Note: Descriptions are shown in the official language in which they were submitted.
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
SPECIFICATION
TITLE
PLANT EXTRACTION METHOD
TECHNICAL FIELD
The present disclosure belongs to the technical field of plant extraction,
specifically
relates to a plant extraction method.
BACKGROUND
Natural plant extracts refer to products obtained by using plants as raw
materials and
specifically obtaining or concentrating one or more active components in
plants without
changing the active structure thereof via physical and chemical extraction and
separation
according to the use of the final extracted product. Plant extracts contain
rich and complex
organic components, wherein the majority of the organic components have
biological activities
such as antibacterial activity, bacteriostatic activity, antioxidative
activity and the activity of
regulating the immunity of body. Being environmentally friendly, healthy,
safe, efficient, and
residue-free, etc., plant extracts are widely used in fields such as
medicines, health care
products, cosmetics, food additives, pesticides, feed and daily necessities
all over the world.
China is the cradle of silkworm industry. Plants of Moraceae have been
considered as precious
materials used as medicine and food since ancient times due to their high
nutritional value and
medicinal value, which is recorded in traditional Chinese medicine classics in
all ages, for
example, as early as in the Compendium of Materia Medica, there are
descriptions of "the
decoction of the mulberry leaf juice can quench thirst in place of tea" and
"drinking after being
cooked and decocted can quench thirst in place of tea". Modem science has also
proved the
nutritional components and the pharmacologically active components of the
plants of
Moraceae as well as the mechanisms of action thereof Researches have indicated
that the
chemical components in the plants of Moraceae mainly include flavone
compounds,
polysaccharide compounds, alkaloids and amino acids, in addition to some
volatile oils, tannin,
succinic acid, adenine, vitamins, and the like, and have antifungal effect,
anti-inflammatory
effect, hypoglycemic effect, antioxidative effect, and the like.
Plant extraction methods may be classified into classical extraction methods
and modern
extraction methods. Classical extraction methods do not require special
instruments, are simple
and easy to operate, have low extraction cost, and mainly include solvent
extraction method,
steam distillation method, and the like. Modern extraction methods are
extraction methods
1
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
based on modem advanced instruments or newly developed extraction methods, and
mainly
include ultrasonic extraction method, microwave extraction method, enzyme
extraction method,
solid phase extraction method, and the like. The components of plant extracts
may be classified
into lipophilic components and hydrophilic components, which may be obtained
by selecting
different solvents and/or extraction methods for different plant extracts. For
example, as for
lipophilic plant components, organic solvents may be used to conduct
percolation, cold
leaching, ultrasonic extraction, microwave extraction, reflux extraction, and
the like; as for
water-soluble components, a commonly used extraction method is water
extraction and alcohol
precipitation method, which is of great significance in terms of the refining
and purification,
the increase of the content of active components, the decrease of dosage of
administration and
the convenience in production and molding of the extracts of traditional
Chinese medicine. The
specific steps include steps such as water extraction, concentration, alcohol
precipitation,
drying, and the like, wherein alcohol precipitation refers to adding ethanol
to the crude
extraction solution until the concentration of ethanol reaches an appropriate
concentration to
allow impurities insoluble in ethanol to precipitate, and then conducting
solid-liquid separation
to achieve the purpose of refining. For example, Xingjie Chang, et al.
extracted the active
components of Forsythia in accordance with the steps of decoction with water,
concentration,
ethanol precipitation and recovery of ethanol (Xingjie Chang, Qian Ding.
Forsythiaside A,
Forsythin and changes of the bacteriostatic activities thereof in the
preparation process of water
extraction and alcohol precipitation of Forsythia [J]. Guiding Journal of
Traditional Chinese
Medicine and Pharmacy, 2018, 24(9):39-41.); Aiying Shen, et al. obtained
mulberry leaf
polysaccharides by using water extraction and ethanol precipitation method
(Aiying Shen, Ziyu
Zhu, Wenliang Zhang. Study on the extraction process of water-soluble
polysaccharides from
mulberry leaves [J]. Acta Sericologica Sinica, 2004, 30(3):277-279.). In order
to further
concentrate and enrich the active components, resin separation, membrane
separation,
ultrafiltration, dialysis and the like may also be conducted after alcohol
precipitation, so as to
achieve the purpose of purification.
The increasing demand of consumers for plant extract products has prompted
great
development of plant extraction process, and the quality of the products has
been improved.
However, compared with developed countries, there are still some prominent
problems in
Chinese plant extracts. Among these, product safety issues such as the residue
of heavy metals
and pesticides have attracted extensive attention. It has been found by
researches that some
plants are capable of enriching the heavy metals (such as copper, lead,
cadmium, zinc, etc.) in
the environment. For example, the research conducted by Xing Zhang, el al.
shows that, in a
case where a mulberry is grown on soil containing the following heavy metals,
i.e., Cu (593.56
2
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
mg/kg), Pb (825.41 mg/kg), Cd (8.11 mg/kg), Zn (705.41 mg/kg), the contents of
heavy metals
measured in roots are up to the following contents, i.e., Cu: 33.13 mg/kg, Pb:
33.13 mg/kg, Cd:
4.53 mg/kg, Zn: 317.72 mg/kg, the contents of heavy metals in leaves are up to
the following
contents, i.e., Cu: 13.18 mg/kg, Pb: 10.32mg/kg, Cd: 1.90 mg/kg, Zn: 186.53
mg/kg, which far
exceeds the relevant regulations (according to the regulations of "Green Trade
Standards of
Medicinal Plants and Preparations for Importing and Exporting", in the raw
materials,
decoction pieces, extracts and preparations of plants, the total content of
heavy metals is 20.0
mg/kg or less, the concent of Pb is 5 mg/kg or less, the content of Cd is 0.3
mg/kg or less, the
content of Hg is 0.2mg/kg or less, the content of Cu is 20.0 mg/kg or less,
and the content of
As is 2.0 mg/kg or less). How to reduce the residues of harmful substances
such as heavy
metals to the greatest extent is an important aspect required to be considered
in the research of
plant extraction methods.
SUMMARY
In general researches and industrial production processes, water-soluble plant
extracts are
generally obtained by water extraction and alcohol precipitation method, and
resin separation,
membrane separation, ultrafiltration, dialysis and the like would be conducted
after alcohol
precipitation for further enrichment and purification when considering
increasing the content of
a specific component. However, as for plant extracts, especially plants like
plants of Moraceae
that are prone to enrich the heavy metals in the environment, using such
extraction method to
obtain extracts is not sufficient to remove the heavy metal residues in the
extracts. It has been
found by the present inventors based on repeated research that, during the
extraction process of
the plant, subjecting the crude extraction solution to resin separation,
concentration and alcohol
precipitation successively is not only capable of improving the separation
efficiency of the
active components, but also capable of reducing the heavy metal residues in
the plant extracts
significantly. Based on such finding, the present inventors provide a novel
plant extraction
method, which is capable of effectively reducing the content of the heavy
metals in the plant
extracts while greatly reducing the amount of ethanol used in the extraction
process, thereby
improving the quality of the product while reducing the production cost, and
improving the
efficiency and safety of industrial production to a certain extent.
In view of this, in the first aspect, the present disclosure provides a plant
extraction
method comprising the following steps of:
step 1): preparing a crude plant extraction solution;
step 2): separating the crude extraction solution via a cation resin to
collect the eluate,
optionally, separating the &nate via an anion resin to collect the effluent;
3
CA 03149142 2022-2-23
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
step 3): concentrating the collection solution obtained in step 2);
step 4): subjecting the concentrated solution obtained in step 3) to alcohol
precipitation;
and
optionally, step 5): concentrating and drying.
1) Preparing the crude plant extraction solution
In the present disclosure, the plant is preferably a plant of Moraceae,
Liliaceae,
Campanulaceae, or Commelinaceae, further preferably a plant of Morus,
Hyacinthus,
Adenophora, or Commelina, and more preferably, the plant is any one or a
combination of
more selected from Morus multicaulis Perrott., Morus alba L., Morus
atropurpurea Roxb,
Morusmizuho Hotta, Morus wittiorum Hand Mazz., Morris laevigata Wall, Morus
nigra Linn.,
Morris cathayana Hemsi., Morus serrata Roxb., Morus mongolica Schneid., Mortis
bombycis
Koidz., Morus notabilis Schmid., Morus nigriformis Koidz., Morus yunnanensis
Koidz.,
Morus australis Poir., Morus mongolica (Bur.) Schneid var. diabolica Koidz.,
Morus alba L.
var. macrophylla loud, Morus alba Var.Pendula Dippel, Moms alba L. var. venosa
Delili, a
mulberry variety bred from the above mulberry species, a hybrid mulberry
obtained from
selective intra-species or inter-species breeding of the above mulberry
species, Hyacinthus
orientalis, Adenophora. triphylla var. japonica, and Commelina cornmuni;
preferably, the plant
is Morus atropurpurea Roxb, Morus multicaulis Perrott., Morus alba L., Morus
serrata Roxb.,
Morus bombycis Koidz., or a hybrid mulberry, the hybrid mulberry is preferably
Yuesang 11,
Guisangyou 62 or Teyou 2. Various parts such as the leaf, root, branch, bark,
bud, stem, and
fruit of the plant may be used.
The plant may be subjected to crude extraction with a solvent such as alcohol-
water,
water, an alkaline aqueous solution or an acidic aqueous solution, preferably,
the solvent used
for the crude extraction is water.
During extraction, it is preferred to crush the plant and then add the
resultant into water
to conduct heat extraction, the extraction duration is preferably 0.5h to 3h
for each extraction,
and the extraction is conducted 1 to 3 times.
In a preferred embodiment, the crushed plant may be added into an extraction
tank to
conduct extraction.
During extraction, the more the addition amount of the solvent, the higher the
extraction
rate of the plant. However, the addition amount of the solvent being excessive
may increase the
difficulty of subsequent separation and purification. The addition amount of
the solvent is
preferably 3 to 20 times and more preferably 4 to 15 times the weight of the
charged plant raw
material, which is capable of obtaining the plant extract to the greatest
extent without
excessively increasing the volume of the solution and increasing the
difficulty of subsequent
4
Date recue/Date received 2023-04-06
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
processing.
Extraction may be conducted by using decocting method, ultrasonic extraction
method or
reflux extraction method, preferably conducted by using decocting method or
reflux extraction
method, and more preferably conducted by using decocting method which has more
mature
industrial equipment.
Optionally, extraction may be conducted repeatedly and the extracting
solutions are
combined.
Preferably, the extracting solution is filtered to remove insoluble matters,
so as to obtain
the crude plant extraction solution.
2) Separation via cation resin and optional anion resin
In the present disclosure, the components in the crude plant extraction
solution are
separated by using ion exchange resin.
In step 2), the crude plant extraction solution is loaded onto the cation
resin and separated
via the cation resin. Preferably, after being packed into the column, the
cation resin is subjected
to activation by being washed with an acidic solution, an alkaline solution
and an acidic
solution successively. =The activation method of the resin is also capable of
realizing the
adjustment of the pH value of the resin environment, thereby optimizing the
adsorption
selectivity of the cation resin and enhancing the separating effect.
Preferably, the resin is washed with an alkaline solution until the pH of the
eluate is 8.0 to
9.5, preferably 8.5 to 9.5.
Preferably, the alkaline solution is ammonia solution, sodium hydroxide
solution,
potassium hydroxide solution or sodium carbonate solution, preferably ammonia
solution or
sodium hydroxide solution. Preferably, the concentration of the alkaline
solution is 0.5 to 4
mol/L, preferably 1 to 2 mol/L.
Preferably, the resin is washed with an acidic solution until the pH of the
eluate is 3.0 to
7.0, preferably 4.5 to 6.5.
Preferably, the acidic solution is selected from hydrochloric acid solution,
phosphoric
acid solution and disodium hydrogen phosphate-citric acid buffer, and is more
preferably
disodium hydrogen phosphate-citric acid buffer. Preferably, the concentration
of the acidic
solution is 0.5 to 4 mol/L, preferably 1 to 2 mol/L.
When disodium hydrogen phosphate-citric acid buffer is used as the acidic
solution, the
pH value of the disodium hydrogen phosphate-citric acid buffer is preferably
4.0 to 6.5, and
more preferably 4.5 to 5Ø
Optionally, the cation resin may also be washed with deionized water having a
volume of
3 to 5 times the column volume after the last washing with the acidic
solution.
5
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
Preferably, the cation resin is one or a combination of more selected from a
strongly
acidic cation exchange resin, a weakly acidic cation exchange resin and a
strongly alkaline
quaternary ammonium-type cation resin.
Preferably, the cation resin is one or a combination of more selected from 732-
type
strongly acidic styrene-based cation exchange resin, 734-type strongly acidic
styrene-based
cation exchange resin, 002SC-type strongly acidic styrene-based cation
exchange resin,
D001-type macroporous and strongly acidic styrene-based cation exchange resin,
D113-type
macroporous and weakly acidic phenylpropene-based cation exchange resin and
D254-type
macroporous and strongly alkaline quaternary ammonium-type cation exchange
resin.
Preferably, the cation resin is 732-type strongly acidic styrene-based cation
exchange
resin, 734-type strongly acidic styrene-based cation exchange resin and D001-
type
macroporous and strongly acidic styrene-based cation exchange resin.
It has been found by the research that, without being bound by any theory, the
loading
process of the cation exchange resin, especially the concentration of the
loading solution and
the amount of resin used, has significant influence on the adsorption and
separation effects of
plant components.
Preferably, the amount of the cation resin used and the charged plant raw
material has a
weight ratio ranging from 1:1 to 1:30, preferably 1:1 to 1:25, and more
preferably 1:2 to 1:20.
After loading the crude plant extraction solution onto the cation resin, the
loaded cation
resin is subjected to elution with an eluent. Preferably, the eluent is a salt
solution or an
alkaline solution containing cations, preferably one or more selected from
sodium chloride,
ammonium chloride, ammonium sulfate, ammonium nitrate, ammonia water,
potassium
chloride and sodium hydroxide.
Preferably, the cations in the eluent have a concentration ranging from 0.04
to 5 mol/L,
preferably 0.2 to 3 mol/L, and more preferably 0.5 to 2.5 mol/L.
Preferably, the flow rate of the eluent is 1 to 15 BV/h, preferably 5 to 10
BV/h.
Preferably, the weight of the eluent used for the separation via cation resin
is 0.1 to 30
times the weight of the charged plant raw material. Preferably, the elution is
conducted with the
eluent, of which the weight is 0.5 to 10 times the weight of the charged plant
raw material.
Collection begins when the eluate flows out of the cation resin. The starting
point of the
collection may be determined according to the pH of the effluent obtained from
the cation resin,
for example, when an alkaline solution such as ammonia water is used for
conducting elution,
collection begins when the pH of the effluent obtained from the cation resin
is higher than 7.
The starting point of the collection may also be determined according to the
properties of the
components to be separated, for example, the starting point of the collection
of the effluent
6
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
may be determined by utilizing a chromogenic reaction or a precipitation
reaction. Also, the
starting point of the collection may be determined by using detection methods
such as high
performance liquid chromatography. Preferably, the collection is terminated
when the volume
of the collection solution reaches 0.1 to 10 times the weight of the charged
plant raw material,
and more preferably, the collection is terminated when the volume of the
collection solution
reaches 0.2 to 5 times the weight of the charged plant raw material.
During the separation via the cation resin, fixed-bed ion exchange process may
be
adopted, and continuous ion exchange process may also be adopted. Preferably,
continuous ion
exchange process with higher degree of automation is used.
In order to improve the separation effect of the cation resin, it is also
possible to conduct
multiple separations via the cation resin, for example, 2 to 5 times of
separation.
Optionally, the collected eluate is separated via an anion resin. Upon
separation via the
anion resin, the anion resin is subjected to activation by being washed with
an alkaline solution,
an acidic solution and an alkaline solution successively after being packed
into the column.
Preferably, the anion resin is washed with an acidic solution until the pH of
the eluate is
3.0 to 7.0, preferably 4.5 to 6.5.
Preferably, the acidic solution is selected from hydrochloric acid solution,
phosphoric
acid solution, and disodium hydrogen phosphate-citric acid buffer, and more
preferably
disodium hydrogen phosphate-citric acid buffer. Preferably, the concentration
of the acidic
solution is 0.5 to 4 mol/L, preferably Ito 2 mol/L. When disodium hydrogen
phosphate-citric
acid buffer is used as the acidic solution, the pH value of the disodium
hydrogen
phosphate-citric acid buffer is preferably 4.0 to 6.5, and more preferably 4.5
to 5Ø
Preferably, the anion resin is washed with an alkaline solution until the pH
of the eluate is
8.0 to 9.5, preferably 8.5 to 9.5.
Preferably, the alkaline solution is ammonia solution, sodium hydroxide
solution,
potassium hydroxide solution or sodium carbonate solution, preferably sodium
hydroxide
solution. Preferably, the concentration of the sodium hydroxide solution is
0.5 to 4 mol/L,
preferably 1 to 2 mol/L.
Preferably, the anion resin is one or a combination of more selected from a
strongly
alkaline anion exchange resin, a weakly alkaline anion exchange resin and a
weakly acidic
anion exchange resin.
Preferably, the anion resin is one or a combination of more selected from 717-
type
strongly alkaline styrene-based anion exchange resin, 711-type strongly
alkaline styrene-based
anion exchange resin, D201-type macroporous and strongly alkaline styrene-
based anion
exchange resin, D218-type macroporous and strongly alkaline acrylic-based
anion exchange
7
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
resin, D301-G-type macroporous and weakly acidic styrene-based anion exchange
resin and
D301-type macroporous and weakly alkaline styrene-based anion exchange resin.
Preferably, the anion resin is 717-type strongly alkaline styrene-based anion
exchange
resin, D201-type macroporous and strongly alkaline styrene-based anion
exchange resin, and
D218-type macroporous and strongly alkaline acrylic-based anion exchange
resin.
Preferably, the amount of the anion resin used and the charged plant raw
material has a
weight ratio ranging from 1:1 to 1:80, preferably 1:1 to 1:64, and more
preferably 1:1 to 1:32.
Collection begins when the liquid flows out of the anion resin. Preferably,
the collection
is terminated when the volume of the collection solution reaches 0.05 to 10
times the weight of
the charged plant raw material, and more preferably, the collection is
terminated when the
volume of the collection solution reaches 0.1 to 5 times the weight of the
charged plant raw
material.
Optionally, in order to improve the separation effect of the anion resin, it
is also possible
to conduct multiple separations via the anion resin, for example, 2 to 4 times
of separation.
Preferably, the extraction method further comprises a step of concentrating
the crude
plant extraction solution prior to the separation in step 2).
Methods for concentrating the crude extraction solution include concentration
by heating,
concentration by nanofiltration membrane, concentration by reverse osmosis
membrane and
the combination thereof
Concentration is preferably conducted via concentration by heating,
concentration by
reverse osmosis membrane, or the combination thereof, so as to increase the
concentration of
the crude plant extraction solution.
Preferably, when using a reverse osmosis membrane and a nanofiltration
membrane to
conduct concentration, in order to improve the efficiency of the
concentration, purities may be
removed by conducting centrifugation, filtration via ultrafiltration membrane
or filtration via
microfiltration membrane prior to the concentration via reverse osmosis
membrane and
nanofiltration membrane.
Preferably, the crude plant extraction solution is concentrated until the mass
concentration of the solid content in the solution is 1% to 15%, preferably 2%
to 10%. The
solid content refers to the solid substance remained after the water in the
solution is removed.
Optionally, the concentrated crude extraction solution may also be subjected
to alcohol
precipitation prior to the resin separation in step 2). During the alcohol
precipitation, ethanol is
added to the crude extraction solution, the mixture is stirred and mixed
evenly, the stirring is
stopped, and the resultant is allowed to stand for a certain period of time to
precipitate the
insoluble matters therein. Preferably, ethanol is added to the crude plant
extraction solution, in
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
which the volume of ethanol is 0.2 to 20 times the volume of the crude plant
extraction
solution, preferably 0.4 to 10 times the volume of the crude plant extraction
solution. More
preferably, alcohol precipitation is conducted by using an alcohol
precipitation tank. Preferably,
the stirring speed in alcohol precipitation is 10 to 600 rpm, preferably 40 to
500 rpm, and more
preferably 80 to 400 rpm.
3) Concentrating the collection solution obtained in step 2)
The methods for concentration treatment in step 3) include concentration by
heating,
concentration by nanofiltration membrane, concentration by reverse osmosis
membrane and
the combination thereof. Preferably, purities may be removed by conducting
centrifugation,
filtration via ultrafiltration membrane or filtration via microfiltration
membrane prior to the
concentration via reverse osmosis membrane and nanofiltration membrane.
Preferably, the specific gravity of the concentrated liquid obtained in step
3) is 1.0 to 1.3.
The specific gravity refers to the mass ratio of the concentrated liquid to
water under the
conditions that the concentrated liquid and the water have the same volume.
4) Alcohol precipitation
In the alcohol precipitation in step 4), the concentrated solution of step 3)
is subjected to
treatment with ethanol. Specifically, ethanol is added to the concentrated
solution of step 3),
the mixture is stirred and mixed evenly, the stirring is stopped, and the
resultant is allowed to
stand for a certain period of time to precipitate the insoluble matters
therein.
Preferably, in step 4), the ethanol used for alcohol precipitation and the
charged plant raw
material has a weight ratio ranging from 1:4 to 1:600, preferably 1:20 to
1:300.
Alcohol precipitation is preferably conducted in alcohol precipitation tank.
Preferably, in the alcohol precipitation in step 4), the stirring speed is 10
to 600 rpm,
preferably 40 to 500 rpm, and more preferably 80 to 400 rpm.
5) Concentrating and drying
Optionally, the extraction method further comprises step 5) of concentrating
and drying.
The solution that has been subjected to alcohol precipitation is filtered to
remove the
insoluble matters, and concentrated under reduced pressure to obtain a plant
extract as an
extractum or dried to obtain a dry product.
In a second aspect, the present disclosure provides a plant extract obtained
according to
the above extraction method.
Preferably, the plant extract obtained according to the above extraction
method of the
present disclosure contains alkaloids with a weight content of 3% or more
(preferably contains
alkaloids with a weight content of 3% to 99%, more preferably contains
alkaloids with a
weight content of 15% to 99%, and further preferably contains alkaloids with a
weight content
9
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
of 45% to 99%, such as 35% to 70% or 60% to 75%), and/or contains
polysaccharides with a
weight content of no more than 70% (preferably contains polysaccharides with a
weight
content of 0.2% to 50%, and more preferably contains polysaccharides with a
weight content of
0.2% to 35%), and/or contains flavones with a weight content of no more than
10% (preferably
contains flavones with a weight content of 0.05% to 5%, and more preferably
contains flavones
with a weight content of 0.05% to 2%), and/or contains amino acids with a
weight content of
no more than 50% (preferably contains amino acids with a weight content of 0%
to 40%, and
more preferably contains amino acids with a weight content of 0% to 25%),
and/or other
components (with a weight content of preferably 0% to 25%, and more preferably
0% to 20%).
The total content of each component is 100%, wherein "each component" refers
to all
components in the plant extract including alkaloids, polysaccharides, flavones
and amino acids.
That is, other components are contained in the plant extract in addition to
alkaloids,
polysaccharides, flavones and amino acids.
Preferably, the plant extract obtained by the above extraction method of the
present
disclosure contains each component in the following weight ratios:
alkaloids 3% to 99%;
polysaccharides 0.2% to 70%;
flavones 0% to 10%;
amino acids 0% to 50%;
other components 0% to 25%.
Preferably, the plant extract obtained by the above extraction method of the
present
disclosure contains each component in the following weight ratios:
alkaloids 5% to 99%;
polysaccharides 0.2% to 50%;
flavones 0.05% to 5%;
amino acids 0% to 40%;
other components 0% to 20%.
Further preferably, the plant extract obtained by the above extraction method
of the
present disclosure contains each component in the following weight ratios:
alkaloids 30% to 99%;
polysaccharides 0.2% to 35%;
flavones 0.05% to 2%;
amino acids 0% to 25%;
other components 0% to 20%.
Preferably, the alkaloids contain 1-deoxynojirimycin (1-DNI) with a weight
content of
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
30% to 99%, preferably 50% to 95%, more preferably 55% to 90%, and more
preferably 60%
to 90%.
In a third aspect, the present disclosure provides a pharmaceutical
composition
comprising the above plant extract and an optional pharmaceutically acceptable
excipient.
The excipient is an inactive component that conforms to the administration
route or the
mode of administration and has no toxic effect on the human body.
The excipient may be a solid excipient or a liquid excipient. Solid
excipients, for
example, include sodium lactate, poloxamer, sodium dodecyl sulfate, sodium
carboxymethyl
cellulose, gelatin, xanthan gum, povidone, starch, magnesium stearate, sodium
carboxymethyl
starch and talc. Liquid excipients, for example, include water, ethanol, syrup
and glycerin.
Preferably, the dosage form of' the pharmaceutical composition includes a
preparation for
oral administration.
Preferably, the dosage form of the pharmaceutical composition includes a
tablet, a
capsule, an oral solution, an oral emulsion, a pill and a granule.
Individual difference may exist in terms of the specific dosage of
administration,
depending on the patient's age, body weight, health condition, diet,
administration route, drugs
used in combination, treatment period, and the like.
In a fourth aspect, the present disclosure provides the use of the above plant
extract or the
above pharmaceutical composition in preparation of a hypoglycemic drug.
In another aspect, the present disclosure provides the use of the above plant
extract or the
above pharmaceutical composition in preparation of a drug for treating
abnormal glucose
tolerance.
In yet another aspect, the present disclosure provides the use of the above
plant extract or
the above pharmaceutical composition in preparation of a drug for preventing
and/or treating a
disease related to abnormal blood glucose. The diseases include but are not
limited to diabetes,
diabetic nephropathy, diabetes foot, eye complications caused by diabetes,
hyperglycemia,
hyperuricemia, hyperlipidemia, alteration of intestinal flora, and
cardiovascular and
cerebrovascular diseases such as cerebral infarction, cerebral hemorrhage,
coronary heart
disease, and hypertension.
The present disclosure further provides the use of the above plant extract or
the above
pharmaceutical composition in preparation of a lipid-lowing drug.
The present disclosure further provides the use of the above plant extract or
the above
pharmaceutical composition in preparation of a drug for regulating intestinal
flora.
In still another aspect, the present disclosure provides a food, a health care
product or a
drink, comprising the above plant extract and an optional excipient acceptable
for food, health
11
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
care product or drink.
The present disclosure further provides the use of the above plant extract in
preparation
of a food, a health care product or a drink. Preferably, the food, the health
care product or the
drink is a food, a health care product or a drink with hypoglycemic effect.
The plant raw material of the present disclosure refers to the plant raw
material used for
extraction, including but not limited to fresh or processed plant or parts of
the plant.
In the present disclosure, the crude plant extraction solution is subjected to
treatment
steps of resin separation, concentration and alcohol precipitation, which has
the following
beneficial effects as compared with the conventional extraction methods.
1. The content of heavy metal is significantly reduced.
2. The weight of the ethanol used in the conventional water extraction and
alcohol
precipitation method is 1/4 to 5 times the weight of the charged plant raw
material, while the
amount of ethanol used in the extraction method of the present disclosure may
be as low as
1/600 of the weight of the charged plant raw material, which greatly reduces
the amount of
ethanol used, reduces the production cost to a certain extent, facilitates
industrial production,
and improves the safety of the production process.
BRIEF DESCRIPTION OF THE DRAWINGS
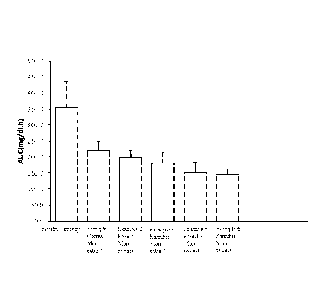
Figure 1 shows the sucrose tolerance test results of the plant extracts
obtained in
Examples 1, 2, 3, 5 and 6 in rats.
DETAILED DESCRIPTION
The present disclosure will be further described in detail with reference to
the
accompanying drawings and examples. The features and advantages of the present
disclosure
will become more clear and definite from these exemplary descriptions.
Herein the specific term "exemplary" means "used as an instance, or an
example, or
explanatory". Any "exemplary" example given here is not necessarily construed
as being
superior to or better than other examples.
In addition, the technical features involved in different embodiments of the
present
disclosure described below can be combined with one another as long as they
are not in
conflict with one another.
The present disclosure encompasses the following detection methods:
1. Content determination of alkaloids:
An appropriate amount of the extract was taken and added with water to
dissolve it by
ultrasound to prepare a test solution. Besides, an appropriate amount of 1-
deoxynojirimycin
12
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
was precisely weighed as a reference sample and added with water to dissolve
it to prepare a
reference solution. Appropriate volumes of the reference solution and the test
solution were
precisely measured respectively, added with sodium bicarbonate solutions, and
mixed well by
shaking. Thereafter, a solution of 9-tluorenylmethoxycarbonyl chloride (FM0C-
C1) in acetone
was added and heated at 30 C for 30 min. Acetic acid was added to terminate
the reaction. The
reactant was mixed well by shaking and filtered. A successive filtrate was
precisely drawn and
injected into a liquid chromatograph. According to the peak area, the content
of the
1-deoxynojirimycin and the content of the total alkaloids in the test sample
were calculated by
the external standard method (calculating chromatographic peaks with the
relative retention
time in the range of 0.4 to 1.7, in terms of 1-deoxynojirimycin) (reference
literature: Xuejun
XIA, Renyun WANG, Yuling LILT. "Determination of mulberry twig alkaloids by RP-
HPLC
with pre-column derivatization" p]. Chinese Journal of New Drugs, 2008,
17(23): 2044-2047).
2. Content determination of amino acids:
An appropriate amount of the extract was taken and added with water to
dissolve it by
ultrasound to prepare a test solution. Besides, an appropriate amount of mixed
amino acid was
precisely weighed as a reference sample, and added with water to dissolve it
to prepare a
reference solution. The remaining operations were the same as those of the
content
determination of alkaloids.
3. Content determination of polysaccharides:
An appropriate amount of the extract was precisely weighed, added with water,
extracted
by ultrasound, and centrifuged at 4000 rpm for 10 min. A supernatant was taken
as a test
solution. 2 ml of the above test solution was measured, and put in a test tube
with stopper, to
which 6 ml of 0.1% anthrone-sulfuric acid reagent was added. The test tube was
heated in a
boiling water bath for 15 min, and left in an ice water bath for 15 min. The
corresponding
reagent was taken as a blank. The absorbance value was immediately measured at
625 nm. The
concentration of the polysaccharides in the test sample relative to the
glucose was calculated
according to the glucose linear regression equation, and its content was
calculated according to
the following equation: Content=C*D*f/W, where W is the sample mass, C is the
polysaccharide concentration relative to the glucose, f is the conversion
factor (3.38), and D is
the dilution factor (reference literature: Zuofa ZHANG, Jie JIN, Liangen SET.
"Method for
content determination of polysaccharide in Rain ulus Mori", China Journal of
Chinese Materia
Medica [J]. 2018, 33(4): 462-464).
4. Content determination of tlavones:
An appropriate amount of the rutin reference sample was weighed, and dissolved
with
60% ethanol to produce a rutin reference stock solution. 0.5 ml, 1.0 ml, 3.0
ml, 5.0 ml, and 7.0
13
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
ml of the rutin reference stock solutions were precisely measured respectively
into 25-ml
volumetric flasks, to which 10 ml of 5% sodium nitrite solution, 10% aluminum
nitrate
solution, and IN NaOH solution were added respectively, and then diluted with
water to the
scale and mixed well by shaking as a reference solution. A blank reference
solution was used as
reference. The absorbance value was measured at 500 nm, and the linear
regression equation
was plotted.
An appropriate amount of the extract was precisely weighed, dissolved with 60%
ethanol
solution by ultrasound, mixed well by shaking, and centrifuged at 4000 rpm for
10 min.
Thereafter, a supernatant was taken as a flavone extracting solution. 2.0 ml
of the flavone
extracting solutions were precisely measured, to which 10 ml of 5% sodium
nitrite solution,
10% aluminum nitrate solution, and IN NaOH solution were added respectively,
then diluted
with water to the scale, mixed well by shaking, left for 15 min, and
centrifuged at 5000 rpm for
5 min. Afterwards, a supernatant was measured for determination. Another 2.0
ml of the
flavone extracting solution was precisely measured, and diluted only with
water to 25 ml as a
blank reference solution in the case of no color reaction. The absorbance
values of reaction
solutions were measured at 500 nm. The flavone concentrations were calculated
according to
the linear regression equation. Then the flavone content in the test sample in
terms of the rutin
was calculated according to the sample weight and the dilution multiple.
5. Content determination of heavy metals
The total content of the heavy metals was determined by the second method in
the
General Rules 0821 of Volume IV of the Chinese Pharmacopoeia 2015 Edition. The
specific
method was as follows:
(1) Preparation of Lead Standard Solution. 0.1599 g of lead nitrate was
weighed and put
in a 1000-m1 volumetric flask. After dissolution with with 5 ml of nitric acid
and 50 ml of
water, the solution was diluted with water to the scale and mixed well by
shaking as a stock
solution. 0.5 ml, 1 ml, 2 ml, 5 ml, and 8 ml of the stock solutions were
precisely measured
respectively into 5-ml volumetric flasks, diluted with water to the scale, and
mixed well by
shaking, thereby obtaining 5 ppm, 10 ppm, 20 ppm, 30 ppm, 40 ppm, 50 ppm, and
80 ppm of
lead standard solutions.
(2) Sample Assay. 2 g of the sample was taken, incinerated slowly until it was
completely
charred, cooled, wetted exactly with 0.5 to 1 ml of sulfuric acid, heated at a
low temperature
until the sulfuric acid was fully removed, added with 0.5 ml of nitric acid,
then evaporated to
dryness, cooled after nitric oxide vapor was fully removed, incinerated at 500
C to 600 C to
make it completely ashed, cooled, added with 2 ml of hydrochloric acid, and
evaporated to
dryness on water bath followed by adding 15 m1 of water. An ammonia solution
was added
14
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
dropwise until the phenolphthalein indicator solution became slightly pink,
and then 2 ml of
acetate buffer (pH3.5) was added. After the materials were dissolved with
gentle heat, the
solution was transferred to Nessler tubes and diluted with water to 25 ml as
test tubes. Another
reagent for formulating the test solution was taken and evaporated to dryness
in a porcelain
dish, then added with 2 ml of acetate buffer (pH3.5) and 15 ml of water,
dissolved with gentle
heat, thereafter transferred to Nessler tubes, to which a certain amount of
the lead standard
solution described in (1) was added respectively, and then diluted with water
to 25 ml as
reference tubes. Next, 2 ml of thioacetamide test solution was added to the
test tubes and the
reference tubes respectively, mixed well by shaking, left for 2 min, and
placed on white paper
at the same time. The test tubes and the reference tubes were observed in
perspective from top
to bottom. The color in the test tubes was compared with the color in the
reference tubes to
determine the content of heavy metals in the samples.
The content of the heavy metals lead, cadmium, mercury and arsenic could also
be
detected by the inductively coupled plasma mass spectrometry (ICP-MS method)
described in
the General Rules 0412 of Volume IV of the Chinese Pharmacopoeia 2015 Edition.
The detection results of the heavy metal content were compared with those
documented
in the Green Trade Standards of Medicinal Plants and Preparations for
Importing and
Exporting. The Standards stipulate that in plant materials, decoction pieces,
extracts and
preparations, the total content of heavy metals<20.0 mg/kg, Pb<5 mg/kg, Cd<0.3
mg/kg,
Hg<0.2mg/kg, Cu<20.0 mg/kg, and As<2.0 mg/kg.
The present inventors have compared the two methods for detecting heavy
metals. The
results show that the results of both methods are consistent, that is, the
heavy metal content of
the plant extracts obtained by the method of the present disclosure is in
compliance with the
provisions of the Green Trade Standards of Medicinal Plants and Preparations
for Importing
and Exporting. Preferably, the heavy metal content of the plant extracts
obtained by the method
of the present disclosure is not more than 20 ppm, more preferably not more
than 10 ppm, and
further preferably not more than 5 ppm.
Example 1
100 g of fresh mulberry (Morus alba L.) was taken and crushed, then added with
300 ml
of alcohol water in 2 times, and extracted by heating reflux for 1 h each
time. The extracting
solutions were combined, and filtered to remove insoluble matters, thereby
obtaining a crude
extraction solution. The crude extraction solution was determined to contain 5
to 10 ppm of
heavy metals, including 5.44 ppm of lead, 0.38 ppm of cadmium, 0.06 ppm of
mercury, and
0.47 ppm of arsenic. The crude extraction solution was concentrated by heating
until the solid
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
content reached 2%, kept at 25 C, and served as a loading solution for the
cation resin column.
g of 732-type strongly acidic styrene-based cation resin was filled in the
column,
washed with 2.5 mol/L hydrochloric acid solution until the pH of the eluate
was 3.5; washed
with 1.5 mol/L sodium hydroxide solution until the pH of the eluate was 8.0;
washed with 2.5
5 mot/
hydrochloric acid solution until the pH of the eluate was 3.5; and then rinsed
with 3
times column volume of deionized water to complete the activation. The
concentrated
extracting solution was loaded, and then eluted with 3L of 0.1 mol/L ammonia
water at an
elution speed of 10 BV/h. The dilate was collected when the effluent from the
cation resin
column was detected to be at pH > 7. When the collection solution was up to
IL, the
collection was stopped. The collection solution was purified directly over the
anion resin
column.
1.25 g of D218-type macroporous and strongly alkaline acrylic-based anion
resin was
filled in the column, washed with 1.5 mol/L sodium hydroxide solution until
the pH of the
eluate was 9.0; washed with 1.5 mol/L hydrochloric acid solution until the pH
of the eluate was
3.5; and washed with 1.5 mol/L sodium hydroxide solution until the pH of the
eluate was 9.0;
and the activation was completed. The eluate collected from the cation resin
was loaded onto
the anion resin. The effluent was collected and the collection was terminated
when the effluent
was up to IL.
The collection solution resulting from the separation via the anion resin
column was
centrifuged to remove impurities, and then concentrated through the reverse
osmosis
membrane. The specific gravity of the concentrated liquid was 1Ø It was
transferred to an
alcohol precipitation tank, and 25 g of anhydrous ethanol was added when the
stiffing paddle
was at 100 rpm. After adding the ethanol, the solution was stopped stirring,
and subjected to
alcohol precipitation for 24 h. The supernatant was taken and vacuum dried to
obtain an
extract.
In addition, fresh Ramulus Mori, Cortex Mori, and Folium Mori (114-orus alba
L.) were
prepared and extracted. The extraction method and parameters were as same as
those described
above. The heavy metal content in the crude extraction solutions obtained from
Ramulus Mori,
Cortex Mori, and Folium Mori was all 5 to 10 ppm, in which the lead content
was 5.51, 5.87,
and 6.12 ppm respectively, the cadmium content was 0.37, 0.35, and 0.41 ppm
respectively, the
mercury content was 0.07, 0.08, and 0.06 ppm respectively, and the arsenic
content was 0.57,
0.55, and 0.61 ppm respectively.
The content of the components and the content of the heavy metals in the
resulting
mulberry, Ramulus Mori, Cortex Mori, and Folium Mori extracts were listed in
Table 1.
Table 1 Content of Components and Content of Heavy Metals in Plant Extracts
16
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
Obtained in Example 1
Extract Mulberry Ramulus Cortex Folium
Content Extract Mori Mori Mori
Extract Extract
Extract
Alkaloids % 45 48 45 30
Polysaccharides % 28 25 27 34
Flavones % 5 4 6 7
Amino acid % 20 17 18 30
1-DNJ % in alkaloids 60 62 61 55
Total heavy metal content <5 <5 <5 <5
(PPnl)
Pb (ppm) 0.74 0.70 0.67 0.66
Cd (ppm) 0.05 0.05 0.04 0.04
Hg (ppm) 0.02 0.01 0.02 0.01
As (ppm) 0.17 0.12 0.13 0.15
Example 2
100 g of fresh Folium Mori (Mortis airopurpurea Rath) was taken and crushed,
then
added with 2000 ml of acid water in 2 times, and extracted by the decocting
method for 1 h
each time. The extracting solutions were combined, and filtered to remove
insoluble matters,
thereby obtaining a crude extraction solution. The crude extraction solution
was determined to
contain 10 to 20 ppm of heavy metals, including 13.6 ppm of lead, 0.84 ppm of
cadmium, 0.16
ppm of mercury, and 0.56 ppm of arsenic. The crude extraction solution was
centrifuged to
remove impurities and then concentrated by filtering through the reverse
osmosis membrane
until the solid content reached 14.5%. The concentrated crude extraction
solution was
transferred to an alcohol precipitation tank, and 80 g (about 100 ml) of
anhydrous ethanol was
added when the stirring paddle was at 300 rpm. After adding the ethanol, the
solution was
stopped stiffing, and subjected to alcohol precipitation for 24 h. The
supernatant was taken as a
loading solution for the cation resin column.
10 g of 734-type strongly acidic styrene-based cation resin was filled in the
column,
washed with 2 mon hydrochloric acid solution until the pH of the eluate was
4.5; washed with
1 mol/L sodium hydroxide solution until the pH of the eluate was 8.5; washed
with 2 moUL
17
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
hydrochloric acid solution until the pH of the eluate was 4.5; and then rinsed
with 5 times
column volume of deionized water to complete the activation. The extracting
solution after
concentration and alcohol precipitation was loaded, and then eluted with 2L of
0.5 mol/L
ammonia water at an elution speed of 8 BV/h. The eluate was collected when the
effluent from
the cation resin column was detected to be at pH > 7. When the collection
solution was up to
800 ml, the collection was stopped. The collection solution was purified
directly over the anion
resin column.
8 g of 717-type strongly alkaline styrene-based anion resin was filled in the
column,
washed with 1.5 mot/ sodium hydroxide solution until the pH of the eluate was
9.0; washed
with 1.5 mol/L hydrochloric acid solution until the pH of the dilate was 3.5;
and washed with
1.5 mol/L sodium hydroxide solution until the pH of the eluate was 9.0 to
complete the
activation. The eluate collected from the cation resin was loaded onto the
anion resin. The
effluent was collected and the collection was terminated when the effluent was
up to 750 ml.
The collection solution resulting from the separation via the anion resin
column was
concentrated by heating. The specific gravity of the concentrated liquid was
1.05. It was
transferred to an alcohol precipitation tank, and 12.5 g of anhydrous ethanol
was added when
the stirring paddle was at 200 rpm. After adding the ethanol, the solution was
stopped stirring,
and subjected to alcohol precipitation for 24 h. The supernatant was taken and
vacuum dried to
obtain an extract.
In addition, fresh Ramulus Mori and Cortex Mori (Morus atropuipurea Ravi))
were
prepared and extracted. The extraction method and parameters were as same as
those described
above. The heavy metal content in the crude extraction solutions obtained from
Ramulus Mori
and Cortex Mori was both 10 to 20 ppm, in which the lead content was 14.5 and
15.8 ppm
respectively, the cadmium content was 0.78 and 0.77 ppm respectively, the
mercury content
was 0.17 and 0.18 ppm respectively, and the arsenic content was 0.57 and 0.55
ppm
respectively.
The content of the components and the content of the heavy metals in the
resulting
Ramulus Mori, Cortex Mori, and Folium Mori extracts were listed in Table 2.
Table 2 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 2
Extract Ramulus Cortex Folium
Content Mori Mori Mori
Extract Extract Extract
Alkaloids % 80 75 60
18
CA 03149142 2022-2-23
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
Polysaccharides % 10 14 17
Flavones % 0.3 0.4 0.5
Amino acid % 5 6 18
1-DNJ % in alkaloids 75 69 70
Total heavy metal content <5 <5 <5
(ppm)
Pb (ppm) 0.65 0.73 1.13
Cd (ppm) 0.03 0.02 0.08
Hg (ppm) 0.01 0.02 0.09
As (ppm) 0.11 0.10 0.25
Example 3
1000 kg of fresh Ramulus Mori (Yuesang 11) was taken and crushed, then added
with
4000L of water, and extracted by heating reflux for 2 h. The extracting
solutions were
combined, and filtered to remove insoluble matters, thereby obtaining a crude
extraction
solution. The crude extraction solution was determined to contain 40 to 80 ppm
of heavy
metals, including 52 ppm of lead, 1.94 ppm of cadmium, 0.88 ppm of mercury,
and 1.11 ppm
of arsenic. The crude extraction solution was concentrated by heating until
the solid content
reached 4%, kept at 50 C, and served as a loading solution for the cation
resin column.
150 kg of D113-type macroporous and weakly acidic phenylpropene-based cation
resin
was filled in the column, washed with 2 mol/L hydrochloric acid solution until
the pH of the
eluate was 4.5; washed with 1 mol/L sodium hydroxide solution until the pH of
the eluate was
8.5; washed with 2 mol/L hydrochloric acid solution until the pH of the eluate
was 4.5; and
then rinsed with 5 times column volume of deionized water to complete the
activation. The
concentrated extracting solution was loaded, and then eluted with 1000L of 2.5
mol/L ammonia
.. water at an elution speed of 6 BV/h. The eluate was collected when the
effluent from the cation
resin column was detected to be at pH > 7. When the collection solution was up
to 900L, the
collection was stopped. The collection solution was purified directly over the
anion resin
column.
62.5 kg of D218-type macroporous and strongly alkaline acrylic-based anion
resin was
.. filled in the column, washed with 1.5 mol/L sodium hydroxide solution until
the pH of the
eluate was 9.0; washed with 1.5 mol/L hydrochloric acid solution until the pH
of the eluate was
3.5; and washed with 1.5 mol/L sodium hydroxide solution until the pH of the
eluate was 9.0 to
19
Date recue/Date received 2023-04-06
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
complete the activation. The eluate collected from the cation resin was loaded
onto the anion
resin. The effluent was collected and the collection was terminated when the
effluent was up to
870L.
The collection solution resulting from the separation via the anion resin
column was
filtered via a micro-filtration membrane to remove impurities, and then
concentrated through
the reverse osmosis membrane. The specific gravity of the concentrated liquid
was 1.1. It was
transferred to an alcohol precipitation tank, and 15 kg of anhydrous ethanol
was added when
the stirring paddle was at 400 rpm. After adding the ethanol, the solution was
stopped stirring,
and subjected to alcohol precipitation for 24 h. The supernatant was taken and
concentrated
under reduced pressure to obtain an extractum.
In addition, fresh Cortex Mori and Folium Mori (Yuesang 11) were prepared and
extracted. The extraction method and parameters were as same as those
described above. The
heavy metal content in the crude extraction solutions obtained from Cortex
Mori and Folium
Mori was both 40 to 80 ppm, in which the lead content was 48 and 53 ppm
respectively, the
.. cadmium content was 1.78 and 1.77 ppm respectively, the mercury content was
0.77 and 0.78
ppm respectively, and the arsenic content was 0.87 and 0.95 ppm respectively.
The content of the components and the content of the heavy metals in the
resulting
Ramulus Mori, Cortex Mori, and Folium Mori extractums were listed in Table 3.
Table 3 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 3
Extract Ramulus Cortex Folium
Content Mori Mori Mori
Extract Extract Extract
Alkaloids % 75 67 50
Polysaccharides % 15 20 27
Flavones % 0.7 0.8 3
Amino acid % 5 6 16
1-DNJ % in alkaloids 72 70 66
Total heavy metal content (ppm) <5 <5 <5
Pb (ppm) 2.71 2.65 2.60
Cd (ppm) 0.03 0.06 0.05
Date recue/Date received 2023-04-06
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
Hg (ppm) 0.24 0.10 0.12
As (pput) 0.72 0.45 0.50
Example 4
1000 kg of air dried Cortex Mori (Guisangyou 62) was taken and crushed, then
added
with 10000L of water in 2 times, and extracted by heating reflux for 2.5 h
each time. The
extracting solutions were combined, and filtered to remove insoluble matters,
thereby obtaining
a crude extraction solution. The crude extraction solution was determined to
contain less than 5
ppm of heavy metals, including 1.57 ppm of lead, 0.23 ppm of cadmium, 0.09 ppm
of mercury,
and 0.58 ppm of arsenic. The crude extraction solution was filtered via a
micro-filtration
membrane to remove impurities, and then concentrated through the reverse
osmosis membrane
until the solid content reached 6%, and served as a loading solution for the
cation resin column.
100 kg of D001-type macroporous and strongly acidic styrene-based cation resin
was
filled in the column. The cation resin was activated according to the method
described in
Example 3. The concentrated extracting solution was loaded, and then eluted
with 500L of 0.2
mol/L ammonium chloride at an elution speed of 5 BV/h. The effluent was
detected with 20%
silicotungstic acid, and started to collect when a white precipitate was
generated. The
collection was terminated when the collection solution reached 200L. The
collection solution
was purified directly over the anion resin column.
32 kg of D201-type macroporous and strongly alkaline styrene-based anion resin
was
filled in the column. The anion resin was activated according to the method
described in
Example 3. The eluate collected from the cation resin was loaded onto the
anion resin. The
effluent was collected and the collection was terminated when the effluent was
up to 100L.
The collection solution resulting from the separation via the anion resin
column was
concentrated by heating. The specific gravity of the concentrated liquid was
1.2. It was
transferred to an alcohol precipitation tank, and 3 kg of anhydrous ethanol
was added when the
stirring paddle was at 350 rpm. After adding the ethanol, the solution was
stopped stirring, and
subjected to alcohol precipitation for 24 h. The supernatant was taken and
concentrated under
reduced pressure to obtain an extractum.
In addition, air dried Ramulus Mori (Guisangyou 62) was prepared and
extracted.
The extraction method and parameters were as same as those described above.
The crude
extraction solution obtained from the Ramulus Mori contained 5 to 10 ppm of
heavy metals, in
which the lead content was 1.66 ppm respectively, the cadmium content was 0.25
ppm
respectively, the mercury content was 0.07 ppm respectively, and the arsenic
content was 0.60
ppm respectively.
21
Date recue/Date received 2023-04-06
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
The content of the components and the content of the heavy metals in the
resulting
Ramulus Mori and Cortex Mori extractums were listed in Table 4.
Table 4 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 4
Extract Ramulus Mori Cortex Mon
Content Extract Extract
Alkaloids % 65 58
Polysaccharides 16 20
Flavones % 0.7 0.5
Amino acid % 17 20
1-DNJ % in alkaloids 69 68
Total heavy metal content (ppm) <5 <5
Pb (ppm) 0.08 0.04
Cd (ppm) 0.04 0
Hg (ppm) 0.01 0.01
As (ppm) 0.21 0.16
Example 5
kg of fresh Ramulus Mori (Teyou 2) was taken and crushed, then added with
150L water in 2 times, and extracted by the decocting method for 3 h each
time. The extracting
solutions were combined, and filtered to remove the insoluble matters. The
extracting solution
was concentrated by heating until the solid content reached 8%. It was
transferred to an alcohol
10 precipitation tank. 2367.9 g of anhydrous ethanol (3L) was added when
the stirring paddle was
at 300 rpm. After adding the ethanol, the solution was stopped stirring, and
subjected to alcohol
precipitation for 24 h. The supernatant was taken as a loading solution for
the cation resin
column.
5 kg of 002SC-type strongly acidic styrene-based cation resin was filled in
the column.
The cation resin was activated according to the method described in Example 3.
The extracting
solution after concentration and alcohol precipitation was loaded, and then
eluted with 100L of
5 mol/L potassium chloride at an elution speed of 5 BV/h. The effluent was
detected with 20%
silicotungstic acid, and started to collect when a white precipitate was
generated. The
collection was terminated when the collection solution reached 25L. The
collection solution
22
Date recue/Date received 2023-04-06
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
was purified directly over the anion resin column.
kg of 711-type strongly alkaline styrene-based anion resin was filled in the
column.
The anion resin was activated according to the method described in Example 3.
The eluate
collected from the cation resin was loaded onto the anion resin. The effluent
was collected and
5 the
collection was terminated when the effluent was up to 15L. The collection
solution was
reloaded onto the cation resin, and separated twice via the cation resin and
anion resin in
sequence according to the methods described above.
The collection solution obtained after three column separation was centrifuged
to remove
impurities, and then concentrated through a reverse osmosis membrane. The
specific gravity
10 of the
concentrated liquid was 1.25. It was transferred to an alcohol precipitation
tank, and
125 g of anhydrous ethanol was added when the stirring paddle was at 1000 rpm.
After adding
the ethanol, the solution was stopped stirring, and subjected to alcohol
precipitation for 24 h.
The supernatant was taken and concentrated under reduced pressure to obtain an
extractum.
addition, fresh Cortex Mori and Folium Mori (Teyou 2) were prepared and
extracted.
The extraction method and parameters were as same as those described above.
The content of
the components and the content of the heavy metals in the resulting Ramulus
Mori, Cortex
Mori, and Folium Mori extractums were listed in Table 5.
Table 5 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 5
Extract Ramulus Cortex Folium
Content Mori Mori Mori
Extract Extract Extract
Alkaloids % 98 95 90
Polysaccharides % 0.2 2 4
Flavones % 0.05 0.1 0.1
Amino acid % 0 1 3
1-DNJ % in alkaloids 99 96 91
Total heavy metal content <5 <5 <5
(Pinn)
Pb (ppm) 0.06 0.05 0.07
Cd (ppm) 0 0 0
23
Date recue/Date received 2023-04-06
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
Hg (ppm) 0 0 0.01
As (ppm) 0.16 0.12 0.15
Example 6
1 kg of fresh mulberry root (Yuesang 11) was taken and crushed, then added
with 6L
alcohol water in 3 times, and extracted by ultrasonic extraction for 1 h each
time. The
extracting solutions were combined, and filtered to remove the insoluble
matters to obtain a
crude extraction solution. The crude extraction solution served as a loading
solution for the
cation resin column.
1 kg of D254-type macroporous and strongly alkaline quatemary ammonium-type
cation
resin was filled in the column. The cation resin was activated according to
the method
described in Example 3. The crude extraction solution was loaded, and then
eluted with 15L of
3 mol/L sodium chloride at an elution speed of 5 BV/h. The effluent was
detected with 20%
silicotungstic acid, and started to collect when a white precipitate was
generated. The
collection was terminated when the collection solution reached 5L. The
collection solution was
purified directly over the anion resin column.
1 kg of D301-type macroporous and weakly alkaline styrene-based anion resin
was filled
in the column. The anion resin was activated according to the method described
in Example 3.
The eluate collected from the cation resin was loaded onto the anion resin.
The effluent was
collected and the collection was terminated when the effluent was up to 5L.
The collection
solution was reloaded onto the cation resin, and separated again via the
cation resin and anion
resin successively according to the methods described above.
The collection solution resulting from two column separation was centrifuged
to remove
impurities, and then concentrated through a reverse osmosis membrane. The
specific gravity of
the concentrated liquid was 1.2. It was transferred to an alcohol
precipitation tank, and 6.3 g of
anhydrous ethanol was added when the stirring paddle was at 600 rpm. After
adding the
ethanol, the solution was stopped stirring, and subjected to alcohol
precipitation for 24 h. The
supernatant was taken and concentrated under reduced pressure to obtain an
extractum. In
addition, fresh Ramulus Mori and Folium Mori (Yuesang 11) were prepared and
extracted. The
extraction method and parameters were as same as those described above. The
content of the
components and the content of the heavy metals in the resulting Ramulus Mori,
Folium Mori,
and mulberry root extractums were listed in Table 6.
Table 6 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 6
24
Date recue/Date received 2023-04-06
CA National Phase of PCT/CN2020/110179 Our
Ref: 37761-37
(6149-2118529US)
Extract Ramulus Folium Mulberry
Content Mori Mori Root
Extract Extract Extract
Alkaloids % 97 85 92
Polysaccharides % 0.3 5 3
Flavones % 0.06 0.3 0.1
Amino acid 'Yo 0 5 2
1-DNJ % in alkaloids 98 83 95
Total heavy metal content <5 <5 <5
(1)Prn)
Pb (ppm) 0.05 0.03 0.05
Cd (ppm) 0 0 0
Hg (ppm) 0 0.02 0
As (ppm) 0.13 0.14 0.14
Example 7
1000 kg of fresh Ramulus Mori (Moms atropurpurea Roxb) was taken and crushed,
then
added with 11500L water, and extracted by heating reflux for 2 h. The
extracting solutions
were combined, and filtered to remove the insoluble matters to obtain a crude
extraction
solution. The crude extraction solution was centrifuged to remove impurities,
then concentrated
through a reverse osmosis membrane until the solid content reached 1%, and
served as a
loading solution for the cation resin column.
150 kg of D001-type macroporous and strongly acidic styrene-based cation resin
was
filled in the column. The cation resin was activated according to the method
described in
Example 3. The concentrated crude extraction solution was loaded, and eluted
with 5000L of
0.04 mol/L ammonium nitrate at an elution speed of 5 BV/h. The effluent was
detected with
20% silicotungstic acid, and started to collect when a white precipitate was
generated. The
collection was terminated when the collection solution reached 1000L.
The collection solution resulting from the cation resin column separation was
concentrated through a nanofiltration membrane. The specific gravity of the
concentrated
liquid was 1.3. It was transferred to an alcohol precipitation tank, and 1.7
kg of anhydrous
ethanol was added when the stirring paddle was at 600 rpm. After adding the
ethanol, the
CA 03149142 2022-2-23
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
solution was stopped stirring, and subjected to alcohol precipitation for 24
h. The supernatant
was taken and concentrated under reduced pressure to obtain an extractum.
In addition, fresh Cortex Mori and Folium Mori (Morus atropurpurea Roxb) were
prepared and extracted. The extraction method and parameters were as same as
those described
above. The content of the components and the content of the heavy metals in
the resulting
Ramulus Mori, Cortex Mori, and Folium Mori extractums were listed in Table 7.
Table 7 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 7
Extract Ramulus Cortex Folium
Content Mori Mori Mori
Extract Extract Extract
Alkaloids % 15 10 8
Polysaccharides % 40 42 45
Flavones % 0.7 0.8 0.6
Amino acid % 40 41 43
1-DNJ % in alkaloids 55 50 49
Total heavy metal content <5 <5 <5
(ppm)
Pb (ppm) 0.12 0.10 0.09
Cd (ppm) 0 0 0
1-1g (ppm) 0.02 0.02 0.01
As (ppin) 0.18 0.11 0.15
Example 8
1000 kg of fresh Ramulus Mori (Yuesang 11) was taken and crushed, then added
with
8000L water in 2 times, and extracted by the decocting method for 2 h each
time. The
extracting solutions were combined, and filtered to remove the insoluble
matters to obtain a
crude extraction solution. The crude extraction solution was filtered via a
micro-filtration
membrane to remove impurities, then concentrated through a reverse osmosis
membrane until
the solid content reached 1%, and served as a loading solution for the cation
resin column.
41.7 kg of 732-type strongly acidic styrene-based cation resin was filled in
the column.
The cation resin was activated according to the method described in Example 3.
The crude
26
Date recue/Date received 2023-04-06
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
extraction solution was loaded, and eluted with 1000L of 0.1 mol/L sodium
chloride at an
elution speed of 5 BV/h. The effluent was detected with 20% silicotungstic
acid, and started to
collect when a white precipitate was generated. The collection was terminated
when the
collection solution reached 500L.
The collection solution resulting from the cation resin column separation was
concentrated
via a nanofiltration membrane. The specific gravity of the concentrated liquid
was 1.25. It was
transferred to an alcohol precipitation tank, and 15 kg of anhydrous ethanol
was added when
the stirring paddle was at 600 rpm. After adding the ethanol, the solution was
stopped stirring,
and subjected to alcohol precipitation for 24 h. The supernatant was taken and
concentrated
under reduced pressure to obtain an extractum.
In addition, fresh Cortex Mori and Folium Mori (Yuesang 11) were prepared and
extracted. The extraction method and parameters were as same as those
described above. The
content of the components and the content of the heavy metals in the resulting
Ramulus Mori,
Cortex Mori, and Folium Mori extractums were listed in Table 8.
Table 8 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 8
Extract Ramulus Cortex Folium
Content Mori Mori Mori
Extract Extract Extract
Alkaloids % 10 8 5
Polysaccharides % 44 50 60
Flavones % 1 1.2 2
Amino acid % 41 37 30
1-DNJ % in alkaloids 54 45 46
Total heavy metal content <5 <5 <5
(PPin)
Pb (ppm) 0.55 0.30 043
Cd (ppm) 0.02 0.01 0.02
Fig (ppm) 0.03 0.01 0.01
As (ppm) 0.11 0.10 0.12
Example 9
27
Date recue/Date received 2023-04-06
CA National Phase of PCT/CN2020/110179 Our
Ref: 37761-37
(6149-2118529US)
100 g of fresh Ramulus Mori (Moms atropurpurea Roxb) was taken and crushed,
then
added with 600 ml water, extracted by heating reflux for 1 h, and filtered to
remove the
insoluble matters to obtain a crude extraction solution. The crude extraction
solution was first
concentrated by heating until the solid content reached 5%, and used as a
loading solution for
the cation resin column.
3.85 g of 732-type strongly acidic styrene-based cation resin was filled in
the column.
The cation resin was activated according to the method described in Example 3.
The crude
extraction solution was loaded, and eluted with 700 mL of 0.15 mol/L ammonium
chloride at
an elution speed of 5 BV/h. The effluent was detected with 20% silicotungstic
acid, and started
to collect when a white precipitate was generated. The collection was
terminated when the
collection solution reached 100 mL.
The collection solution resulting from the cation resin column separation was
concentrated
by heating. The specific gravity of the concentrated liquid was 1.3. It was
transferred to an
alcohol precipitation tank, and 25 g of anhydrous ethanol was added when the
stirring paddle
was at 600 rpm. After adding the ethanol, the solution was stopped stirring,
and subjected to
alcohol precipitation for 24 h. The supernatant was taken and concentrated
under reduced
pressure to obtain an extractum.
In addition, fresh Cortex Mori and Folium Mori (Moms atropurpurea Roxb) were
prepared and extracted. The extraction method and parameters were as same as
those described
above. The content of the components and the content of the heavy metals in
the resulting
Ramulus Mori, Cortex Mori, and Folium Mori extractums were listed in Table 9.
Table 9 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 9
Extract Ramulus Cortex Folium
Content Mori Mori Mori
Extract Extract Extract
Alkaloids ')/G 8 5 3
Polysaccharides % 45 48 50
Flavones % 1.5 2 3
Amino acid % 40 40 41
1-DNJ % in alkaloids 44 40 38
Total heavy metal content <5 <5 <5
28
CA 03149142 2022-2-23
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
(ppm)
Pb (ppm) 0.70 0.67 0.60
Cd (ppm) 0.01 0.02 0.01
Hg (ppm) 0.01 0.02 0.01
As (ppm) 0.14 0.10 0.11
Example 10
1000 kg of fresh Ramulus Mori (Teyou 2) was taken and crushed, then added
with 5000L water, extracted by heating reflux for 1 h, and filtered to remove
the insoluble
matters to obtain a crude extraction solution. The crude extraction solution
was first
concentrated by heating until the solid content reached 10%, and used as a
loading solution for
the cation resin column.
3.5 g of D254 macroporous and strongly alkaline quaternary ammonium-type
cation
resin was filled in the column. The cation resin was activated according to
the method
described in Example 3. The crude extraction solution was loaded, and then
eluted with 200L
of 0.15 mol/L potassium chloride at an elution speed of 5 BV/h. The effluent
was detected with
20% silicotungstic acid, and started to collect when a white precipitate was
generated. The
collection was terminated when the collection solution reached 100L.
The collection solution resulting from the cation resin column separation was
concentrated
by heating. The specific gravity of the concentrated liquid was 1.05. It was
transferred to an
alcohol precipitation tank, and 15 kg of anhydrous ethanol was added when the
stifling paddle
was at 600 rpm. After adding the ethanol, the solution was stopped stirring,
and subjected to
alcohol precipitation for 24 h. The supernatant was taken and concentrated
under reduced
pressure to obtain an extractum.
In addition, fresh Cortex Mori (Teyou 2) was prepared and extracted. The
extraction method and parameters were as same as those described above. The
content of the
components and the content of the heavy metals in the resulting Ramulus Mori,
Cortex Mori
extractums were listed in Table 10.
Table 10 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 10
Extract Ramulus Cortex
Content Mori Mori
Extract Extract
29
Date recue/Date received 2023-04-06
CA National Phase of PCT/CN2020/110179 Our Ref: 37761-37
(6149-2118529US)
Alkaloids % 5 3
Polysaccharides % 47 50
Flavones % 2 3
Amino acid % 41 41
1-DNJ % in alkaloids 47 43
Total heavy metal content <5 <5
(ppm)
Pb (ppm) 0.24 0.27
Cd (ppm) 0 0
Hg (ppm) 0.01 0.02
As (ppm) 0.12 0.14
Example 11
1000 g of fresh Ramulus Mori (Morus hornhycis Koidz.) was taken and crushed,
then
added with 10L acid water in 3 times, extracted by ultrasound for 2 h each
time, and filtered to
remove the insoluble matters to obtain a crude extraction solution. The crude
extraction
solution was filtered via a micro-filtration membrane to remove impurities,
then concentrated
through a reverse osmosis membrane until the solid content reached 4%, and
used as a loading
solution for the cation resin column.
66.67 g of D001-type macroporous and strongly acidic styrene-based cation
resin was
filled in the column. The cation resin was activated according to the method
described in
Example 3. The crude extraction solution was loaded, and then eluted with 12L
of 1.5 mon
ammonia water at an elution speed of 5 BV/h. The eluate was collected when the
effluent from
the cation resin column was detected by the high performance liquid
chromatography to
contain alkaloids. The collection was terminated when the collection solution
was up to 100
mL. The collection solution was purified directly over the anion resin column.
13.3 g of D218-type macroporous and strongly alkaline styrene-based anion
resin was
filled in the column. The anion resin was activated according to the method
described in
Example 3. The eluate collected from the cation resin was loaded onto the
anion resin column.
The effluent was collected and the collection was terminated when the effluent
was up to 50
ml.
The collection solution resulting from the anion resin column separation was
filtered via
a micro-filtration membrane to remove impurities and then concentrated through
a reverse
CA 03149142 2022-2-23
Application No. 3149142 Our
Ref: 37761-37
CA National Phase of PCT/CN2020/110179
(6149-2118529)
osmosis membrane. The specific gravity of the concentrated liquid was 1.15. It
was transferred
to an alcohol precipitation tank, and 25 g of anhydrous ethanol was added when
the stirring
paddle was at 600 rpm. After adding the ethanol, the solution was stopped
stirring, and
subjected to alcohol precipitation for 24 h. The supernatant was taken and
concentrated under
reduced pressure to obtain an extractum.
In addition, fresh Cortex Mori and Folium Mori (Morus bombycis Koidz.) were
prepared
and extracted. The extraction method and parameters were as same as those
described above.
The content of the components and the content of the heavy metals in the
resulting Ramulus
Mori, Cortex Mori, and Folium Mori extractums were listed in Table 11.
Table 11 Content of Components and Content of Heavy Metals in Plant Extracts
Obtained in Example 11
Extract Ramulus Cortex Folium
Content Mori Mori Mori
Extract Extract Extract
Alkaloids % 30 30 -- 15
Polysaccharides 31 34 -- 40
Flavones % 3 2 1
Amino acid % 30 28 39
1-DNJ % in alkaloids 58 55 53
Total heavy metal content <5 <5 <5
(PM)
Pb (ppm) 0.26 0.30 0.25
Cd (ppm) 0.01 0.02 0.01
Hg (ppm) 0.01 0 0.02
As (ppm) 0.11 0.12 0.13
Example 12
100 g of fresh Cortex Mori (Guisangyou 62) was taken and crushed, then added
with
1.2L alcohol water, extracted by the decocting method for 1 h, and filtered to
remove the
insoluble matters to obtain a crude extraction solution. The crude extraction
solution was first
concentrated by heating until the solid content reached 8%, and used as a
loading solution for
the cation resin column.
31
Date recue/Date received 2023-04-06
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
33.34 g of 734-type strongly acidic styrene-based cation resin was filled in
the column.
The cation resin was activated according to the method described in Example 3.
The crude
extraction solution was loaded, and then eluted with 50 mL of 2.5 mol/L
ammonia water at an
elution speed of 5BV/h. The eluate was collected when the effluent from the
cation resin
column was detected to be at pH > 7. The collection was terminated when the
collection
solution was up to 10 mL.
The collection solution resulting from the cation resin column separation was
centrifuged
to remove impurities, and then concentrated through the reverse osmosis
membrane. The
specific gravity of the concentrated liquid was 1.2. It was transferred to an
alcohol precipitation
tank, and 15 g of anhydrous ethanol was added when the stirring paddle was at
600 rpm. After
adding the ethanol, the solution was stopped stirring, and subjected to
alcohol precipitation for
24 h. The supernatant was taken and concentrated under reduced pressure to
obtain an
extractum.
In the Cortex Mori extractum, the alkaloid content was 15%, the polysaccharide
content
was 38%, the flavone content was 2%, and the amino acid content was 40%. In
the alkaloids,
the 1-DNJ content was 52%.
The total heavy metal content was less than 5 ppm, including 0.29 ppm of lead,
0 ppm of
cadmium, 0.02 ppm of mercury, and 0.10 ppm of arsenic.
Example 13
100 g of fresh Ramulus Mori (Morus alba L.) was taken and crushed, then added
with
300 ml alkaline water, extracted by heating reflux for 0.5 h, and filtered to
remove the
insoluble matters to obtain a crude extraction solution. The crude extraction
solution was
centrifuged to remove impurities, then concentrated through a reverse osmosis
membrane until
the solid content reached 6%, and used as a loading solution for the cation
resin column.
3.34 g of 732-type strongly acidic styrene-based cation resin was filled in
the column.
The cation resin was activated according to the method described in Example 3.
The crude
extraction solution was loaded, and then eluted with 3L of 1.0 mol/L ammonia
water at an
elution speed of 5 BV/h. The effluent was detected with 20% silicotungstic
acid, and started to
collect when a white precipitate was generated. The collection was terminated
when the
collection solution reached 400 mL.
The collection solution resulting from the cation resin column separation was
concentrated by heating. The specific gravity of the concentrated liquid was
1.25. It was
transferred to an alcohol precipitation tank, and 5 g of anhydrous ethanol was
added when the
stirring paddle was at 600 rpm. After adding the ethanol, the solution was
stopped stirring, and
32
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
subjected to alcohol precipitation for 24 h. The supernatant was taken and
concentrated under
reduced pressure to obtain an extractum.
In the resulting Ramulus Mori extractum, the alkaloid content was 3%, the
polysaccharide content was 60%, the tlavone content was 5%, and the amino acid
content was
30%. In the alkaloids, the 1-DNJ content was 47%.
The total heavy metal content was less than 5 ppm, including 0.31 ppm of lead,
0 ppm of
cadmium, 0.01 ppm of mercury, and 0.14 ppm of arsenic.
Example 14
100 g of fresh Folium Mori (Morus multicaulis Perrott.) was taken and crushed,
then
added with 500 ml of alcohol water, extracted by the decocting method for 0.5
h, and filtered to
remove the insoluble matters to obtain a crude extraction solution. The crude
extraction
solution was concentrated via a nanoffltration membrane until the solid
content reached 12%,
and used as a loading solution for the cation resin column.
25 g of 732-type strongly acidic styrene-based cation resin was filled in the
column. The
cation resin was activated according to the method described in Example 3. The
crude
extraction solution was loaded, and then eluted with 2L of 2.0 mol/L ammonia
water at an
elution speed of 5 BV/h. The eluate was collected when the effluent from the
cation resin
column was detected by the high performance liquid chromatography to contain
alkaloids. The
collection was terminated when the collection solution was up to 800 mL.
The collection solution resulting from the cation resin column separation was
concentrated by heating. The specific gravity of the concentrated liquid was
1.14. It was
transferred to an alcohol precipitation tank, and 5 g of anhydrous ethanol was
added when the
stirring paddle was at 600 rpm. After adding the ethanol, the solution was
stopped stirring, and
subjected to alcohol precipitation for 24 h. The supernatant was taken and
concentrated under
reduced pressure to obtain an extractum.
In the resulting Folium Mon extractum, the alkaloid content was 10%, the
polysaccharide content was 43%, the flavone content was 1%, and the amino acid
content was
37%. In the alkaloids, the 1-DNJ content was 50%.
The total heavy metal content was less than 5 ppm, including 0.33 ppm of lead,
0.01 ppm
of cadmium, 0.02 ppm of mercury, and 0.15 ppm of arsenic.
Example 15
100 g of fresh Hyacinthus orientalis (Hyacinthus orientalis) bulbs were taken
and
crushed, then added with 700 ml of water in 2 times, and extracted by
ultrasound for 0.5 h. The
33
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
extracting solutions were combined, and filtered to remove the insoluble
matters to obtain a
crude extraction solution. The crude extraction solution was concentrated via
a nanofiltration
membrane until the solid content reached 10%, and used as a loading solution
for the cation
resin column.
3.5 g of 732-type strongly acidic styrene-based cation resin was filled in the
column. The
cation resin was activated according to the method described in Example 3. The
concentrated
crude extraction solution was loaded, and eluted with 10 ml of 1.75 mat sodium
hydroxide
solution at an elution speed of 10 BV/h. The eluate was collected when the
effluent from the
cation resin column was detected to be at pH > 7. The collection was
terminated when the
collection solution was up to 10 ml. The collection solution was purified
directly over the anion
resin column.
4 g of D218-type macroporous and strongly alkaline styrene-based anion resin
was filled
in the column. The anion resin was activated according to the method described
in Example 3.
The eluate collected from the cation resin was loaded onto the anion resin.
The effluent was
collected and the collection was terminated when the effluent was up to 5 ml.
The collection solution resulting from the separation via the anion resin
column was
concentrated by heating. The specific gravity of the concentrated liquid was
1.1. It was
transferred to an alcohol precipitation tank, and 0.4 g of anhydrous ethanol
was added when the
stirring paddle was at 100 rpm. After adding the ethanol, the solution was
stopped stirring, and
subjected to alcohol precipitation for 24 h. The supernatant was taken and
vacuum dried to
obtain a Hyacinthus orientalis bulb extract.
In the Hyacinthus orientalis bulb extract, the alkaloid content was 3%, the
polysaccharide
content was 68%, the tlavone content was 2%, and the amino acid content was
25%.
In the alkaloids, the 1-DNJ content was 30%.
The total heavy metal content was less than 5 ppm, including 0.08 ppm of lead,
0.01 ppm
of mercury, and 0.15 ppm of arsenic, where cadmium was not detected.
Example 16
100 g of fresh Commelina communi (Commelina communi) leaf was taken and
crushed,
then added with 500 ml of alcohol water in 2 times, and extracted by heating
reflux for 1 h
each time. The extracting solutions were combined, and filtered to remove the
insoluble
matters. The extracting solution was concentrated by heating until the solid
content reached
10%, kept at 30 C, and used as a loading solution for the cation resin column.
3.5 g of 732-type strongly acidic styrene-based cation resin was filled in the
column. The
cation resin was activated according to the method described in Example 3. The
concentrated
extracting solution was loaded, and then eluted with 800 ml of 2.3 mol/L
ammonia water at an
34
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
elution speed of 10 BV/h. The eluate was collected when the effluent from the
cation resin
column was detected to be at pH > 7. =The collection was terminated when the
collection
solution was up to 300 ml.
The collection solution resulting from the cation resin column separation was
filtered via
an ultrafiltration membrane to remove purities, and then concentrated through
a reverse
osmosis membrane. The specific gravity of the concentrated liquid was 1.2. It
was transferred
to an alcohol precipitation tank, and 5 g of anhydrous ethanol was added when
the stirring
paddle was at 500 rpm. After adding the ethanol, the solution was stopped
stirring, and
subjected to alcohol precipitation for 24 h. The supernatant was taken and
vacuum dried to
obtain a Commelina communi leaf extract.
In the Commelina communi leaf extract, the alkaloid content was 10%, the
polysaccharide content was 270/0, the tlavone content was 100/0, and the amino
acid content was
50%.
In the alkaloids, the 1-DNJ content was 50%.
The total heavy metal content was less than 5 ppm, including 0.05 ppm of lead
and 0.17
ppm of arsenic, where cadmium and mercury were not detected.
Comparative Example 1
1000 kg of fresh Ramulus Mori (Morus serrata Roxb.) of the same batch as that
used in
Example 3 was taken, and subjected to crude extraction according to the method
described in
Example 3. The crude extraction solution was concentrated by heating. The
specific gravity of
the concentrated liquid was 1.1. It was transferred to an alcohol
precipitation tank, and 62 kg of
anhydrous ethanol was added when the stirring paddle was at 400 rpm. After
adding the
ethanol, the solution was stopped stirring, and subjected to alcohol
precipitation for 24 h. The
supernatant was taken and concentrated under reduced pressure to obtain a
Ramulus Mori
extractum. The alkaloid content was 15%, the polysaccharide content was 40%,
the flavone
content was 5.2%, and the amino acid content was 30%.
In the alkaloids, the 1-DNJ content was 55%.
The total heavy metal content was 30 to 40 ppm, including 29.11 ppm of lead,
1.50 ppm
of cadmium, 0.76 ppm of mercury, and 1.01 ppm of arsenic, in which the content
of the lead,
cadmium and mercury exceeded the content standards.
Without the cation resin column and anion resin column separation steps, this
comparative example exhibited a reduced alkaloid content, a remarkably
increased heavy metal
content, and a 3-fold increase in amount of ethanol, in comparison to Example
3.
35
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
Comparative Example 2
1000 kg of fresh Ramulus Mori (Vforus serrata Roxb.) of the same batch as that
used in
Example 3 was weighed, and subjected to crude extraction, concentration by
heating, and
separation via the cation resin and anion resin according to the method
described in Example 3.
870L of collection solution resulting from the anion resin column separation
was transferred to
an alcohol precipitation tank, and 135 kg of anhydrous ethanol was added when
the stirring
paddle was at 400 rpm. After adding the ethanol, the solution was stopped
stirring, and
subjected to alcohol precipitation for 24 h. The supernatant was taken and
concentrated under
reduced pressure to obtain a Ramulus Mori extractum. The alkaloid content was
62%, the
polysaccharide content was 18%, the flavone content was 1.1%, and the amino
acid content
was 12%.
In the alkaloids, the 1-DNJ content was 68%.
The total heavy metal content was 10 to 20 ppm, including 10.01 ppm of lead,
0.70 ppm
of cadmium, 0.44 ppm of mercury, and 0.83 ppm of arsenic, in which the content
of the lead,
cadmium and mercury exceeded the content standards.
Without the concentration step between the resin separation and the alcohol
precipitation,
this comparative example exhibited an increased heavy metal content and a 8-
fold increase in
amount of ethanol, in comparison to Example 3.
Comparative Example 3
1000 kg of fresh Ramulus Mori (Morus serra(a Roxb.) of the same batch as that
used in
Example 3 was weighed, and subjected to crude extraction, concentration by
heating, and
separation via the cation resin and anion resin according to the method
described in Example 3.
870L of the collection solution resulting from the anion resin column
separation was
concentrated under reduced pressure to obtain a Ramulus Mori extractum. The
alkaloid content
was 52%, the polysaccharide content was 22%, the flavone content was 0.8%, and
the amino
acid content was 20%.
In the alkaloids, the 1-DNJ content was 60%.
The total heavy metal content was 20 to 40 ppm, including 22.15 ppm of lead,
1.45 ppm
of cadmium, 0.65 ppm of mercury, and 0.89 ppm of arsenic, in which the content
of the lead,
cadmium and mercury exceeded the content standards.
Without the alcohol precipitation step after the resin separation, this
comparative
example exhibited a reduced alkaloid content and a remarkably increased heavy
metal content,
in comparison to Example 3.
36
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
Comparative Example 4
1000 kg of fresh Ramulus Mori (Vforus serrata Roxb.) of the same batch as that
used in
Example 3 was taken and crushed, then added with 4-times alcohol water
(4000L), and
extracted by heating reflux for 2 h. The extracting solutions were combined,
and filtered to
remove insoluble matters. The crude extraction solution was concentrated by
heating. The
specific gravity of the concentrated liquid was 1.1. It was transferred to an
alcohol precipitation
tank, and 62 kg of anhydrous ethanol was added when the stirring paddle was at
400 rpm. After
adding the ethanol, the solution was stopped stirring, and subjected to
alcohol precipitation for
24 h. The supernatant was taken and loaded onto the cation resin. According to
the method
described in Example 3, 150 kg of cation resin was filled in the column, and
subjected to cation
resin separation and anion resin separation. The collection solution resulting
from the anion
resin column separation was concentrated under reduced pressure to obtain a
Ramulus Mori
extractum. The alkaloid content was 51%, the polysaccharide content was 25%,
the flavone
content was 0.5%, and the amino acid content was 20%.
In the alkaloids, the 1-DNJ content was 55%.
The total heavy metal content was 10 to 20 ppm, including 11.11 ppm of lead,
0.82 ppm
of cadmium, 0.50 ppm of mercury, and 0.53 ppm of arsenic, in which the content
of the lead,
cadmium and mercury exceeded the content standards.
This comparative example carried out the alcohol precipitation prior to the
cation resin
separation step, and exhibited a reduced alkaloid content, an increased heavy
metal content,
and a 3-fold increase in amount of ethanol, in comparison to Example 3.
Comparative Example 5
1000 kg of fresh Ramulus Mori (Morus serrata Roxb.) of the same batch as that
used in
Example 3 was taken, and subjected to crude extraction and concentration by
heating according
to the method described in Example 3. The concentrated crude extraction
solution was directly
loaded onto the anion resin, and subjected to the anion resin separation
according to the method
described in Example 3. 3000L of effluent was collected. The collection
solution resulting from
the anion resin column separation was centrifuged to remove impurities, and
then concentrated
via a nanofiltration membrane. The specific gravity of the concentrated liquid
was 1.1. It was
transferred to an alcohol precipitation tank, and 46 kg of anhydrous ethanol
was added when
the stirring paddle was at 400 rpm. After adding the ethanol, the solution was
stopped stirring,
and subjected to alcohol precipitation for 24 h. The supernatant was taken and
concentrated
under reduced pressure to obtain a Ramulus Mori extractum.
In the Ramulus Mori extractum, the alkaloid content was 40%, the
polysaccharide
37
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179 Our
Ref: 37761-37
(6149-2118529US)
content was 35%, the flavone content was 1.5%, and the amino acid content was
22%.
In the alkaloids, the 1-DNJ content was 50%.
The total heavy metal content was 30 to 40 ppm, including 30.01 ppm of lead,
1.24 ppm
of cadmium, 0.21 ppm of mercury, and 0.85 ppm of arsenic, in which the content
of the lead,
cadmium and mercury exceeded the content standards.
Without the cation resin separation step, this comparative example exhibited a
reduced
alkaloid content, a remarkably increased heavy metal content, and a 2-fold
increase in amount
of ethanol, in comparison to Example 3.
Test Example 1 Stability Study
The Mulberry extract prepared in Example 1, the Folium Mori extracts prepared
in
Examples 2, 8, and 9, the Ramulus Mori extracts prepared in Examples 3 and 5-
7, and the
Cortex Mori extracts prepared in Examples 4 and 10 were sealed and packaged in
composite
film bags, then left for 24 months at a temperature of 25 C+2 C and a relative
humidity of
RH60 /0 10%, and thereafter the content of alkaloids therein was tested. The
results were listed
in Table 1 below.
Table 1:
Example Ex ample Example Example
Example Example Example Example Example
Content
Example
i% 2 3 4 5 6 7 8 9
10
1
Folium Ramulus Cortex Ramulus Ramulus Ramulus Folium Folium Cortex
Mulberry
Mori Moni Mori Mori Mori Mori
Moni Mori Mori
Items
Extract
Extract Extract Extract Extract Extract
Extract Extract Extract Extract
Total
44.8 59.5 74.3 57.2 97.2 96.7 14.11 4.9 2.9 2.9
alkaloids
1 -DNJ 59.4 68.8 71.3 66.9 911.1 97.5
54.9 45.7 37.7 42.8
It is clear from Table 1 that the plant extracts obtained by the extraction
method of the
present disclosure have good stability.
Test Example 2 Residual Organic Solvents
The gas chromatography was used to detect the residual resin, including n-
hexane,
methyl cyclohexane, divinylbenzene, toluene, benzene, xylene, and styrene. The
plant extracts
prepared in Examples 1 to 9 were tested, and none of them was detected to
contain residual
resin.
38
CA 03149142 2022-2-23
CA National Phase of PCT/CN2020/110179
Our Ref: 37761-37
(6149-2118529US)
Test Example 3 Efficacy Test
Normal male ICR mice were randomly divided into 6 groups (n=10) depending upon
body weight, and fasted overnight before test. One of the groups was orally
administered with
sucrose solutions (4.0 g/kg) as a control group (Normal), while the remaining
5 groups were
orally administered with sucrose as well as the Cortex Mori extract sample
prepared in
Example 1, the Folium Mori extract sample prepared in Example 2, and the
Ramulus Mori
extract samples prepared in Examples 3, 5, and 6 (10 mg/kg for each in terms
of the total
alkaloids) as administration groups. The blood glucose levels prior to the
administration (0 min)
and at 30 min, 60 min, and 120 min after administration were measured. The
time-blood
glucose curve was plotted, and the area under curve (AUC) of blood glucose was
calculated.
The results were as shown in FIG. 1.
The results indicate that the plant extracts obtained by the plant extraction
method of the
present disclosure result in a significant decrease in the elevation of blood
glucose in normal
mice after sucrose loading.
The present disclosure was explained above with reference to the preferred
embodiments,
which are, however, only exemplary and illustrative. On this basis, various
substitutions and
improvements can be made to the present disclosure, and all of them fall
within the scope of
protection for the present disclosure.
39
CA 03149142 2022-2-23