Note: Descriptions are shown in the official language in which they were submitted.
CA 03145331 2021-12-24
POLYOXOMETALATE-BASED ELECTROLYTE CONDUCTOR MATERIAL
AND PREPARATION METHOD AND APPLICATION THEREOF
Field of the Invention
The present invention belongs to the field of battery materials, and
particularly
relates to a polyoxometalate-based electrolyte conductor material and a
preparation
method and application thereof.
Back2round of the Invention
Improving the proton conductivity of electrolytes is the key to improving the
efficiency of fuel cells and secondary batteries. At present, the proton
conductor
material that has been commercialized in proton exchange membrane fuel cells
is
perfluorosulfonic acid (Nafions), which has extremely high proton conductivity
(>
10-2 S cm-1) at a condition of high humidity (RH 100%) and low/medium
temperature
(<373 K). However, Nafions are expensive and have poor stability and
mechanical
property. Therefore, it is an urgent problem to be solved to research and
prepare
conductor materials that can replace Nafions. The development of electrolyte
conductor materials with good conductivity, mechanical property, and
processability
has a profound significance for the development of fuel cells, batteries, and
capacitors.
Polyoxometalates (POMs) are nanoscale early transition metal-oxygen molecule
clusters with a lower effective surface charge density, which endow them with
very
strong proton transmitting capability. In fact, Keggin-type POMs (e.g.,
H3PW12040
and H4SiW12040) exhibit high proton conductivities comparable to Nafions at
high
humidity. Protons are transferred between POMs through the hydrogen-bond
network
formed by their crystal water. Therefore, the conductivity is greatly affected
by
humidity which limits the application of POMs as conductors.
POMs can form organic-inorganic hybrid materials with new functional
performance in combination with different organic components. Therefore,
researchers blend POMs with polymers to prepare a series of conductor
materials with
1
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
somewhat improved stability, but there is a very great gap between them and
the
commercial conductor materials in conductivity. How to prepare electrolyte
conductor
materials with ultra-high conductivity is still a big challenge for
researchers.
Summary of the Invention
Aiming at the defects and deficiencies in the prior art, a primary objective
of the
present invention is to provide a POM-based electrolyte conductor material. In
the
prepared POM-based electrolyte conductor material, a three-dimensional network
for
transferring protons is formed by means of hydrogen-bond interaction between
the
POM and the polymer, thereby realizing effective transfer of protons by means
of the
movement of the polymer chains. This electrolyte conductor material has good
proton
conduction efficiency in low and medium temperature environments, and
meanwhile,
has superior processability, safety, and chemical stability.
Another objective of the present invention is to provide a preparation method
of
the above-described POM-based electrolyte conductor material.
Still another objective of the present invention is to provide an application
of the
above-described POM-based electrolyte conductor material.
The objectives of the present invention are achieved by the following
technical
solutions.
A preparation method of a POM-based electrolyte conductor material includes
the following steps: mixing a POM with a polymer melt to obtain a blend,
subjecting
the blend to reacting under heating and stirring, then cooling to room
temperature
after the end of the reaction to prepare the POM-based electrolyte conductor
material.
Preferably, a mass ratio of the POM to the polymer melt is 1: 9 to 7: 3.
Preferably, the heating and stirring is performed for a time of 5 to 48 hours,
more
preferably 12 hours, at a temperature of 60 to 80 C and a stirring rate of
100 to 700
rpm.
2
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
Preferably, the room temperature is 25 to 35 C.
Preferably, the type of the POM is one of Keggin type POMs, Dawson type
POMs, and Preyssler type POMs.
Preferably, a general chemical formula of the Keggin type POMs is HnXM12040
(M=Mo, W, or V; and X=P or As, and n=3; X=Si or Ge, and n=4; X=B or Al, and
n=5;
or X=Cu or Co, and n=6), and more preferably H3PW12040.
Preferably, a general chemical formula of the Dawson type POMs is HnX2Mt8062
(M=Mo or W; X=P, As, S or V; and n=6).
Preferably, a general chemical formula of the Preyssler type POMs is
HnYX5M300110 (X=P; Y=Bi, Na, Ca, Eu or U; M=W; and n=12).
Preferably, the polymer is a polymer with one or more of hydroxyl, carboxylic
acid group, and amino.
Preferably, the polymer with one or more of hydroxyl, carboxylic acid group,
and
amino is one of polyethylene glycol, polyacrylic acid, polyvinyl alcohol, and
chitosan,
and more preferably polyethylene glycol (PEG).
Preferably, the molecular weight of the polyethylene glycol is an average
molecular weight of 400 to 300,000, and more preferably the polyethylene
glycol is
one or more of polyethylene glycol having an average molecular weight of 400
or
4,000 and six-arm star-shaped polyethylene glycol having an average molecular
weight of 2,400.
A preparation method of a POM-based electrolyte conductor material includes
the following steps: adding a polymer into a solvent to obtain a polymer
solution;
adding a POM into the solvent to obtain a POM solution; mixing the POM
solution
with the polymer solution to obtain a blend, subjecting the blend to reacting
under
heating and stirring, and after the end of the reaction, completely
volatilizing the
3
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
solvent to prepare the POM-based electrolyte conductor material.
Preferably, the polymer is added into the solvent to obtain the polymer
solution,
and a concentration range of the polymer solution is 0.1 g/mL to 1 g/mL.
Preferably, the POM is added into the solvent to obtain the POM solution, and
a
concentration range of the POM solution is 0.1 g/mL to 1 g/mL.
Preferably, a volume ratio of the POM solution to the polymer solution is 1: 9
to
7: 3.
Preferably, the heating and stirring is performed for a time of 5 to 48 hours,
more
preferably 12 hours, at a temperature of 40 to 60 C and a stirring rate of
100 to 700
rpm.
Preferably, the solvent is water or tetrahydrofuran, and more preferably
tetrahydrofuran.
Preferably, the type of the POM is one or more of Keggin type POMs, Dawson
type POMs, and Preyssler type POMs.
Preferably, a general chemical formula of the Keggin type POMs is HnXM12040
(M=Mo, W or V; and X=P or As, and n=3; X=Si or Ge, and n=4; X=B or Al, and
n=5;
or X=Cu or Co, and n=6), and more preferably H3PW12040.
Preferably, a general chemical formula of the Dawson type POMs is HnX2M18062
(M=Mo or W; X=P, As, S or V; and n=6).
Preferably, a general chemical formula of the Preyssler type POMs is
HnYX5M300110 (X=P; Y=Bi, Na, Ca, Eu or U; M=W; and n=12).
Preferably, the polymer is a polymer with one or more of hydroxyl, a
carboxylic
acid group, and amino.
4
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
Preferably, the polymer with one or more of hydroxyl, a carboxylic acid group,
and amino is one of polyethylene glycol, polyacrylic acid, polyvinyl alcohol,
and
chitosan, and more preferably polyethylene glycol.
Preferably, the molecular weight of the polyethylene glycol is an average
molecular weight in a range of 400 to 300,000, and more preferably the
polyethylene
glycol is one or more of polyethylene glycol having an average molecular
weight of
400 and 4,000 and six-arm star-shaped polyethylene glycol having an average
molecular weight of 2,400.
A POM-based electrolyte conductor material is prepared by the preparation
method of the POM-based electrolyte conductor material described above.
The POM-based electrolyte conductor material is applied in the fields of fuel
cells, batteries, and super capacitors.
Compared with the prior art, the present invention has the following
advantages
and beneficial effects:
(1) the POM-based electrolyte conductor material of the present invention has
very high proton conduction efficiency (to 1.01x102 S cm') in low/medium
temperature environments (80 C).
(2) the preparation methods of the present invention are simple, have mild
reaction conditions, are suitable for mass production, and have low cost.
(3) in the systems of the preparation methods of the present invention, the
polyethylene glycol can combine with the POM through the hydrogen bond,
thereby
greatly increasing the proton conduction efficiency, and meanwhile, the
viscosity of
the sample is as high as 273 Pa's, which guarantees the safety of the sample
while
used as an electrolyte.
(4) the POM-based electrolyte conductor material of the present invention has
an
obvious shear thinning behavior which ensures the good processability of the
sample.
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
Brief Description of the Drawin2s
Fig. 1 is small angle X-ray scattering curves of electrolyte conductor
materials
prepared by Examples 1 to 7.
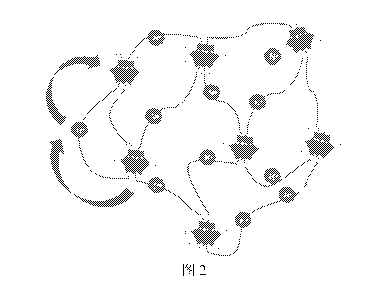
Fig. 2 is a schematic diagram of the structure and proton conduction of an
electrolyte conductor material prepared by an example of the present
invention.
Fig. 3 is a Nyquist diagram of a PEG400 electrolyte conductor material
prepared
by Comparative example 1 at conditions of 25 C, 50 C, and 80 C.
Fig. 4 is a Nyquist diagram of a PEG400-10%PW12 electrolyte conductor
material prepared by Example 1 at conditions of 25 C, 50 C, and 80 C.
Fig. 5 is a Nyquist diagram of a PEG400-20%PW12 electrolyte conductor
material prepared by Example 2 at conditions of 25 C, 50 C, and 80 C.
Fig. 6 is a Nyquist diagram of a PEG400-50%PW12 electrolyte conductor
material prepared by Example 5 at conditions of 25 C, 50 C, and 80 C.
Fig. 7 is a Nyquist diagram of a PEG400-70%PW12 electrolyte conductor
material prepared by Example 7 at conditions of 25 C, 50 C, and 80 C.
Fig. 8 is a Nyquist diagram of a PEG4000-60%PW12 electrolyte conductor
material prepared by Example 8 at conditions of 25 C, 50 C, and 80 C.
Fig. 9 is a Nyquist diagram of a PEG4000-70%PW12 electrolyte conductor
material prepared by Example 9 at conditions of 25 C, 50 C, and 80 C.
Fig. 10 is a Nyquist diagram of a SPEG2400-70%PW12 electrolyte conductor
material prepared by Example 10 at conditions of 25 C, 50 C, and 80 C.
Fig. 11 is a flowing graph of the electrolyte conductor materials prepared by
6
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
Examples 1 to 7 and the electrolyte conductor material prepared by Comparative
example 1.
Detailed Description of the Embodiments
Specific embodiments of the present invention will be further described in
detail
below with reference to the figures and examples. It should be pointed out
that for an
ordinary person skilled in the present field, several modifications and
improvements
can be made on the premise of without departing from the concept of the
present
invention. All these modifications and improvements shall fall within the
scope of
protection of the present invention.
In examples, the room temperature is 27 C, the POM is a Keggin type POM
H3PW12040, and a stirring rate is 300 rpm.
Example 1
1.0 g of POM was dissolved in 9.0 g of polyethylene glycol (PEG) melt having a
relative molecular weight of 400 at 67 C to obtain a blend A; the blend A was
subjected to reacting for 12 hours under heating and stirring at 67 C, and
after the
reaction ended, the temperature was cooled to room temperature to prepare a
transparent POM-based electrolyte conductor material, which was denoted as
PEG400-10%PW12.
Example 2
2.0 g of POM was dissolved in 8.0 g of polyethylene glycol (PEG) melt having a
relative molecular weight of 400 at 67 C to obtain a blend A; the blend A was
subjected to reacting for 12 hours under heating and stirring at 67 C, and
after the
reaction ended, the temperature was cooled to room temperature to prepare a
transparent POM-based electrolyte conductor material, which was denoted as
PEG400-20%PW12.
Example 3
7
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
3.0 g of POM was dissolved in 7.0 g of polyethylene glycol (PEG) melt having a
relative molecular weight of 400 at 67 C to obtain a blend A; the blend A was
subjected to reacting for 12 hours under heating and stirring at 67 C, and
after the
reaction ended, the temperature was cooled to room temperature to prepare a
transparent POM-based electrolyte conductor material, which was denoted as
PEG400-30%PW12.
Example 4
4.0 g of POM was dissolved in 6.0 g of polyethylene glycol (PEG) melt having a
relative molecular weight of 400 at 67 C to obtain a blend A; the blend A was
subjected to reacting for 12 hours under heating and stirring at 67 C, and
after the
reaction ended, the temperature was cooled to room temperature to prepare a
transparent POM-based electrolyte conductor material, which was denoted as
PEG400-40%PW12.
Example 5
5.0 g of POM was dissolved in 5.0 g of polyethylene glycol (PEG) melt having a
relative molecular weight of 400 at 67 C to obtain a blend A; the blend A was
subjected to reacting for 12 hours under heating and stirring at 67 C, and
after the
reaction ended, the temperature was cooled to room temperature to prepare a
transparent POM-based electrolyte conductor material, which was denoted as
PEG400-50%PW12.
Example 6
6.0 g of POM was dissolved in 4.0 g of polyethylene glycol (PEG) melt having a
relative molecular weight of 400 at 67 C to obtain a blend A; the blend A was
subjected to reacting for 12 hours under heating and stirring at 67 C, and
after the
reaction ended, the temperature was cooled to room temperature to prepare a
transparent POM-based electrolyte conductor material, which was denoted as
PEG400-60%PW12.
8
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
Example 7
7.0 g of POM was dissolved in 3.0 g of polyethylene glycol (PEG) melt having a
relative molecular weight of 400 at 67 C to obtain a blend A; the blend A was
subjected to reacting for 12 hours under heating and stirring at 67 C, and
after the
reaction ended, the temperature was cooled to room temperature to prepare a
transparent POM-based electrolyte conductor material, which was denoted as
PEG400-70%PW12.
Example 8
6.0 g of POM was dissolved in 4.0 g of polyethylene glycol (PEG) melt having a
relative molecular weight of 4,000 at 80 C to obtain a blend A; the blend A
was
subjected to reacting for 12 hours under heating and stirring at 80 C, and
after the
reaction ended, the temperature was cooled to room temperature to prepare a
transparent POM-based electrolyte conductor material, which was denoted as
PEG4000-60%PW12.
Example 9
7.0 g of POM was dissolved in 3.0 g of polyethylene glycol (PEG) melt having a
relative molecular weight of 4,000 at 80 C to obtain a blend A; the blend A
was
subjected to reacting for 12 hours under heating and stirring at 80 C, and
after the
reaction ended, the temperature was cooled to room temperature to prepare a
transparent POM-based electrolyte conductor material, which was denoted as
PEG4000-70%PW12.
Example 10
7.0 g of POM was dissolved in 7 mL of tetrahydrofuran to obtain a solution A;
3.0 g of six-arm star-shaped polyethylene glycol having an average molecular
weight
of 2,400 was dissolved in 3 mL of tetrahydrofuran to obtain a solution B; the
solution
A was mixed with the solution B, and the mixture was subjected to reacting for
12
9
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
hours under heating and stirring at 50 C; and after the reaction ended, the
solvent was
volatilized to obtain a POM-based electrolyte conductor material, which was
denoted
as SPEG2400-70%PW12.
Comparative example 1
g of polyethylene glycol having a relative molecular weight of 400 was
subjected to reacting for 12 hours under heating and stirring at 67 C, and
after the
reaction ended, the temperature was cooled to room temperature to obtain a
transparent PEG400 electrolyte conductor material, which was denoted as
PEG400.
Table 1 is test results for conductivity of the electrolyte conductor
materials of
Example 1, Example 2, Example 5, Example 7, Example 8, Example 9, Example 10,
and Comparative example 1 at conditions of 25 C, 50 C, and 80 C (at a
relative
humidity of 45%). A CHI660E electrochemical workstation purchased from CH
Instruments, Inc. was used as a test instrument. During the tests, two
platinum sheets
were used as electrodes, a test frequency range was from 0.01 Hz to 100,000
Hz, EIS
was used for test, and the proton conductivity was calculated by a formula a =
L/(ARb). Rb represented a resistance value, L represented a distance between
the two
platinum sheet electrodes, and A represented an area of the two electrodes.
Table 1 General chart of test results for conductivity
Sample 25 C_ 50 C_ 80 C_
conductivity / S conductivity / S conductivity / S
-1 -1 -
cm cm cm1
PEG400 7.4x106 1.3 x 10-5 2.9x105
PEG400-10%PW12 6.5x10-5 1.8x 10-4 4.2 x10-4
PEG400-20%PW12 1.4 x10-4 4.2x 10-4 1.0x10-3
PEG400-50%PW12 4.0 x10-4 1.2x 10-3 3.5x10-3
PEG400-70%PW12 1.4x10-3 3.6x 10-3 1.0x10-2
PEG4000-60%PW12 1.8 x 10-3 5.3 x 10-3 1.2 x 10-2
PEG4000-70%PW12 1.6 x 10-3 6.5 x 10-3 1.6 x 10-2
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
SPEG2400-70%PW12 6.1 x 10-4 2.4x 10-3 8.8x 10-3
Conductivity values of the electrolyte conductor materials prepared by various
examples are listed in Table 1, and it can be seen that: the conductivities of
various
samples increase as the temperature is increased; with the increase of the
content of
the POM, the conductivity of the prepared electrolyte conductor material is
increased
by three orders of magnitude, wherein the conductivity of the PEG400-70%PW12
sample can reach 1.01x 10-2 S cm-1 at 80 C; an electrolyte conductor material
with
very high proton conduction efficiency can also be obtained by blending a
higher
molecular weight polyethylene glycol with a POM; and the SPEG2400-70%PW12
sample prepared by the solvent method also has higher conductivity.
Fig. 1 shows small angle X-ray scattering curves of the electrolyte conductor
materials prepared by Examples 1 to 7. It can be seen from Fig. 1 that: the
prepared
PEG400-PW12 nanocomposite material has no obvious crystal diffraction peak in
the
small angle X-ray scattering spectrum. It indicates that in the electrolyte
conductor
materials of the present invention, the POM clusters are unifounly dispersed
in the
polymer substrate, which achieves the nanoscale dispersion of the POM, and
guarantees the structural stability of the sample.
Fig. 2 is schematic diagram for the structure and proton conduction of the
electrolyte conductor material prepared by the example of the present
invention.
Wherein, islet shaped structures represent phosphotungstic acids and hydrogen-
bond
interaction between the phosphotungstic acids and the polymer components,
solid
lines connecting different islet structures represent the polymer chains of
polyethylene
glycol, and I-1+ represents protons. It can be seen from Fig. 2 that: the
polyethylene
glycol and the POM form a three-dimensional network through hydrogen bonds,
and
the protons are effectively transferred in virtue of the movement of the
polymer chain.
Fig. 3 is a Nyquist diagram of the PEG400 electrolyte conductor material
prepared by Comparative example 1 at conditions of 25 C, 50 C, and 80 C.
Wherein, the conductivity of the electrolyte conductor material can be
obtained from
the intercept of the Nyquist plot with the real axis.
11
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
Fig. 4 is a Nyquist diagram of the PEG400-10%PW12 electrolyte conductor
material prepared by Example 1 at conditions of 25 C, 50 C, and 80 C.
Wherein,
the conductivity of the electrolyte conductor material can be obtained from
the
intercept of the Nyquist plot with the real axis.
Fig. 5 is a Nyquist diagram of the PEG400-20%PW12 electrolyte conductor
material prepared by Example 2 at conditions of 25 C, 50 C, and 80 C.
Wherein,
the conductivity of the electrolyte conductor material can be obtained from
the
intercept of the Nyquist plot with the real axis.
Fig. 6 is a Nyquist diagram of the PEG400-50%PW12 electrolyte conductor
material prepared by Example 5 at conditions of 25 C, 50 C, and 80 C.
Wherein,
the conductivity of the electrolyte conductor material can be obtained from
the
intercept of the Nyquist plot with the real axis.
Fig. 7 is a Nyquist diagram of the PEG400-70%PW12 electrolyte conductor
material prepared by Example 7 at conditions of 25 C, 50 C, and 80 C.
Wherein,
the conductivity of the electrolyte conductor material can be obtained from
the
intercept of the Nyquist plot with the real axis.
It can be seen from Fig. 4 to Fig. 7 that the conductivity of the conductor is
greatly increased with the addition of the POM. Meanwhile, it indicates that
as the
temperature is increased to 80 C, the conductivity of the sample has an
obvious
rising tendency.
Fig. 8 is a Nyquist diagram of the PEG4000-60%PW12 electrolyte conductor
material prepared by Example 8 at conditions of 25 C, 50 C, and 80 C.
Wherein,
the conductivity of the electrolyte conductor material can be obtained from
the
intercept of the Nyquist plot with the real axis.
Fig. 9 is a Nyquist diagram of the PEG4000-70%PW12 electrolyte conductor
material prepared by Example 9 at conditions of 25 C, 50 C, and 80 C.
Wherein,
the conductivity of the electrolyte conductor material can be obtained from
the
12
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
intercept of the Nyquist plot with the real axis.
It can be seen from Fig. 8 and Fig. 9 that as the temperature is increased to
80 C,
the conductivity of the sample has an obvious rising tendency.
Fig. 10 is a Nyquist diagram of the SPEG2400-70%PW12 electrolyte conductor
material prepared by Example 10 at conditions of 25 C, 50 C, and 80 C.
Wherein,
the conductivity of the electrolyte conductor material can be obtained from
the
intercept of the Nyquist plot with the real axis. It can be seen from Fig. 10
that the
POM-based electrolyte conductor material prepared by the solvent method also
exhibits relatively high conductivity.
Fig. 11 is a flow graph of the electrolyte conductor materials prepared by
Examples 1 to 7 and the electrolyte conductor material of Comparative example
1. It
can be seen from Fig. 11 that the viscosity of the PEG400-70%PW12 sample is as
high
as 273 Pa's at room temperature, which guarantees the safety of the sample
used as an
electrolyte. In addition, the obvious shear thinning behavior of the sample
enables the
sample to have good processability.
From the detailed description of the examples of the present invention with
the
above-described content, it can be understood that the conductivity of the POM-
based
electrolyte conductor material of the present invention is greatly increased
as the
temperature rises, under conditions of the temperature range of 25 C to 80 C
and a
relative humidity of 45%. During the preparation of the materials, the samples
with
the POM in a mass ratio of 70% can all have very high conductivity (at a
temperature
of 80 C, the conductivity of PEG400-70%PW12 is 1.01 x 10-2 S cm-1, the
conductivity
of PEG4000-
70%PW12 is 1.64x 10-2 S cm-1, and the conductivity of
SPEG2400-70%PW12 is 8.8x 10-3 S cm').
What is described above are preferred embodiments of the present invention and
not intended to limit the present invention. It should be pointed out that an
ordinary
person skilled in the present technical field can make several improvements
and
modifications on the premise of without departing from the technical principle
of the
present inventions, and these improvements and modifications shall also be
deemed as
13
Date Recue/Date Received 2021-12-24
CA 03145331 2021-12-24
falling within the scope of protection of the present invention. Therefore,
the content
of without departing from the patented solution of the present invention, any
simple
amendment, equivalent change, and modification made to the above examples
according to the essence of the patented technology of the present invention
shall fall
within the scope of protection of the patent of the present invention.
14
Date Recue/Date Received 2021-12-24