It is to be understood that while several examples of the dendritic
core 12 have been
described, the structure of the dendritic core 12 will depend upon the
polymerization process that is
to be used to generate the multi-arm polymeric hydrogel 10. For example, the
thiocarbonylthio
group-containing dendritic core 12 may be used in RAFT polymerization, while
the ATRP
initiator-containing dendritic core 12 may be used in ATRP, and the NMP
initiator-containing
dendritic core 12 may be used in NMP. Other dendritic cores 12 may be prepared
or obtained and
used in other polymerization processes, such as ROP, etc.
[00104] In the examples of the multi-arm polymeric hydrogel 10 disclosed
herein, the first
acrylamide monomer 16 and the second acrylamide monomer 18 are incorporated
into the arms 14
of the dendritic core 12.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
33
R1
R2
[00105] The first acrylamide monomer 16 has the structure:
wherein R1 and R2 are independently selected from the group consisting of an
alkyl, an alkylamino,
an alkylamido, an alkylthiol, an aryl, a glycol, and optionally substituted
variants thereof R1 and
R2 are selected to provide a more hydrophobic backbone to the arms 14. The R1
and R2 groups
cannot form hydrogen bonds between polymer strands; which may help to increase
the dry storage
ability of the multi-arm polymeric hydrogel 10 without having deleterious
effects on downstream
sequencing operations. In one example, the first acrylamide monomer 16 is NN-
dime thylacrylamide
[00106] The second acrylamide monomer 18 has the structure:
RI 4
A E N3
R3
lo
wherein R3 is hydrogen or an alkyl; R4 is hydrogen or an alkyl; L is a linker
including a linear chain
of 2 atoms to 20 atoms selected from the group consisting of carbon, oxygen,
and nitrogen and
optional substituents on the carbon and any nitrogen atoms in the chain; A is
an N substituted
0
N ________________________________
R5
amide having a structure ,
where R5 is hydrogen or an alkyl; E is a linear chain
of 1 atom to 4 atoms selected from the group consisting of carbon, oxygen and
nitrogen, and
optional substituents on the carbon and any nitrogen atoms in the chain; and Z
is an optional
nitrogen containing heterocycle.
[00107] The azide group of the second acrylamide monomer 18 can
participate in cross-linking
of the multi-arm polymeric hydrogel 10, can attach the multi-arm polymeric
hydrogel 10, e.g., to
the surface of a flow cell (see, e.g., Fig. 2A), and can attach primers (see,
e.g., Fig. 2B and Fig.
2C).
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
34
[00108] When R3 and/or R4 is an alkyl, the number of carbons may range
from 1 to 6 or from 1
to 4.
[00109] In the second acrylamide monomer 18, E may be an optionally
substituted Cl-C4
alkylene, each carbon optionally substituted with one or more substituents
selected from, for
example, Cl-C4 alkyl, -OH, -0C1-C4 alkyl, or =0. As examples, E may be an
unsubstituted Cl-
C4 alkylene, for example CH2, (CH2)2, (CH2)3or (CH2)4
[00110] In other examples, E may include an ether, an ester or an amide.
For example, E may
include -CH2CH2OCH2- , -COCNHCH2- or -CH2COOCH2-.
[00111] In the second acrylamide monomer 18, L may be a linker including
a linear chain that
is a ¨C2-C20 alkylene- or a 3 to 20 atom linear heteroalkylene, each
optionally substituted with
one or more substituents selected from the group consisting of -C1-C4 alkyl, -
OH, -0C1-C4 alkyl,
or =0. L may be a linker with a linear chain that is a ¨C2-C6 alkylene-,
optionally substituted with
one or more ¨C1-C4 alkyl, -OH, -0C1-C4 alkyl, or =0 substituents. L may be
unsubstituted ¨C2-
C6 alkylene- (also drawn as -(CH2)2_6-), for example L may be unsubstituted
¨C3-C4 alkylene-, for
example ¨(CH2)3- or
[00112] In other examples, L may be a linker including a linear chain
that is a 3 to 20 atom
linear heteroalkylene, optionally substituted with one or more substituents
selected from the group
consisting of -C1-C4 alkyl, -OH, -0C1-C4 alkyl, or =0. L may include one or
more ethylene
glycol units. L may be -CH2CH2(OCH2CH2)x-OCH2CH2-, in which x is 0 to 10. In
one example, x
is 1, 2, 3, 4, 5, or 6. L may include one or more amide groups. For example, L
may be ¨C2-C6
alkyl-NHC(0)-C2-C6 alkyl-, or L may be ¨(CH2)2-NHC(0)4CH2)2- or ¨(CH2)3-NHC(0)-
(CH2)2-.
L may include one or more natural or unnatural amino acids, for example L may
include one or
more amino acids selected from the group consisting of glycine, alanine,
valine, isoleucine,
leucine, lysine, serine, threonine, cysteine, asparagine, or glutamine. In
some examples, L may
comprise 1, 2, or 3 amino acid units.
[00113] In the second acrylamide monomer 18, the N substituted amide, A,
may be bonded to
L and Z in two possible configurations, for example the carbonyl carbon of A
may be bonded to L
and the amide nitrogen of A may be bonded to Z. Alternatively, the carbonyl
carbon of A may be
bonded to Z and the amide nitrogen of A may be bonded to L.
[00114] In the second acrylamide monomer 18, Z may include a nitrogen
containing
heterocycle having from 5 to 10 ring members (from 5 to 10 atoms) e.g., a 5 to
10 membered
heterocyclic ring, wherein the ring members are the atoms that form the back
bone of the
heterocyclic ring. Z may include a single cyclic structure or a fused
structure comprising two or
more ring systems. In the case of single cyclic structure, Z may comprise 5 or
6 ring members,
e.g., Z may be a 5 or 6 membered heterocyclic ring. In the case of fused
structure, Z may include 9
or 10 ring members. The nitrogen containing heterocycle may include more than
one heteroatom,
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
for example one or more additional nitrogen heteroatoms, or one or more oxygen
heteroatoms, or
one or more sulphur heteroatoms, or any suitable combination of such
heteroatoms. The nitrogen
containing heterocycle may be aromatic, for example pyridinyl, pyrimidinyl,
pyrrolyl, pyrrazolyl,
imidazolyl, indolyl, quinolinyl, quinazolinyl. The nitrogen containing
heterocycle may be
5 aliphatic, for example a cycloalkyl. The aliphatic nitrogen containing
heterocycle may be saturated
or may include one or more double bonds while not being aromatic. In one
example, the aliphatic
nitrogen containing heterocycle may be pyrrolidinyl, pyridinyl, or
pyrimidinyl.
[00115] One example of the second acrylamide monomer 18 (as shown in Fig.
1A, and does
not include Z) is azido acetamido pentyl acrylamide, and specifically N-(5-
azidoacetamidylpentyl)
10 acrylamide. Variations of N-(5-azidoacetamidylpentyl) acrylamide may
also be used, for example,
the alkyl chain -(CH2)- may range from 1 to 20 and/or each of the -(CH2)- can
be optionally
substituted.
[00116] Some other examples of the second acrylamide monomer 18 including
Z are:
0
H
'===-=,,4õ ..õ - .N . -..........,-,,...õ N...0 0
-1 ii
Hr ..õ.õ..j, ...,...õ...... ..N
. .. :.0
..*:µ,. ...N Ci:\11-r43
N3
15 , ,
0 0
H H
)-LNOorN
N
N
H H
0 N N3 , and 0 N N3
[00117] In the example shown in Fig. 1A, a mixture of the acrylamide
monomers 16, 18 are
polymerized in the presence of the multi-arm component (e.g., the dendritic
core 12). In this
20 example, the multi-arm component is a 4-arm RAFT agent containing four
trithiocarbonate groups,
the first acrylamide monomer 16 is /V,N-dimethylacrylamide, and the second
acrylamide monomer
is azido acetamido pentyl acrylamide 18.
[00118] The mixture of the monomers 16, 18 may include water and a co-
solvent (e.g., N-
methy1-2-pyrollidone (NMP), dimethyl formamide (DMF), dimethyl sulfoxide
(DMSO),
25 acetonitrile (MeCN), methanol (Me0H), ethanol (Et0H), isopropyl alcohol
(IPA), dioxane,
acetone, dimethylacetamide (DMAc), or the like). The mixture may also include
a buffer to at
least substantially prevent undesirable changes in the pH. The pH of the
mixture may be acidic (<
7). Examples of suitable buffers include TRIS (tris(hydroxymethyl)aminomethane
or TRIZMAO),
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
36
Bis-tris methane buffer, ADA buffer (a zwitterionic buffering agent), MES (2-
ethanesulfonic acid),
MOPS (3-(N-morpholino)propanesulfonic acid), or another acidic buffer.
[00119] The polymerization reaction may take place at a temperature
ranging from about 50 C
to about 80 C for a time ranging from about 1 hour to about 48 hours. An
initiator, including azo
initiators, such as azobisisobutyronitrile or 2,2'-Azobis[2-(2-imidazolin-2-
yl)propaneldihydrochloride (one commercially available example is VA-044 from
FujiFilm), may
also be included in the mixture.
[00120] In some examples, the process shown in Fig. lA incorporates the
acrylamide
monomers 16, 18 into each of the arms 14 randomly, although other monomer
incorporation
scenarios (e.g., statistical, alternating, etc.) are possible. Random
incorporation may result in some
blocks of the respective monomers 16 and/or 18. As such, in one example, the
first acrylamide
monomer 16 and the second acrylamide monomer 18 form a random copolymer in
each arm 14 of
the dendritic core 12. The mole ratio of the monomer 16 to the monomer 18 may
range from about
5:95 to about 1:50, or from about 5:95 to about 50:1.
[00121] In another example, the acrylamide monomers 16, 18 may be
incorporated into each of
the arms 14 in controlled blocks. In this example, the block copolymer may be
formed in the
presence of the multi-arm component (e.g., the dendritic core 12). One example
of this method
involves polymerizing a first block with the first acrylamide monomer 16 in
the presence of the
multi-arm component (e.g., the dendritic core 12) to form a modified multi-arm
component (which
includes the first block in each arm 14); and then polymerizing a second block
with the second
acrylamide monomer 18 in the presence of the modified multi-arm component to
form an example
of the multi-arm polymeric hydrogel 10 (which includes both blocks in each arm
14). Another
example of this method involves polymerizing a first block with the second
acrylamide monomer
18 in the presence of the multi-arm component (e.g., the dendritic core 12) to
form a modified
multi-arm component (which includes the first block in each arm 14); and then
polymerizing a
second block with the first acrylamide monomer 16 in the presence of the
modified multi-arm
component to form an example of the multi-arm polymeric hydrogel 10 (which
includes both
blocks in each arm 14). In this example, the first acrylamide monomer 16 and
the second
acrylamide monomer 18 form a block copolymer in each arm 14 of the dendritic
core 12.
[00122] In still other examples, another block may be added to the block
copolymer. This
block may include monomer units not utilized in the other blocks. In one
example, the resulting
block copolymer is a tri-block copolymer.
[00123] In still other examples, the acrylamide monomers 16, 18 may be
incorporated into each
of the arms 14 statistically, where the sequential distribution of the
monomeric units obeys known
.. statistical laws.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
37
[00124] In still further examples, the acrylamide monomers 16, 18 may be
incorporated into
each of the arms 14 so that they are alternating along the length.
[00125] In still other examples, proteins and/or nanoparticles and/or
other polymers may be
conjugated to the end of each arm 14 of the dendritic core 12. These units may
be copolymerized
with the monomer units or may be introduced after polymerization.
[00126] It is to be understood that the arrangement of the recurring "n"
and "m" features in Fig.
lA is representative, and the monomeric subunits 16, 18 may be present in any
order (randomly,
statistically, as alternating units, as a block copolymer, etc.). In an
example, n is an integer ranging
from 1 to 2,500 and m is an integer ranging from 1 to 2,500. In another
example, n + m is an
integer ranging from 2 to 5,000.
[00127] Any example of the hydrogel disclosed herein, including the multi-
arm polymeric
hydrogel 10, may contain a single cross-link per polymer molecule.
[00128] The molecular weight of any example of the hydrogel disclosed
herein, including the
multi-arm polymeric hydrogel 10, may vary depending, at least in part, upon
the starting materials
and the conversion percentage. As one example, the molecular weight of the
multi-arm polymeric
hydrogel 10 is about 850,000 g/mol.
[00129] In other examples, the polymer end groups of the multi-arm
polymeric hydrogel 10
may be cleaved, leaving the arms capped with a suitable end group. Cleavage
may be performed
using any suitable process, such as reaction with peroxide (resulting in an
alcohol end group),
reaction with an azide, radical induced end group removal, UV induced removal,
oxidation-
induced removal, or any other suitable technique. Fig. 1B and Fig. 1C
illustrate two examples in
which the polymer end groups of the multi-arm polymeric hydrogel 10 shown in
Fig. lA have been
cleaved, and the arms have been capped with different ends groups.
[00130] Flow Cell
[00131] The hydrogel disclosed herein may be used in a flow cell 20, an
example of which is
depicted in Fig. 2A. The flow cell 20 includes a substrate 22 and the multi-
arm polymeric
hydrogel 10 on the substrate 22.
[00132] The substrate 22 may be a single layer/material. Examples of
suitable single layer
substrates include epoxy siloxane, glass, modified or functionalized glass,
plastics (including
acrylics, polystyrene and copolymers of styrene and other materials,
polypropylene, polyethylene,
polybutylene, polyurethanes, polytetrafluoroethylene (such as TEFLON from
Chemours), cyclic
olefins/cyclo-olefin polymers (COP) (such as ZEONORO from Zeon), polyimides,
etc.), nylon
(polyamides), ceramics/ceramic oxides, silica, fused silica, or silica-based
materials, aluminum
silicate, silicon and modified silicon (e.g., boron doped p+ silicon), silicon
nitride (Si3N4), silicon
oxide (SiO2), tantalum pentoxide (Ta205) or other tantalum oxide(s) (Ta0x),
hafnium oxide (Hf02),
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
38
carbon, metals, inorganic glasses, or the like. The substrate 22 may also be a
multi-layered
structure. Some examples of the multi-layered structure include glass or
silicon, with a coating
layer of tantalum oxide or another ceramic oxide at the surface. Other
examples of the multi-
layered structure include an underlying support (e.g., glass or silicon)
having a patterned resin
thereon. Still other examples of the multi-layered substrate may include a
silicon-on-insulator
(SOT) substrate.
[00133] In an example, the substrate 22 may have a diameter ranging from
about 2 mm to
about 300 mm, or a rectangular sheet or panel having its largest dimension up
to about 10 feet (¨ 3
meters). In an example, the substrate 22 is a wafer having a diameter ranging
from about 200 mm
to about 300 mm. In another example, the substrate 22 is a die having a width
ranging from about
0.1 mm to about 10 mm. While example dimensions have been provided, it is to
be understood
that a substrate 22 with any suitable dimensions may be used. For another
example, a panel may
be used that is a rectangular support, which has a greater surface area than a
300 mm round wafer.
[00134] In the example shown in Fig. 2A, the flow cell 20 includes flow
channels 24. While
several flow channels 24 are shown, it is to be understood that any number of
channels 24 may be
included in the flow cell 20 (e.g., a single channel 24, four channels 24,
etc.). Each flow channel
24 is an area defined between two bonded components (e.g., the substrate 22
and a lid or two
substrates 22), which can have fluids (e.g., those describe herein) introduced
thereto and removed
therefrom. Each flow channel 24 may be isolated from each other flow channel
24 so that fluid
introduced into any particular flow channel 24 does not flow into any adjacent
flow channel 24.
Some examples of the fluids introduced into the flow channels 24 may introduce
reaction
components (e.g., polymerases, sequencing primers, nucleotides, etc.), washing
solutions,
deblocking agents, etc.
[00135] The flow channel 24 may be defined in the substrate 22 using any
suitable technique
that depends, in part, upon the material(s) of the substrate 22. In one
example, the flow channel 24
is etched into a glass substrate 22. In another example, the flow channel 24
may be patterned into
a resin of a multi-layered substrate 22 using photolithography, nanoimprint
lithography, etc. In
still another example, a separate material (not shown) may be applied to the
substrate 22 so that the
separate material defines the walls of the flow channel 24 and the substrate
22 defines the bottom
of the flow channel 24.
[00136] In an example, the flow channel 24 has a rectilinear
configuration. The length and
width of the flow channel 24 may be smaller, respectively, than the length and
width of the
substrate 22 so that portion of the substrate surface surrounding the flow
channel 24 is available for
attachment to a lid (not shown) or another substrate 22. In some instances,
the width of each flow
channel 24 can be at least about 1 mm, at least about 2.5 mm, at least about 5
mm, at least about 7
mm, at least about 10 mm, or more. In some instances, the length of each lane
20 can be at least
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
39
about 10 mm, at least about 25 mm, at least about 50 mm, at least about 100
mm, or more. The
width and/or length of each flow channel 24 can be greater than, less than or
between the values
specified above. In another example, the flow channel 24 is square (e.g., 10
mm x 10 mm).
[00137] The depth of each flow channel 24 can be as small as a monolayer
thick when
microcontact, aerosol, or inkjet printing is used to deposit a separate
material that defines the flow
channel walls. For other examples, the depth of each flow channel 24 can be
about 1 jun, about 10
jun, about 50 jun, about 100 jun, or more. In an example, the depth may range
from about 10 jun
to about 100 jun. In another example, the depth may range from about 10 gm to
about 30 gm. In
still another example, the depth is about 5 jun or less. It is to be
understood that the depth of each
flow channel 24 be greater than, less than or between the values specified
above.
[00138] Different examples of the architecture within the flow channels
24 of the flow cell 20
are shown Figs. 2B and Fig. 2C.
[00139] In the example shown in Fig. 2B, the flow cell 20 includes a
single layer substrate 22A
and a portion of the flow channel 24 defined in the single layer substrate
22A. In this example, the
multi-arm polymeric hydrogel 10 is positioned within the flow channel 24.
[00140] To introduce the multi-arm polymeric hydrogel 10 (or any example
of the hydrogel
disclosed herein) into the flow channel 24, a mixture of the multi-arm
polymeric hydrogel 10 may
be generated and then applied to the substrate 22 (having the flow channel 24
defined therein). In
one example, the multi-arm polymeric hydrogel 10 may be present in a mixture
(e.g., with water or
with ethanol and water). The mixture may then be applied to the substrate
surfaces (including in
the flow channel(s) 24) using spin coating, or dipping or dip coating, spray
coating, or flow of the
material under positive or negative pressure, or another suitable technique.
These types of
techniques blanketly deposit the catalytic polymeric hydrogel 16' on the
substrate 24 (e.g., in the
flow channel 26 and on the interstitial regions 28). Other selective
deposition techniques (e.g.
involving a mask, controlled printing techniques, etc.) may be used to
specifically deposit the
catalytic polymeric hydrogel 16' in the flow channel 26 and not on the
interstitial regions 28.
[00141] In some examples, the substrate surface (including the portion
that is exposed in the
flow channel 24) may be activated, and then the mixture (including the
hydrogel, such as the multi-
arm polymeric hydrogel 10) may be applied thereto. In one example, a silane or
silane derivative
(e.g., norbornene silane) may be deposited on the substrate surface using
vapor deposition, spin
coating, or other deposition methods. In another example, the substrate
surface may be exposed to
plasma ashing to generate surface-activating agent(s) (e.g., -OH groups) that
can adhere to the
hydrogel, such as the multi-arm polymeric hydrogel 10.
[00142] Depending upon the hydrogel that is used, the applied mixture may
be exposed to a
curing process. In an example, curing may take place at a temperature ranging
from room
temperature (e.g., about 25 C) to about 95 C for a time ranging from about 1
millisecond to about
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
several days. Depending on the material of the hydrogel, other suitable curing
conditions are also
possible.
[00143] Polishing may then be performed in order to remove the hydrogel,
e.g., multi-arm
polymeric hydrogel 10, from the interstitial regions 34 at the perimeter of
the flow channel(s) 24,
5 while leaving the hydrogel on the surface in the flow channel(s) 24 at
least substantially intact.
[00144] The flow cell 20 also includes an amplification primer 26.
[00145] A grafting process may be performed to graft the amplification
primers 26 to the
hydrogel, e.g., the multi-arm polymeric hydrogel 10, in the flow channel 24.
In an example, the
amplification primers 26 can be immobilized to the hydrogel by single point
covalent attachment at
10 or near the 5' end of the primers 26. This attachment leaves i) an
adapter-specific portion of the
primers 26 free to anneal to its cognate sequencing-ready nucleic acid
fragment and ii) the 3'
hydroxyl group free for primer extension. Any suitable covalent attachment may
be used for this
purpose. Examples of terminated primers that may be used include alkyne
terminated primers,
which can attach to the azide moiety of the hydrogel. Specific examples of
suitable primers 26
15 include P5 and P7 primers used on the surface of commercial flow cells
sold by Illumina Inc. for
sequencing on HISEQTM, HISEQXTM, MISEQTM, MISEQDXTM, MNISEQTM, NEXTSEQTm,
NEXTSEQTm DXTM, NOVASEQTM, GENOME ANALYZERTM, ISEQTM, and other instrument
platforms.
[00146] In an example, grafting may involve flow through deposition
(e.g., using a temporarily
20 bound or permanently bonded lid), dunk coating, spray coating, puddle
dispensing, or by another
suitable method that will attach the primer(s) 26 to the hydrogel in the flow
channel 24. Each of
these example techniques may utilize a primer solution or mixture, which may
include the
primer(s) 26, water, a buffer, and a catalyst. With any of the grafting
methods, the primers 26 react
with reactive groups of the hydrogel in the flow channel 24 and have no
affinity for the
25 surrounding substrate 22. As such, the primers 26 selectively graft to
the hydrogel in the flow
channel 24.
[00147] In the example shown in Fig. 2C, the flow cell 20 includes a
multi-layer substrate 22B,
which includes a support 28 and a patterned material 30 positioned on the
support 28. The
patterned material 30 defines depressions 32 separated by interstitial regions
34.
30 [00148] In the example shown in Fig. 2C, the patterned material 30
is positioned on the support
28. It is to be understood that any material that can be selectively
deposited, or deposited and
patterned to form the depressions 32 and the interstitial regions 34 may be
used for the patterned
material 30.
[00149] As one example, an inorganic oxide may be selectively applied to
the support 28 via
35 vapor deposition, aerosol printing, or inkjet printing. Examples of
suitable inorganic oxides
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
41
include tantalum oxide (e.g., Ta205), aluminum oxide (e.g., A1203), silicon
oxide (e.g., SiO2),
hafnium oxide (e.g., Hf02), etc.
[00150] As another example, a resin may be applied to the support 28 and
then patterned.
Suitable deposition techniques include chemical vapor deposition, dip coating,
dunk coating, spin
coating, spray coating, puddle dispensing, ultrasonic spray coating, doctor
blade coating, aerosol
printing, screen printing, microcontact printing, etc. Suitable patterning
techniques include
photolithography, nanoimprint lithography (NIL), stamping techniques,
embossing techniques,
molding techniques, microetching techniques, printing techniques, etc. Some
examples of suitable
resins include a polyhedral oligomeric silsesquioxane resin (POSS)-based
resin, a non-POSS epoxy
resin, a poly(ethylene glycol) resin, a polyether resin (e.g., ring opened
epoxies), an acrylic resin,
an acrylate resin, a methacrylate resin, an amorphous fluoropolymer resin
(e.g., CYTOPO from
Bellex), and combinations thereof
[00151] As used herein, the term "polyhedral oligomeric silsesquioxane"
(POSS) refers to a
chemical composition that is a hybrid intermediate (e.g., RSi015) between that
of silica (5i02) and
silicone (R2Si0). An example of POSS can be that described in Kehagias et al.,
Microelectronic
Engineering 86 (2009), pp. 776-778, which is incorporated by reference in its
entirety. In an
example, the composition is an organosilicon compound with the chemical
formula [RSiO3/210,
where the R groups can be the same or different. Example R groups for POSS
include epoxy,
azide/azido, a thiol, a poly(ethylene glycol), a norbornene, a tetrazine,
acrylates, and/or
.. methacrylates, or further, for example, alkyl, aryl, alkoxy, and/or
haloalkyl groups. The resin
composition disclosed herein may comprise one or more different cage or core
structures as
monomeric units. The polyhedral structure may be a Tg structure, such as:
R7
Si ---- 0 R1
07/ 0 \
R5 ""'S i 0
0
rµ6,
0 R8 ¨si 0
/ 01 / R2
0
Si 0
N'R3 Ts
and represented by: .
This monomeric unit
typically has eight arms of functional groups R1 through Rg.
[00152] The monomeric unit may have a cage structure with 10 silicon atoms
and 10 R groups,
referred to as T10, such as: ,
or may have a cage structure with 12 silicon atoms
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
42
and 12 R groups, referred to as T12, such as: . The POSS-based material may
alternatively include T6, T14, or T16 cage structures. The average cage
content can be adjusted
during the synthesis, and/or controlled by purification methods, and a
distribution of cage sizes of
the monomeric unit(s) may be used in the examples disclosed herein.
[00153] In some of the POSS examples disclosed herein, at least one of R1
through R8 or R10 or
R12 comprises an epoxy. RI through Rg or R10 or R12 may or may not be the
same, and in some
examples at least one of RI through Rg or R10 or R12 comprises epoxy and at
least one other of R1
through Rg or R10 or R12 is a non-epoxy functional group. The non-epoxy
functional group may be
(a) a reactive group that is orthogonally reactive to an epoxy group (i.e.,
reacts under different
conditions than an epoxy group), that serves as a handle for coupling the
resin to an amplification
primer, a polymer, or a polymerization agent; or (b) a group that adjusts the
mechanical or
functional properties of the resin, e.g., surface energy adjustments. In some
examples, the non-
epoxy functional group is selected from the group consisting of an
azide/azido, a thiol, a
poly(ethylene glycol), a norbornene, a tetrazine, an amino, a hydroxyl, an
alkynyl, a ketone, an
aldehyde, an ester group, an alkyl, an aryl, an alkoxy, and a haloalkyl.
[00154] As shown in Fig. 2C, the patterned material 30 includes the
depressions 32 defined
therein, and interstitial regions 34 separating adjacent depressions 32. Many
different layouts of
the depressions 32 may be envisaged, including regular, repeating, and non-
regular patterns. In an
example, the depressions 32 are disposed in a hexagonal grid for close packing
and improved
density. Other layouts may include, for example, rectilinear (rectangular)
layouts, triangular
layouts, and so forth. In some examples, the layout or pattern can be an x-y
format of depressions
32 that are in rows and columns. In some other examples, the layout or pattern
can be a repeating
arrangement of depressions 32 and/or interstitial regions 34. In still other
examples, the layout or
pattern can be a random arrangement of depressions 32 and/or interstitial
regions 34. The pattern
may include spots, pads, wells, posts, stripes, swirls, lines, triangles,
rectangles, circles, arcs,
checks, plaids, diagonals, arrows, squares, and/or cross-hatches.
[00155] The layout or pattern of the depressions 32 may be characterized
with respect to the
density of the depressions 32 (number of depressions 32) in a defined area.
For example, the
depressions 32 may be present at a density of approximately 2 million per mm2.
The density may
be tuned to different densities including, for example, a density of about 100
per mm2, about 1,000
per mm2, about 0.1 million per mm2, about 1 million per mm2, about 2 million
per mm2, about 5
million per mm2, about 10 million per mm2, about 50 million per mm2, or more,
or less. It is to be
further understood that the density of depressions 32 in the patterned
material 30 can be between
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
43
one of the lower values and one of the upper values selected from the ranges
above. As examples,
a high density array may be characterized as having depressions 32 separated
by less than about
100 nm, a medium density array may be characterized as having depressions 32
separated by about
400 nm to about 1 jtm, and a low density array may be characterized as having
depressions 32
separated by greater than about 1 jtm. While example densities have been
provided, it is to be
understood that any suitable densities may be used. The density of the
depressions 32 may depend,
in part, on the depth of the depressions 32. In some instances, it may be
desirable for the spacing
between depressions 32 to be even greater than the examples listed herein.
[00156] The layout or pattern of the depressions 32 may also or
alternatively be characterized
in terms of the average pitch, or the spacing from the center of the
depression 32 to the center of an
adjacent depression 32 (center-to-center spacing) or from the edge of one
depression 32 to the edge
of an adjacent depression 32 (edge-to-edge spacing). The pattern can be
regular, such that the
coefficient of variation around the average pitch is small, or the pattern can
be non-regular in
which case the coefficient of variation can be relatively large. In either
case, the average pitch can
be, for example, about 50 nm, about 0.1 jun, about 0.5 jun, about 1 um, about
5 jun, about 10 um,
about 100 um, or more or less. The average pitch for a particular pattern of
depressions 32 can be
between one of the lower values and one of the upper values selected from the
ranges above. In an
example, the depressions 32 have a pitch (center-to-center spacing) of about
1.5 jun. While
example average pitch values have been provided, it is to be understood that
other average pitch
values may be used.
[00157] The size of each depression 32 may be characterized by its
volume, opening area,
depth, and/or diameter.
[00158] Each depression 32 can have any volume that is capable of
confining a fluid. The
minimum or maximum volume can be selected, for example, to accommodate the
throughput (e.g.,
multiplexity), resolution, labeled nucleotides, or analyte reactivity expected
for downstream uses of
the flow cell 20. For example, the volume can be at least about lx iO3 un3, at
least about lx10-2
um3, at least about 0.1 um3, at least about 1 um3, at least about 10 um3, at
least about 100 um3, or
more. Alternatively or additionally, the volume can be at most about lx104 m3,
at most about
lx iO3 um3, at most about 100 um3, at most about 10 um3, at most about 1 um3,
at most about 0.1
un3, or less.
[00159] The area occupied by each depression opening can be selected
based upon similar
criteria as those set forth above for the volume. For example, the area for
each depression opening
can be at least about lx10 3 [tm2, at least about lx10 2 [un2, at least about
0.1 un2, at least about 1
um2, at least about 10 um2, at least about 100 um2, or more. Alternatively or
additionally, the area
can be at most about lx iO3 um2, at most about 100 um2, at most about 10 um2,
at most about 1
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
44
um2, at most about 0.1 um2, at most about lx10-22, or less. The area occupied
by each
depression opening can be greater than, less than or between the values
specified above.
[00160] The depth of each depression 32 can large enough to house some of
the hydrogel, e.g.,
the multi-arm polymeric hydrogel 10. In an example, the depth may be at least
about 0.1 um, at
least about 0.5 um, at least about 1 um, at least about 10 um, at least about
100 um, or more.
Alternatively or additionally, the depth can be at most about lx iO3 um, at
most about 100 um, at
most about 10 um, or less. In some examples, the depth is about 0.4 um. The
depth of each
depression 32 can be greater than, less than or between the values specified
above.
[00161] In some instances, the diameter or length and width of each
depression 32 can be at
least about 50 nm, at least about 0.1 um, at least about 0.5 um, at least
about 1 um, at least about
10 um, at least about 100 um, or more. Alternatively or additionally, the
diameter or length and
width can be at most about lx iO3 um, at most about 100 um, at most about 10
um, at most about 1
um, at most about 0.5 um, at most about 0.1 um, or less (e.g., about 50 nm).
In some examples,
the diameter or length and width is about 0.4 um. The diameter or length and
width of each
depression 32 can be greater than, less than or between the values specified
above.
[00162] In the example shown in Fig. 2C, the hydrogel (e.g., the multi-
arm polymeric hydrogel
10) is positioned within each of the depressions 32. The multi-arm polymeric
hydrogel 10 or any
other example of the hydrogel disclosed herein may be applied as described in
reference to Fig. 2B,
so that the hydrogel is present in the depressions 32 and not present on the
surrounding interstitial
regions 34.
[00163] In the example shown in Fig. 2C, the primers 26 may be grafted to
the hydrogel within
each of the depressions 32. The primers 26 may be applied as described in
reference to Fig. 2B,
and thus will graft to the hydrogel and not to the surrounding interstitial
regions 34.
[00164] While not shown in Fig. 2A, Fig. 2B, or Fig. 2C, it is to be
understood that the flow
cell 20 may also include a lid attached to the substrate 22. In an example,
the lid may be bonded to
at least a portion of the substrate 22, e.g., at some of the interstitial
regions 34. The bond that is
formed between the lid and the substrate 22 may be a chemical bond, or a
mechanical bond (e.g.,
using a fastener, etc.).
[00165] The lid may be any material that is transparent to an excitation
light that is directed
toward the substrate 22. As examples, the lid may be glass (e.g.,
borosilicate, fused silica, etc.),
plastic, or the like. A commercially available example of a suitable
borosilicate glass is D 263t,
available from Schott North America, Inc. Commercially available examples of
suitable plastic
materials, namely cyclo olefin polymers, are the ZEONORO products available
from Zeon
Chemicals L.P.
[00166] The lid may be bonded to the substrate 22 using any suitable
technique, such as laser
bonding, diffusion bonding, anodic bonding, eutectic bonding, plasma
activation bonding, glass frit
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
bonding, or others methods known in the art. In an example, a spacer layer may
be used to bond
the lid to the substrate 22. The spacer layer may be any material that will
seal at least some of the
substrate 22 and the lid together. In some examples, the spacer layer can be a
radiation-absorbing
material that aids in bonding of the substrate 22 and the lid.
5 [00167] In other examples, the flow cell 20 may also include an
additional patterned or non-
patterned substrate 22 attached to the substrate 22.
[00168] Sequencing Method
[00169] Examples of the flow cell 20 may be used in an ensemble
sequencing technique, such
10 as sequencing by synthesis (SBS). In ensemble sequencing, a template
polynucleotide chain (not
shown) that is to be sequenced may be formed on the flow cell using the
primers 26. At the outset
of template polynucleotide chain formation, library templates may be prepared
from any nucleic
acid sample (e.g., a DNA sample or an RNA sample). The DNA nucleic acid sample
may be
fragmented into single-stranded, similarly sized (e.g., < 1000 bp) DNA
fragments. The RNA
15 nucleic acid sample may be used to synthesize complementary DNA (cDNA),
and the cDNA may
be fragmented into single-stranded, similarly sized (e.g., < 1000 bp) cDNA
fragments. During
preparation, adapters may be added to the ends of these fragments. Through
reduced cycle
amplification, different motifs may be introduced in the adapters, such as
sequencing binding sites,
indices, and regions that are complementary to the primers 26 in the
depressions 32. The final
20 library templates include the DNA or cDNA fragment and adapters at both
ends. In some
examples, the the DNA or cDNA fragments from a single nucleic acid sample have
the same
adapters added thereto. The DNA or cDNA fragment represents the portion of the
final library
template that is to be sequenced.
[00170] A plurality of library templates may be introduced to the flow
cell 20. Multiple library
25 templates are hybridized, for example, to one of two types of primers 26
immobilized in the flow
channel 24 or in the depressions 32.
[00171] Cluster generation may then be performed. In one example of
cluster generation, the
library templates are copied from the hybridized primers by 3' extension using
a high-fidelity DNA
polymerase. The original library templates are denatured, leaving the copies
immobilized in the
30 flow channel 24 or in the depressions 32. Isothermal bridge
amplification or some other form of
amplification may be used to amplify the immobilized copies. For example, the
copied templates
loop over to hybridize to an adjacent, complementary primer 26, and a
polymerase copies the
copied templates to form double stranded bridges, which are denatured to form
two single stranded
strands. These two strands loop over and hybridize to adjacent, complementary
primers 26 and are
35 extended again to form two new double stranded loops. The process is
repeated on each template
copy by cycles of isothermal denaturation and amplification to create dense
clonal clusters. Each
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
46
cluster of double stranded bridges is denatured. In an example, the reverse
strand is removed by
specific base cleavage, leaving forward template polynucleotide strands.
Clustering results in the
formation of several template polynucleotide chains in the flow channel 24 or
in each depression
32. This example of clustering is bridge amplification, and is one example of
the amplification that
.. may be performed. It is to be understood that other amplification
techniques may be used, such as
the exclusion amplification (Examp) workflow (Illumina Inc.).
[00172] A sequencing primer may be introduced that hybridizes to a
complementary sequence
on the template polynucleotide chain. This sequencing primer renders the
template polynucleotide
chain ready for sequencing.
[00173] To initiate sequencing, an incorporation mix may be added to the
flow cell 20. In one
example, the incorporation mix includes a liquid carrier, a polymerase, and
fluorescently labeled
nucleotides. The fluorescently labeled nucleotides may include a 3' OH
blocking group. When
the incorporation mix is introduced into the flow cell 20, the fluid enters
the flow channel 24
and/or the depressions 32 (where the template polynucleotide chains are
present).
[00174] The fluorescently labeled nucleotides are added to the sequencing
primer (thereby
extending the sequencing primer) in a template dependent fashion such that
detection of the order
and type of nucleotides added to the sequencing primer can be used to
determine the sequence of
the template. More particularly, one of the nucleotides is incorporated, by a
respective polymerase,
into a nascent strand that extends the sequencing primer and that is
complementary to the template
polynucleotide chain. In other words, in at least some of the template
polynucleotide chains across
the flow cell 20, respective polymerases extend the hybridized sequencing
primer by one of the
nucleotides in the incorporation mix.
[00175] The incorporation of the nucleotides can be detected through an
imaging event.
During an imaging event, an illumination system (not shown) may provide an
excitation light to
the flow channel 24 and/or depressions 32.
[00176] In some examples, the nucleotides can further include a
reversible termination property
(e.g., the 3' OH blocking group) that terminates further primer extension once
a nucleotide has
been added to the sequencing primer. For example, a nucleotide analog having a
reversible
terminator moiety can be added to the sequencing primer such that subsequent
extension cannot
occur until a deblocking agent is delivered to remove the moiety. Thus, for
examples that use
reversible termination, a deblocking reagent can be delivered to the flow cell
20 after detection
occurs.
[00177] Wash(es) may take place between the various fluid delivery steps.
The SBS cycle can
then be repeated n times to extend the sequencing primer by n nucleotides,
thereby detecting a
sequence of length n.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
47
[00178] While SBS has been described in detail, it is to be understood
that the flow cells 20
described herein may be utilized with other sequencing protocol, for
genotyping, or in other
chemical and/or biological applications. In some instances, the primers of the
flow cell may be
selected to enable simultaneous paired-end sequencing, where both forward and
reverse strands are
present on the hydrogel, allowing for simultaneous base calling of each read.
Sequential and
simultaneously paired-end sequencing facilitates detection of genomic
rearrangements and
repetitive sequence elements, as well as gene fusions and novel transcripts.
In another example,
the flow cells 10 disclosed herein may be used for on-cell library generation.
[00179] To further illustrate the present disclosure, examples are given
herein. It is to be
understood that these examples are provided for illustrative purposes and are
not to be construed as
limiting the scope of the present disclosure.
NON-LIMITING WORKING EXAMPLES
[00180] Example 1
[00181] An example of the multi-arm polymeric hydrogel was prepared using
RAFT
polymerization in accordance with the scheme shown in Fig. 1.
[00182] A comparative polymeric hydrogel (poly(N-(5-
azidoacetamidylpentyl)acrylamide-co-
acrylamide)) was prepared by co-polymerizing acrylamide and N-(5-
azidoacetamidylpentyl)
acrylamide using a free radical synthesis.
[00183] The dispersity of the example multi-arm polymeric hydrogel and the
comparative
polymeric hydrogel was calculated. The results are shown in Fig. 3, where the
median for the
example polymeric hydrogel was about 1.9 and the median for the comparative
polymeric hydrogel
was about 3.3. As depicted, the dispersity of the example multi-arm polymeric
hydrogel was much
lower than the comparative polymeric hydrogel, and thus the example multi-arm
polymeric
hydrogel has a narrower molecular weight distribution than the comparative
polymeric hydrogel.
[00184] Example 2
[00185] The example multi-arm polymeric hydrogel and the comparative
polymeric hydrogel
of Example 1 were coated in flow channels on respective glass (specifically
fused silica) slides,
and 0.1-50 1.1.M oligonucleotide primers were grafted on each of the polymer
layers. The flow cells
were stored at 60 C for 20 days.
[00186] After storage, 300 sequencing cycles were performed in each of
the channels using a
PhiX library.
[00187] The sequencing data collected included phasing (percentage, shown
in Fig. 4 at A),
quality score (percentage greater than Q30, shown in Fig. 4 at B), and error
rate (percentage)
(shown in Fig. 4 at C). Phasing is the rate at which single molecules within a
cluster loose sync
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
48
with each other. Therefore, a lower phasing percentage is more desirable. Q30
is equivalent to the
probability of an incorrect base call 1 in 1000 times. This means that the
base call accuracy (i.e.,
the probability of a correct base call) is 99.9%. A lower base call accuracy
of 99% (Q20) will have
an incorrect base call probability of 1 in 100, meaning that every 100 base
pair sequencing read
will likely contain an error. When sequencing quality reaches Q30, virtually
all of the reads will
be perfect, having zero errors and ambiguities. As shown in Fig. 4, at A, B,
and C, the example
multi-arm polymeric hydrogel performed better than the comparative example
with regard to
phasing, Q30, and error rate. The phasing results for the example multi-arm
polymeric hydrogel
remained at or below 0.19%, even when stored for longer periods, such as 14
days and 20 days. In
contrast, the phasing results for the comparative example multi-arm polymeric
hydrogel increased
to about 0.26% at 14 days and to about 0.39% at 20 days. The Q30 results for
the example multi-
arm polymeric hydrogel remained at or above 85%, even when stored for the
longer periods. In
contrast, the Q30 results for the comparative example multi-arm polymeric
hydrogel decreased to
about 70% at 14 days and to almost 40% at 20 days. The error rate results for
the example multi-
arm polymeric hydrogel remained at or below 2%, even when stored for the
longer periods. In
contrast, the error rate results for the comparative example multi-arm
polymeric hydrogel
decreased to about 2.5% at 14 days and to almost 14% at 20 days. All of these
results indicate that
the example multi-arm polymeric hydrogel is more resistant to irreversible
changes as a result of
dry staging than the comparative example polymeric hydrogel.
[00188] Moreover, the multi-arm polymeric hydrogel architecture may also
minimize
interactions between the multi-arm polymeric hydrogel and DNA during
clustering and/or
sequencing, which may be contributing to the improved sequencing
performance/metrics.
[00189] Example 3
[00190] The example multi-arm polymeric hydrogel and the comparative
polymeric hydrogel
of Example 1 were respectively coated in the depressions of four glass
(specifically fused silica)
flow channels (lanes) of two different patterned flow cells, and 0.1-50 uM
oligonucleotide primers
were grafted on the polymers in the depressions.
[00191] More than 300 sequencing cycles were performed in each of the
flow channels using a
PhiX library.
[00192] The sequencing data collected included quality score (percentage
greater than Q30,
shown in Fig. 5A) and pre-phasing (percentage, shown in Fig. 5B). As shown in
Fig. 5A, the
quality metrics decreased slower for the example multi-arm polymeric hydrogel
than for the
comparative example polymeric hydrogel, resulting in better sequencing runs
especially toward
large number of cycles. The Q30 results for the example multi-arm polymeric
hydrogel remained
at or above 55% for all cycles, and at or above 85% for about 200 cycles. In
contrast, the Q30
CA 03135068 2021-09-24
WO 2021/110700
PCT/EP2020/084163
49
results for the comparative example multi-arm polymeric hydrogel decreased
below 80% at about
175 cycles, and then dropped below 55% at about 240 cycles. As shown in Fig.
5B, pre-phasing
was significantly reduced for the example multi-arm polymeric hydrogel when
compared to the
comparative example polymeric hydrogel, resulting in better sequencing runs.
The average pre-
phasing results for the example multi-arm polymeric hydrogel across the 4
lanes was about 0.11%,
whereas the average pre-phasing results for the comparative example multi-arm
polymeric
hydrogel across the four lanes was about 0.17%.
[00193] Example 4
[00194] The example multi-arm polymeric hydrogel and the comparative
polymeric hydrogel
of Example 1 were respectively coated in depressions of a resin layer of a
multi-layer substrate,
and 0.1-50 uM oligonucleotide primers were grafted on each of the polymer
layers.
[00195] 151 sequencing cycles were performed during read 1 (R1) and read
2 (R2) in each of
the flow channels using a human library with 1% PhiX library.
[00196] The
sequencing data collected included error rate (percentage, shown in Fig. 6A
for
R1 and Fig. 6B for R2). As shown in Fig. 6A and Fig. 6B, the error rate for
the example multi-arm
polymeric hydrogel was significantly reduced compared to the comparative
example polymeric
hydrogel during each read. The mean error rate for the flow cell with the
example multi-arm
polymeric hydrogel was 0.65, compared to 0.93 for the comparative flow cell
with the comparative
polymeric hydrogel.
[00197] Example 5
[00198] The example multi-arm polymeric hydrogel and the comparative
polymeric hydrogel
of Example 1 were respectively coated in the depressions of four glass
(specifically fused silica)
flow channels (lanes) of two different patterned flow cells, and 0.1-50 uM
oligonucleotide primers
were grafted on each of the polymer layers.
[00199] 151 sequencing cycles were performed in each of the flow channels
using a human
library with 1% PhiX library.
[00200] The sequencing data collected included first cycle (C1) intensity,
passing filter (%PF)
(percentage), phasing (%), pre-phasing (%), Q30, and error rate. Passing
filter (PF) is the metric
used to describe clusters which pass a chastity threshold and are used for
further processing and
analysis of sequencing data. A higher %passing filter result indicates an
increased yield of unique
clusters used for sequencing data. Reproducible data was observed across the
lanes of the flow
cells. The sequencing data for one of the lanes of each of the flow cells is
shown in Table 1.
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
TABLE 1
Pre- Error
Cl Phasing Q30
Polymer PF (%) phasing Rate
Intensity (0/0)
(0/0) (%
Example multi-
arm polymeric 239 75.97 0.120 0.105 76.64 0.72
hydrogel
Comparative
polymeric 238 52.18 0.116 0.115 69.84 1.12
hydrogel
5
[00201] As depicted in Table 1, the sequencing results for the example
multi-arm polymeric
hydrogel were better than (e.g., PF%, Q30, error rate) or comparable to (e.g.,
Cl intensity, phasing
and pre-phasing) the comparative example polymeric hydrogel. The mean Cl
intensity for all the
lanes is shown in Fig. 7A. The mean Cl intensity for the example polymeric
hydrogel was about
10 275, while the mean Cl intensity for the comparative example polymeric
hydrogel was about 250.
These results illustrate that the intensity of the example polymeric hydrogel
is as good as, and even
better than the comparative example polymeric hydrogel. The mean error rate
for all the lanes is
shown in Fig. 7B. The mean error rate for the example polymeric hydrogel was
about 1.5 times
less than the mean error rate for the comparative example polymeric hydrogel.
Therefore, over all
15 the lanes, the example multi-arm polymeric hydrogel performed better
than the comparative
example polymeric hydrogel in terms of Cl intensity and error rate.
[00202] Example 6
[00203] The example multi-arm polymeric hydrogel and the comparative
polymeric hydrogel
20 of Example 1 were also used in this example. Each of the hydrogels was
respectively coated in
the depressions of four glass (specifically fused silica) flow channels
(lanes) of two patterned flow
cells, and 0.1-50 uM oligonucleotide primers were grafted on each of the
polymer layers.
[00204] Several sequencing cycles were performed in each of the flow
channels using a human
library with 1% PhiX library.
25 [00205] Duplicate read data was collected for one lane of the example
flow cell and for one
lane of the comparative example flow cell over the sequencing cycles.
Sequencing reads may be
determined to be duplicates if both forward and reverse reads have identical
starting positions. A
lower percentage of duplicates is desirable. The duplicate read results are
shown in Fig. 8A. As
depicted, the example flow cell including the example multi-arm polymeric
hydrogel exhibited few
30 duplicate reads over the sequencing runs. In particular, the percentage
of duplicate reads for the
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
51
example flow cell ranged from about 2.5% to about 12%. In contrast, the
comparative flow cell
had much higher percentages of duplicate reads, ranging from about 10% to
about 24%.
[00206] Pad hopping data was also collected for one lane of the example
flow cell and for one
lane of the comparative example flow cell over the sequencing cycles. Pad
hopping refers to the
process of several adjacent depressions being amplified from the same template
sequence, due to
the template "hopping" to an adjacent depression during cluster generation. A
lower percentage of
pad hopping is desirable. The pad hopping results are shown in Fig. 8B. As
depicted, the example
flow cell including the example multi-arm polymeric hydrogel exhibited little
(e.g., < about 1%) to
no pad hopping over the sequencing runs. In contrast, the comparative flow
cell including the
comparative example polymeric hydrogel exhibited much higher pad hopping
levels, ranging from
about 1% to about 27%.
[00207] The results of all of the examples indicate that the multi-arm
polymeric hydrogel can
be used on a variety of different flow cell architectures, can improve
sequencing metrics, and can
also improve dry storage stability (e.g., sequencing performance is not
deleteriously affected even
after a period of dry storage).
[00208] Additional Notes
[00209] It should be appreciated that all combinations of the foregoing
concepts and additional
concepts discussed in greater detail below (provided such concepts are not
mutually inconsistent)
are contemplated as being part of the inventive subject matter disclosed
herein. In particular, all
combinations of claimed subject matter appearing at the end of this disclosure
are contemplated as
being part of the inventive subject matter disclosed herein. It should also be
appreciated that
terminology explicitly employed herein that also may appear in any disclosure
incorporated by
reference should be accorded a meaning most consistent with the particular
concepts disclosed
herein.
[00210] Reference throughout the specification to "one example", "another
example", "an
example", and so forth, means that a particular element (e.g., feature,
structure, and/or
characteristic) described in connection with the example is included in at
least one example
described herein, and may or may not be present in other examples. In
addition, it is to be
understood that the described elements for any example may be combined in any
suitable manner
in the various examples unless the context clearly dictates otherwise.
[00211] It is to be understood that the ranges provided herein include
the stated range and any
value or sub-range within the stated range, as if such values or sub-ranges
were explicitly recited.
For example, a range from about 200 mm to about 300 mm, should be interpreted
to include not
only the explicitly recited limits of from about 200 mm to about 300 mm, but
also to include
individual values, such as about 240 mm, about 250.5 mm, etc., and sub-ranges,
such as from
CA 03135068 2021-09-24
WO 2021/110700 PCT/EP2020/084163
52
about 225 mm to about 275 mm, etc. Furthermore, when "about" and/or
"substantially" are/is
utilized to describe a value, they are meant to encompass minor variations (up
to +/- 10%) from the
stated value.
[00212] While several examples have been described in detail, it is to be
understood that the
disclosed examples may be modified. Therefore, the foregoing description is to
be considered non-
limiting.
15
25
35