Note: Descriptions are shown in the official language in which they were submitted.
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Modulators of the integrated stress response pathway
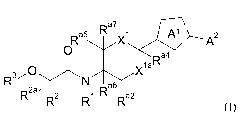
The present invention relates to compounds of formula (I)
Ra5 Ra 7 Al A2
0
vlaRa4
N 6
I Ra
R2a
R2 R1 Ra2 (I)
or pharmaceutically acceptable salts, solvates, hydrates, tautomers or
stereoisomers thereof,
wherein R1, R2, R2a, R3, Ra2, Ra4, Ra5, Ra6, Ra7, xl, x I a, A1
and A2 have the meaning as
indicated in the description and claims. The invention further relates to
pharmaceutical
compositions comprising said compounds, their use as medicament and in a
method for
treating and preventing of one or more diseases or disorders associated with
integrated stress
response.
The Integrated Stress Response (ISR) is a cellular stress response common to
all eukaryotes
(1). Dysregulation of ISR signaling has important pathological consequences
linked inter alia
to inflammation, viral infection, diabetes, cancer and neurodegenerative
diseases.
ISR is a common denominator of different types of cellular stresses resulting
in
phosphorylation of the alpha subunit of eukaryotic translation initiation
factor 2 (eIF2alpha)
on serine 51 leading to the suppression of normal protein synthesis and
expression of stress
response genes (2). In mammalian cells the phosphorylation is carried out by a
family of four
eIF2alpha kinases, namely: PKR-like ER kinase (PERK), double-stranded RNA-
dependent
protein kinase (PKR), heme-regulated eIF2alpha kinase (HRI), and general
control non-
derepressible 2 (GCN2), each responding to distinct environmental and
physiological stresses
(3)-
eIF2alpha together with eIF2beta and eIF2gamma form the eIF2 complex, a key
player of the
initiation of normal mRNA translation (4). The eIF2 complex binds GTP and Met-
tRNA,
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
forming a ternary complex (eIF2-GTP-Met-tRNA1), which is recruited by
ribosomes for
translation initiation (5, 6).
eIF2B is a heterodecameric complex consisting of 5 subunits (alpha, beta,
gamma, delta,
epsilon) which in duplicate form a GEF-active decamer (7).
In response to ISR activation, phosphorylated eIF2alpha inhibits the eIF2B-
mediated
exchange of GDP for GTP, resulting in reduced ternary complex formation and
hence in the
inhibition of translation of normal mRNAs characterized by ribosomes binding
to the 5' AUG
start codon (8). Under these conditions of reduced ternary complex abundance
the translation
of several specific mRNAs including the mRNA coding for the transcription
factor ATF4 is
activated via a mechanism involving altered translation of upstream ORFs
(uORFs) (7, 9, 10).
These mRNAs typically contain one or more uORFs that normally function in
unstressed cells
to limit the flow of ribosomes to the main coding ORF. For example, during
normal
conditions, uORFs in the 5' UTR of ATF occupy the ribosomes and prevent
translation of the
coding sequence of ATF4. However, during stress conditions, i.e. under
conditions of reduced
ternary complex formation, the probability for ribosomes to scan past these
upstream ORFs
and initiate translation at the ATF4 coding ORF is increased. ATF4 and other
stress response
factors expressed in this way subsequently govern the expression of an array
of further stress
response genes. The acute phase consists in expression of proteins that aim to
restore
homeostasis, while the chronic phase leads to expression of pro-apoptotic
factors (1, 11, 12,
13).
Upregulation of markers of ISR signaling has been demonstrated in a variety of
conditions,
among these cancer and neurodegenerative diseases. In cancer, ER stress-
regulated translation
increases tolerance to hypoxic conditions and promotes tumor growth (14, 15,
16), and
deletion of PERK by gene targeting has been shown to slow growth of tumours
derived from
transformed PERKY mouse embryonic fibroblasts (14, 17). Further, a recent
report has
provided proof of concept using patient derived xenograft modeling in mice for
activators of
eIF2B to be effective in treating a form of aggressive metastatic prostate
cancer (28). Taken
together, prevention of cytoprotective ISR signaling may represent an
effective anti-
proliferation strategy for the treatment of at least some forms of cancer.
2
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Further, modulation of ISR signaling could prove effective in preserving
synaptic function
and reducing neuronal decline, also in neurodegenerative diseases that are
characterized by
misfolded proteins and activation of the unfolded protein response (UPR), such
as
amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD),
Alzheimer's disease
.. (AD), Parkinson's disease (PD) and Jakob Creutzfeld (prion) diseases (18,
19, 20). With prion
disease an example of a neurodegenerative disease exists where it has been
shown that
pharmacological as well as genetic inhibition of ISR signaling can normalize
protein
translation levels, rescue synaptic function and prevent neuronal loss (21).
Specifically,
reduction of levels of phosphorylated eIF2alpha by overexpression of the
phosphatase
controlling phosphorylated eIF2alpha levels increased survival of prion-
infected mice
whereas sustained eIF2alpha phosphorylation decreased survival (22).
Further, direct evidence for the importance of control of protein expression
levels for proper
brain function exists in the form of rare genetic diseases affecting functions
of eIF2 and
eIF2B. A mutation in eIF2gamma that disrupts complex integrity of eIF2 and
hence results in
reduced normal protein expression levels is linked to intellectual disability
syndrome (ID)
(23). Partial loss of function mutations in subunits of eIF2B have been shown
to be causal for
the rare leukodystrophy Vanishing White Matter Disease (VWMD) (24, 25).
Specifically,
stabilization of eIF2B partial loss of function in a VWMD mouse model by a
small molecule
related to ISRIB has been shown to reduce ISR markers and improve functional
as well as
pathological end points (26, 27).
Modulators of the eIF2 alpha pathway are described in WO 2014/144952 A2. WO
2017/193030 Al, WO 2017/193034 Al, WO 2017/193041 Al and WO 2017/193063 Al
describe modulators of the integrated stress pathway. WO 2017/212423 Al, WO
2017/212425 Al, WO 2018/225093 Al, WO 2019/008506 Al and WO 2019/008507 Al
describe inhibitors of the ATF4 pathway. WO 2019/032743 Al and WO 2019/046779
Al
relate to eukaryotic initiation factor 2B modulators.
Further documents describing modulators of the integrated stress pathway are
WO
2019/090069 Al, WO 2019/090074 Al, WO 2019/090076 Al, WO 2019/090078 Al, WO
2019/090081 Al, WO 2019/090082 Al, WO 2019/090085 Al, WO 2019/090088 Al, WO
2019/090090 Al. Modulators of eukaryotic initiation factors are described in
WO
2019/183589 Al. WO 2019/118785 A2 describes inhibitors of the integrated
stress response
3
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
pathway. Heteroaryl derivatives as ATF4 inhibitors are described in WO
2019/193540 Al.
Bicyclic aromatic ring derivatives as ATF4 inhibitors are described in WO
2019/193541 Al.
However, there is a continuing need for new compounds useful as modulators of
the
integrated stress response pathway with good pharmacokinetic properties.
Thus, an object of the present invention is to provide a new class of
compounds as modulators
of the integrated stress response pathway, which may be effective in the
treatment of
integrated stress response pathway related diseases and which may show
improved
pharmaceutically relevant properties including activity, solubility,
selectivity, ADMET
properties and/or reduced side effects.
Accordingly, the present invention provides a compound of formula (I)
a5 Ra7
R X11A2
0 a4
vlaR
N 6
I R2a Ra
R2 1 Ra2 (I)
or a pharmaceutically acceptable salt, solvate, hydrate, tautomer or
stereoisomer thereof for
use as a medicament, wherein
X1 is N(Ral);
X1 a is a covalent single bond, CH(Ra3), or CH(Ra3)CH2;
Ral is H, C(0)0C14 alkyl, or C1_4 alkyl, wherein C(0)0C14 alkyl and Ci_4 alkyl
are optionally
substituted with one or more substituents selected from the group consisting
of halogen, OH,
and 0-C1_3 alkyl, wherein the substituents are the same or different;
Ra2, -a3
K
are independently selected from the group consisting of H; OH; 0C1_4 alkyl;
halogen;
C1_4 alkyl; and A2a; and
Ra4, Ras, Ra6, Ra7 are independently selected from the group consisting of H;
halogen; C1_4
alkyl; and A2a,
4
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
provided that only one of Ra2, Ra3; Ra4; Ra5; Ra6; Ra7 is A2a;
or Rai and one of Ra2 and Ra3 form a methylene or ethylene group;
or Rai and Ra6 form an ethylene group;
or Ra2 and Ra5 form a covalent single bond;
or Ra5, Ra7 are joined to form an oxo group;
A1 is C5 cycloalkylene, C5 cycloalkenylene, or a nitrogen ring atom containing
5-membered
heterocyclene, wherein A1 is optionally substituted with one or more R4, which
are the same
or different;
each R4 is independently oxo (=0) where the ring is at least partially
saturated, thiooxo (=S)
where the ring is at least partially saturated, halogen, CN, OR5, or C1_6
alkyl, wherein C1_6
alkyl is optionally substituted with one or more halogen, which are the same
or different;
R5 is H or C1_6 alkyl, wherein C1_6 alkyl is optionally substituted with one
or more halogen,
which are the same or different;
A2 is R6a or A2a;
R6a is OR6a1, SR6al, N(R6a1R6) a2, ;
C1_6 alkyl, C2_6 alkenyl or C2_6 alkynyl, wherein C1_6 alkyl,
C2_6 alkenyl, and C2_6 alkynyl are optionally substituted with one or more
substituents selected
from the group consisting of halogen; CN; OR6a3; and A2a, wherein the
substituents are the
same or different;
R6ad, K-6a2
are independently selected from the group consisting of H; C1_6 alkyl; C2_6
alkenyl;
C2_6 alkynyl; and A2a, wherein C1_6 alkyl; C2_6 alkenyl; and C2_6 alkynyl are
optionally
substituted with one or more substituents selected from the group consisting
of halogen; CN;
OR6a3; 0A2a and A2a, wherein the substituents are the same or different;
R6a3 is H; or C1_4 alkyl, wherein C1_4 alkyl is optionally substituted with
one or more halogen,
which are the same or different;
A2a is
phenyl; C3_7 cycloalkyl; C4_12 bicycloalkyl; or 3- to 7-membered heterocyclyl,
wherein
A2a is optionally substituted with one or more R6, which are the same or
different;
5
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
each R6 is independently R6b; OH; OR6b; halogen; or CN, wherein R6b is
cyclopropyl, Ci_6
alkyl; C2_6 alkenyl; or C2_6 alkynyl, wherein R6b is optionally substituted
with one or more
halogen, which are the same or different; or
two R6 are joined to form together with the atoms to which they are attached a
ring A2b;
2b
A is phenyl; C3_7 cycloalkyl; or 3 to 7 membered heterocyclyl, wherein A2b is
optionally
substituted with one or more R7, which are the same or different;
each R7 is independently C1_6 alkyl, C2_6 alkenyl or C2_6 alkynyl, wherein C1-
6 alkyl, C2-6
alkenyl, and C2_6 alkynyl are optionally substituted with one or more halogen,
which are the
same or different;
RI is H or C1_4 alkyl, preferably H, wherein C1_4 alkyl is optionally
substituted with one or
more halogen, which are the same or different;
R2 is H; F; or C1_4 alkyl, wherein C1_4 alkyl is optionally substituted with
one or more halogen,
which are the same or different; and
R3 is A3, C1_6 alkyl, C2-6 alkenyl, or C2_6 alkynyl, wherein C1_6 alkyl, C2_6
alkenyl, and C2-6
alkynyl are optionally substituted with one or more R8, which are the same or
different; or
R2 and R3 are joined to form together with the oxygen atom and carbon atom to
which they
are attached a ring A3a, wherein A3a is a 7 to 12 membered heterobicyclyl,
wherein 7 to 12
membered heterobicyclyl is optionally substituted with one or more R10, which
are the same
or different;
R2a is H or F, preferably H;
each R8 is independently halogen; CN, C(0)0R9, OR9, C(0)R9, C(0)N(R9R9a),
S(0)2N(R9R9a), S(0)N(R9R9a), S(0)2R9, S(0)R9, N(R9)S(0)2N(R9aR9b), SR9,
N(R9R9a), NO2,
OC(0)R9, N(R9)C(0)R9a,
N(R9)S02R9a , N(R9)S(0)R9a,
N(R9)C(0)N(R9aR9b),
N(R9)C(0)0R9a, OC(0)N(R9R9a), or A3;
R9, R9a, R9b are independently selected from the group consisting of H, C1_6
alkyl, C2_6
alkenyl, and C2_6 alkynyl, wherein C1_6 alkyl, C2_6 alkenyl, and C2_6 alkynyl
are optionally
6
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
substituted with one or more halogen, which are the same or different, or one
OH, or one OCi _
4 alkyl, or one A3;
each A3 is independently phenyl, naphthyl, C3_7 cycloalkyl, 3 to 7 membered
heterocyclyl, or
7 to 12 membered heterobicyclyl, wherein A3 is optionally substituted with one
or more R1 ,
which are the same or different;
each R1 is independently halogen, CN, C(0)0R11, OR11, C(0)R11, C(0)N(R1 1R1
la),
S(0)2N(R
s(0)N(R11R11a), s(0)2R11, S(0)R", N(R11)s(0)2N(RlIaR1 lb ,
)
N(R11Rlla), NO2, OC(0)R11, N(R11)c(o)R1 la, N(R11)s(0)2R1 la, N(R11)s(0)R1la,
N(R1 1)C(0)0R1 1 a, N(R11)C(0)N(R1 1 aR1)OC(0)N(RIIRI
OX0 (=0) where the ring is at
least partially saturated, C1_6 alkyl, C2_6 alkenyl, or C2_6 alkynyl, wherein
C1_6 alkyl, C2-6
alkenyl, and C2_6 alkynyl are optionally substituted with one or more R12,
which are the same
or different;
R11, RI la, RI lb are independently selected from the group consisting of H,
C1_6 alkyl, C2_6
alkenyl, and C2_6 alkynyl, wherein C1_6 alkyl, C2_6 alkenyl, and C2_6 alkynyl
are optionally
substituted with one or more halogen, which are the same or different;
each R12 is independently halogen, CN, C(0)0R13, OR13, C(0)R13,
C(0)N(R13R13a),
S(0)2N(R13R13a), s(0)N(R13R13a), s(0)2R13, S(0)R'3, N(R13)s(0)2N(R13aRl3b
) SR13,
N(R13R13a),
NO2, OC(0)R13, N(R13)C(0)R13a, N(R13)S02R13a, N(R13)S(0)R13a,
N(R13)C(0)N(R13aRl3b), N(R13)C(0)0R13a, or OC(0)N(R13R13a);
R13, R13a, R131 are independently selected from the group consisting of H,
C1_6 alkyl, C2_6
alkenyl, and C2_6 alkynyl, wherein C1_6 alkyl, C2_6 alkenyl, and C2_6 alkynyl
are optionally
substituted with one or more halogen, which are the same or different.
A compound not restricted to the use as a medicament as defined above with
preferences as
defined below and a pharmaceutically acceptable salt, solvate, hydrate,
tautomer or
stereoisomer thereof, is also within the scope of the present invention
provided that the
following compounds or a pharmaceutically acceptable salt, solvate, hydrate,
tautomer or
stereoisomer thereof are excluded:
7
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
o-CH =
7---\ 3
HN.--C 0 HN
---, 0 0 N ..( \ 0
I
N-0 N-0 I CH3
CH3
CAS 1396491-78-3, CAS 1212733-
42-0,
CH3HNO =
_ 7----
0
N &
) 0 HN 0
F 1
-..3,7(
F' \
F
N-0
CH3
N-0 I
CH3
CAS 1212728-70-5, CAS 1212723-
70-0,
,CH3 / CH3/
0 IN7 HN¨C HN
N \ 0
H3C....I,,,,&N) 0
N
N-0 I N-0 I
cH3 CH3
CAS 1212688-74-8, CAS 1212685-
85-2,
OH
0/< 111
HN--(--- HN....\C-0
H3C.7 N &N) 0 0 /
\ 7"" N \ N iN
N-0 N/
CH3 N-0 I
CH3
CAS 1212683-11-8, CAS 1212664-
04-4,
cH3 cH3
HN_.....\C-0
/
) 0 CI
,N ,& ) 0
N \ "". N
H-O I H-O I
CH3 CH3
CAS 1212658-99-5, CAS 1212634-69-9,
= *
CI ,HNO
0 ,HNO
N &N) F/ N .& )
N-0 I N-0 I
CH3 CH3
CAS 1212633-89-0, CAS 1212628-
07-3,
8
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
111
HN
H3C H3C
N
H3C 0 "\rµ NZ' o
N-0 N-0
CH, CH,
CAS 1212619-73-2, CAS 1212618-04-6,
H3C\ CH3
0 HN
/
N
0
N
N-0
CH3
CAS 1212567-76-4, CAS 1212561-17-5,
111OH
HN H3C NN/ _________________________________ HN--C
0
..._ N
N-0
N-0 CH3
CH,
CAS 1212554-50-1, CAS 1212507-42-0.
The excluded compounds represent commercial compounds without indication of
the use.
The present invention also provides preferred compounds of formula (I) or a
pharmaceutically
acceptable salt, solvate, hydrate, tautomer or stereoisomer thereof for use as
a medicament,
wherein
X1 is N(R);
)(la is a covalent single bond, CH(Ra3), or CH(Ra3)CH2;
Rai is H, C(0)0C1_4 alkyl, or C1_4 alkyl, wherein C(0)0C1_4 alkyl and C1_4
alkyl are optionally
substituted with one or more substituents selected from the group consisting
of halogen, OH,
and 0-C1_3 alkyl, wherein the substituents are the same or different;
Ra2, a3
K are independently selected from the group consisting of H; OH;
0C1_4 alkyl; halogen;
C1_4 alkyl; and A2a; and
9
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Ra4; Ra5; Ra6; Ra7 are independently selected from the group consisting of H;
halogen; C1_4
alkyl; and A2a,
provided that only one of Ra2, Ra3; Ra4; Ras; Ra6; Ra7 is A2a;
or Ral and one of Ra2 and Ra3 form a methylene or ethylene group;
or Ral and Ra6 form an ethylene group;
or Ra2 and Ras form a covalent single bond;
or RaS, Ra7 are joined to form an oxo group;
Al is C5 cycloalkylene, C5 cycloalkenylene, or a nitrogen ring atom containing
5-membered
heterocyclene, wherein Al is optionally substituted with one or more R4, which
are the same
or different;
each R4 is independently oxo (=0) where the ring is at least partially
saturated, thiooxo (=S)
where the ring is at least partially saturated, halogen, CN, OR5, or C1_6
alkyl, wherein C1_6
alkyl is optionally substituted with one or more halogen, which are the same
or different;
R5 is H or Ci_6 alkyl, wherein C1_6 alkyl is optionally substituted with one
or more halogen,
which are the same or different;
A2 is R6a or A2a;
R6a is 0R6al, sR6a1; N(R6a)1R6a2µ;
C1_6 alkyl, C2_6 alkenyl or C2_6 alkynyl, wherein C1_6 alkyl,
C2_6 alkenyl, and C2_6 alkynyl are optionally substituted with one or more
substituents selected
from the group consisting of halogen; CN; OR6a3; and A2a, wherein the
substituents are the
same or different;
R6a1; 6
R-a- are independently selected from the group consisting of H; C1_6 alkyl;
C2_6 alkenyl;
C2_6 alkynyl; and A2a, wherein C1_6 alkyl; C2_6 alkenyl; and C2_6 alkynyl are
optionally
substituted with one or more substituents selected from the group consisting
of halogen; CN;
OR6a3; and A2a, wherein the substituents are the same or different;
R6a3 is H; or C1_4 alkyl, wherein C1_4 alkyl is optionally substituted with
one or more halogen,
which are the same or different;
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
A2a is
phenyl; C3_7 cycloalkyl; C4_12 bicycloalkyl; or 3- to 7-membered heterocyclyl,
wherein
A2a is optionally substituted with one or more R6, which are the same or
different;
each R6 is independently R6b; OH; OR61; halogen; or CN, wherein R61 is
cyclopropyl, C1_6
alkyl; C2_6 alkenyl; or C2,6 alkynyl, wherein R6b is optionally substituted
with one or more
halogen, which are the same or different; or
two R6 are joined to form together with the atoms to which they are attached a
ring A2b;
2b
A is phenyl; C3,7 cycloalkyl; or 3 to 7 membered heterocyclyl, wherein A2b is
optionally
substituted with one or more R7, which are the same or different;
each R7 is independently C1_6 alkyl, C2_6 alkenyl or C2_6 alkynyl, wherein
C1_6 alkyl, C2-6
alkenyl, and C2,6 alkynyl are optionally substituted with one or more halogen,
which are the
same or different;
R1 is H or C1_4 alkyl, preferably H, wherein C1_4 alkyl is optionally
substituted with one or
more halogen, which are the same or different;
R2 is H; F; or C1_4 alkyl, wherein C1_4 alkyl is optionally substituted with
one or more halogen,
which are the same or different; and
R3 is A3, C1_6 alkyl, C2_6 alkenyl, or C2_6 alkynyl, wherein C1_6 alkyl, C2_6
alkenyl, and C2-6
alkynyl are optionally substituted with one or more R8, which are the same or
different; or
R2 and R3 are joined to form together with the oxygen atom and carbon atom to
which they
are attached a ring A3a, wherein A3a is a 7 to 12 membered heterobicyclyl,
wherein 7 to 12
membered heterobicyclyl is optionally substituted with one or more R1 , which
are the same
or different;
R2a is H or F, preferably H;
each R8 is independently halogen; CN, C(0)0R9, OR9, C(0)R9, C(0)N(R9R9a),
S(0)2N(R9R9a), S(0)N(R9R9a), S(0)2R9, S(0)R9, N(R9)S(0)2N(R9aR9b), SR9,
N(R9R9a), NO2,
OC(0)R9, N(R9)C(0)R9a, N(R9)S02R9a, N(R9)S(0)R9a, N(R9)C(0)N(R9aR9b),
N(R9)C(0)0R9a, OC(0)N(R9R9a), or A3;
11
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
R9, R9a, R9b are independently selected from the group consisting of H, C1,6
alkyl, C2_6
alkenyl, and C2_6 alkynyl, wherein C1_6 alkyl, C2_6 alkenyl, and C2_6 alkynyl
are optionally
substituted with one or more halogen, which are the same or different, or one
OH, or one OCi_
4 alkyl, or one A3;
each A3 is independently phenyl, naphthyl, C3_7 cycloalkyl, 3 to 7 membered
heterocyclyl, or
7 to 12 membered heterobicyclyl, wherein A3 is optionally substituted with one
or more R10
,
which are the same or different;
each R1 is independently halogen, CN, C(0)0R11, OR", C(0)R11, C(0)N(RIIRIla),
S(0)2N(R11R1 S(0)N(R11R1 S(0)2R11, S(0)R1 1, N(R11)S(0)2N(R1 11R1 lb
) SR",
N(R11R11 a), NO2, OC(0)R11, N(R11)C(0)R11 N(R11)S(0)2R11
N(R11)S(0)R11 a,
N(R11)C(0)0Rila, N(R11)C(0)N(R1 laR1 0c(0)N(R11R1 la\
) OX0 (=0) where the ring is at
least partially saturated, C1_6 alkyl, C2,6 alkenyl, or C2_6 alkynyl, wherein
CI _6 alkyl, C2-6
alkenyl, and C2_6 alkynyl are optionally substituted with one or more R12,
which are the same
or different;
-11b
K
are independently selected from the group consisting of H, C1_6 alkyl, C2_6
alkenyl, and C2_6 alkynyl, wherein C1_6 alkyl, C2_6 alkenyl, and C2_6 alkynyl
are optionally
substituted with one or more halogen, which are the same or different;
each R12 is independently halogen, CN, C(0)0R13, OR13, C(0)R13,
C(0)N(R13R13a),
S(0)2N(R13R13a), S(0)N(R13R13a), S(0)2R13, S(0)R13, N(R13)S(0)2N(R131Rl3b
)
SR13,
N(R13R13a), NO2, OC(0)R13, N(R13)C(0)R13a, N(R13)S02R13a, N(R13)S(0)R13a,
N(R13)C(0)N(Ri3aRi3b), N.-(K 13
)C(0)0R13a, or OC(0)N(Ri3Roa);
R13, R13a, R13b are independently selected from the group consisting of H, Ch6
alkyl, C2_6
alkenyl, and C2_6 alkynyl, wherein C1_6 alkyl, C2_6 alkenyl, and C2_6 alkynyl
are optionally
substituted with one or more halogen, which are the same or different.
The present invention also provides preferred compounds of formula (I),
wherein Xia is
CH(Ra3), Ra7 and R2a are H and thus having formula (I-1)
12
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Ra5 X1 AlA2
0 Ra4
RN Ra3
Da6
R2 Ri Ra2
(I-1)
or a pharmaceutically acceptable salt, solvate, hydrate, tautomer or
stereoisomer thereof,
wherein
X1 is N(R);
Ral is H, or Ci_4 alkyl, wherein C1_4 alkyl is optionally substituted with one
or more
substituents selected from the group consisting of halogen, OH, and 0-C1_3
alkyl, wherein the
substituents are the same or different, preferably Rai is H; and
Ra2, Ra3, Ra4, Ra5, Ra6 are H;
or Ral and one of Ra2 and Ra3 form a methylene or ethylene group;
or Rai and Ra6 form an ethylene group;
A1 is C5 cycloalkylene, C5 cycloalkenylene, or a nitrogen ring atom containing
5-membered
heterocyclene, wherein Al is optionally substituted with one or more R4, which
are the same
or different;
each R4 is independently halogen, CN, OR5, oxo (=0) where the ring is at least
partially
saturated or C1_6 alkyl, wherein C1_6 alkyl is optionally substituted with one
or more halogen,
which are the same or different;
R5 is H or Ch6 alkyl, wherein C1_6 alkyl is optionally substituted with one or
more halogen,
which are the same or different;
A2 is phenyl or 5- to 6-membered aromatic heterocyclyl, wherein A2 is
optionally substituted
with one or more R6, which are the same or different;
13
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
each R6 is independently OH, 0(C1_6 alkyl), halogen, CN, cyclopropyl, C1_6
alkyl, C2-6
alkenyl, or C2_6 alkynyl, wherein cyclopropyl, C1_6 alkyl, C2_6 alkenyl, and
C2_6 alkynyl are
optionally substituted with one or more halogen, which are the same or
different; or
two R6 are joined to form together with atoms to which they are attached a
ring A2b;
2b
A is phenyl; C3,7 cycloalkyl; or 3 to 7 membered heterocyclyl, wherein A2b is
optionally
substituted with one or more R7, which are the same or different;
each R7 is independently C1_6 alkyl, C2,6 alkenyl or C2,6 alkynyl, wherein
Ci_6 alkyl, C2-6
alkenyl, and C2,6 alkynyl are optionally substituted with one or more halogen,
which are the
same or different;
RI is H or C1_4 alkyl, preferably H, wherein C1_4 alkyl is optionally
substituted with one or
more halogen, which are the same or different;
R2 is H or C1_4 alkyl, wherein C1_4 alkyl is optionally substituted with one
or more halogen,
which are the same or different; and
R3 is A3, C1_6 alkyl, C2-6 alkenyl, or C2,6 alkynyl, wherein C1_6 alkyl, C2,6
alkenyl, and C2-6
alkynyl are optionally substituted with one or more R8, which are the same or
different; or
R2 and R3 are joined to form a 7 to 12 membered heterobicyclyl, wherein 7 to
12 membered
heterobicyclyl is optionally substituted with one or more RI , which are the
same or different;
each R8 is independently halogen; CN, C(0)0R9, OR9, C(0)R9, C(0)N(R9R9a),
S(0)2N(R9R9a), S(0)N(R9R9a), S(0)2R9, S(0)R9, N(R9)S(0)2N(R9aR9b), SR9,
N(R9R9a), NO2,
OC(0)R9, N(R9)C(0)R9a, 9
N(R9 )S02Ra , 9
N(R )S(0)R9a ,
N(R9)C(0)N(R9aR9b),
N(R9)C(0)0R9a, OC(0)N(R9R9a), or A3;
R9, R9a, R91' are independently selected from the group consisting of H, C1_6
alkyl, C2_6
alkenyl, and C2_6 alkynyl, wherein C1,6 alkyl, C2,6 alkenyl, and C2,6 alkynyl
are optionally
substituted with one or more halogen, which are the same or different;
each A3 is independently phenyl, naphthyl, C3_7 cycloalkyl, 3 to 7 membered
heterocyclyl, or
7 to 12 membered heterobicyclyl, wherein A3 is optionally substituted with one
or more R11),
which are the same or different;
14
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
each R1 is independently halogen, CN, C(0)0R11, OR11, C(0)R11, C(0)N(R1 1R1
la),
S(0)2N(R11R11 a), S(0)N(R11R11 a), S(0)2R11, S(0)R11, N(R11)S(0)2N(Ri 1 1R1 1
b
) SR",
N(R11R1 ,
)
NO2, OC(0)R11, N(R11)C(0)R1 la, N(R11)S(0)2R1 la, N(R11)S(0)R1 la,
N(R11)C(0)0Rila, N(R11)C(0)N(R1 laR1 oc(o)N(R11R1 la), OX0 (=0) where the
ring is at
least partially saturated, C1_6 alkyl, C2,6 alkenyl, or C2_6 alkynyl, wherein
C _6 alkyl, C2-6
alkenyl, and C2_6 alkynyl are optionally substituted with one or more R12,
which are the same
or different;
R", R1 la, R1 lb are independently selected from the group consisting of H,
C1,6 alkyl, C2_6
alkenyl, and C2_6 alkynyl, wherein C1_6 alkyl, C2_6 alkenyl, and C2_6 alkynyl
are optionally
substituted with one or more halogen, which are the same or different;
each R12 is independently halogen, CN, C(0)0R13, OR13, C(0)R13,
C(0)N(Rt3R13a),
S(0)2N(R13R13a), S(0)N(R13R13a), S(0)2R13, S(0)R13, N(R13)S(0)2N(R131Rl3b
)
SR13,
N(R13R13a), NO2, OC(0)R13, N(R13)C(0)R13a, N(R13)S02R13a, N(R13)S(0)R13a,
N(R13)C(0)N(R13aR1311), Ny¨(K 13
)C(0)0R13a, or OC(0)N(R13R13a);
R13, R13a, R13b are independently selected from the group consisting of H,
C1_6 alkyl, C2_6
alkenyl, and C2_6 alkynyl, wherein C1_6 alkyl, C2_6 alkenyl, and C2_6 alkynyl
are optionally
substituted with one or more halogen, which are the same or different.
Surprisingly, the disclosed example compounds according to the present
invention have
favourable physico-chemical properties and/or selectivity, which combine to
help to achieve
.. beneficial therapeutic efficacy whilst limiting unintended liabilities.
In case a variable or substituent can be selected from a group of different
variants and such
variable or substituent occurs more than once the respective variants can be
the same or
different.
Within the meaning of the present invention the terms are used as follows:
The term "optionally substituted" means unsubstituted or substituted.
Generally -but not
limited to-, "one or more substituents" means one, two or three, preferably
one or two
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
substituents and more preferably one substituent. Generally these substituents
can be the same
or different. The term "one or more substituents" also means by way of example
one, two,
three, four or five, preferanbly by way of example one, two, three or four.
"Alkyl" means a straight-chain or branched hydrocarbon chain. Each hydrogen of
an alkyl
carbon may be replaced by a substituent as further specified.
"Alkenyl" means a straight-chain or branched hydrocarbon chain that contains
at least one
carbon-carbon double bond. Each hydrogen of an alkenyl carbon may be replaced
by a
substituent as further specified.
"Alkynyl" means a straight-chain or branched hydrocarbon chain that contains
at least one
carbon-carbon triple bond. Each hydrogen of an alkynyl carbon may be replaced
by a
substituent as further specified.
"C1_4 alkyl" means an alkyl chain having 1 - 4 carbon atoms, e.g. if present
at the end of a
molecule: methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl,
tert-butyl, or e.g. -
CH2-, -CH2-CH2-, -CH(CH3)-, -CH2-CH2-CH2-, -CH(C2H5)-, -C(CH3)2-, when two
moieties
of a molecule are linked by the alkyl group. Each hydrogen of a C1_4 alkyl
carbon may be
replaced by a substituent as further specified. The term "C1_3 alkyl" is
defined accordingly.
"C1_6 alkyl" means an alkyl chain having 1 - 6 carbon atoms, e.g. if present
at the end of a
molecule: C1_4 alkyl, methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl,
sec-butyl, tert-
butyl, n-pentyl, n-hexyl, or e.g. -CH2-, -CH2-CH2-, -CH(CH3)-, -CH2-CH2-CH2-, -
CH(C2H5)-,
-C(CH3)2-, when two moieties of a molecule are linked by the alkyl group. Each
hydrogen of
a Ci _6 alkyl carbon may be replaced by a substituent as further specified.
"C2_6 alkenyl" means an alkenyl chain having 2 to 6 carbon atoms, e.g. if
present at the end of
a molecule: -CH=CH2, -CH=CH-CH3, -CH2-CH=CH2, -CH=CH-CH2-CH3, -CH=CH-
CH=CH2, or e.g. -CH=CH-, when two moieties of a molecule are linked by the
alkenyl group.
Each hydrogen of a C2_6 alkenyl carbon may be replaced by a substituent as
further specified.
"C2_6 alkynyl" means an alkynyl chain having 2 to 6 carbon atoms, e.g. if
present at the end of
a molecule: -CCH, -CH2-CCH, CH2-CH2-CCH, CH2-CC-CH3, or e.g. -CC- when two
16
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
moieties of a molecule are linked by the alkynyl group. Each hydrogen of a
C2_6 alkynyl
carbon may be replaced by a substituent as further specified.
"C3_7 cycloalkyl" or "C3_7 cycloalkyl ring" means a cyclic alkyl chain having
3 - 7 carbon
atoms, e.g. cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cyclohexenyl,
cycloheptyl.
Preferably, cycloalkyl refers to cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, or
cycloheptyl. Each hydrogen of a cycloalkyl carbon may be replaced by a
substituent as further
specified herein. The term "C3_5 cycloalkyl" or "C3_5 cycloalkyl ring" is
defined accordingly.
"C5 cycloalkylene" refers to a bivalent cycloalkyl with five carbon atoms,
i.e. a bivalent
cyclopentyl ring.
"C5 cycloalkenylene" refers to a bivalent cycloalkenylene, i.e. a bivalent
cyclopentene or
cyclopentadiene.
"C4_12 bicycloalkyl" or "C4_12 bicycloalkyl ring" means a bicyclic fused,
bridged or spiro alkyl
chain having 4 to 12 carbon atoms, e.g. hexahydroindane, Octahydropentalen,
bicycle[2.2.1]heptane or spiro(3.2)hexane. Each hydrogen of a bicycloalkyl
carbon may be
replaced by a substituent as further specified herein.
"Halogen" means fluoro, chloro, bromo or iodo. It is generally preferred that
halogen is fluoro
or chloro.
"3 to 7 membered heterocycly1" or "3 to 7 membered heterocycle" means a ring
with 3, 4, 5, 6
or 7 ring atoms that may contain up to the maximum number of double bonds
(aromatic or
non-aromatic ring which is fully, partially or un-saturated) wherein at least
one ring atom up
to 4 ring atoms are replaced by a heteroatom selected from the group
consisting of sulfur
(including -S(0)-, -S(0)2-), oxygen and nitrogen (including =N(0)-) and
wherein the ring is
linked to the rest of the molecule via a carbon or nitrogen atom. Examples for
a 3 to 7
.. membered heterocycle are aziridine, azetidine, oxetane, thietane, furan,
thiophene, pyrrole,
pyrroline, imidazole, imidazoline, pyrazole, pyrazoline, oxazole, oxazoline,
isoxazole,
isoxazoline, thiazole, thiazoline, isothiazole, isothiazoline, thiadiazole,
thiadiazoline,
tetrahydrofuran, tetrahydrothiophene, pyrrolidine, imidazolidine,
pyrazolidine, oxazolidine,
isoxazolidine, thiazolidine, isothiazolidine, thiadiazolidine, sulfolane,
pyran, dihydropyran,
17
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
tetrahydropyran, imidazolidine, pyridine, pyridazine, pyrazine, pyrimidine,
piperazine,
piperidine, morpholine, tetrazole, triazole, triazolidine, tetrazolidine,
diazepane, azepine or
homopiperazine. The term "5 to 6 membered heterocyclyl" or "5 to 6 membered
heterocycle"
is defined accordingly and and includes 5 to 6 membered aromatic heterocyclyl
or
heterocycle. The term "5 membered heterocyclyl" or "5 membered heterocycle" is
defined
accordingly and includes 5 membered aromatic heterocyclyl or heterocycle.
The term "nitrogen ring atom containing 5-membered heterocyclene" refers to a
bivalent 5-
membered heterocycle, wherein at least one of the five ring atoms is a
nitrogen atom and
wherein the ring is linked to the rest of the molecule via a carbon or
nitrogen atom.
"Saturated 4 to 7 membered heterocyclyl" or "saturated 4 to 7 membered
heterocycle" means
fully saturated "4 to 7 membered heterocyclyl" or "4 to 7 membered
heterocycle".
"4 to 7 membered at least partly saturated heterocyclyl" or "4 to 7 membered
at least partly
saturated heterocycle" means an at least partly saturated "4 to 7 membered
heterocyclyl" or "4
to 7 membered heterocycle".
"5 to 6 membered aromatic heterocyclyl" or "5 to 6 membered aromatic
heterocycle" means a
heterocycle derived from cyclopentadienyl or benzene, where at least one
carbon atom is
replaced by a heteroatom selected from the group consisting of sulfur
(including -S(0)-, -
S(0)2-), oxygen and nitrogen (including =N(0)-). Examples for such
heterocycles are furan,
thiophene, pyrrole, imidazole, pyrazole, oxazole, isoxazole, thiazole,
isothiazole, thiadiazole,
triazole, tetrazole, pyridine, pyrimidine, pyridazine, pyrazine, triazine.
"5 membered aromatic heterocyclyl" or "5 membered aromatic heterocycle" means
a
heterocycle derived from cyclopentadienyl, where at least one carbon atom is
replaced by a
heteroatom selected from the group consisting of sulfur (including -5(0)-, -
S(0)2-), oxygen
and nitrogen (including =N(0)-). Examples for such heterocycles are furan,
thiophene,
pyrrole, imidazole, pyrazole, oxazole, isoxazole, thiazole, isothiazole,
thiadiazole, triazole,
tetrazole.
"7 to 12 membered heterobicycly1" or "7 to 12 membered heterobicycle" means a
heterocyclic system of two rings with 7 to 12 ring atoms, where at least one
ring atom is
18
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
shared by both rings and that may contain up to the maximum number of double
bonds
(aromatic or non-aromatic ring which is fully, partially or un-saturated)
wherein at least one
ring atom up to 6 ring atoms are replaced by a heteroatom selected from the
group consisting
of sulfur (including -S(0)-, -S(0)2-), oxygen and nitrogen (including =N(0)-)
and wherein the
ring is linked to the rest of the molecule via a carbon or nitrogen atom.
Examples for a 7 to 12
membered heterobicycle are indole, indoline, benzofuran, benzothiophene,
benzoxazole,
benzisoxazole, benzothiazole, benzisothiazole, benzimidazole, benzimidazoline,
quinoline,
quinazoline, dihydroquinazoline, quinoline, dihydroquinoline,
tetrahydroquinoline,
decahydroquinoline, isoquinoline, decahydroisoquino line,
tetrahydroisoquinoline,
dihydroisoquinoline, benzazepine, purine or pteridine. The term 7 to 12
membered
heterobicycle also includes spiro structures of two rings like 6-oxa-2-
azaspiro[3,4]octane, 2-
oxa-6-azaspiro[3.3]heptan-6-y1 or 2,6-diazaspiro[3.3]heptan-6-y1 or bridged
heterocycles like
8-aza-bicyclo [3 .2.1] octane or 2,5-diazabicyclo [2.2.2] octan-2-y1 or 3 ,8-
diazabicyclo [3 .2.1]
octane.
"Saturated 7 to 12 membered heterobicycly1" or "saturated 7 to 12 membered
heterobicycle"
means fully saturated "7 to 12 membered heterobicycly1" or "7 to 12 membered
heterobicycle".
"7 to 12 membered at least partly saturated heterobicycly1" or "7 to 12
membered at least
partly saturated heterobicycle" means an at least partly saturated "7 to 12
membered
heterobicycly1" or "7 to 12 membered heterobicycle".
"9 to 11 membered aromatic heterobicycly1" or "9 to 11 membered aromatic
heterobicycle"
means a heterocyclic system of two rings, wherein at least one ring is
aromatic and wherein
the heterocyclic ring system has 9 to 11 ring atoms, where two ring atoms are
shared by both
rings and that may contain up to the maximum number of double bonds (fully or
partially
aromatic) wherein at least one ring atom up to 6 ring atoms are replaced by a
heteroatom
selected from the group consisting of sulfur (including -S(0)-, -S(0)2-),
oxygen and nitrogen
(including =N(0)-) and wherein the ring is linked to the rest of the molecule
via a carbon or
nitrogen atom. Examples for an 9 to 11 membered aromatic heterobicycle are
indole,
indoline, benzofuran, benzothiophene, benzoxazole, benzisoxazole,
benzothiazole,
benzisothiazole, benzimidazole, benzimidazoline, quinoline, quinazoline,
dihydroquinazoline,
dihydroquinoline, tetrahydroquinoline, isoquinoline, tetrahydroisoquinoline,
dihydro-
19
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
isoquinoline, benzazepine, purine or pteridine. The terms "9 to 10 membered
aromatic
heterobicycly1" or "9 to 10 membered aromatic heterobicycle" are defined
accordingly.
Preferred compounds of formula (I) are those compounds in which one or more of
the
residues contained therein have the meanings given above or below, with all
combinations of
preferred substituent definitions being a subject of the present invention.
With respect to all
preferred compounds of the formula (I) the present invention also includes all
tautomeric and
stereoisomeric forms and mixtures thereof in all ratios, and their
pharmaceutically acceptable
salts.
In preferred embodiments of the present invention, the substituents mentioned
below
independently have the following meaning. Hence, one or more of these
substituents can have
the preferred or more preferred meanings given below.
Preferably, X1 is NH or N-C1_4 alkyl, wherein C1_4 alkyl is optionally
substituted with one or
more substituents selected from the group consisting of halogen, OH, and O-
C1_3 alkyl,
wherein the substituents are the same or different; more preferably X1 is NH,
N(CH3),
N(CH2CH3), or N(CH2CH2OCH3); even more preferably NH or N(CH3); even more
preferably NH.
Preferably, Xia is CH(Ra3) or CH(Ra3)CH2, even more preferably CH(Ra3).
Preferably, Ra2, Ra3 are independently selected from the group consisting of
H; OH; 0C1_4
alkyl; halogen; C1_4 alkyl; and A2a; and
Ra4, Ra5, Ra6, Ra7 are independently selected from the group consisting of H;
halogen; C1_4
alkyl; and A2a, provided that only one of Ra2, Ra3, Ra4, Ra5, Ra6, Ra7 is A2a;
or Ra5, Ra7 are joined to form an oxo group.
Preferably, Ra2, Ra3, Ra4, Ra5, Ra6, Ra7 are independently selected from the
group consisting of
H; halogen; C1_4 alkyl; and A2a, provided that only one of Ra2, Ra3, Ra4, Ra5,
Ra6, Ra7 is A2a.
More preferably, Ra2, Ra3, Ra4, Ra5, Ra6, Ra7 are H or Ra2, Ra3, Ra4, Ra6 are
H and Ras, Ra7 are
joined to form an oxo group. Even more preferably Ra2, Ra3, Ra4, Ras, Ra6, Ra7
are H.
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Preferably, Al is a nitrogen ring atom containing 5-membered heterocyclene and
wherein Al
is optionally substituted with one or more R4, which are the same or
different.
More preferaby, Al is a nitrogen ring atom containing 5-membered heterocyclene
selected
from the group of bivalent heterocycles consisting of oxadiazole, imidazole,
imidazolidine,
pyrazole and triazole, preferably oxadiazole, and wherein Al is optionally
substituted with
one or more R4, which are the same or different.
Preferably, Al is unsubstituted or substituted with one or two R4, which are
the same or
different, preferably Al is unsubstituted.
Preferably, R4 is independently oxo (=0) where the ring is at least partially
saturated,
halogen, CN, OR5 or C1_6 alkyl, wherein Ci_6 alkyl is optionally substituted
with one or more
halogen, which are the same or different.
Preferably, R4 is oxo, where the ring is at least partly saturated.
Preferably, Al is
N=N
...:õ.... 3......,!:... >14 3....õ... ....,.:-NN7N..,..:1_, .2,r.s N z
,'....._
0
0
\ N
= \ N = N
>c) ,
_
,-s N
2,--/4 .......,L.
\ N /
0 0
c:\ ,
N N.7:......
or 0 .
More preferably, A1 is
21
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
N¨N
0 '
In one embodiment A2 is R6a.
Preferably, R6a is OR6a1
In an embodiment, R6al, R6a2 are independently selected from the group
consisting of H; C1,6
alkyl; C2_6 alkenyl; C2_6 alkynyl; and A2a, wherein C1,6 alkyl; C2_6 alkenyl;
and C2_6 alkynyl are
optionally substituted with one or more substituents selected from the group
consisting of
halogen; CN; OR6a3; and A2a, wherein the substituents are the same or
different.
R6a1
is preferably A2a or C1_6 alkyl, optionally substituted with one or more
halogen and/or
one A2a and/or one OR6a3. More preferably, R6al is C1,6 alkyl, optionally
substituted with one
or more F and/or one OR6a3.
Preferably, R6a is C1_6 alkyl, optionally substituted with one or more halogen
and/or one A2a
and/or one OR6a3. More preferably, R6a is C1_6 alkyl, optionally substituted
with one or more
halogen and/or one OR6a3.
In one preferred embodiment R6al is unsubstituted C4_6 alkyl; more preferably
3-methylbut-
1y1 or n-butyl. In another preferred embodiment R6al is C2_6 alkyl,
substituted with one or
more halogen, which are the same or different, preferably one or more fluoro;
more preferably
¨6a1
x is 3,3,3-trifluoropropyl, 2-methyl-3,3,3-trifluoropropyl, 4,4,4-trifluorobut-
2-yl, 2,2,3,3,3-
pentafluoropropyl, 3,3-difluorobutyl or 3,3,3-trifluorobutyl. In another
preferred embodiment
R6al is Aza, 012A 2a,
CH2CH2A2a, wherein A2a is unsubstituted or substituted with one or more
halogen, which are the same or different, preferably one or more fluoro; more
preferably R6a1
is cyclobutyl, cyclopentyl, CH2-cyclopropyl, CH2-cyclobutyl, CH2CH2-
cyclopropyl, wherein
R6a1 is substituted with one or more F.
In a particularly preferred embodiment A2 is R6a, R6a is OR6a1 and R6a1 is
CH2CH2CF3 or
CH2CH2OCF3, preferably CH2CH2OCF3.
22
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Preferably, R6a2 is H.
Preferably, R6a is OC1_4 alkyl; 0C1_4 alkyl-OCi_4 alkyl, wherein each C1_4
alkyl is optionally
substituted with one to three F; or OCH2A2a.
In another embodiment A2 is A2a.
Preferably, A2a is phenyl; C3_7 cycloalkyl; or 3- to 7-membered heterocyclyl,
wherein A2a is
optionally substituted with one or more R6, which are the same or different.
Preferably, A2a is phenyl; cyclobutyl; azetidinyl; pyrrolidinyl; or 5- to 6-
membered aromatic
heterocyclyl, preferably pyridyl, pyrazinyl, pyridazinyl, pyrazolyl or 1,2,4-
oxadiazolyl,
wherein A2a is optionally substituted with one or more R6, which are the same
or different.
More preferably, A2a is phenyl, or 5- to 6-membered aromatic heterocyclyl,
preferably
pyridyl, pyrazinyl, pyridazinyl, pyrazolyl or 1,2,4-oxadiazolyl, wherein A2a
is optionally
substituted with one or more R6, which are the same or different.
Even more preferably, A2a is phenyl; cyclobutyl; pyridyl; azetidinyl;
pyrazolyl; or
pyrrolidinyl, wherein A2a is optionally substituted with one or more R6, which
are the same or
different.
Preferably, A2a is C3_7 cycloalkyl, more preferably cyclobutyl, wherein A2a is
optionally
substituted with one or more R6, which are the same or different.
Preferably, A2a is substituted with one or two R6, which are the same or
different.
Preferably, R6 is independently F, Cl, CF3, OCH3, OCF3, OCHF2, CH3, CH2CH3,
CH2CF3, 0-
cyclopropyl, or cyclopropyl. More preferably, R6 is independently F, Cl, CF3,
OCH3, OCF3,
CH3, CH2CH3, or cyclopropyl, preferably F, Cl, CF3, OCH3, CH3, CH2CH3, or
cyclopropyl.
Preferably, R2 is CH3; F; or H, more preferably H.
Preferably, R9, R9a, R9b are independently selected from the group consisting
of H, C1_6 alkyl,
C2_6 alkenyl, and C2_6 alkynyl, wherein C1_6 alkyl, C2_6 alkenyl, and C2_6
alkynyl are optionally
23
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
substituted with one or more halogen, which are the same or different, or one
OH, or one OCi_
4 alkyl, or one A3.
Preferably, R3 is A3.
Preferably, A3 is phenyl, pyridyl, pyrazinyl, pyrimidazyl, cyclopropyl,
cyclobutyl or
cyclohexyl, wherein A3 is optionally substituted with one or more R10, which
are the same or
different. More preferably, A3 is phenyl, wherein A3 is optionally substituted
with one or
more RI , which are the same or different.
Preferably, A3 is substituted with one, two or three, preferably one or two,
(more preferably
two) R10, which are the same or different.
Preferably, R2 and R3 are joined together with the oxygen and carbon atom to
which they are
attached to form a dihydrobenzopyran ring, wherein the ring is optionally
substituted with one
or more Rm, which are the same or different, preferably the ring is
substituted with one or two
RIO.
Preferably, R1 is independently F, Cl, Br, CN, CHF2, CF3, OCH3, OCF3, CH=0,
CH2OH or
CH3; preferably, F, Cl, Br, CF3, OCF3, CH=0, CH2OH or CH3, more preferably F,
Cl, CF3,
CH=0, CH2OH or CH3. More preferably, Rl is independently F or Cl.
Preferably, Rai is H, or C1_4 alkyl, wherein C1_4 alkyl is optionally
substituted with one or
more substituents selected from the group consisting of halogen, OH, and 0-
C1_3 alkyl,
wherein the substituents are the same or different; preferably Rai is H; CH3
or CH2CH3; more
preferably Rai is H.
Compounds of the formula (I) in which some or all of the above-mentioned
groups have the
preferred or more preferred meanings are also an object of the present
invention.
Preferred specific compounds of the present invention are selected from the
group consisting
of
tert-butyl (2R,5S)-5- [2-(4-chloro-3 -fluorophenoxy) acetamido] -2- {5- [4-
(tri fluoromethyl)phenyl] -1,3 ,4- oxadi azol-2-y1} pip eridine-1 - carboxyl
ate
24
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [4-(trifluoromethyl)phenyl] -
1,3 ,4-oxadiazol-
2-yllpiperidin-3-yl] acetamide
tert-butyl (2R,5S)-5-[2-(4-chlorophenoxy)propanamido] -2- {5 - [4-
(trifluoromethyl)phenyl] -
1,3 ,4-oxadiazol-2-y1} piperidine-1 -carboxylate
2-(4-chlorophenoxy)-N-[(3 S,6R)-6- { 5- [4-(trifluoromethyl)phenyl] -1,3 ,4-
oxadiazol-2-
yl } piperidin-3-yl]propanamide
N-[(3R,6S)-645-(4-chloropheny1)-1,3,4-oxadiazol-2-yl]piperidin-3-y1]-2-[(1s,3
s)-3-
(trifluoromethoxy)cyclobutoxy]acetamide
tert-butyl (2R,5S)-5- {2- [(6-chloro-5-fluoropyridin-3 -yl)oxy] acetamido } -2-
[5-(4-
chloropheny1)- 1,3 ,4-oxadiazol-2-yl]piperidine-1 -carboxylate
2- [(6-chloro-5-fluoropyridin-3 -yl)oxy]-N-R3 S ,6R)-6- [5 -(4-chloropheny1)-
1,3 ,4-oxadiazol-2-
yl]piperidin-3-yl] acetamide
tert-butyl (2R,55)-5-[2-(3 ,4-dichlorophenoxy)acetamido] -2- {5-[(1 s,3 s)-3 -
(trifluoromethoxy)cyclobutyl] -1,3 ,4-oxadiazol-2-y1} piperidine- 1 -
carboxylate
2-(3,4-dichlorophenoxy)-N-[(3S,6R)-6- {5 - [(1 s,3 s)-3 -
(trifluoromethoxy)cyclobutyl] -1,3 ,4-
oxadiazol-2-yllpiperidin-3 -yl] acetamide
2- [3 -chloro-4-(trifluoromethyl)phenoxy] -N-[(3 S,6R)-6- { 5- R 1 s,3 s)-3 -
(trifluoromethoxy)cyclobutyl] -1,3 ,4-oxadiazol-2-y1} piperidin-3-yl]acetamide
2- [4-chloro-3 -(difluoromethyl)phenoxy]-N-R3 S,6R)-6- {5 - [2-
(trifluoromethoxy)ethoxy] -
1,3 ,4-oxadiazol-2-ylf piperidin-3 -yllacetamide
2-(4-chloro-3-methylphenoxy)-N-[(3 S,6R)-6- { 5- [2-(trifluoromethoxy)ethoxy] -
1,3 ,4-
oxadiazol-2-yll piperidin-3-yl]acetamide
2-(3,4-dimethylphenoxy)-N-[(3S,6R)-6- {5 - [2-(trifluoromethoxy)ethoxy] -1,3
,4-oxadiazol-2-
yl } piperidin-3-yl] acetamide
N-[(3 S,6R)-6- {5[2-(trifluoromethoxy)ethoxy] -1,3 ,4-oxadiazol-2-y1}
piperidin-3-y1]-2- { [6-
(trifluoromethyppyridin-3 -yl]oxy} acetamide
2- [3 -methoxy-4-(trifluoromethyl)phenoxy] -N- [(3 S,6R)-6- {5 - [2-
(trifluoromethoxy)ethoxy] -
1,3 ,4-oxadiazol-2-ylf piperidin-3 -yllacetamide
tert-butyl (2R,5S)-5-[2-(4-chloro-2-fluorophenoxy)acetamido] -2- [5-(3 ,3 ,3 -
trifluoropropoxy)-
1,3 ,4-oxadiazol-2-yl]piperidine-1 -carboxylate
2-(4-chloro-2-fluorophenoxy)-N-[(3 S ,6R)-6- [5 -(3 ,3 ,3 -trifluoropropoxy)-
1,3 ,4-oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(3 -chloro-4-fluorophenoxy)-N-[(3 S,6R)-6-[5-(3 ,3 ,3 -trifluoropropoxy)-
1,3 ,4-oxadiazol-2-
yl]piperidin-3-yl]acetamide
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
2-[4-(trifluoromethyl)phenoxy]-N-R3S,6R)-6-[5-(3,3,3-trifluoropropoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(3,4-dichlorophenoxy)-N-[(3S,6R)-6-[5-(3,3,3-trifluoropropoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-2,3-difluorophenoxy)-N-[(3S,6R)-645-(3,3,3-trifluoropropoxy)-1,3,4-
oxadiazol-
2-yl]piperidin-3-yl]acetamide
2-(4-chloro-3,5-difluorophenoxy)-N-[(3S,6R)-6-[5-(3,3,3-trifluoropropoxy)-
1,3,4-oxadiazol-
2-yl]piperidin-3-yllacetamide
2-[3-fluoro-4-(trifluoromethyl)phenoxy]-N-R3S,6R)-6-[5-(3,3,3-
trifluoropropoxy)-1,3,4-
oxadiazol-2-yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-2,2-difluoro-N-[(3S,6R)-6-[5-(3,3,3-
trifluoropropoxy)-1,3,4-
oxadiazol-2-yl]piperidin-3-yllacetamide
2-[3-chloro-4-(trifluoromethyl)phenoxy]-N-[(3S,6R)-6-[5-(3,3,3-
trifluoropropoxy)-1,3,4-
oxadiazol-2-yl]piperidin-3-yl]acetamide
2-(3,4,5-trichlorophenoxy)-N-[(3S,6R)-6-[5-(3,3,3-trifluoropropoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-bromophenoxy)-N-[(3S,6R)-6-[5-(3,3,3-trifluoropropoxy)-1,3,4-oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-[3-(trifluoromethyl)phenoxy]-N-R3S,6R)-6-[5-(3,3,3-trifluoropropoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-cyanophenoxy)-N-[(3S,6R)-6-{5-[2-(trifluoromethoxy)ethoxy]-1,3,4-
oxadiazol-2-yllpiperidin-3-yl]acetamide
tert-butyl (2R,45)-4-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-[5-(4-
chloropheny1)-1,3,4-
oxadiazol-2-yl]pyrrolidine-1-carboxylate
2-(4-chloro-3-fluorophenoxy)-N-[(3S,5R)-5-[5-(4-chloropheny1)-1,3,4-oxadiazol-
2-
yl]pyrrolidin-3-yl]acetamide
tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-[5-(3,3,3-
trifluoropropoxy)-
1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3,3,3-trifluoropropoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(6-methylpyridin-3-y1)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3R,65)-6-[5-(4-chloropheny1)-1,3,4-oxadiazol-
2-
yl]piperidin-3-yl]acetamide
26
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
2-(4-chloro-3 -fluorophenoxy)-N- [(3R,6R)-6- [5-(4-chloropheny1)- 1,3 ,4-
oxadiazol-2-
yl]piperidin-3 -yl] acetamide
rac-2-(4-chloro-3 -fluorophenoxy)-N- [(3R,6 S)-6- [5 -(4-chloropheny1)- 1,3 ,4-
oxadiazol-2-yl] -2-
oxopiperidin-3 -yl] acetamide
rac-2-(4-chloro-3-fluorophenoxy)-N-[(3R,6R)-2-oxo-6- {5-[(1 s,3 s)-3 -
(trifluoromethoxy)cyclobutyl] -1,3 ,4-oxadiazol-2-y1} piperidin-3-yl]acetamide
tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido] -2- [5 -(4-
chloropheny1)- 1 ,3 ,4-
oxadiazol-2-yl]piperidine- 1 -carboxylate
2-(4-chloro-3 -fluorophenoxy)-N-[(3 S ,6R)-6- [5-(4-chloropheny1)- 1,3 ,4-
oxadiazol-2-
1 0 .. yl]piperidin-3-yl]acetamide
tert-butyl (2S ,5R)-5 - [2-(4-chloro-3 -fluorophenoxy)acetamido] -2- [5 -(3 ,3
,3 -trifluoropropoxy)-
1 ,3 ,4-oxadiazol-2-yl]piperidine- 1 -carboxylate
2-(4-chloro-3 -fluorophenoxy)-N- [(3R,6 S)-6- [5-(3 ,3 ,3 -trifluoropropoxy)-
1,3 ,4-oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3 -fluorophenoxy)-N-[(3R,6S)-6- {5 - [3 -
(trifluoromethoxy)azetidin- 1 -yl] - 1 ,3 ,4-
oxadiazol-2-yll piperidin-3-yl]acetamide
2-(4-chloro-3 -fluorophenoxy)-N- [(3R,65)-6- [5-(4-chloropheny1)- 1,3 ,4-
oxadiazol-2-yl] - 1 -
methylpiperidin-3 -yl] acetamide
2-(4-chloro-3 -fluorophenoxy)-N-[(3 S ,6R)-6- [5 -(4-chloropheny1)- 1,3 ,4-
oxadiazol-2-yl] -1-
.. methylpiperidin-3-yl]acetamide
2-(4-chloro-3 -fluorophenoxy)-N-[(3 S ,6R)-6- [5-(4-chloropheny1)- 1,3 ,4-
oxadiazol-2-yl] - 1 -
ethylpiperidin-3 -yl] acetamide
2-(4-chloro-3 -fluorophenoxy)-N- [(3R,6 S)-6- [5 -(4-chloropheny1)- 1,3 ,4-
oxadiazol-2-yl] - 1 -
ethylpiperidin-3 -yl] acetamide
2-(4-chloro-3 -fluorophenoxy)-N- [(3R,65)-6- [5-(4-chloropheny1)- 1,3 ,4-
oxadiazol-2-yl] - 1 -(2-
methoxyethyl)piperidin-3 -yl] acetamide
tert-butyl (2R,5 5)-5 - [2-(4-chloro-3 -fluorophenoxy)acetamido] -2- {5 - [(1
s,3 s)-3 -
(trifluoromethoxy)cyclobutyl] -1,3 ,4-oxadiazol-2-ylf piperidine- 1 -
carboxylate
2-(4-chloro-3 -fluorophenoxy)-N-[(3S,6R)-6- {5- [( 1 s,3 s)-3 -
(trifluoromethoxy)cyclobutyl] -
1,3 ,4-oxadiazol-2-y1} piperidin-3 -yl]acetamide
N-[(3 S,6R)-6- [5-(5-chloro- 1 -methyl- 1H-pyrazol-3 -y1)- 1,3 ,4-oxadiazol-2-
yl]piperidin-3 -yl] -2-
(4-chloro-3 -fluorophenoxy)acetamide
2-(4-chloro-3 -fluorophenoxy)-N-[(3S,6R)-6- {5- [6-(trifluoromethyl)pyridin-3 -
yl] - 1 ,3 ,4-
oxadiazol-2-yll piperidin-3-yl]acetamide
27
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(5-chloropyridin-2-y1)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(4-chloro-3-fluoropheny1)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5-[3-(trifluoromethoxy)propy1]-
1,3,4-oxadiazol-
2-yllpiperidin-3-yl]acetamide
tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-{5-[1-(2,2,2-
trifluoroethyl)azetidin-3-y1]-1,3,4-oxadiazol-2-yllpiperidine-1-carboxylate
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5-[1-(2,2,2-trifluoroethypazetidin-
3-y1]-1,3,4-
oxadiazol-2-yllpiperidin-3-yl]acetamide
tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-[5-(3-
cyclopropoxycyclobuty1)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3-cyclopropoxycyclobuty1)-1,3,4-
oxadiazol-
2-yl]piperidin-3-yl]acetamide
2-(3,4-dichlorophenoxy)-N-[(3S,6R)-6-{5-[(1s,3s)-3-
(difluoromethoxy)cyclobuty11-1,3,4-
oxadiazol-2-yllpiperidin-3-yllacetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5-[(trifluoromethoxy)methy1]-1,3,4-
oxadiazol-
2-yllpiperidin-3-yl]acetamide
tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-[5-(3,3,3-
trifluoro-2-
methylpropoxy)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3,3,3-trifluoro-2-methylpropoxy)-
1,3,4-
oxadiazol-2-yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-{5-[(4,4,4-trifluorobutan-2-ypoxy]-
1,3,4-
oxadiazol-2-yllpiperidin-3-yllacetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3,3-difluorobutoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-{5-[(2,2-
difluorocyclopropyl)methoxy]-1,3,4-
oxadiazol-2-yllpiperidin-3-yllacetamide
N-[(3S,6R)-6-(5-butoxy-1,3,4-oxadiazol-2-yl)piperidin-3-y1]-2-(4-chloro-3-
fluorophenoxy)acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-{5-[(3,3-difluorocyclopentypoxy]-
1,3,4-
oxadiazol-2-yllpiperidin-3-yllacetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(2-cyclopropylethoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
28
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3-methylbutoxy)-1,3,4-oxadiazol-
2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-{5-[(2,2-difluorocyclobutypmethoxy]-
1,3,4-
oxadiazol-2-yllpiperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3,3-difluorocyclobutoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(2,2,3,3,3-pentafluoropropoxy)-
1,3,4-
oxadiazol-2-yl]piperidin-3-yllacetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(4,4,4-trifluorobutoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5-[2-(difluoromethoxy)ethoxy]-
1,3,4-oxadiazol-
2-yllpiperidin-3-yl]acetamide
tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-{5-[2-
(trifluoromethoxy)ethoxy]-1,3,4-oxadiazol-2-yllpiperidine-1-carboxylate
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-{5-[2-(trifluoromethoxy)ethoxy]-
1,3,4-
oxadiazol-2-yllpiperidin-3-yllacetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(pentyloxy)-1,3,4-oxadiazol-2-
yl]piperidin-3-
yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3-methoxypropoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(2-cyclopropoxyethoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(2-ethoxyethoxy)-1,3,4-oxadiazol-
2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(2-cyclobutoxyethoxy)-1,3,4-
oxadiazol-2-
yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3R,65)-6-{5-[2-(trifluoromethoxy)ethoxy]-
1,3,4-
oxadiazol-2-yllpiperidin-3-yllacetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5-[(4,4-difluoropentypoxy]-1,3,4-
oxadiazol-2-
yl}piperidin-3-yl]acetamide
2-(3,4-dichlorophenoxy)-N-[(3S,6R)-6-{5-[2-(trifluoromethoxy)ethoxy]-1,3,4-
oxadiazol-2-
y1 fpiperidin-3-yl]acetamide
N-R3S,6R)-6-[5-(2-cyclopropoxyethoxy)-1,3,4-oxadiazol-2-yl]piperidin-3-y1]-2-
(3,4-
dichlorophenoxy)acetamide
29
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [2-(2,2-
difluorocyclopropoxy)ethoxy] -1,3,4-
oxadiazol-2-yll piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-(5- {[2-
(trifluoromethypcyclopropyl]methoxyl -
1,3,4-oxadiazol-2-yl)piperidin-3-yl]acetamide
tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido] -2- {5- [3-
(trifluoromethyl)azetidin-l-yl] -1,3,4-oxadiazol-2-y1} piperidine-l-
carboxylate
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [3 -(trifluoromethypazetidin-l-
yl] -1,3,4-
oxadiazol-2-yll piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [3 -(trifluoromethoxy)azetidin-
l-yl] -1,3,4-
oxadiazol-2-yllpiperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5-[3 -(2,2,2-
trifluoroethyl)azetidin-l-yl] -1 ,3,4-
oxadiazol-2-yllpiperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [3 -methyl-3 -
(trifluoromethoxy)azetidin-l-yl] -
1,3,4-oxadiazol-2-y1} piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-(5- {methyl [2-
(trifluoromethoxy)ethyl] amino } -
1,3,4-oxadiazol-2-yl)piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3-cyclopropoxyazetidin-l-y1)-
1,3,4-
oxadiazol-2-yl]piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [3 -
(trifluoromethoxy)pyrrolidin-l-yl] -1,3,4-
oxadiazol-2-y1 } piperidin-3-yl]acetamide
tert-butyl (2R,55)-5-[2-(4-chloro-3-fluorophenoxy)acetamido] -2- {5- [(1s,3 s)-
3-
(trifluoromethoxy)cyclobutyl] -1,2,4-oxadiazol-3 -yl } piperidine-l-
carboxylate
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [(1s,3 s)-3 -
(trifluoromethoxy)cyclobutyl] -
1,2,4-oxadiazol-3-ylf piperidin-3-yl]acetamide
tert-butyl (2R,5S)-5- {2- [(6-chloro-5-fluoropyridin-3-yl)oxy] acetamido } -2-
[5-(3,4-
dichloropheny1)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate
2- [(6-chloro-5-fluoropyridin-3 -yl)oxy] -N- [(3 S ,6R)-6- [5-(3,4-
dichloropheny1)-1,3,4-
oxadiazol-2-yl]piperidin-3-yl] acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-1-methy1-6- {5- [2-
(trifluoromethoxy)ethoxy] -1,3,4-
oxadiazol-2-yllpiperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-(5- {2- [(1R)-2,2-
difluorocyclopropoxy] ethoxy} -
1,3,4-oxadiazol-2-yl)piperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-(5- {2- [(1 S)-2,2-
difluorocyclopropoxy] ethoxy} -
1,3,4-oxadiazol-2-yl)piperidin-3-yl]acetamide
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-{5-[(1r,3r)-3-
cyclopropoxycyclobuty1]-1,3,4-
oxadiazol-2-yllpiperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-{5-[(1s,3s)-3-
cyclopropoxycyclobuty1]-1,3,4-
oxadiazol-2-yllpiperidin-3-yl]acetamide
__ (2R)-2-(4-chlorophenoxy)-N-[(3S,6R)-6- {5- [4-(trifluoromethyl)phenyl] -
1,3,4-oxadiazol-2-
yl}piperidin-3-yl]propanamide
(2S)-2-(4-chlorophenoxy)-N-[(3S,6R)-6- {5-[4-(trifluoromethyl)pheny1]-1,3,4-
oxadiazol-2-
yl fpiperidin-3-yl]propanamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(4-chloropheny1)-1,3,4-oxadiazol-
2-y1]-2-
__ oxopiperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3R,65)-6-[5-(4-chloropheny1)-1,3,4-oxadiazol-
2-y1]-2-
oxopiperidin-3-yl]acetamide
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [(3 S)-3-
(trifluoromethoxy)pyrrolidin-l-yl] -
1,3,4-oxadiazol-2-yl}piperidin-3-yl]acetamide
__ 2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [(3R)-3-
(trifluoromethoxy)pyrrolidin-l-yl] -
1,3,4-oxadiazol-2-ylf piperidin-3-yl]acetamide
N-[(3S,6R)-6- {5- [2-(trifluoromethoxy)ethoxy] -1,3,4-oxadiazol-2-y1}
piperidin-3-yl] -2- [4-
(trifluoromethyl)phenoxy]acetamide
2-[3-chloro-4-(difluoromethyl)phenoxy]-N-R3S,6R)-6- {5-[2-
(trifluoromethoxy)ethoxy]-
__ 1,3,4-oxadiazol-2-ylfpiperidin-3-yl]acetamide and
2-[3-fluoro-4-(trifluoromethyl)phenoxy]-N-R3S,6R)-6-{5-[2-
(trifluoromethoxy)ethoxy]-
1,3,4-oxadiazol-2-yl}piperidin-3-yl]acetamide.
Where tautomerism, like e.g. keto-enol tautomerism, of compounds of formula
(I) may occur,
__ the individual forms, like e.g. the keto and enol form, are comprised
separately and together
as mixtures in any ratio. Same applies to stereoisomers, like e.g.
enantiomers, cis/trans
isomers, conformers and the like.
Especially, when enantiomeric or diastereomeric forms are given in a compound
according to
__ formula (I) each pure form separately and any mixture of at least two of
the pure forms in any
ratio is comprised by formula (I) and is a subject of the present invention.
31
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
A preferred compound is a compound or a pharmaceutically acceptable salt,
solvate, hydrate,
tautomer or stereoisomer thereof of formula (I) with a relative configuration
as shown in
formula (Ia)
6 Ra7
R.-, X1 Al A2
0 a4
R30y-\
N 6
I Ra
R2a
R2 R1 Ra2 5 (Ia).
Isotopic labeled compounds of formula (I) are also within the scope of the
present invention.
Methods for isotope labeling are known in the art. Preferred isotopes are
those of the elements
H, C, N, 0 and S. Solvates and hydrates of compounds of formula (I) are also
within the
scope of the present invention.
If desired, isomers can be separated by methods well known in the art, e.g. by
liquid
chromatography. Same applies for enantiomers by using e.g. chiral stationary
phases.
Additionally, enantiomers may be isolated by converting them into
diastereomers, i.e.
coupling with an enantiomerically pure auxiliary compound, subsequent
separation of the
resulting diastereomers and cleavage of the auxiliary residue. Alternatively,
any enantiomer of
a compound of formula (I) may be obtained from stereoselective synthesis using
optically
pure starting materials, reagents and/or catalysts.
In case the compounds according to formula (I) contain one or more acidic or
basic groups,
the invention also comprises their corresponding pharmaceutically or
toxicologically
acceptable salts, in particular their pharmaceutically utilizable salts. Thus,
the compounds of
the formula (I) which contain acidic groups can be used according to the
invention, for
example, as alkali metal salts, alkaline earth metal salts or as ammonium
salts. More precise
examples of such salts include sodium salts, potassium salts, calcium salts,
magnesium salts
or salts with ammonia or organic amines such as, for example, ethylamine,
ethanolamine,
triethanolamine or amino acids. Compounds of the formula (I) which contain one
or more
basic groups, i.e. groups which can be protonated, can be present and can be
used according
to the invention in the form of their addition salts with inorganic or organic
acids. Examples
for suitable acids include hydrogen chloride, hydrogen bromide, phosphoric
acid, sulfuric
32
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
acid, nitric acid, methanesulfonic acid, p-toluenesulfonic acid,
naphthalenedisulfonic acids,
oxalic acid, acetic acid, tartaric acid, lactic acid, salicylic acid, benzoic
acid, formic acid,
propionic acid, pivalic acid, diethylacetic acid, malonic acid, succinic acid,
pimelic acid,
fumaric acid, maleic acid, malic acid, sulfaminic acid, phenylpropionic acid,
gluconic acid,
ascorbic acid, isonicotinic acid, citric acid, adipic acid, and other acids
known to the person
skilled in the art. If the compounds of the formula (I) simultaneously contain
acidic and basic
groups in the molecule, the invention also includes, in addition to the salt
forms mentioned,
inner salts or betaines (zwitterions). The respective salts according to the
formula (I) can be
obtained by customary methods which are known to the person skilled in the art
like, for
example by contacting these with an organic or inorganic acid or base in a
solvent or
dispersant, or by anion exchange or cation exchange with other salts. The
present invention
also includes all salts of the compounds of the formula (I) which, owing to
low physiological
compatibility, are not directly suitable for use in pharmaceuticals but which
can be used, for
example, as intermediates for chemical reactions or for the preparation of
pharmaceutically
acceptable salts.
As shown below compounds of the present invention are believed to be suitable
for
modulating the integrated stress response pathway.
The Integrated Stress Response (ISR) is a cellular stress response common to
all eukaryotes
(1). Dysregulation of ISR signaling has important pathological consequences
linked inter alia
to inflammation, viral infection, diabetes, cancer and neurodegenerative
diseases.
ISR is a common denominator of different types of cellular stresses resulting
in
phosphorylation of the alpha subunit of eukaryotic translation initiation
factor 2 (eIF2alpha)
on serine 51 leading to the suppression of normal protein synthesis and
expression of stress
response genes (2). In mammalian cells the phosphorylation is carried out by a
family of four
eIF2alpha kinases, namely: PKR-like ER kinase (PERK), double-stranded RNA-
dependent
protein kinase (PKR), heme-regulated eIF2alpha kinase (HRI), and general
control non-
derepressible 2 (GCN2), each responding to distinct environmental and
physiological stresses
(3).
eIF2alpha together with eIF2beta and eIF2gamma form the eIF2 complex, a key
player of the
initiation of normal mRNA translation (4). The eIF2 complex binds GTP and Met-
tRNA,
33
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
forming a ternary complex (eIF2-GTP-Met-tRNA1), which is recruited by
ribosomes for
translation initiation (5, 6).
eIF2B is a heterodecameric complex consisting of 5 subunits (alpha, beta,
gamma, delta,
epsilon) which in duplicate form a GEF-active decamer (7).
In response to ISR activation, phosphorylated eIF2alpha inhibits the eIF2B-
mediated
exchange of GDP for GTP, resulting in reduced ternary complex formation and
hence in the
inhibition of translation of normal mRNAs characterized by ribosomes binding
to the 5' AUG
start codon (8). Under these conditions of reduced ternary complex abundance
the translation
of several specific mRNAs including the mRNA coding for the transcription
factor ATF4 is
activated via a mechanism involving altered translation of upstream ORFs
(uORFs) (7, 9, 10).
These mRNAs typically contain one or more uORFs that normally function in
unstressed cells
to limit the flow of ribosomes to the main coding ORF. For example, during
normal
conditions, uORFs in the 5' UTR of ATF occupy the ribosomes and prevent
translation of the
coding sequence of ATF4. However, during stress conditions, i.e. under
conditions of reduced
ternary complex formation, the probability for ribosomes to scan past these
upstream ORFs
and initiate translation at the ATF4 coding ORF is increased. ATF4 and other
stress response
factors expressed in this way subsequently govern the expression of an array
of further stress
response genes. The acute phase consists in expression of proteins that aim to
restore
homeostasis, while the chronic phase leads to expression of pro-apoptotic
factors (1, 11, 12,
13).
Upregulation of markers of ISR signaling has been demonstrated in a variety of
conditions,
among these cancer and neurodegenerative diseases. In cancer, ER stress-
regulated translation
increases tolerance to hypoxic conditions and promotes tumor growth (14, 15,
16), and
deletion of PERK by gene targeting has been shown to slow growth of tumours
derived from
transformed PERKY mouse embryonic fibroblasts (14, 17). Further, a recent
report has
provided proof of concept using patient derived xenograft modeling in mice for
activators of
eIF2B to be effective in treating a form of aggressive metastatic prostate
cancer (28). Taken
together, prevention of cytoprotective ISR signaling may represent an
effective anti-
proliferation strategy for the treatment of at least some forms of cancer.
34
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Further, modulation of ISR signaling could prove effective in preserving
synaptic function
and reducing neuronal decline, also in neurodegenerative diseases that are
characterized by
misfolded proteins and activation of the unfolded protein response (UPR), such
as
amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD),
Alzheimer's disease
(AD), Parkinson's disease (PD) and Jakob Creutzfeld (prion) diseases (18, 19,
20). With prion
disease an example of a neurodegenerative disease exists where it has been
shown that
pharmacological as well as genetic inhibition of ISR signaling can normalize
protein
translation levels, rescue synaptic function and prevent neuronal loss (21).
Specifically,
reduction of levels of phosphorylated eIF2alpha by overexpression of the
phosphatase
controlling phosphorylated eIF2alpha levels increased survival of prion-
infected mice
whereas sustained eIF2alpha phosphorylation decreased survival (22).
Further, direct evidence for the importance of control of protein expression
levels for proper
brain function exists in the form of rare genetic diseases affecting functions
of eIF2 and
eIF2B. A mutation in eIF2gamma that disrupts complex integrity of eIF2 and
hence results in
reduced normal protein expression levels is linked to intellectual disability
syndrome (ID)
(23). Partial loss of function mutations in subunits of eIF2B have been shown
to be causal for
the rare leukodystrophy Vanishing White Matter Disease (VWMD) (24, 25).
Specifically,
stabilization of eIF2B partial loss of function in a VWMD mouse model by a
small molecule
related to ISRIB has been shown to reduce ISR markers and improve functional
as well as
pathological end points (26, 27).
The present invention provides compounds of the present invention in free or
pharmaceutically acceptable salt form or in the form of solvates, hydrates,
tautomers or
stereoisomers to be used in the treatment of diseases or disorders mentioned
herein. The same
applies to a pharmaceutical composition of the present invention.
Thus an aspect of the present invention is a compound or a pharmaceutically
acceptable salt,
solvate, hydrate, tautomer or stereoisomer thereof of the present invention
for use as a
medicament as mentioned above. The same applies to a pharmaceutical
composition of the
present invention.
The therapeutic method described may be applied to mammals such as dogs, cats,
cows,
horses, rabbits, monkeys and humans. Preferably, the mammalian patient is a
human patient.
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Accordingly, the present invention provides a compound or a pharmaceutically
acceptable
salt, solvate, hydrate, tautomer or stereoisomer thereof or a pharmaceutical
composition of
the present invention to be used in the treatment or prevention of one or more
diseases or
disorders associated with integrated stress response.
A further aspect of the present invention is a compound or a pharmaceutically
acceptable salt,
solvate, hydrate, tautomer or stereoisomer thereof or a pharmaceutical
composition of the
present invention for use in a method of treating or preventing one or more
disorders or
diseases associated with integrated stress response.
A further aspect of the present invention is the use of a compound or a
pharmaceutically
acceptable salt, solvate, hydrate, tautomer or stereoisomer thereof or a
pharmaceutical
composition of the present invention for the manufacture of a medicament for
the treatment or
prophylaxis of one or more disorders or diseases associated with integrated
stress response.
Yet another aspect of the present invention is a method for treating,
controlling, delaying or
preventing in a mammalian patient in need of the treatment of one or more
diseases or
disorders associated with integrated stress response, wherein the method
comprises
administering to said patient a therapeutically effective amount of a compound
or a
pharmaceutically acceptable salt, solvate, hydrate, tautomer or stereoisomer
thereof ora
pharmaceutical composition of the present invention.
The present invention provides a compound or a pharmaceutically acceptable
salt, solvate,
hydrate, tautomer or stereoisomer thereof or a pharmaceutical composition of
the present
invention to be used in the treatment or prevention of one or more diseases or
disorders
mentioned below.
A further aspect of the present invention is a compound or a pharmaceutically
acceptable salt,
solvate, hydrate, tautomer or stereoisomer thereof or a pharmaceutical
composition of the
present invention for use in a method of treating or preventing one or more
disorders or
diseases mentioned below.
36
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
A further aspect of the present invention is the use of a compound or a
pharmaceutically
acceptable salt, solvate, hydrate, tautomer or stereoisomer thereof or a
pharmaceutical
composition of the present invention for the manufacture of a medicament for
the treatment or
prophylaxis of one or more disorders or diseases mentioned below.
Yet another aspect of the present invention is a method for treating,
controlling, delaying or
preventing in a mammalian patient in need of the treatment of one or more
diseases or
disorders mentioned below, wherein the method comprises administering to said
patient a
therapeutically effective amount of a compound or a pharmaceutically
acceptable salt,
solvate, hydrate, tautomer or stereoisomer thereof or a pharmaceutical
composition of the
present invention.
Diseases or disorders include but are not limited to leukodystrophies,
intellectual disability
syndrome, neurodegenerative diseases and disorders, neoplastic diseases,
infectious diseases,
inflammatory diseases, musculoskeletal diseases, metabolic diseases, ocular
diseases as well
as diseases selected from the group consisting of organ fibrosis, chronic and
acute diseases of
the liver, chronic and acute diseases of the lung, chronic and acute diseases
of the kidney,
myocardial infarction, cardiovascular disease, arrhythmias, atherosclerosis,
spinal cord injury,
ischemic stroke, and neuropathic pain.
Leukodystrophies
Examples of leukodystrophies include, but are not limited to, Vanishing White
Matter Disease
(VWMD) and childhood ataxia with CNS hypo-myelination (e.g. associated with
impaired
function of eIF2 or components in a signal transduction or signaling pathway
including eIF2).
Intellectual disability syndrome
Intellectual disability in particular refers to a condition in which a person
has certain
limitations in intellectual functions like communicating, taking care of him-
or herself, and/or
has impaired social skills. Intellectual disability syndromes include, but are
not limited to,
intellectual disability conditions associated with impaired function of eIF2
or components in a
signal transduction or signaling pathway including eIF2.
37
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Neurodegenerative diseases / disorders
Examples of neurodegenerative diseases and disorders include, but are not
limited to,
Alexander's disease, Alper's disease, Alzheimer's disease, Amyotrophic lateral
sclerosis,
Ataxia telangiectasia, Batten disease (also known as Spielmeyer-Vogt-Sjogren-
Batten
disease), Bovine spongiform encephalopathy (BSE), Canavan disease, Cockayne
syndrome,
Corticobasal degeneration, Creutzfeldt-Jakob disease, frontotemporal dementia,
Gerstmann-
Straussler-Scheinker syndrome, Huntington's disease, HIV-associated dementia,
Kennedy's
disease, Krabbe's disease, Kuru, Lewy body dementia, Machado-Joseph disease
(Spinocerebellar ataxia type 3), Multiple sclerosis, Multiple System Atrophy,
Narcolepsy,
Neuroborreliosis, Parkinson's disease, Pelizaeus-Merzbacher Disease, Pick's
disease, Primary
lateral sclerosis, Prion diseases, Progressive supranuclear palsy, Refsum's
disease, Sandhoffs
disease, Schilder's disease, Subacute combined degeneration of spinal cord
secondary to
Pernicious Anaemia, Schizophrenia, Spinocerebellar ataxia (multiple types with
varying
characteristics), Spinal muscular atrophy, Steele-Richardson-Olszewski
disease, Tabes
dorsalis, and tauopathies.
In particular, the neurodegenerative disease or and disorder is selected from
the group
consisting of Alzheimer's disease, Parkinson's disease and amyotrophic lateral
sclerosis.
Neoplastic diseases
A neoplastic disease may be understood in the broadest sense as any tissue
resulting from
miss-controlled cell growth. In many cases a neoplasm leads to at least bulky
tissue mass
optionally innervated by blood vessels. It may or may not comprise the
formation of one or
more metastasis/metastases. A neoplastic disease of the present invention may
be any
neoplasm as classified by the International Statistical Classification of
Diseases and Related
Health Problems 10th Revision (ICD-10) classes COO-D48.
Exemplarily, a neoplastic disease according to the present invention may be
the presence of
one or more malignant neoplasm(s) (tumors) (ICD-10 classes COO-C97), may be
the presence
of one or more in situ neoplasm(s) (ICD-10 classes DOO-D09), may be the
presence of one or
more benign neoplasm(s) (ICD-10 classes D1O-D36), or may be the presence of
one or more
neoplasm(s) of uncertain or unknown behavior (ICD-10 classes D37-D48).
Preferably, a
neoplastic disease according to the present invention refers to the presence
of one or more
malignant neoplasm(s), i.e., is malignant neoplasia (ICD-10 classes COO-C97).
38
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
In a more preferred embodiment, the neoplastic disease is cancer.
Cancer may be understood in the broadest sense as any malignant neoplastic
disease, i.e., the
presence of one or more malignant neoplasm(s) in the patient. Cancer may be
solid or
hematologic malignancy. Contemplated herein are without limitation leukemia,
lymphoma,
carcinomas and sarcomas.
In particular, neoplastic diseases, such as cancers, characterized by
upregulated ISR markers
are included herein.
Exemplary cancers include, but are not limited to, thyroid cancer, cancers of
the endocrine
system, pancreatic cancer, brain cancer (e.g. glioblastoma multiforme,
glioma), breast cancer
(e.g. ER positive, ER negative, chemotherapy resistant, herceptin resistant,
HER2 positive,
doxorubicin resistant, tamoxifen resistant, ductal carcinoma, lobular
carcinoma, primary,
metastatic), cervix cancer, ovarian cancer, uterus cancer, colon cancer, head
& neck cancer,
liver cancer (e.g. hepatocellular carcinoma), kidney cancer, lung cancer (e.g.
non-small cell
lung carcinoma, squamous cell lung carcinoma, adenocarcinoma, large cell lung
carcinoma,
small cell lung carcinoma, carcinoid, sarcoma), colon cancer, esophageal
cancer, stomach
cancer, bladder cancer, bone cancer, gastric cancer, prostate cancer and skin
cancer (e.g.
melanoma).
Further examples include, but are not limited to, myeloma, leukemia,
mesothelioma, and
sarcoma.
Additional examples include, but are not limited to, Medulloblastoma,
Hodgkin's Disease,
Non-Hodgkin's Lymphoma, multiple myeloma, neuroblastoma, glioma, glioblastoma
multiforme, rhabdomyosarcoma, primary thrombocytosis, primary
macroglobulinemia,
primary brain tumors, malignant pancreatic insulanoma, malignant carcinoid,
urinary bladder
cancer, premalignant skin lesions, testicular cancer, lymphomas, genitourinary
tract cancer,
malignant hypercalcemia, endometrial cancer, adrenal cortical cancer,
neoplasms of the
endocrine or exocrine pancreas, medullary thyroid cancer, medullary thyroid
carcinoma,
melanoma, colorectal cancer, papillary thyroid cancer, hepatocellular
carcinoma, Paget's
Disease of the Nipple, Phyllodes Tumors, Lobular Carcinoma, Ductal Carcinoma,
cancer of
the pancreatic stellate cells, and cancer of the hepatic stellate cells.
39
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Exemplary leukemias include, but are not limited to, acute nonlymphocytic
leukemia, chronic
lymphocytic leukemia, acute granulocytic leukemia, chronic granulocytic
leukemia, acute
promyelocytic leukemia, adult T-cell leukemia, aleukemic leukemia, a
leukocythemic
leukemia, basophylic leukemia, blast cell leukemia, bovine leukemia, chronic
myelocytic
leukemia, leukemia cutis, embryonal leukemia, eosinophilic leukemia, Gross'
leukemia, hairy-
cell leukemia, hemoblastic leukemia, hemocytoblastic leukemia, histiocytic
leukemia, stem
cell leukemia, acute monocytic leukemia, leukopenic leukemia, lymphatic
leukemia,
lymphoblastic leukemia, lymphocytic leukemia, lymphogenous leukemia, lymphoid
leukemia, lymphosarcoma cell leukemia, mast cell leukemia, megakaryocyte
leukemia,
micromyeloblastic leukemia, monocytic leukemia, myeloblasts leukemia,
myelocytic
leukemia, myeloid granulocytic leukemia, myelomonocytic leukemia, Naegeli
leukemia,
plasma cell leukemia, multiple myeloma, plasmacytic leukemia, promyelocytic
leukemia,
Rieder cell leukemia, Schilling's leukemia, stem cell leukemia, subleukemic
leukemia, and
undifferentiated cell leukemia.
Exemplary sarcomas include, but are not limited to, chondrosarcoma,
fibrosarcoma,
lymphosarcoma, melanosarcoma, myxosarcoma, osteosarcoma, Abemethy's sarcoma,
adipose
sarcoma, liposarcoma, alveolar soft part sarcoma, ameloblastic sarcoma,
botryoid sarcoma,
chloroma sarcoma, chorio carcinoma, embryonal sarcoma, Wilms' tumor sarcoma,
endometrial sarcoma, stromal sarcoma, Ewing's sarcoma, fascial sarcoma,
fibroblastic
sarcoma, giant cell sarcoma, granulocytic sarcoma, Hodgkin's sarcoma,
idiopathic multiple
pigmented hemorrhagic sarcoma, immunoblastic sarcoma of B cells, lymphoma,
immunoblastic sarcoma of T-cells, Jensen's sarcoma, Kaposi's sarcoma, Kupffer
cell sarcoma,
angiosarcoma, leukosarcoma, malignant mesenchymoma sarcoma, parosteal sarcoma,
reticulocytic sarcoma, Rous sarcoma, sero cystic sarcoma, synovial sarcoma,
and
telangiectaltic sarcoma.
Exemplary melanomas include, but are not limited to, acral-lentiginous
melanoma,
amelanotic melanoma, benign juvenile melanoma, Cloudman's melanoma, S91
melanoma,
Harding-Passey melanoma, juvenile melanoma, lentigo maligna melanoma,
malignant
melanoma, nodular melanoma, subungal melanoma, and superficial spreading
melanoma.
Exemplary carcinomas include, but are not limited to, medullary thyroid
carcinoma, familial
medullary thyroid carcinoma, acinar carcinoma, acinous carcinoma, adenocystic
carcinoma,
adenoid cystic carcinoma, carcinoma adenomatosum, carcinoma of adrenal cortex,
alveolar
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
carcinoma, alveolar cell carcinoma, basal cell carcinoma, carcinoma
basocellulare, basaloid
carcinoma, basosquamous cell carcinoma, bronchioalveolar carcinoma,
bronchiolar
carcinoma, bronchogenic carcinoma, cerebriform carcinoma, cholangiocellular
carcinoma,
chorionic carcinoma, colloid carcinoma, comedo carcinoma, corpus carcinoma,
cribriform
carcinoma, carcinoma en cuirasse, carcinoma cutaneum, cylindrical carcinoma,
cylindrical
cell carcinoma, duct carcinoma, ductal carcinoma, carcinoma durum, embryonal
carcinoma,
encephaloid carcinoma, epiermoid carcinoma, carcinoma epitheliale adenoides,
exophytic
carcinoma, carcinoma ex ulcere, carcinoma fibrosum, gelatiniforni carcinoma,
gelatinous
carcinoma, giant cell carcinoma, carcinoma gigantocellulare, glandular
carcinoma, granulosa
cell carcinoma, hair-matrix carcinoma, hematoid carcinoma, hepatocellular
carcinoma,
Hurthle cell carcinoma, hyaline carcinoma, hypernephroid carcinoma, infantile
embryonal
carcinoma, carcinoma in situ, intraepidermal carcinoma, intraepithelial
carcinoma,
Krompecher's carcinoma, Kulchitzky-cell carcinoma, large-cell carcinoma,
lenticular
carcinoma, carcinoma lenticulare, lipomatous carcinoma, lobular carcinoma,
lymphoepithelial
carcinoma, carcinoma medullare, medullary carcinoma, melanotic carcinoma,
carcinoma
molle, mucinous carcinoma, carcinoma muciparum, carcinoma mucocellulare,
mucoepidermoid carcinoma, carcinoma mucosum, mucous carcinoma, carcinoma
myxomatodes, nasopharyngeal carcinoma, oat cell carcinoma, carcinoma
ossificans, osteoid
carcinoma, papillary carcinoma, periportal carcinoma, preinvasive carcinoma,
prickle cell
carcinoma, pultaceous carcinoma, renal cell carcinoma of kidney, reserve cell
carcinoma,
carcinoma sarcomatodes, schneiderian carcinoma, scirrhous carcinoma, carcinoma
scroti,
signet-ring cell carcinoma, carcinoma simplex, small-cell carcinoma, solanoid
carcinoma,
spheroidal cell carcinoma, spindle cell carcinoma, carcinoma spongiosum,
squamous
carcinoma, squamous cell carcinoma, string carcinoma, carcinoma
telangiectaticum,
carcinoma telangiectodes, transitional cell carcinoma, carcinoma tuberosum,
tubular
carcinoma, tuberous carcinoma, verrucous carcinoma, and carcinoma villosum.
Infectious diseases
Examples include, but are not limited to, infections caused by viruses (such
as infections by
HIV-1: human immunodeficiency virus type 1; IAV: influenza A virus; HCV:
hepatitis C
virus; DENV: dengue virus; ASFV: African swine fever virus; EBV: Epstein-Barr
virus;
HSV1: herpes simplex virus 1; CHIKV: chikungunya virus; HCMV: human
cytomegalovirus;
SARS-CoV: severe acute respiratory syndrome coronavirus; SARS-CoV-2: severe
acute
41
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
respiratory syndrome coronavirus 2) and infections caused by bacteria (such as
infections by
Legionella, Brucella, Simkania, Chlamydia, Helicobacter and Campylobacter).
Inflammatory diseases
Examples of inflammatory diseases include, but are not limited to,
postoperative cognitive
dysfunction (decline in cognitive function after surgery), traumatic brain
injury, arthritis,
rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis,
multiple sclerosis,
systemic lupus erythematosus (SLE), myasthenia gravis, juvenile onset
diabetes, diabetes
mellitus type 1, Guillain-Barre syndrome, Hashimoto's encephalitis,
Hashimoto's thyroiditis,
ankylosing spondylitis, psoriasis, Sjogren's syndrome, vasculitis,
glomerulonephritis, auto-
immune thyroiditis, Behcet's disease, Crohn's disease, ulcerative colitis,
bullous pemphigoid,
sarcoidosis, ichthyosis, Graves ophthalmopathy, inflammatory bowel disease,
Addison's
disease, Vitiligo, asthma, allergic asthma, acne vulgaris, celiac disease,
chronic prostatitis,
inflammatory bowel disease, pelvic inflammatory disease, reperfusion injury,
sarcoidosis,
transplant rejection, interstitial cystitis, atherosclerosis, and atopic
dermatitis.
Musculoskeletal diseases
Examples of musculoskeletal diseases include, but are not limited to, muscular
dystrophy,
multiple sclerosis, Freidrich's ataxia, a muscle wasting disorder (e.g.,
muscle atrophy,
sarcopenia, cachexia), inclusion body myopathy, progressive muscular atrophy,
motor neuron
disease, carpal tunnel syndrome, epicondylitis, tendinitis, back pain, muscle
pain, muscle
soreness, repetitive strain disorders, and paralysis.
Metabolic diseases
.. Examples of metabolic diseases include, but are not limited to, diabetes
(in particular diabetes
Type II), non-alcoholic steatohepatitis (NASH), non-alcoholic fatty liver
disease (NAFLD),
Niemann-Pick disease, liver fibrosis, obesity, heart disease, atherosclerosis,
arthritis,
cystinosis, phenylketonuria, proliferative retinopathy, and Kearns-Sayre
disease.
Ocular diseases
Examples of ocular diseases include, but are not limited to, edema or
neovascularization for
any occlusive or inflammatory retinal vascular disease, such as rubeosis
irides, neovascular
glaucoma, pterygium, vascularized glaucoma filtering blebs, conjunctival
papilloma;
choroidal neovascularization, such as neovascular age-related macular
degeneration (AMD),
42
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
myopia, prior uveitis, trauma, or idiopathic; macular edema, such as post
surgical macular
edema, macular edema secondary to uveitis including retinal and/or choroidal
inflammation,
macular edema secondary to diabetes, and macular edema secondary to
retinovascular
occlusive disease (i.e. branch and central retinal vein occlusion); retinal
neovascularization
due to diabetes, such as retinal vein occlusion, uveitis, ocular ischemic
syndrome from carotid
artery disease, ophthalmic or retinal artery occlusion, sickle cell
retinopathy, other ischemic or
occlusive neovascular retinopathies, retinopathy of prematurity, or Eale's
Disease; and genetic
disorders, such as VonHippel-Lindau syndrome.
Further diseases
Further diseases include, but are not limited to, organ fibrosis (such as
liver fibrosis, lung
fibrosis, or kidney fibrosis), chronic and acute diseases of the liver (such
as fatty liver disease,
or liver steatosis), chronic and acute diseases of the lung, chronic and acute
diseases of the
kidney, myocardial infarction, cardiovascular disease, arrhythmias,
atherosclerosis, spinal
cord injury, ischemic stroke, and neuropathic pain.
Yet another aspect of the present invention is a pharmaceutical composition
comprising at
least one compound or a pharmaceutically acceptable salt, solvate, hydrate,
tautomer or
stereoisomer thereof of the present invention together with a pharmaceutically
acceptable
carrier, optionally in combination with one or more other bioactive compounds
or
pharmaceutical compositions.
Preferably, the one or more bioactive compounds are modulators of the
integrated stress
reponse pathway other than compounds of formula (I).
"Pharmaceutical composition" means one or more active ingredients, and one or
more inert
ingredients that make up the carrier, as well as any product which results,
directly or
indirectly, from combination, complexation or aggregation of any two or more
of the
ingredients, or from dissociation of one or more of the ingredients, or from
other types of
reactions or interactions of one or more of the ingredients. Accordingly, the
pharmaceutical
compositions of the present invention encompass any composition made by
admixing a
compound of the present invention and a pharmaceutically acceptable carrier.
43
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
A pharmaceutical composition of the present invention may comprise one or more
additional
compounds as active ingredients like a mixture of compounds of formula (I) in
the
composition or other modulators of the integrated stress response pathway.
The active ingredients may be comprised in one or more different
pharmaceutical
compositions (combination of pharmaceutical compositions).
The term "pharmaceutically acceptable salts" refers to salts prepared from
pharmaceutically
acceptable non-toxic bases or acids, including inorganic bases or acids and
organic bases or
acids.
The compositions include compositions suitable for oral, rectal, topical,
parenteral (including
subcutaneous, intramuscular, and intravenous), ocular (ophthalmic), pulmonary
(nasal or
buccal inhalation), or nasal administration, although the most suitable route
in any given case
will depend on the nature and severity of the conditions being treated and on
the nature of the
active ingredient. They may be conveniently presented in unit dosage form and
prepared by
any of the methods well-known in the art of pharmacy.
In practical use, the compounds of formula (I) can be combined as the active
ingredient in
intimate admixture with a pharmaceutical carrier according to conventional
pharmaceutical
compounding techniques. The carrier may take a wide variety of forms depending
on the form
of preparation desired for administration, e.g., oral or parenteral (including
intravenous). In
preparing the compositions for oral dosage form, any of the usual
pharmaceutical media may
be employed, such as water, glycols, oils, alcohols, flavoring agents,
preservatives, coloring
agents and the like in the case of oral liquid preparations, such as, for
example, suspensions,
elixirs and solutions; or carriers such as starches, sugars, microcrystalline
cellulose, diluents,
granulating agents, lubricants, binders, disintegrating agents and the like in
the case of oral
solid preparations such as powders, hard and soft capsules and tablets, with
the solid oral
preparations being preferred over the liquid preparations.
Because of their ease of administration, tablets and capsules represent the
most advantageous
oral dosage unit form in which case solid pharmaceutical carriers are
obviously employed. If
desired, tablets may be coated by standard aqueous or nonaqueous techniques.
Such
compositions and preparations should contain at least 0.1 percent of active
compound. The
44
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
percentage of active compound in these compositions may, of course, be varied
and may
conveniently be between about 2 percent to about 60 percent of the weight of
the unit. The
amount of active compound in such therapeutically useful compositions is such
that an
effective dosage will be obtained. The active compounds can also be
administered
intranasally, for example, as liquid drops or spray.
The tablets, pills, capsules, and the like may also contain a binder such as
gum tragacanth,
acacia, corn starch or gelatin; excipients such as dicalcium phosphate; a
disintegrating agent
such as corn starch, potato starch, alginic acid; a lubricant such as
magnesium stearate; and a
sweetening agent such as sucrose, lactose or saccharin. When a dosage unit
form is a capsule,
it may contain, in addition to materials of the above type, a liquid carrier
such as a fatty oil.
Various other materials may be present as coatings or to modify the physical
form of the
dosage unit. For instance, tablets may be coated with shellac, sugar or both.
A syrup or elixir
may contain, in addition to the active ingredient, sucrose as a sweetening
agent, methyl and
propylparabens as preservatives, a dye and a flavoring such as cherry or
orange flavor.
Compounds of formula (I) may also be administered parenterally. Solutions or
suspensions of
these active compounds can be prepared in water suitably mixed with a
surfactant such as
hydroxypropyl-cellulose. Dispersions can also be prepared in glycerol, liquid
polyethylene
glycols and mixtures thereof in oils. Under ordinary conditions of storage and
use, these
preparations contain a preservative to prevent the growth of microorganisms.
The pharmaceutical forms suitable for injectable use include sterile aqueous
solutions or
dispersions and sterile powders for the extemporaneous preparation of sterile
injectable
solutions or dispersions. In all cases, the form should be sterile and should
be fluid to the
extent that easy syringability exists. It should be stable under the
conditions of manufacture
and storage and should be preserved against the contaminating action of
microorganisms such
as bacteria and fungi. The carrier can be a solvent or dispersion medium
containing, for
example, water, ethanol, polyol (e.g., glycerol, propylene glycol and liquid
polyethylene
glycol), suitable mixtures thereof, and vegetable oils.
Any suitable route of administration may be employed for providing a mammal,
especially a
human, with an effective dose of a compound of the present invention. For
example, oral,
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
rectal, topical, parenteral, ocular, pulmonary, nasal, and the like may be
employed. Dosage
forms include tablets, troches, dispersions, suspensions, solutions, capsules,
creams,
ointments, aerosols, and the like. Preferably compounds of formula (I) are
administered
orally.
The effective dosage of active ingredient employed may vary depending on the
particular
compound employed, the mode of administration, the condition being treated and
the severity
of the condition being treated. Such dosage may be ascertained readily by a
person skilled in
the art.
Starting materials for the synthesis of preferred embodiments of the invention
may be
purchased from commercially available sources such as Array, Sigma Aldrich,
Acros, Fisher,
Fluka, ABCR or can be synthesized using known methods by one skilled in the
art.
In general, several methods are applicable to prepare compounds of the present
invention. In
some cases various strategies can be combined. Sequential or convergent routes
may be used.
Exemplary synthetic routes are described below.
Examples
I Chemical Synthesis
Experimental procedures:
The following Abbreviations and Acronyms are used:
aq aqueous
ACN acetonitrile
Ag0Tf silver trifluoromethanesulfonate
BrCN cyanogen bromide
Brine saturated solution of NaCl in water
BnONH2- HCl 0-benzylhydroxylamine hydrochloride
Boc tert-butoxycarbonyl
Boc20 di-tert-butyl dicarbonate
tBuOK potassium tert-butoxide
CSA (7,7-dimethy1-2-oxobicyclo [2 .2.1] heptan-1 -
yl)methanesulfoni c acid
46
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
CV column volume
DAST N,N-diethylaminosulfur trifluoride
DCM dichloromethane
DCE dichloroethane
DMSO dimethylsulfoxide
DMSO-d6 deuterated dimethylsulfoxide
DIPEA diisopropylethylamine
DMF dimethyl formamide
DMAP N,N-dimethylpyridin-4-amine
ESI+ positive ionisation mode
ESF negative ionisation mode
Et0Ac ethyl acetate
Et0H ethanol
Et20 diethyl ether
H2SO4 sulfuric acid
HATU 1-[bis(dimethylamino)methylidene]-1H-[1,2,3]triazolo[4,5-
b]pyridin-1-
ium 3-oxide hexafluorophosphate
HC1 hydrochloric acid
HPLC high-performance liquid chromatography
h hour(s)
IPA isopropyl alcohol
KHCO3 potassium bicarbonate
LiOH lithium hydroxide
Li0H.H20 lithium hydroxide hydrate
m multiplet
m-CPBA 3-chlorobenzenecarboperoxoic acid
Me0H methanol
MgSO4 magnesium sulphate
min minutes
Ms0H methanesulfonic acid
mL millilitre (s)
N2 nitrogen atmosphere
Na2S03 sodium sulfite
Na2SO4 sodium sulphate
47
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
NaBH4 sodium borohydride
NaHCO3 sodium bicarbonate
NH2-NH2=H20 hydrazine hydrate
NH4C1 ammonium chloride
NiC12=6H20 nickel (II) chloride hexahydrate
NMM 4-methylmorpholine
NMR Nuclear Magnetic Resonance
prep. preparative
r.t. room temperature
Rochelle salt sodium potassium L(+)-tartrate tetrahydrate
RT retention time
satd saturated
SOC12 thionyl chloride
STAB sodium triacetoxyborohydride
T3P propanephosphonic acid anhydride
TsC1 4-methylbenzenesulfonyl chloride
TCDI 1,1'-thiocarbonyldiimidazole
THF tetrahydrofuran
TFA 2,2,2-trifluoroacetic acid
TFAA trifluoroacetic anhydride
TMS-CF3 trimethyl(trifluoromethyl)silane
TMSOI trimethylsulfoxonium iodide
ZnBr2 zinc dibromide
Analytical LCMS conditions are as follows:
System 1 (Si): ACIDIC IPC METHOD (MS18 and MS19)
Analytical (MET/CR/1410) HPLC-MS were performed on a Shimadzu LCMS systems
using
a Kinetex Core shell C18 column (2.1 mm x 50 mm, 5 1,1m; temperature: 40 C)
and a
gradient of 5-100% B (A= 0.1% formic acid in H20; B= 0.1% formic acid in ACN)
over 1.2
min then 100% B for 0.1 min. A second gradient of 100-5% B was then applied
over 0.01 min
with an injection volume of 3 !IL at a flow rate of 1.2 mL/min. UV spectra
were recorded at
215 nm using a SPD-M20A photo diode array detector spectrum range: 200-400 nm.
Mass
spectra were obtained using a 2010EV detector. Data were integrated and
reported using
Shimadzu LCMS-Solutions and PsiPort software.
48
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
System 2 (S2): ACIDIC IPC METHOD (MSQ2 and MSQ4):
Analytical (MET/uPLC/1704) uHPLC-MS were performed on a Waters Acquity uPLC
system using a Waters UPLC BEHTM C18 column (2.1 mm x 50 mm, 1.7 m;
temperature
40 C) and a gradient of 5-100% B (A= 0.1% formic acid in H20: B= 0.1% formic
acid in
ACN) over 1.1 min then 100% B for 0.25 min. A second gradient of 100-5% B was
then
applied over 0.05 min and held for 0.1 min with an injection volume of 1 1_,
at a flow rate of
0.9 mL/min. UV spectra were recorded at 215 nm on a Waters Acquity PDA with a
spectrum
range of 200-400 nm. Mass spectra were obtained using a Waters QDa. Data were
integrated
and reported using Waters MassLynx and OpenLynx software.
System 3 (S3): BASIC IPC METHOD (M516):
Analytical (MET/CR/1602) uHPLC-MS were performed on a Waters Acquity uPLC
system
using Waters UPLCO BEHTM C18 column (2.1 mm x 30 mm, 1.7 p.m; temperature 40
C)
and a gradient of 5-100% B (A: 2 mM ammonium bicarbonate, buffered to pH 10,
B: ACN)
over 0.75 min, then 100% B for 0.1 min. A second gradient of 100-5% B was then
applied
over 0.05 min and held for 0.1 min with an injection volume of 1 L at a flow
rate of 1
mL/min. UV spectra were recorded at 215 nm on a Waters Acquity PDA with a
spectrum
range of 200-400 nm. Mass spectra were obtained using a Waters Quattro Premier
XE. Data
were integrated and reported using Waters MassLynx and OpenLynx software.
System 4(S4): ACIDIC FINAL METHOD (MSQ1 and MSQ2):
Analytical (MET/uPLC/AB101) uHPLC-MS were performed on a Waters Acquity uPLC
system using a Phenomenex Kinetex-XB C18 column (2.1 mm x 100 mm, 1.7 1,1M;
temperature: 40 C) and a gradient of 5-100% B (A = 0.1% formic acid in H20; B
= 0.1%
formic acid in ACN) over 5.3 min then 100% B for 0.5 min. A second gradient of
100-5% B
was then applied over 0.02 min and held for 1.18 min with an injection volume
of 1 !IL at
flow rate of 0.6 mL/min. UV spectra were recorded at 215 nm using a Waters
Acquity PDA
detector spectrum range: 200-400 nm. Mass spectra were obtained using a Waters
SQD
(MSQ1) or Waters Acquity QDA (MSQ2). Data were integrated and reported using
Waters
MassLynx and OpenLynx software.
49
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
System 5 (S5): ACIDIC FINAL METHOD (M518, M519)
Analytical (MET/CR/1416) HPLC-MS were performed on Shimadzu LCMS systems using
a
Waters Atlantis dC18 column (2.1 mm x 100 mm, 3 m; temperature: 40 C) and a
gradient
of 5-100% B (A= 0.1% formic acid in H20; B= 0.1% formic acid in ACN) over 5
min then
.. 100% B for 0.4 min. A second gradient of 100-5% B was then applied over
0.02 min and
held for 1.58 min with an injection volume of 3 L at flow rate of 0.6 mL/min.
UV spectra
were recorded at 215 nm using a SPD-M20A photo diode array detector spectrum
range: 200-
400 nm. Mass spectra were obtained using a 2010EV detector. Data were
integrated and
reported using Shimadzu LCMS-Solutions and PsiPort software.
System 6 (S6): BASIC FINAL METHOD (MS16)
Analytical (MET/uHPLC/AB105) uPLC-MS were performed on a Waters Acquity uPLC
system using a Waters UPLC BEHTM C18 column (2.1 mm x 100 mm, 1.7 m column;
temperature: 40 C) and a gradient of 5-100% (A= 2 mM ammonium bicarbonate,
buffered to
pH 10; B = ACN) over 5.3 min then 100% B for 0.5 min. A second gradient of 100-
5% B was
then applied over 0.02 min and held for 1.18 min with an injection volume of 1
L and at
flow rate of 0.6 mL/min. UV spectra were recorded at 215 nm using a Waters
Acquity photo
diode array detector Spectrum range: 200-400 nm. Mass spectra were obtained
using a Waters
Quattro Premier XE mass detector. Data were integrated and reported using
Waters
MassLynx and OpenLynx software.
Purification methods are as follows:
Method 1: ACIDIC EARLY METHOD
Purifications (P1) LC were performed on a Gilson LC system using a Waters
Sunfire C18
column (30 mm x 100 mm, 10 M; temperature: r.t.) and a gradient of 10-95% B
(A= 0.1%
formic acid in H20; B= 0.1% formic acid in ACN) over 14.44 min then 95% B for
2.11 min.
A second gradient of 95-10% B was then applied over 0.2 min with an injection
volume of
1500 L at flow rate of 40 mL/min. UV spectra were recorded at 215 nm using a
Gilson
detector.
Method 2: ACIDIC STANDARD METHOD
Purifications (P2) LC were performed on a Gilson LC system using a Waters
Sunfire C18
column (30 mm x 10 mm, 10 M; temperature: r.t.) and a gradient of 30-95% B
(A= 0.1%
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
formic acid in water; B= 0.1% formic acid in ACN) over 11.00 min then 95% B
for 2.10 min.
A second gradient of 95-30% B was then applied over 0.2 min with an injection
volume of
1500 i_LL at flow rate of 40 mL/min. UV spectra were recorded at 215 nm using
a Gilson
detector.
Method 3: BASIC EARLY METHOD
Purifications (P3) LC were performed on a Gilson LC system using a Waters X-
Bridge C18
column (30 mm x 100 mm, 10 JIM; temperature: r.t.) and a gradient of 10-95% B
(A= 0.2%
NH4OH in H20; B= 0.2% NH4OH in ACN) over 14.44 min then 95% B for 2.11 min. A
second gradient of 95-10% B was then applied over 0.2 min with an injection
volume of
1500 i_LL at flow rate of 40 mL/min. UV spectra were recorded at 215 nm using
a Gilson
detector.
Method 4: BASIC STANDARD METHOD
Purifications (P4) LC were performed on a Gilson LC system using a Waters X-
Bridge C18
column (30 mm x 10 mm, 10 p,M; temperature: r.t.) and a gradient of 30-95% B
(A= 0.2%
NH4OH in water; B= 0.2% NH4OH in ACN) over 11.00 min then 95% B for 2.10 min.
A
second gradient of 95-30% B was then applied over 0.21 min with an injection
volume of
1500 i_LL at flow rate of 40 mL/min. UV spectra were recorded at 215 nm using
a Gilson
detector.
Method 5: Reverse phase chromatography using acidic pH, standard elution
method
Purifications by FCC on reverse phase silica (acidic pH, standard elution
method) were
performed on Biotage Isolera systems using the appropriate SNAP C18 cartridge
and a
gradient of 10% B (A= 0.1% formic acid in H20; B= 0.1% formic acid in ACN)
over 1.7 CV
then 10-100% B over 19.5 CV and 100% B for 2 CV.
Method 6: Reverse phase chromatography using basic pH, standard elution method
Purifications by FCC on reverse phase silica (basic pH, standard elution
method) were
performed on Biotage Isolera systems using the appropriate SNAP C18 cartridge
and a
gradient of 10% B (A= 0.1% NH3 in H20; B= 0.1% NH3 in ACN) over 1.7 CV then 10-
100%
B over 19.5 CV and 100% B for 2 CV.
51
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Method 7: Reverse phase chromatography using acidic pH, standard elution
method
Purifications by FCC on reverse phase silica (acidic pH, standard elution
method) were
performed on Biotage Isolera systems using the appropriate SNAP C18 cartridge
and a
gradient of 10% B (A= 0.1% TFA in H20; B= 0.1% TFA in ACN) over 1.7 CV then 10-
100%
B over 19.5 CV and 100% B for 2 CV.
Chiral Separation Methods:
Method Cl
Purification method = 15% IPA: 85% heptane; Chiralcel OD-H, 20 x 250 mm, 5 gm
at 18
mL/min. Sample diluent: Me0H, ACN.
Method C2
Purification method = Et0H with Cellulose-4; 21.2 x 250 mm, 5 gm column at 9
mL/min.
Sample diluent: Et0H, Me0H.
Method C3
Purification method = 15% IPA + 0.2% diethylamine: 85% CO2; Chiralpak AD-H, 10
x 250
mm, 5 gm at 15 mL/min. Sample diluent: IPA, Me0H, ACN.
Method C4
Purification method = Me0H + 0.2% diethylamine; Chiralpak AD-H, 20 x 250 mm, 5
gm at
7 mL/min. Sample diluent: Me0H.
Method C5
Purification method = 75:25 CO2:Me0H; Chiralpak AD-H, 10 x 250 mm, 5 gm at 15
mL/min. Sample diluent: Me0H.
Method C6
Purification method = 15% Me0H, 85% CO2; Chiralcel OJ-H, 10 x 250 mm, 5 gm at
15
mL/min. Sample diluent: Me0H, IPA, ACN.
Method C7
Purification method = 80:20 heptane:Et0H; Chiralpak AD-H, 20 x 250 mm, 5gm at
18
mL/min. Sample diluent: Methanol, IPA.
NMR Conditions
Unless otherwise stated, 1H NMR spectra were recorded at 500 MHz, 400 MHz or
250 MHz
on either a Bruker Avance III HD 500 MHz, Bruker Avance III HD 400 MHz
spectrometer or
Bruker Avance III HD 250 MHz spectrometer respectively. Chemical shifts, 6,
are quoted in
parts per million (ppm) and are referenced to the residual solvent peak. The
following
52
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
abbreviations are used to denote the multiplicities and general assignments: s
(singlet), d
(doublet), t (triplet), q (quartet), dd (doublet of doublets), ddd (doublet of
doublet of
doublets), dt (doublet of triplets), dq (doublet of quartets), hep (heptet), m
(multiplet), pent
(pentet), td (triplet of doublets), qd (quartet of doublets), app. (apparent)
and br. (broad).
Coupling constants, J, are quoted to the nearest 0.1 Hz.
General synthesis:
All the compounds have been synthesised with a purity > 95% unless otherwise
specified.
Chemical names are generated by Marvin Sketch, version 19.19.0, from ChemAxon
Ltd.
Scheme for route 1
0
TMSOI, tuOK
0y0
NAO
BnONI-12.HCI 0 Ms0H, KHCO3
a 0
0 Et0Ac, r.t. - reflux Et0Ac, 42 - 52 C
Step a >,0yNH
0 Step b
propanoic acid
Step c H2SO4, Et0Ac, r.t.
-20 C -45 C
Boc20, DMAP
HjL
Y) o Et3N (R)
0,
DCM, r.t.
0,
HOyt,
OH
N"s Step d
0
Intermediate 2 Intermediate 1
Step 1.a: ethyl (2R)-5- [(benzyloxy)imino]-2-{ [(tert-butoxy)carbonyl] amino}-
6-
chlorohexanoate
,0
0
CI
(R)
>,0yNH
DMSO (75 mL) was added to a solution of TMSOI (12.89 g, 58.3 mmol) and 13u0K
(6.27 g,
55.9 mmol) in anhydrous THF (60 mL) and the mixture was stirred at r.t. for 1
h. The reaction
mixture was cooled to -12 C and a solution of ethyl Boc-D-Pyroglutamate (12.5
g, 48.6
mmol) in anhydrous THF (38 mL) was added and stirred at r.t. for 16 h. The
reaction mixture
was diluted with satd aq NH4C1 solution (80 mL), H20 (15 mL) and Et0Ac (200
mL), and the
organic layer was isolated, washed with brine, and concentrated in vacuo to
approximately
100 mL. A solution of BnONH211C1 (8.14 g, 51.0 mmol) in Et0Ac (62 mL), was
added and
the mixture was stirred at reflux for 2 h. The reaction mixture was cooled to
r.t., washed with
53
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
H20 and brine, and the organic layer was concentrated in vacuo to afford the
title compound
(85% purity, 19.5 g, 40.1 mmol, 83% yield) as a colourless oil; 1H NMR (400
MHz,
chloroform-0 6 7.16 - 7.33 (m, 5H), 5.01 - 5.06 (m, 2H), 3.95 - 4.30 (m, 5H),
2.32 - 2.50
(m, 2H), 1.98 -2.13 (m, 1H), 1.75 - 1.92 (m, 1H),1.30 -1.40 (m, 9H), 1.12 -
1.24 (m, 3H),
Step 1.b: ethyl (2R)-5- [(benzyloxy)imino]piperidine-2-carboxylate
0
H
411 0,,N
To a solution of ethyl (21?)-5 -[(b enzyloxy)imino]-2- {Rtert-
butoxy)carbonyl]aminol -6-
chlorohexanoate (85% purity, 19.5 g, 40.1 mmol) in Et0Ac (157 mL) was added
Ms0H (7.8
mL, 0.12 mol) and the mixture was stirred at 42 C for 2 h. The resultant
mixture was added
to a solution of KHCO3 (20.1 g, 0.201 mol) in H20 (100 mL) and stirred at 52
C for 2 h. The
reaction mixture was cooled to r.t. and the organic layer was isolated, washed
with brine,
dried over Na2SO4, and concentrated in vacuo to afford the title compound (85%
purity, 13.0
g, 40.0 mmol) in quantitative yield as a dark orange oil; 11-1 NMR (400 MHz,
chloroform-d) 6
7.20 - 7.34 (m, 5H), 4.99 (d, J= 4.8 Hz, 2H), 4.13 (q, J= 7.1 Hz, 2H), 3.45 -
3.56 (m, 1H),
3.25 (dd, J= 14.9, 9.8 Hz, 1H), 3.08 (dt, J= 14.5, 4.3 Hz, 1H), 2.01 - 2.32
(m, 3H), 1.55 -
1.80 (m, 1H), 1.21 (t, J= 7.1 Hz, 3H).
Intermediate 1 (step 1.c): ethyl (2R,5S)-5-[(benzyloxy)amino]piperidine-2-
carboxylate
oxalic acid
0
H 1
0
H HOyL
OH
0
Intermediate 1
Propanoic acid (23 mL, 0.240 mol) was added to a suspension of NaBH4 (3.03 g,
80.0 mmol)
in Et0Ac (95 mL) and the mixture was stirred at r.t. for 1 h. The resultant
mixture was added
to a solution of ethyl (2R)-5-[(benzyloxy)imino]piperidine-2-carboxylate (85%
purity, 13.0 g,
40.0 mmol) in Et0Ac (95 mL) and H2SO4 (11 mL, 0.20 mol) at -20 C and stirred
at r.t. for
60 h. The reaction mixture was diluted with H20 (75 mL) and neutralised with
aq NH4OH
solution. The organic layer was isolated, washed with brine, dried over
Na2SO4, and
concentrated in vacuo to -75 mL volume. The solution was warmed to 45 C, and
Me0H (30
mL), followed by a solution of oxalic acid (3.60 g, 40.0 mmol) in Me0H (15 mL)
was
added. The mixture was cooled to 0 C, and the resultant precipitate was
isolated via vacuum
54
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
filtration, washing with MeOH:Et0H (1:4) and Et0Ac to afford the title
compound (7.17 g,
19.1 mmol, 48% yield); 1H NMR (500 MHz, DMSO-d6) 6 7.25 - 7.42 (m, 5H), 4.59
(s, 2H),
4.17 -4.24 (m, 2H), 3.92 (dd, J= 12.3, 3.2 Hz, 1H), 3.34 - 3.40 (m, 1H), 3.10
(ddd, J= 15.1,
7.6, 3.9 Hz, 1H), 2.64 (t, J= 11.5 Hz, 1H), 2.13 (dt, J= 10.2, 3.4 Hz, 1H),
1.87 (dd, J= 9.0,
.. 3.8 Hz, 1H), 1.65 (qd, J= 13.2, 3.6 Hz, 1H), 1.40 (qd, J= 12.8, 3.9 Hz,
1H), 1.23 (t, J= 7.1
Hz, 3H); M/Z: 279, [M+H]+, EST, RT = 0.81 (Si).
Intermediate 2 (step 1.d): 1-tert-butyl 2-ethyl (2R,5S)-5-
[(benzyloxy)amino]piperidine-
1,2-dicarboxylate
0y0 0
o__'-'--
H
1 0 Intermediate 2
To a solution of ethyl (2R,5S)-5-[(benzyloxy)amino]piperidine-2-carboxylate
oxalic acid
(2.22 g, 6.03 mmol, Intermediate 1) in anhydrous DCM (30 mL) at 0 C was added
Et3N (3.6
mL, 25.8 mmol), DMAP (76 mg, 0.622 mmol) and Boc20 (4.2 mL, 18.3 mmol) and the
mixture was stirred at r.t. for 17 h. The reaction mixture was diluted with
satd aq NH4C1
solution and DCM, and the organic layer was isolated, washed with H20 and
brine, dried over
Na2SO4, and concentrated in vacuo. The residue was purified by chromatography
on silica gel
(0-20% Et0Ac in heptane) to afford the title compound (86% purity, 1.40 g,
3.18 mmol, 53%
yield) as a colourless oil; 1H NMR (500 MHz, chloroform-d) 6 7.40 - 7.26 (m,
5H), 5.51 -
5.41 (m, 1H), 4.92 -4.80 (m, 1H), 4.79 -4.62 (m, 2H), 4.19 (q, J= 7.0 Hz, 3H),
3.11 (d, J=
45.4 Hz, 2H), 1.96 (s, 2H), 1.73 - 1.60 (m, 1H), 1.55 - 1.49 (m, 1H), 1.46 (s,
9H), 1.27 (t, J=
7.1 Hz, 3H); M/Z: 379, [M+H]+, EST, RT = 1.09 (S2).
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 2
0
oAci 130c 0
30c o 30c o II
N Pd/C, H2 0
pyridine, DMAP
ao 0,, , 0-
)L..õ
Et0H, r.t. DCM, 0 C - r.t. 0
Nõ
140
H2 N'ss'
Step a Step b
Intermediate 2 Intermediate 3 Li0H.H20
Step c THF, Et0H, H20, r.t.
Ipc 0
0
0
Intermediate 4
Intermediate 3 (step 2.a): 1-tert-butyl 2-ethyl (2R,5S)-5-aminopiperidine-1,2-
dicarboxylate
Boc 0
)1)1
(R) 0
H2I\Kµ
Intermediate 3
To a solution of 1-tert-butyl 2-ethyl (2R,55)-5-[(benzyloxy)amino]piperidine-
1,2-
dicarboxylate (82% purity, 24.9 g, 54.0 mmol, Intermediate 2) in anhydrous
Et0H (1 L) was
added 10% Pd/C (2.87 g, 2.70 mmol) and the mixture was stirred at r.t. under
H2 for 24 h. The
reaction mixture was filtered through a pad of Celite and concentrated in
vacuo. The residue
was dissolved in Et20 and washed with 2 M aq HC1 solution. The organic layer
was
discarded, and the aqueous layer was basified using solid NaHCO3 and then
extracted with
IPA:DCM (2:8). The organic extracts were washed with brine, dried over MgSO4,
and
concentrated in vacuo to afford the title compound (11.5 g, 42.2 mmol, 78%
yield) as a pale
yellow oil; 1H NMR (400 MHz, chloroform-d) 1.29 (t, J= 7.1 Hz, 3H), 1.48 (s,
9H), 1.51 ¨
1.70 (m, 4H), 1.93 ¨2.22 (m, 2H), 3.06 ¨ 3.38 (m, 2H), 3.66 ¨ 3.97 (m, 1H),
4.21 (q, J= 7.1
Hz, 2H), 4.56 ¨ 5.01 (m, 1H).
Step 2.b: 1-tert-butyl 2-ethyl (2R,5S)-5-
11(benzyloxy)carbonyljaminolpiperidine-1,2-
dicarboxylate
Boc 0
,N
0 -
To a solution of 1-tert-butyl 2-ethyl (2R,5S)-5-aminopiperidine-1,2-
dicarboxylate (2.50 g,
9.18 mmol), DMAP (90 mg, 0.739 mmol) and pyridine (1.49 mL, 18.4 mmol) in DCM
(45
56
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
mL) at 0 C was added benzyl carbonochloridate (1.99 mL, 13.9 mmol) and the
reaction
mixture was stirred at r.t. for 20 h. Further portions of pyridine (700 L,
8.6 mmol), DMAP
(42 mg, 0.34 mmol) and benzyl carbonochloridate (930 L, 6.5 mmol) were added
at 0 C
and the mixture was stirred at r.t. for 1 h. The reaction mixture was diluted
with H20 (20 mL)
and extracted with DCM (2 x 50 mL). The combined organic extracts were dried
using a
phase separator, concentrated in vacuo and purified by chromatography on
silica gel (0-100%
Et0Ac in heptane) to afford the title compound (84% purity, 3.18 g, 6.57 mmol,
72% yield)
as a colourless oil; 1H NMR (400 MHz, chloroform-0 6 7.36 - 7.21 (m, 5H), 5.16
- 4.92 (m,
3H), 4.87 - 4.50 (m, 1H), 4.13 (q, J= 6.7 Hz, 2H), 3.98 - 3.72 (m, 2H), 3.21 -
2.94 (m, 1H),
2.13 - 1.99 (m, 1H), 1.93 - 1.67 (m, 2H), 1.37 (s, 9H), 1.19 (d, J= 7.1 Hz,
3H), NH proton
not observed; M/Z: 307 [M-Boc+H]+, EST, RT = 1.08 (Si).
Intermediate 4 (step 2.c): (2R,5S)-5-{1(benzyloxy)carbonyljamino}-1-[(tert-
butoxy)carbonyl]piperidine-2-carboxylic acid
Boc 0
,N)L
Intermediate 4
A mixture of 1-tert-butyl 2-ethyl (2R,5S)-5-
{[(benzyloxy)carbonyl]aminolpiperidine-1,2-
dicarboxylate (84% purity, 3.18 g, 6.57 mmol) and Li0H.H20 (311 mg, 7.23 mmol)
in Et0H
(25 mL):THF (25 mL):Water (25 mL) was stirred at r.t. for 24 h. The reaction
mixture was
partitioned between H20 (30 mL) and Et0Ac (30 mL), and the organic layer was
discarded.
The aqueous layer was then acidified using 1 M aq HC1 solution and extracted
with Et0Ac (2
x 50 mL). The combined organic extracts were washed with brine (20 mL), dried
over
MgSO4 and concentrated in vacuo to afford the title compound (86% purity, 2.04
g, 4.64
mmol, 71% yield) as a colorless solid; M/Z: 377 [M-H]+, EST-, RT = 0.90 (S2).
Scheme for route 3
SCX powder, 0
Me0H, H
4 M NH3 in Me0H 40/ B0c20, Et3N, 7 0
0 Step a DCM, r.t.
HO OH
Step b
0
Intermediate 5
57
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 3.a: ethyl (2S,5R)-5-[(benzyloxy)amino]piperidine-2-carboxylate
To a solution of ethyl (2S,5R)-5-[(benzyloxy)amino]piperidine-2-
carboxylate;oxalic acid (10
g, 27.1 mmol) in Me0H (100 mL) was added SCX powder (50 g) and the mixture was
stirred
at r.t. for 10 min. The reaction mixture was filtered under vacuum, washing
with 4 M NH3 in
Me0H, and concentrated in vacuo to afford the title compound (6.22 g, 22.3
mmol, 82%
yield) as a viscous, yellow oil; 114 NMR (400 MHz, DMSO-d6) 6 7.40 - 7.21 (m,
5H), 6.46 (s,
1H), 4.57 (s, 2H), 4.07 (q, J= 7.1 Hz, 2H), 3.19 -3.08 (m, 3H), 2.84 -2.70 (m,
1H), 2.23
(dd, J= 11.7, 9.9 Hz, 1H), 1.92- 1.75 (m, 2H), 1.44- 1.25 (m, 1H), 1.23 - 1.08
(m, 4H);
M/Z: 279 [M+H]+, EST, RT = 0.50 (S2).
Intermediate 5 (step 3.b): 1-tert-butyl 2-ethyl (2S,5R)-5-
1(benzyloxy)amino]piperidine-
1,2-dicarboxylate
oyo 0
Intermediate 5
Boc20 (7.32 g, 33.5 mmol) was added to a solution of ethyl (2S,5R)-5-
[(benzyloxy)amino]piperidine-2-carboxylate (6.22 g, 22.3 mmol) and Et3N (12
mL, 89.4
mmol) in anhydrous DCM (110 mL) and the mixture was stirred at r.t. for 2.5 h.
The reaction
mixture was washed with satd aq NH4C1 solution (100 mL) and brine (100 mL),
dried over
Na2SO4 and concentrated in vacuo. The resultant residue was purified by
chromatography on
silica gel (5-50% Et0Ac in heptane) to afford the title compound (94% purity,
6.82 g, 16.9
mmol, 76% yield) as a colourless oil; 1H NMR (500 MHz, chloroform-d) 6 7.44 -
7.27 (m,
5H), 5.48 (s, 1H), 4.87 (d, J= 9.9 Hz, 1H), 4.79 -4.58 (m, 2H), 4.20 (q, J=
7.0 Hz, 3H), 3.12
(d, J= 44.6 Hz, 2H), 1.97 (s, 2H), 1.77- 1.62 (m, 2H), 1.58 - 1.50 (m, 1H),
1.46 (s, 9H), 1.28
(t, J= 7.1 Hz, 3H); M/Z: 324 [M-tButyl+H]+, ESI+, RT = 1.34 (Si).
58
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 4
Boc 0
11\1)( Boc 0
H HATU, DIPEA IV)L
,N 0 (R) N
0 FN1 H2N DMF, r.t. A .52 0
0 1\1\µµ*
0 Step a el
Intermediate 4
TsCI, K2CO3
Step b
ACN, 80 C
Boc
Boc )11,
F Pd/C, H2
(Ii2 0
F
0 N
H2N\sµ.-<') Et0H, r.t.
Intermediate 6 Step c
Step 4.a: tert-butyl (2R,5S)-5- { (b enzyloxy)carb onyl]
amino}-2-({ [4-
(trifluoromethyl)phenyl] formohydrazido} carb onyl)pip eridine- 1-c arboxylate
BOHF
I II
1ZIIIi1 N'
0 N't,s71\l'N
H
To a solution of (2R,5S)-5- { [(benzyloxy)carb onyl]
amino } -1- [(tert-
butoxy)carbonyl]piperidine-2-carboxylic acid (90% purity, 2.04 g, 4.85 mmol,
Intermediate
4), 4-(trifluoromethyl)benzohydrazide (1.29 g, 6.31 mmol) and HATU (2.21 g,
5.82 mmol) in
anhydrous DMF (24 mL) was added DIPEA (1.7 mL, 9.70 mmol) and the mixture was
stirred at r.t. for 16 h. The reaction mixture was partitioned between Et0Ac
(100 mL) and 1
M aq HC1 solution (50 mL). The organic layer was isolated, washed with brine
(5 x 50 mL),
dried over MgSO4, and concentrated in vacuo. The residue was purified by
chromatography
on silica gel (0-100% Et0Ac in heptane) to afford the title compound (70%
purity, 3.80 g,
4.71 mmol, 97% yield) as an off-white solid; 1H NMR (400 MHz, DMSO-d6) 6 10.59
(s, 1H),
10.01 (s, 1H), 8.07 (d, J= 8.2 Hz, 2H), 7.90 (d, J= 8.3 Hz, 2H), 7.46 ¨ 7.27
(m, 6H), 5.05 (s,
2H), 4.84 ¨4.52 (m, 2H), 4.12 ¨ 3.92 (m, 1H), 3.74 ¨3.46 (m, 2H), 2.23 ¨2.03
(m, 1H), 1.78
¨ 1.62 (m, 1H), 1.63 ¨ 1.49 (m, 1H), 1.38 (s, 9H); M/Z: 465 [M-Boc+H]+, EST,
RT = 1.01
(S2).
59
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 4.b: tert-butyl
(2R,5S)-5-11(benzyloxy)carbonyliamino}-2-{5-[4-
(trilluoromethyl)phenyl]-1,3,4-oxadiazol-2-yllpiperidine-1-carboxylate
Boc N-
I li \
0
F
Ojcs'
H
A suspension of tert-butyl
(2R,5S)-5-{ Rbenzyloxy)carbonyll amino } -2-( { [4-
(trifluoromethyl)phenyl]formohydrazido } carbonyl)piperidine-1 - carboxyl ate
(70% purity, 3.80
g, 4.71 mmol), TsC1 (2.70 g, 14.1 mmol) and K2CO3 (1.95 g, 14.1 mmol) in ACN
(100 mL)
was stirred at 80 C for 3 h. The reaction mixture was partitioned between
Et0Ac (100 mL)
and H20 (100 mL). The organic layer was isolated, washed with brine (50 mL),
dried over
MgSO4, and concentrated in vacuo. The residue was purified by chromatography
on silica gel
(0-100% Et0Ac in heptane). The resultant residue was triturated with Et20, the
solid
discarded, and the filtrate concentrated in vacuo and purified by prep. HPLC
(Method 5) to
afford the title compound (75% purity, 2.90 g, 3.98 mmol, 84% yield) as a pale
yellow oil; 1H
NMR (400 MHz, chloroform-d) 6 8.08 (d, J= 8.1 Hz, 2H), 7.71 (d, J= 8.3 Hz,
2H), 7.34 -
7.21 (m, 5H), 5.78 -5.41 (m, 1H), 5.17 -4.95 (m, 3H), 4.73 (s, 1H), 3.86 (s,
1H), 3.11 (s,
1H), 2.24 - 2.02 (m, 2H), 1.84 (d, J = 22.6 Hz, 2H), 1.41 (s, 9H); M/Z: 447 [M-
Boc+H]+,
EST, RT = 1.22 (S2).
Intermediate 6 (Step 4.c): tert-butyl (2R,5S)-5-amino-2-{544-
(trifluoromethyl)pheny1]-
1,3,4-oxadiazol-2-yllpiperidine-l-carboxylate
Boc N-
I.
F
1-12N\"µ -'-
Intermediate 6
To a solution of
tert-butyl (2R,55)-5-{ [(benzyloxy)carbonyl] amino} -2- {5- [4-
(trifluoromethyl)pheny1]-1,3,4-oxadiazol-2-yl}piperidine-1-carboxylate (75%
purity, 2.90 g,
3.98 mmol) in anhydrous Et0H (80 mL) was added Pd/C (10%, 0.21 g, 0.199 mmol)
and the
mixture was stirred under H2 at r.t. for 24 h. The reaction mixture was
filtered through a pad
of Celite and the filtrate concentrated in vacuo. The residue was partitioned
between Et0Ac
and 2 M aq HC1 solution. The organic layer was isolated and after 20 min
precipitation was
observed. The suspension was filtered under vacuum, washing with H20, to
afford the title
compound as an HC1 salt (0.76 g, 1.68 mmol, 42% yield) as a white solid; 1H
NMR (400
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
MHz, chloroform-d) 6 8.74 (s, 3H), 8.08 (d, J= 8.2 Hz, 2H), 7.73 (d, J= 8.3
Hz, 2H), 5.67 (s,
1H), 4.51 -4.36 (m, 1H), 3.63 (s, 1H), 3.19 (s, 2H), 2.61 -2.47 (m, 1H), 2.31 -
2.14 (m, 2H),
1.47 (s, 9H); M/Z: 413 [M+H]+, EST, RT = 1.08 (S2).
Scheme for route 5
BrjLo
0 4M HCI in 0
CI OH K2003 CI õ/ 1,4- dioxane ci OJL
OH
DMF, 65 C F 50 C
Step a Step b F
Intermediate 7
Step 5.a: tert-butyl 2-[3-chloro-4-(trifluoromethyl)phenoxy]acetate
ci o)-L
A mixture of 3-chloro-4-(trifluoromethyl)phenol (1.00 g, 5.09 mmol), tert-
butyl 2-
bromoacetate (0.83 mL, 5.60 mmol) and K2CO3 (1.41 g, 10.2 mmol) in anhydrous
DMF (5
mL) was stirred at 65 C for 2.5 h. The reaction mixture was cooled to r.t.,
diluted with H20
(30 mL) and extracted with Et0Ac (2 x 30 mL). The combined organic extracts
were washed
with brine (40 mL), dried over Na2SO4 and concentrated in vacuo. The residue
was purified
by chromatography on silica gel (5-50% Et0Ac in heptane) to afford the title
compound (1.35
g, 4.35 mmol, 85% yield) as a colourless oil; 11-1 NMR (500 MHz, chloroform-d)
6 7.61 (d, J
= 8.8 Hz, 1H), 7.02 (d, J= 2.5 Hz, 1H), 6.88 - 6.79 (m, 1H), 4.56 (s, 2H),
1.50 (s, 9H); M/Z:
not observed, EST, RT = 1.16 (S2).
Intermediate 7 (step 5.b): 2-[3-chloro-4-(trifluoromethyl)phenoxy]acetic acid
OH
Intermediate 7
A solution of tert-butyl 2-[3-chloro-4-(trifluoromethyl)phenoxy]acetate (1.35
g, 4.35 mmol)
in 4 M HC1 in 1,4-dioxane (10 mL) was stirred at 50 C for 6 h. The reaction
mixture was
concentrated in vacuo to afford the title compound (1.09 g, 4.28 mmol, 99%
yield) as a white
solid; 1H NMR (400 MHz, DMSO-d6) 6 13.19 (s, 1H), 7.76 (d, J= 8.9 Hz, 1H),
7.31 (d, J=
2.5 Hz, 1H), 7.07 (dd, J= 8.7, 2.4 Hz, 1H), 4.86 (s, 2H); 19F NMR (376 MHz,
DMSO-d6) 6 -
59.84 (2F, s).
61
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
The intermediates in Table 1 were synthesised according to general route 5 as
exemplified by
Intermediate 7 using the corresponding starting materials.
Table 1
Intermediate Structure Name StartingLCMS data NMR data
material
1H NMR (400 MHz,
DMSO-d6) 6 13.24 (s,
1H), 7.74 (t, J= 8.8
Hz, 1H), 7.20 (dd, J=
o 2-[3-fluoro-
13.1, 2.1 Hz, 1H),
F so OJLOH 4- 3-fluoro-4-
7.00 (dd, J= 8.8, 2.0
8 (trifluoromet (trifluoromet
F
Hz, 1H), 4.90 (s, 2H);
hyl)phenoxy hyl)phenol
F
19F NMR (376 MHz,
F ]acetic acid
DMSO-d6) 6 -58.87
(3F, d, J= 12.0 Hz), -
113.67 (1F, q, J-
12.0 Hz).
0
2-(3,4,5- 1H
NMR (400 MHz,
a 0
9
IW J.c) H trichlorophe
noxy)acetic trichlorophe
DMSO-d6) 6 13.15 (s,
1H), 7.31 (s, 2H),
a no!
acid 4.81 (s, 2H).
CI
'H NMR (400 MHz,
DMSO-d6) 6 13.21 (s,
2-(4-chloro-
1H), 7.42 ¨7.27 (m,
F 0 4-chloro-
1H), 7.02 (td, J= 9.4,
2,3-
F I& OJ'LOH difluorophen 2,3- 2.1 Hz, 1H),
4.86 (s,
difluorophen 2H); 19F NMR (376
oxy)acetic
a ol
MHz, DMSO-d6) 6 -
acid
138.49 (1F, d, J-
20.6 Hz), -155.58
(1F, d, J= 20.7 Hz).
1H NMR (400 MHz,
0 2-(4-chloro- DMSO-d6) 6 13.16 (s,
4-chloro-
F si 0j(OH 3,5-
1H), 7.09 ¨ 6.94 (m,
3,5-
11 difluorophen
2H), 4.77 (s, 2H); 19F
difluorophen
a oxy)acetic ol NMR (376 MHz,
F acid
DMSO-d6) 6 -113.48
(2F, s).
2-[(6-chloro- 1H
NMR (500 MHz,
0 5- M/Z: 206, 208 DMSO-d6) 6
13.22 (s,
6-chloro-5-
12
FO
1 JLOH fluoropy fluoropyridi
ridi [M+H]', ESI',
1H), 8.07 (d, J= 2.6
I 11-3- RI = 0.60 Hz, 1H), 7.76
(dd, J=
CIN yl)oxy]acetic n-3-ol (S2) 10.4,
2.6 Hz, 1H),
acid 4.85 (s, 2H).
2 { [6 1H NMR (400 MHz,
- -
o DMSO-d6) 6 13.27 (s,
H (trifluoromet 6- M/Z: 222
-CDH hyl)pyridin-
(trifluoromet [M+H]', ESI', 1H), 8.46 (d, J= 2.9
13 I
Hz, 1H), 7.85 (d, J¨
FN......:.- 3- hyl)pyridin- RT = 0.74
8.7 Hz, 1H), 7.59 (dd,
Fl yl]oxy}aceti 3-ol (S2)
F J= 8.8, 2.9 Hz,
1H),
c acid
4.92 (s, 2H).
62
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
111 NMR (500 MHz,
2-(4-chloro-
DMSO-d6) 6 7.29 (d,
0 M/Z: 199, 201
3- 4-chloro-3-
J= 8.8 Hz, 1H), 6.93
io 0 [M+111 , ESI',
14 J'LOH methylpheno
methylpheno (d, J= 3.0 Hz, 1H),
RT = 0.78
xy)acetic 1
6.76 (dd, J= 8.8, 3.1
01 (S2).
acid
Hz, 1H), 4.66 (s, 2H),
2.28 (s, 3H).
1H NMR (400 MHz,
DMSO-d6) 6 13.11 (s,
0 2-[3-
3-methoxy- 1H), 7.51 (d, J- 8.8
0OH methoxy-4- M/Z: 249
4-
Hz, 1H), 6.78 (d, J-
15 (trifluoromet HI, EST-, RI
(trifluoromet 2.0 Hz, 1H), 6.60 (dd,
hyl)phenoxy = 0.78 (S2).
hyl)phenol J= 8.7, 2.2 Hz, 1H),
]acetic acid
4.80 (s, 2H), 3.87 (s,
3H).
Scheme for route 6
Brj-LoX
N 0 0
OH K2CO3 N
0 j,L TEA N
j-L
0
0
CI DMF, 65 C DCM, 50 C =OH
CI CI
Step a Step b
Intermediate 16
Step 6.a: tert-butyl 2-(4-chloro-3-cyanophenoxy)acetate
0
N
0j-Lo
CI
A mixture of tert-butyl 2-bromoacetate (1.1 mL, 7.16 mmol), 2-chloro-5-
hydroxybenzonitrile
(1.00 g, 6.51 mmol) and K2CO3 (1.80 g, 13.0 mmol) in DMF (6 mL) was stirred at
65 C for 2
h. The reaction mixture was diluted with H20 (30 mL) and extracted with Et0Ac
(2 x 50
mL). The combined organic extracts were washed with brine (30 mL), dried over
Na2SO4,
and concentrated in vacuo to afford the title compound (84% purity, 2.10 g,
6.59 mmol) in
quantitative yield as an orange oil; 'H NMR (400 MHz, DMSO-d6) 6 7.65 (d, J=
9.0 Hz, 1H),
7.61 (d, J= 3.1 Hz, 1H), 7.32 (dd, J= 9.0, 3.1 Hz, 1H), 4.79 (s, 2H), 1.43 (s,
9H); M/Z: 269,
271 [M+Hr, EST, RT = 1.11 (S2).
Intermediate 16 (step 6.b): 2-(4-chloro-3-cyanophenoxy)acetic acid
0
N0
CI
Intermediate 16
To a solution of tert-butyl 2-(4-chloro-3-cyano-phenoxy)acetate (84% purity,
2.50 g, 7.84
mmol) in DCM (5 mL) was added TFA (3.0 mL, 39.2 mmol) and the mixture was
stirred at
63
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
50 C for 2.5 h. The reaction mixture was concentrated in vacuo, and the
residue was
suspended in H20 and stirred at r.t. for 15 min. The resultant precipitate was
filtered under
vacuum, washing with H20, to afford the title compound (94% purity, 1.03 g,
4.58 mmol,
58% yield) as an off white solid; 114 NMR (400 MHz, DMSO-d6) 6 13.13 (s, 1H),
7.69 ¨ 7.56
(m, 2H), 7.32 (dd, J= 9.0, 3.1 Hz, 1H), 4.81 (s, 2H); M/Z: 210, 212 [M-H],
ESL, RT = 0.76
(S2).
Scheme for route 7
o
0 0 0 F 0
K CO
OH2 3 oJ.Le< DAST F 0,
- -0- -
DMF, 65 C DCM, 0 C - r.t.
CI CI CI
Step a Step b
4 M HCI in 1,4-dioxane
Step c 1,4-dioxane
0 C -50 C
0
0j(
OH
CI
Intermediate 17
Step 7.a: tert-butyl 2-(4-chloro-3-formylphenoxy)acetate
0 0
oj=o
To a solution of 2-chloro-5-hydroxybenzaldehyde (1.0 g, 6.39 mmol) in
anhydrous DMF (10
mL) was added K2CO3 (1.77 g, 12.8 mmol) followed by tert-butyl bromoacetate
(1.0 mL,
7.03 mmol) and the mixture was stirred at 65 C for 1 h. The reaction mixture
was cooled to
r.t., poured onto H20 (100 mL) and extracted with Et0Ac (2 x 70 mL). The
combined organic
extracts were washed with brine (100 mL), dried over Na2SO4, and concentrated
in vacuo.
The residue was purified by chromatography on silica gel (10-80% Et0Ac in
heptane) to
afford the title compound (1.70 mg, 6.23 mmol, 98% yield) as a white solid; 1H
NMR (500
MHz, CDC13) 6 10.42 (s, 1H), 7.40 ¨ 7.30 (m, 2H), 7.15 (dd, J= 8.8, 3.2 Hz,
1H), 4.55 (s,
2H), 1.49 (s, 9H); M/Z: no mass ion observed [M+H]+, ESI+, RT = 1.01 (S2).
64
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 7.b: tert-butyl 2-[4-chloro-3-(difluoromethyl)phenoxy]acetate
0
0,0X
CI
To a solution of tert-butyl 2-(4-chloro-3-formylphenoxy)acetate (1.0 g, 3.66
mmol) in
anhydrous DCM (10 mL) at 0 C was added DAST (0.72 mL, 5.49 mmol) dropwise and
the
mixture was stirred at r.t. for 20 h. The reaction mixture was poured onto
satd aq NaHCO3
solution (30 mL) and extracted with DCM (2 x 30 mL). The combined organic
extracts were
dried over Na2SO4, concentrated in vacuo, and purified by chromatography on
silica gel (10-
40% Et0Ac in heptane) to afford the title compound (809 mg, 2.74 mmol, 75%
yield) as a
colourless oil; 1H NMR (500 MHz, chloroform-d) 6 7.32 (d, J= 8.8 Hz, 1H), 7.14
(d, J= 3.0
Hz, 1H), 7.02 -6.76 (m, 2H), 4.53 (s, 2H), 1.49 (s, 9H); 19F NMR (376 MHz,
chloroform-d) 6
-115.44; M/Z: 316, 318 [M+Nal+, EST, RT = 1.08 (S2).
Intermediate 17 (step 7.c): 2-[4-chloro-3-(difluoromethyl)phenoxy]acetic acid
0
OH
CI
Intermediate 17
To a solution of tert-butyl 2-[4-chloro-3-(difluoromethyl)phenoxy]acetate (809
mg, 2.74
mmol) in 1,4-dioxane (10 mL) at 0 C was added 4 M HC1 in 1,4-dioxane (3.4 mL,
13.7
mmol) and the mixture was stirred under N2 at r.t. for 20 h. A further portion
of 4 M HC1 in
1,4-dioxane (3.4 mL, 13.7 mmol) was added and the mixture was stirred at 50 C
for 7 h. A
further portion of 4 M HC1 in 1,4-dioxane (3.4 mL, 13.7 mmol) was added and
the mixture
was stirred at r.t. for 20 h. The reaction mixture was concentrated in vacuo,
triturated using
H20 and dried under vacuum filtration for 1 h to afford the title compound
(574 mg, 2.35
mmol, 86% yield) as a white powder; 1H NMR (400 MHz, DMSO-d6) 6 7.50 (d, J =
8.8 Hz,
1H), 7.30 - 6.97 (m, 3H), 4.78 (s, 2H); M/Z: 235, 237 [M-H], ESL, RT = 1.07
(S2).
The intermediate in Table 2 was synthesised according to general route 7 as
exemplified by
Intermediate 17 using the corresponding starting materials.
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Table 2
Intermediate Structure Name StartingLCMS data 111 NMR
data
material
o 2-[3-chloro-
1H NMR (500 MHz,
CI OJJ 40
OH RI 4- 2-chloro-4-
M[M/Z-:H2]35E,S2 75 13-7 DMS -d6) 13.16 (s,
56 (difluoromet hydroxybenz '
1H), 7.62 ¨ 7.57 (m,
= 0.
hyl)phenoxy aldehyde S2
1H), 7.25 ¨ 6.99 (m,
(
]acetic acid ) 3H), 4.81
(s, 2H).
Scheme for route 8
0
0
OH Cs CO ACN r.t.
OH
then 0.5 M Li0H, F
THF, r.t.
Intermediate 18
Intermediate 18: 2-[4-(trifluoromethyl)phenoxyjacetic acid
0
OOH
Intermediate 18
To a suspension of 4-(trifluoromethyl)phenol (2.00 g, 12.3 mmol) and ethyl 2-
bromoacetate
(1.4 mL, 12.6 mmol) in anhydrous ACN (50 mL) was added Cs2CO3 (6.00 g, 18.4
mmol) and
the mixture was stirred at r.t. for 17 h. The reaction mixture was diluted
with Et0Ac (20 mL),
washed with H20 (2 x 20 mL) and brine (20 mL), dried using a phase separator,
and
concentrated in vacuo. The residue was dissolved in THF (50 mL) and a solution
of 0.5 M aq
LiOH solution (49 mL, 24.7 mmol) was added, and the mixture was stirred at
r.t. for 1.5 h.
The reaction mixture was diluted with H20 (20 mL), extracted with Et0Ac (2 x
20 mL), and
the organic extracts discarded. The aqueous solution was then acidified to pH
1-2 using 1 M
aq HC1 solution and extracted with DCM (3 x 20 mL). The combined organic
extracts were
dried using a phase separator and concentrated in vacuo to afford the title
compound (2.90 g,
12.9 mmol) in quantitative yield as a white solid; 1H NMR (500 MHz, DMSO-d6) 6
13.16 (s,
66
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
1H), 7.65 (d, J= 8.6 Hz, 2H), 7.10 (d, J= 8.6 Hz, 2H), 4.79 (s, 2H); M/Z: 219
[M-HI, ESI-,
RT = 1.03 (Si).
Scheme for route 9
0
oxalyl dichloride
oJcH ______________________________ 0j-L
CI
0 C - r.t.
Step a
Intermediate 19
Intermediate 19: 2-(4-chloro-3-fluorophenoxy)acetyl chloride
F 0j(CI
CI
Intermediate 19
To a solution of 2-(4-chloro-3-fluorophenoxy)acetic acid (5.16 g, 22.7 mmol)
in DCM (45
mL) at 0 C was added oxalyl dichloride (10 mL, 0.115 mol) followed by DMF (81
tiL, 1.11
mmol) and the mixture was stirred at r.t. for 17 h. The reaction mixture was
concentrated in
vacuo to afford the title compound (90% purity, 5.30 g, 21.4 mmol, 94% yield)
as a orange
oil; 1H NMR (400 MHz, chloroform-0 6 7.31 (t, J= 8.6 Hz, 1H), 6.75 (dt, J=
10.2, 2.9 Hz,
1H), 6.66 (ddd, J= 8.9, 2.9, 1.2 Hz, 1H), 4.96 (s, 2H).
The intermediates in Table 3 were synthesised according to the general route 9
as exemplified
by Intermediate 19 using the corresponding starting materials.
Table 3
Interme Structure Name Starting LCMS
111 NMR
diate material data
11-1 NMR (500 MHz,
o 2-(3,4- 2-(3,4-
DMSO-d6) 6 7.53 (d, J=
CI CI dichloropheno dichlorophen 8.9 Hz, 1H),
7.24 (d, J=
xy)acetyl oxy)acetic
2.9 Hz, 1H), 6.96 (dd, J
CI chloride acid
= 8.9, 3.0 Hz, 1H), 4.76
(s, 2H).
2-[(6-chloro-
2-[(6-chloro-
0 5- 5-
fluoropyridi u.n- fl oropyridi
FOL
21 CI 11-3- Used crude
3-
CIN
y
yl)oxy]acetyl l)oxy]acetic
acid
chloride
(Intermediat
67
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
e 12)
Scheme for route 10
F
Boc 0
Boc 04 CI Boc 0
ci
N)L
H2 Pd/C N Intermediate 19 0 (.;/i0
H2Nts`/' Et3N, DCM, r.t. F 00 0,A s.
N'µ
Bn
Step a Intermediate 3 Step b CI
Intermediate 2
Li0H, Et0H,
Step c
H20, r.t.
Boc 0
N)L
0
//i2 OH
F 0,A '9)
CI
Intermediate 22
Intermediate 3 (step 10.a): 1-tert-butyl 2-ethyl (2R,5S)-5-aminopiperidine-1,2-
dicarboxylate
Boc 0
R) 0
H,
Intermediate 3
To a solution of 1-tert-butyl 2-ethyl (2R,55)-5-
[(benzyloxy)amino]piperidine-1,2-
dicarboxylate (93% purity, 8.7 g, 21.3 mmol, Intermediate 2) in anhydrous Et0H
(200 mL)
under N2 was added Pd/C (10%, 2.28 g, 2.14 mmol) and the mixture was stirred
under H2 at
r.t. for 17 h. The reaction mixture was filtered through a pad of Celite and
the filtrate
concentrated in vacuo. The residue was purified using an SCX-2 cartridge,
first flushing with
Me0H and second eluting with 3 M NH3 in Me0H to afford the title compound
(4.88 g, 17.0
mmol, 80% yield) as a pale yellow oil; 1H NMR (400 MHz, chloroform-d) 6 4.98 ¨
4.57 (m,
1H), 4.18 (q, J= 7.1 Hz, 2H), 3.87 ¨ 3.64 (m, 1H), 3.35 ¨2.99 (m, 2H), 2.14 ¨
1.92 (m, 2H),
1.64 ¨ 1.52 (m, 2H), 1.45 (s, 11H), 1.26 (t, J= 7.1 Hz, 3H).
68
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Step 10.b: 1-tert-butyl 2-ethyl (2R,5S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]
piperidine-1,2-dicarboxylate
Boc 0
)1
0 -
CI
To a mixture of 1-tert-butyl 2-ethyl (2R,55)-5-aminopiperidine-1,2-
dicarboxylate (4.89 g,
.. 17.1 mmol) and Et3N (14 mL, 0.103 mol) in DCM (170 mL) at 0 C was added
dropwise a
solution of 2-(4-chloro-3-fluoro-phenoxy)acetyl chloride (4.19 g, 18.8 mmol,
Intermediate
19) in DCM (10 mL) and stirred at r.t. for 48 h. The reaction mixture was
diluted with DCM
(250 mL) and washed with satd aq NaHCO3 solution (2 x 100 mL) and brine (100
mL), dried
over Na2SO4 and concentrated in vacuo. The residue was purified by
chromatography on
silica gel (0-50% Et0Ac in heptane) to afford the title compound (7.14 g, 15.6
mmol, 91%
yield) as a colourless oil; 1H NMR (400 MHz, chloroform-d) 6 7.32 (t, J= 8.6
Hz, 1H), 6.86 ¨
6.72 (m, 2H), 6.69 ¨ 6.63 (m, 1H), 4.98 ¨ 4.66 (m, 1H), 4.45 (s, 2H), 4.29 ¨
4.13 (m, 3H),
4.09 ¨ 3.87 (m, 1H), 3.33 ¨ 3.10 (m, 1H), 2.23 ¨ 2.02 (m, 1H), 2.00 ¨ 1.71 (m,
2H), 1.56 (s,
1H), 1.44 (s, 9H), 1.28 (t, J= 7.2 Hz, 3H); M/Z: 459, 461 [M+H]+, EST, RT =
3.83 (S4).
Intermediate 22 (step 10.c): (2R,5S)-1-[(tert-butoxy)carbonyI]-5-[2-(4-chloro-
3-
fluorophenoxy) acetamido]piperidine-2-carboxylic acid
Boc 0
/11\I
0 OH
Intermediate 22
LiOH (0.78 g, 31.1 mmol) was added to a solution of 1-tert-butyl 2-ethyl
(2R,55)-542-(4-
chloro-3-fluorophenoxy)acetamido] piperidine-1,2-dicarboxylate (7.1 g, 15.6
mmol) in Et0H
(80 mL) and H20 (20 mL) and the mixture was stirred at r.t. for 3 h. The
reaction mixture was
concentrated in vacuo, redissolved in H20 (50 mL), and extracted with DCM (2 x
100 mL).
The aqueous layer was then acidified to pH 2 using 2 M aq HC1 solution and
extracted with
Et0Ac (3 x 100 mL). The combined organic extracts were washed with brine (100
mL), dried
over anhydrous Na2SO4, and concentrated in vacuo to afford the title compound
(87% purity,
5.60 g, 11.3 mmol, 73% yield) as a white solid; 1H NMR (400 MHz, DMSO-d6) 6
8.02 (d, J=
7.3 Hz, 1H), 7.47 (t, J= 8.9 Hz, 1H), 7.03 (dd, J= 11.4, 2.8 Hz, 1H), 6.83
¨6.75 (m, 1H),
69
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
4.59 ¨4.54 (m, 2H), 3.93 (s, 1H), 3.73 (d, J= 54.2 Hz, 1H), 3.13 ¨2.94 (m,
1H), 2.06 ¨ 1.87
(m, 2H), 1.61 (d, J= 12.2 Hz, 1H), 1.56 ¨ 1.43 (m, 1H), 1.37 (s, 10H); M/Z:
429, 431 [M+H],
EST, RT = 0.91 min (Si).
The intermediates in Table 4 were synthesised according to general route 10 as
exemplified
by Intermediate 22 using the corresponding starting materials.
Table 4
Inte
rme Structure Starting LCMS
Name 111 NMR data
diat material data
e
1H NMR (500 MHz,
(5R)-1-[(tert-
DMSO-d6) 6 12.86 (s,
tert-butyl-ethyl
1H), 8.03 (d, J= 7.3 Hz,
butoxy)carbon
(5R)-5-
1H), 7.47 (t, J= 8.9 Hz,
Boc 0 y1]-512-(4 - M/Z: 375,
OH chloro-3-
(benzyloxyamin 1H), 7.03 (dd, J= 11.4,
377 [M-
o)piperidine-
2.8 Hz, 1H), 6.81 (d, J=
23 F a OjN 12-
,
fluorophenoxy Boc+H]'
H )acetamido]pi ESI', RI'= 8.7 Hz' 1H)' 4.70
¨4.45
a l'ILIF peridine-2- dicarboxylate
(m, 3H), 4.00 ¨3.70 (m,
0.91 (S2)
following steps
2H), 3.06 (d, J= 32.9
carboxylic
10.a - 10.c
Hz, 1H), 2.07¨ 1.83 (m,
acid
2H), 1.73 ¨ 1.42 (m, 2H),
1.37 (s, 9H).
1H NMR (500 MHz,
DMSO-d6) 6 12.83 (s,
(2R,55)-1- 1H), 8.04 (d, J= 7.3 Hz,
2-(3,4-
Wert- 1H), 7.53 (d, J= 8.9 Hz,
dichlorophenox M/Z: 347,
Boc o butoxy)carbon
1H), 7.22 (d, J= 2.8 Hz,
y)acetyl 349, 351
(........7 -OH y1]-5-[2-(3,4- 1H), 6.98 ¨6.91 (m, 1H),
chloride [M-
24 a
dichloropheno + 4.68 ¨4.50 (m, 3H), 4.02
40 H xy)acetamido] (Intermediate Boc+H]
,
20) following ESI', RI =
¨3.88 (m, 1H), 3.86 ¨
a piperidine-2-
3.75(m, 1H), 3.14 ¨3.00
steps 10.b and 0.99 (S2)
carboxylic
10.c
(m, 1H), 2.06 ¨ 1.87 (m,
acid
2H), 1.73 ¨ 1.59 (m, 1H),
1.53¨ 1.43 (m, 1H), 1.37
(s, 9H).
1H NMR (500 MHz,
(2S,5R)-1-
DMSO-d6) 6 12.86 (s,
Wert- 1H), 8.03 (d, J= 7.3 Hz,
1-tert-butyl 2-
butoxy)carbon 1H), 7.47 (t, J= 8.9 Hz,
Boc 0 ethyl (2S,5R)-5- M/Z: 375,
y1]-512-(4-
1H), 7.03 (dd, J= 11.4,
OH chlor -3- aminopiperidine 377 [M-
-1,2- tButyl+H]
2.8 Hz, 1H),6.81 (d, J=
25 F '
A(:),ANift<;s) fluorophenoxy
WI H dicarboxylate , ESI', RI
8.7 Hz, 1H), 4.70 ¨4.45
(m, 3H), 4.00 ¨3.70 (m,
)acetamido]pi
a following steps = 0.91 (S2)
peridine-2- 2H), 3.06 (d, J= 32.9
10.b and 10.c
carboxylic
Hz, 1H), 2.07¨ 1.83 (m,
acid
2H), 1.73 ¨ 1.42 (m, 2H),
1.37 (s, 9H).
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 11
Boc 0 Boc 0
0 )40H HNAHTUN,HD I PHE0A
/N)L ...-NH2
0 p N
F A O H
DMF, r.t. F al Or\ j,õ,-.5
H H
CI CI
Intermediate 22 Intermediate 26
Intermediate 26: tert-butyl (2R,5S)-5-12-(4-chloro-3-fluorophenoxy)acetamido]-
2-
(hydrazinecarbonyl)piperidine-l-carboxylate
Boc 0
,IL I\IH,
-
F 0)-LN,õ.===< H
H
CI
Intermediate 26
To a solution of (2R,55)-1- [(tert-butoxy)carbony1]-5-[2-(4-chloro-3-
fluorophenoxy)
acetamido]piperidine-2-carboxylic acid (8.43 g, 19.2 mmol, Intermediate 22)
and HATU
(8.75 g, 23.0 mmol) in DMF (80 mL) was added DIPEA (4.0 mL, 23.0 mmol) and
stirred at
r.t under N2 for 30 min. The resultant solution was added dropwise via a
cannula to a solution
of NH2-NH21120 (1.9 mL, 38.3 mmol) in DMF (40 mL) and stirred at r.t. for 30
min. The
reaction mixture was diluted with Et0Ac (150 mL) and washed with H20 (4 x 100
mL). The
combined organic extracts were dried over MgSO4, concentrated in vacua, and
purified by
chromatography on silica gel (0-10% Me0H in DCM) to afford the title compound
(4.57 g,
10.3 mmol, 54% yield) as a white solid; Ifl NMR (400 MHz, chloroform-d) 6 7.35
¨ 7.27 (m,
2H), 6.88 ¨6.58 (m, 3H), 4.75 (s, 1H), 4.45 (d, J= 3.8 Hz, 2H), 4.12 (s, 2H),
3.88 (s, 2H),
3.09 (d, J= 13.2 Hz, 1H), 2.21 ¨2.12 (m, 1H), 1.86 (s, 2H), 1.69 (ddt, J=
17.8, 14.0, 6.1 Hz,
1H), 1.44 (s, 9H); M/Z: 345, 347 [M-Boc+H]+, EST, RT = 0.82 (S2).
The intermediates in Table 5 were synthesised according to general route 11 as
exemplified
by Intermediate 26 using the corresponding starting materials.
71
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Table 5
Inte
rme Structure Starting LCMS
Name 111 NMR data
diat material data
e
1H NMR (400 MHz,
DMSO-d6) S 9.23 (s,
tert-butyl
(2R,55)-1-[(tert-
1H), 7.97 (d, J= 7.3 Hz,
(2R,5S)-542-
(3A butoxy)carbonyl M/Z: 1H), 7.53 (d, J= 8.8 Hz,
io. dichloropheno -
]-5-[2-(3,4- 361, 363,
1H), 7.23 (d, J= 2.9 Hz,
o
....N....1....yoll.õ _AN, dichlorophenox 365 [M-
1H), 6.96 (dd, J= 9.0,
o R) " N -2-
xy)acetamido]
27 y)acetamido]pip Boc+H]', 2.9 Hz, 1H),
4.65 ¨4.454
ci ojL õ, s) "
(hydrazinecarb eridine-2- ESI', RT (m,
3H), 3.99 ¨3.88 (m,
carboxylic acid = 0.92
1H), 3.88 ¨ 3.76 (m, 1H),
ci onyl)piperidin
e-1-
(Intermediate (S2)
2.03 ¨ 1.90 (m, 1H), 1.87
24) ¨
1.74 (m, 1H), 1.66 ¨
carboxylate
1.50 (m, 2H), 1.36 (s,
9H), 1.29 ¨ 1.24 (m, 1H).
1H NMR (400 MHz,
DMSO-d6) S 9.07 (s,
(2S,5R)-1-[(tert-
1H), 7.96 (d, J= 7.0 Hz,
tert-butyl
butoxy)carbonyl
1H), 7.48 (t, J= 8.9 Hz,
(2S,5R)-542- M/Z:
]-5-[2-(4-chloro-
1H), 7.05 (dd, J= 11.4,
!pc o (4-chloro-3-
3- 345, 347
2.8 Hz, 1H), 6.82 (ddd, J
N ,s.1L ,NH 2 fluorophenoxy [M-
?s, N fluorophenoxy)a =
9.0, 2.8, 1.1 Hz, 1H),
28 0 jNe H )acetamido]-2- Boc+H]',
F
W (hydrazinecarb
H dine-2- cetamido]piperi
ESI', RI 4.63 ¨4.41 (m, 3H), 4.20
(d, J= 3.7 Hz, 2H), 3.98
CI onyl)piperidin = 0.86
e-1- (S2)
carboxylic acid ¨
3.87 (m, 1H), 3.87 ¨
(Intermediate
3.77 (m, 1H), 2.08 ¨ 1.88
carboxylate
25) (m,
1H), 1.88 ¨ 1.73 (m,
1H), 1.69 ¨ 1.46 (m, 2H),
1.36 (s, 9H).
tert-butyl (2R,5S)-5- 1H
NMR (500 MHz,
(2R,55)-5- [(benzyloxy)[(b DMSO-d6) S 7.46 ¨
7.24
Boc 0 [(benzyloxy)[( enzyloxy)carbon (m,
10H), 5.20 (s, 2H),
M/Z: 499
benzyloxy)car yl]amino]-1-
[M+H], 4.93 ¨4.76 (m, 2H), 4.35
(F9 N '
29 101 0, õ= (.< H bonyl]amino]- Rtert-
ESI', RI
¨4.13 (m, 3H), 3.98¨
Nµ 2- butoxy)carbonyl
3.87 (m, 1H), 3.68 ¨3.46
0'L = 1 (S2) .00
(hydrazinecarb ]piidi2- (m,
2H), 3.29 ¨3.04 (m,
oo raz perne-
onyl)piperidin carboxylic acid
1H), 2.07¨ 1.90 (m, 1H),
e-1- (Intermediate 1.87¨ 1.59 (m, 3H), 1.28
carboxylate 44) (s, 9H).
111 NMR (400 MHz,
tert-butyl DMSO-d6) S 9.04 (s,
(2R,5S)-5-
(2R,5S)-5- 1H), 7.46 ¨7.24 (m, 5H),
{[(benzyloxy)ca M/Z: 293
{[(benzyloxy)
5.03 (s, 2H), 4.62 ¨4.37
Toc o rbonyl] amino } - [M-
carbonyl]amin (m,
1H), 4.20 (s, 2H),
,N.,...õõ01 2 -- '
30 o - (R) 1,, N,NH 1Rtert
Boc+H], 4.06 ¨3.82 (m, 1H), 3.76
õ, '=< H butoxy)carbonyl ESI', RI
40 0
onyl)piperidin ]piperidine-2- = 0.79 ¨ 3.48 (m,
1H), 3.26 ¨ (hydrazinecarb
3.13 (m, 1H), 2.12 ¨ 1.93
carboxylic acid (S2).
e-1- (m, 1H), 1.80 ¨ 1.54 (m,
(Intermediate 4)
carboxylate 2H), 1.54¨ 1.44 (m, 1H),
1.42¨ 1.20 (m, 10H)
72
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 12
yoc 0 Boc
0 --N N'NH' BrCN, NaHCO3 0 N ,R) NH' CuBr,
t-BuONO 0 N I ot--13r
F
1,4-dioxane
ACN, r.t., 40 C F
H20, r.t. \A-
CI CI Step b ci
Intermediate 26 Step a
Intermediate 31
Step 12.a: tert-butyl (2R,5S)-2-(5-amino-1,3,4-oxadiazo1-2-y1)-5-[[2-(4-chloro-
3-fluoro-
phenoxy)acetyl] amino] pip eridine- 1-carb oxylate
Boc N¨Nõ
0 )102---N1-12
F
a "IIIP
To a solution of tert-butyl (2R,55)-5 -[2-(4-chloro-3 -
fluorophenoxy)acetamido] -2-
(hydrazinecarbonyl)piperidine-1-carboxylate (3.50 g, 7.87 mmol, Intermediate
26) and
NaHCO3 (991 mg, 11.8 mmol) in H20 (10 mL) and 1,4-dioxane (40 mL) was added
BrCN
(833 mg, 7.87 mmol) and the mixture was stirred at r.t. for 12 h. The reaction
mixture was
diluted with H20 (60 mL) and extracted with Et0Ac (3 x 60 mL). The combined
organic
extracts were dried over MgSO4 and concentrated in vacuo to afford the title
compound (2.30
g, 4.65 mmol, 59% yield) as a white solid; 1H NMR (400 MHz, chloroform-0 6
7.33 (t, J =
8.6 Hz, 1H), 6.88 ¨ 6.64 (m, 3H), 5.48 (s, 1H), 4.95 (s, 2H), 4.53 ¨ 4.40 (m,
2H), 4.22 ¨ 4.01
(m, 2H), 3.15 (s, 1H), 2.23 ¨ 1.83 (m, 4H), 1.46 (s, 9H); M/Z: 470, 472
[M+H]+, EST, RT =
0.87 (S2).
Intermediate 31 (step 12.b): tert-butyl (2R,5S)-2-(5-bromo-1,3,4-oxadiazol-2-
y1)-5-[[2-(4-
chloro-3-fluoro-phenoxy)acetyl] amino] pip eridine-1-carboxylate
Boc N¨N
I
o
F
H
CI
Intermediate 31
To a solution of tert-butyl (2R, 5S)-2-(5-amino-1,3,4-oxadiazol-2-y1)-54[2-(4-
chloro-3-fluoro-
phenoxy)acetyl]amino]piperidine-l-carboxylate (2.30 g, 4.65 mmol) in anhydrous
ACN (35
mL) was added CuBr (3.16 g, 14.0 mmol) and the mixture was stirred at r.t. for
5 min. Tert-
73
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
butyl nitrite (1.9 mL, 14.0 mmol) was added and the mixture was stirred at
r.t. for 16 h then at
40 C for 12 h. The reaction mixture was concentrated in vacuo and the residue
was diluted
with Rochelle salt (150 mL) and extracted with Et0Ac (3 x 150 mL). The
combined organic
extracts were dried over MgSO4, concentrated in vacuo, and purified by
chromatography on
silica gel (12-100% Et0Ac in heptane) to afford the title compound (1.10 g,
2.03 mmol, 43%
yield) as a pale yellow solid; 1H NMR (500 MHz, chloroform-d) 6 7.33 (t, J =
8.6 Hz, 1H),
6.87 ¨6.71 (m, 2H), 6.67 (ddd, J= 8.9, 2.8, 1.1 Hz, 1H), 5.77 ¨ 5.37 (m, 1H),
4.57 ¨4.41 (m,
2H), 4.27 ¨ 3.98 (m, 2H), 3.35 ¨2.95 (m, 1H), 2.29 ¨2.12 (m, 1H), 2.07 ¨ 1.79
(m, 3H), 1.46
(s, 9H); M/Z: 433, 435 [M-Boc+H]+, EST, RT = 1.05 (S2).
The intermediates in Table 6 were synthesised according to general route 12 as
exemplified
by Intermediate 31 using the corresponding starting materials.
Table 6
Inte
rme Structure Starting LCMS
Name 111 NMR
Data
diat material data
111 NMR (400 MHz,
tert-butyl
DMSO-d6) 6 8.10 (d, J=
(2R,5S)-542-
7.0 Hz, 1H), 7.54 (d, J=
tert-butyl
(3,4-
8.9 Hz, 1H), 7.24 (d, J=
(2R,5S)-2-(5- M/Z:
dichloropheno
2.9 Hz, 1H), 6.97 (dd, J
Toc Br bromo-1,3,4-
xy)acetamido] 451, 453,
= 9.0, 2.9 Hz, 1H), 5.58
oxadiazol-2- _2_ 455 [M-
5.46 (m, 1H), 4.65 ¨
32 0 y1)-51243,4- Boc+HL
dichloropheno (hydrazinecarb
onyl)piperidin ESI', RT 4.55 (m, 2H),
3.91 (s,
2H), 3.00 (d, J= 12.3
xy)acetamido] = 1.03
e-1-
Hz, 1H), 2.29 ¨2.21 (m,
piperidine-1- (S2)
carboxylate
1H), 2.07 ¨2.01 (m, 1H),
carboxylate
(Intermediate
1.83 ¨ 1.74 (m, 1H), 1.68
27)
¨ 1.61 (m, 1H), 1.39 (s,
9H).
1H NMR (400 MHz,
tert-butyl
DMSO-d6) 6 8.11 (d, J=
tert-butyl
(2S,5R)-5-[2-
7.0 Hz, 1H), 7.49 (t, J=
(2S,5R)-2-(5-
bromo-1,3,4-
(4-chloro-3- M/Z:
8.9 Hz, 1H), 7.05 (dd, J
Boc N-N
fluorophenoxy 435,437 = 11.4, 2.8 Hz, 1H),6.89
sit oxadiazol-2-
)acetamido]-2- [M-
¨6.77 (m, 1H), 5.51 (s,
r y1)-512-(4-
33 F 0 joel.<
chloro-3-
(hydrazinecarb Boc+H]', 1H), 4.65 ¨ 4.48 (m, 2H),
fluorophenoxy
onyl)piperidin ESI', RT 3.96 ¨ 3.81 (m, 2H), 3.06
ci e-1- =1.17 ¨ 2.93 (m, 1H), 2.31 ¨
)acetamido]pi
carboxylate (S2)
2.16 (m, 1H), 2.10 ¨2.00
peridine-1-
(Intermediate
(m, 1H), 1.85 ¨ 1.71 (m,
carboxylate
28)
1H), 1.70 ¨ 1.59 (m, 1H),
1.39 (s, 9H).
74
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 13
/Boc
0 Boc
F OJL N HATU, DIPEA
OH + _______________________________________________ 0- F 0j- .=2,..7.."
CI H2d H
Step a CI
LiOH=H20
Step b Me0H/H20/THF
I
r.t.
iBoc
F
N
0
H
CI
Intermediate 34
Step 13.a: 1-tert-butyl 2-ethyl (2R,4S)-4-[2-(4-
chloro-3-
fluorophenoxy)acetamido]pyrrolidine-1,2-dicarboxylate
/Boc
R
Nr 0
H
ci
To a solution of 2-(4-chloro-3-fluorophenoxy)acetic acid (250 mg, 1.22 mmol)
in anhydrous
DMF (4 mL) was added HATU (558 mg, 1.47 mmol) and DIPEA (0.32 mL, 1.83 mmol)
and
stirred at r.t. for 10 min. 1-tert-Butyl 2-ethyl (2R,4S)-4-aminopyrrolidine-
1,2-dicarboxylate
(299 mg, 1.22 mmol) was added and the mixture was stirred at r.t. for 45 min.
The reaction
mixture was partitioned between Et0Ac (50 mL) and H20 (30 mL) and the organic
layer was
isolated, washed with brine, dried over MgSO4, and concentrated in vacuo. The
residue was
purified by chromatography on silica gel (0-70% Et0Ac in heptane) to afford
the title
compound (87% purity, 505 mg, 1.02 mmol, 83% yield) as an off-white solid; 1H
NMR (400
MHz, DMSO-d6) 6 8.38 (d, J= 6.7 Hz, 1H), 7.49 (t, J= 8.9 Hz, 1H), 7.06 (dd, J=
11.4, 2.8
Hz, 1H), 6.89 -6.80 (m, 1H), 4.54 (s, 2H), 4.40 - 4.26 (m, 2H), 3.67 (d, J=
11.1 Hz, 3H),
3.64 - 3.52 (m, 1H), 3.28 - 3.16 (m, 1H), 2.30 -2.15 (m, 1H), 2.09 (dt, J=
12.7, 6.1 Hz, 1H),
1.37 (d, J= 22.2 Hz, 9H); M/Z: 331, 333 [M-Boc+H], EST, RT = 0.97 (S2).
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Intermediate 34 (step 13.b): (2R,4S)-1-1(tert-butoxy)carbony1]-4-[2-(4-chloro-
3-
fluorophenoxy)acetamido]pyrrolidine-2-carboxylic acid
Boc
0 N OH
0
CI
Intermediate 34
To a solution of 1-tert-butyl 2-ethyl
(2R,4S)-4-[2-(4-chloro-3-
fluorophenoxy)acetamido]pyrrolidine-1,2-dicarboxylate (87% purity, 505 mg,
1.02 mmol) in
Me0H (2 mL):THF (2 mL):H20 (2 mL) was added Li0H.H20 (53 mg, 1.22 mmol) and
the
mixture was stirred at r.t. for 17 h. The reaction mixture was partitioned
between Et0Ac (30
mL) and 1 M aq HC1 solution (10 mL). The organic layer was isolated, washed
with brine,
dried over MgSO4, and concentrated in vacuo to afford the title compound (80%
purity, 401
mg, 0.770 mmol, 76% yield) as a colourless oil; 11-1 NMR (400 MHz, DMSO-d6) 6
8.37 (d, J
= 6.9 Hz, 1H), 7.54 - 7.45 (m, 1H), 7.06 (dd, J= 11.4, 2.8 Hz, 1H), 6.84 (dt,
J= 9.0, 1.4 Hz,
1H), 4.54 (s, 2H), 4.35 (dq, J= 12.1, 6.5 Hz, 1H), 4.26 - 4.15 (m, 1H), 3.64 -
3.52 (m, 1H),
3.20 (dq, J= 15.0, 5.9, 5.5 Hz, 1H), 2.28 - 2.12 (m, 1H), 2.12 -2.01 (m, 1H),
1.37 (d, J=
15.9 Hz, 9H); M/Z: 317, 319 [M-Boc+H]+, EST, RT = 0.86 (S2).
Scheme for route 14
2-fluoropyridine,
TMS-CF3, Ag0Tf 4 M HCI 0
0 Selectfluor, KF o
in 1,4-dioxane
_____________________________________________________________ HOOF
)OH Et0Ac, r.t. OF
r.t. )<F
Step a Step b
Intermediate 35
Step 14.a: tert-butyl 4-(trifluoromethoxy)butanoate
XooxF
2-Fluoropyridine (1.6 mL, 18.2 mmol) and TMS-CF3 (2.7 mL, 18.2 mmol) were
successively
added dropwise to a solution of tert-butyl 4-hydroxybutanoate (1.0 g, 6.05
mmol), Ag0Tf
(4.69 g, 18.2 mmol), Selectfluor (3.22 g, 9.08 mmol) and KF (1.41 g, 24.2
mmol) in Et0Ac
(50 mL) under N2 in a foil-covered flask and the mixture was stirred at r.t.
for 24 h. The
reaction mixture was filtered through Celite, washing with Et0Ac (30 mL), and
concentrated
in vacuo. The residue was purified by chromatography on silica gel (5-30%
Et0Ac in
heptane) to afford the title compound (90% purity, 330 mg, 1.30 mmol, 21%
yield) as a
76
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
colourless oil; 11-1 NMR (400 MHz, chloroform-d) 6 4.02 (t, J= 6.2 Hz, 2H),
2.37 (t, J= 7.2
Hz, 2H), 1.98 (p, J= 6.7 Hz, 2H), 1.46 (s, 9H); 19F NMR (376 MHz, chloroform-
d) 6 -60.81
(3F, s).
Intermediate 35 (step 14.b): 4-(trifluoromethoxy)butanoic acid
HOOF
Intermediate 35
A solution of tert-butyl 4-(trifluoromethoxy)butanoate (330 mg, 1.45 mmol) in
4 M HC1 in
1,4-dioxane (5 mL) was stirred at r.t. for 2 h. The reaction mixture was
concentrated in vacuo
to afford the title compound (83% purity, 73 mg, 0.352 mmol, 24% yield) as a
yellow oil; 1H
NMR (400 MHz, DMSO-d6) 6 12.20 (s, 1H), 4.32 (td, J= 6.4, 4.2 Hz, 1H), 4.09
(t, J= 6.5
Hz, 2H), 2.32 (td, J = 7.2, 2.8 Hz, 3H), 1.95 - 1.80 (m, 3H), 1.40 (s, 1H);
19F NMR (376
MHz, DMSO-d6) 6 -58.82 (3F, s).
Scheme for route 15
2-fluoropyridine,
TMS-CF3, Ag0Tf 0 4 M HCI HCI
Selectfluor, KF in 1,4- dioxane
___________ 0 0
HN
Et0Ac, r.t. __________________________ 0 F __ F 1,4- dioxane, r.t.
F F
S
Step a tep b
Intermediate 36
Step 15.a: tert-butyl 3-methy1-3-(trifluoromethoxy)azetidine-1-carboxylate
0
F __________________ F
To a solution of tert-butyl 3-hydroxy-3-methylazetidine-1-carboxylate (1.00 g,
5.34 mmol) in
Et0Ac (30 mL) at r.t. under N2 in a foil-covered flask was added Ag0Tf (4.13
g, 16.0 mmol),
KF (1.24 g, 21.4 mmol) and Selectfluor (2.84 g, 8.01 mmol) and stirred at r.t.
for 5 min. 2-
Fluoropyridine (1.4 mL, 16.0 mmol) and TMS-CF3 (2.4 mL, 16.0 mmol) were then
added and
the mixture was stirred at r.t. for 3 h. The reaction mixture was filtered
through Celite,
washing with Et0Ac (100 mL), and the filtrate was concentrated in vacuo. The
residue was
purified by chromatography on silica gel (0-50% Et0Ac in heptane) to afford
the title
compound (163 mg, 0.639 mmol, 12% yield) as a colourless oil; 1H NMR (400 MHz,
DMSO-
d6) 6 4.02 (d, J= 9.5 Hz, 2H), 3.91 (d, J= 9.6 Hz, 2H), 1.67 (s, 3H), 1.39 (s,
9H).
77
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Intermediate 36 (step 15.b): 3-methyl-3-(trifluoromethoxy)azetidine
hydrochloride
HCI FINo
F ________________ F
Intermediate 36
To a solution of tert-butyl 3-methyl-3-(trifluoromethoxy)azetidine-1 -
carboxylate (160 mg,
0.627 mmol) in anhydrous 1,4-dioxane (3 mL) was added 4 M HC1 in 1,4-dioxane
(1.0 mL,
4.00 mmol) and the mixture was stirred at r.t. for 20 h. The reaction mixture
was concentrated
in vacuo to afford the title compound (116 mg, 0.605 mmol, 97% yield) as a
white powder;
1H NMR (400 MHz, DMSO-d6) 6 9.18 (s, 2H), 4.22 (d, J= 12.2 Hz, 2H), 4.07 (d,
J= 12.5
Hz, 2H), 1.74 (s, 3H); M/Z: 156 [M+H]+, EST, RT = 0.37 (S2).
Scheme for route 16
I.
0 a
0 1,r
HN¨
F
HCI ______ \oF H2 N)0 DIPEA, pyridine
* N 401 ____________________ y N o
THF
0 H
Step a
Pd/C, H2
Step b
Et0H, r.t.
F 0
FY C\N
1\11-12
0
Intermediate 37
Step 16.a:
({ [(benzyloxy)carbonyl] amino} amino) [3-(trifluoromethoxy)azetidin-1-
yl] meth anone
F 0
11)
yN 0 io
0 H
To a solution of benzyl hydrazinecarboxylate (406 L, 2.52 mmol) and pyridine
(407 L,
5.03 mmol) in anhydrous THF (5 mL) was added a solution of 4-nitrophenyl
carbonochloridate (558 mg, 2.77 mmol) in anhydrous THF (3 mL) and the mixture
was stirred
under N2 at r.t. for 1 h. The mixture was then added slowly to a solution of 3-
(trifluoromethoxy)azetidine hydrochloride (469 mg, 2.64 mmol) and DIPEA (1.3
mL, 7.55
78
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
mmol) in anhydrous THF (5 mL) and stirred at r.t. under N2 for 30 min. The
reaction mixture
was quenched with satd aq NaHCO3 solution (10 mL) and extracted with Et0Ac (3
x 10 mL).
The combined organic extracts were washed with H20 (30 mL) and brine (30 mL),
dried over
MgSO4, and concentrated in vacuo. The residue was dissolved in Et0Ac (10 mL)
and washed
with satd aq K2CO3 solution (3 x 10 mL). The organic layer was dried over
MgSO4 and
concentrated in vacuo to afford the title compound (92% purity, 770 mg, 2.13
mmol, 85%
yield) as an off-white powder; 1H NMR (500 MHz, DMSO-d6) 6 9.00 (s, 1H), 8.51
(s, 1H),
7.41 -7.26 (m, 5H), 5.21 -5.11 (m, 1H), 5.07 (s, 2H), 4.27 - 4.17 (m, 2H),
3.92 - 3.83 (m,
2H); M/Z: 334 [M+H]+, EST, RT = 0.74 (S2).
Intermediate 37 (step 16.b): 3-(trifluoromethoxy)azetidine-1-carbohydrazide
F 0
F>r c\N
y 1\11-12
0
Intermediate 37
To a solution of ( { [(benzyloxy)carbonyl] amino } amino) [3 -
(trifluoromethoxy)azetidin-1 -
yl]methanone (92% purity, 767 mg, 2.12 mmol) in anhydrous Et0H (21 mL) under
N2 was
added 10% Pd/C (226 mg, 0.212 mmol) and the mixture was stirred under H2 at
r.t. for 4 h.
The reaction mixture was filtered through a pad of Celite, washing with warm
Et0H (3 x 15
mL) and concentrated in vacuo to afford the title compound (88% purity, 269
mg, 1.19 mmol,
56% yield) as a brown solid; 1H NMR (500 MHz, DMSO-d6) 6 7.65 (s, 1H), 5.18 -
5.07 (m,
1H), 4.23 -4.11 (m, 2H), 3.88 (s, 2H), 3.86 -3.76 (m, 2H).
Scheme for route 17
F OH
CDI
_________________________ F '
NH2-N H2. H20 FOyN,,N H2
F F THF, 0 C - r.t. F F 0 DCM, r.t. F F 0
Step a Step b Intermediate 38
Step 17.a: 3,3,3-trifluoropropyl imidazole-l-carboxylate
F F
3,3,3-trifluoropropan-1-ol (1.00 g, 8.77 mmol) in DCM (20 mL) was added to a
solution of
CDI (2.13 g, 13.1 mmol) in THF (50 mL) at 0 C under N2 and the mixture was
stirred at r.t.
for 2 h. The reaction mixture was concentrated in vacuo and the residue was
purified by
79
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
chromatography on silica gel (12-100% Et0Ac in heptane) to afford the title
compound (92%
purity, 827 mg, 3.89 mmol, 44% yield) as a colourless oil; 1H NMR (500 MHz,
DMSO-d6) 6
8.25 (s, 1H), 7.58 (s, 1H), 7.12 ¨7.08 (m, 1H), 4.64 ¨4.58 (m, 2H), 2.87 (tp,
J= 11.3, 5.8 Hz,
2H).
Intermediate 38 (step 17.b): (3,3,3-trifluoropropoxy)carbohydrazide
F)(0yN,Ni H2
F F 0
Intermediate 38
A solution of 3,3,3-trifluoropropyl imidazole-l-carboxylate (92% purity, 8.95
g, 39.6 mmol)
in DCM (140 mL) was treated with NH2NH2J-120 (7.8 mL, 0.158 mol) and the
mixture was
stirred at r.t. for 1 h. The reaction mixture was diluted with IPA (30 mL) and
H20 (50 mL),
and the organic layer was isolated and washed successively with satd aq NaHCO3
solution (50
mL) and brine (50 mL). The organic layer was dried over MgSO4 and concentrated
in vacuo
to afford the title compound (90% purity, 4.09 g, 21.4 mmol, 54% yield) as a
pale yellow oil;
1H NMR (500 MHz, chloroform-0 6 2.28 ¨ 2.57 (m, 2H), 3.69 (s, 2H), 4.19 ¨ 4.36
(m, 2H),
5.99 (s, 1H).
Scheme for route 18
0
H0(
F /P'F
Cul 0 Pd/C, 2
0 H _________________ ci yF ______________
HOOF
ACN, 50 C THF, Ft.
Step a Step b
Intermediate 39
Step 18.a: { [2- (difluoromethoxy)ethoxy] methyl} benzene
(DOF
To a solution of 2-(benzyloxy)ethanol (1.50 g, 9.86 mmol) in anhydrous ACN (20
mL) was
added CuI (469 mg, 2.46 mmol) and the mixture was stirred at 50 C for 5 min.
A solution of
2,2-difluoro-2-(fluorosulfonyl)acetic acid (2.63 g, 14.8 mmol) in anhydrous
ACN (10 mL)
was added dropwise over 25 min and the resultant mixture was stirred at 50 C
for 1 h. The
reaction mixture was concentrated in vacuo and the residue was dissolved in
Et0Ac. The
resultant precipitate was filtered under vacuum, and the filtrate was isolated
and concentrated
in vacuo. The residue was purified by chromatography on silica gel (10-100%
Et0Ac in
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
heptane) to afford the title compound (90% purity, 620 mg, 2.76 mmol, 28%
yield) as a
colourless oil; 1H NMR (400 MHz, chloroform-0 6 7.40 ¨ 7.27 (m, 5H), 6.28 (t,
J= 74.8 Hz,
1H), 4.58 (s, 2H), 4.04 ¨ 3.97 (m, 2H), 3.71 ¨ 3.62 (m, 2H); 19F NMR (376 MHz,
chloroform-
d) 6 -84.27.
Intermediate 39 (step 18.b): 2-(difluoromethoxy)ethan-1-ol
1-10("yF
Intermediate 39
A suspension of {[2-(difluoromethoxy)ethoxy]methylfbenzene (90% purity, 620
mg, 2.76
mmol) and 10% Pd/C (587 mg, 0.552 mmol) in THF (7.5 mL) was stirred under H2
for 18 h.
The reaction mixture was filtered through Celite and washed with THF. The
filtrate was
recharged with 10% Pd/C (1.47 g, 1.38 mmol) and the mixture was stirred under
H2 for 18 h.
The reaction mixture was filtered through Celite, washing with THF, to afford
a crude
solution estimated to be 2.23% of the title compound in THF/toluene
(2.23:95.9:1.83, ¨10
mL); 11-1 NMR (400 MHz, chloroform-d) 6 6.20 (t, J = 74.9 Hz, 1H), 3.89 ¨ 3.81
(m, 2H),
3.76 ¨ 3.56 (m, 2H).
Scheme for route 19
0
io
Et 3N TMSCF3, Nal H0()
DCM, 0 C - r.t.
o
THF, 65 C __________________________________________________________________
F
Step a Step b
1 M NaOH,
Step c 2-methyloxolane
r.t.
Intermediate 40
Step 19.a: 2-(ethenyloxy)ethyl benzoate
0
13C)
To a solution of 2-(ethenyloxy)ethan-1-ol (1.00 g, 11.3 mmol) in DCM (40 mL)
at 0 C was
added Et3N (4.0 mL, 28.4 mmol) followed dropwise by benzoyl chloride (2.0 mL,
17.0 mmol)
and the mixture was stirred at r.t. for 6 h. The reaction mixture was cooled
to 0 C and
quenched with 1 M aq HC1 solution (10 mL). The aqueous solution was extracted
with DCM
81
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
(3 x 20 mL) and the combined organic extracts were washed sequentially with
satd aq
NaHCO3 solution (15 mL) and brine (15 mL), dried over Na2SO4, and concentrated
in vacuo.
The residue was purified by chromatography on silica gel (0-80% Et0Ac in
heptane) to afford
the title compound (760 mg, 3.95 mmol, 35% yield) as a clear oil; 1H NMR (400
MHz,
DMS046) 6 8.00 - 7.93 (m, 2H), 7.70 - 7.61 (m, 1H), 7.58 - 7.49 (m, 2H), 6.55
(dd, J =
14.3, 6.8 Hz, 1H), 4.51 -4.45 (m, 2H), 4.26 (dd, J= 14.3, 1.9 Hz, 1H), 4.05 -
3.99 (m, 3H).
Step 19.b: 2-(2,2-difluorocyclopropoxy)ethyl benzoate
0
0Y-F
To a solution of 2-(ethenyloxy)ethyl benzoate (50 mg, 0.260 mmol) in THF (2
mL) was
added TMSCF3 (77 !IL, 0.520 mmol) and NaI (86 mg, 0.572 mmol) and the mixture
was
stirred under N2 at 65 C for 4 h. The reaction mixture was cooled to r.t.,
diluted with H20 (5
mL) and extracted with Et0Ac (3 x 5 mL). The combined organic extracts were
washed with
brine (5 mL), dried over Na2SO4 and concentrated in vacuo. The residue was
purified by
chromatography on silica gel (0-80% Et0Ac in heptane) to afford the title
compound (62 mg,
0.246 mmol, 94% yield) as a clear oil; 1H NMR (500 MHz, DMSO-d6) 6 8.02 - 7.91
(m, 2H),
7.71 - 7.62 (m, 1H), 7.59 - 7.48 (m, 2H), 4.49 - 4.37 (m, 2H), 4.02 - 3.93 (m,
1H), 3.95 -
3.84 (m, 2H), 1.76 - 1.65 (m, 1H), 1.60 - 1.50 (m, 1H); M/Z: 243 [M+H]+, EST,
RT = 1.22
(Si).
Intermediate 40 (step 19.c): 2-(2,2-difluorocyclopropoxy)ethan-1-ol
F
Intermediate 40
To a solution of 2-(2,2-difluorocyclopropoxy)ethyl benzoate (60 mg, 0.248
mmol) in 2-
methyloxolane (1 mL) was added 1 M aq NaOH solution (0.99 mL, 0.991 mmol) and
the
mixture was stirred at r.t. for 24 h. The reaction mixture was diluted with
H20 (3 mL) and
extracted with Et20 (3 x 3 mL). The combined organic extracts were dried over
MgSO4, and
concentrated in vacuo at r.t. to afford the title compound (17 mg, 0.123 mmol,
50% yield) as a
clear oil; 1H NMR (500 MHz, DMSO-d6) 6 4.72 (t, J= 5.3 Hz, 1H), 3.91 - 3.84
(m, 1H), 3.59
-3.49 (m, 4H), 1.71 -1.59 (m, 1H), 1.54 - 1.43 (m, 1H).
82
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 20
0 Br K+ N 4111 H 0
0 0 0 MeNH-NH2 OyNje\
0
DMF, 90 C 0).YH=r(:) Et0H, r.t. H,N)\/
Br 0
Step a 0 0 0 Step b
0
F am 0,11,c,
ci '10"
Step c
Intermediate 19
DIPEA,
DCM r.t.
v
H H 0
0 o 0
0 OH LiOH
F 1stMeOH, H20, r.t. F
CI 111411111 Step d CI
Intermediate 41
Step 20.a: 1,6-diethyl 2,5-bis(1,3-dioxo-2,3-dihydro-1H-isoindo1-2-
yl)hexanedioate
0
(D
0
0 0
A mixture of (1,3-dioxoisoindolin-2-yl)potassium (4.55 g, 24.4 mmol) and
diethyl 2,5-
dibromohexanedioate (4.0 g, 11.1 mmol) in DMF (40 mL) was stirred at 90 C for
4 h and
then allowed to cool to r.t. The reaction mixture was concentrated in vacuo,
and the residue
was diluted with H20 (150 mL) and extracted with Et0Ac (2 x 100 mL). The
combined
organic extracts were dried over Na2SO4, concentrated in vacuo, and purified
by prep. HPLC
(Method 5) to afford the title compound (91% purity, 2.51 g, 4.64 mmol, 42%
yield) as a
yellow oil; 11-1 NMR (500 MHz, chloroform-d) 6 7.92 ¨ 7.80 (m, 4H), 7.79 ¨
7.70 (m, 4H),
4.95 ¨ 4.89 (m, 1H), 4.82 ¨ 4.74 (m, 1H), 4.23 ¨ 4.07 (m, 4H), 2.38 ¨ 2.21 (m,
4H), 1.20 ¨
1.13 (m, 6H); M/Z: 493 [M+H], EST, RT = 1.02 (Si).
Step 20.b: ethyl 5-amino-6-oxopiperidine-2-carboxylate
0
H2
83
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Methylhydrazine (0.73 mL, 13.72 mmol) was added to a suspension of 1,6-diethyl
2,5-
bis(1,3-dioxo-2,3-dihydro-1H-isoindo1-2-yl)hexanedioate (2.40 g, 4.64 mmol) in
Et0H (25
mL) and the mixture was stirred at reflux for 6 h. The reaction mixture was
concentrated by
half and cooled to 0 C. The solid was filtered under vacuum and the filtrate
concentrated in
vacuo. The residue was dissolved in Et0H (5 mL) and purified using an SCX-2
cartridge, first
flushing with Et0H (2 x 20 mL) and second eluting with 7 M NH3 in Me0H to
afford the title
compound (835 mg, 4.48 mmol, 97% yield) as a tan oil; M/Z: 187 [M+H], EST, RT
= 0.16
(Si).
Step 20.c: ethyl 5-[2-(4-chloro-3-fluorophenoxy)acetamidol-6-oxopiperidine-2-
carboxylate
0
00 N
O N
CI
To a stirred solution of ethyl 5-amino-6-oxo-piperidine-2-carboxylate (700 mg,
3.76 mmol)
and DIPEA (1.3 mL, 7.52 mmol) in DCM (8 mL) at 0 C was added a solution of 2-
(4-chloro-
3-fluoro-phenoxy)acetyl chloride (838 mg, 3.76 mmol) in DCM (2 mL) and stirred
at r.t. for 2
h. The mixture was diluted with DCM (20 mL), and washed with 1 M aq HC1
solution (20
mL). The organic extracts were dried over Na2SO4, concentrated in vacuo, and
purified by
prep. HPLC (Method 5) to afford the title compound (72% purity, 433 mg, 0.836
mmol, 22%
yield) as a white solid; 1H NMR (400 MHz, chloroform-d) 6 7.41 - 7.28 (m, 2H),
6.80 - 6.74
(m, 1H), 6.73 - 6.66 (m, 1H), 6.33 - 6.25 (m, 1H), 4.51 - 4.46 (m, 2H), 4.35 -
4.22 (m, 2H),
4.20 - 4.11 (m, 1H), 2.71 -2.49 (m, 1H), 2.47 - 2.13 (m, 2H), 2.01 - 1.88 (m,
1H), 1.80 -
1.67 (m, 1H), 1.64- 1.50 (m, 1H), 1.35 - 1.26 (m, 2H), (contains 25% methyl
ester impurity);
M/Z: 373, 375 [M+Na], EST, RT = 0.80 (S2).
Intermediate 41 (step 20.d): 5-[2-(4-chloro-3-fluorophenoxy)acetamido]-6-
oxopiperidine-2-carboxylic acid
o
0 OH
0 F j_LN
CI
Intermediate 41
84
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
To
a solution of ethyl 5-[2-(4-chloro-3-fluorophenoxy)acetamido]-6-oxopiperidine-
2-
carboxylate (72% purity, 330 mg, 0.637 mmol) in H20 (3 mL) and Me0H (5 mL) was
added
dropwise 2 M aq LiOH solution (0.38 mL, 0.765 mmol) and the mixture was
stirred at r.t. for
h. The reaction mixture was acidified to pH 5 using 1 M aq HC1 solution (0.6
mL), and then
5 concentrated in vacuo to afford the title compound (55% purity, 397
mg, 0.633 mmol, 99%
yield) as an off-white solid; M/Z: 345, 347, [M+H]+, EST, RT = 0.66, 0.68
(S2).
Scheme for route 21
o
Jil
Boc 0 CI 0 ei
Boc 0 Boc 0
I
ri 4 iL Et0H Li0H.H20,
Pyridine -. OEt
N DCM, r.t. N H20, r.t. N
H
Step a 0 0 Step b 0 0
Intermediate 5
0 40
Intermediate 42
St 4 M HCI in
1,4-dioxane
ep c
DCM, r.t.
.
o ) 10% Pd/C,
H2, 0 HCI
0
ii/s.; OH 0
H 0
N-'4µii0H Boc20
0,No.<2 STAB 0
..
Bocr\ore"</ Et0H, r.t.
DCE, r.t.
H 0 0 0 0
Step e Step d
Intermediate 43
40 40
Step 21.a: 1-tert-butyl 2-ethyl
(2S,5R)-5-
[(benzyloxy)1(benzyloxy)carbonyl[amino]piperidine-1,2-dicarboxylate
Boc 0
li
0 Et
N
0 0
1410
To a mixture of 1-tert-butyl 2-ethyl (2S,5R)-5-[(benzyloxy)amino]piperidine-
1,2-
dicarboxylate (2.30 g, 6.08 mmol, Intermediate 5), DMAP (74 mg, 0.608 mmol)
and pyridine
(0.98 mL, 12.2 mmol) in DCM (23 mL) at 0 C was added benzyl carbonochloridate
(1.3 mL,
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
9.12 mmol) and the mixture was stirred at r.t. for 4 h. The reaction mixture
was poured onto
brine and extracted with DCM. The combined organic extracts were dried over
Na2SO4, and
concentrated in vacuo. The residue was purified by chromatography on silica
gel (0-50%
Et0Ac in heptane) to afford the title compound (91% purity, 2.97 g, 5.27 mmol,
87% yield)
.. as a colorless oil; 1H NMR (400 MHz, chloroform-d) 6 7.45 - 7.27 (m, 10H),
5.28 - 5.17 (m,
2H), 4.92 - 4.84 (m, 2H), 4.56 (s, 1H), 4.28 (s, 1H), 4.19 (q, J= 7.1 Hz, 2H),
3.51 (dd, J=
14.2, 5.0 Hz, 1H), 2.27 - 2.18 (m, 1H), 1.94 - 1.85 (m, 2H), 1.77 - 1.68 (m,
1H), 1.40 (s, 9H),
1.26 (td, J= 7.1, 3.0 Hz, 3H); M/Z: 535 [M+Na], EST, RT = 1.44 (Si).
Intermediate 42 (step 21.b): (2S,5R)-5-1(benzyloxy)1(benzyloxy)carbonyljamino]-
1- [(tert-
1 0 butoxy)carbonyl] piperidine-2-carboxylic acid
3,,c 0
OH
oo
Intermediate 42
To a solution of 1-tert-butyl 2-ethyl
(2S,5R)-5-
Rbenzyloxy)[(benzyloxy)carbonyl]amino]piperidine-1,2-dicarboxylate (91%
purity, 2.97 g,
5.27 mmol) in THF (10 mL) and Et0H (10 mL) was added a solution of Li0H.H20
(249 mg,
5.80 mmol) in H20 (10 mL) and stirred at r.t. for 16 h. The reaction mixture
was acidified to
-pH 4 using 1 M aq HC1 solution and extracted with Et0Ac (2 x 100 mL). The
combined
organic extracts were dried over Na2SO4, concentrated in vacuo, and purified
by
chromatography on silica gel (25-100% Et0Ac in heptane) to afford the title
compound (77%
purity, 2.01 g, 3.19 mmol, 61% yield) as a clear oil; 1H NMR (400 MHz, DMSO-
d6) 6 7.45 -
7.17 (m, 10H), 5.22 - 5.17 (m, 2H), 4.88 -4.77 (m, 2H), 4.66 -4.54 (m, 1H),
4.52 -4.47 (m,
1H), 4.43 -4.32 (m, 1H), 4.26 -4.12 (m, 1H), 3.97 -3.88 (m, 1H), 2.16 -2.02
(m, 1H), 1.83
- 1.66 (m, 3H), 1.30 (s, 9H); M/Z: 507 [M+H]+, EST, RT = 1.28 (Si).
86
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Step 21.c: (2S,5R)-5- [(benzyloxy) [(benzyloxy)carbonyl] amino] piperidine-2-c
arboxylic
acid hydrochloride
HCI 0
H
(R)
eL0
To
a stirred solution of (2S,5R)-5-Rbenzyloxy)[(benzyloxy)carbonyl]amino]-1-
[(tert-
butoxy)carbonyl]piperidine-2-carboxylic acid (77% purity, 200 mg, 0.318 mmol,
Intermediate
42) in DCM (2 mL) at 0 C was added 4 M HC1 in 1,4-dioxane (0.50 mL, 2.0 mmol)
and the
mixture was stirred at r.t for 2 h. The reaction mixture was concentrated in
vacuo to afford the
title compound (58% purity, 180 mg, 0.248 mmol, 78% yield) as a white solid;
M/Z: 385
[M+H]+, EST, RT = 0.97 (Si).
Step 21.d: (2S,5R)-5- [(benzyloxy) [(benzyloxy)carbonyl] amino] -1-ethylpip
eridine-2-
carboxylic acid
0
00
101
To
a suspension of (2S,5R)-5-Rbenzyloxy) [(b enzyloxy)carbonyl] amino]
piperidine-2-
carboxylic acid hydrochloride (58% purity, 169 mg, 0.233 mmol) in DCE (5 mL)
was
added acetaldehyde (36 mg, 0.806 mmol) and stirred at r.t. for 10 min. STAB
(198 mg, 0.93
mmol) was added and the mixture was stirred at r.t. for 16 h. The reaction
mixture was
concentrated in vacuo and purified by prep. HPLC (Method 5) to afford the
title compound
(78 mg, 0.183 mmol, 59% yield) as a beige solid; M/Z: 413 [M+H]+, EST, RT =
1.00 (Si).
87
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Intermediate 43 (step 21.e): (2S,5R)-5-{ Wert-butoxy)carbonyliamino}-1-
ethylpiperidine-
2-carboxylic acid
o
N
'?'=;; OH
Bac
Intermediate 43
To
a solution of (2S,5R)-5-Rbenzyloxy)[(benzyloxy)carbonyl]amino]-1-
ethylpiperidine-2-
carboxylic acid (78 mg, 0.183 mmol) and Boc20 (52 mg, 0.238 mmol) in Et0H (10
mL) was
added 10% Pd/C (10 mg, 0.0940 mmol) and the mixture stirred under H2 at r.t.
for 6 h. The
reaction mixture was filtered through Celite and the filtrate concentrated in
vacuo to afford
the title compound (35% purity, 130 mg, 0.167 mmol, 91% yield) as a colourless
gum; M/Z:
273 [M+Hr, EST, RT = 0.76 (Si).
The intermediate in Table 7 was synthesised according to general route 21 as
exemplified by
Intermediate 42 using the corresponding starting material.
Table 7
Inte
rme Structure Starting LCMS
Name 111 NMR
diat material data
11-1 NMR (400 MHz,
chloroform-d) 6 7.43 ¨
(2R,5S)-5- 1-tert-butyl 2-
7.13 (m, 10H), 5.28 ¨
Boc 0 [(benzyloxy)[( ethyl (2R,55)-5-
benzyloxy)car [(benzyloxy)ami
5.17 (m, 2H), 4.92 ¨4.83
ov 40 bonyllamino]- no]piperidine-
OH M/Z: 483
(m, 2H), 4.66 ¨4.53 (m,
1-Rtert- 1,2-
[M-HI,
1H), 4.37 ¨4.21 (m, 1H),
44
EST-, RI = 4.18 ¨3.89 (m, 2H), 3.51
butoxy)carbon dicarboxylate
o o 1.31 (Si)
(dd, J = 14.2, 4.9 Hz,
yl]piperidine- (Intermediate 2)
2-carboxylic following steps
2.01 1.86 (m, 2H), 1.86
acid 21.a and 21.b
1H), 2.31 ¨ 2.18 (m, 1H),
¨
¨ 1.72 (m, 1H), 1.40 (s,
9H).
88
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 22
Hoyt,
OH (D
H 0
0 0
6s) 0 10 Boc20, H2
N 0,Nor=-c2 K2CO3 io Pd/C
0,
</
DMF Et0H, r.t. BocNire
Step a Step b
Step c
Li0H.H20,
H20, r.t.
0
N'Aµ OH
Boc,No^./
Intermediate 45
Step 22.a: ethyl (2S,510-5-1(benzyloxy)amino]-1-(2-methoxyethyl)piperidine-2-
carboxylate
H 0
00[1,
Ethyl (2S,5R)-5-(benzyloxyamino)piperidine-2-carboxylate oxalic acid (500 mg,
1.36 mmol)
was free-based using a SCX-2 cartridge, first flushing with DCM/Me0H (3:1)
then eluting
with 2 M NH3 in Me0H/DCM (1:3) to afford the amine. The solvent was
concentrated in
vacuo, and the residue was dissoved in anhydrous DMF (5 mL) and transfered to
a microwave
vial. K2CO3 (375 mg, 2.71 mmol) and 1-bromo-2-methoxy-ethane (383 !IL, 4.07
mmol) were
added and the mixture was stirred at 120 C for 1 h. The reaction mixture was
poured onto
brine and extracted with Et0Ac (2 x 50 mL). The combined organic extracts were
washed
with brine, dried over Na2SO4, and concentrated in vacuo. The residue was
purified by
chromatography on silica gel (0-10% 7 M NH3 in Me0H in DCM) to afford the
title
compound (90% purity, 423 mg, 1.13 mmol, 83% yield) as a brown oil; 1H NMR
(400 MHz,
chloroform-0 6 7.40 ¨7.26 (m, 5H), 5.57 (s, 1H), 4.68 (s, 2H), 4.19 (q, J= 7.1
Hz, 2H), 3.49
(td, J= 5.9, 1.8 Hz, 2H), 3.35 ¨ 3.26 (m, 4H), 3.24 ¨ 3.12 (m, 1H), 3.08 (dd,
J= 8.8, 3.7 Hz,
1H), 2.83 ¨2.72 (m, 1H), 2.65 ¨2.55 (m, 1H), 2.18 (dd, J= 11.2, 8.2 Hz, 1H),
1.98 ¨ 1.72
(m, 3H), 1.39 ¨ 1.20 (m, 4H); M/Z: 337 [M+H]+, EST, RT = 0.86 (Si).
89
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Step 22.b: ethyl (2S,5R)-5-{[(tert-butoxy)carbonyl]amino}-1-(2-
methoxyethyl)piperidine-
2-carboxylate
o
? o
Boc.Noe< /
H
A solution of ethyl (2S, 5R)-5- [(benzylo xy)amino] -1 -(2-
methoxyethyl)piperidine-2-
carboxylate (90% purity, 423 mg, 1.13 mmol), Boc20 (321 mg, 1.47 mmol) and 10%
Pd/C
(120 mg, 0.113 mmol) in Et0H (10 mL) was stirred under H2 at r.t. for 16 h.
The reaction
mixture was filtered through a pad of Celite, washing with Et0Ac, and the
filtrate was
concentrated in vacuo to afford the title compound (50% purity, 627 mg, 0.949
mmol, 84%
yield) as a colourless oil; M/Z: 331 [M+H]+, EST, RT = 0.78 (Si).
Intermediate 45 (step 22.c): (2S,5R)-5-{1(tert-butoxy)carbonyljamino}-1-(2-
methoxyethyl)piperidine-2-carboxylic acid
o'
? 0
N , ssiJ
*(.9) OH
Boc.Ni=-<./
H
Intermediate 45
A solution of ethyl (2S,5R)-5-{ [(tert-butoxy)carbonyl] amino 1 -1-(2-
methoxyethyl)piperidine-
2-carboxylate (50% purity, 627 mg, 0.949 mmol) in THF (10 mL) was treated with
a solution
of Li0H.H20 (408 mg, 9.48 mmol) in H20 (10 mL) at r.t. for 16 h. 1 M aq HC1
H20 solution
(7.6 mL, 7.59 mmol) was added dropwise, the organic solvent was extracted and
evaporated
to afford the title compound (286 mg, 0.95 mmol, 99% yield) as a white solid;
M/Z: 303
[M+H]+, EST, RT = 0.79 (Si).
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 23
H di, CI
Bac 0 io CI
Boc N¨N
ii )d
jj, HAN 411"
0 Boc 0
1 II H
?s) H HATU, DIPEA .0 (N'eN'N1 TsCI, DIPEA so
tV õk \ 0
101 0 ...r) _____________________ .= 0,Ne
CI
'N 0
'N
0 oLo 0 oLo
Step a 00 0..L0 Step b
Intermediate 42
Step c Lir
meo 50 oc
Boc C) N¨N Boc N-
I 4
.0, \ 40
...,,s, 0 CI NiCl2.6H20 ,
NaBH4
CI
R) - 0,
HA3 Me0H r.t. SN11.
H
Intermediate 46 Step d
Step 23.a: tert-butyl (2S,5R)-5-1(benzyloxy)1(benzyloxy)carbonyljamino]-2-11(4-
chlorophenyl)formohydrazido]carbonyllpiperidine-1-carboxylate
CI
Boc 0
H
IV sL ,N
1.1 0, ()
1\1*... 0
0 (:)(:)
To a solution
of (2S,5R)-5-Rbenzyloxy)[(benzyloxy)carbonyl]amino]-1- [(tert-
butoxy)carbonyl]piperidine-2-carboxylic acid (77% purity, 1.0 g, 1.59 mmol,
Intermediate
42) in anhydrous DMF (10 mL) at 0 C was added HATU (725 mg, 1.91 mmol)
followed
by DIPEA (0.56 mL, 3.18 mmol) and stirred at r.t for 10 min. 4-
Chlorobenzohydrazide (271
mg, 1.59 mmol) was then added and the mixture was stirred at r.t. for 2 h. The
reaction
mixture was diluted with H20 (80 mL), stirred at r.t. for 20 min, and the
resultant suspension
was filtered under vacuum, washing with H20 (50 mL). The residue was purified
by
chromatography on silica gel (5-80% Et0Ac in heptane) to afford the title
compound (91%
purity, 640 mg, 0.91 mmol, 57% yield) as a white solid; M/Z: 537, 539 [M-
Boc+H]+, EST,
RT = 1.33 (S1).
91
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 23.b: tert-butyl (2S,5R)-5-1(benzyloxy)1(benzyloxy)carbonyl]amino]-2-[5-
(4-
chloropheny1)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate
Boc N¨N
N 4 \
io i") 0 CI
To a solution of tert-butyl (2S,5R)-5-
Rbenzyloxy)[(benzyloxy)carbonyl]amino]-2- {[(4-
chlorophenyl)formohydrazido] carbonyl} piperidine-l-carboxyl ate (91% purity,
640 mg, 0.91
mmol) in ACN (5 mL) was added DIPEA (0.12 mL, 0.669 mmol) and TsC1 (191 mg,
1.0
mmol) and the mixture was stirred at r.t. for 48 h. 15% aq NH4OH solution (20
mL) was
added and the mixture was stirred at r.t. for 15 min. The reaction mixture was
concentrated in
vacuo, diluted with H20 (20 mL) and extracted with DCM (2 x 30 mL). The
combined
organic extracts were washed with brine (30 mL), dried over Na2SO4, and
concentrated in
vacuo. The residue was purified by chromatography on silica gel (10-80% Et0Ac
in heptane)
to afford the title compound (560 mg, 0.877 mmol, 96% yield) as a white gum;
1H NMR (400
MHz, chloroform-d) 6 8.02 ¨ 7.90 (m, 2H), 7.54 ¨ 7.47 (m, 2H), 7.47 ¨ 7.31 (m,
9H), 7.27 ¨
7.19 (m, 1H), 5.52 (s, 1H), 5.35 ¨ 5.20 (m, 2H), 4.99 ¨ 4.88 (m, 2H), 4.32 ¨
4.22 (m, 2H),
3.54 ¨ 3.45 (m, 1H), 2.58 ¨ 2.43 (m, 1H), 2.23 ¨2.11 (m, 1H), 2.09 ¨ 2.01 (m,
2H), 1.41 (s,
9H); M/Z: 641, 643 [M+Na]+, EST, RT = 1.53 (Si).
Step 23.c: tert-butyl (2S,5R)-5- [(benzyloxy)amino]-2-[5-(4-chloropheny1)-
1,3,4-oxadiazol-
2-yl]piperidine-l-carboxylate
Boc N¨N
I \
0,
To a solution of tert-butyl (2S,5R)-5-[(benzyloxy)[(benzyloxy)carbonyl]amino]-
2-[5-(4-
chloropheny1)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate (480 mg, 0.752
mmol) in Me0H
(5 mL) was added 2 M aq LiOH solution (10 mL, 20.0 mmol) and the mixture was
stirred at
r.t. for 15 h. The mixture was then heated to 50 C for 3 h before allowing to
stir at r.t. for 110
h. The reaction mixture was concentrated in vacuo, dissolved in H20 (20 mL)
and acidified to
pH 9 using 1 M aq HC1 solution. The solution was extracted with Et0Ac (2 x 30
mL) and the
combined organic extracts were washed with brine (30 mL), dried over Na2SO4,
and
92
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
concentrated in vacuo. The residue was purified by chromatography on silica
gel (10-100%
Et0Ac in heptane) to afford the title compound (92% purity, 195 mg, 0.370
mmol, 49%
yield); 1H NMR (500 MHz, chloroform-0 6 8.00 ¨ 7.88 (m, 2H), 7.53 ¨ 7.41 (m,
2H), 7.39 ¨
7.23 (m, 5H), 4.81 ¨4.61 (m, 2H), 4.41 (d, J= 72.2 Hz, 2H), 3.14 (d, J= 65.3
Hz, 2H), 2.73
(s, 1H), 2.51 ¨ 2.40 (m, 1H), 2.23 ¨ 2.08 (m, 1H), 1.93 ¨ 1.66 (m, 2H), 1.55 ¨
1.44 (m, 9H);
M/Z: 485, 487 [M+H]+, EST, RT = 1.39 (Si).
Intermediate 46 (step 23.d): tert-butyl (2S,5R)-5-amino-2-[5-(4-chloropheny1)-
1,3,4-
oxadiazol-2-yl]piperidine-1-carboxylate
Boc N¨N
N 4 \
H2N
Intermediate 46
To a solution of tert-butyl (2S,5R)-5-[(benzyloxy)amino] -2- [5 -(4-
chloropheny1)-1,3,4-
oxadiazol-2-yl]piperidine- 1-carboxylate (92% purity, 160 mg, 0.304 mmol) in
Me0H (20
mL) at -10 C was added NiC12.6H20 (291 mg, 1.21 mmol) followed by NaBH4 (344
mg,
9.11 mmol) and the mixture was stirred at r.t. for 5 h. The reaction mixture
was concentrated
in vacuo and purified by prep. HPLC (Method 5) to afford the title compound
(50 mg, 0.132
mmol, 43% yield) as a colourless gum; M/Z: 379, 381 [M+H]+, EST', RT = 0.97
(51).
The intermediate in Table 8 was synthesised according to general route 23 as
exemplified by
Intermediate 46 using the corresponding starting material.
Table 8
Interm Structure Name Starting material LCMS
111 NMR
ediate data
(2R,5S)-5- 'H NMR (500
MHz,
tert-butyl [(benzyloxy)[(ben
chloroform-d) 6 5.91 ¨
(2R,5S)-5- zyloxy)carbonyl]a
5.32 (m, 3H), 4.68 (t, J=
F amino-2-[5- mino]-1-Rtert-
zF (3,3,3- butoxy)carbonyl] 6.2 Hz, 2H),
4.42 ¨4.18
(m, 1H), 3.65 ¨3.49 (m,
Boc trifluoroprop piperidine-2-
47 1H), 3.27
¨2.98 (m, 1H),
oxy)-1,3,4- carboxylic acid
(R) o 2.70 (qt, J= 10.2, 6.2
oxadiazol-2- (Intermediate 44)
Hz, 2H), 2.51 ¨2.34 (m,
yl]piperidine and (3,3,3-
1H), 2.19 ¨2.07 (m, 2H),
-1- trifluoropropoxy)
2.06¨ 1.92 (m, 1H), 1.46
carboxylate carbohydrazide
(s, 9H).
(Intermediate 38)
93
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Scheme for route 24
0 BrCN, NaHCO3 CuBr,
1 tert-buty I nitrite 0
0
liN)---Br
0 '1\1H2 1,4-dioxane, 0 ___ N 0 IN" 2
=
ACN, r.t. 0 R) 0
=
jcs*. 0 H
Step a Step b 411 0
Intermediate 30
<
Step c NaH, THF F
- r.t.
0y0N_Nk
F H2, Pd/C OyO N22.1 F
0
= s)
Et0H, r.t.
0 Ne'
Step d
Intermediate 48
Step 24.a: tert-butyl
(2R,5S)-2-(5-amino-1,3,4-oxadiazol-2-y1)-5-
{1(benzyloxy)carbonyl] amino} piperidine-1-carboxylate
rj
=
0 - 0
OANP"'
To a solution of tert-butyl (2R,5S)-5- {
[(benzyloxy)carbonyl] amino} -2-
(hydrazinecarbonyppiperidine-1-carboxylate (79% purity, 10.2 g, 20.5 mmol,
Intermediate
30) in 1,4-dioxane (70 mL) was added a solution of NaHCO3 (2.58 g, 30.8 mmol)
in H20 (20
mL), followed by BrCN (2.17 g, 20.5 mmol), and the mixture was stirred at r.t.
for 2.5 h. The
reaction mixture was diluted with H20 and the resultant precipitate was
filtered under
vacuum, washing with H20, to afford the title compound in quantitative yield
(84% purity,
11.02 g, 22.2 mmol) as an off-white powder; 1H NMR (400 MHz, DMSO-d6) 6 7.51 -
7.42
(m, 1H), 7.41 -7.27 (m, 5H), 7.05 - 6.95 (m, 2H), 5.30 (s, 1H), 5.09 - 4.97
(m, 2H), 4.11 -
3.98 (m, 1H), 2.86 -2.76 (m, 1H), 2.29 -2.14 (m, 1H), 1.95 - 1.79 (m, 2H),
1.65 - 1.53 (m,
1H), 1.44- 1.30 (m, 10H); M/Z: 318 [M-Boc+H]+, EST, RT = 0.86 (S2).
94
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 24.b: tert-butyl (2R,5S)-5-{1(benzyloxy)carbonyljamino}-2-(5-bromo-1,3,4-
oxadiazol-2-Apiperidine-1-carboxylate
o 0
Br
=
y
o
(R) u
To a solution of tert-butyl
(2R,5S)-2-(5 -amino -1,3 ,4-oxadiazol-2-y1)-5 -
{Rbenzyloxy)carbonyllaminolpiperidine-1-carboxylate (84% purity, 11.02 g, 22.2
mmol) and
CuBr (3 eq, 9.54 g, 66.5 mmol) in anhydrous ACN (400 mL) was added tert-butyl
nitrite
(90%, 17.6 mL, 133.0 mmol) and the mixture was stirred at r.t for 5 h. Further
portions of
CuBr (1.5 eq, 4.77 g, 33.3 mmol) and tert-butyl nitrite (90%, 8.79 mL, 66.5
mmol) were
added and the mixture was stirred at r.t. for 19 h. The reaction mixture was
diluted with
Et0Ac (250 mL) and washed with Rochelle salt (2 x 200 mL) and H20 (3 x 200
mL). The
organic extracts were dried over Na2SO4, concentrated in vacuo, and purified
by
chromatography on silica gel (0-100% Et0Ac in heptane) to afford the title
compound (2.02
g, 4.03 mmol, 18% yield) as a beige solid; 1H NMR (400 MHz, DMSO-d6) 6 7.52
(d, J= 6.2
Hz, 1H), 7.41 - 7.28 (m, 5H), 5.57 - 5.41 (m, 1H), 5.05 (s, 2H), 4.08 - 3.91
(m, 1H), 3.65 -
3.53 (m, 1H), 2.96 -2.84 (m, 1H), 2.33 -2.23 (m, 1H), 1.99 - 1.90 (m, 1H),
1.88 - 1.72 (m,
1H), 1.65 - 1.57 (m, 1H), 1.38 (s, 9H); M/Z: 383 [M-Boc+H]+, EST, RT = 1.09
(S2).
Step 24.c: tert-butyl
(2R,5S)-5-{ [(benzyloxy)carbonyl] amino}-2- {5- [2-
(trifluoromethoxy)ethoxy]-1,3,4-oxadiazol-2-yl} piperidine-l-carboxylate
o 0
y F
0 o 0
0
=
To a solution of 2-(trifluoromethoxy)ethan-l-ol (13% in THF/toluene, 4.50 g,
4.43 mmol) in
anhydrous THF (15 mL) at 0 C was added NaH (60%, 322 mg, 8.06 mmol) and the
mixture
was stirred at 0 C for 10 min. tert-Butyl (2R,5S)-5-
{[(benzyloxy)carbonyl]amino}-2-(5-
bromo-1,3,4-oxadiazol-2-yppiperidine-1-carboxylate (2.02 g, 4.03 mmol) in
anhydrous THF
(10 mL) was added and the mixture was stirred at r.t. for 2 h. The reaction
mixture was
diluted with H20 (50 mL) and extracted with Et0Ac (3 x 100 mL). The combined
organic
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
extracts were dried over MgSO4, concentrated in vacuo, and purified by
chromatography on
silica gel (0-100% Et0Ac in heptane) to afford the title compound (85% purity,
1.60 g, 2.56
mmol, 64% yield) as a yellow oil; 1H NMR (400 MHz, DMSO-d6) 6 7.50 (d, J= 6.1
Hz, 1H),
7.43 ¨ 7.26 (m, 5H), 5.46 ¨ 5.29 (m, 1H), 5.04 (s, 2H), 4.80 ¨ 4.58 (m, 2H),
4.57 ¨ 4.41 (m,
2H), 4.43 ¨4.26 (m, 1H), 3.73 ¨ 3.51 (m, 1H), 2.96 ¨2.80 (m, 1H), 2.32 ¨2.16
(m, 1H), 1.96
¨ 1.73 (m, 2H), 1.69 ¨ 1.49 (m, 1H), 1.37 (s, 9H); M/Z: 531 [M-Boc+H]+, EST,
RT = 3.83
(S4).
Intermediate 48 (step 24.d): tert-butyl
(2R,5S)-5-amino-2-{5-[2-
(trifluoromethoxy)ethoxy]-1,3,4-oxadiazo1-2-yllpiperidine-l-carboxylate
o,o o4F
F
0
H2N\
Intermediate 48
To a solution of
tert-butyl (2R,5S)-5-{ [(benzyloxy)carbonyl] amino} -2- {5- [2-
(tri fluoromethoxy)ethoxy] -1,3 ,4-oxadiazol-2-y1 } piperidine-l-carboxyl ate
(85% purity, 1.60 g,
2.56 mmol) in Et0H (45 mL) under N2 was added 10% Pd/C (3.27 g, 3.08 mmol) and
the
mixture was stirred under H2 at r.t. for 18 h. The reaction mixture was
filtered through a pad
of Celite and concentrated in vacuo to afford the title compound (49% purity,
843 mg, 1.04
mmol, 41% yield) as a light brown oil; 1H NMR (400 MHz, DMSO-d6) 6 5.39 ¨ 5.26
(m, 1H),
4.76 ¨ 4.64 (m, 2H), 4.54 ¨ 4.42 (m, 2H), 4.43 ¨ 4.25 (m, 1H), 3.74 ¨ 3.60 (m,
1H), 3.20 ¨
2.91 (m, 3H), 2.30 ¨2.09 (m, 1H), 1.93 ¨ 1.78 (m, 1H), 1.75 ¨ 1.59 (m, 1H),
1.53 ¨ 1.25 (m,
11H); M/Z: 397 [M+H], EST, RT = 1.76 (S4).
Scheme for route 25
0
H0j44Ø0,kF FF F+F
Boc 0 Boc Ni-N1
F
Intermediate 54
ea 0 HIrciik0
.0 0, C,.?L cI FN:NH2 T3P, DIPEA so (11)4LLN'N 7sCI,, 80 0,
K2CO3
THF, r.t. ACN
40 0 Step a 0.-L-0 Step b 0 0
Intermediate 29 Pd/C H2
Step c
Et0H, r.t.
Boc
F TF
/-
a I O0
HN
Intermediate 49
96
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 25.a: tert-butyl (2R,5S)-5- [(benzyloxy)1(benzyloxy)carbonyljamino]-2-{N'-
[(1s,3s)-3-
(trifluoromethoxy)eyelobutaneearbonyl]hydrazineearbonyllpiperidine-1-
earboxylate
F F
13rioc H
40
==<. 0
401 =''LO
To a solution of (1 s,3s)-3-(trifluoromethoxy)cyclobutane-l-carboxylic acid
(500 mg, 2.63
mmol, Intermediate 54) in THF (20 mL) was added DIPEA (1.4 mL, 7.90 mmol), T3P
(50%
in Et0Ac, 4.7 mL, 7.90 mmol) and
tert-butyl (2R,5S)-5-
Rbenzyloxy)[(benzyloxy)carbonyl] amino] -2-(hydrazinecarbonyl)piperidine-1-
carboxylate
(60% purity, 2.30 g, 2.77 mmol, Intermediate 29) and the mixture was stirred
at r.t. for 18 h.
The reaction mixture was diluted with H20 (50 mL) and extracted with Et0Ac (2
x 100 mL).
The combined organic extracts were dried over Na2SO4, concentrated in vacuo,
and purified
by chromatography on silica gel (0-100% Et0Ac in heptane) to afford the title
compound
(1.60 g, 2.29 mmol, 87% yield) as a white solid; 1H NMR (400 MHz, DMSO-d6) 6
10.01 -
9.69 (m, 2H), 7.49 - 7.21 (m, 10H), 5.21 (s, 2H), 4.92 - 4.74 (m, 3H), 4.52 -
4.17 (m, 2H),
4.01 - 3.70 (m, 2H), 2.76 - 2.68 (m, 1H), 2.29 - 1.99 (m, 2H), 1.93 - 1.47 (m,
4H), 1.42 -
1.34 (m, 2H), 1.30 (s, 9H); M/Z: 687 [M+Na]+, EST, RT = 1.21 (S2).
Step 25.b: tert-butyl (2R,5S)-5-[(benzyloxy)1(benzyloxy)carbonyljamino]-2-{5-
[(1s,3s)-3-
(trifluorornethoxy)eyelobutyl]-1,3,4-oxadiazol-2-yllpiperidine-1-earboxylate
FF
Boc
ciF
(R) -
0
00
A
suspension of tert-butyl (2R,55)-5- Rbenzyloxy)[(benzyloxy)carbonyl]amino]-2-
{N-
[(1s,3s)-3-(trifluoromethoxy)cyclobutanecarbonyl]hydrazinecarbonyllpiperidine-
1-
carboxylate (1.6 g, 2.29 mmol), K2CO3 (2.0 g, 14.4 mmol) and TsC1 (1.40 g,
7.22 mmol) in
ACN (18 mL) was stirred at 80 C for 1.5 h. The reaction mixture was
partitioned between
Et0Ac (100 mL) and H20 (50 mL), and the organic layer was isolated and washed
with brine
97
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
(30 mL). The organic extracts were dried over MgSO4, concentrated in vacuo,
and purified by
chromatography on silica gel (0-50% Et0Ac in heptane) to afford the title
compound (92%
purity, 1.05 g, 1.49 mmol, 62% yield) as a yellow oil; 1H NMR (400 MHz, DMSO-
d6) 6 7.48
¨ 7.27 (m, 10H), 5.36 ¨ 5.27 (m, 1H), 5.21 (s, 2H), 4.96 ¨ 4.83 (m, 3H), 4.19
¨ 4.09 (m, 1H),
4.01 ¨ 3.93 (m, 1H), 3.51 ¨ 3.40 (m, 1H), 2.91 ¨ 2.79 (m, 2H), 2.31 ¨ 2.21 (m,
1H), 2.07 ¨
1.96 (m, 2H), 1.96 ¨ 1.87 (m, 2H), 1.41 (s, 2H), 1.28 (d, J = 5.8 Hz, 9H);
M/Z: 547 [M-
Boc+H]+, EST, RT = 1.27 (S2).
Intermediate 49 (step 25.c): tert-butyl (2R,5S)-5-amino-
2-{5-1(1s,3s)-3-
(trifluoromethoxy)cyclobuty1]-1,3,4-oxadiazol-2-yllpiperidine-l-carboxylate
/F
Boc
1V
(R) 0
Intermediate 49
To a solution of tert-butyl (2R,5S)-5- Rbenzyloxy)[(benzyloxy)carbonyl]amino]-
2- {5-[(1s,3s)-
3 -(trifluoromethoxy)cyclobutyl] -1,3 ,4-oxadi azo1-2-yllpiperidine-l-carboxyl
ate (0.93 g, 1.43
mmol) in anhydrous Et0H (30 mL) was added 10% Pd/C (0.15 g, 0.144 mmol) and
the
resultant mixture was stirred at r.t. under H2 for 24 h. A further portion of
10% Pd/C (0.15 g,
0.144 mmol) was added and the reaction mixture was stirred at r.t. under H2
for 24 h. A
further portion of 10% Pd/C (0.15 g, 0.144 mmol) was added and the reaction
mixture was
stirred at r.t. under H2 for 24 h. The reaction mixture was warmed to 40 C
and filtered
through a pad of Celite, washing copiously with Et0H. The filtrate was
concentrated in vacuo
and purified by chromatography on silica gel (0-20% Me0H in DCM) to afford the
title
compound (321 mg, 0.774 mmol, 54% yield) as a pale yellow oil; 1H NMR (400
MHz,
DMSO-d6) 6 5.39 (s, 1H), 4.90 (p, J= 7.5 Hz, 1H), 3.69 (d, J= 13.1 Hz, 1H),
3.52 ¨ 3.36 (m,
2H), 3.06 ¨2.92 (m, 2H), 2.92 ¨2.76 (m, 2H), 2.32 ¨2.13 (m, 2H), 1.96 ¨ 1.85
(m, 1H), 1.80
¨ 1.59 (m, 1H), 1.49 (d, J= 13.6 Hz, 1H), 1.41 (s, 9H); M/Z: 407 [M+H]+, EST,
RT = 0.71 -
0.76 (S2).
Scheme for route 26
0
.lat.h.or,$)ber_iantnroline /\ P.4 M HCI in
CH2ICI, ZnEt2 \?-0 1,4-dioxane,. H¨I
_____________________________ /\ LI31`1, relloAch 0
0 HIV-0
DOE, 0 C - r.t. THF, r.t.
80 C
Step a Step b Step c
Intermediate 50
98
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 26.a: tert-butyl 3-(ethenyloxy)azetidine-1-carboxylate
A mixture of tert-butyl 3-hydroxyazetidine-1-carboxylate (5.0 g, 28.9 mmol), 1-
(ethenyloxy)butane (56 mL, 0.433 mol), Bathophenanthroline (480 mg, 1.44
mmol),
.. Pd(OAc)2 (981 mg, 1.44 mmol) and Et3N (1.7 mL, 12.1 mmol), split over four
pressure tubes,
was degassed using N2 for 5 min, prior to being sealed and heated at 80 C for
24 h. The
reaction mixture was cooled to r.t. and filtered through a pad of Celite,
washing with Et0Ac.
The filtrate was concentrated in vacuo, and purified by chromatography on
silica gel (0-25%
Et0Ac in heptane) to afford the title compound (93% purity, 3.60 g, 16.8 mmol,
58% yield)
as a yellow oil; 1H NMR (500 MHz, chloroform-0 6 6.37 (dd, J= 14.5, 6.9 Hz,
1H), 4.58 (tt,
J= 6.5, 4.2 Hz, 1H), 4.17 (ddd, J= 9.7, 6.5, 1.0 Hz, 2H), 4.08 (dd, J= 6.9,
2.5 Hz, 1H), 3.96
(dd, J= 14.5, 2.5 Hz, 1H), 3.90 (ddd, J= 9.8, 4.2, 0.9 Hz, 2H), 1.44 (s, 9H);
M/Z: no mas ion
observed [M+H], EST, RT = 0.94 (S2).
Step 26.b: tert-butyl 3-cyclopropoxyazetidine-1-carboxylate
o )>.
4-N-0
To a solution of tert-butyl 3-(ethenyloxy)azetidine-1-carboxylate (93% purity,
3.60 g, 16.8
mmol) and chloro(iodo)methane (9.48 g, 53.8 mmol) in DCE (14 mL) at -5 C was
added a
solution of 0.9 M diethylzinc in hexanes (30 mL, 26.9 mmol) dropwise over 60
mins while
maintaining an internal temperature between 0 and -5 C. The mixture was
warmed to r.t. and
stirred for 30 min. The reaction mixture was cooled to 0 C and quenched using
satd aq
NH4C1 solution (5 mL), followed by NH4OH solution (5 mL). The solution was
extracted
with methyl tert-butyl ether (3 x 20 mL) and the combined organic extracts
were washed with
brine (25 mL), dried over MgSO4, and concentrated in vacuo. The residue was
purified by
chromatography on silica gel (0-25% Et0Ac in heptane) to afford the title
compound (1.05 g,
4.92 mmol, 29% yield) as a colourless oil; 1H NMR (500 MHz, chloroform-d) 6
4.25 (tt, J=
6.6, 4.5 Hz, 1H), 4.02 (ddd, J= 9.4, 6.6, 0.9 Hz, 2H), 3.82 - 3.72 (m, 2H),
3.17 (tt, J= 6.1,
3.0 Hz, 1H), 1.36 (s, 9H), 0.53 (dq, J= 5.0, 3.4, 2.6 Hz, 2H), 0.45 -0.37 (m,
2H).
99
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Intermediate 50 (step 26.c): 3-cyclopropoxyazetidine hydrochloride
HCI
HN 0
Intermediate 50
To a solution of tert-butyl 3-cyclopropoxyazetidine-1-carboxylate (1.05 g,
4.92 mmol) in
THF (3 mL) at 0 C was added 4 M HC1 in 1,4-dioxane (4.9 mL, 19.7 mmol)
dropwise and
the mixture was stirred at r.t. for 3 h. The reaction mixture was concentrated
in vacuo, and
azeotroped using 2-propanol, to afford the title compound (75% purity, 0.75 g,
3.73 mmol,
76% yield) as a beige powder; 1H NMR (500 MHz, methanol-d4) 6 4.62 - 4.52 (m,
1H), 4.35
- 4.27 (m, 2H), 4.04 - 3.97 (m, 2H), 3.40 (tt, J= 6.0, 3.0 Hz, 1H), 0.64 -
0.59 (m, 2H), 0.56 -
0.50 (m, 2H).
Scheme for route 27
0
OH 0
Bathophena nth roline -0-01=
CH2ICI, ZnEt2 0
H2, Pd/C
0 Et3N, Pd(OAc)2 0
0
80 C
DCE, 0 C - r.t jm,\ Et0H, r.t
HO
Step a Step b W Step c
Intermediate 51
Step 27.a: benzyl 3-(ethenyloxy)cyclobutane-1-carboxylate
0
441
A mixture of benzyl 3-hydroxycyclobutane-1-carboxylate (5.0 g, 24.2 mmol), 1-
(ethenyloxy)butane (47 mL, 0.364 mol), Bathophenanthroline (403 mg, 1.21
mmol),
Pd(OAc)2 (824 mg, 1.21 mmol) and Et3N (1.4 mL, 10.2 mmol) was stirred under N2
at 80 C
for 24 h. The reaction mixture was cooled to r.t. and filtered through Celite,
washing with
Et0Ac (100 mL). The filtrate was concentrated in vacuo and the resultant
residue was
purified by chromatography on silica gel (0-50% Et0Ac in heptane) to afford
the title
compound (80% purity, 4.64 g, 16.0 mmol, 66% yield) as a yellow oil; 1H NMR
(400 MHz,
DMSO-d6) 6 7.47 - 7.27 (m, 5H), 6.37 (dd, J= 14.4, 6.9 Hz, 1H), 5.12 (s, 2H),
4.40 - 4.27
(m, 1H), 4.12 (dd, J= 14.4, 1.8 Hz, 1H), 4.00 (dd, J= 6.8, 1.8 Hz, 1H), 2.92 -
2.79 (m, 1H),
2.67 -2.56 (m, 2H), 2.17 -2.04 (m, 2H); M/Z: 233 [M+H], EST, RT = 1.01 (S2).
100
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 27.b: benzyl 3-cyclopropoxycyclobutane-1-carboxylate
o_o_cp.
o
4/
To a solution of benzyl 3-(ethenyloxy)cyclobutane-l-carboxylate (80% purity,
4.64 g, 16.0
mmol) in DCE (40 mL) at 0 C was added chloro(iodo)methane (3.7 mL, 51.1 mmol)
followed by 0.9 M ZnEt2 in hexanes (28 mL, 25.6 mmol) dropwise and the mixture
was
stirred at r.t. for 2 h. The reaction mixture was cooled to 0 C and quenched
using satd aq
NH4C1 solution (30 mL) followed by NH4OH solution (30 mL). The aqueous
solution was
extracted with Et0Ac (3 x 100 mL) and the combined organic extracts were dried
over
Na2SO4 and concentrated in vacuo. The residue was purified by chromatography
on silica gel
(0-50% Et0Ac in heptane) to afford the title compound (90% purity, 1.55 g,
5.66 mmol, 35%
yield) as a colourless oil; 1H NMR (500 MHz, DMSO-d6) 6 7.40- 7.31 (m, 5H),
5.09 (s, 2H),
4.00 - 3.91 (m, 1H), 3.23 - 3.16 (m, 1H), 2.82 - 2.71 (m, 1H), 2.48 - 2.44 (m,
2H), 2.08 -
1.94 (m, 2H), 0.48 - 0.40 (m, 2H), 0.43 - 0.35 (m, 2H); M/Z: 247 [M+H], EST,
RT = 1.00
(S2).
Intermediate 51 (step 27.c): 3-cyclopropoxycyclobutane-1-carboxylic acid
o )>.
-0-0
HO
Intermediate 51
To a solution of benzyl 3-cyclopropoxycyclobutane-1-carboxylate (90% purity,
1.55 g, 5.66
mmol) in anhydrous Et0H (20 mL) was added 10% Pd/C (606 mg, 0.570 mmol) and
the
mixture was stirred at r.t. under H2 for 24 h. The reaction mixtu re was
filtered over Celite,
washing with Et0Ac (50 mL), and the filtrate was concentrated in vacuo to
afford the title
compound (90% purity, 981 mg, 5.65 mmol) in quantitative yield as a colourless
oil; 1H NMR
(400 MHz, DMSO-d6) 6 11.88 (s, 1H), 4.14 - 3.97 (m, 1H), 3.97 - 3.88 (m, 1H),
3.21 -3.17
(m, 1H), 2.43 - 2.38 (m, 2H), 1.99 - 1.88 (m, 2H), 0.48 - 0.35 (m, 4H); M/Z:
157 [M+H]+,
EST, RT = 0.64 (S2).
101
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 28
Boc 0 Boc NN
TCDI
F H F
THF, 70 C
CI Step a CI
Intermediate 26
Step b Mel, K2003
DMF, r.t.
=
Boc NN Boc NN
I
m CPBA N)1
0 0 0
0 \
F DCM, r.t.
NI\µµ
Step c
CI CI
Intermediate 52
Step 28.a: tert-butyl (2R,5S)-542-(4-chloro-3-fluorophenoxy)acetamido]-2-(5-
sulfany1-
1,3,4-oxadiazol-2-yl)piperidine-1-carboxylate
Boc N--"N
o
(R) 0
CI
To a solution of tert-butyl (2R,55)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-
2-
(hydrazinecarbonyl)piperidine-1-carboxylate (300 mg, 0.674 mmol, Intermediate
26) in
anhydrous THF (15 mL) was added TCDI (144 mg, 0.809 mmol) and the mixture was
stirred
at 70 C for 12 h. The reaction mixture was cooled to r.t., diluted with H20
(20 mL) and
extracted with Et0Ac (2 x 30 mL). The combined organic extracts were dried
over MgSO4
and concentrated in vacuo to afford the title compound (85% purity, 360 mg,
0.628 mmol,
93% yield) as a beige powder; 1H NMR (500 MHz, DMSO-d6) 6 8.06 (d, J = 6.9 Hz,
1H),
7.98 (s, 1H), 7.48 (t, J= 8.9 Hz, 1H), 7.18 (s, 2H), 7.05 (dd, J= 11.4, 2.8
Hz, 1H), 6.83 (dd, J
= 9.0, 2.0 Hz, 1H), 5.31 (s, 1H), 3.89 (s, 2H), 2.97 (d, J= 12.0 Hz, 1H), 2.15
(dd, J= 11.6, 6.6
Hz, 1H), 1.94 (d, J= 13.9 Hz, 1H), 1.78 (t, J= 13.5 Hz, 1H), 1.62 (d, J= 12.4
Hz, 1H), 1.39
(s, 9H); M/Z: 485, 487 [M-H], EST-, RT = 0.98 (S2).
102
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 28.b:
tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-[5-
(methylsulfany1)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate
Boc
0 0
CI
Mel (0.43 mL, 6.98 mmol) was added dropwise to a suspension of tert-butyl
(2R,55)-5-[2-(4-
chloro-3-fluorophenoxy)acetamido] -2-(5-sulfany1-1,3,4-oxadiazol-2-
yl)piperidine-1-
carboxylate (85% purity, 2.0 g, 3.49 mmol), K2CO3 (965 mg, 6.98 mmol) and DMF
(15 mL)
and the mixture was stirred at r.t. for 3 h. The reaction mixture was diluted
with 1 M aq
NaOH solution (10 mL) and extracted with DCM (2 x 30 mL). The combined organic
extracts
were dried over MgSO4 and concentrated in vacuo to afford the title compound
(1.80 g, 3.41
mmol, 98% yield) as a white solid; 1H NMR (400 MHz, DMSO-d6) 6 8.11 (d, J= 7.0
Hz,
1H), 7.48 (t, J= 8.9 Hz, 1H), 7.05 (dd, J= 11.4, 2.9 Hz, 1H), 6.90 - 6.72 (m,
1H), 5.48 (s,
1H), 4.69 -4.52 (m, 2H), 3.91 (d, J = 12.7 Hz, 2H), 2.97 (d, J= 12.4 Hz, 1H),
2.70 (s, 3H),
2.33 -2.16 (m, 1H), 2.02 (d, J= 19.2 Hz, 1H), 1.88 - 1.69 (m, 1H), 1.65 (d, J=
13.0 Hz, 1H),
1.39 (s, 9H); M/Z: 401, 403 [M-Boc+H]+, EST, RT = 1.02 (S2).
Intermediate 52 (step 28.c): tert-butyl
(2R,5S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]-2-(5-methanesulfony1-1,3,4-oxadiazol-2-yl)piperidine-
l-
carboxylate
Boc
0 (R) 0
CI
Intermediate 52
A solution of tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-
[5-
(methylsulfany1)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate (1.80 g, 3.41
mmol) and m-
CPBA (60% purity, 2.95 g, 10.2 mmol) in DCM (40 mL) was stirred at r.t. for 48
h. The
reaction mixture was diluted with DCM (20 mL) and satd Na2S03 solution and
stirred at r.t.
for 15 min. The organic layer was isolated using a phase separator and then
concentrated in
vacuo. The residue was purified by chromatography on silica gel (0-40% Et0Ac
in heptane)
103
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
to afford the title compound (89% purity, 1.07 g, 1.79 mmol, 52% yield) as a
light brown
solid; M/Z: 433, 435 [M-Boc+H], ESI+, RT = 0.99 (S2).
Scheme for route 29
Boc o
CI
401 H = CI Boc 0
0 OH HATU, DIPEA N
H 0 (R)
Ale' 2N'N DMF, r.t. 0
0 Step a 40 0
Intermediate 4
TsCI, K2CO3
Step b
ACN, 80 C
Boc NN
Boc
NiC1H0
411ci
R) CI 2.2
NaBH4 - R) 0
0)-LNPµ'µ-'<
H2N"'-< Me0H, r.t.
Intermediate 53 Step c
Step 29.a: tert-butyl (2R,5S)-5-
{[(benzyloxy)carbonyl]amino}-2-IN'-(4-
chlorobenzoyl)hydrazinecarbonyl]piperidine-1-carboxylate
Boc 0
o
NT)
H
To a solution of (2R,5S)-5-{ Rbenzyloxy)carbonyll amino }-
1-[(tert-
butoxy)carbonyl]piperidine-2-carboxylic acid (94% purity, 2.21 g, 5.93 mmol,
Intermediate
4), DIPEA (2.1 mL, 11.9 mmol) and 4-chlorobenzohydrazide (1.11 g, 6.52 mmol)
in
anhydrous DMF (20 mL) was added HATU (2.71 g, 7.11 mmol) and the mixture was
stirred
at r.t. for 20 h. The reaction mixture was diluted with satd aq NaHCO3
solution (20 mL) and
extracted with Et0Ac (2 x 50 mL). The combined organic extracts were washed
with brine,
dried over Na2SO4, and concentrated in vacuo. The residue was purified by
chromatography
on silica gel (0-100% Et0Ac in heptane) to afford the title compound (76%
purity, 1.61 g,
2.43 mmol, 41% yield) as a yellow oil; M/Z: 403, 405 [M-Boc+H]+, ESI+, RT =
0.98 (S2).
104
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 29.b: tert-butyl (2R,5S)-5-1[(benzyloxy)carbonyl] amino}-2- [5-(4-
chlorophenyI)-
1,3,4-oxadiazol-2-yl] pip eridine- 1-carb oxylate
Boc N¨N
I
0
OANr
A mixture of tert-butyl (2R,5S)-5-{ Rbenzyloxy)carbonyll
amino } -2- [N-(4-
chlorobenzoyphydrazinecarbonyl]piperidine-l-carboxylate (76%, 1.61 g, 2.43
mmol), K2CO3
(2.02 g, 14.6 mmol) and TsC1 (1.39 g, 7.30 mmol) in ACN (12 mL) was stirred at
80 C for
1.5 h. The reaction mixture was cooled to r.t., diluted with H20 (10 mL) and
brine (10 mL),
and extracted with Et0Ac (2 x 30 mL). The combined organic extracts were dried
over
Na2SO4, concentrated in vacuo and purified by chromatography on silica gel (0-
50% Et0Ac
in heptane) to afford the title compound (85% purity, 410 mg, 0.719 mmol, 30%
yield) as a
beige gum; M/Z: 485, 487 [M+H]+, EST, RT = 1.71 (Si).
Intermediate 53 (step 29.c): tert-butyl (2R,5S)-5-amino-2- [5-(4-chlorophenyI)-
1,3,4-
oxadiazol-2-yl] piperidine- 1-carb oxylate
Boc NN
(R)
H2Nrs'
Intermediate 53
To a solution of tert-butyl (2R,5S)-5-{Rbenzyloxy)carbonyllamino -2-[5-(4-
chloropheny1)-
1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate (410 mg, 0.845 mmol) in Me0H (40
mL) at 0
C was added NiC121120 (811 mg, 3.38 mmol) followed by NaBH4 (959 mg, 25.36
mmol)
and the mixture was stirred at r.t. for 18 h. The reaction mixture was
concentrated in vacuo,
dissolved in H20 and Et0Ac and the resultant suspension was filtered through
Celite. The
phases were separated and the aqueous layer was further extracted with Et0Ac.
The
combined organic extracts were washed with brine, dried over Na2SO4 and
concentrated in
vacuo. The residue was purified by prep. HPLC (Method 7) to afford the title
compound (142
mg, 0.375 mmol, 44% yield); 11-1 NMR (500 MHz, chloroform-d) 6 8.40 (s, 1H),
7.93 (d, J=
8.5 Hz, 2H), 7.48 (d, J= 8.4 Hz, 2H), 5.65 (s, 1H), 4.31 (d, J= 13.4 Hz, 1H),
3.61 (s, 1H),
3.24 (s, 1H), 2.45 (s, 1H), 2.25 (d, J= 13.3 Hz, 1H), 2.08 (s, 2H), 1.45 (s,
9H); M/Z: 379, 381
[M+H]+, EST, RT = 0.73 (S2).
105
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 30
2-fluoropyridine
TMS-CF3,
0 F OF
40 hre,C( SelectfluorAg0Tf , KF 40 oyo- )<F H2, Pd/C
_______________________________________________________________________
H01(0". I -F
Et0Ac, r.t. Et0H, r.t.
0 0 0
Step a Step b
Intermediate 54
H2 N- NH
iCI-C)
Step c
HATU, DIPEA
DMF, r.t.
0
)<F
H F H2, Pc1,/C
H2N
,NirfCc0)<F
A ,Nyr:t0 F
0 N
Et0H r.t. H 0
0
Intermediate 55 Step d
Step 30.a: benzyl (1s,3s)-3-(trifluoromethoxy)cyclobutane-1-carboxylate
0
0
2-Fluoropyridine (15 mL, 0.180 mol) and TMS-CF3 (27 mL, 0.180 mol) were
successively
added dropwise to a solution of benzyl (1s,3s)-3-hydroxycyclobutane-1-
carboxylate (12.4 g,
59.9 mmol), Ag0Tf (46.3 g, 0.180 mol), Selecfluor (31.8 g, 89.8 mmol) and KF
(13.9 g,
0.240 mol) in Et0Ac (500 mL) and the mixture was stirred at r.t. under N2 for
20 h in a foil
covered flask. The reaction mixture was filtered through Celite, washing with
Et0Ac (100
mL), and concentrated in vacuo. The residue was purified by chromatography on
silica gel (5-
30% Et0Ac in heptane) to afford the title compound (7.47 g, 27.2 mmol, 45%
yield) as a
colourless oil; 1HNMR (400 MHz, chloroform-d) 6 7.43 ¨7.29 (m, 5H), 5.14 (s,
2H), 4.57 (p,
J= 7.5 Hz, 1H), 2.82 ¨ 2.69 (m, 1H), 2.64 (dtd, J= 10.0, 7.3, 2.6 Hz, 2H),
2.53 (qd, J= 9.8,
9.4, 2.0 Hz, 2H); 19F NMR (376 MHz, chloroform-0 6 -59.56.
Intermediate 54 (step 30.b): (1s,3s)-3-(trifluoromethoxy)cyclobutane-1-
carboxylic acid
OF
HOyC( 1F
0
Intermediate 54
A suspension of benzyl (1s,3s)-3-(trifluoromethoxy)cyclobutane-1-carboxylate
(7.85 g, 28.6
mmol) and 5% Pd/C (3.05 g, 1.43 mmol) in Et0H (250 mL) was stirred under H2 at
r.t. for 18
h. The reaction mixture was filtered through Celite and concentrated in vacuo
to afford the
106
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
title compound (5.09 g, 27.6 mmol, 97% yield) as a yellow oil; 1H NMR (400
MHz,
chloroform-0 6 4.60 (p, J= 7.5 Hz, 1H), 2.89 - 2.61 (m, 4H), 2.61 - 2.37 (m,
2H); 19F NMR
(376 MHz, chloroform-d) 6 -59.62 (3F, s).
Step 30.c: ({ [(benzyloxy)carbonyl] amino} amino) [(1s,3s)-3-
(trifluoromethoxy)cyclobutyl] methanone
o N 0 F
:y1=3"
0 N F
H o
To a solution of (1s,3s)-3-(trifluoromethoxy)cyclobutane-1-carboxylic acid
(1.00 g, 5.43
mmol, Intermediate 54) in anhydrous DMF (10 mL) at 0 C was added HATU (2.27
g, 5.97
mmol) followed by DIPEA (1.9 mL, 10.9 mmol) and stirred for 10 min. Benzyl
hydrazinecarboxylate (0.90 g, 5.43 mmol) was added and the mixture was stirred
at r.t. for 20
h. The reaction mixture was diluted with H20 (20 mL) and extracted with Et0Ac
(2 x 50
mL). The combined organic extracts were dried over Na2SO4 and concentrated in
vacuo.
Purification by chromatography on silica gel (15-100% Et0Ac in heptane)
afforded the title
compound (1.03 g, 3.07 mmol, 56% yield) as a white powder; 1H NMR (400 MHz,
DMSO-
d6) 6 9.78 (s, 1H), 9.35 - 8.75 (m, 1H), 7.52 - 7.16 (m, 5H), 5.16 - 4.96 (m,
2H), 4.89 - 4.66
(m, 1H), 2.75 - 2.57 (m, 1H), 2.50 (s, 2H), 2.35 - 2.14 (m, 2H); M/Z: 333
[M+H]+, EST, RT
= 0.88 (S2).
Intermediate 55 (step 30.d): (1s,3s)-3-(trifluoromethoxy)cyclobutane-1-
carbohydrazide
OF
Hyri" hF
1\1 H2N F
0
Intermediate 55
A mixture of
( { [(benzyloxy)carbonyl] amino } amino)[(1s,3s)-3-
(trifluoromethoxy)cyclobutyl]methanone (1.03 g, 3.07 mmol) and 10% Pd/C (100
mg, 3.07
mmol) in Et0H (10 mL) was stirred under H2 at r.t. for 18 h. The reaction
mixture was
filtered through Celite and concentrated in vacuo to afford the title compound
(0.56 g, 2.68
mmol, 87% yield) as a grey solid; 1H NMR (400 MHz, DMSO-d6) 6 9.08 (s, 1H),
4.82 - 4.66
(m, 1H), 4.30 (s, 2H), 2.60 - 2.51 (m, 1H), 2.48 - 2.38 (m, 2H), 2.33 - 2.21
(m, 2H); M/Z:
199 [M+H]+, EST, RT = 0.54 (S2).
107
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 31
0 Boc N¨ Boc N¨
I
F ,J.LCI + R) 0 DI PEA
CI 1-1211"'
0 - 0
F F
DCM, r.t. F
Intermediate 19 Intermediate 6 Step a CI Example 1
Step b ZnBr2, DCM, r.t.
N-
0 R) 0
F F
F OA's
CI N
Example 2
Example 1 (step 31.a): tert-butyl (2R,5S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]-2-
15-[4-(trifluoromethyl)phenyfl-1,3,4-oxadiazol-2-yllpiperidine-1-carboxylate
Boc
0
5 Example 1
To a solution of 2-(4-chloro-3-fluorophenoxy)acetyl chloride (90% purity, 70
mg, 0.282
mmol, Intermediate 19) in DCM (2 mL) was added tert-butyl (2R,55)-5-amino-2-
{544-
(trifluoromethyl)pheny1]-1,3 ,4-oxadi azol-2-y1} pip eridine-1 - carboxyl ate
(123 mg, 0.282
mmol, Intermediate 6) and DIPEA (0.099 mL, 0.565 mmol) and the mixture was
stirred at r.t.
10 for 4 h. The reaction mixture was diluted with H20 (5 mL) and extracted
with DCM (3 x 5
mL). The combined organic extracts were dried over MgSO4, concentrated in
vacuo and
purified by chromatography on silica gel (17-100% Et0Ac in heptane) to afford
the title
compound (78 mg, 0.130 mmol, 46% yield) as a brown powder; M/Z: 499, 501 [M-
Boc+H]+,
EST, RT = 1.20 (S2).
15 Example 2 (step 31.b): 2-(4-chloro-3-fluorophenoxy)-N-R3S,6R)-6-15-[4-
(trifluoromethyflphenyfl-1,3,4-oxadiazol-2-yllpiperidin-3-yflacetamide
N¨N
11-\ L)cl
0 (R)
F ojcõõ.(5
ci
Example 2
108
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
To a solution of tert-butyl (2R,55)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]-2- {5-[4-
(tri fluoromethyl)phenyl] -1,3 ,4-oxadi azol-2-ylf pip eridine-1 - carboxyl
ate (78 mg, 0.130 mmol,
Example 1) in DCM (2 mL) was added ZnBr2 (88 mg, 0.391 mmol) and the mixture
was
stirred at r.t. for 18 h. The reaction mixture was diluted with satd aq NaHCO3
solution (3 mL)
and extracted with DCM:IPA (80:20) (3 x 3 mL). The combined organic extracts
were dried
using a phase separator, concentrated in vacuo, and purified by prep. HPLC
(Method 4) to
afford the title compound (8.0 mg, 0.0152 mmol, 12% yield) as a white powder;
1H NMR
(500 MHz, DMSO-d6) 6 8.26 ¨ 8.17 (m, 2H), 8.04 ¨ 7.94 (m, 3H), 7.50 (t, J= 8.9
Hz, 1H),
7.08 (dd, J= 11.4, 2.8 Hz, 1H), 6.89 ¨ 6.83 (m, 1H), 4.54 (s, 2H), 4.05 ¨ 3.98
(m, 1H), 3.81 ¨
3.69 (m, 1H), 3.07 ¨2.96 (m, 2H), 2.15 ¨2.06 (m, 1H), 1.99 ¨ 1.91 (m, 1H),
1.85 ¨ 1.73 (m,
1H), 1.63 ¨ 1.51 (m, 1H); M/Z: 499, 501 [M+H]+, EST, RT = 2.47 (S4).
Scheme for route 32
0 Boc
Boo \
io 0,A0, /N4-0 F HATU, DIPEA
CI H Nrs DMF, r.t.
2.-
Step a
Intermediate 6 CI
Example 3
Step b TFA, DCM, r.t.
0
Example 4
Example 3 (step 32.a): tert-butyl (2R,5S)-542-(4-chlorophenoxy)propanamido]-2-
{544-
(trifluoromethyl)pheny1]-1,3,4-oxadiazol-2-yl}piperidine-1-carboxylate
Boc
(R) 0
Cl
Example 3
To a solution of 2-(4-chlorophenoxy)propanoic acid (69 mg, 0.343 mmol) in DMF
(1 mL)
was added DIPEA (0.18 mL, 1.03 mmol) and HATU (143 mg, 0.377 mmol) and stirred
at r.t.
for 10 min. tert-butyl (2R,5S)-5- amino-2- {544-(trifluoromethyl)phenyl] -1,3
,4-oxadiazol-2-
yl}piperidine-l-carboxylate (141 mg, 0.343 mmol, Intermediate 6) was added and
the mixture
109
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
was stirred at r.t. for 20 h. The reaction mixture was diluted with H20 (5 mL)
and extracted
with Et0Ac (3 x 5mL). The combined organic extracts were washed with brine (2
x 15 mL),
dried over MgSO4 and concentrated in vacuo. The residue was purified by
chromatography on
silica gel (12-100% Et0Ac in heptane) to afford the title compound (90%
purity, 123 mg,
0.186 mmol, 54% yield) as a clear oil; M/Z: 495, 497 [M-Boc+H]+, EST, RT =
1.22 (S2).
Example 4 (step 32.b): 2-(4-ehlorophenoxy)-N-1(3S,6R)-6-15-[4-
(trifluoromethyl)pheny1]-
1,3,4-oxadiazol-2-yll pip eridin-3-yl] prop an amide
N¨N
0
0j-c,õ,)
C
Example 4
To a solution of tert-butyl (2R,55)-5- [2-(4-chlorophenoxy)propanamido]-2- {5-
[4-
(trifluoromethyl)phenyl] -1,3 ,4-oxadi azol-2-ylf pip eridine-1 - carboxyl ate
(107 mg, 0.181
mmol, Example 3) in DCM (2 mL) was added TFA (70 !IL, 0.947 mmol) and the
mixture was
stirred at r.t. for 18 h. The reaction mixture was concentrated in vacuo,
dissolved in satd aq
NaHCO3 solution (10 mL) and extracted with Et0Ac (3 x 10 mL). The combined
organic
extracts were dried over MgSO4, concentrated in vacuo and triturated using
DMSO:MeCN:H20 (60:30:10), washing with MeCN (1 mL), to afford the title
compound (19
mg, 0.0383 mmol, 21% yield) as a white powder; 11-1 NMR (500 MHz, DMSO-d6) 6
8.25 ¨
8.19 (m, 2H), 8.03 ¨ 7.94 (m, 3H), 7.36 ¨ 7.30 (m, 2H), 6.96 ¨ 6.89 (m, 2H),
4.72 ¨ 4.64 (m,
1H), 4.02 ¨ 3.95 (m, 1H), 3.74 ¨ 3.63 (m, 1H), 3.03 ¨2.91 (m, 2H), 2.13 ¨2.00
(m, 1H), 1.96
¨ 1.82 (m, 1H), 1.82 ¨ 1.69 (m, 1H), 1.60 ¨ 1.46 (m, 1H), 1.43 (d, J= 6.6 Hz,
3H); M/Z: 495,
497 [M+H]+, EST, RT = 2.47 (S4).
The example compound in Table 9 was synthesised according to the synthetic
steps of
general route 32 as exemplified by Example 4 using the corresponding
intermediates.
110
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Table 9
LCMS
Ex Structure Name Intermediates 111 NMR
data
N- 1H NMR (500 MHz,
tert-butyl (2S,5R)-
R3R,65)-6- DMSO-d6) 8.08 ¨ 7.98
5-amino-2-[5-(4-
[5-(4- (m, 2H), 7.72 ¨ 7.66 (m,
chloropheny1)-
chlorophen 2H), 7.62 (d, J= 8.1 Hz,
1,3,4-oxadiazol-2-
y1)-1,3,4- M/Z:
1H), 4.51 ¨4.41 (m, 1H),
3-N
* oxadiazol-
475, 477 4.26 ¨4.20 (m, 1H), 3.78
carboxylate
= 3)LNe 2-
[M+1-1]-', (s, 3H), 3.75 ¨3.66 (m,
Fc7'
yl]piperidi
and 2-[(1s,3s)-3-
(Intermediate 46)
RT
1H), 3.08 (s, 1H), 2.86 ¨
n-3-y1]-2- = 2.32 2.79 (m, 1H), 2.78 ¨2.69
(trifluoromethoxy
[(1s,3s)-3- (S4) (m, 2H), 2.66 ¨ 2.61 (m,
)cyclobutoxy]acet
(trifluorom 1H), 2.17 ¨ 2.12 (m, 1H),
ic acid (described
ethoxy)cyc 2.12 ¨ 2.01 (m, 2H), 1.94
in WO
lobutoxy]a ¨1.84 (m, 1H), 1.77¨
2019032743 Al)
cetamide
1.69 (m, 2H).
Scheme for route 33
0 Boc NN Boc N¨N
II\ I \ 41 _____
SOCl2' DCM, 45 C \ =
CI
OH + (R) -
(5) then
CIN H2N\sµ DIPEA, DCM, r.t.
I
Intermediate 12 Intermediate 53 Step a
CIN
Example 6
Step b ZnBr2, DCM, r.t.
N-1\1
41104
0 (R) 0
01
Example 7
5 Example 6 (step 33.a): tert-butyl (2R,5S)-5-12-[(6-chloro-5-fluoropyridin-3-
yl)oxyjacetamido}-2-[5-(4-chloropheny1)-1,3,4-oxadiazol-2-ylipiperidine-1-
carboxylate
Boc N¨N
I II 0 \
(R)
Cl N
F 0j.c\µµ,=`
Example 6
To a suspension of 2-[(6-chloro-5-fluoropyridin-3-ypoxy]acetic acid (50 mg,
0.243 mmol,
Intermediate 12) in DCM (5 mL) was added thionyl chloride (0.10 mL, 1.37 mmol)
and the
111
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
mixture was stirred at 45 C for 4 h and then at r.t. for 18 h. The reaction
mixture was
concentrated in vacuo and azetroped using heptane. The resultant residue was
dissolved in
DCM (5 mL) and added to a solution of tert-butyl (2R,5S)-5-amino-245-(4-
chloropheny1)-
1,3,4-oxadiazol-2-yl]piperidine-l-carboxylate (71 mg, 0.187 mmol, Intermediate
53) and
DIPEA (0.16 mL, 0.937 mmol) in DCM (5 mL). The reaction mixture was stirred at
r.t. for 2
h, then poured on to satd. aq NaHCO3 solution (10 mL). The aqueous solution
was extracted
with DCM (2 x 20 mL) and the combined organic extracts were dried over Na2SO4
and
concentrated in vacuo. Purification by chromatography on silica gel (20-100%
Et0Ac in
heptane) afforded the title compound (27 mg, 0.0467 mmol, 25% yield) as a
colorless oil; II-I
NMR (400 MHz, chloroform-d) 6 8.00 (d, J= 2.6 Hz, 1H), 7.96 (d, J= 8.6 Hz,
2H), 7.49 (d, J
= 8.5 Hz, 2H), 7.14 (dd, J= 8.8, 2.6 Hz, 1H), 4.60 ¨4.48 (m, 2H), 4.24 ¨ 4.08
(m, 2H), 2.30
(d, J= 13.0 Hz, 1H), 2.14¨ 1.95 (m, 2H), 1.66 (s, 1H), 1.49 (s, 9H), 1.28 ¨
1.22 (m, 2H);
M/Z: 588, 590, 592 [M+Na]+, EST, RT = 1.09 (S2).
Example 7 (step 33.b): 2- [(6-chloro-5-fluoropyridin-3-yl)oxy] -N- [(3S,6R)-6-
[5-(4-
chloropheny1)-1,3,4-oxadiazol-2-yl] pip eridin-3-yl] acetamide
NI¨
I
I H
CIN
Example 7
To a solution of tert-butyl (2R,55)-5- {2-[(6-chloro-5-fluoropyridin-3-
yl)oxy]acetamido}-2-[5-
(4-chloropheny1)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate (48 mg, 0.0830
mmol,
Example 6) in DCM (2 mL) was added ZnBr2 (56 mg, 0.249 mmol) and the reaction
mixture
was stirred at r.t. for 48 h. The reaction mixture was filtered under vacuum,
washing with
DCM, and the filtrate concentrated in vacuo. The residue was purified by prep.
HPLC
(Method 1) to afford the title compound (11 mg, 0.0211 mmol, 25% yield) as a
white solid;
1H NMR (500 MHz, DMSO-d6) 6 8.20 (d, J= 7.7 Hz, 1H), 8.09 (d, J= 2.6 Hz, 1H),
8.03 (d, J
= 8.6 Hz, 2H), 7.75 ¨ 7.68 (m, 2H), 4.68 (s, 2H), 4.38 (s, 1H), 3.90 (s, 1H),
3.22 (d, J= 11.4
Hz, 1H), 2.71 (t, J= 11.1 Hz, 1H), 2.24 (d, J= 11.1 Hz, 1H), 1.99 (d, J= 10.3
Hz, 1H), 1.94 ¨
1.84 (m, 1H), 1.69 ¨ 1.59 (m, 1H); M/Z: 466, 468, 470 [M+H]+, EST, RT = 2.01
(S4).
112
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Scheme for route 34
Boc N¨
O Boc
0 CI al ,A
OH )4'0 HATU, DIPEA
0 (R)
CI ) F F \--F DMF, r.t.
CI WI H2NIV' F F
Step a CI
Intermediate 49 Example 8
ZnBr2
Step b DCM, r.t.
)\--F
0 CI j=LN,
F F
CI WI
Example 9
Example 8 (step 34.a): tert-butyl (2R,5S)-542-(3,4-dichlorophenoxy)acetamido]-
2-15-
[(1s,3s)-3-(trifluoromethoxy)cyclobuty1]-1,3,4-oxadiazol-2-yllpiperidine-1-
carboxylate
Boc N¨ .
0 (R) =-=
FXF-F
CI OJLõ,=-<-
Example 8
To a solution of 2-(3,4-dichlorophenoxy)acetic acid (104 mg, 0.472 mmol) in
DMF (4 mL)
was added HATU (180 mg, 0.472 mmol) and DIPEA (0.21 mL, 1.18 mmol) and stirred
at r.t.
for 10 min. tert-butyl (2R,55)-5-amino-2- {5-[(1s,3s)-3-
(trifluoromethoxy)cyclobuty1]-1,3,4-
oxadiazo1-2-yllpiperidine-1-carboxylate (160 mg, 0.394 mmol, Intermediate 49)
was added
and the mixture was stirred at r.t. for 3 h. The reaction mixture was diluted
with H20 (10 mL)
and extracted with Et0Ac (2 x 20 mL). The combined organic extracts were dried
over
Na2SO4 and concentrated in vacuo to afford the title compound (52% purity, 105
mg, 0.0896
mmol, 23% yield) as yellow oil; M/Z: 509, 511 [M2Butyl+H], EST, RT = 1.17
(S2).
Example 9 (step 34.b): 2-(3,4-dichlorophenoxy)-N-1(3S,6R)-6-15-1(1s,3s)-3-
(trifluoromethoxy)cyclobuty1]-1,3,4-oxadiazol-2-yllpiperidin-3-yljacetamide
o
(17)
F
CI
1\1`
CI
Example 9
113
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
To a solution of tert-butyl (2R,55)-5-[2-(3,4-dichlorophenoxy)acetamido]-2- {5-
[(1s,3s)-3-
(trifluoromethoxy)cyclobuty1]-1,3,4-oxadiazol-2-ylf piperidine-l-carboxylate
(52% purity,
105 mg, 0.0896 mmol, Example 8) in DCM (1 mL) was added ZnBr2 (61 mg, 0.269
mmol)
and the mixture was stirred at r.t. for 6 h. The reaction mixture was diluted
with H20 (10 mL)
and extracted with DCM/IPA (9:1, 2 x 20 mL). The combined organic extracts
were dried
over MgSO4, concentrated in vacuo and purified by prep. HPLC (Method 3) to
afford the title
compound (6.7 mg, 0.0130 mmol, 15% yield) as a white powder; 1H NMR (400 MHz,
DMSO-d6) 6 7.98 (d, J= 8.0 Hz, 1H), 7.55 (d, J= 8.9 Hz, 1H), 7.26 (d, J= 2.9
Hz, 1H), 6.99
(dd, J= 8.9, 2.9 Hz, 1H), 4.98 ¨4.87 (m, 1H), 4.53 (s, 2H), 3.86 (dd, J= 10.5,
2.7 Hz, 1H),
3.71 (s, 1H), 3.45 (d, J= 2.1 Hz, 1H), 2.99 (dd, J= 11.8, 2.9 Hz, 1H), 2.89 ¨
2.80 (m, 3H),
2.43 (s, 3H), 2.00 ¨ 1.97 (m, 1H), 1.92 ¨ 1.88 (m, 1H), 1.69 ¨ 1.64 (m, 1H),
1.54 ¨ 1.49 (m,
1H); M/Z: 509, 511 [M+H]+, EST, RT = 2.42 (S4).
Example compounds in Table 10 were synthesised according to the synthetic
steps of general
route 34 as exemplified by Example 9 using the corresponding intermediates.
Table 10
LCMS
Ex Structure Name Intermediates 111 NMR
data
'H NMR (400 MHz,
tert-butyl
2-[3-chloro- DMSO-d6) 6 8.03 (d,
(2R,55)-5-
4- 8.0 Hz, 1H), 7.79 (d, J=
amino-2-{5-
(trifluoromet
8.9 Hz, 1H), 7.31 (d,
hyl)phenoxy
2.4 Hz, 1H), 7.09 (dd, J
(trifluoromethox
= 8.7, 2.3 Hz, 1H), 5.02
y)cyclobuty1]- M/Z:
¨4.83 (m, 1H), 4.64 (s,
[(3S,6R)-6-
1,3,4-oxadiazol- 543, 545
{5-[(1s 3s)- 2-yl}piperidine-
[M+H]', 2H), 3.87 (dd, J= 10.6,
ab, 1-carboxylate ES I, RT
10 el 033LNõ, F.F
3- 2.7 Hz, 1H), 3.78 ¨3.66
F H (trifluoromet
(m, 1H), 3.45 ¨3.39 (m,
(Intermediate = 2.53
hoxy)cyclob
1H), 3.00 (dd, J= 11.8,
49) and 2-[3- (S4)
uty1]-1,3,4- 3.1 Hz, 1H), 2.91 ¨ 2.79
chloro-4-
oxadiazol-2- (m, 3H), 2.44 (d, J= 11.7
(trifluoromethyl
yl } piperidin- Hz, 3H), 2.11 ¨ 1.97 (m,
)phenoxy]acetic
3- acid
1H), 1.95 ¨ 1.87 (m, 1H),
yl]acetamide
1.75 ¨ 1.63 (m, 1H), 1.55
(Intermediate 7)
¨ 1.47 (m, 1H).
2-[4-chloro- tert-butyl 'H NMR (400
MHz,
3- (2R,5S)-5-
DMSO-d6) 6 8.01 (d, J=
(difluoromet amino-2-{5-[2- M/Z:
8.1 Hz, 1H), 7.57 ¨ 7.45
hyl)phenoxy (trifluoromethox 515, 517
(m, 1H), 7.31 ¨7.02 (m,
]-N- y)ethoxy]-1,3,4-
[M+H]', 2H), 4.74 ¨4.62 (m, 2H),
11 0--4F
[(3S,6R)-6- oxadiazol-2- ESI', RI
4.56 (s, 2H), 4.51 ¨ 4.39
Cy1H F {542- yllpiperidine-1- = 2.27 (m,
2H), 3.82 ¨3.62 (m,
F H (trifluoromet carboxylate
(S4) 2H), 3.05 ¨ 2.90 (m, 1H),
CI 4127 hoxy)ethoxy (Intermediate
2.86 ¨ 2.72 (m, 1H), 2.44
]-1,3,4- 48) and 2-[4-
¨ 2.38 (m, 1H), 2.01 ¨
114
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
oxadiazol-2- chloro-3-
1.83 (m, 2H), 1.73 ¨ 1.56
yllpiperidin- (difluoromethyl) (m,
1H), 1.56 ¨ 1.41 (m,
3- phenoxy]acetic 1H).
yl]acetamide acid
(Intermediate
17)
tert-butyl 'H NMR (500 MHz,
2-(4-chloro- (2R,55)-5-
chloroform-d) S 7.27 (s,
3- amino-2-{5-[2-
1H), 6.84 ¨ 6.80 (m, 1H),
methylpheno (trifluoromethox
6.78 ¨ 6.73 (m, 1H), 6.71
xy)-N- y)ethoxy]-1,3,4-
(dd, J = 8.7, 3.0 Hz, 1H),
N N ,___/ ---E: F M/Z: [(3S,6R)-6-
oxadiazol-2- 4.72 ¨4.67 (m, 2H), 4.45
,..ii 1-- F 479, 481
1542- yllpiperidine-1-
[M+H] ,, (s' 2H)' 4.36 ¨4.31 (m,
12 (trifluoromet
carboxylate 2H), 4.09 ¨4.01 (m, 1H),
0,ANõ. ,
'
ci =H hoxy)ethoxy (Intermediate ESI, RT
= 2.34
3.98 (dd, J = 7.9, 3.4 Hz,
]-1,3,4- 48) and 2-(4- (S4) 1H),
3.30 (dd, J= 12.0,
oxadiazol-2- chloro-3- 3.5
Hz, 1H), 2.62 (dd, J =
yllpiperidin- methylphenoxy)
12.0, 7.8 Hz, 1H), 2.36
3- acetic acid (s,
3H), 2.13 ¨ 2.02 (m,
yl]acetamide (Intermediate
2H), 2.00 ¨ 1.86 (m, 2H),
14) 1.66 ¨ 1.58 (m,
1H).
'H NMR (500 MHz,
chloroform-d) 6 7.08 ¨
7.03 (m, 1H), 6.82 ¨ 6.76
2-(3,4- tert-butyl
(m, 1H), 6.76 ¨6.73 (m,
dimethylphe (2R,5S)-5-
1H), 6.66 (dd, J= 8.2,
noxy)-N- amino-2- {5-[2-
F [(3S,6R)-6- (trifluoromethox 2.7
Hz, 1H), 4.72 ¨4.67
M/Z: 459 (m, 2H), 4.45 (s, 2H),
H
NN -- r--1 \-F- F 1542- y)ethoxy]-1,3,4-
[M+H]', 4.36 ¨ 4.31 (m, 2H), 4.08
0 , 0 (trifluoromet oxadiazol-2-
13 446..sh. 0,)LNõ.0).'
up H hoxy)ethoxy yllpiperidine-1-
]-1,3,4- carboxylate
ESI', RI ¨4.00 (m, 1H), 3.97 (dd,
= 2.26 .1¨
8.2, 3.1 Hz, 1H), 3.30
(S4) (dd, J= 12.0, 3.5
Hz,
oxadiazol-2- (Intermediate
1H), 2.61 (dd, .1= 12.0,
yllpiperidin- 48) and 2-(3,4-
8.0 Hz, 1H), 2.25 (s, 3H),
3- dimethylphenox
2.21 (s, 3H), 2.09 (dd, J
yl]acetamide y)acetic acid
= 11.4, 4.4 Hz, 2H), 1.96
¨1.85 (m, 2H), 1.64 ¨
1.57 (m, 2H).
tert-butyl 'H NMR (500 MHz,
(2R,5S)-5-
N-[(3S,6R)- DMSO-d6) 6 8.47 (d, J=
amino-2- {542-
6-1542- 2.8 Hz, 1H), 8.08 (d, J=
(trifluoromethox
(trifluoromet 8.0
Hz, 1H), 7.87 (d, J=
y)ethoxy]-1,3,4-
hoxy)ethoxy
oxadiazol-2-
8.7 Hz, 1H), 7.56 (dd, J
0 -.<._F_F ]-1,3,4- M/Z: 500 =
8.7, 2.8 Hz, 1H), 4.71
yllpiperidine-1-
H /---/NI-N-c) F
oxadiazol-2- [M+H]', (s, 2H), 4.70 ¨4.66 (m,
carboxylate
14 0j(N . (Y'c'
yllpiperidin- ESI', RI 2H), 4.50 ¨ 4.45 (m, 2H),
(Intermediate
F 1-1 3-y1]-2- {[6- 48) and 2-46-
= 1.84
3.81 ¨3.74 (m, 1H), 3.74
F F N (trifluoromet (S4)
¨3.66 (m, 1H), 2.99 (d, J
(trifluoromethyl
hyl)pyridin- = 11.9 Hz, 1H), 2.81 (s,
)pyridin-3-
3- 1H), 2.47 ¨2.40 (m, 1H),
vl]oxy{ acetic
yl]oxy{aceta - acid
2.01 ¨ 1.86 (m, 2H), 1.71
mide
¨1.60 (m, 1H), 1.54 ¨
(Intermediate
1.43 (m, 1H).
13)
2-[3- tert-butyl 'H NMR (500 MHz,
M/Z:
F methoxy-4- (2R,55)-5-
DMSO-d6) 6 8.00 (d, J=
529, 53+1
N õ.._/ --<- F (trifluoromet amino-2-{5-[2-
[M+H] , 8.1 Hz, 1H), 7.53 (d, J-
15 0 NCy, 1 --ON
H --- 0 F hyl)phenoxy (trifluoromethox ESI+,
RI 8.8 Hz, 1H), 6.81 (d, J=
,0 0,11...Nõ. , ]-N- y)ethoxy]-1,3,4- 2.0
Hz, 1H), 6.63 (dd, J
F 0 H
[(3S,6R)-6- oxadiazol-2- = 2.28 =
8.7, 2.2 Hz, 1H), 4.73
F (S4)
F 1542- yllpiperidine-1-
¨4.65 (m, 2H), 4.59 (s,
115
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
(trifluoromet carboxylate
2H), 4.50 ¨ 4.46 (m, 2H),
hoxy)ethoxy (Intermediate
3.87 (s, 3H), 3.80 ¨3.68
]-1,3,4- 48) and 2-[3- (m, 2H), 3.04 ¨2.95
(m,
oxadiazol-2- methoxy-4-
1H), 2.85 ¨2.78 (m, 1H),
yllpiperidin- (trifluoromethyl
2.46 ¨ 2.41 (m, 1H), 1.99
3- )phenoxy]acetic ¨
1.88 (m, 2H), 1.71 ¨
yl]acetamide acid
1.61 (m, 1H), 1.57 ¨ 1.47
(Intermediate (m, 1H).
15)
tert-butyl
'H NMR (500 MHz,
(2R,55)-5-
DMSO-d6) 6 8.02 (d, J=
N-R3S,6R)- amino-2-{5-[2-
8.0 Hz, 1H), 7.68 (d, J=
6-1542- (trifluoromethox
8.7 Hz, 2H), 7.13 (d, J=
(trifluoromet y)ethoxy]-1,3,4-
04 86 Hz, hoxy)ethoxy oxadiazol-2- M/Z: 499
2H) 4.73 ¨4.65
F ]-1,3,4- yllpiperidine-1- [M+H]-', (m, 2H), 4.59 (s,
2H),
4.51 ¨4.42 (m 2H) 3.81
112 oxadiazol-2- carboxylate ESI', RT"
0,)(Nõ.0
¨ 3.74 (m, 1H), 3.74 -
F ip H yllpiperidin- (Intermediate = 2.18
3.64 (m, 1H), 3.03 ¨2.93
F F 3-y1]-2-[4- 48) and 2-[4- (S4)
(m, 1H), 2.84 ¨2.76 (m,
(trifluoromet (trifluoromethyl
1H), 2.48 ¨2.39 (m, 1H),
hyl)phenoxy )phenoxy]acetic
2.01 ¨ 1.85 (m, 2H), 1.72
]acetamide acid
¨1.59 (m, 1H), 1.56 ¨
(Intermediate
1.44 (m, 1H).
18)
tert-butyl
2-[3-chloro- (2R,5S)-5- 'H NMR (500 MHz,
4- amino-2-{5-[2-
DMSO-d6) 6 8.01 (d, J=
(difluoromet (trifluoromethox 8.1
Hz, 1H), 7.65 ¨ 7.58
hyl)phenoxy y)ethoxy]-1,3,4- (m,
1H), 7.27 ¨6.99 (m,
F ]-N- oxadiazol-2- M/Z: 3H), 4.71 ¨4.64 (m, 2H),
kH F[(3S,6R)-6- yllpiperidine-1-
515, 517 4.59 (s, 2H), 4.51 ¨4.43
113 ci
{542- carboxylate [M+H]', (m, 2H), 3.80 ¨3.74 (m,
0,5L õ.0
F (trifluoromet (Intermediate
ESI', RI 1H), 3.73 ¨3.65 (m, 1H),
hoxy)ethoxy 48) and 2-[3- =
2.24 3.01 ¨2.94 (m, 1H), 2.80
]-1,3,4- chloro-4- (S4) (s, 1H), 2.46 ¨2.39 (m,
oxadiazol-2- (difluoromethyl)
1H), 2.00 ¨ 1.92 (m, 1H),
yllpiperidin- phenoxy]acetic
1.93 ¨ 1.85 (m, 1H), 1.71
3- acid
¨1.59 (m, 1H), 1.55 ¨
yl]acetamide (Intermediate 1.43 (m, 1H).
56)
'H NMR (400 MHz,
tert-butyl
chloroform-d) 6 7.57 (t, J
2-[3-fluoro-
(2R,5 5)-5- =
8.2 Hz, 1H), 6.80 (d, J
4-
amino-2-{5-[2- =
9.8 Hz, 2H), 6.73 (d, J
(trifluoromet
(trifluoromethox =
7.5 Hz, 1H), 4.74 ¨
hyl)phenoxy
y)ethoxy]-1,3,4- 4.66
(m, 2H), 4.53 (s,
]-N-
O oxadiazol-2-
M/Z: 517 2H), 4.37 ¨4.30 (m, 2H),
- 113S 6R) 6
N,--0' F " yllpiperidine-1-
[M+H]', 4.07 (ddt, J = 11.4, 8.0,
0
114 F 0 0 _ ,ANõ.0 carboxylate
ESI', RT 3.9 Hz, 1H),4.01 (dd, J
1110 H (trifluoromet
hoxy)ethoxy (Intermediate = 2.20 = 7.4, 3.5 Hz,
1H), 3.30
F F ]-1,3,4-
48) and 2-[3- (S4) (dd,
J= 12.0, 3.4 Hz,
fluoro-4- 1H),
2.64 (dd, J= 12.0,
oxadiazol-2-
(trifluoromethyl 7.4
Hz, 1H), 2.08 (qt, J
yllpiperidin-
)phenoxy]acetic
10.8, 5.4 Hz, 2H), 1.95
3-
acid
(ddd, J = 16.9, 10.4, 6.1
yl]acetamide
(Intermediate 8) Hz,
2H), 1.71 ¨ 1.58 (m,
1H).
116
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 35
F F
F F
72--F
0 Boc Boc
ojk T3P,
DIPEA 7-2-F
OH + 0 F
DCM, r.t.
CI F121e. Step a
Intermediate 47 CI
Example 16
ZnBr2
Step b
DCM, r.t.
F F
NF
HN
0
ojk (s)
CI
Example 17
Example 16 (step 35.a): tert-butyl (2R,5S)-5-[2-(4-chloro-2-
fluorophenoxy)acetamido]-2-
[5-(3,3,3-trifluoropropoxy)-1,3,4-oxadiazol-2-yl] pip eridine- 1- carb oxylate
Boc
0 (R) 0
`c9
Nr
Cl
Example 16
To a solution of 2-(4-chloro-2-fluorophenoxy)acetic acid (20 mg, 0.0999 mmol)
in DCM (1
mL) was added T3P (50% in Et0Ac, 713 1,1L, 0.120 mmol) and DIPEA (41 pL, 0.233
mmol)
and stirred at r.t. for 10 min. tert-butyl (2R,5 S)-5-amino-2-[5-(3,3,3-
trifluoropropoxy)-1,3,4-
oxadiazol-2-yl]piperidine- 1 -carboxylate (38 mg, 0.10 mmol, Intermediate 47)
was added and
the resultant mixture was stirred at r.t. for 72 h. The reaction mixture was
diluted with H20 (1
mL) and extracted with DCM (2 mL). The combined organic extracts were dried
over Na2SO4
and concentrated in vacuo to afford an orange solid. The crude material was
taken forward
without purification.
117
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Example 17 (Step 35.b): 2-(4-chloro-2-fluorophenoxy)-N-[(3S,6R)-6-[5-(3,3,3-
trifluoropropoxy)-1,3,4-oxadiazol-2-yl] piperidin-3-yl] acetamide
NN jj
Example 17
To a solution of tert-butyl (2R,5S)-5-[2-(4-chloro-2-fluorophenoxy)acetamido] -
2-[5-(3,3,3-
trifluoropropoxy)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate (57 mg, 0.10
mmol, Example
16) in anhydrous DCM (1 mL) was added ZnBr2 (90 mg, 0.40 mmol) and the mixture
was
stirred at r.t. for 18 h. The reaction mixture was diluted with satd aq NaHCO3
solution and
extracted with DCM/IPA (4:1, 2 x 2 mL). The combined organic extracts were
concentrated
in vacuo and purified by prep. HPLC (Method 4) to afford the title compound
(88% purity,
1.7 mg, 0.0032 mmol, 3% yield) as a white solid; 1H NMR (400 MHz, chloroform-
d) 6 1.59 ¨
1.64 (m, 2H), 1.89 ¨ 2.01 (m, 1H), 2.14 (dq, J= 12.8, 4.6, 4.0, 2H), 2.66 (dd,
J= 12.0, 7.9,
1H), 2.68 ¨ 2.79 (m, 2H), 3.34 (dd, J= 12.0, 3.5, 1H), 4.02 (dd, J= 8.1, 3.4,
1H), 4.08 (ddt, J
= 12.3, 8.2, 4.1, 1H), 4.53 (s, 2H), 4.72 (t, J= 6.2, 2H), 6.86 ¨ 6.97 (m,
2H), 7.11 (ddd, J=
8.7, 2.4, 1.7, 1H), 7.19 (dd, J= 10.6, 2.5, 1H); M/Z: 467, 469 [M+H]+, EST, RT
= 1.98 (S4).
Example compounds in Table 11 were synthesised according to the synthetic
steps of general
route 35 as exemplified by Example 17 using the corresponding intermediates.
Table 11
LCMS
Ex Structure Name Intermediates 111 NMR
data
tert-butyl 1H NMR (400
MHz,
2-(3-chloro-
4-
(2R,5S)-5-
chloroform-d) 6 1.59 ¨
amino-2-[5-
1.66 (m, 2H), 1.88 ¨2.01
fluoropheno
xy) -N (3,3,3- (m, 1H), 2.03 ¨2.16 (m,
M/Z:
- trifluoropropoxy 2H), 2.65 (dd, J= 12.1,
[(3S,6R)-6- 467, 469
N-NA
)-1,3,4- 7.6, 1H),
2.73 (qt, J=
trifluoroprop
[5(3,3,3- [M+H]',
18 oxadiazol-2- RT 10.3,
6.2' 2H), 3.32 (dd,
H 0
yl]piperidine-1-
J= 12.0, 3.4, 1H), 4.02
oxy)-1,3,4- = 2.00
0,)(Nõf oxadiazol-2-
carboxylate (dd, J= 7.7,
3.4, 1H),
yl]piperidin-
ci Cs, F
H
(Intermediate (S4)
4.04 ¨ 4.13 (m, 1H), 4.47
F 47) and 2-(3-
(s, 2H), 4.72 (t, J= 6.2,
3-
chloro-4- 2H), 6.77 (d,
J= 7.8,
yl]acetamide
fluorophenoxy)a 1H), 6.83
(dt, J= 9.1,
118
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
cetic acid 3.4, 1H), 7.03 (dd,
J=
5.9, 3.1, 1H), 7.08 ¨ 7.16
(m, 1H).
tert-butyl 1HNMR (400 MHz,
2-[4- (2R,5S)-5- chloroform-d) 6
1.58 ¨
(trifluoromet amino-2-[5-
1.68 (m, 2H), 1.90 ¨2.00
hyl)phenoxy (3,3,3- (m,
1H), 2.04 ¨ 2.16 (m,
]-N- trifluoropropoxy 2H), 2.65 (dd, J=
12.1,
M/Z: 483
H ill_ [(3S,6R)-6- 0 )-1,3,4-
[M+H]', 7.7, 1H), 2.68 ¨ 2.80 (m, [5-(3,3,3- oxadiazol-2- ESI,
RI 2H), 3.32 (dd, J= 12.0,
19 N9'
F ..-. trifluoroprop yl]piperidine-1- 3.4, 1H),4.01 (dd, J =
F 41 I-1 F
oxy)-1,3,4- carboxylate = 2.12
(S4) 7.7, 3.4, 1H), 4.04 ¨ 4.14
F F
oxadiazol-2- (Intermediate (m, 1H), 4.56 (s,
2H),
yl]piperidin- 47) and 2-[4-
4.72 (t, J= 6.2, 2H), 6.78
3- (trifluoromethyl (d,
J= 8.0, 1H), 7.05 (d,
yl]acetamide )phenoxy]acetic J=
8.5, 2H), 7.63 (d, J=
acid 8.5,2H).
1HNMR (400 MHz,
chloroform-d) 6 1.60 ¨
tert-butyl
1.67 (m, 2H), 1.89 ¨2.00
2-(3,4- (2R,55)-5- (m, 1H), 2.10 (dtq,
J=
dichlorophen amino-2-[5- 10.5, 6.9, 3.7,
2H), 2.65
oxy)-N- (3,3,3- M/Z: (dd, J = 12.1,
7.6, 1H),
[(3S,6R)-6- trifluoropropoxy 483, 485, 2.73 (qt, J= 10.3, 6.2,
N¨
H N,_...0 [5-(3,3,3- )-1,3,4- 487 2H),
3.32 (dd, J= 12.0,
20 õ CI \---_, trifluoroprop oxadiazol-2-
[M+H]', 3.5, 1H), 4.02 (dd, J=
CI 0
VI H F F oxy)-1,3,4- yl]piperidine-1-
ESI', RT 7.7, 3.5, 1H), 4.08 (ddq,
CI oxadiazol-2- carboxylate = 2.16 J= 11.5,
8.0, 3.5, 1H),
yl]piperidin- (Intermediate (S4)
4.49 (s, 2H), 4.72 (t, J=
3- 47) and 2-(3,4-
6.2, 2H), 6.75 (d, J= 8.1,
yl]acetamide dichlorophenox 1H), 6.83 (dd, J =
8.9,
y)acetic acid
2.9, 1H), 7.10 (d, J= 2.9,
1H), 7.41 (d, J= 8.9,
1H).
tert-butyl
(2R,5S)-5-
2-(4-chloro- amino-2-[5- 1HNMR (400 MHz,
2,3- (3,3,3- chloroform-d) 6
1.64 ¨
difluorophen trifluoropropoxy
1.72 (m, 2H), 1.91 ¨2.01
oxy)-N- )-1,3,4- M/Z: (m,
1H), 2.05 ¨2.19 (m,
[(3S,6R)-6- oxadiazol-2- 485, 487 2H), 2.63
¨2.79 (m, 3H),
H Nii\lo
F 0 l\(y.., 0 , [5-(3,3,3- Apiperidine-1- [M+H]',
3.33 (dd, J= 12.0, 3.4,
21
F 0
CI j1...No. s)
WI H F trifluoroprop
carboxylate ESI', RI 1H), 3.99 ¨4.15 (m, 2H),
F .
F oxy)-1,3,4- (Intermediate = 2.08 4.55 (s, 2H),
4.72 (t, J=
oxadiazol-2- 47) and 2-(4- (S4)
6.2, 2H), 6.70 ¨ 6.78 (m,
yl]piperidin- chloro-2,3- 1H), 6.88 (d, J=
7.9,
3- difluorophenoxy 1H), 7.16 (ddd, J=
9.2,
yl]acetamide )acetic acid 7.5, 2.5, 1H).
(Intermediate
10)
2-(4-chloro- tert-butyl 1HNMR (400 MHz,
3,5- (2R,5S)-5- chloroform-d) 6
1.61 ¨
difluorophen amino-2-[5-
1.67 (m, 2H), 1.91 ¨2.02
M/Z:
oxy)-N- (3,3,3- (m,
1H), 2.03 ¨2.17 (m,
485, 487
[(3S,6R)-6- trifluoropropoxy [M+H], 2H), 2.58
¨2.80 (m, 3H),
'
22 [5-(3,3,3- )-1,3,4- ESI = 2.12 , RI
3.32 (dd, J= 12.0, 3.4,
'
H N -NA trifluoroprop
oxadiazol-2- 1H), 4.00 ¨4.15 (m, 2H),
0 ,....A.N, s)
1 7-0
oxy)-1,3,4- yl]piperidine-1-
4.48 (s, 2H), 4.72 (t, J=
F
F (y (S4)
.1 H F oxadiazol-2- carboxylate
F 6.2, 2H), 6.60 ¨ 6.68 (m,
CI yl]piperidin- (Intermediate
2H), 6.71 (d, J = 8.0,
F 3- 47) and 2-(4- 1H).
119
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
yl]acetamide chloro-3,5-
difluorophenoxy
)acetic acid
(Intermediate
11)
tert-butyl
(2R,5S)-5-
2-[3-fluoro- ill NMR (400 MHz,
amino-2-[5-
4- chloroform-d) 6 1.66 ¨
(3,3,3-
(trifluoromet 1.74 (m, 2H), 1.89 ¨2.02
trifluoropropoxy
hyl)phenoxy )-1,3,4-
(m, 1H), 2.12 (ddd, .1¨
H N_0 ]-N-
oxadiazol-2- M/Z: 501 12.1, 7.0, 3.3, 2H), 2.61
0 114, 0 N___, [(3S,6R)-6- õ . ,
[M+H]', ¨2.81 (m, 3H), 3.32 (dd,
23 F .)(N, ' F [5-(3,3,3_ Y1 _I pipenaine-1-
ESI', RT J=
12.0, 3.4, 1H), 4.03
H F carboxylate
F WI trifluoroprop = 2.18
(dd, J=7.6, 3.5, 1H),4.05
F F
oxy)-1,3,4- (Intermediate
(S4)
¨4.14 (m, 1H), 4.55 (s,
47) and 213-
oxadiazol-2- 2H), 4.72 (t, J= 6.2, 2H),
fluoro-4-
yl]piperidin- 6.75 (d, J= 7.9, 1H),
(trifluoromethyl
3- 6.82 (d, J= 9.8, 2H),
)phenoxy]acetic
yl]acetamide 7.59 (t, J= 8.2, 1H).
acid
(Intermediate 8)
tert-butyl
(2R,5S)-5-
2-(4-chloro-
amino-2-[5- ill NMR (400 MHz,
3-
(3,3,3-
chloroform-d) 6 1.76 (dt,
fluoropheno
trifluoropropoxy J=
13.9, 7.0, 1H), 1.96 ¨
xy)-2,2-
M/Z:
2.21 (m, 4H), 2.66 ¨2.81
ry ---N difluoro-N-
y io] px iapdei raizdoi n1 -e2--1 - 5[1\403+,145r, (r2.,13, H3).1,
,31.3H1 ) ,( dd 4.02, ./ : H ,__.0
[(3S,6R)-6-
0
(yN: 0 \___)(
24 F 0 0)(,,,,,
N ' F F [5-(3,3,3-
carboxylate ESI', RI 4.14 (m, 2H), 4.73 (t, J=
F F H F trifluoroprop
ci
oxy)-1,3,4-
oxadiazol-2-
(Intermediate = 2.33
6.1, 2H), 6.89 (d, J= 7.3,
47) and 2-(4- (S4) 1H), 7.05 (d, J=
8.9,
chloro-3- 1H), 7.12 (dd, J=
9.3,
yl]piperidin-
3-
fluorophenoxy)- 2.6,
1H), 7.37 ¨7.48 (m,
2,2- 1H).
yl]acetamide
difluoroacetic
acid
tert-butyl 'H NMR (400 MHz,
(2R,5S)-5-
2-[3-chloro- chloroform-d) 6 1.60 ¨
amino-2-[5-
4- 1.67 (m, 2H), 1.90 ¨2.01
(3,3,3-
(trifluoromet (m, 1H), 2.04 ¨2.16 (m,
trifluoropropoxy
hyl)phenoxy 2H), 2.62 ¨2.80 (m, 3H),
M/Z:
H NII-N ]-N-
oxadiazol-2- 517, 519 3.32 (dd, J= 12.0, 3.4,
0,01...., 0 \____ [(3S,6R)-6- 1H), 4.03 (dd, J=
7.6,
JL . , , F
yl]piperidine-1- [M+H]'
25 c' 0 r )( F F [5-(3,3,3-
carboxylate ESI RI + ' 3.5, 1H), 4.09 (ddq, J=
,
F trifluoroprop
11.4, 7.8, 3.5, 1H), 4.55
F (Intermediate = 2.3
F oxy)-1,3,4- (s,
2H), 4.72 (t, J= 6.2,
47) and 2-[3- (S4)
oxadiazol-2- chloro-4-
2H), 6.75 (d, J= 7.9,
yl]piperidin- 1H), 6.92 (dd, J= 8.7,
(trifluoromethyl
3- 2.2, 1H), 7.13 (d, J= 2.4,
)phenoxy]acetic
yl]acetamide 1H), 7.68 (d, J= 8.8,
acid
1H).
(Intermediate 7)
120
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
tert-butyl 'H NMR (400 MHz,
(2R,5S)-5-
chloroform-d) 6 1.58 (dd,
2-(3,4,5- amino-2-[5-
J= 16.6, 8.2, 2H), 1.80 -
trichlorophe (3,3,3-
1.92 (m, 2H), 1.93 -2.06
noxy)-N- trifluoropropoxy M/Z:
(m, 2H), 2.56 (dd, J-
N-N [(3S,6R)-6- )-1,3,4- 517, 519,
11.9, 7.3, 1H), 2.59-
26
[5-(3,3,3- oxadiazol-2- 521, 523
2.70 (m 2H) 3.22 (dd, J
c' Ai c)..)LN0 ' F"A' ' trifluoroprop
yl]piperidine-1- [M+H]', "
H F = 12.0, 3.4, 1H), 3.93
oxy)-1,3,4- carboxylate ESI', RI ci '1111"
(dd, J = 7.5, 3.6, 1H),
ci oxadiazol-2- (Intermediate = 2.39
4.00 (ddt, J = 11.9, 8.1,
yl]piperidin- 47) and 2- (S4)
4.0, 1H), 4.38 (s, 2H),
3- (3,4,5-
4.63 (t, J= 6.2, 2H), 6.63
yl]acetamide trichlorophenox
(d, J= 8.0, 1H), 6.95 (s,
y)acetic acid
2H).
(Intermediate 9)
'H NMR (400 MHz,
tert-butyl chloroform-d) 6 1.57 -2-(4-
(2R,55)-5- 1.63 (m, 2H), 1.88 - 1.99
bromopheno amino-2-[5- (m, 1H), 2.04 -2.15
(m,
xy)-N- (3,3,3- 2H), 2.64 (dd, J= 12.0,
M/Z:
[(3S,6R)-6- trifluoropropoxy 7.8, 1H),
2.73 (qt, J=
H N -NA 493, 495
1 7-0
µ____\ [543,3,3- )-1,3,4-
[M+H]', 10.2, 6.2, 2H), 3.32
(dd,
27 µ A-F trifluoroprop oxadiazol-2- ESI J=
12.0, 3.5, 1H), 4.00
0ke )
'
H F F oxy)-1,3,4- yl]piperidine-1- , RI
(dd, J= 7.9, 3.3, 1H),
Br 140 = 1.98
oxadiazol-2- carboxylate (S4) 4.07 (ddq, J=
11.6, 8.1,
yl]piperidin- (Intermediate 3.4, 1H), 4.49 (s,
2H),
3- 47) and 2-(4-
4.72 (t, J= 6.2, 2H), 6.77
yl]acetamide bromophenoxy) (d, J= 8.0, 1H),
6.80 -
acetic acid
6.90 (m, 2H), 7.40 -7.50
(m, 2H).
'H NMR (400 MHz,
tert-butyl chloroform-d) 6 1.60 -2-[3-
(2R,55)-5- 1.67 (m, 2H), 1.89 -2.00
(trifluoromet amino-2-[5- (m, 1H), 2.03 -2.15
(m,
hyl)phenoxy (3,3,3- 2H), 2.65 (dd, J=
12.0,
]-N- trifluoropropoxy 7.5, 1H), 2.69 - 2.80 (m,
M/Z: 483
NN [(3S,6R)-6- )-1,3,4- [M+H], 2H), 3.32 (dd,
J = 12.0,
'
F 0 (i'f0 \ F ESI, RI
[5-(3,3,3- oxadiazol-2- 3.4, 1H), 4.01 (dd,
J =
28 F '
F 0 0 ,A N õ. .s
H F trifluoroprop yl]piperidine-1-
F
oxy)-1,3,4- carboxylate = 2.11
7.7, 3.4, 1H), 4.09 (dq, J
= 8.1, 4.6, 4.1, 1H), 4.56
(S4)
oxadiazol-2- (Intermediate (s, 2H), 4.72 (t, J=
6.2,
yl]piperidin- 47) and 2-[3- 2H), 6.80 (d, J=
8.1,
3- (trifluoromethyl 1H), 7.14
(dd, J= 8.3,
yl]acetamide )phenoxy]acetic 2.5, 1H), 7.23 (s,
1H),
acid 7.33 (d, J= 7.7,
1H),
7.48 (t, J= 8.0, 1H).
tert-butyl 'H NMR (400 MHz,
2-(4-chloro- (2R,5S)-5-
DMSO-d6) 6 7.99 (d, J=
3- amino-2-{5-[2- 8.1 Hz, 1H),
7.67 (d, J=
cyanopheno (trifluoromethox 9.0 Hz, 1H), 7.59
(d, J =
xy)-N- y)ethoxy]-1,3,4- 3.0 Hz, 1H), 7.34 (dd, J
M/Z:
[(3S,6R)-6- oxadiazol-2- = 9.0, 3.1 Hz, 1H),
4.73
490, 492
F 1542- yllpiperidine-1- [M+H], -
4.65 (m, 2H), 4.59 (s,
N
29 1- )0/¨f F F (trifluoromet carboxylate ESI
2H), 4.51 - 4.43 (m, 2H),
', RI
N 0 j & hoxy)ethoxy (Intermediate 3.77 (ddd, J= 9.1,
5.9,
= 2.04
ci 40H ]-1,3,4- 48) and 2-(4-
(S4) 2.8 Hz, 1H), 3.74 -
3.63
oxadiazol-2- chloro-3- (m, 1H), 3.04 -2.93
(m,
yllpiperidin- cyanophenoxy)a
1H), 2.86 -2.75 (m, 1H),
3- cetic acid
2.46 -2.37 (m, 1H), 2.01
yl]acetamide (Intermediate - 1.83 (m, 2H), 1.72
-
16)
1.58 (m, 1H), 1.56 - 1.42
121
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
(m, 1H).
Scheme for route 36
Boc poc
,
0 0
HN¨NH 1111 CI
H2N,
T3P, DIPEA F s
F =ONõ.. )(1\1µ 0
OH
DMF, r.t.
CI CI
CI Step a
Intermediate 34
TsCI, K2CO3
Step b
ACN, 80 C
,Boc
0
F ,,cs)
0 TFA
F
r t 0 0.44.e,N
0j= s) I
N"
0
40t
CI 401 CI
Example 31 CI Step c Example
30 Cl
Step 36.a: tert-butyl (2R,4S)-4-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-{[(4-
chlorophenyl)formohydrazido]carbonyllpyrrolidine-1-carboxylate
0
Boc
0 HN¨NH Cl
a
To a solution of
(2R,4S)-1-[(tert-butoxy)carbonyl] -4- [2-(4-chloro -3 -
fluorophenoxy)acetamido]pyrrolidine-2-carboxylic acid (80% purity, 200 mg,
0.384 mmol,
Intermediate 34), 4-chlorobenzohydrazide (65 mg, 0.384 mmol) and DIPEA (0.080
mL, 0.461
mmol) in DMF (1 mL) was added T3P (50% in Et0Ac, 0.25 mL, 0.422 mmol) and the
mixture was stirred at r.t. for 1 h. The reaction mixture was diluted with H20
(20 mL) and
extracted with Et0Ac (2 x 20 mL). The combined organic extracts were washed
with brine,
dried over MgSO4, and concentrated in vacuo. The residue was purified by
chromatography
on silica gel (0-100% Et0Ac in heptane) to afford the title compound (89%
purity, 158 mg,
0.247 mmol, 64% yield) as a pale yellow gum; 1H NMR (400 MHz, DMSO-d6) 6 10.52
(d, J
= 48.5 Hz, 1H), 10.05 (d, J= 9.9 Hz, 1H), 8.34 (t, J= 6.4 Hz, 1H), 7.98 ¨ 7.85
(m, 2H), 7.58
122
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
(dd, J= 8.5, 4.7 Hz, 2H), 7.50 (t, J= 8.9 Hz, 1H), 7.08 (d, J= 11.5 Hz, 1H),
6.91 ¨6.80 (m,
1H), 4.55 (s, 2H), 4.52 ¨4.39 (m, 1H), 4.34 (dq, J= 12.6, 4.4 Hz, 1H), 3.73
¨3.59 (m, 1H),
3.25 ¨ 3.11 (m, 1H), 2.31 ¨ 2.11 (m, 2H), 1.40 (s, 9H); M/Z: 469, 471, 473 [M-
Boc+H],
EST, RT = 0.95 (S2).
Example 30 (step 36.b): tert-butyl (2R,4S)-442-(4-chloro-3-
fluorophenoxy)acetamido]-2-
[5-(4-chloropheny1)-1,3,4-oxadiazol-2-yflpyrrolidine-l-carboxylate
Boc
0 r---
/
0
CI
Example 30 CI
A suspension of tert-butyl (2R,45)-4-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-
{[(4-
chlorophenyl)formohydrazido] carbonyl} pyrrolidine-l-carboxylate (89% purity,
158 mg,
0.247 mmol), K2CO3 (205 mg, 1.48 mmol) and TsC1 (0.012 mL, 0.741 mmol) in ACN
(2 mL)
was stirred at 80 C for 2.5 h. The reaction mixture was partitioned between
Et0Ac (30 mL)
and H20 (20 mL). The organic layer was isolated, washed with brine, dried over
MgSO4, and
concentrated in vacuo. The residue was purified by chromatography on silica
gel (0-100%
Et0Ac in heptane) to afford the title compound (119 mg, 0.208 mmol, 84% yield)
as a pale
yellow gum; 1H NMR (400 MHz, DMSO-d6) 6 8.49 (s, 1H), 8.01 (d, J= 8.5 Hz, 2H),
7.70 (d,
J= 8.6 Hz, 2H), 7.50 (t, J= 8.9 Hz, 1H), 7.08 (dd, J= 11.4, 2.8 Hz, 1H), 6.87
(dd, J= 9.0, 1.7
Hz, 1H), 5.30 ¨ 5.18 (m, 1H), 4.66 ¨4.47 (m, 3H), 3.82 ¨ 3.69 (m, 1H), 3.44 ¨
3.40 (m, 1H),
2.44 ¨ 2.35 (m, 2H), 1.42 ¨ 1.18 (m, 9H); M/Z: 595, 597 [M+MeCN+H]+, EST, RT =
1.11
(S2).
Example 31 (step 36.c): 2-(4-chloro-3-fluorophenoxy)-N-[(3S,5R)-5-[5-(4-
chloropheny1)-
1,3,4-oxadiazol-2-yflpyrrolidin-3-yl] acetamide
NN
Ojwõ,
CI
Example 31
A solution of tert-butyl (2R,4S)-4-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-
[5-(4-
chloropheny1)-1,3,4-oxadiazol-2-yl]pyrrolidine-1-carboxylate (119 mg, 0.207
mmol, Example
30) and TFA (0.15 mL, 2.07 mmol) in DCM (2.5 mL) was stirred at r.t. for 4 h.
The reaction
123
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
mixture was partitioned between DCM and satd aq NaHCO3 solution, and the
organic layer
was isolated using a phase separator and concentrated in vacuo. The residue
was purified by
prep. HPLC (Method 3) and then triturated using Et20 to afford the title
compound (44 mg,
0.0965 mmol, 47% yield) as a white solid; Ifl NMR (500 MHz, DMSO-d6) 6 8.26
(d, J= 7.2
Hz, 1H), 8.04 - 7.98 (m, 2H), 7.72 - 7.65 (m, 2H), 7.51 (t, J= 8.9 Hz, 1H),
7.10 (dd, J= 11.4,
2.8 Hz, 1H), 6.90 - 6.85 (m, 1H), 4.65 (s, 1H), 4.55 (s, 2H), 4.46 - 4.37 (m,
1H), 3.16 - 3.07
(m, 2H), 2.87 - 2.79 (m, 1H), 2.44 - 2.38 (m, 1H), 2.19 - 2.10 (m, 1H); M/Z:
451, 453, 455
[M+H]+, EST, RT = 2.26 (S4).
Scheme for route 37
poc 0 Boc 0
0 NOH H HATU, DIPEA 0 (R)
F
0 DMF, r.t. __ F ash , .= H 0
CI WI Step a CI
Intermediate 22 Intermediate 38
TsCI, K2CO3
Step b
ACN, 80 C
N-N Boc N-N
0 ( 0 C \ Th DCM 0 R 0 \
F OANõ.=- TFA F
F F , r.t. F F
I 411111" 0 IW
Example 33 Step c ClC Example 32
Step 37.a: tert-butyl (2R,5S)-5-[[2-(4-chloro-3-fluoro-phenoxy)acetyl]amino]-2-
1(3,3,3-
trifluoropropoxycarbonylamino)carbamoyl] pip eridine- 1- carb oxylate
Boc 0
Y
H 0
CI
To
a solution of (2R, 5 5)-1- [(tert-butoxy)carbonyl] -5- [2-(4-chloro-3 -
fluorophenoxy)
acetamido]piperidine-2-carboxylic acid (500 mg, 1.10 mmol, Intermediate 22) in
anhydrous
DMF (6 mL) was added DIPEA (0.40 mL, 2.29 mmol) and HATU (503 mg, 1.32 mmol)
and
stirred at r.t. for 10 min. (3,3,3-trifluoropropoxy)carbohydrazide (273 mg,
1.43 mmol,
Intermediate 38) was added and the mixture was stirred at r.t. for 12 h. The
reaction mixture
was diluted with Et0Ac (20 mL) and washed with brine (20 mL). The combined
organic
extracts were dried over MgSO4, concentrated in vacuo, and purified by
chromatography on
124
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
silica gel (0-80% Et0Ac in heptane) to afford the title compound (91% purity,
646 mg, 1.00
mmol, 91% yield) as a colourless oil; M/Z: 485, 487 [M-Boc+H]+, EST, RT = 0.96
(S2).
Example 32 (step 37.b): tert-butyl (2R,5S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]-2-
[5-(3,3,3-trifluoropropoxy)-1,3,4-oxadiazol-2-yl] pip eridine- 1-carb oxylate
Bec (R) V--:1
''''µ'õ0
F di
\-----F
F F
CI
Example 32
A suspension of tert-butyl (2R,5S)-5-[[2-(4-chloro-3-fluoro-
phenoxy)acetyl]amino]-2-[(3,3,3-
trifluoropropoxycarbonylamino)carbamoyl]piperidine-1-carboxylate (91% purity,
646 mg, 1.0
mmol), K2CO3 (833 mg, 6.03 mmol) and TsC1 (576 mg, 3.02 mmol) in ACN (5 mL)
was
stirred at 80 C for 3 h. The reaction mixture was diluted with Et0Ac (20 mL)
and washed
with brine (20 mL). The organic layer was dried over MgSO4, concentrated in
vacuo, and
purified by chromatography on silica gel (0-100% Et0Ac in heptane) to afford
the title
compound (252 mg, 0.440 mmol, 44% yield) as a white powder; 11-1 NMR (400 MHz,
DMSO-d6) 6 8.08 (d, J= 7.0 Hz, 1H), 7.48 (t, J= 8.9 Hz, 1H), 7.04 (dd, J=
11.4, 2.9 Hz,
1H), 6.82 (ddd, J= 9.0, 2.8, 1.1 Hz, 1H), 5.38 (s, 1H), 4.65 (t, J= 5.7 Hz,
2H), 4.62 -4.52
(m, 2H), 3.96 - 3.85 (m, 2H), 2.99 - 2.85 (m, 3H), 2.25 - 2.11 (m, 1H), 2.03 -
1.94 (m, 1H),
1.86 - 1.73 (m, 1H), 1.63 (d, J = 13.5 Hz, 1H), 1.37 (s, 9H); M/Z: 467, 469 [M-
Boc+H]+,
EST, RT = 1.07 (S2).
Example 33 (step 37.c): 2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3,3,3-
trifluoropropoxy)-1,3,4-oxadiazol-2-yl] piperidin-3-yl] acetamide
o
F CIiiii
/\---F
F F
Example 33
A solution of tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-
[5-(3,3,3-
trifluoropropoxy)-1,3 ,4-oxadi azol-2-yl] piperidine-1 -carboxyl ate (107 mg,
0.185 mmol,
Example 32) and TFA (0.14 mL, 1.85 mmol) in DCM (2 mL) was stirred at r.t. for
6 h. The
reaction mixture was partitioned between DCM (5 mL) and satd aq NaHCO3
solution (5 mL).
The organic layer was isolated, concentrated in vacuo, and purified by prep.
HPLC (Method
125
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
3) to afford the title compound (15 mg, 0.0298 mmol, 16% yield) as a white
solid; 114 NMR
(400 MHz, DMSO-d6) 6 7.97 (d, J= 8.0 Hz, 1H), 7.50 (t, J= 8.9 Hz, 1H), 7.07
(dd, J= 11.4,
2.8 Hz, 1H), 6.85 (ddd, J= 9.0, 2.9, 1.2 Hz, 1H), 4.74 ¨ 4.58 (m, 2H), 4.53
(s, 2H), 3.79 (dd, J
= 10.5, 2.5 Hz, 1H), 3.76 ¨ 3.62 (m, 1H), 3.06 ¨ 2.80 (m, 3H), 2.47 ¨ 2.41 (m,
1H), 2.05 ¨
1.81 (m, 2H), 1.76 ¨ 1.59 (m, 1H), 1.50 (qd, J= 12.3, 3.7 Hz, 1H); M/Z: 467,
469 [M+H]+,
EST, RT = 3.12 (S6).
Example compounds in Table 12 were synthesized according to the synthetic
steps of general
route 37 as exemplified by Example 33 using the corresponding intermediates.
Table 12
LCMS
Ex Structure Name Intermediates 111 NMR
data
111 NMR (500 MHz,
(2R,55)-1-
chloroform-d) 6 9.13 (d,
Wert-
J = 2.1 Hz, 1H), 8.23
2-(4-chloro- butoxy)carbon
(dd, J= 8.1, 2.3 Hz, 1H),
3- y1]-542-(4-
7.38 ¨7.29 (m, 2H), 6.80
fluoropheno chloro-3-
xy)-N- fluorophenoxy (dd, J= 10.3,
2.8 Hz,
N M/Z: 1H), 6.71 (ddd, J= 8.9,
[(3S,6R)-6-
446, 448
2.8, 1.2 Hz, 2H), 4.48 (s,
0 N [5-(6- acetamido]pip
[M+H]', 2H), 4.21 (dd,
J= 8.0,
34 F 011 .)LNiss'U methylpyridi eridine-2-
H ESI', RT 3.8 Hz,
1H),4.11 (ddq, J
11-3 -y1)- carboxylic
ci = 2.76
= 11.9, 8.0, 3.7 Hz, 1H),
1,3,4- acid
(S4) 3.38 (dd, J=
12.1, 3.4
oxadiazol-2- (Intermediate
Hz, 1H), 2.69 (dd, J
yl]piperidin- 22) and 6-
6.8, 5.3 Hz, 1H), 2.66 (s,
3- methyl-3-
3H), 2.25 ¨2.17 (m, 1H),
yl]acetamide pyridinecarbo
2.17 ¨ 2.09 (m, 1H), 2.09
xylic acid
¨2.00 (m, 1H), 1.72 ¨
hydrazide
1.63 (m, 2H).
1H NMR (500 MHz,
(5R)-1-[(tert-
DMSO-d6) 8.06 ¨7.94
2-(4-chloro-
3-
butoxy)carbon
(m, 3H), 7.69 (d, J= 8.6
y1]-5-[2-(4-
Hz, 2H), 7.50 (t, J= 8.9
fluoropheno
chloro-3- M/Z: Hz, 1H), 7.08
(dd, J=
xy)-N-
fluorophenoxy 465, 467,
11.4, 2.8 Hz, 1H), 6.86
H "1\1\ [(3R,6S)-6-
)acetamido]pi 469
(dd, J= 8.9, 1.9 Hz, 1H),
35 N * CI [544-
r 0 peridine-2-
[M+H]', 4.54 (s, 2H), 3.99 (dd, J
F 0 chlorophenyl
ci )-1,3,4- carboxylic
ESI', RT = 10.6, 2.8 Hz, 1H), 3.83
acid = 2.23
¨3.69 (m, 1H), 3.13 ¨
oxadiazol-2-
(Intermediate (S4)
2.83 (m, 2H), 2.14 ¨2.05
yl]piperidin-
3-
23) and 4-
(m, 1H), 2.00 ¨ 1.87 (m,
chlorobenzohy
1H), 1.86¨ 1.71 (m, 1H),
yl]acetamide
drazide 1.56 (qd, J=
12.5, 3.9
Hz, 1H).
126
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
IHNIVIR (500 MHz,
(5R)-1-[(tert- DMSO-d6) 6 8.19
(s,
2-(4-chloro-
3-
butoxy)carbon
1H), 8.06 ¨ 8.00 (m, 2H),
y1]-542-(4-
8.00 ¨7.93 (m, 1H), 7.74
fluoropheno
chloro-3- M/Z: ¨
7.63 (m, 2H), 7.47 (t, J
xy)-N-
fluorophenoxy 465, 467, =
8.9 Hz, 1H), 7.05 (dd,
, N-N [(3R,6R)-6-
fl \ )acetamido]pi 469 J= 11.4, 2.8 Hz,
1H),
N r) I 0 Ili CI [5-(4-
36 peridine-2- [M+H]-',
6.83 (dd, J = 8.9, 1.8 Hz,
F OjN ) chlorophenyl
a 40 H )-1,3,4- carboxylic
ESL', RI 1H), 4.53 (s, 2H), 4.29 ¨
acid = 2.44
4.17 (m, 1H), 3.90 ¨3.74
oxadiazol-2-
(Intermediate (S4) (m, 1H), 2.85 (dd,
J=
yl]piperidin-
23) and 4-
12.2, 3.5 Hz, 1H), 2.70 ¨
3-
chlorobenzohy
2.59 (m, 1H), 2.14 ¨2.01
yl]acetamide
drazide (m,
1H), 1.96 ¨ 1.82 (m,
1H), 1.80¨ 1.65 (m, 2H).
IHNIVIR (500 MHz,
rac2- -(4 5-[2-(4-
DMSO-d6) 6 8.43 (d, J =
-
chloro-3-
chloro-3- 8.2 Hz, 1H), 8.28 (s, 1H),
fluorophenoxy
fluoropheno 8.11 ¨8.03 (m, 2H), 7.76
)acetamido]-6-
xy)-N- M/Z: ¨7.65 (m, 2H), 7.51 (t, J
oxopiperidine-
H NI, 479, 481, =
8.9 Hz, 1H), 7.12 (dd,
oy(:),?..õ CI [5_0_ 2-carboxylic
483 J= 11.4, 2.8 Hz,
1H),
acid
37 F Oje s) chlorophenyl
[M+H]-', 6.90 (dd, J= 8.9, 1.9 Hz,
IW H )-1,3,4- (Intermediate
ESI+, RI 1H), 4.98 (dd, J= 9.7,
a 41) and 4-
and enantomer at 3Rand 6S oxadiazol-2- = 3.13 4.8
Hz, 1H), 4.65 ¨ 4.55
chlorobenzohy
y1]-2- (S4) (m, 2H), 4.43 ¨4.34 (m,
drazide
oxopiperidin 1H), 2.34 ¨2.27 (m, 1H),
following
-3- 2.21 ¨2.11 (m, 1H), 2.11
steps 37.a and
yl]acetamide 37.b ¨2.04 (m, 1H),
2.02 ¨
1.91 (m, 1H).
5-[2-(4-
chloro-3-
rac -2- (4-
fluorophenoxy
)acetamido]-6- ill NMR (500 MHz,
chloro-3-
oxopiperidine-
DMSO-d6) 6 8.40 (d, J=
fluoropheno
xy) -N-
2-carboxylic 8.2
Hz, 1H), 8.27 ¨ 8.15
acid (m,
1H), 7.54 ¨7.44 (m,
[(3R,6R)-2- M/Z:
(Intermediate
1H), 7.14 ¨ 7.05 (m, 1H),
oxyo 0
pA,F 507, 509
oxo-6-15-
41) and
[M+H]+
6.91 ¨ 6.83 (m, 1H), 4.96
[(1s,3s)-3-
(trifluorometh
38 140 H (1s,3s)-3- ' ¨ 4.88 (m, 1H),
4.88 ¨
ci (trifluoromet ESI+, RI
4.83 (m, 1H), 4.63 ¨4.51
and enanaorner at 3Rand 6R hoxy)cyclob = 3.07
oxy)cyclobuta (m,
2H), 4.41 ¨4.27 (m,
uty1]-1,3,4- (S4)
ne-1-
1H), 3.51 ¨3.39 (m, 1H),
oxadiazol-2-
carbohydrazid
2.90 ¨ 2.80 (m, 2H), 2.57
yl1piperidin-
e ¨2.52 (m, 1H),
2.33 ¨
3-
(Intermediate 1.80 (m, 5H).
yl]acetamide
55) following
steps 37.a and
37.b
tert-butyl (2R,5S)-1- IHNIVIR (500 MHz,
(2R,5S)-5- Wert-
chloroform-d) 6 1.51 (s,
[2-(4-chloro- butoxy)carbon
9H), 1.91 ¨2.16 (m, 3H),
M/Z:
3- y1]-542-(4-
2.25 ¨2.37 (m, 1H), 3.18
Boc N¨N , 465 467,
fluoropheno chloro-3- ¨3.37 (m, 1H), 4.02¨
ri ,I0\
o xy)acetamid fluorophenoxy
4.19 (m, 1H), 4.19 ¨4.29
39 F 0 0,)(Nõ. s) IP a 469 [M-
o]-2-[5-(4- ) B0C+H]
(m" 1H) 4.45 ¨4.57 (m'
H ', ESI-',
a chlorophenyl acetamido]pip 2H), 5.74 (s, 1H),
6.71
RI =
eridine-2- (ddd, J = 8.9, 2.8, 1.1 Hz,
1.39 (Si)
oxadiazol-2- carboxylic
1H), 6.75 ¨ 6.99 (m, 2H),
yl]piperidine acid
7.37 (t, J= 8.6 Hz, 1H),
-1- (Intermediate
7.51 (d, J= 8.6 Hz, 2H),
127
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
carboxylate 22) and 4- 7.93 ¨ 8.04
(m, 2H).
chlorobenzohy
drazide
following
steps 37.a and
37.b
1H NMR (500 MHz,
From tert-
DMSO-d6) 6 = 1.77 (qd,
butyl (2R,5S)- J= 13.2, 12.8,
3.9 Hz,
2-(4-chloro-
3-
5-[2-(4-
1H), 1.95 ¨2.10 (m, 2H),
chloro-3-
2.31 (s, 3H), 2.42 (dd, J
fluoropheno
xy) N fluorophenoxy M/Z:
= 10.9, 3.5 Hz, 1H), 3.01
-
)acetamido]-2- 465, 467, (t, J= 11.8 Hz, 1H), 3.42
H "N\ Ilp [(3S,6 R)-6-
R)-6-
N (,) I 0 CI [5-(4- 469 (dd, J= 12.1,
3.6 Hz,
0
40 F s 0,ANõ, s)
chloropheny1)- [M+H]-', 1H), 4.04 ¨4.19 (m, 1H),
chlorophenyl
H )-1,3,4-
1,3,4- ESI-', RT
4.58 (s, 2H), 4.82 ¨ 4.94
a
oxadiazol-2- = 2.25
(m, 1H), 6.81 ¨6.93 (m,
oxadiazol-2-
yl]piperidine- (S4)
1H), 7.09 (dd, J= 11.3,
yl]piperidin-
3-
1-carboxylate
2.8 Hz, 1H), 7.51 (t, J¨
(Example 39)
8.9 Hz, 1H), 7.69 ¨7.79
yl]acetamide
following step
(m, 2H), 8.01 ¨8.10 (m,
37.c
2H), 8.35 (d, J= 7.8 Hz,
1H), 9.72 (s, 2H).
Scheme for route 38
Boc 0 roc 0 H
)I õLi, ,, , N
, N 0 F
0 - -,;) OH H HATU, DIPEA 0
_ Jt ,,,, ir\-11 Y -rF
+ H2N.N.ti.0Th<F
F Ai ..õõõ- - F a 0 _N 0
0 F DMF, r.t.
H F H
CI 4111111111 CI "111
Step a
Intermediate 25 Intermediate 38
TsCI, K2CO3
Step b ACN, 80 C
H r-N\ roc N¨N\
0,0's) µ 0 V...)µ...._
F & Ojt.,,N0.-<õ,- ZnBr2 F Ai 0.,õ) R)
N
F -4 _____________________________ F
H H
F F F F
CI 111111" DCM, r.t. CI WI'
Example 42 Step c Example 41
Step 38.a: tert-butyl (2S,5R)-5- [2-(4-chloro-3-fluorophenoxy)acetamido]-2-IN'-
[(3,3,3-
trifluoropropoxy)carbonyl]hydrazinecarbonyllpiperidine-l-carboxylate
Boc 0
H
0
F
F =CI 111111111 iii OJI,N,ef- H 0 F
H
To a solution of
(2S,5R)-1-Rtert-butoxy)carbony11-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]piperidine-2-carboxylic acid (450 mg, 1.04 mmol,
Intermediate 25)
128
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
in anhydrous DMF (5 mL) was added HATU (477 mg, 1.25 mmol) and DIPEA (0.36 mL,
2.09 mmol) and the mixture was stirred at r.t. for 10 min. (3,3,3-
trifluoropropoxy)carbohydrazide (90% purity, 260 mg, 1.36 mmol, Intermediate
38) was then
added and the mixture was stirred at r.t. for 18 h. The reaction mixture was
partitioned
.. between Et0Ac (25 mL) and 1 M aq HC1 solution (25 mL). The organic layer
was isolated,
washed with satd aq NaHCO3 (25 mL) and brine (2 x 25 mL), dried over MgSO4 and
concentrated in vacuo. The residue was purified by chromatography on silica
gel (20-100%
Et0Ac in heptane) to afford the title compound (85% purity, 268 mg, 0.389
mmol, 37%
yield) as a clear oil; 1H NMR (400 MHz, DMSO-d6) 6 9.78 (s, 1H), 9.22 (s, 1H),
7.98 (d, J=
7.1 Hz, 1H), 7.48 (m 1H), 7.05 (dd, J= 11.4, 2.8 Hz, 1H), 6.82 (dd, J= 8.9,
1.8 Hz, 1H), 4.74
¨4.44 (m, 3H), 4.22 (m, 2H), 3.87 (m, 2H), 2.63 (m, 2H), 2.02 (m, 2H), 1.85
(m, 1H), 1.58 (s,
2H), 1.37 (s, 9H); M/Z: 485, 487 [M-Boc+H]+, EST, RT = 0.96 (S2).
Example 41 (step 38.b): tert-butyl (2S,5R)-5-12-(4-chloro-3-
fluorophenoxy)acetamidol-2-
[5-(3,3,3-trifluoropropoxy)-1,3,4-oxadiazol-2-yl] pip eridine- 1-carb oxylate
Boc N¨N
11\1 =
0
=
F Ojcre<
F F
CI
Example 41
A suspension of tert-butyl (2S,5R)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-
{N -[(3 ,3 ,3 -
trifluoropropoxy)carbonyl]hydrazinecarbonyllpiperidine-l-carboxylate (85%
purity, 265 mg,
0.385 mmol), K2CO3 (319 mg, 2.31 mmol) and TsC1 (220 mg, 1.16 mmol) in ACN
(2.5 mL)
was stirred at 80 C for 24 h. The reaction mixture was diluted with H20 (10
mL) and
extracted with Et0Ac (3 x 10 mL). The combined organic extracts were washed
with brine (3
x 10 mL), dried over MgSO4, and concentrated in vacuo. The resultant residue
was purified
by chromatography on silica gel (0-100% Et0Ac in heptane) to afford the title
compound
(88% purity, 68 mg, 0.106 mmol, 27% yield) as a brown oil; 1H NMR (500 MHz,
DMSO-d6)
6 8.08 (d, J= 7.0 Hz, 1H), 7.48 (t, J= 8.9 Hz, 1H), 7.04 (dd, J= 11.4, 2.8 Hz,
1H), 6.85 ¨
6.78 (m, 1H), 5.38 (s, 1H), 4.65 (t, J= 5.7 Hz, 2H), 4.62 ¨4.51 (m, 2H), 3.96
¨ 3.84 (m, 2H),
3.02 ¨2.85 (m, 2H), 2.24 ¨2.13 (m, 1H), 2.04 ¨ 1.94 (m, 2H), 1.84 ¨ 1.74 (m,
1H), 1.68 ¨
1.57 (m, 1H), 1.38 (s, 9H); M/Z: 467, 469 [M-Boc+H], EST, RT = 1.31 (Si).
129
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Example 42 (step 38.c): 2-(4-chloro-3-fluorophenoxy)-N-R3R,6S)-6-[5-(3,3,3-
trifluoropropoxy)-1,3,4-oxadiazol-2-yl]piperidin-3-yflacetamide
11¨o
o =;.;) 0
F
F F
CI 41111111-111
Example 42
To a solution of tert-butyl (2S,5R)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-
2-[5-(3,3,3-
trifluoropropoxy)-1,3 ,4-oxadi azo 1-2-yl] piperidine-1 -carboxyl ate (88%
purity, 55 mg, 0.0854
mmol) in DCM (1 mL) was added ZnBr2 (77 mg, 0.341 mmol) and the mixture was
stirred
under N2 at r.t. for 18 h. The reaction mixture was diluted with satd aq
NaHCO3 solution (3
mL) and extracted with DCM:IPA (80:20) (3 x 3 mL). The combined organic
extracts were
washed with brine (5 mL), dried over MgSO4, and concentrated in vacuo. The
resultant
residue was purified by prep. HPLC (Method 3) to afford the title compound (12
mg, 0.0249
mmol, 29% yield) as a white powder; 1H NMR (500 MHz, DMSO-d6) 6 7.96 (d, J=
7.8 Hz,
1H), 7.49 (t, J= 8.8 Hz, 1H), 7.07 (dd, J= 11.4, 2.5 Hz, 1H), 6.87 ¨ 6.81 (m,
1H), 4.64 (t, J=
5.5 Hz, 2H), 4.52 (s, 2H), 3.80 ¨ 3.63 (m, 2H), 3.02 ¨ 2.83 (m, 3H), 2.83 ¨
2.75 (m, 1H), 2.46
¨2.39 (m, 1H), 2.00 ¨ 1.83 (m, 2H), 1.70¨ 1.57 (m, 1H), 1.54 ¨ 1.43 (m, 1H);
M/Z: 467, 469,
[M+H]+, ESI+, RT = 3.12 (S6).
The example compound in Table 13 was synthesized according to the synthetic
steps of
general route 38 as exemplified by Example 42 using the corresponding
intermediates.
Table 13
LCMS
Ex Structure Name Intermediates 1H NMR
data
2-(4- (2S,5R)-1-[(tert- 1H NMR (500 MHz,
chloro-3- butoxy)carbonyl
DMSO-d6) 6 7.96 (d, J=
fluorophen ]-5-[2-(4-chloro-
8.1 Hz, 1H), 7.49 (t, J=
oxy)-N- 3- 8.9 Hz, 1H), 7.07 (dd, J
[(3R,6S)-6- fluorophenoxy)a M/Z:
= 11.4, 2.8 Hz, 1H), 6.85
F
N-N 494 5-[3- cetamido]piperi
(ddd, J= 9.0, 2.8, 1.1 Hz,
, 496
FNI (tnfluorom dine-2-
[M+H] 1H), 5.33 (tt, J= 7.0, 4.1
43
F 0 ji,N ethoxy)aze carboxylic acid
ESI', RT Hz, 1H), 4.51
(s, 2H),
H tidin-1-y1]- (Intermediate
= 3.12
4.50 ¨ 4.42 (m, 2H), 4.24
CI 1,3,4- 25) and 3-
¨4.15 (m, 2H), ( ) 3.76¨
S6
oxadiazol- (trifluoromethox
3.63 (m, 2H), 3.02 ¨ 2.92
2- y)azetidine-1-
(m, 1H), 2.74 ¨2.65 (m,
yl piperidi carbohydrazide
1H), 2.45 ¨2.38 (m, 1H),
11-3- (Intermediate
1.98 ¨ 1.84 (m, 2H), 1.69
yl]acetami 37)
¨ 1.57 (m, 1H), 1.53 ¨
130
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
de 1.42 (m,
1H).
Scheme for route 39
ro
4 \ dik
0 wit. CSA, STAB NI
.õ4
0 = j6s) 0 Wer CI
F 0j.LN-< DOE, r.t. __ ' F OJLN
CI CI
Example 35 Example 44
Example 44: 2-(4-chloro-3-fluorophenoxy)-N- [(3R, 6S)-6- [5-(4-
chloropheny1)-1,3,4-
.. oxadiazol-2-y1]-1-methylpiperidin-3-yl] acetamide
A solution of CSA (200 !IL, 0.0172 mmol), 2-(4-chloro-3-fluorophenoxy)-N-R3R,
6S)-6- [544-
chloropheny1)-1,3,4-oxadiazol-2-yl]piperidin-3-yllacetamide (40 mg, 0.0860
mmol, Example
35) and 1,3,5-trioxane (25 1,1L, 0.215 mmol) in DCE (0.5 mL) was stirred at
r.t. under N2 for
45 min. STAB (55 mg, 0.258 mmol) was added and the reaction mixture was
stirred at r.t. for
3 days. The reaction mixture was diluted with DCM (5 mL) and satd aq NaHCO3
solution (5
mL). The organic layer was isolated, concentrated in vacuo, and purified by
prep. HPLC
(Method 2) to afford the title compound (9.8 mg, 0.0194 mmol, 23% yield) as a
white solid;
1H NMR (500 MHz, DMSO-d6) 6 8.10 (d, J= 8.0 Hz, 1H), 8.06 -7.98 (m, 2H), 7.73 -
7.64
(m, 2H), 7.50 (t, J= 8.9 Hz, 1H), 7.08 (dd, J= 11.4, 2.8 Hz, 1H), 6.86 (dd, J=
8.9, 2.0 Hz,
.. 1H), 4.55 (s, 2H), 4.00 - 3.86 (m, 1H), 3.52 (dd, J= 9.9, 3.0 Hz, 1H), 2.97
(dd, J= 11.0, 3.7
Hz, 1H), 2.14 - 2.04 (m, 4H), 2.02 - 1.81 (m, 3H), 1.57 - 1.39 (m, 1H); M/Z:
479, 481, 482
[M+H]+, EST, RT = 3.00 (S4).
Example compounds in Table 14 were synthesized according to the general route
39 as
exemplified by Example 44 using the corresponding intermediates.
131
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Table 14
Ex Structure Name Intermediates LCMS 111 NMR
data
1H NMR (500 MHz,
2-(4-chloro-
DMSO-d6) 6 1.43 ¨ 1.54
3- (m,
1H), 1.83 ¨ 1.94 (m,
2-(4-chloro-3-
fluoropheno
2H), 1.94 ¨ 2.03 (m, 1H),
xy)-N- fluorophenoxy M/Z:
2.07 ¨ 2.12 (m, 4H), 2.98
)-N-R3S,6R)-
R3S,6R)-6- 479, 481, (dd, J= 11.1,
3.7 Hz,
N.-N 6-[5-(4-
r4c,\ 4 a [544- chlo 483 1H), 3.53 (dd, J=
9.8,
45 o ropheny1)-
chlorophenyl [M+H]', 3.0
Hz, 1H), 3.86 ¨4.00
F 0,)(,
a 101 H )-1,3,4- oxadiazol-2- ESI', RT (m,
1H), 4.56 (s, 2H),
oxadiazol-2- = 2.99
6.83 ¨ 6.91 (m, 1H), 7.09
yl]piperidin-3-
y1]-1- (S4) (dd, J = 11.4, 2.8
Hz,
methylpiperi yl]acetamide
1H), 7.51 (t, J= 8.9 Hz,
(Example 40)
din-3-
1H), 7.64 ¨ 7.73 (m, 2H),
yl]acetamide
8.00 ¨ 8.08 (m, 2H), 8.11
(d, J = 8.0 Hz, 1H).
1H NMR (500 MHz,
DMSO-d6) 6 0.93 (t, J=
7.1 Hz,3H), 1.51 (dt, J=
2-(4-chloro-
3-
15.6, 7.7 Hz, 1H), 1.90
2-(4-chloro-3-
(td, J= 9.4, 8.9, 3.1 Hz,
fluorophenoxy
2H), 2.00 (dt, J= 7.6, 4.0
fluoropheno
)-N-R3S,6R)- Hz, 1H), 2.20 (dd,
J=
xy)-N- M/Z:
6-[5-(4-
11.1, 8.9 Hz, 1H), 2.29
[(3S,6R)-6- 493, 495,
CS ioN\ 111 C I [5-0- chloropheny1)-
1,3,4- 497 (dq, J= 13.9, 7.0
Hz,
1H), 2.45 (dt, J= 14.3,
46 chlorophenyl [M+H]',
F
CI ,, 0 0 c,õ )
H )-1,3,4- oxadiazol-2-
EST+, RI 7.1
Hz, 1H), 2.99 (dd, J
oxadiazol-2- yl]piperidin-3- _ 3.13 =
11.2, 3.5 Hz, 1H),3.87
yl]acetamide (S4)
(dd, J= 8.7, 3.4 Hz, 1H),
(Example 40)
3.90 ¨ 4.00 (m, 1H),4.57
ethylpiperidi
and (s, 2H), 6.87
(ddd, J=
n-3-
acetaldehyde 9.0, 2.8, 1.0 Hz,
1H),
yl]acetamide
7.10 (dd, J= 11.4, 2.8
Hz, 1H), 7.52 (t, J= 8.9
Hz, 1H), 7.63 ¨ 7.72 (m,
2H), 7.97 ¨ 8.07 (m, 3H).
132
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 40
H
o H2N-NoW
) o
H =1\ TsCI, DIPEA
0 ____________________
,N, sik HATU, DIPEA H N N =1\1(13
r _____________________________________________________ m.
CI
BocN..,</ DMF, r.t. ACN, r.t.
BocNimm,"</ 0 Boci\fm/</
Step a H Step b
Intermediate 43
TFA
Step c
DCM, r.t.
0
F 0
) NN CI WI N¨N\I
N = Intermediate 19 .,)
1\1.?,I=c
0 -?.;'s)s 0 CI DIPEA CI
F ojk ==="cl ) H2N
DCM, r.t. F3C,OH
CI Step d 0
Example 47
Step 40.a: tert-butyl N-[(3R,6S)-6-{ [(4-chlorophenyl)formohydrazido]
carbony11-1-
ethylpiperidin-3-yl]carbamate
o
H H CI
Boc 1\1 0
To a solution of (2S, 5R)-5- [(tert-butoxy)carbonyl]amino } -1-ethylpiperidine-
2-carboxylic
acid (50 mg, 0.183 mmol, Intermediate 43) in DMF (5 mL) at 0 C was added 4-
chlorobenzohydrazide (41 mg, 0.238 mmol), followed by HATU (83 mg, 0.220 mmol)
and
DIPEA (0.064 mL, 0.366 mmol), and the mixture was stirred r.t. for 3 h. The
reaction mixture
was diluted with H20 (30 mL) and extracted with Et0Ac (2 x 20 mL). The
combined organic
extracts were washed with brine (30 mL), dried over Na2SO4, and concentrated
in vacuo. The
residue was purified by chromatography on silica gel (10-100% Et0Ac in
heptane) to afford
the title compound (75% purity, 100 mg, 0.177 mmol, 96% yield); M/Z: 425, 427
[M+H]+,
EST, RT = 0.87 (Si).
133
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Step 40.b: tert-butyl N-1(3R,6S)-6-[5-(4-chloropheny1)-
1,3,4-oxadiazol-2-y1]-1-
ethylpiperidin-3-yl]carbamate
) N-N
N k '
0 a
Boc.N
H
To a solution of tert-butyl N-R3R,6S)-6- { [(4-
chlorophenyl)formohydrazido]carbonyll -1-
ethylpiperidin-3-yl]carbamate (75% purity, 100 mg, 0.177 mmol) in anhydrous
ACN (10 mL)
was added TsC1 (101 mg, 0.530 mmol) followed by DIPEA (0.092 mL, 0.530 mmol)
and the
mixture was stirred at r.t. for 16 h. 15% aq NH4OH solution (10 mL) was added
and the
reaction mixture was concentrated in vacuo. The residue was dissolved in H20
(20 mL) and
extracted with DCM (2 x 30 mL). The combined organic extracts were washed with
brine (30
mL), dried over Na2SO4, and concentrated in vacuo. The residue was purified by
chromatography on silica gel (10-100% Et0Ac in heptane) to afford the title
compound (81%
purity, 82 mg, 0.163 mmol, 92% yield) as a white solid; M/Z: 407, 409 [M+H]+,
EST, RT =
1.02 (Si).
Step 40.c: (3R,6S)-6-[5-(4-chloropheny1)-1,3,4-oxadiazol-2-A-1-ethylpiperidin-
3-amine;
trifluoroacetic acid
) N-N
N k ' 41,
FI2Nir.(2'".
F3COH
II
o
To a solution of tert-butyl N-R3R,65)-6-[5-(4-chloropheny1)-1,3,4-oxadiazol-2-
y1]-1-
ethylpiperidin-3-yl]carbamate (81% purity, 82 mg, 0.163 mmol) in DCM (1 mL) at
0 C was
added TFA (1.0 mL, 13.5 mmol) and the mixture was stirred at r.t. for 3 h. The
reaction
mixture was concentrated in vacuo to afford the title compound (28% purity,
160 mg, 0.106
mmol, 65% yield) as a beige gum; M/Z: 307, 309 [M+H]+, EST, RT = 0.84 (51).
134
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Example 47 (step 40.d): 2-(4-chloro-3-fluorophenoxy)-N-[(3R,6S)-645-(4-
chloropheny1)-
1,3,4-oxadiazol-2-y1]-1-ethylpiperidin-3-yl] acetamide
ci
oJF
CI
Example 47
To
a solution of (3R, 65)-6-[5-(4-chloropheny1)-1,3,4-oxadiazol-2-y1]-1-ethyl-
piperidin-3-
amine trifluoroacetic acid (28% purity, 160 mg, 0.106 mmol) in DCM (5 mL) at 0
C was
added DIPEA (0.056 mL, 0.319 mmol) followed by a solution of 2-(4-chloro-3-
fluoro-
phenoxy)acetyl chloride (29 mg, 0.128 mmol, Intermediate 19) in DCM (1 mL),
and the
mixture was stirred at r.t. for 5 h. The reaction mixture was diluted with H20
(10 mL),
extracted with DCM (2 x 10 mL), and the combined organic extracts were dried
over Na2SO4
and concentrated in vacuo. The residue was purified by prep. HPLC (Method 5)
to afford the
title compound (20 mg, 0.0397 mmol, 37% yield) as a white powder;
NMR (400 MHz,
DMSO-d6) 6 8.10 ¨7.89 (m, 3H), 7.76 ¨ 7.60 (m, 2H), 7.51 (t, J= 8.9 Hz, 1H),
7.13 ¨7.05
(m, 1H), 6.91 ¨ 6.83 (m, 1H), 4.56 (s, 2H), 3.99 ¨ 3.83 (m, 2H), 3.03 ¨ 2.94
(m, 1H), 2.49 ¨
2.39 (m, 1H), 2.34 ¨ 2.15 (m, 2H), 2.04 ¨ 1.82 (m, 3H), 1.57 ¨ 1.43 (m, 1H),
0.92 (t, J= 7.1
Hz, 3H); M/Z: 493, 495, 497 [M+H]+, EST, RT = 3.12 (S4).
The example compound in Table 15 was synthesized according to the general
route 40 as
exemplified by Example 47 using the corresponding intermediate.
Table 15
LCMS
Ex Structure Name Intermediates 111 NMR
data
1H NMR (400 MHz,
2-(4-chloro-
3-
DMSO-d6) i 8.06 ¨ 7.95
(2S,5R)-5-
(m, 3H), 7.68 (d, J= 8.7
fluoropheno
xy) -N-
{Rtert-
Hz, 2H), 7.51 (t, J= 8.9
butoxy)carbon M/Z: Hz, 1H), 7.09
(dd, J=
[(3R,6S)-6-
yl]amino}-1- 523, 525,
11.4, 2.8 Hz, 1H), 6.87
[5-(4-
(2- 527
(ddd, J = 9.0, 2.9, 1.2 Hz,
48 N-N chlorophenyl
N . Cl )-1,3,4-
methoxyethyl) [M+H]',
1H), 4.56 (s, 2H), 4.02 ¨
rej110 111
piperidine-2- ESI', RT 3.85 (m,
2H), 3.14 (s,
F 0NX0 oxadiazol-2-
Cl y1]-1-(2- carboxylic = 3.66
3H), 3.06 (dd, J= 11.5,
acid (S4)
3.5 Hz, 1H), 2.62 ¨2.54
methoxyethy
(Intermediate (m, 1H), 2.34
(dd, J=
Dpiperidin-
45)
11.4, 8.4 Hz, 1H), 2.05 ¨
3-
1.94 (m, 1H), 1.94 ¨ 1.77
yl]acetamide
(m, 2H), 1.59 ¨ 1.44 (m,
135
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
1H).
Scheme for route 41
o cF3
,=--0-001
HO
Boc 0 Intermediate 54 Boc 0
1-11(Cio,CF3
,N,611 ,N1-12 ,N
0 - oi2 N HATU, DIPEA 0 A2 N
r.
F 0 OJLN *-/ H DMF, r.t. 5.) F 0 õA õ.. s) ,
0
N
H H
CI Step a CI
Intermediate 26
Step b TsCI, K2CO3
ACN, 80 C
Boc N¨N
II-\L)c. 0
0 ZnBr2 CF3
0 ---.- (R) ,.- \ os.
CF 3 4 _______________________________________ F 0 jc
F so 0j-LNõ,===< DCM, r.t. H
St CI
ep c
CI Example 49
Example 50
Step 41.a: tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-{N'-
[(1s,3s)-3-
(trifluoromethoxy)cyclobutanecarbonyl]hydrazinecarbonyllpiperidine-1-
carboxylate
Boc 0 01
H
0
H
H
CI
To a solution of (1s,3s)-3-(trifluoromethoxy)cyclobutane-1-carboxylic acid
(397 mg, 2.16
mmol, Intermediate 54) and DIPEA (0.94 mL, 5.39 mmol) in anhydrous DMF (12 mL)
was
added HATU (820 mg, 2.16 mmol) and the mixture was stirred at r.t. for 10 min.
A
solution of tert-butyl (2R,5S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]-2-
136
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
(hydrazinecarbonyl)piperidine-l-carboxylate (800 mg, 1.80 mmol, Intermediate
26) in DMF
(7 mL) was added and the mixture was stirred at r.t. for 12 h. The reaction
mixture was
diluted with H20 (50 mL) and extracted with Et0Ac (2 x 50 mL). The combined
organic
extracts were washed with brine (2 x 50 mL), dried over Na2SO4 and
concentrated in vacuo.
The residue was purified by chromatography on silica gel (0-100% Et0Ac in
heptane) to
afford the title compound (86% purity, 970 mg, 1.37 mmol, 76% yield) as an off-
white solid;
1H NMR (400 MHz, DMSO-d6) 6 9.84 (s, 2H), 7.98 (d, J= 7.1 Hz, 1H), 7.47 (t, J=
8.9 Hz,
1H), 7.04 (dd, J= 11.4, 2.9 Hz, 1H), 6.82 (dd, J= 8.9, 1.8 Hz, 1H), 4.86 ¨
4.73 (m, 1H), 4.68
¨4.45 (m, 3H), 3.98 ¨3.75 (m, 2H), 2.73 ¨2.62 (m, 4H), 2.36 ¨ 2.21 (m, 2H),
2.12¨ 1.77 (m,
2H), 1.70¨ 1.50(m, 2H), 1.37 (s, 9H); M/Z: 609, 611 [M-Boc+H]+, EST, RT = 0.99
(S2).
Example 49 (step 41.b): tert-butyl (2R,5S)-5-[2-(4-ehloro-3-
fluorophenoxy)acetamido]-2-
15-[(1s,3s)-3-(trifluoromethoxy)cyclobutyl]-1,3,4-oxadiazol-2-yllpiperidine-1-
carboxylate
Boc N-
O F
F iijca F'"" \F
H
C WI
Example 49
A suspension of tert-butyl (2R,55)-5-[ [2-(4-chloro-3-fluoro-phenoxy)acetyl]
amino] -2-
[ [ [(/S, 35)-3 -(trifluoromethoxy)cyclobutanecarbonyl] amino] carb amo yl]
piperidine-1-
carboxylate (86% purity, 970 mg, 1.37 mmol), K2CO3 (1132 mg, 8.19 mmol) and
TsC1 (781
mg, 4.10 mmol) in ACN (10 mL) was stirred at 80 C for 3 h. The reaction
mixture was
partitioned between Et0Ac (100 mL) and H20 (100 mL), and the organic layer was
isolated,
washed with brine (100 mL), dried over MgSO4, and concentrated in vacuo. The
residue was
purified by chromatography on silica gel (0-100% Et0Ac in heptane) to afford
the title
compound (317 mg, 0.524 mmol, 38% yield) as an off-white powder; 1H NMR (500
MHz,
DMSO-d6) 6 8.12 (d, J= 7.1 Hz, 1H), 7.48 (t, J= 8.9 Hz, 1H), 7.05 (dd, J=
11.4, 2.8 Hz,
1H), 6.82 (dd, J= 8.9, 1.9 Hz, 1H), 5.46 (s, 1H), 4.90 (p, J= 7.5 Hz, 1H),
4.64 ¨ 4.54 (m,
2H), 3.90 (d, J= 12.2 Hz, 2H), 3.44 (tt, J= 9.8, 7.9 Hz, 1H), 3.35 ¨ 3.29 (m,
1H), 2.96 (d, J=
7.8 Hz, 1H), 2.90 ¨2.78 (m, 2H), 2.23 (ddq, J= 13.7, 9.7, 4.4 Hz, 1H), 2.05
(d, J= 11.4 Hz,
1H), 1.75 (m, J= 13.7, 10.1, 4.1 Hz, 1H), 1.64 (d, J= 13.3 Hz, 1H), 1.39 (s,
10H); M/Z: 493,
495 [M-Boc+H]+, EST, RT = 1.12 (S2).
137
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Example 50 (step 41.c): 2-(4-chloro-3-fluorophenoxy)-N-R3S,6R)-6-{5-1(1s,3s)-3-
(trifluoromethoxy)cyclobutyfl-1,3,4-oxadiazol-2-yflpiperidin-3-yflacetamide
FA-F
Ojci,õ.==<
CI
Example 50
To a solution of tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-
2- {5-[(1s,3s)-
3 -(trifluoromethoxy)cyclobutyl] -1,3 ,4-oxadi azol-2-yllpiperidine-l-
carboxylate (317 mg,
0.524 mmol, Example 49) in DCM (10 mL) was added ZnBr2 (714 mg, 3.14 mmol) and
the
mixture was stirred at r.t. under N2 for 20 h. The reaction mixture was
diluted with satd aq
NaHCO3 solution (10 mL) and 20% IPA in DCM (10 mL). The organic layer was
isolated,
concentrated in vacuo, and purified by chromatography on silica gel (0-20%
Me0H in DCM)
to afford the title compound (240 mg, 0.467 mmol, 89% yield) as an off-white
powder;
NMR (400 MHz, DMSO-d6) 6 7.97 (d, J= 8.1 Hz, 1H), 7.50 (t, J= 8.9 Hz, 1H),
7.07 (dd, J=
11.4, 2.8 Hz, 1H), 6.85 (m, J= 9.0, 2.9, 1.2 Hz, 1H), 4.96 ¨4.85 (m, 1H), 4.52
(s, 2H), 3.92 ¨
3.82 (m, 1H), 3.79 ¨ 3.63 (m, 1H), 3.48 ¨ 3.37 (m, 1H), 3.00 (d, J= 12.3 Hz,
1H), 2.91 ¨2.77
(m, 3H), 2.49 ¨2.40 (m, 3H), 2.06 ¨ 1.97 (m, 1H), 1.96 ¨ 1.86 (m, 1H), 1.77 ¨
1.61 (m, 1H),
1.58 ¨ 1.43 (m, 1H); M/Z: 493, 495 [M+H], EST, RT = 1.12 (S4).
Example compounds in Table 16 were synthesised according to the general route
41 as
exemplified by Example 50 using the corresponding intermediates.
Table 16
LCMS
Ex Structure Name Intermediates -111 NMR
data
tert-butyl
(2R,5S)-542-
N-R3S,6R)-
6 [5 (5 (4-chloro-3- 1H NMR (400
MHz,
- - -
fluorophenoxy chloroform-d) 6
7.34 (t, J
chloro-1-
)acetamido]-2- = 8.6 Hz, 1H),
6.88 (s,
methyl-1H- M/Z:
(hydrazinecarb 1H), 6.79 (dd,
J= 10.3, 2.9
lN
, pyrazol-3- 469, 471,
0 Foo\>--0c y1)-1,3,4- onyl)piperidin
e-1-
oxadiazol-2-
M+H Hz, 2H), 6.73 ¨ 6.67 (m,
[ 473] 1H), 4.47 (s, 2H), 4.22 ¨
51 F 0
yl]piperidin-
(Intermediate carboxylate
EST', RT 4.16 (m, 1H),
4.13 ¨ 4.04
= 1.98
(m, 1H), 3.96 (s, 3H), 3.32
chloro-3- (S4)
26) and 5- (dd, J= 12.0,
3.4 Hz, 1H),
chloro-1- 2.70 ¨2.61 (m,
1H), 2.25 ¨
fluoropheno
methyl-1H- 1.99 (m, 4H),
1.72 ¨ 1.62
xy)acetamid
pyrazole-3- (m, 1H).
carboxylic
acid
138
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
'H NMR (500 MHz,
DMSO-d6) 6 9.37 (d, J=
tert-butyl
1.9 Hz, 1H), 8.67 (dd, J =
2-(4-chloro- (2R,5S)-5-[2-
8.1, 1.9 Hz, 1H), 8.17 (d, J
3- (4-chloro-3-
= 8.2 Hz, 1H), 8.03 (d, J=
fluoropheno fluorophenoxy
xy)-N- )acetamido]-2-
8.1 Hz, 1H), 7.51 (t, J=
M/Z: 8.9 Hz, 1H), 7.09
(dd, J=
R3S,6R)-6- (hydrazinecarb
500, 502 11.4, 2.8 Hz, 1H),
6.87
1-N\)___(=>_IF {5-[6- onyl)piperidin
[M+H]', (ddd, J= 9.0, 2.8,
1.1 Hz,
52 " .-.N' (trifluoromet e-1 -
F Ojc õ.k) ESI', RI 1H), 4.55 (s,
2H), 4.09 -
40 H hyl)pyridin- carboxylate
= 2.21 3.99 (m, 1H), 3.83
¨3.71
ci 3-y1]-1,3,4- (Intermediate
(S4) (m, 1H), 3.10 ¨ 2.96
(m,
oxadiazol-2- 26) and 6-
2H), 2.54 (s, 1H), 2.18 ¨
yllpiperidin- (trifluorometh
2.09 (m, 1H), 2.08 (s, 1H),
3- yl)pyridine-3-
2.01 ¨1.91 (m, 1H), 1.81
yl]acetamide carboxylic
acid (qd, J= 12.9, 3.8 Hz,
1H),
1.58 (qd, J= 12.5, 4.0 Hz,
1H).
'H NMR (500 MHz,
DMSO-d6) 6 8.85 (t, J=
tert-butyl
2-(4-chloro- 1.6 Hz, 1H), 8.20 (d, J=
(2R,5S)-5-[2-
3- 1.6 Hz, 2H), 8.01 (d, J=
(4-chloro-3-
fluoropheno 8.1 Hz, 1H), 7.51 (t, J=
fluorophenoxy
xy)-N- 8.9 Hz, 1H), 7.09
(dd, J=
)acetamido]-2- M/Z:
[(3S,6R)-6-
11.4, 2.8 Hz, 1H), 6.87
(hydrazinecarb 466, 468
ci [5(5_
onyl)piperidin [M+H], (ddd, J= 9.0, 2.8,
1.1 Hz,
'
53 F 0j1...,Nõ.,...1 chloropyridi
e-1- EST, RT 1H), 4.54 (s, 2H),
4.07 ¨
.1
ci H n-2-y1)-
1,3,4- '
carboxylate = 2.00 3.99 (m, 1H), 3.82
¨ 3.69
(m, 1H), 3.04 (d, J= 11.5
(Intermediate (S4)
oxadiazol-2- Hz, 1H), 2.97 (s, 1H), 2.57
26) and 5-
yl]piperidin- ¨2.52 (m, 1H), 2.10 (dt, J
chloropyridine
3- = 11.4, 2.9 Hz, 1H), 1.99 ¨
-2-carboxylic
yl]acetamide acid 1.91 (m, 1H), 1.78
(qd, J=
12.8, 3.7 Hz, 1H), 1.57
(qd, J= 12.5, 3.9 Hz, 1H).
tert-butyl 'H NMR (400 MHz,
2-(4-chloro-
3-
(2R,5S)-5-[2- DMSO-d6) 6 8.05 ¨
7.97
(4-chloro-3- (m, 2H), 7.90 ¨ 7.82
(m,
fluoropheno
xy) -N-
fluorophenoxy 2H), 7.50 (t, J= 8.9
Hz,
)acetamido]-2- M/Z: 1H), 7.08 (dd, J=
11.4, 2.8
F [(3S,6R)-6-
(hydrazinecarb 483, 485 Hz, 1H), 6.89 ¨6.83
(m,
" Ni-N\ 110 ci [5-(4-chloro-
N
3-
0 0 onyl)piperidin [M+H]', 1H), 4.53
(s, 2H), 4.04 ¨
54
e-1- ESI', RI 3.94 (m, 1H),
3.81 ¨3.70
ci IW H fluorophenyl
)-1,3,4- carboxylate = 2.39 (m, 1H), 3.08
¨2.94 (m,
(Intermediate (S4) 2H), 2.14 ¨2.05 (m,
1H),
oxadiazol-2-
26) and 4- 1.94 (dd, J= 12.9,
2.8 Hz,
yl]piperidin-
chloro-3- 1H), 1.85 ¨ 1.70 (m,
1H),
3-
fluorobenzoic 1.56 (qd, J= 12.4,
3.8 Hz,
yl]acetamide
acid 1H).
2-(4-chloro- tert-butyl 'H NMR (400 MHz,
3- (2R,5S)-5-[2- methanol-d) 6 8.38 ¨
8.08
fluoropheno (4-chloro-3- (m, 1H), 7.39 (t, J=
8.7
0_/: F xy)-N- fluorophenoxy M/Z: Hz, 1H), 6.95 (dd, J=
H NI "A7-1 \F [(3S,6R)-6- )acetamido]-2- 481, 483 10.9,
2.8 Hz, 1H), 6.84
55 F
{543- (hydrazinecarb [M+H]', (ddd, J= 8.9,
2.8, 1.2 Hz,
03ZNõ. .01
H (trifluoromet onyl)piperidin ESI', RI 1H), 4.54
(s, 2H), 4.20 ¨
ci is
hoxy)propyl] e-1- = 3.22 3.87 (m, 4H), 3.25
¨3.19
-1,3,4- carboxylate (S6) (m, 1H), 3.02 (t,
J= 7.4
oxadiazol-2- (Intermediate Hz, 1H), 2.70 ¨ 2.59
(m,
yllpiperidin- 26) and 4- 1H), 2.26 ¨2.00 (m,
4H),
3- (trifluorometh 1.94¨ 1.79 (m, 1H),
1.73 ¨
139
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
yflacetamide oxy)butanoic 1.58 (m,
1H).
acid
(Intermediate
35)
Scheme for route 42
F F
0
Boc 0 ON
ri L NH
Boc 0 Hrfi<FF
N--Th\IOL , 11\IL
,N
0 (R) N HATU, DIPEA
0 (5) (R)
F 0J.L 2 HO H F 0J.L õ.. 0
DMF, r.t.
CI Step a CI
Intermediate 26
Step b TsCI, K2CO3
ACN, 80 C
H
0 o
7\
0 - .-"=-/n0 TEA
6s)
F F F
DCM, r.t. F 1.1 0j-L
F F
OJLN,
CI
Step c
CI Example 56
Example 57
Step 42.a: tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-{N'-
[1-(2,2,2-
trifluoroethyl)azetidine-3-carbonyl]hydrazinecarbonyllpiperidine-1-carboxylate
Boc 0 Nj FirciN/\1<FF N
0 N'
0
To a solution of 1-(2,2,2-trifluoroethyl)azetidine-3-carboxylic acid (74 mg,
0.405 mmol) in
anhydrous DMF (2 mL) was added HATU (185 mg, 0.486 mmol) followed by DIPEA
(0.14
mL, 0.809 mmol) and the mixture was stirred at r.t. for 10 min. tert-butyl
(2R,5S)-5-[2-(4-
chloro-3-fluorophenoxy)acetamido] -2-(hydrazinecarbonyl)piperidine-1-carboxyl
ate (90%
purity, 200 mg, 0.405 mmol, Intermediate 26) was added and the mixture was
stirred at r.t. for
h. The reaction mixture was diluted with H20 (5 mL) and extracted with Et0Ac
(2 x 20
mL). The combined organic extracts were washed with brine (20 mL), dried over
MgSO4, and
concentrated in vacuo. The residue was purified by chromatography on silica
gel (0-100%
15 Et0Ac in heptane, followed by 0-20% Me0H in Et0Ac) to afford the title
compound (90%
purity, 206 mg, 0.303 mmol, 75% yield) as a pale yellow gum; NMR (400 MHz,
DMSO-
d6) 6 9.81 (s, 2H), 8.05 ¨7.93 (m, 1H), 7.48 (t, J= 8.9 Hz, 1H), 7.05 (dd, J=
11.4, 2.8 Hz,
140
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
1H), 6.83 (dd, J= 9.0, 1.8 Hz, 1H), 4.75 ¨4.48 (m, 3H), 3.99 ¨ 3.76 (m, 2H),
3.55 (t, J= 7.4
Hz, 2H), 3.17 (q, J= 10.2 Hz, 2H), 2.11 ¨2.01 (m, 1H), 1.96 ¨ 1.81 (m, 1H),
1.70 ¨ 1.50 (m,
2H), 1.37 (s, 9H); M/Z: 610, 612 [M+H], EST, RT = 0.85 (S2).
Example 56 (step 42.b): tert-butyl (2R,5S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]-2-
15-[1-(2,2,2-trifluoroethyflazetidin-3-y1]-1,3,4-oxadiazol-2-yllpiperidine-1-
carboxylate
Boc
Ojr\
Example 56
A suspension of tert-butyl (2R,5S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]-2- {N
(2,2,2-trifluoro ethyl)azetidine-3 -carbonyl] hydrazinecarbonyl } piperidine-1
- carboxyl ate (90%
purity, 206 mg, 0.303 mmol), K2CO3 (252 mg, 1.82 mmol) and TsC1 (0.012 mL,
0.910 mmol)
in ACN (1.5384 mL) was stirred at 80 C for 45 min. The reaction mixture was
diluted with
H20 (5 mL) and extracted with Et0Ac (2 x 20 mL). The combined organic extracts
were
washed with brine (20 mL), dried over MgSO4, and concentrated in vacuo. The
residue was
purified by chromatography on silica gel (0-100% Et0Ac in heptane) to afford
the title
compound (110 mg, 0.179 mmol, 59% yield) as a colourless gum; 1H NMR (400 MHz,
DMSO-d6) 6 8.11 (d, J= 7.0 Hz, 1H), 7.48 (t, J= 8.9 Hz, 1H), 7.05 (dd, J=
11.4, 2.8 Hz,
1H), 6.87 ¨ 6.79 (m, 1H), 5.48 (s, 1H), 4.65 ¨ 4.49 (m, 2H), 4.00 ¨ 3.94 (m,
1H), 3.92 (d, J =
11.6 Hz, 1H), 3.78 (t, J= 7.7 Hz, 2H), 3.56 (q, J= 6.8 Hz, 2H), 3.27 (dd, J=
20.3, 10.2 Hz,
2H), 3.09 ¨ 2.93 (m, 1H),2.31 ¨ 2.17 (m, 1H), 2.11 ¨ 2.00 (m, 1H), 1.76 (t, J=
13.7 Hz, 1H),
1.70¨ 1.59 (m, 1H), 1.39 (s, 9H); M/Z: 592, 594 [M+H]+, EST, RT = 0.66 (S2).
Example 57 (step 42.c): 2-(4-chloro-3-fluorophenoxy)-N-R3S,6R)-6-{5-[1-(2,2,2-
trifluoroethyflazetidin-3-y1]-1,3,4-oxadiazol-2-yllpiperidin-3-yfl acetamide
/[1\11-10
Ojr\põ )7F-F
CI
Example 57
A solution of tert-butyl (2R,5S)-5- [2-(4-chloro-3-fluorophenoxy)acetamido]-2-
{5-[1-(2,2,2-
trifluoroethypazetidin-3-yl] -1,3 ,4-oxadi azo 1-2-y1 } piperidine-l-carboxyl
ate (110 mg, 0.179
141
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
mmol, Example 56) and TFA (133 1.1L, 1.79 mmol) in DCM (2 mL) was stirred at
r.t. for 3 h.
The reaction mixture was diluted with satd aq NaHCO3 solution (3 mL) and
extracted with
DCM (2 x 5 mL). The combined organic extracts were dried using a phase
separator,
concentrated in vacuo, and purified by prep. HPLC (Method 3) to afford the
title compound
(24 mg, 0.0478 mmol, 27% yield) as a white solid; 1H NMR (500 MHz, DMSO-d6) 6
7.99 (d,
J= 8.1 Hz, 1H), 7.50 (t, J= 8.9 Hz, 1H), 7.08 (dd, J= 11.4, 2.8 Hz, 1H), 6.86
(ddd, J= 9.0,
2.8, 1.1 Hz, 1H), 4.53 (s, 2H), 4.01 ¨3.92 (m, 1H), 3.88 (ddd, J= 10.4, 6.1,
2.9 Hz, 1H), 3.78
(t, J= 7.7 Hz, 2H), 3.76 ¨ 3.66 (m, 1H), 3.56 (t, J= 6.9 Hz, 2H), 3.28 (q, J=
10.2 Hz, 2H),
3.00 (d, J= 11.5 Hz, 1H), 2.83 (q, J= 6.0 Hz, 1H), 2.48 ¨2.42 (m, 1H), 2.06¨
1.97 (m, 1H),
1.95 ¨ 1.87 (m, 1H), 1.75 ¨ 1.64 (m, 1H), 1.52 (qd, J= 12.5, 3.9 Hz, 1H); M/Z:
492, 494
[M+H]+, EST, RT = 1.88 (S4).
Scheme for route 43
HO
Boc 0 o Intermediate 51 Boc 0 H,ir
/11\loiL ,NH 2 )1oLL ,N
(R) N T3P, DIPEA (R) N
F 0)-LI\H
THF, r.t. F OjLie.,-= 0
CI WI Step a CI
Intermediate 26
TsCI, K2003
Step b
CH3CN, 80 C
c N
N¨N
0
0 ov N0O ZnBr2, DCM 0
F OJL FIF\iiõ,=-
r.t. 0j-FNIõ,.
Step c CI
CI Example 58
Example 59
Step 43.a: tert-butyl (2R,5S)-5-12-(4-chloro-3-fluorophenoxy)acetamido]-2-IN' -
(3-
15 cycloprop oxycyclobutanecarbonyl)hydrazinecarb onyl] piperidine-l-
carboxylate
Boc 0 ¨
1`1)*IJ
0 N
O,=<). H 0
Cl
To a solution of 3-cyclopropoxycyclobutane-l-carboxylic acid (90% purity, 100
mg, 0.576
mmol, Intermediate 51) in THF (5 mL) was added DIPEA (302 !IL, 1.73 mmol), T3P
(50%,
1.0 mL, 1.73 mmol) and tert-butyl (2R,5 S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]-2-
(hydrazinecarbonyl)piperidine-l-carboxylate (256 mg, 0.576 mmol, Intermediate
26) and the
142
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
mixture was stirred at r.t. for 18 h. The reaction mixture was diluted with
H20 (30 mL) and
extracted with Et0Ac (2 x 50 mL). The combined organic extracts were dried
over Na2SO4
and concentrated in vacuo to afford a colourless solid. Purification by
chromatography on
silica gel (0-100% Et0Ac in heptane) afforded the title compound (85% purity,
143 mg,
0.208 mmol, 36% yield) as a white solid; M/Z: 583, 585 [M+H]+, EST, RT = 3.18
(S4).
Example 58 (step 43.b): tert-butyl (2R,5S)-542-(4-chloro-3-
fluorophenoxy)acetamido]-2-
[5-(3-cyclopropoxycyclobuty1)-1,3,4-oxadiazol-2-yl] piperidine- 1 -carboxylate
Boc
(R)
CI
Example 58
To a solution of tert-butyl (2R,55)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-
2-[N-(3-
cyclopropoxycyclobutanecarbonyphydrazinecarb onyl] piperidine-1 - carboxyl ate
(85% purity,
143 mg, 0.208 mmol) in anhydrous ACN (10 mL) was added TsC1 (79 mg, 0.417
mmol) and
K2CO3 (144 mg, 1.04 mmol) and the mixture was stirred at 80 C for 5 h. The
reaction
mixture was diluted with H20 (30 mL) and extracted with Et0Ac (2 x 50 mL). The
combined
organic extracts were dried over Na2SO4 and concentrated in vacuo to afford a
pale orange
oil. Purification by chromatography on silica gel (0-100% Et0Ac in heptane)
afforded the
title compound (70% purity, 111 mg, 0.138 mmol, 66% yield) as a colourless
solid; M/Z: 565,
567 [M+H]+, EST, RT = 3.85 (S4).
Example 59 (step 43.c): 2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-[5-(3-
cycloprop oxycyclobuty1)-1,3,4-oxadiazol-2-yl] pip eridin-3-yl] acetamide
N¨N
Cl
Example 59
To a solution of tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-
2-[5-(3-
cyclopropoxycyclobuty1)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate (70%
purity, 110 mg,
0.136 mmol, Example 58) in DCM (5 mL) was added ZnBr2 (92 mg, 0.409 mmol) and
the
143
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
resultant mixture was stirred at r.t. for 16 h. The reaction mixture was
diluted with NaHCO3
(30 mL), extracted with DCM/IPA (2:1, 2 x 50 mL), and the combined organic
extracts were
dried over Na2SO4 and concentrated in vacuo. The residue was purified by prep.
HPLC
(Method 3) to afford the title compound (26 mg, 0.0559 mmol, 41% yield) as a
white powder;
1H NMR (400 MHz, methanol-d4) 6 7.44 ¨ 7.35 (m, 1H), 6.97 (dd, J= 11.0, 2.8
Hz, 1H), 6.86
(ddd, J= 8.9, 2.8, 1.2 Hz, 1H), 4.55 (s, 2H), 4.27 ¨4.15 (m, 1H), 4.05 ¨ 3.92
(m, 2H), 3.41 ¨
3.34 (m, 2H), 3.25 ¨ 3.19 (m, 1H), 2.81 ¨ 2.60 (m, 3H), 2.40 ¨ 2.26 (m, 2H),
2.23 ¨ 2.06 (m,
2H), 1.94 ¨ 1.81 (m, 1H), 1.73 ¨ 1.59 (m, 1H), 0.60 ¨ 0.42 (m, 4H); M/Z: 465,
467 [M+H]+,
EST, RT = 1.97 (S4).
Example compounds in Table 17 were synthesised according to general route 43
as
exemplified by Example 59 using the corresponding intermediates.
Table 17
LCMS
Ex Structure Name Intermediates 111 NMR
data
tert-butyl
'H NMR (500 MHz,
(2R,5S)-5-[2-
DMSO-d6) 6 8.02 (d, J-
2(3 4-
4 7.4 Hz, 1H), 7.56 (d, J=
- , - (3,
dichloropheno
dichlorophen 8.9 Hz, 1H), 7.26 (d, J¨
xy)acetamido]
oxy)-N- -2-
2.9 Hz, 1H), 6.99 (dd, J¨
[(3S,6R)-6- M/Z: 8.9, 2.9 Hz, 1H), 6.68 (t, J
(hydrazinecarb
F {5-[(1s,3s)- 491, 493,
= 75.4 Hz, 1H), 4.68 (q, J
60 Ho)¨F
(Nic),--V-" 3-
(difluoromet onyl)piperidin
e-1-
carboxylate 495 = 7.5 Hz, 1H),
4.55 (s,
[M+11] ,
2H), 4.05 ¨3.68 (m, 2H),
ci 0,k)
1101 1H hoxy)cyclob
(Intermediate ESIH , RT 3.47 ¨ 3.37
(m, 2H), 3.10 ¨
ci uty1]-1,3,4- 27) and = 2.12
2.98 (m, 1H),2.81 ¨2.73
oxadiazol-2- (S4) (m, 2H), 2.53 ¨2.52 (m,
(1s,3s)-3-
yllpiperidin- 1H), 2.41 ¨ 2.36 (m, 2H),
(difluorometh
3- 2.11 ¨ 1.96 (m, 1H), 1.96 ¨
y oxy)cyclobuta
l]acetamide
ne-1-
1.88 (m, 1H), 1.77 ¨ 1.65
(m, 1H), 1.60 ¨ 1.48 (m,
carboxylic
1H).
acid
tert-butyl
2-(4-chloro- 'H NMR (400 MHz,
(2R,5S)-5-[2-
3- chloroform-d) 6 7.34 (t, J
(4-chloro-3-
fluoropheno = 8.6 Hz, 1H), 6.79 (dd, J
fluorophenoxy
xy)-N- = 10.3, 2.9 Hz, 1H), 6.70
)acetamido]-2- M/Z:
_ ,F [(3S,6R)-6-
(ddd, J= 8.9, 2.9, 1.2 Hz,
{5- (hydrazinecarb 453, 455
2H), 5.17 (s, 2H), 4.47 (s,
61 0
F Ur Rtrifluorome onyl)piperidin [M+H]',
e-1- ESI' , RT
2H), 4.16 (dd, J= 7.8, 3.8
VI H thoxy)methy
1]-1,3,4- carboxylate = 2.05 Hz, 1H),
4.08 (ddq, J-
11.8,7.9, 3.6 Hz, 1H),
ci
(Intermediate (S4)
oxadiazol-2- 3.32 (dd, J= 12.1, 3.2 Hz,
26) and 2-
yllpiperidin- 1H), 2.66 (dd, J= 12.1, 7.7
(trifluorometh
3- Hz, 1H), 2.23 ¨ 1.93 (m,
oxy)acetic
yl]acetamide acid
4H), 1.71 ¨ 1.61 (m, 1H).
144
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Scheme for route 44
HOF
Boc NN Boc NN
NII
NaH
0 ir0 _________________ 0 - 0
F 0)LN,õ..-.5 THF, 0 C, r.t. F Ojc
\µ's
Step a
CI CI
Intermediate 52 Example 62
Step b ZnBr DCM
r.t.
N-N
H
0 -
F
CI
Example 63
Example 62 (step 44.a): tert-butyl (2R,5S)-542-(4-chloro-3-
fluorophenoxy)acetamido]-2-
[5-(3,3,3-trifluoro-2-methylpropoxy)-1,3,4-oxadiazol-2-yl]piperidine-l-
carboxylate
Boc
0 (R)
õ.
CI
Example 62
A suspension of 3,3,3-trifluoro-2-methylpropan-1-ol (34 mg, 0.266 mmol) in
anhydrous THF
at 0 C (1 mL) was treated with NaH (60%, 11 mg, 0.271 mmol) and stirred at 0
C under N2
for 5 min. tert-butyl (2R,55)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido] -2-(5-
methanesulfonyl-1,3 ,4-oxadi azol-2-yl)piperidine-1 -carboxyl ate (71 mg,
0.133 mmol,
Intermediate 52) in anhydrous THF (1 mL) was added and the mixture was stirred
at r.t. for
45 min. The reaction mixture was diluted with satd aq NaHCO3 solution (1 mL)
and extracted
with Et0Ac (2 x 3 mL). The combined organic extracts were dried over Na2SO4
and
concentrated in vacuo to afford the title compound (77 mg, 0.133 mmol) in
quantitative yield.
The crude material was taken forward without purification.
145
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Example 63 (step 44.b): 2-(4-chloro-3-fluorophenoxy)-N-1(3S,6R)-645-(3,3,3-
trifluoro-2-
methylpropoxy)-1,3,4-oxadiazol-2-ylipiperidin-3-yl] acetamide
H
0 - (R)
F Ati
CI
Example 63
To a solution of tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido] -
2-[5-(3,3,3-
trifluoro-2-methylpropoxy)-1,3,4-oxadiazol-2-yl]piperidine-1-carboxylate (77
mg, 0.133
mmol, Example 62) in DCM (2 mL) was added ZnBr2 (180 mg, 0.799 mmol) and the
mixture
was stirred at r.t. for 17 h. The reaction mixture was diluted with satd aq
NaHCO3 solution
(10 mL) and extracted with DCM/IPA (8:2, 2 x 3 mL). The combined organic
extracts were
dried using a phase separator, concentrated in vacuo, and purified by prep.
HPLC (Method 3)
to afford the title compound (94% purity, 7.0 mg, 0.0143 mmol, 11% yield) as a
white
powder; 1H NMR (500 MHz, DMSO-d6) 6 7.95 (d, J= 8.0 Hz, 1H), 7.49 (t, J= 8.9
Hz, 1H),
7.06 (dd, J= 11.4, 2.8 Hz, 1H), 6.85 (ddd, J= 9.0, 2.8, 1.1 Hz, 1H), 4.55 (d,
J= 5.0 Hz, 2H),
4.52 (s, 2H), 3.78 ¨ 3.73 (m, 1H), 3.73 ¨ 3.65 (m, 1H), 3.13 ¨ 3.01 (m, 1H),
3.00 ¨ 2.92 (m,
1H), 2.83 ¨2.76 (m, 1H), 2.46 ¨2.39 (m, 1H), 1.99 ¨ 1.86 (m, 2H), 1.70¨ 1.59
(m, 1H), 1.53
¨ 1.45 (m, 1H), 1.19 (d, J = 7.1 Hz, 3H); M/Z: 481, 483 [M+H]+, EST, RT = 2.15
(S4).
Example compounds in Table 18 were synthesised according to the general route
44 as
exemplified by Example 63 using the corresponding intermediates.
Table 18
Starting LCMS
Ex Structure Name 1H NMR
materials data
2-(4-chloro- tert-butyl 'H NMR (500 MHz,
3- (2R,5S)-5-[2-(4- chloroform-d) 6
7.33 (t, J
fluoropheno chloro-3- = 8.6 Hz, 1H), 6.78 (dd,
xy)-N- fluorophenoxy)a M/Z:
J= 10.3, 2.9 Hz, 1H),
F XF F 481 483 [(3S,6R)-6- cetamido]-2-(5-
6.74 ¨ 6.67 (m, 2H), 5.38
,
H 15-[(4,4,4- methanesulfonyl
M+H] ¨5.16 (m, 1H), 4.46 (s,
['
64 trifluorobuta -1,3,4- ,
2H), 4.06 (ddq, J= 11.4,
F 0 jt,N L. ESI', RT 7.8, 3.4
Hz, 1H), 4.00
H
CI n-2-yl)oxy]- oxadiazol-2-
1,3,4- yl)piperidine-1- = 2.15
S4
¨
3.96 (m, 1H), 3.30 (dt, J
( ) oxadiazol-2- carboxylate = 11.9, 3.7 Hz, 1H),2.80
yl piperidin- (Intermediate ¨ 2.67 (m, 1H), 2.68 ¨
3- 52) and 4,4,4- 2.55 (m, 1H),
2.52 ¨ 2.43
yl]acetamide trifluorobutan- (m, 1H), 2.08 (dt, J-
146
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
2-ol
5.8, 3.1 Hz, 2H), 1.96 ¨
1.88 (m, 1H), 1.63 (d, J=
8.5 Hz, 2H), 1.59 (d, J=
6.3 Hz, 3H).
tert-butyl 111 NMR (400 MHz,
2-(4-chloro- (2R,55)-512-(4-
DMSO-d6) 6 7.96 (d, J=
3- chloro-3- 8.1
Hz, 1H), 7.50 (t, J=
fluoropheno fluorophenoxy)a 8.9
Hz, 1H), 7.07 (dd, J
xy)-N- cetamido]-2-(5-
M/Z: = 11.4, 2.8 Hz, 1H), 6.85
H N,-.1\1 r_F_XF
[(3S,6R)-6- methanesulfonyl 463, 465 (ddd, .1= 9.0, 2.8, 1.1 Hz,
[5-(3,3- -1,3,4- [M+H]',
1H), 4.59 (t, .1= 6.1 Hz,
F OjN, } difluorobuto oxadiazol-2-
ESI', RT 2H), 4.52 (s, 2H), 3.74
a .1 H xy)-1,3,4- yl)piperidine-1- =
2.00 (s, 2H), 3.01 ¨2.94 (m,
oxadiazol-2- carboxylate (S4)
1H), 2.82 ¨2.75 (m, 1H),
yl]piperidin- (Intermediate
2.44 (d, J= 6.5 Hz, 1H),
3- 52) and 3,3-
2.00¨ 1.86 (m, 2H), 1.68
yl]acetamide difluorobutan-1- (t,
J= 19.2 Hz, 5H), 1.55
ol ¨ 1.43 (m, 2H).
1H NMR (400 MHz,
tert-butyl
DMSO-d6) 6 7.95 (d, .1=
2-(4-chloro-
3-
(2R,55)-5-[2-(4- 8.1
Hz, 1H), 7.49 (t, J¨
chloro-3- 8.9
Hz, 1H), 7.07 (dd, J
fluoropheno
xy) -N-
fluorophenoxy)a =
11.4, 2.8 Hz, 1H), 6.85
cetamido]-2-(5- M/Z:
(ddd, J¨ 9.0, 2.8, 1.1 Hz,
F r [(3S,6R)-6-
methanesulfonyl 461, 463 1H), 4.63 ¨4.55 (m, 1H),
H NI -1µ1_0/---<--- {5-[(2,2-
-1,3,4- [M+H]',
4.52 (s, 2H), 4.45 ¨4.37
66 0 Cik, 0 F difluorocycl
j, )
.I Oc H opropyl)met oxadiazol-2- ESI', RT (m,
1H), 3.80 ¨3.64 (m,
yl)piperidine-1- = 1.99
2H), 3.01 ¨2.93 (m, 1H),
a hoxy]-1,3,4-
carboxylate (S4)
2.83 ¨2.74 (m, 1H), 2.45
oxadiazol-2-
(Intermediate ¨
2.37 (m, 1H), 2.37 ¨
yllpiperidin-
52) and (2,2-
2.27 (m, 1H), 2.00 ¨ 1.84
3-
difluorocyclopr (m,
2H), 1.85 ¨ 1.72 (m,
yl]acetamide
opyl)methanol
1H), 1.71 ¨ 1.58 (m, 2H),
1.55 ¨ 1.41 (m, 1H).
1H NMR (500 MHz,
DMSO-d6) 6 7.95 (d, J-
8.1 Hz, 1H), 7.49 (t, J=
tert-butyl
8.9 Hz, 1H), 7.07 (dd, J
(2R,5S)-512-(4-
N-R3S,6R)- = 11.4, 2.8 Hz, 1H), 6.85
chloro-3-
6-(5-butoxy- fluorophenoxy)a
(ddd, J= 9.0, 2.8, 1.1 Hz,
1,3,4- M/Z:
1H), 4.52 (s, 2H), 4.42 (t,
H NI-N cetamido]-2-(5-
%_0/-7--- oxadiazol-2- 427, 429 J= 6.5 Hz, 2H), 3.77 ¨
methanesulfonyl
yl)piperidin- [M+H]', 3.64
(m, 2H), 3.00
67
F aiiiii., OjcõØ..LN 3-y1]-2 -1,3,4-
-(4- ESI', RT ¨ 2.93 (m, 1H),2.79 ¨
a W H
chloro-3- oxadiazol-2-
yl)piperidine-1- = 2.07
2.73 (m, 1H), 2.44 ¨ 2.38
fluoropheno (S4) (m,
1H), 1.99 ¨ 1.85 (m,
carboxylate
xy)acetamid 2H), 1.77 ¨ 1.70 (m, 2H),
(Intermediate
e 1.68 ¨ 1.58 (m, 1H), 1.48
52) and butan-1-
ol (qd, J= 12.3, 3.7
Hz,
1H), 1.40 (h, J= 7.4 Hz,
2H), 0.92 (t, J= 7.4 Hz,
3H).
2-(4-chloro- tert-butyl 111 NMR (400 MHz,
3- (2R,55)-512-(4-
DMSO-d6) 6 7.95 (d, J=
M/Z:
ne..F.F fluoropheno chloro-3- 8.1 Hz, 1H), 7.49 (t, J-
475, 477
xy)-N- fluorophenoxy)a 8.9
Hz, 1H), 7.06 (dd, J
68 j ESI, RT
00)402-0
[(3S,6R)-6- cetamido]-2-(5- [M+H]',
= 11.4, 2.8 Hz, 1H), 6.85
{5-[(3,3- methanesulfonyl
(ddd, .1= 9.0, 2.9, 1.2 Hz,
a W H
difluorocycl -1,3,4- =2.05(S4)
1H), 5.32 (s, 1H), 4.52
opentyl)oxy] oxadiazol-2- (s,
2H), 3.79 ¨ 3.63 (m,
-1,3,4- yl)piperidine-1-
2H), 3.00 ¨2.93 (m, 1H),
147
CA 03137213 2021-10-18
WO 2020/216766 PC T/EP2020/061150
oxadiazol-2- carboxylate
2.81 ¨2.74 (m, 1H), 2.71
yllpiperidin- (Intermediate ¨2.64 (m, 1H), 2.46
¨
3- 52) and 3,3-
2.38 (m, 2H), 2.34 ¨2.19
yl]acetamide difluorocyclope (m,
3H), 2.16 ¨2.07 (m,
ntan-l-ol
1H), 1.99 ¨ 1.85 (m, 2H),
1.70 ¨ 1.58 (m, 1H), 1.48
(qd, J= 12.1, 3.4 Hz,
1H).
'H NMR (400 MHz,
DMSO-d6) 6 7.95 (d, J=
tert-butyl
8.2 Hz, 1H), 7.49 (t, J=
2-(4-chloro- (2R,5S)-5-[2-(4-
8.9 Hz, 1H), 7.07 (dd, J
3- chloro-3-
= 11.4, 2.9 Hz, 1H), 6.85
fluoropheno fluorophenoxy)a
(dd, J= 8.9, 1.7 Hz, 1H),
D.. xy)-N- cetamido]-2-(5- M/Z:
[(3S,6R)-6- methanesulfonyl 439, 441
4.52 (s, 2H), 4.46 (t, J=
NN
69 H -or¨f
0 01 l'O [5-(2- -1,3,4- [M+H]', (m,
2H), 3.00 ¨2.93 (m,
cyclopropyle oxadiazol-2- ESI', RT 6.5
Hz, 2H), 3.78 ¨ 3.64
F 0,)I,No, )
WI thoxy)-1,3,4- yl)piperidine-1- =
2.16 1H), 2.81 ¨ 2.74 (m, 1H),
H
CI
2.42 (d, J= 4.0 Hz, 1H),
oxadiazol-2- carboxylate (S4)
1.98 ¨ 1.86 (m, 2H), 1.67
yl]piperidin- (Intermediate
(q, J= 6.6 Hz, 2H), 1.55
3- 52) and 2-
¨ 1.41 (m, 2H), 0.82 ¨
yl]acetamide cyclopropyletha
0.72 (m, 1H), 0.47 ¨ 0.39
n-l-ol
(m, 2H), 0.15 ¨0.07 (m,
2H).
'H NMR (400 MHz,
tert-butyl
DMSO-d6) 6 7.95 (d, J=
2-(4-chloro- (2R,55)-5-[2-(4- 8.1
Hz, 1H), 7.49 (t, J=
3- chloro-3-
8.9 Hz, 1H), 7.06 (dd, J
fluoropheno fluorophenoxy)a
= 11.4, 2.8 Hz, 1H), 6.85
H N-NA ii----- xy)-N- cetamido]-2-(5-
M/Z: (ddd, J= 9.0, 2.8, 1.1 Hz,
[(3S,6R)-6- methanesulfonyl 441,443
1H), 4.52 (s, 2H), 4.45 (t,
70 0 01,02-0 [5-(3- -1,3,4- [M+H]',
J= 6.5 Hz, 2H), 3.77 ¨
F 0A,N, ) methylbutox oxadiazol-2- ESI', RI
VI y)-1,3,4- yl)piperidine-1- =
2.28 3.63 (m, 2H), 3.00 ¨ 2.93
H
CI (m,
1H), 2.80 ¨2.73 (m,
oxadiazol-2- carboxylate (S4)
1H), 2.46 ¨ 2.37 (m, 1H),
yl]piperidin- (Intermediate
1.99¨ 1.85 (m, 2H), 1.68
3- 52) and 3-
(tq, J= 12.5, 6.6, 5.9 Hz,
yl]acetamide methylbutan-1-
4H), 1.54 ¨ 1.42 (m, 1H),
ol
0.92 (d, J= 6.4 Hz, 6H).
tert-butyl 'H NMR (400 MHz,
2-(4-chloro-
(2R,5S)-5-[2-(4-
DMSO-d6) 6 7.95 (d, J=
3-
chloro-3- 8.0
Hz, 1H), 7.49 (t, J=
fluoropheno
fluorophenoxy)a 8.9
Hz, 1H), 7.06 (dd, J
xy)-N-
cetamido]-2-(5- M/Z: =
11.4, 2.8 Hz, 1H), 6.85
FxF [(3S,6R)-6-
methanesulfonyl 475, 477 (ddd, J= 9.0, 2.8, 1.2 Hz,
71 H "N
{5-[(2,2-
-1,3,4- [M+H]',
1H), 4.60 ¨ 4.48 (m, 4H),
F Oj difluorocycl N,õ )
oxadiazol-2- ESI', RI 3.79 ¨3.63 (m, 3H), 2.96
VI obutyl)meth
oxy]-1,3,4- yl)piperidine-1- = 2.05 (d, J=
11.9 Hz, 1H),
H
0
carboxylate (S4)
2.79 (s, 1H), 2.43 ¨2.38
oxadiazol-2-
(Intermediate (m,
1H), 2.01 ¨ 1.83 (m,
yllpiperidin-
52) and (2,2-
4H), 1.70¨ 1.55 (m, 3H),
3-
difluorocyclobut 1.48 (qd, J= 12.3,
3.7
yl]acetamide
yl)methanol Hz, 1H).
148
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
'H NMR (400 MHz,
tert-butyl
DMSO-d6) 6 7.95 (d, J=
2-(4-chloro-
3-
(2R,5S)-512-(4- 8.2
Hz, 1H), 7.49 (t, J=
chloro-3- 8.9
Hz, 1H), 7.06 (dd, J
fluoropheno
fluorophenoxy)a =
11.4, 2.8 Hz, 1H), 6.85
F xy)-N-
cetamido]-2-(5- M/Z:
(ddd, J= 9.0, 2.8, 1.1 Hz,
r4-F [(3S,6R)-6-
methanesulfonyl 461, 463 1H), 5.20 ¨ 5.09 (m, 1H),
H N -NA P [543,3
72 difluorocycl -
-1,3,4-
[M+H]', 4.52 (s, 2H), 3.80 ¨ 3.64
0 N (,) I 07¨C)
oxadiazol-2- ESL', RI (m,
2H), 3.25 ¨3.14 (m,
F 0 0, .,) obutoxy)-
yl)piperidine-1- = 1.96
2H), 3.02 ¨ 2.85 (m, 3H),
a carboxylate (S4)
2.81 ¨2.73 (m, 1H), 2.45
oxadiazol-2-
(Intermediate ¨2.38 (m, 1H), 2.01
¨
yl]piperidin-
52) and 3,3-
1.84 (m, 2H), 1.70 ¨ 1.59
3-
difluorocyclobut (m,
1H), 1.54 ¨ 1.43 (m,
yl]acetamide
an-l-ol 1H).
tert-butyl
2-(4-chloro-
3-
(2R,5S)-542-(4- 'H NMR (500 MHz,
chloro-3-
chloroform-d) 6 7.33 (t, J
fluoropheno
xy) -N-
fluorophenoxy)a =
8.6 Hz, 1H), 6.78 (dd,
cetamido]-2-(5- J= 10.3, 2.9 Hz,
1H),
[(3S,6R)-6- M/Z:
methanesulfonyl
6.74 ¨ 6.67 (m 2H) 4.94
H 4--1--F [5- 503, 505
-1,3,4-
"
+
o Cy-o F F (2,2,3,3,3-
[M+H], ¨4.86 (m, 2H), 4.46 (s,
73 oxadiazol-2-
2H), 4.10 ¨ 4.00 (m, 2H),
F o11,r. s) pentafluorop
yl)piperidine-1- E_SI+, RT
3.30 (dd, J= 12.0, 3.4
a l'W ropoxy)-
carboxylate 2.29
Hz, 1H), 2.63 (dd, J=
1,3,4- (S4)
(Intermediate
12.1, 7.6 Hz, 1H), 2.13 ¨
oxadiazol-2-
52) and
2.02 (m, 2H), 1.98 ¨ 1.90
yl]piperidin-
2,2,3,3,3- (m,
1H), 1.67 ¨ 1.61 (m,
3-
pentafluoroprop 2H).
yl]acetamide
an-l-ol
tert-butyl
'H NMR (500 MHz,
2-(4-chloro- (2R,55)-5-[2-(4-
3- chloro-3-
DMSO-d6) 6 7.94 (d, J-
7.7 Hz, 1H), 7.49 (t, J=
fluoropheno fluorophenoxy)a
M/Z: 8.9
Hz, 1H), 7.07 (dd, J
F xy)-N- cetamido]-2-(5-
481, 483 =
11.4, 2.8 Hz, 1H), 6.89
r F [(3S,6R)-6- methanesulfonyl
F [M+HC ¨ 6.76 (m, 1H), 4.52 (s,
, a [5-(4,4,4- -1,3,4-
74 , . ' 07--
02H]+, 2H), 4.48 (t, J= 6.3 Hz,
F fit trifluorobuto oxadiazol-2-
ESI+, RT 2H), 3.79 ¨3.64 (m, 2H),
xy)-1,3,4- yl)piperidine-1 -
H = 2.21
3.01 ¨2.89 (m, 1H), 2.77
ci WI' oxadiazol-2- carboxylate
(S4) (s,
1H), 2.45 ¨2.38 (m,
yl]piperidin- (Intermediate
3H), 2.06 ¨ 1.83 (m, 4H),
3- 52) and 4,4,4-
1.71 ¨1.58 (m, 1H), 1.56
yl]acetamide trifluorobutan-
¨ 1.45 (m, 1H).
1-ol
tert-butyl 'H NMR(500 MHz,
(2R,55)-5-[2-(4-
chloroform-d) 6 7.33 (t, J
2-(4-chloro-
chloro-3- =
8.6 Hz, 1H), 6.78 (dd,
3-
fluorophenoxy)a J= 10.3, 2.9 Hz,
1H),
fluoropheno
cetamido]-2-(5-
6.74 (d, J= 7.3 Hz, 1H),
xy)-N-
methanesulfonyl M/Z: 6.69 (ddd, J= 8.9,
2.9,
.4FF [(3S,6R)-6-
-1,3,4-
465, 467 1.2 Hz, 1H), 6.27 (t, J=
H or-I 1542-
75 (difluoromet
oxadiazol-2-
[M+H]+, 73.4 Hz, 1H), 4.69 ¨4.65
0 od..0,¨
yl)piperidine-1- ESI', RI (m, 2H), 4.46
(s, 2H),
F rill 0,, jcs, s) hoxy)ethoxy
carboxylate = 2.23
4.26 ¨4.22 (m, 2H), 4.10
a W'' oxadiazol-2-
(Intermediate (S4) ¨
4.02 (m, 1H), 4.00 (dd,
52) and 2- J=
7.6, 3.5 Hz, 1H), 3.29
yllpiperidin-
3-
(difluoromethox
(dd,J = 12.2, 3.4 Hz, 1H),
y)ethan-l-ol 2.62 (dd, J= 12.0,
7.5
yl]acetamide
(Intermediate Hz,
1H), 2.11 ¨2.03 (m,
39)
2H), 1.98 ¨ 1.89 (m, 1H),
149
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
1.69 - 1.60 (m, 2H).
Scheme for route 45
0--EF
Boc
0
F Boc N--"NF
Br HO
oi2 0
0 (R) 0
F NaH,THF
F 0j-LNõ,.-=
CI Step a
Intermediate 31 CI
Example 76
Step b ZnBr DCM
r.t.
NN OF
0 - (R) 0
CI
Example 77
Example 76 (step 45.a): tert-butyl (2R,5S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]-2-
15-[2-(trifluoromethoxy)ethoxy]-1,3,4-oxadiazol-2-yllpiperidine-1-carboxylate
yoc NN OF
N
0 iO
F õ.=
CI Example 76
To a solution of 2-(trifluoromethoxy)ethanol (139 mg, 1.07 mmol) in anhydrous
THF (6
mL) at 0 C was added NaH (60%, 43 mg, 1.07 mmol) and the mixture was stirred
for 10 min.
A solution of tert-butyl (2R, 55)-2-(5-bromo-1,3,4-oxadiazol-2-y1)-5-[ [2-(4-
chloro-3 -fluoro-
phenoxy)acetyl]amino]piperidine-l-carboxylate (500 mg, 0.890 mmol,
Intermediate 31)
in anhydrous THF (6 mL) was added and the mixture was stirred at r.t. for 2 h.
The reaction
mixture was poured on to water (15 mL) and extracted with Et0Ac (3 x 15 mL).
The
combined organic extracts were dried over MgSO4 and concentrated in vacuo to
afford the
title compound (80% purity, 627 mg, 0.860 mmol, 97% yield) as a yellow oil;
Ifl NMR (400
MHz, chloroform-0 6 7.33 (t, J= 8.6 Hz, 1H), 6.91 ¨ 6.61 (m, 3H), 5.48 (d, J=
25.4 Hz, 1H),
4.77 ¨4.65 (m, 2H), 4.44 (dd, J= 14.2, 4.1 Hz, 2H), 4.38 ¨4.29 (m, 2H), 4.22
¨4.01 (m,
150
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
2H), 3.17 (s, 1H), 2.27 ¨2.06 (m, 1H), 1.91 (d, J= 26.1 Hz, 3H), 1.45 (s, 9H);
M/Z: 483, 485
[M-Boc+H], EST, RT = 1.11 (S2).
Example 77 (step 45.b): 2-(4-chloro-3-fluorophenoxy)-N-R3S,6R)-6-15-[2-
(trifluoromethoxy)ethoxy]-1,3,4-oxadiazol-2-yllpiperidin-3-yflacetamide
61111¨N¨Or¨ F
0 m 0
F õArs. s,
CI
Example 77
To a solution of tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-
2-{5-[2-
(trifluoromethoxy)ethoxy] -1,3 ,4-oxadiazol-2-yllpiperidine-1-carboxylate (80%
purity, 627
mg, 0.860 mmol, Example 76) in DCM (9 mL) was added ZnBr2 (581 mg, 2.58 mmol)
and
the mixture was stirred at r.t. for 12 h. The reaction was poured onto H20 (20
mL) and
extracted with 10% IPA in DCM (3 x 20 mL). The combined organic extracts were
dried over
MgSO4 and concentrated in vacuo. The residue was purified by prep. HPLC
(Method 4) to
afford the title compound (194 mg, 0.394 mmol, 46% yield) as a white solid; 1H
NMR (500
MHz, DMSO-d6) 6 7.99 (d, J= 8.1 Hz, 1H), 7.48 (t, J= 8.9 Hz, 1H), 7.06 (dd, J=
11.4, 2.8
Hz, 1H), 6.84 (m, J= 9.0, 2.8, 1.1 Hz, 1H), 4.70 ¨4.65 (m, 2H), 4.51 (s, 2H),
4.49 ¨4.44 (m,
2H), 3.79 ¨ 3.73 (m, 1H), 3.73 ¨3.65 (m, 1H), 2.96 (dd, J= 11.5, 3.2 Hz, 1H),
2.79 (s, 1H),
2.47 ¨ 2.38 (m, 1H), 1.99 ¨ 1.84 (m, 2H), 1.69 ¨ 1.58 (m, 1H), 1.54¨ 1.44 (m,
1H); M/Z: 483,
485 [M+H]+, EST, RT = 2.19 (S4).
Example compounds in Table 19 were synthesised according to the general route
45 as
exemplified by Example 77 using the corresponding intermediates.
Table 19
Starting LCMS
Ex Structure Name 1H NMR
materials data
2-(4-chloro- tert-butyl
1H NMR (400 MHz,
3- (2R,55)-2-(5- chloroform-d) 6
7.33 (t, J
fluoropheno bromo-1,3,4- M/Z:
= 8.6 Hz, 1H), 6.78 (dd,
H NNr_rj xy)-N- oxadiazol-2-y1)- 441,443 J = 10.3, 2.9 Hz,
1H),
78 [(3S,6R)-6- 5-[[2-(4-chloro-
[M+H] 6.72 ¨ 6.62 (m, 2H), 4.46
[5- 3-fluoro- ESI', RT (s, 2H),
4.21 (t, J= 5.1
c, so H (pentyloxy)- phenoxy)acetyl]
= 2.32 Hz, 2H), 4.11 ¨3.93 (m,
1,3,4- amino]piperidin (S4)
2H), 3.72 (t, J= 5.1 Hz,
oxadiazol-2- e-l-carboxylate
2H), 3.33 (dd, J= 12.0,
yl]piperidin- (Intermediate
3.5 Hz, 1H), 3.17 (s, 3H),
151
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
3- 31) and pentan- 2.60
(dd, J= 11.9, 8.1
yl]acetamide 1-ol Hz, 1H), 2.15 ¨ 1.86 (m,
4H), 1.64 ¨ 1.57 (m, 1H).
tert-butyl
2-(4-chloro-
3-
(2R,55)-2-(5- 'H
NMR (400 MHz,
bromo-1,3,4-
chloroform-d) 6 7.33 (t, J
fluoropheno
oxadiazol-2-y1)- = 8.6 Hz, 1H), 6.81 ¨
, xy)-N- M/Z:
5-[[2-(4-chloro- 6.67 (m, 3H), 4.57 (t, J=
H N...N [(3S,6R)-6- 443, 445
3-fluoro- 6.3
Hz, 2H), 4.46 (s, 2H),
79 o 01-0 [543-yp phenoxy)acetyl]
F [M+H]',
methoxro ESI', RT 4.11 ¨3.92 (m, 2H), 3.52
fiat 0 ,.....,11õ11 amino]piperidin (t, J= 6.0 Hz,
2H), 3.37
poxy)-1,3,4- = 1.86
a V' e-l-carboxylate
¨3.25 (m, 4H), 2.62 (dd,
oxadiazol-2- (S4)
(Intermediate J=
12.0, 7.5 Hz, 1H),
yl]piperidin-
31) and 3-
2.19 ¨ 1.83 (m, 6H), 1.61
3-
methoxypropan- (q, J= 8.3 Hz, 1H).
yl]acetamide
1-ol
2-(4-chloro- tert-butyl 'H NMR (400 MHz,
3- (2R,55)-2-(5-
chloroform-d) 6 7.33 (t, J
fluoropheno bromo-1,3,4- = 8.6 Hz, 1H), 6.81 ¨
xy)-N- oxadiazol-2-y1)- 6.66 (m, 3H), 4.63 ¨4.57
0¨(1
[(3S,6R)-6- 5-[[2-(4-chloro- M/Z:
H N)
(m, 2H), 4.46 (s, 2H),
-N\¨ rj 455, 457
[5-(2- 3-fluoro- [M+H], 4.10 ¨3.96 (m, 2H), 3.90
'
80 F 03)( võk) cyclopropox phenoxy)acetyl]
¨3.84 (m, 2H), 3.37 (tt, J
cil. " yethoxy)- amino]piperidin ESI', RT
= 1.97 =
6.0, 3.0 Hz, 1H), 3.28
1,3,4- e-l-carboxylate (S4) (dd, J= 12.0, 3.4 Hz,
oxadiazol-2- (Intermediate 1H), 2.62 (dd, J=
12.0,
yl]piperidin- 31) and 2- 7.4 Hz, 1H), 2.12¨ 1.86
3- cyclopropoxyet (m,
4H), 1.67 ¨ 1.58 (m,
yl]acetamide han-l-ol 1H), 0.64 ¨0.47 (m, 4H).
'H NMR (400 MHz,
tert-butyl
chloroform-d) 6 7.33 (t, J
2-(4-chloro-
3-
(2R,55)-2-(5- = 8.6
Hz, 1H), 6.81 ¨
bromo-1,3,4-
6.66 (m, 3H), 4.64 ¨4.57
fluoropheno
oxadiazol-2-y1)- (m, 2H), 4.46 (s, 2H),
xy)-N- M/Z:
5-[[2-(4-chloro- 4.10 ¨4.02 (m, 1H), 4.02
[(3S,6R)-6- 443, 445
3-fluoro- ¨
3.97 (m, 1H), 3.84 ¨
81 0 N ) [5-(2-
phenoxy)acetyl] [M+H]',
3.77 (m, 2H), 3.57 (q, J=
F 0 ,..õ1,. N 0. C''..-)...1'
(.I H ethoxyethox ESI', RT
y)-1,3,4- amino]piperidin
= 1.88 7.0
Hz, 2H), 3.28 (dd, J
a e-l-carboxylate =
12.0, 3.4 Hz, 1H), 2.62
oxadiazol-2- (S4)
(Intermediate (dd,
J= 12.0, 7.5 Hz,
yl]piperidin-
31) and 2-
1H), 2.24¨ 1.99 (m, 3H),
3-
ethoxyethan-1- 1.97¨ 1.86 (m, 1H), 1.68
yl]acetamide
ol
¨1.55 (m, 1H), 1.23 (t, J
= 7.0 Hz, 3H).
2-(4-chloro- tert-butyl 'H NMR (400 MHz,
3- (2R,55)-2-(5-
chloroform-d) 6 7.33 (t, J
fluoropheno bromo-1,3,4- = 8.6 Hz, 1H), 6.81 ¨
xy)-N- oxadiazol-2-y1)- 6.66 (m, 3H), 4.61 ¨4.55
M/Z:
[(3S,6R)-6- 5-[[2-(4-chloro- ; 469, 471 (m,
2H), 4.46 (s, 2H),
[5-(2- 3-fluoro-
[M+H]
4.11 ¨3.94 (m, 3H), 3.74
',
82 F , 0.õ.11..õõ.k) cyclobutoxy phenoxy)acetyl]
¨3.68 (m, 2H), 1.77-
H ESI', RT
ci W1 ethoxy)- amino]piperidin
= 2.12
1.60 (m, 2H), 3.28 (dd, J
1,3,4- e-l-carboxylate = 12.0, 3.4 Hz, 1H), 2.62
(S4)
oxadiazol-2- (Intermediate (dd, J= 11.9, 7.5 Hz,
yl]piperidin- 31) and 2- 1H), 2.26 ¨2.16 (m, 2H),
3- cyclobutoxyetha
2.11 ¨ 1.87 (m, 6H), 1.53
yl]acetamide n-l-ol ¨ 1.47 (m, 1H).
152
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
'H NMR (500 MHz,
DMSO-d6) 6 7.97 (d, J=
tert-butyl
2-(4-chloro- 8.0 Hz, 1H), 7.50 (t, J=
(2S,5R)-2-(5-
3-
bromo-1,3,4-
8.9 Hz, 1H), 7.07 (dd, J
fluoropheno = 11.4, 2.8 Hz, 1H), 6.86
oxadiazol-2-y1)-
xy)-N-
(ddd, J= 9.0, 2.8, 1.1 Hz,
5-[2-(4-chloro- M/Z:
; [(3R,65)-6- 3-
1H), 4.71 ¨ 4.67 (m, 2H),
H N..¨ f F {5-[2- fluorophenoxy)a [M+H]483' 485
4.53 (s, 2H), 4.50 ¨ 4.45
).!, /-0 ',
83 7*, 0 (trifluoromet
(m, 2H), 3.80 ¨3.74 (m,
F 0, j ry c, .,õ,0 hoxy)ethoxy
cetamido]piperi ESI', RT
ci 0 H
]-1,3,4- 2.13
1H), 3.74 ¨3.65 (m, 1H),
3.02 ¨ 2.94 (m, 1H), 2.84
carboxylate (S4)
oxadiazol-2- ¨2.77 (m, 1H), 2.47 ¨
(Intermediate
yllpiperidin- 2.39 (m, 1H), 2.00 ¨ 1.93
33) and 2-
3- (m, 1H), 1.93 ¨ 1.86 (m,
(trifluoromethox
yl]acetamide 1H), 1.65 (qd, J= 12.6,
y)ethan-l-o!
3.5 Hz, 1H), 1.50 (qd, J
= 12.4, 3.8 Hz, 1H).
2-(4-chloro- tert-butyl 'H NMR (500 MHz,
3- (2R,55)-2-(5- chloroform-d) 6
7.33 (t, J
fluoropheno bromo-1,3,4- = 8.6 Hz, 1H), 6.78
(dd,
xy)-N- oxadiazol-2-y1)- m/z: J=
10.3, 2.9 Hz, 1H),
.s. r_ J4F R3S,6R)-6- 5-[[2-(4-chloro-
6.74 ¨ 6.66 (m, 2H), 4.52
477, 479
6,=õ0 F { 5- [(4 ,4- 3-fluoro-
[M+H]+, (t, J= 6.0 Hz, 2H), 4.46
84 , 0 difluoropent
phenoxy)acetyl] ESI, RT (s, 2H), 4.10 ¨4.02 (m,
F OjNo, s) +
c, ip H yl)oxy]- amino]piperidin
1,3,4- e-l-carboxylate = 2.20 1H),
3.98 (dd, J= 7.7,
(S4)
3.3 Hz, 1H), 3.30 (dd, J
oxadiazol-2- (Intermediate = 12.0, 3.4 Hz, 1H),
2.62
yllpiperidin- 31) and 4,4- (dd, J= 12.0, 7.6 Hz,
3- difluoropentan-
1H), 2.11 ¨1.89 (m, 8H),
yl]acetamide 1-ol 1.68 ¨ 1.58 (m, 4H).
Scheme for route 46
Boc NN N---"N
ri\i ---Br Flj --Br
ZnBr2 ,N
CI el ojc\sõ,,< DCM, r.t. CI
H Step a H
CI CI
Intermediate 32 OF
HO IF
Step b F
NaH, THE
0 C - r.t.
F
0-----F
N---"N /-----/
`F
0
CI
H
CI
Example 85
153
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Step 46.a:
N- [(3S,6R)-6-(5-bromo-1,3,4-oxadiazol-2-y1)piperidin-3-y1]-2-(3,4-
dichlorophenoxy)acetamide
11-\11
0 (R) 0
CI 01 0j-N,õ.
CI
To a solution of tert-butyl
(2R,5S)-2-(5 -bromo-1,3,4-oxadi azol-2-y1)-5 - [243,4-
dichlorophenoxy)acetamido]piperidine-l-carboxylate (400 mg, 0.712 mmol,
Intermediate 32)
in DCM (10 mL) was added ZnBr2 (642 mg, 2.85 mmol) and the mixture was stirred
at r.t. for
6 h. A further portion of ZnBr2 (642 mg, 2.85 mmol) was added and the mixture
was stirred at
r.t. for 48 h. The reaction mixture was diluted with H20 (50 mL) and extracted
with
DCM/IPA (2:1, 3 x 50 mL). The combined organic extracts were dried over Na2SO4
and
concentrated in vacuo to afford the title compound (80% purity, 269 mg, 0.478
mmol, 67%
yield) as a pale yellow powder; M/Z: 451, 453, 455 [M+H]+, EST, RT = 0.77
(S2).
Example 85 (step 46.b):
2-(3,4-dichlorophenoxy)-N-R3S,6R)-6-15-[2-
(trifluoromethoxy)ethoxy]-1,3,4-oxadiazol-2-yllpiperidin-3-yliacetamide
0--EF
H F
0 - (R)
CI
CI
Example 85
To a solution of 2-(trifluoromethoxy)ethanol (43 L, 0.427 mmol) in anhydrous
THF (3 mL)
at 0 C was added NaH (60%, 17 mg, 0.427 mmol) and the solution was stirred at
r.t. for 5
min.
N-[(3S,6R)-6-(5-bromo-1,3,4-oxadiazol-2-yl)piperidin-3-yll-2-(3,4-
dichlorophenoxy)acetamide (80% purity, 200 mg, 0.355 mmol) was added and the
resultant
mixture was stirred at r.t. for 1 h. The reaction mixture was diluted with H20
(30 mL) and
extracted with Et0Ac (2 x 50 mL). The combined organic extracts were dried
over Na2SO4
and concentrated in vacuo to afford an orange oil. The residue was purified by
chromatography on silica gel (0-100% Et0Ac in heptane), followed by prep. HPLC
(Method
3) to afford the title compound (17 mg, 0.0337 mmol, 10% yield) as a white
powder; NMR
(400 MHz, methanol-d4) 6 7.46 (d, J= 8.9 Hz, 1H), 7.22 (d, J= 2.9 Hz, 1H),
6.98 (dd, J=
8.9, 2.9 Hz, 1H), 4.74 ¨ 4.70 (m, 2H), 4.55 (s, 2H), 4.46 ¨ 4.42 (m, 2H), 4.01
¨ 3.93 (m, 1H),
154
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
3.92 ¨ 3.87 (m, 1H), 3.24 ¨ 3.18 (m, 1H), 2.66 ¨2.57 (m, 1H), 2.19 ¨ 2.06 (m,
2H), 1.91 ¨
1.80 (m, 1H), 1.70¨ 1.58 (m, 1H); M/Z: 499, 501, 503 [M+H]+, EST, RT = 2.30
(S4).
Example compounds in Table 20 were synthesised according to general route 46
as
exemplified by Example 85 using the corresponding intermediates.
Table 20
LCMS
Ex Structure Name Intermediates 111 NMR
data
1H NMR (400 MHz,
tert-butyl
methanol-d4) 6 7.46 (d, J
N-R3S,6R)- (2R,5S)-2-(5-
= 8.9 Hz, 1H), 7.22 (d, J
6-[5-(2- bromo-1,3,4-
= 2.9 Hz, 1H), 6.98 (dd,
cyclopropox oxadiazol-2-y1)- M/Z:
J= 8.9, 2.9 Hz, 1H), 4.62
yethoxY)- 5-[2-(3,4- 471, 473,
¨4.56 (m, 2H), 4.55 (s,
1,3,4- dichlorophenox 475
86 dil-
2tidiM+H]', 2H), 4.01 ¨3.91 (m, 1H),
ei 0j.LNõ. ,) , oxaazo- y)aceamo]pp [
3.91 ¨3.85 (m, 3H), 3.45
01 140 H y312ynpi p- 2e -r (i3A
d in -- c ea rr ibdoi nx ey -1 a1t- e E S I ' , RI
= 2.11 ¨ 3.37 (m, 1H), 3.24 ¨
3.16 (m, 1H), 2.67 ¨2.56
dichlorophen (Intermediate (S4)
(m, 1H), 2.19 ¨2.03 (m,
oxy)acetami 32) and 2-
2H), 1.92¨ 1.77 (m, 1H),
de cyclopropoxyet
1.71 ¨ 1.57 (m, 1H), 0.60
han-l-ol
¨ 0.46 (m, 4H).
tert-butyl 1H NMR (500
MHz,
2-(4-chloro- (2R,55)-2-(5-
DMSO-d6) 6 7.97 (d, J=
3- bromo-1,3,4-
8.1 Hz, 1H), 7.49 (t, J=
fluoropheno oxadiazol-2-y1)-
8.9 Hz, 1H), 7.09 ¨ 7.03
xy)-N- 5-[[2-(4-chloro-
(m, 1H), 6.87 ¨ 6.82 (m,
M/Z:
F [(3S,6R)-6- 3-fluoro-
1H), 4.57 (t, J= 4.4 Hz,
491, 493
{5-[2- [M+H],
(2,2- phenoxy)acetyl] 2H), 4.52 (s, 2H), 4.00 -
H Ni__ 0/ '
87 0 Cy-, 0' difluorocycl amino]piperidin
3.93 (m, 1H), 3.93 ¨3.86
'
opropoxy)et
e-l-carboxylate EST, RI (m, 2H), 3.78 ¨3.72 (m,
= 2.08
c, 411 H hoxy]-1,3,4- (Intermediate
(S4) 1H), 3.72 ¨3.64 (m, 1H),
oxadiazol-2- 31) and 2-(2,2-
3.00 ¨2.93 (m, 1H), 2.82
yllpiperidin- difluorocyclopr ¨2.74
(m, 1H), 2.46 ¨
3- opoxy)ethan-1-
2.38 (m, 1H), 1.98 ¨ 1.85
yl]acetamide ol (Intermediate (m, 2H), 1.75 ¨ 1.43 (m,
40) 4H).
2-(4-chloro- tert-butyl 111 NMR (500
MHz,
3- (2R,55)-2-(5-
chloroform-d) 6 7.33 (t, J
fluoropheno bromo-1,3,4-
= 8.6 Hz, 1H), 6.78 (dd,
xy)-N- oxadiazol-2-y1)- J=
10.3, 2.9 Hz, 1H),
[(3S,6R)-6- 5-[[2-(4-chloro-
M/Z: 6.75 ¨ 6.65 (m, 2H), 4.49
(5- { [2- 3-fluoro- 493,495
¨ 4.42 (m, 3H), 4.31 (dd,
(trifluoromet phenoxy)acetyl] [M+H]', J= 11.2, 7.5 Hz, 1H),
88
64,110N-0/1_, hyl)cyclopro amino]piperidin ESI', RI 4.10 ¨3.96 (m, 2H), 3.29
F F pyl]methoxy e-l-carboxylate = 2.22
(dd, J= 12.0, 3.4 Hz,
c, is H {-1,3,4- (Intermediate (S4)
1H), 2.62 (dd, J= 12.0,
oxadiazol-2- 31) and rac-
7.6 Hz, 1H), 2.13¨ 1.87
yl)piperidin- [(1R,2R)-2-
(m, 4H), 1.85 ¨ 1.77 (m,
3- (trifluoromethyl
1H), 1.73 ¨ 1.66 (m, 1H),
yl]acetamide )cyclopropyl]me
1.64¨ 1.58 (m, 1H), 1.21
155
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
thanol ¨ 1.14 (m, 1H), 0.97 ¨
0.91 (m, 1H); 70:30
mixture of diastereomers.
Scheme for route 47
Boc N-"NHfl Boc Nr-N
0 rs) (R) 0 o rs -0
F 0j'LNõk./ DIPEA F F
DMF, 100 C
CI CI
Intermediate 31 Example 89
Step a
ZnBr2, DCM
Step b r.t.
0
NF
F OANõ,
CI Example 90
Example 89 (step 47.a): tert-butyl (2R,5S)-5-[2-(4-chloro-3-
fluorophenoxy)acetamidol-2-
15-[3-(trifluoromethyl)azetidin-1-y1]-1,3,4-oxadiazol-2-yllpiperidine-1-
carboxylate
Boc NN
I
0 -0
CI Example 89
To a solution of 3-(trifluoromethyl)azetidine hydrochloride (60 mg, 0.371
mmol) in
anhydrous DMF (1 mL) was added DIPEA (0.16 mL, 0.927 mmol) followed by a
solution of
tert-butyl
(2R,5S)-2-(5-bromo-1,3,4-oxadiazol-2-y1)-54 [2-(4-chloro-3-fluoro-
phenoxy)acetyl] amino] piperidine-l-carboxylate (100 mg, 0.185 mmol,
Intermediate 31) in
anhydrous DMF (1 mL) and the mixture was stirred at 100 C for 2 h. The
reaction mixture
was diluted with H20 (10 mL) and extracted with Et0Ac (3 x 10 mL). The
combined organic
extracts were washed with brine (2 x 30 mL), dried over MgSO4, and
concentrated in vacuo
to afford the title compound (87% purity, 117 mg, 0.176 mmol, 95% yield) as a
brown oil;
M/Z: 478, 480 [M+H]+, EST, RT = 1.13 (S2).
156
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Example 90 (Step 47.b): 2-(4-chloro-3-fluorophenoxy)-N-1(3S,6R)-6-{5-13-
(trifluoromethyl)azetidin-1-yl] -1,3,4-oxadiazol-2-yl} pip eridin-3-yl]
aeetamide
11-\11)--NO _______________________
0 (R)NF
=-=
CI
Example 90
To
a solution of tert-butyl (2R,55)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-
{5-[3-
(trifluoromethyl)azetidin-l-yl] -1,3 ,4-oxadiazol-2-ylf piperidine-l-
carboxylate (87% purity,
115 mg, 0.173 mmol) in DCM (2 mL) was added ZnBr2 (117 mg, 0.519 mmol) and the
mixture was stirred at r.t. for 20 h. The reaction mixture was diluted with
satd aq NaHCO3 (3
mL) solution and extracted with DCM:IPA (80:20, 3 x 3 mL). The combined
organic extracts
were dried using a phase separator and concentrated in vacuo. The residue was
purified by
prep. HPLC (Method 4) to afford the title compound (37 mg, 0.0774 mmol, 45%
yield) as a
white powder;
NMR (500 MHz, DMSO-d6) 6 7.96 (d, J= 8.1 Hz, 1H), 7.49 (t, J= 8.9 Hz,
1H), 7.07 (m, 1H), 6.87 ¨ 6.82 (m, 1H), 4.51 (s, 2H), 4.37 ¨ 4.30 (m, 2H),
4.10 ¨ 4.04 (m,
2H), 3.85 ¨ 3.75 (m, 1H), 3.75 ¨ 3.64 (m, 2H), 3.01 ¨2.94 (m, 1H), 2.75 ¨ 2.67
(m, 1H), 2.47
¨2.37 (m, 1H), 1.97 ¨ 1.85 (m, 2H), 1.69 ¨ 1.58 (m, 1H), 1.54 ¨ 1.42 (m, 1H);
M/Z: 480, 482
[M+H]+, EST, RT = 1.97 (S4).
Example compounds in Table 21 were synthesised according to general route 47
as
exemplified by Example 90 using the corresponding intermediates.
Table 21
LCMS
Ex Structure Name Intermediates 111 NMR
data
2-(4-chloro-
3-
tert-butyl 'H NMR (400 MHz,
(2R,5S)-2-(5- chloroform-d) 6
7.33 (t, J
fluoropheno
bromo-1,3,4- = 8.6 Hz,
1H), 6.83 ¨
xy)-N-
oxadiazol-2-y1)-
6.58 (m, 3H), 5.10 (t, J=
[(3S,6R)-6- M/Z:
5-[[2-(4-chloro-
4.7 Hz, 1H), 4.50 ¨4.43
{543-
.0 F (trifluoromet 3-fluoro- 494,496
F
[M+H]', (m, 4H), 4.30
(dd, J =
91
F 0 , phenoxy)acetyl]
hoxy)azetidi ESI', RT 9.8, 4.6 Hz, 2H), 4.10¨
c, n-1-y1]- amino]piperidin
= 2.16
3.93 (m, 2H), 3.32 (dd, J
e-l-carboxylate
= 12.2, 3.4 Hz, 1H), 2.60
1,3,4- (S4)
(Intermediate (dd, J= 12.0,
8.1 Hz,
oxadiazol-2-
31) and 3- 1H),2.08 (d, J = 9.1 Hz,
yl piperidin-
3-
(trifluoromethox
2H), 1.91 (d, J= 8.7 Hz,
y)azetidine 2H), 1.59 (s, 1H).
yl]acetamide
157
CA 03137213 2021-10-18
WO 2020/216766 PC T/EP2020/061150
2-(4-chloro- tert-butyl 5S)-2-(5-
'H NMR (400 MHz,
3- (2R,
methanol-d4) 6 7.44 -
bromo-1,3,4-
fluoropheno
7.37 (m, 1H), 6.96 (dd, J
xy)-N- oxadiazol-2-y1)- 5-[[2-(4-chloro-
M/Z: = 11.0, 2.8 Hz, 1H), 6.85
[(3S,6R)-6- 3-fluoro-
492,494 (ddd, J= 9.0, 2.8, 1.2 Hz,
F F {5-[3-(2,2,2- phenoxy)acetyl]
ty. ESI RI 1] [M+H]', 1H), 4.55 (s, 2H), 4.37 -
92 o' ,IEN,---NiLF trifluoroethy
4.31 (m, 2H), 4.02 -3.91
,
Dazetidin-1- amino]piperidin
H (m,
3H), 3.89 -3.82 (m,
is
y1]-1,3,4- e-l-carboxylate = 2.12
(Intermediate (S4) 1H), 3.25 -3.14 (m, 2H),
ei oxadiazol-2-
2.70 - 2.57 (m, 3H), 2.15
yllpiperidin- 31) and 3-
2- 2, -
2.06 (m, 2H), 1.90 -
3- (2,
trifluoroethyl)az
1.78 (m, 1H), 1.69 - 1.57
yl]acetamide etidine (m, 1H).
tert-butyl
2-(4-chloro- (2R,55)-2-(5-
3- bromo-1,3,4-
'H NMR (400 MHz,
6
fluoropheno oxadiazol-2-y1)-
methanol-d4) 7.35 -
xy)-N- 5-[[2-(4-chloro-
7.23 (m, 1H), 6.84 (dd, J
= 11.0, 2.8 Hz, 1H), 6.73
F--I-[(3S,6R)-6- 3-fluoro-
F {543_ phenoxy)acetyl]
M/Z:
(ddd, J= 8.9, 2.8, 1.2 Hz,
_
methyl-3- amino]piperidin 508,
510 1H), 4.43 (s, 2H), 4.28
[M+H]', (d,
J= 9.1 Hz, 2H), 4.08
93 F (trifluoromet e-l-carboxylate
ci 1.1 H hoxy)azetidi (Intermediate
ESI', RT (d, J= 9.6 Hz 2H), 3.90
n-1-y1]- 31) and 3-
= 2.16 -
3.79 (m, 1H), 3.79-
1,3,4- methyl-3-
(S4)
3.70 (m, 1H), 3.13 -3.05
oxadiazol-2- (trifluoromethox (m,
1H), 2.54 -2.45 (m,
yllpiperidin- y)azetidine
1H), 2.06- 1.93 (m, 2H),
3- hydrochloride
1.78 - 1.68 (m, 4H), 1.57
yl]acetamide (Intermediate - 1.46 (m, 1H).
36)
2-(4-chloro-
tert-butyl
3-
(2R,55)-2-(5- 'H NMR (400 MHz,
fluoropheno
bromo-1,3,4- chloroform-d) 6 7.33 (t, J
xy)-N-
oxadiazol-2-y1)- =
8.6 Hz, 1H), 6.78 (dd,
[(3S,6R)-6-
5-[[2-(4-chloro- J= 10.3, 2.9 Hz, 1H),
M/Z:
3-fluoro- 6.72 - 6.62 (m, 2H), 4.46
496, 498
0-4 (5-
yo",__Nµr-/ F { methyl[2-
phenoxy)acetyl] (s, 2H), 4.21 (t, J= 5.1
[M+H]',
94 amino]piperidin Hz
2H) 4.11 - 3.93 (m,
(trifluoromet
F 0 j(N, e-l-carboxylate ESI', RT
2H'), 3.7'2 (t, J= 5.1 Hz,
C, 0 H hoxy)ethyl]a
mino}-1,3,4- (Intermediate =
2.1 (S4) 2H), 3.33 (dd, J= 12.0,
oxadiazol-2-
31) and 3.5 Hz, 1H), 3.17 (s, 3H),
yl)piperidin-
methyl[2- 2.60 (dd, J= 11.9, 8.1
3-
(trifluoromethox Hz,
1H), 2.15 - 1.86 (m,
yl]acetamide
y)ethyl]amine 4H), 1.64 - 1.57 (m, 1H).
hydrochloride
tert-butyl
2-(4-chloro- (2R,5S)-2-(5-
'H NMR (400 MHz,
3- bromo-1,3,4-
chloroform-d) 6 7.33 (t, J
fluoropheno oxadiazol-2-y1)-
= 8.6 Hz, 1H), 6.78 (dd,
xy)-N- 5-[[2-(4-chloro-
J= 10.3, 2.8 Hz, 1H),
NINI
M/Z:
6.72 - 6.60 (m, 2H), 4.58
k
[(3S,6R)-6- 3-fluoro-
466, 468 -
4.51 (m, 1H), 4.46 (s,
0 (1-, 0'
cyclopropox amino]piperidin
[5-(3- phenoxy)acetyl]
[M+H]', 2H), 4.37 - 4.30 (m, 2H),
0 H yazetidin-1- e-l-
carboxylate ESI', RI 4.12 -3.99 (m, 3H), 3.95
y1)-1,3,4- (Intermediate =
1.96 (dd, J= 8.2, 3.2 Hz, 1H),
01
oxadiazol-2- 31) and 3-
(S4)
3.35 -3.24 (m, 2H), 2.59
yl]piperidin- cyclopropoxyaz (dd,
J= 12.0, 8.0 Hz,
3- etidine
1H), 2.13 - 1.85 (m, 4H),
yl]acetamide hydrochloride
1.62- 1.56 (m, 1H), 0.65
(Intermediate - 0.46 (m, 4H).
158
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
50)
2-(4-chloro- tert-butyl
3- (2R,55)-2-(5-
fluoropheno bromo-1,3,4-
xy)-N- oxadiazol-2-y1)-
[(3S,6R)-6- 5-[[2-(4-chloro- M/Z:
ki Ni...N.,,.......õ,0,1<FF [5_[3_ 3-fluoro-
508, 510
(trifluoromet phenoxy)acetyl] [M+H]-',
96 F
IIIP H hoxy)pyrroli amino]piperidin ESI-', RI
ci din-1-y1]- e-l-carboxylate = 0.70
1,3,4- (Intermediate (S2)
oxadiazol-2- 31) and 3-
yllpiperidin- (trifluoromethox
3- y)pyrrolidine
yflacetamide hydrochloride
Scheme for route 48
Boc 0 Boc 0 Boc
fV)L NH4CI, HATU, N1,24.CN
0 0 R) H2 Et3N, TFAA
rz_...,(R) OH DIPEA
F aii 0.,.}-Thiõ.
DMF, 0 C - r.t.
_____________________________ ' F Oj s)
ilin Ne DCM, 0 C - r.t F la
H H
CI "IP CI gll'illiir Step b CI l
Step a
Intermediate 22
NH2OH.HCI, NaHCO3
Step c
Me0H, r.t.
F 0
F, õ-F
HO-14"a, Fe Boc NH
Boc NH Ircr.0 0 F OH
Intermediate 54 0
Ojc . ' H .. HATU, Et3N F gh 0..õõ.11,,Nõ,(YLNIs)
F am õ, 0
DMF, r.t. H
CI "II
H
CI "IP Step d
pyridine
115 C Step e
1
Boc N H H-C)
0
0 (........, R)
)\--F 2nBr2 F
Ojciõ, s)
F F F Cl. Thiõ..
FE _... 0
H
DCM, r.t.
Cl
Example 97 Step f Example 98
Step 48.a: tert-butyl (2R,5S)-2-carbamoy1-5-[2-
(4-chloro-3-
fluorophenoxy)acetamido]piperidine-l-carboxylate
iiloc 0
0
NJ`
H
CI
To a solution of (2R,5S)-1-Rtert-butoxy)carbonyll-5-[2-(4-chloro-3-
fluorophenoxy)
acetamido]piperidine-2-carboxylic acid (1.86 g, 4.32 mmol, Intermediate 22),
NH4C1 (253
mg, 4.73 mmol) and DIPEA (3.1 mL, 17.7 mmol) in anhydrous DMF (22 mL) at 0 C
was
added HATU (1.80 g, 4.73 mmol) and the mixture was stirred at r.t. for 4 h.
The reaction
159
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
mixture was diluted with Et0Ac (100 mL) and washed with brine (3 x 50 mL). The
combined
organic extracts were dried over MgSO4, and concentrated in vacuo to afford
the title
compound (80% purity, 2.36 g, 4.39 mmol) in quantitative yield as colourless
crystals; 1H
NMR (500 MHz, DMSO-d6) 6 7.99 - 7.93 (m, 1H), 7.47 (t, J = 8.9 Hz, 1H), 7.32
(s, 1H),
7.08 -6.99 (m, 2H), 6.81 (dd, J= 8.9, 1.9 Hz, 1H), 4.63 -4.37 (m, 3H), 3.97 -
3.74 (m, 2H),
3.27 - 3.08 (m, 1H), 1.98 - 1.81 (m, 2H), 1.60 - 1.47 (m, 2H), 1.36 (s, 9H);
M/Z: 330, 332
[M+H]+, EST, RT = 0.88 (S2).
Step 48.b: tert-butyl
(2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-
cyanopiperidine-1-carboxylate
Boc
I
N CN
0 ...0
(R)
F 0j-Nõ,, ==
H
CI
To a solution of
tert-butyl (2R,55)-2-carbamoy1-5-[2-(4-chloro-3-
fluorophenoxy)acetamido]piperidine-l-carboxylate (80% purity, 2.17 g, 4.04
mmol) and Et3N
(2.6 mL, 18.7 mmol) in anhydrous DCM (40 mL) at 0 C was added TFAA (1.2 mL,
8.27
mmol) and the solution was stirred at r.t. for 2 h. The reaction mixture was
cooled to 0 C and
quenched with H20 (20 mL). The solution was diluted with DCM (50 mL) and
washed with
satd aq NaHCO3 solution (20 mL). The organic layer was dried using a phase
separator,
concentrated in vacuo, and purified by chromatography on silica gel (0-50%
Et0Ac in
heptane) to afford the title compound (0.94 g, 2.22 mmol, 55% yield) as a
white powder; 1H
NMR (500 MHz, DMSO-d6) 6 8.06 (d, J= 6.9 Hz, 1H), 7.47 (t, J= 8.9 Hz, 1H),
7.02 (dd, J=
11.4, 2.8 Hz, 1H), 6.80 (dd, J= 8.9, 2.0 Hz, 1H), 5.37 - 5.27 (m, 1H), 4.61 -
4.50 (m, 2H),
4.03 - 3.90 (m, 2H), 3.07 - 2.92 (m, 1H), 2.17 - 2.04 (m, 1H), 1.84 - 1.68 (m,
3H), 1.39 (s,
9H); M/Z: 429, 431 [M+NH4]+, EST, RT = 3.69 (S6).
Step 48.c: tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-(N-
hydroxycarbamimidoyl)piperidine-1-carboxylate
Boc NH
N N.,,,...A OH
(R)
F 0J- 0
H
CI
160
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
To a solution of tert-butyl (2R,55)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-
2-
cyanopiperidine-1-carboxylate (500 mg, 1.21 mmol) in Me0H (6 mL) at 0 C was
added
hydroxylamine hydrochloride (1:1) (125 mg, 1.80 mmol) and NaHCO3 (225 mg, 2.68
mmol)
and the reaction mixture was stirred at r.t. for 40 h. The resulting
suspension was filtered
under vacuum, washing with Me0H, and the filtrate was concentrated in vacuo to
afford the
title compound (69% purity, 620 mg, 0.962 mmol, 79% yield) as a white powder;
1H NMR
(500 MHz, DMSO-d6) 6 8.01 ¨7.90 (m, 1H), 7.48 (t, J= 8.9 Hz, 1H), 7.05 (dd, J=
11.3, 2.9
Hz, 1H), 6.82 (ddd, J= 9.0, 2.8, 0.9 Hz, 1H), 5.22 (s, 1H), 4.67 (s, 1H), 4.62
¨4.51 (m, 2H),
3.95 ¨ 3.78 (m, 2H), 3.19 ¨ 3.11 (m, 1H), 2.00 ¨ 1.82 (m, 2H), 1.75 (d, J =
16.9 Hz, 1H), 1.59
¨ 1.47 (m, 1H), 1.37 (s, 9H); M/Z: 345, 347 [M-Boc+H]+, EST, RT = 0.78 (S2).
Step 48.d: tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-{N-
[(1s,3s)-3-
(trifluoromethoxy)cyclobutanecarbonyloxy] carb amimidoyl} pip eridine-1-carb
oxylate
F F
Boc NH 0
(R) N,Cyff*
õ. 0
To a solution of (1s,3s)-3-(trifluoromethoxy)cyclobutane-l-carboxylic acid (90
mg, 0.489
mmol, Intermediate 54) in anhydrous DMF (2.5 mL) was added Et3N (202 pL, 1.45
mmol)
followed by HATU (200 mg, 0.526 mmol) and stirred at r.t. for 10 min. tert-
butyl (2R,5S)-5-
[2-(4-chloro-3-fluorophenoxy)acetamido] -2-(N-hydroxycarbamimidoyDpiperidine-1-
carboxylate (69% purity, 310 mg, 0.481 mmol) was added and the resultant
mixture was
stirred at r.t. for 17 h. The reaction mixture was diluted with Et0Ac (30 mL)
and washed with
.. brine (3 x 20 mL). The combined organic extracts were dried over MgSO4, and
concentrated
in vacuo to afford the title compound (59% purity, 367 mg, 0.354 mmol, 74%
yield) as an
orange oil; M/Z: 511, 513 [M-Boc+H], EST, RT = 1.12 (S2).
Example 97 (step 48.e): tert-butyl (2R,5S)-542-(4-chloro-3-
fluorophenoxy)acetamido]-2-
15-[(1s,3s)-3-(trifluoromethoxy)eyelobuty1]-1,2,4-oxadiazol-3-yllpiperidine-1-
earboxylate
Boc
0
Example 97
161
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
A solution of tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-2-
{N-[(1s,3s)-3-
(tri fluoromethoxy)cyclobutanecarbonyloxy] carbamimidoyllpiperidine-l-carboxyl
ate (59%
purity, 367 mg, 0.354 mmol) in pyridine (3.5 mL) was stirred at 115 C for 17
h. The reaction
mixture was concentrated in vacuo and purified by chromatography on silica gel
(0-100%
Et0Ac in heptane) to afford the title compound (91% purity, 172 mg, 0.264
mmol, 74%
yield) as a colourless oil; M/Z: 593, 595 [M+H]+, EST, RT = 1.20 (S2).
Example 98 (Step 48.1): 2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-{5-[(1s,3s)-
3-
(trifluoromethoxy)cyclobuty1]-1,2,4-oxadiazol-3-yllpiperidin-3-yflacetamide
H Nr
/ 0
0 (R) N
F
CI
Example 98
To a solution of tert-butyl (2R,5S)-5-[2-(4-chloro-3-fluorophenoxy)acetamido]-
2- {5-[(1s,3s)-
3 -(trifluoromethoxy)cyclobutyll -1,2,4-oxadi azol-3 -yllpiperidine-l-carboxyl
ate (91% purity,
152 mg, 0.233 mmol, Example 97) in anhydrous DCM (1.5 mL) was added ZnBr2 (210
mg,
0.924 mmol) and the resultant mixture was stirred at r.t. under N2 for 17 h.
The reaction
mixture was diluted with satd aq NaHCO3 solution (20 mL) and extracted with
DCM/IPA
80:20 (3 x 50 mL). The combined organic extracts were dried using a phase
separator,
concentrated in vacuo and purified by prep. HPLC (Method 4) to afford the
title compound
(68 mg, 0.138 mmol, 59% yield) as a white solid; 1H NMR (400 MHz, chloroform-
d) 6 7.33
(t, J= 8.6 Hz, 1H), 6.78 (dd, J= 10.3, 2.8 Hz, 1H), 6.70 (ddd, J= 8.9, 2.8,
1.2 Hz, 1H), 6.60 ¨
6.53 (m, 1H), 4.71 (p, J= 7.6 Hz, 1H), 4.46 (s, 2H), 4.10 ¨ 4.00 (m, 1H), 3.94
(dd, J= 9.1, 3.0
Hz, 1H), 3.40 ¨ 3.28 (m, 2H), 2.94 ¨ 2.85 (m, 2H), 2.78 ¨ 2.66 (m, 2H), 2.64 ¨
2.57 (m, 1H),
2.18 ¨2.01 (m, 3H), 1.94 ¨ 1.82 (m, 1H), 1.63 ¨ 1.57 (m, 1H); M/Z: 493, 495
[M+H]+, EST,
RT = 2.32 (S4).
162
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
Scheme for route 49
0 Boc 0
Isobutyl chloroformate Boc 0
F0J-L NMM 0r\ii/11L0
OH + (R) 0
CIN H2N"s4¨< THE, 0 C - r.t.
Intermediate 12 Intermediate 3 Step a
CI N
Li0H.H20,
Step b Me0H, THE,
H20, r.t.
CI 0
H2I\LN 01 CI
CI Boc 0
Boc 0
II H ii I N CI
0 N OH
0
Isobutyl chloroformate
- (R)
NMM
F0J-1\ 0
THF, 0 C - r.t
CIN
Step c
TsCI, K2CO3
Step d
MeCN, 80 C
ci CI
Boc
\
0
CI ZnBr2 NCI
0
F0J-L
N" DCM, r.t.
Step e CIN
Example 99 Example
100
Step 49.a: 1-tert-butyl
2-ethyl (2R,5S)-5-{2-[(6-chloro-5-fluoropyridin-3-
yl)oxy] acetamido} pip eridine-1,2-dic arboxylate
Boc 0
0 -
To a solution of 2-[(6-chloro-5-fluoropyridin-3-ypoxy]acetic acid (400 mg,
1.95 mmol,
Intermediate 12) in anhydrous THF (20 mL) at 0 C was added isobutyl
chloroformate (0.24
mL, 1.85 mmol) followed by NMM (0.21 mL, 1.95 mmol). The mixture was stirred
for 15
min before 1-tert-butyl 2-ethyl (2R,5S)-5-aminopiperidine-1,2-dicarboxylate
(530 mg, 1.95
mmol, Intermediate 3) was added and the resultant mixture was stirred at r.t.
for 1 h. The
reaction mixture was cooled to 0 C, quenched with H20 (0.5 mL) and
concentrated in vacuo.
The residue was partitioned between Et0Ac (10 mL) and H20 (10 mL). The organic
layer
was isolated, washed with satd aq NaHCO3 solution (10 mL) and brine (10 mL),
dried over
Na2SO4, and concentrated in vacuo to afford the title compound (90% purity,
805 mg, 1.58
mmol, 81% yield) as an off-white solid; Ifl NMR (400 MHz, chloroform-d) 6 7.98
(d, J= 2.6
163
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Hz, 1H), 7.12 (dd, J= 8.8, 2.6 Hz, 1H), 6.73 (d, J= 43.3 Hz, 1H), 4.52 (s,
2H), 4.28 ¨4.16
(m, 3H), 4.10 ¨ 3.86 (m, 1H), 3.24 (dd, J= 39.3, 13.1 Hz, 1H), 2.15 (d, J=
15.8 Hz, 1H), 2.01
¨ 1.73 (m, 2H), 1.63 ¨ 1.51 (m, 2H), 1.45 (s, 9H), 1.32 ¨ 1.21 (m, 3H).
Step 49.b: (2R,5S)-1-1(tert-butoxy)carbony1]-5-{2-[(6-chloro-5-fluoropyridin-3-
yl)oxyjacetamido}piperidine-2-carboxylic acid
Boc 0
/N)1
0 (R) OH
F
CIN
A solution of 1-tert-butyl
2-ethyl (2R,55)-5- {2- [(6-chloro-5-fluoropyridin-3 -
ypoxy]acetamido{piperidine-1,2-dicarboxylate (90% purity, 805 mg, 1.58 mmol)
and
Li0H.H20 (81 mg, 1.89 mmol) in THF (2.5 mL)/Me0H (2.5 mL)/H20 (2.5 mL) was
stirred
at r.t. for 6 h. The reaction mixture was concentrated in vacuo and the
residue was partitioned
between Et0Ac (20 mL) and H20 (20 mL). The layers were separated and the
organic layer
discarded. The aqueous layer was cooled to 0 C and acidified to pH 2/3 using
1 M aq HC1
solution. The resultant solution was extracted with Et0Ac (2 x 25 mL) and the
combined
organic extracts were washed with H20 (30 mL), dried over Na2SO4, and
concentrated in
vacuo to afford the title compound (90% purity, 570 mg, 1.19 mmol, 75% yield)
as a white
solid; 1H NMR (400 MHz, DMSO-d6) 6 8.12 (d, J= 6.9 Hz, 1H), 8.03 (d, J= 2.6
Hz, 1H),
7.66 (dd, J= 10.3, 2.3 Hz, 1H), 4.75 ¨4.44 (m, 3H), 3.99 ¨ 3.71 (m, 2H), 3.15
¨2.90 (m,
2H), 2.08 ¨ 1.84 (m, 2H), 1.67¨ 1.55 (m, 1H), 1.54¨ 1.41 (m, 1H), 1.37 (s,
9H).
Step 49.c: tert-butyl (2R,5S)-5-{2-[(6-chloro-5-fluoropyridin-3-
yl)oxyjacetamido}-2-[N'-
(3,4-dichlorobenzoyl)hydrazinecarbonyl]piperidine-1-carboxylate
CI
CI
Boc
0 H
II H 0
F
CI N
To a solution of (2R,5S)-1-Rtert-butoxy)carbonyll-5-{2-[(6-chloro-5-
fluoropyridin-3-
ypoxy]acetamido{piperidine-2-carboxylic acid (90% purity, 530 mg, 1.10 mmol)
in
164
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
anhydrous THF (11 mL) at 0 C was added isobutyl chloroformate (0.14 mL, 1.05
mmol)
followed by NMM (0.12 mL, 1.10 mmol). The mixture was stirred for 15 min
before 3,4-
dichlorobenzohydrazide (226 mg, 1.10 mmol) was added and the resultant mixture
was stirred
at r.t. 1 h. The reaction mixture was cooled to 0 C, quenched with H20 (0.5
mL) and
concentrated in vacuo. The residue was partitioned between Et0Ac (15 mL) and
H20 (15
mL). The organic layer was isolated, washed with satd aq NaHCO3 solution (10
mL) and
brine (10 mL), dried over Na2SO4, and concentrated in vacuo to afford the
title compound
(90% purity, 626 mg, 0.910 mmol, 82% yield) as an off-white solid; 1H NMR (400
MHz,
chloroform-d) 6 8.68 (s, 2H), 8.00 (d, J= 2.6 Hz, 1H), 7.92 (d, J= 1.8 Hz,
1H), 7.66 - 7.59
(m, 1H), 7.56 - 7.50 (m, 1H), 7.13 (dd, J= 8.8, 2.6 Hz, 1H), 5.00 -4.82 (m,
1H), 4.58 - 4.46
(m, 2H), 4.28 -4.08 (m, 2H), 3.33 (d, J= 12.5 Hz, 1H), 2.25 -2.16 (m, 1H),
2.09 - 1.84 (m,
2H), 1.83 - 1.71 (m, 1H), 1.54 - 1.44 (m, 10H).
Example 99 (step 49.d): tert-butyl (2R,5S)-5-{2-[(6-chloro-5-fluoropyridin-3-
yl)oxyjacetamido}-2-[5-(3,4-dichloropheny1)-1,3,4-oxadiazol-2-ylipiperidine-1-
carboxylate
CI
Boc N-
o
FOr\i,õ. -=<
I H
CIN
Example 99
A suspension of tert-butyl (2R,55)-5- {2- [(6-chloro-5-fluoropyridin-3 -
yl)oxy] acetamido}-2-
[N-(3,4-dichlorobenzoyphydrazinecarbonyl]piperidine-l-carboxylate (601 mg,
0.971 mmol),
TsC1 (555 mg, 2.91 mmol) and K2CO3 (805 mg, 5.83 mmol) in anhydrous ACN (10
mL) was
stirred at 80 C for 4 h. The reaction mixture was cooled to r.t. and
partitioned between
Et0Ac (20 mL) and H20 (20 mL). The layers were separated and the aqueous layer
extracted
further with Et0Ac (10 mL). The combined organic extracts were washed with
satd aq
NaHCO3 solution (5 x 20 mL) and brine (20 mL), dried over Na2SO4, and
concentrated in
vacuo. The residue was purified by chromatography on silica gel (40-90% Et0Ac
in heptane)
to afford the title compound (310 mg, 0.490 mmol, 50% yield) as a off-white
solid; 1H NMR
(500 MHz, chloroform-d) 6 8.10 (d, J= 2.0 Hz, 1H), 8.01 (d, J= 2.6 Hz, 1H),
7.87 (dd, J=
8.4, 2.0 Hz, 1H), 7.60 (d, J= 8.4 Hz, 1H), 7.14 (dd, J= 8.8, 2.6 Hz, 1H), 6.77
(s, 1H), 5.93 -
5.44 (m, 1H), 4.62 - 4.48 (m, 2H), 4.29 - 4.19 (m, 1H), 4.17 - 4.02 (m, 1H),
3.43 - 2.98 (m,
1H), 2.38 -2.23 (m, 1H), 2.18 - 1.91 (m, 3H), 1.50 (s, 9H).
165
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Example 100 (step 49.e): 2-[(6-chloro-5-fluoropyridin-3-yl)oxy] -N- [(3S,6R)-6-
[5-(3,4-
dichloropheny1)-1,3,4-oxadiazol-2-yl] pip eridin-3-yl] acetamide
CI
N¨
\
0 (R) 0
F
Example 100
To a solution of tert-butyl (2R,5S)-5- {2-[(6-chloro-5-fluoropyridin-3-
ypoxy]acetamidol-2-[5-
(3 ,4-dichloropheny1)-1,3 piperidine-1 -carboxylate (150 mg, 0.250 mmol,
Example 99) in DCM (3 mL) was added ZnBr2 (225 mg, 0.999 mmol) and the
resultant
mixture was stirred at r.t. for 18 h. The reaction mixture was partitioned
between satd aq
NaHCO3 solution (2 mL) and DCM/IPA (4:1, 2 mL) and the layers were separated
using a
phase separator. The organic layer was concentrated in vacuo and purified by
prep. HPLC
(Method 4) to afford the title compound (28 mg, 0.0554 mmol, 22% yield) as an
off-white
powder;
NMR (500 MHz, chloroform-d) 6 8.17 (d, J= 2.0 Hz, 1H), 8.05 (d, J= 2.6 Hz,
1H), 7.93 (dd, J= 8.4, 2.0 Hz, 1H), 7.63 (d, J = 8.4 Hz, 1H), 7.18 (dd, J=
8.8, 2.6 Hz, 1H),
6.73 (d, J= 7.8 Hz, 1H), 4.57 (s, 2H), 4.27 ¨4.21 (m, 1H), 4.20 ¨4.09 (m, 1H),
3.41 (dd, J=
12.1, 3.3 Hz, 1H), 2.73 (dd, J= 12.1, 7.7 Hz, 1H), 2.29 ¨ 2.20 (m, 1H), 2.20 ¨
2.04 (m, 3H),
1.78 ¨ 1.67 (m, 1H); M/Z: 500, 502, 504, 506 [M+H]+, EST, RT = 2.18 (S4).
Scheme for route 50
F1\11j1,1---0/ F Mel, K2CO3 ,N
I
0 (R) 0 0 R) 0
F io 0,)L
DMF, r t
Cl Cl
Example 77 Example 101
Example 101:
2-(4-chloro-3-fluorophenoxy)-N-1(3S,6R)-1-methyl-6-15-12-
(trifluoromethoxy)ethoxy] -1,3,4-oxadiazol-2-yll pip eridin-3-yl] acetamide
0 (s) 0
Cl
Example 101
166
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
To a solution of 2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6-{5-[2-
(trifluoromethoxy)ethoxy]-
1,3,4-oxadiazol-2-ylfpiperidin-3-yllacetamide (100 mg, 0.197 mmol, Example 77)
and
K2CO3 (57 mg, 0.413 mmol) in DMF (2 mL) was added Mel (134 !IL, 2.16 mmol) and
the
mixture was stirred at r.t. for 5 days. The reaction mixture was quenched with
33% aq
NH4OH solution (1 mL) and stirred for 30 min. The solution was diluted with
H20 (30 mL),
extracted with Et0Ac (2 x 30 mL), and the combined organic extracts were dried
over
Na2SO4, and concentrated in vacuo. Purification by prep. HPLC (Method 5)
afforded the title
compound (36 mg, 0.0725 mmol, 37% yield) as a white powder; 1H NMR (400 MHz,
DMSO-d6) 6 8.05 (d, J= 8.0 Hz, 1H), 7.49 (t, J= 8.9 Hz, 1H), 7.07 (dd, J=
11.4, 2.8 Hz,
1H), 6.89 ¨ 6.81 (m, 1H), 4.72 ¨ 4.63 (m, 2H), 4.53 (s, 2H), 4.49 ¨ 4.42 (m,
2H), 3.91 ¨ 3.80
(m, 1H), 3.29 ¨ 3.20 (m, 1H), 2.96 ¨2.88 (m, 1H), 2.12 ¨ 1.95 (m, 4H), 1.92 ¨
1.80 (m, 2H),
1.78 ¨ 1.67 (m, 1H), 1.49 ¨ 1.35 (m, 1H); M/Z: 497, 499 [M+H], EST, RT = 2.75
(S4).
Scheme for route 51
H
0
F 0A
CI
Example 87
Chiral Separation
F F
H H Nin
0 - (R) 0 N'e9
F F
CI "IP CI "IP
stereochemistry arbitrarily assigned as 1R stereochemistiy arbitrarily
assigned as 1S
Example 102 Example 103
Example 102 and 103 Chiral separation of 2-(4-chloro-3-fluorophenoxy)-N-
[(3S,6R)-6-
15- [2- (2,2- difluor o cyclopr op oxy) ethoxy]
piperidin-3-yl] acetamide
(Example 87)
2-(4-chloro-3-fluorophenoxy)-N-[(3S,6R)-6- {5- [2-(2,2-
difluorocyclopropoxy)ethoxy] -1,3,4-
oxadiazol-2-yllpiperidin-3-yllacetamide (48 mg, 0.0978 mmol, Example 87) was
subjected to
chiral purification using Method C4, affording 2-(4-chloro-3-fluorophenoxy)-N-
[(3S,6R)-6-
(5- {2- [(1R)-2,2-di fluorocyclopropoxy] ethoxy } -1,3 ,4-oxadiazol-2-
yl)piperidin-3 -yl] acetamide
167
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
(98% chiral purity, 13.3 mg, 0.0268 mmol, 27% yield) and 2-(4-chloro-3-
fluorophenoxy)-N-
[(3S,6R)-6-(5- {2-[(1S)-2,2-difluorocyclopropoxy]ethoxy}-1,3,4-oxadiazol-2-
yl)piperidin-3-
yl]acetamide (100% chiral purity, 12.4 mg, 0.025 mmol), 26% yield) as white
powders. The
stereochemistry of each compound was arbitrarily assigned.
Example compounds in Table 22 were chirally purified according to the general
route 51 as
exemplified by Example 102 and 103, using the corresponding intermediates and
methods.
Table 22
Ex Structure Name Intermediate LCMS
111 NMR
and Method data
2-(4-chloro-3-
1H NMR (500 MHz,
fluorophenoxy
2-(4-chloro-3- DMSO-d6) 6 7.96 (d, J=
)-N-R3 S,6R)-
fluorophenoxy
8.1 Hz, 1H), 7.49 (t, J=
6-(5- {2-R1R)-
2,2-
)-N-R3S,6R)- 8.9 Hz, 1H), 7.10 ¨ 7.02
6-{5-[2-(2,2- M/Z: (m, 1H), 6.88 ¨6.81 (m,
,} rdoiflpouxoyro]ecLlopxy
difluorocyclop 491, 493
1H), 4.57 (t, J= 4.3 Hz,
11
102 * {-1,3,4- ropoxy)ethoxy [M+H]-',
2H), 4.52 (s, 2H), 4.01 ¨
CI
oxadiazol-2- ]-1,3,4-
ESI, RT 3.85 (m, 3H), 3.78 ¨3.64
oxadiazol-2- = 2.13 (m, 2H), 3.00 ¨ 2.93 (in,
yl)piperidin-3-
yl{piperidin- (S4) 1H), 2.81 ¨2.74 (m, 1H),
yl]acetamide
3-yl]acetamide
2.46 ¨2.38 (m, 1H), 2.00
(stereochemist
(Example 87) ¨ 1.84 (m, 2H), 1.76 ¨
ry arbitrarily
(Method C4) 1.59 (m, 2H), 1.59 ¨ 1.43
assigned as
(m, 2H).
1R)
2-(4-chloro-3-
1H NMR (500 MHz,
fluorophenoxy
2-(4-chloro-3- DMSO-d6) 6 7.96 (d, J=
)-N-R3 S,6R)-
fluorophenoxy
8.1 Hz, 1H), 7.49 (t, J=
6-(5-12-[(1S)-
2,2-
)-N-R3S,6R)- 8.9 Hz, 1H), 7.10 ¨7.03
6-{5-[2-(2,2- M/Z: (m, 1H), 6.88 ¨6.80 (m,
difluorocyclop
difluorocyclop 491, 493
1H), 4.57 (t, J= 4.4 Hz,
ropoxy]ethoxy
103 0 FN(1?,-- // N } -1,3,4-
ropoxy)ethoxy [M+H]-', 2H), 4.52 (s, 2H), 4.01 ¨
]-1,3,4- ESI, RT 3.85 (m, 3H), 3.79 ¨3.64
F ir6 NAN,
oxadiazol-2-
oxadiazol-2- = 2.13 (m, 2H), 3.01 ¨2.92 (m,
CI
stereochemistry erbIriIy assigned es 15 yl)piperidin-3-
yl}piperidin- (S4) 1H), 2.82 ¨2.74 (m, 1H),
yl]acetamide
3-yl]acetamide
2.47 ¨ 2.38 (m, 1H), 1.99
(stereochemist
(Example 87) ¨ 1.83 (m, 2H), 1.77 ¨
ry arbitrarily
(Method C4) 1.58 (m, 2H), 1.61 ¨ 1.43
assigned as
(m, 2H).
1S)
2-(4-chloro-3- 2-(4-chloro-3- 'H NMR (500 MHz,
fluorophenoxy fluorophenoxy
methanol-d4) 6 7.32 ¨
)-N-R3S,6R)- )-N-R3S,6R)-
7.25 (m, 1H), 6.85 (dd, J
N¨N 6-15-[(1r,30- 6-[5-(3-
= 11.0, 2.8 Hz, 1H), 6.74
rk11)0¨"0"'"D 3- cyclopropoxyc
(ddd, J= 8.9, 2.8, 1.2 Hz,
cyclopropoxyc yclobuty1)-
1H), 4.44 (s, 2H), 4.35¨
104 F C))Nsµ'
yclobuty1]- 1,3,4-
4.26 (m, 1H), 3.92 ¨3.82
CI 41.1V 1,3,4- oxadiazol-2- (m, 2H),
3.61 ¨3.52 (m,
stereochemistry arbitrarily assigned as lrand 3r
oxadiazol-2- yl]piperidin-3-
1H), 3.19 ¨3.17 (m, 1H),
yl{piperidin- yl]acetamide
3.14 ¨3.08 (m, 1H), 2.59
3-yl]acetamide (Example 59) ¨ 2.48 (m, 3H), 2.47 ¨
(stereochemist (Method C3)
2.36 (m, 2H), 2.11 ¨2.04
168
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
ry arbitrarily (m,
1H), 2.03 ¨ 1.96 (m,
assigned as 1 r
1H), 1.81 ¨ 1.72 (m, 1H),
and 3r) 1.60¨ 1.51 (m, 1H), 0.46
¨0.39 (m, 2H), 0.39 ¨
0.32 (m, 2H).
'H NMR (500 MHz,
2-(4-chloro-3- methanol-d4) 6 7.32
¨
fluorophenoxy 7.25 (m, 1H), 6.85 (dd, J
2-(4-chloro-3-
)-N-R3S,6R)- = 11.0, 2.8 Hz, 1H), 6.74
fluorophenoxy
6-{5-[(1s,3s)- (ddd, J= 8.9, 2.8, 1.2 Hz,
)-N-R3 S,6R)-
3-
1H), 4.43 (s, 2H), 4.13 ¨
M/Z:
H NINµ 0 cyclopropoxyc 6-[5-(3-
4.04 (m, 1H), 3.91 ¨3.81
cyclopropoxyc 465, 467
yclobuty1]- yclobuty1)- [M+H], (m,
2H), 3.28 ¨3.22 (m,
'
0 N
105 ' e j1,1`0"L 1,3,4-
2H), 3.13 ¨3.08 (m, 1H),
ci
H oxadiazol-2-
1,3,4- ESI-', RT
IF
oxadiazol-2- = 2.08
2.69 ¨2.61 (m' 2H)' 2.56
stereochemistry arbitrarily assigned as ls and 3s
yl{piperidin- ¨2.48 (m, 1H), 2.26 ¨
yl]piperidin-3- (S4).
3-yl]acetamide 2.18 (m, 2H), 2.10 ¨2.03
yflacetamide
(stereochemist (m, 1H), 2.02 ¨ 1.95 (m,
(Example 59)
ry arbitrarily 1H), 1.80 ¨ 1.70 (m, 1H),
(Method C3)
assigned as is
1.59 ¨ 1.50 (m, 1H), 0.46
and 3s) ¨ 0.39 (m, 2H), 0.39 ¨
0.33 (m, 2H).
(2R)-2-(4-
chlorophenoxy 'H NMR (500 MHz,
)-N-R 2-(4-
3S,6R)- DMSO-d6) 6 8.26 ¨ 8.16
chlorophenoxy
6-1544- (m, 2H), 8.04 ¨7.93 (m,
)-N-R3S,6R)-
(trifluorometh
6-{5-[4-
3H), 7.37 ¨ 7.29 (m, 2H),
yl)pheny1]- M/Z: 6.97 ¨ 6.88 (m, 2H), 4.69
(trifluorometh
N-N 1,3,4- 495, 497 (q,
J= 6.6 Hz, 1H), 4.03
106
0 ("11)-o\ IIP FF oxadiazol-2- Yl)phenyl]-
[WPM+, ¨ 3.95 (m, 1H), 3.73 ¨
F
al r, ,2 yl{piperidin-
1,3,4-
oxadiazol-2-
ESL', RI 3.64 (m, 1H), 2.98 ¨2.92
a 11=P 3- = 2.49 (m,
1H), 2.44 ¨2.37 (m,
yl{piperidin-
stereochemistry arbitrarily assigned as 2R yl]propanamid 3_
(S4) 1H), 2.14 ¨ 2.06 (m, 1H),
e 1.96¨ 1.89 (m, 1H), 1.81
yl]propanamid
(stereochemist ¨1.72 (m, 1H), 1.60 ¨
e (Example 4)
ry arbitrarily 1.50 (m, 1H), 1.43 (d, J=
(Method C5)
assigned as 6.6 Hz, 3H).
2R)
(25)-244-
chlorophenoxy
2-(4- 'H NMR (500 MHz,
)-N-R3S,6R)-
chlorophenoxy DMSO-d6) 6 8.25 ¨8.18
6-1544-
)-N-R3S,6R)- (m, 2H), 8.04 ¨7.94 (m,
(trifluorometh
6-{544-
3H), 7.37 ¨7.29 (m, 2H),
yl)pheny1]- M/Z:
(trifluorometh 6.96 ¨6.89 (m, 2H), 4.68
H N,¨ N\ Aii F 1,3,4- 495, 497
(y-o lir F F oxadiazol-2- yl)pheny1]- [m+H]+, (q,
J= 6.6 Hz, 1H), 4.02
yl{piperidin- ESL, RI 107 0,,. s,
1,3,4- ¨3.96 (m, 1H), 3.72 ¨
100 ' r H oxadiazol-2-
3.63 (m, 1H), 3.03 ¨2.92
a 3- = 2.51
yl{piperidin- (S4) (m, 2H), 2.08 ¨2.01 (m,
stereochemistry arbitrarily assigned as 2S yl]propanamid
3-
1H), 1.89 ¨ 1.81 (m, 1H),
e
yl]propanamid 1.80¨ 1.70 (m, 1H), 1.53
(stereochemist
e (Example 4) ¨ 1.45 (m, 1H), 1.43 (d, J
ry arbitrarily
(Method C5) = 6.6 Hz, 3H).
assigned as
23)
2-(4-chloro-3- Rac-2-(4- 'H NMR (500 MHz,
M/Z:
H NIN\ AL\ fluorophenoxy
chloro-3- DMSO-d6) 6 8.42 (d, J=
479.2
µ11-r ' )-N-R3S,6R)-
fluorophenoxy8.2 Hz, 1H), 8.27 (s, 1H),
108 F ab Oics, si
ty 6-[5-(4- [M+11]+,
)-N-[6-[5-(4-
H +
8.08 ¨ 8.03 (m 2H), 7.72
Cl 111W
chloropheny1)- chloropheny1)- ESI , RI ¨ 7.67 (m, 2H)', 7.51 (t, J
= 3.18
stereochemistry arbitrarily assigned as 38 and 6R
1,3,4- 1,3,4- = 8.9 Hz, 1H), 7.11 (dd,
oxadiazol-2- oxadiazol-2- (S4) J= 11.4, 2.8
Hz, 1H),
169
CA 03137213 2021-10-18
WO 2020/216766 PCT/EP2020/061150
y1]-2- y1]-2- 6.89 (ddd, J= 9.0,
2.8,
oxopiperidin- oxopiperidin- 1.1 Hz, 1H), 4.97
(dd, J
3-yl]acetamide 3-yl]acetamide = 9.5, 4.6 Hz, 1H),
4.63
(stereochemist (Example 37) ¨ 4.55 (m, 2H),
4.37
ry arbitrarily (Method C6) (ddd, J = 11.0,
8.2, 6.1
assigned as 3S Hz,
1H), 2.33 ¨ 2.27 (m,
and 6R)
1H), 2.19 ¨2.10 (m, 1H),
2.09 ¨2.04 (m, 1H), 2.00
¨ 1.92 (m, 1H).
1H NMR (500 MHz,
DMSO-d6) 6 8.42 (d, J=
2-(4-chloro-3-
8.2 Hz, 1H), 8.27 (s, 1H),
fluorophenoxy Rac-2-(4-
8.10 ¨ 8.01 (m, 2H), 7.72
)-N-R3R,6S)- chloro-3-
¨ 7.67 (m, 2H), 7.51 (t, J
6-[5-(4- fluorophenoxy
= 8.9 Hz, 1H), 7.11 (dd,
chloropheny1)- )-N-[6-[5-(4- M/Z:
J= 11.4, 2.8 Hz, 1H),
H N-N 1,3,4- chloropheny1)- 479.2
(3.NCIoxadiazol-2- 1,3,4- [M+H]-', 6.89 (ddd, J= 9.0, 2.8,
109 F 0,5( y1]-2-
N) 1.1 Hz, 1H), 4.97
(dd, J
CI
oxopiperidin- oxadiazol-2- RI
y1]-2- = 3.18 = 9.5, 4.6
Hz, 1H), 4.64
¨4.53 (m, 2H), 4.37
stereochemistry arbitrarily assigned as 3R and 65 3-yl]acetamide
oxopiperidin- (S4)
(ddd, J= 11.1, 8.1, 6.1
(stereochemist 3-yl]acetamide
Hz, 1H), 2.33 ¨2.27 (m,
ry arbitrarily (Example 37)
1H), 2.19 ¨2.10 (m, 1H),
assigned as 3R (Method C6)
2.09 ¨ 2.03 (m, 1H), 1.96
and 65)
(qd, J= 12.6, 3.4 Hz,
1H).
114 NMR (400 MHz,
chloroform-d) 6 7.33 (t, J
2-(4-chloro-3-
= 8.6 Hz, 1H), 6.78 (dd,
fluorophenoxy 2-(4-chloro-3-
J= 10.3, 2.8 Hz, 1H),
)-N-R3S,6R)- fluorophenoxy
6.72 ¨ 6.62 (m, 2H), 5.01
6-{5-[(35)-3- )-N-R3S,6R)-
¨ 4.94 (m, 1H), 4.46 (s,
H /.F (trifluorometh 6-{5-[3- M/Z:
2H), 4.05 (dp, J= 11.8,
1-0/ oxy)pyrrolidin (trifluorometh 508, 510
F jtõ s' -1-y1]-1,3,4- oxy)pyrrolidin [M+1-
1]-', 4.0' 3.4 Hz, 1H), 3.97
Cl 110 oxadiazol-2- -1-y1]-1,3,4- (dd RI '
J= 8.3, 3.3 Hz, 1H),
3.85-3.63 ¨ 3.63 (m, 4H), 3.33
stereochenustry arbitrarily assigned as 3S yl{piperidin-
oxadiazol-2- = 2.07
(dd, J= 11.9, 3.4 Hz,
3-yl]acetamide yl{piperidin- (S4)
1H), 2.60 (dd, J= 12.0,
(stereochemist 3-yl]acetamide
8.1 Hz, 1H), 2.42 ¨ 2.31
ry arbitrarily (Example 96)
(m, 1H), 2.30 ¨2.19 (m,
assigned as (Method C7)
3S)
1H), 2.14 ¨2.02 (m, 2H),
2.00¨ 1.86 (m, 2H), 1.61
¨ 1.52 (m, 1H).
2-(4-chloro-3- 1H NMR (400 MHz,
fluorophenoxy 2-(4-chloro-3-
chloroform-d) 6 7.33 (t, J
)-N-R3S,6R)- fluorophenoxy = 8.6 Hz, 1H), 6.78
(dd,
6-{5-[(3R)-3- )-N-R3S,6R)- J= 10.3, 2.9 Hz,
1H),
(trifluorometh 6-{5-[3- M/Z:
6.73 ¨6.60 (m, 2H), 5.02
H Nc.4))<F oxy)pyrrolidin (trifluorometh 508, 510 ¨4.94 (m, 1H), 4.46
(s,
111
o (11-or- F F -1-y1]-1,3,4-
oxy)pyrrolidin [M+H]-', 2H), 4.10 ¨ 3.93 (m, 2H),
F 0
oxadiazol-2- -1-y1]-1,3,4-
ESI, RT 3.81 ¨3.66 (m, 4H), 3.33
H
yl{piperidin- oxadiazol-2- = 2.07 (dd, J= 11.9,
3.5 Hz,
stereochemistry arbitrarily assigned es 3R 3-yl]acetamide
yl{piperidin- (S4) 1H), 2.60 (dd, J= 11.9,
(stereochemist 3-yl]acetamide 8.1 Hz, 1H), 2.41
¨2.32
ry arbitrarily (Example 96) (m, 1H), 2.30 ¨2.19
(m,
assigned as (Method C7)
1H), 2.15 ¨ 1.87 (m, 4H),
3R) 1.58¨ 1.52 (m,
1H).
170
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
II Assays
HEK-ATF4 High Content Imaging assay
Example compounds were tested in the HEK-ATF4 High Content Imaging assay to
assess
their pharmacological potency to prevent Tunicamycin induced ISR. Wild-type
HEK293 cells
were plated in 384-well imaging assay plates at a density of 12,000 cells per
well in growth
medium (containing DMEM/F12, 10% FBS, 2 mM L-Glutamine, 100 U/mL Penicillin ¨
100
iLtg/mL Streptomycin) and incubated at 37 C, 5% CO2. 24 h later, the medium
was changed to
50 L assay medium per well (DMEM/F12, 0.3% FBS, 2mM L-Glutamine, 100 U/mL
Penicillin ¨ 100 g,/mL Streptomycin). Example compounds were serially diluted
in DMSO,
spotted into intermediate plates and prediluted with assay medium containing
3.3 M
Tunicamycin to give an 11-fold excess of final assay concentration. In
addition to the
example compound testing area, the plates also contained multiples of control
wells for assay
normalization purposes, wells containing Tunicamycin but no example compounds
(High
control), as well as wells containing neither example compound nor Tunicamycin
(Low
control). The assay was started by transferring 5 L from the intermediate
plate into the assay
plates, followed by incubation for 6 h at 37 C, 5% CO2. Subsequently, cells
were fixed (4%
PFA in PBS, 20 min at r.t.) and submitted to indirect ATF4 immunofluorescence
staining
(primary antibody rabbit anti ATF4, clone D4B8, Cell Signaling Technologies;
secondary
antibody Alexa Fluor 488 goat anti-rabbit IgG (H+L), Thermofisher Scientific).
Nuclei were
stained using Hoechst dye (Thermofisher Scientific), and plates were imaged on
an Opera
Phenix High Content imaging platform equipped with 405 nm and 488 nm
excitation. Finally,
images were analyzed using script based algorithms. The main readout HEK-ATF4
monitored
the ATF4 signal ratio between nucleus and cytoplasm. Tunicamycin induced an
increase in
the overall ATF4 ratio signal, which was prevented by ISR modulating example
compounds.
In addition, HEK-CellCount readout was derived from counting the number of
stained nuclei
corresponding to healthy cells. This readout served as an internal toxicity
control. The
example compounds herein did not produce significant reduction in CellCount.
HEK ATF4 Activity of the tested example compounds is provided in Table 23 as
follows:
+++ = IC50 1-500 nM; ++ = IC50 >500-2000 nM; + = IC50 >2000-15000 nM.
171
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Table 23
Example number HEK-AT F4 Activity
2 +++
7 +++
9 +++
+++
11 +++
12 +++
13 +++
14 ++
+++
17
18 ++
19 ++
+++
21 ++
22 ++
23 +++
24 ++
+++
26
27 +++
28
29 ++
31
33 +++
34 ++
+++
36 ++
37 +++
38
39
172
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
42 +++
43 +++
44 +++
45 +++
46 +++
47 +++
48 ++
50 +++
51 ++
52 +++
53 +++
54 +++
55 +++
57 ++
59 ++
60 +++
61 +
63 ++
64 +
65 ++
66 ++
67 +++
68 +
69 +++
70 +++
71 ++
72 +
73 ++
74 +++
75 +++
76 ++
77 +++
78
173
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
79
80 +++
81 ++
82 +++
83 +++
84 +++
85 +++
86 +++
87 +++
88 ++
90 ++
91 +++
92 ++
93 ++
94
95 +++
98 +++
100 +++
101 +++
102 ++
103 +++
105 ++
106
107
108
109 +++
110 +++
111 +++
112 +++
113
174
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
Protocol - Thermodynamic (Equilibrium) solubility in selected buffer
Example compounds were tested in the Thermodynamic (Equilibrium) solubility in
selected
buffer assay. The test compound, in powder form, was weighted into a 4 mL
glass vial and a
calculated volume of selected medium was added to reach the target
concentration of the
solubility test (1 mg/mL). The solution was then stirred overnight at r.t.
protected from the
light. The solution was filtered through a 0.45 gm PTFE membrane at ambient
temperature.
An aliquot of the resulting filtrate was quantified using UPLC-UV method
described below
against a reference solution of the test compound, 0.8 mg/mL in DMSO. Media
compositions:
Phosphate buffer 50 mM (pH = 2.0) 690 mg of NaH2PO4,H20 in 200 mL of
ultrapurified
water adjusted at pH 2 with phosphoric acid 85% ; Acetate buffer 50 mM (pH =
5.5) 820 mg
of anhydrous sodium acetate in 200 mL of ultrapurified water adjusted at pH
5.5 with acetic
acid 99.8% ; Phosphate buffer 50 mM (pH = 7.4) 40.5 mL of Na2HPO4 0.1 M
solution + 9.5
mL of NaH2PO4 0.1 M solution. Analytical conditions: UPLC-UV-MS analyses were
performed with a Waters Acquity UPLC HClass-PDA-QDa system using a reverse
phase
Acquity BEH C18 column (2.1 mm x 50 mm, 1.7 gm; temperature: 40 C) and a
gradient of
10-95% B (A= 0.1% formic acid in H20; B= 0.05% formic acid in ACN) over 1.8
min then
100% B for 0.8 min, with an injection volume of 0.4 gL at flow rate of 0.65
mL/min. UV
chromatograms were recorded at 220 nm, 254 nm and 290 nm using a photo diode
array
detector. Mass spectra were recorded in the 150 to 900 M/Z range at a sampling
rate of 10
scans per sec using a QDa detector. Data were integrated using Empower03
software. Data
Analysis: Equilibrium solubility of the test compound in the selected medium
was calculated
through the ratio of the surface area of the UV chromatographic peak of the
compound in the
filtrate to the surface of the UV chromatographic peak of the compound in the
reference
solution.
Protocol - Measure of the Effect on hERG Channel by Tail Current Recording
Using in Vitro
Rapid ICE
The potency of the example compounds in inhibiting human ERG potassium channel
(hERG)
tail current was assessed in a recombinant HEK293 cell line stably transfected
with hERG
cDNA under an inducible promoter, using Rapid ICE (rapid ion channel
electrophysiology)
assay. Rapid ICE is an automated patch-clamp assay utilizing the QPatch HTX
system
(Sophion Bioscience A/S). Briefly, inducible HEK hERG cells were cultivated in
minimum
essential medium supplemented with 10% FBS, 1% non-essential amino acids, 1%
sodium
pyruvate, 2 mM 1-glutamine, 15 jig/mL blasticidin, and 100 jig/mL hygromycin.
hERG
175
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
channel expression induction was obtained by adding 10 gimL tetracycline for
24, 48, or 72
h before recordings.
On the day of the experiment, cells were detached with TrypLE and prepared to
be loaded on
the instrument. Cells were resuspended in 7 mL of Serum-Free Media containing
25 mM
Hepes and soybean trypsin inhibitor and immediately placed in the cell storage
tank of the
machine. The composition of the extracellular buffer was (mM): NaCl 137, KC1
4, CaCl2 1.8,
MgCl2 1.0, d-glucose 10, N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid
(HEPES) 10,
pH 7.4 with 1 M NaOH. The composition of the intracellular solution was (mM):
KC1 130,
MgCl2 1.0, ethylene glycol-bis(13-aminoethyl ether)-N,N,N,Nr-tetraacetic acid
(EGTA) 5,
MgATP 5, HEPES 10, pH 7.2 with 1 M KOH. The voltage protocol included the
following
steps: step from ¨80 to ¨50 mV for 200 ms, +20 mV for 4.8 s, step to ¨50 mV
for 5 s, then
step to the holding potential of ¨80 mV. Compounds were dissolved in DMSO and
diluted in
extracellular buffer to achieve final test concentrations (0.3, 3, and 30 M)
in 0.3% DMSO.
The voltage protocol was run and recorded continuously during the experiment.
The vehicle,
corresponding to 0.3% DMSO in extracellular buffer was then applied for 3 min,
followed by
the test substance in triplicate. The standard combined exposure time was 5
min. The average
of tail current amplitude values recorded from four sequential voltage pulses
was used to
calculate for each cell the effect of the test substance by calculating the
residual current (%
control) compared with vehicle pretreatment. Data were reported as %
inhibition for each
concentration tested, and IC50 values were estimated using QPatch software. At
least two cells
were tested, and even more if results diverged.
Protocol - LogD 7.4 Assay
Phosphate buffer (1 M) was diluted to 20 mM with deionised water and adjusted
to pH 7.4 (
0.05) with phosphoric acid or sodium hydroxide. A 1:1 mixture of phosphate
buffer (20 mM)
and 1-octanol were saturated by tumbling overnight after which time the two
phases were
separated. Using an automated liquid handler the following procedure was
carried out: 20 mM
DMSO stocks of assay and control compounds were reformatted to provide
cassettes of 4
compounds per well giving a final concentration of 5 mM per compound. In
duplicate 54 of
the cassetted compounds were added to 495 L 1-octanol (saturated with buffer)
followed by
495 L of buffer (saturated with 1-octanol) in a 96-well plate giving final
incubation
concentrations of 25 M (50 M max concentration in either layer if all
compound
partitioned into a single matrix). The layers were mixed by aspirating and
dispensing the
buffer and octanol layers into one another three times. The plate was sealed,
shaken for 120
176
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
minutes and then centrifuged at 25 C for 15 min at 4600 rpm. The 1-octanol
and buffer layer
were sampled separately (ensuring no cross contamination) and the respective
samples diluted
with 60:40 ACN:0.1 M ammonium acetate pH 7.4 (containing external standard;
Sulfisoxazole, assay concentration 120 nM) to obtain final theoretical maximum
concentrations of the 1-octanol layer of 0.025 M, 0.5 M and a buffer layer
concentration of
0.5 M. The second buffer sample was prepared by diluting the assay buffer
layer with
acetonitrile (containing external standard; Sulfisoxazole, assay concentration
120 nM) to give
a theoretical maximum concentration of 20 M. The analytical samples were
analysed by LC-
MS/MS and the LogD calculated as shown:
PA analyte in octanol / PAES
LogD (pH 7.4) = Log( A )
PA analyte in buffer / PrIES
Where PA = Peak area and ES = External standard
Biological Example: Lipophilicity and Solubility
In recent years there have been numerous reports in the medicinal chemistry
literature
associating the clinical success of drug candidates with their physicochemical
properties. The
degree of lipophilicity, in particular, has been highlighted as an important
factor in defining
the overall properties and likely fate of drug candidates (29), and typically
it is beneficial if
the logD lies in the range of 1 to 3. With reference to Table 24 selected
compounds of the
present invention have logD values lying within this range and represent an
improvement
over previously reported and similar analogues in this respect.
Similarly poor solubility of drug candidates has been associated with
increased risk of drug
development failures (30). Also with reference to Table 24 the selected
compounds of the
present invention show a higher aqueous solubility compared to structurally
similar
compounds of the state of the art. Additional solubility data relating to
further example
compounds are shown in Table 25 below.
Table 24
Solubility HEK-
hERG
Example (Itg/mL) Log ATF4
Structure
Activity
number pH pH D Activity
pH 2 **
7.4 5 *
177
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
NN y
W0201904
--<>- F <1 <1 <1 3.6 +++ ++
6779 Ex 1 F
-N Fy:F
50 Ti Y c'-" ¨ 63 78 quant.# 2.7 +++ ++
CI IP H
W0201904 N.-Nµ 0-4F
0 >---/ F 3 3 3 2.9 ++ +++
6779 Ex 30 F0
CI Wil
F
J1,4?....f F
61 90 156 907 2.3 ++
++
*ATF4 Activity category: +++ = IC50 1-500 nM; ++ = IC50 >500-2000 nM; + = IC50
>2000
nM.
**hERG Activity category: +++ = IC50 1-1000 nM; ++ = IC50 >1000-5000 nM; + =
IC50
>5000 nM
# completely dissolved in assay
Table 25
Example Solubility (jug/mL)
number
pH 2.0 pH 5.5 pH 7.4
5 quant.# 215 278
11 617 60 42
12 651 113 105
13 661 318 240
14 616 814 606
34 994 239 153
50 quant.# 78 63
51 945 4 2
52 839 9 7
57 quant.# 663 501
60 886 47 91
178
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
61 907 156 90
68 909 168 102
75 713 26 22
77 810 98 76
84 717 93 62
85 918 9 6
90 quant.#
294 173
95 970 378 230
98 quant.#
61 17
110 982 256 132
111 911 9 6
112 812 97 67
# completely dissolved in assay
Biological Example: hERG selectivity
Drug-induced QT interval prolongation and the appearance of torsade de pointes
(TdP) is well
recognised as a clinical risk. Whilst these effects are often multifactorial
there is a clear
consensus in recognising the role that interactions of drugs with the cardiac
hERG 1( channel
play in the manifestation of these clinical side-effects. In general it is
widely accepted that
minimising the interactions of drug molecules with the hERG 1( channel is
desirable (31).
To this end we have sought to balance the improvements in physicochemical
properties
mentioned above (i.e. logD and solubility ¨ Tables 24 & 25) with selective
modulation of
ATF4 relative to the hERG 1( channel.
Table 26
Example hERG
number Activity*
2 +++
4 +++
7 +++
9
10
179
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
11 +
12 +
13 +
14 +
15 +
29 +
31 +++
33 ++
35 +++
38 +
40 +++
42 ++
43 +
44 +++
45 +++
46 +++
47 +++
50 +
52 +++
53 ++
54 +++
55 +
57 +
59 +
60 +++
61 ++
64 +
65 ++
66 ++
67 ++
68 ++
69 +++
70
180
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
71 ++
72 ++
75 ++
76 +
77 ++
78 ++
79 +
80 +
81 +
82 +
83 +
84 ++
85 ++
86 ++
87 ++
88 ++
90 +
91 ++
92 ++
93 +
94 +
95 ++
98 ++
100 ++
101 +
102 ++
103 +
105 +
106 ++
108 +++
109 +++
110 +
111
181
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
112 +
113 ++
*hERG Activity category: +++ = IC50 1-1000 nM; ++ = IC50 >1000-5000 nM; + =
IC50 >5000
nM
With reference to Tables 23, 25 and 26 selected compounds of the present
invention display
an advantageous balance of properties, especially regarding HEK ATF4 Activity
and/or
solubility and/or selected hERG inhibition.
References
(1) Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The
integrated
stress response. EMBO Rep. 2016 Oct;17(10):1374-1395. Epub 2016 Sep 14.
(2) Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and
translational
control. Biochem Soc Trans. 2006 Feb;34(Pt 1):7-11.
(3) Donnelly N, Gorman AM, Gupta S, Samali A. The eIF2alpha kinases: their
structures and
functions. Cell Mol Life Sci. 201 30ct;70(19):3493-511
(4) Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation
initiation
and principles of its regulation. Nat Rev Mol Cell Biol. 2010 Feb;11(2):113-27
(5) Lomakin IB, Steitz TA. The initiation of mammalian protein synthesis and
mRNA
scanning mechanism. Nature. 2013 Aug 15;500(7462):307-11
(6) Pain VM. Initiation of protein synthesis in eukaryotic cells. Eur J
Biochem. 1996 Mar
15;236(3):747-71
(7) Pavitt GD. Regulation of translation initiation factor eIF2B at the hub of
the integrated
stress response. Wiley Interdiscip Rev RNA. 2018 Nov;9(6):e1491.
(8) Krishnamoorthy T, Pavitt GD, Zhang F, Dever TE, Hinnebusch AG. Tight
binding of the
phosphorylated alpha subunit of initiation factor 2 (eIF2alpha) to the
regulatory
subunits of guanine nucleotide exchange factor eIF2B is required for
inhibition of
translation initiation. Mol Cell Biol. 2001 Aug;21(15):5018-30.
(9) Hinnebusch, A. G., Ivanov, I. P., & Sonenberg, N. (2016). Translational
control by 5'-
untranslated regions of eukaryotic mRNAs. Science, 352(6292), 1413 ¨1416.
182
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
(10) Young, S. K., & Wek, R. C. (2016). Upstream open reading frames
differentially
regulate gene-specific translation in the integrated stress response. The
Journal of
Biological Chemistry, 291(33), 16927 ¨16935.
(11) Lin JH, Li H, Zhang Y, Ron D, Walter P (2009) Divergent effects of PERK
and IRE1
signaling on cell viability. PLoS ONE 4: e4170
(12) Tabas I, Ron D. Nat Cell Biol. 2011 Mar;13(3):184-90. Integrating the
mechanisms of
apoptosis induced by endoplasmic reticulum stress.
(13) Shore GC, Papa FR, Oakes SA. Curr Opin Cell Biol. 2011 Apr;23(2):143-9.
Signaling
cell death from the endoplasmic reticulum stress response.
(14) Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I,
Varia M,
Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C. EMBO
J. 2005 Oct 5;24(19):3470-81 ER stress-regulated translation increases
tolerance to
extreme hypoxia and promotes tumor growth.
(15) Bobrovnikova-Marjon E, Grigoriadou C, Pytel D, Zhang F, Ye J, Koumenis C,
Cavener
D, Diehl JA. Oncogene. 2010 Jul 8;29(27):3881-95 PERK promotes cancer cell
proliferation and tumor growth by limiting oxidative DNA damage.
(16) Avivar-Valderas A, Salas E, Bobrovnikova-Marjon E, Diehl JA, Nagi C,
Debnath J,
Aguirre-Ghiso JA. Mol Cell Biol. 2011 Sep;31(17):3616-29. PERK integrates
autophagy and oxidative stress responses to promote survival during
extracellular
matrix detachment.
(17) Blais, J. D.; Addison, C. L.; Edge, R.; Falls, T.; Zhao, H.; Kishore, W.;
Koumenis, C.;
Harding, H. P.; Ron, D.; Holcik, M.; Bell, J. C. Mol. Cell. Biol. 2006, 26,
9517
¨9532 .PERK-dependent translational regulation promotes tumor cell adaptation
and
angiogenesis in response to hypoxic stress.
(18) Taalab YM, Ibrahim N, Maher A, Hassan M, Mohamed W, Moustafa AA, Salama
M,
Johar D, Bernstein L. Rev Neurosci. 2018 Jun 27;29(4):387-415. Mechanisms of
disordered neurodegenerative function: concepts and facts about the different
roles of
the protein kinase RNA-like endoplasmic reticulum kinase (PERK).
(19) Remondelli P, Renna M. Front Mol Neurosci. 2017 Jun 16;10:187. The
Endoplasmic
Reticulum Unfolded Protein Response in Neurodegenerative Disorders and Its
Potential Therapeutic Significance.
(20) Halliday M, Mallucci GR. Neuropathol Appl Neurobiol. 2015 Jun;41(4):414-
27.Review:
Modulating the unfolded protein response to prevent neurodegeneration and
enhance
memory.
183
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
(21) Halliday M, Radford H, Sekine Y, Moreno J, Verity N, le Quesne J, Ortori
CA, Barrett
DA, Fromont C, Fischer PM, Harding HP, Ron D, Mallucci GR. Cell Death Dis.
2015
Mar 5;6:e1672.Partial restoration of protein synthesis rates by the small
molecule
ISRIB prevents neurodegeneration without pancreatic toxicity.
(22) Moreno JA, Radford H, Peretti D, Steinert JR, Verity N, Martin MG,
Halliday M,
Morgan J, Dinsdale D, Ortori CA, Barrett DA, Tsaytler P, Bertolotti A, Willis
AE,
Bushell M, Mallucci GR. Nature 2012; 485: 507-11. Sustained translational
repression
by eIF2alpha-P mediates prion neurodegeneration.
(23) Skopkova M, Hennig F, Shin BS, Turner CE, Stanikova D, Brennerova K,
Stanik J,
Fischer U, Henden L, Muller U, Steinberger D, Leshinsky-Silver E, Bottani A,
Kurdiova T, Ukropec J, Nyitrayova 0, Kolnikova M, Klimes I, Borck G, Bahlo M,
Haas SA, Kim JR, Lotspeich-Cole LE, Gasperikova D, Dever TE, Kalscheuer VM.
Hum Mutat. 2017 Apr;38(4):409-425. EIF2S3 Mutations Associated with Severe X-
Linked Intellectual Disability Syndrome MEHMO.
(24) Hamilton EMC, van der Lei HDW, Vermeulen G, Gerver JAM, Lourenco CM,
Naidu S,
Mierzewska H, Gemke RJBJ, de Vet HCW, Uitdehaag BMJ, Lissenberg-Witte BI;
VWM Research Group, van der Knaap MS. Ann Neurol. 2018 Aug;84(2):274-288.
Natural History of Vanishing White Matter.
(25) Bugiani M, Vuong C, Breur M, van der Knaap MS. Brain Pathol. 2018
May;28(3):408-
421. Vanishing white matter: a leukodystrophy due to astrocytic dysfunction.
(26) Wong YL, LeBon L, Edalji R, Lim HB, Sun C, Sidrauski C. Elife. 2018 Feb
28;7. The
small molecule ISRIB rescues the stability and activity of Vanishing White
Matter
Disease eIF2B mutant complexes.
(27) Wong YL, LeBon L, Basso AM, Kohlhaas KL, Nikkel AL, Robb HM, Donnelly-
Roberts
DL, Prakash J, Swensen AM, Rubinstein ND, Krishnan S, McAllister FE, Haste NV,
O'Brien JJ, Roy M, Ireland A, Frost JM, Shi L, Riedmaier S, Martin K, Dart MJ,
Sidrauski C. Elife. 2019 Jan 9;8. eIF2B activator prevents neurological
defects caused
by a chronic integrated stress response.
(28) Nguyen HG, Conn CS, Kye Y, Xue L, Forester CM, Cowan JE, Hsieh AC,
Cunningham
JT, Truillet C, Tameire F, Evans MJ, Evans CP, Yang JC, Hann B, Koumenis C,
Walter P, Carroll PR, Ruggero D. Sci Transl Med. 2018 May 2;10(439).
Development
of a stress response therapy targeting aggressive prostate cancer.
(29) Waring M, Expert Opinion on Drug Discovery Volume 5, 2010 - Issue 3, 235-
248.
Lipophilicity in Drug Discovery.
184
CA 03137213 2021-10-18
WO 2020/216766
PCT/EP2020/061150
(30) Alelyunas YW, et.al. Bioorg.Med.Chem.Lett., 20(24) 2010, 7312-7316.
Experimental
solubility profiling of marketed CNS drugs, exploring solubility limit of CNS
discovery candidate.
(31) Redfern WS, et. al., Cardiovascular Research 58(2003), 32-45.
Relationships between
preclinical cardiac electrophysiology, clinical QT interval prolongation and
torsade de
pointes for a broad range of drugs.
185