Note: Descriptions are shown in the official language in which they were submitted.
.4
WO 2015/037248 PCT/JP2014/00.1730
Description
Title of Invention: APPLICATION OF R-KETAMINE AND SALT
THEREOF AS PHARMACEUTICALS
Technical Field
[0001] The present invention relates to a pharmaceutical for prevention
and/or treatment of
psychiatric diseases, preferably diseases exhibiting depressive symptoms. More
specifically, the present invention relates to an antidepressant including R-
ketamine or
a pharmaceutically acceptable salt thereof, and to a pharmaceutical
composition for
prevention and/or treatment of diseases exhibiting depressive symptoms,
including R-
ketamine or a pharmaceutically acceptable salt thereof, and being
substantially free of
S-ketamine or a pharmaceutically acceptable salt thereof.
Background Art
[0002] Along with changes in social life style and aging of society,
various diseases such as
psychiatric diseases and neurological diseases tend to increase as a whole.
For
example, high incidences of depression and schizophrenia, which are major
psychiatric
diseases, have become a serious problem from the viewpoint of medical economy
as
well. In addition, obsessive-compulsive disorder is an anxiety disorder
involving ob-
sessions and compulsions. In treatment of the psychiatric diseases such as
depression,
schizophrenia, anxiety disorders, and autism spectrum disorder, medication is
essential, and an antidepressant (e.g., a tricyclic antidepressant, a
selective serotonin
reuptake inhibitor, and a serotonin and norepinephrine reuptake inhibitor), an
an-
tipsychotic (e.g., a phenothiazine-based compound, a butyrophenone-based
compound,
a benzamide-based compound, an iminodibenzyl compound, a thiepin-based
compound, an indole-based compound, and a serotonin/dopamine receptor
antagonist),
and an anti-anxiety drug are administered. However, those drugs used actually
in a
clinical field are effective for some patients and some symptoms, but patients
for
whom the drugs are ineffective, so-called treatment-resistant patients are
also known to
exist. Thus, there is a strong demand for development of a novel therapeutic
drug. It is
hard to say that the existing drugs exhibit sufficient therapeutic effects on
those psy-
chiatric diseases. In reality, there are substantially no effective prevention
and
treatment methods at present.
[0003] One of the major problems in treatment of depression is that there
are limitations on
effects of the antidepressant and effects of its adjuvant therapy. It takes
several weeks
or more for the current antidepressants to express their drug efficacy. In
addition, there
exist treatment-resistant patients for whom those antidepressants are
ineffective.
Therefore, it is also said that only 50% of patients with depression reach
remission. In
Date recue/ date received 2021-12-23
2
WO 2015/037248 PCT/JP2014/004730
addition, when a dose of the antidepressant is increased for achieving
remission, a
patient suffers from various side effects accordingly. Further, depression is
one of the
cause of suicide. Depression in elderly peoples is known to increase the risk
if incident
dementia, in particular of Alzheimer's disease and vascular dementia (Non
Patent
Literature I).
[0004] In recent research, growing evidence suggests that abnormality in
glutamatergic
transmission, in particular, glutamatergic neurotransmission via an N-
methyl-D-aspartate (hereinafter abbreviated as NMDA) receptor is associated
with
pathophysiology of mood disorders such as major depressive disorder
(hereinafter ab-
breviated as MDD) and bipolar disorder. The MNDA receptor also plays key roles
in
neurobiology and treatment of MDD as well (Non Patent Literature 2).
[0005] It has been reported that an NMDA receptor antagonist ketamine
exhibits rapid and
robust antidepressant effects on treatment-resistant patients with MDD and
depressive
symptoms of treatment-resistant bipolar disorder (Non Patent Literatures 3 to
5). In
addition, it has been reported that ketamine is also effective for treatment-
resistant
obsessive-compulsive disorder and treatment-resistant posttraumatic stress
disorder
(hereinafter abbreviated as PTSD) (Non Patent Literatures 6 to 8). Ketamine
has also
been reported to have an effect of inhibiting suicide ideation (Non Patent
Literature 9).
Further, ketamine treatment in an adult with autism spectrum has been reported
(Non
Patent Literature 10). Ketamine, which was a compound developed as an
anesthetic in
1962, started to be applied clinically in 1965. However, ketamine is
designated as a
controlled substance because of its problems of psychotic symptoms such as
hallu-
cination and delusion, and drug dependence. At present, ketamine is used as an
anesthetic and for treatment of chronic pain in a clinical field.
[0006] It has been reported that clinical antidepressant effects of
ketamine last for a short
period of from 1 to 2 days starting from several hours after its single
administration.
Meanwhile, it has been reported that the effects may last over 2 weeks or more
(Non
Patent Literatures 3, 4, and 11). In addition, it has been reported that
ketamine has psy-
chotomimetic effects as side effects, and antidepressant effects of ketamine
were not
present until after the side effects had disappeared (Non Patent Literatures 3
and 4).
[0007] Ketamine (or sometimes referred to as RS(+/-)-ketamine) is a
racemic mixture
containing equal amounts of R(-)-ketamine and S(+)-ketamine. R(-)-ketamine and
S(+)-ketamine are also called R-isomer and S-isomer of ketamine, respectively.
S(+)-ketamine has approximately 4-fold greater affinity for the NMDA receptor
than
R-isomer (Non Patent Literature 12). Further, S(+)-ketamine has an
approximately 3-
to 4-fold anesthetic effect as compared to R-isomer, and has greater
psychotomimetic
side effects than R-isomer (Non Patent Literature 12). As described above, the
potency
of psychotomimetic effects of ketamine is correlated with the potency of
blockade of
Date recue/ date received 2021-12-23
3
WO 2015/037248 PCT/JP2014/004730
the NMDA receptor (Non Patent Literature 12). A positron emission tomography
(PET) study in healthy volunteers demonstrated that psychotomimetic doses of
S(+)-ketamine (i.e., intravenous infusion of 15 mg for 5 min, then infusion of
the dose
(0.014 to 0.02 mg/Icg/min for 53 mm) increased cerebral metabolic rates of
glucose
(hereinafter abbreviated as CMRglu) markedly in the frontal cortex and
thalamus (Non
Patent Literature 13). In contrast, cquimolar doses of R(-)-ketamine tended to
decrease
CMRglu across brain regions, and did not produce psychotic symptoms, but a
state of
relaxation and a feeling of well being (Non Patent Literature 13).
[0008] As described above, it is generally understood that both analgesic
effects and psy-
chotomimctic effects of ketamine are mediated primarily via the blockade of
the
NMDA receptor. S-isomer of ketamine has high affinity for the NMDA receptor.
Thus,
it is considered that those effects of ketaminc are caused primarily by S-
isomer.
[0009] At present, ketamine is one of the drugs that have attracted
attention for treatment of
treatment-resistant patients with MDD, depressive symptoms of treatment-
resistant
bipolar disorder, treatment-resistant obsessive-compulsive disorder, and
treatment-
resistant PTSD (Non Patent Literatures 5 to 12). A previous case report showed
that
antidepressant effects of S(+)-ketamine (0.25 mg/kg, i.v.) in treatment-
resistant
patients with MDD were weaker than those of RS(+/-)-ketamine (0.5 mg/kg, i.v.)
(Non
Patent Literature 14). Further, an open label study (Non Patent Literature 15)
and a
case report (Non Patent Literature 16) showed that effective oral doses of
RS(+/-)-ketamine and S(+)-ketamine in patients with depression were 0.5 mg/kg
and
1.25 mg/kg, respectively. In addition, intranasal administration of ketamine
showed an-
tidepressant effect in treatment-resistant patients with MDD (Patent
Literature 1 and
Non Patent Literature 17).
Citation List
Patent Literature
[0010] [PTL 1] International Patent Publication No. WO 2007/111880 A2
[PTL 2] US Patent No. 6,040,479 (A)
Non Patent Literature
[0011] [NPL 1] Diniz BD, Butters MA, Albert SM, Dew MA and Reynolds CF
(2013) Late-
life depression and risk of vascular dementia and Alzheimer's disease:
systematic
review and meta-analysis of community-based cohort studies. B. J. Psychiatry
202:
329-335.
[NPL 21 Hashimoto K (2009) Emerging role of glutamate in the pathophysiology
of
major depressive disorder. Brain Res. Rev. 61:105-23.
[NPL 3] Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS,
Krystal JH (2000) Antidepressant effects of ketamine in depressed patients.
Biol.
Date recue/ date received 2021-12-23
4
WO 2015/037248 PCT/JP2014/004730
Psychiatry 47:351-4.
[NPL 4] Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh
DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate
an-
tagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 63:856-
64.
[NPL 5] Diazgranados N. Ibrahim L. Brutsche NE, Newberg A, Kronstein P,
Khalife
S. Kammerer WA, Quezado Z. Luckenbaugh DA, Salvadore G, Machado-Vieira R,
Manji HK, Zarate CA Jr. (2010) A randomized add-on trial of an N-
methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch.
Gen.
Psychiatry 67:793-802.
[NPL 6]Bloch MH, Wasylink S, Landeros-Weisenberger A, Panza KE, Billingslea E,
Leckman JF, Krystal JH, Bhagwagar Z, Sanacora G, Pittenger C (2012) Effects of
ketamine in treatment-refractory obsessive-compulsive disorder. Biol.
Psychiatry
72(10:964-970.
[NPL 7] Rodriguez CI, Kegeles LS, LevinsonA, Feng T, Marcus SM, Vermes D,
Flood P, Simpson HB (2013) Randomized Controlled Crossover Trial of Ketamine
in
Obsessive-Compulsive Disorder: Proof-of-Concept. Neuropsychopharmacology
38:2475-83.
[NPL 8] Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S,
Kirkwood K, Aan Het Rot M, Lapidus KA, Wan LB, losifescu D, Charney DS (2014)
Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress
disorder: a randomized clinical trial. JAMA Psychiatry 71:681-688.
[NPL 9] DiazGranados N, Ibrahim LA, Brutsche NE, Ameli R, Henter ID,
Luckenbaugh DA, Machado-Vieira R, Zarate CA Jr (2010) Rapid resolution of
suicidal ideation after a single infusion of an N-methyl-D-aspartate
antagonist in
patients with treatment-resistant major depressive disorder. J Clin.
Psychiatry
71(12):1605-11.
[NPL 10] Wink LK1, O'Melia AM, Shaffer RC, Pedapati E, Friedmann K, Schaefer
T,
Erickson CA (2014) Intranasal ketamine treatment in an adult with autism
spectrum
disorder. J Clin. Psychiatry 75(8):835-6. doi: 10.4088/JCP.13cr08917.
[NPL 11] Krystal JH, Sanacora G, Duman RS (2013) Rapid-acting glutamatergic an-
tidepressants: the path to ketamine and beyond. Biol. Psychiatry 73:1133-41.
[NPL 12] Domino EF (2010) Taming the ketamine tiger. 1965. Anesthesiology
113:678-86.
[NPL 13] Vollenweider FX, Leenders KL, 0Eye I, Hell D, Angst J (1997)
Differential
psychopathology and patterns of cerebral glucose utilization produced by (S)-
and
(R)-ketamine in healthy volunteers using positron emission tomography (PET).
Eur.
Neuropsychopharmacol. 7: 25-38.
[NPL 14] Paul R, Schaaff N, Padberg F, Moeller Hi, Frodl T (2009) Comparison
of
Date recue/ date received 2021-12-23
5
WO 2015/037248 PCT/JP2014/004730
raccrnic ketamine and S-ketaminc in treatment-resistant major depression:
report from
two cases. World J. Biol.Psychiatry 10: 241-244.
[NPL 15] Paslakis G, Gilles M, Meyer-Lindenberg A, Deuschlc M (2010) Oral
admin-
istration of the NMDA receptor antagonist S-ketarnine as add-on therapy of de-
pression: a case series. Pharmacopsychiatry 43: 33-35.
[NPL 161 Irwin SA, Iglewicz A, Nelcsen RA,Lo JY, Can CH, Romero SD, Lloyd LS
(2013) Daily oral ketamine for the treatment of depression and anxiety in
patients
receiving hospice care: A 28-day open-label proof-of-concept trial. J.
Palliat. Med. 16:
958-965.
[NPL 171 Lapidus KA, Levitch CF, Perez AM, Brallier .1W, Parides MK, Soleimani
L,
Feder A, losifescu DV, Charney DS, Murrough JW (2014) A randomized controlled
trial of intranasal ketamine in major depressive disorder. Biol. Psychiatry
2014 Apr 3.
ph: S0006-3223(14)00227-3.doi: 10.1016/j.biopsych.2014.03.026. [Epub ahead of
print]
[NPL 18] Li SX, Fujita Y, Zhang JC, Ren Q, Ishima T, Wu J, Hashimoto K (2014)
Role of the NMDA receptor in cognitive deficits, anxiety and depressive-like
behavior
in juvenile and adult mice after neonatal dexamethasone exposure. Neurobiol.
Dis. 62:
124-134.
[NPL 19] Golden SA, Covington HE, III, Berton 0, Russo Si (2011) A
standardized
protocol for repeated social defeat stress in mice. Nat. Protoc. 6: 1183-1191.
Summary of Invention
Technical Problem
[0012] It has been reported that the NMDA receptor antagonist ketamine
exhibits rapid an-
tidepressant effects in treatment-resistant patients with depression. The
glutamatcrgic
neurotransmission via the NMDA receptor is considered to be involved in
depression,
ketamine includes optical isomers, i.e., S-isomer and R-isomer, and S-isomer
has
higher affinity for the NMDA receptor than R-isomer. Thus, S-isomer or a
racemic
mixture has been used for research on treatment of depression with ketamine.
However, ketamine has problems of side effects including psychotic symptoms
such as
hallucination and delusion, and dependence, and is designated as a controlled
substance. Accordingly, it is difficult to practically use ketamine in a
clinical field.
[0013] An object of the present invention is to provide a novel compound
having rapid and
long-lasting antidepressant effects on diseases exhibiting depressive
symptoms, such as
depression, bipolar disorder, obsessive-compulsive disorder, PTSD, and autism
spectrum disorder.
Solution to Problem
[0014] The inventors of the present invention have made intensive studies
in order to
Date recue/ date received 2021-12-23
6
WO 2015/037248 PCT/JP2014/004730
achieve the above-mentioned object. In the studies, the inventors have focused
attention on R(-)-ketamine, which has not been used for research on
antidepressant
effects of ketamine heretofore. In addition, in research using a mouse model
of de-
pression, the inventors have found that R(-)-ketamine exhibits more potent
antide-
pressant effects on the depression-like symptoms of the mouse model at a
juvenile
stage than S(+)-ketamine, and the effects last for a longer period. The
inventors also
found in social defeat stress model mice that R(-)-ketamine showed more potent
and
long lasting anti-depressant effect compared to S(+)-ketamine. Furthermore,
admin-
istration of S(+)-ketamine induced some side effects such as a
hyperlocomotion,
prepulse inhibition deficit, and drug dependence, while administration of
R(-)-ketamine did not. Since R-isomer of ketamine has low affinity for an NMDA
receptor as compared to its S-isomer, the R-isomer is considered to have less
psy-
chotomimetic effects as side effects and to hardly produce drug dependence.
The
present invention has been accomplished based on those findings.
[0015] That is, the present invention relates to an agent for prevention
and/or treatment of a
depressive symptom, consisting of R(-)-ketamine or a pharmacologically
acceptable
salt thereof.
[0016] The present invention also relates to the agent, in which the
depressive symptom is a
depressive symptom in depression in children or adults.
[0017] The present invention also relates to a pharmaceutical composition
for prevention
and/or treatment of a depressive symptom, comprising R(-)-ketamine or a
pharmaco-
logically acceptable salt thereof in an effective amount for reducing a
depressive
symptom, and being substantially free of S(+)-ketamine or a pharmacologically
ac-
ceptable salt thereof.
[0018] The present invention also relates to the pharmaceutical
composition, in which the
depressive symptom is a depressive symptom in depression in children or
adults.
[0019] The present invention also relates to a pharmaceutical composition
for prevention
and/or treatment of obsessive-compulsive disorder, comprising R(-)-ketamine or
a
pharmacologically acceptable salt thereof in an effective amount for reducing
a de-
pressive symptom in obsessive-compulsive disorder, and being substantially
free of
S(+)-ketamine or a pharmacologically acceptable salt thereof.
[0020] The present invention also relates to a pharmaceutical composition
for prevention
and/or treatment of posttraumatic stress disorder, comprising R(-)-ketamine or
a phar-
macologically acceptable salt thereof in an effective amount for reducing a
depressive
symptom in posttraumatic stress disorder, and being substantially free of
S(+)-ketamine or a pharmacologically acceptable salt thereof.
[0021] The present invention also relates to a method of treating a
depressive symptom,
comprising administering R(-)-ketamine or a pharmacologically acceptable salt
thereof
Date recue/ date received 2021-12-23
7
WO 2015/037248 PCT/JP2014/004730
to a subject, wherein the subject has been diagnosed with having a depressive
symptom, in an amount effective to treat the depressive symptom.
[0022] The present invention also relates to the method, in which the
subject has been
diagnosed with depression in children or adults
[0023] The present invention also relates to the method, in which the
subject has been
diagnosed with obsessive-compulsive disorder.
[0024] The present invention also relates to the method, in which the
subject has been
diagnosed with posttraumatic stress disorder.
[0025] The present invention also relates to a method of treating a
depressive symptom,
comprising administering a pharmaceutical composition to a subject, wherein
the phar-
maceutical composition comprises R(-)-ketamine or a pharmaceutically
acceptable salt
thereof in an amount effective to treat a depressive symptom, and a
pharmaceutically
acceptable carrier, and being substantially free of S(+)-ketamine or a
pharmaco-
logically acceptable salt thereof, and wherein the subject has been diagnosed
with
having a depressive symptom.
[0026] The present invention also relates to the method, in which the
subject has been
diagnosed with depression in children or adults.
[0027] The present invention also relates to the method, in which the
subject has been
diagnosed with obsessive-compulsive disorder.
[0028] The present invention also relates to the method, in which the
subject has been
diagnosed with posttraumatic stress disorder.
[0029] The present invention also relates to use of R(-)-ketamine for the
manufacture of a
pharmaceutical composition for treating a depressive symptom.
[0030] The present invention also relates to the use, in which the
depressive symptom ac-
companies depression in children or adults.
[0031] The present invention also relates to the use, in which the
depressive symptom ac-
companies obsessive-compulsive disorder.
[0032] The present invention also relates to the use, in which the
depressive symptoms ac-
companies posttraumatic stress disorder.
Advantageous Effects of Invention
[0033] R(-)-ketamine Or a pharmaceutically acceptable salt thereof has
rapid and long-
lasting antidepressant effects and less side effects, and hence is effective
for prevention
and/or treatment of psychiatric diseases exhibiting depressive symptoms.
Accordingly,
the agent consisting of R(-)-ketamine or a pharmacologically acceptable salt
thereof,
and the pharmaceutical composition including R(-)-ketamine or a
pharmacologically
acceptable salt thereof, and being substantially free of S(+)-ketamine or a
pharmaco-
logically acceptable salt thereof are useful as novel pharmaceuticals in the
field of
Date recue/ date received 2021-12-23
8
WO 2015/037248 PCT/JP2014/004730
prevention and/or treatment of psychiatric diseases exhibiting depressive
symptoms.
Brief Description of Drawings
[0034] [fig. l]FIG. 1 is a diagram illustrating the preparation of R(-)- and
S(+)-ketamine hy-
drochloride (Ketamine HC1) from RS(+/-)-ketamine using D(-)- and L(+)-tartaric
acid
(Tartaric acid), respectively.
[fig.2A]FIG. 2A is a diagram illustrating a test protocol for investigating
antide-
pressant effects of R(-)- and S(+)-ketamine. Tests were performed using mice
treated
neonatally with dexarnethasone (hereinafter referred to as DEX-treated mice)
as a new
animal model of depression. In FIG. 2A, DEX means dexamethasone, LMT means a
locomotion test, TST means a tail suspension test, FST means a forced swimming
test,
and SPT means a 1% sucrose preference test. (Example 1)
[fig.2BIFIG. 2B is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the DEX-treated mice investigated by the LMT the day after
the
injection of ketamine. In FIG. 2B, R-Ket and S-Ket represent DEX-treated mouse
groups injected with R(-)-ketamine and S(+)-ketamine, respectively, Saline
represents
a DEX-treated mouse group injected with saline, and Cont represents a control
mouse
group injected with saline. The ordinate axis of FIG. 2B indicates locomotions
(count/60 min). (Example 1)
[fig.2C]FIG. 2C is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the DEX-treated mice investigated by the TST the day (27
hours)
after the injection of ketamine. In FIG. 2C, R-Ket and S-Ket represent DEX-
treated
mouse groups injected with R(-)-ketamine and S(+)-ketamine, respectively,
Saline
represents a DEX-treated mouse group injected with saline, and Cont represents
a
control mouse group injected with saline. The ordinate axis of FIG. 2C
indicates im-
mobility times (sec) in the TST. (Example 1)
[fig.2D1FIG. 2D is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the DEX-treated mice investigated by the FST the day
(29hours) after
the injection of ketamine. In FIG. 2D, R-Ket and S-Ket represent DEX-treated
mouse
groups injected with R(-)-ketamine and S(+)-ketamine, respectively, Saline
represents
a DEX-treated mouse group injected with saline, and Cont represents a control
mouse
group injected with saline. The ordinate axis of FIG. 2D indicates immobility
times
(sec) in the FST. (Example 1)
[fig.2E1FIG. 2E is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the DEX-treated mice investigated by the SPT 2 days after the
injection of ketamine. In FIG. 2E, R-Ket and S-Ket represent DEX-treated mouse
groups injected with R(-)-ketamine and S(+)-ketamine, respectively. Saline
represents
a DEX-treated mouse group injected with saline, and Cont represents a control
mouse
Date recue/ date received 2021-12-23
9
WO 2015/037248 PCT/JP2014/004730
group injected with saline. The ordinate axis of FIG. 2E indicates sucrose
preferences
(%) in the SPT. (Example 1)
[fig.2FTIG. 2F is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the DEX-treated mice investigated by the TST 7 days after the
injection of ketamine. In FIG. 2F, R-Ket and S-Ket represent DEX-treated mouse
groups injected with R(-)-ketamine and S(+)-ketamine, respectively. Saline
represents
a DEX-treated mouse group injected with saline, and Cont represents a control
mouse
group injected with saline. The ordinate axis of FIG. 2F indicates immobility
times
(sec) in the TST. (Example 1)
[fig.2G]FIG. 2G is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the DEX-treated mice investigated by the FST 7 days after the
injection of ketamine. In FIG. 2G, R-Ket and S-Ket represent DEX-treated mouse
groups injected with R(-)-ketamine and S(+)-ketamine, respectively. Saline
represents
a DEX-treated mouse group injected with saline, and Cont represents a control
mouse
group injected with saline. The ordinate axis of FIG. 2G indicates immobility
times
(sec) in the FST. (Example 1)
ffig.3A1FIG. 3A is a diagram illustrating a test protocol for investigating
antide-
pressant effects of R(-)- and S(+)-ketamine in social defeat stress mice. The
social
defeat stress mice were prepared by bringing C57/B6 male mice into contact
with ICR
male mice for 10 consecutive days (Dl to 10). After that, any one of R(-)-
ketamine and
S(+)-ketamine was injected and various tests were performed on day 1, day2,
day 6,
and day 7 (P1, P2, P6, and P7) after the injection. In FIG. 3A, R-Ket and S-
Ket
represent social defeat stress mouse groups injected with R(-)-ketamine and
S(+)-ketamine, respectively. Saline represents a social defeat stress mouse
group
injected with saline. In FIG. 3A, LMT means a locomotion test, TST means a
tail
suspension test, FST means a forced swimming test, and SPT means a 1% sucrose
preference test. (Example 2)
[fig.313.1FIG. 3B is a graph showing results of the antidepressant effects of
R(-)- and
S(+)-ketamine in the social defeat stress mice investigated by the SPT 1 day
(P1) after
the injection of ketamine. In FIG. 3B, R-Ket and S-Ket represent social defeat
stress
mouse groups injected with R(-)-ketamine and S(+)-ketamine, respectively.
Saline
represents a social defeat stress mouse group injected with saline, and
Control
represents a control mouse group injected with saline. The ordinate axis of
FIG. 3B
indicates sucrose preferences (%) in the SPT. (Example 2)
[fig.3C]FIG. 3C is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the social defeat stress mice investigated by the LMT 2
days(P2)
after the injection of ketamine. In FIG. 3C, R-Ket and S-Ket represent social
defeat
stress mouse groups injected with R(-)-ketamine and S(+)-ketamine,
respectively.
Date recue/ date received 2021-12-23
10
WO 2015/037248 PCT/JP2014/004730
Saline represents a social defeat stress mouse group injected with saline, and
Control
represents a control mouse group injected with saline. The ordinate axis of
FIG. 3C
indicates locomotions (count/60min) in the LMT. (Example 2)
[fig.3DWIG. 3D is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the social defeat stress mice investigated by the TST 2 days
(P2) after
the injection of ketamine. In FIG. 3D, R-Ket and S-Ket represent social defeat
stress
mouse groups injected with R(-)-ketamine and S(+)-ketamine, respectively.
Saline
represents a social defeat stress mouse group injected with saline, and
Control
represents a control mouse group injected with saline. The ordinate axis of
FIG. 3D
indicates immobility times (sec) in the TST. (Example 2)
[fig.3E]FIG. 3E is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the social defeat stress mice investigated by the FST 2 days
(P2) after
the injection of ketamine. In FIG. 3E, R-Ket and S-Ket represent social defeat
stress
mouse groups injected with R(-)-ketamine and S(+)-ketamine, respectively.
Saline
represents a social defeat stress mouse group injected with saline, and
Control
represents a control mouse group injected with saline. The ordinate axis of
FIG. 3E
indicates immobility times (sec) in the FST. (Example 2)
[fig.3F1FIG. 3F is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the social defeat stress mice investigated by the SPT 6 days
(P6) after
the injection of ketamine. In FIG. 3F, R-Ket and S-Ket represent social defeat
stress
mouse groups injected with R(-)-ketamine and S(+)-ketamine, respectively.
Saline
represents a social defeat stress mouse group injected with saline, and
Control
represents a control mouse group injected with saline. The ordinate axis of
FIG. 3F
indicates sucrose preferences (%) in the SPT. (Example 2)
lfig.3GWIG. 3G is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the social defeat stress mice investigated by the TST 7 days
(P7) after
the injection of ketamine. In FIG. 3G, R-Ket and S-Ket represent social defeat
stress
mouse groups injected with R(-)-ketamine and S(+)-ketamine, respectively.
Saline
represents a social defeat stress mouse group injected with saline, and
Control
represents a control mouse group injected with saline. The ordinate axis of
FIG. 3G
indicates immobility times (sec) in the TST. (Example 2)
[fig.3H]FIG. 3H is a graph showing results of the antidepressant effects of R(-
)- and
S(+)-ketamine in the social defeat stress mice investigated by the FST 7 days
(P7) after
the injection of ketamine. In FIG. 3H, R-Ket and S-Ket represent social defeat
stress
mouse groups injected with R(-)-ketamine and S(+)-ketamine, respectively.
Saline
represents a social defeat stress mouse group injected with saline, and
Control
represents a control mouse group injected with saline. The ordinate axis of
FIG. 3H
indicates immobility times (sec) in the FST. (Example 2)
Date recue/ date received 2021-12-23
11
WO 2015/037248 PCT/JP2014/004730
[fig.3I]FIG. 31 is a graph showing results of effects of R(-)- and S(+)-
ketamine on the
spine density of the frontal cortex in the social defeat stress mice
investigated 8 days
after the injection of ketamine. In FIG. 31, R-Ket and S-Ket represent social
defeat
stress mouse groups injected with R(-)-ketamine and S(+)-ketamine,
respectively.
Saline represents a social defeat stress mouse group injected with saline, and
Control
represents a control mouse group injected with saline. In addition, mPFC means
the
medial prefrontal cortex. (Example 2)
[fig.311FIG. 3J is a graph showing results of effects of R(-)- and S(+)-
ketamine on the
spine density of the hippocampal dentate gyrus in the social defeat stress
mice in-
vestigated 8 days after the injection of ketamine. In FIG. 3J, R-Ket and S-Ket
represent
social defeat stress mouse groups injected with R(-)-ketamine and S(+)-
ketamine, re-
spectively. Saline represents a social defeat stress mouse group injected with
saline,
and Control represents a control mouse group injected with saline. (Example 2)
[fig.31(]FIG. 3K is a graph showing results of effects of R(-)- and S(+)-
ketamine on the
spine density of the hippocampus CA1 region in the social defeat stress mice
in-
vestigated 8 days after the injection of ketamine. In FIG. 3J, R-Ket and S-Ket
represent
social defeat stress mouse groups injected with R(-)-ketamine and S(+)-
ketamine, re-
spectively. Saline represents a social defeat stress mouse group injected with
saline,
and Control represents a control mouse group injected with saline. (Example 2)
[fig.3L1FIG. 3L is a graph showing results of effects of R(-)- and S(+)-
ketamine on the
spine density of the hippocampus CA3 region in the social defeat stress mice
in-
vestigated 8 days after the injection of ketamine. In FIG. 3J, R-Ket and S-Ket
represent
social defeat stress mouse groups injected with R(-)-ketamine and S(+)-
ketamine, re-
spectively. Saline represents a social defeat stress mouse group injected with
saline,
and Control represents a control mouse group injected with saline. (Example 2)
[fig.3M1FIG. 3M is a graph showing results of effects of R(-)- and S(+)-
ketamine on
the spine density of the nucleus accumbens in the social defeat stress mice
investigated
8 days after the injection of ketamine. In FIG. 3J, R-Ket and S-Ket represent
social
defeat stress mouse groups injected with R(-)-ketamine and S(+)-ketamine, re-
spectively. Saline represents a social defeat stress mouse group injected with
saline,
and Control represents a control mouse group injected with saline. (Example 2)
[fig.31=11FIG. 3N is a graph showing results of effects of R(-)- and S(+)-
ketamine on the
spine density of the striatum in the social defeat stress mice investigated 8
days after
the injection of ketamine. In FIG. 3J, R-Ket and S-Ket represent social defeat
stress
mouse groups injected with R(-)-ketamine and S(+)-ketamine, respectively.
Saline
represents a social defeat stress mouse group injected with saline, and
Control
represents a control mouse group injected with saline. (Example 2)
Ifig.4_IFIG. 4 is a graph showing time-dependent changes in locomotion of
control
Date recue/ date received 2021-12-23
12
WO 2015/037248 PCT/JP2014/004730
mice after the injection of R(-)- and S(+)-ketarnine. In FIG. 4, R-Ket, S-Ket,
and Saline
represent groups injected with R(-)-ketamine, S(+)-ketamine, and saline,
respectively.
The ordinate axis of FIG. 4 indicates locomotion (count/10min). (Example 3)
ffig.5AWIG. 5A is a graph showing changes in prepulse inhibition after the
injection
of R(-)-ketamine in control mice. In FIG. 5A. R-Ket and Saline represent
groups
injected with R(-)-ketamine and saline, respectively. PP69, PP73, PP77, and
PP81
mean that stimuli at 69, 73, 77, and 81 dB for more than 20 milliseconds were
presented 100 milliseconds before a 110-dB pulse, respectively. Data analysis
was
performed by Wilks Lambda, which is multivariate analysis of variance.
(Example 3)
[fig.5131FIG. 5B is a graph showing changes in prepulse inhibition after the
injection of
S(+)-ketamine in the control mice. In FIG. 5B, S-Ket and Saline represent
groups
injected with S(+)-ketamine and saline, respectively. PP69, PP73, PP77, and
PP81
mean that stimuli at 69, 73, 77, and 81 dB for more than 20 milliseconds were
presented 100 milliseconds before a 110-dB pulse, respectively. Data analysis
was
performed by Wilks Lambda, which is multivariate analysis of variance.
(Example 3)
[fig.6A1FIG. 6A is a diagram illustrating a test protocol for investigating
rewarding
effects of R(-)-ketamine, S(+)-ketamine, and RS(+/-)-ketamine on the control
mice
using a conditioned place preference test. 15-minute habituation was performed
for 3
days. Then, 30-minute conditioning was performed on day 4 to day 10, and a be-
havioral evaluation test was performed on day 11. Saline was injected on day
5, day 7,
and day 9. In FIG. 6A, R-Ket, S-Ket, and RS-Ket mean groups injected with
R(-)-ketamine, S(+)-ketamine, and RS(+/-)-ketamine, respectively. In each of
the
groups, the injection was performed three times, i.e., on day 4, day 6, and
day 8. Saline
means a group injected with saline. In the group, the injection was performed
three
times, i.e., on day 5, day 7, and day 9. (Example 3)
[fig.613]FIG. 6B is a graph showing results of the rewarding effects of R(-)-
ketamine
on the control mice using the conditioned place preference test. In FIG. 6B, R-
Ketamine and Saline represent groups injected with R(-)-ketamine and saline,
re-
spectively. The ordinate axis of FIG. 6B indicates conditioned place
preference test
scores (CPP scores). (Example 3)
[fig.6C]FIG. 6C is a graph showing results of the rewarding effects of S(+)-
ketamine
on the control mice using the conditioned place preference test. In FIG. 6C, S-
Ketamine and Saline represent groups injected with S(+)-ketamine and saline,
re-
spectively. The ordinate axis of FIG. 6C indicates conditioned place
preference test
scores (CPP scores). (Example 3)
[fig.6D1FIG. 6D is a graph showing results of the rewarding effects of
RS(+/-)-ketamine on the control mice using the place preference test. In FIG.
6D, RS-
Ketamine and Saline represent groups injected with RS(+/-)-ketamine and
saline, re-
Date recue/ date received 2021-12-23
13
WO 2015/037248 PCT/JP2014/004730
spectively. The ordinate axis of FIG. 6D indicates conditioned place
preference test
scores (CPP scores). (Example 3)
Description of Embodiments
[0035] The present invention relates to an agent for prevention and/or
treatment of a de-
pressive symptom, consisting of R(-)-ketamine or a pharmacologically
acceptable salt
thereof. The present invention also relates to a pharmaceutical composition
for
prevention and/or treatment of a depressive symptom, including R(-)-ketamine
or a
pharmacologically acceptable salt thereof in an effective amount for reducing
a de-
pressive symptom, and being substantially free of S(+)-ketamine or a pharmaco-
logically acceptable salt thereof. The phrase "substantially free of S(+)-
ketamine or a
pharmacologically acceptable salt thereof" means that: S(+)-ketamine or a
pharmaco-
logically acceptable salt thereof is not contained at all; or S(+)-ketamine or
a pharma-
cologically acceptable salt thereof may be contained in such an amount that
its effects
and side effects are not exhibited, or may be contained as such an impurity as
to be
mixed inevitably during the manufacture of the agent and the pharmaceutical
com-
position.
[0036] Further, the present invention relates to a method of treating a
depressive symptom,
comprising administering R(-)-ketamine or a pharmacologically acceptable salt
thereof
to a subject, wherein the subject has been diagnosed with having a depressive
symptom, in an amount effective to treat the depressive symptom. The present
invention also relates to a method of treating a depressive symptom,
comprising ad-
ministering a pharmaceutical composition to a subject, wherein the
pharmaceutical
composition comprises R(-)-ketamine or a pharmaceutically acceptable salt
thereof in
an amount effective to treat a depressive symptom, and a pharmaceutically
acceptable
carrier, and being substantially free of S(+)-ketamine or a pharmacologically
ac-
ceptable salt thereof, and wherein the subject has been diagnosed with having
a de-
pressive symptom.
[0037] Furthermore, the present invention also relates to use of R(-)-
ketamine for the man-
ufacture of a pharmaceutical composition for treating a depressive symptom.
[0038] In the present invention, through the use of a new animal model of
depression, it was
demonstrated that R(-)-ketarnine had rapid and long-lasting antidepressant
effects. The
animal model was prepared by the inventors of the present application based on
their
finding that depression-like behavior was found in mice exposed neonatally to
DEX at
a juvenile stage and an adult stage (Non Patent Literature 18; see Example 1).
The
animal model exhibits depression-like behavior even at a juvenile stage, and
hence is
useful as an animal model of depression in children as well as depression in
adults.
[0039] In the present invention, it was also revealed that R(-)-ketamine
had antidepressant
Date recue/ date received 2021-12-23
14
WO 2015/037248 PCT/JP2014/004730
effects in a social defeat stress model as well. The social defeat stress
model is a
typical animal model of depression, which is used throughout the world (Non
Patent
Literature 19).
[0040] R(-)-ketamine exhibited rapid and long-lasting antidepressant
effects on depression-
like behaviors of the juvenile mice after the neonatal DEX exposure and the
social
defeat stress mouse model through its single administration (see Example 1 and
Example 2). Meanwhile, in locomotion-enhancing effects, disruption of prepulse
in-
hibition, and a dependence test using a conditioned place preference test,
which are
evaluation systems for side effects, significant changes were found in S(+)-
ketamine,
whereas such side effects were not found in R(-)-ketamine (see Examples 4, 5,
and 6).
In addition, in the conditioned place preference test, RS(+/-)-ketamine
increased a con-
ditioned place preference (CPP) score, indicating drug dependence of
RS(+/-)-ketamine. Further, R(-)-ketamine has low affinity for the NMDA
receptor as
compared to S(+)-ketamine, and thus is considered to have less side effects
such as
psychotomimetic effects. Accordingly, R(-)-ketamine can serve as a promising
and
safe antidepressant as compared to S(+)-ketamine and RS(+/-)-ketamine.
[0041] R(-)-ketamine or a pharmacologically acceptable salt thereof may
be used as an an-
tidepressant, specifically, as an agent to be used for treatment and/or
prevention of de-
pressive symptoms such as mood depression, lowering of motivation, anxiety,
the ac-
companying insomnia and anorexia, and suicidal ideation.
[0042] The agent and pharmaceutical composition according to the present
invention are
preferably applicable to diseases exhibiting depressive symptoms, for example.
de-
pression such as MDD or pediatric depression, and bipolar disorder involving a
repeat
of depressive symptoms and manic symptoms as their opposite symptoms, and are
more preferably applicable to depression in children and depression in adults.
In
addition, it has been reported that ketamine is also effective for treatment-
resistant
obsessive-compulsive disorder and treatment-resistant PTSD (Non Patent
Literatures
6, 7, and 8). Thus, the agent and pharmaceutical composition according to the
present
invention are preferably applicable to obsessive-compulsive disorder and PTSD.
Obsessive-compulsive disorder, which is one type of anxiety disorder and is a
disease
with pathological conditions characterized by obsessions and compulsions, is
considered to be associated with depression. Patients with obsessive-
compulsive
disorder have depression as well and exhibit depressive symptoms in addition
to ob-
sessions and compulsions in extremely many cases. Patients with PTSD exhibit
de-
pressive symptoms in many cases. In actuality, an antidepressant such as an
SSRI is
used as a therapeutic drug for PTSD, but its therapeutic effects are weak. The
scope of
the present invention encompasses a pharmaceutical composition for prevention
and/or
treatment of obsessive-compulsive disorder and PTSD, containing R(-)-ketamine
or a
Date recue/ date received 2021-12-23
15
WO 2015/037248 PCT/JP2014/004730
pharmacologically acceptable salt thereof in an effective amount for reducing
symptoms of obsessive-compulsive disorder and PTSD, and being substantially
free of
S(+)-ketamine or a pharmacologically acceptable salt thereof. In addition,
ketamine
treatment in an adult with autism spectrum has been reported (Non Patent
Literature
10). Thus, the agent and pharmaceutical composition according to the present
invention are preferably applicable to autism spectrum disorder. Furthermore,
De-
pression in elderly peoples is known to increase the risk if incident
dementia, in
particular of Alzheimer's disease and vascular dementia (Non Patent Literature
1).
Therefore, the agent and pharmaceutical composition according to the present
invention is a potential preventive or therapeutic drug for dementia including
Alzheimer's disease and vascular dementia.
[0043] The agent and pharmaceutical composition according to the present
invention may be
administered orally or parcnterally. In the oral administration, a known
dosage form
for administration, including a tablet, a capsule, a coated tablet, a troche,
or a liquid
such as a solution or a suspension, may be used. In addition, examples of the
parenteral
administration may include: intravenous, intramuscular, or subcutaneous admin-
istration by injection; transmucosal administration such as transnasal or oral
admin-
istration using a spray, an aerosol, or the like; rectal administration using
a suppository
or the like; and transdermal administration using a patch, a liniment, a gel,
or the like.
Preferred examples thereof may include oral administration, transnasal
administration,
and intravenous administration.
[0044] R(-)-ketamine may be used in both the forms of a free base and a
pharmaceutically
acceptable salt thereof. The pharmaceutically acceptable salt is preferably a
pharma-
ceutically acceptable acid addition salt, more preferably a hydrochloride.
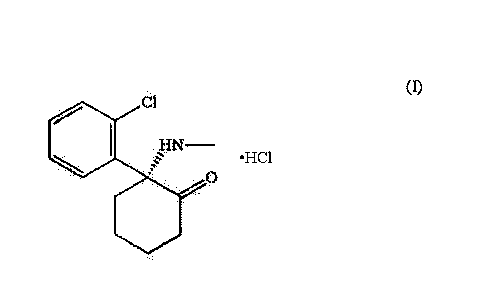
[0045] The chemical structural formula of R(-)-ketamine hydrochloride is
represented by the
following formula (I).
[0046] [Chem.1]
.C1
1-4N
= H CI
0
(I)
[0047] R(-)-ketamine or a pharmacologically acceptable salt thereof may be
subjected to
modification, for example, substitution of a chlorine molecule as a
substituent by
another halogen molecule and/or substitution of a methyl group as a
substitucnt by
Date recue/ date received 2021-12-23
16
WO 2015/037248 PCT/JP2014/004730
another alkyl group, to thereby manufacture a derivative. As a result, a
compound
having more preferred effects may be obtained.
[0048] Further, when the compound according to the present invention is
labeled with an
isotope such as a stable isotope "C or 2H (D), the compound can be measured
for its in
vivo kinetics and quantitatively measured for its affinity for the NMDA
receptor in the
brain, for example.
[0049] The pharmaceutical composition according to the present invention
may contain, in
addition to R(-)-ketamine or a pharmacologically acceptable salt thereof,
other in-
gredients having drug efficacy that are effective for depressive symptoms, the
in-
gredients being other than S(+)-ketamine. In addition, the pharmaceutical
composition
according to the present invention may appropriately contain, in addition to
those in-
gredients having drug efficacy, an appropriate pharmaceutically acceptable
carrier well
known to those of ordinary skill in the art, depending on an administration
form and
the like. Examples of the pharmaceutically acceptable carrier may include an
an-
tioxidant, a stabilizer, a preservative, a taste-masking agent, a colorant, a
solubilizer, a
solubilizing agent, a surfactant, an emulsifier, an antifoaming agent, a
viscosity
adjustor, a gelling agent, an absorption accelerator, a dispersant, an
excipient, and a pH
adjustor.
[00501 When the agent and pharmaceutical composition according to the
present invention
are each prepared as a formulation for injection, it is preferred that the
formulation be
in the form of a solution or a suspension. When the agent and pharmaceutical
com-
position are each prepared as a formulation for transmucosal administration
such as
transnasal or oral administration, it is preferred that the formulation be in
the form of a
powder, a drop, or an aerosol. In addition, when the agent and pharmaceutical
com-
position are each prepared as a formulation for rectal administration, it is
preferred that
the formulation be in the form of a semi-solid formulation such as a cream or
a sup-
pository. Each of those formulations may be prepared by any one of the methods
known to those skilled in the art of pharmacy as disclosed in, for example,
Remington's Pharmaceutical Sciences (Mack Publishing Company, Easton, PA,
1970).
In the formulation for injection, for example, a plasma-derived protein such
as
albumin, an amino acid such as glycine, and a sugar such as mannitol may each
be
added as a carrier, and a buffer, a solubilizing aid, an isotonic agent, and
the like may
also be added. In addition, when the formulation is used as a water-soluble
formulation
or a lyophilized formulation, it is preferred to add a surfactant such as
TweenTm 80 or
TweenTm 20 in order to prevent aggregation. Further, a dosage form for
parenteral ad-
ministration other than the formulation for injection may contain distilled
water or
saline, polyalkylene glycol such as polyethylene glycol, a plant-derived oil,
hy-
drogenated naphthalene, and the like. For example, a formulation for rectal
admin-
Date recue/ date received 2021-12-23
17
istration such as a suppository contains general excipients such as
polyalkylene glycol,
petrolatum, and cacao oil and fat. A vaginal formulation may contain an
absorption ac-
celerator such as a bile salt, an ethylenediamine salt, and a citric acid
salt. A for-
mulation for inhalation may be solid, and may contain an excipient such as
lactose.
Further, a transnasal drop may be a water or oil solution.
[0051] The accurate dosage and dosing regimen of each of the agent and
pharmaceutical
composition according to the present invention may be adjusted depending on
required
amounts, treatment methods, diseases, degrees of necessity, or the like for
individual
treatment targets. The dosage may be specifically determined depending on an
age, a
body weight, a general health condition, a sex, a meal, an administration
time, an ad-
ministration method, an elimination rate, a combination of drugs, a medical
condition
of a patient, and the like, and may be determined in consideration of other
factors.
When the pharmaceutical composition according to the present invention is ad-
ministered for diseases exhibiting depressive symptoms, such as depression,
bipolar
disorder, and obsessive-compulsive disorder, it is preferred that an active
ingredient
contained in the pharmaceutical composition be contained in an effective
amount for
reducing symptoms of the diseases such as depression, bipolar disorder, and
obsessive-
compulsive disorder, preferably depressive symptoms of the diseases. R(-)-
ketamine or
a pharmaceutically acceptable salt thereof can be safely used because of
having less
side effects found in S(+)-ketamine and RS(+/-)-ketamine. Its dosage per day
varies
depending on the condition and body weight of a patient, the kind of a
compound, an
administration route, and the like. For example, in terms of the amount of an
active in-
gredient, it is desired that the dosage in the case of parenteral
administration be from
about 0.01 to 1,000 mg/person/day, preferably from 0.1 to 500 mg/person/day,
and the
dosage in the case of oral administration be from about 0.01 to 500
mg/person/day,
preferably from 0.1 to 100 mg/person/day.
Examples
[0052] The present invention is hereinafter described in more detail by way of
Examples.
However, the present invention is by no means limited to Examples below.
Further,
various modifications are possible without departing from the technical
concept of the
present invention.
Example 1
[0053] A new animal model of depression (Non Patent Literature 18) was used to
investigate
antidepressant effects of R(-)- and S(+)-ketamine on the depression-like
behavior of
the animal model. All tests were performed under the approval of the Animal
Care and
Use Committee of Chiba University.
[0054] 1. Materials and methods
Date recue/ date received 2021-12-23
18
WO 2015/037248
PCT/JP2014/004730
R(-)- and S(+)-ketamine hydrochloride were prepared from RS(+/-)-ketamine
(Ketalar
TM, ketamine hydrochloride, Daiichi Sankyo Co., Ltd., Tokyo, Japan) using D(-)-
and
L(+)-tartaric acid, respectively, by the method disclosed in the previous
report (Patent
Literature 2) (FIG. 1). The purity of each of those isomers was confirmed by
high-
performance liquid chromatography (CHIRALPAKTM IA, column size: 250 x 4.6 mm,
mobile phase; n-hexane/dichloromethane/diethylamine (75/25/0.1), retention
time for
S(+)-ketamine = 6.99 min, retention time for R(-)-ketamine = 10.56 mm, Daicel
Cor-
poration, Tokyo, Japan).
[0055] A new animal model of depression was prepared by exposing mice
neonatally to
dexamethasone (hereinafter abbreviated as DEX). Through the neonatal DEX
exposure, depression-like behavior was observed in each of juvenile mice and
adult
mice. Thus, the mouse model was shown to be able to serve as a novel animal
model
of depression. The mouse model was prepared and reported only recently by the
inventors of the present application and their collaborators (Non Patent
Literature 18).
Specifically, the juvenile mice exposed neonatally to DEX and the adult mice
exposed
neonatally to DEX showed a significant decrease in novel object search time in
a novel
object recognition test as compared to control mice, which indicated a
reduction in
social learning property in the model mice. In addition, in a social memory
test, the
mice exposed neonatally to DEX showed a significant decrease in stimulation
target
follow-up time, which indicated a reduction in social recognition ability. In
an open
field test, a time spent in the center of a field significantly decreased,
which indicated a
reduction in spontaneous activity. In a light-dark box test, a time spent in a
white box
significantly decreased, which indicated that anxiety-like behavior was
caused. In each
of a tail suspension test (TST) and a forced swimming test (FST), an increase
in im-
mobility time was found, which indicated that depression-like behavior was
shown.
Meanwhile, in a locomotion test (LMT), there was no difference in locomotion
between the mice exposed to DEX and the control mice. Further, alterations
were
found in levels of amino acids (glutamate, glutamine, glycine, D-serine, and L-
serine)
in mouse brains after the neonatal DEX exposure (Non Patent Literature 18).
Those
amino acids are known to be associated with NMDA receptor mediated neuro-
transmission. Thus, it is conceivable that alterations in glutamatergic
transmission via
the NMDA receptor after the neonatal DEX exposure may be involved in the de-
pression-like behavior in the juvenile mice and the adult mice (Non Patent
Literature
18).
[0056] The preparation of the animal model of depression and the
administration of the
agent were specifically performed as described below (FIG. 2A). Male and
female ICR
mice (9-week-old, Japan SLC, Inc., Hamamatsu, Japan) were used. The mice were
given free access to water and feed. A breeding procedure consisted of housing
three to
Date recue/ date received 2021-12-23
19
WO 2015/037248 PCT/3P2014/004730
four females with one male, for 14 days. On the final day of this period, the
females
were placed in isolation and checked daily around the expected delivery day.
The day
of birth was defined as day 0. The mice were injected intraperitoneally with
DEX
(Wako Pure Chemical Industries, Ltd., Tokyo, Japan) dissolved in saline on day
1, day
2, and day 3 at doses of 0.5 mg/kg body weight, 0.3 mg/kg body weight, and 0.1
mg/kg
body weight, respectively. In addition, normal controls were injected with
equal
volumes (10 ml/kg) of saline. R(-)- or S(+)-ketamine at a dose of 10 mg/kg
body
weight or vehicle (saline 10 ml/kg) was injected intraperitoneally into male
juvenile
mice on day 36 after the birth.
[0057] The antidepressant effects of the agent were investigated for
juvenile mice by be-
havioral tests such as the TST, the FST, the LMT, and a 1% sucrose preference
test
(SPT) (FIG. 2A). The TST and the FST were performed twice, i.e., the day (27
hours
and 29 hours, respectively) and 7 days after the injection of ketamine, and
the LMT
and the SPT were paiformed on the day and 2 days after the injection of
ketamine. The
TST was performed as described below. First, the mice were taken out from
cages, and
then a small piece of an adhesive tape was bonded onto a portion approximately
2 cm
away from the tip of the tail of the mice. A small hole was opened in the
small piece,
and the mice were each fixed upside down on a hook through the small hole. The
im-
mobility time of each mouse was recorded for 10 minutes. Mice were considered
immobile only when they hung passively and completely motionless. The
immobility
time increases in a depressive state. The FST was performed as described
below. First,
the mice were placed individually in a cylinder (diameter: 23 cm; height: 31
cm)
containing 15 cm of water, maintained at 22 to 24 deg C. The mice were tested
in an
automated forced-swimming apparatus using SCANET MV-40 (MELQUEST Co.,
Ltd., Toyama, Japan). The immobility time was calculated as a value obtained
by sub-
tracting active time from total time, using the analysis software of the
apparatus. Cu-
mulative immobility time was recorded over 6 minutes during a test period. The
LMT
was performed as described below. First, the mice were placed in experimental
cages
(length x width x height: 560 x 560 x 330 mm). The locomotor activity of the
mice
was counted with SCANET MV-40, and the cumulative exercise of the mice was
recorded for 60 minutes. The cages were cleaned between testing session. The
im-
mobility time increases in a depressive state. The SPT was performed by
preparing
general drinking water and a 1% sucrose solution so that the mice had free
access
thereto, and measuring the ratio of the amount of the sucrose solution
consumed. The
consumption of the sucrose solution, which is a reward response, reduces in a
de-
pressive state.
[0058] Statistical analysis was performed by one-way analysis of variance
(one-way
ANOVA), followed by a least significant difference test (LSD test). Data are
presented
Date recue/ date received 2021-12-23
20
WO 2015/037248 PCT/JP2014/004730
as the mean plus minus standard error of the mean (n = 8 to 12 mice/group).
*p<0.05,
p<0.01, and -**p<0.001 indicate significant differences as compared to a DEX-
treated
mouse group injected with saline, and #p<0.05 and "p<0.01 indicate significant
dif-
ferences as compared to a DEX-treated mouse group injected with S(+)-ketamine.
[0059] 2. Results
Significant increases in immobility time in the TST and the FST and a
reduction in
sucrose consumption preference in the SPT were found in the mice exposed
neonatally
to DEX as compared to the control mice. On the other hand, in the LMT, there
was no
difference in locomotion between the DEX-treated mice and the control mice.
[0060] In the LMT performed on the day after the injection of both the
isomers of ketamine,
there was no difference in locomotion among the control mice, the DEX-treated
mice
injected with saline, and the DEX-treated mice injected with R(-)- or S(+)-
ketamine
(FIG. 2B).
[0061] In the TST and FST performed on the day after the injection of
both the isomers of
ketamine, significant increases in immobility time were found in the DEX-
treated mice
injected with saline as compared to the control mice. Each of both the isomers
of
ketamine markedly reduced the immobility time increased in the DEX-treated
mice 27
hours or 29 hours after its injection (FIGS. 2C and 2D). R(-)-ketamine
exhibited
slightly high antidepressant effects as compared to those of S(+)-ketamine,
although no
significant difference was found there between.
[0062] In the SPT performed on 2 days after the injection of both the
isomers of ketamine, a
reduction in sucrose consumption preference was found in the DEX-treated mice
injected with saline as compared to the control mice. Both the isomers of
ketamine sig-
nificantly restored the sucrose consumption preference reduced in the DEX-
treated
mice 48 hours after their injection (FIG. 2E).
[0063] In the TST and FST performed on 7 days after the injection of both
the isomers of
ketamine, significant increases in immobility time were found in the DEX-
treated mice
injected with saline as compared to the control mice. In addition, R(-)-
ketamine sig-
nificantly reduced the immobility time increased in the DEX-treated mice,
whereas
S(+)-ketamine did not reduce the immobility time increased in the DEX-treated
mice.
The differences between R(-)-ketamine and S(+)-ketamine were found to be sta-
tistically significant (FIGS. 2F and 2G).
[0064] The above-mentioned results revealed that R(-)- and S(+)-ketamine
at a dose of 10
mg/kg exhibited antidepressant effects in the juvenile mice after the neonatal
DEX
exposure (days 1 to 3). In the TST and the FST, the antidepressant effects of
both the
isomers of ketamine were found 27 to 29 hours after their single injection. It
is
noteworthy that in the TST and the FST, the antidepressant effects of R(-)-
ketamine
were able to be detected even 7 days after its single injection, whereas the
antide-
Date recue/ date received 2021-12-23
21
WO 2015/037248 PCT/JP2011/004730
pressant effects of S(+)-ketamine were not able to be detected 7 days after
its single
injection. The results show that R(-)-ketamine has more long-lasting
antidepressant
effects than S(+)-isomer. Both the isomers of ketamine are known to exhibit a
rapid in
vivo clearance. Despite the fact that R(-)-ketamine is considered to be
eliminated from
the body by 7 days after its single injection, the antidepressant effects were
found. This
indicates that the differences in antidepressant effects 7 days after the
injection of both
the isomers of ketamine do not result from differences in pharmacokinetics.
Example2
[0065] A social defeat stress model of depression (Non Patent Literature
19) was used to in-
vestigate antidepressant effects of R(-)- and S(+)-ketamine on the depression-
like
behavior of the animal model. All tests were performed under the approval of
the
Animal Care and Use Committee of Chiba University.
[0066] 1. Materials and methods
R(-)- and S(+)-ketamine hydrochloride were prepared from RS(+/-)-ketamine
(Ketalarrm, ketamine hydrochloride, Daiichi Sankyo Co., Ltd., Tokyo, Japan)
using
D(-)- and L(+)-tartaric acid, respectively, by the method disclosed in the
previous
report (Patent Literature 2) (FIG. 1). The purity of each of those isomers was
confirmed by high-performance liquid chromatography (CHIRALPAKTM IA, column
size: 250 x 4.6 mm, mobile phase; n-hexane/dichloromethane/diethylamine
(75/25/0.1), retention time for S(+)-ketamine = 6.99 min, retention time for
R(-)-ketamine = 10.56 mm. Daicel Corporation, Tokyo, Japan).
[0067] A social defeat stress model of depression was prepared by
bringing C57/B6 male
mice into contact with ICR male mice (large aggressive mice) for 10
consecutive days
to apply a stress called a "social defeat stress" in accordance with the
previous report
(Non Patent Literature 19). Depression-like behavior was observed in the mice
that had
received the social defeat stress. Specifically, an increase in immobility
time was found
in each of a tail suspension test (TST) and a forced swimming test (FST). In
addition,
in a 1% sucrose preference test, the ratio of sucrose water drunk
significantly reduced,
suggesting that depression-like behavior (e.g. anhedonia) was shown. On the
other
hand, in a locomotion test (LMT), there was no difference in locomotion
between
social defeat stress mice and control mice.
[0068] The preparation of the animal model of depression and the
administration of the
agent were specifically performed as described below (FIG. 3A). Male C57/B6
mice
(7-week-old, Japan SLC, Inc., Hamamatsu, Japan) and ICR mice (9-week-old,
Japan
SLC, Inc., Hamamatsu, Japan) were used. The mice were given free access to
water
and feed. A social defeat stress was applied by housing one C57/B6 mouse with
one
ICR mouse for 10 days. On day 11, a social interaction test was performed to
select
mice exhibiting depressive symptoms, which were used for the subsequent
behavioral
Date recue/ date received 2021-12-23
22
WO 2015/037248 PCT/JP2014/004730
evaluation. Control mice were injected with vehicle (saline 10 ml/kg) and the
mice ex-
hibiting depressive symptoms were injected intraperitoneally with R(-)- or
S(+)-ketamine at a dose of 10 mg/kg body weight, or vehicle (saline 10
mil/kg).
[0069] The antidepressant effects of the agent were investigated by
behavioral tests such as
the TST, the FST, the LMT, and a 1% sucrose preference test (SPT) (FIG. 3A).
The
1% sucrose preference test (SPT) was performed on 1 day and 6 days after the
injection of ketamine. Each of the TST and the FST was performed on 2 days and
7
days after the injection of ketamine. The TST was performed as described
below. First,
the mice were taken out from cages, and then a small piece of an adhesive tape
was
bonded onto a portion approximately 2 cm away from the tip of the tail of the
mice. A
small hole was opened in the small piece, and the mice were each fixed upside
down
on a hook through the small hole. The immobility time of each mouse was
recorded for
minutes. Mice were considered immobile only when they hung passively and
completely motionless. The immobility time increases in a depressive state.
The FST
was performed as described below. First, the mice were placed individually in
a
cylinder (diameter: 23 cm; height: 31 cm) containing 15 cm of water,
maintained at 22
to 24 deg C. The mice were tested in an automated forced-swimming apparatus
using
SCANET MV-40(MELQUEST Co., Ltd., Toyama, Japan). The immobility time was
calculated as a value obtained by subtracting active time from total time,
using the
analysis software of the apparatus. Cumulative immobility time was recorded
over 6
minutes during a test period. The LMT was performed as described below. First,
the
mice were placed in experimental cages (length x width x height: 560 x 560 x
330
mm). The locomotor activity of the mice was counted with SCANET MV-40, and the
cumulative exercise of the mice was recorded for 60 minutes. The cages were
cleaned
between testing session. The immobility time increases in a depressive state.
The SPT
was performed by preparing general drinking water and a 1% sucrose solution so
that
the mice had free access thereto, and measuring the ratio of the amount of the
sucrose
solution consumed. The consumption of the sucrose solution, which is a reward
response, reduces in a depressive state. The mice were decapitated 5 days
after the
injection of ketamine, and the brain was quickly dissected out and subjected
to Golgi
staining. A spine density was quantitatively evaluated by observation with a
KEYENCE microscope (BZ-9000, Osaka, Japan).
[0070] The statistical analysis of the results of the social defeat stress
model was performed
by one-way analysis of variance (one-way ANOVA), followed by a least
significant
difference test (LSD test). Data are presented as the mean plus minus standard
error of
the mean (n = 8 to 11 mice/group). *p<0.05, ¨p<0.01, and ¨p<0.001 indicate sig-
nificant differences as compared to a social defeat stress mouse group
injected with
saline, and #p<0.05 indicates a significant difference as compared to a social
defeat
Date recue/ date received 2021-12-23
23
WO 2015/037248 PCT/JP2014/004730
stress mouse group injected with S(+)-ketamine.
[0071] 2. Results
Significant increases in immobility time in the TST and the FST and a
significant
reduction in sucrose consumption preference in the SPT were found in the
social defeat
stress mice as compared to the control mice. On the other hand, in the LMT,
there was
no difference in locomotion between the social defeat stress mice and the
control mice.
[0072] In the SPT performed on 1 day after the injection of both the
isomers of ketamine, in
the social defeat stress mice, the sucrose consumption preference
significantly
decreased as compared to the control group, and depressive symptoms were
exhibited.
In the social defeat stress mouse group injected with R(-)- or S(+)-ketamine,
the
sucrose consumption preference significantly increased as compared to the
social
defeat stress mouse group injected with saline, and depressive symptoms were
al-
leviated. In addition, the antidepressant effects of R(-)-ketamine were more
potent than
those of S(+)-ketamine (FIG. 3B).
[0073] In the LMT performed on 2 days after the injection of both the
isomers of ketamine,
there was no difference in locomotion among the normal mice, the social defeat
stress
mice injected with saline, and the social defeat stress mice injected with R(-
)- or
S(+)-ketamine (FIG. 3C).
[0074] In the TST and FST performed on 2 days after the injection of both
the isomers of
ketamine, significant increases in immobility time were found in the social
defeat
stress mice injected with saline as compared to the control mice. Each of both
the
isomers of ketamine markedly reduced the immobility time increased in the
social
defeat stress mice 2 days after its injection (FIGS. 3D and 3E). R(-)-ketamine
exhibited
significant high antidepressant effects as compared to those of S(+)-ketamine
(FIGS.
3D and 3E).
[0075] In the SPT performed on 6 days after the injection of both the
isomers of ketamine, a
reduction in sucrose consumption preference was found in the social defeat
stress mice
injected with saline as compared to the control mice. Both the isomers of
ketamine sig-
nificantly restored the sucrose consumption preference reduced in the social
defeat
stress mice 6 days after their injection. The difference between R(-)-ketamine
and
S(+)-ketamine was found to be statistically significant (FIG. 3F).
[0076] In the TST and FST performed on 7 days after the injection of both
the isomers of
ketamine, significant increases in immobility time were found in the social
defeat
stress mice injected with saline as compared to the control mice. Each of both
the
isomers of ketamine significantly reduced the immobility time increased in the
social
defeat stress mice 7 days after its injection (FIGS. 3G and 3H). R(-)-ketamine
exhibited significantly high antidepressant effects as compared to S(+)-
ketamine
(FIGS. 3G and 3H).
Date recue/ date received 2021-12-23
14
WO 2015/037248 PCT/JP2014/004730
[0077] In the Golgi staining performed 8 days after the injection of both
the isomers of
ketamine, a significant decrease in spine density was found in the frontal
cortex and
hippocampal dentate gyrus of the social defeat stress mice injected with
saline as
compared to the control mice. Each of both the isomers of ketamine
significantly
improved the decreased density of spine in the social defeat stress mice 8
days after its
injection (FIGS. 31 and 3J). In the hippocampal dentate gyrus, R(-)-ketamine
showed
more significant improvement in the restoration of spine density as compared
to
S(+)-ketamine (FIG. 3J). In the hippocampus CA1 region and striatum, no
apparent
change in spine density was observed (FIGS. 3K and 3N), while in hippocampus
CA3
region, a decrease in spine density by social defeat stress was observed,
which was sig-
nificantly improved by R(-)-ketamine and S(+)-ketamine (FIG. 3L). In the
nucleus
accumbens, a significant increase in spine density was observed by social
defeat stress,
which was significantly improved by R(-)-ketamine and S(+)-ketamine (FIG. 3M).
[0078] The above-mentioned results revealed that R(-)- and S(+)-ketamine
at a dose of 10
mg/kg exhibited antidepressant effects in the social defeat stress mice. It is
noteworthy
that in the SPT, the TST, and the FST, the antidepressant effects of R(-)-
ketamine were
significantly potent as compared to the effects of S(+)-ketamine. The results
show that
R(-)-ketamine has more long-lasting antidepressant effects than S(+)-isomer.
Both the
isomers of ketamine are known to exhibit a rapid in vivo clearance. Despite
the fact
that R(-)-ketamine is considered to be eliminated from the body by 7 days
after its
single injection, the antidepressant effects were found. This indicates that
the dif-
ferences in antidepressant effects 7 days after the injection of both the
isomers of
ketamine do not result from differences in pharmacokinetics.
Example3
[0079] Control C57/B6 mice was used to investigate side effects of R(-)-
and S(+)-ketamine.
All tests were performed under the approval of the Animal Care and Use
Committee of
Chiba University.
[0080] 1. Materials and methods
The preparation of R(-)- and S(+)-ketamine hydrochloride and the confirmation
of
their purities were performed by the methods described in Example 2.
[0081] The administration of an agent was performed by the same methods
as the methods
described in Example 2.
[0082] The side effects of R(-)- and S(+)-ketamine were investigated by a
locomotion-
enhancing effect, disruption of prepulse inhibition, and a dependence test
using a con-
ditioned place preference test, which were systems for evaluating side
effects.
[0083] An effect of ketamine on the locomotion of mice was tested using
SCANET MV-40
(MELQUEST Co., Ltd., Toyama, Japan). Specifically, the locomotion was measured
for a total of 180 minutes, i.e., 60 minutes before injection to 120 minutes
after
Date recue/ date received 2021-12-23
25
WO 2015/037248 PCT/JP2014/004730
injection, and calculated as a locomotion per 10 minutes. The statistical
analysis of the
results of the locomotion was performed by repeated one-way analysis of
variance
(repeated one-way ANOVA), followed by a least significant difference test (LSD
test).
Data are presented as the mean plus minus standard error of the mean (n = 7 or
8 mice/
group). **p<0.01 and 'p<0.001 indicate significant differences as compared to
a group
injected with saline.
[0084] The prepulse inhibition test was performed using a startle
response system (SR-LAB,
SanDiego Instruments, San Diego, CA, United States). The analysis of the
results of
prepulse inhibition was performed by multivariate analysis of variance
(MANOVA),
followed by a least significant difference test (LSD test). Data are presented
as the
mean plus minus standard error of the mean (n = 10 to 12 mice/group). *p<0.05
and ¨
p<0.01 indicate significant differences as compared to a group injected with
saline.
[0085] The place preference test was performed using a conditioned place
preference test
apparatus (BrainSienceIdea Co., Ltd., Osaka, Japan). The analysis of the
results of the
place preference test was performed by one-way analysis of variance (one-way
ANOVA), followed by a least significant difference test (LSD test). Data are
presented
as the mean plus minus standard error of the mean (n = 9 or 10 mice/group).
*p<0.05
and 'p<0.01 indicate significant differences as compared to a group injected
with
saline.
[0086] 2. Results
In the measurement of the locomotion after the injection of both the isomers
of
ketamine, a significant increase in locomotion was found 10 minutes and 20
minutes
after the injection in the mice injected with S(+)-ketamine (10 mg/kg or 20
mg/kg) as
compared to the control mice injected with saline. The locomotion was
transiently
enhanced by S(+)-ketamine (10 mg/kg or 20 mg/kg), but returned to a normal
value 30
minutes after the injection. On the other hand, the injection of R(-)-ketamine
(5, 10, or
20 mg/kg) did not affect the locomotion (FIG. 4).
[0087] In the prepulse inhibition test after the injection of both the
isomers of ketamine, the
injection of S(+)-ketamine (5, 10 mg/kg, or 20 mg/kg) disrupted prepulse
inhibition in
a dose-dependent manner (FIG. 5B). On the other hand, the injection of R(-)-
ketamine
(5, 10, or 20 mg/kg) did not disrupt prepulse inhibition (FIG.5A).
[0088] In the conditioned place preference test after the injection of
both the isomers and
racemic mixture of ketamine, the injection of S(+)-ketamine (5, 10 mg/kg, or
20 mg/
kg) increased the CPP score in a dose-dependent manner, indicating drug abuse
potential (FIG. 6C). On the other hand, the injection of R(-)-ketamine (5, 10,
or 20 mg/
kg) did not increase the CPP score (FIG. 6B), indicating no drug abuse
potential. In
addition, the injection of RS(+/-)-ketamine (10 mg/kg) significantly increased
the CPP
score, indicating drug abuse potential (FIG. 6D).
Date recue/ date received 2021-12-23
26
WO 2015/037248 PCT/JP2014/004730
100891 As described above, from the viewpoint of side effects, the
injection of
S(+)-ketamine was found to exhibit a locomotion-enhancing effect, disrupt
prcpulse in-
hibition, and produce drug dependence. In addition, it was suggested that the
injection
of RS(+/-)-ketamine also produced drug dependence. On the other hand, R(-)-
ketarnine
does not exhibit a locomotion-enhancing effect, disrupt prcpulse inhibition,
and
produce drug dependence, for example, and hence is an agent having high safety
as
compared to RS(+/-)-ketamine and S(+)-ketamine that arc clinically used at
present.
Industrial Applicability
[0090] As described above, the agent and pharmaceutical composition for
prevention and/or
treatment of a depressive symptom according to the present invention have
rapid and
long-lasting antidepressant effects and less side effects such as
psychotomimetic
effects, and hence are useful as novel pharmaceuticals in the field of
prevention and/or
treatment of a number of psychiatric diseases exhibiting depressive symptoms.
Date recue/ date received 2021-12-23