Note: Descriptions are shown in the official language in which they were submitted.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 1 -
EXPLOSIVES BASED ON HYDROGEN PEROXIDE WITH IMPROVED SLEEP TIME
FIELD OF THE INVENTION
The present invention relates to improved hydrogen peroxide-based explosives.
The invention has been developed primarily for use as a hydrogen peroxide/fuel-
based
explosive composition for use in mining applications and will be described
hereinafter with
reference to this application. However, it will be appreciated that the
invention is not limited
to this particular field of use.
BACKGROUND OF THE INVENTION
The following discussion of the prior art is provided to place the invention
in an
appropriate technical context and enable the advantages of it to be more fully
understood.
It should be appreciated, however, that any discussion of the prior art
throughout the
specification should not be considered as an express or implied admission that
such prior
art is widely known or forms part of common general knowledge in the field.
Nearly all commercial and mining explosives used in the world today are based
on
ammonium nitrate (AN) or combinations of AN with smaller quantities of other
alkaline
and/or alkaline earth nitrate salts, e.g. sodium nitrate (SN) or calcium
nitrate (ON). AN,
which is a strong oxidiser, has been used as the base of commercial explosives
for at least
the last 50-60 years. Most explosives of this type rely on the energetic
reaction of nitrogen
compounds incorporated within the explosive to provide the necessary explosive
power.
Initially, mining companies used AN as an explosive on its own. However, they
soon realised that the addition of diesel increased the energy output without
a large
increase on costs (ammonium nitrate ¨ fuel oil, now commonly referred to as
'ANFO').
However, the water resistance of ANFO is quite poor, which limited its use in
wet blast
holes. To ameliorate this issue, slurries and watergels were developed.
Slurries typically
comprise AN dissolved/dispersed in water, and other salts such as calcium
nitrate, sodium
nitrate, amine nitrates, perchlorates, etc. and other additives such as guar
gum (as
thickener) and water soluble or insoluble fuels (glycerol, MMAN, diesel, etc).
They can
also be blended with ANFO depending on the characteristics of the ground being
blasted.
Slurries also typically include solid sensitisers (aluminium and high
explosives such as
TNT, RDX, etc) to enable the slurry to detonate and to minimise misfires.
Watergels have
similar compositions to slurries, however, crosslinkers can be added to
enhance the water
resistance of the product.
One of the drawbacks of watergels and slurries is that there is a limit of AN
which
can be incorporated into the solution. This drawback was overcome by the
development
of water-in-oil emulsions. These emulsions can contain AN in high
concentration (see US
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 2 -
Patent No. 3,447,978) as emulsions are manufactured at high temperatures.
Water-in-oil
emulsions are made of a hot aqueous phase (composed of AN, other nitrate
salts,
perchlorate salts, etc.) dispersed into an organic fuel. The aqueous ¨ organic
mixture is
stabilised by the use of an emulsifier. Emulsions can also be blended with
ANFO in
different ratios so suit the ground to be blasted.
Despite the development of AN emulsions, AN slurries, and watergels, however,
there is still a need to develop improved explosives, which are more cost
effective
compared to existing explosive compositions and are capable of being produced
in large
quantities to meet the high demand from industry. It would be advantageous to
use less
AN in the formulation and instead use other types of nitrates to provide
alternatives to the
usage of AN. Additionally, such substitutes should preferably be safer, have a
relatively
low carbon footprint, able to be manufactured nearby the point of use to
minimise the
transport on public roads, able to be manufactured on an as-needs basis to
minimise the
need for stockpiling and to increase safety, allow for the use of existing
delivery equipment,
.. and/or produce a lower amount of (or no) toxic nitrogen oxide fumes (NOR)
upon
detonation, etc. It would also be ideal if there are no onerous regulatory
requirements for
such a substitute, thereby reducing administrative costs. It would also be
preferable for
the explosive composition to be crosslinkable in-situ to increase viscosity
down the
blasthole.
Despite the advances on the types of compositions that can be manufactured
from
ammonium nitrate, one of the disadvantages is that during the detonation NO
fumes can
be generated, due to the presence of nitrogen compounds in the explosive
composition
(from nitrates). These NO fumes are toxic and can affect the health of mine
site personnel.
Therefore, the emission of NO fume after blasting is a safety issue and, in
countries like
Australia, there are now strict regulatory controls in place to manage such
emissions. See
for example "Queensland Guidance Note: Management of oxides of nitrogen in
open cut
blasting" issued by the regulator in Queensland, Australia, 2011. Likewise,
explosive
manufacturers in Australia have also issued a code of practice to manage the
NO fumes
after blasting (AEISG Code of Practice, Prevention and Management of Blast
Generated
NO Gases in Surface Blasting, 2011). Therefore, there is a need to find
explosive
compositions that substantially reduce the production of NOR.
One material that is also an oxidiser and that has the potential to meet at
least
some of these needs is hydrogen peroxide (H202). H202/fuel-based explosives
for mining
operations generally consist of the combination of H202 with about 5 to about
15 percent
of its weight of a liquid carbon-based fuel such as glycerol, fuel oil, and
the like. However,
it has been observed that, in some cases, H202-based explosives are less than
ideal where
sleep-time above 24 hours is required, as it has been observed that the
density changes
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 3 -
over time, which can affect parameters such as the velocity of detonation
(VOD).
It is an object of the present invention to overcome or ameliorate one or more
of
the disadvantages of the prior art, or at least to provide a useful
alternative.
It is an object of preferred forms of the invention to provide a H202/fuel-
based
explosive that has improved sleep time. Preferred explosive compositions of
the invention
have substantially maintained sensitivity, density, and VOD over an extended
sleep time,
which can be in the range of 24 to 48 hours, or even longer. The improved
explosive
compositions of the invention can be safely employed in mines due to extended
sleep-
time, and in some embodiments enable blasts to be undertaken that are not
possible with
prior art H202-based explosive compositions that do not have the extended
sleep time as
per the compositions of the present invention. In particular, compositions of
the invention
enable much larger blasts to be undertaken, as the inventive explosive
compositions
described herein have substantially maintained VOD over an extended period of
time in
the blast hole.
A preferred objective of an embodiment of the present invention is to provide
an
explosive composition which meets one or more of the following objectives: is
conveniently
prepared, has improved density stability over time in situ, can use large
amounts of
sustainable fuels (which lowers the carbon footprint of the explosive), and in
some
preferred embodiments can use large amount of nitrates other than AN (which
lowers the
dependency on AN), and enables much larger blasts due to extended sleep time.
A further preferred objective of the present invention is to substantially
maintain the
density of the explosive composition when loaded into the blast hole, thereby
substantially
maintaining the sleep time for days, and potentially for weeks.
SUMMARY OF THE INVENTION
The present invention relates to explosives for use in commercial,
construction,
civil, agriculture, mining, and similar fields. However, it will be
appreciated that the
invention could be utilised in other related fields.
It has now surprisingly been discovered that H202-based explosive
compositions,
when treated or modified with density stabilisers (i.e., phosphonates),
display improved
sleep time over prior art H202-based explosive compositions. Further, it has
been
surprisingly found that only relatively small quantities of phosphonates
provide the
improved density stability and sleep time. Without wishing to be bound by any
theory, the
inventor considers that the density stabiliser employed in the present
invention acts to
prevent additional bubbles of entrained sensitiser gas to spontaneously form,
whereas
without a density stabiliser existing sensitiser bubbles tend to increase in
volume and
number over time.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 4 -
In the practice of the present invention, phosphonates, usually in liquid
form, are
added to the H202 prior to the addition of fuel. Thickeners may be added to
the combination
of the oxidiser/fuel mixture.
Generally, one or more density stabilisers are incorporated in an amount of up
to
about 15% w/w of the explosives composition, for example about 0.01 `)/0 w/w
to about 10
`)/0 w/w, e.g. about 1 to about 5 `)/0 w/w, such as about 1 to about 3 `)/0
w/w. Thereafter, the
density-stabilised H202-based explosive can be handled, loaded, and fired in
identical
fashion to other explosives.
According to a first aspect, the present invention provides an explosive
composition
comprising:
a. H202;
b. fuel; and
c. one or more density stabilisers.
According to a second aspect, the present invention provides a method of
preparing an explosive composition according to the first aspect, the method
comprising:
combining H202, fuel and one or more density stabilisers and optionally one or
more other
oxidisers and/or a sensitiser.
According to a third aspect, the present invention provides use of an
explosive
composition according to the first aspect to break and move ground, e.g. in
mining
.. operations.
According to a fourth aspect, the present invention provides the use of one or
more
density stabilisers to improve the sleep time of an explosive composition in
reactive or
metalliferous ground, wherein the explosive composition comprises H202 and
fuel.
In a related aspect, the present invention consists of a method of treating an
explosive composition to improve its long-term stability (sleep time), the
method
comprising the step of combining a density stabiliser with said explosive
composition to
stabilise the density of the explosive composition, wherein the explosive
composition
comprises H202 and fuel. In a related aspect, the present invention comprises
the use of
a density stabiliser for improving the long-term stability of an explosive
composition. The
density stabiliser is used in a density-stabilising concentration.
The present invention relates to an explosive which substantially avoids the
release
of unwanted NO fumes upon detonation into the atmosphere surrounding the
blasting
site. A preferred objective of the present invention is to reduce and
preferably eliminate
nitrogen containing ingredients from the explosive composition. It will be
appreciated that
with little or no nitrogen present in the explosive virtually no NO is
released into the
atmosphere, or a substantially reduced amount is released. The present
invention relates
to explosives for use in commercial, construction, agriculture, mining, and
similar fields.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 5 -
However, it will be appreciated that the invention could be utilised in other
related fields.
The density is maintained by including a density stabiliser. The density
stabiliser
retains the density of the explosive composition to within +/- 0.1, 0.2, 0.3,
0.4, 0.5, 0.6, 0.7,
0.8, 0.9, 1.0, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 14, 16, 18, 20, 21, 22, 23, 24,
25, 26, 27, 28, 29,
30, 35, 40, or 50% of its initial density, and/or the density of the explosive
composition
within 5, 10, 15, 20, 30 or 60, 90 or 120 minutes of being loaded into the
blast hole. The
density is preferably maintained (or stabilised) over a period of up to 1, 2,
3, 4, 5, 6, 7, 8,
9, 10, 11, 12, 13, or 14 days. The density stabiliser preferably maintains the
VOD to within
+/- 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1.0, 2, 3, 4, 5, 6, 7, 8, 9,
10, 12, 14, 16, 18, 20,
21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 35, 40, or 50% of the initial VOD, or
the VOD of the
explosive composition within 5, 10, 15, 20, 30, 60, 90 or 120 minutes of being
loaded into
the blast hole. The VOD is preferably maintained over a period of up to 1, 2,
3, 4, 5, 6, 7,
8, 9, 10, 11, 12, 13, or 14 days.
The compositions of the invention have a predictable and controllable change
in
density and VOD over time. For example, at certain concentrations of density
stabiliser,
the density reduces by around 5% per day to around 10% per day, causing a
corresponding drop of around 5% to 10% per day in VOD. This enables the drill
and blast
engineer to provide a predetermined VOD after a certain sleep time, as the
quantity of
density stabiliser can be selected to control change in density over that
sleep time. It will
be appreciated that, in some prior art compositions, the density reduces by
around 10%
per day to around 30% per day, causing a corresponding drop of around 10% to
30% per
day in VOD.
Preferably the composition further includes other additives, such as fuel,
water,
thickeners, emulsifiers, mechanical sensitisation, chemically-derived
sensitisation,
injected gases, etc, as discussed further below. In one preferred embodiment
the
composition comprises no components which lead to the production of NO in the
after-
blast fumes. However, in other embodiments components are added which result
in
minimal NO in the after-blast fumes.
Whilst the preferred explosive oxidiser of the invention is hydrogen peroxide,
it will
be appreciated that other oxidiser salts or peroxide derivatives can be used
with the
invention, as partial replacements of H202. Non-limiting examples include
nitrates salts,
perchlorates salts, sodium/potassium peroxide, etc.
DEFINITIONS
In describing and claiming the present invention, the following terminology
will be
used in accordance with the definitions set out below. It is also to be
understood that the
terminology used herein is for the purpose of describing particular
embodiments of the
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 6 -
invention only and is not intended to be limiting. Unless defined otherwise,
all technical
and scientific terms used herein have the same meaning as commonly understood
by one
having ordinary skill in the art to which the invention pertains.
Unless the context clearly requires otherwise, throughout the description and
the
claims, the words 'comprise', 'comprising', and the like are to be construed
in an inclusive
sense as opposed to an exclusive or exhaustive sense; that is to say, in the
sense of
'including, but not limited to'.
Other than in the operating examples, or where otherwise indicated, all
numbers
expressing quantities of ingredients or reaction conditions used herein are to
be
understood as modified in all instances by the term 'about'. The examples are
not intended
to limit the scope of the invention. In what follows, or where otherwise
indicated, r /0' will
mean 'weight /0', 'ratio' will mean 'weight ratio' and 'parts' will mean
'weight parts'.
Unless the context clearly indicates otherwise, all references to a component
being
present at a certain A, w/w are with respect to the entire explosive
composition. For
example, an explosive composition comprising 2-25 A, w/w hydrogen peroxide
refers to
an explosive composition comprising 2-25 g hydrogen peroxide per 100 g of the
explosive
composition.
The term H202 is an abbreviation for hydrogen peroxide.
The term AN means ammonium nitrate.
ON means calcium nitrate tetra hydrate.
CAN means calcium ammonium nitrate
SN is an abbreviation for sodium nitrate.
ANFO is an abbreviation for ammonium nitrate fuel oil.
Amine nitrates is an abbreviation for monomethylamine or ethyl amine or propyl
amine nitrate.
Sensitiser means an additive that introduces voids in the composition.
Sensitisers
enable and increase the sensitivity to detonation of energetic materials. The
sensitiser can
be chemically generated voids (gas bubbles) or can enclose or entrap a gas
(examples of
which include ceramic/glass microballoons, EPS and polyurethane foams).
GMB is an abbreviation for glass micro balloons.
EPS is an abbreviation for expanded polystyrene.
TNT means trinitrotoluene.
HMX refers to octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine.
RDX refers to 1,3,5-trinitroperhydro-1,3,5-triazine.
VOD refers to velocity of detonation in m/sec.
OB means oxygen balance.
The term g/cm3 is has the same meaning as g/ml.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 7 -
Phosphonates are organic compounds with C-P bonds, such as C-PO(OH)2 or
C-PO(OR)2 groups. "Phosphonate" as used herein also includes phosphonate salts
comprising phosphonate anions with counter-cations (e.g. sodium salts).
"Phosphonate"
includes mono-phosphonates as well as bis-phosphonates and higher
phosphonates. The
R group of a phosphonate is not limited to alkyl and can, for example, include
heteroatoms
(e.g. N).
DTPMPA.Na.x mean diethylenetriamine pentamethylene phosphonic acid sodium
salt (C9H28_xN3015P5Nax, CAS no. 22042-96-2).
The term phosphate refers to chemical derivatives of phosphoric acid. The
.. phosphate ion (P043-) is the conjugate base of phosphoric acid and can form
many
different salts. Organophosphates have the general structure 0=P(OR)3, wherein
the R
groups can be the same or different. R includes alkyl and aryl. Phosphates
comprise
COP bonds and lack the C-P bonds present in phosphonates.
The term "stannate" refers to compounds containing tin (II), (IV) or (VI) and
oxygen.
Sleep time is understood as the time between explosives being loaded into a
blast
hole and their initiation. The period is typically days.
The terms 'preferred', 'preferably' and 'suitably' refer to embodiments of the
invention that may afford certain benefits, under certain circumstances.
However, other
embodiments may also be preferred, under the same or other circumstances.
Furthermore, the recitation of one or more preferred embodiments does not
imply that
other embodiments are not useful, and is not intended to exclude other
embodiments from
the scope of the invention.
The terms 'a', 'an' and 'the' mean 'one or more', unless expressly specified
otherwise. The terms 'an embodiment', 'embodiment', 'embodiments', 'the
embodiment',
'the embodiments', 'an embodiment', 'some embodiments', 'an example
embodiment', 'at
least one embodiment', 'one or more embodiments' and 'one embodiment' mean
'one or
more (but not necessarily all) embodiments of the present invention(s)' unless
expressly
specified otherwise.
The terms "subterranean" or "sub-surface" refers to areas below exposed earth
and areas below earth covered by water such as fresh water and salt water.
The term "optionally substituted" as used throughout the specification denotes
that
the group may or may not be further substituted or fused (so as to form a
condensed
polycyclic system), with one or more non-hydrogen substituent groups. In
certain
embodiments the substituent groups are one or more groups independently
selected from
the group consisting of halogen, =0, =S, -ON, -NO2, -CF3, -00F3, alkyl,
alkenyl, alkynyl,
haloalkyl, haloalkenyl, haloalkynyl, heteroalkyl, cycloalkyl, cycloalkenyl,
heterocycloalkyl,
heterocycloalkenyl, aryl, heteroaryl, cycloalkylalkyl, heterocycloalkylalkyl,
heteroarylalkyl,
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 8 -
arylalkyl, cycloalkylalkenyl, heterocycloalkylalkenyl, arylalkenyl,
heteroarylalkenyl,
cycloalkylheteroalkyl, heterocycloalkylheteroalkyl, arylheteroalkyl,
heteroarylheteroalkyl,
hydroxy, hydroxyalkyl, alkyloxy, alkyloxyalkyl, alkyloxycycloalkyl,
alkyloxyheterocycloalkyl,
alkyloxyaryl, alkyloxyheteroaryl, alkyloxycarbonyl, alkylaminocarbonyl,
alkenyloxy,
alkynyloxy, cycloalkyloxy, cycloalkenyloxy, heterocycloalkyloxy,
heterocycloalkenyloxy,
aryloxy, phenoxy, benzyloxy, heteroaryloxy, arylalkyloxy, amino, alkylamino,
acylamino,
aminoalkyl, arylamino, sulfonylamino, sulfinylamino, sulfonyl, alkylsulfonyl,
arylsulfonyl,
aminosulfonyl, sulfinyl, alkylsulfinyl, arylsulfinyl, aminosulfinylaminoalkyl,
-C(=0)0H, -
C(=0)Re, -C(=0)0Re, C(=0)NReRf, C(=NOH)Re, C(=NRe)NRfRg, NReRf, NReC(=0)Rf,
NReC(=0)0Rf, NReC(=0)NRfRg, NReC(=NRf)NRgRh, NReS02Rf, -SR, SO2NReRf, -0Re,
OC(=0)NReRf, OC(=0)Re and acyl,
wherein Re, Rf, Rg and Rh are each independently selected from the group
consisting of H, C1-C12alkyl, Ci-Ci2haloalkyl, 02-Ci2alkenyl, 02-C12alkynyl,
Ci-
Cioheteroalkyl, 03-C12cycloalkyl, 03-C12cycloalkenyl, Ci-Ci2heterocycloalkyl,
Ci-
Ci2heterocycloalkenyl, 06-Ci8aryl, Ci-Cisheteroaryl, and acyl, or any two or
more of Ra,
Rb, RC and Rd, when taken together with the atoms to which they are attached
form a
heterocyclic ring system with 3 to 12 ring atoms.
In some embodiments each optional substituent is independently selected from
the
group consisting of: halogen, =0, =S, -ON, -NO2, -CF3, -0CF3, alkyl, alkenyl,
alkynyl,
haloalkyl, haloalkenyl, haloalkynyl, heteroalkyl, cycloalkyl, cycloalkenyl,
heterocycloalkyl,
heterocycloalkenyl, aryl, heteroaryl, hydroxy, hydroxyalkyl, alkyloxy,
alkyloxyalkyl,
alkyloxyaryl, alkyloxyheteroaryl, alkenyloxy, alkynyloxy, cycloalkyloxy,
cycloalkenyloxy,
heterocycloalkyloxy, heterocycloalkenyloxy, aryloxy,
heteroaryloxy, arylalkyl,
heteroarylalkyl, arylalkyloxy, amino, alkylamino, acylamino, aminoalkyl,
arylamino,
sulfonyl, alkylsulfonyl, arylsulfonyl, aminosulfonyl, aminoalkyl, -COOH, -SH,
and acyl.
Examples of particularly suitable optional substituents include F, CI, Br, I,
CH3,
0H20H3, OH, 00H3, CF3, 00F3, NO2, NH2, and ON.
In the definitions of a number of substituents below it is stated that "the
group may
be a terminal group or a bridging group". This is intended to signify that the
use of the
term is intended to encompass the situation where the group is a linker
between two other
portions of the molecule as well as where it is a terminal moiety. Using the
term alkyl as
an example, some publications would use the term "alkylene" for a bridging
group and
hence in these other publications there is a distinction between the terms
"alkyl" (terminal
group) and "alkylene" (bridging group). In the present application no such
distinction is
made and most groups may be either a bridging group or a terminal group.
"Acyl" means an R-C(=0)- group in which the R group may be an alkyl,
cycloalkyl,
heterocycloalkyl, aryl or heteroaryl group as defined herein. Examples of acyl
include
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 9 -
acetyl and benzoyl. The group may be a terminal group or a bridging group. If
the group
is a terminal group it is bonded to the remainder of the molecule through the
carbonyl
carbon.
"Acylamino" means an R-C(=0)-NH- group in which the R group may be an alkyl,
cycloalkyl, heterocycloalkyl, aryl or heteroaryl group as defined herein. The
group may be
a terminal group or a bridging group. If the group is a terminal group it is
bonded to the
remainder of the molecule through the nitrogen atom.
"Alkenyl" as a group or part of a group denotes an aliphatic hydrocarbon group
containing at least one carbon-carbon double bond and which may be straight or
branched
preferably having 2-12 carbon atoms, more preferably 2-10 carbon atoms, most
preferably
2-6 carbon atoms, in the normal chain. The group may contain a plurality of
double bonds
in the normal chain and the orientation about each is independently E or Z.
The alkenyl
group is preferably a 1-alkenyl group. Exemplary alkenyl groups include, but
are not
limited to, ethenyl, propenyl, butenyl, pentenyl, hexenyl, heptenyl, octenyl
and nonenyl.
The group may be a terminal group or a bridging group.
"Alkenyloxy" refers to an alkenyl-O- group in which alkenyl is as defined
herein.
Preferred alkenyloxy groups are Ci-C6alkenyloxy groups. The group may be a
terminal
group or a bridging group. If the group is a terminal group it is bonded to
the remainder of
the molecule through the oxygen atom.
"Alkyl" as a group or part of a group refers to a straight or branched
aliphatic
hydrocarbon group, preferably a 01-012 alkyl, more preferably a Ci-Cio alkyl,
most
preferably 01-06 unless otherwise noted. Examples of suitable straight and
branched
01-06 alkyl substituents include methyl, ethyl, n-propyl, 2-propyl, n-butyl,
sec-butyl, t-butyl,
hexyl, and the like. The group may be a terminal group or a bridging group.
"Alkylamino" includes both mono-alkylamino and dialkylamino, unless specified.
"Mono-alkylamino" means an Alkyl-NH- group, in which alkyl is as defined
herein.
"Dialkylamino" means a (alkyl)2N- group, in which each alkyl may be the same
or different
and are each as defined herein for alkyl. The alkyl group is preferably a Ci-
06a1ky1 group.
The group may be a terminal group or a bridging group. If the group is a
terminal group it
is bonded to the remainder of the molecule through the nitrogen atom.
"Alkylaminocarbonyl" refers to a group of the formula (Alkyl)x(H)yNC(=0)- in
which
alkyl is as defined herein, x is 1 or 2, and the sum of X+Y = 2. The group may
be a terminal
group or a bridging group. If the group is a terminal group it is bonded to
the remainder of
the molecule through the carbonyl carbon.
"Alkyloxy" refers to an alkyl-0- group in which alkyl is as defined herein.
Preferably
the alkyloxy is a Ci-06a1ky10xy. Examples include, but are not limited to,
methoxy and
ethoxy. The group may be a terminal group or a bridging group.
CA 03142594 2021-12-03
WO 2020/243788 PC T/AU2020/050573
- 10 -
"Alkyloxyalkyl" refers to an alkyloxy-alkyl- group in which the alkyloxy and
alkyl
moieties are as defined herein. The group may be a terminal group or a
bridging group.
If the group is a terminal group it is bonded to the remainder of the molecule
through the
alkyl group.
"Alkyloxyaryl" refers to an alkyloxy-aryl- group in which the alkyloxy and
aryl
moieties are as defined herein. The group may be a terminal group or a
bridging group.
If the group is a terminal group it is bonded to the remainder of the molecule
through the
aryl group.
"Alkyloxycarbonyl" refers to an alkyl-O-C(=0)- group in which alkyl is as
defined
herein. The alkyl group is preferably a 01-06 alkyl group. Examples include,
but are not
limited to, methoxycarbonyl and ethoxycarbonyl. The group may be a terminal
group or a
bridging group. If the group is a terminal group it is bonded to the remainder
of the
molecule through the carbonyl carbon.
"Alkyloxycycloalkyl" refers to an alkyloxy-cycloalkyl- group in which the
alkyloxy
and cycloalkyl moieties are as defined herein. The group may be a terminal
group or a
bridging group. If the group is a terminal group it is bonded to the remainder
of the
molecule through the cycloalkyl group.
"Alkyloxyheteroaryl" refers to an alkyloxy-heteroaryl- group in which the
alkyloxy
and heteroaryl moieties are as defined herein. The group may be a terminal
group or a
bridging group. If the group is a terminal group it is bonded to the remainder
of the
molecule through the heteroaryl group.
"Alkyloxyheterocycloalkyl" refers to an alkyloxy-heterocycloalkyl- group in
which
the alkyloxy and heterocycloalkyl moieties are as defined herein. The group
may be a
terminal group or a bridging group. If the group is a terminal group it is
bonded to the
remainder of the molecule through the heterocycloalkyl group.
"Alkylsulfinyl" means an alkyl-S-(=0)- group in which alkyl is as defined
herein.
The alkyl group is preferably a 01-06 alkyl group. Exemplary alkylsulfinyl
groups include,
but not limited to, methylsulfinyl and ethylsulfinyl. The group may be a
terminal group or
a bridging group. If the group is a terminal group it is bonded to the
remainder of the
molecule through the sulfur atom.
"Alkylsulfonyl" refers to an alkyl-S(=0)2- group in which alkyl is as defined
above.
The alkyl group is preferably a Ci-06a1ky1 group. Examples include, but not
limited to
methylsulfonyl and ethylsulfonyl. The group may be a terminal group or a
bridging group.
If the group is a terminal group it is bonded to the remainder of the molecule
through the
sulfur atom.
"Alkynyl" as a group or part of a group means an aliphatic hydrocarbon group
containing a carbon-carbon triple bond and which may be straight or branched
preferably
CA 03142594 2021-12-03
WO 2020/243788 PC T/AU2020/050573
-11 -
having from 2-12 carbon atoms, more preferably 2-10 carbon atoms, more
preferably 2-6
carbon atoms in the normal chain. Exemplary structures include, but are not
limited to,
ethynyl and propynyl. The group may be a terminal group or a bridging group.
"Alkynyloxy" refers to an alkyny1-0- group in which alkynyl is as defined
herein.
Preferred alkynyloxy groups are C1-C6alkynyloxy groups. The group may be a
terminal
group or a bridging group. If the group is a terminal group it is bonded to
the remainder of
the molecule through the oxygen atom.
"Aminoalkyl" means an NH2-alkyl- group in which the alkyl group is as defined
herein. The group may be a terminal group or a bridging group. If the group is
a terminal
group it is bonded to the remainder of the molecule through the alkyl group.
"Aminosulfonyl" means an NH2-S(=0)2- group. The group may be a terminal group
or a bridging group. If the group is a terminal group it is bonded to the
remainder of the
molecule through the sulfur atom.
"Aryl" as a group or part of a group denotes (i) an optionally substituted
monocyclic,
or fused polycyclic, aromatic carbocycle (ring structure having ring atoms
that are all
carbon) preferably having from 5 to 12 atoms per ring. Examples of aryl groups
include
phenyl, naphthyl, and the like; (ii) an optionally substituted partially
saturated bicyclic
aromatic carbocyclic moiety in which a phenyl and a C5_7cycloalkyl or
C5_7cycloalkenyl
group are fused together to form a cyclic structure, such as
tetrahydronaphthyl, indenyl or
indanyl. The group may be a terminal group or a bridging group. Typically an
aryl group
is a 06-018 aryl group.
"Arylalkenyl" means an aryl-alkenyl- group in which the aryl and alkenyl are
as
defined herein. Exemplary arylalkenyl groups include phenylallyl. The group
may be a
terminal group or a bridging group. If the group is a terminal group it is
bonded to the
remainder of the molecule through the alkenyl group.
"Arylalkyl" means an aryl-alkyl- group in which the aryl and alkyl moieties
are as
defined herein. Preferred arylalkyl groups contain a 0i_5a1ky1 moiety.
Exemplary arylalkyl
groups include benzyl, phenethyl, 1-naphthalenemethyl and 2-naphthalenemethyl.
The
group may be a terminal group or a bridging group. If the group is a terminal
group it is
bonded to the remainder of the molecule through the alkyl group.
"Arylalkyloxy" refers to an aryl-alkyl-0- group in which the alkyl and aryl
are as
defined herein. The group may be a terminal group or a bridging group. If the
group is a
terminal group it is bonded to the remainder of the molecule through the
oxygen atom.
"Arylamino" includes both mono-arylamino and di-arylamino unless specified.
Mono-arylamino means a group of formula aryINH-, in which aryl is as defined
herein.
Di-arylamino means a group of formula (aryl)2N- where each aryl may be the
same or
different and are each as defined herein for aryl. The group may be a terminal
group or a
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 12 -
bridging group. If the group is a terminal group it is bonded to the remainder
of the
molecule through the nitrogen atom.
"Arylheteroalkyl" means an aryl-heteroalkyl- group in which the aryl and
heteroalkyl
moieties are as defined herein. The group may be a terminal group or a
bridging group.
If the group is a terminal group it is bonded to the remainder of the molecule
through the
heteroalkyl group.
"Aryloxy" refers to an aryl-0- group in which the aryl is as defined herein.
Preferably the aryloxy is a 06-C18aryloxy, more preferably a 06-Cioaryloxy.
The group may
be a terminal group or a bridging group. If the group is a terminal group it
is bonded to the
remainder of the molecule through the oxygen atom.
"Arylsulfonyl" means an aryl-S(=0)2- group in which the aryl group is as
defined
herein. The group may be a terminal group or a bridging group. If the group is
a terminal
group it is bonded to the remainder of the molecule through the sulfur atom.
A "bond" is a linkage between atoms in a compound or molecule. The bond may
be a single bond, a double bond, or a triple bond.
"Cycloalkenyl" means a non-aromatic monocyclic or multicyclic ring system
containing at least one carbon-carbon double bond and preferably having from 5-
10
carbon atoms per ring. Exemplary monocyclic cycloalkenyl rings include
cyclopentenyl,
cyclohexenyl or cycloheptenyl. The cycloalkenyl group may be substituted by
one or more
substituent groups. A cycloalkenyl group typically is a 03-012 alkenyl group.
The group
may be a terminal group or a bridging group.
"Cycloalkyl" refers to a saturated monocyclic or fused or spiro polycyclic,
carbocycle preferably containing from 3 to 9 carbons per ring, such as
cyclopropyl,
cyclobutyl, cyclopentyl, cyclohexyl and the like, unless otherwise specified.
It includes
monocyclic systems such as cyclopropyl and cyclohexyl, bicyclic systems such
as decalin,
and polycyclic systems such as adamantane. A cycloalkyl group typically is a
03-012 alkyl
group. The group may be a terminal group or a bridging group.
"Cycloalkylalkyl" means a cycloalkyl-alkyl- group in which the cycloalkyl and
alkyl
moieties are as defined herein. Exemplary monocycloalkylalkyl groups
include
cyclopropylmethyl, cyclopentylmethyl, cyclohexylmethyl and cycloheptylmethyl.
The
group may be a terminal group or a bridging group. If the group is a terminal
group it is
bonded to the remainder of the molecule through the alkyl group.
"Cycloalkylalkenyl" means a cycloalkyl-alkenyl- group in which the cycloalkyl
and
alkenyl moieties are as defined herein. The group may be a terminal group or a
bridging
group. If the group is a terminal group it is bonded to the remainder of the
molecule through
the alkenyl group.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 13 -
"Cycloalkylheteroalkyl" means a cycloalkyl-heteroalkyl- group in which the
cycloalkyl and heteroalkyl moieties are as defined herein. The group may be a
terminal
group or a bridging group. If the group is a terminal group it is bonded to
the remainder of
the molecule through the heteroalkyl group.
"Cycloalkyloxy" refers to a cycloalkyl-O- group in which cycloalkyl is as
defined
herein. Preferably the cycloalkyloxy is a Cl-C6cycloalkyloxy. Examples
include, but are
not limited to, cyclopropanoxy and cyclobutanoxy. The group may be a terminal
group or
a bridging group. If the group is a terminal group it is bonded to the
remainder of the
molecule through the oxygen atom.
"Cycloalkenyloxy" refers to a cycloalkeny1-0- group in which the cycloalkenyl
is as
defined herein. Preferably the cycloalkenyloxy is a Cl-C6cycloalkenyloxy. The
group may
be a terminal group or a bridging group. If the group is a terminal group it
is bonded to the
remainder of the molecule through the oxygen atom.
"Haloalkyl" refers to an alkyl group as defined herein in which one or more of
the
hydrogen atoms has been replaced with a halogen atom selected from the group
consisting of fluorine, chlorine, bromine and iodine. A haloalkyl group
typically has the
formula CnH(2n+1-m)Xm wherein each X is independently selected from the group
consisting
of F, Cl, Br and I. In groups of this type n is typically from 1 to 10, more
preferably from 1
to 6, most preferably 1 to 3. m is typically 1 to 6, more preferably 1 to 3.
Examples of
haloalkyl include fluoromethyl, difluoromethyl and trifluoromethyl.
"Haloalkenyl" refers to an alkenyl group as defined herein in which one or
more of
the hydrogen atoms has been replaced with a halogen atom independently
selected from
the group consisting of F, Cl, Br and I.
"Haloalkynyl" refers to an alkynyl group as defined herein in which one or
more of
the hydrogen atoms has been replaced with a halogen atom independently
selected from
the group consisting of F, Cl, Br and I.
"Halogen" represents chlorine, fluorine, bromine or iodine.
"Heteroalkyl" refers to a straight- or branched-chain alkyl group preferably
having
from 2 to 24 carbons, 2 to 18 carbons, 2 to 14 carbons, 2 to 12 carbons, 2 to
6 carbons in
the chain, in which one or more of the carbon atoms (and any associated
hydrogen atoms)
are each independently replaced by a heteroatomic group selected from S, 0, P
and NR'
where R' is selected from the group consisting of H, optionally substituted Cl-
Ci2alkyl,
optionally substituted 03-Ci2cyc10a1ky1, optionally substituted 06-Ci8ary1,
and optionally
substituted 01-C18heteroaryl. Exemplary heteroalkyls include alkyl ethers,
secondary and
tertiary alkyl amines, amides, alkyl sulfides, and the like. Examples of
heteroalkyl also
include hydroxyC1-C6alkyl, Cl-C6alkyloxyCi-C6alkyl, aminoC1-C6alkyl, 01-
C6alkylaminoC1-
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 14 -
C6alkyl, and di(Ci-C6alkyl)aminoCi-C6alkyl. The group may be a terminal group
or a
bridging group.
"Heteroalkyloxy" refers to a heteroalkyl-O- group in which heteroalkyl is as
defined
herein. Preferably the heteroalkyloxy is a 02-C6heteroalkyloxy. The group may
be a
terminal group or a bridging group.
"Heteroaryl" either alone or part of a group refers to groups containing an
aromatic
ring (preferably a 5 or 6 membered aromatic ring) having one or more
heteroatoms as ring
atoms in the aromatic ring with the remainder of the ring atoms being carbon
atoms.
Suitable heteroatoms include nitrogen, oxygen and sulphur. The group may be a
monocyclic or bicyclic heteroaryl group. Examples of heteroaryl include
thiophene,
benzothiophene, benzofuran, benzimidazole, benzoxazole,
benzothiazole,
benzisothiazole, naphtho[2,3-b]thiophene, furan, isoindolizine, xantholene,
phenoxatine,
pyrrole, imidazole, pyrazole, pyridine, pyrazine, pyrimidine, pyridazine,
tetrazole, indole,
isoindole, 1H-indazole, purine, quinoline, isoquinoline, phthalazine,
naphthyridine,
quinoxaline, cinnoline, carbazole, phenanthridine, acridine, phenazine,
thiazole,
isothiazole, phenothiazine, oxazole, isooxazole, furazane, phenoxazine, 2-, 3-
or 4-
pyridyl, 2-, 3-, 4-, 5-, or 8- quinolyl, 1-, 3-, 4-, or 5- isoquinolinyl 1-, 2-
, or 3- indolyl, and 2-
or 3-thienyl. A heteroaryl group is typically a Ci-Cisheteroaryl group. The
group may be
a terminal group or a bridging group.
"Heteroarylalkyl" means a heteroaryl-alkyl group in which the heteroaryl and
alkyl
moieties are as defined herein. Preferred heteroarylalkyl groups contain a
lower alkyl
moiety. Exemplary heteroarylalkyl groups include pyridylmethyl. The group may
be a
terminal group or a bridging group. If the group is a terminal group it is
bonded to the
remainder of the molecule through the alkyl group.
"Heteroarylalkenyl" means a heteroaryl-alkenyl- group in which the heteroaryl
and
alkenyl moieties are as defined herein. The group may be a terminal group or a
bridging
group. If the group is a terminal group it is bonded to the remainder of the
molecule through
the alkenyl group.
"Heteroarylheteroalkyl" means a heteroaryl-heteroalkyl- group in which the
heteroaryl and heteroalkyl moieties are as defined herein. The group may be a
terminal
group or a bridging group. If the group is a terminal group it is bonded to
the remainder of
the molecule through the heteroalkyl group.
"Heteroaryloxy" refers to a heteroaryl-O- group in which the heteroaryl is as
defined
herein. Preferably the heteroaryloxy is a Ci-Cisheteroaryloxy. The group may
be a
terminal group or a bridging group. If the group is a terminal group it is
bonded to the
remainder of the molecule through the oxygen atom.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 15 -
"Heterocyclic" refers to saturated, partially unsaturated or fully unsaturated
monocyclic, bicyclic or polycyclic ring system containing at least one
heteroatom selected
from the group consisting of nitrogen, sulfur and oxygen as a ring atom.
Examples of
heterocyclic moieties include heterocycloalkyl, heterocycloalkenyl and
heteroaryl.
"Heterocycloalkenyl" refers to a heterocycloalkyl group as defined herein but
containing at least one double bond. A heterocycloalkenyl group typically is a
02-
Ci2heterocycloalkenyl group. The group may be a terminal group or a bridging
group.
"Heterocycloalkyl" refers to a saturated monocyclic, bicyclic, or polycyclic
ring
containing at least one heteroatom selected from nitrogen, sulfur, oxygen,
preferably from
1 to 3 heteroatoms in at least one ring. Each ring is preferably from 3 to 10
membered,
more preferably 4 to 7 membered. Examples of suitable heterocycloalkyl
substituents
include pyrrolidyl, tetrahydrofuryl, tetrahydrothiofuranyl, piperidyl,
piperazyl,
tetrahydropyranyl, morphilino, 1,3-diazapane, 1,4-diazapane, 1,4-oxazepane,
and
1,4-oxathiapane. A heterocycloalkyl group typically is a 02-
Ci2heterocycloalkyl group.
The group may be a terminal group or a bridging group.
"Heterocycloalkylalkyl" refers to a heterocycloalkyl-alkyl- group in which the
heterocycloalkyl and alkyl moieties are as defined herein. Exemplary
heterocycloalkylalkyl
groups include (2-tetrahydrofuryl)methyl, (2-tetrahydrothiofuranyl) methyl.
The group may
be a terminal group or a bridging group. If the group is a terminal group it
is bonded to the
remainder of the molecule through the alkyl group.
"Heterocycloalkylalkenyl" refers to a heterocycloalkyl-alkenyl- group in which
the
heterocycloalkyl and alkenyl moieties are as defined herein. The group may be
a terminal
group or a bridging group. If the group is a terminal group it is bonded to
the remainder of
the molecule through the alkenyl group.
"Heterocycloalkylheteroalkyl" means a heterocycloalkyl-heteroalkyl- group in
which
the heterocycloalkyl and heteroalkyl moieties are as defined herein. The group
may be a
terminal group or a bridging group. If the group is a terminal group it is
bonded to the
remainder of the molecule through the heteroalkyl group.
"Heterocycloalkyloxy" refers to a heterocycloalkyl-O- group in which the
heterocycloalkyl is as defined herein. Preferably the heterocycloalkyloxy is a
Ci-
C6heterocycloalkyloxy. The group may be a terminal group or a bridging group.
If the
group is a terminal group it is bonded to the remainder of the molecule
through the oxygen
atom.
"Heterocycloalkenyloxy" refers to a heterocycloalkenyl-O- group in which
heterocycloalkenyl is as defined herein. Preferably the Heterocycloalkenyloxy
is a 01-06
Heterocycloalkenyloxy. The group may be a terminal group or a bridging group.
If the
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 16 -
group is a terminal group it is bonded to the remainder of the molecule
through the oxygen
atom.
"Hydroxyalkyl" refers to an alkyl group as defined herein in which one or more
of
the hydrogen atoms has been replaced with an OH group. A hydroxyalkyl group
typically
has the formula CnH(2n+1-x)(OH)x. In groups of this type n is typically from 1
to 10, more
preferably from 1 to 6, most preferably 1 to 3. x is typically 1 to 6, more
preferably 1 to 3.
"Sulfinyl" means an R-S(=0)- group in which the R group may be OH, alkyl,
cycloalkyl, heterocycloalkyl; aryl or heteroaryl group as defined herein. The
group may be
a terminal group or a bridging group. If the group is a terminal group it is
bonded to the
remainder of the molecule through the sulfur atom.
"Sulfinylamino" means an R-S(=0)-NH- group in which the R group may be OH,
alkyl, cycloalkyl, heterocycloalkyl; aryl or heteroaryl group as defined
herein. The group
may be a terminal group or a bridging group. If the group is a terminal group
it is bonded
to the remainder of the molecule through the nitrogen atom.
"Sulfonyl" means an R-S(=0)2- group in which the R group may be OH, alkyl,
cycloalkyl, heterocycloalkyl; aryl or heteroaryl group as defined herein. The
group may be
a terminal group or a bridging group. If the group is a terminal group it is
bonded to the
remainder of the molecule through the sulfur atom.
"Sulfonylamino" means an R-S(=0)2-NH- group. The group may be a terminal
group or a bridging group. If the group is a terminal group it is bonded to
the remainder of
the molecule through the nitrogen atom.
The prior art referred to in this specification is incorporated herein by
reference.
BRIEF DESCRIPTION OF THE DRAWINGS
Preferred embodiments of the invention will now be described, by way of
example
only, with reference to the accompanying drawings in which:
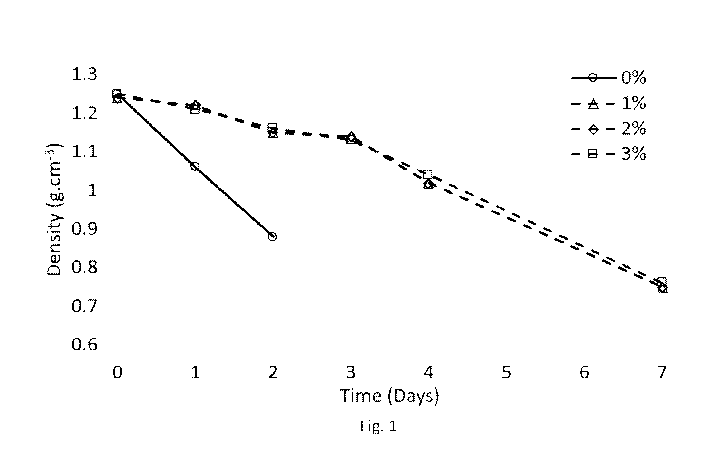
Figure 1 is a graph showing the results of Example 1, showing the change of
gel
density (g.cm-3) over time (days) with range of density-stabilised H202
employing the
phosphonate, DTMPMA .Na.X (Y() w/w).
Figure 2 is a graph showing the results of Example 1, showing loss of gel
density
(`)/0 to initial) compared to initial density over time (days) with range of
density-stabilised
H202 employing the phosphonate, DTMPMA .Na.X ( /0 w/w).
Figure 3 is a graph showing the results of Example 2, showing averaged
Velocity
of Detonation (VOD) for varying densities of 3% w/w DTMPMA enhanced
H202/glycerol-
based explosive formula in unconfined detonations (n=3) in 47 mm diameter
tubing,
sensitised with 3MTm K15 Glass Micro-Balloons, and initiated with a 25 g
Pentex D Booster.
Two VOD monitors, VOD1 (dotted line) and VOD2 (dashed line) were attached to
each
CA 03142594 2021-12-03
WO 2020/243788 PC T/AU2020/050573
- 17 -
shot, average for VOD data displayed as solid line.
Figure 4 is a graph showing the change in gel density (g.cm-3) over ten days
between 0 - 5 % w/w PA. Error bars are Standard Deviation (n=4). 3, 4, & 5%
w/w PA
formulations collapsed at 5 days.
Figure 5 is a graph showing the loss of gel density ( /0) compared to initial
density
over 10 days between 0 - 5 % w/w PA. Error bars are Standard Deviation (n=4).
3, 4, &
5% w/w PA formulations collapsed at 5 days.
Figure 6 is a graph showing the change in gel density (g.cm-3) over 13 days
between 0 - 2 % w/w PA. Error bars are Standard Deviation (n=4).
Figure 7 is a graph showing the loss of gel density ( /0) compared to initial
density
over 13 days between 0 - 2 % w/w PA. Error bars are Standard Deviation (n=4).
DETAILED DESCRIPTION OF THE INVENTION
The present invention provides an explosive composition comprising:
a. H202;
b. fuel; and
c. one or more density stabilisers.
In one embodiment, the compositions of the invention are formulated as
watergels. In an alternative embodiment, the compositions of the invention are
formulated as emulsions.
Hydrogen peroxide (H202)
The preferred concentration of H202 in the composition of the invention is
between
about 2% to 85% by weight. By way of example only, a concentrated H202
solution can
be sourced (70% w/w) and diluted down to 25% w/w for use in the composition.
Other
possibilities will be apparent to the skilled person. Preferably the H202
concentration in
the composition is around 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16,
17, 18, 19, 20,
21, 22, 23, 24, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75, 80, or 85% (w/w).
Preferably the
H202 concentration in the composition is around between about 2 to 3, 3 to 5,
5 to 10, 10
to 15, 15 to 20, 20 to 25, 25 to 30,30 to 35,35 to 40,40 to 45,45 to 50,50 to
55,55 to
60, 60 to 65, 65 to 70, 70 to 75, 75 to 80, or 80 to 85 % (w/w).
It will be understood that the % w/w of hydrogen peroxide present in the
composition refers the amount of pure hydrogen peroxide. As hydrogen peroxide
is
provided in the form of an aqueous solution having an H202 concentration of
less than 100
%, for example, having an H202 concentration of 50 % w/w, or 35 % w/w, or 30 %
w/w, the
skilled person will readily understand the need and manner by which they can
adjust the
amount of diluted H202 solution required to ensure the explosive compositions
of the
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 18 -
invention comprise 2 to 25 `)/0 w/w H202. To take an example for the avoidance
of doubt,
if a composition of the invention contains 20% of a 50% w/w solution of H202,
the
composition contains 10% w/w H202. The skilled person will also appreciate
that the 2 to
85 `)/0 w/w concentration of H202 is the final H202 concentration in the
explosive
composition, and thus account must be taken of the diluting effects of any
other
components (e.g., fuels, oxidisers, thickeners, etc.) added to the composition
during
formulation.
Water
The explosive compositions described herein may comprise water. In one
embodiment, the explosive composition may comprise less than 50 `)/0 w/w of
water, or 40
`)/0 w/w or less of water, or 30 `)/0 w/w or less of water, for example 25
`)/0 w/w or less, 20 `)/0
w/w or less, 15% w/w or less or 10 `)/0 w/w or less. In one embodiment, the
explosive
composition may comprise 5 `)/0 w/w or more of water, for example 10 % w/w or
more. The
composition may thus comprise between 5 and 50 `)/0 w/w water, or between 5
and 20 `)/0
w/w water, or between 15 and 30 `)/0 w/w water, or between 10 and 40 `)/0 w/w
water, or 50,
45, 40, 35, 30, 25, 20, 15, 10, 5 or 1 `)/0 w/w water.
Sensitisers
The explosive composition according to the invention may comprise one or more
sensitisers dispersed in the composition to produce voids which improve
sensitivity to
detonation. In addition, H202 may itself act as both a sensitiser and an
oxidiser.
Alternatively, H202 itself may act as the sensitiser and no other sensitisers
may be used.
Sensitisers include gas bubbles generated in situ or injected air or air/gas
entrapped material. Another example of sensitisation is the combination of
both gas
bubbles (chemically generated and or injected) and air entrapped material.
The explosive compositions of the present invention comprise a discontinuous
gaseous component to sensitise the composition.
The present invention relies on sensitisation of a H202-based composition to
result
in an explosive composition, and to control key factors such as explosive
sensitivity,
density, velocity of detonation (VOD) and the delivery of the energy.
Preferably the explosive composition of the invention is adapted to retain the
sensitiser in a substantially homogenous dispersion (e.g. by a thickener or an
emulsifier in
the case of a watergel or an emulsion, respectively). It will be appreciated
that a variety
of techniques can be utilised to achieve this property, as discussed further
below.
Preferably a minimum concentration of sensitiser is included into the
composition
to cause it to be explosive. Preferably the sensitiser is included in a
detonation-sensitive
concentration or amount. The sensitiser is also preferably maintained in a
detonation-
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 19 -
sensitive dispersion/distribution throughout the composition. Preferably the
final density of
the composition is controlled into an initial preferred pre-determined
explosive range.
Preferably the final density is controlled with sensitiser to about 0.6 to
about 1.15 g/ml.
Preferably the density of the composition is formulated to be around 0.1, 0.2,
0.3, 0.4, 0.5,
0.6, 0.7, 0.8, 0.9, 1, 1.1, 1.2, 1.3, or 1.4 g/ml. Preferably the density of
the composition is
formulated to be initially between around 0.1 to 0.2, 0.2 to 0.3, 0.3 to 0.4,
0.4 to 0.5, 0.5 to
0.6, 0.6t0 0.7, 0.7t0 0.8, 0.8t0 0.9, 0.9t0 1.0, 1.0t0 1.1, 1.1 to 1.2, 1.2t0
1.3, 1.3t0 1.4,
or 1.4 to 1.5 g/ml. However, it will be appreciated that for some applications
other high
density additives can specifically be included to increase the density, up to
1.6, 1.7, 1.8,
1.9 or 2.0 g/ml. Preferably the density is maintained or stabilised as
discussed above over
an extended period of time, thereby increasing sleep time compared to the
explosive
composition not including a density stabiliser as discussed herein.
The skilled person will appreciate that a mathematical conversion will be
required
to convert the weight of mechanical sensitisers, such as ceramic/glass/plastic
micro
balloons or expanded polystyrene spheres or the amount of chemical to be
decomposed
into bubbles to yield a certain density, to volume (for void spaces). However,
irrespective
of the type of sensitisation, it will be appreciated that the final density is
controlled to a
predetermined value to yield an explosive composition and to thereby control
the
parameters discussed above.
Many advantages result from the inventive explosive compositions taught
herein.
For example, certain formulations of the compositions of the invention are
more cost
effective compared to existing explosive compositions, and are capable of
being produced
in large quantities to meet the demand from the mining industry. The explosive
compositions of the invention utilise H202, which is a sustainably-produced
material that
has a relatively low carbon footprint compared to other types oxidisers used
in the art. The
explosive compositions of the invention can also be formulated into slurry,
prilled, beaded,
or emulsion form. It will also be appreciated that the compositions of the
invention produce
reduced amounts of NOR, and in preferred forms of the invention no NO at all.
Other
advantages include the stabilisation of density over an extended period of
time (compared
to not including a density stabiliser as described herein), thereby improving
sleep time and
enabling blasts to be conducted that are not possible with H202-based
explosive
compositions that do not include a density stabiliser.
Once the explosive is sensitised, it can be initiated by a primer/booster,
which as
the skilled person will be aware is an explosive which generates a high
detonation pressure
which then initiates detonation of the sensitised explosive.
The introduction of voids into the composition can be provided by a variety of
techniques (by entrapping gas bubbles when mixing, by using gas bubbles
chemically
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 20 -
generated in situ, by injecting gas bubbles, or mixing the composition with
gas entrapped
material), which are all applicable to the present invention.
Examples of air entrapped material for sensitisation for hydrogen peroxide-
based
explosives that can be used in conjunction with gas bubbles are glass or
plastic
microballoons, expanded polystyrene beads, polyurethane foam, etc.
Preferably the void component is incorporated into the compositions of the
present
invention as fine gas bubbles dispersed throughout the composition. Hollow gas-
filled
compressible particles such as micro balloons, or porous particles, or
mixtures thereof can
also be included.
The discontinuous phase of fine gas bubbles may be incorporated into the
compositions of the present invention by mechanical agitation, injection by
bubbling the
gas through the composition, or by in situ generation of the gas by chemical
means.
Suitable chemicals for the in situ generation of gas bubbles include H202
itself
which can be decomposed with manganese (Mn) salts, yeast, iodide salts, etc;
nitrogen-
based compounds such as, for example, sodium nitrite, nitrosoamines such as,
for
example, N,N'dinitrosopentamethylenetetramine; boron-based compounds such as,
for
example, sodium borohydride; carbonates such as, for example, sodium
carbonate.
Decomposition in situ of a portion of the hydrogen peroxide with permanganates
(or the
like) forms oxygen gas bubbles. Decomposition of carbonates with acid in situ
to forms
carbon dioxide bubbles.
Examples of suitable hollow particles include small hollow microspheres of
glass
and resinous materials such as phenol-formaldehyde, poly(vinylidene
chloride)/poly(acrylonitrile) copolymers and ureaformaldehyde. Examples of
suitable
hollow particles include Q-Cel, Cenospheres, Expancel, 3M, Extendospheres,
etc.
Examples of porous materials include expanded minerals such as perlite, fly
ash or hollow
particles that are a by-product of coal fired power stations
Typically, sufficient void space/gas bubbles (potentially also including
hollow
particles and/or porous particles) are used in the compositions of the present
invention to
give an explosive composition having a density in the range of from 0.1 to 1.4
g/cm3. In
preferred embodiments, the sensitisation is provided entirely from gas
bubbles, with the
proviso that there are no hollow gas-filled compressible particles.
Using conventional mixing techniques to provide bubbles in emulsion explosive
compositions often produce bubbles with a range of bubble sizes. For example,
the
bubbles often have diameters up to 2000 microns and average bubble diameters
of less
than 50 microns are also common. By choice of suitable surfactants bubbles of
smaller or
larger diameters can be produced. Thus, by choice of an appropriate surfactant
at a
desired concentration the mean gas bubble diameter in the discontinuous gas
phase may
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
-21 -
be controlled, and bubbles of 50 to 200 microns are possible. It will be
appreciated that
the bubble size influences the overall density, and if low densities are
required 50 to 100
microns gas bubbles are preferred. For emulsified explosives the density range
is
preferably around 0.60 ¨ 1.20 g/ml, and for watergels the density range is
preferably
between 0.2 ¨ 1.2 g/ml. In an emulsified system the gas bubbles are preferably
10 ¨ 100
times larger than the disperse phase droplets. The oily phase is likely to be
in contact the
gas bubble, whereas the oxidiser (or discontinuous phase) does not.
As discussed above, the introduction of gas bubbles can be provided by a
variety
of techniques, which are all applicable to the present invention.
In one embodiment the bubbles may be 'trapped' during the preparation of the
explosive composition or by their formation through a chemical reaction. In US
Patent No.
3,400,026 a formulation which uses protein in solution (albumin, collagen, soy
protein, etc.)
in order to favour the formation of bubbles and their stabilisation is
described. US Patent
No. 3,582,411 describes a watergel explosive formulation which contains a
foaming agent
of the guar gum type modified by hydroxy groups. In US Patent No. 3,678,140 a
process
for the incorporation of air by means of the use of protein solution is
described, by passing
the composition through a series of openings at pressures from 40 to 200 psi
and
simultaneously introducing air through eductors.
Incorporation of gas bubbles by means of their generation as a result of a
chemical
reaction is also described in the prior art. Wherein in situ generation of gas
bubbles is
provided by the decomposition of chemicals compounds, the decomposition
suitably
produces 02, 002, N2, H2, or combinations thereof.
Various gases in bubble form have been used to sensitise blasting agents, for
example nitrogen, carbon dioxide, oxygen, and hydrogen. It is also known to
directly inject
air or gas into the explosive mixture. Suitable gases for injection include
air, oxygen,
nitrogen, carbon dioxide, hydrogen, and noble gases (such as Argon).
Alternatively, hollow gas-filled compressible particles such as glass or
plastic micro
balloons, or porous particles, or expanded polystyrene (EPS) or mixtures
thereof are
included. In related embodiments the compressible material is any low-density
material
which has a specific gravity < 1.0 g/cm3. In brief summary, examples of glass
balloons can
be seen in US Patent No.'s 4,326,900 and 3,447,978, and plastic micro balloons
in US
Patent No.'s 4,820,361 and 4,547,234. These balloons are typically 0.05 mm in
diameter
and have a bulk density of 100 g/L. Use of expanded polystyrene can be seen
for example
in US Patent No.'s 5,470,407 and 5,271,779.
In one embodiment, the compressible material is gas-filled and selected from
small
hollow microspheres of ceramic, glass or resinous materials or porous
materials, and
combinations thereof, such as perlite or fly ash.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 22 -
Preferably the microspheres/micro balloons contain gas such as pentane, etc.
Suitably the microspheres are sized between about 20 to 2000 micron and have a
bulk
density of less than 1000 g/L.
In alternative embodiments, the compressible material is a cellular material,
such
as expanded polystyrene (EPS), polyurethane foam, cotton seeds, expanded pop
corn,
husks, and combinations thereof.
Examples of suitable hollow particles include small hollow microspheres of
ceramic, glass and resinous materials such as phenol-formaldehyde,
poly(vinylidene
chloride)/poly(acrylonitrile) copolymers and ureaformaldehyde. Examples of
suitable
hollow particles include Q-Cel, Envirospheres , Cenospheres , Expancel , 3M,
Extendospheres , etc. Examples of porous materials include expanded minerals
such as
perlite, fly ash. A further example of a porous material is hollow particles
that are a by-
product of coal fired power stations.
Typically, sufficient bubbles and/or hollow particles and/or porous particles
are
used in the compositions of the present invention to give an explosive
composition having
a density in the range of from 0.3 to 1.4 g/cm3.
For example, an explosive composition of the invention may have a density of
up
to 1.4g/cm3, up to 1.3g/cm3, up to 1.2g/cm3, up to 1.1g/cm3, up to 1.0g/cm3,
etc. An
explosive composition of the invention may have a density of from 0.3 g/cm3,
from 0.4
g/cm3, from 0.5 g/cm3, etc. Using conventional mixing techniques to provide
bubbles in
emulsion explosive compositions often produce bubbles with a range of bubble
sizes. For
example, the bubbles often have diameters up to 2000 microns and average
bubble
diameters of less than 300 microns are also common. By choice of suitable
surfactants
bubbles of smaller or larger diameters can be produced. Thus by choice of an
appropriate
surfactant at a desired concentration the mean gas bubble diameter in the
discontinuous
gas phase may be controlled, and bubbles of 50 to 300 microns are possible.
For
emulsified explosives the density range is suitably around 0.60 ¨ 1.30 g/cm3,
and for
watergels the density range is suitably between 0.2 ¨ 1.40 g/cm3. In an
emulsified system
the gas bubbles are suitably 10 ¨ 100 times larger than the disperse phase
droplets. The
oily phase is likely to be in contact the gas bubble, whereas the oxidiser (or
discontinuous
phase) does not.
Other types of sensitising materials can be used in the compositions of the
invention, e.g. TNT, HMX, RDX, aluminium powder and silicon powder and
combinations
thereof (e.g. TNT, HMX, RDX and aluminium powder and combinations thereof).
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 23 -
Density stabilisers
The explosive compositions of the present invention comprise at least one
density
stabiliser.
Generally, one or more density stabilisers are incorporated in an amount of up
to
about 15 % w/w of the explosives composition, for example about 0.01 % w/w to
about 10
% w/w, e.g. about 1 to about 5 % w/w, such as about 1 to about 3 % w/w. The
one or
more density stabilisers are preferably present in a concentration of about
0.01, 0.05, 0.1,
0.25, 0.5, 0.75, 1, 1.25, 1.5, 1.75, 2, 2.25, 2.5, 2.75, 3, 3.25, 3.5, 3.75,
4, 4.25, 4.5, 4.75,
5, 5.25, 5.5, 5.75, 6, 6.25, 6.5, 6.75, 7, 7.25, 7.5, 7.75, 8, 8.25, 8.5,
8.75, 9, 9.25, 9.5, 9.75,
10, 11, 12, 13, 14 or 15% w/w. The one or more density stabilisers are
preferably present
in a concentration of around 0.01 to 0.05, 0.05 to 0.1, 0.1 to 0.5, 0.5 to 1,
1 to 1.5, 1.5 to
2, 2 to 2.5, 2.5 to 3, 3 to 3.5, 3.5 to 4, 4 to 4.5, 4.5 to 5, 5 to 5.5, 5.5
to 6, 6 to 6.5, 6.5 to 7,
7 to 7.5, 7.5 to 8, 8 to 8.5, 8.5 to 9, 9 to 9.5, 9.5 to 10, 10 to 11, 11 to
12, 12 to 13, 13 to
.. 14 or 14 to 15 % w/w.
Preferably the density stabiliser is present at about 0.01 % w/w to about 10 %
w/w,
e.g. about 1 to about 5 % w/w, such as about 1 to about 3 % w/w.
Preferred density stabilisers are phosphonates.
Phosphonates
Preferably the phosphonate(s) are in liquid form (e.g. dissolved in solution).
The phosphonate may have 1, 2, 3, 4, 5 or 6 pendant phosphonate groups, or
more
than 6 groups. In some embodiments, the phosphonate may have 1, 2, 3, 4, 5, 6,
7, 8, 9
or 10 pendant phosphonate groups. Preferably the phosphonate has at least 3
pendant
phosphonate groups, more preferably 5 pendant phosphonate groups.
In certain embodiments, the phosphonate has the structure X-(P03Y2)n, where X
is
selected from the group consisting of an optionally substituted alkyl,
optionally substituted
heteroalkyl, optionally substituted cycloalkyl, optionally substituted
heterocycloalkyl,
optionally substituted alkenyl; optionally substituted aryl, optionally
substituted heteroaryl;
Y is H or a water-soluble cation; and n is 1 to 10 (i.e., 1, 2, 3, 4, 5, 6, 7
,8, 9, 10). Suitable
optional substituents can be selected from the group consisting of -OH, -COOH,
halogen,
-NH2, -SH.In preferred embodiments, the phosphonate has the structure X-
(P03Y2)n,
where X is an optionally substituted heteroalkyl, optionally substituted
heterocycloalkyl or
an optionally substituted heteroaryl having at least two nitrogen atoms,
preferably at least
three nitrogen atoms, more preferably three nitrogen atoms; Y is H or a water-
soluble
cation; and n is 1 to 10 (i.e., 1, 2, 3, 4, 5, 6, 7 ,8, 9, 10). Suitable
optional substituents can
be selected from the group consisting of -OH, -COOH, halogen, -N H2, -SH.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 24 -
In some preferred embodiments the phosphonates suitable for use in the
invention
are amine based, more preferably tertiary amine based.
Suitable water soluble-cations for phosphonate anions include alkali metals
(e.g.
lithium, sodium, potassium), ammonium, substituted ammonium and alkaline earth
metals
(e.g. calcium, magnesium). Preferred compounds have n = 1 to 2 and preferably
Y is
hydrogen, ammonium, sodium or potassium or mixtures thereof. In one preferred
embodiment, the density stabiliser is a phosphonate which is
diethylenetriamine
pentamethylene phosphonic acid sodium salt (DTPMPA.Na.x, C9H28_xN3015P5Nax) ¨
see
Formula I.
Na+-0 0- Na+
\a.
Na+ "
U
Nato
0=p-O-Na+
P N
0 0" Na+
Na+ 0 L.,
0-Na+
Na '0
Formula I
Examples of other suitable phosphonates include those falling within the scope
of
Formula II:
ox
0, /
OX X0, ,OX
N 0
X0, m çOX
OX
P ox
e \ox,
x6
wherein n = 1-4; and X = H or a water-soluble cation.
Formula ll
The various X groups may be the same or different.
CA 03142594 2021-12-03
WO 2020/243788
PCT/AU2020/050573
- 25 -
Examples of other suitable phosphonates include:
Phosphonate (number of Structure
phosphonate groups)
Glyphosate (1)
OH OH
HO/
Foscarnet (1)
0 0
HO4i-4.
61-1 OH
Perzinfotel (1) a
ii
HO-P -OH
)
t.,,. I 0
N
r
\ , 0
NH
Selfotel (1)
1-10., ..0
, i(
OH '' NH
HO, r.,/ j
6
N- 0
(phosphonomethyl)iminodiac
Fia.)N) 0
etic acid (PMIDA) (1)
,.., N
i '----- OH
HO-P -OH
011
2-carboxyethyl phosphonic
0
,
acid (CEPA) (1) HOiiP._
/ OH
\
HO µ')
0
CA 03142594 2021-12-03
WO 2020/243788
PCT/AU2020/050573
- 26 -
Phosphonate (number of Structure
phosphonate groups)
vinylphosphonic acid (1)
OH
2-phosphonobutane-1,2,4-
0
tricarboxylic acid (PBTC) (1)
OHOH
R\
(i0H
P¨OH
(5/
HO'. '0
aminomethylphosphonic acid
0
(AMPA) (1) HO,
H2N /
OH
2-hydroxyphosphonoacetic
acid (HPAA) (1) 0
HOiOH
HO
hydroxyethylidene-1 ,1 -
0 OH 0
diphosphonic acid (HEDP) (2) I 1
HO¨P P¨OH
OH OH
hydroxyethylamino-
di(methylene phosphonic
HS OH
acid) (HEMPA) (2)
P N
\\ L,scr OH
0
OH
CA 03142594 2021-12-03
WO 2020/243788
PCT/AU2020/050573
- 27 -
Phosphonate (number of Structure
phosphonate groups)
N,N- HO
bis(phosphonomethyl)glycine
(BPMG) (2) HO'"
/OH
s-
7 OH
HO 0
aminotris(methylenephospho
0 OH
nic acid) (ATMP) (3) HO, /
HO -P
'0
OH IN¨'
\ OH
OH
ethyienediamine
OH
tetra(methylene phosphonic HO,
Põ N
acid) (EDIMP) (4) HO' c;, OH
0
0 pNE
t1/43 n
N
\OH
HO-P=0
OH
hexamethylene diamine tetra
HO
(methylene phosphonic acid)
P N OH
P,
(HMDTMP) (4) H0*- OH
HO, P
N
HO
O=P-OH
OH
CA 03142594 2021-12-03
WO 2020/243788
PCT/AU2020/050573
- 28 -
Phosphonate (number of Structure
phosphonate groups)
polyamino polyether 0
0
p
methylene phosphonic acid µ1 ( "OH
HO-
(PAPEMP) (4) HO
OH /
\ /0
HO" OH
HO' OH
tetramethylenediaminetetra(
methylenephosphonic acid) 0
(TDTMP) (4) HO¨P HO
0
OH
\ 0
\
OH H0,12,
H0 N-C)
hexamethylenediaminetetra(
HO\
methylenephosphonic acid) PH
(HDTMP) (4) HO' \\c) 'OH
0
I 9µ ,OH
OH
HO-P=0
6H
CA 03142594 2021-12-03
WO 2020/243788
PCT/AU2020/050573
- 29 -
Phosphonate (number of Structure
phosphonate groups)
bis(hexamethylene triamine
0
penta (methylene phosphonic VOH
acid)) (BHMTPMP) (5)
y õ7-,õ õ
HOIDI
0 ,OH
P-OH
0
HO 11
0 ,OH
P- OH
0
diethylenetriamine
penta(methylene phosphonic HO, OH
acid) (DTPMP) (5) .P P
HO' \\ , 6 OH
0
HO, µ,7 0=P-OH
P N
HO' \\(:) OH
Fici OH
Phytic acid (6)
OH \\ OH
HO-P=0 0- ku
6,
HO'? 'T HdOH
Hd09
0 0=P -OH
OH
and salts, solvates, dimers, stereoisomers thereof.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 30 -
Without being bound by any one theory, the inventor contemplates that suitable
phosphonates for use in the invention provide density stabilisation via
chelation or
sequestration of impurities inherently present within the explosive
compositions
contemplated herein (i.e., providing "internal" stability). Additionally,
suitable
phosphonates for use in the invention provide density stabilisation via
chelation or
sequestration of impurities that arise when the explosive composition is
loaded into the
blasthole and exposed to rock (i.e., providing "external" stability). It will
be appreciated that
the explosives of the invention can be used in a wide range of surface and
subsurface
applications, and in a range of different types of rock having different
metalliferous
minerals. One or more phosphonates described herein can be selected for use
depending
on the type of impurity (metal ion) present in the rock to be blasted and/or
whether the
application is in hot reactive ground, which can affect the solubility of
metal ions and/or the
pH.
Composition stabilisers
Other composition stabilisers can also be used with the present invention.
Suitable composition stabilisers may be selected from the group consisting of
phosphates, stannates and sulfites.
Suitable composition stabilisers also include EDTA and nitrates (e.g. sodium
nitrate
or potassium nitrate).
For example, stannates, sulphites, and nitrates, either as a separate entity
or as a
component of the density stability system such as, for example, a mixture of
phosphonates
and nitrates.
Typically, the other composition stabiliser component(s) of the compositions
of the
present invention are incorporated in an amount of up to about 15 `)/0 w/w,
for example
about 0.01 `)/0 w/w to about 10% w/w, e.g. about 1 to about 5% w/w, such as
about 1 to
about 3 `)/0 w/w of the total composition.
Fuels for watergels
The explosive compositions of the invention may comprise one or more fuels.
H202-based watergels can be prepared with either water-miscible or water
immiscible fuels.
The skilled person will appreciate that there are many options available for
use as
a fuel. For example, depending on their origin, the fuel may be a product of
vegetable
origin, such as sugars, molasses, vegetable oils or alcohols. Such fuels may
be regarded
as sustainable fuels. Other fuels can be sourced from the petrochemical
industry, as for
example diesel, paraffinic oils or mineral oil, organic acids, ethers, esters,
amine nitrates,
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
-31 -
urea, hexamine, etc. Other fuels may be silicone oils, etc. Suitable fuels for
use in the
compositions of the invention are glycerol, sugar, syrup, alcohol, carbon,
ground coal,
waxes, oils such as corn, cottonseed, olive, peanut, or fatty acid oils.
Suitable sustainable
fuels for use in the compositions of the invention may include, sugar
molasses, vegetable
oil, alcohol, oils such as corn, cottonseed, olive, peanut, fatty acid oils,
or gums. Other
fuels may be selected from ethylene glycol, glycerol, propylene glycol, and/or
formamide
Preferably, the sustainable fuel is glycerol. The composition may comprise
between 15
and 25% w/w sustainable fuel, e.g., between 15 and 20%, or between 20 and 25%
w/w.
The composition may alternatively comprise less than 40% w/w sustainable fuel,
less than
30%, less than 25%, or less than 20% w/w sustainable fuel, e.g., 5%, 10%, 15%,
20%,
25%, 30%, 35% or 40% w/w sustainable fuel. Alternatively, the above fuels can
also split
into water-soluble and water-insoluble fuels.
Water-miscible fuels which can be used with the present invention can be
selected
from the group consisting of: glycerol, sugar, amine nitrates, hexamine and
urea.
Water immiscible fuels which can be used with the present invention can be
selected from the group consisting of: aliphatic, alicyclic and aromatic
compounds and
mixtures thereof which are in the liquid state at the formulation temperature.
Suitable
organic fuels may be chosen from fuel oil, diesel oil, distillate, kerosene,
naphtha, waxes,
(e.g. microcrystalline wax, paraffin wax and slack wax) paraffin oils,
benzene, toluene,
xylenes, asphaltic materials, polymeric oils such as the low molecular weight
polymers of
olefins, vegetable oils, animal oils, fish oils, and other mineral,
hydrocarbon or fatty oils,
and mixtures thereof. Preferred organic fuels are liquid hydrocarbons
generally referred to
as petroleum distillates such as gasoline, kerosene, fuel oils, paraffin oils
and vegetable
oils or mixture thereof.
Typically, the water miscible or water-immiscible fuel of the watergel
composition
of the present invention comprises from 5 to 30% by weight and preferably 10
to 25% by
weight of the total composition. Preferably the fuel is included in a
concentration of about
5, 7, 8, 10, 12, 15, 20, 25, 30, 35, 40, 45, or 50% (w/w). Preferably the fuel
is included in
a concentration of between about 5 to 10, 10 to 15, 15 to 20, 20 to 25, 25 to
30, 30 to 35,
35 to 40, 40 to 45, or 45 to 50% (w/w).
In one embodiment, the water-immiscible fuel is included at 7 to 25% w/w of
the
total composition.
In one embodiment, the water-miscible fuel is included at 8 to 25% w/w of the
total
composition.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 32 -
Fuels for emulsions
The explosive compositions of the invention may comprise one or more fuels.
H202-based emulsions can be prepared with water-immiscible fuels.
The fuel can be any fuel such as diesel fuel, and/or oil distillates.
Alternatively, it
can be paraffinic, mineral, olefinic, naphthenic, animal, vegetable, fish and
silicone oils.
Other types of fuels are benzene, toluene, xylenes, asphaltic materials and
the likes. The
fuel may be a sustainable fuel. Suitable sustainable fuels for use in
emulsions may include
vegetable oil, oils such as corn, cottonseed, olive, peanut, or fatty acid
oils. The
composition may comprise between 15 and 25% w/w sustainable fuel, e.g.,
between 15
and 20%, or between 20 and 25 `)/0 w/w. The composition may alternatively
comprise less
than 40% w/w sustainable fuel, less than 30%, less than 25%, or less than 20%
w/w
sustainable fuel, e.g., 5%, 10%, 15%, 20%, 25%, 30%, 35% or 40% w/w
sustainable fuel.
The water-immiscible organic phase component of the composition of the present
invention comprises the continuous "oil" phase of the water-in-oil emulsion
and is the fuel.
Suitable organic fuels include aliphatic, alicyclic and aromatic compounds and
mixtures
thereof which are in the liquid state at the formulation temperature. Suitable
organic fuels
may be chosen from recycled lubricant distillates, recycled oil distillates,
fuel oil, diesel oil,
distillate, kerosene, naphtha, waxes, (e.g. microcrystalline wax, paraffin wax
and slack
wax) paraffin oils, benzene, toluene, xylenes, asphaltic materials, polymeric
oils such as
the low molecular weight polymers of olefins, vegetable oils, animal oils,
fish oils, and other
mineral, hydrocarbon or fatty oils, and mixtures thereof. Preferred organic
fuels are liquid
hydrocarbons generally referred to as petroleum distillates such as gasoline,
kerosene,
fuel oils, paraffin oils and vegetable oils or mixture thereof.
Typically, the organic fuel or continuous phase of the H202-based emulsion
composition of the present invention comprises from 2 to 20% by weight and
preferably 3
to `)/0 20% by weight of the total composition. Preferably the organic fuel is
included in a
concentration of about 2, 4, 6, 8, 10, 12, 14, 16, 18, or 20 c)/0 (w/w).
Preferably the organic
fuel is included in a concentration of between about 2 to 4, 4 to 6, 6 to 8, 8
to 10, 10 to 12,
12 to 14, 14 to 16, 16 to 18, or 18 to 20% (w/w).
Secondary fuels for watergels and emulsions
If desired, other optional fuel materials, hereinafter referred to as
secondary fuels,
may be incorporated into the compositions of the present invention.
Examples of secondary fuels include finely divided solids. Examples of
secondary
fuels also include water-miscible organic liquids. Examples of solid secondary
fuels include
sulfur; aluminium; and carbonaceous materials such as gilsonite, comminuted
coke or
charcoal, carbon black, resin acids such as abietic acid, vegetable products
such as
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 33 -
starch, nut meal, grain meal and wood pulp and combinations thereof. Examples
of
secondary fuels include sugars such as glucose and dextrose. Examples of
secondary
fuels further include recycled plastic waste.
Typically, the optional secondary fuel component of the compositions of the
present
invention comprise from 0 to 20% w/w of the total composition, e.g. at 0.1 to
12% w/w.
Thickeners
The explosive compositions of the invention may comprise one or more
thickeners.
More particularly, the watergel explosive compositions of the invention may
comprise one
or more thickeners.
Because bubbles of gas and materials enclosing gas have a relatively low
density,
they will tend to migrate towards the surface of the column of explosive if
the viscosity of
the H202-based explosive composition is not capable of maintaining the
sensitising
material homogeneously dispersed throughout. Migration of the sensitising
material
towards the surface is undesirable as it may render the explosive too
insensitive to
initiation, and therefore the explosive composition may not deliver the energy
and gases
needed to break and move the rock as required or even worst, the explosive may
undergo
a misfire. One way to ameliorate this issue is to formulate the explosive
composition into
a watergel. These types of compositions can be formulated with different
levels of viscosity
by using a thickener. Viscosities can be selected to generally retain the
sensitising
material in a homogeneously dispersed state throughout the composition.
If desired the aqueous solution of the compositions of the present invention
may
comprise thickeners which optionally may be crosslinked. Any conventional
thickener may
be used with the present invention. The thickeners, when used in the
compositions of the
present invention, are suitably polymeric materials, especially gum materials
typified by
the galactomannan gums such as locust bean gum or xanthan gum or alginate gum
or
derivates of alginate gum or guar gum or derivatives thereof such as
hydroxypropyl guar
gum. The thickener may be selected from gums including natural gums, such guar
gum,
xanthan gum, sodium alginate, carboxymethylcellullose, methylcellulose and the
like.
Other useful, but less preferred, gums are the so-called biopolymeric gums
such as the
heteropolysaccharides prepared by the microbial transformation of carbohydrate
material,
for example the treatment of glucose with a plant pathogen of the genus
Xanthomonas
typified by Xanthomonas campestris. Other useful thickeners include synthetic
polymeric
materials and in particular synthetic polymeric materials which are derived,
at least in part,
from the monomer acrylamide. An example of a synthetic thickener is
polyacrylamide.
Inorganic thickeners, such as fumed silica, clays and carbosil, may also be
used, or a
combination thereof. Suitably the thickener is selected from locust bean gum,
guar gum,
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 34 -
hydroxypropyl guar gum, sodium alginate and heteropolysaccharides, and
combinations
thereof.
Typically, the thickener component of the compositions of the present
invention
comprises from 0 to 5% by weight of the total composition, e.g. from 0.5 to 5%
w/w, e.g.
from 0 to 2% w/w of the total composition, e.g. from 0.1 to 2% by weight of
the total
composition.
Crosslinkers
Crosslinkers can also be used with the present invention.
Thickeners in combination with crosslinkers can improve the water resistance
and
mechanical strength of the explosive. It is convenient for this purpose to use
conventional
crosslinking agents such as zinc chromate or a dichromate either as a separate
entity or
as a component of a redox system such as, for example, a mixture of potassium
dichromate and potassium antimony tartrate. Salts of Ca, Ti, Sb can also be
used as
crosslin kers.
In one embodiment the crosslinker is selected from salts containing zinc,
calcium,
titanium, antimony, chromium, borate and dichromate and combinations thereof.
Typically, the crosslinker component of the compositions of the present
invention
comprises from 0 to 3% w/w, e.g. from 0 to 0.1% w/w of the total composition,
e.g. from
0.1 to 1% w/w of the total composition, e.g. from 1 to 2% w/w of the total
composition, e.g.
from 2 to 3% w/w of the total composition.
Emulsifiers
The explosive compositions of the invention, when prepared as emulsion form,
may
comprise one or more emulsifiers.
H202-based emulsion compositions are made of a discontinuous phase of
oxidising
material that is dispersed in a continuous phase of an organic fuel in the
presence of one
or more emulsifiers. The emulsifier is adapted or chosen to maintain phase
separation.
The emulsifier component of the composition of the present invention may be
chosen from the wide range of emulsifiers known in the art for the preparation
of water-in-
oil emulsion explosive compositions. Examples of such emulsifiers include
polyisobutylene
succinic anhydride (PIBSA) reacted with amines; other emulsifiers examples are
alcohol
alkoxylates, phenol alkoxylates, poly(oxyalkylene) glycols, poly(oxyalkylene)
fatty acid
esters, amine alkoxylates, fatty acid esters of sorbitol and glycerol, fatty
acid salts, sorbitan
esters, poly(oxyalkylene) sorbitan esters, fatty amine alkoxylates,
poly(oxyalkylene) glycol
esters, fatty acid amides, fatty acid amide alkoxylates, fatty amines,
quaternary amines,
alkyloxazolines, alkenyloxazolines, imidazolines, alkyl-sulfonates,
alkylarylsulfonates,
alkylsulfosuccinates, alkylphosphates, alkenylphosphates, phosphate esters,
lecithin,
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 35 -
copolymers of poly(oxyalkylene) glycols and poly(12-hydroxystearic acid), and
mixtures
thereof.
Among the preferred emulsifiers are the 2-alkyl- and 2-alkeny1-4,4'-bis
(hydroxymethyl) oxazoline, the fatty acid esters of sorbitol, lecithin,
copolymers of
poly(oxyalkylene) glycols and poly(12-hydroxystearic acid), and mixtures
thereof, and
particularly sorbitan mono-oleate, sorbitan sesquioleate, 2-oleyl- 4,4'-bis
(hydroxymethyl)
oxazoline, mixture of sorbitan sesquioleate, lecithin and a copolymer of
poly(oxyalkylene
glycol and poly (12-hydroxystearic acid), and mixtures thereof.
Typically, the emulsifier component of the composition of the present
invention
comprises up to 5% by weight of the total composition. Higher proportions of
the emulsifier
may be used and may serve as a supplemental fuel for the composition but in
general it is
not necessary to add more than 5% by weight of emulsifier to achieve the
desired effect.
One of the advantages of the compositions of the present invention is that
stable emulsions
can be formed using relatively low levels of emulsifier and for reasons of
economy it is
preferable to keep the amount of emulsifier used to the minimum required to
have the
desired effect. The preferred level of emulsifier used is in the range from
0.1 to 2.0% by
weight of the total composition.
Surfactants
The explosive compositions of the invention when formulated as watergels may
comprise one or more surfactants. In particular, one or more surfactants may
be employed
when the explosive composition comprises a diesel-like fuel.
The surfactant component of the composition of the present invention may be
chosen from the wide range of surfactants known in the art for the preparation
of watergels
and water-in-oil emulsion explosive compositions. Examples of such surfactants
include
Sodium Lauryl Sulphate, Betaine CAB30, Sodium Coco Sulphate (Sodium Mono-012-
018-Alkyl Sufate), Alpha Olefin Su!phonate 46, Coconut diethanolamide, APG0810
(Octyldecyl glucoside), and Cocamidopropyl Betaine.
Typically, the surfactant component of the composition of the present
invention
comprises up to about 0.5% by weight of the total composition, with about 0.25
c)/0 w/w.
used for Cocamidopropyl Betaine. Other oxidisers for watergel and emulsion
H202-based
explosive compositions
It lies within the invention that there may also be incorporated into the H202-
based
watergel/emulsion compositions hereinbefore described one or more other
substances or
mixtures of substances which are themselves suitable as explosive materials.
As a typical example of such a modified compositions reference is made to
compositions wherein there is added to and mixed with an watergel/emulsion
composition
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 36 -
as hereinbefore described up to 90% w/w of an oxidizing salt such as ammonium
nitrate
or an explosive composition comprising a mixture of an oxidizing salt such as
ammonium
nitrate and fuel oil and commonly referred to by those skilled in the art as
"ANFO". The
compositions of "ANFO" are well known and have been described at length in the
literature
relating to explosives.
In particular, the explosive compositions of the invention optionally comprise
one
or more other oxidisers (e.g. one other oxidiser, e.g. two other oxidisers).
Any suitable
oxidiser can be used. For example, the one or more other oxidiser(s) are
suitably selected
from the group consisting of nitrate salts, perchlorate salts, sodium peroxide
and
potassium peroxide and optionally nitric acid.
Nitrate salts may be selected from the group consisting of ammonium nitrate,
sodium nitrate, calcium ammonium nitrate, calcium nitrate, potassium nitrate,
barium
nitrate and magnesium nitrate.
Perchlorate salts may be selected from the group consisting of ammonium
perchlorate, sodium perchlorate, potassium perchlorate, barium perchlorate,
magnesium
perchlorate and calcium perchlorate (e.g. ammonium perchlorate and sodium
perchlorate).
In one embodiment the one or more other oxidiser(s) are selected from the
group
consisting of nitrate salts and perchlorate salts. In one embodiment the one
or more other
oxidiser(s) are selected from nitrate salts. In one embodiment the one or more
other
oxidiser(s) are selected from the group consisting of AN, CAN and SN. In one
embodiment
the one or more other oxidiser(s) are selected from the group consisting of
CAN, CN and
SN. In one embodiment the one or more other oxidiser(s) are selected from the
group
consisting of CAN and SN. In one embodiment the other oxidiser is CAN. In one
embodiment the other oxidiser is SN. In one embodiment the other oxidiser is
CN. In one
embodiment, the one or more other oxidiser(s) do not include AN. In other
words, in one
embodiment, the explosive composition is devoid of AN.
The compositions of the invention comprise from greater than 0 and up to about
90
c)/0 w/w of one or more other oxidisers, such as from about 0.1% to about 75%
w/w. For
example, compositions of the invention may comprise from greater than 0, from
0.1%, from
1%, from 10%, from 20%, from 30%, from 40%, from 50%, or from 60% w/w up to 90
`)/0
w/w of one or more other oxidisers, e.g., compositions of the invention may
comprise from
1 to 20 c)/0, from 20 to 40 `)/0, from 15 to 35 `)/0, from 35 to 55 `)/0, from
30 to 70 `)/0, from 40 to
70 `)/0, or from 50 to 80 `)/0 w/w of the one or more other oxidisers. For
example,
compositions of the invention may comprise up to 90%, 80%, 75%, 70%, 65%, 60%,
50%,
40%, 30%, 20% w/w, etc of one or more other oxidisers, or may comprise about
90%,
80%, 75%, 70%, 65%, 60%, 55%, 50%, 45%, 40%, 30%, or 20% w/w of one or more
other
oxidisers. It will be understood that the explosive compositions herein
comprise one or
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 37 -
more oxidisers according to the foregoing amounts or ranges in total, and as
such, where
more than one oxidiser is used, each oxidiser may be present in any suitable
amount within
the foregoing amounts or ranges such that the total mass of the oxidisers adds
up to the
specified amount or range.
It will be appreciated that the oxidiser can be in the form of a mixture of
solid and
liquids. To explain, typically the oxidiser will be solubilised in water when
used at a
relatively low concentration, and if present at higher concentrations beyond
the solubility
of the oxidiser, then the oxidiser will be solubilised and in a solid form. In
some
embodiments, the oxidiser is fully solubilised (or substantially fully
solubilised) in the
composition. In such embodiments, excess solid oxidiser, e.g., in the form of
prills, may
be added. In
other embodiments, the oxidiser is only partially solubilised in the
composition, in which case solid oxidiser (e.g., in the form of solid prills)
may be added
just prior to detonation such that there is insufficient time for the prills
to solubilise
substantially. The oxidiser can be in a liquid :solid ratio of between 100:0
to 20:80, and any
ratio in between. For example, the liquid:solid ratio may be between 100:0 and
70:30, or
between 80:20 and 60:40, or between 70:30 and 40:60, or between 5:50 and
30:70, or of
100:0, 70:30, 60:40, 50:50, 45:55, 40:60; or 20:80.
It also lies within the invention to have as a further explosive component of
the
composition well known explosive materials comprising one or more of for
example
trinitrotoluene, nitroglycerine or pentaerythritol tetranitrate.
It will also be appreciated that these other oxidisers can be used to
partially replace
H202 in the H202 compositions. Examples of such oxidisers are nitrate salts,
perchlorate
salts, sodium / potassium peroxide, etc.
Ratios of oxidisers : fuel
In one embodiment, the explosive composition may comprise a ratio of H202:one
or more other oxidisers in the range between 100:1 to 30:70.
In one embodiment, the explosive composition may comprise a ratio of H202 (or
H202 + one or more oxidisers):fuel in the range between 87:13 to 64:36.
In one embodiment, the explosive composition may comprise a ratio of H202 (or
H202 + one or more oxidisers):fuel:water in the range between 60:20:20 to
72:24:4.
Energy deferments
The explosive compositions of the invention may optionally comprise one or
more
energy deferments. Energy deferments include metal oxides such as aluminium
oxide.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 38 -
Energy diluents
The explosive compositions of the invention may optionally comprise one or
more
energy diluents.
In the context of this invention, energy diluents are inert materials that
have minimal
contribution to the detonation process and can be used to replace part of the
energetic
material in the composition and therefore reduce the energy output of the H202-
based
explosive.
In some cases these energy diluents may increase, decrease or not alter the
density of the H202-based composition. In some cases, these energy diluting
agents are
able to reduce the density of the H202-based composition without increasing
the sensitivity.
Examples of these diluents materials are EPS (with particle size larger than
2mm
in diameter), granulated/shredded rubber (from tyres), cotton seeds, saw dust,
husk,
expanded popcorn, plastic beads, wool meal, bagasse, peanut and oat husks,
peanut
shells etc. US Patent No. 5,409,556 describes some example of these energy
reducing
agents. In
one embodiment the energy diluting agent is selected from
granulated/shredded tyres, rubber, expanded rice, expanded popcorn, expanded
wheat,
and combinations thereof. These materials could also be used in combination
with
sensitisers to offer more flexibility (as shown in US patent 5,470,407) as far
as the
performance properties of the H202-based explosive is concerned.
Therefore, another advantage of the H202-based explosive is that the
performance
properties of the explosive can be altered to suit the characteristics of the
blasting site.
Possible variations of this general procedure will be evident to those skilled
in the
art of the preparation of emulsion explosive compositions.
Watergel or emulsion H202-based explosive compositions made according to the
present invention include energy diluents in concentration between 0 ¨ 800% by
volume
(i.e. the volume can be increased by 8x).. As a result, the use of the
additives (sensitiser
and energy diluents), provides a better control of the density, VOD and energy
delivery in
the ground being blasted.
Therefore, an additional advantage of the H202-based explosive is that it
could be
used in a range of density between about 0.1 g/ml to about 1.4 g/ml (e.g.
between about
0.3 g/cm3 to about 1.4 g/cm3.
In one preferred embodiment the H202-based explosive compositions of the
invention comprise the following components: H202:fuel:water in the range
between
25%:5%:70% to 73%: 1 1 %: 1 6%.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 39 -
Density of the explosive compositions
Suitably the final density is controlled with sensitiser to around 0.3 to 1.4
g/cm3.
Suitably the density of the composition is formulated to be around 0.3, 0.4,
0.5, 0.6, 0.7,
0.8, 0.9, 1, 1.1, 1.2, 1.3, or 1.4 g/cm3. Suitably the final density of the
composition is
.. formulated to be between around 0.3 to 0.4, 0.4 to 0.5, 0.5 to 0.6, 0.6 to
0.7, 0.7 to 0.8, 0.8
to 0.9, 0.9 to 1.0, 1.0 to 1.1, 1.1 to 1.2, 1.2 to 1.3, or 1.3 to 1.4 g/cm3.
In some embodiments
the density is controlled to a predetermined target value by selection of the
ratios of the
components of the composition. For example, by balancing the concentration of
a
component which reduces the density, such as hollow microspheres, and one that
has a
.. relatively high density, such as nitrate prills.
pH of the explosive compositions
The pH of the emulsion explosive compositions of the present invention is not
narrowly critical. However, in general the pH is between 0 and 8 and suitably
the pH is
between 1 and 6, and may be controlled by suitable addition of conventional
additives, for
.. example inorganic or organic acids and salts.
Viscosity of the H202-based compositions
The viscosity of the H202-based compositions (watergel or emulsion type) will
be
discussed in terms of apparent viscosity. Where used herein the term "apparent
viscosity"
refers to viscosity measure using a Brookfield RVT viscometer, #7 spindle at
50 r.p.m.
It is preferred in the process of the present invention that the explosive
composition
of the water-in-oil emulsion explosive particles have an apparent viscosity
greater than
10Pa*s (Pascal*second) prior to the entrainment of gas bubbles. Apparent
viscosity is
more preferably in the range 10 to 50 Pa*s. A more preferred viscosity range
for the
entrainment of gas bubbles by mechanical mixing is from 10 to 35Pa*s. The
range 10 to
.. 25Pa*s provides the most efficient entrainment of gas bubbles by mechanical
mixing.
Preferably the explosive composition of the invention can be easily pumped.
Oxygen balance of the explosive compositions
"Oxygen balance" (OB) is a term of the art which is used to indicate the
degree to
which an explosive can be oxidised. An OB close to zero is preferred when
formulating
mining explosives, such that no reactant is in excess during the detonation
process, and
therefore the expected products are nitrogen, water and carbon dioxide. If the
oxygen
balance is far from zero, some part of the reactant materials will not react
and instead,
those unreacted material absorb/sink heat from the detonation reaction, which
in turn will
cause the explosive to underperform. For example, some prior art compositions
are
unsuitable for combustion, as they lack fuel (and therefore the OB is too
positive) and the
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 40 -
composition cannot burn.
Suitably the amount of fuels materials in the explosive composition can be
adjusted
so the composition has a final oxygen balance between +10 and -10, e.g.
between +5 and
-5.
PREPARATION OF COMPOSITION
According to a second aspect, the present invention provides a method of
preparing an explosive composition according to the first aspect, the method
comprising:
combining H202, and fuel and one or more density stabilisers, and optionally
one or more
other oxidisers and/or, a sensitiser, and one or more density stabilisers.
The H202-based compositions of the present invention may be prepared by a
number of methods.
In one preferred method of manufacture, the H202-based watergel type
compositions may be prepared by combining H202 with a density stabiliser,
water miscible
fuels, and thickeners until the thickener starts increasing the viscosity of
said composition.
Once the watergel is formed, solid ingredients (fuels, energy diluting agents,
etc) are
optionally mixed into said watergel. Sensitisers can be mixed into said
watergel capable
in an amount capable to sensitise the watergel. Finally, sensitising agents
can be mixed
into the oxidiser component prior to mixing into said watergel.
In one preferred method of manufacture the H202-based emulsion type
compositions may be prepared by: combining hydrogen peroxide with a density
stabiliser,
the water-immiscible organic phase, a water-in-oil emulsifier, with rapid
mixing to form a
water-in-oil emulsion; then mixing until the emulsion is uniform. Once the
emulsion is
formed, solid ingredients (fuels, energy diluting agents, etc) are optionally
mixed into said
watergel. Sensitisers are mixed into said emulsion in an amount capable of
sensitising
said watergel. Finally, sensitising agents can be mixed into the oxidiser
component prior
to mixing into said emulsion.
Preparation of watergel H202-based explosive composition
Watergel explosive compositions made according to the present invention
preferably include H202 in concentrations between 10 ¨ 64% by weight.
It will also be appreciated that other oxidisers can be combined with H202, as
discussed above. For example nitrate salts, perchlorate salts, amine nitrates,
sodium/potassium peroxide, etc., can be also incorporated in combination with
H202.
The skilled person will appreciate that there are many options that are
available for
use as a fuel. For example the fuel may be a product of vegetable origin, such
as sugars
or molasses, alcohols, organic acids, ethers, esters, urea, hexamines,etc.
Alternatively, it
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
-41 -
may be a product derived from crude oil such a diesel, paraffinic oils or
mineral oil, etc.
Other fuels may be silicone oils, etc.
Secondary fuels may be a solid hydrocarbon, such as coal and recycled plastic
waste. It may also be a metallic fuel, such as aluminium / silicon, etc, or
gilsonite,
comminuted coke or charcoal, carbon black, resin acids such as abietic acid,
vegetable
products such as starch, nut meal, grain meal and wood pulp; or nitrogen
compounds such
as amides, amines, etc.
Preferably the amount of these fuels materials in the formulation can be
adjusted
so the H202-based composition has an oxygen balance between 3 and -10 and the
H202-
based composition can be easily pumped. The preferred fuels are oil
distillates, diesel-like
hydrocarbons, glycerol, sugar, syrup, alcohol, carbon, ground coal, waxes,
oils such as
corn, cottonseed, olive, peanut, or fatty acid oils.
It will be appreciated that for an H202-based composition in accordance with
the
invention to be functional, it is important that gas bubbles are homogeneously
distributed
throughout the composition. It is also important that once distributed
throughout, the gas
bubbles should be maintained in a homogenous distribution throughout the
composition ,
i.e. little or no segregation or settling, and that the density be maintained
or stabilised to
increase the sleep time. In accordance with the present invention this may be
achieved
by formulating the explosive as a stable watergel and including a density
stabiliser.
Formation of watergel compositions is conventional in the art and one skilled
in the art will
be familiar with the various forms that may be produced. Typically this will
involve the use
of a thickener that acts on the liquid oxidant component of the composition.
Herein the
term "thickener" is also intended to include gelling agents, crosslinking
agents, and the
like.
As discussed above, any conventional thickener may be used with the present
invention. The thickener may be selected from natural gums, such guar gum,
xanthan gum,
sodium alginate, carboxymethylcellullose, methylcellulose and the like.
Synthetic
thickeners, such polyacrylamide, may also be used. Inorganic thickeners, such
as fumed
silica, clays and carbosil, may also be used, or a combination thereof.
Crosslinkers can also used with the present invention. Thickeners in
combination
with crosslinkers can improve the water resistance and mechanical strength of
the H202-
based explosive. Examples of crosslinkers are those from antimony, calcium,
titanium,
chromium, borate salts and dichromate salts, etc.
Various additional ingredients, familiar to those skilled in the art, may be
employed
in the formulation of the invention.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 42 -
Preparation of water-in-oil H202-based explosive composition
Water-in-oil explosive compositions made according to the present invention
include hydrogen peroxide in concentration between 10¨ 85% by weight. It will
also be
appreciated that other oxidisers can be combined with H202, as discussed
above. For
example nitrate salts, perchlorate salts, amine nitrates, sodium / potassium
peroxide, etc.,
can be also incorporated in combination with H202.
The fuel can be any fuel such as diesel fuel, recycled oil distillates, and
diesel-like
distillates. Alternatively it can be paraffinic, mineral, olefinic, naphtenic,
animal, vegetable,
fish and silicone oils. Other types of fuels are benzene, toluene, xylenes,
asphaltic
materials and the likes.
Secondary fuels may be a solid hydrocarbon, such as coal and recycled plastic
waste. It may also be a metallic fuel, such as aluminium / silicon, etc, or
gilsonite,
comminuted coke or charcoal, carbon black, resin acids such as abietic acid,
vegetable
products such as starch, nut meal, grain meal and wood pulp; or nitrogen
compounds such
as amides, amines, etc.
Preferably the amount of these fuels materials in the formulation can be
adjusted
so the H202-based composition has an oxygen balance between 3 and -10 and the
H202-
based composition can be easily pumped.
In relation to sensitisation, similar considerations apply to water-in-oil
explosive
compositions as the watergel explosive compositions discussed above, namely
preferably
the gas bubbles are homogeneously distributed throughout the composition. In
accordance with the present invention this is achieved by formulating the
explosive as a
stable water¨in¨oil emulsion. Formation of emulsified explosives is
conventional in the art
and one skilled in the art will be familiar with the various forms may be
produced. Typically
this will involve the use of an emulsifier, which is adapted to keep the
oxidiser dispersed
throughout the continuous organic phase (fuel).
Emulsifiers commonly used in emulsion explosive compositions include sorbitan
mono oleate, sorbitan sesquioleate, poly isobutylene succinic anhydrides
(PIBSA) and
amino derivatives of PIBSA, PIB-lactone and its amino derivatives, fatty acid
salts, lecithin,
etc.
USE OF THE COMPOSITIONS
According to a third aspect, the present invention provides use of an
explosive
composition according to the first aspect to break and move ground, e.g. in
mining
operations.
According to a fourth aspect, the present invention provides the use of one or
more
density stabilisers to improve the sleep time of an explosive composition in
reactive or
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 43 -
metalliferous ground wherein the explosive composition comprises H202 and
fuel.
FURTHER EMBODIMENTS OF THE INVENTION
According to an embodiment of the present invention, a method of preparing an
explosive composition is provided comprising: combining hydrogen peroxide a
density
stabiliser and a sensitiser, wherein the sensitiser comprises a compressible
material
and/or bubbles of gas. It will also be appreciated that the invention relates
to a method of
preparing an explosive composition comprising combining hydrogen peroxide and
one or
more compounds which produce a sensitiser.
According to a further embodiment of the present invention, use of an
explosive
composition is provided comprising hydrogen peroxide and a density stabiliser
and a
sensitiser, wherein the sensitiser comprises a compressible material and/or
bubbles of
gas. It will be appreciated that the composition of the invention can be used
for many
purposes, but in particular to break and move ground in mining operations.
According to yet a further embodiment, the present invention provides a
sensitised
and sleep-time enhanced explosive composition that delays auto-sensitisation
comprising
H202, compressible material and/or bubbles of gas, and a density stabiliser.
In some embodiments the present invention consists essentially of H202, fuel,
density stabiliser, and a sensitiser, wherein the sensitiser comprises a
compressible
material and/or bubbles of gas. In other embodiments the present invention
consists
essentially of H202, density stabiliser, fuel, a sensitiser, a thickener
and/or crosslinker,
wherein the sensitiser comprises a compressible material and/or bubbles of
gas. In other
embodiments the present invention consists essentially of H202, fuel, density
stabiliser, a
sensitiser, fuel, surfactant/emulsifier, a thickener and/or crosslinker,
wherein the sensitiser
comprises a compressible material and/or bubbles of gas.
In certain aspects, the present invention provides an explosive composition
comprising: from about 2 to about 25 `)/0 w/w H202; from greater than 0 and up
to about 90
c)/0 w/w one or more other oxidisers, and a density stabiliser.
In one embodiment, there is provided an explosive composition comprising: from
about 2 to about 25 `)/0 w/w H202; and from greater than 0 and up to about 90
`)/0 w/w of
one or more of other oxidisers; and from about 15 to about 25% w/w of fuels,
preferably
sustainable fuels, and a density stabiliser.
According to a preferred embodiment, the present invention provides an
explosive
composition comprising: from about 2 to about 25 `)/0 w/w H202; from greater
than 0 and
up to about 90 `)/0 w/w of one or more other oxidisers; a fuel phase; a
thickener and/or
crosslinker; a secondary fuel; a sensitiser, and a density stabiliser.
In some embodiments, the composition comprises from about 5 to about 25 `)/0
w/w
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 44 -
H202. Preferably the one or more other oxidiser(s) is a salt or acid selected
from the group
consisting of nitrate salts, perchlorate salts, peroxide salts, or nitric
acid. For example, the
one or more other oxidisers may be selected from the group consisting of
nitrate salts,
perchlorate salts, sodium peroxide, potassium peroxide and optionally nitric
acid. The
perchlorate salts may be selected from ammonium perchlorate and sodium
perchlorate.
Preferably the salts are selected from ammonium nitrate (AN), calcium nitrate
(ON),
calcium ammonium nitrate (CAN), sodium nitrate (SN), NI-140104, NaCI04, Na202,
K202 or
mixtures thereof. For example, the nitrate salts may be selected from ammonium
nitrate,
calcium nitrate and sodium nitrate. By way of further example, the nitrate
salts may be
selected from calcium ammonium nitrate, calcium nitrate and sodium nitrate. In
one
embodiment, the explosive composition is devoid of AN. The one or more other
oxidisers
in the explosive composition may be selected from calcium nitrate and sodium
nitrate.
Preferably the explosive composition contains from 0.1 to 75% w/w of one or
more other
oxidisers. In one embodiment, the explosive composition contains from 0.1 to
75% w/w of
dissolved salts. In a preferred embodiment, at least some of at least one of
the one or
more other oxidisers is not fully dissolved in the explosive composition but
is present as a
solid oxidiser, e.g., in the form of powder or prills. In such an embodiment,
the one or more
other oxidisers that is at least partially present as a solid may be selected
from the group
consisting of AN, SN, ON, CAN, or mixtures thereof. The composition may
comprise a
solid nitrate oxidiser, for example, in an amount of from contains from 0.1 to
70% w/w. The
composition may comprise water. The solid nitrate oxidiser may be selected
from the
group of AN, SN, CAN or mixtures thereof.
In one embodiment, there is provided an explosive composition comprising:
= from about 2 to about 85 `)/0 w/w hydrogen peroxide; and
= from about 2 to about 25 `)/0 w/w of fuels, preferably sustainable fuels;
and
= from about 0.25 to about 5 of DTPMPA.Na.x
According to a preferred embodiment, the present invention provides an
explosive
composition comprising:
= from about 2 to about 25 `)/0 w/w hydrogen peroxide; and from greater
than 0 and
up to about 90 `)/0 w/w of one or more other oxidisers; or from about 2 to
about 85
% w/w hydrogen peroxide;
= from about 0.25 to about 3 c)/0 w/w of DTPMPA.Na.x;
= a fuel phase;
= a thickener and/or crosslinker;
= a secondary fuel; and
= a sensitiser.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 45 -
Preferably the composition comprises 50 c)/0 w/w or less of water, or 30 c)/0
w/w or
less of water, or 25 c)/0 w/w or less of water. The explosive composition may
further
comprise one or more other components selected from the group consisting of
sensitisers,
fuels, secondary fuels, water, thickeners, crosslinkers, emulsifiers, energy
diluents and
optionally other additives.
Preferably the explosive composition comprises a sensitiser. Preferably the
sensitiser comprises a compressible material and/or bubbles of gas, or
comprises a gas
entrapped material. The bubbles of gas may be formed in situ and consist of
N2, 02, 002,
NO, or H2 bubbles or a mixture thereof. The gas entrapped material may be
selected from
glass microballoons, ceramic microballoons, plastic microballoons or EPS with
a particle
size smaller than 2 mm. The explosive composition preferably has a density
controlled by
adding a sufficient amount of sensitiser such that the composition is
detonation-sensitive.
The density may be controlled to around 0.3 to 1.4 g/cm3, or may be formulated
to around
0.3 to 1.4 g/cm3.
The composition may comprise a fuel, or it may comprise a fuel and a secondary
fuel. The fuel may be a water soluble fuel. The water soluble fuel may be
selected from an
amine nitrate or urea or a mixture thereof. The explosive composition may
contain from
0.1 to 30% w/w of water soluble fuel. The composition may contain between 13 ¨
25%
w/w of the fuel phase. Preferably the fuel phase comprises one or more
components
selected from the group consisting of gums, glycerol, ethylene glycol,
propylene glycol,
sugar molasses, formamide or mixtures thereof. For example, the fuel phase may
comprise one or more components selected from the group consisting of gums,
glycerol,
ethylene glycol, propylene glycol, formamide or mixtures thereof. The
composition may
comprise a sustainable fuel. The sustainable fuel may be present in the
composition in an
amount of between 15 and 25 c)/0 w/w.
Preferably the composition is a watergel composition, in which case the
composition may comprise a thickener or crosslinker. The composition may be a
watergel
composition comprising a thickener and a crosslinker. The thickener may be
suspended
in the fuel. The thickener may be selected from the group consisting of guar
gum, xanthan
gum, sodium alginate, polyacrylamides, and polyvinyl alcohols. The composition
may
comprise a crosslinker selected from the group of antimony salts, chromic
salts,
phosphoric acid or mixtures thereof. The fuel phase may comprise one or more
water
insoluble fuels selected from the group consisting of diesel, oils, vegetable
oils, or mixtures
thereof. Accordingly, the explosive composition may be formulated as an
emulsion, in
which case it may comprise an emulsifier. The emulsifier may be mixed in the
fuel. The
emulsifier may be selected from the group consisting of PIBSA-amine
derivatives, SMO,
lecithin or a mixture thereof.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 46 -
Preferably the composition is formulated to have an oxygen balance between +10
to -10, e.g., the composition may have an oxygen balance of between +5 and -5.
The
explosive composition may contain from 1 to 800% v/v of an energy reducing
agent (i.e.,
diluent material). The energy diluent material may be selected from the group
consisting
of EPS, crumb rubber tyre, popcorn, and plastic beads. The hydrogen peroxide,
one or
more other oxidisers and a fuel containing thickeners may be mixed until a
thick material
is formed, with a viscosity between 5 ¨ 50 Pa*s. The composition may have a
viscosity of
from 5 to 50 Pa*s.
Many advantages result from the inventive explosive compositions taught
herein.
For example, certain formulations of the compositions of the invention may be
more
convenient to prepare, more cost effective compared to existing explosive
compositions,
safer to produce and to store, and/or capable of being produced in large
quantities to meet
the demand from the mining industry. Added safety and broader application is
provided
by the use of a density stabiliser to extend sleep-time. The present invention
is therefore
a significant advance in the art. The explosive compositions of the invention
utilise H202,
which is a sustainably-produced material that has a relatively low carbon
footprint
compared to other types oxidisers used in the art. The composition may also
use
sustainable fuels, as opposed to current technology used in the mining
industry. To
explain, current explosive compositions use a low concentration of fuel, which
is typically
sourced from the petrochemical industry. In contrast, the inventive explosive
compositions
disclosed herein are able to incorporate relative amounts of recycled fuel to
commercially
available or prior art explosive compositions. Accordingly, the recycled fuels
from the
petrochemical industry is a significant advance in the art. Additionally use
of recycled fuels
in the composition means that the amount of oxidiser material in the
formulation can be
balanced without affecting the detonation properties.
The present invention is counterintuitive to the common knowledge in the art.
To
explain, it is currently believed that it is impossible or very difficult to
stabilise the density
of compositions that has a relatively high concentration of H202. However,
surprisingly,
the present invention provides the ability to enhance the density stability of
compositions
that contain up to 42% w/w of H202. This aspect of the present invention is a
significant
advance in the art. The present invention also provides the ability to
incorporate a
relatively high amount of nitrates by making a watergel or emulsion, which
already
comprises H202/nitrate in the aqueous phase, with a further solid nitrate
phase in the form
of prills. Use of oxidiser in solid form enables some control over the density
of the overall
composition, and therefore provides some control over the VOD, as will be
discussed
below.
The explosive compositions of the invention may also be formulated into
emulsion
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 47 -
form. It will also be appreciated that the inventive compositions of the
invention may
produce low amounts of NOR, and in some forms of the invention no NO at all.
The compositions of the invention are contemplated to provide several
advantages
over the prior art, such as better stability over time than explosive
compositions comprising
a higher percentage of H202. This is advantageous in the context of both
safety, storage,
and application. More specifically, the "sleep time" (i.e. the time over which
an explosive
deteriorates in situ such that its velocity of detonation decreases below a
defined useful
limit of such an explosive composition when it is in contact with rocks) is
expected to be
greater than an explosive comprising a higher percentage of H202. By way of
example, a
density-stabilised H202 composition according to the invention has been made
and found
to have a sleep time that is in excess of compositions without density
stabilisers, and
comparable / compatible for application in a commercially viable product, for
example, a
sleep time beyond 24 hours or more. It is therefore contemplated that larger
blasts are
possible because there is a longer time (e.g. several days) over which
explosives can be
loaded into many holes before the first-loaded explosive becomes unstable in
its hole.
More holes can therefore be loaded before detonation.
Another advantage is that these compositions detonate when density stabliisers
are used. This is unexpected because of concerns that density-stabilised
compositions
may not detonate. There are also safety advantages contemplated in using
density
stabilised compositions.
The present invention relates to a peroxide-based explosive composition that
is
preferably prepared as watergel or water-in-oil emulsion and is sensitised.
Numerous embodiments are described in this patent application and are
presented
for illustrative purposes only. The described embodiments are not intended to
be limiting
in any sense. The invention is widely applicable to numerous embodiments, as
is readily
apparent from the disclosure herein.
Table 1 lists the components of explosive systems discussed herein and
provides
typical ranges for each:
Explosive technology
Component Watergel Emulsion
(in % by weight of total
composition except where
indicated otherwise)
H202 From 2 to 65 From 10 to 80
Density stabiliser From 0.1 to 5 From 0.1 to 5
CA 03142594 2021-12-03
WO 2020/243788
PCT/AU2020/050573
- 48 -
One or more other oxidiser From 0 to 90 From 0 to 90
Sensitiser (Y() by volume) From 0.5 to 800* From 0.5 to 800*
Fuels From 2 to 25 From 2 to 25
Secondary fuels From 0.1 to 11 From 0.1 to 11
Water From 5 to 40 From 5 to 40
Thickeners From 0.5 to 5 N/A
Emulsifiers N/A From 0.5 to 5
Additives 0.1 to 5 0.1 to 5
Energy diluents (Y() by From 0.1 to 300** From 0.1 to 300**
volume)
Oxygen Balance From 5 to -5 From 5 to -5
Final densities (g/m1) 0.3 to 1.4 0.3 to 1.4
Table 1: components for explosive systems discussed herein with typical ranges
for
each. NOTE: it will be appreciated that the volume can be increased by 8x (*),
and 3x (**),
respectively.
Typical components for each type of explosive technology are listed in Table
2:
Explosive technology
Component Watergel Water-in-oil emulsion
Oxidiser(s) hydrogen peroxide (H202) hydrogen peroxide (H202)
optionally nitrate salts and optionally nitrate salts and
/or perchlorate salts and/or /or perchlorate salts and/or
sodium / potassium sodium
potassium
peroxide peroxide
Sensitiser gas bubbles (chemically gas bubbles (chemically
generated or injected generated or injected
bubbles) and/or gas bubbles) and/or gas
entrapped compressible entrapped
compressible
materials materials
Fuel Water miscible fuels, water Water miscible fuels,
water
immiscible fuels, water immiscible fuels, water
soluble fuels or water- soluble fuels or water-
insoluble fuels insoluble fuels
Surfactant water soluble surfactants or water soluble
surfactants or
fuel-soluble surfactants fuel-soluble surfactants
Density stabiliser e.g., Phosphonates e.g., Phosphonates.
CA 03142594 2021-12-03
WO 2020/243788
PCT/AU2020/050573
- 49 -
Composition Phosphates, Stannates, Phosphates, Stannates, etc
stabiliser etc.
Additives crosslinkerss, catalysts for crosslinkers, catalysts
for
gassing, pH adjusters, gassing, pH adjusters,
thickeners emulsifiers
Energy
diluents Granulated / shredded Granulated / shredded
(optional) rubber, expanded popcorn, rubber, expanded popcorn,
expanded rice, plastic expanded rice, plastic
beads, EPS >5 mm beads, EPS >5 mm
Energy deferments Metal oxides Metal oxides
(optional)
Table 2: Typical components of the present invention for each type of
explosive
technology.
EXAMPLES
The present invention can be used for a variety of forms of explosives
provided of
course that the principles of the invention as described herein are observed.
The invention
is further illustrated with reference to the following examples.
Example 1
Hydrogen peroxide/fuel-based hydrogel formulations, containing a glycerol fuel
phase, were calculated and hand-made containing 0 - 3 % w/w DTMPMA .Na.X
material
(See Table 3 below).
DTMPMA .Na.X was first suspended in the oxidiser phase, then mixed with the
fuel phase of the formulation. Plastic pots (-58 mL) were used to store the
gels (n=4) on
laboratory benches at room temperature (25-35 C). Formulation density vs
DTMPMA
.Na.X % w/w were determined for the range of 0 - 3 % w/w. Over seven days,
density
measurements were taken and change in density was calculated. Tests were
terminated
when gels displayed compromised structure due to large gas bubble generation.
Several samples were prepared in accordance with Table 3:
Component (w/w %)
Composition (w/w % phosphonate)
0 1 2 3
hydrogen peroxide (100% w.w.) 41.8 41.5 41.1 40.8
water 41.8 41.5 41.1 40.8
glycerol fuel 13.4 13 12.8 12.4
xanthan gum-based explosive 3 3 3 3
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 50 -
compositions
diethylenetriamine pentamethylene 0 1 2 3
phosphonic acid sodium salt
(DTPMPA.Na.x)
Table 3: Explosive compositions prepared according to the present invention.
The results are shown in Figure 1 and Figure 2. Whilst 0% w/w DTMPMA .Na.X
formulation degraded within two days, the tests were allowed to continue until
decomposition of all gels was observed.
Time Density (g.cm-3) at Composition
(days) (% w/w phosphonate)
0 1 2 3
0 1.25 1.25 1.25 1.25
1 1.06 1.21 1.21 1.21
2 0.88 1.16 1.16 1.16
3 1.13 1.13 1.13
4 1.02 1.02 1.04
7 0.76 0.76 0.76
Table 4: Density (g.cm-3) vs time (days) for explosive compositions having
varying levels
of phosphonates. Note, 0 % w/w phosphonate mixture collapsed by day 3.
Time (days) Density loss (% to initial) at Composition
(% w/w phosphonate)
0 1 2 3
0 0 0 0 0
1 15.2 1.2 2.2 3
2 29.6 4 5.2 7
3 7.4 8.2 9.4
4 16.6 16.6 16.6
7 40 40 40
Table 5: Density loss ( /0 to initial) vs time for explosive compositions
having varying levels
of phosphonates. Note, 0 % w/w phosphonate mixture collapsed by day 3.
Formulations without density-stabilised hydrogen peroxide degraded within 48
hours, whereas density-stabilised hydrogen peroxide compositions at the same
time
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 51 -
period exhibited approx. 7 percent density loss to the initial density. This
is a significant
and surprising improvement. This improvement to sleep time means that more
blast holes
can be loaded during a planned blast saving time and money, that the
detonation
performance of the product has enhanced reliability, and that the product has
improved
safety due to the reduced likelihood of density loss.
Example 2
Detonation performance analysis was carried out in field-range unconfined
tests to
assess the explosive capability and characteristics of density-stabilised
hydrogen peroxide
compositions 81.35% w/w hydrogen peroxide (50 % w/w), 12.65 % w/w glycerol
fuel, 3 %
w/w xanthan gum, and 3 % w/w DTPMPA.Na.x formulation, incorporating varying %
w/w
GMB (3MTm K15 Glass Bubbles) for required density (See table 4 below).
Component (w/w %) Density (g.cm-3)
0.83 1.0 1.03 1.05 1.08
hydrogen peroxide (100% w/w) 39.4 39.8 40 40 40.06
water 39.4 39.8 40 40 40.06
glycerol fuel 12.25 12.35 12.4 12.45
12.46
xanthan gum-based explosive 2.9 2.9 2.925 2.9525 2.96
compositions
diethylenetriamine 2.9 2.9 2.925 2.9525 2.96
pentamethylene phosphonic acid
sodium salt (DTPMPA.Na.x)
GMB, (3MTm K15) 3.15 2.25 1.75 1.6 1.5
Average Velocity of Detonation 3628 4210 4337 4334 4401
(m.s-1)
Table 6: Detonation testing of explosive compositions prepared according to
the present
invention.
Triplicate samples were prepared of explosive compositions.
The results are shown in Figure 3.
As can be seen from these examples, the density loss and instability of the
hydrogen peroxide-based explosives was reduced, with the addition of
phosphonates,
increasing the sleep-time of the formulations. The use of hydrogen
peroxide/fuel-based
explosives prepared with the addition of phosphonates results in a substantial
improvement in the art of hydrogen peroxide/fuel-based explosives.
Importantly, the
addition of density stabilisers as described herein does not adversely impact
on the
detonation performance of the explosive composition.
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 52 -
Detonation testing
Selected compositions were tested to determine detonation. 47 mm diameter,
clear
high density (150 m) polyethylene lay-flat tube by 1000 mm in length and
sealed on one
end were used for gels. Detonation was initiated with a 25 g Pentex D Booster.
Duplicate VOD was measured using a Time Domain Reflectometry (TRD)-based
VOD instrument featuring a sample rate of under 4uS and a nomlnal resoiution
of 90 Pica
seconds. The VOD data indicate an acceptable detonation performance for mining
applications.
Example 3
Stabilised-hydrogen peroxide/fuel-based hydrogel formulations, containing a
glycerol fuel phase, were calculated and hand-made containing 0 - 2 A, w/w
Phytic Acid
(PA). PA was first suspended in the oxidiser phase, then mixed with the fuel
phase (3%
xanthan gum) of the formulation. Plastic pots (-58 mL) were used to store the
gels (n=4)
on laboratory benches at room temperature (20-25 C). Product density vs PA A,
w/w were
established for the range of 0 - 5 A, PA. Over 13 days, density measurements
were taken
and change in density was calculated. Tests were terminated when gels
displayed
compromised structure due to large gas bubble generation. Whilst 3-5% w/w PA
degraded
within 5 days, the tests were allowed to continue until decomposition of all
gels was
observed.
Over two tests, of 10 days and 13 days respectively, it was observed that all
gels
decreased in density. All initial gel densities were approx. 1.22 g.cm-3. With
increasing %
w/w PA an associated increase in gel density was observed (Figure 4 & Figure
5). Gels
containing 3-5% w/w PA degraded within five days. At approximately seven days
the rate
of degradation of the nil-PA control appears to change, which may be an
indicator of HP
degradation nearing completion. Whilst 0.25% w/w PA displayed the least
density loss
over the time course (Figure 6 & Figure 7), statistically significant loss
over 13 days was
not determined between nil-PA and 0.25% w/w PA gels (p-value = 0.19). Over the
measurement time, individual pot measurement standard deviations of replicants
did not
exceed 0.02 mL.
Formulations containing PA at concentrations of 0.25 - 0.75% w/w displayed
increased gel stability over 13 days. However, PA at concentrations above
about 0.75 A,
accelerated decomposition. After 13 days of room temperature bench-top
storage, all gels,
including mixtures containing PA, had lost at least 18% gel density when
compared to
initial density.
The skilled addressee will understand that the invention comprises the
embodiments and features disclosed herein as well as all combinations and/or
CA 03142594 2021-12-03
WO 2020/243788 PCT/AU2020/050573
- 53 -
permutations of the disclosed embodiments and features.
Although the invention has been described with reference to specific examples,
it
will be appreciated by those skilled in the art that the invention may be
embodied in many
other forms. In particular features of any one of the various described
examples may be
provided in any combination in any of the other described examples.