Note: Descriptions are shown in the official language in which they were submitted.
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
PROTEIN PREPARATION AND PACKAGING METHODS, SYSTEMS AND
RELATED DEVICES
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application claims priority to U.S. Provisional Application
62/899,068 filed
September 11, 2019, and entitled "Protein Preparation and Packaging Methods,
Systems and
Related Devices". This application claims priority to and is a continuation-in-
part application of
U.S. Application 16/419,359 filed May 22, 2019, and entitled "Protein
Preparation and
Packaging Methods, Systems, and Related Devices", which is a continuation-in-
part application
of U.S. Application 15/932,235 filed February 16, 2018, and entitled "Modified
Atmosphere and
High-Pressure Pasteurization Protein Preparation Packaging Methods, Systems
and Related
Devices," which claims priority to U.S. Provisional Application 62/459,888
filed February 16,
2017, and entitled "Protein Preparation Systems, Devices and Related Methods."
All of the
above applications are hereby incorporated by reference in their entirety
under 35 U.S.C.
119(e).
TECHNICAL FIELD
[002] The disclosure relates to devices, systems, and methods for the
preparation and
storage of proteins. Namely, the disclosure relates to a packaging system,
devices and methods
that allow for a significant reduction in pathogens, extended shelf-life, and
increased food safety
of various proteins, such as fresh beef, lamb, pork, poultry, fish, fowl and
bison.
BACKGROUND
[003] Prior art retail protein presentation methods and devices often
present a number of
shortcomings. These shortcomings can include pathogens and limited shelf life
for fresh meat,
such as beef, lamb, pork, poultry, fish, fowl, bison and the like, as well as
other meat alternative
forms of protein known in the art (hereinafter generally referred to as
"protein"). Also, less
skilled labor in the protein sector, increasing overhead, lack of
traceability, and regimented
advertisement campaigns create difficulties that are not in tune to market
opportunities.
[004] Under prior art approaches, protein suppliers generally fabricate
carcasses into so-
called "subprimals" which are typically cryovac or vacuum packaged. In this
subprimal state the
-1-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
protein typically has a shelf life of approximately the following: beef/lamb
40 days, pork 15
days and chicken 7 days. These proteins are also often contaminated with
various pathogens
which can be harmful to the consumer if not cooked properly.
[005] There is a need in the art for improved methods of protein
preparation.
BRIEF SUMMARY
[006] Described herein are various embodiments relating to devices, systems
and
methods for protein processing, packaging and preparation. Although multiple
embodiments,
including various devices, systems, and methods are described herein as a
"system," this is in no
way intended to be restrictive or limiting.
[007] In one example, a system for retail protein preparation, including: a
modified
atmosphere device configured to seal the protein in a modified atmosphere; and
a high-pressure
pasteurization device configured to pasteurize the sealed protein.
Implementations may include
one or more of the following features. The system where the modified
atmosphere includes
carbon monoxide. The system where the modified atmosphere includes carbon
dioxide. The
system where the modified atmosphere includes nitrogen. The system where the
modified
atmosphere includes carbon dioxide, carbon monoxide, and nitrogen. The system
where the
modified atmosphere does not include oxygen. Other embodiments include
corresponding
computer systems, apparatus, and computer programs recorded on one or more
computer storage
devices, each configured to perform the actions of the methods.
Implementations of the
described techniques may include hardware, a method or process, or computer
software on a
computer-accessible medium.
[008] Another example includes a method for fresh retail protein
preparation, including
operating a packaging system including a modified atmosphere device configured
to expose the
protein to a modified atmosphere and seal the protein in a container. The
method of this example
also includes a high-pressure pasteurization device constructed and arranged
to pasteurize the
sealed protein within the sealed container, where the system is configured to
perform steps
including a modified atmosphere step, and a high-pressure pasteurization step,
where the protein
is sealed in a modified atmosphere and exposed to high pressure
pasteurization.
[009] Implementations according to this and other examples may include one
or more of
the following features. The method where the modified atmosphere includes
carbon monoxide.
-2-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
The method where the modified atmosphere includes carbon dioxide. The method
where the
modified atmosphere includes nitric oxide. The method where the modified
atmosphere includes
carbon dioxide, carbon monoxide and nitrogen. The method where the modified
atmosphere does
not include oxygen.
[010] Another example includes a method for high-pressure pasteurization of
protein,
including at least one modified atmosphere step where the protein is sealed in
a modified
atmosphere, and at least high-pressure pasteurization step performed on the
sealed modified
atmosphere protein.
[011] Yet a further example includes a method of packaging protein in a
modified
atmosphere for high-pressure pasteurization, including several steps a
preparation step, including
a physical preparation sub-step and a chemical preparation sub-step, a
modified atmosphere step
including a modified atmosphere introduction sub-step and a sealing sub-step,
and a high-
pressure pasteurization step including a high pressure pasteurization sub-
step, where the protein
is sealed and high-pressure pasteurized in a container with a modified
atmosphere including
carbon monoxide, carbon dioxide, and nitrogen without substantial oxygen.
[012] Implementations of these examples may include one or more of the
following
features. The method where the modified atmosphere step includes a modified
atmosphere sub-
step, and a sealing sub-step. The method where the modified atmosphere
includes carbon
monoxide, carbon dioxide, and nitrogen. The method where the modified
atmosphere includes
carbon monoxide, carbon dioxide, and nitrogen. The method where the modified
atmosphere
includes about 0.4% carbon monoxide. The method where the modified atmosphere
includes
about 20% carbon dioxide. The method where the modified atmosphere includes
more than 79%
nitrogen. The method where the high-pressure pasteurization step includes a
coding/dating sub-
step and a scanning sub-step. The method where the high-pressure
pasteurization step includes
an high pressure pasteurization ("HPP") sub-step. The method where the HPP sub-
step is
performed on the sealed modified atmosphere protein at about 87,000 psi. The
method where the
HPP sub-step is performed on the sealed modified atmosphere protein for about
3 minutes. The
method where the HPP sub-step is performed on the sealed modified atmosphere
protein for
between about 1 second and about 3600 seconds. The method where the HPP sub-
step is
performed on the sealed modified atmosphere protein at between about 43,500
and about 87,000
psi.
-3-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
[013] Another example includes a method for packaging proteins including a
preparation step including: providing a protein; exposing the protein to an
aqueous ozone
solution; placing the protein in a container. The method may also include a
modified atmosphere
step including introducing a modified atmosphere into the container and
sealing the container.
The method may also include a high-pressure pasteurization step including
exposing the
container to high pressure pasteurization. The method may also include storing
the container.
[014] Implementations may include one or more of the following features.
The method
where the aqueous ozone solution is about .5 to about 4 ppm aqueous ozone. The
method where
the protein is exposed to the aqueous ozone solution for 1 to 10 seconds. The
method further
including portioning the protein after exposing to the aqueous ozone solution.
The method
further including coding and dating the container. The method where the high-
pressure
pasteurization is at least about 72,000 psi. The method where the high-
pressure pasteurization is
at least about 3 minutes.
[015] Another example, includes a method for extending the shelf life of a
food product
including: providing a food product, exposing the food product to an aqueous
ozone solution,
placing the food product into a package, flushing the package with a modified
atmosphere,
sealing the package, and exposing the package to high pressure pasteurization.
[016] Implementations may include one or more of the following features.
The method
where the food product is exposed to the aqueous ozone solution for 1 to 10
seconds. The
method where the aqueous ozone solution includes .5 to 4 ppm of aqueous ozone.
The method
where the modified atmosphere is substantially without oxygen. The method
where the aqueous
ozone solution is exposed to the food product via spray nozzles. The method
where the high-
pressure pasteurization is at least 72,000 psi for at least 3 minutes. The
method where the shelf-
life of the food product is extended by at least 60 days.
[017] In another example, a system for processing proteins including: an
aqueous ozone
application unit; a packager in communication with the aqueous ozone
application unit; a
modified atmosphere injector in communication with the packager; a sealer in
communication
with the packager and modified atmosphere injector; and a high-pressure
pasteurization tank in
connection with the packager. In this example, a protein is exposed to aqueous
ozone in the
aqueous ozone application unit; the protein is placed into a package by the
packager; the package
is flushed with a modified atmosphere by the modified atmosphere injector; the
package is sealed
-4-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
by the sealer while flushed with the modified atmosphere; and the protein is
exposed to high-
pressure pasteurization in the high-pressure pasteurization tank.
[018] Implementations may include one or more of the following features.
The system
where the aqueous ozone solution is .5 to 4 ppm of aqueous ozone. The system
where the protein
is exposed to aqueous ozone for 1 to 10 seconds. The system where the self-
life of the protein is
extended by at least 30 days. The system where the self-life of the protein is
extended by at least
45 days. The system where the self-life of the protein is extended by at least
60 days. The system
where the protein is beef.
[019] In various implementations featuring automation, a system of one or
more
components including computers can be configured to perform particular
operations or actions
by virtue of having software, firmware, hardware, or a combination of them
installed on the
system that in operation causes or cause the system to perform the actions.
One or more
computer programs can be configured to perform particular operations or
actions by virtue of
including instructions that, when executed by data processing apparatus, cause
the apparatus to
perform the actions.
[020] Ranges can be expressed herein as from "about" one particular value,
and/or to
"about" another particular value. When such a range is expressed, a further
aspect includes from
the one particular value and/or to the other particular value. Similarly, when
values are expressed
as approximations, by use of the antecedent "about," it will be understood
that the particular
value forms a further aspect. It will be further understood that the endpoints
of each of the ranges
are significant both in relation to the other endpoint, and independently of
the other endpoint. It
is also understood that there are a number of values disclosed herein, and
that each value is also
herein disclosed as "about" that particular value in addition to the value
itself. For example, if the
value "10" is disclosed, then "about 10" is also disclosed. It is also
understood that each unit
between two particular units are also disclosed. For example, if 10 and 15 are
disclosed, then 11,
12, 13, and 14 are also disclosed.
[021] While multiple implementations are disclosed, still other
implementations of the
disclosure will become apparent to those skilled in the art from the following
detailed
description, which shows and describes illustrative embodiments of the
disclosed apparatus,
systems and methods. As will be realized, the disclosed apparatus, systems and
methods are
capable of modifications in various obvious aspects, all without departing
from the spirit and
-5-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
scope of the disclosure. Accordingly, the drawings and detailed description
are to be regarded as
illustrative in nature and not restrictive.
BRIEF DESCRIPTION OF THE DRAWINGS
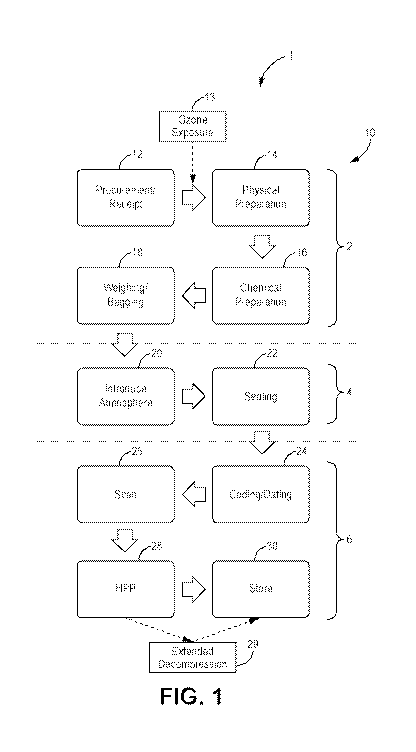
[022] FIG. 1 is an illustrative flow diagram of the protein packaging
process, according
to exemplary implementations.
[023] FIG. 2A is a perspective view of a aqueous ozone application unit,
according to
one implementation.
[024] FIG. 2B is a top-view floorplan of a facility capable of performing
the protein
packaging process, according to one implementation.
[025] FIG. 3 is a perspective view of a several components utilized in the
process,
including a weighing device, according to one implementation.
[026] FIG. 4 is a perspective view of a modified atmosphere device
comprising a
conduit and bagging chute, according to one implementation.
[027] FIG. 5 is an end-long view of the modified atmosphere device of FIG.
4.
[028] FIG. 6 is a perspective view of a bagged and sealed protein in a
modified
atmosphere on a conveyor belt, according to one implementation.
[029] FIG. 7 is a perspective view of a conveyor and high pressure
pasteurization
device, according to one implementation.
[030] FIG. 8 is a further perspective view of a high pressure
pasteurization device,
according to one implementation.
[031] FIG. 9 is yet a further side view of a high pressure pasteurization
device,
according to one implementation.
DETAILED DESCRIPTION
[032] The various embodiments disclosed or contemplated herein are directed
to
systems, methods and devices for packaging of protein in an air-tight bag or
other container,
wherein the protein is exposed to a modified atmosphere within the bag and the
bag is exposed to
high-pressure pasteurization ("HPP"). In some implementations the protein is
additionally
exposed to aqueous ozone prior to packaging. In various implementations, the
HPP has an
extended decompression time. In various implementations, a variety of
automated or semi-
-6-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
automated components can be used to execute a variety of steps and sub-steps
to prepare such
packaged protein. These implementations can improve shelf-life, aesthetic
quality, and other
features and properties of the prepared and packaged protein, as will be
described in detail
herein.
[033] FIGS. 1-9 depict several exemplary implementations of the protein
packaging
process 1 executed via the operation of a packaging system 10 comprising
several components,
some of which may be automated or semi-automated. The various implementations
relate to
packaging a protein such as meat sealed in an air-tight container containing a
modified
atmosphere and exposed to HPP (in some implementations HPP includes exposing
the product to
isostatic pressures of up to about 600 MPa/87,000 psi or more) for improved
shelf-life and other
advantages, as is described in detail herein.
[034] Through the combination of the aqueous ozone solution, modified
atmosphere
and the use of HPP with or without an extended decompression period, the
various
implementations allow for a significant reduction in pathogens, extended shelf-
life, and/or
improved aesthetic qualities of the packaged protein. In some of these
implementations, the
process 1 and system 10 include steps for tracking and tracing such that the
final packaged
protein can be traced back to the source, thereby adding another important
food safety element in
the supply network. It is understood that these edible proteins are considered
a commodity
market at one stage or another in the process or path to the end user.
[035] Turning to the drawings in greater detail, in the implementation of
FIG. 1, the
process 1 or method 1 comprises various optional steps and optional sub-steps
that can be
performed in any order. It is understood that in various implementations, a
packaging system 10
is constructed and arranged to perform this process 1 by utilizing several
components and
associated devices. Various implementations of this system 10 are depicted in
FIGS. 2A-9.
[036] The disclosed implementations involve several optional steps which
may be
performed in any order. Additional steps and/or substep may be included, while
other steps
and/or substeps may be omitted, depending on the specific implementation. One
example
packaging system 10, shown in FIG. 1, is provided to illustrate optional steps
and substeps, but is
in no way intended to limit the embodiments to this particular implementation.
[037] In these implementations, and as shown in FIG. 1, the steps in
exemplary
implementations of the process include:
-7-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
1. an optional preparation step 2,
2. an optional modified atmosphere step 4; and
3. an optional high-pressure pasteurization step 6.
Other steps and substeps may be included. In various aspects each of these
steps 2, 4, 6 can
comprise various optional sub-steps, as shown in the implementation of FIG. 1.
[038] In the optional preparation step 2, according to various
implementations like that
of FIG. 1, a protein (shown in FIG. 6 at 70) can be procured, received,
treated, and packaged in
an atmosphere-resistant bag, package or other container, as described herein.
In alternate
implementations, the protein may be pre-bagged or otherwise contained in an
air-tight container
for processing in the modified atmosphere 4 and pasteurization 6 steps. In
various
implementations, the protein may be exposed to an aqueous ozone solution
during the
preparation step 2.
[039] FIG. 1 shows one exemplary implementation of the disclosed method 1
as
implemented on a packaging system 10 during the optional preparation step 2.
The protein
(shown in FIG. 6 at 70) may enter the system 10, through an optional
procurement/receipt sub-
step (box 12). In this procurement/receipt sub-step (box 12), according to
certain
implementations, a procurement order is issued, such as from a central
processing component or
computer (not shown). In these implementations, the procurement order can
trigger delivery and
receipt of the protein¨such as meat¨for cataloging via supply-chain and/or
inventory systems
as would be known and understood in the art.
[040] In various implementations, the protein for processing can be one or
more of fresh
beef, lamb, pork, poultry, fish, fowl, bison and the like. In various
implementations, more than
one protein¨such as a blend of chicken and beef¨may be used. For example, more
than one
protein may be used for preparing fajitas, stir fry and/or other preparations
as would be
understood and appreciated in the art.
[041] In various implementations, the preparation step 2 includes an
optional ozone
exposure sub-step (box 13). The protein prior to physical preparation (box
14), chemical
preparation (box 16) and/or bagging (step 18)¨as described further below¨may
be exposed to
an aqueous/liquid ozone solution. In various alternative implementations, the
protein is exposed
to ozone after and/or during various other steps and substeps including but
not limited to
procurement (box 12), physical preparation (box 14), chemical preparation (box
16), and of
-8-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
weighing/bagging (box 18). In various implementation, the aqueous ozone
solution may kill,
eliminate, or otherwise render inactive various microorganisms¨such as
lactobacillus and other
bacteria. The aqueous ozone exposure sub-step (box 13) may be useful in
targeting those
bacteria/pathogens that are unaffected by HPP, modified atmosphere, and/or
other preparation
steps.
[042] In the ozone exposure sub-step (box 13) the protein may be sprayed,
dipped,
submerged, or otherwise exposed to an aqueous ozone solution. In various
implementations, the
aqueous ozone solution contains about 0 to about 100 PPM of aqueous/liquid
ozone. In some
implementations, the aqueous ozone solution contains about .5 to 4 PPM of
aqueous/liquid
ozone. In various alternative implementations the aqueous ozone solution
contains about 5 PPM
of ozone.
[043] In various implementations the aqueous ozone solution is at about 33
to 212 F. In
some implementations, the temperature of the aqueous ozone solution is at
ambient or room
temperature. The protein may be exposed to the liquid ozone solution for about
1 to about 10
seconds or longer.
[044] In some implementations, the aqueous ozone solution may be applied to
the
protein via an aqueous ozone application unit 34. In various of these
implementations the
aqueous ozone application unit 34 has spray nozzle(s) 36, as shown in FIG. 2A
and 2B. FIG. 2A
depicts one exemplary implementation of a nozzle applicator 34 where the
aqueous ozone
solution is applied via multiple spray nozzles 36 positioned above a conveyor
38. In these and
other implementation, the protein is placed on the conveyor 38 and passes
under the nozzles 36.
Of course other implementations are possible and would be recognized by those
of skill in the
art. In various implementations, the aqueous ozone solution is applied at a
pressure of about 1 to
50 psi. In some implementations, the ozone solution is applied at a pressure
of about 35 psi.
[045] Use of the aqueous ozone exposure sub-step (box 13) with the
packaging system
has been shown to reduce the bacterial load to zero or near 0 over a 60 day
period.
Additionally, use of aqueous ozone exposure has been shown to extend the shelf-
life of various
proteins to at least about 100 days. In one specific example, a sample of beef
was exposed to
ozone (as described above) and HPP (87,000 psi for 3 min) then tested for
bacterial load after 60
days. In this example, the sample was found to have less than 10 cfu/g in
tests for E. coli and
-9-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
lactic acid bacteria. Additionally, the aerobic plate count was less than 10
cfu/g and also, Listeria
monocytogenes was not detected per 25g.
[046] In another example, a sample of beef was exposed to ozone (as
described above)
and HPP (72,000 psi for 3 min) then tested for bacterial load after 60 days.
In this example, the
sample was found to have less than 10 cfu/g in tests for E. coli and lactic
acid bacteria.
Additionally, the aerobic plate count was less than 10 cfu/g and also,
Listeria monocytogenes
was not detected per 25g.
[047] The results of these tests show that exposure of protein to aqueous
ozone, as
described herein, is useful in reducing the bacterial load of the proteins and
therefore extending
the shelf-life and increasing the food safety of the proteins over time.
Further examples and data
are given in the Experimental section below.
[048] Turning back to the preparation step 2 of the implementation of FIG.
1 and as
further shown in FIG. 2B, the entering protein can be vacuum packed, frozen,
and/or fresh. An
optional physical preparation sub-step (box 14) can be performed on proteins
in any state.
During such a physical preparation sub-step (box 14), it is understood that
various preparatory
techniques can be employed to prepare the protein for processing in subsequent
steps and/or sub-
steps of the system 10. These sub-steps may be used for conversion from
subprimal or subprime
material to retail presentation through understood techniques such as boning,
trimming and
portioning. For example, subprimal beef chuck eye roll may be cut into roast
and trimmings.
[049] As would be further appreciated, in certain implementations during
the
preparation step 2, according to certain aspects, a chemical preparation sub-
step (box 16) is
performed. During the chemical preparation sub-step (box 16), in these
aspects, marinades, other
treatment(s), and/or seasoning techniques may be applied to the protein. In
various
implementations, the chemical preparation sub-step (box 16) is performed prior
to optional
weighing and bagging in a weighing/bagging sub-step (box 18). It is understood
that in these
implementations¨during the chemical preparation sub-step (box 16)¨various
flavored and/or
neutral marinades may be introduced and/or utilized to prepare the product to
a desired flavor
and/or color.
[050] In some implementations, the weighing/bagging sub-step (box 18)
completes the
preparation step 2. In further implementations, the optional weighing/bagging
sub-step (box 18)
is performed at any point in the process 1. While it is apparent that weighing
is generally
-10-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
optional, in various implementations, the product must be bagged or otherwise
inserted into an
air-tight container during this weighing/bagging sub-step (box 18)¨or
preparation step 2
generally¨to ready the protein for the modified atmosphere step 4 and/or HPP
step.
[051] In one illustrative example, the system 10 is constructed and
arranged such that
the pre-bagged protein is about 16 oz. In other examples, the protien is about
6 oz, 8 oz, 10 oz,
12 oz or more. In further examples, the protein weight is between about 1 oz
and 64 oz. In
additional implementations, the protein is more than 64 oz. In yet further
examples, the protein
comprises a variety of individual pieces that in sum weigh about a specified
amount, such as
shrimp and/or fajita cuts. It is understood that a variety of sizes and
weights are possible,
depending on the final retail application.
[052] In certain implementations, the barrier film or barrier bag (shown in
FIG. 6 at 69)
used in the weighing/bagging sub-step (box 18) can be a nylon bag, a 3-ply
bag, though other
high barrier product. Other implementations are possible, including metallic,
Saran , PET, and
others known and understood by those of skill in the art to have the proper
gas permeability to
retain the introduced modified atmosphere.
[053] As is shown in FIG. 2B, the preparation step 2 and corresponding sub-
steps can
be performed, for example by way of an arrangement of tables 40 conveyors 42,
graders 44
tumblers 46 and/or weighing devices 48. It is understood myriad configurations
are possible, as
would be understood by the skilled artisan.
[054] In these implementations, following the preparation step 2, a
modified atmosphere
step 4 is performed. In some implementations, the preparation step 2 is not
performed or is
performed simultaneous to or after the modified atmosphere step 4. The
modified atmosphere
step 4 generally relates to the introduction of a modified atmosphere ("MA")
to the protein
within the bag. In various implementations, the modified atmosphere is a
combination of carbon
monoxide, carbon dioxide, and nitrogen. As will be apparent to one of skill in
the art, many
additional atmospheric and gaseous compositions are possible.
[055] In one illustrative example, the prepared, portioned, and weighed
protein¨such
as at the end of the preparation step 2 and weighing/bagging sub-step (box
18)¨is exposed to a
modified atmosphere via a modified atmosphere introduction sub-step (box
20)¨of the modified
atmosphere step 4¨and then packaged or sealed in a sealing sub-step (box 22),
as shown in FIG.
1.
-11-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
[056] As shown in FIG. 2B, the modified atmosphere introduction sub-step
(box 20)
and the sealing sub-step (box 22) can be performed in rapid succession via an
automatic bagger
or MA device 50 and subject to further processing. It is understood that the
introduction of the
modified atmosphere to the protein and sealing of the package or bag can be
performed in a
variety of alternative ways. In certain implementations, and as shown in FIGS.
3-5, the MA
device 50 is configured to package the protein/product in a barrier film
within a bagging chute
53, where the bag is filled with a modified atmosphere via a conduit 51. Other
filling and
bagging devices, methods, and systems can be utilized in alternate
implementations, as would be
understood.
[057] In certain implementations, oxygen is flushed from the protein, and
the modified
atmosphere includes about 0.4% carbon monoxide, about 20% carbon dioxide, and
the remainder
(more than 79% or about 80%) is nitrogen. It is understood that in these and
other
implementations, it may be desirable to exclude oxygen from the modified
atmosphere.
[058] In further implementations, the carbon monoxide concentration can be
about 0.1%
or less, and can increase to 0.2%, 0.3% or more, or can exceed 0.5%, 1.0% or
up to 100% of the
atmosphere.
[059] Similarly, the modified atmosphere can include less than 20% carbon
dioxide,
down to 0.1% or less. In alternate implementations, the modified atmosphere
can include more
than 20% carbon dioxide, such as 25%, 30%, 40%, 50% or more, up to 100%. In
all of these
implementations, nitrogen can comprise the remainder of the modified
atmosphere.
[060] In certain implementations, ranges from about 0% to about 100% nitric
oxide
and/or carbon dioxide can also be introduced into the modified atmosphere
mixture.
Alternatively, other inert gases may be introduced into the modified
atmosphere. However, in
exemplary implementations, the modified atmosphere of many implementations
does not contain
oxygen, as would be readily understood by one of skill in the art.
[061] In some implementations, the gas or gases in the modified atmosphere
can be
adjusted or modified based on the product or cut of meat/protein being
packaged. In one specific
example, for raw red meat the modified atmosphere mixture includes about 60-
80% oxygen and
20-40% carbon dioxide. In another specific example, the modified atmosphere
for raw light
poultry includes about 40-100% carbon dioxide and 0-60% nitrogen. In another
example, for raw
dark poultry the modified atmosphere includes 70% oxygen and 30% carbon
dioxide. In another
-12-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
example, for sausage the modified atmosphere includes 20-30% carbon dioxide
and 70-80%
nitrogen. In another example, the modified atmosphere for sliced and cooked
meat includes 30%
carbon dioxide and 70% nitrogen. Of course other modified atmosphere
compositions are
possible as would be recognized.
[062] Continuing with the implementations of FIGS. 1-2, and FIGS. 6-9, the
protein
sealed in a modified atmosphere ("MA protein" ¨ shown in FIG. 6 at 70) can be
taken through a
HPP step 6 comprising one or more post-sealing sub-steps via conveyors 42 and
other devices
used in the industry and understood in the art. In various alternative
implementations, the HPP
step is performed before the modified atmosphere step 4, or the modified
atmosphere step 4 is
omitted from the process 1.
[063] In certain implementations, during the high-pressure pasteurization
step 6 a sub-
step of performing HPP is required, but several other optional sub-steps
relating to processing
can also be performed.
[064] For example, after the protein has been sealed in a package (with or
without
modified atmostphere) (as shown in FIG. 1 box 22), the protein can optionally
be bag dated or
coded in a coding/dating sub-step (box 24). In another optional sub-step the
protein is scanned or
X-rayed in a scanning sub-step (box 26 and in FIG. 2B at 52). Various of these
step and substep
may ensure quality and/or compliance¨such as with USDA regulations. It is
understood that
various white-labeling and/or other marketing badges may also be applied to
the bag at or
between any of these optional sub-steps of the process 1. Alternate
implementations do not
include these dating, coding, scanning, and x-raying sub-steps. In a further
implementations the
process 1 comprises additional packaging, labeling and quality-control sub-
steps as would be
understood in the art. It is further understood that these optional substeps
of dating, coding,
scanning, and x-raying can be performed at any point in the process 1 and may
be performed at
multiple points in the process.
[065] Turning to FIGS. 7-9, the protein (whether or not packaged in
modified
atmosphere) is exposed to HPP in a HPP sub-step, as is shown in FIG. 1 at box
28 and in FIGS.
7-9 at 54.
[066] In one implementation, the HPP sub-step (box 28) is performed at up
to about
87,000 psi for a duration of about 3 minutes or more. Various alternative
implementations can
utilize HPP of 300-600 MPa/43,500-87,000 psi or more, over durations of from
less than about a
-13-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
minute to more than about ten minutes, more than about 20 minutes, more than
about 30
minutes, more than about 60 minutes or longer. The conditions and parameters
of the HPP sub-
step (box 28) may depend on the environment, conditions, and other parameters
as would be
recognized. Various implementations can perform the HPP sub-step (box 28) from
about 1
second to about 3600 seconds or more at between about 43,500 and about 87,000
psi or more.
[067] In various implementations, HPP (box 28) has process parameters
between about
50,000 to 87,000 psi for between 3 to 5 minutes. In another implementation,
the HPP (box 28) is
conducted at 60,000 psi for 4 minutes.
[068] In these and other implementation the HPP sub-step (box 28) may
include an
extended decompression step (box 29). In various implementations, after the
HPP time has
elapsed the pressure is released and the package/item is returned to
atmospheric pressure. This
decompression time can last from about less than one second to more than about
10 minutes. In
some implementations, the decompression time is at least about 5 min. In some
implementations,
the decompression time is at least about 8 minutes or more.
[069] The extended decompression step (box 29) may provide additional time
that the
product/protein is exposed to pressures above atmospheric pressure. By
exposing the protein to
pressures of longer periods it is understood that the the amount of bacterial
killed may increase.
Additionally, allowing additional time for decompression after HPP has been
shown to improve
the food quality as well as maintain the aesthetic appearance to the
protein/product, such as the
food color, when compared to products subject to HPP where the high pressure
is released and
the product returned to atmospheric pressure instantly or over a very short
time period, such as a
few seconds.
[070] In certain implementations, the temperature for all steps and
substeps of the
process 1 is kept below about 50 degrees Fahrenheit, though alternate
implementations may vary
from freezing to room-temperature or higher.
[071] It will be appreciated by the skilled artisan that the HPP sub-step
(box 28) does
not cause the rupture of the bag in these implementations because the pressure
is being applied to
the bag or other air-tight or sufficiently gaseous-impermeable container
uniformly.
[072] In various implementations, the high-pressure pasteurized packages
are
subsequently dried and packed in a case and palletized in a storage sub-step,
as is shown in FIG.
1 at box 30 and in FIG. 2B at 56.
-14-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
[073] In certain implementations, the system 10 can further comprise a
water bath. For
example, a 180 degree Fahrenheit ( F) water bath may be used, or any other
bath from about 33
degrees Fahrenheit ( F) or more. In various of these implementations, the
system 10 is able to
pull a vacuum (shown in FIG. 2B at 58) on the sealed bag 69. These
implementations may result
in a freezable product that can be provided to commercial outlets. In some of
these
implementations freezing may affect the color of the protein, wherein the
protein turns an
undesirable color.
[074] Alternatively, however, as would be understood by one of skill in the
art, the
sealed protein 70 can either be exposed to MA or be vacuum packed rather than
or in addition to
being frozen. Accordingly, in certain implementations of the system, an
alternate route or series
of steps and substeps can be performed such that processing for vacuum packing
and MA
processing can both be performed in the same facility at substantially the
same time as the
process 1.
[075] In various implementations, the finished product bags will be about 1
lb. each,
and can be packaged in 40 lb. boxes on 1800 lb. pallets, so as to present an
economically viable
shipping method. Other configurations are of course possible, as would be
appreciated by one of
skill in the art.
[076] The product treated with the process 1 described herein may remain
edible in the
fresh state and have a shelf-life as follows: beef/lamb about 60 days, pork
about 45 days and
chicken about 30 days. In some implementations, the product is to remain
refrigerated at about
28 to 36 degrees Fahrenheit ( F) during this period. In various
implementations, the product can
be stored at about 2-6 C (35-43 F). Use of the aqueous ozone exposure sub-step
(box 13) and/or
extended decompression sub-step (box 29) may further extend shelf-life.
[077] In various implementations, the disclosed system 10 and associated
devices, and
methods also provide an extended protein shelf life for retailers, and a safer
product for end
consumers. Given the differences in advertising cycles and shelf life in the
current retail
environment, retailers typically purchase advertising at least a month prior
to the actual purchase
of proteins. Typically, these ads are driven on the basis of seasonal trends,
and tend to "lock" the
retailer into a sales promotion for the designated period. The presently-
disclosed system 10 and
associated , devices and methods may allow a retailer to defer or minimize
this marketing
decision, thus allowing retailers to select less-expensive cuts of product
when suppliers have
-15-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
excess, thereby keeping costs down and creating efficiency. As described
herein, protein from
various market buys can be held for a period of time, for example about 30 to
50 days, then
processed using the presently disclosed system 10 and associated methods and
devices. By
processing with the disclosed system 10 the protein is provided with an
additional shelf life of up
to about 60 days or longer. These improvements will be appreciated by those of
skill in the art in
light of the present disclosure.
[078] It is understood that the improved product presentation of the
protein according to
various implementations will provide numerous benefits to end retailers, who
will have a clean,
extended shelf-life product that does not require trimming, boning, packaging
and the like.
Various of these retailers will therefore enjoy less overhead, while reducing
the need for skilled
labor. The traceable, and in some implementations privately labeled, product
can be placed
directly in a fresh protein counter. These packaged protein units, utilizing
the disclosed system
10, may also benefit end consumers, who in turn will be purchasing a high
quality portion of
protein, which is safe, has normal aesthetic qualities, and can be traced back
to its source facility.
Additionally, for retailers the disclosed system 10 provides proteins and
other products that
require no or minimal product rework and and decrease shrinkage. As such the
overall number of
preparation steps for the proteins carried by the retailer may be reduced.
Further, as discussed
above, a retailer can take advantage of avoiding the peak times of the year
for buying particular
products, while still being able to sell into the seasonal trends.
EXPERIMENTAL
[079] Various implementations of the above described process were carried
out on
various cuts of meat and then subject to testing for bacterial load. TESTS 1-
15 were conducted
with the following process parameters: (box 13) Ozone application at > 5 ppm;
(box 28) HPP at
60,000 psi for 240 seconds with a water temperature of 40 F; (box 29) HPP
extended
decompression time of 486 seconds; and (box 20) Modified Atmosphere Packaging
with a gas
mixture of 80% N2, 19.6% CO2, and 0.4% CO. Testing was conducted prior to
processing, after
processing, and at subsequent approximately 10-day intervals.
[080] TEST 1: Chuck Blade Steak
Test Date Coliforms E.Coli Aerobic Anerobic Listeria
(CFU/g) Generic Plate Count Plate Count
(CFU/g)
1 - before 8/19/2019 <10 <10 540,000 <10 ND
-16-
CA 03141532 2021-11-22
WO 2020/236218
PCT/US2019/062753
treatment
2 - after 8/19/2019 <10 <10 540 <10 ND
treatment
3 8/27/2019 <10 <10 26,000 <10 ND
4 9/6/2019 <10 <10 N/A N/A ND
[081] TEST 2: Clod Heart Steak
Test Date Coliforms E.Coli Aerobic Anerobic
Listeria
(CFU/g) Generic Plate Count Plate Count
(CFU/g)
1 8/19/2019 100 <10 57,000 <10 ND
2 8/19/2019 <10 <10 30 10 ND
3 8/27/2019 <10 <10 60 <10 ND
4 9/6/2019 <10 <10 60,000 42,000 ND
[082] TEST 3: Beef Ribeye Steak
Test Date Coliforms E.Coli Aerobic Anerobic
Listeria
(CFU/g) Generic Plate Count Plate Count
(CFU/g)
1 8/19/2019 90 <10 5,800 <10 ND
2 8/19/2019 <10 <10 <10 <10 ND
3 8/27/2019 <10 <10 50 <10 ND
4 9/6/2019 <10 <10 <10 <10 ND
[083] TEST 4: Beef sirloin tri tip
Test Date Coliforms E.Coli Aerobic Anerobic
Listeria
(CFU/g) Generic Plate Count Plate Count
(CFU/g)
1 8/19/2019 <10 <10 350,000 210,000 ND
2 8/19/2019 <10 <10 500 140 ND
3 8/27/2019 <10 <10 240,000 <10 ND
4 9/6/2019 <10 <10 320,000 150,000 ND
[084] TESTS: Beef Top Butt
Test Date Coliforms E.Coli Generic
Aerobic Anerobic Listeria
(CFU/g) (CFU/g) Plate Count Plate Count
1 8/19/2019 <10 <10 660,000 970,000 ND
2 8/19/2019 <10 <10 10 <10 ND
3 8/27/2019 <10 <10 7,800 8,400 ND
-17-
CA 03141532 2021-11-22
WO 2020/236218
PCT/US2019/062753
4 9/6/2019 <10 <10 540,000 > 250,000 ND
[085] TEST 6: Beef Eye of Round
Test Date Coliforms E.Coli Generic Aerobic Anerobic
Listeria
(CFU/g) (CFU/g) Plate Count Plate Count
1 8/19/2019 <10 <10 360,000 800,000
ND
2 8/19/2019 <10 <10 20 <10 ND
3 8/27/2019 <10 <10 14,000 <10 ND
4 9/6/2019 <10 <10 NA NA ND
[086] TEST 7: Beef Inside Round Steak
Test Date Coliforms E.Coli Generic Aerobic Anerobic
Listeria
(CFU/g) (CFU/g) Plate Count Plate Count
1 8/19/2019 10 <10 5,600 5,700 ND
2 8/19/2019 <10 <10 <10 10 ND
3 8/27/2019 <10 <10 7,200 <10 ND
4 9/6/2019 <10 <10 NA NA ND
[087] TEST 8: Beef Bottom Sirloin Flap Meat
Test Date Coliforms E.Coli Generic Aerobic Anerobic
Listeria
(CFU/g) (CFU/g) Plate Count Plate Count
1 8/19/2019 <10 <10 530,000 1,200,000
ND
2 8/19/2019 <10 <10 10 <10 ND
3 8/27/2019 <10 <10 < 10 <10 ND
4 9/6/2019 <10 <10 NA NA ND
[088] TEST 9:
Test Date Coliforms E.Coli Generic Aerobic Anerobic
Listeria
(CFU/g) (CFU/g) Plate Count Plate Count
1 8/19/2019 10 <10 1,200 550 ND
2 8/19/2019 <10 <10 10 <10 ND
3 8/27/2019 <10 <10 40 <10 ND
4 9/6/2019 <10 <10 4,500 70 ND
[089] TEST 10: Pork Boneless Loin
Test Time Date Coliforms E.Coli Generic Aerobic
Anerobic Listeria
(CFU/g) (CFU/g)
Plate Count Plate Count
1 0 - before 4/19/2019 10 <10 1,200 550 ND
treatment
-18-
CA 03141532 2021-11-22
WO 2020/236218
PCT/US2019/062753
2 0 - after 4/19/2019 <10 <10 <10
<10 ND
treatment
3 1 8/27/2019 <10 <10 20 < 10
ND
4 2 9/6/2019 <10 <10 290,000 190,000 ND
[090] TEST 11: Pork Bone In Loin
Test Time Date Coliforms E.Coli
Generic Aerobic Anerobic Listeria
(CFU/g) (CFU/g)
Plate Count Plate Count
1 0 - before 8/19/2019 <10 <10 470
<10 ND
treatment
2 0 - after 8/19/2019 <10 <10 30
<10 ND
treatment
3 1 8/27/2019 <10 <10 10 <10
ND
4 2 9/6/2019 <10 <10 NA NA ND
[091] TEST 12: Pork Loin Ground
Test Time Date Coliforms E.Coli
Generic Aerobic Anerobic Listeria
(CFU/g) (CFU/g)
Plate Count Plate Count
1 0 - before 8/19/2019 <10 <10 320
<10 ND
treatment
2 0 - after 8/19/2019 <10 <10 <10
<10 ND
treatment
3 1 8/27/2019 <10 <10 50 <10
ND
4 2 9/6/2019 <10 <10 33,000 18,000 ND
[092] TEST 13: Chicken Thigh
Test Time Date Coliforms Salmonella Aerobic
Anerobic Listeria
(CFU/g)
Plate Count Plate Count
1 0 - before 8/19/2019 <10 ND
130,000 70,000 ND
treatment
2 0 - after 8/19/2019 <10 ND 140
<10 ND
treatment
3 1 8/27/2019 <10 ND 900 <10
ND
[093] TEST 14: Chicken Breast
Test Time Date Coliforms Salmonella Aerobic
Anerobic Listeria
(CFU/g)
Plate Count Plate Count
1 0 - before 8/19/2019 <10 ND 120
<10 ND
treatment
2 0 - after 8/19/2019 <10 ND <10
<10 ND
treatment
-19-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
3 1 8/27/2019 <10 ND <10
<10 ND
[094] TEST 15: Ground Chicken
Test Time Date Coliforms Salmonella Aerobic
Anerobic Listeria
(CFU/g) Plate Count Plate Count
1 0 - before 8/19/2019 <10 ND 40
<10 ND
treatment
2 0 - after 8/19/2019 <10 ND 10
<10 ND
treatment
3 1 8/27/2019 <10 ND 10
<10 ND
[095] Tests A-E tested the application of ozone (box 13) to meat products
and varying
HPP (box 28) process parameters over time.
[096] TEST A: Beef Not Ground
Days Date E. Coli Aerobic Anerobic Listeria
General CFU/g Plate Count plate count
0 - before 2/27/2019 <10 130 430 ND
0 - after 2/27/2019 <10 <10 <10 ND
22 3/20/2019 <10 <10 <10 ND
43 4/12/2019 <10 170,000 <10 ND
50 4/19/2019 <10 <10 <10 ND
60 4/29/2019 <10 <10 <10 ND
70 5/9/2019 <10 >3,000,000 <10 ND
81 5/20/2019 <10 1,300,000 <10 ND
* HPP 72K for 3 min; Ozone application
[097] TEST B: Chicken Breast
Days Date E. Coli Aerobic Anerobic Salmonella
Listeria
General CFU/g Plate Count plate count
0 2/27/2019 <10 <10 <10 ND ND
22 2/27/2019 <10 800,000 160,000 ND ND
* HPP 72K for 3 min; Ozone application
[098] TEST C: Chicken Breast
Days Date E. Coli Aerobic Anerobic Salmonella
Listeria
General CFU/g Plate Count plate count
0 - before 2/27/2019 <10 >3,000,000 2,600,000 ND
ND
0- after 2/27/2019 <10 80 <10 ND ND
22 3/20/2019 <10 990,000 600,000 ND ND
* HPP 70K for 3 min; Ozone application
[099] TEST D: Beef - not ground
-20-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
Days Date E. Coli Aerobic Anerobic Listeria
General CFU/g Plate Count plate count
0 - before 2/27/2019 <10 50 20 ND
0 - after 2/27/2019 <10 30 50 ND
22 3/20/2019 <10 <10 120 ND
43 4/12/2019 <10 10 <10 ND
50 4/19/2019 <10 70 <10 ND
60 4/29/2019 <10 <10 <10 ND
70 5/9/2019 <10 <10 <10 ND
81 5/20/2019 <10 <10 <10 ND
* HPP 87K for 3 min; Ozone Application
[0100] Tests E-L related to applying various process parameters to a 1/4
chicken. TEST
E reflects microbial values of the chicken before any processing via the
process 1. TEST F
reflects microbial values of the chicken after an ozone application step (box
13). TESTS G-L
reflect microbial values after treatment with HPP (box 28) with the processing
parameters
indicated.
[0101] TEST E: 1/4 Chicken
Days Date E. Coli Aerobic Plate Anerobic
Salmonella Listeria
General CFU/g Count plate count
0 2/28/2019 <10 290,000 <10 ND ND
* before treatment
[0102] TEST F: 1/4 Chicken
Days Date E. Coli Aerobic Plate Anerobic
Salmonella Listeria
General CFU/g Count plate count
0- 2/28/2019 <10 70,000 <10 ND ND
after
12 3/11/2019 <10 2,900,000 950,000 ND ND
* Ozone Application
[0103] TEST G: 1/4 Chicken
Days Date E. Coli Aerobic Plate Anerobic
Salmonella Listeria
General CFU/g Count plate count
0- 2/28/2019 <10 140 30 ND ND
after
12 3/11/2019 <10 1,600,000 <10 ND ND
* HPP 50K for 4 min
[0104] TEST H: 1/4 Chicken
Days Date E. Coli Aerobic Plate Anerobic
Salmonella Listeria
-21-
CA 03141532 2021-11-22
WO 2020/236218
PCT/US2019/062753
General CFU/g Count plate count
0 2/28/2019 <10 140 30 ND ND
12 3/11/2019 <10
2,800,000 100,000 ND ND
* HPP 50 K for 5 min
[0105] TEST I: 1/4 Chicken
Days Date E. Coli Aerobic Plate Anerobic
Salmonella Listeria
General CFU/g Count plate count
0 2/28/2019 <10 60 <10 ND ND
12 3/11/2019 <10
150,000 140,000 ND ND
20 3/19/2019 <10 >3,000,000 4,400 ND ND
* HPP 60K for 4 min
[0106] TEST J: 1/4 Chicken
Days Date E. Coli Aerobic Plate Anerobic
Salmonella Listeria
General CFU/g Count plate count
0 2/28/2019 <10 60 <10 ND ND
12 3/11/2019 <10
110,000 89,000 ND ND
20 3/19/2019 <10
>3,000,000 470,000 ND ND
* HPP 60K for 5 min
[0107] TEST K: 1/4 Chicken
Days Date E. Coli Aerobic Plate Anerobic
Salmonella Listeria
General CFU/g Count plate count
0 2/28/2019 <10 10 <10 ND ND
12 3/11/2019 <10 170,000 <10 ND ND
20 3/19/2019 <10
>3,000,000 21,000 ND ND
* HPP 70K for 4 min
[0108] TEST L: 1/4 Chicken
Days Date E. Coli Aerobic Plate Anerobic
Salmonella Listeria
General CFU/g Count plate count
0 2/28/2019 <10 10 <10 ND ND
12 3/11/2019 <10 19,000 14,000 ND ND
* HPP 70K for 5 min
[0109] Another set of tests were conducted on beef sirloin flap meat
using various
process parameters and including or omitted various steps and/or substeps. In
tests using
Modified Atmosphere Packaging ("MAP") a gas mixture of 80% N, 19.6% CO2, and
0.4% CO
was used.
Process Total Coliforms E. Coli Aerobic Plate
(CFU/g) (CFU/g) Count (CFU/g)
-22-
CA 03141532 2021-11-22
WO 2020/236218 PCT/US2019/062753
Vacuum Packed 200 <10 7700
Ozone Applied; Vacuum 560 <10 7700
Packed
Ozone Applied; 90 <10 6800
MAP
Ozone applied; MAP; <10 <10 680
HPP 50K for 4 min
Ozone applied; MAP; <10 <10 350
HPP 50K for 5 min
Ozone applied; <10 <10 2800
MAP; HPP 50K for 6
min
Ozone applied; MAP; <10 <10 470
HPP 60K for 4 min
[0110] Although the disclosure has been described with reference to
preferred
embodiments, persons skilled in the art will recognize that changes may be
made in form and
detail without departing from the spirit and scope of the disclosed apparatus,
systems and
methods.
-23-