Note: Descriptions are shown in the official language in which they were submitted.
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
GENETIC RISK ANALYSIS FOR ATTENTION DEFICIT/HYPERACTIVITY
DISORDERS AND BEHAVIORAL MANAGEMENT THEREOF
FIELD OF INVENTION
[0001] The present invention is directed to assessing severity index for
genetic risks of
attention deficit/hyperactivity disorders and the methods of behavioral
management thereof.
RELATED APPLICATIONS
[0002] This Application claims the benefit of provisional United States Patent
Application
Serial No. 62/835,193, filed April 17, 2019, entitled "Genetic Risk Analysis
For Attention
Deficit Disorders And Behavioral Management Thereof', which is commonly
assigned to the
Assignee of the present invention and is hereby incorporated in reference in
its entirety for all
purposes.
[0003] This Application is also related to PCT Patent Publ. No.
WO/2016/007927, published
January 14, 2016, entitled "Genetic Addiction Risk Analysis For RDS Severity
Index," to Blum
("Blum '927 PCT Application"), which is commonly assigned to the Assignee of
the present
invention and is hereby incorporated in reference in its entirety for all
purposes, including the
sequence listing provided therein.
[0004] This Application is also related to PCT Patent Appl. No.
PCT/U520/23437, filed March
18, 2020, entitled "Genetic Addiction Risk Analysis For Post-Traumatic Stress
Disorders And
Behavioral Management Thereof," to Blum, which is commonly assigned to the
Assignee of
the present invention and is hereby incorporated in reference in its entirety
for all purposes.
BACKGROUND OF THE INVENTION
Characteristics of attention deficit/hyperactivity disorder (ADHD)
[0005] Attention deficit/hyperactivity disorder (ADHD) is a complex disorder
having multiple
causes including genetics as impacted by one's environment. The condition is
usually
diagnosed in childhood, when difficulties arise during play and school, and it
is marked by lack
of concentration, short attention span, and physical restlessness [APA 1994;
APA 2000].
1
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
ADHD often is blamed on bad parenting, or a "bad" attitude. However, brain-
imaging studies
have shown that children with this disorder have an underlying neurological
dysfunction, which
likely accounts for their behavior [Zametkin 1990; Lou 1998].
[0006] In the simplest terms, the brains of these children have yet to come
fully "on-line." It is
conjectured that while certain important brain pathways are working normally,
cortical regions
involved in attention, impulse control, and stimulus integration abilities,
have yet to become
fully active. ADHD is a widespread affliction that we are just beginning to
understand. People
with ADHD suffer from overload [Miller 2008]. That is, they have heightened
awareness of
incoming stimuli, particularly sight, sound, and touch. They are so bombarded
by the normal
stimuli in their environment that they cannot filter out the background noise,
and they have
trouble focusing or concentrating on a problem or a task. Because of their
inability to focus,
those with ADHD have trouble completing what they start. They have
difficulties with making
plans and even more difficulty in carrying out plans in an orderly fashion.
[0007] People with ADHD tend to be disorganized. Children have messy rooms;
adults have
cluttered desks; daily activities tend to be chaotic. Attics and basements are
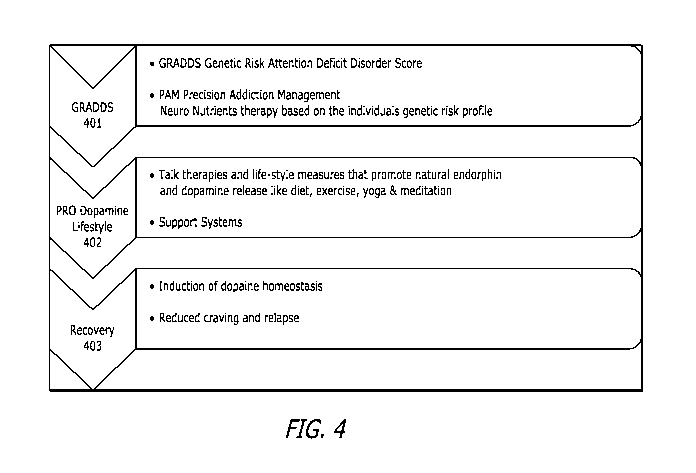
likely to be filled
with partly completed sewing projects, woodworking projects, repairs, and
notebooks; desk
drawers are likely to be cluttered with unfinished letters, outlines, and
project plans. Many
people with the disorder are highly intelligent, but they tend to be
underachievers because they
cannot concentrate or sustain interest. As a result, family, friends,
teachers, and coworkers
become impatient and expect them to fail. People with ADHD also have trouble
adapting to
change. Their life is so full of tumult that even a minor additional change in
their routine can
be upsetting or can even create a crisis, e.g., a parent goes away on a trip,
a new teacher takes
over a class, the family moves to a new city, or a pet dies.
[0008] ADHD afflicted people live under stress so severe they cannot tolerate
frustration, and
when they are frustrated, they are likely to become angry. The anger tends to
come suddenly
2
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
and explosively, accompanied by slamming doors, harsh words, tantrums, and
leaving
important meetings in a frenzy. Children get into fights; adults lose jobs and
alienate friends.
Afterwards, they may be sorry, but the damage is done. With their high level
of frustration,
people with ADHD are impatient. They hate to wait in line, and delays of any
kind can make
them frantic. Whatever is going on ¨ a trip, a movie, a class, a discussion ¨
they want it to go
quickly and be finished. Their impatience makes people with ADHD impulsive. As
children,
they leap into action without thinking of consequences. As adults, they drive
too fast, use power
tools carelessly, and plunge into activities without thinking of the danger.
The result is they
often hurt themselves or others. People with ADHD have trouble with their
orientation to time
and space. They may have trouble differentiating their right hand from their
left; they may have
difficulty following a set of instructions, reading a map, or telling time. As
babies or children
they constantly are on the move, squirming, twisting, and getting into
everything. As adults,
they are restless, easily bored, rebellious when asked to follow a routine,
and always on the
move. It is noteworthy that some of these characteristics are tied to comorbid
Oppositional
Defiant Disorder (ODD) and conduct disorder (CD), separate from ADHD per se
[Biederman
11 2007].
[0009] The diagnosis of ADHD is based on criteria outlined by the Diagnostic
and Statistical
Manual of the American Psychiatric Association ("DSM-IV") [APA 1994]. TABLE I
lists
these criteria. There have been a number of similar criteria set out in
earlier versions of the
DSM. While the names have changed somewhat, all have embraced the letters ADD
in one
form or another, representing the core of the disorder ¨ attention deficit
disorder. The subtypes
in the DMS-IV are ADHD-I representing predominately the inattentive type, ADHD-
H
representing predominately the hyperactive-impulsive type, and ADHD-C,
representing the
combined type.
3
CA 03137128 2021-10-18
WO 2020/215026
PCT/US2020/028870
TABLE I
DSM-IV diagnostic criteria for attention-deficit/hyperactivity disorder
A. Either (1) or (2)
1. six (or more) of the following symptoms of inattention have
persisted for at least 6
months to a degree that is maladaptive and inconsistent with developmental
level:
Inattention
a. often fails to give close attention to details or makes careless mistakes
in
schoolwork, work or other activities
b. often has difficulty sustaining attention in tasks or play activities
c. often does not seem to listen when spoken to directly
d. often does not follow through on instructions and fails to finish
schoolwork,
chores, or duties in the workplace (not due to oppositional behavior or
failure
to understand instructions)
e. often has difficulty organizing tasks and activities
f. often avoids, dislikes, or is reluctant to engage in tasks that require
sustained
mental effort (such as schoolwork or homework)
g. often loses things necessary for tasks or activities (e.g., toys, school
assignments, pencils, books, or tools)
h. is often easily distracted by extraneous stimuli
i. is often forgetful in daily activities
2. six (or more) of the following symptoms of hyperactivity-impulsivity have
persisted for at least 6 months to a degree that is maladaptive and
inconsistent with
developmental level:
Hyperactivity
a. often fidgets with hands or feet or squirms in seat
b. often leaves seat in classroom or in other situations in which remaining
seated
is expected
c. often runs about or climbs excessively in a situation in which it is
inappropriate (in adolescents or adults, may be limited to subjective feelings
of restlessness)
d. often has difficulty playing or engaging in leisure activities quietly
e. is often "on the go" or often acts as if "driven by a motor"
f. often talks excessively
Impulsivity
g. often blurts out answers before questions have been completed
h. often has difficulty awaiting turn
i. often interrupts or intrudes on others (e.g., butts into conversations
or games)
B. Some hyperactivity-impulsive or inattentive symptoms that caused impairment
were
present before age 7 years
C. Some impairment from the symptoms is present in two or more settings (e.g.,
at school
[or work] and at home)
D. There must be clear evidence of clinically significant impairment in
social, academic, or
occupational functioning
4
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
E. The symptoms do not occur exclusively during the course of a Pervasive
Developmental
Disorder, Schizophrenia, or other Psychotic Disorder and are not better
accounted for by
other mental disorder (e.g., Mood Disorder, Anxiety Disorder, Dissociative
Disorder, or a
Personality Disorder).
Code based on type:
314.01 Attention-Deficit/Hyperactivity Disorder, Combined Type: if both
Criteria Al
and A2 are met for the past 6 months
314.00 Attention-Deficit/Hyperactivity Disorder, Predominately Inattentive
Type: if
Criteria Al is met but Criteria A2 is not met for the past 6 months
314.01 Attention-Deficit/Hyperactivity Disorder, Predominately Hyperactive-
Impulsive Type: if Criteria A2 is met but Criteria Al is not met for the past
6 months
[0010] There has been increased interest in ADHD as a heritable
neuropsychiatric condition
linked to pathogenesis of brain dopamine [Shaw 2007; Swanson 2007; Volkow
2007]. As
discussed herein, ADHD as an important putative complex subtype of a general
condition or
umbrella disorder known as reward deficiency syndrome (RD S) [Blum 11 1996].
"RDS" refers
to the breakdown of a cascade of neurotransmitters in the brain in which one
reaction triggers
another ¨ the reward cascade [Blum II 1990] ¨ and resultant aberrant conduct
[Blum I 1996].
At the level of individual neurons, the reward cascade is catalyzed by a
number of specific
neurotransmitters, each of which binds to certain types of receptors and
serves a specific
function. The binding of the neurotransmitter to neuronal receptors triggers a
reaction that is
part of the cascade. Disruption of these intercellular cascades results in
aberrant behavior of
one form or another in RDS, including ADHD.
[0011] RDS has genetic and environmental influences, and it predisposes
individuals to high
risk for multiple addictive, impulsive, and compulsive behaviors. Depending on
genes that
control different parts of the reward neurotransmitter pathways, a person may
display anything
from mild anxiety, irritability, hyperactivity, or risk taking, to compulsive
shopping, gambling,
sexual behaviors, drug addiction, alcoholism, smoking, and even eating
disorders. Of all of
these conditions, one that is especially controversial and receives
considerable media coverage,
is ADHD [APA 1994; APA2000].
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0012] According to CHADD (Children and Adults with ADHD), 3.5 million school
age
children have ADHD [CHADD 2007]. ADHD usually persists throughout a person's
lifetime.
It is not limited to children. Approximately one-half to two¨thirds of
children with ADHD will
continue to have significant problems with ADHD symptoms and behaviors as
adults, where it
impacts their lives on the job, within the family, and in social
relationships. ADHD is
recognized as a disability under federal legislation (the Rehabilitation Act
of 1973; the
Americans with Disabilities Act; and the Individuals with Disabilities
Education Act).
Appropriate and reasonable accommodations are sometimes made at school for
children with
ADHD, and in the workplace for adults with ADHD, which help the individual to
work more
efficiently and productively. While teachers are not equipped to make a
definitive diagnosis,
they are a meaningful source of initiation of the process to attain a sound
diagnosis [Biederman
2006]. However, less than half of those individuals who have been targeted by
teachers receive
appropriate diagnosis and corrective intervention. Of those who are diagnosed,
few are
receiving appropriate multi-modal treatment apart from pharmacological
manipulation.
Moreover, pediatricians report that approximately 4% of their patients have
ADHD. Boys are
four times more likely to have this illness than girls.
[0013] Twin studies indicate that 75%-90% of ADHD is caused by genetic
factors. If one
person in a family is diagnosed with ADHD there is a 25%-35% probability that
another family
member also has ADHD, compared to a 4%-6% probability for someone in the
general
population. Between 10% and 35% of children with ADHD have a first degree
relative with
past or present ADHD. Approximately one-half of parents who had ADHD have a
child with
the disorder. There may be non-genetic factors as well, including prenatal
exposure to nicotine
by mothers who smoked, anoxia in the neonatal period of infancy, and childhood
exposure to
high quantities of lead.
6
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
Science of reward deficiency syndrome
[0014] RDS results from a dysfunction in the "brain reward cascade," a complex
interaction
among brain neurotransmitters in reward centers of the brain, which directly
links abnormal
craving behavior with a defect in at least the DRD2 dopamine receptor gene
[Blum I 1990].
Dopamine is a powerful brain neurotransmitter that controls feelings of well
being [Blum II
1990; Blum 1991; Blum I 1996]. Dopamine interacts with other powerful brain
chemicals and
neurotransmitters (e.g., serotonin and the opioids), which themselves are
associated with
control of moods. In individuals possessing an abnormality in the DRD2
dopamine receptor
gene, the brain lacks sufficient numbers of dopamine receptor sites to use the
normal amount
of dopamine in reward centers and thus reduces the amount of dopamine produced
in this area.
In individuals not possessing the variant in the dopamine receptor gene, but
who have engaged
in risky behaviors (such as cocaine abuse, extremely low caloric diet, high
levels of stress over
an extended period of time), the brain functions as though it had the
DRD2genetic variant (or
other specific gene variants) [Faraone 2003].
[0015] The overall effect is inadequate dopaminergic activity in brain reward
centers. This
defect drives individuals to engage in activities that will increase brain
dopamine function.
Consuming large quantities of alcohol or carbohydrates (carbohydrate bingeing)
stimulates the
brain's production of, and utilization of, dopamine. So too does the intake of
crack/cocaine and
the abuse of nicotine. Also, it has been found that the genetic abnormality is
associated with
aggressive behavior, which also stimulates the brain's use of dopamine [Blum
II 1996; Blum
2000].
[0016] RDS can be manifested in relatively mild or severe forms that follow as
a consequence
of an individual's biochemical inability to derive reward from ordinary,
everyday activities. At
least one genetic aberration has been identified that leads to an alteration
in the reward
pathways of the brain [Bowirrat 2005]. It is a variant form of the gene for
the dopamine
7
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
D2 receptor, called the Al allele. This genetic variant also is associated
with a spectrum of
impulsive, compulsive, and addictive behaviors. The concept of the RDS unites
those disorders
and may explain how simple genetic anomalies give rise to complex aberrant
behaviors. While
this polymorphic gene may play a significant role in ADHD predisposition, it
must be tied to
a certain subset of additional genes for the clinical expression of ADHD. This
is called
polygenic inheritance. Recent associations of certain alleles of both the
dopamine D4 and
dopamine D2genes and novelty seeking behavior have confirmed previous work
suggesting
polygenic inheritance [Comings 1996; Lee 2003].
Biology of reward
[0017] The reward system in the brain was discovered by accident in the 1950s
by James Olds
[Olds 1956]. Olds had been studying brain mechanisms of attention using
laboratory rats, when
he mistakenly placed electrodes in a region of the limbic system. When the
electrodes were
attached so that the animals could self-stimulate this region by pressing a
lever, rats would
press the lever almost nonstop, as much as 5,000 times an hour. The animals
would stimulate
themselves to the exclusion of everything else except sleep. They also would
endure
tremendous pain and deprivation for an opportunity to press the lever. Olds
had clearly found
an area in the limbic system that provided a powerful reward for these
animals.
[0018] Later research on human subjects revealed that the electrical
stimulation of the medial
hypothalamus in the limbic system produced a feeling of quasi-orgasmic sexual
arousal. If
certain other areas of the brain were stimulated, an individual experienced a
type of light-
headedness that banished negative thoughts [Olds 1956; Blum 2000]. These
discoveries
demonstrated that pleasure is a distinct neurological function that is linked
to a complex reward
and reinforcement system. During the past several decades, research has been
able to better
define some of the brain regions and neurotransmitters involved in reward
[Blum I 1996; Blum
2000]. A neuronal circuit deep in the brain involving the limbic system, the
nucleus accumbens,
8
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
and the globus pallidus, appears to be critical in the expression of reward
[Wise 1984].
Although each substance of abuse or each addictive behavior may act on
different parts of this
circuit, the end result is the same: Dopamine appears to be the primary
neurotransmitter
released in brain reward sites [Koob 1988].
Cascade Theory of Reward
[0019] Considerable attention has been devoted to the investigation of the
neurochemical and
neuroanatomical systems that underlie a variety of substance-seeking
behaviors. In healthy
people, neurotransmitters work together in a pattern of stimulation or
inhibition, the effects
spreading downward, like a cascade, from stimulus input to complex patterns of
response
leading to feelings of well-being (the "Cascade Theory Of Reward") [Stein
1986; Blum II
1990; Cloninger 1993]. Although this neurotransmitter system is very complex
and still not
completely understood, the main central reward areas in the human brain's
mesolimbic system
are summarized below.
[0020] As can be seen in FIGS. 1A-1B, the following interactions take place in
brain reward
areas [Blum 1991; Stein 1986]: (1) Serotonin in the hypothalamus indirectly
activates opiate
receptors and causes a release of enkephalins in the ventral tegmental region
A10. The
enkephalins inhibit the firing of gamma-aminobutyric acid neurotransmitter
(GABA), which
originates in the substantia nigra A9 region. (2) GABA' s normal role, acting
through GABA
B receptors, is to inhibit and control the amount of dopamine released at the
ventral tegmental
regions for action at the nucleus accumbens. When dopamine is released in the
nucleus
accumbens, it activates dopamine D2 receptors, a key reward site. This release
also is regulated
by enkephalins acting through GABA. The supply of enkephalins is controlled by
the amount
of the neuropeptidases, which destroy them. (3) Dopamine also may be released
into the
amygdala. From the amygdala, dopamine exerts an effect on neurons within the
hippocampus
(i.e., the dopamine stimulates the hippocampus and the CA and cluster cells
stimulate
9
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
dopamine D2 receptors). (4) An alternate pathway involves norepinephrine in
the locus
ceruleus whose fibers project into the hippocampus at a reward area centering
around cluster
cells, which have not been precisely identified (designated as CAx). When GABA
A receptors
in the hippocampus are stimulated, they cause the release of norepinephrine.
[0021] It is to be noted that the putative glucose receptor in the
hypothalamus is intricately
involved and links the serotonergic system with opioid peptides leading to the
ultimate release
of dopamine at the nucleus accumbens. In the brain reward cascade these
interactions may be
viewed as activities of subsystems of a larger system, taking place
simultaneously or in
sequence, merging in cascade fashion toward anxiety, anger, low self-esteem,
or other
unpleasant feelings, or toward craving of a substance that will reduce or
eliminate the feelings
(e.g., alcohol, carbohydrates, alcohol, and drugs) [Blum 11 1990].
[0022] The notion of dopamine as the final common pathway for a number of
diverse drugs of
abuse is supported by the findings of Ortiz and associates [Ortiz 1995]. They
demonstrated that
chronic administration of cocaine, morphine, or alcohol resulted in several
biochemical
adaptations in the mesolimbic dopamine system. They suggested that these
adaptations may
underlie changes in the structural and functional properties of the neuronal
pathway of this
system related to substance abuse [011at 1990; also see Imperato 1988].
[0023] Genetic anomalies, long-term continuing stress, or long-term abuse of
substances can
lead to a self-sustaining pattern of abnormal craving behavior in both animals
and humans.
Research on nonhuman animals has provided support for the cascade theory of
reward and its
genetic links. Thus, Li and colleagues [Russell 1988; Zhou 1991; McBride 1993;
McBride 1994; Li 2006] developed strains of alcohol-preferring (P) and non-
preferring (NP)
rat lines. They found that the P rats have the following neurochemical
profile: lower serotonin
neurons in the hypothalamus; higher levels of enkephalin in the hypothalamus
(due to a lower
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
release); more GABA neurons in the nucleus accumbens; reduced dopamine supply
at the
nucleus accumbens; and reduced densities of dopamine D2 receptors in the
mesolimbic areas.
[0024] In terms of genetics, especially as related to ADHD, a number of genes
have been
associated, and these candidate genes are all involved in the reward cascade.
Comings II
2000 described a subset of at least 42 gene variants, which associate with
ADHD and contribute
to the overall variance. Interestingly, these genes constitute the basis for
the reward cascade
including certain neurotransmitters but not limited to dopaminergic,
serotonergic,
enkephalinergic, catecholaminergic, cholinergic, GABAergic, androgen
receptors, as well as
other putative transmitters, hormones, and their receptors and enzymes (both
anabolic and
catabolic).
[0025] In recent years, a number of reviews of the neurochemical basis of ADHD
have
emphasized the involvement of multiple neurotransmitters and emphasized that
one single
genetic defect cannot explain all of the data. Polygenic inheritance is
uniquely capable of
answering the question of how to account for both the range of comorbid
disorders in ADHD
and their interaction, but it fails to provide us with a true model of subsets
of genes and their
contribution to the variance of the disorder in question. One example of
polygenic inheritance
for ADHD was tested by Comings I 2000. They found that three dopaminergic
genes, DRD2,
DAT1, and DBH, differentially associated with ADHD probands. Their results
showed that
these three genes were additive in their effect. Thus, individuals who had
three out of three
markers had the highest ADHD score; those with two of three had the next
highest score; then
one of three; and those with none of the three markers had the lowest ADHD
score [Comings
1996]. Moreover, this additive effect was also seen for a number of other
related ADHD
behaviors (i.e., stuttering, obsessive compulsive disorder [OCD], tics,
conduct disorder [CD])
and supports the polygenic hypothesis of ADHD. In other words, the different
associated
11
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
behaviors are due to similar sets of genes in that certain psychiatric
disorders have a number
of genes in common.
[0026] This suggests a four-part cascade sequence leading to a reduction of
net dopamine
release in a key reward area. Additional support for this idea came when
investigators found
that by administering substances that increase the serotonin supply at the
synapse, or by
stimulating dopamine D2 receptors directly, they could reduce craving for
alcohol [McBride
1994]. Specifically, D2 receptor agonists reduced alcohol intake in high
alcohol preferring rats,
whereas D2 dopamine receptors antagonists increased alcohol drinking in these
inbred animals
[Dyr 1993].
Science of ADHD
Neuropsychogenetics of ADHD
[0027] In ADHD, the picture emerges of individuals suffering from overload,
trying to adjust
to a world that is too bright, too loud, too abrasive, and too rapidly
changing for comfort. Early
speculation about the causes of ADHD focused on such factors as marital
disorder, poor
parenting, brain damage, psychiatric illness, or alcoholism or drug abuse in
the family.
Associated behaviors included CD and anti-social personality. Later these
behaviors were
shown to be linked hereditarily to substance use disorder (SUP). Most
recently, research has
begun to show a significant association between these behavioral disorders,
ADHD, and
specific genetic anomalies.
[0028] This leads to the inquiry as to what is the cause or basis of ADHD. It
is an impulse
disorder with genetic components that results from imbalances of
neurotransmitters. Its effects
can be eased by treatment and counseling. The biological basis for this
disorder has been
established by a number of investigators [Comings 1991; Biederman 1992]. In
one study
individuals with ADHD were found to have abnormal brain wave patterns [Lubar
1991]. Their
beta waves (brain waves associated with concentration) are low, and their
theta waves
12
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
(associated with relaxation) are high, suggesting a state of drowsiness and
daydreaming. It is
not surprising, therefore, that activities associated with beta waves, e.g.,
watchful anticipation
and problem solving, are difficult for individuals with ADHD to sustain. They
like activities
that permit them to stay in a theta state with a minimum of outside
stimulation [Lubar 1991].
It may be that people with ADHD are afflicted with a defective filtering
system such that their
brainstem reticular formation does not block out irrelevant stimuli. These
people appear to be
aware of every sound, every object, every touch, and they all merge in
disorganized behaviors
that are difficult to tolerate. Non-essential stimuli get the same attention
as those essential to
work or relating to other people. At a deeper level, ADHD is a problem of
communication
among brain cells, or neurons, possibly involving the neurotransmitters that
carry inter-neural
messages. These brain messengers may be either in short supply for certain
behaviors such as
cravings (probably due to inadequate serotonergic and or dopaminergic
function) or other
attentional deficits, or they may be the result of too much norepinephrine
rather than too little.
If the messengers that inhibit incoming stimuli are deficient, too many
signals get through and
create confusion.
[0029] At a still deeper level, the problem lies in the genes that lay down
the blueprint for
manufacturing neurotransmitters. People with ADHD have at least one defective
gene, the
DRD2 gene that makes it difficult for neurons to respond to dopamine, the
neurotransmitter
that is involved in feelings of pleasure and the regulation of attention.
Studies on genetic
anomalies have implicated other dopaminergic genes such as the DRD4 receptor
gene, the
dopamine beta hydroxylase (Df3H) gene, and the dopamine transporter genes as
causative
factors in ADHD [Cook 1995; Waldman 1998], as well as gene variants involved
in multiple
neurotransmitter pathways.
[0030] Support for the role of genetics in ADHD includes evidence showing that
it runs in
families. For example, a number of studies have shown that fathers and/or
mothers of ADHD
13
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
children tend to have antisocial personality and alcoholism. As early as 1971,
James Morrison
and Mark Stewart examined parents of 59 hyperactive children and 41 control
children. In 21
of the families, at least 1 parent was alcoholic or had antisocial personality
and other related
behaviors. By contrast, only 4 of the control families were so affected. In a
family study of
parents and siblings of felons, there was an increased frequency of antisocial
personality,
alcoholism, and drug addiction in male relatives of hyperactive children
[Cantwell 1972].
[0031] Numerous studies indicate that 20%-30% of siblings of ADHD children
also have
ADHD. This is 2-7 times the frequency found in non-ADHD children. These
siblings also
were 5 times more likely to have major depression than control children
[Weiner 1977; August
1983]. Other studies showed that 22% of brothers and 8% of sisters of
hyperactive children
were hyperactive themselves. Interestingly, however, when ADD is considered
without
hyperactivity, the number of brothers and sisters affected was the same
[Cantwell 1976]. In
another study of ADHD children it was found that if neither parent had the
syndrome, 11% of
the siblings had ADHD. If one parent had ADHD, 34% of the siblings had ADHD
[Pauls
1986].
[0032] The observed fact that ADHD parents have an ADHD child does not prove
that the
problem is genetic. The question can be asked, was the behavior learned? One
answer to the
question is to look at siblings and half-siblings, both raised in the same
environment. If ADHD
is learned, the frequency should be the same for both. In actuality, half-
siblings who have only
half the genetic similarity show a significantly decreased frequency of ADHD
[Safer 1973]. In
a study of twins [Willerman 1973], it was found that if one identical twin had
ADHD, the other
also had ADHD. If non-identical twins had ADHD, only 17% of the other twins
had ADHD.
This finding was confirmed in other independent studies.
[0033] Another approach is to look at the parents of ADHD children given up
for adoption. If
ADHD is a genetic disorder, the parents of children with the problem should
show a higher
14
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
frequency of ADHD, antisocial personality, or alcoholism than the adopting
parents. In a study
of ADHD children of ADHD parents who gave up their children at birth for
adoption, it was
found that the rate of antisocial personality, alcoholism, and ADHD was higher
in the
biological parents than in the adopting parents. In a study by Comings et at.
[Comings 1991],
the investigators found that the Al allele of the dopamine D2 receptor gene
was present in 49%
of a sample of ADHD children compared to only 27% of controls.
[0034] To some extent, people with ADHD can learn to cope. They can avoid
situations that
generate stress; avoid crowds and noisy environments; give themselves plenty
of time and
avoid tight deadlines; and avoid rapid changes in their environment. The most
destructive
coping strategy is self-medication with alcohol or drugs. Such substances give
the illusion that
they are making life easier and more pleasant, for the symptoms seem to
disappear. But the
addiction quickly takes over, and life becomes a nightmare [Faraone 1991].
Then, when they
withdraw from alcohol or drugs, the ADHD problems return in full force.
[0035] The inherent tragedy here is that the ADHD person may be genetically at
risk of
developing an addiction. Possibly the same neurochemical imbalance in their
brain that
produces ADHD also produces a predisposition to addiction, Tourette syndrome,
ODD, CD,
and as well as other related behaviors [Comings 1991; Blum II 1996; Miller
2008].
Behavioral and electrophysiological diagnostic tools
[0036] The following assists in the understanding the need for behavioral and
electrophysiological diagnostic tools and treatment thereof of ADHD.
[0037] In clinical settings, a number of rating scales have been utilized with
mixed results for
the diagnosis of ADHD. One set of commonly employed tools involves the
Conners' Rating
Scales [Conners 2006], an instrument that uses observer ratings and self-
reports to help
evaluate problem behaviors in children and adolescents. Another alternative
utilized in a
clinical setting to assist in properly diagnosing ADHD is a continuous
performance test called
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
T.O.V.A. (Test of Variables of Attention) [TOVA 2006]. The latest version of
this test is
computerized, and it is designed to identify a minimum of four types of
attention failures. One
type is marked by omission abnormalities when the patient's attention failure
is measured by
missing information. The problem with relying on this parameter is that
omission errors have
been associated with a wide spectrum, including schizophrenia and petit mal
seizure disorder,
in which the attention failure is marked by neurological absences. The second
type is marked
by commission abnormalities associated with impulsive behaviors, and it
frequently is co-
morbid with a cluster of anxiety disorders (e.g., obsessive compulsive
behaviors, panic, and
oppositional defiance). The third type is marked by abnormalities in reaction
time. It is believed
that this type is not specific for ADHD and is associated with slowing of
response times as seen
in classic psychomotor retardation, dysthymia, and major depression. The
fourth type is
response variability (either fast or slow). Of all the above, this is more
closely related to ADHD
and is also common in adults that have obesity, alcoholism, and/or craving
disorders. It is this
fourth type that is most likely linked to dopaminergic deficiency. However, it
is important to
note that results of T.O.V.A. tests have been associated with a number of
false negative
diagnoses.
[0038] To test the relationship between response variability and dopaminergic
deficiency, a
study was embarked upon by the inventor of the present invention that examined
associations
between dopamine D2receptor variants and T.O.V.A. scores (including response
variability),
as well as a measure of brain electrical activity, the P300 event related
brain potential [Noble
1994]. 100 patients entering the PATH Medical Clinic, New York City, were
studied for a
variety of medical complaints including neuropsychiatric, cardiovascular, and
oncological
problems. Each patient was given the T.O.V.A. and brain electrical activity
mapping. When all
the T.O.V.A. scores were summed (<1 standard deviation above the norm) a
significant linear
trend was observed, whereby increasing abnormal T.O.V.A. scores were
associated with a
16
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
percentage of patients having an abnormal prolonged P300 latency (normal being
300 plus
age). Moreover, significant differences were found between the various scores
(inattention,
impulsivity, response time, and variability) and abnormal P300 latency
[Braverman 2006]. In
contrast, only variability response was significant for P300 amplitude. This
site-specific
association may be attributable to dopaminergic variants. It is well known
that the DRD2 gene
Al allele is associated with abnormalities in both the P300 latency and
amplitude in well-
screened alcoholics [Noble 1994]. Thus, the clinicians must be cautious in
terms of utilizing
only one diagnostic tool to diagnose ADHD. In additional embodiments, the
methods can be
employed together with one or more additional tests, including both Conners
and T.O.V.A., as
well as gene testing.
ADHD is a common disorder
[0039] Estimates of the frequency of the various types of ADHD, based on
population surveys,
have shown variable results. A fairly common range is illustrated in TABLE II.
The advantage
of population based samples, in contrast to clinic based samples, is that
individuals in the
community who have not sought medical attention are included in the sample. In
most
locations, far fewer than 16%, and usually less than 4%, of the children in a
given population
receive treatment for a form of ADHD. This is contrary to the notion that the
ADHD is over
diagnosed and overtreated. In fact, the majority of symptomatic children are
not treated. Other
associated disorders include CD and ODD.
TABLE II
Prevalence of various types of ADHD in the general population
Hyperactive/Impulsive 2.6
Inattentive 8.8
Combined 4.7
Total 16.1
M/F ratio 4:1
[Wolraich 1998].
17
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0040] While many of these children can be handled by appropriate teaching
methods and do
not require treatment, these figures suggest that ADHD-I at least, is probably
under diagnosed
and under treated. While the sex ratio for ADD-H and ADHD-C is 4:1, the sex
ratio for ADD-
I is closer to 1:1. This is a reflection of the fact that ADHD in girls tends
to present as the
inattentive type while boys are more likely to present as the hyperactive-
impulsive or combined
type. Symptoms of hyperactivity and impulsivity in school are obvious and
disruptive, whereas
symptoms of inattention are more subtle and non-disruptive; consequently, boys
tend to be
diagnosed and treated more than girls.
ADHD is a spectrum disorder
[0041] It has been known for many years that if an individual inherits enough
genes to develop
any given behavioral disorder, the risk of developing a second behavioral
disorder is two to
four times greater than for the general population. This is likely due to the
fact that different
behavioral disorders share some gene variants in common. Thus, the more a
person exceeds
the required threshold number of gene variants, the greater the likelihood of
developing more
than one behavioral problem, thus the term spectrum disorders. Some of the
most common
coexisting or comorbid spectrum disorders seen in individuals with ADHD are
ODD, CD,
major depressive disorder, anxiety disorders, OCD, bipolar disorder, learning
disorders, and
substance abuse disorder including alcoholism and drug addiction.
ADHD has lifelong effects
[0042] Having pointed out that much of the poor outcome in ADHD children is
due to the
comorbid presence of CD, the studies of a 1985 report of Howell and coworkers
[Howell 1985]
should still be presented. While this longitudinal study did not distinguish
between ADHD and
ADHD plus CD, it did something no other study has done. The study compared the
outcome
of three groups of children instead of just ADHD children and controls.
Children in the early
grade school years were evaluated on a continuum of ADHD symptoms and divided
into three
18
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
groups, those scoring in the highest 10% (ADHD group) those in the lowest 10%
(low ADHD
group) and the rest ("normal" group). They were then re-evaluated after they
graduated from
high school.
[0043] The remarkable finding was that in virtually every aspect of their life
the low ADHD
group performed best, the normal individuals were intermediate and the ADHD
group
performed worst. This should not be taken to suggest that children with ADHD
always
underachieve. Again, it should be emphasized there are many examples in which
the restless,
workaholic, always-have-to-be-doing-something, I-need-to-be-my-own-boss,
characteristics
of ADHD subjects result in very successful lives. Thus, in the right
combination, some of the
symptoms that are being discussed in a negative light can be used to great
advantage [Comings
2005].
Genes and ADHD
[0044] It has been proposed that ADHD is a polygenic disorder due to the
additive effect of
genes affecting dopamine, norepinephrine, serotonin, GABA, and other
neurotransmitters [see,
e.g., Comings 12000]. Some of the specific loci involved are dopamine genes
DRD1, DRD2,
DRD4, DRD5, dopamine¨beta-hydroxylase, and the dopamine transporter;
norepinephrine and
epinephrine genes ADRA2A, ADRA2C, PNMT, norepinephrine transporter, MAOA,
catechol-O-methyltransferase (COMT); serotonin genes TD02, HTR1A, HTR1DA,
serotonin
transporter; GABA genes GABRB3; androgen receptor and other genes. This model
is
consistent with present knowledge about ADHD including the following [Comings
II 2000]:
(a) the increased frequency of ADHD in the relatives of ADHD probands, (b) the
presence of
a wide spectrum of comorbid behaviors (depression, anxiety, learning, CD, ODD,
and
substance abuse disorders) in ADHD probands and their relatives on both
parental sides, (c)
the close relationship to Tourette syndrome, (d) the failure to find the genes
for Tourette
syndrome using linkage analysis, (e) the brain imaging studies showing
hypometabolism of the
19
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
frontal lobes, (f) the relationship between dopamine D2receptor density and
regional blood
flow, (g) the correlation between cerebral spinal fluid homovanilic acid
levels and
DRD2 genotypes, (h) the correlation between tics and receptor density in
Tourette syndrome,
(i) the dopamine D2 motor hyperactivity of dopamine transporter and dopamine
D3 receptor
gene knockout mice, (j) the Le Moat 1991 and Shaywitz 1976 dopamine deficiency
animal
models of ADHD, (k) the norepinephrine models of ADHD, (1) the failure to
explain ADHD
on the basis of any single neurotransmitter defect, (m) the response of ADHD
to dopamine and
alpha-adrenergic agonists, (n) the small percentage of the variance of
specific behaviors
accounted for by each gene, and numerous other aspects of ADHD.
[0045] In one study [Brookes 2006], 1,038 single-nucleotide polymorphisms
(SNPs) spanning
51 candidate genes involved in the regulation of neurotransmitter pathways,
particularly
dopamine, norepinephrine, and serotonin pathways, in addition to circadian
rhythm genes,
revealed interesting results. The analyses involved within-family tests of
association in a
sample of 776 DSM-IV ADHD combined-type cases ascertained for an international
multi-
centre ADHD gene project. The researchers found nominal significance with one
or more SNPs
in 18 genes, including the two most replicated findings in the literature:
DRD4 and DAT1.
Gene-wide tests, adjusted for the number of single nucleotide polymorphisms
(SNPs) analyzed
in each gene, identified associations with the following: serotonergic (TPH2),
adrenergic
(ARRB2, ADRB2), dopaminergic (DAT1), neurotransmitter metabolizing (MAO),
pituitary
development (HES1), enkephalinergic (PNMT), and synapase regulator
(synaptophysin II [syp
II]) gene polymorphisms.
Molecular genetics and ADHD
[0046] ADHD is not caused by poor parenting, family problems, poor teachers or
schools, too
much TV, food allergies or excess sugar. Instead, it is caused by biological
and genetic factors
that influence neurotransmitter activity in certain parts of the brain
[Wallis. 2008]. Studies at
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
the National Institute of Mental Health using positron emission tomography
(PET) scans to
observe the brain at work have shown a link between a person's ability to pay
continued
attention and the level of activity in the brain. In people with ADHD, the
brain areas that control
attention used less glucose, indicating that they were less active. It appears
from this research
that a lower level of activity in some parts of the brain may cause
inattention and other ADHD
symptoms.
A dopamine model
[0047] Defects in dopamine metabolism have long been implicated in the
etiology of ADHD.
There are many reasons for this [Comings 1991; Kirley 2003]: (1) Le Moat 1991
showed that
lesions of the dopaminergic neurons of the ventral tegmental area resulted in
hyperactivity,
hyper-responsivity, poor response to stress, and a spectrum of other
disorders. (2) Shaywitz
1976 showed that chemical destruction of frontal lobe dopaminergic neurons
shortly after birth
produced an animal model of ADHD that responded to stimulants. (3)
Catecholamines in the
cerebral spinal fluid (C SF) of children with Tourette syndrome showed
significantly lower
levels of homovanillic acid. Some have also reported low CSF homovanillic acid
in children
with ADHD, while more recent studies have shown a positive correlation between
CSF
homovanillic acid and scores of hyperactivity and conduct disorder ADHD. (4)
Brain imaging
studies showed defects in the dopamine-rich striatum in ADHD [Krause 2003].
(5)
Furthermore, brain imaging studies indicate hypofunctionality of the frontal
lobes in ADHD
and Tourette syndrome. (6) Other studies have shown hyperactivity in knockout
mice missing
the dopamine transporter or DRD3 genes. (7) Further evidence demonstrated the
effectiveness
of dopaminergic agonists in the treatment of ADHD [la Fougere 2006]. The
following are
some of the specific dopaminergic genes that have been implicated in the
etiology of ADHD.
See FIGS. IA-1B.
21
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
Dopamine D2 receptor gene (DRD2)
[0048] The first molecular genetic studies of ADHD were reported in 1991 by
Comings et at.
following the discovery by Blum and associates linking DRD2 Al allele to
severe alcoholism
[Blum III 1990; Comings 1991]. They examined the prevalence of the Taq Al
allele of the
DRD2 gene in impulsive, compulsive, addictive behaviors. These results
suggested that genetic
variants at the DRD2 locus played a role in a range of impulsive, compulsive,
addictive
disorders, including ADHD. The prevalence of the D2A1 allele in these
disorders ranged from
42.3 to 54.5%. While it was clear that the DRD2 was not a major gene causing
these conditions,
since it was usually not even present in half of the cases, it was also clear
that the prevalence
of the D2A1 allele was approximately two-fold higher than in controls.
[0049] An indication of the importance of the dopamine D2 receptor in Tourette
syndrome
comes from SPECT (single photon emission computed tomography) studies of
monozygotic
twins discordant for tic severity. For example, differences in D2receptor
density in the head of
the caudate nucleus predicted differences in phenotypic severity with the
almost unheard of
correlation coefficient of r = 0.99, p < 0.001, suggesting that striatal
dopamine D2receptor
density accounted for 98% of the variance of tic severity [Wolf 1996].
[0050] In a subsequent study of individuals who smoked at least one pack of
cigarettes per day
and were unable to quit on their own, it was found that 48% carried the Taq I
D2A1 allele and
had trouble sleeping. The prevalence Taq I D2A1 allele was even higher in a
large group of
pathological gamblers. It was also verified in post-traumatic stress disorder.
[0051] The initial interpretation was that the DRD2 gene modified the effect
of an unidentified
major gene for Tourette syndrome and ADHD. The important feature is that the
DRD2 gene
accounted for less than 5% of the variance of a number of quantitative traits
relating to ADHD
and other behaviors. As the number of genes showing a similar modest effect
were identified
(see below), and as the failure to find any gene causing a major effect
continued, we and others
22
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
began to favor the polygenic mode of inheritance for ADHD, Tourette syndrome,
and other
psychiatric disorders [Noble 2003].
[0052] Moreover, recent work indicates that other RDS related behaviors
including adolescent
excessive internet video gaming are significantly associated with the DRD2 Al
allele.
Interestingly, in both Borderline Personality Disorder as well as healthy
individuals, the
presence of the DRD2 Al allele correlated with the commission of more time
violations on a
test sensitive to the integrity of the frontal lobes, and especially in the
healthy subjects, with
longer execution times. This work suggests that the DRD2 gene could exert an
effect on
executive functioning controlled by frontal brain systems.
Dopamine D2 receptors, regional blood flow, and response to methylphenidate
[0053] In reviews of published articles that examined striatal dopamine
transporter (DAT)
density in ADHD patients, Krause et al. [Krause 2003; Krause 2006] cited
numerous
neuroimaging findings of elevation in that region. Additionally, Krause et al.
[Krause
2005] investigated whether availability of striatal DAT may have an influence
on the response
of adult ADHD patients to methylphenidate, as measured with SPECT scans. They
found that
ADHD individuals with low DAT availability failed to respond to
methylphenenidate therapy.
[0054] Also using SPECT technology, Volkow and colleagues [Volkow 1995]
examined the
relationship between the effects of methylphenidate on regional blood flow and
the density of
dopamine D2 receptors in various regions of the brain. In some subjects,
methylphenidate
increased regional blood flow while in others it decreased blood flow. The
changes in the
frontal, temporal and cerebellar metabolism were related to the density of
D2receptors ¨ the
higher the density the greater the increases in blood flow. Methylphenidate
decreased the
relative metabolic activity of the basal ganglia. These results are
consistent, indicating that
genetic defects in dopamine metabolism, resulting in a hypo-dopaminergic state
in the limbic
system and frontal lobes, result in a compensatory increase in dopaminergic
activity in the basal
23
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
ganglia, and that methylphenidate reverses these through a combination of
enhancing brain
dopamine activity by inhibition of the dopamine transporter, with a secondary
decrease in
dopaminergic activity in the basal ganglia and a decrease in basal ganglia
blood flow.
[0055] These studies are also consistent with the results of Castellanos and
colleagues
[Castellanos 1998] showing a positive correlation between the response to
methylphenidate
and CSF levels of homovanillic acid, a metabolite of dopamine whose levels in
the CSF are
related to D2 receptor density.
[0056] One of the intriguing aspects of the Volkow 1995 study was the finding
that
methylphenidate consistently increased cerebellar metabolism, despite the
paucity of
D2 receptors in this structure. This is consistent with the increasing
evidence that the
cerebellum plays an important role in attention, learning, and memory.
[0057] In support of the above studies, Noble et at. [Noble 1997] also found
an association
between the Taq I D2A1 genotype and regional blood flow. Using PET and 18F-
deoxyglucose,
they observed that Al carriers showed a significantly lower relative glucose
metabolism in the
putamen, nucleus accumbens, frontal and temporal gyri and medial prefrontal,
occipito-
temporal and orbital cortices than those with the A22 genotype. Noble and Blum
and associates
had previously shown that Taq I D2A1 carriers had a significantly decreased
dopamine
D2 receptor in the basal ganglia. In a different PET study, Farde et at.
[Farde 1997] observed
a significant decrease in dopamine D2 receptor density in individuals with
detachment, social
isolation, and lack of intimate friendships.
Heterosis at the DRD2 gene
[0058] Within the past decade, Comings et at. have examined the role of the
DRD2 gene in a
range of behaviors, and have noticed a persistent tendency for quantitative
behavioral scores
to be highest in 12 heterozygotes, lowest in 11 homozygotes, and intermediate
in 22
24
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
homozygotes. Most often the relationship is 12>>22>11 or 12 >>11 = 22. The
presence of a
greater effect in heterozygotes than either homozygote is termed heterosis.
[0059] Strong support for heterosis at the DRD2 gene comes from research by
Jonsson et at.
1996. They compared the CSF levels of the dopamine breakdown product
homovanillic acid
to the DRD2 genotype using the Tag I D2A1 polymorphism. There was a remarkable
similarity
to the profile for the inattention score in the Tourette syndrome subjects,
with 12 heterozygotes
showing the highest inattention score, and the Sinsson 1996 subjects who were
12
heterozygotes had the lowest levels of CSF homovanillic acid. The highest
levels of
homovanillic acid were seen in the 11 homozygotes, with the levels in 22
homozygotes being
intermediate. This suggests that subjects with the lowest levels of CSF
homovanillic acid had
the most symptoms of ADHD. While this is consistent with some studies showing
a
significantly lower level of CSF homovanillic acid in children with ADHD and
Tourette
syndrome, it seems to conflict with the studies of Castellanos 1998 showing a
positive
correlation among some aspects of symptom severity and response to
methylphenidate, and
CSF homovanillic acid levels. However, these studies only examined children
with ADHD and
did not include controls. While it is yet to be studied, those individuals
carrying the Tag I D2A1
allele may not be those who respond best to methylphenidate.
[0060] Recent PET and SPECT studies of the relationship between the Tag I
genotypes of the
DRD2 gene and number of dopamine D2 receptors in the striatum, support the
effect of
molecular heterosis producing the lowest level of D2 receptors in 12
heterozygotes, the highest
levels in 11 homozygotes and high levels in 22 homozygotes. These combined
results provide
the first illustration of a direct connection between a genotype, a
neurotransmitter level
(dopamine), and ADHD symptoms. While the studies of homovanillic acid levels
in ADHD
have been variable, these results suggest that some ADHD is associated with
low CSF levels
of homovanillic acid and this in turn is related to heterozygosity for the
DRD2 Tag I alleles. In
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
contrast, Noble 1994 found that the lowest level of D2 density was found in
the 11
homozygote.
[0061] In an attempt to further our understanding of the role of genes in ADHD
as a subtype
behavior of RDS, a research study was performed involving generational family-
based subjects
genotyped for three dopaminergic genes.
Dopamine transporter gene
[0062] The dopamine transporter is responsible for moving dopamine across the
presynaptic
membrane back into the nerve cell from which it was released. In a recent
review of the
literature [Comings 2005], the DAT lgene was considered an important candidate
gene for
ADHD, because it is a major dopaminergic gene, and it is the site of action of
methylphenidate
and dexedrine, widely used in the treatment of ADHD. These stimulant
medications inhibit the
transport process, resulting in an increase in synaptic dopamine. Cook 1995
reported a
significant positive association between the 10 allele of the DAT1 gene and 49
cases of ADHD
using the haplotype relative risk procedure. When eight cases of
undifferentiated ADD were
added, the results were unchanged. Using the family based haplotype relative
risk
procedure, Gill 1997 also found a significant preferential transmission of the
10 allele in 40
parent-child sets.
[0063] Comings 2001 also observed a significant association between the 10
allele and ADHD
and a range of other behavioral variables in Tourette syndrome probands. For
example, in a
group of 352 Tourette syndrome probands and control subjects, the mean
cumulative ADHD
score based on counts of DSM-III ADHD criteria, was 25.44 for those that were
10/10
homozygotes versus 20.42 for those that were not 10/10 homozygotes. Consistent
with these
results, Malison 1995, using SPECT imaging, reported a significant increase in
the level of
dopamine transporter protein in the striatum of Tourette syndrome subjects
compared to
controls.
26
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0064] Knockout mice missing the DAT1 genes are very hyperactive. While these
mice show
increased motor activity in open field studies, they were dramatically more
hyperactive in
smaller spaces. This suggests that the stress of being confined contributes to
the hyperactivity.
This is analogous to the contribution of the DRD2 gene to both hyperactivity
and poor response
to stress in humans. Studies of the DAT knockout mice showed a five-fold
increase in brain
dopamine levels, down-regulation of D2 receptors, uncoupling of D2receptor
function, and a
57% decrease in body size. While the presence of hyperactivity in the absence
of DAT1 genes
may seem to conflict with the above results, suggesting hyperactivity in the
presence of
increased activity of the human DAT1 gene, the presence of compensatory and
plastic changes
in other dopaminergic systems occurring when major defects of the dopamine
transporter are
present from conception, may account for the differences. Alternatively,
because of complex
inhibitory and stimulatory loops, both increases and decreases (too much or
too little) in the
amount of receptor or transport protein may result in similar symptoms. In
contrast to the above
results, LaHoste 1996 did not find a significant increase in the frequency of
the DAT1 10 allele
in their group of ADHD subjects. They showed, instead, an increase in the
prevalence of the 7
allele of the DRD4 gene.
[0065] Waldman 1998 have also examined the role of the DAT1 gene in ADHD. In
their first
report, they used the transmission disequilibrium technique (a family-based
association test to
examine the linkage between a genetic marker and a trait) to determine the
role of the
DAT1 gene in ADHD, ODD and CD in 123 families. They found a significant
association
between the DAT1 10 allele and ODD, CD, and hyperactivity-impulsivity. After
controlling
for the level of hyperactivity-impulsivity symptoms, the association with ODD
and CD was no
longer significant, suggesting that the relationship between childhood ODD and
CD was
mediated through its effect on hyperactivity and impulsivity. In a subsequent
report, they
examined 74 ADHD probands, 79 siblings, and a control sample of 49 twins. The
mean scores
27
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
for hyperactivity/impulsivity, inattentiveness, ODD, CD, and depression and
dysthymia were
progressively lower across these three groups. The inclusion of parents
allowed family based
association studies. It was of interest that the greatest power came from
discordant siblings.
Twelve of the 41 siblings were discordant for the high risk DAT1 alleles (10
repeat), and in 10
of these, the siblings carrying the high risk alleles had significantly higher
scores for
hyperactive-impulsive symptoms and for inattentive symptoms. The transmission
disequilibrium test also showed association and linkage of the 10 repeat with
the combined
form of ADHD. Of the 10 studied, eight were positive for a role of the DAT1
gene in ADHD.
[0066] Winsberg 1999 examined the correlation between response to
methylphenidate
treatment and DATlgenotype in a series of 30 African-American children with
ADHD. Of the
responders, only 31% carried the 10/10 genotype while 86% of the non-
responders carried the
10/10 genotype, suggesting that in this population 10/10 homozygosity is
associated with a
poor response to stimulant treatment. Although these interesting
pharmacogenomic findings
have been confirmed by some [Kirley 2003], they await further replication.
[0067] A prior meta-analysis concluded that there is a significant association
between ADHD
and dopamine system genes, such as DAT1, but even more robust with regard to
the DRD4 and
DRD5 genes [Li 2006]. Of further interest, Mill 2006 presented evidence that
polymorphisms
in the DRD4 and DAT1 genes were associated with variation (compromise) in
intellectual
functioning among children diagnosed as having ADHD. The same authors further
showed
from longitudinal evidence that these polymorphisms predicted which children
with ADHD
were at greatest risk for poor adult prognosis [see also Heiser 2004; Madras
2005; Larsson
2006].
Generational association studies of dopaminergic genes in RDS probands and
family members
[0068] At this point, it is important to emphasize that polymorphisms of the
dopamine
D2 receptor gene are associated with RDS and a number of related impulsive,
addictive, and
28
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
compulsive behaviors. In an unpublished study with Joel Lubar from the
University of
Tennessee, Knoxville, and Judith Lubar at the Southwestern Biofeedback and
Neurobehavioral
Clinic, the authors genotyped 51 subjects from four generations derived from
two multiply-
affected families All subjects were genotyped for three of the dopaminergic
genes (DRD2,
DATi, and DBH). In this study 80% of all subjects (40 of 50) carried the DRD2
Taq 1A1 allele.
When compared with "highly screened controls called super controls" (1/30 or
3.3% of the
controls carried the DRD2 Al allele), a highly significant association was
observed. It is
noteworthy that as the number of RDS behaviors increased in the subjects, the
prevalence of
the DRD2 Al allele also increased. This work allows one to utilize genotyping
to access certain
personality factors such as ADHD and other related RDS behaviors.
The role of polygenes as a diagnostic indicator
[0069] While there is much evidence for the involvement of the dopaminergic
system and
specific genes involved and treatment possibilities, other models including
genes related to
dopamine D4, dopamine D5, dopa decarboxylase gene, norepinephrine, adrenergic
2a and 2c,
COMT, tryptophan 2,3-dioxygenase, and GABA should also be considered [see
Comings
20011.
[0070] In terms of polygenic inheritance, others have observed that several
genes are
associated with ADHD, including DAT1, DBH, DRD4, DRD5 and 5HT1B. Moreover,
linkage
studies using affected sibling pairs and extended pedigrees have identified
several
chromosomal regions containing putative ADHD susceptibility genes. Chromosomal
regions
highlighted by replication across studies are accumulating evidence with
increasing sample
size and include chromosomes 5p13, 6q12, 16p13, and 17p11 [Arcos-Burgos 2004;
Asherson
2005].
[0071] Kent et at. [Kent 2005] found evidence to support the hypothesis that
the gene BDNF
(brain-derived neurotrophic factor) located at 11p13 and encoding for a
precursor peptide
29
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
(proBDNF), is associated with ADHD. Additionally, Turic and others [Turic I
2005] found
evidence that genes related to glutamate function such as SLC1A3 (Solute
Carrier Family 1,
member 3) in a family based study may contribute to susceptibility to ADHD.
Other genes that
have been associated with ADHD susceptibility include the calcyon gene
(DRD11p) [Laurin
2005]; beta hydroxylase gene [Inkster 2004]; NR4A2 gene [Smith 2005]; and the
COMT gene
[Turic 11 2005].
[0072] Understanding the genetic meaning of carrying the DRD2 and DAT1
polymorphisms
to assist in the diagnosis of ADHD is of paramount importance. One must first
consider the
difference between a single-gene-single-cause concept as in the situation with
Cystic Fibrosis
or Huntington's disease, or even Muscular Dystrophy, compared to multiple
genes involved in
complex disorders such as ADHD [Comings 1996]. With regard to psychiatric
genetic
anomalies such as schizophrenia, bipolar disorder, Alzheimer disease, RDS,
among other
related behaviors, dopaminergic allelic presence does not necessarily diagnose
the disorder. On
the other hand if an individual carries one or more of these associated
polymorphisms, the
scientific evidence supports a diagnosis of predisposition and high
probability that the subject
is at greater risk for having the disorder in question or may at some time in
the future present
with typical clinical symptoms. Moreover, we do know from the use of Bayes
theory to predict
outcomes, that carriers born with the dopamine D2 receptor Al allele have a
74% chance that
they would have RDS behavior [Blum III 1990; Blum III 1996].
[0073] This predisposition diagnosis is typical in that the same parameters
and limitations that
have been placed on other diseases such as so called oncogenes for cancer, as
well as the gene
for diabetes, are the same for RDS. There is a tendency in psychiatric
genetics to think in terms
of the single-gene¨single-disorder model and to lose sight of the fact that
polygenic inheritance
has its own distinct set of rules. There are some distinct issues that are
relevant to the genetics
of ADHD. A major point is that polygenic inheritance is far more complex than
single gene
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
inheritance. The ultimate truth about the role of any one gene involved in
polygenic inheritance
may require a summation across many different studies and the examination of
the additive
genes involved in both childhood and adult ADHD and their comorbid disorders.
Once the
gene map of ADHD is uncovered, it will provide improved diagnosis (prevent
over-diagnosis)
and treatment (non-drug, non-addictive, efficacious and safe) of these very
common disorders
and demonstrate for all but the most recalcitrant critic that these are real
biological entities.
[0074] Comings [Comings 2001] summarized the role of multiple genes in ADHD
providing
a polygenic model for the etiology of ADHD including the following salient
points modified
herein:
= Multiple dopaminergic genes and other genes each contributing to a small
percentage
of the total variance.
= The co-morbidity between ADHD and substance abuse (common sets of genes
affecting the frontal lobes and the reward pathways).
= The central role of the frontal lobes and ADHD and related disorders.
= The evidence from animals that defects of dopamine metabolism in the
frontal lobes
are important in ADHD.
= The secondary hypersensitivity of dopamine receptors in the basal ganglia
leading to
hyperactivity and tics.
= The close relationship between ADHD and Tourette syndrome.
= The role of norepinephrine genes in learning and language disorders
involving parietal
lobe attention centers.
= The role of serotonergic and GABAergic genes in the reward cascade.
= The role of enkephalinergic genes as they relate to dopamine release.
[0075] As stated above, attention deficit/hyperactivity disorder (ADHD) is a
highly heritable
childhood behavioral disorder affecting 5% of children and 2.5% of adults.
Common genetic
variants contribute substantially to ADHD susceptibility, but no variants have
been robustly
associated with ADHD. However a 2019 genome-wide association meta-analysis of
20,183
individuals diagnosed with ADHD and 35,191 controls that identifies variants
surpassing
genome-wide significance in 12 independent loci, finding important new
information about the
underlying biology of ADHD. Associations are enriched in evolutionarily
constrained genomic
regions and loss-of-function intolerant genes and around brain-expressed
regulatory marks.
Analyses of three replication studies: a cohort of individuals diagnosed with
ADHD, a self-
31
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
reported ADHD sample and a meta-analysis of quantitative measures of ADHD
symptoms in
the population, support these findings while highlighting study-specific
differences on genetic
overlap with educational attainment. Strong concordance with GWAS of
quantitative
population measures of ADHD symptoms supports that clinical diagnosis of ADHD
is an
extreme expression of continuous heritable traits.
[0076] This analysis reveals the following list: ADRA2A, COMT, DRD1, DRD4,
HTR1B,
LPHN3, MAOA, NOS1, SLC6A2/NET1, SLC6A3/DAT1, SLC6A4/5HTT, SNAP25, and
TPH2. This is not an exhaustive lists because there could be even more gene
polymorphisms
that could contribute to the overall phenotype ADD/ADHD types.
Treatments for ADHD
[0077] The website for the American Academy of Child and Adolescent Psychiatry
(AACP)
states, "The goal of any type of ADHD treatment is to reduce symptoms and help
the child
function at a normal level. Treatment may include medication, therapy, family
support,
educational support, or a combination of these" [AA CP ADHD Guide].
[0078] Symptoms of ADHD often are treated with drugs, an approach that
conforms to
mainstream medical and regulatory guidelines. Common conventional therapies
are targeted at
suppressing symptoms by inhibiting, blocking, or (conversely) amplifying
production,
reception and/or disposal of various neurotransmitters (e.g., serotonin with
selective serotonin
reuptake inhibitors). These therapies carry some associated undesirable risks.
When
pharmacological agents are administered to children, reactions often are
polarized. Some critics
object to the prospect of millions of children who are prescribed controlled
substances that are
potentially addictive and injurious to the brain. Others support the
opportunity given to people
diagnosed with ADHD (including adults) for receiving the clinical attention
they deserve,
including effective treatment, despite side effects. Whatever treatment option
is chosen, in
order to provide an effective outcome for individuals with ADHD, it is
important to recognize
32
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
the following: First, individuals may be born with a predisposition to
behavioral symptoms
associated with ADHD and other RDS disorders. Second, these various RDS
disorders involve
complex interactions of neurotransmitters. Third, ADHD may be the precursor
for multiple
addictions including alcohol, drugs, food, sex, and even gambling. And fourth,
there is an
association between a severe form of alcoholism and defects in the D2 gene in
the reward area
of the brain and other dopaminergic genes (i.e., the dopamine transporter gene
and the
dopamine beta-hydroxylase gene) [Blum 11996; Pohjalainen 1998; Bowirrat 2005].
While the
genetics are far more complex than these genes, carriers of dopaminergic gene
variants, or
genetic deficits including these or other gene subsets, can develop behavioral
manifestations
of RD S.
Pharmacological treatments
Stimulants
[0079] Pharmacological treatment with psychostimulants is the most widely
studied treatment
for ADHD. Stimulant treatment has been used for childhood behavioral disorders
since 1933.
While stimulant treatments are highly effective for 75%-90% of children with
ADHD, at least
four separate psychostimulant medications consistently reduce the core
features of ADHD in
literally hundreds of randomized controlled trials: methylphenidate,
dextroamphetamine,
pemoline, and a mixture of amphetamine salts.
[0080] These medications are metabolized, leave the body fairly quickly, and
work for up to
four hours. (Widely prescribed drugs, Concerta and Adderall, are believed to
last 6-12 hours.)
These medications have their greatest effects on symptoms of hyperactivity,
impulsivity, and
inattention, and the associated features of defiance, aggression, and
oppositionality. They also
improve classroom performance and behavior, promoting increased interaction
with teachers,
parents and peers.
33
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0081] Many double blind studies over the past 40 years have uniformly agreed
that stimulants
such as methylphenidate, dextro-amphetamine, as well as other substances, are
very effective
in the treatment of 70%-80% of children and adults with ADHD. One of the myths
of ADHD
is that ADHD children show a paradoxical effect of being calmed by stimulants,
while
"normal" individuals are stimulated by them. However, studies have shown that
the activity
levels are decreased and attention levels are increased by stimulants in
individuals with and
without ADHD. The difference is that since the levels of hyperactivity and
inattention are much
higher in ADHD subjects, the improvement is relatively much greater, giving
the impression
that they respond, while non-ADHD subjects do not.
[0082] It is known that like the effect of serotonin re-uptake inhibitors on
the serotonin
transporters, stimulants inhibit both dopamine transporters and norepinephrine
transporters.
Since hyperactivity is related to excessive dopamine activity in the basal
ganglia, on the surface
this would seem to make things worse instead of better. However, FIGS. 2A-2D
show how the
stimulants work in ADHD. This results in a decrease in dopaminergic
stimulation in the basal
ganglia where the density of the D2 receptors is the highest. Of particular
interest, there are few
D2 receptors in the prefrontal lobe. Thus, dopamine activity in the prefrontal
lobes is increased
instead of decreased. This is consistent with a model of ADHD in which there
is too little
dopamine in the frontal lobes, resulting in symptoms of prefrontal lobe
deficits and too much
dopamine in the basal ganglia, such as motor hyperactivity and not
infrequently, motor tics.
The stimulants correct both the prefrontal lobe deficiency of dopamine and the
basal ganglion
excess of dopamine.
[0083] Despite this indication of how uniquely suited stimulant medications
are to the
treatment of ADHD, they can have undesirable side effects such as insomnia,
decreased
appetite, stomachaches, headaches, and jitteriness. Some children may develop
tics. Other side
effects include rebound hyper-activity and psychosis. Pemoline has been
associated with
34
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
hepatotoxicity, so monitoring of liver function is necessary. Additionally,
many still worry that
ADHD children are receiving a form of "speed." Studies have shown that in
order to obtain a
"high," stimulants need to reach the brain very quickly. This requires
intravenous or nasal
administration, or the use of doses that exceed therapeutic recommendations.
At therapeutic
oral doses, the stimulants used for treatment of ADHD do not cause a euphoric
high. Perhaps
the best indicator of this is that one of the hardest parts of the treatment
for ADHD children is
to get them to take their medication. This, however, is no guarantee that
these drugs are never
abused. It is important that children and adolescents with ADHD not have free
access to their
medications, since it is clear that these drugs can be abused when given
nasally, or
intravenously, or in high doses. Keeping track of the medications helps to
ensure that they are
not sold for illicit use.
[0084] In addition to the use of stimulant medications, a second class of
medications that works
primarily on norepinephrine pathways (e.g., clonidine, guanifacine, and
atomoxetine, can also
be quite effective [Perwien 2006; Spencer 2001; Spencer 2006]. Clonidine and
guanifacine are
especially useful in treating individuals with both ADHD and chronic tics
(Tourette syndrome)
since clonidine and guanifacine uniquely treat both ADHD and Tourette
syndrome. Physicians
are often reluctant to treat individuals with both ADHD and Tourette syndrome
with stimulants,
for fear of exacerbating the tics. However, consistent with the above
mechanism of action of
stimulants, significant exacerbation is unusual, and often the tics are
unchanged or improve
following stimulant treatment [Gadow 1992].
[0085] As discussed and described above, it is often the comorbid disorders
such as ODD and
CD that cause the greatest distress to parents of children with ADHD. Though
experience of
the inventor, the atypical neuroleptics such as risperidone, olanzipine, and
molindone, can be
very effective in the treatment of these comorbid conditions.
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
Other medications
[0086] For children with ADHD who do not respond to stimulants (10%-30%) or
cannot
tolerate the side effects, other alternatives may be available. However, other
competitive
solutions also have been tried with mixed results. The anti-depressant
bupropion has been
found to be superior to placebo, although the response is not as strong as
stimulants. Well-
controlled trials have shown tricyclic antidepressants to be superior to
placebo but less effective
than stimulants. Reports of sudden death of a few children in the early 1990s
on the tricyclic
compound desipramine led to great caution with the use of tricyclics in
children.
[0087] Clonidine can be an effective mode of treatment of ADHD. Since it also
treats motor
and vocal tics, it is especially useful in the treatment of Tourette-syndrome
children who also
have ADHD. Neuroleptics have been found to be occasionally effective, yet the
risk of
movement disorders, such as tardive dyskinesia, makes their use problematic.
Lithium,
fenfluramine, or benzodiazepines have not been found to be effective
treatments for ADHD,
nor have serotonin re-uptake inhibitors such as fluoxetine.
[0088] Another drug being tested is lisdexamfetamine dimesylate (LDX), a
therapeutically
inactive prodrug in which d-amphetamine is covalently bound to 1-lysine, a
naturally occurring
amino acid. Pharmacologically active d-amphetamine is released from LDX
following oral
ingestion. A phase 2, randomized, double-blind, placebo- and active-controlled
crossover study
compared the efficacy and safety of LDX (30, 50, or 70 mg) with placebo, with
mixed
amphetamine salts (extended-release 10, 20, or 30 mg) included as a reference
arm of the study,
in 52 ADHD children aged 6-12 years in an analog classroom setting [Biederman
2007] . The
primary efficacy measure was the Swanson, Kotkin, Agler, M-Flynn, and Pelham
(SKAMP)
Rating Scale. Secondary efficacy measures included the Permanent Product
Measure of
Performance (PERMP) Derived Measures, and the Clinical Global Impression (CGI)
Scale.
Results showed that LDX treatment significantly improved scores on SKAMP-
deportment,
36
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
SKAMP-attention, PERMP-attempted, PERMP-correct, and CGI-improvement from
baseline.
Adverse events were similar for both active treatments. In a laboratory
classroom environment,
LDX significantly improved ADHD symptoms versus placebo in school-age children
with
ADHD.
Over prescription of stimulants
[0089] Concerns have been raised that children, particularly active boys, are
being
overdiagnosed with ADHD and thus are receiving psychostimulants unnecessarily.
While
recent reports suggest that overprescription and overdiagnosis are unfounded,
a more important
issue is that fewer children (2%-3% of school-aged children) are being treated
for ADHD than
suffer from it [Faraone 2003]. Treatment rates are lower for selected groups
such as girls,
minorities, and children receiving care through public service systems.
However, there have
been major increases in the number of stimulant prescriptions since 1989, and
methylphenidate
is being manufactured at 2.5 times the rate of a decade ago [Comings 2005].
Nonetheless, some
of the increase in use may reflect inappropriate diagnosis and treatment. In
one study, the rate
of stimulant use was twice the rate of parent-reported ADHD, based on
standardized
psychiatric interview [Comings 2005].
[0090] Moreover, in 2005, 4.4% of children (ages 0-19) and 0.8% of adults
(ages 20 and older)
used ADHD medications. During the period between 2000 and 2005, treatment
prevalence
increased rapidly (11.8% per year). In addition, global use of ADHD
medications rose threefold
from 1993 to 2003, whereas global spending (US$2.4 billion in 2003) rose 9-
fold, adjusting
for inflation.
[0091] While a number of stimulant drugs are utilized to treat ADHD symptoms,
a promising
alternative approach involves a natural polypharmacy directed at correction
and control of
neurochemistry and dopamine D2receptor proliferation, while minimizing side
effects [Blum
2006]. It also involves a noninvasive DNA based diagnostic test for the
determination of
37
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
predisposing sets of polymorphic genes and their interaction (known as
epistasis). However,
this treatment approach also can be accomplished in combination with known FDA-
approved
stimulants.
The polypharmacy and multigenetic approach
[0092] The polygenic inheritance of ADHD and its comorbid disorders makes the
need for
more than one medication (polypharmacy) easy to understand as an optimal
treatment of
complex cases. Thus, the involvement of variant dopamine genes resulting in
ADHD and tics
may require dopaminergic agonists (methylphenidate or dexedrine) or
antagonists (haloperidol,
pimozide, risperidone, etc.). The involvement of variant norepinephrine or
epinephrine genes
resulting in ADHD and behavioral dysregulation, may require a2-adrenergic
agonists (e.g.,
clonidine, guanifacine, venlafaxine, and atomoxetine). The involvement of
variant serotonergic
genes resulting in depression and anxiety disorders may require selective
serotonin re-uptake
inhibitors (e.g., fluoxetine, sertraline, paroxetine, and fluvoxamine). The
involvement of other
variant genes resulting in ODD, CD, and other behaviors, may require
medications such as
valproic acid, molindone, and risperidone [Biederman 11 2007].
[0093] Parents often raise legitimate concerns when their children are placed
on any
medication, let alone two or more. Explaining ADHD in terms of a complex set
of different
genes affecting different neurotransmitters often helps to moderate these
concerns. To this
effect, the utilization of certain specific ingredients, which modify the
brain reward cascade by
targeting serotonergic, opioidergic, GABAergic, catecholaminergic, and
acetylcholinergic
pathways, can alter behaviors known to be associated with ADHD. Such a
polypharmacy
approach may include the utilization of a nutraceutical (nutrigenomic)
approach targeted at
enhancing slow dopamine release in the nucleus accumbens. One available
nutraceutical
combines the following: select amino acids (5-hydroxytyptophan, dl-
Phenylalanine, 1-tyrosine,
1-glutamine); herbals (Rhodiola rosea, ferulic acid, ginkgo-biloba, ginseng,
gotu kola,
38
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
huperzine A); trace metals (chromium and zinc); macro minerals (calcium,
magnesium,
manganese); vitamins (ascorbic acid, d-alpha tocopheryl, niacin, pyridoxal-
phosphate, B12);
and co-factors (biotin, folic acid, dimethylethanoiamine).
[0094] In an early study of healthy volunteers, a combination of amino acids
and herbals
showed positive results [Defrance 1977]. The researchers observed a
significant amplitude
enhancement of the P300 component of the cognitive event-related brain
potentials, as well as
improvement in cognitive processing speeds, after the subjects were given the
amino acid
formula. These improvements in normal volunteers are consistent with the
observed facilitation
of recovery of individuals with RDS, including substance abuse and ADHD, as
well as with
dopaminergic involvement in short term memory [Kimberg 1997].
Combination therapy: a long-term approach
[0095] The short-term safety and tolerability of psychostimulants has been
reasonably well
studied, and the risks associated with these compounds in the short term are
generally
acceptable. However, the amount of long-term effectiveness and safety data
related to
psychostimulant therapy is relatively small. Data that do exist suggest that
long-term treatment
with psychostimulants in appropriately diagnosed patients may be associated
with salutary
effects as well as relatively modest risks.
[0096] ADHD has an early onset and requires an extended course of treatment.
Research is
needed to examine the long-term safety of treatment and to investigate whether
other forms of
treatment could be combined with psychostimulants to lower their doses as well
as to reduce
other problem behaviors found with ADHD. One important treatment goal is to
develop a side-
effect free natural product to augment psychostimulants with the ultimate goal
of reducing the
need for psychostimulants. Core to this therapeutic strategy would be to
develop a product with
mechanisms of action that would both increase the release of dopamine, and
induce long term
39
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
D2 receptor proliferation. Such a novel combination therapy would mimic
stimulants like
methylphenidate, and thus an additive and/or synergistic action should be
expected.
[0097] In fact, combined therapies might be used to improve overall
functioning by targeting
symptoms of disorders that often accompany ADHD, such as CD, SUD, and learning
disabilities. Moreover, because stimulants also can be abused, and because
children with
ADHD are at increased risk for substance-seeking behavior, concerns have been
raised about
the potential for abuse of stimulants by children taking medication or
migrating to other drugs
of abuse. In this regard, critics argue that many children who do not have
true ADHD are
medicated as a way to control non-ADHD disruptive behaviors. However,
ironically,
organizations like CHADD recommend the use of stimulants for school-aged
children,
comparing the pills to eyeglasses, braces, and allergy medications [CHADD
2007].
[0098] In this regard, the use of methylphenidate and amphetamine, which are
the mainstay
for the treatment of ADHD, has raised concerns because of their reinforcing
effects. That is,
the chronic use of these medicines during childhood or adolescence might
induce changes in
the brain that could facilitate drug abuse in adulthood. This concern was
recently addressed by
Thanos and colleagues [Thanos 2007]. They measured the effects of chronic
treatment (8
months) with oral methylphenidate (1 or 2 mg/kg), which was initiated in
periadolescent rats
(postnatal day 30). Following this treatment, the rats were tested on cocaine
self-
administration. In addition, at 2 and 8 months of treatment, the investigators
measured
dopamine D2receptor (D2R) availability in the striatum using [(11)C]raclopride
microPET
(muPET) imaging.
[0099] Animals treated for 8 months with 2 mg/kg of methylphenidate showed
significantly
reduced rates of cocaine self-administration at adulthood compared to vehicle-
treated rats. D2R
availability in the striatum was significantly lower in rats after 2 months of
treatment with
methylphenidate (1 and 2 mg/kg) but significantly higher after 8 months of
methylphenidate
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
treatment than in the vehicle-treated rats. In vehicle-treated rats, D2R
availability decreased
with age, whereas it increased in rats treated with methylphenidate. Because
low D2R levels
in the striatum are associated with a propensity for self-administration of
drugs both in
laboratory animals and in humans, this effect could underlie the lower rates
of cocaine self-
administration observed in the rats given 8 months of treatment with
methylphenidate. Eight-
month treatment with oral methylphenidate beginning in adolescence decreased
cocaine self-
administration (1 mg/kg) during adulthood which could reflect the increases in
D2R
availability observed at this life stage since D2R increases are associated
with reduced
propensity for cocaine self-administration.
[0100] In contrast, 2-month treatment with methylphenidate started also at
adolescence
decreased D2R availability, which could raise concern that at this life stage,
short treatments
could possibly increase vulnerability to drug abuse during adulthood. These
findings indicate
that methylphenidate effects on D2R expression in the striatum are sensitive
not only to length
of treatment but also to the developmental stage at which treatment is given.
The authors
suggested that future studies evaluating the effects of different lengths of
treatment on drug
self-administration are required to assess optimal duration of treatment
regimes to minimize
adverse effects on the propensity for drug self-administration in humans.
[0101] Little is known about the risks and characteristics of ADHD patients
who misuse or
divert their stimulant medications. As part of a 10-year longitudinal study of
youths with
ADHD, Wilens et at. [Wilens 2006] evaluated medication diversion or misuse in
a young
ADHD population. The investigators used structured psychiatric interviews for
diagnosis, and
a self-report questionnaire regarding medication use in medicated subjects
with ADHD
compared with controls without ADHD receiving psychotropic medications for non-
ADHD
treatment. Of 98 subjects receiving psychotropic medications (mean age of 20.8
5 years), 55
(56%) were ADHD subjects and 43 (44%) were controls receiving medications for
other
41
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
purposes. The authors found that 11% of the ADHD group reported selling their
medications
compared with no subjects in the control group. An additional 22% of the ADHD
group
reported misusing their medications compared with 5% of the control subjects,
and that those
with CD or SUP accounted for the misuse and diversion. A minority of subjects
reported
escalating their doses and concomitant use with alcohol and drugs.
Interestingly, the data
indicated that the majority of ADHD individuals, particularly those without CD
or SUP, used
their medications appropriately. The authors' findings also highlighted the
need to monitor
medication use in ADHD individuals with CD or SUP and to carefully select
agents with a low
likelihood of diversion or misuse in this group. Based on this report,
therefore, it may be helpful
for individuals to be tested for candidate genes to determine a predisposition
of substance
seeking-behavior.
[0102] In terms of methamphetamine utilization, there are concerns related to
its genotoxic
effects. A study was conducted to investigate the index of cerebral and
peripheral DNA damage
in young and adult rats after acute and chronic methylphenidate exposure. The
researchers used
single cell gel electrophoresis (Comet assay) to measure early DNA damage in
hippocampus,
striatum, and total blood, as well as a micronucleus test in total blood
samples. Their results
showed that methylphenidate increased the peripheral index of early DNA damage
in young
and adult rats, which was more pronounced with chronic treatment and in the
striatum
compared to the hippocampus. Neither acute nor chronic methylphenidate
treatment increased
micronucleus frequency in young or in adult rats. Peripheral DNA damage was
positively
correlated with striatal DNA damage. These results suggest that
methylphenidate may induce
central and peripheral early DNA damage, but this early damage may be repaired
[Andreazza
2007].
42
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
Alternative treatments
[0103] Because of the concern about the use of medications, many parents seek
alternative
methods of treatment of ADHD. Most clinicians agree that a combination of
medication and
behavioral modification is the most effective approach to the treatment of
ADHD, even though
the medications appear to contribute greater benefits. Children with ADHD may
also respond
well to adjustments in their education setting, e.g., taking advantage of an
individualized
educational plan. The following are some additional alternatives that are most
often used.
EEG biofeedback
[0104] Electroencephalographic (EEG) biofeedback usually utilizes the feedback
from a game
played on a TV screen to attempt to train the brain to alter the levels of
alpha, beta and delta
waves. This tactic has the advantage that no drugs are used and appears to be
effective in some
cases. The disadvantage is that it can be expensive. Satisfactory double blind
testing and
evaluation of its effectiveness has been very difficult, and the effects may
not be long lasting.
Herbal remedies
[0105] Numerous herbal remedies have been used by ADHD patients. Sometimes
they seem
to be effective, sometimes not, or their effectiveness may be short-lived.
Many parents turn to
them because they are perceived as "natural." However, to be effective they
must contain an
active ingredient for which the identity is usually not known. In addition, a
wide range of other
ingredients may be present that are not necessary or may cause unknown, or
worse yet,
undesirable side effects. As physicians and pharmacologists, we suggest that
using pure
medications with known doses, known mechanisms of action and known side
effects is always
preferable.
Nutraceuticals
[0106] In contrast to herbal remedies, the composition of other nutraceuticals
is more precisely
known. They usually consist of amino acids, vitamins, minerals, and other
known compounds.
43
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
Because they are closer to food substances than drugs, they do not have the
same rigorous
restrictions by the Federal Drug Administration that drugs do and can be
purchased over the
counter. Because a number of amino acids have direct or indirect effects on
the levels of
specific neurotransmitters, they have the potential of helping to control some
of the symptoms
of ADHD. Nutraceuticals have the advantage that double-blind studies [Blum I
1988] can be
easily carried out. It is not unlikely that some combinations of the above
compounds, carefully
tested in double-blind studies, may play a supporting role in controlling some
of the symptoms
of ADHD [Blum 11988; Blum 11 1988; Blum 2000; Blum 2006; Blum 1991; Chen
2004].
Diets and vitamin supplements
[0107] Still further prior studies compared attentional abilities of two
groups of children with
ADHD, one group after treatment with Ritalin, and the other after treatment
with dietary
supplements (a mix of vitamins, minerals, phytonutrients, amino acids,
essential fatty acids,
phospholipids, and probiotics). Both groups showed significant improvement.
These findings
support the effectiveness of food supplement treatment in improving attention
and self-control
in children with ADHD and suggest food supplement treatment of ADHD may be of
equal
efficacy to Ritalin treatment.
Dopaminergic and serotonergic releaser combination therapy
[0108] Another treatment for substance-seeking behaviors consists of agonist
therapy (not
antagonist therapy). This strategy involves administration of stimulant-like
medications (e. g. ,
monoamine releasers) to alleviate withdrawal symptoms and prevent relapse. A
major
limitation of this approach is that many candidate medicines possess
significant abuse potential
because of activation of mesolimbic dopamine neurons in central nervous system
reward
circuits. Previous data suggest that serotonin neurons can provide regulatory
influence over
mesolimbic dopamine neurons. Thus, it might be predicted that the balance
between dopamine
44
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
and serotonin transmission is important to consider when developing
medications with reduced
stimulant side effects.
[0109] Several issues have been disclosed and discussed herein related to the
putative
mechanisms related to ADHD behaviors. The potential development of dual
dopamine/serotonin releasers for the treatment of substance use disorders has
otherwise been
discussed [Rothman 2007]. In this regard, there is evidence supporting the
existence of a dual
deficit in dopamine and serotonin function during withdrawal from chronic
cocaine or alcohol
abuse [Rothman 2007].
[0110] Rothman and associates further summarize studies that have tested the
hypothesis that
serotonin neurons can dampen the effects mediated by mesolimbic dopamine. For
example, it
has been shown that pharmacological manipulations that increase extracellular
serotonin
attenuate stimulant effects produced by dopamine release, such as locomotor
stimulation and
self-administration behavior.
[0111] Finally, they discuss their recently published data about PAL-287
(naphthylisopropylamine), a novel non-amphetamine dopamine/serotonin-releasing
agent that
suppresses cocaine self-administration but lacks positive reinforcing
properties [Hiebel 2007].
[0112] Using this concept, the Synaptamine Complex (5G8839)TM was developed
[Chen
2004]. TABLE III provides details about the ingredients of the synaptamine
complex, as well
as proposed brain targets and behavioral changes.
Table III
Synaptamine complex review
Supplemental Restored Addictive Amino acid Expected
ingredient brain substance deficiency behavior
chemical abuse symptoms change
D-Phenyl al anine or Enkephalins Heroin, Most reward
Reward
DL-Phenylalanine Endorphins alcohol, deficiency stimulation.
marijuana, syndrome (RD S) Anti-craving.
sweets, conditions sensitive Mild
CA 03137128 2021-10-18
WO 2020/215026
PCT/US2020/028870
Supplemental Restored Addictive Amino acid Expected
ingredient brain substance deficiency behavior
chemical abuse symptoms change
starches, to physical or antidepression.
chocolate, emotional pain. Mild improved
tobacco Crave comfort and energy and
pleasure. Desire focus. D-
certain food or Phenylalanine
drugs. D- promotes pain
phenylalanine is a relief, increases
known pleasure.
enkephalinase
inhibitor.
L-Phenylalanine or Norepinephrin Caffeine, Most RD S Reward
L-Tyrosine e Dopamine speed, conditions. stimulation.
cocaine, Depression, low Anti-craving.
marijuana, energy. Lack of Anti-depression.
aspartame, focus and Increased
chocolate, concentration. energy.
alcohol, Attention-deficit Improved
tobacco, disorder. mental focus.
sweets,
starches
L-Tryptophan or 5 Serotonin Sweets, Low self-esteem. Anti-craving.
hydroxytryptophan alcohol, Obsessive/compulsi Anti-depression.
(5HTP) starches, ye behaviors. Anti-insomnia.
ecstasy, Irritability or rage. Improved
marijuana, Sleep problems. appetite control.
chocolate, Afternoon or Improvement in
tobacco evening cravings, all mood and
Negativity. Heat other serotonin
intolerance. deficiency
Fibromyalgia, SAD symptoms.
(winter blues).
GABA (Gamma- GABA Valium, Feeling of being Promotes
amino butyric alcohol, stressed-out. calmness.
acid) marijuana, Nervous. Tense Promotes
tobacco, muscles. Trouble relaxation.
sweets, relaxing.
starches
L-Glutamine GABA (mild Sweets, Stress. Mood Anti-craving,
enhancement) starches, swings. anti-stress.
Fuel source alcohol Hypoglycemia. Levels blood
for entire sugar and mood.
brain GABA (mild
enhancement).
Fuel source for
entire brain.
46
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0113] To assist in amino-acid nutritional therapy, the use of a multi-
vitamin/mineral formula
is recommended. Many vitamins and minerals serve as co-factors in
neurotransmitter synthesis.
They also serve to restore general balance, vitality and well-being to the
reward deficiency
syndrome (RDS) patient who typically is in a state of poor nutritional health.
The utilization of
GABA is limited due to its polar nature and ability to cross the blood brain
barrier and
glutamate is used in a low level only to prevent over-inhibition of enkephalin
breakdown and
subsequent inhibition of gabaergic spiny neurons of the sub stania nigra.
[0114] As early as 2008 XV World Congress of Psychiatric genetics held in New
York City, a
number of new gene loci presented at the congress included: Nosl exon lf-VNTF;
NTF3;
CNTFR; NTRK2; rs2242447 (noradrenergic transporter gene); HTR1B; beta-tubulin
111;
MAP2; ADRA2A; and linkage to chromosome 3, 9, and 16 among others. Many of
these risk
alleles have been incorporated in the panels of the present invention.
Novel Nutrigenomic Investigations
[0115] Attention Deficit-Hyperactivity Disorder (ADHD) is a serious
neuropsychiatric
condition that affects approximately 8.7% of the adolescent population [Visser
2014] and 4.4%
of the adult population [Kessler 2006] in the United States. The world-wide
prevalence of
ADHD is estimated at 5.29% [Polanczyk 2007]. The disorder is characterized by
impairments
in attention, self-regulation (hyperactivity-impulsivity) and executive
function [Barkley 1997],
as well as problems with working memory (WM) [Barkley 1997; Alderson 2013;
Lenartowicz
2014]. These impairments cause significant underachievement in academic,
occupational and
interpersonal areas of life [Weiss 1993].
[0116] Prior research has focused on the contribution of neuroanatomic,
neurotransmitter and
genetic mechanisms to the pathophysiology of ADHD. Neuroimaging research
reveals that
ADHD is associated with dysfunction in prefrontal, cingulate and striatal
brain regions [Bush
1999; Bush 2005].
47
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0117] Bledsoe et at. [Bledsoe 2013] using MRI, reported that ADHD children
had reduced
right, rostral anterior cingulate cortical thickness, which correlated with
parental-teacher
reports of the severity of their ADHD symptoms. Thus, children with thinner
cortical tissue
were rated as having more severe ADHD symptoms. These findings are consistent
with Makris
et at. [Makris 2007], who used structural MRI and found cortical thinning in
the attention and
executive function network of ADHD adults. They noted reduced cortical
thickness in the right
dorsolateral prefrontal, anterior cingulate and inferior parietal areas.
[0118] Dopamine (DA) neurons project from the substantia nigra to the basal
ganglia, and
support motor function, and they also project from the ventral mesencephalon
to the forebrain,
and play a vital role in motivation, reward, learning and WM [Girault 2004].
Synaptic levels
of dopamine are influenced by the dopamine transporter (DAT), a protein that
removes
dopamine from the synapse and absorbs it into the presynaptic neuron. DAT
density was 70%
greater in adults with ADHD, compared to controls [Dougherty 1999], which was
consistent
with lower post synaptic levels of dopamine in ADHD. Using Single Photon
Emission
Computerized Tomography (SPECT), with ([Tc-99m]TRODAT-1), a radio-ligand
specific
for0 the dopamine transporter, researchers demonstrated that treatment with
methylphenidate
reduced DAT receptor binding sites which produced clinical improvement in ADHD
adults
[Dresel 2000].
[0119] These researchers also reported increased striatal DAT receptor
binding, in adult
ADHD, which was reduced by methylphenidate treatment. [Krause 2000]. Volkow et
at.
[Volkow 2001], working with Positron Emission Tomography (PET) and
[11C]raclopride, a
D2 receptor radio-ligand, and normal participants, demonstrated that oral
methylphenidate
increased extracellular dopamine in the brain. This is significant in light of
the ability of
methylphenidate to block the dopamine transporter and amplify the effect of
dopamine in
supporting attention [Volkow 2001]. Notably, Badgaiyan et at. [Badgalyan 2015]
in a PET
48
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
study reported that ADHD adults have reduced tonic (resting) release and
increased phasic,
task-related release of dopamine in the right caudate nucleus. The increase in
phasic DA release
may compensate for the reduced tonic baseline, as may be needed, in ADHD.
These studies
collectively support the role of dopamine dysregulation in the pathophysiology
of ADHD.
[0120] Dopamine plays a central role in cognitive functions [Nieoullon 2002]
and WM
[Takahashi 2012] and hippocampal D2 receptor availability correlates
positively with memory,
executive function and verbal fluency [Takahashi 2007]. Aalto et at. [Aalto
2005] used
[11C]FLB457, a high affinity dopamine2 receptor ligand, in a PET study of
vigilance and WM
with normal participants. They found that their visual, WM task increased D2
receptor binding
in the bilateral, ventrolateral frontal cortex as well as in the left medial
temporal cortex,
including the amygdala and hippocampus. This result is consistent with
Kemppainen et at.
[Kemppainen 2003] who reported reduced hippocampal D2 receptor activity in
Alzheimer's
Disease patients, which correlated with the patients' reduced memory and
naming
performance. The consistency in these two studies lies in the relationship
between D2 receptor
activation and WM performance. Seamans et at. [Seamans 2004] discuss the
complex role of
dopamine as a neuromodulator of prefrontal, cognitive function and suggest
that dopamine
modulates the breadth of information stored in prefrontal, WM networks.
[0121] Blum et at. [Blum I 1996] proposed that ADHD and other impulsive and
compulsive
disorders, including substance use disorder (SUP), may be subsumed under
Reward
Deficiency Syndrome (RDS). RDS disorders have a common proposed etiology in
reduced
sensitivity of the brain's reward circuitry to pleasurable environmental
stimulation. Blum et at.
[Blum I 1996] attributed RDS to a variant in a gene (Al allele) that codes the
DA D2 receptor.
Individuals with the Al allele have a decreased density of D2 receptors and a
relative inability
to experience pleasure associated with ordinary stimulation and activities.
The relationship
between Al allele of the D2 receptor, RDS and ADHD is summarized in Blum I
1996.
49
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
Individuals with two copies of the Al allele are at greater risk for
alcoholism, SUP and ADHD
compared to those with one or no Al alleles. The occurrence of the Al allele
of the D2 receptor
gene correctly classified 77% of alcoholics, while the absence of this allele
accurately classified
72% of non-alcoholic research participants [Blum III 1990]. Comings, et at.
[Comings 1991]
found that the Al, D2 receptor allele was significantly more prevalent in ADHD
(46.2%) as
compared to controls (24.5%). This allele was also more prevalent in patients
with alcoholism,
Tourette' s Syndrome and autism. ADHD is clearly a polygenic disorder and has
a heritability
of .75 [Takahashi 2005]. Although no single gene has a large, deterministic
role, genes
affecting dopamine activity make an important contribution to the expression
of ADHD.
[0122] Blum et at. [Blum I 2016; Blum 11 2016] have summarized Blum' s work,
over the last
fifty years, in developing a pro-dopamine, nutrigenomic complex (KB220Z), to
stabilize the
activity of dopamine in RDS. This compound, which includes dopamine precursor
amino acids
and natural ingredients, was designed to correct the dysregulation of dopamine
in the brain's
mesolimbic reward system. The goal for this compound is dopamine homeostasis,
relieving the
cravings associated with addiction and the drive to action associated with
impulsive disorders,
including ADHD, that are subsumed under RDS. Previously, DeFrance et at.
[DeFrance.
1977], using normal participants, had demonstrated that an amino acid mixture
increased the
amplitude of the P300 evoked potential and decreased processing time in
spatial orienting and
continuous performance tests. The improvement in function in this early study
is similar to that
which would be expected from KB220Z with RDS disorders.
[0123] Consumption of KB220Z is expected to improve cognitive functions that
utilize the D2
receptor. McLaughlin et at. [McLaughlin 2017] reported substantial
improvements in semantic
verbal fluency in an elderly male with mild memory impairment, following
consumption of
KB220Z. The participant demonstrated an average, baseline Animal Naming score
of 14,
placing him in the 30th percentile, for his age and gender, for semantic
verbal fluency.
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
Following a single, acute dose of KB220Z the patient's verbal fluency score
increased to 19
animal names, placing him in the 76%. Following discontinuance of KB220Z, the
patient's
verbal memory performance decreased to 13 animal names. Notably, with
resumption of
KB220Z, the patient's verbal fluency score improved to 24 animal names,
placing his verbal
semantic memory performance in the 98th percentile. These clinical results
suggest that
activation of the participant's D2 receptors was associated with a dramatic
improvement in his
semantic verbal fluency.
[0124] Steinberg et at. [Steinberg 2016] used quantitative EEG analysis (QEEG)
and Low-
resolution Electromagnetic Tomography (LORETA) to measure the effect of KB220Z
on WM
and brain electrical activity in an elderly adult with ADHD. The subject had
long-standing
issues with attention, organization, difficulties with sustained mental effort
and procrastination.
He was tested during baseline and following consumption of a daily dose (1
ounce) of KB220Z.
The tasks included a resting EC condition as well as a WM task. The WM task
required the
participant to memorize and repeat random sequences of letters and numbers, in
ascending
order (numbers) and alphabetical order (letters). QEEG for the EC, resting
condition, revealed
that KB220Z produced an increase in absolute power in theta (4-8 Hz), alpha (8-
12 Hz) and
beta (12-25 Hz) frequency bands, in frontal (Fz), central (Cz) and parietal
(Pz), midline
locations. Right hemisphere EEG activity also increased in these bands in
frontal (F4) and
parietal (P4) locations.
[0125] LORETA was also used to study the sources of the EEG signals. LORETA
produces
measures of current source density, which are estimates of current flow
originating from the
Brodmann areas of interest. Data are expressed in standard deviation (z score
units) that
represent current flow for the participant compared to an age and gender
matched, normative
EEG database. During the WM task we observed that KB220Z increased the z
scores for theta
(4-7 Hz), low alpha (8-10 Hz) and high alpha (11-13 Hz) current source density
in the anterior
51
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
cingulate, dorsal cingulate and posterior cingulate cortices (Brodmann areas
32, 24 and 31,
respectively). Thus, pro-dopamine regulation increased EEG activity in areas
of the brain
known to support attention and WM. With KB220Z, the participant demonstrated
an
improvement in WM, from 13 to 14 correct letter-number sequences. This
improvement in
WM is consistent with the KB220Z's effect in activating DA and EEG activity,
in the attention
and WM areas of the brain. It has been confirmed by the present inventor that
the participant
in Steinberg 2016 had the Al allele of the D2 receptor gene.
[0126] In McLaughlin 2017 and Steinberg 2016, the participants were tested
under baseline
(no active agent, no placebo) and treatment (KB220Z) conditions, and the
participants were
aware of which condition was being used on each trial, raising the possibility
of expectancy
effects. The purpose is to replicate these findings using a double-blind,
placebo-controlled,
cross-over study, which protects the data from experimenter bias. The use of a
placebo control
allows for assessment of the physiological effects of KB220Z, beyond the
impact of the
participant's expectations [Jensen 2002; Onton 2005]
The development of the genetic addiction risk score (GARS)
[0127] There are many examples of association studies involving genes and
polymorphisms
especially of the ten reward genes measured in the GARS test. Alleles of genes
that affect the
synthesis, degradation, reception, and transport of neurotransmitters (like
enkephalin,
serotonin, GABA, and dopamine) and enzymes like Monoamine Oxidase (MOA) A and
COMT in the reward pathway of the brain were candidates for selection for the
GARS test if
they contributed to hypodopaminergia. Comings and Blum proposed that
functional defects in
the genes for these neurotransmitters result in dopamine deficit, later
identified as RDS. They
suggested that individuals with hypodopaminergia are at risk for seeking
reward from RDS
behaviors to satisfy their lack of natural rewards [Comings I 2000]. Some
examples of
52
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
functional research and studies that associated RDS behaviors with the risk
alleles of the genes
and second messengers that comprise the GARS test follow.
The development of precision addiction management
[0128] Blum et at. proposed that KB220Z; a mild neuro-nutrient formulation,
can stimulate
the D2 receptor [Blum I 2008; Blum II 2008]. Blum's group advocates
instigating dopamine
release, to cause the induction of D2-directed mRNA to direct the
proliferation of D2 receptors
in the brain [Blum 2012]. For example, DNA-directed compensatory
overexpression of the
DRD2 receptors (a form of gene therapy), resulted in a significant reduction
in alcohol craving
behavior in alcohol-preferring rodents [Thanos 2005] and self-administration
of cocaine
[Thanos 2008]. Thus, based on this model enhanced bioavailability of D2
receptors was shown
to reduce craving.
[0129] Studies that showed rats with depleted neostriatal dopamine display
increased
sensitivity to dopamine agonists estimated to be 30-100 x in the 6-
hydroxydopamine (6-
OHDA) rotational model [Mandel 1993] were the basis for "denervation
supersensitivity"
[Blum 2009]. Denervation supersensitivity was identified as a putative
physiological
mechanism to help explain the enhanced sensitivity following intense acute
dopaminergic D2
receptor activation in the face of hypodopaminergia. In contrast, promotion of
long-term
(chronic low vs. intense acute) dopaminergic activation by lower potency
dopaminergic
repletion therapy has been shown in clinical and neuro imaging studies, to be
an effective
modality when used to treat RDS behaviors including Substance Use Disorders
(SUP),
Attention Deficit/Hyperactivity Disorder (ADHD), obesity and others, without
side effects
[Blum 12016; Blum 11 2016].
[0130] An unprecedented number of clinical studies validating this patented
nutrigenomic
technology for re-balancing brain chemistry, and optimizing dopamine
sensitivity and function
have been published. Here clinicians and neuroscientists are encouraged to
continue to embrace
53
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
the concept of "dopamine homeostasis" and search for safe, effective,
validated and authentic
means to achieve a lifetime of recovery, instead of reverting to anti-
dopaminergic agents. Anti-
dopaminergic agents are doomed to fail because chronic use continues and
exacerbates
hypodopaminergia while promoting powerful D2 agonists like bromocriptine and L-
Dopa
compromises needed balance [Blum 2017]. Increased resting state functional
connectivity as
well as an increased neuronal recruitment has been demonstrated acutely on
fMRI in both
animal and humans within 15 (animal) to 60 (human) minutes post administration
of neuro
nutrient therapy. These studies demonstrate neuronal dopamine firing in brain
areas involved
in reward processing and possible induced neuroplasticity and "dopamine
homeostasis" [Blum
2015; Febo 2017]. The comprehensive role of dopamine as the mesolimbic system
neurotransmitter underlying motivational function supports the low potency
dopaminergic
repletion therapy concept; sustainable, mild activation of D2 receptors [Blum
2012].
[0131] Accordingly, a need still remains for behavioral and
electrophysiological diagnostic
tools for ADHD and for the behavioral management thereof.
SUMMARY OF THE INVENTION
[0132] The present invention relate to methods and kits for assessing severity
index for genetic
risks of attention deficit/hyperactivity disorders and behavioral management
thereof.
[0133] In general, in one embodiment, the invention features a method that
includes the step
of obtaining a biological sample from a subject. The method further includes
the step of
performing an allelic analysis on the biological sample to detect the presence
of a plurality of
predetermined alleles in the biological sample. The plurality of predetermined
alleles include
one or more alleles of BAIAP2. The one or more BAIAP2 alleles include one or
more of
polymophisms rs8079626, rs8079781, rs7210438, and rs4969385. The plurality of
predetermined alleles further include one or more alleles of CHRNA4. The one
or more
CHRNA4 alleles include one or more of polymorphisms rs2273505 and rs3787141.
The
54
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
plurality of predetermined alleles further include one or more alleles of
COMT. The one or
more COMT alleles include one or more of polymorphisms rs6269, rs4818, rs4633,
rs933271,
rs1544325, rs740603, rs740601, rs4646316, rs174696, rs165774, rs9332377,
rs165599,
rs2020917, and rs4680. The plurality of predetermined alleles further include
one or more
alleles of DAT1. The one or more DAT1 alleles include one or more of
polymorphisms
rs460700, rs37020, rs13161905, rs27048, rs6347, rs11133767, rs40184,
rs2975292,
rs2652511, VNTR IN 3-UTR/10-repeat allele, and 40bp repeat (exon 15). The
plurality of
predetermined alleles further include one or more alleles of DBH. The one or
more DBH alleles
include polymorphism rs1108580. The plurality of predetermined alleles further
include one
or more alleles of DRD1. The one or more DRD1 alleles include polymorphism
rs4532. The
plurality of predetermined alleles further include one or more alleles of
DRD2. The one or
more DRD2 alleles include polymorphism rs1800497. The plurality of
predetermined alleles
further include one or more alleles of DRD3. The one or more DRD3 alleles
include
polymorphism rs6280. one or more alleles of DRD4. The one or more DRD4 alleles
include
one or more of polymorphisms rs1800955, rs4646984, rs3758653, rs936465, VNTR
in exon
3/7 repeat allele, VNTR in exon 3/5 repeat allele, and 7-11 repeats of 48bp
(intron 3). The
plurality of predetermined alleles further include one or more alleles of
DRD5. The one or
more DRD5 alleles include one or more of polymorphisms VNTR in exon 8/3-repeat
allele and
dinucleotide repeat/ 148-bp allele. The plurality of predetermined alleles
further include one
or more alleles of HTR1B. The one or more HTR1B alleles include polymorphism
rs6296. The
plurality of predetermined alleles further include one or more alleles of
OPRM1. The one or
more OPRM1 alleles include polymorphism rs1799971. The plurality of
predetermined alleles
further include one or more alleles of SNAP25. The one or more SNAP25 alleles
include one
or more of polymorphisms rs66039806, rs362549, rs362987 and rs362998. The
plurality of
predetermined alleles further include one or more alleles of HTTLPR. The one
or more
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
HTTLPR alleles include polymorphism rs25531. The plurality of predetermined
alleles further
include one or more alleles of MAOA. The one or more MAOA alleles include
polymorphism
30 bp repeat (promoter, X chrom only). The plurality of predetermined alleles
further include
one or more alleles of GABRB3. The one or more GABRB3 alleles include
polymorphism
CA-Repeat (171-201 bases, X chrom only).
[0134] Implementations of the invention can include one or more of the
following features:
[0135] The method can further include identifying each of the alleles in the
plurality of
predetermined alleles that was detected to be present in the biological
sample. The method can
further include assigning a count for each of the alleles in the plurality of
pre-determined alleles
that was detected to be present in the biological sample. The count for a
particular allele is the
number of the particular allele detected to be present in the biological
sample. The method can
further include determining a risk score for the subject based upon the count.
The risk score
can be the sum of the counts. The risk score can identify a severity of the
genetic risk for
attention deficit/hyperactivity disorder (ADHD). The
method can further include
administering treatment based upon the severity of the genetic addition risk
identified for the
subject. The treatment can include providing precision addictive/behavioral
management based
upon the severity of the genetic risk for ADHD. The precision
addictive/behavioral
management can include providing one or more neuro nutrient treatments that
are targeted to
the subject based upon the identification of the alleles in the plurality of
predetermined alleles
that was detected to be present in the biological sample.
[0136] The plurality of predetermined alleles can further include at least one
of: (i) the one or
more DRD4 alleles further includes polymorphism rs4646983; (ii) one or more
alleles of
SEMA3A, wherein the one or more SEMA3A alleles includes polymorphism
rs139438618;
and (iii) one or more allelles of Amelo.
56
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0137] The plurality of predetermined alleles can include two or more alleles
of BAIAP2. The
two or more BAIAP2 alleles can include two or more of the polymophisms
rs8079626,
rs8079781, rs7210438, and rs4969385. The plurality of predetermined alleles
can further
include two or more alleles of CHRNA4. The two or more CHRNA4 alleles can
include the
polymorphisms rs2273505 and rs3787141. The plurality of predetermined alleles
can further
include two or more alleles of COMT. The two or more COMT alleles can include
two or more
of the polymorphisms rs6269, rs4818, rs4633, rs933271, rs1544325, rs740603,
rs740601,
rs4646316, rs174696, rs165774, rs9332377, rs165599, rs2020917, and rs4680. The
plurality
of predetermined alleles can further include two or more alleles of DAT1. The
two or more
DAT1 alleles can include two or more of the polymorphisms rs460700, rs37020,
rs13161905,
rs27048, rs6347, rs11133767, rs40184, rs2975292, rs2652511, VNTR IN 3-UTR/10-
repeat
allele, and 40bp repeat (exon 15). The plurality of predetermined alleles can
further include
two or more alleles of DRD4. The two or more DRD4 alleles can include two or
more of the
polymorphisms rs1800955, rs4646984, rs3758653, rs936465, VNTR in exon 3/7
repeat allele,
VNTR in exon 3/5 repeat allele, and 7-11 repeats of 48bp (intron 3). The
plurality of
predetermined alleles can further include two or more alleles of DRD5. The two
or more DRD5
alleles can include the polymorphisms VNTR in exon 8/3-repeat allele and
dinucleotide repeat/
148-bp allele. The plurality of predetermined alleles can further include two
or more alleles of
SNAP25. The two or more SNAP25 alleles can include two or more of the
polymorphisms
rs66039806, rs362549, rs362987 and rs362998.
[0138] The one or more BAIAP2 alleles can include all of the polymophisms
rs8079626,
rs8079781, rs7210438, and rs4969385. The one or more CHRNA4 alleles can
include all of
the polymorphisms rs2273505 and rs3787141. The one or more COMT alleles can
include all
of the polymorphisms rs6269, rs4818, rs4633, rs933271, rs1544325, rs740603,
rs740601,
rs4646316, rs174696, rs165774, rs9332377, rs165599, rs2020917, and rs4680. The
one or
57
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
more DAT1 alleles can include all of the polymorphisms rs460700, rs37020,
rs13161905,
rs27048, rs6347, rs11133767, rs40184, rs2975292, rs2652511, VNTR IN 3-UTR/10-
repeat
allele, and 40bp repeat (exon 15). The one or more DRD4 alleles can all of
include the
polymorphisms rs1800955, rs4646984, rs3758653, rs936465, VNTR in exon 3/7
repeat allele,
VNTR in exon 3/5 repeat allele, and 7-11 repeats of 48bp (intron 3). The one
or more DRD5
alleles can include all of the polymorphisms VNTR in exon 8/3-repeat allele
and dinucleotide
repeat/ 148-bp allele. The one or more SNAP25 alleles can include all of the
polymorphisms
rs66039806, rs362549, rs362987 and rs362998.
[0139] The plurality of predetermined alleles can further include (i) the DRD4
allele of the
polymorphism rs4646983; the SEMA3A allele of polymorphism rs139438618; and
(iii) an
allelle of Amelo.
[0140] In the plurality of predetermined alleles, the one or more BAIAP2
alleles only include
the polymophisms rs8079626, rs8079781, rs7210438, and rs4969385. In the
plurality of
predetermined alleles, the one or more CHRNA4 alleles only include the
polymorphisms
rs2273505 and rs3787141. In the plurality of predetermined alleles, the one or
more COMT
alleles only include the polymorphisms rs6269, rs4818, rs4633, rs933271,
rs1544325,
rs740603, rs740601, rs4646316, rs174696, rs165774, rs9332377, rs165599,
rs2020917, and
rs4680. In the plurality of predetermined alleles, the one or more DAT1
alleles only include
the polymorphisms rs460700, rs37020, rs13161905, rs27048, rs6347, rs11133767,
rs40184,
rs2975292, rs2652511, VNTR IN 3-UTR/10-repeat allele, and 40bp repeat (exon
15). In the
plurality of predetermined alleles, the one or more DBH alleles only include
the polymorphism
rs1108580. In the plurality of predetermined alleles, the one or more a DRD1
alleles only
include the polymorphism rs4532. In the plurality of predetermined alleles,
the one or more
DRD2 alleles only include the polymorphism rs1800497. In the plurality of
predetermined
alleles, the one or more DRD3 alleles only include the polymorphism rs6280. In
the plurality
58
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
of predetermined alleles, the one or more DRD4 alleles only include the
polymorphisms
rs1800955, rs4646984, rs3758653, rs936465, VNTR in exon 3/7 repeat allele,
VNTR in exon
3/5 repeat allele, and 7-11 repeats of 48bp (intron 3). In the plurality of
predetermined alleles,
the one or more DRD5 alleles only include the polymorphisms VNTR in exon 8/3-
repeat allele
and dinucleotide repeat/ 148-bp allele. In the plurality of predetermined
alleles, the one or
more HTR1B alleles only include the polymorphism rs6296. In the plurality of
predetermined
alleles, the one or more OPRM1 alleles only include the polymorphism
rs1799971. In the
plurality of predetermined alleles, the one or more SNAP25 alleles only
include the
polymorphisms rs66039806, rs362549, rs362987 and rs362998. In the plurality
of
predetermined alleles, the one or more HTTLPR alleles only include the
polymorphism
rs25531. In the plurality of predetermined alleles, the one or more MAOA
alleles only include
the polymorphism 30 bp repeat (promoter, X chrom only). In the plurality of
predetermined
alleles, the one or more GABRB3 alleles only include the polymorphism CA-
Repeat (171-201
bases, X chrom only).
[0141] In the plurality of predetermined alleles, the one or more BAIAP2
alleles only include
the polymophisms rs8079626, rs8079781, rs7210438, and rs4969385. In the
plurality of
predetermined alleles, the one or more CHRNA4 alleles only include the
polymorphisms
rs2273505 and rs3787141. In the plurality of predetermined alleles, the one or
more COMT
alleles only include the polymorphisms rs6269, rs4818, rs4633, rs933271,
rs1544325,
rs740603, rs740601, rs4646316, rs174696, rs165774, rs9332377, rs165599,
rs2020917, and
rs4680. In the plurality of predetermined alleles, the one or more DAT1
alleles only include
the polymorphisms rs460700, rs37020, rs13161905, rs27048, rs6347, rs11133767,
rs40184,
rs2975292, rs2652511, VNTR IN 3-UTR/10-repeat allele, and 40bp repeat (exon
15). In the
plurality of predetermined alleles, the one or more DBH alleles only include
the polymorphism
rs1108580. In the plurality of predetermined alleles, the one or more a DRD1
alleles only
59
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
include the polymorphism rs4532. In the plurality of predetermined alleles,
the one or more
DRD2 alleles only include the polymorphism rs1800497. In the plurality of
predetermined
alleles, the one or more DRD3 alleles only include the polymorphism rs6280. In
the plurality
of predetermined alleles, the one or more DRD4 alleles only include the
polymorphisms
rs1800955, rs4646983, rs4646984, rs3758653, rs936465, VNTR in exon 3/7 repeat
allele,
VNTR in exon 3/5 repeat allele, and 7-11 repeats of 48bp (intron 3). In the
plurality of
predetermined alleles, the one or more DRD5 alleles only include the
polymorphisms VNTR
in exon 8/3-repeat allele and dinucleotide repeat/ 148-bp allele. In the
plurality of
predetermined alleles, the one or more HTR1B alleles only include the
polymorphism rs6296.
In the plurality of predetermined alleles, the one or more OPRM1 alleles only
include the
polymorphism rs1799971. In the plurality of predetermined alleles, the one or
more SNAP25
alleles only include the polymorphisms rs66039806, rs362549, rs362987 and
rs362998. In the
plurality of predetermined alleles, the one or more HTTLPR alleles only
include the
polymorphism rs25531. In the plurality of predetermined alleles, the one or
more MAOA
alleles only include the polymorphism 30 bp repeat (promoter, X chrom only).
In the plurality
of predetermined alleles, the one or more GABRB3 alleles only include the
polymorphism CA-
Repeat (171-201 bases, X chrom only).
[0142] The plurality of predetermined alleles further include the SEMA3a
allele that is only
the polymorphism rs139438618, and at least one of the alleles of Amelo.
[0143] The plurality of predetermined alleles can only include (a) the one or
more alleles of
BAIAP2; (b) the one or more alleles of CHRNA4; (c) the one or more alleles of
COMT; (d)
the one or more alleles of DAT1; (e) one or more alleles of DBH; (f) the one
or more alleles
of DRD1; (g) the one or more alleles of DRD2; (h) the one or more alleles of
DRD3; (i) the
one or more alleles of DRD4; (j) the one or more alleles of DRD5; (k) the one
or more alleles
of HTR1B; (1) the one or more alleles of OPRM1; (m) the one or more alleles of
SNAP25; (n)
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
the one or more alleles of HTTLPR; (o) the one or more alleles of MAOA; and
(p) the one or
more alleles of GABRB3.
[0144] The plurality of predetermined alleles can only include (a) the one or
more alleles of
BAIAP2; (b) the one or more alleles of CHRNA4; (c) the one or more alleles of
COMT; (d)
the one or more alleles of DAT1; (e) one or more alleles of DBH; (f) the one
or more alleles
of DRD1; (g) the one or more alleles of DRD2; (h) the one or more alleles of
DRD3; (i) the
one or more alleles of DRD4; (j) the one or more alleles of DRD5; (k) the one
or more alleles
of HTR1B; (1) the one or more alleles of OPRM1; (m) the one or more alleles of
SNAP25; (n)
the one or more alleles of HTTLPR; (o) the one or more alleles of MAOA; (p)
the one or more
alleles of GABRB3; (q) the one or more alleles of SEMA3A; and (r) one or more
alleles of
Amelo.
[0145] The risk score in a first pre-determined range can identify a lower
increased genetic risk
for ADHD. The risk score in a second pre-determined range can identify a
higher increased
genetic risk for ADHD. For a higher increased genetic risk for ADHD identified
subject, the
treatment can include providing precision addictive/behavioral management
tailored for
persons with higher increased genetic risk for ADHD.
[0146] The first pre-determined range can be between 0% and 33% of the number
of alleles in
the plurality of predetermined alleles. The second pre-determined range can be
between 33%
and 100% of the number of alleles in the plurality of predetermined alleles.
[0147] The risk score in a third pre-determined range can identify a moderate
increased genetic
risk for ADHD.
[0148] For a moderate increased genetic risk for ADHD identified subject, the
treatment can
include providing the precision addictive/behavioral management tailored for
persons with
moderate increased genetic risk for ADHD.
61
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0149] The first pre-determined range can be between 0% and 33% of the number
of alleles in
the plurality of predetermined alleles. The third pre-determined range can be
between 33%
and 67% of the number of alleles in the plurality of predetermined alleles.
The second pre-
determined range can be between 67% and 100% of the number of alleles in the
plurality of
predetermined alleles.
[0150] For a lower increased genetic risk for ADHD identified subject, the
treatment can
include providing the precision addictive/behavioral management tailored for
persons with
lower increased genetic risk for ADHD.
[0151] The treatment can further include promoting a pro-dopamine lifestyle
for the subject.
[0152] The pro-dopamine lifestyle can be selected from a group consisting of
talk therapies,
life-style measures, support systems, mindfulness training, and neurofeedback.
[0153] The life-style measures can include a measure selected from a group
consisting of diet,
exercise, yoga, and meditation.
[0154] The treatment can further include a drug screen to monitor outcomes of
the subject.
[0155] The drug screen can include a urine drug screen.
[0156] The subject can regain dopamine homeostasis.
[0157] The method can reduce one or more of stress, craving, and relapse of
the subject.
[0158] The can further include the step of identifying each of the alleles in
the plurality of
predetermined alleles that was detected to be present in the biological
sample. The can further
include the step of assigning a count for each of the alleles in the plurality
of pre-determined
alleles that was detected to be present in the biological sample. The count
for a particular allele
can be the number of the particular allele detected to be present in the
biological sample. The
can further include the step of determining a risk score for the subject based
upon the count.
The risk score can be the sum of the counts. The risk score can identify a
severity of the genetic
risk for a reward deficiency syndrome behavior. The risk score in a first pre-
determined range
62
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
can identify a lower increased genetic risk for the reward deficiency syndrome
behavior. The
risk score in a second pre-determined range can identify a higher increased
genetic risk for the
reward deficiency syndrome behavior. The can further include the step of, for
a higher
increased genetic risk for reward deficiency syndrome behavior identified
subject, the
treatment includes treating the subject for the reward deficiency syndrome
behavior and further
includes entry of the subject in a residential treatment program for the
reward deficiency
syndrome behavior of the subject and medically monitoring the reward
deficiency syndrome
behavior of the subject.
[0159] The reward deficiency syndrome behavior can be selected from a group
consisting of
addictive behaviors, impulse behaviors, obsessive compulsive behaviors,
personality disorder
behaviors, and combinations thereof.
[0160] The step of performing the allelic analysis can include utilizing a kit
for detecting the
presence of the plurality of predetermined alleles in the biological sample.
[0161] In general, in another embodiment, the invention features a kit that
can be utilized to
performing the allelic analysis for detecting the present of the plurality of
predetermined alleles
in the biological sample in the methods described above.
BRIEF DESCRIPTION OF THE DRAWINGS
[0162] FIGS. 1A-1B are (in combination) an illustration of the interactions
taking place in
brain reward regions.
[0163] FIGS. 2A-2D are diagrammatic representations of the mechanisms of
action of
stimulants in treating ADHD. FIG. 2A shows the basal unstimulated state with
dopamine
stored in the vesicles and low levels of dopamine in the synapse. FIG. 2B
shows the result of
stimulation of the dopamine neuron with the vesicles releasing dopamine into
the synapse and
re-uptake of dopamine into the presynaptic neuron by the dopamine
transporters. FIG. 2C
shows that in the presence of stimulants, the function of the dopamine
transporters is partially
63
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
blocked and the basal level of dopamine increases in the synapse. This results
in the occupation
of the presynaptic dopamine D2 receptors by dopamine. FIG. 2D shows that, when
the nerve
is now stimulated, because of the occupation of the presynaptic D2 receptors,
the amount of
dopamine released from the vesicles is decreased.
[0164] FIG. 3 is a graph of an example of PCR amplification of variants of
dopamine receptor
D4 (DRD4).
[0165] FIG. 4 is a schematic of the precision behavior management process (or
protocol).
DETAILED DESCRIPTION
[0166] The present invention relate to methods and kits for assessing severity
index for genetic
risks of attention deficit/hyperactivity disorders. The present invention
further relates to
methods for behavioral management thereof. In some embodiments, the methods
and kits
provide a risk analysis score (termed a "genetic risk attention deficit
disorder score" (or
"GRADDS")). The method for behavioral management of those depending upon his
or her
GRADDS is terms the precision behavioral management (or "PBM") protocol.
[0167] ADHD is a complex disorder, usually appearing first in childhood, and
having multiple
causes including genetics as impacted by one's environment. Thus, to dispel
myths about
ADHD, this requires examination of the additive effects of multiple genes.
Further, and
because polygenic inheritance is far more complex than single gene
inheritance, an ultimate
understanding of the role of any one gene involved in polygenic inheritance
will require a
summation across many different studies. While the use of psychostimulants has
resulted in
attenuation of behavioral symptoms in a high percentage of ADHD children,
parents have been
concerned about potential side effects. Thus, Applicant has derived novel
concept of an
adjunctive polypharmacy approach for the prevention and treatment of ADHD
rather than
single neurochemical and/or neurogenetic targets (e.g., D1-D5, DAT1, DBH,
COMT, 5HT1B,
NR4A2, SLC1A3, BDNF, as well as loci at 4q13.2, 5q33.3, 11q22 and 17p11).
64
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0168] Because of advances in molecular pharmacology, nutrition, and molecular
genetics, the
legacy of RDS and subtype ADHD behavior will be reduced. To advance these
goals, ADHD
can be diagnosed using specific DNA polymorphic analysis coupled with
electrophysiological
and computerized testing, especially in young children. In this regard,
Larsson 2006 suggested
that the finding of persistent cross-subtype (i.e., combined) and persistent
subtype-specific
genetic influences (i.e., primarily hyperactive-impulsive and inattentive
disorders) are in line
with a genetic basis for the DSM-IV classification of ADHD subtypes (Table I).
Finally, rather
than a single pharmaceutical treatment approach, DNA-based personalized
nutraceutical
therapies can then be implemented in combination with a pro-dopamine lifestyle
to lead to
recovery of the individual.
Genetic Risk Profile for Attention¨Deficit Disorder Of All Types
[0169] The importance of genetic risk testing has great benefit based on the
fact that the current
diagnosis is not objective and relies mostly on teacher¨student and parent
relationships and
observations. There is indeed misdiagnosis and a real need for an objective
assessment tool. In
embodiments of the present invention a detailed panel can be utilized to
capture variable risk
for ADD and ADHD types.
[0170] In general, the performing an allelic analysis on a biological sample
(i.e., the GRADDS
testing), which includes testing for the following panel of alleles:
(a) one or more alleles of BAIAP2, which one or more BAIAP2 alleles include
at
least one or more of polymophisms rs8079626, rs8079781, rs7210438, and
rs4969385;
(b) one or more alleles of CHRNA4, which one or more CHRNA4 alleles include
at least one or more of polymorphisms rs2273505 and rs3787141;
(c) one or more alleles of COMT, which one or more COMT alleles include at
least
one or more of polymorphisms rs6269, rs4818, rs4633, rs933271, rs1544325,
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
rs740603, rs740601, rs4646316, rs174696, rs165774, rs9332377, rs165599,
rs2020917, and rs4680;
(d) one or more alleles of DAT1, which one or more DAT1 alleles include at
least
one or more of polymorphisms rs460700, rs37020, rs13161905, rs27048,
rs6347, rs11133767, rs40184, rs2975292, rs2652511, VNTR IN 3-UTR/10-
repeat allele, and 40bp repeat (exon 15);
(e) one or more alleles of DBH, which one or more DBH alleles include
polymorphism rs1108580;
(f) one or more alleles of DRD1, which one or more DRD1 alleles include
polymorphism rs4532;
(g) one or more alleles of DRD2, which one or more DRD2 alleles include
polymorphism rs1800497;
(h) one or more alleles of DRD3, which one or more DRD3 alleles include
polymorphism rs6280;
(i) one or more alleles of DRD4, which one or more DRD4 alleles include one
or
more of polymorphisms rs1800955, rs4646984, rs3758653, rs936465, VNTR
in exon 3/7 repeat allele, VNTR in exon 3/5 repeat allele, and 7-11 repeats of
48bp (intron 3);
(j) one or more alleles of DRD5, which one or more DRD5 alleles include one
or
more of polymorphisms VNTR in exon 8/3-repeat allele and dinucleotide
repeat/ 148-bp allele;
(k) one or more alleles of HTR1B, which one or more HTR1B alleles include
polymorphism rs6296;
(1) one or more alleles of OPRIVI1, which one or more OPRIVI1 alleles
include
polymorphism rs1799971;
66
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
(m) one or more alleles of SNAP25, which one or more SNAP25 alleles include
at
least one or more of polymorphisms rs66039806, rs362549, rs362987 and
rs362998;
(n) one or more alleles of HTTLPR, which one or more HTTLPR alleles include
polymorphism rs25531;
(o) one or more alleles of MAOA, which one or more MAOA alleles include
polymorphism 30 bp repeat (promoter, X chrom only); and
(p) one or more alleles of GABRB3, which one or more GABRB3 alleles include
polymorphism CA-Repeat (171-201 bases, X chrom only).
[0171] For example, the detailed panel can include each of the alleles set
forth in TABLE IV:
TABLE IV
Variation Sequence
Gene SNP Class ID No.
BAIAP2 rs8079626 SNV
BAIAP2 rs8079781 SNV
BAIAP2 rs7210438 SNV
BAIAP2 rs4969385 SNV
CHRNA4 rs2273505 SNV
CHRNA4 rs3787141 SNV
COMT rs6269 SNV 1
COMT rs4818 SNV 2
COMT rs4633 SNV 3
COMT rs933271 SNV 4
COMT rs1544325 SNV 5
COMT rs740603 SNV 6
COMT rs740601 SNV 7
COMT rs4646316 SNV 8
COMT rs174696 SNV 9
COMT rs165774 SNV 10
COMT rs9332377 SNV 11
COMT rs165599 SNV 12-15
COMT rs2020917 SNV 16
COMT rs4680 SNV 17
DAT 1 rs460700 SNV
DAT 1 rs37020 SNV
DAT 1 rs13161905 SNV
DAT 1 rs27048 SNV 18
67
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
DAT 1 rs6347 SNV 19
DAT 1 rs11133767 SNV
DAT 1 rs40184 SNV 20
DAT 1 rs2975292 SNV
DAT 1 rs2652511 SNV
DAT 1 VNTR IN 3-UTR/10-repeat allele VNTR
DAT1 40bp repeat (Exon 15) VNTR
DBH rs1108580 SNV
DRD1 rs4532 SNV 21
DRD2 rs1800497 SNV 22
DRD3 rs6280 SNV 23
DRD4 rs1800955 SNV 24
DRD4 rs4646984 SNV
DRD4 rs3758653 SNV 25
DRD4 rs936465 SNV
DRD4 VNTR in exon 3/7 repeat allele VNTR
DRD4 VNTR in exon 3/5 repeat allele VNTR
DRD4 7-11 repeats of 48bp (Intron 3) VNTR
DRD5 VNTR in exon 8/3- repeat allel VNTR
DRD5 Dinucleotide repeat/ 148-bp allele DINUCL
HTR1B rs6296 SNV 26
OPRM1 rs1799971 SNV 27
SNAP25 rs66039806 SNV
SNAP25 rs362549 SNV
SNAP25 rs362987 SNV
SNAP25 rs362998 SNV
HTTLPR 5-HTTLPR including rs25531 VNTR 28
30 bp repeat (promoter, X chrom 29
MAOA only*) VNTR
CA-Repeat (171-201 bases, X
GABRB3 chrom only*) DINUCL
[0172] A "single-nucleotide polymorphism" (also "SNP") is a substitution of a
single
nucleotide that occurs at a specific position in the genome. For example, at a
specific base
position in the human genome, the C nucleotide may appear in most individuals,
but in a
minority of individuals, the position is occupied by an A. This means that
there is a SNP at this
specific position, and the two possible nucleotide variations ¨ C or A ¨ are
said to be the
"alleles" for this specific position.
[0173] A "single-nucleotide variant" (also "SNV") is a variation in a single
nucleotide without
any limitations of frequency and may arise in somatic cells.
68
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0174] A "variable number tandem repeat" (or "VNTR") is a location in a genome
where a
short nucleotide sequence is organized as a tandem repeat.
[0175] A "dinucleotide" (or "DINUCL") is a variation of a nucleotide having
two units.
[0176] Further, for example, the panel can include each of the alleles in
TABLE IV (and, if
desired, additional alleles).
[0177] In some embodiments of the, the panel can exclude one, two, or three of
the
genes/alleles listed above (a)-(p). For instance, the panel can include the
genes (and one or
more the particular alleles) in (a)-(1) and (n)-(p) in the absence of (m)
(i.e., without HTR1B
allele having polymorphism rs6296 and even without any HTR1B alleles at all).
[0178] Additional alleles of can be added to the panel. These be for an allele
of the one of the
same gene listed above in (a)-(p), such as rs4646983 of DRD4. These can also
be for allele of
a different gene, such one or more alleles of SEMA3A, which can include
polymorphism
rs139438618 or such as one or more alleles of Amelo (for sex determination),
which can
include Amelo-F and/or Amelo-R.
[0179] The sequences of these alleles are described in further detail herein,
including as set
forth in SEQ. ID NOS. 1-29, and further including in Blum '927 PCT Application
and the
various references identified in the Reference section below and discussed
herein (including
Bonvicini 2016, Hawi 2003, Hasler 2017, Faraone 2010, and Bhaduri 2009).
[0180] Further, TABLE V provides sequences of certain polymorphisms used in
methods of
the present invention.
Gene SNP Sequence Chromosome
AAACATTTATTTGGCCTTCG
CAGTG[ A/Es. AAATCTAATTT Chr.17: 81053072 on
BAIAP2 rs8079626 CTTGAGTCTAAGAA GRCh38
TCCGCCTCTCCATCAACGGA
GGCCC V:TiGTCTAGGTTGT Chr.17: 81076348 on
BAIAP2 rs8079781 GGAGGATGCTTGAG GRCh38
GGCTCATGGGCCTCGGTTTT
GCTGC[CIT_ AGTGGTCCGCG Chr.17: 81086380 on
BAIAP2 rs7210438 CTGCGTTGGGTTCA GRCh38
69
CA 03137128 2021-10-18
WO 2020/215026
PCT/US2020/028870
TCAGCCCAGGGTGACTAAG
TGGGAG-C/T1ATTCTGTGGG Chr.17: 81095090 on
BAIAP2 rs4969385 TCTTAAATGCTTTCA GRCh38
GCAGGGCGACCTCAGTCAC
AGTGCA CSTIGATGGCCACG
CHRNA4 rs2273505 CCCTCACCTACCACG Chr.20 : 63359526
TGCCAGCGGCCGCCTCTGCC
AGCTA[CIT CACTTTGCAGC Chr.20: 63358042 on
CHRNA4 rs3787141 CGTGTTTGCAAACC GRCh38
GCATTTCTGAACCTTGCCCC
TCTGC[A/S AACACAAGGGG Chr.22: 19962429 on
COMT rs6269 GCGATGGTGGCACT GRCh3
GCCTGCTGTCACCAGGGGC
GAGGCT[CiSIATCACCATCG Chr.22: 19963684 on
COMT rs4818 AGATCAACCCCGACT GRCh3
CCAAGGAGCAGCGCATCCT
GAACCA C/TIGTGCTGCAGC Chr.22: 19962712 on
COMT rs4633 ATGCGGAGCCCGGGA GRCh3
GTGGTTACTTTCTGGAGAGA
GCATG 1T/CIGGCATGCAGGA Chr.22: 19943884 on
COMT rs933271 GC TGGAGGGGGGGT GRCh3
GAGACAAATTAGAAATGTC
AGTCTG[A/GIAGAGAGTGGT Chr.22: 19944145 on
COMT rs1544325 AGGTAGCCAGATACT GRCh3
TGTGAGGCACTGAGGATGC
CCTCAC V(IiICGTGCATCTG Chr.22: 19957654 on
COMT rs740603 CATGTGGCGTGCATG GRCh3
AACACAGAGCTGCCCTCTCT
GAATC -A/C CCGAACCGCCC Chr.22: 19963240 on
COMT rs740601 ACCTTGGGGCCCTG GRCh38
CCCAGACCAGACACCAGGG
CAGAAA1C/TIGGCACAGGAC Chr.22: 19964609 on
COMT rs4646316 CAAGGAGATGGGGTG GRCh38
CTGCGTCCGGCCGTATTCCA
GCTTT[C/TIAAAACAACAAA Chr.22: 19965653 on
COMT rs174696 AAACAACAAAAACT GRCh38
AAACTGGACACTGCTGTTA
GCAGCC AlSIGACTAGGAGC Chr.22: 19965038 on
COMT rs165774 ACGAGGGGCACAGCC GRCh38
AACCCCTCTCCTTGGGTGCC
TCTCC ITCATAGGCCTGA Chr.22: 19968169 on
COMT rs9332377 GTTCCTGGCACTG GRCh38
TGTTAGCCCCATGGGGACG
ACTGCC(A/Gj GCCTGGGAAA Chr.22: 19969258 on
COMT rs165599 CGAAGAGGAGTCAGC GRCh38
CTGGGGAGAAGTTGGGAAG
TCTGGC[TfciAGTGGGGCCG Chr.22: 19941361 on
COMT rs2020917 GTGCCTGGTGACCTC GRCh38
CA 03137128 2021-10-18
WO 2020/215026
PCT/US2020/028870
CCAGCGGATGGTGGATTTC
GCTGGC11.:01TGAAGGACAA
COMT rs4680 GGTGTGCATGCCTGA
ATTTCCCTAATTAAACACCC
TATGAIA/S]CACCCCAGGCC Chr.5: 1429854 on
DAT 1 rs460700 TGGGCTGCATGTGC GRCh38
AGGCAGCCATGGGCACTGG
CTCTGGI&T1 GTGGCACTGG Chr.5: 1418259 on
DAT 1 rs37020 GCAGAGGCCTGAGCT GRCh38
TTCACAACCCCCTGAGTGTG
TCCCC[CT CATCCCATGTGC Chr.5: 1417097 on
DAT 1 rs13161905 GCCCTGCTGCATG GRCh38
GCTTCCTGCTACCAGCAGGC
AGACT CII IGGATGGAGGTG Chr.5: 1412530 on
DAT 1 rs27048 GAGGGGACGAGAGT GRCh38
CCATCGCCACGCTCCCTCTG
TCCTCP/V:3. GCCTGGGCCGT Chr.5: 1411297 on
DAT 1 rs6347 GGTCTTCTTCATCA GRCh38
TCCAGGCCCCAGGAGCTGC
CGCAGC 'A/CIGGCAGTGGAA Chr.5: 1401465 on
DAT 1 rs11133767 GGAAGGCACGTTCAG GRCh38
AAATCAAGTAATGATTGATT
TGTAG- A/GIAGTTTGAGTGA Chr.5: 1394962 on
DAT 1 rs40184 GGCATCGGATCCCC GRCh38
GGCACCTGGACATGGCACC
TATGAG[C/OjCTGCAAAGCA Chr.5: 1419817 on
DAT 1 rs2975292 GTTGGGTGGGGGTGG GRCh38
CTGGACATCCTGGGCCTTGG
CGGCC -C/I1GGGGGCTCCAT Chr.5: 1446274 on
DAT 1 rs2652511 TCCTCCGCGCGCTG GRCh38
GCGACCCCAAGGATTACCT
CATTGA -A/G1GTAAGGGGTG Chr.9:133639992 on
DBH rs1108580 GCCGCGAGTACCCAG Build GRCh38
GGGGCTCTGACACCCCTCA
AGTTCC 'C.T AAGCAGGGAA
DRD1 rs4532 TAGGGGTCAGTCAGA
TGGACGTCCAGCTGGGCGC
CTGCCT[Cf ' ' GACCAGCACT
DRD2 rs1800497 TTGAGGATGGCTGTG
GCCCCACAGGTGTAGTTCA
GGTGGC C,IIIACTCAGCTGG
DRD3 rs6280 CTCAGAGATGCCATA
GGGCAGGGGGAGCGGGCGT
GGAGGG 1CITIGCGCACGAGG
DRD4 rs1800955 TCGAGGCGAGTCCGC
Chr.11 NC 000011.10
DRD4 rs4646984 (637305..640706)
71
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
CCTCTTTGGTGAAGAGTCCA
TAGAAX:T TCTCTGCTGCG Chr.11: 636399 on
DRD4 rs3758653 CTTTGC'AAGCACTT GRCh38
GGCTGCCTTGGGGCGAGAA
AGTACA ACTCAGCAAC Chr.11: 643568 on
DRD4 rs936465 AGCTCAGCAGGCACC GRCh38
CGGAGACTCGCACTTTGACT
TGGTT[C ACATACACAGG Chr.6:77462543 on
HTR1B rs6296 AGATCCGGATTCGC Build GRCh38
GGTCAACTTGTCCCACTTAG
ATGGCIA Ci )ACCTGTCCGAC
OPRM1 rs1799971 CCATGCGGTCCGAA
GACCTGGAAACGTACTGAG
CCCTGG A:(31ACTTTCATTCC Chr.20: 10289242 on
SNAP25 rs362549 TCTAGAAAATTTGT GRCh38
GTCTGGATTATGATATATCG
GTATT C TCTAATGCCTCG Chr.20: 10296804 on
SNAP25 rs362987 ACTTAAAACTCAT GRCh38
AAAAAGCCTGGGGCAATAA
TCAGGA GGAGTGGTGG Chr.20: 10296973 on
SNAP25 rs362998 CCAGCCAGCCTGCTC GRCh38
CTCGCGGCATCCCCCCTGCA
5-HTTLPR CCCCC A:(31GCATCCCCCCT
including GCAGCCCCCCCAGC (for
HTTLPR rs25531 rs25531)
30 bp repeat
(promoter, X ACCGGCACCGGCACCAGTA
MAOA chrom only*) CCCGCACCAGT
[0181] In one embodiment, the PCR sequences for the DRD5 Dinucleotide repeat/
148-bp
allele are provided in Table 1 of Hawi 2003. (50 CGTGTATGATCCCTGCAG30; 50
GCTCATGAGAAGAATGGAGTG30). Dinucleotide repeat microsatellite polymorphism
(CT/GT/GA) located 18.5 kb from the 5' end of the gene is highly polymorphic
with at least
12 possible alleles.
[0182] In one embodiment, the polymorphism rs139438618 of SEMA is disclosed in
Zhou
2017.
[0183] The allelic analysis can be performed on a biological sample for the
panel of
genes/alleles using techniques known in the art, such as allelic analysis
techniques that are
similar to those described in Blum '927 PCT Application.
72
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0184] The information received from the analysis is both qualitative and
quantitative, in that
the biological sample provides an overall score (basically, a count of the
number of alleles in
the panel that were determined to be present) and also provides for which
particular alleles
were determined to be present. Such information will be utilized for
identification and
treatment of persons with ADHD.
[0185] As for the count of the number of alleles that are determined to be
present, the following
are the general ranges for a panel that contains X alleles in the panel:
Zero to 33% of X: Lower increased risk for ADHD
33% to 67% of X: Moderate increased risk for ADHD
67% to 100% of X: High increased risk for ADHD
[0186] For example, if the panel set forth above in TABLE IV is performed on a
subject
(which panels has 53 total alleles), the ranges would be as follows:
Score of Zero to 17: Lower increased risk for ADHD
Score of 18-35: Moderate increased risk for ADHD
Score of 36-54: High increased risk for ADHD
[0187] From a qualitative point of view, the particular alleles tested
positive would be also
pertinent, as these can be utilized to tune the treatment that is provided to
the subject. I.e., the
combination of alleles is relevant to precision behavioral management and
treatment.
Precision Behavioral Management (PBM)
[0188] The present invention includes therapeutic method for treating ADHD
that includes the
above-described allelic analysis of the gene/allele panel (which again is
testing for genetic risk
predisposition) and can include customization of neuronutrient supplementation
to target the
individual genetic allele variation(s), based on the testing results, and
thereby deliver precision
behavioral manamgent (PBM) to patients. Since part of the proposed GRADDS
panel contains
73
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
GARS, Pro-Dopamine Regulation for ADHD could be developed and utilized in
treatment and
possibly even prophylaxsis's.
[0189] As discussed above, the genotyping testing taught and described for
identified SNPs in
individuals can be used for targeting precision nutrigenomics treatment.
[0190] FIG. 3 is an example of how simple genotyping for identified SNPs in
individuals can
be used to identify targets for precision nutrigenomics treatment. FIG. 3 is a
graph of an
example of PCR amplification of variants of dopamine receptor D4 (DRD4). DRD4
(Dopamine
Receptor 4) variants detected via polymerase chain reaction (PCR)
amplification with multiple
control samples. 2R to 8R = six different 48 base pair (bp) repeat sequences.
2R repeats = 48
bp twice, 3R = thrice and so forth. Peak height (y-axis) indicates
fluorescence signal amplitude,
peak location (x-axis) indicates fragment size (bp). Fragment sizes are shown
below the peaks
(base pairs). Humans carry two copies of this variant and their lengths are
from 2R to 11R.
Carrying one or both variants at 7R+ increases the risk of developing RDS.
This is one of the
eleven established risk variants assessed by the GARS test.
[0191] Multiple repeats of DRD4 variants are associated with disorders within
the RDS
spectrum [Huang 2002; Dragan 2009; Gervasini 2018]. In FIG. 3, six different
48 bp repeat
sequences are identified, from 2 repeats (2R) to 8R. The DRD4, DRD2, catechol-
0-
methyltransferase (COMT) are among genes within the mesolimbic reward pathway
with SNPs
that contribute to RDS,
[0192] FIG. 4 is a schematic illustrating various elements related precision
addiction
management (PAM) and shows the interrelatedness of genetic testing (i.e., the
GRADDS
testing), utilizing the testing with above-described gene/allele panel and a
customized
polymorphic matched nutraceutical therapeutics.
74
CA 03137128 2021-10-18
WO 2020/215026
PCT/US2020/028870
Coupling Genetic Testing with Precision Addiction Management (PAM)
[0193] In step 401, the genetic testing (i.e., the GRADDS testing) such as
described and taught
above is performed. From this testing, precision addictive/behavioral
management (PAM) is
designed, which can include, for example, neuro nutrient therapy based upon a
particular
individual's genetic risk profile.
[0194] In the GRADDS testing, the panel can be used to identify the overall
count as well as a
qualitative analysis as to what alleles in the panel were determined to be
present. The treatment
can then be tailored for the individual using the results of this testing.
[0195] This type of precision can be accomplished for each gene based allelic
polymorphism
primarily as it relates to major neurotransmitter pathways such serotonergic,
endorphinergic,
GABAergic, Glutaminergic, Cholinergic, and dopaminergic. This will enable the
novel
development of Precision Behavioral Management for ADHD treatment never before
utilized.
[0196] By way of example, consider six different nutraceuticals of Maker's
Nutrition
(Hauppauge, NY), namely: (a) endogen tablets, (b) equigen tablets, (c) gabagen
tablets, (d)
metagen tablets, (e) serogen tables, and (f) polygen tablets. The general
composition of these
nutraceutical tablets are shown in TABLE VI below:
TABLE VI
Ingredient Endogen Equigen Gabagen Metagen Serogen Polygen
Thiamine (as 15 mg 15 mg 15 mg 15 mg 15 mg 15 mg
Thiamin HCL)
Vitamin B6 (as 50 mg 50 mg 50 mg 50 mg 50 mg 50 mg
Pyridoxa1-5-
Phosphate)
Vitamin B6 (as 10 mg 10 mg 10 mg 10 mg 10 mg 10 mg
Pyridoxine HCL)
Chromium 200
mcg 200 mcg 200 mcg 600 mcg 600 mcg 600 mcg
(as Chromium
Polynicotinate)
DL- 2200
mg 2200 mg 2000 mg 2200 mg 2000 mg 2200 mg
Phenylalanine
(DLPA)
L-Tyrosine, USP 650 mg 700 mg 650 mg 700 mg 650 mg 700 mg
CA 03137128 2021-10-18
WO 2020/215026
PCT/US2020/028870
Larch 425 mg 125 mg 430 mg 50 mg
360 mg 50 mg
Arabinogalactans
N-Acetyl 200 mg 250 mg 200 mg 150
mg 200 mg 250 mg
Cystenine
(NAC)
Passion Flower 200 mg 300 mg 325 mg 325
mg 300 mg 325 mg
Extract 3.5 (4%
Vitexins)
Rhodiola Rosea 200 mg 250 mg 200 mg 250 mg 250 mg 250 mg
Extract (3%
Rosavin, 1%
Salidroside)
Griffonia Seed 100 mg 100 mg 100 mg 150
mg 150 mg 150 mg
SE 99% 5-
Hydroxy-
tryptophan
CoQ 10 30 mg 50 mg 50 mg 50 mg 30 mg
50 mg
White Bark Pine 25 mg 25 mg 25 mg 25 mg 25 mg 25 mg
L-Glutamine 25 mg 50 mg 75 mg 50 mg 50 mg
25 mg
Organic Aloe 15 mg 15 mg 15 mg 15 mg 15 mg
15 mg
Vera Gel Powder
200:1 #700
NADH 10 mg 15 mg 10 mg 15 mg 10 mg
10 mg
Kosher GTF 0.4 mg 0.4 mg 0.4 mg 0.4
mg 0.4 mg 0.4 mg
Chromium Yeast
Other ingredients in include Lecithin, Dicalcium Phosphate, Microchrystalline
Cellulose,
Croscarmellose Sodium, Magnesium Dioxide, and Pharmaceutical Glaze. Amounts
are
calculated on a four-tablet basis.
[0197] TABLE VII provides the targets and mechanism of actions for certain of
the
ingredients in these tablets:
TABLE VII
Ingredient Therapeutic Target Mechanism of
Actions
L-Phenylalanine Dopamine Synthesis 20%
of this precursor amino-acid is
converted to dopamine
D-Phenylalanine Enkephalin/Endorphin Inhibition of the
carboxypeptidase
Catabolism
(enkephalinase); thereby, increasing
opioid peptide levels in brain
L-Tyrosine Dopamine Synthesis Rate-limiting step in the
synthesis of
dopamine
L-Glutamate GABA Synthesis Supplied in small amount to assist
in
balance of over-inhibiting GABA by
natural opioid peptides
Chromium Salts Serotonin Synthesis
Chromium is known to increase the
sensitivity of the insulin receptor
thereby, reducing the carbohydrate
ratio by one-third in the blood; This
76
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
effect causes gut tryptophan to
increase in the brain with a
concomitant increase in serotonin
synthesis
5-Hy droxytry ptophane Serotonin Synthesis Involved in the synthetic
pathway to
produce serotonin
Rhodiola rosea Enzyme Inhibitor Rhodiola rosea has been shown to
Increasing inhibit COMT activity thereby,
Catecholamines increasing DA in the synapse as
well
as inhibiting MAO-A in the
mitochondria, which increases
vesicular DA in pre-synaptic neuron
Pyridoxine Phosphate Enzyme Catalyst Assists in the synthesis of
dopamine
Passion Flower Benzodiazepine Receptor By stimulating the benzodiazepine
Stimulant receptor, there is a reduction in
anxiety due to stress from
detoxification
[0198] By the allelic analysis utilizing the gene/allele panels described and
taught above, a
particular regime can then be selected aimed at addressing the therapeutic
targets identified by
this analysis. I.e.õ if the analysis favors that the therapy should include
promoting GABA
synthesis and serotonin synthesis, this would favor utilizing metagen tablets
that have 700 mg
L-Tyrosine, 150 mg Griffonia Seed SE 99% 5-Hydroxy-tryptophan, and 600 mcg
chromium,
which are at the higher ranges for these ingredients (as comparted to the
other tablets).
Promoting a Pro-Dopamine Lifestyle
[0199] Referring back to FIG. 4, step 402 is directed to providing the
individual a pro-
dopamine lifestyle, which can include talk therapies, life-style measures to
promote natural
endorphin and dopamine release (such as diet, exercise, yoga, meditation,
etc.), and support
systems.
[0200] A comprehensive treatment program that teaches a pro-dopamine lifestyle
and uses
urine drug screens (like the Comprehensive Analysis of Reported Drugs (CARD))
to monitor
outcomes, and as a basis for therapeutic interactions, can be utilized in
embodiments of the
present invention. Further a pro-dopamine lifestyle with gentle prolonged D2
agonist therapy
can be utilized to overcome DNA polymorphisms by promoting positive epigenetic
effects
77
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
Holistic modalities like exercise, low glycemic index diet, mindfulness
training,
neurofeedback, yoga, and meditation are known to support reward
neurotransmission and
naturally release dopamine the product of reward neurotransmission. These
holistic pro
dopamine modalities supported fellowship, can be used to induce feelings of
well-being and
thereby reduce craving and relapse.
[0201] These basic concepts underpin translational addiction-related research
that assist the
multitude of victims of genetically induced ADHD become the recipients of
better therapeutic
relapse-preventive tactics.
[0202] For example, the function of DAT1 is to clear excess dopamine released
from the pre-
neuron into the synapse and prevent uptake into the receptors on the next
neuron. Much
research, both biochemical and structural, has been performed to obtain clues
about the
mechanism of reuptake. The activity of clearing dopamine from the synapse is
dependent on
the variant form of this gene. So under normal conditions, the dopamine active
transporter
protein pumps the chemical messenger dopamine out of the synaptic cleft back
into the cytosol
of the pre-neuron cell. The DAT1 gene is located on chromosome 5 at p15. The
gene has a
variable number tandem repeats (VNTR) at the 3 'end of the gene and another in
the intron 8
region.
[0203] The importance here is that differences in the VNTR, for example, lOR
vs. 9R have
been shown to affect the basal level of expression (activity) of the
transporter. Indeed it has
been demonstrated that the 9R is a risk form because it has a much higher
ability to clear DA
from the synaptic cleft compared to the 1OR allele. Therefore, carriers of the
9R are more prone
to ADHD due to hypodopaminergia (low dopamine function). The regional brain
distribution
of the DAT includes high dopamine-containing neurons in the old reptilian
limbic system
similar to the DRD2 receptor distribution. The maximum expression of the DAT1
gene is found
in a parts of the brain called the substantia nigra and ventral tegmentum area
[brain regions
78
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
containing large amounts of the inhibitory chemical messenger GABA that fine-
tunes
dopamine release at the reward site]. It is also interesting that DAT is co-
localized with the D2
receptors.
[0204] Carriers of the 9R high activity of the DAT1 gene, requires a blocking
action. Therefore
based on this result the precision pro-dopamine variant can be chosen that
contains a higher
amount above normal of for example, L-Tyrosine. The rationale behind this
enhancement is
two ¨ fold: (1) L-Tyrosine is the rate limiting step in the brain synthesis of
dopamine. As such,
utilizing a higher mount of L-Tyrosine and subsequent increased amount of
needed dopamine
in the synapse, and (2) L-Tyrosine is also known to inhibit the action of the
9R DAT1 (high
activity) and as such reduce synaptic dopamine clearance.
Recovery
[0205] In step 403 of FIG. 4, such activities of steps 401 and 402 are
performed in tandem
leading the subject regaining dopamine homeostasis. Detection of a
predisposition (and thus
an early diagnosis) through genetic testing couples with pharmacogenetic and
pharmacogenomic monitoring, and appropriate urine drug screening, and
treatment with pro-
dopamine regulators can reduce stress, craving, and relapse and enhance well-
being in the
recovery community.
[0206] While embodiments of the invention have been shown and described,
modifications
thereof can be made by one skilled in the art without departing from the
spirit and teachings of
the invention. The embodiments described and the examples provided herein are
exemplary
only, and are not intended to be limiting. Many variations and modifications
of the invention
disclosed herein are possible and are within the scope of the invention. The
scope of protection
is not limited by the description set out above, but is only limited by the
claims which follow,
that scope including all equivalents of the subject matter of the claims.
79
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0207] The disclosures of all patents, patent applications, and publications
cited herein are
hereby incorporated herein by reference in their entirety, to the extent that
they provide
exemplary, procedural, or other details supplementary to those set forth
herein.
[0208] Amounts and other numerical data may be presented herein in a range
format. It is to
be understood that such range format is used merely for convenience and
brevity and should
be interpreted flexibly to include not only the numerical values explicitly
recited as the limits
of the range, but also to include all the individual numerical values or sub-
ranges encompassed
within that range as if each numerical value and sub-range is explicitly
recited. For example,
a numerical range of approximately 1 to approximately 4.5 should be
interpreted to include not
only the explicitly recited limits of 1 to approximately 4.5, but also to
include individual
numerals such as 2, 3, 4, and sub-ranges such as 1 to 3, 2 to 4, etc. The same
principle applies
to ranges reciting only one numerical value, such as "less than approximately
4.5," which
should be interpreted to include all of the above-recited values and ranges.
Further, such an
interpretation should apply regardless of the breadth of the range or the
characteristic being
described.
[0209] Unless defined otherwise, all technical and scientific terms used
herein have the same
meaning as commonly understood to one of ordinary skill in the art to which
the presently
disclosed subject matter belongs. Although any methods, devices, and materials
similar or
equivalent to those described herein can be used in the practice or testing of
the presently
disclosed subject matter, representative methods, devices, and materials are
now described.
[0210] Following long-standing patent law convention, the terms "a" and "an"
mean "one or
more" when used in this application, including the claims.
[0211] Unless otherwise indicated, all numbers expressing quantities of
ingredients, reaction
conditions, and so forth used in the specification and claims are to be
understood as being
modified in all instances by the term "about." Accordingly, unless indicated
to the contrary,
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
the numerical parameters set forth in this specification and attached claims
are approximations
that can vary depending upon the desired properties sought to be obtained by
the presently
disclosed subject matter.
[0212] As used herein, the term "about" and "substantially" when referring to
a value or to an
amount of mass, weight, time, volume, concentration or percentage is meant to
encompass
variations of in some embodiments 20%, in some embodiments 10%, in some
embodiments
5%, in some embodiments 1%, in some embodiments 0.5%, and in some
embodiments
0.1% from the specified amount, as such variations are appropriate to perform
the disclosed
method.
[0213] As used herein, the term "substantially perpendicular" and
"substantially parallel" is
meant to encompass variations of in some embodiments within 10 of the
perpendicular and
parallel directions, respectively, in some embodiments within 5 of the
perpendicular and
parallel directions, respectively, in some embodiments within 1 of the
perpendicular and
parallel directions, respectively, and in some embodiments within 0.5 of the
perpendicular
and parallel directions, respectively.
[0214] As used herein, the term "and/or" when used in the context of a listing
of entities, refers
to the entities being present singly or in combination. Thus, for example, the
phrase "A, B, C,
and/or D" includes A, B, C, and D individually, but also includes any and all
combinations and
subcombinations of A, B, C, and D.
REFERENCES
[0215] Aalto, S., et at., "Frontal and temporal dopamine release during
working memory and
attention tasks in healthy humans: A Positron Emission Tomography study using
the high-
affinity dopamine D2 receptor ligand [11CFLB457]," Journal of Neuroscience,
2005;Mar:25(10):2471-2477 ("Aalto 2005") .
81
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0216] Alderson, R. M., et at., "Attention-Deficit Hyperactivity Disorder
(ADHD) and
working memory in adults: A meta-analytic review," Neuropsychology,
2013;27(3):287-302
("Alderson 2013").
[0217] Andreazza, A. C., et at., "DNA damage in rats after treatment with
methylphenidate," Prog Neuropsychopharmacol
Biol Psychiatry, 2007;31:1282-8
("Andreazza 2007").
[0218] American Academy of Child Adolescent Psychiatry (AACP), "A guide for
families/getting treatment,"
http://www.aacap.org/cs/adhd a guide for families/
getting treatment ("AACP ADHD Guide").
[0219] American Psychiatric Association, Diagnostic and statistical manual of
the American
Psychiatric Association. Washington, DC, 2000 ("APA 2000").
[0220] American Psychiatric Association, Diagnostic and statistical manual of
mental
disorders (DSM-IV) Washington, D.C, 1994 ("APA 1994").
[0221] Arcos-Burgos, M., et at., "Attention-deficit/ hyperactivity disorder in
a population
isolate: linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11," Am J Hum
Genet., 2004;75:998-
1014 ("Arcos-Burgos 2004").
[0222] Asherson, P., et at., "Unravelling the complexity of attention-deficit
hyperactivity
disorder: a behavioural genomic approach," Br J Psychiatry, 2005;187:103-5
("Asherson
2005").
[0223] August, G. J., et at., "Familial subtypes of childhood hyperactivity,"
J Nery Ment
Dis., 1983;171:362-8 ("August 1983").
[0224] Badgaiyan R. D., et at., "Attenuated tonic and enhanced phasic release
of dopamine in
Attention Deficit Hyperactivity Disorder," PLoSOne, 2015;10:e0137326
("Badgaiyan 2015").
82
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0225] Barkley, R., "Behavioral inhibition, sustained attention, and executive
functions:
Constructing a unifying theory of ADHD," Psychological Bulletin.,
1997;121(1):65-94
("Barkley 1997").
[0226] Bhaduri, N., et al., "Study on DBH genetic polymorphisms and plasma
activity in
attention deficit hyperactivity disorder patients from Eastern India," Cell
Mot Neurobiol.,
2010Mar;30(2)265-74 ("Bhaduri 2010").
[0227] Biederman J., et al., "Lisdexamfetamine Dimesylate and mixed
amphetamine salts
extended-release in children with ADHD: A double-blind, placebo-controlled,
crossover
analog classroom study," Biol Psychiatry, 2007;62:970-6 ("Biederman II 200
7").
[0228] Biederman, J., et al., "Effect of comorbid symptoms of oppositional
defiant disorder on
responses to atomoxetine in children with ADHD: a meta-analysis of controlled
clinical trial
data," Psychopharmacology (Berl), 2007b; 190:31-41 ("Biederman II 200 7").
[0229] Biederman J., et al., "Comparison of parent and teacher reports of
attention-
deficit/hyperactivity disorder symptoms from two placebo-controlled studies of
atomoxetine
in children," Biol Psychiatry., 2006;60:1106-10 ("Biederman 2006").
[0230] Biederman, J., et al., "Further evidence for family-genetic risk
factors in attention
deficit hyperactivity disorder. Patterns of comorbidity in probands and
relatives psychiatrically
and pediatrically referred samples, Arch Gen Psychiatry, 1992;49:728-38
("Biederman
1992").
[0231] Bledsoe, J. C., et al., "Anterior cingulate cortex and symptom severity
in Attention-
Deficit/Hyperactivity Di s order," Journal of Abnormal Psychology, 2013;122(2)
:558-565
("Bledsoe 2013").
[0232] Blum, K., et al., "Pro-dopamine regulator, KB220Z, attenuates hoarding
and shopping
behavior in a female, diagnosed with SUP and ADHD, "JBehav Addict., 2018Mar;
7(1):192-
203 ("Blum 2018").
83
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0233] Blum, K., et at., "Dopamine homeostasis' requires balanced
polypharmacy: Issue with
destructive, powerful dopamine agents to combat America's drug epidemic," J
Syst Integr
Neurosci., 2017;3:6,10.15761/JSIN.1000183 ("Blum 2017").
[0234] Blum, K., et at., "Fifty years in the development of a Glutaminergic-
Dopaminergic
optimization complex (KB220) to balance brain reward circuitry in Reward
Deficiency
Syndrome: A pictorial," Austin Addiction Sc., 2016; 1(2): 1-11 ("Blum 12016").
[0235] Blum, K., et at., "Fifty Years in the development of a glutaminergic-
dopaminergic
optimization complex (KB220) to balance brain reward circuitry in reward
deficiency
syndrome: a pictorial," Austin Addict Sci., 2016;1:1006 ("Blum 11 2016").
[0236] Blum, K., et al., "rsfIVIRI effects of KB220Z on neural pathways in
reward circuitry of
abstinent genotyped heroin addicts," Postgrad
Med., 2015; 127:232-41,
10.1080/00325481.2015.994879 ("Blum 2015").
[0237] Blum, K., et at., "Neurogenetics and nutrigenomics of neuro-nutrient
therapy for
reward deficiency syndrome (RDS): clinical ramifications as a function of
molecular
neurobiological mechanisms, J Addict Res Ther, 2012;3:139,10.4172/2155-
6105.1000139
("Blum 2012").
[0238] Blum, K., et al., "Neurogenetics of dopaminergic receptor
supersensitivity in activation
of brain reward circuitry and relapse: proposing 'deprivation-amplification
relapse therapy'
(DART)," Postgrad Med., 2009; 121: 176-96,10.3810/pgm.2009.11.2087 ("Blum
2009").
[0239] Blum, K., et at., "Attention-deficit-hyperactivity disorder and reward
deficiency
syndrome," Neuropsychiatric Disease and Trament., 2008;4(5): 893 -917 ("Blum I
2008").
[0240] Blum, K., et at., "Activation instead of blocking mesolimbic
dopaminergic reward
circuitry is a preferred modality in the long term treatment of reward
deficiency syndrome
(RDS): a commentary," Theor Blot Med Model., 2008;5:24:10, 1186/1742-4682-5-24
("Blum
11 2008").
84
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0241] Blum, K., et at., "DNA based customized nutraceutical 'gene therapy'
utilizing a
genoscore: a hypothesized paradigm shift of a novel approach to the diagnosis,
stratification,
prognosis and treatment of inflammatory processes in the human," Med
Hypotheses, 2006a;66: 1008-18 ("Blum 2006").
[0242] Blum, K., et at., "Reward deficiency syndrome: a biogenetic model for
the diagnosis
and treatment of impulsive, addictive, and compulsive behaviors," J
Psychoactive
Drugs, 2000;32(Suppl i¨iv):1-112 ("Blum 2000").
[0243] Blum, K., et at., "Reward deficiency syndrome," Am Sc., 1996;84:132
("Blum I
1996").
[0244] Blum, K., et at., "The D2 dopamine receptor gene as a determinant of
reward deficiency
syndrome.," J R Soc Med., 1996b;89:396-400 ("Blum 11 1996").
[0245] Blum, K., et at., "Alcohol and the addictive brain: new hope for
alcoholics from
biogenetic research," New York: Free Press; 1991, 320 ("Blum 1991").
[0246] Blum, K., et al., "Ethanol and neuromodulator influences. A cascade
model of reward,"
in 011at H. et at., (editors), Alcohol and behaviour: Basic and clinical
aspects, Utrecht,
Netherlands:VSP;1990 ("Blum I 1990").
[0247] Blum, K., et at., "Ethanol and Neuromodulator influences. A cascade
model of reward.
In: 011at H., et at., (editors), Alcohol and Behaviour: Basic and Clinical
Aspects Progress in
Alcohol Research, Utrecht, Netherlands: VSP;1990 ("Blum 11 1990").
[0248] Blum, K., et at., "Allelic association of human dopamine D2 receptor
gene in
alcoholism, JAMA, 1990;263(15):2055-2060 ("Blum III 1990").
[0249] Blum, K., et at., "Enkephalinase inhibition and precursor amino acid
loading improves
inpatient treatment of alcohol and polydrug abusers: double-blind placebo-
controlled study of
the nutritional adjunct SAAVE," Alcohol., 1988;5:481-93 ("Blum 11988").
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0250] Blum, K., et at., "Neurogenetic deficits caused by alcoholism:
restoration by SAAVE,
a neuronutrient intervention adjunct," J Psychoactive Drugs, 1988;20:297-313
("Blum II
1988").
[0251] Bonvicini, C., et at., "Attention-deficit hyperactivity disorder in
adults: A systematic
review and meta-analysis of genetic, pharmacogenetic and biochemical studies,"
Mot
Psychiatry, 2016Jul ;21(7): 872-884 ("Bonvicini 2016").
[0252] Bowirrat, A., et at., "Relationship between dopaminergic
neurotransmission,
alcoholism, and Reward Deficiency Syndrome," Am J Med Genet B Neuropsychiatr
Genet., 2005;132:29-37 ("Bowirrat 2005").
[0253] Braverman, ER., et at., "Delayed P300 latency correlates with abnormal
Test of
Variables of Attention (TOVA) in adults and predicts early cognitive decline
in a clinical
setting," Adv Ther., 2006;23:582-600 ("Braverman 2006").
[0254] Brookes, K., et at., "The analysis of 51 genes in DSM-IV combined type
attention
deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other
genes," Mot
Psychiatry, 2006;11:934-53 ("Brookes 2006").
[0255] Bush, G., et al., "Functional neuroimaging of Attention-
Deficit/Hyperactivity Disorder:
A review and suggested future directions," Biological Psychiatry, 2005;57:1273-
1284 ("Bush
2005").
[0256] Bush, G., et at., "Anterior cingulate cortex dysfunction in Attention-
Deficit/Hyperactivity Disorder revealed by fM1t1 and the counting stroop,"
Biol Psychiatry,
1999;45:1542-1552 ("Bush 1999").
[0257] Cantwell, D. P., "Genetic factors in the hyperkinetic syndrome," J Am
Acad Child
Psychiatry, 1976;15:214-23 ("Cantwell 1976").
[0258] Cantwell, D. P., "Psychiatric illness in the families of hyperactive
children," Arch Gen
Psychiatry, 1972;27:414-7 ("Cantwell 1972").
86
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0259] Castellanos, F. X., et at., "Lack of an association between a dopamine-
4 receptor
polymorphism and attention-deficit/ hyperactivity disorder: genetic and brain
morphometric
analyses," Mot Psychiatry, 1998;3 :431-4 ("Castellanos 1998").
[0260] CHADD, CHADD 's educators manual, Landover, MD, 2007 ("CHADD 2007").
[0261] Chen, T. J., et at., "Narcotic antagonists in drug dependence: pilot
study showing
enhancement of compliance with SYN-10, amino-acid precursors and enkephalinase
inhibition
therapy," Med Hypotheses, 2004;63 :538-48 ("Chen 2004").
[0262] Cloninger, C. R., et at., "A psychobiological model of temperament and
character," Arch Gen Psychiatry, 1993;50:975-90 ("Cloninger 1993").
[0263] Comings, D. E., et at., "Neurogenetic interactions and aberrant
behavioral comorbidity
of attention deficit hyperactivity disorder (ADHD): dispelling myths," Theor
Biol Med
Model, 2005;2:50,10.1186/1742-4682-2-50 ("Comings 2005").
[0264] Comings, D. E., "Clinical and molecular genetics of ADHD and Tourette
syndrome.
Two related polygenic disorders," Ann N Y Acad Sci., 2001;931:50-83 ("Comings
2001").
[0265] Comings, D. E., et at., "Reward deficiency syndrome: genetic aspects of
behavioral
disorders," Prog Brain Res., 2000;126:325-41,10.1016/S0079-6123(00)26022-6
("Comings I
2000").
[0266] Comings, D. E., et at., "Multivariate analysis of associations of 42
genes in ADHD,
ODD and conduct disorder," Clin Genet., 2000;58:31-40 ("Comings 11 2000").
[0267] Comings, D. E., et at., "Polygenic inheritance of Tourette syndrome,
stuttering,
attention deficit hyperactivity, conduct, and oppositional defiant disorder:
the additive and
subtractive effect of the three dopaminergic genes ¨ DRD2, D beta H, and
DAT1," Am J Med
Genet., 1996;67:264-88 ("Comings 1996").
[0268] Comings, D. E., et at., "The dopamine D2 receptor locus as a modifying
gene in
neuropsychiatric disorders," JAMA, 19910ct2;266(13)1:1793-800 ("Comings
1991").
87
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0269] Conners, C. K, CRS-R (Conners' Rating Scales-Revised), Minneapolis, MN:
Pearson
Assessment Group; 2006 ("Conners 2006").
[0270] Cook, E. H., Jr., et at., "Association of attention-deficit disorder
and the dopamine
transporter gene," Am J Hum Genet., 1995;56:993-8 ("Cook 1995").
[0271] DeFrance, J. F., et at., "Enhancement of attentional processing by
Kantroll in healthy
humans: A pilot study," Clin Electroencephalography, 1977;28:68-75 ("DeFrance
1977").
[0272] Dougherty, D. D., et at., "Dopamine transporter density in patients
with Attention
Deficit Hyperactivity Disorder," Lancet., 1999Dec18-25;354(9196): 2132-3
("Dougherty
1999").
[0273] Dragan, W. L., et at., "The association between dopamine D4 receptor
exon III
polymorphism and intensity of PTSD symptoms among flood survivors," Anxiety
Stress
Coping, 2009;22:483-95,10.1080/10615800802419407 ("Dragan 2009").
[0274] Dresel, S., et at., "Attention Deficit Hyperactivity Disorder: binding
of99mTcTRODAT-
1 to the dopamine transporter before and after methylphenidate treatment,"
European Journal
of Nuclear Medicine, 2000;27(10): 1518-1524 ("Dresel 2000").
[0275] Dyr, W., et at., "Effects of D1 and D2 dopamine receptor agents on
ethanol
consumption in the high-alcohol-drinking (HAD) line of rats, Alcohol,
1993;10:207-12 ("Dyr
1993").
[0276] Faraone, S. V., "Molecular Genetics of Attention Deficit Hyperactivity
Disorder,"
Psychiatr Clin North Am., 2010Mar;33 (1) : 159-180 ("Faraone 2010").
[0277] Faraone, S. V., "Report from the 4th international meeting of the
attention deficit
hyperactivity disorder molecular genetics network," Am J Med Genet B
Neuropsychiatr
Genet., 2003;121:55-9 ("Faraone 2003").
[0278] Faraone, S. V., et at., "A family-genetic study of girls with DSM-III
attention deficit
disorder," Am J Psychiatry, 1991;148:112-7 ("Faraone 1991").
88
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0279] Farde, L., et at., "D2 dopamine receptors and
personality
traits," Nature, 1997;385(6617): 590 ("Farde 1997").
[0280] Febo, M., et at., "Dopamine homeostasis: brain functional connectivity
in reward
deficiency syndrome," Front Biosci. (Landmark Ed.), 2017;22:669-
91,10.2741/4509 ("Febo
2017").
[0281] Gadow, K. D., et al., "Methylphenidate in hyperactive boys with
comorbid tic disorder:
II.
Short-term behavioral effects in school settings," J Am Acad Child Adolesc
Psychiatry, 1992;31:462-71 ("Gadow 1992").
[0282] Gervasini, G., et at., "Effect of dopamine receptor D4 (DRD4)
haplotypes on general
psychopathology in patients with eating disorders," Gene, 2018;10.1016/j
.gene.2018.02.035
("Gervasini 2018").
[0283] Gill, M., et at., "Confirmation of association between attention
deficit hyperactivity
disorder and a dopamine transporter polymorphism," Mot Psychiatry, 1997;2:311-
3 ("Gill
1997").
[0284] Girault, J. A., et at., "The neurobiology of dopamine signaling," Arch
Neurol.,
2004; 61(5): 641-4 ("Girault 2004").
[0285] Hasler, R., et at., "Inter-hemispherical asymmetry in default-mode
functional
connectivity and BAIAP2 gene are associated with anger expression in ADHD
adults,"
Psychiatry Research: Neuroimaging, 2017Nov;269:54-61 ("Hasler 2017").
[0286] Hawi, Z., et at., "Linkage disequilibrium mapping at DAT1, DRD5 and DBH
narrows
the search for ADHD susceptibility alleles at these loci," Mot Psychiatry,
2003Mar;8(3):299-
308 ("Hawi 2003")
[0287] Heiser, P., et at., "Molecular genetic aspects of attention-
deficit/hyperactivity
disorder," Neurosci Biobehav Rev., 2004;28:625-41 ("Heiser 2004").
89
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0288] Hiebel, A. C., et at., "Probes for narcotic receptor mediated
phenomena. 34. synthesis
and structure-activity relationships of a potent mu-agonist delta-antagonist
and an exceedingly
potent antinociceptive in the enantiomeric C9-substituted 5-(3-hydroxypheny1)-
N-
phenylethylmorphan series," J Med Chem., 2007;50:65-75 ("Hiebel 2007").
[0289] Howell, D. C., et at., "Fifteen-year follow-up of a behavioral history
of attention deficit
disorder," Pediatrics, 1985;76:185-90 ("Howell 1985").
[0290] Huang, Y., et at., "Transmission disequilibrium test of DRD4 exon III
48bp variant
number tandem repeat polymorphism and tic disorder," Zhonghua Yi Xue Yi Chuan
Xue Za
Zhi, 2002;19:100-3 ("Huang 2002").
[0291] Imperato, A., et at., "Effects of locally applied D-1 and D-2 receptor
agonists and
antagonists studied with brain dialysis," Eur J Pharmacol., 1988;156:385-93
("Imperato
1988").
[0292] Inkster, B., et at., "Linkage disequilibrium analysis of the dopamine
beta-hydroxylase
gene in persistent attention deficit hyperactivity disorder," Psychiatr
Genet., 2004;14:117-20
("Inkster 2004").
[0293] Jensen, 0., et at., "Frontal theta activity in humans increases with
memory load in a
working memory task," Eur. J. Neuroscience, 2002;15:1395-1399 ("Jensen 2002").
[0294] Jonsson, E., et at., "Dopamine-related genes and their relationships to
monoamine
metabolites in CSF," Blot Psychiatry, 1996;40:1032-43 ("Jonsson 1996").
[0295] Kemppainen, N., et at., "Hippocampal dopamine D2 receptors correlate
with memory
functions in Alzheimer's disease," Eur J Neurosci., 2003Jul;18(1):149-154
("Kemppainen
2003").
[0296] Kent, L., et al., "Association of the paternally transmitted copy of
common Valine allele
of the Va166Met polymorphism of the brain-derived neurotrophic factor (BDNF)
gene with
susceptibility to ADHD,"Mol Psychiatry, 2005;10:939-43 ("Kent 2005").
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0297] Kessler, R. C., et at., "The prevalence and correlates of adult ADHD in
the United
States: Results from the National Comorbidity Survey Replication," Am J
Psychiatry,
2006Apr; 163 (4): 716-23 ("Kessler 2006").
[0298] Kimberg, D. Y., et al., "Effects of bromocriptine on human subjects
depend on working
memory capacity," Neuroreport., 1997;8:3581-5 ("Kimberg 1997").
[0299] Kirley, A., et at., "Association of the 480 bp DAT I allele with
methylphenidate
response in a sample of Irish children with ADHD," Am J Med Genet B
Neuropsychiatr
Genet., 2003;121:50-4 ("Kirley 2003").
[0300] Koob, G. F., et at., "Cellular and molecular mechanisms of drug
dependence," Science, 1988;242(4879):715-723 ("Koob 1988").
[0301] Krause, J., et at., "ADHD in adolescence and adulthood, with a special
focus on the
dopamine transporter and nicotine. Dialogues," Clin Neurosci., 2006;8:29-36
("Krause
2006").
[0302] Krause, J., et at., "Influence of striatal dopamine transporter
availability on the response
to methylphenidate in adult patients with ADHD, "Europ Arch Psychiatry Clin
Neurosci," 2005;255:428-31 ("Krause 2005").
[0303] Krause, J., et at., "The dopamine transporter and neuroimaging in
attention deficit
hyperactivity disorder," Neurosci Biobehav Rev., 2003;27:605-13 ("Krause
2003").
[0304] Krause, K. H., et at., "Increased striatal dopamine transporter in
adult patients with
Attention Deficit Hyperactivity Disorder: Effects of methylphenidate as
measured by single
photon emission computerized tomography," Neurosci Lett., 2000May12;285(2):107-
110
("Krause 2000").
[0305] la Fougere, C., et at., "Value of 99mTc-TRODAT-1 SPECT to predict
clinical response
to methylphenidate treatment in adults with attention deficit hyperactivity
disorder," Nuclear
Med Commun., 2006;27:733-7 ("la Fougere 2006").
91
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0306] LaHoste, G. J., et at., "Dopamine D4 receptor gene polymorphism is
associated with
attention deficit hyperactivity disorder," Mot Psychiatry, 1996;1:121-4
("LaHoste 1996").
[0307] Larsson, H., et at., "Genetic contributions to the development of ADHD
subtypes from
childhood to adolescence," J Am Acad Child Adolesc Psychiatry, 2006;45:973-81
("Larsson
2006").
[0308] Laurin, N., et at., "Association of the calcyon gene (DRD HP) with
attention
deficit/hyperactivity disorder," Mot Psychiatry, 2005;10:1117-25 ("Laurin
2005").
[0309] Le Moal, M., et at., "Mesocorticolimbic dopaminergic network:
functional and
regulatory roles," Physiol Rev., 1991;71:155-234 ("Le Moat 1991").
[0310] Lee, H. J., et at., "D2 and D4 dopamine receptor gene polymorphisms and
personality
traits in a young Korean population," Am J Med Genet B Neuropsychiatr Genet.,
2003;121:44-
9 ("Lee 2003").
[0311] Lenartowicz, A. G., et at., "Electroencephalography correlates of
spatial working
memory deficits in Attention-Deficit/Hyperactivity Disorder: Vigilance,
encoding, and
maintenance," Journal of Neuroscience, 2014;34(4): 1171-1182 ("Lenartowicz
2014").
[0312] Li, D., et at., "Meta-analysis shows significant association between
dopamine system
genes and attention deficit hyperactivity disorder (ADHD)," Hum Mot Genet.,
2006;15:2276-
84 ("Li 2006").
[0313] Lou, H. C., et at., "The striatum in a putative cerebral network
activated by verbal
awareness in normals and in ADHD children," Eur J Neurol., 1998;5:67-74 ("Lou
1998").
[0314] Lubar, J. F., "Discourse on the development of EEG diagnostics and
biofeedback for
attention-deficit/hyperactivity disorders.," Biofeedback Self Regul.,
1991;16:201-25 ("Lubar
1991").
[0315] Madras, B. K., et at., "The dopamine transporter and attention-
deficit/hyperactivity
disorder," Biol Psychiatry, 2005;57:1397-409 ("Madras 2005").
92
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0316] Makris, N., et at., "Cortical thinning of the attention and executive
function networks
in adults with Attention-Deficit\Hyperactivity Disorder," Cerebral Cortex,
2007;17(6):1364-
1375 ("Makris 2007").
[0317] Malison, R. T., et at., "123Ibeta-CIT SPECT imaging of striatal
dopamine transporter
binding in Tourette' s disorder," Am J Psychiatry, 1995;152:1359-61 ("Mat/son
1995").
[0318] Mandel, R. J., et at., "A quantitative estimate of the role of striatal
D-2 receptor
proliferation in dopaminergic behavioral supersensitivity: the contribution of
mesolimbic
dopamine to the magnitude of 6-0HDA lesion-induced agonist sensitivity in the
rat," Behav
Brain Res., 1993;59:53-64,10.1016/0166-4328(93)90151-F ("Mandel 1993").
[0319] McBride, W. J., et at., "Regional serotoninlA receptors in the CNS of
alcohol-
preferring and -nonpreferring rats," Pharmacol Biochem Behay., 1994;49:7-12
("McBride
1994").
[0320] McBride, W. J., et al., "Densities of dopamine D2 receptors are reduced
in CNS regions
of alcohol-preferring P rats," Alcohol, 1993;10:387-90 ("McBride 1993").
[0321] McLaughlin, T., et at., "Improvement of long-term memory as measured by
semantic
verbal fluency with acute intake of a pro-dopamine regulator (liquid nano
variant KB220Z) in
an elderly male: Are we targeting dopamine tone?" I Syst Integ Neurosci.,
2017May;3(3),
10.15761/JSIN.1000165 ("McLaughlin 2017").
[0322] Mill, J., et at., "Prediction of heterogeneity in intelligence and
adult prognosis by
genetic polymorphisms in the dopamine system among children with attention-
deficit/hyperactivity disorder: evidence from 2
birth cohorts," Arch Gen
Psychiatry, 2006;63:462-9 ("Mill 2006").
[0323] Miller, D., et at., Overload: Attention deficit disorder and the
addictive brain, Salt Lake
City, Utah: Woodland Publishing Company; 2008 ("Miller 2008").
93
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0324] Nieoullon, A., "Dopamine and the regulation of cognition and
attention," Progress in
Neurobiology, 2002;67:53-83 ("Nieoullon 2002").
[0325] Noble, E. P, "D2 dopamine receptor gene in psychiatric and neurologic
disorders and
its phenotypes," Am J Med Genet B Neuropsychiatr Genet., 2003;116:103-25
("Noble 2003").
[0326] Noble, E. P., et al., "D2 dopamine receptor polymorphism and brain
regional glucose
metabolism," Am J Med Genet., 1997;74:162-6 ("Noble 1997").
[0327] Noble, E. P., et al., "Prolonged P300 latency in children with the D2
dopamine receptor
Al allele," Am J Hum Genet., 1994;54:658-68 ("Noble 1994").
[0328] Noble, E. P., et al., "Allelic association of the D2 dopamine receptor
gene with
receptor-binding characteristics in alcoholism," Arch Gen Psychiatry,
1991Jul;48(7):648-54
("Noble 1991").
[0329] Olds, J., "A preliminary mapping of electrical reinforcing effects in
the rat brain," J
Comp Physiol Psychol, 1956;49:281-5 ("Olds 1956").
[0330] 011at, H., et al., Progress in alcohol research, Utrecht,
Netherlands:VSP; 1990,
("Alcohol and behaviour: basic and clinical aspects"), 285 ("011at 1990").
[0331] Onton, J., et al., "Frontal midline EEG dynamics during working
memory,"
Neuroimage, 2005;27:341-356 ("Onton 2005").
[0332] Ortiz, J., et al., "Biochemical actions of chronic ethanol exposure in
the mesolimbic
dopamine system," Synapse, 1995;21:289-98 ("Ortiz 1995").
[0333] Pauls, D. L., et al., "The inheritance of Gilles de la Tourette's
syndrome and associated
behaviors. Evidence for autosomal dominant transmission," N Engl J Med.
1986;315:993-7
("Pauls 1986").
[0334] Perwien, A. R., et al., "Atomoxetine treatment in children and
adolescents with
attention-deficit hyperactivity disorder: what are the long-term health-
related quality-of-life
outcomes?" J Child Adolesc Psychopharmacol., 2006;16:713-24 ("Penvien 2006").
94
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0335] Pohjalainen, T., et al., "The Al allele of the human D2 dopamine
receptor gene predicts
low D2 receptor availability in healthy volunteers," Mot Psychiatry, 1998;3
:256-60
("Pohjalainen 1998").
[0336] Polanczyk, G., et at., "The world-wide prevalence of ADHD: A systematic
review and
metaregres si on analysis," Am J Psychiatry, 2007June; 164 : 6,942-948
("Polanczyk 2007").
[0337] Rothman, R. B., et at., "Dual dopamine/serotonin releasers as potential
medications for
stimulant and alcohol addictions," AAPS1, 2007;9:E1-10 ("Rothman 2007").
[0338] Russell, V. A., et at., "Effect of ethanol on 3Hdopamine release in rat
nucleus
accumbens and striatal slices," Neurochem Res., 1988;13:487-92 ("Russell
1988").
[0339] Safer, D. J., "A familial factor in minimal brain dysfunction," Behav
Genet., 1973;3:175-86 ("Safer 1973").
[0340] Seamans, J. K., et al., "The principal features and mechanisms of
dopamine modulation
in the prefrontal cortex," Prog Neurobiol., 20045ep;74(1):1-58 ("Seamans
2004").
[0341] Seeman, P., et at., "Anti-hyperactivity medication: methylphenidate and
amphetamine," Mot Psychiatry, 1998;3 :386-96 ("Seeman 1998").
[0342] Shaw, P., et at., "Polymorphisms of the dopamine d4 receptor, clinical
outcome, and
cortical structure in attention-deficit/
hyperactivity disorder," Arch Gen
Psychiatry, 2007;64:921-31 ("Shaw 2007").
[0343] Shaywitz, B. A., et at., "Selective brain dopamine depletion in
developing rats: an
experimental model of minimal brain dysfunction," Science, 1976;191(4224):305-
8
("Shaywitz 1976").
[0344] Smith, K. M., et at., "Identification and characterization of human
NR4A2
polymorphisms in attention deficit hyperactivity disorder," Am J Med Genet B
Neuropsychiatr
Genet., 2005;133:57-63 ("Smith 2005").
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0345] Spencer, T. J., et at., "Atomoxetine and adult attention-
deficit/hyperactivity disorder:
the effects of comorbidity," J Clin Psychiatry, 2006;67:415-20 ("Spencer
2006").
[0346] Spencer, T., et at., "An open-label, dose-ranging study of atomoxetine
in children with
attention deficit hyperactivity disorder," J Child Adolesc Psychopharmacol.,
2001;11:251-65
("Spencer 2001").
[0347] Stein, L., et at., "Second messengers, natural rewards, and drugs of
abuse," Clin
Neuropharmacol,. 1986;9(Suppl 4):205-7 ("Stein 1986").
[0348] Steinberg, B., et at., "Low-resolution electromagnetic tomography
(LORETA) of
changed brain function provoked by pro-dopamine regulator (KB220Z) in one
adult ADHD
case," Open J Clin Med Case Rep., 2016;1121 ("Steinberg 2016").
[0349] Swanson, J. M., et at., "Etiologic subtypes of attention-
deficit/hyperactivity disorder:
brain imaging, molecular genetic and environmental factors and the dopamine
hypothesis," Neuropsychol Rev., 2007;17:39-59 ("Swanson 2007").
[0350] Takahashi, H., et at., "Functional significance of central D1 receptors
in cognition:
Beyond working memory," J. Cereb Blood Flow & Metab. 2012Jul;32(7):1248-1258
("Takahashi 2012").
[0351] Takahashi, H., et at., "Memory and frontal lobe functions: Possible
relations with
dopamine D2 receptors in the hippocampus," Neuroimage, 2007Feb15;34(4):1643-9
("Takahashi 2007").
[0352] Takahashi, H. et at., "SV. Attention-deficit hyperactivity disorder,"
Thelancet.com,
2005,Jul 16;366:237-246 ("Takahashi 2005").
[0353] Thanos, P. K., et at., "D2R DNA transfer into the nucleus accumbens
attenuates cocaine
self-administration in rats." Synapse, 2008;62:481-6,10.1002/syn.20523
("Thanos 2008").
96
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0354] Thanos, P. K., et at., "Effects of chronic oral methylphenidate on
cocaine self-
administration and striatal dopamine D2 receptors in rodents," Pharmacol
Biochem
Behay., 2007;87:426-33 ("Thanos 2007").
[0355] Thanos, P. K., et al., "Dopamine D2R DNA transfer in dopamine D2
receptor-deficient
mice: effects on ethanol drinking," Life Sc., 2005;77:130-9,10.1016/j
.1fs.2004.10.061
("Thanos 2005").
[0356] TOVA (Test of Variables of Attention), Los Alamitos, CA: The TOVA
Company;
2006 ("TOVA 2006").
[0357] Turic, D., et at., "A family based study implicates solute carrier
family 1-member 3
(SLC1A3) gene in attention-deficit/hyperactivity disorder," Blot Psychiatry,
2005a;57:1461-
6 ("Turic 12005").
[0358] Turic, D., et at., "A family based study of catechol-O-
methyltransferase (COMT) and
attention deficit hyperactivity disorder (ADHD)," Am J Med Genet B
Neuropsychiatr
Genet., 2005b;133:64-7 ("Turic 11 2005").
[0359] Visser, S. N., et at., "Trends in the parent-report of health care
provider-diagnosed and
medicated attention-deficit/hyperactivity disorder: United States, 2003-2011,"
J Am Acad
Child Adolsec Psychiatry, 2014Jan;53 (1): 34-46. e2 .PMID :24342384 ("Visser
2014").
[0360] Volkow, N. D., et at., "Depressed dopamine activity in caudate and
preliminary
evidence of limbic involvement in adults with attention-deficit/hyperactivity
disorder," Arch
Gen Psychiatry, 2007;64:932-40 ("Volkow 2007").
[0361] Volkow, N. D., et al., "Therapeutic doses of oral methylphenidate
significantly increase
extracellular dopamine in the human brain," Journal of Neuroscience, 2001;21:
1-5 ("Volkow
2001").
97
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0362] Volkow, N. D., et at., "Is methylphenidate like cocaine? Studies on
their
pharmacokinetics and distribution in the human brain," Arch Gen Psychiatry,
1995;52:456-63
("Volkow 1995").
[0363] Waldman, I. D., et at., "The relation between childhood antisocial
behavior and the
dopamine transporter gene (DAT1). Mediation via hyperactivity-impulsivity," Am
J Med Gen
B Neuropsychiatr Genet, 1998;81:451-556 ("Waldman 1998").
[0364] Wallis, D., et at., "Genetics of attention deficit/hyper-activity
disorder, J Pediatr
Psychol., 2008Jun3;Epub ahead of print ("Wallis 2008").
[0365] Weiss, G., et at., Hyperactive children grown up, 2nd. Ed. New York:
Guilford Press.
1993 ("Weiss 1993").
[0366] Welner, Z., et at., "A controlled study of siblings of hyperactive
children," J Nery Ment
Dis., 1977;165:110-7 ("Weiner 1977").
[0367] Wilens, T. E., et at., "Characteristics of adolescents and young adults
with ADHD who
divert or misuse their prescribed medications," J Am Acad Child Adolesc
Psychiatry, 2006;45:408-14 ("Wilens 2006").
[0368] Willerman, L., "Activity level and hyperactivity in twins," Child Dev.,
1973;44:288-
93 ("Willerman 1973").
[0369] Winsberg, B. G., et at., "Association of the dopamine transporter gene
(DAT1) with
poor methylphenidate response," J Am Acad Child Adolesc Psychiatry,
1999;38:1474-7
("Winsberg 1999").
[0370] Wise, R. A., et at., "Brain reward circuitry: four circuit elements
'wired' in apparent
series," Brain Res Bull., 1984;12:203-8 ("Wise 1984").
[0371] Wolf, S. S., et at., "Tourette syndrome: prediction of phenotypic
variation in
monozygotic twins by caudate nucleus D2 receptor binding. Science,"
1996;273(5279):1225-
7 ("Wolf 1996").
98
CA 03137128 2021-10-18
WO 2020/215026 PCT/US2020/028870
[0372] Wolraich, M. L., et at., "Examination of DSM-IV criteria for attention
deficit/hyperactivity disorder in a county-wide sample," J Dev Behav Pediatr.,
1998;19:162-8
("Wolraich 1998").
[0373] Zametkin, A. J., et at., "Cerebral glucose metabolism in adults with
hyperactivity of
childhood onset," N Engl J Med., 1990;323:1361-6 ("Zametkin 1990").
[0374] Zhou, F. C., et at., "Immunostained serotonergic fibers are decreased
in selected brain
regions of alcohol-preferring rats," Alcohol, 1991;8:425-31 ("Zhou 1991").
[0375] Zhou, H. et at., "Genetic Risk Variants Associated With Comorbid
Alcohol
Dependence and Major Depression," AMA Psychiatry, 2017;74(12):1234-1241 ("Zhou
2017").
99