Note: Descriptions are shown in the official language in which they were submitted.
APPLICATION OF:
Deanna Montrose (Phoenix, AZ)
FOR UNIIED STATES LETTERS PATENT ON
TITLE: SKIN THERAPY SYSTEMS
1
Date Recue/Date Received 2023-05-30
APPLICATION FOR PATENT
TITLE: SKIN THERAPY SYSTEMS
Inventor: Deanna Montrose (Phoenix, AZ)
CROSS-REFERENCES TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional Patent
Application Serial
No. 62/830,196, filed on April 5, 2019, and is a Continuation-In-Part of U.S.
Reissue
application entitled SKIN THERAPY SYSTEMS, assigned application number
15/979,459, filed on May 14, 2018, which claims priority to U.S. Provisional
Patent
Application Serial No. 62/505,700, filed on May 12, 2017, and is a
Continuation-In-
Part of U.S. Reissue application entitled SKIN THERAPY SYSTEMS, assigned
application number 14/972,811, filed on December 17, 2015, now abandoned. To
the
extent that the present disclosure conflicts with the referenced applications,
however,
the present disclosure is to be given priority.
BACKGROUND
[0002] Wax-based therapeutic compositions applied externally to the
skin may be
used to condition and soften skin, relieve joint pain, and improve a variety
of skin
conditions. Wax-based therapeutic compositions may be heated to melt the wax
and
to heat the skin or body part to which it is applied for pain relief. Current
methods of
melting bulk quantities of wax-based therapeutic compositions may take
approximately two to three hours, impeding the delivery of the therapy to a
person.
Further, repeated heating-cooling cycles of wax-based therapeutic compositions
for
2
Date Recue/Date Received 2023-05-30
skin therapy may reduce the effectiveness of the composition over time.
Additionally, overheating and uneven heating and cooling of the wax-based
therapeutic compositions poses a significant safety risk for burning the
person's skin.
SUMMARY OF THE TECHNOLOGY
[0003] A convenient and hygienic skin therapy system comprising an
encaser, one or
more therapeutic compositions, a sealing means and optional accessories is
disclosed.
A method of using such a skin therapy system is also provided. The skin
therapy
system and the use thereof provides an effective, efficient and safe
therapeutic
approach.
BRIEF DESCRIPTION OF THE DRAWING
[0004] A more complete understanding of the present technology may be
derived by
referring to the detailed description when considered in connection with the
following
illustrative figures. In the following figures, like reference numbers refer
to similar
elements and steps throughout the figures.
[0005] The figures described are for illustration purposes only and are
not intended to
limit the scope of the present disclosure in any way. Various aspects of the
present
technology may be more fully understood from the detailed description and the
accompanying drawing figures, wherein:
[0006] FIG. 1 illustrates an exemplary therapy system comprising a
glove;
[0007] FIG. 2 shows a top view illustrating the therapy system
comprising a glove;
[0008] FIG. 3 illustrates an exemplary therapy system comprising a boot;
[0009] FIG. 4A shows a perspective view illustrating a container for
holding and
heating the therapy system;
3
Date Recue/Date Received 2023-05-30
[0010] FIG. 4B shows a planar view illustrating the container for
holding and heating
of the therapy system;
[0011] FIG. 5 shows a cross-sectional view along section 5-5 of FIG. 4B;
[0012] FIG. 6A shows a planar view illustrating the container for
holding and heating
the therapy system;
[0013] FIG. 6B shows a perspective view of the therapy system as a pre-
packaged kit;
[0014] FIG. 7 shows a perspective view illustrating the therapy system
comprising a
glove;
[0015] FIG. 8 shows a diagrammatic view illustrating heating of a
therapeutic
composition;
[0016] FIG. 9 illustrates a method of using the therapy system;
[0017] FIG. 10 is a flow chart illustrating a method of heating the
therapy system;
[0018] FIGS. 11A and 11B show additional dimensions and details of the
therapy
system comprising a glove and a boot;
[0019] FIGS. 12A and 12B illustrate an exemplary thermochromatic ink
applied to the
encaser of the therapy system;
[0020] FIG. 13 illustrates a therapy system with thermochromatic ink
indicating
uneven heat in the therapeutic composition;
[0021] FIG. 14 illustrates an exemplary therapy system with heat-sealed
edges;
[0022] FIG. 15 is an exemplary cross-section view of a skin therapy
system, which
illustrates various methods of skin therapy; and
[0023] FIG. 16 is an exemplary cross-section view of a skin therapy
system, which
illustrates various methods of transdermal delivery of a medicinal.
4
Date Recue/Date Received 2023-05-30
DETAILED DESCRIPTION
[0024] The present technology may be described in terms of functional
block
components and various processing steps. Such functional blocks may be
realized by
any number of components configured to perform the specified functions and
achieve
the various results. For example, methods and systems according to various
aspects of
the present technology may employ various materials for containing heat-stable
therapeutic compositions which may be practiced in conjunction with any number
of
compositions and procedures for treating pain and inflammation of joints and
other
body parts and the systems described are merely exemplary applications for the
technology. Various representative implementations of the present technology
may be
applied to any portion of the human body for the treatment of skin, pain,
injury,
and/or inflammatory medical conditions.
[0025] The particular implementations shown and described are
illustrative of the
technology and its best mode and are not intended to otherwise limit the scope
of the
present technology in any way. For the sake of brevity, conventional
manufacturing,
connection, preparation, sterilization, and other functional aspects of the
system may
not be described in detail. Furthermore, the connecting lines shown in the
various
figures are intended to represent exemplary functional relationships and/or
steps
between the various elements. Many alternative or additional functional
relationships
or physical connections may be present in a practical system.
[0026] Various aspects of the exemplary embodiments provide methods,
apparatus,
and systems for making and using a skin therapy system 100 and may comprise
one
Date Recue/Date Received 2023-05-30
or more of an encaser 120, encaser liner 116, therapeutic composition 106,
and/or
temperature indicator 118. The encaser 120 comprises an internal volume for
receiving a person's body part, such as a hand, foot, and/or elbow. The
encaser 120
may comprise a fastener that may be configured to attach or hold the encaser
120 to
the desired portion of the body. The encaser liner 116 forms a second internal
volume
for receiving the body part and is positioned within the internal volume of
the encaser
120 and helps with the substantially even distribution of the therapeutic
composition
106 throughout the encaser 120 in a space formed between an inner surface of
the
encaser 120 and an outer surface of the encaser liner 116. The therapeutic
composition 106 may comprise a wax with any number of cosmetic and/or
pharmaceutical compositions that may treat problems of the skin and/or pain,
injury,
and/or inflammation of the body. The temperature indicator 118 may comprise
any
temperature activated device, ink, or other indicator that may be applied to
the
encaser 120, the encaser liner 116, and/or the therapeutic composition 106
that
functions to indicate the temperature of the therapeutic composition 106 to
ensure
safe use of the therapy system. A detailed description of various embodiments
is
provided as a specific enabling disclosure that may be generalized to any
application
of the disclosed systems and methods.
[0027] Various embodiments of the skin therapy system 100 may be
configured as a
sealed, reusable container for a therapeutic composition 106 disposed inside
the
encaser 120. For example, an outer peripheral edge of the encaser liner 116
may be
coupled to an inner surface of the encaser 120 along an outer peripheral edge
of the
encaser 120 such that the encaser liner 116 retains the general shape of the
encaser
6
Date Recue/Date Received 2023-05-30
120 while also creating a fillable space or region between an inner surface of
the
encaser 120 and the outer surface of the encaser liner 116. The therapeutic
composition 106 may then be disposed within the fillable space prior to use.
The
encaser liner 116 remains stretched to edges of the encaser 120 regardless of
the
composition and/or temperature of the therapeutic composition 106. This
configuration prevents the contraction, or "balling up" of the encaser liner
116 when
the body part is removed from the encaser 120 and/or when the therapeutic
composition 106 cools after use.
[0028] The sealed configuration of the skin therapy system 100 allows
it to be heated
in a variety of apparatus while avoiding contamination of the enclosed encaser
liner
116 and therapeutic composition 106. In today's clinical and/or cosmetic
settings,
such as hospitals, spas, and cosmetology salons, infection from methicillin-
resistant
Staphylococcus aureus (MRSA) can be deadly for patients and clients. Unlike
conventional wax treatment systems, the encaser liner 116 is not dipped into a
communal container of liquid paraffin (sometimes referred to as a "paraffin
pot").
Instead, the fillable space is filled with the therapeutic composition 106 and
sealed
into place.
[0029] The encaser 120 may be formed into the shape of a desired body
part such as:
a hand, a foot, an elbow, a knee, a patch or strip suitable to attach to the
torso or skull,
and/or any other body part. Alternatively, the encaser 120 may be formed in
the shape
of a glove, a mitten, a muff, a fingerstall, a sock, a slipper, a shoe, a
booty, a bonnet, a
strip, a knee support, a skullcap, and/or a mask in the form of all or part of
the face or
head, or any other suitable forms and shapes. In one embodiment, the encaser
120 is
7
Date Recue/Date Received 2023-05-30
hand shaped. In another embodiment, the encaser 120 is foot shaped.
[0030] The encaser 120 may comprise any suitable encaser materials to
contain the
therapeutic composition 106 such as carbon-fiber, plastic,
transparent/translucent
polymer such as polyethylene, plastic film, metal foil, depending on design
requirements and heating sources. The encaser 120 may be made of elastic
material
that allows for expanding or stretching. The material used to make the encaser
120 is
heat-durable and does not release toxic chemicals upon heating.
[0031] The material of the encaser 120 may be formed of at least two
layers of the
encaser material. For example, the encaser material may be adhered together to
appear as a single layer. In some embodiments, a temperature indicator 118
such as a
thermochromatic material comprising ink and/or other markings such as a
trademark
may be printed, stamped, and/or adhered to one layer of the encaser material.
The
encaser material may then be adhered to another layer of encaser material such
that
the thermochromatic material or other marking may be sealed between the two
layers
of the encaser material.
[0032] The encaser 120 may also be configured to accommodate body parts
suffering
from injury or restricted movement. For example, the encaser 120 may be formed
for
a hand in which the user may not have full range of motion or restricted
movements
in the fingers such that the fingers may not be comfortably placed in a
standard glove
configuration. In this embodiment, the encaser 120 may accommodate two or more
fingers in a single section while allowing one or more other fingers to be
positioned
within their own section. The encaser 120 may also comprise elements, such as
a
8
Date Recue/Date Received 2023-05-30
strap, configured to help position a finger or body part in a desired position
during
treatment.
[0033] In some embodiments, the body part shaped encaser 120 may be
unisized,
such that it references to the average body part size acceptable in the
industry. In one
embodiment, the encaser 120 may be unisized for women's hands or feet. In one
embodiment, the encaser 120 may be unisized for men's hands or feet. In one
embodiment, the encaser 120 may be stretchable such that it can fit for any
size of
body part of a man and/or a woman. The encaser 120 may also be manufactured in
a
series of sizes that are standard in the industry, such like the standard
sizes for gloves,
shoes, and other similar products. The encaser 120 may have in-folds, which
provide
a three-dimensional shape that better accommodates the shape and dimension of
a
body part upon usage. In one embodiment, the encaser 120 is foot-shaped, with
an in-
fold providing a sole of a slipper, a boot, a shoe when unfolded.
[0034] FIG. 1 shows a schematic diagram depicting multiple embodiments
of a
single-use body part-shaped encaser 120 to exemplify the skin therapy system
100.
Single-use body part-shaped encaser 120 utilizes at least one single-use glove
102 or,
alternately, at least one single-use boot 104. Such a single-use glove 102 or
single-use
boot 104 comprises a hand or foot encaser 120 for encasing at least one human
hand
or foot. Each single-use glove 102 or each single- use boot 104, contains at
least one
therapeutic composition 106, which may be quickly heated (from about one to
about
four minutes) by various healing sources including, but not limited to, a
microwave
oven 108.
9
Date Recue/Date Received 2023-05-30
[0035] In
some embodiments, each single-use glove 102 or single-use boot 104 is
structured and arranged to be a sanitary one-time-use disposable product as
further
described herein. Upon reading this specification those of ordinary skill in
the art will
appreciate that, under appropriate circumstances, other device configurations,
such as,
for example, arm wraps, leg wraps, etc., may suffice. Various embodiments of
skin
therapy system 100, as described herein, may function to warm the skin to help
soften
dead skin (thus facilitating exfoliation). In addition, various embodiments of
skin
therapy system 100 may function to warm joints and assist with circulation.
[0036] As
an example, FIG. 2 shows a top view, illustrating a single-use glove 102
of the skin therapy system 100. Single-use glove 102 may comprise therapeutic
composition 106 positioned in each finger portion 111 and thumb portion 115
and at
the palm region 123, as shown. In one embodiment, the palm region 123 of
single use
glove 102 comprises at least one first quantity of therapeutic composition
106. As
shown in FIG. 2, the therapeutic composition 106 is placed within the interior
of the
single-use glove 102 in the palm region 123. In another embodiment, each
single-use
glove 102 comprises at least one second quantity of therapeutic composition
106
placed within the interior of the glove in each of the finger portions 111 and
thumb
portion 115, as shown. The therapeutic composition 106 in the palm region and
the
hand digit portions 111 and 115 may be the same or different.
[0037] As
shown in FIG. 2, single-use glove 102 comprises one substantially flexible
containment wall 107 having an interior portion 113 structured and arranged to
contain at least one palm composition 130, a finger composition 132, and a
thumb
composition 134. Containment wall 107 further comprises an access opening,
which
Date Recue/Date Received 2023-05-30
is a hand aperture 140 in the single-use glove 102 embodiment. Hand aperture
140
may permit a user to insert a hand into the single-use glove 102, during use.
Hand
aperture 140 may comprise a width, when flat, of about seven inches. Single-
use
glove 102 may further comprise at least one temperature indicator 118,
optionally
positioned at any finger-tip, at the thumb-tip, at the palm area, or anywhere
the
temperature indicator 118 may sense the temperature of the therapeutic
composition
106 enclosed in the single-use glove 102.
[0038] FIG. 3 shows a top view illustrating a single-use boot 104 of
the skin therapy
system 100. In one embodiment, the single-use boot 104 comprises a therapeutic
composition 106 positioned in the ankle region 145, and/or toe region, of the
single-
use boot 104. Therapeutic composition 106 in ankle region 145 of single-use
boot
104 may comprise at least one ankle composition 136. In addition, the single-
use
boot 104 comprises at least one foot aperture 142. Foot aperture 142 permits
the user
to insert a foot into the single-use boot 104, during use. Foot aperture 142
may
comprise a width, when flat, of about ten inches.
[0039] FIGS. 11A and 11B depict exemplary dimensions and details of an
embodiment of a single-use glove 102 and single-use boot 104 for the skin
therapy
system 100:
FIG 11A ¨ material thickness per sheet: 0.0875 mm and two sheets
will be seamed together along profile; and
FIG 11B ¨ material thickness per sheet: 0.0875 mm and two sheets
will be seamed together along profile.
11
Date Recue/Date Received 2023-05-30
Upon reading this specification, those with ordinary skill in the art will now
appreciate that, under appropriate circumstances, considering such issues as
design
preference, user preferences, marketing preferences, cost, structural
requirements,
available materials, technological advances, etc., other glove and boot
material
arrangements that meet or exceed criteria set forth herein may suffice. In
addition to
customized manufacturing a single-use glove 102 and single-use boot 104
encaser,
the single-use boot 104 (without any therapeutic composition) is also
commercially
available for purchase, for example, the Sani-boot made by Keystone, or
similar
products through Pro-safety of Milwaukee WI.
[0040]
The therapeutic compositions 106 applicable for the skin therapy system 100
may be wax-based, liquid-based, or gel-based; all of which are to be spread
evenly
inside the encaser in a pre-determined amount prior to skin application. For a
wax-
based composition, the solidified composition is spread into a thin layer in a
body
part shaped encaser 120 with the shape essentially the same as the encaser
120. For a
liquid based composition, a predetermined amount of the composition sufficient
to
cover targeted skin area is enclosed in a body part shaped encaser. The
therapeutic
composition 106 is contained inside the encaser 120 without the risk of
leaking, spill,
evaporation, or cross-contamination, and can readily be applied to the skin
area
without the need of further spreading while providing nearly even and direct
contact
to skin under treatment. The therapeutic compositions 106 applicable for the
skin
therapy system 100 may comprise various ingredients, which are selected
according
to their physical, chemical, or pharmaceutical characteristics suitable for
the skin
therapy system 100 and therapeutic targets. The wax-based, liquid-based, or
gel-
12
Date Recue/Date Received 2023-05-30
based therapeutic composition 106 is pre-packaged into the encaser of the skin
therapy system 100 and may or may not require heating prior to skin
application. In
other embodiments, the wax-based, liquid-based, or gel-based therapeutic
composition may be installed into the encaser 120 customarily prior to a
therapy
session. The written description below provides further details of therapeutic
compositions 106.
[0041]
Each encaser 120 of the skin therapy system 100 may comprise one or more
therapeutic compositions 106. In some embodiments, the therapeutic composition
106 is positioned in a single-use body part shaped encaser 120 at time of
manufacturing. In some embodiments, the therapeutic composition 106 is
uniformly
positioned in a single-use body part shaped encaser 120, such that the
therapeutic
composition 106 is spread about evenly as a thin layer throughout the encaser
120. In
some embodiments, the therapeutic composition 106 in a body part shaped
encaser
120 is a contiguous thin layer of solidified or gel-like form extending evenly
to each
interior portion 113 or region of an encaser 120. Depending on the therapeutic
composition 106 (solid or gel, wax-, mud- or clay- based mixture), the
thickness of
the contiguous thin layer of the composition may be between about 0.1 inch to
about
1.5 inches. In some embodiments, a solid form therapeutic composition 106 of a
quantity may be molded into a certain shape of certain size before positioned
in a
body part shaped encaser 120. In other embodiments, the therapeutic
composition
106 of a quantity may be added in to a body part shaped encaser 120 when the
therapeutic composition 106 is in a liquefied state, which then may solidify
or
undergo gelification with or without pressing or molding the composition into
the
13
Date Recue/Date Received 2023-05-30
shape of the encaser 120 upon cooling or sitting. Upon reading this
specification,
those with ordinary skill in the art will now appreciate that, under
appropriate
circumstances, considering such issues as design preference, user preferences,
marketing preferences, cost, structural requirements, available materials,
technological advances, etc., other therapeutic substance insert arrangements
such as,
for example, pre-coating of the internal glove, linked portions insertable
into the
glove, etc., may suffice.
[0042] In some embodiments a quantity of a first therapeutic
composition 106 is
placed in each of the fingers and thumb portion 111, 115 of a hand shaped
encaser
120, and a quantity of a second therapeutic composition 106 is placed in the
palm
region 123 of a single-use body part shaped encaser 120; wherein the first
therapeutic
composition 106 heats up at a slightly slower rate than the second therapeutic
composition 106 placed in the palm region 123 such that, upon heating, all of
therapeutic composition 106 placed into a hand shaped encaser 120 will heat up
or
melt about equally and reach a predetermined temperature at about the same
time.
Even heating without overheating any portion of a therapeutic composition 106
may
be important for even liquefaction of solid form therapeutic composition 106,
and the
safe use of the skin therapy system 100 to prevent skin injury due to
excessive or
uneven heat during the direct skin-composition contact in a skin therapy.
[0043] As exemplified in FIG. 2, therapeutic composition 106 in each
finger portion
111 may comprise at least one finger composition 132, or alternately, in thumb
portion 115 at least one thumb composition 134. Finger composition 132 may
comprise enough therapeutic composition 106, when melted or heated, to
14
Date Recue/Date Received 2023-05-30
substantially coat each finger portion 111 of single-use glove 102. In one
embodiment, finger composition 132 comprises about one-half ounce of
therapeutic
composition 106. Thumb composition 134 may comprise enough therapeutic
composition 106, when melted or heated, to substantially coat the thumb
portion 115
of single-use glove 102. In one embodiment, thumb composition 134 comprises
about one ounce of therapeutic composition 106. In one embodiment, finger
composition 132 and thumb composition 134 comprise at least one insert 135,
which
may be a bar as shown, or pellets, or a thin layer of solidified or gel like
therapeutic
composition 106. In one embodiment, finger composition 132 comprises an insert
135 such as a bar having a length of about three inches, a width of about one-
half
inch, and a thickness of about one-half inch. In one embodiment, thumb
composition
134 comprises an insert 135 such as a bar having a length of about two-and one-
half
inches, a width of about one inch, and a thickness of about one- half inch.
[0044]
Therapeutic composition 106 in the palm region 123 comprises at least one
palm composition 130. Palm composition 130 may comprise enough therapeutic
composition 106, when melted or heated, to substantially coat the palm region
123 of
single-use glove 102. In one embodiment, palm composition 130 comprises at
least
one insert of the therapeutic composition 106, which may be in a form of a
circular
disc, as shown, or a bar, or pellets, or an evenly spread out solidified or
gel like thin
layer. In one embodiment, palm composition 130 comprises two-and-one-quarter
ounces of therapeutic composition 106. In one embodiment, palm composition 130
in
a form of circular disk comprises a diameter of about four inches, and a
thickness of
about one-half inch.
Date Recue/Date Received 2023-05-30
[0045] As exemplified in FIG. 3, the therapeutic composition 106 in
ankle region 145
of single-use boot 104 may comprise at least one ankle composition 136. In one
embodiment, ankle composition 136 comprises enough therapeutic composition
106,
when melted, to substantially coat single-use boot 104. Ankle composition 136
may
comprise about six ounces of therapeutic composition 106. In one embodiment,
ankle composition 136 is in a form of at least one insert, which may be a
circular
disc, as shown, or a bar, or pellets, or an evenly spread out solidified or
gel like thin
layer. The ankle composition 136 in a form of circular disc comprises a
diameter of
about eight inches and a thickness of about one-quarter inch. In some
embodiments,
the therapeutic composition 106 is inserted as a contiguous thin layer of
solidified or
gel like form extending evenly throughout the ankle region 145, a toe portion
of the
encaser 120 and up to a bottom of a top section 125 proximate foot aperture
142.
[0046] Hand aperture 140 or foot aperture 142 may be sealed or
partially sealed to
prevent spilling of therapeutic composition 106 outside of the body part
shaped
encaser 120 during heating and application. In some embodiments, the apertures
140
and 142 are sealed by folding, a tongue in groove locking mechanism, removable
tape or adhesive strips, removable adhesive, sewing, iron-on or heat seals,
such that
air or steam in the encaser may be released when the seal is removed and the
encaser
120 is heated. In some embodiments, the apertures 140 and 142 are partially
sealed to
permit venting of any accumulated gases associated with heating of therapeutic
composition 106. Other venting arrangements such as, for example, one-way
vents,
slits, re-sealable portals, etc., may also be applicable. In some embodiments,
the
apertures 140 and 142 are vacuum sealed, such that the encaser 120 stays air-
free
16
Date Recue/Date Received 2023-05-30
during heating so that heat and moisture in the encaser are retained, and the
explosion
or expanding of the encaser 120 during heating can be avoided. The seal of a
body
part shaped encaser 120 may be removed by cutting, trimming, or tearing after
heating and prior to skin application.
[0047] In other embodiments, a closure 150, as best shown in FIGS. 4A-
6A, of a
sealing box 114 may be used to hold and seal hand aperture 140 and foot
aperture
142 (shown in FIG. 6A). The top section 125 of the gloves 102 and boots 104
may
have an extended length along respective wrist (glove) and ankle (boot)
portions as
shown in FIG. 2 and FIG. 3. Once therapeutic composition 106 is positioned in
single-use wax-based encaser 120, each device may be folded over on the opened
end
(aperture) and placed in the sealing box 114, with the folded portion of the
aperture
140, 142 aligned with the flap of the closure 150 of the sealing box 114 as
shown in
FIGS. 4A, 4B and 5. The flap of the closure 150 is elevated with respect to
the rest of
single-use body part shaped device 120 when the closure 150 is in a closed
position
as shown in FIG. 5. The elevated position of the hand aperture 140 or foot
aperture
142 provided by the elevated flap closure 150 when the sealing box 114 is
closed
seals the aperture 140, 142 and prevents spilling of therapeutic composition
106
outside of single-use body part shaped device 120 during heating by further
utilizing
gravity. Closure 150 also permits venting of any accumulated gases associated
with
heating of therapeutic composition 106.
[0048] The sealing box 114 may further comprise at least one window 152
in
addition to at least one closure 150 (FIG. 4A). Window 152 permits viewing of
temperature indicator 118 (described below), during heating, to visually
determine
17
Date Recue/Date Received 2023-05-30
the proper temperature. Window 152 also permits viewing therapeutic
composition
106, during heating, to visually determine complete melting of therapeutic
composition 106. Upon reading this specification, those with ordinary skill in
the art
will now appreciate that, under appropriate circumstances, considering such
issues as
design preference, user preferences, marketing preferences, cost, structural
requirements, available materials, technological advances, etc., other viewing
arrangements such as, for example, multiple smaller portals, multiple windows,
slits,
pop-up notifiers, sound notifiers, etc., may suffice.
[0049] In some embodiments, one or more single-use therapy encasers 120
is placed
in one sealing box 114. In other embodiments, an individual single-use encaser
120 is
placed in one sealing box 114. The sealing box 114 may be for single-use or
may be
used repeatedly. The external sealing box 114 can be microwavable. In some
embodiments, the microwavable sealing box 114 may comprise at least one
microwave-safe material selected from cardboard, a wood-pulp material, carbon-
fiber, microwavable plastics, ceramics, wood derivative materials, and any
combination thereof.
[0050] The encaser liner 116 is used to help apply the therapeutic
composition 106 to
the user's skin during treatment. For example, the encaser liner 116 may be
configured to allow at least a portion of the therapeutic composition 106 to
migrate
from the finable space through the encaser liner 116 itself and to the user's
skin. The
encaser liner 116 may have the same shape and dimension as the encaser 120 or
be
sized slightly smaller, such that the encaser liner 116 can be inserted into
the encaser
120 with ease.
18
Date Recue/Date Received 2023-05-30
[0051] The encaser liner 116 may be made of material selected from
paper, textile,
non-woven fabrics, plastic fabrics, non-woven polypropylene fabrics, and any
combination thereof. The encaser liner 116 may be opaque or may have any level
of
transparency and may have any tint of color. The addition of the encaser liner
116
provides a range of functions such as heat insulation, even heating,
overheating spot
prevention, moisture retaining, distribution, absorbency, resilience, stretch,
softness,
strength, cushioning, padding, filtering and sterility. Additionally, the
encaser liner
116 provides a medium support or a holding agent for any form of the
therapeutic
composition 106 including, but not limited to, mud-based, clay-based, wax-
based,
liquid-based and gel-based compositions, such that the therapeutic composition
106
has reduced mobility within fillable space of the encaser 120. In one
embodiment, the
encaser liner 116 is made of paper sheet. In another embodiment, the encaser
liner
116 is made of non-woven polypropylene fabric configured to allow one or more
ingredients of the therapeutic composition 106 to migrate from the fillable
space to
the user's skin during use.
[0052] A single-use body part shaped encaser 120 may further comprise a
temperature indicator 118 for visually indicating the temperature range of a
therapeutic composition 106 during and after heating. This feature is provided
to
assist in preventing overheating of therapeutic composition 106 and to monitor
the
temperature of the skin therapy system 100 during the therapy. For example,
126 F is
a recognized temperature safety limit in the industry. A therapeutic
composition 106
with a temperature above 126 F is not suitable for direct application on top
of skin.
The temperature indicator 118 may be a coating, a strip, a sticker, a label, a
tape, or
19
Date Recue/Date Received 2023-05-30
any other form that is applicable. The temperature indicator 118 may be
reversible or
irreversible depending on the indications desired to be given. Single-use body
part
shaped encaser 120 comprises at least one temperature indicator 118. In some
embodiments, the temperature indicator 118 comprises at least one
thermochromatic
coating structured and arranged to visibly indicate internal therapeutic
composition
temperature of a respective body part shaped encaser 120 comprising
therapeutic
composition 106. In some embodiments, one or more temperature indicator 118 is
located on the interior side of the encaser 120. In some embodiments, the
temperature
indicator 118 may be applied as a thermochromatic patch or sticker to the
exterior
surface of the encaser 120. The temperature indicator 118 functions to
visually
indicate the approximate temperature of therapeutic composition 106 such that
the
user is warned if the temperature is above or under a desired range of
temperature. In
various embodiments, the visual indication may be through a change of color,
the
disappearance of color, the appearance of color, a showing of a number
presenting a
temperature, a level of temperature, a range of temperature, and/or other
number or
text that conveys information about the temperature to the user. Other
temperature
indicators may include pop-up notifiers or sound notifiers as known in the
art.
[0053] In
various embodiments, the single-use glove 102, as illustrated in FIG.2,
comprises at least one temperature indicator 118. The temperature indicator(s)
118
may be positioned anywhere on the surface of the encaser, including on each
fingertip, at the thumb-tip, and at the palm area of single-use glove 102.
Similarly,
and referring to FIG. 3, the single-use boot 104 may also comprise at least
one
temperature indicator 118, positioned anywhere including at the ankle area and
at the
Date Recue/Date Received 2023-05-30
toe area of single-use boot 104. In one example, both the thermochromatic
coating
110 and the thermochromatic sticker 112 may change to at least one warning-
temperature color, when therapeutic composition 106 exceeds at least one ideal
temperature range. Upon reading this specification, those with ordinary skill
in the art
will now appreciate that, under appropriate circumstances, considering such
issues as
design preference, user preferences, marketing preferences, cost, structural
requirements, available materials, technological advances, etc., other
temperature
arrangements such as, for example, greater or lesser temperatures, varying
ranges of
temperatures, more or fewer temperature indicators, etc., may suffice.
[0054] Referring to FIGS. 12A-B and 13, in various embodiments, the
temperature
indicator 118 may comprise a temperature activated ink. In various
embodiments,
the temperature activated ink may be sprayed, printed, stamped, and/or
otherwise
applied between two substrates of film that comprise the encaser 120. The two
substrates of film may then be laminated together to form the material for the
encaser 120. The use of the temperature indicator 118 comprising the
temperature
activated ink embedded between the two layers of laminated film that comprise
the
encaser 120 may allow the skin therapy system 100 to be heated in hot water
baths in
hospitals or other clinical setting and prevent the temperature activated ink
from
washing or fading away.
[0055] In some embodiments, as shown in FIG. 12A, temperature indicator
118
comprising the temperature activated ink may be applied onto the film in
discrete
patches, such as a design and/or trademark throughout the therapy system 100.
FIG.
12B illustrates another embodiment in which the temperature activated ink may
be
21
Date Recue/Date Received 2023-05-30
applied evenly onto the film. A change in the color of the temperature
indicator 118
may indicate that the therapeutic composition 106 inside the encaser 120 may
be too
hot for safe use and may cause burns to the skin. In that case, the user may
allow the
therapeutic composition 106 to cool to a safe temperature as indicated by a
return of
temperature indicator 118 to its previous color before applying the skin
therapy
system 100 to the skin. As shown in FIG. 13, uneven heating of the therapeutic
composition 106 may become apparent to the user when the temperature indicator
118 has one color in some areas of the skin therapy system 100 and a different
color
1300 (including no color) in other areas. This safety feature may be useful to
users
with insensitivity in their fingertips caused by nerve damage, diabetic
neuropathy,
and other conditions that render the user unable to feel their skin burn right
away.
[0056] Once a single-use body part shaped encaser 120 comprising a
therapeutic
composition 106 is applied to the targeted skin area, a fastener may attach
and
stabilize the encaser to the skin area for a period of therapy time. The
fastener may be
chosen from adhesive tape, straps, strings, elastic material, fabric tape,
tubing, or
other functional devices. The fastener may or may not be included in the skin
therapy
system. In one embodiment, a body shaped encaser 120 comprises a fastener.
[0057] FIG. 7 shows a perspective view of an exemplary single-use glove
102 of the
skin therapy system 100, and FIG. 9 shows a method using the single-use glove
102
wherein the single-use glove 102 comprises at least one aperture seal 144. In
one
embodiment, aperture seal 144 further comprises at least one strap 146, which
includes at least one adhesive strip 148. In use, after a user inserts a hand,
or
alternately a foot, into single-use body part shaped encaser 120, strap 146
can be
22
Date Recue/Date Received 2023-05-30
wrapped around and affixed to a wrist or an ankle using adhesive strip 148 to
ensure
attachment and stability of the skin therapy system 100 during therapy. Strap
146 and
strip 148 may vary in width and length. In one embodiment, strap 146 has a
length of
about seven inches and a width of about one-half inch. hi some embodiments,
strap
146 is attached to single-use body shaped encaser 120 through at least one
seam.
Alternately, strap 146 may be attached to single-use body shaped encaser 120
by heat
welding or mechanical fastener. In one embodiment, adhesive strip 148
comprises a
length of from about one-half-inch to about four-inches.
[0058] The skin therapy system 100, as disclosed herein, may further
comprise one or
more external padding, outer pouch, coverlet, harness, heating or temperature
maintaining element, stand-alone user instruction, or instruction attached to
the single
use body part shaped encaser. The instruction may be in a print, a writing, a
disk, or
any other suitable medium. In one embodiment, one or more heating or
temperature
maintaining elements is attached to the exterior surface of the body part
shaped
encaser of the skin therapy system 100.
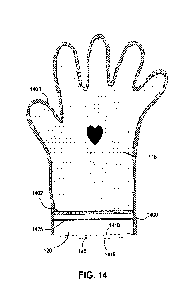
[0059] Referring now to FIG. 14, the encaser liner 116 may be
positioned between a
first substrate of film 1410 and a second substrate of film 1415, which form
the
encaser 120. The encaser liner 116 may extend to the edges of the first
substrate of
film 1410 and the second substrate of film 1415. Outer peripheral edges of the
encaser liner 116, the first substrate of film 1410, and the second substrate
of film
1415 may be sealed together to form a first hermetic seal 1400 along the outer
edges
of the skin therapy system 100. This also forms the fillable space between an
inner
surface of the first and second substrates of film 1410, 1415 and the encaser
liner 116.
23
Date Recue/Date Received 2023-05-30
The fillable space may be filled with the therapeutic composition 106 and
subsequently sealed in place with at least one additional hermetic seal 1402
positioned near a wrist portion of the encaser 120. The hermetic seals 1400,
1402 are
used to keep the therapeutic composition 106 within the fillable space and
around the
encaser liner 116 during and between uses.
[0060]
Access to the internal volume of the encaser 120 is accomplished via the hand
aperture 140 that allows the user to insert their hand into the encaser liner
116. The
hand aperture 140 may be positioned at an end most location of the encaser 120
to
allow a user's hand to be inserted into and removed from the internal volume
of the
encaser 120 similar to a regular glove.
[0061] A
closure element 1425 may be positioned between the hand aperture 140 and
the additional hermetic seal 1402 to form a temporary seal to the internal
volume of
the encaser 120. For example, the closure element 1425 may comprise a tongue
in
groove closure and/or other suitable closure that can be used to reseal the
encaser 120
between uses. This temporary seal may be used to help prevent moisture
intrusion,
bacterial contamination, and the accumulation of particulates within the
internal
volume.
[0062]
The therapeutic composition 106 of the skin therapy system 100 may be solid,
semi-solid or liquid at the room-temperature. In some embodiments, the
therapeutic
composition 106 is mud-based. In some embodiments, the therapeutic composition
106 is clay-based. In some embodiments the therapeutic composition 106 is wax-
based, and the wax may be in a solid, semi-solid or liquid state. In some
embodiments, therapeutic composition 106 does not comprise wax, and is mostly
in a
24
Date Recue/Date Received 2023-05-30
liquid state. The therapeutic composition 106 may be pre-packaged or packaged
prior
to the commencement of a therapy session such that it is enclosed in the body
part
shaped encaser 120 of the skin therapy system 100, as disclosed herein. Upon
application, the targeted skin area is in direct contact with the therapeutic
composition 106. The formulation of the therapeutic composition 106 may vary
depending on the skin condition to be treated, therapeutic purposes, or
specific
portions of a body part shaped encaser 120 of the skin therapy system 100, for
example, fingers versus palm, toes versus ankles.
[0063] Various embodiments of the therapeutic composition 106 of the
skin therapy
system 100 may comprise a hot lotion and/or a cold lotion. For example, the
therapeutic composition 106 may comprise shea butter and/or any other
moisturizer,
emollient, and/or humectant suitable for improving the condition of the skin.
In
another embodiment, the therapeutic composition 106 may comprise glycolic acid
for
a glycolic peel of the skin.
[0064] Various embodiments of the therapeutic composition 106 of the
skin therapy
system 100 may comprise a wax-based composition. The wax may be selected from
paraffin wax, soy wax, beeswax, and palm wax. In one embodiment, the
therapeutic
composition 106 is paraffin based. Paraffin utilized in the present
embodiments may
soften, hydrate and protect the skin and may also be used as a treatment for
some skin
disorders. The paraffin may be selected from paraffin wax, liquid paraffin oil
(also
called mineral oil, nujol, adepsine oil, alboline, glymol, medicinal paraffin,
or saxol),
semi-solid paraffin (also called petroleum jelly, petrolatum, white petrolatum
or soft
paraffin), and any derivatives thereof. The paraffin wax based therapeutic
Date Recue/Date Received 2023-05-30
composition 106 has a melting point temperature in the range between about 46
C
and about 68 C, between about 44 C and about 60 C, between about 42 C and
about 55 C, or between about 39 C and about 50 C.
[0065] In a traditional commercial setting for skin therapy, the
customary melt time
for standard paraffin is approximately 10-15 minutes or longer in a standard
non-
commercial microwave (750-1000 watts) depending on the quantity being heated.
Such a period of heating time is too long and thus not ideal. Further, as
shown in FIG.
8, it was observed that uneven heating occurs in therapeutic composition 106
when
the therapeutic composition 106 is enclosed in a body part shaped encaser 120
such
as a glove or a booty, when using microwave heating. This uneven heating
causes the
fingers and thumb in a glove to have a substantially higher temperature than
the palm
area when the same therapeutic composition 106 is used in all areas. Not to be
bound
by theory, it is believed that due to the dielectric heating effect of
microwaves 202
from microwave producer 200, variations in volumes and surface areas of
therapeutic
composition 106 in various areas of single-use glove 102 cause variations in
microwave absorption. In order to compensate for the variation in microwave
absorption, and thus the particular amount of heat absorbed, between finger
composition 132, thumb composition 134, and palm composition 130, the
formulations of the therapeutic composition 106 were further modified such
that the
uniform melting of the therapeutic composition 106 in a body part shaped
encaser
120 is achieved.
[0066] To reduce the melting time of a paraffin based therapeutic
composition 106,
various nut or seed oils including safflower oil, vitamin E oil, coconut oil,
among
26
Date Recue/Date Received 2023-05-30
other oils, were tested for their effects of paraffin wax melting time after
being mixed
with the paraffin wax. Theoretically, the use of oils helps to lower the
initial viscosity
of the paraffin composition and accelerates the melting process. However, not
all
tested oils can achieve that purpose. An ideal melting time for the
therapeutic
composition 106 of the skin therapy system 100 disclosed here in is between
about 1
to 2 minutes depending on the heating sources.
[0067]
During testing, many different types of oils, once mixed with the paraffin
wax, made the melted paraffin wax runny and would not allow the wax to re-form
or
re-solidify. Some seed or nut oils don't even mix well with paraffin. In
addition, re-
solidification or gelification may occur for easy application and removal of
the skin
therapy system 100 during and after the therapy. Surprisingly, the mixing of
coconut
oil with the paraffin is homogenized and, at certain range of ratio, the
mixture
allowed re-solidification or gelification of the paraffin-based therapeutic
composition
106, when achieving a comparatively shortened melt time. This property for a
therapeutic composition 106 may be achieved due to coconut oil forming a more
solid state in comparison to other oils at typical room temperatures (below
about 80
degrees Fahrenheit). Shortened time of melting and re-solidification or
gelification is
desirable, in that it shortens the preparation time and enables the formation
and
shaping of the therapeutic composition 106 into a body part shape much easier
and
faster either during the therapy or before and after the therapeutic
composition 106 is
enclosed in a body part shaped encaser 120 of the skin therapy system 100 as
disclosed herein. In one embodiment, a paraffin based therapeutic composition
106 of
the skin therapy system 100 as disclosed herein comprises paraffin and one or
more
27
Date Recue/Date Received 2023-05-30
nut oils including coconut oil. In one embodiment, a paraffin based
therapeutic
composition 106 of the skin therapy system 100 may comprise paraffin and
coconut
oil.
[0068] Continued experimentation with formulations by adding and
changing
combinations of paraffin and coconut oil revealed, surprisingly, that the best
combination for the therapeutic composition to hold up on the skin with a body
part
shaped, shell-like effect while still melting in under 2 minutes is to mix the
paraffin
with coconut oil at a ratio of from about 1:3 to about 3:1, by weight of the
therapeutic
composition 106. Some variations are utilized in such a mixture as described
herein
to assist even-melting in a given glove or boot or other body part shaped
encaser 120
with or without an encaser liner 116 as disclosed herein.
[0069] In one embodiment, the therapeutic composition 106 of the skin
therapy
system 100 may comprise paraffin and at least one of a seed oil and a nut oil.
In
some embodiments, the concentration of paraffin may be in a range of about 25
wt%
to about 75 wt%. In some embodiments, the at least one of the seed oil and nut
oil in
the therapeutic composition 106 may comprise coconut oil. In various
embodiments,
the coconut oil may be at a concentration in a range of about 25 wt% to about
75
wt%. In some embodiments, the therapeutic composition 106 of the skin therapy
system 100 comprises from about 30 wt% to about 60 wt% of coconut oil and from
about 40 wt% to about 70 wt% of paraffin. In one embodiment, the therapeutic
composition 106 of the skin therapy system 100 comprises from about 35 wt% to
about 55 wt% of coconut oil and from about 45 wt% to about 65 wt% of paraffin.
In
28
Date Recue/Date Received 2023-05-30
one embodiment, the therapeutic composition 106 of the skin therapy system 100
comprises about 50 wt% of coconut oil and about 50 wt% of paraffin.
[0070] To achieve a simultaneously complete melting of all compositions
in a body
part shaped encaser 120, other alterations to change the latent heat of fusion
of
composition in fingers, toes, palm and anchor of the encaser 120 were tested,
for
example by using two or more different therapeutic compositions 106 at
different
portions of a body part shaped encaser 120. Without being bound by theory,
more
heat absorption is required in areas previously overheated is required and
less heat
absorption is required in areas previously under-heated to effect a change in
the state
of matter from solid to liquid. As such, having various latent heats, each
therapeutic
composition 106 may melt and achieve a temperature within the ideal
temperature
range after the same amount of time exposed to a heating source such as
microwave.
In one embodiment, the palm composition 130 for a hand shaped encaser
comprises
at least about 25 wt% to about 75 wt% of paraffin and 25 wt% to about 75 wt%
of
coconut oil; and the finger composition 132 and thumb composition 134 for a
hand
shaped encaser comprise at least 50 wt% to 70 wt% of paraffin and at least
about 30
wt% to about 50 wt% of coconut oil.
[0071] Various embodiments of the therapeutic composition 106 may
comprise
various cannabinoid-containing extracts of Cannabis (Cannabis indica and/or
Cannabis sativia) plants for transdermal delivery of the cannabinoids. In some
embodiments the cannabinoid may comprise the non-psychotropic alkaloid
cannabidiol (also referred to as CBD). In other embodiments, the cannabinoid
may
29
Date Recue/Date Received 2023-05-30
comprise the non-psychotropic cannabidiolic acid (CBDA). CBDA may be
converted to CBD through heating which causes decarboxylation of CBDA.
[0072] Accordingly, the therapeutic composition 106 may comprise a
broad-spectrum
CBD, a full-spectrum CBD, an isolate of a CBD, a CBDA, or any combination
thereof. In some embodiments, the therapeutic composition 106 comprises a
broad-
spectrum CBD oil. The broad-spectrum CBD may contain THC. Since the melting
point of is above 70 C , the THC remains in the paraffin wax when the
therapeutic
composition 106 is heated between 35 C (113 F) to about 55 C (131 F). An
advantage of using an isolate CBD is targeting a condition with a specific
cannabinoid, without any THC in the isolate CBD.
[0073] Transdermal delivery of CBD through the CBD-containing
therapeutic
composition 106 may provide the benefits of topically administered CBD such as
alleviating wound-related pain, accelerating wound healing, reducing the need
for
opioid analgesics for controlling pain in skin conditions. CBD-containing
therapeutic
composition 106 may also improve conditions of deeper tissues such as
peripheral
neuropathic pain, fibromyalgia, osteoarthritis, and musculoskeletal pain. CBD
may
also provide anti-aging and antioxidant benefits to the skin. CBD may also
have anti-
inflammatory properties that may improve skin conditions such as acne,
psoriasis,
and/or eczema. The anti-inflammatory properties of CBD may compliment the anti-
inflammatory properties of paraffin itself in the therapeutic composition 106.
The
benefits of topical CBD are further evidenced by its lack of systemic side
effects, its
ease of be self-administration by the consumer, and its rapid onset of
analgesia.
Date Recue/Date Received 2023-05-30
[0074]
The addition of CBD to the therapeutic composition 106 may retain the
desired melting temperature of the therapeutic composition 106 of
approximately
about 45 C (113 F) to about 55 C (131 F), as discussed below. In various
embodiments, the CBD may be stable within a homogenous mixture of the
therapeutic composition 106 such that the CBD binds well with seed and/or nut
oils,
such as coconut oil. For example, the therapeutic composition 106 comprising
coconut oil, paraffin, and CBD may be mixed and may maintain efficient
dispersion,
avoiding phase separation.
[0075] In
some embodiments, the therapeutic composition 106 can be produced by
mixing of coconut oil with the paraffin at a temperature greater than 50 C but
less
than 80 C until it homogenized. The CBD oil contains CBD in a concentration of
20
mg/ml to 100 mg/ml. The CBD oil is heated to a temperature of greater than 65
C,
which is the melting point of CBD. The heated CBD oil is then added to the
homogenized mixture of coconut oil and paraffin, which is heated to a
temperature of
greater than 65 C. At this temperature, the mixture is homogenized, and the
CBD oil
can be blended into the coconut oil. Optional additives including, but not
limited to,
fragrances, colors, emollients, essential oils, oil soluble vitamins and/or
anti-oxidants,
known in the art, can be added to the homogenized mixture of CBD, coconut oil,
and
paraffin to create the therapeutic composition 106. The volume between the
encaser
120 and the encase liner 116 is filled with the therapeutic composition 106 at
a
temperature above 60 C.
[0076]
The CBD-containing therapeutic composition 106 may comprise a
therapeutically effective amount of CBD. In some embodiments, the CBD may
31
Date Recue/Date Received 2023-05-30
comprise a commercially available CBD oil and/or CBD wax. In an exemplary
embodiment, the CBD-containing therapeutic composition 106 may comprise at
least
approximately 2 milligrams of CBD per kilogram of CBD-containing therapeutic
composition 106. In another exemplary embodiment, the amount of CBD in the
CBD-containing therapeutic composition 106 may be approximately 2 to
approximately 100 milligrams per kilogram. In another exemplary embodiment,
the
amount of CBD in the CBD-containing therapeutic composition 106 may be
approximately 50 milligrams within each skin therapy system 100.
[0077] In some embodiments, the therapeutic composition 106 comprises
paraffin,
coconut oil, and CBD. For example, the therapeutic composition 106 can
comprise
paraffin, and coconut oil in a ratio in a range from 4:1 to 2:1 and CBD in a
range of 5
mg/kg of a combination of paraffin and coconut oil to 100 mg/kg of a
combination of
paraffin and coconut oil. In some examples, the amount of CBD in a single
encaser
120 is 5 mg and total of 10 mg for a pair of encasers 120.
[0078] In some embodiments, a paraffin based therapeutic composition 106
of the skin
therapy system 100 can comprise paraffin, coconut oil and CBD. In some
embodiments
[0079] In some examples, the amount of CBD in a single encaser 120 is 5
mg and total
of 10 mg for a pair of encasers 120. In some examples, the amount of CBD in a
single encaser 120 is 25 mg and total of 50 mg for a pair of encasers 120. In
some
examples, the amount of CBD in a single encaser 120 is 50 mg and total of 100
mg
for a pair of encasers 120. In one example, the amount of CBD in a single
encaser
120 is 100 mg and total of 200 mg for a pair of encasers 120.
32
Date Recue/Date Received 2023-05-30
[0080]
Without being bound by theory, the CBD is transported in the coconut oil
through the encase liner 116 and into the skin when the temperature of the
therapeutic
composition 106 is between 48 C (119 F) to about 51 C (124 F) . The heat
is the transportation mechanism for moving the CBD from the therapeutic
composition 106 to the skin surface of the user.
[0081]
Various embodiments of the CBD-containing therapeutic composition 106
may further comprise various additives such as steroids. For example, the
steroid may
be 1% hydrocortisone.
[0082]
Therapeutic composition 106 as disclosed herein may further comprise at least
one essential oil. For medical purposes, from about six to about twelve drops
of
medical grade essential oils may be added to the therapeutic composition 106.
A drop
of essential oils, as defined herein using the AFNOR-ISO standard for
quantifying
essential oils, is about 1120th of one milliliter when utilizing the standard
of 20 drops
per milliliter of essential oil. When the standard for a specific essential
oil is different
due to viscosity, a single-drop volume may be adjusted accordingly. In one
embodiment, the essential oils are added and mixed into the therapeutic
composition
106 before the therapeutic composition 106 is enclosed in a body part shaped
encaser
120 of the skin therapy system 100. In one embodiment, the essential oils are
added
into the therapeutic composition 106 prepackaged in a body part shaped encaser
120
of the skin therapy system 100 upon applying the same to a targeted skin area,
such
that different essential oils may be used for a specific condition of a
specific
individual under the therapy. Essential oils and aromatic oils of the
therapeutic
composition 106 may be selected from peppermint oil, cinnamon leaf oil,
lemongrass
33
Date Recue/Date Received 2023-05-30
oil, clove oil, castor oil, orange oil, eucalyptus oil, tea tree oil,
wintergreen oil,
patchouli oil, lavender, bergamot, sandalwood, chamomile, aldehyde C16,
a-terpineol, amyl cinnamic aldehyde, amyl salicylate, anisic aldehyde, benzyl
alcohol, benzyl acetate, cinnamaldehyde, cinnamic alcohol, carvacrol, carveol,
citral,
citronellal, citronellol, p-cymene, diethyl phthalate, dimethyl salicylate,
dipropylene
glycol, eucalyptol, eugenol, iso-eugenol, galaxolide, geraniol, guaiacol,
ionone,
menthol, methyl salicylate, methyl anthranilate, methyl ionone, a-
phellandrene,
pennyroyal oil, perillaldehyde, 1- or 2-phenyl ethyl alcohol, 1- or 2-phenyl
ethyl
propionate, piperonal, piperonyl acetate, piperonyl alcohol, D-pulegone,
terpinen-4-ol, terpinyl acetate, 4-tert butylcyclohexyl acetate, thyme oil,
thymol,
metabolites of trans-anethole, vanillin, ethyl vanillin, and combinations
thereof.
[0083] In one embodiment, an essential oil mixture for pain relieving
comprises
peppermint oil, cinnamon leaf oil, clary sage, and orange oil. In one
embodiment, an
essential oil mixture for anti-fungal and anti-bacterial effects comprises tea
tree oil,
clove oil, lemon oil, eucalyptus oil and patchouli oil. In one embodiment, an
essential
oil mixture for relaxation comprises lavender, bergamot, sandalwood and
chamomile.
Additionally, aromatherapy oils may also be utilized to add further
therapeutic
effects. In one embodiment, the therapeutic composition 106, comprising
paraffin
and coconut oil, may further comprise at least one aromatic oil.
[0084] The paraffin based therapeutic composition 106 may further
comprise
optional additives including, but not limited to fragrances, colors,
emollients, and
antioxidants, known in the art. The antioxidant may be natural or synthetic.
Suitable
antioxidants include, but are not limited to, ascorbic acid and its salts,
ascorbyl
34
Date Recue/Date Received 2023-05-30
palmitate, ascorbyl stearate, anoxomer, N-acetylcysteine, benzyl
isothiocyanate, m-
aminobenzoic acid, o-aminobenzoic acid, p-aminobenzoic acid (PABA), butylated
hydroxyanisole (BHA), butylated hydroxytoluene (BHT), caffeic acid,
canthaxantin,
alpha-carotene, beta-carotene, beta-carotene, beta-apo-carotenoic acid,
carnosol,
carvacrol, catechins, cetyl gallate, chlorogenic acid, citric acid and its
salts, clove
extract, coffee bean extract, p-coumaric acid, 3,4-dihydroxybenzoic acid, N,N'-
di phenyl-p-phenyl en edi amin e (DPPD), dilauryl thi odipropionate, di
stearyl
thiodipropionate, 2,6-di-tert-butylphenol, dodecyl gallate, edetic acid,
ellagic acid,
erythorbic acid, sodium erythorbate, esculetin, esculin, 6-ethoxy-1,2-dihydro-
2,2,4-
trimethylquinoline, ethyl gallate, ethyl maltol, ethylenediaminetetraacetic
acid
(EDTA), eucalyptus extract, eugenol, ferulic acid, flavonoids (e.g., catechin,
epicatechin, epicatechin gallate, epigallocatechin (EGC), epigallocatechin
gallate
(EGCG), polyphenol epigallocatechin-3-gallate), flavones (e.g., apigenin,
chrysin,
luteolin), flavonols (e.g., datiscetin, myricetin, daemfero), flavanones,
fraxetin,
fumaric acid, gallic acid, gentian extract, gluconic acid, glycine, gum
guaiacum,
hesperetin, alpha-hydroxybenzyl phosphinic acid, hydroxycinammic acid,
hydroxyglutaric acid, hydroquinone, N-hydroxysuccinic acid, hydroxytryrosol,
hydroxyurea, rice bran extract, lactic acid and its salts, lecithin, lecithin
citrate; R-
alpha-lipoic acid, lutein, lycopene, malic acid, maltol, 5-methoxy tryptamine,
methyl
gallate, monoglyceride citrate; monoisopropyl citrate; morin, beta-
naphthoflavone,
nordihydroguaiaretic acid (NDGA), octyl gallate, oxalic acid, palmityl
citrate,
phenothiazine, phosphatidylcholine, phosphoric acid, phosphates, phytic acid,
phytylubichromel, pimento extract, propyl gallate, polyphosphates, quercetin,
trans-
Date Recue/Date Received 2023-05-30
resveratrol, rosemary extract, rosmarinic acid, sage extract, sesamol,
silymarin,
sinapic acid, succinic acid, stearyl citrate, syringic acid, tartaric acid,
thymol,
tocopherols (i.e., alpha-, beta-, gamma- and delta-tocopherol), tocotrienols
(i.e.,
alpha-, beta-, gamma- and delta-tocotrienols), tyrosol, vanilic acid, 2,6-di-
tert-buty1-
4-hydroxym ethylphenol (i.e., Ionox 100),
2,4-(tris-3',5'-bi-tert-buty1-4'-
hydroxybenzy1)-mesitylene (i.e., Ionox 330), 2,4,5-trihydroxybutyrophenone,
ubiquinone, tertiary butyl hydroquinone (TBHQ), thiodipropionic acid,
trihydroxy
butyrophenone, tryptamine, tyramine, uric acid, vitamin K and derivatives,
vitamin
Q10, wheat germ oil, zeaxanthin, or combinations thereof. One skilled in the
art will
appreciate that the antioxidants incorporated into the composition (including
those
listed herein) encompass all potential salt and ester forms of the
antioxidants in
addition to the pure forms of the compound. In some embodiments, the
antioxidant
may comprise a vitamin E compound such as tocopheryl acetate, tocopheryl
linoleate, tocopherol nicotinate, tocopheryl succinate, ascorbyl tocopheryl
phosphate,
dioleyl tocopheryl methylsilanol, tocophersolan, and tocopheryl
linoleate/oleate. In
one embodiment, included in the vitamin E oil are traces of safflower oil, and
other
oils. In another embodiment, the vitamin E formula further comprises the
largest
amount of sunflower seed oil followed by safflower seed oil, tocopheryl
acetate, rice
bran oil, almond oil, apricot oil, wheat germ oil and lecithin. In one
embodiment of
the therapeutic composition 106 comprising paraffin at a concentration in a
range of
about 25 wt% to about 75 wt% and coconut oil at a concentration in a range of
about
25 wt% to about 75 wt%, the therapeutic composition 106 further comprises from
about 2 wt% to 7 wt% of a mixture of antioxidant.
36
Date Recue/Date Received 2023-05-30
[0085] The therapeutic composition 106 of the skin therapy system 100
may be a
liquid-based composition, such that a solid to liquid, then back to solid
phase changes
are not involved. In some embodiments, a pre-heating or pre-cooling step
before
application may be required. In some other embodiments, a pre-heating or pre-
cooling step before application may not be required. In one embodiment, the
liquid-
based composition comprises a therapeutic composition 106 comprising alpha
hydroxy acid including lactic acid and glycolic acid; and/or beta hydroxy acid
including salicylic acid, and any combination thereof. The liquid based
composition
comprising alpha hydroxy acid including lactic acid and glycolic acid; and/or
beta
hydroxy acid including salicylic acid may further comprise essential oils,
fragrances,
colors, emollients, anti-oxidants, and other additives including absorbent,
adsorbent,
pH controller, and substances for rehydration known in the art. In one
embodiment, a
liquid based therapeutic composition 106 for the skin therapy system 100
comprises
lactic acid, glycolic acid, salicylic acid, lemon oil, polyquaternium-10, PEG-
40
hydrogenated castor oil, sodium hydroxide, and one or more antioxidant
including
vitamin E. Various suitable essential oils and anti-oxidants for a liquid-
based
therapeutic composition 106 are described above in detail.
[0086] This technology also provides a method relating to providing
skin treatment
utilizing a therapeutic composition 106 contained in a body part shaped
encaser 120
amenable to various heating elements to provide liquefaction before use
without
burning the skin. The method of using the skin therapy system 100 as disclosed
herein for skin treatment generally comprises the steps of heating the encaser
120
containing a therapeutic composition 106 using one or more heating elements,
37
Date Recue/Date Received 2023-05-30
applying the unsealed encaser 120 to targeted skin area by attaching the
encaser 120
to a body part, and removing the encaser 120 from the targeted skin area at
the end of
the therapy.
[0087] Some paraffin based therapeutic compositions 106 need to be
heated to melt
prior to application. Paraffin wax typically has a melting point temperature
in the
range between about 46 C (114.8 F) and about 68 C (154.4 F). Petroleum
jelly
based therapeutic compositions 106 have a melting-point usually within a few
degrees of human body temperature, which is approximately 37 C (98.6 F).
Liquid
paraffin-based therapeutic composition 106 and other liquid based composition
may
need to be pre-heated to body temperature for the comfortable feel to the skin
upon
application. Depending on the storage condition, room temperature, and
specific
therapeutic temperature requirement, the therapeutic composition 106 enclosed
in a
body part shaped encaser 120 of the skin therapy system 100 as disclosed
herein may
require a heating process by a heating element. Suitable heat element may be
selected
from microwave oven, stove, hot towel cabinet, heating coils, heating pad,
heater,
heating lamp, warmer, radiator, boiler, steamer (such as a towel steamer),
warm
water bath, hydrocollator, and any other device or equipment known in the art.
In one
embodiment, the heating element may be portable.
[0088] In some embodiments, the heating element is comprised in the
skin therapy
system 100. The heating temperature may be provided by a heating element
included
in the skin therapy system 100, and the temperature of the therapeutic
composition
106 may be indicated by touching or temperature indicator attached to the body
part
shaped encaser 106 of the skin therapy system 100. With a heating time of the
38
Date Recue/Date Received 2023-05-30
therapeutic composition 106 between 1-5 minutes, or preferably 1-2 minutes,
the
melting temperature of a therapeutic composition 106 ranges from about 45 C
(113
F) to about 55 C (131 F). With a heating time of the therapeutic composition
106
between 1-2 minutes, the melting temperature of a therapeutic composition 106
ranges from about 48 C (119 F) to about 51 C (124 F). In one embodiment,
the
therapeutic composition 106 may comprise paraffin at a concentration of about
25-75
wt%, by weight of the composition, and coconut oil at a concentration of about
25-75
wt% by weight of the composition and have a melting temperature between about
48
C to about 51 C, with an even melting of the composition taking place in
about 1-5
minutes.
[0089]
After a predetermined temperature range of a therapeutic composition 106 of
the skin therapy system 100 is reached, the sealed encaser 120 containing the
therapeutic composition 106 is opened by cutting, unzipping or tearing the
closure
1425 of the encaser 120. The body part is inserted into the encaser 120 such
that the
targeted skin area is in direct contact with the therapeutic composition 106,
through
touching, dipping, or being covered by the therapeutic composition 106. The
encaser
120 is then attached to the body part using adhesive tape, strap, string
elastic band, or
tubing for stabilization during the therapy. The application may last for 10
minutes,
20 minutes, 30 minutes, 60 minutes, 120 minutes or longer, or any range of
duration
in between. When the therapy ends at a time point, the encaser 120 is released
from
the body part by removing the body part from the encaser 120 comprising the
therapeutic composition 106.
39
Date Recue/Date Received 2023-05-30
[0090]
The method of using the skin therapy system 100 as disclosed herein for skin
treatment may further comprise assembling the body part shaped encaser 120.
Referring to the flow diagram of FIG. 10 as an example in which a microwave
oven
is used as a heating element in method 300, the following steps may be
followed. In
initial step 302 of method 300 a first wax-based composition is formulated to
be
heatable by a microwave energy producer. Next, as indicated I n step 304 a
second
microwave-heatable wax-based composition is formulated to be heatable by a
microwave energy producer. Next, as indicated in step 306 at least one body
part
shaped encaser structured and arranged to encase a body part of a human body
is
provided. As indicated in Step 308 one or more microwave-heatable wax-based
composition is encased by spreading the composition into a thin layer in the
encaser.
As indicated in Step 310 each one of such microwave-heatable wax-based
composition is formulated to comprise at least one first substance, and at
least one
second substance; wherein such first substance comprises wax elements; wherein
the
second substance comprises oil elements. Step 312 indicates that a first
microwave-
heatable wax-based composition is formulated to comprise a first ratio X of
wax
elements to the oil elements, and a second microwave-heatable wax-based
composition is formulated to comprise a second ratio Y of wax elements to the
oil
elements. The latent heat of fusion of the resulting first microwave-heatable
wax-
based composition may be substantially different from the latent heat of
fusion of the
second microwave-heatable waxy composition. Finally, as indicated in Step 314,
a
skin treatment utilizing such wax-based compositions amenable to microwave
heating to provide liquefaction before use without skin burning may be
provided by
Date Recue/Date Received 2023-05-30
adjusting placement and amount of a first microwave-heatable wax-based
composition and a second microwave-heatable wax-based composition within a
body
part shaped encaser to equalize the melting of the wax-based composition to
assist
prevention of injuring skin tissues of the body part to be treated.
[0091] As
an additional example, FIG. 6B shows an illustrative set of instructions for
using a pre-packaged kit/apparatus according to an embodiment of the present
technology. In one embodiment of a method of use, single-use wax-based encaser
120 may be provided as a prepackaged kit 400 (See Fig 4A, FIG. 4B and FIG. 6A
and FIG. 6B) comprising a microwavable sealing box 114 comprising at least one
single-use glove 102 or single-use boot 104 comprising one or more therapeutic
composition 106 and at least one set of instructions 410.
[0092] In an exemplary method of manufacture, the temperature indicator
118, such as
temperature activated ink, may be sprayed, printed, stamped, and/or otherwise
applied to at least one of the first and second substrates of film 1410, 1415
that folln
the encaser 120. The encaser liner 116 may be layered between the first and
second
substrates of film 1410, 1415. A hot compress having an outline of a desired
body
part shape may be applied to the layered stack of the first and second
substrates of
film 1410, 1415 and the encaser liner 116. The hot compress may melt the
layered
stack forming the first hermetic seal 1400 and affixing the encaser liner's
116
position within the internal volume of the encaser 102 while also creating a
first
fillable space between the first substrate of film 1410 and a first layer of
the encaser
liner 116 (top layer) and a second fillable space between the second substrate
of film
1415 and a second layer of the encaser liner 116 (bottom layer).
41
Date Recue/Date Received 2023-05-30
[0093] The therapeutic composition 106 may be poured or otherwise placed
into the
two fillable spaces (top and bottom layers). Once complete, a second hermetic
seal
may be formed between the first substrate of film 1410 and the first layer of
the
encaser liner 116 to seal the therapeutic composition 106 in place within the
first
fillable space (top layer). Similarly, a third hermetic seal may be formed
between the
second substrate of film 1415 and the second layer of the encaser liner 116 to
seal
the therapeutic composition 106 within the second fillable space (bottom
layer).
Finally, a closure element 1425 may be included between the hand aperture 140
and
the additional hermetic seal 1402 to seal off the entire internal volume of
the encaser
120 and the internal volume of the encaser liner 116 from the surrounding
environment.
[0094] With reference to FIG. 15, an exemplary cross-section view of a
skin therapy
system 100 illustrates various methods of skin therapy. Various embodiments
provide a skin therapy system 100 comprising an encaser 120, an encaser liner
116,
and a therapeutic composition 106 positioned in a fillable space between the
encaser
120 and the encaser liner 116. The fillable space is a volume defined by the
inner
surface of the encaser 120 and the outer surface of the encaser liner 116,
which are
connected at the cuff of the encaser 120. In some embodiments, the fillable
space is
a volume defined by the inner surface of the encaser 120 and the outer surface
of the
encaser liner 116, which are connected at the cuff of the encaser 120 and
connected
at the fingertips (and thumb lip) of the encaser 120. In these embodiments, a
fillable
space is located on both of the outer surfaces of the encaser liner 116 and
the encaser
liner 116 is held in place by the connections to the encaser 120 the
fingertips when
42
Date Recue/Date Received 2023-05-30
user pulls a body part (such as, for example a hand) out of the skin therapy
system
100.
[0095] The therapeutic composition 106 is held in the fillable space by
the encaser liner
116 and the therapeutic composition 106 is not in contact with a user's skin
1450.
The encaser liner 116 can made of non-woven polypropylene fabric (or an
equivalent material), which is permeable to one or more ingredients of the
therapeutic composition 106 at an elevated temperature in a range from 45 C
(113
F) to 55 C (131 F).
[0096] For example, a therapeutic composition 106 comprises paraffin
and coconut oil,
as described herein, if the therapeutic composition 106 is temperature in a
range
from 45 C (113 F) to 55 C (131 F), a portion of the coconut oil is
thermally
transported 1140 through the encaser liner 116 and onto the skin 1450 of a
body part
1460. In
another example, a therapeutic composition 106 comprises paraffin,
coconut oil, and vitamin E, as described herein, if the therapeutic
composition 106 is
temperature in a range from 45 C (113 F) to 55 C (131 F), a portion of the
coconut oil and the vitamin E is thermally transported 1140 through the
encaser liner
116 and onto the skin 1450 of a body part 1460.
Other examples include a
therapeutic composition 106 comprises paraffin, coconut oil, and an essential
oil, as
descried herein, if the therapeutic composition 106 is temperature in a range
from 45
C (113 F) to 55 C (131 F), a portion of the coconut oil and the essential
oil is
thermally transported 1140 through the encaser liner 116 and onto the skin
1450 of a
body part 1460. In these examples, the paraffin is blocked by the encaser
liner 116
and the paraffin does not make contact with the skin 1450.
43
Date Recue/Date Received 2023-05-30
[0097] As illustrated in FIG. 15, the body part 1460 is defined by the
skin 1450 on
either side of the internal structure 1455 of the body part 1460. For example,
the
body part can be a finger, a thumb, a hand, a toe, a foot, a part of a leg, or
a part of
an arm.
[0098] Various embodiments provide methods of treating the skin. For
example, a
method can include providing a skin therapy system 100 comprising an encaser
120,
an encaser liner 116, and a therapeutic composition 106 positioned in a
fillable space
between the encaser 120 and the encaser liner 116 and heating the therapeutic
composition 106 at an elevated temperature in a range from 35 C to 55 C. The
method can include inserting a body part 1460 into an opening of the encaser
120
and contacting skin surface 1450 surrounding the body part 1460with the
encaser
liner 116. The method can include keeping the skin surface 1450 in contact
with the
encaser liner 116 while the therapeutic composition 106 at the elevated
temperature
and thermally transporting 1440 at least one ingredient from the therapeutic
composition on to the skin 1450. The method can include blocking the paraffin
from
reaching the skin 1450. The at least one ingredient can be coconut oil. The at
least
one ingredient can be vitamin E. The at least one ingredient can be lanolin.
The
treating the skin can be adding moisture to dry skin 1450. The treating the
skin can
reducing cracks in the skin 1450. The treating the skin can be relieving pain
from
arthritis. The treating the skin can be a skin therapy.
[0099] Finally moving to FIG. 16, an exemplary cross-section view of the
skin therapy
system 100 illustrates various methods of transdermal delivery of a medicinal.
Some
embodiments employ various materials for containing heat-stable therapeutic
44
Date Recue/Date Received 2023-05-30
compositions 106 which may be practiced in conjunction with any number of
compositions and procedures for treating pain and inflammation of joints and
other
body parts and the systems described are merely exemplary applications for the
technology. Various representative implementations of these embodiments may be
applied to any portion of the human body for the treatment of skin, pain,
injury,
and/or inflammatory medical conditions.
[00100] In some embodiments, the treating the skin can be reducing
inflammation in the
internal structure 1455 of the body part 1460. Examples of the internal
structure
1455 can be a muscle, a tendon, a ligament, or connecting tissue. In
some
embodiments, the thermally transporting 1444 at least one ingredient from the
therapeutic composition on to the skin 1450 can include transporting the at
least one
ingredient through the skin 1450 and into the internal structure 1455 of the
body part
1460. Some embodiments can include treating a sprain in the internal structure
1455 of a body part 1460, which can include thermally transporting 1444 at
least one
ingredient from the therapeutic composition into the internal structure 1455
of the
body part 1460. In some examples of these embodiments, the therapeutic
composition 106 can include an medically active ingredient and the medically
active
ingredient can be thermally transported 1444 into the internal structure 1445
of the
body part 1460 and treating at least one of a muscle, a tendon, a ligament, or
connecting tissue. A medically active ingredient can be at least one of an
anti-
inflammatory, an antioxidant, a steroid, an essential oil, and combinations
thereof.
[00101] For example, a therapeutic composition 106 comprises paraffin,
coconut oil, and
a medically active ingredient, as described herein, if the therapeutic
composition 106
Date Recue/Date Received 2023-05-30
is temperature in a range from 45 C (113 F) to 55 C (131 F), a portion of
the
coconut oil is thermally transported 1140 through the encaser liner 116 and
onto the
skin 1450 of a body part 1460 and the medically active ingredient is thermally
transported 1111 into the skin 1450 and can be thermally transport 1444 into
the
internal structure 1455 of the body part 1460. In another example, a
therapeutic
composition 106 comprises paraffin, coconut oil vitamin E, and a medically
active
ingredient, as described herein, if the therapeutic composition 106 is
temperature in a
range from 45 C (113 F) to 55 C (131 F), a portion of the coconut oil and
the
vitamin E is thermally transported 1140 through the encaser liner 116 and onto
the
skin 1450 of a body part 1460 and the medically active ingredient is thermally
transported 1444 into the skin 1450 and can be thermally transport 1444 into
the
internal structure 1455 of the body part 1460. Other examples include a
therapeutic
composition 106 comprises paraffin, coconut oil, an essential oil, and a
medically
active ingredient, as descried herein, if the therapeutic composition 106 is
temperature in a range from 45 C (113 F) to 55 C (131 F), a portion of the
coconut oil and the essential oil is thermally transported 1140 through the
encaser
liner 116 and onto the skin 1450 of a body part 1460 and the medically active
ingredient is thermally transported 1444 into the skin 1450 and can be
thermally
transport 1444 into the internal structure 1455 of the body part 1460. In
these
examples, the paraffin is blocked by the encaser liner 116 and the paraffin
does not
make contact with the skin 1450.
[00102] In some embodiments, the and a medically active ingredient is
CBD. Without
being bound by theory, the CBD is transported in the coconut oil through the
encase
46
Date Recue/Date Received 2023-05-30
liner 116 and into the skin when the temperature of the therapeutic
composition 106
is between 48 C (119 F) to about 51 C (124 F). The heat is the
transportation
mechanism for moving the CBD from the therapeutic composition 106 to the skin
surface of the user. The heat provided to the skin 1450 by the heated
therapeutic
composition 106 increases the permeability of the skin 1450, which allows the
CBD
to penetrate deeper into the skin 1450 (and in some cases into the internal
structure
1455) and last longer in the tissue. This enables a larger amount of CBD to
reach a
treatment as compared to rubbing a CBD oil with the same concentration on to
one's
skin at room temperature.
[00103] Method of treating inflammation comprising proving an encaser 120
having a
therapeutic composition 106 comprising comprises paraffin, coconut oil, and
CBD,
the therapeutic composition 106 positioned between the encase 120 and an
encase
liner 122. The method can comprise heating the therapeutic composition 106 to
a
temperature in the between 48 C (119 F) to about 51 C (124 F) then
contacting the
encase liner 122 to a surface of skin. The method can comprise applying heat
to the
surface of the skin to increase permeability of the skin and transporting an
oil
comprising CBD from the therapeutic composition 106 into the skin. The amount
of
CBD in the therapeutic composition 106 can be from 5 mg to 100 mg. The oil can
also comprise coconut oil. The ratio of coconut oil to CBD in the therapeutic
composition 106 is in a range from 40:1 to 5:1 by weight.
[00104] In some embodiments, a method of relieving inflammation and/or
pain includes
provide a skin therapy system 100 comprising an encaser 120, an encaser liner
116,
and a therapeutic composition 106 comprising an therapeutic amount of CBD
47
Date Recue/Date Received 2023-05-30
positioned in a fillable space between the encaser 120 and the encaser liner
116 and
thermally transporting the therapeutic amount of CBD to a targeted
inflammation
site in the internal structure 1455 of the body part 1460
[00105] In some embodiments, a method of relieving stress, anxiety,
and/or PSD
includes provide a skin therapy system 100 comprising an encaser 120, an
encaser
liner 116, and a therapeutic composition 106 comprising an therapeutic amount
of
CBD positioned in a fillable space between the encaser 120 and the encaser
liner 116
and thermally transporting the therapeutic amount of CBD into at least one
blood
vessel in the internal structure 1455 of the body part 1460.
[00106] In some embodiments, a method of delivering antioxidants includes
provide a
skin therapy system 100 comprising an encaser 120, an encaser liner 116, and a
therapeutic composition 106 comprising an therapeutic amount of CBD positioned
in
a fillable space between the encaser 120 and the encaser liner 116 and
thermally
transporting the therapeutic amount of CBD into at the internal structure 1455
of the
body part 1460.
[00107] In the foregoing description, the technology has been described
with reference
to specific exemplary embodiments. Various modifications and changes may be
made, however, without departing from the scope of the present technology as
set
forth. The description is to be regarded in an illustrative manner, rather
than a
restrictive one and all such modifications are intended to be included within
the
scope of the present technology. Accordingly, the scope of the technology
should be
determined by the generic embodiments described and their legal equivalents
rather
than by merely the specific examples described above. For example, the steps
recited
48
Date Recue/Date Received 2023-05-30
in any method or process embodiment may be executed in any appropriate order
and
are not limited to the explicit order presented in the specific examples.
Additionally,
the components and/or elements recited in any system embodiment may be
combined in a variety of permutations to produce substantially the same result
as the
present technology and are accordingly not limited to the specific
configuration
recited in the specific examples.
[00108] Benefits, other advantages and solutions to problems have been
described above
with regard to particular embodiments. Any benefit, advantage, solution to
problems
or any element that may cause any particular benefit, advantage or solution to
occur
or to become more pronounced, however, is not to be construed as a critical,
required or essential feature or component.
[00109] The terms "comprises," "comprising," or any variation thereof,
are intended to
reference a non-exclusive inclusion, such that a process, method, article,
composition or apparatus that comprises a list of elements does not include
only
those elements recited, but may also include other elements not expressly
listed or
inherent to such process, method, article, composition or apparatus. Other
combinations and/or modifications of the above-described structures,
arrangements,
applications, proportions, elements, materials or components used in the
practice of
the present technology, in addition to those not specifically recited, may be
varied or
otherwise particularly adapted to specific environments, manufacturing
specifications, design parameters or other operating requirements without
departing
from the general principles of the same.
49
Date Recue/Date Received 2023-05-30