Note: Descriptions are shown in the official language in which they were submitted.
WO 2021/034349
PCT/US2020/020991
METHODS FOR PREVENTING DENGUE AND HEPATITIS A
FIELD OF THE INVENTION
[0001] The present invention relates to a method for administering a unit dose
of a dengue vaccine composition
to a subject or a subject population simultaneously on the same day with a
hepatitis A vaccine. The unit dose
according to this invention provides immune responses against all serotypes of
dengue virus, i.e. DENY-1, DENV-2,
DENV-3 and DENV-4 and against hepatitis A virus.
BACKGROUND OF THE INVENTION
[0002] Vaccines for protection against viral infections have been effectively
used to reduce the incidence of
human disease. One of the most successful technologies for viral vaccines is
to immunize animals or humans with a
weakened or attenuated virus strain (a "live attenuated virus"). Due to
limited replication after immunization, the
attenuated virus strain does not cause disease. However, the limited viral
replication is sufficient to express the full
repertoire of viral antigens and can generate potent and long-lasting immune
responses to the virus. Thus, upon
subsequent exposure to a pathogenic virus strain, the immunized individual is
protected from the disease. These live
attenuated viral vaccines are among the most successful vaccines used in
public health.
[0003] Dengue disease is a mosquito-borne disease caused by infection with a
dengue virus. Dengue virus
infections can lead to debilitating and painful symptoms, including a sudden
high fever, headaches, joint and muscle
pain, nausea, vomiting and skin rashes. To date, four serotypes of dengue
virus have been identified: dengue-I
(DENY-I), dengue-2 (DENV-2), dengue-3 (DENV-3) and dengue-4 (DENV-4). Dengue
virus serotypes 1-4 can also
cause dengue hemorrhagic fever (DHF) and dengue shock syndrome (MS). In the
most severe cases, DHF and DSS
can be life threatening. Dengue viruses cause 50-100 million cases of
debilitating dengue fever, 500,000 cases of
DHF/DSS, and more than 20,000 deaths each year, a large portion of which are
children. All four dengue virus
serotypes are endemic throughout the tropical regions of the world and
constitute the most significant mosquito-
borne viral threat to humans there. Dengue viruses are transmitted to humans
primarily by Aedes aegypti
mosquitoes, but also by Aedes alboattus mosquitoes. Infection with one dengue
virus serotype results in life-long
protection from re-infection by that serotype, but does not prevent secondary
infection by one of the other three
dengue virus serotypes. In fact, previous infection with one dengue virus
serotype may lead to an increased risk of
severe disease (DHF/DSS) upon secondary infection with a different serotype.
[0004] To date, only one vaccine, a tetravalent dengue vaccine based on a
yellow fever backbone, CYD-TDV
(Dengvaxia , Sanofi Pasteur, Lyon, France), has been licensed in several
countries based on the clinical
demonstration of an overall vaccine efficacy (VE) against virologically-
confirmed dengue (VCD) of 56-61% in children
in Asia and Latin America (Capeding MR et al. Clinical efficacy and safety of
a novel tetravalent dengue vaccine in
healthy children in Asia: a phase 3, randomised, observer-masked, placebo-
controlled trial. Lancet 2014, 384:1358-
65; Villar LA et al. Safety and immunogenicity of a recombinant tetravalent
dengue vaccine in 9-16 year olds: a
randomized, controlled, phase II trial in Latin America. Pediatr Infect Dis J
2013, 32:1102-9). However, clinical trials
have shown that Dengvaxia can enhance, rather than reduce, the risk of severe
disease due to dengue infection in
individuals who had not been previously infected by a dengue virus
(seronegative populations). Therefore,
Dengvaxia is only recommended for use in individuals who had been previously
infected with at least one dengue
virus serotype (seropositive populations). More specifically, according to the
European Medicine Agencys European
Public Assessment report (EPAR) for the product, Dengvaxia is only for use in
people from 9 to 45 years of age
1
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
who have been infected with dengue virus before and who live in areas where
this infection is endemic. Endemic
areas are areas where the disease occurs regularly throughout the year. See
also Sridhar S et al. Effect of Dengue
Serostatus on Dengue Vaccine Safety and Efficacy. N Engl MS 2018, 379:327-40;
and World Health Organization.
Dengue vaccine: WHO position paper ¨ September 2018. Wkly. Epidemiol. Rec.
2018, 93:457-476. S.R. Hadinegoro
et al. report in the New England Journal of Medicine, Vol. 373, page 1195, in
"Efficacy and Long-Term Safety of a
Dengue Vaccine in Regions of Endemic Disease" a pooled risk of hospitalization
for virologically-confirmed dengue
disease among those under the age of 9 years of 1.58 indicating an increased
risk for the vaccinated group with
respect to severe dengue. This leaves a substantial unmet need for an
effective vaccine with a good safety profile in
both dengue-naive and seropositive individuals, including those dengue-naive
populations living in endemic areas,
younger individuals who may not have developed any seropositive response to
dengue or been exposed to dengue,
and travelers and individuals from non-endemic regions. There is also a need
for outbreak control or travel
vaccination, offering a reduction in the risk of dengue after only one dose.
[0005] One further disadvantage of the only currently approved dengue vaccine,
Dengvaxia@, is that it must only
be given to people who have had a positive test result showing a previous
infection with dengue virus (EPAR), i.e.
individuals with known serostatus fix dengue. Thus, individuals with unknown
serostatus for dengue cannot be
vaccinated with Dengvaxia@.
[0006] There is hence a need for a dengue vaccine and corresponding method of
inoculation that stimulates an
immune response to all dengue serotypes, preferably a balanced immune response
to all serotypes, and protects
against dengue disease of any severity (including DSS, DHF), both in
seronegative and seropositive populations,
which is safe for a larger group of ages, in particular also for subjects of 9
years and younger. The development of a
safe and effective vaccine capable of protecting all populations, including
both seronegative and seropositive
populations, and in particular children and young adults and elderly subjects
in endemic settings and for the purpose
of traveling, represents an important approach to the prevention and control
of this global disease.
[0007] There is thus a medical need for a dengue vaccine and corresponding
method of inoculation which, as well
as being safe and efficacious irrespective of serostatus and in a broad age
group. There is a need for a dengue
vaccine and corresponding method of inoculation that avoids costly and time
consuming serostatus tests or
seroprevalence considerations. There is a need for a dengue vaccine and
corresponding method of inoculation that
can be used in an outbreak situation. Furthermore there is a medical need for
a dengue vaccine which as well as
being safe and effective can also be administered to individuals with unknown
dengue serostatus, children under 9
years and seronegative individuals.
[0008] There is also a need for a vaccine that is administered in fewer doses
than the current Dengvaxia@ dosing
schedule of 3 doses, 6 months apart, such as a vaccine that can be
administered in only two doses or one dose to be
efficacious.
[0009] The above objects are commensurate with the research priorities
provided by the WHO in the Dengue
Vaccine: WHO position paper ¨ September 2018 (Wkly. Epidemiol. Rec. 2018,
93:457-476).
[0010] Hepatitis A is a liver disease caused by the hepatitis A virus (HAV).
The virus is primarily spread when an
uninfected (and unvaccinated) person ingests food or water that is
contaminated with the feces of an infected
person. The disease is closely associated with unsafe water or food,
inadequate sanitation and poor personal
hygiene. The virus can also be transmitted through close physical contact with
an infectious person. Unlike hepatitis
B and C, hepatitis A infection does not cause chronic liver disease and is
rarely fatal, but it can cause debilitating
2
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
symptoms and fulminant hepatitis (acute liver failure), which is often fatal.
Hepatitis A occurs sporadically and in
epidemics worldwide, with a tendency for cyclic recurrences.
[0011] The hepatitis A virus is one of the most frequent causes of foodborne
infection. Epidemics related to
contaminated food or water can erupt explosively, such as the epidemic in
Shanghai in 1988 that affected about
300,000 people. Hepatitis A viruses persist in the environment and can
withstand food-production processes routinely
used to inactivate and/or control bacterial pathogens_ The disease can lead to
significant economic and social
consequences in communities. It can take weeks or months for people recovering
from the illness to return to work,
school, or daily life. The impact on food establishments identified with the
virus, and local productivity in general, can
be substantial_ In developing countries with poor sanitary conditions and
hygienic practices, most children (90%)
have been infected with the hepatitis A virus before the age of 10 years_
[0012] The number of people traveling internationally has grown substantially
in recent decades. According to the
United Nations World Tourism Organization (UNWTO), over 1.1 billion tourists
travelled abroad in 2014. The risk of
becoming ill during international travel depends on many factors, such as the
region of the world visited, the length
of the trip, and the diversity of planned activities. Vaccine recommendations
are a prominent part of health
preparations before international travel. Vaccination against hepatitis A
virus is commonly recommended for travelers
to at-risk areas around the world including Asia, Africa, and Latin America.
[0013] For routine hepatitis A vaccination, a two-dose schedule is
recommended, particularly in travelers at
substantial risk of contracting hepatitis A and in immunocompromised
individuals. However, in healthy individuals,
comparable effectiveness has been achieved with a single dose. The vaccination
schedule for children/adolescents
(12 months through 18 years of age) as well as for adults (a19 years of age)
consists of a primary dose
administered intramuscularly, and a further booster dose administered
intramuscularly 6 to 18 months later.
[0014] Available hepatitis A vaccines include HAVRDM and VAQTA .
[0015] Hence, there is a need for a safe and effective method of
simultaneously preventing dengue disease and
hepatitis A. In particular, there is a need for hepatitis A and dengue
vaccines which provide non-inferiority when
administered simultaneously to a subject or subject population and a suitable
administration schedule for achieving
synergy.
[0016] Furthermore, there is a need of effectively and safely preventing
dengue disease and hepatitis A in
subjects being unaware of their hepatitis A and/or dengue serostatus, in
particular in subjects from non-endemic
countries which travel into dengue and hepatitis A endemic countries.
OBJECTS AND SUMMARY
[0017] It is an object of the present invention to provide a safe and
effective protection against dengue disease
and hepatitis A.
[0018] It is an object of the present invention to provide a method of
administration for preventing hepatitis A
and dengue disease which is useful in typical vaccination settings wherein the
subjects are unaware of their
serostatus for dengue and/or hepatitis A and a corresponding serotest is
unavailable, unpractical or unreliable.
[0019] It is an object of the present invention to provide a safe and
effective protection against dengue disease
and hepatitis A for travelers from hepatitis A and dengue non-endemic
countries, in particular for travelers being
3
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
vaccinated in travel clinics. In this context it is beneficial if multiple
during the same medical appointments are
avoided and vaccination can be conducted simultaneously for more than one
disease.
[0020] It is an object of the present invention to provide a safe and
effective vaccine for preventing hepatitis A
and dengue disease in a subject or subject population and a corresponding
method of preventing hepatitis A and
dengue disease in a subject or a subject population from a dengue-endemic and
dengue non-endemic region and for
a broad range of ages, in particular for subjects between 2 to 60 years of
age, preferably for subjects between 18
and 60 years of age, and independent of previous exposure to any dengue virus
serotype and/or to hepatitis A virus
and independent of corresponding serapositivity or seronegativity with respect
to dengue and/or hepatitis A before
vaccination.
[0021] It is an object of the invention to provide vaccines and a
corresponding method of preventing hepatitis A
and dengue disease which avoids testing for individual dengue and/or hepatitis
A serostatus before individual
administration of a hepatitis A and a dengue vaccine to a subject or subject
population, or analysis of seroprevalence
rates of dengue and/or hepatitis A in subjects or subject populations to be
vaccinated.
[0022] It is an object of the present invention to provide a dengue vaccine
and a hepatitis A vaccine which can be
safely co-administered with TDV as travel vaccines before an international
travel of a subject to FlANT and dengue
endemic countries and a method of safely administering these vaccines.
[0023] Therefore, the present invention S directed to a method of preventing
dengue disease as well as hepatitis
A.
[0024] The present invention is further directed to a method of preventing
hepatitis A and dengue disease in a
subject or subject population, the method comprising simultaneously on the
same day administering a hepatitis A
vaccine and a unit dose of a dengue vaccine composition, wherein said unit
dose comprises a tetravalent dengue
virus composition including four live, attenuated dengue virus strains.
DEFINMONS
[0025] In describing the present invention, the following terms are to be used
as indicated below. As used herein,
the singular forms "a," "an," and "the" include plural references unless the
context clearly indicates otherwise.
[0026] As used herein, the terms "unit dose of a dengue vaccine composition",
"unit dose" and "unit dose of the
invention as described herein" refer to the amount of a dengue vaccine which
is administered to a subject in a single
dose. In one embodiment, one unit dose is present in a vial and this unit dose
is administered to a subject, e.g.
optionally after reconstitution. In one embodiment, more than one unit dose of
the dengue vaccine composition may
be present in a vial so that with the content of one vial more than one
subject can be vaccinated.
[0027] A "lyophilized unit dose" or "unit dose in lyophilized form" refers to
the unit dose that is obtained by
subjecting a given volume of the liquid dengue vaccine composition, such as
0.5 mL, to lyophilization. Thus, the
aqueous formulations of the dengue vaccine composition being produced by
combining the pharmaceutically
acceptable excipienis and the dengue virus composition comprising the four
dengue virus strains, preferably TDV-1
to TDV-4, is subjected to lyophilization to obtain the lyophilized unit dose.
[0028] A "reconstituted unit dose" or "unit dose in reconstituted form" is
obtained from the lyophilized dose by
reconstitution with a pharmaceutically acceptable diluent. The diluent does
not contain dengue virus. The
4
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
reconstituted unit dose is a liquid which can be administered to a subject,
for example by injection, such as
subcutaneous injection.
[0029] As used herein, the term 'upon reconstitution with 0.5 mL" is not
limiting the reconstitution to be
performed using 0.5 mL of the diluent, but refers to the concentration of the
dengue viruses that will be present in
the reconstituted unit dose when 0.5 mL diluent are used for reconstitution.
While using a different volume for
reconstitution (e.g. 0.8 mL) will result in a different concentration of
dengue viruses in the reconstituted unit dose,
the administration of the total volume of the unit dose (e.g. 0.8 mL) will
result in the same total amount of dengue
virus being administered.
[0030] As used herein, a "concentration of at least X log10 pfu/0.5 mL" refers
to the concentration of a dengue
serotype in 0.5 mL, but is not limiting the unit dose to be 0.5 mL If the unit
dose has a volume different than 0.5
mL, or is lyophilized from a volume different than 0.5 mL, or is reconstituted
with a volume different than 0.5 mL,
said concentration will differ from the "concentration of at least X log10
pfu/0.5 mL". However, if the unit dose has a
volume of 0.5 mL, or is lyophilized from a volume of 0.5 mL, or is
reconstituted with a volume of 0.5 mL, said
concentration will be the "concentration of at least X log10 pfu/0.5 mL".
Thus, while the concentration may differ,
the total amount of virus in the unit dose remains the same.
[0031] As used herein, the term "dengue serotype refers to a species of dengue
virus which is defined by its cell
surface antigens and therefore can be distinguished by serological methods
known in the art. At present, four
serotypes of dengue virus are known, i.e. dengue serotype 1 (DEW-1), dengue
serotype 2 (DENV-2), dengue
serotype 3 (DENV-3) and dengue serotype 4 (DENV-4).
[0032] As used herein, the term 'tetravalent dengue virus composition" refers
to a dengue virus composition
comprising four different immunogenic components from the four different
dengue serotypes DEW-I, DENV-2,
DENV-3 and DENV-4, preferably comprising four different live, attenuated
dengue viruses, each representing one
dengue serotype, and which aims to stimulate immune responses to all four
dengue serotypes.
[0033] As used herein, the term "live attenuated dengue virus" refers to a
viable dengue virus which is mutated
to provide reduced virulence. The live attenuated dengue virus can be a dengue
virus in which all components are
derived from the same dengue serotype or it can be a chimeric dengue virus
having parts from two or more dengue
serotypes or a mixed chimeric dengue virus having parts from other
fiaviviruses.
[0034] A "virus strain" and in particular a "dengue virus strain" is a genetic
subtype of a virus, in particular of a
dengue virus, which is characterized by a specific nucleic acid sequence. A
dengue serotype may comprise different
strains with different nucleic acid sequences which have the same cell surface
antigens. A dengue virus strain can be
a dengue virus in which all components are derived from the same dengue
serotype or it can be a chimeric dengue
virus having parts from two or more dengue serotypes.
[0035] As used herein, "TDV-2" refers to a molecularly characterized and
cloned dengue serotype 2 strain derived
from the live attenuated DEN-2 PDK-53 virus strain. The PDK-53 strain is
described for example in Bhamarapravati et
al. (1987) Bulletin of the World Health Organization 65(2): 189-195. In one
embodiment, the TDV-2 strain served as
a backbone for the chimeric TDV-1, TDV-3 and TDV-4 strains into which parts
from the TDV-1, TDV-3 and TDV-4
strains were introduced.
[0036] A "non-chimeric dengue virus" or "non-chimeriC dengue serotype strain"
or "non-chimeric dengue strain"
comprises only parts from one dengue serotype. In particular, a non-chimeric
dengue virus does not include parts
5
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
from a different flavivirus such as yellow fever virus, Zika virus, West Nile
virus, Japanese encephalitis virus, St. Louis
encephalitis virus, tick-borne encephalitis virus. TDV-2 is an example of a
non-chimeric dengue virus.
[0037] A 'chimeric dengue virus" or "chimeric dengue serotype strain" or
"chimeric dengue strain" comprises
pads from at least two different dengue serotypes. As used herein, the
chimeric dengue virus does not include parts
from a different flavivirus such as yellow fever virus, Zika virus, West Nile
virus, Japanese encephalitis virus, St. Louis
encephalitis virus, tick-borne encephalitis virus. In particular, the chimeric
dengue virus described herein does not
include path from the yellow fever virus. As used herein, a "chimeric dengue
serotype 2/1 strain" or "DENV-2/1
chimera" or "TDV-1" refers in a dengue virus chimeric construct which
comprises parts from both DENV-2 and DENV-
1. In particular, in the chimeric dengue serotype 2/1 strain the prM and E
proteins from DEN V-1 replace the prM and
E proteins from DENV-2 as detailed below. As used herein, a "chimeric dengue
serotype 2/3 strain" or "DENV-213
chimera" or "TDV-3" refers in a dengue virus chimeric construct which
comprises parts from both DENV-2 and DEW/-
3. In particular, in the chimeric dengue serotype 2/3 strain the prM and E
proteins from DENV-3 replace the prM and
E proteins from DENV-2 as detailed below. As used herein, a "chimeric dengue
serotype 2/4 strain" or "DENV-2/4
chimera" or "TDV-4" refers to a dengue virus chimeric construct which
comprises parts from both DENV-2 and DENY-
4. In particular, in the chimeric dengue serotype 2/4 strain the prM and E
proteins from DENV-4 replace the prM and
E proteins from DENV-2 as detailed below. A mixed chimeric dengue virus has
parts from other flaviviruses.
[0038] As used herein, "-DV" refers to a tetravalent live attenuated dengue
vaccine that comprises a mixture of
the four live attenuated dengue virus strains TDV-1, TDV-2, TDV-3 and TDV-4
expressing surface antigens from the
four dengue serotypes DENV-1, DENV-2, DENV-3 and DENV-4, respectively. In one
embodiment (e.g. also in the
examples), TDV-1 has the nucleotide sequence according to SEQ ID No. 1 and/or
the amino acid sequence according
to SEQ ID No. 2. In one embodiment, 1DV-2 has the nucleotide sequence
according to SEQ ID No. 3 and/or the
amino acid sequence according to SEQ ID No. 4. In one embodiment, TDV-3 has
the nucleotide sequence according
to SEQ ID No. 5 and/or the amino acid sequence according to SEQ ID No. 6. In
one embodiment, TDV-4 has the
nucleotide sequence according to SEQ ID No. 7 and/or the amino acid sequence
according to SEQ ID No. 8.
[0039] As used herein, the term "dengue disease" refers to the disease which
is caused by infection with dengue
virus. Symptoms of dengue disease include sudden high fever, headaches, joint
and muscle pain, nausea, vomiting
and skin rashes. The term dengue disease also includes the more severe forms
of dengue hemorrhagic fever (DHF)
and dengue shock syndrome (DSS). Symptoms of DHF include increased vascular
permeability, hypovolemia and
abnormal blood clotting mechanisms. Subjects with DHF may present with severe
manifestations of plasma leakage
and hemorrhage. When a subject with DHF experiences shock he or she will be
categorized as having DSS.
Symptoms of DSS include bleeding that may appear as tiny spots of blood on the
skin and larger patches of blood
under the skin. Prolonged shock is the main factor associated with
complications including massive gastrointestinal
hemorrhage that can lead to death. As used herein, Dl-IF cases are defined as
VCD cases meeting WHO 1997 DI-IF
criteria. In the context of preventing dengue disease in elderly subject, the
term "preventing dengue disease"
preferably includes preventing DHF and/or DSS. In the context of preventing
dengue disease in elderly subjects, the
term "preventing dengue disease" preferably includes preventing severe end-
organ manifestations of dengue such as
hepatornegaly and acute renal failure.
[0040] As used herein, "preventing dengue disease" refers to preventing a
subject from developing one or more
symptoms of dengue disease because of an infection with a dengue virus. In
particular, preventing dengue disease is
achieved by vaccinating or inoculating a subject with a dengue vaccine
composition, such as the reconstituted unit
dose described herein. As used herein, the term "prophylactically treating
dengue disease" is equivalent to
6
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
"preventing dengue disease". In a particular embodiment, preventing dengue
disease includes preventing DHS
and/or DSS.
[0041] As used herein, the terms "virologically-confirmed dengue disease",
"VCD case", or "VCD fever" refer to
febrile illness or illness clinically suspected to be dengue disease with a
positive serotype-specific reverse
transcriptase polymerase chain reaction (RT-PCFt). The term "virologically
confirmable dengue" disease refers to a
subject having febrile illness or illness clinically suspected to be dengue
disease, wherein testing the subject, e.g.
using RT-PCFt, would confirm the presence of at least one dengue serotype.
Severe forms of VCD fever will be
identified as follows: Dengue Hemorrhagic Fever (DHF) was defined according to
the WHO 1997 criteria. Severe
dengue was defined through an assessment of an independent Dengue Case
Adjudication Committee which will
assess all hospitalized VCD cases (severe / non-severe) based on criteria
redefined in a charter. All non-hospitalized
cases are considered non-severe.
[0042] As used herein, the term "febrile illness" is defined as temperature 1-
38 C on any 2 of 3 consecutive days.
[0043] As used herein, the terms "virologically-confirmed dengue disease with
hospitalization", is considered to
be a surrogate for severe dengue and the "incidence of virologically-confirmed
dengue disease with hospitalization" is
used as a safety parameter. As used herein, the 'relative risk with respect to
virologically-confirmed dengue disease
with hospitalization" means the number of events of virologically confirmed
dengue disease with hospitalization
divided by the number of subjects treated with the unit dose as disclosed
herein over the number of events of
virologically confirmed dengue disease with hospitalization divided by the
number of subjects treated with placebo. If
the "relative risk with respect to virologically-confirmed dengue disease with
hospitalization" is 1 or lower the vaccine
provides for the same or less risk for virologically-confirmed dengue disease
with hospitalization as placebo and is
considered "safe". In this context the risk of virologically-confirmed dengue
disease with hospitalization may be also
0.9 or less, 0.8 or less, 0.7 or less, 0.6 or less, 0.5 or less, 0.4 or less,
0.3 or less, 0.2 or less, or Di or less, in
particular when determined from 30 days after a second administration until 12
months after a second
administration, in particular when determined in age groups selected from the
age group of 4 to 16 year old
subjects, the age group of 4 to under 9 year old subjects, the age group of 2
to under 9 year old subjects, the age
group of 4 to 5 year old subjects, the age group of 6 to 11 year old subjects,
and the age group of 12 to 16 year old
subjects.
[0044] As used herein, alternatively a vaccine is considered 'safe" when the
vaccine efficacy (VE) with respect to
virologically-confirmed dengue disease with hospitalization is 0% or higher.
This means that the vaccine provides for
the same likelihood or less for virologically-confirmed dengue disease with
hospitalization as placebo. In particular
considered "safe" is the combined vaccine efficacy against virologically-
confirrned dengue with hospitalization against
all four serotypes with a 2-sided 95% confidence interval, wherein the lower
bound is more than 25%, in particular
when measured against placebo in a subject population of at least 1,500 or at
least 2,000 healthy subjects (in
particular when measured in age groups selected in particular from the age
group of 4 to 16 year old subjects, the
age group of 4 to under 9 year old subjects, the age group of 2 to under 9
year old subjects, the age group of 4 to 5
year old subjects, the age group of 6 to 11 year old subjects, and the age
group of 12 to 16 year old subjects) being
seronegative against all serotypes at baseline or being seropositive against
at least one serotype at baseline, in
particular when said unit dose Or said placebo is administered at least twice
within less than 6 months, such as within
3 months, about from first administration or from 30 days after the second or
last administration of the
administration schedule until at least 12 months, until 12 to 18 months, until
12 months, or until 18 months after the
second or last administration of the administration schedule. In particular,
the lower bound may be more than 30%,
more than 40%, more than 50%, more than 60%, more than 65%, more than 66%,
more than 67%, more than
7
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
68% more than 70%, or more than 75%. In particular, the 2-sided 95% confidence
interval of the combined
vaccine efficacy against virologically-confirmed dengue with hospitalization
against all four serotypes when
comparing senapositive and seronegative subjects provides for lower bounds of
the 2-sided confidence interval which
are within 10% points or within 15% points or within 20% points. In a
particular embodiment "safe" means providing
a combined vaccine efficacy against virologically-confirmed dengue with
hospitalization against all four serotypes with
a 2-sided 95% confidence interval, wherein the lower bound is more than 65%,
when measured against placebo in a
subject population of at least 5,000 healthy 4 to 16 year old subjects
irrespective of serostatus at baseline from first
administration of the administration schedule until 12 to 18 months after the
last administration of the administration
schedule.
[0045] If one of the criteria as defined above for the term "safe" is
fulfilled, the vaccine S considered safe within
the meaning of this invention. In this context, safe in particular refers to a
vaccine that is safe for all subjects
irrespective of their serostatus at baseline. This means that the vaccine can
be administered without the need to
determine the occurrence of a previous dengue infection in the subject before
administration. Preferably, the vaccine
is safe as defined above with respect to all age groups starting from 4 years
of age and preferably irrespective of the
serostatus, in particular from 4 years of age to 60 years of age, or 4 years
of age to 16 years of age. Relevant
subgroups in this context are under 9 years of age, from 2 years of age to
under 9 years of age, from 4 years of age
to under 9 years of age, 4 to 5 years of age, 6 to 11 years of age and 12 to
16 years of age or any age group within
4 to 16 years of age. For further definitions of VE against virologically-
confirmed dengue disease with hospitalization
reference is made to the disclosure below with respect to certain methods of
treatment
[0046] As used herein, "vaccine efficacy" or "VE" measure the proportionate
reduction in cases among vaccinated
persons. Vaccine efficacy (VE) is measured by calculating the risk of disease
among vaccinated and unvaccinated
persons and determining the percentage reduction in risk of disease among
vaccinated persons relative to
unvaccinated persons. The greater the percentage reduction of illness in the
vaccinated group, the greater the
vaccine efficacy. For example, a VE of 90% indicates a 90% reduction in
disease occurrence among the vaccinated
group, or a 90% reduction from the number of cases you would expect if they
have not been vaccinated_ The
vaccine efficiency is calculated by the formula: 100 * (1 ¨ FIR), wherein HR
is the Hazard Ratio which is defined as
the Hazard rate of vaccine (Ay) divided by the Hazard rate of placebo (k),
i.e. HR = /iv/M. /iv denote the hazard
rate for the subjects vaccinated with a tetravalent dengue vaccine composition
as disclosed herein and Ac denote the
hazard rate for unvaccinated sub"-
_______________________________________________________________________________
____________ is, i.e. subjects receiving placebo. The hazard rate ratio HR is
estimated from a
Cox proportional hazard model with study vaccine as a factor, adjusted for
age, and stratified by region. As used
herein the term"combined vaccine efficacy against all four serotypes" is
defined as the vaccine efficacy in relation to
the risk of dengue disease irrespective of the serotype being responsible for
the virologically-confirmed dengue
disease and the subject baseline serostatus. A vaccine is considered
"effective" in case the combined vaccine efficacy
is above 300Jo. In this context the combined vaccine efficacy may be also 40%
or more, 50% or more, 60% or more,
70% or more, 72010 or more, or 80% or more, in particular when determined from
30 days after a second
administration until 12 months after a second administration or 18 months
after a second vaccination, in particular
when determined in age groups selected from the age group of 4 to 16 year old
subjects, the age group of 4 to
under 9 year old subjects, the age group of 2 to under 9 year old subjects,
the age group of 4 to 5 year old subjects,
the age group of 6 to 11 year old subjects, and the age group of 12 to 16 year
old subjects. In this context, effective
in particular refers to a vaccine that is effective for all subjects
irrespective of their serostatus at baseline. Preferably,
the vaccine is effective with respect to all age groups starting from 4 years
of age and preferably irrespective of the
serostatus, in particular from 4 years of age to 60 years of age or from 4
years of age to 16 years of age and
irrespective of the serostatus. Relevant subgroups in this context are under 9
years of age, from 2 years of age to
8
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
under 9 years of age, from 4 years of age to under 9 years of age, 4 to 5
years of age, 6 to 11 years of age and 12
to 16 years of age or any age group within 4 to 16 years of age. In certain
embodiments "effective" means providing
a combined vaccine efficacy against all four serotypes, in preventing
virologically confirmable dengue disease with a
2-sided 95% confidence interval, wherein the lower bound is more than 60%,
when measured against placebo in a
subject population of at least 5,000 healthy subjects irrespective of
serostatus at baseline and 4 to 16 years of age,
from the first administration of the administration schedule until 18 months
after the last administration of the
administration schedule. Further specific efficacies can be defined. As used
herein, "combined vaccine efficacy
against all four serotypes in seronegative subjects" refers to the efficacy
measured in subjects which are
seronegative at baseline. As used herein, "vaccine efficacy against a specific
serotype, e.g. serotype 1" refers to the
efficacy in relation to a specific serotype being responsible for the
virologically-confirmed dengue disease. As used
herein, "combined vaccine efficacy against all four serotypes against
virologically-confirmed dengue with
hospitalization" refers to the efficacy wherein only virologically-confirmed
dengue cases with hospitalization are
considered. Such vaccine efficacies can be determined with respect to subjects
being seronegative or seropositive at
baseline and for different age groups.
[0047] As used herein, the 'relative risk" means the number of events of
virologically confirmed dengue disease
divided by the number of subjects treated with the unit dose as disclosed
herein over the number of eyefuls of
virologically confirmed dengue disease divided by the number of subjects
treated with placebo. As used herein the
term"combined relative risk against all four serotypes" is defined as the
relative risk in relation to the risk of dengue
disease irrespective of the serotype being responsible for the virologically-
confirmed dengue disease and the subject
baseline serostatus.
[0048] As used herein, "vaccinating" or "inoculating" refers to the
administration of a vaccine to a subject, with
the aim to prevent the subject, from developing one or more symptoms of a
disease. As used herein, "vaccinating
against dengue disease" or "inoculating against dengue disease" refers to the
administration of a dengue vaccine
composition in a subject, with the aim to prevent the subject, from developing
one or more symptoms of dengue
disease. In principle the method comprises a primary vaccination and
optionally one or more booster vaccinations.
The primary vaccination is defined as the primary administration schedule for
administering the composition or unit
dose as disclosed herein to establish a protective immune response and e.g.
consists of two administrations e.g.
within three months. Whenever an administration is mentioned within this
disclosure such administration refers to
the primary vaccination unless it is specified as booster vaccination. The
booster vaccination refers to an
administration or administration schedule which takes place after the primary
vaccination e.g. at least 1 year, or 4 to
4.5 years, or even 5 or 10 years after the last administration, e.g. the
second administration, of the primary
vaccination schedule. The booster administration attempts at enhancing or
reestablishing the immune response of
the primary vaccination.
[0049] As used herein, the terms "subject" or "subjects" are limited to human
subjects (e.g. infants, children or
adults). The terms "elderly subject" or "elderly subjects" refer to subjects
with an age of more than 68 years, such as
61 years to 100 years, 61 years to 90 years, 61 years to 80 years, 61 years to
75 years, or 61 years to 70 years.
[0050] As used herein, "subject population" refers to a group of subjects. The
subject population may refer to
least 40 subjects, at least 50 subjects, at least 60 subjects, at least 100
subjects or at least 1000 subjects and is
defined by certain parameters. The parameters that may be used to define a
subject population include, but are not
limited to, the age of the subjects, whether the subjects are from a dengue
endemic region or from a dengue non-
endemic region and the serostatus of the subjects.
9
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[0051] As used herein, "endemic region" refers to a region where a disease or
infectious agent is constantly
present and/or usually prevalent in a population within this region. As used
herein, "non-endemic region" refers to a
region from which the disease is absent or in which it is usually not
prevalent. Accordingly, a "dengue endemic
region" refers to geographic areas in which an infection with dengue virus is
constantly maintained at a baseline
level. A "dengue non-endemic region" is a geographic area in which an
infection with dengue virus is not constantly
maintained at a baseline level. Accordingly, subject populations or subjects
'from a dengue endemic region" or "from
a dengue non-endemic region" refer to subject populations or subjects living
in geographic areas as defined above.
Whether a geographic area or a subject population is dengue-endemic or not can
be determined by different
calculatory methods such as the ones described in Bhatt et al. (2013) Nature
496 (7446): 504507 and
supplementary material and in Stanaway et al. (2016) Lancet Infect Dis. 16(6):
712-723 and supplementary material.
Overviews of dengue endemic regions and dengue epidemiology are regularly
published, for example, by the WHO or
CDC. Typical dengue-endemic regions are in Latin America, Southeast Asia and
the Pacific islands and dengue
endemic countries include, but are not limited to, Australia, Brazil,
Bangladesh, Colombia, China, Dominican Republic,
Indonesia, India, Mexico, Malaysia, Nicaragua, Nigeria, Pakistan, Panama,
Philippines, Puerto Rico, Singapore, Sri
Lanka, Thailand and Vietnam. The area's force of infection is measured by
seroprevalence surveys provided as
seroprevalence rate_ Areas with very high force of infection are considered to
have a seroprevalence rate of more
than 80%. As used herein the term "region" when it concerns seroprevalence
rates refers to a geographic area
where the seroprevalence rate could be determined or is known, e.g. a village,
a town, a city, a region, a county, a
state, a province or parts of the foregoing or a whole country.
[0052] As used herein, "serostatus" refers to the amount of antibodies a
subject has with respect to a certain
infectious agent, in particular dengue virus. As used herein, "seronegative"
or "seronaive" means that the subject
does not have neutralizing antibodies against any one of dengue serotypes DENV-
1, DENV-2, DENV-3 and DENV-4 in
the serum. A seronegative or seronaive subject or subject population is
defined by a neutralizing antibody titer of less
than 10 for each one of the four dengue serotypes. A subject or subject
population having a neutralizing antibody
titer of equal to or more than 10 for at least one dengue serotype is defined
as being "seropositive" with respect to
said dengue serotype. Serostatus at baseline refers to the serostatus before
the administration of a dengue vaccine
composition as described herein.
[0053] As used herein, a "neutralizing antibody titer" refers to the amount of
antibodies in the serum of a subject
that neutralize the respective dengue serotype. The neutralizing antibody
titer against DEW-1, DENV-2, DENV-3 and
DENV-4 is determined in a serum sample of the subject using known methods such
as the plaque reduction
neutralization test (PRINT) as described in the WHO Guidelines (World Health
Organization Department of
Immunization Vaccines Biologicals (2007) Guidelines for plaque reduction
neutralization testing of human antibodies
to dengue viruses, WHO/NB/07.07) or a microneutralization (MNT50) assay as
described herein. As used herein, the
"ratio of not more than 20 for the neutralizing antibody titer of dengue
serotype 2 to the neutralizing antibody titer of
dengue serotype 4" means that the neutralizing antibody titer of dengue
serotype 2 is divided by the neutralizing
antibody titer of dengue serotype 4 and that the ratio obtained hereby is no
more than 20. In other words, the
neutralizing antibody titer of dengue serotype 2 is not more than 20-times
higher than the neutralizing antibody titer
of dengue serotype 4 in the subject.
[0054] As used herein, the terms "geometric mean neutralizing antibody titer"
and "GMT" refer to the geometric
mean value of the titer of neutralizing antibodies against the corresponding
dengue serotype in the serum of subjects
in a subject population. The geometric mean value is calculated by a well-
known formula. As used herein, the "ratio
of not more than 20 for the GMT of dengue serotype 2 to the GMT of dengue
serotype 4" means that the geometric
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
mean neutralizing antibody titer of dengue serotype 2 (GMT DENV-2) is divided
by the geometric mean neutralizing
antibody titer of dengue serotype 4 (GMT DENV-4) and that the ratio obtained
hereby is no more than 20. In other
words, the geometric mean neutralizing antibody titer of dengue serotype 2 is
not more than 20-times higher than
the geometric mean neutralizing antibody titer of dengue serotype 4 in the
subject population.
[0055] As used herein, an "immune response" refers to a subject's response to
the administration of the dengue
vaccine. In particular, the immune response includes the formation of
neutralizing antibodies to one or more dengue
serotypes. It may also include the stimulation of a cell-mediated response or
the formation of antibodies to non-
structural proteins such as NS1. An immune response is stimulated by the
administration of a unit dose of the
invention as described herein, if the titer of neutralizing antibodies against
at least one dengue virus serotype and
preferably against all four dengue virus serotypes is increased after said
administration of said unit dose An immune
response is stimulated by the administration of a unit dose of the invention
as described herein, if the secretion of
interferon gamma by peripheral blood mononuclear cells stimulated with
peptides from dengue virus proteins is
increased after said administration of said unit dose. An immune response is
stimulated by the administration of a
unit dose of the invention as described herein, if the titer of antibodies to
non-structural proteins such as NS1 is
increased after said administration of said unit dose. In a particular
embodiment, the administration of a
reconstituted unit dose of the present invention as described herein
stimulates the formation of neutralizing
antibodies to one or more dengue serotypes, a cell-mediated response and the
formation of antibodies to non-
structural proteins such as N51.
[0056] As used herein, a "balanced immune response" means that the immune
response to the four dengue
serotypes is sufficient to provide protection against infection by all four
dengue serotypes and preferably the immune
response to the four dengue serotypes has a similar strength. In particular,
the neutralizing antibody titer against the
four dengue serotypes at day 180 or day 365 after administration of a first
reconstituted unit dose of the invention as
described herein is similar, i.e. it differs by less than factor 30, by less
than factor 25 or by less than factor 20.
[0057] The õtotal concentration in pfu/0.5 ml" which serves as a base value
for the calculation of the percentage
concentration for each individual component of a tetravalent dengue vaccine is
shown for one exemplary tetravalent
vaccine composition comprising dengue serotype 1 in a concentration of 3.60
log1Opfu/0.5 ml, a dengue serotype 2
concentration of 4.00 loglOpfu/0.5 ml, a dengue serotype 3 concentration of
4.60 log1Opfu/0.5 ml and a dengue
serotype 4 concentration of 5.11 log1Opfu/0.5 ml.
Primarily, the logarithmic values of the concentrations are converted into
numerical values. The results of this
conversion are 4x103 pfu/D.5 ml for serotype 1, lx104 pfu/0.5m1 for serotype
2, 4x104 pfu/0.5 ml for serotype 3 and
1.3x105 pfu/0.5 ml for serotype 4. The total concentration in pfu/0.5 ml is
the sum of the preceding numerical values
resulting in 1.84 x105 pfu/0.5 ml.
[0058] The "percentage concentration" for each of the serotypes 1, 2, 3 and 4
is obtained by dividing the
numerical concentration value (expressed as pfu/0.5 ml) of an individual
serotype by the total concentration
(expressed in pfu/0.5 ml) and multiplying the result by 100 i.e.:
Percentage concentration of serotype 1 = (4x103 pfu/0.5 ml 1.84 x105 pfu/0.5
ml) x 100 = 2%
Percentage concentration of serotype 2 = (1x104 pfu/0.5m1 1.84 x105 pfu/0.5
ml) x 100 = 5%
Percentage concentration of serotype 3 = (4x104 pfu/0.5 ml + 1.84 x105 pfu/0.5
ml) x 100 = 22%
Percentage concentration of serotype 4= (1.3x105 pfu/0.5 ml 1.84 x105
pfu/0.5 ml) x 100 = 71%.
The percentage concentrations are rounded to whole numbers.
11
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[0059] As used herein "simultaneous" administration means an administration of
at least two different vaccines
such as a dengue vaccine and a hepatitis A vaccine on the same day. "On the
same day" has the ordinary meaning
of within 24 hours, such as e.g. within one calendar day. The simultaneous
administration may be administered by
the same medical practitioner, such as during the same medical appointment.
[0060] As used herein "sequential" administration means an administration of
at least two different vaccines,
such as a dengue vaccine and a yellow fever vaccine, or a dengue vaccine and a
hepatitis A vaccine on different or
subsequent days, such as within 90 days, but in a combined administration
schedule.
[0061] As used herein, the term "chronic disease or condition" includes those
diseases and conditions which
persist in an elderly subject for three months or more. In particular, it
includes diabetes, hypertension, allergies,
previous strokes, ischemic heart disease, chronic renal impairment and chronic
obstructive pulmonary disease.
[0062] As used herein, the term "impaired immune system" means that at least
one function of at least one
component of the immune system is weaker than in younger subjects, i.e. in
subjects with an age of less than 60
years. These functions include a lower antioxidant response of monocytes
against oxidative stress induced by dengue
virus and lower T cell responses and cybakine production in response to dengue
virus infection.
[0063] As used herein, "solicited systemic adverse events" in children under 6
years are defined as fever,
irritability/fussiness, drowsiness and loss of appetite that occurred within
14 days after each vaccination, and in
children of 6 years or more are defined as fever, headache, asthenia, malaise
and myalgia that occurred within 14
days after each vaccination.
[0064] As used herein, "solicited local adverse events" are injection site
pain, injection site erythema and injection
site swelling that occurred within 7 days after each vaccination.
[0065] As used herein, "unsolicited adverse events" are any adverse events
(AEs) that are not solicited local or
systemic AEs, as defined above.
[0066] As used herein, a "serious adverse event" or "SAE" is any untoward
medical occurrence or effect that at
any dose results in death, is life-threatening, requires inpatient
hospitalization or prolongation of existing
hospitalization, results in persistent or significant disability / incapacity,
is a congenital anomaly / birth defect or is
medically important due to other reasons than the above mentioned criteria.
[0067] The relationship of each AE, including solicited systemic AEs
(solicited local AEs are considered as related)
to trial vaccine(s) will be assessed using the following categories: As used
herein, "IP-Related AE" or "vaccine related
AE" means Mat there is suspicion that there is a relationship between the
vaccine and the AE (without determining
the extent of probability); there is a reasonable possibility that the vaccine
contributed to the AE. As used herein,
"Non-IP Related" or "non-vaccine related" means that there is no suspicion
that there is a relationship between the
vaccine and the AE; there are other more likely causes and administration of
the vaccine is not suspected to have
contributed to the AE.
[0068] As used herein, a subject or subject population being "2 to 60 years of
age" "or 18 to 60 years of age"
refers to a subject or subject population being 2 to 60 years of age or 18 to
60 years of age on the first day of the
administration of the dengue vaccine composition as described herein_
[0069] As used herein "%-points" refers to the difference of two %-values in a
%-value. For example two values
12
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
in % which are within 5 'Ye-points refers to e.g. one value at 1% and a second
value at 6%.
[0070] As used herein, the term "determination of the previous dengue
infection in the subject before
administration" means that a previous dengue infection has to be assessed
before vaccination in that there is a
laboratory confirmed history of dengue or through an appropriately validated
serological test e.g. by the method as
disclosed herein such as the Misff50 test described in Example 2 or any
serotesting with adequate performance in
term of specificity and cross 'reactivity based on the locale disease
epidemiology.
[0071] As used herein % w/v refers to % mg/ml wherein e.g. 150 mg/ml are 15%
w/v.
[0072] As used herein, the term "hepatitis A virus" may be abbreviated as
"HAV".
[0073] As used herein, the term "placebo" may be abbreviated as "Pbo".
[0074] As used herein, "hepatitis A seronegative at baseline" or "hepatitis A
naive (at baseline)" each mean that a
subject does not have a predefined amount of anti-hepatitis A antibodies in
the serum. Quantitatively, the hepatitis A
seronegativity of a subject is defined as an anti-hepatitis A antibody level
of < 10 mIll/ml. When anti-hepatitis A
antibody levels are determined by ELISA, the lower level of quantification is
12.5 mIU/m1 which is effectively the
lower anti-IIAV antibody level for determining seronegativity. Subjects having
anti-hepatitis A antibody levels of
12.5 mIU/m1 are defined as hepatitis A seropositive. An ELISA for determining
the anti-hepatitis A antibodies is for
example disclosed in Beck et al. ] Travel Med 2004; 11:201-207.
[0075] As used herein, "at baseline" refers to the time point of the last
measurement of a subject's serostatus
prior to the first vaccination.
[0076] As used herein, the unit "mIU/mr refers to milli-international unit per
milliliter. This concentration unit
refers to a quantity of anti-hepatitis A antibodies in a subject's serum (e.g.
when measured prior or after
vaccination). As used herein, the "viral antigen activity of hepatitis A
vaccines" of the present invention is expressed
in terms of a standard recommendation of the WHO using an enzyme-linked
immunosorbent assay (ELISA).
According to this recommendation of the WHO (see WHO Information Sheet
"Observed Rate of Vaccine Reactions ¨
Hepatitis A Vaccine", published June 2012), the viral antigen activity of a
hepatitis A vaccine is expressed in terms of
ELISA Units (ELU.). The viral antigen activity of a hepatitis A vaccine can
for example be determined by an ELISA
according to Andre FE., Hepburn A. D'Horkft E., "Inactivated candidate
vaccines for hepatitis", A. Prog Med Virol
1990; 37:72-95.
[0077] As used herein, the term "CCID" refers to the quantity of virus (e.g.
vaccinal virus) infecting 50% of the
cell culture. The CCID50 assay is a limit dilution assay with statistical
titer calculation (Morrison D et al, J Infect Dis.
2010; 201(3):370-7)).
[0078] "Non-inferiority", as used herein, with respect to a simultaneous on
the same day administration of a
hepatitis A vaccine and a tetravalent dengue vaccine is in particular
concluded, if the seroproteclion rate (SPR)
difference between the SPR of a subject group receiving HAV and placebo
(simultaneously on the same day, i.e.
control subject population) and the SPR of a subject group receiving HAV and
TDV (simultaneously on the same day)
has an upper bound of a two-sided 95% confidence interval which is lower than
the non-inferiority margin set at
10%, wherein seroprotection rates are based on measurements on day 30 after
the simultaneous administration on
day 1, calculated using the Newcombe score method. A non-inferiority clinical
study is a study designed to provide a
comparison between at least two methods of treatments, in the present case
between a simultaneous administration
13
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
of a dengue vaccine and a hepatitis A vaccine and a mono-administration of
either a dengue vaccine or a hepatitis A
vaccine.
[0079] As used herein, the term "seroprotection rate", abbreviated nSPR", is
defined by the
proportion/percentage of HAV or DEN-naive subjects at baseline who are
seroprotected against HAV or DENV,
respectively, at day 30 (month 1) after the first vaccination.
[0080] As used herein, the term "control subject population" refers to a group
of subjects which does not receive
a simultaneous administration of a hepatitis A vaccine and a unit dose of a
dengue vaccine composition, but a single
verum (such as a hepatitis A vaccine or a unit dose of a dengue vaccine
composition) and a placebo on the same day
in a clinical study setting as e.g. in a non-inferiority clinical study.
[0081] As used herein, the term "synergism" or "synergy" is defined as an
effect of simultaneously on the same
day administering the hepatitis A vaccine and the unit dose of the dengue
vaccine composition to a subject or
subject population, wherein said administering provides a higher anti-
hepatitis A antibody concentration and/or a
higher mean titer of neutralizing antibodies against each of the dengue virus
serotypes than the corresponding
simultaneous administration of a hepatitis A vaccine and a placebo on the same
day and/or the simultaneous
administration of a unit dose of the dengue vaccine composition and a placebo
on the same clay (mono-
administrations). Such higher antibody concentrations after simultaneous
administration in comparison to the mono-
administrations are signs in favor of the simultaneous adminisLidlion.
BRIEF DESCRIPTION OF TFIE DRAWINGS
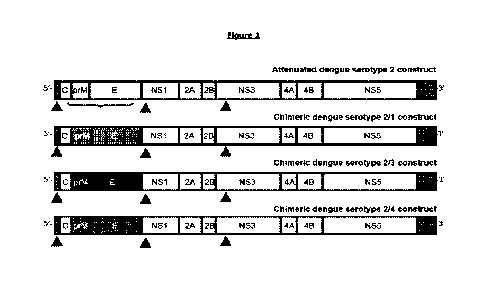
[0082] Figure 1: Genetic structure of the four
dengue strains contained in TDV. The solid red triangles
indicate the three attenuating mutations present in the 51NICR, NS1 and NS3
proteins. The TDV-1, TDV-3 and TDV-4
strains are chimeric viruses where the prM and E genes from dengue serotype 1,
3 and 4, respectively, are inserted
into the TDV-2 backbone.
[0083] Figure 2: Schematic drawing illustrating the
microneutralization test (MNT) used in determine the
titer of neutralizing antibodies.
[0084] Figure 3: Flow diagram of the clinical trial of Example 3.
[0085] Figure 4: Cumulative incidence of A)
virologically-confirmed dengue cases and B) hospitalized
virologically-confirmed dengue cases over time during Part 1 study period by
baseline serostatus (safety set data;
data presented truncated at Month 18). Tables show numbers of participants
under follow-up at various time points
to end of Part 1 study period.
[0086] Figure 5: Study design of phase III study described in example 3.
[0087] Figure 6: Scheme of the trial design of the simultaneous HAV and TDV
administration study described in
Example 4.
DETAILED DESCRIPTION
Dengue virus strains
14
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[0088] The dengue virus is a single stranded, positive sense RNA virus of the
family flaviviridae. The taxonomy is
outlined in Table 1. The family flaviviridae includes three genera,
flavivirus, hepacivirus and pestivirus. The genus
flavivirus contains highly pathogenic and potentially hemorrhagic fever
viruses, such as yellow fever virus and
dengue virus, encephalitic viruses, such as Japanese encephalitis virus,
Murray Valley encephalitis virus and West
Nile virus, and a number of less pathogenic viruses.
Table 1. Dengue Virus Taxonomy of the GMO Parental
Strain
Family Flaviviridae
Genus Flavivirus
Species Dengue virus
Strains Dengue Serotype 2 (Strain 16681),
Strain DEN-2 PDK-53
GMO parent TDV-2
[0089] The flavivirus genonie comprises in S. to 3 direction (see Figure 1):
- a 5'-noncoding region (5'-NCR),
- a capsid protein (C) encoding region,
- a pre-membrane protein (prM) encoding region,
- an envelope protein (E) encoding region,
- a region encoding nonstructural proteins (NSI, NS2A, N528, N53, NS4A,
NS4B, N55) and
- a 3' noncoding region (3'-NCR).
[0090] The viral structural proteins are C, prM and E, and the nonstructural
proteins are NSI to NS5. The
structural and nonstructural proteins are translated as a single polyprotein
and processed by cellular and viral
proteases.
[0091] The unit dose of the invention as described herein comprises a dengue
virus composition that comprises
four live attenuated dengue virus strains (tetravalent dengue virus
composition) representing dengue serotype 1,
dengue serotype 2, dengue serotype 3 and dengue serotype 4. Preferably the
composition comprises chimeric
dengue viruses and optionally at least one non-chimet dengue virus, in
particular a molecularly characterized and
cloned dengue serotype 2 strain derived from the live attenuated DEN-2 PDK-53
virus strain (TDV-2), and three
chimeric dengue strains derived from the TDV-2 strain by replacing the
structural proteins prM and E from TDV-2
with the corresponding structural proteins from the other dengue serotypes,
resulting in the following chimeric
dengue strains:
- a DEN V-2/1 chimera (TDV-1),
- a DEN V-2/3 chimera (TDV-3) and
- a DEN V-2/4 chimera (TDV-4).
[0092] The genetically modified tetravalent dengue vaccine TDV is based on a
molecularly characterized and
cloned dengue-2 virus strain (TDV-2). This attenuated TDV-2 strain was
generated by cDNA cloning of the
attenuated laboratory-derived DEN-2 PDK-53 virus strain that was originally
isolated at Mahidol University, Bangkok,
Thailand (Kinney et al. (1997) Virology 230(2): 300-308). DEN-2 PDK-53 was
generated by 53 serial passages in
primary dog kidney (PDK) cells at 32 C (Bhamarapravati et al. (1987) Bull.
World Hearth Organ. 65(2): 189-195).
[0093] The attenuated DEN-2 PDK-53 strain (the precursor of TDV-2) was derived
from the wild type virus strain
DEN-2 16681 (SEQ ID NO 11) and differs in nine nucleotides from the wild type
as follows (Kinney et al. (1997)
Virology 230(2): 300-308):
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
(i) 51-noncoding region (NCR)-57 (nt-57 C-to-T): major attenuation locus
(ii) prM-29 Asp-to-Val (nt-524 A-to-T)
(iii) nt-2055 C-to-T (E gene) silent mutation
(iv) NS1-53 Gly-to-Asp (nt-2579 G-to-A): major attenuation locus
(v) NS2A-181 Leu-to-Phe (nt-4018 C-to-T)
(vi) N53-250 Glu-to-Val (nt-5270 A-to-T): major attenuation locus
(vii)nt-5547 (N53 gene) T-to-C silent mutation
(viii) NS4A-75 Gly-to-Ala (nt-6599 (3-to-C)
* nt-8571 C-to-T (NS5 gene) silent mutation
[0094] The three nucleotide changes located in the 5' noncoding region (NCR)
(nucleotide 57) (mutation (I)), the
NS-1 (amino acid 828 of SEQ ID NO. 4) (mutation (iv)) and NS-3 genes (amino
acid 1725 of SEQ ID NO. 4)
(mutation (vi)) form the basis for the attenuation phenotype of the DEN-2 PDK-
53 strain (Butrapet et al. (2000) J.
Viral. 74(7): 3111-3119) (Table 2). These three mutations are referred to
herein as the "attenuating mutations" and
are comprised in WV-1, TDV-2, TDV-3 and TDV-4.
Table 2. Attenuating mutations in the common genetic backbone of all TDV
strains
Location of Mutation Nudeotide Change in
TDV-2 Amino Acid Change in TDV-2
5' Noncoding Region (5'NCR) 57 C to T
Not applicable (silent)
Nonstructural Protein 1 (NS1) 2579(3 to A
828 (Sly to Asp
Nonstructural Protein 3 (NS3) 5270 A to T
1725 Glu to Val
[0095] In one embodiment, TDV-2 comprises in addition to the three attenuating
mutations one or more
mutations selected from:
a) a mutation in the prM gene at nucleotide 524 from adenine to thymidine
resulting in an amino acid change
at position 143 from asparagine to valine, and/or
b) a silent mutation in the E gene at nucleotide 2055 from cytosine to
thymidine, and/or
c) a mutation in the NS2A gene at nucleotide 4018 from cytosine to thymidine
resulting in an amino acid
change at position 1308 from leucine to phenylalanine, and/or
d) a silent mutation in the NS3 gene at nucleotide 5547 from thymidine to
cytosine, and/or
e) a mutation in the NS4A gene at nucleotide 6599 from guanine to cytosine
resulting in an amino acid change
at position 2168 from glycine to alanine, and/or
f) a silent mutation in the prM gene at nucleotide 900 from thymidine to
cytosine.
The silent mutation in the NS5 gene at nucleotide 8571 from cytosine to
thymidine of DEN-2 PDK-53 is not present in
the TDV-2 strain.
[0096] In another embodiment, TDV-2 comprises in addition to the three
attenuating mutations one or more
mutations selected from:
g) a mutation in the prM gene at nucleotide 592 from adenine to guanine
resulting in an amino acid change at
position 166 from lysine to glutamine, and/or
h) a mutation in the NS5 gene at nucleotide 8803 from adenine to guanine
resulting in an amino acid change
at position 2903 from isoleucine to valine.
[0097] In another embodiment, TDV-2 comprises in addition to the three
attenuating mutations the mutations a)
and g), preferably the mutations a), g), c), e) and h), more preferably the
mutations a), g), c), e), h) and b), even
16
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
more preferably the mutations a), g), c), e), h), b) and d), and most
preferably the mutations a) to h). The
nucleotide positions and amino acids positions of TDV-2 refer to the
nucleotide sequence as shown in SEQ ID NO. 3
and amino acid sequence as shown in SEQ ID NO. 4.
[0098] The dengue virus structural envelope (E) protein and pre-membrane (prM)
protein have been identified as
the primary antigens that elicit a neutralizing protective antibody response
(Plotkin 2001). For creation of the
tetravalent dengue vaccine (TDV), TDV-2 was modified by replacing the nucleic
acid sequence encoding the DENV-2
prM and E glycoproteins with the nucleic acid sequence encoding the
corresponding wild type prM and E
glycoproteins from the DENV-1, DENV-3, and DENV-4 wild type strains DENV-1
16007, DENV-3 16562 or DENV-4
1036 virus, respectively, (see Table 3) using standard molecular genetic
engineering methods (Huang et al. (2003) J.
Virol. 77(21): 11436-11447).
Table 3.Viral origin of prfl/E gene regions of the TDV virus strains
Nudeotide
Amino acid
Virus Strain Origin Source
Reference
sequence
sequence
DENV-1 16007 Thailand, 1964 DHF/DSS
Halstead and SEQ ID NO. 9 SEQ ID NO. 10
patient
Simasthien, 1970
DENV-2 16681 Thailand, 1964 DHF/DSS
Halstead and SEQ ID NO. 11 SEQ ID NO. 12
patient
Simasthien, 1970
DENV-3 16562 Philippines, 196, DHF patient
Halstead and SEQ ID NO. 13 SEQ ID NO. 14
Simasthien, 1970
DENV-4 1036 Indonesia, 1976 DF patient Gubler
et al., 1979 SEQ ID NO. 15 SEQ ID NO. 16
[0099] A diagram of the four TDV strains comprised in the dengue vaccine
composition is shown in Figure 1.
[00100] The chimeric dengue strains TDV-1, TDV-3 and TDV-4 express the surface
antigens prM and E of the
DEW-1, DENV-3 or DENV-4 viruses, as depicted in Table 3 respectively, and
retain the genetic alterations
responsible for the attenuation of TDV-2. Thus, each of the TDV-1, TDV-3 and
TDV-4 strains comprises the
attenuating mutations described in Table 2.
[00101] In one embodiment, WV-1 comprises in addition to the three attenuating
mutations one or more
mutations selected from:
c) a mutation in the NS2A gene at nucleotide 4018 from cytosine to thymidine
resulting in an amino acid
change at position 1308 from leucine to phenylalanine, and/or
d) a silent mutation in the N53 gene at nucleotide 5547 from thymidine to
cytosine, and/or
e) a mutation in the NS4A gene at nucleotide 6599 from guanine to cytosine
resulting in an amino acid change
at position 2168 from glycine to alanine, and/or
i) a silent mutation in the E gene at nucleotide 1575 from thymidine to
cytosine, and/or
j) a silent mutation in the junction site between the prM-E gene and the DEN-2
PDK-53 backbone at nucleotide
453 from adenine to guanine, and/or
k) a mutation in the junction site between the prM-E gene and the DEN-2 PDK-53
backbone at nucleotides
2381/2382 from thymidine-guanine to cytosine-cytosine resulting in an amino
acid change at position 762 from
valine to alanine.
17
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00102] In another embodiment, TDV-1 comprises in addition to the three
attenuating mutations one or more
mutations selected from:
I) a mutation in the NS2A gene at nucleotide 3823 from adenine to cytosine
resulting in an amino acid change
at position 1243 from isoleucine to leucine, and/or
m) a mutation in the NS28 gene at nucleotide 4407 from adenine to thymidine
resulting in an amino acid
change at position 1437 from glutamine to asparagine, and/or
n) a silent mutation in the N548 gene at nucleotide 7311 from adenine to
guanine.
[00103] In another embodiment, the TDV-1 strain comprises in addition to the
three attenuating mutations the
mutations I) and m), preferably the mutations l), m), c) and e), even more
preferably the mutations l), m), c), e), d)
and n), and most preferably the mutations l), m), c), e), d), n), i), j) and
k). The nucleotide positions and amino
acids positions of WV-1 refer to the nucleotide sequence as shown in SEQ ID
NO. 1 and amino acid sequence as
shown in SEQ ID NO. 2.
[00104] In one embodiment, TDV-3 comprises in addition to the three
attenuating mutations one or more
mutations selected from:
c) a mutation in the NS2A gene at nucleotide 4012 from cytosine to thymidine
resulting in an amino acid
change at position 1306 from leucine to phenylalanine, and/or
d) a silent mutation in the NS3 gene at nucleotide 5541 from thymidine to
cytosine, and/or
e) a mutation in the NS4A gene at nucleotide 6593 from guanine to cytosine
resulting in an amino acid change
at position 2166 from glycine to alanine, and/or
j) a silent mutation in the junction site between the prM-E gene and the DEN-2
PDK-53 backbone at nucleotide
453 from adenine to guanine, and/or
k) a mutation in the junction site between the prM-E gene and the DEN-2 PDK-53
backbone at nucleotides
2375/2376 from thymidine-guanine to cytosine-cytosine resulting in an amino
acid change at position 760 from
valine to alanine, and/or
co a silent mutation in the prM gene at nucleotide 552 from cytosine to
thymidine, and/or
p) a mutation in the E gene at nucleotide 1970 from adenine to thymidine
resulting in an amino acid change at
position 625 from histidine to leucine.
[00105] In another embodiment, TEN-3 comprises in addition to the three
attenuating mutations one or more
mutations selected from:
q) a mutation in the E gene at nucleotide 1603 from adenine to thymidine
resulting in an amino acid change at
position 503 from threonine to serine, and/or
r) a silent mutation in the NS5 gene at nucleotide 7620 from adenine to
guanine.
[00106] In another embodiment, 1DV-3 comprises in addition to the three
attenuating mutations the mutations p)
and q), preferably the mutations p), q), c) and e), even more preferably the
mutations p), q), c), e), d) and r), and
most preferably the mutations p), q), c), e), d), r), j), k) and o). The
nucleotide positions and amino acids positions
of TDV-3 refer to the nucleotide sequence as shown in SEQ ID NO. 5 and amino
acid sequence as shown in SEQ ID
NO. 6.
[00107] In one embodiment, 1DV-4 comprises in addition to the three
attenuating mutations one or more
mutations selected from:
0 a mutation in the NS2A gene at nucleotide 4018 from cytosine to thymidine
resulting in an amino acid
change at position 1308 from leucine to phenylalanine, and/or
18
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
d) a silent mutation in the NS3 gene at nucleotide 5547 from thymidine to
cytosine, and/or
e) a mutation in the NS4A gene at nucleotide 6599 from guanine to cytosine
resulting in an amino acid change
at position 2168 from glycine to alanine, and/or
j) a silent mutation in the junction site between the prM-E gene and the DEN-2
PDK-53 backbone at nucleotide
453 from adenine to guanine, and/or
k) a mutation in the junction site between the prM-E gene and the DEN-2 PDK-53
backbone at nucleotides
2381/2382 from thymidine-guanine to cytosine-cytosine resulting in an amino
acid change at position 762 from
valine to alanine, and/or
s) a mutation in the C gene at nucleotide 396 from adenine to cytosine
resulting in an amino acid change at
position 100 from arginine to serine, and/or
t) a silent mutation in the E gene at nucleotide 1401 from adenine to guanine,
and/or
u) a mutation in the E gene at nucleotide 2027 from cytosine to thymidine
resulting in an amino acid change at
position 644 from alanine to valine, and/or
v) a mutation in the E gene at nucleotide 2275 from adenine to cytosine
resulting in an amino acid change at
position 727 from methionine to leucine.
[00108] In another embodiment, TDV-4 comprises in addition to the three
attenuating mutations one or more
mutations selected from:
w) a silent mutation in the C gene at nucleotide 225 from adenine to
thymidine, and/or
x) a mutation in the NS2A gene at nucleotide 3674 from adenine to guanine
resulting in an amino acid change
at position 1193 from asparagine to glycine, and/or
y) a mutation in the NS2A gene at nucleotide 3773 from adenine to an
adenine/guanine mix resulting in an
amino acid change at position 1226 from lysine to a lysine/asparagine mix,
and/or
z) a silent mutation in the NS3 gene at nucleotide 5391 from cytosine to
thymidine, and/or
aa) a mutation in the NS4A gene at nucleotide 6437 from cytosine to thymidine
resulting in an amino acid
change at position 2114 from ala nine to valine, and/or
It) a silent mutation in the NS4B gene at nucleotide 7026 from thymidine to a
thymidine/cytosine mix, and/or
cc) a silent mutation in the NS5 gene at nucleotide 9750 from adenine to
cytosine.
[00109] In another embodiments, TDV-4 comprises in addition to the three
attenuating mutations the mutation s),
u) and v), preferably the mutations s), u), v), c), e), x), y) and aa), even
more preferably the mutations s), u), v), c),
e), x), y), aa) and w), even more preferably the mutations s), u), v), c), e),
x), y), aa), w), d), 4, bb) and cc), and
most preferably the mutations s), u), v), c), e), x), y), aa), w), d), A bb),
cc), j), k) and t). The nucleotide positions
and amino acids positions of TDV-4 refer to the nucleotide sequence as shown
in SEQ ID NO. 7 and amino acid
sequence as shown in SEQ ID NO. 8.
[00110] In a preferred embodiment, TDV-1 has the nucleotide sequence of SEQ ID
NO. 1, TDV-2 has the
nucleotide sequence of SEQ ID NO. 3, TDV-3 has the nucleotide sequence of SEQ
ID NO. 5, and/or TDV-4 has the
nucleotide sequence of SEQ ID NO. 7. In a further preferred embodiment, TDV-1
has the amino acid sequence of
SEQ ID NO. 2, TDV-2 has the amino acid sequence of SEQ ID NO. 4, TDV-3 has the
amino acid sequence of SEQ ID
NO. 6, and mV-4 has the amino acid sequence of SEQ ID NO. 8. In a further
preferred embodiment, TDV-1 has a
nucleotide sequence encoding the amino acid sequence of SEQ ID NO. 2, TDV-2
has a nucleotide sequence encoding
the amino acid sequence of SEQ ID NO. 4, TDV-3 has a nucleotide sequence
encoding the amino acid sequence of
SEQ ID NO. 6, and TDV-4 has a nucleotide sequence encoding the amino acid
sequence of SEQ ID NO. 8.
Table 4.Sequences of the TDV virus strains
19
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
SEQ ID NO. dengue virus strain sequence type
SEQ ID NO. 1 TDV-1 nucleotide
sequence
SEQ ID NO. 2 TDV-1 amino acid
sequence
SEQ ID NO. 3 TDV-2 nucleotide
sequence
SEQ ID NO. 4 TDV-2 amino acid
sequence
SEQ ID NO. 5 TDV-3 nucleotide
sequence
SEQ ID NO. 6 TDV-3 amino acid
sequence
SEQ ID NO. 7 TDV-4 nucleotide
sequence
SEQ ID NO. 8 TDV-4 amino acid
sequence
[00111] Thus, in a particularly preferred embodiment, the unit dose of the
invention as described herein comprises
the live attenuated dengue virus strains TDV-1, TDV-2, TDV-3 and TDV-4,
wherein TDV-1, TDV-3 and TDV-4 are
based on TDV-2 and comprise the prM and E regions of DENV-1, -3 and -4,
respectively. In another particularly
preferred embodiment, TDV-1 is characterized by the nucleotide sequence
according to SEQ ID No. 1 and the amino
acid sequence according to SEQ ID No. 2, TDV-2 is characterized by the
nucleotide sequence according to SEQ ID
No. 3 and the amino acid sequence according to SEQ ID No. 4, TDV-3 is
characterized by the nucleotide sequence
according to SEQ ID No. 5 and the amino acid sequence according to SEQ ID No.
6 and TDV-4 is characterized by
the nucleotide sequence according to SEQ ID No. 7 and the amino acid sequence
according to SEQ ID No. 8.
[00112] The E protein of DENV-3 has two fewer amino acids than the E protein
of DENV-2. Therefore, the
nucleotides and encoded amino acid backbone of TDV-2 starting after the E
region of DENV-3 at nucleotide 2374 of
SEQ ID NO. 5 and amino acid 760 of SEQ ID NO. 6 are 6 nucleotides less and 2
amino acids less than the original
TDV-2 nucleotide and amino acid positions, respectively.
Deng' lea varrine rnmpostinn
[00113] The present invention is in part directed to a unit dose of a dengue
vaccine composition as described. The
dengue vaccine composition comprises a tetravalent dengue virus composition,
also referred to as dengue virus
composition, and pharmaceutically acceptable excipients.
lienaue virus composition virus concentrations and %-concentrations
[00114] The present invention is in part directed to a unit dose of a dengue
vaccine composition, wherein the
dengue vaccine composition comprises a tetravalent dengue virus composition
including four live attenuated dengue
virus strains:
(i) a dengue serotype 1 preferably in a concentration of at least 3.3 log10
pfu/0.5 mL,
(ii) a dengue serotype 2 preferably in a concentration of at least 2.7 log10
pfu/0.5 mL,
(iii) a dengue serotype3 preferably in a concentration of at least 4.0 10g10
pfu/0.5 mL, and
(iv) a dengue serotype 4 preferably strain in a concentration of at least 4.5
log10 pfu/0.5 mL.
[00115] In one embodiment, the dengue vaccine composition comprises a
tetravalent dengue virus composition
including four live attenuated dengue virus strains:
(i) a dengue serotype 1 preferably in a concentration of at least 3.3 log10
pfu/0.5 mL to 3.8 log10 pfu/0.5 mL,
(ii) a dengue serotype 2 preferably in a concentration of at least 2.7 log10
pfu/0.5 mL,
(iii) a dengue serotype 3 preferably in a concentration of at least 4.0 log10
pfu/0.5 mL, and
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
(iv) a dengue serotype 4 preferably strain in a concentration of at least 4.5
log10 pfu/0.5 ml or 4.6 log10
pfu/0.5 mL, optionally to 6.2 log10 pfu/0.5 ml.
[00116] The present invention is further in part directed to a unit dose of a
dengue vaccine composition, wherein
the dengue vaccine composition comprises a tetravalent dengue virus
composition including four live attenuated
dengue virus strains:
(i) a chimeric dengue serotype 2/1 strain in a concentration of at least
3.3 l0g10 pfu/0.5 mL,
(ii) a dengue serotype 2 strain in a concentration of at least 2.7 log10
pfu/0.5 mL,
(iii) a chimeric dengue serotype 2/3 strain in a concentration of at least 4.0
log10 pfu/0.5 mL, and
(iv) a chimeric dengue serotype 2/4 strain in a concentration of at least 4.5
log10 pfu/0.5 mL
[00117] In one embodiment, the dengue vaccine composition comprises a
tetravalent dengue virus composition
including four live attenuated dengue virus strains:
(i) a chimeric dengue serotype 2/1 strain in a concentration of at least 3.3
1og10 pfu/0.5 mL to 3.8 log10
pfu/0.5 ml,
(ii) a dengue serotype 2 strain in a concentration of at least 2.7 log10
pfu/0.5 mL,
(iii) a chimeric dengue serotype 2/3 strain in a concentration of at least 4.0
10g10 pfu/0.5 mL, and
(iv) a chimeric dengue serotype 2/4 strain in a concentration of at least 4.5
10910 pfu/0.5 mL or at least 4.6
log10 pfu/0.5 mL to optionally 6.2 log10 pfu/0.5 ml.
[00118] Preferably, the chimeric dengue serotype 2/1 strain is TDV-1, the
dengue serotype 2 strain is TDV-2, the
chimeric dengue serotype 2/3 strain is TDV-3 and the chimeric dengue serotype
2/4 strain is TDV-4.
[00119] In one embodiment, the dengue vaccine composition comprises a
tetravalent dengue virus composition
including four live attenuated dengue virus strains wherein:
(i) the dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 10910 pfu/0.5
mL to 5.3 bg10 pfu/0.5 mL,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 2.7 1og10 pfu/0.5 mL to 5.0
10g10 pfu/0.5 mL,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 1og10 pfu/0.5
mL to 6.0 log10 pfu/0.5 mL, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 4.5 log10 pfu/0.5
mL to 6.5 log10 pfu/0.5 mL.
[00120] In one such embodiment, the dengue vaccine composition comprises a
tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
(i) the dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 1og10 pfu/0.5
mL to 5.0 log10 pfu/0.5 mL,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 2.7 log10 pfu/0.5 mL to 4.9
log10 pfu/0.5 mL,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 1og10 pfu/0.5
mL to 5.7 log10 pfu/0.5 mL, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 4.5 log10 pfu/0.5
mL to 6.2 log10 pfu/0.5 mL.
21
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00121] In a further such embodiment, the dengue vaccine composition comprises
a tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
(i) a dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 log10 pfu/dose
to 5.0 log10 pfu/dose,
(ii) a dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 2.7 log10 pfu/dose to 4.9 bg10
pfu/ dose,
(iii) a dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 log10 pfu/dose
to 5.7 10g10 pfu/ dose, and
(iv) a dengue serotype 4 (e.g. chimeric dengue serotype 2)4 strain) has a
concentration of 4.5 log10 pfu/dose
to 5.5 log10 pfu/dose .
[00122] In a further such embodiment, the dengue vaccine composition comprises
a tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
(i) a dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 log10 pfu/dose
to 4.1 log10 pfu/dose,
(ii) a dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 2.7 10g10 pfu/dose to 3.6 log10
pfu/dose ,
(iii) a dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 log10 pfu/dose
to 4.7 log10 pfu/dose, and
(iv) a dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 4.5 log10 pfu/dose
to 5.3 log10 pfu/dose.
[00123] In a further such embodiment, the dengue vaccine composition comprises
a tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
(i) a dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 log10 pfu/0.5 mL
to 3.6 log10 pfu/0.5 m1_,
(ii) a dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 2.7 log10 pfu/0.5 mL to 4.0
log10 pfu/0.5 mL,
(iii) a dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 log10 pfu/0.5 mL
to 4.6 log10 pfu/0.5 mL, and
(iv) a dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 4.5 1og10 pfu/0.5 ml
or 4.6 log10 pfu/0.5 mL to 5.1 log10 pfu/0.5 mL.
[00124] In another embodiment, the dengue vaccine composition comprises a
tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
(i) the dengue serotype 1 (e.g. chimeric dengue serotype
2/1 strain) has a concentration of 4.3 log10 pfu/0.5
mL to 4.4 log10 pfu/0.5 mL,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 3.7 log10 pfu/0.5 mL to 3.8
log10 pfu/0.5 mL,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.5 log10 pfu/0.5
mL to 5.0 bg10 pfu/0.5 mL, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 5.5 log10 pfu/0.5
mL to 5.6 log10 pfu/0.5 mL.
[00125] In a particularly preferred embodiment, the dengue vaccine composition
comprises a tetravalent dengue
virus composition including four live attenuated dengue virus strains wherein:
22
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
(i) the dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 4.4 log10 pfu/0.5
mL,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 3.8 10g10 pfu/0.5 mL,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.5 10g10 pfu/0.5
mL, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 5.6 log10 pfu/0.5
mL
[00126] In another particularly preferred embodiment, the dengue vaccine
composition comprises a tetravalent
dengue virus composition including four live attenuated dengue virus strains
wherein:
(0 the dengue serotype 1 (e_g_ chimeric dengue serotype 2/1 strain) has a
concentration of 3.6 log10 pfu/0.5
mL,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 4.0 log10 pfu/0.5 mL,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.6 log10 pfu/0.5
mL, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 5.1 log10 pfu/0.5
mL
[00127] In another preferred embodiment, the dengue vaccine composition
comprises a tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein the
arithmetic sum of all four serotypes is
less than 6.7 log10 pfu/0.5 mL, preferably less than 5.5 log10 pfu/0.5 mL. In
certain such embodiments, the
arithmetic sum of all four serotypes is at least 4.6 10g10 pfu/0.5 mL In a
preferred embodiment, the dengue vaccine
composition comprises a tetravalent dengue virus composition including four
live attenuated dengue virus strains
wherein the arithmetic sum of all four serapes is in the range of 4.6 log10
pfu/0.5 mL to 6.7 10g10 pfu/0.5 mL,
preferably in the range of 4.6 log10 pfu/0.5 mL to 5.5 log10 pfu/0.5 mL
[00128] Preferably, in said embodiments the chimeric dengue serotype 2/1
strain is TDV-1, the dengue serotype 2
strain is TDV-2, the chimeric dengue serotype 2/3 strain is TDV-3 and the
chimeric dengue serotype 2/4 strain is
TDV-4. More preferably, TDV-1 is characterized by the nucleotide sequence
according to SEQ ID No. 1 and the amino
acid sequence according to SEQ ID No. 2, TDV-2 is characterized by the
nucleotide sequence according to SEQ ID
No. 3 and the amino acid sequence according to SEQ ID No. 4, TDV-3 is
characterized by the nucleotide sequence
according to SEQ ID No. 5 and the amino acid sequence according to SEQ ID No.
6 and TDV-4 is characterized by
the nucleotide sequence according to SEQ ID No. 7 and the amino acid sequence
according to SEQ ID No. 8.
[00129] The present invention is in part directed to a unit dose of a dengue
vaccine composition, wherein the
dengue vaccine composition comprises a tetravalent dengue virus composition
including four live attenuated dengue
virus strains:
(i) a dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) in a
concentration of at least 33 log10
pfu/dose,
(ii) a dengue serotype 2 (e.g. dengue serotype 2 strain) in a concentration of
at least 2.7 log10 pfu/dose,
(iii) a dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) in a
concentration of at least 4.0 log10
pfu/dose, and
(iv) a dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) in a
concentration of at least 4.5 10910
pfu/dose.
23
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00130] In one embodiment, the dengue vaccine composition comprises a
tetravalent dengue virus composition
including four live attenuated dengue virus strains wherein:
(i) the dengue serotype 1 (e.g. chimeric dengue serotype
2/1 strain) has a concentration of 3.3 logle pfu/dose
to 5.3 log10 pfu/dose,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 22 log10 pfu/dose to 5.0
log10 pfu/dose,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 log1D pfu/dose
to 6.0 log10 pfu/dose, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2)4 strain) has a
concentration of 4.5 log10 pfu/dose
to 6.5 log10 pfu/dose.
[00131] In one such embodiment, the dengue vaccine composition comprises a
tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
(i) the dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 log10 pfu/dose
to 5.0 log10 pfu/dose,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 22 log10 pfu/dose to 4.9
log10 pfu/dose,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 log10 pfu/dose
to 5.7 log10 pfu/dose, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 4.5 log10 pfu/dose
to 6.2 log10 pfu/dose.
[00132] In a further such embodiment, the dengue vaccine composition comprises
a tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
(i) a dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 log10 pfu/dose
to 5.0 log10 pfu/dose,
(ii) a dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 2.7 log10 pfu/dose to 4.9 10g10
pfu/dose,
(iii) a dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 log10 pfu/dose
to 5.7 log10 pfu/dose, and
(iv) a dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 4.5 log10 pfu/dose
to 5.5 log10 pfu/dose.
[00133] In a further such embodiment, the dengue vaccine composition comprises
a tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
(i) a dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 log10 pfu/dose
to 4.1 log10 pfu/dose,
(ii) a dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 2.7 log10 pfu/dose to 3.6 log10
pfu/ dose,
(iii) a dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 log10 pfu/dose
to 4.7 log10 pfu/dose, and
(iv) a dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 4.5 log10 pfu/dose
to 5.3 log10 pfu/dose.
[00134] In a further such embodiment, the dengue vaccine composition comprises
a tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
24
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
(i) a dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 log10 pfu/dose
to 3.6 log10 pfu/dose,
(ii) a dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 2.7 log10 pfu/dose to 4.0 log10
pfu/dose,
(iii) a dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 log10 pfu/dose
to 4.6 log10 pfu/dose, and
(iv) a dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 4.5 log10 pfu/dose
4.6 log10 pfu/dose to 51 log10 pfu/dose.
[00135] In another embodiment, the dengue vaccine composition comprises a
tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein:
(i) the dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain)
has a concentration of 4.3 log10 pfu/dose
to 4.4 log10 pfu/dose,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 3.7 log10 pfu/dose to 3.8
10g10 pfu/dose,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.5 log10 pfu/dose
to 5.0 kig10 pfu/dose, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 5.5 log10 pfu/dose
to 5.6 log10 pfu/dose.
[00136] In a particularly preferred embodiment, the dengue vaccine composition
comprises a tetravalent dengue
virus composition including four live attenuated dengue virus strains wherein:
(i) the dengue sal:type 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 4.4 log10
pfu/dose,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 3.8 log10 pfu/dase,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.5 log10
pfu/dose, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 5.6 10910
pfu/dose.
[00137] In another particularly preferred embodiment, the dengue vaccine
composition comprises a tetravalent
dengue virus composition including four live attenuated dengue virus strains
wherein:
(i) the dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.6 log10
pfu/dose,
(ii) the dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 4.0 log10 pfu/dose,
(iii) the dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.6 log10
pfu/dose, and
(iv) the dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 5.1 log10
pfu/dose.
[00138] In another preferred embodiment, the dengue vaccine composition
comprises a tetravalent dengue virus
composition including four live attenuated dengue virus strains wherein the
arithmetic sum of all four serotypes is
less than 6.7 log10 pfu/dose, preferably less than 5.5 log10 pfu/dose. In
certain such embodiments, the arithmetic
sum of all four serotypes is at least 4.6 log10 pfu/dose. in a preferred
embodiment, the dengue vaccine composition
comprises a tetravalent dengue virus composition including four live
attenuated dengue virus strains wherein the
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
arithmetic sum of all four serotypes is in the range of 4.6 log10 pfu/dose to
6.7 log10 pfu/dose, preferably in the
range of 4.6 log10 pfu/dose to 5.5 log10 pfu/dose.
[00139] In one embodiment in the composition (i), (ii), (iii), and (iv)
provide a total concentration of pfu/0.5 mL
and based on said concentration, the concentration of (iii) at least 10% of
the total concentration in pfu/0.5 mL.
[00140] In one embodiment in the composition (i), (ii), (iii), and (iv)
provide a total concentration of pfu/0.5 mL
and based on said total concentration the concentration of (ii) in pfu/0.5 mL
is less than 10%, and the concentration
of (iv) in pfu/0.5 mL is at least 509'u, and the concentration of (i) in
pfu/0.5 mL is at least 1%, and the concentration
of (iii) in pfu/0.5 mL is at least 6%, or at least 8%, or at least 10%, or at
least 12')/0, or at least 14%, or at least
16%, or at least 18%.
[00141] It is preferred that the concentration in the reconstituted unit dose
of (iii) in pfu/0.5 mL is at least 10%.
[00142] In one embodiment in the composition (i), (ii), (iii), and (iv)
provide a total concentration of pfu/0.5 mL
and based on said total concentration the concentration of (ii) in pfu/0.5 mL
is less than 2%, the concentration of
(iv) in pfu/0.5 mL is at least 50%, the concentration of (i) in pfu/0.5 mL is
at least 1%, and the concentration of (iii)
in pfu/0.5 mL is at least 6%.
[00143] Preferably, in said embodiments the chimeric dengue serotype 2/1
strain is TDV-1, the dengue serotype 2
strain is TDV-2, the chimeric dengue serotype 2/3 strain is TDV-3 and the
chimeric dengue serotype 2/4 strain is
TDV-4. More preferably, TDV-1 is characterized by the nucleotide sequence
according to SEQ ID No. 1 and the amino
acid sequence according to SEQ ID No. 2, TDV-2 is characterized by the
nucleotide sequence according to SEQ ID
No. 3 and the amino acid sequence according to SEQ ID No. 4, TDV-3 is
characterized by the nucleotide sequence
according to SEQ ID No. 5 and the amino acid sequence according to SEQ ID No.
6 and TDV-4 is characterized by
the nucleotide sequence according to SEQ ID No. 7 and the amino acid sequence
according to SEQ ID No. 8.
[00144] The concentration of the different dengue viruses is preferably
determined by an immuno-focus assay
known in the art. For example, the concentration may be determined by an
immuno-focus assay wherein serial
dilutions of dengue virus are applied to monolayers of adherent cells, such as
Vero cells. After a period of time which
allows infectious viruses to bind to the cells and to be taken up by the
cells, an overlay containing thickening agents,
such as agarose or carboxymethylcellulose, is added to prevent diffusion of
viruses so that progeny viruses can only
infect cells adjacent to the original infected cells. After a period of
incubation to allow viral replication, cells are fixed
and stained using serotype-specific anti-dengue monoclonal antibodies and a
secondary antibody such as an
antibody labeled with alkaline phosphatase. The foci are stained by adding a
suitable substrate for- the enzyme
attached to the secondary antibody, such as 5-bromo-4-chbro-3-indolyl-
phosphate/nitro blue tetrazolium
phosphatase substrate. The number of plaques on the plate corresponds to the
plaque forming units of the virus in
the solutions applied to the cells. For example, a concentration of 1,000
pfu/pl indicates that 1 pl of the solution
applied to the cells contains enough viruses to produce 1,000 plaques in a
cell monolayer.
[00145] The dengue vaccine composition comprises a tetravalent dengue virus
composition including four live
attenuated dengue virus strains, wherein a chimeric dengue serotype 2/1
strain, a dengue serotype 2 strain, a
chimeric dengue serotype 2/3 strain, and a chimeric dengue serotype 2/4 strain
provide a total concentration in
pfu/0.5 mL. The term "total concentration in pfu/0.5 mL" or "total
concentration in pfu/dose" is the sum of the
concentrations of the dengue serotype 1 (e.g. chimeric dengue serotype 2/1
strain), dengue serotype 2 (e.g. the
dengue serotype 2 strain), the dengue serotype 3 (e.g. chimeric dengue
serotype 2/3 strain) and the dengue
26
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
serotype 4 (e.g. chimeric dengue serotype 2/4 strain), preferably the sum of
the concentrations of TDV-1, TDV-2,
TDV-3 and TDV-4, and is defined as 100% of the dengue virus concentration as
determined by pfu (plaque forming
units) in 0.5 mL or in a dose.
[00146] In one embodiment, the dengue vaccine composition comprises a
tetravalent dengue virus composition
including four live attenuated dengue virus strains, wherein a dengue serotype
1 (e.g. chimeric dengue serotype 211
strain), a dengue serotype 2 (e.g. dengue serotype 2 strain), a dengue
serotype 3 (e.g. chimeric dengue serotype
2/3 strain), and a dengue serotype 4 (e.g. chimeric dengue serotype 2/4
strain) provide a total concentration in
pfu/0.5 mL, wherein based on said total concentration the concentration of a
dengue serotype 2 (e.g. dengue
serotype 2 strain) measured in pfu/0.5 mL is less than 10% of the total
concentration, or less than 8%, or less than
6% of the total concentration, and wherein the concentration of a dengue
serotype 4 (e.g. chimeric dengue serotype
2/4 strain) measured in pfu/0.5 mL is at least 50% or at least 60% or at least
65% of the total concentration. In one
embodiment, based on said total concentration the concentration of a dengue
serotype 2 (e.g. dengue serotype 2
strain) measured in pfu/0.5 mL is 0.3 to 10% or 0.5 to 8% of the total
concentration and the concentration of a
dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) measured in
pfu/0.5 mL is 50% to 90% or 60% to
88% of the total concentration. This means that the concentration of the
dengue serotype 2 (e.g. dengue serotype 2
strain) is lower than the concentration of the dengue serotype 4 (e.g.
chimeric dengue serotype 2/4 strain).
[00147] In one such embodiment, the concentration of a dengue serotype 1 (e.g.
chimeric dengue serotype 211
strain) measured in pfu/0.5 mL is at least 1% of the total concentration,
and/or the concentration of a dengue
serotype 3 (e.g. chime-ic dengue serotype 2/3 strain) measured in pfu/0.5 mL
is at least 6% of the total
concentration, or at least 7% or 8%, 10%, 12%, 14%, 16% or 18% of the total
concentration. In one such
embodiment, the concentration of a dengue serotype 2 (e.g. chimeric dengue
serotype 2/1 strain) measured in
pfu/0.5 mL is 1% to 7% or 2% to 6% or 2.0% to 5.0% of the total concentration,
and/or the concentration of a
dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) measured in
pfu/0.5 mL is 6% to 25% or 7% to 25%
or 10% to 25% or 18% to 25% of the total concentration. This means that the
concentration of the dengue serotype
1 (e.g. chimeric dengue serotype 2/1 strain) is lower than the concentration
of the dengue serotype 3 (e.g. chimeric
dengue serotype 2/3 strain).
[00148] In a preferred embodiment, the concentration of a dengue serotype 2
strain, such as TDV-2, measured in
pfu/0.5 mL is less than 10% of the total concentration, preferably less than
6% or less than 2%, the concentration of
a dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain), such as TDV-4,
measured in pfu/0.5 mL is at least
50% of the total concentration, preferably at least 65%, the concentration of
a dengue serotype 1 (e.g. chimeric
dengue serotype 2/1 strain), such as TDV-1, measured in pfu/0.5 mL is at least
1% of the total concentration,
preferably between 1% and 7% or 2.0% to 5.0%, and the concentration of a
dengue serotype 3 (e.g. chimeric
dengue serotype 2/3 strain), such as TDV-3, measured in pfu/0.5 mL is at least
6% of the total concentration,
preferably between 6% and 25% or 10% to 25% or 18% to 25%.
[00149] In a further preferred embodiment, a dengue virus composition
comprising a dengue serotype 1 (e.g.
chimeric dengue serotype 2/1 strain), a dengue serotype 2 (e.g. dengue
serotype 2 strain), a dengue serotype 1
(e.g. chimeic dengue serotype 2(3 strain), and a dengue serotype 4 (e.g.
chimeric dengue serotype 2/4 strain), such
as TDV-1, TDV-2, TDV-3 and TDV-4, is provided, wherein the concentration of
the dengue serotype 1 (e.g. chimeric
dengue serotype 2/1 strain) measured in pfu/0.5 mL is at least 1% of the total
concentration, preferably between
1% and 7% or 2.0% and 5.0%, the concentration of the dengue serotype 2 (e.g.
dengue serotype 2 strain)
measured in pfu/0.5 mL is less than 10% of the total concentration, preferably
less than 6% or less than 2% and the
concentration of the dengue serotype 3 (e.g. chimeric dengue serotype 2/3
strain) measured in pfu/0.5 mL is at least
27
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
6% of the total concentration, preferably between 6% and 25% or 10% to 25% or
18% to 25%. It is particularly
preferred that the dengue serotype 4 (e.g. chimeric dengue serotype 2/4
strain) has the highest concentration of all
four dengue serotypes.
[00150] In a further preferred embodiment, the dengue vaccine composition
comprises a tetravalent dengue virus
composition including four live attenuated dengue virus strains, wherein the
concentration of the dengue serotype 1
(e.g. chimeric dengue serotype 2/1 strain) measured in pfu/0.5 mL is 1% to 7%
of the total concentration, the
concentration of the dengue serotype 2 (e.g. dengue serotype 2 strain)
measured in pfu/0.5 mL is less than 8% of
the total concentration, such as in the range of 1% to 8% of the total
concentration, the concentration of the dengue
serotype 3 (e.g. chimeric dengue serotype 2/3 strain) measured in p1u/0.5 mL
is at least 10% of the total
concentration, and the concentration of the dengue serotype 4 (e.g. chimeric
dengue serotype 2/4 strain) measured
in pfu/0.5 mL is at least 65% of the total concentration, such as in the range
of 65% to 80%. In certain such
embodiments, the arithmetic sum of all four serotypes is in the range of 4.6
log10 pfu/0.5 mL to 6.7 log10 pfu/0.5
mL, preferably in the range of 4.6 log10 pfu/0.5 mL to 5.5 log10 pfu/0.5 mL.
[00151] In a further preferred embodiment the dengue serotype 1 (e.g. chimeric
dengue serotype 2/1 strain) such
as TDV-1 and the dengue serotype 2 (e.g. dengue serotype 2 strain) such as TDV-
2 are present each in a
concentration based on the total concentration in pfu/0.5 mL which is within
5%-points of each other and/or are
together less than about 10% of the total concentration in pfu/0.5 mL. In
certain such embodiments the dengue
serotype 3 (e.g. chimeric dengue serotype 2/3 strain) such as TDV-3 is
preferably at least about 10% of the total
concentration in pfu/0.5 mL and more preferably the dengue serotype 4 (e.g.
chimeric dengue serotype 2/4 strain)
such as TDV-4 is at least about 70% of the total concentration in pfu/0.5 mL
In certain such embodiments the
dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) such as TDV-4
represents the highest concentration in
the composition of all four serotypes, preferably with at least about 70% of
the total concentration in pfu/0.5 mL,
dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) such as TDV-3
represents the second highest
concentration in the composition of all four serotypes, preferably with at
least about 10% of the total concentration
in pfu/0.5 mL, and dengue serotype 1 (e.g. chimeric dengue serotype 2/1
strain) such as TDV-1 and dengue
serotype 2 (e.g. dengue serotype 2 strain) such as TDV-2 each represent lower
concentrations than the
concentration of serotype 3 (e.g. chimeric dengue serotype 2/3 strain) such as
TDV-3, and optionally together
represent less than about 10% of the total concentration in pfu/0.5 mL
[00152] Preferably, in said embodiments the chimeric dengue serotype 2/1
strain is TDV-1, the dengue serotype 2
strain is TDV-2, the chimeric dengue serotype 2/3 strain is TDV-3 and the
chimeric dengue serotype 2/4 strain is
TDV-4. More preferably, TDV-1 is characterized by the nucleotide sequence
according to SEQ ID No. 1 and the amino
acid sequence according to SEQ ID No. 2, 1DV-2 is characterized by the
nucleotide sequence according to SEQ ID
No. 3 and the amino acid sequence according to SEQ ID No. 4, TDV-3 is
characterized by the nucleotide sequence
according to SEQ ID No. 5 and the amino acid sequence according to SEQ ID No.
6 and TDV-4 is characterized by
the nucleotide sequence according to SEQ ID No. 7 and the amino acid sequence
according to SEQ ID No. 8.
[00153] According to a further embodiment, the chimeric dengue serotype 2/4
strain, preferably TDV-4, has the
highest concentration in the dengue vaccine composition, followed by the
chimeric dengue serotype 2/3 strain,
preferably TDV-3, followed by the chimeric dengue serotype 2/1 strain,
preferably TDV-1, followed by the dengue
serotype 2 strain, preferably TDV-2. It is particularly preferred that the
dengue serotype 2 strain has the lowest
concentration of the four strains present in the dengue vaccine composition.
28
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00154] Whenever reference is made to a concentration/0.5m1, this does not
limit the volume of the unit dose
described herein to 0.5m1. 0.5m1 is the reference volume for the determination
of the concentrations of the virus
strains in the composition in pfu/ml. The volume and/or amount per unit dose
is described in the respective chapter.
Pharmaceutically acceptable excioients
[00155] The present invention is in part directed to a unit dose of a dengue
vaccine composition, wherein the
dengue vaccine composition comprises one or more pharmaceutically acceptable
excipients. In one embodiment, the
dengue vaccine composition comprises a non-reducing sugar, a surfactant, a
protein and an inorganic salt.
Preferably, the non-reducing sugar is trehalose, the surfactant is poloxamer
407, the protein is human serum
albumin and the inorganic salt is sodium chloride.
[00156] In one embodiment, the unit dose of a dengue vaccine composition
comprises the following
pharmaceutically acceptable excipients:
- from about 10 % w/v to about 20 % w/v o,o-trehalose dihydrate or an
equimolar amount of other forms of
a,o-trehalose,
- from about 0.5 % w/v to about 1.5 A) w/v poloxamer
407,
- from about 0.05 A) w/v to about 2 % w/v human serum albumin, and
- from about 70 mM to 140 mM sodium chloride.
[00157] In one embodiment, the unit dose of a dengue vaccine composition
comprises the following
pharmaceutically acceptable excipients when measured in 0.5 ml:
- from about 10 A) w/v to about 20 % w/v oda-trehalose or an equimolar
amount of other forms of ato-
trehalose,
- from about 0.5 % w/v to about 1.5 % w/v poloxamer 407,
- from about 0.05 % w/v to about 2 % w/v human serum albumin, and
- from about 70 mM to 140 mM sodium chloride, and preferably
- has a pH of 7 to 8.5.
[00158] In one embodiment, the unit dose of a dengue vaccine composition
comprises the following
pharmaceutically acceptable excipients when measured in 0.5m1:
- from about 143 mg/ml to about 185 mg/ml an-trehalose
dihydrate or an equimolar amount of other forms
of o,o-trehalose,
- from about 9.1 mg/ml to about 12.4 mg/ml poloxamer 407,
- from about 0.88 % mg/ml to about 1.32 mg/ml human serum albumin, and
- from about 70 mM to 140 mM sodium chloride, and
preferably
- has a pH of 7 to 8.5.
[00159] In a preferred embodiment, the lyophilized unit dose of the invention
as described herein comprises the
following pharmaceutically acceptable excipients:
- about 15 % w/v o,a-trehalose dihydrate,
- about 1 % w/v poloxamer 407,
- about 0.1 % w/v human serum albumin, and
- about 100 mM sodium chloride.
29
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00160] In a preferred embodiment, the lyophilized unit dose of the invention
as described herein comprises the
following pharmaceutically acceptable excipients when measured in 0.5m1:
- about 15 % w/v o,a-trehalose,
- about 1 % w/v poloxamer 407,
- about 0.1 % w/v human serum albumin, and
- about 100 mM sodium chloride.
[00161] In a preferred embodiment, the lyophilized unit dose of the invention
as described herein comprises the
following pharmaceutically acceptable excipients:
- about 82.9 mg a,a-trehalose dihydrate,
- about 5 mg poloxamer 407,
- about 0.5 mg human serum albumin, and
- about 50 pmoles sodium chloride.
[00162] In a preferred embodiment, the reconstituted unit dose of the
invention as described herein comprises the
following pharmaceutically acceptable excipients:
- about 15 % w/v o,a-trehalose dihydrate,
- about 1 % w/v poloxamer 4137,
- about 0.1 % w/v human serum albumin, and
- about 137 mM sodium chloride, and preferably
- has a pH of 7 to 8.5
[00163] In a preferred embodiment, the reconstituted unit dose of the
invention as described herein comprises the
following pharmaceutically acceptable excipients when measured in 0.5 ml:
- about 15 % w/v ota-trehalose,
- about 1 % w/v poloxamer 407,
- about 0.1 % w/v human serum albumin, and preferably
- about 137 mM sodium chloride and preferably
- has a pH of 7 to 8.5.
[00164] In a preferred embodiment, the reconstituted unit dose of the
invention as described herein comprises the
following pharmaceutically acceptable excipients:
- about 82.9 mg o,o-trehalose dihydrate,
- about 5 mg poloxamer 407,
- about 0.5 mg human serum albumin, and preferably
- about 68.5 pmoles sodium chloride, and preferably
- has a pH of 7 to 8.5.
[00165] The human serum albumin may be a native or recombinant human serum
albumin (rHSA). The poloxamer
407 may be e.g. Pluronic F127.
[00166] In one embodiment, the unit dose further comprises a buffer. The
buffer may be phosphate buffered
saline (PBS). The buffer may include at least one of sodium chloride (NaCI),
monosodium dihydrogen phosphate
(NaH2PO4), disodium hydrogen phosphate (Na2HPO4), potassium chloride (KCl),
and potassium dihydrogen
phosphate (KH2PO4). In a preferred embodiment, the buffer may include disodium
hydrogen phosphate (Na2HPa4),
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
potassium chloride (KCI), and potassium dihydrogen phosphate (KH2PO4). The
buffer may have a pH in the range of
7.0 to 8.5 at 25 C.
Unit dose
[00167] The present invention is directed in part to a unit dose of a dengue
vaccine composition comprising a
tetravalent dengue virus composition as described herein and pharmaceutically
acceptable excipients as described
herein.
[00168] The present invention is directed in part to a unit dose of a dengue
vaccine composition as described
above e.g. of
(i) a dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) with a
concentration of at least 33 log10
pfu/0.5 mL,
(ii) a dengue serotype 2 (e.g. dengue serotype 2 strain) with a concentration
of at least 2.7 log10 pfu/0.5 mt.,
(iii) a dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) with a
concentration of at least 4.0 log10
pfu/0.5 mL, and
(iv) a dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) with a
concentration of at least 4.5 10910
pfu/0.5 mL.
[00169] Preferably, the chimeric dengue serotype 2/1 strain is TDV-1, the
dengue serotype 2 strain is TDV-2, the
chimeric dengue serotype 2/3 strain is TDV-3, and the chimeric dengue serotype
2/4 strain is TDV-4. More
preferably, TDV-1 is characterized by the nucleotide sequence according to SEQ
ID No. 1 and the amino acid
sequence according to SEQ ID No. 2, TDV-2 is characterized by the nucleotide
sequence according to SEQ ID No. 3
and the amino acid sequence according to SEQ ID No. 4, TDV-3 is characterized
by the nucleotide sequence
according to SEQ ID No. 5 and the amino acid sequence according to SEQ ID No.
6 and TDV-4 is characterized by
the nucleotide sequence according to SEQ ID No. 7 and the amino acid sequence
according to SEQ ID No. 8.
[00170] In one embodiment, the unit dose is lyophilized. In one such
embodiment, the lyophilized unit dose is
obtained by subjecting a volume of 0.5 mL of the aqueous dengue vaccine
composition produced by combining
pharmaceutically acceptable excipients as described herein and the dengue
vaccine composition as described herein
comprising the four dengue virus strains, in particular TDV-1 to TDV-4, to
lyophilization. In a preferred embodiment
the residual moisture content as determined by Karl Fischer Determination is
equal to or less than 5.0%, preferably
equal to or less than 3%.
[00171] In another embodiment, the unit dose is reconstituted. The
reconstituted unit dose is obtained by
subjecting the lyophilized unit dose to reconstitution with a pharmaceutically
acceptable diluent, preferably before
administration of the dengue vaccine. In one such embodiment, reconstitution
will be accomplished by adding a
pharmaceutically acceptable diluent, such as water for injection, phosphate
buffered saline or an aqueous sodium
chloride solution, to the lyophilized unit dose. In one embodiment, an aqueous
sodium chloride solution, such as a 37
mM aqueous sodium chloride solution, is added to the lyophilized unit dose for
reconstitution. In one such
embodiment, the lyophilized unit dose will be reconstituted with 0.3 to 0.8
mL, or 0.4 to 0.7 mL, or 0.5 mL of diluent.
In a preferred embodiment, the lyophilized unit dose is reconstituted with 0.3
to 0.8 mL, 0.4 to 0.7 mL or 0.5 mL of
37 mM aqueous sodium chloride solution. In a more preferred embodiment, the
lyophilized unit dose is reconstituted
with 0.5 mL of 37 mM aqueous sodium chloride solution. The reconstituted unit
dose can subsequently be
administered subcutaneously.
31
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00172] It is preferred that the unit dose in lyophilized form is the final
product after manufacture of the unit dose
and the storage form of the unit dose, wherein the unit dose in reconstituted
form is prepared before administration
of the unit dose to a subject.
[00173] The present invention is, moreover, directed in part to a unit dose of
a dengue vaccine composition
comprising:
a tetravalent virus composition including four live attenuated dengue virus
strains, wherein the unit dose is
lyophilized and upon reconstitution with 0.5 mL of a pharmaceutically
acceptable diluent comprises:
(i) a dengue serotype 1, such as a chimeric dengue serotype 2/1 strain, in a
concentration of at least 3.3 log10
pfu/0.5 ml,
(ii) a dengue serotype 2, such as a dengue serotype 2 strain, in a
concentration of at least 2.7 log10 pfu/0.5
ml,
(iii) a dengue serotype 3, such as a chimeric dengue serotype 2/3 strain, in a
concentration of at least 4.0 log10
pfu/0.5 ml, and
(iv) a dengue serotype 4, such as a chimeric dengue serotype 2/4 strain, in a
concentration of at least 4.5
log10 pfu/0.5 ml.
[00174] In one embodiment, the reconstituted unit dose has a volume of e.g.
0.5 mL, wherein upon reconstitution
with a pharmaceutically acceptable diluent (i), (ii), (iii), and (iv) provide
a total concentration of pfu/0.5 mL and
based on said concentration, the concentration of (iii) at least 10% of the
total concentration in pfu/0.5 mL.
[00175] In another embodiment the reconstituted unit dose has a volume of e.g.
0.5 mL, wherein upon
reconstitution with a pharmaceutically acceptable diluent (i), (ii), (iii),
and (iv) provide a total concentration of
pfu/0.5 mL and based on said total concentration the concentration of (ii) in
p4i/0.5 mL is less than 10%, and the
concentration of (iv) in pfu/0.5 mL is at least 50%, and the concentration of
(i) in pfu/0.5 mL is at least 1%, and the
concentration of (iii) in pfu/0.5 mL is at least 6%, or at least 8%, or at
least 10%, or at least 12%, or at least 14%,
or at least 16%, or at least 18%.
[00176] It is preferred that the concentration in the reconstituted unit dose
of (iii) in pfu/0.5 mL is at least 10%.
[00177] In one embodiment the reconstituted unit dose has a volume of e.g. 0.5
mL, wherein upon reconstitution
with a pharmaceutically acceptable diluent (i), (ii), (iii), and (iv) provide
a total concentration of pfu/0.5 mL and
based on said total concentration the concentration of (ii) in pfu/0.5 mL is
less than 2%, the concentration of (iv) in
pfu/0.5 mL is at least 50%, the concentration of (i) in pfu/0.5 mL is at least
1%, and the concentration of (iii) in
pfu/0.5 mL is at least 6%.
[00178] In one embodiment, the present invention is directed to a lyophilized
unit dose of a dengue vaccine
composition comprising upon reconstitution with 0.5 mL of a pharmaceutically
acceptable diluent a dengue serotype
1 (e.g. chimeric dengue serotype 2/1 strain) with a concentration of at least
3.3 10g10 pfu/0.5 mL, a dengue
serotype 2 (e.g. dengue serotype 2 strain) with a concentration of at least
2.7 log10 pfu/0.5 mL, a dengue serotype
3 (e.g. chimeric dengue serotype 2/3 strain) with a concentration of at least
4.0 log10 pfu/0.5 mL, and a dengue
serotype 4 (e.g. chimeric dengue serotype 2/4 strain) with a concentration of
at least 4.5 log10 pfu/0.5 mL and
pharmaceutically acceptable excipients as described herein, wherein the unit
dose is preferably formulated in 0.5 mL
32
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
before lyophilization. Preferably, the chimeric dengue serotype 2/1 strain is
TDV-1, the dengue serotype 2 strain is
TDV-2, the chimeric dengue serotype 2/3 strain is TDV-3 and the chimeric
dengue serotype 2/4 strain is TDV-4. More
preferably, TDV-1 is characterized by the nucleotide sequence according to SEQ
ID No.. 1 and the amino acid
sequence according to SEQ ID No. 2, TDV-2 is characterized by the nucleotide
sequence according to SEQ ID No. 3
and the amino acid sequence according to SEQ ID No. 4, TDV-3 is characterized
by the nucleotide sequence
according to SEQ ID No. 5 and the amino acid sequence according to SEQ ID No.
6 and TDV-4 is characterized by
the nucleotide sequence according to SEQ ID No. 7 and the amino acid sequence
according to SEQ ID No. 8.
[00179] In one such embodiment, the lyophilized unit dose is obtained by
lyophilizing 0.5 mL of a dengue vaccine
composition comprising a dengue serotype 1 (e.g. chimeric dengue serotype 2/1
strain) in a concentration of 3.3
log10 pfu/dose to 5.0 log10 pfu/0.5 mL, a dengue serotype 2 (e.g. dengue
serotype 2 strain) in a concentration of
2.7 log10 pfu/dose to 4.9 log10 pfu/0.5 mL, a dengue serotype 3 (e.g. chime-lc
dengue serotype 2/3 strain) in a
concentration of 4.0 log10 pfu/dose to 5.7 log10 pfu/0.5 mL, and a dengue
serotype 4 (e.g. chimeric dengue
serotype 2/4 strain) in a concentration of 4.5 log10 pfu/dose to 5.5 log10
pfu/0.5 mL and pharmaceutically
acceptable excipients as described herein. Preferably, the chimeric dengue
serotype 2/1 strain is TDV-1, the dengue
serotype 2 strain is 1DV-2, the chime-ic dengue se-otype 2/3 strain is TDV-3
and the chimeric dengue serotype 2/4
strain is TDV-4.
[00180] In one such embodiment, the lyophilized unit dose is obtained by
lyophilizing 0.5 mL of a dengue vaccine
composition comprising a dengue serotype 1 (e.g. chimeric dengue serotype 2/1
strain) in a concentration of 3.3
log10 pfu/0.5 mL to 3.6 log10 pfu/0.5 mL, a dengue serotype 2 (e.g. dengue
serotype 2 strain) in a concentration of
2.7 log10 pfu/0.5 mL to 4.0 log10 pfu/0.5 m1_, a dengue serotype 3 (e.g.
chimeric dengue serotype 2/3 strain) in a
concentration of 4.0 log1D pfu/0.5 mL to 4.6 10g10 pfu/0.5 mL, and a dengue
serotype 4 (e.g. chimeric dengue
serotype 2/4 strain) in a concentration of 4.5 log10 pfu/0.5 mL or 4.6 log10
pfu/0.5 mL to 5.1 log10 pfu/0.5 mL and
pharmaceutically acceptable excipients as described herein. Preferably, the
chimeric dengue serotype 2/1 strain is
TDV-1, the dengue serotype 2 strain is TDV-2, the chimeric dengue serotype
2/3 strain is TDV-3 and the chimeric
dengue serotype 2/4 strain is TDV-4.
[00181] In certain embodiments, the lyophilized unit dose refers to 0.5 mL
before lyophilization, wherein TDV-2
and TDV-4 are present in certain relative amounts, based on the total
concentration of TDV-1, TDV-2, TDV-3 and
TDV-4 in pfu/0.5 mL, and the concentration of TDV-2 measured in pfu/0.5 mL is
less than 10% or less than 8% or
less than 6%, and the concentration of TDV-4 measured in pfu/0.5 mL is at
least 50% or at least 65%. In some of
these embodiments, the concentration of TDV-1 measured in pfu/0.5 mL is at
least 1% and/or the concentration of
TDV-3 measured in pfu/0.5 mL is at least 6%, 7%, 8%, 10%, 12%, 14%, 16% or at
least 18%.
[00182] In certain embodiments, the reconstituted unit dose has a volume of
0.5 mL and TDV-2 and TDV-4 are
present in certain relative amounts, based on the total concentration of mV-1,
TDV-2, TDV-3 and TDV-4 in pfu/0.5
m1_, and the concentration of TDV-2 measured in pfu/0.5 mL is less than 10% or
less than 8% or less than 6%, and
the concentration of TDV-4 measured in pfu/0.5 mL is at least 50% or at least
65%. In some of these embodiments,
the concentration of TDV-1 measured in pfu/0.5 mL is at least 1% and/or the
concentration of TDV-3 measured in
pfu/0.5 mL is at least 6%, 7%, 8%, 10%, 12%, 14%, 16% or at least 18%.
[00183] In a further piefei
_______________________________________________________________________________
____________________ ed embodiment, the reconstituted unit dose has a volume
of 0.5 mL and comprises a
tetravalent dengue virus composition including four live attenuated dengue
virus strains, wherein the concentration
of the dengue serotype 1 (e.g. dengue serotype 2/1 strain) measured in pfu/0.5
mL is 1% to 7% of the total
concentration, the concentration of the dengue serotype 2 (e.g. dengue
serotype 2 strain) measured in pfu/0.5 mL is
33
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
less than 8% of the total concentration, such as in the range of 1% to 8% of
the total concentration, the
concentration of the dengue serotype 3 (e.g. dengue serotype 2/3 strain)
measured in pfu/0.5 mL is at least 1130/0 of
the total concentration, and the concentration of the dengue serotype 4 (e.g.
dengue serotype 2/4 strain) measured
in pfu/0.5 mL is at least 65% of the total concentration, such as in the range
of 65% to 80%. In certain such
embodiments, the arithmetic sum of all four serotypes is in the range of 4.6
log10 pfu/0.5 mL to 6.7 log10 pfu/0.5
mL, preferably in the range of 4.6 log10 pfu/0.5 mL to 5.5 log10 p41/0.5 mL.
[00184] In a further preferred embodiment, the reconstituted unit dose has a
volume of 0.5 mL and comprises a
tetravalent dengue virus composition including four live attenuated dengue
virus strains, wherein the dengue
serotype 1 (e.g. chimeric dengue serotype 2/1 strain) such as TDV-1 and the
dengue serotype 2 (e.g. dengue
serotype 2 strain) such as TDV-2 are present each in a concentration based on
the total concentration in pfu/0.5 mL
which is within 5%-points of each other and/or are together less than about
10% of the total concentration in
pfu/0.5 ml... In certain such embodiments the dengue serotype 3 (e.g. chimeric
dengue serotype 2/3 strain) such as
TDV-3 is preferably at least about 10 k of the total concentration in pfu/0.5
mL and more preferably the dengue
serotype 4 (e.g. chimeric dengue serotype 2/4 strain) such as TDV-4 is at
least about 70% of the total concentration
in pfu/0.5 mi.. In certain such embodiments the dengue serotype 4 (e.g.
chimeric dengue serotype 2/4 strain) such
as TDV-4 represents the highest concentration in the composition of all four
serotypes, preferably with at least about
70% of the total concentration in pfu/i).5 mL, dengue serotype 3 (e.g.
chimeric dengue serotype 2/3 strain) such as
TDV-3 represents the second highest concentration in the composition of all
four serotypes, preferably with at least
about 10% of the total concentration in pfu/0.5 mL, and dengue serotype 1
(e.g. chimeric dengue serotype 2/1
strain) such as TDV-1 and dengue serotype 2 (e.g. dengue serotype 2 strain)
such as TDV-2 each represent lower
concentrations than the concentration of serotype 3 (e.g. chimeric dengue
serotype 2/3 strain) such as TDV-3, and
optionally together represent less than about 10% of the total concentration
in pfu/0.5 mL.
[00185] The lyophilized unit dose reconstituted in 0.5 mL will provide the
above concentrations for the four dengue
serotypes. While the unit dose of a dengue vaccine composition as described
herein refers to the concentrations of
the dengue serotypes in 0.5 mL, the lyophilized unit dose can be reconstituted
with other volumes of a
pharmaceutically acceptable diluent, such as an aqueous sodium chloride
solution, without changing the absolute
virus amount administered or the ratios of the viruses to one another.
[00186] In certain embodiments, the lyophilized unit dose of the invention is
prepared from a solution comprising
a non-reducing sugar, a surfactant, a protein and an inorganic salt.
[00187] In certain embodiments, the lyophilized unit dose of the invention is
prepared from a solution comprising
trehalose, poloxamer 407, human serum albumin and sodium chloride.
[00188] In certain embodiments, the lyophilized unit dose of the invention is
prepared from a solution comprising
about 10 % w/v to about 20 % w/v a,a-trehalose dihydrate or an equimolar
amount of other forms of a,a-trehalose,
from about 0.5 % w/v to about 1.5 A) w/v poloxamer 407, from about 0.05 % w/v
to about 2 % w/v human serum
albumin, and about 70 mM to about 120 mM sodium chloride
[00189] In preferred embodiments, the lyophilized unit dose of the invention
as described herein is prepared from
a solution comprising about 15 % w/v a,a-trehalose dihydrate, about 1 % w/v
poloxamer 407, about 0.1 % w/v
human serum albumin and about 100 mM sodium chloride.
34
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00190] In one embodiment, the solution from which the lyophilized unit dose
is prepared further comprises a
buffer. The buffer may be phosphate buffered saline (PBS). The buffer may
include at least one of sodium chloride
(NaCI), monosodium dihydrogen phosphate (NaH2PO4), disodium hydrogen phosphate
(Na2HPO4), potassium chloride
(KCI), and potassium dihydrogen phosphate (KH2PO4). In a preferred embodiment,
the buffer may include disodium
hydrogen phosphate (Na2HPO4), potassium chloride (KCI), and potassium
dihydrogen phosphate (K112PO4). The
buffer may have a pH in the range of about 7.0 to about 8.5 at 25 C or a pH of
about 6.8 to about 7.6 at 25 C,
preferably a pH of about 7.2 at 25 C.
[00191] In preferred embodiments, the reconstituted unit dose of the invention
as described herein comprising
about 15 0/0 w/v ci,a-trehalase dihydrate, about 1 A) w/v poloxamer 407,
about 0.1 Ai w/v human serum albumin
and about 137 mM sodium chloride. The reconstituted unit dose may have a pH of
about 7.0 to about 8.5 at 25 C,
preferably a pH of about 7.2 at 25 C.
[00192] The unit dose of the invention as described herein activates multiple
arms of the immune system ¨
neutralizing antibodies, cellular immunity and anti-NS1 antibodies ¨ in both
seronegative and seropositive subject
populations or in bath seronegative and seropositive subjects. Thus, the unit
dose of the invention as described
herein protects both dengue seronegative and dengue seropositive subject
populations or subjects against dengue
disease.
[00193] In one embodiment, one unit dose is present in a container, preferably
a vial, and said unit dose is
administered to a subject after reconstitution. In one embodiment, more than
one unit dose of the dengue vaccine
composition may be present in a container, preferably a vial, so that with the
content of one container, preferably a
vial, more than one subject can be vaccinated. In one embodiment, the
container comprising more than one unit
doses of the invention as described herein is used for providing the
reconstituted unit dose to be used in the
methods of the invention as described herein.
[00194] The certain embodiments, the container comprising the unit dose of the
invention is part of a kit. Thus,
the invention is directed in part to a kit for preparing a reconstituted unit
dose comprising a lyophilized unit dose of
the present invention as described herein, and a pharmaceutically acceptable
diluent for reconstitution.
[00195] In certain embodiments, the diluent for reconstitution provided in a
container, preferably a vial, or a pre-
filled syringe. In some embodiments, the diluent for reconstitution is
selected from water for injection, phosphate
buffered saline or an aqueous sodium chloride solution. In a preferred
embodiment, the diluent for reconstitution is
to 40 mM sodium chloride, such as 37 mM sodium chloride.
30
[00196] In certain embodiments, the kit
may further comprise a hepatitis A vaccine, such as HAVRIXO or
VAQTA@. In some embodiments, the hepatitis A vaccine may be in a separate
container, such as a vial. In another
embodiment, the hepatitis A vaccine and the unit dose of the invention may be
in the same container. Thus, the
invention is directed in part to a combined dengue/hepatitis A vaccine,
wherein the unit dose of the invention as
described herein is combined with a hepatitis A vaccine. Such a combined
dengue/hepatitis A vaccine comprises the
unit dose of the invention as described herein and a hepatitis A vaccine, such
as HAVRIXO or VAQTAC), in the same
formulation. In certain embodiments, the invention is directed to a kit
comprising such a combined dengue/hepatitis
A vaccine and a unit dose of the invention as described herein.
Hepatitis A varrine
[00197] In certain embodiments, the hepatitis A vaccine is an inactivated
hepatitis A vaccine.
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00198] In certain embodiments, the hepatitis A vaccine comprises a hepatitis
A virus derived from a hepatitis A
virus strain HM-175.
[00199] In certain embodiments, wherein the hepatitis A vaccine comprises an
inactivated hepatitis A virus and the
inacfivated hepatitis A virus is derived from a wild-type hepatitis A virus
strain HM-175.
[00200] In certain embodiments, the inactivated hepatitis A virus is adsorbed
on a carrier aluminum. In some of
these embodiments, the aluminum is aluminum hydroxide or aluminum
hydroxyphosphate sulfate.
[00201] In certain embodiments, wherein the hepatitis A vaccine comprises a
phosphate-buffered saline solution
and excipients dissolved therein in the form of an amino acid and in the form
of polysorbate. In such embodiments,
the amino acid is present at a concentration of 0.2 to 0.8% w/v and/or the
polysorbate is present at a concentration
of 0.01 to 0.09 mg/ml.
[00202] In certain embodiments, the hepatitis A vaccine includes a hepatitis A
virus expressing a viral antigen in a
concentration ranging from 500 ELISA Units (EL.U.) to 2000 ELISA Units (ELU.),
preferably from 700 ELU_ to 1600
EL.U., most preferably from 1300 to 1550 FLU__ Alternatively, the
concentration ranges from 500 ELU. to 900 FLU..
In a further embodiment, the concentration ranges from 200 to 400 ELU.
[00203] In certain embodiments, the hepatitis A vaccine is included in a
liquid 1 ml dose or in a 0.5 ml dose.
[00204] An example of such an hepatitis A vaccine is HAVRIX , from
GlaxoSmithKline, which is a sterile
suspension of inactivated virus for intramuscular administration. HAVRIXO
makes use of the hepatitis A virus strain
HM-175 which is derived from a wild-type hepatitis A virus (HAV) HM-175 of
which the complete nucleotide sequence
is disclosed in Cohen et al., Journal of Virology, Vol. 61, No. 1, published
Jan. 1987, p. 50 to 59 (in particular, the
entire sequence of the wild-type hepatitis A virus HM-175 is provided in
Figure 1 of said publication).
[00205] The virus (strain HM175) is propagated in MRC-5 human diploid cells.
After removal of the cell culture
medium, the cells are lysed to form a suspension. This suspension is purified
through uttrafittration and gel
permeation chromatography procedures. Treatment of this lysate with formalin
ensures viral inactivation. Viral
antigen activity is referenced to a standard using an enzyme linked
immunosorbent assay (ELISA), and is therefore
expressed in terms of ELISA Units (ELU.). Each 1-mL dose for adults
18 years of age) of vaccine contains 1140
EL.U. of viral antigen, adsorbed on 0.5 mg of aluminum as aluminum hydroxide.
Each 0.5-mL dose for children and
adolescents (12 months through 18 years of age) of vaccine contains 720 ELU.
of viral antigen, adsorbed onto 0.25
mg of aluminum as aluminum hydroxide. HAVRDC) contains the following
excipienis: Amino acid supplement (0.304
w/v) in a phosphate-buffered saline solution and polysorbate 20 (0.05 mg/mL).
From the manufacturing process,
HAVR1YO also contains residual MRC-5 cellular proteins (not more than 5
pg/mL), formalin (not more than 0.1
mg/mL), and neomycin sulfate (not more than 40 ng/mL), an aminoglycoside
antibiotic included in the cell growth
media. HAVRIX is formulated without preservatives.
[00206] Another useful hepatitis A vaccine is VAQTA from Merck Sharp & Dohrne
Corp., which is an inactivated
whole virus vaccine derived from hepatitis A virus grown in cell culture in
human MRC-5 diploid fibroblasts. It
contains inactivated virus of a strain, which was originally derived by
further serial passage of a proven attenuated
strain. The virus is grown, harvested, purified by a combination of physical
and high performance liquid
chromatographic techniques developed at the Merck Research Laboratories,
formalin inactivated, and then adsorbed
onto amorphous aluminum hydroxyphosphate sulfate. VAQTAO is a sterile
suspension for intramuscular injection.
One milliliter of the vaccine contains approximately 50 U of hepatitis A virus
antigen, which is purified and formulated
36
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
without a preservative. Within the limits of current assay variability, the 50
Ii dose of VAQTA contains less than 0.1
pg of non-viral protein, less than 4 x 1D-6 pg of DNA, less than 104 pg of
bovine albumin, and less than 0.8 pg of
formaldehyde. Other process chemical residuals are less than 10 pads per
billion (ppb), including neomycin. Each
0.5-mL pediatric dose contains 25 U of hepatitis A virus antigen and adsorbed
onto approximately 0.225 mg of
aluminum provided as amorphous aluminum hydroxyphosphate sulfate, and 35 pg of
sodium borate as a pH
stabilizer, in 0.9% sodium chloride. Each 1-mL adult dose contains 50 U of
hepatitis A virus antigen and adsorbed
onto approximately 0.45 mg of aluminum provided as amorphous aluminum
hydroxyphosphate sulfate, and 70 pg of
sodium borate as a pH stabilizer, in 0.9% sodium chloride.
Yellow Fever varrine
[00207] YF-VAX , a yellow fever vaccine from Sanofi, for subcutaneous use, is
prepared by culturing the YF-17D
strain of yellow fever virus in living avian leukosis virus-free (ALV-free)
chicken embryos. The vaccine contains
sorbitol and gelatin as a stabilizer and is lyophilized. No preservative is
added. YF-VAX is formulated to contain not
less than 4/4 log10 pfu per 0.5 mLdose throughout the life of the product.
Combined Vaccine Composition
[00208] The present invention is also directed in part to a combined vaccine
composition comprising a hepatitis A
antigen as in HAVRDC or VAQTAO, and a dengue antigen such as the tetravalent
dengue vaccine, TDV, as
disclosed herein or any other suitable tetravalent live attenuated dengue
virus vaccine.
[00209] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
dengue vaccine composition comprises a tetravalent dengue virus composition
including four live attenuated dengue
virus strains:
(i) a dengue serotype 1 preferably in a concentration of at least 3.3 log10
pfu/0.5 mL,
(ii) a dengue serotype 2 preferably in a concentration of at least 2.7 log10
pfu/0.5 mL,
(iii) a dengue serotype3 preferably in a concentration of at least 4.0 log10
pfu/0.5 mL, and
(iv) a dengue serotype 4 preferably strain in a concentration of at least 4.5
log10 pfu/0.5 mL.
[00210] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
dengue vaccine composition comprises a tetravalent dengue virus composition
including four live attenuated dengue
virus strains:
(i) a chimeric dengue serotype 2/1 strain in a concentration of at least 3.3
log10 pfu/0.5 mL to 3.8 log10
pfu/0.5 ml,
(ii) a dengue serotype 2 strain in a concentration of at least 17 log10
pfu/0.5 mL,
(iii) a chimeric dengue serotype 2/3 strain in a concentration of at least 4.0
10g10 pfu/0.5 mL, and
(iv) a chimeric dengue serotype 2/4 strain in a concentration of at least 4.5
log10 pfu/0.5 mL or at least 4.6
log10 pfu/0.5 mL to optionally 6.2 log10 pfu/0.5 mi.
[00211] Preferably, in said embodiments the chimeric dengue serotype 2/1
strain is 1DV-1, the dengue serotype 2
strain is TOV-2, the chimeric dengue serotype 2/3 strain is TDV-3 and the
chimeric dengue serotype 2/4 strain is
TDV-4. More preferably, TDV-1 is characterized by the nucleotide sequence
according to SEQ ID No. 1 and the amino
acid sequence according to SEQ ID No. 2, TDV-2 is characterized by the
nucleotide sequence according to SEQ ID
No. 3 and the amino acid sequence according to SEQ ID No. 4,1DV-3 is
characterized by the nucleotide sequence
according to SEQ ID No. 5 and the amino acid sequence according to SEQ ID No.
6 and TDV-4 is characterized by
the nucleotide sequence according to SEQ ID No. 7 and the amino acid sequence
according to SEQ ID No. 8.
37
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00212] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein upon
reconstitution of the dengue vaccine composition with a pharmaceutically
acceptable diluent (I), (ii), (iii), and (iv)
provide a total concentration of pfu/(15 mL and based on said total
concentration of pfu/0.5 ml the concentration of
(ii) in pfu/0.5 mL is less than 10%, and the concentration of (iv) in pfu/0.5
mL is at least 509'o, and the concentration
of (i) in pfu/0.5 mL is at least 1%, and the concentration of (iii) in pfu/0.5
mL is at least 6%, at least 8%, or at least
10%, or at least 12%, or at least 14%, or at least 16%, or at least 18%.
[00213] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
dengue vaccine composition comprises one or more pharmaceutically acceptable
excipients. In one embodiment, the
dengue vaccine composition comprises a non-reducing sugar, a surfactant, a
protein and an inorganic salt.
Preferably, the non-reducing sugar is trehalose, the surfactant is poloxamer
407, the protein is human serum
albumin and the inorganic salt is sodium chloride.
[00214] Furthermore, any vaccine excipients or combinations thereof known to
the person skilled in the art, e.g.
disclosed in WO 2018/027075 Al, can be used for the combined vaccine
composition.
[00215] In one embodiment, the unit dose of a dengue vaccine composition
comprises the following
pharmaceutically acceptable excipients:
- from about 10 % w/v to about 20 % w/v a,a-trehalose dihydrate or an
equimolar amount of other forms of
a,o-trehalose,
- from about 0.5 % w/v to about 1.5 % w/v poloxamer 107,
- from about 0.05 % w/v to about 2 % w/v human serum albumin, and
- from about 70 mM to 140 mM sodium chloride.
[00216] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
dengue vaccine composition comprises other dengue vaccines such as Dengvaxia0.
Dengvaxia0 is a tetravalent
dengue vaccine with mixed chimeric dengue viruses based on a yellow fever
backbone, CYD-TDV (Dengvaxia0,
Sanofi Pasteur, Lyon, France), and has been licensed in several countries
based on the clinical demonstration of an
overall vaccine efficacy (VE) against virologically-confirmed dengue (VCD) of
56-61% in children in Asia and Latin
America (Capeding MR et al. Clinical efficacy and safety of a novel
tetravalent dengue vaccine in healthy children in
Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet
2014, 384:1358-65; ViIlar LA et al.
Safety and immunogenicity of a recombinant tetravalent dengue vaccine in 9-16
year olds: a randomized, controlled,
phase II trial in Latin America. Pediatr Infect Dis ) 2013, 32:1102-9). The
preparation of these particular strains
CYD1, CYD2, CYD3 and CYD4 has been described in detail in international patent
applications WO 98/37911, WO
03/101397, W007/021672, WO 08/007021, WO 08/047023 and WO 08/065315, to which
reference may be made for
a precise description of the processes for their preparation. The
corresponding nucleotide sequences of the prM-E
regions of CYD1, CYD2, CYD3 and CYD4 are provided in W02016034629 and SEQID
NOs are set out in Table 16 of
this reference.
[00217] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
quantity of a chimeric dengue virus within CYD-TDV comprised in a vaccine
composition of the present invention lies
within a range of about 105 CCID50 to about 106 CCID50. The quantity of a live
attenuated chimeric dengue virus of
each of serotypes 1 to 4 comprised in the CYD dosage form, e.g. Dengvaxia , is
preferably equal.
[00218] In such embodiments, the CYD-TDV is dissolved/dissolvable in a
solution containing 0.4% NaCI.
38
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00219] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
dengue vaccine composition comprises other dengue vaccines such as TV003 or
TV005. TV003, developed by the
U.S. National Institute of Allergy and Infectious Diseases, comprises vaccine
components rDEN1A.30, rDEN2J4A30,
rDEN3e30/31 and rDEN4A301, wherein each of these components is present at a
concentration of 3 logioPFU. TV005
is similar to TV003 with the difference that the concentration of rDEN2/4A30
in TV005 is 4 logioPFU. The vaccines
TV003 and TV005 and their vaccine components as well as their production are
described in more detail in WO
2008/022196 A2 and S.S. Whitehead, Expert Rev Vaccines, 2016, 15(4): 509 to
517. Using recombinant DNA
technology, two attenuation strategies were utilized for the vaccine
components of TV003 or TWOS: deletions in the
3' untranslated region and structural gene chimerization. For example, the
component rDEN4A30 contains all the
structural and non-structural proteins of a wild type DENV-4, but is
attenuated by a 30-nucleotide deletion in the 3'
untranslated region (denoted "A301. The other vaccine components are also
attenuated due to the 30-nucleotide
deletion in the 3' untranslated region. In addition, rDEN3A30/31 includes a 31
nucleotide deletion in the 3'
untranslated region (shown in detail in Fig. 1c and Fig. 13 of WO 2008/022196
Al). The rDEN2/4A30 component was
created by substituting the prM and E genes of DENV-2 into the rDEN4A30
genome. The complete genomic
sequences of dengue strains which can be used to produce TV003 or TV005 are
available under the Genbank
accession numbers in Table A of WO 2008/022196 Al.
[00220] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
hepatitis A vaccine is an inactivated hepatitis A vaccine.
[00221] In certain embodiments, wherein the hepatitis A vaccine comprises a
hepatitis A virus derived from a
hepatitis A virus strain HM-175.
[00222] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
hepatitis A vaccine comprises an inactivated hepatitis A virus and the
inactivated hepatitis A virus is derived from a
wild-type hepatitis A virus strain HM-175.
[00223] In certain embodiments, the invention is directed to the combined
vaccine composition, the inactivated
hepatitis A virus is adsorbed on a carrier aluminum. In some of these
embodiments, the aluminum is aluminum
hydroxide or aluminum hydroxyphosphate sulfate.
[00224] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
hepatitis A vaccine comprises a phosphate-buffered saline solution and
excipients dissolved therein in the form of an
amino acid and in and in the form of polysorbate. In such embodiments, the
amino acid is present at a concentration
of 0.2 to 0.8% w/v and/or the polysorbate is present at a concentration of
0.01 to 0.09 mg/ml.
[00225] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
hepatitis A vaccine includes a hepatitis A virus expressing a viral antigen in
a concentration ranging from 500 ELISA
Units (FLU.) to 2000 ELISA Units (FLU.), preferably from 700 FLU. to 1600
FLU., most preferably from 1300 to
1550 EL.U.. Alternatively, the concentration ranges from 500 FLU. to 900 FLU..
In a further embodiment, the
concentration ranges from 200 to 400 EL.U..
[00226] In certain embodiments, the invention is directed to the combined
vaccine composition, wherein the
combined vaccine is included in a dose comprising a liquid, wherein the liquid
has a volume of 0.5 ml, 1 ml, or 1.5
ml.
39
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00227] In certain embodiments, the combined vaccine composition is provided
in one single vial in a liquid form
or in a dehydrated form, such as a lyophilized form.
[00228] In certain embodiments, the combined vaccine composition is obtained
from mixing a unit dose of a
dengue vaccine composition and a dose of a hepatitis A vaccine in a syringe.
[00229] The invention is also directed in part to a method of administering
any of the above combined vaccine
compositions to a subject or subject population.
[00230] In certain embodiments, the invention is directed to said methods,
wherein the combined vaccine
composition is administered subcutaneously or intramuscularly.
Method of preventing dengue disease and hepatitis A, rerresponding I ISPS, and
rniffsponding kit
[00231] The present invention is directed to a method of preventing hepatitis
A and dengue disease.
[00232] The present invention is directed in part to a method of preventing
hepatitis A and dengue disease in a
subject or subject population, the method comprising simultaneously on the
same day administering a hepatitis A
vaccine, such as HAVRIX or VAQTA , and a unit dose of a dengue vaccine
composition, wherein said unit dose
comprises a tetravalent dengue virus composition including four live,
attenuated dengue virus strains.
[00233] In certain embodiments, the invention is directed to said method,
wherein the hepatitis A vaccine, such as
HAVRIX , comprises an inactivated virus. Preferably, the hepatitis A vaccine
comprises an inactivated hepatitis A
virus and the inactivated hepatitis A virus is derived from a hepatitis A
virus strain HM-175.
[00234] In certain embodiments, the hepatitis A vaccine, such as HAVRIX , is
derived from a hepatitis A virus
strain HM-175.
[00235] In certain embodiments, the invention is directed to said methods,
wherein the hepatitis A vaccine, such
as HAVRIX , which is preferably a virus derived from a hepatitis A virus
strain HM-175, is adsorbed on aluminum.
According to some of these embodiments, the aluminum is aluminum hydroxide or
aluminum hydroxyphosphate
sulfate.
[00236] In certain embodiments, the invention is directed to said method,
wherein the hepatitis A vaccine, such as
HAVFtlYC), which is preferably derived from a hepatitis A virus strain HM-175,
comprises a phosphate-buffered saline
solution and excipients dissolved therein in the form of an amino acid and in
and in the form of polysorbate.
[00237] In certain embodiments, the invention is directed to said method,
wherein the hepatitis A vaccine, such as
HAVRIX , includes a hepatitis A virus expressing a viral antigen in a
concentration ranging from 500 ELISA Units
(EL.U.) to 2000 ELISA Units (ELU.), preferably from 700 EL.U. to 1600 EL.U.,
most preferably from 1300 to 1550
EL.U.. Alternatively, the concentration ranges from 500 EL.U. to 900 EL.U.. In
a further embodiment, the
concentration ranges from 200 to 400 EL.U..
[00238] For example, viral antigen activity of a hepatitis A vaccine can be
measured according to a method
disclosed in Andre FE., Hepburn A., D'Hondt E., "Inactivated candidate
vaccines for hepatitis", A. Prog Med Virol
1990; 37:72-95.
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00239] In certain embodiments, the invention is directed to said method,
wherein the dengue vaccine
composition upon reconstitution with 0.5 mL of a pharmaceutically acceptable
diluent comprises
(i) a chimeric dengue serotype 2/1 strain in a concentration of at least
3.3 log10 pfu/0.5 mL,
(ii) a dengue serotype 2 strain in a concentration of at least 2.7 log10
pfu/0.5 mL,
(iii) a chimeric dengue serotype 2/3 strain in a concentration of at least 4.0
log10 pfu/0.5 mL, and
(iv) a chimeric dengue serotype 2/4 strain in a concentration of at least 4.5
log10 pfu/0.5 mL.
[00240] According to some of these embodiments, upon reconstitution of the
dengue vaccine composition with a
pharmaceutically acceptable diluent, (i), (ii), (iii), and (iv) provide a
total concentration of pfu/0.5 mL and based on
said total concentration of pfu/0.5 ml the concentration of (ii) in pfu/0.5 mL
is less than 10%, and the concentration
of (iv) in pfu/0.5 mL is at least 50%, and the concentration of (i) in pfu/0.5
mL is at least 1%, and the concentration
of (iii) in pfu/0.5 mL is at least 6%, at least 8%, or at least 10%, or at
least 12%, or at least 14%, or at least 16%,
or at least 18')/0.
[00241] In certain embodiments, the invention is directed to said methods,
wherein the subject population or
subject is seronegative with respect to all dengue serotypes. According to
some of these embodiments, the subject
population or subject is seronegative with respect to hepatitis A at baseline.
[00242] In certain embodiments the invention S directed to said methods,
wherein the unit dose of the invention
as described herein and the hepatitis A vaccine, such as lIAVR.1X or VAQTA ,
are administered on day 0/1.
[00243] In certain embodiments, the invention is directed to said methods,
wherein the unit dose of the invention
as described herein is administered by subcutaneous injection and wherein the
hepatitis A vaccine, such as HAVRa
or VAQTA , is administered by intramuscular injection. According to some
embodiments, the injections are
administered to the arm, preferably to the deltoid region of the arm.
According to some of these embodiments, the
subcutaneous injection of the unit dose of the invention as described herein
and the intramuscular injection of the
hepatitis A vaccine, such as HAVRD or VAQTA , are administered to different
anatomical sites, such as to
opposite arms.
[00244] In certain embodiments, the invention is directed to said methods,
wherein two unit doses of the dengue
vaccine composition of the invention as described herein are administered. In
some embodiments, the two unit doses
of the invention as described herein are administered within 12 month or more,
or within 6 month, or within three
months, such as at day 0/1 and day 90. According to some of these embodiments,
a further third unit dose of the
invention as described herein is administered after the second administration.
Such a third administration may be
administered between 6 to 12 months after the first administration, such as 12
months after the first administration,
or later than 12 month after the first administration, such as 12 months (1
year) after the second administration or
even 5 years or longer after the first or second administration and may act as
a booster.
[00245] In certain embodiments, the invention is directed to said methods,
wherein two unit doses of the
invention as described herein and one dose of a hepatitis A vaccine, such as
HAVRIX or VAQTA , are
administered, in particular according to the following schedule
- a first simultaneous administration of the first unit dose and said
hepatitis A vaccine on day 0/1, and
- a second administration of the second unit dose after said first
simultaneous administration, such as about 3
months later such as on day 90.
41
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00246] In certain embodiments, the invention is directed to said method,
wherein the unit dose of the invention
as described herein is administered subcutaneously to a subject or subject
population and the hepatitis A vaccine,
such as HAVR1)00 or VAQTA , is administered intramuscularly to a subject or
subject population, and wherein the
subject or the subject population is seronegative with respect to all dengue
serotypes. In other embodimenls, the
subject or subject population is seropositive with respect to at least one
dengue serotype.
[00247] In certain embodiments, the invention is directed to said method,
wherein the unit dose of the invention
as described herein and the hepatitis A vaccine, such as HAVRE or VAQTA , are
administered to a subject or
subject population from a dengue endemic region. In certain embodiments, the
unit dose of the invention as
described herein is administered subcutaneously and the hepatitis A vaccine,
such as HAVRDO or VAQTA , is
administered intramuscularly to a subject or subject population from a dengue
endemic region.
[00248] In certain embodiments, the invention is directed to said method,
wherein the subject or subject
population is from a dengue non-endemic region, preferably from a dengue non-
endemic and a hepatitis A non-
endemic region.
[00249] According to some embodiments, a second dose of a hepatitis A vaccine,
such as HAVRIXO or VAQTA ,
is administered. The second dose of the hepatitis A vaccine may be
administered after the first administration of the
hepatitis A vaccine. Such a second administration may act as a booster and may
be administered 6 to 12 months or 6
to 18 months, such as 9 months after the first administration of the hepatitis
A vaccine, such as on day 270.
[00250] In certain embodiments, the invention is directed to said method,
wherein the unit dose of the invention
as described herein is administered subcutaneously and wherein the hepatitis A
vaccine, such as HAVRIX or
VAQTA , is administered intramuscularly to a subject or subject population of
more than 17 years, or more than 18
years, or 18 to 60 years of age. In further embodiments, the subjects or
subject population are adults of more than
21 years, or 21 to 60 years, or 21 to 45 years of age. In some embodiments,
the subject or subject population is
from a dengue endemic region. In another embodiment, the subject or subject
population is from a dengue non-
endemic region, preferably from a dengue non-endemic and a hepatitis A non-
endemic region. According to certain
embodiments, the subject or subject population is seronegative for all four
dengue serotypes.
[00251] In certain embodiments, the invention is directed to said method,
wherein the method does not include a
step of determination whether there was a previous dengue infection and/or a
previous hepatitis A infection in the
subject population or in the subject before the administration of the
hepatitis A vaccine and before the administration
of the unit dose of the dengue vaccine composition or wherein the hepatitis A
serostatus and/or the dengue
serostatus of the subject population or of the subject is unknown before the
administration of the hepatitis A vaccine
and before the administration of the unit dose of the dengue vaccine
composition. According to certain
embodiments, the method does not include a step of determination whether there
was a previous dengue infection
and/or a previous hepatitis A infection in the subject population or in the
subject at any time before, during and after
the steps of administration of the hepatitis A vaccine and of the unit dose of
the dengue vaccine composition or
wherein the hepatitis A serostatus and/or the dengue serostatus of the subject
population or of the subject is
unknown at any time before, during or after the steps of administration of the
hepatitis A vaccine and of the unit
dose of the dengue vaccine composition.
[00252] In certain embodiments, the invention is directed to said method,
wherein the method comprises a
primary vaccination consisting of the steps of:
42
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
(A) selecting a subject for administration of the unit doses of the
tetravalent dengue virus composition and the
hepatitis A vaccine in need for protection against dengue infection and
hepatitis A infection without determination
whether there was a previous dengue infection and/or a previous hepatitis A
infection, and
(B) administering simultaneously on the same day a first unit dose of the
tetravalent dengue virus composition
and a hepatitis A vaccine to the subject, and optionally
(C) administering at least one further unit dose of the tetravalent dengue
virus composition to the subject
within 3 to 12 months of administration of the first unit dose and optionally
(D) administering at least one further dose of the hepatitis A vaccine to
the subject within 6 to 18 months of
administration of the first unit dose.
.
[00253] In certain embodiments, the invention is directed to said method, the
method comprises a primary
vaccination consisting of the steps of:
(A) selecting a subject for administration of the unit doses of the
tetravalent dengue virus composition and the
hepatitis A vaccine in need for protection against dengue infection and
hepatitis A infection, and
(B) administering simultaneously on the same day a first unit dose of the
tetravalent dengue virus composition
and a hepatitis A vaccine to the subject, and
(C) administering two further unit doses of the tetravalent dengue virus
composition to the subject at about 6
and about 12 months of administration of the first unit dose and administering
a further hepatitis A vaccine to the
subject at either about 6 or about 12 months of administration of the first
unit dose. In some of these embodiments,
step (A), the selecting of the subject, is can-led out without determination
whether there was a previous hepatitis A
infection.
[00254] In certain embodiments, the invention is directed to said method,
wherein upon reconstitution of the unit
dose with a pharmaceutically acceptable diluent (i), (ii), (iii), and (iv)
provide a total concentration of pfu/0.5 mL and
based on said total concentration of pfu/0.5 ml the concentration of (11) in
pfu/0.5 mL is less than 10%, and the
concentration of (iv) in pfu/0.5 mL is at least 50%, and the concentration or
(i) in pfu/0.5 mL is at least 1%, and the
concentration of (iii) in pfu/0.5 mL is at least 6%, at least 8%, or at least
10%, or at least 12%, or at least 14%, or
at least 16%, or at least 18%.
[00255] In certain embodiments, the method provides compatibility between the
dengue vaccine composition and
the hepatitis A vaccine. Compatibility means in particular that the immune
response after simultaneous
administration is not inferior in comparison with a mono-administration of
these vaccines.
[00256] In certain embodiments, the method provides synergy between the dengue
vaccine composition and the
hepatitis A vaccine. Synergy means in particular that the immune response
after simultaneous administration is
better for one or both vaccines in comparison with a mono administration of
these vaccines.
[00257] In certain eribodiments, the invention is directed ba said method,
wherein the method provides non-
inferiority in a non-inferiority clinical study including at least 60 or at
least 120 healthy subjects divided into one
subject population and into one control subject population, wherein the
subject population receives simultaneously
on the same day the hepatitis A vaccine and the unit dose of the dengue
vaccine composition and the control subject
population receives simultaneously on the same day a hepatitis A vaccine and a
placebo administration
[00258] In certain embodiments, the invention is directed to said methods,
wherein the hepatitis A vaccine
provides a hepatitis A seroprotection rate of at least 95% or of at least 98%
on day 30 after an administration (on
43
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
day 0/1) to a subject population of at least 30 or at least 50 healthy
subjects receiving simultaneously on the same
day the hepatitis A vaccine and the unit dose of the dengue vaccine
composition and being seronegative with respect
to hepatitis A at baseline and being seronegative with respect to all dengue
virus serotypes at baseline.
[00259] In certain embodiments, the invention is directed to said method,
wherein the method provides a hepatitis
A seroprotection rate difference with respect to a hepatitis A mono-
administration, the difference being determined in
a non-inferiority clinical study including at least 60 or at least 120 healthy
subjects being seronegative with respect to
hepatitis A at baseline and seronegative with respect to all dengue virus
serotypes at baseline,
the healthy subjects being divided into
a) a subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the same day
an administration (on day 0/1) of the hepatitis A vaccine and the unit dose of
the dengue vaccine
composition, and
b) a control subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the
same day an administration (on day 0/1) of a hepatitis A vaccine and a
placebo,
wherein the difference is determined between the hepatitis A seroprotection
rate of the control subject
population on day 30 after the administration (on day 0/1) and the hepatitis A
seroprotection rate of the subject
population on day 30 after the administration (on day 0/1), and
wherein the difference has an upper bound within a two-sided 95% confidence
interval which is lower than
10%.
[00260] In certain embodiments, the invention is directed to said method,
wherein the hepatitis A vaccine provides
a hepatitis A seroprotection rate of at least 95% or of at least 98% or of at
least 99% on day 30 after an
administration (on day 0/1) to a subject population of at least 30 or at least
50 healthy subjects receiving
simultaneously on the same day the hepatitis A vaccine and the unit dose of
the dengue vaccine composition and
being seronegative with respect to hepatitis A at baseline, wherein the
healthy subjects include healthy subject(s)
which are seropositive with respect to at least one dengue virus serotype at
baseline and healthy subject(s) which
are seronegative with respect to all dengue virus serotypes at baseline.
[00261] In certain embodiments, the invention is directed to said method,
wherein the method provides a hepatitis
A seroprotection rate difference with respect to a hepatitis A mono-
administration, the difference being determined in
a non-inferiority clinical study including at least 60 or at least 120 healthy
subjects being seronegative with respect to
hepatitis A at baseline, wherein the healthy subjects include healthy
subject(s) which are seropositive with respect to
at least one dengue virus serotype at baseline and healthy subject(s) which
are seronegative with respect to all
dengue virus serotypes at baseline,
the healthy subjects being divided into
a) a subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the same day
an administration (on day 0/1) of the hepatitis A vaccine and the unit dose of
the dengue vaccine
composition, wherein the subject population includes healthy subject(s) which
are seropositive with respect
to at least one dengue virus serotype at baseline and healthy subject(s) which
are seronegative with respect
to all dengue virus serotypes at baseline, and
b) a control subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the
same day an administration (on day 0/1) of a hepatitis A vaccine and a
placebo, wherein the control subject
population includes healthy subject(s) which are seropositive with respect to
at least one dengue virus
serotype at baseline and healthy subject(s) which are seronegative with
respect to all dengue virus
serotypes at baseline,
44
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
wherein the difference is determined between the hepatitis A seroprotection
rate of the control subject
population on day 30 after the administration (on day 0/1) and the hepatitis A
seroprotection rate of the subject
population on day 30 after the administration (on day 0/1), and
wherein the difference has an upper bound within a two-sided 95% confidence
interval which is lower than
10%.
[00262] In certain embodiments, the invention is directed to said method,
wherein the subject or subject
population is exposed to a hepatitis A virus outbreak and/or a dengue virus
outbreak.
In certain embodiments, the invention is directed to said method, wherein the
method provides an anti-hepatitis A
virus antibody Geometric Mean Concentration (GMC) of at least 70 mIU/m1 or at
least 80 mIU/m1 or at least 90
mIU/m1 on day 30 after an administration (on day 0/1) to a subject population
of at least 30 or at least 50 healthy
subjects receiving simultaneously on the same day the hepatitis A vaccine and
the unit dose of the dengue vaccine
composition and being seronegative with respect to hepatitis A at baseline and
being seronegative with respect to all
dengue virus serotypes at baseline.
[00263] An ELISA for determining the anti-hepatitis A antibodies is for
example disclosed in Beck et al. Travel
Med 2004; 11:201-207.
[00264] In certain embodiments, the invention is directed to said method,
wherein the simultaneous administration
of the hepatitis A vaccine and the unit dose of the dengue vaccine composition
to the subject or the subject
population does not provide serious adverse events related to the simultaneous
administration. Additionally, there
are no deaths related to the simultaneous administration.
[00265] In certain embodiments, the invention is directed to said methods,
wherein the method provides the
Geometric Mean Titer (GMT) of neutralizing antibodies measured by M11150 of
- at least 110 or at least 110 or at least 150 for dengue serotype 1,
- at least 3000 or at least 3500 or at least 3900 for dengue serotype 2,
- at least 100 or at least 120 or at least 140 for dengue serotype 3, and/or
- at least 80 or at least 110 or at least 140 for dengue serotype 4,
on day 30 after an administration (on day 0/1) to a subject population of at
least 30 or at least 50 healthy subjects
receiving simultaneously on the same day the hepatitis A vaccine and the unit
dose of the dengue vaccine
composition and being seronegative with respect to hepatitis A at baseline and
being seronegative with respect to all
dengue virus serotypes at baseline.
[00266] In some embodiments, the geometric mean neutralizing antibody titers
(GMTs) of a subject population or
the neutralizing antibody titers of a subject are determined in accordance
with a microneutralization test, for example
according to the method described in Example 2.
[00267] The present invention is directed in part to a method of preventing
hepatitis A and dengue disease in a
subject or subject population, the method comprising simultaneously on the
same day administering a hepatitis A
vaccine, and a unit dose of a dengue vaccine composition, wherein said unit
dose comprises a tetravalent dengue
virus composition including four live, attenuated dengue virus strains,
wherein the four live, attenuated dengue virus
strains are different from the ones used in the unit dose as defined above.
45
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00268] In one embodiment of the invention, the method is directed to a
simultaneous on the same day
administration of a hepatitis A vaccine with other dengue vaccines such as
Dengvaxia . Dengvaxia is a tetravalent
dengue vaccine based on a yellow fever backbone, CYD-TDV (Dengvaxia , Sanofi
Pasteur, Lyon, France), and has
been licensed in several countries based on the clinical demonstration of an
overall vaccine efficacy (VE) against
virologically-confirmed dengue (VCD) of 56-61% in children in Asia and Latin
America (Capeding MR et al. Clinical
efficacy and safety of a novel tetravalent dengue vaccine in healthy children
in Asia: a phase 3, randomised,
observer-masked, placebo-controlled trial. Lancet 2014, 384:1358-65; Villar LA
et al. Safety and immunogenicity of a
recombinant tetravalent dengue vaccine in 9-16 year olds: a randomized,
controlled, phase II trial in Latin America.
Pediatr Infect Dis J 2013, 32:1102-9). The preparation of these particular
strains CYD1, CYD2, CYD3 and CYD4 has
been described in detail in international patent applications WO 98/37911, WO
03/101397, W007/021672, WO
08/007021, WO 08/047023 and WO 08/065315, to which reference may be made for a
precise description of the
processes for their preparation. The corresponding nucleotide sequences of the
prM-E regions of CYD1, CYD2, CYD3
and CYD4 are provided in W02016034629 and SEQID NOs are set out in Table 16 of
this reference.
[00269] In one such embodiment, the method comprises a vaccination consisting
of the steps of:
(A)
selecting a subject for administration of the
equal doses of the CYD-TDV composition, such as Dengvaxia ,
and the hepatitis A vaccine, such as HAVRDCO or VAQTA , in need for protection
against dengue infection and
hepatitis A infection, and
(B)
administering a first dose of
the CYD-TDV composition, such as Dengvaxia , and the hepatitis A vaccine,
such as HAVRIXO or VAQTA to the subject at month 0,
(C)
administering a further dose of the CYD-TDV
composition, such as Dengvaxia , and optionally the hepatitis
A vaccine, such as HAVRIXO or VAQTA to the subject within 3 to 11 months, in
particular at about month 6 of the
administration of the first CYD-TDV dose, and
(D)
administering a final dose of
the CYD-TDV, such as Dengvaxia , and optionally the hepatitis A vaccine,
such as HAVRIXO or VAQTA to the subject at about month 12.
[00270] In certain embodiments, the subject is from 2 to 60 years of age.
[00271] In particular embodiments, the subject is 2 to 18 years of age, or 4
to 16 years of age, or 18 to 60 years
of age.
[00272] Preferably, the exact quantity of each component of the CYD-TDV to be
administered may vary according
to the age and the weight of the subject being vaccinated, the frequency of
administration as well as the other
ingredients in the composition. The quantity of a chimeric dengue virus within
CYD-TDV comprised in a dose of a
vaccine composition lies within a range of about 105 CCID50 to about 106
CCID50 . The quantity of a live attenuated
chimeric dengue virus of each of serotypes 1 to 4 comprised in the CYD dosage
form, e.g. Dengvaxia , is preferably
equal. Advantageously, a vaccine composition, as described in this section,
comprises an effective amount of a
dengue antigen as defined herein.
[00273] In certain embodiments, the invention is directed to said method,
wherein the dengue vaccine
composition comprises other dengue vaccines such as TV003 or TV005. TV003,
developed by the U.S. National
Institute of Allergy and Infectious Diseases, comprises vaccine components
rDEN1A.30, rDEN2/4A30, rDEN3A30/31
and rDEN4A30, wherein each of these components is present at a concentration
of 3 logioPFU. TV005 is similar to
TV003 with the difference that the concentration of rDEN2/4A30 in TV005 is 4
logioPFU.The vaccines TV003 and
TV005 and their vaccine components as well as their production are described
in more detail in WO 2008/022196 A2
and S.S. Whitehead, Expert Rev Vaccines, 2016, 15(4): 509 to 517. Wing
recombinant DNA technology, two
46
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
attenuation strategies were utilized for the vaccine components of TV003 or
WOOS: deletions in the 3' untranslated
region and structural gene chimerization. For example, the component rDEN4A30
contains all the structural and non-
structural proteins of a wild type DENV-4, but is attenuated by a 30-
nucleotide deletion in the 3' untranslated region
(denoted "A301. The other vaccine components are also attenuated due to the 30-
nucleotide deletion in the 3'
untranslated region. In addition, rDEN3A30/31 includes a 31 nucleotide
deletion in the 3' untranslated region (shown
in detail in Fig. lc and Fig. 13 of WO 2008/022196 A2). The rDEN2/4A30
component was created by substituting the
prM and E genes of DENV-2 into the rDEN4630 genome. The complete genomic
sequences of dengue strains which
can be used to produce TV003 or TV005 are available under the Genbank
accession numbers in Table A of WO
2008/022196 Al.
[00274] In certain embodiments, the invention is directed to said methods,
wherein the unit dose disclosed herein,
which in particular comprises a chimeric dengue serotype 2/1 strain, a live
attenuated dengue serotype 2 strain, a
chimeric dengue serotype 2/3 strain and a chimeric dengue serotype 2/4 strain,
and Dengvaxiag disclosed herein
and the hepatitis A vaccine disclosed herein are simultaneously on the same
day administered to the subject or to
the subject population.
[00275] In certain embodiments, the invention is directed to said methods,
wherein the unit dose disclosed herein,
which in particular comprises a chimeric dengue serotype 2/1 strain, a live
attenuated dengue serotype 2 strain, a
chimeric dengue serotype 2/3 strain and a chimeric dengue serotype 2/4 strain,
and the hepatitis A vaccine disclosed
herein are simultaneously on the same day administered to the subject or to
the subject population on day 0/1 as a
first administration and Dengvaxia() disclosed herein is subsequently
administered to the subject or to the subject
population within three months from the first administration, such as on day
90 from the first administration, as a
second administration. Alternatively, Dengvaxia disclosed herein and the
hepatitis A vaccine disclosed herein are
simultaneously on the same day administered to the subject or to subject
population on day 0/1 as a first
administration and the unit dose disclosed herein, which in particular
comprises a chimeric dengue serotype 2/1
strain, a live attenuated dengue serotype 2 strain, a chimeric dengue serotype
2/3 strain and a chimeric dengue
serotype 2/4 strain, is administered subsequently to the subject or to the
subject population within three months
from the first administration, such as on day 90 from the first
administration, as a second administration.
[00276] In certain embodiments, the invention is directed to said methods,
wherein the unit dose disclosed herein,
which in particular comprises a chimeric dengue serotype 2/1 strain, a live
attenuated dengue serotype 2 strain, a
chimeric dengue serotype 2/3 strain and a chimeric dengue serotype 2/4 strain,
and TV003 or TV005 disclosed
herein and the hepatitis A vaccine disclosed herein are simultaneously on the
same day administered to the subject
or to the subject population.
[00277] In certain embodiments, the invention is directed to said methods,
wherein the unit dose disclosed herein,
which in particular comprises a chimeric dengue serotype 2/1 strain, a live
attenuated dengue serotype 2 strain, a
chimeric dengue serotype 2/3 strain and a chimeric dengue serotype 2/4 strain,
and the hepatitis A vaccine disclosed
herein are simultaneously on the same day administered to the subject or the
subject population on day 0/1 as a first
administration and wherein TV003 or TV005 disclosed herein is subsequently
administered to the subject or to the
subject population within three months from the first administration, such as
on day 90 from the first administration,
as a second administration. Alternatively, TV003 or TV005 disclosed herein and
the hepatitis A vaccine disclosed
herein are simultaneously on the same day administered to the subject or to
the subject population on day 0/1 as a
first administration and the unit dose disclosed herein, which in particular
comprises a chimeric dengue serotype 2/1
47
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
strain, a live attenuated dengue serotype 2 strain, a chimeric dengue serotype
2/3 strain and a chimeric dengue
serotype 2/4 strain, is administered subsequently to the subject or to the
subject population within three months
from the first administration, such as on day 90 from the first
administration, as the second administration.
[00278] The above method is also to be considered in the context of a use of
the unit close of dengue vaccine as
disclosed herein for such methods of preventing dengue disease and hepatitis A
or in the context of the use of the
unit dose of dengue vaccine for the manufacture of a medicament for such
methods of preventing dengue disease
and hepatitis A.
[00279] Furthermore, the present invention is directed to a kit against
hepatitis A and dengue disease comprising
a box containing at least
(a) a first container holding a hepatitis A vaccine, as defined above such
as HAVRDC , and
(b) a second container holding a unit dose of a dengue vaccine composition
as defined above, wherein
said unit dose comprises a tetravalent dengue virus composition including four
live, attenuated dengue virus strains.
Method of preventing dengue disease and yellow fever and I ISPS
[00280] The present invention is directed in part to a method of preventing
dengue disease as well as yellow fever
in a subject. Thus, in certain embodiments the invention is directed to a
method of preventing dengue disease in a
subject, comprising administering to the subject a reconstituted unit dose of
the invention as described herein,
wherein the method further comprises preventing yellow fever in the subject by
concomitant administration of a
yellow fever vaccine, in particular YF-17D, to the subject.
[00281] The present invention is directed in part to a method of preventing
dengue disease as well as yellow fever
in a subject population. Thus, in certain embodiments the invention is
directed to a method of preventing dengue
disease in a subject population, comprising administering to the subject
population a reconstituted unit dose of the
invention as described herein, wherein the method further comprises preventing
yellow fever in the subject
population by concomitant administration of a yellow fever vaccine, in
particular YF-17D, to the subject population.
[00282] In certain embodiments the invention is directed to said methods,
wherein the unit dose of the invention
as described herein and the yellow fever vaccine, in particular YF-17D, are
administered simultaneously. In some of
these embodiments the simultaneous administration is on day 0 or day 90,
preferably on day 0. In other
embodiments the administration of the unit dose of the invention as described
herein and the yellow fever vaccine, in
particular YF-17D, are done sequentially such as wherein the yellow fever
vaccine is administered before or after the
unit dose of dengue vaccine as described herein, such as within about 6 weeks,
or such as within about 4 weeks, or
such as within about 2 weeks, or such as about within 1 week.
[00283] In certain embodiments the invention is directed to said methods,
wherein the reconstituted unit dose of
the invention as described herein is administered and the yellow fever
vaccine, in particular YF-17D, are administered
by subcutaneous injection. According to some embodiments, the subcutaneous
injections are administered to the
arm, preferably to the deltoid region of the arm. According to some of these
embodiments the subcutaneous
injections of the unit dose of the invention as described herein and yellow
fever vaccine, in particular YF-17D, are
administered to different anatomical sites, such as to opposite arms, in
particular when the vaccines are administered
simultaneously.
48
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00284] In certain embodiments the invention is directed to said methods,
wherein two unit doses of the invention
as described herein are administered. In some embodiments the two unit doses
of the invention as described herein
are administer& within 12 month or more, or within 6 month, or within three
months, such as at day 0/1 and day
90. According to some of these embodiments a further third unit dose of the
invention as described herein is
administered after the second. Such a third administration may act as a
booster and may be administered between 6
to 12 months after the first administration, such as 12 months after the first
administration, or later than 12 month
after the first administration, such as 12 months (1 year) after the second
administration or even 5 years or longer
after the first or second administration .
[00285] In certain embodiments the invention is directed to said methods,
wherein two reconstituted unit doses of
the invention as described herein and one dose of a yellow fever vaccine, in
particular YF-17D, are administered, in
particular according to the following schedule
- an administration of said yellow fever vaccine on day 0,
- a first administration of the first reconstituted unit dose after said
yellow fever vaccine administration, such
as 3 months later and preferably on day 90, and
- a second administration of the second reconstituted unit dose after said
first administration of the
reconstituted unit dose, such as 3 months later and preferably on day 180.
[00286] In certain embodiments the invention is directed to said methods,
wherein two reconstituted unit doses of
the invention as described herein and one dose of a yellow fever vaccine, in
particular YF-17D, are administered, in
particular according to the following schedule
- a first administration of the first reconstituted unit dose on day 0,
- a second administration of the second reconstituted unit dose after said
first administration of the
reconstituted unit dose, such as 3 months later and preferably on day 90, and
- an administration of said yellow fever vaccine after said second
administration of the reconstituted unit
dose, such as 3 months later and preferably on day 180.
[00287] In certain embodiments the invention is directed to said methods,
wherein two reconstituted unit doses of
the invention as described herein and one dose of a yellow fever vaccine, in
particular YF-17D, are administered, in
particular according to the following schedule
- a simultaneous administration of the first reconstituted unit dose and
said yellow fever vaccine on day 0,
and
- a second administration of the second reconstituted unit dose after said
simultaneous administration, such
as 3 months later and preferably on day 90.
[00288] In a preferred embodiment, the yellow fever vaccine and unit dose of
the invention as described herein
are administered simultaneously on day 0 or simultaneously on day 90.
[00289] In certain embodinbents, the invention is directed to said methods,
wherein the subject or subject
population is seronegative to all dengue serotypes. In certain embodiments,
the invention is directed to said
methods, wherein the reconstituted unit dose of the invention as described
herein is administered subcutaneously to
a subject or subject population and the yellow fever vaccine, in particular YF-
17D vaccine, is administered
subcutaneously to a subject or subject population, and wherein the subject or
the subject population is seronegative
with respect to all dengue serotypes. In other embodiments, the subject or
subject population is seropositive with
respect to at least one dengue serotype.
[00290] In certain embodiments, the invention is directed to said methods,
wherein the unit dose of the invention
as described herein and the yellow fever vaccine, in particular YF-17D, are
administered to a subject or subject
49
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
population from a dengue endemic region. In certain embodiments, the
reconstituted unit dose of the invention as
described herein and the yellow fever vaccine, in particular YF-17D, are
administered subcutaneously to a subject or
subject population from a dengue endemic region. In other embodiments, the
subject or subject population is from a
dengue non-endemic region. Such a subject population or such a subject may be
vaccinated according to the present
invention in the context of traveling to a dengue endemic region and yellow
fever endemic region.
[00291] In certain embodiments the invention is directed to said methods,
wherein the reconstituted unit dose of
the invention as described herein and of the yellow fever vaccine, in
particular YF-17D, are administered
subcutaneously to a subject or subject population of more than 17 years, or
more than 18 years, or 18 to 60 years of
age. In further embodiments, the subjects or subject population are adults of
more than 21 years, or 21 to 60 years,
or 21 to 45 years of age. In some embodiments, the subject or subject
population is from a dengue endemic region.
In another embodiment, the subject or subject population is from a dengue non-
endemic region, preferably from a
dengue non-endemic and yellow fever non-endemic region. According to some of
these embodiments, the subject or
subject population are seronegative for all four dengue serotypes.
[00292] The above method is also to be considered in the context of a use of
the unit dose of dengue vaccine as
disclosed herein for such methods or in the context of the use of the unit
dose of dengue vaccine for the
manufacture of a medicament for such methods.
Method of preventing and uses, method of inoculating agairrait dengi IP
disease and itses
[00293] The present invention is directed in part to a method of preventing
dengue disease (in particular
virologically confirmable dengue, VCD) in a subject. Thus, in certain
embodiments the invention is directed to a
method of preventing dengue disease in a subject, comprising administering to
the subject, a unit dose/tetravalent
dengue virus composition, in particular a reconstituted unit dose of the
invention as described herein.
[00294] The present invention is directed in part to a method of preventing
dengue hemorrhagic fever (DHF) and
dengue shock syndrome (DSS). Thus, in certain embodiments the invention is
directed to a method of preventing
dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), comprising
administering to the subject a
reconstituted unit dose/tetravalent dengue virus composition of the invention
as described herein.
[00295] The present invention is therefore directed to a method of inoculating
a subject against virologically
confirmable dengue disease with a tetravalent dengue virus composition
including four live attenuated dengue virus
strains representing serotype 1, serotype 2, serotype 3 and serotype 4,
wherein in particular the tetravalent dengue
virus composition includes a chimeric dengue serotype 2/1 strain and a dengue
serotype 2 strain and a chime-ic
dengue serotype 2/3 strain and a chimeric dengue serotype 2/4 strain, wherein
in particular the dengue serotype 2
strain is derived from the wild type virus strain DEN-2 16681 (SEQ ID NO 11)
and differs in at least three nucleotides
from the wild type as follows:
a) 5'-noncoding region (NCR)-57 (nt-57 C-to-T): major attenuation locus
b) NS1-53 Gly-to-Asp (nt-2579 G-to-A): major attenuation locus
c) N53-250 (nt-5270 A-to-T): major attenuation locus; and
wherein the three chimeric dengue strains are derived from the serotype 2
strain by replacing the structural proteins
prM and E from serotype 2 strain with the corresponding structural proteins
from the other dengue serotypes,
resulting in the following chimeric dengue strains:
- a DENV-2/1 chimera,
- a DEN V-2/3 chimera and
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
- a DENV-2/4 chimera.
[00296] Further information regarding the serotypes of the tetravalent
composition can be derived from section
"Dengue virus strains" above.
[00297] The tetravalent dengue virus composition for such a method may be in
the form of a unit dose
comprising:
(i) a dengue serotype 1 in a concentration of at least 3.3 log10 pfu/0.5
ml,
(ii) a dengue serotype 2, in a concentration of at least 2.7 log10 pfu/0.5
ml,
(iii) a dengue serotype 3, in a concentration of at least 4.0 10g10 pfu/0.5
ml, and
(iv) a dengue serotype 4, in a concentration of at least 4.5 log10 pfu/0.5
ml.
[00298] The present invention is in particular directed to such a method
wherein the unit dose is lyophilized and
upon reconstitution with 0.5 mL of a pharmaceutically acceptable diluent
comprises:
(i) a dengue serotype 1 in a concentration of at lest 33 10g10 pfu/0.5 ml,
(ii) a dengue serotype 2, in a concentration of at least 2.7 log10 pfu/0.5
ml,
(iii) a dengue serotype 3, in a concentration of at least 4.0 log10 pfu/0.5
ml, and
(iv) a dengue serotype 4, in a concentration of at least
4.5 log10 pfu/0.5 ml.
[00299] Further information regarding the tetravalent composition or the unit
dose can be derived from section
"Dengue vaccine composition" and "Unit dose" above.
[00300] The present invention is therefore directed in a method and
corresponding use, the method comprising a
primary vaccination with only two administrations of the unit dose comprising
the steps of:
(A) administering a first unit dose of the tetravalent dengue virus
composition to the subject, and
(B) administering a second unit does of the tetravalent dengue virus
composition to the subject within 3 months
of administration of the first unit dose
According to this embodiment the administration of only two doses within 3
months is sufficient to provide effective
protection against a subsequent dengue infection.
[00301] Such method preferably provides a combined vaccine efficacy against
all four serotypes in preventing
virologically confirmable dengue disease with a 2-sided 95% confidence
interval, wherein the lower bound is more
than 60%, when measured against placebo in a subject population of at least
5,000 healthy subjects in-espective of
serostatus at baseline and 14 to 16 years of age, from the first
administration of the administration schedule until 18
months after the second administration of the administration schedule.
[00302] Such method also preferably provides a combined vaccine efficacy
against all four serotypes, in preventing
virologically confirmable dengue disease with a 2-sided 95% confidence
interval, wherein the lower bound is more
than 45 A), when measured against placebo in a subject population of at least
1,500 or at least 2,000 healthy
subjects seronegative against all serotypes at baseline and 14 to 16 years of
age, from 30 days after the second
administration of the administration schedule until 18 months after the second
administration of the administration
schedule.
51
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00303] According to certain embodiments the method of inoculation against the
virologically confirmable dengue
disease is due to a dengue serotype 2, and/or due to a dengue serotype 1. The
method has very high efficacy
against dengue serotype 2 and dengue serotype 1 and the highest efficacy
against dengue serotype 2.
[00304] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
serotype 1, in preventing virologically confirmable dengue disease with a 2-
sided 95% confidence interval, wherein
the lower bound is more than 25%, when measured against placebo in a subject
population of at least 1,500, or at
least 2,000, or at least 5,000 healthy subjects (or at least 10,000, or at
least 15,000 healthy subjects) irrespective of
serostatus at baseline and 4 In 16 years of age from 30 days post second
administration until 12 to 18 months (e.g.
at 12 or at 18 months) after the second administration. In certain such
embodiments, the lower bound is more than
30%, is more than 35% is more than 40%, is more than 45%, is more than 50%, or
is more than 54%.In certain
such embodiments the subject population of at least 1,500 is seronegative
against all serotypes at base line and the
lower bound is more than 35%. In certain such embodiments the seronegative and
seropositive population each
provide a vaccine efficacy against serotype 1 with a 2-sided 95% confidence
interval, wherein the lower bounds are
within 10 %-points.
[00305] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
serotype 1, in preventing virologically confirmable dengue disease, when
measured against placebo in a subject
population of at least 1,500, or at least 2,000, or at least 5,000 healthy
subjects (or at least 10,000, or at least
15,000 healthy subjects) in-espective of serostatus at baseline and 4 to 16
years of age from 30 days post second
administration until 12 to 18 months (e.g. at 12 or at 18 months) after the
second administration. In certain such
embodiments, the vaccine efficacy is more than 40%, is more than 50%, is more
than 60%, or is more than 65%. In
certain such embodiments the subject population of at least 1,500 is
seronegative against all serotypes at base line.
In certain such embodiments the seronegative and seropositive population each
provide a vaccine efficacy against
serotype 1 which are within 5 %-points.
[00306] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
serotype 2, in preventing virologically confirmable dengue disease with a 2-
sided 95% confidence interval, wherein
the lower bound is more than 25%, when measured against placebo in a subject
population of at least 1,500, or at
least 2,000, or at least 5,000 healthy subjects (or at least 10,000, or at
least 15,000 healthy subjects) irrespective of
serostatus at baseline and 4 to 16 years of age from 30 days post second
administration until 12 to 18 months (e.g.
at 12 or at 18 months) after the second administration. In certain such
embodiments, the lower bound is more than
50%, is more than 60%, is more than 70%, is more than 80%, or is more than
85%. In certain such embodiments
the subject population of at least 1,500, is seronegative against all
serotypes. In certain such embodiments the
seronegative and seropositive population each provide a vaccine efficacy
against serotype 2 with a 2-sided 95%
confidence interval, wherein the lower bounds are within 5 %-points.
[00307] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
serotype 2, in preventing virologically confirmable dengue disease, when
measured against placebo in a subject
population of at least 1,500, or at least 2,000, or at least 5,000 healthy
subjects (or at least 10,000, or at least
15,000 healthy subjects) irrespective of serostatus at baseline and 4 to 16
years of age from 30 days post second
administration until 12 to 18 months (e.g. at 12 or at 18 months) after the
second administration. In certain such
embodiments, the vaccine efficacy is more than 60%, is more than 70%, is more
than 80%, or is more than 90%. In
certain such embodiments the subject population of at least 1,500 is
seronegative against all serotypes at base line.
In certain such embodiments the seronegative and seropositive population each
provide a vaccine efficacy against
serotype 2 which are within 5 %-paints.
52
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00308] The efficacy of the method is further described in more detail below
in this the section.
[00309] In certain embodiments the unit dose is reconstituted and administered
by subcutaneous injection.
According to some of these embodiments, the subcutaneous injection is
administered to the arm, preferably to the
deltoid region of the arm.
[00310] According to one embodiment such a method does not include a step of
determination whether there was
a previous dengue infection in the subject before administration of the unit
dose or wherein the serostatus of the
subject is unknown before administration of the unit dose.
[00311] According to one embodiment such a method does not include a step of
determination of a previous
dengue infection in the subjects preferably at any time before, during or
after the steps of administration or wherein
the serostatus of the subject is unknown preferably at any time before, during
or after the steps of administration.
[00312] The method according to the invention does not require the testing of
the serostatus before vaccination
and thus allows immediate treatment and outbreak control. According to certain
embodiments the use is for a
method wherein the subject is exposed to a dengue outbreak. In certain such
embodiments the outbreak is due to a
dengue serotype 2, and/or due to a serotype 1.
[00313] According to one embodiment such a method the subject is from a region
wherein the seroprevalence rate
is unknown and/or wherein the seroprevalence rate is below 80%, or below 70%,
or below 60%.
[00314] According to one embodiment of such a method the subject is
seronegative at baseline and is from a
region or travels to a region wherein the seroprevalence rate is high with
respect to serotype 1 and/or serotype 2 is.
80%, or 90% or above.
[00315] According this embodiment the vaccine and corresponding method is safe
for seronegative and
seropositive subjects and thus does not require an analysis of the serostatus
or a determination of a previous dengue
infection or a high seroprevalence rate in the region. Such a method
preferably provides a combined vaccine efficacy
against virologically-confirmed dengue with hospitalization against all four
serotypes with a 2-sided 95% confidence
interval, wherein the lower bound is more than 65%, when measured against
placebo in a subject population of at
least 5,000 healthy 4 to 16 year old subjects irrespective of serostatus at
baseline, preferably in at least 1,500
healthy 4 to 16 year old subjects seronegative at baseline, from first
administration of the administration schedule
until 12 to 18 months after the second administration of the administration
schedule. Preferably, the 2-sided 95%
confidence interval of the combined vaccine efficacy against virologically-
confirmed dengue with hospitalization
against all four serotypes when comparing seropositive and seronegative
subjects provides for lower bounds of the
2-sided confidence interval which are within 10% points or within 15% points
or within 20% points. The method is
preferably safe with respect to serotype 1 and serotype 2 which may therefore
be used in outbreak situations due to
serotype 1 and/or serotype 2 or even for seronegative subjects (e.g.
travelers) or subjects with unknown serostatus
in regions with very high seroprevalence rates (>80%) due to serotype 1 and/or
serotype 2.
[00316] The safety of the method is further described in more detail in the
section "method of preventing, method
of inoculating".
[00317] According to one embodiment such a method does not include the active
surveillance with respect to
febrile illness of the subject after the administration Cl the first- and
second-unit dose. During active surveillance any
subject with febrile illness (defined as fever L=38 C on any 2 of 3
consecutive days) will be asked to return to the site
53
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
for dengue fever evaluation by the Investigator. Subjects/guardians will be
contacted at least weekly to ensure
robust identification of febrile illness by reminding subjects/guardians of
their obligation to return to the site in case
of febrile illness. This contact will be implemented through appropriate
methods that may differ in each trial site (eg,
phone calls, text messaging, home visits, school-based surveillance).
[00318] According to one embodiment such a method does not include vaccine
immunogenicity analysis including
GMTs for dengue neutralizing antibodies.
[00319] According to one embodiment such a method does not include a
reactogenicity analysis. Such a
reactogenicity analysis relates to solicited local AEs (injection site pain,
injection site erythema, and injection site
swelling) and solicited systemic AEs (child < 6 years: fever,
irritability/fussiness, drowsiness and loss of appetite;
child 6 years: asthenia, fever, headache, malaise and myalgia) which will e.g.
be assessed for 7 days and 14 days,
respectively, following each vaccination (vaccination day included) via
collection of diary cards.
[00320] According to one embodiment the method does not include an active
surveillance, an immunogenicity
analysis and a reactogenicity analysis.
[00321] According to such embodiments the vaccine and the corresponding method
of inoculation are safe and
therefore do not require further steps of surveillance or analysis.
[00322] In view of the above the method according to one embodiment comprises
a primary vaccination consisting
of the steps of:
(A)
selecting a subject for
administration of the unit doses of the tetravalent dengue virus composition
in need
for protection against dengue infection without determination of a previous
dengue infection, and
(B) administering a first unit dose of the tetravalent dengue virus
composition to the subject, and
(C)
administering a second unit
dose of the tetravalent dengue virus composition to the subject within 3
months
of administration of the first unit dose.
Therefore the method of inoculating is finalized without determination of a
previous dengue infection. The method
further optionally comprises at least 1 years after the administration of the
second unit dose a booster dose of the
unit dose.
[00323] Selecting the subject may indude all types of considerations but
preferably not the determination of a
previous dengue infection. The selection may include consideration of the age,
health conditions, and threat of
infection. The threat of infection includes consideration of the
seroprevalence rate in the region in which the subject
normally lives or intends to travel, the serotype specific seroprevalence rate
and an outbreak situation or serotype
specific outbreak situations. The subject may be selected due to its exposure
to serotype 1 and/or serotype 2 or due
to the fact it requires protection against a specific dengue serotype, i.e.
serotype 1 and/or serotype 2.
[00324] According to the invention the method is applicable to subjects of all
kinds of ages. According to one
embodiment the subject is under 9 years of age, or 4 to 5 years of age, or 6
to 11 years of age or 12W 16 years, or
6 to 16 years of age or 4 to 16 years of age, or 2 to 17 years of age, or 9
years of age, or over 9 years of age, or 9
to 17 years of ageõ or 18 to 45 years of age, or 46 to 60 years of age, or
over 60 years of age.
[00325] In particular the present invention is directed to such a method
wherein the method which is safe
54
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00326] In particular the present invention is directed to such a method
providing a combined vaccine efficacy
against virologically-confirmed dengue with hospitalization against all four
serotypes with a 2-sided 95% confidence
interval, wherein the lower bound is more than 65%, when measured against
placebo in a subject population of at
least 5,000 healthy 4 to 16 year old subjects irrespective of serostatus at
baseline from first administration of the
administration schedule until 12 to 18 months after the last administration of
the administration schedule.
[00327] In particular the present invention is directed to such a method
wherein the method which is effective.
[00328] In particular the present invention is directed to such a method
providing a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with a 2-sided 95% confidence
interval, wherein the lower bound is more than 60%, when measured against
placebo in a subject population of at
least 5,000 healthy subjects irrespective of serostatus at baseline and 14 to
16 years of age, from the first
administration of the administration schedule until 18 months after the last
administration of the administration
schedule.
[00329] In certain embodiments, the invention is directed to said methods,
wherein the subject is seronegative to
all dengue serotypes.
[00330] The present invention is directed in part to a method of preventing
dengue disease (in particular
virologically confirmable dengue, VCD) in a subject population. Thus, in
certain embodiments the invention is
directed to a method of preventing dengue disease in a subject population,
comprising administering to the subject
population a unit dose, in particular a reconstituted unit dose of the
invention as described herein.
[00331] The present invention is in part directed to said method for
preventing dengue disease (in particular
virologically confirmable dengue, VCD) in a subject population comprising
administering to the subject population at
least a first reconstituted unit dose of the invention as described herein,
wherein certain ratios of geometric mean
neutralizing antibody titers (GMTs) at day 180 or 365 after administration of
said first unit dose to the subject
population are achieved. According to some embodiments, the geometric mean
neutralizing antibody titer for dengue
serotype 2 (GMT DENV-2) and the geometric mean neutralizing antibody titer for
dengue serotype 4 (GMT DENV-4)
when tested in at least 40, or at least 50, or at least 60 subjecis at day 180
or day 365 after at least a first
administration of said reconstituted unit dose of the invention as described
herein, and optionally a second
administration of a reconstituted unit dose of the invention as described
herein 90 days after said first administration,
provide a ratio of GMT DENV-2: GMT DENV-4 of not more than 50, or not more
than 40, or not more than 30, or not
more than 20. In some of these embodiments, the ratio of GMT DENV-2 : GMT DEW-
1 is not more than 20, or not
more than 18, or not more than 15 at day 180 or 365 after administration of
said first reconstituted unit dose, and/or
the ratio of GMT DENV-2 : GMT DENV-3 is not more than 20, or not more than 18,
or not more than 15 at day 180
or 365 after administration of said first reconstituted unit dose.
[00332] The present invention is in part directed to said method for
preventing dengue disease (in particular
virologically confirmable dengue, VCD) in a subject comprising administering
to the subject at least a first
reconstituted unit dose of the invention as described herein, wherein certain
ratios of neutralizing antibody titers at
day 180 or 365 after administration of said first unit dose to the subject are
achieved. According to some
embodiments, the neutralizing antibody titer for dengue serotype 2 and the
neutralizing antibody titer for dengue
serotype 4 at day 180 or day 365 after at least a first administration of the
reconstituted unit dose of the invention as
described herein, and optionally a second administration of a reconstituted
unit dose of the invention as described
herein 90 days after said first administration, provide a ratio of
neutralizing antibody titer for DENV-2 : neutralizing
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
antibody titer for GMT DENV-4 of not more than 50, or not more than 40, or not
more than 30, or not more than 20.
In some of these embodiments, the ratio of the neutralizing antibody titers of
DENV-2 : DENV-1 is not more than 20,
or not more than 18, or not more than 15 at day 180 or 365 after
administration of said first reconstituted unit dose,
and/or the ratio of the neutralizing antibody titers of DENV-2 : DENV-3 is not
more than 20, or not more than 18, or
not more than 15 at day 180 or 365 after administration of said first
reconstituted unit dose.
[00333] The geometric mean neutralizing antibody titers (GMTs) of a subject
population or the neutralizing
antibody titers of a subject are determined in accordance with the
microneutralization test disclosed herein, for
example according to the method described in Example 2. Without wishing to be
bound to any theory, it is presently
understood that a method inducing a more balanced immune response due to the
administration of the reconstituted
unit dose of the invention as described herein, in terms of less differences
between the geometric mean neutralizing
antibody titers (GMTs) against the four dengue serotypes or the neutralizing
antibody titers against the four dengue
serotypes, is beneficial to the subject or subject population to be
vaccinated. In particular, it is understood that a
much greater response to any one of the four serotypes, such as to DENV-2 in
comparison to the other serotypes, is
less beneficial.
[00334] The present invention is in part directed to said method for
preventing dengue disease (in particular
virologically confirmable dengue, VCD) in a subject or subject population
wherein the method provides a
seropositivity rate in a subject population of at least 50 subjects including
the administration of two unit doses
subcutaneously at day I and at day 90, wherein the subjects of the subject
population are seronegative to all dengue
serotypes at baseline. In certain such embodiments, at least 80% of the
subject population are seropositive for all
four dengue serotypes at least one month after administration of the first
unit dose, such as at day 30, and/or at
least 80% of the subject population are seropositive for all four dengue
serotypes before or at the time of the
administration of the second unit dose, such as at day 90, and/or at least 80
k, or at least 85%, or at least 90%, or
at least 95% of the subject population are seropositive for all four dengue
serotypes after the administration of the
second unit dose, such as at day 120, and/or at least 80 k, or at least 850/0
,or at least 90% of the subject
population are seropositive for all four dengue serotypes after the
administration of the second unit close, such as at
day 270.
[00335] The present invention is in part directed to said method for
preventing dengue disease (in particular
virologically confirmable dengue, VCD) in a subject or subject population
wherein the method provides a
seropositivity rate in a subject population of at least 100 subjects including
administration of two unit doses
subcutaneously at day 1 and at day 90, wherein the subjects of the subject
population comprises from 20% to 10%
subjects who are seronegative to all dengue serotypes and from 60% to 80%
subjects who are seropositive to at
least one dengue serotype at base line, wherein at day 120 and/or day 270 the
seropositivity rate for all four dengue
serotypes in the seronegative part of the subject population and the
seropositivity rate for all four dengue serotypes
in the seropositive part of the subject population do not deviate more than
10%-points and/or wherein at day 120
the seropositivity rate for all four dengue serotypes in the seronegative part
of the subject population and the
seropositivity rate for all four dengue serotypes in the seropositive part of
the subject population do not deviate more
than 5%-points.
[00336] The present invention is in part directed to a method of preventing
virologically confirmable dengue
disease in a subject or subject population comprising administering to the
subject or subject population a
reconstituted unit dose of a tetravalent dengue virus composition including
four live, attenuated dengue serotypes, in
particular the virus strains as described herein.
56
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00337] The present invention is in part directed to a method of preventing
virologically confirmable dengue
disease with hospitalization in a subject or subject population comprising
administering to the subject or subject
population a reconstituted unit dose of a tetravalent dengue virus composition
including four live, attenuated dengue
serotypes, in particular the virus strains as described herein.
[00338] In certain embodiments, the method includes a reconstituted unit
dose/tetravalent dengue virus
composition of a dengue vaccine composition administered for preventing dengue
disease in a subject or a subject
population, the reconstituted unit dose comprising: a tetravalent virus
composition including four live attenuated
dengue virus strains, wherein a unit dose is lyophilized and upon
reconstitution with 0.5 mL of a pharmaceutically
acceptable diluent the reconstituted unit dose is obtained which comprises:
(0 a dengue serotype 1, such as a chimeric dengue serotype 2/1 strain, in a
concentration of at least 3.3
log10 pfu/0.5 ml,
(ii) a dengue serotype 2, such as a dengue serotype 2 strain, in a
concentration of at least 2.7 log10 pfu/0.5
ml,
(iii) a dengue serotype 3, such as a chimeric dengue serotype 2/3 strain, in a
concentration of at least 4.0
log10 pfu/0.5 ml, and
(vi) a dengue serotype 4, such as a chimeric dengue serotype 2/4 strain, in a
concentration of at least 4.5 log10
pfu/0.5 ml.
[00339] It is preferred that the reconstituted unit dose/tetravalent dengue
virus composition is used in the method
of preventing dengue disease of the present invention, wherein upon
reconstitution of the unit dose with a
pharmaceutically acceptable diluent (i), (ii), (iii), and (iv) provide a total
concentration of pfu/0.5 mL and based on
said total concentration the concentration of (ii) in pfu/0.5 mL is less than
2%, the concentration of (iv) in pfu/0.5
mL is at least 50%, the concentration of (i) in pfu/0.5 mL is at least 1%, and
the concentration of (iii) in pfu/0.5 mL
is at least 6% and wherein the subject or subject population is of 18 to 60
years of age.
[00340] In another preferred embodiment, the reconstituted unit
dose/tetravalent dengue virus composition is
used in the method of preventing dengue disease of the present invention,
wherein upon reconstitution with a
pharmaceutically acceptable diluent (i), (ii), (iii), and (iv) provide a total
concentration of pfu/0.5 mL and based on
said total concentration the concentration of (ii) in pfu/0.5 mL is less than
10%, and the concentration of (iv) in
pfu/0.5 mL is at least 50%, and the concentration of (i) in pfu/0.5 mL is at
least 1%, and the concentration of (iii) in
pfu/0.5 mL is at least 8% and wherein the subject or subject population is of?
to 17 years of age.
[00341] In certain embodiments, the invention is directed to said methods,
wherein said unit dose comprises a
tetravalent dengue virus composition including four live attenuated dengue
serotypes, in particular the virus strains
described herein wherein the serotypes have certain concentrations as
described herein with respect to the virus
composition and unit dose such as:
(i) a dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) has a
concentration of 3.3 log10 pfu/dose
to 5.0 log10 pfu/dose, or 3.3 log10 pfu/0.5 mL to 5.0 log10 pfu/0.5 mL
(ii) a dengue serotype 2 (e.g. dengue serotype 2 strain) has a concentration
of 2.713910 Vu/dose to 4.9 log10
pfu/0.5 dose, or 2.7 10g10 pfu/0.5m1 to 4.9 10g10 pfu/0.5m1
(iii) a dengue serotype 3 (e.g. chimeric dengue serotype 2/3 strain) has a
concentration of 4.0 logic) pfu/dose
to 5.7 log10 pfu/0.5 dose, or 4.0 log10 pfu/0.5 mL to 5.7 log10 pfu/0.5 mL and
(iv) a dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain) has a
concentration of 4.5 log10 pfu/dose
to 5.5 log10 pfu/0.5 dose, or 4.5 log10 pfu/0.5 mL to 5.5 log10 pfu/0.5 mL
57
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
In preferred such embodiments, the subject or subject population is of 2 to 17
years of age, such as 4 to 16 years of
age, and preferably less than 9 years of age. In other preferred embodiments,
the subject or subject population is 4-
years of age, 6-11 years of age or 12-16 years of age.
[00342] In certain embodiments, the invention is directed to said methods,
wherein said unit dose upon
5
reconstitution with 0.5 mL of a
pharmaceutically acceptable diluent has a concentration of 3.3 10g10 pfu/0.5
mL to
3.6 log10 pfu/0.5 mL for dengue serotype 1 (e.g. chimeric dengue serotype 2/1
strain), has a concentration of 2.7
log10 pfu/0.5 mL to 4.0 10g10 pfu/0.5 mL for dengue serotype 2 (e.g. dengue
serotype 2 strain), has a
concentration of 4.0 log10 pfu/0.5 mL to 4.6 log10 pfu/0.5 mL for dengue
serotype 3 (e.g. chimeric dengue serotype
2/3 strain) and has a concentration of 4.5 log10 pfu/0.5 mL or 4.6 log10
pfu/0.5 mL to 5.1 log10 pfu/0.5 mL for
dengue serotype 4 (e.g. chimeric dengue serotype 2/4 strain). In preferred
such embodiment, the subject or subject
population is of 2 to 17 years of age, such as 4 to 16 years of age, and
preferably less than 9 years of age. In other
preferred embodiment, the subject or subject population is 4-5 years of age, 6-
11 years of age or 12-16 years of
age.
[00343] In certain embodiments, the invention is directed to said methods,
wherein the concentration of the
dengue serotype 1 (e.g. chimeric dengue serotype 2/1 strain) measured in
pfu/0.5 mL is 1% to 7% of the total
concentration, the concentration of the dengue serotype 2 (e.g. dengue
serotype 2 strain) measured in pfu/0.5 mL is
less than 8% of the total concentration, such as in the range of 1% to 8% of
the total concentration, the
concentration of the dengue serotype 3 (e.g. chimeric dengue serotype 2/3
strain) measured in pfu/0.5 mL is at least
10% of the total concentration, and the concentration of the dengue serotype 4
(e.g. chimeric dengue serotype 2/4
strain) measured in pfu/0.5 mL is at least 65% of the total concentration,
such as in the range of 65% to 80%. In
certain such embodiments, the arithmetic sum of all four serotypes is in the
range of 4.6 log10 pfu/0.5 mL to 6.7
log10 pfu/0.5 mL, preferably in the range of 4.6 log10 pfu/0.5 mL to 5.5 log10
pfu/0.5 mL Preferably, in said
embodiments the subject or subject population is of 2 to 17 years of age, such
as 4 to 16 years of age, and even
more preferably less than 9 years of age. In other preferred embodiment, the
subject or subject population is 4-5
years of age, 6-11 years of age 01 12-16 years of age.
[00344] In a further preferred embodiment, the invention is directed to said
methods, wherein the dengue
serotype 1 (e.g. chimeric dengue serotype 2/1 strain) such as TDV-1 and the
dengue serotype 2 (e.g. dengue
serotype 2 strain) such as Tr:IV-2 are present each in a concentration based
on the total concentration in pfu/0.5 mL
which is within 5%-point of each other and/or are together less than about 10%
of the total concentration in
pfu/0.5 mL In certain such embodiments the dengue serotype 3 (e.g. chimeric
dengue serotype 2/3 strain) such as
TDV-3 is preferably at least about 10% of the total concentration in pfu/0.5
mL and more preferably the dengue
serotype 4 (e.g. chimeric dengue serotype 2/4 strain) such as TDV-4 is at
least about 70% of the total concentration
in pfu/0.5 mL In certain such embodiments the dengue serotype 4 (e.g. chimeric
dengue serotype 2/4 strain) such
as TDV-4 represents the highest concentration in the composition of all four
serotypes, preferably with at least about
70% of the total concentration in pfu/0.5 mL, dengue serotype 3 (e.g. chimeric
dengue serotype 2/3 strain) such as
TDV-3 represents the second highest concentration in the composition of all
four serotypes, preferably with at least
about 10% of the total concentration in pfu/0.5 mL, and dengue serotype 1
(e.g. chimeric dengue serotype 2/1
strain) such as WV-1 and dengue serotype 2 (e.g. dengue serotype 2 strain)
such as TDV-2 each represent lower
concentrations than the concentration of serotype 3 (e.g. chimeric dengue
serotype 2/3 strain) such as TDV-3, and
optionally together represent less than about 10% of the total concentration
in pfu/0.5 mt.
[00345] Preferably, the chimeric dengue serotype 2/1 strain is WV-1, the
dengue serotype 2 strain is TDV-2, the
chimeric dengue serotype 2/3 strain is TDV-3 and the chimeric dengue serotype
2/4 strain is TDV-4. More preferably,
58
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
TDV-1 is characterized by the nucleotide sequence according to SEQ ID No. 1
and the amino acid sequence
according to SEQ ID No. 2, TDV-2 is characterized by the nucleotide sequence
according to SEQ ID No. 3 and the
amino acid sequence according to SEQ ID No. 4, TDV-3 is characterized by the
nucleotide sequence according to
SEQ ID No. 5 and the amino acid sequence according to SEQ ID No. 6 and TDV-4
is characterized by the nucleotide
sequence according to SEQ ID No. 7 and the amino acid sequence according to
SEQ ID No. 8.
[00346] In certain embodiments, the invention is directed to said methods,
wherein the reconstituted unit close of
the invention as described herein is administered by subcutaneous injection.
According to some of these
embodiments, the subcutaneous injection is administered to the arm, preferably
to the deltoid region of the arm.
[00347] In certain embodiments, the invention is directed to said methods,
wherein the reconstituted unit dose is
administered to a subject of unknown serostatus and/or wherein no test has
been carried out to determine whether
the subject is seropositive or seronegative (before) the unit dose as
described herein is administered. In certain
embodiments, the invention is directed to said methods which do not include a
step of determination of a previous
dengue infection in the subject or subjects. In certain embodiments, the
invention is directed to said methods which
do not include the analysis of the seroprevalence rate in the region or is
conducted in a region with a seroprevalence
of below 80%, below 70% or below 60%. In certain embodiments the invention is
directed to a method wherein the
serostatus of the subject is unknown. In such embodiments the serostatus is
not determined at any time before and
after administration in relation to this method. In certain embodiments of the
invention the method is used in an
outbreak situation. In certain embodiments, the invention is directed to said
methods being conducted outside a
clinical trial
[00348] In certain embodiments, the invention is directed to said methods,
wherein the subject, or subject
population is seronegative to all dengue serotypes.
[00349] In certain embodiments, the invention is directed to said methods,
wherein two unit doses of the
invention as described herein are administered. In some embodiments the two
unit doses are administered within 12
months or more, or within six months, or within three months, and optionally
at least 4 weeks apart such as at day 0
and day 90 or at day 1 and day 90. According to some of these embodiments, a
further third unit dose of the
invention as described herein is administered after the second administration.
Such a third administration may act as
a booster and may be administered between 6 to 12 months after the first
administration, such as 12 months after
the first administration, or later than 12 month after the first
administration, such as 12 months (1 year) after the
second administration or even 5 years or longer after the first or second
administration.
[00350] In certain embodiments, the method of the invention comprises or
consists of a single unit dose of the
invention being administered.
[00351] In certain embodiments, the invention is directed to said methods,
wherein the reconstituted unit dose of
the invention as described herein is administered subcutaneously to a subject
or subject population that is
seronegative with respect to all dengue serotypes. In other embodiments, the
subject or subject population is
seropositive with respect to at least one dengue serotype_
[00352] In certain embodiments, the invention is directed to said methods,
wherein the unit dose of the invention
as described herein is administered to a subject or subject population from a
dengue endemic region. In some of
these embodiments, the subject or subject population is from Singapore,
Dominican Republic, Panama, Philippines,
Colombia, Puerto Rico or Thailand, in particular from Singapore, Dominican
Republic, Panama, or Philippines. In a
59
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
preferred embodiment, the subject or subject population is from Asia Pacific
or from Latin America. In some other of
these embodiments, the subject or subject population is from Thailand, Sri
Lanka, Philippines, Panama, Nicaragua,
Dominican Republic, Colombia or Brazil. In other embodiments, the subject, or
subject population is from a dengue
non-endemic region. Such a subject population or such a subject may be
vaccinated according to the present
invention in the context of traveling to a dengue endemic region. In certain
embodiments, the reconstituted unit
dose of the invention as described herein is administered subcutaneously to a
subject, or subject population that is
from a dengue endemic region or a dengue non-endemic region.
[00353] In certain embodiments, the invention is directed to said methods,
wherein the reconstituted unit close of
the invention as described herein is administered subcutaneously to a subject
or subject population of 2 to 60 years
of age. In some embodiments, the subjects or subject population are adults of
more than 17 years, or more than 18
years, or 18 to 60 years. In further specific embodiments, the subjects or
subject population are adults of more than
21 years, or 21 to 60 years, or 21 to 45 years of age.
[00354] In certain embodiments, the invention is directed to said methods,
wherein the reconstituted unit dose of
the invention as described herein is administered subcutaneously to children
and adolescents of 2 to 17 years of age.
In some embodiments, the subjects or subject population are less than 9 years
of age, or less than 4 years of age. In
some embodiments, the subjects or subject population are from 2 to 9 years of
age, or from 2 to 5 years of age, or
from 4 to 9 years of age or from 6 to 9 years of age. In other embodiment, the
subject or subject population is 4 to
16 years of age. In some such embodiments, the subject or subject population
is 4-5 years of age, 6-11 years of age
or 12-16 years of age. Optionally, the subject or subject population is
seronegative with respect to all dengue
serotypes.
[00355] In certain embodiments, the invention is directed to said methods,
wherein the unit dose of the invention
as described herein is administered to a pediatric subject or pediatric
subject population of less than 2 years of age,
preferably of 2 months to 2 years or 2 months to 1.5 years or 2 months to 1
year. According to some of these
embodiments, the pediatric subject or pediatric subject population is
seronegative and from a dengue endemic
region.
[00356] In certain embodiments, the invention is directed to said methods,
wherein the reconstituted unit dose of
the invention as described herein is administered to a pediatric subject or
pediatric subject population of less than 2
years of age, preferably of 2 months to 2 years or 2 months to 1.5 years or 2
months to 1 year, preferably by
subcutaneous injection. According to some of these embodiments, the pediatric
subject or pediatric subject
population is seronegative and from a dengue endemic region.
[00357] In a certain embodiments, the invention is directed to said methods,
wherein the subject or subject
population is 4-5 years of age and from Asia Pacific, 6-11 years of age and
from Asia Pacific, or 12-16 years of age
and from Asia Pacific. In other embodiments, the subject or subject population
is 4-5 years of age and from Latin
America, 6-11 years of age and from Latin America, or 1246 years of age and
from Latin America.
[00358] In a certain embodiments, the invention is directed to said methods,
wherein the subject or subject
population is 4-5 years of age and seropositive for at least 1 dengue
serotype, 6-11 years of age and seropositive for
at least 1 dengue serotype, or 12-16 years of age and seropositive for at
least 1 dengue serotype. In other
embodiments, the subject or subject population is 4-5 years of age and
seronegative for all dengue serotypes, 6-11
years of age and seronegative for all dengue serotypes, or 12-16 years of age
and seronegative for all dengue
serotypes.
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00359] In a certain embodiments, the invention is directed to said methods,
wherein the subject or subject
population is from Asia Pacific or Latin America and seropositive for at least
one dengue serotype at baseline. In
other embodiments, the subject or subject population is from Asia Pacific or
Latin America and seronegative for at all
dengue serotype at baseline.
[00360] In certain embodiments, the invention is directed to sad methods,
wherein the subject or subject
population is from Asia Pacific, seropositive for at least one dengue serotype
at baseline and 4-5 years of age, 6-11
years of age, or 12-16 years of age. In other embodiments, the subject or
subject population is from Asia Pacific,
seronegative for all dengue serotypes at baseline and 4-5 years of age, 6-11
years of age, or 12-16 years of age. In
yet other embodiments, the subject or subject population is from Latin
America, seropositive for at least one dengue
serotype at baseline and 4-5 years of age, 6-11 years of age, or 12-16 years
of age. In other embodiments, the
subject or subject population is from America, seronegative for all dengue
serotypes at baseline and 4-5 years of
age, 6-11 years of age, or 12-16 years of age.
[00361] In certain embodiments, the invention is directed to sad methods,
wherein the subject or subject
population had prior vaccination against Yellow Fever. In other embodiments,
the subject or subject population had
prior vaccination against Japanese Encephalitis. In yet other embodiments, the
subject or subject population had no
prior vaccination against Yellow Fever. In other embodiments, the subject or
subject population had no prior
vaccination against Japanese Encephalitis. Prior vaccination indicates a
vaccination prior to 30 days after a second
administration, such as within 4 months after the first administration, with
the reconstituted unit dose as described
herein. For example for vaccine efficacy (VE) as determined in Example 6 from
30 days post-second vaccination, a
prior vaccination of Yellow Fever is defined as a Yellow Fever vaccination
occurring before 30 days post-second
vaccination. In certain embodiments, the subject or subject population
received DengvaxiaCt within the
administration regimen as described herein or within 4.5 years after
administration of the first dose.
[00362] Particularly unbalanced titers of neutralizing antibodies against the
four dengue serotypes are observed in
seronegative populations or subjects after administration of the commercially
available dengue vaccine. The present
invention shows that in particular seronegative subjects show a more balanced
immune response to the four dengue
serotypes after administration of the reconstituted unit dose of the invention
as described herein. It is therefore
contemplated that the unit dose of the invention as described herein and
methods of the present invention as
described herein may provide a more robust immune response in a subject
population including both seropositive
and seronegative subjects. This balanced response and balanced efficacy and
safety is required to allow inoculation
without prior serastatus analysis which is a major advantage in vaccination
programs and in particular in outbreak
situations_
[00363] The present invention is directed in part to a method of preventing
virologically confirmable dengue
disease in a subject comprising administering to the subject a tetravalent
dengue virus composition including four
dengue virus strains representing serotype 1, serotype 2, serotype 3 and
serotype 4, wherein the virus strains are
optionally live, attenuated dengue virus strains.
[00364] The present invention is directed in part to a method of preventing
virologically confirmable dengue
disease in a subject consisting of administering to the subject a tetravalent
dengue virus composition including four
dengue virus strains representing serotype 1, serotype 2, serotype 3 and
serotype 4, wherein the virus strains are
optionally live, attenuated dengue virus strains.
61
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00365] In certain embodiments, the invention is directed to said methods,
wherein there is no step of determining
the serostatus of the subject at baseline, in other words, said methods do not
comprise a determination of a previous
dengue infection of the subject at baseline before the administration of the
tetravalent dengue virus composition. In
particular, such methods are safe and effective. Thus, in certain such
embodiments, the subject has not been tested
for the presence a previous dengue infection.
[00366] In certain embodiments, the invention is directed to said methods,
wherein the vaccine administration is
safe irrespective of whether there is a determination that the subject had a
previous dengue infection before the
administration of the tetravalent dengue virus composition. In particular,
such methods are also effective.
[00367] In certain embodiments, the invention is directed to said methods,
wherein the method is safe and/or
effective.
[00368] In certain embodiments, the invention is directed to said methods,
wherein the composition includes at
least one chimeric dengue virus. In certain such embodiments, the invention is
directed to said methods, wherein the
composition includes at least one non-chimeric dengue virus and at least one
chimeric dengue virus, in particular a
chimeric dengue serotype 2/1 strain and a dengue serotype 2 strain and a
chimeric dengue serotype 2/3 strain and a
chimeric dengue serotype 2/4 strain. The details of the composition are
described above.
[00369] The-Sore, in certain embodiments, the invention is directed to said
methods having a vaccine efficacy,
preferably a combined vaccine efficacy against all four serotypes, in
preventing virologically confirmable dengue
disease with a 2-sided 95% confidence interval, wherein the lower bound is
more than 25%, when measured against
placebo in a subject population of at least 5,000 healthy subjects (or at
least 10,000, or at least 15,000 healthy
subjects) irrespective of serostatus at baseline and e.g. 14 to 16 years of
age, wherein a reconstituted unit
dose/tetravalent dengue virus composition as described herein or placebo is
administered e.g. at least twice within
less than 6 months, such as within 3 months, after first administration or 30
days after the second/last administration
until at least 12 to 18 months (e.g. at 12 or at 18 months) after the second/
last administration. In embodiments, the
invention is directed to said methods having a vaccine efficacy, preferably a
combined vaccine efficacy against all
four serotypes, in preventing virologically confirmable dengue disease with a
2-sided 95% confidence interval,
wherein the lower bound is more than 25%, when measured against placebo in a
subject population of at least 5,000
healthy subject (or at least 10,000, or at least 15,000 healthy subjects)
irrespective of serostatus at baseline,
wherein a reconstituted unit dose or tetravalent dengue virus composition as
described herein or placebo is
administered at least once, until 15 to 21 months (e.g. 15 or 21 months) after
the first administration of the
administration schedule. In certain such embodiments, the lower bound is more
than 30 k, more than 40%, more
than 50%, more than 55%, more than 60%, more than 65%, more than 70% or more
than 72%. Preferably said
reconstituted unit dose or placebo is administered subcutaneously within about
3 months, such as on days 0 and 90.
[00370] Therefore, in certain embodiments, the invention is directed to said
methods having a combined vaccine
efficacy against all four serotypes, in preventing virologically confirmable
dengue disease with a 2-sided 95%
confidence interval, wherein the lower bound is more than 60%, when measured
against placebo in a subject
population of at least 5,000 healthy subjects (or at least 10,000, or at least
15,000 healthy subjects) irrespective of
serostatus at baseline and 4 in 16 years of age, wherein a reconstituted unit
dose/tetravalent dengue virus
composition as described herein or placebo is administered e.g. at least twice
within less than 6 months, such as
within 3 months, after the first administration until 18 months after the last
administration. In these embodiments,
the lower bound is e.g. more than 62%, more than 64%, more than 66%, more than
68%, or more than 69%.
62
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00371] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy, preferably a
combined vaccine efficacy against all four serotypes, in preventing
virologically confirmable dengue disease of more
than 30%, when measured against placebo in a subject population of at least
5,000 healthy subjects (or at least
10,0001 or at least 15,000 healthy subjects) irrespective of serostatus at
baseline and e.g. 14 to 16 years of age,
wherein a reconstituted unit dose or tetravalent dengue virus composition as
described herein or placebo is
administered at least twice within less than 6 months, such as within 3
months, after first administration or 30 days
after the second administration/last administration until at least 12 months
or until 12 to 18 months (e.g. at 12 or at
18 months) after the second administration/last administration. In certain
embodiments, the invention is directed to
said methods having a vaccine efficacy, preferably a combined vaccine efficacy
against all four serotypes, in
preventing virologically confirmable dengue disease of more than 30%, when
measured against placebo in a subject
population of at least 5,000 healthy subjects (or at least 10,000, or at least
15,000 healthy subjects) irrespective of
serostatus at baseline, wherein a reconstituted unit dose or tetravalent
dengue virus composition as described herein
or placebo is administered at least once, until 15 months after the first
administration of the administration schedule.
In certain such embodiments, the vaccine efficacy is more than 40%, more than
50%, more than 55%, more than
60%, more than 65%, more than 70%, more than 75%, more than 78 k, more than
79% or about 80%. Preferably
said reconstituted unit dose or placebo is administered subcutaneously within
about 3 month, such as on days 0 and
90.
[00372] Therefore, in certain embodiments, the invention is directed to said
methods having a combined vaccine
efficacy against all four serotypes, in preventing virologically confirmable
dengue disease of more than 66%, when
measured against placebo in a subject population of at least 5,000 healthy
subjects (or at least 10,000, or at least
15,000 healthy subjects) irrespective of serostatus at baseline and 14 to 16
years of age, wherein a reconstituted
unit dose/tetravalent dengue virus composition as described herein or placebo
is administered e.g. at least twice
within less than 6 months, such as within 3 months, after the first
administration until 18 months after the last
administration. In these embodiments, the vaccine efficacy is e.g. more than
68%, more than 70%, more than 72%,
or more than 74%.
[00373] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy, preferably a
combined vaccine efficacy against all four serotypes, in preventing
virologically confirmable dengue disease with
hospitalization with a 2-sided 95% confidence interval, wherein the lower
bound is more than 0%, when measured
against placebo in a subject population of at least 5,000 healthy subjects (or
at least 10,000, or at least 15,000
healthy subjects) irrespective of serostatus at baseline, wherein a
reconstituted unit dose or tetravalent dengue virus
composition as described herein or placebo is administered at least twice
within less than 6 months, such as within 3
months, 30 days after the second administration until at least 18 months after
the second administration. In certain
such embodiments, the lower bound is more than 10%, is more than 20%, is more
than 30%, is more than 40%, is
more than 50%, is more than 55%, is more than 60%, is more than 65%, is more
than 70% or is more than 80%,
or more than 90%.
[00374] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four dengue serotypes in seronegative subjects with a 2-sided 95%
confidence interval, wherein the lower
bound is more than 25%, when measured against placebo in a subject population
of at least 1,500 or at least 2,000
healthy subjects being seronegative against all serotypes at baseline, wherein
said unit dose/tetravalent dengue virus
composition or said placebo is administered at least twice within less than 6
months, such as within 3 months, about
30 days after the second administration of the administration schedule until
at least 12 months or until 12 to 18
63
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
months (e.g. at 12 or at 18 months) after the second administration of the
administration schedule. In certain such
embodiments, the lower bound is more than 30%, is more than 40%, is more than
50%, or is more than 55%.
[00375] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with a 2-sided 95% confidence
interval, wherein the lower bound S more than 25%, when measured against
placebo in a subject population of at
least 1,500 or at least 2,000 or at least 5,000 healthy subjects (or at least
10,000, or at least 15,000 healthy
subjects) being seronegative against all serotypes at baseline and 4 to 16
years of age, wherein a reconstituted unit
dose/tetravalent dengue virus composition as described herein or placebo is
e.g. administered at least twice within
less than 6 months, such as within 3 months, from 30 days post last
administration until 12 to 18 months (e.g. at 12
months or at 18 months) after the last administration. In certain such
embodiments, the lower bound S more than
30%, is more than 35%, is more than 40%, or is more than 45%.
[00376] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with a 2-sided 95% confidence
interval, wherein the lower bound is more than 25%, when measured against
placebo in a subject population of at
least 5,000 healthy subjects (or at least 10,000, or at least 15,000 healthy
subjects) being seropositive at baseline
and 4 to 16 years of age, wherein a reconstituted unit dose/tetravalent dengue
virus composition as described herein
or placebo is e.g. administered at least twice within less than 6 months, such
as within 3 months, from 30 days post
last administration until 12 to 18 months (e.g. at 12 months or at 18 months)
after the last administration. In certain
such embodiments, the lower bound is more than 40%, is more than 45%, is more
than 50%, is more than 60%, or
is more than 65%.
[00377] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with a 2-sided 95% confidence
interval, wherein the lower bound is more than 25%, when measured against
placebo in a subject population of at
least 5,000 healthy subjects (or at least 10,000, or at least 15,000 healthy
subjects) being seropositive at baseline
being or seronegative against all serotypes at baseline and 4 to 16 years of
age, wherein a reconstituted unit
close/tetravalent dengue virus composition as described herein Or placebo is
e.g. administered at least twice within
less than 6 months, such as within 3 months, from 30 days post last
administration until 12 to 18 months (e.g. at 12
months or at 18 months) after the last administration. In certain such
embodiments, the difference between the
lower bound provided by the seropositive subjects at baseline and the subjects
seronegative against all serotypes at
baseline is no more than 15%-points.
[00378] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four dengue serotypes in seronegative subjects of more than 30%,
when measured against placebo in a
subject population of at least 1,500 or at least 2,000 healthy subjects being
seronegative against all serotypes at
baseline, wherein said unit dose/tetravalent dengue virus composition or said
placebo is administered at least twice
within less than 6 months, such as within 3 months, 30 days after the second
administration until at least 12 months
or until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration. In certain such embodiments,
the combined vaccine efficacy against all four dengue serotypes in
seronegative subjects is more than 40%, is more
than 50%, is more than 60%, S more than 65%, or is more than 70%.
[00379] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease, when measured against placebo in
a subject population of at least 1,500 or at least 2,000 or at least 5,000
healthy subjects (or at least 10,000, or at
64
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
least 15,000 healthy subjects) being seronegative against all serotypes at
baseline and 4 to 16 years of age, wherein
a reconstituted unit dose/tetravalent dengue virus composition as described
herein or placebo is e.g. administered at
least twice within less than 6 months, such as within 3 months, from 30 days
post last administration until 12 to 18
months (e.g. at 12 months or at 18 months) after the last administration. In
certain such embodiments the said
vaccine efficacy is more than 30 k, is more than 40%, is more than 50%, is
more than 55%, is more than 60%, or is
more than 65%.
[00380] In certain embodiments, the invention S directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease, when measured against placebo in
a subject population of at least 5,000 healthy subjects (or at least 10,000,
or at least 15,000 healthy subjects) being
seropositive at baseline and 4 to 16 years of age, wherein a reconstituted
unit dose/tetravalent dengue virus
composition as described herein or placebo is e.g. administered at least twice
within less than 6 months, such as
within 3 months, from 30 days post last administration until 12 to 18 months
(e.g. at 12 months or at 18 months)
after the last administration. In certain such embodiments the said vaccine
efficacy is more than 40%, is more than
50%, is more than 60%, is more than 65%, is more than 70%, or is more than
75%.
[00381] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with a 2-sided 95% confidence
interval, wherein the lower bound S more than 25%, when measured against
placebo in a subject population of at
least 5,000 healthy subjects (or at least 10,000, or at least 15,000 healthy
subjects) being seropositive at baseline
being or seronegative against all serotypes at baseline and 4 to 16 years of
age, wherein a reconstituted unit
dose/tetravalent dengue virus composition as described herein or placebo is
e.g. administered at least twice within
less than 6 months, such as within 3 months, from 30 days post last
administration until 12 to 18 months (e.g. at 12
months or at 18 months) after the last administration. In certain such
embodiments, the difference between the
lower bound provided by the seropositive subjects at baseline and the subjects
seronegative against all serotypes at
baseline is no more than 15%-points, or S no more than 10%-points.
[00382] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four dengue serotypes with a 2-sided 95% confidence interval,
wherein the lower bound is more than
25%, when measured against placebo in a subject population of at least 1,000
healthy subjects 4 to 5 years of age
at the time of randomization and irrespective of serostatus at baseline,
wherein said unit dose/tetravalent dengue
virus composition or said placebo is administered at least twice within less
than 6 months, such as within 3 months,
about 30 days after the second administration of the administration schedule
until at least 12 months or until 12 to
18 months (e.g. at 12 or at 18 months) after the second administration of the
administration schedule. In certain
such embodiments, the lower bound is more than 30%, is more than 40%, is more
than 45%.
[00383] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four dengue serotypes of more than 30%, when measured against
placebo in a subject population of at
least 1,000 healthy subjects 4 to 5 years of age at the time of randomization
and irrespective of serostatus at
baseline, wherein said unit dose/tetravalent dengue virus composition or said
placebo is administered at least twice
within less than 6 months, such as within 3 months, 30 days after the second
administration until at least 12 months
or until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration. In certain such embodiments,
the combined vaccine efficacy against all four dengue serotypes is more than
40%, is more than 50%, is more than
60%, is more than 65%, or is more than 70%.
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00384] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four dengue serotypes with a 2-sided 95% confidence interval,
wherein the lower bound is more than
25%, when measured against placebo in a subject population of at least 1,000
healthy subjects 6 to 11 years of age
at the time of randomization and irrespective of serostatus at baseline,
wherein said unit dose/tetravalent dengue
virus composition or said placebo is administered at least twice within less
than 6 months, such as within 3 months,
about 30 days after the second administration of the administration schedule
until at least 12 months or until 12 to
18 months (e.g. at 12 or at 18 months) after the second administration of the
administration schedule. In certain
such embodiments, the lower bound is more than 30%, is more than 40%, is more
than 50%, is more than 60%, or
is more than 70%.
[00385] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four dengue serotypes of more than 30%, when measured against
placebo in a subject population of at
least 1,000 healthy subjects 6 to 11 years of age at the time of randomization
and irrespective of serostatus at
baseline, wherein said unit dose/tetravalent dengue virus composition or said
placebo is administered at least twice
within less than 6 months, such as within 3 months, 30 days after the second
administration until at least 12 months
or until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration. In certain such embodiment,
the combined vaccine efficacy against all four dengue serotypes is more than
40%, is more than 50%, is more than
60%, is more than 70%, is more than 75%, or is more than 80%.
[00386] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four dengue serotypes with a 2-sided 95% confidence interval,
wherein the lower bound is more than
25%, when measured against placebo in a subject population of at least 1,000
healthy subjects 12 to 16 years of
age at the time of randomization and irrespective of serostatus at baseline,
wherein said unit dose/tetravalent
dengue virus composition or said placebo is administered at least twice within
less than 6 months, such as within 3
months, about 30 days after the second administration of the administration
schedule until at least 12 months or
until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration of the administration schedule. In
certain such embodiments, the lower bound is more than 30%, is more than 40%,
is more than 50%, is more than
60%, is more than 65%, or is more than 68%.
[00387] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four dengue serotypes of more than 30%, when measured against
placebo in a subject population of at
least 1,000 healthy subjects 12 to 16 years of age at the time of
randomization and irrespective of serostatus at
baseline, wherein said unit dose/tetravalent dengue virus composition or said
placebo is administered at least twice
within less than 6 months, such as within 3 months, 30 days after the second
administration until at least 12 months
or until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration. In certain such embodiments,
the combined vaccine efficacy against all four dengue serotypes is more than
40%, is more than 50%, is more than
60%, is more than 70%, is more than 75%, or is more than 80%.
[00388] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
dengue serotype 1 with a 2-sided 95% confidence interval, wherein the lower
bound is more than 25%, when
measured against placebo in a subject population of at least 5,000 healthy
subjects, or at least 10,000 healthy
subjects, or at least 15,000 healthy subjects irrespective of serostatus at
baseline, wherein said unit dose/tetravalent
dengue virus composition or said placebo is administered at least twice within
less than 6 months, such as within 3
months, about 30 days after the second administration of the administration
schedule until at least 12 months or
until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration of the administration schedule. In
certain such embodiments, the lower bound is more than 30%, is more than 40%,
or is more than 50%.
66
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00389] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
serotype 1, in preventing virologically confirmable dengue disease with a 2-
sided 95% confidence interval, wherein
the lower bound S more than 25%, when measured against placebo in a subject
population of at least 1,500, or at
least 2,000, or at least 5,000 healthy subjects (or at least 10,000, or at
least 15,000 healthy subjects) irrespective of
serostatus at baseline and 4 to 16 years of age, wherein a reconstituted unit
dose/tetravalent dengue virus
composition as described herein or placebo is e.g. administered at least twice
within less than 6 months, such as
within 3 months, from 30 days post last administration until 12 to 18 months
(e.g. at 12 or at 18 months) after the
last administration. In certain such embodiments, the lower bound is more than
30%, is more than 35% is more
than 40 k, is more than 45%, is more than 503'o, or is more than 54 40.In
certain such embodiments the subject
population of at least 1,500 is seronegative against all serotypes at base
line and the lower bound is more than 35%.
In certain such embodiments the seronegative and seropositive population each
provide a vaccine efficacy against
serotype 1 with a 2-sided 95% confidence interval, wherein the lower bounds
are within 10 %-points.
[00390] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
dengue serotype 1 of more than 30%, when measured against placebo in a subject
population of at least 5,000
healthy subjects, or at least 10,000 healthy subjects, or at least 15,000
healthy subjects irrespective of serostatus at
baseline, wherein said unit dose/tetravalent dengue virus composition or said
placebo is administered at least twice
within less than 6 months, such as within 3 months, 30 days after the second
administration until at least 12 months
or until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration. In certain such embodiments,
the vaccine efficacy against dengue serotype 1 is more than 40%, is more than
50%, is more than 60%, is more
than 65%, or is more than 70%.
[00391] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
serotype 1, in preventing virologically confirmable dengue disease, when
measured against placebo in a subject
population of at least 1,500, or at least 2,000, or at least 5,000 healthy
subjects (or at least 10,000, or at least
15,000 healthy subjects) irrespective of serostatus at baseline and 4 to 16
years of age, wherein a reconstituted unit
close/tetravalent dengue virus composition as described herein or placebo is
e.g. administered at least twice within
less than 6 months, such as within 3 months, from 30 days post last
administration until 12 to 18 months (e.g. at 12
or at 18 months) after the last administration. In certain such embodiments,
the vaccine efficacy is more than 40%,
is more than 50%, is more than 60%, or is more than 65%. In certain such
embodiments the subject population of
at least 1,500 is seronegative against all serotypes at base line. In certain
such embodiments the seronegative and
seropositive population each provide a vaccine efficacy against serotype 1
which are within 5 k-points.
[00392] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
dengue serotype 2 with a 2-sided 95% confidence interval, wherein the lower
bound is more than 25%, when
measured against placebo in a subject population of at least 5,000 healthy
subjects, or at least 10,000 healthy
subjects, or at least 15,000 healthy subjects irrespective of serostatus at
baseline, wherein said unit dose/tetravalent
dengue virus composition or said placebo is administered at least twice within
less than 6 months, such as within 3
months, about 30 days after the second administration of the administration
schedule until at least 12 months or
until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration of the administration schedule. In
certain such embodiments, the lower bound is more than 30%, is more than 40%,
is more than 50, is more than 60,
is more than 70, is more than 80, or is more than 90%.
[00393] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
serotype 2, in preventing virologically confirmable dengue disease with a 2-
sided 95% confidence interval, wherein
the lower bound is more than 25%, when measured against placebo in a subject
population of at least 1,500, or at
67
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
least 2,000, or at least 5,000 healthy subject (or at least 10,000, or at
least 15,000 healthy subjects) irrespective of
serostatus at baseline and 4 to 16 years of age, wherein a reconstituted unit
dose/tetravalent dengue virus
composition as described herein or placebo is e.g. administered at least twice
within less than 6 months, such as
within 3 months, from 30 days post last administration until 12 to 18 months
(e.g. at 12 or at 18 months) after the
last administration. In certain such embodiments, the lower bound is more than
50%, is more than 60%, is more
than 70%, is more than 80%, or is more than 85%. In certain such embodiments
the subject population of at least
1,500, is seronegative against all serotypes. In certain such embodiments the
seronegative and seropositive
population each provide a vaccine efficacy against serotype 2 with a 2-sided
95% confidence interval, wherein the
lower bounds are within 5 %-points.
[00394] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
dengue serotype 2 of more than 30%, when measured against placebo in a subject
population of at least 5,000
healthy subject, or at least 10,000 healthy subjects, or at least 15,000
healthy subjects irrespective of serostatus at
baseline, wherein said unit dose/tetravalent dengue virus composition or said
placebo is administered at least twice
within less than 6 months, such as within 3 months, 30 days after the second
administration until at least 12 months
or until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration. In certain such embodiments,
the vaccine efficacy against dengue serotype 2 is more than 40%, is more than
50%, is more than 60%, is more
than 70%, is more than 80, or is more than 90%.
[00395] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
serotype 2, in preventing virologically confirmable dengue disease, when
measured against placebo in a subject
population of at least 1,500, or at least 2,000, or at least 5,000 healthy
subjects (or at least 10,000, or at least
15,000 healthy subjects) irrespective of serostatus at baseline and 4 to 16
years of age, wherein a reconstituted unit
dose/tetravalent dengue virus composition as described herein or placebo is
e.g. administered at least twice within
less than 6 months, such as within 3 months, from 30 days post last
administration until 12 to 18 months (e.g. at 12
or at 18 months) after the last administration. In certain such embodiments,
the vaccine efficacy is more than 60%,
is more than 70%, is more than WM, or is more than 90%. In certain such
embodiments the subject population of
at least 1,500 is seronegative against all serotypes at base line. In certain
such embodiments the seronegative and
seropositive population each provide a vaccine efficacy against serotype 2
which are within 5 %-points.
[00396] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
dengue serotype 3 with a 2-sided 95% confidence interval, wherein the lower
bound is more than 25%, when
measured against placebo in a subject population of at least 5,000 healthy
subjects, or at least 10,000 healthy
subjects, or at least 15,000 healthy subjects in-espective of serostatus at
baseline, wherein said unit dose/tetravalent
dengue virus composition or said placebo is administered at least twice within
less than 6 months, such as within 3
months, about 30 days after the second administration of the administration
schedule until at least 12 months after
the second administration of the administration schedule. In certain such
embodiments, the lower bound is more
than 30%, is more than 40%.
[00397] In certain embodiments, the invention is directed to said methods
having a vaccine efficacy against
dengue serotype 3 of more than 30%, when measured against placebo in a subject
population of at least 5,1300
healthy subjects, or at least 10,000 healthy subjects, or at least 15,000
healthy subjects irrespective of serostatus at
baseline, wherein said unit dose/tetravalent dengue virus composition or said
placebo is administered at least twice
within less than 6 months, such as within 3 months, 30 days after the second
administration until at least 12 months
after the second administration. In certain such embodiments, the vaccine
efficacy against dengue serotype 3 is
more than 40%, is more than 50%, is more than 55%, or is more than 60%.
68
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00398] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with hospitalization with a 2-sided
95% confidence interval, wherein the lower bound is more than 25%, when
measured against placebo in a subject
population of at least 5,000 healthy subjects (or at least 10,000, or at least
15,000 healthy subjects) irrespective of
serostatus at baseline and 4 to 16 years of age, wherein a reconstituted unit
dose/tetravalent dengue virus
composition as described herein or placebo is e.g. administered at least twice
within less than 6 months, such as
within 3 months, from first administration until 12 lo 18 months (e.g. at 12
months or at 18 months) after the last
administration, or from 30 clays post last administration until 12 to 18
months (e.g. at 12 or at 18 months) after the
last administration. In certain such embodiments, the lower bound is more than
10%, is more than 20%, is more
than 30%, is more than 40%, is more than 50%, is more than 55%, is more than
60%, is more than 65%, is more
than 66%, is more than 67%, is more than 70%, is more than 75%, is more than
77%, or is more than 80%.
[00399] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with hospitalization, when measured
against placebo in a subject population of at least 5,000 healthy subjects (or
at least 10,000, or at least 15,000
healthy subjects) irrespective of serostatus at baseline and 4 to 16 years of
age, wherein a reconstituted unit
dose/tetravalent dengue virus composition as described herein or placebo is
e.g. administered at least twice within
less than 6 months, such as within 3 months, from first administration until
12 to 18 months (e.g. at 12 months or at
18 months) after the last administration, or from 30 days post last
administration until 12 to 18 months (e.g. at 12 or
at 18 months) after the last administration. In certain such embodiments, the
vaccine efficacy is more than is more
than 70%, is more than 75%, is more than 80%, or is more than 82%, or is more
than 85%, more than 88%.
[00400] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against virologically-confirmed dengue with hospitalization against all four
serotypes with a 2-sided 95% confidence
interval, wherein the lower bound is more than 25%, when measured against
placebo in a subject population of at
least 1,500 or at least 2,000 healthy subjects being seronegative against all
serotypes at baseline, wherein said unit
close/tetravalent dengue virus composition or said placebo is administered at
least twice within less than 6 months,
such as within 3 months, about 30 days after the second administration of the
administration schedule until at least
12 months or until 12 to 18 months (e.g. at 12 or at 18 months) after the
second administration of the
administration schedule. In certain such embodiments, the lower bound is more
than 30%, is more than 40%, is
more than 50%, is more than 60%, is more than 70%, or is more than 75%.
[00401] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with hospitalization with a 2-sided
95% confidence interval, wherein the lower bound is more than 25%, when
measured against placebo in a subject
population of at least 5,000 healthy subjects (or at least 10,000, or at least
15,000 healthy subjects) being
seronegative against all serotypes at baseline and 4 to 16 years of age,
wherein a reconstituted unit dose/tetravalent
dengue virus composition as described herein or placebo is e.g. administered
at least twice within less than 6
months, such as within 3 months, from 30 days post last administration until
12 to 18 months (e.g. at 12 months or
at 18 months) after the last administration. In certain such embodiments, the
lower bound is more than 60%, is
more than 65%, is more than 66%, is more than 67%, is more than 70%, is more
than 75%, is more than 77% or is
more than 80%.
[00402] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against virologically-confirmed dengue with hospitalization against all four
serotypes of more than 30%, when
measured against placebo in a subject population of at least 1,500 or at least
2,000 healthy subjects, healthy
69
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
subjects being seronegative against all serotypes at baseline, wherein said
unit dose/tetravalent dengue virus
composition or said placebo is administered at least twice within less than 6
months, such as within 3 months, 30
days after the second administration until at least 12 months or until 12 to
18 months (e.g. at 12 or at 18 months)
after the second administration. In certain such embodiments, the combined
vaccine efficacy against virologically-
confirmed dengue with hospitalization against all four serotypes is more than
4004, is more than 50%, is more than
60%, is more than 70%, is more than 80%, or is more than 90%.
[00403] In certain embodiments, the invention S directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with hospitalization, when measured
against placebo in a subject population of at least 5,000 healthy subjects (or
at least 10,000, or at least 15,000
healthy subjects) being seronegative against all serotypes at baseline and 4
to 16 years of age, wherein a
reconstituted unit dose/tetravalent dengue virus composition as described
herein or placebo is e.g. administered at
least twice within less than 6 months, such as within 3 months, from 30 days
post last administration until 12 to 18
months (e.g. at 12 months or at 18 months) after the last administration. In
certain such embodiments, the said
vaccine efficacy is more than 60 /0, S more than 65%, is more than 66%, is
more than 67%, is more than 70%, is
more than 75%, is more than 77%, is more than 80, or is more than 85%.
[00404] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against virologically-confirmed dengue with hospitalization against all four
serotypes with a 2-sided 95 k confidence
interval, wherein the lower bound is more than 25%, when measured against
placebo in a subject population of at
least 1,500 or at least 2,000 healthy subjects being seropositive at baseline,
wherein said unit dose/tetravalent
dengue virus composition or said placebo is administered at least twice within
less than 6 months, such as within 3
months, about 30 days after the second administration of the administration
schedule until at least 12 months or
until 12 to 18 months (e.g. at 12 or at 18 months) after the second
administration of the administration schedule. In
certain such embodiments, the lower bound is more than 30%, is more than 40 k,
is more than 50%, is more than
60%, is more than 70%, or is more than 800/o.
[00405] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with hospitalization with a 2-sided
95% confidence interval, wherein the lower bound is more than 25%, when
measured against placebo in a subject
population of at least 5,000 healthy subjects (or at least 10,000, or at least
15,000 healthy subjects) being
seropositive at baseline and 4 to 16 years of age, wherein a reconstituted
unit dose/tetravalent dengue virus
composition as described herein or placebo is e.g. administered at least twice
within less than 6 months, such as
within 3 months, from 30 days post last administration until 12 to 18 months
(e.g. at 12 months or at 18 months)
after the last administration. In certain such embodiments, the lower bound is
more than 60%, is more than 65%, is
more than 70%, is more than 75%, or is more than 80%.
[00406] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against virologically-confirmed dengue with hospitalization against all four
serotypes of more than 30%, when
measured against placebo in a subject population at least 1,500 or of at least
2,000 healthy subjects, healthy
subjects being seropositive at baseline, wherein said unit dose/tetravalent
dengue virus composition or said placebo
is administered at least twice within less than 6 months, such as within 3
months, 30 days after the second
administration until at least 12 months or until 12 to 18 months (e.g. at 12
or at 18 months) after the second
administration. In certain such embodiments, the combined vaccine efficacy
against virologically-confirmed dengue
with hospitalization against all four serotypes is more than 40%, is more than
50%, is more than 60%, is more than
70%, is more than 80%, or is more than 90%.
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00407] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with hospitalization, when measured
against placebo in a subject population of at least 5,000 healthy subjects (or
at least 10,000, or at least 15,000
healthy subjects) being seropositive at baseline and 4 to 16 years of age,
wherein a reconstituted unit
dose/tetravalent dengue virus composition as described herein or placebo is
e.g. administered at least twice within
less than 6 months, such as within 3 months, from 30 days post last
administration until 12 to 18 months (e.g. at 12
months or at 18 months) after the last administration. In certain such
embodiments, the vaccine efficacy is more
than 75%, is more than 70%, is more than 80%, is more than 85%, or is more
than 90%.
[00408] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serapes, in preventing virologically confirmable dengue
disease with hospitalization with a 2-sided
95% confidence interval, wherein the lower bound is more than 25%, when
measured against placebo in a subject
population of at least 5,000 healthy subjects (or at least 10,000, or at least
15,000 healthy subjects) being
seropositive at baseline being or seronegative against all serotypes at
baseline and 4 to 16 years of age, wherein a
reconstituted unit dose/tetravalent dengue virus composition as described
herein or placebo is e.g. administered at
least twice within less than 6 months, such as within 3 months, from 30 days
post last administration until 12 to 18
months (e.g. at 12 months or at 18 months) after the last administration. In
certain such embodiments, the
difference between the lower bound provided by the seropositive subjects at
baseline and the subjects seronegative
against all serotypes at baseline is no more than 15%-points.
[00409] In ca-Lain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes, in preventing virologically confirmable dengue
disease with hospitalization, when measured
against placebo in a subject population of at least 5,000 healthy subjects (or
at least 10,000, or at least 15,000
healthy subjects) being seropositive at baseline being or seronegative against
all serotypes at baseline and 4 to 16
years of age, wherein a reconstituted unit dose/tetravalent dengue virus
composition as described herein or placebo
is e.g. administered at least twice within less than 6 months, such as within
3 months, from 30 days post last
administration until 12 to 18 months (e.g. at 12 months or at 18 months) after
the last administration. In certain
such embodiments, the difference between the vaccine efficacy provided by the
seropositive subjects at baseline and
the subjects seronegative against all serotypes at baseline is no more than
10%-points or no more than 5%-points.
[00410] In certain embodiments, the invention is directed to said methods
having a relative risk, preferably a
combined relative risk against all four serotypes, with a 2-sided 95%
confidence interval, wherein the upper bound is
less than 0.75, when measured against placebo in a subject population of at
least 5,000 healthy subjects (or at least
10,000, or at least 15,000 healthy subjects) irrespective of serostatus at
baseline, wherein a reconstituted unit
dose/tetravalent dengue virus composition as described herein or placebo is
administered at least twice within less
than 6 months, such as within 3 months, 30 days after the second
administration until at least 12 months after the
second administration. In certain such embodiments, the upper bound is less
than 0.70, less than 0.65, less than
0.60, less than 0.55, less than 0.50, less than 0.45, less than 0.40, less
than 0.35, less than 0.30 or less than 0.28.
Preferably said reconstituted unit dose or placebo is administered
subcutaneously within about 3 month, such as on
days 0 and 90.
[00411] In certain embodiments, the invention is directed to said methods
having a relative risk, preferably a
combined relative risk against all four serotypes, of less than 0.70, when
measured against placebo in a subject
population of at least 5,000 healthy subjects (or at least 10,000, or at least
15,000 healthy subjects) irrespective of
serostatus at baseline, wherein a reconstituted unit dose/tetravalent dengue
virus composition as described herein or
placebo is administered at least twice within less than 6 months, such as
within 3 months, 30 days after the second
71
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
administration until at least 12 months after the second administration. In
certain such embodiment, the relative risk
is less than 0.65, less than 0.60, less than 0.55, less than 0.50, less than
0.45, less than 0.40, less than 0.35, less
than 0.30, less than 0.25 or less than a23. Preferably said reconstituted unit
dose or placebo is administered
subcutaneously within about 3 month, such as on days 0 and 90.
[00412] In certain embodiments, the invention is directed to said methods,
wherein virologically confirmable
dengue disease occurs in less than 25% of the subjects, when measured against
placebo in a subject population of
at least 5,000 healthy subject (or at least 10,000, or at least 15,000 healthy
subjects) in-espective of serostatus at
baseline, wherein a reconstituted unit dose/tetravalent dengue virus
composition as described herein or placebo is
administered at least twice within less than 6 months, such as within 3
months, 30 days after the second
administration until at least 12 months or at least 18 months after the second
administration. In certain such
embodiment, virologically confirmable dengue disease occurs in less than 2.0%
of the subjects, less than 15% of
the subjects, less than 1.0% of the subjects, less than 0.8% of the subject,
or less than 0.6% of the subject.
Preferably said reconstituted unit dose or placebo is administered
subcutaneously within about 3 month, such as on
days 0 and 90.
[00413] In ca-Lain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes with a 2-sided 95% confidence interval, wherein the
lower bound is more than 61.0%, or
more than 65.0 or more than 70.0% or more than 72.0% when measured against
placebo in a subject population of
at least 5,000 healthy subjects (or at least 10,000, or at least 15,000
healthy subjects) from endemic irrespective of
serostatus at baseline and being selected from the group consisting of 4 to 16
year aid subject at the time of
randomization, wherein said unit dose/tetravalent dengue virus composition or
said placebo is administered at least
twice within 6 months or less, about 30 days after the last administration of
the administration schedule until at least
12 or 13 months after the last administration of the administration schedule.
[00414] In certain embodiments, the invention is directed to said methods
having a combined vaccine efficacy
against all four serotypes of more than 66 '1/4, or of more than 70%, or of
more than 75%, or of more than 77%, or
of more than 80.0%, when measured against placebo in a subject population of
at least 5,000 healthy subjects (or at
least 10,000, or at least 15,000 healthy subjects) from endemic areas
irrespective of Ser0S-13tuS at baseline and being
selected from the group consisting of 4 to 16 year old subjects at the time of
randomization, wherein said unit
dose/tetravalent dengue virus composition or said placebo is administered at
least twice within 6 months or less,
about 30 days after the last administration of the administration schedule
until at least 12 months or 13 month after
the last administration of the administration schedule.
[00415] In certain embodiments, the invention is directed to said methods,
wherein the combined vaccine efficacy
against all four serotypes is measured about 30 days after the last
administration of the administration schedule until
12 or 13 months after the last administration of the administration schedule.
[00416] In certain embodiment, the invention is directed to said methods,
wherein said unit dose or said placebo
is administered at least twice within three months, in particular at about day
1 and about day 90, and wherein the
combined vaccine efficacy against all four serotypes is measured 30 days after
the second administration until 12 or
13 months after the second administration of the administration schedule.
[00417] In certain embodiments, the invention is directed to said methods,
wherein said methods are effective and
safe. In some of these embodiments, the subject or subject population is under
9 years of age, under 4 years of age,
72
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
or under 2 years of age or from 2 to 9 years of age, or from 2 to 5 years of
age, or from 4 to 9 years of age or from
6 to 9 years of age. Optionally the subject is seronegative with respect to
all dengue serotypes.
[00418] In certain embodiments, the invention is directed to said methods,
wherein said methods having a relative
risk for virologically confirmed dengue with hospitalization of 1 or less, or
0.8 or less, or 0.6 or less, when measured
against placebo in a subject population of at least 5,000 healthy subjects (or
at least 10,000, or at least 15,000
healthy subjects). In some of these embodiments, the subject or subject
population is under 9 years of age, under 4
years of age, or under 2 years of age or from 2 to 9 yea rS of age, or from
2t0 5 years of age, or from 4 to 9 years of
age or from 6 to 9 years of age. Optionally the subject is seronegative with
respect to all dengue serotypes.
[00419] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population are 4 to 16 years of age. In some of such embodiments, the
healthy subjects of the subject
population are 4 to 5 years of age, 6 to 11 years of age, or 12 to 16 years of
age.
[00420] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population are defined as being healthy in view of the exclusion
criteria specified in Example 6.
[00421] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population are from Asia Pacific or Latin America.
[00422] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population are seropositive with respect to at least one serotype. In
other embodiments, the healthy subjects
of the subject population are seronegative with respect to all serotypes.
[00423] In certain embodiments, the invention is directed In said methods,
wherein the healthy subjects of the
subject population are 4-5 years of age and from Asia Pacific, 6-11 years of
age and from Asia Pacific, or 12-16 years
of age and from Asia Pacific. In other embodiments, the healthy subjects of
the subject population are 4-5 years of
age and from Latin America, 6-11 years of age and from Latin America, or 12-16
years of age and from Latin
America.
[00424] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population are 4-5 years of age and seropositive for at least 1 dengue
serotype, 6-11 years of age and
seropositive for at least 1 dengue serotype, or 12-16 years of age and
seropositive for at least 1 dengue serotype. In
other embodiments, the healthy subjects of the subject population are 4-5
years of age and seronegative for all
dengue serotypes, 6-11 years of age and seronegative for all dengue serotypes,
or 12-16 years of age and
seronegative for all dengue serotypes.
[00425] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population are from Asia Pacific or Latin America and seropositive for
at least one dengue serotype at
baseline. In other embodiments, the healthy subjects of the subject population
are from Asia Pacific or Latin America
and seronegative for at all dengue serotype at baseline.
[00426] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population are from Asia Pacific, seropositive for at least one dengue
serotype at baseline and 4-5 years of
age, 6-11 years of age, or 1246 years of age. In other embodiments, the
healthy subjects of the subject population
are from Asia Pacific, seronegative for all dengue serotypes at baseline and 4-
5 years of age, 6-11 years of age, or
73
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
1246 years of age. In yet other embodiments, the healthy subjects of the
subject population are from Latin America,
seropositive for at least one dengue serotype at baseline and 4-5 years of
age, 6-11 years of age, or 12-16 years of
age. In other embodiments, the healthy subjects of the subject population are
from America, seronegative for all
dengue serotypes at baseline and 4-5 years of age, 6-11 years of age, or 12-16
years of age.
[00427] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population had prior vaccination against Yellow Fever. In other
embodiments, the healthy subjects of the
subject population had no prior vaccination against Yellow Fever. Prior
vaccination indicates a vaccination prior to the
first vaccination with the reconstituted unit dose as described herein. For
example for vaccine efficacy (VE) as
determined in Example 6 from 30 days post-second vaccination, a prior
vaccination of Yellow Fever is defined as a
Yellow Fever vaccination occurring before 30 days post-second vaccination.
[00428] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population had prior vaccination against Japanese Encephalitis. In
other embodiments, the healthy subjects
of the subject population had no prior vaccination against Japanese
Encephalitis.
[00429] In certain embodiments, the invention is directed to said methods,
wherein the healthy subjects of the
subject population received Dengvaxia within the administration regimen as
described herein or within 4.5 years
after administration of the first dose. In certain embodiments, the invention
is directed to said methods, wherein the
occurrence of vaccine related serious adverse events is less than 0.1%.
[00430] In certain embodiments, the invention is directed to said methods,
wherein the occurrence of vaccine
related unsolicited adverse events occurring within 4 weeks of administration
is less than 2%.
[00431] In certain embodiments, the invention is directed to said methods,
wherein the occurrence of vaccine
related solicited adverse events occurring within 2 weeks of administration is
less than 35%.
[00432] In certain embodiments, the invention is directed to said methods,
wherein the occurrence of vaccine
related solicited local reactions occurring within 1 weeks of administration
is less than 40%.
[00433] In certain embodiments, the invention is directed to said methods,
wherein the method does not increase
the risk of virologically-confirmed dengue with hospitalization in the
individual, such as in a seronegative individual.
[00434] The above methods are also to be considered in the context of a unit
dose for use in such methods or in
the context of a use of such a unit dose for use in the manufacture of a
medicament for such methods.
[00435] In certain embodiments, a tetravalent dengue vaccine such as Dengvaxia
is used for inoculating against
dengue disease. Dengvaxia is a tetravalent dengue vaccine based on a yellow
fever backbone, CID-TDV
(Dengvaxia , Sanofi Pasteur, Lyon, France), and has been licensed in several
countries based on the clinical
demonstration of an overall vaccine efficacy (VE) against virologically-
confirmed dengue (VCD) of 56-61% in children
in Asia and Latin America (Capeding MR et al. Clinical efficacy and safety of
a novel tetravalent dengue vaccine in
healthy children in Asia: a phase 3, randomised, observer-masked, placebo-
controlled trial. Lancet 2014, 384:1358-
65; Villar LA et al. Safety and immunogenicity of a recombinant tetravalent
dengue vaccine in 9-16 year olds: a
randomized, controlled, phase II trial in Latin America. Pediatr Infect Dis )
2013, 32:1102-9). The preparation of
these particular strains CYD1, CYD2, CYD3 and CYD4 has been described in
detail in international patent applications
WO 98/37911, WO 03/101397, W007/021672, WO 08/007021, WO 08/047023 and WO
08/065315, to which
reference may be made for a precise description of the processes for their
preparation. The corresponding nucleotide
74
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
sequences of the prM-E regions of CYD1, CYD2, CYD3 and CYD4 are provided in
W02016034629 and SEQID NOs are
set out in Table 16 of this reference.
[00436] In certain embodiments, the invention is directed to said methods,
wherein the unit dose disclosed herein,
which in particular comprises a chimeric dengue serotype 2/1 strain, a live
attenuated dengue serotype 2 strain, a
chimeric dengue serotype 2/3 strain and a chimeric dengue serotype 2/4 strain,
and Dengvaxia@ disclosed herein
are administered simultaneously on the same day to the subject or to the
subject population.
[00437] In certain embodiments, the invention is directed to said methods,
wherein the unit dose disclosed herein,
which in particular comprises a chimeric dengue serotype 2/1 strain, a live
attenuated dengue serotype 2 strain, a
chimeric dengue serotype 2/3 strain and a chimeric dengue serotype 2/4 strain,
is administered to the subject or to
the subject population on day 0/1 as a first administration and Dengvaxia
disclosed herein is subsequently
administered to the subject or to the subject population within three months
from the first administration, such as on
day 90 from the first administration, as a second administration.
Alternatively, Dengvaxia0 disclosed herein is
administered to the subject or to the subject population on day 0/1 as a first
administration and the unit dose
disclosed herein, which in particular comprises a chimeric dengue serotype 2/1
strain, a live attenuated dengue
serotype 2 strain, a chimeric dengue serotype 2/3 strain and a chimeric dengue
serotype 2/4 strain, is administered
subsequently to the subject or the subject population within three months from
the first administration, such as on
day 90 from the first administration, as a second administration.
[00438] In certain embodiments, the invention is directed to said method,
wherein the dengue vaccine
composition comprises other dengue vaccines such as TV003 or TV005. TV003,
developed by the U.S. National
Institute of Allergy and Infectious Diseases, comprises vaccine components
rDEN1A30, rDEN2/4A30, rDEN3A30/31
and rDEN4A30, wherein each of these components is present at a concentration
of 3 logioPFU. 11/005 is similar to
TV003 with the difference that the concentration of rDEN2/4A30 in TV005 is 4
logmPFU. The vaccines TV003 and
TV005 and their vaccine components as well as their production are described
in more detail in WO 2008/022196 A2
and S.S. Whitehead, Expert Rev Vaccines, 2016, 15(4): 509 to 517. Wing
recombinant DNA technology, two
attenuation strategies were utilized for the vaccine components of 11/003 or
TV005: deletions in the 3' untranslated
region and structural gene chimerization. For example, the component rDEN4A30
contains all the structural and non-
structural proteins of a wild type DENV-4, but is attenuated by a 30-
nucleotide deletion in the 3' untranslated region
(denoted "A30'). The other vaccine components are also attenuated due to the
30-nucleotide deletion in the 3'
untranslated region. In addition, rDEN3A30/31 includes a 31 nucleotide
deletion in the 3' untranslated region (shown
in detail in Fig. 1c and Fig. 13 of WO 2008/022196 A2). The rDEN2/4630
component was created by substituting the
prM and E genes of DENV-2 into the rDEN4A30 genome. The complete gem:link
sequences of dengue strains which
can he used to produce TV003 or TVOOS are available under the Genbank
accession numbers in Table A of WO
2008/022196 Al.
[00439] In certain embodiments, the invention is directed to said methods,
wherein the unit dose disclosed herein,
which in particular comprises a chimeric dengue serotype 2/1 strain, a live
attenuated dengue serotype 2 strain, a
chimeric dengue serotype 2/3 strain and a chimeric dengue serotype 2/4 strain,
and TV003 or 11/005 disclosed
herein are administered simultaneously on the same day to the subject or to
the subject population.
[00440] In certain embodiments, the invention is directed to said methods,
wherein the unit dose disclosed herein,
which in particular comprises a chimeric dengue serotype 2/1 strain, a live
attenuated dengue serotype 2 strain, a
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
chimeric dengue serotype 2/3 strain and a chimeric dengue serotype 2/4 strain,
is administered to the subject or the
subject population on day 0/1 as a first administration and wherein TV003 or
TV005 disclosed herein is subsequently
administered to the subject or to the subject population within three months
from the first administration, such as on
day 90 from the first administration, as a second administration.
Alternatively, 1V003 or TV005 disclosed herein is
administered to the subject or to subject population on day 0/1 as a first
administration and the unit dose disclosed
herein, which in particular comprises a chimeric dengue serotype 2/1 strain, a
live attenuated dengue serotype 2
strain, a chimeric dengue serotype 2/3 grain and a chimeric dengue serotype
2/4 strain, is administered
subsequently to the subject or to the subject population within three months
from the first administration, such as on
day 90 from the first administration, as a second administration.
EXAMPLES
[00441] The following Examples are included to demonstrate certain aspects and
embodiments of the invention as
described in the claims. It should be appreciated by those of skill in the
art, however, that the following description is
illustrative only and should not be taken in any way as a restriction of the
invention.
Example 1: Preparation of the denaue virus strains.
[00442] The methods used to generate the chimeric dengue strains TDV-1, -3 and
-4 were standard molecular
cloning and DNA engineering methods and are describeet al. (2003)3. Virology
77(21): 11436-11447. The following
well-known methods were used to construct and introduce the prM-E genes of
dengue serotypes 1, 3 and 4 into the
TDV-2 backbone: Reverse-transcriptase PCR (RT-PCR), PCR, restriction enzyme
digestion, DNA fragment ligation,
bacterial transformations by electroporation, plasmid DNA preparations, in
vitro transcription by T7 RNA polymerase,
and transfection of Vero cells by electroporation.
[00443] After growing and purifying the different dengue serotypes separately
as described in Huang et al. (2013)
PLOS Neglected Dis, 7(5):e2243, they are mixed in certain concentrations
provided in Example 4. The mixture of
dengue serotypes is present in a dengue vaccine composition and combined with
a composition of pharmaceutically
acceptable excipients resulting in a dengue vaccine composition comprising 15%
w/v a,a trehalcise dihydraim, 1%
w/v poloxamer 407, 0.1% w/v human serum albumin and 100 mM sodium chloride.
The dengue vaccine composition
is lyophilized and represents a lyophilized unit dose of TDV. The lyophilized
unit dose is reconstituted with 37 mM
aqueous sodium chloride solution and the reconstituted unit dose comprises 15%
w/v a,a trehalose dihydrate, 1%
w/v poloxamer 407, 0.1% w/v human serum albumin and 137 mM sodium chloride.
Example 2: Microneutralization test
[00444] Immunogenicity was measured by a microneutralization assay to each one
of the four dengue serotypes
with titers defined as the dilution resulting in a 50% reduction in plaque
values (MNT50). Briefly, on day 1 Vero cells
were seeded on 96-well assay plates in DMEM and 10% FBS at a density of 2.5 x
105cells/m1 and incubated at 37 C
for 24 hours. On day 2 serial dilutions of the heat-inactivated antibody-
containing test and control sera samples
(dilutions range 1:10 to 1:20480) were prepared and mixed with a constant
concentration of dengue viruses, in
particular DENV-1 strain 16007, DENV-2 strain 16681, DENV-3 strain 16562 and
DENV-4 strain 1036, (target 60-80
pfu/well) in a 96 well microtiter plate and incubated overnight at 2-8 C to
enable the neutralization of the virus by
the antibodies present in the sera. After the incubation the mixture of virus
and antibodies was transferred onto the
96 well plates with Vero cells and the plates were incubated at 37 C for 90-
120 minutes to infect the Vero cells. A
1% methylcellulose overlay in DMEM was applied to the plate to restrict spread
of progeny virus and the plate was
76
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
incubated for 46-70 hours at 340C depending on the Dengue serotype:
DEW! - 66 2 hours
DENV2 - 70 2 hours
DENV3 - 66 2 hours
DENV4 - 46 2 hours
[00445] After the incubation the cells were washed twice with PBS and fixed by
adding cold methanol and
incubating for 60 minutes at a temperature of S -20 C. After fixing the plates
were dried and washed three times
with washing buffer (lx PBS, pH 7.4 with 0.5% Tween), before 50 pl of serotype-
specific anti-dengue monoclonal
antibodies in blocking solution (2.5% nonfat dry milk in PBST) per well were
added and incubated with the cells for
18 4 hours at 2-8 C.
[00446] The monoclonal antibodies were made as described in Gentry et al.
(1982) Am. J. Trop. Med. Hyg. 31,
548-555; Henchal et al. (1985) Am. J. Trap. Med. Hyg. 34, 162-169; and Henchal
et al. (1982) Am. J. Trop. Med.
Hyg. 31(4):830-6). Briefly, the anti-DENV-1 HBD was made against dengue 1
strain Hawaii, Envelope, the anti-DENV-
2 was made against dengue 2 strain New Guinea C, Envelope, isotype 1, the anti-
DENV-3 HBD was made against
dengue 3 strain H87, Envelope, isotype 2A, and the anti-DENV-4 HBD was made
against dengue 4 strain H241,
Envelope, isotype 2A.
[00447] After incubation, the plates were washed three times with washing
buffer and 50 pl of a secondary
peroxidase labelled goat anti-mouse IgG (H + L) (KPL Catit074-1806) in
blocking solution was added and incubated
for 90 to 120 minutes at 37 C. Then the plates were washed three times with
washing buffer and 50 pl of precipitant
substrate (2-amino-9-ethyl carbazole (AEC) tablet in 2.5 ml DMSO, 47.5 ml 50mM
acetate buffer and 250 pl
hydrogen peroxide) were added and the mixture was incubated for 20 minutes at
room temperature. Finally, the
substrate was removed, the plates were rinsed with dH20 and dried.
[00448] Sample titers are calculated using the linear regression method and
reported as MIIT50 titers for each
sample. Clinical data are reported as a geometric mean titer for all the
individual MN1-50 titers in each treatment
group. Briefly, the number of infectious foci in each well was counted and the
titer of neutralizing antibodies was
determined by comparing the percent reduction of infectious rod centers in
wells containing antibody (test samples)
in comparison to wells containing virus alone. The MNT50 was calculated using
the following linear regression
equation:
MN1-50 = 10"1(50-c)/m1) where c = y intercept of regression line and m = slope
of regression line
[00449] Each test sample was tested in triplicates and the titer was
calculated from the average of the triplicates.
A schematic drawing of the steps performed in this test is provided in Figure
2.
examole 3: Phase III clinical trial in children
[00450] A Phase III, double-blind, randomized, and placebo-controlled trial in
20100 subjects aged 4 to 16 years
living in Thailand, Sri Lanka, Philippines, Panama, Nicaragua, Dominican
Republic, Colombia or Brazil was performed
evaluating the efficacy, safety and immunogenicity of a tetravalent dengue
vaccine referred to hereinafter as TDV
(TDV-1, TDV-2, 1DV-3 and TDV-4 as described herein). The trial includes 3
parts. Part 1 evaluates vaccine efficacy
(VE) and lasts until both of the following 2 criteria are fulfilled: (i) 120
cases of dengue fever are confirmed and (ii)
minimum duration of subject follow-up of 12 months post-second vaccination.
Part 2 is for an additional 6 months to
77
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
evaluate VE and for secondary efficacy analyses. Part 3 will evaluate long-
term safety by following participants for
side effects and will last an additional 3 years.
[00451] Part 1: Active surveillance for the primary assessment of efficacy in
all subjects. During this time subjects
were contacted at least weekly to ensure identification of febrile illness
that could potentially be due to dengue. This
part commenced on the day of vaccination and finished once both of the
following 2 criteria were fulfilled: (i) 120
cases of dengue fever are confirmed and (ii) minimum duration of subject
follow-up of 12 months post-second
vaccination. The end of Part 1 was defined for each subject so that the
duration of follow up after the second
vaccination was approximately the same for all subjects. Virologically-
confirmed cases in Part 1 count towards the
primary efficacy objective if occurring at least 30 days post-second
vaccination. Part 1 was finished 12 months post-
second vaccination
[00452] Part 2: Active surveillance for an additional 6 months for each
subject following the completion of Part 1,
I, i.e. 18 month post second vaccination. During this time subjects were
contacted at least weekly to ensure
identification of febrile illness that could potentially be due to dengue.
Virologically-confirmed cases in Parts 1 and 2
contribute towards the secondary efficacy objectives.
[00453] Part 3: Modified active surveillance for the acatagsment of safety in
all subjects following the completion of
Part 2 and lasting 3 years for each subject. The modified surveillance during
Part 3 will maintain at least weekly
contacts through Part 3 of the trial, but the intensity of investigation will
be modified based on the need for
hospitalization. Surveillance will identify febrile illness of any severity
that could potentially be due to dengue.
[00454] Criteria for Inclusion include:
= The subject was aged 4 to 16 years inclusive, at the time of randomization.
= Individuals who were in good health at the time of entry into the trial
as determined by medical history,
physical examination (including vital signs) and clinical judgment of the
Investigator.
= The subject and/or the subject's parent/guardian signed and dated an
assent/written informed consent form
where applicable, and any required privacy authorization prior to the
initiation of any trial procedunes, after
the nature of the trial has been explained according to local regulatory
requirements.
= Individuals who can comply with trial procedures and are available for
the duration of follow-up.
[00455] Exclusion criteria include:
1. Febrile illness (temperature -38 C) or moderate or severe acute illness or
infection at the time of
randomization.
2. History or any illness that, in the opinion of the Investigator, might
interfere with the results of the trial or
pose an additional risk to the subject due to participation in the trial,
including but not limited to:
a. Known hypersensitivity or allergy to any of the vaccine component
b. Female subjects (post-menarche) who are pregnant or breastfeeding.
c. Individuals with any serious chronic or progressive disease according to
judgment of the
Investigator (e.g., neoplasm, insulin-dependent diabetes, cardiac, renal or
hepatic disease,
neurologic or seizure disorder or (5uillain-Barre syndrome).
d. Known or suspected impairment/alteration of immune function, including:
i. Chronic use of oral steroids (equivalent to 20 mg/day prednisone 12 weeks/
mg/kg
body weight/day prednisone L.2 weeks) within 60 days prior to Day 1 (Month 0)
(use of
78
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
intranasal, or topical corticosteroids is allowed).
ii. Receipt of parenteral steroids (equivalent to 20 mg/day prednisone .1.2
weeks /
mg/kg body weight/day prednisone weeks) within 60 days prior to Day 1 (Month
0).
iii. Administration of immunoglobulins and/or any blood products within the 3
months prior to
Day 1 (Month 0) or planned administration during the trial.
iv. Receipt of immunostimulants within 60 days prior to Day 1 (Month 0).
v. Immunosuppressive therapy such as anti-cancer chemotherapy or radiation
therapy within
6 months prior to Day 1 (Month 0).
vi. Human Immunodeficiency Virus (HIV) infection or HIV-related disease.
vii. Genetic immunodeficiency.
3. Receipt of any other vaccine within 14 clays (for inactivated vaccines)
or 28 days (for live vaccines) prior to
Day 1 (Month 0) or planning to receive any vaccine within 28 days after Day 1
(Month 0).
4. Participation in any clinical trial with another investigational product 30
days prior to Day 1 (Month 0) or
intent to participate in another clinical trial at any time during the conduct
of this trial.
5. Previous participation in any clinical trial of a dengue candidate vaccine,
or previous receipt of a dengue
vaccine.
6. First degree relatives of individuals involved in trial conduct.
7. Females of childbearing potential who are sexually active, and who have not
used any of the acceptable
contraceptive method for at least 2 months prior to Day 1 (Month 0)-
8. Females of childbearing potential who are sexually active, and who refuse
to use an acceptable
contraceptive method up to 6 weeks post-second vaccination.
9. Deprived of freedom by administrative or court order, or in an emergency
setting, or hospitalized
involuntarily.
10. Current alcohol abuse or drug addiction that may interfere with the
subject's ability to comply with trial
procedures.
11. Identified as an employee of the Investigator or trial center, with direct
involvement in the proposed trial or
other trials under the direction of that Investigator or trial center.
[00456] Eligible subjects were randomized (2:1) into two treatment groups:
groups 1 received one subcutaneous
(SC) dose of TDV in the upper arm on Day 1 and on Day 90, and group 2 received
one subcutaneous dose of
placebo in the upper arm on Day 1 and on Day 90. Randomization was stratified
by region (Asia Pacific and Latin
America) and age range (children aged 4-5 years, 641 years, and 12-16 years)
to ensure each age range has the
appropriate ratio of TDV to placebo in each region. After randomization
dropouts were not replaced. Study Day 1 is
defined to be the date of the first dose administration of TDV or placebo. The
TDV was prepared as described in
Example I. Each subcutaneous dose of TDV was 0.5 mL and the concentration of
the four dengue serotypes in the
TDV vaccine in each dose was 3.6 log10 PFU/dose, 4.0 logio PFU/dose, 4.6 log10
PFU/dose and 5.1 log10 PFU/dose of
TDV-1, TDV-2, TDV-3 and TDV-4, respectively.
[00457] The õtotal concentration in pfu/0.5 mr which saves as a base value for
the calculation of the percentage
concentration for each individual component of a tetravalent dengue vaccine is
shown for one exemplary tetravalent
vaccine composition comprising dengue serotype 1 in a concentration of 3.60
1og10pfu/0.5 ml, a dengue serotype 2
concentration of 4.00 10g10pfu/0.5 ml, a dengue serotype 3 concentration of
4.60 log1Opfu/0.5 ml and a dengue
serotype 4 concentration of 5.11 10910Pfu/0.5 ml.
Primarily, the logarithmic values of the concentrations are converted into
numerical values. The results of this
conversion are 4x103 pfu/0.5 ml for serotype 1, 1x104 pfu/0.5m1 for serotype
2, 4x104 pfu/0.5 ml for serotype 3 and
79
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
1.3x105 pfu/0.5 ml for serotype 4. The total concentration in pfu/0.5 ml is
the sum of the preceding numerical values
resulting in 1.84 x105 pfu/0.5 ml.
[00458] The "percentage concentration" for each of the serotypes 1, 2, 3 and 4
is obtained by dividing the
numerical concentration value (expressed as pfu/0.5 ml) of an individual
serotype by the total concentration
(expressed in pfu/0.5 ml) and multiplying the result by 100 i.e.:
Percentage concentration of serotype 1 = (4x1C.P pfu/0.5 ml 1.84 x1(t
pfu/0.5 ml) x 100 = 2%
Percentage concentration of serotype 2 = (1x104 pfu/0.5m1 + 1.84 x105 pfu/0.5
ml) x 100 = 5%
Percentage concentration of serotype 3 = (4x104 pfu/0.5 ml 1.84 x105 pfu/0.5
ml) x 100 = 22%
Percentage concentration of serotype 4 = (1.3x105 p41/0.5 ml 1.84 x105
pfu/0.5 ml) x 100 = 71%.
The percentage concentrations are rounded to whole numbers.
[00459] Primary Outcome Measures included the vaccine efficacy (VE) of two
doses of TDV in preventing
virologically-confirrned dengue (VCD) fever induced by any dengue serotype
[time frame: 30 days post-second
vaccination (Day 120) until the end of Part 1]. VE is defined as 1 - (1w/Ac),
wherein Av and Ac denote the hazard
rates for the TDV and placebo groups, respectively. A virologically-confirmed
dengue case is defined as febrile illness
(defined as temperature 1-38 C on any 2 of 3 consecutive days) or illness
clinically suspected to be dengue by the
Investigator with a positive serotype-specific reverse transcriptase
polymerase chain reaction (RT-PCR). A febrile
illness will require an interval of at least 14 days from a previous febrile
illness to avoid overlap of acute and
convalescent visits from one episode with those from a second episode.
[00460] Secondary Outcome Measures include:
1) VE of two doses of TDV in preventing virologically-confirmed dengue fever
induced by each dengue
serotype [time frame: from 30 days post-second vaccination (Day 120) until the
end of Part 2].
2) VE of two doses of TDV in preventing virologically-confirmed dengue
fever induced by any dengue serotype
in participants dengue seronegative at baseline [time frame: from 30 days post-
second vaccination (Day 120)
until the end of Part 2 (up to 21 months)].
3) VE of two doses of TDV in preventing virologically-confirmed dengue
fever induced by any dengue serotype
in participants dengue seropositive at baseline [ time frame: from 30 days
post-second vaccination (Day 120)
until the end of Part 2].
4) VE of two doses of TDV in preventing hospitalization due to
virologically-confirmed dengue fever induced by
any dengue serotype [time frame: from 30 days post-second vaccination (Day
120) until the end of Part 2].
5) VE of two doses of TDV in preventing virologically-confirmed severe
dengue fever induced by any dengue
serotype [time frame: from 30 days post-second vaccination (Day 120) until the
end of Part 2].
6) Percentage of participants with solicited local injection site adverse
events (AEs) in the safety subset [time
frame: Days 1 through 7 after each vaccination] and severity of solicited
local injection AEs. Solicited local AEs at
injection site are defined as pain, erythema and swelling that occurred within
7 days after each vaccination.
7) Percentage of participants with solicited systemic adverse events (AB) in
the safety subset [time frame
Days 1 through 14 after each vaccination] and severity of solicited systemic
AEs. Solicited systemic AEs in
children (< 6 years) are defined as fever, irritability/fussiness, drowsiness
and loss of appetite that occurred
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
within 14 days after each vaccination. Solicited systemic AEs in children (a 6
years) are defined as fever,
headache, asthenia, malaise and myalgia that occurred within 14 days after
each vaccination.
8) Percentage of participants with any unsolicited adverse events (AEs) in
the safety subset [time frame: Days
1 through 28 after each vaccination]. Unsolicited Aft are any AEs that are not
solicited local or systemic AEs, as
defined above.
9) Percentage of participants with serious adverse events (SAEs) during
Parts 1 and 2 [time frame: from Day 1
until the end of Parts 1 and 2]. A serious adverse event (SAE) is any untoward
medical occurrence or effect that
at any dose results in death, is life-threatening, requires inpatient
hospitalization or prolongation of existing
hospitalization, result in persistent or significant disability / incapacity,
is a congenital anomaly / birth defect or is
medically important due to other reasons than the above mentioned criteria.
10) Percentage of participant with fatal SAEs and SAEs related to study drug
during the first and second half of
Part 3 [time frame: for 3 years (18 month halves) beginning at the end of Part
2 (approximately 21 months after
the first vaccination)].
11) Percentage of participants with a seropositive response for each of the
four dengue serotypes in the
immunogenicity subset [time frame: Day 1 and months 1, 3, 4, 9, 15 and then
annually (up to 3 years)].
Seropositive response is defined as a reciprocal neutralizing titer a 10. The
four DENV serotypes are DEN-1, DEN-
2, DEN-3 and DEN-4.
12) Percentage of participants with a seropositive response for multiple
dengue serotypes in the immunogenicity
subset [time frame: Day 1 and months 1, 3, 4, 9, 15 and then annually (up to 3
years)].
13) Geometric Mean Titers (GMTs) of neutralizing antibodies for each of the
four dengue serotypes in the
immunogenicity subset [time frame: Day 1 and months 1, 3, 4, 9, 15 and then
annually (up to 3 years)]. GMTs of
neutralizing antibodies will be measured via microneutralization test (MNT) as
described in Example 2.
a) Study population
[00461] After screening, 20,099 participants were randomized, and 20,071
received at least one injection. In total,
97.4% of placebo participant (n/N: 6,521/6,698) and 97.3% of vaccinees (n/N:
13,038/13,401) completed Part 1 of
the study (Figure 3). Reasons for study withdrawals included AEs, participants
lost to follow-up, pregnancy, protocol
violations, and withdrawal by participants (or parent/guardians). Baseline
characteristics were similar across both
treatment groups (Table 5). Mean age of study participants was 9.6 years, with
baseline seronegativity of 27.7%,
and enrollment was broadly balanced across regions (46.5% in Asia, 53.5% in
Latin America). The highest
seronegative rate was in Panama (62.2%), followed by Sri Lanka (385%),
Thailand (34.4%), Brazil (28.8%),
Nicaragua (22.3%), Colombia (15.4%), the Philippines (12.4%), and the
Dominican Republic (2.8%).
Table 5. Baseline characteristics of study population (number, Wu)
TDV
Placebo Total
Number of Participants 12,704
6,317 19,021
Mean Age (Years, SD) 9.6 (3.35)
9.6(334) 9.6 (3.35)
Baseline Seronegativea 3,533
(27.8) 1,726 (27.3) 5,259 (27.7)
Female 6,314
(49.7) 3,098 (49.0) 9,412 (49.5)
81
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Male 6,390
(50.3) 3,219 (51.0) 9,609 (50.5)
Asia Pacific 5,896
(46.4) 2,942 (46.6) 8,838 (46.5)
Baseline Seronegative 1,503
(25.5) 773 (26.3) 2,276 (25.8)
Latin America 6,808
(53.6) 3,375 (53.4) 10,183 (53.5)
Baseline Seronegative 2,030
(29.8) 953 (28.2) 2,983 (29.3)
Safety see
Number of Participants 13,380
6,687 20,071
Mean Age (Years, SD) 9.6 (336)
9.6 (134) 9.6(135)
Baseline Seronegativea 3,714
(27.8) 1,832 (27.4) 5,547 (27.6)
Female 6,651
(49.7) 3,276 (49.0) 9,929 (49.5)
Male 6,729
(50.3) 3,411 (51.0) 10,142 (50.5)
Safety Set of' SIIb8b
Number of Participants 2,663
1,329 3,993
Baseline Seronegativea 740 (27.8)
369 (27.8) 1,109 (27.8)
aSeronegative for all serotypes; seropositive defined as reciprocal
neutralizing antibody titer -10; SD, standard
!_;leviation.
unumbers of participants in TVD plus placebo groups are not equal to total
numbers shown because misallocated
participants (i.e. those who received both TVD and placebo due to an
administrative error) are not included in the
TEN and placebo group data
b) Febrile illnesses and VCD
[00462] During Part 1, 5,754 and 4,663 episodes of febrile illness were
reported in Asian and Latin American sites,
respectively. Acute samples were obtained in 99.5% and 96.6% of these cases,
with 98.3% and 85.1% of samples
taken within five days, in Asia and Latin America, respectively. There were
278 VCD cases (76 hospitalized) in the
safety set during the entire Part 1 period, of which 210(58 hospitalized) were
30 days post-second vaccination in the
PPS (Table 6; Table 8) and were included in primary endpoint analysis.
c) Distribution of VCD included in primary endpoint analysis
[00463] DEW-1 was reported in all countries with VCD and included all the 21
cases in Panama. In Sri Lanka, 54
of 60 VCD were DENV-2, and 87 of 109 VCD in the Philippines were DENV-3. All
seven DENV-4 VCD were reported in
the Philippines. No VCD were reported in Nicaragua or the Dominican Republic.
Of the associated 58 hospitalized
VCD, 43 were reported in Sri Lanka. A total of two severe dengue (both DENV-3)
and five dengue hemorrhagic fever
(DHF; three DENV-2; two DENV-3) cases were reported (Table 7). These seven
were also the only such cases in the
entire part 1 safety set.
d) Vaccine efficacy
[00464] VE against VCD of any serotype was 80.2% (95% CI: 73.3-85.3; P<0.001).
A similar efficacy of 81%
(95% CI: 64.1-90.0) between the doses and from first dose onwards in the
safety set (Table 6) suggests that the
vaccine was efficacious after the first dose. Exploratory analysis of the
secondary efficacy endpoints showed a trend
of differential efficacy by serotype, with the highest efficacy against DENV-2
(97.7%), followed by DENV-1 (73.7%),
DENV-4 (63.2% with CI containing zero), and DENV-3 (62.6 A); Table 7).
Overall, efficacy was similar in baseline
seronegatives (74.9%) and seropositives (82.2%; Figure 4 above); however, this
varied by serotype. Efficacy against
DENV-2 was not impacted by serostatus; efficacy against DENV-1 was slightly
higher in baseline seropositives
(79.8%; 95% CI: 51.3-91.6) than baseline seronegatives (67.2%; 95% CI: 23.2-
86.0). No efficacy was observed
against DENV-3 in baseline seronegatives (-38.7%; 95% CI: -335.7-55.8)
compared to baseline seropositives
(71.3%; 95% CI: 54.2-82.0). Efficacy by serostatus could not be calculated for
DENV-4 because no cases were
82
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
observed in baseline seronegatives. In the primary endpoint timeframe of the
PPS, only five VCD requiring
hospitalization were reported in the vaccine group compared with 53 cases in
the placebo group, with a VE of 95.4%
(95% CI: 88.4-98.2; 97.2 /0 for baseline seronegatives and 94.4% for baseline
seropositives; Table 7; Figure 4
below), consistent with a VE of 93.3% (95% CI: 86.7-96.7) in the safety set
from first dose onwards.
[00465] The primary vaccine efficacy (VE) of two doses of TDV in preventing
virologically-confirmed dengue (VCD)
fever induced by any dengue serotype is shown in Table 6.
Table 6. Vaccine efficacy of TDV in preventing virologically-confirmed dengue
(VCD) fever against any
serotype from 30 days post-second vaccination until end of part 1 Per Protocol
Set (PPS), i.e. 12
months post-second vaccination. Safety set analysis from first dose to end of
Part 1 study period, i.e.
12 months post-second vaccination
Placebo
TDV (PPS)
n= 6317
n= 12,704
number of subject evaluated
6,316 12,700
number of subjects with febrile illness
1,712 3,195
number of febrile illness cases
2,591 4,692
virologically confirmed dengue fever (n [%])
149 [2.4] 61 [0.5]
Person-years at risk
5,670.1 11,578.7
incident density
2.6 0.5
relative risk
0.20
95% CI of relative risk
(0.15, 0.27)
vaccine efficacy (0/0)
80.2
95% CI of vaccine efficacy
(73.3, 85.3)
p-value for vaccine efficacy
<0.001
Placebo
TDV (Safety Set)*
number of subject evaluated
6,687 13,380
virologically confirmed dengue fever (n [ /0])
199 [3.0] 78 [0.6]
Person-years at risk
8,072.0 16,351.5
incident density
2.5 0.5
vaccine efficacy (0/0)
80.9
95% CI of vaccine efficacy
(75.2, 85.3)
Note 1: Percentage of virologically confirmed dengue (VCD) fever are based on
number of subjects evaluated.
Note 2: Person-years at risks is defined as cumulative time in years until
start of VCD fever or until end of Part 1
study period or discontinuation date, whichever comes first. Incident density
is defined as the number of cases per
100 person-years at risk. Percentages are based on total number (denominator)
of analysis set participants evaluated
and may not be equal to the total number of participants in the per protocol
analysis set.
One participant had two instances of VCD during Part 1, only the first VCD was
included in efficacy calculation
Note 3: Vaccine efficacy (VE) and 2-sided 95% CIs are estimated from a Cox
proportional hazard model with TDV as
a factor, adjusted for age and stratified by region.
Note 4: Statistical significance will be concluded if the lower bound of the
95% CI for VE is above 25%. Since the
hypotheses will be tested in a confirmatory manner at a 2-sided significance
level of 5%, the cakulated p-value
should be compared with 0.025.
Note 5: Relative risk is calculated as the number of events divided by the
number of subjects evaluated in the TDV
group, over the number of events divided by the number of subjects evaluated
in the placebo group.
[00466] For the efficacy evaluation shown in Table 6, a case of VCD was
defined as febrile illness (defined as fever
83
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
38 C on any 2 of 3 consecutive days) with a positive serotype-specific RT-PCR
(i.e., positive dengue detection RT-
PCR) and occurring at any time starting from 30 days post-second vaccination
(Day 120 [Month 4]) through the end
of Part 1. The analysis was performed on the Per-Protocol Set (PPS) and Safety
Set.
[00467] As used herein, the "Per-Protocol Set (PPS)" consist of all subjects
in the Full Analysis Set (FAS) consisting
of all randomized subjects who received at least one dose of TDV or placebo
who had no major protocol violations.
Major protocol violations are not receiving both doses of TDV or placebo
administration, not receiving both doses in
the correct interval, not having the correct administration of TDV or placebo,
use of prohibited medications / vaccines
by the subject, the subject meets any of the exclusion criteria of 2d, 3, 4 or
5 defined above or product preparation
error.
[00468] The p-value is obtained by solving the critical value Z in the
following equation:
Upper bound of 1-sided (1-p%) CI of HR=0.75, wherein HR is the hazard ratio
and defined as HR= AV/AC.
eA[/Ys+Z*CE] = 0.75, wherein rdefines the treatment and ST the related
standard error.
The 1-sided p-value is 1-(area to the left of the critical value Z from a
standard normal distribution). Since the
hypotheses will be tested in a confirmatory manner 2-sided at a significance
level of 5%, the calculated 1-sided p-
value should be compared with 0.025.
[00469] In summary in Part 1 of this study, a high vaccine efficacy of 80.2%
against virologically-confirmed
dengue of any serotype in children 1-16 years of age was found. It included an
efficacy of 74.9% in baseline
seronegatives and a robust 95.4% reduction in hospitalizations. Onset of
protection could be seen after the first dose
with 81% efficacy between doses. Overall, these results suggest a potential
benefit for each vaccine recipient
regardless of prior dengue exposure or age. This finding is significant
because vaccine development against dengue
has been challenging, especially for dengue naive individuals, and dengue
remains one of the WHO's top ten threats
to global hearth in 2019.19 Furthermore, the onset of protection after the
first dose has potential utility in the context
of outbreak control or travel vaccination, offering a reduction in the risk of
dengue after only one dose.
[00470] Severe forms of dengue were assessed as follows: Dengue Hemorrhagic
Fever (DHF) as defined by the
1997 WHO definition. Severe Dengue through the Dengue Case Adjudication
Committee. The Dengue Case
Adjudication Committee (DCAC) consisted of four members: a voting chairperson,
two voting members, and an
independent non-voting statistician. The three DCAC voting members are all
physicians and clinical dengue experts.
DCAC members are not study investigators and do not have any conflict of
interest that would bias their review of
the trial data. All non-hospitalized cases were considered non-severe The DCAC
severe dengue case criteria applied
in a blinded manner to virologically-confirmed hospitalized dengue cases are
as follows: 1) bleeding abnormality, for
a case to be considered severe there needs to be a significant intervention
required in response to the bleeding
episode such as blood transfusion, nasal packing, hormonal therapy, or,
bleeding occurred into critical organs such as
the brain; 2) plasma leakage, for a case to be considered severe there needs
to be evidence of both plasma leakage
and functional impairment (plasma leakage includes clinical evidence,
radiological evidence, or hematocrit elevated
>200/0 above normal levels or baseline; functional impairment defined as shock
or respiratory distress); 3) liver, for a
case to be considered severe there needs to be evidence of both hepatitis and
functional impairment (hepatitis
defined as an aspartate aminotransferase [AST] or ala nine aminotransferase
[ALT] >10 upper limit of normal range
[ULN]; functional impairment defined as prothrombin [F1] >1.5 ULN or
hypoalbuminemia); 4) renal, serum
creatinine >2.5 times ULN or requiring dialysis; 5) cardiac, abnormalities
intrinsic to the heart (i.e. not resulting from
intravascular volume depletion) and with evidence of functional impairment
(examples of intrinsic abnormality:
myccarditis, pericarditis, and myopericarditis; example of functional
impairment new conduction abnormality
resulting in irregular heart rhythm [i.e. not transient first-degree heart
block]); 6) central nervous system, any
84
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
abnormality with the exception of a simple febrile convulsion or a brief
delirium; 7) shock, all shock cases considered
severe. At least 1 functional impairment (of criterion 3,4,5,6), needs to be
present but the totality of data were
considered by the members in their assAssment.
[00471] Further results of part 1 and part 2 are presented in Tables 7a to c.
CA 03147807 2022-2-11
C
U)
A
A
---I
CO
0
--4
N.)
C
NJ
N)
N)
A
A
Table 7a: Distribution of cases contributing to primary endpoint by per
protocol set subgroup (30 days after second vaccination until end of Part 1,
i.e. 12
0
months after second vaccination)
b.)
a
TIN TDV
Placebo Placebo Vaccine
Efficacy N.
Imt
Dengue Cases Incidence Density Dengue Cases
Incidence Density (95% CI) o
La
.4..
vcD cases
roe
a.
Baseline Seropositivea 41/9,165 (0.4%)
0.5 110/4,587 (2.4%) 2.7 82.2% (74.5%-
87.6%)
Baseline Seronegativea 20/3,531 (0.6%)
0.6 39/11726 (2.3%) 2.5 74.9% (57.0%-
85.4%)
DENV-1 16/12,700 (0.1%)
0.1 30/6,316 (0.5%) 0.5 73.7% (51.7%-
85.7%)
DENV-2 3/12,700 (<0.1%)
<0.1 64/6,316 (1.0%) 1.1 97.7% (92.7%-
99.3%)
DENV-3 39/12,700 (0.3%)
0.3 51/6,316 (0.8%) 0.9 62.6% (43.3%-
75.4%)
DENV-4d 3/12,700 (<0.1%)
<0.1 4/6,316 (<0.1%) <0.1 63.2% (44.6
441.8%)
4-5 Years Old 13/1,619 (0.8%)
0.9 23/801 (2.9%) 3.2 72.8% (46.29'-
86.2%)
6-11 Years Old 34/7,009 (0.5%)
0.5 85/3,491 (2.4%) 2.7 80.7% (71.3%-
87.0%)
12-16 Years Old 14/4,072 (0.3%)
0.4 41/2,024 (2.0%) 2.2 83.3% (69.3%-
90.9%)
Asia 54/5,894 (0.9%)
1.0 127/2,942 (4.3%) 4.9 79.5% (71.8%-
85.1%)
cie
Latin America 7/6,806 (0.1%)
0.1 22/3,374 (0.7%) 0.7 84.3% (63.1%-
93.3%)
Baseline Seropositivea 4/9,165 (<0.1%)
<0.1 35/4,587 (0.8%) 0.8 94.4% (84.3%-
98.0%)
Baseline Seronegativea 1/3,531 (<0.1%)
<0.1 18/1,726 (1.0%) 1.2 97.2% (79.1%-
99.6%)
All participants 1/12,700 (<0.1%)
<0.1 4/6,316 (<0.1%) <0.1 87.3% (-13.5%-
98.6%)
All participants 1/12,700 (<0.1%)
<0.1 1/6,316 (<0.1%) <0.1 50.8% (-686.9%-
96.9%)
VCD, virologically-confirmed dengue; DHF, dengue hemorrhagic fever
'Seronegative for all serotypes; baseline saropositive defined as reciprocal
neutralizing antibody titer a10 to one or more serotypes.
mo
bVCD cases meeting WHO 1997 DHF criteria; incidence density defined as the
number of cases per 100 person-years at risk; percentages are based on total
number (denominator) of n
per protocol set participants evaluated.
?
two severe VCD were not classified as DHF.
CA
IS
d The number of cases identified was sufficient to provide reasonably precise
estimates of vaccine efficacy against all individual serotypes, except DENV-4.
0
t4
0
0
he
d
1/4.0
4.
=11.
C
0,
A
-1
co
0
-,
N,
0
,,
N
N
-
Table 7b: Distribution of cases contributing to secondary endpoint by per
protocol set subgroup (30 days after second vaccination until end of Part 2,
i.e. 18
months after second vaccination)
0
w
a
TDV TDV
Placebo Placebo Vaccine
Efficacy ra
1.1
Dengue Cases Incidence
Density Dengue Cases Incidence Density (95% CI)
er>
La
a
w
Overall
73. 3% (66.5%-78.8%) kro
Baseline Seropositivea 75
0.6 150 2.4
76.1% (685%-81.9%)
Baseline Seronegative 39
0.8 56 2.4
66.2% (49.1%-77.5%)
DENV-1 38
0.2 62 0.7
69.8% (54.8%-79.9%)
Baseline Seropositivea 21
0.2 37 0.6
72.0 (52.2%-83.6%)
Baseline Seronegativea 17
0.3 25 1
67.8 (40.3%-82.6%)
DENV-2 8
<0.1 80 0.9
95.1% (89.9%-97.6%)
Baseline Seropositives 7
<0.1 54 0.9
93.7 (86.1%-97.1%)
Baseline Seronegativea 1
<0.1 26 1.1
98.1 (85.8%-99.7%)
jt>5Pjt4h*ed,YPA'aOeS,*:.:)Y.::..j..''..'...'.'.,j.,'.,,:):.,j.,,55'.,:)'):::):
::')H9')j.,',:)'...'.'..j..'...'..'..j.,j):.,,','.,,:):::)5'.,'..,'.,'..,'.,'..
. .,H'H:,:,:czzcc::::c:cc:;zcc:::::c:cc:;zcc::::::c:cc:czc::
co
-4 Overall 13
<0.1 66 0.8 90.4% (82.6%-
94.7%)
Baseline Seropositive 8
<0.1 45 0.7
91.4% (81.7%-9529%)
Baseline Seronegative 5
0.1 21 0.9
88.1% (68.5%-95.5%)
VCD, virologically-oonfirmed dengue;
Seronegative for all serotypes; baseline seroposiliye defined as reciprocal
neutralizing antibody titer al to one or more serotypes.
V
tin
?
cn
b 0
0
t 4
0
Z S
N
a
k 4
a
1.1
C
U)
CO
N)
0
NJ
N)
N)
Table 7c: Distribution of cases contributing to secondary endpoint by safety
set (first vaccination until end of Part 2, i.e. 21 months after first
vaccination) 0
C:p
TDV TDV
Placebo Placebo Vaccine
Efficacy kJ
Dengue Cases Incidence
Density Dengue Cases Incidence Density (95% CI)
c..!e
Overall
75.3 % (69.5%-80.0%)
Overall in betweena
81.0% (64.1%-
90.0%)
Baseline Seropositiveb 89
0.5 187 2.3
77.2% (70.6%-82.3%)
Baseline Seronegativeb 42
0.7 70 2.3
70.6% (56.9%-79.9%)
DENV4 41 0.2
78 0.7
73.9% (61.90/0-82.1%)
DENV-2 14
<0.1 109 1.0
93.7% (89.0%-96.4%)
Hospitailad viCoases .........
.......... ......... ........... .........
.........
Overall 17
<0.1 81 0.7 89.7% (82.6%-
93.9%)
VCD, virologically-confirmed dengue;
In between: VCD after first vaccination and before second vaccination
bSeronegative for all serotypes; baseline seroposItive defined as reciprocal
neutralizing antibody titer aio to one or more serotypes.
oe
oo
Table 7d; Dengvaxia VCD (first vaccination until 25 months after first
vaccination (i.e.13 month after third vaccination), ITT from CYD15, 9 to 16
years of age)a
111
Vaccine Efficacy
(95% CI)
Baseline seropositive. 83.7% (622%-93.7%)
=:baseline Seronegativeb 43.2% (-61 5%-a .0%)
DENV-1 58.8% (40.2%-65.9%)
DENV4 50.2%(31.8%-63.6%)
OveraltHospitalized VCD 80.3% (E4.7%-89.5%):
(I)
'Luis Villar et al Efficacy of a tetravalent dengue vaccine in Children in
Latin America: N Engl J of Med 2015 Vol. 372 No2, 113-123
t4e
C:p
WO 2021/034349
PCT/US2020/020991
[00472] Clinical signs and symptoms of virologically-confimiecl dengue cases
during Part 1 study period in safety set
data are shown in Table 8.
Table 8: Clinical signs and symptoms of virologically-confirmed dengue cases
during Part 1 study period
(safety set data)
TM/
Placebo Relative
(N=13,380)
(N=6687) Risk
Median Duration of Febrile Illness (days; 95% Or
6.0 (5.7-7.4) 6.0 (5.9-6.8)
Number of Hospitalized VCD Cases
9 67
NSian burafaon of lbSIahzathn (days; '95% C
SO (2S¨S.4) Sfl (4..&-&#) -
Evidence of Bleeding (Wu, n/N)
3..8% (308) 3.5% (7/2-130- ) 1.1.0
Plasma Leakage%1 nfN)
2.6% (Z178) 63% (131200) 039
Plasma Leakage - Pleural Effusion ( /0, n/N)
1.3% (1/78) 1.5% (3/200)
Plasma Leakage - Radiological Signs (0/0, n/N)
40.0% (2/5) 19.6% (10/51)
Platelet Count '100x109 (%, n/N)C
e..4% (5/78) 22.0% (44/260¨ ) 029.
ALT or AST a-10CICI U/L ( /0, n/NY
0% (0/78) 0% (0/200)
VCD, virologically-confirmed dengue; ALT, alanine aminotransferase; AST,
aspartate aminotransferase
aDuration of febrile illness defined as start date of earliest symptom to end
date of latest symptom plus one day
(symptoms considered include fever and any general symptoms).
Hematocrit increase defined as maximum hematocrit between Day 3 and Day 7
inclusive, from onset of fever -20 /0
increase over minimum hernatocrit before Day 3 or after Day 7 from onset of
fever.
`For platelet, ALT, and AST data, acsPssments within 14 days of onset of
febrile illness have been considered.
N refers to number of VCD cases with available data for the specific parameter
e) Immunogen icity
[00473] The highest geometric mean titers (Gl 11-s) were observed against DENV-
2 regardless of baseline serostatus
(Table 10). A very high tetravalent seropctsitivity rate (99.5%) in baseline
seronegatives one month after the second dose
(Tables 9 and 10) was observed.
[00474] Seropositivity rate ( /0 of seropositive subjects) for each of the
four dengue serotypes is determined at
prevaccination on Day 1 (Month 0), post-first vaccination on Day 30 (Month 1),
prevaccination on Day 90 (Month 3), post-
second vaccination on Day 120 (Month 4), Day 270 (Month 9), Day 450 (Month
15), and then annually. Seropositivity
rates ( /0 participants, 95% CI) by dengue serotype per protocol set for
immunogenicity data for Day 0, Day 30, Day 90,
Day 120, and Day 270 are shown in Table 9.
[00475] Seropositivity rates ( /0 participants, 95 k CI) by dengue serotype
against three or more serotypes (trivalent)
and against all four serotypes (tetravalent) per protocol set for
immunogenicity data for Day 0, Day 30, Day 90, Day 120,
and Day 270 are shown in Table 9. The tetravalent sercipositivity rates were
high (>91%) in baseline seronegatives six
months after second dose.
89
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table 9; Seropositivity rates (% participants, 95% CI) by dengue serotype (per
protocol set for
immunogenidty data)
BASELINE 5EROPOSITIVE
BASELINE SERONEGATIVE Seropo
TDV Placebo TDV
Placebo sitivity
N=1,816 N=902 N=702
N=345 rates
rirtilti t -------------------------------------------------------------------
------------------------------------------------- (%
89.1 (87.6-90.5) 90.6 (88.5-92.4) 0(0-0.5)
0(0-1.1)
99.5 (99.1-99.8) 88.6 (86.3-90.7)
94.1 (92.0-95.8) 4.9 (2.8-7.8) particip
99.3 (98.8-99.6) 90.2 (88.1-92.1)
91.6(89.3-93.5) 6.1 (3.8-9.2) ants.
>99.9 (99.7-100) 90.3 (88.1-92.3)
99.5 (98.6-99.9) 8.3(5.5-11.9)
99.6(99.1-99.8) 89.8 (87.5-91.8)
95.1 (93.0-96.6) 9.0(6.0-12.8) CI) by
-------------------------------------------------------------------------------
------------------------------------------------ dengue
96.5 (95.6-973) 97.2 (95.9-98.2) 0(0-0.5)
0(0-1.1) serotyp
99.9 (99.6-100) 93.3 (91.444.9)
98.6 (97.4-99.4) 10.7(7.5-14.5) e (Per
>99.9 (99.7-100) 94.0 (92.2-953)
990(9&O-996) 12.2 (8.9-16.1) proboco
99.9 (99.6-100) 93.6 (91.7-95.2)
100(99.4-100) 14.7(11.0-19.1)
I set for
100(99.8-100) 94.6 (92.8-96.1) 100(99.4-100)
18.3(14.1-232)
immun
DEN::::::: ------------- ::::::::::::::::::: ----------------------------------
--------------------------------------- :::::::::::::::::::: :::::::
:::::::::::::: ::::::::: ::::::::::::::::::: ::::::::::::::::::::: ::::::::
:::::::::::::::::::::::: :::::::::::::: ::::::::::::::: ::::::::
.........___............. _... ...___
...._...................___................ __.......
88.1 (86.5-89.6) 88.0 (85.7-90.1) 0(0-0.5)
0(0-1.1) ogen icit
99.8 (99.4-99.9) 87.6 (85.1-89.7)
96.1 (94.3-97.4) 4.0(2.1-6.7) y data;
99.5 (99.1-99.8) 87.3 (84.9-89.4)
94.4(92.5-96.0) 2.0(0.8-4.1) seropos
99.8 (99.5-100) 87.9 (85.5-90.1)
100(99.4-100) 5.1 (2.9-8.2) dye
993 (99.4-99.9) 87.1 (84.6-894)
96.4 (94.6-973) 7.7(4.9-11.3) defined
as a
88.1 (86.5-89.6) 87.4 (85.0-89.5) 0 (0-0.5)
0(0-1.1) recipro
99.6(99.2-99.9) 86.6 (84.1-88.8)
90.5 (88.0-92.6) 1.8(0.7-3.9)
99.3 (98.8-99.7) 86.9 (84.5-89.0)
92.0(89.8-93.9) 2.9 (1.4-5.3) cal
>99.9 (99.7-100) 88.3 (85.9-90.4)
99.8 (99.1-100) 4.8 (2.7-7.8) neutrali
99.7 (99.3-99.9) 87.6 (85.1,89.9)
97.0(95.4-98.2) 6.3 (3.9-9.7) zing
antibod
Three or More-Serotypes ::::: """"" 87.5 (85.9-89.0) 87.3
(84.9-89.4) --------- 0 (0-0.5) 0(0-1.1) y titer
99.8 (99.5-100) 87.2 (84.7-89.4)
96.5 (94.8-97.8) 1.2 (0.3-3.1) 10;
99.7 (99.3-99.9) 87.7 (85.3-89.7)
94.9 (93.0-96.4) 1.7 (0.6-3.7) baselin
99.9 (99.6-100) 88.4 (86.010.5)
99.8 (99.1-100) 4.2 (2.2-7.0) e
99.7 (99.1-99.9) 87.3 (84.7,89.5)
97.5 (96.0-98.6) 5.7 (3.3-8.9)
AU Four Sentyp ----------------------------------------------------------------
---------------------------------------
_______________________________________________________________________________
________________________________________________ servile
gative
83.5 (81.7-85.2) 83.5 (80.9-85.8) 0(0-0.5)
0(0-1.1)
99.1 (98.5-99.5) 82.9 (80.2-85.4)
85.3 (82.4-87.9) 0.9 (0.2-2.6) defined
98.6(97.9-99.1) 83.6 (81.0-86.0)
84.3 (81.4-86.9) 1.4(0.5-3.3) as
99.8 (99.5-100) 85.2 (82.6-87.6)
99.5 (98.6-99.9) 3.5 (1.8-6.2) serone
99.2 (98.7-99.6) 84.6 (81.9-87.0)
91.3 (88.7-93.4) 5.3 (3.1-8.5) gative
to all serotype; baseline seropositive defined as seropositive to one or more
serotypes; N refers to number of participants
in the analysis set; number of participants evaluated at each time point may
vary)
[00476] Geometric mean titers (GMTs) of neutralizing antibodies
(microneutralization test [MNT]) for each dengue
serotype are determined at pre-vaccination on Day 1 (Month 0), post-first
vaccination on Day 30 (Month 1), pre-
vaccination on Day 90 (Month 3), post-second vaccination on Day 120 (Month 4),
Day 270 (Month 9), Day 450 (Month
15), and then annually. Geometric mean titers (95% CI) by dengue serotype per
protocol set for immunogenicity data for
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Day 0, Day 30, Day 90, Day 120, and Day 270 are shown in Table 10.
Table 10: Geometric mean titers (95% CI) by dengue serotype (per protocol set
for immunogenidty data)
BASELINE SEROPOSITIVE
BASELINE SERONEGATIVE
TDV Placebo
TDV Placebo
N=1,816 N-902
N=702 N=345
Day 1 410(365-461) 445(377-524)
5.0(5.0-5.0) 5.0(5.0-5.0)
Day 30 2,404(2,204-2,622)
430(361-512) 118 (106-131) 5.8(5.3-6.3)
Day 90 1,945(1,791-2,112)
410(349-481) 91(82-102) 5.9(5.4-6.3)
Day 120 2,115(1,957-2,286)
451 (381-534) 184 (169-201) 6.3(5.7-7.0)
Day 270 1,447(1,329-1,574)
415(350-492) 87(79-97) 6.3(5.7-6.9)
Day 1 745 (674-825) 802 (697-924)
5.0 (5.0-5.0) 5.0 (5.0-5.0)
Day 30 6,697(6,301-7,117)
744(635-870) 6,277 (5,648-6,977) 6.6(6.0-7.3)
Day 90 4,826(4,571-5,096)
729(629-845) 1,682(1,544-1,834) 7.0(6.3-7.9)
Day 120 4,897(4,646-5,163)
766(655-896) 1,730(1,614-1,855) 7.7(6.7-8.8)
Day 270 3,692(2,496-2,898)
776 (665-906) 929 (856-1,010) 8.7 (7.4-10.2)
Day 1 357 (321-398) 356 (305-415)
5.0 (5.0-5.0) 5.0 (5.0-5.0)
Day 30 2,255(2,094-2,428)
349 (298-409) 194 (173-218) 5.5 (5.2-5.9)
Day 90 1,563(1,453-1,682)
321 (277-374) 94(85-104) 5.5(5.1-5.9)
Day 120 1,761 (1,646-1,885)
353(301-414) 228 (212-246) 6.0(5.4-6.6)
Day 270 1,089(1,009-1,175)
307 (261-360) 72(66-78) 6.3(5.7-7.0)
Day 1 218(198-241) 234(203-270)
5.0(5.0-5.0) 5.0(5.0-5.0)
Day 30 1,303 (1,221-1,391)
222 (191-258) 111 (98-125) 5.4 (5.0-5.7)
Day 90 1,002 (940-1,069) 215 (187-248)
63 (57-70) 5.5 (5.1-5.9)
Day 120 1,129(1,066-1,196)
241 (208-280) 144(134-155) 5.8(5.3-6.4)
Day 270 778 (730-830) 229 (197-266)
64 (59-70) 6.2 (5.6-6.9)
[00477] Vaccine viremia is assessed by three PCRs: dengue detection RT-PCR,
vaccine screening PCR and TDV
sequencing in subjects with febrile illness within 30 days after each
vaccination.
f) Safety
[00478] Rates of serious adverse events (SAEs) were similar in the vaccine and
placebo groups (3.1% and 3.8% of
participants, respectively; Table 11). One vaccine and four placebo recipients
experienced SAEs considered to be related
to receiving blinded investigational product by the investigator (two
experienced hypersensitivity, two were diagnosed
with dengue, and one with DHF). There were five deaths during Part 1, and all
were considered unrelated to the
investigational product or study procedures. Total rates of unsolicited AEs
were similar between the vaccine and placebo
groups. The most commonly (113/0 of vaccine-recipients) reported unsolicited
AE5 within four weeks of any dose by
preferred term were pyrexia (vaccine group 1.5%; placebo 1.4%),
nasopharyngitis (vaccine 2.7%; placebo 3.0%), upper
respiratory tract infection (vaccine 2.6%; placebo 2.9%), and viral infection
(vaccine 1.1%; placebo 0.9%). Solicited local
reactions were reported more frequently in the vaccine group.
Table lla: Overview of safety data. Subjects with at least one adverse event
after any vaccine dose. Data
presented as number of events (percentage of subjects; number [n] of
subjects/total [N] subjects) unless
otherwise stated (safety set data)
91
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
7131f
Placebo
SAES
3.1% (409/13,380) 3.8% (255/6,687)
Non-IP-Relabeda SAES
3.0% (408/13,380) 3.8% (251/6,687)
IP-Relatecla SAEs
<0.1% (1/13,380) <0.1% (4/6,687)
SAES Leading to II' Withdrawal and / or Trial Discontinuation
0.1% (18/13,380) 0.1% (3/6,687)
Deaths
<0.1% (4/13,380) <0.1% (1/6,687)
IP-Related Deaths
0% (0/13,380) 0% (0/6,687)
: ::::::::::::::::: __________
: :
Unsolicited AEs Occurring Within 4 Weeks of Any Dose
18.4% (490/2,663) 18.8% (250/1,329)
IP-Relateda Unsolicited AEs Occurring Within 4 Weeks of Any
1.0% (27/2,663)
1.6% (21/1,329)
Dose
Solicited Systemic AEs Occurring Within 2 Weeks of Any Dose?
410% (1,107/2,635) 38.0% (501/1,317)
IP-Relateda Solicited Systemic AEs Occurring Within 2 Weeks of
31.2% (821/2,635) 28.2% (371/1,317)
Any Dose
Solicited Local Reactions Occurring Within 1 Week of Any
36.7% (967/2,633) 25.7% (338/1,317)
Dose`
AE, adverse event; SAE, serious adverse event; IP, investigational product/TEN
aIP-related, defined as related to the investigational product as assessed by
investigator
bonly participants with diary data available were evaluated
call injection site (solicited local) reactions considered to be IP-related
92
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table lib; Number of participants (%) with serious adverse events after any
vaccination during Part 1 by
MedDRA (Medical Dictionary for Regulatory Activities )System Organ aass in the
order of decreasing
frequency (safety set data presented by TDV and placebo group for events that
occurred in >3 partidpants
due to risk of unblinding).
TDV
Placebo Total*
MedDM System Organ Class
N=13,380
N=6,687 N=20,071
-Any Serious Adverse -
.................................................
Infections and infestations 235
(1.8) 179 (2.7) 414 (2.1)
-------------------------------------------------------------------------
11710.7) 3.7. COM ........ 124%6) ----------
Gastrointestinal disorders 23
(0.2) 9 (0.1) 32 (0.2)
Respiratory, thoracic and mediastinal disorders
14(0.1) 6 (<0.1) 20 (<0.1)
eft ry:
Blood and lymphatic system disorders 8
(<0.1) 2 (<0.1) 10 (<0.1)
Pregnancy, puerperium and pen natal coricrtilds 8 (011)
............................ 2 (<13:1) ----- :10 (all) --------
Skin and subcutaneous tissue disorders 7
(<0.1) 3 (<0.1) 10 (<0.1)
General disorders and adminisbation site conditions 5
(<0.1) 3 (<0.1) 8 (<0.1)
Metabolism and nutrition disorders 6
(<0.1) 1 (<0.1) 7 (<0.1)
Musculoskeletal and cam ----------------- ective* :tissue ---------------------
--------- 5 (<04 CitYsi) ..............
Social circumstances 2
(<0.1) 4 (<0.1) 6 (<0.1)
-Congenial; familial-and genetic disoraers :::: 3 CallY ---------------------
--------------------------------------------
Neoplasms benign, malignant and unspecified (including
3 (<0.1)
1 (<0.1) 4 (<0.1)
cysts and polyps)
- - - - - - - - -
- - - - - ------------- -
Endoodrte disorders ......................................................
_.............. ::: .._................... ---- ::: 3 (<111)
Hepatobiliary disorders
3 (<0.1)
Vascular disorders
3 (<0.1)
--Cardiactlisonlers- ------------------------------- ::::::
- ::: ------ 2 (<ta) -- :: ---
Eye disorders
2 (<0.1)
!.!!! ::::::::::::::::::::: :::::::::::::::
:::::::::::::::::: :::::::: ....... ::::::::::: f<GLII
:!790ren-.7 ?. ---------------------------------------------------------------
--------------------------------------------
Product issues
1 (<0.1)
.._.._ _ _
_ _
*Total column includes participants who received both TAK-003 and placebo due
to administration error and are
excluded from the TAK-003 and placebo groups. N in column header refers to
number of participants in the safety set
93
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table 11c: Number of participants (%) with unsolicited adverse events of any
severity up to 28-days
after any vaccination by MedDRA System Organ Class in the order of decreasing
frequency (Subset of
safety set data presented by TIN and placebo group for events that occurred in
>6 participants due to
risk of unblincling).
11)V
Placebo Total
MedDRA System Organ Class
N=2,663 N=1,329 N=3,993
-- OhSal ited Aitbie .................... EVeritS ---------
249 (iggy:---736(i8:43¨i
Infections and infestations
368 (13.8) 190 (14_3) 558 (14_0)
Injury, pcisoning and procedural complications --------------------------------
------- 21W8) 22(1+7) 43 (LI)
Gastrointestinal disorders
33(1.2) 9 (0.7) 42 (1.1)
--------------
--- it .11 .. --- it fti ,stv ....... it+ irt fit
6efteral iiithiders¨ and adininiSiiiiiithis¨rte:conditions'
:::::::::: --- ----------------------------- :::::::::
Skin and subcutaneous tissue disorders
27 (1.0) 7 (0.5) 34(0.9)
(t .a) --- :::::: --- :: :::::
IIIIIIIII
Nervoussystemhsorders ---------------------------------------------------------
----------------------------------------------
Respiratory, thoracic and mediastinal disorders
18 (0.7) 10 (0.8) 28 (0.7)
mood ------------------- and lymphatic-- systerndisorders¨ -------------
:::::::::::::::::: ----------- :::::::::::::: 6 -- 5(0.4) 11 If (tLS)
Musculoskeletal and connective tissue disorders
6 (0.2) 5 (0.4) 11 (0.3)
Psychiatric disorders
3 (<0.1)
Rroductive system and breast disorders
- (c0i}
Ear and labyrinth disorders
2 (<0.1)
taidair¨diSOrderk --------------------------------------------------------- ---
--- --- -------- -------------
õ::õ::, ::::::::::::::::::
1.111111
Congenital, familial and genetic disorders
1 (<0.1)
1:EfldiSorders ----------------------------------------------------------------
------- ::::::::: )
Renal and urinary disorders
1 (<0.1)
crot31 column includes participants who received both TAK-003 and placebo due
to administration error and are
excluded from the TAK-003 and placebo groups. N in column header refers to
number of participants in the subset of
safety set.
15
94
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table 11d: Summary of diary reported injection site reactions up to 7 days and
systemic adverse
events up to 14 days after any vaccination (Subset of safety set data). Data
presented as number of
participants with events / number of evaluated participants in the analysis
set (% of evaluated
participants with events).
Solicited Events TIN
Placebo
Any
106/331 (32.0) 43/169 (25.4)
Erythema 5/331
(1.5) 1/169 (0.6)
Injection site reactions (Age 6 years)
-------------------------------------------------------------------------------
--------------------- ..,ne.õ 4 A, 0 Ink
nick- iutpwri
Pain
853/2302 (37.1) 293/1148 (25.5)
-ErAherna ............................................ 33/230.1 (1.A)
:::::::::::::::::::::::::: ::: 1/1147(<0,1)
Swelling
33/2300 (1.4) 6/1147 (0.5)
Any
88/331 (26.6) 35/169 (20.7)
Drc)wsiness
45/331 (13.6) 21/169 (12.4)
"Loss of.Appetit ------------------------------------- 57/331 0:7.j21
:::: z2/169 taw
Fever (Body temperature > = 38 C or 100.4 F)
45/327(13.8) 23/169 (13.6)
Any
941/2302 (40.9) 422/1147(36.8)
i4Ieatic:lieEEEE
/!.1-!1t'
Asthenia
404/2302 (175) 187/1147 (16.3)
malarse --------------------------------------------- 510/2301-(222)-
225/1147 (19.7)
Myalgia
554/2302 (24.1) 216/1147 (18.8)
[00479] Additionally, a study to assess the efficacy of a booster dose as a
follow-on study of the above-described
phase III study, such that booster will be given at 4 to 4.5 years post the
second dose in a large enough subset of
the above-described phase III study, wherein said subset e.g. includes at
least 20 subjects or at least 200 subjects,
is possible.
95
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Fxample rnnrnmitant administration of a hepatitis A varrine and a dengiie
varrine
4.1 Introduction. Puroose and Objectives of the Study
[00480] A randomized, observer blind, phase 3 trial was conducted in 900
healthy adult subjects aged 18 to 60
years (distributed across the entire age range) in non-endemic countries for
dengue disease and hepatitis A virus
(HAV) to investigate the immunogenicity and safety of two doses of tetravalent
dengue vaccine TDV (subcutaneous
(SC) injection), and of the simultaneous on the same day administration of a
single dose of HAV vaccine (containing
an inactivated HAV; intramuscular (IM) injection) and TDV (SC injection).
[00481] A purpose of the study was to acc.Pcs whether HAV vaccine can be
safely administered simultaneously on
the same day with TDV as travel vaccines before an international travel of a
subject to HAV and dengue (DENV)-
endemic countries.
[00482] The primary objective of this study was demonstrate non-inferiority
(NI) of the immune response to one
dose of HAV vaccine simultaneously administered on the same day with one dose
TDV on the same day, compared
to one dose HAV vaccine simultaneously on the same day administered with
placebo on the same day, in DENV/HAV-
naive subjects one month after vaccination.
[00483] The secondary objectives of this study were to describe WV-induced
immunogenicity after a single dose
of TDV in DENV/HAV-naive subjects; to describe TDV-induced immunogenicity
after two doses of TDV administered
90 clays apart in DENV/HAV-naive subJects; to describe 11AV vaccine-induced
immunogenicity in DENV/HAV-naive
subjects; and to accPcc the safety profile after each vaccine injection in all
trial groups.
4.2 Eligibility Criteria
[00484] Criteria for inclusion include:
1. The participant is aged 18 to 60 years, inclusive.
2. Participants who are in good health at the time of entry into the trial as
determined by medical history,
physical examination (including vital signs) and the clinical judgment of the
Investigator.
3. The participant signs and dates a written informed consent form and any
required privacy authorization
prior to the initiation of any trial procedures, after the nature of the trial
has been explained according to
local regulatory requirements.
4. Participants who can comply with trial procedures and are available for
the duration of follow-up.
[00485] Exclusion criteria include:
1. Participants with an elevated oral temperature (38 C or 100.4 F) within 3
days of the intended date of
vaccination.
2. Known hypersensitivity or allergy to any of the vaccine components
(including excipients of the
investigational vaccines or placebo).
3. Participants with behavioral or cognitive impairment or psychiatric disease
that, in the opinion of the
Investigator, may interfere with the participant's ability to participate in
the trial.
4. Participants with any history of progressive or severe neurologic disorder,
seizure disorder or neuro-
inflammatory disease (e.g., Guillain-Barre syndrome).
96
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
5. Participants with history or any illness that, in the opinion of the
Investigator, might interfere with the
results of the trial or pose additional risk to the participant due to
participation in the trial.
6. Known or suspected impairment/alteration of immune function, including:
1. Chronic use of oral steroids (equivalent to 20 mg/day prednisone
weekfl2 mg/kg body
weight/day prednisone a-2 weeks) within 60 days prior to Day 1 (MO) (use of
inhaled, intranasal, or
topical corticosteroids is allowed).
2. Receipt of parenteral steroids (equivalent to 20 mg/day prednisone 12
weeks/ 2 mg/kg body
weight/day prednisone weeks) within 60
days prior to Day 1 (MO).
3. Administration of immunoglobulins and/or any blood products within the 3
months prior to Day 1
(MO) or planned administration during the trial.
4. Receipt of immunostimulants within 60 days
prior to Day 1(M0).
5. Immunosuppressive therapy such as anti-cancer chemotherapy or radiation
therapy within 6
months prior to Day 1 (MO).
6. Human immunodeficiency virus (HIV) infection
or HIV-related disease.
7. Hepatitis A virus (HAV) infection.
8. Hepatitis C virus infection.
9. Genetic immunodeficiency.
7. Abnormalities of splenic or thymic function.
B. Participants with a known bleeding diathesis, or any condition that may be
associated with a prolonged
bleeding time.
9. Participants with any serious chronic or progressive
disease according to judgment of the Investigator (e.g.,
neoplasm, insulin dependent diabetes, cardiac, renal or hepatic disease).
10. Participants with body mass index (BMI) greater than or equal to 35 kg/m"-
2 (=weight in kg/[height in
meters2]).
11. Participants participating in any clinical trial with another
investigational product 30 days prior to Day 1 (MO)
or intent to participate in another clinical trial at any time during the
conduct of this trial.
12. Participants who received any other vaccine within 14 days (for
inactivated vaccines) or 28 days (for live
vaccines) prior to enrollment in this trial or who are planning to receive any
vaccine within 28 days of trial
vaccine administration.
13. Previous HAV vaccination (in a clinical trial or with an approved
product).
14. Participants involved in the trial conduct or their first degree
relatives.
15. Participants with history of substance or alcohol abuse within the past 2
years.
16. Female participants who are pregnant or breastfeeding.
17. Females of childbearing potential who are sexually active, and who have
not used any of the acceptable
contraceptive methods for at least 2 months prior to Day 1 (MO).
1. Of childbearing potential is defined as status post onset of menarche
and not meeting any of the
following conditions: bilateral tubal ligation (at least 1 year previously),
bilateral oophorecb3my (at
least 1 year previously) or hysterectomy
2. Acceptable birth control methods are defined as one or more of the
following:
97
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
i. Hormonal contraceptive (such as oral, injection, transdermal patch,
implant, cervical ring).
ii. Barrier method (condom with spermicide or diaphragm with spermicide) each
and every time during
intercourse.
iii. Intrauterine device (IUD). iv. Monogamous relationship with vasectomized
partner (partner must have
been vasectomized for at least 6 months prior to Day 1 [MO]).
Other contraceptive methods may be considered in agreement with the Sponsor
and implemented only after
approval of a substantial amendment by the regulatory authorities and by the
appropriate ethics committee.
18. Females of childbearing potential who are sexually active, and who refuse
to use an acceptable
contraceptive method up to 6 weeks after the last dose of trial vaccine (Day
90 [M3]). In addition, they
must be advised not to donate ova during this period.
19. Any positive or indeterminate pregnancy test.
20. Previous and planned vaccination (during the trial conduct) against any
flaviviruses including dengue, yellow
fever (YF), Japanese Encephalitis (JE) viruses or tick-borne encephalitis.
21. Previous participation in any clinical trial of a dengue or other
flavivirus (e.g., West Nile [WN] virus)
candidate vaccine, except for participants who received placebo in those
trials.
22. Participants with a current or previous infection with a flavivirus such
as dengue, Zika, YF, 31E, WN fever,
tick-borne encephalitis or Murray Valley encephalitis and participants with a
history of prolonged year)
habitation in a dengue endemic area.
23. Participants with contraindications, warnings and/or precautions to
vaccination with the HAY vaccine as
specified within the product information.
4.3 Study Design & Vaccinations
[00486] Eligible subjects were randomized equally (1:1:1 ratio) to one of the
following 3 trial groups (300 subjects
per group):
- Group 1: HAV vaccine (IM) and placebo (SC), simultaneously on the same day
administered on day 1 (month 0);
placebo (SC) administered at day 90 (month 3).
- Group 2: TDV (SC) and placebo (IM), simultaneously on the same day
administered on day 1 (month 0); TDV
(SC) administered at day 90 (month 3).
- Group 3: TDV (SC) and HAV vaccine (IM), simultaneously on the same day
administered on day 1 (month 0);
TDV (SC) administered at day 90 (month 3).
A more detailed scheme of the study design is shown in Figure 6. Up to 28 days
prior to the first vaccination,
enrolment was carried out and blood samples were taken for screening anti-HAV
antibodies. On day 1, pre-
vaccination blood samples were taken. On day 30 (after the first vaccination
on day 1) post-vaccination blood
samples were taken. On day 120 (after the first vaccination on day 1) another
blood sample was taken. Safety
follow-up took place on day 270 (after the first vaccination on day 1).
[00487] The TDV was prepared as described in Example 1. Each subcutaneous dose
of the TDV had a volume of
0.5 ml and the concentration of the four dengue serotypes in the TDV in each
dose was 5.1 10910pfu/0.5 ml, 4.5
1og10pfu/0.5 ml, 5.4 10g10pfu/0.5 ml and 5.9 log10pfu/0.5 ml of TDV-1, TDV-2,
TDV-3 and TDV-4, respectively. Each
subcutaneous dose comprises the TDV dispersed in 0.5 ml of an aqueous solution
containing Pluronic F127 (10.6
mg/ml), trehalose dihydrate (170 mg/ml) and human serum albumin (1.08 mg/m1).
98
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00488] The HAV vaccine includes an inactivated hepatitis A virus, derived
from a hepatitis A virus strain HM-175
(see definitions above), and is commercially available under the tradename
HAVRIX as described above. The
intramuscular dose of the liAV vaccine administered to groups 1) and 3) was 1
ml and each 1 ml close has a viral
antigen activity of about 1440 ELU., wherein the viral antigen is adsorbed on
0.5 mg of aluminum in the form of
aluminum hydroxide. The hepatitis A vaccine contains excipients in the form of
an amino acid supplement (about
(13% vilv) and in the form of polysorbate (about 0.05 mg/ml) dissolved in a
phosphate-buffered saline solution.
[00489] Simultaneously on the same day administered trial vaccines were
injected to opposite arms. Normal saline
solution for injection (0.9% NaCI) was used as placebo. A blood sample for an
anti-HAV antibody test were collected
at screening from all subjects to exclude subjects who are positive for anti-
HAV antibodies up to 28 days prior b,
vaccination (see Figure 6). All subjects were followed-up for 6 months after
the second vaccination at day 90 (month
3), so the trial duration was 270 days or 9 months for each subject (not
including the screening period). Outside the
context of this trial, subjects in Groups 1 and 3 will be offered a HAV
vaccine booster dose after the completion of
trial procedures at day 270 (month 9).
[00490] Dengue neutralizing antibodies (microneutralization test (MNT50)) were
measured using blood samples
collected at pre-first trial vaccination (day 1 (month 0)), 1 month post first
trial vaccination (day 30 (month 1)), and
1 month post second trial vaccination (day 120 (month 4)). Blood samples for
the measurement of anti41AV
antibodies (enzyme-linked immunosorbent assay (ELISA)) were collected at pre-
first trial vaccination (day 1 (month
0)) and 1 month post first trial vaccination (day 30 (month 1)).
4.4 Primary Endpoint
[00491] The primary endpoint included the proportion of HAWDENV-naive subjects
at baseline who are
seroprotected against HAV at day 30 (month 1) as measured by enzyme-linked
immunosorbent assay (ELISA)). In
other words, the primary endpoint includes the seroprotection rates (SPRs).
Seroprotection is defined as serum anti-
HAV antibody levels L-1.0 mIU/mL Immunological naivety to HAV/DENV is defined
as anti-HAV antibody levels <10
mIU/mL and reciprocal neutralizing titers for all 4 dengue serotypes <10-
4.5 Secondary Endpoints
[00492] a) Secondary Immunogenicity Endpoints
[00493] The secondary endpoints included the geometric mean titers of
neutralizing antibodies (GMTs)
(microneutralization test (MNT50)) for each of the 4 dengue serotypes at day
30 (month 1) and day 120 (month 4)
which were determined in HAV/DENV-naive subjects at baseline; the proportion
of 11AV/DENV-naive subjects at
baseline who are seropositive for each of the 4 dengue serotypes at day 30
(month 1) and day 120 (month 4)
(seroprotection rate); and geometric mean concentrations (GMC) of anti-HAV
antibodies at day 30 (month 1) in
subjects I1AV/DENV-naive at baseline.
[00494] Seropositivity for dengue virus is defined as a reciprocal
neutralizing titer flO fix any of the four dengue
serotypes within the secondary immunogenicity endpoints.
[00495] b) Secondary Safety Endpoints
[00496] Secondary safety endpoints included the frequency and severity of
solicited local adverse events (AE) for 7
99
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
days after each trial vaccination; the frequency and severity of solicited
systemic AEs for 14 days after each trial
vaccination; the percentage of subjects with any unsolicited AEs for 28 days
after each trial vaccination; the
percentage of subjects with serious adverse events (SAE) throughout the trial;
and the percentage of subjects with
medically attended adverse events (MAAE) throughout the trial.
4.6 Analysis Sets of the Study
[00497] Table 12 below displays each analysis set of the present study. In
total, 1199 subjects belonging to the
group "all screened" included all subjects who signed the informed consent,
regardless of whether the subjects were
screen failures. After initial screening, 900 subjects were included into the
"randomized set" which includes all
randomized subjects, regardless of whether any dose of the IPs was received.
The safety set, consisting of 897
subjects, includes all randomized subjects who received
dose of the IPs. The immunogenicity subjects
included a
total of 359 subjects and is subdivided into the following four subsets. The
HAV-full analysis set (HAV-FAS) includes
all randomized subjects in the immunogenicity subset who received 1- 1 dose of
the trial vaccine with available day 1
and day 30 anti-HAV antibody measurements. The HAV-per-protocol set (HAV-PPS)
includes all I-1AV- and DENV-
naive subjects from the HAV-FAS who have no major protocol violations. The mV-
full analysis set (TDV-FAS)
includes all randomized subjects in the immunogenicity subset who received a-
1 dose of trial vaccine and with
available day 1 and 1 post-dose measurements. The TDV-per-protocol set (TDV-
PPS), consisting of 197 subjects,
includes all HAV- and DENV-naive subjects from the TDV-FAS who have no major
protocol violations.
Table 12. Analysis sets of the study.
HAV/Pbo
TDV/ Pbo HAV/TDV Total
All Screened' NA
NA NA 1199
Randomized See 30()
300 300 900
Safety Set (55)3 299
300 298 897
__________________________________
...............................................................................
...............................................................................
...............................................................................
.......................................
= HAV Full
Analysis Set (HAV-FAS)4 115 117 114 346
= HAY Per-
Protocol Set (HAV-PPS)5 75 71 81 227
= TDV-FAS6
116 117 115 348
= TDV-PPS7
67 63 67 197
E:E:ESAiblilittiE:aiiabiaied for primary non-Inferiority
;;; ;
;;;;;;;;;;;;;;; ...... ;;;;;;;;;;;;;;;;;;;;;;;;;; ; ;; ....... ; .
,;;;;;;;;;;;;;;;; ; .......... ; . ;;;;; .......
'All Screened: All subjects who signed the informed consent, regardless of
whether subjects were screen failures
2Randomized Set: All randomized subjects, regardless of whether any dose of
the trial vaccines was received.
3Safety Set: All randomized subjects who received a- 1 dose of trial vaccines.
4HAV-FAS: All randomized subjects in the immunogenicity subset who recdved> 1
dose of trial vaccine, with available Day 1 and
Day 30 HAV measurements.
5HAV-PPS: All HAV & DENV-naive subjects from the HAV-FAS who have no major
protocol violations.
frTDV-FAS: All randomized subjects in the immunogenicity subset who received 1
dose of trial vaccine and available Day 1 and
1 post-dose measurement.
7TDV-PPS: All HAV & DENV-n=ive subjects from the -WV-FAS who have no major
protocol violations.
a Subject excluded from TDV-PPS but induded into analysis for primary non-
inferiority objective had their Day 30 measurement
outside the protocol defined visit window.
100
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00498] From a total number of 359 subjects in the immunogenicity subset
(including all subjects which received
= 1 vaccination), 13 subjects have been excluded from HAV-FAS because of
not providing a valid baseline and post-
dosing measurement (on day 30) for HAV. Furthermore, HAV-PPS includes all HAV-
and DENV-naive subjects of I-1AV-
FAS who had no major protocol violations. The subjects analyzed for primary
non-inferiority objective are based on
the HAV-PPS subjects of the HAV/Pbo and HAVADV group, wherein based on HAV-PPS
of these two groups
(HAV/Pbo and HAVIITDV), some subjects were not included in the 30 days
analysis (6 subjects of the HAV/Pbo
group and 2 Subjects of the HAV/TDV group), since these subjects had their day
30 measurement outside the visit
window defined in the protocol. Therefore, a total of 69 subjects was included
into the TDV/Pbo group and a total of
79 subjects was included in the HAVaDV group for analyzing the primary non-
inferiority objective.
[00499] From a total number of 359 subjects in the immunogenicity subset
(including all subjects which received
1 vaccination), a total of 11 subjects have been excluded from TDV-FAS,
because they did not provide a valid
baseline and at least one post-dosing measurement (day 30 and/or day 120) for
TDV. Furthermore, a total number
of 151 subjects had been excluded from the TDV-PPS for not being HAV & DENV-
naive at baseline or for not
receiving both vaccinations 1 and 2 or if vaccination 2 (usually on day 90) is
outside the window -15/ 25 days or if
major protocol violations occur.
4.7 HAV Baseline Serostatus and Dernoaraohic & Baseline Characteristics
[00500] The safety set evaluated for baseline HAV antibody levels included a
total of 362 subjects of which 27.3%
were HAV seropositive at baseline (see Table 13).
[00501] HAS-MS included a tab! of 346 subjects evaluated for baseline HAV
antibody levels (see Table 13). HAV-
naivety was defined as anti-HAV antibody (ab) level of < 10 mIU/ml. However,
the ELISA used for serological
analysis could not be validated below levels of 12.5 mIU/ml. The qualitative
screening test had a specification that
effectively amounted to a lower limit of quantification of 70 mIU/ml. In view
of these criteria, 72.5% of the subjects
of said 1-LAS-FAS evaluated -for baseline HAV antibody levels were HAV naive
at baseline (see Table 13).
Table 13. HAV baseline serostatus in the safety set
and in the HAV-FAS
-------------------------------------------------------------------------------
------ 11 V/Trrif -- TOW
antibody .................. levels ................................... 119
121 122::::::
362
= HAV
seropositive at baseline 31 (26.1%) 38 (31.4%) 30
(24.6%) 99 (27.3%)
HAV-FASEvaluated For
::: 114(100%) ::::346(100%)
= HAV
seronegative at baseline <12.5 mILI/rni 86 (74.8%) 7-9 (67.5%) 86
(74.4%) 251 (72.5%)
(a)
= HAV
seropositive at baseline (b) 29 (25.2%) 38 (32.5%) 28
(24.6%) 95 (27.5%)
=
baseline 12.5-70 mIU/ml 18 23 13 54
=
baseline 70-1000 mIU/ml 11 14 14 39
=
baseline >1000 0 1 1 2
(a) HAV-naivety was defined as anti-HAV ab level of< 10 mIU/ml; The EUSA used
for serological analysis could not be
validated below levels of 12.5 mIU/ml.
101
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
(b) The qualitative screening test had a specification that effectively
amounted to a lower limit of quantification of 70
mw/m1
[00502] The HAV-PPS includes a total number of 227 subjects (see Table 14).
The mean age of the total number
of subjects of the HAV-PPS, which are DEW- and HAV-naive was 34.8. 31.3% of
the total number of subjects of the
HAV-PPS were female (see Table 14). In total, 97.8% of the total subjects of
the HAV-PPS were of an ethnicity which
is NOT Hispanic or Latino, and, in particular, 89.9% of the HAV-PPS
participants were of race "white European",
especially in order to reflect the situation of travelers of HAV- and dengue
non-endemic countries (see Table 14).
[00503] The safety set includes a total number of 897 subjects (see Table 14).
The mean age of the total number
of subjects of the safety set was 35_4 years of which 31.3% were female. In
total, 97.7% of the total subjects of the
HAV-PPS were of an ethnicity which is NOT Hispanic or Latino, and, in
particular, 87.1% of the HAV-PPS participants
were of race "white EuropeanTM, especially in order to reflect the situation
of travelers of HAV- and dengue non-
countries (see Table 14).
Table 14. Demographic and baseline characteristics
(HAV-PPS and Safety Set)
NAV/ Pbo
TIW/Pbo HAV/TDV Total
fin"rn tin" .. vinas ---- N
75
71 81 --------- 227 ....
-naive) -----------------------------------------------------------------------
-------------------------------------------------
Age Years Mean (SD)
34.3 (11.68) 35.5 (11.24) 34.8(11.70) 34.8 (11.51)
Gender Female n (c/o)
24 (32.0%) 30 (42.3%) 17 (21.0%) 71 (31.3%)
Ethnicity NOT Hispanic or
n ( /0)
/2(96.0%) 71 (10(10%) 79 (97.5%) 222 (97.8%)
Latino
Race White European n (0/0)
64(85.3%) 64 (90.1%) 76(93.8%) 204 (89.9%)
HAV/Pbo
TDV/Pbo HAV/TDV Total
:
_______________________________________________________________________________
_______________________________________________
Age Years Mean (SD)
34.7 (12.04) 36.0(11.88) 35.5 35.4(11.96)
(11.96 /0)
Gender Female n ( /0)
107 (35.8%) 120 (40.0%) 90(302%) 317 (35.3%)
Ethnicity NOT Hispanic or n (%)
289 (96.7%) 293 (97.7%) 294 (98.7%)
876 (97.7%)
Latino
Race White European n (%)
255 (85.3%) 265 (88.3%) 261 (87.6%)
781 (87.1%)
4.8 Study Results
[00504] a) Primary endpoint and sensitivity analyses
102
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00505] The present study was successful in meeting the primary objective of
non-inferiority for the simultaneous
on the same day administration of HAV and TDV. Table 15 displays the
seroprotection rates (SPRs) of groups 1
(received HAV/Pbo) and 3 (received HAV/TDV) on day 30 after the first
vaccination (on day 1), the SPR differences
between the HAV/Pbo group and the HAV/TDV group on day 30, and the confidence
intervals (CIs) of these SPR
differences for HAV and DENV-baseline naive subjects. These values (SPRs, SPR
differences; CIs) were used for the
primary endpoint evaluation of the study. Table 15 further shows these values
for the results of three sensitivity
analyses (also used for non-inferiority assessments), wherein the subjects had
different, i.e. mixed, HAV/TDV
serostatuses at baseline. Non-inferiority between the hepatitis A vaccine and
the tetravalent dengue vaccine, when
simultaneously on the same day administered, in the present study is
concluded, if the seroprotection rate (SPR)
difference between group 1 (received HAV and placebo on the same day 1) and
group 3 (received HAV and TDV on
the same day 1) has an upper bound of a two-sided 95% confidence interval,
calculated using the Newcombe score
method, which is lower than the 10% non-inferiority margin. This criterion is
fulfilled for each of the groups in Table
15. In the primary endpoint group, the upper bound of the 95% CI of the SPR
difference is 4.31% which is less
than the non-inferiority margin of 10% (see second line from above in Table
15).
[00506] As mentioned above, sensitivity analyses 1 to 3 were used to evaluate
populations that included subjects
who were seropositive for dengue and/or for hepatitis A at baseline, in
particular reflecting "real life" travel clinic
settings in non-endemic countries in which subjects, i.e. travelers who plan
to go to dengue and HAV endemic
countries, are not always aware of their HAV and/or dengue serostatus before
requesting pre-travel vaccinations.
[00507] The object of non-inferiority of the simultaneous on the same day
administration was also met in
sensitivity analyses 1 to 3 (upper bounds of the 95% CI of the SPR
differences: 3.21%; 2.93%; and
2.55% which are each less than the non-inferiority margin of 10%).
Furthermore, the SPRs of thellAV/TDV
groups (98.8%; 99.0%; 99.1%, respectively, see Table 15) were respectively
higher than the SPRs of the 1-IAV/Pbo
group (96.2%; 96.9%; 97.2%, respectively, see Table 15).
[00508] Therefore, due to the non-inferiority of the simultaneous on the same
day administration of the HAV
vaccine and TDV to subjects with mixed baseline serostatus (and baseline
naivety) with respect to HAV and all
dengue serotypes, there is no need for determining or knowing the subject's
baseline serostatus with respect to each
of the two diseases, prior to simultaneously on the same day administering the
1-1Av vaccine and TDV.
103
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table 15. Primary endpoint: Non-inferiority (NI)
assessments & sensitivity analysis
HAV/Pbo HAV/TDV
95% cv
Analysis Analysis Set (Group 1) (Group3)
SPR difference (of SPR
SPR% (n/n) SPR% (n/n)
difference)
................... .......... .................. ............ .
. .. ....... ............. .........
.. . ............ .................... ........ ................
.............. ............... .............. .. .
anas..õ HAVRPS :,: includes. ........ ....... ... oit;. .
. .
"" "" .................. wo .......... ........ .
....... ............ .
... ........ -------------------------
................ !!(+.a.7%. 441)!
!!!-401.1010..!,-.R; -- .......... su .....................
(07f. 49):: ............ (n17.9). ........
...........::::::: ......... ::::::::::::::::: ..............
::::::::::::::: ................ .....
................... .......... ................... .................. .......
............. ......... ................. .................... ........
....................... .............. ............... .............. .....
HAV PPS ¨ includes
Sensitivity 96.2% 98.8%
baseline HAY-, DENV-,
-2.61 (-9.46, 3.21)
Analysis 1 and DENV+ subjecls (76/79) (83/84)
HAV PPS ¨ includes
Sensitivity baseline HAV-; HAV+ 96.9% 99.0%
-2.09
(-7.8Z /93)
Analysis 2 (12.5-70mIU/m1); DENY-; (93/96) (96/97)
DEN1V+
HAV PPS ¨ includes
Sensitivky 97.2%
baseline HAV-; HAV+; (109/110)
-1.92 (-7.14, 2.55)
Analysis 3 (103/106)
DENV-; DENV+
= Confidence Interval
Non-inferiority Assessment: Seroprotection Rates (SPRs) for HAV Group 1 (HAV +
placebo simultaneous on the same day
administration ) vs Group 3 (HAV + 11W simultaneous on the same day
administration)
Rates difference for primary comparison (G-oup 1 ¨ Group 3) are presented
together with 95% CI calculated using
Newcombe score method
NI of simultaneous on the same day administration of HAV and TDV to HAV alone
will be concluded if the upper bound of
the 95% CI is less than NI margin of 10%.
[00509] b) Secondary irrimunogenicity endpoints
[00510] Table 16 shows GMTs (with respect to each of the four dengue virus
serotypes DENY-1 to DENV-4) when
measured on day 1 pre-first vaccination, on day 30 after the first vaccination
(on day 1), and on day 120 after the
first vaccination of the subjects (on day 1) of the DENV-PPS including the
groups receiving HAV/TDV, WV and
placebo, as well as HAV and placebo (Pbo), respectively. Table 16 shows
positive trends in favor of the simultaneous
on the same day administration group (received HAV/TDV) with respect to all
dengue GMTs.
[00511] Said positive trend in favor of the simultaneous on the same day
administration and the day 30 synergism
of the simultaneously on the same day administered vaccines is also confirmed
in Table 17.
104
CA 03147807 2022-2-11
C
u.,
1-,
A
-1
co
0
-.,
N,
0
.
N
N
-
Table 16. Immunogenicity of DENV-PPS: GMTs of DENV MNT50. In particular, DENV
GMTs against each serotype with respect to mean titers of neutralizing
0
antibodies measured by MNT50 for each dengue serotype by trial visit are
shown. 0
64
kJ
DENV-1
DENV-2 DENV-3
DENV-4 1.1.
C
.... . .. .................. . ....:::.::::::.: -.... .-
........E.:.:õ..........
,::.:.:::.:.:.:7...........:.::.:::.:.:::.:.....,....::.....7......E.::::::::
7 ... ... ... ...... .::7:::.:.......
NAV/ .....TOW
HAV/TDV FIAV/p
/DV/p- HAVITDV ---- - ---HAVip - ..TDV/p -HAVEIDV.. '
HAV P:=::: - P= = - - :: = ::
: :: . === === ==== :::::::=.:-.. . . .. - .
.......:::::::. - .... - . -:.-:.:-:..... - . - . - .. -
...... : .... : ........ : ::
=
= == == = -:--::=:: : :: : :: : :. :: : ==== : :::::::::::::: :: : - 2-2..2.22
- . - ::::::::: . - - ................. - - -:.-:-:-:-:-:-:-:-:-:
..........:::::.::::::.........:::.:.::.:.:.:.::...... .....::::.::::::::..:
........: .:::.:.::.:::.:.::...................:::::::::::::.:::.:.: .:
..............:.........................:)).:))..:).:.:......:
............................................ ...........
n 67 63 67 67
63 67 67 63 67 67
63 67
Day 1 GMT 5.0 5.0 5.0 5.0
5.0 5.0 5.0 5.0 5.0 5.0
5.0 5.0
(SD') (1.00) (1.00) (1.00) (1.00)
(1.00) (1.00) (1.00) (1.00) (1.00) (1.00)
(1.00) (1.00)
n 62 60 65 62
60 65 62 60 65 62
60 65
Day 30 GMT 50 1082 1525 60
2897 9 39600 53 95.4 140.5 5 0 74
3 142.1
cn n 50 55 62 50
55 62 50 55 62 50
55 62
Day 120 GMT 5.0 171.3 173.7 5.7
2064.1 1764.3 5.0 83.8 92.6 5.0
56.1 81.4
(SD) (1.00) (6.23) (4.28) (1.72)
(3.60) (4.03) (1.00) (3.66) (2.80) (1.00)
(3.19) (3.49)
1 SD = standard deviation.
00
ri
cit
b.*
e
no
em.
I
ti.=
C
Ne
ie
-
WO 2021/034349
PCT/US2020/020991
Table 17. Secondary endpoint: HAV Geometric Mean Concentrations (GMCs)
HAV/Pbo ------------------------------------------------------------------
TDV/Pbo :::::::: ---------------------------- :::::::HAV/TDV
Daseime: ----------------------------------------- :::::::::::::::::
::::::::::::: :::::::::::::::
= n
75 71 81 227
= Geometric Mean (SD')
6.3 (1.00) 6.3 (1.00) 63 (1.00) 63 (1.00)
= 95% Cl2
(NE, NE) (NE, NE) (NE, NE) (NE, NE)
= Median
6.3 63 6.3 6.3
= Min-max
6,6 6,6 6,6 6,6
= n
69 66 79 214
----------------------- ::::::: ----------------- Mean (344
86.8 (161) :::: 93A (2A):::: 39S(427.)::::-
...............................................................................
........................................
:::::::::: ------------ :::::::::::::::::::::: :::::
=....................... :::::::: -- .............. ....... ::::::::
................... ::::::::::::
= 95% CI
(61.8, 105.0) (6.4, 7.2) (76.1, 113.6) (32.5, 48.0)
= Median
81.5 63 94.5 52.4
= Min-max
6, 1044 6, 16 6, 1859 6, 1859
SD = standard deviation.
a CT = confidence interval.
[00512] c) Secondary safety endpoints
[00513] The safety set was investigated for solicited adverse events,
solicited systemic adverse events, unsolicited
adverse everrls and serious adverse events throughout the study. Tables 18a to
21c show the study results of the
safety set with respect to each secondary safety endpoint.
106
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
[00514] Table 18a.
Frequency of Solicited Local
AEs and Solidted Systemic Ms after first
vaccination ¨ Safety Set
HAV/Pbo (N=270) TDV/Pbo (N=271) HAV/TDV (N=257)
. .......
Solicited Local
::::::::::: ------------------------------------------------------------
:::::::::::::::::::::::::::::::: .. ::::::::::::
14 /289 mai m :::::::::::
:::::::::::: :19s1285 (68.581
.............
.
ken Systemic :::::::::::::
Jart4ow ,4 oat :tartnrar : t: :::::::::: ..
05.,a) ::::::: .. kL) ..
Related to IP 98/289 (33.9) 106/292 (36.3) 117/285
(41.1)
Not related to IP 41/289 (14.2)
26/292 (8.9) 24/285 (8.4)
Note: For solicited AEs, excluding prolonged solicited AEs, percentages are
calculated based on number of subjects with non-
missing data (n) evaluated in each trial group. Subjects with 1 or more AEs
for a particular category of AEs are counted only once
using the most related event
Table 18b.
Frequency of Solidted Local
AEs and Solicited Systemic AEs after second vaccination ¨
Safety Set
Pbo
TI)V TDV
(N=270)
(N=271) (N=257)
Solicited Local
28/255 (11.0)
100/264 (37.9) 103/251 (41.0)
(within 7 days)
Solicited Systemic
73/254 (28.7)
82/263 (31.2) 85/251 (33.9)
(within 14 days)
Related to IP 49/254 (19.3) 60/263 (22.8) 61/251
(24.3)
Not related to II' 24/254 (9.4)
22/263 (8.4) 24/251 (9.6)
Not: For solicited AB, excluding prolonged solicited Arms, percentages are
calculated based on number of subjects with non-
missing data (n) evaluated in each trial group. Subjects with 1 or more AEs
for a particular category of te are counted only once
using the most related event
107
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table 18c.
Frequency of Solicited I nral AEs and Solidted Systemic
AEs after any vaccination ¨
Safety Set
HAV/Pbo (n=299)
TDV/Pbo (n=300) HAV/TDV (n=298)
Solicited Local
151/289 (52.2)
175/292 (59.9) 216/285 (75.8)
(within 7 days)
Solicited Systemic
161/289 (55.7)
167/292 (57.2) 167/285 (58.6)
(within 14 days)
Related to IP 121/289 (41.9) 133/292 (45.5)
141/285 (49.5)
Not related to IP 401289 (13.8) 34/292
(11.6) 26/285 (9.1)
Note: For solicited AEs, excluding prolonged solicited AEs, percentages are
calculated based on number of subjects with me-
missing data (n) evaluated in each trial group. Subjects with 1 or more AEs
for a particular category of AEs are counted only once
using the most related event
Table 19a.
Overview of Unsolicited AE up to 28 Days Post-vaccination
(after first vaccination) ¨
Safety Set
HAV/Pbo
TDV/Pbo HAV/TDV
N=299
11=300 N=298
[Any AE], n (%) 43 (14.4%)
51 (17.0%) 56 (18.8%)
1 ............. õ,...... _ _ _ . .õ,
.... _ _ . .......õ,.
.................õ,............ ,.... .. _ ::
I IIIIIIIIIIIIIIIIIIIIIIIIIIII
Moderate 11 (3.7%)
18(50%) 12 (4.0%)
Severe 0 1 (0.3%)
1 (0.3%)
Notes:
= This summary includes all unsolicited AEs with a date of onset within 28
days after each trial vaccination.
= N is the number of subjects who received the specific vaccination.
Percentages are calculated based on N for corresponding
column.
= Table shows the number cf subjects that reported unsolicited AE
= Subject with 1 or more AEs for a particular category of adverse event are
counted only once using the most related/most
severe/most serious event.
108
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table 19b. Overview of Unsolicited AE up to 28 Days
Post-vaccination (after second vaccination) ¨
Safety Set
Pbo WV
TDV
N=270 N =271
N=257
Any AE, n ( /0) 39 (14.4%) 27
(10.01)/0) 30 (11.7%)
Mild 17 (6.3%) 14 (5.2%)
15 (5.8%)
Moderate 22 (8.1%) 13 (4.8%)
13 (5.1%)
Severe 0 0
2(0.8%)
Nos:
= This summary includes all unsolicited AEs with a date of onset within 28
days after each trial vaccination.
= N is the number of subjects who received the specific vaccination.
Percentages are calculated based on N for corresponding
column.
= Table shows the totals of AE that were experienced by the number of
subjects that reported unsolidted AE
Subjects with 1 or more AEs for a particular category of adverse event are
counted only once using the most related/most
severe/most serious event.
Table 20. Most Common Unsolicited AEs Up to 28 Days
Post-Vacdnation (after Any Vaccination)*
¨ Safety Set
NAV/ Pbo
TDV/Pbo I1AV/TDV
AE, n (0/0)
N=299
N=300 N=298
Nasopharyngitis 9 (3.0%)
8 (2.7%) 11 (3.7%)
* Reported by 2.0 % of subjects.
109
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table 21a.
Safety: Overview of Serious Adverse
Events (SAEs) After any dose ¨ Safety Set
Number of
events, HAV/Pbo TDV/Pbo
HAV/TDV Total
number (%) (n=299) (n=300)
(n=298) (N=597)
subjects with Evenla Subjects
Events Subjects Events Subjects Events Subjects
SAE
2 8
7 17
SAES ¨ any 3 10
10 23
(0.7%) (2.7%)
(2.3%) (2.8%)
*After 14 dose up 3
2 5
0 0 3
3 6
to 2nd dose (1.0%)
(01%) (0.8%)
= After 2"d dose 2
5 5 12
3 7 7 up to trial
end (I).7%) (1.8%) (1.9%) 17 (2.0%)
SAEs ¨ related 0 0 0 0
0 0 0 0
ME ¨
premature 1
1
vaccine and/or 0 0 1
0 0 1
(0.3%)
(0.2%)
trial
discontinuation
Deaths 0 0 0 0
0 0 0 0
p = Placebo
110
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table 21b. Safety: Serious Adverse Events by System
Organ Class and Preferred Term after first
and after second dose - Safety Set
Number (%) subjects with SAE
HAV/Pbo
TDV/Pbo HAV/TDV
SOC
(N=299) (N=300) (N=298)
After e dose up to ea dose ¨ any SAE
0 3 (1.0) 2(0-7)
Gastrointestinal disorders Crohn's
disease* 0 1 (0.3) 0
Injury, poisoning and procedural
complications
Intentional
overdose
0 0 1 (0.3)
Neoplasms benign, malignant and unspecified
Bladder cancer
stage II
0 1(0.3) 0
Nervous system disorders Loss of
0 1 (0.3) 0
consciousness'*
Psychiatric disorders
Intentional self- 0 0 110.3)
injury
Pbo
TDV TDV
(N=270)
(N=271) (N=257)
After ed dose up to end of trial¨ any SAE
2(02) 5(1.8) 5(1.9)
Cardiac disorders
Supraventricular 0 0 1 (0.4)
tachycardia
Gastrointestinal disorders Abdominal
pain 0 1 (0.4) 0
Abdominal
1 (0.4) 0 0
strangulated
hernia
Intestinal
0 0 1 (0.4)
ischaemia
Mesenteric vein
0 0 1 (0.4)
thrombosis
Oesophagitis
0 1 (0.4) 0
* Subject had a history of irritable bowel syndrome
**Occurred >2 months after vaccination
111
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
Table 21c. Safety: Serious Adverse Events by System
Organ Class and Preferred TermAfter first
and after second dose - Safety Set ¨ Continued
Number (%) subjects
with SAE
Pbo TDV TDV
(N=270)
(N=271) (N=257)
SOC PT
Infections and Appendicitis
0 1 (0.4) 0
infestations
Wound infection
1 (0.4) 0 0
Injury, poisoning and
procedural complications
Abdominal injury
0 1 (0.4) 0
Cervical vertebral fracture
0 0 1 (0.4)
Fractured coccyx
1 (0.4) 0 0
Joint dislocation
0 1 (0.4) 0
Lower limb fracture
0 0 1 (0.4)
Thermal bum
0 1 (0.4) 0
Neoplasms benign,
malignant and Invasive ductal breast
unspecified carcinoma
0 1 (0.4) 0
Prostate cancer
0 0 1 (0.4)
Respiratory, thoracic and Acute respiratory
mediastinal disorders distress syndrome
0
0 1(0.4)
112
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
FIRST ITEM LIST OF THE INVENTION
1. A method of preventing hepatitis A and dengue disease in a subject or
subject population, the method
comprising simultaneously on the same day administering a hepatitis A vaccine
and a unit dose of a dengue vaccine
composition, wherein said unit dose comprises a tetravalent dengue virus
composition including four live, attenuated
dengue virus strains.
2. The method according to item 1, wherein the hepatitis A vaccine is an
inactivated virus vaccine.
3.
The method according to item 1 or 2, wherein the
dengue vaccine composition upon reconstitution with 0.5
mL of a pharmaceutically acceptable diluent comprises
(i) a chimeric dengue serotype 211 strain in a concentration of at least 33
log10 pfu/0.5 mL,
(ii) a dengue serotype 2 strain in a concentration of at least 2.7 log10
pfu/0.5 mL,
(iii) a chimeric dengue serotype 2/3 strain in a concentration of at least 4.0
log10 pfu/0.5 mL, and
(iv) a chimeric dengue serotype 2/4 strain in a concentration of at least 4.5
log10 pfu/0.5 mL
4.
The method according to any
one of the preceding items, wherein the subject population or subject is
seronegative to all dengue serotypes.
5.
The method according to any one of the preceding
items, wherein said unit dose of the dengue vaccine
composition is administered by subcutaneous injection and said hepatitis A
vaccine is administered by intramuscular
injection, and wherein said injections are preferably administered to the arm,
more preferably to the deltoid region of
the arm.
6.
The method according to item 5, wherein said unit
dose of the dengue vaccine composition and said
hepatitis A vaccine are administered to different anatomical sites, such as to
opposite arms.
7. The method according to any one of the preceding items, wherein two of
said unit doses of the dengue
vaccine composition are administered within 12 months or more, or within six
months, or within three months.
8. The method according to item 7 comprising the administration of two of
said unit doses of the dengue
vaccine composition and one dose of said hepatitis A vaccine, in particular
according to the following schedule
- a first simultaneous adminisU .. a lion of the first
unit dose of the dengue vaccine composition and said hepatitis A
vaccine on day 0, and
- a second administration of the second unit dose of the dengue vaccine
composition after said first simultaneous
administration, such as about 3 months later.
9. The method according to any one of the preceding items, wherein the
subject population or subject is of 2
to 60 years of age.
10. The method according to any one of the preceding items, wherein the
subject population or subject is from
a dengue endemic region.
113
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
11. The method according to any one of items 1 to 9, wherein the subject
population or subject is from a
dengue non-endemic region, preferably from a dengue non-endemic and hepatitis-
A non-endemic region.
12. The method according to any one of the preceding items, wherein the
hepatitis A vaccine comprises a
hepatitis A virus derived from a hepatitis A virus strain 1-1M-175.
13. The method according to any one of the preceding items, wherein the
hepatitis A vaccine comprises an
inactivated hepatitis A virus and the inactivated hepatitis A virus is derived
from a hepatitis A virus strain HM-175.
14.
The method according to any one of the preceding
items, wherein the hepatitis A vaccine comprises an
inactivated hepatitis A virus and wherein the inactivated hepatitis A virus is
adsorbed on aluminum.
15. The method according to item 14, wherein the aluminum is aluminum
hydroxide or aluminum
hydroxyphosp hate sulfate.
16. The method according to any one of the preceding items, wherein the
hepatitis A vaccine comprises an
inactivated hepatitis A virus and wherein the hepatitis A vaccine comprises a
phosphate-buffered saline solution and
excipients dissolved therein in the form of an amino acid and in and in the
form of polysorbate.
17.
The method according to any one of the preceding
items, wherein the hepatitis A vaccine includes a
hepatitis A virus expressing a viral antigen in a concentration ranging from
500 ELLSA Units (FLU.) to 2000 ELISA
Units (FLU.).
18. The method according to any one of the preceding items, wherein the
method does not include a step of
determination whether there was a previous dengue infection and/or a previous
hepatitis A infection in the subject
population or in the subject before the administration of the hepatitis A
vaccine and before the administration of the
unit dose of the dengue vaccine composition or
wherein the hepatitis A serostatus and/or the dengue serostatus of the subject
population or of the subject is
unknown before the administration of the hepatitis A vaccine and before the
administration of the unit dose of the
dengue vaccine composition.
19. The method according to item 18, wherein the method does not include a
step of determination whether
there was a previous dengue infection and/or a previous hepatitis A infection
in the subject population or in the
subject at any time before, during and after the steps of administration of
the hepatitis A vaccine and of the unit
dose of the dengue vaccine composition or
wherein the hepatitis A serostatus and/or the dengue serostatus of the subject
population or of the subject is
unknown at any time before, during or after the steps of administration of the
hepatitis A vaccine and of the unit
dose of the dengue vaccine composition.
20.
The method according to any of the preceding
items, wherein the method comprises a primary vaccination
consisting of the steps of:
(A)
selecting a subject for
administration of the unit doses of the tetravalent dengue virus composition
and the
hepatitis A vaccine in need for protection against dengue infection and
hepatitis A infection without determination
whether there was a previous dengue infection and/or a previous hepatitis A
infection, and
114
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
(B) administering a first unit dose of the tetravalent dengue virus
composition and a hepatitis A vaccine to the
subject, and optionally
(C) administering at least one further unit close of the tetravalent dengue
virus composition to the subject
within 3 to 12 months of administration of the first unit dose, and optionally
(D)
administering at least one further dose of the
hepatitis A vaccine to the subject within 6 to 18 months of
administration of the first unit dose.
21.
The method according to any
one of the preceding items, wherein the method comprises a primary
vaccination consisting of the steps of:
(A)
selecting a subject for administration of the
unit doses of the tetravalent dengue virus composition and the
hepatitis A vaccine in need for protection against dengue infection and
hepatitis A infection, and
(B) administering a first unit dose of the tetravalent dengue virus
composition and a hepatitis A vaccine to the
subject, and
(C) administering two further unit doses of the tetravalent dengue virus
composition to the subject at about 6
and about 12 months of administration of the first unit dose and administering
a hepatitis A vaccine to the subject at
either about 6 or about 12 months of administration of the first unit dose.
22.
The method according to item
21, wherein step (A) is carried out without determination whether there was
a previous hepatitis A infection.
23.
The method according to any
one of items 3 to 22, wherein upon reconstitution of the dengue vaccine
composition with a pharmaceutically acceptable diluent (i), (ii), (iii), and
(iv) provide a total concentration of pfu/0.5
mL and based on said total concentration of pfu/0.5 ml the concentration of
(ii) in pfu/0.5 mL is less than 10%, and
the concentration of (iv) in pfu/0.5 mL is at least 50%, and the concentration
of (i) in pfu/0.5 mL is at least 1%, and
the concentration of (iii) in pfu/0.5 mL is at least 6%, at least 8%, or at
least 10%, or at least 12%, or at least 14%,
or at least 16%, or at least 18%.
24.
The method according to any
one of the preceding items, wherein the method provides compatibility
between the dengue vaccine composition and the hepatitis A vaccine.
25.
The method according to any
one of the preceding items, wherein the method provides synergy between
the dengue vaccine composition and the hepatitis A vaccine.
26.
The method according to any
one of the preceding items, wherein the method provides non-inferiority in a
non-inferiority clinical study including at least 60 or at least 120 healthy
subjects divided into one subject population
and into one control subject population, wherein the subject population
receives simultaneously on the same day the
hepatitis A vaccine and the unit dose of the dengue vaccine composition and
the control subject population receives
simultaneously on the same day a hepatitis A vaccine and a placebo
administration.
27.
The method according to any one of the preceding
items, wherein the hepatitis A vaccine provides a
hepatitis A seroprotection rate of at least 95% or of at least 98% on day 30
after an administration (on day 0/1) to a
subject population of at least 30 or at least 50 healthy subjects receiving
simultaneously on the same day the
hepatitis A vaccine and the unit dose of the dengue vaccine composition and
being seronegative with respect to
hepatitis A at baseline and being seronegative with respect to all dengue
virus serotypes at baseline.
115
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
28. The method according to any one of the preceding items, wherein the
method provides a hepatitis A
seroprotection rate difference with respect to a hepatitis A mono-
administration, the difference being determined in a
non-inferiority clinical study including at least 60 or at least 120 healthy
subjects being seronegative with respect to
hepatitis A at baseline and seronegative with respect to all dengue virus
serotypes at baseline,
the healthy subjects being divided into
a) a subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the same day
an administration (on day 0/1) of the hepatitis A vaccine and the unit dose of
the dengue vaccine
composition, and
b) a control subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the
same day an administration (on day 0/1) of a hepatitis A vaccine and a
placebo,
wherein the difference is determined between the hepatitis A seroprotection
rate of the control subject
population on day 30 after the administration (on day 0/1) and the hepatitis A
seroprotection rate of the subject
population on day 30 after the adminiblration (on day 0/1), and
wherein the difference has an upper bound within a two-sided 95% confidence
interval which is lower than
10%.
29. The method according to any one of items 1 to 26, wherein the hepatitis
A vaccine provides a hepatitis A
seroprotection rate of at least 95% or of at least 98% or of at least 99% on
day 30 after an administration (on day
0/1) to a subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the same day
the hepatitis A vaccine and the unit dose of the dengue vaccine composition
and being seronegative with respect to
hepatitis A at baseline, wherein the healthy subjects include healthy
subject(s) which are seropositive with respect to
at least one dengue virus serotype at baseline and healthy subject(s) which
are seronegative with respect to all
dengue virus serotypes at baseline.
30. The method according to any one of items 1 to 26, wherein the method
provides a hepatitis A
seroprotection rate difference with respect to a hepatitis A mono-
administration, the difference being determined in a
non-inferiority clinical study including at least 60 or at least 120 healthy
subjects being seronegative with respect to
hepatitis A at baseline, wherein the healthy subjects include healthy
subject(s) which are seropositive with respect to
at least one dengue virus serotype at baseline and healthy subject(s) which
are seronegative with respect to all
dengue virus serotypes at baseline,
the healthy subjects being divided into
a) a subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the same day
an administration (on day 0/1) of the hepatitis A vaccine and the unit dose of
the dengue vaccine
composition, wherein the subject population includes healthy subject(s) which
are seropositive with respect
to at least one dengue virus serotype at baseline and healthy subject(s) which
are seronegative with respect
to all dengue virus serotypes at baseline, and
b) a control subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the
same day an administration (on day 0/1) of a hepatitis A vaccine and a
placebo, wherein the control subject
population includes healthy subject(s) which are seropositive with respect to
at least one dengue virus
serotype at baseline and healthy subject(s) which are seronegative with
respect to all dengue virus
serotypes at baseline,
116
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
wherein the difference is determined between the hepatitis A semprotection
rate of the control subject
population on day 30 after the administration (on day 0/1) and the hepatitis A
seroprotection rate of the subject
population on day 30 after the administration (on day 0/1), and
wherein the difference has an upper bound within a two-sided 95% confidence
interval which is lower than
10%.
31. The method according to any one of the preceding
items, wherein the subject or subject population is
exposed to a hepatitis A virus outbreak and/or a dengue virus outbreak.
11) 32. The method according to any of the preceding items, wherein the
method provides an anti-hepatitis A virus
antibody Geometric Mean Concentration (GMC) of at least 70 mIU/m1 or at least
80 mIU/m1 or at least 90 mIU/m1 on
day 30 after an administration (on day 0/1) to a subject population of at
least 30 or at least 50 healthy subjects
receiving simultaneously on the same day the hepatitis A vaccine and the unit
dose of the dengue vaccine
composition and being seronegative with respect to hepatitis A at baseline and
being seronegative with respect to all
dengue virus serotypes at baseline.
33. The method according to any one of the preceding items, wherein the
simultaneous on the same day
administration of the hepatitis A vaccine and the unit dose of the dengue
vaccine composition to the subject or the
subject population is safe.
34. The method according to any one of the preceding items, wherein there
are no serious adverse events
related to the simultaneous on the same day administration.
35. The method according to any one of the preceding items, wherein the
method provides the Geometric Mean
Titer (GMT) of neutralizing antibodies measured by MUT50 of
- at least 110 or at least 140 or at least 150 for dengue serotype 1,
- at least 3000 or at least 3500 or at least 3900 for dengue seratype 2,
- at least 100 or at least 120 or at least 140 for dengue serotype 3,
and/or
- at least 80 or at least 110 or at least 140 for dengue serotype 4,
on day 30 after an administration (on day 0/1) to a subject population of at
least 30 or at least 50 healthy subjects
receiving simultaneously on the same day the hepatitis A vaccine and the unit
dose of the dengue vaccine
composition and being seronegative with respect to hepatitis A at baseline and
being seronegative with respect to all
dengue virus serotypes at baseline.
36. The method according to any one of the preceding items, wherein the
subject or subject population is 18 to
60 years of age.
37. A kit against hepatitis A and dengue disease
comprising
a box containing at least
(a) a first container holding a hepatitis A vaccine, and
(b) a second container holding a unit dose of a
dengue vaccine composition, wherein said unit dose
comprises a tetravalent dengue virus composition including four live,
attenuated dengue virus strains.
117
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
SECOND ITEM LIST OF THE INVENTION
1. Use of a hepatitis A vaccine for the manufacture of
a medicament for preventing hepatitis A in a subject or
subject population and a use of a unit dose of a dengue vaccine composition
for the manufacture of medicament for
preventing dengue disease in the subject or subject population, the prevention
of hepatitis A and dengue disease
comprising simultaneously on the same day administering the hepatitis A
vaccine and the unit dose of a dengue
vaccine composition, wherein said unit dose comprises a tetravalent dengue
virus composition including four live,
attenuated dengue virus strains.
2. Use according to item 1, wherein the hepatitis A vaccine is an
inactivated virus vaccine.
3. Use according to item 1 or 2, wherein the dengue
vaccine composition upon reconstitution with 0.5 mL of a
pharmaceutically acceptable diluent comprises
(i) a chimeric dengue serotype 2/1 strain in a
concentration of at least 3.3 log10 pfu/0.5 mL,
(ii) a dengue serotype 2 strain in a concentration of at least 2.7 log10
pfu/0.5 mL,
(iii) a chimeric dengue serotype 2/3 strain in a concentration of at least 4.0
log10 pfu/0.5 mL, and
(iv) a chimeric dengue serotype 2/4 strain in a concentration of at least 4.5
log10 pfu/0.5 mL
4. Use according to any one of the preceding items,
wherein the subject population or subject is seronegative
to all dengue serotypes.
5. Use according to any one of the preceding items,
wherein said unit dose of the dengue vaccine composition
is administered by subcutaneous injection and said hepatitis A vaccine is
administered by intramuscular injection, and
wherein said injections are preferably administered to the arm, more
preferably to the deltoid region of the arm.
6. Use according to item 5, wherein said unit dose of
the dengue vaccine composition and said hepatitis A
vaccine are administered to different anatomical sites, such as to opposite
arms.
7. Use according to any one of the preceding items,
wherein two of said unit doses of the dengue vaccine
composition are administered within 12 months Of more, or within six months,
or within three months.
8. Use according to item 7 comprising the
administration of two of said unit doses of the dengue vaccine
composition and one dose of said hepatitis A vaccine, in particular according
to the following schedule
- a first simultaneous administration of the first unit
dose of the dengue vaccine composition and said hepatitis A
vaccine on day 0, and
- a second administration of the second unit dose of the
dengue vaccine composition after said first simultaneous
administration, such as about 3 months later.
9. Use according to any one of the preceding items,
wherein the subject population or subject is of 2 to 60
years of age.
10. Use according to any one of the preceding items,
wherein the subject population or subject is from a
dengue endemic region.
118
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
11. Use according to any one of items 1 to 9, wherein the subject
population or subject is from a dengue non-
endemic region, preferably from a dengue non-endemic and hepatitis-A non-
endemic region.
12. Use according to any one of the preceding items, wherein the hepatitis
A vaccine comprises a hepatitis A
virus derived from a hepatitis A virus strain HM-175.
13. Use according to any one of the preceding items, wherein the hepatitis
A vaccine comprises an inactivated
hepatitis A virus and the inactivated hepatitis A virus is derived from a
hepatitis A virus strain 1*1-175.
14. Use according to any one of the preceding items, wherein the hepatitis
A vaccine comprises an inactivated
hepatitis A virus and wherein the inactivated hepatitis A virus is adsorbed on
aluminum.
15. Use according to item 14, wherein the aluminum is aluminum hydroxide or
aluminum hydroxyphosphate
sulfate.
16. Use according to any one of the preceding items, wherein the hepatitis
A vaccine comprises an inactivated
hepatitis A virus and wherein the hepatitis A vaccine comprises a phosphate-
buffered saline solution and excipients
dissolved therein in the form of an amino acid and in and in the form of
polysorbate.
17. Use according to any one of the preceding items, wherein the hepatitis
A vaccine includes a hepatitis A virus
expressing a viral antigen in a concentration ranging from 500 ELISA Units
(ELU.) to 2000 ELISA Units (ELU.).
18. Use according to any one of the preceding items, wherein the method
does not include a step of
determination whether there was a previous dengue infection and/or a previous
hepatitis A infection in the subject
population or in the subject before the administration of the hepatitis A
vaccine and before the administration of the
unit dose of the dengue vaccine composition or
wherein the hepatitis A serostatus and/or the dengue serostatus of the subject
population or of the subject is
unknown before the administration of the hepatitis A vaccine and before the
administration of the unit dose of the
dengue vaccine composition.
19. Use according to item 18, wherein the method does not include a step of
determination whether there was
a previous dengue infection and/or a previous hepatitis A infection in the
subject population or in the subject at any
time before, during and after the steps of administration of the hepatitis A
vaccine and of the unit dose of the
dengue vaccine composition or
wherein the hepatitis A serostatus and/or the dengue serostatus of the subject
population or of the subject is
unknown at any time before, during or after the steps of administration of the
hepatitis A vaccine and of the unit
dose of the dengue vaccine composition.
20. Use according to any of the preceding items, wherein the method
comprises a primary vaccination
consisting of the steps of:
(A) selecting a subject for administration of the unit doses of the
tetravalent dengue virus composition and the
hepatitis A vaccine in need for protection against dengue infection and
hepatitis A infection without determination
whether there was a previous dengue infection and/or a previous hepatitis A
infection, and
(B) administering a first unit dose of the tetravalent dengue virus
composition and a hepatitis A vaccine to the
subject, and optionally
119
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
(C) administering at least one further unit dose of the tetravalent dengue
virus composition to the subject
within 3 to 12 months of administration of the first unit dose, and optionally
(D) administering at least one further dose of the hepatitis A vaccine to
the subject within 6 to 18 months of
administration of the first unit dose.
21. Use according to any one of the preceding items, wherein the method
comprises a primary vaccination
consisting of the steps of:
(A)
selecting a subject for
administration of the unit doses of the tetravalent dengue virus composition
and the
hepatitis A vaccine in need for protection against dengue infection and
hepatitis A infection, and
(B)
administering a first unit dose of the
tetravalent dengue virus composition and a hepatitis A vaccine to the
subject, and
(C)
administering two further
unit doses of the tetravalent dengue virus composition to the subject at about
6
and about 12 months of administration of the first unit dose and administering
a hepatitis A vaccine to the subject at
either about 6 or about 12 months of administration of the first unit dose.
22. Use according to item 21, wherein step (A) is carried out without
determination whether there was a
previous hepatitis A infection.
23. Use according to any one of items 3 to 22, wherein upon reconstitution
of the dengue vaccine composition
with a pharmaceutically acceptable diluent (i), (ii), (iii), and (iv) provide
a total concentration of pfu/0.5 mL and
based on said total concentration of pfu/0.5 ml the concentration of (ii) in
pfu/0.5 mL is less than 10%, and the
concentration of (iv) in pfu/0.5 mL is at least 50%, and the concentration of
(i) in pfu/0.5 mL is at least 1%õ and the
concentration of (iii) in pfu/0.5 mL is at least 6%, at least 8%, or at least
10%, or at least 12%, or at least 14%, or
at least 16%, or at least 18%.
24. Use according to any one of the preceding items, wherein the method
provides compatibility between the
dengue vaccine composition and the hepatitis A vaccine.
25. Use according to any one of the preceding items, wherein the method
provides synergy between the
dengue vaccine composition and the hepatitis A vaccine.
26. Use according to any one of the preceding items, wherein the method
provides non-inferiority in a non-
inferiority clinical study including at least 60 or at least 120 healthy
subjects divided into one subject population and
into one control subject population, wherein the subject population receives
simultaneously on the same day the
hepatitis A vaccine and the unit dose of the dengue vaccine composition and
the control subject population receives
simultaneously on the same day a hepatitis A vaccine and a placebo
administration.
27. Use according to any one of the preceding items, wherein the hepatitis
A vaccine provides a hepatitis A
seroprotection rate of at least 95% or of at least 98% on day 30 after an
administration (on day 0/1) to a subject
population of at least 30 or at least 50 healthy subjects receiving
simultaneously on the same day the hepatitis A
vaccine and the unit dose of the dengue vaccine composition and being
seronegative with respect to hepatitis A at
baseline and being seronegative with respect to all dengue virus serotypes at
baseline.
120
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
28. Use according to any one of the preceding items,
wherein the method provides a hepatitis A seroprotection
rate difference with respect to a hepatitis A mono-administration, the
difference being determined in a non-inferiority
clinical study including at least 60 or at least 120 healthy subjects being
seronegative with respect to hepatitis A at
baseline and seronegative with respect to all dengue virus serotypes at
baseline,
the healthy subjects being divided into
c) a subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the same day
an administration (on day 0/1) of the hepatitis A vaccine and the unit dose of
the dengue vaccine
composition, and
d) a control subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the
same day an administration (on day 0/1) of a hepatitis A vaccine and a
placebo,
wherein the difference is determined between the hepatitis A seroprotection
rate of the control subject
population on day 30 after the administration (on day 0/1) and the hepatitis A
seroprotection rate of the subject
population on day 30 after the administration (on day 0/1), and
wherein the difference has an upper bound within a two-sided 95% confidence
interval which is lower than
10%.
29. Use according to any one of items 1 to 26, wherein
the hepatitis A vaccine provides a hepatitis A
seroprotection rate of at least 95% or of at least 98% or of at least 99% on
day 30 after an administration (on day
0/1) to a subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the same day
the hepatitis A vaccine and the unit dose of the dengue vaccine composition
and being seronegative with respect to
hepatitis A at baseline, wherein the healthy subjects include healthy
subject(s) which are seropositive with respect to
at least one dengue virus serotype at baseline and healthy subject(s) which
are seronegative with respect to all
dengue virus serotypes at baseline.
30. Use according to any one of items 1 to 26, wherein the method provides
a hepatitis A seroprotection rate
difference with respect to a hepatitis A mono-administration, the difference
being determined in a non-inferiority
clinical study including at least 60 or at least 120 heathy subjects being
seronegative with respect to hepatitis A at
baseline, wherein the healthy subjects include healthy subject(s) which are
seropostive with respect to at least one
dengue virus serotype at baseline and healthy subject(s) which are
seronegative with respect to all dengue virus
serotypes at baseline,
the healthy subjects being divided into
c) a subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the same day
an administration (on day 0/1) of the hepatitis A vaccine and the unit dose of
the dengue vaccine
composition, wherein the subject population includes heathy subject(s) which
are seropositive with respect
to at least one dengue virus serotype at baseline and healthy subject(s) which
are seronegative with respect
to all dengue virus serotypes at baseline, and
d) a control subject population of at least 30 or at least 50 healthy subjects
receiving simultaneously on the
same day an administration (on day 0/1) of a hepatitis A vaccine and a
placebo, wherein the control subject
population includes healthy subject(s) which are seropositive with respect to
at least one dengue virus
serotype at baseline and healthy subject(s) which are seronegative with
respect to all dengue virus
serotypes at baseline,
wherein the difference is determined between the hepatitis A seroprotection
rate of the control subject
population on day 30 after the administration (on day 0/1) and the hepatitis A
seroprotection rate of the subject
population on day 30 after the administration (on day 0/1), and
121
CA 03147807 2022-2-11
WO 2021/034349
PCT/US2020/020991
wherein the difference has an upper bound within a two-sided 95% confidence
interval which is lower than
10%.
31. Use according to any one of the preceding items, wherein the subject or
subject population is exposed to a
hepatitis A virus outbreak and/or a dengue virus outbreak.
32. Use according to any of the preceding items, wherein the method
provides an anti-hepatitis A virus antibody
Geometric Mean Concentration (GMC) of at least 70 rnIU/m1 or at least 80
mIU/m1 or at least 90 mIU/m1 on day 30
after an administration (on day 0/1) to a subject population of at least 30 or
at least 50 healthy subjects receiving
simultaneously on the same day the hepatitis A vaccine and the unit dose of
the dengue vaccine composition and
being seronegative with respect to hepatitis A at baseline and being
seronegative with respect to all dengue virus
serotypes at baseline.
33. Use according to any one of the preceding items, wherein the
simultaneous on the same day administration
of the hepatitis A vaccine and the unit dose of the dengue vaccine composition
to the subject or the subject
population is safe.
34. Use according to any one of the preceding items, wherein there are no
serious adverse events related to the
simultaneous on the same day administration.
35. Use according to any one of the preceding items, wherein the method
provides the Geometric Mean Titer
(GMT) of neutralizing antibodies measured by MNT50 of
- at least 110 or at least 140 or at least 150 for dengue serotype 1,
- at least 3000 or at least 3500 or at least 3900 for dengue serotype 2,
- at least 100 or at least 120 or at least 140 for dengue serotype 3, and/or
- at least 80 or at least 110 or at least 140 for dengue serotype 4,
on day 30 after an administration (on day 0/1) to a subject population of at
least 30 or at least 50 healthy subjects
receiving simultaneously on the same day the hepatitis A vaccine and the unit
dose of the dengue vaccine
composition and being seronegative with respect to hepatitis A at baseline and
being seronegative with respect to all
dengue virus serotypes at baseline.
36. Use according to any one of the preceding items,
wherein the subject or subject population is 18 to 60
years of age.
37. A kit against hepatitis A and dengue disease comprising
a box containing at least
(a) a first container holding a hepatitis A vaccine, and
(b) a second container holding a unit dose of a dengue vaccine composition,
wherein said unit dose
comprises a tetravalent dengue virus composition including four live,
attenuated dengue virus strains.
122
CA 03147807 2022-2-11